
GR-MD-02 for Indication of NASH Cirrhosis: NASH-CX Clinical Trial Results Supplemental Information to Corporate Presentation February 6, 2018 NASDAQ: GALT www.galectintherapeutics.com 2017 Galectin Therapeutics | NASDAQ: GALT For more information, see galectintherapeutics.com Exhibit 99.2

Forward Looking Statements This presentation contains, in addition to historical information, forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events or future financial performance, and use words such as “may,” “estimate,” “could,” “expect” and others. They are based on our current expectations and are subject to factors and uncertainties which could cause actual results to differ materially from those described in the statements. These statements include those regarding potential therapeutic benefits of our drugs, expectations, plans and timelines related to our clinical trials, potential partnering opportunities and estimated spending for 2018 and beyond. Factors that could cause our actual performance to differ materially from those discussed in the forward-looking statements include, among others, our trials may not lead to positive outcomes or regulatory approval. We may experience delays in our trials, which could include enrollment delays. Future phases or future clinical studies may not begin or produce positive results in a timely fashion, if at all, and could prove time consuming and costly. Plans regarding development, approval and marketing of any of our drugs are subject to change at any time based on the changing needs of our company as determined by management and regulatory agencies. Strategies and spending projections may change. We may be unsuccessful in developing partnerships with other companies or obtaining capital that would allow us to complete our clinical trials or further develop and/or fund any future studies or trials. To date, we have incurred operating losses since our inception, and our future success may be impacted by our ability to manage costs and finance our continuing operations. For a discussion of additional factors impacting our business, see our Annual Report on Form 10-K for the year ended December 31, 2016, and our subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause our views to change, we disclaim any obligation to update forward-looking statements.
Developing Treatments Where Galectin-3 Protein is Implicated in Disease Clinical Phase Studies With Galectin-3 Inhibitor GR-MD-02 Primary Program is in NASH Cirrhosis (topic of this presentation) Combination Cancer Immunotherapy Investigator initiated phase 1b clinical trial of GR-MD-02 in combination with KEYTRUDA in advanced melanoma and other malignancies Encouraging early data with 5 of 8 responders (2 CR and 3 PR) in advanced melanoma Psoriasis and Atopic Dermatitis Small open label studies show clinically significant effect, demonstrating activity of drug in human disease
NASH Cirrhosis Development Program: Summary Gal-3 null mice are resistant to development of NASH 1 and liver fibrosis 1, 2 GR-MD-02 is a glycopolymer (polysaccharide), considered a Nonbiological Complex Drug (NBCD) that binds to galectin-3 protein, has strong patent protection, and is administered intravenously GR-MD-02 has robust efficacy in pre-clinical models of NASH and toxic cirrhosis, with action at multiple pathophysiological processes 3, 4 Well tolerated and safe in preclinical toxicology and clinical trials (2 P1, P2a and P2b) NASH-CX phase 2b clinical trial showed clinically meaningful positive results of GR-MD-02 in patients with NASH cirrhosis without esophageal varices NASH-CX trial identified endpoints and patient population that can form the basis of phase 3 trials in NASH cirrhosis without esophageal varices 1 Journal of Hepatology 2011;54:975-983 2 PNAS 2006;103:5060-5065 3 Traber PG and Zomer E.PLOS ONE 2013;8:e83481 4 Traber PG, Chou H, Zomer E, Hong F, Klyosov A Fiel M-I, Friedman, SL. PLOS ONE 2013;8:e75361.
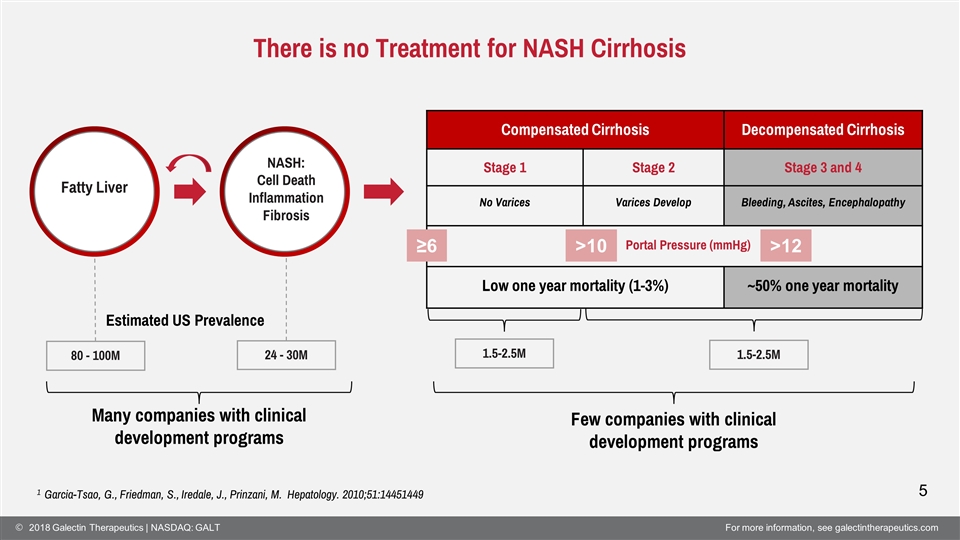
80 - 100M 24 - 30M 1.5-2.5M 1.5-2.5M Estimated US Prevalence There is no Treatment for NASH Cirrhosis Fatty Liver NASH: Cell Death Inflammation Fibrosis 5 1 Garcia-Tsao, G., Friedman, S., Iredale, J., Prinzani, M. Hepatology. 2010;51:14451449 Compensated Cirrhosis Decompensated Cirrhosis Stage 1 Stage 2 Stage 3 and 4 No Varices Varices Develop Bleeding, Ascites, Encephalopathy Low one year mortality (1-3%) ~50% one year mortality ≥6 >10 >12 Many companies with clinical development programs Few companies with clinical development programs Portal Pressure (mmHg)
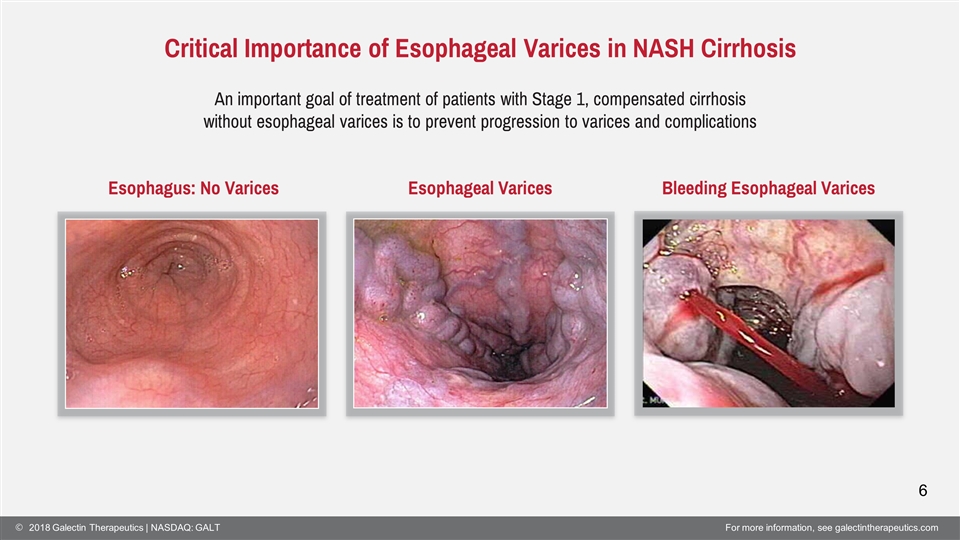
Critical Importance of Esophageal Varices in NASH Cirrhosis Esophagus: No Varices Esophageal Varices Bleeding Esophageal Varices An important goal of treatment of patients with Stage 1, compensated cirrhosis without esophageal varices is to prevent progression to varices and complications 6
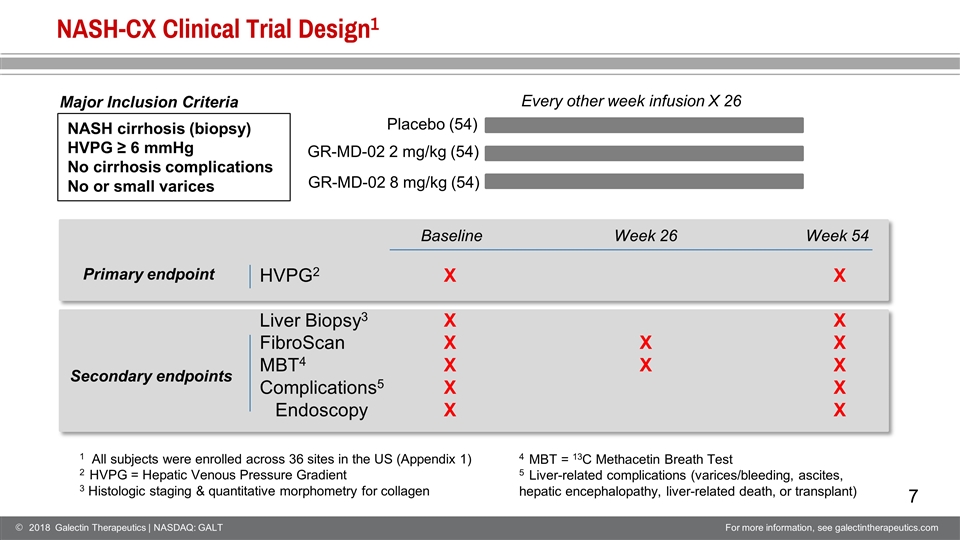
NASH-CX Clinical Trial Design1 Every other week infusion X 26 Placebo (54) GR-MD-02 2 mg/kg (54) GR-MD-02 8 mg/kg (54) NASH cirrhosis (biopsy) HVPG ≥ 6 mmHg No cirrhosis complications No or small varices HVPG2 Liver Biopsy3 FibroScan MBT4 Complications5 Endoscopy X X X X X X X X X X X X X X 1 All subjects were enrolled across 36 sites in the US (Appendix 1) 2 HVPG = Hepatic Venous Pressure Gradient 3 Histologic staging & quantitative morphometry for collagen Primary endpoint Secondary endpoints Baseline Week 26 Week 54 4 MBT = 13C Methacetin Breath Test 5 Liver-related complications (varices/bleeding, ascites, hepatic encephalopathy, liver-related death, or transplant) Major Inclusion Criteria
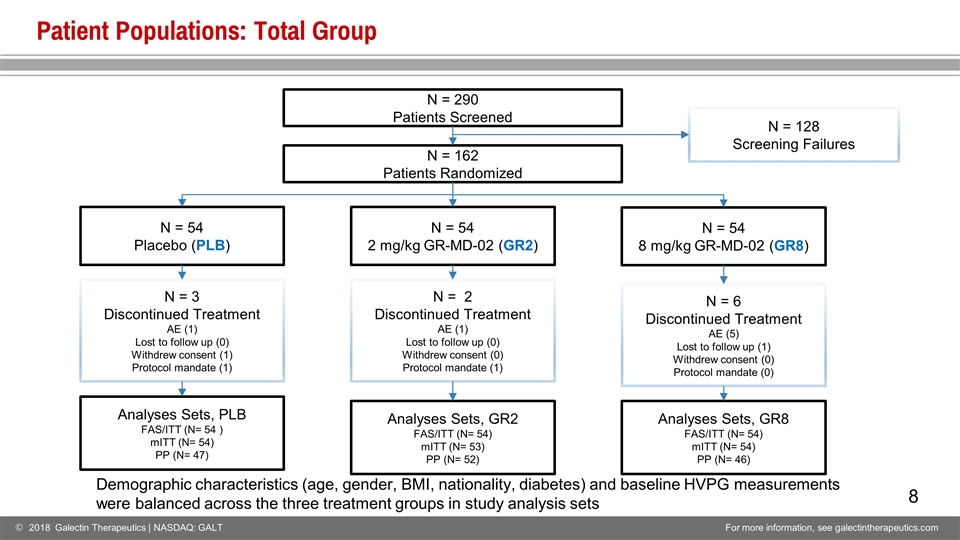
Patient Populations: Total Group N = 290 Patients Screened N = 162 Patients Randomized N = 128 Screening Failures N = 54 Placebo (PLB) N = 54 2 mg/kg GR-MD-02 (GR2) N = 54 8 mg/kg GR-MD-02 (GR8) N = 3 Discontinued Treatment AE (1) Lost to follow up (0) Withdrew consent (1) Protocol mandate (1) N = 2 Discontinued Treatment AE (1) Lost to follow up (0) Withdrew consent (0) Protocol mandate (1) N = 6 Discontinued Treatment AE (5) Lost to follow up (1) Withdrew consent (0) Protocol mandate (0) Analyses Sets, PLB FAS/ITT (N= 54 ) mITT (N= 54) PP (N= 47) Analyses Sets, GR8 FAS/ITT (N= 54) mITT (N= 54) PP (N= 46) Analyses Sets, GR2 FAS/ITT (N= 54) mITT (N= 53) PP (N= 52) Demographic characteristics (age, gender, BMI, nationality, diabetes) and baseline HVPG measurements were balanced across the three treatment groups in study analysis sets
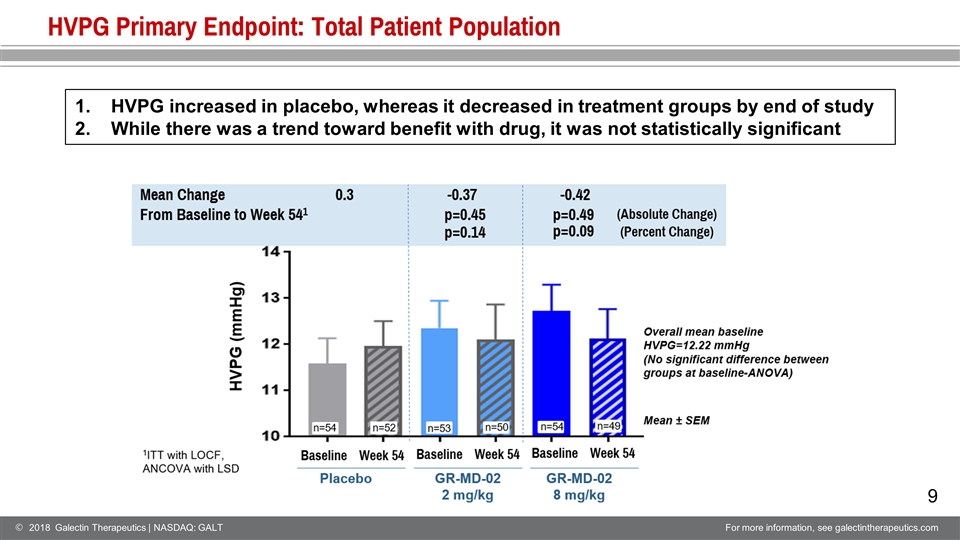
HVPG Primary Endpoint: Total Patient Population HVPG increased in placebo, whereas it decreased in treatment groups by end of study While there was a trend toward benefit with drug, it was not statistically significant

HVPG Primary Endpoint: Mild Portal Hypertension in Total Population Group Evaluation of mild portal hypertension was a pre-determined statistical analysis There was a statistically significant effect of both doses of GR-MD-02 There was no effect on those with high portal pressure (data not shown)
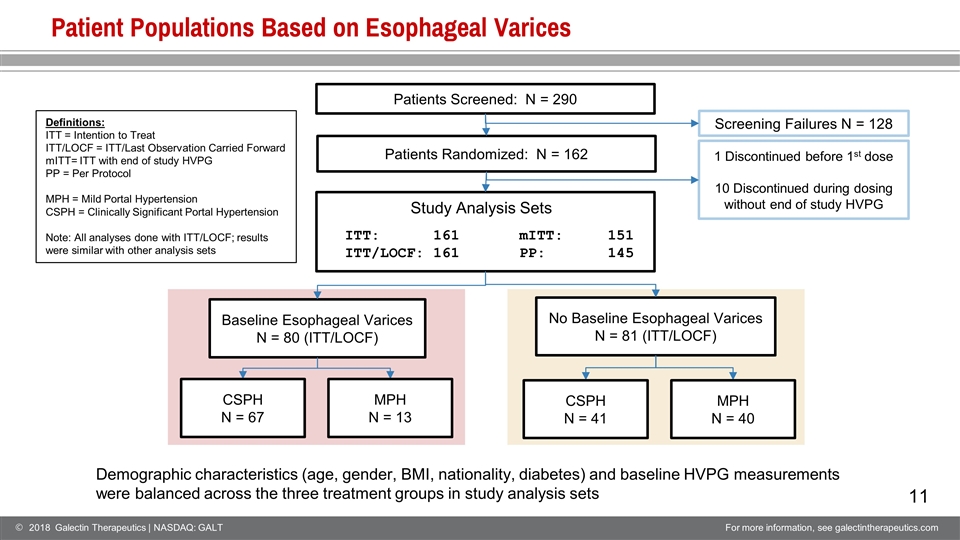
Patient Populations Based on Esophageal Varices Patients Screened: N = 290 Screening Failures N = 128 Baseline Esophageal Varices N = 80 (ITT/LOCF) No Baseline Esophageal Varices N = 81 (ITT/LOCF) 1 Discontinued before 1st dose 10 Discontinued during dosing without end of study HVPG CSPH N = 67 MPH N = 13 CSPH N = 41 MPH N = 40 Patients Randomized: N = 162 Demographic characteristics (age, gender, BMI, nationality, diabetes) and baseline HVPG measurements were balanced across the three treatment groups in study analysis sets ITT: 161 ITT/LOCF: 161 mITT: 151 PP: 145 Definitions: ITT = Intention to Treat ITT/LOCF = ITT/Last Observation Carried Forward mITT= ITT with end of study HVPG PP = Per Protocol MPH = Mild Portal Hypertension CSPH = Clinically Significant Portal Hypertension Note: All analyses done with ITT/LOCF; results were similar with other analysis sets Study Analysis Sets
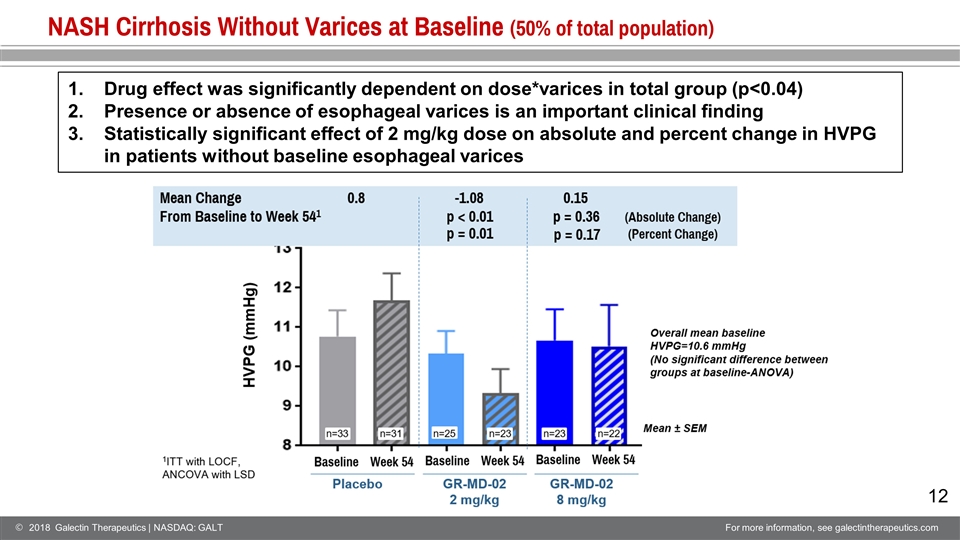
NASH Cirrhosis Without Varices at Baseline (50% of total population) Drug effect was significantly dependent on dose*varices in total group (p<0.04) Presence or absence of esophageal varices is an important clinical finding Statistically significant effect of 2 mg/kg dose on absolute and percent change in HVPG in patients without baseline esophageal varices
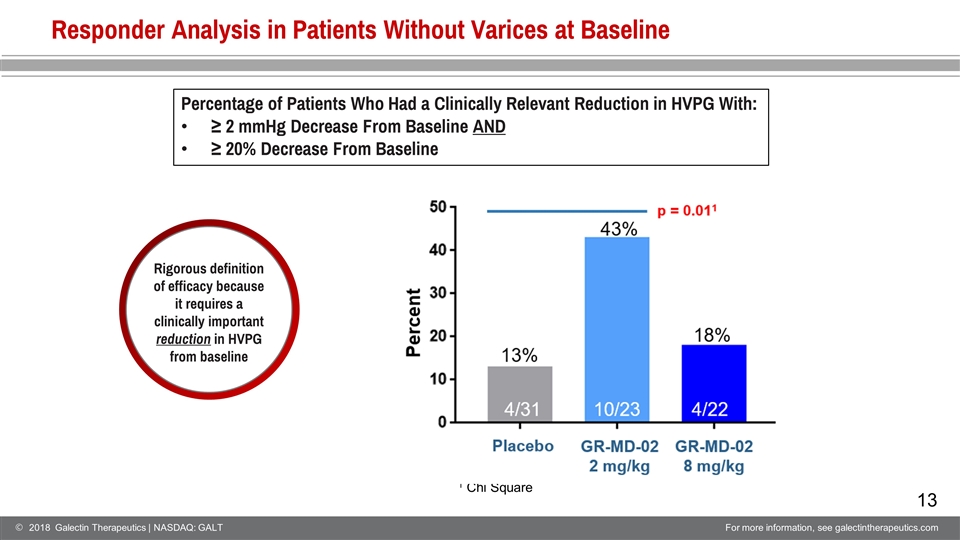
Responder Analysis in Patients Without Varices at Baseline 1 Chi Square Percentage of Patients Who Had a Clinically Relevant Reduction in HVPG With: ≥ 2 mmHg Decrease From Baseline AND ≥ 20% Decrease From Baseline Rigorous definition of efficacy because it requires a clinically important reduction in HVPG from baseline
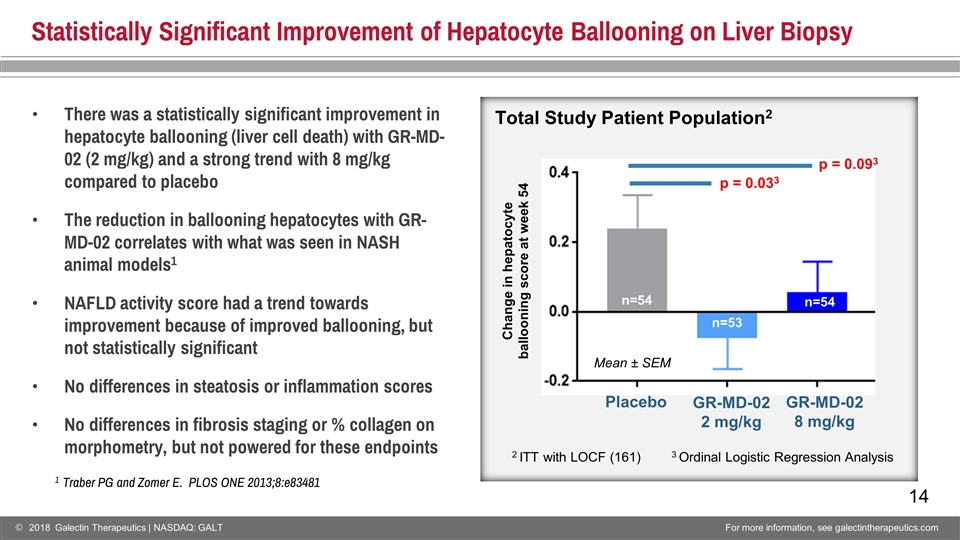
Statistically Significant Improvement of Hepatocyte Ballooning on Liver Biopsy Change in hepatocyte ballooning score at week 54 Mean ± SEM n=54 n=54 n=53 Total Study Patient Population2 2 ITT with LOCF (161) 1 Traber PG and Zomer E. PLOS ONE 2013;8:e83481 3 Ordinal Logistic Regression Analysis p = 0.033 p = 0.093 Placebo GR-MD-02 2 mg/kg GR-MD-02 8 mg/kg There was a statistically significant improvement in hepatocyte ballooning (liver cell death) with GR-MD-02 (2 mg/kg) and a strong trend with 8 mg/kg compared to placebo The reduction in ballooning hepatocytes with GR-MD-02 correlates with what was seen in NASH animal models1 NAFLD activity score had a trend towards improvement because of improved ballooning, but not statistically significant No differences in steatosis or inflammation scores No differences in fibrosis staging or % collagen on morphometry, but not powered for these endpoints
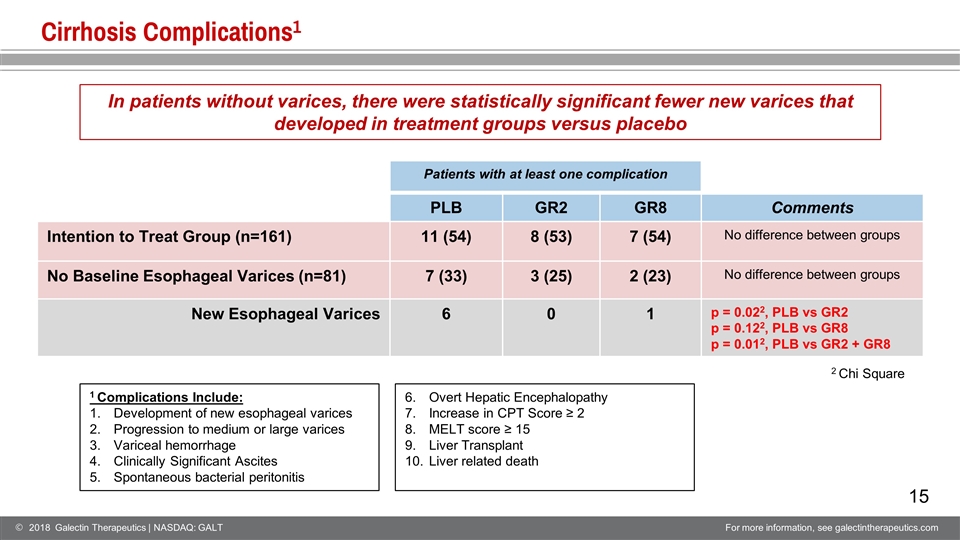
Cirrhosis Complications1 Patients with at least one complication PLB GR2 GR8 Comments Intention to Treat Group (n=161) 11 (54) 8 (53) 7 (54) No difference between groups No Baseline Esophageal Varices (n=81) 7 (33) 3 (25) 2 (23) No difference between groups New Esophageal Varices 6 0 1 p = 0.022, PLB vs GR2 p = 0.122, PLB vs GR8 p = 0.012, PLB vs GR2 + GR8 2 Chi Square In patients without varices, there were statistically significant fewer new varices that developed in treatment groups versus placebo 1 Complications Include: Development of new esophageal varices Progression to medium or large varices Variceal hemorrhage Clinically Significant Ascites Spontaneous bacterial peritonitis Overt Hepatic Encephalopathy Increase in CPT Score ≥ 2 MELT score ≥ 15 Liver Transplant Liver related death
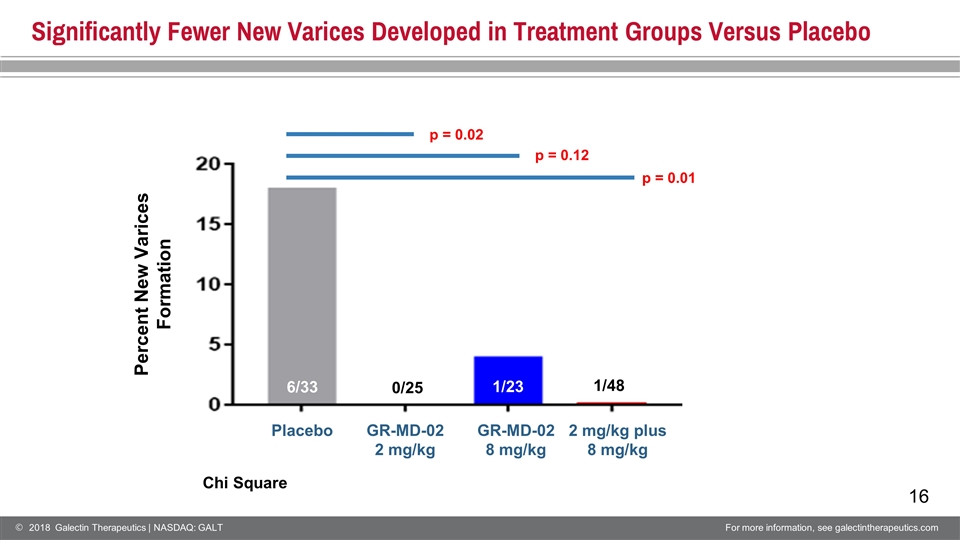
Significantly Fewer New Varices Developed in Treatment Groups Versus Placebo Placebo GR-MD-02 2 mg/kg GR-MD-02 8 mg/kg 2 mg/kg plus 8 mg/kg 6/33 1/23 0/25 1/48 Percent New Varices Formation p = 0.02 p = 0.12 p = 0.01 Chi Square
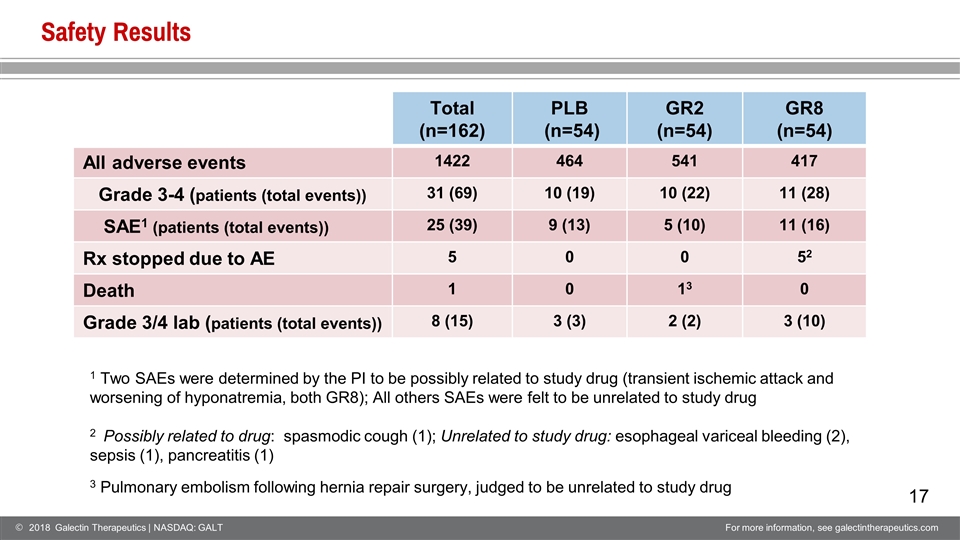
Safety Results Total (n=162) PLB (n=54) GR2 (n=54) GR8 (n=54) All adverse events 1422 464 541 417 Grade 3-4 (patients (total events)) 31 (69) 10 (19) 10 (22) 11 (28) SAE1 (patients (total events)) 25 (39) 9 (13) 5 (10) 11 (16) Rx stopped due to AE 5 0 0 52 Death 1 0 13 0 Grade 3/4 lab (patients (total events)) 8 (15) 3 (3) 2 (2) 3 (10) 1 Two SAEs were determined by the PI to be possibly related to study drug (transient ischemic attack and worsening of hyponatremia, both GR8); All others SAEs were felt to be unrelated to study drug 2 Possibly related to drug: spasmodic cough (1); Unrelated to study drug: esophageal variceal bleeding (2), sepsis (1), pancreatitis (1) 3 Pulmonary embolism following hernia repair surgery, judged to be unrelated to study drug

GR-MD-02 Was Safe and Well Tolerated No differences between treatment groups in the number of patients with treatment emergent adverse events (AEs), grade 3/4 AEs, serious adverse events (SAE), or grade 3/4 laboratory abnormalities All but 2 SAEs were unrelated to study drug; 2 patients in 8 mg/kg group had SAEs that were possibly related to study drug There was one death due to complications of a surgical procedure that was unrelated to study drug There was a low patient dropout rate of 6% which suggests the drug was well tolerated. Only one patient was removed from study for an AE possibly related to study drug
Major Conclusions from NASH-CX Clinical Trial Results GR-MD-02 had a statistically significant and clinically meaningful effect in improving HVPG versus placebo in patients with NASH cirrhosis who did not have baseline esophageal varices Important drug effect in the total study population on liver biopsy, with a statistically significant improvement in hepatocyte ballooning (cell death) Statistically significant reduction in the development of new esophageal varices in drug-treated patients compared to placebo While there was a drug effect in both dosage groups on liver biopsy and in the mild portal hypertension group, there was a consistently greater and statistically significant effect of the 2 mg/kg dose GR-MD-02 appears to be safe and well tolerated in this one year, phase 2b clinical trial We believe this is the first large, randomized clinical trial of any drug to demonstrate a clinically meaningful improvement in portal hypertension or liver biopsy in patients with NASH cirrhosis without varices
Summary Conclusions and Next Steps NASH-CX is the first clinical trial to show positive results in compensated NASH cirrhosis without esophageal varices Clinically meaningful effect in reducing portal pressure Improvement in liver cell death, a key component of NASH Reduction in the development of new esophageal varices Drug was safe and well-tolerated These results will propel development program to next stage Ongoing data analysis (pharmacokinetics of drug levels, serum biomarkers) and preparation of clinical study report Phase 3 clinical trial being designed to seek approval from FDA We believe program will be eligible for FDA “Breakthrough” designation; will be submitted when clinical study report completed Ongoing discussions with Pharma for potential partnerships

Appendix NASDAQ: GALT www.galectintherapeutics.com 2017 Galectin Therapeutics | NASDAQ: GALT For more information, see galectintherapeutics.com

22 Appendix 1: Deep Gratitude to Patient Volunteers and Clinical Study Sites





















