MT103 (MEDI-538) Induces B-cell Depletion, Clearance of Bone Marrow Infiltration and Clinical Responses in Heavily Pre-treated NHL Patients: First Data from Phase I Dose-escalation Study MT103-104
R. C. Bargou1, P. Kufer2, P. Kirchinger2, R. Noppeney3, M. Schuler4, A. Viardot5, K. Weigang-Köhler6, F. Zettl7, C. Gerecke8, M. Libicher9, H. Einsele1, G. Riethmüller10, R. Lutterbüse2, M. Klinger2, P. A. Baeuerle2, B. Schlereth2, A. Wolf2, C. Reinhardt2
1Medizinische Klinik und Poliklinik II, Universitätsklinikum Würzburg;2Micromet, Inc., Carlsbad, CA, USA;3Klinik für Hämtologie, Essen, Germany;4III. Medizinische Klinik und Poliklinik, Mainz, Germany;5Universitätsklinikum Ulm, Germany;6Institut für medizinische Onkologie und Hämtologie, Nürnberg, Germany;7Medizinische Universität, Göttingen, Germany;8Medizinische Klinik für Hämatologie und Onkologie, Berlin, Germany;9Universitätsklinikum, Heidelberg, Germany;10Institut für Immunologie, München, Germany
Introduction
MT103/MEDI-538 is a murine recombinant single-chain antibody derivative that combines in one molecule the binding specificity for both the pan-B cell antigen CD19 and the epsilon chain of the T cell receptor/CD3 complex1-4.
MT103 is non-glycosylated and has a molecular weight of approximately 54 kDa. MT103 belongs to a series of compounds called BiTE®, which are designed to direct T cells against tumor cells5. The specific therapeutic action of MT103 results from target cell-specific cytotoxicity. Cytotoxic T cells are efficiently recruited via the anti-CD3 domain whereas malignant B cells are targeted by the highly specific interaction with CD19, a marker not expressed on tissues other than B cells.
MT103 is an extremely potent molecule with half-maximal target cell lysis in vitro in the 10 to 100 pg/mL range. As the cell binding affinities of MT103/MEDI-538 are demonstrated to be at least two orders of magnitude lower, the data indicate that binding of only a few molecules on target and effector cells is sufficient to induce cell death. Recent studies have shown that MT103-activated T cells are capable of serial tumor cell killing6, translating into activity at very low effector-target cell ratios. Cytolytic synapse formation is critical for BiTE® activity7.
Here we show first data from an ongoingPhase 1 dose escalations study with continuously infused MT103 in patients with refractory non-Hodgkin lymphoma (NHL).
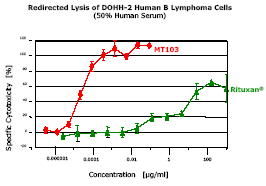
High Potency of Redirected Tumor Cell Lysisin vitro2
Results from a FACS-based cytotoxicity assay are shown. ADCC/CDC by Rituxan® and T-cell-mediated cytotoxicity by MT103 were assayed under identical conditions using unstimulated PBMC from the same healthy human donor. Human B lymphoma line DOHH-2 was used as target cells, which expresses both CD20 and CD19, the respective targets for Rituxan® and MT103. Fifty percent human serum was added to supply human complement for Rituxan® CDC activity. The difference in specific tumor cell lysis of Rituxan® and MT103, as defined by half-maximal cell lysis (ED50), was >100,000 fold. Error bars show standard deviations of triplicate determinations. The ED50 for redirected tumor cell lysis by MT103 was approximately 50 pg/ml.
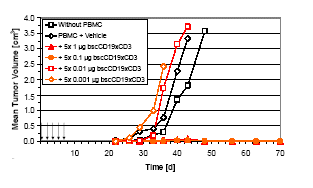
High Anti-tumor Activity of MT103 in SCID Mouse Model4
Cells of human pre-B lymphoma line NALM-6 were mixed with unstimulated human PBMC (1:1,000) and subcutaneously injected in NOD/SCID mice. Tumor outgrowth was observed after 25-30 days in the absence and presence of PBMC, and at doses of 1 and 10 ng MT103. Complete inhibition of tumor outgrowth was achieved at MT103 doses of 100 ng and 1 µg given i.v. for the first five days after tumor inculation (arrows). No relapses were observed in these two groups (N=8).
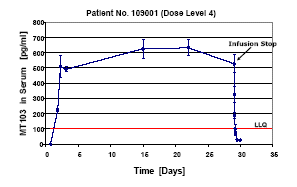
Steady-state Serum Levels of MT103 in Patients by Continuous Infusion
Patients with relapsed indolent lymphoma were treated in the phase I study MT103-104 according to a 3+3 dose escalation design with continuously infused MT103 for 4-8 weeks. As shown for one patient at dose level 4 (15 µg/m2/24 h), steady state serum levels of 500-600 pg/ml were achieved for the entire 4-week treatment period. A highly sensitive cell-based bioactivity assay was employed for determination of MT103 serum levels. LLQ: lower limit of quantitation (red line). Error bars give standard deviations of triplicate determinations.
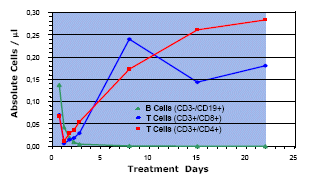
B Cell Depletion and T Cell Activation by MT103
All patients at dose level 4 in phase I study MT103-104 showed complete depletion of peripheral B and B lymphoma cells, and some patients at dose levels 2 (1.5 µg/m2/24 h) and 3 (5 µg/m2/24 h). A representative patient from dose level 4 is shown. Concomittant with B cell depletion an increase of both peripheral CD8- and CD4-positive T cell counts was observed in most cases. In vitro experiments have shown that both T cell populations can contribute to redirected lysis by MT103 and are induced to proliferate in response to MT103 when target cells are present. B cell and T cell counts in peripheral PBMC preparations of patients were determined by FACS analysis.
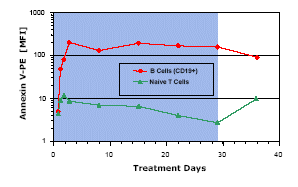
Apoptosis of B Lymphoma Cells Induced by MT103
A reason for B cell depletion in response to MT103 was investigated by staining B cells with the apopotosis marker annexin V (PE-labeled), followed by FACS analysis. While B cells showed a close to 100-fold increase in annexin V positivity, it only transiently doubled for naive T cells. A constantly high level of apoptosis of B cells was seen during the entire 4-week treatment with MT103 for this one patient.
References
| 1. | | Löffler et al. (2000),Blood95:2098. |
| |
| 2. | | Dreier et al. (2002),Intl J Cancer100:690 |
| |
| 3. | | Löffler et al. (2003),Leukemia17:900 |
| |
| 4. | | Dreier et al. (2003),J Immunol170:4397 |
| 5. | | Wolf et al. (2005),Drug Disc Today10:1237 |
| |
| 6. | | Hoffmann et al. (2005),Intl J Cancer115:98 |
| |
| 7. | | Offner et al. (2006),Mol Immunol43:763 |
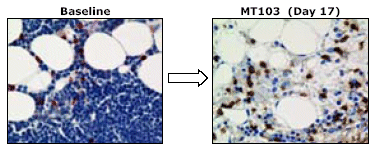
Clearance of Bone Marrow From Lymphoma Cells
Patient #1 at Dose Level 4 (15 µg/m2/24 hrs)
Previous treatments included CHOP, Chlorambucil, Fludarabine, Dexa-BEAM, Endoxan and repeated Rituxan
A bone marrow biopsy was obtained from one patient at dose level 4 and stained for B lymphoma (blue) and T cells (brown). Seventeen days following the beginning of continuous MT103 treatment, a second biopsy was taken and analyzed by immunohistochemistry.
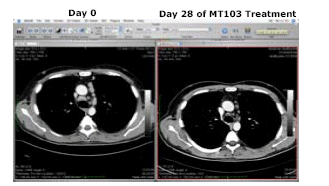
Clinical Efficacy by Single-agent MT103 Treatment
A computer tomography is shown for one patient at dose level 4 before treatment (day 0), and after a 4-week continuous infusion of MT103 (day 28). The reduction in tumor lesions was calculated as partial response according to standardized Cheson criteria.
| | | | | | | | | | | | | | | |
| Cohort | | Dose Level | | Disease | | Treatment | | Best Response | |
| | | | | | | Duration | | | | | | |
| 1 | | 0.5 µg/m2/24 | | Binet C Immunocytoma | | 4 Weeks | | | | SD | | | | |
| | | hours | | Stage IV A Mantle Cell | | 4 Weeks | | PD | | | | | | |
| | | | | Stage IV CLL | | 8 Weeks | | | | | | MR |
| 2 | | 1.5 µg/m2/24 | | Stage IV B Mantle Cell | | 8 Weeks | | | | SD | | | | |
| | | hours | | Stage IV B Mantle Cell | | 4 Weeks | | | | SD | | | | |
| | | | | Stage IV E Mantle Cell | | 23 Days | | PD | | | | | | |
| 2b | | 1.5 µg/m2/24 | | Stage IV A Mantle Cell | | 25 Days | | | | SD | | | | |
| | | hours | | Stage IV CLL | | 4 Weeks | | | | SD | | | | |
| | | | | Stage IV B Follicular | | 4 Weeks | | | | SD | | | | |
| 3 | | 5 µg/m2/24 | | Stage IV Mantle Cell | | 4 Weeks | | | | SD | | | | |
| | | hours | | Stage IV B Follicular | | 4 Weeks | | | | SD | | | | |
| | | | | Stage III Mantle Cell | | 4 Weeks | | | | SD | | | | |
| 4 | | 5 µg/m2/24 | | Stage III SLL/CLL | | 18 Days | | | | | | PR |
| | | hours on Day 1 | | Stage IV Mantle Cell | | 4 Weeks | | | | SD | | | | |
| | | | | Stage IV Follicular | | 8 Weeks | | | | | | PR |
| | | 15 µg/m2/24 | | Stage IV Follicular | | 8 Weeks | | | | | | CR | |
| | | hours from Day | | Stage IV A Marginal Zone | | 3 Days | | | | N/A* | | | | |
| | | 2 onward | | Stage II Follicular | | 4 Weeks | | PD | | | | | | |
| | | | | Stage II A Mantle Cell | | 1.5 Days | | | | N/A* | | | | |
Summary of the ongoing phase I study MT103-104.
PD: Progressive Disease
SD: Stable Disease
MR: Minimal Response
PR: Partial Response
CR: Complete Response
N/A: Not assessible because of too short treatment
Safety and Tolerability of Continuously Infused MT103
The most common adverse events (AEs) occuring in 7 or more patients were:
Leukopenia (14 pts.; 74%), lymphopenia (12 pts.; 63%), abnormal hepatic function (9 pts.; 47%), enzyme abnormality (9 pts.; 47%), pyrexia (8 pts.; 42%) diarrhea (7 pts.; 37%) and hyperglycemia (7 pts.; 37%).
Nineteen AEs were grade 3 or higher. The most common grade 3 or higher AEs occuring in 3 or more patients were:
Lymphopenia (12 pts.; 63%), leukopenia (9 pts.; 47%), neutropenia (3 pts.; 16%) and enzyme abnormality (3 pts.; 16%).
The most common serious adverse events (SAEs) were:
Lymphopenia (5 pts.; 21%), leukopenia (4 pts.; 21%) and overdose (3 pts.; 16%). To date, no anti-MT103 antibodies have been detected.
Conclusion
First results from ongoing phase I study MT103-104 indicate that low doses of continuously infused MT103/MEDI-538 as a single-agent therapy have clinical activity in heavily pre-treated NHL patients.