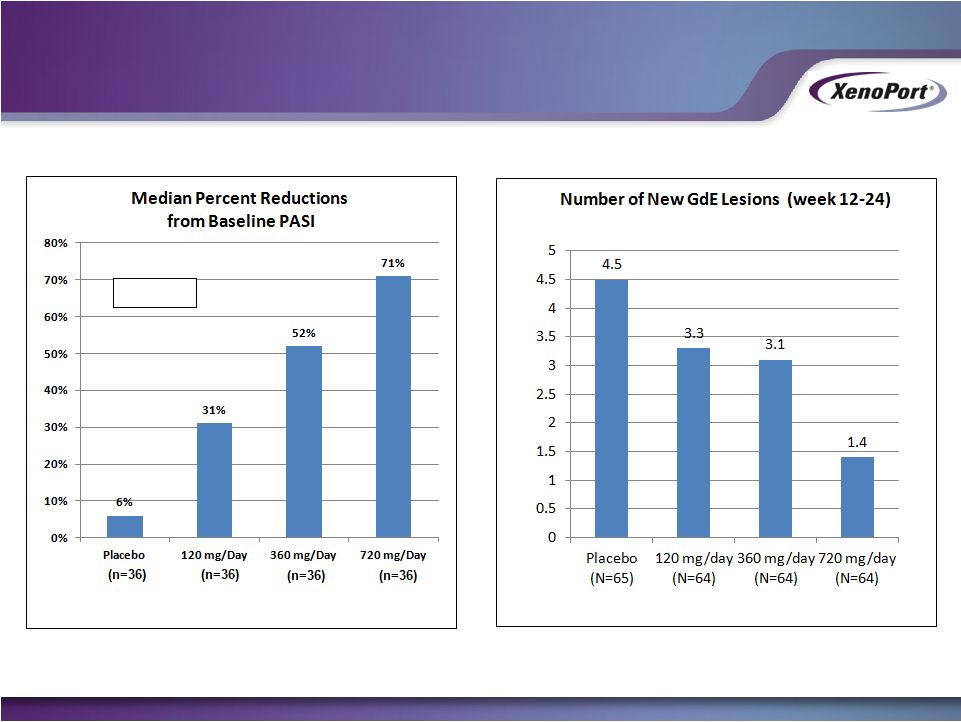
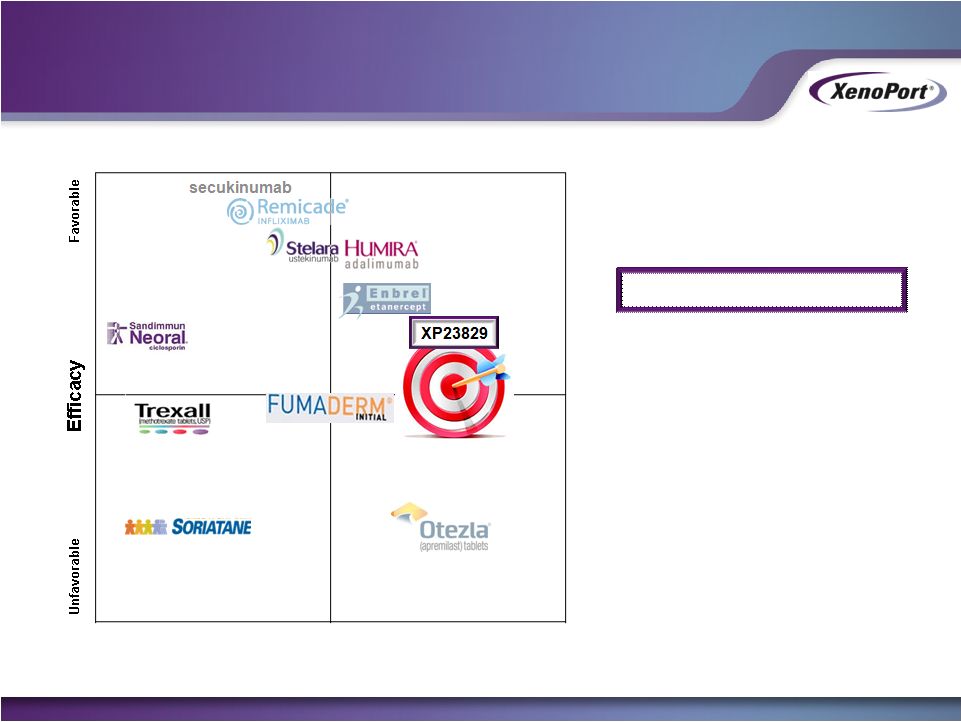
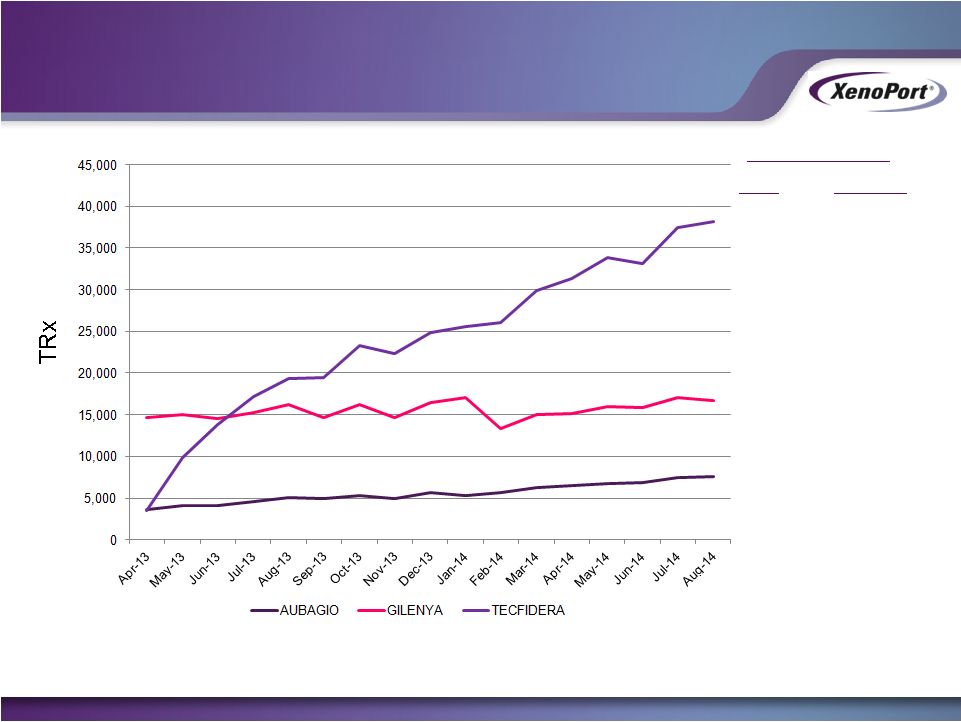
Free signup for more
- Track your favorite companies
- Receive email alerts for new filings
- Personalized dashboard of news and more
- Access all data and search results
Filing tables
XNPT similar filings
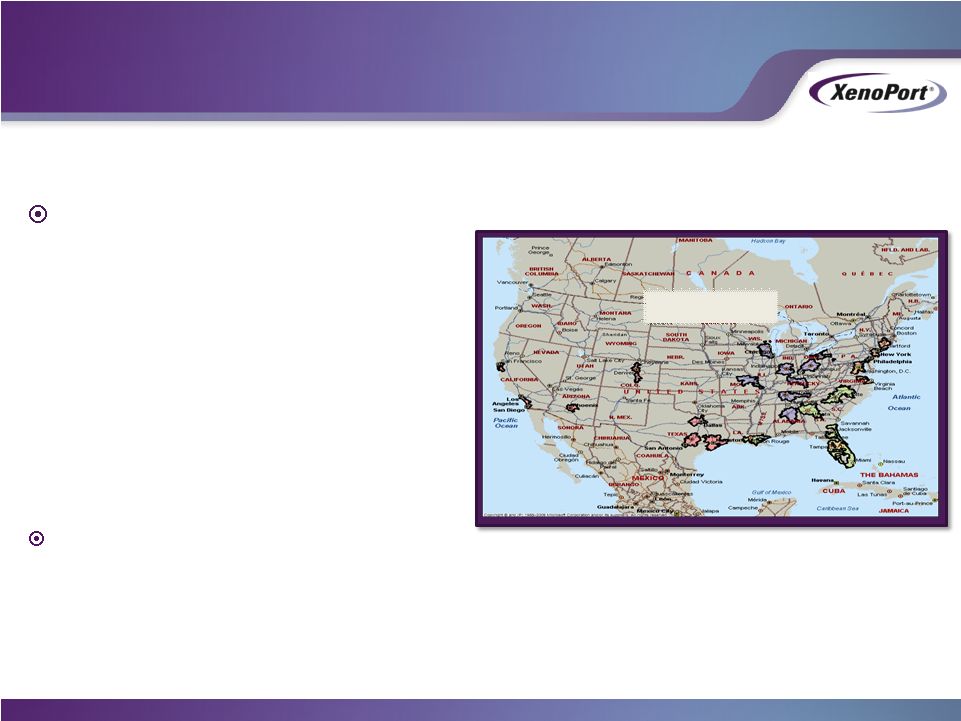
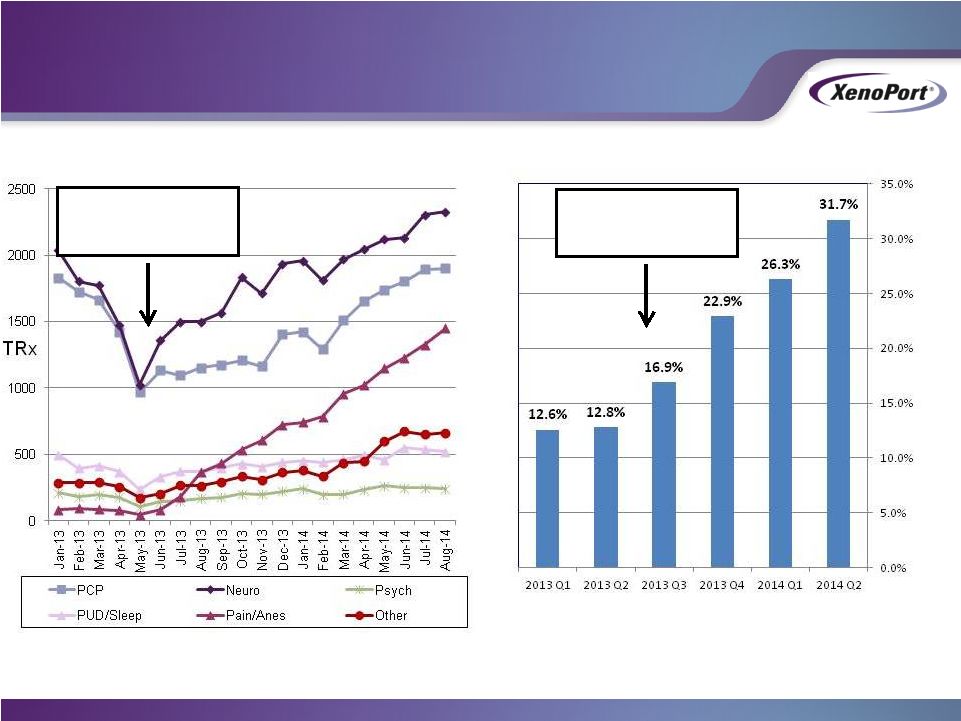
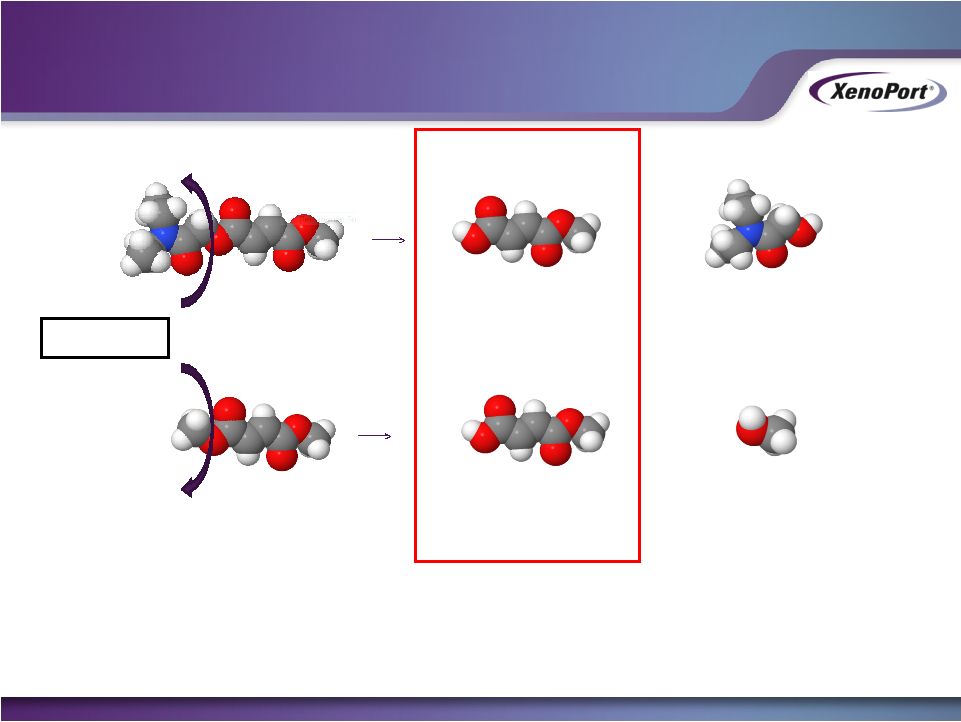
- 12 Jan 15 XenoPort Provides Highlights of HORIZANT Prescription Trends and Update on Development for Alcohol Use Disorder
- 26 Nov 14 Entry into a Material Definitive Agreement
- 4 Nov 14 XenoPort Reports Third Quarter Financial Results
- 16 Oct 14 Regulation FD Disclosure
- 6 Aug 14 XenoPort Reports Second Quarter Financial Results
- 24 Jun 14 Completion of Acquisition or Disposition of Assets
- 16 Jun 14 Departure of Directors or Certain Officers
Filing view
External links