Free signup for more
- Track your favorite companies
- Receive email alerts for new filings
- Personalized dashboard of news and more
- Access all data and search results
Filing tables
GXDX similar filings
- 26 Jan 11 Genoptix Announces Agreement to be Acquired by Novartis
- 21 Dec 10 Departure of Directors or Certain Officers
- 4 Nov 10 Genoptix Reports Operating Results for the Third Quarter and First Nine
- 21 Sep 10 Regulation FD Disclosure
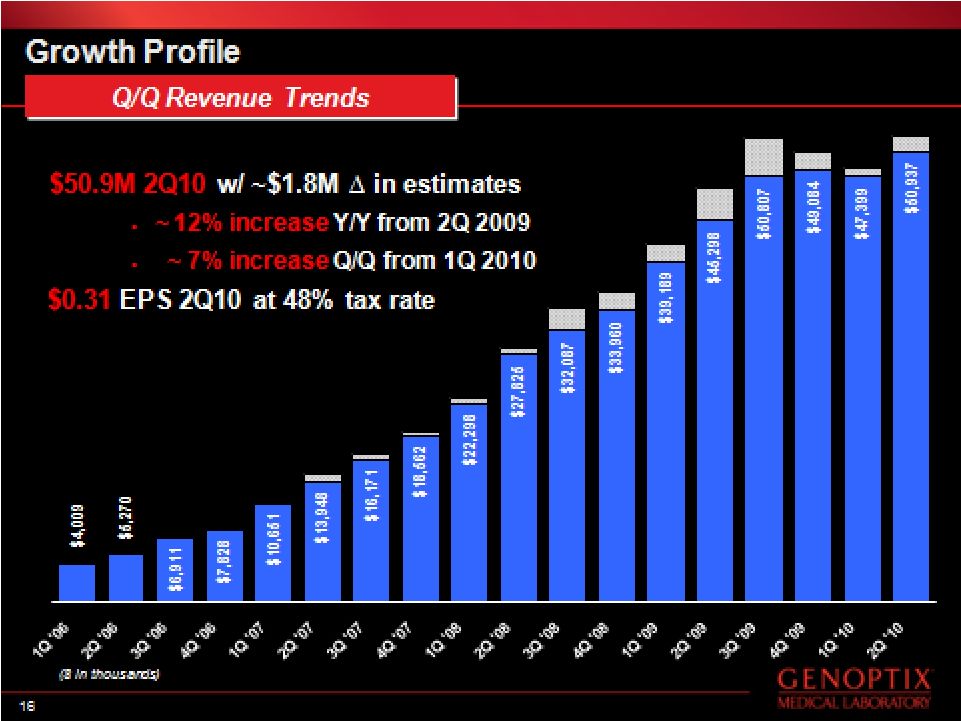
- 29 Jul 10 Genoptix Reports Operating Results for the Second Quarter 2010
- 1 Jun 10 Submission of Matters to a Vote of Security Holders
- 6 May 10 Genoptix Reports Solid Results for the First Quarter 2010
Filing view
External links