Exhibit 99.1

Corporate Overview February 2017 A Translational Medicine Company NASDAQ: CAPR

2 This presentation contains forward - looking statements and information that are based on the beliefs of the management of Capricor Therapeutics, Inc . (Capricor) as well as assumptions made by and information currently available to Capricor . All statements other than statements of historical fact included in this presentation are forward - looking statements, including but not limited to statements identified by the words “anticipates,” “believes,” “estimates,” and “expects” and similar expressions . Such forward - looking statements also include any expectation of or dates for commencement of clinical trials, IND filings, similar plans or projections and other matters that do not relate strictly to historical facts . These statements reflect Capricor’s current views with respect to future events, based on what we believe are reasonable assumptions ; however, the statements are subject to a number of risks, uncertainties and assumptions . There are a number of important factors that could cause actual results or events to differ materially from those indicated by such forward - looking statements . More information about these and other risks that may impact Capricor's business are set forth in Capricor's Annual Report on Form 10 - K for the year ended December 31 , 2015 , as filed with the Securities and Exchange Commission on March 30 , 2016 , in its Registration Statement on Form S - 3 , as filed with the Securities and Exchange Commission on September 28 , 2015 and in its Quarterly Report on Form 10 - Q for the period ended September 30 , 2016 as filed with the Securities and Exchange Commission on November 14 , 2016 . Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those in the forward - looking statements . Further, Capricor’s management does not intend to update these forward - looking statements and information after the date of this presentation . Forward - Looking Statements

3 Transformative Therapies from Bench to Bedside Capricor’s mission is to serve patients through products that promote the body’s own repair systems to address cardiac and other medical conditions

4 Investment Highlights Innovative, Proprietary Therapeutic Platforms Capital Efficiency Strong Scientific Foundation & Leadership Team • First - in - class biologics with potential to reverse “irreversible ” damage to the heart and other organs • Product candidates based on cells and exosomes • Developing commercial manufacturing process • Janssen collaboration and license option • Translational approach to product development based on the research of leading academic scientists and institutions • Management has deep domain expertise • Successful record of securing non - dilutive capital Technical and Strategic Execution Potentially Transformative Newsflow in 2017 • Multiple clinical readouts expected • D ecision on Janssen license option expected

5 Capricor’s Innovative Product Pipeline Product Candidate Indication/ Population Development Phase Status Preclinical I II III CAP - 1002 ( allogeneic CDCs) Duchenne Muscular Dystrophy ▪ To report to p - line six - month results from Phase I/II HOPE clinical trial in early 2Q 2017 Post - Myocardial Infarction ▪ To report top - line 12 - month results from Phase II ALLSTAR clinical trial in 4Q 2017 Advanced Heart Failure ▪ Presented positive 12 - month DYNAMIC results at TCT CAP - 2003 (CDC e xosomes) Ocular GVHD ▪ Plan to submit IND in 2H 2017

6 Estimated Global Patient Populations for CAP - 1002 Population US 15 K Europe 20 K WW incidence ~ 1 per 3,600 male births Population US 6 M Europe 15 M Japan 1 M Duchenne Muscular Dystrophy Heart Failure CAP - 1002 has FDA orphan drug designation for the treatment of DMD.

7 The Power of Capricor’s Technology Scar formation results from heart muscle death, and the amount of scar correlates with outcomes. Neither time nor current treatment options will lead to the replacement of scar tissue with muscle. Capricor’s technology offers transformative treatment potential through therapeutic regeneration. Supported by results from clinical trials in heart attack and advanced heart failure populations. cardiosphere - derived cells (CDCs) c ardiac muscle damage, e.g. due to heart attack

8 Clinical Validation Provided by the CADUCEUS Trial – Randomized trial in patients who had experienced a large heart attack (N=25; two centers) – One - time intracoronary delivery of autologous CDCs vs. usual care controls First in - human evidence for therapeutic heart regeneration Lancet , 2012, 21(6): 1121 - 1135 . CADUCEUS was sponsored by Cedars - Sinai Medical Center, with Johns Hopkins University significant decrease in scar mass Treatment with CDCs significant increase in healthy muscle
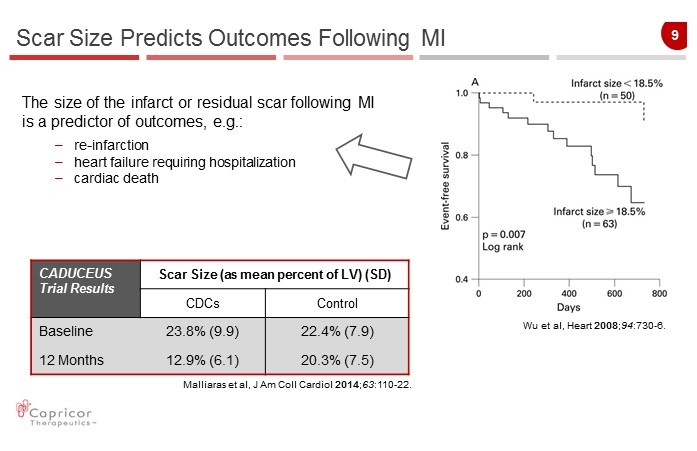
9 Scar Size Predicts Outcomes Following MI CADUCEUS Trial Results Scar Size (as mean p ercent of LV) (SD) CDCs Control Baseline 23.8 % (9.9) 22.4% (7.9) 12 Months 12.9% (6.1) 20.3% (7.5) The size of the infarct or residual scar following MI is a predictor of outcomes, e.g.: – re - infarction – heart failure requiring hospitalization – cardiac death Wu et al, Heart 2008 ; 94 :730 - 6 . Malliaras et al, J Am Coll Cardiol 2014 ; 63 :110 - 22.

10 Cell Therapy Programs for Adult Heart Disease

11 Phase I/II ALLSTAR trial in post - myocardial infarction Phase I DYNAMIC trial in advanced heart failure ALLSTAR & DYNAMIC Trials with “Off - the - Shelf” CAP - 1002 Clinical trials of CAP - 1002 in prevalent cardiovascular disorders: – Well - tolerated in completed studies; no immune - related safety observations – Positive and concordant efficacy results reported, with support for dose - response Phase I portion – completed & results reported Phase II portion – enrollment completed Completed & results reported
12 Enrollment in Phase II ALLSTAR Trial has Completed – Similar to CADUCEUS trial, but using CAP - 1002 (allogeneic) – Randomized, double - blind , placebo - controlled Phase II trial of CAP - 1002 134 patients who had experienced a large heart attack were enrolled Each received a one - time infusion of CAP - 1002 (25M cells) or placebo Trial being conducted at 30 centers in U.S. & Canada Expect to report top - line 12 - month data in 4Q 2017 p rimary efficacy analysis based on scar size at 12 - month follow - up – Phase I results support safety, with evidence of scar size reduction Supported in part by loan award from CIRM

13 0 20 40 60 80 100 Baseline 6 Months 12 Months % of Subjects (N=12) NYHA Class by Study Visit I II III IV Positive Results with CAP - 1002 in Advanced Heart Failure – DYNAMIC demonstrated an efficacy signal despite its small sample size ( N=14). – Concordant improvements from baseline in functional status and ventricular function were observed, and were especially evident at six months, – A dose effect was also seen, with the highest dose (75M cells) yielding the greatest benefit to NYHA Class . DYNAMIC was an open - label , dose - escalation clinical trial in patients with dilated cardiomyopathy. NYHA Class III or ambulatory Class IV HF of ischemic or non - ischemic origin and baseline LV ejection fraction ≤ 35%. One - time triple coronary infusion at one of four doses (37.5 , 50, 62.5, or 75 million cells); six and 12 - month follow - up. -50 -25 0 25 50 LVEF LVFS LVEDV LVESV Dynamics & Dimensions Month 6 Month 12 Median (IQR) % Change p=0.02 p=0.02 p=0.08 p=0.11 p=0.15 p=0.28 p=0.09 p=0.27 p=0.01 p=0.58 1 2 3 4 Baseline Month 3 Month 6 Month 12 37.5 50 62.5 75 NYHA Class by Dose (mean)

14 – In 2014, Capricor granted Janssen Biotech an exclusive option to enter into an exclusive license agreement for worldwide rights to CAP - 1002 for certain CV indications – Option period to end 60 days following Capricor’s delivery of interim six - month ALLSTAR data to Janssen – Under a potential license agreement for CAP - 1002: Janssen Holds a License Option on CAP - 1002 Janssen Development and registration Product manufacture Global commercialization Capricor License fee Milestone payments Sales royalties u p to $325 million l ow double - digit percent

15 Manufacturing Process Development with Janssen We expect to be able to generate thousands of doses of CAP - 1002 from each donor heart via commercial process in development

16 Cell Therapy Program for Duchenne Muscular Dystrophy
17 Heart Failure is #1 Cause of Death in DMD – DMD results from mutation in dystrophin gene – By early adulthood, nearly all people with DMD have clinical manifestations of cardiac disease – No approved therapies for the heart disease associated with DMD

18 Menon et al, Pediatr Cardiol 2014 ; 35 :1279 - 85; Tandon et al, J Am Heart Assoc 2015 ; 4 :e001338 . Heart Failure is the End Result of Progressive Scarring in DMD scarring in heart muscle increases linearly with age and strongly correlates with LV ejection fraction m ay be clinically silent due to functional compensation until sufficient to cause HF

19 Concept of Using CAP - 1002 to Treat DMD Heart Disease What happens in DMD CDCs’ Actions Oxidative/ nitrosative stress Anti - oxidative Inflammation Anti - inflammatory Apoptosis Anti - apoptotic Remodeling Anti - remodeling Loss of myocytes Regenerative Diverse effects of CDCs support their potential to retard or reverse the multiple pathological processes that occur in DMD
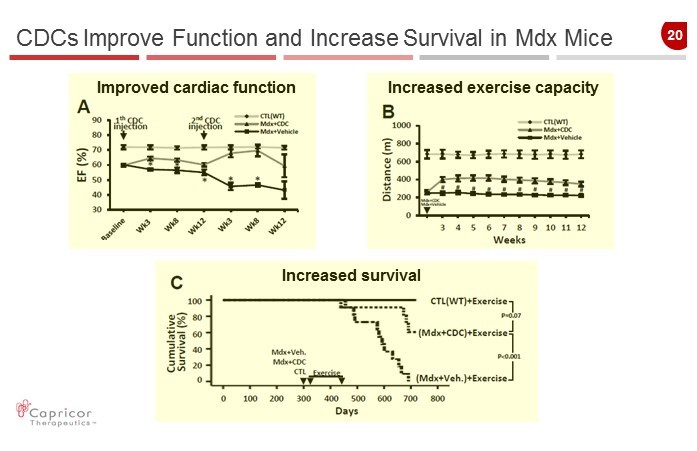
20 CDCs Improve Function and Increase Survival in Mdx Mice Improved cardiac function Increased exercise capacity Increased survival

21 Randomized Phase I / II HOPE - Duchenne Trial of CAP - 1002 Being conducted in 25 boys and young men with DMD - associated cardiomyopathy Ambulatory and non - ambulatory patients are eligible Single infusion of CAP - 1002 or usual care only Exploratory efficacy assessments to be conducted at six and 12 months Ages 12+ Stable steroids LV scar in 4+ segments* EF >35% 1 x CAP - 1002 (75M cells) Usual Care * Assessed by late gadolinium enhancement (LGE) cardiac MRI. 12 6 To report top - line six - month results in early 2Q 2017
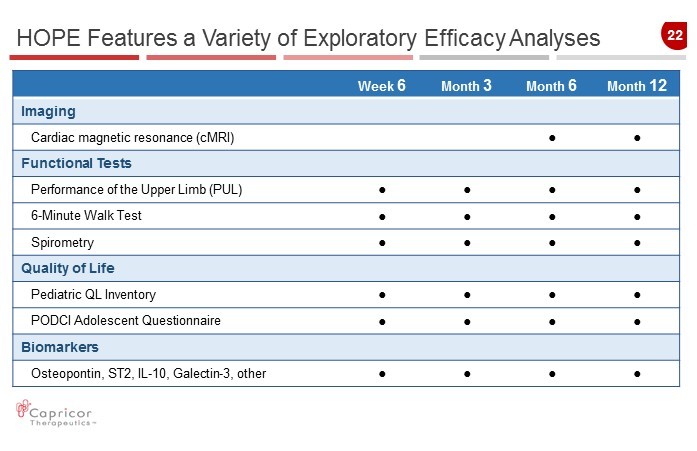
22 HOPE Features a Variety of Exploratory Efficacy Analyses Week 6 Month 3 Month 6 Month 12 Imaging Cardiac magnetic resonance ( cMRI ) Ɣ Ɣ Functional Tests Performance of the Upper Limb (PUL) Ɣ Ɣ Ɣ Ɣ 6 - Minute Walk Test Ɣ Ɣ Ɣ Ɣ Spirometry Ɣ Ɣ Ɣ Ɣ Quality of Life Pediatric QL Inventory Ɣ Ɣ Ɣ Ɣ PODCI Adolescent Questionnaire Ɣ Ɣ Ɣ Ɣ Biomarkers Osteopontin , ST2, IL - 10, Galectin - 3, other Ɣ Ɣ Ɣ Ɣ

23 Cardiac MRI to Assess Several Parameters Left Ventricular Month 6 Month 12 Myocardial composition Scar ( late gadolinium enhancement) Ɣ Ɣ Viable mass Ɣ Ɣ Dynamics Ejection fraction Ɣ Ɣ Regional wall motion Ɣ Ɣ Circumferential strain Ɣ Ɣ Dimensions End - diastolic volume Ɣ Ɣ End - systolic volume Ɣ Ɣ Stroke volume Ɣ Ɣ

24 Initial HOPE Data to be Reported in Early 2Q 2017 ► Expected to be reported: – Safety and tolerability – Exploratory efficacy measures (no powering for statistical significance) ► Independent Data Safety Monitoring Board (DSMB) has met four times during study per a predefined schedule – Trial was recommended to continue following each review

25 Exosome Program “Next - Generation” Class of Biological Therapies

26 Exosomes – a Cell - Free Regenerative Medicine Platform Gallet et al, Eur Heart J 2016 ; 38 :201 - 211.

27 Exosomes – a Cell - Free Regenerative Medicine Platform Gallet et al, Eur Heart J 2016 ; 38 :201 - 211. also active in non - cardiac inflammatory disease models

28 Cash Runway Through Key 2017 Events Capricor has received over $30 million in competitive grant and loan awards Cash and cash equivalents $20.5 million (as of 9/30/16) Net cash used in operations $11.6 million (nine months ended 9/30/16) Shares outstanding 21.4 million (February 13, 2017 )

29 Cash Runway Through Key 2017 Events Capricor has received over $30 million in competitive grant and loan awards Cash and cash equivalents $20.5 million (as of 9/30/16) Net cash used in operations $11.6 million (nine months ended 9/30/16) Shares outstanding 21.4 million (February 13, 2017 )

30 Recent and Upcoming Milestones Clinical Programs CAP - 1002 in Duchenne Heart Disease x 3Q 2016: Announced completion of enrollment in HOPE trial Early 2Q 2017: To report six - month top - line results of HOPE trial CAP - 1002 in Adult Heart Disease x 4Q 2016: Announced completion of ALLSTAR enrollment x 4Q 2016: Presented positive 12 - month DYNAMIC results at TCT conference Mid - 2017: Expect Janssen decision on license option Preclinical CAP - 2003 x 3Q 2016: Reported positive effects in oGVHD - relevant model 2H 2017: Expect to submit IND application for oGVHD

www.capricor.com A Translational Medicine Company NASDAQ: CAPR