As filed with the U.S. Securities and Exchange Commission on September 29, 2003
Registration No. 333-108165
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
AMENDMENT NO. 1
TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Xcel Pharmaceuticals, Inc.
(Exact name of Registrant as specified in its charter)
| Delaware | | 2834 | | 33-0949894 |
(State or other jurisdiction of incorporation or organization) | | (Primary standard industrial classification code number) | | (IRS employer identification no.) |
6363 Greenwich Drive, Suite 100
San Diego, CA 92122
(858) 202-2700
(Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
Michael T. Borer
President and Chief Executive Officer
6363 Greenwich Drive, Suite 100
San Diego, CA 92122
(858) 202-2700
(Name, address, including zip code, and telephone number, including area code, of agent for service)
David R. Snyder, Esq. Christopher M. Forrester, Esq. Pillsbury Winthrop LLP 101 West Broadway, Suite 1800 San Diego, California 92101-4700 Phone: (619) 234-5000 Fax: (619) 236-1995 Counsel to the Registrant | | Craig S. Andrews, Esq. Jeffrey C. Thacker, Esq. Heller Ehrman White & McAuliffe LLP 4350 La Jolla Village Drive, 7th Floor San Diego, CA 92122-1246 Phone: (858) 450-4500 Fax: (858) 450-8499 Counsel to the Underwriters |
Approximate date of commencement of proposed sale to the public:As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ¨
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If delivery of the prospectus is expected to be made pursuant to Rule 434, please check the following box. ¨
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, or until this Registration Statement shall become effective on such date as the Commission, acting pursuant to Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the U.S. Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
PRELIMINARY PROSPECTUS (Subject to Completion)
Dated September 29, 2003.
Shares

Common Stock
We are selling shares of common stock. This is an initial public offering of our common stock. Prior to this offering, there has been no public market for our common stock. See “Underwriting” for a discussion of the factors considered in determining the initial public offering price. We have applied to have the shares approved for quotation on The Nasdaq National Market under the symbol XCEL. We currently estimate that the initial public offering price of our common stock will be between $ and $ per share.
Our business and an investment in our common stock involve significant risks. These risks are described under the caption “Risk Factors” beginning on page 8 of this prospectus.
Neither the U.S. Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| | | Per Share
| | Total
|
| | |
Public offering price | | $ | | | $ | |
| | |
Underwriting discount and commissions | | $ | | | $ | |
| | |
Proceeds, before expenses, to us | | $ | | | $ | |
The underwriters also may purchase up to shares of our common stock from us at the public offering price, less the underwriting discounts and commissions, to cover over-allotments.
The underwriters expect to deliver the shares in New York, New York on or about , 2003.
SG Cowen
CIBC World Markets
Thomas Weisel Partners LLC
U.S. Bancorp Piper Jaffray
, 2003
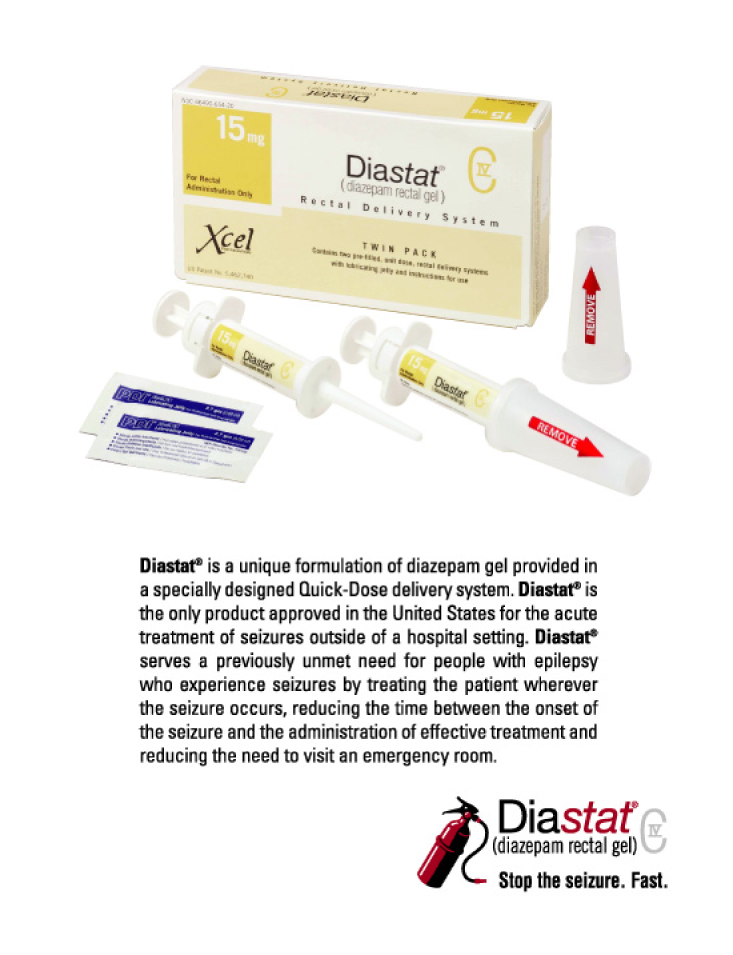
TABLE OF CONTENTS
i
Prospectus Summary
This summary highlights information contained elsewhere in this prospectus that we believe is the most important information to be considered by a potential investor in evaluating whether to purchase our common stock. This summary may not contain all of the information that is important to you, and we encourage you to read this prospectus in its entirety before making your investment decision. The information contained in this summary is qualified in its entirety by, and should be read in conjunction with, the detailed information and financial statements, including the notes thereto, appearing elsewhere in this prospectus. You should read this summary, together with the more detailed information, including “Risk Factors” and our financial statements and related notes, before making your investment decision.
Our Company
We are a specialty pharmaceutical company focused on prescription products that treat disorders of the central nervous system, or the CNS. The CNS market consists of three major segments: neurology, pain and psychiatry. Sales of products used to treat CNS disorders in the United States totaled $39.5 billion in 2002 and grew at an average annual rate of 19% from 1998 to 2002. We currently focus on neurology, a segment where a limited number of high-prescribing physicians write a significant number of the prescriptions for neurology products. The top two areas of practice focus of neurologists are epilepsy and migraine. Sales of products used to treat epileptic seizures in the United States totaled $5.4 billion in 2002 and grew at an average annual rate of 24% from 1998 to 2002. Sales of products used to treat migraines in the United States totaled $1.8 billion in 2002 and grew at an average annual rate of 12% from 1998 to 2002.
Our product portfolio includes four commercial products, Diastat® and Mysoline®, which are used to treat epilepsy, and Migranal® and D.H.E. 45®, which are used to treat migraine, and one product candidate, MT 300™, a potential treatment for migraine. Our 96-person nationwide field sales organization promotes our commercial products to high-prescribing epilepsy and migraine specialists. We intend to increase prescription demand for our key products, Diastat, Migranal and, subject to regulatory approval and commercial launch, MT 300, through targeted sales and marketing efforts and to develop enhancements for our current products. In addition, we intend to leverage our presence in the CNS market through the acquisition, development and commercialization of additional late-stage development product candidates and through the acquisition of additional commercial products.
The concentration of high-prescribing physicians in the neurology market enables us to promote our products effectively with a small, dedicated sales and marketing organization and a limited corporate infrastructure. During 2002, the 14,800 neurologists and pediatric neurologists in the United States collectively wrote prescriptions for $1.5 billion of the products used to treat epileptic seizures and $354 million of the products used to treat migraines. Our field sales organization promotes our commercial products to 6,900 of the highest-prescribing physicians in this group. These 6,900 high-prescribing neurologists collectively wrote 93% of the prescriptions for epilepsy and migraine products among all 14,800 neurologists and pediatric neurologists in 2002. We have designed our corporate infrastructure to support our field sales organization, to identify and pursue the acquisition of additional late-stage development product candidates and commercial products, and to lead our product development and enhancement efforts. We utilize third parties to manufacture and distribute our products and to support our product development and enhancement efforts.
Diastat is the only drug approved in the United States for the acute treatment of seizures outside of a hospital setting. Total retail prescriptions for Diastat, which was launched in October 1997, have grown from 3,100 in the first quarter of 1998 to 29,300 in the second quarter of 2003. Diastat is covered by a patent until September 2013 and is the subject of an orphan drug exclusivity designation until July 2004. Mysoline has been
1
used since the early 1950s for the chronic treatment of seizures associated with epilepsy and is highly complementary to, and benefits from, our marketing efforts for Diastat.
Migranal, a nasal spray, and D.H.E. 45, an injection, contain the active ingredient dihydroergotamine and are used for the acute treatment of migraines. Our product candidate, MT 300, an injection in a pre-filled syringe, contains an improved, highly purified formulation of dihydroergotamine that is suitable for at-home use. Of the 16 million people who receive a prescription drug for the treatment of migraines each year, approximately 25%, or 4 million, of them do not experience effective relief from first-line therapies. The leading first-line migraine therapies prescribed by neurologists are the triptan class of drugs. Triptans generally are prescribed as the first-line therapy for migraines because they are fast-acting compared to other available therapies. Migranal, D.H.E. 45 and MT 300 have a mechanism of action that is broader than the triptan class of drugs. Migranal and D.H.E. 45 are safe and effective second-line therapies for patients who do not experience effective relief from the triptan class of drugs. In clinical studies, MT 300 has consistently demonstrated its safety and effectiveness in treating migraine.
Migranal was launched by Novartis Pharmaceuticals Corporation and its affiliates, or Novartis, in January 1998. Prior to our relaunch of promotional activity in October 2002, Migranal had received no significant promotional activity since 1999. As a result of our sales force relaunching promotional activity, the historical decline of Migranal total retail prescriptions has slowed by 53% to a 6.4% decline for the nine months since our relaunch compared to a 13.5% decline for the nine months before our relaunch. The decline of new prescriptions, which is a leading indicator of product demand, has slowed by 97% to a 0.4% decline for the nine months since our relaunch compared to a 11.6% decline for the nine months before our relaunch. Migranal is covered by a patent until December 2009. D.H.E. 45 has been used since the mid-1940s and has been a standard of care for many years for the acute treatment of migraine. MT 300 is covered by a patent until March 2020. A new drug application to market MT 300 for the treatment of migraine was submitted to the U.S. Food and Drug Administration, or FDA, by Pozen Inc., or Pozen, in December 2002 and is under review by the FDA. Subject to regulatory approval and commercial launch, we intend to promote the benefits of MT 300 to our targeted physicians to grow our injectable dihydroergotamine franchise.
We believe that the changing pharmaceutical industry landscape will present significant opportunities for us to acquire additional commercial products and late-stage development product candidates. Larger pharmaceutical companies are shifting their sales and marketing efforts away from smaller products toward products with higher revenue potential and new product launches. As a result, an increasing number of smaller commercial products that may have growth potential are receiving little or no attention and present potentially attractive acquisition opportunities for us. Biotechnology companies developing CNS product candidates also are looking for partners to assist with the commercialization of their product candidates and associated costs, including late-stage development costs. We continually evaluate opportunities to acquire rights to these types of commercial products and product candidates, although we have no specific agreements with respect to additional acquisitions. We are pursuing product enhancements for Diastat and Migranal, and on our behalf, Pozen is developing a product enhancement for MT 300. Subject to regulatory approval, we believe these product enhancements will provide additional patient, physician and distribution channel benefits.
We face many challenges as an emerging specialty pharmaceutical company. For instance, we have a short operating history, rely on sales of only four commercial products, have limited experience in acquiring and developing products in the late stages of development and our product candidate, MT 300, may not be approved by the FDA. In addition, we face generic competition for Mysoline and D.H.E. 45. Also, if our competitors can design products that do not infringe our patents, they may compete with Diastat (following expiration of our orphan drug exclusivity in July 2004) and with Migranal.
2
We were founded in January 2001. We acquired Diastat from two affiliates of Elan Corporation, plc, or Elan, in March 2001 and Mysoline from Elan in April 2001. We acquired Migranal and D.H.E. 45 from Novartis in June 2002. We licensed the exclusive commercial rights in the United States to MT 300 from Pozen in September 2003. We began selling Diastat and Mysoline in April 2001 and Migranal and D.H.E. 45 in July 2002. Our management has over 40 years of collective experience acquiring, developing and marketing prescription products in the specialty pharmaceutical industry, and have completed over 20 product transactions involving more than 30 products, including all of our current commercial products and our product candidate.
Our Strategy
Our objective is to capitalize on our management team’s experience in acquiring, developing and marketing pharmaceutical products. We intend to become a leading specialty pharmaceutical company focused on disorders of the CNS in the United States. Specifically, our strategy is to:
| | • | | Increase prescription demand for our key products, Diastat, Migranal and, subject to regulatory approval and commercial launch, MT 300. We believe there are significant opportunities to increase prescription demand for Diastat, Migranal and, subject to regulatory approval and commercial launch, MT 300. Diastat is the only product approved in the United States for the acute treatment of seizures associated with epilepsy outside of a hospital setting. Diastat is safe and effective and has significant quality-of-life and cost advantages. We intend to increase prescription demand for Diastat by promoting to our targeted physicians the benefits of including Diastat as part of the standard treatment regimen for their epilepsy patients. Migranal, a nasal spray, is safe and effective for the acute treatment of migraine. MT 300, an injection in a pre-filled syringe, is a potential treatment for migraine. We intend to increase prescription demand for Migranal and, subject to regulatory approval and commercial launch, MT 300, by promoting to our targeted physicians the benefits of prescribing Migranal and MT 300 for their migraine patients who do not experience effective relief with first-line migraine therapies. |
| | • | | Leverage our presence in the CNS market through the acquisition of additional late-stage development product candidates. We intend to build a product pipeline by acquiring additional late-stage development product candidates that, subject to regulatory approval and commercial launch, can be marketed to our targeted physician audiences. We target product candidates that, at a minimum, are in Phase II clinical studies. |
| | • | | Leverage our presence in the CNS market through the acquisition of additional commercial products. We intend to enhance our growth and leverage our organization by acquiring additional commercial products that have been approved by the FDA that can be marketed to our targeted physician audiences. |
| | • | | Maximize the value of our products by developing product enhancements. We intend to expand sales of our current products by developing product enhancements, such as new delivery systems, new formulations and new dosage strengths, and by conducting targeted post-approval clinical studies. We are developing product enhancements for Diastat and Migranal, and on our behalf, Pozen is developing a product enhancement for MT 300. Subject to regulatory approval and commercial launch, we believe these product enhancements will provide additional patient, physician and distribution channel benefits. |
3
Corporate Information
Our headquarters are located at 6363 Greenwich Drive, Suite 100, San Diego, California 92122 and our telephone number is (858) 202–2700. Our web site address iswww.xcelpharmaceuticals.com. The information on our web site is not a part of this prospectus.
We own the U.S. registered trademarks Diastat, Mysoline, Migranal, D.H.E. 45 and Xcel Pharmaceuticals®. We also own the Canadian registered trademark Diastat. All other trademarks and trade names referred to in this prospectus are the property of their respective owners.
About This Prospectus
You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with information that is different. We are offering to sell and seeking offers to buy shares of our common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of our common stock.
4
The Offering
Common stock offered hereby | | shares |
| |
Common stock to be outstanding after the offering | | shares |
| |
Use of proceeds | | For general corporate purposes, including acquisitions of products and product candidates and working capital, and retirement of debt. See “Use of Proceeds.” |
| |
Proposed Nasdaq National Market symbol | | XCEL |
The common stock to be outstanding after this offering is based on 23,528,980 shares of common stock outstanding as of June 30, 2003, assuming conversion of all outstanding shares of our convertible preferred stock. This table excludes:
| | • | | 1,039,192 shares of our common stock issuable upon exercise of options outstanding under our 2001 Stock Plan as of June 30, 2003 at a weighted average exercise price of $4.86 per share; and |
| | • | | 2,000,000 shares to be available for issuance under our 2003 Stock Incentive Plan to be adopted in connection with this offering, including 181,828 additional shares of our common stock reserved for issuance as of June 30, 2003 under our 2001 Stock Plan. |
Except as otherwise indicated, information in this prospectus is based on the following assumptions:
| | • | | the conversion of all outstanding shares of our convertible preferred stock into 18,750,000 shares of common stock upon the closing of this offering; |
| | • | | the filing of an amended and restated certificate of incorporation upon the closing of this offering; |
| | • | | no exercise of the underwriters’ over-allotment option; and |
| | • | | no exercise of stock options after June 30, 2003. |
In addition, this prospectus excludes the effect of a paid-in-kind dividend on our convertible preferred stock at an annual rate of 7% of the original issuance price of the preferred stock, which begins to accrue as of October 1, 2003 and is payable in shares of our preferred stock. The number of shares to be paid is determined by dividing the aggregate amount of the then-accrued dividend on the applicable series of preferred stock by the original issue price per share with respect to the applicable series of preferred stock as to which such dividend has accrued. The dividend will cease to accrue as of the conversion of the preferred stock to common stock upon the closing of this offering. We estimate that if this offering closes on or before , the aggregate effect of the dividend will be that the number of shares of common stock into which the preferred stock will convert as of the closing of this offering will increase from 18,750,000 to .
5
Summary Financial Data
The following table sets forth our summary financial data. You should read this information together with the financial statements and related notes appearing elsewhere in this prospectus and the information under “Selected Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Statement of Operations Data:
| | | January 24, 2001
(Inception)
Through December 31,
2001(1)
| | | Year Ended December 31, 2002(2)
| | | Six Months Ended June 30,
|
| | | | | 2002
| | | 2003
|
| | | (in thousands, except per share data) |
Net sales | | $ | 14,124 | | | $ | 50,055 | | | $ | 23,044 | | | $ | 39,142 |
Total operating costs and expenses | | | 20,317 | | | | 42,946 | | | | 17,398 | | | | 25,582 |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
Operating income (loss) | | | (6,193 | ) | | | 7,109 | | | | 5,646 | | | | 13,560 |
Other income (expense) (3) | | | (3,323 | ) | | | (7,032 | ) | | | (3,290 | ) | | | 33,808 |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
Income (loss) before income taxes | | | (9,516 | ) | | | 77 | | | | 2,356 | | | | 47,368 |
Income tax expense (benefit) | | | — | | | | (3,384 | ) | | | (2,692 | ) | | | 18,237 |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
Net income (loss) | | $ | (9,516 | ) | | $ | 3,461 | | | $ | 5,048 | | | $ | 29,131 |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
Net income (loss) per common share: | | | | | | | | | | | | | | | |
Pro forma—basic and diluted (4) | | $ | (2.12 | ) | | | | | | | | | | | |
| | |
|
|
| | | | | | | | | | | |
Basic (4) | | | | | | $ | 2.13 | | | $ | 3.87 | | | $ | 11.38 |
| | | | | | |
|
|
| |
|
|
| |
|
|
Diluted (4) | | | | | | $ | 0.17 | | | $ | 0.27 | | | $ | 1.28 |
| | | | | | |
|
|
| |
|
|
| |
|
|
Weighted average common shares outstanding: | | | | | | | | | | | | | | | |
Pro forma—basic and diluted (4) | | | 4,487 | | | | | | | | | | | | |
| | |
|
|
| | | | | | | | | | | |
Basic (4) | | | | | | | 1,625 | | | | 1,304 | | | | 2,561 |
| | | | | | |
|
|
| |
|
|
| |
|
|
Diluted (4) | | | | | | | 20,867 | | | | 19,034 | | | | 22,847 |
| | | | | | |
|
|
| |
|
|
| |
|
|
| | | | |
Balance Sheet Data: | | | | | | | | | | | | | | | |
| | | |
| | | | | | | | | As of June 30, 2003
|
| | | | | | | | | Actual
| | | Pro Forma
As
Adjusted(5)
|
| | | | | | | | | (in thousands) |
Cash and cash equivalents | | $ | 29,694 | | | $ | |
Working capital | | | 15,578 | | | | |
Total assets | | | 227,161 | | | | |
Long-term debt, including current portion | | | 62,000 | | | | |
Retained earnings | | | 23,076 | | | | |
Total stockholders’ equity | | | 123,977 | | | | |
| (1) | | We acquired Diastat on March 31, 2001 and Mysoline on April 1, 2001 and the related operations are included in our results since April 1, 2001. |
| (2) | | We acquired Migranal and D.H.E. 45 on June 25, 2002 and the related operations are included in our results since July 1, 2002. |
6
| (3) | | On March 31, 2003, with a payment of $89.5 million, we retired $99.0 million in product acquisition notes payable to Elan that we incurred in connection with our acquisition of Diastat and Mysoline, repaid the $10.0 million outstanding on our credit facility with Elan, terminated our royalty-based financing arrangement with Elan and repurchased 3,000,000 shares of our Series A convertible preferred stock owned by Elan. Concurrently, we issued $62.0 million of senior debt to Regiment Capital III, L.P., or Regiment, and raised net proceeds of $25.9 million through the issuance of 4,000,000 shares of Series C convertible preferred stock to investors other than Elan. We recognized a pre-tax gain of $37.4 million on the retirement of the product acquisition notes payable and $246,000 on the termination of the royalty-based financing arrangement. |
| (4) | | See note 1 to our financial statements for an explanation of the number of shares used in computing net income (loss) per common share. |
| (5) | | The pro forma as adjusted balance sheet data gives effect to: |
| | • | | the conversion of all outstanding shares of our convertible preferred stock into 18,750,000 shares of common stock upon the closing of this offering; and |
| | • | | the sale of shares of common stock in this offering at an assumed initial offering price of $ per share, which is the midpoint of our expected public offering range, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. |
7
RISK FACTORS
An investment in our common stock involves significant risks. You should consider carefully the following risks described below and the other information included in this prospectus, including our financial statements and related notes, before you decide to buy our common stock. Our business, operating results and financial condition could be harmed by any of the following risks. The trading price of our common stock could decline due to any of these risks, and you could lose part or all of your investment.
Risks Related to Our Business
We have a short operating history and rely on sales from our four commercial products for all of our revenues.
We were formed in January 2001. We acquired Diastat and Mysoline in 2001 and Migranal and D.H.E. 45 in 2002. All of our revenues come from sales of these products. Until such time as we obtain marketing approval from the FDA for MT 300 or we acquire additional commercial products, we will continue to rely on these products for all of our revenues. Accordingly, our success depends on our ability to maintain and increase sales of our commercial products. Our sales and marketing efforts may not be successful in maintaining and increasing the prescriptions for our commercial products. Sales of our commercial products also are subject to the following risks, among others:
| | • | | the ability of our competitors to price products below a price at which we can competitively sell these products; |
| | • | | physician or public perception that these products are not safe or effective; |
| | • | | the introduction of new competitive generic or branded products; and |
| | • | | a suspension or reduction in sales of these products as a result of an adverse event experienced by a patient. |
Our growth depends, in part, on our ability to acquire additional products.
We engage only in limited efforts to develop product enhancements for our products and will rely primarily on acquisitions from other companies to obtain additional commercial products and late-stage development product candidates. Our inability to acquire additional commercial products or product candidates could limit our growth. These product acquisitions may be carried out through the purchase of assets, joint ventures, licenses or acquisitions of other companies. We face many risks in this business strategy, including:
| | • | | we may not successfully identify commercial products or product candidates to acquire; |
| | • | | we will compete with other entities, many of which are larger and have greater resources, for these opportunities; |
| | • | | we may not be successful in negotiating favorable acquisition terms for any commercial products or product candidates that we may identify for acquisition; |
| | • | | we may not succeed in raising physician and patient awareness and interest in commercial products that we own or acquire sufficiently to increase sales of those products; and |
| | • | | we may be required to absorb large and immediate write-offs, incur debt, assume liabilities or amortize expenses in connection with our acquisition of commercial products or product candidates, any of which could harm our business. |
If we are unable to identify and acquire additional commercial products or product candidates, our ability to grow our business will be harmed. In addition, if we are unable to increase sales of our existing commercial
8
products or commercial products that we may acquire in an amount sufficient to cover our fixed expenses and generate an acceptable return on our investment, our business could be harmed.
We must integrate any commercial products or product candidates we acquire into our business.
We anticipate that the integration of newly acquired commercial products, product candidates or companies into our business will require significant management attention and may require expansion of our organization. To manage any acquisitions we complete, we must maintain adequate operational, financial and management information systems and motivate and manage our employees. Any failure by us to integrate our acquisitions could harm our business.
We may not be able to acquire or develop late-stage development products.
We intend to expand our business in part through the acquisition of product candidates that are in the late stages of clinical development. Although we always are evaluating opportunities to acquire late-stage product candidates, MT 300 is the only late-stage product that we have acquired to date, and it had finished clinical development at the time of our acquisition.
The process of developing product candidates involves a high degree of risk and may take several years. Product candidates that appear promising in the early phases of development may fail to reach the market for several reasons, including:
| | • | | clinical trials may show the product candidates to be ineffective or to have harmful side effects; |
| | • | | product candidates may fail to receive regulatory approvals required to bring the products to market; |
| | • | | manufacturing costs or other factors may make product candidates uneconomical; and |
| | • | | the proprietary rights of others and their competing products and technologies may prevent the product candidates from being commercialized. |
Success in preclinical and early clinical trials does not ensure that large-scale clinical trials will be successful. Clinical results are frequently susceptible to varying interpretations that may delay, limit or prevent regulatory approvals. The length of time necessary to complete clinical trials and to submit an application for marketing approval for a final decision by a regulatory authority varies significantly and may be difficult to predict.
Developing pharmaceutical product candidates is very expensive and will have a significant impact on our ability to generate profits. Factors affecting our product development expenses include, but are not limited to:
| | • | | the number and outcome of clinical trials conducted by us and/or our collaborators; |
| | • | | the number of products we may have in late-stage clinical development; |
| | • | | in-licensing or other partnership activities, including the timing and amount of related development funding, licensee fees or milestone payments; and |
| | • | | future levels of revenue. |
Our future product development efforts also could result in large and immediate write-offs, incurrence of debt and contingent liabilities or amortization of expenses related to intangible assets, any of which could harm our business.
9
We intend to rely heavily on third-party contractors and collaborators for our product development and product enhancement activities.
We rely, and expect to continue to rely, on third parties to assist us in developing our product candidates and product enhancements. We are subject to a number of risks associated with our dependence on these third parties, including:
| | • | | We may not have day-to-day control over the activities of our third-party contractors or collaborators; |
| | • | | Third parties may not fulfill their obligations to us or otherwise meet our expectations; |
| | • | | We may not realize the contemplated or expected benefits from third-party collaborative or other arrangements; |
| | • | | Business combinations and changes in the contractual or collaborative party’s business strategy may adversely affect its willingness or ability to complete its obligations to us; |
| | • | | The contractor or collaborative party may have the right to terminate its arrangements with us on limited or no notice and for reasons outside of our control; |
| | • | | The contractual or collaborative party may develop or have rights to competing products or product candidates and withdraw support or cease to perform work on our products; and |
| | • | | Disagreements may arise regarding breach of the arrangement, ownership of proprietary rights, clinical results or regulatory approvals. |
Any of these factors could lead to delays in the development of our product candidates or product enhancements, and disagreements with our contractors or collaborators could require or result in litigation or arbitration, which would be time-consuming and expensive. Our ultimate success may depend upon the success and performance on the part of these third parties. If we fail to maintain these relationships or establish new relationships as required, development of our product candidates and product enhancements will be delayed.
Product enhancements that we may develop may not be approved by the FDA and, even if approved, may not be successful commercially.
We are developing product enhancements for Diastat and Mysoline and, on our behalf, Pozen is developing a product enhancement for MT 300. We continuously evaluate other potential product enhancements such as new delivery systems, new formulations and new dosage strengths for all of our products. Any product enhancement we develop will be subject to regulatory approval before we can market the product enhancement. The process of obtaining regulatory approval for product enhancements may be lengthy, and we may not be able to obtain regulatory approval for our product enhancements. We are awaiting a response from the FDA regarding marketing approval of our product candidate, MT 300, for the treatment of migraines.
We also likely will seek patent protection for any product enhancement we develop. The process of obtaining a patent is lengthy and expensive, and we cannot guarantee that we will be able to obtain a patent for our product enhancements. Even if we successfully obtain patents for our product enhancements, our competitors may allege that we have sought unlawfully to expand our patent protection for our product enhancements and seek damages or invalidation of our patents.
We intend to rely on third parties to help develop, test and manufacture product enhancements. We may not be able to identify adequate development partners, and even if identified, we may not be able to enter into agreements with these entries on commercially reasonable terms, or at all. Even if we are successful in developing product enhancements, obtaining regulatory approval for those product enhancements and securing patent protection for the product enhancements, if our product enhancements are not accepted by physicians, we may not recover our investment in our product enhancements.
10
If medical doctors do not prescribe our products or the medical profession does not accept our products or the products that we may develop, our ability to generate product revenue in the future will be harmed.
Our business is dependent on market acceptance among physicians, healthcare payors, patients and the medical community. Medical doctors’ willingness to prescribe our commercial products depends on many factors, including:
| | • | | convenience and ease of administration; |
| | • | | prevalence and severity of adverse side effects; |
| | • | | availability of alternative treatments; |
| | • | | effectiveness of our marketing strategy and the pricing of our products; |
| | • | | publicity concerning our products or competitive products; and |
| | • | | our ability to obtain third-party coverage or reimbursement. |
Even though we have received regulatory approval for our commercial products, and even if we receive regulatory approval and satisfy the above criteria for any of our product candidates, physicians may not prescribe our products if we do not promote our products effectively. Factors that could affect our success in marketing our products includes:
| | • | | the effectiveness of our sales force; |
| | • | | the effectiveness of our production and marketing capabilities; |
| | • | | the success of competing products; and |
| | • | | the availability and extent of reimbursement from third-party payors. |
If any of our products fail to achieve market acceptance, we may not be able to market and sell the products successfully, which would limit our ability to generate revenue and could harm our business.
We may need to raise additional funds to pursue our growth strategy or continue our operations.
We do not have a sufficient operating history to know with certainty whether our existing financial resources and the proceeds of the sale of our common stock in this offering will be sufficient to finance our anticipated growth. Depending on the acquisition opportunities available and our use of our financial resources to satisfy existing capital and operating needs, we may need to raise additional funds to finance these transactions or continue our operations, either through the public or private sale of our debt or equity securities or by borrowing funds. We may not be able to borrow money on commercially reasonable terms, or at all, and any sale of our debt or equity securities may result in dilution to our stockholders. In addition, our loan and security agreements with Regiment limit our ability to incur debt in excess of certain limits without Regiment’s consent. Further, the rights, preferences and privileges of the securities we may sell may be senior to those held by our current stockholders. If adequate funds are not available to us, when needed and on commercially reasonable terms, our ability to expand our business through the acquisition of additional commercial products, product candidates or companies or to respond to competitive pressures would be limited.
We rely on third-party manufacturers and a third-party distributor for our products.
Diastat is manufactured by DPT Laboratories, Inc., or DPT, and Migranal and D.H.E. 45 are manufactured by Novartis. We have entered into an agreement with Yamanouchi Pharma Technologies, Inc., or Yamanouchi, pursuant to which, upon approval by the FDA of Yamanouchi’s manufacturing facilities, Yamanouchi will manufacture Mysoline for us.
11
If we were to experience any interruption in the manufacturing of our products, we might not be able to locate an alternative manufacturer in a timely fashion or on commercially reasonable terms. In addition, although we believe we have adequate finished goods inventories of Mysoline to supply our customers during the FDA approval process for Yamanouchi’s manufacturing facilities, if the FDA does not approve Yamanouchi’s manufacturing facilities in the time we expect, we may run short of products and be unable to meet the needs of our customers. In the event of any interruption of the manufacturing of our products, we could experience reduced sales and increased expenses associated with identifying and qualifying alternate manufacturers. The following factors, among others, could impact the supply of our products from our third-party manufacturers:
| | • | | an interruption in the supply of products from a third party as a result of the lack of required governmental or regulatory approvals, including as a result of deficiencies in the current Good Manufacturing Practices, or cGMP, identified by a governmental or regulatory agency following an inspection of a manufacturer’s facilities; |
| | • | | an interruption in the supply of products from a third-party manufacturer as a result of the unavailability of raw materials or component parts; and |
| | • | | an interruption in the supply of products from third-party manufacturers as a result of the occurrence of a labor interruption or natural disaster. |
Our products are stored and distributed by Integrated Commercial Solutions, Inc., or ICS. Any damage or destruction to a material amount of our inventory while stored at ICS, for example, as a result of a fire or other natural disaster affecting the facility where our products are stored, may cause us to experience an interruption in our ability to supply our customers.
Failure to comply with government regulations could impair our ability to operate our business.
Our business and products are subject to extensive and rigorous regulation by federal and state agencies. We and our contract manufacturers are regulated by the FDA, as well as by various other governmental agencies including, in the case of Diastat, the Drug Enforcement Agency, or DEA. These regulatory authorities govern the development, testing, manufacture, safety, effectiveness, labeling, storage, record keeping, approval, export, advertising and promotion of our products.
The FDA has approved our commercial products, Diastat, Mysoline, Migranal and D.H.E. 45, for marketing and is considering whether to grant marketing approval for our product candidate, MT 300. Changes that we may propose to an approved product, such as adding a new indication, may require additional regulatory approval before the change can be implemented. Our products are sometimes used by physicians for indications other than those approved by the FDA, and we cannot be certain that the FDA will not object to this off-label use. For example, Mysoline is used off-label for essential tremor and Diastat is used off-label for febrile seizures. We do not promote any of our products for off-label use; however, we have sponsored continuing medical education teleconferences in which experts present information on the off-label use of Diastat for febrile seizures. Failure to maintain all necessary approvals may result in a default under our loan and security agreements with Regiment. In addition, our failure to obtain and maintain such approvals would be a violation of FDA regulations and may cause the FDA to object to the continued marketing of our products.
We and our contract manufacturers and distributor, our products, the facilities at which the products are manufactured and stored and other aspects of our business, such as our promotional activities, must continue to comply with the FDA’s regulatory requirements, including, in the case of manufacturers, compliance with cGMP. In addition, because Diastat is regulated as a controlled substance, entities in the chain of distribution for Diastat, including our third-party distributor and our third-party manufacturer of Diastat, must maintain security, control and accounting mechanisms in order to prevent loss and diversion of controlled substances. The FDA and the DEA regularly inspect manufacturing and other pharmaceutical facilities to evaluate compliance with these requirements. Violation of regulatory requirements may result in adverse consequences, including the regulatory
12
agency delaying its approval or refusing to approve a product, the suspension or withdrawal of an approved product from the market, recalls, fines, operating restrictions, injunctions or criminal prosecution, any of which could harm our business.
We cannot predict what impact changes in regulations, enforcement positions, statutes or legal interpretations, when and if promulgated, adopted or enacted, may have on our business in the future.
If third-party reimbursement ceases to be available for our products, demand for our products may decline, which could result in downward price pressure for our products.
Our ability to sell our products depends in part on the extent to which reimbursement for the costs of our products is available from government health administration authorities, private health insurers and others. Third-party insurance coverage may not be adequate for us to maintain price levels sufficient to realize an appropriate return on our investment in our products. Government authorities, private insurers and other third-party payors are attempting to contain health care costs by:
| | • | | generally limiting the coverage and level of reimbursement for prescription products; |
| | • | | seeking to switch to over-the-counter use for drugs that are currently available by prescription only; |
| | • | | refusing, in some cases, to provide any coverage for uses of approved products for indications for which the FDA has not granted marketing approval; |
| | • | | controlling the pharmaceutical products that are on their formulary lists; and |
| | • | | requiring or encouraging, through more favorable reimbursement levels or otherwise, the substitution of generic alternatives for branded products. |
In addition, many managed care organizations are considering formulary contracts primarily with pharmaceutical companies that can offer a full line of products for a given therapy sector or disease. Our products may not be included on the formulary lists of managed care organizations. In this event, third-party reimbursement for our products likely would not be available and physicians may not prescribe our products.
The competition between pharmaceutical companies to get products approved for reimbursement also may result in downward pricing pressure in the industry or in the markets in which our products compete. We may not be successful in any efforts we take to mitigate the effect of a decline in average selling prices for our products. Any decline in our average selling prices could reduce our revenues and harm our gross margins.
New legislation and regulatory proposals may harm our ability to raise capital and increase our revenues.
A number of legislative and regulatory proposals aimed at changing the health care system, including the cost of prescription products and changes in the levels at which consumers and healthcare providers are reimbursed for purchases of pharmaceutical products, have been proposed. While we cannot predict with certainty when or whether any of these pending legislation or regulatory proposals will be signed into law or the effect that it may have on our business, the nature of these changes, as well as the adoption of any of these changes, may exacerbate industry-wide pricing pressures, which could reduce our revenues and harm our gross margins.
We receive approximately 90% of our revenues from only three wholesalers.
We sell our commercial products to wholesale drug distributors who generally sell the products to retail pharmacies, hospitals and other institutional customers. We do not promote our products to these wholesalers, and they do not determine the prescription demand for our products. However, since our inception on January 24, 2001, we have received approximately 90% of our revenues from the pharmaceutical industry’s three largest
13
wholesalers: Cardinal Health, Inc., McKesson Corporation and AmerisourceBergen Corporation. During the six months ended June 30, 2003, we received 28% of our revenues from Cardinal, 33% of our revenues from McKesson and 27% of our revenues from AmerisourceBergen. For the year ended December 31, 2002, we received 39% of our revenue from Cardinal, 26% of our revenue from McKesson and 24% of our revenue from AmerisourceBergen. For the period of January 24, 2001 to December 31, 2001, we received 19% of our revenues from Cardinal, 38% of our revenues from McKesson and 27% of our revenues from AmerisourceBergen. Our business could be harmed if any of these wholesalers were unable or unwilling to pay us for our products and we were unable to collect the amounts owing to us, or any of these wholesalers refused to distribute our products.
In connection with our refinancing of existing product financing indebtedness, we have borrowed $62.0 million from Regiment pursuant to loan and security agreements containing various operating covenants.
In connection with this loan, we have granted Regiment a security interest in all of our assets. Regiment is also protected by various loan covenants and other rights that, if breached by us, even in the absence of a payment default, will entitle Regiment to foreclose on its security interest and acquire our assets without payment or refund to us. These covenants include, among others, maintaining certain leverage ratios, fixed charge coverage ratios and minimum cash on hand amounts. We also have agreed to limit our borrowings from third parties without Regiment’s consent. Any such foreclosure would severely harm us and the interests of our stockholders and could result in a complete loss of your investment. For a more complete description of the terms and provisions of the loan and security agreements, see “Business—Material Agreements—Debt and Security Agreements” beginning on page 44.
We may face competition for Diastat.
Our patent for Diastat expires in 2013. Our orphan drug exclusivity expires in July 2004. Following expiration of our patent we may face generic competition for Diastat. Even prior to the expiration of our patent, we may face competition if, after the expiration of our orphan drug exclusivity designation, other drug companies can develop products using methods and technologies that are beyond the scope of our patent. If competing products are developed and marketed, we may experience downward price pressure and reduced sales of Diastat. Given that we rely on sales of Diastat for a substantial portion of our revenues, any decline in Diastat sales or downward price pressure on Diastat as a result of competing drugs could harm our business significantly.
We face significant competition for Migranal.
Our patent for Migranal expires in December 2009. Following expiration of our patent we may face generic competition for Migranal. Even prior to the expiration of our patent, we may face competition if other drug companies can develop products using methods and technologies that are beyond the scope of our patent. If competing products are developed and marketed, we may experience downward price pressure and reduced sales of Migranal. Given that we rely on sales of Migranal as one of our two lead-marketed products, any decline in Migranal sales or downward price pressure on Migranal as a result of competing drugs could harm our business significantly.
In addition, we compete with alternative therapies for Migranal. Physicians generally prescribe, as a first-line migraine therapy, the triptan-based class of drugs. These drugs are promoted heavily by large pharmaceutical companies with greater resources than us to neurologists and primary care physicians. However, in many cases, triptan-based drugs do not treat patients effectively. Marketed products in the triptan class include, but are not limited to, Imitrex, Zomig, Maxalt, Amerge and Axert. We promote Migranal, a dihydroergotamine, primarily as an alternative therapy when triptan-based drugs are not effective. Given that we rely on sales of Migranal as one of our two lead-marketed products, our sales of Migranal may decline or fail to increase, either of which could significantly harm our business, in the event that physicians do not prescribe Migranal when triptans are not effective.
14
We compete with generic substitutes for Mysoline and D.H.E. 45.
We have no patent protection on Mysoline or D.H.E. 45. We compete with manufacturers of generic substitutes for Mysoline. The generic manufacturers for Mysoline include Watson Pharmaceuticals, Inc., Danbury Pharmaceuticals, Inc., Lannett Company, Inc. and Qualitest Pharmaceuticals, Inc. Generic substitutes for Mysoline currently sell at prices that are less than the price of Mysoline, which has resulted in declining prescription trends. In the second quarter of 2003, the FDA approved abbreviated new drug applications for generic substitutes of D.H.E. 45 being developed by Paddock Laboratories, Inc., or Paddock Laboratories, and Bedford Laboratories, Inc., or Bedford Laboratories. In the third quarter of 2003, Paddock Laboratories and Bedford Laboratories launched their generic substitutes for D.H.E. 45. We expect these generic substitutes to result in declining prescription trends and affect adversely our future net sales of D.H.E. 45. In addition, subject to regulatory approval and commercial launch, we intend to promote the benefits of MT 300 to our targeted physicians to grow our injectable dihydroergotamine franchise, which we also expect to affect adversely our future net sales of D.H.E. 45. We rely on sales of Mysoline and D.H.E. 45 for a substantial portion of our revenues and any significant decline in sales, downward price pressure or further downward prescription trends for these products could harm our business significantly.
Our revenues and operating results may fluctuate in future periods and we may fail to meet expectations, which may cause the price of our common stock to decline.
Variations in our quarterly operating results are difficult to predict and may fluctuate significantly from period to period. We are a relatively new company and our sales prospects are uncertain. We have only limited experience selling our products and cannot predict with any certainty the timing or level of sales of our products in the future. If our quarterly sales or operating results fall below the expectations of investors or securities analysts, the price of our common stock could decline substantially. In addition to the other factors discussed under these “Risk Factors,” specific factors that may cause fluctuations in our operating results include:
| | • | | demand and pricing for our products, including any change in wholesaler purchasing patterns for our products; |
| | • | | physician and patient acceptance of, and prescription rates for, our products; |
| | • | | government or private healthcare reimbursement policies; |
| | • | | any interruption in the manufacturing or distribution of our products; |
| | • | | our operating expenses, many of which are relatively fixed; |
| | • | | timing and size of any new acquisitions we may complete; and |
| | • | | product returns and rebates. |
As a result of these factors, we believe that period-to-period comparisons of our operating results are not a good indication of our future performance. Forecasting our revenues is complicated by difficulties estimating inventory levels at our material wholesalers and retail pharmacies, the timing of purchases by wholesalers and retailers to replenish inventory and the occurrence and amount of product returns.
Impairment of our significant intangible assets may reduce our profitability.
The costs of our acquired product rights are recorded as intangible assets and amortized over the period that we expect to benefit from the assets. As of June 30, 2003, intangible assets comprised approximately 78% of our total assets and approximately 1.4 times our stockholders’ equity. We evaluate periodically the recoverability and the amortization period of our intangible assets. Any impairment of our intangible assets will harm our profitability.
15
As described above under “We compete with generic substitutes for Mysoline and D.H.E. 45,” in the third quarter of 2003, generic substitutes for D.H.E. 45 were introduced into the U.S. market and, subject to regulatory approval and commercial launch of MT 300, we intend to promote the benefits of MT 300 to our targeted physicians to grow our injectable dihydroergotamine franchise. As a result, future net sales of D.H.E. 45 could be affected adversely. At this time, we do not have sufficient information to determine the impact of these events on our net cash flows from our injectable dihydroergotamine products, D.H.E. 45 and MT 300. However, a decline in the future undiscounted cash flows below the net book value of our D.H.E. 45 and MT 300 injectable dihydroergotamine product rights would result in a reduction in the carrying value of these product rights and could also result in a reduction in the amortization period. A reduction in the carrying value of the product rights would result in an immediate charge to our earnings. A reduction in the amortization period would result in additional annual product rights amortization expense.
We are recording stock-based compensation expense relating to stock option grants that results in a charge to our earnings.
Stock-based compensation represents an expense associated with the recognition of the difference between the deemed fair market value of common stock at the time of an option grant and the option exercise price. Stock-based compensation generally is amortized over the vesting period of the options. We have deferred $1.5 million in stock-based compensation related to option grants to our employees and members of our board of directors, which is being amortized to selling, general and administrative expense on a straight-line basis as the options are earned, generally over a period of four years. Also, we have granted options to consultants which, for compensation purposes, must be re-measured at each reporting date during the vesting period, which may require us to record additional non-cash selling, general and administrative expense.
We depend on protection of our intellectual property rights.
Diastat is protected by a U.S. patent until September 2013; Migranal is protected by a U.S. patent until December 2009; and MT 300 is protected by a U.S. patent until March 2020. Other drug companies may be able to develop generic versions of Diastat, after the expiration of our orphan drug exclusivity designation in 2004 but prior to September 2013, or Migranal prior to December 2009 if they can design products that do not infringe our patents. Mysoline and D.H.E. 45 are not covered by patents. In addition, we own registered U.S. trademarks for Diastat, Mysoline, Migranal and D.H.E. 45 and a registered trademark for Diastat in Canada. We also have trade secrets and other proprietary information in connection with the manufacture of our products.
We may not be successful in securing or maintaining proprietary or patent protection for our products and, even if we are successful, we may not be able to protect our intellectual property rights against third party infringement. The validity of patents and other intellectual property rights can be subject to expensive litigation, and our patents and intellectual property rights may be challenged. In addition, our competitors may develop products similar to ours using methods and technologies that are beyond the scope of our intellectual property protection, which could reduce our sales. If we are unsuccessful in defending our intellectual property rights, third parties may be able to copy our products, which would impair our ability to meet our sales projections.
A third party could claim that our intellectual property rights infringe its intellectual property rights. If our intellectual property rights ultimately were determined to infringe a third party’s rights, we could be liable for royalties on past sales and could be required to enter into licenses to continue to manufacture and sell our products. These licenses would require us to pay future royalty payments to the third party. Alternatively, such third party could refuse to grant us a license and require us to cease infringing a claim such that we would have to remove our product from the market. If we become involved in any dispute regarding our intellectual property rights, regardless of whether we prevail, we could be required to engage in costly, distracting and time-consuming litigation that could harm our business.
16
We may face liability and indemnity claims that could result in unexpected costs and damage to our reputation.
Our business exposes us to potential liability risks that arise from the testing, manufacture and sale of our products. Some plaintiffs have received substantial damage awards against pharmaceutical companies based upon claims for injuries allegedly caused by the use of their products. Although currently we maintain product liability insurance of $10.0 million, there is no guarantee that any claims brought against us would be within our existing or future insurance policy coverage or limits. Any judgment against us that is in excess of our policy limits would have to be paid from our cash reserves, which would reduce our capital resources. Further, we may not have sufficient capital resources to pay a judgment, in which case our creditors could levy against our assets. Also, it may be necessary for us to recall products that do not meet approved specifications, which would result in adverse publicity, potentially significant costs in connection with the recall and a loss of revenues. Any product liability claim or series of claims brought against us could harm our business significantly, particularly if such claims resulted in adverse publicity or damage awards outside or in excess of our insurance policy limits.
We rely for our success on our ability to hire and retain key personnel.
Our success depends on our ability to retain the services of Michael T. Borer, our President and Chief Executive Officer, and Cam L. Garner, our Chairman of the Board. If we lose the services of either of these individuals, we may not be able to achieve our business objectives in a timely manner or at all. In particular, the loss of services of either of these individuals could affect adversely our product acquisition efforts, ability to bring product candidates to market, sales prospects or financial operations. We are not aware that either of these individuals, or any of our executive officers, has plans to retire or leave our company in the near future. We do not have employment contracts or key life insurance policies with any of our executive officers. We may not be able to recruit and retain qualified personnel in the future due to intense competition for personnel among pharmaceutical businesses. Recruiting and retaining experienced sales personnel is equally difficult but essential to the success of our business.
Some of our charter and other contractual and statutory provisions may prevent a change of control that could be beneficial to our stockholders.
Some provisions of our charter documents and our loan and security agreements with Regiment could make it more difficult for a third party to acquire us without separate approval of our board of directors and Regiment. As a result of these provisions, we or Regiment could delay, deter or prevent a takeover attempt or third party acquisition that our stockholders consider to be in their best interests, including a takeover attempt that results in a premium over the market price for the shares held by our stockholders. These provisions include:
| | • | | a classified board of directors; |
| | • | | the ability of the board of directors to designate the terms, and issue new series, of preferred stock; |
| | • | | advance notice requirements for nominations for election to the board of directors; |
| | • | | special voting requirements for the amendment of our charter and bylaws; and |
| | • | | Regiment’s right to approve transactions that would constitute a change of control as defined in our loan and security agreements with Regiment. |
We also are subject to anti-takeover provisions of Delaware law which could delay or prevent a change of control. Together, these charter, contractual and statutory provisions may make the removal of management more difficult and may discourage transactions that otherwise could involve payment of a premium over prevailing market prices for our common stock.
17
Risks Related to this Offering
We have broad discretion to use the offering proceeds, and the manner in which we invest these proceeds may not yield a favorable return on your investment.
The net proceeds of this offering are not allocated for specific uses other than general corporate purposes, including product acquisitions and working capital, and the retirement of long-term debt. Our management has broad discretion over how these proceeds are used and could spend the proceeds in ways with which you may not agree. The proceeds may not be invested ultimately in a manner that yields a favorable or any return on your investment.
Our stock price may be volatile and you may not be able to sell your shares at or above the offering price.
Our common stock has not been publicly traded, and an active trading market may not develop or be sustained after this offering. The market prices for securities of specialty pharmaceutical companies in general have been highly volatile and may continue to be highly volatile in the future. You may not be able to sell your shares at or above the offering price. The price at which our common stock will trade after this offering may fluctuate substantially as a result of several factors, including:
| | • | | actual or anticipated fluctuations in our operating results; |
| | • | | changes in, or our failure to meet, investors’ and securities analysts’ expectations; |
| | • | | announcements of technological innovations or new commercial products; |
| | • | | introduction of new products and services by us or our competitors; |
| | • | | developments concerning proprietary rights, including patents; |
| | • | | regulatory developments and related announcements in the United States and foreign countries; |
| | • | | general stock market conditions. |
As a new investor, you will incur immediate dilution as a result of this offering.
The initial public offering price will be substantially higher than the net tangible book value per share of our outstanding common stock. As a result, investors purchasing common stock in this offering will incur immediate dilution of $ per share, based on an expected offering price of $ , which is the mid-point of our expected offering range. This dilution is due in large part to earlier investors in our company having paid substantially less than the initial public offering price when they purchased their shares. Investors who purchase shares of common stock in this offering will contribute approximately % of the total amount we have raised to fund our operations, but will own only approximately % of our common stock. The exercise of outstanding options and future equity issuances, including future public offerings or future private placements of equity securities and any additional shares issued in connection with acquisitions, may result in further economic dilution to investors.
Future sales of our common stock may depress our stock price.
Sales of a substantial number of shares of our common stock in the public market after this offering or after the expiration of contractually or legally required holding periods could cause the market price of our common stock to decline. After this offering, we will have approximately shares of common stock outstanding. All of the shares sold in this offering will be freely tradable unless they are purchased by our affiliates, as defined under the Securities Act of 1933. The remaining shares of common stock outstanding immediately after this offering will be subject to the restrictions on transfer contained in the lock-up agreements described under “Underwriting.” Immediately after the expiration of the 180-day period set forth in the lock-up agreements,
18
approximately million shares, which will be outstanding immediately after this offering, will become available for sale, subject to certain restrictions. Six months following the completion of this offering, the holders of 18,750,000 shares of common stock issuable upon conversion of our convertible preferred stock have the right to cause us to register these shares under the Securities Act of 1933. Registration of shares of common stock pursuant to the exercise of demand registration rights, piggyback registration rights or S-3 registration rights under the Securities Act of 1933 would result in these shares becoming freely tradable without restriction under such Act immediately upon the effectiveness of such registration. Once we register these shares, they can be freely sold in the public market upon issuance, subject to the lock-up agreements described under “Underwriting.”
Our executive officers and directors and their affiliates will exercise control over matters requiring stockholder approval.
After this offering, our executive officers and directors and their affiliates will together control approximately % of our outstanding common stock. As a result, these stockholders collectively will be able to control all matters requiring approval of a majority of our stockholders, including the election of directors and significant corporate transactions. The concentration of ownership may delay, prevent or deter a change in control of our company, could deprive our stockholders of an opportunity to receive a premium for their common stock as part of a sale of our company or our assets and might affect the prevailing market price of our common stock.
We may become involved in securities class action litigation that could divert management’s attention and harm our business.
The stock market has from time to time experienced significant price and volume fluctuations that have affected the market prices for the common stock of pharmaceutical companies. These broad market fluctuations may cause the market price of our common stock to decline. In the past, following periods of volatility in the market price of a particular company’s securities, securities class action litigation has often been brought against the company. We may become involved in this type of litigation in the future. Litigation often is expensive and diverts management’s attention and resources, which could harm our business.
19
INFORMATION REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements. You may find these forward-looking statements under “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business,” as well as elsewhere in this prospectus. These statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performances or achievements expressed or implied by any forward-looking statements. Forward-looking statements include, but are not limited to, statements about:
| | • | | our ability to successfully market and sell our products; |
| | • | | our ability to leverage our existing sales force; |
| | • | | our ability to acquire and assimilate new product acquisitions; |
| | • | | our ability to develop product enhancements; |
| | • | | our estimates regarding reimbursement rates or methods; and |
| | • | | our estimates regarding our capital requirements and our need for additional financing. |
In some cases, forward-looking statements can be identified by terms such as “intends,” “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “predicts” and “potential,” as well as similar expressions intended to identify forward-looking statements. These statements reflect our views with respect to future events and are based on assumptions and subject to risks and uncertainties. Given these uncertainties, you should not place undue reliance on these forward-looking statements. We discuss many of these risks in this prospectus in greater detail under “Risk Factors.” These forward-looking statements represent our estimates and assumptions only as of the date of this prospectus.
You should read this prospectus and the documents that we reference in this prospectus completely and with the understanding that our actual future results may be different materially from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
In this prospectus, we rely on and refer to information, statistics and forecasts regarding the markets in which we compete. We obtained this information and these statistics and forecasts from various third party sources and publications that are not produced for the purposes of securities offerings or economic analysis. We have not verified independently the data and make no representation as to the accuracy of the data we have included.
20
USE OF PROCEEDS
The net proceeds we will receive from the sale of the shares of common stock offered by us in this offering at the initial public offering price are estimated to be $ or $ million if the underwriters’ over-allotment option is exercised in full. This is based on an assumed initial public offering price of $ per share, which is the midpoint of our expected public offering range, and after deducting the underwriting discounts and commissions and the estimated offering expenses payable by us.
We intend to use the net proceeds of this offering for general corporate purposes, including acquisitions of products and product candidates and working capital, and the retirement of long-term debt with Regiment. Our debt was incurred in March 2003 to assist in the retirement of our product acquisition notes payable with Elan, has a current coupon rate of 11% and matures in March 2008. The actual amounts and timing of the use of proceeds for purposes other than the repayment of our long-term debt will vary significantly depending on a number of factors, including the amount of cash used in or generated by our operations. We continually evaluate opportunities to acquire commercial products or product candidates, although currently we have no specific agreements with respect to any additional acquisitions of commercial products or product candidates. We intend to invest the net proceeds of this offering after our repayment of long-term debt in short-term, interest-bearing investment grade securities until they are used.
DIVIDEND POLICY
We have never declared or paid cash dividends on our capital stock and do not anticipate paying cash dividends in the foreseeable future. We currently intend to retain our earnings, if any, for the development of our business. Furthermore, our loan and security agreements with Regiment prohibit the payment of cash dividends.
21
CAPITALIZATION
The following table sets forth our capitalization as of June 30, 2003:
| | • | | on an actual basis; and |
| | • | | on a pro forma as adjusted basis to give effect to: |
| | • | | the conversion of all outstanding shares of our convertible preferred stock into 18,750,000 shares of common stock upon the closing of this offering; |
| | • | | the sale of shares of common stock by us in the offering at an assumed initial public offering price of $ per share, which is the midpoint of our expected public offering range for our common stock in this offering; |
| | • | | deducting the underwriting discounts and commissions and estimated offering expenses; and |
| | • | | the filing of our amended and restated certificate of incorporation upon the closing of this offering increasing our authorized shares of common stock to 100,000,000 and changing our authorized shares of preferred stock to 5,000,000. |
You should read this table together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” our financial statements and the related notes appearing elsewhere in this prospectus.
| | | As of June 30, 2003
| |
| | | Actual
| | | Pro Forma
As Adjusted
| |
| | | (in thousands, except share data) | |
Cash and cash equivalents | | $ | 29,694 | | | $ | | |
| | |
|
|
| |
|
|
|
Long-term debt, including current portion | | $ | 62,000 | | | $ | | |
| | |
|
|
| |
|
|
|
Stockholders’ equity: | | | | | | | | |
| | |
Convertible preferred stock; $0.0001 par value; 52,200,000 shares authorized actual and no shares authorized pro forma as adjusted; 18,750,000 shares issued and outstanding actual and no shares issued and outstanding pro forma as adjusted | | | 3 | | | | — | |
| | |
Preferred stock; $0.0001 par value; no shares authorized, issued or outstanding actual; 5,000,000 shares authorized and no shares issued or outstanding pro forma as adjusted | | | — | | | | — | |
| | |
Common stock; $0.0001 par value; 62,200,000 shares authorized actual; 100,000,000 shares authorized pro forma as adjusted; 4,778,980 shares issued and outstanding actual and shares issued and outstanding pro forma as adjusted | | | 1 | | | | | |
Additional paid-in capital | | | 101,748 | | | | | |
Deferred stock-based compensation | | | (851 | ) | | | (851 | ) |
Retained earnings | | | 23,076 | | | | 23,076 | |
| | |
|
|
| |
|
|
|
Total stockholders’ equity | | | 123,977 | | | | | |
| | |
|
|
| |
|
|
|
Total capitalization | | $ | 185,977 | | | $ | | |
| | |
|
|
| |
|
|
|
The actual and pro forma as adjusted information set forth in the table excludes:
| | • | | 1,039,192 shares of our common stock issuable upon exercise of options outstanding under our 2001 Stock Plan as of June 30, 2003 at a weighted average exercise price of $4.86 per share; and |
| | • | | 2,000,000 additional shares to be available for future issuances under our 2003 Stock Incentive Plan to be adopted in connection with this offering, including 181,828 additional shares of our common stock reserved for grant as of June 30, 2003 under our 2001 Stock Plan. |
From July 1, 2003 through August 31, 2003, we granted options to purchase 97,200 shares of our common stock at an exercise price of $5.00 per share.
22
DILUTION
Our net tangible book value as of June 30, 2003 was $(56,442,392), or $(2.40) per share. Net tangible book value per share is determined by dividing the amount of our total assets less total liabilities and intangible assets by the number of shares of common stock outstanding, assuming conversion of all outstanding shares of convertible preferred stock into shares of common stock upon the closing of this offering.
Dilution in net tangible book value per share represents the difference between the amount per share paid by purchasers of common stock in this offering and the net tangible book value per share of common stock immediately after the closing of this offering. After giving effect to the sale of the shares of common stock offered by us in this offering, our net tangible book value at June 30, 2003 would have been $ or $ per share. This assumes an initial public offering price of $ per share, which is the midpoint of our expected public offering range, after deducting the underwriting discounts and commissions and our estimated offering expenses. This represents an immediate increase in net tangible book value of $ per share to the existing stockholders and an immediate dilution of $ per share to new investors purchasing shares in this offering. The following table illustrates this per share dilution:
Assumed initial public offering price | | | | | $ | | |
| | | | | |
|
|
|
Net tangible book value per share at June 30, 2003 | | (2.40 | ) | | | | |
| | |
Increase per share attributable to this offering | | | | | | | |
| | |
|
| | | | |
Net tangible book value per share after this offering | | | | | | ( | ) |
| | | | | |
|
|
|
Dilution per share to new investors | | | | | $ | | |
| | | | | |
|
|
|
If the underwriters exercise their over-allotment option in full, the net tangible book value per share would increase to $ per share, and the dilution per share to new investors would be $ .
The following table summarizes, as of June 30, 2003, and assuming the conversion of all outstanding shares of convertible preferred stock, the total number of shares of common stock purchased from us, the total consideration paid to us and the average price per share paid by existing stockholders and by new investors purchasing shares in this offering. The information on the table assumes an initial public offering price of $ per share, which is the midpoint of our expected public offering range, before deducting the underwriting discounts and commissions and our estimated offering expenses.
| | | Shares Purchased
| | | Total Consideration
| | | Average Price Per Share
|
| | | Number
| | Percent
| | | Amount
| | Percent
| | |
Existing stockholders | | 23,528,980 | | | | | $ | 103,692,991 | | | | | $ | 4.41 |
New investors | | | | | | | | | | | | | | |
| | |
| |
|
| |
|
| |
|
| | | |
Total | | | | 100.0 | % | | $ | | | 100.0 | % | | | |
| | |
| |
|
| |
|
| |
|
| | | |
If the underwriters exercise their over-allotment option in full, our existing stockholders would own % and our new investors would own % of the total number of shares of our common stock outstanding after this offering.
The preceding information excludes:
| | • | | 1,039,192 shares of our common stock issuable upon exercise of options outstanding under our 2001 Stock Plan as of June 30, 2003 at a weighted average exercise price of $4.86 per share; and |
| | • | | an additional 2,000,000 shares available for future issuance under our 2003 Stock Incentive Plan to be adopted in connection with this offering, including 181,828 additional shares of our common stock reserved for grant as of June 30, 2003 under our 2001 Stock Plan. |
Because the exercise price of the outstanding options are below the initial offering price, investors purchasing shares of common stock in this offering will suffer additional dilution when and if these options are exercised.
23
SELECTED FINANCIAL DATA
We were formed on January 24, 2001. We acquired Diastat on March 31, 2001 and Mysoline on April 1, 2001. Prior to the acquisition of Diastat and Mysoline, we had no substantive operations. The selected financial data reflects our results from January 24, 2001 through December 31, 2002 and for the six months ended June 30, 2002 and 2003 and the contribution of Diastat and Mysoline to Elan’s results for the years ended December 31, 1998 through 2000 and for the three months ended March 31, 2001. The contribution of Diastat and Mysoline to Elan’s results is presented in accordance with the requirements of the U.S. Securities and Exchange Commission as representing the predecessor to our business. The selected financial data should be read in conjunction with the financial statements and related notes thereto and other financial information included elsewhere in this prospectus, as well as “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Historical results are not necessarily indicative of our future results.
Our balance sheet data as of December 31, 2001 and 2002 and our operations data for the period from January 24, 2001 (inception) through December 31, 2001 and the year ended December 31, 2002 were derived from our financial statements that have been audited by Deloitte & Touche LLP, independent auditors, and are included elsewhere in this prospectus. Our balance sheet data as of June 30, 2003 and our operations data for the six months ended June 30, 2002 and 2003 were derived from our unaudited financial statements included elsewhere in this prospectus. The unaudited financial statements were prepared on the same basis as the audited financial statements and, in the opinion of management, include all adjustments, consisting only of normal recurring accruals, necessary to present fairly the data for such period.
The product contribution data represents the contribution of the products while they were owned by Elan and includes certain allocations of general costs to the products. The product contribution data for the year ended December 31, 2000 and for the three months ended March 31, 2001 were derived from the statements of product contribution that have been audited by Deloitte & Touche LLP and are included elsewhere in this prospectus. The product contribution data for the year ended December 31, 1999 was derived from the statement of product contribution that has been audited by Deloitte & Touche LLP and is not included in this prospectus. The product contribution data for the year ended December 31, 1998 was derived from an unaudited statement of product contribution, which is not included in this prospectus. The unaudited statement of product contribution was prepared on the same basis as the audited statements of product contribution and, in the opinion of management, includes all adjustments, consisting only of normal recurring accruals, necessary to present fairly the product contribution data for such period. Diastat was approved by the FDA in July 1997 and was launched by Elan in October 1997. Elan acquired Mysoline in February 1998. The contribution of Mysoline to Elan’s results is included beginning February 1998.
24
| | | Product Contribution Data of
Diastat and Mysoline
| | Operations Data of Xcel
| |
| | | (representing our predecessor business) | | | | | | | | | | | | |
| | | Year Ended December 31,
| | Three Months
Ended
March 31,
2001
| | January 24
(Inception)
Through December 31,
2001(1)
| | | Year Ended December 31,
2002(2)
| | | Six Months Ended June 30,
| |
| | | | | | |
| | | 1998
| | 1999
| | 2000
| | | | | 2002
| | | 2003
| |
| | | (in thousands, except per share data) | |
Net sales | | $ | 17,497 | | $ | 33,575 | | $ | 42,131 | | $ | 8,773 | | $ | 14,124 | | | $ | 50,055 | | | $ | 23,044 | | | $ | 39,142 | |
Operating costs and expenses: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Cost of sales | | | 3,262 | | | 5,571 | | | 7,381 | | | 1,608 | | | 3,285 | | | | 8,835 | | | | 4,448 | | | | 5,637 | |
Selling, general and administrative | | | 8,004 | | | 12,963 | | | 13,382 | | | 2,269 | | | 11,224 | | | | 20,275 | | | | 9,123 | | | | 13,847 | |
Research and development | | | 444 | | | 1,651 | | | 2,072 | | | 354 | | | — | | | | — | | | | — | | | | — | |
Product development | | | — | | | — | | | — | | | — | | | — | | | | 588 | | | | 129 | | | | 1,051 | |
Costs of abandoned initial public offering (3) | | | — | | | — | | | — | | | — | | | — | | | | 991 | | | | — | | | | — | |
Product acquisition charge (4) | | | — | | | — | | | — | | | — | | | — | | | | 3,512 | | | | — | | | | — | |
Product rights amortization | | | — | | | — | | | — | | | — | | | 5,808 | | | | 8,745 | | | | 3,698 | | | | 5,047 | |
| | |
|
| |
|
| |
|
| |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total operating costs and expenses | | | 11,710 | | | 20,185 | | | 22,835 | | | 4,231 | | | 20,317 | | | | 42,946 | | | | 17,398 | | | | 25,582 | |
| | |
|
| |
|
| |
|
| |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Operating income (loss) | | $ | 5,787 | | $ | 13,390 | | $ | 19,296 | | $ | 4,542 | | | (6,193 | ) | | | 7,109 | | | | 5,646 | | | | 13,560 | |
| | |
|
| |
|
| |
|
| |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Other income (expense): | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Interest expense | | | | | | | | | | | | | | | (3,996 | ) | | | (7,327 | ) | | | (3,504 | ) | | | (3,818 | ) |
Interest income | | | | | | | | | | | | | | | 673 | | | | 295 | | | | 214 | | | | 96 | |
Gain on retirement of debt (5) | | | | | | | | | | | | | | | — | | | | — | | | | — | | | | 37,437 | |
Other, net | | | | | | | | | | | | | | | — | | | | — | | | | — | | | | 93 | |
| | | | | | | | | | | | | | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total other income (expense) | | | | | | | | | | | | | | | (3,323 | ) | | | (7,032 | ) | | | (3,290 | ) | | | 33,808 | |
| | | | | | | | | | | | | | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income (loss) before income taxes | | | | | | | | | | | | | | | (9,516 | ) | | | 77 | | | | 2,356 | | | | 47,368 | |
Income tax expense (benefit) | | | | | | | | | | | | | | | — | | | | (3,384 | ) | | | (2,692 | ) | | | 18,237 | |
| | | | | | | | | | | | | | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net income (loss) | | | | | | | | | | | | | | $ | (9,516 | ) | | $ | 3,461 | | | $ | 5,048 | | | $ | 29,131 | |
| | | | | | | | | | | | | | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net income (loss) per common share: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Pro forma—basic and diluted (6) | | | | | | | | | | | | | | $ | (2.12 | ) | | $ | 2.13 | | | $ | 3.87 | | | $ | 11.38 | |
| | | | | | | | | | | | | | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Basic (6) | | | | | | | | | | | | | | | | | | $ | 0.17 | | | $ | 0.27 | | | $ | 1.28 | |
| | | | | | | | | | | | | | | | | | |
|
|
| |
|
|
| |
|
|
|
Diluted (6) | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Weighted average common shares outstanding: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Pro forma—basic and diluted (6) | | | | | | | | | | | | | | | 4,487 | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
|
|
| | | | | | | | | | | | |
Basic (6) | | | | | | | | | | | | | | | | | | | 1,625 | | | | 1,304 | | | | 2,561 | |
| | | | | | | | | | | | | | | | | | |
|
|
| |
|
|
| |
|
|
|
Diluted (6) | | | | | | | | | | | | | | | | | | | 20,867 | | | | 19,034 | | | | 22,847 | |
| | | | | | | | | | | | | | | | | | |
|
|
| |
|
|
| |
|
|
|
| | | As of December 31,
| | | As of June 30, 2003
|
| | | 2001(1)
| | | 2002(2)
| | | Actual(5)
| | Pro Forma As
Adjusted(7)
|
| Balance Sheet Data: | | | | | | | | (in thousands) | | |
Cash and cash equivalent | | $ | 19,487 | | | $ | 16,561 | | | $ | 29,694 | | $ | |
Working capital | | | 16,093 | | | | 9,678 | | | | 15,578 | | | |
Total assets | | | 171,906 | | | | 215,647 | | | | 227,161 | | | |
Long-term debt, including current portion | | | 99,000 | | | | 109,000 | | | | 62,000 | | | |
Retained earnings (accumulated deficit) | | | (9,516 | ) | | | (6,055 | ) | | | 23,076 | | | |
Total stockholders’ equity | | | 60,382 | | | | 86,711 | | | | 123,977 | | | |
| (1) | | We acquired Diastat on March 31, 2001 and Mysoline on April 1, 2001, and the related operations are included in our results since April 1, 2001. |
| (2) | | We acquired Migranal and D.H.E. 45 on June 25, 2002, and the related operations are included in our results since July 1, 2002. |
| (3) | | In December 2002, we abandoned our efforts related to a proposed initial public offering we were pursuing and expensed legal, accounting and other costs related to filing a registration statement with the U.S. Securities and Exchange Commission. |
25
| (4) | | In December 2002, we incurred a charge related to our acquisition of Diastat and Mysoline from Elan. Please see note 4 to our financial statements for further explanation. |
| (5) | | On March 31, 2003, with a payment of $89.5 million, we retired $99.0 million in product acquisition notes payable to Elan that we incurred in connection with our acquisition of Diastat and Mysoline, repaid the $10.0 million outstanding on our credit facility with Elan, terminated our royalty-based financing arrangement with Elan and repurchased 3,000,000 shares of our Series A convertible preferred stock owned by Elan. Concurrently, we issued $62.0 million of senior debt to Regiment and raised net proceeds of $25.9 million through the issuance of 4,000,000 shares of Series C convertible preferred stock to investors other than Elan. We recognized a pre-tax gain of $37.4 million on the retirement of the product acquisition notes payable and $246,000 on the termination of the royalty-based financing arrangement. |
| (6) | | See note 1 to our financial statements for an explanation of the number of shares used in computing net income (loss) per common share. |
| (7) | | The pro forma as adjusted balance sheet data gives effect to: |
| | • | | the conversion of all outstanding shares of our convertible preferred stock into 18,750,000 shares of common stock upon the closing of this offering; and |
| | • | | the sale of shares of common stock in this offering at the initial offering price of $ per share, which is the midpoint of our expected public offering range, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. |
26
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion contains predictions, estimates and other forward-looking statements that involve a number of risks and uncertainties, including those discussed under “Risk Factors” and elsewhere in this prospectus. These risks could cause our actual results to differ materially from any future performance suggested below. We undertake no obligation to update these forward-looking statements to reflect events or circumstances arising after the date of this prospectus. You should read this discussion together with the financial statements, related notes and other financial information included elsewhere in this prospectus.
Overview
We are a specialty pharmaceutical company focused on prescription products that treat disorders of the CNS. We currently focus on neurology, a segment where a limited number of high-prescribing physicians write a significant number of the prescriptions for neurology products. Our portfolio includes four commercial products, Diastat and Mysoline, which are used to treat epilepsy, and Migranal and D.H.E. 45, which are used to treat migraine, and one product candidate, MT 300, a potential treatment for migraine. Diastat is the only drug approved in the United States for the acute treatment of seizures outside of a hospital setting. Mysoline is an oral prescription medication that is used for the chronic treatment of seizures associated with epilepsy. Migranal, a nasal spray, and D.H.E. 45, an injection, contain the same active ingredient, dihydroergotamine, and are prescribed for the treatment of migraines. MT 300, an injection in a convenient pre-filled syringe, is a highly purified formulation of dihyroergotamine that will, subject to regulatory approval, expand our injectable dihydroergotamine franchise. Our 96-person nationwide field sales organization promotes our commercial products to high-prescribing epilepsy and migraine specialists. Third parties manufacture our products and provide our distribution services.
We were formed in January 2001. On March 30, 2001, we raised net proceeds of $69.6 million from the issuance of 14,000,000 shares of Series A convertible preferred stock. We then acquired Diastat and Mysoline from Elan and began selling these products in April 2001. We had no substantive operations prior to these activities. From April 1, 2001 through December 31, 2001, we focused on hiring and training our field sales force, hiring our corporate administrative employees, building our corporate infrastructure, promoting Diastat and Mysoline and pursuing new product acquisition opportunities. In June 2002, we acquired Migranal and D.H.E. 45 from Novartis. The activities of these products are included in our results of operation since July 1, 2002. In September 2003, we licensed exclusive commercial rights in the United States to MT 300 from Pozen. Pozen submitted a new drug application to market MT 300 for the treatment of migraine to the FDA in December 2002, which is under review by the FDA.
Comparing the results of our commercial products while owned by Elan and Novartis to our results since we acquired the products would not be meaningful. This is due to the significant differences in our organizational structure, marketing and promotional strategies and corporate resources compared to those of Elan and Novartis. Elan and Novartis are multi-national organizations with large portfolios of products. Diastat, Mysoline, Migranal and D.H.E. 45 received varying levels of sales and marketing attention by Elan and Novartis based on the promotional priorities of their respective corporate strategies. In addition, Elan and Novartis have significant internal resources for performing other administrative activities to support their products. We are a specialty pharmaceutical company with only four products and one product candidate and fewer internal resources than Elan or Novartis. We seek to promote our products through targeted marketing efforts. We also seek to leverage our resources by adding new products to our portfolio without increasing materially our marketing and support infrastructure.
In addition, our emphasis in 2001 was on transitioning responsibilities for Diastat and Mysoline from Elan and building our corporate infrastructure, while allowing our wholesalers to fulfill demand for Diastat and Mysoline primarily from their existing inventories of Diastat and Mysoline. This further contributes to the lack of
27
comparability of the activities of Diastat and Mysoline after our acquisition of these products from Elan to the historical net sales and related costs of Diastat and Mysoline while owned by Elan. For the same reasons, our results for 2001 were not indicative of our future results.
This management’s discussion and analysis of financial condition and results of operations includes an analysis of our results of operations and our liquidity and capital resources for the period from our inception through December 31, 2002 and for the six months ended June 30, 2003. In addition, the discussion includes a separate analysis of the contribution of Diastat and Mysoline to the results of their prior owner, Elan, for the year ended December 31, 2000 and the three months ended March 31, 2001 as representing our predecessor business in accordance with the requirements of the U.S. Securities and Exchange Commission. During this period, the operations of Diastat and Mysoline were included in Elan’s consolidated results and had not been reported on separately. The analysis of the contribution to Elan’s results should be read together with the audited statements of product contribution for the year ended December 31, 2000 and the three months ended March 31, 2001 included elsewhere in this prospectus.
Xcel Pharmaceuticals, Inc.
Critical Accounting Policies
The following are accounting policies that we believe to be critical to our financial condition, results of operations and cash flows and require significant judgment and estimates in their application. Our critical accounting estimates and related assumptions are evaluated regularly as conditions warrant and adjusted as new information or changed circumstances require such revision. Application of these policies is often subjective and complex because we need to make estimates about the effect of matters that are inherently uncertain. For a summary of all of our significant accounting policies, including critical accounting policies discussed below, see note 1 to our financial statements.
Revenue Recognition. We sell our commercial products to wholesale drug distributors who generally sell the products to retail pharmacies, hospitals and other institutional customers. Product sales are recognized when the products are received by the wholesaler, which represents the point when the risks and rewards of ownership have been transferred to the customer. We invoice wholesalers at our wholesale list price. Sales are shown net of discounts, rebates, chargebacks and returns, which are estimated based on experience with the products and other related factors. We periodically review our reserves for rebates, chargebacks and returns and make adjustments, if necessary, based on actual experience. While we believe we can make reliable estimates for all adjustments to revenues, it is possible that actual amounts realized could vary from our estimates and that the amounts of such changes could affect our operating results. Chargebacks represent the difference between the wholesale list price and the contractual sales price. Our customers generally may return product upon the expiration date of the product.
Impairment of Long-Lived Assets. We currently are amortizing our property and equipment and product rights over estimated useful lives ranging from three to 20 years. We periodically evaluate the recoverability of our long-lived assets, as well as the related useful lives, to determine whether facts and circumstances warrant adjustments to the carrying values and/or estimates of the useful lives. This evaluation is performed using the estimated projected future undiscounted cash flows associated with the asset over its remaining useful life compared to the asset’s carrying amount to determine if a write-down is required. Facts and circumstances that determine the useful lives of our products are inherently uncertain and include, but are not limited to, generic competition, competition from products prescribed for similar indications, physician loyalty and prescribing patterns and the sensitivity of our products to promotional efforts. When impairment is indicated for long-lived assets, the amount of impairment loss is the excess of net book value over fair value as approximated using discounted cash flows. If we determine that an asset is impaired, we will be required to record a charge for the excess of net book value over the fair value or accelerate the amortization.
28
Inventory Obsolescence. Inventories consist of purchased pharmaceutical products, product components and product samples used in our sales and marketing efforts. Inventories are stated at the lower of cost or market. Cost is determined using the first-in, first-out method. Our commercial products have shelf lives ranging from 24 to 36 months. We evaluate our inventory levels based on forecasts of sales, promotional focus, levels of products held by our customers and other factors. If our estimates or forecasts change, we may be required to record charges for write-downs of excess or obsolete inventories.
Allowance for Doubtful Accounts. We maintain an allowance for doubtful accounts for estimated losses resulting from our customers’ inability to make required payments. We estimate the amount of the allowance based on historical levels of bad debts and the financial strength of our customers, among other factors. If the financial condition of our customers were to deteriorate, our actual losses may exceed the estimates and we would be required to record a charge for additional allowances.
Results of Operations
Six Months Ended June 30, 2003 Compared to Six Months Ended June 30, 2002
Net sales. Net sales were $39.1 million for the six months ended June 30, 2003, an increase of $16.1 million, or 70%, over $23.0 million for the six months ended June 30, 2002. Of this increase, $14.6 million was attributable to net sales of Migranal and D.H.E. 45, which we acquired in June 2002. The remainder of the increase of $1.5 million was due to a 21% increase in net sales of Diastat offset by a 16% decrease in net sales of Mysoline. The increase in net sales of Diastat was due to a 14% increase in our average selling price and a 6% increase in unit sales. The decrease in net sales of Mysoline was due to a 33% decline in unit sales, partially offset by a 35% increase in our average selling price. The decline in Mysoline unit sales was due to the continued impact of generic competition.
During the six months ended June 30, 2003, we received 28% of our revenues from Cardinal Health, Inc., 33% of our revenues were from McKesson Corporation and 27% of our revenues from AmerisourceBergen Corporation, as compared to 54%, 22% and 13% of our revenues, respectively, for the six months ended June 30, 2002.
In the second quarter of 2003, the FDA approved abbreviated new drug applications for two generic substitutes for D.H.E. 45. In the third quarter of 2003, Paddock Laboratories and Bedford Laboratories launched their generic substitutes for D.H.E. 45. In addition, subject to approval of MT 300 by the FDA and commercial launch of MT 300, we intend to promote the benefits of MT 300 to our targeted physicians to grow our injectable dihydroergotamine franchise. As a result, future net sales of D.H.E. 45 could be affected adversely.
Cost of sales. Cost of sales was $5.6 million for the six months ended June 30, 2003, an increase of $1.2 million, or 26.7%, over $4.4 million for the six months ended June 30, 2002. Cost of sales was 14.4% of net sales during the six months ended June 30, 2003 as compared to 19.3% during the same period in 2002, a decrease of 4.9%. This percentage decrease was attributable primarily to our acquisition of Migranal and D.H.E. 45 in June 2002, which have lower cost of sales as a percentage of net sales than Diastat and Mysoline.
Selling, general and administrative expenses. Selling, general and administrative expenses were $13.8 million for the six months ended June 30, 2003, an increase of $4.7 million, or 51.8%, over $9.1 million for the six months ended June 30, 2002. This increase was due primarily to $1.7 million of costs associated with our efforts to launch and promote Migranal and D.H.E. 45, acquired in June 2002, and $2.0 million of headcount-related costs resulting principally from a 14-person expansion of our sales force in the first quarter of 2003. As of June 30, 2003, we had 125 employees.
Product development expense. Product development expense was $1.1 million for the six months ended June 30, 2003, an increase of $922,000 over $129,000 for the six months ended June 30, 2002. The increase
29
represents costs incurred to evaluate and develop enhancements for our products. Currently, we are pursuing opportunities that we believe will provide additional patient, physician and distribution channel benefits for Diastat and Migranal. These endeavors could increase substantially our future product development expenses and reduce our future operating results. In addition, our business strategy is to pursue late-stage development product candidates that can be marketed to our current targeted physician audiences. The future acquisition and development of product candidates could increase significantly our future development expenses and reduce our future operating results until these products are approved, marketed and generating revenues, which is not assured. If product candidates that we may acquire do not receive FDA approval, or even after receiving FDA approval do not develop substantial sales, we may not recover our investment in these product candidates.
Product rights amortization expense. Product rights amortization expense was $5.0 million for the six months ended June 30, 2003, an increase of $1.3 million over $3.7 million for the six months ended June 30, 2002. The increase was due to the amortization of the $49.0 million acquisition cost for Migranal and D.H.E. 45, which we acquired in June 2002.
As described above under “Net sales,” in the third quarter of 2003, generic substitutes for D.H.E. 45 were introduced into the U.S. market and, subject to FDA approval and commercial launch, we intend to promote the benefits of MT 300 to our targeted physicians to grow our injectable dihydroergotamine franchise. As a result, future net sales of D.H.E. 45 could be affected adversely. At this time, we do not have sufficient information to determine the future impact of these events on the net cash flows from our injectable dihydroergotamine products, D.H.E. 45 and MT 300. However, a decline in the undiscounted cash flows below the net book value of our D.H.E. 45 and MT 300 injectable dihydroergotamine product rights would result in a reduction in the carrying value of the these product rights and could also result in a reduction in the amortization period. A reduction in the carrying value of the product rights would result in an immediate charge to our earnings. A reduction in the amortization period would result in additional annual product rights amortization expense.
Interest expense. Interest expense was $3.8 million for the six months ended June 30, 2003, an increase of $314,000 over $3.5 million for the six months ended June 30, 2002. We issued $99.0 million in product acquisition notes payable to Elan in March 2001 that we incurred in connection with our acquisition of Diastat and Mysoline and drew $10.0 million on our credit facility with Elan in June 2002, each with an interest rate of 7%. In March 2003, we retired this debt and entered into a $62.0 million senior term loan with Regiment. Our debt with Regiment has a current coupon rate of 11% and had an effective interest rate of 12.3% through June 30, 2003 when considering the amortization of a portion of the $2.4 million in issuance costs we incurred to obtain this debt. The increase was the result of interest accrued on the $10.0 million credit facility during the first three months of 2003, for which there was no comparable expense in the first six months of 2002, and additional interest associated with the $62.0 million senior term loan in the second quarter of 2003. We intend to repay a portion of our outstanding debt with Regiment using a portion of the proceeds from this offering.
Gain on debt retirement. On March 31, 2003, we entered into an agreement with Elan to payoff the $10.0 million outstanding on the credit facility and retire the $99.0 million in product acquisition notes payable for a combined payment of $71.5 million. We recognized a gain on the retirement of the product acquisition notes payable of $37.4 million.
Income taxes. The provision for income taxes for the six months ended June 30, 2003 was an expense of $18.2 million, reflecting an effective income tax rate of 38.5%, as compared to a benefit of $2.7 million for the six months ended June 30, 2002. The benefit in 2002 arose primarily from the reversal in June 2002 of the valuation allowance that we recorded against our net deferred tax assets as of December 31, 2001.
30
Year Ended December 31, 2002 Compared to Period From January 24, 2001 (Inception) Through December 31, 2001
Net sales. Net sales were $50.1 million for the year ended December 31, 2002, an increase of $35.9 million, or 254%, over $14.1 million for the period from April 1, 2001 through December 31, 2001. Net sales of Migranal and D.H.E. 45, which we acquired in June 2002, contributed $9.9 million to the increase in net sales for the year ended December 31, 2002. In addition, net sales of Diastat and Mysoline increased $26.0 million in 2002. The increase was the result of a full year of net sales of Diastat and Mysoline, which we acquired in March and April 2001, and, as described below, a closer alignment in 2002 of wholesaler purchases with prescriptions filled by patients. Wholesalers purchase product based on prescription demand and the amount of product previously purchased. During the period from April 1, 2001 through December 31, 2001, wholesalers fulfilled prescription demand for Diastat and Mysoline primarily from their existing inventories. In 2002, wholesalers’ purchases of Diastat and Mysoline were aligned more closely with the demand generated from prescriptions written by physicians and filled by patients. In addition, our average selling prices increased 6% for Diastat and 39% for Mysoline during the year ended December 31, 2002. There were no price increases or decreases in 2001.
During the year ended December 31, 2002, 39% of our revenues were from Cardinal Health, Inc., 26% of our revenues were from McKesson Corporation and 24% of our revenues were from AmerisourceBergen Corporation, as compared to 19%, 38% and 27% of our revenues, respectively, during the period ended December 31, 2001.
Cost of sales. Cost of sales was $8.8 million for the year ended December 31, 2002, an increase of $5.5 million, or 169%, over $3.3 million for the period ended December 31, 2001. Cost of sales was 17.7% of net sales during the year ended December 31, 2002 as compared to 23.3% during the period ended December 31, 2001, a decrease of 5.6%. This percentage decrease was attributable primarily to our acquisition of Migranal and D.H.E. 45 in June 2002 which have lower cost of sales as a percentage of net sales than Diastat and Mysoline.
Selling, general and administrative expenses. Selling, general and administrative expenses were $20.3 million for the year ended December 31, 2002, an increase of $9.1 million, or 80.6%, over $11.2 million for the period ended December 31, 2001. The increase was attributable primarily to the effect of a full year of expense in 2002 and additional sales and marketing expenses associated with our acquisition of Migranal and D.H.E. 45 in June 2002. In 2001, we incurred costs primarily in recruiting, hiring and training our field sales force, hiring our corporate administrative employees, developing our corporate infrastructure and promoting our products. During the period from April 1, 2001 through December 31, 2001, we hired our field sales organization and home-office personnel, with 99 total employees as of December 31, 2001. As of December 31, 2002, we had 111 employees.
Product rights amortization expense. Product rights amortization expense was $8.7 million for the year ended December 31, 2002, an increase of $2.9 million, or 50.6%, over $5.8 million for the period ended December 31, 2001. In March and April 2001, we acquired the rights to Diastat and Mysoline for an initial cost of $160.6 million. The product acquisition agreements provided for quarterly purchase price adjustments during 2001 to the extent reported product revenues differed from product revenues based on total prescriptions for Diastat and Mysoline. The purchase price adjustments totaled $12.4 million, reducing our net cost of these product rights to $148.2 million. We accounted for the change in amortization related to these purchase price adjustments prospectively from the date of adjustment. In June 2002, we acquired the rights to Migranal and D.H.E. 45 for $49.0 million. The increase in product rights amortization expense was due to a full year of amortization of the acquisition cost of Diastat and Mysoline and the amortization of the acquisition cost for Migranal and D.H.E. 45.
Costs of abandoned initial public offering. Due to difficult conditions in the U.S. equity markets, in December 2002, we decided to abandon our effort to pursue a public offering in 2002 and expensed $991,000 in deferred costs incurred in connection with this effort.
31
Product acquisition charge. Under the terms of the Diastat and Mysoline acquisition agreements, Elan was responsible for the payment and processing of rebates, chargebacks and returns on Diastat and Mysoline sold by Elan prior to our acquisition of these products up to contractually specified amounts and time periods. As of December 31, 2002, the contractually specified periods had ended. In December 2002, we recorded a charge of $3.5 million, representing the activity in excess of the contractually specified amounts or beyond the specified time periods related to Diastat and Mysoline sold by Elan.
Interest expense. Interest expense was $7.3 million for the year ended December 31, 2002, an increase of $3.3 million over $4.0 million for the period ended December 31, 2001. We issued $99.0 million in product acquisition notes payable to Elan in March 2001 that we incurred in connection with our acquisition of Diastat and Mysoline and drew $10.0 million on our credit facility with Elan in June 2002, each with an interest rate of 7%. Of the $3.3 million increase, $2.9 million was attributable to a full year of interest accrued on the $99.0 million of product acquisition notes payable and $385,000 was a result of interest accrued on the $10.0 million credit facility.
Income taxes. We recorded an income tax benefit of $3.4 million for the year ended December 31, 2002. We did not provide for any current or deferred federal or state income tax provision or benefit for the period ended December 31, 2001. A valuation allowance was recognized to offset fully our net deferred tax assets as of December 31, 2001 as realization of such assets was uncertain. The benefit in 2002 arose primarily from the reversal of this valuation allowance. As of December 31, 2002, we had federal and California income tax loss carry-forwards of $6.4 million.
Liquidity and Capital Resources
As of June 30, 2003, we had cash and cash equivalents of $29.7 million and working capital of $15.6 million. As of December 31, 2002, we had cash and cash equivalents of $16.6 million and working capital of $9.7 million. As of December 31, 2001, we had cash and cash equivalents of $19.5 million and working capital of $16.1 million.
Net cash provided by operating activities was $18.3 million for the six months ended June 30, 2003, an increase of $7.1 million over $11.2 million for the six months ended June 30, 2002. Net cash provided by operating activities was $17.9 million for the year ended December 31, 2002, an increase of $17.8 million over $138,000 for the period ended December 31, 2001. The increases in the respective periods were due to net cash inflows resulting from net sales of Migranal and D.H.E. 45 acquired in June 2002 and increased net cash inflows from the combined increase in net sales of Diastat and Mysoline.
Net cash used in investing activities during the six months ended June 30, 2003 was $1.2 million, which related primarily to purchases of property and equipment and costs associated with the transfer of the manufacturing technology for Mysoline to a new third-party manufacturer. Net cash used in investing activities in 2002 was $53.3 million, which resulted primarily from our payment of the cash component of our purchase of the Migranal and D.H.E. 45 product rights in June 2002 of $47.7 million, the payment of $1.8 million for finished product inventories acquired in connection with the acquisition of Migranal and D.H.E. 45 product rights and the payment of $5.2 million for finished product inventories acquired in connection with the acquisition of Diastat and Mysoline product rights offset by $1.5 million of cash received from purchase price adjustments related to Diastat and Mysoline. Net cash used in investing activities in 2001 was $50.7 million and resulted primarily from payments of $60.5 million related to our acquisition of Diastat and Mysoline product rights offset by $10.9 million of cash received from purchase price adjustments related to Diastat and Mysoline.
Net cash used by financing activities for the six months ended June 30, 2003 was $4.0 million. Under an agreement with Elan, effective March 31, 2003, with a cash payment to Elan of $89.5 million, we retired the $99.0 million of product acquisition notes payable due Elan that we incurred in our acquisition of Diastat and Mysoline, repaid the $10.0 million credit line note payable to Elan and repurchased 3,000,000 shares of our
32
Series A convertible preferred stock held by Elan. To assist in financing this transaction, we issued $62.0 million of senior debt to Regiment and raised net proceeds of $25.9 million in private equity through the issuance of 4,000,000 shares of our Series C convertible preferred stock to investors other than Elan. We recognized a gain of $37.4 million on the retirement of the product acquisition notes payable. In addition, we terminated the financing agreement we entered into with Elan in June 2001 whereby Elan had agreed to make payments to us based on the net sales of Elan’s product Zanaflex and we agreed to make payments to Elan based on the net sales of Mysoline. We recognized a gain of $246,000 in connection with the termination of this financing arrangement. Neither Elan nor we have any further rights or obligations under the financing agreement. The transaction with Elan culminated from discussions that began in the third quarter of 2002. At that time, Elan publicly disclosed its intention to liquidate several of its non-core assets in an effort to meet its debt requirements. We looked at this as a potential opportunity to retire our long-term debt with Elan at a discount and eliminate our related-party relationship with Elan through the acquisition of our preferred stock held by Elan. We approached Elan in the third quarter of 2002 with our offer to retire our outstanding debt and repurchase the preferred stock. Elan agreed to our proposal contingent on our ability to obtain financing to facilitate the transaction. As described above, in March 2003, we obtained the additional financing and completed the transaction with Elan.
Interest on the $62.0 million in senior debt with Regiment is due monthly on the outstanding balance at prime (with a minimum of 6.5%) plus 4.5%, which resulted in a coupon rate of 11% through June 30, 2003. Quarterly principal payments of $2.0 million are due June 30, 2004 through December 31, 2007, with a final balloon payment of $32.0 million due on March 31, 2008. Debt issuance costs of $2.4 million we incurred in connection with this transaction were deferred and are being amortized to interest expense ratably over the term of the loan, which resulted in an effective interest rate of 12.3% through June 30, 2003. We also make monthly administrative fee payments of $15,000 and will be required to make a $930,000 payment on each anniversary of the loan agreement until the loan is repaid. The loan is secured by an interest in all of our assets, contains restrictions on our ability to dispose of or acquire assets and incur additional debt and requires us to maintain certain covenants, including certain leverage ratios, fixed charge coverage ratios and minimum cash on hand amounts. To date, we have remained in compliance with all covenants. We intend to repay a portion of our outstanding debt with Regiment using a portion of the proceeds from this offering.
In September 2003, we licensed the exclusive commercial rights in the United States to MT 300 from Pozen. We paid an upfront fee of $2.0 million to Pozen with potential additional milestone payments of $8.0 million due upon certain regulatory approvals and the achievement of a predetermined net sales threshold on MT 300. We also will pay royalties to Pozen on the combined net sales of MT 300 and D.H.E. 45 once MT 300 is commercialized. Royalty rates on the combined annual net sales of MT 300 and D.H.E. 45 will commence in the low double digits and increase based on the achievement of predetermined net sales thresholds.
Net cash provided by financing activities for the year ended December 31, 2002 was $32.4 million. This cash resulted primarily from net proceeds of $22.4 million associated with the issuance of 3,750,000 shares of our Series B convertible preferred stock in June 2002 and $10.0 million in borrowings against the Elan credit line facility in June 2002. Net cash provided by financing activities was $70.1 million for the period ended December 31, 2001. This cash resulted primarily from the net proceeds of $69.6 million received from the issuance of 14,000,000 shares of our Series A convertible preferred stock in March 2001.
Under the terms of the Diastat and Mysoline acquisition agreements, Elan was responsible for the payment and processing of rebates, chargebacks and returns on Diastat and Mysoline sold by Elan prior to our acquisition of these products up to contractually specified amounts and time periods. As of December 31, 2002, the contractually specified time periods had ended. As of June 30, 2003, we recorded an estimated liability of $4.1 million related to these contractual obligations as a product acquisition payable. In addition, Elan continues to process and pay rebates on our sales of Diastat and Mysoline inventories that we acquired from Elan in connection with the acquisition of the products. As of June 30, 2003, we recorded an estimated liability of $5.8 million related to these contractual obligations as a rebate reimbursement payable. On August 5, 2003, we paid Elan $9.6 million in satisfaction of the $4.1 million product acquisition liability and the $5.8 million rebate
33
reimbursement payable accrued as of June 30, 2003. Payment of agreed upon liabilities subsequent to June 30, 2003 are due to Elan 30 days after our receipt of an invoice from Elan.
We expect that cash from operations will be adequate to fund our operations and working capital requirements for at least the next 12 months. In the event that we make significant future acquisitions of commercial products or product candidates, we expect that we will need to raise additional funds. Adequate funds for these purposes, whether through the financial markets or from other sources, may not be available when needed or on terms acceptable to us. Insufficient funds may cause us to delay, scale back or abandon some or all of our future product acquisition projects.
Our future capital requirements and adequacy of our available funds will depend on many factors, including the effectiveness of our sales and marketing activities; the timing and magnitude of product acquisitions; the size and scope of our efforts to develop new commercial products, product candidates and product enhancements; the status of competitive products; and the establishment, continuation or termination of third-party manufacturing agreements.
Recent Accounting Pronouncements
In December 2002, the Financial Accounting Standards Board issued SFAS No. 148 as an amendment to SFAS No. 123. SFAS No. 148 provides alternative methods of transition for a voluntary change to the fair value based method of accounting for stock-based employee compensation. In addition, SFAS No. 148 amends the disclosure requirements of SFAS No. 123 to require prominent disclosures in both annual and interim financial statements about the method of accounting for stock-based compensation and the effect of the method used on reported results. The voluntary transition and amended disclosure requirements are effective for fiscal years ending after December 15, 2002. The interim reporting requirements are effective for interim periods beginning after December 15, 2002. We account for employee stock-based compensation under the intrinsic value method in accordance with APB No. 25. We did not voluntarily change our method of accounting, but did implement the disclosure requirements.
Quantitative and Qualitative Disclosure About Market Risks
We invest our excess cash in highly liquid interest-bearing instruments with original maturities of three months or less. These instruments are subject to interest rate risk. The amount that we earn on these instruments will decline if interest rates decline. However, due to the short-term nature of these holdings, an immediate 10% decline in interest rates would not have a material impact on our financial condition, results of operations or cash flows.
Inflation
Our operations have not been affected materially by inflation. We cannot assure you that our operations will not be affected by inflation in the future.
34
Product Contribution of Diastat and Mysoline
to Elan’s Results of Operations
The following discussion analyzes the contribution of Diastat and Mysoline to Elan’s results for the year ended December 31, 2000 and three months ended March 31, 2001 and is not intended to be a complete presentation of these products’ results of operations. Elan has not prepared financial statements of these products, which would be intended to report a complete presentation of financial position, results of operations and cash flows. The results of these products were derived from the internal financial statements of Elan and incorporate certain cost allocations which may not be indicative of the costs had these products been operated on a stand- alone basis. This discussion is based on our understanding of contribution of Diastat and Mysoline to Elan’s results of operations for the periods presented.
Net sales. Net sales were $42.1 million in 2000. In 2000, there was a 6% increase in the price of Diastat and a 15% increase in the price of Mysoline. Diastat was approved by the FDA in July 1997 and launched by Elan in October 1997. Mysoline was acquired by Elan in February 1998.
During the three months ended March 31, 2001, net sales were $8.8 million. On an annualized basis, this would project to a decline of 16.7% compared to annual net sales in 2000. This projected decline was primarily due to the timing of wholesaler buying and Elan reducing its sales and marketing emphasis on Diastat and Mysoline and targeting its marketing and promotional efforts on larger products within its overall product portfolio. There were no price increases or decreases in 2001. As discussed further below, selling, general and administrative expenses as a percentage of net sales decreased from 31.8% in 2000 to 25.9% in the three months ended March 31, 2001.
Cost of sales. Cost of sales as a percentage of net sales was 17.5% in 2000 and 18.3% during the three months ended March 31, 2001. Cost of sales consists primarily of the cost to manufacture Diastat and Mysoline and royalties under separate royalty agreements ranging from 5% to 10% of net product sales. Third parties manufactured Diastat and Mysoline for Elan. The increase in cost of sales was primarily due to increases in the manufacturing costs during these periods.
Selling, general and administrative expenses. Selling, general and administrative expenses as a percentage of net sales were 31.8% in 2000 and 25.9% during the three months ended March 31, 2001. The annual decline as a percentage of net sales was due to a decrease in sales and marketing activities in promoting Diastat and Mysoline, primarily related to a reduction in the field sales force promotion. As discussed above, Diastat was launched by Elan in October 1997 and received extensive marketing and promotion for a limited time following launch. As Elan acquired or developed additional products to add to its product portfolio, the sales and marketing resources committed to Diastat and Mysoline declined compared to Elan’s other products.
Research and development expenses. Research and development expenses as a percentage of net sales were 4.9% in 2000 and 4% during the three months ended March 31, 2001. These costs primarily relate to certain Diastat clinical trials that were nearing completion by March 31, 2001.
35
BUSINESS
Overview
We are a specialty pharmaceutical company focused on prescription products that treat disorders of the CNS. The CNS market consists of three major segments: neurology, pain and psychiatry. Sales of products used to treat CNS disorders in the United States totaled $39.5 billion in 2002 and grew at an average annual rate of 19% from 1998 to 2002. We currently focus on neurology, a segment where a limited number of high-prescribing physicians write a significant number of the prescriptions for neurology products. The top two areas of practice focus of neurologists are epilepsy and migraine.
Our product portfolio includes four commercial products, Diastat and Mysoline, which are used to treat epilepsy, and Migranal and D.H.E. 45, which are used to treat migraine, and one product candidate, MT 300, a potential treatment for migraine. Our 96-person nationwide field sales organization promotes our commercial products to high-prescribing epilepsy and migraine specialists. We intend to increase prescription demand for our key products, Diastat, Migranal and, subject to regulatory approval and commercial launch, MT 300 through targeted sales and marketing efforts and to develop enhancements for our current products. In addition, we intend to leverage our presence in the CNS market through the acquisition, development and commercialization of additional late-stage development product candidates and through the acquisition of additional commercial products.
The concentration of high-prescribing physicians in the neurology market enables us to promote our products effectively with a small, dedicated sales and marketing organization and a limited corporate infrastructure. During 2002, the 14,800 neurologists and pediatric neurologists in the United States collectively wrote prescriptions for $1.5 billion of the products used to treat epileptic seizures and $354 million of the products used to treat migraines. Our field sales organization promotes our commercial products to 6,900 of the highest-prescribing physicians in this group. These 6,900 high-prescribing neurologists collectively wrote 93% of the prescriptions for epilepsy and migraine products among all 14,800 neurologists and pediatric neurologists in 2002. We have designed our corporate infrastructure to support our field sales organization, to identify and pursue the acquisition of additional late-stage development product candidates and commercial products and to lead our product development and enhancement efforts. We utilize third parties to manufacture and distribute our products and to support our product development and enhancement efforts.
Our epilepsy products, Diastat and Mysoline, are used in the treatment of seizures principally associated with epilepsy. We acquired Diastat and Mysoline in 2001 from Elan. Diastat is currently the only drug approved in the United States for the acute treatment of seizures outside of a hospital setting and is the subject of a patent that expires in September 2013. Mysoline has been used since the early 1950s for the chronic treatment of seizures associated with epilepsy and is highly complementary to our marketing efforts for Diastat.
Our commercial migraine products, Migranal and D.H.E. 45, contain the active ingredient dihydroergotamine and represent safe and effective second-line therapies for the acute treatment of migraine. We acquired Migranal and D.H.E. 45 in 2002 from Novartis. Migranal is the subject of a patent that expires in December 2009. D.H.E. 45 has been used since the mid-1940s, has been a standard of care for many years for the acute treatment of migraine. Our product candidate, MT 300, is a potential treatment for migraine. MT 300 is an improved and highly purified formulation of dihydroergotamine in a convenient pre-filled syringe that is suitable for at-home use. MT 300 is covered by a patent that expires in March 2020. A new drug application to market MT 300 for the treatment of migraines was submitted to the FDA by Pozen in December 2002 and is under review by the FDA. We licensed exclusive commercial rights in the United States to MT 300 from Pozen in September 2003. Subject to regulatory approval and commercial launch, we intend to promote the benefits of MT 300 to our targeted physicians to grow our injectable dihydroergotamine franchise.
We believe that the changing pharmaceutical industry landscape will present significant opportunities for us to acquire additional commercial and late-stage development product candidates. Larger pharmaceutical
36
companies are shifting their sales and marketing efforts away from smaller commercial products toward products with higher revenue potential and new product launches. As a result, an increasing number of smaller products and late-stage development product candidates that may have growth potential are receiving little or no attention and present potentially attractive acquisition opportunities for us. Biotechnology companies developing CNS product candidates also are looking for partners to assist with the commercialization of their product candidates and associated costs, including late-stage development costs. We continually evaluate opportunities to acquire rights to these types of commercial products and product candidates, although currently we have no specific agreements with respect to such acquisitions. We are pursuing product enhancements for Diastat and Migranal and, on our behalf, Pozen is developing a product enhancement for MT 300. Subject to regulatory approval and commercial launch, we believe these product enhancements will provide additional patient, physician and distribution channel benefits.
Our Strategy
Our objective is to capitalize on our management team’s experience acquiring, developing and marketing pharmaceutical products. We intend to become a leading specialty pharmaceutical company focused on disorders of the CNS in the United States. Specifically, our strategy is to:
| | • | | Increase prescription demand for our key products, Diastat and Migranal and, subject to regulatory approval and commercial launch, MT 300. We believe there are significant opportunities to increase prescription demand for Diastat, Migranal and MT 300. Diastat is the only product approved in the United States for the acute treatment of seizures associated with epilepsy outside of a hospital setting. Diastat is safe and effective and has significant quality-of-life and cost advantages. Although one-third of our targeted physician audience wrote 67% of all Diastat prescriptions during 2002, the remaining two-thirds of these physicians did not prescribe Diastat. We intend to increase prescription demand for Diastat by promoting to our targeted physicians the benefits of including Diastat as part of the standard treatment regimen for their epilepsy patients. Migranal, a nasal spray, is safe and effective for the acute treatment of migraine. MT 300, an injection in a pre-filled syringe, is a potential treatment for migraines. We intend to increase prescription demand for Migranal and, subject to regulatory approval and commercial launch, MT 300 by promoting to our targeted physicians the benefits of prescribing Migranal and MT 300 for their migraine patients who do not experience effective relief with first-line migraine therapies. |
| | • | | Leverage our presence in the CNS market through the acquisition of additional late-stage development product candidates. We intend to build a product pipeline by acquiring additional late-stage development product candidates that, subject to regulatory approval and commercial launch, can be marketed to our targeted physician audiences. We target product candidates that, at a minimum, are in Phase II clinical studies. We have developed valuable relationships with third-party consultants and contract research organizations that we intend to utilize to support our advancement of late-stage development product candidates through the development and approval process. |
| | • | | Leverage our presence in the CNS market through the acquisition of additional commercial products. We intend to enhance our growth and leverage our organization by acquiring additional FDA-approved, commercial products that can be marketed to our targeted physician audiences. We believe that a growing number of opportunities to acquire neurology products will arise due to the increasing emphasis by larger pharmaceutical companies on products with higher revenue potential and new product launches. |
| | • | | Maximize the value of our products by developing product enhancements. We intend to expand sales of our current products by developing product enhancements, such as new delivery systems, new formulations and new dosage strengths, and by conducting targeted post-approval clinical studies. We currently are developing product enhancements for Diastat and Migranal, and on our behalf, Pozen is developing a product enhancement for MT 300. Subject to regulatory approval and commercial launch, we believe these product enhancements will provide additional patient, physician |
37
| | and distribution channel benefits. We believe that product enhancements represent attractive ways to expand our business because they typically are less costly, less risky and require less time than most other product development efforts. We have relationships with third parties to support development of our product enhancements. |
Our Market Focus—Disorders of the Central Nervous System
Our market focus is on disorders of the CNS, which includes neurologic disorders, pain and psychiatric disorders. Our current focus within the CNS market is neurology, or the treatment of diseases that affect the nervous system. We currently focus on neurology, a segment where a limited number of high-prescribing physicians write a significant number of prescriptions for neurology products. The top two areas of practice focus of neurologists are epilepsy and migraine. Our commercial products, Diastat and Mysoline, are used in the treatment of seizures principally associated with epilepsy. Sales of products in the United States used to treat epileptic seizures totaled $5.4 billion in 2002 and grew at an average annual rate of 24% from 1998 to 2002. Our commercial products, Migranal and D.H.E. 45, are used in the treatment of migraines. Our product candidate, MT 300, is under review by the FDA as a potential treatment for migraine. Sales of products used to treat migraines in the United States totaled $1.8 billion in 2002 and grew at an average annual rate of 12% from 1998 to 2002.
Epilepsy
Epilepsy is a chronic disorder characterized by abnormal electrical discharges in the brain. These abnormal electrical discharges result in seizures, the clinical manifestation of epilepsy. Epilepsy is one of the most common neurologic disorders in the United States, affecting 2.5 million people in 2000 with an estimated 181,000 new cases diagnosed each year. Most seizures end within two minutes; however, those that do not are unpredictable in length. The longer a seizure lasts, the more difficult it becomes to treat and the greater the risk of injury and death to the patient.
A wide variety of antiepileptic drugs are available to prevent and treat seizures. These drugs are typically administered orally on a daily basis. Most patients receive a regimen of a single drug or a combination of several antiepileptic drugs. Nevertheless, approximately 30% of, or 750,000, patients with epilepsy have inadequate seizure control or are resistant to drug therapy and suffer from seizures, which we refer to as breakthrough seizures. These patients may have frequent visits to the emergency room and admissions to the hospital because of their breakthrough seizures. Even patients for whom antiepileptic drugs provide adequate control are still at risk of breakthrough seizures.
If uncontrolled, breakthrough seizures can progress into a single prolonged seizure, a cluster of seizures or status epilepticus, a prolonged seizure or series of intermittent seizures without recovery of consciousness that lasts more than 30 minutes. Status epilepticus occurs an estimated 152,000 times each year in the United States and is a neurologic emergency. Estimates of the risk of death from status epilepticus vary widely, but have been as high as 53%. While status epilepticus can be devastating, even brief seizures have significant consequences, including the potential for physical injury and brain damage to the patient and the emotional distress inflicted upon family members and caregivers witnessing the event.
As a result of the significant risks of injury and death associated with prolonged seizures and status epilepticus, the goal of therapy is to stop the seizure as quickly as possible. Unfortunately, effective treatments for prolonged seizures in the United States historically have been available only in a hospital setting. Depending on the distance between the location where the seizure occurs and the nearest hospital, the delay between the onset of a prolonged seizure and the initiation of treatment can be significant. In one study, the time from the onset of the seizure to the initiation of treatment at the hospital averaged 85 minutes. Once in the emergency room, physicians tend to treat seizures aggressively, usually with benzodiazepines delivered intravenously. When the seizure has stopped, the patient may remain heavily sedated for many hours as a result of the seizure itself and the longer-term effects of the medication used to stop the seizure.
38
In the 1980s, several patient advocacy groups and a nationwide group of neurologists specializing in the treatment of epilepsy began lobbying drug manufacturers to develop an alternative treatment for seizures that could be administered by caregivers outside a hospital setting. Initial work focused on developing an alternative to the intravenous route for delivering benzodiazepines. Oral or sublingual administration was considered impractical for seizure patients because of the dangers of the patient choking on the medication, as well as the difficulty of administering the medication if the patient will not, or cannot, cooperate. Intramuscular administration through injection was also rejected because it results in erratic drug absorption and has been associated with a condition known as necrosis, or tissue death, at the injection site. For several years, physicians in Europe had been using diazepam, a benzodiazepine in a liquid solution delivered rectally, to treat prolonged seizures by a caregiver at home. One limitation of this approach, however, is that it is difficult to contain the liquid solution while the seizing patient’s body absorbs the drug. As a result, U.S. efforts focused on developing a better delivery system that would permit more complete absorption of the drug by the patient. Diastat, approved by the FDA in 1997, was the culmination of these efforts.
Migraine
Migraine is a neurologic disorder characterized by recurrent headache attacks where the pain most often occurs on one side of the head. Migraines are usually accompanied by various combinations of symptoms, including nausea and vomiting, distorted vision, and sensitivity to light and sound. The biochemical mechanism underlying migraines remains to be fully understood. However, recent advances in migraine research have identified the neurotransmitter, serotonin (5-hydroxytryptamine, or 5-HT), as a key mediator in the generation of migraine. There is much evidence to suggest that the principal factor in migraine and its associated symptoms are blood flow changes in the brain. Migraines affect approximately 28 million people in the United States, nearly 13% of the total U.S. population. Migraines are episodic and vary among patients. The average migraine patient experiences two migraines per month.
A variety of migraine drugs are available to abort migraines. These drugs can be administered orally, nasally or via injection and are taken on an as-needed basis. Two important prescription drug classes for the abortive treatment of migraines are triptans and ergotamines. Triptans and ergotamines relieve the migraine pain by mediating the constriction of blood vessels in the brain, referred to as vasoconstriction. However, triptans and ergotamines can cause side effects, including nausea, dizziness, neck or chest tightness, warm or cold sensations and muscle weakness. Dihydroergotamine is a derivative of ergotamine that exhibits a more selective vasoconstriction that results in reduction of the migraine pain with fewer side effects than ergotamines. Of the 16 million people who receive a prescription drug for migraines each year, approximately 25%, or 4 million, of them do not experience effective relief from first-line therapies. The leading first-line migraine therapies prescribed by neurologists are the triptan class of drugs. Triptans generally are prescribed as the first-line therapy for migraine because they are fast-acting compared to other available therapies. Migranal and D.H.E. 45, both of which are dihydroergotamines, have a mechanism of action that is broader than the triptan class of drugs and therefore represent safe and effective second-line therapies for patients who are not experiencing effective relief from triptan-based drugs. Migranal is delivered through a nasal spray, while D.H.E. 45 can be administered via intravenous, intramuscular of subcutaneous injection. Our product candidate, MT 300, is a potential treatment for migraine. MT 300 is an improved and highly purified formulation of dihydroergotamine in a convenient pre-filled syringe that is suitable for at-home use. A new drug application to market MT 300 for the treatment of migraine was submitted to the FDA by Pozen in December 2002 and is under review by the FDA.
39
Our Commercial Products
Our four commercial products include two products, Diastat and Mysoline, which are used to treat epilepsy, and two products, Migranal and D.H.E. 45, which are used to treat migraine. Our strategy is to increase prescription demand for Diastat and Migranal by promoting these products to our targeted physicians. Diastat is patented into September 2013, and Migranal is patented into December 2009. Mysoline and D.H.E. 45 are not protected by patents. The following table provides more information on each of our commercial products:
Commercial
Product
| | Indication
| | Administration / Delivery
| | Patent
Expiration
|
Diastat | | Epilepsy | | Rectally administered gel for the acute treatment of epilepsy patients age two years and above experiencing seizures. | | 2013 |
| | | |
Migranal | | Migraine | | Nasal spray for the acute treatment of migraines. | | 2009 |
| | | |
D.H.E. 45 | | Migraine | | Intravenous, intramuscular or subcutaneous injection for the acute treatment of migraines. | | N/A |
| | | |
Mysoline | | Epilepsy | | Oral tablets used alone or in combination with other therapies in the chronic control of epileptic seizures for patients age eight and above; also used off-label to treat essential tremor. | | N/A |
Diastat
Diastat is the only drug approved in the United States for the acute treatment of seizures outside of a hospital setting. Diastat is a rectally administered gel for patients age two and above experiencing breakthrough seizures. We acquired our rights to Diastat from Elan in March 2001. Diastat serves a previously unmet need for patients suffering from a breakthrough seizure by treating the patient immediately at the location where the seizure occurs. For most patients, the ability to receive treatment wherever the seizure occurs significantly shortens the time between the onset of the seizure and the administration of effective treatment. This, in combination with Diastat’s rapid onset of action, generally between five and 15 minutes, results in an increased likelihood of shortening the seizure, thereby reducing the risks of irreparable injury and death to the patient. Diastat also reduces the need for patients and their families to visit an emergency room. When administered within five to ten minutes of the onset of a seizure, Diastat has been shown to reduce the number of emergency room visits by 67%. Diastat has an excellent safety profile, with the most noticeable side effect being drowsiness. When compared to the costs of treating a prolonged seizure in an emergency room, Diastat also represents a significant cost savings to patients, government entities and private health insurers.
Diastat provides significant quality of life benefits for epilepsy patients and their families. Diastat enables patients and their families to enjoy greater freedom to travel away from home and to live away from large metropolitan areas where most hospitals are located because they have a treatment option in the event of a breakthrough seizure. Diastat also provides family members and caregivers with greater comfort knowing that their loved one has an effective treatment that can be administered at the location where the seizure occurs.
During 2002, one-third of our targeted prescriber audience of 6,900 neurologists and pediatric neurologists wrote 67% of the total Diastat prescriptions. The remaining two-thirds did not prescribe Diastat. We believe that through focused and consistent promotion of the benefits of including Diastat as part of the standard treatment regimen for their epilepsy patients, we can continue to increase the use of Diastat among our targeted prescriber audience.
Retail prescriptions for Diastat have grown steadily since its launch by Elan in October 1997 and have continued to grow since we acquired Diastat in March 2001. Total retail prescriptions increased 29.4% during the 12 months after our re-launch of promotion in October 2001. Retail prescriptions for Diastat have continued their growth trends into 2003, with total retail prescriptions in June 2003 reaching an all-time high of 9,962 while increasing 25.9% during the 12 months ended June 30, 2003.
40
Migranal
Migranal is a nasal spray used to treat migraine. We acquired our rights to Migranal from Novartis in June 2002. Migraines affect approximately 28 million people in the United States, nearly 13% of the total U.S. population. Sales of products used to treat migraines in the United States totaled $1.8 billion in 2002 and grew at an average annual rate of 12% from 1998 to 2002. Of the 16 million people who receive a prescription drug for migraines each year, approximately 25%, or 4 million people, do not experience effective relief from first-line therapies. The leading first-line migraine therapies prescribed by neurologists are the triptan class of drugs. Migranal has a mechanism of action that is broader than the triptan class of drugs. Our strategy is to promote Migranal to our targeted physicians as a safe and effective therapeutic option for migraine patients who do not experience effective relief with first-line migraine therapies.
Migranal was initially launched by Novartis in January 1998, but had no significant promotional activity from 1999 until our relaunch in October 2002. As a result of our sales force relaunching promotional activity, the historical decline of Migranal total retail prescriptions has slowed by 53% to a 6.4% decline for the nine months since our relaunch compared to a 13.5% decline for the nine months before our relaunch. The decline of new retail prescriptions, which is a leading indicator of demand, has slowed by 97% to a 0.4% decline for the nine months since our relaunch compared to a 11.6% decline for the nine months before our relaunch. We believe that through focused and consistent promotion of the benefits of using Migranal as an alternative therapy for patients who do not experience effective relief with triptans, we can increase the use of Migranal among our targeted prescriber audience.
D.H.E. 45
D.H.E. 45 is an injectable product that can be administered intravenously, intramuscularly or subcutaneously to treat migraines. We acquired our rights to D.H.E. 45 from Novartis in June 2002. D.H.E. 45 has been used since the mid-1940s and has been a standard of care for many years for the acute treatment of migraine. The majority of D.H.E. 45 ampules are administered in headache clinics, medical offices or emergency rooms. We promote D.H.E. 45 together with Migranal as a proven treatment for migraine patients who do not experience effective relief from triptan-based drugs. D.H.E. 45 is not protected by a patent and two abbreviated new drug applications for generic equivalents to D.H.E. 45 were approved by the FDA in the second quarter of 2003. D.H.E. 45 has experienced slowly declining prescription trends for many years. In the third quarter of 2003, generic substitutes for D.H.E. 45 were launched by Paddock Laboratories and Bedford Laboratories. In addition, in September 2003, we acquired exclusive commercial rights in the United States to sell MT 300, a potential treatment for migraine. MT 300 is an improved and highly purified formulation of dihydroergotamine in a convenient pre-filled syringe that is suitable for at-home use. A new drug application to market MT 300 for the treatment of migraine was submitted to the FDA by Pozen in December 2002 and is under review by the FDA. Subject to regulatory approval and commercial launch, we intend to promote the benefits of MT 300 to our targeted physicians to grow our injectable dihydroergotamine franchise. We expect the sales of generic substitutes for D.H.E. 45 and the anticipated approval and launch of MT 300 to increase the rate of prescription decline and to affect adversely our future net sales of D.H.E. 45.
Mysoline
Mysoline, our brand name for primidone, is an oral prescription medication that has been used since the early 1950s for the chronic treatment of seizures associated with epilepsy. We acquired our rights to Mysoline from Elan in April 2001. It is used alone and in combination with other anticonvulsant drugs. Mysoline is also a frequently prescribed therapy for the treatment of essential tremor (although Mysoline is not indicated for essential tremor). Essential tremor is considered the most common neurologic movement disorder.
Mysoline is not protected by a patent and several generic versions of the product are available. Mysoline is, however, included in the FDA list of narrow therapeutic index drugs. These drugs are characterized by sensitivity
41
to slight formulation changes between different manufacturers that can lead to different therapeutic effects and adverse events in patients. In the case of Mysoline and most other antiepileptic drugs, the difficulty of finding a drug regimen that can control seizures effectively and the potential adverse consequences that can result from any breakthrough seizure leads many neurologists to prescribe branded drugs in lieu of generic substitutes. Despite the introduction of generic competition in June 2001, Mysoline 50 milligram tablets have retained 31% of the 50 milligram primidone market as of the end of the second quarter of 2003. In comparison, generic versions of the 250 milligram primidone tablet were introduced in 1981 and Mysoline 250 milligram tablets have retained 16% of the 250 milligram primidone market as of the end of the second quarter of 2003. However, as a result of the generic competition, we have experienced declining prescription trends for Mysoline.
Our Product Candidate
Our product candidate is MT 300, a treatment for migraine for which marketing approval is pending. The following table provides more information on our product candidate.
Product
Candidate
| | Potential
Indication
| | Administration / Delivery
| | Patent
Expiration
|
MT 300 | | Migraine | | Subcutaneous injection via a pre-filled syringe for the acute treatment of migraines | | 2020 |
MT 300
MT 300 is an injectable product candidate in a pre-filled syringe for treating migraine. We licensed the exclusive commercial rights in the United States to MT 300 from Pozen in September 2003. MT 300 is an improved formulation of dihydroergotamine in a convenient pre-filled syringe that is suitable for at-home use. In clinical trials, MT 300 has been studied in over 800 patients. In the two Phase III clinical trials, MT 300 demonstrated a statistically significant improvement in the percentage of patients achieving pain relief at two hours as well as sustained pain relief at 24 hours when compared to placebo. A new drug application to market MT 300 for the treatment of migraine was submitted to the FDA by Pozen in December 2002 and is under review by the FDA. Subject to regulatory approval and commercial launch, we intend to promote the benefits of MT 300 to our targeted physicians to grow our injectable dihydroergotamine franchise. We expect the anticipated approval and launch of MT 300 to increase the rate of prescription decline and to affect adversely our future net sales of D.H.E. 45.
Product Development
Our strategic objectives include building our product pipeline by acquiring additional late-stage development product candidates that, subject to regulatory approval and commercial launch, can be marketed to our targeted physician audiences. We have the exclusive commercial rights in the United States to MT 300. MT 300 is under review by the FDA to be marketed as a treatment for migraine. We target product candidates that are in Phase II clinical studies up through the filing of new drug applications with the FDA. Our management team has significant experience in managing neurology product candidates through the development process and successful regulatory approval. We have developed valuable relationships with third-party consultants and contract research organizations that we intend to utilize to support our advancement of late-stage development product candidates through the development and approval process. The future acquisition and development of product candidates should increase significantly our development expenses and reduce our operating results until such product candidates are approved, marketed and generate revenue, which is not assured. If product candidates that we may acquire do not receive regulatory approval, or even after receiving regulatory approval, do not develop substantial sales, we may not recover our investment in these product candidates.
42
Currently, we are pursuing opportunities to develop product enhancements for Diastat and Migranal, and on our behalf, Pozen is developing a product enhancement for MT 300. Subject to regulatory approval and commercial launch, we believe these product enhancements will provide additional patient, physician and distribution channel benefits. For competitive reasons, we do not disclose the specifics about our product enhancement programs. However, potential enhancements could include new delivery systems, new formulations and new dosage strengths. We also intend to evaluate opportunities to conduct targeted post-approval clinical studies for our products. We believe these types of product enhancements can offer important patient benefits and attractive opportunities to increase the use of our products. The development of product enhancements typically is less costly, less risky and requires less time than most other development efforts. We rely on third parties to assist us in our development of product enhancements.
Sales and Marketing
During 2002, the 14,800 neurologists and pediatric neurologists in the United States collectively wrote prescriptions for $1.5 billion of the products used to treat epileptic seizures and $354 million of the products used to treat migraine. Our field sales organization promotes our products to 6,900 of the highest prescribing physicians in this group. These 6,900 high-prescribing neurologists collectively wrote 93% of the prescriptions for migraine and epilepsy products among all 14,800 neurologists and pediatric neurologists in 2002. This concentration of high volume prescribers enables us to promote effectively our products with a small, dedicated sales and marketing organization.
Our field sales professionals are positioned in major metropolitan areas across the United States. Our two regional directors and ten area business managers have an average of ten years pharmaceutical experience, which includes five years pharmaceutical management experience. Our 84 sales representatives have an average of seven years sales experience, which includes four years pharmaceutical sales experience. Each member of our sales team undergoes a rigorous training program focused on our product offerings, disease background, competitive products and our sales techniques. Our program includes significant field-based learning to provide a comprehensive understanding and perspective as to the CNS market and the needs of both physicians and patients.
We have created an incentive bonus program for our sales force based on performance, with no limit on the total bonuses that can be earned. We focus on cultivating a relationship of trust and confidence with our targeted physician audience. In addition, we use a variety of marketing programs to promote our products, including promotional materials, industry publications and educational conferences and seminars. We continuously educate our representatives, as well as physicians and medical personnel, on the clinical merits of our products.
Material Agreements
Product Acquisition Agreements:
Diastat and Mysoline
We acquired rights to our epilepsy products pursuant to agreements that we entered into with Elan in March and April 2001. Under these agreements, we acquired exclusive worldwide rights to Diastat and exclusive rights in the United States to Mysoline. We also acquired equipment that is used by our third-party manufacturer in the manufacture of Diastat. The equipment included molds that are unique to the manufacture of Diastat and other items that are used generally in the manufacture of pharmaceutical products. The purchase price for these assets included the assumption of third-party agreements and royalty obligations of Elan pertaining to the products. The purchase price for these assets consisted of a net cost of $148.7 million of which we borrowed $99.0 million from Elan and included a $1.0 million obligation we recorded for returns, chargebacks and rebates related to sales of the products prior to our acquisition. The $99.0 million that we borrowed from Elan was repaid in full in March 2003 with a cash payment of $61.5 million.
43
The agreements with Elan contain customary representation, warranty, indemnity and confidentiality provisions. The third-party agreements that we assumed relate primarily to the manufacture of Diastat and Mysoline and are discussed in more detail under “—Manufacturing.” The third-party royalty obligations that we assumed range in amounts from 5% to 10% of net sales on these products. We also acquired existing inventory of both Diastat and Mysoline from Elan for an additional $5.2 million.
Migranal and D.H.E. 45
We acquired rights to our migraine products pursuant to agreements that we entered into with Novartis in June 2002. Under these agreements, we acquired exclusive rights in the United States to Migranal and D.H.E. 45. The net cost for these assets was $49.0 million. Included in the net cost is a $1.1 million obligation we recorded for returns, chargebacks and rebates related to sales of the products prior to our acquisition. The agreements with Novartis contain customary representation, warranty, indemnity and confidentiality provisions. In connection with the acquisition of these products, we entered into a supply agreement with Novartis to manufacture Migranal and D.H.E. 45. The supply agreement is discussed in more detail under “—Manufacturing.” We also acquired existing inventory of both Migranal and D.H.E. 45 from Novartis for an additional $1.8 million.
MT 300
We licensed exclusive commercial rights in the United States to MT 300 pursuant to an agreement that we entered into with Pozen in September 2003. We paid an upfront fee of $2.0 million to Pozen. Potential milestone payments of up to $8.0 million may be due to Pozen upon certain future regulatory approvals and the achievement of a predetermined net sales threshold on MT 300. We also will pay royalties to Pozen on combined net sales of MT 300 and D.H.E. 45 once MT 300 is commercialized. Royalty rates on the combined annual net sales of MT 300 and D.H.E. 45 will commence in the low double-digits and increase based on the achievement of predetermined net sales thresholds. The agreements with Pozen contain customary representation, warranty, indemnity and confidentiality provisions. Our rights to MT 300 can be sublicensed to third parties in connection with co-promotion activities we may undertake, but otherwise are not generally sublicensable under the agreement. We did not assume any manufacturing or supply agreements in connection with the agreement with Pozen. We are working to enter into supply agreements for the commercial manufacture of MT 300. Additional details are discussed under “—Manufacturing.”
Debt and Security Agreements:
Elan Agreements
In connection with the purchase of Diastat and Mysoline from Elan, we entered into a loan agreement under which Elan provided us with $99.0 million in product financing. Under the loan agreement, Elan also agreed to provide us with a $10.0 million credit line, which we borrowed in full in June 2002. The $99.0 million in product financing owed to Elan and the $10.0 million in credit line borrowings were repaid in full in March 2003 through the payment of $71.5 million. We recorded a gain of $37.4 million on the retirement of the product financing.
Under the terms of the product acquisition agreements, Elan was responsible for the payment and processing of returns, chargebacks and rebates on Diastat and Mysoline sold by Elan prior to our acquisition of the products up to contractually specified amounts and time periods. As of December 31, 2002, the contractually specified time periods had ended. In addition, Elan continues to process and pay rebates on our sales of Diastat and Mysoline inventories we acquired from Elan in connection with the acquisition of the products. As of June 30, 2003, we have recorded an estimated liability to Elan of $9.9 million related to these contractual obligations. On August 5, 2003, we paid Elan $9.6 million in satisfaction of the $9.9 million of contractual obligations accrued as of June 30, 2003. Payment of agreed upon liabilities subsequent to June 30, 2003 are due to Elan 30 days after receipt of an invoice from Elan.
44
In June 2001, we entered into an agreement with Elan whereby Elan agreed to make payments to us through December 31, 2010 based on the net sales of Elan’s product Zanaflex. In exchange, we and Elan amended the original product acquisition agreements to provide for payments from us to Elan through December 31, 2015 based on the net sales of Mysoline. This product financing had an imputed interest rate of approximately 7% based on estimates of the total net payments over the term of the financing. In March 2003, these agreements were mutually terminated with no future obligations remaining on either party. We recorded a gain on termination of $246,000.
Senior Debt Agreement
In March 2003, we raised proceeds of $62.0 million through the issuance of senior secured debt to Regiment. We are obligated to make monthly payments of interest only at prime (with a minimum of 6.5%) plus 4.5% on the outstanding balance of this loan through March 31, 2004. This resulted in a coupon rate of 11% through June 30, 2003. Quarterly principal payments of $2.0 million are due June 30, 2004 through December 31, 2007 with a final balloon payment of $32.0 million due on March 31, 2008. We intend to repay a portion of this debt with a portion of the proceeds from this offering.
To secure our obligations under the loan agreement, we entered into a security agreement pursuant to which we granted a security interest in all of our assets to Regiment. Under the loan and security agreements, Regiment also is protected by various financial and operating covenants and other rights that, if breached by us, even in the absence of a payment default, would entitle Regiment to foreclose on its security interest without payment or refund to us. Our covenants under the loan and security agreements include not taking, without Regiment’s consent, any of the following actions:
| | • | | engaging in a line of business outside of the development, marketing, manufacturing, distribution and selling of pharmaceutical products affecting the CNS; |
| | • | | selling, leasing or disposing of all or any part of our assets or business; |
| | • | | acquiring all or substantially all of the assets of others; |
| | • | | merging with or consolidating into other parties; or |
| | • | | making certain distributions to our stockholders. |
In addition, except for specific exceptions set forth in the loan and security agreements, including an exception allowing us to incur subordinated debt in connection with permitted acquisitions, we cannot have debt outstanding to anyone other than to Regiment. We will also be in default if we fail to cure a breach of our representations, warranties and covenants under the agreements within any applicable cure period. If we fail to cure any breach, Regiment may exercise its rights and remedies under the agreements, including accelerating the due date of all our obligations and foreclosing on the collateral, without payment or refund.
Competition
Many companies, including large pharmaceutical firms with financial and marketing resources and development capabilities substantially greater than ours, are engaged in developing, marketing and selling products that compete with those we offer. We believe that competition among prescription pharmaceuticals aimed at the specialty markets, including those for CNS and neurologic disorders, will be based on, among other things, product efficacy, safety, reliability, availability and price. As specialty markets continue to evolve, there will be numerous companies and products that will continue to offer competitive advantages to already existing products in the marketplace. There are several companies in the United States that are developing, marketing and selling pharmaceuticals to treat neurologic conditions, including Novartis AG, Pfizer Inc., Teva Pharmaceuticals Industries and Biogen, Inc.
We also compete with other pharmaceutical companies for commercial product acquisitions. These competitors include King Pharmaceuticals, Inc., aaiPharma Inc., Endo Pharmaceuticals Holdings Inc., Cephalon,
45
Inc., Forest Laboratories, Inc., Watson Pharmaceuticals, Inc. and other companies attempting to acquire branded pharmaceutical products. We face competition for acquisitions of late-stage development product candidates from these companies as well as other pharmaceutical or biotechnology companies, most of which have substantially greater resources and product development capabilities than we do.
Diastat
Diastat is protected by a patent in the United States that expires in September 2013 and has orphan drug exclusivity that expires on July 29, 2004. Other drug companies may be able to obtain FDA approval to market generic versions of Diastat after July 2004 if they can design products that do not infringe our patent for Diastat and they are able to meet the FDA’s approval requirements.
There are no other FDA-approved competing drugs to Diastat. However, there are a few drugs that are used to abort seizures outside of a hospital setting without being indicated for that use. These products include sublingual Ativan or Intensol (lorazepam), intranasal midazolam and intravenous diazepam solution administered rectally. Lorazepam is indicated for the management of anxiety disorders but also is used for seizures. To be efficacious, sublingual lorazepam must be held in the buccal area of the patient’s mouth for two minutes. For a seizing patient, this is not practical. Intranasal midazolam is indicated for use in pediatric patients for sedation. It is difficult to administer in a seizing patient because a large volume of the drug is required and the drug can run down the throat and can cause choking. Finally, diazepam solution administered rectally is difficult to administer due to the length of time it takes to set up the catheter, draw up solution into a syringe and final administration of the drug to the patient. In addition, dosing errors often occur as the caregiver tends to overcompensate and administer more drug than is required to counteract the loss of solution in the catheter and the loss of drug that tends to leak out of the seizing patient. Overdosage could lead to respiratory depression.
A potential competing product to Diastat is injectable diazepam in a 10 milligram auto-injector being developed by King Pharmaceuticals. King Pharmaceuticals obtained injectable diazepam through its acquisition of Meridian Medical Technologies and filed an abbreviated new drug application with the FDA in November 2001.
Migranal, D.H.E. 45 and MT 300
Migranal is protected by a patent in the United States that expires in December 2009. D.H.E. 45 is not protected by a patent and two abbreviated new drug applications for generic equivalents to D.H.E. 45 were approved by the FDA in the second quarter of 2003. In the third quarter of 2003, Paddock Laboratories and Bedford Laboratories launched their generic substitutes for D.H.E. 45. The generic substitutes are expected to result in downward price pressure and prescription declines for D.H.E. 45, particularly if third-party payors refuse to reimburse patients for branded products or if physicians begin to prescribe, or pharmacists begin to administer, generic products more frequently than the branded counterparts, which we expect to affect adversely our future net sales of D.H.E. 45. MT 300, an improved formulation of dihydroergotamine in a pre-filled syringe, is protected by a patent in the United States that expires in March 2020. We also expect the anticipated approval and launch of MT 300 to increase the rate of prescription decline and to affect adversely our future net sales of D.H. E. 45.
A variety of migraine drugs are available to abort migraines. These drugs can be administered orally, nasally or via injection and are taken on an as needed basis. Two drug classes for the abortive treatment of migraines are triptans and ergotamines. Marketed products in the triptan class include, but are not limited to, Imitrex, Zomig, Maxalt, Amerge and Axert. Most patients who visit a neurologist for migraine receive a prescription for a triptan, the class of drugs used predominantly as first-line migraine therapy. Products in the ergotamine class include, but are not limited to, Cafergot and Wigraine.
46
Mysoline
We have no patent protection on Mysoline and we compete with manufacturers of generic substitutes for Mysoline. These generic manufacturers include Watson Pharmaceuticals, Inc., Danbury Pharmaceuticals, Inc., Lannett Company, Inc. and Qualitest Pharmaceuticals, Inc. Generic substitutes for Mysoline currently sell at prices less than the price of Mysoline. Any significant decline in the prices for generic substitutes for Mysoline would result in downward price pressure on Mysoline, particularly if third-party payors refuse to reimburse patients for branded products or if physicians begin to prescribe, or pharmacists begin to administer, generic products more frequently than the branded counterparts. Although the patent for Mysoline has expired, Mysoline is included in the FDA list of narrow therapeutic index drugs. These drugs are characterized by sensitivity to slight formulation changes between different manufacturers that can lead to different therapeutic effects or adverse events in patients. In the case of Mysoline and most other antiepileptic drugs, the difficulty of finding a drug regimen that can effectively control seizures and the potential adverse consequences that result from any breakthrough seizure leads many neurologists to prescribe branded drugs in lieu of generic substitutes.
For the chronic treatment of epilepsy, Mysoline competes with many marketed products that are available in oral dosage forms that are taken on a daily basis to prevent epileptic seizures. These products include, but are not limited to, Neurontin, Topamax, Depakote, Lamictal, Trileptal and Keppra. Many physicians also use Mysoline to treat a movement disorder called essential tremor even though Mysoline is not indicated for this use. The principal other drug used to treat essential tremor is Inderal (propranolol).
Manufacturing
We rely on third parties for the manufacture and distribution of our products. Our products are supplied under long-term supply agreements and we do not maintain alternative manufacturing sources for any of our products. Our third-party manufacturers and distributors are also subject to extensive governmental regulation. The FDA mandates that drugs be manufactured, packaged and labeled in conformity with cGMP. In complying with cGMP regulations, manufacturers must continue to expend time, money and effort in production, record keeping and quality control to ensure that products they produce meet applicable specifications and other requirements to ensure product safety and efficacy. The manufacture of Diastat is also subject to the Controlled Substances Act and related regulations, which are administered by the DEA. We intend to continue to outsource the manufacture and distribution of our products for the foreseeable future.
We currently rely on DPT as our single supplier of Diastat. We have an agreement with DPT through December 2006. We have an agreement with Yamanouchi for five years from the FDA approval date for Yamanouchi to manufacture Mysoline, which we expect to occur in early 2004. We believe that we have adequate finished goods inventories of Mysoline to supply our customers until Yamanouchi receives FDA approval. We currently rely on Novartis as our single supplier of finished ampules for Migranal and D.H.E. 45. We have an agreement with Novartis through June 2005. Pozen has been working with a single third-party manufacturer for MT 300 throughout the product candidate’s development. We are working to enter into a commercial supply agreement with this manufacturer.
Under each of our agreements with our third-party manufacturers, the manufacturers:
| | • | | are required to supply products to us based on purchase orders we provide to them; |
| | • | | provide representations and warranties regarding the compliance with cGMP of the products they make for us; |
| | • | | are required to operate their facilities in compliance with all legal and regulatory requirements; |
| | • | | are permitted to terminate the agreement only in the event that we materially breach the agreement or become insolvent; and |
| | • | | have agreed to assist us with the transfer of the manufacturing process for our products to another manufacturer prior to termination or expiration of the agreements. |
47
Government Regulation
Our business and our products are subject to extensive and rigorous regulation at both the federal and state levels. At the federal level, we are regulated principally by the FDA as well as by the DEA, the Federal Trade Commission, the U.S. Department of Agriculture, the Occupation Safety and Health Administration and the U.S. Environmental Protection Agency, or the EPA.
The FDA administers the Food, Drug and Cosmetic Act, or FDCA, and related regulations, which govern, among other things, the development, testing, approval, safety, effectiveness, manufacture, labeling, storage, recordkeeping, export, advertising and promotion of prescription drugs, a category that includes our products. Failure to comply with FDA requirements may subject us and/or our contract manufacturers to administrative or judicial sanctions, including FDA refusal to approve pending applications, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, fines, injunctions and/or criminal prosecution.
Although our existing commercial products have been approved for marketing by the FDA, we are subject to a number of post-approval requirements. If we seek to make certain changes to an approved product, such as promoting or labeling a product for a new indication, making certain manufacturing changes or product enhancements or adding labeling claims, we will need FDA review and approval before the change can be implemented. While physicians may use products for indications that have not been approved by the FDA, we may not label or promote the product for an indication that has not been approved. Securing FDA approval for new indications or product enhancements and, in some cases, for manufacturing and labeling claims, is generally a time-consuming and expensive process that may require us to conduct clinical studies under FDA’s investigational new drug regulations. Even if such studies are conducted, the FDA may not approve any change in a timely fashion, or at all. In addition, certain adverse experiences associated with use of the products must be reported to the FDA, and FDA rules govern how we can label, advertise or otherwise promote our products.
In addition, we and the third-party manufacturers on which we rely for the manufacture of our products are subject to requirements that drugs be manufactured, packaged and labeled in conformity with cGMP. To comply with cGMP requirements, manufacturers must continue to spend time, money and effort to meet organization and personnel, facilities, equipment, production and process, labeling and packaging, quality control, recordkeeping and other requirements. The FDA periodically inspects drug manufacturing facilities to evaluate compliance with cGMP requirements.
We also intend to acquire late-stage development product candidates that, at a minimum, are in Phase II clinical studies. Even if we are successful in acquiring such product candidates, we will not be able to market these product candidates until they have been approved by the FDA. The steps required before a drug may be marketed in the United States include:
| | • | | pre-clinical laboratory tests, animal studies, and formulation studies; |
| | • | | submission to the FDA of an investigational new drug exemption for human clinical testing, which must become effective before human clinical trials may begin; |
| | • | | adequate and well-controlled clinical trials to establish the safety and efficacy of the drug for each indication; |
| | • | | submission to the FDA of a new drug application for approval; |
| | • | | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the drug is produced to assess compliance with cGMP; and |
| | • | | FDA review and approval of the new drug application. |
Clinical trials involve administration of the investigational drug to human subjects under the supervision of qualified investigators and are conducted under protocols detailing the objectives of the study, the parameters to
48
be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol must be submitted to the FDA as part of the investigational new drug exemption process and must be reviewed and approved by an independent Institutional Review Board before it can begin. Clinical trials typically are conducted in three sequential phases, but the phases may overlap or be combined. Phase I usually involves the initial introduction of the investigational drug into people to evaluate its safety, dosage tolerance, pharmacodymanics and, if possible, to gain an early indication of its effectiveness. Phase II usually involves trials in a limited patient population to evaluate dosage tolerance and appropriate dosage, identify possible adverse effects and safety risks and evaluate preliminarily the efficacy of the drug for specific indications. Phase III trials usually further evaluate clinical efficacy and test further for safety by using the drug in its final form in an expanded patient population. We cannot guarantee that testing for our development product candidates will be completed successfully within any specified period of time, if at all. Many products that initially appear promising are found, after clinical evaluation, not to be safe or effective. Also, we, or the FDA, may suspend clinical trials at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk.
Assuming successful completion of the required clinical testing, the results of the preclinical studies and of the clinical studies, together with other detailed information, including information on the manufacture and composition of the drug, are submitted to the FDA in the form of a new drug application requesting approval to market the product for one or more indications. Before approving an application, the FDA usually will inspect the facility or the facilities at which the drug is manufactured and will not approve the product unless compliance with cGMP is satisfactory. The FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
The FDA granted Diastat orphan drug exclusivity when the drug was approved in 1997. Orphan exclusivity is available for drugs that are intended to treat a condition that affects fewer than 200,000 individuals in the United States, that are the subject of an orphan drug designation and that receive the first FDA approval for the indication for which the drug has orphan designation. When orphan exclusivity is granted, the FDA may not approve any other application to market the same drug for the same indication, except in specific limited circumstances, for a period of seven years. Orphan drug exclusivity does not prevent a competitor from obtaining approval for a different drug for the same indication, or for the same drug for a different indication. Diastat’s orphan drug exclusivity will expire July 29, 2004.
Diastat is also regulated as a controlled substance under the Controlled Substances Act and related regulations, which are administered by the DEA. These regulations require registration of facilities that manufacture, distribute, dispense, import or export controlled substances; establish labeling and packaging requirements; establish production and manufacturing quotas; require extensive recordkeeping and reporting; and impose other requirements to ensure appropriate security, control and accounting mechanisms to prevent drug loss and diversion. The DEA conducts periodic inspections of establishments that handle controlled substances. Failure of our manufacturers or distributors to comply with DEA requirements could result, among other consequences, in DEA failure to renew registrations, suspension or revocation of existing registrations, civil penalties, forfeitures or criminal prosecution.
Intellectual Property
We rely on patent, trademark, copyright, trade secret and other laws to protect the proprietary aspects of our business. Diastat is protected by a patent in the United States that expires in September 2013. We also have a patent application for Diastat pending in Canada. Migranal is protected by a patent in the United States that expires in December 2009. MT 300 is protected by a U.S. patent until March 2020 and is the subject of an additional U.S. patent application. Mysoline and D.H.E. 45 are not protected by a patent. Our competitors may develop products similar to ours using methods and technologies that are beyond the scope of our intellectual property protection, which could reduce our sales. If we are unsuccessful in defending our intellectual property rights, third parties may be able to copy our products, which could impair our ability to meet our sales projections.
49
We also own registered U.S. trademarks on Diastat, Mysoline, Migranal and D.H.E. 45 and a registered trademark on Diastat in Canada. We also have a trademark application pending for DiaSTAT in Canada. We will continue to assess appropriate occasions for seeking patent and other intellectual property protections for our intellectual property.
In addition, we have trade secrets and other proprietary information in connection with the manufacture of our products. We routinely require our employees, customers and potential business partners to enter into confidentiality and nondisclosure agreements before we will disclose any sensitive aspects of our products or business plans. In addition, we require employees to agree to surrender to us any proprietary information, inventions or other intellectual property relating to our business that they generate or come to possess while employed by us. Despite our efforts to protect our proprietary rights through confidentiality and license agreements, unauthorized parties may attempt to copy or otherwise obtain and use our products and intellectual property. These precautions may not prevent misappropriation or infringement of our intellectual property.
Third parties may infringe or misappropriate our intellectual property. In addition, other parties may assert infringement claims against us. Although we have not received notice of any alleged infringement, our products may infringe issued patents that may relate to our products. In addition, because patent applications in the United States filed before November 29, 2000 are not published until the patent is issued and, typically, patent applications issued after November 29, 2000 are not published until 18 months after they are filed, applications may have been filed that relate to our products. We may be subject to legal proceedings and claims from time to time in the ordinary course of our business, including claims of alleged infringement of the patents, trademarks and other intellectual property rights of third parties. Intellectual property litigation is expensive and time-consuming and could divert our management’s attention from running our business. This litigation could also require us to enter into license or royalty agreements. These agreements, if required, may not be available on commercially reasonable terms, or at all. Our failure or inability to license new product rights on a timely basis could harm our business.
Employees
As of June 30, 2003, we had 125 full-time employees, comprising 104 in sales and marketing, 19 in administrative and support services and two in product development and enhancement activities. We utilize third-parties to manufacture and distribute our products and to support our development of product candidates and product enhancements. None of our employees are covered by a collective bargaining agreement. We believe our relations with our employees are good.
Legal Proceedings
We are not a party to any legal proceedings. However, we may be subject to various claims and legal actions arising in the ordinary course of business.
Facilities
Our corporate headquarters are located in San Diego, California, where we occupy approximately 11,000 square feet under a lease expiring in June 2006 at a monthly cost of $26,000, subject to an annual increase of approximately 4%.
50
MANAGEMENT
Executive Officers, Officers and Directors
Our executive officers, officers and directors and their ages as of September 30, 2003 were as follows:
Name
| | Age
| | Position(s)
|
Michael T. Borer | | 44 | | President, Chief Executive Officer and Director |
John R. Cook | | 40 | | Senior Vice President, Corporate Operations and Secretary |
James L. Fares | | 41 | | Senior Vice President, Commercial Operations |
Wendy L. Martin, M.D. | | 49 | | Vice President, Clinical and Product Development |
George M. Stuart | | 40 | | Vice President, Finance, Chief Financial Officer and Treasurer |
David A. Gonyer, R.Ph. | | 39 | | Vice President, Marketing |
Craig C. Chambliss | | 38 | | Vice President, Sales |
James C. Blair, Ph.D. (1) | | 64 | | Director |
Carlos A. Ferrer | | 49 | | Director |
Cam L. Garner (1) | | 55 | | Chairman of the Board |
David F. Hale (2) | | 54 | | Director |
William R. Ringo, Jr. (2) | | 57 | | Director |
Corey A. Seale (2) | | 45 | | Director |
Sigrid Van Bladel, Ph.D. (1) | | 38 | | Director |
| (1) | | Member of Compensation Committee |
| (2) | | Member of Audit Committee |
Michael T. Borer, one of our founders, has served as our Chief Executive Officer and President since our inception. Prior to founding us, Mr. Borer served as Senior Vice President and Chief Financial Officer of Dura Pharmaceuticals, Inc. from 1998 to 2001. Prior to serving as Dura’s Senior Vice President and Chief Financial Officer, Mr. Borer provided services to Dura in several capacities, including as Director of Finance from 1994 to 1996 and General Manager of Dura’s Health Script division from 1996 to 1998. From 1981 to 1993, he held various positions, the last of which was senior manager, with Deloitte & Touche LLP.
John R. Cook, one of our founders, has served as our Senior Vice President, Corporate Operations, since our inception. Prior to founding us, Mr. Cook served as Dura’s Vice President, Associate General Counsel from 1999 to 2001. Prior to joining Dura, Mr. Cook spent 10 years with the law firm Brobeck, Phleger & Harrison LLP, most recently as a partner, where he represented many life science companies.
James L. Fares, one of our founders, has served as our Senior Vice President, Commercial Operations, since February 2001. Prior to founding us, Mr. Fares served as Vice President and General Manager of the neurology division of Elan Pharmaceuticals, Inc. from 1998 to 2001. In 1991, Mr. Fares joined Athena Neurosciences, Inc., where he held various sales and marketing management positions. Athena was acquired by Elan in 1996. Before joining Athena, Mr. Fares spent five years with Merck & Co., Inc. in various sales and marketing management positions.
Wendy L. Martin, M.D., has served as our Vice President, Clinical and Product Development since December 2002. Dr. Martin has over ten years of experience in clinical research and pharmaceutical product development in neurology and pain. Prior to joining us, Dr. Martin worked at Elan from 1999 to 2002, most recently serving as Senior Director of Clinical Development, Pain and Epilepsy. Prior to joining Elan, Dr. Martin provided services in several capacities to Glaxo Wellcome (Glaxo SmthKline) from 1992 to 1999, including serving as Senior Clinical Research Physician.
51
George M. Stuart, one of our founders, has served as our Vice President, Finance, since April 2001 and our Chief Financial Officer since December 2001. Prior to founding us, Mr. Stuart served as Director of Corporate Accounting with Ligand Pharmaceuticals Incorporated, from 1999 to 2001. From 1986 to 1999, Mr. Stuart held various positions with Deloitte & Touche LLP, the last of which was senior manager, where he specialized in emerging growth companies and U.S. Securities and Exchange Commission registrants. Mr. Stuart has been a Certified Public Accountant in California since 1988.
David A. Gonyer, R.Ph., one of our founders, has served as our Vice President, Marketing since August 2003. Prior to his promotion to this position, he served as Director, Marketing since our inception. Prior to founding us, Mr. Gonyer served as Director, Marketing at Dura Pharmaceuticals Inc. from 1997 to 2001. Prior to joining Dura, Mr. Gonyer served in various marketing and sales roles at Eli Lilly & Co. from 1987 to 1996.
Craig C. Chambliss has served as our Vice President, Sales since August 2003. Prior to his promotion to this position, he served as National Sales Director since August 2001. Prior to joining us, Mr. Chambliss served as Director, Business Development and Strategic Marketing at Elan Pharmaceuticals, Inc. from 2000 to 2001. From 1998 to 2000, Mr. Chambliss served as Director, Marketing at Ther-Rx Corporation. Prior to joining Ther-Rx, he served in various sales and marketing management roles at ALZA Pharmaceuticals from 1994 to 1998.
James C. Blair, Ph.D., has served as one of our directors since March 2001. Since 1985, Dr. Blair has served as a managing member of Domain Associates, LLC, a venture capital management company. Dr. Blair is currently a director of Vista Medical Technologies, Inc., as well as 11 private companies in the healthcare industry, including four medical device companies, two molecular diagnostic companies, two other specialty pharmaceutical companies and three biopharmaceutical companies.
Carlos A. Ferrer, has served as one of our directors since March 2003. Mr. Ferrer co-founded Ferrer Freeman & Company, LLC, or FFC, in 1995 to provide private equity capital exclusively for companies in the health care industry. Prior to founding FFC, Mr. Ferrer was a managing director at Credit Suisse First Boston. From 1982 through 1995, he founded and served as head of its Health Care Investment Banking Group. He is vice chairman of the board of the Cancer Research Institute, and a member of the board of directors of AmeriGroup Corporation, Concentra Managed Care, intelliClaim, Inc., OrthoRx, Inc. and Ardent Health Services, LLC.
Cam L. Garner, one of our founders, has served as the Chairman of our Board of Directors since our inception. Prior to founding us, Mr. Garner was the Chairman of Dura’s Board of Directors, a position he held from 1995 to 2001. Mr. Garner was also Dura’s Chief Executive Officer from 1989 to 2001 and Dura’s President from 1990 to 1998. Mr. Garner serves as Chairman for Favrille, Inc. and a director of CancerVax Corporation, SkinMedica, Inc. and Pharmion Corporation.
David F. Hale, has served as one of our directors since November 2001. Mr. Hale has served as President and Chief Executive Officer of CancerVax Corporation since October 2000 and as a director of CancerVax Corporation since December 2000. Prior to joining CancerVax, Mr. Hale served as President and Chief Executive Officer and as a director of Women First HealthCare, Inc. from 1998 until 2000, and as Chairman, President and Chief Executive Officer of Gensia, Inc. from 1987 to 1997, and Chairman of Viagene from 1987 to 1994. Mr. Hale is currently Chairman of the Board of SkinMedica, Inc. and LMA North America, Inc. and serves on the boards of directors of other private companies and not-for-profit organizations.
William R. Ringo, Jr., has served as one of our directors since July 2001. Mr. Ringo served as Executive Chairman and interim Chief Executive Officer of InterMune Pharmaceuticals, Inc. from June 2003 until September 2003 and currently is the Chairman of InterMune. Mr. Ringo is a founding member of Barnard Life Science Health Care Consultants. In 2001, Mr. Ringo retired from Eli Lilly and Company after serving 28 years in various positions, the most recent of which were President of Oncology and Critical Care Products, a position
52
he held since 1999, and President of Internal Medicine Products from 1998 to 1999. Mr. Ringo also serves as a member of the board of directors of Praecis Pharmaceuticals Incorporated, La Jolla Pharmaceutical Company, Inspire Pharmaceuticals, Inc. and Encysive Pharmaceuticals, as well as several private companies in the healthcare industry.
Corey A. Seale, has served as one of our directors since February 2003. Mr. Seale is Administrator of Moreno Valley Hospital, a part of Valley Health System. Mr. Seale served as Chief Executive Officer of Fallbrook Hospital, a for-profit hospital owned by Community Health Systems, Inc. from 1995 to 2003. Mr. Seale joined Fallbrook Hospital in 1990 as Chief Financial Officer and was named Chief Executive Officer in 1995. Mr. Seale has over 20 years of financial and audit experience in both private and publicly-traded companies. Mr. Seale also serves as a member of the board of directors of Community Bancorp.
Sigrid Van Bladel, Ph.D., has served as one of our directors since March 2001. In 1994, Dr. Van Bladel joined New Enterprise Associates, a venture capital firm, where she is currently a partner focusing on biopharmaceuticals and medical device companies. Dr. Van Bladel serves on the boards of several private companies in the healthcare industry. Prior to joining New Enterprise Associates, Dr. Van Bladel worked as a management consultant at McKinsey and Company, Inc. and as a research associate with the National Science Foundation of Belgium.
Board of Directors and Committees
Following this offering, our board of directors will consist of eight directors divided into three classes, with each class serving for a term of three years. At each annual meeting of stockholders, directors will be elected for a three-year term to succeed the directors whose terms are expiring. Messrs. Hale and Ringo will be class I directors whose terms will expire in 2004; Drs. Blair and Van Bladel and Mr. Ferrer will be class II directors whose terms will expire in 2005 and Messrs. Borer, Garner and Seale will be class III directors whose terms will expire in 2006.
Our board has a compensation committee and an audit committee, and we will establish a nominating and corporate governance committee, each of which are or will be governed by a written charter. The compensation committee is responsible for making recommendations to the board regarding salaries, incentives and other forms of compensation for directors, officers and employees. The compensation committee is empowered to engage the necessary internal and external resources to fulfill its duties. Drs. Blair and Van Bladel and Mr. Garner are the current members, and Mr. Garner is the chairman of our compensation committee.
The audit committee reviews our annual audit and meets with our independent auditors to review our internal controls and financial management practices. Each year, subject to stockholder approval in years following 2003, the audit committee recommends to the board the selection of our independent auditors. The audit committee is empowered to engage the necessary internal and external resources to fulfill its duties. Messrs. Seale, Hale and Ringo are the current members of our audit committee, and Mr. Seale is the chairman of our audit committee.
The nominating and corporate governance committee will oversee corporate governance matters and make recommendations to the board regarding board organization and procedures, evaluate the board’s performance and effectiveness and nominate candidates for election to the board. The nominating and corporate governance committee will be empowered to engage the necessary internal and external resources to fulfill its duties. The nominating and corporate governance committee will consider those persons recommended by stockholders for nomination to the board if the names of such persons are submitted in writing in accordance with the rules and regulations of the U.S. Securities and Exchange Commission and our bylaws.
The board may establish, from time to time, other committees to facilitate the management of our business.
53
As of , 2003, the board has determined that a majority of the members of the board are “independent” within the meaning of the rules and regulations of the U.S. Securities and Exchange Commission and the rules of Nasdaq presently in effect. In addition, the audit committee has determined that Mr. Seale qualifies as an audit committee finance expert within the meaning of the rules and regulations of the U.S. Securities and Exchange Commission and the rules of Nasdaq presently in effect.
As of September , 2003, our board approved a code of business conduct to govern the affairs of our chief executive officer and our chief financial officer. We have established a system for reporting violations of our code of business conduct.
Director Compensation
Except for the grant of stock options to our non-employee directors, we do not compensate our directors for their services as directors. Our policy is to make an initial grant of options to purchase 30,000 shares to each non-employee director on the date of the director’s election to the board and to make an additional grant to purchase 7,500 shares on each anniversary of their initial election to the board. All of the initial grants to our directors vest over a four-year period, with 25% of the options vesting on the first anniversary of the date of grant and the remaining options vesting in equal monthly installments during the next 36 months. Options granted to directors on each anniversary of their initial election vest in 12 monthly installments commencing on the date of grant. We also reimburse each of our directors for out-of-pocket expenses incurred in connection with attending board meetings.
We have issued options to each of Drs. Blair and Van Bladel and Mr. Garner to purchase 30,000, 7,500 and 7,500 shares of our common stock at a purchase price of $0.50, $10.70 and $5.00 per share, respectively. We have issued options to Mr. Ringo to purchase 30,000, 7,500 and 7,500 shares of our common stock at a purchase price of $0.50, $8.00 and $5.00 per share, respectively. We have issued options to Mr. Hale to purchase 30,000 and 7,500 shares of our common stock at a purchase price of $1.00 and $5.00 per share, respectively. We have issued options to each of Messrs. Seale and Ferrer to purchase 30,000 shares of our common stock at a purchase price of $5.00 per share.
From July through December 2001, Mr. Garner, Domain Associates, L.L.C., of which Dr. Blair is a principal and controlling person, and Mr. Hale exercised options to purchase an aggregate of 90,000 shares of our common stock at their respective purchase prices of $0.50 per share for Mr. Garner and Domain Associates, L.L.C. and $1.00 per share for Mr. Hale. In June 2003, Domain Associates, L.L.C. exercised 7,500 shares of our common stock at the exercise price of $5.00 per share. These exercises were made under an “early exercise” right permitting the optionee to exercise the option prior to the time that the options vest for tax reasons. However, prior to the time that the options fully vest, we can repurchase these shares if, in the cases of Messrs. Garner and Hale’s shares, they cease to provide services to us and, in the case of Domain Associates, L.L.C.’s shares, Dr. Blair ceases to provide services to us.
Compensation Committee Interlocks and Insider Participation
The members of our compensation committee are Drs. Blair and Van Bladel and Mr. Garner. No interlocking relationship exists, or has existed in the past, between our board or compensation committee and the board or compensation committee of any other company.
Executive Officers
Our chief executive officer serves at the discretion of our board and holds office until his or her successor is appointed or until his or her earlier resignation or removal. Our remaining executive officers and officers report to our chief executive officer. There are no family relationships among any of our directors, executive officers or officers.
54
Executive Compensation
The following table provides summary information concerning compensation earned by or paid to our chief executive officer and each of our other executive officers whose total annual salary and bonus exceeded $100,000 for services rendered in all capacities to us during the period ended December 31, 2001 and the year ended December 31, 2002. These individuals are referred to as our named executive officers.
Summary Compensation Table
| | | | | Annual Compensation (1)
| | | Long-Term
Compensation
Awards
|
Name and Principal Position(s)
| | Year
| | Salary($)
| | Bonus($)
| | Other Annual
Compensation($)
| | | Securities
Underlying
Options(#)
|
Michael T. Borer | | 2002 | | $ | 256,058 | | $ | 100,000 | | | — | | | — |
President and Chief Executive Officer | | 2001 | | | 182,692 | | | — | | | — | | | — |
| | | | | |
John R. Cook | | 2002 | | | 204,846 | | | 60,000 | | | — | | | 40,000 |
Senior Vice President, Corporate Operations and Secretary | | 2001 | | | 146,154 | | | — | | | — | | | — |
| | | | | |
James L. Fares | | 2002 | | | 230,452 | | | 70,000 | | | — | | | 20,000 |
Senior Vice President, Commercial Operations | | 2001 | | | 164,423 | | | — | | $ | 142,847 | (2) | | 50,000 |
| | | | | |
Wendy L. Martin, M.D. (3) | | 2002 | | | 9,554 | | | — | | | — | | | 100,000 |
Vice President, Clinical and Product Development | | 2001 | | | — | | | — | | | — | | | — |
| | | | | |
George M. Stuart | | 2002 | | | 176,000 | | | 50,000 | | | — | | | 15,000 |
Vice President, Finance, Chief Financial Officer and Treasurer | | 2001 | | | 110,769 | | | — | | | — | | | 25,000 |
| (1) | | No salaries were paid for the period from inception through March 2001. Annual 2001 salaries for the named individuals were: Mr. Borer, $250,000; Mr. Cook, $200,000; Mr. Fares, $225,000; and Mr. Stuart, $160,000. |
| (2) | | Represents relocation expenses paid by us on behalf of Mr. Fares. |
| (3) | | Dr. Martin began her employment with us in December 2002. Her annualized salary for 2003 is $207,000. |
Stock Options
The following tables summarize option grants and exercises during the year ended December 31, 2002 to or by our named executive officers, and the value of the options held by such persons as of December 31, 2002, including the potential realizable value over the ten-year term of the options, based on assumed rates of stock appreciation of 5% and 10%, compounded annually. These assumed rates of appreciation comply with the rules of the U.S. Securities and Exchange Commission and do not represent our estimate of future stock price. Actual gains, if any, on stock option exercises will be dependent on the future performance of our common stock. The assumed 5% and 10% rates of stock appreciation are based on an assumed initial public offering price of $ per share, which is the midpoint of our expected offering range. We have not granted any stock appreciation rights.
From January 1, 2003 through June 30, 2003, we granted options to purchase up to an aggregate of 340,466 shares, net of cancellations, under our 2001 Stock Plan. All options were granted at exercise prices at or above the fair market value of our common stock on the date of grant, as determined in good faith by our board of directors. Option shares generally vest over four years.
55
2002 Option Grants
Name
| | Number of
Securities
Underlying
Options
Granted(#)
| | Percentage of Total
Options Granted to
Employees in Fiscal
Year(%)
| | | Exercise or
Base Price($/sh)
| | Expiration
Date
| | Potential Realizable
Value at Assumed
Annual Rates of Stock
Price Appreciation for Option Term($)
|
| | | | | | 5%
| | 10%
|
Michael T. Borer | | — | | — | % | | $ | — | | — | | — | | — |
John R. Cook | | 40,000 | | 8.8 | | | | 8.00 | | 2012 | | | | |
James L. Fares | | 20,000 | | 4.4 | | | | 8.00 | | 2012 | | | | |
Wendy L. Martin | | 100,000 | | 22.0 | | | | 5.00 | | 2012 | | | | |
George M. Stuart | | 15,000 | | 3.3 | | | | 8.00 | | 2012 | | | | |
Aggregate Option Exercises in 2002 and Option Values at December 31, 2002
The following table describes for the named executive officers their option exercises for the year ended December 31, 2002, and exercisable and unexercisable options held by them as of December 31, 2002. The value realized and the value of unexercised in-the-money options at December 31, 2002 are based on an assumed initial public offering price of $ per share, which is the midpoint of our expected offering range, less the per share exercise price, multiplied by the number of shares issued or issuable, as the case may be, upon exercise of the option. All options were granted under our 2001 Stock Plan.
Name
| | Shares
Acquired
on Exercise(#)
| | Value
Realized($)
| | Number of Securities
Underlying Unexercised
Options at December 31,
2002(#)
| | Value of Unexercised In-the-Money Options at December 31, 2002($)
|
| | | | Exercisable
| | Unexercisable
| | Exercisable
| | Unexercisable
|
Michael T. Borer | | — | | — | | — | | — | | $ | — | | — |
John R. Cook | | — | | — | | 40,000 | | — | | | | | — |
James L. Fares | | — | | — | | 70,000 | | — | | | | | — |
Wendy L. Martin | | — | | — | | 100,000 | | — | | | | | — |
George M. Stuart | | — | | — | | 40,000 | | — | | | | | — |
Stock Plans
We adopted our 2001 Stock Plan in March 2001. Our 2003 Stock Incentive Plan will replace our 2001 Stock Plan immediately upon the closing of this offering, at which time we will transfer all remaining shares reserved for grant under the 2001 Stock Plan to the 2003 Stock Incentive Plan. Thereafter, we will cease making grants under the 2001 Stock Plan. The aggregate number of shares available for grant under the 2003 Stock Incentive Plan, including all shares transferred from our 2001 Stock Plan, initially will be 2,000,000. Under the terms of the 2003 Stock Plan, the number of shares subject to the plan will increase each year in an amount equal to the lesser of 1% of our then outstanding capital stock or an amount determined by our board. The plans are designed to assist us in recruiting, retaining and motivating our employees, non-employee directors and independent contractors. Under these plans, we can grant:
| | • | | restricted shares, which are shares of our common stock granted to an individual that vest over a period of time and may also be restricted as to sale, assignment or other transfer; |
| | • | | stock units, which are unfunded contractual rights to receive an amount based on the value of our common shares; |
| | • | | incentive stock options, which are options to purchase shares of our common stock that are designed to receive favorable tax treatment under the Internal Revenue Code of 1986, or the Code; |
56
| | • | | non-qualified stock options, which are options to purchase shares of our common stock that are not designed to receive favorable tax treatment under the Code; and |
| | • | | stock appreciation rights, which are rights to receive the appreciation, if any, in the value of a share of our common stock between the date of grant and the date of exercise. |
Our compensation committee administers the plan and has the authority to determine to whom options will be granted and shares will be sold, the number of shares and the exercise and vesting terms.
Options granted under the plan are subject to the following terms:
| | • | | options are exercisable within the times or upon the events as determined by the compensation committee and described in each grant; each option must become exercisable within ten years from the date of grant, and no incentive stock option granted to a 10% or greater stockholder shall be exercisable after the expiration of five years from the date the option was granted; |
| | • | | the exercise price of any non-qualifying option must be at least 85%, or for non-employee directors at least 100%, of the fair market value of the shares on the date the option is granted and the exercise price of an incentive stock option must be at least 100%, or for 10% stockholders, at least 110% of the fair market value on the date the option is granted; |
| | • | | unless the stock option grant states differently, options granted under the plan terminate and may not be exercised if the optionee ceases to render service to us or our affiliates; and |
| | • | | upon the occurrence of a change of control event, the surviving entity must either assume or substitute for the options granted under the plan or pay cash or cash equivalents in settlement of the full value of outstanding options or, in the alternative, the options become fully vested and exercisable. |
As of June 30, 2003:
| | • | | options to purchase 1,039,192 shares were issued and outstanding; and |
| | • | | options to purchase 258,980 shares had been exercised. |
Employment Agreements and Change in Control Arrangements
We do not have any employment agreements with our executive officers. Messrs. Borer, Cook, Fares and Stuart have purchased common stock from us pursuant to agreements that, except as described below, permit us to repurchase any unvested shares at the initial purchase price of $0.0025 per share if the individual’s service with us is terminated. These individuals have acquired a vested interest in 60% of their common stock through June 30, 2003, and the remaining shares of common stock will vest in successive equal monthly installments over the following 19 months so long as the individual continues to be a service provider to us. An individual will continue to be a service provider if he continues to provide periodic services to us as an employee, director or consultant. Our repurchase right also will terminate immediately with respect to all unvested shares of an individual if:
| | • | | we are acquired by or merge into another company; |
| | • | | the individual dies or suffers a permanent or total disability; |
| | • | | we terminate the individual’s employment for any reason other than his conviction of a felony, act of fraud or embezzlement or willful failure to perform his duties; or |
| | • | | the individual terminates his service because his responsibilities, base salary or total cash compensation are reduced, we relocate our corporate offices or we breach our agreement with the individual. |
57
Limitation of Liability and Indemnification Matters
Our certificate of incorporation limits the liability of our directors to the maximum extent permitted by Delaware law. Delaware law provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except liability for:
| | • | | any breach of their duty of loyalty to the corporation or its stockholders; |
| | • | | acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law; |
| | • | | unlawful payments of dividends or unlawful stock repurchases or redemptions; or |
| | • | | any transaction from which the director derived an improper personal benefit. |
This limitation of liability does not apply to liabilities arising under the federal securities laws and does not affect the availability of equitable remedies such as injunctive relief or rescission.
Our certificate of incorporation and bylaws provide that we will indemnify our directors and executive officers and may indemnify our other officers and employees and other agents to the fullest extent permitted by law. Our certificate of incorporation and bylaws also permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in such capacity, regardless of whether the bylaws would permit indemnification.
We have entered into agreements to indemnify each of our directors and executive officers, in addition to the indemnification provided for in our certificate of incorporation and bylaws. In addition, we maintain directors’ and officers’ liability insurance. We believe that these provisions and agreements are necessary to attract and retain qualified persons as directors and executive officers.
58
RELATED-PARTY TRANSACTIONS
Since our inception, there has not been any transaction or series of transactions to which we were or are a party in which the amount involved exceeded or exceeds $60,000 and in which any director, executive officer, holder of more than 5% of any class of our voting securities or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest, other than the following:
In January 2001, we sold and issued 1,800,000 shares of our common stock to Mr. Borer, 700,000 shares of our common stock to Mr. Garner and 1,100,000 shares of our common stock to Mr. Cook. In February 2001, we sold and issued 650,000 shares of our common stock to Mr. Fares. In March 2001, we sold and issued 150,000 shares of our common stock to Mr. Stuart. All of these sales occurred at a price of $0.0025 per share.
During 2001, we paid $142,847 in relocation related expenses on behalf of Mr. Fares.
In March 2001, we sold 14,000,000 shares of our Series A convertible preferred stock for net proceeds of $69.6 million. In June 2002, we sold 3,750,000 shares of our Series B convertible preferred stock for net proceeds of $22.4 million. In March 2003, we sold 4,000,000 shares of our Series C convertible preferred stock for net proceeds of $25.9 million. Upon completion of this offering, each share of our convertible preferred stock will convert into one share of our common stock. Several of our officers and directors purchased shares of our convertible preferred stock in these offerings. These affiliates purchased the shares at the same price and on the same terms and conditions as the unaffiliated investors in the private financings. The following table summarizes purchases, valued in excess of $60,000, of shares of our convertible preferred stock by our directors, executive officers and 5% stockholders:
Purchaser
| | Number of
Series A
Shares
| | Number of
Series B
Shares
| | Number of
Series C
Shares
|
Domain Partners V, L.P. and its affiliate (1)(2) | | 5,118,000 | | 1,166,667 | | |
New Enterprise Associates 10, Limited Partnership and its affiliate (3) | | 5,000,000 | | 2,500,000 | | |
FFC Partners II, L.P. and its affiliate (4) | | | | | | 3,692,308 |
Michael T. Borer | | 100,000 | | | | 73,230 |
John R. Cook | | 19,000 | | | | |
Cam L. Garner | | 60,000 | | | | |
David F. Hale | | 20,000 | | | | 40,000 |
George M. Stuart | | | | | | 10,000 |
| (1) | | Includes 5,000,000 Series A shares beneficially owned by Domain Partners V, L.P. and 118,000 Series A shares beneficially owned by DP V Associates, L.P., an affiliate of Domain Partners V. |
| (2) | | Includes 1,139,743 Series B shares beneficially owned by Domain Partners V, L.P. and 26,924 Series B shares beneficially owned by DP V Associates, L.P., an affiliate of Domain Partners V. |
| (3) | | Includes 4,997,400 Series A shares beneficially owned by New Enterprise Associates 10, Limited Partnership and 2,600 Series A shares beneficially owned by NEA Ventures 2001, L.P., an affiliate of New Enterprise Associates 10. |
| (4) | | Includes 3,639,896 Series C shares beneficially owned by FFC Partners II, L.P. and 52,412 Series C shares beneficially owned by FFC Executive Partners II, L.P., an affiliate of FFC Partners II. |
Stock option grants to our directors are described under “Management—Director Compensation.”
We have entered into indemnification agreements with all of our officers and directors. Some of our stockholders are entitled to have their shares registered by us for resale.
On March 31, 2003, with a payment of $89.5 million, we retired $99.0 million in product acquisition notes payable to Elan that we incurred in connection with our acquisition of Diastat and Mysoline, repaid the
59
$10.0 million outstanding on our credit facility with Elan, terminated our royalty-based financing arrangement with Elan and repurchased 3,000,000 shares of our Series A convertible preferred stock owned by Elan. Concurrently, we issued $62.0 million of senior debt to Regiment and raised net proceeds of $25.9 million through the issuance of 4,000,000 shares of Series C convertible preferred stock to investors other than Elan. We recognized a pre-tax gain of $37.4 million on the retirement of the product acquisition notes payable and $246,000 on the termination of the royalty-based financing arrangement. For more information on our former relationship with Elan, see “Business—Material Agreements” beginning on page 43.
60
PRINCIPAL STOCKHOLDERS
The following table sets forth information regarding beneficial ownership of our common stock as of June 30, 2003 by:
| | • | | each person or entity, or group of affiliated persons, known to us to own beneficially more than 5% of our common stock; |
| | • | | each of our named executive officers; and |
| | • | | all of our executive officers and directors as a group. |
Beneficial ownership is determined in accordance with the rules of the U.S. Securities and Exchange Commission and includes voting and investment power with respect to our shares. Except as otherwise indicated and subject to applicable community property laws, each person has sole investment and voting power with respect to the shares shown. Ownership information is based upon information furnished by the individuals or entities. Percentage ownership before the offering is based on 23,528,980 shares of common stock outstanding as of June 30, 2003, assuming conversion of all of our convertible preferred stock, and after the offering, is based on shares of our common stock outstanding assuming our sale of shares in this offering. Unless otherwise indicated, the address for the following stockholders is Xcel Pharmaceuticals, Inc., 6363 Greenwich Drive, Suite 100, San Diego, CA 92122.
In computing the number of shares beneficially owned by a person and the percentage ownership of that person, shares of common stock subject to options held by that person that are currently exercisable or exercisable within 60 days of June 30, 2003 are deemed outstanding. These shares, however, are not deemed outstanding for the purposes of computing the percentage ownership of any other person.
| | | Shares Beneficially Owned Prior to Offering
| | Shares Beneficially Owned After Offering
|
Name of Beneficial Owner
| | Number
| | Percentage
| | Number
| | Percentage
|
Domain Partners V, L.P. and affiliates (1) One Palmer Square, Suite 515 Princeton, NJ 08542 | | 6,329,667 | | 26.89 | | 6,329,667 | | |
New Enterprise Associates and affiliates (2) 1119 St. Paul Street Baltimore, MD 21202 | | 7,545,000 | | 32.01 | | 7,545,000 | | |
FFC Partners II, L.P. and affiliates (3) 10 Glenville Street Greenwich, CT 06831 | | 3,722,308 | | 15.80 | | 3,722,308 | | |
Michael T. Borer | | 1,973,230 | | 8.39 | | 1,973,230 | | |
John R. Cook (4) | | 1,169,000 | | 4.96 | | 1,169,000 | | |
James L. Fares (5) | | 759,000 | | 3.21 | | 759,000 | | |
George M. Stuart (4) | | 214,000 | | * | | 214,000 | | * |
Wendy L. Martin (5) | | 100,000 | | * | | 100,000 | | * |
James C. Blair, Ph.D. (1) | | 6,329,667 | | 26.89 | | 6,329,667 | | |
Carlos A. Ferrer (3) | | 3,722,308 | | 15.80 | | 3,722,308 | | |
Cam L. Garner (6) | | 730,000 | | 3.10 | | 730,000 | | |
David F. Hale (7) | | 97,500 | | * | | 97,500 | | * |
William R. Ringo, Jr. (8) | | 37,500 | | * | | 37,500 | | * |
Corey A. Seale (9) | | 35,000 | | * | | 35,000 | | * |
Sigrid Van Bladel, Ph.D. (2) | | 7,545,000 | | 32.01 | | 7,545,000 | | |
All Directors and Executive Officers as a Group (12 Persons)(10) | | 22,712,205 | | 94.60 | | 22,712,205 | | |
61
| (1) | | Includes 6,139,743 shares owned by Domain Partners V, L.P. and 144,924 shares owned by DP V Associates, L.P. James C. Blair, Ph.D, one of our directors, is a general partner of One Palmer Square Associates V, L.L.C., the general partner of Domain Partners V, L.P. and DP V Associates, L.P. Dr. Blair shares voting and investment power with respect to these shares and disclaims beneficial ownership of these shares except to the extent of his proportionate interest therein. Also includes 37,500 shares owned by Domain Associates, L.L.C. Domain Associates, L.L.C. is the manager of Domain Partners V, L.P. and DP V Associates, L.P. and has the same managing members as One Palmer Square Associates V, L.P. Also includes 7,500 shares subject to an option that is exercisable at any time issued to Domain Associates, L.L.C. Dr. Blair is a managing member of Domain Associates, L.L.C., shares voting and investment power with respect to these shares, and disclaims beneficial ownership of these shares except to the extent of his proportionate interest therein. Mr. Blair’s business address is the address listed above for Domain Partners V, L.P. |
| (2) | Includes 7,497,400 shares held by New Enterprise Associates 10, Limited Partnership, 2,600 shares held by NEA Ventures 2001, L.P. and 45,000 shares subject to options that are exercisable at any time issued to Sigrid Van Bladel, Ph.D., a member of our board of directors. Dr. Van Bladel is a partner of New Enterprise Associates but does not have voting or dispositive power with respect to the shares held by New Enterprise Associates 10, Limited Partnership or NEA Ventures 2001, L.P. Therefore, Dr. Van Bladel disclaims beneficial ownership of these shares, except to the extent of her proportionate interest therein. The New Enterprise Associates entities disclaim beneficial ownership of the 45,000 shares subject to the options held by Dr. Van Bladel. Dr. Van Bladel’s business address is the address listed above for New Enterprise Associates. |
| (3) | Includes 3,639,896 shares owned by FFC Partners II, L.P. and 52,412 shares owned by FFC Executive Partners, II, L.P. Carlos A. Ferrer, one of our directors, is a general partner of FFC Partners II, L.P., the general partner of FFC Executive Partners, II, L.P. Mr. Ferrer shares voting and investment power with respect to these shares and disclaims beneficial ownership of these shares except to the extent of his proportionate interest therein. Also includes 30,000 shares subject to an option that is exercisable at any time issued to FFC Partners II, L.P. Mr. Ferrer is a managing member of FFC Partners II, L.P., shares voting and investment power with respect to these shares, and disclaims beneficial ownership of these shares except to the extent of his proportionate interest therein. Mr. Ferrer’s business address is the address listed above for FFC Partners II, L.P. |
| (4) | Includes 50,000 shares subject to an option that is exercisable at any time. |
| (5) | Includes 100,000 shares subject to an option that is exercisable at any time. |
| (6) | Includes 15,000 shares subject to an option that is exercisable at any time. |
| (7) | Includes 7,500 shares subject to an option that is exercisable at any time. |
| (8) | Includes 37,500 shares subject to an option that is exercisable at any time. |
| (9) | Includes 30,000 shares subject to an option that is exercisable at any time. |
| (10) | Includes the shares and information contained in notes 1 through 9 above. |
62
DESCRIPTION OF CAPITAL STOCK
The following information describes our common stock and preferred stock and provisions of our certificate of incorporation and our bylaws as in effect upon the closing of this offering. This description is only a summary. You should also refer to the certificate of incorporation and bylaws which have been filed with the U.S. Securities and Exchange Commission as exhibits to our registration statement, of which this prospectus forms a part.
Upon completion of this offering, and after giving effect to the conversion of all outstanding convertible preferred stock into common stock, our authorized capital stock will consist of 100,000,000 shares of common stock, $0.0001 par value, and 5,000,000 shares of preferred stock, $0.0001 par value. As of June 30, 2003, there were 23,528,980 shares of our common stock outstanding held of record by 82 stockholders, assuming conversion of our outstanding convertible preferred stock which will occur upon the closing of this offering.
Common Stock
Subject to preferences that may be applicable to any preferred stock outstanding at the time, the holders of common stock are entitled to the following:
Dividends. Holders of common stock are entitled to receive dividends out of assets legally available for the payment of dividends at the times and in the amounts as the board of directors from time to time may determine, subject to any preferential dividend rights of any outstanding preferred stock.
Voting. Holders of common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders, including the election of directors. Cumulative voting for the election of directors is not authorized by our amended and restated certificate of incorporation, which means that the holders of a majority of the shares voted can elect all of the directors then standing for election.
Preemptive rights, conversion and redemption. The common stock is not entitled to preemptive rights and is not subject to conversion or redemption.
Liquidation, dissolution and winding-up. Upon our liquidation, dissolution or winding-up, the holders of common stock are entitled to share ratably in all assets remaining after payment of liabilities and the liquidation preferences of any preferred stock.
Each outstanding share of common stock is, and all shares of common stock to be outstanding upon completion of this offering will be, upon payment therefore, duly and validly issued, fully paid and non-assessable.
Options
As of June 30, 2003, options to purchase a total of 1,039,192 shares of common stock were outstanding, all of which are subject to lock-up provisions under the terms of the 2001 Stock Plan under which these options were granted. Options to purchase a total of 181,828 shares of common stock remain available for grant under the 2001 Stock Plan. Following this offering, options to purchase, or other equity-based awards with respect to, 2,000,000 shares of our common stock will be available under our 2003 Stock Incentive Plan, and we will cease issuing options under our 2001 Stock Plan.
Preferred Stock and Bylaw Provisions
Our board of directors is authorized, without action by the stockholders, to designate and issue up to 5,000,000 shares of preferred stock in one or more series. Our board of directors can also fix the rights, preferences and privileges of the shares of each series and any qualifications, limitations or restrictions on these shares.
63
Our board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of common stock. The issuance of preferred stock, while providing desirable flexibility in connection with possible acquisitions and other corporate purposes, may:
| | • | | delay, defer or prevent a change in control; |
| | • | | discourage bids for the common stock at a premium over the market price of our common stock; |
| | • | | affect adversely the voting and other rights of the holders of our common stock; and |
| | • | | discourage acquisition proposals or tender offers for our shares and, as a consequence, inhibit fluctuations in the market price of our shares that could result from actual or rumored takeover attempts. |
Upon the closing of this offering, there will be no shares of our preferred stock outstanding. We have no current plans to issue any shares of preferred stock.
Our bylaws provide that:
| | • | | all stockholder action must be taken at a stockholders’ meeting; |
| | • | | special meetings of stockholders may only be called by the chairman of the board, the chief executive officer or a majority of the board of directors; and |
| | • | | our board of directors is divided into three classes, with each class serving a staggered three-year term. |
The provisions described above, together with the ability of our board of directors to issue preferred stock and the terms of our senior debt owed to Regiment, may have the effect of deterring a hostile takeover or delaying a change in our control or management.
Registration Rights
Upon completion of this offering, the holders of 18,750,000 shares of common stock issuable upon conversion of the convertible preferred stock have the right to cause us to register these shares under the Securities Act of 1933 as follows:
| | • | | Demand Registration Rights. At any time after six months following this offering, the holders of at least 5,625,000 of these shares may request that we register all or part of their shares so long as the aggregate proceeds from the sale of the shares is expected to total at least $10.0 million. |
| | • | | Piggyback Registration Rights. The holders of these shares may request that we register their shares any time we file a registration statement for our own account or for the account of others. |
| | • | | S-3 Registration Rights. The holders of these shares have the right to request registrations on Form S-3 if we are eligible to use Form S-3, have not already effected two S-3 registrations within the preceding 12 months, and if the aggregate proceeds from the registration will be at least $2.5 million. |
Registration of shares of common stock pursuant to the exercise of demand registration rights, piggyback registration rights or S-3 registration rights under the Securities Act of 1933 would result in these shares becoming freely tradable without restriction under such Act immediately upon the effectiveness of such registration. Under the terms of our registration rights agreements with these stockholders, these stockholders will not be entitled to exercise any registration rights for at least 180 days after the date of this offering.
We will pay all registration expenses, other than underwriting discounts and commissions, in connection with any demand, piggyback or S-3 registration. The registration rights terminate five years following
64
completion of this offering, or, with respect to each holder of registrable securities, when the holder can sell all of the holder’s shares in any 90-day period under Rule 144 under the Securities Act of 1933.
Delaware Anti-Takeover Law
We are subject to Section 203 of the Delaware General Corporation Law which, subject to some exceptions, prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years following the date that such stockholder became an interested stockholder, unless:
| | • | | prior to such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; |
| | • | | upon consummation of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owns at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares outstanding those shares owned (a) by persons who are directors and also officers and (b) by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| | • | | on or subsequent to such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock that is not owned by the interested stockholder. |
In general, Section 203 defines an interested stockholder as any entity or person who, together with his or her affiliates and associates owns, or within three years did own, beneficially 15% or more of the outstanding voting stock of the corporation. The term business combination includes mergers, asset sales and other similar transactions resulting in a financial benefit to an interested stockholder.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock will be Mellon Investor Services.
Listing
We have applied to have our common stock included for quotation on The Nasdaq National Market under the symbol XCEL.
65
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering there has been no public market for our common stock, and we cannot predict the effect, if any, that market sales of shares or the availability of shares for sale will have on the market price prevailing from time to time. As described below, only a limited number of shares will be available for sale shortly after this offering due to contractual and legal restrictions on resale. Nevertheless, sales of substantial amounts of our common stock in the public market after the restrictions lapse, or the perception that such sales may occur, could cause the market price of our common stock to decline.
When this offering is completed, we will have a total of shares of common stock outstanding, assuming no exercise of outstanding options prior to completion of this offering. The shares offered by this prospectus will be freely tradable, unless they are purchased by our affiliates as defined in Rule 144 under the Securities Act of 1933. The remaining shares are restricted, which means they were originally sold in offerings that were not subject to a registration statement filed with the U.S. Securities and Exchange Commission. These restricted shares may be resold only through registration under the Securities Act of 1933 or under an available exemption from such registration, such as provided through Rule 144, Rule 144(k) or Rule 701.
Eligibility of Restricted Shares for Sale in the Public Market
All of our officers and directors and all of our stockholders are subject to lock-up provisions under which they have agreed not to transfer or dispose of, directly or indirectly, any shares of common stock, or any securities convertible into or exercisable or exchangeable for shares of common stock, for a period of 180 days after the date of this prospectus. Transfers or dispositions can be made sooner with the prior written consent of SG Cowen Securities Corporation.
Rule 144
In general, under Rule 144 a person, or persons whose shares are aggregated, who has beneficially owned restricted securities for at least one year, including the holding period of any prior owner of the shares who is not an affiliate, is entitled to sell within any three-month period a number of our shares of common stock that does not exceed the greater of:
| | • | | 1% of the then outstanding shares of our common stock, which will equal approximately shares upon completion of this offering; or |
| | • | | the average weekly trading volume of our common stock on The Nasdaq National Market during the four calendar weeks preceding the date on which a notice of sale is filed with the U.S. Securities and Exchange Commission. |
Sales under Rule 144 are subject to restrictions relating to manner of sale, notice and the availability of current public information about us. Under Rule 144 and subject to volume limitations and certain other restrictions, approximately million of the restricted shares will be eligible for sale beginning 180 days after the date of this prospectus, and the remaining restricted shares will become eligible for sale at various times thereafter. Additional restricted shares will become available for sale beginning 180 days after the date of this prospectus under Rule 701 as described below.
Rule 144(k)
A person who is not deemed an affiliate of ours at any time during the 90 days preceding a sale and who has beneficially owned shares for at least two years, including the holding period of any prior owner of the shares who is not an affiliate, will be entitled to sell shares following this offering under Rule 144(k) without regard to the volume limitations, manner of sale provisions, public information requirements or notice requirements of
66
Rule 144. However, affiliates must always sell pursuant to the volume limitations, manner of sale provisions, public information requirements and notice requirements under Rule 144, even after the applicable holding periods have been satisfied.
Rule 701
In general, under Rule 701 of the Securities Act of 1933 as currently in effect, any of our employees, consultants or advisors who purchases securities, including options, from us before the date of this prospectus through our 2001 Stock Plan or through some other written agreement is eligible to resell those shares, including shares issued upon the exercise of options, 90 days after the effective date of this offering in reliance on Rule 144, but without compliance with the holding period, public information and volume restrictions contained in Rule 144. As of June 30, 2003, 258,980 of our outstanding shares of common stock had been issued in reliance on Rule 701 as a result of the exercise of stock options. All of these shares are subject to contractual 180-day lock-up restrictions, and all of these shares will be eligible for sale upon expiration of such 180-day lock-up restrictions. In addition, as of June 30, 2003, options to purchase a total of 1,039,192 shares of common stock were outstanding.
Registration of Option Shares
Following this offering, we intend to file a registration statement under the Securities Act of 1933 covering shares of common stock issued upon the exercise of or subject to outstanding options issued or issuable under our 2001 Stock Plan and our 2003 Stock Incentive Plan. Based on the number of shares issued upon the exercise of or subject to outstanding options at June 30, 2003 under our 2001 Stock Plan and the 2,000,000 shares of common stock that will be available for issuance under our 2003 Stock Incentive Plan following the closing of this offering, this registration statement would cover approximately shares. This registration statement will automatically become effective upon filing. Accordingly, shares registered under this registration statement will, subject to vesting restrictions with us and Rule 144 volume limitations applicable to our affiliates, be available for sale in the open market immediately after the expiration of the contractual 180-day lock-up restrictions described above.
67
MATERIAL U.S. FEDERAL INCOME TAX CONSIDERATIONS FOR NON U.S. HOLDERS OF OUR COMMON STOCK
The following is a general discussion of the material U.S. federal income and estate tax consequences of the ownership and disposition of our common stock applicable to Non-U.S. Holders. A “Non-U.S. Holder” is a beneficial owner of our common stock that holds our common stock as a capital asset and who is generally an individual, corporation, estate or trust other than:
| | • | | an individual who is a citizen or resident of the United States for U.S. federal income tax purposes; |
| | • | | a corporation (or entity treated as a corporation for U.S. federal income tax purposes) created or organized in the United States or under the laws of the United States or of any subdivision thereof; |
| | • | | an estate whose income is includible in gross income for U.S. federal income tax purposes regardless of source; and |
| | • | | a trust subject to the primary supervision of a court within the United States and the control of one or more U.S. persons. |
The following discussion does not consider specific facts and circumstances that may be relevant to a particular Non-U.S. Holder’s tax position and does not consider U.S. state and local or non-U.S. tax consequences. Further, it does not consider Non-U.S. Holders subject to special tax treatment under the federal income tax laws (including partnerships or other pass-through entities and their owners, banks and insurance companies, tax-exempt entities, dealers in securities, holders of securities held as part of a “straddle,” “hedge,” “conversion transaction” or other risk-reduction transaction, certain U.S. expatriates (including certain former citizens or residents of the United States) and persons who hold or receive common stock as compensation). This disclosure does not address the U.S. federal income tax consequences to a Non-U.S. holder who owns, directly or indirectly, 5% or more of our common stock. The following discussion is based on provisions of the Code, as amended, applicable Treasury regulations, and administrative and judicial interpretations as of the date of this prospectus, all of which are subject to change, possibly on a retroactive basis, and any change could affect the continuing validity of this discussion.
The following summary is included herein for general information. Accordingly, each prospective Non-U.S. Holder is urged to consult a tax advisor with respect to the federal, state, local or non-U.S. tax consequences of holding and disposing of our common stock.
U.S. Trade or Business Income
For purposes of the following discussion, dividends and gains on the sale, exchange or other disposition of our common stock will be considered to be “U.S. trade or business income” if such income or gain is (i) effectively connected with the conduct of a U.S. trade or business or (ii) in the case of a treaty resident, attributable to a permanent establishment (or, in the case of an individual, a fixed base) in the United States. Generally, U.S. trade or business income is subject to U.S. federal income tax on a net income basis at regular graduated tax rates. Any U.S. trade or business income received by a Non-U.S. Holder that is a corporation may, under specific circumstances, be subject to an additional “branch profits tax” at a 30% rate or a lower rate that an applicable income tax treaty may specify.
Dividends
Dividends paid to a Non-U.S. Holder of common stock generally will be subject to withholding of U.S. federal income tax at a 30% rate unless the dividends are U.S. trade or business income and the Non-U.S. Holder files a properly executed IRS Form W-8ECI with the withholding agent.
The 30% withholding rate may be reduced if the Non-U.S. Holder is eligible for the benefits of an income tax treaty that provides for a lower rate. Generally, to claim the benefits of an income tax treaty, a Non-U.S.
68
Holder of common stock will be required to provide a properly executed IRS Form W-8BEN and satisfy applicable certification and other requirements, including, in certain cases, the request to obtain and furnish an IRS taxpayer identifying number. A Non-U.S. Holder of common stock that is eligible for a reduced rate of U.S. withholding tax under an income tax treaty may obtain a refund or credit of any excess amounts withheld by filing an appropriate claim for a refund with the IRS. A Non-U.S. Holder should consult its tax advisor on its entitlement to benefits under a relevant income tax treaty.
��
Disposition of Common Stock
A Non-U.S. Holder generally will not be subject to U.S. federal income tax in respect of gain recognized on a sale or exchange of common stock unless:
| | • | | the gain is U.S. trade or business income; |
| | • | | the Non-U.S. Holder is an individual who is present in the United States for 183 or more days in the taxable year of the sale or exchange and meets other requirements; or |
| | • | | we are or have been a “U.S. real property holding corporation” (a “USRPHC”) for U.S. federal income tax purposes at any time during the shorter of the five-year period ending on the date of disposition and the Non-U.S. Holder’s holding period for the common stock. |
The tax relating to stock in a USRPHC does not apply to a Non-U.S. Holder whose holdings, actual and constructive, at all times during the applicable period, amount to 5% or less of the common stock, provided that the common stock is regularly traded on an established securities market. Generally, a corporation is a USRPHC if the fair market value of its “U.S. real property interests” equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests and its other assets used or held for use in a trade or business. We believe that we have not been and are not currently a USRPHC for U.S. federal income tax purposes, nor do we anticipate becoming a USRPHC in the future. However, no assurance can be given that we will not be a USRPHC when a Non-U.S. Holder sells its shares of common stock.
Federal Estate Taxes
Common stock owned or treated as owned by an individual who is a Non-U.S. Holder at the time of death will be included in the individual’s gross estate for U.S. federal estate tax purposes and may be subject to U.S. federal estate tax, unless an applicable estate tax treaty provides otherwise.
Information Reporting Requirements and Backup Withholding Tax
Dividends
We must report annually to the IRS and to each Non-U.S. Holder any dividend income that is subject to withholding or that is exempt from U.S. withholding tax pursuant to an income tax treaty. Copies of these information returns may also be made available under the provisions of a specific treaty or agreement to the tax authorities of the country in which a Non-U.S. Holder resides. Dividends paid to Non-U.S. Holders of common stock generally will be exempt from backup withholding if the Non-U.S. Holder provides a properly executed IRS Form W-8BEN or otherwise establishes an exemption.
Disposition of Common Stock
The payment of the proceeds from the disposition of common stock to or through the U.S. office of any broker, U.S. or foreign, will be subject to information reporting and possible backup withholding unless the owner certifies as to its non-U.S. status under penalties of perjury or otherwise establishes an exemption, provided that the broker does not have actual knowledge (or reason to know) that the holder is a U.S. person or that the conditions of any other exemption are not, in fact, satisfied. The payment of the proceeds from the
69
disposition of common stock to or through a non-U.S. office of a non-U.S. broker will not be subject to information reporting or backup withholding unless the non-U.S. broker has certain types of relationships with the U.S. (a “U.S. related person”). In the case of the payment of the proceeds from the disposition of common stock to or through a non-U.S. office of a broker that is either a U.S. person or a U.S. related person, the Treasury regulations require information reporting (but not backup withholding) on the payment unless the broker has documentary evidence in its files that the owner is a Non-U.S. Holder and the broker does not have actual knowledge (or reason to know) to the contrary. Non-U.S. Holders should consult their own tax advisors on the application of information reporting and backup withholding to them in their particular circumstances (including upon their disposition of common stock).
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a Non-U.S. Holder will be refunded or credited against the holder’s U.S. federal income tax liability, if any, if the holder provides the required information to the IRS.
70
UNDERWRITING
We and the underwriters named below have entered into an underwriting agreement with respect to the shares being offered. Subject to the terms and conditions of the underwriting agreement, the underwriters named below have severally agreed to purchase from us the number of shares of our common stock set forth opposite their names on the table below at the public offering price, less the underwriting discounts and commissions set forth on the cover page of this prospectus. SG Cowen Securities Corporation, CIBC World Markets Corp., Thomas Weisel Partners LLC and U.S. Bancorp Piper Jaffray Inc. are acting as representatives of the underwriters named below:
Name
| | Number of Shares
|
SG Cowen Securities Corporation | | |
CIBC World Markets Corp. | | |
Thomas Weisel Partners LLC | | |
U.S. Bancorp Piper Jaffray Inc. | | |
| | |
|
Total | | |
| | |
|
The underwriting agreement provides that the obligations of the underwriters to purchase the shares of common stock offered hereby are conditional and may be terminated at their discretion based on their assessment of the state of the financial markets. The obligations of the underwriters also may be terminated upon the occurrence of other events specified in the underwriting agreement. The underwriters are committed severally to purchase all of the shares of common stock being offered by us if any shares are purchased.
The underwriters propose to offer the shares of common stock to the public at the public offering price set forth on the cover of this prospectus. The underwriters may offer the common stock to securities dealers at the price to the public less a concession not in excess of $ per share. Securities dealers may reallow a concession not in excess of $ per share to other dealers. After the shares of common stock are released for sale to the public, the underwriters may vary the offering price and other selling terms from time to time.
We have granted to the underwriters an option, exercisable not later than 30 days after the date of this prospectus, to purchase up to an aggregate of additional shares of common stock at the public offering price set forth on the cover page of this prospectus less the underwriting discounts and commissions. The underwriters may exercise this option only to cover over allotments, if any, made in connection with the sale of the common stock offered hereby. If the over allotment option is exercised in full, the underwriters will purchase additional common shares from us in approximately the same proportion as shown in the table above.
The following table shows the underwriting discounts and commissions that we will pay to the underwriters in connection with this offering. These amounts are shown assuming no exercise and full exercise of the underwriters’ option to purchase additional shares of common stock.
| | | Per Share
| | Total
|
| | | Without
Over Allotment
| | With
Over Allotment
| | Without
Over Allotment
| | With
Over Allotment
|
Public offering price | | $ | | | $ | | | $ | | | $ | |
Underwriting discounts and commissions payable by us | | | | | | | | | | | | |
Proceeds, before expenses, to us | | | | | | | | | | | | |
We estimate that the total expenses of this offering, excluding underwriting discounts and commissions, will be approximately $ .
71
We have agreed to indemnify the underwriters against certain civil liabilities, including liabilities under the Securities Act and liabilities arising from breaches of representations and warranties contained in the underwriting agreement, and to contribute to payments the underwriters may be required to make in respect of any such liabilities.
All of our directors and executive officers, and security holders have agreed with SG Cowen Securities Corporation, or are otherwise subject to arrangements providing, that for a period of 180 days following the date of this prospectus, they will not offer, sell, assign, transfer, pledge, contract to sell, or otherwise dispose of or hedge any shares of our common stock or any securities convertible into or exchangeable for shares of common stock. SG Cowen Securities Corporation may, in its sole discretion, at any time without prior notice, release all or any portion of the shares from the restrictions in any such agreement. We have entered into a similar agreement with SG Cowen Securities Corporation, provided we may, without the consent of SG Cowen Securities Corporation, grant options and sell shares pursuant to our stock plans. There are no agreements between SG Cowen Securities Corporation and any of our stockholders, optionholders or affiliates releasing them from these lock-up agreements prior to the expiration of the 180-day period.
The underwriters may engage in over-allotment, stabilizing transactions, syndicate covering transactions, penalty bids, and passive market making in accordance with Regulation M under the Securities Exchange Act of 1934, as amended. Over-allotment involves syndicate sales in excess of the offering size, which creates a syndicate short position. Covered short sales are sales made in an amount not greater than the number of shares available for purchase by the underwriters under the over-allotment option. SG Cowen Securities Corporation may close out a covered short sale by exercising its over-allotment option or purchasing shares in the open market. Naked short sales are sales made in an amount in excess of the number of shares available under the over-allotment option. The underwriters must close out any naked short sale by purchasing shares in the open market. Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. Syndicate covering transactions involve purchases of the shares of common stock in the open market after the distribution has been completed in order to cover syndicate short positions. Penalty bids permit the representatives to reclaim a selling concession from a syndicate member when the shares of common stock originally sold by such syndicate member is purchased in a syndicate covering transaction to cover syndicate short positions. Penalty bids may have the effect of deterring syndicate members from selling to people who have a history of quickly selling their shares. In passive market making, market makers in the shares of common stock who are underwriters or prospective underwriters may, subject to certain limitations, make bids for or purchases of the shares of common stock until the time, if any, at which a stabilizing bid is made. These stabilizing transactions, syndicate covering transactions and penalty bids may cause the price of the shares of common stock to be higher than it would otherwise be in the absence of these transactions. These transactions may be effected on the Nasdaq National Market or otherwise and, if commenced, may be discontinued at any time.
Prior to this offering, there has been no public market for our common stock. The initial public offering price was determined by negotiation between us and the representatives. The principal factors considered in determining the public offering price included the following:
| | • | | the information included in this prospectus and otherwise available to the representatives; |
| | • | | market conditions for initial public offerings; |
| | • | | the history of and the prospects for the industry in which we compete; |
| | • | | the ability of our management; |
| | • | | the prospects for our future earnings; |
| | • | | our current financial condition; |
| | • | | the general condition of the securities markets at the time of this offering; and |
| | • | | the recent market prices of, and the demand for, publicly traded common stock of generally comparable companies. |
72
LEGAL MATTERS
Selected legal matters with respect to the validity of the common stock offered by this prospectus are being passed upon for us by Pillsbury Winthrop LLP, San Diego, California. An attorney of Pillsbury Winthrop LLP owns 2,000 shares of our Series C convertible preferred stock, which will be converted automatically into an equal number of shares of common stock upon the closing of this offering. An entity in which attorneys and former attorneys of Pillsbury Winthrop LLP are members owns an aggregate of 20,000 shares of our Series A convertible preferred stock, which will be converted automatically into an equal number of shares of common stock upon the closing of this offering. Selected legal matters in connection with this offering will be passed upon for the underwriters by Heller Ehrman White & McAuliffe LLP, San Diego, California.
EXPERTS
The financial statements of Xcel Pharmaceuticals, Inc. as of December 31, 2001 and 2002, and for the period January 24, 2001 (inception) through December 31, 2001 and the year ended December 31, 2002, included in this prospectus and from which the Selected Financial Data as of December 31, 2001 and 2002 and for the period January 24, 2001 (inception) through December 31, 2001 and the year ended December 31, 2002, included in this prospectus have been derived, and the related financial statement schedule included elsewhere in the registration statement have been audited by Deloitte & Touche LLP, independent auditors, as stated in their reports appearing herein and elsewhere in the registration statement. Such financial statements, financial statement schedule and Selected Financial Data have been included herein and elsewhere in the registration statement in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
The Diastat and Mysoline Statements of Product Contribution for the year ended December 31, 2000 and for the period January 1, 2001 through March 31, 2001 of Elan Corporation, plc, included in this prospectus, and from which the Selected Financial Data for the period December 31, 2000 and for the period January 1, 2001 through March 31, 2001, included in this prospectus have been derived, have been audited by Deloitte & Touche LLP, independent auditors, as stated in their report appearing herein. Such financial statements and Selected Financial Data have been included herein in reliance upon the reports of such firm given upon their authority as experts in accounting and auditing.
The Migranal and D.H.E. 45 Statements of Product Contribution for the years ended December 31, 2000 and 2001 and for the period January 1, 2002 through June 25, 2002 of Novartis Pharmaceuticals Corporation included in this prospectus have been so included in reliance on the report of PricewaterhouseCoopers LLP, independent accountants, given on the authority of said firm as experts in auditing and accounting.
73
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the U.S. Securities and Exchange Commission a Registration Statement on Form S-1 under the Securities Act of 1933 with respect to the common stock offered hereby. This prospectus, which constitutes a part of the Registration Statement, does not contain all of the information set forth in the Registration Statement or the exhibits and schedules which are part of the Registration Statement. For further information with respect to Xcel and the common stock offered by this prospectus, we refer you to the Registration Statement and the exhibits and schedules filed as part of the Registration Statement. You may read and copy any document we file at the U.S. Securities and Exchange Commission’s public reference room at 450 Fifth Street, N.W., Washington, D.C. 20549. Please call the U.S. Securities and Exchange Commission at 1–800–SEC–0330 for further information on the public reference rooms. Our U.S. Securities and Exchange Commission filings are also available to the public from the U.S. Securities and Exchange Commission’s web site at http://www.sec.gov. Upon completion of this offering, we will become subject to the information and periodic reporting requirements of the Securities and Exchange Act, as amended, and, in accordance therewith, will file periodic reports, proxy statements and other information with the U.S. Securities and Exchange Commission. These periodic reports, proxy statements and other information will be available for inspection and copying at the U.S. Securities and Exchange Commission’s public reference rooms and the web site of the U.S. Securities and Exchange Commission referred to above.
74
XCEL PHARMACEUTICALS, INC.
INDEX TO FINANCIAL STATEMENTS
F-1
XCEL PHARMACEUTICALS, INC.
INDEPENDENT AUDITORS’ REPORT
To the Board of Directors and Stockholders of Xcel Pharmaceuticals, Inc.:
We have audited the accompanying balance sheets of Xcel Pharmaceuticals, Inc. (the “Company”) as of December 31, 2001 and 2002 and the related statements of operations, stockholders’ equity, and cash flows for the period January 24, 2001 (inception) through December 31, 2001 and the year ended December 31, 2002. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such financial statements present fairly, in all material respects, the financial position of Xcel Pharmaceuticals, Inc. as of December 31, 2001 and 2002 and the results of its operations and its cash flows for the period January 24, 2001 (inception) through December 31, 2001 and for the year ended December 31, 2002 in conformity with accounting principles generally accepted in the United States of America.
DELOITTE & TOUCHE LLP
San Diego, California
January 31, 2003 (March 31, 2003 as to the first paragraph in Note 11 and August 7, 2003 as to the second paragraph in Note 11)
F-2
XCEL PHARMACEUTICALS, INC.
BALANCE SHEETS
| | | December 31,
| | | June 30, 2003
| |
| | | 2001
| | | 2002
| | |
| | | | | | | | | (unaudited) | |
| | | (in thousands, except share amounts) | |
Assets | | | | | | | | | | | | |
Current assets: | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 19,487 | | | $ | 16,561 | | | $ | 29,694 | |
Accounts receivable, net | | | 2,915 | | | | 4,616 | | | | 5,599 | |
Inventories | | | 3,891 | | | | 6,925 | | | | 6,671 | |
Product acquisition receivable | | | 1,521 | | | | — | | | | — | |
Prepaid expenses and other current assets | | | 803 | | | | 622 | | | | 981 | |
Deferred tax asset, net | | | — | | | | 2,765 | | | | 2,765 | |
| | |
|
|
| |
|
|
| |
|
|
|
Total current assets | | | 28,617 | | | | 31,489 | | | | 45,710 | |
Deferred tax asset, net | | | — | | | | 912 | | | | — | |
Furniture and equipment, net | | | 916 | | | | 567 | | | | 1,031 | |
Product rights, net | | | 142,373 | | | | 182,588 | | | | 177,541 | |
Deferred debt issuance costs, net | | | — | | | | — | | | | 2,258 | |
Other long-term assets | | | — | | | | 91 | | | | 621 | |
| | |
|
|
| |
|
|
| |
|
|
|
Total assets | | $ | 171,906 | | | $ | 215,647 | | | $ | 227,161 | |
| | |
|
|
| |
|
|
| |
|
|
|
Liabilities and Stockholders’ Equity | | | | | | | | | | | | |
Current liabilities: | | | | | | | | | | | | |
Current portion of long-term debt | | $ | — | | | $ | 1,875 | | | $ | 2,000 | |
Accounts payable | | | 1,231 | | | | 1,653 | | | | 2,582 | |
Income taxes payable | | | — | | | | 296 | | | | 3,672 | |
Accrued expenses and other current liabilities | | | 11,293 | | | | 17,987 | | | | 21,878 | |
| | |
|
|
| |
|
|
| |
|
|
|
Total current liabilities | | | 12,524 | | | | 21,811 | | | | 30,132 | |
Long-term debt, less current portion | | | 99,000 | | | | 107,125 | | | | 60,000 | |
Deferred tax liability, net | | | — | | | | — | | | | 13,052 | |
| | |
|
|
| |
|
|
| |
|
|
|
Total liabilities | | | 111,524 | | | | 128,936 | | | | 103,184 | |
| | |
|
|
| |
|
|
| |
|
|
|
Commitments and contingencies (notes 3, 4, 5, 8 and 11) | | | | | | | | | | | | |
Stockholders’ equity: | | | | | | | | | | | | |
Series A convertible preferred stock, $0.0001 par value; 33,000,000, 33,000,000 and 33,600,000 shares authorized, respectively; 14,000,000, 14,000,000 and 11,000,000, shares issued and outstanding, respectively (liquidation preference of $140,000, $140,000 and $110,000, respectively) | | | 1 | | | | 1 | | | | 1 | |
Series B convertible preferred stock, $0.0001 par value; zero, 9,000,000 and 9,000,000 shares authorized, respectively; zero, 3,750,000 and 3,750,000 shares issued and outstanding, respectively (liquidation preference of zero, $45,000 and $45,000, respectively) | | | — | | | | 1 | | | | 1 | |
Series C convertible preferred stock, $0.0001 par value; zero, zero and 9,600,000 shares authorized, respectively; zero, zero and 4,000,000 shares issued and outstanding, respectively (liquidation preference of zero, zero and $52,000, respectively) | | | — | | | | — | | | | 1 | |
Common stock, $0.0001 par value; 43,000,000, 52,000,000 and 62,200,000 shares authorized, respectively; 4,728,975, 4,772,962 and 4,778,980 shares issued and outstanding, respectively | | | 1 | | | | 1 | | | | 1 | |
Additional paid-in capital | | | 71,272 | | | | 93,792 | | | | 101,748 | |
Deferred stock-based compensation | | | (1,376 | ) | | | (1,029 | ) | | | (851 | ) |
Retained earnings (accumulated deficit) | | | (9,516 | ) | | | (6,055 | ) | | | 23,076 | |
| | |
|
|
| |
|
|
| |
|
|
|
Total stockholders’ equity | | | 60,382 | | | | 86,711 | | | | 123,977 | |
| | |
|
|
| |
|
|
| |
|
|
|
Total liabilities and stockholders’ equity | | $ | 171,906 | | | $ | 215,647 | | | $ | 227,161 | |
| | |
|
|
| |
|
|
| |
|
|
|
The accompanying notes are an integral part of these financial statements.
F-3
XCEL PHARMACEUTICALS, INC.
STATEMENTS OF OPERATIONS
| | | Period from January 24, 2001 (inception) Through December 31, 2001
| | | Year Ended
December 31, 2002
| | | Six Months Ended June 30,
| |
| | | | | 2002
| | | 2003
| |
| | | | | | | | | (unaudited) | | | (unaudited) | |
| | | (in thousands, except per share amounts) | |
Net sales | | $ | 14,124 | | | $ | 50,055 | | | $ | 23,044 | | | $ | 39,142 | |
Operating costs and expenses: | | | | | | | | | | | | | | | | |
Cost of sales | | | 3,285 | | | | 8,835 | | | | 4,448 | | | | 5,637 | |
Selling, general and administrative | | | 11,224 | | | | 20,275 | | | | 9,123 | | | | 13,847 | |
Product development | | | — | | | | 588 | | | | 129 | | | | 1,051 | |
Costs of abandoned initial public offering | | | — | | | | 991 | | | | — | | | | — | |
Product acquisition charge | | | — | | | | 3,512 | | | | — | | | | — | |
Product rights amortization | | | 5,808 | | | | 8,745 | | | | 3,698 | | | | 5,047 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total operating costs and expenses | | | 20,317 | | | | 42,946 | | | | 17,398 | | | | 25,582 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Operating income (loss) | | | (6,193 | ) | | | 7,109 | | | | 5,646 | | | | 13,560 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Other income (expense): | | | | | | | | | | | | | | | | |
Interest expense | | | (3,996 | ) | | | (7,327 | ) | | | (3,504 | ) | | | (3,818 | ) |
Interest income | | | 673 | | | | 295 | | | | 214 | | | | 96 | |
Gain on debt retirement | | | — | | | | — | | | | — | | | | 37,437 | |
Other, net | | | — | | | | — | | | | — | | | | 93 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total other income (expense) | | | (3,323 | ) | | | (7,032 | ) | | | (3,290 | ) | | | 33,808 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income (loss) before income taxes | | | (9,516 | ) | | | 77 | | | | 2,356 | | | | 47,368 | |
Income tax expense (benefit) | | | — | | | | (3,384 | ) | | | (2,692 | ) | | | 18,237 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net income (loss) | | $ | (9,516 | ) | | $ | 3,461 | | | $ | 5,048 | | | $ | 29,131 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net income (loss) per common share: | | | | | | | | | | | | | | | | |
Pro forma basic and diluted | | $ | (2.12 | ) | | | | | | | | | | | | |
| | |
|
|
| | | | | | | | | | | | |
Basic | | | | | | $ | 2.13 | | | $ | 3.87 | | | $ | 11.38 | |
| | | | | | |
|
|
| |
|
|
| |
|
|
|
Diluted | | | | | | $ | 0.17 | | | $ | 0.27 | | | $ | 1.28 | |
| | | | | | |
|
|
| |
|
|
| |
|
|
|
Weighted average common shares outstanding: | | | | | | | | | | | | | | | | |
Pro forma basic and diluted | | | 4,487 | | | | | | | | | | | | | |
| | |
|
|
| | | | | | | | | | | | |
Basic | | | | | | | 1,625 | | | | 1,304 | | | | 2,561 | |
| | | | | | |
|
|
| |
|
|
| |
|
|
|
Diluted | | | | | | | 20,867 | | | | 19,034 | | | | 22,847 | |
| | | | | | |
|
|
| |
|
|
| |
|
|
|
The accompanying notes are an integral part of these financial statements.
F-4
XCEL PHARMACEUTICALS, INC.
STATEMENTS OF STOCKHOLDERS’ EQUITY
| | | Convertible Preferred Stock
| | Common Stock
| | Additional
Paid-In
Capital
| | | Deferred
Stock-based
Compensation
| | | Retained
Earnings
(Accumulated
Deficit)
| | | Total
| |
| | | Shares
| | | Amount
| | Shares
| | Amount
| | | | |
| | | (in thousands, except per share amounts) | |
Proceeds from issuance of common stock to founders | | — | | | $ | — | | 4,520,000 | | $ | 1 | | $ | 10 | | | $ | — | | | $ | — | | | $ | 11 | |
Net proceeds from issuance of Series A preferred stock | | 14,000,000 | | | | 1 | | — | | | — | | | 69,633 | | | | — | | | | — | | | | 69,634 | |
Net proceeds from exercise of stock options | | — | | | | — | | 208,975 | | | — | | | 121 | | | | — | | | | — | | | | 121 | |
Deferred stock-based compensation | | — | | | | — | | — | | | — | | | 1,508 | | | | (1,508 | ) | | | — | | | | — | |
Amortization of deferred stock-based compensation | | — | | | | — | | — | | | — | | | — | | | | 132 | | | | — | | | | 132 | |
Net loss | | — | | | | — | | — | | | — | | | — | | | | — | | | | (9,516 | ) | | | (9,516 | ) |
| | |
|
| |
|
| |
| |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Balance at December 31, 2001 | | 14,000,000 | | | | 1 | | 4,728,975 | | | 1 | | | 71,272 | | | | (1,376 | ) | | | (9,516 | ) | | | 60,382 | |
Net proceeds from issuance of Series B preferred stock | | 3,750,000 | | | | 1 | | — | | | — | | | 22,440 | | | | — | | | | — | | | | 22,441 | |
Net proceeds from exercise of stock options | | — | | | | — | | 43,987 | | | — | | | 25 | | | | — | | | | — | | | | 25 | |
Deferred stock-based compensation | | — | | | | — | | — | | | — | | | 55 | | | | (55 | ) | | | — | | | | — | |
Amortization of deferred stock-based compensation | | — | | | | — | | — | | | — | | | — | | | | 402 | | | | — | | | | 402 | |
Net income | | — | | | | — | | — | | | — | | | — | | | | — | | | | 3,461 | | | | 3,461 | |
| | |
|
| |
|
| |
| |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Balance at December 31, 2002 | | 17,750,000 | | | | 2 | | 4,772,962 | | | 1 | | | 93,792 | | | | (1,029 | ) | | | (6,055 | ) | | | 86,711 | |
Net proceeds from issuance of Series C preferred stock (unaudited) | | 4,000,000 | | | | 1 | | — | | | — | | | 25,904 | | | | — | | | | — | | | | 25,905 | |
Repurchase of Series A preferred stock (unaudited) | | (3,000,000 | ) | | | — | | — | | | — | | | (18,000 | ) | | | — | | | | — | | | | (18,000 | ) |
Net proceeds from exercise of stock options (unaudited) | | — | | | | — | | 6,018 | | | — | | | 36 | | | | — | | | | — | | | | 36 | |
Deferred stock-based compensation (unaudited) | | — | | | | — | | — | | | — | | | 16 | | | | (16 | ) | | | — | | | | — | |
Amortization of deferred stock-based compensation (unaudited) | | — | | | | — | | — | | | — | | | — | | | | 194 | | | | — | | | | 194 | |
Net income (unaudited) | | — | | | | — | | — | | | — | | | — | | | | — | | | | 29,131 | | | | 29,131 | |
| | |
|
| |
|
| |
| |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Balance at June 30, 2003 (unaudited) | | 18,750,000 | | | $ | 3 | | 4,778,980 | | $ | 1 | | $ | 101,748 | | | $ | (851 | ) | | $ | 23,076 | | | $ | 123,977 | |
| | |
|
| |
|
| |
| |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
The accompanying notes are an integral part of these financial statements.
F-5
XCEL PHARMACEUTICALS, INC.
STATEMENTS OF CASH FLOWS
| | | Period from
January 24,
2001 (inception)
Through
December 31, 2001
| | | Year Ended
December 31,
2002
| | | Six Months Ended June 30 ,
| |
| | | | | 2002
| | | 2003
| |
| | | | | | | | | (unaudited) | | | (unaudited) | |
| | | (in thousands) | |
Operating Activities: | | | | | | | | | | | | | | | | |
Net income (loss) | | $ | (9,516 | ) | | $ | 3,461 | | | $ | 5,048 | | | $ | 29,131 | |
Adjustments to reconcile net income (loss) to net cash provided by operating activities: | | | | | | | | | | | | | | | | |
Product rights amortization | | | 5,808 | | | | 8,745 | | | | 3,698 | | | | 5,047 | |
Stock-based compensation | | | 132 | | | | 402 | | | | 220 | | | | 194 | |
Depreciation | | | 189 | | | | 450 | | | | 178 | | | | 174 | |
Deferred income taxes | | | — | | | | (3,677 | ) | | | (2,693 | ) | | | 13,964 | |
Amortization of debt issuance costs | | | — | | | | — | | | | — | | | | 119 | |
Gain on debt retirement | | | — | | | | — | | | | — | | | | (37,437 | ) |
Gain on termination of product financing | | | — | | | | — | | | | — | | | | (246 | ) |
Changes in assets and liabilities, net of assets acquired and liabilities assumed related to product acquisitions: | | | | | | | | | | | | | | | | |
Accounts receivable | | | (2,915 | ) | | | (1,701 | ) | | | 1,844 | | | | (983 | ) |
Inventories | | | 1,279 | | | | (1,237 | ) | | | 43 | | | | 254 | |
Prepaid expenses and other current assets | | | (803 | ) | | | 90 | | | | (677 | ) | | | (359 | ) |
Accounts payable | | | 1,231 | | | | 422 | | | | (80 | ) | | | 929 | |
Income taxes payable | | | — | | | | — | | | | — | | | | 3,376 | |
Accrued expenses and other current liabilities | | | 4,733 | | | | 10,986 | | | | 3,637 | | | | 4,130 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net cash provided by operating activities | | | 138 | | | | 17,941 | | | | 11,218 | | | | 18,293 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Investing Activities: | | | | | | | | | | | | | | | | |
Purchase of product rights | | | (60,500 | ) | | | (47,703 | ) | | | (47,703 | ) | | | — | |
Payment for inventories acquired in product acquisitions | | | — | | | | (6,967 | ) | | | (6,967 | ) | | | — | |
Proceeds from product purchase price adjustments | | | 10,881 | | | | 1,521 | | | | 1,521 | | | | — | |
Purchase of furniture and equipment | | | (1,105 | ) | | | (101 | ) | | | (53 | ) | | | (638 | ) |
Payments for manufacturing transfer | | | — | | | | — | | | | — | | | | (530 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net cash used in investing activities | | | (50,724 | ) | | | (53,250 | ) | | | (53,202 | ) | | | (1,168 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Financing Activities: | | | | | | | | | | | | | | | | |
Net proceeds from issuance of convertible preferred stock | | | 69,634 | | | | 22,441 | | | | 22,465 | | | | 25,905 | |
Repurchase of convertible preferred stock | | | — | | | | — | | | | — | | | | (18,000 | ) |
Proceeds from issuance of long-term debt | | | — | | | | 10,000 | | | | 10,000 | | | | 62,000 | |
Deferred debt issue costs | | | — | | | | — | | | | — | | | | (2,370 | ) |
Payment of long-term debt | | | — | | | | — | | | | — | | | | (71,563 | ) |
Proceeds from issuance of common stock to founders | | | 11 | | | | — | | | | — | | | | — | |
Net proceeds from exercise of stock options | | | 121 | | | | 25 | | | | 24 | | | | 36 | |
Proceeds from product financing | | | 500 | | | | 1,000 | | | | — | | | | — | |
Payment on product financing | | | (193 | ) | | | (1,083 | ) | | | (83 | ) | | | — | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net cash provided by (used in) financing activities | | | 70,073 | | | | 32,383 | | | | 32,406 | | | | (3,992 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net increase (decrease) in cash and cash equivalents | | | 19,487 | | | | (2,926 | ) | | | (9,578 | ) | | | 13,133 | |
Cash and cash equivalents, beginning of period | | | — | | | | 19,487 | | | | 19,487 | | | | 16,561 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Cash and cash equivalents, end of period | | $ | 19,487 | | | $ | 16,561 | | | $ | 9,909 | | | $ | 29,694 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Supplemental Disclosures of Cash Flow Information: | | | | | | | | | | | | | | | | |
Cash paid for interest | | $ | 3,990 | | | $ | 7,315 | | | $ | 3,500 | | | $ | 3,695 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Cash paid for income taxes | | $ | — | | | $ | — | | | $ | — | | | $ | 897 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Non-cash Investing and Financing Activities: | | | | | | | | | | | | | | | | |
Notes issued to acquire products | | $ | 99,000 | | | | | | | | | | | | | |
| | |
|
|
| | | | | | | | | | | | |
Obligation for product inventories | | $ | 5,170 | | | | | | | | | | | | | |
| | |
|
|
| | | | | | | | | | | | |
Product acquisition receivable | | $ | 1,521 | | | | | | | | | | | | | |
| | |
|
|
| | | | | | | | | | | | |
The accompanying notes are an integral part of these financial statements.
F-6
XCEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
1. Summary of Significant Accounting Policies
Description of Business
Xcel Pharmaceuticals, Inc., a Delaware corporation (the “Company”), is a specialty pharmaceutical company focused on the treatment of disorders of the central nervous system (“CNS”). The Company’s portfolio includes four commercial products, Diastat and Mysoline, which are used to treat epilepsy, and Migranal and D.H.E. 45, which are used to treat migraine, and one product candidate, MT 300, a potential treatment for migraine. The Company’s 96-person nationwide field sales organization promotes the Company’s commercial products to the highest prescribing epilepsy and migraine specialists. The Company’s strategy is to increase prescription demand for Diastat, Migranal and, subject to regulatory approval and commercial launch, MT 300 through targeted sales and marketing efforts, to leverage the Company’s presence in the CNS market through the acquisition of additional late-stage development product candidates and marketed products, and to develop enhancements for the Company’s current products.
The Company was formed on January 24, 2001. On March 31, 2001 and April 1, 2001, respectively, the Company acquired its initial products, Diastat® and Mysoline® from two affiliates of Elan Corporation, plc (Elan Corporation, plc and its affiliates collectively are referred to as “Elan”). The Company had no substantive operations prior to the acquisition of these products. The Company acquired exclusive worldwide rights to Diastat. Diastat is the only drug approved in the United States (“U.S.”) for the acute treatment of seizures outside of a hospital setting. The Company acquired exclusive rights in the U.S. to Mysoline. Mysoline is an oral prescription medication that has been used since the early 1950s for the chronic treatment of seizures associated with epilepsy. In June 2002, the Company acquired exclusive rights in the U.S. to Migranal® and D.H.E. 45® from Novartis Pharmaceuticals Corporation (“Novartis”). Migranal, a nasal spray, and D.H.E. 45, an injection, contain the same active ingredient, dihydroergotamine, and are prescribed for the treatment of migraine.
In September 2003, the Company licensed the exclusive commercial rights in the U.S. to MT 300 from Pozen Inc (“Pozen”). MT 300, an injection in a convenient pre-filled syringe, is a highly purified formulation of dihyroergotamine. Pozen submitted a new drug application with the U.S. Food and Drug Administration (“FDA”) in December 2002 for marketing approval of MT 300 for the treatment of migraines, which is under review by the FDA. Subject to regulatory approval and commercial launch, the Company intends to promote the benefits of MT 300 to its targeted physicians to grow its injectable dihydroergotamine franchise.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect certain reported amounts and disclosures. Accordingly, actual results could differ from those estimates.
Interim Unaudited Financial Information
The financial statements as of June 30, 2003 and for the six months ended June 30, 2002 and 2003 are unaudited; however, in the opinion of management, all adjustments (consisting solely of normal recurring adjustments) necessary for a fair presentation of the unaudited financial statements for this interim period have been included. The results of the six months ended June 30, 2003 are not necessarily indicative of results to be obtained for the full year.
F-7
XCEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
Revenue Recognition
The Company sells its commercial products to wholesale drug distributors who generally sell the products to retail pharmacies, hospitals and other institutional customers. Product sales are recognized when the products are received by the wholesaler, which represents the point when the risks and rewards of ownership have been transferred to the customer. The Company invoices wholesalers at its wholesale list price. Sales are shown net of discounts, rebates, chargebacks, and returns, which are estimated based on historical experience or projections. The Company periodically reviews its reserves for rebates, chargebacks and returns and makes adjustments, if necessary, based on actual experience. Chargebacks represent the difference between the wholesale list price and the contractual sales price. The Company’s customers may generally return product upon the expiration date of the product.
Net Sales
Net sales is detailed as follows (in thousands):
| | | January 24, 2001
(Inception) through
December 31, 2001
| | Year Ended
December 31,
2002
| | Six Months Ended June 30,
|
| | | | | 2002
| | 2003
|
| | | | | | | (unaudited) | | (unaudited) |
Diastat | | $ | 7,040 | | $ | 25,482 | | $ | 14,106 | | $ | 17,008 |
Mysoline | | | 7,084 | | | 14,674 | | | 8,938 | | | 7,521 |
Migranal | | | — | | | 2,664 | | | — | | | 3,891 |
D.H.E. 45 | | | — | | | 7,235 | | | — | | | 10,722 |
| | |
|
| |
|
| |
|
| |
|
|
Net sales | | $ | 14,124 | | $ | 50,055 | | $ | 23,044 | | $ | 39,142 |
| | |
|
| |
|
| |
|
| |
|
|
The following table presents a summary of sales to significant customers that individually accounted for more than 10% of net sales:
| | | January 24, 2001
(Inception) through
December 31, 2001
| | Year Ended
December 31, 2002
| | Six Months Ended June 30,
|
| | | | | 2002
| | 2003
|
| | | | | | | (unaudited) | | (unaudited) |
Cardinal Health, Inc. | | 19% | | 39% | | 54% | | 28% |
McKesson Corporation | | 38% | | 26% | | 22% | | 33% |
AmerisourceBergen Corporation | | 27% | | 24% | | 13% | | 27% |
Cost of Sales
Cost of sales includes primarily third-party product manufacturing and distribution costs and royalties due to third parties on net product sales. The Company makes royalty payments of 10% of net sales on one of its products and 5% of net sales on a second product. The royalty requirements were assumed with the acquisition of the products. Royalty expense totaled $1.1 million and $3.3 million for the period ended December 31, 2001 and the year ended December 31, 2002, respectively, and $1.8 million and $2.1 million for the six months ended June 30, 2002 and 2003, respectively.
Product Development
Product development expenses consist primarily of costs incurred to develop enhancements for the Company’s products. The Company expenses all product development costs as incurred.
F-8
XCEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
Costs of Abandoned Initial Public Offering
In 2001 and 2002, the Company incurred $991,000 in legal, accounting and other costs related to filing a registration statement with the U.S. Securities and Exchange Commission in anticipation of an initial public offering of the Company’s common stock. These costs were capitalized to prepaid expenses and other current assets as deferred initial public offering costs. Due to difficult conditions in the U.S. equity markets, in December 2002, the Company decided to abandon its efforts to pursue a public offering in 2002 and expensed these costs.
Cash and Cash Equivalents
The Company considers only those investments that are highly liquid and readily convertible to cash with an original maturity of three months or less to be cash equivalents.
Concentrations of Risk
In the third quarter of 2003, two generic substitutes for D.H.E. 45 were introduced into the U.S. market. In addition, subject to regulatory approval and commercial launch, the Company intends to promote the benefits of MT 300 to its targeted physicians to grow its injectable dihydroergotamine franchise. As result, future net sales of D.H.E. 45 could be affected adversely. At this time, the Company does not have sufficient information to determine the future impact of these events on the net cash flows from the Company’s injectable dihydroergotamine products, D.H.E. 45 and MT 300. However, a decline in the future undiscounted cash flows of D.H.E. 45 and MT 300 below the net book value of the Company’s D.H.E. 45 and MT 300 injectable dihydroergotamine product rights would result in a reduction in the carrying value of these product rights and could also result in a reduction in the amortization period.
The Company extends credit on an uncollateralized basis to wholesalers throughout the United States. The Company has not experienced significant credit losses on its accounts. The Company’s three largest customers accounted for approximately 89%, 90% and 88% of accounts receivable as of December 31, 2001 and 2002 and June 30, 2003, respectively. The Company maintains an allowance for doubtful accounts for estimated losses resulting from its customers’ inability to make required payments. The Company estimates the amount of the allowance based on historical levels of bad debts and the financial strength of its customers, among other factors.
Segment Reporting
The Company operates in a single segment, the sale and marketing of prescription drugs.
Inventories
Inventories consist of purchased pharmaceutical products, product components, and product samples used in the sales and marketing efforts. Inventories are stated at the lower of cost or market. Cost is determined using the first-in, first-out method. The Company’s products have shelf lives ranging from 24 to 36 months. The Company evaluates its inventory levels based on forecasts of future sales, promotional focus, levels of products held by its customers, among other factors. Samples are expensed upon distribution.
Furniture and Equipment
Furniture and equipment is stated at cost. Depreciation is provided on a straight-line basis over the estimated useful lives of the assets, which range from three to five years.
F-9
XCEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
Product Rights
The costs of acquiring the Company’s products were capitalized and are being amortized on a straight-line basis over the estimated periods to be benefited, which range from seven to 20 years. Product rights include trade names, trademarks, patents and other intangible assets. The useful lives of these assets are estimated by management based on factors such as market size and growth trends, barriers to competitive entry, stability of therapeutic class and strength of competing products. Amortization of product rights totaled $5.8 million and $8.7 million for the period ended December 31, 2001 and the year ended December 31, 2002, respectively, and $3.7 million and $5.0 million for the six months ended June 30, 2002 and 2003, respectively. Estimated annual amortization for 2003 is $10.1 million and for each of the years in the period from 2004 to 2007 is $10.2 million.
Impairment of Long-Lived Assets
The Company periodically evaluates the recoverability of its long-lived assets as well as the related useful lives to determine whether facts and circumstances warrant adjustments to the carrying values and/or estimates of useful lives. This evaluation is performed using the estimated projected future undiscounted cash flows associated with the asset over its remaining useful life compared to the asset’s carrying amount to determine if a write-down is required. Facts and circumstances that determine the useful lives of the Company’s products are inherently uncertain and include, but are not limited to, generic competition, competition from products prescribed for similar indications, physician loyalty and prescribing patterns, and the sensitivity of the Company’s products to promotional efforts. When impairment is indicated for long-lived assets, the amount of impairment loss is the excess of net book value over fair value as approximated using discounted cash flows.
Manufacturing Transfer Costs
The Company incurs costs when transferring the manufacturing of certain of its products from one third-party manufacturer to a new third-party manufacturer. The costs pertain primarily to third-party project management, preparation of stability batches and preparation of the supplemental filing with the FDA. The Company capitalizes these costs and will amortize the costs over the term of the supply agreement with the new manufacturer. These capitalized costs are included in other long-term assets and totaled $621,000 and $91,000 as of June 30, 2003 and December 31, 2002, respectively.
Fair Value of Financial Instruments
The carrying amount of cash, cash equivalents, receivables, accounts payable and accrued expenses and other current liabilities are considered to be representative of their respective fair values because of the short-term nature of those instruments. It is not practicable to estimate the fair value of the Company’s long-term debt because of the lack of quoted market prices and the inability to estimate fair value without incurring excessive costs. However, management believed that the carrying amount recorded as of December 31, 2001 and 2002 and June 30, 2003 approximated the corresponding fair value.
Net Income (Loss) Per Common Share
Basic net income per common share is calculated based on the weighted average number of common shares outstanding during the period that are vested and not subject to repurchase. Diluted net income per common share gives effect to all potential dilutive stock equivalents during the period such as convertible preferred stock, unvested outstanding common shares subject to repurchase and outstanding stock options.
F-10
XCEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
Below is the calculation of basic and diluted net income per common share for the year ended December 31, 2002 and the six months ended June 30, 2002 and 2003 (in thousands, except per share amounts).
| | | | | Six Months Ended
June 30,
|
| | | Year Ended
December 31,
2002
| | 2002
| | 2003
|
| | | | | (unaudited) | | (unaudited) |
Net income | | $ | 3,461 | | $ | 5,048 | | $ | 29,131 |
| | |
|
| |
|
| |
|
|
Weighted average common shares outstanding—basic | | | 1,625 | | | 1,304 | | | 2,561 |
Dilutive stock equivalents: | | | | | | | | | |
Series A convertible preferred stock | | | 14,000 | | | 14,000 | | | 13,252 |
Series B convertible preferred stock | | | 1,957 | | | 125 | | | 3,750 |
Series C convertible preferred stock | | | — | | | — | | | 997 |
Common shares—subject to repurchase | | | 3,139 | | | 3,451 | | | 2,213 |
Stock options | | | 146 | | | 154 | | | 74 |
| | |
|
| |
|
| |
|
|
Weighted average common shares outstanding—diluted | | | 20,867 | | | 19,034 | | | 22,847 |
| | |
|
| |
|
| |
|
|
Net income per common share: | | | | | | | | | |
Basic | | $ | 2.13 | | $ | 3.87 | | $ | 11.38 |
| | |
|
| |
|
| |
|
|
Diluted | | $ | 0.17 | | $ | 0.27 | | $ | 1.28 |
| | |
|
| |
|
| |
|
|
None of the Company’s outstanding common shares were vested as of December 31, 2001. Under the provisions of Statement of Financial Accounting Standards (“SFAS”) No. 128, “Earnings Per Share,” such shares are considered contingently returnable and are included in basic earnings per share only when issuance of the shares is no longer contingent. Because all of the outstanding common shares as of December 31, 2001 were unvested and subject to repurchase, net loss per common share is not meaningful. Pro forma basic and diluted net loss per common share for the period ended December 31, 2001 is computed assuming that all of the Company’s common shares are fully vested and no longer subject to repurchase as of December 31, 2001. Basic and diluted pro forma net loss per share amounts are equivalent for the period ended December 31, 2001 as the inclusion of common stock equivalents in the number of shares used for the diluted computation would be anti-dilutive. Pro forma weighted average common shares outstanding for the period ended December 31, 2001 excludes 14,000,000 shares of common stock issuable upon the conversion of Series A convertible preferred stock and 345,500 shares of common stock issuable upon the exercise of stock options.
F-11
XCEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
Accounting for Stock-Based Compensation
The Company accounts for stock options granted to its employees and members of its board of directors under the intrinsic value method in accordance with Accounting Principles Board Opinion No. 25 (“APB No. 25”), “Accounting for Stock Issued to Employees,” and related interpretations, and with the disclosure requirements of SFAS No. 123, “Accounting for Stock-Based Compensation,” as amended by SFAS No. 148, “Accounting for Stock-Based Compensation—Transition and Disclosure.” The following table illustrates the effect on net income (loss) and net income (loss) per share if the Company had applied the fair value recognition provisions of SFAS No. 123 to stock-based employee compensation (in thousands, except per share amounts).
| | | January 24,
2001
(Inception)
Through
December 31,
2001
| | | Year Ended
December 31,
2002
| | | Six Months Ended June 30,
| |
| | | | | 2002
| | | 2003
| |
| | | | | | | | | (unaudited) | | | (unaudited) | |
Net income (loss), as reported | | $ | (9,516 | ) | | $ | 3,461 | | | $ | 5,048 | | | $ | 29,131 | |
Add: Stock-based employee compensation expense included in reported net income (loss), net of related tax effects | | | 94 | | | | 214 | | | | 109 | | | | 110 | |
Deduct: Total stock-based employee compensation expense determined under the fair value based method, net of related tax effects | | | (134 | ) | | | (333 | ) | | | (162 | ) | | | (228 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Pro forma net income (loss) | | $ | (9,556 | ) | | $ | 3,342 | | | $ | 4,995 | | | $ | 29,013 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net income (loss) per share: | | | | | | | | | | | | | | | | |
Pro forma basic and diluted—as reported | | $ | (2.12 | ) | | | | | | | | | | | | |
| | |
|
|
| | | | | | | | | | | | |
Pro forma basic and diluted—pro forma | | $ | (2.13 | ) | | | | | | | | | | | | |
| | |
|
|
| | | | | | | | | | | | |
Basic—as reported | | | | | | $ | 2.13 | | | $ | 3.87 | | | $ | 11.38 | |
| | | | | | |
|
|
| |
|
|
| |
|
|
|
Basic—pro forma | | | | | | $ | 2.06 | | | $ | 3.83 | | | $ | 11.33 | |
| | | | | | |
|
|
| |
|
|
| |
|
|
|
Diluted—as reported | | | | | | $ | 0.17 | | | $ | 0.27 | | | $ | 1.28 | |
| | | | | | |
|
|
| |
|
|
| |
|
|
|
Diluted—pro forma | | | | | | $ | 0.16 | | | $ | 0.26 | | | $ | 1.27 | |
| | | | | | |
|
|
| |
|
|
| |
|
|
|
The Company accounts for stock options granted to non-employee consultants in accordance with the provisions of SFAS No. 123 and Emerging Issues Task Force (“EITF”) Issue No. 96-18, “Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services,” which require that such equity instruments are recorded at their fair value on the measurement date. The measurement of stock-based compensation is subject to periodic adjustment as the underlying equity instruments vest.
F-12
XCEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
Income Taxes
The Company accounts for income taxes in its annual reporting based on SFAS No. 109, “Accounting for Income Taxes.” SFAS No. 109 is an asset and liability approach that requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the Company’s financial statements or tax returns. Measurement of the deferred items is based on enacted tax laws. Valuation allowances are established, when necessary, to reduce future income tax assets to the amount expected to be realized. During interim periods, the Company accounts for income taxes based on APB No. 28, “InterimFinancial Reporting.” Under APB No. 28, the Company makes its best estimate of the effective tax rate the Company expects to be applicable for the full fiscal year, which is used in providing for income taxes for the current year-to-date period.
New Accounting Pronouncement
In December 2002, the Financial Accounting Standards Board issued SFAS No. 148 as an amendment to SFAS No. 123. SFAS No. 148 provides alternative methods of transition for a voluntary change to the fair value based method of accounting for stock-based employee compensation. In addition, SFAS No. 148 amends the disclosure requirements of SFAS No. 123 to require prominent disclosures in both annual and interim financial statements about the method of accounting for stock-based compensation and the effect of the method used on reported results. The voluntary transition and amended disclosure requirements are effective for fiscal years ending after December 15, 2002. The interim reporting requirements are effective for interim periods beginning after December 15, 2002. The Company accounts for employee stock-based compensation under the intrinsic value method in accordance with APB No. 25. The Company did not voluntarily change its method of accounting, but did implement the disclosure requirements.
Reclassifications
Certain prior period amounts have been reclassified to conform with the current period presentation.
F-13
XCEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
2. Balance Sheet Details (in thousands)
| | | December 31,
| | | June 30,
2003
| |
| | | 2001
| | | 2002
| | |
| | | | | | | | | (unaudited) | |
Accounts receivable: | | | | | | | | | | | | |
Trade accounts receivable | | $ | 3,015 | | | $ | 4,772 | | | $ | 5,755 | |
Allowance for doubtful accounts | | | (100 | ) | | | (156 | ) | | | (156 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Accounts receivable, net | | $ | 2,915 | | | $ | 4,616 | | | $ | 5,599 | |
| | |
|
|
| |
|
|
| |
|
|
|
Inventories: | | | | | | | | | | | | |
Raw materials | | $ | — | | | $ | 884 | | | $ | 461 | |
Finished goods | | | 3,891 | | | | 5,492 | | | | 5,786 | |
Samples | | | — | | | | 549 | | | | 424 | |
| | |
|
|
| |
|
|
| |
|
|
|
Total inventories | | $ | 3,891 | | | $ | 6,925 | | | $ | 6,671 | |
| | |
|
|
| |
|
|
| |
|
|
|
Furniture and equipment: | | | | | | | | | | | | |
Office furniture and equipment | | $ | 252 | | | $ | 293 | | | $ | 341 | |
Manufacturing equipment | | | 500 | | | | 508 | | | | 1,014 | |
Computer hardware and software | | | 353 | | | | 405 | | | | 489 | |
Accumulated depreciation | | | (189 | ) | | | (639 | ) | | | (813 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Furniture and equipment, net | | $ | 916 | | | $ | 567 | | | $ | 1,031 | |
| | |
|
|
| |
|
|
| |
|
|
|
Product rights: | | | | | | | | | | | | |
Trademarks, trade names, patent and other | | $ | 148,181 | | | $ | 194,141 | | | $ | 194,141 | |
Accumulated amortization | | | (5,808 | ) | | | (14,353 | ) | | | (19,200 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Trademarks, tradenames, patent and other, net | | $ | 142,373 | | | $ | 179,788 | | | $ | 174,941 | |
| | |
|
|
| |
|
|
| |
|
|
|
Migranal patent | | $ | — | | | $ | 3,000 | | | $ | 3,000 | |
Accumulated amortization | | | — | | | | (200 | ) | | | (400 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Migranal patent, net | | $ | — | | | $ | 2,800 | | | $ | 2,600 | |
| | |
|
|
| |
|
|
| |
|
|
|
Total product rights | | $ | 148,181 | | | $ | 197,141 | | | $ | 197,141 | |
Total accumulated amortization | | | (5,808 | ) | | | (14,553 | ) | | | (19,600 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Product rights, net | | $ | 142,373 | | | $ | 182,588 | | | $ | 177,541 | |
| | |
|
|
| |
|
|
| |
|
|
|
Accrued expenses and other current liabilities: | | | | | | | | | | | | |
Product return allowance | | $ | 1,132 | | | $ | 4,382 | | | $ | 5,079 | |
Product acquisition payable | | | — | | | | 3,912 | | | | 4,155 | |
Rebate reimbursement payable | | | — | | | | 3,036 | | | | 5,757 | |
Rebate obligation | | | 1,512 | | | | 2,320 | | | | 2,345 | |
Employee compensation and benefits | | | 1,604 | | | | 1,422 | | | | 1,401 | |
Accrued royalties | | | 603 | | | | 862 | | | | 1,501 | |
Chargeback obligation | | | 488 | | | | 451 | | | | 463 | |
Product financing obligation | | | 313 | | | | 242 | | | | — | |
Obligation for product inventories | | | 5,170 | | | | — | | | | — | |
Other | | | 471 | | | | 1,360 | | | | 1,177 | |
| | |
|
|
| |
|
|
| |
|
|
|
Total accrued expenses and other current liabilities | | $ | 11,293 | | | $ | 17,987 | | | $ | 21,878 | |
| | |
|
|
| |
|
|
| |
|
|
|
F-14
XCEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
3. Long-Term Debt
Long-term debt as of December 31, 2001 and 2002 and June 30, 2003 is comprised of the following (in thousands):
| | | December 31,
| | | June 30, 2003
| |
| | | 2001
| | 2002
| | |
| | | | | | | | (unaudited) | |
Product acquisition notes payable | | $ | 99,000 | | $ | 99,000 | | | $ | — | |
Credit line note payable | | | — | | | 10,000 | | | | — | |
Senior term loan | | | — | | | — | | | | 62,000 | |
Less current portion | | | — | | | (1,875 | ) | | | (2,000 | ) |
| | |
|
| |
|
|
| |
|
|
|
Long-term debt, less current portion | | $ | 99,000 | | $ | 107,125 | | | $ | 60,000 | |
| | |
|
| |
|
|
| |
|
|
|
Product Acquisition and Credit Line Notes Payable
Under an agreement with Elan, effective March 31, 2003, the Company retired the $99.0 million of product acquisition notes payable due Elan it incurred in connection with its acquisition of Diastat and Mysoline and repaid the $10.0 million outstanding on its credit line note payable with Elan with a cash payment to Elan of $71.5 million. The Company recognized a gain on the retirement of the product acquisition notes payable of $37.4 million. The product acquisition notes accrued interest at 7% per annum with payments of interest only due quarterly through March 31, 2004. Interest and principal payments were then due quarterly through December 31, 2007 with a final balloon payment due March 31, 2008. The credit line note accrued interest at 7% per annum with payments of interest only due quarterly through March 31, 2003. Interest and principal payments were then due quarterly through March 31, 2007. The Company’s ability to access further borrowings under the credit line facility with Elan terminated in June 2002.
In connection with this debt, the Company had granted to Elan a security interest in all the assets the Company purchased from Elan. Elan was also protected by various loan covenants and other rights that, if breached by the Company, even in the absence of a payment default, would have entitled Elan to foreclose on its security interest and reacquire the purchased assets without payment or refund to the Company. These covenants included not taking any of the following actions without Elan’s consent: engaging in a material line of business outside of the pharmaceutical business; selling, leasing or disposing of substantially all of the Company’s assets or any of the collateral; acquiring all or substantially all of the assets of others; merging with or consolidating into other parties; and making certain distributions to its stockholders. Beginning with the quarter ended June 30, 2002, the Company was also required to comply with certain financial covenants requiring the maintenance of minimum levels of cash, working capital, and earnings before interest, taxes, depreciation and amortization. As of December 31, 2002, the Company was in compliance with these covenants. The Company had also agreed to limit its borrowings from third parties without Elan’s consent. Generally, the Company could not have had debt outstanding, other than the debt owed to Elan and debt incurred for product acquisitions, in excess of $6.5 million. The terms of the repayment agreement released all security and collateral interests granted to Elan under the product acquisition financing agreements. The Company has no further obligations under these agreements.
Senior Term Loan
On March 31, 2003, concurrent with the repayment of its debt to Elan, the Company entered into a $62.0 million senior term loan agreement with a new lender. Interest on the term loan is due monthly on the
F-15
XCEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
outstanding balance at prime (with a minimum of 6.5%) plus 4.5%. The coupon rate was 11% through June 30, 2003. Quarterly principal payments of $2.0 million are due June 30, 2004 through December 31, 2007 with a final balloon payment of $32.0 million due on March 31, 2008. Pursuant to the terms of the agreement, the Company also makes monthly administrative fee payments of $15,000 and will be required to make a $930,000 payment on each anniversary of the loan agreement until the loan is repaid. Debt issuance costs incurred in connection with this transaction were $2.4 million, which are being amortized to interest expense ratably over the term of the loan. The Company will repay a portion of this debt with a portion of the proceeds from its initial public offering.
The term loan agreement is secured by an interest in all of the Company’s assets. The lender is protected by various financial and operating covenants. The financial covenants include maintaining certain leverage ratios, fixed charge coverage ratios and minimum cash on had amounts. In addition, the agreement contains certain restrictions on asset acquisitions, disposals and indebtedness. A breach of the covenants and restrictions would entitle the lender to foreclose on its security interest without payment or refund to the Company, even in the absence of a payment default.
As of June 30, 2003, principal payments under long-term debt are due as follows (in thousands):
| Year ending December 31, | | |
2004 | | $ | 6,000 |
2005 | | | 8,000 |
2006 | | | 8,000 |
2007 | | | 8,000 |
2008 | | | 32,000 |
| | |
|
|
Total | | $ | 62,000 |
| | |
|
|
Interest expense in connection with long-term debt agreements was $4.0 million and $7.3 million for the period ended December 31, 2001 and the year ended December 31, 2002, respectively; and $3.5 million and $3.8 million for the six months ended June 30, 2002 and 2003, respectively.
F-16
XCEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
4. Product Agreements
Diastat and Mysoline
On March 31, 2001 and April 1, 2001, the Company entered into product acquisition agreements with Elan for the purchase of Diastat and Mysoline, respectively. Under the terms of the agreements, the Company made initial cash payments of $61.0 million and incurred $99.0 million in long-term debt for the acquisition of the product rights and certain manufacturing equipment. The Company allocated $500,000 of the purchase price to the manufacturing equipment. The product acquisition agreements provided for quarterly purchase price adjustments through the quarter ended December 31, 2001 to the extent the Company’s reported product revenues differed from product revenues based on total prescriptions for the Company’s products. The Company accounted for the change in amortization related to these purchase price adjustments prospectively from the date of adjustment. The Company recorded a $12.4 million reduction in the purchase price related to this provision with $10.9 million and $1.5 million of cash received during the period ended December 31, 2001 and the year ended December 31, 2002, respectively. As part of the product acquisition agreements, the Company also acquired $5.2 million in product inventories, with payment made in March 2002.
Under the terms of the acquisition agreements, Elan was responsible for the payment and processing of rebates, chargebacks, and returns on Diastat and Mysoline sold by Elan prior to the acquisition of the products by the Company up to contractually specified amounts and time periods. As of January 1, 2003, Elan is no longer responsible for the payment and processing of rebates, chargebacks, and returns related to sales of Diastat and Mysoline by Elan prior to the Company’s acquisition of these products. The Company recorded a $1.0 million liability upon the acquisition of the products representing the Company’s estimate of rebates, chargebacks and returns that would be incurred after the contractual processing periods that related to product sold by Elan.
Diastat and Mysoline were the Company’s initial products. Prior to the acquisition of Diastat and Mysoline, the Company had no substantive operations. The results of Diastat and Mysoline are included in the Company’s statements of operations since April 1, 2001. The purchase price allocation was as follows (in thousands):
Tradenames, trademarks, patent, other product rights | | $ | 148,181 | |
Product inventories | | | 5,170 | |
Manufacturing equipment | | | 500 | |
| | |
|
|
|
Total assets | | | 153,851 | |
Liability for rebates, chargebacks, and returns | | | (1,035 | ) |
| | |
|
|
|
Total acquisition | | $ | 152,816 | |
| | |
|
|
|
The product rights are being amortized over a period of 20 years. The unaudited pro forma summary below presents certain financial information as if the acquisition of Diastat and Mysoline had occurred as of January 1, 2001. These pro forma results have been prepared for comparative purposes and do not purport to be indicative of what would have occurred had the acquisition been made on January 1, 2001. Additionally, these pro forma results are not indicative of future results (in thousands, except per share data):
| | | Year Ended
December 31,
2001
| |
| | | (unaudited) | |
Net sales | | $ | 22,897 | |
| | |
|
|
|
Net loss | | $ | (8,522 | ) |
| | |
|
|
|
Pro forma basic and diluted net loss per share | | $ | (1.90 | ) |
| | |
|
|
|
F-17
XCEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
In December 2002, Elan reported $3.9 million of rebates, chargebacks and returns that were processed and paid by Elan that were above the designated caps or after the contractually specified periods. While such amounts are subject to audit by the Company, the Company recorded a product acquisition charge of $3.5 million for the year ended December 31, 2002 representing the $3.9 million liability less $400,000 which was included by the Company in the initial product acquisition accrual. During the six months ended June 30, 2003, the Company accrued $243,000 for additional returns and chargebacks reported by Elan that were above the designated caps or after the contractually specified periods. The Company had accrued for this in the initial product acquisition accrual. These additional returns and chargebacks increased the product acquisition liability to $4.1 million as of June 30, 2003.
In addition, Elan has processed and paid rebates on sales made by the Company on inventories the Company acquired from Elan in connection with the acquisition of the products. During 2001, 2002 and the six months ended June 30, 2003, the Company recorded an estimated liability for these rebates with a corresponding reduction to gross sales upon the sale of the products by the Company. In December 2002, Elan reported $3.0 million of rebates that had been processed and paid on the Company’s behalf. The Company recorded the amount as a rebate reimbursement payable as of December 31, 2002. During the six months ended June 30, 2003, the Company accrued $2.8 million for additional rebates Elan processed and paid on the Company’s behalf resulting in a $5.8 million rebate reimbursement payable as of June 30, 2003. On August 5, 2003, the Company paid Elan $9.6 million in satisfaction of the $4.1 million product acquisition liability and $5.8 million rebate reimbursement payable accrued by the Company as of June 30, 2003. Payment of agreed upon liabilities subsequent to June 30, 2003 are due to Elan 30 days after the Company’s receipt of an invoice from Elan.
In March 2003, the Company also terminated the financing agreement the Company and Elan entered into in June 2001, whereby Elan agreed to make payments to the Company based on the net sales of Elan’s product Zanaflex and the Company agreed to make payments to Elan based on the net sales of Mysoline. The Company recognized a gain of $246,000 in connection with the termination of this agreement. Neither Elan nor the Company has any further rights or obligations under the financing agreement.
Under this agreement, the Company had agreed to establish and maintain a well-trained sales force and to use commercially reasonable efforts to co-promote Zanaflex in a tertiary position for a 12-month period that ended June 30, 2002. The payments from Elan to the Company were based on 5% to 10% of Zanaflex net sales subject to contractually agreed upon maximum annual payments ranging from $1.5 million to $4.5 million. The payments from the Company to Elan were 20% of the first $8.0 million of Mysoline annual net sales and 5% of Mysoline annual net sales between $8.0 million and $25.0 million.
The Company accounted for this transaction as a financing. The factors considered in making this determination included the following: the disproportionate level of payments the Company was to receive compared to the nominal co-promotion support required by the agreement (as indicated by the fact that the agreement did not require any minimum sales presentations, reports of co-promotion support or other measurable performance criteria) and the Company did not provide any co-promotion support for Zanaflex. In addition, the Company believed the payment streams were highly predictable and therefore implicitly limited Elan’s rate of return based on the royalty rates, royalty caps and sales thresholds, the fact that the payments the Company were to receive were non-refundable and were payable irrespective of whether the Company co-promoted Zanaflex, and the requirement that both parties were to pay royalties on a substitute product if the initial products were unavailable for sale. This financing had an imputed interest rate of approximately 7% based on the Company’s estimates of the total net payments over the term of the financing. The Company periodically reviewed the actual payment streams and its estimates of projected payments to determine whether any adjustment to the imputed interest rate was appropriate.
F-18
XCEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
During the period ended December 31, 2001, the Company received $500,000 from Elan and made payments of $193,000 to Elan under this financing arrangement. During the year ended December 31, 2002, the Company received $1.0 million from Elan and made payments of $1.1 million to Elan. The net obligation and accrued interest payable of $313,000 and $242,000 as of December 31, 2001 and 2002, respectively, is included on the balance sheets in product financing within other current liabilities. The Company recorded accrued interest expense of $6,000 and $11,000 for the period ended December 31, 2001 and the year ended December 31, 2002, respectively, which is included in interest expense in the statements of operations.
Migranal and D.H.E. 45
On June 25 2002, the Company entered into an asset purchase agreement with Novartis for the purchase of Migranal and D.H.E. 45. Under the terms of the agreement, the Company made a cash payment of $47.7 million for the acquisition of the product rights. The Company also acquired $1.8 million in product inventories under a separate supply agreement.
Under the terms of the acquisition agreement, Novartis was responsible for the payment and processing of rebates, chargebacks, and returns related to sales of the products by Novartis prior to the acquisition of the products by the Company through contractually specified time periods. As of January 1, 2003, Novartis is no longer responsible for the payment and processing of rebates, chargebacks, and returns related to sales of Migranal and D.H.E. 45 by Novartis prior to the Company’s acquisition of these products. The Company recorded a $1.1 million liability upon the acquisition of the products as an estimate of its liability for such items after the contractually specified periods that related to product sold by Novartis. However, the Company’s actual results could differ from this estimate.
The acquisition of Migranal and D.H.E. 45 expanded the Company’s product base in the treatment of CNS disorders. The acquisition also leverages the Company’s sales efforts as many of the Company’s targeted prescribers of Diastat and Mysoline also prescribe Migranal and D.H.E. 45. The results of Migranal and D.H.E. 45 are included in the Company’s statements of operations since July 1, 2002. The purchase price allocation was as follows (in thousands):
Tradenames, trademarks, other product rights | | $ | 45,960 | |
Migranal patent | | | 3,000 | |
| | |
|
|
|
Total product rights | | | 48,960 | |
Product inventories | | | 1,798 | |
| | |
|
|
|
Total assets | | | 50,758 | |
Liability for rebates, chargebacks, and returns | | | (1,125 | ) |
| | |
|
|
|
Total acquisition | | $ | 49,633 | |
| | |
|
|
|
F-19
XCEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
The patent is being amortized over a period of seven years, while the remaining product rights are being amortized over a period of 20 years. This results in a weighted average amortization period for the Migranal and D.H.E. 45 product rights of 18.1 years. The unaudited pro forma summary below presents certain financial information as if the acquisition of Migranal and D.H.E. 45 had occurred as of January 1, 2001 and January 1, 2002. These pro forma results have been prepared for comparative purposes and do not purport to be indicative of what would have occurred had the acquisition been made on January 1, 2001 or January 1, 2002. Additionally, these pro forma results are not indicative of future results (in thousands, except per share data):
| | | Year Ended December 31,
|
| | | 2001
| | | 2002
|
| | | (unaudited) | | | (unaudited) |
Net sales | | $ | 26,053 | | | $ | 57,513 |
| | |
|
|
| |
|
|
Net income (loss) | | $ | (2,495 | ) | | $ | 5,431 |
| | |
|
|
| |
|
|
Diluted net income per share | | | | | | $ | 0.24 |
| | | | | | |
|
|
Pro forma basic and diluted net loss per share | | $ | (0.56 | ) | | | |
| | |
|
|
| | | |
MT 300
On September 3, 2003, the Company acquired the exclusive commercial rights in the U.S. to MT 300 from Pozen. MT 300, an injection in a pre-filled syringe, was developed by Pozen for the treatment of migraine and is a highly purified formulation of dihydroergotamine that is patented through March 2020. Pozen submitted a new drug application to the FDA in December 2002 to market MT 300 for the treatment of migraine, which is under review by the FDA. The Company paid an upfront fee of $2.0 million to Pozen with potential additional milestone payments of $8.0 million due upon certain regulatory approvals and the achievement of a predetermined net sales threshold on MT 300. The Company will also pay royalties to Pozen on the combined net sales of MT 300 and D.H.E. 45 once MT 300 is commercialized. Royalty rates on the combined annual net sales of MT 300 and D.H.E. 45 will commence in the low double digits and increase based on the achievement of predetermined net sales thresholds.
5. Stockholders’ Equity
Convertible Preferred Stock
On March 30, 2001, the Company raised net proceeds of $69.6 million through the issuance of 14,000,000 shares of Series A preferred stock at $5.00 per share. On June 24, 2002, the Company raised net proceeds of $22.4 million through the issuance of 3,750,000 shares of Series B preferred stock at $6.00 per share. On March 31, 2003, the Company raised net proceeds of $25.9 million through the issuance of 4,000,000 shares of Series C preferred stock at $6.50 per share. Each share of preferred stock is entitled to one vote. The preferred stock is convertible into common stock on a one-for-one basis and automatically converts upon a public offering of the Company’s common stock at a per share price of not less than $10.00 and an aggregate offering price of not less than $60.0 million.
In addition, the Series A, B and C preferred stockholders have a liquidation preference of $10.00 per share, $12.00 per share and $13.00 per share, respectively, in the event of a liquidation, dissolution or winding up of the Company, anti-dilution protection in the event of certain future issuances of equity at a price below the conversion price, an annual dividend of $0.35 per share, $0.42 per share and $0.455 per share for the Series A, B and C preferred stockholders, respectively, payable in preferred stock to the extent the preferred stock has not been converted on or before September 30, 2003, protective provisions requiring the approval of a majority of the
F-20
XCEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
preferred stockholders for various events impacting the rights of the preferred stockholders, and rights of first refusal for certain preferred stockholders on future sales of equity.
Upon completion of the distribution in full of the preferred liquidation preferences described above, the remaining assets of the Company would be distributed ratably among the holders of common stock until such holders have received an aggregate distribution of $60.0 million. Any remaining assets would be ratably distributed among the common and preferred stockholders.
On March 31, 2003, the Company repurchased the 3,000,000 shares of Series A preferred stock owned by Elan for $18.0 million. As of December 31, 2001 and 2002, respectively, Elan had a 16.0% and 13.0% ownership interest in the Company based on outstanding common and preferred stock. In June 2001, the Company entered into a letter agreement with Elan pursuant to which Elan agreed to purchase an amount of shares in connection with an offering of the Company’s common stock, including an initial public offering, such that, following the offering, Elan would own not less than 15.0% of the Company’s outstanding capital stock on a fully-diluted basis. This letter agreement was also terminated on March 31, 2003.
Common Stock
Each share of common stock is entitled to one vote. The holders of common stock are also entitled to receive dividends whenever funds are legally available and when declared by the board of directors, subject to the prior rights of holders of all preferred stock.
During the period from inception through March 2, 2001, the Company issued 4,520,000 shares of common stock to its founders at $0.0025 per share under restricted stock purchase agreements. Under the terms of the purchase agreements, the Company has the option to repurchase unvested shares of stock upon the termination of the holder’s services to the Company at the purchase price. The number of shares subject to repurchase is reduced by 1/4th of the initial number subject to repurchase after one year of service and 1/48th of such number for each subsequent month that services are provided to the Company. Issued shares of common stock subject to repurchase totaled 4,728,975, 2,376,100 and 1,811,190 as of December 31, 2001 and 2002 and June 30, 2003, respectively.
6. Stock Options
2001 Stock Plan
The Company’s 2001 Stock Plan (the “2001 Plan”) provides for the grant of incentive and nonqualified stock options to officers, directors, employees, and consultants. The Company reserved 1,480,000 shares of common stock for issuance under the 2001 Plan. The Company’s board of directors has the sole authority to determine the meaning and application of the terms of the 2001 Plan, the persons to whom option grants are made, the nature and amount of option grants, the price to be paid upon exercise of each option, the period within which options may be exercised, and the other terms and conditions of grants. The 2001 Plan will terminate in March 2011. The stock options have a ten-year term and can be exercised anytime after the grant date, with 1/4 th of the shares vesting after one year of service and1/48th of the shares vesting each subsequent month that services are provided to the Company. Unvested shares acquired pursuant to option exercises are subject to repurchase by the Company upon termination of the holder’s service with the Company at the lower of fair value or the purchase price. As of December 31, 2001 and 2002 and June 30, 2003, 208,975, 188,608 and 155,587 shares acquired pursuant to options granted were subject to repurchase.
F-21
XCEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
The Company has granted stock options as follows:
| | | Shares
| | | Weighted Average Exercise Price
|
Granted | | 563,625 | | | $ | 0.64 |
Canceled | | (9,150 | ) | | $ | 0.52 |
Exercised | | (208,975 | ) | | $ | 0.58 |
| | |
|
| | | |
Outstanding and exercisable at December 31, 2001 | | 345,500 | | | $ | 0.68 |
Granted | | 455,053 | | | $ | 7.91 |
Canceled | | (51,822 | ) | | $ | 7.49 |
Exercised | | (43,987 | ) | | $ | 0.56 |
| | |
|
| | | |
Outstanding and exercisable at December 31, 2002 | | 704,744 | | | $ | 4.86 |
Granted (unaudited) | | 360,700 | | | $ | 5.00 |
Canceled (unaudited) | | (20,234 | ) | | $ | 7.18 |
Exercised (unaudited) | | (6,018 | ) | | $ | 6.10 |
| | |
|
| | | |
Outstanding and exercisable at June 30, 2003 (unaudited) | | 1,039,192 | | | $ | 4.86 |
| | |
|
| | | |
Shares reserved for issuance under the 2001 Plan were 925,525, 522,294 and 181,828 as of December 31, 2001 and 2002 and June 30, 2003, respectively.
The following table sets forth the range of exercise prices, number of shares, weighted average exercise price, and remaining contractual lives by similar price and grant date as of December 31, 2002 and June 30, 2003.
| | | | | Outstanding
|
Range of Exercise Price
| | Outstanding at December 31,
2002
| | Weighted Average
Remaining
Contractual Life
| | Weighted Average
Exercise Price
|
$0.50–$1.00 | | 285,315 | | 8.6 years | | $ | 0.71 |
$5.00–$8.00 | | 274,685 | | 9.7 years | | $ | 6.26 |
$9.45–$10.70 | | 144,744 | | 9.2 years | | $ | 10.38 |
| | |
| | | | | |
Total | | 704,744 | | 9.1 years | | $ | 4.86 |
| | |
| | | | | |
| | |
| | | | | Outstanding
|
Range of Exercise Price
| | Outstanding at June 30, 2003
| | Weighted Average
Remaining
Contractual Life
| | Weighted Average
Exercise Price
|
$0.50–$1.00 | | 283,349 | | 8.1 years | | $ | 0.71 |
$5.00–$8.00 | | 620,350 | | 9.2 years | | $ | 5.55 |
$9.45–$10.70 | | 135,493 | | 8.7 years | | $ | 10.36 |
| | |
| | | | | |
Total | | 1,039,192 | | 8.9 years | | $ | 4.86 |
| | |
| | | | | |
Stock-based Compensation
From the Company’s inception through January 30, 2002, the Company issued certain stock options to its employees and members of its board of directors under the 2001 Plan with exercise prices below the deemed fair value of the Company’s common stock at the date of grant. In accordance with the requirements of APB 25, the Company recorded deferred stock-based compensation for the difference between the exercise price of the stock
F-22
XCEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
options and the deemed fair value of the Company’s stock at the date of grant. This deferred stock-based compensation is amortized to selling, general and administrative expense on a straight-line basis over the period during which the Company’s right to repurchase the stock lapses or the options become vested, generally four years. The Company recorded deferred stock-based compensation related to these options in the amount of $1.5 million, net of cancellations, of which $94,000 and $365,000 was amortized to expense for the period ended December 31, 2001 and the year ended December 31, 2002, respectively, and $186,000 and $178,000 was amortized to expense for the six months ended June 30, 2002 and 2003, respectively.
Pro forma information regarding net income (loss) and net income (loss) per common share is required by SFAS No. 123 as if the Company had accounted for its employee stock options under the fair value method. The table in the Company’s policy for accounting for stock-based compensation in note 1 illustrates the pro forma net income (loss) and net income (loss) per share as if the Company had accounted for stock option grants to employees under the fair value recognition provisions of SFAS No. 123. For purposes of SFAS No. 123 pro forma disclosures, the estimated fair value of the options is amortized to expense over the options’ vesting period. The estimated weighted average fair value at grant date was $2.87 per share and $2.43 per share for options granted for the period ended December 31, 2001 and the year ended December 31, 2002, respectively, and $2.94 per share and $1.75 per share for the six months ended June 30, 2002 and 2003, respectively. The fair value was estimated at the dates of grant using the Black-Scholes option valuation model with the following weighted-average assumptions:
| | | 2001
| | 2002
| | 2003
|
Risk free interest rate | | 4.50% | | 3.10% | | 2.75% |
Dividend yield | | — | | — | | — |
Volatility | | 50% | | 40% | | 40% |
Weighted average expected life | | 4 years | | 4 years | | 4 years |
The Black-Scholes option valuation model was developed for use in estimating the fair value of traded options that have no vesting restrictions and are fully transferable. In addition, option valuation models require the input of highly subjective assumptions including the expected stock price volatility. Because the Company’s employee stock options have characteristics significantly different from those of traded options, and because changes in the subjective input assumptions can materially affect the fair value estimate, in management’s opinion, the existing models do not necessarily provide a reliable single measure of the fair value of its employee stock options.
Stock-based compensation expense related to stock options granted to non-employee consultants is recognized pursuant to the provisions of EITF 96-18 as the stock options are earned. The Company granted options to purchase 13,000, 20,000 and 10,500 shares of common stock during the period ended December 31, 2001, the year ended December 31, 2002 and the six months ended June 30, 2003, respectively, to non-employee consultants for services rendered that generally vest over two to four years. The Company believes that the fair value of the stock options is more reliably measurable than the fair value of the services received. The fair value of the stock options granted is calculated at each reporting date using the Black-Scholes option pricing model as prescribed by SFAS No. 123 using the previously detailed assumptions. The stock-based compensation expense will fluctuate as the deemed fair value of the common stock fluctuates. In connection with the grant of stock options to non-employee consultants, the Company deferred and amortized to selling, general and administrative expense $38,000 and $37,000 for the period ended December 31, 2001 and the year ended December 31, 2002, respectively and $34,000 and $16,000 for the six months ended June 30, 2002 and 2003, respectively.
F-23
XCEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
7. Income Taxes
The income tax expense (benefit) for the year ended December 31, 2002 consisted of the following (in thousands):
| | | Year Ended December 31, 2002
| |
Current: | | | | |
Federal | | $ | — | |
State | | | 293 | |
| | |
|
|
|
Total current | | | 293 | |
| | |
|
|
|
Deferred: | | | | |
Federal | | | (2,765 | ) |
State | | | (912 | ) |
| | |
|
|
|
Total deferred | | | (3,677 | ) |
| | |
|
|
|
Total income tax benefit | | $ | (3,384 | ) |
| | |
|
|
|
A reconciliation of the income tax expense computed using the federal statutory rate on pre-tax income and the income tax benefit as recognized in the statement of operations for the year ended December 31, 2002 is as follows (in thousands):
| | | Year Ended
December 31, 2002
| |
Income tax computed using federal statutory rate of 34% | | $ | 26 | |
State income tax | | | 3 | |
Permanent items | | | 182 | |
Adjustment of estimated income tax accruals | | | 395 | |
Change in valuation allowance, federal only | | | (3,990 | ) |
| | |
|
|
|
Income tax benefit | | $ | (3,384 | ) |
| | |
|
|
|
The Company did not recognize any current or deferred federal or state income tax expense or benefit for the period ended December 31, 2001. A valuation allowance was recognized to fully offset the income tax benefit and net deferred tax assets as of December 31, 2001 as realization of such benefit was uncertain. The Company reversed the valuation allowance in 2002 based on management’s estimate of future taxable income.
The Company had federal and California income tax loss carryforwards of approximately $4.0 million and $6.4 million as of December 31, 2001 and 2002, respectively. The federal and California tax loss carryforwards will begin to expire in 2021 and 2013, respectively, unless previously utilized. Due to changes in the California tax law during 2002, the net operating losses generated in 2001 cannot be utilized until 2004. The Tax Reform Act of 1986 limits the use of net operating loss carryforwards in the case of an “ownership change” of a corporation. Any ownership changes, as defined, may restrict utilization of the Company’s carryforwards.
F-24
XCEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
Significant components of the Company’s deferred tax assets and liabilities as of December 31, 2001 and 2002 are as shown below (in thousands).
| | | December 31,
|
| | | 2001
| | | 2002
|
Deferred tax assets: | | | | | | | |
Net operating loss carryforward | | $ | 3,676 | | | $ | 1,960 |
Accrued expenses | | | 950 | | | | 2,590 |
Uniform capitalization | | | 156 | | | | 205 |
Other, net | | | 323 | | | | 263 |
| | |
|
|
| |
|
|
Total deferred tax assets | | | 5,105 | | | | 5,018 |
| | |
|
|
| |
|
|
Deferred tax liabilities: | | | | | | | |
Purchased intangible assets | | | 1,052 | | | | 1,271 |
Fixed assets | | | 9 | | | | — |
Prepaid expenses | | | 54 | | | | 70 |
| | |
|
|
| |
|
|
Total deferred tax liabilities | | | 1,115 | | | | 1,341 |
| | |
|
|
| |
|
|
Net deferred tax assets | | | 3,990 | | | | 3,677 |
Valuation allowance for deferred tax assets | | | (3,990 | ) | | | — |
| | |
|
|
| |
|
|
| | | $ | — | | | $ | 3,677 |
| | |
|
|
| |
|
|
As of June 30, 2003, the Company had current net deferred tax assets of $2.8 million and long-term net deferred tax liabilities of $13.1 million. The long-term net deferred tax liability represents the deferred tax liability of $14.0 million resulting from the Company’s $37.4 million gain on the retirement of long-term debt (see note 3) net of long-term net deferred tax assets of $912,000.
8. Commitments and Contingencies
Office Lease
The Company leases its facility under a non-cancelable operating lease that expires May 31, 2006. Rent expense was $172,000 and $295,000 for the period ended December 31, 2001 and the year ended December 31, 2002, respectively, and $153,000 and $145,000 for the six months ended June 30, 2002 and 2003, respectively.
The total minimum future commitments under the lease are as follows (in thousands):
| Year ending December 31, | | |
2003 | | $ | 293 |
2004 | | | 305 |
2005 | | | 317 |
2006 | | | 135 |
| | |
|
|
Total | | $ | 1,050 |
| | |
|
|
Product Manufacturing Agreements
The Company uses third-party contractors to manufacture and package its products. The agreements extend through various periods ranging from June 2005 to December 2009. The manufacturing agreements provide for
F-25
XCEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
procurement of raw materials, manufacture and release of finished products and stability testing at contractually agreed upon prices that increase annually based on changes to the Consumer Price Index. The Company has no minimum purchase requirements under these arrangements. During 2002, the Company entered into a supply agreement with a new manufacturer for Mysoline that expires in December 2009. The Company is in the process of transferring the manufacturing of Mysoline to the new supplier.
9. Retirement Plan
In 2001, the Company established a tax qualified defined contribution 401(k) plan for which generally all employees are eligible. The annual contribution, if any, to the trust is at the discretion of the board of directors of the Company. The board of directors approved a contribution of $18,000 to the plan for 2001, which was funded during the year ended December 31, 2002 and a contribution of $43,000 to the plan for 2002, which was funded during the six months ended June 30, 2003.
10. Quarterly Financial Information (Unaudited)
The following table sets forth summary quarterly financial information for the period January 24, 2001 (inception) through December 31, 2001, the year ended December 31, 2002 and the six months ended June 30, 2003 (in thousands, except per share amounts).
2001
| | Inception
Through
March 31
| | June 30
| | | September 30
| | | December 31
| |
Net sales | | $ | — | | $ | 1,795 | | | $ | 3,854 | | | $ | 8,475 | |
Operating loss | | $ | — | | $ | (2,608 | ) | | $ | (3,160 | ) | | $ | (425 | ) |
Net loss | | $ | — | | $ | (2,858 | ) | | $ | (4,594 | ) | | $ | (2,064 | ) |
Pro forma net loss per common share: | | | | | | | | | | | | | | | |
Basic and diluted | | $ | — | | $ | (0.63 | ) | | $ | (1.00 | ) | | $ | (0.44 | ) |
Weighted average shares | | | 4,520 | | | 4,520 | | | | 4,590 | | | | 4,664 | |
| | | | |
2002
| | March 31
| | June 30
| | | September 30
| | | December 31
| |
Net sales | | $ | 11,629 | | $ | 11,415 | | | $ | 11,693 | | | $ | 15,318 | |
Operating income (loss) | | $ | 2,722 | | $ | 2,924 | | | $ | 1,815 | | | $ | (352 | ) |
Net income (loss) | | $ | 1,088 | | $ | 3,960 | | | $ | (36 | ) | | $ | (1,551 | ) |
Net income (loss) per common share: | | | | | | | | | | | | | | | |
Basic | | $ | 0.84 | | $ | 2.67 | | | $ | (0.02 | ) | | $ | (0.74 | ) |
Weighted average shares | | | 1,297 | | | 1,485 | | | | 1,791 | | | | 2,099 | |
Diluted | | $ | 0.06 | | $ | 0.21 | | | $ | (0.02 | ) | | $ | (0.74 | ) |
Weighted average shares | | | 19,069 | | | 19,167 | | | | 1,791 | | | | 2,099 | |
| | | | |
2003
| | March 31
| | June 30
| | | | | | | |
Net sales | | $ | 19,085 | | $ | 20,057 | | | | | | | | | |
Operating income | | $ | 6,461 | | $ | 7,099 | | | | | | | | | |
Net income | | $ | 24,644 | | $ | 4,487 | | | | | | | | | |
Net income per common share: | | | | | | | | | | | | | | | |
Basic | | $ | 10.23 | | $ | 1.65 | | | | | | | | | |
Weighted average shares | | | 2,409 | | | 2,713 | | | | | | | | | |
Diluted | | $ | 1.09 | | $ | 0.19 | | | | | | | | | |
Weighted average shares | | | 22,576 | | | 23,598 | | | | | | | | | |
F-26
XCEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
During the quarter ended June 30, 2002, the Company recognized a tax benefit of $2.7 million resulting from the reversal of the valuation allowance the Company had recorded against the Company’s deferred tax assets as of December 31, 2001 (see note 7). During the quarter ended December 31, 2002, the Company recorded pre-tax charges of $991,000 related to the costs incurred in an abandoned initial public offering (see note 1) and $3.5 million related to the Company’s acquisition of Diastat and Mysoline (see note 4). During the quarter ended March 31, 2003, the Company recorded a pre-tax gain of $37.4 million on the retirement of long-term debt (see note 3).
11. Subsequent Events
March 2003
On March 31, 2003, with a payment of $89.5 million, the Company retired its $99.0 million of product acquisition notes payable due Elan it incurred in connection with its acquisition of Diastat and Mysoline, repaid the $10.0 million outstanding on its credit facility with Elan, repurchased Elan’s 3,000,000 shares of the Company’s Series A convertible preferred stock and terminated a royalty-based financing arrangement. The Company recorded a gain of $37.4 million on the retirement of the product acquisition notes payable and $246,000 on the termination of the royalty-based financing arrangement. Concurrently, the Company raised $62.0 million of senior debt with a new lender and issued 4,000,000 shares of its Series C convertible preferred stock to investors other than Elan resulting in net proceeds of $25.9 million. See notes 3, 4 and 5 for further description of these transactions.
August 2003
On August 5, 2003, the Company paid Elan $9.6 million in satisfaction of the $4.1 million product acquisition liability and $5.8 million rebate reimbursement payable accrued by the Company as of June 30, 2003. Payment of agreed upon liabilities subsequent to June 30, 2003 are due to Elan 30 days after the Company’s receipt of an invoice from Elan. See note 4 for further description of these liabilities.
September 2003
On September 3, 2003, the Company acquired the exclusive commercial rights in the U.S. to MT 300 from Pozen. See note 4 for further description.
F-27
DIASTAT AND MYSOLINE PRODUCTS
INDEPENDENT AUDITORS’ REPORT
To the Board of Directors of Xcel Pharmaceuticals, Inc.:
We have audited the accompanying Statements of Product Contribution for the Diastat and Mysoline products (the “Products”) of Elan Corporation, plc (“Elan”) for the year ended December 31, 2000 and the three months ended March 31, 2001. These special purpose financial statements are the responsibility of Elan’s management. Our responsibility is to express an opinion on these special purpose financial statements based on our audits.
We conducted our audits in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
As discussed in Note 1, the accompanying Statements of Product Contribution are not intended to be a complete presentation of the Products’ results of operations. Accordingly, the resulting Statements of Product Contribution are not necessarily indicative of the costs and expenses that would have resulted if the Products had been operated as a separate independent entity. The operations covered by the Statements of Product Contribution have no separate legal status or existence.
In our opinion, such statements present fairly, in all material respects, the product contribution of the Products for the year ended December 31, 2000 and the three months ended March 31, 2001 in conformity with accounting principles generally accepted in the United States of America.
DELOITTE & TOUCHE LLP
San Diego, California
December 14, 2001
F-28
DIASTAT AND MYSOLINE PRODUCTS
STATEMENTS OF PRODUCT CONTRIBUTION
| | | Year Ended
December 31,
2000
| | Three Months
Ended March 31,
2001
|
| | | (in thousands) |
Net sales | | $ | 42,131 | | $ | 8,773 |
Costs and expenses: | | | | | | |
Cost of sales | | | 7,381 | | | 1,608 |
Selling, general and administrative | | | 13,382 | | | 2,269 |
Research and development | | | 2,072 | | | 354 |
| | |
|
| |
|
|
Total costs and expenses | | | 22,835 | | | 4,231 |
| | |
|
| |
|
|
Product contribution | | $ | 19,296 | | $ | 4,542 |
| | |
|
| |
|
|
The accompanying notes are an integral part of the Statements of Product Contribution.
F-29
DIASTAT AND MYSOLINE PRODUCTS
NOTES TO STATEMENTS OF PRODUCT CONTRIBUTION
1. Description of Business and Basis of Presentation
Affiliates of Elan Corporation, plc (“Elan”) sold all of their rights, title and interest in Diastat® and Mysoline® (the “Products”) to Xcel Pharmaceuticals, Inc. (“Xcel”) on March 31, 2001 and April 1, 2001, respectively. Diastat was approved by the U.S. Food and Drug Administration (“FDA”) in July 1997 and launched by Elan in October 1997. Diastat is the only drug approved in the United States for the acute treatment of seizures outside of a hospital setting. Mysoline was acquired by Elan in February 1998. Mysoline is an oral prescription medication that has been used since the early 1950’s for the chronic treatment of seizures associated with epilepsy.
The accompanying Statements of Product Contribution were prepared for the purpose of complying with the rules and regulations of the U.S. Securities and Exchange Commission for inclusion in the Registration Statement on Form S-1 of Xcel, and are not intended to be a complete presentation of the Products’ results of operations. The operations covered by these special purpose financial statements have no separate legal status or existence. Elan has not prepared financial statements of the Products, which would be intended to report a complete presentation of financial position, results of operations and cash flows in accordance with accounting principles generally accepted in the United States of America. All of the estimates in the Statements of Product Contribution as described in Note 2 are based on assumptions Elan’s management believes are reasonable. Accordingly, the accompanying Statements of Product Contribution do not purport to present the results of operations of the Products that would have resulted if the Products had been operated as a separate independent entity.
2. Summary of Significant Accounting Policies
Use of Estimates
The preparation of these special purpose financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect certain reported amounts. Accordingly, actual results could differ from those estimates.
Revenue Recognition
The Products are sold to wholesale drug distributors who generally sell the Products to retail pharmacies, hospitals, and other institutional customers. Revenues are recognized when the Products are received by the wholesaler, which represents the point when the risks and rewards of ownership are transferred to the customer. Elan invoices wholesalers at its wholesale list price. Sales are shown net of discounts, rebates, chargebacks, and returns specifically identifiable to the Products. The reserve for rebates, chargebacks, and returns is estimated based on historical experience and other relevant information in accordance with accounting principles generally accepted in the United States of America. Chargebacks represent the difference between the wholesale list price and the estimated contractual sales price.
Cost of Sales
Cost of sales includes primarily third-party product manufacturing costs, distribution costs, and royalties specifically identifiable to the Products. Distribution costs principally include freight and warehousing charges. Royalties are due third parties under separate royalty arrangements ranging from 5% to 10% of net sales.
F-30
DIASTAT AND MYSOLINE PRODUCTS
NOTES TO STATEMENTS OF PRODUCT CONTRIBUTION
Selling, General and Administrative Expenses
Selling, general and administrative expenses include the costs of selling, marketing and promoting the Products specifically identifiable to the Products. In addition, such costs include an allocation from Elan Pharmaceuticals, a division of Elan, for company-wide sales and marketing costs and operations management costs that are not specifically identifiable to the Products. Such costs are allocated to the Products based on annual plans of action for sales force utilization and marketing focus.
Selling, general and administrative expenses also include an allocation of administrative costs, such as finance, human resources, legal, information systems, and other corporate affairs of Elan Pharmaceuticals.
Research and Development Expense
Research and development expense includes the costs of certain clinical trials and other development activities specifically identifiable to the Products. In addition, such costs include an allocation from Elan Pharmaceuticals for company-wide research and development costs and research administration costs that are not specifically identifiable to the Products. Such costs are allocated to the Products based on actual time spent on the Products.
F-31
MIGRANAL AND D.H.E. 45 PRODUCTS
REPORT OF INDEPENDENT ACCOUNTANTS
To the Board of Directors of Novartis Pharmaceuticals Corporation:
We have audited the accompanying historical statements of product contribution for the years ended December 31, 2000 and 2001 and the period January 1, 2002 through June 25, 2002 of the Migranal and D.H.E. 45 Products of Novartis Pharmaceuticals Corporation (the “Products” or the “Company”). These historical statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these historical statements based upon our audits.
We conducted our audits of these statements in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the statements of product contribution are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in these historical statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
As discussed in Note 1, the accompanying historical statements of product contribution were prepared for the purpose of complying with the rules and regulations of the U.S. Securities and Exchange Commission and are not intended to be a complete presentation of the Product’s results of operation.
In our opinion, the historical statements referred to above present fairly, in all material respects, the product contribution of the Products for the years ended December 31, 2000 and 2001 and the period January 1, 2002 through June 25, 2002 in conformity with the accounting principles generally accepted in the United States of America.
PricewaterhouseCoopers LLP
Florham Park, NJ
August 6, 2002
F-32
MIGRANAL AND D.H.E. 45 PRODUCTS
STATEMENTS OF PRODUCT CONTRIBUTION
| | | Year Ended
December 31,
| | Period January 1 to
June 25,
|
| | | 2000
| | 2001
| | 2001
| | 2002
|
| | | | | | | (unaudited) | | |
| | | (in thousands) |
Net Sales | | $ | 13,080 | | $ | 11,929 | | $ | 5,826 | | $ | 7,458 |
Costs and expenses: | | | | | | | | | | | | |
Cost of sales | | | 1,758 | | | 1,435 | | | 756 | | | 802 |
Selling, general and administrative | | | 185 | | | 43 | | | 16 | | | 47 |
Litigation and product liability | | | 1,421 | | | 732 | | | 205 | | | 1,891 |
| | |
|
| |
|
| |
|
| |
|
|
Total costs and expenses | | | 3,364 | | | 2,210 | | | 977 | | | 2,740 |
| | |
|
| |
|
| |
|
| |
|
|
Product contribution | | $ | 9,716 | | $ | 9,719 | | $ | 4,849 | | $ | 4,718 |
| | |
|
| |
|
| |
|
| |
|
|
The accompanying notes are an integral part of the Statements of Product Contribution.
F-33
MIGRANAL AND D.H.E. 45 PRODUCTS
NOTES TO STATEMENTS OF PRODUCT CONTRIBUTION
1. Description of Business and Basis of Presentation
On June 25, 2002, Novartis Pharma AG and Novartis Pharmaceuticals Corporation (collectively “the Company”) sold all of their rights, title, and interest for Migranal Nasal Spray (“Migranal”) and D.H.E. 45 Injection (“DHE 45”) (the “Products”) within the United States of America to Xcel Pharmaceuticals, Inc. DHE 45 and Migranal were approved in the mid 1940s and December 1997, respectively, for the treatment of moderate to severe migraine headaches.
Historically, financial statements were not prepared for the Products, as the Company did not maintain the Products as a separate business unit. These statements have been developed from the historical accounting records of the Company and represent the revenues and directly related expenses, only, of the Products and do not purport to represent all the costs, expenses and results associated with a stand alone, separate company. As a result, there are no allocations for costs such as corporate overhead, taxes and interest, among others. All of the estimates in the financial statements, as described in note 2, are based on assumptions that Company management believes are reasonable. However, these estimates are not necessarily indicative of the revenues and directly related expenses that would have resulted if the Products had been operated as a separate entity. The accompanying historical Statements of Product Contribution were prepared for the purpose of complying with the rules and regulations of the U.S. Securities and Exchange Commission and are not intended to be a complete presentation of the Products results of operations.
The Statements of Product Contribution have been prepared in accordance with accounting principles generally accepted in the United States of America.
2. Summary of Significant Accounting Policies
Use of Estimates
The preparation of the Statements of Product Contribution in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect certain reported amounts. Actual results could differ from those estimates. Such estimates include Medicaid rebates, managed care rebates, chargebacks and sales returns.
Interim Unaudited Financial Information
The Statement of Product Contribution for the period January 1, 2001 through June 25, 2001 is unaudited; however, in the opinion of the management of the Company, all adjustments (consisting solely of normal recurring adjustments) necessary for a fair presentation of the unaudited financial statements for this interim period have been included. The results of this interim period are not necessarily indicative of results to be obtained for a full year.
Revenue Recognition
Sales and related cost of sales are recognized as revenue and expense when goods are shipped and title, risk of loss and ownership passes to the customer. Provision is made at the time of sale for discounts and estimated sales allowances and returns.
Rebates, Chargebacks, Cash Discounts and Sales Returns
The Company entered into contracts with certain managed care organizations to provide access to the Products through formularies. Based on the managed care organization’s market share performance and
F-34
MIGRANAL AND D.H.E. 45 PRODUCTS
NOTES TO STATEMENTS OF PRODUCT CONTRIBUTION—(Continued)
utilization of the Products, the organization received managed care rebates from the Company. In addition, the Company was bound by certain laws and regulations to provide the Products at a discounted rate to Medicaid recipients. Medicaid rebates were paid to each state in the United States based on claims filed by pharmacies that provide the Products to the Medicaid recipients at the reduced rate. Managed care and Medicaid rebates were charged to the Products monthly, on an accrual basis, determined by estimating the actual usage of the Products by Medicaid recipients and managed care participants covered by the contracted prices.
Chargebacks are amounts credited to wholesalers to reimburse the wholesaler for sales to third parties at reduced prices based on contracts the Company negotiated. Chargebacks were accrued based on historical experience.
The Company offered a cash discount to customers if invoices were paid within a certain time period. The cash discounts were applied to each product on the invoice proportionately.
Anticipated sales returns were charged to the Products based upon historical experience.
Cost of Sales
Cost of sales includes the costs to manufacture the Products, which consists of raw materials, direct labor, plant overhead and manufacturing variances. Certain of these costs were specifically identifiable to the Products, and the remaining costs were allocated based on production measures. Management believes this method was a reasonable basis for allocating these costs. Certain efforts in the manufacturing of the Products were performed by an affiliate of the Company and a mark up was charged for these efforts.
Selling, General and Administrative Expenses
Selling, general and administrative expenses include the direct costs of advertising, promoting and distributing the Products. During the periods presented, the Company did not maintain a sales force dedicated to the Products. However, the Company did incur certain general advertising and promotion expenses, primarily involving samples, professional advertising and other promotional activities. The Company did not incur any research and development costs during the periods.
Significant Customers
The Products are subject to certain customer concentration risk related to net sales. Three customers represented the following percentages of total sales of the Products:
| | | Year Ended
December 31,
| | Period January 1
to June 25,
|
| | | 2000
| | 2001
| | 2001
| | 2002
|
| | | | | | | (unaudited) | | |
Customer A | | 30% | | 30% | | 30% | | 22% |
Customer B | | 18% | | 22% | | 22% | | 22% |
Customer C | | 18% | | 20% | | 24% | | 21% |
3. Related Party Transactions
The Company purchased certain raw materials from Novartis Pharma AG, a related party. Included in cost of sales are $128 and $80 of material cost associated with these purchases for the years ended December 31,
F-35
MIGRANAL AND D.H.E. 45 PRODUCTS
NOTES TO STATEMENTS OF PRODUCT CONTRIBUTION—(Continued)
2000 and 2001, respectively, and $48 and $61 for the period January 1, 2001 to June 25, 2001 and the period January 1, 2002 to June 25, 2002, respectively.
4. Litigation and Product Liability
The Products are, from time to time, involved in litigation, including product liability claims, arising out of the ordinary course of business. Litigation and product liability expenses include those costs, consisting only of legal expenses and settlement payments, related to product liability cases involving the Products during the periods. The majority of such costs are the legal expenses incurred by the Company in vigorously defending these cases. The Company has not recorded any expenses related to loss contingencies related to any litigation. The Company is responsible for all litigation and product liability claims related to its ownership of the Products.
F-36
XCEL PHARMACEUTICALS, INC.
UNAUDITED PRO FORMA STATEMENT OF OPERATIONS
Novartis Pharmaceuticals Corporation (“Novartis”) sold all of their rights, title and interest in Migranal® and D.H.E. 45® (the “Products”) on June 25, 2002 to Xcel Pharmaceuticals, Inc. (the “Company”). The Products are prescribed for the treatment of migraine.
The following Unaudited Pro Forma Statement of Operations for the year ended December 31, 2002 has been prepared to give effect to the Company’s acquisition of the Products, as if the acquisition had occurred on January 1, 2002. The pro forma adjustments are based upon available information and certain assumptions that the Company believes are reasonable in the circumstances.
The Unaudited Pro Forma Statement of Operations should be read in conjunction with the Company’s historical Financial Statements and related Notes thereto, Management’s Discussion and Analysis of Financial Condition and Results of Operations, the Migranal and D.H.E. 45 Statements of Product Contribution, and other financial information included elsewhere in this prospectus. The operating results of the Products for the period January 1, 2002 through June 25, 2002 are derived from the internal financial statements of Novartis and represent the revenues and directly related expenses, only, of the Products and do not purport to represent all the costs, expenses and results associated with a stand alone, separate company. The Unaudited Pro Forma Statement of Operations and related notes are provided for information purposes only and do not purport to be indicative of the results which would have actually been obtained had the Products been acquired on the date indicated or which may be expected to occur in the future.
F-37
XCEL PHARMACEUTICALS, INC.
UNAUDITED PRO FORMA STATEMENT OF OPERATIONS
(in thousands, except per share data)
| | | The Company for the Year
Ended
December 31,
2002
| | | The Products
for the
Period from January 1, 2002 through June 25,
2002
| | Pro Forma
Adjustments
| | | Pro
Forma
| |
Net sales | | $ | 50,055 | | | $ | 7,458 | | $ | — | | | $ | 57,513 | |
Operating costs and expenses: | | | | | | | | | | | | | | | |
Cost of sales | | | 8,835 | | | | 802 | | | — | | | | 9,637 | |
Selling, general and administrative | | | 20,275 | | | | 1,938 | | | — | | | | 22,213 | |
Product development | | | 588 | | | | — | | | — | | | | 588 | |
Costs of abandoned initial public offering | | | 991 | | | | — | | | — | | | | 991 | |
Product acquisition charge | | | 3,512 | | | | — | | | — | | | | 3,512 | |
Product rights amortization | | | 8,745 | | | | — | | | 1,349 | | | | 10,094 | |
| | |
|
|
| |
|
| |
|
|
| |
|
|
|
Total operating costs and expenses | | | 42,946 | | | | 2,740 | | | 1,349 | | | | 47,035 | |
| | |
|
|
| |
|
| |
|
|
| |
|
|
|
Operating income (loss) | | | 7,109 | | | | 4,718 | | | (1,349 | ) | | | 10,478 | |
| | |
|
|
| |
|
| |
|
|
| |
|
|
|
Other income (expense): | | | | | | | | | | | | | | | |
Interest expense | | | (7,327 | ) | | | — | | | — | | | | (7,327 | ) |
Interest income | | | 295 | | | | — | | | — | | | | 295 | |
| | |
|
|
| |
|
| |
|
|
| |
|
|
|
Total other income (expense) | | | (7,032 | ) | | | — | | | — | | | | (7,032 | ) |
| | |
|
|
| |
|
| |
|
|
| |
|
|
|
Income (loss) before income taxes | | | 77 | | | | 4,718 | | | (1,349 | ) | | | 3,446 | |
Income tax expense (benefit) | | | (3,384 | ) | | | — | | | 1,399 | | | | (1,985 | ) |
| | |
|
|
| |
|
| |
|
|
| |
|
|
|
Net income (loss) | | $ | 3,461 | | | $ | 4,718 | | $ | (2,748 | ) | | $ | 5,431 | |
| | |
|
|
| |
|
| |
|
|
| |
|
|
|
Net income per common share: | | | | | | | | | | | | | | | |
Basic | | $ | 2.13 | | | | | | | | | | $ | 3.34 | |
| | |
|
|
| | | | | | | | |
|
|
|
Diluted | | $ | 0.17 | | | | | | | | | | $ | 0.24 | |
| | |
|
|
| | | | | | | | |
|
|
|
Weighted average common shares outstanding: | | | | | | | | | | | | | | | |
Basic | | | 1,625 | | | | | | | | | | | 1,625 | |
| | |
|
|
| | | | | | | | |
|
|
|
Diluted | | | 20,867 | | | | | | | 1,875 | | | | 22,742 | |
| | |
|
|
| | | | |
|
|
| |
|
|
|
See Notes to Unaudited Pro Forma Statement of Operations.
F-38
XCEL PHARMACEUTICALS, INC.
NOTES TO UNAUDITED PRO FORMA STATEMENT OF OPERATIONS
1. Basis of Presentation
The results for the Company are for the year ended December 31, 2002 as presented in the Company’s audited historical financial statements for that period included elsewhere in this prospectus. The results for the Products are for the period January 1, 2002 through June 25, 2002 as presented in the audited Migranal and D.H.E. 45 Statements of Product Contribution for that period included elsewhere in this prospectus.
2. Pro forma Adjustments
Product Rights Amortization
As described in Note 1 of the Company’s Notes to Financial Statements, the Company is amortizing the product rights acquired over the estimated period to be benefited of seven to 20 years. The adjustment represents the additional amortization the Company would have recorded had the Company acquired the Products on January 1, 2002.
Income Taxes
The adjustment represents the application of the Company’s statutory tax rate for 2002 of 41.5% to the incremental pro forma income before income taxes.
Weighted Average Common Shares Outstanding
The Company paid for the Products through the use of cash generated from its operations and the issuance of 3,750,000 shares of Series B convertible preferred stock. The preferred stock was issued on June 24, 2002. The adjustment represents the additional weighted average common shares the Company would have included in the computation of fully diluted net income per common share had the Company acquired the Products on January 1, 2002.
F-39
Shares

Common Stock
PROSPECTUS
SG Cowen
CIBC World Markets
Thomas Weisel Partners LLC
U.S. Bancorp Piper Jaffray
, 2003
Until , all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This requirement is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the costs and expenses, other than underwriting discounts and commissions, payable by the registrant in connection with the sale of common stock being registered. All amounts are estimates except the registration fee, the NASD filing fee and The Nasdaq National Market entry fee.
| | | Amount
to be Paid
|
SEC Registration Fee | | $ | 6,068 |
NASD Filing Fee | | | 8,000 |
Nasdaq National Market Listing Fee | | | 130,000 |
Printing and Engraving | | | * |
Legal Fees and Expenses | | | * |
Accounting Fees and Expenses | | | * |
Blue Sky Fees and Expenses | | | * |
Transfer Agent Fees | | | * |
Director & Officer Liability Insurance (1933 Act Premiums) | | | * |
Miscellaneous | | | * |
| | |
|
|
Total | | $ | |
| | |
|
|
| * | | To be filed by amendment. |
Item 14. Indemnification of Directors and Officers
Section 145 of the Delaware General Corporation Law authorizes a court to award, or a corporation’s board of directors to grant, indemnity to directors and officers in terms sufficiently broad to permit such indemnification under certain circumstances for liabilities (including reimbursement for expenses incurred) arising under the Securities Act of 1933, as amended (the “Securities Act”).
As permitted by Delaware General Corporation Law, the Registrant’s certificate of incorporation includes a provision that eliminates the personal liability of its directors for monetary damages for breach of fiduciary duty as a director, except to the extent that exculpation from liability is not permitted under the Delaware General Corporation Law as in effect at the time such liability is determined.
As permitted by the Delaware General Corporation Law, the bylaws of the registrant provide for indemnification of the registrant’s directors, officers, employees and other agents to the extent and under the circumstances permitted by the Delaware General Corporation Law.
The registrant has also entered into agreements with certain of its directors and executive officers that will require the registrant, among other things, to indemnify them against certain liabilities that may arise by reason of their status or service as directors and executive officers to the fullest extent not prohibited by law.
The registrant has purchased directors and officers liability insurance.
Reference is also made to the Underwriting Agreement, which provides for the indemnification of officers, directors and controlling persons of the registrant against certain liabilities.
II-1
Item 15. Recent Sales of Unregistered Securities
During the past three years, the following securities were sold or issued by the Registrant without registration under the Securities Act:
| | 1. | | In January, February and March 2001, the Registrant issued an aggregate of 4,520,000 shares of common stock to nine individuals, at a per share price of $0.0025. Aggregate proceeds from this offering were $11,300. |
| | 2. | | In March 2001, the Registrant issued an aggregate of 14,000,000 shares of its Series A preferred stock to 36 accredited investors for an aggregate purchase price of $70.0 million. |
| | 3. | | In June 2002, the Registrant issued an aggregate of 3,750,000 shares of its Series B preferred stock to 4 accredited investors for an aggregate purchase price of $22.5 million. |
| | 4. | | In March 2003, the Registrant issued an aggregate of 4,000,000 shares of its Series C preferred stock to 15 accredited investors for an aggregate purchase price of $26.0 million. |
| | 5. | | Through August 22, 2003, the Registrant granted options under its stock option plan to purchase an aggregate of 1,390,353 shares, net of cancellations, of its common stock, of which 258,980 options have been exercised at a per share weighted average exercise price of $0.70 per share for an aggregate exercise price of approximately $181,286, to employees, outside directors and consultants of the Registrant. |
The sales of the above securities described in paragraphs 1, 2, 3, 4 and, with respect to paragraph 5, options with respect to 32,000 shares issued to certain consultants (the “Consultant Options”) and options with respect to 75,000 shares that were issued to certain corporate entities under the control of certain directors (the “Director Entity Options”) were exempt from registration under the Securities Act in reliance on Section 4(2) of the Securities Act, or Regulation D promulgated thereunder. The issuance of options and the subsequent exercise of such options, other than the Consultant Options and the Director Entity Options, which are exempt from registration under the Securities Act pursuant to Section 4(2) of the Securities Act or Regulation D promulgated thereunder, were exempt from such registration pursuant to Rule 701 promulgated under Section 3(b) of the Securities Act. The recipients of securities in the transactions noted in all of the paragraphs above represented their intention to acquire the securities for investment only and not with view to or for sale in connection with any distribution thereof and appropriate legends were affixed to the share certificates and instruments issued in such transactions. All recipients had adequate access, through their relationship with the Registrant, to information about the Registrant.
Item 16. Exhibits and Financial Statement Schedule
(a) Exhibits
Exhibit
Number
| | Description
|
| |
| 1.1 | | Form of Underwriting Agreement* |
| |
| 3.1 | | Amended and Restated Certificate of Incorporation of the Registrant (in effect).† |
| |
| 3.2 | | Form of Amended and Restated Certificate of Incorporation of the Registrant (to be adopted upon the closing of this offering)† |
| |
| 3.3 | | Bylaws of Registrant (in effect)† |
| |
| 3.4 | | Form of Bylaws of the Registrant (to be adopted upon the closing of this offering)† |
| |
| 3.5 | | Audit Committee Charter of the Registrant |
| |
| 4.1 | | Form of Stock Certificate† |
| |
| 4.2 | | Form of Restricted Stock Purchase Agreement between the Registrant and Michael T. Borer, John R. Cook, James L. Fares, Cam L. Garner and George M. Stuart† |
| |
| 4.3 | | Investors’ Rights Agreement, dated as of March 30, 2001, by and among the Registrant and the parties named therein† |
II-2
| |
| 4.4 | | 2001 Stock Plan† |
| |
| 4.5 | | Form of 2003 Stock Incentive Plan† |
| |
| 5.1 | | Opinion of Pillsbury Winthrop LLP* |
| |
| 10.1 | | Omnibus Amendment and Termination Agreement by and among the Registrant, Elan Pharmaceuticals, Inc., Elan Pharma International Limited and Elan International Services, Ltd. dated as of March 31, 2003.† |
| |
| 10.2 | | Amended and Restated Diastat Asset Purchase Agreement dated as of March 31, 2001, by and among Elan Pharmaceuticals, Inc., Elan Pharma International Limited and the Registrant.**, † |
| |
| 10.3 | | Amended and Restated Mysoline Asset Purchase Agreement dated as of April 1, 2001, by and among Elan Pharma International Limited and the Registrant.**, † |
| |
| 10.4 | | Asset Purchase Agreement dated as of June 25, 2002 by and among the Registrant, Novartis AG and Novartis Pharmaceuticals Corporation.** |
| |
| 10.5 | | Financing Agreement by and among the Registrant and Regiment Capital III, L.P. dated as of March 28, 2003† |
| |
| 10.6 | | Form of Directors and Officers’ Indemnity Agreement.† |
| |
| 10.7 | | Collaboration and License Agreement by and between the Registrant and Pozen Inc. dated as of September 3, 2003.** |
| |
| 23.1 | | Consent of Deloitte & Touche LLP, Independent Auditors. |
| |
| 23.2 | | Consent of Pillsbury Winthrop LLP (contained in their opinion filed as Exhibit 5.1). |
| |
| 23.3 | | Consent of PricewaterhouseCoopers LLP, Independent Accountants. |
| |
| 24.1 | | Power of attorney.† |
| * | | To be filed by amendment |
| ** | | The Registrant has applied (or will apply) for Confidential Treatment with respect to portions of this Exhibit. An unredacted version of this Exhibit has been filed separately with the U.S. Securities and Exchange Commission. |
II-3
(b) Financial Statement Schedule
SCHEDULE II
XCEL PHARMACEUTICALS, INC.
VALUATION AND QUALIFYING ACCOUNTS
In thousands
| | | Balance at
Beginning of
Period
| | Additions
| | Deductions(a)
| | | Balance at End of
Period
|
Period January 24, 2001 (inception) through December 31, 2001: | | | | | | | | | | | | | |
Allowance for doubtful accounts | | $ | — | | $ | 100 | | $ | — | | | $ | 100 |
Accrued product returns | | | — | | | 1,132 | | | — | | | | 1,132 |
Year ended December 31, 2002: | | | | | | | | | | | | | |
Allowance for doubtful accounts | | | 100 | | | 92 | | | (36 | ) | | | 156 |
Accrued product returns | | | 1,132 | | | 3,312 | | | (62 | ) | | | 4,382 |
Six months ended June 30, 2003 (unaudited): | | | | | | | | | | | | | |
Allowance for doubtful accounts | | | 156 | | | — | | | — | | | | 156 |
Accrued product returns | | | 4,382 | | | 1,852 | | | (1,155 | ) | | | 5,079 |
(a) Provision charged to earnings
Item 17. Undertakings
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the U.S. Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933, as amended and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
The undersigned registrant hereby undertakes to provide the underwriter at the closing specified in the underwriting agreements, certificates in such denominations and registered in such names as required by the underwriter to permit prompt delivery to each purchaser.
II-4
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the Registrant has duly caused this Amendment No. 1 to this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of San Diego, State of California, on the twenty-ninth day of September 2003.
| XCEL PHARMACEUTICALS, INC. |
| |
By: | | /s/ MICHAEL BORER
|
| | | Michael T. Borer Chief Executive Officer, President and Director |
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed below by the following persons in the capacities and on the dates indicated.
Name
| | Title
| | Date
|
| | |
/s/ MICHAEL BORER
Michael T. Borer | | Chief Executive Officer, President and Director (Principal Executive Officer) | | September 29, 2003 |
| | |
/s/ GEORGE STUART*
George M. Stuart | | Vice President, Finance, Chief Financial Officer and Treasurer (Principal Financial Officer and Principal Accounting Officer) | | September 29, 2003 |
| | |
/s/ JAMES C. BLAIR*
James C. Blair, Ph.D. | | Director | | September 29, 2003 |
| | |
/s/ CARLOS A. FERRER*
Carlos A. Ferrer | | Director | | September 29, 2003 |
| | |
/s/ CAM GARNER*
Cam L. Garner | | Chairman of the Board and Director | | September 29, 2003 |
| | |
/s/ DAVID F. HALE*
David F. Hale | | Director | | September 29, 2003 |
| | |
/s/ WILLIAM R. RINGO, JR.*
William R. Ringo, Jr. | | Director | | September 29, 2003 |
| | |
/s/ COREY A. SEALE*
Corey A. Seale | | Director | | September 29, 2003 |
| | |
/s/ SIGRID VAN BLADEL*
Sigrid Van Bladel, Ph.D. | | Director | | September 29, 2003 |
| |
| *by: | | /s/ MICHAEL T. BORER |
| |
|
| | | Michael T. Borer Attorney-in-fact |
II-5
EXHIBIT INDEX
Exhibit
Number
| | Description
|
| |
| 1.1 | | Form of Underwriting Agreement.* |
| |
| 3.1 | | Amended and Restated Certificate of Incorporation of the Registrant (in effect).† |
| |
| 3.2 | | Form of Amended and Restated Certificate of Incorporation of the Registrant (to be adopted upon the closing of this offering).† |
| |
| 3.3 | | Bylaws of Registrant (in effect).† |
| |
| 3.4 | | Form of Bylaws of the Registrant (to be adopted upon the closing of this offering).† |
| |
| 3.5 | | Audit Committee Charter of the Registrant. |
| |
| 4.1 | | Form of Stock Certificate.† |
| |
| 4.2 | | Form of Restricted Stock Purchase Agreement between the Registrant and Michael T. Borer, John R. Cook, James L. Fares, Cam L. Garner and George M. Stuart.† |
| |
| 4.3 | | Investors’ Rights Agreement, dated as of March 30, 2001, by and among the Registrant and the parties named therein.† |
| |
| 4.4 | | 2001 Stock Plan.† |
| |
| 4.5 | | Form of 2003 Stock Incentive Plan.† |
| |
| 5.1 | | Opinion of Pillsbury Winthrop LLP.* |
| |
| 10.1 | | Omnibus Amendment and Termination Agreement by and among the Registrant, Elan Pharmaceuticals, Inc., Elan Pharma International Limited and Elan International Services, Ltd. dated as of March 31, 2003.† |
| |
| 10.2 | | Amended and Restated Diastat Asset Purchase Agreement dated as of March 31, 2001, by and among Elan Pharmaceuticals, Inc., Elan Pharma International Limited and the Registrant.**,† |
| |
| 10.3 | | Amended and Restated Mysoline Asset Purchase Agreement dated as of April 1, 2001, by and among Elan Pharma International Limited and the Registrant.**,† |
| |
| 10.4 | | Asset Purchase Agreement dated as of June 25, 2002 by and among the Registrant, Novartis AG and Novartis Pharmaceuticals Corporation.** |
| |
| 10.5 | | Financing Agreement by and among the Registrant and Regiment Capital III, L.P. dated as of March 28, 2003.† |
| |
| 10.6 | | Form of Directors and Officers’ Indemnity Agreement.† |
| |
| 10.7 | | Collaboration and License Agreement by and between the Registrant and Pozen Inc. dated as of September 3, 2003.** |
| |
| 23.1 | | Consent of Deloitte & Touche LLP, Independent Auditors. |
| |
| 23.2 | | Consent of Pillsbury Winthrop LLP (contained in their opinion filed as Exhibit 5.1). |
| |
| 23.3 | | Consent of PricewaterhouseCoopers LLP, Independent Accountants. |
| |
| 24.1 | | Power of attorney.† |
| * | | To be filed by amendment. |
| ** | | The Registrant has applied (or will apply) for Confidential Treatment with respect to portions of this Exhibit. An unredacted version of this Exhibit has been filed separately with the U.S. Securities and Exchange Commission. |

