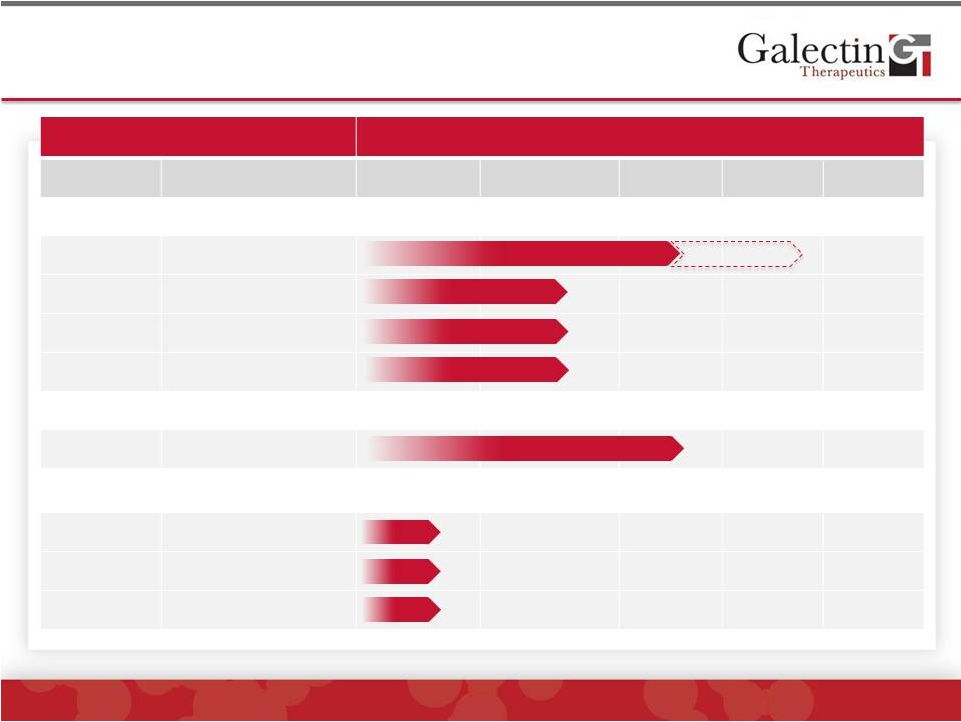
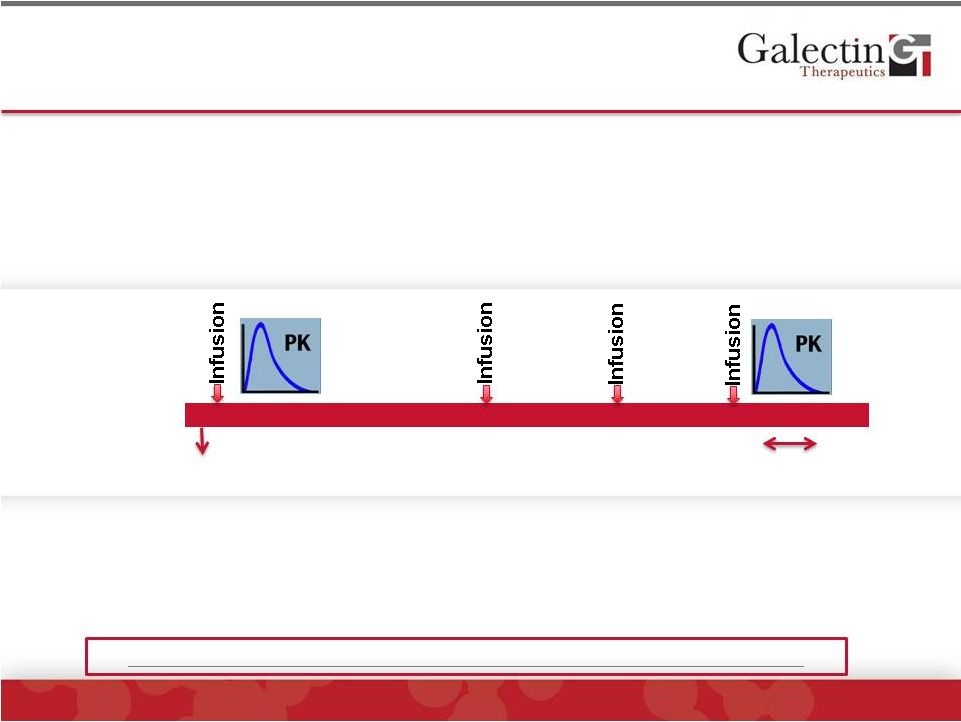
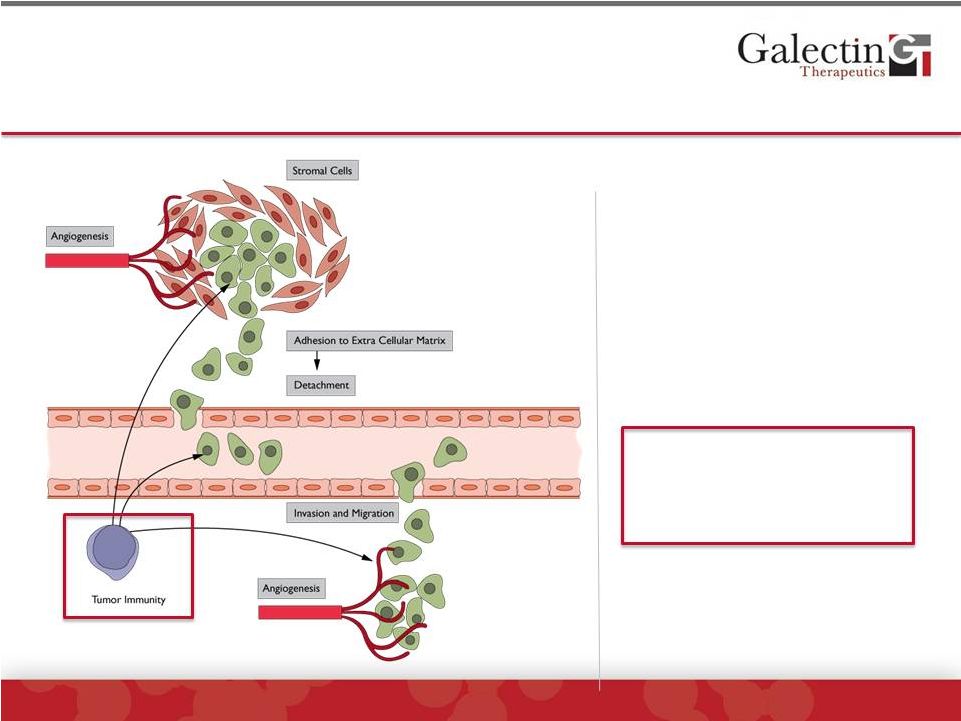
Forward-Looking Statement Disclaimer 2 © 2014 Galectin Therapeutics | NASDAQ:GALT This presentation contains, in addition to historical information, forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events or future financial performance, and use words such as “may,” “estimate,” “could,” “expect” and others. They are based on our current expectations and are subject to factors and uncertainties which could cause actual results to differ materially from those described in the statements. These statements include those regarding potential therapeutic benefits of our drugs, expectations, plans and timelines related to our clinical trials, potential partnering opportunities and estimated spending for 2014. Factors that could cause our actual performance to differ materially from those discussed in the forward-looking statements include, among others, our trials may not lead to positive outcomes or regulatory approval. We may experience delays in our trials, which could include enrollment delays. Future phases or future clinical studies may not begin or produce positive results in a timely fashion, if at all, and could prove time consuming and costly. Plans regarding development, approval and marketing of any of our drugs are subject to change at any time based on the changing needs of our company as determined by management and regulatory agencies. Strategies and spending projections may change. We may be unsuccessful in developing partnerships with other companies or obtaining capital that would allow us to further develop and/or fund any studies or trials. We are currently the subject of litigation, which may impact our human and capital resources. To date, we have incurred operating losses since our inception, and our future success may be impacted by our ability to manage costs and finance our continuing operations. For a discussion of additional factors impacting our business, see our Annual Report on Form 10-K for the year ended December 31, 2013, and our subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause our views to change, we disclaim any obligation to update forward-looking statements. |