QuickLinks -- Click here to rapidly navigate through this documentAs filed with the Securities and Exchange Commission on August 26, 2003
Registration No. 333-
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
TolerRx, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | | 2834 | | 04-3522987 |
(State or other jurisdiction of
incorporation or organization) | | (Primary Standard Industrial
Classification Code Number) | | (I.R.S. Employer
Identification No.) |
300 Technology Square
Cambridge, Massachusetts 02139
(617) 452-1300
(Address, including zip code, and telephone number, including
area code, of registrant's principal executive offices) |
Douglas J. Ringler, V.M.D.
President and Chief Executive Officer
TolerRx, Inc.
300 Technology Square
Cambridge, Massachusetts 02139
(617) 452-1300
(Name, address, including zip code, and telephone number, including
area code, of agent for service) |
copies to: |
Julio E. Vega, Esq.
Bingham McCutchen LLP
150 Federal Street
Boston, Massachusetts 02110
Telephone: (617) 951-8000
Facsimile: (617) 951-8736 | | David W. Pollak, Esq.
Morgan, Lewis & Bockius LLP
101 Park Avenue
New York, New York 10178
Telephone: (212) 309-6000
Facsimile: (212) 309-6001 |
Approximate date of commencement of proposed sale to public:As soon as practicable after the effective date hereof.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act, check the following box. o
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration number of the earlier effective registration statement for the same offering. o
If delivery of the Prospectus is expected to be made pursuant to Rule 434, please check the following box. o
CALCULATION OF REGISTRATION FEE
|
Title of each class of
securities to be registered
| | Amount to
be registered
| | Proposed maximum
offering price
per share
| | Proposed maximum
aggregate
offering price(1)
| | Amount of
registration fee(2)
|
|---|
|
| Common Stock, $0.001 par value per share | | | | | | $75,000,000 | | $6,068 |
|
(1) Estimated solely for the purpose of calculating the amount of the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended.
(2) Calculated pursuant to Rule 457(a) based on an estimate of the proposed maximum aggregate offering price.
THE REGISTRANT HEREBY AMENDS THIS REGISTRATION STATEMENT ON SUCH DATE OR DATES AS MAY BE NECESSARY TO DELAY ITS EFFECTIVE DATE UNTIL THE REGISTRANT SHALL FILE A FURTHER AMENDMENT WHICH SPECIFICALLY STATES THAT THIS REGISTRATION STATEMENT SHALL THEREAFTER BECOME EFFECTIVE IN ACCORDANCE WITH SECTION 8(A) OF THE SECURITIES ACT OF 1933 OR UNTIL THIS REGISTRATION STATEMENT SHALL BECOME EFFECTIVE ON SUCH DATE AS THE COMMISSION, ACTING PURSUANT TO SAID SECTION 8(A), MAY DETERMINE.
Subject to completion August 26, 2003
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and we are not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
Preliminary prospectus
shares

Common stock
This is an initial public offering of shares of common stock by TolerRx, Inc. The estimated initial public offering price is between $ and $ per share.
We have applied for listing of our common stock on the Nasdaq National Market under the symbol TLRX.
|
|---|
| | Per share
| | Total
|
|---|
|
|---|
Initial public offering price |
|
$ |
|
|
$ |
|
Underwriting discounts and commissions |
|
$ |
|
|
$ |
|
Proceeds to TolerRx, before expenses |
|
$ |
|
|
$ |
|
|
The underwriters may also purchase up to shares of common stock from us at the public offering price, less the underwriting discounts and commissions, within 30 days from the date of this prospectus. The underwriters may exercise this option only to cover over-allotments, if any. If the underwriters exercise this option in full, the total underwriting discounts and commissions will be $ , and our total proceeds, before expenses, will be $ .
The underwriters are offering our common stock as set forth under "Underwriting." Delivery of the shares will be made on or about , 2003.
Investing in our common stock involves a high degree of risk. See "Risk factors" beginning on page 5.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
Joint Book-Running Managers
| JP Morgan | | SG Cowen |
Leerink Swann & Company
|
, 2003
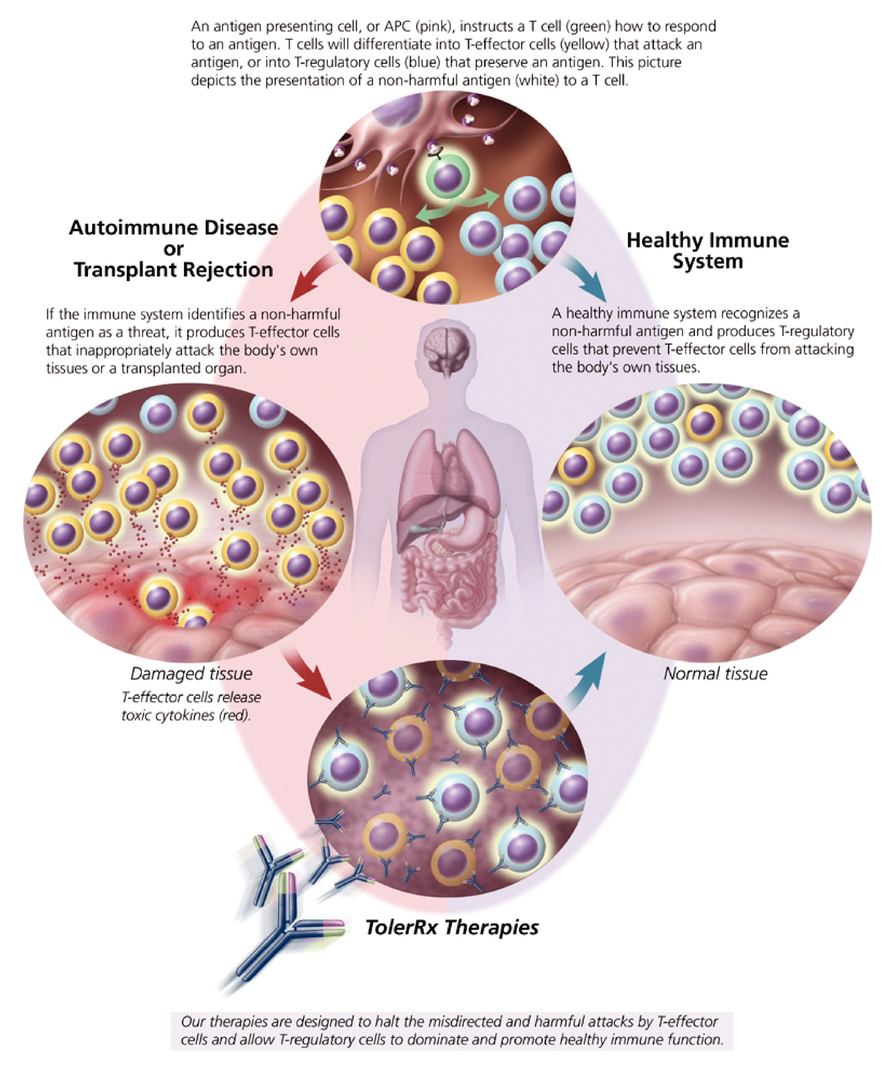
You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with different information. We are not making an offer of these securities in any state where the offer is not permitted. You should not assume that the information provided by this prospectus is accurate as of any date other than the date on the front of this prospectus.
Through and including (the 25th day after the date of this prospectus), all dealers that buy, sell, or trade our common stock, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to the dealers' obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
Table of contents
| | Page
|
|---|
| Prospectus summary | | 1 |
| Risk factors | | 5 |
| Special note regarding forward-looking statements | | 18 |
| Use of proceeds | | 19 |
| Dividend policy | | 19 |
| Capitalization | | 20 |
| Dilution | | 21 |
| Selected consolidated financial data | | 22 |
Management's discussion and analysis of
financial condition and results of operations | | 23 |
| Business | | 34 |
| Management | | 51 |
| Related party transactions | | 59 |
| Principal stockholders | | 61 |
| Description of capital stock | | 63 |
| Shares eligible for future sale | | 67 |
Certain U.S. federal income tax considerations
for Non-U.S. Holders of our common stock | | 70 |
| Underwriting | | 73 |
| Legal matters | | 76 |
| Experts | | 76 |
| Where you can find additional information | | 76 |
| Index to consolidated financial statements | | F-1 |
The names "TolerMab", "TolerRx", and the TolerRx logo are our trademarks. All other trademarks or tradenames referred to in this prospectus are the property of their respective owners.
i
Prospectus summary
This summary highlights selected information contained elsewhere in this prospectus. This summary does not contain all the information you should consider before investing in shares of our common stock. You should read this entire prospectus carefully, including "Risk factors" beginning on page 5 and our consolidated financial statements and the notes to those financial statements beginning on page F-1, before making an investment decision.
Our business
We are a biopharmaceutical company focused on the discovery, development, and commercialization of novel therapies to treat patients with diseases of the immune system. Our therapies are based upon a unique understanding of the way the immune system recognizes and avoids attacking the body's own tissues and proteins, as well as other antigens that are not harmful to the body. This state of non-aggressive responsiveness to an antigen is called immunological tolerance and is a natural part of a properly functioning immune system. Therapies that induce immunological tolerance represent a fundamentally new approach to treating and potentially curing autoimmune diseases, organ transplant rejection, and other conditions associated with adverse or undesirable immune responses.
We have established a pipeline of five products, two in human clinical trials and three in preclinical development. Our most advanced products are:
• TRX4
Our TRX4 monoclonal antibody is in an investigator-sponsored Phase II clinical trial of 80 patients in Europe with new-onset Type I diabetes. All of the patients have completed treatment and are currently in an 18-month follow-up phase. We plan to file an Investigational New Drug application for TRX4 with the U.S. Food and Drug Administration first for psoriasis and psoriatic arthritis by early 2004, and later for additional studies in new-onset Type I diabetes based on the results of the ongoing European trial; and
• TRX1
Our TRX1 monoclonal antibody is being developed in collaboration with Genentech to induce tolerance in transplantation, autoimmune diseases, and clinical situations where the immune system attacks therapeutic proteins or biologic drugs. In July 2003, we completed a Phase I clinical trial in the United Kingdom, which demonstrated that TRX1 was generally well tolerated.
We also have ongoing research and development programs focused on generating new monoclonal antibody and small molecule products for the treatment of immunological diseases.
In addition, we have developed our TolerMab antibody technology, which is intended to enable us to modify antibodies to induce immunological tolerance to themselves. One of the limitations with current monoclonal antibody therapies is that they can trigger an immune system response which renders such therapies ineffective. We believe that our TolerMab technology will improve the side effect profile and enhance the long-term effectiveness of antibody therapy to treat patients with chronic disease. We are evaluating two TolerMab antibodies in preclinical development.
1
Our strategy
Our goal is to become the recognized leader in the discovery, development, and commercialization of products that treat patients with diseases of the immune system, such as Type I diabetes, psoriasis, psoriatic arthritis, multiple sclerosis, rheumatoid arthritis, transplant rejection, and immune system neutralization of therapeutic proteins. Our strategy to achieve this goal is to:
• develop and commercialize our lead products to provide breakthrough therapies that induce immunological tolerance;
• generate new products for our pipeline based on our understanding of T-regulatory cells;
• leverage collaborative partnerships;
• retain commercialization rights to certain products;
• exploit knowledge of immunological tolerance to optimize clinical development; and
• commercialize the TolerMab antibody technology internally and through partners.
Collaboration with Genentech
In December 2002, we entered into a collaboration agreement with Genentech for the development and commercialization of our TRX1 monoclonal antibody. To date, we have received an aggregate of $8.0 million from Genentech in the form of a license fee, an equity investment, and a milestone payment. We may receive up to approximately $80 million in additional payments if all development and regulatory approval milestones for TRX1 in multiple disease indications are achieved. If TRX1 receives marketing approval, we will be entitled to receive royalties on worldwide net sales. In addition, in lieu of receiving royalties on sales of TRX1 in the United States, we have the option to participate in a cost and profit sharing relationship with Genentech and to support the commercialization of TRX1 through our own medical science liaisons who would educate the medical profession about our product. For further information regarding our collaboration with Genentech, including any payments thereunder, see "Risk factors" on page 7 and "Business" on page 34.
Corporate information
We were formed on July 6, 2000, as a Delaware corporation. Our principal executive offices are located at 300 Technology Square, Cambridge, Massachusetts 02139, and our telephone number is (617) 452-1300. Our web site address is www.tolerrx.com. Information contained on our web site is not a part of this prospectus.
2
The offering
| Common stock offered by TolerRx: shares | | |
Common stock to be outstanding after this offering: shares |
|
|
Use of proceeds |
|
|
We intend to use the proceeds from this offering for research and development activities relating to drug discovery and development programs, our collaboration with Genentech, and other general corporate and working capital purposes. See "Use of proceeds." |
Proposed Nasdaq National Market symbol: TLRX |
|
|
The number of shares of our common stock that will be outstanding after this offering is based on the number of shares outstanding as of June 30, 2003, unless otherwise indicated herein, without taking into consideration a reverse stock split which we intend to effect prior to this offering, assumes the conversion of all outstanding shares of our redeemable convertible preferred stock into common stock on a one-for-one basis, and excludes:
• 956,750 shares of our common stock issuable upon the exercise of stock options outstanding as of June 30, 2003 at a weighted average exercise price of $0.15 per share, of which options to purchase 42,750 shares of our common stock were then exercisable;
• 311,250 shares of our common stock issuable upon the exercise of warrants to purchase common stock at a weighted average exercise price of $0.81 per share; and
• 2,706,000 shares of our common stock reserved for future grant under our 2000 Equity Incentive Plan, as amended.
Unless specifically stated otherwise, the information in this prospectus:
• assumes no exercise of the underwriters' over-allotment option;
• assumes an initial public offering price of $ per share, the midpoint of the initial public offering price range indicated on the cover of this prospectus;
• reflects the automatic conversion of all shares of our redeemable convertible preferred stock into 61,035,000 shares of our common stock upon the completion of this offering;
• does not reflect a reverse stock split which we intend to effect prior to this offering; and
• reflects the filing, prior to the completion of this offering, of our restated certificate of incorporation, referred to in this prospectus as our certificate of incorporation, and the adoption of our amended and restated by-laws, referred to in this prospectus as our by-laws, implementing the provisions described under "Description of capital stock."
3
Summary consolidated financial information
The following table contains our summary consolidated financial information. You should read this information together with our consolidated financial statements and related notes to those statements, "Selected consolidated financial data," and "Management's discussion and analysis of financial condition and results of operations" included elsewhere in this prospectus.
Pro forma per share amounts in the table below reflect the conversion of all issued and outstanding shares of our redeemable convertible preferred stock into common stock as if the shares had been converted immediately upon their issuance.
| |
|---|
| |
| | Year ended
December 31,
| | Six months ended
June 30,
| |
|---|
| | Inception
through
December 31,
2000
| |
|---|
(in thousands, except share
and per share data)
| |
|---|
| | 2001
| | 2002
| | 2002
| | 2003
| |
|---|
| |
|---|
| Statement of operations data: | | | | | | | | | | | | | | | | |
| Revenues | | $ | — | | $ | — | | $ | 27 | | $ | — | | $ | 750 | |
| Operating expenses | | | | | | | | | | | | | | | | |
| | Research and development | | | 232 | | | 3,797 | | | 10,908 | | | 4,883 | | | 5,236 | |
| | General and administrative | | | 125 | | | 1,370 | | | 2,774 | | | 1,330 | | | 1,556 | |
| | Stock-based compensation | | | 9 | | | 627 | | | 613 | | | 432 | | | 607 | |
| | |
| |
| | | Total operating expenses | | | 366 | | | 5,794 | | | 14,295 | | | 6,645 | | | 7,399 | |
| | |
| |
| | | Loss from operations | | | (366 | ) | | (5,794 | ) | | (14,268 | ) | | (6,645 | ) | | (6,649 | ) |
| Interest income | | | — | | | 165 | | | 250 | | | 139 | | | 215 | |
| Interest expense | | | (1 | ) | | (40 | ) | | (295 | ) | | (117 | ) | | (156 | ) |
| | |
| |
| Net loss | | | (367 | ) | | (5,669 | ) | | (14,313 | ) | | (6,623 | ) | | (6,590 | ) |
| Accretion of redeemable convertible preferred stock | | | (10 | ) | | (737 | ) | | (2,224 | ) | | (934 | ) | | (2,411 | ) |
| |
| |
|---|
| Net loss attributable to common stockholders | | $ | (377 | ) | $ | (6,406 | ) | $ | (16,537 | ) | $ | (7,557 | ) | $ | (9,001 | ) |
| |
| |
|---|
| Net loss attributable to common stockholders per share, basic and diluted | | $ | (1.52 | ) | $ | (8.91 | ) | $ | (9.33 | ) | $ | (5.19 | ) | $ | (3.92 | ) |
| |
| |
|---|
| Pro forma net loss per share, basic and diluted | | | | | | | | $ | (0.51 | ) | | | | $ | (0.10 | ) |
| |
| |
|---|
| Shares used in computing net loss per share, basic and diluted | | | 248,119 | | | 719,094 | | | 1,773,180 | | | 1,455,871 | | | 2,295,342 | |
| |
| |
|---|
| Shares used in computing pro forma net loss per share, basic and diluted | | | | | | | | | 27,903,802 | | | | | | 63,330,342 | |
| |
The following table is a summary of our balance sheet as of June 30, 2003:
• on an actual basis;
• on a pro forma basis to give effect to the automatic conversion of all outstanding shares of our redeemable convertible preferred stock into common stock upon the closing of this offering; and
• on a pro forma as adjusted basis to give effect to the sale of the shares of common stock offered by this prospectus at an assumed initial public offering price of $ per share, the midpoint of the range set forth on the cover of this prospectus, and after deducting underwriting discounts and commissions and estimated offering expenses.
|
|---|
June 30, 2003
(in thousands)
| | Actual
| | Pro forma
| | Pro forma
as adjusted
|
|---|
|
| Balance sheet data: | | | | | | | | | |
| Cash and cash equivalents | | $ | 35,628 | | $ | 35,628 | | $ | |
| Working capital | | | 31,178 | | | 31,178 | | | |
| Total assets | | | 42,593 | | | 42,593 | | | |
| Long-term debt, net of current portion | | | 486 | | | 486 | | | |
| Redeemable convertible preferred stock | | | 63,110 | | | — | | | |
| Accumulated deficit | | | (29,583 | ) | | (29,583 | ) | | |
| Stockholders' equity (deficit) | | | (30,328 | ) | | 32,782 | | | |
|
4
Risk factors
An investment in our common stock involves significant risks. Before making an investment decision, you should carefully consider the following risk factors in addition to the other information in this prospectus. The risks and uncertainties described are not the only ones facing us. Our business, results of operations, and financial condition may be materially and adversely affected by any of the following risks.
Risks related to our business
We have a history of substantial net losses and cannot predict when we will become profitable, if at all.
We were incorporated in July 2000 and have a limited operating history upon which an investor may evaluate our operations and future prospects. We have incurred net losses since our inception, including net losses of $14.3 million for the year ended December 31, 2002, and $6.6 million for the six months ended June 30, 2003. As of June 30, 2003, we had an accumulated deficit of $29.6 million. We expect to continue to incur significant operating losses in future periods and cannot predict when we will become profitable, if at all. We expect to make substantial expenditures to further our drug discovery and development programs. We expect that our rate of spending will accelerate as a result of the increased costs and expenses associated with our collaboration with Genentech and with our internal drug discovery and development programs. In the event that we exercise our cost and profit sharing option with Genentech, we will be required to pay a percentage of the costs of development and commercialization of TRX1, which amounts are uncertain and are expected to be substantial. Our revenue and profit potential is unproven, and our limited operating history makes our future operating results difficult to predict. Additionally, our quarterly revenues and operating results are difficult to predict and may fluctuate significantly from quarter to quarter for numerous reasons, including the timing of the receipt of milestone payments and royalties, neither of which are within our control. Consequently, an investor in our common stock may be unable to evaluate our future operations.
None of our products has received marketing approval, and we may not successfully develop any approved products in the future.
We plan to develop and commercialize products that we discover independently or acquire from third parties. To date, however, our most advanced products are in early clinical development, and we have not developed any products that have received marketing approval. Our ability to develop and commercialize products in the future will depend on our:
• successfully completing preclinical and clinical testing;
• obtaining necessary regulatory approvals;
• producing and manufacturing products;
• effectively deploying sales and marketing resources;
• entering into third-party collaborations for our products; and
5
• obtaining intellectual property protection for our products.
Some of the material risks that we may encounter in conducting internal drug development programs include the following:
• preclinical development and clinical trials for our potential products are expensive and time consuming and their outcome is uncertain;
• we have little experience in conducting clinical development activities and often rely on external clinical investigators;
• since we may be subject to extensive and changing government regulatory requirements, we may be unable to obtain government approval of any products in a timely manner;
• we will rely on third-party manufacturers to produce our products; and
• we currently have no distribution, sales, or marketing capabilities.
Our products are based on an understanding of the immune system that could ultimately prove to be incorrect.
Our success as a company depends on our ability to develop novel therapeutics that induce, maintain, or remove immunological tolerance and have clinically beneficial effects in a number of disease settings. In order to accomplish this, we must identify cells, cellular pathways, and molecular targets that are involved in the development and maintenance of immunological tolerance. The science of discovering therapeutics based on the identification of genes, cells, and cellular pathways that may be involved in the induction of immunological tolerance is relatively new and evolving rapidly. Our approach may fail to produce therapeutically relevant results, hindering our ability to pursue our clinical and regulatory strategy and sustain our growth. Immunological tolerance may be linked to one or a combination of several genes, cells, and cellular pathways. Therefore, a product that modifies the effect of any one gene, cell, or target may not have a clinical effect in patients.
Therapies that modulate the immune system may have harmful and unintended effects.
While we believe that our products will be safe, durable, and effective treatments for diseases of the immune system, our most advanced products to date are in early clinical development. There are potential risks involved with promoting immunological tolerance and interfering with normal immunological function, including unintended tolerization to viruses and cancers and increased susceptibility to the reactivation of chronic latent viral infections such as Epstein-Barr virus, other herpes viruses, and human papilloma virus.
Because many of the products that we develop will be based on new technologies and novel therapeutic approaches, the market may not be receptive.
The commercial success of any products we develop, if approved for marketing, will depend upon their acceptance by the medical community and private and government third-party payors as clinically useful, cost-effective, and safe. Many of the products that we are developing are based upon new technologies, such as our TolerMab antibody technology, and new therapeutic approaches based on short courses of therapy with long-term and durable
6
clinical benefits, such as the induction and maintenance of immunological tolerance. As a result, it may be more difficult for us to achieve market and third-party payor acceptance of our products, particularly the first products that we introduce to the market. Our efforts to educate the medical and third-party payor communities on these potentially unique approaches may require greater resources than would be typically required for products based on conventional technologies or therapeutic approaches. The safety, efficacy, convenience, and cost-effectiveness of our products as compared to competitive products will also affect market acceptance.
We rely significantly on Genentech for the development and commercialization of one of our lead products, TRX1. If our collaboration is terminated or does not yield favorable results, our business would be harmed significantly.
We have entered into a collaboration agreement with Genentech pursuant to which Genentech and we will conduct preclinical and clinical trials, and Genentech will seek regulatory approval and undertake the commercialization of our TRX1 monoclonal antibody and other CD4-targeting products. The collaboration agreement allows Genentech full discretion in electing how to pursue the development of TRX1. As a result, we cannot control the amount or timing of resources Genentech dedicates to the collaboration. Our receipt of revenues from achievement of drug development and regulatory approval milestones or royalties on sales under the collaboration agreement is dependent upon Genentech's activities and its development, manufacturing, and marketing resources. If we are unable to achieve any of the development or regulatory approval milestones, or the commercialization of TRX1 is not successful, we may not derive any additional revenue from the collaboration. Moreover, certain products that Genentech discovers may be competitive with TRX1. Accordingly, we cannot be certain that Genentech will proceed with the development of TRX1 or will pursue their existing or alternative products in preference to or in lieu of TRX1.
We are relying on Genentech to fund a substantial portion of the research and development expenses of TRX1 over the next several years. Genentech may terminate our collaboration agreement at any time, with or without cause, with 90 days notice, and in such event we would lose all right to any further payments under the agreement. If Genentech terminates or breaches the collaboration agreement, or otherwise does not conduct its collaborative activities in a timely manner, the development or commercialization of TRX1 could be delayed or terminated, or we could be required to undertake unforeseen additional responsibilities or devote unbudgeted additional resources to such development or commercialization.
We will require substantial funds in addition to the proceeds of this offering that we may not be able to raise.
Based on our current plans, we believe that our cash and cash equivalents, together with the estimated net proceeds from this offering, will be sufficient to meet our capital requirements for the next three years. However, after that, or in the event of unforeseen developments relating to our business, we will require substantial additional funds to develop and commercialize our products. Additional financing may not be available when we need it or, if available, it may not be on terms that are favorable to us. If we are unable to obtain adequate funding on a timely basis, we may be required to cease one or more of our drug discovery or development programs. We could be required to seek funds through arrangements with
7
collaborators or others that may require us to relinquish certain rights to our technologies or products, which we would have otherwise retained.
We may not be able to expand our current collaboration, enter into additional collaborations, or achieve the milestones needed to fund our growth.
We currently have no collaboration agreements other than with Genentech. We expect to receive revenue from Genentech and from future collaboration agreements for the research and development of products depending on the achievement of specific milestones and the development and commercialization of successful products. If we are unable to achieve the development and regulatory milestones specified in the Genentech agreement or expand the Genentech collaboration to include additional products or targets, or if we are unable to enter into new collaboration agreements, we may not generate sufficient revenues to successfully fund our research and development programs. Whether we generate sufficient revenues will depend, in part, on our efforts as well as the efforts of our collaborators. If we or any of our collaborators fail to meet specific milestones or to advance the products in accordance with the terms of a collaboration agreement, then that collaboration agreement may be terminated, which could result in a decrease in our future revenues.
Clinical trials for our products are expensive and time consuming and their outcome is uncertain. If our clinical trials are unsuccessful, or if they experience significant delays, our ability to commercialize products will be impaired.
Before we can obtain marketing approval for the commercial sale of any product, we must complete preclinical development and extensive clinical trials in humans to demonstrate a product's safety and efficacy. Each of these trials requires the investment of substantial time and expense.
The preclinical laboratory testing, formulation, development, manufacturing, and clinical trials of any product we develop independently or in collaboration with third parties, as well as the distribution and marketing of these products, are regulated by numerous federal, state, and local governmental authorities in the United States, principally the U.S. Food and Drug Administration, or FDA, and by similar agencies in other countries. It may take us several years to complete our testing, and failure can occur at any stage of testing. Interim results of preclinical or clinical studies do not necessarily predict their final results, and acceptable results in early studies might not be seen in later studies. Any preclinical or clinical test may fail to produce results that meet FDA requirements. Preclinical and clinical data can be interpreted in different ways, which could delay, limit, or prevent marketing approval. Negative or inconclusive results from a preclinical study or clinical trial, adverse medical events during a clinical trial, or safety issues resulting from products of the same class of drug could cause a preclinical study or clinical trial to be repeated, halted, or terminated, even if other studies or trials relating to the same drug are successful.
We may not complete our planned preclinical or clinical trials on schedule or at all. We may not be able to demonstrate the safety and efficacy of our products in long-term clinical trials which may result in a delay or failure to commercialize our products. As a result, we may have to expend substantial additional funds to obtain access to resources, or delay or modify our plans significantly. Our product development costs will increase if we experience delays in clinical testing or subsequent approvals. Significant clinical trial delays could allow our
8
competitors to bring products to market before we do, and impair our ability to commercialize our products or potential products.
If we are unable to obtain and maintain patent protection for our discoveries, the value of our technology and products will be adversely affected.
We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent they are covered by valid and enforceable patents or are effectively maintained as trade secrets. To date, we have licenses to approximately 95 issued U.S. and foreign patents. In addition, we own or have licenses to approximately 45 pending U.S. and foreign patent applications. Because the patent position of pharmaceutical and biotechnology companies involves complex legal, scientific, and factual questions, the issuance, scope, and enforceability of patents cannot be predicted with certainty. In addition, there is some uncertainty as to whether human clinical data will be required for issuance of patents for human therapeutics. Patents, if issued, may be challenged, invalidated, or circumvented. Thus, any patents that we own or license from others may not provide adequate protection against competitors. Our pending patent applications, those we file in the future, or those we may license from third parties, may not result in patents being issued. If issued, they may not provide us with proprietary protection or competitive advantages against others with similar products and technology. Furthermore, others may independently develop similar products or technology or duplicate any technology that we have developed. Moreover, the laws of many foreign countries do not protect our intellectual property rights to the same extent as do the laws of the United States.
We may incur substantial costs or lose important rights as a result of litigation or other proceedings relating to patent and other intellectual property rights.
The defense and prosecution of intellectual property rights, U.S. Patent and Trademark Office interference proceedings, and related legal and administrative proceedings in the United States and elsewhere are costly and time consuming and their outcome is uncertain. Litigation may be necessary to:
• assert claims of infringement;
• enforce our issued and licensed patents;
• protect trade secrets or know-how; or
• determine the enforceability, scope, and validity of the proprietary rights of others.
If we become involved in any litigation, interference, or other administrative proceedings, we will incur substantial expense and it will divert the efforts of our scientific and management personnel. Uncertainties resulting from the initiation and continuation of litigation, interference, or other administrative proceedings could have a material adverse effect on our ability to compete in the marketplace pending resolution of the disputed matters. An adverse determination may subject us to significant liabilities or require us to seek licenses that may not be available from third parties on commercially reasonable terms, if at all. We or our collaborators may be restricted or prevented from developing and commercializing our products, if any, in the event of an adverse determination in a judicial or administrative
9
proceeding, or if we fail to obtain necessary licenses. Any of these outcomes would significantly harm our business.
As is commonplace in the biotechnology and pharmaceutical industries, we employ individuals who were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. To the extent our employees are involved in research areas which are similar to those areas in which they were involved at their former employers, we may be subject to claims that such employees or we have inadvertently or otherwise used or disclosed the alleged trade secrets or other proprietary information of the former employers. Litigation may be necessary to defend against such claims.
We rely on third-party licenses for the development and commercialization of our products. If any of these licenses are terminated, we may not be able to develop and commercialize the products covered by them.
We license from third parties intellectual property and technologies that are critical to the development of our products, including our lead products TRX4 and TRX1. We hold many of these licenses pursuant to license agreements that may be terminated by the licensors if we commit a material breach or if we become insolvent or file for bankruptcy. If any of these license agreements are terminated, we may not be able to develop and commercialize our products.
Third parties may own or control patents or patent applications and require us to seek licenses, which could increase our development and commercialization costs or prevent us from developing, partnering, or marketing our products.
We may not have rights under some patents or patent applications related to our products. Third parties may own or control such patents and patent applications in the United States and abroad. For example, we are currently developing recombinant antibody products that include both humanized and chimeric antibodies. We are aware of third-party patents issued in the United States and abroad that are directed at recombinant antibodies, including humanized and chimeric antibodies and their production. In such cases, in order to develop, manufacture, sell, or import some of our proposed products, we or our collaborators may choose to seek, or be required to seek, licenses under third-party patents issued in the United States and abroad or those that might issue from United States and foreign patent applications. In such event, we may be required to pay license fees or royalties or both to the licensor. If licenses are not available to us on acceptable terms, we or our collaborators may not be able to develop, manufacture, sell, import, or export these products.
We may have conflicts with our partners over rights to intellectual property or development strategies, which could harm the development of our products.
We may disagree with our existing or future collaborators, such as research and development partners, manufacturing partners, or sales and marketing partners, over rights to our intellectual property. We may also have disagreements with our partners over our drug development strategy and programs. In addition to diverting the efforts of our management and scientific personnel, such conflicts could reduce our ability to enter into future collaboration arrangements and negatively impact our relationship with existing collaborators,
10
which could reduce our revenues, delay or terminate our drug discovery programs, and result in time consuming and expensive litigation or arbitration.
We have only limited experience in regulatory affairs, and some of our products may be based on new technologies and discoveries. These factors may affect our ability or the time we require to obtain necessary marketing approvals.
We have only limited experience in drafting and filing the submissions necessary to gain marketing approval for our products. Moreover, certain of the products that are likely to result from our research and development programs may be based on new technologies and new therapeutic approaches that have not been extensively tested in humans. The regulatory requirements governing these types of products may be more rigorous than for conventional products. As a result, we may experience a longer regulatory process in connection with any products that we develop based on these new technologies or new therapeutic approaches.
Failure to attract, motivate, and retain skilled personnel and academic collaborators and to effectively manage our growth may have an adverse effect on our business.
Our success depends on our continued ability to attract, motivate, and retain highly qualified management and scientific personnel and our ability to develop and maintain important relationships with leading academic and other research institutions and scientists. Competition for personnel and relationships in our industry is intense. If we lose the services of our management or scientific personnel or if our relationships with academic and other research institutions and scientists are compromised, our drug discovery and development programs may be significantly delayed, and our research and development objectives may be impeded. We are particularly dependent on Dr. Douglas J. Ringler, our President and Chief Executive Officer. Dr. Ringler's employment is at-will, and the loss of his services could delay or prevent the achievement of our research, development, and business objectives.
In addition, we have experienced, and expect to continue to experience, growth in the number of our employees and the scope of our operations. To compete effectively, we will have to expand, improve, and effectively use our operating, management, and financial systems to accommodate our growth. Failure to effectively anticipate, implement, or manage the changes required to sustain our growth may have an adverse effect on our business.
Because we have limited manufacturing capabilities, we will be dependent on third parties to manufacture products for us.
We have no clinical grade or commercial scale manufacturing capabilities. We currently rely upon third parties to produce material for preclinical and clinical testing purposes. We expect to continue to rely on third parties, including Genentech, to produce materials required for clinical trials and for the commercial production of certain of our products.
There are a limited number of third-party manufacturers that operate under the FDA's current Good Manufacturing Practices regulations and that have the necessary expertise and capacity to manufacture our products. As a result, we may experience some difficulty finding manufacturers for our anticipated future needs. If we are unable to arrange for third-party manufacturing of our products, or to do so on commercially reasonable terms, we may not be able to complete development of our products.
11
Reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured products ourselves, including reliance on the third party for regulatory compliance and quality assurance, the possibility of breach of the manufacturing agreement by the third party because of factors beyond our control, and the possibility of termination or nonrenewal of the agreement by the third party, based on its own business priorities, at a time that is costly or inconvenient for us.
We may in the future elect to manufacture certain of our products without the assistance of third parties. We would need to invest substantial additional funds and recruit qualified personnel in order to build or lease and operate any manufacturing facilities.
We may seek to expand our business through possible acquisitions of complementary products, technologies, or organizations, and we may have difficulty integrating those assets into our business and operations.
In the future, we may from time to time seek to expand our business through acquisitions. Our acquisition of products, technologies, companies, and businesses and our expansion of operations would involve risks such as the following:
• the potential inability to successfully integrate acquired operations and businesses and to realize anticipated synergies, economies of scale, or other expected values;
• unanticipated costs associated with the acquisition;
• adverse market reaction;
• the incurrence of expenses related to transactions that may or may not be consummated; and
• the inability to effectively manage and coordinate operations at multiple venues, which, among other things, could divert our management's attention from other important business matters.
In addition, our financial condition and the reported results of our operations could be negatively affected by any acquisition or expansion of operations, which could result in dilutive issuances of equity securities, the incurrence of additional debt, write-offs relating to in-process research and development, and the creation of intangible assets and goodwill.
We face a risk of product liability claims and may not be able to obtain adequate insurance.
Our business exposes us to the risk of product liability claims that is inherent in the manufacturing, testing, and marketing of human therapeutic products. Although we have product liability insurance that we believe is appropriate for our business at this time, this insurance is subject to deductibles and coverage limitations, and the market for such insurance is becoming more restrictive. We may not be able to obtain or maintain adequate protection against potential liabilities. If we are unable to sufficiently insure against potential product liability claims, we will be exposed to significant liabilities, which may materially and adversely affect our business and financial position. These liabilities could prevent or interfere with our product development and commercialization efforts.
12
Risks related to our industry
We face growing and new competition, which may result in others discovering, developing, or commercializing products before or more successfully than us.
The fields of biotechnology and pharmaceutical development are highly competitive, and there are often several companies developing products to the same targets. Many of our competitors are substantially larger than we are, and these competitors have substantially greater capital resources, research and development staffs, and facilities than we have. Furthermore, many of our competitors are more experienced in research, drug discovery, development, and commercialization, obtaining marketing approvals, product manufacturing, and marketing.
Many other companies have previously developed an antibody or small molecule directed to the same target on white blood cells as our TRX1 antibody, and both Genmab and IDEC Pharmaceuticals are conducting clinical trials with antibodies that are directed to the same target as TRX1. Our TRX4 monoclonal antibody is directed to the same target as products being developed or sold by Protein Design Labs and Johnson & Johnson. MedImmune is developing an antibody directed to the the same target as our TolerMab TRX3 antibody. These products could represent competition for us in terms of enrolling patients in clinical trials or eventual commercialization of our products, if approved. These competitors may also own or control intellectual property which may be necessary for the commercialization of our products, and we may not be able to obtain a license to such intellectual property on favorable terms, or at all.
The pharmaceutical and biotechnology industries are highly concentrated, and any further consolidation could reduce the number of our potential collaborators and may affect our ability to execute our business strategies.
There are a limited number of large pharmaceutical and biotechnology companies, and these companies represent the market of potential collaborators for our drug discovery and development programs. The number of our potential collaborators could decline even further through consolidation among these companies. If the number of our potential collaborators declines, their relative negotiating power with us may increase. If we are unable to enter into additional collaborations on commercially acceptable terms, we may not be able to execute our business strategies.
If we fail to obtain an adequate level of reimbursement for our products by third-party payors, there may be no commercially viable markets for our products.
Our ability or the ability of our collaborators to commercialize our products, if any, may depend in part on the extent to which government health administration authorities, private health insurers, and other organizations will reimburse consumers for the cost of treatment. These third-party payors continually attempt to contain or reduce the costs of healthcare by challenging both the need, and the prices charged, for medical products and services. In certain foreign countries, particularly the countries of the European Union, the pricing of prescription pharmaceuticals is subject to governmental control. Significant uncertainty exists as to the reimbursement status of newly approved therapeutics. We may not be able to sell our products profitably if reimbursement is unavailable or limited in scope or amount.
13
In both the United States and certain foreign jurisdictions, there have been a number of legislative and regulatory proposals to change the healthcare system. Further proposals are likely. The potential for adoption of these proposals affects or will affect our ability to market our products.
The manufacture and storage of pharmaceutical and biological products is subject to environmental regulation and risk.
Because of the biological nature of antibodies and their manufacturing process, the biotechnology industry is subject to extensive environmental regulation and the risk of incurring liability for damages or the cost of remedying environmental problems. If we fail to comply with environmental regulations to use, discharge, or dispose of hazardous materials appropriately or otherwise to comply with the conditions attached to our operating licenses, the licenses could be revoked, and we could be subject to criminal sanctions and substantial liability or could be required to suspend or modify our operations.
Environmental laws and regulations can require us to undertake or pay for investigation, clean-up, and monitoring of environmental contamination identified at properties that we may own or operate or that we formerly owned or operated. Further, they can require us to undertake or pay for such actions at offsite locations where we may have sent hazardous substances for disposal. These obligations are often imposed without regard to fault. In the event we are found to have violated environmental laws or regulations, our reputation will be harmed, and we may incur substantial monetary liabilities.
Guidelines and recommendations concerning the healthcare industry can negatively affect the use of our products.
Government agencies promulgate regulations and guidelines directly applicable to us and to our products. In addition, professional societies, practice management groups, and private health and science organizations involved in various diseases from time to time may also publish guidelines or recommendations to the healthcare and patient communities. Recommendations of government agencies or these other groups or organizations may relate to such matters as usage, dosage, route of administration, and use of concomitant therapies. Recommendations or guidelines that are followed by patients and healthcare providers could result in decreased use of our products.
Risks related to this offering
There may not be an active, liquid trading market for our common stock.
Prior to this offering, there has been no public market for our common stock. An active, liquid trading market for our common stock may not develop or be maintained following this offering. As a result, you may not be able to sell your shares of our common stock quickly or at the market price. The initial public offering price of our common stock was determined by negotiation between us and representatives of the underwriters based upon a number of factors and may not be indicative of prices that will prevail following the completion of this offering. The market price of our common stock may decline below the initial public offering price, and you may not be able to resell your shares of our common stock at or above the initial offering price.
14
Our stock price will likely be volatile, your investment could decline in value, and we may incur significant costs from class action litigation.
The trading price of our common stock is likely to be volatile and subject to wide price fluctuations in response to various factors, including:
• actual or anticipated variations in our quarterly operating results;
• introductions or announcements of technological innovations or new products by us, our collaborators, or our competitors;
• the loss of a significant collaborator;
• disputes or other developments relating to proprietary rights, including patents, litigation matters, and our ability to patent our products and technologies;
• changes in our financial estimates by securities analysts;
• conditions or trends in the pharmaceutical and biotechnology industries;
• additions or departures of key personnel;
• announcements by us or our competitors of significant acquisitions, strategic partnerships, clinical trial results, joint ventures, or capital commitments;
• regulatory developments in the United States and abroad;
• public concern or opinion as to new drug discovery techniques; and
• economic and political factors.
In addition, the stock market in general, and the market for biotechnology and pharmaceutical companies in particular, have experienced significant price and volume fluctuations that have often been unrelated or disproportionate to operating performance. These broad market and industry factors may seriously harm the market price of our common stock, regardless of our operating performance. In the past, following periods of volatility in the market price of a company's securities, securities class action litigation has often been instituted. A securities class action suit against us could result in potential liabilities, substantial costs, and the diversion of our management's attention and resources, regardless of the outcome.
Future sales of our common stock may depress our stock price.
The market price of our common stock could decline as a result of sales of substantial amounts of our common stock in the public market after the closing of this offering, or the perception that these sales could occur. In addition, these factors could make it more difficult for us to raise funds through future offerings of common stock. There will be shares of our common stock outstanding immediately after this offering, based on the number of shares of our common stock outstanding as of August 22, 2003. All of the shares of our common stock sold in this offering will be freely transferable without restriction or further registration under the Securities Act of 1933, except for any shares of our common stock purchased by our executive officers, directors, principal stockholders, and some related parties. For more information, see "Shares eligible for future sale."
15
Although all of our stockholders have agreed with our underwriters to be bound by a 180-day lock-up agreement that prohibits these holders from selling or transferring their stock except in limited circumstances, J.P. Morgan Securities Inc. and SG Cowen Securities Corporation, on behalf of the underwriters, at their discretion, can waive the restrictions of the lock-up agreement at an earlier time without prior notice or announcement and allow our stockholders to sell their shares of our common stock. If the restrictions of the lock-up agreement are waived, shares of our common stock will be available for sale into the market, subject only to applicable securities rules and regulations, which would likely reduce the market price for shares of our common stock.
After this offering, we intend to register shares of our common stock that are reserved for issuance upon the exercise of options granted or reserved for grant under our equity incentive plan and our employee stock purchase plan. Once we register these shares of our common stock, stockholders can sell them in the public market upon issuance, subject to restrictions under the securities laws and any applicable lock-up agreements. In addition, some of our existing stockholders will be entitled to register their shares of our common stock after this offering.
You will experience immediate and substantial dilution as a result of this offering and may experience additional dilution in the future.
If you purchase common stock in this offering, you will pay more for your shares than the amounts paid by existing stockholders for their shares. As a result, you will incur immediate and substantial dilution of $ per share, representing the difference between our pro forma net tangible book value per share after giving effect to this offering and the initial public offering price. In addition, purchasers of shares of our common stock in this offering will have contributed approximately % of the aggregate price paid by all purchasers of our stock, but will own only approximately % of the shares of our common stock outstanding after the offering based on the number of shares of our common stock and redeemable convertible preferred stock outstanding as of June 30, 2003. If the holders of outstanding options exercise those options at prices below the initial public offering price, you will incur further dilution. We may also acquire other companies or technologies or finance strategic alliances by issuing equity, which may result in additional dilution to our stockholders.
Our executive officers, directors, and current principal stockholders own a large percentage of our voting common stock and could limit new stockholders from influencing corporate decisions.
Immediately after this offering, our executive officers, directors, current principal stockholders, and their respective affiliates will beneficially own, in the aggregate, approximately % of our outstanding common stock. These stockholders, as a group, would be able to control substantially all matters requiring approval by our stockholders, including mergers, sales of assets, the election of all directors, and approval of other significant corporate transactions, in a manner with which you may not agree or that may not be in the best interest of other stockholders.
16
Provisions of Delaware law and our charter documents could delay or prevent an acquisition of us, even if the acquisition would be beneficial to our stockholders.
Provisions of Delaware law and our certificate of incorporation and by-laws could hamper a third party's acquisition of us, or discourage a third party from attempting to acquire control of us. Stockholders who wish to participate in these transactions may not have the opportunity to do so. These provisions could also limit the price that investors might be willing to pay in the future for shares of our common stock.
These provisions include:
• the application of a Delaware law prohibiting us from entering into a business combination with the beneficial owner of 15% or more of our outstanding voting stock for a period of three years after such 15% or greater owner first reached that level of stock ownership, unless we meet specified criteria;
• our ability to issue preferred stock with rights senior to those of the common stock without any further vote or action by the holders of our common stock;
• the requirement that our stockholders provide advance notice when nominating our directors; and
• the inability of our stockholders to convene a stockholders' meeting without the chairperson of the board, the president, or a majority of the board of directors first calling the meeting.
Our management will have broad discretion in the use of net proceeds from this offering.
As of the date of this prospectus, we cannot specify with certainty the amounts we will spend on particular uses from the net proceeds we will receive from this offering. Our management will have broad discretion in the application of the net proceeds but currently intends to use the net proceeds as described in "Use of proceeds." The failure by our management to apply these funds effectively could affect our ability to continue to develop our business.
We have never paid dividends on our capital stock, and we do not anticipate paying dividends in the foreseeable future.
We have paid no dividends on any of our classes of capital stock to date, and we currently intend to retain our future earnings, if any, to fund the development and growth of our business. In addition, the terms of any future debt or credit facility may preclude us from paying any dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
17
Special note regarding forward-looking statements
This prospectus contains forward-looking statements. The forward-looking statements are principally contained in the sections entitled "Prospectus summary," "Business," and "Management's discussion and analysis of financial condition and results of operations." These statements involve known and unknown risks, uncertainties, and other factors which may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by the forward-looking statements. For this purpose, any statement that is not a statement of historical fact should be considered a forward-looking statement. These forward-looking statements include statements about the following:
• our product research and development efforts, the clinical indications that we are pursuing or may pursue, and the implications of preclinical and clinical results, in particular with respect to TRX4 and TRX1;
• the commercialization of our products;
• our collaboration agreement with Genentech, including potential payments thereunder;
• our intentions regarding the establishment of collaborations;
• anticipated operating losses, future revenues, capital expenditures, debt and lease obligations, and need for additional financing;
• anticipated regulatory filing dates and clinical trial initiation dates for our products;
• the ability to obtain marketing approval for our products; and
• the benefits to be derived from collaborations with partners, license agreements, and other collaborative efforts, including those relating to the development and commercialization of our products.
In some cases, you can identify forward-looking statements by terms such as "may," "will," "should," "could," "would," "believe," "anticipate," "plan," "expect," "intend," "estimate," "potential," and similar expressions to help identify forward-looking statements.
Forward-looking statements reflect our current views with respect to future events and are subject to risks and uncertainties. Given these uncertainties, you should not place undue reliance on these forward-looking statements. We discuss many of the risks associated with our business in greater detail under the heading "Risk factors." All forward-looking statements represent our estimates and assumptions only as of the date of this prospectus.
You should read this prospectus and the documents that we reference in this prospectus and have filed as exhibits to the registration statement, of which this prospectus is part, completely and with the understanding that our actual future results may be materially different from what we expect. You should assume that the information appearing in this prospectus is accurate as of the date on the front cover of this prospectus only. Our business, financial condition, results of operations, and prospects may change. We may not update these forward-looking statements, even though our situation may change in the future, unless we have obligations under federal securities laws to update and disclose material developments related to previously disclosed information. We qualify all of our forward-looking statements by these cautionary statements.
18
Use of proceeds
We estimate that the net proceeds from this offering will be $ million, or $ million if the underwriters exercise their over-allotment option in full, based on an assumed initial public offering price of $ per share and after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
We intend to use the net proceeds of this offering primarily for research and development activities relating to our internal drug discovery and development programs, our collaboration with Genentech, and other general corporate and working capital purposes, which may include investing in complementary products, licensing additional technologies, or making potential acquisitions.
The foregoing use of the net proceeds from this offering represents our current intentions based upon our present plans and business condition. We retain broad discretion in the allocation and use of the net proceeds of this offering, and a change in our plans or business condition could result in the application of the net proceeds from this offering in a manner other than as described in this prospectus. Pending the uses described above, we intend to invest the net proceeds from this offering in short-term, investment grade, interest-bearing securities.
Dividend policy
We have never declared or paid dividends on our capital stock. We currently intend to retain our future earnings, if any, to support the growth and development of our business, and we do not anticipate paying any dividends in the foreseeable future. Any future determination to pay dividends will be at the discretion of our board of directors and will depend on our financial condition, results of operations, capital requirements, and other factors that our board of directors may deem relevant.
19
Capitalization
The following table describes our capitalization as of June 30, 2003:
• on an actual basis;
• on a pro forma basis giving effect to the automatic conversion upon the closing of this offering of all outstanding shares of our redeemable convertible preferred stock into an aggregate of 61,035,000 shares of common stock;
• on a pro forma as adjusted basis to give effect to the sale of shares of our common stock offered at an assumed initial public offering price of $ per share, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
You should read this table in conjunction with "Management's discussion and analysis of financial condition and results of operations" and our consolidated financial statements and the related notes included elsewhere in this prospectus.
|
|---|
June 30, 2003
(in thousands, except share data)
| | Actual
| | Pro forma
| | Pro forma
as adjusted
|
|---|
|
|---|
| Long-term debt, net of current portion | | $ | 486 | | $ | 486 | | $ | |
| | |
| | | | | | |
| Redeemable convertible preferred stock: | | | | | | | | | |
| Series A convertible preferred stock, $0.001 par value; 9,035,000 shares authorized; 9,035,000 issued and outstanding actual; no shares authorized, issued or outstanding, pro forma and pro forma as adjusted | | | 7, 108 | | | — | | | |
| Series B convertible preferred stock, $0.001 par value; 17,146,250 shares authorized; 17,000,000 issued and outstanding actual; no shares authorized, issued or outstanding, pro forma and pro forma as adjusted | | | 19,439 | | | — | | | |
| Series C convertible preferred stock, $0.001 par value; 40,000,000 shares authorized; 35,000,000 issued and outstanding actual; no shares authorized, issued or outstanding, pro forma and pro forma as adjusted | | | 36,563 | | | — | | | |
| | |
|
| | | | 63,110 | | | — | | | |
| | |
|
| Stockholders' equity (deficit): | | | | | | | | | |
| Common stock, $0.001 par value; 75,000,000 shares authorized actual; 4,202,250 issued, 3,962,250 outstanding actual; shares authorized, 65,237,250 issued and 64,997,250 outstanding, pro forma; issued and outstanding, pro forma as adjusted | | | 4 | | | 65 | | | |
| Additional paid-in capital | | | — | | | 63,049 | | | |
| Deferred compensation | | | (749 | ) | | (749 | ) | | |
| Accumulated deficit | | | (29,583 | ) | | (29,583 | ) | | |
| | |
| | | | | | |
| | Total stockholders' equity (deficit) | | | (30,328 | ) | | 32,782 | | | |
| | |
|
| | | Total capitalization | | $ | 33,268 | | $ | 33,268 | | $ | |
|
Excludes (i) an aggregate of 956,750 shares of common stock issuable pursuant to stock options outstanding as of June 30, 2003 at a weighted average exercise price per share of $0.15, and (ii) 311,250 shares of common stock issuable pursuant to warrants outstanding as of June 30, 2003, at a weighted average exercise price per share of $0.81.
20
Dilution
The historical net tangible book value of our common stock as of June 30, 2003 was $ million, or $ per share. The historical net tangible book value per share of our common stock is the difference between our tangible assets and our liabilities, divided by the number of common shares outstanding. The pro forma net tangible book value of our common stock as of June 30, 2003 was $ million, or $ per share. The pro forma net tangible book value per share of our common stock is the difference between our tangible assets and our liabilities, divided by the number of shares of our common stock outstanding as of June 30, 2003, after giving effect to the automatic conversion of all outstanding shares of our redeemable convertible preferred stock into 61,035,000 shares of our common stock upon the completion of this offering. For new investors in our common stock, dilution is the per share difference between the initial public offering price of our common stock and the pro forma net tangible book value of our common stock immediately after completing this offering. Dilution in this case results from the fact that the per share offering price of our common stock is substantially in excess of the per share price paid by our current stockholders.
As of June 30, 2003, after giving effect to the sale of the shares of our common stock offered by this prospectus at an assumed initial public offering price of $ per share and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, the pro forma net tangible book value per share of our common stock would have been $ per share. Therefore, new investors in our common stock would have paid $ for a share of common stock having a pro forma net tangible book value of approximately $ per share after this offering. That is, their investment would have been diluted by approximately $ per share. At the same time, our current stockholders would have realized an increase in pro forma net tangible book value of $ per share after this offering without further cost or risk to themselves. The following table illustrates this per share dilution:
|
|---|
| Assumed initial public offering price per share | | $ | |
| | Pro forma net tangible book value per share before this offering | | $ | |
| | Increase in pro forma net tangible book value per share attributable to investors in this offering | | $ | |
| Pro forma net tangible book value per share after this offering | | $ | |
| Dilution per share to new investors | | $ | |
|
The following table sets forth, as of June 30, 2003, on a pro forma basis to give effect to the conversion of all shares of our redeemable convertible preferred stock into 61,035,000 shares of common stock, the number of shares of common stock purchased in this offering, the total consideration paid, and the average price per share paid by existing and new stockholders, before deducting underwriting discounts and commissions and our estimated offering expenses:
|
|---|
| | Shares purchased
| | Total consideration
| |
|
|---|
| | Average price
per share
|
|---|
| | Number
| | Percent
| | Amount
| | Percent
|
|---|
|
|---|
| Existing stockholders | | | | | % | $ | | | | % | $ | |
| New investors | | | | | | | | | | | | |
| | |
|
| | Total | | | | 100 | % | $ | | | 100 | % | | |
|
Excludes (i) an aggregate of 956,750 shares of common stock issuable pursuant to stock options outstanding as of June 30, 2003 at a weighted average exercise price per share of $0.15, and (ii) 311,250 shares of common stock issuable pursuant to warrants outstanding as of June 30, 2003, at a weighted average exercise price per share of $0.81.
21
Selected consolidated financial data
The following selected consolidated financial data as of December 31, 2001 and 2002, and for the period from inception (July 6, 2000) through December 31, 2000, and for the years ended December 31, 2001 and December 31, 2002, are derived from our audited consolidated financial statements appearing elsewhere in this prospectus. The selected consolidated financial data as of December 31, 2000, is derived from our audited financial statements, which are not included in this prospectus. The selected consolidated financial data for the six months ended June 30, 2002 and 2003 are derived from our unaudited financial statements appearing elsewhere in this prospectus. The unaudited financial statements include all adjustments, consisting of normal recurring accruals, which we consider necessary for a fair presentation of our financial position and results of operations for these periods.
Operating results for the six months ended June 30, 2003 are not necessarily indicative of the results that may be expected for the year ending December 31, 2003 or for any other periods in the future. The data below should be read in conjunction with, and are qualified by reference to, "Management's discussion and analysis of financial condition and results of operations" and our consolidated financial statements and related notes included elsewhere in this prospectus.
| |
| |
| | Year ended December 31,
| | Six months ended
June 30,
| |
|---|
| | Inception
through
December 31,
2000
| |
|---|
(in thousands, except share and per share data)
| |
|---|
| | 2001
| | 2002
| | 2002
| | 2003
| |
|---|
| |
| Statement of operations data: | | | | | | | | | | | | | | | | |
| Revenues | | $ | — | | $ | — | | $ | 27 | | $ | — | | $ | 750 | |
| Operating expenses | | | | | | | | | | | | | | | | |
| | Research and development | | | 232 | | | 3,797 | | | 10,908 | | | 4,883 | | | 5,236 | |
| | General and administrative | | | 125 | | | 1,370 | | | 2,774 | | | 1,330 | | | 1,556 | |
| | Stock-based compensation | | | 9 | | | 627 | | | 613 | | | 432 | | | 607 | |
| |
| |
|---|
| | | Total operating expenses | | | 366 | | | 5,794 | | | 14,295 | | | 6,645 | | | 7,399 | |
| |
| |
|---|
| | | Loss from operations | | | (366 | ) | | (5,794 | ) | | (14,268 | ) | | (6,645 | ) | | (6,649 | ) |
| Interest income | | | — | | | 165 | | | 250 | | | 139 | | | 215 | |
| Interest expense | | | (1 | ) | | (40 | ) | | (295 | ) | | (117 | ) | | (156 | ) |
| |
| |
|---|
| Net loss | | | (367 | ) | | (5,669 | ) | | (14,313 | ) | | (6,623 | ) | | (6,590 | ) |
| Accretion of redeemable convertible preferred stock | | | (10 | ) | | (737 | ) | | (2,224 | ) | | (934 | ) | | (2,411 | ) |
| |
| |
|---|
| Net loss attributable to common stockholders | | $ | (377 | ) | $ | (6,406 | ) | $ | (16,537 | ) | $ | (7,557 | ) | $ | (9,001 | ) |
| Net loss attributable to common stockholders per share, basic and diluted | | $ | (1.52 | ) | $ | (8.91 | ) | $ | (9.33 | ) | $ | (5.19 | ) | $ | (3.92 | ) |
| |
| |
|---|
| Shares used in computing net loss per share, basic and diluted | | | 248,119 | | | 719,094 | | | 1,773,180 | | | 1,455,871 | | | 2,295,342 | |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | December 31,
| |
| |
|---|
| | June 30,
2003
| |
|---|
(in thousands)
| | 2000
| | 2001
| | 2002
| |
|---|
| |
| Balance sheet data: | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 1,582 | | $ | 16,529 | | $ | 37,891 | | $ | 35,628 | |
| Working capital | | | 1,434 | | | 14,573 | | | 36,432 | | | 31,178 | |
| Total assets | | | 1,790 | | | 20,155 | | | 47,837 | | | 42,593 | |
| Long-term debt, net of current portion | | | — | | | 523 | | | 1,021 | | | 486 | |
| Redeemable convertible preferred stock | | | 1,958 | | | 23,625 | | | 60,639 | | | 63,110 | |
| Accumulated deficit | | | (367 | ) | | (6,036 | ) | | (21,609 | ) | | (29,583 | ) |
| Stockholders' deficit | | | (364 | ) | | (6,013 | ) | | (21,937 | ) | | (30,328 | ) |
| |
22
Management's discussion and analysis of financial condition
and results of operations
The following discussion of our financial condition and results of operations should be read in conjunction with the consolidated financial statements and the related notes to those statements and other financial information included elsewhere in this prospectus. This discussion contains forward-looking statements that involve risks and uncertainties. As a result of many factors, such as those set forth under "Risk factors" and elsewhere in this prospectus, our actual results may differ materially from those anticipated in these forward-looking statements.
Overview
Since our incorporation in July 2000, we have devoted substantially all of our resources to the discovery and development of novel therapies to treat patients with diseases of the immune system. Our therapies are based upon a unique understanding of the way the immune system recognizes and avoids attacking the body's own tissues and proteins, as well as other antigens that are not harmful to the body. This state of non-aggressive responsiveness to an antigen is called immunological tolerance and is a natural part of a properly functioning immune system. Therapies that induce immunological tolerance represent a fundamentally new approach to treating and potentially curing autoimmune diseases, rejection of transplanted organs, and other conditions associated with adverse or undesirable immune responses. We have established a pipeline of five monoclonal antibody products, two in human clinical trials and three in preclinical development, and have ongoing efforts to discover and develop other potential products.
In December 2002, we entered into a collaboration agreement with Genentech, Inc. for the development and commercialization of our TRX1 monoclonal antibody. Under this agreement, we are co-developing TRX1 with Genentech, and Genentech will be responsible for the manufacturing and marketing of TRX1. As part of the collaboration, Genentech paid us $4.5 million in the form of an upfront license fee and a milestone payment, and invested $3.5 million in our offering of Series C convertible preferred stock. Genentech may also make payments to us based on the achievement of additional development and regulatory approval milestones, and for research reimbursement. In addition, we are entitled to royalties on the worldwide net sales of TRX1 and, in the United States, we have the option to participate in a cost and profit sharing relationship with Genentech for TRX1 instead of receiving royalties on domestic net sales.
During the period from inception to December 31, 2002, we were considered to be a development stage enterprise as defined in Statement of Financial Accounting Standards No. 7. In 2003, we have generated significant revenues from planned principal operations and are, therefore, considered to be in the operating stage for the six months ended June 30, 2003.
Since our inception, we have incurred substantial losses, and as of June 30, 2003, we had an accumulated deficit of $29.6 million. These losses and accumulated deficit have resulted from the significant costs incurred in the research and development of our products and technologies, including payroll and payroll-related costs, manufacturing costs of preclinical and clinical grade materials, facility and facility-related costs, preclinical study costs, clinical trial
23
costs, general and administrative costs, and non-cash stock-based compensation expense. We expect that our losses will continue for the foreseeable future as we continue to expand our research, development, manufacturing, clinical trial activities, and infrastructure in support of these activities. Because a substantial portion of our revenues for the foreseeable future will depend on achieving development and clinical milestones, our revenue may vary substantially from year to year and quarter to quarter. Our operating expenses may also vary substantially from year to year and quarter to quarter based on the timing of manufacturing activities, clinical trial patient accruals, and collaborative activities. We believe that period-to-period comparisons of our results of operations are not meaningful and should not be relied on as indicative of our future performance.
Financial operations overview
Revenues. We do not currently have any commercial products for sale. To date, our revenues have been derived solely from our collaboration agreement with Genentech. We have received an aggregate of $4.5 million in a license fee and a milestone payment from Genentech. These payments were deferred and are being recognized as revenue ratably over our expected period of performance, which is three years. Any additional revenues that we may receive in the future are expected to consist primarily of license fees, milestone payments, and research reimbursement payments to be received from Genentech and from other collaborative partners with whom we may enter into agreements.
Research and development expense. Research and development expense consists primarily of salaries and related expenses for personnel, costs of contract manufacturing services, costs of facilities and equipment, fees paid to professional service providers in conjunction with independently monitoring our clinical trials and acquiring and evaluating data in conjunction with our clinical trials, fees paid to research organizations in conjunction with preclinical animal studies, costs of materials used in research and development, consulting, license, and sponsored research fees paid to third parties, depreciation of capital resources used to develop our products, and the legal costs of pursuing patent protection of our intellectual property. We expense research and development costs, both internal and external, as incurred. We expect our research and development expense to increase as we continue to develop our product candidates. From inception through June 30, 2003, we spent an aggregate of $20.2 million, excluding stock-based compensation expense of $1.8 million, on research and development.
General and administrative expense. General and administrative expense consists primarily of salaries and other related costs for personnel serving executive, business development, finance, accounting, intellectual property, administrative, and human resource functions. Other costs include facility costs not otherwise included in research and development expense, insurance, and professional fees for legal and accounting services. We expect that our general and administrative expense will increase as we add personnel, increase investor relations activities, obtain insurance coverage appropriate for a public company, and become subject to public company reporting obligations. From inception through June 30, 2003, we spent an aggregate of $5.8 million, excluding stock-based compensation expense of $39,000, on general and administrative expense.
24
Stock-based compensation expense. We have recorded stock-based compensation expense in connection with the grant of stock options to employees and the grant of stock options and restricted stock to non-employees.
Deferred stock-based compensation for options granted to employees is the difference between the deemed fair value of our common stock and the exercise price on the date such options were granted. We recorded deferred stock-based compensation and additional paid-in capital of $23,000, $356,000, and $526,000 in the years ended December 31, 2001 and 2002, and the six months ended June 30, 2003, respectively, related to stock options granted to employees. These amounts were recorded as a component of stockholders' deficit and are being amortized as charges to operations over the vesting periods of the options. We recorded amortization of deferred stock-based compensation of $1,000, $46,000, $23,000, and $109,000 for the years ended December 31, 2001 and 2002, and the six months ended June 30, 2002 and 2003, respectively. For options granted to employees through June 30, 2003, we expect to record additional amortization of deferred stock-based compensation as follows: $109,000 during the remainder of 2003, $228,000 in 2004, $226,000 in 2005, $175,000 in 2006, and $11,000 in 2007. We will record additional deferred stock-based compensation for additional options granted prior to this offering. We expect that the deferred stock-based compensation associated with additional options granted prior to this offering could be significant.
We recorded stock-based compensation expense of $626,000, $568,000, $409,000, and $498,000 for the years ended December 31, 2001 and 2002, and the six months ended June 30, 2002 and 2003, respectively, for stock options granted and restricted stock issued to non-employees, including our scientific founders, in accordance with Statement of Financial Accounting Standards No. 123 based on the fair value of the equity instruments issued. Stock-based compensation for options and restricted stock issued to non-employees is periodically remeasured as the underlying stock awards vest in accordance with Emerging Issues Task Force Issue No. 96-18 and as a result, the compensation expense to be recognized in the future may increase due to a corresponding increase in our stock price.
Interest income and interest expense. Interest income consists of interest earned on our cash and cash equivalents. Interest expense consists of interest incurred on lines of credit and the amortization of debt issuance costs.
Accretion of redeemable convertible preferred stock to redemption value. We accrete our redeemable convertible preferred stock to equal the redemption value at the earliest redemption date. To date, the accretion primarily consists of the increase in the redemption value of the redeemable convertible preferred stock. Our redeemable convertible preferred stock accretion decreases the amount of stockholders' equity available to common stockholders and increases the loss per share of common stock. Upon completion of this offering, the carrying value of the redeemable convertible preferred stock, which includes the accretion to the redemption value and offering costs, will be reclassified to stockholders' equity as the redeemable convertible preferred shares will automatically convert into common shares. Accordingly, there will be no further accretion to the redemption value and offering costs as these shares will no longer be outstanding after this offering.
25
Results of operations
Six months ended June 30, 2003 compared to six months ended June 30, 2002
Revenues. Revenues for the six months ended June 30, 2003 were $750,000, representing the recognition of the license fees and milestone payment received from our collaboration agreement with Genentech. We did not record any revenues during the six months ended June 30, 2002.
Research and development expense. Research and development expense increased by $353,000, or 7%, to $5.2 million for the six months ended June 30, 2003 from $4.9 million for the six months ended June 30, 2002. Of this increase, $616,000 was attributable to higher personnel and personnel-related costs, $690,000 was due to higher facility and facility-related costs, and $410,000 was related to higher clinical trial costs. These increases were partially offset by $1.3 million less in manufacturing costs as the majority of costs associated with manufacturing TRX1 for clinical trials was incurred in 2002 and $325,000 as a result of the accelerated depreciation of leasehold improvements for our previous facility. During the remainder of 2003, and thereafter, we expect that research and development expense will increase substantially as we increase the number of products and indications for which we conduct clinical trials.
General and administrative expense. General and administrative expense increased $225,000, or 17%, to $1.6 million for the six months ended June 30, 2003 from $1.3 million for the six months ended June 30, 2002. Of this increase, $275,000 was due to higher personnel and personnel-related costs and $272,000 was related to higher facility and facility-related costs. These increases were partially offset by a decrease in consulting fees of $270,000 related to a market positioning study done in 2002. During the remainder of 2003, and thereafter, we expect that general and administrative expense will increase substantially as we add personnel, increase investor relations activities, obtain insurance coverage appropriate for a public company, and become subject to public company reporting obligations.
Stock-based compensation expense. Stock-based compensation expense increased $175,000, or 41%, to $607,000 for the six months ended June 30, 2003 from $432,000 for the six months ended June 30, 2002. The increase was primarily due to an increase in the stock price used to measure stock-based compensation associated with restricted stock awards to non-employees and additional stock option grants to employees.
Interest income and interest expense. Interest income increased $76,000, or 55%, to $215,000 for the six months ended June 30, 2003 from $139,000 for the six months ended June 30, 2002. Interest expense increased $39,000, or 33%, to $156,000 for the six months ended June 30, 2003 from $117,000 for the six months ended June 30, 2002. The increase in interest income was attributable to higher average cash balances, and the increase in interest expense was related to additional borrowings under our lines of credit.
Fiscal year ended December 31, 2002 compared to fiscal year ended December 31, 2001
Revenues. Revenues for the year ended December 31, 2002 were $27,000, representing the recognition of amounts received from our collaboration agreement with Genentech that was executed in December 2002. We did not record any revenues during the fiscal year ended December 31, 2001.
26
Research and development expense. Research and development expense increased $7.1 million, or 187%, to $10.9 million for the fiscal year ended December 31, 2002 from $3.8 million for the fiscal year ended December 31, 2001. Of this increase, $893,000 was attributable to higher personnel and personnel-related costs, $1.8 million was due to higher facility and facility-related costs, $3.3 million was related to higher manufacturing costs, $350,000 was due to clinical study costs related to our Phase I trial for TRX1, and $515,000 was attributable to higher preclinical study costs related to our development programs.
General and administrative expense. General and administrative expense increased $1.4 million, or 102%, to $2.8 million for the fiscal year ended December 31, 2002 from $1.4 million for the fiscal year ended December 31, 2001. Of this increase, $459,000 was due to higher personnel and personnel-related costs, $635,000 was attributable to higher facility and facility-related costs, and $270,000 was due to consulting fees for a market positioning study.
Stock-based compensation expense. Stock-based compensation expense decreased $14,000, or 2%, to $613,000 for the fiscal year ended December 31, 2002 from $627,000 for the fiscal year ended December 31, 2001. The decrease was primarily due to the vesting in 2001 of 415,000 shares of restricted stock issued to non-employees, including our scientific founders, partially offset by an increase in the fair value of our capital stock in 2002.
Interest income and interest expense. Interest income increased $85,000, or 52%, to $250,000 for the fiscal year ended December 31, 2002 from $165,000 for the fiscal year ended December 31, 2001. Interest expense increased $256,000 to $296,000 for the fiscal year ended December 31, 2002 from $40,000 for the fiscal year ended December 31, 2001. The increase in interest income was due to increased balances as a result of the proceeds received from our Series B convertible preferred stock offering. The increase in interest expense was primarily attributable to borrowings under our lines of credit and the amortization of debt issuance costs.
Fiscal year ended December 31, 2001 compared to the period from inception (July 6, 2000) to December 31, 2000
Revenues. We did not record any revenues in the year ended December 31, 2001 or in the period from inception to December 31, 2000.
Research and development expense. Research and development expense increased $3.6 million to $3.8 million for the fiscal year ended December 31, 2001 from $232,000 for the period from inception to December 31, 2000. The increase was primarily attributable to an increase in personnel and all activities associated with our research and development programs, as scientific operations commenced in December 2000 with the first closing of our Series A convertible preferred stock offering.
General and administrative expense. General and administrative expense increased $1.2 million to $1.4 million for the fiscal year ended December 31, 2001 from $125,000 for the period from inception to December 31, 2000. This increase was primarily due to the full year of corporate operations in 2001 and higher compensation and benefits expenses related to new hires and higher facilities and related costs as corporate operations increased in December 2000 after the first closing of our Series A convertible preferred stock offering.
27
Stock-based compensation expense. Stock-based compensation expense increased $618,000 to $627,000 for the fiscal year ended December 31, 2001 from $9,000 for the period from inception through December 31, 2000. The increase was primarily due to an increase in the stock price used to measure stock-based compensation.
Interest income and interest expense. Interest income was $165,000 for the fiscal year ended December 31, 2001. We did not record any interest income in the period from inception to December 31, 2000. Interest expense was $40,000 for the fiscal year ended December 31, 2001 and $1,000 for the period from inception to December 31, 2000. The increase in interest expense for the fiscal year ended December 31, 2001 was attributable to borrowings under our lines of credit.
Liquidity and capital resources
We have funded our operations principally with the proceeds of $58.0 million from three redeemable convertible preferred stock offerings over the period from 2000 through 2003 as follows:
|
|---|
| |
| | Number of
shares
| | Price per
share
| |
|
|---|
Issue
| | Year of issuance
| | Amount
|
|---|
|
|---|
| Series A | | 2000 and 2001 | | 9,035,000 | | $ | 0.664 | | $ | 6,000,000 |
| Series B | | 2001 | | 17,000,000 | | | 1.00 | | | 17,000,000 |
| Series C | | 2002 and 2003 | | 35,000,000 | | | 1.00 | | | 35,000,000 |
| | | | |
|
| | | | | 61,035,000 | | | | | $ | 58,000,000 |
|
Each share of redeemable convertible preferred stock is convertible into one share of our common stock.
During 2002, we had access to an equipment line of credit and a leasehold improvement line of credit for $1.25 million and $1.75 million, respectively. We also had an $800,000 line of credit that expired in 2001. We borrowed an aggregate of $3.8 million under all of these lines of credit. The borrowings bear interest at approximately 7% and are repayable over 24 to 36 month periods. At the end of the term, we are required to pay an additional 9% to 9.5% of the original advance amount. The lines of credit are collateralized by our assets. As of June 30, 2003 and December 31, 2002, approximately $1.8 million and $2.6 million, respectively, were outstanding under these lines of credit.
At June 30, 2003, cash and cash equivalents were $35.6 million compared to $37.9 million at December 31, 2002. Our cash and cash equivalents are highly liquid investments with a maturity of three months or less at date of purchase and consist of time deposits and investments in money market funds with commercial banks and financial institutions. Also, we maintain cash balances with financial institutions in excess of insured limits. We do not anticipate any losses with respect to such cash balances.
Net cash used in operating activities was $1.9 million and $4.4 million for the six months ended June 30, 2003 and 2002, respectively. Net cash used in operating activities for the six months ended June 30, 2003 reflects the net loss of $6.6 million partially offset by non-cash charges for depreciation and amortization of $397,000 and stock-based compensation of $607,000 and the
28
collection of $4.5 million from Genentech. Net cash provided by investing activities during the six months ended June 30, 2003 was $200,000 and consisted of a decrease in restricted cash of $517,000 for the release of a portion of our facility lease covenant partially offset by expenditures of $317,000 for property and equipment. Net cash used in financing activities for the six months ended June 30, 2003 was $586,000, which consisted primarily of $780,000 of repayments related to our lines of credit partially offset by $131,000 of allowances received from our landlord, and $60,000 from the final closing of our Series C convertible preferred stock offering.
Net cash used in operating activities was $12.5 million and $3.4 million for the years ended December 31, 2002 and 2001, respectively. The net loss for 2002 of $14.3 million was partially offset by non-cash charges for depreciation and amortization of $1.1 million and stock-based compensation of $613,000. The net loss for 2001 of $5.7 million was partially offset by non-cash charges for depreciation and amortization of $168,000 and stock-based compensation of $627,000. Net cash used in investing activities for the fiscal year ended December 31, 2002 was $4.7 million and consisted of purchases of property and equipment. Net cash from financing activities for the year ended December 31, 2002, was $38.5 million, which consisted primarily of $34.8 million in net proceeds from the issuance of Series C convertible preferred stock, $2.7 million from our lines of credit, and $2.1 million in allowances received from our landlord for the build out of our new facility, partially offset by repayments of $1.0 million related to our lines of credit.
In addition to our lines of credit, we also have contractual obligations related to our facility lease, research services agreements, consulting agreements, and license agreements. The following table summarizes our contractual obligations at June 30, 2003 and the effects such obligations are expected to have on our liquidity and cash flows in future periods.
|
|---|
(in thousands)
| | Total
| | July to
December
2003
| | 2004
| | 2005
| | 2006
| | 2007
| | 2008
| | After 2008
|
|---|
|
|---|
| Short and long-term debt | | $ | 2,245 | | $ | 867 | | $ | 1,069 | | $ | 309 | | $ | — | | $ | — | | $ | — | | $ | — |
| Operating lease obligations | | | 19,962 | | | 1,082 | | | 2,164 | | | 2,164 | | | 2,164 | | | 2,259 | | | 2,315 | | | 7,814 |
| Other contractual obligations | | | 2,381 | | | 605 | | | 932 | | | 306 | | | 327 | | | 65 | | | 42 | | | 104 |
| | |
|
| | | $ | 24,588 | | $ | 2,554 | | $ | 4,165 | | $ | 2,779 | | $ | 2,491 | | $ | 2,324 | | $ | 2,357 | | $ | 7,918 |
|
|---|
We expect to incur losses from operations for the foreseeable future. We expect to incur increasing research and development expenses, including expenses related to the hiring of personnel and additional clinical trials. We expect that our general and administrative expenses will increase in the future as we expand our finance and administrative staff, add infrastructure, and incur additional costs related to being a public company, including directors' and officers' insurance, investor relations programs, and increased professional fees. Our future capital requirements will depend on a number of factors, including the continued progress of our research and development of products, the timing and outcome of clinical trials and regulatory approvals, payments received or made under the Genentech agreement and any future collaborative agreements that we may enter into, the costs involved in preparing, filing, prosecuting, maintaining, defending, and enforcing patent claims and other intellectual property rights, the acquisition of licenses to new products or compounds, the status of competitive products, the availability of financing, and our collaborators' success in developing markets for our products. We believe our existing cash and cash equivalents, together with the
29
net proceeds of this offering, will be sufficient to fund our operating expenses, debt repayments, and capital equipment requirements for at least the next three years.
Currently we have no availability under our credit facilities or other committed sources of capital. To the extent our capital resources are insufficient to meet future capital requirements, we will need to raise additional capital or incur additional indebtedness to fund our operations. We cannot assure you that additional equity or debt financing will be available on acceptable terms, if at all. If adequate funds are not available, we may be required to delay, reduce the scope of, or eliminate our research and development programs, reduce our commercialization efforts, or obtain funds through arrangements with collaborative partners or others that may require us to relinquish rights to certain product candidates that we might otherwise seek to develop or commercialize independently. Any future equity funding may dilute the ownership of our equity investors.
Critical accounting policies and estimates
Our discussion and analysis of our financial condition and results of operations are based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, and expenses. On an ongoing basis, we evaluate our estimates and judgments, including those related to revenue recognition, accrued expenses, and the fair valuation of stock related to stock-based compensation. We based our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
We believe the following critical accounting policies affect our more significant judgments and estimates used in the preparation of our financial statements.
Revenue recognition. We recognize revenue relating to our collaborative arrangements in accordance with the SEC's Staff Accounting Bulletin No. 101, or SAB 101, "Revenue Recognition in Financial Statements." Revenues under such collaborative arrangements may include non-refundable license fees, milestones, and research and development payments.
Where we have continuing performance obligations under the terms of a collaborative arrangement, non-refundable license fees are recognized as revenue over the period in which we expect to complete our performance obligations. We have estimated the period over which we will complete our performance obligations under our collaboration agreement with Genentech to be three years. If this estimated performance period changes, then we will record an adjustment to the cumulative revenue recognized under the agreement and will record the remaining unrecognized non-refundable license fees over this new performance period. Significant judgments and estimates are involved in determining the estimated development period and different assumptions could yield materially different results.
Revenues from milestones related to an arrangement under which we have continuing performance obligations, if deemed substantive, are recognized as revenue upon achievement of the milestones. A milestone is considered substantive, if all of the following conditions are
30
met: the milestone is non-refundable; achievement of the milestone was not reasonably assured at the inception of the arrangement; substantive effort is involved to achieve the milestone; and the amount of the milestone appears reasonable in relation to the effort expended, the other milestones in the arrangement, and the related risk associated with the achievement of the milestone. If any of these conditions are not met, the milestone payment is deferred and recognized as revenue as we complete our performance obligations. Revenues from milestones related to an arrangement under which we have no continuing performance obligations are recognized as revenue upon achievement of the milestones.
Payments received from collaborative partners for research and development efforts performed by us are recognized as revenue over the contract term as the related costs are incurred. We recognize such revenue only if we believe that collection of these amounts is reasonably assured. This assessment involves judgment on our part. If we do not believe that collection of amounts billed, or amounts to be billed to our collaborators, is reasonably assured, then we defer revenue recognition. To date we have not received or recognized any research and development payments.
Accrued expenses. As part of the process of preparing financial statements, we are required to estimate accrued expenses. This process involves identifying services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for such service as of each balance sheet date in our financial statements. Examples of estimated accrued expenses include professional service fees, such as lawyers and accountants, contract service fees, such as amounts paid to clinical monitors, data management organizations, and investigators in conjunction with clinical trials, and fees paid to contract manufacturers in conjunction with the production of clinical materials. In connection with such service fees, our estimates are most affected by our understanding of the status and timing of services provided relative to the actual level of services incurred by such service providers. The majority of our service providers invoice us monthly in arrears for services performed. In the event that we do not identify certain costs that have begun to be incurred or we under or over estimate the level of services performed or the costs of such services, our reported expenses for such period could be overstated or understated. The date on which certain services commence, the level of services performed on or before a given date, and the cost of such services are often judgmental. We make these judgments based upon the facts and circumstances known to us in accordance with generally accepted accounting principles.
Stock-based compensation. We have elected to follow APB Opinion No. 25,Accounting for Stock Issued to Employees, and related interpretations, in accounting for our stock-based compensation plans, rather than the alternative fair value accounting method provided for under SFAS No. 123,Accounting for Stock-Based Compensation. In the notes to our financial statements, we provide pro forma disclosures in accordance with SFAS No. 123 and related pronouncements. We account for transactions in which services are received in exchange for equity instruments based on the fair value of such services received from non-employees or of the equity instruments issued, whichever is more reliably measured, in accordance with SFAS No. 123 and Emerging Issues Task Force ("EITF") Issue No. 96-18,Accounting for Equity Instruments that Are Issued to Other than Employees for Acquiring, or in Conjunction with Selling, Goods or Services. The factor which most affects charges or credits to operations related to stock-based compensation is the fair value of the common stock underlying stock options for which stock-based compensation is recorded. If our estimates of the fair value of
31
these equity instruments are too high or too low, it would have the effect of overstating or understating expenses, respectively. Because shares of our common stock have not been publicly traded, market factors historically considered in valuing stock and stock option grants include pricing of private sales of our redeemable convertible preferred stock and the effect of events that have occurred between the time of such grants, economic trends, perspective provided by investment banks, and the comparative rights and preferences of the security being granted compared to the rights and preferences of our other outstanding equity.
Recent accounting pronouncements
In June 2002, the Financial Accounting Standards Board ("FASB") issued SFAS No. 146,Accounting for Costs Associated with Exit and Disposal Activities. SFAS No. 146 requires that a liability for costs associated with an exit or disposal activity be recognized and measured initially at fair value only when the liability is incurred. The provisions of SFAS No. 146 are effective for exit disposal activities that are initiated after December 31, 2002. SFAS No. 146 did not have a material impact on our financial position, results of operations, or cash flows.
In November 2002, the EITF reached a consensus on Issue No. 00-21,Revenue Arrangements with Multiple Deliverables. EITF Issue No. 00-21 provides guidance on how to account for arrangements that involve the delivery or performance of multiple products, services, and/or rights to use assets. The provisions of EITF Issue No. 00-21 will apply to revenue arrangements entered into in fiscal periods beginning after June 15, 2003. We do not expect the adoption of EITF Issue No. 00-21 to have a material impact on our financial position, results of operations, or cash flows.
In December 2002, the FASB issued SFAS No. 148,Accounting for Stock-Based Compensation, Transition and Disclosure. SFAS No. 148 provides alternative methods of transition for a voluntary change to the fair value based method of accounting for stock-based employee compensation. SFAS No. 148 also requires that disclosures of the pro forma effect of using the fair value method of accounting for stock-based employee compensation be displayed more prominently and in a tabular format. Additionally, SFAS No. 148 requires disclosures of the pro forma effect in interim financial statements. The transition and annual disclosure requirements of SFAS No. 148 are effective for fiscal years ending after December 15, 2002, and have been adopted by us, and we have elected to continue to account for employee stock options under APB No. 25.
In November 2002, the FASB issued FASB Interpretation ("FIN") No. 45,Guarantor's Accounting and Disclosure Requirements for Guarantees, Including Guarantees of Indebtedness of Others, and interpretation of FASB Statements No. 5, 57, and 107 and Rescission of FASB Interpretation No. 34. This interpretation expands the disclosure requirements of guarantee obligations and requires the guarantor to recognize a liability for the fair value of the obligation assumed under a guarantee. In general, FIN No. 45 applies to contracts or indemnification agreements that contingently require the guarantor to make payments to the guaranteed party based on changes in an underlying instrument that is related to an asset, liability, or equity security of the guaranteed party. Other guarantees are subject to the disclosure requirements of FIN No. 45 but not to the recognition provisions. The disclosure requirements of FIN No. 45 are effective for financial statement periods ending after December 15, 2002. The adoption of this standard did not have a material impact on our financial statements at December 31, 2002. In
32
addition, the impact of new arrangements entered into since January 1, 2003 is not significant. We have incorporated the disclosure requirements of FIN No. 45 into the footnotes to our financial statements.
In January 2003, the FASB issued FIN No. 46,Consolidation of Variable Interest Entities, and interpretation of ARB 51. The primary objectives of FIN No. 46 are to provide guidance on the identification of entities for which control is achieved through means other than through voting rights (variable interest entities) and to determine when and which business enterprise should consolidate the variable interest entities. This new model for consolidation applies to an entity which either (1) the equity investors (if any) do not have a controlling financial interest or (2) the equity investment at risk is insufficient to finance the entity's activities without receiving additional subordinated financial support from other parties. FIN No. 46 also requires enhanced disclosure requirements related to variable interest entities. FIN No. 46 applies immediately to variable interest entities created after January 31, 2003, and to variable interest entities in which an enterprise obtains an interest after the date. It applies in the first fiscal year or interim period beginning after June 15, 2003 to variable interest entities in which an enterprise holds a variable interest that it acquired before February 1, 2003. The adoption of this standard did not have an impact on our consolidated financial statements at December 31, 2002.
In April 2003, the FASB issued SFAS No. 149,Amendment of Statement 133 on Derivative Instruments and Hedging Activities. SFAS No. 149 amends and clarifies financial accounting and reporting for derivative instruments, including certain derivative instruments embedded in other contracts (collectively referred to as derivatives) and for hedging activities under FASB Statement No. 133,Accounting for Derivative Instruments and Hedging Activities. We do not expect that the adoption of SFAS No. 149 will have a material effect on our financial position, results of operations, or cash flows.
In May 2003, the FASB issued SFAS No. 150,Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity. SFAS No. 150 establishes standards for how an issuer classifies and measures certain financial instruments with characteristics of both liabilities and equity. It requires that an issuer classify a financial instrument that is within its scope as a liability (or an asset in some circumstances). Many of those instruments were previously classified as equity. This statement is effective for financial instruments entered into or modified after May 31, 2003, and otherwise is effective at the beginning of the first interim period beginning after June 15, 2003. We do not anticipate that the adoption of SFAS No. 150 will have a material effect on our financial statements.
Quantitative and qualitative disclosures about market risk
Our exposure to market risk is currently confined to our cash and cash equivalents and restricted cash that have maturities of less than three months. We currently do not hedge interest rate exposure. We have not used derivative financial instruments for speculation or trading purposes. Because of the short-term maturities of our cash and cash equivalents, we do not believe that an increase in market rates would have any significant impact on the realized value of our investments.
33
Business
Overview
We are a biopharmaceutical company focused on the discovery, development, and commercialization of novel therapies that harness the newly-discovered, natural mechanisms of immunological tolerance. Our goal is to utilize these mechanisms to design therapies that reprogram the immune system in order to treat autoimmune disease patients, organ transplant recipients, and patients suffering from other harmful immune system responses. Our therapies are designed to provide long-term efficacy after a short course of treatment without compromising the immune system's ability to protect the body against invading bacteria, viruses, and fungi.
We have established a pipeline of two monoclonal antibodies in human clinical trials and three monoclonal antibodies in preclinical development. Our TRX4 antibody is in an investigator-sponsored Phase II trial of 80 patients in Europe with new-onset Type I diabetes in which all patients have completed treatment and are currently in the follow-up stage. We intend to file an Investigational New Drug, or IND, application with the U.S. Food and Drug Administration, or FDA, for TRX4 by early 2004 to expand our indications to include psoriasis and psoriatic arthritis. Based on the results of the ongoing European trial, we also intend to file an IND or IND supplement to conduct additional studies in new-onset Type I diabetes. Our TRX1 antibody is being developed, in collaboration with Genentech, to induce immunological tolerance in transplantation, autoimmune disease, and clinical situations where the immune system attacks a therapeutic protein. We completed a Phase I clinical trial of TRX1 in the United Kingdom in July 2003. Our research and development programs are designed to generate new products based on our unique understanding of the mechanisms of immunological tolerance and the cells that mediate tolerance. We have also developed the TolerMab antibody technology which modifies monoclonal antibodies so they can generate tolerance to themselves. TolerMab antibodies are designed to allow for repetitive dosing in immunocompetent patients with chronic diseases without eliciting the interfering immune system response often encountered with traditional long-term antibody therapy.
The immune system and immunological tolerance
The human immune system protects the body against infection by bacteria, viruses, and fungi predominantly through the action of a group of blood cells collectively called white blood cells or leukocytes. A subset of leukocytes known as T lymphocytes, or T cells, have a surface receptor that recognizes a specific antigen presented to them by other cells of the immune system called antigen presenting cells, or APCs. APCs are found throughout the body and function as sentinels guarding against invasion by pathogenic organisms. APCs continuously sample their surrounding environment for antigens which they process, transport, and present to T cells. When T cells recognize a specific antigen presented by APCs, the APCs also instruct the T cells whether the antigen is dangerous or harmful to the body and should be eliminated through a classical immune response, or whether it is non-threatening and should be preserved through a tolerogenic immune response. In a normal immune system, leukocytes eliminate pathogens and other dangerous antigens without inflicting damage to the body's own healthy cells and tissues.
34
The Classical Immune Response. A classical immune response is initiated when APCs present antigens to T cells in the presence of alarm signals from the body's own cells and from foreign invaders, such as molecules associated with inflammation and cell death. This type of antigen presentation results in T cell activation and the generation of a subtype of T cells known as T-effector cells. These T-effector cells orchestrate the immunological processes that eliminate the antigen and cells which contain the antigen on their surface.
The Tolerogenic Immune Response. Immunological tolerance is a normal state in which the immune system recognizes an antigen, or tissue bearing an antigen, as non-dangerous and thus prevents a harmful or misdirected attack. A tolerogenic immune response begins through a similar pathway as the classical immune response with antigen presentation by APCs and T cell activation. Without alarm signals, or in the presence of certain tolerance-promoting molecules, antigen presentation by APCs to T cells results in the generation of a newly discovered subtype of T cells known as T-regulatory cells. These T-regulatory cells are potent mediators of immunological tolerance in the body and can actively suppress T-effector cells.
The classical immune response and the tolerogenic immune response can be considered competing and reciprocal immune responses to a specific antigen. When the classical immune response is dominant, T-effector cells mediate the elimination of the antigen and cells which contain the antigen on their surface. In contrast, when the tolerogenic immune response is dominant, T-regulatory cells mediate the preservation of the antigen and cells which contain the antigen on their surface.
The problems associated with immune system diseases and current treatments
A normally functioning immune system makes the appropriate determination between permitting the classical immune response or the tolerogenic immune response to dominate upon presentation of an antigen. In autoimmune disease, T cells are incorrectly instructed to respond to the body's own, or self, antigens that are non-threatening as though they are harmful or dangerous. This leads to the generation of T-effector cells specific for self antigens which dominate over T-regulatory cells, and the immune system harmfully attacks its own tissue. For example, in Type I diabetes, the immune system attacks and destroys the insulin-producing cells of the pancreas. As a result of this attack, the Type I diabetes patient loses the ability to secrete insulin and to control glucose levels, requiring life-long insulin injections. People with Type I diabetes are at an increased risk of visual impairment and blindness, serious foot problems, kidney disease, and damage to the nervous system. Other conditions associated with adverse, harmful, or misdirected immune responses to self tissues include psoriasis, psoriatic arthritis, rheumatoid arthritis, multiple sclerosis, and systemic lupus erythematosis.
Historically, autoimmune diseases have been treated with drugs that suppress the entire immune system. These immunosuppressive drugs must be administered continuously as discontinuation of these drugs results in relapse of disease. These therapies do not specifically target the cause of a disease and can have numerous harmful effects, including rendering the patient susceptible to life-threatening infections, malignancies, and metabolic disorders.
In organ transplant patients, the immune system often rejects the transplanted organ instead of tolerating it as the body's own tissue. Accordingly, transplant patients generally must receive multiple immunosuppressive drugs. This course of therapy causes numerous side effects for transplant recipients, including an increased risk of infection as well as an increased risk of
35
cancer after transplant. In order to further reduce the risk of organ rejection, tremendous efforts are made to match potential donors with recipients. Despite this regimen, approximately 30% of patients experience an acute rejection episode.
Therapeutic proteins are increasingly used to treat diseases, including interferon beta for multiple sclerosis, Factor VIII for hemophilia A, and monoclonal antibodies for various disorders. However, these proteins frequently elicit an immune response that can reduce their efficacy or preclude repeat dosing. Betaseron, an interferon beta product marketed by Schering AG and Berlex Laboratories, has been reported to generate an undesirable immune response in approximately 45% of multiple sclerosis patients. Approximately 30% to 40% of patients with severe hemophilia A develop neutralizing antibodies to Factor VIII replacement therapy. The use of Johnson & Johnson's approved monoclonal antibody, Orthoclone OKT3, to treat transplant rejection has been limited by the immune response against it and first dose reactions such as fever, chills, and pulmonary and gastrointestinal symptoms.
Despite progress in the use of immunosuppressive drugs and therapeutic proteins, there is still a significant, unmet clinical need for more effective and safer therapies for patients with diseases of the immune system.
The TolerRx solution
Our solution is to harness the body's natural tolerance mechanisms to develop therapies that reprogram the immune system and provide a long-term, durable effect after a short course of therapy.
T cells are at the heart of most immune responses. We have focused our research efforts on a new class of T cells, called T-regulatory cells, which can actively suppress the generation and function of T-effector cells. T-regulatory cells are among the most powerful mechanisms used by the body to establish and maintain immunological tolerance. T-regulatory cells, and their interaction with other cells of the immune system, represent a new discovery relating to the function of a normal immune system. We believe that T-regulatory cells provide novel mechanisms and targets for use in the development of new products to treat patients with diseases of the immune system. Additionally, we are developing surrogate markers and prognostic indicators of T-regulatory cell dominance and tolerance which we believe could serve as early efficacy endpoints in clinical development and allow us to improve our clinical outcomes.
Our therapies are designed to change the immune system's perception of autoimmune disease related antigens and transplanted organ antigens so that T-regulatory cells are induced, tolerogenic mechanisms become dominant, and normal immunological defenses against invading pathogens are preserved. As these therapies induce natural mechanisms of the immune system, we believe they will be safe, durable, and effective, and in some cases may provide a cure. For example, we intend to develop therapies for new-onset Type I diabetes that induce the immune system to suppress the T-effector cells that cause injury to the insulin-producing cells in the pancreas and thereby promote tolerance to these insulin-producing cells. In addition, we intend to develop therapies that will allow transplanted organs to be accepted without the chronic use of immunosuppressive drugs.
36
Our strategy
Our goal is to become the recognized leader in the discovery, development, and commercialization of products to treat patients with diseases of the immune system. Our strategy to achieve this goal is to:
Develop and commercialize our lead products to provide breakthrough therapies that induce immunological tolerance. We are evaluating TRX4 in an investigator-sponsored Phase II clinical trial of 80 patients in Europe with new-onset Type I diabetes. We are also developing TRX4 for psoriasis and psoriatic arthritis. In collaboration with Genentech, we are developing our TRX1 monoclonal antibody to induce tolerance in transplantation, autoimmune disease, and clinical situations where the immune system attacks therapeutic proteins or biologic drugs. We completed a Phase I clinical trial of TRX1 in the United Kingdom in July 2003, and TRX1 appeared to be generally well tolerated. We believe that our lead products will provide breakthrough therapies for immune system diseases by establishing or re-establishing long-lasting immunological tolerance to the disease causing antigens after only a short course of treatment.
Generate new products for our pipeline based on our understanding of T-regulatory cells. We have performed extensive studies comparing T-regulatory cells to T-effector cells in order to identify genes, proteins, and signaling pathways that are unique to or preferentially expressed by each cell population. These genes, proteins, and pathways represent target opportunities for novel immunological therapies. Targets that can inhibit T-effector cells and not T-regulatory cells, or targets that activate T-regulatory cells, could lead to tolerance promoting therapies. Conversely, therapies that block the function of T-regulatory cells and not T-effector cells may be utilized to temporarily disrupt tolerance mechanisms that a solid tumor or chronic viral infection tends to induce, thereby allowing the immune system to remove the cancer cells or virus. We plan to expand our pipeline by generating monoclonal antibody and small molecule products directed against our newly discovered targets.
Leverage collaborative partnerships. Leveraging the resources of collaborative partners is an important element of our strategy to exploit the products and targets being generated from our research and development programs. In these collaborations, we seek to maintain co-development, profit sharing, and commercialization opportunities for our products. In December 2002, we entered into a collaboration agreement with Genentech for the development and commercialization of TRX1. We believe that this collaboration allows us to enhance the development of TRX1 through the funding of research and by providing us with Genentech's expertise in manufacturing, preclinical and clinical development, regulatory affairs, and marketing.
Retain commercialization rights to certain products. We intend to maximize the value of our product pipeline by retaining rights to certain products for internal development and commercialization. We have retained all rights for the internal development and commercialization of TRX4, TolerMab TRX4, and TolerMab TRX3.
Exploit knowledge of immunological tolerance to optimize clinical development. We are utilizing our knowledge of T-regulatory cells to identify surrogate markers of tolerance that can serve as early endpoints in our clinical trials. With further development, we believe these surrogate markers will serve as prognostic indicators that will allow us to assess the severity of
37
a patient's condition, adjust a patient's dosing regimen, and monitor a patient's progress towards the establishment of immunological tolerance. We expect that this approach will improve our clinical outcomes and accelerate the development of our drugs.
Commercialize the TolerMab antibody technology internally and through partners. We intend to make our TolerMab antibody technology the standard for enabling long-term, repeated dosing of antibodies in immunocompetent patients with chronic diseases. We plan to enter into agreements for TolerMab versions of partners' antibodies in exchange for license fees, milestone payments, and royalties. In addition, we intend to use the TolerMab technology to develop products for our internal pipeline, such as TolerMab TRX3 and TolerMab TRX4.
Drug development programs
We currently have five monoclonal antibodies in our pipeline, two in human clinical trials and three in preclinical development, as follows:
|
|---|
Product
| | Indication
| | Development stage
| | Development and
commercialization rights
|
|---|
|
| TRX4 | | Type I Diabetes | | Phase II(1) | | TolerRx |
|
|
Psoriasis/Psoriatic Arthritis |
|
Preclinical |
|
|
|
| TRX1 | | Transplantation,
Autoimmune Disease, and
Tolerance to Therapeutic Proteins | |
Phase I | | Genentech/TolerRx |
|
| TRX2 | | Transplantation | | Preclinical | | TolerRx(2) |
|
| TolerMab TRX3 | | Autoimmune Disease | | Preclinical | | TolerRx |
|
| TolerMab TRX4 | | Autoimmune Disease | | Preclinical | | TolerRx |
|
(1) Investigator-sponsored trial in Europe.
(2) Genentech has been granted an exclusive right of first negotiation.
TRX4
TRX4 is a humanized monoclonal antibody that binds to a receptor found on all T cells called CD3, which is involved in normal T cell signaling. TRX4 is designed to block the function of T-effector cells that attack the body and cause autoimmune disease. Because T-effector cells and T-regulatory cells function differently, TRX4 is expected to have a favorable effect on T-regulatory cells, thereby promoting a state of immunological tolerance. We are developing TRX4 to treat patients with Type I diabetes, psoriasis, and psoriatic arthritis.
38
CD3 is a well validated target for monoclonal antibody therapeutics. The first monoclonal antibody approved by the FDA was the murine anti-CD3 antibody, Orthoclone OKT3, by Johnson & Johnson. OKT3 is used to deplete T cells in order to reverse organ transplant rejection. However, the use of OKT3 has been limited due to early adverse effects, including fevers, chills, and pulmonary and gastrointestinal symptoms. Additionally, the clinical utility of OKT3 has been limited by the immune response that the patient generates against it.
TRX4 is currently being studied in an investigator-sponsored Phase II clinical trial in Europe in patients with new-onset Type I diabetes. As of March 2003, all 80 patients had completed treatment in this randomized, double-blind, placebo-controlled trial and are currently in an 18-month follow-up phase. The trial is being conducted by investigators associated with the Brussels Free University, the Juvenile Diabetes Research Foundation Center for Beta Cell Therapy, Belgium Diabetes Registry, and Hospital Necker in Paris, France, with financial support from the Juvenile Diabetes Research Foundation. Based on the results of this trial, we intend to submit an IND to the FDA to further develop TRX4 for the treatment of Type I diabetes.
In Type I diabetes, the pancreas produces little or no insulin as a result of the immune system attacking and destroying the insulin-producing cells in the pancreas. According to the Juvenile Diabetes Research Foundation, approximately one million people in the United States have Type I diabetes and 30,000 new patients are diagnosed each year, including over 13,000 children. TRX4 has been designed to treat patients with Type I diabetes by blocking T-effector cells from attacking insulin-producing cells. Published studies indicate that blocking these T cells early in the disease process with anti-CD3 therapy will decrease injury to the insulin producing cells and preserve the normal function of these cells. In one study, 75% of treated patients showed improvement or lack of disease progression one year after a single therapeutic course. We believe anti-CD3 therapy in new-onset Type I diabetes has the potential to have a durable therapeutic effect, a characteristic that no approved treatment regime can claim in this indication. We expect our therapy to provide efficacy and an improved quality of life for these patients by decreasing their dependence on insulin injections and restoring normal glucose control.
We intend to file an IND in the United States by early 2004 to expand our TRX4 clinical indications and commence a clinical trial of TRX4 in patients with psoriasis or psoriatic arthritis.
Psoriasis is an immune-mediated, genetic disease of the skin that affects about two percent of the U.S. population. The most severe and disabling cases of psoriasis require systemic medications such as methotrexate and cyclosporine, which have severe side effects including decreased kidney function, high blood pressure, and high cholesterol. Several new treatments for psoriasis include biologics, such as Amevive®, which must be injected or infused into the body rather than taken orally.
Psoriatic arthritis is a form of joint disease that affects approximately 25% of psoriasis patients. First-line therapies for psoriatic arthritis are nonsteroidal anti-inflammatory drugs, or NSAIDs, including aspirin and ibuprofen, which provide some relief of joint pain, inflammation, and stiffness. NSAIDs have been reported to trigger flares of psoriasis and cause stomach problems including ulcers and gastrointestinal bleeding. Patients taking NSAIDs often continue to have
39
their disease progress, and they typically begin using a class of second-line therapies known as disease-modifying antirheumatic drugs, such as methotrexate or sulfasalizine. Many people cease using these drugs because of side effects that can include nausea, vomiting, and loss of appetite. The third-line therapies are biologics, including drugs which block an inflammatory cytokine called tumor necrosis factor alpha. These biologic drugs provide relief only of the symptoms of the disease and do not affect the underlying cause. Current biologic therapy for psoriatic arthritis must be administered chronically because symptoms return if treatment is ended.
Published studies have indicated that anti-CD3 therapy has the potential for therapeutic benefit in psoriasis and psoriatic arthritis. In one study, anti-CD3 therapy resulted in a statistically significant reduction in the number of tender and swollen joints 30 days and 90 days after therapy. The anti-CD3 therapy also resulted in an improvement in the accompanying skin disease.
We are developing a TolerMab form of TRX4 as a potential second generation product. TolerMab TRX4 is in preclinical development. We own all rights for the development and commercialization of TRX4 and TolerMab TRX4.
TRX1
In December 2002, we entered into a collaboration agreement with Genentech for the development and commercialization of products targeting the CD4 molecule, including TRX1. TRX1 is a humanized monoclonal antibody that binds to the CD4 receptor found on both T-effector cells and T-regulatory cells. Because T-effector cells utilize different signaling pathways upon antigen presentation as compared to T-regulatory cells, some of which are associated with the CD4 receptor, TRX1 is expected to block the activation and function of T-effector cells and to favor the dominance of T-regulatory cells. TRX1 is being developed to induce immunological tolerance in transplantation, autoimmune diseases, and in settings requiring the chronic administration of therapeutic proteins.
In July 2003, we completed a single-dose, placebo-controlled, double-blind Phase I trial in the United Kingdom with TRX1. The trial evaluated doses of 1, 5 and 10 mg/kg of TRX1 in normal healthy volunteers. The endpoints of the trial were designed to assess the safety and pharmacokinetic parameters of TRX1. In the study, TRX1 appeared to be well tolerated, did not deplete T cells, and first dose side effects, such as fevers, chills, and hypotension were not observed. At the highest dosing group of 10 mg/kg, a rash was observed in two of three TRX1-treated volunteers at 17 and 20 days after dosing, which required symptomatic therapy to resolve the rash. Rash was not observed at the 1 and 5 mg/kg dosing groups. Based upon the pharmacokinetic profile of TRX1, we believe that the effective tolerizing dose of TRX1 will be lower than 10 mg/kg, thereby reducing the risk of rash.
In nonhuman primates, we have demonstrated that a short course of TRX1 can induce a long-term, antigen-specific tolerance to a normally immunogenic biological protein drug without compromising normal immune function. We believe that TRX1 could be applicable in situations where administration of a biologic protein therapy induces an interfering immune response, such as Factor VIII therapy in hemophilia A, interferon beta therapy in multiple sclerosis, enzyme replacement therapy, and therapies involving repeated administration of antibodies. For example, approximately 30% to 40% of patients with severe hemophilia A
40
develop neutralizing antibodies to Factor VIII, requiring clinical tolerization strategies involving increased Factor VIII dosing and expenses of approximately $1 million per patient during one year of treatment. In collaboration with Genentech, we are evaluating the potential use of TRX1 to induce tolerance to therapeutic proteins or biologic drugs.
In addition, we are evaluating TRX1 in nonhuman primates as a tolerance induction therapy in kidney transplantation. The objective is to prevent transplant rejection, and decrease or eliminate the need for chronic, immunosuppressive, maintenance therapy. From July 2001 to June 2002, approximately 25,000 patients in the U.S. received an organ transplant, approximately 80,000 people were on a waiting list to receive a transplant, and approximately 6,300 people died while waiting for a transplant. Our preclinical studies are examining the parameters required for successful induction of transplantation tolerance and are expected to provide the foundation for the design of human clinical trials using TRX1 in transplantation.
As part of our collaboration with Genentech, we are evaluating TRX1 in preclinical models as a primary therapy for patients with autoimmune diseases. Our objective will be to induce tolerance to autoimmune disease causing antigens.
TRX2
TRX2 is a humanized monoclonal antibody that binds to the CD8 receptor found on a subset of T cells. TRX2 is currently in preclinical development in a renal transplantation study in nonhuman primates as combination therapy with TRX1. We believe that TRX2 could be synergistic with TRX1 for the induction of immunological tolerance in organ transplantation due to the role that T cells bearing the CD8 receptor have in the early rejection of transplants. TRX2 is designed to deplete these T cells at the time of transplant so that they do not cause rejection of the transplant before immunological tolerance can be established by TRX1. In addition to the current agreement with Genentech that covers uses of TRX1 in combination therapy with other therapeutics including TRX2, we have also granted Genentech a right of first negotiation for TRX2 and any rights we may own or control that solely relate to anti-CD8 antibodies if we decide to seek a partner for such products.
TolerMab antibody technology
One of the problems associated with monoclonal antibody drugs is that the patient's immune system may incorrectly respond to the antibody as dangerous and eliminate it from the body, thereby decreasing its effectiveness. While humanized and fully human antibodies generate less of an immune response than mouse or rat antibodies, published data regarding certain antibodies currently marketed or under development indicate that the body will still generate an immune response to these antibodies. We believe that this immunogenicity will continue to be recognized as a significant clinical issue. For example, the fully human antibody Humira®, marketed by Abbott Laboratories, has been reported to elicit a 12% rate of immunogenicity in rheumatoid arthritis trials, and previously, it has been reported that the humanized antibody Campath® from ILEX Oncology had a greater than 50% rate of neutralizing antibody formation in rheumatoid arthritis patients.
Using our TolerMab technology, we intend to modify monoclonal antibodies to induce immunological tolerance to themselves while maintaining their therapeutic benefits. We have
41
two TolerMab antibodies in preclinical development, TolerMab TRX3 and TolerMab TRX4. The TolerMab technology is expected to provide the following benefits:
• effective, long-term, repeated dosing for the treatment of immunocompetent patients with chronic disease;
• lower first dose side effects than traditional antibodies that deplete their target cells; and
• no or lower rates of interfering immune responses.
Our proprietary TolerMab technology involves attaching two small amino acid sequences, or modifiers, to the antibody. Each modifier can move in and out of an antibody binding site. When a modifier blocks a binding site, the antibody is hindered from binding to target sites. However, binding to the target and antibody function occur when a modifier moves out of the binding site. The result is that the binding rate of the antibody to the target is decreased, resulting in an excess of free, non-cell bound antibody rather than cell bound antibody immediately after dosing. The immune system recognizes the high concentration of the unbound antibody as non-dangerous and triggers the induction of immunological tolerance to TolerMab antibodies. We believe that TolerMab antibodies bind to their target and are effective in the body after this tolerance induction is established. TolerMab antibodies are designed to be used repeatedly without the interference of a neutralizing immune response.
TolerMab TRX3
TolerMab TRX3 is a monoclonal antibody that binds to the CD2 receptor found on T cells and other subsets of lymphocytes. TolerMab TRX3 is in preclinical development for treatment of autoimmune diseases. Activated T cells have elevated levels of CD2 on their surface and have been shown to play an important role in autoimmune and inflammatory diseases such as psoriasis. Inhibiting the function of activated T cells by targeting the CD2 antigen is expected to have therapeutic benefit. Biogen's Amevive®, which was recently approved for the treatment of patients with psoriasis, is a fusion protein that is directed at the CD2 antigen.
TolerMab TRX3 has been designed to have similar therapeutic benefits as other CD2-related therapies but without immunogenicity. In a recent clinical trial of psoriasis patients, MedImmune reported that as many as 50% of patients generated an undesirable immune response to its humanized anti-CD2 monoclonal antibody MEDI-507. We own all rights for the development and commercialization of TolerMab TRX3.
Research and discovery
In our research and discovery programs, we have discovered, isolated, and characterized cells that mediate immunological tolerance. We have developed a unique understanding of how the immune system operates, both normally and in disease states. Our research and development efforts have produced and will continue to develop:
• a database consisting of over 100 potential targets, including components of signaling pathways, co-stimulatory molecules, cell surface molecules, and cytokines that selectively block or enhance the function of T-effector cells and T-regulatory cells;
• screening assays, or tests, for the identification of new tolerance promoting therapies;
42
• animal models that allow for a direct analysis of the efficacy of tolerance promoting therapies; and
• in vivo surrogate markers and prognostic indicators for tolerance to rapidly identify the most appropriate dosing strategies.
We have research and discovery programs to identify novel targets involved in immunological tolerance mediated by T-regulatory cells. We believe that research on targets found to be associated with T-effector cells rather than T-regulatory cells, or that activate T-regulatory cells, could lead to tolerance-inducing therapies to treat patients with immune system diseases.
The majority of our research to date has been focused on the therapeutic induction of immunological tolerance. However, in some diseases, the immune system fails to identify and eliminate a danger to the body. Certain tumors and viruses, such as hepatitis C and HIV, evade detection and removal by the immune system. These tumors and viruses appear to have defense mechanisms that enable them to induce T-regulatory cells and a tolerogenic immune response instead of a classical immune response. In these diseases, our goal is to discover therapies that block the function of T-regulatory cells rather than T-effector cells in order to disrupt the specific tolerance mechanisms that have been generated and unleash the body's immune system to eliminate the tumor or virus.
Overall, we have identified and are validating potentially relevant targets and have filed several patent applications to protect these discoveries. We have generated multiple monoclonal antibodies and fusion proteins against many of our cell surface targets. We also intend to develop small molecule therapeutics to selected intracellular targets. We will use our development experience to advance these new drug candidates.
Collaboration with Genentech
In December 2002, we entered into a collaboration agreement with Genentech for the development and commercialization of products that target the CD4 molecule, including our TRX1 monoclonal antibody. To date, we have received a total of $8.0 million from Genentech in the form of a license fee, an equity investment, and a milestone payment. Subject to the achievement of all development and regulatory approval milestones regarding multiple indications for products that target the CD4 molecule, we may receive up to approximately $80 million of additional funding from Genentech. If TRX1 receives marketing approval, Genentech would be responsible for commercialization and would record all revenue related to sales of TRX1, and we would receive royalty payments on net sales of TRX1. In lieu of receiving royalties on sales of TRX1 in the United States, we have the option to participate in a cost and profit sharing relationship and to support the commercialization of TRX1 through our own medical science liaisons who would educate the medical profession about our product, subject to Genentech's approval. We have also granted Genentech a right of first negotiation for products targeting the CD8 molecule on T cells, including our TRX2 monoclonal antibody. Genentech has the right to terminate our collaboration agreement at any time, with or without cause, 90 days after providing written notice. Our collaboration with Genentech, including the receipt of any payments from Genentech, is subject to further risks set forth in "Risk factors" contained elsewhere in this prospectus, in particular, the risks relating to our collaboration with Genentech appearing on pages 7 and 8.
43
Patent and proprietary rights
We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary rights are covered by valid and enforceable patents or trademarks or are effectively maintained as trade secrets. Accordingly, patent, trademark, and other proprietary rights are an essential element of our business. To date, we have licenses to approximately 95 issued U.S. and foreign patents. In addition, we own or have licenses to approximately 45 pending U.S. and foreign patent applications.
As our research and development efforts yield novel targets, small molecule drug candidates, and therapeutic antibodies, we intend to apply for broad composition of matter patents covering such targets, drug candidates, and antibodies. In addition, we intend to file patent applications claiming new methods of treating diseases for our identified drug candidates and antibodies. As we develop new technologies or novel improvements in our existing technologies, we will file for both composition of matter and method of use patents, as appropriate.
We are party to various agreements that give us rights to use technologies developed in collaborative research and development programs. These collaborations are intended to further our understanding of the mechanisms of tolerance induction and maintenance. We believe that this knowledge will lead to the discovery of novel targets, small molecule drug candidates, and therapeutic antibodies. We, either individually or jointly with our collaborators, in accordance with the terms of our agreements, may seek patent protection for these discoveries.
As a general matter, obtaining patents in the biopharmaceutical field is highly uncertain and involves complex legal, scientific, and factual matters. Obtaining a patent in the United States in the biopharmaceutical field can be expensive and can require several years. Failure to receive patents under the applications that we have filed, or that others have filed on our behalf, and may file from time to time could be harmful to us. Our patent filings in the United States may be subject to interference or re-examination proceedings. The defense and prosecution of interference and re-examination proceedings and related legal and administrative proceedings in the United States involve complex legal, scientific, and factual questions.
We also file patent applications outside of the United States. The laws of some foreign countries may not protect our proprietary rights to the same extent as do the laws of the United States. Third parties may attempt to oppose the issuance of our patents in foreign countries by initiating opposition proceedings. Additionally, if an opposition proceeding is initiated against any of our patent filings in a foreign country, that proceeding could have an adverse effect on the corresponding patents that are issued or pending in the United States. If we become involved in any interference, re-examination, opposition, or litigation proceedings in the United States or foreign countries regarding patent or other proprietary rights, those proceedings may result in substantial cost to us, regardless of the outcome. In addition, these proceedings may have a material adverse effect on our ability to develop, manufacture, market, or license our technologies or products, or to maintain or form strategic alliances.
Although we plan to aggressively prosecute our patent applications and defend our patents against third-party infringement, our patent applications may not result in the issuance of patents or, if issued, our patents may be challenged, invalidated, or circumvented. Moreover, our patents, to the extent they are or will be issued, may not provide us protection against competitors with other technologies. Our technologies and potential products may conflict
44
with patents that have been or may be granted to competitors, universities, or others. In particular, patent applications or patents for innovative and broadly applicable technologies, such as our TolerMab antibody platform, are sometimes challenged by third parties as obvious or as obvious extensions of technologies previously developed by those third parties.
As the biopharmaceutical industry expands and more patents are issued, the risk increases that our technologies and potential products may give rise to claims that they infringe the patents of others. Third parties claiming infringement of their proprietary rights could bring legal actions against us claiming damages and seeking to enjoin our commercialization of a product or our use of a technology. If any actions based on these claims are successful, in addition to any potential liability for damages, we could be required to obtain a license in order to continue to use a technology or to manufacture or market a product, or could be required to cease using those products or technologies. Any claim, with or without merit, could result in costly litigation and divert the efforts and attention of our scientific and management personnel. We may not prevail in any action, and any license required under any patent may not be made available on acceptable terms, if at all.
In addition to patent protection, we may rely on trade secrets, proprietary know-how, and continuing technological advances to develop and maintain our competitive position. To maintain the confidentiality of our trade secrets and proprietary information, all of our employees are required to enter into and adhere to a confidentiality and invention assignment agreement as a condition of employment. Additionally, these agreements require that our employees do not bring to us, or use without proper authorization, any third-party proprietary technology. We also require all of our consultants and collaborators that have access to proprietary property to execute confidentiality and invention assignment agreements in our favor before beginning their relationship with us. While such arrangements are intended to enable us to better control the use and disclosure of our proprietary property and provide for our ownership of proprietary technology developed on our behalf, they may not provide us with meaningful protection for such property and technology in the event of unauthorized use or disclosure.
Manufacturing and supply
We currently have no manufacturing facilities for clinical or commercial production of any monoclonal antibodies. We rely on third parties to manufacture our products for development and clinical trials. Under our collaboration agreement with Genentech, we expect that Genentech will manufacture the TRX1 monoclonal antibody for clinical and commercial use.
Government regulation
U.S. regulatory requirements
The U.S. Food and Drug Administration, or FDA, and comparable regulatory agencies in foreign countries regulate and impose substantial requirements upon the research, development, preclinical and clinical testing, labeling, manufacture, quality control, storage, approval, advertising, promotion, marketing, distribution, and export of pharmaceutical products, including biologics, as well as significant reporting and record-keeping obligations. State governments may also impose obligations in these areas.
45
In the United States, pharmaceutical products are regulated by the FDA under the federal Food, Drug, and Cosmetics Act, or FDCA, and other laws, including in the case of biologics, the Public Health Service Act. We believe, but cannot be certain, that our products will be regulated as biologics and drugs by the FDA. The process required by the FDA before biologics or drugs may be marketed in the United States generally involves the following:
• preclinical laboratory and animal tests performed under the FDA's Good Laboratory Practices regulations to assess potential safety and effectiveness;
• submission and approval of an IND which must become effective before clinical trials may begin in the United States;
• obtaining approval of Institutional Review Boards to protect the welfare and rights of human subjects in clinical trials;
• adequate and well-controlled human clinical trials to establish the safety and efficacy of the product in the product's intended use;
• development of manufacturing processes which conform to FDA-mandated current Good Manufacturing Practices, or cGMPs;
• laboratory evaluation of the product's formulation and stability; and
• FDA review and approval of either a Biologics License Application, or BLA, or a New Drug Application, or NDA, prior to any commercial sale or shipment of a product.
The testing and approval process requires substantial time, effort, and financial resources, and we cannot be certain that any approval will be granted on a timely basis, if at all.
The results of the preclinical tests, together with initial specified manufacturing information, the proposed clinical trial protocol, and information about the participating investigators, are submitted to the FDA as part of an IND, which must be approved before we may begin human clinical trials. Additionally, an independent Institutional Review Board at each medical site proposing to conduct the clinical trials must review and approve each study protocol and oversee conduct of the trial. An IND becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day period, raises concerns or questions about the conduct of the trials as outlined in the IND and imposes a clinical hold. If the FDA imposes a clinical hold, the IND sponsor must resolve the FDA's concerns before clinical trials can begin. Preclinical tests and studies can take several years to complete, and there is no guarantee that an IND we submit based on such tests and studies will become effective within any specific time period, if at all.
Human clinical trials are typically conducted in three sequential phases that may overlap:
• Phase I: The drug is initially introduced into healthy human subjects or patients and tested for safety and dosage tolerance. Absorption, metabolism, distribution, and excretion testing is generally performed at this stage.
• Phase II: The drug is studied in controlled, exploratory therapeutic trials in a limited number of subjects with the disease or medical condition for which the new drug is intended to be used in order to identify possible adverse effects and safety risks, to determine the preliminary or potential efficacy of the product for specific targeted diseases or medical conditions, and to determine dosage tolerance and the optimal effective dose.
46
• Phase III: When Phase II studies demonstrate that a specific dosage range of the drug is likely to be effective and the drug has an acceptable safety profile, controlled, large-scale therapeutic Phase III trials are undertaken at multiple study sites to demonstrate clinical efficacy and to further test for safety in an expanded patient population.
We cannot be certain that we will successfully complete Phase I, Phase II, or Phase III testing of our products within any specific time period, if at all. Furthermore, the FDA, the Institutional Review Board or we may suspend or terminate clinical trials at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk.
Results of preclinical studies and trials, as well as detailed information about the manufacturing process, quality control methods, and product composition, among other things, are submitted to the FDA as part of a BLA or NDA seeking approval to market and commercially distribute the product on the basis of a determination that the product is safe and effective for its intended use. BLAs are used for products that are regulated as biologics, such as antibodies, and NDAs are used for products that are regulated as drugs, such as synthetic chemicals. Before approving a BLA or NDA, the FDA will inspect the facilities at which the product is manufactured and will not approve the product unless cGMP compliance is satisfactory. If applicable regulatory criteria are not satisfied, the FDA may deny the BLA or NDA or require additional testing or information. As a condition of approval, the FDA also may require post-marketing testing or surveillance to monitor the product's safety or efficacy. Even after a BLA or NDA is approved, the FDA may impose additional obligations or restrictions (such as labeling changes), or even suspend or withdraw product approval on the basis of data that arise after the product reaches the market, or if compliance with regulatory standards is not maintained. We cannot be certain that any BLA or NDA we submit will be approved by the FDA on a timely basis, if at all. Also, any such approval may limit the indicated uses for which the product may be marketed. Any refusal to approve, delay in approval, suspension or withdrawal of approval, or restriction on indicated uses could have a material adverse impact on our business.
Each BLA or NDA must be accompanied by a user fee, pursuant to the requirements of the Prescription Drug User Fee Act, or PDUFA, and its amendments. According to the FDA's fee schedule, effective on October 1, 2003 for the fiscal year 2004, the user fee for an application requiring clinical data, such as a BLA or NDA, is $573,500. The FDA adjusts the PDUFA user fees on an annual basis. PDUFA also imposes an annual product fee for prescription drugs and biologics ($36,080 for the fiscal year 2004), and an annual establishment fee ($226,800) on facilities used to manufacture prescription drugs and biologics. We are not at the stage of development with our products where we are subject to these fees, but they are significant expenditures that will be incurred in the future and must be paid at the time of application submission to FDA.
Satisfaction of FDA requirements typically takes several years. The actual time required varies substantially, based upon the type, complexity, and novelty of the pharmaceutical product, among other things. Government regulation imposes costly and time-consuming requirements and restrictions throughout the product life cycle and may delay product marketing for a considerable period of time, limit product marketing, or prevent marketing altogether. Success in preclinical or early stage clinical trials does not assure success in later stage clinical trials. Data obtained from preclinical and clinical activities is not always conclusive and may be
47
susceptible to varying interpretations that could delay, limit, or prevent marketing approval. Even if a product receives marketing approval, the approval is limited to specific clinical indications. Further, even after marketing approval is obtained, the discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market.
After product approval, there are continuing significant regulatory requirements imposed by the FDA, including record-keeping requirements, obligations to report adverse experiences, and restrictions on advertising and promotional activities. Quality control and manufacturing procedures must continue to conform to cGMPs, and the FDA periodically inspects facilities to assess cGMP compliance. Additionally, post-approval changes in manufacturing processes or facilities, product labeling, or other areas require FDA review and approval. Failure to comply with FDA regulatory requirements may result in enforcement action by the FDA, including product recalls, suspension or revocation of product approval, seizure of product to prevent distribution, impositions of injunctions prohibiting product manufacture or distribution, and civil and criminal penalties. Maintaining compliance is costly and time-consuming. Nonetheless, we cannot be certain that we, or our present or future suppliers or third-party manufacturers, will be able to comply with all FDA regulatory requirements, and potential consequences of noncompliance could have a material adverse impact on our business.
FDA's policies may change, and additional government regulations may be enacted that could delay, limit, or prevent regulatory approval of our products or affect our ability to manufacture, market, or distribute our products after approval. Moreover, increased attention to the containment of healthcare costs in the United States and in foreign markets could result in new government regulations that could have a material adverse effect on our business. Our failure to obtain coverage, an adequate level of reimbursement, or acceptable prices for our future products could diminish any revenues we may be able to generate. Our ability to commercialize future products will depend in part on the extent to which coverage and reimbursement for the products will be available from government and health administration authorities, private health insurers, and other third-party payors. European Union and U.S. government and other third-party payors increasingly are attempting to contain healthcare costs by consideration of new laws and regulations limiting both coverage and the level of reimbursement for new drugs. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative or administrative action, either in the United States or abroad.
Our activities also may be subject to state laws and regulations that affect our ability to develop and sell our products. We are also subject to numerous federal, state, and local laws relating to such matters as safe working conditions, clinical, laboratory, and manufacturing practices, environmental protection, fire hazard control, and disposal of hazardous or potentially hazardous substances. We may incur significant costs to comply with such laws and regulations now or in the future, and the failure to comply may have a material adverse impact on our business.
Foreign regulatory requirements
Outside the United States, our ability to market our products will also be contingent upon receiving marketing authorizations from the appropriate regulatory authorities and compliance with applicable post-approval regulatory requirements. Although the specific requirements and
48
restrictions vary from country to country, as a general matter, foreign regulatory systems include risks similar to those associated with FDA regulation, described above. Under EU regulatory systems, marketing authorizations may be submitted either under a centralized or decentralized procedure. Under the centralized procedure, a single application to the European Medicines Evaluation Agency (EMEA) leads to an approval granted by the European Commission which permits the marketing of the product throughout the EU. We assume that the centralized procedure will apply to our products that are developed by means of a biotechnology process. The decentralized procedure provides for mutual recognition of nationally approved decisions and is used for products that do not qualify under the centralized procedure. Under the decentralized procedure, the holder of a national marketing authorization may submit further applications to the competent authorities of the remaining member states which will then be requested to recognize the original authorization based upon an assessment report prepared by the original authorizing competent authority. The recognition process should take no longer than 90 days, but if one member state makes an objection, which under the legislation can only be based on a possible risk to human health, we have the option to withdraw the application from that country or take the application to arbitration by the Committee for Proprietary Medicinal Products (CPMP) of the EMEA. If a referral for arbitration is made, the procedure is suspended, and in the intervening time, the only EU country in which the product can be marketed will be the country where the original authorization has been granted, even if all the other designated countries are ready to recognize the product. The opinion of the CPMP, which is binding, could support or reject the objections or alternatively could reach a compromise position acceptable to all EU countries concerned. Arbitration can be avoided if the application is withdrawn in the objecting country, but once the application has been referred to arbitration, it cannot be withdrawn. The arbitration procedure may take an additional year before a final decision is reached and may require the delivery of additional data.
As with FDA approval, we may not be able to secure regulatory approvals in Europe in a timely manner, if at all. Additionally, as in the United States, post-approval regulatory requirements, such as those regarding product manufacture, marketing, or distribution, would apply to any product that is approved in Europe, and failure to comply with such obligations could have a material adverse effect on our ability to successfully commercialize any product.
Competition
The development and commercialization of new drugs is competitive. We currently face and expect to continue to face competition from pharmaceutical and biotechnology companies, academic and scientific institutions, governmental agencies, and public and private research organizations. Most pharmaceutical and large biotechnology companies have internal research and development departments that are focused on immunological diseases. We believe that several pharmaceutical and biotechnology companies have begun to employ, or are investigating the importance of, immunological tolerance and T-regulatory cells in connection with their internal drug discovery efforts.
Although we compete generally with biotechnology and pharmaceutical companies, those biotechnology and pharmaceutical companies pursuing projects in immunological tolerance or developing antibodies directed at the same targets as our products will be our most direct competitors. We are aware that Genmab and IDEC Pharmaceuticals are developing antibodies
49
directed at the CD4 antigen; Johnson & Johnson and Protein Design Labs are developing antibodies directed at the CD3 antigen; MedImmune is developing an antibody directed at the CD2 antigen; and Lorantis is pursuing research efforts to develop new therapies involving immunological tolerance. Some of these companies, as well as other potential competitors, have significantly greater financial, operational, sales, and marketing resources than us.
Competitors may also develop products that are more effective, safer, or less costly than any that we might develop, or they may obtain FDA approval for their products more rapidly than we may obtain approval for ours. In addition, we compete with these entities, as well as academic and research institutions, contract research companies, and other firms, to hire qualified scientists and other personnel.
Employees
As of July 31, 2003, we had 37 full-time employees, 29 in research and development and 8 in business development, finance, and administration. None of our employees is represented by a collective bargaining agreement nor have we experienced any work stoppages. We believe that relations with our employees are good.
Facilities
Our corporate headquarters is located in Cambridge, Massachusetts. We lease this 36,000 square foot facility for a term which expires in May 2012. We believe that our existing facility is adequate to meet our current requirements and that we should be able to obtain additional space if needed.
Legal proceedings
We are not currently a party to any material legal proceedings.
50
Management
Executive officers and directors
Our executive officers and directors and their ages are as follows:
|
|---|
Name
| | Age
| | Position
|
|---|
|
|---|
| Douglas J. Ringler, V.M.D. | | 46 | | President, Chief Executive Officer, and Director |
| Douglas E. Onsi | | 34 | | Chief Financial Officer |
| Louis Vaickus, M.D. | | 50 | | Vice President of Clinical Development and Medical Affairs |
| Paul Ponath, Ph.D. | | 42 | | Vice President of Research |
| Christopher K. Mirabelli, Ph.D. | | 49 | | Chairman of the Board of Directors |
| Wayne T. Hockmeyer, Ph.D.(1)(2) | | 58 | | Director |
| Yasunori Kaneko, M.D. | | 50 | | Director |
| Daniel L. Kisner, M.D.(1)(2) | | 56 | | Director |
| David J. McLachlan(1) | | 65 | | Director |
| James E. Niedel, M.D., Ph.D. | | 58 | | Director |
Herman Waldmann, Ph.D., FRCPath,
MRCP, FRS, FMedSci | | 58 | | Director |
| Harold R. Werner | | 55 | | Director |
|
(1) Member of Audit Committee of the Board of Directors.
(2) Member of Compensation Committee of the Board of Directors.
Dr. Douglas J. Ringler is a co-founder of TolerRx and has served as our President and Chief Executive Officer, and as a director, since our inception in July 2000. Dr. Ringler has over 18 years of experience as an academician, scientist, and executive in biomedical research focusing on the research and development of therapies designed to alter immunological responses. From August 1998 to July 2000, Dr. Ringler was a consultant to the biotechnology industry. Dr. Ringler was a founding scientific and executive officer of LeukoSite, Inc., a biotechnology company, from September 1993 to July 1998. Dr. Ringler holds a B.S. and V.M.D. from the University of Pennsylvania and completed his postdoctoral training as a Research Fellow at Harvard Medical School.
Mr. Douglas E. Onsi has served as our Chief Financial Officer since June 2003, having served as our Vice President of Business Development and Finance from February 2003 to June 2003 and our Senior Director and Head of Business Development from August 2001 to February 2003. From January 2000 to August 2001, he was Senior Director of Business Development and Marketing at InfoLibria, Inc., an internet infrastructure company. From February 1999 to January 2000, Mr. Onsi was Assistant Director of Business Development at LeukoSite, Inc. and then at Millennium Pharmaceuticals, Inc. after it acquired LeukoSite. From September 1993 to February 1999, he was an associate at Bingham Dana LLP. Mr. Onsi holds a B.S. in genetics from Cornell University and a J.D. from the University of Michigan Law School.
Dr. Louis Vaickus has served as our Vice President of Clinical Development and Medical Affairs since July 2002. Dr. Vaickus has over 25 years of experience in the preclinical, clinical, and post-marketing research and development of biologics and small molecules as a research
51
scientist, academic physician, and pharmaceutical executive. From March 1998 to July 2002, Dr. Vaickus served as Vice President of Clinical Research and later as Senior Vice President of Medical Affairs at Sepracor, Inc., a biotechnology company. From June 1993 to March 1998, Dr. Vaickus served as an Associate Medical Director, Medical Director, and later Medical Marketing Director for the Rebif® Multiple Sclerosis Strategic Business Unit at Serono, Inc., a biotechnology company. Dr. Vaickus holds an M.D. from Loyola University's Stritch School of Medicine and completed his postdoctoral Research Fellowship in Immunology at the Mayo Clinic in Rochester, Minnesota. Dr. Vaickus is board certified in internal medicine, hematology, and medical oncology.
Dr. Paul Ponath has served as our Vice President of Research since February 2003 and as our Director of Research, Immunology from February 2001 to February 2003. Prior to joining us, Dr. Ponath was Senior Director, Discovery Research at ChemoCentryx, Inc., a biotechnology company, from July 2000 to February 2001. From September 1993 to December 2000, he served in several positions, including Senior Director of Immunobiology, at LeukoSite, Inc., which was later acquired by Millennium Pharmaceuticals, Inc. Dr. Ponath holds a B.A. in zoology and a Ph.D. in microbiology from the University of Texas, Austin. He completed his postdoctoral training as a Research Fellow and later as Investigator at the Arthritis Foundation at Harvard University.
Directors
Dr. Christopher K. Mirabelli has served as one of our directors since December 2000. He is currently a general partner of HealthCare Ventures, a position he has held since 2000. From July 1993 to December 1999, Dr. Mirabelli served as Chairman of the Board, President, and Chief Executive Officer of LeukoSite, Inc. Dr. Mirabelli served as President of Pharmaceutical Research and Development and a member of the Millenium Pharmaceuticals, Inc. board of directors from December 1999 to June 2000 following the acquisition of LeukoSite by Millennium Pharmaceuticals, Inc. In 1988, Dr. Mirabelli was a founder of Isis Pharmaceuticals, Inc., where he served until July 1993 in several positions, including Executive Vice President. Dr. Mirabelli holds a B.S. in biology from SUNY–Fredonia and a Ph.D. in molecular pharmacology from Baylor College of Medicine.
Dr. Wayne T. Hockmeyer has served as one of our directors since April 2001. Dr. Hockmeyer founded MedImmune, Inc., a biotechnology company, in 1988, where he served as President and Chief Executive Officer until October 2000 and has served as a member of the board of directors since 1988 and as Chairman of the Board since 1993. Dr. Hockmeyer currently serves as the President and Chairman of the Board of MedImmune Ventures, Inc. He is a member of the Board of Directors of Advancis Pharmaceutical Corporation, Diversa Corporation, GenVec, Inc., and InterMune Inc., each of the foregoing a biotechnology company. Dr. Hockmeyer holds a bachelor's degree from Purdue University and a Ph.D. from the University of Florida.
Dr. Yasunori Kaneko has served as one of our directors since September 2001. Dr. Kaneko currently serves as managing director of Skyline Ventures, a position he has held since 1999. Dr. Kaneko served as Vice President of Business Development for Tularik Inc., a biopharmaceutical company, from 1992 to 1999, having served as Chief Financial Officer from 1993 to 1996. Dr. Kaneko served as Chief Financial Officer of Isis Pharmaceuticals, Inc. from 1991 to 1992. Dr. Kaneko earned his undergraduate and medical degrees at Keio University
52
School of Medicine, Tokyo, Japan and holds an M.B.A. from Stanford University Graduate School of Business.
Dr. Daniel L. Kisner has served as one of our directors since April 2001. Dr. Kisner is currently Chairman of the Board of Caliper Technologies, Inc., a biotechnology company, where he served as President and Chief Executive Officer from February 1999 to June 2003. From 1994 to 1999, Dr. Kisner was President and Chief Operating Officer at Isis Pharmaceuticals, Inc., having served as Executive Vice President from 1991 to 1994. Dr. Kisner serves as a member of the board of directors of Sequenom, Inc., a biotechnology company. Dr. Kisner holds a B.A. from Rutgers University and an M.D. from Georgetown University. He is board certified in Medical Oncology.
Mr. David J. McLachlan has served as one of our directors since August 2003. Mr. McLachlan currently serves as Senior Advisor to the Chairman and Chief Executive Officer of Genzyme Corporation, a position he has held since July 1999. From December 1989 to June 1999, Mr. McLachlan served as Executive Vice President and Chief Financial Officer of Genzyme. Mr. McLachlan currently serves as a member of the board of directors of Skyworks Solutions, Inc., a wireless semiconductor company, Dyax Corporation, a biotechnology company, and HearUSA, Inc., a hearing care company. Mr. McLachlan holds a B.A. from Harvard University and an M.B.A. from Harvard Business School.
Dr. James E. Niedel has served as one of our directors since December 2002. Since May 2002, Dr. Niedel has served as a venture partner for healthcare technology investments at the Sprout Group. From December 2000 to August 2001, Dr. Niedel served as Chief Science and Technology Officer for GlaxoSmithKline and as a director of Glaxo Wellcome plc from June 1995 to December 2000. Dr. Niedel served as Vice President of Research and Senior Vice President of Research and Development for Glaxo, Inc., from December 1988 to December 1994. Dr. Niedel currently serves as a member of the board of directors of Sirna Therapeutics, Inc. and Closure Medical, Inc. He holds a B.S. in psychology and chemistry from the University of Wisconsin and an M.D. and Ph.D. in biochemistry from the University of Miami.
Dr. Herman Waldmann is a co-founder of TolerRx, has served as one of our directors since our inception in July 2000, and is Chairman of our Scientific Advisory Board. Dr. Waldmann has been a Professor of Pathology, Head of the Sir William Dunn School of Pathology at the University of Oxford and Clinical Director of the Therapeutic Antibody Centre (TAC), University of Oxford since 1994. Prior to that, Dr. Waldmann was a professor at the University of Cambridge. Dr. Waldmann holds a Ph.D. from the University of Cambridge and is a Fellow of the Royal College of Pathology, a Member of the Royal College of Physicians, a Fellow of the Royal Society, and a Fellow of the Academy of Medical Sciences.
Mr. Harold R. Werner has served as one of our directors since December 2000. In 1985 he was a founder of HealthCare Ventures where he currently serves as a General Partner. Mr. Werner currently serves on the board of directors of GenVec, Inc., a biotechnology company. Mr. Werner received his B.S. and M.S. degrees from Princeton University and an M.B.A. from the Harvard Business School.
53
Scientific advisory board
We have a Scientific Advisory Board (SAB) to advise us on scientific and medical matters. The members of our SAB collectively have considerable experience in the areas of immunology and of the principal molecules and disease targets of interest. Our SAB meets as a group at least once per year, and individual members meet with our scientists on anad hoc basis several times per year. All of our SAB members have entered into consulting agreements with us and own some form of our equity. The members of our SAB include:
|
|---|
Member
| | Affiliation
|
|---|
|
|---|
| Herman Waldmann, Ph.D., FRCPath, MRCP, FRS, FMedSci | | Professor of Pathology, Head of the Sir William Dunn School of Pathology, University of Oxford; Clinical Director of the Therapeutic Antibody Centre, University of Oxford |
Lucienne Chatenoud, M.D., D.Sc. |
|
Professor in Immunology, Universite René Descartes, Hôpital Necker-Enfants Malades |
Mark I. Greene, M.D., Ph.D., F.R.C.P. |
|
John Eckman Professor of Medical Science, Abramson Investigator, University of Pennsylvania |
Stuart J. Knechtle, M.D. |
|
Professor of Surgery, University of Wisconsin |
David Wofsy, M.D. |
|
George A. Zimmerman Distinguished Professor of Medicine and Microbiology/Immunology, University of California at San Francisco |
|
Consultants and collaborators
Our key consultants and collaborators include:
|
|---|
Consultant or collaborator
| | Affiliation
|
|---|
|
|---|
| Geoffrey Hale, Ph.D. | | Professor of Therapeutic Immunology, University of Oxford |
Stephen Cobbold, Ph.D. |
|
Reader in Cellular Immunology, University of Oxford |
Bart Keymeulen, M.D., Ph.D. |
|
Juvenile Diabetes Research Foundation Center for Beta Cell Therapy in Europe, Brussels Free University—VUB |
Daniel Pipeleers, M.D., Ph.D. |
|
Director, Juvenile Diabetes Research Foundation Center for Beta Cell Therapy in Europe, Brussels Free University—VUB |
|
Election of directors
Our current board of directors consists of nine directors, with members serving until the annual meeting of stockholders at which their respective successors are elected and qualified.
54
Compensation of directors
Our directors may be reimbursed for expenses incurred in attending board and committee meetings. Each outside director who is not an affiliate of a significant stockholder is granted an award of stock or options upon his or her election to the board of directors for 100,000 shares, which award vests in a series of three successive, equal annual installments, commencing on the first anniversary of the grant date. In addition, each such director is granted for each complete year of service an award of stock or options for 15,000 shares, which award vests on the first anniversary of the grant date. Pursuant to a consulting agreement entered into in October 2000, one of our directors, Herman Waldmann, is paid $100,000 annually for consulting services, which include, among other things, serving as a member of our board of directors.
Board committees
Compensation committee
Our compensation committee consists of Wayne T. Hockmeyer and Daniel L. Kisner, each of whom is an independent director. Our compensation committee reviews the salaries and other compensation of our executive officers, including equity-based compensation, and makes recommendations to our board of directors. In addition, the compensation committee reviews and administers our incentive compensation and other stock-based plans.
Audit committee
Our audit committee consists of Wayne T. Hockmeyer, Daniel L. Kisner, and David J. McLachlan, each of whom is an independent director. We believe that each of the members of the audit committee is financially sophisticated and is able to read and understand our consolidated financial statements. We believe that Mr. McLachlan is an "audit committee financial expert" as defined in recently adopted SEC Rules. Our audit committee makes recommendations to our board of directors regarding the selection of our independent auditors and reviews the professional services provided by our independent auditors, the independence of our auditors, the professional fees payable to our auditors, our annual financial statements, and our system of internal accounting procedures and controls.
Compensation committee interlocks and insider participation
None of our executive officers serves as a member of the board of directors or compensation committee of any other company that has one or more executive officers serving as a member of our board of directors or compensation committee. Prior to the formation of the compensation committee, the board of directors as a whole made decisions relating to compensation of our executive officers.
55
Executive compensation
Summary compensation table
The following table sets forth information, for the fiscal year ended December 31, 2002, with respect to the compensation paid to our Chief Executive Officer and each of our other three most highly compensated executive officers whose salary and bonus exceeded $100,000.
|
|---|
| | Salary ($)
| | Bonus ($)
| | Restricted
stock awards (#)(2)
|
|---|
| | Annual
compensation(1)
| | Long-term
compensation awards
|
|---|
Name and principal position
|
|---|
|
|---|
Douglas J. Ringler
President and Chief Executive Officer | | $ | 247,500 | | $ | 61,875 | | — |
Douglas E. Onsi
Chief Financial Officer |
|
|
167,200 |
|
|
23,450 |
|
— |
|
|
|
|
|
|
|
|
|
Paul Ponath
Vice President of Research |
|
|
172,300 |
|
|
29,300 |
|
— |
Louis Vaickus
Vice President of Clinical Development
and Medical Affairs |
|
|
120,729(3 |
) |
|
56,400 |
|
0(4) |
|
|---|
|
|
|
|
|
|
|
|---|
(1) Salary includes amounts, if any, deferred pursuant to our 401(k) plan. The compensation in this table does not include medical, group life, or other benefits which are available to our salaried employees, and perquisites and other benefits, securities, or property which do not exceed the lesser of $50,000 or 10% of the person's salary and bonus reported in this table.
(2) The remaining number of shares of restricted common stock that had not vested and the value of this stock as of December 31, 2002, was as follows: Dr. Ringler, 466,395 shares, $ ; Dr. Ponath, 75,000 shares, $ ; Mr. Onsi, 93,750 shares, $ ; and Dr. Vaickus, 225,000 shares $ . The value is based on the midpoint of estimated public offering price range set forth on the cover page of this prospectus less the purchase price paid. The holders of these shares of restricted stock will be entitled to receive any dividends we pay on our common stock.
(3) Dr. Vaickus joined us as an executive officer in July 2002, and this is the amount he earned through December 2002.
(4) In September 2002, we sold 225,000 shares of restricted stock to Dr. Vaickus, subject to our right to repurchase at $0.16 per share, the then-current fair market value of the common stock as determined by our board of directors. Our repurchase right lapses 25% one year from the date Dr. Vaickus commenced employment and 25% annually thereafter.
2000 Equity Incentive Plan
In August 2003, our board of directors approved our Amended and Restated 2000 Equity Incentive Plan, to become effective on the closing of this offering. The 2000 Equity Incentive Plan provides for the grant of incentive stock options, within the meaning of Section 422 of the Internal Revenue Code, to employees and non-qualified stock options, awards of common stock, and opportunities to make direct purchases of common stock to our employees, directors, and consultants.
The aggregate number of shares of our common stock that may be issued under the 2000 Equity Incentive Plan is , which number represents 15% of the total number of shares of our common stock that will be outstanding upon completion of this offering. However, in no
56
event may more than shares of our common stock, which represents 25% of the total number of shares of common stock that will be outstanding upon completion of this offering, be issued pursuant to the exercise of incentive stock options granted under the 2000 Equity Incentive Plan. The aggregate number of shares of common stock that may be granted in any calendar year to any one person pursuant to the 2000 Equity Incentive Plan may not exceed 30% of the aggregate number shares of our common stock that may be issued pursuant to the 2000 Equity Incentive Plan.
The 2000 Equity Incentive Plan is administered by the compensation committee of our board of directors. Subject to the provisions of the 2000 Equity Incentive Plan, the compensation committee has the discretion to determine when awards are made, which directors, employees, or consultants receive awards, whether an award will be in the form of an incentive stock option, a nonqualified stock option, or restricted stock, the number of shares subject to each award, and all other relevant terms of the award. The committee also has broad discretion to construe and interpret the 2000 Equity Incentive Plan and adopt rules and regulations thereunder.
Generally, options granted to employees and consultants under the 2000 Equity Incentive Plan vest over a four-year period from the date of grant. The 2000 Equity Incentive Plan also contains an automatic grant feature pursuant to which outside directors are granted stock or options for 100,000 shares, representing approximately 0.15% of the company prior to this offering, upon election to the board of directors, which initial shares vest over a three-year period, and stock or options for 15,000 shares, representing approximately 0.02% of the company prior to this offering, on the anniversary of each year of service on the board of directors, which additional shares vest one year from the date of grant.
In the event we are sold to an acquiror that does not have an equity incentive plan under which options outstanding under the 2000 Equity Incentive Plan may be assumed or pursuant to which options may be issued in substitution of or in exchange for all such options outstanding under our 2000 Equity Incentive Plan, then the vesting of all outstanding options shall automatically accelerate by one year.
Our board of directors may amend, modify, or terminate our 2000 Equity Incentive Plan at any time, subject to applicable rules and law and the rights of holders of outstanding awards. Our 2000 Equity Incentive Plan will automatically terminate in August 2010, unless our board of directors terminates it prior to that time.
2003 Employee Stock Purchase Plan
In August 2003, our board of directors and stockholders approved our 2003 Employee Stock Purchase Plan, to become effective upon the closing of this offering. The 2003 Employee Stock Purchase Plan authorizes the issuance of up to a total of 150,000 shares of our common stock to eligible employees. The 2003 Employee Stock Purchase Plan shall terminate in 2008.
The 2003 Employee Stock Purchase Plan, which is intended to qualify under Section 423 of the Internal Revenue Code, will be implemented by a series of six-month offering periods. New offering periods, other than the first offering period, are expected to commence on January 1 or July 1 of each year and end on the next following June 30 or December 31, respectively. Each offering period will generally consist of a consecutive six-month purchase period, and at
57
the end of each six-month period an automatic purchase will be made for participants. The initial offering and initial purchase periods are expected to commence on the date of this offering. The 2003 Employee Stock Purchase Plan will be administered by the board of directors or by a committee appointed by the board. Employees are eligible to participate if we employ them for at least 20 hours per week and more than five months per year. Eligible employees may purchase common stock through payroll deductions only after the effectiveness of an appropriate registration statement, which in any event may not exceed 10% of an employee's compensation, at a price equal to the lower of 85% of the fair market value of the common stock at the beginning of each offering period or at the end of each purchase period. All eligible employees shall be required to participate in the initial offering period under the 2003 Employee Stock Purchase Plan. However, participating employees may end their participation at any time during the initial offering period and participation ends automatically on termination of employment.
Under the 2003 Employee Stock Purchase Plan, no employee shall be granted an option under the plan if immediately after the grant the employee would own stock, including any outstanding options to purchase stock, equaling 5% or more of the total voting power or value of all classes of our stock. In addition, the 2003 Employee Stock Purchase Plan provides that no employee shall be granted an option under the 2003 Employee Stock Purchase Plan if the option would permit the employee to purchase stock under all of our employee stock purchase plans in an amount that exceeds $25,000 of the fair market value of such stock for each calendar year in which the option is outstanding at any time and that no employee may purchase more than 2,500 shares of common stock under the plan in any one purchase period. The board of directors may, at its discretion, prior to the beginning of an offering period, subject the shares acquired (or to be acquired) by employees for such offering period to certain transfer restrictions.
In the event of a merger, consolidation, or other acquisition event resulting in any change of control of us, each right to purchase stock under the 2003 Employee Stock Purchase Plan will be assumed, or an equivalent right will be substituted by, the successor corporation. Any ongoing offering period, however, will be shortened so that employees' rights to purchase stock under the 2003 Employee Stock Purchase Plan are exercised prior to the transaction, unless the employee has withdrawn, in the event that the successor corporation refuses to assume each purchase right or to substitute an equivalent right. The board of directors has the power to amend or terminate the 2003 Employee Stock Purchase Plan and to change or terminate offering periods as long as any action does not adversely affect any outstanding rights to purchase stock. Our board of directors may amend or terminate the 2003 Employee Stock Purchase Plan or an offering period even if it would adversely affect outstanding options in order to avoid our incurring adverse accounting charges.
401(k) plan
During 2001, we adopted an employee savings and retirement plan, qualified under Section 401(k) of the Internal Revenue Code. All of our full time employees who have attained the age of 21 are eligible to participate in our 401(k) plan after 3 months of employment. Pursuant to the 401(k) plan, employees may elect to reduce their current compensation by up to the statutorily prescribed annual limit and have the amount of the reduction contributed to the 401(k) plan.
58
Related party transactions
Other than compensation agreements and other arrangements which are described as required in "Management" and the transactions described below, since July 6, 2000, the date of our formation, there has not been, and there is not currently proposed to be, any transaction or series of similar transactions to which we were or will be a party in which the amount involved exceeded or will exceed $60,000 and in which any director, executive officer, holder of 5% or more of any class of our capital stock, or any member of their immediate family had or will have a direct or indirect material interest.
We believe that we have executed all of the transactions set forth below on terms no less favorable to us than we could have obtained from unaffiliated third parties. It is our intention to ensure that all future transactions between us and our officers, directors, and principal stockholders and their affiliates are approved by a majority of the board of directors, including a majority of the independent and disinterested members of the board of directors, and are on terms no less favorable to us than those that we could obtain from unaffiliated third parties.
Private placements of securities
In December 2000 and March and July 2001, we issued and sold an aggregate of 9,035,000 shares of Series A convertible preferred stock at a price of $0.664 per share. In September 2001, we issued and sold an aggregate of 17,000,000 shares of Series B convertible preferred stock at a price of $1.00 per share. In November and December 2002 and January 2003, we issued and sold an aggregate of 35,000,000 shares of Series C convertible preferred stock at a price of $1.00 per share.
The following table summarizes, on a common stock equivalents basis, the participation by our 5% stockholders and stockholders associated with some of our directors in these private placements.
|
Purchaser(1)
| | Total common stock
equivalents
| | Aggregate consideration paid
| | Investment participation
|
|---|
|
| Stockholders associated with Directors: | | | | | | | |
| HealthCare Ventures VI, L.P.(2) | | 20,483,249 | | $ | 17,599,278 | | Series A, B and C |
| Skyline Venture Partners Group(3) | | 11,000,000 | | $ | 11,000,000 | | Series B and C |
| Sprout Group | | 6,930,000 | | $ | 6,930,000 | | Series C |
5% Stockholders |
|
|
|
|
|
|
|
| Rho Ventures Group(4) | | 5,900,000 | | $ | 5,900,000 | | Series B and C |
| Artal Services N.V | | 5,000,000 | | $ | 5,000,000 | | Series C |
| Genentech, Inc | | 3,500,000 | | $ | 3,500,000 | | Series C |
|
(1) See "Principal stockholders" for more detail on shares held by these purchasers.
(2) Dr. Christopher K. Mirabelli, one of our directors, is a general partner of HealthCare Ventures VI, L.P., and Mr. Harold R. Werner, one of our directors, is a general partner of HealthCare Ventures VI, L.P. Consideration paid to us by HealthCare Ventures VI, L.P. for our redeemable convertible preferred stock during 2000, 2001 and 2002 was $5,699,278, $6,900,000, and $5,000,000, respectively.
59
(3) Dr. Yasunori Kaneko, one of our directors, is a general partner at the Skyline Venture Partners Group. Consideration paid to us by the Skyline Venture Partners Group for our redeemable convertible preferred stock during 2001 and 2002 was $4,000,000 and $7,000,000, respectively.
(4) Consideration paid to us by Rho Ventures Group for our redeemable convertible preferred stock during 2001 and 2002 was $2,500,000 and $3,400,000, respectively.
In connection with the above transactions, we entered into agreements with all of the investors that invested in the above-listed private placements of redeemable convertible preferred stock providing for registration rights with respect to these shares. For more information, please see "Description of capital stock—Registration rights."
Transactions with our directors
In October 2000, we entered into a consulting agreement with one of our directors, Herman Waldmann, pursuant to which Dr. Waldmann provides consulting and technical advisory services relating to our research and development, serves as chair of our Scientific Advisory Board, and serves as a member of our board of directors. Dr. Waldmann is paid $100,000 annually for services provided under that agreement. Either we or Dr. Waldmann may terminate the agreement for any reason upon 30 days prior written notice.
Indemnification
Our certificate of incorporation and by-laws provide that we shall indemnify our directors and officers to the fullest extent permitted by Delaware law.
We have entered into indemnification agreements with Douglas J. Ringler, Herman Waldmann, Christopher K. Mirabelli, Wayne T. Hockmeyer, Yasunori Kaneko, Daniel L. Kisner, David J. McLachlan, James E. Niedel, and Harold R. Werner, as directors. These agreements provide that, among other things, we will indemnify the director against all expenses, fines, and judgments incurred due to any proceeding arising from his service as a director. We will not indemnify a director or officer, however, unless he acted in good faith, reasonably believed his conduct was in, or not opposed to, our best interests, and had no reason to believe his conduct was unlawful.
60
Principal stockholders
The following table provides information regarding the beneficial ownership of our common stock as of August 22, 2003, and as adjusted to reflect the completion of the sale of our common stock offered in this prospectus by:
• each person who beneficially owns more than 5% of the outstanding shares of our common stock;
• each of our executive officers listed in the Summary Compensation Table;
• each of our directors; and
• all of our executive officers and directors as a group.
The amounts and percentages of common stock beneficially owned are reported on the basis of regulations of the Securities and Exchange Commission governing the determination of beneficial ownership of securities. Under the rules of the Commission, a person is deemed to be a "beneficial owner" of a security if that person has or shares "voting power," which includes the power to vote or to direct the voting of such security, or "investment power," which includes the power to dispose of or to direct the disposition of such security. A person is also deemed to be a beneficial owner of any securities of which that person has a right to acquire beneficial ownership within 60 days. Under these rules, more than one person may be deemed a beneficial owner of the same securities and a person may be deemed to be the beneficial owner of securities as to which such person has no economic interest.
There were 65,547,875 shares of our common stock outstanding before this offering as of August 22, 2003, assuming the conversion of our redeemable convertible preferred stock into common stock on a one-for-one basis upon completion of this offering. The number of shares of our common stock that will be outstanding after this offering reflects the sale of the shares of our common stock offered by this prospectus and assumes no exercise of the underwriters' over-allotment option.
|
|---|
| |
| | Percent owned
|
|---|
| | Shares
beneficially
owned(1)
|
|---|
Name and address
| | Before the
offering
| | After the
offering
|
|---|
|
| 5% Stockholders: | | | | | | |
HealthCare Ventures VI, L.P.(2) |
|
20,483,249 |
|
31.2 |
% |
|
| Entities affiliated with Skyline Venture Partners(3) | | 11,000,000 | | 16.8 | % | |
| Entities affiliated with Sprout Group(4) | | 7,070,000 | | 10.8 | % | |
| Entities affiliated with Rho Capital Partners(5) | | 5,900,000 | | 9.0 | % | |
| Artal Services N.V.(6) | | 5,000,000 | | 7.6 | % | |
| Genentech, Inc.(7) | | 3,500,000 | | 5.3 | % | |
Directors and officers: |
|
|
|
|
|
|
| Douglas J. Ringler | | 1,190,000 | | 1.8 | % | |
| Christopher K. Mirabelli(8) | | 20,483,249 | | 31.2 | % | |
| Wayne T. Hockmeyer(9) | | 35,000 | | * | | |
| Yasunori Kaneko(10) | | 11,000,000 | | 16.8 | % | |
| Daniel L. Kisner(11) | | 55,000 | | * | | |
| David J. McLachlan | | 100,000 | | * | | |
| James E. Niedel(12) | | 7,070,000 | | 10.8 | % | |
| Herman Waldmann | | 1,190,000 | | 1.8 | % | |
| Harold R. Werner(13) | | 20,483,249 | | 31.2 | % | |
| Douglas E. Onsi | | 125,000 | | * | | |
| Paul Ponath | | 100,000 | | * | | |
| Louis Vaickus | | 225,000 | | * | | |
All executive officers and directors as a group
(12 persons) |
|
41,573,249 |
|
63.4 |
% |
|
|
* Less than 1% of the outstanding common stock.
61
(1) Beneficial ownership is determined in accordance with Rule 13d-3 promulgated by the Commission under the Securities and Exchange Act of 1934, as amended. Shares of common stock issuable pursuant to options, warrants, and convertible securities, to the extent such securities are currently exercisable or convertible within 60 days of August 22, 2003, are treated as outstanding for computing the percentage of the person holding such securities but are not treated as outstanding for computing the percentage of any other person. Unless otherwise noted, each person or group identified possesses sole voting and investment power with respect to shares, subject to community property laws where applicable.
(2) The address for HealthCare Ventures VI, L.P. is 44 Nassau Street, Princeton, NJ 08542.
(3) Consists of 6,342,097 shares held by Skyline Venture Partners Qualified Purchaser Fund III, L.P., 2,762,829 shares held by Skyline Venture Partners Qualified Purchaser Fund II, L.P., 157,903 shares held by Skyline Venture Partners III, L.P., 237,171 shares held by Skyline Venture Partners II, L.P., and 1,500,000 shares held by Skyline Expansion Fund, L.P. The address for Skyline Venture Partners is 125 University Ave., Palo Alto, CA 94301.
(4) Consists of 6,573,220 shares held by Sprout Capital IX, L.P., 330,875 shares held by Sprout IX Plan Investors, L.P., 25,905 shares held by Sprout Entrepreneurs Fund, L.P., and 140,000 shares held by Dr. James E. Niedel. Dr. Niedel is a venture partner, and not an employee, of the Sprout Group, and in such capacity, he may be deemed to share voting and investment power with respect to the shares held by entities affiliated with the Sprout Group. Dr. Niedel disclaims beneficial ownership of the shares held by entities affiliated with the Sprout Group. The address for the Sprout Group is 11 Madison Avenue, New York, NY 10010.
(5) Consists of 1,812,479 shares held by Rho Ventures IV Gmbh & Co. Beiteligungs KG, 1,739,182 shares held by Rho Ventures IV (QP) L.P., 1,609,598 shares held by Rho Management Trust I, and 738,741 shares held by Rho Ventures IV, L.P. The address for Rho Capital Partners is 152 W. 57th Street, 23rd Floor, New York, NY 10019.
(6) The address for Artal Services N.V. is Woluwe Garden, Woluwedal 28, Sint-Stevens-Woluwe 1932, Belgium.
(7) The address for Genentech, Inc. is 1 DNA Way, South San Francisco, CA 94080-4990.
(8) Consists of 20,483,249 shares held by HealthCare Ventures VI, L.P. Dr. Mirabelli is a General Partner of HealthCare Ventures VI, L.P. In such capacity, he may be deemed to share voting and investment power with respect to the shares owned by HealthCare Ventures VI, L.P. Dr. Mirabelli disclaims beneficial ownership of the shares held by HealthCare Ventures VI, L.P., except to the extent of his proportionate pecuniary interest therein.
(9) This number does not include 125,000 shares held by the John T. Hockmeyer Irrevocable Trust. Dr. Hockmeyer disclaims beneficial ownership of the shares held by the trust.
(10) Consists of 11,000,000 shares held by entities affiliated with Skyline Venture Partners. Dr. Kaneko is a Managing Director of Skyline Venture Partners. In such capacity, he may be deemed to share voting and investment power with respect to the shares owned by the entities affiliated with Skyline Venture Partners. Dr. Kaneko disclaims beneficial ownership of the shares held by Skyline Venture Partners, except to the extent of his proportionate pecuniary interest therein.
(11) Consists of 30,000 shares held by Dr. Kisner individually, and 12,500 shares held by the Griffen Daniel Kisner Irrevocable Trust and 12,500 shares held by the Jordan Renee Kisner Irrevocable Trust, both of which Dr. Kisner is joint trustee with Carmen Rosette Garcia.
(12) Consists of 140,000 shares held by Dr. Niedel individually and 6,930,000 shares held by entities affiliated with Sprout Group. Dr. Niedel is a venture partner, and not an employee, of the Sprout Group, and in such capacity, he may be deemed to share voting and investment power with respect to the shares held by entities affiliated with the Sprout Group. Dr. Niedel disclaims beneficial ownership of the shares held by entities affiliated with the Sprout Group.
(13) Consists of 20,483,249 shares held by HealthCare Ventures VI, L.P. Mr. Werner is a General Partner of HealthCare Ventures VI, L.P. In such capacity, he may be deemed to share voting and investment power with respect to the shares owned by HealthCare Ventures VI, L.P. Mr. Werner disclaims beneficial ownership of the shares held by HealthCare Ventures VI, L.P., except to the extent of his proportionate pecuniary interest therein.
62
Description of capital stock
General
Upon the closing of this offering, our board of directors will be authorized to issue 50,000,000 shares of common stock, par value $0.001 per share, of which shares will be issued and outstanding, and 5,000,000 shares of undesignated preferred stock, par value $0.001 per share, of which no shares will be issued or outstanding.
The following summary description of our capital stock, certificate of incorporation, and by-laws, is not intended to be complete, and assumes the filing as of the closing of the offering of our certificate of incorporation and the adoption of our bylaws. This description is qualified by reference to the provisions of applicable Delaware law and to our certificate of incorporation and by-laws filed as exhibits to the registration statement of which this prospectus is a part.
Common stock
As of August 22, 2003, there were 65,547,875 shares of our common stock outstanding, held of record by 64 stockholders, after giving effect to the automatic conversion of all of our outstanding shares of our redeemable convertible preferred stock on a one-for-one basis into 61,035,000 shares of our common stock upon the completion of this offering.
Upon the closing of this offering, our board of directors will be authorized to issue up to 50,000,000 shares of common stock, $0.001 par value per share. Holders of our common stock are entitled to one vote per share for each share held of record on all matters to be voted upon by the stockholders. Accordingly, holders of a majority of the shares of our common stock entitled to vote in any election of directors may elect all of the directors standing for election. Subject to preferences that may be applicable to any outstanding preferred stock, holders of our common stock are entitled to receive ratably such dividends as may be declared from time to time by our board of directors out of funds legally available for that purpose. In the event of our liquidation, dissolution, or winding up, the holders of our common stock are entitled to share ratably in all assets remaining after payment of liabilities, subject to prior distribution rights of preferred stock, if any, then outstanding. The holders of our common stock have no cumulative voting rights, preemptive or conversion rights or other subscription rights. There are no redemption or sinking fund provisions applicable to our common stock. The outstanding shares of our common stock are, and the shares offered in this offering will be, when issued and paid for, fully paid and nonassessable.
Preferred stock
Upon the closing of this offering, our board of directors will be authorized to issue up to 5,000,000 shares of preferred stock, $0.001 per share. The board of directors will be authorized, subject to any limitations prescribed by law, without further vote or action by the stockholders, to issue from time to time shares of preferred stock in one or more series. Each such series of preferred stock shall have such number of shares, designations, preferences, voting powers, qualifications, and special or relative rights or privileges as shall be determined by the board of
63
directors, which may include, among others, dividend rights, voting rights, redemption and sinking fund provisions, liquidation preferences, conversion rights, and preemptive rights.
The rights of the holders of our common stock will be subject to, and may be adversely affected by, the rights of holders of any preferred stock that may be issued in the future. Such rights may include voting and conversion rights which could adversely affect the holders of our common stock. Satisfaction of any dividend preferences of outstanding preferred stock would reduce the amount of funds available, if any, for the payment of dividends on common stock. Holders of our preferred stock would typically be entitled to receive a preference payment in the event of our liquidation, dissolution or winding up before any payment is made to the holders of our common stock. Additionally, the issuance of our preferred stock could have the effect of making it more difficult for a third party to acquire, or of discouraging a third party from attempting to acquire, a majority of our outstanding voting stock. Upon the closing of the offering, there will be no shares of preferred stock outstanding, and we have no present plans to issue any shares of preferred stock after this offering.
Registration rights
Certain persons and entities have rights with respect to the registration of common stock under the Securities Act. Immediately after the closing of this offering, those rights will cover 61,246,250 shares of common stock, which will include 211,250 shares of common stock issuable upon exercise of warrants (assuming such warrants are exercised). In general, if we propose to register any of our securities under the Securities Act, either for our own account or for the account of other security holders, subject to certain exceptions, we must, upon the written request of a holder of registrable shares, use our best efforts to cause to be registered under the Securities Act all of the registrable shares requested to be registered, provided, however, that we are not required to register registrable securities in excess of the amount, if any, of common stock which the principal underwriter of an underwritten offering shall agree to include in such offering. In addition, the holders of 61,035,000 registrable shares are entitled to demand registration rights pursuant to which they may require us to file a registration statement under the Securities Act on Form S-1, S-2, or S-3 at our expense with respect to their registrable shares, and we are required to use our best efforts to effect that registration, provided that we are not required to effect more than two such registrations annually and we are not required to effect a requested registration within six months following this offering. Upon receipt of any such request from such holders, we will be required to use our best efforts to effect such registration, subject to certain conditions and limitations.
Anti-takeover effects of Delaware law and certain charter and by-law provisions
Upon completion of this offering, we will be subject to the provisions of Section 203 of the Delaware General Corporation Law. Subject to certain exceptions, Section 203 of the Delaware General Corporation Law prohibits a publicly-held Delaware corporation from engaging in a "business combination" with an "interested stockholder" for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the interested stockholder attained such status with the approval of our board of directors or unless the business combination is approved in a prescribed manner. A "business combination" includes certain mergers, asset sales or other transactions resulting in a financial benefit to the
64
interested stockholder. Subject to various exceptions, an "interested stockholder" is a person who, together with his or her affiliates and associates, owns, or within the past three years did own, 15% or more of a corporation's voting stock. This statute could prohibit or delay the accomplishment of mergers or other takeover or change in control attempts with respect to us and, accordingly, may discourage attempts to acquire us.
In addition, some provisions of our certificate of incorporation and by-laws may be deemed to have an anti-takeover effect and may delay, defer, or prevent a tender offer or takeover attempt that a stockholder might deem to be in his or her best interest. The existence of these provisions could limit the price that investors might be willing to pay in the future for shares of our common stock. These provisions include:
Stockholder action; special meeting of stockholders. Our certificate of incorporation and by-laws provide that stockholders may not take action by written consent, but only at a duly called annual or special meeting of stockholders, and that special meetings of our stockholders may be called only by the chairman of the board of directors, the president, or a majority of the board of directors. Thus, without approval by the chairman of the board of directors, the president, or a majority of the board of directors, stockholders may take no action between meetings. These provisions may have the effect of delaying until the next annual stockholders' meeting stockholder actions that are favored by the holders of a majority of our outstanding voting securities, including actions to remove directors. These provisions may also discourage another person or entity from making a tender offer for our common stock, because such person or entity, even if it acquired all or a majority of our outstanding voting securities, would be able to take action as a stockholder (such as electing new directors or approving a merger) only at a duly called stockholders' meeting, and not by written consent.
Advance notice requirements for stockholder proposals and director nominations. Our certificate of incorporation and by-laws provide that a stockholder seeking to bring business before an annual meeting of stockholders, or to nominate candidates for election as directors at an annual meeting of stockholders, must provide timely notice of this intention in writing. To be timely, a stockholder's notice must be delivered to our secretary not less than 120 nor more than 150 days prior to the first anniversary of the date of our proxy statement delivered to stockholders in connection with the preceding year's annual meeting, or if the date of the annual meeting is more than 30 days before or more than 60 days after such anniversary, or if no proxy statement was delivered to our stockholders in connection with the preceding year's annual meeting, such notice must be delivered not earlier than 90 days prior to such annual meeting and not later than the later of (i) 60 days prior to the annual meeting or (ii) 10 days following the date on which public announcement of the date of such annual meeting was made. With respect to special meetings of stockholders, such notice must be delivered to our secretary not more than 90 days prior to such meeting and not later than the later of (i) 60 days prior to such meeting or (ii) 10 days following the date on which public announcement of the date of such meeting is first made. The notice must contain, among other things, certain information about the stockholder delivering the notice and, as applicable, background information about each nominee or a description of the proposed business to be brought before the meeting. These provisions may preclude stockholders from bringing matters before an annual meeting of stockholders or from making nominations for directors at an annual or special meeting of stockholders.
65
Authorized but unissued shares. The authorized but unissued shares of common stock and preferred stock are available for future issuance without stockholder approval, subject to any limitations imposed by the Nasdaq National Market. These additional shares may be utilized for a variety of corporate acquisitions and employee benefit plans. The existence of authorized but unissued and unreserved common stock and preferred stock could make more difficult or discourage an attempt to obtain control of us by means of a proxy contest, tender offer, merger, or otherwise.
Super-majority voting. Delaware law provides generally that the affirmative vote of a majority of the shares entitled to vote on any matter is required to amend a corporation's certificate of incorporation or by-laws, unless a corporation's certificate of incorporation or by-laws require a greater percentage. We have provisions in our certificate of incorporation which require the affirmative vote of the holders of at least 67% of our authorized voting stock to amend or repeal certain provisions of our certificate of incorporation which include, but are not limited to provisions which would reduce or eliminate the number of authorized common or preferred shares and all indemnification provisions. Such 67% stockholder vote would in either case be in addition to any separate class vote that might in the future be required pursuant to the terms of any preferred stock at the time any such amendments are submitted to stockholders. Our by-laws may also be amended or repealed by a majority vote of our board of directors.
Board discretion in considering certain offers. Our certificate of incorporation empowers our board of directors, when considering a tender offer or merger or acquisition proposal, to take into account factors in addition to potential economic benefits to stockholders. Such factors may include (i) comparison of the proposed consideration to be received by stockholders in relation to the then-current market price of our capital stock, our estimated current value in a freely negotiated transaction, and our estimated future value as an independent entity, and (ii) the impact of such a transaction on our employees, suppliers, and customers and its effect on the communities in which we operate.
Limitation of liability. Our certificate of incorporation contains certain provisions permitted under Delaware General Corporation Law relating to the liability of directors. These provisions eliminate a director's personal liability for monetary damages resulting from a breach of fiduciary duty, except in certain circumstances involving certain wrongful acts, such as the breach of a director's duty of loyalty or acts or omissions that involve intentional misconduct or a knowing violation of law. These provisions do not limit or eliminate our rights or the rights of any stockholder to seek non-monetary relief, such as an injunction or rescission, in the event of a breach of a director's fiduciary duty. These provisions will not alter a director's liability under federal securities laws. Our certificate of incorporation and by-laws also contain provisions indemnifying our directors and officers to the fullest extent permitted by the Delaware General Corporation Law. We believe that these provisions will assist us in attracting and retaining qualified individuals to serve as directors.
Transfer agent and registrar
Upon the closing of this offering, the transfer agent and registrar for the common stock will be .
Nasdaq national market listing
We have filed an application for our common stock to be quoted on the Nasdaq National Market under the symbol TLRX.
66
Shares eligible for future sale
Sales of restricted shares
Upon the completion of this offering, based upon the number of shares of our common stock outstanding as of June 30, 2003, and assuming the automatic conversion of all outstanding shares of our redeemable convertible preferred stock into 61,035,000 shares of our common stock upon the completion of this offering we will have shares of our common stock outstanding. Of these shares, shares of our common stock to be sold in this offering, will be freely tradable without restriction or further registration under the Securities Act, except that any shares of our common stock purchased by our affiliates, as that term is defined in Rule 144 under the Securities Act, may generally only be sold in compliance with the limitations of Rule 144 described below.
The remaining shares of our common stock outstanding upon completion of this offering are deemed "restricted shares" under Rule 144 or Rule 701 under the Securities Act. None of these restricted shares of our common stock will be eligible for sale in the public market on the date of this prospectus. Ninety days from the date of this prospectus, shares of our common stock will be eligible for sale in the public market pursuant to Rule 701, subject to the lock-up agreements. Upon expiration of the lock-up agreements described below, 180 days after the date of this prospectus, an additional shares of our common stock will be eligible for sale in the public market pursuant to Rule 144.
Rule 144 makes available an exemption from the registration requirements of the Securities Act. In general, under Rule 144, a person (or persons whose shares are aggregated) who owns shares that were acquired from the issuer or an affiliate of the issuer at least one year prior to the proposed sale will be entitled to sell in any three-month period a number of shares that does not exceed the greater of:
• one percent of the then outstanding shares of our common stock (approximately shares of our common stock immediately after the completion of this offering); or
• the average weekly trading volume in our common stock on the Nasdaq National Market during the four calendar weeks preceding the date on which notice of such sale is filed, provided certain requirements concerning availability of public information, manner of sale, and notice of sale are satisfied.
In addition, our affiliates must comply with the restrictions and requirements of Rule 144, other than the one-year holding period requirement, in order to publicly sell shares of our common stock which are not restricted securities. A stockholder who is not one of our affiliates and has not been our affiliate for at least three months prior to the sale and who has beneficially owned restricted shares of our common stock for at least two years may resell the shares without limitation. In meeting the one and two year holding periods described above, a holder of restricted shares of our common stock can include the holding periods of a prior owner who was not our affiliate. The one and two year holding periods described above do not begin to run until the full purchase price or other consideration is paid by the person acquiring the restricted shares of our common stock from the issuer or one of our affiliates.
67
Rule 701 provides that currently outstanding shares of our common stock acquired under our employee compensation plans may be resold beginning 90 days after the date of this prospectus by:
• persons, other than our affiliates, subject only to the manner of sale provisions of Rule 144; and
• our affiliates under Rule 144, without compliance with its one-year minimum holding period, subject to certain limitations.
Options
Rule 701 also provides that the shares of our common stock acquired upon the exercise of currently outstanding options or pursuant to other rights granted under our 2000 Equity Incentive Plan may be resold beginning 90 days after the date of this prospectus by:
• persons, other than our affiliates, subject only to the manner of sale provisions of Rule 144; and
• our affiliates under Rule 144, without compliance with its one-year minimum holding period, subject to certain limitations.
At August 22, 2003, approximately 32,500 shares of our common stock were issuable pursuant to vested options under our 2000 Equity Incentive Plan, of which all vested options are subject to lock-up agreements with the underwriters. These shares will become eligible for sale in the public market in accordance with Rule 701 under the Securities Act beginning 180 days after the date of this prospectus.
Following the date of this prospectus, we intend to file one or more registration statements on Form S-8 under the Securities Act to register up to shares of our common stock issuable under our 2000 Equity Incentive Plan. These registration statements will become effective upon filing.
Lock-up agreements
We and our executive officers, directors, and all our security holders have agreed that, during the period beginning from the date of this prospectus and continuing to and including the date 180 days after the date of this prospectus, none of us will, without the prior written consent of J.P. Morgan Securities Inc. and SG Cowen Securities Corporation on behalf of the underwriters, directly or indirectly:
• offer, pledge, announce the intention to sell, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any shares of our common stock or any securities convertible into or exercisable or exchangeable for common stock (including common stock which may be deemed to be beneficially owned by any of the security holders) except for securities which may be issued upon exercise of a stock option or warrant and except for the participation by our employees in our 2003 Employee Stock Purchase Plan (provided in each case that the holder of our equity has entered into a similar lock-up agreement), or enter into any swap or other agreement that transfers, in whole or in
68
part, any of the economic consequences of ownership of our common stock, regardless of whether any such transactions are to be settled by delivery of such common stock or such other securities, in cash or otherwise; or
• make any demand for, or exercise any right with respect to, the registration of any shares of common stock or any security convertible into or exercisable or exchangeable for common stock.
For more information on the lock-up agreements see "Underwriting."
69
Certain U.S. federal income tax considerations for
Non-U.S. Holders of our common stock
The following is a general discussion of certain material U.S. federal income and estate tax consequences of the ownership and disposition of our common stock by a "Non-U.S. Holder." For purposes of this discussion, a "Non-U.S. Holder" is a beneficial owner of our stock who is treated for the relevant U.S. federal tax purposes as a non-resident alien, or a foreign partnership, corporation, estate, or trust. Because U.S. federal tax law uses different tests in determining whether an individual is a nonresident alien for income and estate tax purposes, some individuals may be "Non-U.S. Holders" for purposes of the federal income tax discussion below, but not for purposes of the federal estate tax discussion, andvice versa.
This discussion is based on the U.S. Internal Revenue Code of 1986, as amended (the "Code"), judicial decisions, and administrative regulations and interpretations in effect as of the date of this prospectus, all of which are subject to change, possibly with retroactive effect. This discussion does not address all aspects of U.S. federal income and estate taxation that may be relevant to Non-U.S. Holders in light of their particular circumstances, including, without limitation, Non-U.S. Holders that are controlled foreign corporations, passive foreign investment companies, pass-through entities, or U.S. expatriates, and Non-U.S. Holders that hold their common stock through pass-through entities. This discussion also does not address any tax consequences arising under the laws of any state, local, or non-U.S. jurisdiction.
You should consult your own tax advisor regarding the federal income and estate tax consequences of holding and disposing of our common stock in light of your particular situation, as well as any consequences under state, local, or foreign law.
Dividends
Distributions on our common stock will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined for U.S. federal income tax purposes. In general, we will be required to withhold U.S. federal income tax at a 30% rate, or such lower rate as may be specified by an applicable income tax treaty, on dividends paid to a Non-U.S. Holder. To obtain a reduced rate of withholding under a treaty, you must provide us with appropriate documentation (typically, a properly-executed IRS Form W-8BEN certifying your entitlement to benefits under the treaty). Treasury Regulations provide special rules to determine whether, for purposes of determining the applicability of a tax treaty, dividends paid to a Non-U.S. Holder that is an entity should be treated as paid to the entity or to those holding an interest in that entity.
We generally will not be required to withhold income taxes from dividends that are effectively connected with your conduct of a trade or business within the United States, so long as you provide us with appropriate documentation (typically, a properly-executed IRS Form W-8ECI, stating that the dividends are so connected). Instead, such dividends will be subject to regular U.S. income tax, on a net income basis, generally at the same rates applicable to a comparable U.S. holder. If you are a foreign corporation, your effectively-connected dividends may also be subject to an additional "branch profits tax," which is imposed under certain circumstances at a
70
rate of 30% (or such lower rate as may be specified by an applicable treaty), subject to certain adjustments.
Gain on disposition of common stock
A Non-U.S. Holder generally will not be subject to U.S. federal income tax with respect to gain realized on a sale or other disposition of our common stock. However, you will be taxable on such a gain if (i) the gain is effectively connected with a trade or business that you conduct in the United States (in the event certain tax treaty provisions apply, the gain must also be attributable to a permanent establishment in the U.S. in order to be subject to tax), (ii) you are a nonresident alien individual, you are present in the United States for 183 or more days in the taxable year of the disposition, and certain other conditions are met, or (iii) our stock is treated as a United States real property interest in your hands, within the meaning of Section 897(c) of the Code.
Subject to the exception noted below, our stock will generally be treated as a U.S. real property interest if we are or have been a "United States real property holding corporation" within the meaning of Section 897(c) at any time that you held the stock within five years before the disposition. We believe that we are not, and we do not anticipate becoming, a United States real property holding corporation. Moreover, even if we are treated as a United States real property holding corporation, so long as our common stock is regularly traded on an established securities market, the common stock will not be treated as a U.S. real property interest in the hands of any Non-U.S. Holder who has owned no more than five percent of the common stock (assuming for this purpose any options or shares of redeemable convertible preferred stock that you own have been exercised or converted) during the five years preceding a disposition.
Information reporting requirements and backup withholding
Generally, we must report to the U.S. Internal Revenue Service the amount of dividends we pay to you, your name and address, and the amount of any tax withheld. A similar report is sent to you. Pursuant to tax treaties or other information-sharing agreements, the U.S. Internal Revenue Service may make its reports available to tax authorities in your country of residence.
We generally will not be required to apply backup withholding to dividends we pay to you if you have provided an appropriate certification of your federal taxpayer identification number, or of the fact that you are not a U.S. person, unless we or our paying agent otherwise have knowledge that you are a U.S. person.
Under current U.S. federal income tax law, information reporting and backup withholding imposed at a rate of 28% (increasing to 31% in 2011) will apply to the proceeds of a disposition of our common stock effected by or through a U.S. office of a broker unless the disposing holder certifies as to its non-U.S. status or otherwise establishes an exemption. Generally, U.S. information reporting and backup withholding will not apply to a payment of disposition proceeds where the transaction is effected outside the United States through a non-U.S. office of a non-U.S. broker. However, U.S. information reporting requirements (but not backup withholding) will apply to a payment of disposition proceeds where the transaction is effected outside the United States by or through an office outside the United States of a
71
broker that fails to maintain documentary evidence that the holder is a Non-U.S. Holder and that certain conditions are met, or that the holder otherwise is entitled to an exemption, and the broker is (i) a U.S. person, (ii) a foreign person which derived 50% or more of its gross income for certain periods from the conduct of a trade or business in the United States, (iii) a "controlled foreign corporation" for U.S. federal income tax purposes, or (iv) a foreign partnership (a) at least 50% of the capital or profits interest in which is owned by U.S. persons, or (b) that is engaged in a U.S. trade or business. Backup withholding will apply to a payment of disposition proceeds if the broker has actual knowledge that the holder is a U.S. person.
Backup withholding is not an additional tax. Rather, the amount of tax withheld will be treated as a payment against your actual U.S. federal income tax liability (if any), and if the withholding results in an overpayment of tax, a refund may be obtained, provided that the required information is furnished to the U.S. Internal Revenue Service.
Federal estate tax
An individual Non-U.S. Holder who is treated as the owner of, or has made certain lifetime transfers of, an interest in our common stock will be required to include the value thereof in his gross estate for U.S. federal estate tax purposes, and may be subject to U.S. federal estate tax unless an applicable estate tax treaty provides otherwise.
72
Underwriting
J.P. Morgan Securities Inc. and SG Cowen Securities Corporation are acting as joint book-running managers for this offering.
We and the underwriters named below anticipate entering into an underwriting agreement covering the common stock to be offered in this offering. J.P. Morgan Securities Inc., SG Cowen Securities Corporation, and Leerink Swann & Company, Inc. are acting as representatives of the underwriters. Each underwriter has agreed to purchase the number of shares of common stock set forth opposite its name in the following table.
|
|---|
Name
| | Number of shares
|
|---|
|
|---|
| J.P. Morgan Securities Inc. | | |
| SG Cowen Securities Corporation | | |
| Leerink Swann & Company, Inc. | | |
| |
|
|---|
| Total | | |
|
The underwriting agreement provides that if the underwriters take any of the shares presented in the table above, then they must take all of these shares. No underwriter is obligated to take any shares allocated to a defaulting underwriter except under limited circumstances. The underwriting agreement provides that the obligations of the underwriters are subject to certain conditions precedent, including the absence of any material adverse change in our business and the receipt of certain certificates, opinions, and letters from us, our counsel, and our independent auditors.
The underwriters are offering the shares of common stock, subject to the prior sale of shares, and when, as and if such shares are delivered to and accepted by them. The underwriters will initially offer to sell shares to the public at the initial public offering price shown on the cover page of this prospectus. The underwriters may sell shares to securities dealers at a discount of up to $ per share from the initial public offering price. Any such securities dealers may resell shares to certain other brokers or dealers at a discount of up to $ per share from the initial public offering price. After the initial public offering, the underwriters may vary the public offering price and other selling terms.
If the underwriters sell more shares than the total number shown in the table above, the underwriters have the option to buy up to an additional shares of common stock from us to cover such sales. They may exercise this option during the 30-day period from the date of this prospectus. If any shares are purchased with this option, the underwriters will purchase shares in approximately the same proportion as shown in the table above.
The following table shows the per share and total underwriting discounts and commissions that we will pay to the underwriters. These amounts are shown assuming both no exercise and full exercise of the underwriters' option to purchase additional shares.
|
|---|
| | No exercise
| | Full exercise
|
|---|
|
|---|
| Per share | | $ | | | $ | |
| Total | | $ | | | $ | |
|
73
The representatives have advised us that, on behalf of the underwriters, they may make short sales of our common stock in connection with this offering, resulting in the sale by the underwriters of a greater number of shares than they are required to purchase pursuant to the underwriting agreement. The short position resulting from those short sales will be deemed a "covered" short position to the extent that it does not exceed the shares subject to the underwriters' over-allotment option and will be deemed a "naked" short position to the extent that it exceeds that number. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the trading price of the common stock in the open market that could adversely affect investors who purchase shares in this offering. The underwriters may reduce or close out their covered short position either by exercising the over-allotment option or by purchasing shares in the open market. In determining which of these alternatives to pursue, the underwriters will consider the price at which shares are available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. Any "naked" short position will be closed out by purchasing shares in the open market. Similar to the other stabilizing transactions described below, open market purchases made by the underwriters to cover all or a portion of their short position may have the effect of preventing or retarding a decline in the market price of our common stock following this offering. As a result, our common stock may trade at a price that is higher than the price that otherwise might prevail in the open market.
The representatives have advised us that, pursuant to Regulation M under the Securities Act, they may engage in transactions, including stabilizing bids or the imposition of penalty bids, that may have the effect of stabilizing or maintaining the market price of the shares of common stock at a level above that which might otherwise prevail in the open market. A "stabilizing bid" is a bid for or the purchase of shares of common stock on behalf of the underwriters for the purpose of fixing or maintaining the price of the common stock. A "penalty bid" is an arrangement permitting the representatives to claim the selling concession otherwise accruing to an underwriter or syndicate member in connection with the offering if the common stock originally sold by that underwriter or syndicate member is purchased by the representatives in the open market pursuant to a stabilizing bid or to cover all or part of a syndicate short position. The representatives have advised us that stabilizing bids and open market purchases may be effected on the Nasdaq National Market, in the over-the-counter market or otherwise and, if commenced, may be discontinued at any time.
One or more of the underwriters may facilitate the marketing of this offering online directly or through one of its affiliates. In those cases, prospective investors may view offering terms and a prospectus online and, depending upon the particular underwriter, place orders online or through their financial advisors. Other than the prospectus in electronic format, the information on those web sites is not part of this prospectus or registration statement of which the prospectus forms a part, has not been approved or endorsed by us or any underwriter in its capacity as an underwriter, and should not be relied on by investors.
We estimate that the total expenses of this offering, excluding underwriting discounts and commissions, will be approximately $ million.
We have agreed to indemnify the underwriters against certain liabilities, including civil liabilities under the Securities Act of 1933 and liabilities arising from breaches of
74
representations and warranties contained in the underwriting agreement, and to contribute to payments the underwriters may be required to make in respect of any such liabilities.
We and our executive officers, directors, and all of our stockholders have agreed that, during the period beginning from the date of this prospectus and continuing to and including the date 180 days after the date of this prospectus, subject to certain exceptions, none of us will, directly or indirectly, offer, pledge, announce the intention to sell, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any shares of common stock or any of our securities which are convertible into or exercisable or exchangeable for common stock (including common stock which may be deemed to be beneficially owned by any of the security holders) except for securities which may be issued upon exercise of a stock option or warrant and except for the participation by our employees in our 2003 Employee Stock Purchase Plan (provided in each case that the holder of our equity has entered into a similar lock-up agreement), without the prior written consent of J.P. Morgan Securities Inc. and SG Cowen Securities Corporation.
At our request, the underwriters have reserved shares of common stock for sale to our directors, officers, employees, business associates, and family members associated with the foregoing who have expressed an interest in participating in this offering. We expect these persons to purchase no more than % of the common stock offered in this offering. The number of shares available for sale to the general public will be reduced to the extent such persons purchase such reserved shares.
We have applied to have our common stock listed for quotation on the Nasdaq National Market under the symbol TLRX.
It is expected that delivery of the shares will be made to investors on or about , 2003.
There has been no public market for our common stock prior to this offering. We and the underwriters will negotiate the initial offering price. In determining the price, we and the underwriters expect to consider a number of factors in addition to prevailing market conditions, including:
• the information set forth in this prospectus and otherwise available to the representatives;
• the history and the prospects for the industry in which we compete;
• the ability of our management;
• our prospects for future earnings,
• the present state of our development and our current financial position;
• the general condition of the securities markets at the time of this offering; and
• the recent market prices of, and the demand for, publicly traded common stock of generally comparable companies.
We and the underwriters will consider these and other relevant factors in relation to the price of similar securities of generally comparable companies. Neither we nor the underwriters can assure investors that an active trading market will develop for our common stock, or that our common stock will trade in the public market at or above the initial offering price.
75
Legal matters
The validity of our common stock offered hereby will be passed upon by Bingham McCutchen LLP, Boston, Massachusetts. Legal matters in connection with this offering will be passed upon for the underwriters by Morgan, Lewis & Bockius LLP, New York, New York.
Experts
The financial statements as of December 31, 2001 and 2002 and for the years then ended and for the period from inception (July 6, 2000) through December 31, 2000 included in this prospectus have been so included in reliance on the report of PricewaterhouseCoopers LLP, independent accountants, given on the authority of said firm as experts in auditing and accounting.
Where you can find additional information
We have filed with the Securities and Exchange Commission a registration statement on Form S-1, including exhibits and schedules, under the Securities Act with respect to the shares of our common stock to be sold in the offering. This prospectus does not contain all of the information set forth in the registration statement. For further information with respect to us and the shares to be sold in the offering, reference is made to the registration statement and the exhibits and schedules attached to the registration statement. Statements contained in this prospectus as to the contents of any contract, agreement, or other document referred to are not necessarily complete. As a result of this offering, we will become subject to the information and reporting requirements of the Securities Exchange Act of 1934, as amended, and will file annual, quarterly, and current reports, proxy statements, and other information with the Securities and Exchange Commission.
You may read and copy all or any portion of the registration statement or any reports, statements, or other information that we file at the Securities and Exchange Commission's Public Reference Room at 450 Fifth Street, N.W., Washington, D.C. 20549. You can request copies of these documents, upon payment of a duplicating fee, by writing to the Securities and Exchange Commission. Please call the Securities and Exchange Commission at 1-800-SEC-0330 for further information on the operation of the Public Reference Room. Our Securities and Exchange Commission filings, including the registration statement, are also available to you on the Securities and Exchange Commission's web site http://www.sec.gov.
76
TolerRx, Inc.
Index to consolidated financial statements
| Report of Independent Auditors | | F-2 |
Financial Statements: |
|
|
| |
Consolidated balance sheets |
|
F-3 |
| |
Consolidated statements of operations |
|
F-4 |
| |
Consolidated statement of changes in stockholders' deficit |
|
F-5 |
| |
Consolidated statements of cash flows |
|
F-6 |
| |
Notes to consolidated financial statements |
|
F-7 |
F-1
REPORT OF INDEPENDENT AUDITORS
To the Board of Directors and Stockholders of
TolerRx, Inc.
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of operations, stockholders' deficit and cash flows present fairly, in all material respects, the financial position of TolerRx, Inc. and its subsidiaries at December 31, 2002 and 2001, and the results of its operations and its cash flows for the years then ended and for the period from inception (July 6, 2000) through December 31, 2000 in conformity with accounting principles generally accepted in the United States of America. These financial statements are the responsibility of the Company's management; our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with auditing standards generally accepted in the United States of America, which require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
/s/ PricewaterhouseCoopers LLP
August 22, 2003
Boston, Massachusetts
F-2
TolerRx, Inc.
Consolidated balance sheets
| |
|---|
| | December 31,
| |
| |
| |
|---|
| |
| | Pro forma
stockholders'
equity
June 30, 2003
| |
|---|
| | June 30,
2003
| |
|---|
| | 2001
| | 2002
| |
|---|
| |
|---|
| |
| |
| | (unaudited)
| | (unaudited)
| |
|---|
| Assets | | | | | | | | | | | | | |
| Current assets | | | | | | | | | | | | | |
| | Cash and cash equivalents | | $ | 16,528,741 | | $ | 37,890,749 | | $ | 35,627,616 | | | | |
| | Restricted cash | | | — | | | 517,500 | | | 517,500 | | | | |
| | Accounts receivable | | | — | | | 2,500,000 | | | — | | | | |
| | Prepaid expenses and other current assets | | | 65,198 | | | 299,723 | | | 416,162 | | | | |
| | |
| | | | |
| | | | Total current assets | | | 16,593,939 | | | 41,207,972 | | | 36,561,278 | | | | |
| Property and equipment, net | | | 1,445,777 | | | 5,070,774 | | | 4,990,743 | | | | |
| Long-term deposits | | | 38,921 | | | — | | | — | | | | |
| Restricted cash | | | 2,070,000 | | | 1,552,500 | | | 1,035,000 | | | | |
| Other assets | | | 6,796 | | | 6,032 | | | 5,649 | | | | |
| | |
| | | | |
| | | | Total assets | | $ | 20,155,433 | | $ | 47,837,278 | | $ | 42,592,670 | | | | |
| | |
| | | | |
| Liabilities, redeemable convertible preferred stock and stockholders' equity (deficit) | | | | | | | | | | | | | |
| Current liabilities | | | | | | | | | | | | | |
| | Accounts payable | | $ | 559,259 | | $ | 834,731 | | $ | 257,217 | | | | |
| | Accrued expenses | | | 1,100,774 | | | 1,258,025 | | | 1,951,276 | | | | |
| | Current portion of long-term debt | | | 360,756 | | | 1,534,824 | | | 1,300,342 | | | | |
| | Deferred rent | | | — | | | 315,116 | | | 374,580 | | | | |
| | Deferred revenue | | | — | | | 833,333 | | | 1,500,000 | | | | |
| | |
| | | | |
| | | | Total current liabilities | | | 2,020,789 | | | 4,776,029 | | | 5,383,415 | | | | |
| Long-term debt | | | 523,005 | | | 1,020,542 | | | 486,335 | | | | |
| Long-term deferred rent | | | — | | | 1,699,315 | | | 1,718,037 | | | | |
| Long-term deferred revenue | | | — | | | 1,639,270 | | | 2,222,604 | | | | |
| Commitments (Note 13) | | | | | | | | | | | | | |
| Redeemable convertible preferred stock | | | | | | | | | | | | | |
| | Series A redeemable convertible preferred stock, $0.001 par value; 9,035,000 shares authorized, issued and outstanding at December 31, 2001 and 2002 and June 30, 2003 (unaudited), and none pro forma (unaudited) (liquidation preference of $6,881,386 at December 31, 2002) | | | 6,316,259 | | | 6,833,453 | | | 7,107,816 | | | | |
| | Series B redeemable convertible preferred stock, $0.001 par value; 17,146,250 shares authorized; 17,000,000 issued and outstanding at December 31, 2001 and 2002 and June 30, 2003 (unaudited), and none pro forma (unaudited) (liquidation preference of $18,742,290 at December 31, 2002) | | | 17,308,842 | | | 18,705,771 | | | 19,438,965 | | | | |
| | Series C redeemable convertible preferred stock, $0.001 par value; 40,000,000 shares authorized; 34,930,000 and 35,000,000 issued and outstanding at December 31, 2002 and June 30, 2003 (unaudited), respectively, and none pro forma (unaudited) (liquidation preference of $35,236,597 at December 31, 2002) | | | — | | | 35,099,676 | | | 36,563,259 | | | | |
| | |
| | | | |
| | | | Total redeemable convertible preferred stock | | | 23,625,101 | | | 60,638,900 | | | 63,110,040 | | | | |
| Stockholders' equity (deficit) | | | | | | | | | | | | | |
| | Common stock, $0.001 par value; 75,000,000 shares authorized; 3,941,000, 4,189,750, and 4,202,250 shares issued at December 31, 2001 and 2002, and June 30, 2003 (unaudited), respectively, and 3,941,000, 3,944,750 and 3,962,250 outstanding at December 31, 2001 and 2002, and June 30, 2003 (unaudited), respectively; 65,237,250 issued and 64,997,250 outstanding pro forma at June 30, 2003 (unaudited) | | | 3,941 | | | 3,945 | | | 3,962 | | $ | 64,997 | |
| | Additional paid-in capital | | | 40,494 | | | — | | | — | | | 63,049,005 | |
| | Deferred stock-based compensation | | | (21,478 | ) | | (331,670 | ) | | (748,577 | ) | | (748,577 | ) |
| | Accumulated deficit | | | (6,036,419 | ) | | (21,609,053 | ) | | (29,583,146 | ) | | (29,583,146 | ) |
| | |
| |
| | | | Total stockholders' equity (deficit) | | | (6,013,462 | ) | | (21,936,778 | ) | | (30,327,761 | ) | $ | 32,782,279 | |
| | |
| |
| | | | Total liabilities, redeemable convertible preferred stock and stockholders' equity (deficit) | | $ | 20,155,433 | | $ | 47,837,278 | | $ | 42,592,670 | | | | |
| |
The accompanying notes are an integral part of these consolidated financial statements.
F-3
TolerRx, Inc.
Consolidated statements of operations
| |
| | Period from
July 6, 2000
(Date of
Inception)
through
December 31,
2000
| |
| |
| |
| |
| |
|---|
| | Year Ended
December 31,
| | Six Months Ended
June 30,
| |
|---|
| | 2001
| | 2002
| | 2002
| | 2003
| |
|---|
| |
| |
| |
| |
| | (unaudited)
| |
|---|
| Revenues | | $ | — | | $ | — | | $ | 27,397 | | $ | — | | $ | 750,000 | |
| | |
| |
| Operating expenses | | | | | | | | | | | | | | | | |
| Research and development(1) | | | 232,286 | | | 3,796,718 | | | 10,908,492 | | | 4,882,979 | | | 5,236,345 | |
| General and administrative(1) | | | 125,381 | | | 1,369,774 | | | 2,773,675 | | | 1,330,417 | | | 1,555,576 | |
| Stock-based compensation | | | 8,502 | | | 627,304 | | | 613,238 | | | 431,670 | | | 606,922 | |
| | |
| |
| | | Total operating expenses | | | 366,169 | | | 5,793,796 | | | 14,295,405 | | | 6,645,066 | | | 7,398,843 | |
| | |
| |
| | | Loss from operations | | | (366,169 | ) | | (5,793,796 | ) | | (14,268,008 | ) | | (6,645,066 | ) | | (6,648,843 | ) |
| Interest income | | | — | | | 164,878 | | | 250,400 | | | 138,822 | | | 214,759 | |
| Interest expense | | | (931 | ) | | (40,046 | ) | | (295,522 | ) | | (116,516 | ) | | (155,557 | ) |
| | |
| |
| Net loss | | | (367,100 | ) | | (5,668,964 | ) | | (14,313,130 | ) | | (6,622,760 | ) | | (6,589,641 | ) |
| Accretion of redeemable convertible preferred stock | | | (9,799 | ) | | (737,420 | ) | | (2,223,799 | ) | | (933,751 | ) | | (2,410,933 | ) |
| | |
| |
| Net loss attributable to common stockholders | | $ | (376,899 | ) | $ | (6,406,384 | ) | $ | (16,536,929 | ) | $ | (7,556,511 | ) | $ | (9,000,574 | ) |
| | |
| |
| Net loss attributable to common stockholders per share | | | | | | | | | | | | | | | | |
| Basic and diluted | | $ | (1.52 | ) | $ | (8.91 | ) | $ | (9.33 | ) | $ | (5.19 | ) | $ | (3.92 | ) |
| | |
| |
| Unaudited pro forma net loss attributable to common stockholders per share | | | | | | | | | | | | | | | | |
| Basic and diluted | | | | | | | | $ | (0.51 | ) | | | | $ | (0.10 | ) |
| | | | | | | | |
| | | | |
| |
| Weighted-average shares outstanding | | | | | | | | | | | | | | | | |
| Basic and diluted | | | 248,119 | | | 719,094 | | | 1,773,180 | | | 1,455,871 | | | 2,295,342 | |
| | |
| |
| Unaudited pro forma weighted average shares outstanding | | | | | | | | | | | | | | | | |
| Basic and diluted | | | | | | | | | 27,903,802 | | | | | | 63,330,342 | |
| | | | | | | | |
| | | | |
| |
(1)Excludes stock-based compensation as follows: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Research and development | | $ | 8,502 | | $ | 626,777 | | $ | 603,439 | | $ | 426,729 | | $ | 577,768 | |
| | General and administrative | | | — | | | 527 | | | 9,799 | | | 4,941 | | | 29,154 | |
| |
| |
|---|
| | | $ | 8,502 | | $ | 627,304 | | $ | 613,238 | | $ | 431,670 | | $ | 606,922 | |
| |
The accompanying notes are an integral part of these consolidated financial statements.
F-4
TolerRx, Inc.
Consolidated statement of changes in stockholders' deficit
| |
| |
| |
| | Additional
paid-in
capital
| | Deferred
stock-based
compensation
| |
| |
| |
|---|
| | Common stock
| | Accumulated
deficit
| |
| |
|---|
| | shares
| | par value
| | Total
| |
|---|
| |
| Issuance of common stock | | 3,336,000 | | $ | 3,336 | | $ | 942 | | $ | — | | $ | — | | $ | 4,278 | |
| Accretion of redeemable preferred stock to redemption value | | | | | | | | (9,444 | ) | | | | | (355 | ) | | (9,799 | ) |
| Non-employee stock compensation | | | | | | | | 8,502 | | | | | | | | | 8,502 | |
| Net loss | | | | | | | | | | | | | | (367,100 | ) | | (367,100 | ) |
| |
| |
|---|
| Balance at December 31, 2000 | | 3,336,000 | | | 3,336 | | | — | | | — | | | (367,455 | ) | | (364,119 | ) |
| Issuance of common stock | | 605,000 | | | 605 | | | 54,045 | | | | | | | | | 54,650 | |
| Issuance of warrants in connection with equipment line and lease | | | | | | | | 75,087 | | | | | | | | | 75,087 | |
| Accretion of redeemable preferred stock to redemption value | | | | | | | | (737,420 | ) | | | | | | | | (737,420 | ) |
| Non-employee stock compensation | | | | | | | | 625,874 | | | | | | | | | 625,874 | |
| Deferred compensation related to employee stock option grants | | | | | | | | 22,908 | | | (22,908 | ) | | | | | — | |
| Amortization of deferred compensation | | | | | | | | | | | 1,430 | | | | | | 1,430 | |
| Net loss | | | | | | | | | | | | | | (5,668,964 | ) | | (5,668,964 | ) |
| |
| |
|---|
| Balance at December 31, 2001 | | 3,941,000 | | | 3,941 | | | 40,494 | | | (21,478 | ) | | (6,036,419 | ) | | (6,013,462 | ) |
| Issuance of common stock | | 3,750 | | | 4 | | | 371 | | | | | | | | | 375 | |
| Non-employee stock compensation | | | | | | | | 567,737 | | | | | | | | | 567,737 | |
| Accretion of redeemable preferred stock to redemption value | | | | | | | | (964,295 | ) | | | | | (1,259,504 | ) | | (2,223,799 | ) |
| Deferred compensation related to employee stock option grants | | | | | | | | 355,693 | | | (355,693 | ) | | | | | — | |
| Amortization of deferred compensation | | | | | | | | | | | 45,501 | | | | | | 45,501 | |
| Net loss | | | | | | | | | | | | | | (14,313,130 | ) | | (14,313,130 | ) |
| |
| |
|---|
| Balance at December 31, 2002 | | 3,944,750 | | | 3,945 | | | — | | | (331,670 | ) | | (21,609,053 | ) | | (21,936,778 | ) |
| Issuance of common stock (unaudited) | | 17,500 | | | 17 | | | 2,652 | | | | | | | | | 2,669 | |
| Non-employee stock compensation (unaudited) | | | | | | | | 497,856 | | | | | | | | | 497,856 | |
| Accretion of redeemable preferred stock to redemption value (unaudited) | | | | | | | | (1,026,481 | ) | | | | | (1,384,452 | ) | | (2,410,933 | ) |
| Deferred compensation related to employee stock option grants (unaudited) | | | | | | | | 525,973 | | | (525,973 | ) | | | | | — | |
| Amortization of deferred compensation | | | | | | | | | | | 109,066 | | | | | | 109,066 | |
| Net loss (unaudited) | | | | | | | | | | | | | | (6,589,641 | ) | | (6,589,641 | ) |
| |
| |
|---|
| Balance at June 30, 2003 (unaudited) | | 3,962,250 | | $ | 3,962 | | $ | — | | $ | (748,577 | ) | $ | (29,583,146 | ) | $ | (30,327,761 | ) |
| |
The accompanying notes are an integral part of these consolidated financial statements.
F-5
TolerRx, Inc.
Consolidated statements of cash flows
| |
| | Period from
July 6, 2000
(Date of
inception)
through
December 31,
2000
| |
| |
| |
| |
| |
|---|
| | Year Ended
December 31,
| | Six Months Ended
June 30,
| |
|---|
| | 2001
| | 2002
| | 2002
| | 2003
| |
|---|
| |
| |
| |
| |
| | (unaudited)
| |
|---|
| Cash flows from operating activities | | | | | | | | | | | | | | | | |
| Net loss | | $ | (367,100 | ) | $ | (5,668,964 | ) | $ | (14,313,130 | ) | $ | (6,622,760 | ) | $ | (6,589,641 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities | | | | | | | | | | | | | | | | |
| | Depreciation and amortization | | | 1,680 | | | 167,825 | | | 1,065,195 | | | 707,909 | | | 397,410 | |
| | Noncash interest expense | | | — | | | 9,722 | | | 22,481 | | | 11,240 | | | 11,238 | |
| | Stock-based compensation | | | 8,502 | | | 627,304 | | | 613,238 | | | 431,670 | | | 606,922 | |
| | Amortization of deferred rent | | | — | | | — | | | (58,085 | ) | | (4,848 | ) | | (52,501 | ) |
| | Changes in assets and liabilities | | | | | | | | | | | | | | | | |
| | | (Increase) decrease in accounts receivable | | | — | | | — | | | (2,500,000 | ) | | — | | | 2,500,000 | |
| | | (Increase) decrease in deposits | | | (38,921 | ) | | — | | | 38,921 | | | 38,921 | | | — | |
| | | (Increase) in prepaid expenses and other assets | | | (48,607 | ) | | (23,387 | ) | | (233,761 | ) | | (160,503 | ) | | (116,056 | ) |
| | | Increase (decrease) in accounts payable | | | 121,662 | | | 437,597 | | | 275,472 | | | 923,161 | | | (577,514 | ) |
| | | Increase in accrued expenses | | | 74,635 | | | 1,026,139 | | | 157,251 | | | 265,900 | | | 693,251 | |
| | | Increase in deferred revenue | | | — | | | — | | | 2,472,603 | | | — | | | 1,250,001 | |
| | |
| |
| | | | Net cash used in operating activities | | | (248,149 | ) | | (3,423,764 | ) | | (12,459,815 | ) | | (4,409,310 | ) | | (1,876,890 | ) |
| | |
| |
| Cash flows from investing activities | | | | | | | | | | | | | | | | |
| Purchases of property and equipment | | | (122,063 | ) | | (1,493,219 | ) | | (4,690,192 | ) | | (3,660,340 | ) | | (317,379 | ) |
| Changes in restricted cash | | | — | | | (2,070,000 | ) | | — | | | — | | | 517,500 | |
| | |
| |
| | | | Net cash provided by (used in) investing activities | | | (122,063 | ) | | (3,563,219 | ) | | (4,690,192 | ) | | (3,660,340 | ) | | 200,121 | |
| | |
| |
| Cash flows from financing activities | | | | | | | | | | | | | | | | |
| Proceeds from issuance of Series A redeemable convertible preferred stock, net of issuance costs | | | 1,948,061 | | | 3,978,602 | | | — | | | — | | | — | |
| Proceeds from issuance of Series B redeemable convertible preferred stock, net of issuance costs | | | — | | | 16,951,219 | | | — | | | — | | | — | |
| Proceeds from issuance of Series C redeemable convertible preferred stock, net of issuance costs | | | — | | | — | | | 34,790,000 | | | — | | | 60,207 | |
| Proceeds from issuance of common stock | | | 4,278 | | | 54,650 | | | 375 | | | 375 | | | 2,669 | |
| Cash received from landlord related to lease | | | — | | | — | | | 2,072,516 | | | 641,294 | | | 130,687 | |
| Borrowings under line of credit | | | — | | | 1,129,500 | | | 2,670,500 | | | 1,599,635 | | | — | |
| Payments under line of credit | | | — | | | (180,374 | ) | | (1,021,376 | ) | | (307,830 | ) | | (779,927 | ) |
| | |
| |
| | | | Net cash provided by (used in) financing activities | | | 1,952,339 | | | 21,933,597 | | | 38,512,015 | | | 1,933,474 | | | (586,364 | ) |
| | |
| |
| Net increase (decrease) in cash and cash equivalents | | | 1,582,127 | | | 14,946,614 | | | 21,362,008 | | | (6,136,176 | ) | | (2,263,133 | ) |
| Cash and cash equivalents, beginning of period | | | — | | | 1,582,127 | | | 16,528,741 | | | 16,528,741 | | | 37,890,749 | |
| | |
| |
| Cash and cash equivalents, end of period | | $ | 1,582,127 | | $ | 16,528,741 | | $ | 37,890,749 | | $ | 10,392,565 | | $ | 35,627,616 | |
| | |
| |
| Cash paid during the year for interest | | $ | — | | $ | 33,545 | | $ | 139,541 | | $ | 51,977 | | $ | 71,155 | |
Noncash activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Warrants issued in connection with lines of credit | | $ | — | | $ | 67,438 | | $ | — | | $ | — | | $ | — | |
| Warrants issued in connection with lease agreement | | $ | — | | $ | 7,649 | | $ | — | | $ | — | | $ | — | |
| |
|---|
The accompanying notes are an integral part of these consolidated financial statements.
F-6
TolerRx, Inc.
Notes to consolidated financial statements
Information as of June 30, 2003 and for the six months ended
June 30, 2002 and 2003 is unaudited.
1. Nature of the business
TolerRx, Inc. (the "Company") was incorporated as a Delaware corporation on July 6, 2000. The Company was formed to discover, develop and commercialize therapies to treat patients with diseases of the immune system. During the period from inception (July 6, 2000) through December 31, 2002, the Company was considered to be a development stage enterprise as defined in Statement of Financial Accounting Standards ("SFAS") No. 7. In 2003, the Company has generated significant revenues from planned principal operations and is, therefore, considered to be in the operating stage for the six months ended June 30, 2003.
The Company is subject to risks common in the biotechnology industry, including but not limited to, raising additional capital, development by its competitors of new technological innovations, ability to complete preclinical and clinical development of products and receive timely regulatory approval of products, market acceptance of the Company's products, protection of proprietary technology, healthcare cost containment initiatives, and compliance with governmental regulations, including those of the U.S. Department of Health and Human Services and the U.S. Food and Drug Administration.
As shown in the financial statements, as of December 31, 2002, the Company has incurred a cumulative net loss of approximately $20.3 million since inception on July 6, 2000, has an accumulated deficit of $21.6 million, and has had no revenues from the sale of products. The Company intends to seek the additional financing it needs to fund its operations. There can be no assurance that such financing will be available with terms acceptable to the Company.
2. Summary of significant accounting policies
Significant accounting policies followed by the Company in the preparation of its financial statements are as follows:
Principles of consolidation
These consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary. All significant intercompany transactions and balances have been eliminated.
Unaudited interim results
The accompanying consolidated balance sheet as of June 30, 2003, the consolidated statements of operations and cash flows for the six months ended June 30, 2002 and 2003, and the consolidated statement of stockholders' deficit for the six months ended June 30, 2003, are unaudited. The unaudited interim financial statements have been prepared on the same basis as the annual financial statements and, in the opinion of management, reflect all adjustments, which include only normal recurring adjustments, necessary to present fairly the Company's financial position as of June 30, 2003 and results of operations and cash flows for the six
F-7
months ended June 30, 2002 and 2003. The financial data and other information disclosed in these notes to financial statements related to the six months ended June 30, 2002 and 2003 are unaudited. The results for the six months ended June 30, 2003 are not necessarily indicative of the results to be expected for the year ending December 31, 2003 or for any other interim period or for any other future year.
Pro forma stockholders' equity information (unaudited)
Immediately prior to the effective date of an initial public offering, the Company's outstanding redeemable convertible preferred stock will automatically convert into shares of common stock. The pro forma effects of the conversion of all outstanding shares of redeemable convertible preferred stock into 61,035,000 shares of common stock are unaudited, do not take into account a reverse stock split which the Company intends to effect prior to the closing of an initial public offering and have been reflected in the accompanying pro forma stockholders' equity as of June 30, 2003.
Use of estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Research and development
Internal and external research and development costs, including costs of funded research and development arrangements, are expensed in the period incurred.
Revenue recognition
The Company recognizes revenue from nonrefundable, upfront license fees over the related performance period or at the time the Company has no remaining performance obligations. Revenue from milestone payments for which the Company has no continuing performance obligations is recognized upon achievement of the related milestone. When the Company has continuing performance obligations, the Company recognizes milestone payments as revenue upon the achievement of the milestone only if all of the following conditions are met:
• the milestone payments are nonrefundable;
• achievement of the milestone was not reasonably assured at the inception of the arrangement;
F-8
• there is substantial effort involved in achieving the milestone; and
• the amount of the milestone is reasonable in relation to the level of effort associated with achievement of the milestone.
If any of these conditions are not met, the milestone payments are deferred and recognized as revenue over the term of the arrangement as the Company completes its performance obligations.
Cash, cash equivalents and marketable securities
The Company considers all highly liquid investments with an original maturity, or a remaining maturity at the time of purchase, of three months or less to be cash equivalents. The Company invests excess cash primarily in money market funds, which have strong credit ratings. These investments are subject to minimal credit and market risks.
Property and equipment
Property and equipment are stated at historical cost, less accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful lives of the assets as follows:
|
| Computer equipment and software | | 3 years |
| Office equipment, lab equipment, and furniture and fixtures | | 5 years |
| Leasehold improvements | | Life of lease |
|
The Company accounts for impairment of property and equipment in accordance with SFAS No. 144,Accounting for the Impairment of Disposal of Long-Lived Assets, which the Company adopted in 2002. Recoverability of assets to be held and used is measured by comparing the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of the asset exceeds its estimated undiscounted future net cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the asset. The carrying value of the asset is reviewed on a regular basis for the existence of facts, both internally and externally, that may suggest impairment. The Company did not recognize impairment charges in any of the periods presented.
Repairs and maintenance costs are expensed as incurred.
Income taxes
Deferred taxes are determined based on the difference between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect in the years in which the differences are expected to reverse. Valuation allowances are provided if, based upon the
F-9
weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
Financial instruments
The carrying amounts of the Company's financial instruments, which include cash equivalents, accounts receivable, accounts payable, accrued expenses and long-term debt, approximate their fair values due to their short maturities.
Concentration of credit risk
Financial instruments which potentially expose the Company to concentrations of credit risk consist primarily of the Company's investment in cash equivalents and accounts receivable. The Company invests its cash in investment grade securities to mitigate its risk.
Redeemable convertible preferred stock
The carrying value of redeemable convertible preferred stock is increased by periodic accretions so that the carrying amount will equal the redemption amount at the earliest redemption date. These increases are effected through charges to additional paid-in capital to the extent there is any, and, thereafter, to accumulated deficit.
Comprehensive income
SFAS No. 130,Reporting Comprehensive Income, requires disclosure of all components of comprehensive income and loss on an annual and interim basis. Comprehensive income and loss is defined as the change in equity of a business enterprise during a period from transactions and other events and circumstances from nonowner sources. Other than the reported net loss for all periods presented, there were no components of comprehensive income or loss which require disclosure.
Accounting for stock-based compensation
Employee stock awards under the Company's compensation plan are accounted for in accordance with Accounting Principles Board Opinion No. 25,Accounting for Stock Issued to Employees ("APB No. 25"), and related interpretations. Stock-based awards to nonemployees are accounted for under the provisions of SFAS No. 123 and Emerging Issues Task Force ("EITF") Issue No. 96-18,Accounting for Equity Instruments that are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services. Employee stock awards are measured at the date of grant and the resulting compensation is recorded as deferred compensation and is recognized over the employees' vesting period, typically four years. Stock-based awards to non-employees are measured at the date of grant and subsequently marked to market until the award vests in full.
F-10
The Company has computed the pro forma disclosures required under SFAS No. 123,Accounting for Stock-Based Compensation, for options granted using the Black-Scholes option-pricing model prescribed by SFAS No. 123.
Had compensation cost for the 2000 Equity Incentive Plan been determined consistent with SFAS No. 123, the Company's net loss attributable to common stockholders would have been the following pro forma amounts:
| |
| | Period from July 6, 2000 through December 31, 2000
| | Year ended December 31,
| | Six months ended June 30,
| |
|---|
| | 2001
| | 2002
| | 2002
| | 2003
| |
|---|
| |
| |
| |
| |
| | (unaudited)
| |
|---|
| Net loss attributable to common stockholders | | | | | | | | | | | | | | | | |
| Net loss—as reported | | $ | (376,899 | ) | $ | (6,406,384 | ) | $ | (16,536,929 | ) | $ | (7,556,511 | ) | $ | (9,000,574 | ) |
| Add: stock-based compensation expense included in reported net loss | | | — | | | 1,430 | | | 45,501 | | | 22,750 | | | 109,066 | |
| Deduct: stock-based compensation expense determined under fair value method | | | (203 | ) | | (1,737 | ) | | (45,981 | ) | | (23,230 | ) | | (109,305 | ) |
| |
| |
|---|
| Net loss—pro forma | | $ | (377,102 | ) | $ | (6,406,691 | ) | $ | (16,537,409 | ) | $ | (7,556,991 | ) | $ | (9,000,813 | ) |
| |
| |
|---|
| Net loss per share (basic and diluted) | | | | | | | | | | | | | | | | |
| | As reported | | $ | (1.52 | ) | $ | (8.91 | ) | $ | (9.33 | ) | $ | (5.19 | ) | $ | (3.92 | ) |
| |
| |
|---|
| | Pro forma | | $ | (1.52 | ) | $ | (8.91 | ) | $ | (9.33 | ) | $ | (5.19 | ) | $ | (3.92 | ) |
| |
|---|
The assumptions used are as follows:
| |
| | Year ended December 31,
| | Six months ended June 30,
| |
|---|
| | 2000
| | 2001
| | 2002
| | 2002
| | 2003
| |
|---|
| |
| |
| |
| |
| | (unaudited)
| |
|---|
| Risk-free interest rate | | 5.02 | % | 5.02 | % | 4.36 | % | 4.36 | % | 2.69 | % |
| Expected dividend yield | | — | | — | | — | | — | | — | |
| Expected lives | | 5 | | 5 | | 5 | | 5 | | 5 | |
| Expected volatility | | 70 | % | 70 | % | 70 | % | 70 | % | 70 | % |
F-11
Recent accounting pronouncements
In June 2002, the Financial Accounting Standards Board ("FASB") issued SFAS No. 146,Accounting for Costs Associated with Exit and Disposal Activities. SFAS No. 146 requires that a liability for costs associated with an exit or disposal activity be recognized and measured initially at fair value only when the liability is incurred. The provisions of SFAS No. 146 are effective for exit or disposal activities that are initiated after December 31, 2002. SFAS No. 146 did not have a material impact on the Company's financial position, results of operations, or cash flows.
In November 2002, the EITF reached a consensus on Issue No. 00-21,Revenue Arrangements with Multiple Deliverables. EITF Issue No. 00-21 provides guidance on how to account for arrangements that involve the delivery or performance of multiple products, services and/or rights to use assets. The provisions of EITF Issue No. 00-21 will apply to revenue arrangements entered into in fiscal periods beginning after June 15, 2003. The Company does not expect the adoption of EITF Issue No. 00-21 to have a material impact on its financial position, results of operations, or cash flows.
In December 2002, the FASB issued SFAS No. 148,Accounting for Stock-Based Compensation, Transition and Disclosure. SFAS No. 148 provides alternative methods of transition for a voluntary change to the fair value based method of accounting for stock-based employee compensation. SFAS No. 148 also requires that disclosures of the pro forma effect of using the fair value method of accounting for stock-based employee compensation be displayed more prominently and in a tabular format. Additionally, SFAS No. 148 requires disclosures of the pro forma effect in interim financial statements. The transition and annual disclosure requirements of SFAS No. 148 are effective for fiscal years ending after December 15, 2002 and have been adopted by the Company, which has elected to continue to account for employee stock options under APB No. 25.
In November 2002, the FASB issued FASB Interpretation ("FIN") No. 45,Guarantor's Accounting and Disclosure Requirements for Guarantees, Including Guarantees of Indebtedness of Others, and interpretation of FASB Statements No. 5, 57, and 107 and Rescission of FASB Interpretation No. 34. This interpretation expands the disclosure requirements of guarantee obligations and requires the guarantor to recognize a liability for the fair value of the obligation assumed under a guarantee. In general, FIN No. 45 applies to contracts or indemnification agreements that contingently require the guarantor to make payments to the guaranteed party based on changes in an underlying instrument that is related to an asset, liability, or equity security of the guaranteed party. Other guarantees are subject to the disclosure requirements of FIN No. 45 but not to the recognition provisions. The disclosure requirements of FIN No. 45 are effective for financial statement periods ending after December 15, 2002. The adoption of this standard did not have a material impact on the Company's financial statements at December 31, 2002. In addition, the impact of new arrangements entered into since January 1,
F-12
2003 is not significant. The Company has incorporated the disclosure requirements of FIN No. 45 in Note 13.
In January 2003, the FASB issued FIN No. 46,Consolidation of Variable Interest Entities, and interpretation of ARB 51. The primary objectives of FIN No. 46 are to provide guidance on the identification of entities for which control is achieved through means other than through voting rights (variable interest entities), and to determine when and which business enterprise should consolidate the variable interest entities. This new model for consolidation applies to an entity which either (1) the equity investors (if any) do not have a controlling financial interest or (2) the equity investment at risk is insufficient to finance the entity's activities without receiving additional subordinated financial support from other parties. FIN No. 46 also requires enhanced disclosure requirements related to variable interest entities. FIN No. 46 applies immediately to variable interest entities created after January 31, 2003, and to variable interest entities in which an enterprise obtains an interest after that date. It applies in the first fiscal year or interim period beginning after June 15, 2003 to variable interest entities in which an enterprise holds a variable interest that it acquired before February 1, 2003. The adoption of this standard did not have an impact on the Company's consolidated financial statements at December 31, 2002.
In April 2003, the FASB issued SFAS No. 149,Amendment of Statement 133 on Derivative Instruments and Hedging Activities. SFAS No. 149 amends and clarifies financial accounting and reporting for derivative instruments, including certain derivative instruments embedded in other contracts (collectively referred to as derivatives) and for hedging activities under FASB Statement No. 133,Accounting for Derivative Instruments and Hedging Activities. The adoption of SFAS No. 149 is not expected to have a material effect on the Company's financial position, results of operations, or cash flows.
In May 2003, the FASB issued SFAS No. 150,Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity.SFAS No. 150 establishes standards for how an issuer classifies and measures certain financial instruments with characteristics of both liabilities and equity. It requires that an issuer classify a financial instrument that is within its scope as a liability (or an asset in some circumstances). Many of those instruments were previously classified as equity. This statement is effective for financial instruments entered into or modified after May 31, 2003, and otherwise is effective at the beginning of the first interim period beginning after June 15, 2003. The adoption of SFAS No. 150 is not expected to have a material effect on the Company's consolidated financial statements.
3. Net loss per common share and unaudited pro forma net loss per common share
Basic and diluted net loss per common share is calculated by dividing the net loss attributable to common stockholders by the weighted-average number of unrestricted common shares
F-13
outstanding during the period. Diluted net loss per common share is the same as basic net loss per common share, since the effects of potentially dilutive securities are antidilutive for all periods presented.
The following sets forth the computation of basic and diluted net loss per common share:
| |
| | Year ended December 31,
| | Six months ended June 30,
| |
|---|
| | 2000
| | 2001
| | 2002
| | 2002
| | 2003
| |
|---|
| |
| |
| |
| |
| | (unaudited)
| |
|---|
| Basic and diluted net loss per common share | | | | | | | | | | | | | | | | |
| | Net loss attributable to common shareholders | | $ | (376,899 | ) | $ | (6,406,384 | ) | $ | (16,536,929 | ) | $ | (7,556,511 | ) | $ | (9,000,574 | ) |
| Basic and diluted weighted average | | | | | | | | | | | | | | | | |
| | Number of common shares outstanding | | | 248,119 | | | 719,094 | | | 1,773,180 | | | 1,455,871 | | | 2,295,342 | |
| Basic and diluted net loss per common share | | $ | (1.52 | ) | $ | (8.91 | ) | $ | (9.33 | ) | $ | (5.19 | ) | $ | (3.92 | ) |
| |
The following potentially dilutive, common share equivalents were excluded from the calculation of diluted net loss per common share because their effect was antidilutive:
|
| | Year ended December 31,
| | Six months ended June 30,
|
|---|
| | 2000
| | 2001
| | 2002
| | 2002
| | 2003
|
|---|
|
| |
| |
| |
| | (unaudited)
|
|---|
| Options | | 242,500 | | 94,500 | | 325,700 | | 185,750 | | 956,750 |
| Redeemable convertible preferred stock | | 8,012,050 | | 26,035,000 | | 60,965,000 | | 26,035,000 | | 61,035,000 |
| Warrants | | — | | 311,250 | | 311,250 | | 311,250 | | 311,250 |
| Restricted stock | | 2,726,083 | | 2,591,062 | | 2,003,542 | | 2,209,052 | | 1,592,781 |
|
Unaudited pro forma net loss per share
Unaudited pro forma net loss per share is computed using the weighted average number of common shares outstanding, including the pro forma effects of automatic conversion of all outstanding redeemable convertible preferred stock and accretion to redemption value through each balance sheet date into shares of the Company's common stock effective upon the assumed closing of the Company's proposed initial offering as if such conversions had occurred at the date of original issuance.
F-14
The following sets forth the computation of pro forma net loss per common share:
| |
| | Year
ended
December 31,
2002
| | Six months
ended
June 30,
2003
| |
|---|
| |
| Pro forma net loss | | | | | | | |
| | Net loss attributable to common stockholders | | $ | (16,536,929 | ) | $ | (9,000,574 | ) |
| | Accretion of redeemable convertible preferred stock | | | 2,223,799 | | | 2,410,933 | |
| | |
| |
| Pro forma net loss | | $ | (14,313,130 | ) | $ | (6,589,641 | ) |
| | |
| |
| Shares used in computing pro forma basic and diluted net loss per common share | | | | | | | |
| | Weighted-average number of common shares outstanding | | | 1,773,180 | | | 2,295,342 | |
| | Weighted-average impact of assumed conversion of all redeemable convertible preferred stock | | | 26,130,622 | | | 61,035,000 | |
| | |
| |
| | | | 27,903,802 | | | 63,330,342 | |
| |
Potentially dilutive common share equivalents of 2,640,492 and 2,860,781 at December 31, 2002 and June 30, 2003, respectively, related to the assumed exercise of the outstanding warrants, stock options and restricted stock were excluded from the pro forma diluted calculation because their effect was antidilutive.
4. Collaboration agreement
Genentech
In December 2002, the Company entered into a collaboration agreement with Genentech, Inc. for the development and commercialization of one of its products, TRX1. Genentech was required to pay a $2.5 million nonrefundable upfront payment upon execution of the agreement and $3.5 million for the purchase of 3.5 million shares of Series C convertible preferred stock which represented approximately 5 percent of the Company's capital stock on a fully diluted basis. The upfront fee was deferred and is being recognized ratably over the period of the Company's performance obligation, currently estimated to be three years. In February 2003, the Company received a $2.0 million milestone payment, which is being recognized as revenue over the three-year performance obligation.
The Company may also receive royalties upon product sales and additional payments upon the achievement of specific product development milestones as specified in the agreement. At its option, the Company may participate in a profit/loss sharing agreement in the United States in lieu of royalties on product sales in the United States. If the Company elects to exercise the
F-15
profit loss sharing option, the two parties will be required to split cumulative and future costs based upon a predetermined ratio.
5. Property and equipment
|
| | December 31,
|
|---|
| | 2001
| | 2002
|
|---|
|
| Computer equipment | | $ | 70,982 | | $ | 114,813 |
| Office equipment, lab equipment, and furniture and fixtures | | | 591,954 | | | 1,644,118 |
| Leasehold improvements | | | 831,807 | | | 3,892,437 |
| Construction-in-progress | | | 120,539 | | | — |
| | |
|
| | | | 1,615,282 | | | 5,651,368 |
| | |
|
| Less—accumulated depreciation and amortization and amortization | | | 169,505 | | | 580,594 |
| | |
|
| | | $ | 1,445,777 | | $ | 5,070,774 |
|
Depreciation expense was $1,680, $167,825 and $1,065,195 for the period from July 6, 2000 (date of inception) through December 31, 2000, and years ended December 31, 2001 and 2002, respectively.
During 2002, in connection with the Company's move to its new facility, the Company wrote off certain fully depreciated leasehold improvements.
6. Accrued expenses
|
| | December 31,
| |
|
|---|
| | June 30,
2003
|
|---|
| | 2001
| | 2002
|
|---|
|
| | (unaudited)
|
|---|
| Accrued licensing and research and development fees | | $ | 787,202 | | $ | 736,618 | | $ | 1,257,211 |
| Accrued payroll and payroll related costs | | | 61,537 | | | 173,306 | | | 317,556 |
| Accrued interest | | | 4,426 | | | 137,926 | | | 211,090 |
| Accrued professional fees | | | 137,764 | | | 116,974 | | | 102,155 |
| Other | | | 109,845 | | | 93,201 | | | 63,264 |
| | |
|
| | | $ | 1,100,774 | | $ | 1,258,025 | | $ | 1,951,276 |
|
7. Long-term debt
During 2002, the Company had access to an equipment and a leasehold improvement line of credit for $1.25 million and $1.75 million, respectively. The Company also had an $800,000 line of credit that expired in 2001. The Company drew $3,800,000 and repaid $1,201,750 related to
F-16
all of these lines of credit through the year ended December 31, 2002. The lines of credit bear interest at approximately 7 percent, and are collateralized by the assets of the Company. At the end of the term, the Company will be required to pay an additional 9.0 percent to 9.5 percent of the original advance amounts, which is being recorded as additional interest expense over the term of the line of credit. The balance outstanding at December 31, 2002 is payable as follows:
|
| |
|
|---|
| | Amount
|
|---|
|
| 2003 | | $ | 1,718,870 |
| 2004 | | | 1,069,156 |
| 2005 | | | 308,798 |
| | |
|
| | | | 3,096,824 |
| Less: amounts representing interest | | | 541,458 |
| | |
|
| Present value of future minimum payments | | | 2,555,366 |
| Less: amounts due within one year | | | 1,534,824 |
| | |
|
| Long term portion | | $ | 1,020,542 |
|
In connection with the $800,000 line of credit, the Company issued warrants to purchase approximately 65,000 shares of common stock at an exercise price of $0.10 per share. The warrants are immediately exercisable and expire ten years from the date of issuance. The relative fair value of the warrants of approximately $3,200 has been recorded as a debt discount and is being charged to interest expense over the repayment term of the underlying line of credit. In connection with the $1.25 million and $1.75 million lines of credit, the Company issued warrants to purchase approximately 146,000 shares of Series B convertible preferred stock at an exercise price of $1.00 per share. The warrants are immediately exercisable and expire ten years from the date of issuance. The relative fair value of the warrants of approximately $64,200 has been recorded as a debt discount and is being charged to interest expense over the repayment term of the underlying lines of credit. As of December 31, 2002, all of the warrants remained unexercised.
F-17
8. Redeemable convertible preferred stock ("preferred stock")
The following table summarizes the activity for the preferred stock:
| | Series A
| | Series B
| | Series C
| |
|
|---|
| | Shares
| | Amount
| | Shares
| | Amount
| | Shares
| | Amount
| | Total
|
|---|
|
|---|
|
| Issuance of Series A redeemable convertible preferred stock, net of issuance costs of $51,940 | | 3,012,050 | | $ | 1,948,061 | | — | | $ | — | | — | | $ | — | | $ | 1,948,061 |
| Accretion to redemption value | | | | | 9,799 | | | | | | | | | | | | | 9,799 |
|
|---|
| Balance at December 31, 2000 | | 3,012,050 | | | 1,957,860 | | — | | | — | | — | | | — | | | 1,957,860 |
Issuance of Series A redeemable convertible preferred stock, net of issuance costs of $20,637 |
|
6,022,950 |
|
|
3,978,602 |
|
|
|
|
|
|
|
|
|
|
|
|
3,978,602 |
| Issuance of Series B redeemable convertible preferred stock, net of issuance costs of $48,781 | | | | | | | 17,000,000 | | | 16,951,219 | | | | | | | | 16,951,219 |
| Accretion to redemption value | | | | | 379,797 | | | | | 357,623 | | | | | | | | 737,420 |
|
|---|
| Balance at December 31, 2001 | | 9,035,000 | | | 6,316,259 | | 17,000,000 | | | 17,308,842 | | — | | | — | | | 23,625,101 |
Issuance of Series C redeemable convertible preferred stock, net of issuance costs of $140,000 |
|
|
|
|
|
|
|
|
|
|
|
34,930,000 |
|
|
34,790,000 |
|
|
34,790,000 |
| Accretion to redemption value | | | | | 517,194 | | | | | 1,396,929 | | | | | 309,676 | | | 2,223,799 |
|
|---|
| Balance at December 31, 2002 | | 9,035,000 | | | 6,833,453 | | 17,000,000 | | | 18,705,771 | | 34,930,000 | | | 35,099,676 | | | 60,638,900 |
Issuance of Series C redeemable convertible preferred stock, net of issuance cost $9,793 (unaudited) |
|
|
|
|
|
|
|
|
|
|
|
70,000 |
|
|
60,207 |
|
|
60,207 |
| Accretion to redemption value (unaudited) | | | | | 274,363 | | | | | 733,194 | | | | | 1,403,376 | | | 2,410,933 |
|
|---|
| Balance at June 30, 2003 (unaudited) | | 9,035,000 | | $ | 7,107,816 | | 17,000,000 | | $ | 19,438,965 | | 35,000,000 | | $ | 36,563,259 | | $ | 63,110,040 |
|
|---|
The preferred stock has the following terms:
Voting
The holders of the preferred stock are entitled to vote, together with the holders of common stock, on all matters submitted to stockholders for a vote. Each preferred stockholder is entitled to the number of votes equal to the number of shares of common stock into which each preferred share is convertible at the time of such vote. In addition, the holders of the Series A preferred stock have the right to elect two members of the Board of Directors of the Company and the holders of the Series B preferred stock and Series C preferred stock each have the right to elect one member of the Board of Directors.
Dividends
The holders of shares of preferred stock shall be entitled to receive, when and as declared or paid by the Board of Directors on any shares of preferred stock, out of funds legally available
F-18
for that purpose, dividends at the rate of 8 percent per annum compounding annually and which will accrue on a quarterly basis commencing on the original issuance date of such series of stock prior to the payment of dividends and other distributions on any other class of securities of the Company. Dividends hereunder shall be payable, as accrued, on any liquidation, upon any event of sale, or upon meeting any redemption date. Whenever any dividend may be declared or paid on any shares of preferred stock, the Board of Directors shall also declare and pay a dividend on the same terms, at the same rate, and in like kind upon each other share of the preferred stock then outstanding, so that all outstanding shares of preferred stock will participate equally with each other.
Liquidation preference
In the event of any liquidation, dissolution, or winding-up of the affairs of the Company, the holders of the then outstanding preferred stock shall receive for each share an amount equal to the original purchase price plus 8 percent per annum, compounded annually, plus all declared but unpaid dividends, thereon (other than the foregoing accruing, compounding dividends), payable in preference and priority to any payments made to the holders of the then outstanding common stock. The Company's Series A preferred stock, Series B preferred stock, and Series C preferred stock shall rank equally with each other.
In the event of a liquidation, after payments shall have been made first to the preferred stockholders and then to the holders of each junior class or series of capital stock (other than common stock) of the full amount to which they shall be entitled, the holders of the preferred stock as a class shall be entitled to share ratably with the common stockholders in all the remaining assets of the Company available for distribution to its stockholders until the preferred stockholders have received an aggregate distribution equal to two times their respective preferred amounts.
Conversion
Each share of preferred stock, at the option of the holder, is convertible into a number of fully paid shares of common stock as determined by dividing the respective original purchase price by the conversion price in effect at the time. Any preferred stockholder who exercises the right to convert shares of preferred stock into shares of common stock shall be entitled to payment of all declared but unpaid dividends payable with respect to such preferred stock.
In the event of an underwritten public offering at a price of $2.00 per share and with net proceeds of $30 million, each share of preferred stock then outstanding will automatically convert into shares of common stock as determined by dividing the respective original purchase price by the conversion price in effect at the time.
F-19
Redemption
Any holder of the outstanding preferred stock may, by written request, delivered after November 1, 2007, require the Company to redeem the preferred stock by paying in cash a sum equal to the original purchase price of the preferred stock plus 8 percent per annum thereon, compounded annually, for each share of preferred stock from the original issuance date until the redemption date, plus, in each case, an amount equal to any declared but unpaid dividends thereon, up to 25 percent of the preferred stock at the time that such request is made, and in each subsequent year thereafter up to 25 percent of the preferred stock on the redemption date plus that number of shares of preferred stock that such requesting holder could have required the Company to have redeemed in the year or years following the redemption date but elected not to have redeemed.
If the Company does not have sufficient funds legally available to redeem all shares of preferred stock for which redemption has been requested, then the Company shall redeem such shares ratably.
9. Common stock
Each share of common stock is entitled to one vote. The holders of common stock are also entitled to receive dividends whenever funds are legally available and when declared by the Board of Directors, subject to the prior rights of holders of all classes of stock outstanding.
10. Equity incentive plan
During 2000, the Company adopted the 2000 Equity Incentive Plan (the "Plan"). The Plan provides for the granting of incentive and nonqualified stock options and restricted stock to employees, directors, and consultants of the Company. Incentive stock options may not be granted at an exercise price less than the fair market value of the Company's common stock at the date of grant and for a term not to exceed ten years. For holders of more than 10 percent of the Company's total combined voting power of all classes of stock, incentive stock options may not be granted at less than 110 percent of the fair market value of the Company's common stock at the date of grant and for a term not to exceed five years. The exercise price under each nonqualified stock option may be granted at less than fair market value but not less than par value. As of December 31, 2002, the Company had reserved an aggregate of 5,085,000 shares of common stock for the issuance of options and restricted stock under the Plan.
F-20
The following table summarizes the activity of the Plan:
|
|---|
| | 2000
| | 2001
| | 2002
| | June 30, 2003
|
|---|
| | Number of
options
| | Weighted
average
exercise
price
| | Number of
options
| | Weighted
average
exercise
price
| | Number of
options
| | Weighted
average
exercise
price
| | Number of
options
| | Weighted
average
exercise
price
|
|---|
|
|---|
| |
| |
| |
| |
| |
| |
| | (unaudited)
|
|---|
| Outstanding, beginning of period | | — | | $ | — | | 242,500 | | $ | 0.06 | | 94,500 | | $ | 0.13 | | 325,750 | | $ | 0.13 |
| Granted | | 242,500 | | | 0.06 | | 477,000 | | | 0.12 | | 244,500 | | | 0.16 | | 649,000 | | | 0.16 |
| Exercised | | — | | | — | | (605,000 | ) | | — | | (3,750 | ) | | 0.10 | | (12,500 | ) | | 0.15 |
| Canceled | | — | | | — | | (20,000 | ) | | 0.09 | | (9,500 | ) | | 0.14 | | (5,500 | ) | | 0.16 |
| |
|
|---|
| Outstanding, end of period | | 242,500 | | $ | 0.06 | | 94,500 | | $ | 0.13 | | 325,750 | | $ | 0.15 | | 956,750 | | $ | 0.15 |
| |
|
|---|
Options exercisable at end of period |
|
— |
|
|
|
|
2,500 |
|
|
|
|
22,125 |
|
|
|
|
42,750 |
|
|
|
| |
|
|---|
Options available for future grant |
|
1,842,000 |
|
|
|
|
829,500 |
|
|
|
|
3,349,550 |
|
|
|
|
2,706,000 |
|
|
|
| |
|
|---|
The weighted average fair value of options granted at fair value during the year ended December 31, 2000 and 2001 was $0.01 and $0.12, respectively. The weighted average fair value of options granted at less than deemed fair value was $0.51, $0.79 and $0.81 for the years ended December 31, 2001 and 2002 and the six months ended June 30, 2003, respectively.
The following table summarizes information about stock options outstanding at December 31, 2002:
|
|
|---|
Exercise
Price
| | Options
outstanding
| | Weighted-average remaining contractual life
| | Options
exercisable
|
|---|
| $0.06 | | 2,500 | | 7.84 | | 2,500 |
| $0.10 | | 21,750 | | 8.19 | | 3,000 |
| $0.13 | | 11,500 | | 8.59 | | 2,875 |
| $0.15 | | 52,500 | | 8.79 | | 13,750 |
| $0.16 | | 237,500 | | 9.46 | | — |
| | |
|
| | | 325,750 | | | | 22,125 |
|
In 2000, an employee purchased 1,190,000 shares of stock, of which 1,066,042 were restricted. One-fourth of the restricted stock vests after the first year. The remaining restricted stock vests quarterly over the remaining three years. At December 31, 2002, approximately 466,393 shares remained restricted.
In 2001, certain employees and directors exercised options to purchase 605,000 shares of restricted stock. In 2002, certain employees and directors purchased 245,000 shares of restricted stock under the Plan. The stock has ratable annual vesting over four years. At December 31, 2002, 600,000 shares remained restricted.
F-21
During the years ended December 31, 2001 and 2002 and the six months ended June 30, 2003, in connection with the grant of stock options and restricted stock to employees, the Company recorded deferred stock compensation of approximately $22,900, $355,700 and $526,000, respectively, representing the difference between the exercise price and the deemed fair market value of the Company's common stock on the date the stock options or restricted stock were granted. In the years ended 2001 and 2002 and the six months ended June 30, 2003, the Company issued 45,000, 489,500 and 649,000, respectively, of stock options and restricted stock with an exercise price below the deemed fair market value. During the years ended December 31, 2001 and 2002 and the six months ended June 30, 2002 and 2003, the Company recorded amortization of deferred stock compensation of approximately $1,400, $45,500, $22,800 and $109,100, respectively. At December 31, 2002 and June 30, 2003, $331,700 and $748,600, respectively, remains unamortized.
In November 2000, the Company issued 2,146,000 shares of common stock to nonemployee founders of the Company, of which approximately 1,660,000 were restricted. One fourth of the restricted stock vests after the first year. The remaining restricted stock vests quarterly over the remaining three years. The Company recognized approximately $624,300, $556,800, $405,100 and $493,500 of compensation expense for the years ended December 31, 2001 and 2002 and the six months ended June 30, 2002 and 2003, respectively, related to this stock. At December 31, 2002, 764,550 shares remain restricted.
In addition, the Company granted certain consultants nonqualified stock options to purchase a total of 29,500 shares of common stock in 2001 and 2002. The Company recorded compensation expense of approximately $1,600, $10,900, $3,800 and $4,300 related to these options for the year ended December 31, 2001 and 2002 and the six months ended June 30, 2002 and 2003, respectively.
Subsequent to June 30, 2003, the Company increased the shares available under the Plan to 7,245,000, granted options to purchase 3,923,500 shares of common stock and sold 240,000 shares of restricted common stock to various employees and nonemployees.
In August 2003, the Company's board of directors and stockholders approved the 2003 Employee Stock Purchase Plan. To date, no shares have been issued under this plan.
F-22
11. Income taxes
The Company's deferred tax assets consist of the following:
| |
| | December 31,
| |
|---|
| | 2001
| | 2002
| |
|---|
| |
| Net operating loss carryforwards | | $ | 1,530,428 | | $ | 280,961 | |
| Research and development credits | | | 110,122 | | | 351,777 | |
| Capitalized start-up costs | | | 623,132 | | | 1,699,632 | |
| Capitalized research and development costs | | | — | | | 4,680,598 | |
| Deferred revenue | | | — | | | 995,877 | |
| Other | | | 57,672 | | | 61,834 | |
| | |
| |
| Total gross deferred tax asset | | | 2,321,354 | | | 8,070,679 | |
| Valuation allowance | | | (2,321,354 | ) | | (8,070,679 | ) |
| | |
| |
| Net deferred tax asset | | $ | — | | $ | — | |
| |
The Company has provided a valuation allowance for the full amount of its net deferred tax assets since realization of any future benefit from deductible temporary differences and net operating loss and tax credit carryforwards cannot be sufficiently assured at December 31, 2002.
At December 31, 2002, the Company had federal and state net operating loss carryforwards of approximately $709,000 and $638,000, respectively, available to reduce future taxable income and which begin to expire in 2020 and 2006, respectively. The Company also had federal and state research and development tax credit carryforwards of approximately $251,000 and $153,000, respectively, available to reduce future tax liabilities which begin to expire in 2015.
Under provisions of the Internal Revenue Code, certain substantial changes in the Company's ownership may limit the amount of the net operating loss carryforwards and research and development credit carryforwards which could be utilized annually to offset future taxable income and taxes payable.
The Company's effective income tax rate differs from the statutory federal income tax rate as follows:
|
| |
| |
| |
|
|---|
| | 2000
| | 2001
| | 2002
|
|---|
|
| Tax provision benefit | | | | | | |
| | U.S. statutory rate | | (34.0 | )% | (34.0 | )% | (34.0)% |
| State income taxes, net of federal benefit | | (6.2 | ) | (6.6 | ) | (6.8) |
| Research and development credits | | — | | (1.7 | ) | (1.2) |
| Deductions subject to deferred tax valuation allowance | | 40.2 | | 42.3 | | 42.0 |
| | |
|
| Effective tax rate | | 0.0 | % | 0.0 | % | 0.0% |
|
F-23
12. 401(k) plan
During 2001, the Company adopted an employee savings and retirement plan, qualified under Section 401(k) of the Internal Revenue Code. All of the Company's full-time employees who have attained the age of 21 are eligible to participate in the 401(k) plan after three months of employment. Pursuant to the 401(k) plan, employees may elect to reduce their current compensation by up to the statutorily prescribed annual limit and have the amount of the reduction contributed to the 401(k) plan. To date no contributions have been made to the Plan by the Company.
13. Commitments and contingencies
The Company leases its office and lab space under a noncancelable operating lease. Total rent expense was approximately $1,336,000 and $234,000 for the years ended December 31, 2002 and 2001, respectively.
Future minimum lease payments under noncancelable operating leases at December 31, 2002 are as follows:
|
| | Operating
leases
|
|---|
Year ending December 31,
|
|---|
|
| 2003 | | $ | 2,164,473 |
| 2004 | | | 2,164,473 |
| 2005 | | | 2,164,473 |
| 2006 | | | 2,164,473 |
| 2007 and thereafter | | | 12,386,898 |
| | |
|
| | | $ | 21,044,790 |
|
The Company recognizes rent expense on a straight-line basis over the term of the lease. Amounts recorded as expense in excess of cash paid are recorded as deferred rent. Cash paid in excess of rent expense is recorded as an offset to deferred rent.
In November 2001, in connection with its operating lease, the Company issued a warrant to purchase 100,000 shares of common stock at an exercise price of $1.00 per share. The warrant, of which 20,000 shares were immediately exercisable, has annual vesting over 4 years. At December 31, 2002, 60,000 shares remain unvested. The warrant expires ten years from the date of issuance. The value of the warrant of approximately $7,800 has been recorded as an asset and is being expensed as additional rent expense over the ten-year term of the lease. As of December 31, 2002, the warrant remained unexercised.
In 2002, the Company received approximately $2,073,000 from its landlord for the purchase of certain equipment and leasehold improvements. Approximately $270,000 of this amount is to be repaid over a thirty-six month period with an interest rate of 10 percent. There is no provision for repayment of the remaining allowance. These amounts have been classified as
F-24
deferred rent in the accompanying consolidated balance sheets. The allowance that is not required to be repaid is being amortized over the life of the lease. For the year ended December 31, 2002, the Company recorded amortization of approximately $105,000 related to this allowance. In addition, the Company was required to enter into a $2,070,000 letter of credit agreement for the benefit of the landlord. The letter of credit is collateralized by $2,070,000 of cash, which has been classified as restricted cash in the accompanying consolidated balance sheet at December 31, 2002.
The Company has entered into various consulting agreements with nonemployees. Under these agreements, the Company is obligated to pay annual fees and has granted options, or the option to purchase restricted stock (Note 10) to the consultants. The Company recorded approximately $184,600, and $240,000, for the annual fees for the years ended December 31, 2001 and 2002, respectively. In addition, the Company is obligated to pay annual fees of approximately $240,000 through 2005.
The Company has entered into several license agreements with various third parties. In connection with these agreements, the Company is required to pay certain upfront and annual fees, which totaled approximately $230,000 and $146,000 for the years ended December 31, 2001 and 2002, respectively. Under the license agreements, the Company is obligated to pay annual fees of approximately $60,000 through 2007 in order to maintain the licenses. In addition, the Company may be required to pay milestones and royalties related to the licensed technology in the future.
In connection with its research and development efforts, the Company entered into three research agreements with an academic institution which is also a stockholder. Under these agreements, the Company is obligated to pay approximately $920,000 and $636,000 for 2003 and 2004, respectively in exchange for research services and the right to license certain technologies. The Company records these fees ratably as research and development expense over the year.
Guarantor agreements
As permitted under Delaware law, the Company has contractual agreements and charter and by-law provisions whereby it indemnifies its officers and directors for certain events or occurrences while the officer or director is, or was serving, at the Company's request in such capacity. The term of the indemnification period is for the officers' or directors' lifetime. The maximum potential amount of future payments the Company could be required to make under these indemnification agreements is unlimited; however, the Company has a director and officer insurance policy that limits its exposure and enables it to recover a portion of any future amounts paid. Because the Company has no history of claims against its officers and directors and because it maintains insurance policy coverage, the Company believes the estimated fair value of these indemnification obligations is minimal.
F-25
The Company enters into standard indemnification agreements in the ordinary course of business. Pursuant to these agreements, the Company indemnifies, holds harmless, and agrees to reimburse the indemnified party for losses suffered or incurred by the indemnified party, generally business partners or customers for certain reasons, such as in connection with any U.S. patent, or any copyright or other intellectual property infringement claim by any third party with respect to the Company's products. The term of these indemnification agreements is generally perpetual any time after execution of the agreement. The maximum potential amount of future payments the Company could be required to make under these indemnification agreements is unlimited. The Company has never incurred costs to defend lawsuits or settle claims related to these indemnification agreements. As a result, the Company believes the estimated fair value of these agreements is minimal.
14. Segment information
The Company operates in one segment, which is the business of developing, manufacturing and marketing drugs for human health care. The chief operating decision-makers review the profit and loss of the Company on an aggregate basis and manage the operations of the Company as a single operating segment. All the Company's assets are located in the United States, and the Company derives all of its revenue from its collaboration agreement with Genentech in the United States.
F-26
_____________ shares

Common stock
Prospectus
Joint Book-Running Managers
| JP Morgan | | SG Cowen |
Leerink Swann & Company
|
, 2003
You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with information different from that contained in this prospectus. We are offering to sell, and seeking offers to buy, shares of our common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of shares of our common stock.
No action is being taken in any jurisdiction outside the United States to permit a public offering of shares of our common stock or possession or distribution of this prospectus in that jurisdiction. Persons who come into possession of this prospectus in jurisdictions outside the United States are required to inform themselves about and to observe any restrictions as to this offering and the distribution of this prospectus applicable to that jurisdiction.
Until _____________, all dealers that buy, sell or trade in shares of our common stock, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers' obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
Part II
Information not required in prospectus
Item 13. Other expenses of issuance and distribution
Expenses of the Registrant in connection with the issuance and distribution of the securities being registered, other than the underwriting discount and commissions, are estimated as follows:
| SEC Registration Fee | | $ | 6,068 |
| NASD Fees | | | 8,000 |
| Nasdaq National Market Listing Fees | | | 5,000 |
| *Printing and Engraving Expenses | | | |
| *Legal Fees and Expenses | | | |
| *Accountants' Fees and Expenses | | | |
| *Expenses of Qualification Under State Securities Laws, Including Attorneys' Fees | | | |
| *Transfer Agent and Registrar's Fees | | | |
| *Miscellaneous Costs | | | |
| | *Total | | | |
* To be provided by amendment.
Item 14. Indemnification of officers and directors
Section 145 of the Delaware General Corporation Law empowers a Delaware corporation to indemnify its officers, directors and certain other persons to the extent and under the circumstances set forth therein.
The Registrant's restated certificate of incorporation and restated bylaws contain provisions that the Registrant will indemnify to the fullest extent authorized by Delaware law, and advance expenses to, any person who was or is a party or is threatened to be made a party to any action, suit or proceeding, by reason of being or having been a director or officer of the Registrant or serving or having served at the request of the Registrant as a director, trustee, officer, employee, or agent of another corporation or of a partnership, joint venture, trust or other enterprise, including service with respect to an employee benefit plan, whether the basis of such proceeding is alleged action or failure to act in an official capacity as a director, trustee, officer, employee, or agent. The Registrant's restated by-laws provide that it may indemnify and advance expenses to any of its employees or agents on the same basis on which it is required to indemnify its officers and directors.
The Registrant has entered into indemnification agreements with Douglas J. Ringler, Herman Waldmann, Christopher K. Mirabelli, Wayne T. Hockmeyer, Yasunori Kaneko, Daniel L. Kisner, David J. McLachlan, James E. Niedel, and Harold R. Werner. These agreements provide that the Registrant will indemnify the director to the fullest extent permitted by law, in connection with any proceeding arising from service as a director, against all expenses (including attorney's fees and all other costs, expenses and obligations incurred in connection with such proceeding), judgments, fines, amounts paid in settlement (if such settlement is approved in advance by the
II-1
Registrant), and taxes incurred as a result of any payments received pursuant to such indemnification agreement.
The Registrant will agree to indemnify the underwriters and their controlling persons, and the underwriters will agree to indemnify the Registrant and its controlling persons, including directors and executive officers of the Registrant against certain liabilities, including liabilities under the Securities Act. Reference is made to the form of the Underwriting Agreement that will be filed as part of the Exhibits hereto.
For information regarding our undertaking to submit to adjudication the issue of indemnification for violation of the securities laws, see Item 17 hereof.
Item 15. Recent sales of unregistered securities
Described below is information regarding all unregistered securities of the Registrant sold by the Registrant within the past three years.
1. As of August 22, 2003, the Registrant had granted restricted stock and options to purchase an aggregate of 6,568,750 shares of common stock under its 2000 Equity Incentive Plan, as amended, of which 1,732,875 shares have been exercised or sold at exercise prices ranging from $0.001 to $0.25 per share. These grants and sales were made in reliance upon Rule 701 promulgated under the Securities Act and are deemed to be exempt transactions as sales of an issuer's securities pursuant to a written plan or contract relating to the compensation of such individuals, and upon Section 4(2) of the Securities Act as transactions not involving any public offering.
2. In July and August 2000, the Registrant issued and sold an aggregate of 2,380,000 shares of common stock at a purchase price of $0.001 per share to Douglas J. Ringler, Herman Waldmann, Geoffrey Hale, and Stephen Cobbold. In November 2000, the Registrant issued and sold 400,000 shares of common stock at a purchase price of $0.001 per share to Oxford University. The issuance and sale of such shares of common stock were made in reliance upon Section 4(2) of the Securities Act.
3. In the period between December 2000 and July 2001, the Registrant issued and sold an aggregate of 9,035,000 shares of Series A convertible preferred stock at a purchase price of $0.664 per share to HealthCare Ventures VI, L.P. and Johnson & Johnson Development Corporation. The issuance and sale of such shares of Series A convertible preferred stock were made in reliance upon Rule 506 of Regulation D promulgated under the Securities Act, and Section 4(2) of the Securities Act.
4. In February 2001, the Registrant issued to Silicon Valley Bank a warrant to purchase an aggregate of 65,000 shares of common stock at an exercise price of $0.10 per share. The issuance and sale of such warrant, and the shares of common stock issuable upon exercise thereof, were made in reliance upon Section 4(2) of the Securities Act.
5. In September 2001, the Registrant issued and sold an aggregate of 17,000,000 shares of Series B convertible preferred stock at a purchase price of $1.00 per share to HealthCare Ventures VI, L.P., Johnson & Johnson Development Corporation, Priceworth Investments Limited, Vertex Technology Fund (III) Limited, Vertex Life Science, Inc., Rho Ventures IV, L.P., Rho Ventures IV Gmbh & Co. Beiteligungs KG, Rho Ventures IV (QP) L.P., Rho Management
II-2
Trust I, Skyline Venture Partners II, L.P., Skyline Venture Partners Qualified Purchaser Fund II, L.P., Skyline Venture Partners III, L.P., Skyline Venture Partners Qualified Purchaser Fund III, L.P., and The DC Investment Trust FBO Lee Casty. The issuance and sale of such shares of Series B convertible preferred stock were made in reliance upon Rule 506 of Regulation D promulgated under the Securities Act, and Section 4(2) of the Securities Act.
6. In November 2001, the Registrant issued to the Massachusetts Institute of Technology a warrant to purchase an aggregate of 100,000 shares of common stock at an exercise price of $1.00 per share in connection with a lease facility. The issuance and sale of such warrant, and the shares of common stock issuable upon the exercise thereof, were made in reliance upon Section 4(2) of the Securities Act.
7. In December 2001, the Registrant issued to Silicon Valley Bank a warrant to purchase an aggregate of 73,125 shares of Series B convertible preferred stock at an exercise price of $1.00 per share. The issuance and sale of such warrant, and the shares of Series B convertible preferred stock issuable upon the exercise thereof, were made in reliance upon Section 4(2) of the Securities Act.
8. In December 2001, the Registrant issued to GATX Ventures, Inc. a warrant to purchase an aggregate of 73,125 shares of Series B convertible preferred stock at an exercise price of $1.00 per share. The issuance and sale of such warrant, and the shares of Series B convertible preferred stock issuable upon the exercise thereof, were made in reliance upon Section 4(2) of the Securities Act.
9. In the period between November 2002 and January 2003, the Registrant issued and sold an aggregate of 35,000,000 shares of Series C convertible preferred stock at a purchase price of $1.00 per share to Skyline Venture Partners II, L.P., Skyline Venture Partners Qualified Purchaser Fund II, L.P., Skyline Venture Partners III, L.P., Skyline Venture Partners Qualified Purchaser Fund III, L.P., Skyline Expansion Fund, L.P., Rho Ventures IV, L.P., Rho Ventures IV Gmbh & Co. Beiteligungs KG, Rho Ventures IV (QP) L.P., Rho Management Trust I, Vertex Technology Fund (III) Limited, Vertex Life Science, Inc., The Yasuda Enterprise Development II, L.P., Artal Services N.V., HealthCare Ventures VI, L.P., Lehman Brothers P.A. LLC, Lehman Brothers Partnership Account 2000/2001, L.P., Lehman Brothers Offshore Partnership Account 2000/2001, L.P., Lehman Brothers Healthcare Venture Capital L.P., Sprout Capital IX, L.P., Sprout IX Plan Investors, L.P., Sprout Entrepreneurs Fund, L.P., Genentech, Inc., Mizuho Capital Co., Ltd., Aozora Investment I Venture Capital Limited Partnership, Duke University Special Ventures Fund, Inc., and James Niedel. The issuance and sale of such shares of Series C convertible preferred stock were made in reliance upon Rule 506 of Regulation D promulgated under the Securities Act, and Section 4(2) of the Securities Act.
Item 16. Exhibits and financial statements
(a) Exhibits:
|
|---|
| Exhibit
number
| | Description
|
|---|
|
|---|
| | 1.1* | | Proposed Form of Underwriting Agreement |
| | | | |
II-3
| | 3.1 | | Form of Fourth Restated Certificate of Incorporation of the Registrant, to be effective upon the closing of this offering |
| | 3.2 | | Form of Amended and Restated By-Laws of the Registrant, to be effective upon the closing of this offering |
| | 4.1* | | Specimen certificate for shares of common stock |
| | 5.1* | | Opinion of Bingham McCutchen LLP, counsel to the Registrant |
| | 10.1 | | Amended and Restated 2000 Equity Incentive Plan |
| | 10.2 | | 2003 Employee Stock Purchase Plan |
| | 10.3 | | Consulting Agreement, dated as of October 27, 2000, between the Registrant and Herman Waldmann |
| | 10.4 | | Second Amended and Restated Stockholders Agreement, dated as of November 1, 2002, between the Registrant and the stockholders of the Registrant listed therein, as amended |
| | 10.5 | | Lease Agreement, dated November 20, 2001, between the Registrant and Massachusetts Institute of Technology |
| | 10.6† | | Collaboration Agreement, dated December 23, 2002, by and between Genentech, Inc. and the Registrant |
| | 10.7† | | License Agreement, dated December 8, 2000, between the Registrant and Cambridge University Technical Services Limited, as amended by that certain Agreement, dated July 23, 2001, and as further amended by that certain Amendment Agreement, dated as of December 31, 2001, and that certain Amendment Agreement, dated December 19, 2002 |
| | 10.8† | | CD2 Antibody License Agreement, dated September 25, 2001, between the Registrant and Cambridge University Technical Services Limited, as amended by that certain Amendment Agreement, dated December 31, 2001 |
| | 10.9† | | License Agreement, dated December 5, 2000, among Isis Innovation Limited, the Registrant and the Chancellor Masters and Scholars of the University of Oxford, as amended by that certain Amendment Agreement, dated December 19, 2002 |
| | 10.10† | | License Agreement, dated February 1, 2001, between the Registrant and Isis Innovation Limited, as amended by that certain Amendment Agreement, dated December 19, 2002 |
| | 10.11† | | License Agreement, dated September 28, 2001, between the Registrant and BTG International Limited |
| | 10.12 | | Registration Rights Agreement, dated as of February 23, 2001, by and between Silicon Valley and the Registrant |
| | 10.13 | | Registration Rights Agreement, dated as of December 13, 2001, by and among Silicon Valley Bank, GATX Ventures, Inc., and the Registrant |
| | 10.14 | | Form of Indemnification Agreement entered into with each of our directors |
| | 10.15 | | Form of Stock Restriction Agreement entered into with certain of our officers and directors |
| | 10.16 | | Warrant to purchase 100,000 shares of common stock, $0.001 par value per share, dated November 20, 2001, issued to Massachusetts Institute of Technology |
| | 10.17 | | Warrant to purchase 65,000 shares of common stock, $0.001 par value per share, dated February 23, 2001, issued to Silicon Valley Bank |
| | 10.18 | | Warrant to purchase 73,125 shares of Series B Preferred Stock, dated December 13, 2001, issued to Silicon Valley Bank |
| | | | |
II-4
| | 10.19 | | Warrant to purchase 73,125 shares of Series B Preferred Stock, dated December 13, 2001, issued to GATX Ventures, Inc. |
| | 21.1 | | List of Subsidiaries |
| | 23.1* | | Consent of Bingham McCutchen LLP (included in Exhibit 5) |
| | 23.2 | | Consent of PricewaterhouseCoopers LLP |
| | 24.1 | | Power of Attorney (included in signature pages to Registration Statement) |
* To be filed by amendment.
† Confidential Treatment requested as to certain portions.
(b) Financial statement schedules:
All financial statement schedules have been omitted because either they are not required, are not applicable, or the information is otherwise set forth in the Financial Statements and notes thereto.
Item 17. Undertakings
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers, and controlling persons of the Registrant pursuant to the foregoing provisions described in Item 14 above, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer, or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer, or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes:
(1) To provide to the Underwriters at the closing specified in the Underwriting Agreement, certificates in such denominations and registered in such names as required by the Underwriters to permit prompt delivery to each purchaser.
(2) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4), or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(3) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-5
Signatures
Pursuant to the requirements of the Securities Act of 1933, the Registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Cambridge, Commonwealth of Massachusetts, on this 26th day of August, 2003.
| | TOLERRX, INC. |
|
By: |
|
/s/ DOUGLAS J. RINGLER
Douglas J. Ringler, V.M.D.
President, Chief Executive Officer, and Director |
Power of Attorney
Pursuant to the requirements of the Securities Act, this Registration Statement has been signed by the following persons in the capacities indicated on the 26th day of August, 2003. Each person whose signature appears below hereby constitutes and appoints Douglas J. Ringler, V.M.D. and Douglas E. Onsi, or either of them, as such person's true and lawful attorney-in-fact and agent with full power and substitution for such person and in such person's name, place and stead, in any and all capacities, to sign and to file with the Securities and Exchange Commission, any and all amendments and post-effective amendments to this Registration Statement, with exhibits thereto and other documents in connection therewith, including any registration statements or amendments thereto filed pursuant to Rule 462(b) under the Securities Act, granting unto said attorney-in-fact and agent full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as such person might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent or any substitute therefor, may lawfully do or cause to be done by virtue thereof.
Signature
| | Title
|
|---|
|
|
|
/s/ DOUGLAS J. RINGLER
Douglas J. Ringler, V.M.D. | | President, Chief Executive Officer, and Director |
/s/ DOUGLAS E. ONSI
Douglas E. Onsi |
|
Chief Financial Officer |
/s/ CHRISTOPHER K. MIRABELLI
Christopher K. Mirabelli, Ph.D. |
|
Chairman of the Board of Directors |
/s/ WAYNE T. HOCKMEYER
Wayne T. Hockmeyer, Ph.D. |
|
Director |
| | | |
II-6
/s/ YASUNORI KANEKO
Yasunori Kaneko, M.D. |
|
Director |
/s/ DANIEL L. KISNER
Daniel L. Kisner, M.D. |
|
Director |
/s/ DAVID J. MCLACHLAN
David J. McLachlan |
|
Director |
/s/ JAMES E. NIEDEL
James E. Niedel, M.D., Ph.D. |
|
Director |
/s/ HERMAN WALDMANN
Herman Waldmann, Ph.D. |
|
Director |
/s/ HAROLD R. WERNER
Harold R. Werner |
|
Director |
II-7
Exhibit index
|
|---|
| Exhibit
number
| | Description
|
|---|
|
|---|
| | 1.1* | | Proposed Form of Underwriting Agreement |
| | 3.1 | | Form of Fourth Restated Certificate of Incorporation of the Registrant, to be effective upon the closing of this offering |
| | 3.2 | | Form of Amended and Restated By-Laws of the Registrant, to be effective upon the closing of this offering |
| | 4.1* | | Specimen certificate for shares of common stock |
| | 5.1* | | Opinion of Bingham McCutchen LLP, counsel to the Registrant |
| | 10.1 | | Amended and Restated 2000 Equity Incentive Plan |
| | 10.2 | | 2003 Employee Stock Purchase Plan |
| | 10.3 | | Consulting Agreement, dated as of October 27, 2000, between the Registrant and Herman Waldmann |
| | 10.4 | | Second Amended and Restated Stockholders Agreement, dated as of November 1, 2002, between the Registrant and the stockholders of the Registrant listed therein, as amended |
| | 10.5 | | Lease Agreement, dated November 20, 2001, between the Registrant and Massachusetts Institute of Technology |
| | 10.6† | | Collaboration Agreement, dated December 23, 2002, by and between Genentech, Inc. and the Registrant |
| | 10.7† | | License Agreement, dated December 8, 2000, between the Registrant and Cambridge University Technical Services Limited, as amended by that certain Agreement, dated July 23, 2001, and as further amended by that certain Amendment Agreement, dated as of December 31, 2001, and that certain Amendment Agreement, dated December 19, 2002 |
| | 10.8† | | CD2 Antibody License Agreement, dated September 25, 2001, between the Registrant and Cambridge University Technical Services Limited, as amended by that certain Amendment Agreement, dated December 31, 2001 |
| | 10.9† | | License Agreement, dated December 5, 2000, among Isis Innovation Limited, the Registrant and the Chancellor Masters and Scholars of the University of Oxford, as amended by that certain Amendment Agreement, dated December 19, 2002 |
| | 10.10† | | License Agreement, dated February 1, 2001, between the Registrant and Isis Innovation Limited, as amended by that certain Amendment Agreement, dated December 19, 2002 |
| | 10.11† | | License Agreement, dated September 28, 2001, between the Registrant and BTG International Limited |
| | 10.12 | | Registration Rights Agreement, dated as of February 23, 2001, by and between Silicon Valley and the Registrant |
| | 10.13 | | Registration Rights Agreement, dated as of December 13, 2001, by and among Silicon Valley Bank, GATX Ventures, Inc., and the Registrant |
| | 10.14 | | Form of Indemnification Agreement entered into with each of our directors |
| | 10.15 | | Form of Stock Restriction Agreement entered into with certain of our officers and directors |
| | | | |
II-8
| | 10.16 | | Warrant to purchase 100,000 shares of common stock, $0.001 par value per share, dated November 20, 2001, issued to Massachusetts Institute of Technology |
| | 10.17 | | Warrant to purchase 65,000 shares of common stock, $0.001 par value per share, dated February 23, 2001, issued to Silicon Valley Bank |
| | 10.18 | | Warrant to purchase 73,125 shares of Series B Preferred Stock, dated December 13, 2001, issued to Silicon Valley Bank |
| | 10.19 | | Warrant to purchase 73,125 shares of Series B Preferred Stock, dated December 13, 2001, issued to GATX Ventures, Inc. |
| | 21.1 | | List of Subsidiaries |
| | 23.1* | | Consent of Bingham McCutchen LLP (included in Exhibit 5) |
| | 23.2 | | Consent of PricewaterhouseCoopers LLP |
| | 24.1 | | Power of Attorney (included in signature pages to Registration Statement) |
* To be filed by amendment.
† Confidential Treatment requested as to certain portions.
II-9
QuickLinks
Table of contentsProspectus summaryThe offeringSummary consolidated financial informationRisk factorsSpecial note regarding forward-looking statementsUse of proceedsDividend policyCapitalizationDilutionSelected consolidated financial dataManagement's discussion and analysis of financial condition and results of operationsBusinessManagementRelated party transactionsPrincipal stockholdersDescription of capital stockShares eligible for future saleCertain U.S. federal income tax considerations for Non-U.S. Holders of our common stockUnderwritingLegal mattersExpertsWhere you can find additional informationIndex to consolidated financial statementsREPORT OF INDEPENDENT AUDITORSTolerRx, Inc. Consolidated balance sheetsTolerRx, Inc. Consolidated statements of operationsTolerRx, Inc. Consolidated statements of cash flowsTolerRx, Inc. Notes to consolidated financial statementsInformation as of June 30, 2003 and for the six months ended June 30, 2002 and 2003 is unaudited.Part II Information not required in prospectusSignaturesPower of AttorneyExhibit index

