UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2013.
Or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to .
Commission File Number 000-51171
ZALICUS INC.
(Exact name of registrant as specified in its charter)
| | |
| Delaware | | 04-3514457 |
(State or other jurisdiction of incorporation or organization) | | (IRS Employer Identification Number) |
| |
245 First Street Third Floor Cambridge, Massachusetts | | 02142 |
| (Address of Principal Executive Offices) | | (Zip Code) |
Registrant’s telephone number, including area code: (617) 301-7000
Securities registered pursuant to Section 12(b) of the Exchange Act:
| | |
Title of Each Class | | Name of Exchange on Which Registered |
Common Stock, par value $0.001 | | The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Exchange Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. Check One:
| | |
| Large Accelerated Filer: ¨ | | Accelerated Filer: x |
| |
| Non-Accelerated Filer: ¨ | | Smaller Reporting Company: ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No x
The aggregate market value of voting common equity of the registrant held by non-affiliates of the registrant was approximately $74,398,690.80 on June 28, 2013. For purposes of the foregoing sentence, the term “affiliate” includes each director and executive officer of the registrant and affiliates of such persons. The computation of the aggregate market value is based upon the closing price of the common stock as reported on the NASDAQ Capital Market on June 28, 2013.
As of March 12, 2014, the registrant had 26,108,910 shares of common stock, par value $0.001 per share, outstanding.
Specified portions of the registrant’s definitive Proxy Statement relating to the registrant’s Annual Meeting of Stockholders, which is expected to be filed pursuant to Regulation 14A within 120 days after the end of the registrant’s fiscal year ended December 31, 2013 are incorporated by reference in Part III of this Annual Report on Form 10-K.
ZALICUS INC.
ANNUAL REPORT
ON FORM 10-K
INDEX
2
PART I
FORWARD-LOOKING STATEMENTS
This annual report on Form 10-K includes statements with respect to Zalicus Inc. (“Zalicus”) and its subsidiaries (“we”, “our” or “us”), which constitute “forward-looking statements” within the meaning of the safe harbor provisions of the United States Private Securities Litigation Reform Act of 1995. Words such as “believe,” “anticipate,” “expect,” “estimate,” “intend,” “plan,” “project,” “will be,” “will continue,” “will result,” “seek,” “could,” “may,” “might,” or any variations of such words or other words with similar meanings are intended to identify such forward-looking statements. Forward-looking statements in this annual report on Form 10-K include, without limitation, statements regarding our future expectations; any projections of financing needs, revenue, expenses, earnings or losses from operations, or other financial items; any statements of the plans, strategies and objectives of management for future operations; any statements concerning product candidate research, development and commercialization plans and timelines; any statements regarding safety and efficacy of product candidates; any statements regarding our plans for the outlicensing of our clinical or pre-clinical product candidates and our seeking of collaborations; any statements regarding timing of initiating and completing clinical and pre-clinical trials and studies; any statements of expectation or belief; and any statements regarding other matters that involve known and unknown risks, uncertainties and other factors that may cause actual results, levels of activity, performance or achievements to differ materially from results expressed in or implied by this annual report on Form 10-K.
The risks, uncertainties and assumptions referred to above include risks that are described in this annual report on Form 10-K in the section entitled “Risk Factors” and elsewhere and that are otherwise described from time to time in our Securities and Exchange Commission reports filed after this report. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this annual report on Form 10-K. We specifically disclaim any obligation to update these forward-looking statements in the future, except as required by law.
On September 18, 2013, our board of directors unanimously approved a 1-for-6 reverse stock split of our common stock, which we effected on October 3, 2013. All share and per share amounts of common stock, options and warrants in this Annual Report on Form 10-K, including those amounts included in the accompanying financial statements, have been restated for all periods to give retroactive effect to the reverse stock split.
Overview
We are a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain. We have a portfolio of proprietary product candidates targeting pain and have entered into multiple revenue-generating collaborations with large pharmaceutical companies relating to other products, product candidates and drug discovery technologies. We also apply our expertise in the discovery and development of selective ion channel modulators and our combination high throughput screening technology, or cHTSTM to discover new product candidates for our portfolio or for our collaborators in the areas of pain and oncology.
Our most advanced product candidate is Z944, a novel, oral, state-dependent, selective T-type calcium channel modulator we are seeking to develop for the treatment of pain indications. T-type calcium channels have been recognized as key targets for therapeutic intervention in a broad range of cell functions and have been implicated in pain signaling. During 2013, we completed a Phase 1b clinical trial utilizing Laser-Evoked-Potentials, or LEP, to provide both objective and subjective assessments of the activity of Z944 in induced pain states. On November 1, 2013, we announced positive results from this Phase 1b clinical trial. Based on these results, Zalicus is currently planning to evaluate multiple modified-release formulations of Z944 in a Phase 1 pharmacokinetic and safety study in the first half of 2014, with the goal of advancing a modified-release formulation of Z944 toward Phase 2 clinical development in an appropriate pain indication by late 2014.
3
We have also been performing discovery research and preclinical development activities on our proprietary selective ion channel modulators targeting the Nav1.7 sodium channel for the treatment of pain. This discovery research and preclinical development has been conducted as part of a research collaboration with Hydra Biosciences, Inc., or Hydra, a recognized leader in novel ion channel discovery and development. This collaboration expired in February 2014 in accordance with its terms.
Until November 2013, we had also been advancing the development of Z160, a novel, oral N-type calcium channel modulator we were seeking to develop for the treatment of chronic pain. In December 2012 and August 2012, we initiated Phase 2 clinical trials of Z160 for the treatment of neuropathic pain, including post-herpetic neuralgia, or PHN, a painful neuropathic condition resulting from an outbreak of the herpes zoster virus, otherwise known as shingles, and lumbosacral radiculopathy, or LSR, a common neuropathic back pain condition resulting from the compression or irritation of the nerves exiting the lumbar region of the spine, respectively. Patient enrollment completed for both studies in September 2013. Results from both Phase 2 trials of Z160 were released on November 11, 2013 and Z160 did not meet the primary endpoint in either of these Phase 2 trials. Based on the results, we have terminated further development of Z160.
Until September 2012, we had also been advancing the development of Synavive, a product candidate to treat immuno-inflammatory disorders. In June 2011, we initiated a Phase 2b clinical trial evaluating Synavive in patients with rheumatoid arthritis, which we refer to as the SYNERGY trial. Results from the SYNERGY trial were announced in September 2012, and while Synavive achieved a statistically significant improvement in signs and symptoms of rheumatoid arthritis, as measured by DAS28-CRP, compared to placebo, the primary end point of the trial, Synavive did not demonstrate a meaningful clinical benefit measured by DAS28-CRP, compared to 2.7 mg of prednisolone or 5 mg of prednisone, key secondary endpoints of the SYNERGY trial. Based on the data from the SYNERGY trial, we terminated further development of Synavive.
We have also been using our cHTS platform to perform our obligations with our collaboration partners, including the Novartis Institutes of Biomedical Research, Inc., or Novartis, and other pharmaceutical companies who have adopted cHTS as an important addition to their oncology discovery efforts. Our cHTS platform technology profiles the activity of individual agents and combinations in cell-based phenotypic assays at high throughput to discover synergistic multi-target drug candidates that may generate novel intellectual property, inform clinical development and expand development pipelines. Our proprietary software solution, Chalice™, works with the cHTS technology platform to extract valuable insights, discoveries, and data trends from high-throughput combination activity profiling experiments.
On December 21, 2009, we completed a merger, which we refer to as the Neuromed Merger, with Neuromed Pharmaceuticals Inc., or Neuromed, pursuant to which Neuromed Pharmaceuticals Ltd. became a wholly-owned subsidiary of Zalicus. On September 8, 2010, we changed our name from CombinatoRx, Incorporated to Zalicus Inc. We also changed the name of our subsidiaries, including Neuromed Pharmaceuticals Ltd., which is now named Zalicus Pharmaceuticals Ltd, and which we refer to herein as Zalicus Canada.
Our Proprietary Product Candidates
All of our proprietary product candidates are focused in the area of pain, which is an area of continuing unmet medical need with potentially large commercial markets.
Our Ion Channel Product Candidates for Pain
We are focused on discovering and developing new compounds that selectively target neuronal calcium and sodium channels for the treatment of pain. The current treatment paradigms for pain rely on four major classes of medicines: opiates, non-steroidal anti-inflammatory drugs, or NSAIDs, gabapentanoids, a class of anti-convulsants with analgesic properties and antidepressants, all of which have significant safety, tolerability and dependence issues. We are using our experience with ion channel research and development in our efforts to discover and develop novel proprietary compounds to potentially treat pain by selectively modulating T-type
4
calcium channels or Nav1.7 sodium channels. We believe our ion channel modulator programs have the potential to produce a new class of analgesics for the treatment of acute, chronic or visceral pain, with the potential for safety and efficacy advantages over existing analgesics.
Background of Clinical Pain.Pain results from sensory nerve stimulation often associated with actual or potential tissue damage. Specific nerve fibers carry the pain signal across the nervous system to the brain, where it is recognized as pain. Pain is generally characterized on two dimensions, intensity and duration. Pain intensity is typically expressed as mild, moderate or severe. Mild pain results from relatively common conditions such as headaches, sprains or strains. Moderate pain results from conditions such as surgery, severe strains or sprains. Severe pain results from serious underlying illnesses such as osteoarthritis, lower back pain, post-herpetic neuralgia, diabetic neuropathy, inflammatory bowel syndrome, cancer or AIDS. Pain duration is expressed as acute or chronic. Acute pain often subsides in a short period of time and is typically associated with tissue injury such as surgery, a cut, a joint dislocation, or pressure on a nerve. Chronic pain persists for long periods of time and may involve underlying changes in the nervous system producing unusual sensitivity to touch, shooting or visceral pains, aching and other often disabling pain symptoms.
Background on Pain Treatment Market.NSAIDs, including COX-2 inhibitors, used to treat mild to moderate pain, are widely prescribed within the pain pharmaceutical market. NSAIDs are drugs with analgesic, fever-reducing and anti-inflammatory effects. As a class, NSAIDs are usually prescribed as first-line treatment; however, their relatively low potency may result in insufficient pain control for the patient. The long-term use of NSAIDs may result in side effects such as gastrointestinal bleeding, liver and kidney damage and cardiovascular-related complications.
Opioids have long been prescribed to treat moderate to severe pain and are regarded as the most potent class of analgesics. When used for extended periods, however, opioids can lead to side-effects, the development of tolerance, dependence and addiction. Tolerance means that increasing doses of opioids are required to maintain effective pain relief. Dependence means reliance on the drug and the existence of significant withdrawal symptoms upon cessation of drug administration. Addiction refers to drug-seeking behaviors characterized by a continued craving for the opioid and the need to use it for effects other than pain relief. As a consequence of these and other serious side effects, opioids are usually prescribed when other treatments for chronic pain have failed. In general, the more severe or chronic the pain, the more likely an opioid will be prescribed.
Despite the availability of many drugs to treat chronic pain, the results of a 2006 survey of chronic pain sufferers conducted by the American Pain Foundation, found that approximately 51% of the respondents felt that they had little or no control over their pain. We believe that this lack of adequate pain control, particularly in patients with moderate to severe chronic pain, represents a significant therapeutic gap in current pain management.
Our Ion Channel Programs
T-type calcium channels and sodium channels in cells are important to the regulation of the body’s nervous and cardiovascular systems. We are developing novel proprietary compounds to treat pain and potentially other diseases by selectively blocking ion channels in the neuronal cells responsible for pain signal transmission. We believe that our ion channel programs have the potential to produce a new class of analgesics for the treatment of acute, chronic or visceral pain, with potential safety and efficacy advantages over existing analgesics.
Overview of Calcium Channel Biology
Calcium ions play critical roles in the biochemistry, physiology and anatomy of cells and organisms. The rapid entry of calcium ions into cells is mediated by several classes of calcium channels that can be regulated by voltage, endogenous ligands or other cellular signaling events. The class called voltage gated calcium channels respond to electrical signals by the opening of a calcium-selective pore in the cell membrane. Calcium channels are involved in a large number of normal physiological processes including muscle contraction, hormone secretion, gene expression, and electrical signaling in the nervous system.
5
In order to carry out the multiple physiological functions that calcium channels help regulate, the human genome encodes ten distinct types of voltage gated calcium channels. These ten different calcium channels have been traditionally classified into five designations; L-type (four gene subtypes), T-type (three gene subtypes), and one gene subtype for each of the R-type, P/Q-type and N-type. Of particular relevance for pharmaceutical development, each of the different types of voltage gated calcium channels is known to perform distinct physiological functions and offers the opportunity to target specific drugs to specific calcium channels and human disease indications.
For example, L-type calcium channels are responsible for triggering contraction of both heart muscle and blood vessel smooth muscle. As such, L-type channels have been the selective target for drugs treating cardiovascular disease including hypertension and cardiac arrhythmias. These well-known calcium channel modulators have been on the market for over 40 years and provide proof-of-concept that calcium channels represent a valid target for therapeutic intervention and may address large commercial opportunities. One of the founders of Neuromed Pharmaceuticals Ltd., or Neuromed, which we acquired in December 2009, Dr. Terrance Snutch, was the first to describe that the various calcium channels in the nervous system are encoded by a family of distinct genes. In addition to being the first to clone these important clinical targets, we recognized the potential pharmaceutical significance of the N-type and T-type calcium channels, devised innovative screening platforms and invented our initial proprietary calcium channel modulator product candidates. Building on this work, we are currently pursuing a program, Z944, targeting all three T-type calcium channel gene subtypes.
Z944 - our T-type Calcium Channel Program
Our T-type calcium channel program is focused on developing drug candidates to treat a variety of chronic pain conditions. Our clinical-stage product candidate Z944 is a novel, oral, state-dependent, selective T-type calcium channel modulator that has demonstrated preclinical efficacy in inflammatory and visceral pain models, and that we are seeking to develop for the treatment of pain. During 2012 and early 2013, we completed Phase 1 single ascending dose and multiple ascending dose clinical studies of Z944. During 2013, we completed a Phase 1b clinical trial utilizing LEP to provide both objective and subjective assessments of the activity of Z944 in induced pain states. On November 1, 2013, we announced positive results from the Phase 1b clinical trial. Based on these results, Zalicus is planning to evaluate multiple modified-release formulations of Z944 in a Phase 1 pharmacokinetic and safety study in the first half of 2014, with the goal of advancing a modified-release formulation of Z944 toward Phase 2 clinical development in an appropriate pain indication by late 2014.
T-type Background.The wide distribution of T-type calcium channels found in neurons in the brain, heart, endocrine cells and other tissues provides for the possibility of developing therapeutics for multiple indications, including treatment of pain, epilepsy or tremor. We have identified and cloned three gene targets for T-type calcium channels that we believe have therapeutic potential for pain. Modulating T-type calcium channels in animal models has been shown to produce relief of acute and chronic pain from mild to moderate intensities. We have discovered proprietary T-type calcium channel modulators such as Z944 that show efficacy in animal models of inflammatory pain.
Potential Benefits of Oral T-type Calcium Channel Modulators.We believe that oral T-type calcium channel modulators such as Z944 have the potential to become a new class of analgesics. Limitations with existing pain therapies may be addressed by our T-type calcium channel blocker product candidate Z944. Z944 has the potential to improve:
| | • | | Efficacy.Despite the availability of many drugs to treat chronic pain, results of a 2006 survey by the American Pain Foundation of chronic pain sufferers found that 51% of the respondents felt that they had little or no control over their pain. For some patients, chronic pain may not be under control due to ineffective medications or the inability to tolerate effective doses of currently available analgesics. Deletion of either Cav3.1 or Cav3.2 (two of the three T-type channel subtypes) in animals has the effect of eliminating T-type calcium channel activity using these gene products, resulting in animals with normal general behaviors, but with attenuated responses to painful stimuli. |
6
| | • | | Selectivity. Our discovery screening process selects for ion channel modulators that are ‘state-dependent.’ This means that our compounds preferentially block only those ion channels that are in the hyperexcited state, a state in which they exist when transmitting pain. Ion channels that are not transmitting pain signals are not blocked, thereby allowing neuronal signaling to proceed normally. Current standards of care for pain are relatively indiscriminant for which neurons are affected, leading to unwanted side effects. We believe that our T-type calcium modulator Z944, being state-dependent, will allow for a more targeted approach to pain treatment, thereby improving on the safety and tolerability of current pain therapies. |
| | • | | Safety Profile.NSAIDs and opioid analgesics used over an extended period of time may lead to significant on and off-target side effects, providing the opportunity for the introduction of a new class of analgesics with a more favorable safety profile. We have tested Z944 in human clinical trials in 87 subjects and found it to be generally well tolerated and to exhibit a good safety profile with no serious drug-related adverse effects. |
| | • | | Tolerance and Addiction.Patients often require increasing doses of opioids to maintain effective pain relief, commonly referred to as tolerance. Patients who abruptly stop using opioids commonly experience withdrawal symptoms. Some patients using opioids experience euphoria and can progress to addictive behaviors and abuse. Based on our current understanding of Z944, we would not expect it to be scheduled as a controlled substance in the United States. |
| | • | | Drug Delivery.Z944 is a small molecule that is expected to be orally administered using tablet or capsule formulations. |
Overview of Sodium Channel Biology
In addition to our research and development on T-type calcium channels being targeted for pain, we have been actively pursuing discovery of another class of ion channel modulators for pain. Voltage gated sodium channels such as the Nav1.7 channel regulate pain signaling by mediating sodium ion currents that contribute to the excitability of both peripheral pain-sensing neurons and also neurons within the spinal cord that relay pain signals to the brain. Several distinct types of voltage gated sodium channels are important for setting the threshold and influencing the frequency, sustainability and intensity of pain signaling.
Of the ten sodium channel genes found in humans, the Nav1.7 and Nav1.8 types are of particular interest related to multiple chronic pain conditions as they are validated in humans. For example, naturally occurring loss-of-function mutations of the SCN9A gene which encodes the pore forming subunit of the Nav1.7 channel leads to the complete absence of pain sensation while other gain-of-function Nav1.7 mutations of the SCN9A gene cause severe chronic pain syndromes such as primary erythromelalgia. Further, in animal models it has been shown that the suppression of either Nav1.7 or Nav1.8 channels reduces various kinds of acute and neuropathic pain.
Sodium Channel Program
Addressing these attractive genetically-validated targets for pain intervention, we have utilized our expertise in ion channels to research and develop novel, orally available agents that block the functioning of sodium channels relevant to pain signaling. Our goal is to specifically target the increased firing and hypersensitivity in peripheral and spinal cord neurons that express Nav1.7 and Nav1.8 and that are associated with acute and chronic neuropathic pain.
We have generated a pipeline of preclinical agents shown to affect the Nav1.7 sodium channels. A number of these compounds have been found to both reduce the excitability of neurons and to reverse pain hypersensitivity in animal models of acute and neuropathic pain. We hope that targeting sodium channels in the peripheral and central neuronal signaling pathways through a unique mechanism of action has the potential to lead to novel classes of safe and effective pain therapeutics.
7
On February 8, 2012, we entered into a research collaboration agreement with Hydra, to advance the development of our preclinical Nav1.7 sodium channel modulator product candidates for the treatment of pain. The collaboration brought together our portfolio of novel, preclinical ion channel product candidates, representing multiple sodium channel modulators, with Hydra’s expertise in novel ion channel discovery and preclinical drug development with the goal of advancing Nav1.7 sodium channel drug candidates toward clinical development for the treatment of pain. Under the terms of the research collaboration agreement, we funded research and development activities at Hydra for a two year period until February 2014, during which time Hydra performed the discovery and preclinical development work necessary to advance our preclinical Nav1.7 sodium channel product candidates. Under the agreement, we retained all intellectual property and commercial rights to our Nav1.7 sodium channel product candidates that were studied under the agreement. The research collaboration agreement with Hydra expired in February 2014, in accordance with its terms.
Our cHTS Drug Discovery Technology
Combination approaches are an emerging clinical standard in the treatment of cancer. We believe that our cHTS platform has been validated through our research collaborations with Novartis and other pharmaceutical companies (described in greater detail below), who have adopted cHTS as an important addition to their oncology discovery and translational medicine efforts.
The cHTS platform technology profiles the activity of individual agents and combinations in cell-based phenotypic assays at high throughput to discover synergistic multi-target drug candidates. The technology generates novel intellectual property and provides insights for both drug discovery and clinical translational medicine efforts. We perform fee-for-service activities and also seek strategic partnerships to leverage the power of the cHTS technology platform in the area of oncology.
Our proprietary cHTS technology platform provides a solution to quantitatively assess how drugs or other probes of biological targets interact with diverse genetic backgrounds using whole cell-based phenotypic screening assays. This technology offers collaboration partners a high-throughput capability to identify synergistic interactions between small-molecule drugs or therapeutic antibodies and proteins that can be developed as therapeutic candidates. The platform can also identify compounds that antagonize the activity of drugs in relevant indications. Combinations are now emerging as the standard of care in indications such as cancer, inflammation, diabetes, and infectious disease. Unfortunately, the standard approach of developing combination therapies at the clinical stage misses the opportunity to identify novel multi-target mechanisms that better address the systems biology of disease and the emergence of resistance. In addition to the ability to identify synergistic multi-target drug candidates that generate novel intellectual property and expand development pipelines, our cHTS technology can also be deployed to define genetic determinants of chemical and multi-target drug sensitivity to identify likely responder populations and companion diagnostics. This approach provides collaboration partners with an opportunity to optimize the value of their existing product candidates, and improve their return on investment in less ideal candidates, by identifying synergistic combinations that amplify drug activity and potency within an indication, expand therapeutic utility into new indications, and extend patent estates through new compositions and use.
We also receive interest from collaboration partners to profile how their drugs or clinical candidates interact with standard-of-care agents in particular indications. We have engaged with multiple large pharmaceutical companies to characterize the activity of very large sets of combinations across multiple cancer and tissue types to better understand how various drugs and combinations of interest interact with diverse cellular networks. We expect this type of collaboration leveraging the power of the cHTS technology to produce future value for us and our strategic partners.
Our proprietary software solution, Chalice, was developed by us to enable the visualization and analysis of combination activity profiling data. This software provides a unique and generalized capability to assemble combination test data into matrices for visualization and analysis, and has utility across diverse therapeutic indications. Chalice allows researchers to apply specialized capabilities to quantitatively assess the strength of
8
combination interactions. The platform requires only test article identification, concentration, and measurable response data to produce a rich set of combination results suitable for the analysis of a wide variety of data types including: biochemical assays (enzyme inhibition, gene expression, etc.), cell-based assays (proliferation, apoptosis, etc.), andin vivo biomarker or activity assays. In addition, test article and experimental data attributes can be utilized to visualize combination activity profiles to facilitate meta-analyses and drive hypothesis generation. For example, visualizing combination synergy measures with the oncogenic status of cell-lines tested may suggest possible predictors of response that could then be validated. The Chalice software works with the cHTS technology platform to extract valuable insights, discoveries, and data trends from high-throughput combination activity profiling experiments.
Our Partnered Products, Product Candidates and Collaborations
We have entered into and intend to continue to seek collaborations with pharmaceutical and biotechnology companies to support the development and commercialization of our product candidates and to obtain access to additional development, commercial or financial resources. We also plan to continue to engage in selected discovery research collaborations to utilize our proprietary discovery research platforms to discover new product candidates for ourselves or our collaborators and to generate ongoing revenue.
Active Collaborations
Novartis
In May 2009, we entered into a research collaboration and license agreement with Novartis, focused on the discovery of novel anti-cancer combinations. Through the collaboration, we are using our proprietary cHTS platform to screen a unique library of molecules, including Novartis compounds, in multiple cell lines representing a broad spectrum of cancers to potentially discover novel single agent and combination therapies to treat various cancers.
Under the terms of the collaboration agreement, we received an initial payment of $4.0 million and will receive annual research support payments of up to $3.0 million, plus certain expenses. In addition, the collaboration agreement may provide us with up to $58.0 million for each combination product candidate advanced by Novartis upon achievement of certain clinical, regulatory and commercial milestones as follows:
| | • | | Up to $5.0 million in clinical development milestones. |
| | • | | Up to $23.0 million in regulatory milestones. |
| | • | | Up to $30.0 million in commercial milestones. |
We did not recognize any milestone payments under this arrangement in the years ended December 31, 2013 and 2012. The research program had an initial two-year term that could be extended by Novartis for three additional one-year periods. We also entered into a software license agreement with Novartis, where we provided Novartis with a non-exclusive license to use our proprietary Chalice software in connection with the collaboration and other Novartis research programs for approximately five years, with options to renew for multiple additional five-year terms. In January 2011, Novartis elected to extend the research program for an additional year, into May 2012 and in April 2012, we entered into an amendment with Novartis to extend the research program for an additional year, into May 2013. In April 2013, we entered into a second amendment to the collaboration agreement with Novartis, to extend the funded research program under the collaboration agreement until October 31, 2014 and provide for up to $3.0 million in funded research payments under the collaboration agreement. In April 2013, we entered into an amendment to the software license with Novartis to extend the term of the license until October 31, 2014, and to amend Novartis’ option to extend the term of the software license to a single, one-year option, exercisable upon the payment of amended additional software licensing fees.
The library to be screened under the collaboration consists of certain Novartis oncology compounds and compounds from our library of approved drugs and other molecules. Novartis will own and have an exclusive
9
license to intellectual property generated under the collaboration to research, develop and commercialize their approved or active development-stage compounds. We will own and have an exclusive license to intellectual property generated under the collaboration to research, develop and commercialize compounds from our library. Intellectual property generated under the collaboration using certain compounds from the Novartis library will be jointly owned by Novartis and us and non-exclusively licensed to allow each party to research, develop and commercialize product candidates. Under the collaboration agreement, Novartis retains an option, exercisable once per year of the research collaboration, to exclusively license a portion of this jointly owned intellectual property if certain conditions are met. Novartis also has a right of first negotiation to exclusively license the intellectual property owned by us that was discovered as a part of the collaboration, under terms to be negotiated by the parties at such time.
The collaboration agreement may be terminated by either party after ninety days’ notice upon an unremedied material breach and upon thirty days’ notice in the event of bankruptcy of the other party. Novartis may terminate the collaboration agreement after sixty days’ notice in the event of a change in control or liquidation of us, as defined in the collaboration agreement.
Amgen Inc.
In December 2009, we entered into a research collaboration agreement with Amgen Inc., or Amgen, focused on identifying synergistic combinations for two oncology targets of interest to Amgen. Under the agreement, we received a $750,000 payment in January 2010 to fund the initial research plan, and Amgen also agreed to reimburse us for laboratory supplies consumed. The initial research plan ended in September 2010, and Amgen elected for us to perform follow-up research at an annual rate of $300,000 per full-time employee equivalent, plus the reimbursement of laboratory supplies. Under the terms of the research collaboration agreement, Amgen will also pay us a $1.0 million milestone payment for each investigational new drug application, or IND, filing by Amgen for a product candidate with new intellectual property generated by the collaboration. We did not recognize any milestone payments under this arrangement in the years ended December 31, 2013 and 2012. We also entered into a software license agreement with Amgen in May 2011, where we provided Amgen with a non-exclusive license to use our proprietary Chalice software in connection with the collaboration and other Amgen research programs for one year. We entered into amendments to this software license agreement with Amgen in April 2012, January 2013 and May 2013, pursuant to which we have provided Amgen with the same non-exclusive license until May 2014. Through December 31, 2013, we have received $2.9 million in funding, expense reimbursement and software license fees under these agreements.
Prednisporin – Fovea Pharmaceuticals SA, a subsidiary of Sanofi
On January 30, 2006, we entered into a research and license agreement with Fovea Pharmaceuticals SA, or Fovea. Under the terms of the agreement, Fovea agreed to conduct, at its own expense, preclinical and clinical development of combination drug candidates it selected from our portfolio of product candidates for certain ophthalmic indications, including creating ophthalmic formulations for these selected drug candidates. Fovea was acquired by Sanofi SA in October 2009 and is now a subsidiary of Sanofi focused on ophthalmic diseases.
On July 22, 2009, we amended and restated the research and license agreement with Fovea. Under the amended and restated agreement, we granted Fovea an exclusive worldwide license to certain drug combinations to treat allergic and inflammatory diseases of the front of the eye. Fovea has advanced one such combination, Prednisporin™ (FOV1101), through Phase 2b clinical development for allergic conjunctivitis.
We have received payments totaling $1.5 million related to Prednisporin (FOV1101) and are eligible to receive up to an additional $39.0 million from Fovea upon achievement of certain clinical and regulatory milestones for Prednisporin (FOV1101) and each other product candidate subject to the research and license agreement as follows:
| | • | | Up to $3.0 million in clinical development milestones. |
| | • | | Up to $21.0 million in regulatory milestones. |
10
| | • | | A $15.0 million milestone for the FDA approval of a product candidate to treat keratoconjunctivitis sicca, commonly known as dry eye syndrome. |
Sanofi, Fovea’s parent company, has publicly announced its intention not to develop Prednisporin/FOV1101 itself and is planning to sublicense its rights to a third party. As of February 28, 2014, Sanofi has not sublicensed Prednisporin/FOV1101 to a third party.
Prednisporin (FOV1101), is not currently being developed and is not planned to be developed, to treat dry eye syndrome, nor are there any other product candidates subject to the Fovea agreement that are currently being developed for dry eye syndrome. As a result, we believe that there is a remote likelihood that this milestone will be achieved. We did not recognize any milestone payments under this arrangement in the years ended December 31, 2013 and 2012.
The agreement has no definite term; however, Fovea’s royalty payment obligations terminate on the later of 15 years from the date of the first commercial sale of an exclusively licensed combination and the expiration of all patents covering a royalty bearing product under the license agreement, each on a country-by-country basis. The agreement may be terminated by either party upon an unremedied material breach. In addition, if Fovea fails to develop a product candidate it selects pursuant to specified diligence milestones, after discussions between the parties, the agreement may be terminated by us. We may terminate the agreement if Fovea fails to make required undisputed payments and either party may terminate the agreement upon the insolvency of the other party.
Previous Collaborations
Exalgo – Mallinckrodt Inc., a subsidiary of Covidien plc
In June 2009, prior to our merger with Neuromed, Neuromed entered into an asset purchase agreement with Mallinckrodt Inc., or Mallinckrodt, then a subsidiary of Covidien plc, or Covidien, to sell all of the tangible and intangible assets associated with Exalgo, including the rights to develop and commercialize the product candidate in the United States. Exalgo® is an extended release formulation of hydromorphone, an opioid analgesic that has been used in an immediate release formulation to treat pain for many years and is intended for use in the management of moderate to severe pain in opioid tolerant patients requiring continuous, around-the-clock opioid analgesia for an extended period of time. Under the asset purchase agreement, Mallinckrodt was responsible for all commercialization activities for Exalgo in the United States, including marketing and sales, and for all post-approval regulatory activities. We acquired the rights and assumed the obligations under the asset purchase agreement as a result of our merger with Neuromed in December 2009.
Neuromed acquired the United States rights to Exalgo in 2007 and successfully advanced it through Phase 3 clinical development, the submission of a new drug application in May 2009 and the approval by the United States Food and Drug Administration, or FDA, of the 8, 12 and 16 mg dosage strengths of Exalgo on March 1, 2010. As part of the agreement with Mallinckrodt, Neuromed received upfront and initial milestone payments of $15.0 million, and we received a $40.0 million milestone payment following FDA approval of Exalgo in 2010. Exalgo was commercially launched in the United States by Mallinckrodt on April 26, 2010, and the 32 mg dosage strength of Exalgo was approved by the FDA in August 2012 and launched by Mallinckrodt in September 2012. We received tiered royalties on Mallinckrodt’s net sales of Exalgo on a quarterly basis. Mallinckrodt was to continue to pay these royalties on net sales for as long as it is selling Exalgo, although the royalty rate was to be reduced by 50% upon the earlier to occur of the first sale of a generic version of Exalgo or June 11, 2024. Following the settlement of patent litigation between Mallinckrodt and Watson Pharmaceuticals, Inc., now Actavis plc, or Actavis, Actavis was permitted by Mallinckrodt to introduce a generic version of Exalgo at approved dosage strengths on November 15, 2013, subject to FDA approval. In February 2013, Mallinckrodt and Actavis entered into a separate settlement agreement that would permit Actavis to introduce a generic version of the 32mg dosage strength of Exalgo starting on May 15, 2014, subject to FDA approval of the Actavis generic. As of February 28, 2014, Actavis has not introduced a generic version of Exalgo at any of the approved dosage strengths. We recognized $16.0 million in royalty revenue from Exalgo through December 31, 2013.
11
In connection with the asset purchase agreement we also entered into a development and transition services agreement, pursuant to which we have performed certain clinical development and regulatory activities primarily relating to the FDA approval of Exalgo and certain post-approval activities. These activities were at Mallinckrodt’s cost and expense, capped at $16.0 million. Through the year ended December 31, 2012, we received $8.8 million in funding and expense reimbursement under this agreement, and the tasks required to be completed by us under the agreement are complete.
On July 1, 2013, Mallinckrodt plc, the successor to Mallinckrodt, Inc., was spun off from Covidien as an independent company. This transaction did not impact sales of Exalgo by Mallinckrodt.
On January 31, 2014, Zalicus Canada and Mallinckrodt Medical Imaging – Ireland, an affiliate of Mallinckrodt, entered into a royalty purchase agreement, relating to the asset purchase agreement. Under the terms of the royalty purchase agreement, in exchange for the payment of $7.2 million from Mallinckrodt to Zalicus on January 31, 2014, we terminated any further rights we had to the payment of royalties on net sales of Exalgo by Mallinckrodt.
USAMRIID
In December 2008, we entered into a cooperative research and development agreement with the United States Army Medical Research Institute for Infectious Diseases, or USAMRIID, focused on discovering combinations of agents to prevent or treat Ebola, Marburg and Lassa virus infections. Under the agreement, which expired in September 2011, we and USAMRIID undertook a joint research project, and we were eligible to receive up to approximately $1.4 million in funding. In May 2010, Zalicus entered into a cooperative research and development agreement with USAMRIID focused on discovering combinations of agents to prevent or treat Alphavirus infections, which are mosquito-borne viruses whose infection can cause severe and fatal encephalitis in humans. Under this agreement, which expired in April 2013, we and USAMRIID undertook a joint research project, under which we were eligible to receive up to approximately $1.1 million in funding. In September 2012, we entered into a cooperative research and development agreement with USAMRIID focused on discovering chemotherapeutic interventions to prevent and/or treat encephalitic and viral hemorrhagic fever virus infections. This agreement had an initial one-year term that was extended by USAMRIID for an additional one-year period. Zalicus and USAMRIID have mutually agreed to terminate activities under the cooperative research and development agreement as of February 28, 2014. Through December 31, 2013, we have received approximately $2.9 million in funding from these three agreements with USAMRIID.
Patents and Other Proprietary Rights
Our success depends, in part, on our ability and the ability of our collaborators to obtain and maintain intellectual property protection for drug candidates, technology and know-how, to operate without infringing the proprietary rights of others and to prevent others from infringing on our proprietary rights.
Our policy is to seek to protect our proprietary chemical compounds, compositions, formulations and methods of using them by, among other methods, filing United States and foreign patent applications directed to our product candidates and discovery programs. We also rely on trade secrets, know-how and continuing technological innovation to develop and maintain our proprietary position.
As of March 1, 2014, our worldwide patent estate for our product candidate Z944, our Nav1.7 channel program and our licensed programs for Prednisporin/FOV1101 and with USAMRIID includes 10 issued patents and 44 pending patent applications. Of the issued patents, 6 are issued in the United States. Of the pending patent applications, 8 are United States non-provisional or provisional applications.
To maximize the protection and potential value of our intellectual property relating to ion channels, we are building a portfolio which currently includes 9 patent families, including 2 issued patents and 21 pending patent applications worldwide, which cover a variety of new chemical entities and their methods of use as well as
12
formulations of these molecules. Issued and pending patents in the ion channel space, including our most advanced ion channel product Z944, contain claims which cover a broad genre of compounds using several chemical scaffolds, as well as claims to specific compounds, the use of such compounds in certain ion channel related disorders such as pain and compositions and methods of use of formulations, preferred routes of administration, dosages and other properties.
One issued United States patent, which expires in 2029, covers the pharmaceutical composition and certain methods of use of our T-type calcium channel modulator product candidate Z944. One issued United States patent, which expires in 2029, covers the methods of using Z944 to treat pain. We also have one pending United States patent application relating to other T-type calcium channel modulators, which if issued as a patent, would be expected to expire between in 2029 and 2030. This application includes claims covering compositions and methods of our other T-type calcium channel modulator product candidates.
Three pending United States and three pending PCT patent applications relate to our Nav1.7 sodium channel modulators which, if issued as patents, would be expected to expire between 2030 and 2034. These applications include claims covering the pharmaceutical composition and methods of use for our Nav1.7 sodium channel modulator product candidates.
One issued United States patent, which expires in June 2029, and is licensed to Fovea, covers the pharmaceutical composition and methods of use of Prednisporin in certain ophthalmic diseases. For combination product candidates such as Prednisporin, it is our current practice to seek the issuance of extensive claims in our patent applications directed to the following:
| | • | | pharmaceutical compositions comprising the active pharmaceutical ingredients in the combination; |
| | • | | pharmaceutical compositions comprising structural, functional, or mechanistic analogs of the active pharmaceutical ingredients in the combination; |
| | • | | methods of treating diseases by administering the active pharmaceutical ingredients in the combination or their analogs; |
| | • | | pharmaceutical compositions or kits or packages, including the active pharmaceutical ingredients in the combination or their analogs and instructions for the treatment of diseases; and |
| | • | | compositions and methods of use for formulations, preferred routes of administration, dosages and other properties for our more advanced product candidates. |
In addition to seeking patent protection in the United States, we generally file patent applications in major European countries, Canada, Japan, China and additional countries on a selective basis in order to further protect the inventions that we or our collaboration partners consider important to the development and commercialization of our product candidates. As we develop novel formulations of our product candidates and learn more about the most promising doses, pharmacokinetic and pharmacodynamic parameters for our drug candidates, we intend to file additional patent applications to augment the core composition of matter and method of use patents we have been issued or are currently seeking.
In all of our activities, we rely on proprietary materials and information, trade secrets, and know-how to conduct research and development activities and to attract and retain collaborative partners, licensees, and customers. We attempt to protect our trade secrets by entering into confidentiality agreements with third parties, employees, and consultants. Our employees and consultants are also asked to sign agreements requiring that they assign to us present and future interests in patents and other intellectual property arising from their work for us. We also require all employees to sign an agreement not to engage in any conflicting employment or activity during their employment with us, and not to disclose or misuse our confidential information.
In certain instances, a patent term can be extended to recapture a portion of the term effectively lost as a result of the FDA regulatory review period. The duration of foreign patents varies in accordance with provisions
13
of applicable local law, but typically is 20 years from the earliest effective filing date. Our patent estate, based on patents existing now and patents we expect will be issued based on pending applications, will expire on dates ranging from 2018 to 2034.
The actual protection afforded our product candidates by a patent varies from country to country and depends upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory related extensions, the availability of legal remedies in a particular country and the validity and enforceability of the patent.
Government Regulation
The FDA and comparable regulatory authorities in other countries impose substantial requirements upon companies involved in the clinical development, manufacture, marketing and distribution of drugs. These agencies and other federal, state, provincial and local entities regulate research and development activities and the testing, manufacturing, quality control, safety, effectiveness, labeling, storage, record keeping, approval, advertising and promotion, and export and import of pharmaceutical products such as those that we are developing.
United States Food and Drug Administration Approval Process
United States Government Regulation
In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, and the agency’s implementing regulations. If we fail to comply with the applicable United States requirements at any time during the product development process, clinical testing, and the approval process or after approval, we, our product candidates or, once approved, our products may become subject to administrative or judicial sanctions. These sanctions could include the FDA’s refusal to approve pending applications, license suspension or revocation, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, civil penalties or criminal prosecution. Any FDA enforcement action could have a material adverse effect on us.
The process required by the FDA before a drug may be marketed in the United States generally involves the following:
| | • | | completion of extensive pre-clinical laboratory tests, pre-clinical animal studies and formulation studies all performed in accordance with the FDA’s good laboratory practice regulations; |
| | • | | submission to the FDA’s Center for Drug Evaluation and Research, or CDER, of an IND, which must become effective before human clinical trials may begin. The IND contains the plan for the clinical trial. CDER specialists carefully review the IND to determine whether there are any flaws in the initial studies and whether the overall development plan is feasible and reasonably designed to minimize health risks to the participants; |
| | • | | performance of adequate and well-controlled clinical trials to establish the safety and efficacy of the product candidate for each proposed indication; |
| | • | | submission to the FDA of a new drug application, or NDA; |
| | • | | the FDA may require compliance with risk evaluation and mitigation strategies, or REMS; |
| | • | | the FDA may request feedback by an advisory committee on the application and ask for their advice regarding approval, risk/benefit, risk management, and/or specific labeling claims. FDA is not obligated to follow recommendations of an Advisory Committee, but may consider such advice prior to completion of its own internal decision making; |
| | • | | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product candidate is produced to assess compliance with current good manufacturing practice regulations; and |
14
| | • | | FDA review and approval of the NDA before any commercial marketing, sale or shipment of the product. |
Pre-clinical Tests
Pre-clinical tests include laboratory evaluations of product chemistry, formulation and stability, as well as animal studies to evaluate efficacy and toxicity. The results of the pre-clinical tests, together with manufacturing information and analytical data, are submitted to the FDA as part of an IND to allow the product candidate to be studied in a clinical trial in human subjects. The IND automatically becomes effective 30 days after receipt by the FDA unless the FDA, within the 30-day time period, raises concerns or questions about the conduct of the clinical trial, such as subjecting human research subjects to unreasonable health risks. In that case, the IND sponsor and the FDA must resolve any outstanding FDA concerns before the clinical trials can begin. Submission of an IND may result in the FDA not allowing the trials to commence or not allowing the trial to commence on the terms originally specified in the IND. A separate submission to an existing IND must also be made for each successive clinical trial conducted during product development, and the FDA must grant permission, either explicitly or implicitly (by not objecting), before each clinical trial can begin. Outside the United States, preclinical data to support the conduct of a clinical trial is submitted to regulatory authorities in each country, often as part of a clinical trial application, or CTA.
Clinical Trials
Clinical trials involve the administration of the investigational product to human subjects under the supervision of qualified medical investigators. Clinical trials are conducted under protocols detailing, among other things, the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol must be submitted to the FDA as part of the IND. Outside the United States, protocols are submitted to regulatory authorities in each country, often as part of a CTA. An independent Institutional Review Board, or IRB, for each medical center proposing to conduct a clinical trial must also review and approve a plan for any clinical trial before it can begin at that center and the IRB must monitor the trial until it is completed. The FDA or other local regulatory authority, the IRB or the sponsor may suspend or discontinue a clinical trial at any time for various reasons, including a finding that the subjects are being exposed to an unacceptable health risk. Clinical testing also must satisfy extensive good clinical practice requirements and the requirements for informed consent.
During the conduct of a clinical trial, a company is required to monitor the investigators’ compliance with the clinical study protocol and other FDA or local regulatory requirements, including the requirements to submit reports to the sponsor, the IRB, and the FDA or other regulatory authority, and to keep detailed records regarding study findings and use and disposition of the study drug. Although monitoring can help reduce the risk of inadequate compliance by study investigators, it cannot eliminate this risk entirely. Inadvertent regulatory noncompliance by the investigator, or intentional investigator misconduct, can jeopardize the usefulness of study results and, in rare circumstances, require a company to repeat a study. A company must report to the FDA or other local regulatory authority any adverse event that is both unexpected and serious and for which there is a reasonable possibility that the event may have been caused by the investigational drug. In addition, a company must within seven days report to the FDA or other local regulatory authority any unexpected fatal or life-threatening event that may have been caused by the drug. The FDA or other local regulatory authority may stop a clinical trial by placing a “clinical hold” on such trials because of concerns about the safety of the product candidate being tested. Such holds can cause substantial delay and in some cases may require abandonment of a product candidate.
15
For the purposes of an NDA submission and approval, clinical trials are typically conducted in the following four sequential phases, which may overlap:
| | |
| Phase 1 | | Trials are initially conducted with relatively few subjects to test the drug candidate for safety, dosage tolerance, absorption, bioavailability, metabolism, distribution and excretion in healthy humans, or, on occasion, in patients with the disease or condition under trial to gain an early indication of its effectiveness or tolerance in a patient population. |
| |
| Phase 2 | | Trials are generally conducted with a relatively small number of subjects to: |
| |
| | – evaluate dosage tolerance and appropriate dosage; |
| |
| | – identify possible adverse effects and safety risks; and |
| |
| | – evaluate the efficacy of the drug for specific indications in patients with the disease or condition under trial. |
| |
| Phase 3 | | Trials, commonly referred to as “pivotal” or “registration” trials, are typically conducted when phase 2 clinical trials demonstrate that a dose range of the drug candidate is effective and has an acceptable safety profile. Phase 3 clinical trials are undertaken with large numbers of patients (several hundred to several thousand) to further evaluate dosage, to provide substantial evidence of clinical efficacy and to further test for safety in an expanded and diverse patient population at multiple, geographically dispersed clinical trial sites. |
| |
| Phase 4 | | Post-approval trials, to further assess the drug’s safety and effectiveness, are sometimes required by the FDA as a condition of approval. |
When two or more drugs are combined in a single dosage form, as Prednisporin is, the data submitted to the FDA must ordinarily show that each component makes a contribution to the claimed effects and that the dosage of each component (amount, frequency, duration) is such that the combination is safe and effective for a significant patient population requiring such concurrent therapy as defined in the labeling for the drug. This FDA policy may necessitate more elaborate and expensive clinical trials for combination candidates than would be required for a single-agent pharmaceutical because the trials may need to be designed to study the combined agent, each drug as a single agent and a placebo.
When FDA approval is sought for a new drug, the sponsor must demonstrate that the drug is safe and effective for the proposed use. The FDA’s specific requirements for safety data will be determined on a case-by-case basis for each product candidate.
Special Protocol Assessment Process
In the United States, certain protocols for clinical trials can be submitted to the FDA for special protocol assessment, or SPA. Under a SPA, the applicant and the FDA reach an agreement on the design and size of the clinical trial. This agreement can be in writing and cannot be changed after the clinical trial begins except with written agreement of the applicant and the FDA or if the director of the FDA reviewing division determines that “a substantial scientific issue essential to determining the safety or effectiveness of the drug” was identified after testing began.
New Drug Applications
The results of the pre-clinical testing and of the clinical trials, together with other detailed information, including extensive manufacturing information and information on the composition of the product, are submitted to the FDA in the form of an NDA requesting approval to market the product for one or more specified indications. The FDA reviews an NDA to determine, among other things, whether a product is safe and effective for its intended use.
16
Submission of a NDA does not assure FDA approval for marketing. After the application is submitted, the FDA initially determines whether all pertinent data and information have been submitted before accepting the application for filing. After the application is accepted for filing, the FDA begins its substantive review. The FDA typically will request a review of the data in the NDA and recommendation regarding approval by an advisory committee consisting of outside experts. The FDA may accept or reject the advisory committee’s recommendations, or accept them with modifications. The application review process generally takes a year or longer to complete, although reviews of drugs that meet a medical need for serious or life-threatening diseases may be accelerated or prioritized for a six-month review. The FDA may deny approval of an application. Any such denial may require extensive additional testing, which could take years to complete, in order to make the application approvable, or the denial may be based on considerations that cannot be favorably resolved through additional testing. In some circumstances, the FDA may approve an application even though some unanswered questions remain about the product, if the applicant agrees to conduct post-marketing studies. The FDA may impose other conditions of approval as well. Expedited or accelerated approvals may require additional larger confirmatory clinical studies to be conducted following approval.
Product approval may be withdrawn if compliance with regulatory requirements is not maintained or if post-marketing adverse events associated with the product are reported that cannot be addressed satisfactorily through changes to the product’s labeling or warnings to healthcare professionals. The FDA requires reporting of certain safety and other information that becomes known to a manufacturer of an approved product. A company may become aware of such information from reports of adverse events suspected to be related to the product, voluntarily provided to the company and/or to the FDA by physicians and other healthcare professionals, or from published scientific data. In some circumstances, the FDA may require the company to make changes to its approved product labeling or to issue safety warnings to healthcare professionals or the public, which may have a negative impact on product sales. In addition, the Amendments Act of 2007 provides the FDA with expanded authority over drug products after approval, including the authority to require post-approval studies and clinical trials, labeling changes based on new safety information, and compliance with risk evaluation and mitigation strategies, or REMS, approved by the FDA. The FDA’s exercise of this authority could result in delays or increased costs during the period of product candidate development, clinical trials and regulatory review and approval, increased costs to assure compliance with new post-approval regulatory requirements, and potential restrictions on the sale of approved products, which could lead to lower product revenues to us or our collaborators. Manufacturing and sales may also be disrupted or delayed in the event of failure to comply with all required current good manufacturing practice, or cGMP, as determined by FDA investigators in periodic inspections of manufacturing facilities. Upon approval, a drug may only be marketed for the approved indications, in the approved dosage forms, and at the approved dosage. The nature of marketing claims that we will be permitted to make in the labeling and advertising of our products will be limited to those specified in an FDA approval.
Other United States Regulatory Requirements
Any end products manufactured or distributed by us or our collaborators and licensors pursuant to FDA approvals are subject to continuing regulation by the FDA, including recordkeeping and reporting requirements. Adverse event experience with the product must be reported to the FDA in a timely fashion and the FDA may mandate a pro-active search for these adverse effects. Drug manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with ongoing regulatory requirements, including cGMPs, which impose certain procedural and documentation requirements upon them and their third-party manufacturers. Failure to comply with the statutory and regulatory requirements can subject a manufacturer to possible legal or regulatory action.
In addition to regulations enforced by the FDA, we are also subject to regulation under the Occupational Safety and Health Act, the Toxic Substances Control Act, the Resource Conservation and Recovery Act, and other present and potential future federal, state, provincial and local statutes and regulations. Our research and
17
development involves the controlled use of hazardous materials, chemicals, and various radioactive compounds. Although we believe that our safety procedures for storing, handling, using, and disposing of such materials comply with the standards prescribed by applicable regulations, the risk of accidental contaminations or injury from these materials cannot be completely eliminated. In the event of such an accident, we could be held liable for any damages that result, and any such liability could materially affect our ongoing business.
International Regulation
Outside the United States, our ability to market a product is contingent upon receiving marketing authorization from the appropriate regulatory authorities. The requirements governing the conduct of clinical trials, marketing authorization, pricing, and reimbursement vary widely from country to country. At present, foreign marketing authorizations are applied for at a national level, although within the European Union, centralized procedures are available to companies wishing to market a product in more than one European Union member state. If the regulatory authorities are satisfied that adequate evidence of safety, quality, and efficacy has been presented, a marketing authorization will be granted. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country.
Third Party Reimbursement and Pricing Controls
Sales of pharmaceutical products depend in significant part on the availability of coverage and adequate reimbursement from government and other third-party payers, including the United States Medicare and Medicaid programs. Third-party payers are increasingly challenging the pricing of pharmaceutical products and may not consider our product candidates or those of its collaborators cost-effective or may not provide coverage of, and adequate reimbursement for, any future product candidates, in whole or in part.
Competition
The development and commercialization of pharmaceutical products is highly competitive. We compete against a wide range of pharmaceutical, biotechnology and life science companies that have greater resources than us, including existing research and development programs in the markets we plan to target. We must compete with these companies both in regard to the discovery technology we use to identify potential product candidates and in regard to the development and commercialization of our product candidates themselves.
Product candidates for pain, if approved, will compete with existing proprietary and generic opioid and non-opioid analgesics as well as new molecules, formulations and technologies that may be developed or commercialized in the future for the treatment of pain. Any of these drugs and drug delivery technologies may receive government approval or gain market acceptance more rapidly than our product candidates, may offer therapeutic, safety or cost advantages over our product candidates or may cure their targeted diseases or their underlying causes completely. As a result, our product candidates, even if they are approved, may become non-competitive or obsolete.
In regard to our product candidates, we file patent applications on the composition and use of the molecules we discover. If we obtain the patent protection we are seeking for our product candidates, we believe that this will give us the exclusive rights to market products covered by our patents. We intend to seek regulatory approval for our product candidates as new drugs, and the expense and time involved in seeking regulatory approval for a new drug may deter potential competitors.
The target markets for our product candidates and those of our collaborators, are all very competitive, with existing approved products holding substantial market share and other product candidates being developed by other pharmaceutical or biotechnology companies.
18
Principal competitive factors impacting drug development and commercialization include:
| | • | | improved patient outcomes; |
| | • | | demonstrable safety of product candidates; |
| | • | | acceptance of products by physicians and other healthcare providers; |
| | • | | research and drug development capabilities; |
| | • | | government and third-party reimbursements of approved therapies; |
| | • | | intellectual property positions; |
| | • | | sales and marketing capabilities; and |
| | • | | availability of capital resources to fund research, development and commercialization activities. |
In regard to our ion channel discovery efforts, we have protected our trade secrets around our assays and experimental techniques in order to give us a competitive advantage in discovering compounds that modulate ion channels. Many large and small companies have sought to discover and develop ion channel modulators for the treatment of pain and other diseases, and may be more successful in discovering ion channel modulators using similar or different techniques.
In regard to our cHTS discovery technology and Chalice software, we protect our trade secrets in order to give us the exclusive right to use our technologies. Many companies have already developed and employ high throughput screening technologies. Should these companies seek to apply these technologies to the discovery of combination drugs, our drug discovery technology may be rendered obsolete or noncompetitive.
Many of the companies competing against us have financial and other resources substantially greater than our own. In addition, many of our competitors have significantly greater experience in clinical testing, obtaining FDA and other regulatory approvals and in the manufacture and commercialization of products.
Manufacturing
We have no manufacturing capabilities. We rely and plan to continue to rely on third parties to manufacture our compounds, formulations and product candidates for research, development, preclinical and clinical trials. We believe that there are several manufacturing sources available to us on commercially reasonable terms to meet our preclinical and clinical requirements.
We plan to rely on third parties to manufacture commercial quantities of products we successfully develop, if any. Among the conditions for FDA or other regulatory approval of a pharmaceutical product is the requirement that the manufacturer’s quality control and manufacturing procedures conform to cGMP, which must be followed at all times. The FDA typically inspects manufacturing facilities every two years, and other regulators inspect manufacturing facilities as well. In complying with cGMP regulations, pharmaceutical manufacturers must expend resources and time to ensure compliance with product specifications as well as production, record keeping, quality control, reporting, and other requirements. We plan to seek suitable third-party manufacturing arrangements for the commercial production of a product candidate.
Employees
As of March 1, 2014, we employed 27 persons. A total of 4 of our employees hold Ph.D. or M.D. degrees. Approximately 20 employees are engaged in research and development, and 7 employees are engaged in finance, human resources, legal, and other administrative functions. Our workforce is non-unionized, and we believe that our relations with employees are good.
19
Segment and Geographic Information
For additional segment and geographic information, please See “Management’s Discussion and Analysis” under the heading “Overview” and Note 12, “Segment and Geographic Information”, to our consolidated financial statements for information about our operating segments and financial information about geographic areas.
Corporate and Available Information
We were incorporated in Delaware in 2000. Our principal executive offices are located at 245 First Street, Third Floor, Cambridge, Massachusetts 02142. We have one subsidiary: Zalicus Pharmaceuticals Ltd. Our Internet website is www.zalicus.com. We make available free of charge through our website our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Sections 13(a) and 15(d) of the Securities Exchange Act of 1934, as amended. We have made these reports available through our website at the same time that they become available on the Securities and Exchange Commission’s website. The public may read and copy any materials that we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet site (http://www.sec.gov) that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC.
Our code of conduct and ethics, corporate governance guidelines, and the charters of the Audit Committee, Compensation Committee and Nominating and Corporate Governance Committee are all available on the corporate governance section of our website at www.zalicus.com/investors. Stockholders may request a free copy of any of these documents by writing to Zalicus Inc., 245 First Street, Third Floor, Cambridge, Massachusetts 02142, Attn: Chief Financial Officer.
“Zalicus”, “cHTS”, “Chalice” and “Synavive” are trademarks of Zalicus or its subsidiaries. Other trademarks, service marks and trade names referred to in this Form 10-K are the property of their respective owners.
20
Risks Related to Discovery, Development and Commercialization of Drug Products
Zalicus’s approach to the discovery and clinical development of drugs is unproven and may never lead to commercially viable products.
Zalicus’s various approaches to drug discovery and development using our ion channel expertise or our cHTS technology platform are complex and unproven. Previously unrecognized or unexpected defects in or limitations to Zalicus’s drug discovery technologies or drug development strategies may emerge, which we may also be unable to overcome or mitigate. None of the product candidates identified or developed to date through the application of Zalicus’s business model and drug discovery technologies, has been effective in Phase 2 clinical trials, been approved by any regulatory agency for commercial sale or been commercialized.
All of our product candidates are in an early stage of development and their risk of failure is high. The data supporting Zalicus’s drug discovery and development programs, including the product candidate Z944, is derived from either laboratory and pre-clinical studies and limited early stage clinical trials that were not designed to be statistically significant. We cannot predict when or if any one of Zalicus’s product candidates will prove effective or safe in humans or will receive regulatory approval. If we are unable to discover or successfully develop drugs that are effective and safe in humans, we will not have a viable business.
Our future success depends on the successful development of product candidates, such as Z944, identified by our ion channel drug discovery technology. The scientific evidence to support the feasibility of developing drugs that modulate ion channels to treat pain conditions is limited, and many companies with more resources than us have not been able to successfully develop drugs that modulate ion channels to treat pain conditions.
For these and other reasons, Zalicus’s approach to drug discovery and development may not be successful and Zalicus’s current business model may not generate viable products or revenue. Even if Zalicus’s approach is theoretically viable, we may not complete the significant research and development or obtain the financial resources and personnel required to further develop and apply Zalicus’s discovery technology, advance promising product candidates into and through clinical trials, and obtain the regulatory approvals required for commercialization around the world.
We depend almost entirely on the success of one product candidate, Z944, which is still in Phase 1 clinical development. We cannot be certain that we will be able to obtain regulatory approval for, or successfully commercialize, Z944 or any of our other product candidates.
We currently have only one product candidate, Z944, in clinical development, and our business depends almost entirely on its successful clinical development, regulatory approval and commercialization. We currently have no drug products for sale and may never be able to develop marketable drug products. Z944, which we currently plan to evaluate a modified-release formation of in a Phase 1 pharmacokinetic and safety study in the first half of 2014, will require substantial additional clinical development, testing, and regulatory approval before we are permitted to commence its commercialization. Our other product candidates are still in pre-clinical development stages. None of our product candidates have advanced into a pivotal study, and it may be years before such a study is initiated, if ever. The clinical trials of our product candidates are, and the manufacturing and marketing of our product candidates will be, subject to extensive and rigorous review and regulation by numerous government authorities in the United States and in other countries where we intend to test and, if approved, market any product candidate. Before obtaining regulatory approvals for the commercial sale of any product candidate, we must successfully meet a number of critical developmental milestones, including:
| | • | | demonstrating through clinical trials that the product candidate is safe and effective in patients for the intended indication; |
| | • | | developing dosages that will be tolerated, safe and effective; |
| | • | | determining the appropriate delivery mechanism; |
21
| | • | | demonstrating that the product candidate formulation will be stable for commercially reasonable time periods; and |
| | • | | completing the development and scale-up to permit manufacture of our product candidates in commercial quantities and at acceptable prices. |
The time necessary to achieve these developmental milestones for any individual product candidate is long and uncertain, and we may not successfully complete these milestones for Z944 or any other product candidates that we may develop. We have not yet completed development of any product. We may not be able to finalize the design or formulation of any product candidate. In addition, we may select components, solvents, excipients or other ingredients to include in our product candidates that have not been previously approved for use in pharmaceutical products, which may require us to perform additional studies and may delay clinical testing and regulatory approval of our product candidates.
We are continuing to test and develop our product candidates and may explore possible design or formulation changes to address safety, efficacy, manufacturing efficiency and performance issues. We may not be able to complete development of any product candidates that will be safe and effective and that will have a commercially reasonable treatment and storage period. If we are unable to complete development of Z944, or any other product candidates that we may develop, we will not be able to commercialize and earn revenue from them.
Negative or inconclusive results from our product candidates may adversely affect our business and financial prospects.
We are planning to advance clinical development of our most advanced product candidate Z944 in a Phase 2 clinical study. Positive results from our prior preclinical assessments of Z944 and Phase 1b clinical trials of Z944 may not necessarily be predictive of the results from future Phase 2 clinical trials of Z944. Many companies in the pharmaceutical and biotechnology industries, including Zalicus, have suffered significant setbacks in clinical trials after achieving positive results in early stage development, and we cannot be certain that we will not face similar setbacks. These setbacks have been caused by, among other things, failure to achieve clinical trial endpoints with statistical significance or at all due to greater than expected placebo effects, insufficient statistical powering of clinical trials, selection of incorrect dosages of a product candidate to study, or selection of inappropriate indications or patient populations to study. Moreover, pre-clinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that believed their product candidates performed satisfactorily in pre-clinical studies and clinical trials nonetheless failed to obtain FDA approval for those product candidates. If we fail to produce positive results in our planned clinical trials of Z944, the development timeline and regulatory approval and commercialization prospects for our most advanced product candidate, and, correspondingly, our business and financial prospects, may be materially adversely affected.
We may not be able to initiate and complete clinical trials for our product candidates.
Conducting clinical studies for any of our product candidates requires finding appropriate clinical sites and clinical investigators, securing approvals for such studies from the independent review board at each such site and local regulatory authorities and enrolling sufficient numbers of patients on a timely basis. We may not be able to arrange for appropriate clinical trials for our product candidates, secure the necessary approvals or enroll the necessary number of participants on a timely basis. We cannot guarantee that outside clinical investigators conducting clinical trials will conduct them in compliance with applicable United States or foreign regulations or the relevant clinical trial protocol. Clinical sites may fail the FDA’s or other regulatory agencies’ inspections or reviews, and our trials could be halted for these or other reasons. Zalicus contracts with third-party clinical research organizations and other parties to conduct virtually all aspects of Zalicus’s Phase 2 and other Phase 1 clinical trials for Zalicus’s product candidates, including Z944. These organizations may not adequately or completely perform their contractual obligations regarding the clinical trials, or may not diligently or completely perform their tasks with respect to clinical trials under their supervision. As a result of these risks, our clinical
22
trials may be extended, delayed or terminated, which could delay the receipt of clinical results for our product candidates, which could delay, impede or stop the development, regulatory approval or successful commercialization of our product candidates.
We may not be able to create commercially viable pharmaceutical formulations of our product candidates.
We have developed or are developing proprietary formulations of our product candidate Z944. Developing such proprietary formulations is costly and difficult, and we have limited experience in developing formulations ourselves. We are relying on and expect to rely on third-party suppliers to develop and test the pharmaceutical formulations of Zalicus’s product candidates as well as manufacture materials for clinical trials of our product candidates. These third parties may not be successful in developing or manufacturing these novel formulations of our product candidates or may experience delays in doing so that could delay clinical trials, and ultimately our ability to obtain approval of Zalicus’s product candidates, including our product candidate and Z944. Defects in the formulation, delivery method or packaging of any of Zalicus’s product candidates could delay our ability to conduct clinical trials or require us to repeat clinical trials using a revised formulation, delivery method or packaging. If we are unsuccessful in creating commercially viable formulations, delivery methods or packaging, we may never generate product revenue or be profitable.
We may be unable to find safe and effective doses for Zalicus’s product candidates without extensive clinical trials and substantial additional costs, if at all.
We must select the doses, including the amount, frequency and duration, of the active pharmaceutical ingredients included in Zalicus’s product candidates. Our clinical trials in humans may show that the doses we select based on Zalicus’s in vitro screening, animal testing or early clinical trials do not achieve the desired therapeutic effect in humans, or achieve this effect only in a small part of the population. Even if the doses we select show efficacy in humans, the resulting doses of active pharmaceutical ingredients may not have acceptable safety profiles for targeted indications. Furthermore, even if we believe that pre-clinical and clinical studies adequately demonstrate that the doses we select for Zalicus’s product candidates are safe and effective in humans, the FDA or other regulatory agencies in foreign jurisdictions may conclude that the clinical trials do not support this conclusion. We may be required to conduct additional clinical studies and provide more evidence substantiating the safety and effectiveness of the doses selected in a significant patient population. If we need to adjust the doses of our product candidates, we may need to conduct additional clinical trials. We may also be required to make different doses available for different types of patients. All of this may result in significant delays and additional costs or prevent commercialization of Zalicus’s product candidates.
We may fail to select or capitalize on the most scientifically, clinically or commercially promising or profitable product candidates.
We may make incorrect determinations as to which product candidates should proceed to initial clinical trials, later stage clinical development and potential commercialization. Our decisions to allocate finite research, management and financial resources toward particular product candidates or therapeutic areas may not lead to the development of viable commercial products and may divert resources from better opportunities. Similarly, our decisions to delay or terminate drug development programs may also be incorrect and could cause us to miss valuable opportunities.
If we undertake business combinations, acquisitions or similar strategic transactions, they may dilute stockholder value, cause us to incur increased transaction costs, disrupt our business, divert management’s attention or be difficult to integrate.
On a regular basis, we consider various business combination transactions, collaborations, license agreements and strategic transactions with third parties, including transactions which may result in us acquiring,
23
or being acquired by, a third party. The consummation or performance of any future business combination, collaboration or strategic transaction may involve risks, such as:
| | • | | a change of control of our company if a transaction results in us being acquired by a third party; |
| | • | | additional dilution to our existing stockholders if we use our common stock as consideration for any acquisitions; |
| | • | | higher than expected transaction costs; |
| | • | | diversion of managerial resources from day-to-day operations; |
| | • | | challenges associated with integrating acquired technologies and operations of acquired companies; |
| | • | | exposure to unforeseen liabilities; |
| | • | | misjudgment with respect to value, return on investment or strategic fit; and |
| | • | | difficulties in the assimilation of different cultures and practices, as well as in the assimilation and retention of broad and geographically dispersed personnel and operations;. |
As a result of these risks, we may not be able to achieve the expected benefits of any such strategic transaction. If we are unsuccessful in completing, including because we are unable to obtain any required stockholder approval, or integrating any acquisition, we may be required to reevaluate that component of our strategy only after we have incurred substantial expenses and devoted significant management time and resources in seeking to complete and integrate the acquisition. In addition, even if we successfully divest some or all of our assets in a strategic transaction with a third party, the price paid by the third party for those assets may be at a significant discount compared to their potential value if we had instead developed and commercialized such assets ourselves.
A material component of our business strategy is to establish and maintain collaborative relationships to fund research, development and commercialization of product candidates by us or by our collaborators. If we or any collaborator terminates or fails to perform any obligations under our collaboration agreements, the development and commercialization of product candidates under these agreements could be delayed or terminated.
A material component of our business strategy is to establish and maintain collaborative arrangements with pharmaceutical and biotechnology companies and to fund research development and commercialization of drug products for the treatment of diseases. We have established collaborative royalty and milestone-based agreements with Sanofi for Prednisporin and funded discovery research agreements with Novartis and others. If any of our product candidates continue to advance through preclinical or clinical development, we intend to continue to seek collaborative relationships to obtain discovery or clinical development funding and expertise, as well as domestic or international sales, marketing and distribution capabilities.
The process of establishing collaborative relationships is difficult, time-consuming and involves significant uncertainty. Moreover, it may be difficult to maintain or perform under collaboration arrangements, as our funding resources may be limited or our collaborators may seek to renegotiate or terminate their relationships due to unsatisfactory research or clinical results, changes in the competitive landscape for a particular therapeutic area, a change in business strategy, a change of control or other reasons.
If we or any collaborator fails to fulfill any responsibilities in a timely manner, or at all, our research, clinical development or commercialization efforts related to that collaboration could be delayed or terminated. Additionally it may become necessary for us to assume responsibility for activities that would otherwise have been the responsibility of our collaborator. Further, if we are unable to establish and maintain collaborative relationships on acceptable terms, we may have to delay or discontinue further development of one or more of our product candidates, undertake development and commercialization activities at our own expense or find alternative sources of funding.
24
Our collaborations typically involve a complex allocation of responsibilities, costs and benefits and provide for milestone payments to us upon the achievement of specified clinical and regulatory milestones. Our collaborations also may provide us with royalty-based revenue if product candidates are successfully commercialized. Under the Sanofi, Novartis and other collaborations, we will rely on our collaborators to provide resources to develop new product candidates and to potentially achieve these milestones and commercialize any new products. We may not be able to achieve any of the milestones provided in the Novartis, Sanofi or other collaboration agreements or derive any license or royalty revenue with respect to our collaborations.
We have no sales or distribution capabilities and may not obtain the collaboration, development, commercialization, manufacturing or other third-party relationships required to develop, commercialize and manufacture some or all of our product candidates.
We have no sales or distribution capabilities and lack many of the internal resources, capabilities and experience necessary to clinically develop, formulate, manufacture, test, market and sell pharmaceuticals. As a result, to succeed in our business plan, we will be dependent on the efforts of third parties. We depend on collaborators, licensees, clinical research organizations and other third parties to formulate product candidates and to conduct clinical trials for all of our product candidates. We also rely on third-party manufacturers to manufacture all clinical trial supplies of our product candidates.
Our third-party manufacturers may encounter difficulties performing their obligations in a timely manner and in accordance with applicable governmental regulations, including problems involving: inconsistent production yields; poor quality control and assurance or inadequate process controls; and lack of compliance with regulations set forth by the FDA or other foreign regulatory agencies. We typically engage only a single contract manufacturer to make any product candidate which can exacerbate the impact of any such difficulties.
We expect to be able to develop and commercialize many of our product candidates only with the participation of pharmaceutical or biotechnology company collaborators or by out-licensing rights to the product candidates. Pharmaceutical and biotechnology companies and others may be reluctant to collaborate with Zalicus or to license rights to Zalicus’s product candidates due to the unproven nature of Zalicus’s drug discovery and development approach, concerns regarding the pricing of and reimbursement for Zalicus’s product candidates if they are successfully developed, or other factors.
We cannot guarantee that we will be able to successfully negotiate agreements for relationships with collaborators, partners, licensees, clinical investigators, manufacturers and other third parties on favorable terms, if at all. If we are unable to obtain these agreements, we may not be able to clinically develop, formulate, manufacture, test, obtain regulatory approvals for or commercialize our product candidates. We expect to expend substantial funds and management time and effort to enter into relationships with these third parties and, if we successfully enter into such relationships, to manage these relationships. However, we cannot control the amount or timing of resources our contract partners will devote to our research and development programs, product candidates or potential product candidates, and we cannot guarantee that these parties will succeed in a timely fashion, if at all.
We may not be able to gain market acceptance of our product candidates, which would prevent us from becoming profitable.
We cannot be certain that any of our product candidates, if approved, will gain market acceptance among physicians, patients, healthcare payors, pharmaceutical companies or others. Demonstrating the safety and efficacy of our product candidates and obtaining regulatory approvals will not guarantee future revenue. Sales of medical products largely depend on the reimbursement of patients’ medical expenses by government healthcare programs and private health insurers. Governments and private insurers closely examine medical products to determine whether they should be covered by reimbursement and if so, the level of reimbursement that will apply. We cannot be certain that third-party payors will sufficiently reimburse sales of our products, or enable us
25
to sell our products, if approved, at profitable prices. Sales of medical products also depend on physicians’ willingness to prescribe the treatment, which is likely to be based on a determination by these physicians that the products are safe, therapeutically effective and cost-effective. We cannot predict whether physicians, other healthcare providers, government agencies or private insurers will determine our products are safe, therapeutically effective and cost effective relative to competing treatments.
Disputes under key agreements could delay or prevent development or commercialization of our product candidates.
Any agreements we have or may enter into with third parties, such as collaboration, license, formulation supplier, manufacturing, testing, clinical research organization or clinical trial agreements, may give rise to disputes regarding the rights and obligations of the parties to such agreements. Disagreements could develop over rights to ownership or use of intellectual property, the scope and direction of research and development, the approach for regulatory approvals or commercialization strategy. We intend to conduct research programs in a range of therapeutic areas, but our pursuit of these opportunities could result in conflicts with the other parties to these agreements who may be developing or selling pharmaceuticals or conducting other activities in these same therapeutic areas. Any disputes or commercial conflicts could lead to the termination of these agreements, delay progress of our product development programs, compromise our ability to renew agreements or obtain future agreements, lead to the loss of intellectual property rights or result in costly litigation.
Risks Related to Financial Results, Need for Additional Financing and Debt Arrangements
We may be unable to raise the substantial additional capital that we will need to sustain our operations.
We will need substantial additional funds to support our planned operations. Based on current operating plans, we expect our resources to be sufficient to fund our operations into the first half of 2015. We may, however, raise additional funds before that time if our research and development expenses exceed current expectations or our collaboration funding is less than current assumptions or expectations. This could occur for many reasons, including:
| | • | | our product candidates require more extensive clinical or pre-clinical testing, clinical trials take longer to complete than we currently expect or our clinical trials are not successful; |
| | • | | we advance more of our product candidates than expected into costly later stage clinical trials; |
| | • | | we advance more of our pre-clinical product candidates than expected into early stage clinical trials; |
| | • | | our revenue generating collaboration agreements are terminated; |
| | • | | we determine or are required to conduct more discovery research than expected to develop additional product candidates; |
| | • | | some or all of our product candidates fail in clinical or pre-clinical studies or prove to be less commercially promising than we expect or we are forced to seek additional product candidates; |
| | • | | we are required, or consider it advisable, to acquire or license rights from one or more third parties; |
| | • | | we determine to enter into a business combination or acquire or license rights to additional product candidates or new technologies. |
While we expect to seek additional funding through public or private financings, we may not be able to obtain financing on acceptable terms, or at all. In addition, the terms of any financings may be dilutive to, or otherwise adversely affect, holders of Zalicus common stock. We may also seek additional funds through arrangements with collaborators or others. These arrangements would generally require us to relinquish rights to some of our technologies, product candidates or products, and we may not be able to enter into such agreements on acceptable terms, if at all. The arrangements also may include issuances of equity, which may also be dilutive to, or otherwise adversely affect, holders of Zalicus common stock. There can be no assurance that we will be
26
able to access equity or credit markets in order to finance our operations or expand development programs for any of our product candidates, or that there will not be a further deterioration in financial markets and confidence in economies. We may also have to scale back or further restructure our operations. If we are unable to obtain additional funding on a timely basis, we may be required to curtail or terminate some or all of our research or development programs.
Zalicus has a history of operating losses. We expect to incur significant operating losses and may never be profitable. Zalicus common stock is a highly speculative investment.
Zalicus commenced operations in March 2000 and has no approved products of its own and has generated no direct product revenue. Zalicus has incurred operating losses since Zalicus’s inception in 2000. As of December 31, 2013, Zalicus had an accumulated deficit of $379.6 million. Our cash and cash equivalents are sufficient to fund operations into the first half of 2015. We have spent, and expect to continue to spend, significant resources to fund research and development of our product candidates and to enhance our drug discovery technologies. We expect to incur substantial operating losses over the next several years due to our ongoing research, development, pre-clinical testing, and clinical trial activities. As a result, our accumulated deficit will continue to increase.
Our product candidates are in the early stages of development and may never result in any revenue. We will not be able to generate product revenue unless and until one of our product candidates successfully completes clinical trials and receives regulatory approval. We may seek to obtain revenue from collaboration or licensing agreements with third parties. Our current collaboration and license agreements may not provide us with material, sustainable ongoing future revenue, and we may not be able to enter into additional collaboration agreements. Even if we eventually generate product revenues, we may never be profitable, and if we ever achieve profitability, we may not be able to sustain it.
Risks Related to Regulatory Approvals
The regulatory approval process is costly and lengthy and we may not be able to successfully obtain all required regulatory approvals.
The pre-clinical development, clinical trials, manufacturing, marketing, testing and labeling of pharmaceuticals and medical devices are all subject to extensive regulation by numerous governmental authorities and agencies in the United States and other countries. We or our collaborators must obtain regulatory approval for product candidates before marketing or selling any of them. The approval process is typically lengthy and expensive, and approval is never certain. It is not possible to predict how long the approval processes of the FDA or any other applicable federal or foreign regulatory authority or agency for any of our products will take or whether any such approvals ultimately will be granted. The FDA and foreign regulatory agencies have substantial discretion in the drug approval process, and positive results in pre-clinical testing or early phases of clinical studies offer no assurance of success in later phases of the approval process. Generally, pre-clinical and clinical testing of products can take many years and require the expenditure of substantial resources, and the data obtained from these tests and trials can be susceptible to varying interpretations that could delay, limit or prevent regulatory approval. Any delay in obtaining, or failure to obtain, approvals could prevent or adversely affect the marketing of our products or our collaborator’s products and our ability to generate product revenue. The risks associated with the approval process include delays or rejections in the regulatory approval process based on the failure of clinical or other data to meet expectations, or the failure of the product or medical device to meet a regulatory agency’s requirements for safety, efficacy and quality. In addition, regulatory approval, if obtained, may significantly limit the indicated uses for which a product may be marketed.
We or our collaborators may delay, suspend or terminate clinical trials to obtain marketing authorization of any of our product candidates or products at any time for reasons including:
| | • | | ongoing discussions with the FDA or comparable foreign authorities regarding the scope or design of clinical trials; |
27
| | • | | delays or the inability to obtain required approvals from institutional review boards or other governing entities at clinical sites selected for participation in our clinical trials; |
| | • | | delays in enrolling patients and volunteers into clinical trials; |
| | • | | lower than anticipated retention rates of patients and volunteers in clinical trials; |
| | • | | the need to repeat clinical trials as a result of inconclusive or negative results or poorly executed testing; |
| | • | | lack of effectiveness of a product candidate in other clinical trials; |
| | • | | lack of sufficient funds for further clinical development; |
| | • | | insufficient supply or deficient quality of product candidate materials or other materials necessary to conduct clinical trials; |
| | • | | unfavorable regulatory inspection of a manufacturing, testing, labeling or packaging facility for drug substance or drug product; |
| | • | | unfavorable regulatory inspection and review of a clinical or pre-clinical trial site or records of any clinical or pre-clinical investigation; |
| | • | | serious and unexpected drug-related side effects or serious adverse safety events experienced by participants in clinical trials or by patients following commercialization; or |
| | • | | the placement of a clinical hold on a product candidate in an ongoing clinical trial. |
Positive or timely results from pre-clinical studies and early clinical trials do not ensure positive or timely results in late stage clinical trials or product approval by the FDA or any other regulatory authority. Product candidates that show positive pre-clinical or early clinical results often fail in later stage clinical trials. Data obtained from pre-clinical and clinical activities is susceptible to varying interpretations, which could delay, limit, or prevent regulatory approvals.
We may not be able to conduct clinical trials at preferred sites, enlist clinical investigators, enroll sufficient numbers of patients, or begin or successfully complete clinical trials in a timely fashion, if at all. Any failure to perform may delay or terminate the trials. Our current clinical trials may be insufficient to demonstrate that our potential products are active, safe, or effective and as a result we may decide to abandon further development of such product candidates. Additional clinical trials may be required if clinical trial results are negative or inconclusive, which will require us to incur additional costs and significant delays. If we do not receive the necessary regulatory approvals, we will not be able to generate product revenues and will not become profitable. We may encounter significant delays in the regulatory process that could result in excessive costs that may prevent us from continuing to develop our product candidates. In addition, the failure to comply with applicable regulatory requirements may result in criminal prosecution, civil penalties, product recalls, withdrawal of product approval, mandatory restrictions or other actions that could impair our ability to conduct our business.
Even if we receive regulatory approvals for marketing Zalicus’s product candidates, if we fail to comply with continuing regulatory requirements, we could lose regulatory approvals, and our business would be adversely affected.
The FDA and other regulatory authorities continue to review therapeutic products even after they receive initial approval. If we or our collaborators receive approval to commercialize any product candidates, the manufacturing, testing, marketing, sale and distribution of these drugs will be subject to continuing regulation, including compliance with quality systems regulations, good manufacturing practices, adverse event reporting requirements and prohibitions on promoting a product for unapproved uses. Furthermore, heightened Congressional scrutiny on the adequacy of the FDA’s drug approval process and the agency’s efforts to assure the safety of marketed drugs has resulted in the enactment of legislation, the FDA Amendments Act of 2007, addressing, among other things, drug safety issues. This law provides the FDA with expanded authority over
28
drug products after approval, including the authority to require post-approval studies and clinical trials, labeling changes based on new safety information, and compliance with Risk Evaluation and Mitigation Strategies, or REMS, approved by the FDA. The FDA’s exercise of this authority could result in delays or increased costs during the period of product candidate development, clinical trials and regulatory review and approval, increased costs to assure compliance with new post-approval regulatory requirements, and potential restrictions on the sale of approved products, which could lead to lower product revenues to us or our collaborators. Enforcement actions resulting from failure to comply with government requirements could result in fines, suspension of approvals, withdrawal of approvals, recalls of products, product seizures, operating restrictions, and civil or criminal penalties. These enforcement actions could affect the manufacturing, testing, marketing, sale and distribution of our products.
FDA approval of our drug candidates would subject Zalicus and our collaborators to ongoing FDA obligations and continued regulatory review, such as continued safety reporting requirements, and we and our collaborators may also be subject to additional FDA post-marketing obligations or new regulations, all of which may result in significant expense and limit our ability to commercialize our drug candidates.
Any regulatory approvals that we or our collaborators receive for our drug candidates may be subject to limitations on the indicated uses for which the drug may be marketed or contain requirements for potentially costly post-marketing follow-up studies. In addition, the labeling, packaging, adverse event reporting, storage, advertising, promotion and record keeping for any drug candidate the FDA may approve, is subject to extensive regulatory requirements. The subsequent discovery of previously unknown problems with a drug, including but not limited to adverse events of unanticipated severity or frequency, or the discovery that adverse events previously observed in pre-clinical research or clinical trials that were believed to be minor actually constitute much more serious problems, may result in restrictions on the marketing of the drug, and could include withdrawal of the drug from the market.
The FDA’s policies may change, and additional government regulations may be enacted that could prevent or delay regulatory approval of our drug candidates or may adversely impact the commercialization of our product candidates. If we are not able to maintain regulatory compliance, including compliance with a required REMS program, we may be subject to fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution. Any of these events could prevent us from marketing our drug candidates and our business could suffer accordingly. We cannot predict the likelihood, nature or extent of adverse government regulation that may arise from future legislation or administrative action, either in the United States or abroad.
Legislative or regulatory reform of the health care system in the United States and foreign jurisdictions may affect our ability to profitably sell our products, if approved.
Our and our collaborators’ ability to commercialize our future products successfully will depend in part on the extent to which reimbursement for the products will be available from government and health administration authorities, private health insurers and other third-party payors. The continuing efforts of the United States and foreign governments, insurance companies, managed care organizations and other payors of health care services to contain or reduce health care costs may adversely affect our ability to set prices for our products which we believe are fair, and our ability to generate revenues and achieve and maintain profitability.
Specifically, in both the United States and some foreign jurisdictions, there have been a number of legislative and regulatory proposals to change the health care system in ways that could affect our ability to sell our products profitably. The Affordable Health Choices Act was enacted in 2009 and Congress is considering a number of proposals that are intended to reduce or limit the growth of health care costs and which could significantly transform the market for pharmaceuticals products. We expect further federal and state proposals and health care reforms to continue to be proposed by legislators, which could limit the prices that can be charged for the products we develop and may limit our commercial opportunity. In the United States, the
29
Medicare Prescription Drug, Improvement, and Modernization Act of 2003, also called the Medicare Modernization Act, or MMA, changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on average sales prices for drugs. In addition, this legislation provided authority for limiting the number of drugs that will be covered in any therapeutic class. As a result of this legislation and the expansion of federal coverage of drug products, we expect that there will be additional pressure to contain and reduce costs. These cost reduction initiatives and other provisions of this legislation could decrease the coverage and price that we receive for any approved products and could seriously harm our business. While the MMA applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates, and any reduction in reimbursement that results from the MMA may result in a similar reduction in payments from private payors.
The continuing efforts of government and other third-party payors to contain or reduce the costs of health care through various means may limit our commercial opportunity. It will be time consuming and expensive for us or our partners to go through the process of seeking reimbursement from Medicare and private payors. Our product candidates may not be considered cost effective, and government and third-party private health insurance coverage and reimbursement may not be available to patients for any of our future products or sufficient to allow us or our collaborators to sell our products on a competitive and profitable basis. Our results of operations could be adversely affected by the MMA and additional prescription drug coverage legislation, by the possible effect of this legislation on amounts that private insurers will pay and by other health care reforms that may be enacted or adopted in the future. In addition, increasing emphasis on managed care in the United States will continue to put pressure on the pricing of pharmaceutical products. Cost control initiatives could decrease the price that we or any potential collaborators could receive for any of our future products and could adversely affect our profitability.
In some foreign countries, including major markets in the European Union and Japan, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take 6 to 12 months or longer after the receipt of regulatory marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we or our collaborators may be required to conduct a clinical study that compares the cost-effectiveness of our product candidates to other available therapies. Such pharmacoeconomic studies can be costly and the results uncertain. Our business could be harmed if reimbursement of our or our collaborators’ products are unavailable or limited in scope or amount or if pricing is set at unsatisfactory levels.
Federal laws or regulations on drug importation could make lower cost versions of our future products available, which could adversely affect our revenues, if any.
The prices of some drugs are lower in other countries than in the United States because of government regulation and market conditions. Under current law, importation of drugs into the United States is generally not permitted unless the drugs are approved in the United States and the entity that holds that approval consents to the importation. Various proposals have been advanced to permit the importation of drugs from other countries to provide lower cost alternatives to the products available in the United States. In addition, the MMA requires the Secretary of Health and Human Services to promulgate regulations for drug reimportation from Canada into the United States under some circumstances, including when the drugs are sold at a lower price in Canada than in the United States.
If the laws or regulations are changed to permit the importation of drugs into the United States in circumstances not now permitted, such a change could have an adverse effect on our business by making available lower priced alternatives to our future products. Failure to obtain regulatory and pricing approvals in foreign jurisdictions could delay or prevent commercialization of our products abroad.
If we succeed in developing any products, we intend to market them in the European Union and other foreign jurisdictions. In order to do so, we must obtain separate regulatory approvals and comply with numerous
30
and varying regulatory requirements. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval abroad may differ from that required to obtain FDA approval. The foreign regulatory approval process may include all of the risks associated with obtaining FDA approval. We may not obtain foreign regulatory approvals on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or by the FDA. We and our collaborators may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize our products in any market outside the United States. The failure to obtain these approvals could materially adversely affect our business, financial condition and results of operations. Even if we are successful at obtaining these approvals, regulatory agencies in foreign countries, where the pricing of prescription drugs is controlled by the government, could determine pricing for Zalicus’s products in a manner adverse to us.
Risks Related to Intellectual Property
Our success depends upon our ability to obtain and maintain intellectual property protection for our products and technologies.
Our success will depend on our ability to obtain and maintain adequate protection of our intellectual property, including our proprietary products or product candidates. We intend to apply for patents with claims covering our products, product candidates, technologies and processes, when and where we deem it appropriate to do so and plan to take other steps to protect our intellectual property. We have applied for patent protection covering our clinical and pre-clinical product candidates in the United States, and some, but not all, foreign countries. In countries where we have not and do not seek patent protection, third parties may be able to manufacture and sell our products without our permission, and we may be unable to stop them from doing so.
Similar to other biotechnology companies, our patent position is generally uncertain and involves complex legal and factual questions. In addition, the laws of some countries do not protect proprietary rights to the same extent as the laws of the United States, and other biotechnology companies have encountered significant problems in protecting and defending their proprietary rights in non-United States jurisdictions. Whether filed in the United States or abroad, our patent applications may be challenged or may fail to result in issued patents. In addition, our existing patents and any future patents may not be sufficiently broad to prevent others from practicing our technologies or from developing or commercializing competing products. Furthermore, others may independently develop or commercialize similar or alternative technologies or drugs, or design around our patents. Our patents may be challenged, invalidated or fail to provide any competitive advantages.
The United States Patent and Trademark Office and similar agencies in foreign jurisdictions may not agree with our view that our combination product candidates are patentable or novel and non-obvious, and on this basis may deny patent protection. Even if we receive patent protection, others, may attempt to invalidate our patent or trade secret rights. Even if our patent or trade secret rights are not directly challenged, disputes among third parties could lead to the weakening or invalidation of intellectual property rights.
If we do not obtain or are unable to maintain adequate patent or trade secret protection for products in the United States, competitors could duplicate them without repeating the extensive testing that we will be required to undertake to obtain approval of the products by the FDA and other regulatory authorities. Regardless of any patent protection, under the current statutory framework the FDA is prohibited by law from approving any generic version of any of Zalicus’s product candidates for five years after it has approved Zalicus’s product candidates. Upon the expiration of that period, or if that time period is altered, the FDA could approve a generic version of Zalicus’s product unless we have patent protection sufficient to enforce our rights. Without sufficient patent protection, the applicant for a generic version of Zalicus’s product would be required only to conduct a relatively inexpensive study to show that its product is bioequivalent to Zalicus’s product and would not have to repeat the studies that we conducted to demonstrate that the product is safe and effective. In the absence of adequate patent protection in other countries, competitors may similarly be able to obtain regulatory approval of products that duplicate Zalicus’s products.
31
We may not be able to develop or commercialize our product candidates due to intellectual property rights held by third parties.
If a third party holds a patent to a formulation technology related to Zalicus’s planned formulation of a product candidate, we may not be able to develop or commercialize such product candidates without first obtaining a license to such patent, or waiting for the patent to expire. Our business will be harmed if we are unable to use the optimal formulation of our product candidates. This may occur because the formulations or methods of use are covered by one or more third-party patents, and a license to such patents is unavailable or is only available on terms that are unacceptable.
Confidentiality agreements with employees and others may not adequately prevent disclosure of trade secrets and other proprietary information.
In our activities, we rely substantially upon proprietary materials, information, trade secrets and know-how to conduct our research and development activities, and to attract and retain collaborators, licensees and customers. We take steps to protect our proprietary rights and information, including the use of confidentiality and other agreements with our employees and consultants and in our academic and commercial relationships.
However, these steps may be inadequate, agreements may be violated, or there may be no adequate remedy available for a violation of an agreement. Our proprietary information may be inadvertently disclosed or we may lose the protection of our trade secrets. Our competitors may independently develop substantially equivalent proprietary information or may otherwise gain access to our trade secrets, which could adversely affect our ability to compete in the market.
Litigation or third-party claims of intellectual property infringement could require substantial time and money to resolve. Unfavorable outcomes in these proceedings could limit our intellectual property rights and our activities.
We may need to resort to litigation to enforce or defend our intellectual property rights, including any patents issued to us. If a competitor or collaborator files a patent application claiming technology also invented by us, in order to protect our rights, we may have to participate in an expensive and time consuming interference proceeding before the United States Patent and Trademark Office. We cannot guarantee that our product candidates will be free of claims by third parties alleging that we have infringed their intellectual property rights. Third parties may assert that we are employing their proprietary technologies without authorization and they may resort to litigation to attempt to enforce their rights. Third parties may have or obtain patents in the future and claim that the use of our technology or any of our product candidates infringes their patents. We may not be able to develop or commercialize product candidates because of patent protection others have. Our business will be harmed if we cannot obtain a necessary or desirable license, can obtain such a license only on terms we consider to be unattractive or unacceptable, or if we are unable to redesign our product candidates or processes to avoid actual or potential patent or other intellectual property infringement.
Our efforts to obtain, protect and defend our patent and other intellectual property rights, whether we are successful or not, may require us to incur substantial costs, including the diversion of management and technical personnel. An unfavorable ruling in patent or intellectual property litigation could subject us to significant liabilities to third parties, require us to cease developing, manufacturing or selling the affected products or using the affected processes, require us to license the disputed rights from third parties, or result in awards of substantial damages against us. In addition, defending patent or other intellectual property litigation, whether we are successful or not, can be very expensive and may require us to incur substantial costs, including the diversion of management and technical personnel. During the course of any patent litigation, there may be public announcements of the results of hearings, motions, and other interim proceedings or developments in the litigation. If securities analysts or investors regard these announcements as negative, the market price of Zalicus common stock may decline. General proclamations or statements by key public figures may also have a negative impact on the perceived value of our intellectual property.
32
There can be no assurance that we would prevail in any intellectual property infringement action, will be able to obtain a license to any third-party intellectual property on commercially reasonable terms, successfully develop non-infringing alternatives on a timely basis, or license non-infringing alternatives, if any exist, on commercially reasonable terms. Any significant intellectual property impediment to our ability to develop and commercialize our products could seriously harm our business and prospects.
Risks Related to the Biotechnology and Pharmaceutical Industry
Our industry is highly competitive and our competitors and potential competitors may develop products and technologies that make ours less attractive or obsolete.
The development and commercialization of pharmaceutical products is highly competitive. Many companies, universities, and research organizations developing competing product candidates have greater resources and significantly greater experience in research and development, formulation, manufacturing, marketing, sales, distribution, financial and technical regulatory matters than we have. In addition, many competitors have greater name recognition and more extensive collaborative relationships. Our competitors could commence and complete clinical testing of their product candidates, obtain regulatory approvals, and begin commercial-scale manufacturing of their products faster than we are able to for our products. They could develop drug discovery technologies or products that would render Zalicus’s drug discovery technologies and our product candidates, and those of our collaborators, obsolete and noncompetitive.
Zalicus’s drug discovery technologies compete against well-established techniques to discover new drugs. If we are unable to compete effectively against these existing techniques and the companies that support them, then we may not be able to commercialize our product candidates or achieve a competitive position in the market. In addition, any product candidates that we do discover will face competition from existing pharmaceuticals. Our competitors’ success is discovering new drugs and marketing existing drugs which compete with our product candidates would adversely affect our ability to generate revenues.
Our competitors and our cHTS collaborators already have high throughput screening technologies and if they employ these technologies to discover combination drugs, they may render Zalicus’s technologies or Zalicus’s approach to combination drug discovery and development obsolete or noncompetitive, which could materially affect the revenue generated by our cHTS business.
We may have significant product liability exposure which may harm our business and our reputation.
We face exposure to product liability and other claims if our product candidates, products or processes are alleged to have caused harm. These risks are inherent in the testing, manufacturing, and marketing of human therapeutic products and medical devices. We maintain product liability insurance covering our clinical trials of our product candidates. We may not have sufficient insurance coverage, and we may not be able to obtain sufficient coverage at a reasonable cost, if at all. Our inability to obtain product liability insurance at an acceptable cost or to otherwise protect against potential product liability claims could prevent or inhibit the commercialization of any products or product candidates that we develop. If we are sued for any injury caused by our products, product candidates or processes, our liability could exceed our product liability insurance coverage and our total assets. Claims against us, regardless of their merit or potential outcome, may also divert significant management time and resources, generate negative publicity or hurt our ability to obtain physician endorsement of our products or expand our business.
We use and generate materials that may expose us to expensive and time-consuming legal claims.
Our development programs involve the use of hazardous materials, chemicals, and biological materials. We are subject to foreign, federal, state and local laws and regulations governing the use, manufacture, storage, and disposal of materials and waste products. We believe that our safety procedures for handling these materials comply with the standards prescribed by laws and regulations. However, we may incur significant costs to
33
comply with current or future environmental laws and regulations. In addition, we cannot completely eliminate the risk of contamination or injury from hazardous materials. In the event of an accident, an injured party may seek to hold us liable for any damages that result. Any liability could exceed the limits or fall outside the coverage of our insurance, and we may not be able to maintain insurance on acceptable terms, if at all.
Risks Related to an Investment in Our Common Stock
Our recently completed 1-for-6 stock split could adversely impact our company and the trading of our common stock.
On October 3, 2013, we filed a certificate of amendment to our Sixth Amended and Restated Certificate of Incorporation with the Secretary of State of the State of Delaware to effect a 1-for-6 reverse stock split of our common stock. While the reverse stock split enabled us to regain compliance with The NASDAQ Capital Market’s minimum bid price listing requirement, it may also result in certain adverse impacts to our company and the trading of our common stock. Additionally, the liquidity of our common stock could be adversely affected by the reduced number of shares resulting from the reverse stock split, which, in turn, could result in greater volatility in the price per share of our common stock. As a result and notwithstanding our reverse stock split and our regained compliance with the NASDAQ Capital Market’s minimum bid price listing requirement, we may not be able to maintain a price per share of our common stock in excess of $1.00 per share or the additional criteria for continued listing of our common stock set forth by The NASDAQ Capital Market. The occurrence of any future non-compliance with the NASDAQ Capital Market’s minimum bid price or other listing requirements may have a material adverse effect on our stock price, our business and our prospects.
Our common stock has a volatile public trading price.
The market price for our common stock has been volatile, and market prices for securities of companies comparable to us have been highly volatile. In addition, the stock market as a whole and biotechnology and other life science stocks in particular have experienced significant recent price volatility. Like our common stock, these stocks have experienced significant price and volume fluctuations for reasons unrelated to the operating performance of the individual companies. Factors giving rise to this volatility may include:
| | • | | disclosure of actual or potential clinical or preclinical results with respect to product candidates we are developing, including Z944; |
| | • | | disclosure by our collaboration partners, including Sanofi and Novartis regarding our collaborations and the related product candidates, including Prednisporin; |
| | • | | regulatory developments in both the United States and abroad; |
| | • | | developments concerning proprietary rights, including patents and litigation matters; |
| | • | | disclosure of new collaborations or other strategic transactions; |
| | • | | public concern about the safety or efficacy of our product candidates or technology, their components, or related technology or new technologies generally; |
| | • | | public announcements by our competitors or others regarding new products or new product candidates; |
| | • | | general market conditions and comments by securities analysts and investors; and |
| | • | | our recent 1-for-6 reverse stock split. |
Fluctuations in our operating losses could adversely affect the price of our common stock.
Our operating losses may fluctuate significantly on a quarterly basis. Some of the factors that may cause our operating losses to fluctuate on a period-to-period basis include the status of our clinical and pre-clinical development programs, level of expenses incurred in connection with our clinical and pre-clinical development programs, restructuring costs, implementation or termination of collaboration, licensing, manufacturing or other
34
material agreements with third parties, non-recurring revenue or expenses under any such agreement, and compliance with regulatory requirements. Period-to-period comparisons of our historical and future financial results may not be meaningful, and investors should not rely on them as an indication of future performance. Our fluctuating losses may fail to meet the expectations of securities analysts or investors. Our failure to meet these expectations may cause the price of our common stock to decline.
Anti-takeover provisions in our charter documents and provisions of Delaware law may make an acquisition more difficult and could result in the entrenchment of management.
We are incorporated in Delaware. Anti-takeover provisions of Delaware law and our charter documents may make a change in control or efforts to remove management more difficult. Also, under Delaware law, our board of directors may adopt additional anti-takeover measures. The existence of the following provisions of Delaware law and our sixth amended and restated charter, as amended, or our amended and restated bylaws could limit the price that investors might be willing to pay in the future for shares of our common stock.
Our charter authorizes our board of directors to issue up to 5,000,000 shares of preferred stock and to determine the terms of those shares of stock without any further action by our stockholders. If the board of directors exercises this power to issue preferred stock, it could be more difficult for a third party to acquire a majority of our outstanding voting stock and vote the stock they acquire to remove management or directors.
Our charter also provides staggered terms for the members of our board of directors. Under Section 141 of the Delaware General Corporation Law and our charter, our directors may be removed by stockholders only for cause and only by vote of the holders of 75% of voting shares then outstanding. These provisions may prevent stockholders from replacing the entire board in a single proxy contest, making it more difficult for a third party to acquire control without the consent of our board of directors. These provisions could also delay the removal of management by the board of directors with or without cause. In addition our amended and restated bylaws limit the ability our stockholders to call special meetings of stockholders.
Our equity incentive plans generally permit our board of directors to provide for acceleration of vesting of options granted under these plans in the event of certain transactions that result in a change of control. If our board of directors uses its authority to accelerate vesting of options, this action could make an acquisition more costly, and it could prevent an acquisition from going forward.
Under Section 203 of the Delaware General Corporation Law, a corporation may not engage in a business combination with any holder of 15% or more of its capital stock until the holder has held the stock for three years unless, among other possibilities, the board of directors approves the transaction in advance.
| Item 1B. | Unresolved Staff Comments |
None.
We currently lease approximately 23,000 square feet of laboratory and office space in Cambridge, Massachusetts. We believe that our current facility is sufficient for our current operations.
None.
| Item 4. | Mine Safety Disclosures |
None.
35
PART II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Market Price of and Dividends on Our Common Stock and Related Stockholder Matters.
Our common stock is listed for quotation on the NASDAQ Capital Market under the symbol “ZLCS.” On October 3, 2013, we effected a 1-for-6 reverse stock split of our common stock. The following table sets forth the high and low sale prices per share of our common stock as reported on the NASDAQ Capital Market for the periods indicated, adjusted for the reverse stock split.
| | | | | | | | |
| | | Common Stock Price | |
| | | High | | | Low | |
Fiscal year ended December 31, 2013 | | | | | | | | |
First quarter | | $ | 4.98 | | | $ | 3.60 | |
Second quarter | | $ | 4.20 | | | $ | 2.88 | |
Third quarter | | $ | 7.20 | | | $ | 2.82 | |
Fourth quarter | | $ | 8.28 | | | $ | 0.88 | |
Fiscal year ended December 31, 2012 | | | | | | | | |
First quarter | | $ | 7.62 | | | $ | 5.88 | |
Second quarter | | $ | 8.70 | | | $ | 4.56 | |
Third quarter | | $ | 8.88 | | | $ | 4.44 | |
Fourth quarter | | $ | 4.56 | | | $ | 2.58 | |
On March 12, 2014, the reported last sale price of our common stock on the NASDAQ Capital Market was $1.35 per share. As of March 12, 2014, there were approximately 47 holders of record of our common stock.
We have never paid cash dividends on our common stock. We currently do not anticipate paying cash dividends on our common stock in the foreseeable future. We currently intend to retain earnings, if any, to finance the growth and development of our business. Payment of future dividends, if any, will be at the discretion of our board of directors and will depend on our financial condition, results of operations, capital requirements, restrictions contained in current or future financing instruments and other factors that our board of directors deems relevant. In connection with the loan and security agreement with Oxford, we have issued to Oxford warrants to purchase our common stock each time we have borrowed funds under the loan and security agreement. Specifically, on December 22, 2010, we issued Oxford a warrant to purchase 10,791 shares of our common stock with a per share exercise price of $8.34; on June 27, 2011, we issued Oxford a warrant to purchase 18,876 shares of our common stock with a per share exercise price of $13.50; and on December 16, 2011, we issued to Oxford a warrant to purchase 37,373 shares of our common stock with a per share exercise price of $6.84. The warrants are exercisable, in whole or in part, immediately, upon issuance and may be exercised on a cashless basis. The warrants will terminate on the earlier of December 22, 2017 and the closing of a merger or consolidation transaction in which Zalicus is not the surviving entity. In reliance on Rule 506 of Regulation D, the “safe harbor” for the private offering exemption of Section 4(2) of the Securities Act and because the offering satisfied all of the requirements thereunder, these warrants were not registered under the Securities Act.
36
Securities Authorized For Issuance Under Equity Compensation Plans
| | | | | | | | | | | | |
Plan Category | | Number of Securities
to be Issued upon
Exercise of
Outstanding
Options, Warrants
or Rights(1) | | | Weighted Average
Exercise Price of
Outstanding
Options,
Warrants or Rights(2) | | | Number of
Securities
Remaining
Available for
Future Issuance
Under Equity
Compensation
Plan (Excluding
Securities
Reflected in
Column(a))(1)(3) | |
| | | (a) | | | (b) | | | (c) | |
Equity compensation plans approved by security holders | | | 1,754,241 | | | $ | 7.31 | | | | 1,137,977 | |
Equity compensation plans not approved by security holders | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
Total | | | 1,754,241 | | | $ | 7.31 | | | | 1,137,977 | |
| | | | | | | | | | | | |
| (1) | As of December 31, 2013. |
| (2) | For outstanding restricted stock units, the exercise price was deemed to be $0. |
| (3) | Our Amended and Restated 2004 Incentive Plan (the “2004 Plan”) contains an “evergreen provision” that allows for an annual increase in the number of shares of common stock available for issuance under the 2004 Plan, which annual increase is and will be added on the first day of each fiscal year from 2011 through 2015, inclusive, and will be equal to the least of: (i) 666,666 shares of common stock, (ii) 4% of the outstanding shares on that date, or (iii) such lesser amount determined by the board of directors. The Compensation Committee of the board of directors elected not to increase the number of shares of common stock available for issuance under the 2004 Plan for 2014. |
37
Comparative Stock Performance Graph
The information contained in the performance graph shall not be deemed to be “soliciting material” or to be “filed” with the Securities and Exchange Commission, and such information shall not be incorporated by reference into any future filing under the Securities Act or Exchange Act, except to the extent that Zalicus specifically incorporates it by reference into such filing.
The comparative stock performance graph below compares the cumulative total stockholder return (assuming reinvestment of dividends, if any) from investing $100 on December 31, 2008, and plotted at the close of the last trading day of the fiscal year ended December 31, 2013, in each of (i) Zalicus common stock, (ii) the NASDAQ Global Stock Market Index, which is referred to as the NASDAQ Stock Market Index, and (iii) the NASDAQ Global Stock Market Biotechnology Index, which is referred to as the NASDAQ Biotechnology Index; except, in the case of the NASDAQ Stock Market Index and the NASDAQ Biotechnology Index, the stock performance graph below reflects an investment date of December 31, 2008.
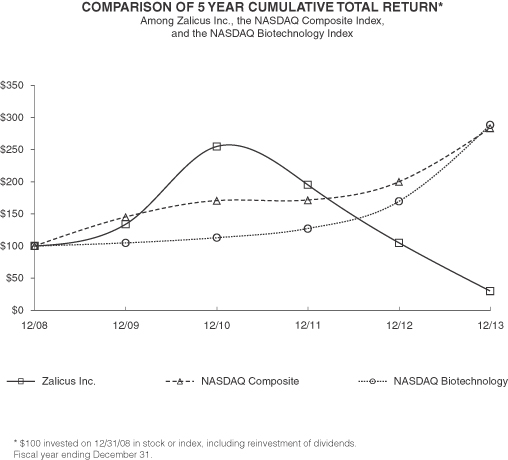
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Cumulative Total Return | |
| | | 12/08 | | | 12/09 | | | 12/10 | | | 12/11 | | | 12/12 | | | 12/13 | |
ZALICUS INC. | | | 100.00 | | | | 133.87 | | | | 254.84 | | | | 195.16 | | | | 104.84 | | | | 29.84 | |
NASDAQ COMPOSITE | | | 100.00 | | | | 144.88 | | | | 170.58 | | | | 171.30 | | | | 199.99 | | | | 283.39 | |
NASDAQ BIOTECHNOLOGY | | | 100.00 | | | | 104.67 | | | | 112.89 | | | | 127.04 | | | | 169.50 | | | | 288.38 | |
38
| Item 6. | Selected Financial Data |
The historical financial data set forth below should be read in conjunction with our “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and the related notes thereto appearing elsewhere in this Annual Report on Form 10-K. The selected financial data in this section are not intended to replace the financial statements. We have derived the statement of operations data for the years ended December 31, 2013, 2012 and 2011 and the balance sheet data as of December 31, 2013 and 2012 from our consolidated financial statements included elsewhere in this annual report, which have been audited by Ernst & Young LLP, our independent registered public accounting firm. We derived the statement of operations data for the years ended December 31, 2010 and 2009 and the balance sheet data as of December 31, 2011, 2010 and 2009 from our audited consolidated financial statements, which are not included herein. See the notes to the financial statements for an explanation of the method used to determine the number of shares used in determining basic and diluted net loss per common share.
| | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2013 | | | 2012 | | | 2011 | | | 2010 | | | 2009 | |
| | | (in thousands, except share and per share amounts) | |
Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | |
Revenue: | | | | | | | | | | | | | | | | | | | | |
Royalties and milestones | | $ | 6,661 | | | $ | 5,220 | | | $ | 2,521 | | | $ | 41,615 | | | $ | — | |
cHTS services and other collaborations | | | 8,070 | | | | 7,330 | | | | 5,663 | | | | 5,126 | | | | 17,273 | |
| | | | | | | | | | | | | | | | | | | | |
Total revenue | | | 14,731 | | | | 12,550 | | | | 8,184 | | | | 46,741 | | | | 17,273 | |
| | | | | | | | | | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | |
Research and development | | | 33,964 | | | | 41,423 | | | | 35,294 | | | | 23,011 | | | | 21,244 | |
General and administrative | | | 7,523 | | | | 8,977 | | | | 10,400 | | | | 12,115 | | | | 17,081 | |
Amortization of intangible asset | | | 8,722 | | | | 3,892 | | | | 5,141 | | | | 18,736 | | | | 520 | |
Impairment charge | | | 1,732 | | | | — | | | | — | | | | — | | | | — | |
Gain on legal settlement | | | — | | | | — | | | | — | | | | — | | | | (3,700 | ) |
Restructuring | | | — | | | | 1,094 | | | | — | | | | — | | | | 2,736 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
Total operating expenses | | | 51,941 | | | | 55,386 | | | | 50,835 | | | | 53,862 | | | | 37,881 | |
| | | | | | | | | | | | | | | | | | | | |
Loss from operations | | | (37,210 | ) | | | (42,836 | ) | | | (42,651 | ) | | | (7,121 | ) | | | (20,608 | ) |
Interest income | | | 46 | | | | 150 | | | | 136 | | | | 132 | | | | 257 | |
Interest expense | | | (1,467 | ) | | | (2,173 | ) | | | (976 | ) | | | (12 | ) | | | (28 | ) |
(Loss) gain on revaluation of contingent consideration | | | — | | | | — | | | | — | | | | (29,286 | ) | | | 12,068 | |
Other income (expense) | | | 14 | | | | 91 | | | | 20 | | | | 32 | | | | (281 | ) |
Gain on bargain purchase | | | — | | | | — | | | | — | | | | — | | | | 9,809 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
Net (loss) income before benefit for income taxes | | | (38,617 | ) | | | (44,768 | ) | | | (43,471 | ) | | | (36,255 | ) | | | 1,217 | |
Benefit for income taxes | | | — | | | | 441 | | | | 1,428 | | | | 1,210 | | | | 67 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
Net (loss) income from continuing operations | | | (38,617 | ) | | | (44,327 | ) | | | (42,043 | ) | | | (35,045 | ) | | | 1,284 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
Discontinued operations: | | | | | | | | | | | | | | | | | | | | |
Loss from operations of discontinued subsidiary | | | — | | | | — | | | | — | | | | — | | | | (1,536 | ) |
Gain on disposal of discontinued operations | | | — | | | | — | | | | — | | | | — | | | | 15,640 | |
| | | | | | | | | | | | | | | | | | | | |
Gain on discontinued operations | | | — | | | | — | | | | — | | | | — | | | | 14,104 | |
| | | | | | | | | | | | | | | | | | | | |
Net (loss) income | | $ | (38,617 | ) | | $ | (44,327 | ) | | $ | (42,043 | ) | | $ | (35,045 | ) | | $ | 15,388 | |
| | | | | | | | | | | | | | | | | | | | |
Net (loss) income per share—basic: | | | | | | | | | | | | | | | | | | | | |
From continuing operations | | $ | (1.68 | ) | | $ | (2.27 | ) | | $ | (2.59 | ) | | $ | (2.54 | ) | | $ | 0.21 | |
From discontinued operations | | | — | | | | — | | | | — | | | | — | | | | 2.26 | |
| | | | | | | | | | | | | | | | | | | | |
Net (loss) income per share—basic | | $ | (1.68 | ) | | $ | (2.27 | ) | | $ | (2.59 | ) | | $ | (2.54 | ) | | $ | 2.47 | |
| | | | | | | | | | | | | | | | | | | | |
Net (loss) income per share—diluted: | | | | | | | | | | | | | | | | | | | | |
From continuing operations | | $ | (1.68 | ) | | $ | (2.27 | ) | | $ | (2.59 | ) | | $ | (2.54 | ) | | $ | 0.20 | |
From discontinued operations | | | — | | | | — | | | | — | | | | — | | | | 2.26 | |
| | | | | | | | | | | | | | | | | | | | |
Net (loss) income per share—diluted | | $ | (1.68 | ) | | $ | (2.27 | ) | | $ | (2.59 | ) | | $ | (2.54 | ) | | $ | 2.46 | |
| | | | | | | | | | | | | | | | | | | | |
Weighted-average number of common shares used in net (loss) income per share calculation: | | | | | | | | | | | | | | | | | | | | |
Basic | | | 22,932,675 | | | | 19,552,828 | | | | 16,224,532 | | | | 13,777,274 | | | | 6,223,007 | |
Diluted | | | 22,932,675 | | | | 19,552,828 | | | | 16,224,532 | | | | 13,777,274 | | | | 6,248,540 | |
| | | | | | | | | | | | | | | | | | | | |
Net (loss) income | | $ | (38,617 | ) | | $ | (44,327 | ) | | $ | (42,043 | ) | | $ | (35,045 | ) | | $ | 15,388 | |
Other comprehensive (loss) income, net of tax: | | | | | | | | | | | | | | | | | | | | |
Unrealized gain (loss) on investments | | | (10 | ) | | | 18 | | | | 12 | | | | (18 | ) | | | (75 | ) |
| | | | | | | | | | | | | | | | | | | | |
Comprehensive (loss) income | | $ | (38,627 | ) | | $ | (44,309 | ) | | $ | (42,031 | ) | | $ | (35,063 | ) | | $ | 15,313 | |
| | | | | | | | | | | | | | | | | | | | |
39
| | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2013 | | | 2012 | | | 2011 | | | 2010 | | | 2009 | |
| | | (in thousands) | |
Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
Cash, cash equivalents and short-term investments | | $ | 17,958 | | | $ | 34,590 | | | $ | 47,874 | | | $ | 44,619 | | | $ | 23,330 | |
Working capital | | | 5,850 | | | | 18,738 | | | | 35,663 | | | | 38,803 | | | | 18,350 | |
Total assets | | | 32,106 | | | | 61,375 | | | | 79,883 | | | | 82,669 | | | | 88,152 | |
Long-term debt, less current portion | | | 2,132 | | | | 8,772 | | | | 15,099 | | | | 2,523 | | | | — | |
Accumulated deficit | | | (379,640 | ) | | | (341,023 | ) | | | (296,696 | ) | | | (254,653 | ) | | | (219,608 | ) |
Total stockholders’ equity | | | 14,182 | | | | 31,026 | | | | 43,913 | | | | 62,997 | | | | 52,913 | |
40
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with our financial statements and related notes appearing elsewhere in this annual report. The following discussion contains forward-looking statements that involve risks and uncertainties. Our actual results and the timing of certain events could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including those discussed below and elsewhere in this annual report, particularly under the heading “Risk Factors.”
Overview
We are a biopharmaceutical company that discovers and develops novel treatments for patients suffering from pain. We have a portfolio of proprietary product candidates targeting pain and have entered into multiple revenue-generating collaborations with large pharmaceutical companies relating to other products, product candidates and drug discovery technologies. We also apply our expertise in the discovery and development of selective ion channel modulators and our combination high throughput screening technology, or cHTS to discover new product candidates for our portfolio or for our collaborators in the areas of pain and oncology.
Our most advanced product candidate is Z944, a novel, oral, state-dependent, selective T-type calcium channel modulator we are seeking to develop for the treatment of pain indications. T-type calcium channels have been recognized as key targets for therapeutic intervention in a broad range of cell functions and have been implicated in pain signaling. During 2013, we completed a Phase 1b clinical trial utilizing Laser-Evoked-Potentials, or LEP, to provide both objective and subjective assessments of the activity of Z944 in induced pain states. On November 1, 2013, we announced positive results from this Phase 1b clinical trial. Based on these results, Zalicus is currently planning to evaluate multiple modified-release formulations of Z944 in a Phase 1 pharmacokinetic and safety study in the first half of 2014, with the goal of advancing a modified-release formulation of Z944 toward Phase 2 clinical development in an appropriate pain indication by late 2014.
We have also been performing discovery research and preclinical development activities on our proprietary selective ion channel modulators targeting the Nav1.7 sodium channel for the treatment of pain. This discovery research and preclinical development has been conducted as part of a research collaboration with Hydra Biosciences, Inc., or Hydra, a recognized leader in novel ion channel discovery and development. This collaboration expired in February 2014 in accordance with its terms.
Until November 2013, we had also been advancing the development of Z160, a novel, oral N-type calcium channel modulator we were seeking to develop for the treatment of chronic pain. In December 2012 and August 2012, we initiated Phase 2 clinical trials of Z160 for the treatment of neuropathic pain, including, post-herpetic neuralgia, or PHN, a painful neuropathic condition resulting from an outbreak of the herpes zoster virus, otherwise known as shingles, and lumbosacral radiculopathy, or LSR, a common neuropathic back pain condition resulting from the compression or irritation of the nerves exiting the lumbar region of the spine, respectively. Patient enrollment completed for both studies in September 2013. Results from both Phase 2 trials of Z160 were released on November 11, 2013 and Z160 did not meet the primary endpoint in either of these Phase 2 trials. Based on the results, we have terminated further development of Z160.
Until September 2012, we had also been advancing the development of Synavive, a product candidate to treat immuno-inflammatory disorders. In June 2011, we initiated a Phase 2b clinical trial evaluating Synavive in patients with rheumatoid arthritis, which we refer to as the SYNERGY trial. Results from the SYNERGY trial were announced in September 2012, and while Synavive achieved a statistically significant improvement in signs and symptoms of rheumatoid arthritis, as measured by DAS28-CRP, compared to placebo, the primary end point of the trial, Synavive did not demonstrate a meaningful clinical benefit measured by DAS28-CRP, compared to 2.7 mg of prednisolone or 5 mg of prednisone, key secondary endpoints of the SYNERGY trial. Based on the data from the SYNERGY trial, we have terminated further development of Synavive.
41
We have also been performing discovery research and preclinical development activities on our proprietary selective ion channel modulators targeting the Nav1.7 sodium channel as well as sodium or T-type calcium channels. This discovery research and preclinical development has been conducted as part of a research collaboration with Hydra a recognized leader in novel ion channel discovery and development, which expired in February 2014.
We have also been using our cHTS platform to perform our obligations with our collaboration partners, including Novartis Institutes of Biomedical Research, Inc., or Novartis, and other pharmaceutical companies who have adopted cHTS as an important addition to their oncology discovery efforts.
On December 21, 2009, we completed a merger, which we refer to as the Neuromed Merger, with Neuromed Pharmaceuticals Inc., or Neuromed, pursuant to which Neuromed Pharmaceuticals Ltd. became a wholly-owned subsidiary of Zalicus. On September 8, 2010, we changed our name from CombinatoRx, Incorporated to Zalicus Inc. We also changed the name of our subsidiaries, including Neuromed Pharmaceuticals Ltd., which is now named Zalicus Pharmaceuticals Ltd., and which we refer to herein as Zalicus Canada.
The United States commercial rights to Exalgo were acquired by Mallinckrodt from Neuromed in June 2009 pursuant to an asset purchase agreement. Exalgo is an extended release formulation of hydromorphone, an opioid analgesic that has been used in an immediate release formulation to treat pain for many years, and is intended for use in the management of moderate to severe pain in opioid tolerant patients requiring continuous, around-the-clock opioid analgesia for an extended period of time. Under the asset purchase agreement, Mallinckrodt is responsible for all commercialization activities for Exalgo in the United States, including marketing and sales, and for all post-approval regulatory activities. We received a $40.0 million milestone payment following FDA approval of Exalgo in March of 2010, and received tiered royalties on net sales of Exalgo by Mallinckrodt following its commercial launch in April 2010. We recognized $16.0 million in revenue related to these royalties through December 31, 2013. Following the settlement of the Exalgo litigation between Mallinckrodt and Watson Pharmaceuticals, Inc., now Actavis Inc., or Actavis, that became effective in January 2012, Actavis was able to introduce a generic version of the 8, 12 and 16 mg dosage strengths of Exalgo starting on November 15, 2013, subject to FDA approval. In August 2012, the FDA approved the 32 mg dosage strength of Exalgo, which is not subject to Mallinckrodt’s settlement with Actavis and on which our royalties for net sales of Exalgo at such dosage will not be reduced as a result of Mallinckrodt’s settlement with Actavis. In February 2013, Mallinckrodt and Actavis entered into a separate settlement agreement that would allow Actavis to introduce a generic version of the 32mg dosage strength of Exalgo starting on May 15, 2014, subject to FDA approval.
On January 31, 2014, we and Mallinckrodt Medical Imaging—Ireland entered into the Royalty Purchase Agreement relating to the asset purchase agreement. Under the terms of the Royalty Purchase Agreement, in exchange for the payment of $7.2 million from Mallinckrodt to Zalicus on January 31, 2014, we terminated any further rights we had to the payment of royalties on net sales of Exalgo by Mallinckrodt. As a result, we recorded an impairment charge of $1.7 million related to the Exalgo intangible asset in the year ended December 31, 2013.
As of December 31, 2013, we had an accumulated deficit of $379.6 million. We had a net loss of $38.6 million and $44.3 million for the years ended December 31, 2013 and 2012, respectively.
Our management currently uses consolidated financial information in determining how to allocate resources and assess performance. We have determined that we conduct operations in one business segment. For each of the years ended December 31, 2013, 2012 and 2011, all of our revenues were from customers located in the United States. As of December 31, 2013, all of our long-lived assets were located in the United States.
Critical Accounting Policies
Our discussion and analysis of our financial condition and results of operations are based on our financial statements, which have been prepared in accordance with United States generally accepted accounting principles, or GAAP. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of
42
the financial statements and the reported amounts of revenues and expenses during the reporting periods. On an ongoing basis, we evaluate our estimates and judgments, including those related to revenue recognition, intangible assets, stock-based compensation, accrued expenses and income taxes. We base our estimates on historical experience, known trends and events and various other factors that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
We believe that several accounting policies are important to understanding our historical and future performance. We refer to these policies as “critical” because these specific areas generally require us to make judgments and estimates about matters that are uncertain at the time we make the estimate, and different estimates—which also would have been reasonable—could have been used, which would have resulted in different financial results. We believe the following critical accounting policies affect our more significant judgments and estimates used in the preparation of our financial statements. It is important that the discussion of our operating results that follows be read in conjunction with the critical accounting policies discussed below.
Revenue Recognition
Collaboration Revenue
We apply the authoritative guidance for revenue recognition related to multiple-element revenue arrangements, under which each deliverable within a multiple-element revenue arrangement is accounted for as a separate unit of accounting if both of the following criteria are met: (1) the delivered item or items have value to the customer on a standalone basis and (2) for an arrangement that includes a general right of return relative to the delivered item(s), delivery or performance of the undelivered item(s) is considered probable and substantially in our control. We consider a deliverable to have standalone value if we sell the item separately or if the item is sold by another vendor or could be resold by the customer. Deliverables not meeting the criteria for being a separate unit of accounting are accounted for on a combined basis.
In the event we enter into a contract in which the deliverables are required to be separated, we will allocate arrangement consideration to each deliverable in an arrangement based on its relative selling price. We determine selling price using vendor-specific objective evidence (“VSOE”), if it exists; otherwise, we use third-party evidence (“TPE”). If neither VSOE nor TPE of selling price exists for a deliverable, we use estimated selling price (“ESP”) to allocate the arrangement consideration. We apply appropriate revenue recognition guidance to each unit of accounting.
Contingent consideration from research and development activities that is earned upon the achievement of a substantive milestone is recognized in its entirety in the period in which the milestone is achieved. At the inception of each arrangement that includes milestone payments, we evaluate whether each milestone is substantive. This evaluation includes an assessment of whether (a) the consideration is commensurate with either (1) the entity’s performance to achieve the milestone, or (2) the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from the entity’s performance to achieve the milestone, (b) the consideration relates solely to past performance and (c) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement. We evaluate factors such as the scientific, clinical, regulatory, commercial and other risks that must be overcome to achieve the respective milestone, the level of effort and investment required and whether the milestone consideration is reasonable relative to all deliverables and payment terms in the arrangement in making this assessment.
We have concluded that all of the clinical development milestones pursuant to our research and development arrangements are substantive. Clinical development milestones are typically payable when a product candidate advances into a defined phase of clinical research or completes such phase. For example, a milestone may be due upon the initiation of a Phase 3 clinical trial for a particular product candidate, the last phase of clinical development, which if the product candidate is proven to be safe and effective, may lead to its marketing approval by the FDA or other global regulatory authorities.
43
We have also concluded that all of the regulatory milestones pursuant to our research and development arrangements are substantive. Regulatory milestones are typically payable when a product candidate is ultimately approved for marketing by the FDA or other global regulatory authorities after it is deemed to be safe and efficacious to treat a defined disease or condition. For example, a milestone may be due upon our receipt of marketing approval in the United States. Revenues from clinical development and regulatory milestones, if they are nonrefundable and deemed substantive, are recognized upon successful accomplishment of the milestones. Milestones that are not considered substantive are accounted for as license payments and are evaluated as such in accordance with our accounting policy for multiple element arrangements.
We have entered into collaborative research and development agreements and service agreements with other pharmaceutical and biotechnology companies, government agencies and charitable foundations. These agreements are generally in the form of research and development and license agreements. The agreements are primarily for early-stage compounds and are generally focused on specific disease areas. The agreements provide for nonrefundable up-front payments, milestone payments upon achieving significant milestone events and in some cases ongoing research funding. The agreements also contemplate royalty payments on sales if and when the product receives marketing approval by the FDA or other regulatory agencies.
Our collaboration agreements typically include one or more of the following deliverables: research and development services, screening services, licenses to our high throughput screening analysis software and licenses to specific pharmaceutical compounds. The arrangements do not contain any substantive performance conditions or refund rights. We evaluate our arrangements with software license components in order to determine whether the arrangement should be accounted for under revenue recognition guidance for software or if other applicable revenue guidance should be applied. Revenue is recognized when there is persuasive evidence that an arrangement exists, delivery has occurred, the price is fixed or determinable, and collection is reasonably assured. Consideration allocated to licenses that do not have standalone value or amounts allocated to units of accounting that will be delivered or provided over a period of time, where we have a continuing obligation to perform services, are deferred and recognized over the performance period. Revenues for research and development and screening services are recognized as services are performed. Royalty revenue is recognized based upon net sales of licensed products as provided by the relevant license and is recognized in the period the sales occur. The periods over which revenue is recognized are subject to estimates by management and may change over the course of a collaborative agreement. Such changes would impact the amount of revenue we recognize in each financial reporting period.
Government Contracts and Grants
Revenue under government grants or cost reimbursement contracts is recognized as we perform the underlying research and development activities.
Intangible Assets
We had a significant intangible asset in our rights to Exalgo milestones and royalties as a result of the acquisition of Neuromed. Our intangible asset had a finite life and was amortized based upon the pattern in which we expected to utilize the economic benefits. Determining the estimated life of a finite-lived intangible asset requires us to make significant judgments and estimates, and changes in those estimates could materially impact our results of operations.
The value of the intangible asset was initially determined by a risk-adjusted, discounted cash flow approach. We assessed the potential impairment of the intangible asset whenever events or changes in circumstances indicated that the carrying value may not be recoverable. In connection with this assessment, we considered the cash flow forecast for projected royalties and any other factors that that could impact the amount of cash flows we might receive in future periods. During the third quarter of 2013, events and circumstances indicated that our intangible asset might be impaired. However, our estimate of the fair value of the asset as of September 30, 2013
44
exceeds its carrying value and therefore no impairment was recognized. As of December 31, 2013, we determined that the carrying value of our intangible asset exceeded its estimated fair value. We determined the estimated fair value of our Exalgo intangible asset at December 31, 2013 based on the proceeds from the execution of a royalty purchase agreement, pursuant to which, in exchange for the payment of $7.2 million from an affiliate of Mallinckrodt to Zalicus, we terminated any further rights we had to the payment of royalties on net sales of Exalgo by Mallinckrodt. As a result, in the year ended December 31, 2013, we recorded an impairment charge of $1.7 million in the consolidated statements of operations and comprehensive loss.
Stock-Based Compensation
We measure the compensation cost of stock-based compensation at the grant date, based on the fair value of the award, including estimated forfeitures, and we recognize that cost as an expense ratably over the associated employee service period, which generally is the vesting period of the equity award. For our awards with performance conditions, we make estimates regarding the likelihood of satisfaction of the performance condition that affect the period over which the expense is recognized.
We calculate the grant date fair value of stock options using the Black-Scholes option pricing model. The Black-Scholes option pricing model requires us to make certain assumptions and estimates concerning our stock price volatility, the rate of return of risk-free investments, the expected term of the awards, and our anticipated dividends. We have determined that we have sufficient trading history as a public company to estimate stock price volatility and began to use our own historical volatility to develop such estimate. We utilize the expected terms from an analysis of peer group companies to support the expected term used in the Black-Scholes model, as we do not believe we have sufficient historical data to support this assumption.
In determining the amount of expense to be recorded, we also are required to exercise judgment to estimate forfeiture rates for awards, based on the probability that employees will complete the required service period. If actual forfeitures differ significantly from our estimates, if any of our estimates or assumptions prove incorrect, or if the likelihood of achievement of a performance condition changes, our results could be materially affected.
We recognized stock compensation expense of $2.0 million, $1.8 million and $2.2 million for the years ended December 31, 2013, 2012 and 2011, respectively. As of December 31, 2013, there was approximately $2.0 million of total stock-based compensation expense not yet recognized relating to non-vested awards granted under our equity incentive plans. This expense is net of estimated forfeitures and is expected to be recognized over a weighted-average period of approximately 1.8 years. The amount of stock-based compensation expense to be recorded in any future period cannot be accurately predicted due to the uncertainty of future grant levels and actual forfeitures to be recorded. Additionally, changes to the assumptions used in the Black-Scholes model could cause a material change in the amount of stock-based compensation expense to be recorded in future reporting periods.
Accrued Expenses
As part of the process of preparing our consolidated financial statements, we are required to estimate certain accrued expenses. This process involves identifying services that third parties have performed on our behalf and estimating the amount of service performed and the associated cost incurred for these services as of the balance sheet date in our consolidated financial statements. Examples of estimated accrued expenses for our business are professional service fees, such as attorneys and accountants, contract service fees, such as amounts due to clinical research organizations who are supporting clinical trials for our product candidates preclinical and toxicology research services providers and formulation development providers. In connection with these service fees, our estimates are most affected by our understanding of the status and timing of services provided relative to the actual level of services incurred by the service providers. In the event that we do not identify certain costs that have been incurred or we under- or over-estimate the level of services or the costs of such services, our reported expenses for a reporting period could be understated or overstated.
45
Income Taxes
In preparing our consolidated financial statements, we estimate our income tax liability in each of the jurisdictions in which we operate by estimating our actual current tax expense together with assessing temporary differences resulting from differing treatment of items for tax and financial reporting purposes. These differences result in deferred tax assets and liabilities, which are included in our consolidated balance sheets. Significant management judgment is required in assessing the realizability of our deferred tax assets. In performing this assessment, we consider whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. In making this determination, under the applicable financial accounting standards, we are allowed to consider the scheduled reversal of deferred tax liabilities, projected future taxable income, and the effects of tax planning strategies. Our estimates of future taxable income include, among other items, our estimates of future income tax deductions related to the exercise of stock options. In the event that actual results differ from our estimates, we adjust our estimates in future periods and we may need to establish a valuation allowance, which could materially impact our financial position and results of operations.
We account for uncertain tax positions using a “more-likely-than-not” threshold for recognizing and resolving uncertain tax positions. The evaluation of uncertain tax positions is based on factors that include, but are not limited to, changes in tax law, the measurement of tax positions taken or expected to be taken in tax returns, the effective settlement of matters subject to audit, new audit activity and changes in facts or circumstances related to a tax position. We evaluate uncertain tax positions on a quarterly basis and adjust the level of the liability to reflect any subsequent changes in the relevant facts surrounding the uncertain positions. Our liabilities for uncertain tax positions can be relieved only if the contingency becomes legally extinguished through either payment to the taxing authority or the expiration of the statute of limitations, the recognition of the benefits associated with the position meet the “more-likely-than-not” threshold or the liability becomes effectively settled through the examination process. We consider matters to be effectively settled once the taxing authority has completed all of its required or expected examination procedures, including all appeals and administrative reviews; we have no plans to appeal or litigate any aspect of the tax position; and we believe that it is highly unlikely that the taxing authority would examine or re-examine the related tax position.
Results of Operations
Years Ended December 31, 2013 and 2012
Revenue. For the year ended December 31, 2013, we recorded $14.7 million of revenue from Exalgo royalties and cHTS services and other collaborations. Royalty revenue from Mallinckrodt on net sales of Exalgo for the year ended December 31, 2013 was $6.7 million. For the year ended December 31, 2013, we recorded an aggregate of $8.0 million of revenue from cHTS services and other collaborations. For the year ended December 31, 2012, we recorded $12.6 million of revenue from Exalgo royalties and cHTS services and other collaborations, which was comprised of royalty revenue from Mallinckrodt on net sales of Exalgo of $5.2 million and revenue from our cHTS services and other collaborations of $7.4 million. The increase in revenue for the year ended December 31, 2013 was primarily due to a $1.4 million increase in Exalgo royalties and a $0.8 million and $0.9 million increase in the recognition of cHTS revenue from amended and new services collaborations with Novartis and Amgen, respectively, offset by a $1.0 million decrease in recognition of cHTS revenue from other research agreements. In light of the January 2014 royalty purchase agreement entered into with an affiliate of Mallinckrodt, we do not expect any royalty revenue for the year ended December 31, 2014.
Research and Development Expense. Research and development expense for the year ended December 31, 2013 was $34.0 million compared to $41.4 million for the year ended December 31, 2012. The $7.4 million decrease was primarily due to a $11.6 million decrease in expenses related to Synavive, a $2.6 million decrease in expenses related to the clinical development of Z944, a $1.5 million decrease in cHTS collaboration discovery costs, preclinical program and unallocated clinical and preclinical program costs and a $0.9 million decrease in infrastructure and support costs and non-cash stock-based compensation expense, offset by a $8.3 million
46
increase in expenses related to the clinical development of Z160 and a $0.7 million increase in ion channel discovery costs and other clinical programs. The $11.6 million decrease in expenses related to Synavive is due to the termination of development activities related to Synavive in the third quarter of 2012. The $8.3 million increase in expenses related to Z160 is due to the initiation and completion of Phase 2 clinical development during 2013. The $2.6 million decrease in expenses related to Z944 is due to the timing of conducting our Phase 1 clinical trials in 2012. The $0.9 million decrease in infrastructure and support costs and non-cash stock-based compensation expense is primarily related to 2012 severance costs incurred in connection with the termination of personnel in our clinical and commercial development departments. During the fourth quarter of 2013, we terminated all development activities related to Z160. Accordingly, we expect research and development expense to decrease significantly for the year ending December 31, 2014, compared to the year ended December 31, 2013.
The table below summarizes our allocation of research and development expenses to our discontinued clinical program, Z160, our internal clinical program, Z944, our discontinued clinical program, Synavive, our preclinical programs and our drug discovery platforms for the years ended December 31, 2013 and 2012. Our internal project costing methodology does not allocate all of the personnel and other indirect costs from all of our research and development departments to specific clinical and preclinical programs, and such unallocated costs are further summarized in the table below. Other clinical program costs consist primarily of the personnel and other expenses for our clinical operations department, the majority of which supported the development of our clinical product candidates, Z160, Z944 and Synavive. Preclinical program costs consist of the personnel and other expenses allocated to our internally funded preclinical programs. cHTS collaboration discovery costs consist of the personnel and other expenses allocated to all of our cHTS services. Ion channel program discovery costs consist of the personnel and other expenses allocated to our ion channel drug discovery programs, including expenses incurred under our collaboration agreement with Hydra, other than the preclinical development costs associated with preclinical product candidates.
Unallocated clinical and preclinical program costs consist primarily of the personnel and other expenses for our formulations, pharmacology and discovery departments, the majority of which supported the development of our clinical product candidates, including Z160 and Z944, as well as our preclinical product candidates from our ion channel program. Infrastructure and support costs consist of facility costs, depreciation and amortization and costs for research and development support personnel such as our informatics and facilities departments.
Due to the uncertainty in drug development and the stage of development of our pre-clinical and clinical programs, we are unable to predict the nature, specific timing and estimated costs to complete the development of our product candidates or the timing of when material cash inflows may commence.
| | | | | | | | |
| | | Year Ended
December 31, | |
| | | 2013 | | | 2012 | |
| | | (in thousands) | |
Z160 | | $ | 19,047 | | | $ | 10,750 | |
Z944 | | | 2,018 | | | | 4,569 | |
Synavive | | | 125 | | | | 11,686 | |
Other clinical program costs | | | 854 | | | | 427 | |
| | | | | | | | |
Total clinical program costs | | | 22,044 | | | | 27,432 | |
| | | | | | | | |
Preclinical program costs | | | 3 | | | | 122 | |
cHTS collaboration discovery costs | | | 3,072 | | | | 4,272 | |
Ion channel discovery costs | | | 3,615 | | | | 3,299 | |
Unallocated clinical and preclinical program costs | | | 1,438 | | | | 1,593 | |
Infrastructure and support costs | | | 3,468 | | �� | | 4,338 | |
Noncash employee and non-employee stock-based compensation expense | | | 324 | | | | 367 | |
| | | | | | | | |
Total research and development costs | | $ | 33,964 | | | $ | 41,423 | |
| | | | | | | | |
47
General and Administrative Expense. General and administrative expense for the year ended December 31, 2013 was $7.5 million compared to $9.0 million for the year ended December 31, 2012. The $1.5 million decrease was primarily due to lower personnel costs during the year ended December 31, 2013. We expect our general and administrative expense for the year ending December 31, 2014 to decrease, compared to the year ended December 31, 2013.
Amortization of Intangible Asset.For the years ended December 31, 2013 and 2012, we recorded $8.7 million and $3.9 million, respectively, of amortization expense related to the Exalgo intangible asset. The increase in amortization expense relates to the manner in which the Exalgo intangible asset is being amortized, which reflected an estimate of the future undiscounted cash flows we expected to receive over the remaining useful life of Exalgo. There will be no amortization expense related to the Exalgo intangible asset for the year ended December 31, 2014 as a result of the January 2014 royalty purchase agreement entered into with an affiliate of Mallinckrodt.
Impairment Charge.Impairment charge of $1.7 million for the year ended December 31, 2013 is the result of our revised estimate of the fair value of our Exalgo intangible asset as of December 31, 2013 based on the January 2014 royalty purchase agreement entered into with an affiliate of Mallinckrodt.
Interest Income. Interest income, for the years ended December 31, 2013 and 2012, was less than $0.1 million and $0.2 million, respectively.
Interest Expense. Interest expense, for the years ended December 31, 2013 and 2012, was $1.5 million and $2.2 million, respectively. The interest expense relates to our term loans with Oxford Finance LLC, or Oxford. We prepaid our outstanding term loans with Oxford in the amount of approximately $8.6 million on January 31, 2014 and as a result expect interest expense for the year ending December 31, 2014 to be significantly less than for the year ended December 31, 2013.
Benefit for Income Taxes.In the years ended December 31, 2013 and 2012, we recognized a tax benefit of $0 million and $0.4 million, respectively. The income tax benefit for the year ended December 31, 2012 was related to the reversal of an unrecognized tax benefit for an uncertain tax position due to the expiration of the relevant statute of limitations.
Years Ended December 31, 2012 and 2011
Revenue.For the year ended December 31, 2012, we recorded $12.6 million of revenue from royalties paid by Mallinckrodt on net sales of Exalgo and from our research and development collaborations with Novartis and other companies as well as from grants from governmental agencies. Royalty revenue from Mallinckrodt on net sales of Exalgo for the year ended December 31, 2012 was $5.2 million. For the year ended December 31, 2011, we recorded $8.2 million of revenue from royalties paid by Mallinckrodt on net sales of Exalgo and from our research and development collaborations with Novartis, Amgen Inc., or Amgen, and other companies as well as from grants from governmental agencies. The increase in revenue for the year ended December 31, 2012 was primarily due to a $2.7 million increase in Exalgo royalties from Mallinckrodt and a $1.3 million increase in the recognition of collaboration revenue from Novartis as a result of the extension of the research term.
Research and Development Expense. Research and development expense for the year ended December 31, 2012 was $41.4 million compared to $35.3 million for the year ended December 31, 2011. The $6.1 million increase was due to a $7.3 million increase in expenses related to the clinical development of Z160, a $1.4 million increase in expenses related to the clinical development of Z944 and a $0.3 million increase in cHTS collaboration discovery costs and unallocated clinical and preclinical program costs, offset by a $1.1 million decrease in ion channel discovery costs, a $0.8 million decrease in other clinical programs and preclinical programs, a $0.6 million decrease in infrastructure and support costs and non-cash stock-based compensation expense and a $0.4 million decrease in expenses related to Exalgo and Synavive. The $7.3 million increase in expenses related to Z160 was due to the continuation of the multiple Phase 1 clinical trials and the initiation of
48
Phase 2 clinical development. The $1.4 million increase in expenses related to Z944 was due to our conducting Phase 1 clinical trials throughout 2012. During the third quarter of 2012, we terminated all development activities related to Synavive.
The table below summarizes our allocation of research and development expenses to our discontinued clinical program, Z160, our internal clinical program, Z944, our partnered product Exalgo and our discontinued clinical program Synavive, our preclinical programs and our drug discovery platforms for the years ended December, 2012 and 2011.
| | | | | | | | |
| | | Year Ended
December 31, | |
| | | 2012 | | | 2011 | |
| | | (in thousands) | |
Z160 | | $ | 10,750 | | | $ | 3,477 | |
Z944 | | | 4,569 | | | | 3,135 | |
Exalgo and Synavive | | | 11,686 | | | | 12,083 | |
Other clinical program costs | | | 427 | | | | 816 | |
| | | | | | | | |
Total clinical program costs | | | 27,432 | | | | 19,511 | |
| | | | | | | | |
Preclinical program costs | | | 122 | | | | 459 | |
cHTS collaboration discovery costs | | | 4,272 | | | | 4,163 | |
Ion channel discovery costs | | | 3,299 | | | | 4,404 | |
Unallocated clinical and preclinical program costs | | | 1,593 | | | | 1,437 | |
Infrastructure and support costs | | | 4,338 | | | | 4,569 | |
Noncash employee and non-employee stock-based compensation expense | | | 367 | | | | 751 | |
| | | | | | | | |
Total research and development costs | | $ | 41,423 | | | $ | 35,294 | |
| | | | | | | | |
General and Administrative Expense. General and administrative expense for the year ended December 31, 2012 was $9.0 million compared to $10.4 million for the year ended December 31, 2011. The $1.4 million decrease was primarily due to legal and other costs incurred during the year ended December 31, 2011 relating to strategic transactions that were evaluated and not executed, as well as overall lower expenses in the year ended December 31, 2012.
Amortization of Intangible Asset.For the years ended December 31, 2012 and 2011, we recorded $3.9 million and $5.1 million, respectively, of amortization expense related to the Exalgo intangible asset. The decrease in amortization expense related to the manner in which the Exalgo intangible asset is being amortized, which reflected an estimate of the future undiscounted cash flows we expected to receive over the remaining life of Exalgo.
Interest Income. Interest income, for the years ended December 31, 2012 and 2011, was $0.2 and $0.1 million, respectively.
Interest Expense. Interest expense, for the years ended December 31, 2012 and 2011, was $2.2 million and $1.0 million, respectively. The interest expense relates to our term loans with Oxford Finance LLC, or Oxford.
Benefit for Income Taxes.In the years ended December 31, 2012 and 2011, we recognized a tax benefit of $0.4 million and $1.4 million, respectively. The income tax benefit for the years ended December 31, 2012 and 2011 was related to the reversal of an unrecognized tax benefit for an uncertain tax position due to the expiration of the relevant statute of limitations.
Liquidity and Capital Resources
Since our inception in March 2000, we have funded our operations principally through private and public offerings of our equity securities and, to a lesser extent, from debt financing, payments from our collaboration
49
partners and proceeds from litigation. As of December 31, 2013, we had cash and cash equivalents of approximately $19.8 million, which includes $1.8 million of restricted cash. Our funds are comprised of cash and cash equivalents and as such, we do not believe there is significant risk in our investment portfolio as of December 31, 2013.
We expect our resources to be sufficient to fund our planned operations into the first half of 2015. However, we may require significant additional funds earlier than we currently expect if our research and development expenses exceed our current expectations or our collaboration funding is less than our current expectations. We may seek additional funding through collaboration agreements and public or private financings of debt or equity capital. However, funding may not be available to us on acceptable terms or at all. If we are unable to obtain funding on a timely basis, we may be required to significantly curtail one or more of our research or development programs or our operations. We also could be required to seek funds through arrangements with collaborators or others that may require us to relinquish rights to some of our technologies or product candidates which we would otherwise pursue on our own.
Operating Activities.Our operating activities used cash of $29.8 million, $38.5 million and $33.4 million for the years ended December 31, 2013, 2012 and 2011, respectively. The $8.7 million decrease in net cash used in operating activities for the year ended December 31, 2013 is primarily attributed to a $7.4 million decrease in research and development expense primarily related to the termination of development activities related to Synavive during 2012 and the timing of conducting our Phase 1 Z944 clinical trials, offset by increased expense related to the initiation and completion of Phase 2 Z160 clinical trials during 2013. We expect our cash used in operating activities for the year ending December 31, 2014 to decrease significantly as a result of lower research and development expense.
The $5.1 million increase in net cash used in operating activities for the year ended December 31, 2012 compared to the year ended December 31, 2011 is primarily attributed to a $6.1 million increase in research and development expense related to the advancement of Synavive, Z160 and Z944 into clinical trials throughout the year ended December 31, 2012.
Investing Activities.Our investing activities provided cash of $29.9 and $15.3 million for the years ended December 31, 2013 and 2012, respectively and used cash of $3.6 million for the year ended December 31, 2011. The cash provided by investing activities for both the years ended December 31, 2013 and 2012 was primarily due to the timing of purchases and maturities of our short-term investments. The cash used in investing activities for the year ended December 31, 2011 was primarily due to net purchases of short-term investments of $3.3 million and purchases of fixed assets of $0.4 million.
Financing Activities.Our financing activities generated cash proceeds of $13.3 million, $24.9 million and $36.9 million during the years ended December 31, 2013, 2012, and 2011, respectively. The cash provided by financing activities for the year ended December 31, 2013 was primarily related to the issuance of 4,876,186 shares of our common stock resulting in net proceeds of $19.8 million, offset by $6.5 million in repayments of our term loans. The cash provided by financing activities for the year ended December 31, 2012 was primarily related to the issuance of 4,524,989 shares of our common stock resulting in net proceeds of $29.1 million, offset by $4.3 million in repayments of our outstanding term loans. The proceeds during the year ended December 31, 2011 were primarily related to $19.2 million of net proceeds from our 2011 equity offering, $17.0 million of net proceeds from Term Loan B and C and $1.1 million of proceeds from the exercise of stock options, offset by $0.4 million in repayment of our term loans.
Oxford Loan and Security Agreement and Exalgo Royalty Sale
On December 22, 2010, we entered into a loan and security agreement with Oxford, pursuant to which Oxford agreed to lend us up to $20.0 million. Upon entering into the loan and security agreement, we borrowed $3.0 million from Oxford under Term Loan A. Under the terms of the loan and security agreement, we were
50
eligible, in our sole discretion, to borrow from Oxford up to an additional $8.5 million, at any time on or before July 15, 2011 under Term Loan B, and up to an additional $8.5 million, at any time on or before January 15, 2012 under Term Loan C, which we refer to collectively with Term Loan A and Term Loan B, the Term Loans. Our wholly owned subsidiary, Zalicus Pharmaceuticals Ltd., which we refer to as Zalicus Canada, is also a party to the loan and security agreement as a co-borrower. Our obligations under the loan and security agreement are secured by a first priority security interest in substantially all of our assets, including those of Zalicus Canada, other than intellectual property. We borrowed $8.5 million under Term Loan B on June 27, 2011 and borrowed $8.5 million under Term Loan C on December 16, 2011.
We were required to make interest only payments on the Term Loans on a monthly basis for the first six full calendar months subsequent to the funding of the applicable Term Loan. After the interest only period, we were required to make payments of outstanding principal and interest on each Term Loan in 36 equal monthly installments. Each Term Loan becomes due and payable 42 months after the date of the funding of each Term Loan. Interest on each Term Loan accrued at an annual fixed rate equal to 10.26%, 10.25% and 10.51%, for Term Loan A, Term Loan B and Term Loan C, respectively.
Upon the last payment date of the amounts borrowed under the loan and security agreement, whether on the maturity date of one of the Term Loans, on the date of any prepayment or on the date of acceleration in the event of a default, we were required to pay Oxford a final payment fee equal to 1.5% of any of the Term Loans borrowed.
Upon the occurrence of an event of default, which as defined in the loan and security agreement includes such matters as payment defaults, breaches of covenants, a material adverse change in the collateral, our business, operations or condition (financial or otherwise) and certain levies, attachments and other restraints on our business, the interest rate will be increased by five percentage points and all outstanding obligations will become immediately due and payable. The loan and security agreement also contains a subjective acceleration clause, which provided Oxford the ability to demand repayment of the loan early upon a material adverse change, as defined in the loan and security agreement. The portion of the Term Loans that is not due within 12 months of December 31, 2013 had been classified as long-term, as we believed a material adverse change was remote.
On January 31, 2014, we prepaid our outstanding Term Loans of approximately $8.6 million, including accrued interest, to Oxford under the loan and security agreement. The proceeds from the January 2014 royalty purchase agreement of $7.2 million, along with the royalty payment of approximately $2.0 million from Mallinckrodt for royalties earned on net sales of Exalgo for the quarter ended December 31, 2013, were sufficient to prepay the remaining obligation of approximately $8.6 million under the loan and security agreement. As a result, the loan and security agreement terminated effective January 31, 2014, and Oxford has released all security interests in our tangible and intangible property. As part of the sale of our Exalgo royalty stream pursuant to the royalty purchase agreement, we terminated any further rights we had to the payment of royalties on net sales of Exalgo by Mallinckrodt.
Equity Offerings
On February 9, 2011, we entered into an equity distribution agreement with Wedbush Securities Inc., or Wedbush, under which we could, from time to time, utilize our effective shelf registration statement to offer and sell our common stock having aggregate sales proceeds of up to $20.0 million through Wedbush, or to Wedbush, for resale. We agreed to pay Wedbush a commission, or allow a discount, of 3.0% of the gross proceeds from each sale. Effective March 31, 2011, we terminated this equity distribution agreement with Wedbush. Prior to terminating the agreement, we sold an aggregate of 1,480,800 shares of our common stock pursuant to the agreement for aggregate net proceeds of approximately $19.2 million.
On January 10, 2012, we entered into an equity distribution agreement with Wedbush under which we could, from time to time, offer and sell our common stock having aggregate sales proceeds of up to $15.0 million through Wedbush, or to Wedbush, for resale. We agreed to pay Wedbush a commission, or allow a discount, as
51
the case may be, equal to 2.5% of the gross offering proceeds from each sale. Effective March 28, 2012, we terminated this equity distribution agreement with Wedbush. Prior to terminating the agreement, we sold an aggregate of 2,342,996 shares of our common stock pursuant to the agreement for aggregate net proceeds of approximately $14.5 million.
On June 19, 2012, we entered into an equity distribution agreement with Wedbush under which we could, from time to time, issue and sell shares of our common stock having an aggregate offering price of up to $15.0 million from time to time through Wedbush acting as agent and/or principal. We agreed to pay Wedbush a commission, or allow a discount, of 2.0% of the gross proceeds from each sale. Effective August 1, 2012, we terminated this equity distribution agreement with Wedbush. Prior to terminating the agreement, we sold an aggregate of 2,181,993 shares of our common stock pursuant to the agreement for aggregate net proceeds of approximately $14.6 million.
On May 8, 2013, we entered into a purchase agreement with Lincoln Park Capital Fund, LLC, or LPC, pursuant to which we have the right to sell to LPC up to $25.0 million of shares of our common stock, subject to certain limitations and conditions set forth in the purchase agreement, over the period from May 8, 2013 to May 1, 2015. As consideration for entering into the purchase agreement, we also issued LPC 133,333 shares of common stock, for which we did not receive any cash proceeds. Under the purchase agreement, on any business day and as often as every other business day over the 24-month term of the purchase agreement, and up to an aggregate amount of an additional $23.0 million shares of our common stock (subject to certain limitations), we have the right, from time to time, at our sole discretion and subject to certain conditions to direct LPC to purchase up to 83,333 shares of our common stock. The purchase price of shares of common stock pursuant to the purchase agreement will be based on prevailing market prices of our common stock at the time of sales without any fixed discount, and we will control the timing and amount of any sales of common stock to LPC, but in no event will shares be sold to LPC on a day the closing price of our common stock is less than $1.00 per share, subject to adjustment. In addition, we may direct LPC to purchase additional amounts as accelerated purchases if on the date of a regular purchase the closing sale price of our common stock is not below $3.00 per share. There is no upper limit on the price per share that LPC could be obligated to pay for our common stock under the purchase agreement. We have the right to terminate the purchase agreement at any time, at no cost or penalty.
From the effective date of the purchase agreement through December 31, 2013, we issued an aggregate of 4,876,186 shares of common stock to LPC, including the 133,333 shares of common stock issued to LPC as consideration for entering into the purchase agreement, under the purchase agreement for gross proceeds of $19.8 million. Between January 1 and March 13, 2014, we did not issue any additional shares of common stock to LPC under the purchase agreement.
Reverse Stock Split
On October 22, 2012, we received a deficiency notice from NASDAQ notifying us that we were not in compliance with NASDAQ’s Marketplace Rule 5450(a)(1) (the “Rule”) because the bid price for our common stock, over the last 30 consecutive business days, had closed below the minimum $1.00 per share requirement for continued listing on the NASDAQ Global Market. The notification had no immediate effect on the listing of our common stock.
In accordance with Marketplace Rule 5810(c)(3)(A), we had a period of 180 calendar days, or until April 22, 2013, to regain compliance with the Rule. If at any time before April 22, 2013, the bid price of our common stock had closed at or above $1.00 per share for a minimum of 10 consecutive business days, NASDAQ would have provided written notification that we had achieved compliance with the Rule. We were not able to maintain the minimum closing bid price by April 22, 2013, and as a result, we voluntarily applied to transfer the listing of our common stock from the NASDAQ Global Market to the NASDAQ Capital Market. In connection with the transfer to the NASDAQ Capital Market, which occurred on April 23, 2013, we were granted an additional 180 days, or until October 21, 2013, to regain compliance by maintaining a minimum closing bid price of at least $1.00 for ten consecutive business days.
52
At our 2013 annual meeting of stockholders held on June 6, 2013, our shareholders approved a proposal to authorize an amendment to our sixth amended and restated certificate of incorporation to effect a reverse stock split of our issued and outstanding shares of common stock that may be implemented by our Board of Directors at their discretion at any time prior to the 2014 annual meeting of stockholders, pursuant to which any whole number of outstanding shares between and including 5 and 10 would be combined and reclassified into one share of our common stock. On September 18, 2013, the Board of Directors approved a 1-for-6 reverse stock split. We filed a certificate of amendment to our Sixth Amended and Restated Certificate of Incorporation with the Secretary of State of the State of Delaware that became effective on October 3, 2013, to effect a 1-for-6 reverse stock split of our common stock. On October 14, 2013, we received a letter from the listing qualifications department staff of the NASDAQ Capital Market that the closing bid price for our common stock had been at or above $1.00 per share for 10 consecutive business days and that we had regained compliance with Listing Rule 5450(a)(1).
Contractual Obligations and Commitments
The following table summarizes our contractual obligations at December 31, 2013 and the effects such obligations are expected to have on our liquidity and cash flows in future periods. The following table does not include our debt obligations to Oxford as of December 31, 2013, as the term loans were prepaid in full by Zalicus on January 31, 2014.
| | | | | | | | | | | | | | | | | | | | |
Contractual Obligations | | Total | | | 2014 | | | 2015
through
2016 | | | 2017
through
2018 | | | 2019
and
After | |
Capital lease obligations(1) | | $ | 17 | | | $ | 17 | | | $ | 0 | | | $ | 0 | | | $ | — | |
Operating lease obligations: | | | | | | | | | | | | | | | | | | | | |
Cambridge facility(2) | | | 3,791 | | | | 1,229 | | | | 2,460 | | | | 102 | | | | — | |
Purchase obligations(3) | | | 1,313 | | | | 1,313 | | | | 0 | | | | 0 | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total contractual obligations | | $ | 5,121 | | | $ | 2,559 | | | $ | 2,460 | | | $ | 102 | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | |
| (1) | On May 31, 2011, we entered into a capital lease for certain information technology equipment. Under the terms of the lease agreement, we are obligated to make fixed monthly lease payments, including interest, through May 2014. |
| (2) | On October 18, 2005, we entered into a lease agreement for approximately 40,000 square feet of office and laboratory space in Cambridge, Massachusetts. On March 9, 2006, we entered into an amendment to the lease agreement to lease an additional approximately 23,000 square feet of laboratory space. On August 3, 2009, we entered into a second amended lease agreement to reduce our leased space to approximately 23,000 square feet of office and laboratory space. The lease term, as amended, extends through January 2017. Our rent obligations under the lease, as amended, are reflected as operating lease obligations in the table above. The amounts in the table above do not include management fees payable to our landlord for our leased space, which are meant to cover our allocable share of property taxes, utilities and other common area costs. Our payment obligations under the amended lease are supported by a standby letter of credit totaling $1.8 million. |
| (3) | On February 8, 2012, we entered into a research collaboration agreement with Hydra Biosciences Inc., to advance the development of our preclinical ion channel modulator product candidates for the treatment of pain. Under the terms of the research collaboration agreement, we funded research and development activities at Hydra for a two-year period, which ended on February 8, 2014, during which time Hydra performed the discovery and preclinical development work necessary to advance our preclinical ion channel product candidates toward clinical development. |
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements or relationships with unconsolidated entities of financial partnerships, such as entities often referred to as structured finance or special purpose entities.
53
Climate Change
Recent scientific studies have suggested that emissions of certain gases, commonly referred to as “greenhouse gases,” may be contributing to warming of the earth’s atmosphere. As a result, there have been a variety of regulatory developments, proposals or requirements and legislative initiatives that have been introduced in the United States (as well as other parts of the world) that are focused on restricting the emission of carbon dioxide, methane and other greenhouse gases. Based upon our existing and currently expected future operations, we do not believe that climate change itself, or any current or future legislation aimed at preventing its advance, will have a significant effect on our operations.
| Item 7A. | Quantitative and Qualitative Disclosure about Market Risk |
We are exposed to market risk related to changes in interest rates and changes in the exchange rate of the United States dollar to the Canadian dollar. As of December 31, 2013, we had unrestricted cash and cash equivalents of $18.0 million consisting of cash and highly liquid short-term investments. Our cash is deposited in and invested through highly rated financial institutions in the United States and Canada.
Transactions by our subsidiary, Zalicus Canada, may be denominated in Canadian dollars, however, the entity’s functional currency is the United States dollar. Exchange gains or losses resulting from the translation between the currency in which a transaction is denominated and functional currency of Zalicus Canada are included in net loss for our consolidated financial statements. Fluctuations in exchange rates, primarily between the United States dollar and the Canadian dollar, may adversely affect our results of operations, financial position and cash flows. We do not hedge this exposure.
| Item 8. | Financial Statements and Supplementary Data |
The information called for by this item is indexed on page F-1 of this Annual Report on Form 10-K and is contained on pages F-2 through F-30.
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
| Item 9A. | Controls and Procedures |
| (a) | Evaluation of Disclosure Controls and Procedures |
As required by Rule 13a-15(b) of the Securities Exchange Act of 1934, as amended (the “1934 Act”), the company’s management, including the Chief Executive Officer and the Chief Financial Officer, conducted an evaluation as of the end of the period covered by this Annual Report on Form 10-K of the effectiveness of the design and operation of the company’s disclosure controls and procedures. Based on that evaluation, the company’s Chief Executive Officer and Chief Financial Officer concluded that the company’s disclosure controls and procedures are effective at the reasonable assurance level in ensuring that information required to be disclosed by the company in the reports that it files or submits under the 1934 Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms.
| (b) | Management’s Report on Internal Control Over Financial Reporting |
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) or 15d-15(f) promulgated under the 1934 Act as a process designed by, or under the supervision of, the issuer’s principal executive and principal financial officers, or persons performing similar functions, and effected by the issuer’s board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting
54
and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
| | (1) | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the issuer; |
| | (2) | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the issuer are being made only in accordance with authorizations of management and directors of the issuer; and |
| | (3) | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the issuer’s assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2013. In making this assessment, the company’s management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) inInternal Control-Integrated Framework (1992). Based on this assessment, our management concluded that, as of December 31, 2013, our internal control over financial reporting is effective based on those criteria.
Our independent registered accounting firm has issued an audit report on our internal control over financial reporting. The report appears below:
55
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Zalicus Inc.
We have audited Zalicus Inc.’s internal control over financial reporting as of December 31, 2013, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (1992 framework) (the COSO criteria). Zalicus Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Zalicus Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2013, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Zalicus Inc. as of December 31, 2013 and 2012, and the related consolidated statements of operations and comprehensive loss, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2013 and our report dated March 14, 2014 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Boston, Massachusetts
March 14, 2014
56
Changes in Internal Control
There has been no change to the company’s internal control over financial reporting during the last quarter of the fiscal year covered by this Annual Report on Form 10-K that has materially affected, or is reasonably likely to materially affect, the company’s internal control over financial reporting.
| Item 9B. | Other Information |
None.
PART III
| Item 10. | Directors and Executive Officers |
Information concerning our directors and executive officers will appear in our Proxy Statement for the 2014 Annual Meeting of Stockholders, to be filed pursuant to Regulation 14A on or before April 30, 2014, under the captions “Election of Directors” and “Executive Officers.” Such information is incorporated herein by reference.
Information concerning compliance with Section 16(a) of the Act of 1934 will appear in the company’s Proxy Statement for the 2014 Annual Meeting of Stockholders, to be filed pursuant to Regulation 14A on or before April 30, 2014, under the caption “Section 16(a) Beneficial Ownership Reporting Compliance.” Such information is incorporated herein by reference.
Information about our Audit Committee, including the members of the Committee, and our Audit Committee financial experts, will appear in our Proxy Statement for the 2014 Annual Meeting of Stockholders, to be filed pursuant to Regulation 14A on or before April 30, 2014, under the captions “The Audit Committee” and “Audit Committee Financial Experts.” Such information is incorporated herein by reference.
Information concerning our Code of Ethics and Conduct will appear in our Proxy Statement for the 2014 Annual Meeting of Stockholders, to be filed pursuant to Regulation 14A on or before April 30, 2014, under the caption “Code of Ethics and Conduct.” Such information is incorporated herein by reference.
| Item 11. | Executive Compensation |
Information in response to this item will appear in our Proxy Statement for the 2014 Annual Meeting of Stockholders, to be filed pursuant to Regulation 14A on or before April 30, 2014, under the captions “Executive Compensation,” “Director Compensation” and “Report of the Compensation Committee on Executive Compensation.” Such information is incorporated herein by reference.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
Information concerning security ownership of certain beneficial owners and management will appear in our Proxy Statement for the 2014 Annual Meeting of Stockholders, to be filed pursuant to Regulation 14A on or before April 30, 2014, under the caption “Security Ownership of Certain Beneficial Owners and Management.” Such information is incorporated herein by reference.
| Item 13. | Certain Relationships and Related Transactions |
Information concerning certain relationships and related transactions will appear in the company’s Proxy Statement for the 2014 Annual Meeting of Stockholders, to be filed pursuant to Regulation 14A on or before April 30, 2014, under the caption “Certain Relationships and Related Transactions.” Such information is incorporated herein by reference.
57
| Item 14. | Principal Accountant Fees and Services |
Information concerning principal accounting fees and services will appear in our Proxy Statement for the 2014 Annual Meeting of Stockholders, to be filed pursuant to Regulation 14A on or before April 30, 2014, under the caption “Independent Public Accountants.” Such information is incorporated herein by reference.
PART IV
| Item 15. | Exhibits, Financial Statement Schedules |
(a)(1)Financial Statements.
The consolidated financial statements filed as part of this Annual Report on Form 10-K are listed and indexed at page F-1.
(a)(2)Financial Statement Schedules.
Certain schedules are omitted because they are not applicable, or not required, or because the required information is included in the consolidated financial statements or notes thereto.
(a)(3)Exhibits.
The Exhibits listed in the Exhibit Index immediately preceding the Exhibits are filed as a part of this Annual Report on Form 10-K.
58
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | |
| ZALICUS INC. |
| |
| By: | | /s/ MARK CORRIGAN |
| | Mark Corrigan President and Chief Executive Officer |
Date: March 14, 2014
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the date indicated.
| | | | |
Signature | | Title | | Date |
| | |
/s/ MARK CORRIGAN Mark Corrigan | | President and Chief Executive Officer (Principal Executive Officer) | | March 14, 2014 |
| | |
/s/ JUSTIN A. RENZ Justin A. Renz | | Executive Vice President and Chief Financial Officer (Principal Financial and Accounting Officer) | | March 14, 2014 |
| | |
/s/ SALLY CRAWFORD Sally Crawford | | Director | | March 14, 2014 |
| | |
/s/ FRANK HAYDU Frank Haydu | | Director | | March 14, 2014 |
| | |
/s/ WILLIAM HUNTER William Hunter | | Director | | March 14, 2014 |
| | |
/s/ MICHAEL KAUFFMAN Michael Kauffman | | Director | | March 14, 2014 |
| | |
/s/ W. JAMES O’SHEA W. James O’Shea | | Director | | March 14, 2014 |
59
Zalicus Inc.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
F-1
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Zalicus Inc.
We have audited the accompanying consolidated balance sheets of Zalicus Inc. (the “Company”) as of December 31, 2013 and 2012, and the related consolidated statements of operations and comprehensive loss, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2013. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Zalicus Inc. at December 31, 2013 and 2012, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2013, in conformity with U.S. generally accepted accounting principles.
We have also audited, in accordance with standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of December 31, 2013, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (1992 framework) and our report dated March 14, 2014, expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Boston, Massachusetts
March 14, 2014
F-2
Zalicus Inc.
Consolidated Balance Sheets
(in thousands except per share data)
| | | | | | | | |
| | | December 31, | |
| | | 2013 | | | 2012 | |
Assets | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 17,958 | | | $ | 4,531 | |
Restricted cash | | | 50 | | | | 50 | |
Short-term investments | | | — | | | | 30,059 | |
Accounts receivable | | | 2,511 | | | | 3,045 | |
Prepaid expenses and other current assets | | | 223 | | | | 684 | |
| | | | | | | | |
Total current assets | | | 20,742 | | | | 38,369 | |
Property and equipment, net | | | 2,363 | | | | 3,535 | |
Intangible asset, net | | | 7,200 | | | | 17,654 | |
Restricted cash and other assets | | | 1,801 | | | | 1,817 | |
| | | | | | | | |
Total assets | | $ | 32,106 | | | $ | 61,375 | |
| | | | | | | | |
Liabilities and stockholders’ equity | | | | | | | | |
Current liabilities: | | | | | | | | |
Accounts payable | | $ | 2,237 | | | $ | 3,261 | |
Accrued expenses and other current liabilities | | | 3,339 | | | | 4,841 | |
Deferred revenue | | | 2,392 | | | | 4,918 | |
Current portion of term loan payable | | | 6,640 | | | | 6,327 | |
Current portion of lease incentive obligation | | | 284 | | | | 284 | |
| | | | | | | | |
Total current liabilities | | | 14,892 | | | | 19,631 | |
Term loan payable, net of current portion | | | 2,132 | | | | 8,772 | |
Deferred revenue, net of current portion | | | — | | | | 600 | |
Deferred rent, net of current portion | | | 309 | | | | 457 | |
Lease incentive obligation, net of current portion | | | 591 | | | | 875 | |
Other long-term liabilities | | | — | | | | 14 | |
Stockholders’ equity: | | | | | | | | |
Preferred stock, $0.001 par value; 5,000 shares authorized; no shares issued and outstanding | | | — | | | | — | |
Common stock, $0.001 par value; 200,000 shares authorized; 26,090 and 21,170 shares issued and outstanding at December 31, 2013 and 2012, respectively | | | 26 | | | | 21 | |
Additional paid-in capital | | | 393,796 | | | | 372,018 | |
Accumulated other comprehensive income | | | — | | | | 10 | |
Accumulated deficit | | | (379,640 | ) | | | (341,023 | ) |
| | | | | | | | |
Stockholders’ equity | | | 14,182 | | | | 31,026 | |
| | | | | | | | |
Total liabilities and stockholders’ equity | | $ | 32,106 | | | $ | 61,375 | |
| | | | | | | | |
See accompanying notes.
F-3
Zalicus Inc.
Consolidated Statements of Operations and Comprehensive Loss
(in thousands, except share and per share amounts)
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2013 | | | 2012 | | | 2011 | |
Revenue: | | | | | | | | | | | | |
Royalties | | $ | 6,661 | | | $ | 5,220 | | | $ | 2,521 | |
cHTS services and other collaborations | | | 8,070 | | | | 7,330 | | | | 5,663 | |
| | | | | | | | | | | | |
Total revenue | | | 14,731 | | | | 12,550 | | | | 8,184 | |
| | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | |
Research and development | | | 33,964 | | | | 41,423 | | | | 35,294 | |
General and administrative | | | 7,523 | | | | 8,977 | | | | 10,400 | |
Amortization of intangible | | | 8,722 | | | | 3,892 | | | | 5,141 | |
Impairment charge | | | 1,732 | | | | — | | | | — | |
Restructuring | | | — | | | | 1,094 | | | | — | |
| | | | | | | | | | | | |
Total operating expenses | | | 51,941 | | | | 55,386 | | | | 50,835 | |
| | | | | | | | | | | | |
Loss from operations | | | (37,210 | ) | | | (42,836 | ) | | | (42,651 | ) |
Interest income | | | 46 | | | | 150 | | | | 136 | |
Interest expense | | | (1,467 | ) | | | (2,173 | ) | | | (976 | ) |
Other income | | | 14 | | | | 91 | | | | 20 | |
| | | | | | | | | | | | |
Net loss before benefit for income taxes | | | (38,617 | ) | | | (44,768 | ) | | | (43,471 | ) |
Benefit for income taxes | | | — | | | | 441 | | | | 1,428 | |
| | | | | | | | | | | | |
Net loss | | $ | (38,617 | ) | | $ | (44,327 | ) | | $ | (42,043 | ) |
| | | | | | | | | | | | |
Net loss per share—basic and diluted | | $ | (1.68 | ) | | $ | (2.27 | ) | | $ | (2.59 | ) |
| | | | | | | | | | | | |
Weighted average number of common shares used in net loss per share calculation—basic and diluted | | | 22,932,675 | | | | 19,552,828 | | | | 16,224,532 | |
| | | | | | | | | | | | |
Net loss | | $ | (38,617 | ) | | $ | (44,327 | ) | | $ | (42,043 | ) |
Other comprehensive loss: | | | | | | | | | | | | |
Unrealized (loss) gain on investments | | | (10 | ) | | | 18 | | | | 12 | |
| | | | | | | | | | | | |
Comprehensive loss | | $ | (38,627 | ) | | $ | (44,309 | ) | | $ | (42,031 | ) |
| | | | | | | | | | | | |
See accompanying notes.
F-4
Zalicus Inc.
Consolidated Statements of Stockholders’ Equity
(in thousands, except share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock | | | Additional
Paid-in
Capital | | | Accumulated
Other
Comprehensive
Income (Loss) | | | Accumulated
Deficit | | | Total | |
| | | Shares | | | Par
Value | | | | | |
Balance at December 31, 2010 | | | 14,852,136 | | | $ | 15 | | | $ | 317,655 | | | $ | (20 | ) | | $ | (254,653 | ) | | $ | 62,997 | |
Comprehensive loss | | | — | | | | — | | | | — | | | | 12 | | | | (42,043 | ) | | | (42,031 | ) |
Exercise of stock options | | | 175,711 | | | | — | | | | 1,124 | | | | — | | | | — | | | | 1,124 | |
Vesting of restricted stock units | | | 31,250 | | | | — | | | | — | | | | — | | | | — | | | | — | |
Stock-based compensation expense | | | — | | | | — | | | | 2,196 | | | | — | | | | — | | | | 2,196 | |
Issuance of common stock, net of issuance costs | | | 1,480,800 | | | | 1 | | | | 19,198 | | | | — | | | | — | | | | 19,199 | |
Issuance of warrants in connection with term loan | | | — | | | | — | | | | 428 | | | | — | | | | — | | | | 428 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2011 | | | 16,539,897 | | | | 16 | | | | 340,601 | | | | (8 | ) | | | (296,696 | ) | | | 43,913 | |
Comprehensive loss | | | — | | | | — | | | | — | | | | 18 | | | | (44,327 | ) | | | (44,309 | ) |
Exercise of stock options | | | 25,090 | | | | — | | | | 124 | | | | — | | | | — | | | | 124 | |
Vesting of restricted stock units | | | 31,250 | | | | — | | | | — | | | | — | | | | — | | | | — | |
Stock-based compensation expense | | | — | | | | — | | | | 1,832 | | | | — | | | | — | | | | 1,832 | |
Issuance of common stock, net of issuance costs | | | 4,524,989 | | | | 5 | | | | 29,107 | | | | — | | | | — | | | | 29,112 | |
Severance payment settled in stock | | | 48,611 | | | | — | | | | 354 | | | | — | | | | — | | | | 354 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2012 | | | 21,169,837 | | | $ | 21 | | | $ | 372,018 | | | $ | 10 | | | $ | (341,023 | ) | | $ | 31,026 | |
Comprehensive loss | | | — | | | | — | | | | — | | | | (10 | ) | | | (38,617 | ) | | | (38,627 | ) |
Exercise of stock options | | | 2,916 | | | | — | | | | 7 | | | | — | | | | — | | | | 7 | |
Vesting of restricted stock units | | | 31,250 | | | | — | | | | — | | | | — | | | | — | | | | — | |
Stock-based compensation expense | | | — | | | | — | | | | 2,001 | | | | — | | | | — | | | | 2,001 | |
Issuance of common stock, net of issuance costs | | | 4,876,186 | | | | 5 | | | | 19,770 | | | | — | | | | — | | | | 19,775 | |
Severance payment settled in stock | | | 9,722 | | | | — | | | | — | | | | — | | | | — | | | | — | |
Partial shares paid out in connection with 1-for-6 stock split | | | (26 | ) | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2013 | | | 26,089,885 | | | $ | 26 | | | $ | 393,796 | | | $ | — | | | $ | (379,640 | ) | | $ | 14,182 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
See accompanying notes.
F-5
Zalicus Inc.
Consolidated Statements of Cash Flows
(in thousands)
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2013 | | | 2012 | | | 2011 | |
Operating activities | | | | | | | | | | | | |
Net loss | | $ | (38,617 | ) | | $ | (44,327 | ) | | $ | (42,043 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | |
Depreciation and amortization | | | 10,048 | | | | 5,373 | | | | 7,205 | |
Noncash impairment charge | | | 1,732 | | | | — | | | | — | |
Noncash restructuring charge | | | — | | | | (7 | ) | | | — | |
Noncash interest expense | | | 174 | | | | 266 | | | | 152 | |
Noncash rent expense | | | (284 | ) | | | (284 | ) | | | (283 | ) |
Stock-based compensation expense | | | 2,001 | | | | 1,832 | | | | 2,196 | |
Loss (gain) on fixed assets | | | 19 | | | | (8 | ) | | | 42 | |
Loss (gain) on foreign exchange | | | — | | | | 7 | | | | (53 | ) |
Decrease in deferred rent | | | (148 | ) | | | (148 | ) | | | (138 | ) |
Changes in assets and liabilities: | | | | | | | | | | | | |
Decrease (increase) in accounts receivable | | | 534 | | | | (1,159 | ) | | | (281 | ) |
Decrease (increase) in prepaid expenses and other assets | | | 472 | | | | 756 | | | | (460 | ) |
(Decrease) increase in accounts payable | | | (1,024 | ) | | | 1,518 | | | | (97 | ) |
(Decrease) increase in accrued restructuring | | | (34 | ) | | | 29 | | | | — | |
(Decrease) increase in accrued expenses and other long-term liabilities | | | (1,482 | ) | | | (1,506 | ) | | | 72 | |
(Decrease) increase in deferred revenue | | | (3,126 | ) | | | (831 | ) | | | 312 | |
| | | | | | | | | | | | |
Net cash used in operating activities | | | (29,735 | ) | | | (38,489 | ) | | | (33,376 | ) |
| | | | | | | | | | | | |
Investing activities | | | | | | | | | | | | |
Purchases of property and equipment | | | (180 | ) | | | (48 | ) | | | (350 | ) |
Proceeds from sales of property and equipment | | | 7 | | | | 308 | | | | 43 | |
Purchases of short-term investments | | | (31,848 | ) | | | (110,481 | ) | | | (160,196 | ) |
Sales and maturities of short-term investments | | | 61,907 | | | | 125,546 | | | | 156,883 | |
| | | | | | | | | | | | |
Net cash provided by (used in) investing activities | | | 29,886 | | | | 15,325 | | | | (3,620 | ) |
| | | | | | | | | | | | |
Financing activities | | | | | | | | | | | | |
Net proceeds from term loan | | | — | | | | — | | | | 16,979 | |
Repayment of term loan | | | (6,496 | ) | | | (4,293 | ) | | | (364 | ) |
Proceeds from issuance of common stock, net of issuance costs | | | 19,775 | | | | 29,112 | | | | 19,199 | |
Proceeds from exercise of stock options | | | 7 | | | | 124 | | | | 1,124 | |
| | | | | | | | | | | | |
Net cash provided by financing activities | | | 13,286 | | | | 24,943 | | | | 36,938 | |
| | | | | | | | | | | | |
Effect of exchange rate changes on cash and cash equivalents | | | (10 | ) | | | 2 | | | | (12 | ) |
| | | | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | | | 13,427 | | | | 1,781 | | | | (70 | ) |
Cash and cash equivalents at beginning of the period | | | 4,531 | | | | 2,750 | | | | 2,820 | |
| | | | | | | | | | | | |
Cash and cash equivalents at end of the period | | $ | 17,958 | | | $ | 4,531 | | | $ | 2,750 | |
| | | | | | | | | | | | |
Supplemental disclosure of cash flow information | | | | | | | | | | | | |
Cash paid for interest | | $ | 1,289 | | | $ | 1,851 | | | $ | 649 | |
| | | | | | | | | | | | |
Supplemental disclosure of noncash investing and financing activities | | | | | | | | | | | | |
Fair value of warrants issued in connection with issuance of term loans | | $ | — | | | $ | — | | | $ | 428 | |
| | | | | | | | | | | | |
Assets acquired under capital lease | | $ | — | | | $ | — | | | $ | 159 | |
| | | | | | | | | | | | |
See accompanying notes.
F-6
Notes to Consolidated Financial Statements
(in thousands, except share and per share amounts)
| 1. | | Nature of the Business |
On September 8, 2010, CombinatoRx, Incorporated changed its name to Zalicus Inc. In conjunction with the name change, the trading symbol for its common stock on the NASDAQ Global Market changed from “CRXX” to “ZLCS”. Zalicus Inc. was formed as a Delaware corporation on March 28, 2000. Zalicus Inc., and its subsidiaries (“Zalicus” or the “Company”), is a biopharmaceutical company developing drug candidates with a focus on the treatment of pain. To date, the Company has devoted substantially all of its resources to the development of its drug discovery technologies and the research and development of its drug candidates, including conducting preclinical and clinical trials and seeking intellectual property protection for its technology and product candidates.
On December 21, 2009, the Company completed a merger, referred to as the Neuromed Merger, with Neuromed Pharmaceuticals Inc., or Neuromed, pursuant to which Neuromed Pharmaceuticals Ltd., became a wholly-owned subsidiary of Zalicus. Neuromed Pharmaceuticals Ltd. is now named Zalicus Pharmaceuticals Ltd. and is referred to as Zalicus Canada.
The Company is subject to risks common to companies in the life science industry. All of its current product candidates are in preclinical or clinical development. If it does not successfully commercialize any of its product candidates, it will be unable to generate product revenue or achieve profitability.
The Company has a limited operating history and has incurred losses from operations since inception, resulting in an accumulated deficit of $379,640 at December 31, 2013. The Company’s cash and cash equivalents are expected to be sufficient to fund operations through at least December 31, 2014. The Company may seek additional funding through public or private equity or debt financings and collaboration agreements. Additional funding, if needed, may not be available to the Company on acceptable terms or at all. Any additional equity financing would be dilutive to existing stockholders, and any debt financing, if available, may involve restrictive covenants that could adversely impact how the Company conducts its business. If the Company is unable to obtain funding on a timely basis, it may be required to significantly curtail its business or one or more of its research or development programs. The Company also could be required to seek funds through arrangements with collaborators or others that may require the Company to relinquish rights to some of its technologies or product candidates which the Company would otherwise develop and pursue on its own.
| 2. | | Summary of Significant Accounting Policies |
Basis of Presentation and Principles of Consolidation
The accompanying consolidated financial statements have been prepared under U.S. generally accepted accounting principles, or GAAP. The consolidated financial statements include the accounts of the Company and its wholly-owned, controlled subsidiary. All intercompany transactions have been eliminated in consolidation.
Reverse Stock Split
The Company effected a 1-for-6 reverse stock split of its outstanding common stock on October 3, 2013. The accompanying consolidated financial statements and notes to the consolidated financial statements give retroactive effect to the reverse stock split for all periods presented. The shares of common stock retained a par value of $0.001 per share. Accordingly, stockholders’ equity reflects the reverse stock split by reclassifying from common stock to additional paid-in capital an amount equal to the par value of the decreased shares resulting from the reverse stock split. As a result of the reverse stock split, the Company regained compliance with the $1.00 per share minimum bid price requirement for continued listing on The NASDAQ Capital Market, effective October 14, 2013.
F-7
Reclassifications
Certain amounts in the prior year financial statements have been reclassified to conform to the current period presentation. During 2013, the Company changed its presentation of revenue to present royalty income separately from collaboration revenue and to combine government grant revenue with collaboration revenue. As a result, the Company reclassified (i) $5,220 and $2,521 from collaboration revenue to royalties for the years ended December 31, 2012 and 2011, respectively, and (ii) $453 and $589 from government contracts and grants to cHTS services and other collaborations for the years ended December 31, 2012 and 2011, respectively. The reclassifications had no effect on the Company’s previously reported consolidated balance sheet as of December 31, 2012 or statements of cash flows for the years ended December 31, 2012 and 2011.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Key estimates include the timing of clinical trial expenditures, estimates of the fair value of an intangible asset and stock compensation expense. Actual results could differ from those estimates.
Foreign Currency Transactions
The functional currency of the Company’s foreign subsidiary is the United States dollar. Foreign currency transaction gains and losses are recorded in the consolidated statement of operations. Net foreign exchange losses of $0 and $7 were recorded in other expense in the years ended December 31, 2013 and 2012, respectively, and a gain of $53 was recorded in other expense in the year ended December 31, 2011.
Comprehensive Loss
Comprehensive loss is the change in equity of a company during a period from transactions and other events and circumstances, excluding transactions resulting from investments by owners and distributions to owners. Comprehensive loss includes net loss and unrealized gain on investments for all periods presented.
In February 2013, the Financial Accounting Standards Board, or FASB, issued amended accounting guidance for reporting accumulated other comprehensive loss. The amendments require a company to provide information about the amounts reclassified out of accumulated other comprehensive income by component. In addition, a company is required to present, either on the face of the statement where net income is presented or in the notes, significant amounts reclassified out of accumulated other comprehensive income by the respective line items of net income. The Company adopted this guidance on January 1, 2013. The Company did not recognize any realized gains or losses from sales of its available-for-sale securities and did not reclassify any amounts out of accumulated other comprehensive income during the year ended December 31, 2013.
Revenue Recognition
Multiple-Element Arrangements
Under the authoritative guidance for revenue recognition related to multiple-element revenue arrangements, each deliverable within a multiple-element revenue arrangement is accounted for as a separate unit of accounting if both of the following criteria are met: (1) the delivered item or items have value to the customer on a standalone basis and (2) for an arrangement that includes a general right of return relative to the delivered item(s), delivery or performance of the undelivered item(s) is considered probable and substantially in the Company’s control. The Company considers a deliverable to have standalone value if the Company sells this item separately or if the item is sold by another vendor or could be resold by the customer. Deliverables not meeting the criteria for being a separate unit of accounting are accounted for on a combined basis.
In the event the Company enters into a contract in which the deliverables are required to be separated, the Company will allocate arrangement consideration to each deliverable in an arrangement based on its relative
F-8
selling price. The Company determines selling price using vendor-specific objective evidence (“VSOE”), if it exists; otherwise, the Company uses third-party evidence (“TPE”). If neither VSOE nor TPE of selling price exists for a deliverable, the Company uses estimated selling price (“ESP”) to allocate the arrangement consideration. The Company applies appropriate revenue recognition guidance to each unit of accounting.
Milestones
Contingent consideration from research and development activities that is earned upon the achievement of a substantive milestone is recognized in its entirety in the period in which the milestone is achieved. At the inception of each arrangement that includes milestone payments, the Company evaluates whether each milestone is substantive. This evaluation includes an assessment of whether: (a) the consideration is commensurate with either (1) the entity’s performance to achieve the milestone, or (2) the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from the entity’s performance to achieve the milestone, (b) the consideration relates solely to past performance and (c) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement. The Company evaluates factors such as the scientific, clinical, regulatory, commercial and other risks that must be overcome to achieve the respective milestone, the level of effort and investment required and whether the milestone consideration is reasonable relative to all deliverables and payment terms in the arrangement in making this assessment.
Collaborative Research and Development and Service Agreements
The Company has entered into collaborative research and development agreements and service agreements with other pharmaceutical and biotechnology companies, government agencies and charitable foundations. These agreements are generally in the form of research and development and license agreements. The agreements are primarily for early-stage compounds and are generally focused on specific disease areas. The agreements generally provide for nonrefundable up-front payments, milestone payments upon achieving significant milestone events and in some cases ongoing research funding. The agreements also contemplate royalty payments on sales if and when the product receives marketing approval by the FDA or other regulatory agencies.
The Company’s collaboration agreements typically include one or more of the following deliverables: research and development services, screening services, licenses to the Company’s high throughput screening analysis software and licenses to specific pharmaceutical compounds. The arrangements do not contain any substantive performance conditions or refund rights. The Company evaluates its arrangements with software license components in order to determine whether the arrangement should be accounted for under revenue recognition guidance for software or if other applicable revenue guidance should be applied. Revenue is recognized when there is persuasive evidence that an arrangement exists, delivery has occurred, the price is fixed or determinable, and collection is reasonably assured. Consideration allocated to licenses that do not have standalone value or amounts allocated to units of accounting that will be delivered or provided over a period of time, where the Company has a continuing obligation to perform services, are deferred and recognized over the performance period. Revenues for research and development and screening services are recognized as services are performed. Royalty revenue is recognized based upon net sales of licensed products as provided by the relevant license and is recognized in the period the sales occur. The periods over which revenue is recognized are subject to estimates by management and may change over the course of a collaborative agreement.
Government Contracts and Grants
Revenue under government grants or cost reimbursement contracts is recognized as the Company performs the underlying research and development activities.
Concentrations of Credit Risk
Financial instruments that potentially expose the Company to concentrations of credit risk consist of cash, cash equivalents, short-term investments and accounts receivable. The Company maintains its cash and cash
F-9
equivalents at high-quality financial institutions. The Company limits the amount of investment in any one type of investment, thereby reducing credit risk concentrations. The Company does not believe there is significant concentration of credit risk related to accounts receivable since its customers are primarily large well-capitalized pharmaceutical companies, foundations or government agencies.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original or remaining maturity of three months or less at the date of purchase to be cash equivalents, except for those funds managed by the Company’s investment manager, which are classified as short-term investments. Cash equivalents consist primarily of money market instruments.
Fair Value Measurements
The carrying values of the Company’s cash equivalents and term loan payable approximate their fair values.
The Company has certain assets and liabilities recorded at fair value, which have been classified as Level 1, 2 or 3 within the fair value hierarchy as described in the accounting standards for fair value measurements.
| | • | | Level 1 – Quoted market prices in active markets for identical assets or liabilities. Assets utilizing Level 1 inputs include money market funds, U.S. government securities and bank deposits. |
| | • | | Level 2 – Inputs other than Level 1 inputs that are either directly or indirectly observable, such as quoted market prices, interest rates and yield curves. Assets utilizing Level 2 inputs include U.S. agency securities, including direct issuance bonds and corporate bonds. |
| | • | | Level 3 – Unobservable inputs developed using estimates and assumptions developed by the Company, which reflect those that a market participant would use. |
During the years ended December 31, 2013 and 2012, there were no transfers between levels.
The Company had no assets or liabilities measured at fair value on a recurring basis in the accompanying consolidated balance sheet as of December 31, 2013. The following table summarizes the assets measured at fair value on a recurring basis in the accompanying consolidated balance sheet as of December 31, 2012:
| | | | | | | | | | | | | | | | |
| | | Fair Value Measurement as of December 31, 2012 | | | | |
| | | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
Assets: | | | | | | | | | | | | | | | | |
Short-term investments | | $ | 8,524 | | | $ | 21,535 | | | $ | — | | | $ | 30,059 | |
| | | | | | | | | | | | | | | | |
The Company’s Level 2 securities are valued using third-party pricing sources. These sources generally use interest rates and yield curves observable at commonly quoted intervals of similar assets as observable inputs for pricing.
The disclosed fair value of the Company’s term loan payable is determined using current applicable rates for similar instruments as of the balance sheet date. The carrying value of the Company’s term loan payable approximates fair value because of the length of the remaining term of the loan and an interest rate yield that is near current market rate yields. The disclosed fair value of the Company’s term loan payable is a Level 3 liability within the fair value hierarchy.
During the year ended December 31, 2013, the Company recorded an impairment charge of $1,732 in the consolidated statement of operations and comprehensive loss to reduce the carrying amount of the Exalgo intangible asset to its estimated fair value of $7,200 at December 31, 2013, which is based upon the sale of the
F-10
asset in January 2014 (see Note 5). The estimated fair value of the Exalgo intangible asset as of December 31, 2013is a Level 3 measurement and was determined after considering the proceeds from the sale of the asset in January 2014 of $7,200.
Property and Equipment
Property and equipment are recorded at cost and depreciated over their estimated useful lives using the straight-line method. Upon retirement or sale, the cost of assets disposed of and the related accumulated depreciation are removed from the accounts, and any resulting gain or loss is credited or charged to the statement of operations. Repairs and maintenance costs are expensed as incurred.
Accrued Clinical Expenses
As part of the process of preparing the Company’s financial statements, the Company is required to estimate its accrued expenses. This process involves reviewing open contracts and purchase orders, communicating with Company personnel to identify services that have been performed on its behalf and estimating the level of service performed and the associated cost incurred for the service when the Company has not yet been invoiced or otherwise notified of actual cost. Payments under some of the contracts the Company has with third parties depend on factors, such as the milestones accomplished, successful enrollment of certain numbers of patients, site initiation and the completion of clinical trial milestones. Examples of estimated accrued clinical expenses include:
| | • | | fees paid to investigative sites and laboratories in connection with clinical studies; |
| | • | | fees paid to clinical research organizations, or CROs, in connection with clinical studies, if CROs are used; and |
| | • | | fees paid to contract manufacturers in connection with the production of clinical study materials. |
In accruing clinical expenses, the Company estimates the time period over which services will be performed and the level of effort to be expended in each period. If possible, the Company obtains information regarding unbilled services directly from the service providers. However, the Company may be required to estimate the cost of these services based on information available to it. If the Company underestimates or overestimates the cost associated with a trial or service at a given point in time, adjustments to research and development expenses may be necessary in future periods. Historically, the Company’s estimated accrued clinical expenses have approximated actual expenses incurred.
Research and Development Expenses
Research and development expenses include all direct costs, including cash compensation, stock-based compensation and benefits for research and development personnel, supplies and materials, external costs including costs of clinical trials, formulation, manufacturing, preclinical programs, collaboration expenses, external consultants, infrastructure costs and overhead related to the development of drug candidates. These costs have been charged to research and development expense as incurred.
Nonrefundable advance payments for goods or services to be received in the future for use in research and development activities are deferred and recorded as a prepaid expense. The prepaid amounts are expensed as the related goods are delivered or the services are performed. If expectations change such that the Company does not expect it will need the goods to be delivered or the services to be rendered, prepaid nonrefundable advance payments would be charged to expense.
Impairment of Long-Lived Assets
The Company continually monitors whether events or circumstances have occurred that indicate that the carrying value of long-lived assets, including property and equipment and definite-lived intangible assets, may no
F-11
longer be recoverable or that the estimated remaining useful life of these assets may warrant revision. The carrying value for long-lived assets is reviewed for impairment when events or changes in circumstances indicate the carrying value of the assets may not be recoverable. An impairment loss would be recognized when estimated undiscounted future cash flows from the use of the asset and its eventual disposition are less than the asset’s carrying value. Any write-downs are treated as permanent reductions in the carrying value of the assets.
Accounting for Stock-Based Compensation
The Company recognizes as expense the estimated grant date fair value of all share-based payments to employees. The Company accounts for transactions in which services are received from non-employees in exchange for equity instruments based on the fair value of such services received or of the equity instruments issued, whichever is more reliably measured.
Income Taxes
Deferred taxes are determined based on the difference between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect in the years in which the differences are expected to reverse. Valuation allowances are provided, if, based upon the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized. The Company accounts for uncertain tax positions using a “more-likely-than-not” threshold for recognizing and resolving uncertain tax positions. The evaluation of uncertain tax positions is based on factors including, but not limited to, changes in tax law, the measurement of tax positions taken or expected to be taken in tax returns, the effective settlement of matters subject to audit, new audit activity and changes in facts or circumstances related to a tax position. The Company’s evaluation was performed for the tax years ended December 31, 2000 through December 31, 2013, the tax years which remain subject to examination by major tax jurisdictions as of December 31, 2013. The Company may from time to time be assessed interest or penalties by major tax jurisdictions, although any such assessments historically have not impacted the financial results of the Company. In the event the Company would receive an assessment for interest and/or penalties, it would be classified as general and administrative expense in the consolidated financial statements.
Recent Accounting Pronouncements
Unrecognized Tax Benefits
In July 2013, the FASB issued an accounting standards update clarifying the presentation of an unrecognized tax benefit when a net operating loss (“NOL”) carryforward, a similar tax loss, or a tax credit carryforward exists. The updated guidance requires the netting of unrecognized tax benefits against a deferred tax asset for a loss or other carryforward when settlement of the liability for an unrecognized tax benefit in this manner is available. The update is effective prospectively for reporting periods beginning after December 15, 2013, and early adoption and retrospective adoption are permitted. Because the guidance relates only to the presentation of unrecognized tax benefits, the Company does not expect the adoption in January 2014 to have a material effect on the Company’s results of operations or financial position.
| 3. | | Short-Term Investments |
Short-term investments consist primarily of investments with original maturities greater than ninety days and less than one year when purchased and also investments in money market funds. The Company classifies these investments as available-for-sale. Unrealized gains and losses are included in other comprehensive loss.
F-12
The Company had $0 of available-for-sale securities at December 31, 2013. Available-for-sale securities at December 31, 2012 consist of the following:
| | | | | | | | | | | | | | | | |
| | | Amortized
Cost | | | Unrealized
Gains | | | Unrealized
Losses | | | Fair
Value | |
December 31, 2012— | | | | | | | | | | | | | | | | |
Corporate debt securities | | $ | 21,525 | | | $ | 10 | | | $ | — | | | $ | 21,535 | |
Treasury money market funds | | | 8,524 | | | | — | | | | — | | | | 8,524 | |
| | | | | | | | | | | | | | | | |
| | $ | 30,049 | | | $ | 10 | | | $ | — | | | $ | 30,059 | |
| | | | | | | | | | | | | | | | |
The amortized cost and estimated fair value of investments in debt securities, which excludes money market funds, at December 31, 2012, by contractual maturity, were as follows:
| | | | | | | | |
| | | December 31, 2012 | |
| | | Cost | | | Estimated
Fair Value | |
Maturing in one year or less | | $ | 21,525 | | | $ | 21,535 | |
| | | | | | | | |
Maturing in more than one year | | $ | — | | | $ | — | |
| | | | | | | | |
The cost of securities sold is determined based on the specific identification method for purposes of recording realized gains and losses. Gross realized gains and losses on the sales of investments have not been material to the Company’s results of operations for all periods presented. As a matter of investment policy, the Company does not invest in auction rate securities.
| 4. | | Property and Equipment |
Property and equipment consist of the following:
| | | | | | | | | | |
| | | Estimated Useful Life (Years) | | December 31, | |
| | | | 2013 | | | 2012 | |
Leasehold improvements | | Lesser of useful life or life of lease | | $ | 5,596 | | | $ | 5,571 | |
Laboratory equipment | | 5 | | | 5,949 | | | | 6,036 | |
Computer equipment | | 3 | | | 843 | | | | 805 | |
Construction in progress | | — | | | 39 | | | | 5 | |
Capitalized software | | 5 | | | 815 | | | | 815 | |
Furniture and fixtures | | 3 | | | 497 | | | | 496 | |
| | | | | | | | | | |
| | | | | 13,739 | | | | 13,728 | |
Less: accumulated depreciation | | | | | (11,376 | ) | | | (10,193 | ) |
| | | | | | | | | | |
| | | | $ | 2,363 | | | $ | 3,535 | |
| | | | | | | | | | |
Depreciation expense, including amortization of assets recorded under capital leases, for the years ended December 31, 2013, 2012 and 2011 was approximately $1,326, $1,481 and $2,064, respectively.
The intangible asset relates to rights to receive milestone payments and royalties from Mallinckrodt Inc. for the commercial rights to Exalgo that were acquired as part of the merger with Neuromed Pharmaceuticals Ltd., or Neuromed (see Note 8). The intangible asset was initially recorded on December 21, 2009 at a value of $45,943 with an ongoing useful life of five years, representing the remaining patent life of Exalgo. The intangible asset was being amortized in a manner which reflected estimates of future undiscounted cash flows expected to be generated from Exalgo. At December 31, 2011, the Company evaluated the Exalgo intangible asset based on
F-13
estimates of future undiscounted cash flows and determined that the remaining useful life should be increased to four years. The Company recorded amortization expense of $8,722, $3,892 and $5,141 for the years ended December 31, 2013, 2012 and 2011, respectively.
On January 31, 2014, Zalicus Canada and Mallinckrodt Medical Imaging – Ireland, an affiliate of Mallinckrodt plc, entered into a Royalty Purchase Agreement (the “Royalty Purchase Agreement”) relating to the Asset Purchase Agreement, dated as of June 11, 2009, between Zalicus and Mallinckrodt Inc. (collectively with Mallinckrodt Medical Imaging – Ireland and Mallinckrodt plc, “Mallinckrodt”), as amended (the “Asset Purchase Agreement”). Under the terms of the Royalty Purchase Agreement, in exchange for the payment of $7,200 from Mallinckrodt to the Company on January 31, 2014, the Company has terminated any further rights it has to the payment of royalties on net sales of Exalgo by Mallinckrodt for any period subsequent to December 31, 2013.
As of December 31, 2013, the Company concluded that facts and circumstances existed which indicated that the carrying value of the intangible asset exceeded its estimated fair value. The Company determined the estimated fair value of the intangible asset as of December 31, 2013 based on the $7,200 in proceeds from the Royalty Purchase Agreement. As a result, during the year ended December 31, 2013, the Company recorded an impairment charge of $1,732 in the consolidated statements of operations and comprehensive loss.
As of December 31, 2013 and 2012, the gross carrying amount of the intangible asset was $7,200 and $45,943, respectively and accumulated amortization was $0 and $28,289, respectively.
On February 8, 2012, the Company committed to closing its discovery research operations in Vancouver, British Columbia, Canada. The Company closed its Vancouver discovery research operations to conserve capital while still advancing the Company’s preclinical ion channel modulator program. The closing of the Vancouver operations resulted in a workforce reduction of 16 employees. The Company completed the restructuring during the fourth quarter of 2012.
As a result of the closing of its Vancouver operations, the Company recorded a net restructuring charge of $1,094 in the year ended December 31, 2012. The restructuring charge was primarily associated with cash payments for severance and other personnel-related expenses. Severance payments continued into the first quarter of 2013. Costs associated with the restructuring are included in operating expenses in the statement of operations for the year ended December 31, 2012 and in current liabilities on the balance sheet at December 31, 2012.
The following table summarizes the activity of accrued restructuring expense as of December 31, 2013 related to the closing of the Company’s Vancouver discovery research operations:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Balance at
December 31, 2011 | | | Charges | | | Payments | | | Other | | | Balance at
December 31, 2012 | | | Payments | | | Balance at
December 31, 2013 | |
Termination benefits | | $ | — | | | $ | 1,101 | | | $ | (1,067 | ) | | $ | — | | | $ | 34 | | | $ | (34 | ) | | $ | — | |
Facilities | | | — | | | | (7 | ) | | | — | | | | 7 | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total | | $ | — | | | $ | 1,094 | | | $ | (1,067 | ) | | $ | 7 | | | $ | 34 | | | $ | (34 | ) | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
On December 22, 2010, the Company entered into a loan and security agreement (the “Loan and Security Agreement”) with Oxford Finance Corporation (the “Lender”), pursuant to which the Lender agreed to lend the Company up to $20,000. Upon entering into the Loan and Security Agreement, the Company borrowed $3,000 from the Lender (“Term Loan A”). Under the terms of the Loan and Security Agreement, the Company was eligible, in its sole discretion, borrow from the Lender up to an additional $8,500, at any time on or before
F-14
July 15, 2011 (“Term Loan B”) and up to an additional $8,500, at any time on or before January 15, 2012 (“Term Loan C”, collectively with Term Loan A and Term Loan B, the “Term Loans”). The Company’s wholly owned subsidiary, Zalicus Pharmaceuticals Ltd. (the “Subsidiary”), is also a party to the Loan and Security Agreement as a co-borrower. The Company’s obligations under the Loan and Security Agreement are secured by a first priority security interest in substantially all of the assets of the Company and the Subsidiary, other than intellectual property. The Company borrowed $8,500 under Term Loan B on June 27, 2011 and borrowed $8,500 under Term Loan C on December 16, 2011.
The Company was required to make interest only payments on the Term Loans on a monthly basis for the first six full calendar months subsequent to the funding of the applicable Term Loan. After the interest only period, the Company was required to make payments of outstanding principal and interest on each Term Loan in 36 equal monthly installments. Each Term Loan becomes due and payable 42 months after the date of the funding of each Term Loan. Interest on each Term Loan accrued at an annual fixed rate equal to 10.26%, 10.25% and 10.51% for Term Loan A, Term Loan B and Term Loan C, respectively.
Upon the last payment date of the amounts borrowed under the Loan and Security Agreement, whether on the maturity date of one of the Term Loans, on the date of any prepayment or on the date of acceleration in the event of a default, the Company was required to pay the Lender a final payment fee equal to 1.5% of any of the Term Loans borrowed. The Company recorded the final payment fee as interest expense over the term of the loan.
Upon the occurrence of an event of default, including payment defaults, breaches of covenants, a material adverse change in the collateral, the Company’s business, operations or condition (financial or otherwise) and certain levies, attachments and other restraints on the Company’s business, the interest rate will be increased by five percentage points and all outstanding obligations will become immediately due and payable. The Loan and Security Agreement also contains a subjective acceleration clause, which provides the Lender the ability to demand repayment of the loan early upon a material adverse change, as defined in the Loan and Security Agreement. The portion of the Term Loans that is not due within 12 months of December 31, 2013 has been classified as long-term, as the Company believed a material adverse change is remote.
In connection with the Loan and Security Agreement with Oxford, the Company issued to Oxford warrants to purchase its common stock each time the Company borrowed funds under the loan and security agreement. Specifically, on December 22, 2010, the Company issued Oxford a warrant to purchase 10,791 shares of its common stock with a per share exercise price of $8.34; on June 27, 2011, the Company issued Oxford a warrant to purchase 18,876 shares of its common stock with a per share exercise price of $13.50; and on December 16, 2011, the Company issued to Oxford a warrant to purchase 37,373 shares of its common stock with a per share exercise price of $6.84. The warrants are exercisable, in whole or in part, immediately, upon issuance and may be exercised on a cashless basis. The warrants will terminate on the earlier of December 22, 2017 and the closing of a merger or consolidation transaction in which Zalicus is not the surviving entity.
The fair values of the warrants issued in connection with Term Loan A, Term Loan B and Term Loan C were $81, $231 and $209, respectively and were recorded as a discount to the respective Term Loan. The Company also reimbursed the Lender certain costs associated with the Loan and Security Agreement of $42 which was also recorded as a discount to the respective Term Loans. The discounts are being amortized to interest expense over the 42 month period that applicable Term Loans are outstanding using the effective interest method.
On January 31, 2014, the Company prepaid its outstanding indebtedness of $8,647, including accrued interest, to the Lender under the Loan and Security Agreement. The proceeds from the Royalty Purchase Agreement of $7,200, along with the royalty payment of approximately $2,006 from Mallinckrodt for royalties earned on net sales of Exalgo for the quarter ended December 31, 2013, were sufficient to prepay the remaining balance of $8,647 under the Loan and Security Agreement. As a result, the Loan and Security Agreement is terminated effective January 31, 2014, and the Lender has released all security interests in the Company’s tangible and intangible property.
F-15
| 8. | | Research and Development Agreements |
Mallinckrodt Inc., a subsidiary of Covidien plc
In June 2009, Neuromed entered into the Asset Purchase Agreement with Mallinckrodt, then a subsidiary of Covidien plc, to sell all of the tangible and intangible assets associated with Exalgo, including the rights to develop and commercialize the product candidate in the United States. As part of the agreement, Neuromed received upfront payments of $15,000. The Company received a milestone payment of $40,000 following FDA approval of Exalgo in March 2010 and was eligible for tiered royalties on Mallinckrodt’s net sales of Exalgo. Mallinckrodt was obligated to pay these royalties on net sales for as long as it sold Exalgo although the royalty rate will be reduced upon the earlier to occur of generic competition or June 11, 2024. For the years ended December 31, 2013, 2012 and 2011, total revenue recognized from Mallinckrodt represented 45.2%, 42.0% and 35.0% of total revenue, respectively.
Mallinckrodt launched the commercial sale of Exalgo in the second quarter of 2010. The Company recognized $6,661, $5,220 and $2,521 of revenue related to royalties from Mallinckrodt’s sale of Exalgo during the years ended December 31, 2013, 2012 and 2011, respectively. The Company received payments related to Exalgo royalties totaling $6,120, $4,593 and $2,058 during the years ended December 31, 2013, 2012 and 2011, respectively.
Pursuant to the Royalty Purchase Agreement between the Company and Mallinckrodt, in exchange for the payment of $7,200 from Mallinckrodt to the Company on January 31, 2014, the Company has terminated any further rights it has to the payment of royalties on net sales of Exalgo by Mallinckrodt for any period subsequent to December 31, 2013.
Neuromed also entered into a development and transition services agreement with Mallinckrodt, pursuant to which the Company performed development and regulatory activities relating to the FDA approval of Exalgo and some post-approval activities. These activities were at Mallinckrodt’s cost and expense, capped at $16,000. Under the development and transition services agreement, which expired in November 2011, $8,763 was billed and received. The Company recorded $56 and $340 of revenue and received payments related to development and transition services totaling $100 and $371 during the years ended December 31, 2012 and 2011, respectively.
Fovea Pharmaceuticals SA
On January 30, 2006, the Company entered into a research and license agreement with Fovea Pharmaceuticals SA (“Fovea”). Under the terms of the agreement, Fovea agreed to conduct, at its own expense, preclinical and clinical development of combination drug candidates it selected from the Company’s portfolio of product candidates for certain ophthalmic indications, including creating ophthalmic formulations for these selected drug candidates. Fovea was acquired by Sanofi SA in October 2009 and is now a subsidiary of Sanofi.
On July 22, 2009, the Company and Fovea amended and restated the research and license agreement. Under the amended and restated agreement, the Company granted Fovea an exclusive worldwide license to certain drug combinations to treat allergic and inflammatory diseases of the front of the eye. Fovea has advanced one such combination, Prednisporin (FOV1101), through Phase 2b clinical development for allergic conjunctivitis and is currently seeking to continue the development of Prednisporin (FOV1101) through a sublicense.
The Company has received payments totaling $1.5 million related to Prednisporin (FOV1101) and is eligible to receive up to an additional $39.0 million from Fovea upon achievement of certain clinical and regulatory milestones for Prednisporin (FOV1101) and each other product candidate subject to the research and license agreement as follows:
| | • | | Up to $3.0 million in clinical development milestones. |
| | • | | Up to $21.0 million in regulatory milestones. |
| | • | | A $15.0 million milestone for the FDA approval of a product candidate to treat keratoconjunctivitis sicca, commonly known as dry eye syndrome. |
F-16
The most advanced product candidate subject to the Fovea agreement, Prednisporin (FOV1101), is not currently being developed, or planned to be developed, to treat dry eye syndrome, nor are there any other product candidates subject to the Fovea agreement that are currently being developed for dry eye syndrome. As a result, the Company believes that there is a remote likelihood that this milestone will be achieved. The Company did not recognize any milestone payments under this arrangement in the years ended December 31, 2013, 2012 and 2011.
Novartis
In May 2009, Zalicus entered into a research collaboration and license agreement (“Collaboration Agreement”) with Novartis Institutes of BioMedical Research, Inc., (“Novartis”), focused on the discovery of novel anti-cancer combinations. Through the collaboration, the Company is using its proprietary cHTS platform to screen a unique library of molecules, including Novartis compounds, in multiple cell lines representing a broad spectrum of cancers to potentially discover novel single agent and combination therapies to treat various cancers.
Under the terms of the Collaboration Agreement, the Company received an initial payment of $4,000 and will receive annual research support payments of up to $3,000, plus certain expenses. In addition, the Collaboration Agreement may provide the Company with up to $58,000 for each combination product candidate advanced by Novartis upon achievement of certain clinical, regulatory and commercial milestones as follows:
| | • | | Up to $5,000 in clinical development milestones. |
| | • | | Up to $23,000 in regulatory milestones. |
| | • | | Up to $30,000 in commercial milestones. |
The Company did not recognize any milestone payments under this arrangement in the years ended December 31, 2013, 2012, or 2011.
The Collaboration Agreement had an initial two-year term that could be extended by Novartis for three additional one-year periods. In January 2011, Novartis elected to extend the research program for an additional contract year, into May 2012. The Company also entered into a software license agreement with Novartis (“Software License”), where the Company provided Novartis with a non-exclusive license to use its proprietary Chalice™ software in connection with the collaboration and other Novartis research programs for approximately five years.
In April, 2012, Novartis and the Company entered into an amendment to the Collaboration Agreement to extend the funded research program pursuant to the Collaboration Agreement for an additional year until May 1, 2013. The amendment also changed the basis for up to $3,000 of annual research support payments under the Collaboration Agreement from payments to fund the Company’s full-time equivalent employees to payments based on the Company’s combination screening deliverables. The amendment was not a material modification of the arrangement pursuant to the accounting guidance for multiple-element arrangements.
In April 2013, Novartis and the Company entered into a second amendment to the Collaboration Agreement, to extend the funded research program under the Collaboration Agreement until October 31, 2014. The second amendment provides for up to an additional $3,000 in funded research payments under the Collaboration Agreement.
In April 2013, Novartis and the Company entered into an amendment to the Software License, to extend the term of the license until October 31, 2014, and to amend Novartis’ option to extend the term of the Software License to a single, one-year option, exercisable upon the payment of amended additional software licensing fees.
The library to be screened under the collaboration will consist of certain Novartis oncology compounds and compounds from the Company’s library of approved drugs and other molecules. Novartis will own and have an
F-17
exclusive license to intellectual property generated under the collaboration to research, develop and commercialize their approved or active development-stage compounds. The Company will own and have an exclusive license to intellectual property generated under the collaboration to research, develop and commercialize compounds from the Company’s library. Intellectual property generated under the collaboration using certain compounds from the Novartis library will be jointly owned by Novartis and the Company and non-exclusively licensed to allow each party to research, develop and commercialize product candidates. Under the Collaboration Agreement, Novartis retains an option, exercisable once per year of the Collaboration Agreement, to exclusively license a portion of this jointly owned intellectual property if certain conditions are met. Novartis also has a right of first negotiation to exclusively license the intellectual property owned by the Company that was discovered as a part of the collaboration, under terms to be negotiated by the parties at such time.
The Collaboration Agreement may be terminated by either party after ninety days’ notice upon an unremedied material breach and upon thirty days’ notice in the event of bankruptcy of the other party. Novartis may terminate the Collaboration Agreement after sixty days’ notice in the event of a change in control or liquidation of the Company, as defined in the Collaboration Agreement.
The Company is recognizing the total consideration under the agreement ratably over the extended Software License term. The Company recorded $4,833, $4,000 and $2,667 of revenue related to the research and license agreement for the years ended December 31, 2013, 2012 and 2011, respectively, representing 32.8%, 31.9% and 32.6% of total revenue for the years ended December 31, 2013, 2012 and 2011, respectively. The Company received payments related to the research and license agreement totaling $2,190 and $3,400 during the years ended December 31, 2013 and 2012, respectively. As of December 31, 2013 and 2012, the Company had unbilled receivables for this agreement totaling $263 and $450, respectively.
Amgen Inc.
In December 2009, Zalicus entered into a research collaboration agreement with Amgen Inc. (“Amgen”), focused on identifying synergistic combinations for two oncology targets of interest to Amgen. Under the agreement, the Company received a $750 payment in January 2010 to fund the initial research plan, and Amgen also agreed to reimburse the Company for laboratory supplies consumed. The initial research plan ended in September 2010, and Amgen elected for the Company to do follow-up research at an annual rate of $300 per full-time employee equivalent, plus the reimbursement of laboratory supplies. Amgen will also pay the Company a $1,000 milestone payment for each investigational new drug application filing by Amgen for a product candidate with new intellectual property generated by the collaboration. The Company did not recognize any milestone payments under this arrangement in the years ended December 31, 2013, 2012, and 2011. The Company also entered into a software license agreement with Amgen in May 2011, pursuant to which the Company provided Amgen with a non-exclusive license to use its proprietary Chalice software in connection with the collaboration and other Amgen research programs for one year. The Company entered into an amendment to this software license agreement with Amgen in August 2012, pursuant to which the Company provided Amgen with the same non-exclusive license for an additional year. Through December 31, 2013, the Company has received $2,855 in funding, expense reimbursement and software license fees under these agreements.
The Company recorded $1,183, $285 and $347 of revenue related to the agreements with Amgen for the years ended December 31, 2013, 2012 and 2011, respectively. The Company received payments related to the agreements with Amgen totaling $1,349, $153 and $378 during the years ended December 31, 2013, 2012 and 2011, respectively.
USAMRIID
In December 2008, the Company entered into a cooperative research and development agreement with the United States Army Medical Research Institute for Infectious Diseases, or USAMRIID, focused on discovering agents to prevent or treat Ebola, Marburg and Lassa virus infections, which was extended in October 2010.
F-18
Under the agreement, which expired in September 2011, the Company and USAMRIID undertook a joint research project, and the Company was eligible to receive up to $1,387 in funding. The Company received approximately $1,364 in funding from this agreement.
In May 2010, Zalicus entered into a cooperative research and development agreement with USAMRIID focused on discovering agents to prevent or treat Alphavirus infections. Under the agreement, which expired in April 2013, the Company and USAMRIID undertook a joint research project, and the Company was eligible to receive up to approximately $1,056 in funding. The Company received approximately $1,056 in funding from this agreement.
In September 2012, the Company entered into a cooperative research and development agreement with USAMRIID focused on discovering chemotherapeutic interventions to prevent and/or treat encephalitic and viral hemorrhagic fever virus infections. This agreement had an initial one-year term that was extended by USAMRIID for an additional one-year period. Zalicus and USAMRIID have mutually agreed to terminate the activities under the cooperative research and development agreement as of February 28, 2014. Under this agreement, the Company and USAMRIID undertook a joint research project, and the Company was eligible to receive up to approximately $353 in funding in the first year and $382 in funding in the second year. Through December 31, 2013, the Company has received approximately $458 in funding from this agreement.
The Company recorded $596, $453 and $560 of revenue related to the cooperative research and development agreements with USAMRIID for the years ended December 31, 2013, 2012 and 2011, respectively. The Company received payments related to the agreements totaling $557, $443 and $638 during the years ended December 31, 2013, 2012 and 2011, respectively. As of December 31, 2013 and December 31, 2012, the Company had unbilled receivables for these agreements totaling $19 and $59, respectively.
Other Research Agreements
The Company recorded revenue of $1,458, $2,536 and $1,749 related to other cHTS research agreements for the years ended December 31, 2013, 2012 and 2011, respectively. The Company received payments related to these agreements totaling $1,923, $1,870 and $1,770 during the years ended December 31, 2013, 2012 and 2011, respectively.
| 9. | | Research Collaboration Agreement |
In February 2012, the Company entered into a research collaboration agreement with Hydra Biosciences, Inc. (“Hydra”) to advance the development of its preclinical ion channel modulator product candidates for the treatment of pain. The collaboration brings together the Company’s portfolio of novel, preclinical ion channel product candidates, with Hydra’s expertise in novel ion channel discovery and preclinical drug development with the goal of advancing ion channel drug candidates into clinical development for the treatment of pain. Under the terms of the research collaboration agreement, the Company funded research and development activities at Hydra for a two-year period until February 2014, during which time Hydra performed the discovery and preclinical development work necessary to advance the Company’s preclinical ion channel product candidates toward clinical development. In addition, the Company will retain all intellectual property and commercial rights to its ion channel product candidates that are studied under the research collaboration agreement. The Company recorded research and development expense of $2,775 and $2,477 in the years ended December 31, 2013 and 2012, respectively, relating to this agreement and anticipates incurring payments of $1,313 over the remaining term of the agreement. The research collaboration agreement with Hydra expired in February 2014 in accordance with its terms.
Each share of the Company’s common stock, par value $0.001 per share (“Common Stock”), is entitled to one vote. The holders of common stock are also entitled to receive dividends whenever funds are legally available and when declared by the Board of Directors, subject to the prior rights of holders of all classes of stock outstanding.
F-19
Equity Financings
Equity Distribution Agreements
On February 9, 2011, the Company entered into an equity distribution agreement with Wedbush Securities Inc. (“Wedbush”), pursuant to which the Company could issue and sell shares of its Common Stock having an aggregate offering price of up to $20,000 from time to time through Wedbush acting as agent and/or principal. The Company agreed to pay Wedbush a commission, or allow a discount, of 3.0% of the gross proceeds from each sale. On March 31, 2011, the Company terminated its equity distribution agreement with Wedbush. The Company sold an aggregate of 1,480,800 shares of Common Stock for net proceeds of approximately $19,199.
On January 10, 2012, the Company entered into an equity distribution agreement with Wedbush, pursuant to which the Company could issue and sell shares of its Common Stock having an aggregate offering price of up to $15,000 from time to time through Wedbush acting as agent and/or principal. The Company agreed to pay Wedbush a commission, or allow a discount, of 2.5% of the gross proceeds from each sale. On March 28, 2012, the Company terminated its equity distribution agreement with Wedbush. The Company sold an aggregate of 2,342,996 shares of Common Stock for net proceeds of approximately $14,484.
On June 19, 2012, the Company entered into an equity distribution agreement with Wedbush, pursuant to which the Company could issue and sell shares of its Common Stock having an aggregate offering price of up to $15,000 from time to time through Wedbush acting as agent and/or principal. The Company agreed to pay Wedbush a commission, or allow a discount, of 2.0% of the gross proceeds from each sale. In August 2012, the Company terminated its equity distribution agreement with Wedbush. The Company sold an aggregate of 2,181,993 shares of Common Stock for net proceeds of approximately $14,628.
Purchase Agreement
On May 8, 2013, the Company entered into a purchase agreement (the “Purchase Agreement”) with Lincoln Park Capital Fund, LLC (“LPC”), pursuant to which the Company has the right to sell to LPC up to $25,000 of shares of the Company’s Common Stock, subject to certain limitations and conditions set forth in the Purchase Agreement, over the period from May 8, 2013 to May 1, 2015.
As consideration for entering into the Purchase Agreement, the Company also issued LPC 133,333 shares of Common Stock. The Company did not receive any cash proceeds from the issuance of these 133,333 shares. Under the Purchase Agreement, on any business day and as often as every other business day over the 24-month term of the Purchase Agreement, and up to an aggregate amount of an additional $23,000 (subject to certain limitations) in shares of Common Stock, the Company has the right, from time to time, at its sole discretion and subject to certain conditions to direct LPC to purchase up to 83,333 shares of Common Stock. The purchase price of shares of Common Stock pursuant to the Purchase Agreement will be based on prevailing market prices of Common Stock at the time of sales without any fixed discount, and the Company will control the timing and amount of any sales of Common Stock to LPC, but in no event will shares be sold to LPC on a day the Common Stock closing price is less than $1.00 per share, subject to adjustment. In addition, the Company may direct LPC to purchase additional amounts as accelerated purchases if on the date of a regular purchase the closing sale price of the Common Stock is not below $3.00 per share. There is no upper limit on the price per share that LPC could be obligated to pay for Common Stock under the Purchase Agreement. The Company has the right to terminate the Purchase Agreement at any time, at no cost or penalty
From the effective date of the Purchase Agreement through December 31, 2013, the Company issued a total of 4,876,186 shares of Common Stock to LPC, including the 133,333 shares of Common Stock issued to LPC as consideration for entering into the Purchase Agreement, for aggregate gross proceeds of $19,811.
Between January 1 and March 13, 2014, the Company did not issue any additional shares of Common Stock to LPC under the Purchase Agreement.
F-20
Reserved Shares
The Company has reserved a total of 2,960,818 shares of common stock for the exercise of stock options and warrants at December 31, 2013. The Company issued warrants to purchase 1,560 shares of common stock to General Electric Capital Corporation with an exercise price of $40.50 per share that expire on September 15, 2014 and June 28, 2015; and warrants to purchase 10,791, 18,876 and 37,373 shares of common stock to Oxford at exercise prices of $8.34, $13.50 and $6.84 per share, respectively. The Oxford warrants expire on December 22, 2017.
| 11. | | Stock Compensation Plans |
Summary of Stock Compensation Plans
In 2000, the Company adopted the 2000 Stock Plan (“2000 Plan”), as amended, under which 504,761 shares of the Company’s common stock were reserved for issuance to employees, officers, directors, advisors and consultants. Options granted under the 2000 Plan may be incentive stock options or non-statutory stock options. As of December 31, 2013, there were no options available for future issuance under the 2000 Plan.
In December 2004, the Board of Directors and stockholders adopted the 2004 Incentive Plan (“2004 Plan”), which was effective upon the Company’s initial public offering on November 9, 2005. On December 21, 2009, the Compensation Committee of the Board of Directors, in conjunction with the Company’s Annual Meeting, authorized an increase in the number of shares of common stock reserved for issuance. As of December 21, 2009, 3,333,333 shares of common stock were reserved for issuance under the 2004 Plan. The 2004 Plan includes an “evergreen provision” that allows for an annual increase in the number of shares of common stock available for issuance under the 2004 Plan, which annual increase will be added on the first day of each fiscal year from 2011 through 2015, inclusive, and will be equal to the least of (i) 666,667 shares of common stock, (ii) 4% of the outstanding shares on that date or (iii) such lesser amount determined by the Board of Directors. The Compensation Committee of the Board of Directors elected not to increase the number of shares of common stock available for issue under the 2004 Plan in each of the years subsequent to 2009. The 2004 Plan provides for the grant of incentive stock options, non-statutory stock options, restricted stock and unrestricted stock awards, stock appreciation rights, cash awards, performance awards and restricted stock units. Awards under the 2004 Plan may be granted to employees, directors, consultants and advisors. As of December 31, 2013, there were 1,137,977 shares available for future issuance under the 2004 plan.
The Board of Directors, or the Compensation Committee of the Board of Directors, administers the 2000 Plan and the 2004 Plan and has sole discretion to grant options to purchase shares of the Company’s common stock and other stock-based awards or to delegate to certain officers of the Company the ability to make specified grants. The Compensation Committee or the respective officers of the Company determine the exercise price and the period over which options become exercisable. However, incentive stock options may not be granted at less than 100% of the fair market value of the Company’s common stock as determined by the Compensation Committee at the time of grant, or for a term in excess of ten years. For holders of more than 10% of the Company’s total combined voting power of all classes of stock, incentive stock options may not be granted at less than 110% of the fair market value of the Company’s common stock at the date of grant, and for a term not to exceed five years.
The Company recognized stock compensation expense of $2,001, $1,832 and $2,196 for the years ended December 31, 2013, 2012 and 2011, respectively.
F-21
Stock Options
A summary of the status of the Company’s stock option plans at December 31, 2013 and changes during the year then ended are presented in the table and narrative below:
| | | | | | | | | | | | | | | | |
| | | Options | | | Weighted-
Average
Exercise Price | | | Weighted
Average
Remaining
Contractual
Term | | | Aggregate
Intrinsic Value | |
Outstanding at December 31, 2012 | | | 1,314,196 | | | $ | 9.37 | | | | | | | | | |
Granted | | | 604,901 | | | | 4.53 | | | | | | | | | |
Exercised | | | (2,916 | ) | | | 2.46 | | | | | | | | | |
Cancelled | | | (234,856 | ) | | | 9.44 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Outstanding at December 31, 2013 | | | 1,681,325 | | | $ | 7.63 | | | | 7.19 | | | $ | — | |
| | | | | | | | | | | | | | | | |
Vested or expected to vest at December 31, 2013 | | | 1,376,512 | | | $ | 8.29 | | | | 6.80 | | | $ | — | |
| | | | | | | | | | | | | | | | |
Exercisable at December 31, 2013 | | | 854,469 | | | $ | 9.50 | | | | 5.82 | | | $ | — | |
| | | | | | | | | | | | | | | | |
The aggregate intrinsic value in the table above represents the value (the difference between the Company’s closing common stock price on the last trading day of the year ended December 31, 2013 and the exercise price of the options, multiplied by the number of in-the-money options) that would have been received by the option holders had all option holders exercised their options on December 31, 2013. As of December 31, 2013, there was $1,892 of total unrecognized stock-based compensation expense related to stock options granted under the plans. The expense is expected to be recognized over a weighted-average period of 1.88 years. The weighted-average grant date fair value of options for the years ended December 31, 2013, 2012 and 2011 was $3.69, $5.32 and $11.47, respectively. The intrinsic value of stock options exercised for the years ended December 31, 2013, 2012 and 2011 was $3, $43 and $1,381, respectively, and represents the difference between the exercise price of the option and the market price of the Company’s common stock on the dates exercised.
Stock-based compensation expense for stock options with performance-based vesting criteria is only recognized when it is probable that the vesting criteria will be achieved. On February 8, 2011, the Company issued 235,000 stock options, which are included in the table above, with performance-based vesting criteria. The fair value of the options granted was determined using the Black-Scholes pricing model. On April 7, 2011, the performance-based vesting criteria were modified by the Compensation Committee of the Company’s Board of Directors. The Company calculated the fair value of the stock options at the modification date using the Black-Sholes pricing model. During 2012, 166,666 of these stock options were cancelled upon the termination of an executive. Also included in the cancelled options in the table above are 34,167 stock options that expired on January 1, 2013 as a result of performance-based vesting criteria not being achieved, and an additional 34,167 stock options that expired on July 1, 2013 as a result of separate performance-based vesting criteria not being achieved. Accordingly, for the years ended December 31, 2013, 2012 and 2011, the Company did not recognize any stock compensation expense related to these stock options.
The table above also includes 297,500 stock options issued on January 3, 2013 with performance-based vesting criteria. The fair value of the options granted was determined using the Black-Scholes pricing model. In November 2013, the Company learned that the vesting criteria would not be achieved with respect to these outstanding performance-based options, which was confirmed by the Compensation Committee of the Board of Directors, so the stock options would expire on July 1, 2014. Accordingly, for the year ended December 31, 2013, the Company did not recognize any stock compensation expense related to these stock options.
Valuation of Stock Options
The Company calculates the grant date fair value of stock options using the Black-Scholes option pricing model. The Black-Scholes option pricing model requires the Company to make certain assumptions and
F-22
estimates concerning its stock price volatility, the expected term of the awards, the rate of return of risk-free investments, and anticipated dividends. The Company determined that it has sufficient trading history as a public company to estimate stock price volatility and began to use its own historical volatility to develop such estimate. The Company utilizes the expected terms from an analysis of peer group companies to support the expected term used in the Black-Scholes model, as it does not believe it has sufficient historical data to support this assumption. The risk-free interest rates used are based on the United States Treasury yield curve in effect for periods corresponding with the expected life of the stock option. The Company has estimated forfeitures based upon an average of its historical data of option cancellations and employee turnover rates. Changes in estimated forfeitures are recognized through a cumulative true-up adjustment in the period of change. The resulting grant date fair value is recorded as compensation cost on a straight-line basis over the requisite service period, which generally equals the option vesting period.
During the years ended December 31, 2013, 2012 and 2011, respectively, the weighted-average assumptions used in the Black-Scholes model were as follows:
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2013 | | | 2012 | | | 2011 | |
Volatility factor | | | 106.47 | % | | | 102.40 | % | | | 102.93 | % |
Risk-free interest rate | | | 1.03 | % | | | 1.30 | % | | | 2.21 | % |
Dividend yield | | | — | % | | | — | % | | | — | % |
Expected term (in years) | | | 6.0 | | | | 6.0 | | | | 5.6 | |
Restricted Stock Units
During 2010, the Company issued performance-based Restricted Stock Units (“RSUs”) and RSUs with time-based vesting criteria to certain employees and directors. During 2013, the Company issued RSUs with time-based vesting criteria to its Chief Executive Officer. The fair value of RSUs is based on the closing price of the Company’s common stock on the award date. Expense for performance-based RSUs is recognized when it is probable the performance goal will be achieved. On March 1, 2010, the performance goal was achieved for the RSUs granted in 2010 such that all of the awards are expected to ultimately vest. The expense for this grant will be recognized on a straight-line basis over the four-year vesting period. A summary of the status of non-vested RSUs as of December 31, 2013 is as follows:
| | | | | | | | | | | | | | | | |
| | | Restricted Stock
Units | | | Weighted-
Average
Grant Date Fair
Value | | | Weighted
Average
Remaining
Contractual
Term | | | Aggregate
Intrinsic Value | |
Non-vested at December 31, 2012 | | | 62,500 | | | $ | 5.70 | | | | | | | | | |
Granted | | | 41,666 | | | | 4.56 | | | | | | | | | |
Vested | | | (31,250 | ) | | | 5.70 | | | | | | | | | |
Cancelled | | | — | | | | — | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Non-Vested at December 31, 2013 | | | 72,916 | | | $ | 5.05 | | | | 1.17 | | | $ | 81 | |
| | | | | | | | | | | | | | | | |
As of December 31, 2013, there was $129 of total unrecognized stock-based compensation expense related to non-vested RSUs granted under the 2004 Plan. The expense is expected to be recognized over a weighted-average period of one year. The total fair value of RSUs vested for the years ended December 31, 2013, 2012 and 2011 was $139, $219 and $403, respectively.
On February 22, 2012, the Company granted 58,333 shares of common stock to a former executive as part of a separation agreement. The shares were granted from the 2004 Plan. The shares were issued over the period from September 4, 2012 through February 1, 2013. The grant date fair value of the shares was $354 and was recorded as a settlement of a previously accrued liability.
F-23
| 12. | | Segment and Geographic Information |
Operating segments are defined as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision maker, or decision-making group, in deciding how to allocate resources and in assessing performance. The Company views its operations and manages its business in one operating segment. The Company operates in two geographic segments: the United States and Canada. As of December 31, 2013, $2,363 and $0 of the Company’s property and equipment was located in the United States and Canada, respectively. As of December 31, 2012, $3,535 and $0 of the Company’s property and equipment assets was located in the United States and Canada, respectively. For the years ended December 31, 2013, 2012 and 2011, none of the Company’s revenues were generated from customers located outside the United States.
The benefit for income taxes for the years ended December 31, 2013, 2012 and 2011 are as follows, in thousands:
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2013 | | | 2012 | | | 2011 | |
U.S.: | | | | | | | | | | | | |
Current | | $ | — | | | $ | — | | | $ | 34 | |
Deferred | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
Total U.S. | | | — | | | | — | | | | 34 | |
| | | | | | | | | | | | |
Foreign: | | | | | | | | | | | | |
Current | | | — | | | | 441 | | | | 1,394 | |
Deferred | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
Total foreign | | | — | | | | 441 | | | | 1,394 | |
| | | | | | | | | | | | |
Benefit for income taxes | | $ | — | | | $ | 441 | | | $ | 1,428 | |
| | | | | | | | | | | | |
A reconciliation of the expected income tax benefit computed using the federal statutory income tax rate to the Company’s effective income tax rate is as follows for the years ended December 31, 2013, 2012 and 2011:
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2013 | | | 2012 | | | 2011 | |
Income tax computed at federal statutory tax rate | | | 34.0 | % | | | 34.0 | % | | | 34 | % |
State taxes, net of federal benefit | | | 0.9 | % | | | 2.6 | % | | | 2.4 | % |
Change in valuation allowance | | | (35.7 | )% | | | (36.5 | )% | | | (36.2 | )% |
Stock-based compensation | | | (0.8 | )% | | | (0.6 | )% | | | (5.0 | )% |
Research and development credits | | | 0.2 | % | | | 0.7 | % | | | 1.6 | % |
Permanent differences | | | (0.2 | )% | | | (0.2 | )% | | | 0.1 | % |
Impact of state rate change | | | — | % | | | — | % | | | 0.1 | % |
Other | | | 1.6 | % | | | 1.0 | % | | | 6.2 | % |
| | | | | | | | | | | | |
Total | | | — | % | | | 1.0 | % | | | 3.2 | % |
| | | | | | | | | | | | |
The Company has incurred net operating losses since inception. At December 31, 2013, the Company had domestic federal and state net operating loss carryforwards of approximately $91,469 and $63,554, respectively, available to reduce future taxable income, which expire at various dates through 2033. At December 31, 2013, the Company also had federal and state research and development tax credit carryforwards of approximately $1,518 and $1,068, respectively, available to reduce future tax liabilities and which expire at various dates through 2033 and 2028, respectively. The Company also has foreign net operating loss carryforwards, subject to
F-24
certain limitations on use, of approximately $105,921, which expire at various dates through 2033. The Company also has foreign research and development tax credit carryforwards of $7,277, which never expire. The domestic net operating loss carryforwards included $3,349 of federal and $308 of state net operating losses that are attributable to stock option exercises which will be recorded as an increase in additional paid-in-capital once they are “realized”. Utilization of the domestic federal net operating loss and research and development credit carryforwards may be subject to a substantial annual limitation under Internal Revenue Code Section 382 of the Internal Revenue Code of 1986 due to ownership change limitations that could occur in the future.
Deferred taxes consist of the following:
| | | | | | | | |
| | | As of December 31, | |
| | | 2013 | | | 2012 | |
Net operating loss carryforwards | | $ | 60,839 | | | $ | 47,924 | |
Research and development credits/Investment tax credits | | | 14,224 | | | | 13,306 | |
Capitalized research and development costs | | | 5,630 | | | | 6,940 | |
Stock-based compensation | | | 4,127 | | | | 3,733 | |
Depreciation and amortization | | | 1,164 | | | | 1,150 | |
Accrued expenses | | | 58 | | | | 205 | |
Deferred revenue | | | 236 | | | | 1,178 | |
Other | | | (583 | ) | | | (493 | ) |
| | | | | | | | |
Deferred tax asset | | | 85,695 | | | | 73,943 | |
Deferred tax asset valuation allowance | | | (85,695 | ) | | | (73,943 | ) |
| | | | | | | | |
Net deferred tax asset | | $ | — | | | $ | — | |
| | | | | | | | |
Management of the Company has evaluated the positive and negative evidence bearing upon the realizability of its deferred tax assets. Management has determined at this time that it is more likely than not that the Company will not recognize the benefits of its federal and state deferred tax assets and, as a result, a valuation allowance of $85,695 and $73,943 has been established at December 31, 2013 and 2012, respectively. The change in the valuation allowance was $11,752 for the year ended December 31, 2013.
As a result of the acquisition of Neuromed, the Company recorded a liability for unrecognized tax benefits of $2,685 and interest and penalties of $560 in other long-term liabilities. During the years ended December 31, 2013, 2012 and 2011, the Company reversed $0, $441 and $1,395 of unrecognized tax benefits, respectively, and $0, $65 and $379 of related accrued interest and penalties, respectively, due to the expiration of the statute of limitations for certain tax years. The Company has not, as yet, conducted a study of its domestic research and development credit carryforwards. This study may result in an increase or decrease to the Company’s research and development credit carryforwards; however, until a study is completed and any adjustment is known, no amounts are being presented as an uncertain tax position. A full valuation allowance has been provided against the Company’s research and development credits, and if an adjustment is required, this adjustment would be offset by an adjustment to the valuation allowance. As a result, there would be no impact to the consolidated balance sheet, statement of operations and comprehensive loss or cash flows if an adjustment were required.
F-25
There were no uncertain tax positions at December 31, 2013. The following is a reconciliation of the Company’s gross uncertain tax positions at December 31, 2012 and 2011:
| | | | |
December 31, 2010 | | $ | 1,878 | |
Decrease related to positions taken in prior years | | | — | |
| | | | |
Expiration of statute of limitations | | | (1,395 | ) |
Foreign exchange revaluation | | | (52 | ) |
| | | | |
December 31, 2011 | | $ | 431 | |
| | | | |
Decrease related to positions taken in prior years | | | — | |
Expiration of statute of limitations | | | (441 | ) |
Foreign exchange revaluation | | | 10 | |
| | | | |
December 31, 2012 | | $ | — | |
| | | | |
The tax years 2000 through 2013 remain open to examination by major taxing jurisdictions to which the Company is subject, which are primarily in the United States and Canada, as carryforward attributes generated in years past may still be adjusted upon examination by the Internal Revenue Service, Revenue Canada or state or provincial tax authorities if they have or will be used in a future period. The Company is currently not under examination by the Internal Revenue Service or any other jurisdictions for any tax years. For the years ended December 31, 2013, 2012 and 2011, the Company recognized $0, $0 and $74 of interest expense, respectively, related to uncertain tax positions.
Accrued expenses consist of the following:
| | | | | | | | |
| | | As of December 31, | |
| | | 2013 | | | 2012 | |
Accrued clinical trial costs | | $ | 803 | | | $ | 1,535 | |
Accrued payroll and related benefits | | | 656 | | | | 1,696 | |
Accrued professional fees | | | 290 | | | | 179 | |
Accrued research collaboration expense | | | 1,015 | | | | 515 | |
Accrued other expenses | | | 575 | | | | 916 | |
| | | | | | | | |
| | $ | 3,339 | | | $ | 4,841 | |
| | | | | | | | |
On October 18, 2005, the Company entered into a lease agreement for approximately 40,000 square feet of office and laboratory space located in Cambridge, Massachusetts. The initial term of the lease commenced on September 1, 2006 for the office space and December 1, 2006 for the laboratory space and was to extend until November 30, 2016 with two five-year renewal options. The Company had the right to use and controlled physical access to the leased premises beginning on December 6, 2005. Thus, the effective lease term began on that date. In March 2006, the Company amended (the “First Amendment”) the October 18, 2005 operating lease agreement. The First Amendment provided for 23,199 square feet of additional laboratory space. The Company committed to lease this additional laboratory space through January 2017. In addition, the First Amendment extended the original lease term of the existing space an additional two months through January 2017.
Additionally, the lease, as amended, contains rent escalation, rent holiday, and leasehold improvement incentives. Rent escalation and rent holiday are being accounted for as rent expense under the straight-line method. In connection with the lease, the Company received approximately $6,900 in leasehold improvement incentives from the landlord. These leasehold improvement incentives are being accounted for as a reduction in
F-26
rent expense ratably over the lease term. The balance from these leasehold improvement incentives is included in current portion of lease incentive obligation and lease incentive obligation, net of current portion in the balance sheets at December 31, 2013 and 2012. Leasehold improvements are amortized using the straight-line method over the estimated useful lives of the assets or the term of the lease, whichever is shorter.
On August 3, 2009, the Company entered into a Second Amendment (the “Amendment”) to the Office and Laboratory Lease Agreement (the “Lease”). Prior to the Amendment of the Lease, which, as amended, expires in January 2017, the Company leased approximately 63,000 square feet of office and laboratory space.
In accordance with the terms of the Amendment, the Company and the landlord agreed that the Company’s occupancy of approximately 18,000 square feet of leased premises (the “Office Premises”) would cease as of June 16, 2009, and that the Company would be liable for rent payments and occupancy costs on the Office Premises through September 30, 2009. In addition, the Company and the Landlord agreed that the Company’s occupancy and liability for rent payments and occupancy costs of approximately 22,000 square feet of leased premises (the “Lab Premises”) would cease as of September 15, 2009.
Under the terms of the Lease, as amended by the Amendment, the Company continues to lease and occupy approximately 23,000 square feet of office and laboratory space with a lease term until January 2017. Under the Amendment, as consideration for the right to cease occupancy of the Office Premises and Lab Premises, the Company paid the Landlord $500 on October 1, 2009 and $1,000 in the year ended December 31, 2010, respectively.
In connection with the Amendment, the Company’s Letter of Credit for the benefit of the Landlord was reduced from $4,000 to $2,500, effective December 1, 2009 and was reduced to $1,800 effective as of December 1, 2010. The certificate of deposit that secures the letter of credit is included in restricted cash on the consolidated balance sheet in the amount of $1,800 for each of the years ended December 31, 2013 and 2012.
In connection with the merger with Neuromed in 2009, the Company assumed a sublease renewal and amendment agreement relating to the Company’s office and laboratory facility in Vancouver, British Columbia, Canada. Under the terms of this sublease the Company leased approximately 24,600 square feet of office and laboratory space, which was reduced to approximately 12,000 square feet. The Company renewed the sublease on December 14, 2011 through December 31, 2012. On February 9, 2012, the Company terminated the sublease, effective August 9, 2012.
The Company also leases certain office equipment under various operating leases. Total rent expense was $1,082, $1,322 and $1,486 for the years ended December 31, 2013, 2012 and 2011, respectively.
Future minimum lease payments under noncancelable operating leases at December 31, 2013 are as follows:
| | | | |
2014 | | $ | 1,229 | |
2015 | | | 1,230 | |
2016 | | | 1,230 | |
2017 | | | 102 | |
2018 | | | — | |
Thereafter | | | — | |
| | | | |
Total | | $ | 3,791 | |
| | | | |
F-27
On May 31, 2011 the Company entered into a capital lease for certain information technology equipment. At December 31, 2013 and 2012, $159 was recorded in equipment, and $142 and $89 was recorded in accumulated depreciation, respectively, related to this capital lease. A liability of $14 and $68 was recorded in accrued expenses and other current liabilities and other long-term liabilities at December 31, 2013 and 2012, respectively. Future minimum payments are as follows:
| | | | |
2014 | | $ | 14 | |
Total | | | 14 | |
Less interest | | | — | |
| | | | |
Capital lease obligation | | $ | 14 | |
| | | | |
Basic and diluted net loss per common share is calculated by dividing net loss applicable to common stockholders by the weighted-average number of common shares outstanding during the period, without consideration for common stock equivalents. The Company’s potentially dilutive shares, which include outstanding stock options, unvested restricted stock units, warrants and stock issuance commitments, are considered to be common stock equivalents and are only included in the calculation of diluted net loss per share when their effect is dilutive.
The following potentially dilutive securities outstanding prior to the use of the treasury stock method have been excluded from the computation of diluted weighted-average shares outstanding for the years ended December 31, 2013, 2012 and 2011, as they would be anti-dilutive.
| | | | | | | | | | | | |
| | | As of December 31, | |
| | | 2013 | | | 2012 | | | 2011 | |
Options outstanding | | | 1,681,325 | | | | 1,314,196 | | | | 1,262,231 | |
Unvested restricted stock or restricted stock units | | | 72,916 | | | | 62,500 | | | | 94,792 | |
Warrants outstanding | | | 68,600 | | | | 68,600 | | | | 68,600 | |
Outstanding stock issuance commitments (See Note 11) | | | — | | | | 9,722 | | | | — | |
| 17. | | Employee Benefit Plans |
In May 2001, the Company adopted the Zalicus Inc. 401(k) Plan (“401(k) Plan”). The 401(k) Plan allows employees to make pre-tax contributions up to the maximum allowable amount set by the IRS. Under the 401(k) Plan, the Company may make discretionary contributions as approved by the Board of Directors. During 2013, 2012 and 2011, the Company made contributions of $200, $237 and $240, respectively.
Effective December 1, 2007, the Company approved the Zalicus Nonqualified Deferred Compensation Plan (the “NQ Plan”), a non-qualified tax-deferred compensation plan in which certain senior managers and officers of the Company may participate. The NQ Plan provides a tax-favorable vehicle for deferring cash compensation, including base salary and bonus awards. Under the NQ Plan, each year a participant may defer up to 25% of his or her base salary and up to 100% of his or her annual cash bonus pay. The participant will at all times be vested in the portion of his or her account attributable to the compensation the participant has elected to defer under the NQ Plan. The Company has established a special account for each participant, however, the Company’s obligation to pay the balance credited to such account will at all times be an unfunded and unsecured obligation of the Company and rank on parity with other unsecured and unsubordinated indebtedness of the Company from time to time outstanding.
The Company may also credit to the account of each eligible participant who makes deferrals a matching contribution in an amount equal to 100% of the deferrals contributed by the participant for such plan year, up to a maximum amount equal to: (i) four percent (4%) of each such participant’s cash compensation for such year, less
F-28
(ii) the amount of matching contributions made to the Company’s qualified 401(k) Plan for such year on behalf of such participant. In order to be eligible for a matching contribution for a given year, a participant must be employed by the Company on the date the matching contribution is credited to the NQ Plan, which is currently planned to be the January following a participant’s election, and have deferred the maximum amount permitted under the Company’s tax-qualified Section 401(k) Plan for such year. A participant will become 100% vested in any employer contributions credited to his or her account upon the participant’s death, disability or a change in control (as defined in the NQ Plan). Deferred balances are credited to each participant’s account under the NQ Plan and will be credited, at periodic intervals, with earnings that track the actual rate of return for such period realized by the investment fund or funds or index or indices selected by such participant from the range of investment vehicles offered under the NQ Plan. Deferred amounts are paid, at the participant’s option, either in a lump sum or in annual installments over a period of up to ten years upon separation from service or up to five years for scheduled in-service withdrawals. The Company contributed approximately $89 to the NQ Plan as of December 31, 2013. As of December 31, 2013, there were no participants enrolled in the NQ Plan.
F-29
| 18. | | Quarterly Financial Information (unaudited) |
| | | | | | | | | | | | | | | | |
| | | First Quarter
Ended
March 31,
2013 | | | Second Quarter
Ended
June 30,
2013 | | | Third Quarter
Ended
September 30,
2013 | | | Fourth Quarter
Ended
December 31,
2013 | |
Revenue | | $ | 3,674 | | | $ | 3,891 | | | $ | 3,400 | | | $ | 3,766 | |
Operating expenses: | | | | | | | | | | | | | | | | |
Research and development | | | 7,079 | | | | 9,845 | | | | 9,343 | | | | 7,697 | |
General and administrative | | | 2,042 | | | | 2,059 | | | | 2,077 | | | | 1,345 | |
Amortization of intangible asset | | | 2,181 | | | | 2,180 | | | | 2,180 | | | | 2,181 | |
Impairment charge | | | — | | | | — | | | | — | | | | 1,732 | |
| | | | | | | | | | | | | | | | |
Total operating expenses | | | 11,302 | | | | 14,084 | | | | 13,600 | | | | 12,955 | |
Loss from operations | | | (7,628 | ) | | | (10,193 | ) | | | (10,200 | ) | | | (9,189 | ) |
Interest income | | | 23 | | | | 14 | | | | 6 | | | | 3 | |
Interest expense | | | (440 | ) | | | (393 | ) | | | (342 | ) | | | (292 | ) |
Other (expense) income | | | (2 | ) | | | 10 | | | | 7 | | | | (1 | ) |
| | | | | | | | | | | | | | | | |
Net loss before benefit for income taxes | | | (8,047 | ) | | | (10,562 | ) | | | (10,529 | ) | | | (9,479 | ) |
Benefit for income taxes | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | |
Net loss | | $ | (8,047 | ) | | $ | (10,562 | ) | | $ | (10,529 | ) | | $ | (9,479 | ) |
| | | | | | | | | | | | | | | | |
Net loss per share—basic and diluted | | $ | (0.38 | ) | | $ | (0.49 | ) | | $ | (0.46 | ) | | $ | (0.37 | ) |
| | | | | | | | | | | | | | | | |
Weighted-average number of common shares used in net loss per share calculation—basic and diluted | | | 21,204,220 | | | | 21,704,432 | | | | 22,931,109 | | | | 25,840,014 | |
| | | | | | | | | | | | | | | | |
Comprehensive loss | | $ | (8,053 | ) | | $ | (10,567 | ) | | $ | (10,528 | ) | | $ | (9,479 | ) |
| | | | | | | | | | | | | | | | |
| | | | |
| | | First Quarter
Ended
March 31,
2012 | | | Second Quarter
Ended
June 30,
2012 | | | Third Quarter
Ended
September 30,
2012 | | | Fourth Quarter
Ended
December 31,
2012 | |
Revenue | | $ | 2,320 | | | $ | 2,924 | | | $ | 3,518 | | | $ | 3,788 | |
Operating expenses: | | | | | | | | | | | | | | | | |
Research and development | | | 10,582 | | | | 9,861 | | | | 12,050 | | | | 8,930 | |
General and administrative | | | 2,663 | | | | 2,308 | | | | 2,201 | | | | 1,805 | |
Amortization of intangible asset | | | 973 | | | | 973 | | | | 974 | | | | 972 | |
Restructuring | | | 1,101 | | | | 28 | | | | (17 | ) | | | (18 | ) |
| | | | | | | | | | | | | | | | |
Total operating expenses | | | 15,319 | | | | 13,170 | | | | 15,208 | | | | 11,689 | |
Loss from operations | | | (12,999 | ) | | | (10,246 | ) | | | (11,690 | ) | | | (7,901 | ) |
Interest income | | | 41 | | | | 44 | | | | 35 | | | | 30 | |
Interest expense | | | (591 | ) | | | (563 | ) | | | (532 | ) | | | (487 | ) |
Other income | | | 7 | | | | — | | | | 13 | | | | 71 | |
| | | | | | | | | | | | | | | | |
Net loss before benefit for income taxes | | | (13,542 | ) | | | (10,765 | ) | | | (12,174 | ) | | | (8,287 | ) |
Benefit for income taxes | | | — | | | | 441 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | |
Net loss | | $ | (13,542 | ) | | $ | (10,324 | ) | | $ | (12,174 | ) | | $ | (8,287 | ) |
| | | | | | | | | | | | | | | | |
Net loss per share—basic and diluted | | $ | (0.78 | ) | | $ | (0.54 | ) | | $ | (0.59 | ) | | $ | (0.39 | ) |
| | | | | | | | | | | | | | | | |
Weighted-average number of common shares used in net loss per share calculation—basic and diluted | | | 17,298,345 | | | | 18,955,010 | | | | 20,760,483 | | | | 21,164,974 | |
| | | | | | | | | | | | | | | | |
Comprehensive loss | | $ | (13,520 | ) | | $ | (10,331 | ) | | $ | (12,171 | ) | | $ | (8,287 | ) |
| | | | | | | | | | | | | | | | |
F-30
EXHIBIT INDEX
| | | | | | | | | | | | | | | | | | |
Exhibit No. | | Description | | Incorporated by Reference to: | |
| | | Form or
Schedule | | | Exhibit
No. | | | Filing
Date with
SEC | | | SEC File
Number | |
| 2.1 | | Agreement and Plan of Merger, dated as of June 30, 2009, by and among the Registrant, PawSox, Inc., Neuromed Pharmaceuticals Inc., Neuromed Pharmaceuticals Ltd. and the Stockholder Representative named therein. | | | 8-K | | | | 2.1 | | | | 07/01/2009 | | | | 000-51171 | |
| | | | | |
| 2.2 | | Escrow Agreement, dated as of June 30, 2009, by and among the Registrant, Computershare Trust Company, N.A. and the Stockholder Representative. | | | 8-K | | | | 2.2 | | | | 07/01/2009 | | | | 000-51171 | |
| | | | | |
| 3.1 | | Sixth Amended and Restated Certificate of Incorporation of the Registrant. | | | S-1/A | | | | 3.2 | | | | 11/04/2005 | | | | 333-121173 | |
| | | | | |
| 3.2 | | Certificate of Amendment to the Registrant’s Sixth Amended and Restated Certificate of Incorporation. | | | 8-K | | | | 3.1 | | | | 12/21/2009 | | | | 000-51171 | |
| | | | | |
| 3.3 | | Certificate of Amendment to the Registrant’s Sixth Amended and Restated Certificate of Incorporation. | | | 8-K | | | | 3.1 | | | | 09/09/2010 | | | | 000-51171 | |
| | | | | |
| 3.4 | | Certificate of Amendment to the Registrant’s Sixth Amended and Restated Certificate of Incorporation. | | | 8-K | | | | 3.1 | | | | 10/02/2013 | | | | 000-51171 | |
| | | | | |
| 3.5 | | Amended and Restated By-Laws of the Registrant. | | | 8-K | | | | 3.2 | | | | 09/09/2010 | | | | 000-51171 | |
| | | | | |
| 4.1 | | Specimen Common Stock Certificate of the Registrant. | | | 10-Q | | | | 4.1 | | | | 11/10/2010 | | | | 000-51171 | |
| | | | | |
| 4.2 | | Warrant issued to General Electric Capital Corporation on September 15, 2004, to purchase up to 1,482 shares of the Registrant’s Common Stock. | | | S-1 | | | | 10.7 | | | | 12/10/2004 | | | | 333-121173 | |
| | | | | |
| 4.3 | | Warrant issued to General Electric Capital Corporation on June 28, 2005, to purchase 78.5 shares of the Registrant’s Common Stock. | | | 10-K | | | | 4.5 | | | | 03/20/2006 | | | | 000-51171 | |
| | | | | |
| 4.4 | | Form of Warrant issued to Oxford Finance Corporation on December 22, 2010. | | | 8-K | | | | 4.1 | | | | 12/22/2010 | | | | 000-51171 | |
| | | | | |
| #10.1 | | 2000 Stock Option Plan, as amended. | �� | | S-1 | | | | 10.1 | | | | 12/10/2004 | | | | 333-121173 | |
| | | | | |
| #10.2 | | Amended and Restated 2004 Incentive Plan. | | | S-8 | | | | 4.1 | | | | 04/16/2010 | | | | 333-166118 | |
| | | | | |
| #10.3 | | Form Incentive Stock Option Agreement under the 2004 Incentive Plan. | | | 10-K | | | | 10.3 | | | | 03/20/2006 | | | | 000-51171 | |
| | | | | |
| #10.4 | | Form Non-Qualified Option Agreement under the 2004 Incentive Plan. | | | 10-K | | | | 10.4 | | | | 03/20/2006 | | | | 000-51171 | |
| | | | | |
| #10.5 | | Nonqualified Deferred Compensation Plan, effective December 1, 2007. | | | S-8 | | | | 4.1 | | | | 11/30/2007 | | | | 333-147745 | |
| | | | | |
| #10.6 | | Employment Letter Agreement with Jason Cole, dated as of January 23, 2006. | | | 10-K | | | | 10.15 | | | | 03/20/2006 | | | | 000-51171 | |
| | | | | |
| #10.7 | | Amendment to Employment Agreement with Jason Cole, dated December 15, 2008. | | | 10-K | | | | 10.13 | | | | 03/16/2009 | | | | 000-51171 | |
| | | | | |
| #10.8 | | Amendment to Employment Agreement with Jason Cole, dated January 4, 2013. | | | 8-K | | | | 10.2 | | | | 01/09/2013 | | | | 000-51171 | |
| | | | | |
| #10.9 | | Amended and Restated Restricted Stock Award Agreement, dated September 5, 2008, by and between Jason Cole and the Registrant. | | | 10-Q | | | | 10.25 | | | | 11/10/2008 | | | | 000-51171 | |
| | | | | | | | | | | | | | | | | | |
Exhibit No. | | Description | | Incorporated by Reference to: | |
| | | Form or
Schedule | | | Exhibit
No. | | | Filing
Date with
SEC | | | SEC File
Number | |
| | | | | |
| #10.10 | | Employment Letter Agreement with Justin Renz, dated as of August 31, 2006. | | | S-4 | | | | 10.14 | | | | 08/07/2009 | | | | 333-161146 | |
| | | | | |
| #10.11 | | Letter Agreement Re: Severance Arrangements with Justin Renz, dated December 12, 2008. | | | S-4 | | | | 10.16 | | | | 08/07/2009 | | | | 333-161146 | |
| | | | | |
| #10.12 | | Letter Agreement Re: Severance Arrangements with Justin Renz, dated January 4, 2013. | | | 8-K | | | | 10.1 | | | | 01/09/2013 | | | | 000-51171 | |
| | | | | |
| #10.13 | | Employment Agreement with Mark H. N. Corrigan, dated as of March 8, 2010. | | | 8-K | | | | 10.1 | | | | 03/08/2010 | | | | 000-51171 | |
| | | | | |
| #10.14 | | Form Incentive Stock Option Agreement with Mark H. N. Corrigan, dated as of January 15, 2010. | | | 8-K | | | | 10.1 | | | | 01/20/2010 | | | | 000-51171 | |
| | | | | |
| #10.15 | | Form Nonstatutory Stock Option Agreement with Mark H. N. Corrigan, dated as of January 15, 2010. | | | 8-K | | | | 10.2 | | | | 01/20/2010 | | | | 000-51171 | |
| | | | | |
| #10.16 | | Form Restricted Stock Unit Agreement between the Registrant and Mark Corrigan, dated as of January 15, 2010. | | | 8-K | | | | 10.3 | | | | 01/20/2010 | | | | 000-51171 | |
| | | | | |
| #10.17 | | Restricted Stock Unit Agreement between the Registrant and Mark Corrigan, dated as of January 3, 2013. | | | 10-K | | | | 10.17 | | | | 03/07/2013 | | | | 000-51171 | |
| | | | | |
| #10.18 | | Revised Offer Letter from Neuromed Pharmaceuticals accepted by Christopher C. Gallen, dated May 27, 2004. | | | S-4 | | | | 10.45 | | | | 08/07/2009 | | | | 333-161146 | |
| | | | | |
| #10.19 | | Separation Agreement, dated as of February 22, 2012 between the Registrant and Christopher C. Gallen | | | 10-K | | | | 10.20 | | | | 03/09/2012 | | | | 000-51171 | |
| | | | | |
| #10.20 | | Neuromed Pharmaceuticals Inc. Special Equity Incentive Plan. | | | S-4/A | | | | 10.51 | | | | 09/16/2009 | | | | 333-161146 | |
| | | | | |
| #10.21 | | The Registrant’s Form of Restricted Stock Unit Agreement for awards granted under the Neuromed Pharmaceuticals Inc. Special Equity Incentive Plan (Directors). | | | S-4/A | | | | 10.52 | | | | 09/16/2009 | | | | 333-161146 | |
| | | | | |
| #10.22 | | The Registrant’s Form of Restricted Stock Unit Agreement for awards granted under the Neuromed Pharmaceuticals Inc. Special Equity Incentive Plan (Executives). | | | S-4/A | | | | 10.53 | | | | 09/16/2009 | | | | 333-161146 | |
| | | | | |
| #10.23 | | The Registrant’s Form Stock Option Agreement for performance-based stock options, dated as of February 8, 2011. | | | 10-Q | | | | 10.24 | | | | 05/05/2011 | | | | 000-51171 | |
| | | | | |
| #10.24 | | The Registrant’s Form Stock Option Agreement for performance-based stock options, dated as of February 8, 2011. | | | 10-Q | | | | 10.25 | | | | 05/05/2011 | | | | 000-51171 | |
| | | | | |
| #10.25 | | The Registrant’s Form Stock Option Agreement for performance-based stock options, dated January 3, 2013. | | | 10-K | | | | 10.25 | | | | 03/07/2013 | | | | 000-51171 | |
| | | | | |
| 10.26 | | Form of Indemnification Agreement for Directors. | | | 8-K | | | | 10.1 | | | | 12/21/2009 | | | | 000-51171 | |
| | | | | |
| 10.27 | | Second Amended and Restated Research and License Agreement, dated July 22, 2009, between Fovea Pharmaceuticals SA and the Registrant. | | | 8-K | | | | 10.1 | | | | 07/23/2009 | | | | 000-51171 | |
| | | | | | | | | | | | | | | | | | |
Exhibit No. | | Description | | Incorporated by Reference to: | |
| | | Form or
Schedule | | | Exhibit
No. | | | Filing
Date with
SEC | | | SEC File
Number | |
| | | | | |
| †10.28 | | Research Collaboration and License Agreement, dated as of May 1, 2009, by and between the Registrant and Novartis Institutes for BioMedical Research, Inc. | | | 10-Q | | | | 10.43 | | | | 05/11/2009 | | | | 000-51171 | |
| | | | | |
| 10.29 | | Amendment to Research Collaboration and License Agreement, dated April 12, 2012, by and between Registrant and Novartis Institutes for BioMedical Research, Inc. | | | 10-Q | | | | 10.30 | | | | 05/04/2012 | | | | 000-51171 | |
| | | | | |
| 10.30 | | Amendment No. 2 to Research Collaboration and License Agreement, dated April 30, 2013, by and between Registrant and Novartis Institutes for BioMedical Research, Inc. | | | 8-K | | | | 10.1 | | | | 05/01/2013 | | | | 000-51171 | |
| | | | | |
| 10.31 | | Software License Agreement, dated as of May 1, 2009, by and between the Registrant and Novartis Institutes for BioMedical Research, Inc. | | | 10-Q | | | | 10.44 | | | | 05/11/2009 | | | | 000-51171 | |
| | | | | |
| 10.32 | | Amendment No. 1 to Software License Agreement, dated as of April 30, 2013, by and between the Registrant and Novartis Institutes for BioMedical Research, Inc. | | | 8-K | | | | 10.2 | | | | 05/01/2013 | | | | 000-51171 | |
| | | | | |
| †10.33 | | Asset Purchase Agreement, dated as of June 11, 2009, between Neuromed Development Inc. and Mallinckrodt Inc. and amendments thereto. | | | S-4/A | | | | 10.35 | | | | 10/13/2009 | | | | 333-161146 | |
| | | | | |
| †10.34 | | Development and Transition Services Agreement, dated as of June 11, 2009, by and among Neuromed Development Inc., Neuromed Pharmaceuticals Ltd. and Mallinckrodt Inc. | | | S-4/A | | | | 10.40 | | | | 10/13/2009 | | | | 333-161146 | |
| | | | | |
| 10.35 | | Office and Laboratory Lease Agreement, dated as of October 18, 2005, by and between MA-Riverview, 245 First Street, L.L.C. and the Registrant. | | | S-1/A | | | | 10.48 | | | | 10/24/2005 | | | | 333-121173 | |
| | | | | |
| 10.36 | | First Amendment to Office and Laboratory Lease Agreement, dated as of March 9, 2006, by and between MA-Riverview, 245 First Street, L.L.C. and the Registrant. | | | 10-K | | | | 10.44 | | | | 03/20/2006 | | | | 000-51171 | |
| | | | | |
| 10.37 | | Second Amendment to Office and Laboratory Lease Agreement, dated as of August 3, 2009, by and between MA-Riverview, 245 First Street, L.L.C. and the Registrant. | | | 8-K | | | | 10.1 | | | | 08/04/2009 | | | | 000-51171 | |
| | | | | |
| 10.38 | | Sub-Lease Agreement between Neuromed Pharmaceuticals Inc. and Discovery Parks, Inc. | | | S-4/A | | | | 10.56 | | | | 10/09/2009 | | | | 333-161146 | |
| | | | | |
| 10.39 | | Sub-Lease Renewal and Amendment Agreement between Neuromed Pharmaceuticals Ltd. and Discovery Parks, Inc., dated as of November 2, 2009. | | | 10-K | | | | 10.54 | | | | 03/26/2010 | | | | 000-51171 | |
| | | | | |
| 10.40 | | Sub-Lease Renewal Agreement between Zalicus Pharmaceuticals Ltd. and Discovery Parks, Inc., dated as of September 16, 2010. | | | 10-Q | | | | 10.45 | | | | 11/10/2010 | | | | 000-51171 | |
| | | | | |
| 10.41 | | Loan and Security Agreement dated as of December 22, 2010, by and among the Registrant and Oxford Finance Corporation. | | | 8-K | | | | 10.1 | | | | 12/22/2010 | | | | 000-51171 | |
| | | | | | | | | | | | | | | | | | |
Exhibit No. | | Description | | Incorporated by Reference to: | |
| | | Form or
Schedule | | | Exhibit
No. | | | Filing
Date with
SEC | | | SEC File
Number | |
| | | | | |
| 10.42 | | Consent and First Loan Modification Agreement dated as of February 15, 2012, by and among the Registrant and Oxford Finance LLC. | | | 10-K | | | | 10.41 | | | | 03/02/2012 | | | | 000-51171 | |
| | | | | |
| 10.43 | | Equity Distribution Agreement dated as of February 9, 2011 between the Registrant and Wedbush Securities Inc. | | | 8-K | | | | 10.1 | | | | 02/09/2011 | | | | 000-51171 | |
| | | | | |
| 10.44 | | Equity Distribution Agreement dated as of January 10, 2012 between the Registrant and Wedbush Securities Inc. | | | 8-K | | | | 10.1 | | | | 01/11/2012 | | | | 000-51171 | |
| | | | | |
| 10.45 | | Equity Distribution Agreement dated as of June 29, 2012 between the Registrant and Wedbush Securities Inc. | | | 8-K | | | | 10.1 | | | | 06/20/2012 | | | | 000-51171 | |
| | | | | |
| 10.46 | | Purchase Agreement dated as of May 8, 2013 between the Registrant and Lincoln Park Capital Fund, LLC. | | | 8-K | | | | 10.1 | | | | 05/08/2013 | | | | 000-51171 | |
| | | | | |
| 10.47 | | Royalty Purchase Agreement dated as of January 31, 2014 between Zalicus Pharmaceuticals Ltd. and Mallinckrodt Medical Imaging—Ireland. | | | 8-K | | | | 10.1 | | | | 02/03/2014 | | | | 000-51171 | |
| | | | | |
| *21.1 | | List of subsidiaries | | | | | | | | | | | | | | | | |
| | | | | |
| *23.1 | | Consent of Ernst & Young LLP | | | | | | | | | | | | | | | | |
| | | | | |
| *31.1 | | Certification of Chief Executive Officer pursuant to Exchange Act rules 13a-14 or 15d-14. | | | | | | | | | | | | | | | | |
| | | | | |
| *31.2 | | Certification of Chief Financial Officer pursuant to Exchange Act rules 13a-14 or 15d-14. | | | | | | | | | | | | | | | | |
| | | | | |
| *32.1 | | Certifications of Chief Executive Officer and Chief Financial Officer pursuant to Exchange Act rules 13a-14(b) or 15d-14(b) and 18 U.S.C. Section 1350. | | | | | | | | | | | | | | | | |
| | | | | |
| *101.INS | | XBRL Instance Document | | | | | | | | | | | | | | | | |
| | | | | |
| *101.SCH | | XBRL Taxonomy Extension Schema Document | | | | | | | | | | | | | | | | |
| | | | | |
| *101.CAL | | XBRL Taxonomy Extension Calculation Document | | | | | | | | | | | | | | | | |
| | | | | |
| *101.DEF | | XBRL Taxonomy Extension Definition Linkbase Document | | | | | | | | | | | | | | | | |
| | | | | |
| *101.LAB | | XBRL Taxonomy Extension Labels Linkbase Document | | | | | | | | | | | | | | | | |
| | | | | |
| *101.PRE | | XBRL Taxonomy Extension Presentation Link Document | | | | | | | | | | | | | | | | |
| † | Confidential treatment requested as to certain portions, which portions are omitted and filed separately with the Securities and Exchange Commission. |
| # | Management contract or compensatory plan or arrangement. |
