Table of Contents
As filed pursuant to Rule 424(b)(5)
Under the Securities Act of 1933
Registration No. 333-183285
The information in this preliminary prospectus is not complete and may be changed. A registration statement relating to the notes has become effective under the Securities Act of 1933, as amended. This preliminary prospectus supplement and the accompanying prospectus are not an offer to sell these securities and it are not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
Subject to completion, dated August 13, 2012
Preliminary prospectus supplement

DaVita Inc.
$1,000,000,000
% Senior Notes due 2022
Issue Price %
Interest payable and
We are offering $ million aggregate principal amount of % senior notes due 2022, or the notes. The notes will mature on , 2022. We will pay interest on the notes on and of each year. Interest will accrue on the notes from , 2012 and the first interest payment date will be , 2013.
The notes are being offered to finance a portion of the cash consideration for our merger with HealthCare Partners Holdings, LLC, or HCP. Upon consummation of the offering of the notes, we will deposit the net proceeds (after deducting the underwriting discount) from this offering, together with additional amounts needed to redeem the notes at the special mandatory redemption price described below, into escrow as described in “Description of Notes—Escrow of proceeds; release conditions.” If the conditions to our merger with HCP and certain other conditions are not satisfied on or prior to November 30, 2012, subject to up to three one-month extensions as described herein (which we sometimes refer to as the Escrow End Date), or if we notify the escrow agent that we will not pursue consummation of the merger, the amount deposited in escrow will be applied to redeem all of the notes offered hereby at a special mandatory redemption price equal to 100% of the issue price of the notes, plus accrued and unpaid interest from the date of initial issuance, or the most recent date to which interest has been paid or duly provided for, as the case may be, to but excluding the special mandatory redemption date. If the conditions to our merger with HCP and certain other conditions are satisfied on or before the Escrow End Date, the amounts deposited in escrow will be released to us and applied to finance a portion of the cash consideration for the merger. See “Use of Proceeds” and “Description of Notes—Escrow of proceeds; release conditions” and “Special mandatory redemption.”
We may redeem some or all of the notes at any time on or after , 2017 at redemption prices described in this prospectus supplement and prior to such date at a “make-whole” redemption price described in this prospectus supplement. At any time prior to , 2015, we may also redeem up to 35% of the notes with the net cash proceeds we receive from certain equity offerings at the redemption price set forth in this prospectus supplement. If a change of control occurs as described in this prospectus supplement under the heading “Description of Notes—Change of control”, we may be required to offer to purchase the notes from the holders.
Except as described under “Description of Notes—Escrow of proceeds; release conditions,” the notes will be our unsecured senior obligations and will rank equally with our existing and future unsecured senior indebtedness. The notes will be guaranteed by certain of our domestic subsidiaries. The guarantees will rank equally with all existing and future unsecured senior indebtedness of the guarantors. The notes and guarantees will be effectively subordinated to all of our and the guarantors’ existing and future secured debt (including our senior secured credit facilities) to the extent of the value of the collateral securing such debt and structurally subordinated to all existing and future liabilities of any of our subsidiaries that do not guarantee the notes. The notes will be issued only in registered form in minimum denominations of $2,000 and integral multiples of $1,000 in excess thereof.
Investing in the notes involves risks. See “Risk Factors” beginning on page S-35.
| Public offering price(1) | Underwriting discount | Proceeds, before expenses, to us(1) | ||||||||||
Per note | % | % | % | |||||||||
Total | $ | $ | | | $ | |||||||
| (1) | Plus accrued interest from , 2012, if settlement occurs after that date. |
The notes will not be listed on any securities exchange or quotation system. Currently, there is no public market for the notes.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement or the accompanying prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The notes will be ready for delivery in book-entry form through the facilities of The Depository Trust Company for the accounts of its participants, including Euroclear Bank S.A./N.V. and Clearstream Banking, société anonyme, on or about , .
Joint Book-Running Managers
J.P. Morgan | ||||||||||||||
Barclays | ||||||||||||||
BofA Merrill Lynch | ||||||||||||||
Credit Suisse | ||||||||||||||
Goldman, Sachs & Co. | ||||||||||||||
Morgan Stanley | ||||||||||||||
| SunTrust Robinson Humphrey | ||||||||||||||
Wells Fargo Securities | ||||||||||||||
Co-Managers
Credit Agricole CIB | Mitsubishi UFJ Securities | Scotiabank | SMBC Nikko |
, 2012.
Table of Contents
Prospectus Supplement
| Page | ||||
| S-1 | ||||
| S-2 | ||||
| S-3 | ||||
| S-5 | ||||
| S-35 | ||||
| S-74 | ||||
| S-77 | ||||
| S-78 | ||||
Unaudited Pro Forma Condensed Consolidated Financial Information | S-79 | |||
| S-88 | ||||
| S-90 | ||||
Management’s Discussion and Analysis of Financial Condition and Results of Operations | S-91 | |||
| S-149 | ||||
| S-172 | ||||
| S-188 | ||||
| S-190 | ||||
| S-191 | ||||
| S-193 | ||||
| S-248 | ||||
| S-251 | ||||
| S-255 | ||||
| S-260 | ||||
| S-261 | ||||
| S-261 | ||||
| S-261 | ||||
| S-262 | ||||
| F-1 | ||||
| Prospectus | Page | |||
| 1 | ||||
| 2 | ||||
| 3 | ||||
| 5 | ||||
| 5 | ||||
| 5 | ||||
| 6 | ||||
| 6 | ||||
| 6 | ||||
| 6 | ||||
| 7 | ||||
| 7 | ||||
S-i
Table of Contents
ABOUT THIS PROSPECTUS SUPPLEMENT
This document is in two parts. The first part is this prospectus supplement, which contains the terms of this offering of notes. The second part, the accompanying prospectus dated August 13, 2012, gives more general information, some of which may not apply to this offering.
This prospectus supplement and the information incorporated by reference in this prospectus supplement may add to, update or change the information in the accompanying prospectus. If information in this prospectus supplement is inconsistent with information in the accompanying prospectus, this prospectus supplement will apply and will supersede that information in the accompanying prospectus.
It is important for you to read and consider all information contained or incorporated by reference in this prospectus supplement and the accompanying prospectus in making your investment decision.
No person is authorized to give any information or to make any representations other than those contained or incorporated by reference in this prospectus supplement or the accompanying prospectus and, if given or made, such information or representations must not be relied upon as having been authorized. This prospectus supplement and the accompanying prospectus do not constitute an offer to sell or the solicitation of an offer to buy any securities other than the securities described in this prospectus supplement or an offer to sell or the solicitation of an offer to buy such securities in any circumstances in which such offer or solicitation is unlawful. Neither the delivery of this prospectus supplement and the accompanying prospectus, nor any sale made hereunder, shall under any circumstances create any implication that there has been no change in our affairs since the date of this prospectus supplement, or that the information contained or incorporated by reference in this prospectus supplement or the accompanying prospectus is correct as of any time subsequent to the date of such information.
The distribution of this prospectus supplement and the accompanying prospectus and the offering of the notes in certain jurisdictions may be restricted by law. This prospectus supplement and the accompanying prospectus do not constitute an offer, or an invitation on our behalf or on behalf of the underwriters or any one of them, to subscribe to or purchase any of the notes, and may not be used for or in connection with an offer or solicitation by anyone, in any jurisdiction in which such an offer or solicitation is not authorized or to any person to whom it is unlawful to make such an offer or solicitation. See “Underwriting.”
In this prospectus supplement, unless otherwise stated or the context otherwise requires:
| • | the terms “we,” “us,” “our,” “DaVita” and “Company” refer to DaVita Inc. and, in some instances, its consolidated subsidiaries; |
| • | the term “Financings” refers to this offering of notes and the use of proceeds therefrom, and the expected amendment of and initial borrowings under our senior secured credit facilities; |
| • | the term “HCP” refers to HealthCare Partners Holdings, LLC, together with its consolidated subsidiaries and affiliated physician groups (unless the context otherwise requires); |
| • | the term “Merger” refers to DaVita’s agreement to acquire HCP through a merger of Seismic Acquisition LLC, a California limited liability company and a wholly owned subsidiary of DaVita, with and into HCP, with HCP continuing as the surviving entity in the Merger; |
| • | the term “senior secured credit facilities” means our existing senior secured credit facilities or our amended senior secured credit facilities that we expect will become effective, pursuant to an amendment to our existing senior secured credit facilities prior to the consummation of the Merger, or both, as the context requires. |
If we use a capitalized term in this prospectus supplement and do not define the term in this document, it is defined in the accompanying prospectus.
S-1
Table of Contents
Industry and market data contained or incorporated by reference in this prospectus supplement were obtained through company research, surveys and studies conducted by third parties and industry and general publications or based on our experience in the industry. We have not independently verified market and industry data from third-party sources. While we believe internal company surveys and assumptions are reliable and market definitions are appropriate, neither these surveys and assumptions nor these definitions have been verified by any independent sources and we cannot assure that they are accurate. Our internal company reports have not been verified by any independent source. Statements as to our industry position are based on market data currently available to us. The information in this prospectus supplement concerning HCP is based on information provided to us by HCP’s management. We have not independently verified this information, and, accordingly, the accuracy of this information is not guaranteed. While we are not aware of any misstatements regarding the industry data presented herein, this information involves risks and uncertainties and is subject to change based on various factors, including those discussed under the heading “Risk Factors” in this prospectus supplement.
S-2
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus supplement and the documents deemed to be incorporated by reference in this prospectus supplement contains or may contain statements that are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended and Section 21E of the Securities Exchange Act of 1934, as amended. We intend these forward-looking statements to be covered by the safe harbor provisions for such statements contained in these documents. All statements that do not concern historical facts are forward-looking statements and include, among other things, statements about our expectations, beliefs, intentions and/or strategies for the future. These forward-looking statements include statements regarding anticipated refinancing transactions, our future operations, financial condition and prospects, expectations for treatment growth rates, revenue per treatment, expense growth, levels of the provision for uncollectible accounts receivable, operating income, cash flow, operating cash flow, estimated tax rates, capital expenditures, the development of new centers and center acquisitions, government and commercial payment rates, revenue estimating risk and the impact of our related level of indebtedness on our financial performance, including earnings per share. These statements can sometimes be identified by the use of forward looking words such as “may,” “believe,” “will,” “should,” “could,” “would,” “expect,” “project,” “estimate,” “anticipate,” “plan,” “continue,” “seek,” “forecast,” or “intend” or other similar words or expressions of the negative thereof.
These statements involve substantial known and unknown risks and uncertainties that could cause our actual results to differ materially from those described in the forward-looking statements, including, but not limited to:
| • | risks resulting from uncertainties associated with government regulations, |
| • | general economic and other market conditions, |
| • | competition, |
| • | accounting estimates, |
| • | variability of our cash flows, |
| • | the concentration of profits generated from commercial payor plans, |
| • | continued downward pressure on average realized payment rates from commercial payors, which may result in the loss of revenue or patients, |
| • | a reduction in the number of patients under higher-paying commercial plans, |
| • | a reduction in government payment rates under the Medicare end stage renal disease, or ESRD, program or other government-based programs, |
| • | the impact of health care reform legislation that was enacted in the U.S. in March 2010, |
| • | changes in pharmaceutical or anemia management practice patterns, payment policies, or pharmaceutical pricing, |
| • | our ability to maintain contracts with physician medical directors, |
| • | legal compliance risks, including our continued compliance with complex government regulations, |
| • | current or potential investigations by various governmental entities and related government or private-party proceedings, |
| • | continued increased competition from large and medium-sized dialysis providers that compete directly with us, |
| • | the emergence of new models of care introduced by the government or private sector, such as accountable care organizations, independent practice associations, or IPAs, and integrated delivery systems, and changing affiliation models for physician plans, such as employment by hospitals, that may erode our patient base and reimbursement rates, |
S-3
Table of Contents
| • | our ability to complete any acquisitions or mergers, including the consummation of the Merger, or dispositions that we might be considering or announce, or to integrate and successfully operate any business we may acquire, including the HCP business, or to expand our operations and services to markets outside the U.S., or to businesses outside of dialysis, |
| • | the risk that the Merger could compromise or diminish HCP’s distinctive physician-owned, physician-led culture and business model, including the potential impact on current employees, affiliated physicians and physician groups and IPA consolidation opportunities, |
| • | the risk that the cost of providing services under HCP’s agreements will exceed its compensation, |
| • | the risk that laws regulating the corporate practice of medicine could restrict the manner in which HCP conducts its business, |
| • | the risk that reductions in reimbursement rates and future regulations may negatively impact HCP’s business, revenue and profitability, |
| • | the risk that HCP may not be able to successfully establish a presence in new geographic regions, |
| • | the risk that reductions in the quality ratings of health maintenance organization plan customers of HCP could have an adverse effect on HCP’s business, |
| • | the fact that HCP faces certain competitive threats that could reduce its profitability, |
| • | the risk that health plans that acquire health maintenance organizations may not be willing to contract with HCP or may be willing to contract only on less favorable terms, and |
| • | the risk that a disruption in HCP’s healthcare provider networks could have an adverse effect on HCP’s operations and profitability. |
The forward-looking statements included or incorporated by reference in this prospectus supplement are only made as of the date of this prospectus supplement or the respective document incorporated by reference herein, as applicable. Except as required by law, we undertake no obligation to update or revise these statements, whether as a result of changes in underlying factors, new information, future events or otherwise. See “Where You Can Find More Information.”
S-4
Table of Contents
This summary may not contain all the information that may be important to you. You should read this entire prospectus supplement and the accompanying prospectus, together with the information incorporated by reference herein and therein, including our financial statements and related notes, before making an investment decision. In this summary, we have presented certain financial measures, such as free cash flow, net debt, pro forma Adjusted EBITDA, Adjusted EBITDA, total care dollars under management and metrics derived therefrom, that are non-GAAP financial measures. We are presenting these non-GAAP financial measures because we believe that they provide us and readers of this prospectus supplement with useful supplemental information. We do not intend for these non-GAAP financial measures to be a substitute for any GAAP financial information. See “DaVita Summary Historical Financial and Operating Data” and “HCP Summary Historical Financial and Operating Data” for a reconciliation of these non-GAAP financial measures to their most comparable measure calculated and presented in accordance with GAAP.
DaVita
We are a leading provider of kidney dialysis services in the U.S. for patients suffering from chronic kidney failure, also known as end stage renal disease, or ESRD. As of June 30, 2012, DaVita provided dialysis and other related services through a network of 1,884 outpatient dialysis centers located in the U.S. throughout 43 states and the District of Columbia, serving a total of approximately 149,000 patients. In addition, as of June 30, 2012, DaVita provided outpatient dialysis and administrative service to a total of 19 outpatient dialysis centers located in four countries outside the U.S. DaVita centers offer outpatient hemodialysis treatments and other ESRD-related services such as the administration of physician-prescribed pharmaceuticals, including erythropoietin, or EPO, vitamin D analogs and iron supplements. DaVita also provides services for home dialysis patients, vascular access, disease management services and laboratory services related to ESRD. As of June 30, 2012, DaVita also provides acute inpatient dialysis services in approximately 960 hospitals and related laboratory services throughout the U.S. DaVita is a Delaware corporation, incorporated in 1994.
DaVita’s U.S. dialysis and related lab services business accounted for approximately 92% of DaVita’s consolidated net operating revenues for the twelve months ended June 30, 2012. Other ancillary services and strategic initiatives accounted for approximately 8% of our consolidated net operating revenues for the same period and relate primarily to DaVita’s core business of providing kidney dialysis services. For the twelve months ended June 30, 2012, DaVita generated consolidated net operating revenues of $7,365 million, Adjusted EBITDA of $1,585 million, and net income attributable to DaVita of $519 million. For an explanation of Adjusted EBITDA and a reconciliation of Adjusted EBITDA to net income, see “DaVita Summary Historical Financial and Operating Data” beginning on page S-30.
We provide our services through the following business segments:
Dialysis and Related Lab Services. Our network of 1,884 outpatient dialysis centers located in the U.S. and 19 outpatient dialysis centers located outside the U.S. are designed specifically for outpatient hemodialysis. In the twelve months ended June 30, 2012 our overall network of outpatient dialysis centers increased by 14% primarily as a result of acquisitions and the opening of new centers, net of center closures and divestitures. A large portion of this increase was driven from the acquisition of DSI Renal Inc., or DSI, a medium sized dialysis provider that we acquired in September 2011, that contributed a net 83 outpatient dialysis centers.
Throughout the U.S. we also provided hospital inpatient hemodialysis services, excluding physician services, to patients in approximately 960 hospitals as of June 30, 2012. We render these services for a contracted per-treatment fee that is individually negotiated with each hospital. When a hospital requests our services, we typically administer the dialysis treatment at the patient’s bedside or in a dedicated treatment room in the hospital, as needed. In the twelve months ended June 30, 2012 hospital inpatient hemodialysis services accounted for approximately 4.5% of our total U.S. dialysis treatments.
S-5
Table of Contents
We also own two separately incorporated, licensed, clinical laboratories, which specialize in ESRD patient testing. These specialized laboratories provide routine laboratory tests for dialysis and other physician-prescribed laboratory tests for ESRD patients. Our laboratories provide these tests predominantly for our network of ESRD patients throughout the U.S. These tests are performed to monitor a patient’s ESRD condition, including the adequacy of dialysis, as well as other medical conditions. Our laboratories utilize information systems which provide information to certain members of the dialysis centers’ staff and medical directors regarding critical outcome indicators.
As of June 30, 2012, we operated or provided management and administrative services to 24 outpatient dialysis centers located in the U.S. and three outpatient dialysis centers located outside of the U.S. in which we either own a minority equity investment or which are wholly-owned by third parties. These services are provided pursuant to management and administrative services agreements. Management fees are established by contract and are recognized as earned typically based on a percentage of revenues or cash collections generated by the centers.
Ancillary Services and Strategic Initiatives. Our ancillary services and strategic initiatives consist of pharmacy services, infusion therapy services, disease management services, vascular access services, ESRD clinical research programs, physician services, direct primary care and our international dialysis operations.
DaVita’s Industry
The loss of kidney function is normally irreversible. Kidney failure may be caused by Type I and Type II diabetes, high blood pressure, polycystic kidney disease, long-term autoimmune attack on the kidney and prolonged urinary tract obstruction. Patients suffering from ESRD generally require dialysis at least three times a week for the rest of their lives. Treatment options that we provide for ESRD are hemodialysis and peritoneal dialysis. Hemodialysis, the most common form of ESRD treatment, uses an artificial kidney, called a dialyzer, to remove toxins, fluids and salt from the patient’s blood. The procedure is typically performed at a freestanding center, a hospital-based outpatient center, or at the patient’s home. Peritoneal dialysis uses the patient’s peritoneal or abdominal cavity to eliminate fluid and toxins and is typically performed in the patient’s home.
The dialysis industry is characterized by:
Stable and Growing Patient Base. The nature of ESRD allows for significant demand stability due to a lack of clinical need controversy and limited treatment alternatives for patients. In addition, patients require treatment at least three times a week for the rest of their lives, regardless of seasonality or macroeconomic conditions. According to U.S. Renal Data System, there were approximately 399,000 ESRD dialysis patients in the U.S. in 2009 and the underlying ESRD dialysis patient population grew at an approximate compound annual growth rate, or CAGR, of 3.9% from 2000 to 2009, the latest period for which such data is available. The growth rate is attributable to the aging of the population, increased incidence rates for diseases that cause kidney failure such as diabetes and hypertension, lower mortality rates for dialysis patients and growth rates of minority populations with higher than average incidence rates of ESRD.
Competitive Landscape. The dialysis industry has consolidated significantly over time, but still remains highly competitive. The two largest dialysis companies account for approximately 70% of the U.S. dialysis patient population based upon management estimates, with DaVita serving approximately 33% of that population. The remainder of the industry is highly fragmented, comprised of regional chains, local hospital based dialysis facilities and physician and other independently-owned centers.
Universal Medicare Reimbursement. Since 1972, the federal government has provided health care coverage for ESRD patients under the Medicare ESRD program, regardless of age or financial circumstances. ESRD is the
S-6
Table of Contents
first and only disease state eligible for dialysis and dialysis-related lab services and for all benefits available under the Medicare program. Although Medicare reimbursement limits the allowable charge per treatment, it provides industry participants with a relatively predictable and recurring revenue stream for dialysis services provided to patients without commercial insurance. For DaVita, revenue attributable to Medicare and Medicare-assigned plans represented 59% of dialysis and related lab services revenues for the twelve months ended June 30, 2012.
Significant Government Responsibility. Because of universal Medicare reimbursement for dialysis treatment, the federal government provides significant oversight and regulation of the dialysis sector on a federal, state and local level. A primary concern is the significant, yet fragmented, presence of approximately 825 independent providers of dialysis treatments, whose survival depends on adequate Medicare reimbursement rates. Given patient dependence on dialysis for sustaining life and the critical financial role undertaken by the government, we believe there is likely to be some protection from government rate cuts or any cuts that would make it difficult for small and regional providers to continue to offer dialysis services to their patients.
Bundled Reimbursement System. Since January 2011, ESRD payments have been made under a single bundled payment rate that provides for an annual inflation adjustment, based upon a market basket index, less a productivity improvement factor. The bundled payment rate provides a fixed payment rate to encompass all goods and services provided during the dialysis treatment, including pharmaceuticals that were historically separately reimbursed to the dialysis providers, such as Epogen®, or EPO, vitamin D analogs and iron supplements, irrespective of the level of pharmaceuticals administered or additional services performed. Most lab services that used to be paid directly to laboratories are also included in the new bundled payment. The bundled payment rate is also adjusted for certain patient characteristics, a geographic usage index and certain other factors.
Also, beginning January 1, 2014, certain oral-only ESRD drugs (currently paid separately to pharmacies under Medicare Part D) will be included in the ESRD bundled payment to dialysis facilities. It is currently unclear how the Centers for Medicare and Medicaid Services, or CMS, will “price” the oral-only drugs for inclusion in the ESRD bundle in 2014.
Although Medicare reimbursement limits the allowable charge per treatment, it provides industry participants with a relatively predictable and recurring revenue stream for dialysis services provided to patients without commercial insurance. For the twelve months ended June 30, 2012, 90% of our total patients were under government-based programs, with approximately 80% of our patients under Medicare and Medicare-assigned plans.
DaVita’s Competitive Strengths
Superior Clinical Outcomes. We believe that the clinical outcomes of our patient population compare favorably with other dialysis providers and generally exceed the dialysis outcome quality indicators of the National Kidney Foundation. To better assess overall outcomes improvement we have developed our own index, which we refer to as the DaVita Quality Index, or DQI. DQI takes into account outcomes associated with adequacy of dialysis, anemia management, cardiovascular and bone disease, nutrition, and vascular access. The DQI methodology awards points for the percentage of patients exceeding a specified goal and deducts points for the percentage of patients falling below a certain level, providing an objective measure of our total patient care. We believe that DQI correlates with patient survival and likelihood of hospitalization. We believe that our strong clinical outcomes have led to improved quality of life for our patients, lower mortality rates, reduced hospitalizations, and greater satisfaction with care. We believe that this, in turn, has reduced overall patient costs for the payors. In addition, we have an active national physician council, consisting of twenty physicians across the country, that advises our senior management on all clinical issues impacting our operations. DaVita and its affiliated physicians collaborated to achieve outstanding clinical outcomes in 2011. As just one example, our patients’ 2010 gross mortality rate improved for the fifth straight year to 15%, a 16% improvement from our 2005 mortality rate of 19%.
S-7
Table of Contents
National scale. DaVita has a network of 1,884 outpatient dialysis and administrative centers located in the U.S. throughout 43 states and the District of Columbia, serving a total of approximately 149,000 patients. This scale allows DaVita to provide its patient base with convenient locations and access to a full range of services; benefit from economies of scale in purchases of pharmaceuticals and other medical supplies and services; enhance relationships with managed care payors by offering an extensive set of related services to lower the overall cost of patient care; leverage information technology and compliance systems; provide a greater depth and breadth of services; strengthen its medical director recruitment and retention initiatives; and develop the expertise and obtain the resources needed to continue to expand the business through denovo center expansion and selected acquisitions.
Strong operating track record. DaVita has demonstrated strong and resilient financial performance even through the recent macroeconomic downturn as demand for care is steady, predictable and independent of the many macroeconomic factors affecting the broader economy. DaVita’s growth has been underpinned by the stable volume growth of the underlying dialysis patient population, which increased at a CAGR of 3.9% from 2000 through 2009, the latest period for which such data is available. Since June 30, 2009, DaVita’s quarterly organic growth has ranged between 3.7% and 5.5%. From June 30, 2009 to June 30, 2012, DaVita’s net operating revenue and Adjusted EBITDA have grown at CAGRs of 8.8% and 10.0%, respectively.
Strong and stable free cash flow. The stability of demand and reimbursement for DaVita’s services, consistent historical Adjusted EBITDA margins of approximately 20%–22% since fiscal year ended December 31, 2009 and efficient management of working capital have resulted in strong operating cash flow. DaVita has increased its net cash provided by operating activities from $705 million in the twelve months ended June 30, 2009 to $1,180 million for the twelve months ended June 30, 2012, representing a CAGR of 19%. In addition, DaVita’s centers require limited and predictable maintenance capital expenditures once they are operational, resulting in strong and stable free cash flow generation, which allows DaVita to fund its growth-related investments and reduce indebtedness. DaVita’s maintenance capital expenditures have ranged from $104–$259 million, or approximately 2%–4% of consolidated net operating revenues, between the twelve months ended June 30, 2009 and the twelve months ended June 30, 2012. DaVita has increased its free cash flow from $527 million in the twelve months ended June 30, 2009 to $817 million for the twelve months ended June 30, 2012, representing a CAGR of 16%. For an explanation of free cash flow and a reconciliation to operating cash flow, see “DaVita Summary Historical Financial and Operating Data” beginning on page S-30.
Comprehensive compliance program.DaVita’s dialysis operations are subject to extensive federal, state and local government regulations. Management has designed and implemented a company-wide, corporate compliance program as part of DaVita’s commitment to comply fully with all applicable laws and regulations and to maintain the high standards of conduct DaVita expects from all of its employees, whom DaVita refers to as its teammates. To increase awareness of the necessity of complying with all applicable laws and regulations, DaVita has developed ongoing training programs for its teammates through its in-house training program, DaVita University. In addition, DaVita has well-established guidelines around physician roles and responsibilities and requires that its physicians attest to their adherence to these guidelines on a periodic basis. DaVita’s compliance programs are overseen by the Chief Compliance Officer who reports directly to the Chief Executive Officer and to the Compliance Committee of the Board of Directors.
Experienced management team.DaVita’s management team has extensive experience and expertise in the dialysis industry with an average of 15 years of industry experience. Under management’s guidance, DaVita has enjoyed consistent improvements in clinical outcomes, improving contract negotiation results with managed care payors, strong organic growth and successful acquisition and denovo growth. DaVita’s consolidated net operating revenues, Adjusted EBITDA and number of U.S. centers in operation grew from $1.3 billion, $188 million and 572, respectively, in 1999 when DaVita’s current Chief Executive Officer, Kent Thiry, joined DaVita as CEO, to $7.4 billion, $1.6 billion and 1,884, respectively, as of and for the twelve months ended June 30, 2012.
S-8
Table of Contents
DaVita’s Strategy
DaVita plans to continue to grow its business and improve its financial performance by implementing its business strategy, the key elements of which are:
Continuous improvement in patient care.DaVita believes its reputation for providing quality patient care is a key factor in attracting patients and qualified medical directors as well as in maintaining and building relationships with referring physicians and managed care and government payors. DaVita strives to deliver best-in-class clinical outcomes as well as increase patient involvement in their care. For example, DaVita’s “At Home Initiative” is committed to leading the introduction and promotion of effective home hemodialysis and peritoneal dialysis solutions for healthier, more independent dialysis patients who prefer to dialyze at home. Moreover, DaVita is committed to continuous improvement in its medical and clinical processes through quality management programs to monitor and enhance the level of services it delivers. Through these quality management programs supervised by the Office of the Chief Medical Officer and the Directors of Clinical Services, DaVita continuously works to promote its high standards of patient care. These efforts include further development and implementation of patient care policies and procedures, clinical education and training programs, clinical guidelines and protocols and audits of the quality of services rendered at each of DaVita’s centers. Although it is difficult to reliably measure clinical performance across the dialysis industry, DaVita believes its clinical outcomes compare favorably with other dialysis providers in the U.S.
Developing and maintaining strong relationships with physicians. DaVita continuously seeks to develop relationships with nephrologists. DaVita believes that collaborating with these physicians leads to enhanced quality of care, patient satisfaction and physician satisfaction. DaVita intends to sustain and strengthen its physician relationships by emphasizing DaVita’s high quality of care and state-of-the-art centers, expanding its broad array of services and technologies, developing and offering quality training programs and continuing to involve DaVita’s physicians in establishing clinical guidelines and protocols.
Expansion of operations. DaVita intends to continue to expand its operations by building out its existing centers, as well as developing and/or acquiring new centers both domestically and internationally. DaVita will continue to evaluate acquisition and denovo opportunities that it identifies as complementary to its existing base of operations or as compelling for new geographic expansion. DaVita believes that its enhanced geographic presence makes it a more attractive partner for national managed care payors.
Integrated kidney care. DaVita maintains an integrated approach to managing the overall health of kidney disease patients through the development and administration of DaVita’s ancillary service offerings, including DaVita Rx, Lifeline and VillageHealth. DaVita Rx, DaVita’s pharmacy services offering, provides oral medications to DaVita’s ESRD patients with the main objectives of (i) providing patients a convenient way to fill their prescription needs by delivering the prescriptions to the center where they are treated and (ii) improving clinical outcomes by facilitating increased patient compliance. Lifeline, DaVita’s vascular access services offering, provides management and administrative services to physician-owned vascular access clinics that provide surgical and interventional radiology services for dialysis patients. VillageHealth, DaVita’s disease management services offering, provides advanced care management services to health plans and government agencies for employees/members diagnosed with ESRD.
Effective teammate retention and satisfaction. DaVita’s dialysis business requires nurses and other teammates with specialized training for treating patients with complex care needs. Recruitment and retention of nurses are continuing concerns for health care providers due to short supply. DaVita has an active program of investing in its teammates. As a result of these efforts DaVita’s teammate turnover has improved from 25% to 18% for the quarter ended June 30, 2012 compared to the quarter ended December 31, 2007. This has been a major contributor to DaVita’s improving productivity and effective cost control. To meet DaVita’s recruitment and retention targets, DaVita offers its teammates expanded training opportunities, tuition reimbursements and other incentives.
S-9
Table of Contents
HCP
HCP’s Business
HCP is a patient- and physician-focused, integrated health care delivery and management company with nearly three decades of providing coordinated, outcomes-based medical care in a cost-effective manner. Through capitation contracts with some of the nation’s leading health plans, as of June 30, 2012, HCP had approximately 669,400 current members under its care in southern California, central and south Florida and southern Nevada. Of these, approximately 190,700 individuals were patients enrolled in Medicare Advantage. The remaining approximately 478,700 individuals were managed care members whose health coverage is provided through their employer or who have individually acquired health coverage directly from a health plan or as a result of their eligibility for Medicaid benefits. In addition, during 2011, HCP provided care to over 412,000 fee-for-service patients.
The patients of HCP’s affiliated physicians, physician groups and IPAs benefit from an integrated approach to medical care that places the physician at the center of patient care. As of June 30, 2012, HCP delivered services to its members via a network of over 1,800 affiliated group and other network primary care physicians, 139 network hospitals, and several thousand affiliated group and network specialists. Together with hundreds of case managers, registered nurses and other care coordinators, these medical professionals utilize a comprehensive data analysis engine, sophisticated risk management techniques and clinical protocols to provide high-quality, cost effective care to HCP’s members.
Approximately 94% of HCP’s revenues are derived from multi-year capitation contracts with health plans. Under these contracts, HCP’s health plan customers delegate full responsibility for member care to physicians and health care facilities that are part of HCP’s network. In return, HCP receives a per-member per-month, or PMPM, fee for each HCP member. As a result, HCP has financial and clinical accountability for a population of members. In California, HCP does not assume direct financial risk for institutional (hospital) services, but is responsible for managing the care dollars associated with both the professional (physician) and institutional services being provided for the PMPM fee attributable to both professional and institutional services. In those cases and as a result of its managed care-related administrative services agreements with hospitals, HCP recognizes the surplus of institutional revenues less institutional expense as HCP revenues. In addition to revenues recognized for financial reporting purposes, HCP measures its total care dollars under management which includes the PMPM fee payable to third parties for institutional (hospital) services where HCP manages the care provided to its members by hospitals and other institutional providers, which fees are not included in GAAP revenues. For the twelve months ended June 30, 2012, HCP’s total consolidated operating revenues were $2.6 billion, total care dollars under management were $3.4 billion, net income was $450 million and Adjusted EBITDA was $561 million. Total care dollars under management and Adjusted EBITDA are non-GAAP measures. For a description of how HCP calculates total care dollars under management and Adjusted EBITDA and a reconciliation to revenues and net income, respectively, see “HCP Summary Historical Financial and Operating Data” beginning on page S-33.
We believe that HCP is well positioned to profitably leverage marketplace demands for greater provider accountability, measurable quality results and cost effective medical care. We believe that HCP’s business model is likely to continue to be an attractive alternative for health plans looking for high quality, cost effective delivery systems, physicians seeking an attractive practice environment and patients interested in a highly integrated approach to managing their medical care. Additionally, we believe that the scale of HCP’s business allows it to spread capitation risk over a large population of members, invest in comprehensive analytic and health care information tools as well as clinical and quality measurement infrastructure, and recognize administrative and operating efficiencies. For these reasons, we believe that HCP offers patients, physicians and health plans a proven platform for addressing many of the most pressing challenges facing the U.S. health care system, including rising medical costs.
S-10
Table of Contents
HCP Industry Overview
U.S. healthcare spending has increased steadily over the past twenty years. These increases have been driven, in part, by the aging population of the baby boomer generation, lack of healthy lifestyle both in terms of exercise and diet, rapidly increasing costs in medical technology and pharmaceutical research, and provider reimbursement structures that may promote volume over quality in a fee-for-service environment. These factors, as well as the steady growth of the U.S. population, have made the healthcare industry a growing market. In 2009, CMS reported that health care accounted for 17.3% of the U.S. economy. According to CMS the increase in health spending, from $2.3 trillion in 2008 to $2.5 trillion in 2009, was the largest one-year jump since 1960. Comprising an estimated 14% of the federal budget and more than one-fifth of total national health expenditures in 2010, Medicare is frequently the focus of discussions on how to moderate the growth of both federal spending and health care spending in the U.S.
Growth in Medicare spending is expected to continue due to demographics. According to the U.S. Census Bureau from 1970 through 2011, the overall U.S. population is expected to have grown 52% while the number of Medicare enrollees will have grown approximately 130% over that time period. As an increasing number of the baby boomers become eligible for Medicare, the senior market is expected to grow to 79 million by 2030, more than double the number in 2000. UnitedHealth estimates that over the next decade 10,000 people per day will become newly eligible for Medicare. This translates into a Medicare population that makes up more than 20% of the total U.S. population by the year 2025, compared to less than 15% currently.
Medicare Advantage is an alternative to the traditional fee-for-service Medicare program, which permits Medicare beneficiaries to receive benefits from a managed care health plan. Medicare Advantage plans contract with CMS to provide benefits at least comparable to those offered under the traditional fee-for-service Medicare program in exchange for a fixed monthly premium payment per member from CMS. The monthly premium varies based on the county in which the member resides, as adjusted to reflect the plan members’ demographics and the members’ risk scores. Individuals who elect to participate in the Medicare Advantage program typically receive greater benefits than traditional fee-for-service Medicare Part B beneficiaries, including additional preventive services, vision, dental and prescription drug benefits, and typically have lower deductibles and co-payments than traditional fee-for-service Medicare.
Managed care health plans were developed, primarily during the 1980s, in an attempt to mitigate the rising cost of providing healthcare benefits to populations covered by traditional health insurance. These managed care health plans enroll members through their employers, under federal Medicare benefits or through state Medicaid programs. As a result of the prevalence of these health plans, many seniors now becoming eligible for Medicare have been interacting with managed care companies through their employers for the last 30 years. Individuals turning 65 now are likely to be far more familiar with the managed care setting than previous Medicare populations. According to the Kaiser Family Foundation, in 2012, Medicare Advantage represents only 27% of total Medicare members, creating a significant opportunity for additional Medicare Advantage penetration of newly eligible seniors.
In an effort to reduce the number of uninsured and to begin to control healthcare expenditures, President Obama signed the Medicare and Medicaid regulations and the Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010, or the Health Reform Acts, into law in March 2010, which were affirmed, in substantial part, by the U.S. Supreme Court in June 2012. The Health Reform Acts provide for a reduction of up to 32 million uninsured by 2019, while potentially increasing Medicaid coverage by up to 16 million and net commercial coverage by 16 million. CMS projects that the total number of uninsured Americans will fall to 24 million in 2019 from 52 million in 2011. These previously uninsured Americans and potentially newly eligible Medicaid beneficiaries represent a significant new market opportunity for health plans. We believe that health plans looking to cover these newly eligible individuals under fixed premium arrangements will seek provider arrangements that can effectively manage the cost and quality of the care being provided to these newly eligible individuals.
S-11
Table of Contents
In 2006, Medicare began to pay Medicare Advantage health plans under a bidding process. Plans bid against county-level benchmarks established by Medicare based on the prior year’s Medicare Advantage county payment rate and increased by the projected national growth rate in per capita Medicare spending. Those payment rates were at least as high as per capita fee-for-service Medicare spending in each county and often substantially higher because Congress set floors to raise the lowest rates to stimulate plan growth in areas where plans historically had not found it profitable to enter. If a plan’s bid is higher than the benchmark, enrollees pay the difference in the form of a monthly premium. If the bid is lower than the benchmark, the Medicare program retains 25% of the difference as savings and the plan receives 75% as a rebate, which must be returned to enrollees in the form of additional benefits or reduced premiums. Plan payments are also adjusted based on enrollees’ risk profiles. The formula for base payment is a combination of the base rate for the enrollee’s county of residence, multiplied by the enrollee’s risk score.
One of the primary ways in which the Healthcare Reform Acts will fund increased health insurance coverage is through cuts in Medicare Advantage reimbursement. County benchmarks are transitioning to a system in which each county’s benchmark in 2017 will be a certain percentage (ranging from 95% to 115%) of fee-for-service. Medicare Payment Advisory Commission, or MedPAC, estimated that 2012 Medicare Advantage benchmarks, bids, and payments will average 112%, 98%, and 107% of fee-for-service spending, respectively. As a result, plans on average would have to bid 36% lower than fee-for-service or 43% lower than the Medicare Advantage benchmark for CMS to begin to save money on Medicare Advantage. As result of the transition of county benchmarks to 95% to 115% of fee-for-service, Medicare Advantage benchmarks on average are expected to be reduced to parity with fee-for-service as compared to 112% of fee for-service today. Given that CMS will retain 25% of the difference of any plans bid below benchmark, the overall Medicare Advantage program should realize savings as compared to fee-for-service in 2017, which would result in lower payments to Medicare Advantage plans and to HCP.
Many health plans recognize both the opportunity for growth from senior members as well as the potential risks and costs associated with managing additional senior members. In California, Florida, Nevada and numerous other markets, many health plans subcontract a significant portion of the responsibility for managing patient care to integrated medical systems such as HCP. These integrated health care systems, whether medical groups or IPAs, offer a comprehensive medical delivery system and sophisticated care management know-how and infrastructure to more efficiently provide for the health care needs of the population enrolled with that health plan. While reimbursement models for these arrangements vary around the country, health plans in California, Florida and Nevada often prospectively pay the integrated health care system a fixed PMPM amount, or capitation payment, which is often based on a percentage of the amount received by the health plan. The capitation payment is for much—and sometimes virtually all—of the care needs of the applicable membership. Capitation payments to integrated health care systems, in the aggregate, represent a prospective budget from which the system manages care-related expenses on behalf of the population enrolled with that system. To the extent that these systems manage care-related expenses under the capitated levels, the system realizes an operating profit. On the other hand, if care-related expenses exceed projected levels, the system will realize an operating deficit. Since premiums paid represent a significant amount per person, there is a significant revenue opportunity for an integrated medical system like HCP that is able to effectively manage its costs under a capitated arrangement. This is particularly the case for Medicare Advantage members for which revenue to a system can be substantial given the higher expected morbidity and cost associated with a Medicare Advantage member.
Integrated medical systems, such as HCP, that have scale are positioned to spread an individual member’s cost experience across a wider population and realize the benefits of pooling medical risk among large numbers. In addition, integrated medical systems with years of managed care experience can utilize their sizeable medical claims data to identify specific medical care and quality management strategies and interventions for potential high cost cases and aggressively manage them to improve the health of its population base and, thus, lower cost.
S-12
Table of Contents
Many integrated medical systems, like HCP, have also established physician performance metrics that allow them to monitor quality and service outcomes achieved by participating physicians in order to reward efficient, high quality care delivered to members and initiate improvement efforts for physicians whose results can be enhanced.
HCP’s Competitive Strengths
We believe that HCP distinguishes itself through its ability to demonstrably improve medical outcomes and patient satisfaction while effectively managing costs. HCP achieves this result through the following key strengths:
| • | Clinically based utilization management models.HCP’s clinical leadership and affiliated group and network physicians devote significant efforts to ensuring that HCP’s members receive the most appropriate care in the most appropriate setting. HCP believes this results in significant differences compared to a typical unmanaged patient population. For example, during fiscal 2010, HCP’s inpatient acute bed days in California were 864 days per 1,000 members for its Medicare Advantage members, as compared to an average of 1,706 days per 1,000 patients for Medicare’s fee-for-service program during the same period. Similarly, HCP’s 30 day “all cause” hospital re-admission rate in California during fiscal 2010 was 14%, which HCP believes was lower than the Medicare fee-for-service benchmark. HCP has achieved similarly favorable outcomes in Nevada and Florida when compared to benchmarks. |
| • | Service commitment.HCP is committed to maximizing its patients’ satisfaction levels with HCP and their physicians. HCP regularly conducts comprehensive satisfaction surveys of its members and actively monitors survey results at the individual physician level. In its most recent survey conducted during the second quarter of 2012, 91.6% of patients surveyed gave their HCP physician top satisfaction scores. We believe that HCP’s high rates of patient satisfaction lead to greater member retention. Because of the number of HCP commercial health plan customers, if an employer changes health plans, members can often move to another plan and still retain their participation with HCP. HCP believes the longevity of the patient-physician relationship provides it with additional leverage with the health plans and helps to ensure the stability of the relationship between the health plans and HCP. |
| • | Long standing relationships with health plans. We believe that HCP’s scale, combined with its strong reputation and high quality patient care, makes it an attractive partner for health plans compared to smaller provider groups that may have a higher risk of default and may not have the same resources to devote to integrated care techniques. We believe that HCP is a leader in managing global capitation arrangements by assuming both professional (physician) and institutional (hospital) risk and has the critical mass necessary to diversify these risks across a large membership base. HCP’s scale and resources enable it to invest in continuous innovation to improve the clinical outcomes of its members. We believe that health plans in the regions in which it operates appreciate HCP’s ability to manage global risk because these arrangements eliminate the volatility of medical costs, the largest cost component for health plans. HCP, or its predecessor companies, have longstanding relationships with its health plan customers, with these relationships having an average tenure of approximately 20 years. For example, HCP has had a relationship spanning approximately 28 years with UnitedHealthcare, one of HCP’s largest customers. HCP also provides care to a significant portion of Humana’s Medicare Advantage membership in the central Florida region. HCP is not aware of any health plan customer that has not renewed its contract with HCP. |
| • | Proprietary database of long-tenured patient data. HCP has nearly three decades of experience in managing complex disease cases for its population of patients. As a result, HCP has developed a rich |
S-13
Table of Contents
dataset of patient care experiences and outcomes which permits HCP to proactively monitor and intervene in improving the care of its members. HCP uses this proprietary database to: |
| • | identify patients with high-cost or high-utilization disease categories; |
| • | provide direct feedback to their physicians and other care-givers with point of care reminders and other notifications of patient’s needs; |
| • | reduce variation in practice patterns, provide immediate feedback to physicians and improve the overall quality of care; |
| • | benchmark HCP’s performance across its organization and against published metrics to establish a best practices approach to health care; and |
| • | accurately model historical utilization and cost patterns and, from that, seek to project future patterns, allowing HCP to better assess risk and negotiate health plan contracts. |
| • | Experienced management team.HCP’s senior management team possesses substantial experience within the healthcare industry, with average experience of nearly 35 years. The management team has overseen significant growth in its business and demonstrated the ability to produce strong financial performance. HCP’s senior management team is expected to continue with HCP after the Merger. |
| • | Strong financial performance.Consistent revenue and EBITDA growth over the prior 14 years, coupled with negative working capital and low maintenance capital expenditures over this period of less than one percent of revenue, have enabled HCP to achieve attractive historical cash flows. In the twelve months ended June 30, 2012, HCP generated cash flows from operating activities of $512 million. HCP’s ability to generate strong and consistent cash flow from operations has enabled it to invest in its operations and pursue attractive growth opportunities. |
| • | Scalable and portable business model. We believe that HCP’s strong clinical outcomes, reputation with health plans and health care providers and its ability to successfully manage complex regulatory, reimbursement, clinical and operating environments associated with practicing medicine are key reasons that medical groups and IPAs are interested in joining HCP’s network. HCP has the capacity to extend its network and systems to encompass additional medical groups and IPAs with only limited incremental capital expenditures. |
HCP’s Strategy
HCP intends to continue to increase its membership, and generate incremental revenue and earnings opportunities in existing and new markets. HCP expects to accomplish this through pursuing the following activities:
Continue to Provide High Quality Care to Patients While Minimizing Costs. HCP intends to continue to improve quality care and strong medical outcomes for its patients while managing health care costs and minimizing the level of unnecessary care by investing in the following programs and initiatives:
| • | Integrated care teams. HCP has re-engineered the patient care process to enhance the patient care experience through the use of integrated care teams. These include care teams of physicians, nurses and medical assistants who have direct contact with and deep personal knowledge of a panel of assigned patients. Patients have direct phone and/or email access to these teams for appointments and information flow. Teams are supported by a multi-disciplined support center, 24 hours a day, seven days per week, that handles customer service issues, claims and benefit questions as well as medical questions and the triaging of medical conditions to the appropriate resource after office hours. |
S-14
Table of Contents
| • | Disease management programs.HCP proactively manages its patients with specific disease conditions, including chronic obstructive pulmonary disease, chronic kidney disease, ESRD and diabetes, among others, through a combination of direct clinical intervention and treatment, and patient education. These programs are designed to reduce the escalation of the severity of the medical conditions, thereby reducing hospital admissions and medical claims costs, as well as improving the overall quality of life for patients with these conditions. |
| • | Hospitalists.HCP utilizes hospitalists in all of its markets to more efficiently use HCP’s primary care physicians and to provide more individualized and focused attention for hospitalized patients. These specifically trained physicians monitor and manage on a 24 hours a day, seven days per week basis all aspects of care during a patient’s hospital stay, in many cases on-site at the hospital. We believe this results in more efficient, and generally shorter hospital stays, as well as reduced levels of readmissions. |
| • | Comprehensive care centers.HCP offers comprehensive care centers that are typically located within existing medical clinics and practice locations. These comprehensive care centers provide customized interventions for high-risk patients with multiple chronic diseases. These comprehensive services are designed to prevent these chronic disease conditions from becoming more severe. |
| • | Home care program.The most ill, highest risk patient population typically accounts for a disproportionate level of hospitalizations and emergency room visits. HCP’s home care program brings personalized care to its most frail and ill patients in their home. This program is designed to reduce inpatient acute admissions and emergency room visits for the patients under HCP’s care. |
| • | Same or next day access.Most physicians who depend on fee-for-service reimbursement have fully booked schedules so that when a patient calls with symptoms that are troublesome, but not life-threatening, the patient may be told to go to the emergency room, an extremely high cost and inefficient setting for delivery of care. To mitigate this problem, HCP keeps open a significant block of its physicians’ schedules for same or next day access. This allows patients with non life-threatening problems to be seen in a physician’s office on the same or next day after they call. We believe this program not only improves the quality of care, but also enhances patient satisfaction and retention. |
| • | Urgent care centers.HCP owns and operates freestanding urgent care centers to provide access for patients who require immediate care. These centers create a more appropriate clinical alternative to emergency room visits, which are typically expensive and may lead to unnecessary inpatient admissions. |
Organically Grow by Adding Physicians, Physician Groups and IPAs in Existing and Adjacent Markets. Consistent with HCP’s historical growth model, HCP plans to continue to organically grow its network in and adjacent to its existing markets by adding physicians, physician groups and IPAs, particularly those with strong senior enrollment and an acceptance of integrated care management and evidence-based medicine techniques. We believe that HCP’s strong relationships with many leading health plans, extensive provider networks, and reputation for providing quality care, make it an attractive partner for a wide range of physician groups and IPAs. We believe that there are many of these physician groups and IPAs in its existing and adjacent markets that have experience in managed care. As such, HCP believes that the growth opportunity from organically adding physician groups and IPAs is significant in its primary and adjacent markets.
Opportunistically Expand into New Markets. HCP intends to continue to expand its business model into new markets in a disciplined and opportunistic manner. HCP has acquired or has become affiliated with a number of medical groups, IPAs and physician practices in the past and is currently reviewing a number of acquisitions and affiliation candidates of various sizes both within and outside its existing geographic markets.If a significant portion of the opportunities currently being reviewed were consummated, HCP could be required to raise up to $1 billion in additional financing.
S-15
Table of Contents
Pursue New Product Offerings. HCP also intends to pursue new product offerings. In HCP’s existing markets, HCP intends to contract with health plans that undertake to manage the care of members who are dually eligible for both Medicare and Medicaid benefits, and who are currently receiving care through a traditional fee-for-service model. Health plans receive a higher premium from CMS for dual-eligible patients under a Medicare Advantage program, as these patients typically have higher medical costs. For example, these patients experience 80% higher medical costs than the average Medicare patient and have a 47% higher rate of diabetes; over half of these patients are under treatment for five or more chronic conditions. As a result of CMS authorized demonstration projects, several states are exploring enrolling these dual-eligible patients in managed care plans, and California announced an intention to launch a demonstration project in 2013. Given the high level of chronic disease states among this population and the higher associated costs, HCP believes there is a sizeable new revenue opportunity to apply its integrated care management model to serving dual-eligible patients. In addition, HCP has been selected by CMS Innovation Center to be among the 32 “Pioneer Accountable Care Organizations,” or Pioneer ACO, in each of HCP’s three markets. HCP is the only such Pioneer ACO in more than one state. Pioneer ACOs contract with CMS on a direct basis, not through health plans, to manage the care of Medicare fee-for-service patients attributable to these organizations. The Pioneer ACO program presents an opportunity for HCP to bring the benefit of its integrated care programs to a fee-for-service patient population. Because Medicare fee-for-service is not part of HCP’s health plan customers business, this new product offering will not compete with HCP’s customers.
S-16
Table of Contents
The Merger
Rationale for the Merger
DaVita believes that the Merger with HCP can open a large new market for DaVita—the integrated healthcare services market that HCP serves—offering considerable growth opportunities beyond domestic dialysis. The combination offers the potential to create an industry leading company that may be well positioned to capitalize on anticipated trends in U.S. healthcare, including growth in managed healthcare services, especially to the Medicare-eligible population.
As a significant participant in healthcare delivery with a proven track record, HCP is a recognized leader in its field and should allow DaVita to significantly expand the range of services it provides with only limited additional operational resources required. HCP’s industry leadership provides it substantial credibility with governmental entities, physician groups, large hospital systems and payors across the U.S.
There are many similarities in the values and cultures of DaVita and HCP, including a strong common culture of putting the patient first. In the case of HCP, this is demonstrated by its commitment to and the success of its integrated care model, which has had high quality clinical outcomes and has been able to effectively manage its costs under capitated arrangements. DaVita believes that HCP’s business model is in the right place to capitalize on long-term trends in healthcare in the U.S. —the need to more effectively manage the cost of providing healthcare services, especially to the Medicare-eligible population, while continuing to deliver high quality care. In addition, DaVita believes that HCP’s experience may be able to help DaVita achieve attractive reimbursement for globally capitated kidney care.
Merger Agreement
On May 20, 2012, we entered into a merger agreement, or Merger Agreement, providing for our acquisition of HCP pursuant to the Merger of a newly formed wholly owned subsidiary of DaVita into HCP. Under the Merger Agreement, HCP will be the surviving entity in the Merger and will become a wholly owned subsidiary of DaVita. Following the Merger, DaVita will be renamed “DaVita HealthCare Partners Inc.”
If the Merger is completed, the total merger consideration to be paid to the holders of HCP common units and vested and unvested options to purchase HCP common units, or the HCP options, is an aggregate of $3.6 billion in cash and approximately 9.4 million shares of DaVita common stock, subject to certain adjustments.
In addition to the merger consideration payable at the closing of the Merger and amounts that may be released over time from the escrow accounts as further described below in “—Merger Agreement Escrows”, HCP members and holders of HCP options may receive up to $275.0 million of additional cash consideration in the form of two separate earn-out payments of $137.5 million in cash that are based on the financial performance of HCP and the achievement of certain financial targets for fiscal years 2012 and 2013.
The completion of the Merger is subject to various customary conditions, including, among others, (i) obtaining the approval of HCP’s members, (ii) subject to certain materiality exceptions, the accuracy of the representations and warranties made by DaVita and HCP, respectively, and compliance by DaVita and HCP with their respective obligations under the Merger Agreement, and (iii) declaration of the effectiveness by the Securities and Exchange Commission of the registration statement on Form S-4 filed by DaVita regarding the shares of DaVita common stock to be issued in the Merger.
The Merger must be approved by a vote of the majority of the HCP members. The board of managers of HCP made a recommendation to the HCP members to approve the principal terms of the Merger and the Merger
S-17
Table of Contents
Agreement and the holders of approximately 74% of the outstanding HCP common units has entered into a voting agreement with DaVita pursuant to which it has agreed to vote in favor of the principal terms of the Merger and the Merger Agreement. Accordingly, pursuant to such voting agreement the HCP member approval is assured.
The Merger Agreement contains certain termination rights for each of DaVita and HCP and provides that DaVita is required to pay HCP a $125.0 million termination fee in the event that the Merger Agreement is terminated under certain circumstances. Specifically, in the event that DaVita cannot obtain the financing required for the Merger, each party to the Merger generally has the right to terminate the Merger Agreement and HCP may be entitled to the termination fee.
The Merger Agreement provides that at the closing the DaVita board of directors will be increased in size by one member, and Dr. Robert Margolis, Chairman and Chief Executive Officer of HCP, will be appointed to fill the newly created directorship as “Co-Chairman”. In addition, for a minimum period of four consecutive annual meetings of stockholders of DaVita, Dr. Margolis will hold the office of “Co-Chairman” until the expiration of his term of office or until his successor is duly elected and qualified, subject to his earlier death, resignation, disqualification, or removal in accordance with DaVita’s bylaws and/or applicable law.
Merger Agreement Escrows
Approximately $575 million of the closing merger consideration will be withheld from payment and contributed to escrow accounts that support a potential working capital adjustment, certain indemnification obligations, certain contingent payments, and certain costs and expenses that may be incurred by the HCP member representative designated in the Merger Agreement. Beginning on the second anniversary of the closing, funds in escrow, to the extent not previously released or reserved for certain indemnity claims, will be released on various dates, with the final release to occur on or about October 15, 2017.
Employment Agreements
Concurrently with the execution of the Merger Agreement, each of Dr. Margolis, Mr. Mazdyasni, Dr. Chin, Dr. Thomas Paulsen, Executive Medical Director, California of HCP, Zan Calhoun, Chief Operating Officer of HCP, and Lorie Glisson, Chief Executive Officer—JSA Healthcare, entered into an employment agreement with HCP and DaVita that will become effective upon the consummation of the Merger.
Financing of the Merger
We expect to finance the cash portion of the Merger consideration through a combination of available cash, the net proceeds of the notes offered hereby, and additional borrowings under our senior secured credit facilities, which senior secured credit agreement is expected to be amended to permit or facilitate, among other things, the additional borrowings under the senior secured credit facilities, the Merger and this note offering. There is no financing condition to the Merger; however, DaVita must use its reasonable best efforts to arrange and obtain the financing required to consummate the Merger.
We currently intend to enter into an amendment to our senior secured credit facilities to provide for additional borrowings in an aggregate principal amount of $3,000 million, comprised of:
| • | a new five year Term Loan A-3 facility in an aggregate principal amount of $1,350 million, and |
| • | a new seven year Term Loan B-2 facility in an aggregate principal amount of $1,650 million. |
The proceeds from these additional borrowings, together with available cash, will be used to finance a portion of the cash portion of the Merger consideration, to repay approximately $198 million of our Term Loan
S-18
Table of Contents
A-2 outstanding under our existing senior secured credit agreement, to repay the net amount of HCP indebtedness as a result of the Merger, and pay related fees and expenses.
We intend to borrow all $3,000 million of the term loans and issue $1,000 million of the notes offered hereby. Based upon the amount of available cash, and the proceeds of the notes and secured debt expected to be available to the Company, after giving pro forma effect to the Financing and the Merger as if they had occurred on June 30, 2012, we do not anticipate borrowing any amounts under our revolving credit facility.
The terms and conditions of the amended senior secured credit facilities have not been finalized and are subject to change. We may not finalize the terms until prior to the consummation of the Merger, but after the issuance of the notes offered hereby.
We expect that our amended senior secured credit facilities will be guaranteed by a substantial portion of our direct or indirect wholly owned domestic subsidiaries and will be secured by substantially all of our and our subsidiary guarantors’ assets. In particular, these facilities will be secured by first priority pledges of 100% of the equity interests owned by us and the subsidiary guarantors in our direct domestic subsidiaries and 65% of the equity interests of our and the subsidiary guarantors’ direct foreign subsidiaries, if any.
We expect that our amended senior credit facilities will contain limits and restrictions on certain of our business activities. In addition, we expect that the amended senior secured credit facilities will require compliance on a quarterly basis with certain financial covenants.
As a result of the borrowings that we will incur to finance the Merger, the aggregate amount of our indebtedness and annual debt expense will increase substantially following the Merger. See “Risk Factors,” “Capitalization” and “DaVita Inc. and HealthCare Partners Holdings, LLC Unaudited Pro Forma Condensed Consolidated Financial Statements.”
Sources and Uses
We estimate the net proceeds from this offering, after deducting the underwriting discount and other estimated expenses payable by us, will be approximately $983 million and will be deposited into an escrow account upon the closing of this offering. Funds held in escrow will be released upon the consummation of the Merger and satisfaction of customary conditions, and we intend to use the escrowed proceeds from this offering, together with proceeds from our anticipated amended senior secured credit facilities and cash on hand, to finance the aggregate cash consideration for the Merger and pay related fees and expenses. The following table illustrates the expected sources and uses of funds from the Financing. No assurances can be given that the information in the following table will not change depending on the nature of our financing arrangements and/or whether the Merger will be consummated in accordance with the anticipated timing or at all. See “Risk Factors—Risks Relating to the Merger.”
Sources of Funds | ||||
| (in millions) | ||||
Amended senior secured credit facilities(1) | �� | $ | 3,000 | |
Notes offered hereby | 1,000 | |||
Equity consideration(2) | 907 | |||
Cash from balance sheet | 67 | |||
|
| |||
Total sources | $ | 4,974 | ||
|
| |||
Uses of Funds | ||||
| (in millions) | ||||
| Cash portion of purchase price(3) | $ | 3,592 | ||
Equity portion of purchase price(2) | | 907 | | |
| Repayment of Term Loan A-2 | 198 | |||
| Repayment of HCP’s existing debt(4) | 187 | |||
Estimated fees and expenses | 90 | |||
|
| |||
| Total uses | $ | 4,974 | ||
|
| |||
| (1) | Assumes that such amounts are obtained through the issuance of additional term loans under the amended senior secured credit facilities. The terms of the amended senior secured credit facilities have not yet been finalized and are subject to change. |
S-19
Table of Contents
| (2) | Based upon the issuance of 9,380,312 shares of Davita Inc. common stock valued at the closing market price on August 10, 2012 as reported by the New York Stock Exchange, or NYSE. |
| (3) | The cash portion of the purchase price for HCP consists of $3.66 billion in cash less an estimated negative working capital adjustment of $68 million. |
| (4) | Represents HCP’s debt to be repaid at the closing of the Merger (based upon HCP’s existing debt net of available cash, in each case as of June 30, 2012). |
S-20
Table of Contents
Combined Company Condensed Organizational Chart
The following condensed organizational chart shows our corporate structure after giving effect to the Merger. It does not show our actual corporate structure and is intended solely to illustrate the general ownership structure after the acquisition of HCP, including the subsidiaries and affiliates that serve as guarantors and non-guarantors of the notes.
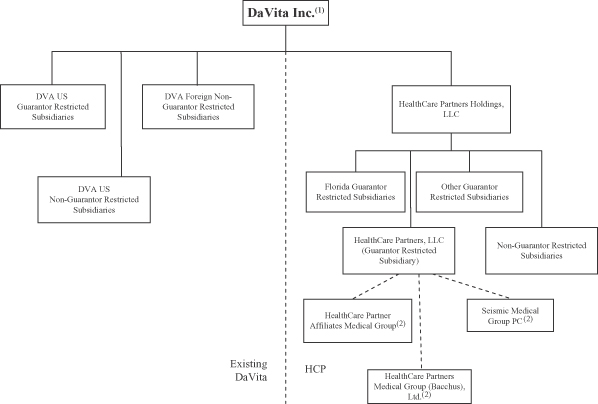
| (1) | Following the Merger, DaVita will be renamed “DaVita HealthCare Partners Inc.” |
| (2) | Non-owned, non-subsidiary, non-guarantor affiliates included in HCP consolidated financial statements and subject to management agreements. |
S-21
Table of Contents
THE OFFERING
The summary below describes some of the terms of the notes and the related indenture and escrow agreement. Certain of the terms and conditions described below are subject to important limitations and exceptions. For a more detailed description of the terms and conditions of the notes and the related indenture and escrow agreement, see the section entitled “Description of Notes.” As used in this section, references to the “we,” “us,” “our,” “DaVita” and “Company” mean DaVita Inc. and not any of its subsidiaries, unless otherwise expressly stated or the context otherwise requires.
Issuer | DaVita Inc. |
Notes Offered | $1,000,000,000 aggregate principal amount of % Senior Notes due 2022. |
Maturity Date | The notes will mature on , 2022. |
Interest | The notes will bear interest at a rate of % per year. Interest will accrue from , 2012. |
Interest Payment Dates | and of each year, commencing , 2013. |
Guarantees | Except as described below under “Escrow of Proceeds; Special Mandatory Redemption”, the notes initially will be guaranteed by each of our domestic restricted subsidiaries that guarantee our senior secured credit facilities. Upon consummation of the Merger, HCP and each of its subsidiaries that guarantee our senior secured credit facilities will also guarantee the notes. |
Ranking | Except as described under “Description of Notes—Escrow of proceeds; release conditions,” the notes will be unsecured senior obligations of the Company and will rank senior in right of payment to all of the Company’s existing and future unsecured debt, if any, that is expressly subordinated in right of payment to the notes. The notes will rank equally in right of payment with all of the Company’s existing and future unsecured senior debt, will be effectively subordinated to all of the Company’s existing and future secured debt (including its senior secured credit facilities) to the extent of the value of the collateral securing such debt and will be structurally subordinated to all existing and future liabilities of the Company’s subsidiaries that do not guarantee the notes. |
| The guarantees will be unsecured senior obligations of the guarantors and will rank senior in right of payment to all their existing and future unsecured debt, if any, that is expressly subordinated in right of payment to the guarantees. The guarantees will rank equally in right of payment with all of the guarantors’ existing and future unsecured senior debt, will be effectively subordinated to all of the guarantors’ existing and future secured debt (including their guarantees of our senior secured credit facilities) to the extent of the value of the collateral securing such debt and will be structurally subordinated to all existing and future liabilities of any of the guarantors’ subsidiaries that do not guarantee the notes. |
S-22
Table of Contents
| As of June 30, 2012, after giving pro forma effect to the Financings and the Merger as if they had occurred on that date, the Company and the guarantors would have had total secured debt of approximately $5,650 million and approximately $284 million of additional secured debt available to be borrowed under our amended senior secured credit facilities (after giving effect to outstanding letters of credit of approximately $66 million), and the notes and the guarantees would have been structurally subordinated to $510 million of liabilities, including $64 million of indebtedness and the rest being primarily trade payables, of non-guarantor subsidiaries. |
| HCP provides services to certain affiliated physician groups that are not owned by HCP, will not constitute “Subsidiaries” (as defined in the indenture governing the notes) and will not guarantee the notes, even though the accounts of these groups are consolidated with the financial statements of HCP and would be consolidated with the financial statements of the Company following the Merger. Pursuant to management agreements between HCP and these affiliated physician groups, a substantial portion of the aggregate net revenues of these groups is payable to subsidiaries of HCP and will be payable to entities that will be guarantors of the notes as compensation for management and administrative services under management services agreements. See “HCP’s Business—Government Regulations—Corporate Practice of Medicine and Fee Splitting.” As of June 30, 2012, after giving pro forma effect to the Financing and the Merger as if they had occurred on that date, our consolidated balance sheet would have included third party liabilities of these affiliated physician groups, in the amount of approximately $305 million and assets of these affiliated physician groups in the amount of approximately $510 million after elimination of intercompany receivables (or approximately 3% of our pro forma consolidated total assets at that date). The pro forma consolidated net operating revenues and Adjusted EBITDA of DaVita for the twelve months ended June 30, 2012, giving effect to the Financing and the Merger as if they had occurred on July 1, 2011, would have been $9,929 million and $2,167 million, respectively. The pro forma consolidated net operating revenues and Adjusted EBITDA of DaVita, excluding HCP’s affiliated physician groups and DaVita’s existing non-guarantor Subsidiaries, for the twelve months ended June 30, 2012, giving effect to the Financing and the Merger as if they had occurred on July 1, 2011, would have been $6,623 million and $1,744 million, respectively. Substantially all of the difference between pro forma consolidated Adjusted EBITDA of $2,167 million and the pro forma consolidated Adjusted EBITDA excluding HCP’s affiliated physician groups and DaVita’s existing non-guarantor subsidiaries of $1,744 million for the twelve months ended June 30, 2012 is attributable to the exclusion of the existing non-guarantor subsidiaries of DaVita. |
S-23
Table of Contents
| The consolidated net operating revenues and Adjusted EBITDA of HCP for the twelve months ended June 30, 2012 were $2,564 million and $561 million, respectively. Excluding HCP’s affiliated physician groups, but inclusive of the management fees earned by HCP from the affiliated physician groups of $725 million, the net operating revenue and Adjusted EBITDA of HCP for the twelve months ended June 30, 2012 would have been $1,731 million and $557 million, respectively. Excluding the management fees earned by HCP from the affiliated physician groups, HCP net operating revenue for the twelve months ended June 30, 2012 would have been $1,006 million. |
Escrow of Proceeds; Special Mandatory Redemption | Upon consummation of the offering of the notes, we will deposit the net proceeds from this offering (after deducting the underwriting discount), together with additional amounts needed to redeem the notes at the redemption price set forth below, into escrow as described in “Description of Notes—Escrow of proceeds; release conditions.” |
| If the conditions to our merger with HCP and certain other conditions are not satisfied on or prior to the Escrow End Date, or if we notify the escrow agent that we will not pursue consummation of the Merger, the amount deposited in escrow will be applied to redeem all of the notes offered hereby at a special mandatory redemption price equal to 100% of the issue price of the notes plus accrued and unpaid interest from the date of initial issuance, or the most recent date to which interest has been paid or duly provided for, as the case may be, to but excluding the special mandatory redemption date. See “Description of Notes—Special mandatory redemption.” |
| If the conditions to our merger with HCP and certain other conditions are satisfied on or before the Escrow End Date, the amounts deposited in escrow will be released to us and applied to finance a portion of the cash consideration for the Merger. See “Use of Proceeds” and “Description of Notes—Escrow of proceeds; release conditions.” |
Optional Redemption | At any time prior to , 2015, the Company may redeem up to 35% of the notes with the net cash proceeds of certain equity offerings at the redemption price set forth under “Description of Notes—Optional redemption.” |
| At any time prior to , 2017, the Company may also redeem the notes, in whole or in part, at a “make whole” redemption price, plus accrued and unpaid interest to the date of redemption, as set forth under “Description of Notes—Optional redemption.” |
| On and after , 2017, the Company may redeem the notes, in whole or in part, at the redemption prices set forth under “Description of Notes—Optional redemption”. |
S-24
Table of Contents
Change of Control | If specific kinds of changes of control occur and the Company has not previously exercised its right to redeem all of the outstanding notes as described under “Description of Notes—Optional redemption” or “Description of Notes—Special mandatory redemption,” the Company must offer to purchase the notes at a price equal to 101% of the principal amount thereof plus any accrued and unpaid interest. The amount required to be escrowed by the Company as described above under “Escrow of Proceeds; Special Mandatory Redemption” is less than the amount required to pay such price in full. |
Covenants | The indenture governing the notes, which we refer to as the indenture, will, among other things, restrict our ability and the ability of our restricted subsidiaries (as defined) to: |
| • | incur additional indebtedness and issue certain preferred stock; |
| • | make certain distributions, investments and other restricted payments; |
| • | sell certain assets; |
| • | agree to restrictions on the ability of restricted subsidiaries to make payments to us; |
| • | create certain liens; |
| • | merge, consolidate or sell substantially all of our assets; and |
| • | enter into certain transactions with affiliates. |
| These covenants are subject to important exceptions and qualifications described under the heading “Description of Notes.” |
Use of Proceeds | Upon consummation of the Merger and satisfaction of certain other conditions, we intend to use the net proceeds from this offering, together with proceeds from our anticipated amended senior secured credit facilities and available cash, to finance the aggregate cash consideration for the Merger and pay related fees and expenses. Substantially simultaneously with the consummation of the Merger, we intend to use the proceeds from additional borrowings under our amended senior secured credit facilities and available cash to repay approximately $198 million of our Term Loan A-2 outstanding under our existing senior secured credit agreement, to repay the net amount of HCP indebtedness as a result of the Merger, and pay related fees and expenses. See “Use of Proceeds.” If the Merger is not consummated on or prior to the Escrow End Date or the Merger Agreement is terminated at any time prior thereto, we will be required to redeem all of the notes as described under “Description of Notes—Escrow of proceeds; release conditions” and “Description of Notes—Special mandatory redemption.” Pending such uses, the net proceeds may be invested in short-term, investment-grade, interest bearing securities. See “Use of Proceeds.” |
S-25
Table of Contents
No Public Market | The notes are a new series of securities for which there is currently no established trading market. The underwriters have advised us that they presently intend to make a market in the notes. However, you should be aware that they are not obligated to make a market and may discontinue their market-making activities at any time without notice. As a result, a liquid market for the notes may not be available if you try to sell your notes. We do not intend to apply for a listing of the notes on any securities exchange or any automated dealer quotation system. |
Form | The notes will be represented by registered global notes registered in the name of Cede & Co., the nominee of the depository, The Depository Trust Company, or DTC. Beneficial interests in the notes will be shown on, and transfers will be effected through, records maintained by DTC and its participants. |
Risk Factors | See “Risk Factors” beginning on page S-35 of this prospectus supplement for important information regarding us, HCP and an investment in the notes. |
S-26
Table of Contents
Summary Unaudited Pro Forma Financial and Other Data
The following summary unaudited pro forma condensed consolidated statements of income and balance sheet data were derived from DaVita’s unaudited pro forma condensed consolidated financial information included elsewhere in this prospectus. The pro forma other financial data and operating data were derived from historical operating data of each of DaVita and HCP. The unaudited pro forma condensed consolidated statements of income and balance sheet data are based on the audited financial statements for the year ended December 31, 2011 of each of DaVita and HCP and unaudited financial information for the six months ended June 30, 2012 of DaVita and HCP included elsewhere and/or incorporated by reference in this prospectus, and the unaudited financial information for trailing twelve months ended June 30, 2012. The unaudited pro forma condensed consolidated financial information gives effect to the Merger and related borrowings as if each had occurred on January 1, 2011, in the case of income statement data and other financial data derived therefrom, and gives effect to the Merger and related borrowings on June 30, 2012, in the case of balance sheet data and other financial data derived therefrom. The unaudited financial data has been prepared on a basis consistent with DaVita’s and HCP’s annual audited financial statements. In the opinion of management, such unaudited financial data reflects all adjustments, consisting only of normal and recurring adjustments, necessary for a fair presentation of the results for those periods.
The summary unaudited pro forma condensed consolidated financial information has been derived from estimates and financial data that may change materially between the date of this prospectus supplement and the consummation of the Merger. The summary unaudited pro forma financial information below does not purport to represent what DaVita’s results of operations or financial data would actually have been had the Merger and related borrowings in fact occurred on the dates specified, nor does it purport to project our results of operations or financial position for any future period or at any future date. Because the information below is a summary, you should read the following information in conjunction with the other information contained under the captions “DaVita Inc. and HealthCare Partners Holdings, LLC Unaudited Pro Forma Condensed Consolidated Financial Statements,” DaVita’s and HCP’s historical financial statements and the accompanying notes thereto, and other financial and statistical data included elsewhere or incorporated by reference in this prospectus. For information regarding the pro forma adjustments in the following summary unaudited pro forma condensed consolidated financial information, see “DaVita Inc. and HealthCare Partners Holdings, LLC Unaudited Pro Forma Condensed Consolidated Financial Statements,” “DaVita Selected Historical Financial and Other Data” and “HCP Selected Historical Financial and Other Data” beginning on page S-84 , page S-88 and page S-90, respectively.
S-27
Table of Contents
Unaudited Pro Forma Condensed Consolidated Statement of Income
| Pro forma year ended December 31, 2011 | Pro forma six months ended June 30, 2012 | Pro forma twelve months ended June 30, 2012 | ||||||||||
| (dollars in millions) | ||||||||||||
Net dialysis patient service revenues, less provision for uncollectable accounts | $ | 6,273 | $ | 3,465 | $ | 6,745 | ||||||
Integrated care revenue | 2,375 | 1,294 | 2,511 | |||||||||
Other revenues(1) | 566 | 360 | 673 | |||||||||
|
|
|
|
|
| |||||||
Net operating revenues | 9,214 | 5,119 | 9,929 | |||||||||
|
|
|
|
|
| |||||||
Operating expenses and charges: | ||||||||||||
Patient care costs | 6,402 | 3,515 | 6,802 | |||||||||
General and administrative | 896 | 513 | 993 | |||||||||
Depreciation and amortization | 425 | 234 | 453 | |||||||||
Provision for uncollectible accounts | 7 | 4 | 8 | |||||||||
Equity investment income | (34 | ) | (17 | ) | (38 | ) | ||||||
Goodwill impairment charge | 24 | — | — | |||||||||
Legal proceeding contingency accrual and related expenses(2) | — | 78 | 78 | |||||||||
|
|
|
|
|
| |||||||
Total operating expenses and charges | 7,720 | 4,327 | 8,296 | |||||||||
|
|
|
|
|
| |||||||
Operating income | 1,494 | 792 | 1,633 | |||||||||
Debt expense | (434 | ) | (212 | ) | (430 | ) | ||||||
Other income | 11 | 5 | 11 | |||||||||
|
|
|
|
|
| |||||||
Income from continuing operations before income taxes | 1,071 | 585 | 1,214 | |||||||||
Income tax expense | 390 | 220 | 444 | |||||||||
|
|
|
|
|
| |||||||
Income from continuing operations | 681 | 365 | 770 | |||||||||
Discontinued operations: | ||||||||||||
Income from operations of discontinued operations, net of tax | 1 | — | 1 | |||||||||
Loss on disposal of discontinued operations, net of tax | (5 | ) | — | (5 | ) | |||||||
|
|
|
|
|
| |||||||
Net income | 677 | 365 | 766 | |||||||||
Less: Net income attributable to noncontrolling interests | (95 | ) | (49 | ) | (104 | ) | ||||||
|
|
|
|
|
| |||||||
Net income attributable to DaVita Inc. | $ | 582 | $ | 316 | $ | 662 | ||||||
|
|
|
|
|
| |||||||
Other financial data and ratios: | ||||||||||||
Adjusted EBITDA(3) | 2,063 | 1,052 | 2,167 | |||||||||
Net debt(4) | 8,062 | 8,157 | 8,167 | |||||||||
Ratio of net debt to Adjusted EBITDA | 3.91x | 3.77x | ||||||||||
| (1) | Other revenues for DaVita include revenues from our ancillary services and strategic initiatives and fees for providing management and administrative services. Other revenues for HCP include revenues primarily from consulting services and fees from providing management and administrative services. |
| (2) | Represents a legal proceeding contingency accrual and related expenses that resulted from an agreement we reached in principle to settle the Woodard Private Civil Suit. See “DaVita’s Business—Legal Proceedings” beginning on page S-169. |
| (3) | We present Adjusted EBITDA because it is one of the components used in the calculations of the leverage ratio that is included in the covenants contained in our existing senior secured credit agreement, and we expect similar covenants to be included in our amended senior secured credit agreement; however, the terms of the amended senior secured credit agreement have not yet been finalized. Adjusted EBITDA is defined as |
S-28
Table of Contents
| net income attributable to DaVita Inc. before income taxes, debt expense, depreciation and amortization, noncontrolling interests, and equity investment income, net and we further adjust for non-cash charges, stock-based compensation, pro forma amounts for acquisitions and asset sales as if they had been consummated on the first day of each period, and non-cash gains and credits. Management uses Adjusted EBITDA and similar calculations as measures to assess operating and financial performance including compliance with the financial covenants contained in its indentures and its senior secured credit agreement. Adjusted EBITDA is not a measure of financial performance computed in accordance with GAAP and should not be considered in isolation or as a substitute for operating income, net income, cash flows from operations, or other statement of operations or cash flow data prepared in conformity with GAAP, or as measures of profitability or liquidity. In addition the calculation of Adjusted EBITDA is susceptible to varying interpretations and calculation, and the amounts presented may not be comparable to similarly titled measures of other companies. Adjusted EBITDA may not be indicative of historical operating results, and we do not mean for it to be predictive of future results of operations or cash flows. The following table contains a reconciliation of Adjusted EBITDA to net income attributable to DaVita Inc.: |
| Pro forma year ended December 31, 2011 | Pro forma six months ended June 30, 2012 | Pro forma twelve months ended June 30, 2012 | Pro forma Guarantors twelve months ended June 30, 2012(d) | |||||||||||||
| (dollars in millions) | ||||||||||||||||
Net income attributable to DaVita Inc.(a) | $ | 582 | $ | 316 | $ | 662 | $ | 633 | ||||||||
Debt expense(b) | 434 | 212 | 430 | 407 | ||||||||||||
Income taxes | 390 | 220 | 444 | 382 | ||||||||||||
Depreciation and amortization | 425 | 234 | 453 | 255 | ||||||||||||
Stock compensation expense | 56 | 28 | 57 | 57 | ||||||||||||
Goodwill impairment | 24 | — | — | — | ||||||||||||
Noncontrolling interests and equity income, net | 95 | 49 | 104 | — | ||||||||||||
Other items(c) | 57 | (7 | ) | 17 | 10 | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Adjusted EBITDA | $ | 2,063 | $ | 1,052 | $ | 2,167 | $ | 1,744 | ||||||||
|
|
|
|
|
|
|
| |||||||||
| (a) | Net income attributable to DaVita Inc. for the six and twelve months ended June 30, 2012, includes an after-tax legal proceeding contingency accrual and related expenses of $78.0 million recorded in the second quarter of 2012. |
| (b) | Debt expense includes interest expense, amortization of deferred financing costs and the amortization of debt discount. |
| (c) | Represents pro forma acquisition EBITDA, non-cash gains or losses, other valuation adjustments and interest income. |
| (d) | Pro forma amounts for DaVita, excluding HCP’s affiliated physician groups and DaVita’s existing non-guarantor subsidiaries, giving effect to the Merger and the Financings as if they had occurred on July 1, 2011. |
| (4) | Net debt is defined as total debt, plus outstanding letters of credit, excluding debt discounts, or premiums and less cash and cash equivalents. |
S-29
Table of Contents
DaVita Summary Historical Financial and Operating Data
The following summary historical financial information was derived from DaVita’s audited historical financial statements for the years ended December 31, 2009, 2010, and 2011 and unaudited financial information for the six months ended June 30, 2011 and 2012 and the trailing twelve months ended June 30, 2012, incorporated by reference in this prospectus. Effective January 1, 2012, DaVita adopted FASB’s ASU No 2011-07Health Care Entities—Presentation and Disclosure of Patient Service Revenue, Provision for Bad Debts, and the Allowance for Doubtful Accounts. Upon adoption of this standard, DaVita was required to change the presentation of its provision for uncollectible accounts related to patient service revenue as a deduction from patient service operating revenues. These consolidated financial results have been revised for all prior periods presented to reflect the retrospective application of adopting these new presentation and disclosure requirements for the provision for uncollectible accounts. You should read the information set forth below in conjunction with DaVita’s historical consolidated financial statements and related notes, incorporated herein by reference, and “DaVita Selected Historical Financial and Other Data” and “Unaudited Pro Forma Condensed Consolidated Financial Information” included in this prospectus beginning on pages S-88 and S-79, respectively.
| Year ended December 31, | Six months ended June 30, | Twelve months ended June 30, | ||||||||||||||||||||||
| 2009 | 2010 | 2011 | 2011 | 2012 | 2012 | |||||||||||||||||||
| (audited) | (unaudited) | |||||||||||||||||||||||
| (dollars in millions) | ||||||||||||||||||||||||
Statement of operations data: | ||||||||||||||||||||||||
Net dialysis patient service revenues, less provision for uncollectible accounts | $ | 5,601 | $ | 5,877 | $ | 6,273 | $ | 2,992 | $ | 3,465 | $ | 6,745 | ||||||||||||
Other revenue | 343 | 395 | 519 | 232 | 332 | 620 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Net operating revenues | 5,944 | 6,272 | 6,792 | 3,224 | 3,797 | 7,365 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Operating expenses and charges: | ||||||||||||||||||||||||
Patient care costs | 4,242 | 4,467 | 4,681 | 2,277 | 2,575 | 4,979 | ||||||||||||||||||
General and administrative | 531 | 579 | 691 | 315 | 422 | 798 | ||||||||||||||||||
Depreciation and amortization | 228 | 234 | 267 | 126 | 154 | 294 | ||||||||||||||||||
Provision for uncollectible accounts | 5 | 4 | 7 | 3 | 4 | 8 | ||||||||||||||||||
Goodwill impairment charge(1) | — | — | 24 | 24 | — | — | ||||||||||||||||||
Legal proceeding contingency accrual and related expenses(2) | — | — | — | — | 78 | 78 | ||||||||||||||||||
Equity investment income | (2 | ) | (9 | ) | (9 | ) | (4 | ) | (5 | ) | (10 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Total operating expenses and charges | 5,004 | 5,275 | 5,661 | 2,742 | 3,228 | 6,147 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Operating income | 940 | 997 | 1,131 | 482 | 569 | 1,218 | ||||||||||||||||||
Debt expense | (186 | ) | (182 | ) | (241 | ) | (118 | ) | (122 | ) | (245 | ) | ||||||||||||
Refinancing and debt redemption charges(3) | — | (74 | ) | — | — | — | — | |||||||||||||||||
Other income | 4 | 3 | 3 | 1 | 2 | 3 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Income from continuing operations before income taxes | 758 | 744 | 893 | 365 | 449 | 976 | ||||||||||||||||||
Income tax expense | 278 | 260 | 316 | 130 | 164 | 349 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Income from continuing operations | 480 | 484 | 577 | 235 | 285 | 627 | ||||||||||||||||||
Discontinued operations(4) | — | — | (4 | ) | 1 | — | (4 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Net income | 480 | 484 | 573 | 236 | 285 | 623 | ||||||||||||||||||
Less: Net income attributable to noncontrolling interests | (57 | ) | (78 | ) | (95 | ) | (41 | ) | (49 | ) | (104 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Net income attributable to DaVita Inc. | $ | 423 | $ | 406 | $ | 478 | $ | 195 | $ | 236 | $ | 519 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
S-30
Table of Contents
| Year ended December 31, | Six months ended June 30, | Twelve months ended June 30, | ||||||||||||||||||||||
| 2009 | 2010 | 2011 | 2011 | 2012 | 2012 | |||||||||||||||||||
| (audited) | (unaudited) | |||||||||||||||||||||||
| (dollars in millions) | ||||||||||||||||||||||||
Balance sheet data (at end of period): | ||||||||||||||||||||||||
Cash and cash equivalents | $ | 539 | $ | 860 | $ | 394 | $ | 730 | $ | 273 | ||||||||||||||
Working capital | 1,256 | 1,699 | 1,128 | 1,478 | 943 | |||||||||||||||||||
Total assets | 7,558 | 8,114 | 8,892 | 8,193 | 9,255 | |||||||||||||||||||
Total debt | 3,632 | 4,309 | 4,505 | 4,286 | 4,498 | |||||||||||||||||||
Total shareholders’ equity(5) | 2,135 | 1,978 | 2,141 | 1,881 | 2,379 | |||||||||||||||||||
Other financial data: | ||||||||||||||||||||||||
Adjusted EBITDA(6) | $ | 1,225 | $ | 1,288 | $ | 1,534 | $ | 660 | $ | 740 | $ | 1,585 | ||||||||||||
Net cash provided by operating activities | 667 | 840 | 1,180 | 534 | 534 | 1,180 | ||||||||||||||||||
Net debt(7) | 3,142 | 3,503 | 4,171 | 3,610 | 4,281 | 4,281 | ||||||||||||||||||
Ratio of net debt to Adjusted EBITDA(6)(7) | 2.56x | 2.72x | 2.72x | — | — | 2.70x | ||||||||||||||||||
Operating data: | ||||||||||||||||||||||||
Maintenance capital expenditures(8) | 114 | 159 | 224 | 88 | 122 | 259 | ||||||||||||||||||
Centers | 1,530 | 1,612 | 1,820 | 1,669 | 1,903 | 1,903 | ||||||||||||||||||
Patients | 118,000 | 125,000 | 143,000 | 131,000 | 150,000 | 150,000 | ||||||||||||||||||
U.S. Dialysis treatments | 16,985,000 | 17,964,000 | 19,599,000 | 9,364,000 | 10,766,000 | 21,001,000 | ||||||||||||||||||
| (1) | Operating expenses and charges in 2011 include $24 million of a non-cash goodwill impairment charge related to our infusion therapy business. |
| (2) | Represents a legal proceeding contingency accrual and related expenses that resulted from an agreement we reached in principle to settle the Woodard Private Civil Suit. See “DaVita’s Business—Legal Proceedings” beginning on page S-169. |
| (3) | In 2010, we incurred $74 million of refinancing and debt redemption charges in conjunction with the extinguishment of our prior senior secured credit facilities and the redemption of $200 million of our previously outstanding 6 5/8% senior notes. |
| (4) | During 2011, we divested a total of 28 outpatient dialysis centers in conjunction with a consent order issued by the Federal Trade Commission on September 30, 2011 in order for us to complete the acquisition of DSI. In addition, we also completed the sale of two additional centers that were previously pending state regulatory approval in conjunction with the acquisition of DSI on October 31, 2011. The operating results of the historical DaVita divested centers are reflected as discontinued operations in our consolidated financial statements for all periods presented. In addition, the operating results for the DSI divested centers are reflected as discontinued operation in our consolidated financial statements beginning September 1, 2011. |
| (5) | Share repurchases consisted of 3,794,686 shares of DaVita common stock for $323 million in 2011, 8,918,760 shares of DaVita common stock for $618 million in 2010, 2,902,619 shares of DaVita common stock for $153 million in 2009, and 3,710,086 shares of DaVita common stock for $316 million in the first six months of 2011. Shares issued in connection with stock awards amounted to 1,260,259 in 2011, 1,771,384 in 2010 and 2,104,304 in 2009. |
S-31
Table of Contents
| (6) | We present Adjusted EBITDA because it is one of the components used in the calculations of the leverage ratio that is included in the covenants contained in our existing senior secured credit agreement, and we expect similar covenants to be included in our amended senior secured credit agreement; however, the terms of the amended senior secured credit agreement have not yet been finalized. Adjusted EBITDA is defined as net income attributable to DaVita Inc. before income taxes, debt expense, depreciation and amortization, noncontrolling interests, and equity investment income, net, and we further adjust for non-cash charges, stock-based compensation, pro forma amounts for acquisitions and assets sales as if they had been consummated on the first day of each period, and non-cash gains and credits. Management uses Adjusted EBITDA and similar calculations as measures to assess operating and financial performance including compliance with the financial covenants contained in our indentures and our senior secured credit agreement. Adjusted EBITDA is not a measure of financial performance computed in accordance with GAAP and should not be considered in isolation or as a substitute for operating income, net income, cash flows from operations, or other statement of operations or cash flow data prepared in conformity with GAAP, or as measures of profitability or liquidity. In addition the calculation of Adjusted EBITDA is susceptible to varying interpretations and calculation, and the amounts presented may not be comparable to similarly titled measures of other companies. Adjusted EBITDA may not be indicative of historical operating results, and we do not intend for it to be predictive of future results of operations or cash flows. Adjusted EBITDA reconciled to net income attributable to DaVita is as follows: |
| Year ended December 31, | Six months ended June 30, | Twelve months ended June 30, | ||||||||||||||||||||||
| 2009 | 2010 | 2011 | 2011 | 2012 | 2012 | |||||||||||||||||||
| (dollars in millions) | ||||||||||||||||||||||||
Net income attributable to DaVita Inc(a). | $ | 423 | $ | 406 | $ | 478 | $ | 195 | $ | 236 | $ | 519 | ||||||||||||
Income tax expense | 278 | 260 | 316 | 130 | 164 | 349 | ||||||||||||||||||
Debt expense(b) | 186 | 182 | 241 | 118 | 122 | 245 | ||||||||||||||||||
Depreciation and amortization | 228 | 234 | 267 | 126 | 154 | 294 | ||||||||||||||||||
Noncontrolling interests and equity investment income, net | 55 | 75 | 95 | 41 | 49 | 104 | ||||||||||||||||||
Non-cash charges(c) | 53 | 62 | 56 | 30 | 25 | 55 | ||||||||||||||||||
Non-cash goodwill impairment charge | — | — | 24 | 24 | — | — | ||||||||||||||||||
Debt refinancing and redemption charges | — | 74 | — | — | — | — | ||||||||||||||||||
Pro forma amounts for acquisitions and assets sales | 9 | 22 | 89 | 18 | 22 | 62 | ||||||||||||||||||
Non-cash gains and credits | (7 | ) | (27 | ) | (32 | ) | (22 | ) | (32 | ) | (43 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Adjusted EBITDA | $ | 1,225 | $ | 1,288 | $ | 1,534 | $ | 660 | $ | 740 | $ | 1,585 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
| (a) | Net income for the quarter and twelve months ended June 30, 2012, includes an after-tax legal proceeding contingency accrual and related expenses of $78.0 million recorded in the second quarter of 2012. |
| (b) | Debt expense is defined as interest expense plus the amortization of deferred financing costs and amortization of debt discounts or premiums. |
| (c) | Includes stock-based compensation expense, impairments and valuation adjustments and other non-cash charges and losses. |
| (7) | Net debt is defined as total debt, plus outstanding letters of credit, excluding debt discounts, or premiums and less cash and cash equivalents. |
| (8) | Maintenance capital expenditures represent routine capital expenditures to maintain the current operations of the business and include such expenditures for system development, information technology equipment, and dialysis machines. |
We have presented free cash flow in this prospectus supplement. Free cash flow represents net cash provided by operating activities less income distributions to noncontrolling interests and capital expenditures for routine maintenance and information technology. We believe free cash flow is a useful adjunct to cash flow from operating activities and other measurements under GAAP, since free cash flow is a meaningful measure of our ability to fund acquisition and development activities and meet our debt service requirements. In addition, free cash flow excluding income distributions to noncontrolling interests provides an investor with an understanding of free cash flows that are attributable to DaVita Inc. Free cash flow is not a measure of financial performance under GAAP and should not be considered as an alternative to cash flows from operating, investing or financing activities, as an indicator of cash flows or as a measure of liquidity.
| Twelve months ended | ||||||||
| June 30, 2012 | June 30, 2009 | |||||||
| (dollars in millions) | ||||||||
Cash provided by operating activities | $ | 1,180 | $ | 705 | ||||
Less: Income distributions to noncontrolling interests | (105 | ) | (58 | ) | ||||
|
|
|
| |||||
Cash provided by operating activities attributable to DaVita Inc. | 1,075 | 647 | ||||||
Less: Expenditures for routine maintenance and information technology | (258 | ) | (120 | ) | ||||
|
|
|
| |||||
Free cash flow | $ | 817 | $ | 527 | ||||
|
|
|
| |||||
S-32
Table of Contents
HCP Summary Historical Financial and Operating Data
The following summary historical financial information was derived from HCP’s audited historical financial statements for the years ended December 31, 2009, 2010, and 2011, unaudited financial information for the six months ended June 30, 2011 and 2012, and the unaudited financial information for the twelve months ended June 30, 2012. The unaudited pro forma financial information gives effect to the Financing and the Merger as if it had occurred on that date. You should read the information set forth below in conjunction with HCP’s historical financial statements and related notes thereto included in this prospectus and the discussion under “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in this prospectus beginning on page S-91. The combined statement of operations and balance sheet data presented below are derived from the consolidated financial statements of HCP.
| Year ended December 31, | Six months ended June 30, | Twelve Months ended June 30, | ||||||||||||||||||||||
| 2009 | 2010 | 2011 | 2011 | 2012 | 2012 | |||||||||||||||||||
| (audited) | (unaudited) | |||||||||||||||||||||||
| (dollars in millions, except operating data) | ||||||||||||||||||||||||
Statement of operations data: | ||||||||||||||||||||||||
Medical revenues | $ | 1,731 | $ | 2,049 | $ | 2,375 | $ | 1,158 | $ | 1,294 | $ | 2,511 | ||||||||||||
Other operating revenues | 46 | 40 | 47 | 22 | 28 | 53 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Total operating revenues | 1,777 | 2,089 | 2,422 | 1,180 | 1,322 | 2,564 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Operating expenses and charges: | ||||||||||||||||||||||||
Medical expenses | 930 | 1,034 | 1,165 | 569 | 620 | 1,216 | ||||||||||||||||||
Hospital expenses | 212 | 222 | 248 | 121 | 155 | 282 | ||||||||||||||||||
Clinic support and other operating costs | 226 | 263 | 308 | 148 | 165 | 325 | ||||||||||||||||||
General and administrative expenses | 136 | 178 | 207 | 101 | 110 | 216 | ||||||||||||||||||
Depreciation and amortization | 26 | 29 | 31 | 16 | 16 | 31 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Total operating expenses | 1,530 | 1,726 | 1,959 | 955 | 1,066 | 2,070 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Equity earnings of unconsolidated joint ventures | 12 | 15 | 25 | 9 | 12 | 28 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Operating income | 259 | 378 | 488 | 234 | 268 | 522 | ||||||||||||||||||
Interest income | 6 | 6 | 7 | 3 | 4 | 8 | ||||||||||||||||||
Interest expense | (6 | ) | (5 | ) | (16 | ) | (9 | ) | (6 | ) | (13 | ) | ||||||||||||
Gain on sale of investments | 2 | — | 1 | 1 | — | — | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Total other income (expense) | 2 | 1 | (8 | ) | (5 | ) | (2 | ) | (5 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Income before income taxes | 261 | 379 | 480 | 229 | 266 | 517 | ||||||||||||||||||
Provision for income taxes | 41 | 49 | 71 | 37 | 33 | 67 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Net income | $ | 220 | $ | 330 | $ | 409 | $ | 192 | $ | 233 | $ | 450 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Balance sheet data (end of period): | ||||||||||||||||||||||||
Cash and cash equivalents | 358 | 361 | 395 | 183 | 355 | |||||||||||||||||||
Working capital. | 179 | 360 | 304 | 192 | 341 | |||||||||||||||||||
Total assets. | 911 | 1,286 | 1,366 | 1,188 | 1,415 | |||||||||||||||||||
Total debt | 220 | 218 | 556 | 571 | 542 | |||||||||||||||||||
Member’s equity. | 340 | 566 | 188 | 29 | 248 | |||||||||||||||||||
Other financial data: | ||||||||||||||||||||||||
Total care dollars under management(1) | 2,388 | 2,792 | 3,212 | 1,582 | 1,752 | 3,382 | ||||||||||||||||||
Adjusted EBITDA(2) | 293 | 414 | 527 | 255 | 288 | 561 | ||||||||||||||||||
Capital expenditures | 12 | 21 | 23 | 11 | 10 | 22 | ||||||||||||||||||
Net cash provided by operating activities | 286 | 343 | 509 | 181 | 184 | 512 | ||||||||||||||||||
Operating data: | ||||||||||||||||||||||||
Managed care members | 589,900 | 658,000 | 667,700 | 659,200 | 669,400 | |||||||||||||||||||
Medical clinic locations | 99 | 129 | 152 | 138 | 157 | |||||||||||||||||||
Full time physicians | 570 | 715 | 794 | 734 | 818 | |||||||||||||||||||
IPA Primary care physicians | 1,268 | 1,291 | 1,458 | 1,414 | 1,454 | |||||||||||||||||||
Ratio of operating income to total care dollars under management | 10.8 | % | 13.5 | % | 15.2 | % | 14.8 | % | 15.3 | % | 15.4 | % | ||||||||||||
S-33
Table of Contents
| (1) | In California, as a result of its managed care administrative services agreement with hospitals, HCP does not assume the direct financial risk for institutional (hospital) services, but is responsible for managing the care dollars associated with both the professional (physician) and institutional services being provided for the PMPM fee attributable to both professional and institutional services. In those cases, HCP recognizes the surplus of institutional revenue less institutional expense as HCP revenue. In addition to revenues recognized for financial reporting purposes, HCP measures its total care dollars under management, which includes the PMPM fee payable to third parties for institutional (hospital) services where HCP manages the care provided to its members by the hospitals and other institutions, which are not included in GAAP revenues. HCP uses total care dollars under management as a supplement to GAAP revenues as it allows HCP to measure profit margins on a comparable basis across both the global capitation model (where HCP assumes the full financial risk for all services, including institutional services) and the risk sharing models (where HCP operates under managed care administrative services agreements where HCP does not assume the full risk). HCP believes that presenting amounts in this manner is useful because it presents its operations on a unified basis without the complication caused by models that HCP has adopted in its California market as a result of various regulations related to the assumption of institutional risk. Total care dollars under management is not a measure of financial performance computed in accordance with GAAP and should not be considered in isolation or as a substitute for revenues calculated in accordance with GAAP. Total care dollars under management includes PMPM payments to third parties that are not recorded in HCP’s accounting records and have not been reviewed and are not otherwise subject to procedures by HCP’s independent auditors. The following table reconciles Total Care Dollars Under Management to medical revenues for the periods indicated. “Total Care Dollars Under Management” is a non-GAAP measure. |
| Year ended December 31, | Six months ended June 30, | Twelve months ended June 30, | ||||||||||||||||||||||
| 2009 | 2010 | 2011 | 2011 | 2012 | 2012 | |||||||||||||||||||
| (in millions) | ||||||||||||||||||||||||
Medical revenues | $ | 1,731 | $ | 2,049 | $ | 2,375 | $ | 1,158 | $ | 1,294 | $ | 2,511 | ||||||||||||
Less: Risk share revenue, net | (30 | ) | (87 | ) | (127 | ) | (52 | ) | (61 | ) | (136 | ) | ||||||||||||
Add: Institutional capitation amounts | 687 | 831 | 964 | 476 | 519 | 1,007 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Total care dollars under management | $ | 2,388 | $ | 2,793 | $ | 3,212 | $ | 1,582 | $ | 1,752 | $ | 3,382 | ||||||||||||
| (2) | HCP uses Adjusted EBITDA and similar calculations as measures to assess operating and financial performance, including compliance with the financial covenants contained in its senior secured credit agreement. Adjusted EBITDA is defined as net income attributable to HCP before income taxes, net debt expense, depreciation and amortization, stock-based compensation, and any impairment charges. Adjusted EBITDA is not a measure of financial performance computed in accordance with GAAP and should not be considered in isolation or as a substitute for operating income, net income, cash flows from operations, or other statement of operations or cash flow data prepared in conformity with GAAP, or as measures of profitability or liquidity. In addition, the calculation of Adjusted EBITDA is susceptible to varying interpretations and calculation, and the amounts presented may not be comparable to similarly titled measures of other companies. Adjusted EBITDA may not be indicative of historical operating results, and HCP does not mean for it to be predictive of future results of operations or cash flows. Adjusted EBITDA reconciled to net income to HCP is as follows: |
| Guarantors | ||||||||||||||||||||||||||||
| Year ended December 31, | Six months ended June 30, | Twelve months ended June 30, | Twelve months ended June 30,(a) | |||||||||||||||||||||||||
| 2009 | 2010 | 2011 | 2011 | 2012 | 2012 | 2012 | ||||||||||||||||||||||
| (dollars in millions) | ||||||||||||||||||||||||||||
Net income | $ | 220 | $ | 330 | $ | 409 | $ | 192 | $ | 233 | $ | 450 | $ | 451 | ||||||||||||||
Income tax expense | 41 | 49 | 71 | 37 | 33 | 67 | 64 | |||||||||||||||||||||
Debt expense | 6 | 5 | 16 | 9 | 6 | 13 | 13 | |||||||||||||||||||||
Depreciation and amortization | 26 | 29 | 31 | 16 | 16 | 31 | 28 | |||||||||||||||||||||
Stock-based compensation | 6 | 7 | 7 | 4 | 4 | 8 | 8 | |||||||||||||||||||||
Interest income | (6 | ) | (6 | ) | (7 | ) | (3 | ) | (4 | ) | (8 | ) | (7 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Adjusted EBITDA | $ | 293 | $ | 414 | $ | 527 | $ | 255 | $ | 288 | $ | 561 | $ | 557 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
| (a) | This information consists of combined amounts for HCP, excluding HCP’s affiliated physician groups, but inclusive of management fee revenue therefrom. |
S-34
Table of Contents
Any investment in the notes involves a high degree of risk. You should carefully consider the risks described below together with all the other information contained in, or incorporated by reference into, this prospectus supplement and the accompanying prospectus, before making a decision to invest in the notes. Some of these factors relate principally to our business. The risks and uncertainties described below are not the only ones facing us. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also have a material adverse effect on our business and operations.
If any of the matters included in the following risks were to occur, our business, financial condition, results of operations, cash flows or prospects could be materially adversely affected. In such case, you may lose all or part of your original investment.
Risks Relating to Our Business
If the average rates that commercial payors pay us decline significantly, it would have a material adverse effect on our revenues, earnings and cash flows.
Approximately 34% of our dialysis and related lab services revenues for the six months ended June 30, 2012 were generated from patients who have commercial payors as the primary payor. The majority of these patients have insurance policies that pay us on terms and at rates that are generally significantly higher than Medicare rates. The payments we receive from commercial payors generate nearly all of our profit and all of our nonacute dialysis profits come from commercial payors. We continue to experience downward pressure on some of our commercial payment rates and it is possible that commercial payment rates could be materially lower in the future. The downward pressure on commercial payment rates is a result of general conditions in the market, recent and future consolidations among commercial payors, increased focus on dialysis services and other factors.
We are continuously in the process of negotiating our existing or potentially new agreements with commercial payors who tend to be aggressive in their negotiations with us. Sometimes many significant agreements are up for renewal or being renegotiated at the same time. In the event that our continual negotiations result in overall commercial rate reductions in excess of overall commercial rate increases, the cumulative effect could have a material adverse effect on our financial results. Consolidations have significantly increased the negotiating leverage of commercial payors. Our negotiations with payors are also influenced by competitive pressures. Some of our contracted rates with commercial payors may decrease or we may experience decreases in patient volume as our negotiations with commercial payors continue. In addition to downward pressure on contracted commercial payor rates, payors have been attempting to impose restrictions and limitations on non-contracted or out-of-network providers. In some circumstances for some commercial payors, our centers are designated as out-of-network providers. Rates for out-of-network providers are on average higher than rates for in-network providers. We believe commercial payors have or will begin to restructure their benefits to create disincentives for patients to select or remain with out-of-network providers and to decrease payment rates for out-of-network providers. Decreases in out-of-network rates and restrictions on out-of-network access, our turning away new patients in instances where we are unable to come to agreement on rates, or decreases in contracted rates could result in a significant decrease in our overall revenues derived from commercial payors. If the average rates that commercial payors pay us decline significantly, or if we see a decline in commercial patients, it would have a material adverse effect on our revenues, earnings and cash flows.
If the number of patients with higher-paying commercial insurance declines, then our revenues, earnings and cash flows would be substantially reduced.
Our revenue levels are sensitive to the percentage of our patients with higher-paying commercial insurance coverage. A patient’s insurance coverage may change for a number of reasons, including changes in the patient’s or a family member’s employment status. Currently, for a patient covered by an employer group health plan,
S-35
Table of Contents
Medicare generally becomes the primary payor after 33 months, or earlier, if the patient’s employer group health plan coverage terminates. When Medicare becomes the primary payor, the payment rate we receive for that patient shifts from the employer group health plan rate to the lower Medicare payment rate. We have seen an increase in the number of patients who have government-based programs as their primary payors which we believe is largely a result of improved mortality and recent economic conditions which have a negative impact on the percentage of patients covered under commercial insurance plans. To the extent there are sustained or increased job losses in the U.S., independent of whether general economic conditions might be improving, we could experience a continued decrease in the number of patients covered under commercial plans. We could also experience a further decrease if changes to the healthcare regulatory system result in fewer patients covered under commercial plans or an increase of patients covered under more restrictive commercial plans with lower reimbursement rates. In addition, our continuous process of negotiations with commercial payors under existing or potentially new agreements could result in a decrease in the number of patients under commercial plans to the extent that we cannot reach agreement with commercial payors on rates and other terms, resulting in termination or non-renewals of existing agreements or our inability to enter into new ones. If there is a significant reduction in the number of patients under higher-paying commercial plans relative to government-based programs that pay at lower rates, it would have a material adverse effect on our revenues, earnings and cash flows.
Changes in the structure of, and payment rates under the Medicare ESRD program, including the Budget Control Act of 2011 and other healthcare reform initiatives, could substantially reduce our revenues, earnings and cash flows.
Approximately 49% of our dialysis and related lab services revenues for the six months ended June 30, 2012 was generated from patients who have Medicare as their primary payor. Prior to January 1, 2011, the Medicare ESRD program paid us for dialysis treatment services at a fixed composite rate. The Medicare composite rate was the payment rate for a dialysis treatment including the supplies used in those treatments, specified laboratory tests and certain pharmaceuticals. Certain other pharmaceuticals, including EPO, vitamin D analogs and iron supplements, as well as certain specialized laboratory tests, were separately billed.
In July 2008, the Medicare Improvements for Patients and Providers Act of 2008 was passed by Congress. This legislation introduced a new payment system for dialysis services beginning in January 2011 whereby payment for dialysis treatment and related services is now made under a bundled payment rate which provides a fixed rate to encompass all goods and services provided during the dialysis treatment, including pharmaceuticals that were historically separately reimbursed to the dialysis providers, such as EPO, vitamin D analogs and iron supplements, as well as laboratory testing. In August 2010, CMS published the final rule implementing the bundled payment in the Federal Register. The initial 2011 bundled rate included reductions of 2% from the prior reimbursement and further reduced overall rates by 5.94% tied to an expanded list of case-mix adjustors which can be earned back based upon the presence of certain patient characteristics and co-morbidities at the time of treatment. There are also other provisions which may impact payment including an outlier pool and a low volume facility adjustment.
Another important provision in the law is an annual adjustment, or market basket update, to the base ESRD Prospective Payment Rate, or PPS. Absent action by Congress the PPS base rate will be automatically updated by a formulaic inflation adjustment.
On November 1, 2011, CMS issued the final ESRD PPS rule for 2012, which increased the base rate by 2.1%, representing a market base of increase of 3.0% less a productivity adjustment of 0.9%. The increase in the final base rate for 2012 (2.1%) is slightly greater than the increase of 1.8% stated in the proposed 2012 ESRD PPS rule published in July 2011, and was made irrespective of the Medicare Payment Advisory Commission, or MedPAC, recommendation for a reduced increase. The MedPAC focus on such a reduction indicates further scrutiny of the annual update is possible.
On July 11, 2012, CMS issued the proposed ESRD PPS rule for 2013. As currently proposed, the base rate will increase by 2.5%, resulting from a market basket increase of 3.2% less a productivity adjustment of 0.7%.
S-36
Table of Contents
This increase in the ESRD PPS base rate will be further reduced by the Budget Control Act of 2011 sequestration, discussed below. The proposed rule implements the reduction in bad debt payments to dialysis facilities (as well as to all other providers eligible for bad debt payments) mandated under the Middle Class Tax Relief and Job Creation Act of 2012 and adds new quality reporting measures.
The new payment system presents operating, clinical and financial risks. For example, with regard to the expanded list of case-mix adjustors, there is a risk that our dialysis centers or billing and other systems may not accurately document and track the appropriate patient-specific characteristics, resulting in a reduction or overpayment in the amounts of the payments that we would otherwise be entitled to receive.
Beginning January 1, 2014, certain oral-only ESRD drugs (currently paid separately to pharmacies under Medicare Part D) will be included in the ESRD bundled payment to dialysis facilities. CMS delayed the inclusion of these oral only ESRD drugs until 2014 in order to assess how to reimburse for these oral drugs and services. It is currently unclear how CMS will “price” the oral-only drugs for inclusion in the ESRD bundle in 2014. Inadequate pricing could have a significant negative financial impact on our dialysis facilities given the volume and value of these drugs.
We expect to continue experiencing increases in operating costs that are subject to inflation, such as labor and supply costs, regardless of whether there is a compensating inflation-based increase in Medicare payment rates or in payments under the new bundled payment rate system.
On August 2, 2011, President Obama signed into law the “Budget Control Act of 2011” (Public Law 112-25), which raised the debt ceiling and put into effect a series of actions to reduce the federal budget deficit over ten years. The law created a Joint Congressional Committee charged with producing legislation reducing federal spending by at least $1.2 trillion. As a result of the committee’s failure to act, the federal government is facing a $1.2 trillion sequester (across-the-board cuts in discretionary programs). However, Medicare providers face a maximum of no more than a 2% reduction in reimbursements in fiscal year 2013.
We also cannot predict whether we will be able to comply with the CMS rules related to the bundled payment system as processes and systems are modified substantially to capture all required data. To the extent we are not able to adequately bill and collect for certain payment adjustors and are not able to offset the mandated reductions in reimbursement or if we face regulatory enforcement actions and penalties as a result of alleged improper billing of governmental programs, it could have a material adverse effect on our revenues, earnings and cash flows. For additional details regarding the risks we face for failing to adhere to our Medicare and Medicaid regulatory compliance obligations, see the risk factor below under the heading “If we fail to adhere to all of the complex government regulations that apply to our business, we could suffer severe consequences that would substantially reduce our revenues, earnings and cash flows”.
Health care reform could substantially reduce our revenues, earnings and cash flows.
In March 2010, broad health care reform legislation was enacted in the U.S. Although many of the provisions of the new legislation do not take effect immediately, and may be modified before they are implemented, the reforms could have an impact on our business in a number of ways. We cannot predict how employers, private payors or persons buying insurance might react to these changes or what form many of these regulations will take before implementation. In March 2012, the Department of Health and Human Services, or HHS, issued two final proposed rules related to the establishment of health care insurance exchanges due to be operating by 2014 that will provide a marketplace for eligible individuals to purchase health care insurance. The first relates to the standards and requirements applicable to the exchanges, employers and qualified health plans that are marketed in the exchange. The second rule finalizes the provisions governing the risk adjustment program that includes reinsurance, risk corridors and risk adjustment. The final exchange rules clarify the requirements related to implementation of such exchanges, outline areas of state flexibility in their implementation of such exchanges and provide standards for certain risk adjustment mechanisms. We believe the
S-37
Table of Contents
establishment of health care insurance exchanges could result in a reduction in patients covered by commercial insurance or an increase of patients covered through the exchanges under more restrictive commercial plans with lower reimbursement rates. To the extent that the implementation of such exchanges results in a reduction in patients covered by commercial insurance or a reduction in reimbursement rates for our services from commercial and/or government payors, our revenues, earnings and cash flows could be adversely affected.
In October 2011, CMS issued a final rule concerning the Medicare Shared Savings Program established by the health care reform legislation, which under the statute was required to be implemented no later than January 1, 2012. The Medicare Shared Savings Program, which is now operational provides financial incentives to health care providers and suppliers that work together to furnish coordinated, high-quality care to Medicare beneficiaries through accountable care organizations, or ACOs.
The CMS Center for Innovation (Innovation Center) is in various stages of development in working with various healthcare providers to implement ACOs and other innovative models of care for Medicare and Medicaid beneficiaries. We are currently uncertain of the extent to which these models of care including ACOs, Bundled Payments for Care Improvement Initiative, the Comprehensive Primary Care Initiative, the Duals Demonstration, or other models, will impact the health care market. As a provider of dialysis services, we may choose to participate in one or several of these models either as a partner with other providers or independently. We are currently seeking a renal specific coordinated care pilot with the Innovation Center. Even if we do not participate in these programs, some of our patients may be assigned to a pilot, in which case the quality and cost of care that we furnish will be included in an ACO’s or other program’s calculations regardless of our participation in the program. As new models of care emerge, we may be at risk for losing our Medicare patient base, which would have a materially adverse effect on our revenues, earnings and cash flow. Furthermore, further initiatives in the government or private sector may arise, including the development of models similar to ACOs, independent practice associations and integrated delivery systems or evolutions of those concepts which could adversely impact our business.
In addition, the Health Reform Acts introduced severe penalties for the knowing and improper retention of overpayments collected from government payors. As a result, we made initial significant investments in additional resources to accelerate the time it takes to identify and process overpayments and we may be required to make additional investments in the future. Acceleration in our ability to identify and process overpayments could result in us refunding overpayments to government or other payors sooner than we have in the past, which could have a material adverse effect on our operating cash flows. The failure to return identified overpayments within the specified time frame is now a violation of the federal False Claims Act, or FCA.
The Health Reform Acts also reduced the timeline to file Medicare claims, which now must be filed with the government within one calendar year after the date of service. To comply with this reduced timeline, we must deploy significant resources and may change our claims processing methods to ensure that our Medicare claims are filed in a timely fashion. Failure to file a claim within the one year window could result in payment denials, adversely affecting our revenues, earnings and cash flows.
Effective March 2011, CMS instituted new screening procedures and a new $500 enrollment fee for providers enrolling and re-enrolling in government health care programs. A provider is subject to screening upon initial enrollment and each time the provider re-validates its enrollment application. Screening includes verification of enrollment information and review of various federal databases to ensure the provider has valid tax identification NPI numbers and is not excluded from participation in federal and state healthcare programs. We expect this screening process to delay the Medicare contractor approval process, potentially causing a delay in reimbursement. The enrollment fee is also applicable upon initial enrollment, re-validation, and each time an existing provider adds a new facility location. This fee is an additional expense that must be paid for each center every three years and could be more significant if other government and commercial payors follow this trend. Ultimately, we anticipate the new screening and enrollment requirements will require additional personnel and financial resources and will potentially delay the enrollment and revalidation of our centers which in turn will delay payment.
S-38
Table of Contents
Other reform measures allow CMS to place a moratorium on new enrollment of providers and to suspend payment to providers upon a credible allegation of fraud from any source. These types of reform measures, or others, depending upon the scope and breadth of the implementing regulations, could adversely impact our revenues, earnings and cash flows.
There are numerous steps required to implement the broad healthcare reform legislation adopted by Congress, and Congress may seek to alter or eliminate some of the provisions described above. Numerous legal challenges have also been raised to the healthcare reform legislation that could alter or eliminate certain provisions. The United States Supreme Court reviewed state actions challenging the constitutionality of the health insurance mandate and the Medicaid expansion program. The Court upheld the mandate under Congress’ taxing power and upheld the Medicaid expansion program. However, the Court found that the federal government cannot withhold all of a state’s Medicaid funding for the state’s failure or refusal to expand its Medicaid program as contemplated by the reform legislation, effectively leaving the Medicaid expansion decision up to the individual states. Several states have announced they do not intend to expand their Medicaid programs. Further, various health insurance reform proposals are also emerging at the state level. There is a considerable amount of uncertainty as to the prospective implementation of the federal healthcare reform legislation and what similar measures might be enacted at the state level. The enacted reforms as well as future legislative changes could have a material adverse effect on our results of operations, including lowering our reimbursement rates and increasing our expenses. The Healthcare Reform Acts added several new tax provisions that, among other things, impose various fees and excise taxes, and limit compensation deductions for health insurance providers and their affiliates. To date, the IRS has not issued regulations for many of these provisions. In the event that we, or any of our current or future subsidiaries, were to become subject to these rules, our cash flow and tax liabilities could be negatively impacted.
Changes in state Medicaid or other non-Medicare government-based programs or payment rates could reduce our revenues, earnings and cash flows.
Approximately 16% of our dialysis and related lab services revenues for the six months ended June 30, 2012 was generated from patients who have state Medicaid or other non-Medicare government-based programs, such as Medicare-assigned plans or the VA, as their primary coverage. As state governments and governmental organizations face increasing budgetary pressure, we may in turn face reductions in payment rates, delays in the timing of payments, limitations on eligibility or other changes to the applicable programs. For example, some programs, such as certain state Medicaid programs and the VA, have recently considered, proposed or implemented rate reductions.
On December 17, 2010, the Department of Veterans Affairs published a final rule in which it materially changed the payment methodology and ultimately the amount paid for dialysis services furnished to veterans in non-VA centers such as ours. In the final rule, the VA adopted the bundled payment system implemented by Medicare and estimated a reduction of 39% in payments for dialysis services to veterans at non-VA centers. Approximately 2% of our dialysis and related lab services revenues for the six months ended June 30, 2012 was generated by the VA. The new VA payment methodology will have a significant negative impact on our revenues, earnings and cash flows as a result of the reduction in rates or as a result of the decrease in the number of VA patients we serve. We recently executed contractual agreements with the VA and there is some uncertainty as to when this rule will take effect for the patients covered by these contracts. While at this time the contracts remain in force, these agreements provide for the right of the VA to terminate the agreement without cause on short notice. Further, patients who are not covered by the contractual arrangements will likely be reimbursed at Medicare rates beginning with the date of implementation of the rule. If the VA proceeds with payment rate reductions or fails to renew our existing contracts, we might have to cease accepting patients under this program and could even be forced to close centers.
State Medicaid programs are increasingly adopting Medicare-like bundled payment systems, but sometimes these new payment systems are poorly defined and could include all drugs (even those oral-only drugs that
S-39
Table of Contents
Medicare will not include in the bundled payment until 2014) and are implemented without any claims processing infrastructure, or patient or facility adjusters. If these new payment systems are implemented without any adjusters and claims processing changes, Medicaid payments will be substantially reduced and the costs to submit such claims may increase. In addition, some state Medicaid program eligibility requirements mandate that citizen enrollees in such programs provide documented proof of citizenship. If our patients cannot meet these proof of citizenship documentation requirements, they may be denied coverage under these programs. These Medicaid payment and enrollment changes, along with similar changes to other non-Medicare government programs could reduce the rates paid by these programs for dialysis and related services, delay the timing of payment for services provided, and further limit eligibility for coverage which could adversely affect our revenues, earnings and cash flows.
Changes in clinical practices, payment rates or regulations impacting EPO and other pharmaceuticals could reduce our revenues, earnings and cash flows.
Historically, Medicare and most Medicaid programs paid for EPO outside of the composite rate. This separate payment has long been the subject of discussions regarding appropriate dosing and payment in an effort to reduce escalating expenditures for EPO. Since January 1, 2011, Medicare has bundled EPO into the prospective payment system such that dosing variations will not change the amount paid to a dialysis facility. Although some Medicaid programs and other payors suggest movement towards a bundled payment system inclusive of EPO, some non-Medicare payors continue to pay for EPO separately from the treatment rate. The administration of EPO and other pharmaceuticals that are separately billable accounted for approximately 5% of our dialysis and related lab services revenues for the six months ended June 30, 2012, with EPO alone accounting for approximately 3% of our dialysis and related lab services revenues for the same period. Changes in physician clinical practices that result in further decreased utilization of prescribed pharmaceuticals or changes in payment rates for those pharmaceuticals could reduce our revenues, earnings and cash flows.
Since late 2006, there has been significant media discussion and government scrutiny regarding anemia management practices in the U.S. which has created confusion and concern in the nephrology community. In late 2006, the U.S. House of Representatives Ways and Means Committee held a hearing on the issue of the utilization of ESAs, which include EPO, and in 2007, the FDA required changes to the labeling of EPO and Aranesp® to include a black box warning, the FDA’s strongest form of warning label. An FDA advisory panel on ESA use met in October 2010, which meeting was similar to the prior meeting held in 2007 in that there was significant discussion and concern about the safety of ESAs. The panel concluded it would not recommend a change in ESA labeling. However, the FDA is not bound by the panel’s recommendation. In June 2011, the FDA required that the black box warning be slightly revised and also include more conservative dosing recommendations for patients with chronic kidney disease. In addition, in June 2011, CMS opened a National Coverage Analysis, or NCA, for ESAs. Further in January 2011, CMS convened a meeting of the Medicare Evidence Development and Coverage Advisory Committee, or MEDCAC, to evaluate evidence for the pending NCA. In June 2011, CMS determined not to issue a national coverage determination for ESAs due to a lack of available evidence to establish coverage criteria or limitations.
The forgoing congressional and agency activities and related actions could result in further restrictions on the utilization and reimbursement for ESAs. Commercial payors have also increasingly examined their administration policies for EPO and, in some cases, have modified those policies. Further changes in labeling of EPO and other pharmaceuticals in a manner that alters physician practice patterns or accepted clinical practices, changes in private and governmental payment criteria, including the introduction of EPO administration policies or the conversion to alternate types of administration of EPO or other pharmaceuticals that result in further decreases in utilization of EPO for patients covered by commercial payors or increased utilization of EPO for patients for whom the cost of EPO is included in a bundled reimbursement rate, or further decreases in reimbursement for EPO and other pharmaceuticals that are not included in a bundled reimbursement rate, could have a material adverse effect on our revenues, earnings and cash flows.
S-40
Table of Contents
Changes in EPO pricing could materially reduce our earnings and cash flows and affect our ability to care for our patients.
In November 2011, we entered into a seven year Sourcing and Supply Agreement with Amgen USA Inc. Under the agreement we committed to purchase EPO in amounts necessary to meet no less than 90% of our requirements for erythropoiesis stimulating agents. The agreement replaces in its entirety the prior one-year supply agreement between us and Amgen that expired on December 31, 2011. As long as certain conditions are met by us, the agreement limits Amgen’s ability to unilaterally decide to increase the price for EPO. Future increases in the cost of EPO without corresponding increases in payment rates for EPO from commercial payors and without corresponding increases in the Medicare bundled rate could have a material adverse effect on our earnings and cash flows and ultimately reduce our income. Our agreement with Amgen for EPO provides for discounted pricing and rebates for EPO. Some of the rebates are subject to various conditions including but not limited to future pricing levels of EPO by Amgen and data submission by us. In addition, the rebates are subject to certain limitations. We cannot predict whether, over the seven year term of the agreement, we will continue to receive the rebates for EPO that we have received in the past, or whether we will continue to achieve the same levels of rebates within that structure as we have historically achieved. In the initial years of the agreement, however, the total rebate opportunity is less than what was provided in the agreement that expired at the end of 2011, however, the opportunity for us to earn discounts and rebates increases over the term of the agreement. Factors that could impact our ability to qualify for rebates provided for in our agreement with Amgen in the future include, but are not limited to, our ability to track certain data elements. We cannot predict whether we will be able to meet the applicable qualification requirements for receiving rebates. Failure to meet certain targets and earn the specified rebates could have a material adverse effect on our earnings and cash flows.
We are the subject of a number of inquiries by the federal government and two private civil suits, any of which could result in substantial penalties or awards against us, imposition of certain obligations on our practices and procedures, exclusion from future participation in the Medicare and Medicaid programs and, in certain cases, criminal penalties.
We are the subject of a number of inquiries by the federal government. We have received subpoenas or other requests for documents from the federal government in connection with the 2005 U.S. Attorney investigation, the Woodard private civil suit, the Vainer private civil suit, the 2010 U.S. Attorney physician relationship investigation, the 2011 U.S. Attorney physician relationship investigation and the 2011 U.S. Attorney Medicaid investigation. Certain current and former members of the Board and executives have been subpoenaed to testify before the grand jury in Colorado, and other Company representatives may also receive subpoenas for testimony related to the 2011 U.S. Attorney physician relationship investigation. After investigation, the government did not intervene and is not actively pursuing either the Woodard or the Vainer private civil suits mentioned above. In each of these private civil suits, a relator has filed a complaint against us in federal court under thequi tam provisions of the FCA and is pursuing the claims independently. The parties are engaged in active litigation in the Vainer private civil suit. In the Woodward private civil suit, though we have reached an agreement in principle to settle all allegations relating to claims arising out of this suit, it is still subject to the parties being able to enter into a mutually acceptable settlement agreement and receive the requisite approval of the federal government and the court to fully and finally resolve this matter. We are cooperating with the OIG and those offices of the U.S. Attorney still actively pursuing the matters mentioned above and are producing the requested records. Although we cannot predict whether or when proceedings might be initiated by the federal government, the scope of such proceedings or when these matters may be resolved, it is not unusual for investigations such as these to continue for a considerable period of time through the various phases of document and witness requests and on-going discussions with regulators. Responding to the subpoenas or investigations and defending ourselves in the private civil suits will continue to require management’s attention and significant legal expense. Any negative findings could result in substantial financial penalties or awards against us, imposition of certain obligations on our practices and procedures, exclusion from future participation in the Medicare and Medicaid programs and, in certain cases, criminal penalties (see the discussion below under the captions “Management’s Discussion and Analysis of Financial Condition and Results of Operations—
S-41
Table of Contents
Operating expenses and charges— Legal proceeding contingency accrual and related expenses” and “Legal Proceedings” for additional details regarding these matters). To our knowledge, no proceedings have been initiated by the federal government against us at this time.
Continued inquiries from various governmental bodies with respect to our utilization of EPO and other pharmaceuticals will require management’s attention, cause us to incur significant legal expense and could result in substantial financial penalties against us, repayment obligations or exclusion from future participation in the Medicare and Medicaid programs, and could have a material adverse effect on our revenues, earnings and cash flows.
In response to clinical studies which identified risks in certain patient populations related to the utilization of EPO and other ESAs, i.e., Aranesp®, and in response to changes in the labeling of EPO and Aranesp®, there has been substantial media attention and government scrutiny resulting in hearings and legislation regarding pharmaceutical utilization and reimbursement. Although we believe our anemia management practices and other pharmaceutical administration practices have been compliant with existing laws and regulations, as a result of the current high level of scrutiny and controversy, we may be subject to increased inquiries from a variety of governmental bodies and claims by third parties. Additional inquiries from or audits by various agencies and claims by third parties with respect to these issues would continue to require management’s attention and significant legal expense and any negative findings could result in substantial financial penalties or repayments, imposition of certain obligations on our practices and procedures and the attendant financial burden on us to comply, or exclusion from future participation in the Medicare and Medicaid programs, and could have a material adverse effect on our revenues, earnings and cash flows.
If we fail to adhere to all of the complex government regulations that apply to our business, we could suffer severe consequences that would substantially reduce our revenues, earnings, cash flows and stock price.
Our dialysis operations are subject to extensive federal, state and local government regulations, including Medicare and Medicaid payment rules and regulations, federal and state anti-kickback laws, the Stark Law physician self-referral prohibition and analogous state referral statutes, Federal Acquisition Regulations, the FCA and federal and state laws regarding the collection, use and disclosure of patient health information and the storage, handling and administration of pharmaceuticals. The Medicare and Medicaid reimbursement rules related to claims submission, enrollment and licensing requirements, cost reporting, and payment processes impose complex and extensive requirements upon dialysis providers. A violation or departure from any of these requirements may result in government audits, lower reimbursements, significant fines and penalties, the potential loss of certification and recoupments or voluntary repayments.
The regulatory scrutiny of healthcare providers, including dialysis providers continues to increase. For example, CMS has indicated that after implementation of the Medicare bundled payment system, it will monitor the use of EPO and other pharmaceuticals. In addition, Medicare has increased the frequency and intensity of its certification inspections of dialysis centers. For example, we are required to provide substantial documentation related to the administration of pharmaceuticals, including EPO, and, to the extent that any such documentation is found insufficient, we may be required to refund to government or commercial payors any amounts received for such administration, and be subject to substantial penalties under applicable laws or regulations. In addition, Medicare contractors have increased their prepayment and post-payment reviews.
We endeavor to comply with all of the requirements for receiving Medicare and Medicaid payments, to structure all of our relationships with referring physicians to comply with state and federal anti-kickback laws and physician self-referral law (Stark Law), and for storing, handling and administering pharmaceuticals. However, the laws and regulations in these areas are complex, require considerable resources to monitor and implement and are subject to varying interpretations. For example, if an enforcement agency were to challenge the level of compensation that we pay our medical directors or the number of medical directors whom we engage, we could be required to change our practices, face criminal or civil penalties, pay substantial fines or otherwise
S-42
Table of Contents
experience a material adverse effect as a result of a challenge to these arrangements. In addition, amendments to the FCA impose severe penalties for the knowing and improper retention of overpayments collected from government payors. These amendments could subject our procedures for identifying and processing overpayments to greater scrutiny. We have made significant investments in additional resources to decrease the time it takes to identify and process overpayments and we may be required to make additional investments in the future. An acceleration in our ability to identify and process overpayments could result in us refunding overpayments to government or other payors sooner than we have in the past. A significant acceleration of these refunds could have a material adverse affect on our operating cash flows. Additionally, amendments to the federal anti-kickback statute in the health reform law make anti-kickback violations subject to FCA prosecution, includingqui tam or whistleblower suits.
If any of our operations are found to violate these or other government regulations, we could suffer severe consequences that would have a material adverse effect on our revenues, earnings, cash flows and stock price, including:
| • | Suspension or termination of our participation in government payment programs; |
| • | Refunds of amounts received in violation of law or applicable payment program requirements; |
| • | Loss of required government certifications or exclusion from government payment programs; |
| • | Loss of licenses required to operate health care facilities or administer pharmaceuticals in some of the states in which we operate; |
| • | Reductions in payment rates or coverage for dialysis and ancillary services and related pharmaceuticals; |
| • | Fines, damages or monetary penalties for anti-kickback law violations, Stark Law violations, FCA violations, civil or criminal liability based on violations of law, or other failures to meet regulatory requirements; |
| • | Enforcement actions by governmental agencies and/or claims for monetary damages by patients who believe protected health information has been used or disclosed in violation of federal or state patient privacy laws, including the federal Health Insurance Portability and Accountability Act of 1996 (HIPAA); |
| • | Mandated changes to our practices or procedures that significantly increase operating expenses; |
| • | Imposition of and compliance with Corporate Integrity Agreements that could subject us to ongoing audits, reporting, increased scrutiny of our billing and business practices and potential additional fines; |
| • | Termination of relationships with medical directors; and |
| • | Harm to our reputation, which could impact our business relationships, ability to obtain financing and access to new opportunities. |
Delays in state Medicare and Medicaid certification of our dialysis centers could adversely affect our revenues, earnings and cash flows.
Before we can begin billing for patients treated in our outpatient dialysis centers who are enrolled in government-based programs, we are required to obtain state and federal certification for participation in the Medicare and Medicaid programs. As state agencies responsible for surveying dialysis centers on behalf of the state and Medicare program face increasing budgetary pressure, certain states are having difficulty keeping up with certifying dialysis centers in the normal course resulting in significant delays in certification. If state governments continue to have difficulty keeping up with certifying new centers in the normal course and we continue to experience significant delays in our ability to treat and bill for services provided to patients covered under government programs, it could cause us to incur write-offs of investments or accelerate the recognition of
S-43
Table of Contents
lease obligations in the event we have to close centers or our centers’ operating performance deteriorates, and it could have an adverse effect on our revenues, earnings and cash flows.
If our joint ventures were found to violate the law, we could suffer severe consequences that would have a material adverse effect on our revenues, earnings and cash flows.
As of June 30, 2012, we owned a controlling interest in numerous dialysis-related joint ventures, which represented approximately 19% of our dialysis and related lab services revenues for the six months ended June 30, 2012. In addition, we also owned minority equity investments in several other dialysis related joint ventures. We anticipate that we will continue to increase the number of our joint ventures. Many of our joint ventures with physicians or physician groups also have the physician owners providing medical director services to those centers or other centers we own and operate. Because our relationships with physicians are governed by the federal anti-kickback statute, we have sought to structure our joint venture arrangements to satisfy as many safe harbor requirements as we believe are reasonably possible. However, our joint venture arrangements do not satisfy all elements of any safe harbor under the federal anti-kickback statute (and possibly the Stark Law). The subpoena and related requests for documents we received from the U.S. Attorney’s Office for the Eastern District of Missouri in the 2005 U.S. Attorney investigation, the OIG’s Office in Dallas in the 2010 U.S. Attorney physician relationship investigation and the U.S. Attorney’s Office for the District of Colorado in the 2011 U.S. Attorney physician relationship investigation, included requests for documents related to our joint ventures. We were advised by the U.S. Department of Justice that it is conducting civil and grand jury investigations into our financial relationships with physicians.
If our joint ventures are found to be in violation of the anti-kickback statute or the Stark Law provisions, we could be required to restructure the joint ventures or refuse to accept referrals for designated health services from the physicians with whom the joint venture centers have a financial relationship.
We also could be required to repay amounts received by the joint ventures from Medicare and certain other payors to the extent that these arrangements are found to give rise to prohibited referrals, and we could be subject to monetary penalties, exclusion from government healthcare programs and, if criminal proceedings are brought against us, criminal penalties. If our joint venture centers are subject to any of these penalties, we could suffer severe consequences that would have a material adverse effect on our revenues, earnings and cash flows.
There are significant estimating risks associated with the amount of dialysis revenues and related refund liabilities that we recognize and if we are unable to accurately estimate our revenues and related refund liabilities, it could impact the timing and the amount of our revenues recognition or have a significant impact on our operating results.
There are significant estimating risks associated with the amount of dialysis and related lab services revenues and related refund liabilities that we recognize in a reporting period. The billing and collection process is complex due to ongoing insurance coverage changes, geographic coverage differences, differing interpretations of contract coverage, and other payor issues. Determining applicable primary and secondary coverage for approximately 149,000 U.S. patients at any point in time, together with the changes in patient coverage that occur each month, requires complex, resource-intensive processes. Errors in determining the correct coordination of benefits may result in refunds to payors. Revenues associated with Medicare and Medicaid programs are also subject to estimating risk related to the amounts not paid by the primary government payor that will ultimately be collectible from other government programs paying secondary coverage, the patient’s commercial health plan secondary coverage or the patient. Collections, refunds and payor retractions typically continue to occur for up to three years and longer after services are provided. We generally expect our range of dialysis and related lab services revenues estimating risk to be within 1% of revenues for the segment, which can represent as much as 6% of consolidated operating income. If our estimates of dialysis and related lab services revenues and related refund liabilities are materially inaccurate, it could impact the timing and the amount of our revenues recognition and have a significant impact on our operating results.
S-44
Table of Contents
The ancillary services we provide or the strategic initiatives, including our international dialysis operations, that we invest in now or in the future may generate losses and may ultimately be unsuccessful. In the event that one or more of these activities is unsuccessful, we may have to write off our investment and incur other exit costs.
Our ancillary services and strategic initiatives currently include pharmacy services, infusion therapy services, disease management services, vascular access services, ESRD clinical research programs, physician services and our international dialysis operations. We expect to add additional service offerings and pursue additional strategic initiatives in the future as circumstances warrant, which could include healthcare services not related to dialysis. Many of these initiatives require or would require investments of both management and financial resources and can generate significant losses for a substantial period of time and may not become profitable. There can be no assurance that any such strategic initiative will ultimately be successful. Any significant change in market conditions, or business performance, or in the political, legislative or regulatory environment, may impact the economic viability of any of these strategic initiatives. For example, during 2011 and 2010, several of our strategic initiatives generated net operating losses and some are expected to generate net operating losses in 2012. If any of our ancillary services or strategic initiatives, including our international dialysis operations, do not perform as planned, we may incur a material write-off or an impairment of our investment, including goodwill, in one or more of these activities or we could incur significant termination costs if we were to exit a certain line of business. As an example, during the second quarter of 2011 we recorded a goodwill impairment charge of $24 million related to a decrease in the implied fair value of goodwill below its carrying amount associated with our infusion therapy business.
If a significant number of physicians were to cease referring patients to our dialysis centers, whether due to regulatory or other reasons, it would have a material adverse effect on our revenues, earnings and cash flows.
We believe that physicians prefer to have their patients treated at dialysis centers where they or other members of their practice supervise the overall care provided as medical director of the center. As a result, the primary referral source for most of our centers is often the physician or physician group providing medical director services to the center. Neither our current nor former medical directors have an obligation to refer their patients to our centers. If a medical director agreement terminates, whether before or at the end of its term, and a new medical director is appointed, it may negatively impact the former medical director’s decision to treat his or her patients at our center. If we are unable to enforce noncompetition provisions contained in the terminated medical director agreements, former medical directors may choose to provide medical director services for competing providers or establish their own dialysis centers in competition with ours. Also, if the quality of service levels at our centers deteriorates, it may negatively impact patient referrals and treatment volumes.
Our medical director contracts are for fixed periods, generally three to ten years, and at any given time a large number of them could be up for renewal at the same time. Medical directors have no obligation to extend their agreements with us, and there are a number of factors, including opportunities presented by our competitors or different affiliation models in the changing healthcare environment, such as an increase in the number of physicians becoming employed by hospitals, that could negatively impact their decisions to extend their agreements with us. In addition, we may take actions to restructure existing relationships or take positions in negotiating extensions of relationships to assure compliance with the anti-kickback statute, Stark Law and other similar laws. These actions also could negatively impact the decision of physicians to extend their medical director agreements with us or to refer their patients to us. If the terms of any existing agreement are found to violate applicable laws, we may not be successful in restructuring the relationship which could lead to the early termination of the agreement, or cause the physician to stop referring patients to our dialysis centers. If a significant number of physicians were to cease referring patients to our dialysis centers, whether due to regulatory or other reasons, then our revenues, earnings and cash flows would be substantially reduced.
S-45
Table of Contents
Current economic conditions as well as further disruptions in the financial markets could have a material adverse effect on our revenues, earnings and cash flows and otherwise adversely affect our financial condition.
Current economic conditions could adversely affect our business and our profitability. Among other things, the potential decline in federal and state revenues that may result from such conditions may create additional pressures to contain or reduce reimbursements for our services from Medicare, Medicaid and other government sponsored programs. Increasing job losses or slow improvement in the unemployment rate in the U.S. as a result of current or recent economic conditions has and may continue to result in a smaller percentage of our patients being covered by an employer group health plan and a larger percentage being covered by lower paying Medicare and Medicaid programs. Employers may also begin to select more restrictive commercial plans with lower reimbursement rates. To the extent that payors are negatively impacted by a decline in the economy, we may experience further pressure on commercial rates, a further slowdown in collections and a reduction in the amounts we expect to collect. In addition, uncertainty in the financial markets could adversely affect the variable interest rates payable under our credit facilities or could make it more difficult to obtain or renew such facilities or to obtain other forms of financing in the future, if at all. Any or all of these factors, as well as other consequences of the current economic conditions which cannot currently be anticipated, could have a material adverse effect on our revenues, earnings and cash flows and otherwise adversely affect our financial condition.
We may engage in acquisitions, mergers or dispositions, including the Merger, which may affect our results of operations, debt-to-capital ratio, capital expenditures or other aspects of our business.
We may engage in acquisitions, mergers or dispositions, including the Merger, which may affect our results of operations, debt-to-capital ratio, capital expenditures, or other aspects of our business. There can be no assurance that we will be able to identify suitable acquisition targets or merger partners or that, if identified, we will be able to acquire these targets on acceptable terms or agree to terms with merger partners. There can also be no assurance that we will be successful in completing any acquisitions, mergers or dispositions that we might be considering or announce, or integrating any acquired business into our overall operations or operate them successfully as stand-alone businesses, or that any such acquired business will operate profitably or will not otherwise adversely impact our results of operations. Further, we cannot be certain that key talented individuals at the business being acquired will continue to work for us after the acquisition or that they will be able to continue to successfully manage or have adequate resources to successfully operate any acquired business.
If we are not able to continue to make acquisitions, or maintain an acceptable level of non-acquired growth, or if we face significant patient attrition to our competitors or a reduction in the number of our medical directors, it could adversely affect our business.
The dialysis industry is highly competitive, particularly in terms of acquiring existing dialysis centers. We continue to face increased competition in the U.S. dialysis industry from large and medium-sized providers which compete directly with us for acquisition targets as well as for individual patients and medical directors. In addition, as we continue our international dialysis expansion into various international markets, we will face competition from large and medium-sized providers for these acquisition targets as well. Acquisitions, patient retention and medical director retention are an important part of our growth strategy. Because of the ease of entry into the dialysis business and the ability of physicians to be medical directors for their own centers, competition for growth in existing and expanding markets is not limited to large competitors with substantial financial resources. Occasionally, we have experienced competition from former medical directors or referring physicians who have opened their own dialysis centers. In addition, Fresenius, our largest competitor, manufactures a full line of dialysis supplies and equipment in addition to owning and operating dialysis centers. This may give it cost advantages over us because of its ability to manufacture its own products. If we are not able to continue to make acquisitions, continue to maintain acceptable levels of non-acquired growth, or if we face significant patient attrition to our competitors or a reduction in the number of our medical directors, it could adversely affect our business.
S-46
Table of Contents
If businesses we acquire have liabilities that we are not aware of, we could suffer severe consequences that would substantially reduce our earnings and cash flows.
Our business strategy includes the acquisition of dialysis centers and businesses that own and operate dialysis centers, as well as other ancillary and non-dialysis services and strategic initiatives, including the Merger. Businesses we acquire may have unknown or contingent liabilities or liabilities that are in excess of the amounts that we originally estimated. Although we generally seek indemnification from the sellers of businesses we acquire for matters that are not properly disclosed to us, we are not always successful. In addition, even in cases where we are able to obtain indemnification, we may discover liabilities greater than the contractual limits or the financial resources of the indemnifying party. In the event that we are responsible for liabilities substantially in excess of any amounts recovered through rights to indemnification, we could suffer severe consequences that would substantially reduce our earnings and cash flows.
Expansion of our operations to and offering our services in markets outside of the U.S. subjects us to political, legal, operational and other risks that could adversely affect our business, results of operations and cash flows.
We are undertaking an expansion of our operations and beginning to offer our services outside of the U.S., which increases our exposure to the inherent risks of doing business in international markets. Depending on the market, these risks include, without limitation, those relating to:
| • | changes in the local economic environment; |
| • | political instability, armed conflicts or terrorism; |
| • | social changes; |
| • | intellectual property legal protections and remedies; |
| • | trade regulations; |
| • | procedures and actions affecting approval, production, pricing, reimbursement and marketing of products and services; |
| • | foreign currency; |
| • | repatriating or moving to other countries cash generated or held abroad, including considerations relating to tax-efficiencies and changes in tax laws; |
| • | export controls; |
| • | lack of reliable legal systems which may affect our ability to enforce contractual rights; |
| • | changes in local laws or regulations; |
| • | potentially longer payment and collection cycles; |
| • | financial and operational, and information technology systems integration; and |
| • | failure to comply with U.S. or local laws that prohibit us or our intermediaries from making improper payments to foreign officials for the purpose of obtaining or retaining business. |
Additionally, some factors that will be critical to the success of our international business and operations will be different than those affecting our domestic business and operations. For example, conducting international operations requires us to devote significant management resources to implement our controls and systems in new markets, to comply with local laws and regulations and to overcome the numerous new challenges inherent in managing international operations, including those based on differing languages, cultures and regulatory environments, and those related to the timely hiring, integration and retention of a sufficient number of skilled personnel in an environment with which we are not familiar to carry out operations.
S-47
Table of Contents
We anticipate expanding our international operations through acquisitions of varying sizes or through organic growth, which could increase these risks. Additionally, though we might invest material amounts of capital and incur significant costs in connection with the growth and development of our international operations, there is no assurance that we will be able to operate them profitably anytime soon, if at all. As a result, we would expect these costs to be dilutive to our earnings over the next several years as we start-up or acquire new operations.
These risks could have a material adverse effect on our financial condition, results of operations and cash flows.
The level of our current and future debt could have an adverse impact on our business, and our ability to generate cash to service our indebtedness depends on many factors beyond our control.
We have substantial debt outstanding and we may incur additional indebtedness in the future. The high level of our indebtedness, among other things, could:
| • | make it difficult for us to make payments on our debt securities; |
| • | increase our vulnerability to general adverse economic and industry conditions; |
| • | require us to dedicate a substantial portion of our cash flow from operations to payments on our indebtedness, thereby reducing the availability of our cash flow to fund working capital, capital expenditures, acquisitions and investments and other general corporate purposes; |
| • | limit our flexibility in planning for, or reacting to, changes in our business and the markets in which we operate; |
| • | place us at a competitive disadvantage compared to our competitors that have less debt; and |
| • | limit our ability to borrow additional funds. |
Our ability to make payments on our indebtedness and to fund planned capital expenditures and expansion efforts, including any strategic acquisitions we may make in the future, will depend on our ability to generate cash. This, to a certain extent, is subject to general economic, financial, competitive, regulatory and other factors that are beyond our control.
We cannot provide assurance that our business will generate sufficient cash flow from operations in the future or that future borrowings will be available to us in an amount sufficient to enable us to service our indebtedness or to fund other liquidity needs. The borrowings under our amended senior secured credit facilities are guaranteed by a substantial portion of our direct and indirect wholly owned domestic subsidiaries and are secured by a substantial portion of DaVita’s and its guarantors’ assets.
Increases in interest rates may increase our interest expense and adversely affect our earnings and cash flow and our ability to service our indebtedness.
A portion of our outstanding debt bears interest at variable rates. We are subject to LIBOR-based interest rate volatility from a floor of 1.50% to a cap of 4.00% on $1.25 billion notional amounts of our Term Loan B outstanding debt as a result of several interest rate cap agreements that were entered into in January 2011. The remaining $474 million of outstanding debt on the Term Loan B is subject to LIBOR-based interest rate volatility above a floor of 1.50%. At June 30, 2012, we were also subject to LIBOR-based interest rate volatility above a floor of 1.00% on $199 million of outstanding debt associated with our Term Loan A-2.
We also have approximately $350 million of additional borrowings available of which approximately $49 million was committed for outstanding letters of credit, under our amended senior secured credit facilities that are subject to LIBOR-based interest rate volatility. We may also incur additional variable rate debt in the
S-48
Table of Contents
future. Increases in interest rates would increase our interest expense of the variable portion of our indebtedness, which could negatively impact our earnings and cash flow and our ability to service our indebtedness which would be particularly significant in the event of rapid and substantial increases in interest rates.
At June 30, 2012, if interest rates were to hypothetically increase by 100 basis points it would increase our interest expense by approximately $0.5 million, which increase solely relates to our Term Loan A-2 that is subject to LIBOR-based interest rate volatility above a floor of 1.00%.
However, interest expense would not be impacted by any LIBOR-based interest rate volatility associated with our other Term Loans since all of our Term Loan A is economically fixed and our Term Loan B is subject to LIBOR-based interest rate volatility above a floor of 1.50%, as described above. The current LIBOR rate in effect, plus a hypothetical increase of 100 basis points, is currently less than our Term Loan B floor of 1.50%. Therefore, LIBOR-based interest rates would have to increase above a floor of 1.50% for the Term Loan B to have a negative impact on our financial results.
If there are shortages of skilled clinical personnel or if we experience a higher than normal turnover rate, we may experience disruptions in our business operations and increases in operating expenses.
We are experiencing increased labor costs and difficulties in hiring nurses due to a nationwide shortage of skilled clinical personnel. We compete for nurses with hospitals and other health care providers. This nursing shortage may limit our ability to expand our operations. In addition, changes in certification requirements or increases in the required staffing levels for skilled clinical personnel can impact our ability to maintain sufficient staff levels to the extent our teammates are not able to meet new requirements or competition for qualified individuals increases. If we are unable to hire skilled clinical personnel when needed, or if we experience a higher than normal turnover rate for our skilled clinical personnel, our operations and treatment growth will be negatively impacted, which would result in reduced revenues, earnings and cash flows.
Our business is labor intensive and could be adversely affected if we were unable to maintain satisfactory relations with our employees or if union organizing activities were to result in significant increases in our operating costs or decreases in productivity.
Our business is labor intensive, and our results are subject to variations in labor-related costs, productivity and the number of pending or potential claims against us related to labor and employment practices. If political efforts at the national and local level result in actions or proposals that increase the likelihood of union organizing activities at our facilities or if union organizing activities increase for other reasons, or if labor and employment claims, including the filing of class action suits, trend upwards, our operating costs could increase and our employee relations, productivity, earnings and cash flows could be adversely affected.
Upgrades to our billing and collections systems and complications associated with upgrades and other improvements to our billing and collections systems could have a material adverse effect on our revenues, cash flows and operating results.
We are continuously performing upgrades to our billing systems and expect to continue to do so in the near term. In addition, we continuously work to improve our billing and collections performance through process upgrades, organizational changes and other improvements. We may experience difficulties in our ability to successfully bill and collect for services rendered as a result of these changes, including a slow-down of collections, a reduction in the amounts we expect to collect, increased risk of retractions from and refunds to commercial and government payors, an increase in our provision for uncollectible accounts receivable and noncompliance with reimbursement regulations. The failure to successfully implement the upgrades to the billing and collection systems and other improvements could have a material adverse effect on our revenues, cash flows and operating results.
S-49
Table of Contents
Our ability to effectively provide the services we offer could be negatively impacted if certain of our suppliers are unable to meet our needs or if we are unable to effectively access new technology, which could substantially reduce our revenues, earnings and cash flows.
We have significant suppliers that are either the sole or primary source of products critical to the services we provide, including Amgen, Baxter Healthcare Corporation, NxStage Medical, Inc. and others or to which we have committed obligations to make purchases including Gambro Renal Products and Fresenius. If any of these suppliers are unable to meet our needs for the products they supply, including in the event of a product recall, or shortage, and we are not able to find adequate alternative sources, or if some of the drugs that we purchase are not reimbursed or not adequately reimbursed by commercial payors or through the bundled payment rate by Medicare, our revenues, earnings and cash flows could be substantially reduced. In addition, the technology related to the products critical to the services we provide is subject to new developments and may result in superior products. If we are not able to access superior products on a cost-effective basis or if suppliers are not able to fulfill our requirements for such products, we could face patient attrition which could substantially reduce our revenues, earnings and cash flows.
We may be subject to liability claims for damages and other expenses not covered by insurance that could reduce our earnings and cash flows.
The administration of dialysis and related services to patients may subject us to litigation and liability for damages. Our business, profitability and growth prospects could suffer if we face negative publicity or we pay damages or defense costs in connection with a claim that is outside the scope of any applicable insurance coverage, including claims related to adverse patient events, contractual disputes and professional and general liability claims. In addition, we have received several notices of claims from commercial payors and other third parties related to our historical billing practices and the historical billing practices of the centers acquired from Gambro Healthcare and other matters related to their settlement agreement with the Department of Justice. Although the ultimate outcome of these claims cannot be predicted, an adverse result with respect to one or more of these claims could have a material adverse effect on our financial condition, results of operations, and cash flows. We currently maintain programs of general and professional liability insurance. However, a successful claim, including a professional liability, malpractice or negligence claim which is in excess of our insurance coverage could have a material adverse effect on our earnings and cash flows.
In addition, if our costs of insurance and claims increase, then our earnings could decline. Market rates for insurance premiums and deductibles have been steadily increasing. Our earnings and cash flows could be materially and adversely affected by any of the following:
| • | the collapse or insolvency of our insurance carriers; |
| • | further increases in premiums and deductibles; |
| • | increases in the number of liability claims against us or the cost of settling or trying cases related to those claims; and |
| • | an inability to obtain one or more types of insurance on acceptable terms. |
Provisions in our charter documents, compensation programs and Delaware law may deter a change of control that our stockholders would otherwise determine to be in their best interests.
Our charter documents include provisions that may deter hostile takeovers, delay or prevent changes of control or changes in our management, or limit the ability of our stockholders to approve transactions that they may otherwise determine to be in their best interests. These include provisions prohibiting our stockholders from acting by written consent; requiring 90 days advance notice of stockholder proposals or nominations to our Board of Directors; and granting our Board of Directors the authority to issue preferred stock and to determine the rights and preferences of the preferred stock without the need for further stockholder approval.
S-50
Table of Contents
Most of our outstanding employee stock options include a provision accelerating the vesting of the options in the event of a change of control. We also maintain a change of control protection program for our employees who do not have a significant number of stock awards, which has been in place since 2001, and which provides for cash bonuses to the employees in the event of a change of control. Based on the market price of our common stock and shares outstanding on June 30, 2012, these cash bonuses would total approximately $364 million if a change of control transaction occurred at that price and our Board of Directors did not modify this program. These change of control provisions may affect the price an acquirer would be willing to pay for our Company.
We are also subject to Section 203 of the Delaware General Corporation Law that, subject to exceptions, would prohibit us from engaging in any business combinations with any interested stockholder, as defined in that section, for a period of three years following the date on which that stockholder became an interested stockholder.
These provisions may discourage, delay or prevent an acquisition of our Company at a price that our stockholders may find attractive. These provisions could also make it more difficult for our stockholders to elect directors and take other corporate actions and could limit the price that investors might be willing to pay for shares of our common stock.
Risks Relating to HCP
As a healthcare company, HCP is subject to many of the same risks to which DaVita is subject.
As a participant in the healthcare industry, HCP is subject to many of the same risks that DaVita is subject to as described in the DaVita risk factors, included elsewhere in or incorporated by reference into this prospectus supplement, any of which could materially and adversely affect HCP’s revenues, earnings or cash flows. Among these risks are the following:
| • | the healthcare business is heavily regulated and changes in laws, regulations, or government programs could have a material impact on HCP’s business; |
| • | failure to comply with complex governmental regulations could have severe consequences to HCP, including, without limitation, exclusion from governmental payor programs like Medicare and Medicaid; |
| �� | HCP could become the subject of governmental investigations, claims, and litigation; |
| • | HCP may be unable to continue to make acquisitions or to successfully integrate such acquisitions into its business, and such acquisitions may include liabilities of which HCP was not aware; and |
| • | as a result of the broad scope of HCP’s medical practice, including its affiliated physician groups in California and Nevada, HCP is exposed to medical malpractice claims, as well as claims for damages and other expenses, that may not be covered by insurance. |
Under most of HCP’s agreements with health plans, HCP assumes some or all of the risk that the cost of providing services will exceed its compensation.
Substantially all of HCP’s revenue is derived from PMPM fees, paid by health plans under capitation agreements with HCP or its affiliated physician groups. In Florida, HCP contracts directly with health plans under global capitation arrangements to assume financial responsibility for both professional and institutional services. In Nevada, HCP contracts directly with health plans under capitation arrangements to assume financial responsibility for professional services, but does not generally assume institutional risk. Under such contracts, the health plan establishes pools for both professional services and institutional services based on a contractual PMPM fee, and the health plan then pays both professional and institutional expenses and remits the residual amounts to HCP. In California, HCP utilizes a capitation model in several different forms. While there are variations specific to each arrangement, HealthCare Partners Affiliates Medical Group, or HCPAMG, generally
S-51
Table of Contents
contracts with health plans to receive a PMPM fee for professional services and assumes the financial responsibility for professional services only. In some cases, the health plans separately enter into capitation contracts with third parties (typically hospitals) who receive directly a portion of the PMPM fee and assume contractual financial responsibility for hospital services. In other cases, the health plan does not pay any portion of the PMPM fee to the hospital, but rather administers claims for hospital expenses itself. In both scenarios, HCP enters into managed care-related administrative services agreements or similar arrangements with those third parties (hospitals) under which HCP agrees to be responsible for utilization review, quality assurance, and other managed care-related administrative functions. As compensation for such administrative services, HCP is entitled to share up to 100% of the amount by which the hospital capitation revenue exceeds hospital expenses; any such risk-share amount to which HCP is entitled is recorded as medical revenues.
To the extent that members require more care than is anticipated, aggregate PMPM payments may be insufficient to cover the costs associated with treatment. If medical expenses exceed estimates, except in very limited circumstances, HCP will not be able to increase the PMPM fee received under these risk agreements during their then-current terms.
If HCP or its affiliated physician groups enter into capitation contracts with unfavorable economic terms, or a capitation contract is amended to include unfavorable terms, HCP could, directly or indirectly through its contracts with HCPAMG, suffer losses with respect to such contract. Since HCP does not negotiate with CMS or any health plan regarding the benefits to be provided under their Medicare Advantage or other managed care plans, HCP often has just a few months to familiarize itself with each new annual package of benefits it is expected to offer.
Relatively small changes in HCP’s or HCPAMG’s ratio of medical expense to revenue can create significant changes in HCP’s financial results. Accordingly, the failure to adequately predict and control medical expenses and to make reasonable estimates and maintain adequate accruals for incurred but not reported claims, may have a material adverse effect on HCP’s financial condition, results of operations or cash flows.
Historically, HCP’s and HCPAMG’s medical expenses as a percentage of revenue have fluctuated. Factors that may cause medical expenses to exceed estimates include:
| • | the health status of members; |
| • | higher than expected utilization of new or existing healthcare services or technologies; |
| • | an increase in the cost of healthcare services and supplies, including pharmaceuticals, whether as a result of inflation or otherwise; |
| • | changes to mandated benefits or other changes in healthcare laws, regulations, and practices; |
| • | periodic renegotiation of provider contracts with specialist physicians, hospitals, and ancillary providers; |
| • | periodic renegotiation of contracts with HCP’s affiliated primary care physicians; |
| • | changes in the demographics of the participating members and medical trends; |
| • | contractual or claims disputes with providers, hospitals, or other service providers within a health plan’s network; and |
| • | the occurrence of catastrophes, major epidemics, or acts of terrorism. |
S-52
Table of Contents
Risk-sharing arrangements that HCP-affiliated physician groups (including HCPAMG) have with health plans and hospitals could result in their costs exceeding the corresponding revenues, which could reduce or eliminate any shared risk profitability.
Most of the agreements between health plans and HCP and its affiliated physician groups, including HCPAMG, contain risk-sharing arrangements under which the physician groups can earn additional compensation from the health plans by coordinating the provision of quality, cost-effective healthcare to members. However, such arrangements may require the physician group to assume a portion of any loss sustained from these arrangements, thereby reducing HCP’s net income. Under these risk-sharing arrangements, HCP and its affiliated physician groups are responsible for a portion of the cost of hospital services or other services that are not capitated. The terms of the particular risk-sharing arrangement allocate responsibility to the respective parties when the cost of services exceeds the related revenue, which results in a “deficit,” or permit the parties to share in any “surplus” amounts when actual costs are less than the related revenue. The amount of non-capitated and hospital costs in any period could be affected by factors beyond the control of HCP, such as changes in treatment protocols, new technologies, longer lengths of stay by the patient, and inflation. To the extent that such non-capitated and hospital costs are higher than anticipated, revenue may not be sufficient to cover the risk-sharing deficits the health plans and HCP are responsible for, which could reduce HCP’s revenues and profitability. Certain of HCP’s agreements with health plans stipulate that risk-sharing pool deficit amounts are carried forward to offset any future years’ surplus amounts HCP would otherwise be entitled to receive. HCP accrues for any such risk-sharing deficits.
Health plans often insist on withholding negotiated amounts from professional PMPM payments, which the health plans are permitted to retain, in order to cover HCP’s share of any risk-sharing deficits. Whenever possible, HCP seeks to contractually reduce or eliminate its liability for risk-sharing deficits. Notwithstanding the foregoing, risk-sharing deficits could have a significant impact on future profitability.
Renegotiation, renewal, or termination of capitation agreements with health plans could have a significant impact on HCP’s future profitability.
Under most of HCP’s and its affiliated physician groups’, including HCPAMG’s, capitation agreements with health plans, the health plan is generally permitted to modify the benefit and risk obligations and compensation rights from time to time during the terms of the agreements. If a health plan exercises its right to amend its benefit and risk obligations and compensation rights, HCP and its affiliated physician groups, including HCPAMG, are generally allowed a period of time to object to such amendment. If HCP or its affiliated physician group so objects, under some of the risk agreements, the relevant health plan may terminate the applicable agreement upon 60 to 90 days written notice. In addition, in connection with the Merger, HCP must obtain the consent of certain health plans to assign certain capitation agreements, which could result in health plans attempting to renegotiate or threatening to cancel such contracts. Depending on the health plan at issue and the amount of revenue associated with the health plan’s risk agreement, the renegotiated terms or termination may have a material adverse effect on HCP’s and DaVita’s future revenues and profitability.
Laws regulating the corporate practice of medicine could restrict the manner in which HCP is permitted to conduct its business and the failure to comply with such laws could subject HCP to penalties or require a restructuring of HCP.
Some states have laws that prohibit business entities, such as HCP, from practicing medicine, employing physicians to practice medicine, exercising control over medical decisions by physicians (also known collectively as the corporate practice of medicine) or engaging in certain arrangements, such as fee-splitting, with physicians. In some states these prohibitions are expressly stated in a statute or regulation, while in other states the prohibition is a matter of judicial or regulatory interpretation. Of the three states in which HCP currently operates, California and Nevada prohibit the corporate practice of medicine.
In California and Nevada, HCP operates by maintaining long-term contracts with its affiliated physician groups, including HCPAMG, which are each owned and operated by physicians and which employ or contract
S-53
Table of Contents
with additional physicians to provide physician services. Under these arrangements, HCP provides management services, receives a management fee for providing non-medical management services, does not represent that it offers medical services, and does not exercise influence or control over the practice of medicine by the physicians or the affiliated physician groups.
In addition to the above management arrangements, HCP has certain contractual rights relating to the orderly transfer of equity interests in certain of its California and Nevada affiliated physician groups through succession agreements and other arrangements with their physician equityholders. However, such equity interests cannot be transferred to or held by HCP or by any non-professional organization. Accordingly, neither HCP nor HCP’s subsidiaries directly own any equity interests in any physician groups in California and Nevada. In the event that any of these affiliated physician groups fails to comply with the management arrangement or any management arrangement is terminated and/or HCP is unable to enforce its contractual rights over the orderly transfer of equity interests in its affiliated physician groups, such events could have a material adverse effect on HCP’s business, financial condition or results of operations.
HCP may be required to restructure its relationship with its affiliated physician groups if HCP’s management services agreements with such affiliated physician groups or HCP’s succession agreements and other related arrangements with equityholders of any such affiliated physician groups are deemed invalid under prohibitions against the corporate practice of medicine in California and Nevada.
Some of the relevant laws, regulations, and agency interpretations relating to the corporate practice of medicine have been subject to limited judicial and regulatory interpretation. Moreover, state laws are subject to change and regulatory authorities and other parties, including HCP’s group physicians, may assert that, despite these arrangements, HCP is engaged in the prohibited corporate practice of medicine.
In light of the above, it is possible that a state regulatory agency or a court could determine that HCP’s agreements with physician equityholders of certain managed California and Nevada affiliated physician groups as described above, either independently or coupled with the management services agreements with such affiliated physician groups, confer impermissible control over the business and/or medical operations of such affiliated physician groups, that the management fee payable under such arrangements results in profit sharing or that HCP is the beneficial owner of the affiliated physician groups’ equity interests in violation of the corporate practice of medicine doctrine. If there were a determination that a corporate practice of medicine violation existed or exists, these arrangements could be deemed invalid, potentially resulting in a loss of revenues and results of operations derived from such affiliated physician groups. In addition, HCP’s California and Nevada affiliated physician groups and HCP, as well as those physician equityholders of affiliated physician groups who are subject to succession agreements with HCP, could be subject to criminal or civil penalties or an injunction for practicing medicine without a license or aiding and abetting the unlicensed practice of medicine.
A determination that a corporate practice of medicine violation existed could also force a restructuring of HCP’s management arrangements with affiliated physician groups in California and/or Nevada. Such a restructuring might include revisions of the management services agreements, which might include a modification of the management fee, and/or establishing an alternative structure, such as obtaining a California Knox-Keene license (a managed care plan license issued pursuant to the California Knox-Keene Health Care Service Plan Act of 1975, or Knox-Keene Act) or its Nevada equivalent, which would permit HCP to contract with a physician network without violating the corporate practice of medicine prohibition. There can be no assurance that such a restructuring would be feasible, or that it could be accomplished within a reasonable time frame without a material adverse effect on HCP’s operations and financial results.
S-54
Table of Contents
If HCP’s agreements or arrangements with any physician equityholder(s) of affiliated physicians, physician groups, or IPAs are deemed invalid under state law, including laws against the corporate practice of medicine, or Federal Law, or are terminated as a result of changes in state law, or if there is a change in accounting principles or the interpretation thereof by the Financial Accounting Standards Board, or FASB, affecting consolidation of entities, it could impact HCP’s consolidation of total revenues derived from such affiliated physician groups.
HCP’s financial statements are consolidated and include the accounts of its majority-owned subsidiaries and certain non-owned HCP-affiliated physician groups, which consolidation is effectuated in accordance with applicable accounting rules. In the event of a change in accounting principles promulgated by FASB or in FASB’s interpretation of its principles, or if there were an adverse determination by a regulatory agency or a court or if there were a change in state or federal law relating to the ability to maintain present agreements or arrangements with such physician groups, HCP may not be permitted to continue to consolidate the total revenues of such organizations. A change in accounting for consolidation with respect to HCP’s present agreement or arrangements would diminish HCP’s reported revenues but would not adversely affect its results of operations, while regulatory or legal rulings or changes in law interfering with HCP’s ability to maintain its present agreements or arrangements could diminish both revenues and results of operations.
If HCPAMG and HCP’s affiliated physician groups are not able to satisfy the California Department of Managed Health Care’s financial solvency requirements, HCP could become subject to sanctions and its ability to do business in California could be limited or terminated.
The California Department of Managed Health Care, or DMHC, has instituted financial solvency regulations. The regulations are intended to provide a formal mechanism for monitoring the financial solvency of capitated physician groups. Under the regulations, HCPAMG and HCP’s affiliated physician groups are required to, among other things:
| • | Maintain, at all times, a minimum “cash-to-claims ratio” (where “cash-to-claims ratio” means the organization’s cash, marketable securities, and certain qualified receivables, divided by the organization’s total unpaid claims liability). The regulations currently require a cash-to-claims ratio of 0.75. |
| • | Submit periodic reports to the DMHC containing various data and attestations regarding performance and financial solvency, including incurred but not reported calculations and documentation, and attestations as to whether or not the organization was in compliance with the Knox-Keene Act requirements related to claims payment timeliness, had maintained positive tangible net equity (i.e., at least $1.00), and had maintained positive working capital (i.e., at least $1.00). |
In the event that a physician organization is not in compliance with any of the above criteria, the organization would be required to describe in a report submitted to the DMHC the reasons for non-compliance and actions to be taken to bring the organization into compliance. Further, under these regulations, the DMHC can make public some of the information contained in the reports, including, but not limited to, whether or not a particular physician organization met each of the criteria. In the event HCP or its affiliated physician groups are not able to meet certain of the financial solvency requirements, and fail to meet subsequent corrective action plans, HCP could be subject to sanctions, or limitations on, or removal of, its ability to do business in California.
Reductions in Medicare Advantage health plan reimbursement rates stemming from recent healthcare reforms and any future related regulations may negatively impact HCP’s business, revenue and profitability.
A significant portion of HCP’s revenue is directly or indirectly derived from the monthly premium payments paid by CMS to health plans for medical services provided to Medicare Advantage enrollees. As a result, HCP’s business and results of operations are, in part, dependent on government funding levels for Medicare Advantage programs. Any changes that limit or reduce Medicare Advantage reimbursement levels,
S-55
Table of Contents
such as reductions in or limitations of reimbursement amounts or rates under programs, reductions in funding of programs, expansion of benefits without adequate funding, elimination of coverage for certain benefits, or elimination of coverage for certain individuals or treatments under programs, could have a material adverse effect on HCP’s business.
The Health Reform Acts contain a number of provisions that negatively impact Medicare Advantage plans, including the following:
| • | Medicare Advantage benchmarks for 2011 were frozen at 2010 levels. Beginning in 2012, Medicare Advantage benchmark rates are being phased down from current levels to levels that are between 95% and 115% of fee-for-service costs, depending on a plan’s geographic area. Medicare Advantage plans receiving certain quality ratings by CMS will be eligible for bonus rate increases. |
| • | Rebates received by Medicare Advantage plans that “underbid” based on payment benchmarks will be reduced, with larger reductions for plans failing to receive certain quality ratings. |
| • | The Secretary of the Department of Health and Human Services, or HHS, is granted explicit authority to deny Medicare Advantage plan bids that propose significant increases in cost sharing or decreases in benefits. |
| • | Beginning in 2014, Medicare Advantage plans with medical loss ratios below 85% will be required to pay a rebate to the Secretary of HHS. The Secretary of HHS will halt enrollment in any plan failing to meet this ratio for three consecutive years, and terminate any plan failing to meet the ratio for five consecutive years. If an HCP-contracting Medicare Advantage plan experiences a limitation on enrollment or is otherwise terminated from the Medicare Advantage program, HCP may suffer materially adverse consequences to its business or financial condition. |
| • | Since January 1, 2011, cost-sharing for certain services (such as chemotherapy and skilled nursing care) has been limited to the cost-sharing permitted under the original fee-for-service Medicare program. |
| • | Prescription drug plans are now required to cover all drugs on a list developed by the Secretary of HHS, and the Medicare Part D premium subsidy for high-income beneficiaries has been reduced by 25%. |
| • | Beginning in 2014, CMS is required to increase coding intensity adjustments for Medicare Advantage plans, which is expected to reduce CMS payments to Medicare Advantage plans, which in turn will likely reduce the amounts payable to HCP and its affiliated physicians, physician groups, and IPAs under its capitation agreements. |
In addition to the above, the Health Reform Acts establish a new Independent Payment Advisory Board, or IPAB, to recommend ways to reduce Medicare spending if the increase in Medicare costs per capita exceeds certain targets, which will be implemented unless Congress passes alternative legislation that achieves the same savings. The Health Reform Acts mandate that if targets are not met, the IPAB’s recommendations are to include ways to reduce payments to Medicare Advantage plans and Medicare Part D prescription drug plans related to administrative expenses (including profits) and performance bonuses. Also, the Budget Control Act of 2011, or BCA, mandates a 2% decrease in Medicare Advantage spending in order to bring Medicare spending for Medicare Advantage beneficiaries more in line with Medicare fee-for-service spending. Additional steps could be taken by government agencies and plan providers to further restrict, directly or indirectly, the reimbursements available to plan service providers like HCP.
Finally, it is possible that the impact of the Health Reform Acts could cause a reduction in enrollment in Medicare Advantage plans, which, in turn, would reduce HCP’s revenues and net income. For example, the Congressional Budget Office, or CBO, expects that, after reaching a high of 25% participation in Medicare Advantage plans in 2012, such participation will decline to 17% in 2020. The CBO predicts that this, together with other changes under the Reform Act, will result in reductions in Medicare Advantage spending by CMS of up to an aggregate of $131.9 billion over 10 years.
S-56
Table of Contents
Although the Health Reform Acts provide for reductions in payments to Medicare Advantage plans, the Health Reform Acts also provide for bonus payments to Medicare Advantage plans rated four or five stars based on quality measures. In November 2011, CMS announced a three-year demonstration project with an alternative bonus structure that awards bonuses to plans with three or more stars. The Government Accountabilty Office, or GAO, and MedPAC have criticized the demonstration project. If Congress acts to curb the CMS initiated bonus structure, HCP’s revenues would decrease.
HCP’s operations are dependent on competing health plans and, at times, their and HCP’s economic interests may diverge.
For the year ended December 31, 2011, 70% of HCP’s consolidated medical revenues was earned through contracts with three health plans.
HCP expects that, going forward, substantially all of its revenue will continue to be derived from these and other health plans. Each health plan may immediately terminate any of HCP’s contracts and/or any individual credentialed physician upon the occurrence of certain events. They may also amend the material terms of the contracts under certain circumstances. Failure to maintain the contracts on favorable terms, for any reason, would materially and adversely affect HCP’s results of operations and financial condition. A material decline in the number of members could also have a material adverse effect on HCP’s results of operations.
Notwithstanding each health plan’s and HCP’s current shared interest in providing service to HCP’s members who are enrolled in the subject health plans, the health plans may have different and, at times, opposing economic interests from those of HCP. The health plans provide a wide range of health insurance services across a wide range of geographic regions, utilizing a vast network of providers. As a result, they and HCP may have different views regarding the proper pricing of services and/or the proper pricing of the various service providers in their provider networks, the cost of which HCP bears to the extent that the services of such service providers are utilized. These health plans may also have different views than HCP regarding the efforts and expenditures that they, HCP, and/or other service providers should make to achieve and/or maintain various quality ratings. In addition, several health plans have purchased or announced their intent to purchase IPAs or HMOs. If health plans with which HCP contracts make significant purchases, they may not continue to contract with HCP or contract on less favorable terms. Similarly, as a result of changes in laws, regulations, consumer preferences, or other factors, the health plans may find it in their best interest to provide health insurance services pursuant to another payment or reimbursement structure. In the event HCP’s interests diverge from the interests of the health plans, HCP may have limited recourse or alternative options in light of its dependence on these health plans. There can be no assurances that HCP will continue to find it mutually beneficial to work with the health plans. As a result of various restrictive provisions that appear in some of the managed care agreements with health plans, HCP may, at times, have limitations on its ability to cancel an agreement with a particular health plan and immediately thereafter contract with a competing health plan with respect to the same service area.
HCP and its affiliated physicians, physician groups, including HCPAMG, and IPAs and other physicians may be required to continue providing services following termination or renegotiation of certain agreements with health plans.
There are circumstances under federal and state law pursuant to which HCP and its affiliated physician groups, including HCPAMG, IPAs, and other physicians could be obligated to continue to provide medical services to HCP members in their care following a termination of their applicable risk agreement with health plans and termination of the receipt of payments thereunder. In certain cases, this obligation could require the physician group or IPA to provide care to such member following the bankruptcy or insolvency of a health plan. Accordingly, the obligations to provide medical services to HCP members (and the associated costs) may not terminate at the time the applicable agreement with the health plan terminates, and HCP may not be able to recover its cost of providing those services from the health plan, which could have a material adverse effect on HCP’s financial condition, results of operations, and/or cash flows.
S-57
Table of Contents
HCP operates only in Florida, California, and Nevada. HCP may not be able to successfully establish a presence in new geographic regions.
HCP derives substantially all of its revenue from operations exclusively in California, Nevada, and Florida (California, Nevada, and Florida are hereinafter referred to as, the Existing Geographic Regions). As a result, HCP’s exposure to many of the risks described herein are not mitigated by a greater diversification of geographic focus. Furthermore, due to the concentration of HCP’s operations in the Existing Geographic Regions, HCP’s business may be adversely affected by economic conditions, natural disasters (such as earthquakes or hurricanes), or acts of war or terrorism that disproportionately affect the Existing Geographic Regions as compared to other states and geographic markets.
To expand the operations of its network outside of the Existing Geographic Regions, HCP must devote resources to identifying and exploring such perceived opportunities. Thereafter, HCP must, among other things, recruit and retain qualified personnel, develop new offices, establish potentially new relationships with one or more health plans, and establish new relationships with physicians and other healthcare providers. The ability to establish such new relationships may be significantly inhibited by competition for such relationships and personnel in the health care marketplace in the targeted new geographic regions. In addition, if HCP were to seek expansion outside of the Existing Geographic Regions, HCP would be required to comply with laws and regulations of states that may differ from the ones in which it currently operates, and could face competitors with greater knowledge of such local markets. HCP anticipates that any geographic expansion may require it to make a substantial investment of management time, capital, and/or other resources. There can be no assurance that HCP will be able to establish profitable operations or relationships in any new geographic markets.
Reductions in the quality ratings of the health plans HCP serves could have an adverse effect on its results of operations, financial condition, and/or cash flow.
As a result of the Health Reform Acts, HCP anticipates that the level of reimbursement each health plan receives from CMS will be dependent, in part, upon the quality rating of the Medicare plan that such health plan serves. Such ratings are expected to impact the percentage of any cost savings rebate and any bonuses earned by such health plan. Since a significant portion of HCP’s revenue for 2012 is expected to be calculated as a percentage of CMS reimbursements received by these health plans with respect to HCP members, reductions in the quality ratings of a health plan that HCP serves could have an adverse effect on its results of operations, financial condition, and/or cash flows. In addition, CMS has announced its intention to terminate any plan that has a rating of less than three stars for three consecutive years. Medicare Advantage plans with five stars are permitted to conduct enrollment throughout the year and enrollees in plans with 4.5 or fewer stars are permitted to change plans during the year. None of the plans with which HCP contracts are five-star plans. Given each health plan’s control of its plans and the many other providers that serve such plans, HCP believes that it will have limited ability to influence the overall quality rating of any such plan. Accordingly, since low quality ratings can potentially lead to the termination of a plan that HCP serves, HCP may not be able to prevent the potential termination of a contracting plan or a shift of patients to other plans based upon quality issues which could, in turn, have an adverse effect on HCP’s results of operations, financial condition, and/or cash flows.
HCP’s records and submissions to a health plan may contain inaccurate or unsupportable information regarding risk adjustment scores of members, which could cause HCP to overstate or understate its revenue and subject it to various penalties.
HCP, on behalf of itself and its affiliated physicians, physician groups, including HCPAMG, and IPAs, submits to health plans claims and encounter data that support the risk adjustment factor, or RAF, scores attributable to members. These RAF scores determine, in part, the revenue to which the health plan and, in turn, HCP is entitled for the provision of medical care to such members. The data submitted to CMS by each health plan is based on medical charts and diagnosis codes prepared and submitted by HCP. Each health plan generally relies on HCP to appropriately document and support such RAF data in HCP’s medical records. Each health plan also relies on HCP to appropriately code claims for medical services provided to members. HCP may
S-58
Table of Contents
periodically review medical records and may find inaccurate or unsupportable coding or otherwise inaccurate records. Erroneous claims and erroneous encounter records and submissions could result in inaccurate PMPM fee revenue and risk adjustment payments, which may be subject to correction or retroactive adjustment in later periods. This corrected or adjusted information may be reflected in financial statements for periods subsequent to the period in which the revenue was recorded. HCP might also need to refund a portion of the revenue that it received, which refund, depending on its magnitude, could damage its relationship with the applicable health plan and could have a material adverse effect on HCP’s results of operations, financial condition or cash flows.
CMS audits Medicare Advantage plans for documentation to support RAF-related payments for members chosen at random. The Medicare Advantage plans ask providers to submit the underlying documentation for members that they serve. It is possible that claims associated with members with higher RAF scores could be subject to more scrutiny in a CMS audit. HCP has experienced increases in RAF scores attributable to its members, and thus there is a possibility that a Medicare Advantage plan may seek repayment from HCP as a result of CMS payment adjustments to the Medicare Advantage plan. The plans also may hold HCP liable for any penalties owed to CMS for inaccurate or unsupportable RAF scores provided by HCP.
CMS has indicated that, starting with payment year 2011, payment adjustments will not be limited to RAF scores for the specific Medicare Advantage enrollees for which errors are found but may also be extrapolated to the entire Medicare Advantage plan subject to a particular CMS contract. Although CMS has described its audit process as plan-year specific and has stated that it will not extrapolate audit results for plan years prior to 2011, CMS has not specifically stated that payment adjustments as a result of one plan year’s audit will not be extrapolated to prior plan years. There can be no assurance that a health plan will not be randomly selected or targeted for review by CMS or that the outcome of such a review will not result in a material adjustment in HCP’s revenue and profitability, even if the information HCP submitted to the plan is accurate and supportable. Since the CMS rules, regulations, and statements regarding this audit program are still not well defined and, in some cases, have not been published in final form, there is also a risk that CMS may adopt new rules and regulations that are inconsistent with their existing rules, regulations, and statements.
A failure to estimate incurred but not reported medical benefits expense accurately could adversely affect HCP’s profitability.
Medical claims expense includes estimates of future medical claims that have been incurred by the patient but for which the provider has not yet billed HCP. These claim estimates are made utilizing actuarial methods and are continually evaluated and adjusted by management, based upon HCP’s historical claims experience and other factors. Adjustments, if necessary, are made to medical claims expense when the assumptions used to determine HCP’s claims liability changes and when actual claim costs are ultimately determined.
Due to the inherent uncertainties associated with the factors used in these estimates and changes in the patterns and rates of medical utilization, materially different amounts could be reported in HCP’s financial statements for a particular period under different conditions or using different, but still reasonable, assumptions. It is possible that HCP’s estimates of this type of claim may be inadequate in the future. In such event, HCP’s results of operations could be adversely impacted. Further, the inability to estimate these claims accurately may also affect HCP’s ability to take timely corrective actions, further exacerbating the extent of any adverse effect on HCP’s results.
HCP faces certain competitive threats which could reduce HCP’s profitability and increase competition for patients.
HCP faces certain competitive threats based on certain features of the Medicare programs, including the following:
| • | As a result of the direct and indirect impacts of the Health Reform Acts, many Medicare beneficiaries may decide that an original fee-for-service Medicare program is more attractive than a Medicare Advantage plan. As a result, enrollment in the health plans HCP serves may decrease. |
S-59
Table of Contents
| • | Managed care companies offer alternative products such as regional preferred provider organizations, or PPOs, and private fee-for-service plans. Medicare PPOs and private fee-for-service plans allow their patients more flexibility in selecting physicians than Medicare Advantage health plans, which typically require patients to coordinate care with a primary care physician. The Medicare Prescription Drug, Improvement, and Modernization Act of 2003 has encouraged the creation of regional PPOs through various incentives, including certain risk corridors, or cost-reimbursement provisions, a stabilization fund for incentive payments, and special payments to hospitals not otherwise contracted with a Medicare Advantage plan that treat regional plan enrollees. The formation of regional Medicare PPOs and private fee-for-service plans may affect HCP’s relative attractiveness to existing and potential Medicare patients in their service areas. |
| • | The payments for the local and regional Medicare Advantage plans are based on a competitive bidding process that may indirectly cause a decrease in the amount of the PMPM fee or result in an increase in benefits offered. |
| • | The annual enrollment process and subsequent “lock-in” provisions of the Health Reform Acts may adversely affect HCP’s level of revenue growth as it will limit the ability of a health plan to market to and enroll new Medicare beneficiaries in its established service areas outside of the annual enrollment period. |
| • | Commencing in 2012, CMS will allow Medicare beneficiaries who are enrolled in a Medicare Advantage plan with a quality rating of 4.5 stars or less to enroll in a five-star rated Medicare Advantage plan at any time during the benefit year. None of the plans HCP serves are five-star rated. Therefore, HCP may face a competitive disadvantage in recruiting and retaining Medicare beneficiaries. |
In addition to the competitive threats intrinsic to the Medicare programs, competition among health plans and among healthcare providers may also have a negative impact on HCP’s profitability. For example, California, Nevada, and Florida have become increasingly attractive to health plans that may compete with HCP, including the health plans with which HCP and its affiliated physicians, physician groups, and IPAs currently compete. HCP may not be able to continue to compete profitably in the healthcare industry if additional competitors enter the same market. If HCP cannot compete profitably, the ability of HCP to compete with other service providers that contract with competing health plans may be substantially impaired. Similarly, California, Nevada, and Florida have also become increasingly attractive to HCP’s competitors due to the large populations of Medicare beneficiaries. HCP may not be able to continue to compete effectively if additional competitors enter the same regions.
HCP competes directly with various regional and local companies that provide similar services in HCP’s Existing Geographic Regions. HCP’s competitors vary in size and scope and in terms of products and services offered. HCP believes that some of its competitors and potential competitors may be significantly larger than HCP and have greater financial, sales, marketing, and other resources. Furthermore, it is HCP’s belief that some of its competitors may make strategic acquisitions or establish cooperative relationships among themselves.
A disruption in HCP’s healthcare provider networks could have an adverse effect on HCP’s operations and profitability.
In any particular service area, healthcare providers or provider networks could refuse to contract with HCP, demand higher payments, or take other actions that could result in higher healthcare costs, disruption of benefits to HCP’s members, or difficulty in meeting applicable regulatory or accreditation requirements. In some service areas, healthcare providers or provider networks may have significant market positions. If healthcare providers or provider networks refuse to contract with HCP, use their market position to negotiate favorable contracts, or place HCP at a competitive disadvantage, then HCP’s ability to market products or to be profitable in those service areas could be adversely affected. HCP’s provider networks could also be disrupted by the financial insolvency of a large provider group. Any disruption in HCP’s provider networks could result in a loss of members or higher healthcare costs.
S-60
Table of Contents
HCP’s revenues and profits could be diminished if HCP fails to retain and attract the services of key primary care physicians.
Key primary care physicians with large patient enrollment could retire, become disabled, terminate their provider contracts, get lured away by a competing independent physician association or medical group, or otherwise become unable or unwilling to continue practicing medicine or contracting with HCP or its affiliated physicians, physician groups, or IPAs, including as a result of HCP no longer being physician-owned after the Merger. Moreover, given limitations relating to the enforcement of post-termination noncompetition covenants in California, it would be difficult to restrict a primary care physician from competing with HCP’s affiliated physicians, physician groups, or IPAs. As a result, members who have been served by such physicians could choose to enroll with competitors’ physician organizations or could seek medical care elsewhere, which could reduce HCP’s revenues and profits. Moreover, HCP may not be able to attract new physicians to replace the services of terminating physicians or to service its growing membership.
HCP regularly explores potential acquisitions, which if consummated could affect its financial condition, results of operations or other aspects of its business.
HCP regularly explores potential acquisitions, which if consummated could affect its financial condition, results of operations or other aspects of its business. There can be no assurance that HCP will be able to identify suitable acquisition candidates or that, if identified, HCP would be able to consummate an acquisition on acceptable terms. There can also be no assurance that HCP will be successful in completing any acquisitions that it might be considering, or integrating any acquired business into its overall operations, or that any such acquired business will operate profitably or will not otherwise adversely impact HCP’s results of operations.
Participation in Accountable Care Organization programs is subject to federal regulation, is new and subject to evolving regulatory development, and supervision and may result in financial liability.
The Health Reform Acts establish a Medicare shared savings program for ACOs, which took effect in January 2012. Participating ACOs that meet specified quality performance standards will be eligible to share in any savings below a specified benchmark amount. The Secretary of HHS is also authorized, but not required, to use capitation payment models with ACOs. The continued development and expansion of ACOs will have an uncertain impact on HCP’s business, revenue, and profitability.
As an initial step in the formation and development of ACOs, CMS has issued contracts for participation in a “Pioneer ACO” program. HCP, through certain of its subsidiaries, was awarded contracts to participate as a Pioneer ACO in California, Nevada, and Florida. HCP is in the process of implementing such operations. The Pioneer ACO program provides for a three-year participation with opportunities for upside incentives and downside risk liability for an assigned population of Medicare fee-for-service patients. It is the responsibility of HCP’s subsidiary ACOs to provide care to, and manage the health of, a patient population in California, Nevada, and Florida drawn from the traditional Medicare fee-for-service program, using a panel of specified physicians and healthcare facilities. The Pioneer ACO program requires participants to report on ACO operations, utilize healthcare information technology, and attempt to improve the quality of patient care.
The ACO programs are new and therefore operational and regulatory guidance is limited. It is possible that the operations of HCP’s subsidiary ACOs may not fully comply with current or future regulations and guidelines applicable to ACOs, may not achieve quality targets or cost savings, or may not attract or retain sufficient physicians or patients to allow HCP to meet its objectives. Additionally, poor performance could put the HCP ACOs at financial risk and obligation to CMS. Traditionally, other than fee-for-service billing by the medical clinics and healthcare facilities operated by HCP, HCP has not directly contracted with CMS and has not operated any health plans or provider sponsored networks. Therefore, HCP may not have the necessary experience, systems, or compliance to successfully achieve a positive return on its ACOs’ investment or to avoid financial or regulatory liability. To date, demonstration projects using healthcare delivery models substantially similar to an ACO have not resulted in savings. HCP believes that its historical experience with fully delegated managed care will be applicable to operation of its subsidiary ACOs, but there can be no such assurance.
S-61
Table of Contents
California hospitals may terminate their agreements with HCPAMG or reduce the fees they pay to HCP.
In California, HCPAMG maintains significant hospital arrangements designed to facilitate the provision of coordinated hospital care with those services provided to members by HCPAMG and its affiliated physicians, physician groups, and IPAs. Through contractual arrangements with certain key hospitals, HCPAMG provides utilization review, quality assurance, and other management services related to the provision of patient care services to members by the contracted hospitals and downstream hospital contractors. In the event that any one of these key hospital agreements is amended in a financially unfavorable manner or is otherwise terminated, such events could have a material adverse effect on HCP’s business, financial condition, and results of operations.
HCP’s professional liability and other insurance coverages may not be adequate to cover HCP’s potential liabilities.
HCP maintains professional liability insurance and other insurance coverage through California Medical Group Insurance Company, Risk Retention Group, an Arizona corporation in which HCP is a part owner. HCP believes such insurance is adequate based on industry standards. Nonetheless, potential liabilities may not be covered by insurance, insurers may dispute coverage or may be unable to meet their obligations, or the amount of insurance coverage and/or related reserves may be inadequate. There can be no assurances that HCP will be able to obtain insurance coverage in the future, or that insurance will continue to be available on a cost-effective basis, if at all. Moreover, even if claims brought against HCP are unsuccessful or without merit, HCP would have to defend itself against such claims. The defense of any such actions may be time-consuming and costly and may distract HCP management’s attention. As a result, HCP may incur significant expenses and may be unable to effectively operate HCP’s business.
Changes in the rates or methods of third-party reimbursements may adversely affect HCP operations.
HCP derives a substantial portion of its revenue from direct billings to governmental healthcare programs, such as Medicare and Medicaid, and private health insurance companies and/or health plans, including but not limited to those participating in the Medicare Advantage program. As a result, any negative changes in governmental capitation or fee-for-service rates or methods of reimbursement for the services HCP provides could have a significant adverse impact on HCP’s revenue and financial results.
Medicare program reimbursements for physician services as well as other services to Medicare beneficiaries who are not enrolled in Medicare Advantage plans are based upon the fee-for-service rates set forth in the Medicare Physician Fee Schedule, which relies, in part, on a target-setting formula system called the Sustainable Growth Rate, or SGR. Each year, on January 1st, the Medicare program updates the Medicare Physician Fee Schedule reimbursement rates. Many private payors use the Medicare Physician Fee Schedule to determine their own reimbursement rates. Based on the SGR, the annual fee schedule update is adjusted to reflect the comparison of actual expenditures to target expenditures. Because one of the factors for calculating the SGR is linked to the growth in the U.S. gross domestic product, or GDP, the SGR formula may result in a negative payment update if growth in Medicare beneficiaries’ use of services exceeds GDP growth, a situation which has occurred every year since 2002 and the reoccurrence of which HCP cannot predict.
CMS determined that, effective January 1, 2012, the SGR formula results in a payment cut of approximately 27 percent. Congress, however, enacted the Temporary Payroll Tax Cut Continuation Act of 2011, which blocked this cut through the end of February 2012. In February 2012, Congress passed the Middle Class Tax Relief and Job Creation Act of 2012, or Tax Relief Act, which blocks the cut through the end of 2012. While Congress has repeatedly intervened to mitigate the negative reimbursement impact associated with the SGR formula, there is no guarantee that Congress will continue to do so in the future. Moreover, the existing methodology may result in significant yearly fluctuations in the Medicare Physician Fee Schedule amounts, which may be unrelated to changes in the actual costs of providing physician services. Unless Congress enacts a change to the SGR methodology, the uncertainty regarding reimbursement rates and fluctuation will continue to
S-62
Table of Contents
exist. Moreover, if Congress does change the SGR methodology or substitute a new system for physician fee-for-service payments, it may require reductions in other Medicare programs including Medicare Advantage to offset such additional costs.
Another provision that affects physician payments under the Medicare Physician Fee Schedule is an adjustment under the Medicare statute to reflect the geographic variation in the cost of delivering physician services, by comparing those costs to the national average. Medicare payments to physicians under the Medicare Physician Fee Schedule are geographically adjusted to reflect the varying cost of delivering physician services across areas. The adjustments are made by indices, known as the Geographic Practice Cost Indices, or GPCI, that reflect how each geographic area compares to the national average. In 2003, Congress established that for three years there would be a “floor” of 1.0 on the “work” component of the Medicare Physician Fee Schedule formula used to determine physician payments, which meant that physician payments would not be reduced in a geographic area just because the relative cost of physician work in that area fell below the national average. Congress extended the GPCI work floor several times since its enactment in 2003. The Tax Relief Act provides another extension through 2012. Although Congress has extended the GPCI work floor several times, there is no guarantee that Congress will block the adjustment in the future, which could result in a decrease in payments HCP receives for physician services.
Congress has a strong interest in reducing the federal debt, which may lead to new proposals designed to achieve savings by altering payment policies. The BCA established a Joint Select Committee on Deficit Reduction, which had the goal of achieving a reduction in the federal debt level of at least $1.2 trillion. As a result of the Joint Select Committee’s failure to draft a proposal by the BCA’s deadline, automatic cuts in various federal programs will commence in January 2013. Although the Medicaid program is exempt from these cuts, Medicare payments to providers are not exempt. The BCA does, however, provide that the Medicare cuts to providers may not exceed 2%. At this time it is unclear how this automatic reduction may be applied to various Medicare healthcare programs, including physician reimbursement. Therefore it is not possible at this time to estimate what impact, if any, the BCA will have on HCP’s business or results of operations.
As noted, the cuts described above will occur automatically as a matter of law. Certain members of Congress, however, want to achieve even greater reductions in the federal debt, and they want to change entitlement programs, such as Medicare. It is difficult to assess whether and to what extent Congress will alter Medicare payment policies.
Because governmental healthcare programs generally reimburse on a fee schedule basis rather than on a charge-related basis, HCP generally cannot increase its revenues from these programs by increasing the amount it charges for its services. Moreover, if HCP’s costs increase, HCP may not be able to recover its increased costs from these programs. Government and private payors have taken and may continue to take steps to control the cost, eligibility for, use, and delivery of healthcare services as a result of budgetary constraints, cost containment pressures and other reasons. HCP believes that these trends in cost containment will continue. These cost containment measures, and other market changes in non-governmental insurance plans have generally restricted HCP’s ability to recover, or shift to non-governmental payors, any increased costs that HCP experiences. HCP’s business and financial operations may be materially affected by these developments.
HCP’s business model depends on numerous complex management information systems, and any failure to successfully maintain these systems or implement new systems could materially harm HCP’s operations and result in potential violations of healthcare laws and regulations.
HCP depends on a complex, specialized, and integrated management information system and standardized procedures for operational and financial information, as well as for HCP’s billing operations. HCP may be unable to enhance its existing management information systems or implement new management information systems where necessary. Additionally, HCP may experience unanticipated delays, complications, or expenses in implementing, integrating, and operating its systems. HCP’s management information systems may require modifications, improvements, or replacements that may require both substantial expenditures as well as
S-63
Table of Contents
interruptions in operations. HCP’s ability to implement these systems is subject to the availability of information technology and skilled personnel to assist HCP in creating and implementing these systems.
HCP’s failure to successfully implement and maintain all of its systems could have a material adverse effect on its business, financial condition, and results of operations. For example, HCP’s failure to successfully operate its billing systems could lead to potential violations of healthcare laws and regulations. If HCP is unable to handle its claims volume, or if HCP is unable to pay claims timely, HCP may become subject to a health plan’s corrective action plan or de-delegation until the problem is corrected, and/or termination of the health plan’s agreement with HCP. This could have a material adverse effect on HCP’s operations and profitability. In addition, if HCP’s claims processing system is unable to process claims accurately, the data HCP uses for its IBNR estimates could be incomplete and HCP’s ability to accurately estimate claims liabilities and establish adequate reserves could be adversely affected. Finally, if HCP’s management information systems are unable to function in compliance with applicable state or federal rules and regulations, including, without limitation, medical information confidentiality laws such as the Health Insurance Portability and Accountability Act of 1996, or HIPAA, possible penalties and fines as a result of this lack of compliance could have a material adverse effect on HCP’s business, financial condition, and results of operations.
Federal and state privacy and information security laws are complex, and HCP may be subject to government or private actions due to privacy and security breaches.
HCP must comply with numerous federal and state laws and regulations governing the collection, dissemination, access, use, security and privacy of protected health information, or PHI, including the Health Insurance Portability and Accountability Act of 1996 and its implementing privacy and security regulations, as amended by the federal Health Information Technology for Economic and Clinical Health Act, or HITECH Act and collectively referred to as HIPAA. In the event that HCP’s non-compliance with existing or new laws and regulations related to PHI results in privacy or security breaches, HCP could be subject to monetary fines, civil suits, civil penalties or criminal sanctions and requirements to disclose the breach publicly.
HCP may be impacted by eligibility changes to government and private insurance programs.
Due to potential decreased availability of healthcare through private employers, the number of patients who are uninsured or participate in governmental programs may increase. The Health Reform Acts will increase the participation of individuals in the Medicaid program in states that elect to participate in the expanded Medicaid coverage. A shift in payor mix from managed care and other private payors to government payors or the uninsured may result in a reduction in the rates of reimbursement or an increase in uncollectible receivables or uncompensated care, with a corresponding decrease in net revenue. Changes in the eligibility requirements for governmental programs such as the Medicaid program under the Health Reform Acts and state decisions on whether to participate in the expansion of such programs also could increase the number of patients who participate in such programs or the number of uninsured patients. Even for those patients who remain with private insurance, changes in those programs could increase patient responsibility amounts, resulting in a greater risk for uncollectible receivables. These factors and events could have a material adverse effect on HCP’s business, financial condition, and results of operations.
Negative publicity regarding the managed healthcare industry generally or HCP in particular could adversely affect HCP’s results of operations or business.
Negative publicity regarding the managed healthcare industry generally, or the Medicare Advantage program or HCP in particular, may result in increased regulation and legislative review of industry practices that further increase HCP’s costs of doing business and adversely affect HCP’s results of operations or business by:
| • | requiring HCP to change its products and services; |
| • | increasing the regulatory, including compliance, burdens under which HCP operates, which, in turn, may negatively impact the manner in which HCP provides services and increase HCP’s costs of providing services; |
S-64
Table of Contents
| • | adversely affecting HCP’s ability to market its products or services through the imposition of further regulatory restrictions regarding the manner in which plans and providers market to Medicare Advantage enrollees; or |
| • | adversely affecting HCP’s ability to attract and retain members. |
Risks Relating to the Merger
Under the accounting rules applicable to the contingent consideration, DaVita must determine the fair value of the contingent consideration on a quarterly basis, which could result in DaVita recording changes in the fair value as an expense in its financial statements, and any such expense may have an adverse impact on DaVita’s earnings and DaVita’s ability to predict the amount of earnings.
A portion of the merger consideration is contingent upon HCP’s performance following the closing of the Merger. The accounting rules applicable to the contingent consideration require that DaVita determine the fair value of the contingent consideration on a quarterly basis. To the extent that the fair value in any quarter exceeds the prior quarter’s determination, DaVita will be required to record the increase in fair value as an expense in its financial statements. Any such expense will reduce DaVita’s net income in the quarter in which it is recognized. These requirements will also limit DaVita’s ability to predict its earnings in the quarters in which it must assess the fair value of the contingent consideration, and have not been included in any of DaVita’s existing earnings guidance.
The Merger is subject to the receipt of approvals, waivers or consents from regulatory authorities and third parties that may impose conditions that could have an adverse effect on DaVita, and DaVita may terminate the Merger Agreement if holders of more than 5% of the outstanding HCP common units validly exercise dissenters’ rights.
Before the Merger can be completed, various approvals, waivers or consents must be obtained from regulatory authorities. These authorities may impose conditions on the completion of the Merger or require changes to the terms of the Merger. Although DaVita and HCP do not currently expect that any such conditions or changes will be imposed, there can be no assurance that they will not be, and such conditions or changes could have the effect of delaying completion and closing of the Merger or imposing additional costs on or limiting the revenues of DaVita following the Merger. See “HCP’s Business—Government Regulations” beginning on page S-184. In addition, HCP must obtain the consent of third parties to assign certain contracts, including contracts with health plans. In addition, DaVita may terminate the Merger Agreement if, at the time of termination, holders of more than 5% of the outstanding HCP common units have validly exercised their dissenters’ rights (and not withdrawn such exercise or otherwise become ineligible to effect such exercise) in respect of the transactions.
HCP operates in a different line of business from DaVita’s historical business, and the Merger is significantly larger than any other acquisition DaVita has made to date. DaVita may face challenges managing HCP as a new business and may not realize anticipated benefits.
The Merger is the largest acquisition DaVita has attempted to date and will result in DaVita being significantly engaged in a new line of business. Upon entering into a new line of business, DaVita may not have the expertise, experience, and resources to pursue all of its businesses at once, and it may be unable to successfully operate the businesses. The administration of the businesses will require implementation of appropriate operations, management, and financial reporting systems and controls. DaVita may experience difficulties in effectively implementing these and other systems. The management of HCP will require the focused attention of DaVita’s management team, including a significant commitment of its time and resources. The need for management to focus on these matters could have a material and adverse impact on DaVita’s revenues and operating results. If the HCP operations are less profitable than DaVita currently anticipates or if DaVita does not have the experience, the appropriate expertise, or the resources to pursue all businesses in the combined company, the results of operations and financial condition may be materially and adversely affected.
S-65
Table of Contents
HCP will become a subsidiary of DaVita following the Merger. If HCP’s liabilities are greater than expected, or if there are unknown HCP obligations, DaVita’s business could be materially and adversely affected.
As a result of the Merger, HCP will become a subsidiary of DaVita and HCP’s liabilities, including contingent liabilities, will be consolidated with DaVita’s. DaVita may learn additional information about HCP’s business that adversely affects DaVita, such as unknown liabilities, issues relating to internal controls over financial reporting or issues that could affect DaVita’s ability to comply with other applicable laws, including healthcare laws and regulations. As a result, DaVita cannot assure you that the Merger will be successful or will not, in fact, harm its business. Among other things, if HCP’s liabilities are greater than expected, or if there are obligations of HCP of which DaVita is not aware at the time of completion of the Merger, DaVita’s business could be materially and adversely affected.
DaVita has limited indemnification rights in connection with matters affecting HCP. HCP may also have other unknown liabilities which DaVita will be responsible for after the Merger. If DaVita is responsible for liabilities not covered by indemnification rights or substantially in excess of amounts covered through any indemnification rights, DaVita could suffer severe consequences that would substantially reduce its revenues, earnings and cash flows.
If we fail to successfully integrate HCP into our internal control over financial reporting or if the current internal control of HCP over financial reporting were found to be ineffective, the integrity of DaVita’s and/or HCP’s financial reporting could be compromised which could result in a material adverse effect on our reported financial results.
As a private company, HCP has not been subject to the requirements of the Securities Exchange Act of 1934, as amended, with respect to internal control over financial reporting, and for a period of time after the consummation of the Merger our management evaluation and auditor attestation regarding the effectiveness of our internal control over financial reporting will be permitted to exclude the operations of HCP. The integration of HCP into our internal control over financial reporting will require significant time and resources from our management and other personnel and will increase our compliance costs. If we fail to successfully integrate these operations into our internal control over financial reporting, our internal control over financial reporting may not be effective. Failure to achieve and maintain an effective internal control environment could have a material adverse effect on our ability to accurately report our financial results and the market’s perception of our business and our stock price. In addition, if HCP’s internal control over financial reporting were found to be ineffective, the integrity of HCP’s past financial reporting could be adversely impacted.
Risks Relating to Investment in the Notes
The notes will be unsecured.
Except as described under “Description of Notes—Escrow of proceeds; release conditions,” the notes will not be secured by any of our or our subsidiaries’ assets. The indenture governing the notes permits us and our subsidiaries to incur secured debt, including pursuant to our existing and future senior secured credit facilities and other forms of secured debt. As a result, the notes and the guarantees will be effectively subordinated to all of our and the guarantors’ existing and future secured obligations to the extent of the value of the assets securing such obligations. As of June 30, 2012, after giving pro forma effect to the Financings and the Merger as if they had occurred on that date, DaVita and the guarantors would have had total secured debt of approximately $5,650 million and approximately $284 million of additional secured debt available to be borrowed under our amended senior secured credit facilities (after giving effect to outstanding letters of credit of approximately $66 million), and the notes and the guarantees would have been structurally subordinated to $510 millionof liabilities, including $64 million of indebtedness and the rest being primarily trade payables, of non-guarantor subsidiaries.
If we or the subsidiary guarantors were to become insolvent or otherwise fail to make payment on the notes or the guarantees, except as described under “Description of Notes—Escrow of proceeds; release conditions,”
S-66
Table of Contents
holders of any of our and the subsidiary guarantors��� secured obligations would be paid first out of the proceeds of the assets securing such obligations before the holders of the notes would receive any payments from such proceeds. You may therefore not be fully repaid, or repaid at all, if we or the subsidiary guarantors become insolvent or otherwise fail to make payment on the notes.
The indentures governing the notes offered hereby, our outstanding 6 3/8% Senior Notes due 2018, or our 2018 Notes, and our outstanding 6 5/8% Senior Notes due 2020, or our 2020 Notes, and the agreement governing the existing senior secured credit facilities contain, and we expect that the agreement governing the amended senior secured credit facilities will contain, various covenants which limit our management’s discretion in the operation of our business.
The indentures governing the notes offered hereby, the 2018 Notes and the 2020 Notes restrict, among other things, our ability and the ability of our restricted subsidiaries to:
| • | incur additional indebtedness and issue certain preferred stock; |
| • | make certain distributions, investments and other restricted payments; |
| • | sell certain assets; |
| • | agree to restrictions on the ability of restricted subsidiaries to make payments to us; |
| • | create liens; |
| • | merge, consolidate or sell substantially all of our assets; and |
| • | enter into certain transactions with affiliates. |
In addition, the agreement governing our existing senior secured credit facilities requires us to comply with, and we expect that the agreement governing our amended senior secured credit facilities will require us to comply with, certain financial ratios and negative covenants. Our ability to comply with the ratios and covenants may be affected by events beyond our control.
Any failure to comply with the restrictions of the indenture governing the notes offered hereby, the 2018 Notes or the 2020 Notes, or of the existing or amended senior secured credit facilities or any other subsequent financing agreements, may result in an event of default under those agreements. Such a default will generally allow the creditors under the applicable agreement to declare the debt outstanding thereunder to be due and payable immediately, and such default and acceleration may cause other debt to become immediately due and payable as a result of cross-acceleration or cross-default provisions in such other indebtedness. In addition, lenders may have the right in these circumstances to terminate any commitments they have to provide further borrowings. Our assets and cash flow may not be sufficient to fully repay borrowings under our outstanding debt agreements if accelerated upon an event of default.
Federal and state statutes may allow courts, under specific circumstances, to void the guarantees and require noteholders to return payments received from guarantors.
Under federal bankruptcy law and comparable provisions of state fraudulent transfer laws, a guarantee could be deemed a fraudulent transfer if the guarantor received less than a reasonably equivalent value in exchange for giving the guarantee and
| • | was insolvent on the date that it gave the guarantee or became insolvent as a result of giving the guarantee, or |
| • | was engaged in business or a transaction, or was about to engage in business or a transaction, for which property remaining with the guarantor was an unreasonably small capital, or |
| • | intended to incur, or believed that it would incur, debts that would be beyond the guarantor’s ability to pay as those debts matured. |
S-67
Table of Contents
A court would likely find that a guarantor did not receive reasonably equivalent value or fair consideration for its guarantee if the guarantor did not substantially benefit directly or indirectly from the issuance of the guarantees. A guarantee could also be deemed a fraudulent transfer if it was given with actual intent to hinder, delay or defraud any entity to which the guarantor was or became, on or after the date the guarantee was given, indebted.
The measures of insolvency for purposes of the foregoing laws will vary depending upon the law applied in any proceeding with respect to the foregoing. Generally, however, a guarantor would be considered insolvent if:
| • | the sum of its debts, including contingent liabilities, is greater than all its assets, at a fair valuation, or |
| • | the present fair saleable value of its assets is less than the amount that would be required to pay its probable liability on its existing debts, including contingent liabilities, as they become absolute and mature, or |
| • | it could not pay its debts as they become due. |
We cannot predict:
| • | what standard a court would apply in order to determine whether a guarantor was insolvent as of the date it issued the guarantee or whether, regardless of the method of valuation, a court would determine that the guarantor was insolvent on that date; or |
| • | whether a court would determine that the payments under the guarantee constituted fraudulent transfers or conveyances on other grounds. |
The indenture governing the notes offered hereby will contain a “savings clause” intended to limit each subsidiary guarantor’s liability under its guarantee to the maximum amount that will result in the obligations of such subsidiary guarantor under its guarantee of the notes not constituting a fraudulent conveyance or fraudulent transfer under applicable law. However, as was demonstrated in a recent bankruptcy case originating in the State of Florida which was affirmed by the Eleventh Circuit Court of Appeals on other grounds, this provision may not be effective to protect the subsidiary guarantees from being voided under fraudulent conveyance or fraudulent transfer laws. Accordingly, there can be no assurance that this provision will be upheld as intended.
If a guarantee is deemed to be a fraudulent transfer, it could be voided altogether, or it could be subordinated to all other debts of the guarantor. In such case, any payment by the guarantor pursuant to its guarantee could be required to be returned to the guarantor or to a fund for the benefit of the creditors of the guarantor. If a guarantee is voided or held unenforceable for any other reason, holders of the notes offered hereby would cease to have a claim against the subsidiary guarantor based on the guarantee and would be creditors only of the Company and any guarantor whose guarantee was not similarly voided or otherwise held unenforceable.
We may not have sufficient funds to purchase notes upon a change of control.
If there is a change of control (as defined in the indenture governing the notes) and we have not previously exercised our right to redeem all of the outstanding notes as described under “Description of Notes—Optional redemption” or “Description of Notes—Special mandatory redemption,” each holder of notes may require us to purchase all or a portion of its notes at a purchase price equal to 101% of the principal amount thereof, plus accrued interest to the date of purchase. Certain agreements governing our existing and future indebtedness (including the agreements governing our existing and anticipated amended senior credit facilities) restrict or may restrict our ability to purchase the notes upon a change of control. As a result, in order to purchase notes following a change of control, we may be required to seek the consent of holders of our other indebtedness to purchase the notes or to refinance our outstanding indebtedness, which we might not be able to do, and even if we were able to refinance our other indebtedness, any financing might be on terms unfavorable to us. Under those circumstances, if we do not obtain the consent of the holders of our other indebtedness or refinance our other indebtedness, we will or may be prohibited from purchasing notes.
S-68
Table of Contents
We cannot assure you that we will have the financial ability to purchase outstanding notes upon the occurrence of a change of control. This risk is increased by the fact that the indentures governing the 2018 Notes and the 2020 Notes contain change of control provisions requiring us to offer to repurchase all of the outstanding 2018 Notes and the 2020 Notes upon the occurrence of change of control events substantially similar to those that would require us to offer to repurchase the notes offered hereby. The amount required to be escrowed by the Company as described below under “Description of Notes–Escrow of proceeds; release conditions” is less than the amount required to pay for all of the outstanding notes upon the occurrence of a change of control.
In addition, our existing senior secured credit facilities provide, and we expect that our amended senior secured credit facilities will provide, that the occurrence of certain kinds of change of control events will constitute a default under our senior secured credit facilities. Any future agreements to which we become a party may contain similar restrictions and provisions. In the event a change of control under the indenture governing the notes offered hereby occurs at a time when we are prohibited from purchasing notes pursuant to such agreements and we do not obtain a consent or repay the borrowings, we will remain prohibited from purchasing notes. Our failure to purchase tendered notes would constitute an event of default under the indenture which may, in turn, constitute a default under our other debt agreements. See “Description of Notes—Change of control” and “Events of default.” Likewise, any failure or inability to repurchase the 2018 Notes or the 2020 Notes upon the occurrence of change of control events specified in the indentures governing those notes could have similar consequences.
Courts interpreting change of control provisions under New York law (which will be the governing law of the indenture governing the notes) have not provided clear and consistent meanings of such change of control provisions, leading to subjective judicial interpretation. In addition, a court case in Delaware has questioned whether a change of control provision contained in an indenture could be unenforceable on public policy grounds. No assurances can be given that another court would enforce the change of control provisions in the indenture governing the notes as written for the benefit of the holders of the notes, or as to how these change of control provisions would be impacted were we to become a debtor in a bankruptcy case.
Furthermore, the change of control provisions of the notes may not provide holders of the notes protection in the event of in the event of highly leveraged transactions, reorganizations, restructurings, mergers, or similar transactions involving us that may adversely affect holders of notes. In particular, such a transaction may not give rise to a change of control, in which case we would not be required to make an offer to purchase the notes as required by the indenture governing the notes.
In addition, under the indenture governing the notes offered hereby, a change of control will occur when a majority of the members of our board of directors are not “continuing directors” (as defined in the indenture). In a decision in connection with a proxy contest, the Court of Chancery of Delaware held that the occurrence of a “change of control” under a similar indenture provision may nevertheless be avoided if the existing directors were to approve the slate of new director nominees (who would constitute a majority of the new board of directors) as “continuing directors” solely for purposes of avoiding the triggering of such change of control clause, provided the incumbent directors give their approval in the good faith exercise of their fiduciary duties. Therefore, in certain circumstances involving a significant change in the composition of our board of directors, including in connection with a proxy contest where our board of directors does not endorse a dissident slate of directors but approves them as “continuing directors,” holders of the notes may not be entitled to require us to make an offer to purchase the notes as required by the indenture governing the notes.
Moreover, a change of control will occur under the indenture governing the notes when there is a sale, lease, transfer, conveyance or other disposition (other than by way of merger or consolidation) of “all or substantially all” of the assets of DaVita and its restricted subsidiaries (as defined in the indenture), taken as a whole. Although there is a limited body of case law interpreting the phrase “substantially all,” there is no precise established definition of the phrase under applicable law. Accordingly, in certain circumstances there may be uncertainty as to whether a particular transaction would involve a disposition of “substantially all” of the
S-69
Table of Contents
property of DaVita and its restricted subsidiaries. As a result, it may be unclear whether a change of control has occurred under the indenture governing the notes and whether a holder of notes offered hereby may require us to make an offer to repurchase the notes under those circumstances.
Investors may find it difficult to trade the notes.
The notes are a new issue of securities and there is currently no public market for the notes. We do not intend to apply for a listing of the notes on any securities exchange or quotation system. Although the underwriters have informed us that they intend to make a market in the notes, they are under no obligation to do so and may discontinue any market making activities at any time without notice. Any such market making will be subject to the limitations imposed by the Securities Act of 1933, as amended and the Securities Exchange Act of 1934, as amended.
We also cannot assure you that you will be able to sell your notes at a particular time or that the prices that you receive when you sell will be favorable. We also cannot assure you as to whether a trading market for the notes will develop or as to the liquidity of any trading market for the notes which may develop. Future trading prices of the notes will depend on many factors, including:
| • | our operating performance, prospects and financial condition or the operating performance, prospects and financial condition of companies in our industry generally; |
| • | prevailing interest rates and other economic conditions; |
| • | the interest of securities dealers in making a market for the notes; and |
| • | the market for similar securities. |
It is possible that the market for the notes will be subject to disruptions. Any disruptions may have a negative effect on the holders of the notes, regardless of our prospects and financial performance.
Changes in credit ratings issued by nationally recognized statistical rating organizations could adversely affect our cost of financing and the market price of the notes.
Credit rating agencies rate our debt securities on factors that include our operating results, actions that we take, their view of the general outlook for our industry and their view of the general outlook for the economy. Actions taken by the rating agencies can include maintaining, upgrading, or downgrading the current rating or placing us on a watch list for possible future downgrading. Downgrading the credit rating of our debt securities or placing us on a watch list for possible future downgrading would likely increase our cost of financing, limit our access to the capital markets and have an adverse effect on the market price of the notes.
Not all of our subsidiaries or other entities included in our consolidated financial statements guarantee our obligations under the notes, and the assets of the non-guarantor subsidiaries and other entities may not be available to make payments on the notes.
Certain of our domestic subsidiaries will not be guarantors of the notes. In addition, while we currently do not have significant foreign operations, the notes will not be guaranteed by any of our existing or future foreign subsidiaries. Payments on the notes are only required to be made by the subsidiary guarantors and us. As a result, no payments are required to be made from the assets of subsidiaries that do not guarantee the notes. As of June 30, 2012, after giving pro forma effect to the Financings and the Merger as if they had occurred as of that date, our non-guarantor subsidiaries would have had aggregate total indebtedness and other liabilities including trade debt of approximately $510 million on their respective balance sheets and would have represented approximately 16.0% of our total assets as of June 30, 2012.
In the event of a bankruptcy, liquidation or reorganization of any of the non-guarantor subsidiaries, holders of their indebtedness and other obligations, including their trade creditors, will be entitled to payment of their
S-70
Table of Contents
claims from the assets of those subsidiaries before any assets are made available for distribution to us. As a result, the notes are structurally subordinated to all the liabilities of the non-guarantor subsidiaries. The indenture governing the notes offered hereby will permit, our existing senior secured credit facilities permit, and our amended senior secured credit facilities are expected to permit the incurrence of certain additional indebtedness by our non-guarantor subsidiaries in the future.
HCP provides services to certain affiliated physician groups that are not owned by HCP, will not constitute “Subsidiaries” (as defined in the indenture governing the notes) and will not guarantee the notes, even though the accounts of these groups are consolidated with the financial statements of HCP and would be consolidated with the financial statements of the Company following the Merger. Pursuant to management agreements between HCP and these affiliated physician groups, a substantial portion of the aggregate net revenues of these groups is payable to subsidiaries of HCP and will be payable to entities that will be guarantors of the notes as compensation for management and administrative services under management services agreements. See “HCP’s Business—Government Regulations—Corporate Practice of Medicine and Fee Splitting.” As of June 30, 2012, after giving pro forma effect to the Financing and the Merger as if they had occurred on that date, our consolidated balance sheet would have included third party liabilities of these affiliated physician groups, in the amount of approximately $305 million and assets of these affiliated physician groups in the amount of approximately $510 million after elimination of intercompany receivables (or approximately 3% of our consolidated total assets at that date). The pro forma consolidated net operating revenues and Adjusted EBITDA of DaVita for the twelve months ended June 30, 2012, giving effect to the Financing and the Merger as if they had occurred on July 1, 2011, would have been $9,929 million and $2,167 million, respectively. The pro forma consolidated net operating revenues and Adjusted EBITDA of DaVita, excluding HCP’s affiliated physician groups and DaVita’s existing non-guarantor Subsidiaries, for the twelve months ended June 30, 2012, giving effect to the Financing and the Merger as if they had occurred on July 1, 2011, would have been $6,623 million and $1,744 million, respectively. Substantially all of the difference between pro forma consolidated Adjusted EBITDA of $2,167 million and the pro forma consolidated Adjusted EBITDA excluding HCP’s affiliated physician groups and DaVita’s existing non-guarantor subsidiaries of $1,744 million for the twelve months ended June 30, 2012 is attributable to the exclusion of the existing non-guarantor subsidiaries of DaVita. The consolidated net operating revenues and Adjusted EBITDA of HCP for the twelve months ended June 30, 2012 were $2,564 million and $561 million, respectively. Excluding HCP’s affiliated physician groups, but inclusive of the management fees earned by HCP from the affiliated physician groups of $725 million, the net operating revenue and Adjusted EBITDA of HCP for the twelve months ended June 30, 2012 would have been $1,731 million and $557 million, respectively. Excluding the management fees earned by HCP from the affiliated physician groups, HCP net operating revenue for the twelve months ended June 30, 2012 would have been $1,006 million.
If the conditions to our Merger with HCP and certain other conditions are not satisfied on or prior to the Escrow End Date, or in certain other circumstances, we will be required to redeem all of the notes. If this occurs, you may not obtain your expected return on the notes.
Upon consummation of the offering of the notes, we will deposit the net proceeds (after deducting the underwriting discount from this offering), together with additional amounts needed to redeem the notes into escrow at the special mandatory redemption price as described in “Description of Notes—Escrow of proceeds; release conditions.” If the conditions to our Merger with HCP and certain other conditions are not satisfied on or prior to the Escrow End Date, or if we notify the escrow agent that we will not pursue consummation of the Merger, the amounts deposited in escrow will be applied to redeem all of the notes offered hereby at a special mandatory redemption price equal to 100% of the issue price of the notes, plus accrued and unpaid interest from the date of initial issuance, or the most recent date to which interest has been paid or duly provided for, as the case may be, to but excluding the special mandatory redemption date. See “Description of Notes—Special mandatory redemption.” There can be assurance that the conditions to our Merger with HCP or these other conditions, many of which are beyond our control, will be satisfied. Likewise, the Merger Agreement may be terminated under various circumstances specified therein or may be terminated or amended voluntarily by agreement of us and HCP. If we are not able to satisfy the conditions to the Merger and these other conditions prior to the Escrow End Date, or we give the escrow agent the notice described above prior to that date, the
S-71
Table of Contents
amounts deposited in escrow will be applied to effect this special mandatory redemption. Following such redemption, you may not be able to reinvest the proceeds from the redemption in an investment that yields a return comparable to the return on the notes. Additionally, you may suffer a loss on your investment if you purchased the notes at a price greater than the special optional redemption price. As a result, you may not obtain your expected return on the notes or you may suffer a loss on your investment in the notes.
We will not be required to redeem the notes if, between the date of this prospectus supplement and the consummation of the Merger with HCP, we or HCP experiences adverse changes in our respective business or financial condition.
Your decision to invest in the notes is made at the time of this offering of the notes. You will have no rights under the special mandatory redemption provisions as long as the conditions to the Merger with HCP and certain other conditions are satisfied or waived and none of the other events that would require us to effect a special optional redemption as described in the preceding risk factor occurs, in each case on or prior to the Escrow End Date. Likewise, we will not be required to redeem the notes, nor will you have any right to require us to repurchase your notes, if, between the closing of the notes offering and the closing of the Merger, we or HCP experience adverse changes in our respective businesses, results of operations, financial condition or prospects, or if the terms of the Merger change.
If a bankruptcy or reorganization case is commenced, bankruptcy laws may prevent the release of the escrowed funds.
If we or any of our subsidiaries commences a bankruptcy or reorganization case, or one is commenced against us or any of our subsidiaries, while amounts remain in the escrow account described under “Description of Notes—Escrow of proceeds; release conditions,” applicable bankruptcy laws may prevent the escrow agent from releasing, the escrowed funds. or applying those funds to effect a special mandatory redemption of the notes or otherwise applying those funds for the benefit of the holders of the notes. The court adjudicating that case might find that such escrow account is the property of the bankruptcy estate. Although the amounts in the escrow account will be pledged as collateral for payment, if required, of the special mandatory redemption price, the automatic stay provisions of the federal bankruptcy laws generally prohibit secured creditors from foreclosing upon or disposing of a debtors’ property without bankruptcy court approval. As a result, holders of the notes may not be able to have the escrow funds applied at the time or in the manner contemplated by the indenture and could suffer a loss as a result.
Our substantial indebtedness could adversely affect our financial health and prevent us from fulfilling our obligations under the notes.
As of June 30, 2012, after giving pro forma effect to the Financings and the Merger as if each of the Financings and the Merger had occurred on that date, we would have had approximately $8,308 million in outstanding debt excluding the debt discounts associated with our term loans on our consolidated balance sheet and approximately $284 million of available unused borrowing capacity under the revolving portion of our amended senior secured credit facilities (after giving effect to outstanding letters of credit of approximately $66 million) and for the twelve months then ended our debt expense, calculated on a pro forma basis as if the Financings and the Merger had occurred as of the first day of such twelve month period, would have been approximately $430 million. Our substantial indebtedness could have important consequences to you. For example, it could:
| • | make it more difficult for us to satisfy our obligations with respect to the notes, |
| • | increase our vulnerability to general adverse economic and industry conditions, |
| • | require us to dedicate a substantial portion of our cash flow from operations to payments on our indebtedness, thereby reducing the availability of our cash flow to fund working capital, capital expenditures, acquisitions and investments and other general corporate purposes, |
S-72
Table of Contents
| • | expose us to interest rate fluctuations because the interest on the debt under our amended senior secured credit facilities may be at variable rates, |
| • | limit our flexibility in planning for, or reacting to, changes in our business and the markets in which we operate, |
| • | place us at a competitive disadvantage compared to our competitors that have less debt, and |
| • | limit our ability to borrow additional funds. |
In addition, we may incur substantial additional indebtedness in the future. The terms of the indentures governing the notes, the 2008 Notes or the 2010 Notes, the existing senior secured credit facilities and the amended senior secured credit facilities will allow us to incur substantial additional debt. If new debt is added to current debt levels, the related risks described above could intensify. If additional debt financing is not available when required or is not available on acceptable terms, we may be unable to grow our business, take advantage of business opportunities, respond to competitive pressures or refinance maturing debt, any of which could have a material adverse effect on our operating results and financial condition.
We will require a significant amount of cash to service our indebtedness. Our ability to generate cash depends on many factors beyond our control.
Our ability to make interest and principal payments on our indebtedness, including the notes, and to fund planned capital expenditures and expansion efforts, including any strategic acquisitions we may make in the future, will depend on our ability to generate cash. This, to a certain extent, is subject to general economic, financial, competitive, regulatory and other factors that are beyond our control.
We cannot assure you that our business will generate sufficient cash flow from operations in the future, that our currently anticipated growth in revenue and cash flow will be realized on schedule or that future borrowings will be available to us in an amount sufficient to enable us to service our indebtedness, including the notes, to refinance our indebtedness when it matures or to fund other liquidity needs. We may need to refinance all or a portion of our indebtedness, including the notes, on or before maturity. Our existing senior secured credit facilities are secured and our amended senior secured credit facilities will be secured by substantially all of our and the subsidiary guarantors’ assets. In particular, our existing senior secured credit facilities are secured by first priority pledges of 100% of the equity interests owned by us in our direct and indirect domestic subsidiaries and 65% of the equity interests of our direct foreign subsidiaries and the amended or any successor credit facility is likely to be secured on a similar basis. As such, our ability to refinance the notes or seek additional financing could be limited by the fact that these assets have been pledged to secure borrowings under our credit facilities. We cannot assure you that we will be able to service or refinance our indebtedness on commercially reasonable terms or at all.
S-73
Table of Contents
Rationale for the Merger
DaVita believes that the Merger with HCP can open a large new market for DaVita—the integrated healthcare services market that HCP serves—offering considerable growth opportunities beyond domestic dialysis. The combination offers the potential to create an industry leading company that may be well positioned to capitalize on anticipated trends in U.S. healthcare, including growth in managed healthcare services, especially to the Medicare-eligible population.
As a significant participant in healthcare delivery with a proven track record, HCP is a recognized leader in its field and should allow DaVita to significantly expand the range of services it provides with only limited additional operational resources required. HCP’s industry leadership provides it substantial credibility with governmental entities, physician groups, large hospital systems and payors across the U.S.
There are many similarities in the values and cultures of DaVita and HCP, including a strong common culture of putting the patient first. In the case of HCP, this is demonstrated by its commitment to and the success of its integrated care model, which has had high quality clinical outcomes and has been able to effectively manage its costs under capitated arrangements. DaVita believes that HCP’s business model is in the right place to capitalize on long-term trends in healthcare in the U.S.—the need to more effectively manage the cost of providing healthcare services, especially to the Medicare-eligible population, while continuing to deliver high quality care. In addition, DaVita believes that HCP’s experience may be able to help DaVita achieve attractive reimbursement for globally capitated kidney care.
Merger Agreement
On May 20, 2012, we entered into a Merger Agreement providing for our acquisition of HCP pursuant to the Merger of a newly formed wholly owned subsidiary of DaVita into HCP. Under the Merger Agreement, HCP will be the surviving entity in the Merger and will become a wholly owned subsidiary of DaVita. Following the Merger, DaVita will be renamed “DaVita HealthCare Partners Inc.”
If the Merger is completed, the total merger consideration to be paid to the holders of HCP common units and vested and unvested options to purchase HCP common units is an aggregate of $3.6 billion in cash and approximately 9.4 million shares of DaVita common stock, subject to certain adjustments.
In addition to the merger consideration payable at the closing of the Merger and amounts that may be released over time from the escrow accounts as further described below in “—Merger Agreement Escrows”, HCP members and holders of HCP options may receive up to $275.0 million of additional cash consideration in the form of two separate earn-out payments of $137.5 million in cash that are based on the financial performance of HCP and the achievement of certain financial targets for fiscal years 2012 and 2013.
The completion of the Merger is subject to various customary conditions, including, among others, (i) obtaining the approval of HCP’s members, (ii) subject to certain materiality exceptions, the accuracy of the representations and warranties made by DaVita and HCP, respectively, and compliance by DaVita and HCP with their respective obligations under the Merger Agreement, and (iii) declaration of the effectiveness by the Securities and Exchange Commission of the registration statement filed by DaVita regarding the shares of DaVita common stock to be issued in the Merger.
The Merger must be approved by a vote of the majority of the HCP members. The board of managers of HCP made a recommendation to the HCP members to approve the principal terms of the Merger and the Merger Agreement and the holders of approximately 74% of the outstanding HCP common units has entered into a voting agreement with DaVita pursuant to which it has agreed to vote in favor of the principal terms of the Merger and the Merger Agreement. Accordingly, pursuant to such voting agreement the HCP member approval is assured.
S-74
Table of Contents
The Merger Agreement contains certain termination rights for each of DaVita and HCP and provides that DaVita is required to pay HCP a $125.0 million termination fee in the event that the Merger Agreement is terminated under certain circumstances. Specifically, in the event that DaVita cannot obtain the financing required for the Merger, each party to the Merger generally has the right to terminate the Merger Agreement and HCP may be entitled to the termination fee.
The Merger Agreement provides that at the closing the DaVita board of directors will be increased in size by one member, and Dr. Robert Margolis, Chairman and Chief Executive Officer of HCP, will be appointed to fill the newly created directorship as “Co-Chairman”. In addition, for a minimum period of four consecutive annual meetings of stockholders of DaVita, Dr. Margolis will hold the office of “Co-Chairman” until the expiration of his term of office or until his successor is duly elected and qualified, subject to his earlier death, resignation, disqualification, or removal in accordance with DaVita’s bylaws and/or applicable law.
Merger Agreement Escrows
Approximately $575 million of the closing merger consideration will be withheld from payment and contributed to escrow accounts that support a potential working capital adjustment, certain indemnification obligations, certain contingent payments, and certain costs and expenses that may be incurred by the HCP member representative designated in the Merger Agreement. Beginning on the second anniversary of the closing, funds in escrow, to the extent not previously released or reserved for certain indemnity claims, will be released on various dates, with the final release to occur on or about October 15, 2017.
Employment Agreements
Concurrently with the execution of the Merger Agreement, each of Dr. Margolis, Mr. Mazdyasni, Dr. Chin, Dr. Thomas Paulsen, Executive Medical Director, California of HCP, Zan Calhoun, Chief Operating Officer of HCP, and Lorie Glisson, Chief Executive Officer—JSA Healthcare, entered into an employment agreement with HCP and DaVita that will become effective upon the consummation of the Merger.
Financing of the Merger
We expect to finance the cash portion of the Merger consideration through a combination of available cash, the net proceeds of the notes offered hereby, and additional borrowings under our senior secured credit facilities, which senior secured credit agreement is expected to be amended to permit or facilitate, among other things, the additional borrowings under the senior secured credit facilities, the Merger and this note offering. There is no financing condition to the Merger; however, DaVita must use its reasonable best efforts to arrange and obtain the financing required to consummate the Merger.
We currently intend to enter into an amendment to our senior secured credit facilities to provide for additional borrowings in an aggregate principal amount of $3,000 million, comprised of:
| • | a new five year Term Loan A-3 facility in an aggregate principal amount of $1,350 million, and |
| • | a new seven year Term Loan B-2 facility in an aggregate principal amount of $1,650 million. |
The proceeds from these additional borrowings, together with available cash, will be used to finance a portion of the cash portion of the Merger consideration, to repay approximately $198 million of our Term Loan A-2 outstanding under our existing senior secured credit agreement, to repay the net amount of HCP indebtedness as a result of the Merger, and pay related fees and expenses.
We intend to borrow all $3,000 million of the term loans and issue $1,000 million of the notes offered hereby. Based upon the amount of available cash, and the proceeds of the notes and secured debt expected to be available to the Company, after giving pro forma effect to the Financing and the Merger as if they had occurred on June 30, 2012, we do not anticipate borrowing any amounts under our revolving credit facility.
S-75
Table of Contents
The terms and conditions of the amended senior secured credit facilities have not been finalized and are subject to change. We may not finalize the terms until prior to the consummation of the Merger, but after the issuance of the notes offered hereby.
We expect that our amended senior secured credit facilities will be guaranteed by a substantial portion of our direct or indirect wholly owned domestic subsidiaries and will be secured by substantially all of our and our subsidiary guarantors’ assets. In particular, these facilities will be secured by first priority pledges of 100% of the equity interests owned by us and the subsidiary guarantors in our direct domestic subsidiaries and 65% of the equity interests of our and the subsidiary guarantors direct foreign subsidiaries, if any.
We expect that our amended senior credit facilities will contain limits and restrictions on certain of our business activities. In addition, we expect that the amended senior secured credit facilities will require compliance on a quarterly basis with certain financial covenants.
As a result of the borrowings that we will incur to finance the Merger, the aggregate amount of our indebtedness and annual debt expense will increase substantially following the Merger. See “Risk Factors,” “Capitalization” and “Unaudited Pro Forma Condensed Consolidated Financial Information.”
S-76
Table of Contents
We estimate the net proceeds from this offering, after deducting the underwriting discount and other estimated expenses payable by us, will be approximately $983.0 million. The net proceeds, after deducting the underwriting discount, will be deposited into an escrow account upon the closing of this offering. Funds held in escrow will be released upon the consummation of the Merger and satisfaction of certain other conditions and we intend to use the escrowed proceeds from this offering, together with proceeds from our anticipated amended senior secured credit facilities and cash on hand, to finance the aggregate cash consideration of the Merger and pay related fees and expenses. The following table illustrates the sources and uses of funds from the Financing.
Sources of Funds | ||||
| (in millions) | ||||
Amended senior secured credit facilities(1) | $ | 3,000 | ||
Notes offered hereby | 1,000 | |||
Equity consideration(2) | 907 | |||
Cash from balance sheet | 67 | |||
|
| |||
Total sources | $ | 4,974 | ||
|
| |||
Uses of Funds | ||||
| (in millions) | ||||
Cash portion of purchase price(3) | $ | 3,592 | ||
Equity portion of purchase price(2) | 907 | |||
Repayment of Term Loan A-2 | 198 | |||
Repayment of HCP’s existing debt(4) | 187 | |||
Estimated fees and expenses | 90 | |||
|
| |||
Total uses | $ | 4,974 | ||
|
| |||
| (1) | Assumes that such amounts are obtained through the issuance of additional term loans under the amended senior secured credit facilities. The terms of the amended senior secured credit facilities have not yet been finalized and are subject to change. |
| (2) | Based upon the issuance of 9,380,312 shares of Davita Inc. common stock valued at the closing market price on August 10, 2012 as reported by the NYSE. |
| (3) | The cash portion of the purchase price for HCP consists of $3.66 billion in cash less an estimated negative working capital adjustment of $68 million. |
| (4) | Represents HCP’s debt to be repaid at the closing of the Merger (based upon HCP’s existing debt net of available cash, in each case as of June 30, 2012). |
If the Merger is not consummated on or prior to the Escrow End Date, or if we notify the escrow agent that we will not pursue consummation of the Merger, the amount deposited in escrow will be applied to redeem all of the notes offered hereby at a special mandatory redemption price equal to 100% of the issue price of the notes, plus accrued and unpaid interest from the date of initial issuance, or the most recent date to which interest has been paid or duly provided for, as the case may be, to but excluding the special mandatory redemption date. If the conditions to the Merger are satisfied on or before the Escrow End Date, the amounts deposited in escrow will be released to us and applied to finance a portion of the cash consideration for the merger. See “Use of Proceeds” and “Description of Notes—Special mandatory redemption.” Funds held in the escrow until released from escrow will be invested in be invested in short-term, investment-grade, interest bearing securities.
S-77
Table of Contents
The following table sets forth our capitalization as of June 30, 2012:
| • | on an actual basis, |
| • | on an as adjusted basis to give effect to the Financings, as if they had occurred on that date, and |
| • | on a further adjusted basis to give effect to the Merger as if it had occurred on that date. |
You should read the following table in conjunction with the financial statements incorporated by reference in this prospectus supplement and the related notes thereto and the section of the prospectus supplement entitled “Use of Proceeds.”
| As of June 30, 2012 | ||||||||||||
| Actual | As Adjusted | As Further Adjusted | ||||||||||
| (Unaudited, dollars in millions) | ||||||||||||
Cash and cash equivalents | $ | 273 | $ | (67 | ) | $ | 206 | (5) | ||||
Senior debt: | ||||||||||||
Senior secured credit facilities: | ||||||||||||
Revolving credit facility(1) | — | — | ||||||||||
Term loans | ||||||||||||
Existing Term Loan A and Term Loan B | 2,649 | 2,649 | ||||||||||
Term Loan A-2 | 198 | (198 | ) | |||||||||
New Term Loans(2) | 3,000 | 3,000 | ||||||||||
Other | 36 | 36 | ||||||||||
|
|
|
| |||||||||
Total secured debt | $ | 2,883 | $ | 5,685 | ||||||||
Unsecured debt: | ||||||||||||
Existing notes | 1,550 | 1,550 | ||||||||||
New notes offered hereby | — | 1,000 | 1,000 | |||||||||
Other | 72 | 72 | ||||||||||
|
|
|
| |||||||||
Total unsecured debt | $ | 1,622 | $ | 2,622 | ||||||||
|
|
|
| |||||||||
Total debt(3) | $ | 4,505 | $ | 8,307 | ||||||||
Net debt(4) | 4,281 | 8,167 | ||||||||||
Total DaVita Inc. shareholders’ equity | 2,379 | 896 | 3,275 | |||||||||
|
|
|
| |||||||||
Total capitalization | $ | 7,157 | $ | 11,788 | ||||||||
|
|
|
| |||||||||
| (1) | As of June 30, 2012, after giving pro forma effect to the Financings and the Merger as if they had occurred on that date, DaVita and the guarantors would have had approximately $284 million available for future borrowings under the revolving credit facility (after giving effect to letters of credit of approximately $66 million). |
| (2) | Excludes upfront fees payable to lenders, if any. |
| (3) | Total debt refers to total funded debt outstanding and excludes the unamortized balance of debt discounts associated with our Term Loans. |
| (4) | Net debt is total debt including our outstanding letters of credit (approximately $49 million on an actual basis and approximately $66 million on an as adjusted basis to give effect to the Financings and the Merger) less cash and cash equivalents. |
| (5) | As adjusted balance excludes the effect of expenses. |
S-78
Table of Contents
UNAUDITED PRO FORMA CONDENSED CONSOLIDATED FINANCIAL INFORMATION
The following table presents unaudited pro forma condensed consolidated financial information about the financial condition and results of operations of DaVita after giving effect to the Merger. The unaudited pro forma condensed consolidated income statement data for the six months ended June 30, 2012 and the year ended December 31, 2011 give effect to the Merger as if the Merger had taken place on January 1, 2011. The unaudited pro forma condensed consolidated balance sheet data gives effect to the Merger as if it had taken place on June 30, 2012.
The following unaudited pro forma condensed consolidated financial information has been prepared by applying the purchase method of accounting with DaVita treated as the acquirer and does not give effect to any potential cost savings or other operating efficiencies that could result from the Merger. In addition, DaVita’s fair value of consideration paid to HCP unitholders will be allocated to the assets acquired and liabilities assumed based upon their estimated fair values as of the date of the Merger. The allocation is dependent upon certain valuations and other studies that have not progressed to the state where there is sufficient information to make a definitive allocation. Accordingly, the purchase price allocation pro forma adjustments are preliminary and have been made solely for the purpose of providing unaudited pro forma condensed consolidated financial information in this prospectus supplement. The actual number of shares of DaVita common stock issued in respect of each unit of HCP in the Merger will be established shortly before completion of the Merger.
You should read the information set forth below in conjunction with each of DaVita’s and HCP’s consolidated financial statements and the related notes, “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “DaVita Selected Historical Financial and Other Data,” “HCP Selected Historical Financial and Other Data” and “Unaudited Pro Forma Condensed Consolidated Financial Information.” The unaudited pro forma condensed consolidated financial information set forth below has been presented for informational purposes only and is not necessarily indicative of what the consolidated financial condition or results of operations actually would have been had the Merger been completed as of the dates indicated. In addition, the unaudited pro forma condensed consolidated financial information presented below does not purport to project the consolidated financial condition or operating results for any future period.
S-79
Table of Contents
Unaudited pro forma condensed consolidated statement of income
Year ended December 31, 2011
| Pro forma adjustment(i) | ||||||||||||||||
| Historical DaVita | Historical HCP | Merger and related financing | Pro forma consolidated | |||||||||||||
| (dollars in millions, except per share data) | ||||||||||||||||
Net dialysis patient service revenues, less provision for uncollectable accounts of $190 | $ | 6,273 | $ | — | $ | 6,273 | ||||||||||
Integrated care revenue | — | 2,375 | 2,375 | |||||||||||||
Other revenues(1) | 519 | 47 | 566 | |||||||||||||
|
|
|
|
|
| |||||||||||
Net operating revenues | 6,792 | 2,422 | 9,214 | |||||||||||||
|
|
|
|
|
| |||||||||||
Operating expenses and charges: | ||||||||||||||||
Patient care costs | 4,681 | 1,721 | 6,402 | |||||||||||||
General and administrative | 691 | 207 | (2 | )(a) | 896 | |||||||||||
Depreciation and amortization | 267 | 31 | 143 | (a) | 425 | |||||||||||
| (16 | )(a) | |||||||||||||||
Provision for uncollectible accounts | 7 | — | 7 | |||||||||||||
Equity investment income | (9 | ) | (25 | ) | (34 | ) | ||||||||||
Goodwill impairment charge | 24 | — | 24 | |||||||||||||
|
|
|
|
|
| |||||||||||
Total operating expenses and charges | 5,661 | 1,934 | 7,720 | |||||||||||||
|
|
|
|
|
| |||||||||||
Operating income | 1,131 | 488 | 1,494 | |||||||||||||
Debt expense | (241 | ) | (16 | ) | (169 | )(b) | (434 | ) | ||||||||
| (12 | )(b) | |||||||||||||||
| 17 | (b) | |||||||||||||||
| (13 | )(b) | |||||||||||||||
Other income | 3 | 8 | 11 | |||||||||||||
|
|
|
|
|
| |||||||||||
Income from continuing operations before income taxes | 893 | 480 | 1,071 | |||||||||||||
Income tax expense | 316 | 71 | 3 | (c) | 390 | |||||||||||
|
|
|
|
|
| |||||||||||
Income from continuing operations | 577 | 409 | 681 | |||||||||||||
Discontinued operations: | ||||||||||||||||
Income from operations of discontinued operations, net of tax | 1 | — | 1 | |||||||||||||
Loss on disposal of discontinued operations, net of tax | (5 | ) | — | (5 | ) | |||||||||||
|
|
|
|
|
| |||||||||||
Net income | 573 | 409 | 677 | |||||||||||||
Less: Net income attributable to noncontrolling interests | (95 | ) | — | (95 | ) | |||||||||||
|
|
|
|
|
| |||||||||||
Net income attributable to DaVita Inc. | $ | 478 | $ | 409 | $ | 582 | ||||||||||
|
|
|
|
|
| |||||||||||
Earnings per share: | ||||||||||||||||
Basic income from continuing operations per share attributable to DaVita Inc. | $ | 5.09 | $ | 5.63 | ||||||||||||
|
|
|
| |||||||||||||
Basic net income per share attributable to DaVita Inc. | $ | 5.05 | $ | 5.59 | ||||||||||||
|
|
|
| |||||||||||||
Diluted income from continuing operations per share attributable to DaVita Inc. | $ | 4.99 | $ | 5.53 | ||||||||||||
|
|
|
| |||||||||||||
Diluted net income per share attributable to DaVita Inc. | $ | 4.96 | $ | 5.49 | ||||||||||||
|
|
|
| |||||||||||||
Weighted average shares for earnings per share: | ||||||||||||||||
Basic | 94,658,027 | 9,380,312 | 104,038,339 | |||||||||||||
|
|
|
| |||||||||||||
Diluted | 96,532,110 | 9,380,312 | 105,912,422 | |||||||||||||
|
|
|
| |||||||||||||
Amounts attributable to DaVita Inc.: | ||||||||||||||||
Income from continuing operations | $ | 482 | $ | 586 | ||||||||||||
Discontinued operations | (4 | ) | (4 | ) | ||||||||||||
|
|
|
| |||||||||||||
Net income | $ | 478 | $ | 582 | ||||||||||||
|
|
|
| |||||||||||||
| (1) | Other revenues for DaVita include revenues from our ancillary services and strategic initiatives and fees for providing management and administrative services and the other revenues for HCP include revenues primarily from consulting services and fees from providing management and administrative services. |
S-80
Table of Contents
Notes to unaudited pro forma condensed consolidated statement of income
Year ended December 31, 2011
| (a) | Reflects net amortization expense associated with the customer relationships, non-compete agreements and other intangible assets. The customer relationships are being amortized over seventeen years, the non-compete agreements are being amortized over three and seven years and the other intangible assets are being amortized over five to ten years, as set forth in the table below: |
| Amount | Life | Amortization | ||||||||||
| (dollars in millions) | ||||||||||||
Customer relationships | $ | 940 | 17 | $ | 55 | |||||||
Non-compete agreements | $ | 220 | 3-7 | 33 | ||||||||
Other intangibles | $ | 478 | 5-10 | 55 | ||||||||
|
| |||||||||||
| $ | 143 | |||||||||||
|
| |||||||||||
Historical HCP amortization expense of $16 million will be replaced with the estimated $143 million of amortization expense as a result of recording the acquisition at fair value.
Transaction costs of $2 million that were recognized during the year ended December 31, 2011 have been reversed since they represent non-recurring charges directly related to the transaction.
| (b) | Reflects adjustments to interest expense to reflect: (i) increase in annual interest expense of $169 million associated with borrowings under the amended senior secured credit facilities and the incurrence of the additional senior indebtedness, (ii) the amortization of deferred financing costs and debt discount associated with the Financings of approximately $12 million, (iii) reduced interest expense associated with the repayment of HCP’s outstanding debt of approximately $14 million, and (iv) the amortization of previously recognized deferred financings costs and interest expense with respect to DaVita’s previously outstanding Term Loan A-2 of $3 million. Pro forma debt expense assumes a weighted average effective interest rate of 4.48% including the impact of our anticipated new swap agreements for the year ending December 31, 2011. Pro forma debt expense assumes the interest rates on the financings that we expect to obtain at the time of closing, actual interest rates may vary. An increase or decrease of 0.125% in the interest rate applicable to the additional $3,802 million of indebtedness at closing of the Merger would result in an approximate change of approximately $5 million in debt expense annually (assuming that (A) DaVita will enter into swap agreements with respect to the additional borrowings under the amended senior secured credit facilities with a notional amount of $1,350 million and associated debt expense of $13 million and (B) the prevailing LIBOR rate on the closing date of the Merger will be less than the assumed interest rate floor of 1.00% associated with the portion of the additional borrowings under the amended senior secured credit facilities that will not be subject to the swap agreements referred to in clause (A)). |
| (c) | Reflects the adjustment to the income tax expense amount of $3 million based on the overall pro forma pre-tax income at 40.0% after considering noncontrolling interests. |
| (i) | The unaudited pro forma condensed consolidated statement of income for the year ended December 31, 2011 gives effect to the acquisition of HCP and related borrowings as if each had occurred on January 1, 2011. |
S-81
Table of Contents
Unaudited pro forma condensed consolidated statement of income
six months ended June 30, 2012
| Pro forma adjustment(i) | ||||||||||||||||
| Historical DaVita | Historical HCP | Merger and related financing | Pro forma consolidated | |||||||||||||
| (dollars in millions, except per share data) | ||||||||||||||||
Dialysis patient service operating revenue, less provision for uncollectable accounts of $107 | $ | 3,465 | $ | — | $ | 3,465 | ||||||||||
Integrated care revenue | — | 1,294 | 1,294 | |||||||||||||
Other revenues(1) | 332 | 28 | 360 | |||||||||||||
|
|
|
|
|
| |||||||||||
Net operating revenues | 3,797 | 1,322 | 5,119 | |||||||||||||
|
|
|
|
|
| |||||||||||
Operating expenses and charges: | ||||||||||||||||
Patient care costs | 2,575 | 940 | 3,515 | |||||||||||||
General and administrative | 422 | 110 | (19 | )(a) | 513 | |||||||||||
Depreciation and amortization | 154 | 16 | 72 | (a) | 234 | |||||||||||
| (8 | )(a) | |||||||||||||||
Provision for uncollectible accounts | 4 | — | 4 | |||||||||||||
Equity investment income | (5 | ) | (12 | ) | (17 | ) | ||||||||||
Legal proceeding contingency accrual and related expenses | 78 | 78 | ||||||||||||||
|
|
|
|
|
| |||||||||||
Total operating expenses | 3,228 | 1,054 | 4,327 | |||||||||||||
|
|
|
|
|
| |||||||||||
Operating income | 569 | 268 | 792 | |||||||||||||
Debt expense | (122 | ) | (6 | ) | (83 | )(b) | (212 | ) | ||||||||
| (6 | )(b) | |||||||||||||||
| 11 | (b) | |||||||||||||||
| (6 | )(b) | |||||||||||||||
Other income, net | 2 | 4 | 5 | |||||||||||||
|
|
|
|
|
| |||||||||||
Income before income taxes | 449 | 266 | 585 | |||||||||||||
Income tax expense | 164 | 33 | 23 | (c) | 220 | |||||||||||
|
|
|
|
|
| |||||||||||
Net income | 285 | 233 | 365 | |||||||||||||
Less: Net income attributable to noncontrolling interests | (49 | ) | — | (49 | ) | |||||||||||
|
|
|
|
|
| |||||||||||
Net income | $ | 236 | $ | 233 | $ | 316 | ||||||||||
|
|
|
|
|
| |||||||||||
Earnings per share: | ||||||||||||||||
Basic | $ | 2.51 | $ | 3.06 | ||||||||||||
|
|
|
| |||||||||||||
Diluted | $ | 2.46 | $ | 3.01 | ||||||||||||
|
|
|
| |||||||||||||
Weighted average shares for earnings per share: | ||||||||||||||||
Basic | 93,970,295 | 9,380,312 | 103,350,607 | |||||||||||||
|
|
|
| |||||||||||||
Diluted | 95,865,605 | 9,380,312 | 105,245,917 | |||||||||||||
|
|
|
| |||||||||||||
| (1) | Other revenues for DaVita include revenues from our ancillary services and strategic initiatives and fees for providing management and administrative services and the other revenues for HCP include revenues primarily from consulting services and fees from providing management and administrative services. |
S-82
Table of Contents
Notes to unaudited pro forma condensed consolidated statement of income
six months ended June 30, 2012
| (a) | Reflects net amortization expense associated with the customer relationships, non-compete agreements and other intangible assets. The customer relationships are being amortized over seventeen years, the non-compete agreements are being amortized over seven and three years and the other intangible assets are being amortized over five to ten years, as set forth in the table below: |
| Amount | Life | Amortization | ||||||||||
| (dollars in millions) | ||||||||||||
Customer relationships | $ | 940 | 17 | $ | 28 | |||||||
Non-compete agreements | $ | 220 | 3-7 | 16 | ||||||||
Other intangibles | $ | 478 | 5-10 | 28 | ||||||||
|
| |||||||||||
| $ | 72 | |||||||||||
|
| |||||||||||
Historical HCP amortization of $8 million will be replaced with the estimated $72 million of amortization expense as a result of recording the acquisition at fair value.
Transaction costs of $19 million that were recognized during the first six months of 2012 have been revered since they represent non-recurring charges directly related to the transaction.
| (b) | Reflects adjustments to interest expense to reflect: (i) increase in annual interest expense of $83 million associated with borrowings under the amended senior secured credit facilities and the incurrence of the additional senior indebtedness, (ii) the amortization of deferred financing costs and debt discount associated with the Financings of approximately $6 million, (iii) reduced interest expense associated with the repayment of HCP’s outstanding debt of approximately $6 million, and (iv) the amortization of previously recognized deferred financings costs and interest expense with respect to DaVita’s previously outstanding Term Loan A-2 of $5 million. Pro forma debt expense assumes a weighted average effective interest rate of 4.49% including the impact of our anticipated new swap agreements for the six months ended June 30, 2012. Pro forma debt expense assumes the interest rates on the financings that we expect to obtain at the time of closing, actual interest rates may vary. An increase or decrease of 0.125% in the interest rate applicable to the additional $3,802 million of indebtedness at closing of the Merger would result in an approximate change of approximately $2 million in debt expense for the six month period (assuming that (A) DaVita will enter into swap agreements with respect to the additional borrowings under the amended senior secured credit facilities with a notional amount of $1,350 million and associated debt expense of $6 million and (B) the prevailing LIBOR rate on the closing date of the Merger will be less than the assumed interest rate floor of 1.00% associated with the portion of the additional borrowings under the amended senior secured credit facilities that will not be subject to the swap agreements referred to in clause (A)). |
| (c) | Reflects the adjustment to the income tax expense amount of $23 million based on the overall impact of the pro forma pre-tax income at 41.0% after considering noncontrolling interests. |
| (i) | The unaudited pro forma condensed consolidated statement of income for the six months ended June 30, 2012 gives effect to the acquisition of HCP and related borrowings as if each had occurred on January 1, 2011. |
S-83
Table of Contents
DAVITA INC. AND HEALTHCARE PARTNERS HOLDINGS, LLC
UNAUDITED PRO FORMA CONDENSED CONSOLIDATED
FINANCIAL STATEMENTS
Unaudited pro forma condensed consolidated balance sheet
As of June 30, 2012
| Pro forma adjustments(i) | ||||||||||||||||||||
| Historical DaVita | Historical HealthCare Partners | DaVita and HealthCare Partners Merger(a) | Related Borrowings | Pro forma Consolidated | ||||||||||||||||
| (dollars in millions) | ||||||||||||||||||||
Assets | ||||||||||||||||||||
Cash and cash equivalents | $ | 273 | $ | 355 | $ | (3,592 | ) | $ | 3,712 | (b) | $ | 206 | ||||||||
| (542 | )(f) | |||||||||||||||||||
Short-term investments | 9 | 180 | 189 | |||||||||||||||||
Accounts receivable, net | 1,250 | 172 | 1,422 | |||||||||||||||||
Inventories | 78 | — | 78 | |||||||||||||||||
Other receivables | 211 | — | 211 | |||||||||||||||||
Other current assets | 46 | 106 | 152 | |||||||||||||||||
Income tax receivable | 12 | 1 | (e) | 19 | ||||||||||||||||
| 4 | (d) | |||||||||||||||||||
| 2 | (f) | |||||||||||||||||||
Deferred income taxes | 300 | 7 | (7 | ) | 300 | |||||||||||||||
|
|
|
|
|
| |||||||||||||||
Total current assets | 2,179 | 820 | 2,577 | |||||||||||||||||
Property and equipment, net | 1,586 | 79 | 1,665 | |||||||||||||||||
Amortizable intangibles, net | 162 | 158 | 1,638 | 52 | (d) | 1,850 | ||||||||||||||
| (158 | ) | (2 | )(e) | |||||||||||||||||
Notes receivable, net | — | 8 | 8 | |||||||||||||||||
Equity investments | 28 | — | 4 | 32 | ||||||||||||||||
Long-term investments | 12 | — | 12 | |||||||||||||||||
Other long-term assets | 30 | 62 | (4 | ) | (5 | )(f) | 83 | |||||||||||||
Goodwill | 5,258 | 288 | 3,361 | 8,716 | ||||||||||||||||
| 158 | ||||||||||||||||||||
| (288 | ) | |||||||||||||||||||
| (68 | ) | |||||||||||||||||||
| 7 | ||||||||||||||||||||
|
|
|
|
|
| |||||||||||||||
| $ | 9,255 | $ | 1,415 | $ | 14,943 | |||||||||||||||
|
|
|
|
|
| |||||||||||||||
Liabilities and shareholders’ equity | ||||||||||||||||||||
Accounts payable | $ | 299 | $ | 222 | $ | 521 | ||||||||||||||
Other liabilities | 395 | — | $ | 144 | 539 | |||||||||||||||
Accrued compensation and benefits | 436 | — | 436 | |||||||||||||||||
Medical claims and related payables | — | 228 | 228 | |||||||||||||||||
Current portion of long-term debt | 106 | 30 | (2 | )(b) | 188 | |||||||||||||||
| 84 | (c) | |||||||||||||||||||
| (30 | )(f) | |||||||||||||||||||
|
|
|
|
|
| |||||||||||||||
Total current liabilities | 1,236 | 480 | 1,912 | |||||||||||||||||
Long-term debt | 4,393 | 512 | (196 | )(b) | 8,086 | |||||||||||||||
| 4,000 | (c) | |||||||||||||||||||
| (84 | )(c) | |||||||||||||||||||
| (27 | )(d) | |||||||||||||||||||
| (512 | )(f) | |||||||||||||||||||
Other long-term liabilities | 147 | 107 | 116 | 370 | ||||||||||||||||
Alliance and product supply agreement, net | 17 | — | 17 | |||||||||||||||||
Deferred income taxes | 431 | 68 | 200 | 631 | ||||||||||||||||
| (68 | ) | |||||||||||||||||||
|
|
|
|
|
| |||||||||||||||
Total liabilities | 6,224 | 1,167 | 11,016 | |||||||||||||||||
S-84
Table of Contents
| Pro forma adjustments(i) | ||||||||||||||||||||
| Historical DaVita | Historical HealthCare Partners | DaVita and HealthCare Partners Merger(a) | Related Borrowings | Pro forma Consolidated | ||||||||||||||||
| (dollars in millions, except per share data) | ||||||||||||||||||||
Noncontrolling interests subject to put provisions | 523 | — | 523 | |||||||||||||||||
Shareholders’ equity: | ||||||||||||||||||||
Preferred stock ($0.001 par value; 5,000,000 shares authorized; none issued) | — | — | ||||||||||||||||||
Common stock ($0.001 par value; 450,000,000 shares authorized; 134,862,283 shares issued; 94,486,725 and 103,867,037 shares outstanding historically and on a pro forma basis, respectively) | — | — | — | |||||||||||||||||
Additional paid-in capital | 565 | — | 536 | 1,101 | ||||||||||||||||
Retained earnings | 3,431 | — | (1 | )(e) | 3,420 | |||||||||||||||
| (7 | )(d) | |||||||||||||||||||
| (3 | )(f) | |||||||||||||||||||
Treasury stock, at cost (40,375,558 shares historically and 30,995,246 on a pro forma basis) | (1,598 | ) | — | 371 | (1,227 | ) | ||||||||||||||
Accumulated other comprehensive loss | (19 | ) | — | (19 | ) | |||||||||||||||
|
|
|
|
|
| |||||||||||||||
Total DaVita Inc. shareholders’ equity | 2,379 | — | 3,275 | |||||||||||||||||
Members equity | — | 248 | (248 | ) | — | |||||||||||||||
Noncontrolling interests not subject to put provisions | 129 | — | 129 | |||||||||||||||||
|
|
|
|
|
| |||||||||||||||
Total equity | 2,508 | 248 | 3,404 | |||||||||||||||||
|
|
|
|
|
| |||||||||||||||
Total liabilities plus shareholders’ equity | $ | 9,255 | $ | 1,415 | $ | 14,943 | ||||||||||||||
|
|
|
|
|
| |||||||||||||||
S-85
Table of Contents
Notes to unaudited pro forma condensed consolidated balance sheet
| (a) | The purchase of HCP for approximately $4,749 million comprised of $3,592 million in cash (which includes the effect of an estimated negative working capital adjustment of $68 million), a $275 million contingent earn-out consideration with a preliminary estimated fair value of $250 million and $907 million from the issuance of 9,380,312 shares of DaVita common stock valued at the closing market price as reported by the NYSE as of August 10, 2012. The purchase price is subject to a final working capital true-up adjustment at closing (as described under the Merger Agreement). |
| (dollars in millions) | ||||
Customer relationships | $ | 940 | ||
Practice management tools | 250 | |||
Non-compete agreements | 220 | |||
Trade names | 220 | |||
Provider network | 8 | |||
Net tangible liabilities | (198 | ) | ||
Deferred income taxes | (200 | ) | ||
Other closing liabilities | (10 | ) | ||
Goodwill | 3,519 | |||
|
| |||
| $ | 4,749 | |||
|
| |||
Goodwill is also being adjusted for the elimination of HCP’s historical amounts for goodwill and deferred taxes in the amount of $288 million and $61 million, respectively.
Assuming the Company’s stock price were to either decrease or increase by 15% as compared to the price on August 10, 2012, then the overall purchase price would be adjusted by approximately $136 million and would result in a corresponding adjustment to goodwill.
Of the total contingent earn-out consideration, $134 million is expected to be short-term and the balance of $116 million is considered to be long-term.
The $275 million total contingent earn-out consideration as described above can be earned in two tranches. The first tranche consists of $137.5 million if the EBITDA for HCP for 2012 is equal or greater than $550 million and the second tranche consists of $137.5 million if the earn-out EBITDA for HCP for 2013 is equal or greater than $600 million. We have estimated the preliminary fair value of the contingent earn-out consideration to be $250 million as of the expected closing date. The contingent earn-out consideration will subsequently be remeasured to fair value at each reporting date until the contingency is resolved with changes in the liability due to the re-measurement recorded in earnings. Therefore, if HCP achieves both of these earn-out EBITDA targets, we would be required to record a charge to earnings in the amount of $25 million, primarily in 2013, representing the difference between the preliminary fair value of the contingent earn-out consideration of $250 million as compared to the total potential pay-out of $275 million.
Conversely, if the fair value of the contingent earn-out consideration were to decrease below the preliminary fair value amount of $250 million, we would then record a gain to earnings.
For purposes of this pro forma presentation, we estimate that the amounts for tangible assets and liabilities reflected on HCP’s consolidated balance sheet approximate the fair values of such assets and liabilities, and accordingly, such amounts have not been adjusted in the accompanying pro forma financial information. Our projections and underlying assumptions concerning the initial purchase price allocations and fair values of HCP’s identifiable assets, liabilities and contingent earn-out consideration represent our current best estimates and are based upon the information available to us at this time. However, these estimates are preliminary and subject to change based upon completion of final valuation analyses. Additionally, the final purchase price is subject to a working capital true-up adjustment. Accordingly, the final amounts will differ from the amounts shown above.
This includes the reclassification of equity investments of $4 million for consistent presentation.
S-86
Table of Contents
| (b) | Net proceeds of cash as a result of the Financings related to the acquisition of HCP as follows: net proceeds from borrowings under the amended senior credit facilities of $3,000 million, plus the net proceeds of $1,000 million from the incurrence of additional senior financing reduced by an estimated $90 million of fees and the pay-off of Term Loan A-2 for $198 million. |
| (c) | The borrowing under the amended senior secured credit facilities and from the incurrence of the additional senior financing is set forth in the table below: |
| (dollars in millions) | ||||
Senior secured credit facilities | ||||
Term Loan A-3 | $ | 1,350 | ||
Term Loan B-2 | 1,650 | |||
|
| |||
Total new Term Loans | 3,000 | |||
Additional Senior Financing | 1,000 | |||
|
| |||
Total borrowings | $ | 4,000 | ||
|
| |||
Of the $4,000 million of total borrowings, $84 million is expected to be short-term and $3,916 million is expected to be long-term.
| (d) | Fees and expenses totaling $90 million are expected to be paid from the proceeds of the borrowings and as described above and are as follows: |
| (dollars in millions) | ||||
Deferred financing costs | $ | 52 | ||
Debt discount | 27 | |||
Transaction costs (expensed) | 11 | |||
|
| |||
| $ | 90 | |||
|
| |||
We estimate that all of the deferred financing costs of $52 million associated with the amended senior secured credit facilities and the incurrence of additional senior financing will be capitalized and that no existing deferred financing costs associated with the existing senior secured credit facilities, except for the Term Loan A-2, will be written off. However, these amounts are subject to change depending upon the final calculations that will determine the actual amount of deferred financing costs that will be capitalized or expensed.
| (e) | Write-off of the existing deferred financing costs associated with the extinguishment of the Term Loan A-2 of $2 million. |
| (f) | In conjunction with the Merger, it is anticipated that all of HCP’s outstanding debt totaling $542 million will be paid-off at close and the related deferred financing costs of $5 million will be written-off. |
| (i) | The unaudited pro forma condensed consolidated balance sheet as of June 30, 2012 gives effect to the acquisition of HCP and related borrowings as if each had occurred on June 30, 2012. |
S-87
Table of Contents
DAVITA SELECTED HISTORICAL FINANCIAL AND OTHER DATA
The following selected consolidated financial data should be read in conjunction with DaVita’s financial statements for the years ended December 31, 2009, 2010 and 2011 and unaudited financial information for the six months ended June 30, 2012 and 2011, and related notes thereto incorporated by reference in this prospectus supplement. The consolidated statement of operations data and balance sheet data presented below are derived from DaVita’s consolidated financial statements included or incorporated by reference in this prospectus supplement. Effective January 1, 2012, DaVita adopted FASB’s ASU No 2011-07 Health Care Entities – Presentation and Disclosure of Patient Service Revenue, Provision for Bad Debts, and the Allowance for Doubtful Accounts. Upon adoption of this standard, DaVita was required to change the presentation of its provision for uncollectible accounts related to patient service revenue as a deduction from patient service operating revenues. These consolidated financial results have been revised for all prior periods presented to reflect the retrospective application of adopting these new presentation and disclosures requirement for the provision for uncollectible accounts.
| Year ended December 31, | Six months ended June 30, | |||||||||||||||||||||||||||
| 2007 | 2008 | 2009 | 2010 | 2011 | 2011 | 2012 | ||||||||||||||||||||||
| (audited) | (unaudited) | |||||||||||||||||||||||||||
| (dollars in millions) | ||||||||||||||||||||||||||||
Statement of operations data: | ||||||||||||||||||||||||||||
Net dialysis patient service revenues, less provision for uncollectible accounts | $ | 4,970 | $ | 5,247 | $ | 5,601 | $ | 5,877 | $ | 6,273 | $ | 2,992 | $ | 3,465 | ||||||||||||||
Other revenue | 154 | 264 | 343 | 395 | 519 | 232 | 332 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Net operating revenues | 5,124 | 5,511 | 5,944 | 6,272 | 6,792 | 3,224 | 3,797 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Operating expenses and charges: | ||||||||||||||||||||||||||||
Patient care costs | 3,584 | 3,915 | 4,242 | 4,467 | 4,681 | 2,277 | 2,575 | |||||||||||||||||||||
General and administrative | 491 | 508 | 531 | 579 | 691 | 315 | 422 | |||||||||||||||||||||
Depreciation and amortization | 193 | 216 | 228 | 234 | 267 | 126 | 154 | |||||||||||||||||||||
Provision for uncollectible accounts | 3 | 4 | 5 | 4 | 7 | 3 | 4 | |||||||||||||||||||||
Valuation gain on the Product Supply Agreement(1) | (55 | ) | — | — | — | — | — | — | ||||||||||||||||||||
Goodwill impairment charge(1) | — | — | — | — | 24 | 24 | — | |||||||||||||||||||||
Legal proceeding contingency accrual and related expenses(2) | — | — | — | — | — | — | 78 | |||||||||||||||||||||
Equity investment income | (1 | ) | (1 | ) | (2 | ) | (9 | ) | (9 | ) | (4 | ) | (5 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Total operating expenses and charges | 4,215 | 4,642 | 5,004 | 5,275 | 5,661 | 2,742 | 3,228 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Operating income | 909 | 869 | 940 | 997 | 1,131 | 482 | 569 | |||||||||||||||||||||
Debt expense(3) | (257 | ) | (225 | ) | (186 | ) | (182 | ) | (241 | ) | (118 | ) | (122 | ) | ||||||||||||||
Refinancing and debt redemption charges(4) | — | — | — | (74 | ) | — | — | — | ||||||||||||||||||||
Other income(5) | 22 | 13 | 4 | 3 | 3 | 1 | 2 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Income from continuing operations before income taxes | 674 | 657 | 758 | 744 | 893 | 365 | 449 | |||||||||||||||||||||
Income tax expense | 245 | 236 | 278 | 260 | 316 | 130 | 164 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Income from continuing operations | 429 | 421 | 480 | 484 | 577 | 235 | 285 | |||||||||||||||||||||
Discontinued operations(6) | — | — | — | — | (4 | ) | 1 | — | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Net income | 429 | 421 | 480 | 484 | 573 | 236 | 285 | |||||||||||||||||||||
Less: Net income attributable to noncontrolling interests | (47 | ) | (47 | ) | (57 | ) | (78 | ) | (95 | ) | (41 | ) | (49 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Net income attributable to DaVita Inc. | $ | 382 | $ | 374 | $ | 423 | $ | 406 | $ | 478 | $ | 195 | $ | 236 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Balance sheet data (as end of period): | ||||||||||||||||||||||||||||
Cash and cash equivalents | 447 | 411 | 539 | 860 | 394 | 730 | 273 | |||||||||||||||||||||
Working capital | 890 | 965 | 1,256 | 1,699 | 1,128 | 1,478 | 943 | |||||||||||||||||||||
Total assets | 6,944 | 7,286 | 7,558 | 8,114 | 8,892 | 8,193 | 9,255 | |||||||||||||||||||||
Total debt | 3,707 | 3,695 | 3,632 | 4,309 | 4,505 | 4,286 | 4,498 | |||||||||||||||||||||
Total shareholder’s equity(7) | 1,504 | 1,768 | 2,135 | 1,978 | 2,141 | 1,881 | 2,379 | |||||||||||||||||||||
Operating data: | ||||||||||||||||||||||||||||
Maintenance capital expenditures | 114 | 105 | 114 | 159 | 224 | 88 | 122 | |||||||||||||||||||||
Centers | 1,359 | 1,449 | 1,530 | 1,612 | 1,820 | 1,669 | 1,903 | |||||||||||||||||||||
Patients | 107,000 | 112,000 | 118,000 | 125,000 | 143,000 | 131,000 | 150,000 | |||||||||||||||||||||
U.S. Dialysis treatments | 15,296,000 | 16,192,000 | 16,985,000 | 17,964,000 | 19,599,000 | 9,364,000 | 10,766,000 | |||||||||||||||||||||
S-88
Table of Contents
| (1) | Operating expenses and charges in 2011 include $24 million of a non-cash goodwill impairment charge related to our infusion therapy business and $55 million in 2007 of valuation gains on the alliance and product supply agreement with Gambro Renal Products, Inc. Operating expenses and charges in 2007 also include $7 million of gains from insurance settlements related to Hurricane Katrina and a fire that destroyed one center. |
| (2) | Represents a legal proceeding contingency accrual and related expenses that resulted from an agreement we reached in principle to settle the Woodard Private Civil Suit. See “DaVita’s Business—Legal Proceedings” beginning on page S-169. |
| (3) | Debt expense in 2007 includes the write-off of approximately $4 million of deferred financing costs associated with our principal prepayments on our term loans. |
| (4) | In 2010, we incurred $74 million of refinancing and debt redemption charges in conjunction with the extinguishment of our previously existing senior secured credit facilities and the redemption of $200 million of our previously outstanding 6 5/8% senior notes. |
| (5) | Other income, net, includes $6 million of gains in 2007 from the sale of investment securities. |
| (6) | During 2011, we divested a total of 28 outpatient dialysis centers in conjunction with a consent order issued by the Federal Trade Commission on September 30, 2011 in order for us to complete the acquisition of DSI. In addition, we also completed the sale of two additional centers that were previously pending state regulatory approval in conjunction with the acquisition of DSI on October 31, 2011. The operating results of the historical DaVita divested centers are reflected as discontinued operations in our consolidated financial statements for all periods presented. In addition, the operating results for the DSI divested centers are reflected as discontinued operation in our consolidated financial statements beginning September 1, 2011. |
| (7) | Share repurchases consisted of 3,794,686 shares of DaVita common stock for $323 million in 2011, 8,918,760 shares of DaVita common stock for $618 million in 2010, 2,902,619 shares of DaVita common stock for $153 million in 2009, 4,788,881 shares of DaVita common stock for $233 million in 2008, 111,300 shares of DaVita common stock for $6 million in 2007 and 3,710,086 shares of DaVita common stock for $316 million in the first six months of 2011. Shares issued in connection with stock awards amounted to 1,260,259 in 2011, 1,771,384 in 2010, 2,104,304 in 2009, 1,314,074 in 2008, and 2,480,899 in 2007. |
S-89
Table of Contents
HCP SELECTED HISTORICAL FINANCIAL AND OTHER DATA
The following selected combined and consolidated financial data should be read in conjunction with HCP’s financial statements for the years ended December 31, 2009, 2010 and 2011, and unaudited financial information for the six months ended June 30, 2012 and 2011, and related notes thereto included in this prospectus and the discussion under “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in this prospectus. The combined statement of operations and balance sheet data presented below, are derived from the combined and consolidated financial statements of HCP.
| Year ended December 31, | Six months ended June 30, | |||||||||||||||||||||||||||
| 2007 | 2008 | 2009 | 2010 | 2011 | 2011 | 2012 | ||||||||||||||||||||||
| (audited) | (unaudited) | |||||||||||||||||||||||||||
| (dollars in millions, except operating data) | ||||||||||||||||||||||||||||
Statement of operations data: | ||||||||||||||||||||||||||||
Medical revenues | $ | 1,187 | $ | 1,557 | $ | 1,731 | $ | 2,049 | $ | 2,375 | $ | 1,158 | $ | 1,294 | ||||||||||||||
Other operating revenues | 42 | 46 | 46 | 40 | 47 | 22 | 28 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Total operating revenues | 1,229 | 1,603 | 1,777 | 2,089 | 2,422 | 1,180 | 1,322 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Operating expenses and charges: | ||||||||||||||||||||||||||||
Medical expenses | 605 | 814 | 930 | 1,034 | 1,165 | 569 | 620 | |||||||||||||||||||||
Hospital expenses | 122 | 191 | 212 | 222 | 248 | 121 | 155 | |||||||||||||||||||||
Clinic support and other operating costs | 175 | 197 | 226 | 263 | 308 | 148 | 165 | |||||||||||||||||||||
General and administrative expenses | 120 | 142 | 136 | 178 | 207 | 101 | 110 | |||||||||||||||||||||
Depreciation and amortization | 19 | 24 | 26 | 29 | 31 | 16 | 16 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Total operating expenses | 1,041 | 1,368 | 1,530 | 1,726 | 1,959 | 955 | 1,066 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Equity earnings of unconsolidated joint ventures | 12 | 11 | 12 | 15 | 25 | 9 | 12 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Operating income | 200 | 246 | 259 | 378 | 488 | 234 | 268 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Interest income | 6 | 6 | 6 | 6 | 7 | 3 | 4 | |||||||||||||||||||||
Interest expense | (20 | ) | (14 | ) | (6 | ) | (5 | ) | (16 | ) | (9 | ) | (6 | ) | ||||||||||||||
Investment impairment | — | (5 | ) | — | — | — | — | — | ||||||||||||||||||||
Gain on sale of investments | — | — | 2 | — | 1 | 1 | — | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Total other income (expense) | (14 | ) | (13 | ) | 2 | 1 | (8 | ) | (5 | ) | (2 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Income before income taxes | 186 | 233 | 261 | 379 | 480 | 229 | 266 | |||||||||||||||||||||
Provision for income taxes | 9 | 30 | 41 | 49 | 71 | 37 | 33 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Net income | $ | 177 | $ | 203 | $ | 220 | $ | 330 | $ | 409 | $ | 192 | $ | 233 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Balance sheet data (end of period): | ||||||||||||||||||||||||||||
Cash and cash equivalents | 154 | 214 | 358 | 361 | 395 | 183 | 355 | |||||||||||||||||||||
Working capital. | 28 | 72 | 179 | 360 | 304 | 192 | 341 | |||||||||||||||||||||
Total assets. | 590 | 748 | 911 | 1,286 | 1,366 | 1,188 | 1,415 | |||||||||||||||||||||
Total debt | 260 | 223 | 220 | 218 | 556 | 571 | 542 | |||||||||||||||||||||
Member’s equity. | 84 | 225 | 340 | 566 | 188 | 29 | 248 | |||||||||||||||||||||
Other financial data: | ||||||||||||||||||||||||||||
Capital expenditures | 12 | 28 | 12 | 21 | 23 | 11 | 10 | |||||||||||||||||||||
Net cash provided by operating activities | 218 | 233 | 286 | 343 | 509 | 181 | 184 | |||||||||||||||||||||
Operating data: | ||||||||||||||||||||||||||||
Managed care members | 469,700 | 586,500 | 589,900 | 658,000 | 667,700 | 659,200 | 669,400 | |||||||||||||||||||||
Medical clinic locations | 82 | 90 | 99 | 129 | 152 | 138 | 157 | |||||||||||||||||||||
Full time physicians | 461 | 496 | 570 | 715 | 794 | 734 | 818 | |||||||||||||||||||||
IPA Primary care physicians | 875 | 1,178 | 1,268 | 1,291 | 1,458 | 1,414 | 1,454 | |||||||||||||||||||||
S-90
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
DaVita
Forward-looking statements
This Management’s Discussion and Analysis of Financial Condition and Results of Operations contains forward-looking statements within the meaning of the federal securities laws. All statements that do not concern historical facts are forward-looking statements and include, among other things, statements about our expectations, beliefs, intentions and/or strategies for the future. These forward-looking statements include statements regarding our future operations, financial condition and prospects, expectations for treatment growth rates, revenue per treatment, expense growth, levels of the provision for uncollectible accounts receivable, operating income, cash flow, operating cash flow, estimated tax rates, capital expenditures, the development of new centers and center acquisitions, government and commercial payment rates, revenue estimating risk and the impact of our related level of indebtedness on our financial performance, including earnings per share and the anticipated timing of the closing of the HCP transaction. These statements involve substantial known and unknown risks and uncertainties that could cause our actual results to differ materially from those described in the forward-looking statements, including, but not limited to, risks resulting from uncertainties associated with governmental regulations, general economic and other market conditions, competition, accounting estimates, the variability of our cash flows, the concentration of profits generated from commercial payor plans, continued downward pressure on average realized payment rates from commercial payors, which may result in the loss of revenue or patients, a reduction in the number of patients under higher-paying commercial plans, a reduction in government payment rates under the Medicare ESRD program or other government-based programs, the impact of health care reform legislation that was enacted in the U.S. in March 2010, changes in pharmaceutical or anemia management practice patterns, payment policies, or pharmaceutical pricing, our ability to maintain contracts with physician medical directors, legal compliance risks, including our continued compliance with complex government regulations, current or potential investigations by various governmental entities and related government or private-party proceedings, the impact of our agreement in principle to settle all allegations relating to claims arising out of previously disclosed litigation filed in 2002 in the U.S. District Court in the Eastern District of Texas to resolve the federal program claims regarding EPO relating to historical EPO practices dating back to 1997, continued increased competition from large and medium-sized dialysis providers that compete directly with us, the emergence of new models of care introduced by the government or private sector, such as accountable care organizations, independent practice association and integrated delivery systems, and changing affiliation models for physicians, such as employment by hospitals, that may further erode our patient base and reimbursement rates, our ability to complete any acquisitions, mergers or dispositions that we might be considering or announce, including the HCP transaction, or integrate and successfully operate any business we may acquire, expansion of our operations and services to markets outside the U.S., or to businesses outside of dialysis and the other risk factors set forth in our Annual Report on Form 10-K for the fiscal year ended December 31, 2011 and Part II, Item 1A. of our Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2012 each as filed with the Securities and Exchange Commission. We base our forward-looking statements on information currently available to us, and we undertake no obligation to update or revise any forward-looking statements, whether as a result of changes in underlying factors, new information, future events or otherwise.
The following should be read in conjunction with our consolidated financial statements and “Item 1. Business” of our Annual Report Form 10-K for the fiscal year ended December 31, 2011.
Overview
We are a leading provider of kidney dialysis services, primarily in the U.S., through a network of approximately 1,809 outpatient dialysis centers and approximately 900 hospitals, located in the U.S. throughout 43 states and District of Columbia, serving a total of approximately 142,000 patients. We estimate that we have approximately a 32% market share in the U.S. based upon the number of patients that we serve. In 2011, our overall network of dialysis centers increased by 208 centers primarily as a result of acquisitions and from
S-91
Table of Contents
opening new centers. In September 2011 we acquired DSI, a medium sized dialysis provider, for approximately $723 million in net cash plus the assumption of certain liabilities. DSI contributed a net 83 dialysis centers, after which we agreed to divest a total of 30 dialysis centers in order to complete the acquisition of DSI. In addition, the overall number of patients that we serve in the U.S. increased by approximately 13% as compared to 2010.
In addition, as of December 31, 2011, we provided dialysis and administrative services to a total of 11 outpatient dialysis centers located in three countries outside of the U.S. Our international dialysis operations are currently in a start-up phase in which we primarily commenced operations during the fourth quarter of 2011. The total operating revenues generated from our international operations were not material during 2011 and are included as a component of our ancillary services and strategic initiatives. Therefore, all references in this document to dialysis and related lab services continue to refer only to our U.S. dialysis and related lab services business for the year ended December 31, 2011.
Our national scale and size, among other things, allows us to provide industry-leading quality care with superior clinical outcomes that attracts patients and referring physicians, as well as qualified medical directors, provides our patient base with convenient locations and access to a full range of services and provides us the ability to effectively control certain costs while maintaining strong compliance programs.
Our stated mission is to be the provider, partner and employer of choice. We believe our attention to these three stakeholders—our patients, our business partners, and our teammates—represents the major driver of our long-term performance, although we are subject to the impact of external factors such as government policy and physician practice patterns. Accordingly, two principal non-financial metrics we track are quality clinical outcomes and teammate turnover. We have developed our own composite index for measuring improvements in our clinical outcomes, which we refer to as the DaVita Quality Index, or DQI. Our clinical outcomes as measured by DQI have improved over each of the past three years which we believe directly decreases patient mortalities. Although it is difficult to reliably measure clinical performance across our industry, we believe our clinical outcomes compare favorably with other dialysis providers in the U.S. and generally exceed the dialysis outcome quality indicators of the National Kidney Foundation. In addition, over the past several years our teammate turnover has remained relatively constant, which we believe was a major contributor to our continued clinical performance improvements and also a major driver of our ability to maintain or improve clinical hours per treatment. We will continue to focus on these stakeholders and our clinical outcomes as we believe these are fundamental long-term value drivers.
Our overall financial performance was strong for 2011 and was characterized by strong treatment volume growth, primarily from acquisitions and non-acquired growth rates and by decreased operating costs from a decline in the utilization of physician-prescribed pharmaceuticals due to continued evolution of clinical practices and physicians responding to the new FDA label for EPO.
Our major financial operating performance indicators in 2011 as compared to 2010 were as follows:
| • | consolidated revenue growth of approximately 8.5%; |
| • | an increase of approximately 9.1% in the overall number of treatments that we provided; |
| • | normalized non-acquired treatment growth of 4.6%; |
| • | consolidated operating income growth of approximately 13.4%, which includes the impact of a noncash goodwill impairment charge of 2.4%; |
| • | effective operating cost control initiatives; and |
| • | strong operating cash flows of $1,180 million. |
However, we believe that 2012 will continue to be challenging as we implement some additional Medicare billing requirements and as we implement system and process upgrades to enhance our ability to capture certain patient characteristics that can impact our overall reimbursements from Medicare. In addition, there remains
S-92
Table of Contents
significant scrutiny and uncertainty around the utilization of physician-prescribed pharmaceuticals, which along with pharmaceutical cost increases and changes in certain government policies, can have a significant impact on our operating results. We are also committed to our international expansion plans that will continue to require a significant investment in 2012. In addition, if the percentage of our patients with commercial payors continues to deteriorate this would also impact our operating results.
Approximately 93% of our 2011 consolidated operating revenues were derived directly from our dialysis and related lab services business. Approximately 81% of our 2011 dialysis and related lab services revenues were derived from outpatient hemodialysis services in the 1,776 U.S. centers that we consolidate. Other dialysis services, which are operationally integrated with our dialysis operations, are peritoneal dialysis, home-based hemodialysis, hospital inpatient hemodialysis services and management and administrative services. These services collectively accounted for the balance of our 2011 dialysis and related lab services revenues.
Our other business operations include ancillary services and strategic initiatives which are primarily aligned with our core business of providing dialysis services to our network of patients. These consist primarily of pharmacy services, infusion therapy services, disease management services, vascular access services, ESRD clinical research programs, physician services and our international dialysis operations. These services generated approximately $514 million of operating revenues in 2011, representing approximately 7% of our consolidated operating revenues. We currently expect to continue to invest in our ancillary services and strategic initiatives including our continued expansion into certain international markets as we work to develop successful new business operations in the U.S. as well as outside the U.S. However, any significant change in market conditions, business performance or in the regulatory environment may impact the economic viability of any of these strategic initiatives. Any unfavorable changes in these strategic initiatives could result in a write-off or an impairment of some or all of our investments, including goodwill, which occurred in 2011 when we recorded a non-cash goodwill impairment charge relating to our infusion therapy business, and could also result in significant termination costs if we were to exit a certain line of business.
The principal drivers of our dialysis and related lab services revenues are:
| • | the number of treatments, which is primarily a function of the number of chronic patients requiring approximately three treatments per week, as well as, to a lesser extent, the number of treatments for peritoneal dialysis services and home-based dialysis and hospital inpatient dialysis services; and |
| • | average dialysis revenue per treatment. |
The total patient base is a relatively stable factor, which we believe is influenced by a demographically growing need for dialysis services, our relationships with referring physicians, together with the quality of our clinical care which can lead to reduced patient mortalities, and our ability to open and acquire new dialysis centers. In 2011, we were able to increase our overall network of patients that we serviced in the U.S. by approximately 13% as compared to 2010.
Our average dialysis and related lab services revenue per treatment in 2011 was primarily driven by our mix of commercial and government (principally Medicare and Medicaid) patients, commercial and government payment rates, our billing and collecting operations performance, and the mix and intensity of physician-prescribed pharmaceuticals that are separately billable. Beginning in 2011, with the implementation of Medicare’s new single bundled payment rate system, the intensities of physician-prescribed pharmaceuticals had a lesser impact on our average dialysis and related lab services revenue per treatment since payment for these pharmaceuticals is included in a single bundled payment. In addition, some of our commercial contracts covering certain patients also pay us under a single bundled payment rate for all dialysis services provided.
On average, payment rates from commercial payors are significantly higher than Medicare, Medicaid and other government program payment rates, and therefore the percentage of commercial patients to total patients represents a major driver of our total average dialysis revenue per treatment. The percentage of commercial patients covered under contracted plans as compared to commercial patients with out-of-network providers can
S-93
Table of Contents
also significantly affect our average dialysis revenue per treatment. In 2011, the growth of our government-based patients continued to outpace the growth of our commercial patients, which has been a trend that we have experienced for the past several years. We believe the growth in our government-based patients is driven primarily by improved mortality and the current economic environment, which impacts the number of individuals that are covered under commercial insurance plans. This trend has negatively impacted our average dialysis revenue per treatment as a result of receiving a larger proportion of our revenue from government-based payors, such as Medicare, that reimburse us at lower payment rates.
The following table summarizes our dialysis and related lab services revenues for the year ended December 31, 2011:
| Revenues | ||||
Medicare and Medicare-assigned plans | 58 | % | ||
Medicaid and Medicaid-assigned plans | 5 | % | ||
Other government-based programs | 3 | % | ||
|
| |||
Total government-based programs | 66 | % | ||
Commercial (including hospital dialysis services) | 34 | % | ||
|
| |||
Total dialysis and related lab services revenues | 100 | % | ||
|
| |||
Government payment rates in the U.S. are principally determined by federal Medicare and state Medicaid policy. These payment rates have historically had limited potential for rate increases and are sometimes at risk of reduction as federal and state governments face increasing budget pressures. On January 1, 2011 we implemented Medicare’s new payment system in which all ESRD payments are made under a single bundled payment rate that, beginning in 2012, provides for an annual inflation adjustment based upon a market basket index, less a productivity adjustment. Also beginning in 2012, the rule provides for up to a 2% annual payment withhold that can be earned back by the facilities that meet certain defined clinical performance standards. The new payment system reimburses providers based upon a single bundled or average payment for each Medicare treatment provided. This new bundled payment amount is designed to cover all dialysis services which were historically included in the composite rate and all separately billable ESRD services such as pharmaceuticals and laboratory costs. In the past the amount of services that were separately billable accounted for approximately 30% of our total dialysis and related lab services revenues. The new bundled payment rate is adjusted for certain patient characteristics, a geographic wage index and certain other factors. The initial 2011 bundled payment rate included reductions of 2.0% from the prior reimbursement and further reduced overall rates by 5.94% tied to an expanded list of case mix adjustors which can be earned back based upon the presence of certain patient characteristics and co-modalities at the time of treatment. There are also other provisions which may impact payment including an outlier pool and a low volume facility adjustment with regard to the expanded list of case-mix adjusters. These are difficult and, in some cases, have not been possible for our dialysis centers to document and track, which has resulted in lower reimbursement amounts than we would otherwise have received.
On April 1, 2011, CMS released an interim final rule correcting the 3.1% transition adjustment factor to properly update the number of ESRD facilities that elected to opt fully into the new Prospective Payment System, or PPS. This new rule was prospective and as a result, effective April 1, 2011 we began recognizing revenues in accordance with the new rule, which resulted in an increase in Medicare revenue per treatment of approximately 3.1% in comparison to our levels recorded in the first quarter of 2011. This reduced our transition adjustment to zero for the balance of 2011 and to an aggregate of approximately 0.75% for 2011.
On November 1, 2011, CMS issued the final ESRD PPS rule for 2012. The base rate increased by 2.1%, representing a market basket increase of 3.0% less a productivity adjustment of 0.9%. The increase in the final base rate for 2012 (2.1%) is slightly greater than the increase of 1.8% stated in the proposed 2012 ESRD PPS
S-94
Table of Contents
rule published in July 2011, and higher than the 1.0% increase recommended by MedPAC. In 2012 and beyond, the ESRD PPS system includes additional quality measures that could result in decreased payments if a dialysis facility fails to meet the standards.
On July 2, 2012 CMS issued its proposed ESRD PPS rule for 2013. The proposed base rate reflects a 3.1% market basket increase reduced by a productivity adjustment of 0.7%, resulting in a proposed update of 2.5%. CMS also proposes additional quality measures and modifications to existing quality measures for 2015.
Also, beginning January 1, 2014, certain oral-only ESRD drugs (currently paid separately to pharmacies under Medicare Part D) will be included in the ESRD bundled payment to dialysis facilities. It is currently unclear how CMS will “price” the oral-only drugs for inclusion in the ESRD bundle in 2014.
We believe the new payment system presents additional operating clinical and financial risks that our dialysis centers or billing and other systems may not accurately document and track the appropriate patient-specific characteristics, resulting in a reduction or overpayment in the amounts of the payments that we would otherwise be entitled to receive.
Dialysis payment rates from commercial payors can vary and a major portion of our commercial rates are set at contracted amounts with payors and are subject to intense negotiation pressure. Our commercial payment rates also include payments for out-of-network patients that on average are higher than our in-network contract rates. In 2011, we were successful in increasing some of our commercial payment rates which contributed to an increase in our average dialysis revenue per treatment, which helped offset some of the overall decline in our average dialysis revenue per treatment. In 2011, we also entered into some commercial contracts covering certain patients that will primarily pay us a single bundled payment rate for all dialysis services provided to these patients. We are continuously in the process of negotiating agreements with our commercial payors, and payors are aggressive in their negotiations. If our negotiations result in overall commercial rate reductions in excess of overall commercial rate increases, this would have a material adverse effect on our operating results. In addition, if there are sustained or increased job losses in the U.S. as a result of current economic conditions, or depending upon changes to the healthcare regulatory system, we could experience a decrease in the number of patients under commercial plans.
Approximately 4% of our dialysis and related lab services revenues for the year ended December 31, 2011, were from physician-prescribed pharmaceuticals that are separately billable, with EPO accounting for approximately 3% of our dialysis and related lab services revenues. The impact of physician-prescribed pharmaceuticals on our overall revenues that are separately billable in 2011 has significantly decreased from prior years primarily as a result of implementing Medicare’s new single bundled payment system as well as some additional commercial contracts that pay us a single bundled payment rate. Therefore, in 2010 and prior, changes in physician practice patterns, pharmaceutical protocols, pharmaceutical intensities and changes in commercial and governmental payment rates for EPO had a greater significant influence on our revenues.
Our operating performance with respect to dialysis services billing and collection can also be a significant factor in the average dialysis and related lab services revenue per treatment we actually realize. Over the past several years we have invested heavily in upgrades to our systems and processes that we believe have helped improve our operating performance and reduced our regulatory compliance risks and we expect to continue to improve these systems and processes. In 2011, we continued to upgrade our information technology systems and implemented process changes and will continue to do so in 2012 to improve our ability to capture the necessary patient characteristics and certain other factors under Medicare’s new bundled payment system. We believe this will help us capture additional reimbursement amounts from Medicare and enhance our overall billing and collection performance. However, as we continue to make upgrades to our systems and processes, or as payors change their systems and requirements, our collection performance as well as our dialysis and related lab services revenue per treatment could be negatively impacted.
S-95
Table of Contents
Our revenue recognition involves significant estimation risks. Our estimates are developed based on the best information available to us and our best judgment as to the reasonably assured collectability of our billings as of the reporting date based upon our actual historical collection experience. Changes in estimates are reflected in the then-current period financial statements based upon on-going actual experience trends, or subsequent settlements and realizations depending on the nature and predictability of the estimates and contingencies.
Our annual average dialysis and related lab services revenue per treatment was approximately $330, $337 and $340 for 2011, 2010 and 2009, respectively. In 2011, the average dialysis and related lab services revenue per treatment decreased by approximately $7 per treatment primarily due to a decline in our Medicare reimbursements as a result of operating in the new single bundled payment system, a decline in the commercial payor mix, and a decline in the intensities of physician-prescribed pharmaceuticals, partially offset by an increase in some of our commercial payment rates. In 2010, the average dialysis and related lab services revenue per treatment decreased by approximately $3 per treatment primarily due to a decline in the intensities of physician-prescribed pharmaceuticals, and a decline in the commercial payor mix, partially offset by an increase of 1.0% in the Medicare composite rate and an increase in some of our commercial payment rates. Commercial payment rates, changes in the mix and intensities of physician-prescribed pharmaceuticals that are billed separately, government payment policies regarding reimbursement amounts for dialysis treatments and pharmaceuticals under the new Medicare bundled payment rate system including our ability to capture all patient characteristics, and changes in the mix of government and commercial patients may materially impact our average dialysis and related lab services revenue per treatment in the future.
The principal drivers of our dialysis and related lab services patient care costs are clinical hours per treatment, labor rates, vendor pricing of pharmaceuticals, utilization levels of pharmaceuticals, business infrastructure, including the operating costs of our dialysis centers, and compliance costs. However, other cost categories can also represent significant cost variability, such as employee benefit costs and insurance costs. Our average clinical hours per treatment in 2011 were relatively flat or increased slightly compared to 2010, which continues to be impacted by our ability to maintain or reduce clinical teammate turnover and improve training and processes. We are always striving for improved productivity levels, however, changes in federal and state policies or regulatory billing requirements can adversely impact our ability to achieve optimal productivity levels. In addition, improvements in the U.S. economy could stimulate additional competition for skilled clinical personnel and result in higher teammate turnover which would adversely affect productivity levels. In 2011 and 2010, we experienced an increase in our clinical labor rates of approximately 2.0% in both years, as clinical labor rates have increased consistent with general industry trends, mainly due to the demand for skilled clinical personnel, along with general inflation increases. However, in 2011, we continued to implement certain cost control initiatives to minimize increases in our clinical labor rates. In addition, in 2011, we experienced an approximately 5.0% increase in our EPO costs. Our new agreement with Amgen requires us to purchase EPO in amounts necessary to meet no less than 90% of our requirements for erythropoiesis stimulation agents and provides for discounted pricing and rebates, which are subject to various conditions including future pricing levels of EPO and data submission, which could negatively impact our earnings if we are unable to continue to qualify for discount pricing and rebates. In the initial years of the agreement the total rebate opportunity is less than what was provided for in the agreement that expired at the end of 2011, however, the opportunity for us to earn discounts and rebates increases over the term of the agreement. In 2011, we also experienced increases in our infrastructure and operating costs of our dialysis centers, primarily due to the number of new centers opened, and general increases in rent, utilities and repairs and maintenance.
General and administrative expenses represented 9.9% of our operating revenues in 2011 as compared to 9.0% in 2010. This represents a fairly significant increase in the dollar amount of our general and administrative expenses primarily related to strengthening our dialysis business, improving our regulatory compliance and other operational processes, responding to certain legal and compliance matters, professional fees associated with information technology matters and international growth initiatives, transaction and integration costs associated with the acquisition of DSI and supporting the growth in some of our ancillary services and strategic initiatives. We expect that these levels of expenditures on general and administrative expenses in 2012 will continue and
S-96
Table of Contents
could possibly increase as we seek out new business opportunities within the dialysis industry or to other healthcare services outside of dialysis, making additional investments in our existing long-term initiatives, such as our ancillary services and strategic initiatives, and the expansion of our international operations, as well as investments in improving our information technology infrastructure and the level of support required for our regulatory compliance and legal matters.
Results of operations for the fiscal year ended December 31, 2011 vs. December 31, 2010 and December 31, 2010 vs. December 31, 2009
We operate principally as a dialysis and related lab services business in the U.S. but also operate other ancillary services and strategic initiatives. These ancillary services and strategic initiatives consist primarily of pharmacy services, infusion therapy services, disease management services, vascular access services, ESRD clinical research programs, physician services and our international dialysis operations. The U.S. dialysis and related lab services business qualifies as a separately reportable segment and all of the other ancillary services and strategic initiatives have been combined and disclosed in the other segments category.
Following is a summary of consolidated operating results for reference in the discussion that follows. The operating results of DSI are included in our operating results effective September 1, 2011.
| Year ended December 31, | ||||||||||||||||||||||||
| 2011 | 2010 | 2009 | ||||||||||||||||||||||
| (dollar amounts rounded to nearest million) | ||||||||||||||||||||||||
Total net operating revenues | $ | 6,792 | $ | 6,272 | $ | 5,944 | ||||||||||||||||||
Add: Provision for uncollectible accounts related to patient service revenues | 190 | 166 | 156 | |||||||||||||||||||||
|
|
|
|
|
| |||||||||||||||||||
Consolidated operating revenues | $ | 6,982 | 100 | % | $ | 6,438 | 100 | % | $ | 6,100 | 100 | % | ||||||||||||
|
|
|
|
|
| |||||||||||||||||||
Dialysis and related lab services patient service operating revenues | $ | 6,463 | $ | 6,043 | $ | 5,758 | ||||||||||||||||||
Less: Provision for uncollectible accounts related to patient service revenues | (190 | ) | 3 | % | (166 | ) | 3 | % | (156 | ) | 3 | % | ||||||||||||
|
|
|
|
|
| |||||||||||||||||||
Net patient service operating revenues | 6,273 | 5,877 | 5,602 | |||||||||||||||||||||
Other revenues | 519 | 395 | 342 | |||||||||||||||||||||
|
|
|
|
|
| |||||||||||||||||||
Total net operating revenues | $ | 6,792 | $ | 6,272 | $ | 5,944 | ||||||||||||||||||
|
|
|
|
|
| |||||||||||||||||||
Operating expenses and charges: | ||||||||||||||||||||||||
Patient care costs | 4,681 | 67 | % | 4,467 | 69 | % | 4,242 | 70 | % | |||||||||||||||
General and administrative | 691 | 10 | % | 579 | 9 | % | 532 | 9 | % | |||||||||||||||
Depreciation and amortization | 267 | 4 | % | 234 | 4 | % | 228 | 4 | % | |||||||||||||||
Provision for uncollectible accounts | 7 | — | 4 | — | 5 | — | ||||||||||||||||||
Goodwill impairment charge | 24 | — | — | — | — | — | ||||||||||||||||||
Equity investment income | (9 | ) | — | (9 | ) | — | (2 | ) | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Total operating expenses and charges | 5,661 | 84 | %(1) | 5,275 | 84 | %(1) | 5,005 | 85 | %(1) | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Operating income | $ | 1,131 | 16 | % | $ | 997 | 16 | % | $ | 940 | 15 | % | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
| (1) | The percentages include total operating expenses and charges and the provisions for uncollectible accounts related to patient service revenues. |
S-97
Table of Contents
The following table summarizes consolidated net operating revenues:
| Year ended | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| (dollar amounts rounded to nearest million) | ||||||||||||
Dialysis and related lab services patient service operating revenues | $ | 6,474 | $ | 6,052 | $ | 5,774 | ||||||
Less: Provision for uncollectible accounts related to patient service revenues | (190 | ) | (166 | ) | (156 | ) | ||||||
|
|
|
|
|
| |||||||
Dialysis and related lab services net patient service operating revenues | 6,284 | 5,886 | 5,618 | |||||||||
Other revenues | 11 | 11 | 9 | |||||||||
|
|
|
|
|
| |||||||
Total net dialysis and related lab services operating revenues | 6,295 | 5,897 | 5,627 | |||||||||
Other—Ancillary services and strategic initiatives | 507 | 378 | 328 | |||||||||
Other Ancillary services and strategic initiatives net patient service operating revenues | 7 | 6 | 6 | |||||||||
|
|
|
|
|
| |||||||
Total net segment operating revenues | 6,809 | 6,281 | 5,961 | |||||||||
Elimination of intersegment revenues | (17 | ) | (9 | ) | (17 | ) | ||||||
|
|
|
|
|
| |||||||
Consolidated net operating revenues | $ | 6,792 | $ | 6,272 | $ | 5,944 | ||||||
|
|
|
|
|
| |||||||
Consolidated operating revenues | $ | 6,982 | $ | 6,438 | $ | 6,100 | ||||||
|
|
|
|
|
| |||||||
The following table summarizes consolidated operating income:
| Year ended | ||||||||||||
| 2011(1) | 2010 | 2009 | ||||||||||
| (dollar amounts rounded to nearest million) | ||||||||||||
Dialysis and related lab services | $ | 1,225 | $ | 1,039 | $ | 994 | ||||||
Other—ancillary services and strategic initiatives loss | (54 | ) | (6 | ) | (12 | ) | ||||||
|
|
|
|
|
| |||||||
Total segment operating income | 1,171 | 1,034 | 982 | |||||||||
Reconciling items: | ||||||||||||
Stock-based compensation | (49 | ) | (46 | ) | (44 | ) | ||||||
Equity investment income | 9 | 9 | 2 | |||||||||
|
|
|
|
|
| |||||||
Consolidated operating income | 1,131 | 997 | 940 | |||||||||
Reconciliation of non-GAAP measure: | ||||||||||||
Add: Goodwill impairment charge | 24 | — | — | |||||||||
|
|
|
|
|
| |||||||
Non-GAAP consolidated operating income | $ | 1,155 | $ | 997 | $ | 940 | ||||||
|
|
|
|
|
| |||||||
| (1) | For the year ended December 31, 2011 we have excluded a non-cash goodwill impairment charge from operating expenses and operating income because management believes that this presentation enhances a user’s understanding of our normal consolidated operating income by excluding a non-cash goodwill impairment charge that resulted from a decrease in the implied fair value of goodwill below its carrying amount associated with HomeChoice Partners (HomeChoice), which provides infusion therapy services, during the second quarter of 2011 and is therefore more meaningful and comparable to our prior period results and more indicative of our normal consolidated operating income. |
Consolidated operating revenues
Consolidated operating revenues for 2011 increased by approximately $544 million or approximately 8.5% from 2010. This increase was primarily due to an increase in dialysis and related lab services revenues of approximately $422 million, principally due to strong volume growth from additional treatments from non-acquired growth and acquisitions including the acquisition of DSI, partially offset by a decline of $7 in the
S-98
Table of Contents
average dialysis revenue per treatment, primarily from a decrease in our Medicare revenues as a result of operating in the new single bundled payment system, as described above. Consolidated operating revenues also increased as a result of an increase of approximately $130 million in the ancillary services and strategic initiatives net revenues driven primarily from growth in our pharmacy services and from our disease management services.
Consolidated operating revenues for 2010 increased by approximately $337 million or approximately 5.5% from 2009. This increase was primarily due to an increase in our dialysis and related lab services revenues of approximately $279 million, principally due to an increase in the number of treatments from non-acquired growth and acquisitions, partially offset by a decline of $3 in the average dialysis revenue per treatment, as described above, and an increase of approximately $50 million in the ancillary services and strategic initiatives net revenues driven primarily from growth in our pharmacy services and from our infusion therapy services.
Consolidated operating income
Consolidated operating income of $1,131 million for 2011 increased by approximately $134 million, or 13.4%, from 2010 which includes the $24 million HomeChoice goodwill impairment charge. Excluding this item, consolidated operating income would have increased by $158 million, or 15.8%, primarily due to an increase in the dialysis and related lab services operating revenues as a result of strong volume growth in revenue from additional treatments as a result of non-acquired growth and acquisitions, partially offset by a decline in our average dialysis revenue per treatment of approximately $7, as described below. Consolidated operating income also increased as a result of overall lower pharmaceutical costs mainly from a decline in the intensities of physician-prescribed pharmaceuticals, additional operating income from the acquisition of DSI and from cost control initiatives. However, consolidated operating income was negatively impacted by higher labor and benefit costs, an increase in our professional fees for compliance and legal initiatives, and for information technology matters, transaction and integration costs associated with the acquisition of DSI, an increase in EPO pharmaceutical costs and an increase in expenses associated with our international expansion.
Consolidated operating income of $997 million for 2010 increased by approximately $57 million, or 6.1%, from 2009. This increase was primarily attributable to an increase in revenue as a result of additional treatments from non-acquired growth and acquisitions in dialysis and related lab services, partially offset by a decline in our average dialysis revenue per treatment of approximately $3, as described below. Operating income also increased as a result of continued cost control initiatives, improved productivity, overall lower pharmaceutical costs and lower operating losses in our ancillary services and strategic initiatives, partially offset by the negative impact on our operating margin from a decline in the intensities of physician-prescribed pharmaceuticals, higher labor costs and increases in other operating costs of our dialysis centers.
S-99
Table of Contents
Operating segments
Dialysis and Related Lab Services
| Year ended | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| (dollar amounts rounded to nearest million, except per treatment data) | ||||||||||||
Net operating revenues | $ | 6,295 | $ | 5,896 | $ | 5,627 | ||||||
Add: Provision for uncollectible accounts | 190 | 166 | 156 | �� | ||||||||
|
|
|
|
|
| |||||||
Dialysis and related lab services operating revenues | $ | 6,485 | $ | 6,062 | $ | 5,783 | ||||||
|
|
|
|
|
| |||||||
Patient service operating revenues | $ | 6,474 | $ | 6,052 | $ | 5,774 | ||||||
Less: Provision for uncollectible accounts related to patient service revenues | (190 | ) | (166 | ) | (156 | ) | ||||||
|
|
|
|
|
| |||||||
Net patient service operating revenues | 6,284 | 5,886 | 5,618 | |||||||||
Other revenues | 11 | 11 | 9 | |||||||||
|
|
|
|
|
| |||||||
Total net operating revenues | $ | 6,295 | $ | 5,897 | $ | 5,627 | ||||||
|
|
|
|
|
| |||||||
Segment operating income | $ | 1,225 | $ | 1,039 | $ | 994 | ||||||
|
|
|
|
|
| |||||||
Dialysis treatments | 19,599,472 | 17,963,862 | 16,984,959 | |||||||||
Average dialysis treatments per treatment day | 62,618 | 57,393 | 54,352 | |||||||||
Average dialysis revenue per treatment (including lab services) | $ | 330 | $ | 337 | $ | 340 | ||||||
Operating revenues
Dialysis and related lab services operating revenues for 2011 increased by approximately $422 million or approximately 7.0% from 2010. The increase in net operating revenues was primarily due to strong volume growth from additional treatments of approximately 9.1% due to an increase in non-acquired treatment growth at existing and new centers and growth through acquisitions, which includes additional treatments associated with the acquisition of DSI. However, this increase was partially offset by a decrease in the average dialysis revenue per treatment of approximately $7, or 2.1%. The decrease in the average dialysis revenue per treatment in 2011, as compared to 2010, was primarily due to a decline in our Medicare reimbursements as a result of operating in the new single bundled payment system, continued decline in the commercial payor mix and a decline in the intensities of physician-prescribed pharmaceuticals, partially offset by an increase in some of our commercial payment rates.
Dialysis and related lab services operating revenues for 2010 increased by approximately $279 million or approximately 4.8% from 2009. The increase in net operating revenues was primarily due to an increase in the number of treatments of approximately 5.8% due to an increase in non-acquired treatment growth at existing and new centers and growth through acquisitions. However, this increase was partially offset by a decrease in the average dialysis revenue per treatment of approximately $3, or 0.9%. The decrease in the average dialysis revenue per treatment in 2010, as compared to 2009, was primarily due to a decline in the intensities of physician-prescribed pharmaceuticals and a decline in the commercial payor mix, partially offset by a 1% increase in the Medicare composite rate and an increase in some of our commercial payment rates.
S-100
Table of Contents
The following table summarizes our dialysis and related lab services revenues by modality for the year ended December 31, 2011:
| Revenue percentages | ||||
Outpatient hemodialysis centers | 81 | % | ||
Peritoneal dialysis and home-based hemodialysis | 14 | % | ||
Hospital inpatient hemodialysis | 5 | % | ||
|
| |||
Total dialysis and related lab services revenues | 100 | % | ||
|
| |||
Approximately 66% of our total dialysis and related lab services revenues for the year ended December 31, 2011 were from government-based programs, principally Medicare, Medicaid, and Medicare-assigned plans, representing approximately 89% of our total patients. Over the last several years, we have been experiencing growth in our government-based patients that has been outpacing the growth in our commercial patients which has negatively impacted our average dialysis and related lab services revenue per treatment. Our overall percentage of patients and revenues associated with commercial payors continued to decline in 2011. Less than 1% of our dialysis and related lab services revenues are due directly from patients. No single commercial payor accounted for more than 6% of total dialysis and related lab services revenues for the year ended December 31, 2011.
On average we are paid significantly more for services provided to patients covered by commercial healthcare plans in the U.S. than we are for patients covered by Medicare, Medicaid or other government plans such as Medicare-assigned plans. Patients covered by commercial health plans transition to Medicare coverage after a maximum of 33 months. As a patient transitions from commercial coverage to Medicare or Medicaid coverage, the payment rates normally decline substantially. Medicare payment rates are insufficient to cover our costs associated with providing dialysis treatments, and therefore we lose money on each Medicare treatment.
Nearly all of our net earnings from our dialysis and related lab services are derived from commercial payors, some of which pay at established contract rates and others which pay negotiated payment rates based on our usual and customary fee schedule for our out-of-network patients. If we experience a net overall reduction in our contracted and non-contracted commercial rates as a result of these negotiations or restrictions, it could have a material adverse effect on our operating results.
Our average dialysis and related lab services revenue per treatment can be significantly impacted by several major factors, including our commercial payment rates, government payment policies regarding reimbursement amounts for dialysis treatments and pharmaceuticals under the new Medicare bundled payment rate system, including our ability to capture all patient characteristics, changes in the mix of government and commercial patients, and changes in the mix and intensities of physician-prescribed pharmaceuticals that are billed separately.
Operating expenses and charges
Patient care costs. Dialysis and related lab services patient care costs are those costs directly associated with operating and supporting our dialysis centers and consist principally of labor, pharmaceuticals, medical supplies and operating costs of the dialysis centers. The dialysis and related lab services patient care costs on a per treatment basis were $217 and $232 for 2011 and 2010, respectively. The $15 decrease in the per treatment costs in 2011 as compared to 2010 was primarily attributable to a decline in the intensities of physician-prescribed pharmaceuticals, continued cost control initiatives, partially offset by higher labor and benefit costs, and higher EPO costs.
S-101
Table of Contents
Dialysis and related lab services patient care costs on a per treatment basis were $232 and $235 for 2010 and 2009, respectively. The $3 decrease in the per treatment costs in 2010 as compared to 2009 was primarily attributable to a decline in the intensities of physician-prescribed pharmaceuticals, a decrease in our overall pharmaceutical costs and continued improvements in productivity, partially offset by higher labor rates.
General and administrative expenses. Dialysis and related lab services general and administrative expenses for the years ended 2011, 2010 and 2009 were approximately $551 million, $471 million and $428 million, respectively. The increase of approximately $80 million in 2011 as compared to 2010 was primarily due to increases in labor and benefit costs, an increase in our professional expenses for legal and compliance initiatives and for information technology matters as well as transaction and integration costs associated with the acquisition of DSI. The increase in general and administrative expenses of approximately $43 million in 2010 as compared to 2009 was primarily due to increases in labor and benefit costs, partially offset by the timing of certain other expenditures.
Depreciation and amortization. Dialysis and related lab services depreciation and amortization expenses for 2011, 2010 and 2009 were approximately $260 million, $227 million and $221 million, respectively. The increase of approximately $33 million in depreciation and amortization for dialysis and related lab services in 2011 and $6 million in 2010 were primarily due to growth through new center developments and acquisitions.
Provision for uncollectible accounts receivable. The provision for uncollectible accounts receivable for dialysis and related lab services was 2.9% for 2011, 2.7% for 2010, and 2.7% for 2009. The increase in the provision for uncollectible accounts during 2011 was primarily the result of a slowdown in our historical collection experience from some of our non-government payors. The current provision level of 3.0% at the end of 2011 may increase if we encounter problems with our billing and collection process as a result of sustained weakness in the U.S. economy.
Segment operating income
Dialysis and related lab services operating income for 2011 increased by approximately $186 million as compared to 2010. The increase in the operating income for 2011 as compared to 2010 was primarily due to strong treatment growth as a result of additional dialysis treatments from non-acquired growth and acquisitions, partially offset by a decrease in the average dialysis revenue per treatment of approximately $7 as described above. The dialysis and related lab services operating income also increased as a result of a decline in the intensities of physician-prescribed pharmaceuticals, and additional operating income from the acquisition of DSI. However, the dialysis and related lab services operating income was negatively impacted by higher labor and benefit costs, an increase in the cost of EPO, an increase in our professional fees in conjunction with compliance and legal initiatives and for information technology matters as well as transaction and integration costs associated with the acquisition of DSI.
Dialysis and related lab services operating income for 2010 increased by approximately $45 million as compared to 2009. The increase in the operating income for 2010 as compared to 2009 was primarily due to growth in the number of dialysis treatments from non-acquired growth and acquisitions, partially offset by a decrease in the average dialysis revenue per treatment of approximately $3 as described above. The dialysis and related lab services operating income also increased as a result of certain cost control initiatives, improved productivity, and overall lower pharmaceutical costs. However, the dialysis and related lab services operating income was negatively impacted by an operating margin decrease due to a decline in the intensities of physician-prescribed pharmaceuticals, higher labor costs and an increase in other operating costs of our dialysis centers.
S-102
Table of Contents
Other—Ancillary services and strategic initiatives
| Year ended | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| (dollar amounts rounded to nearest million) | ||||||||||||
Revenues | $ | 514 | $ | 384 | $ | 334 | ||||||
|
|
|
|
|
| |||||||
Segment operating loss | $ | (54 | ) | $ | (6 | ) | $ | (12 | ) | |||
|
|
|
|
|
| |||||||
Net operating revenues
The ancillary services and strategic initiatives net operating revenues for 2011 increased by approximately $130 million or 33.9% as compared to 2010, primarily from growth in pharmacy services, and from our disease management services.
The ancillary services and strategic initiatives net operating revenues for 2010 increased by approximately $50 million or 15.0% as compared to 2009, primarily from growth in pharmacy services, and from our infusion therapy services, partially offset by a decline in our net operating revenues in our disease management services as a result of discontinuing the full service health care plans at the end of 2009.
Operating expenses
Ancillary services and strategic initiatives operating expenses for 2011 increased by approximately $179 million from 2010, which includes the $24 million HomeChoice goodwill impairment charged as described below. Excluding this item, ancillary services and strategic initiatives adjusted operating expenses would have increased by $155 million. This increase in operating expenses was primarily due to an increase in volume in our pharmacy business, an increase in expenses associated with our international dialysis expansion and an increase in labor and benefit costs.
Ancillary services and strategic initiatives operating expenses for 2010 increased by approximately $43 million from 2009, primarily due to an increase in volume in our pharmacy business and an increase in labor costs, partially offset by lower operating costs of our disease management services as a result of discontinuing the full service health care plans at the end of 2009.
Goodwill
In the second quarter of 2011, we determined that circumstances indicated it was more likely than not that the fair value of one of our ancillary businesses, HomeChoice, which provides infusion therapy services, was less than its carrying amount. The primary factor informing our conclusion was the recent decline in the operating performance of HomeChoice caused mainly by rapid expansion. This led management to revise its view of HomeChoice’s organizational growth capability and scale back significantly its current plans for HomeChoice’s future growth initiatives and to update HomeChoice’s forecasts and current operating budgets accordingly. These revisions reflected the current and expected future cash flows that we believed market participants would use in determining the fair value or the HomeChoice business. As a result, in the second quarter of 2011, we estimated that the carrying amount of goodwill related to HomeChoice exceeded its implied fair value by $24 million, resulting in a pre-tax goodwill impairment charge of that amount. As of December 31, 2011, after giving effect to this impairment charge, we have approximately $32 million of HomeChoice goodwill remaining. During the fourth quarter of 2011, we finalized our calculation of this impairment charge, which did not change the goodwill impairment charge previously recorded.
Segment operating loss
Ancillary services and strategic initiatives operating losses for 2011 increased by approximately $48 million from 2010, which includes the $24 million HomeChoice goodwill impairment charge, as described above. Excluding this item, ancillary services and strategic initiatives adjusted operating losses would have increased by
S-103
Table of Contents
$24 million. This increase in operating losses was primarily due to an increase in expenses associated with our international dialysis expansion and a deterioration in the operating performance of our infusion therapy services, partially offset by an increase in the operating performance of our pharmacy business, and in our vascular access services.
Ancillary services and strategic initiatives operating losses for 2010 decreased by approximately $6 million from 2009. The decrease in operating losses was primarily due to volume growth in revenues associated with our pharmacy business, and a decrease in operating losses in our disease management business as a result of discontinuing the full service health care plans at the end of 2009.
Corporate level charges
Stock-based compensation. Stock-based compensation of approximately $49 million for 2011 increased by approximately $3 million from 2010. Stock-based compensation of approximately $46 million for 2010 increased by approximately $2 million from 2009. The increase in 2011 resulted from an increase in the overall grant date fair value for the grant years that contributed expense to 2011, driven by both an increase in the grant date fair value of 2011 grants over that for recent years and an increase in the number of awards granted in 2011 over 2010. The increase in 2010 resulted principally from an increase in the overall grant date fair value for the grant years that contributed expense to 2010, driven in part by a substantial increase in the grant date fair value of 2010 grants over that for recent years offset by a significant reduction in the number of awards granted in 2010.
Debt expense. Debt expense for 2011, 2010, and 2009 consisted of interest expense of approximately $231 million, $172 million, and $176 million, respectively, including the amortization and accretion of debt discounts and premiums and the amortization of deferred financing costs of approximately $10 million in 2011, $9 million in 2010 and $10 million in 2009. The increase in interest expense in 2011 as compared to 2010 was primarily related to additional borrowings under our Senior Secured Credit Facilities that were issued in the fourth quarter of 2010 and additional borrowings associated with the new Term Loan A-2 that contain significantly higher interest rates than our previous facility. In addition, debt expense in 2011 was also impacted by the amount of interest rate swaps that resulted in a higher overall weighted average effective interest rate on the Term Loan A and from the amortization of an interest rate cap premium associated with our Term Loan B. However, debt expense in 2011 benefited from lower rates and lower outstanding balances associated with our new senior notes that were issued in the fourth quarter of 2010. Our overall weighted average effective interest rate in 2011 was 5.28% as compared to 4.68% in 2010.
The decrease in interest expense in 2010 as compared to 2009 was primarily related to lower average outstanding principal balances on our previously outstanding Term Loan A, lower average outstanding principal balances on our previously outstanding senior notes, lower interest rates associated with the issuance of our New Senior Notes and a decrease in our weighted average effective interest rate on the Term Loan B as a result of lower notional amounts of fixed rate swap agreements that contained higher rates. Our overall weighted average effective interest rate in 2010 was 4.68% as compared to 4.86% in 2009. However, interest expense in the fourth quarter of 2010 was negatively affected by the refinancing of our Senior Secured Credit Facilities that occurred on October 20, 2010, as the interest rates under our new Senior Secured Credit Facilities are substantially higher than the interest rates under the previous facility. Our overall weighted average effective interest rate in the fourth quarter of 2010 was 4.86%.
Equity investment income. Equity investment income was approximately $9.0 million in 2011 as compared to $9.0 million in 2010 and $2.4 million in 2009. Equity investment income in 2011 as compared to 2010 was flat but was impacted by an increase in the profitability of certain of our nonconsolidated joint ventures, offset by a decrease in the operating performance of certain other joint ventures. The increase in equity investment income in 2010 as compared to 2009 was primarily due to an increase in the profitability of our nonconsolidated joint ventures.
S-104
Table of Contents
Other income. Other income was approximately $3 million, $3 million, and $4 million in 2011, 2010, and 2009, respectively, and consisted principally of interest income. Other income in 2011 was slightly down from 2010, primarily as a result of lower average interest rates and lower average cash balances. The decrease in 2010 as compared to 2009 was primarily the result of lower average interest rates, partially offset by higher average cash balances.
Provision for income taxes. The provision for income taxes for 2011 represented an effective annualized tax rate of 35.4%, compared with 35.0% and 36.7% in 2010 and 2009, respectively. The effective tax rate in 2011 was higher primarily due to non-deductible transaction costs associated with the DSI acquisition.
Impairments and valuation adjustments. We perform impairment or valuation reviews for our property and equipment, amortizable intangible assets with finite useful lives, equity investments in non-consolidated businesses, and our investments in ancillary services and strategic initiatives at least annually and whenever a change in condition indicates that an impairment review is warranted. Such changes include shifts in our business strategy or plans, the quality or structure of our relationships with our partners, or when a center experiences deteriorating operating performance. Goodwill is also assessed at least annually for possible valuation impairment using fair value methodologies. These types of adjustments are charged directly to the corresponding operating segment that incurred the charge. Except for the HomeChoice goodwill charge in 2011, there were no other significant impairments or valuation adjustments recognized during the periods presented.
Noncontrolling interests
Net income attributable to noncontrolling interests for 2011, 2010 and 2009 was approximately $95 million, $79 million and $57 million, respectively. The increases in noncontrolling interests in 2011 and 2010 were primarily due to increases in the number of new joint ventures and increases in the profitability of our dialysis-related joint ventures. The percentage of dialysis and related lab services net operating revenues generated from dialysis-related joint ventures was approximately 18% in both 2011 and 2010.
Accounts receivable
Our accounts receivable balances at December 31, 2011 and 2010 represented approximately 61 days of revenue for both periods, net of bad debt allowance. Our days outstanding in 2011, represent solid improved cash collections that enabled us to keep pace with our growth in revenue. However, our cash collections in early 2012 could be negatively impacted as Medicare upgrades its systems to meet their new billing requirements.
As of December 31, 2011 and 2010, approximately $188 million and $153 million in unreserved accounts receivable, respectively, representing approximately 16% and 15% of our total accounts receivable balance, respectively, were more than six months old. During 2011, we experienced improved cash collections from certain government payors and certain commercial payors. There were no significant unreserved balances over one year old. Less than 1% of our revenues are classified as “patient pay”. Substantially all revenue realized is from government and commercial payors, as discussed above.
Amounts pending approval from third-party payors as of December 31, 2011 and 2010, other than the standard monthly billing, consisted of approximately $57 million in 2011 and $46 million in 2010, associated with Medicare bad debt claims, classified as “other receivables”. Currently, a significant portion of our Medicare bad debt claims are typically paid to us before the Medicare fiscal intermediary audits the claims. However, the payment received from Medicare is subject to adjustment based upon the actual results of the audits. Such audits typically occur one to four years after the claims are filed. As a kidney dialysis provider, our revenue is not subject to cost report settlements, except for potentially limiting the collectability of these Medicare bad debt claims.
S-105
Table of Contents
Results of operations for the six months ended June 30, 2012
We operate principally as a dialysis and related lab services business in the U.S. but also operate other ancillary services and strategic initiatives. These ancillary services and strategic initiatives consist of pharmacy services, infusion therapy services, disease management services, vascular access services, ESRD clinical research programs and physician services, direct primary care and our international dialysis operations. The U.S. dialysis and related lab services business qualifies as a separately reportable segment and all references to dialysis and related lab services continue to refer only to our U.S. dialysis and related lab services business. All of the other ancillary services and strategic initiatives operating segments, including our international dialysis operations, have been combined and disclosed in the other segments category.
Our consolidated operating results for the second quarter of 2012 compared with the prior sequential quarter and the same quarter of 2011 as well as the six months ended June 30, 2012 compared to the same periods in 2011 were as follows:
| Three months ended | Six months ended | |||||||||||||||||||||||||||||||||||||||
| June 30, 2012 | March 31, 2012 | June 30, 2011 | June 30, 2012 | June 30, 2011 | ||||||||||||||||||||||||||||||||||||
| (dollar amounts rounded to nearest million) | ||||||||||||||||||||||||||||||||||||||||
Total net operating revenues | $ | 1,930 | $ | 1,867 | $ | 1,661 | $ | 3,797 | $ | 3,224 | ||||||||||||||||||||||||||||||
Add: Provision for uncollectible accounts related to patient service revenues | 54 | 53 | 48 | 107 | 88 | |||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||
Consolidated operating revenues | $ | 1,984 | 100 | % | $ | 1,920 | 100 | % | $ | 1,709 | 100 | % | $ | 3,904 | 100 | % | $ | 3,312 | 100 | % | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||
Patient service operating revenues | $ | 1,809 | $ | 1,763 | $ | 1,583 | $ | 3,572 | $ | 3,080 | ||||||||||||||||||||||||||||||
Less: Provision for uncollectible accounts related to patient service revenues | (54 | ) | 3 | % | (53 | ) | 3 | % | (48 | ) | 3 | % | (107 | ) | 3 | % | (88 | ) | 3 | % | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||
Net patient service operating revenues | 1,755 | 1,710 | 1,535 | 3,465 | 2,992 | |||||||||||||||||||||||||||||||||||
Other revenues | 175 | 157 | 126 | 332 | 232 | |||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||
Total net operating revenues | 1,930 | 1,867 | 1,661 | 3,797 | 3,224 | |||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||
Operating expenses and charges: | ||||||||||||||||||||||||||||||||||||||||
Patient care costs | 1,312 | 66 | % | 1,263 | 66 | % | 1,163 | 68 | % | 2,575 | 66 | % | 2,277 | 69 | % | |||||||||||||||||||||||||
General and administrative | 215 | 11 | % | 207 | 11 | % | 164 | 10 | % | 422 | 11 | % | 315 | 10 | % | |||||||||||||||||||||||||
Depreciation and amortization | 78 | 4 | % | 76 | 4 | % | 64 | 4 | % | 154 | 4 | % | 126 | 4 | % | |||||||||||||||||||||||||
Provision for uncollectible accounts | 2 | — | 2 | — | 2 | — | 4 | — | 3 | — | ||||||||||||||||||||||||||||||
Goodwill impairment charge | — | — | — | — | 24 | 1 | % | — | — | 24 | 1 | % | ||||||||||||||||||||||||||||
Equity investment income | (3 | ) | — | (3 | ) | — | (2 | ) | — | (5 | ) | — | (4 | ) | — | |||||||||||||||||||||||||
Legal proceeding contingency accrual and related expenses | 78 | 4 | % | — | — | — | — | 78 | 2 | % | — | — | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||
Total operating expenses and charges | 1,682 | 87 | %(1) | 1,546 | 83 | %(1) | 1,415 | 86 | %(1) | 3,228 | 85 | %(1) | 2,742 | 85 | %(1) | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||
Operating income | $ | 248 | 13 | % | $ | 321 | 17 | % | $ | 247 | 14 | % | $ | 569 | 15 | % | $ | 482 | 15 | % | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||
| (1) | The percentages include total operating expenses and charges and the provision for uncollectible accounts related to patient service revenues. |
S-106
Table of Contents
The following table summarizes consolidated net operating revenues for our U.S. dialysis and related lab services segment as well as our other ancillary services and strategic initiatives:
| Three Months Ended | Six Months Ended | |||||||||||||||||||
| June 30, 2012 | March 31, 2012 | June 30, 2011 | June 30, 2012 | June 30, 2011 | ||||||||||||||||
| (dollar amounts rounded to nearest million) | ||||||||||||||||||||
Dialysis and related lab services patient service operating revenues | $ | 1,813 | $ | 1,767 | $ | 1,585 | $ | 3,580 | $ | 3,085 | ||||||||||
Less: Provision for uncollectible accounts related to patient service revenues | (54 | ) | (53 | ) | (48 | ) | (107 | ) | (88 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Dialysis and related lab services net patient service operating revenues | $ | 1,759 | $ | 1,714 | $ | 1,537 | $ | 3,473 | $ | 2,997 | ||||||||||
Other revenues | 3 | 3 | 3 | 6 | 5 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total net dialysis and related lab services operating revenues | 1,762 | 1,717 | 1,540 | 3,479 | 3,002 | |||||||||||||||
Other—Ancillary services and strategic initiatives | 170 | 153 | 121 | 323 | 226 | |||||||||||||||
Other—Ancillary services and strategic initiatives net patient service operating revenues | 5 | 3 | 2 | 8 | 3 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total net segment operating revenues | 1,937 | 1,873 | 1,663 | 3,810 | 3,231 | |||||||||||||||
Elimination of intersegment revenues | (7 | ) | (6 | ) | (2 | ) | (13 | ) | (7 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Consolidated net operating revenues | $ | 1,930 | $ | 1,867 | $ | 1,661 | $ | 3,797 | $ | 3,224 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Consolidated operating revenues | $ | 1,984 | $ | 1,920 | $ | 1,709 | $ | 3,904 | $ | 3,312 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
The following table summarizes consolidated operating income:
| Three months ended | Six months ended | |||||||||||||||||||
| June 30, 2012 | March 31, 2012 | June 30, 2011 | June 30, 2012 | June 30, 2011 | ||||||||||||||||
| (dollar amounts rounded to nearest million) | ||||||||||||||||||||
Dialysis and related lab services | $ | 286 | $ | 354 | $ | 288 | $ | 641 | $ | 540 | ||||||||||
Other—Ancillary services and strategic initiatives | (19 | ) | (17 | ) | (30 | ) | (37 | ) | (39 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total segment operating income | 267 | 337 | 258 | 604 | 501 | |||||||||||||||
Reconciling items: | ||||||||||||||||||||
Stock-based compensation | (12 | ) | (13 | ) | (13 | ) | (24 | ) | (23 | ) | ||||||||||
Other corporate general and administrative expenses | (10 | ) | (6 | ) | — | (16 | ) | — | ||||||||||||
Equity investment income | 3 | 3 | 2 | 5 | 4 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Consolidated operating income | 248 | 321 | 247 | 569 | 482 | |||||||||||||||
Reconciliation of non-GAAP measure: | ||||||||||||||||||||
Add: | ||||||||||||||||||||
Legal proceeding contingency accrual and related expenses | 78 | — | — | 78 | — | |||||||||||||||
Goodwill impairment charge | — | — | 24 | — | 24 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Non-GAAP consolidated operating income(1) | $ | 326 | $ | 321 | $ | 271 | $ | 647 | $ | 506 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
| (1) | For the three and six months ended June 30, 2012 and for the three and six months ended June 30, 2011, we have excluded a legal proceeding contingency accrual and related expenses and a non-cash goodwill impairment charge from operating expenses and operating income, respectively, because management believes that these presentations enhance a user’s understanding of our normal consolidated operating income by excluding an unusual charge for a legal proceeding contingency accrual and related expenses that |
S-107
Table of Contents
| resulted from an agreement we reached in principle to settle the Woodard Private Civil Suit (see Note 7 to the condensed consolidated financial statements) in our quarterly report on Form 10-Q for the quarterly period ended June 30, 2012 as incorporated by reference and a non-cash goodwill impairment charge that resulted from a decrease in the implied fair value of goodwill below its carrying amount associated with HomeChoice Partners, which provides infusion therapy services, during the second quarter of 2011, and therefore these adjusted consolidated operating income amounts are meaningful and comparable to our prior period results and more indicative of our normal consolidated operating income. |
Consolidated operating revenues
Consolidated operating revenues for the second quarter of 2012 increased by approximately $64 million, or approximately 3.3%, as compared to the first quarter of 2012. The increase in consolidated operating revenues was primarily due to an increase in dialysis and related lab services operating revenues of approximately $46 million, principally due to strong volume growth from additional treatments from non-acquired growth and acquisitions. Our average dialysis revenue per treatment was flat in the second quarter of 2012 as compared to the first quarter of 2012. The increase in the consolidated operating revenues was also due to an increase of approximately $19 million in the ancillary services and strategic initiatives revenues primarily from growth in our pharmacy services.
Consolidated operating revenues for the second quarter of 2012 increased by approximately $275 million, or approximately 16.1%, as compared to the second quarter of 2011. The increase in consolidated operating revenues was primarily due to an increase in dialysis and related lab services operating revenues of approximately $228 million, principally due to strong volume growth from additional treatments from non-acquired treatment growth in existing and new centers and growth through acquisitions. The increase in consolidated operating revenues was also due to an increase of approximately $52 million in the ancillary services and strategic initiatives revenues primarily from growth in our pharmacy services.
Consolidated operating revenues for the six months ended June 30, 2012 increased by approximately $592 million, or approximately 17.9%, as compared to the same period in 2011. The increase in consolidated operating revenues was primarily due to an increase in dialysis and related lab services operating revenues of approximately $496 million, principally due to strong volume growth from additional treatments from non-acquired treatment growth in existing and new centers and growth through acquisitions, and as a result of one additional treatment day for the six months ended June 30, 2012. The increase in consolidated operating revenues was also due to an increase of approximately $4 in the average dialysis revenue per treatment, as described below, and from an increase of approximately $102 million in the ancillary services and strategic initiatives revenues primarily from growth in our pharmacy services.
Consolidated operating income
Consolidated operating income for the second quarter of 2012 decreased by approximately $73 million, or approximately 22.7%, as compared to the first quarter of 2012, including the legal proceeding contingency accrual and related expenses of approximately $78 million. Excluding this item, adjusted consolidated operating income would have increased by $5 million. The increase in adjusted operating income was primarily due to strong volume growth in the number of treatments, lower payroll taxes, lower accruals for self-insurance reserves and improvements in productivity, partially offset by an increase in our pharmaceuticals costs, an increase in labor and benefit costs and an increase in expenses associated with our annual leadership meeting.
Consolidated operating income for the second quarter of 2012 increased by approximately $1 million, or approximately 0.4%, as compared to the second quarter of 2011, including the legal proceeding contingency accrual and related expenses of $78 million in the second quarter of 2012 and the $24 million goodwill impairment charge in the second quarter of 2011. Excluding these items from their respective periods adjusted consolidated operating income would have increased by $55 million. The increase in adjusted operating income
S-108
Table of Contents
was primarily due to strong volume growth from additional treatments from non-acquired treatment growth in existing and new centers and growth through acquisitions. In addition, adjusted consolidated operating income also increased as a result of overall lower pharmaceutical costs mainly from a decline in the intensities of physician-prescribed pharmaceuticals, lower accruals for self-insurance reserves and improvements in productivity. Consolidated operating income for the second quarter of 2012 was negatively impacted by higher labor and related payroll taxes, higher benefit costs, an increase in our professional fees for legal and compliance matters, an increase in our other direct operating expenses associated with our dialysis centers, an increase in acquisition-related expenses and an increase in the operating losses associated with our ancillary services and strategic initiatives.
Consolidated operating income for the six months ended June 30, 2012 increased by approximately $87 million, or approximately 18.0%, as compared to the same period in 2011, including the legal proceeding contingency accrual and related expenses of $78 million for the six months ended June 30, 2012 and the $24 million goodwill impairment charge for the six months ended June 30, 2011. Excluding these items from their respective periods adjusted consolidated operating income would have increased by $141 million. The increase in adjusted operating income was primarily due to strong volume growth from additional treatments as a result of non-acquired growth in existing and new centers, growth through acquisitions and as a result of one additional treatment day for the six months ended June 30, 2012, as well as from an increase in the average dialysis revenue per treatment of approximately $4, as described below. In addition, consolidated operating income also increased as a result of overall lower pharmaceutical costs mainly from a decline in the intensities of physician-prescribed pharmaceuticals and improvements in productivity. Consolidated operating income for the six months ended June 30, 2012 was negatively impacted by higher labor and related payroll taxes, higher benefit costs, an increase in our professional fees for legal and compliance matters, an increase in our other direct operating expenses associated with our dialysis centers, an increase in acquisition-related expenses and an increase in the operating losses associated with our ancillary services and strategic initiatives.
Operating segments
U.S. dialysis and related lab services
| Three months ended | Six months ended | |||||||||||||||||||
| June 30, 2012 | March 31, 2012 | June 30, 2011 | June 30, 2012 | June 30, 2011 | ||||||||||||||||
| (dollar amounts rounded to nearest million, except per treatment data) | ||||||||||||||||||||
Net operating revenues | $ | 1,762 | $ | 1,717 | $ | 1,540 | $ | 3,479 | $ | 3,002 | ||||||||||
Add: Provision for uncollectible accounts | 54 | 53 | 48 | 107 | 88 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Dialysis and related lab services operating revenues | $ | 1,816 | $ | 1,770 | $ | 1,588 | $ | 3,586 | $ | 3,090 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Patient service operating revenues | $ | 1,813 | $ | 1,767 | $ | 1,585 | $ | 3,580 | $ | 3,085 | ||||||||||
Less: Provision for uncollectible accounts related to patient service revenues | (54 | ) | (53 | ) | (48 | ) | (107 | ) | (88 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net patient service operating revenues | 1,759 | 1,714 | 1,537 | 3,473 | 2,997 | |||||||||||||||
Other revenues | 3 | 3 | 3 | 6 | 5 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total net operating revenues | $ | 1,762 | $ | 1,717 | $ | 1,540 | $ | 3,479 | $ | 3,002 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Segment operating income | $ | 286 | $ | 354 | $ | 288 | $ | 641 | $ | 540 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Dialysis treatments | 5,451,901 | 5,314,275 | 4,769,661 | 10,766,176 | 9,364,211 | |||||||||||||||
Average dialysis treatments per treatment day | 69,896 | 68,132 | 61,150 | 69,014 | 60,414 | |||||||||||||||
Average dialysis revenue per treatment (including lab services) | $ | 333 | $ | 332 | $ | 332 | $ | 333 | $ | 329 | ||||||||||
S-109
Table of Contents
Operating revenues
Dialysis and related lab services’ operating revenues for the second quarter of 2012 increased by approximately $46 million, or approximately 2.6%, as compared to the first quarter of 2012. The increase in operating revenues was primarily due to an increase in the number of treatments as a result of non-acquired treatment growth in existing and new centers and from growth through acquisitions. Our average dialysis revenue per treatment in the second quarter of 2012 was flat as compared to the first quarter of 2012 but benefited from an increase as a result of an increase in some of our commercial payment rates, partially offset by a slight decline in our government reimbursements.
Dialysis and related lab services’ operating revenues for the second quarter of 2012 increased by approximately $228 million, or approximately 14.4%, as compared to the second quarter of 2011. The increase in operating revenues in the second quarter of 2012 was principally due to strong volume growth from additional treatments. The increase in the number of treatments was primarily attributable to non-acquired treatment growth at existing and new centers and growth through acquisitions. The average dialysis revenue per treatment benefited from an increase in our Medicare reimbursements, and an increase in some of our commercial payment rates, principally offset by a decline in our commercial payor mix and a decline in the intensities of physician-prescribed pharmaceuticals.
Dialysis and related lab services’ operating revenues for the six months ended June 30, 2012, increased by approximately $496 million, or approximately 16.1%, in the first quarter of 2012, as compared to the same period in 2011. The increase in operating revenues in the first six months of 2012 was principally due to strong volume growth from additional treatments of approximately 15.0%, and an increase in the average dialysis revenue per treatment of approximately $4, or approximately 1.0%. The increase in the number of treatments was primarily attributable to non-acquired treatment growth at existing and new centers and growth through acquisitions and as a result of one additional treatment day for the six months ended June 30, 2012. The increase in the average dialysis revenue per treatment was primarily due to an increase in our Medicare reimbursements, an increase in some of our commercial payment rates, partially offset by a decline in our commercial payor mix and a decline in the intensities of physician-prescribed pharmaceuticals.
Operating expenses and charges
Patient care costs.Dialysis and related lab services’ patient care costs on a per treatment basis for the second quarter of 2012 increased by $2 compared to the first quarter of 2012. The increase was primarily the result of higher pharmaceutical costs, higher labor and benefit costs and an increase in expenses associated with our annual leadership meeting, partially offset by lower payroll taxes, lower accruals for self-insurance reserves and improvements in productivity.
Dialysis and related lab services’ patient care costs on a per treatment basis for the second quarter of 2012 decreased by approximately $9 as compared to the second quarter of 2011. The decrease was primarily attributable to lower pharmaceutical costs mainly from a decline in the intensities of physician-prescribed pharmaceuticals, lower accruals for self-insurance reserves and improvements in productivity, partially offset by higher labor and benefit costs.
Dialysis and related lab services’ patient care costs on a per treatment basis for the six months ended June 30, 2012 decreased by approximately $10 as compared to the same period in 2011. The was primarily attributable to lower pharmaceutical costs mainly from a decline in the intensities of physician-prescribed pharmaceuticals, lower accruals for self-insurance reserves and improvements in productivity, partially offset by higher labor and benefit costs.
General and administrative expenses.Dialysis and related lab services’ general and administrative expenses of approximately $157 million decreased by approximately $4 million in the second quarter of 2012 as compared to the first quarter of 2012. The decrease was primarily due to lower professional fees for legal and compliance matters, lower integration costs and lower payroll taxes, partially offset by higher labor and benefit costs.
S-110
Table of Contents
Dialysis and related lab services general and administrative expenses for the second quarter of 2012 increased by approximately $29 million as compared to the second quarter of 2011. The increase was primarily due to higher labor and benefit costs and an increase in professional fees in conjunction with legal and compliance matters.
Dialysis and related lab services general and administrative expenses for the six months ended June 30, 2012 increased by approximately $68 million as compared to the same period in 2011. The increase was primarily due to higher labor and benefit costs and an increase in professional fees in conjunction with legal and compliance matters. Dialysis and related lab services general and administrative expenses, as a percentage of dialysis and related lab services’ revenue, was 8.6% for the second quarter of 2012, 9.1% for the first quarter of 2012 and 8.0% for the second quarter of 2011.
Depreciation and amortization. Depreciation and amortization for dialysis and related lab services was approximately $76 million for the second quarter of 2012, $74 million for the first quarter of 2012 and $63 million for the second quarter of 2011. The increases in depreciation and amortization in the second quarter of 2012, as compared to both the first quarter of 2012 and the second quarter of 2011, was primarily due to growth in newly developed centers and from acquired centers.
Depreciation and amortization for dialysis and related lab services was approximately $149 million for the six months ended June 30, 2012, as compared to $123 million for the same period in 2011. The increase was primarily due to the same factors, as described above.
Provision for uncollectible accounts.The provision for uncollectible accounts receivable for dialysis and related lab services was 3.0% for both the second quarter of 2012 and the first quarter of 2012, and was 3.0% for the second quarter of 2011. We assess our level of the provision for uncollectible accounts based upon our historical cash collection experience and trends, and have and will continue to adjust the provision as necessary as a result of changes in our cash collections.
Legal proceeding contingency accrual and related expenses: We reached an agreement in principle to settle all allegations relating to claims arising out of the previously disclosed litigation filed in 2002 in the U.S. District Court in the Eastern District of Texas (Settlement). In connection with the Settlement we accrued a charge of approximately $78 million in the second quarter of 2012 that consist of $55 million for the settlement plus attorney fees and other related expenses. We expect that the Settlement will resolve federal program claims regarding EPO that were or could have been raised in the complaint relating to historical EPO practices dating back to 1997. The Settlement is subject to certain conditions, such as court approval. Until the conditions and documentation are completed, there can be no assurance that this matter will in fact be resolved pursuant to the terms of the Settlement. See Note 7 to the condensed consolidated financial statements for additional details.
Segment operating income
Dialysis and related lab services’ operating income for the second quarter of 2012 decreased by approximately $68 million, or approximately 19.2%, as compared to the first quarter of 2012, including the legal proceeding contingency accrual and related expenses of $78 million, as discussed above. Excluding this item, dialysis and related lab services adjusted operating income would have increased by $10 million. The increase in adjusted operating income was primarily due to strong volume growth, lower payroll taxes, lower accruals for self-insurance reserves, lower professional fees for legal and compliance matters, lower integration costs and from improvements in productivity. However, operating income was negatively impacted by an increase in pharmaceutical costs, higher labor and benefit costs and an increase in our expenses associated with our annual leadership meeting.
Dialysis and related lab services’ operating income for the second quarter of 2012 decreased by approximately $2 million, or approximately 0.7%, as compared to the second quarter of 2011, including the legal proceeding contingency accrual and related expenses of $78 million, as discussed above. Excluding this item,
S-111
Table of Contents
dialysis and related lab services adjusted operating income would have increased by $76 million. The increase in adjusted operating income was primarily attributable to strong volume growth in revenues from additional treatments as a result of non-acquired treatment growth and growth through acquisitions. Dialysis and related lab services’ operating income also increased as a result of lower overall pharmaceutical costs mainly from a decline in the intensities of physician-prescribed pharmaceuticals, lower accruals for self-insurance reserves and improvements in productivity, but was negatively impacted by higher labor costs and related payroll taxes, additional benefit costs and an increase in professional fees in conjunction with legal and compliance matters.
Dialysis and related lab services’ operating income for the six months ended June 30, 2012 increased by approximately $101 million, or approximately 18.7%, as compared to the same period in 2011, including the legal proceeding contingency accrual and related expenses of $78 million, as discussed above. Excluding this item, dialysis and related lab services adjusted operating income would have increased by $179 million. The increase in adjusted operating income was primarily attributable to strong volume growth in revenue from additional treatments as a result of non-acquired treatment growth and growth through acquisitions and as a result of one additional treatment day for the six months ended June 30, 2012. In addition, operating income also increased as a result of an increase in the average dialysis revenue per treatment of approximately $4, as described above. Dialysis and related lab services was also impacted by the same additional factors discussed above for the second quarter of 2012 as compared to the second quarter of 2011.
Other—Ancillary services and strategic initiatives
| Three months ended | Six months ended | |||||||||||||||||||
| June 30, 2012 | March 31, 2012 | June 30, 2011 | June 30, 2012 | June 30, 2011 | ||||||||||||||||
| (dollar amounts rounded to nearest million) | ||||||||||||||||||||
U.S. revenues | ||||||||||||||||||||
Net patient service revenues | $ | 2 | $ | 2 | $ | 2 | $ | 4 | $ | 3 | ||||||||||
Other revenues | 169 | 151 | 121 | 320 | 226 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total | 171 | 153 | 123 | 324 | 229 | |||||||||||||||
International revenues | ||||||||||||||||||||
Net patient service revenues | 3 | 1 | — | 4 | — | |||||||||||||||
Other revenues | 1 | 2 | — | 3 | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total | 4 | 3 | — | 7 | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total net operating revenues | $ | 175 | $ | 156 | $ | 123 | $ | 331 | $ | 229 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Segment operating loss | $ | (19 | ) | $ | (17 | ) | $ | (30 | ) | $ | (37 | ) | $ | (39 | ) | |||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net operating revenues
The ancillary services and strategic initiatives’ net operating revenues for the second quarter of 2012 increased by approximately $19 million as compared to the first quarter of 2012. The increase was primarily due to an increase in revenues in our pharmacy services due to volume growth, an increase in revenues associated with our disease management services and with our ESRD clinical research programs.
The ancillary services and strategic initiatives’ net operating revenues for the second quarter of 2012 increased by approximately $52 million, as compared to the second quarter of 2011. The increase was primarily due to volume growth in our pharmacy services, an increase in revenues in our disease management services, along with increases in revenues associated with our infusion therapy services, and our ESRD clinical research programs.
S-112
Table of Contents
The ancillary services and strategic initiatives’ net operating revenues for the six months ended June 30, 2012, increased by approximately $102 million as compared to the same period in 2011. The increase primarily due to the same factors as discussed for the increase in the second quarter of 2012 as compared to the second quarter of 2011.
Operating expenses
Ancillary services and strategic initiatives’ operating expenses for the second quarter of 2012 increased by approximately $21 million as compared to the first quarter of 2012. The increase in operating expenses was primarily due to volume growth associated with our pharmacy services, an increase in labor and benefit costs, an increase in claims expense in our disease management services and an increase in our professional fees associated with our international expansion.
Ancillary services and strategic initiatives’ operating expenses for the second quarter of 2012 increased by approximately $41 million as compared to the second quarter in 2011, which includes the $24 million of goodwill impairment charge. Excluding this item, ancillary services and strategic initiatives adjusted operating expenses would have increased by $65 million. The increase in adjusted operating expenses was primarily due to volume growth in our pharmacy services, an increase in claims expense, an increase in labor and benefit costs and an increase in our professional fees associated with our international expansion.
Ancillary services and strategic initiatives’ operating expenses for the six months ended June 30, 2012 increased by approximately $100 million as compared to the same period in 2011, which includes the $24 million of goodwill impairment charge recorded in the second quarter of 2011. Excluding this item, ancillary services and strategic initiatives adjusted operating expenses would have increased by approximately $124 million primarily due to the same factors as discussed for the increase in the second quarter of 2012 as compared to the second quarter of 2011.
Goodwill impairment
In the second quarter of 2011, we recorded a pre-tax non-cash impairment charge of $24 million as a result of a decrease in the implied fair value of goodwill below its carrying amount associated with our infusion therapy business.
Segment operating results
Ancillary services and strategic initiatives’ operating losses for the second quarter of 2012 increased by approximately $2 million as compared to the first quarter of 2012. The increase in operating losses was primarily due to additional losses associated with our international expansion and additional losses in our disease management services due to an increase in claim expenses, partially offset by improved performance in our pharmacy services.
Ancillary services and strategic initiatives’ operating losses for the second quarter of 2012 decreased by approximately $11 million, as compared to the second quarter of 2011, which includes the $24 million goodwill impairment charge. Excluding this item, ancillary services and strategic initiatives adjusted operating losses would have increased by $13 million. The increase in adjusted operating losses was primarily due to a decline in operating performance in our disease management services, additional operating losses associated with our new direct primary care services, as well as additional operating losses associated with our international expansion.
Ancillary services and strategic initiatives’ operating losses for the six months ended June 30, 2012 decreased by approximately $2 million, as compared to the same period in 2011, which includes the $24 million goodwill impairment charge recorded in the second quarter of 2011. Excluding this item, ancillary services and strategic initiatives adjusted operating losses would have increased by $22 million. The increase in adjusted operating losses was primarily due to the same factors as discussed for the increase in operating losses in the second quarter of 2012 as compared to the second quarter of 2011.
S-113
Table of Contents
Corporate-level charges
Stock-based compensation. Stock-based compensation of approximately $11.8 million in the second quarter of 2012 decreased from approximately $12.6 million in the first quarter of 2012 and from approximately $13.3 million in the second quarter of 2011. These decreases were primarily due to fewer stock-based awards granted in the first half of 2012 than in the same period in prior years.
Other corporate general and administrative expenses.Other corporate general and administrative expenses of $10.4 million increased by approximately $4 million in the second quarter of 2012 as compared to the first quarter of 2012, primarily due to additional acquisition related expenses associated with the proposed acquisition of HCP. Other corporate general and administrative expenses increased by $16 million for the six months ended June 30, 2012 as compared to the same period in 2011, due to the same factors as described above.
Other income.Other income for the second quarter of 2012 decreased by approximately $0.2 million as compared to the first quarter of 2012 and increased by approximately $0.3 million as compared to the second quarter of 2011.
Debt expense.Debt expense of $60.7 million decreased by approximately $0.7 million in the second quarter of 2012 as compared to the first quarter of 2012 and increased by $0.8 million as compared to the second quarter of 2011. The decrease in debt expense in the second quarter of 2012 as compared to the first quarter of 2012 was primarily due to lower average outstanding principal balances during the quarter. The increase in debt expense in the second quarter of 2012 as compared to the second quarter of 2011 was primarily due to additional borrowings associated with the new Term Loan A-2 which bear higher interest rates. The overall weighted average effective interest rate for both the second quarter of 2012 and for the first quarter of 2012 was 5.27%, compared to 5.33% for the second quarter of 2011.
For the six months ended June 30, 2012, debt expense increased by approximately $3.6 million, as compared to the same period in 2011. The increase was primarily attributable to the same factors that were discussed above for the increase in debt expense for the second quarter of 2012 as compared to the second quarter of 2011.
Equity investment income. Equity investment income was approximately $2.6 million for the second quarter of 2012, as compared to $2.6 million for the first quarter of 2012 and $2.4 million for the second quarter of 2011. The increases in equity income in the second quarter of 2012, as compared to the second quarter of 2011, were primarily due to improvements in the operating performance of certain joint ventures.
Noncontrolling interests
Net income attributable to noncontrolling interests. Net income attributable to noncontrolling interests was $24.7 million for the second quarter of 2012, as compared to $24.8 million for the first quarter of 2012 and $20.7 million for the second quarter of 2011. The increase in net income attributable to noncontrolling interests in the second quarter of 2012 as compared to the second quarter of 2011 was primarily due to an increase in the overall number of joint ventures and an increase in the overall profitability of our dialysis joint ventures.
Accounts receivable
Our accounts receivable balances at June 30, 2012 and March 31, 2012 were $1,250 million and $1,267 million, respectively, which represented approximately 60 days and 63 days of revenue, respectively, which is net of bad debt provision. The decrease in day sales outstanding (DSO), was primarily the result of improved cash collections from Medicare. Our DSO calculation is based on the current quarter’s average revenues per day. There were no significant changes during the second quarter of 2012 from the first quarter of 2012 in the amount of unreserved accounts receivable over one year old or the amounts pending approval from third-party payors.
S-114
Table of Contents
Liquidity and capital resources
Liquidity and capital resources.Cash flow from operations during the second quarter of 2012 was $202 million, compared to $204 million during the second quarter of 2011. Cash flow from operations in the second quarter of 2012 benefited from improved cash collections and the timing of certain working capital items, but was negatively impacted by an increase in income tax payments. Non-operating cash outflows for the second quarter of 2012 included capital asset expenditures of $138 million, including $71 million for new center developments and relocations and $67 million for maintenance and information technology. In addition, we spent $214 million for acquisitions. We paid distributions to noncontrolling interests of $24 million. Non-operating cash outflows for the second quarter of 2011 included capital asset expenditures of $87 million, including $39 million for new center developments and relocations and $48 million for maintenance and information technology. In addition, we spent $70 million for acquisitions. We paid distributions to noncontrolling interests of $24 million and repurchased 3.4 million shares of our common stock for $291 million.
Cash flow from operations during the six months ended June 30, 2012 was $534 million, compared to $534 million for the six months ended June 30, 2011. Cash flows from operations in 2012 benefited from improved cash collections, an increase in cash earnings and the timing of certain working capital items, but was offset by an increase in income tax payments. Non-operating cash outflows for the six months ended June 30, 2012 included capital asset expenditures of $251 million, including $129 million for new center developments and relocations and $122 million for maintenance and information technology. In addition, we spent $347 million for acquisitions. We paid distributions to noncontrolling interests of $50 million. Non-operating cash outflows for the first six months of 2011 included capital asset expenditures of $155 million, including $67 million for new center developments and relocations and $88 million for maintenance and information technology. In addition, we spent $151 million for acquisitions. We paid distributions to noncontrolling interests of $46 million and we repurchased 3.4 million shares of our common stock for approximately $291 million.
During the second quarter of 2012, we acquired a total of 33 dialysis centers, opened 14 dialysis centers and merged and sold four centers located in the U.S. In addition, we also opened and acquired a total of four centers outside of the U.S. During the second quarter of 2011, we acquired and opened a total of 27 dialysis centers located in the U.S.
During the first six months of 2012 we acquired a total of 61 dialysis centers, opened 27 dialysis centers and merged or sold four centers located in the U.S. In addition, we also acquired and opened a total of eight centers outside the U.S. For the six months ended June 30, 2011, we acquired and opened a total of 60 dialysis centers, merged two centers and sold one center located in the U.S.
During the first six months of 2012, we made mandatory principal payments under our Senior Secured Credit Facilities totaling $25.0 million on the Term Loan A, $1.0 million on the Term Loan A-2 and $8.8 million on the Term Loan B.
As of June 30, 2012, we maintained a total of nine interest rate swap agreements with amortizing notional amounts totaling $925 million. These agreements had the economic effect of modifying the LIBOR variable component of our interest rate on an equivalent amount of our Term Loan A to fixed rates ranging from 1.59% to 1.64%, resulting in an overall weighted average effective interest rate of 4.11%, including the Term Loan A margin of 2.50%. The swap agreements expire by September 30, 2014 and require monthly interest payments. During the six months ended June 30, 2012, we accrued net charges of $6.5 million from these swaps which are included in debt expense. As of June 30, 2012, the total fair value of these swap agreements was a liability of $22.7 million. We estimate that approximately $11.4 million of existing unrealized pre-tax losses in other comprehensive income at June 30, 2012 will be reclassified into income over the next twelve months.
As of June 30, 2012, we maintained five interest rate cap agreements with notional amounts totaling $1.25 billion. These agreements have the economic effect of capping the LIBOR variable component of our interest
S-115
Table of Contents
rate at a maximum of 4.00% on an equivalent amount of our Term Loan B debt. The cap agreements expire on September 30, 2014. As of June 30, 2012, the total fair value of these cap agreements was an asset of $0.3 million. During the six months ended June 30, 2012, we recorded $0.7 million, net of tax, as a decrease to other comprehensive income due to unrealized valuation changes in the cap agreements.
As a result of the swap and cap agreements, our overall weighted average effective interest rate on the Senior Secured Credit Facilities was 4.61%, based upon the current margins in effect of 2.50% for the Term Loan A, 3.50% for the Term Loan A-2 and 3.00% for the Term Loan B, as of June 30, 2012.
As of June 30, 2012, interest rates on our Term Loan A-2 and Term Loan B debt are set at their interest rate floors. Interest rates on our senior notes and Term Loan A are fixed and economically fixed, respectively, while rates on $1.25 billion of our Term Loan B are subject to interest rate caps.
Our overall weighted average effective interest rate during the second quarter of 2012 was 5.27% and as of June 30, 2012 was 5.28%.
As of June 30, 2012, we had undrawn revolving credit facilities totaling $350 million of which approximately $49 million was committed for outstanding letters of credit.
We believe that we will have sufficient liquidity and will generate significant operating cash flows to fund our scheduled debt service and other obligations for the foreseeable future, including the next 12 months, under the terms of our debt agreements. Our primary sources of liquidity are cash from operations and cash from borrowings.
Acquisition of DSI Renal Inc.
On September 2, 2011, we completed our acquisition of all of the outstanding common stock of CDSI I Holding Company, Inc., the parent company of dialysis provider DSI Renal Inc., or DSI, pursuant to an agreement and plan of merger for approximately $723 million in net cash, plus the assumption of certain liabilities totaling approximately $6.5 million, subject to certain post-closing adjustments. DSI had 113 outpatient dialysis centers that provided services to approximately 8,000 patients in 23 states. We also incurred approximately $22 million in transaction and integration costs during the year ended December 31, 2011 associated with this acquisition that are included in general and administrative expenses.
Pursuant to a consent order issued by the Federal Trade Commission on September 2, 2011, we agreed to divest a total of 30 outpatient dialysis centers and several home-based dialysis programs in order to complete the acquisition of DSI. In conjunction with the consent order, on September 30, 2011, we completed the sale of 28 outpatient dialysis centers to Dialysis Newco, Inc., or Dialysis Newco, a portfolio company of Frazier Healthcare VI, L.P. and New Enterprise Associates 13, Limited Partnership pursuant to an asset purchase agreement dated August 26, 2011. Effective October 31, 2011, we also completed the sale of two additional outpatient dialysis centers to Dialysis Newco that were previously pending state regulatory approval. We anticipate receiving total net cash consideration of approximately $82.0 million for all of the outpatient dialysis centers that were divested.
Acquisition of HealthCare Partners Holdings, LLC
On May 20, 2012, we entered into a definitive merger agreement to acquire HCP, the country’s largest operator of medical groups and physician networks. HCP is a patient- and physician-focused integrated health care delivery and management company providing coordinated, outcomes-based medical care in a cost-effective manner. For the year ended December 31, 2011, HCP generated approximately $2.4 billion in revenues and approximately $488 million in operating income.
S-116
Table of Contents
The total purchase price to be paid by the Company. will consist of $3.66 billion in cash and approximately 9.38 million shares of Company common stock, subject to post-close adjustments. In addition to the total merger consideration payable at close, the Company will pay to the owners of HCP a total of up to $275 million of additional cash consideration in the form of two separate earn-out payments if certain financial performance targets are achieved by HCP in 2012 and 2013. We still expect the transaction to close early in the fourth quarter of this year.
2011 capital structure changes and other items
On August 26, 2011, we entered into an Increase Joinder Agreement under our existing Senior Secured Credit Agreement. Pursuant to the Increase Joinder Agreement, we increased the revolving credit facility by $100 million, to a total of $350 million, and entered into an additional $200 million Term Loan A-2. The new Term Loan A-2 required a principal payment of $0.5 million on December 31, 2011 and thereafter requires annual principal payments of $2.0 million with the balance of $191.5 million due in 2016, and bears interest at LIBOR (floor of 1.00%) plus an interest rate margin of 3.50% subject to a rating based step-down to 3.25%.
During the year ended December 31, 2011 we made mandatory principal payments under our Senior Secured Credit Facilities totaling $50 million on the Term Loan A, $0.5 million on Term Loan A-2 and $17.5 million on the Term Loan B.
2010 capital structure changes
On October 20, 2010, we entered into a $3,000 million Senior Secured Credit Agreement, or the Credit Agreement, consisting of a five year $250 million revolving line of credit, a five year $1,000 million Term Loan A and a six year $1,750 million Term Loan B. We also have the right to request an increase to the borrowing capacity to a total aggregate principal amount of not more than $4,000 million subject to bank participation. The revolving line of credit and the Term Loan A initially bore interest at LIBOR plus an interest rate margin of 2.75% until June 30, 2011, when the interest rate margin was reduced to 2.50%. The interest rate margin is still subject to adjustment depending upon our leverage ratio and can range from 2.25% to 2.75%. The Term Loan A requires annual principal payments of $50 million in 2011, $50 million in 2012, $100 million in 2013, and $150 million in 2014, with the balance of $650 million due in 2015. The Term Loan B bears interest at LIBOR (floor of 1.50%) plus 3.00% subject to a ratings based step-down to 2.75%. The Term Loan B requires annual principal payments of $17.5 million in each year from 2011 through 2015 with the balance of $1,663 million due in 2016. The borrowings under the Credit Agreement are guaranteed by substantially all of our direct and indirect wholly-owned domestic subsidiaries and are secured by substantially all of DaVita’s and its guarantors’ assets. The Credit Agreement contains customary affirmative and negative covenants such as various restrictions on investments, acquisitions, the payment of dividends, redemptions and acquisitions of capital stock, capital expenditures and other indebtedness, as well as limitations on the amount of tangible net assets in non-guarantor subsidiaries. However, many of these restrictions will not apply as long as our leverage ratio is below 3.50:1.00. In addition, the Credit Agreement requires compliance with financial covenants including an interest coverage ratio and a leverage ratio that determines the interest rate margins as described above.
On October 20, 2010, we also issued $775 million aggregate principal amount of 6 3/8% senior notes due 2018 and $775 million aggregate principal amount of 6 5/8% senior notes due 2020 (the New Senior Notes). The New Senior Notes will pay interest on May 1 and November 1, of each year beginning May 1, 2011. The New Senior Notes are unsecured senior obligations and rank equally to other unsecured senior indebtedness. The New Senior Notes are guaranteed by substantially all of our direct and indirect wholly-owned domestic subsidiaries. We may redeem some or all of the 6 3/8% senior notes at any time on or after November 1, 2013 at certain redemption prices and may redeem some or all of the 6 5/8% senior notes at any time on or after November 1, 2014 at certain redemption prices.
We received total proceeds of $4,300 million from these transactions, $2,750 million from the borrowings on Term Loan A and Term Loan B and an additional $1,550 million from the issuance of the New Senior Notes.
S-117
Table of Contents
We used a portion of the proceeds to pay-off the outstanding principal balances of our existing senior secured credit facilities plus accrued interest totaling $1,795 million and to purchase pursuant to a cash tender offer $558 million of the outstanding principal balances of our $700 million 6 5/8% senior notes due 2013 and $731 million of the outstanding balances of our $850 million 7 1/4% senior subordinated notes due 2015 (the Existing Notes), plus accrued interest totaling $1,297 million. The total amount paid for the Existing Notes was $1,019.06 per $1,000 principal amount of the 6 5/8% senior notes and $1,038.75 per $1,000 principal amount of the 7 1/4% senior subordinated notes. This resulted in us paying a cash tender premium of $39 million in order to extinguish this portion of the Existing Notes. On November 19, 2010, we redeemed the remaining outstanding balance of the existing 6 5/8% senior notes of $142 million at 101.656% per $1,000 and the remaining outstanding balance of the existing 7 1/4% senior subordinated notes of $119 million at 103.625% per $1,000 plus accrued interest totaling $265 million. In addition, we paid a call premium totaling $7 million. We also paid an additional $74 million in fees, discounts and other expenses. As a result of the above transactions, we received approximately $823 million in excess cash which we have been using for general purposes and other opportunities, including share repurchases, acquisitions and other growth investments.
In connection with these transactions, we expensed debt refinancing and redemption charges totaling $70.3 million in the fourth quarter of 2010, which includes the write off of certain existing deferred financing costs and other new financing costs, the cash tender and call premiums, as described above and other expenses.
On June 7, 2010, we redeemed $200 million aggregate principal amount of our outstanding 6 5/8% senior notes due 2013, at a price of 101.656% plus accrued interest. As a result of this transaction, we expensed debt redemption charges of $4.1 million, which includes the call premium and the net write-off of other finance costs.
Interest rate swaps and caps
In January 2011, we entered into nine interest rate swap agreements with amortizing notional amounts totaling $1.0 billion that went effective on January 31, 2011, as a means of hedging our exposure to and volatility from variable-based interest rate changes as part of our overall risk management strategy. As of December 31, 2011, we maintained a total of nine interest rate swap agreements with amortizing notional amounts totaling $950 million. These agreements had the economic effect of modifying the LIBOR variable component of our interest rate on an equivalent amount of our Term Loan A to fixed rates ranging from 1.59% to 1.64%, resulting in an overall weighted average effective interest rate of 4.11%, including the Term Loan A margin of 2.50%. The swap agreements expire by September 30, 2014 and require monthly interest payments. During the year ended December 31, 2011, we accrued net charges of $12.6 million from these swaps which are included in debt expense. As of December 31, 2011, the total fair value of these swap agreements was a liability of $23.1 million. We estimate that approximately $10.9 million of existing unrealized pre-tax losses in other comprehensive income at December 31, 2011 will be reclassified into income over the next year.
In addition, in January 2011, we also entered into five interest rate cap agreements with notional amounts totaling $1.25 billion that went effective on January 31, 2011. These agreements have the economic effect of capping the LIBOR variable component of our interest rate at a maximum of 4.00% on an equivalent amount of our Term Loan B debt. The cap agreements expire on September 30, 2014. As of December 31, 2011, the total fair value of these cap agreements was an asset of $1.4 million. During the year ended December 31, 2011, we recorded $5.2 million, net of tax, as a decrease to other comprehensive income due to unrealized valuation changes in the cap agreements, net of the amortization of the interest rate cap premiums that were reclassified into net income.
As a result of the swap and cap agreements, our overall weighted average effective interest rate on the Senior Secured Credit Facilities was 4.61%, based upon the current margins in effect of 2.50% for the Term Loan A, 3.50% for the Term Loan A-2 and 3.00% for the Term Loan B, as of December 31, 2011.
S-118
Table of Contents
As of December 31, 2011, interest rates on our Term Loan A-2 and Term Loan B are set at their LIBOR floors plus their interest rate margins. Interest rates on our senior notes and Term Loan A are fixed and economically fixed, respectively, with rates on the $1.25 billion of our Term Loan B subject to interest rate caps.
Our overall weighted average effective interest rate in 2011 was 5.28% and as of December 31, 2011 was 5.27%.
Senior Secured Credit Facilities and senior and senior subordinated notes
During 2010, we made mandatory principal payments totaling $65.6 million on our prior outstanding Term Loan A. We did not make any principal payments on our prior Term Loan B during 2010, nor were we required to.
All of the outstanding balances under the prior Term Loan A, Term Loan B and the senior and senior subordinated notes were extinguished as part of our debt refinancing transactions that occurred on October 20, 2010, as described above.
Stock repurchases
During 2010, we repurchased a total of 8,918,760 shares of our common stock for $618.5 million, or an average price of $69.35 per share, pursuant to previously announced authorizations by the Board of Directors.
During 2011, we repurchased a total of 3,794,686 shares of our common stock for $323.3 million, or an average price of $85.21 per share, pursuant to a previously announced authorization by the Board of Directors on November 3, 2010, that authorized an additional $800 million of share repurchases of our common stock. As a result of these transactions, the total outstanding authorization for share repurchases as of June 30, 2011 was $358.2 million. We have not repurchased any additional shares of our common stock from January 1, 2012 through June 30, 2012. This stock repurchase program has no expiration date.
Stock-based compensation
Stock-based compensation recognized in a period represents the amortization during that period of the estimated grant-date fair value of current and prior stock-based awards over their vesting terms, adjusted for expected forfeitures. Shares issued upon exercise of stock awards are generally issued from shares in treasury. We have used the Black-Scholes-Merton valuation model for estimating the grant-date fair value of stock-settled stock appreciation rights granted in all periods. During the six months ended June 30, 2012, we granted 0.3 million stock-settled stock appreciation rights with an aggregate grant-date fair value of $5.8 million and a weighted-average expected life of approximately 3.5 years, and also granted 11,000 stock units with an aggregate grant-date fair value of $0.9 million and a weighted-average expected life of approximately 1.6 years.
For the six months ended June 30, 2012 and 2011, we recognized $24.3 million and $23.1 million, respectively, in stock-based compensation expense for stock appreciation rights, stock units and discounted employee stock plan purchases, which are primarily included in general and administrative expenses. The estimated tax benefits recorded for stock-based compensation through June 30, 2012 and 2011 was $9.2 million and $8.8 million, respectively. As of June 30, 2012, there was $74.5 million of total estimated unrecognized compensation cost related to nonvested stock-based compensation arrangements under our equity compensation and stock purchase plans. We expect to recognize this cost over a weighted average remaining period of 1.3 years.
During the six months ended June 30, 2012 and 2011, we received $2.0 million and $5.2 million, respectively, in cash proceeds from stock option exercises and $27.6 million and $33.8 million, respectively, in actual tax benefits upon the exercise of stock awards.
S-119
Table of Contents
Other items
On July 22, 2010, we entered into a First National Service Provider Agreement, or the Agreement, with NxStage Medical Inc., or NxStage. Under the terms of the Agreement we have the ability to continue to purchase NxStage System One hemodialysis machines and related supplies at discount prices. In addition, we may, in lieu of cash rebate, vest in warrants to purchase NxStage common stock based upon achieving certain System One home patient growth targets by June 30, 2011, 2012 and 2013. The warrants are exercisable for up to a cumulative total of 5.5 million shares of common stock over the three years at an initial exercise price of $14.22 per share. From the period July 1, 2010 through June 30, 2011, we earned warrants to purchase 250,000 shares of NxStage common stock. In October 2011 we exercised our right to purchase 250,000 shares of NxStage common stock at $14.22 per share, for a total of approximately $3.6 million and in February 2012, we sold all 250,000 shares for approximately $5.2 million.
In July 2010, we announced that we will construct a new corporate headquarters in Denver, Colorado. In July 2010, we acquired the land and existing improvements for approximately $12 million. Effective December 18, 2010, we entered into a construction agreement for the construction of the new building. We currently estimate the total construction costs and other project costs of the building will be approximately $95 million. Construction began in early 2011, and is still estimated to be complete in the second half of 2012. Through December 31, 2011, we have paid construction costs and architecture and other design fees totaling approximately $49 million.
Off-balance sheet arrangements and aggregate contractual obligations
In addition to the debt obligations reflected on our balance sheet, we have commitments associated with operating leases and letters of credit, as well as potential obligations associated with our equity investments in nonconsolidated businesses and to dialysis centers that are wholly-owned by third parties. Substantially all of our facilities are leased. We have potential acquisition obligations for several joint ventures and for some of our non-wholly-owned subsidiaries in the form of put provisions. If these put provisions were exercised, we would be required to purchase the third-party owners’ noncontrolling interests at either the appraised fair market value or a predetermined multiple of earnings or cash flow attributable to the noncontrolling interests put to us, which is intended to approximate fair value. The methodology we use to estimate the fair values of noncontrolling interests subject to put provisions assumes either the higher of a liquidation value of net assets or an average multiple of earnings, based on historical earnings, patient mix and other performance indicators, as well as other factors. The estimated fair values of the noncontrolling interests subject to put provisions is a critical accounting estimate that involves significant judgments and assumptions and may not be indicative of the actual values at which the noncontrolling interests may ultimately be settled, which could vary significantly from our current estimates. The estimated fair values of noncontrolling interests subject to put provisions can fluctuate and the implicit multiple of earnings at which these noncontrolling interests obligations may be settled will vary significantly depending upon market conditions including potential purchasers’ access to the capital markets, which can impact the level of competition for dialysis and non-dialysis related businesses, the economic performance of these businesses and the restricted marketability of the third-party owners’ noncontrolling interests. The amount of noncontrolling interests subject to put provisions that contractually employ a predetermined multiple of earnings rather than fair value are immaterial. For additional information see Note 10 to the condensed consolidated financial statements in our quarterly report on Form 10-Q for the quarterly period ended June 30, 2012 as incorporated by reference.
We also have certain potential commitments to provide operating capital to several dialysis centers that are wholly-owned by third parties or centers in which we own a minority equity investment as well as to physician-owned vascular access clinics that we operate under management and administrative services agreements.
S-120
Table of Contents
The following is a summary of these contractual obligations and commitments as of June 30, 2012 (in millions):
| Remainder of 2012 | 1-3 years | 4-5 years | After 5 years | Total | ||||||||||||||||
Scheduled payments under contractual obligations: | ||||||||||||||||||||
Long-term debt | $ | 41 | $ | 301 | $ | 2,537 | $ | 1,558 | $ | 4,437 | ||||||||||
Interest payments on the senior notes | 50 | 202 | 202 | 279 | 733 | |||||||||||||||
Interest payments on the Term Loan B(1) | 40 | 155 | 137 | — | 332 | |||||||||||||||
Interest payments on Term Loan A-2(2) | 4 | 18 | 16 | — | 38 | |||||||||||||||
Capital lease obligations | 1 | 7 | 7 | 53 | 68 | |||||||||||||||
Operating leases | 143 | 503 | 430 | 806 | 1,882 | |||||||||||||||
Construction of the new corporate headquarters | 20 | — | — | — | 20 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
| $ | 299 | $ | 1,186 | $ | 3,329 | $ | 2,696 | $ | 7,510 | |||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Potential cash requirements under existing commitments: | ||||||||||||||||||||
Letters of credit | $ | 49 | $ | — | $ | — | $ | — | $ | 49 | ||||||||||
Noncontrolling interests subject to put provisions | 307 | 90 | 73 | 53 | 523 | |||||||||||||||
Pay-fixed swaps potential obligations | 6 | 17 | — | — | 23 | |||||||||||||||
Operating capital advances | 4 | — | — | — | 4 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
| $ | 366 | $ | 107 | $ | 73 | $ | 53 | $ | 599 | |||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
| (1) | Assuming no changes to LIBOR-based interest rates as the Term Loan B currently bears interest at LIBOR (floor of 1.50%) plus an interest rate margin of 3.00%. |
| (2) | Assuming no changes to LIBOR-based interest rates as the Term Loan A-2 currently bears interest at LIBOR (floor of 1.00%) plus an interest rate margin of 3.50%. |
The pay-fixed swap obligations represent the estimated fair market values of our interest rate swap agreements that are based upon valuation models utilizing the income approach and commonly accepted valuation techniques that use inputs from closing prices for similar assets and liabilities in active markets as well as other relevant observable market inputs and other current market conditions that existed as of June 30, 2012. This amount represents the estimated potential obligation that we would be required to pay based upon the estimated future settlement of each specific tranche over the term of the swap agreements, assuming no future changes in the forward yield curve. The actual amount of our obligation associated with these swaps in the future will depend upon changes in the LIBOR-based interest rates that can fluctuate significantly depending upon market conditions, and other relevant factors that can affect the fair market value of these swap agreements.
In addition to the above commitments, we are obligated to purchase a certain amount of our hemodialysis products and supplies at fixed prices through 2015 from Gambro Renal Products, Inc. in connection with the Alliance and Product Supply Agreement. Our total expenditures for the six months ended June 30, 2012 on such products were approximately 2% of our total operating costs. In addition, we are obligated to purchase a certain amount of dialysis equipment, parts and supplies from Fresenius Medical Care, or Fresenius, through 2013. Our total expenditures for the six months ended June 30, 2012 on such products were approximately 2% of our total operating costs.
The actual amount of purchases in future years from Gambro Renal Products and Fresenius will depend upon a number of factors, including the operating requirements of our centers, the number of centers we acquire, growth of our existing centers, and in the case of the Alliance and Product Supply Agreement, Gambro Renal Products’ ability to meet our needs.
In November 2011, we entered into a seven year Sourcing and Supply Agreement with Amgen USA Inc. that expires on December 31, 2018. Under the terms of the agreement we will purchase EPO in amounts necessary to meet no less than 90% of our requirements for erythropoiesis stimulating agents. The actual amount of EPO that we will purchase from Amgen will depend upon the amount of EPO administered during dialysis as prescribed by physicians and the overall number of patients that we serve.
S-121
Table of Contents
Settlements of approximately $14 million of existing income tax liabilities for unrecognized tax benefits are excluded from the above table as reasonably reliable estimates of the timing cannot be made.
Contingencies
The information in Note 16 to the consolidated financial statements in our Annual Report on Form 10-K is incorporated by reference in response to this item.
Critical accounting estimates and judgments
Our consolidated financial statements and accompanying notes are prepared in accordance with U.S. generally accepted accounting principles. These accounting principles require us to make estimates, judgments and assumptions that affect the reported amounts of revenues, expenses, assets, liabilities, contingencies and temporary equity. All significant estimates, judgments and assumptions are developed based on the best information available to us at the time made and are regularly reviewed and updated when necessary. Actual results will generally differ from these estimates. Changes in estimates are reflected in our financial statements in the period of change based upon on-going actual experience trends, or subsequent settlements and realizations depending on the nature and predictability of the estimates and contingencies. Interim changes in estimates are applied prospectively within annual periods. Certain accounting estimates, including those concerning revenue recognition and accounts receivable, impairments of long-lived assets, accounting for income taxes, quarterly and annual variable compensation accruals, purchase accounting valuation estimates, fair value estimates and stock-based compensation are considered to be critical to evaluating and understanding our financial results because they involve inherently uncertain matters and their application requires the most difficult and complex judgments and estimates.
Revenue recognition and accounts receivable. There are significant estimating risks associated with the amount of revenue that we recognize in a given reporting period. Payment rates are often subject to significant uncertainties related to wide variations in the coverage terms of the commercial healthcare plans under which we receive payments. In addition, ongoing insurance coverage changes, geographic coverage differences, differing interpretations of contract coverage, and other payor issues complicate the billing and collection process. Net revenue recognition and allowances for uncollectible billings require the use of estimates of the amounts that will ultimately be realized considering, among other items, retroactive adjustments that may be associated with regulatory reviews, audits, billing reviews and other matters.
Revenues associated with Medicare and Medicaid programs are recognized based on (a) the payment rates that are established by statute or regulation for the portion of the payment rates paid by the government payor (e.g., 80% for Medicare patients) and (b) for the portion not paid by the primary government payor, the estimated amounts that will ultimately be collectible from other government programs paying secondary coverage (e.g., Medicaid secondary coverage), the patient’s commercial health plan secondary coverage, or the patient. Effective January 1, 2011, our reimbursements from Medicare became subject to certain variations under Medicare’s new single bundled payment rate system whereby our reimbursements can be adjusted for certain patient characteristics and certain other factors. Our revenue recognition depends upon our ability to effectively capture, document and bill for Medicare’s base payment rate and these other factors. In addition, as a result of the potential range of variations that can occur in our reimbursements from Medicare under the new single bundled payment rate system, our revenue recognition is now subject to a greater degree of estimating risk.
Commercial healthcare plans, including contracted managed-care payors, are billed at our usual and customary rates; however, revenue is recognized based on estimated net realizable revenue for the services provided. Net realizable revenue is estimated based on contractual terms for the patients under healthcare plans with which we have formal agreements, non-contracted healthcare plan coverage terms if known, estimated secondary collections, historical collection experience, historical trends of refunds and payor payment adjustments (retractions), inefficiencies in our billing and collection processes that can result in denied claims for
S-122
Table of Contents
payments, slow down in collections, a reduction in the amounts that we expect to collect and regulatory compliance issues. Determining applicable primary and secondary coverage for our more than 142,000 patients at any point in time, together with the changes in patient coverages that occur each month, requires complex, resource-intensive processes. Collections, refunds and payor retractions typically continue to occur for up to three years or longer after services are provided.
We generally expect our range of dialysis and related lab services revenues estimating risk to be within 1% of its revenue, which can represent as much as 6% of consolidated operating income. Changes in estimates are reflected in the then-current financial statements based on on-going actual experience trends, or subsequent settlements and realizations depending on the nature and predictability of the estimates and contingencies. Changes in revenue estimates for prior periods are separately disclosed and reported if material to the current reporting period and longer term trend analyses, and have not been significant.
Lab service revenues for current period dates of services are recognized at the estimated net realizable amounts to be received.
Impairments of long-lived assets. We account for impairments of long-lived assets, which include property and equipment, equity investments in non-consolidated businesses, amortizable intangible assets with finite useful lives and goodwill, in accordance with the provisions of applicable accounting guidance. Impairment reviews are performed at least annually and whenever a change in condition occurs which indicates that the carrying amounts of assets may not be recoverable.
Such changes include changes in our business strategies and plans, changes in the quality or structure of our relationships with our partners and deteriorating operating performance of individual dialysis centers or other operations. We use a variety of factors to assess the realizable value of assets depending on their nature and use. Such assessments are primarily based upon the sum of expected future undiscounted net cash flows over the expected period the asset will be utilized, as well as market values and conditions. The computation of expected future undiscounted net cash flows can be complex and involves a number of subjective assumptions. Any changes in these factors or assumptions could impact the assessed value of an asset and result in an impairment charge equal to the amount by which its carrying value exceeds its actual or estimated fair value.
Accounting for income taxes. We estimate our income tax provision to recognize our tax expense for the current year, and our deferred tax liabilities and assets for future tax consequences of events that have been recognized in our financial statements, measured using enacted tax rates and laws expected to apply in the periods when the deferred tax liabilities or assets are expected to be realized. We are required to assess our tax positions on a more-likely-than-not criteria and to also determine the actual amount of benefit to recognize in the financial statements. Deferred tax assets are assessed based upon the likelihood of recoverability from future taxable income and, to the extent that recovery is not likely, a valuation allowance is established. The allowance is regularly reviewed and updated for changes in circumstances that would cause a change in judgment about the realizability of the related deferred tax assets. These calculations and assessments involve complex estimates and judgments because the ultimate tax outcome can be uncertain and future events unpredictable.
Variable compensation accruals. We estimate variable compensation accruals quarterly based upon the annual amounts expected to be earned and paid out resulting from the achievement of certain teammate-specific and/or corporate financial and operating goals. Our estimates, which include compensation incentives for bonuses, and other awards, are updated periodically based on changes in our economic condition or cash flows that could ultimately impact the actual final award. Actual results reflected in each fiscal quarter may vary due to the subjectivity involved in anticipating fulfillment of specific and/or corporate goals, as well as the final determination and approval of amounts by our Board of Directors.
S-123
Table of Contents
Purchase accounting valuation estimates. We make various assumptions and estimates regarding the valuation of tangible and intangible assets, liabilities, noncontrolling interests and contractual as well as non-contractual contingencies associated with our acquisitions. These assumptions can have a material effect on our balance sheet valuations and the related amount of depreciation and amortization expense that will be recognized in the future.
Fair value estimates. We have recorded certain assets, liabilities and noncontrolling interests (temporary equity) subject to put provisions at fair value. The FASB defines fair value which is measured based upon certain valuation techniques that include inputs and assumptions that market participants would use in pricing assets, liabilities and noncontrolling interests subject to put provisions. We have measured the fair values of our applicable assets, liabilities and noncontrolling interests subject to put provisions based upon certain market inputs and assumptions that are either observable or unobservable in determining fair values and have also classified these assets, liabilities and noncontrolling interests subject to put provisions into the appropriate fair value hierarchy levels. The fair value of our investments available for sale are based upon quoted market prices from active markets and the fair value of our swap and cap agreements were based upon valuation models utilizing the income approach and commonly accepted valuation techniques that use inputs from closing prices for similar assets and liabilities in active markets as well as other relevant observable market inputs at quoted intervals such as current interest rates, forward yield curves, implied volatility and credit default swap pricing. For our noncontrolling interests subject to put provisions we have estimated the fair values of these based upon either the higher of a liquidation value of net assets or an average multiple of earnings based on historical earnings, patient mix and other performance indicators, as well as other factors. During the second quarter of 2010, we refined the methodology used to estimate the fair value of noncontrolling interests subject to put provisions by eliminating an annual inflation factor that was previously applied to the put provisions until they became exercisable. We believe that eliminating an annual inflation factor will result in a better representation of the estimated actual fair value of the noncontrolling interests subject to put provisions. The estimate of the fair values of the noncontrolling interests subject to put provisions involves significant judgments and assumptions and may not be indicative of the actual values at which the noncontrolling interests may ultimately be settled, which could vary significantly from our current estimates. The estimated fair values of the noncontrolling interests subject to put provisions can also fluctuate and the implicit multiple of earnings at which these noncontrolling interests obligations may be settled will vary depending upon market conditions including potential purchasers’ access to the capital markets, which can impact the level of competition for dialysis and non-dialysis related businesses, the economic performance of these businesses and the restricted marketability of the third-party owners’ noncontrolling interests.
Stock-based compensation. Stock-based compensation recognized in a period represents the straight-line amortization during that period of the estimated grant-date fair value of stock-based awards over their vesting terms, adjusted for expected forfeitures. We estimate the grant-date fair value of stock awards using complex option pricing models that rely heavily on estimates from us about uncertain future events, including the expected term of the awards, the expected future volatility of our stock price, and expected future risk-free interest rates.
Significant new accounting standards
On January 1, 2012, we adopted FASB’s Accounting Standard Update (ASU) No. 2011-08,Intangibles—Goodwill and Other. This standard amends the two-step goodwill impairment test required under the prior accounting guidance. This amendment allows reporting entities the option to first assess certain qualitative factors to ascertain whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount to determine if the two-step impairment test is necessary. If an entity concludes that certain events or circumstances demonstrate that it is more likely than not that the fair value of a reporting unit is less than its carrying amount, then the an entity is required to proceed to step one of the two-step goodwill impairment test. This standard was effective on January 1, 2012. The adoption of this standard did not have a material impact on our consolidated financial statements.
S-124
Table of Contents
On January 1, 2012, we adopted FASB’s ASU No. 2011-07,Health Care Entities-Presentation and Disclosure of Patient Service Revenue, Provision for Bad Debts, and the Allowance for Doubtful Accounts.This standard amends the prior presentation and disclosure requirements for Health Care Entities that recognize significant amounts of patient service revenues at the time the services are rendered without assessing the patient’s ability to pay. This standard requires health care entities to reclassify the provision for bad debts from an operating expense to a deduction from patient service revenues. In addition, this standard requires more disclosure on the policies for recognizing revenue, assessing bad debts, as well as quantitative and qualitative information regarding changes in the allowance for doubtful accounts. This standard was applied retrospectively to all prior periods presented and was effective on January 1, 2012. Upon adoption of this standard, we changed our presentation of our provision for uncollectible accounts related to patient service revenues as a deduction from our patient service operating revenues and enhanced our disclosures as indicated above. See Note 4 to the condensed consolidated financial statements for further details in our quarterly report on Form 10-Q for the period ended June 30, 2012 as incorporated by reference.
On January 1, 2012, we adopted FASB’s ASU No. 2011-05 as amended by ASU No. 2011-12,Comprehensive Income—Presentation of Comprehensive Income. This standard amends the prior presentation requirements for comprehensive income by eliminating the presentation of the components of other comprehensive income within the statement of equity. This standard allows two options on how to present the various components of comprehensive income. These options are either to report the components of comprehensive income separately on the income statement or to present total other comprehensive income and the components of other comprehensive income in a separate statement. This standard does not change the items that must be reported in other comprehensive income or when an item must be reclassified into net income. The FASB temporarily deferred the requirement to present separate line items on the statement of income for the amounts that are realized and reclassified out of accumulated other comprehensive income into net income. No timetable has been set for FASB’s reconsideration of this item. This standard, except for the requirements that were deferred, as stated above, was applied retrospectively and was effective on January 1, 2012. Upon adoption of this standard, we presented total other comprehensive income and the components of other comprehensive income in a separate statement of comprehensive income.
On January 1, 2012, we adopted FASB’s ASU No. 2011-04,Fair Value Measurement.This standard amends the current fair value measurement and disclosure requirements to improve comparability between U.S. GAAP and International Financial Reporting Standards, or IFRS. The intent of this standard is to update the disclosures that describe several of the requirements in U.S. GAAP for measuring fair value and to enhance disclosures about fair value measurements which will improve consistency between U.S. GAAP and IFRS. This standard does not change the application of the requirements on fair value measurements and disclosures. This was applied prospectively and was effective on January 1, 2012. The adoption of this standard did not have a material impact on our consolidated financial statements.
HCP
Overview and Recent Developments
HCP is a patient- and physician-focused, integrated health care delivery and management company with nearly three decades of providing coordinated, outcomes-based medical care in a cost-effective manner. Through capitation contracts with some of the nation’s leading health plans, as of June 30, 2012, HCP had approximately 669,400 current members under its care in southern California, central and south Florida and southern Nevada. Of these, approximately 190,700 individuals represented patients enrolled in Medicare Advantage. The remaining approximately 478,700 individuals represented managed care members whose health coverage is provided through their employer or who have individually acquired health coverage directly from a health plan or as a result of their eligibility for Medicaid benefits. In addition, during 2011, HCP provided care to over 412,000 fee-for-service patients.
S-125
Table of Contents
The patients of HCP’s affiliated physicians, physician groups and IPAs benefit from an integrated approach to medical care that places the physician at the center of patient care. As of June 30, 2012, HCP delivered services to its members via a network of over 1,800 affiliated group and other network primary care physicians, 139 network hospitals, and several thousand affiliated group and network specialists. Together with hundreds of case managers, registered nurses and other care coordinators, these medical professionals utilize a comprehensive data analysis engine, sophisticated risk management techniques and clinical protocols to provide high-quality, cost effective care to HCP’s members. HCP monitors certain control metrics, such as the number of inpatient acute bed days per 1,000 patients and hospital readmission rates, as they are contributors to quality clinical outcomes and HCP’s financial performance. HCP endeavors to stay informed of any changes to the Medicare and Medicare Advantage programs as such changes may affect its financial performance. Additionally, in an effort to identify changes or trends with respect to its commercial, senior and Medicaid payer classifications, HCP closely monitors the number of managed care members who have enrolled with an HCP employed or affiliated physician as their primary care physician.
On April 16, 2012, HCP acquired the assets of Cardiovascular Consultants of Nevada, Inc., or Cardiovascular Consultants, a physician group practice providing primarily cardiology and related services in southern Nevada and surrounding communities, for $15.0 million.
Key Financial Measures and Indicators
Operating Revenues
General. HCP’s consolidated revenues consist primarily of (1) medical revenues, including revenues attributable to capitation arrangements contracts with health plans and, to a lesser extent, revenues from fee-for-services arrangements and (2) other operating revenues, each as described in more detail below.
Medical Revenues. Medical revenues consist primarily of fees for medical services provided under capitated contracts with various health plans or under fee-for-service arrangements with privately insured individuals. Capitation revenue derived from health plans typically results from either (1) premium payments by CMS to HCP’s health plan customers under Medicare Advantage with respect to seniors, disabled and other eligible persons (which are referred to herein as HCP’s senior membership), (2) premium payments by state governments to HCP’s health plan customers under Medicaid managed care programs (which are referred to herein as HCP’s Medicaid membership), and (3) premium payments from public and private employers and individuals to HCP’s health plan customers with respect to their employees (which are referred to herein as HCP’s commercial membership). Capitation payments under health plan contracts are made monthly based on the number of enrollees selecting an HCP affiliated group physician employed or affiliated with one of HCP’s medical group entities as their primary health care provider. The amount of monthly capitation HCP receives from health plans on behalf of a member generally does not vary during a given calendar year, regardless of the level of actual medical services utilized by the member. Due to differing state laws affecting health care entities, HCP’s capitation contracts fall into two general categories. As described in more detail below, in central Florida and southern Nevada, HCP utilizes a global capitation model in which it assumes the financial responsibility for both professional (physician) and institutional (or hospital) services for covered benefits. In southern California, HCP utilizes variants of a different model for capitation under which it is directly financially responsible for covered professional services, but indirectly financially responsible for covered institutional expenses. HCP’s affiliated medical groups also receive specified incentive payments from health plans based on specified performance and quality criteria. These amounts are accrued when earned, and the amounts can be reasonably estimated.
| • | Global Capitation Model. HCP records the aggregate global capitation PMPM fee as revenue and the amounts paid with respect to claims as medical expenses or hospital expenses, as applicable, in its combined financial statements (see “—Operating Expenses—Medical Expenses” and “—Operating Expenses—Hospital Expenses” below). Revenue with respect to both professional and institutional capitation is recorded in the month in which enrollees are entitled to receive health care. In HCP’s central Florida market, HCP also receives capitation revenue and is liable for corresponding expenses |
S-126
Table of Contents
for prescription drug activity rendered on behalf of HCP’s senior members through the Part D component under the Medicare Advantage program. |
| • | Risk-Sharing Model. As compensation under its various managed care-related administrative services agreements with hospitals, HCP is entitled to receive up to 100% of the amount by which the hospital capitation revenue received from health plans exceeds hospital expenses, and any such risk-share amount to which HCP is entitled is recorded as medical revenues. In addition, pursuant to such managed care-related administrative services agreements, HCP agrees to be responsible should the third party incur hospital expenses in excess of hospital capitation revenue. As with global capitation, revenue with respect to professional capitation is reported in the month in which enrollees are entitled to receive health care. Risk-share revenues (that is, the portion of the excess of hospital capitation revenue to which HCP is entitled less hospital expenses), in contrast, are based on the number of enrollees and estimates of hospital utilization and associated costs incurred by assigned health plan enrollees, and the amounts accrued when earned can be reasonably estimated. Differences between actual contract settlements and estimated receivables and payables are recorded in the year of final settlement. |
| • | Retroactive Revenue-Adjustments.The Medicare Advantage revenue received by HCP’s health plan customers is adjusted periodically to give effect to the relative clinical and demographic profile of the members for whom HCP is financially responsible. The model employed by CMS bases a portion of the total reimbursement payments on various clinical and demographic risk factors, including hospital inpatient diagnoses, additional diagnosis data from ambulatory treatment settings, hospital outpatient department and physician visits, gender, age and Medicaid eligibility. Under this methodology, health plans must capture, collect and submit diagnosis code information to CMS. Capitation payments under this methodology are paid at interim rates during the year and retroactive adjustments occur in subsequent periods (generally in the third quarter of the same year, with a final adjustment in the third quarter of the following year) after the data is compiled by CMS. HCP estimates the amount of such adjustments in revenues during the first and second quarters of any given year and adjusts its estimates during the third quarter, upon receipt of payments from CMS. Differences between actual contract settlements and estimated revenues are recorded in the year of final settlement. To date, all such adjustments have resulted in increases in revenue. See “—Critical Accounting Policies and Estimates” below for further information on HCP’s estimations. |
| • | Fee-for-Service Revenues.Fee-for-service revenues are recorded when the services are provided. Such revenues are based on a negotiated fixed-fee schedule with the applicable health plan. |
Other Operating Revenues.In addition to the revenues discussed above, other operating revenues primarily represents (1) revenues received by The Camden Group, a medical consulting firm and HCP’s wholly owned subsidiary; and (2) management fees HCP receives with respect to its role as the manager of Magan Medical Group, Magan JV or Magan, an unconsolidated joint venture with Magan Medical Clinic, Inc., located in southern California, in which HCP owns a 50% interest.
Operating Expenses
General.HCP’s largest expense is the cost of medical services provided pursuant to its capitation contracts, which is recorded in HCP’s financial statements in medical expenses, hospital expenses and clinical support and other operating costs, as further described below. Under both the global capitation and the risk-share capitation models, costs of medical services are recognized in the month in which the related services are provided. In addition, medical expenses and hospital expenses include an estimate of such expenses that have been incurred but not yet reported. For further information on how HCP estimates such claims, see “—Critical Accounting Policies and Estimates–Medical Claims Liability and Related Payable, Medical Expense and Hospital Expense” below.
S-127
Table of Contents
Medical Expenses. Medical expenses consist of payments for professional and ancillary services to independent primary care physicians, specialists, ancillary providers and hospitals (including, with respect to hospitals, for outpatient services) pursuant to agreements with those entities. The structure of such expenses can consist of, among other things, sub-capitation and fee-for-service payments. In addition, medical expenses include compensation and related expenses incurred with respect to HCP’s affiliated group primary care physicians and specialists, registered nurses, physician assistants and hospitalists.
Hospital Expenses. Hospital expenses consist of payments for institutional services to contracted and non-contracted hospitals for both inpatient and outpatient services, skilled nursing facilities, and to other institutional providers. Hospital expenses are only incurred in connection with the services HCP provides in central Florida and southern Nevada. In those regions, as described above, HCP enters into contracts with health plans pursuant to which it assumes the risk for institutional hospital services. In California, in contrast, HCP’s medical groups are not permitted to contract with health plans to directly assume the risk for institutional services. Accordingly, the risk-share revenue that HCP records in California is net of reported claims and estimates of hospital utilization and associated costs incurred by assigned health plan enrollees, and no portion of institutional hospital costs incurred with respect to HCP’s California operations is included in hospital expenses.
Clinic Support and Other Operating Costs. Clinic support and other operating costs primarily consist of the costs incurred with respect to compensation of administrative and other support staff employed at HCP’s medical clinics, clinic rent and utilities, medical supplies and other direct costs incurred to support clinic operations. Also included in clinic support costs are direct costs incurred to support The Camden Group.
Earnings of Unconsolidated Joint Venture.As discussed above, HCP is a 50% owner of the Magan JV with Magan Medical Clinic, Inc. HCP accounts for this interest under the equity method of accounting, meaning that its assets and liabilities are not consolidated with HCP’s, but HCP records its pro rata share of Magan JV’s earnings as earnings of an unconsolidated joint venture.
Other Income (Expense)
Interest Income and Expense.Interest income consists of earnings on HCP’s invested cash. Interest expense consists of amounts of interest incurred related to HCP’s existing credit facility.
Gain on Sale of Investments.Gain on sale of investments represents principally gains realized upon the sale of investment securities and fixed assets, and in 2009, from divestures of certain operating divisions.
Provision for Income Taxes.HCP’s provision for income taxes relates to taxes recorded for those entities included in its consolidated financial statements that are organized as C-corporations which file separate tax returns. No provision for income taxes has been made for HCP’s limited liability companies or partnerships, as taxes on profits of those entities are the responsibility of the individual partners and limited liability company members.
2012 performance: HCP uses Adjusted EBITDA and similar calculations as measures to assess operating and financial performance, including compliance with financial covenants contained in its senior secured credit agreement. HCP generated $288 million of Adjusted EBITDA during the six months ended June 30, 2012. HCP’s results during the first quarter of 2012 benefited primarily from a lower incidence of high-cost hospital cases relative to historical norms, which is not necessarily indicative of HCP’s normal operating results and Adjusted EBITDA. HCP’s results during the second quarter of 2012 are more consistent with HCP’s expected operating results. For an explanation of Adjusted EBITDA and Adjusted EBITDA to net income, see “HCP Summary Historical Financial and Operating Data” included in this prospectus, beginning on page S-33.
S-128
Table of Contents
Results of Operations
Comparison of 2011 and 2010
HCP’s consolidated operating results for the year ended December 31, 2011, as compared to the year ended December 31, 2010 were as follows:
| 2011 | 2010 | $ increase | % change | |||||||||||||
| (dollars in millions) | ||||||||||||||||
Operating revenues: | ||||||||||||||||
Medical revenues | $ | 2,375.1 | $ | 2,048.6 | $ | 326.5 | 15.9 | % | ||||||||
Other operating revenues | 46.8 | 39.9 | 6.9 | 17.3 | % | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Total operating revenues | 2,421.9 | 2,088.5 | 333.4 | 16.0 | % | |||||||||||
Operating expense: | ||||||||||||||||
Medical expenses | 1,165.8 | 1,034.1 | 131.7 | 12.7 | % | |||||||||||
Hospital expenses | 247.6 | 222.4 | 25.2 | 11.3 | % | |||||||||||
Clinic support and other operating costs | 307.6 | 262.6 | 45.0 | 17.1 | % | |||||||||||
General and administrative expense | 206.9 | 178.0 | 28.9 | 16.2 | % | |||||||||||
Depreciation and amortization | 30.6 | 28.6 | 2.0 | 7.0 | % | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Total expenses | 1,958.5 | 1,725.7 | 232.8 | 13.5 | % | |||||||||||
Equity in earnings of unconsolidated joint venture | 24.6 | 15.1 | 9.5 | 62.9 | % | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Operating income | $ | 488.0 | $ | 377.9 | $ | 110.1 | 29.1 | % | ||||||||
Overview
Net income in 2011 was $408.6 million, as compared to $329.9 million in 2010, an increase of $78.7 million or 23.9%. HCP’s operating revenues in 2011 were $2,421.9 million, as compared to $2,088.5 million in 2010, an increase of $333.4 million or 16.0%. The increase in revenue was attributable to an increase in average senior enrollment of 7.4%, partially due to the full year impact in 2011 of the acquisition of all of the outstanding shares of Talbert Medical Group, Inc. and their subsidiary entities (collectively, referred to herein as Talbert), which occurred in May 2010, and from increased average capitation premium PMPM rates on HCP’s commercial and senior membership of 9.9% and 8.1%, respectively.
Medical and hospital expenses were $1,413.4 million in 2011, as compared to $1,256.5 million in 2010, an increase of $156.9 million, or 12.5%. The increase in medical and hospital expenses was primarily attributable to membership growth and medical costs that are subject to inflation.
General and administrative expenses were $206.9 million in 2011, as compared to $178.0 million in 2010, an increase of $28.9 million or 16.2%.
S-129
Table of Contents
Membership Information
The table set forth below provides (i) the total number of managed care members to whom HCP provided healthcare services as of December 31, 2011 and 2010, and (ii) the aggregate member months for 2011 and 2010. Member months represent the aggregate number of months of healthcare services HCP has provided to managed care members during a period of time.
| Members at December 31, | Member months in | Percentage increase in member months between years | ||||||||||||||||||
| Payor classification | 2011 | 2010 | 2011 | 2010 | ||||||||||||||||
Commercial | 418,658 | 421,933 | 5,023,789 | 4,943,479 | 1.6 | % | ||||||||||||||
Senior | 172,121 | 158,699 | 1,986,592 | 1,850,325 | 7.4 | % | ||||||||||||||
Medicaid | 26,245 | 25,357 | 306,321 | 195,992 | 56.3 | % | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
| 617,024 | 605,989 | 7,316,702 | 6,989,796 | 4.7 | % | |||||||||||||||
The increase in member months for 2011 as compared to 2010 was primarily the result of the full year impact of the acquisition of Talbert. Excluding the impact of this acquisition, member months were unchanged in 2011, as compared to 2010. Within the corresponding lines of business, excluding the impact of the Talbert acquisition, commercial member months decreased by 1.9% in 2011, as compared to 2010, primarily due to general economic conditions and commercial employers making health insurance selections other than health maintenance organization coverage for their employee base; whereas senior member months increased by 4.9% in 2011 as compared to 2010, resulting from strong membership increases during the open enrollment period, and increased number of independent physicians affiliated with HCP.
In addition, through the Magan JV, HCP provided care to 50,628 and 52,038 members as of December 2011 and 2010, respectively.
Revenues
The following table provides a breakdown of HCP’s sources of operating revenues:
| Year ended December 31, | ||||||||||||||||
| 2011 | 2010 | $ increase | % increase | |||||||||||||
| (dollar amounts in millions) | ||||||||||||||||
Commercial managed care revenues | $ | 627.5 | $ | 556.6 | $ | 70.9 | 12.7 | % | ||||||||
Senior managed care revenues | 1,626.5 | 1,393.4 | 233.1 | 16.7 | % | |||||||||||
Medicaid managed care revenues | 19.2 | 10.8 | 8.4 | 77.8 | % | |||||||||||
Fee-for-service | 101.9 | 87.8 | 14.1 | 16.1 | % | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Medical revenues | 2,375.1 | 2,048.6 | 326.5 | 15.9 | % | |||||||||||
Other operating revenues | 46.8 | 39.9 | 6.9 | 17.3 | % | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Total operating revenues | $ | 2,421.9 | $ | 2,088.5 | $ | 333.4 | 16.0 | % | ||||||||
HCP’s commercial managed care revenues increased by $70.9 million, or 12.7% in 2011, as compared to 2010. Excluding the impact of the Talbert acquisition, HCP’s commercial managed care revenue increased by $46.3 million, or 8.6% in 2011, as compared to 2010, primarily from annual premium increases from HCP’s commercial payors and from improved inpatient utilization impacting shared risk settlements in HCP’s California market, partially offset by the impact of a reduction in commercial member months. The full year impact of the Talbert acquisition, inclusive of improvements in contractual reimbursement terms with HCP’s commercial payors, contributed $24.6 million of the increase in revenues in 2011, as compared to 2010.
Under risk-sharing programs with commercial health plans, HCP shares in the risk for hospitalization services and have the opportunity to earn additional incentive revenues based on the utilization of hospital and
S-130
Table of Contents
related services. Estimated shared-risk revenues are recorded based upon hospital utilization and associated costs incurred by corresponding health plan enrollees, including an estimate of costs which have been incurred but not reported as of the measurement date. Differences between actual contract settlements and estimated receivables are recorded in the year of final settlement. During 2011 and 2010, HCP recorded favorable changes of estimates related to its prior years’ commercial shared risk settlements in the amount of $20.9 million and $18.7 million, respectively.
HCP’s senior managed care revenue increased by $233.1 million, or 16.7% in 2011, as compared to 2010. Excluding the impact of the Talbert acquisition, HCP’s senior managed care revenue increased by $180.9 million, or 13.4% in 2011, as compared to 2010, primarily from RAF coding driving higher capitation premiums, from improved inpatient utilization impacting shared risk settlements in its California market, and from increased revenues associated with a 7.4% increase in senior member months from 2010 to 2011. The full year impact of the Talbert acquisition, inclusive of improvements in contractual reimbursement terms with HCP’s senior payors, contributed $52.2 million of the increase in revenues in 2011, as compared to 2010.
Periodically, HCP receives retroactive adjustments to the capitation premiums paid to HCP based on the updated RAF scores of its senior managed care enrollees. The factors considered in these updates include changes in demographic factors, changes in risk adjustment scores resulting from time lags in medical encounter data submitted to senior health plans and CMS and updated customer information. HCP records any corresponding retroactive revenues in the year of receipt. During 2011 and 2010, HCP recorded approximately $14.3 million and $19.5 million, respectively, of additional senior managed care revenue related to prior year premium risk adjustments. In addition, during 2011 and 2010, HCP recorded favorable changes of estimates related to its prior year senior shared risk settlements in the amount of $16.6 million and $12.9 million, respectively.
The increase in HCP’s Medicaid managed care operations in 2011 as compared to 2010 of $8.4 million results from the full year impact of the Talbert acquisition; prior to the acquisition of Talbert, HCP did not provide care to Medicaid managed care patients. The increase in fee-for-service revenues was primarily attributable to acquisitions in HCP’s Nevada market, and from the full year impact of the Talbert acquisition.
Medical Expenses, Hospital Expenses, and Clinic Support and Other Operating Costs
The following table reflects HCP’s medical expenses, hospital expenses and clinic support costs:
| Year ended December 31, | ||||||||||||||||
| 2011 | 2010 | $ increase | % change | |||||||||||||
| (dollar amounts in millions) | ||||||||||||||||
Medical expenses | $ | 1,165.8 | $ | 1,034.1 | $ | 131.7 | 12.7 | % | ||||||||
Hospital expenses | 247.6 | 222.4 | 25.2 | 11.3 | % | |||||||||||
Clinic support and other operating costs | 307.6 | 262.6 | 45.0 | 17.1 | % | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Total | $ | 1,721.0 | $ | 1,519.1 | $ | 201.9 | 13.3 | % | ||||||||
HCP’s medical expenses increased by $131.7 million, or 12.7%, in 2011, as compared to 2010. Of this increase, $101.0 million was attributable to increased payments to contracted and affiliated physicians, of which $53.8 million, or 39%, results from increased managed care membership growth in 2011, and $47.2 million, or 34%, results from increased performance incentive payments to contracted physicians and from medical costs that are subject to inflation. $36.0 million, or 27%, of the increase was attributable to increases in employed clinician compensation, resulting from increases in the number of employed clinicians due to HCP’s acquisitions, expansion of programs to improve utilization rates of its high risk patients, and medical costs that are subject to inflation. Additionally, during the years ended December 31, 2011 and 2010, HCP recorded favorable changes in estimates to prior year medical claims and related payables in the amount of $5.3 million and $5.1 million, respectively.
S-131
Table of Contents
HCP’s hospital expenses, which represent hospital costs incurred on behalf of global capitated managed care members in its Florida and Nevada markets, increased by $25.2 million, or 11.3%, in 2011, as compared to 2010. This increase was attributable to increases in managed care membership, which contributed $15.2 million of the increase, and an increase of $10.1 million attributable to medical costs that are subject to inflation and slight increased acuity of the corresponding patient population.
HCP’s clinic support and other operating costs increased by $45.0 million, or 17.1%, in 2011, as compared to 2010. This increase was attributable to increases in the average number of employed staff and related clinic support costs resulting from acquisitions in HCP’s California and Nevada markets, increases in performance bonus payments to staff, and cost inflation.
Other Operating Expenses
HCP’s general and administrative costs increased by $28.9 million, or 16.2%, in 2011, as compared to 2010. This increase was primarily attributable to additional costs incurred to support newly acquired entities principally in HCP’s California and Nevada markets.
HCP’s 2011 depreciation and amortization expense of $30.6 million consists of $13.5 million of depreciation expense for equipment and leasehold improvements, compared to $13.5 million in 2010, and $17.1 million of amortization expense associated with intangible assets acquired in various acquisitions, compared to $15.1 million of intangible asset amortization expense in 2010.
Other Items
HCP’s share of earnings from joint venture relationships increased from $15.1 million in 2010 to $24.6 million in 2011, an increase of $9.5 million, or 62.9%. This increase was primarily attributable to improved inpatient utilization for the corresponding patient population, increased senior managed care membership, as well as approximately $4.0 million related to favorable changes in estimates related to risk sharing settlements with health plans.
HCP recognized other expense of $7.9 million in 2011, as compared to other income of $0.6 million in 2010. The increase in other expense was primarily attributable to increased interest expense incurred during 2011, as compared to 2010, and from the January 2011 refinancing of HCP’s credit facility to repurchase certain preferred equity interests in HCP.
Income Taxes
HCP’s provision for income taxes relates to taxes recorded for those entities included in its consolidated financial statements that are organized as C-corporations which file separate tax returns. No provision for income taxes has been made for its limited liability companies or partnerships, as taxes on any profits of those entities are the responsibility of the individual partners and limited liability company members.
HCP’s provision for income taxes increased from $48.6 million in 2010 to $71.5 million in 2011, an increase of $22.9 million, or 47.1%. The increase was primarily attributable to additional expense recognized to increase HCP’s reserve for uncertain tax positions of $13.5 million, and taxes resulting from increased taxable income in HCP’s subsidiaries taxed as corporations.
Acquisitions
On October 1, 2011, HCP acquired certain assets of Outcome Based Delivery Systems, LLC, or OBDS, a physician group practice and management services organization serving the south Florida and southern Nevada markets. Additionally, on May 1, 2010, HCP acquired Talbert, a multi-specialty medical group providing comprehensive health care services to managed care enrollees in southern California.
S-132
Table of Contents
Comparison of 2010 and 2009
HCP’s consolidated operating results for the year ended December 31, 2010, as compared to the year ended December 31, 2009 were as follows:
| Year ended December 31, |
|
| ||||||||||||||
| 2010 | 2009 | $ increase | % change | |||||||||||||
| (dollars in millions) | ||||||||||||||||
Operating revenues: | ||||||||||||||||
Medical revenues | $ | 2,048.6 | $ | 1,730.7 | $ | 317.9 | 18.4 | % | ||||||||
Other operating revenues | 39.9 | 46.1 | (6.2 | ) | -13.4 | % | ||||||||||
|
|
|
|
|
|
|
| |||||||||
Total operating revenues | 2,088.5 | 1,776.8 | 311.7 | 17.5 | % | |||||||||||
Operating expense: | ||||||||||||||||
Medical expenses | 1,034.1 | 930.2 | 103.9 | 11.2 | % | |||||||||||
Hospital expenses | 222.4 | 211.5 | 10.9 | 5.2 | % | |||||||||||
Clinic support and other operating costs | 262.6 | 225.5 | 37.1 | 16.5 | % | |||||||||||
General and administrative expense | 178.0 | 136.3 | 41.7 | 30.6 | % | |||||||||||
Depreciation and amortization | 28.6 | 26.0 | 2.6 | 10.0 | % | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Total expenses | 1,725.7 | 1,529.5 | 196.2 | 12.8 | % | |||||||||||
Equity in earnings of unconsolidated joint venture | 15.1 | 11.5 | 3.6 | 31.3 | % | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Operating income | $ | 377.9 | $ | 258.8 | $ | 119.1 | 46.0 | % | ||||||||
Overview
Net income in 2010 was $329.9 million, as compared to $220.3 million in 2009, an increase of $109.6 million or 49.7%. HCP’s operating revenues in 2010 were $2,088.5 million, as compared to $1,776.8 million in 2009, an increase of $311.7 million or 17.5%. The increase in revenue was attributable to an increase in average senior enrollment of 10.3%, partially due to the Talbert acquisition, and to increased average capitation premium PMPM rates on HCP’s commercial and senior membership of 6.6% and 3.8%, respectively.
Medical and hospital expenses were $1,256.5 million in 2010, as compared to $1,141.7 million in 2009, an increase of $114.8 million or 10.1%. Membership growth and increased medical costs subject to inflation contributed to this increase.
General and administrative expenses were $178.0 million in 2010, as compared to $136.3 million in 2009, an increase of $41.7 million or 30.6%, due primarily to costs incurred supporting acquired entities.
Membership Information
The table set forth below provides (i) the total number of managed care members to whom HCP provided healthcare services as of December 31, 2010 and 2009, and (ii) the aggregate member months for 2010 and 2009.
| Members at December 31, | Member months in | Percentage increase in member months between years | ||||||||||||||||||
| Payor classification | 2010 | 2009 | 2010 | 2009 | ||||||||||||||||
Commercial | 421,933 | 394,099 | 4,943,479 | 4,826,413 | 2.4 | % | ||||||||||||||
Senior | 158,699 | 141,652 | 1,850,325 | 1,677,734 | 10.3 | % | ||||||||||||||
Medicaid | 25,357 | 0 | 195,992 | 0 | 100 | % | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
| 605,989 | 535,751 | 6,989,796 | 6,504,147 | 7.5 | % | |||||||||||||||
In addition, through HCP’s joint venture relationship with Magan Medical Group, HCP provided care to 52,308 and 54,158 members as of December 2010 and 2009, respectively.
S-133
Table of Contents
The increase in member months for 2010 as compared to 2009 is primarily the result of the acquisition of Talbert, partially offset by the full year impact of the sale of operations associated with approximately 25,000 managed care members to HCP’s Magan JV effective July 1, 2009. Excluding these events, HCP’s member months remained flat from 2009 to 2010. Within the corresponding lines of business, excluding the Talbert and Magan transactions, commercial member months decreased by 1.4% in 2010 as compared to 2009, primarily due to general economic conditions and commercial employers making health insurance selections other than health maintenance organization coverage for their employee base; whereas senior member months increased by 5.3% in 2010 as compared to 2009, resulting from strong membership increases during the open enrollment period, and increased number of independent physicians joining HCP’s network.
Revenues
The following table provides a breakdown of HCP’s sources of operating revenues:
| Year ended December 31, | ||||||||||||||||
| 2010 | 2009 | $ increase | % increase | |||||||||||||
| (dollar amounts in millions) | ||||||||||||||||
Commercial managed care revenues | $ | 556.6 | $ | 478.6 | $ | 78.0 | 16.3 | % | ||||||||
Senior managed care revenues | 1,393.4 | 1,190.6 | 202.8 | 17.0 | % | |||||||||||
Medicaid managed care revenues | 10.8 | 0.0 | 10.8 | 100.0 | % | |||||||||||
Fee-for-service | 87.8 | 61.5 | 26.3 | 42.8 | % | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Medical revenues | 2,048.6 | 1,730.7 | 317.9 | 18.4 | % | |||||||||||
Other operating revenues | 39.9 | 46.1 | (6.2 | ) | -13.4 | % | ||||||||||
|
|
|
|
|
|
|
| |||||||||
Total operating revenues | $ | 2,088.5 | $ | 1,776.8 | $ | 311.7 | 17.5 | % | ||||||||
HCP’s commercial managed care revenue increased by $78.0 million, or 16.3%, in 2010, as compared to 2009. Excluding the impact of the Talbert acquisition, HCP’s commercial managed care revenue increased by $57.8 million, or 12.1%, in 2010, as compared to 2009, primarily from annual premium increases from its commercial payors and from improved inpatient utilization impacting shared risk settlements in its California market, partially offset by the impact of a reduction in commercial member months. The Talbert acquisition contributed $20.2 million of the increase in commercial managed care revenues in 2010, as compared to 2009.
Differences between actual contract settlements and estimated receivables are recorded in the year of final settlement. During 2010 and 2009, HCP recorded favorable changes of estimates related to its prior years’ commercial shared risk settlements in the amount of $18.7 million and $11.0 million, respectively.
HCP’s senior managed care revenue increased by $202.8 million, or 17.0%, in 2010, as compared to 2009. Excluding the impact of the Talbert acquisition, HCP’s senior managed care revenue increased by $164.0 million, or 13.8%, from 2009, resulting from improved MRA coding driving higher capitation premiums, from improved inpatient utilization impacting shared risk settlements in its California market, and from increased revenues associated with a 2.4% increase in senior member months in 2010, as compared to 2009, excluding the impact of the Talbert acquisition. The Talbert acquisition contributed $38.8 million of the increase in senior managed care revenues in 2010.
During 2010 and 2009, HCP recorded $19.5 million and $15.5 million, respectively, of additional senior managed care revenue related to prior year premium risk adjustments. In addition, during 2010 and 2009, HCP recorded favorable changes of estimates related to its prior year senior shared risk settlements in the amount of $12.9 million and $9.0 million, respectively.
The increase in HCP’s Medicaid managed care operations in 2010 as compared to 2009 of $10.8 million results exclusively to the Talbert acquisition, prior to which HCP did not provide care to Medicaid managed care patients. The increase in fee-for-service revenues results primarily from acquisitions in HCP’s Nevada market and from the Talbert acquisition.
S-134
Table of Contents
Medical Expenses, Hospital Expenses, and Clinic Support and Other Operating Costs
The following table reflects HCP’s medical expenses, hospital expenses and clinic support costs:
| Year ended December 31, | ||||||||||||||||
| 2010 | 2009 | $ increase | % change | |||||||||||||
| (dollar amounts in millions) | ||||||||||||||||
Medical expenses | $ | 1,034.1 | $ | 930.2 | $ | 103.9 | 11.2 | % | ||||||||
Hospital expenses | 222.4 | 211.5 | 10.9 | 5.2 | % | |||||||||||
Clinic support and other operating costs | 262.6 | 225.5 | 37.1 | 16.5 | % | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Total | $ | 1,519.1 | $ | 1,367.2 | $ | 151.9 | 11.1 | % | ||||||||
HCP’s medical expenses increased by $103.9 million, or 11.2%, in 2010, as compared to 2009. Of this increase, $66.0 million was attributable to increased payments to contracted and affiliated physicians resulting principally from increased membership volume in 2010. $43.0 million of the increase was attributable to increases in employed clinician compensation, resulting from increases in number of employed clinicians due to acquisitions, expansion of programs to improve utilization rates by HCP’s high risk patients, and medical costs subject to inflation. Additionally, during the years ended December 31, 2010 and 2009, HCP recorded favorable changes in estimates to prior year medical claims and related payables of $5.1 million and $ 4.2 million, respectively.
HCP’s hospital expenses, which represent hospital costs incurred on behalf of global capitated managed care members in its Florida and Nevada markets, increased by $10.9 million, or 5.2%, in 2010, as compared to 2009. This increase was primarily attributable to increases in managed care membership. Excluding changes in membership levels, hospital expenses were essentially flat in 2010, as compared to 2009.
HCP’s clinic support and other operating costs increased by $37.1 million, or 16.5%, in 2010, as compared to 2009. This increase was primarily attributable to increases in the average number of employed staff and related clinic support costs resulting from acquisitions in HCP’s California and Nevada markets, increases in performance bonus payments to staff, and cost inflation.
Other Operating Expenses
HCP’s general and administrative costs increased by $41.7 million, or 30.6%, in 2010, as compared to 2009. This increase was primarily attributable to additional costs incurred to support newly acquired entities principally in HCP’s California and Nevada markets.
HCP’s 2010 depreciation and amortization expense of $28.6 million consists of $13.5 million of depreciation expense for equipment and leasehold improvements, compared to $13.0 million in 2009, and $15.1 million of amortization expense associated with intangible assets acquired in various acquisitions in 2010, compared to $13.0 million of intangible asset amortization in 2009.
Other Income
HCP recognized other income of $0.6 million in 2010 compared to other income of $1.7 million in 2009. The decrease in other income was primarily attributable to recognition of gains of $1.8 million in 2009 on the sale of HCP’s interests in a wholly owned third party administrator and a minority-owned software firm, offset by increases in net interest activity.
Income Taxes
HCP’s provision for income taxes increased from $40.3 million in 2009 to $48.6 million in 2010, an increase of $8.3 million, or 20.6%. This increase was primarily attributable to taxes resulting from increased taxable income in its subsidiaries taxed as corporations.
S-135
Table of Contents
Acquisitions
On May 1, 2010, HCP acquired Talbert, a multi-specialty medical group providing comprehensive health care services to managed care enrollees in southern California, as described in “—Overview”, above.
On July 1, 2009, HCP acquired all tangible assets and administrative operations of Fremont Medical Center, Inc., a medical practice based in southern Nevada, providing medical services to patients on primarily a fee-for-service basis, for $9.5 million.
Comparison of the Six Months Ended June 30, 2012 and 2011
HCP’s consolidated operating results for the six months ended June 30, 2012 compared to the six months ended June 30, 2011 were as follows:
| Six months ended June 30, | ||||||||||||||||
| 2012 | 2011 | $ increase | % change | |||||||||||||
| (dollars in millions) | ||||||||||||||||
| Operating revenues: | ||||||||||||||||
Medical revenues | $ | 1,295.1 | $ | 1,158.6 | $ | 136.5 | 11.8 | % | ||||||||
Other operating revenues | 28.1 | 21.9 | 6.2 | 28.3 | % | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Total operating revenues | 1,323.2 | 1,180.5 | 142.7 | 12.1 | % | |||||||||||
Operating expense: | ||||||||||||||||
Medical expenses | 619.9 | 569.7 | 50.2 | 8.8 | % | |||||||||||
Hospital expenses | 155.4 | 121.7 | 33.7 | 27.7 | % | |||||||||||
Clinic support and other operating costs | 165.2 | 147.7 | 17.5 | 11.8 | % | |||||||||||
General and administrative expense | 109.8 | 101.0 | 8.8 | 8.7 | % | |||||||||||
Depreciation and amortization | 15.9 | 15.9 | 0.0 | 0.0 | % | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Total expenses | 1,066.2 | 956.0 | 110.2 | 11.5 | % | |||||||||||
Equity in earnings of unconsolidated joint venture | 11.5 | 8.7 | 2.8 | 32.2 | % | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Operating income | $ | 268.5 | $ | 233.2 | $ | 35.3 | 15.1 | % | ||||||||
Overview
Net income for the six months ended June 30, 2012 was $232.7 million, as compared to $192.1 million for the six months ended June 30, 2011, an increase of $40.6 million or 21.1%. HCP’s operating revenues for the six months ended June 30, 2012 were $1,323.2 million, as compared to $1,180.5 million for the six months ended June 30, 2011, an increase of $142.7 million or 12.1%. The increase in revenue was attributable to an increase in average senior enrollment of 11.4%, and to increases average capitation premium PMPM rates on HCP’s commercial membership of 6.9%.
Medical and hospital expenses were $775.3 million for the six months ended June 30, 2012, as compared to $691.4 million for the six months ended June 30, 2011, an increase of $83.9 million or 12.1%. Membership growth and medical cost inflation contributed to this increase.
General and administrative expenses were $109.8 million for the six months ended June 30, 2012, as compared to $101.0 million for the six months ended June 30, 2011, an increase of $8.8 million or 8.7%, resulting from $2.4 million of advisory and related costs during the six months ended June 30, 2012 incurred to support activities related to the transaction with DaVita Inc., and from costs incurred supporting acquired entities.
S-136
Table of Contents
Membership information
The table set forth below provides (i) the total number of managed care enrollees to whom HCP was providing healthcare services as of June 30, 2012 and 2011, and (ii) the aggregate member months for the six months ended June 30, 2012 and 2011, respectively.
| Members at June 30, | Member months in six months ended June 30, | Percentage increase in member months between years | ||||||||||||||||||
| Payer classification | 2012 | 2011 | 2012 | 2011 | ||||||||||||||||
Commercial | 410,742 | 418,791 | 2,473,451 | 2,503,696 | -1.2 | % | ||||||||||||||
Senior | 182,477 | 163,497 | 1,090,663 | 979,428 | 11.4 | % | ||||||||||||||
Medicaid | 27,076 | 25,247 | 160,696 | 150,760 | 6.6 | % | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
| 620,295 | 607,535 | 3,724,810 | 3,633,884 | 2.5 | % | |||||||||||||||
In addition, through our joint venture relationship with Magan Medical Group, HCP provided care to 49,080 and 51,654 members as of June 30, 2012 and 2011, respectively.
The increase in member months for the six months ended June 30, 2012 compared to the six months ended June 30, 2011 is the result of the conversion of senior patients not previously contracted with HCP through globally capitation relationships into contractual relationships under global capitation, increased enrollment growth from the OBDS acquisition and positive growth trends in all three geographic markets. Commercial member months decreased by 1.2% from the six months ended June 30, 2012, as compared to the six months ended June 30, 2011, primarily due to general economic conditions and commercial employers making health insurance selections other than managed care coverage for their employee base.
Revenues
The following table provides a breakdown of HCP’s sources of operating revenues:
| Six months ended June 30, | ||||||||||||||||
| 2012 | 2011 | $ increase | % increase | |||||||||||||
| (dollars in millions) | ||||||||||||||||
Commercial managed care revenues | $ | 333.9 | $ | 308.9 | $ | 25.0 | 8.1 | % | ||||||||
Senior managed care revenues | 894.0 | 793.0 | 101.0 | 12.7 | % | |||||||||||
Medicaid managed care revenues | 7.2 | 8.7 | (1.5 | ) | -17.2 | % | ||||||||||
Fee for service | 60.0 | 48.0 | 12.0 | 25.0 | % | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Medical revenues | 1,295.1 | 1,158.6 | 136.5 | 11.8 | % | |||||||||||
Other operating revenues | 28.1 | 21.9 | 6.2 | 28.3 | % | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Total operating revenues | $ | 1,323.2 | $ | 1,180.5 | $ | 142.7 | 12.1 | % | ||||||||
HCP’s commercial managed care revenue increased by $25.0 million, or 8.1%, from the six months ended June 30, 2011, as compared to the six months ended June 30, 2012, resulting primarily from annual premium increases from its commercial payers of approximately 6.9%, from improved inpatient utilization impacting shared risk settlements in its California market, partially offset by the impact of a reduction in commercial member months. During the quarter ended March 31, 2012, HCP recognized $2.5 million of retroactive commercial capitation payments relating to 2011. During the six months ended June 30, 2012 and 2011, HCP recorded favorable changes of estimates related to its prior years’ commercial shared risk settlements in the amount of $4.0 million and $3.3 million. HCP’s senior managed care revenue increased by $101.0 million, or 12.7%, from the six months ended June 30, 2011, as compared to the six months ended June 30, 2012, resulting primarily from increased revenue associated with an 11.4% increase in senior member months from the six months ended June 30, 2011, as compared to the six months ended June 30, 2012 and from improved inpatient
S-137
Table of Contents
utilization impacting shared risk settlements in its California market. During the six months ended June 30, 2012 and 2011, HCP recorded favorable changes of estimates related to its prior years’ senior shared risk settlements in the amount of $7.2 million and $3.3 million, respectively. HCP’s managed care revenues during the quarter ended March 31, 2012 benefited from a lower incidence of high-cost hospital cases relative to historical norms.
Medical Expenses, Hospital Expenses, and Clinic Support and Other Operating Costs
The following table reflects our medical expenses, hospital expenses and clinic support costs:
| Six months ended June 30, |
|
| ||||||||||||||
| 2012 | 2011 | $ increase | % change | |||||||||||||
| (dollars in millions) | ||||||||||||||||
Medical expenses | $ | 619.9 | $ | 569.7 | $ | 50.2 | 8.8 | % | ||||||||
Hospital expenses | 155.4 | 121.7 | 33.7 | 27.7 | % | |||||||||||
Clinic support and other operating costs | 165.2 | 147.7 | 17.5 | 11.8 | % | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Total | $ | 940.5 | $ | 839.1 | $ | 101.4 | 12.1 | % | ||||||||
HCP’s medical expenses increased by $50.2 million, or 8.8%, from the six months ended June 30, 2011, as compared to the six months ended June 30, 2012. Of this increase, $43.3 million was attributable to increased payments to contracted and affiliated physicians resulting principally from increased membership volume from the six months ended June 30, 2011, as compared to the six months ended June 30, 2012. $11.9 million of the increase was attributable to increases in employed clinician compensation, resulting from increases in number of employed clinicians due to acquisitions, to support expansion of high risk programs, to improve utilization rates of HCP’s patients, and from medical cost inflation. Additionally, during the six months ended June 30, 2012 and June 30, 2011, HCP recorded favorable changes in estimates to prior year medical claims and related payables of $5.0 million and $2.6 million, respectively, adjusted through the respective six month expense.
HCP’s hospital expenses, which represent hospital costs incurred on behalf of globally capitated managed care members in its Florida and Nevada markets, increased by $33.7 million, or 27.7%, from the six months ended June 30, 2011, as compared to the six months ended June 30, 2012, of which $27.7 million was attributable to senior membership increases in its Florida and Nevada markets, and the remaining $6.0 million primarily attributable to general medical cost inflation increases.
HCP’s clinic support and other operating costs increased by $17.5 million, or 11.8%, from the six months ended June 30, 2011, as compared to the six months ended June 30, 2012. This increase was attributable to increases in the average number of employed staff and related clinic support costs resulting from acquisitions in HCP’s Florida and Nevada markets, and to cost inflation.
Other operating expenses
HCP’s general and administrative costs increased by $8.8 million, or 8.7%, from the six months ended June 30, 2011, as compared to the six months ended June 30, 2012. This increase was primarily attributable $2.4 million incurred during the six months ended June 30, 2012 for advisory services related to the transaction with DaVita Inc., additional costs incurred to support newly acquired entities, and to cost inflation.
HCP depreciation and amortization expense for the six months ended each of June 30, 2012 and 2011 of $15.9 million consists of $7.9 million of depreciation expense in equipment and leasehold improvements, compared to $7.3 million during the six months ended June 30, 2011, and $8.0 million of amortization of intangible assets acquired in various acquisitions, compared to $8.6 million of intangible asset amortization during the six months ended June 30, 2011.
S-138
Table of Contents
Other Income (Expense)
HCP recognized other expense of $2.8 million for the six months ended June 30, 2012, as compared to other expense of $4.4 million in during the six months ended June 30, 2011. The change in other expense is primarily attributable to write-off during the six months ended June 30, 2011 of $1.9 million of previously unamortized deferred financing costs, in connection with our January 2011 refinancing and increase of HCP’s credit facility (see “—Liquidity and Capital Resources” below).
Income taxes
HCP’s provision for income taxes decreased from $36.9 million for the six months ended June 30, 2011, as compared to $32.9 million for the six months ended June 30, 2012, a decrease of $4.0 million, or 10.8%. This decrease is primarily attributable to a reduction in the amount of tax expense recognized to increase our reserves for uncertain tax positions, from $16.9 million for the six months ended June 30, 2011, as compared to $2.8 million for the six months ended June 30, 2012, partially offset from taxes resulting from increased taxable income in HCP’s subsidiaries taxed as corporations.
Acquisitions
Effective April 16, 2012, HCP acquired certain assets of Cardiovascular Consultants of Nevada, Inc. for $15.0 million.
Comparison of the Quarter Ended June 30, 2012 and 2011
HCP’s consolidated operating results for the quarter ended June 30, 2012, as compared to the quarter ended June 30, 2011 were as follows:
| Quarter ended June 30, | ||||||||||||||||
| 2012 | 2011 | $ increase | % change | |||||||||||||
| (dollars in millions) | ||||||||||||||||
Operating revenues: | ||||||||||||||||
Medical revenues | $ | 642.3 | $ | 583.0 | $ | 59.3 | 10.2 | % | ||||||||
Other operating revenues | 14.4 | 11.2 | 3.2 | 28.6 | % | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Total operating revenues | 656.7 | 594.2 | 62.5 | 10.5 | % | |||||||||||
Operating expense: | ||||||||||||||||
Medical expenses | 312.7 | 285.2 | 27.5 | 9.6 | % | |||||||||||
Hospital expenses | 77.1 | 61.4 | 15.7 | 25.6 | % | |||||||||||
Clinic support and other operating costs | 83.5 | 76.1 | 7.4 | 9.7 | % | |||||||||||
General and administrative expense | 55.1 | 49.9 | 5.2 | 10.4 | % | |||||||||||
Depreciation and amortization | 8.5 | 7.7 | 0.8 | 10.4 | % | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Total expenses | 536.9 | 480.3 | 56.6 | 11.8 | % | |||||||||||
Equity in earnings of unconsolidated joint venture | 6.5 | 3.8 | 2.7 | 71.1 | % | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Operating income | $ | 126.3 | $ | 117.7 | $ | 8.6 | 7.3 | % | ||||||||
Overview
Net income for the quarter ended June 30, 2012 was $111.6 million, as compared to $104.9 million for the quarter ended June 30, 2011, an increase of $6.7 million or 6.4%. HCP’s operating revenues for the quarter ended June 30, 2012 were $656.7 million, as compared to $594.2 million for the quarter ended June 30, 2011, an increase of $62.5 million or 10.5%. The increase in revenue was attributable to an increase in average senior enrollment of 11.5%, and to increases average capitation premium PMPM rates on HCP’s commercial membership of 5.7%.
S-139
Table of Contents
Medical and hospital expenses were $389.8 million for the quarter ended June 30, 2012, as compared to $346.6 million for the quarter ended June 30, 2011, an increase of $43.2 million or 12.5%. Membership growth and medical cost inflation contributed to this increase.
General and administrative expenses were $55.1 million for the quarter ended June 30, 2012, as compared to $49.9 million for the quarter ended June 30, 2011, an increase of $5.2 million or 10.4%, resulting from $1.7 million of advisory and related costs during the quarter ended June 30, 2012 incurred to support activities related to the transaction with DaVita Inc., and from costs incurred supporting acquired entities.
Membership information
The table set forth below provides (i) the total number of managed care enrollees to whom HCP was providing healthcare services as of June 30, 2012 and 2011, and (ii) the aggregate member months for the quarter ended June 30, 2012 and 2011, respectively. Member months represent the aggregate number of months of healthcare services HCP has provided to managed care enrollees during a period of time.
| Members at June 30, | Member months in quarter ended June 30, | Percentage increase in member months between years | ||||||||||||||||||
| Payer classification | 2012 | 2011 | 2012 | 2011 | ||||||||||||||||
Commercial | 410,742 | 418,791 | 1,236,341 | 1,254,482 | -1.4 | % | ||||||||||||||
Senior | 182,477 | 163,497 | 546,310 | 489,907 | 11.5 | % | ||||||||||||||
Medicaid | 27,076 | 25,247 | 80,667 | 75,874 | 6.3 | % | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
| 620,295 | 607,535 | 1,863,318 | 1,820,263 | 2.4 | % | |||||||||||||||
In addition, through our joint venture relationship with Magan Medical Group, HCP provided care to 49,080 and 51,654 members as of June 30, 2012 and 2011, respectively.
The increase in member months for the quarter ended June 30, 2012, as compared to the quarter ended June 30, 2011 is the result of the conversion of senior patients not previously contracted with HCP through globally capitation relationships into contractual relationships under global capitation, as well as from increased enrollment growth from the OBDS acquisition. Commercial member months decreased by 1.4% from the quarter ended June 30, 2012, as compared to the quarter ended June 30, 2011, primarily due to general economic conditions and commercial employers making health insurance selections other than managed care coverage for their employee base.
Revenues
The following table provides a breakdown of HCP’s sources of operating revenues:
| Quarter ended June 30, | ||||||||||||||||
| 2012 | 2011 | $ increase | % increase | |||||||||||||
| (dollars in millions) | ||||||||||||||||
Commercial managed care revenues | $ | 162.3 | $ | 157.4 | $ | 4.9 | 3.1 | % | ||||||||
Senior managed care revenues | 444.8 | 397.1 | 47.7 | 12.0 | % | |||||||||||
Medicaid managed care revenues | 3.5 | 4.8 | (1.3 | ) | -27.1 | % | ||||||||||
Fee for service | 31.7 | 23.7 | 8.0 | 33.8 | % | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Medical revenues | 642.3 | 583.0 | 59.3 | 10.2 | % | |||||||||||
Other operating revenues | 14.4 | 11.2 | 3.2 | 28.6 | % | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Total operating revenues | $ | 656.7 | $ | 594.2 | $ | 62.5 | 10.5 | % | ||||||||
HCP’s commercial managed care revenue increased by $4.9 million, or 3.1%, from the quarter ended June 30, 2011, as compared to the quarter ended June 30, 2012, resulting from annual premium increases from its
S-140
Table of Contents
commercial payers of approximately 5.7%, partially offset from a decrease of revenue of $1.0 million from shared risk settlements in its California market and by the impact of the reduction in commercial member months. During the quarters ended June 30, 2012 and 2011, HCP recorded favorable changes of estimates related to its prior years’ commercial shared risk settlements in the amount of $1.3 million and $1.7 million, respectively.
HCP’s senior managed care revenue increased by $47.7 million, or 12.0%, from the quarter ended June 30, 2011, as compared to the quarter ended June 30, 2012, resulting primarily from increased revenue associated with an 11.5% increase in senior member months from the quarter ended June 30, 2011, as compared to the quarter ended June 30, 2012. During the quarters ended June 30, 2012 and 2011, HCP recorded favorable changes of estimates related to its prior years’ senior shared risk settlements in the amount of $5.9 million and $2.0 million, respectively.
Medical Expenses, Hospital Expenses, and Clinic Support and Other Operating Costs
The following table reflects our medical expenses, hospital expenses and clinic support costs:
| Quarter ended June 30, | ||||||||||||||||
| 2012 | 2011 | $ increase | % change | |||||||||||||
| (dollars in millions) | ||||||||||||||||
Medical expenses | $ | 312.7 | $ | 285.2 | $ | 27.5 | 9.6 | % | ||||||||
Hospital expenses | 77.1 | 61.4 | 15.7 | 25.6 | % | |||||||||||
Clinic support and other operating costs | 83.5 | 76.1 | 7.4 | 9.7 | % | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Total | $ | 473.3 | $ | 422.7 | $ | 50.6 | 12.0 | % | ||||||||
HCP’s medical expenses increased by $27.5 million, or 9.6%, from the quarter ended June 30, 2011, as compared to the quarter ended June 30, 2012. Of this increase, $20.5 million was attributable to increased payments to contracted and affiliated physicians resulting principally from increased membership volume from the quarter ended June 30, 2011, as compared to the quarter ended June 30, 2012. $7.0 million of the increase was attributable to increases in employed clinician compensation, resulting from increases in number of employed clinicians due to acquisitions, to support expansion of high risk programs to improve utilization rates of HCP’s patients, and from medical cost inflation. Additionally, during the quarters ended June 30, 2012 and 2011, HCP recorded favorable changes in estimates to prior year medical claims and related payables of $0 and $1.3 million, respectively.
HCP’s hospital expenses, which represent hospital costs incurred on behalf of globally capitated managed care members in its Florida and Nevada markets, increased by $15.7 million, or 25.6%, from the quarter ended June 30, 2011, as compared to the quarter ended June 30, 2012, of which $14.0 million was attributable to senior membership increases in its Florida and Nevada markets, and the remaining $1.7 million primarily attributable to general medical cost inflation increases.
HCP’s clinic support and other operating costs increased by $7.4 million, or 9.7%, from the quarter ended June 30, 2011, as compared to the quarter ended June 30, 2012. This increase was attributable to increases in the average number of employed staff and related clinic support costs resulting from acquisitions in HCP’s Florida and Nevada markets, and to cost inflation.
Other operating expenses
HCP’s general and administrative costs increased by $5.2 million, or 10.4%, from the quarter ended June 30, 2011, as compared to the quarter ended June 30, 2012. This increase was primarily attributable $1.7 million incurred during the quarter ended June 30, 2012 for advisory services related to the transaction with DaVita Inc., additional costs incurred to support newly acquired entities, and to cost inflation.
S-141
Table of Contents
HCP depreciation and amortization expense for the quarter ended June 30, 2012 and 2011 of $8.5 million and $7.7 million, respectively, consists of $4.0 million of depreciation expense in equipment and leasehold improvements for the quarter ended June 30, 2012, as compared to $3.6 million during the quarter ended June 30, 2011, and $4.5 million of amortization of intangible assets acquired in various acquisitions for the quarter ended June 30, 2012, as compared to $4.1 million of intangible asset amortization during the quarter ended June 30, 2011.
Other Income (Expense)
HCP recognized other expense of $1.3 million for the quarter ended June 30, 2012, as compared to other expense of $1.4 million in during the quarter ended June 30, 2011.
Income taxes
HCP’s provision for income taxes increased from $11.5 million for the quarter ended June 30, 2011, as compared to $13.4 million for the quarter ended June 30, 2012, an increase of $1.9 million, or 16.5%, resulting primarily from increased taxable income in HCP’s subsidiaries taxed as corporations.
Total Care Dollars Under Management
As described above, HCP recognizes revenue generally under two capitation models: the global capitation model and the risk-share capitation model. Under the global capitation model, HCP records as revenue the aggregate PMPM capitation payments under its contracts with health plans, which incorporates both professional and institutional components of capitation funding. Amounts paid with respect to health care services provided to its enrollees are recorded as medical expenses or hospital expenses, as applicable, in its consolidated financial statements. In HCP’s California market, in contrast, it records as medical revenue (1) 100% of capitation payments it receives with respect to professional services and (2) only the risk-share revenue that it receives with respect to hospital services, which is based on hospital funding on behalf of, less estimated hospital utilization and associated costs incurred by, assigned health plan enrollees. See “—Key Financial Measures and Indicators—Operating Revenues—Medical Revenues”.
Accordingly, in order to reflect the total dollar amount that would represent medical revenues as if all of HCP’s operations were operated under the global capitation model, HCP also presents “Total Care Dollars Under Management”. HCP believes that presenting amounts in this manner is useful because it presents its operations on a unified basis without the complication caused by models that HCP has adopted in its California market as a result of various regulations related to the assumption of institutional risk.
The following table reconciles Total Care Dollars Under Management to medical revenues for the periods indicated. “Total Care Dollars Under Management” is a non-GAAP measure.
| Year ended December 31, | Six months ended June 30, | |||||||||||||||||||
| 2009 | 2010 | 2011 | 2011 | 2012 | ||||||||||||||||
| (dollars in millions) | ||||||||||||||||||||
Medical revenues | $ | 1,730.7 | $ | 2,048.6 | $ | 2,375.1 | $ | 1,158.6 | $ | 1,295.1 | ||||||||||
Less: Risk share revenue, net | (30.1 | ) | (87.3 | ) | (126.9 | ) | (52.2 | ) | (61.8 | ) | ||||||||||
Add: Institutional capitation amounts | 687.0 | 830.7 | 963.9 | 475.4 | 518.5 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total care dollars under management | $ | 2,387.6 | $ | 2,792.0 | $ | 3,212.1 | $ | 1,581.8 | $ | 1,751.8 | ||||||||||
Liquidity and Capital Resources
Cash, cash equivalents and short-term investments at December 31, 2011 and 2010 totaled $569.6 million and $540.4 million, respectively. Working capital totaled $304.1 million at December 31, 2011, compared to
S-142
Table of Contents
$360.1 million at December 31, 2010, a decrease of $56.0 million, or 15.6%. HCP’s members’ equity at December 31, 2011 was $188.1 million, compared to $566.0 million at December 31, 2010. In January 2011, HCP repurchased 24.1 million Class A Preferred Units from investment funds affiliated with Summit Partners, LP in exchange for $540.0 million in cash and 436,550 Class B common units valued at $10.0 million and an assumed tax liability of $37.0 million. This transaction was financed from a combination of cash on hand and cash provided by a new and expanded credit facility which replaced HCP’s previously existing credit facility.
HCP has historically financed its operations primarily through internally generated funds. HCP generates cash primarily from fees for medical services provided under capitated contracts with various health plans or under fee-for-service arrangements, and its primary use of cash is the payment of medical costs. HCP generally invests cash in short-term fixed income securities that have final maturities of five years or less and average maturity of two years or less. Professional portfolio managers operating under documented guidelines manage HCP’s investments. Additionally, as of December 31, 2011, HCP had invested $340.9 million in a portfolio of highly liquid money market securities, and HCP’s fixed income investments consisted solely of investment-grade debt securities. All of HCP’s investments are classified as current assets, except for auction rate securities with an estimated fair value of $3.0 million at December 31, 2011, which are classified as non-current assets.
During 2011, HCP’s cash and cash equivalents increased by $33.4 million. In 2011, net cash provided by operating activities was $509.3 million, which was attributable principally to net income of $408.6 million. The following non-cash expenses also contributed to the cash provided by operating activities: depreciation and amortization of $30.6 million, amortization of debt issuance costs of $3.1 million, and share-based compensation of $7.5 million. HCP’s cash provided by operating activities was also sourced from increases in accounts payable, accrued compensation and other liabilities of $56.0 million and in medical claims and capitation payable of $35.4 million, partially offset by decreases in amounts payable to health plans of $19.7 million.
In 2010, net cash provided by operating activities was $343.1 million, which was attributable principally to net income of $329.9 million. The following non-cash expenses also contributed to the cash provided by operating activities: depreciation and amortization of $28.6 million, amortization of debt issuance costs of $0.7 million, and share-based compensation of $7.4 million. HCP’s cash provided by operating activities was also sourced from increases in accounts payable, accrued compensation and other liabilities of $36.9 million and in medical claims and capitation payable of $46.4 million, partially offset by decreases in amounts payable to health plans of $56.3 million.
Net cash used in investing activities during 2011 was $56.5 million, which resulted primarily from cash used to finance acquisitions, net of cash acquired, of $39.8 million and from capital expenditures of $23.2 million, offset by investments in marketable securities and other items.
Net cash used in investing activities during 2010 was $225.6 million, which resulted primarily from net purchases of marketable securities of $175.7 million, and from cash used to finance acquisitions, net of cash acquired, of $30.7 million.
Net cash used in financing activities during 2011 was $419.5 million. As discussed, during January 2011 HCP repurchased 24.1 million Class A Preferred Units from investment funds affiliated with Summit Partners, LP in exchange for $540.0 million in cash (as well as non-cash activities of 436,550 Class B common units valued at $10.0 million and an assumed tax liability of $37.0 million). This transaction was partially financed from $585.0 million of proceeds from HCP’s credit facility. The proceeds from the credit facility were also utilized to extinguish HCP’s previous credit facility, with a then outstanding term loan balance of $217.0 million. HCP also made payments during 2011 of $29.3 million on the term loan under its new credit facility. During 2011, HCP issued cash distributions to its members of $211.0 million to fund their respective pass-through tax liabilities associated with their ownership interests, and repurchased for $6.4 million vested options to acquire HCP common units.
S-143
Table of Contents
Net cash used in financing activities during 2010 was $113.9 million, which consisted of cash distributions to members of HCP of $111.1 million and principal payments on the term loan of its credit facility of $2.8 million.
During the six months ended June 30, 2012, HCP’s cash and cash equivalents decreased by $39.4 million. Cash provided by operating activities during the six months ended June 30, 2012 was $184.1 million, as compared to $180.7 million during the six months ended June 30, 2011. Cash used in investing activities during the six months ended June 30, 2012 was $31.7 million, as compared to $15.3 million during the six months ended June 30, 2011, for which the increase is primarily attributable to an increase in cash used to finance acquisitions of $7.4 million and to increases in cash used to purchase marketable securities. Cash used in finance activities during the six months ended June 30, 2012 was $191.7 million, as compared to $343.8 million during the six months ended June 30, 2011. The decrease is primarily attributable to net cash utilized during January 2011 related to the repurchase of Class A Preferred Units and concurrent refinancing of HCP’s credit facility, offset by an increase in distributions to members from $150.4 million during the six months ended June 30, 2011, as compared to $176.9 million during the six months ended June 30, 2012.
Credit Facility
On January 6, 2011, HCP extinguished its then existing credit facility in full and entered into a new credit facility, or HCP Credit Facility, which includes a term loan in the amount of $585.0 million and a revolving line of credit in the amount of $15.0 million, with various lenders. Principal payments on the term loan are due quarterly until maturity on January 6, 2016. The term loan bears interest at the Eurodollar rate (defined as the British Bankers Association London interbank offered rate for deposits in U.S. dollars) plus a margin ranging from 1.50% to 2.50%, based on defined financial ratios. The average interest rate on the HCP Credit Facility during 2011 was 2.07%. The HCP Credit Facility is secured by substantially all of HCP’s assets.
At June 30, 2012, HCP had issued several standby letters of credit in a collective amount of $17.0 million, primarily to secure medical service obligations and workers’ compensation claim liabilities. There were no amounts drawn under these letters of credit.
The HCP Credit Facility includes usual and customary covenants, including restrictive financial covenants. As of June 30, 2012, HCP was in compliance with all covenants under the HCP Credit Facility. The HCP Credit Facility will be repaid and terminated as of the closing of the Merger.
Critical Accounting Policies and Estimates
The preparation of the consolidated financial statements of HCP in accordance with U.S. generally accepted accounting principles requires its management to make a number of estimates and assumptions relating to the reported amount of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and to the reported amounts of revenues and expenses during the period. HCP bases its estimates on historical experience and on various other assumptions that HCP believes are reasonable under the circumstances. Changes in estimates are recorded if and when better information becomes available. Actual results could significantly differ from those estimates under different assumptions and conditions. HCP believes that the accounting policies discussed below are those that are most important to the presentation of its financial condition and results of operations and that require its management’s most difficult, subjective and complex judgments.
Variable Interest Entities
Accounting Standards Codification (ASC) Section 810-10-15-14 stipulates that generally any entity with (a) insufficient equity to finance its activities without additional subordinated financial support or (b) equity holders that, as a group, lack the characteristics that evidence a controlling financial interest, is considered a
S-144
Table of Contents
Variable Interest Entity, or VIE. All VIEs in which HCP owns a majority voting interest and all VIEs for which HCP is the primary beneficiary are included in HCP’s consolidated financial statements. HCP determines whether it is the primary beneficiary of a VIE through a qualitative analysis that identifies which variable interest holder has the controlling financial interest in the VIE. The variable interest holder who has both of the following has the controlling financial interest and is the primary beneficiary: (1) the power to direct the activities of the VIE that most significantly impact the VIE’s economic performance and (2) the obligation to absorb losses of, or the right to receive benefits from, the VIE that could potentially be significant to the VIE. In performing this analysis, HCP management considered all relevant facts and circumstances, including: the design and activities of the VIE, the terms of the contracts the VIE has entered into, the nature of the VIE’s variable interests issued and how they were negotiated with or marketed to potential investors, and which parties participated significantly in the design or redesign of the entity. HCP’s consolidated financial statements include HCPAMG, HealthCare Partners Medical Group, Inc., and Healthcare Partners Medical Group (Bacchus), Ltd.
Revenue Recognition
HCP’s medical revenue consists primarily of fees for medical services provided under shared risk capitated (or per person) contracts with various health plans in its southern California market, and fees for medical and hospital services under global capitation contracts in its central Florida and southern Nevada markets. Capitation revenue under health plan contracts is paid monthly based on the number of enrollees assigned to HCP’s employed and affiliated physicians.
Capitation revenue paid by health plans is recognized in the month in which HCP is obligated to provide medical and hospital services. Capitation revenue may be subsequently adjusted to reflect changes in enrollment as a result of enrollee retroactive terminations or additions. Such retroactive terminations or additions have not had a material effect on capitation revenue.
Revenue that HCP receives with respect to its senior membership is adjusted periodically to give effect to the relative risk profile of the senior members for whom HCP is financially responsible. In the Balanced Budget Act of 1997, Congress created a rate-setting methodology that included a provision requiring CMS to implement a risk adjustment payment system for Medicare health plans. Risk adjustment uses health status indicators to improve the accuracy of risk adjusted payments and establish incentives for plans to enroll and treat less healthy Medicare beneficiaries. Under this methodology, health plans must capture, collect and submit diagnosis code information to CMS. Capitation premiums under this methodology are paid at interim rates during the year and retroactive adjustments occur in subsequent periods (generally in the third quarter of the same year, with a final adjustment in the third quarter of the following year) after data is compiled by CMS. Positive or negative payment adjustments are made for enrollees with conditions requiring more or less health care services than assumed in the interim payments. HCP includes in the first two quarters of each year an estimate of the retroactivity adjustment, based on each enrollee’s anticipated payment rates derived from qualifying diagnoses coded on behalf of enrollees as compiled in its data bases. HCP anticipates that, over the coming years, as HCP continues to collect more historical data and refine its estimation practices, HCP will record larger estimates in the first and second quarter of any given year.
HCP also has the potential to earn additional revenue or incur losses under health plan contracts by sharing in the risk of hospitalization based upon inpatient and related services utilized by its members. HCP estimates risk pool revenues based on estimated hospital utilization and associated costs compared to contracted rates. Differences between actual and estimated settlements are recorded when the final outcome is known. HCP also receives incentives under “pay for performance” programs for quality medical care based on various criteria. HCP estimates revenues under these programs based on historical performance and contractual guarantees.
Medical Claims Liability and Related Payable, Medical Expense and Hospital Expense
Medical expense and hospital expense are recognized in the period in which services are provided and include an estimate of claims which have been incurred but not reported as of the balance sheet date. Medical
S-145
Table of Contents
claims expenses consist of payments for professional services to contracted independent physician associations, medical groups and physicians that are not affiliated with HCP. Hospital expenses consist of payments for institutional services to contracted hospitals. In addition, as explained above under “—Key Financial Measures and Indicators—Operating Revenues,” under HCP’s risk-share capitation model, risk-share revenues (that is, the portion of the excess of hospital capitation revenue to which HCP is entitled over hospital expenses) is based on the number of enrollees and estimates of hospital utilization and associated costs incurred by assigned health plan enrollees, including an estimate of institutional claims which have been incurred but not reported.
The IBNR component of total medical claims liability and related payables is based on historical utilization and payment trends, medical inflation, enrollment levels, product line and other relevant information. Estimating IBNR is complex and involves a significant amount of judgment. Accordingly, it represents HCP’s most critical accounting estimate. Such estimates are analyzed and reviewed on a monthly basis, and adjustments to monthly accrual estimates are reflected in current operations. Changes in this estimate can materially affect, either favorably or unfavorably, HCP’s combined operating results and overall financial position.
HCP’s IBNR estimate methodology utilizes standard lag-based actuarial models to develop completion factors. The model considers claims payment data for the most recent 24 months. The completion factor is a measure of how complete the claims paid to date are relative to the estimate of the total claims for services rendered for a given reporting period. For any given month of service, the corresponding completion factor is divided into claims paid to date to estimate the amount of incurred claims for the given month. Although the completion factor is generally reliable for older service periods, it is more volatile, and hence less reliable, for more recent measurement periods given inherent billing and processing lags involved in the administration of claim payments. As a result, for the most recent two to three service months, the estimate for incurred claims is developed from a trend factor analysis based on per member per month claims trends experienced in the preceding months, as adjusted for other relevant factors.
Each month, HCP re-examines the previously established medical claims liability and related payable estimates based on actual claim submissions and other relevant changes in facts and circumstances. As the liability estimates recorded in prior periods become more exact, HCP increases or decreases the amount of the estimates, and include the changes in medical expenses in the period in which the change is identified. In every reporting period, HCP’s operating results include the effects of more completely developed medical claims liability and related payable estimates associated with prior periods.
HCP believes that the amount of its medical claims liability estimate is adequate to provide for its ultimate liability for incurred claims as of the balance sheet dates; however, the amount of actual future claim payments may differ from its estimate. Assuming a hypothetical 1% variance in HCP’s estimate of accrued medical claims, HCP’s medical expense for the years ended December 31, 2011 and 2010 would increase or decrease by approximately $0.9 million and $0.8 million, respectively.
HCP also regularly evaluates the potential need to establish premium deficiency reserves for the probability that anticipated future health care costs could exceed future capitation payments from health plans. To date, HCP has determined that no premium deficiency reserves have been necessary.
Goodwill and Intangible Assets
HCP’s goodwill balance was $287.7 million and $278.6 million at June 30, 2012 and December 31, 2011, respectively. Goodwill represents the excess cost of a business acquisition over the fair value of the net assets acquired. As of December 31, 2011, HCP early adopted ASU 2011-08, Testing Goodwill for Impairment. As such, HCP assessed qualitative factors to determine whether it is more likely than not that fair value of the reporting units is less than the carrying value. As a result of this assessment, it was determined that performing the two-step impairment test was not necessary. HCP’s analysis indicated that goodwill had not been impaired as of December 31, 2011.
S-146
Table of Contents
HCP’s intangible assets were $157.8 million and $157.4 million at June 30, 2012 and December 31, 2011, respectively. Identifiable intangible assets with definite useful lives consist primarily of customer relationships and trade names, and are amortized over periods between two and 26 years. Intangible assets are measured for impairment when events or changes in business conditions suggest that the carrying value of an asset may not be recovered. No intangible assets were deemed to be impaired at June 30, 2012 and December 31, 2011.
In addition to the initial consideration paid at the close of each business combination, the stock or asset purchase agreements for completed acquisitions frequently provide for additional future consideration to be paid, which is generally based upon the achievement of certain operating results of the acquired organization as of defined measurement dates. Pursuant to GAAP, the estimated fair value of contingent consideration is accrued at the acquisition date. Subsequent changes, if any, to the estimated fair value of contingent consideration are recognized as part of on-going operations.
Recent Accounting Pronouncements
In May 2011, the Financial Accounting Standards Board (FASB) issued ASU Number 2011-04,Fair Value Measurement, which modifies the fair value measurement and disclosure guidance. This guidance results in new disclosures primarily related to Level 3 measurements, including quantitative disclosure about unobservable inputs and assumptions, a description of the valuation processes, and a narrative description of the sensitivity of the fair value to changes in unobservable inputs. This guidance is effective for reporting periods (including interim periods) beginning after December 15, 2011, and HCP adopted this guidance for the interim period ending March 31, 2012. The adoption of this ASU did not have a material impact on HCP’s financial position, results of operations, or cash flows, but changed certain disclosures.
In June 2011, the FASB issued ASU Number 2011-05,Presentation of Comprehensive Income, which changed the disclosure requirements for the presentation of other comprehensive income (OCI) in the financial statements, including eliminating the option to present OCI in the statement of stockholders’ equity. OCI and its components will be required to be presented for both interim and annual periods either in a single financial statement, the statement of comprehensive income, or in two separate but consecutive financial statements consisting of a statement of income followed by a separate statement presenting OCI. HCP adopted this ASU for the interim period ending March 31, 2012, which is the period for which it became effective.
In July 2011, the FASB issued an ASU modifying the presentation and disclosure of patient service revenue, provision for bad debts, and the allowance for doubtful accounts. This guidance changes the presentation on the statement of operations by requiring the reclassification of the provision for bad debts associated with patient service revenues from an operating expense to a deduction from patient service revenue (net of contractual allowances and discounts). Additionally, the amendment requires disclosures regarding HCP’s policy for recognizing patient service revenue and assessing bad debts. Qualitative and quantitative information about changes in the allowance for doubtful accounts is required. This guidance is effective for annual periods beginning after December 15, 2012, and interim and annual periods thereafter, and should be applied retrospectively to all prior periods presented. The adoption of this accounting standards update will not have a material impact on HCP’s statement of financial position, operating results, or cash flows.
S-147
Table of Contents
Contractual Obligations and Reserves
The below table summarizes by maturity HCP’s significant contractual obligations and reserves as of June 30, 2012:
| Total | Remainder of 2012 | 2013-2014 | 2015-2016 | 2017 and beyond | ||||||||||||||||
| (dollar amounts in thousands) | ||||||||||||||||||||
Long term debt | $ | 541,234 | $ | 14,625 | $ | 73,125 | $ | 453,375 | $ | 109 | ||||||||||
Operating leases | 192,381 | 19,184 | 71,686 | 51,423 | 50,088 | |||||||||||||||
Capital leases | 332 | 198 | 134 | 0 | 0 | |||||||||||||||
Deferred compensation liabilities (1) | 43,504 | 0 | 0 | 0 | 43,504 | |||||||||||||||
Acquisition earn-out payments (2) | 10,408 | 10,408 | 0 | 0 | 0 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total contractual obligations | $ | 787,859 | $ | 44,415 | $ | 144,945 | $ | 504,798 | $ | 93,701 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
| (1) | Due to uncertainty regarding payment timing, obligations for deferred compensation liabilities have been categorized in the “2017 and beyond” category. |
| (2) | In addition to the initial consideration paid pursuant to certain stock and asset purchase agreements entered into, additional contingent consideration is to be paid based upon the achievement of certain operating results. |
HCP has typically paid 90% to 95% of medical claims within six months of the date incurred and approximately 98% of medical claims within nine months of the date incurred. HCP believes medical claims liabilities are short-term in nature and therefore do not meet the listed criteria for classification as contractual obligations and, accordingly, have been excluded from the table above.
Off-Balance Sheet Arrangements
As part of HCP’s ongoing business, it does not participate or knowingly seek to participate in transactions that generate relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities, or SPE, which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes. As of June 30, 2012, HCP was not involved in any SPE transactions.
S-148
Table of Contents
We are a leading provider of kidney dialysis services in the U.S. for patients suffering from chronic kidney failure, also known as end stage renal disease, or ESRD. As of June 30, 2012, DaVita provided dialysis and other related services through a network of 1,884 outpatient dialysis centers located in the U.S. throughout 43 states and the District of Columbia, serving a total of approximately 149,000 patients. In addition, as of June 30, 2012, DaVita provided outpatient dialysis and administrative service to a total of 19 outpatient dialysis centers located in four countries outside the U.S. DaVita centers offer outpatient hemodialysis treatments and other ESRD-related services such as the administration of physician-prescribed pharmaceuticals, including erythropoietin, or EPO, vitamin D analogs and iron supplements. DaVita also provides services for home dialysis patients, vascular access, disease management services and laboratory services related to ESRD. As of June 30, 2012, DaVita also provides acute inpatient dialysis services in approximately 960 hospitals and related laboratory services throughout the U.S. DaVita is a Delaware corporation, incorporated in 1994.
DaVita’s U.S. dialysis and related lab services business accounted for approximately 92% of DaVita’s consolidated net operating revenues for the twelve months ended June 30, 2012. Other ancillary services and strategic initiatives accounted for approximately 8% of our consolidated net operating revenues for the same period and relate primarily to DaVita’s core business of providing kidney dialysis services. For the twelve months ended June 30, 2012, DaVita generated consolidated net operating revenues of $7,365 million, Adjusted EBITDA of $1,585 million, and net income attributable to DaVita of $519 million. For an explanation of Adjusted EBITDA and a reconciliation of Adjusted EBITDA to net income, see “DaVita Summary Historical Financial and Operating Data” beginning on page S-30.
We provide our services through the following business segments:
Dialysis and Related Lab Services. Our network of 1,884 outpatient dialysis centers located in the U.S. and 19 outpatient dialysis centers located outside the U.S. are designed specifically for outpatient hemodialysis. In the twelve months ended June 30, 2012 our overall network of outpatient dialysis centers increased by 14% primarily as a result of acquisitions and the opening of new centers, net of center closures and divestitures. A large portion of this increase was driven from the acquisition of DSI Renal Inc., or DSI, a medium sized dialysis provider that we acquired in September 2011, that contributed a net 83 outpatient dialysis centers.
Throughout the U.S. we also provided hospital inpatient hemodialysis services, excluding physician services, to patients in approximately 960 hospitals as of June 30, 2012. We render these services for a contracted per-treatment fee that is individually negotiated with each hospital. When a hospital requests our services, we typically administer the dialysis treatment at the patient’s bedside or in a dedicated treatment room in the hospital, as needed. In the twelve months ended June 30, 2012 hospital inpatient hemodialysis services accounted for approximately 4.5% of our total U.S. dialysis treatments.
We also own two separately incorporated, licensed, clinical laboratories, which specialize in ESRD patient testing. These specialized laboratories provide routine laboratory tests for dialysis and other physician-prescribed laboratory tests for ESRD patients. Our laboratories provide these tests predominantly for our network of ESRD patients throughout the U.S. These tests are performed to monitor a patient’s ESRD condition, including the adequacy of dialysis, as well as other medical conditions. Our laboratories utilize information systems which provide information to certain members of the dialysis centers’ staff and medical directors regarding critical outcome indicators.
As of June 30, 2012, we operated or provided management and administrative services to 24 outpatient dialysis centers located in the U.S. and three outpatient dialysis centers located outside of the U.S. in which we either own a minority equity investment or which are wholly-owned by third parties. These services are provided
S-149
Table of Contents
pursuant to management and administrative services agreements. Management fees are established by contract and are recognized as earned typically based on a percentage of revenues or cash collections generated by the centers.
Ancillary Services and Strategic Initiatives. Our ancillary services and strategic initiatives consist of pharmacy services, infusion therapy services, disease management services, vascular access services, ESRD clinical research programs, physician services, direct primary care and our international dialysis operations.
DaVita’s Industry
The loss of kidney function is normally irreversible. Kidney failure may be caused by Type I and Type II diabetes, high blood pressure, polycystic kidney disease, long-term autoimmune attack on the kidney and prolonged urinary tract obstruction. Patients suffering from ESRD generally require dialysis at least three times a week for the rest of their lives. Treatment options that we provide for ESRD are hemodialysis and peritoneal dialysis. Hemodialysis, the most common form of ESRD treatment, uses an artificial kidney, called a dialyzer, to remove toxins, fluids and salt from the patient’s blood. The procedure is typically performed at a freestanding center, a hospital-based outpatient center, or at the patient’s home. Peritoneal dialysis uses the patient’s peritoneal or abdominal cavity to eliminate fluid and toxins and is typically performed in the patient’s home.
The dialysis industry is characterized by:
Stable and Growing Patient Base. The nature of ESRD allows for significant demand stability due to a lack of clinical need controversy and limited treatment alternatives for patients. In addition, patients require treatment at least three times a week for the rest of their lives, regardless of seasonality or macroeconomic conditions. According to U.S. Renal Data System, there were approximately 399,000 ESRD dialysis patients in the U.S. in 2009 and the underlying ESRD dialysis patient population grew at an approximate compound rate, or CAGR, of 3.9% from 2000 to 2009, the latest period for which such data is available. The growth rate is attributable to the aging of the population, increased incidence rates for diseases that cause kidney failure such as diabetes and hypertension, lower mortality rates for dialysis patients and growth rates of minority populations with higher than average incidence rates of ESRD.
Competitive Landscape. The dialysis industry has consolidated significantly over time, but still remains highly competitive. The two largest dialysis companies account for approximately 70% of the U.S. dialysis patient population based upon management estimates, with DaVita serving approximately 33% of that population. The remainder of the industry is highly fragmented, comprised of regional chains, local hospital based dialysis facilities and physician and other independently-owned centers.
Universal Medicare Reimbursement. Since 1972, the federal government has provided health care coverage for ESRD patients under the Medicare ESRD program, regardless of age or financial circumstances. ESRD is the first and only disease state eligible for dialysis and dialysis-related lab services and for all benefits available under the Medicare program. Although Medicare reimbursement limits the allowable charge per treatment, it provides industry participants with a relatively predictable and recurring revenue stream for dialysis services provided to patients without commercial insurance. For DaVita, revenue attributable to Medicare and Medicare-assigned plans represented 59% of dialysis and related lab services revenues for the twelve months ended June 30, 2012.
Significant Government Responsibility. Because of universal Medicare reimbursement for dialysis treatment, the federal government provides significant oversight and regulation of the dialysis sector on a federal, state and local level. A primary concern is the significant, yet fragmented, presence of approximately 825 independent providers of dialysis treatments, whose survival depends on adequate Medicare reimbursement rates. Given patient dependence on dialysis for sustaining life and the critical financial role undertaken by the government, we believe there is likely to be some protection from government rate cuts or any cuts that would make it difficult for small and regional providers to continue to offer dialysis services to their patients.
S-150
Table of Contents
Bundled Reimbursement System. Since January 2011, ESRD payments have been made under a single bundled payment rate that provides for an annual inflation adjustment, based upon a market basket index, less a productivity improvement factor. The bundled payment rate provides a fixed payment rate to encompass all goods and services provided during the dialysis treatment, including pharmaceuticals that were historically separately reimbursed to the dialysis providers, such as Epogen®, or EPO, vitamin D analogs and iron supplements, irrespective of the level of pharmaceuticals administered or additional services performed. Most lab services that used to be paid directly to laboratories are also included in the new bundled payment. The bundled payment rate is also adjusted for certain patient characteristics, a geographic usage index and certain other factors.
Also, beginning January 1, 2014, certain oral-only ESRD drugs (currently paid separately to pharmacies under Medicare Part D) will be included in the ESRD bundled payment to dialysis facilities. It is currently unclear how CMS will “price” the oral-only drugs for inclusion in the ESRD bundle in 2014.
Although Medicare reimbursement limits the allowable charge per treatment, it provides industry participants with a relatively predictable and recurring revenue stream for dialysis services provided to patients without commercial insurance. For the twelve months ended June 30, 2012, 90% of our total patients were under government-based programs, with approximately 80% of our patients under Medicare and Medicare-assigned plans.
DaVita’s Competitive Strengths
Superior Clinical Outcomes. We believe that the clinical outcomes of our patient population compare favorably with other dialysis providers and generally exceed the dialysis outcome quality indicators of the National Kidney Foundation. To better assess overall outcomes improvement we have developed our own index, which we refer to as the DaVita Quality Index, or DQI. DQI takes into account outcomes associated with adequacy of dialysis, anemia management, cardiovascular and bone disease, nutrition, and vascular access. The DQI methodology awards points for the percentage of patients exceeding a specified goal and deducts points for the percentage of patients falling below a certain level, providing an objective measure of our total patient care. We believe that DQI correlates with patient survival and likelihood of hospitalization. We believe that our strong clinical outcomes have led to improved quality of life for our patients, lower mortality rates, reduced hospitalizations, and greater satisfaction with care. We believe that this, in turn, has reduced overall patient costs for the payors. In addition, we have an active national physician council, consisting of twenty physicians across the country, that advises our senior management on all clinical issues impacting our operations. DaVita and its affiliated physicians collaborated to achieve outstanding clinical outcomes in 2011. As just one example, our patients’ 2010 gross mortality rate improved for the fifth straight year to 15%, a 16% improvement from our 2005 mortality rate of 19%.
National scale. DaVita has a network of 1,884 outpatient dialysis and administrative centers located in the U.S. throughout 43 states and the District of Columbia, serving a total of approximately 149,000 patients. This scale allows DaVita to provide its patient base with convenient locations and access to a full range of services; benefit from economies of scale in purchases of pharmaceuticals and other medical supplies and services; enhance relationships with managed care payors by offering an extensive set of related services to lower the overall cost of patient care; leverage information technology and compliance systems; provide a greater depth and breadth of services; strengthen its medical director recruitment and retention initiatives; and develop the expertise and obtain the resources needed to continue to expand the business through denovo center expansion and selected acquisitions.
Strong operating track record. DaVita has demonstrated strong and resilient financial performance even through the recent macroeconomic downturn as demand for care is steady, predictable and independent of the many macroeconomic factors affecting the broader economy. DaVita’s growth has been underpinned by the
S-151
Table of Contents
stable volume growth of the underlying dialysis patient population, which increased at a CAGR of 3.9% from 2000 through 2009, the latest period for which such data is available. Since June 30, 2009, DaVita’s quarterly organic growth has ranged between 3.7% and 5.5%. From June 30, 2009 to June 30, 2012, DaVita’s net operating revenue and Adjusted EBITDA have grown at CAGRs of 8.8% and 10.0%, respectively.
Strong and stable free cash flow. The stability of demand and reimbursement for DaVita’s services, consistent historical Adjusted EBITDA margins of approximately 20%–22% since fiscal year ended December 31, 2009 and efficient management of working capital have resulted in strong operating cash flow. DaVita has increased its net cash provided by operating activities from $705 million in the twelve months ended June 30, 2009 to $1,180 million for the twelve months ended June 30, 2012, representing a CAGR of 19%. In addition, DaVita’s centers require limited and predictable maintenance capital expenditures once they are operational, resulting in strong and stable free cash flow generation, which allows DaVita to fund its growth-related investments and reduce indebtedness. DaVita’s maintenance capital expenditures have ranged from $104–$259 million, or approximately 2%–4% of consolidated net operating revenues, between the twelve months ended June 30, 2009 and the twelve months ended June 30, 2012. DaVita has increased its free cash flow from $527 million in the twelve months ended June 30, 2009 to $817 million for the twelve months ended June 30, 2012, representing a CAGR of 16%. For an explanation of free cash flow and a reconciliation to operating cash flow, see “DaVita Summary Historical Financial and Operating Data” beginning on page S-30.
Comprehensive compliance program.DaVita’s dialysis operations are subject to extensive federal, state and local government regulations. Management has designed and implemented a company-wide, corporate compliance program as part of DaVita’s commitment to comply fully with all applicable laws and regulations and to maintain the high standards of conduct DaVita expects from all of its employees, whom DaVita refers to as its teammates. To increase awareness of the necessity of complying with all applicable laws and regulations, DaVita has developed ongoing training programs for its teammates through its in-house training program, DaVita University. In addition, DaVita has well-established guidelines around physician roles and responsibilities and requires that its physicians attest to their adherence to these guidelines on a periodic basis. DaVita’s compliance programs are overseen by the Chief Compliance Officer who reports directly to the Chief Executive Officer and to the Compliance Committee of the Board of Directors.
Experienced management team. DaVita’s management team has extensive experience and expertise in the dialysis industry with an average of 15 years of industry experience. Under management’s guidance, DaVita has enjoyed consistent improvements in clinical outcomes, improving contract negotiation results with managed care payors, strong organic growth and successful acquisition and denovo growth. DaVita’s consolidated net operating revenues, Adjusted EBITDA and number of U.S. centers in operation grew from $1.3 billion, $188 million and 572, respectively, in 1999 when DaVita’s current Chief Executive Officer, Kent Thiry, joined DaVita as CEO, to $7.4 billion, $1.6 billion and 1,884, respectively, as of and for the twelve months ended June 30, 2012.
S-152
Table of Contents
DaVita’s Strategy
DaVita plans to continue to grow its business and improve its financial performance by implementing its business strategy, the key elements of which are:
Continuous improvement in patient care.DaVita believes its reputation for providing quality patient care is a key factor in attracting patients and qualified medical directors as well as in maintaining and building relationships with referring physicians and managed care and government payors. DaVita strives to deliver best-in-class clinical outcomes as well as increase patient involvement in their care. For example, DaVita’s “At Home Initiative” is committed to leading the introduction and promotion of effective home hemodialysis and peritoneal dialysis solutions for healthier, more independent dialysis patients who prefer to dialyze at home. Moreover, DaVita is committed to continuous improvement in its medical and clinical processes through quality management programs to monitor and enhance the level of services it delivers. Through these quality management programs supervised by the Office of the Chief Medical Officer and the Directors of Clinical Services, DaVita continuously works to promote its high standards of patient care. These efforts include further development and implementation of patient care policies and procedures, clinical education and training programs, clinical guidelines and protocols and audits of the quality of services rendered at each of DaVita’s centers. Although it is difficult to reliably measure clinical performance across the dialysis industry, DaVita believes its clinical outcomes compare favorably with other dialysis providers in the U.S.
Developing and maintaining strong relationships with physicians. DaVita continuously seeks to develop relationships with nephrologists. DaVita believes that collaborating with these physicians leads to enhanced quality of care, patient satisfaction and physician satisfaction. DaVita intends to sustain and strengthen its physician relationships by emphasizing DaVita’s high quality of care and state-of-the-art centers, expanding its broad array of services and technologies, developing and offering quality training programs and continuing to involve DaVita’s physicians in establishing clinical guidelines and protocols.
Expansion of operations. DaVita intends to continue to expand its operations by building out its existing centers, as well as developing and/or acquiring new centers both domestically and internationally. DaVita will continue to evaluate acquisition and denovo opportunities that it identifies as complementary to its existing base of operations or as compelling for new geographic expansion. DaVita believes that its enhanced geographic presence makes it a more attractive partner for national managed care payors.
Integrated kidney care.DaVita maintains an integrated approach to managing the overall health of kidney disease patients through the development and administration of DaVita’s ancillary service offerings, including DaVita Rx, Lifeline and VillageHealth. DaVita Rx, DaVita’s pharmacy services offering, provides oral medications to DaVita’s ESRD patients with the main objectives of (i) providing patients a convenient way to fill their prescription needs by delivering the prescriptions to the center where they are treated and (ii) improving clinical outcomes by facilitating increased patient compliance. Lifeline, DaVita’s vascular access services offering, provides management and administrative services to physician-owned vascular access clinics that provide surgical and interventional radiology services for dialysis patients. VillageHealth, DaVita’s disease management services offering, provides advanced care management services to health plans and government agencies for employees/members diagnosed with ESRD.
Effective teammate retention and satisfaction. DaVita’s dialysis business requires nurses and other teammates with specialized training for treating patients with complex care needs. Recruitment and retention of nurses are continuing concerns for health care providers due to short supply. DaVita has an active program of investing in its teammates. As a result of these efforts DaVita’s teammate turnover has improved from 25% to 18% for the quarter ended June 30, 2012 compared to the quarter ended December 31, 2007. This has been a major contributor to DaVita’s improving productivity and effective cost control. To meet DaVita’s recruitment and retention targets, the Company offers its teammates expanded training opportunities, tuition reimbursements and other incentives.
S-153
Table of Contents
Services We Provide
Dialysis and Related Lab Services
Outpatient dialysis services
As of June 30, 2012, we operated or provided administrative services through a network of 1,884 outpatient dialysis centers located in the U.S. and 19 outpatient dialysis centers located outside the U.S. that are designed specifically for outpatient hemodialysis. For the twelve months ended June 30, 2012, our overall network of outpatient dialysis centers increased by 234 primarily as a result of acquisitions and the opening of new centers, net of center closures and divestitures, representing a total increase of approximately 14%. A large portion of this increase was driven from the acquisition of DSI, a medium sized dialysis provider that we acquired in September 2011, that contributed a net 83 outpatient dialysis centers.
As a condition of our enrollment in Medicare, we contract with a nephrologist or a group of affiliated nephrologists to provide medical director services at each of our centers. In addition, other nephrologists may apply for practice privileges to treat their patients at our centers. Each center has an administrator, typically a registered nurse, who supervises the day-to-day operations of the center and its staff. The staff of each center typically consists of registered nurses, licensed practical or vocational nurses, patient care technicians, a social worker, a registered dietician, biomedical technician support and other administrative and support personnel.
Many of our outpatient dialysis centers offer certain support services for dialysis patients who prefer and are able to perform either home-based hemodialysis or peritoneal dialysis in their homes. Home-based hemodialysis support services consist of providing equipment and supplies, training, patient monitoring, on-call support services and follow-up assistance. Registered nurses train patients and their families or other caregivers to perform either home-based hemodialysis or peritoneal dialysis.
Under Medicare regulations, we cannot promote, develop or maintain any kind of contractual relationship with our patients that would directly or indirectly obligate a patient to use or continue to use our dialysis services, or which would give us any preferential rights other than those related to collecting payments for our services. Our total patient turnover which is based upon all causes averaged approximately 30% per year for the last two years. However, for the twelve months ended June 30, 2012, the overall number of patients to whom we furnished services in the U.S. increased by approximately 14%, primarily from new centers and acquisitions, as well as continued growth within the industry and lower mortality rates.
Hospital inpatient hemodialysis services
As of June 30, 2012, we provided hospital inpatient hemodialysis services, excluding physician services, to patients in approximately 960 hospitals throughout the U.S. We render these services for a contracted per-treatment fee that is individually negotiated with each hospital. When a hospital requests our services, we typically administer the dialysis treatment at the patient’s bedside or in a dedicated treatment room in the hospital, as needed. Hospital inpatient hemodialysis services are required for patients as discussed above. For the twelve months ended June 30, 2012, hospital inpatient hemodialysis services accounted for approximately 4.5% of our total U.S. dialysis treatments.
ESRD laboratory services
We own two separately incorporated, licensed, clinical laboratories which specialize in ESRD patient testing. These specialized laboratories provide routine laboratory tests for dialysis and other physician-prescribed laboratory tests for ESRD patients. Our laboratories provide these tests predominantly for our network of ESRD patients throughout the U.S. These tests are performed to monitor a patient’s ESRD condition, including the adequacy of dialysis, as well as other medical conditions. Our laboratories utilize information systems which provide information to certain members of the dialysis centers’ staff and medical directors regarding critical outcome indicators.
S-154
Table of Contents
Management services
As of June 30, 2012, we operated or provided management and administrative services to 24 outpatient dialysis centers located in the U.S. and three outpatient dialysis centers located outside of the U.S. in which we either own a minority equity investment or are wholly-owned by third parties. These services are provided pursuant to management and administrative services agreements. Management fees are established by contract and are recognized as earned typically based on a percentage of revenues or cash collections generated by the centers.
Ancillary services and strategic initiatives
Ancillary services and strategic initiatives, which include our international dialysis operations, as described above, accounted for approximately 8% of our total consolidated net operating revenues for the twelve months ended June 30, 2012, consist primarily of the following:
| • | Pharmacy services. DaVita Rx is a pharmacy that provides oral medications to DaVita’s patients with ESRD. The main objectives of the pharmacy are to improve clinical outcomes by facilitating increased patient compliance and to provide our patients a convenient way to fill their prescription needs by delivering the prescriptions to the center where they are treated. Revenues are recognized as prescriptions are filled and shipped to patients. |
| • | Infusion therapy services. HomeChoice Partners provides comprehensive personalized infusion therapy services to patients typically in their own homes as a cost-effective alternative to inpatient hospitalization. Intravenous and nutritional support therapies are typically managed by registered and/or board-certified professionals including pharmacists, nurses and dieticians in collaboration with the patient’s physician in support of the patient’s ongoing health care needs. Revenues are recognized in the period when infusion therapy services are provided. |
| • | Disease management services. VillageHealth provides advanced care management services to health plans and government agencies for employees/members diagnosed with Chronic Kidney Disease, CKD or ESRD. Through a combination of clinical coordination, medical claims analysis and information technology, we endeavor to assist our customers and patients in obtaining superior renal health care and improved clinical outcomes, as well as helping to reduce overall medical costs. Revenues are typically based upon an established contract fee and are recognized as earned over the contract period and can include additional fees for cost savings recognized by certain customers. |
| • | Vascular access services. Lifeline provides management and administrative services to physician-owned vascular access clinics that provide surgical and interventional radiology services for dialysis patients. Lifeline also is the majority-owner of one vascular access clinic. Management fees generated from providing management and administrative services are recognized as earned typically based on a percentage of revenues or cash collections generated by the clinics. Revenues associated with the vascular access clinic that is majority-owned are recognized in the period when physician services are provided. |
| • | ESRD clinical research programs. DaVita Clinical Research conducts research trials principally with dialysis patients and provides administrative support for research conducted by DaVita-affiliated nephrology practices. Revenues are based upon an established fee per study, as determined by contract with drug companies and other sponsors and are recognized as earned according to the contract terms. |
| • | Physician services. DaVita Nephrology Partners offers practice management and administrative services to physicians who specialize in nephrology under management and administrative services agreements. Practice management and administrative services typically include operations management, IT support, billing and collections, credentialing and coding, and other support functions. Management fees generated from providing practice management and administrative services to physician practices are recognized as earned typically based upon cash collections generated by the practices. |
S-155
Table of Contents
| • | Direct Primary Care. Paladina Health is a healthcare services organization that operates membership-based primary care clinics mainly through employer-based on-site and near-site clinics. The clinics offer patients more personalized and improved access to primary care physicians, including unlimited visits and same-day or next-day appointments. Physicians focus on clinical outcomes and patient satisfaction. Revenues are recognized over the membership period. |
Quality Care
As of June 30, 2012, we employ 234 clinical service teammates. The primary focus of this group is assuring and facilitating processes that aim to achieve superior clinical outcomes at our centers.
Our physician leadership in the Office of the Chief Medical Officer, or OCMO, includes nine senior nephrologists, led by our Chief Medical Officer, with a variety of academic, clinical practice, and clinical research backgrounds. Our Physician Council is an advisory body to senior management, currently composed of ten physicians with extensive experience in clinical practice in addition to the members of OCMO and currently six Group Medical Directors.
Sources of Revenue—Concentrations and Risks
Our dialysis and related lab services business net operating revenues represent approximately 92% of our consolidated net operating revenues for the twelve months ended June 30, 2012, with the balance of our revenues from ancillary services and strategic initiatives which also includes our international dialysis operations. Our dialysis and related lab services revenues are derived primarily from our core business of providing kidney dialysis services, the administration of pharmaceuticals, related laboratory services and to a lesser extent management fees generated from providing management and administrative services to certain outpatient dialysis centers.
The sources of our dialysis and related lab services revenues are principally from government-based programs, including Medicare and Medicare-assigned plans, Medicaid and Medicaid-assigned plans and commercial insurance plans.
The following table summarizes our dialysis and related lab services revenues by source for the twelve months ended June 30, 2012:
| Revenue percentages | ||||
Medicare and Medicare-assigned plans | 59 | % | ||
Medicaid and Medicaid-assigned plans | 5 | % | ||
Other government-based programs | 2 | % | ||
|
| |||
Total government-based programs | 66 | % | ||
Commercial (including hospital inpatient dialysis services) | 34 | % | ||
|
| |||
Total dialysis and related lab services revenues | 100 | % | ||
|
| |||
The following table summarizes our dialysis and related lab services revenues by modality for the twelve months ended June 30, 2012:
| Revenue percentages | ||||
Outpatient hemodialysis centers | 80 | % | ||
Peritoneal dialysis and home-based hemodialysis | 15 | % | ||
Hospital inpatient hemodialysis | 5 | % | ||
|
| |||
Total dialysis and related lab services revenues | 100 | % | ||
S-156
Table of Contents
Medicare revenue
Under the Medicare ESRD program, payment rates for dialysis are established by the U.S. Congress. On January 1, 2011 we implemented Medicare’s new payment system in which all ESRD payments are made under a single bundled payment rate that, beginning in 2012, will provide for an annual inflation adjustment based upon a market basket index, less a productivity adjustment that was determined to be 2.1% by the CMS for 2012. Also beginning in 2012, the rule provides for up to a 2% annual payment withhold that can be earned back by the facilities that meet certain defined clinical performance standards. The new payment system reimburses providers based upon a single bundled or average payment for each Medicare treatment provided. This new bundled payment amount is designed to cover all dialysis services which were historically included in the composite rate and all separately billable ESRD services such as pharmaceuticals and laboratory costs. In the past the amount of services that were separately billable accounted for approximately 30% of our total dialysis and related lab services revenues. The new bundled payment rate is adjusted for certain patient characteristics, a geographic wage index and certain other factors. The initial 2011 bundled payment rate included reductions of 2.0% from the prior reimbursement and further reduced overall rates by 5.94% tied to an expanded list of case mix adjustors which can be earned back based upon the presence of certain patient characteristics and co-modalities at the time of treatment. There are also other provisions that may impact payment including an outlier pool and a low volume facility adjustment. With regard to the expanded list of case-mix adjusters, these are difficult and in some cases, have not been possible for our dialysis centers to document and track, which has resulted in lower reimbursement amounts than we would otherwise have received.
ESRD patients receiving dialysis services become eligible for primary Medicare coverage at various times, depending on their age or disability status, as well as whether they are covered by an employer group health plan. Generally, for a patient not covered by an employer group health plan, Medicare becomes the primary payor either immediately or after a three-month waiting period. For a patient covered by an employer group health plan, Medicare generally becomes the primary payor after 33 months, which includes a three month waiting period, or earlier if the patient’s employer group health plan coverage terminates. When Medicare becomes the primary payor, the payment rate we receive for that patient shifts from the commercial insurance plan rate to the Medicare payment rate.
Medicare pays 80% of the amount set by the Medicare system for each covered treatment. The patient is responsible for the remaining 20%. In most cases, a secondary payor, such as Medicare supplemental insurance, a state Medicaid program or a commercial health plan, covers all or part of these balances. Some patients, who do not qualify for Medicaid but otherwise cannot afford secondary insurance, can apply for premium payment assistance from charitable organizations through a program offered by the American Kidney Fund. We and other dialysis providers support the American Kidney Fund and similar programs through voluntary contributions. If a patient does not have secondary insurance coverage, we are generally unsuccessful in our efforts to collect from the patient the 20% portion of the ESRD composite rate that Medicare does not pay. However, we are able to recover some portion of this unpaid patient balance from Medicare through an established cost reporting process by identifying these Medicare bad debts on each center’s Medicare cost report.
In 2011, we experienced a decrease in our operating costs primarily from a decline in the utilization of physician-prescribed pharmaceuticals due to continued evolution of clinical practices which helped minimize the overall negative financial impact that we incurred from reductions in our reimbursement amounts for services we provide to Medicare patients. However, certain operating expenditures, such as labor and supply costs, are subject to inflation, and without a compensating inflation-based increase in the new bundled payment rate system, could significantly impact our operating results. Our operating results can also be impacted by the cost of physician-prescribed pharmaceuticals.
In 2011, we operated a Medicare Advantage ESRD Special Needs Plan in partnership with a payor that works with CMS to provide ESRD patients full service health care. We are at risk for all medical costs of the program in excess of the capitation payments. We also participated in a CKD/ESRD demonstration program until
S-157
Table of Contents
April 2011, when we terminated the program. We were paid a management fee for program enrollees relating to CKD and ESRD disease states, which are subject to retraction if certain medical cost savings targets were not met. The demonstration program is still in the process of evaluating costs to determine whether these targets were actually met.
Medicaid revenue
Medicaid programs are state-administered programs partially funded by the federal government. These programs are intended to provide health coverage for patients whose income and assets fall below state-defined levels and who are otherwise uninsured. These programs also serve as supplemental insurance programs for co-insurance payments due from Medicaid-eligible patients with primary coverage under Medicare. Some Medicaid programs also pay for additional services, including some oral medications that are not covered by Medicare. We are enrolled in the Medicaid programs in the states in which we conduct our business.
Commercial revenues
Before a patient becomes eligible to have Medicare as their primary payor for dialysis services, a patient’s commercial insurance plan, if any, is responsible for payment of such dialysis services. Although commercial payment rates vary, average commercial payment rates are generally significantly higher than Medicare rates. The payments we receive from commercial payors generate nearly all of our profits. Payment methods from commercial payors include a single lump-sum per treatment, referred to as bundled rates, and in some cases separate payments for treatments and pharmaceuticals, if used as part of the treatment, referred to as fee for service rates. Commercial payment rates are the result of negotiations between us and insurers or third-party administrators. Our out-of-network payment rates are on average higher than in-network payment rates. In 2011, we continued to enter into some commercial contracts, covering certain patients that will primarily pay us a single bundled payment rate for all dialysis services provided to these patients. However, some of the contracts will pay us for certain other services and pharmaceuticals in addition to the bundled payment. These contracts typically contain annual price escalator provisions. We are continuously in the process of negotiating agreements with our commercial payors and if our negotiations result in overall commercial rate reductions in excess of our commercial rate increases, our revenues and operating results could be negatively impacted. In addition, if there are sustained or increased job losses in the U.S. as a result of current economic conditions, or depending upon changes to the healthcare regulatory system, we could experience a decrease in the number of patients covered under commercial plans.
Approximately 34% of our dialysis and related lab services revenues and approximately 10% of our patients were associated with commercial payors for the twelve months ended June 30, 2012. Our commercial patients as a percentage of our total patients declined from June 30, 2011 to June 30, 2012, from approximately 11% to 10%. Less than 1% of our dialysis and related lab services revenues are due directly from patients. No single commercial payor accounted for more than 6% of total dialysis and related lab services revenues for the twelve months ended June 30, 2012.
Revenue from EPO and other pharmaceuticals
Approximately 5% of our total dialysis and related lab services revenues for the twelve months ended June 30, 2012 are associated with the administration of physician-prescribed pharmaceuticals that are separately billable, which help improve clinical outcomes when included with the dialysis treatment. These pharmaceuticals include EPO, vitamin D analogs and iron supplements.
EPO is an erythropoiesis stimulating agent, or ESA, genetically-engineered form of a naturally occurring protein that stimulates the production of red blood cells. EPO is used in connection with all forms of dialysis to treat anemia, a medical complication most ESRD patients experience. The administration of EPO, when separately billable, accounted for approximately 3% of our dialysis and related lab services revenues for the twelve months ended June 30, 2012.
S-158
Table of Contents
These percentages represent a significant decrease from prior years in the amount of revenue that we generated from separately billable pharmaceuticals as a result of implementing Medicare’s new single bundled payment rate system, whereby pharmaceuticals, including EPO, are now included in a single bundled payment. In addition, we also entered into some commercial contracts covering certain patients that also pay us under a single bundled rate for all dialysis services provided to these patients.
EPO is produced by a single manufacturer, Amgen. Any interruption of supply or product cost increases could adversely affect our operations. As an example, in 2011 we experienced an increase in the cost of EPO of approximately 5%. In November 2011, we entered into a seven year Sourcing and Supply Agreement with Amgen USA Inc., Amgen that expires on December 31, 2018. Under the terms of the agreement we will purchase EPO in amounts necessary to meet no less than 90% of our requirements for erythropoiesis stimulations agents. The agreement replaces in its entirety the prior one-year supply agreement between us and Amgen that expired on December 31, 2011. As long as certain conditions are met by us, the agreement limits Amgen’s ability to unilaterally decide to increase the price it charges us for EPO. The agreement, among other things, provides for discounted pricing and rebates for EPO. Some of the rebates are subject to various conditions including future pricing levels of EPO by Amgen and data submission by us. In the initial years of the agreement the total rebate opportunity is less than what was provided for in the agreement that expired as the end of 2011, however, the opportunity for us to earn discounts and rebates increases over the term of the agreement.
Since late 2006, there has been significant media discussion and government scrutiny regarding anemia management practices in the U.S., which has created confusion and concern in the nephrology community. In late 2006, the U.S. House of Representatives Ways and Means Committee held a hearing on the issue of the utilization of ESAs, which include EPO, and in 2007, the FDA required changes to the labeling of EPO and Aranesp® to include a black box warning, the FDA’s strongest form of warning label. An FDA advisory panel on ESA use met in October 2010, which meeting was similar to the prior meeting held in 2007 in that there was significant discussion and concern about the safety of ESAs. The panel concluded it would not recommend a change in ESA labeling. However, the FDA is not bound by the panel’s recommendation. In June 2011, the FDA required that the black box warning be slightly revised and also include more conservative dosing recommendations for patients with chronic kidney disease. In addition, in June 2011, CMS opened a National Coverage Analysis, or NCA, for ESAs. Further, in January 2011, CMS convened a meeting of the Medicare Evidence Development and Coverage Advisory Committee, or MEDCAC, to evaluate evidence for the pending NCA. In June 2011, CMS determined not to issue a national coverage determination for ESAs due to a lack of available evidence to establish coverage criteria or limitations.
The forgoing congressional and agency activities and related actions could result in further restrictions on the utilization and reimbursement for ESAs. Commercial payors have also increasingly examined their administration policies for EPO and, in some cases, have modified those policies. Further changes in labeling of EPO and other pharmaceuticals in a manner that alters physician practice patterns or accepted clinical practices, changes in private and governmental payment criteria, including the introduction of EPO administration policies or the conversion to alternate types of administration of EPO or other pharmaceuticals that result in further decreases in utilization of EPO for patients covered by commercial payors or increased utilization of EPO for patients for whom the cost of EP is include in a bundled reimbursement rate, or further decreases in reimbursement for EPO and other pharmaceuticals that are not included in a bundled reimbursement rate, could have a material adverse effect on our revenues, earnings and cash flows.
Physician Relationships
An ESRD patient generally seeks treatment at an outpatient dialysis center near his or her home where his or her treating nephrologist has practice privileges. Our relationships with local nephrologists and our ability to meet their needs and the needs of their patients are key factors in the success of our dialysis operations. As of June 30, 2012, approximately 4,200 nephrologists currently refer patients to our outpatient dialysis centers. As is typical in the dialysis industry, one or a few physicians, including the outpatient dialysis center’s medical
S-159
Table of Contents
director, usually account for all or a significant portion of an outpatient dialysis center’s patient base. If a significant number of physicians, including an outpatient dialysis center’s medical directors, were to cease referring patients to our outpatient dialysis centers, our business could be adversely affected.
Participation in the Medicare ESRD program requires that dialysis services at an outpatient dialysis center be under the general supervision of a medical director who is a licensed physician. We have engaged physicians or groups of physicians to serve as medical directors for each of our outpatient dialysis centers. At some outpatient dialysis centers, we also separately contract with one or more other physicians to serve as assistant or associate medical directors or to direct specific programs, such as home dialysis training programs. As of June 30, 2012, we have contracts with approximately 1,800 individual physicians and physician groups to provide medical director services.
Medical directors enter into written contracts with us that specify their duties and fix their compensation generally for periods of ten years. The compensation of our medical directors is the result of arm’s length negotiations and generally depends upon an analysis of various factors such as the physician’s duties, responsibilities, professional qualifications and experience, among others.
Our medical director contracts generally include covenants not to compete. Also, when we acquire an outpatient dialysis center from one or more physicians or where one or more physicians own minority interests in our outpatient dialysis centers, these physicians have agreed to refrain from owning interests in other competing outpatient dialysis centers within a defined geographic area for various time periods. These non-compete agreements restrict the physicians from owning or providing medical director services to other outpatient dialysis centers, but do not prohibit the physicians from referring patients to any outpatient dialysis center, including competing centers. Many of these non-compete agreements continue for a period of time beyond expiration of the corresponding medical director agreements, although some expire at the same time as the medical director agreement. Occasionally, we experience competition from a new outpatient dialysis center established by a former medical director following the termination of his or her relationship with us.
Corporate Compliance Program
Our dialysis operations are subject to extensive federal, state and local government regulations. Management has designed and implemented a company-wide corporate compliance program as part of our commitment to comply fully with all applicable laws and regulations and to maintain the high standards of conduct we expect from all of our teammates. We continuously review this program and enhance it as necessary. The primary purposes of the program include:
| • | Increasing, through training and education, the awareness of our teammates and affiliated professionals of the necessity of complying with all applicable laws and regulations; |
| • | Auditing and monitoring the activities of our dialysis centers, laboratories and billing offices on a regular basis to identify potential instances of noncompliance in a timely manner; |
| • | Establishing guidelines around physicians roles and responsibilities that require our physicians attest to their adherence to these guidelines on a periodic basis; and |
| • | Ensuring that we take steps to resolve instances of noncompliance or to address areas of potential noncompliance as promptly as we become aware of them. |
When evaluating the effectiveness of our corporate compliance program, we take into consideration a number of factors, including favorable results under various government inquiries and adherence to industry standards.
We have a code of conduct that each of our teammates and affiliated professionals must follow and we have a confidential toll-free hotline (888-458-5848) for teammates and patients to report potential instances of
S-160
Table of Contents
noncompliance. Our Chief Compliance Officer administers the compliance program. The Chief Compliance Officer reports directly to our Chief Executive Officer, our Chief Operating Officer and to the Compliance Committee of our Board of Directors.
Insurance
We maintain insurance for property and general liability, professional liability, directors’ and officers’ liability, workers compensation and other coverage in amounts and on terms deemed adequate by management based on our actual claims experience and expectations for future claims. Future claims could, however, exceed our applicable insurance coverage. Physicians practicing at our dialysis centers are required to maintain their own malpractice insurance and our medical directors are required to maintain coverage for their individual private medical practices. Our liability policies cover our medical directors for the performance of their duties as medical directors at our outpatient dialysis centers.
Capacity and Locations
We are able to increase our capacity by extending hours at our existing centers, expanding our existing centers, relocating our centers, developing new centers and by acquiring centers. The development of a typical outpatient dialysis center by us generally requires approximately $2.5 million for leasehold improvements, equipment and first-year working capital. Based on our experience, a new center typically opens within a year after the property lease is signed, normally achieves operating profitability in the second year after certification and normally reaches maturity within three to five years. Acquiring an existing outpatient dialysis center requires a substantially greater initial investment, but profitability and cash flow are generally initially more predictable. To a limited extent, we enter into agreements to provide management and administrative services to outpatient dialysis centers in which we either own a minority equity investment, or are wholly-owned by third parties in return for management fees, which are typically based on a percentage of revenues or cash collections of the managed operations.
The table below shows the growth of our company by number of dialysis centers.
| Six months ended June 30, 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | |||||||||||||||||||
Number of centers at beginning of year | 1,820 | 1,612 | 1,530 | 1,449 | 1,359 | 1,300 | ||||||||||||||||||
Acquired centers | 64 | 178 | (1) | 41 | 19 | 20 | 16 | |||||||||||||||||
Developed centers | 32 | 65 | 65 | 78 | 86 | 64 | ||||||||||||||||||
Net change in centers with management and administrative services agreements* | (9 | ) | 4 | — | 8 | (2) | 1 | (15 | )(3) | |||||||||||||||
Sold and closed centers** | (1 | ) | (32 | )(1) | (10 | ) | (8 | ) | (9 | ) | (4 | ) | ||||||||||||
Closed centers*** | (3 | ) | (7 | ) | (14 | ) | (16 | ) | (8 | ) | (2 | ) | ||||||||||||
Number of centers at end of year | 1,903 | 1,820 | 1,612 | 1,530 | 1,449 | 1,359 | ||||||||||||||||||
| (1) | In 2011, we acquired 113 dialysis centers and divested a total of 30 centers in connection with the acquisition of DSI. |
| (2) | During 2009, we made minority equity investments in 6 centers and we entered into 2 additional management and administrative service agreements. |
| (3) | In November 2007, one of our management and administration service agreements was terminated, in which we provided management and administrative services to 20 dialysis centers. |
| * | Represents dialysis centers in which we either own a minority equity investment, or are wholly-owned by third parties. |
| ** | Represents dialysis centers that were sold and/or closed in which patients were not retained. |
| *** | Represents dialysis centers that were closed and the majority of patients were retained and transferred to one of our other existing outpatient dialysis centers. |
S-161
Table of Contents
As of June 30, 2012, we operated or provided administrative services to a total of 1,903 outpatient dialysis centers, of which 1,884 are located in the U.S. and 19 are located in four countries outside of the U.S. A total of 1,876 are consolidated in our financial statements. Of the remaining 27 unconsolidated outpatient dialysis centers, we own a minority equity investment in 19 centers and provide management and administrative services to eight centers, of which three centers are located outside of the U.S., that are wholly-owned by third parties. The locations of the 1,876 outpatient dialysis centers consolidated in our financial statements at June 30, 2012 were as follows:
State | Centers | State | Centers | State | Centers | |||||||||||
California | 218 | New York | 40 | Kansas | 23 | |||||||||||
Texas | 159 | Minnesota | 39 | Nevada | 18 | |||||||||||
Florida | 147 | Kentucky | 34 | Nebraska | 15 | |||||||||||
Georgia | 108 | Oklahoma | 31 | Massachusetts | 13 | |||||||||||
Ohio | 86 | Colorado | 33 | Mississippi | 11 | |||||||||||
Pennsylvania | 76 | New Jersey | 33 | District of Columbia | 10 | |||||||||||
Illinois | 72 | Wisconsin | 37 | Idaho | 9 | |||||||||||
North Carolina | 62 | Louisiana | 27 | Utah | 4 | |||||||||||
Michigan | 68 | South Carolina | 27 | New Mexico | 4 | |||||||||||
Virginia | 56 | Arizona | 25 | West Virginia | 4 | |||||||||||
Maryland | 52 | Washington | 27 | South Dakota | 3 | |||||||||||
Indiana | 48 | Connecticut | 22 | New Hampshire | 2 | |||||||||||
Tennessee | 54 | Iowa | 20 | North Dakota | 2 | |||||||||||
Missouri | 50 | Oregon | 20 | Rhode Island | 1 | |||||||||||
Alabama | 47 | Arkansas | 23 | |||||||||||||
International | Centers | |||||||||||||||
India | 11 | |||||||||||||||
Germany | 2 | |||||||||||||||
Saudi Arabia | 3 | |||||||||||||||
Teammates
As of June 30, 2012, we had approximately 43,000 teammates:
• Licensed professional staff (nurses, dieticians and social workers) | 19,000 | |||
• Other patient care and center support staff and laboratory personnel | 18,000 | |||
• Corporate, billing and regional administrative staff | 6,000 | |||
Our dialysis business requires nurses with specialized training for treating patients with complex care needs. Recruitment and retention of nurses are continuing concerns for healthcare providers due to short supply. We have an active program of investing in our professional healthcare teammates to help ensure we meet our recruitment and retention targets, including expanded training opportunities, tuition reimbursements and other incentives.
Government Regulation
Our dialysis operations are subject to extensive federal, state and local governmental regulations. These regulations require us to meet various standards relating to, among other things, government payment programs, dialysis facilities and equipment, management of centers, personnel qualifications, maintenance of proper records and quality assurance programs and patient care.
S-162
Table of Contents
Because we are subject to a number of governmental regulations, our business could be adversely impacted by:
| • | Loss or suspension of federal certifications; |
| • | Loss or suspension of licenses under the laws of any state or governmental authority from which we generate substantial revenues; |
| • | Exclusion from government healthcare programs including Medicare and Medicaid; |
| • | Significant reductions or lack of inflation-adjusted increases in payment rates or reduction of coverage for dialysis and ancillary services and related pharmaceuticals; |
| • | Fines, damages and monetary penalties for anti-kickback law violations, Stark Law violations, submission of false claims, civil or criminal liability based on violations of law or other failures to meet regulatory requirements; |
| • | Claims for monetary damages from patients who believe their protected health information has been used or disclosed in violation of federal and state patient privacy laws; |
| • | Mandated changes to our practices or procedures that significantly increase operating expenses; or |
| • | Refunds of payments received from government payors and government health care program beneficiaries because of any failures to meet applicable requirements. |
We expect that our industry will continue to be subject to substantial regulation, the scope and effect of which are difficult to predict. Our activities could be reviewed or challenged by regulatory authorities at any time in the future. This regulation and scrutiny could have a material adverse impact on us.
Licensure and certification
Our dialysis centers are certified by CMS, as is required for the receipt of Medicare payments. In some states, our dialysis centers also are required to secure additional state licenses and permits. Governmental authorities, primarily state departments of health, periodically inspect our centers to determine if we satisfy applicable federal and state standards and requirements, including the conditions of participation in the Medicare ESRD program.
To date, we have not experienced significant difficulty in maintaining our licenses or our Medicare and Medicaid authorizations. However, we have experienced delays in obtaining certifications from CMS.
CMS continues to study the regulations applicable to Medicare certification to provide dialysis services. On April 15, 2008, CMS issued new regulations for Medicare-certified ESRD facilities to provide dialysis services, referred to as Conditions for Coverage. The Conditions for Coverage were effective October 14, 2008, with some provisions having a phased in implementation date of February 1, 2009. The regulations are patient, quality and outcome focused. Among other things, they establish performance expectations for facilities and staff, eliminate certain procedural requirements, and promote continuous quality improvement and patient safety measures. We have established an interdisciplinary work group that includes a comprehensive auditing process to monitor our continued compliance with the Conditions of Coverage.
Federal anti-kickback statute
The “anti-kickback” statute contained in the Social Security Act imposes criminal and civil sanctions on persons who receive, make, offer or solicit payments in return for:
| • | The referral of a Medicare or Medicaid patient for treatment; |
| • | The ordering or purchasing of items or services that are paid for in whole or in part by Medicare, Medicaid or similar federal and state programs; or |
| • | Arranging for or recommending the ordering or purchasing of such items. |
S-163
Table of Contents
Federal criminal penalties for the violation of the anti-kickback statute include imprisonment, fines and exclusion of the provider from future participation in the Medicare and Medicaid programs. Violations of the anti-kickback statute are punishable by imprisonment for up to five years and fines of up to $25,000 or both. Larger fines can be imposed upon corporations under the provisions of the U.S. Sentencing Guidelines and the Alternate Fines Statute. Individuals and entities convicted of violating the anti-kickback statute are subject to mandatory exclusion from participation in Medicare, Medicaid and other federal healthcare programs for a minimum of five years. Civil penalties for violation of this law include up to $50,000 in monetary penalties per violation, repayments of up to three times the total payments between the parties and suspension from future participation in Medicare and Medicaid. Court decisions have also held that the statute is violated whenever one of the purposes of remuneration is to induce referrals. The Health Reform Acts amended the anti-kickback statute to lower the standard of proof for the intent requirement that the government must make in order to obtain a conviction. The government does not have to prove that the defendant knew of the existence of the anti-kickback statute or had the specific intent to violate it. In addition, the Health Reform Acts amended the anti-kickback statute to provide that any claims submitted from an arrangement that violates the anti-kickback statute are false claims, under the False Claims Act.
The Department of Health and Human Services regulations create exceptions or “safe harbors” for some business transactions and arrangements. Transactions and arrangements structured within these safe harbors are deemed to not violate the anti-kickback statute. A business transaction or arrangement must satisfy every element of a safe harbor to be protected by that safe harbor. Transactions and arrangements that do not satisfy all elements of a relevant safe harbor do not necessarily violate the statute, but can be subject to greater scrutiny by enforcement agencies.
Our medical directors refer patients to our centers, and these arrangements, by which we pay them for their medical director services, must be in compliance with the federal anti-kickback statute. Among the available safe harbors is one for personal services furnished for fair market value. However, most of our agreements with our medical directors do not satisfy all seven of the requirements of the personal services safe harbor. We believe that because of the nature of our medical directors’ duties, it is impossible to satisfy the anti-kickback safe-harbor requirement that services provided under an agreement on a part-time basis must specify the schedule of intervals of service, and their precise length and the exact charge for such intervals. Accordingly, while we believe that our agreements with our medical directors satisfy as many of the elements of this safe harbor as we believe is reasonably possible, our arrangements do not qualify for safe harbor protection, as precise scheduling is not possible. We also note that there is little guidance available as to what constitutes fair market value for medical director services. We believe that our agreements do not violate the federal anti-kickback statute; however, since the arrangements do not satisfy all of the requirements for safe harbor protection, these arrangements could be challenged.
We own a controlling interest in numerous dialysis related joint ventures. For the twelve months ended June 30, 2012, these joint ventures represented approximately 19% of our dialysis and related lab services revenues. In addition, we also own minority equity investments in several other dialysis related joint ventures. Our relationships with physicians and other referral sources relating to these joint ventures are required to comply with the anti-kickback statute. Although there is a safe harbor for certain investment interests in “small entities,” it is not clear if any of our joint ventures satisfies all of the requirements for protection by this safe harbor. Under current law, physician joint ventures are not prohibited but instead require a case-by-case evaluation under the anti-kickback statute. We have structured our joint ventures to satisfy as many safe harbor requirements as we believe are reasonably possible. We believe that these investments are offered on a fair market value basis and provide returns to the physician investors only in proportion to their actual investment in the venture. We believe that our agreements do not violate the federal anti-kickback statute; however, since the arrangements do not satisfy all of the requirements for safe harbor protection, these arrangements could be and have been challenged.
S-164
Table of Contents
As of June 30, 2012, we lease space for approximately 600 of our centers from entities in which physicians hold ownership interests and we sublease space to referring physicians at approximately 200 of our dialysis centers. These arrangements must be in compliance with the anti-kickback statute. We believe that we meet the elements of the safe harbor for space rentals in all material respects.
Some medical directors and other referring physicians may own our common stock. We believe that these interests materially satisfy the requirements of the safe harbor for investments in large publicly traded companies for the anti-kickback statute.
Because we are purchasing and selling items and services in the operation of our centers that may be paid for, in whole or in part, by Medicare or a state healthcare program and because we acquire certain items and services at a discount, we must structure these arrangements in compliance with the federal anti-kickback statute. Subject to certain requirements and limitations, discounts representing reductions in the amounts we are charged for items or services based on arm’s-length transactions can qualify for safe harbor protection if we fully and accurately report the discounts in the applicable Medicare cost reports. While some of the safe harbor criteria are subject to interpretation, we believe that our vendor contracts with discount provisions are in compliance with the anti-kickback statute.
Stark Law
Another federal law, known as the “Stark Law”, prohibits a physician who has a financial relationship, or who has an immediate family member who has a financial relationship, with entities providing designated health services, or DHS, from referring Medicare patients to such entities for the furnishing of such services, unless an exception applies. Stark Law DHS include home health services, outpatient prescription drugs, inpatient and outpatient hospital services and clinical laboratory services. The Stark Law also prohibits the DHS entity receiving a prohibited referral from filing a claim or billing for the services arising out of the prohibited referral. The prohibition applies regardless of the reasons for the financial relationship and the referral; unlike the federal anti-kickback statute, intent to induce referrals is not required. Sanctions for violation of the Stark Law include denial of payment for claims for services provided in violation of the prohibition, refunds of amounts collected in violation, a civil penalty of up to $15,000 for each service arising out of the prohibited referral, exclusion from the federal healthcare programs, including Medicare and Medicaid and a civil penalty of up to $100,000 against parties that enter into a scheme to circumvent the Stark Law prohibition. Stark Law violations also can form the basis for False Claims Act liability. The types of financial arrangements between a physician and a DHS entity that trigger the self-referral prohibitions of the Stark Law are broad and include direct and indirect ownership and investment interests and compensation arrangements.
CMS has adopted implementing regulations under the Stark Law, collectively, Stark Regulations. CMS has not yet adopted implementing regulations regarding application of the Stark Law to Medicaid, but has indicated that it anticipates issuing additional regulations regarding the application of the Stark Law to Medicaid referrals.
The definition of DHS under the Stark Law excludes services paid under a composite rate, even if some of the components bundled in the composite rate are DHS. Although the new ESRD bundled payment system is no longer titled a “composite rate”, we believe that the former composite rate payment system and the current bundled system are both composite systems excluded from the Stark Law. Since most services furnished to Medicare beneficiaries provided in our dialysis centers are reimbursed through a composite or bundled rate, the services performed in our facilities generally are not DHS, and the Stark Law referral prohibition does not apply to those services. The definition of DHS also excludes inpatient dialysis performed in hospitals that are not certified to provide ESRD services. Consequently, our arrangements with such hospitals for the provision of dialysis services to hospital inpatients do not trigger the Stark Law referral prohibition.
S-165
Table of Contents
In addition, although prescription drugs are DHS, there is an exception in the Stark Regulations for EPO and other specifically enumerated dialysis drugs when furnished in or by an ESRD facility, in compliance with the anti-kickback statute and applicable billing requirements. The exception is available only for drugs included on a list of CPT/HCPCS codes published by CMS, and in the case of home dialysis, the exception applies only to EPO, Aranesp® and equivalent drugs dispensed by the facility for use at home. While we believe that most drugs furnished by our dialysis centers are covered by the exception, dialysis centers may administer drugs that are not on the list of CPT/HCPCS codes and therefore do not meet this exception. In order for a physician who has a financial relationship with a dialysis center to order one of these drugs from the center and for the center to obtain Medicare reimbursement, another exception must apply.
We have entered into several types of financial relationships with referring physicians, including compensation arrangements. We believe that the compensation arrangements under our medical director agreements satisfy the personal services compensation arrangement exception to the Stark Law. While we believe that compensation under our medical director agreements, which is the result of arm’s length negotiations, results in fair market value payments for medical director services, an enforcement agency could nevertheless challenge the level of compensation that we pay our medical directors. If the arrangement does not meet a Stark Law exception, we could in the future be required to change our practices, face civil penalties, pay substantial fines, return certain payments received from Medicare and beneficiaries or otherwise experience a material adverse effect as a result of a challenge to payments made pursuant to referrals from these physicians under the Stark Law.
Some of our dialysis centers are leased from entities in which referring physicians hold interests and we sublease space to referring physicians at some of our dialysis centers. The Stark Law provides an exception for lease arrangements if specific requirements are met. We believe that our leases and subleases with referring physicians satisfy the requirements for this exception.
Some medical directors and other referring physicians may own our common stock. We believe that these interests satisfy the Stark Law exception for investments in large publicly traded companies.
Some of our referring physicians also own equity interests in entities that operate our dialysis centers. None of the Stark Law exceptions applicable to physician ownership interests in entities to which they make DHS referrals applies to the kinds of ownership arrangements that referring physicians hold in several of our subsidiaries that operate dialysis centers. Accordingly, these dialysis centers cannot bill Medicare for DHS referrals from physician owners. If the dialysis centers bill for DHS referred by physician owners, the dialysis center would be subject to the Stark Law penalties described above.
While we believe that most of our operations do not implicate the Stark Law, particularly under the new ESRD bundled payment system, and that to the extent that our dialysis centers furnish DHS, they either meet an exception or do not bill for services that do not meet a Stark Law exception, if CMS determined that we have submitted claims in violation to the Stark Law, we would be subject to the penalties described above. In addition, it might be necessary to restructure existing compensation agreements with our medical directors and to repurchase or to request the sale of ownership interests in subsidiaries and partnerships held by referring physicians or, alternatively, to refuse to accept referrals for DHS from these physicians. Any such penalties and restructuring could have a material adverse effect on our operations.
If any of our business transactions or arrangements, including those described above, were found to violate the federal anti-kickback statute of Stark Law, we could face criminal, civil or administrative sanctions, including possible exclusion from participation in Medicare, Medicaid and other state and federal healthcare programs. Any findings that we have violated these laws could have a material adverse impact on our operations.
S-166
Table of Contents
Fraud and abuse under state law
Many states in which we operate dialysis centers have statutes prohibiting physicians from holding financial interests in various types of medical facilities to which they refer patients. Some of these statutes could be interpreted as prohibiting physicians who hold shares of our publicly traded stock from referring patients to our dialysis centers if the centers use our laboratory subsidiary to perform laboratory services for their patients. Some states also have laws similar to the federal anti-kickback statute that may affect our ability to receive referrals from physicians with whom we have financial relationships, such as our medical directors. Some state anti-kickback statutes also include civil and criminal penalties. Some of these statutes include exemptions applicable to our medical directors and other physician relationships or for financial interests limited to shares of publicly traded stock. Some, however, include no explicit exemption for medical director services or other services for which we contract with and compensate referring physicians or for joint ownership interests of the type held by some of our referring physicians or for financial interests limited to shares of publicly traded stock. If these statutes are interpreted to apply to referring physicians with whom we contract for medical director and similar services, to referring physicians with whom we hold joint ownership interests or to physicians who hold interests in DaVita limited solely to publicly traded stock, we may be required to terminate or restructure some or all of our relationships with or refuse referrals from these referring physicians and could be subject to civil and administrative sanctions, refund requirements and exclusions from government healthcare programs, including Medicare and Medicaid. Such events could negatively affect the decision of referring physicians to refer patients to our centers.
The False Claims Act
The FCA is a means of policing false bills or false requests for payment in the healthcare delivery system. In part, the FCA authorizes the imposition of up to three times the government’s damages and civil penalties on any person who:
| • | knowingly presents or causes to be presented to the federal government, a false or fraudulent claim for payment or approval; |
| • | knowingly makes, uses or causes to be made or used, a false record or statement to get a false or fraudulent claim paid or approved by the federal government; |
| • | conspires to defraud the federal government by getting a false or fraudulent claim allowed or paid; or |
| • | knowingly makes, uses or causes to be made or used, a false record or statement to conceal, avoid or decrease an obligation to pay or transmit money or property to the federal government. |
In addition, recent amendments to the FCA impose severe penalties for the knowing and improper retention of overpayments collected from government payors. Within 60 days of identifying an overpayment, a provider is required to notify CMS or the Medicare Administrative Contractor of the overpayment and the reason for it and return the overpayment. These amendments could subject our procedures for identifying and processing overpayments to greater scrutiny. We have made significant investments in additional resources to accelerate the time it takes to identify and process overpayments and we may be required to make additional investments in the future. An acceleration in our ability to identify and process overpayments could result in us refunding overpayments to government or other payors sooner than we have in the past. A significant acceleration of these refunds could have a material adverse affect on our operating cash flows.
The penalties for a violation of the FCA range from $5,500 to $11,000 for each false claim plus three times the amount of damages caused by each such claim, which generally means the amount received directly or indirectly from the government. The federal government has used the FCA to prosecute a wide variety of alleged false claims and fraud allegedly perpetrated against Medicare and state healthcare programs, including coding errors, billing for services not rendered, the submission of false cost reports, billing for services at a higher payment rate than appropriate, billing under a comprehensive code as well as under one or more component
S-167
Table of Contents
codes included in the comprehensive code and billing for care that is not considered medically necessary. The Health Reform Acts provide that a violation of the federal anti-kickback statute can form the basis for liability under the FCA. Some courts have held that filing claims or failing to refund amounts collected in violation of the Stark Law can form the basis for liability under the FCA. In addition to the provisions of the FCA, which provide for civil enforcement, the federal government can use several criminal statutes to prosecute persons who are alleged to have submitted false or fraudulent claims for payment to the federal government.
The Health Insurance Portability and Accountability Act of 1996
The Health Insurance Portability and Accountability Act of 1996 and its implementing privacy and security regulations, as amended by the federal Health Information Technology for Economic and Clinical Health Act, or HITECH Act and collectively referred to as HIPAA, requires us to provide certain protections to patients and their health information under the Protected Health Information, or PHI. HIPAA requires us to afford patients certain rights regarding their PHI, and to limit uses and disclosure of their PHI existing in any media form (electronic and hardcopy). HIPAA also requires us to implement administrative, physical, and technical safeguards with respect to electronic PHI. We believe our HIPAA Privacy and Security Program sufficiently addresses HIPAA requirements. Penalties for impermissible use or disclosure of PHI were increased by the HITECH Act by imposing a tiered penalties ranging up to $50,000 per violation and up to $1.5 million per year for the same type of violation. In addition, if PHI of 500 or more individuals is improperly used or disclosed, we would be required to report the improper use or disclosure to the Department of Health and Human Services , which would post the violation on its website. If there were improper use or disclosure of PHI of more than 500 individuals in the same jurisdiction, we would be required to report the improper use or disclosure to the media. Improper use or disclosure could result in significant fines and reputational damage.
Other regulations
Our operations are subject to various state hazardous waste and non-hazardous medical waste disposal laws. These laws do not classify as hazardous most of the waste produced from dialysis services. Occupational Safety and Health Administration regulations require employers to provide workers who are occupationally subject to blood or other potentially infectious materials with prescribed protections. These regulatory requirements apply to all healthcare facilities, including dialysis centers, and require employers to make a determination as to which employees may be exposed to blood or other potentially infectious materials and to have in effect a written exposure control plan. In addition, employers are required to provide or employ hepatitis B vaccinations, personal protective equipment and other safety devices, infection control training, post-exposure evaluation and follow-up, waste disposal techniques and procedures and work practice controls. Employers are also required to comply with various record-keeping requirements. We believe that we are in material compliance with these laws and regulations.
A few states have certificate of need programs regulating the establishment or expansion of healthcare facilities, including dialysis centers. We believe that we are in material compliance with all applicable state certificate of need laws.
Competition
The U.S. dialysis industry has consolidated significantly over time but still remains highly competitive, particularly in terms of acquiring existing outpatient dialysis centers. We continue to face increased competition in the U.S. dialysis industry from large and medium-sized providers who compete directly with us for the acquisition of dialysis businesses, relationships with physicians to act as medical directors and for individual patients. In addition, as we continue our international dialysis expansion into various international markets, we will face competition from large and medium-sized providers for these acquisition targets as well. Acquisitions, developing new outpatient dialysis centers, patient retention and physician relationships are an important part of
S-168
Table of Contents
our growth strategy and our business could be adversely affected if we are not able to continue to make acquisitions on reasonable terms, experience significant patient attrition to our competitors and are not able to maintain or establish new relationships with physicians. Competition for qualified physicians to act as medical directors and for inpatient dialysis services agreements with hospitals is also intense. Occasionally, we have also experienced competition from former medical directors or referring physicians who have opened their own dialysis centers. In addition, we experience competitive pressures in connection with negotiating contracts with commercial healthcare payors.
The two largest dialysis companies, Fresenius Medical Care, or Fresenius, and our company, account for approximately two-thirds of outpatient dialysis patients in the U.S. with our company serving approximately 33% of the total outpatient dialysis patients. Approximately 46% of the centers not owned by us or Fresenius are owned or controlled by hospitals or non-profit organizations. Hospital-based and non-profit dialysis units typically are more difficult to acquire than physician-owned centers. Because of the ease of entry into the dialysis business and the ability of physicians to be medical directors for their own centers, competition for growth in existing and expanding markets is not limited to large competitors with substantial financial resources.
Fresenius also manufactures a full line of dialysis supplies and equipment in addition to owning and operating outpatient dialysis centers. This may give them cost advantages over us because of their ability to manufacture their own products. However, Fresenius has been one of our largest suppliers of dialysis products. In January 2010, we entered into an agreement with Fresenius which committed us to purchase a certain amount of dialysis equipment, parts and supplies from them through 2013. In addition, in August 2006 in connection with the DVA Renal Healthcare acquisition, we also entered into a product supply agreement with Gambro Renal Products that requires us to purchase a certain amount of our hemodialysis non-equipment product supplies, such as dialyzers, at fixed prices through 2015. Our purchases of products in these categories generally offered by both Fresenius and Gambro Renal Products represent approximately 4% of our total operating expenses. For the twelve months ended June 30, 2012, we purchased hemodialysis products and supplies from Gambro Renal Products representing approximately 2% of our total operating expenses.
Legal Proceedings
Inquiries by the Federal Government and Certain Related Civil Proceedings
2005 U.S. Attorney Investigation: In March 2005, the Company received a subpoena from the U.S. Attorney’s Office for the Eastern District of Missouri in St. Louis. The subpoena required production of a wide range of documents relating to the Company’s operations, including documents related to, among other things, pharmaceutical and other services provided to patients, relationships with pharmaceutical companies, and financial relationships with physicians and joint ventures. The subpoena covers the period from December 1, 1996 through March 2005. In October 2005, the Company received a follow-up request for additional documents related to specific medical director and joint venture arrangements. In February 2006, the Company received an additional subpoena for documents, including certain patient records relating to the administration and billing of Epogen® (EPO). In May 2007, the Company received a request for documents related to durable medical equipment and supply companies owned and operated by the Company. The Company cooperated with the inquiry and has produced the requested documents. The subpoenas were issued in connection with a joint civil and criminal investigation. It is possible that criminal proceedings may be initiated against the Company in connection with this investigation. The Company has not received a communication from the St. Louis U.S. Attorney’s Office on this matter for nearly three years.
Woodard Private Civil Suit: In February 2007, the Company received a request for information from the Office of Inspector General, U.S. Department of Health and Human Services, or OIG, for documents relating to EPO claims submitted to Medicare. In August 2007, the Company received a subpoena from the OIG seeking similar documents. The requested documents relate to services provided from 2001 to 2004 by a number of the Company’s centers. The request and subpoena were sent from the OIG’s offices in Houston and Dallas, Texas.
S-169
Table of Contents
The Company cooperated with the inquiry and has produced all previously requested documents to date. The Company was contacted by the U.S. Attorney’s Office for the Eastern District of Texas, which stated that this was a civil investigation related to EPO claims. On July 6, 2009, the U.S. District Court for the Eastern District of Texas lifted the seal on the civilqui tam complaint related to these previous requests for information. The Company was subsequently served with a complaint by the relator, Ivey Woodard, purportedly on behalf of the federal government, under thequi tam provisions of the federal False Claims Act. The government did not intervene and is not actively pursuing this matter. The relator has been pursuing the claims independently and the parties have been engaged in active litigation. The complaint contains allegations relating to the Company’s EPO practices for the period from 1992 through 2010 and seeks monetary damages and civil penalties as well as costs and expenses. The court has ruled that claims earlier than 1996 are beyond the statute of limitations. The Company believes that there is some overlap between the subject of this complaint and the review of EPO utilization in the 2005 U.S. Attorney investigation described above. The Company publicly disclosed on July 3, 2012 that it had reached an agreement in principle to settle all allegations relating to claims arising out of this matter. In connection with this settlement, the Company accrued a charge of $78.0 million that consists of $55.0 million for the settlement plus attorney fees and related expenses. The Company expects that the settlement will resolve federal program claims regarding EPO that were or could have been raised in the complaint relating to historical EPO practices dating back to 1997. The settlement is subject to certain conditions, such as court approval. Until the conditions and documentation are completed, there can be no assurance that this matter will in fact be resolved pursuant to the terms of the agreement in principle to settle.
Vainer Private Civil Suit: In December 2008, the Company received a subpoena for documents from the OIG relating to the pharmaceutical products Zemplar, Hectorol, Venofer, Ferrlecit and EPO, as well as other related matters. The subpoena covers the period from January 2003 to December 2008. The Company was in contact with the U.S. Attorney’s Office for the Northern District of Georgia and the U.S. Department of Justice in Washington, DC, since November 2008 relating to this matter, and were advised that this was a civil inquiry. On June 17, 2009, the Company learned that the allegations underlying this inquiry were made as part of a civil complaint filed by individuals and brought pursuant to thequi tam provisions of the federal False Claims Act. On April 1, 2011, the U.S. District Court for the Northern District of Georgia ordered the case to be unsealed. At that time, the Department of Justice and U.S. Attorney’s Office filed a notice of declination stating that the U.S. would not be intervening and not pursuing the relators’ allegation in litigation. On July 25, 2011, the relators, Daniel Barbir and Dr. Alon Vainer, filed their amended complaint in the U.S. District Court for the Northern District of Georgia, purportedly on behalf of the federal government. The allegations in the complaint relate to the Company’s drug administration practices for Vitamin D and iron agents for a period from 2003 through 2010. The complaint seeks monetary damages and civil penalties as well as costs and expenses. The Company is vigorously defending this matter and intends to continue to do so. The Company can make no assurances as to the time or resources that will be needed to devote to this litigation or its final outcome.
2010 U.S. Attorney Physician Relationship Investigation: In May 2010, the Company received a subpoena from the OIG’s office in Dallas, Texas. The civil subpoena covers the period from January 1, 2005 to May 2010, and seeks production of a wide range of documents relating to the Company’s operations, including documents related to, among other things, financial relationships with physicians and joint ventures. Some of the requested documents overlap with documents requested pursuant to the subpoena in the 2011 U.S. Attorney Physician Relationship Investigation described below. The Company is cooperating with the government and is producing the requested documents. The Company can make no assurances as to the time or resources that will be needed to devote to this litigation or its final outcome.
2011 U.S. Attorney Physician Relationship Investigation: In August 2011, the Company announced it had learned that the U.S. Attorney’s Office for the District of Colorado would be looking into certain activities of the Company in connection with information being provided to a grand jury. The Company announced further that it understood that this investigation was at a very preliminary stage, and while its precise scope was unclear, it appeared to overlap, at least in part, with the 2010 U.S. Attorney Physician Relationship Investigation described above. Subsequent to the Company’s announcement of this 2011 U.S. Attorney Physician Relationship
S-170
Table of Contents
Investigation, it received a subpoena for documents which substantially overlaps with the subpoena in the 2010 U.S. Attorney Physician Relationship Investigation described above and covers the period from January 2006 to September 2011. The Company is cooperating with the government and is producing the requested documents. Certain current and former members of the Board and executives received subpoenas in November 2011 and thereafter to testify before the grand jury, and other Company representatives may also receive subpoenas for testimony related to this matter. It is possible that criminal proceedings may be initiated against the Company in connection with this investigation. The Company can make no assurances as to the time or resources that will be needed to devote to this litigation or its final outcome.
2011 U.S. Attorney Medicaid Investigation: In October 2011, the Company announced that it would be receiving a request for documents, which could include an administrative subpoena from the Office of Inspector General for the U.S. Department of Health and Human Services. Subsequent to the Company’s announcement of this 2011 U.S. Attorney Medicaid Investigation, the Company received a request for documents in connection with the inquiry by the U.S. Attorney’s Office for the Eastern District of New York. The request relates to payments for infusion drugs covered by Medicaid composite payments for dialysis. The Company believes this inquiry is civil in nature. The Company does not know the time period or scope. The Company understands that certain other providers that operate dialysis clinics in New York may be receiving or have received a similar request for documents. The Company is cooperating with the government and is producing the requested documents.
Except for the private civil complaints filed by the relators as described above, to our knowledge, no proceedings have been initiated against us at this time in connection with any of the inquiries by the federal government. Although we cannot predict whether or when proceedings might be initiated or when these matters may be resolved, it is not unusual for inquiries such as these to continue for a considerable period of time through the various phases of document and witness requests and on-going discussions with regulators. Responding to the subpoenas or inquiries and defending us in the relator proceedings will continue to require management’s attention and significant legal expense. Any negative findings in the inquiries or relator proceedings could result in substantial financial penalties or awards against us, exclusion from future participation in the Medicare and Medicaid programs and, to the extent criminal proceedings may be initiated against us, possible criminal penalties. At this time, we cannot predict the ultimate outcome of these inquiries, or the potential outcome of the relators’ claims (except as described above), or the potential range of damages, if any.
Other
We have received several notices of claims from commercial payors and other third parties related to historical billing practices and claims against DVA Renal Healthcare (formerly known as Gambro Healthcare), a subsidiary of ours, related to historical Gambro Healthcare billing practices and other matters covered by its 2004 settlement agreement with the Department of Justice and certain agencies of the U.S. government. We have received no further indication that any of these claims are active, and some of them may be barred by applicable statutes of limitations. To the extent any of these claims might proceed, we intend to defend against them vigorously; however, we may not be successful and these claims may lead to litigation and any such litigation may be resolved unfavorably. At this time, we cannot predict the ultimate outcome of this matter or the potential range of damages, if any.
A wage and hour claim, which has been styled as a class action, is pending against us in the Superior Court of California. We were served with the complaint in this lawsuit in April 2008, and it has been amended since that time. The lawsuit, as amended, alleges that we failed to provide meal periods, failed to pay compensation in lieu of providing rest or meal periods, failed to pay overtime, and failed to comply with certain other California Labor Code requirements. In September 2011, the court denied the plaintiffs’ motion for class certification. Plaintiffs have appealed that decision. We intend to continue to vigorously defend against these claims. Any potential settlement of these claims is not anticipated to be material to our consolidated financial statements.
S-171
Table of Contents
In October 2007, we were contacted by the Attorney General’s Office for the State of Nevada. The Attorney General’s Office informed us that it was conducting a civil and criminal investigation of our operations in Nevada and that the investigation related to the billing of pharmaceuticals, including EPO. In February 2008, the Attorney General’s Office informed us that the civil and criminal investigation had been discontinued. The Attorney General’s Office further advised us that Nevada Medicaid intended to conduct audits of ESRD dialysis providers in Nevada and such audits would relate to the issues that were the subjects of the investigation. To our knowledge, no court proceedings have been initiated against us at this time. Any negative audit findings could result in a substantial repayment by us. At this time, we cannot predict the ultimate outcome of this matter or the potential range of damages, if any.
In June 2004, DVA Renal Healthcare was served with a complaint filed in the Superior Court of California by one of its former employees who worked for its California acute services program. The complaint, which is styled as a class action, alleges, among other things, that DVA Renal Healthcare failed to provide overtime wages, defined rest periods and meal periods, or compensation in lieu of such provisions and failed to comply with certain other California Labor Code requirements. The parties reached an agreement last year to fully resolve this matter for an amount that did not materially impact our financial results. That settlement has now received final approval from the court. Settlement payments have been made to the class members, and a final accounting hearing is scheduled to take place this fall.
In addition to the foregoing, we are subject to claims and suits, including from time to time, contractual disputes and professional and general liability claims, as well as audits and investigations by various government entities, in the ordinary course of business. We believe that the ultimate resolution of any such pending proceedings, whether the underlying claims are covered by insurance or not, will not have a material adverse effect on our financial condition, results of operations or cash flows.
HCP’S BUSINESS
HCP is a patient- and physician-focused, integrated health care delivery and management company with nearly three decades of providing coordinated, outcomes-based medical care in a cost-effective manner. Through capitation contracts with some of the nation’s leading health plans, as of June 30, 2012, HCP had approximately 669,400 current members under its care in southern California, central and south Florida and southern Nevada. Of these, approximately 190,700 individuals were patients enrolled in Medicare Advantage. The remaining approximately 478,700 individuals were managed care members whose health coverage is provided through their employer or who have individually acquired health coverage directly from a health plan or as a result of their eligibility for Medicaid benefits. In addition, during 2011, HCP provided care to over 412,000 fee-for-service patients.
The patients of HCP’s affiliated physicians, physician groups and IPAs benefit from an integrated approach to medical care that places the physician at the center of patient care. As of June 30, 2012, HCP delivered services to its members via a network of over 1,800 affiliated group and other network primary care physicians, 139 network hospitals, and several thousand affiliated group and network specialists. Together with hundreds of case managers, registered nurses and other care coordinators, these medical professionals utilize a comprehensive data analysis engine, sophisticated risk management techniques and clinical protocols to provide high-quality, cost effective care to HCP’s members.
Approximately 94% of HCP’s revenues are derived from multi-year capitation contracts with health plans. Under these contracts, HCP’s health plan customers delegate full responsibility for member care to physicians and health care facilities that are part of HCP’s network. In return, HCP receives a PMPM fee for each HCP member. As a result, HCP has financial and clinical accountability for a population of members. In California,
S-172
Table of Contents
HCP does not assume direct financial risk for institutional (hospital) services, but is responsible for managing the care dollars associated with both the professional (physician) and institutional services being provided for the PMPM fee attributable to both professional and institutional services. In those cases and as a result of its managed care-related administrative services agreements with hospitals, HCP recognizes the surplus of institutional revenues less institutional expense as HCP revenues. In addition to revenues recognized for financial reporting purposes, HCP measures its total care dollars under management which includes the PMPM fee payable to third parties for institutional (hospital) services where HCP manages the care provided to its members by hospitals and other institutional providers, which fees are not included in GAAP revenues. For the twelve months ended June 30, 2012, HCP’s total consolidated operating revenues were $2.6 billion, total care dollars under management were $3.4 billion, net income was $450 million and Adjusted EBITDA was $561 million. Total care dollars under management and Adjusted EBITDA are non-GAAP measures. For a description of how HCP calculates total care dollars under management and Adjusted EBITDA and a reconciliation to revenues and net income, respectively, see “HCP Summary Historical Financial and Operating Data.”
We believe that HCP is well positioned to profitably leverage marketplace demands for greater provider accountability, measurable quality results and cost effective medical care. We believe that HCP’s business model is likely to continue to be an attractive alternative for health plans looking for high quality, cost effective delivery systems, physicians seeking an attractive practice environment and patients interested in a highly integrated approach to managing their medical care. Additionally, we believe that the scale of HCP’s business allows it to spread capitation risk over a large population of members, invest in comprehensive analytic and health care information tools as well as clinical and quality measurement infrastructure, and recognize administrative and operating efficiencies. For these reasons, we believe that HCP offers patients, physicians and health plans a proven platform for addressing many of the most pressing challenges facing the U.S. health care system, including rising medical costs.
HCP’s Industry
U.S. healthcare spending has increased steadily over the past twenty years. These increases have been driven, in part, by the aging population of the baby boomer generation, lack of healthy lifestyle both in terms of exercise and diet, rapidly increasing costs in medical technology and pharmaceutical research, and provider reimbursement structures that may promote volume over quality in a fee-for-service environment. These factors, as well as the steady growth of the U.S. population, have made the healthcare industry a growing market. In 2009, CMS reported that health care accounted for 17.3% of the U.S. economy. According to CMS, the increase in health spending, from $2.3 trillion in 2008 to $2.5 trillion in 2009, was the largest one-year jump since 1960. Comprising an estimated 14% of the federal budget and more than one-fifth of total national health expenditures in 2010, Medicare is frequently the focus of discussions on how to moderate the growth of both federal spending and health care spending in the U.S.
Growth in Medicare spending is expected to continue due to demographics. According to the U.S. Census Bureau, from 1970 through 2011, the overall U.S. population is expected to have grown 52% while the number of Medicare enrollees will have grown approximately 130% over that time period. As an increasing number of the baby boomers become eligible for Medicare, the senior market is expected to grow to 79 million by 2030, more than double the number in 2000. UnitedHealth estimates that over the next decade 10,000 people per day will become newly eligible for Medicare. This translates into a Medicare population that makes up more than 20% of the total U.S. population by the year 2025, compared to less than 15% currently.
Medicare Advantage is an alternative to the traditional fee-for-service Medicare program, which permits Medicare beneficiaries to receive benefits from a managed care health plan. Medicare Advantage plans contract with CMS to provide benefits at least comparable to those offered under the traditional fee-for-service Medicare program in exchange for a fixed monthly premium payment per member from CMS. The monthly premium varies based on the county in which the member resides, as adjusted to reflect the plan members’ demographics and the members’ risk scores. Individuals who elect to participate in the Medicare Advantage program typically
S-173
Table of Contents
receive greater benefits than traditional fee-for-service Medicare Part B beneficiaries, including additional preventive services, vision, dental and prescription drug benefits, and typically have lower deductibles and co-payments than traditional fee-for-service Medicare.
Managed care health plans were developed, primarily during the 1980s, in an attempt to mitigate the rising cost of providing healthcare benefits to populations covered by traditional health insurance. These managed care health plans enroll members through their employers, under federal Medicare benefits or through state Medicaid programs. As a result of the prevalence of these health plans, many seniors now becoming eligible for Medicare have been interacting with managed care companies through their employers for the last 30 years. Individuals turning 65 now are likely to be far more familiar with the managed care setting than previous Medicare populations. According to the Kaiser Family Foundation, in 2012, Medicare Advantage represents only 27% of total Medicare members, creating a significant opportunity for additional Medicare Advantage penetration of newly eligible seniors.
In an effort to reduce the number of uninsured and to begin to control healthcare expenditures, President Obama signed the Health Reform Acts into law in March 2010, which were affirmed, in substantial part, by the U.S. Supreme Court in June 2012. The Health Reform Acts provide for a reduction of up to 32 million uninsured by 2019, while potentially increasing Medicaid coverage by up to 16 million and net commercial coverage by 16 million. CMS projects that the total number of uninsured Americans will fall to 24 million in 2019 from 52 million in 2011. These previously uninsured Americans and potentially newly eligible Medicaid beneficiaries represent a significant new market opportunity for health plans. We believe that health plans looking to cover these newly eligible individuals under fixed premium arrangements will seek provider arrangements that can effectively manage the cost and quality of the care being provided to these newly eligible individuals.
In 2006, Medicare began to pay Medicare Advantage health plans under a bidding process. Plans bid against county-level benchmarks established by Medicare based on the prior year’s Medicare Advantage county payment rate and increased by the projected national growth rate in per capita Medicare spending. Those payment rates were at least as high as per capita fee-for-service Medicare spending in each county and often substantially higher because Congress set floors to raise the lowest rates to stimulate plan growth in areas where plans historically had not found it profitable to enter. If a plan’s bid is higher than the benchmark, enrollees pay the difference in the form of a monthly premium. If the bid is lower than the benchmark, the Medicare program retains 25% of the difference as savings and the plan receives 75% as a rebate, which must be returned to enrollees in the form of additional benefits or reduced premiums. Plan payments are also adjusted based on enrollees’ risk profiles. The formula for base payment is a combination of the base rate for the enrollee’s county of residence, multiplied by the enrollee’s risk score.
One of the primary ways in which the Healthcare Reform Acts will fund increased health insurance coverage is through cuts in Medicare Advantage reimbursement. County benchmarks are transitioning to a system in which each county’s benchmark in 2017 will be a certain percentage (ranging from 95% to 115%) of fee-for-service. MedPAC estimated that 2012 Medicare Advantage benchmarks, bids, and payments will average 112%, 98%, and 107% of fee-for-service spending, respectively. As a result, plans on average would have to bid 36% lower than fee-for-service or 43% lower than the Medicare Advantage benchmark for CMS to begin to save money on Medicare Advantage. As result of the transition of county benchmarks to 95% to 115% of fee-for-service, Medicare Advantage benchmarks on average are expected to be reduced to parity with fee-for-service as compared to 112% of fee-for-service today. Given that CMS will retain 25% of the difference of any plans bid below benchmark, the overall Medicare Advantage program should realize savings as compared to fee-for-service in 2017, which would result in lower payments to Medicare Advantage plans and to HCP.
Many health plans recognize both the opportunity for growth from senior members as well as the potential risks and costs associated with managing additional senior members. In California, Florida, Nevada and numerous other markets, many health plans subcontract a significant portion of the responsibility for managing patient care to integrated medical systems such as HCP. These integrated health care systems, whether medical
S-174
Table of Contents
groups or IPAs, offer a comprehensive medical delivery system and sophisticated care management know-how and infrastructure to more efficiently provide for the health care needs of the population enrolled with that health plan. While reimbursement models for these arrangements vary around the country, health plans in California, Florida and Nevada often prospectively pay the integrated health care system a fixed PMPM amount, or capitation payment, which is often based on a percentage of the amount received by the health plan. The capitation payment is for much—and sometimes virtually all—of the care needs of the applicable membership. Capitation payments to integrated health care systems, in the aggregate, represent a prospective budget from which the system manages care-related expenses on behalf of the population enrolled with that system. To the extent that these systems manage care-related expenses under the capitated levels, the system realizes an operating profit. On the other hand, if care-related expenses exceed projected levels, the system will realize an operating deficit. Since premiums paid represent a significant amount per person, there is a significant revenue opportunity for an integrated medical system like HCP that is able to effectively manage its costs under a capitated arrangement. This is particularly the case for Medicare Advantage members for which revenue to a system can be substantial given the higher expected morbidity and cost associated with a Medicare Advantage member.
Integrated medical systems, such as HCP, that have scale are positioned to spread an individual member’s cost experience across a wider population and realize the benefits of pooling medical risk among large numbers. In addition, integrated medical systems with years of managed care experience can utilize their sizeable medical claims data to identify specific medical care and quality management strategies and interventions for potential high cost cases and aggressively manage them to improve the health of its population base and, thus, lower cost. Many integrated medical systems, like HCP, have also established physician performance metrics that allow them to monitor quality and service outcomes achieved by participating physicians in order to reward efficient, high quality care delivered to members and initiate improvement efforts for physicians whose results can be enhanced.
Healthcare Reform
The U.S. healthcare system, including the Medicare Advantage program, is subject to a broad array of new laws and regulations as a result of the Health Reform Acts. The Health Reform Acts are considered by some to be the most dramatic change to the U.S. healthcare system in decades. The Supreme Court recently found that the individual mandate to obtain health insurance coverage under this legislation is constitutional and also found that the expanded Medicaid benefit included in the legislation is constitutional if states can opt out of the expanded Medicaid benefit without losing their funding under the current Medicaid program. This legislation made significant changes to the Medicare program and to the health insurance market overall. The Health Reform Acts reflect sweeping legislation that, once fully implemented, may have a significant impact on the U.S. health care system generally and the operations of HCP’s business. There are numerous steps required to implement the Health Reform Acts, and Congress may seek to alter or eliminate some of their provisions.
One provision of the Health Reform Acts requires CMS to establish a Medicare Shared Savings Program, or MSSP, that promotes accountability and coordination of care through the creation of Accountable Care Organizations, or ACOs, beginning no later than January 1, 2012. The program allows certain providers and suppliers (including hospitals, physicians and other designated professionals) to voluntarily form ACOs and work together along with other ACO participants to invest in infrastructure and redesign delivery processes to achieve high quality and efficient delivery of services.
In addition, beginning January 1, 2012, CMS authorized 32 organizations to participate in the “Pioneer” ACO program, which is similar to, but separate from, the ACOs created under the MSSP regulations. HCP has been designated as a Pioneer ACO in each of its three geographic regions—Florida, California and Nevada. The Pioneer ACO designation is designed for health care organizations and providers, like HCP, that are already experienced in coordinating care for patients across care settings. It allows designated provider groups to move more rapidly from a shared savings payment model to a population-based payment model on a track consistent
S-175
Table of Contents
with, but separate from, the MSSP. The Pioneer ACO program is designed to work in coordination with private payors by aligning provider incentives. This alignment of provider incentives is intended to improve quality and medical outcomes for patients across the ACO, and achieve cost savings for Medicare, employers and patients. The Pioneer ACOs began operating January 1, 2012. As the initial participants for the MSSP, Pioneer ACOs face significant uncertainty. CMS authorized an additional 27 ACOs in April 2012 to begin services April 1, 2012 and an additional 88 ACOs in July 2012 to begin services as of July 1, 2012. See “—Government Regulations” below for a discussion of some of these issues.
Payor Environment
Government Programs
HCP derives a significant portion of its revenues from services rendered to beneficiaries of Medicare, including Medicare Advantage, Medicaid, and other governmental healthcare programs.
Medicare.The Medicare program was established in 1965 and became effective in 1967 as a federally funded U.S. health insurance program for persons aged 65 and older, and it was later expanded to include individuals with ESRD and certain disabled persons, regardless of income or age. Since its formation, Medicare has grown to a $549 billion program in 2011, covering approximately 49 million Americans and, based on the growing number of eligible beneficiaries and increases in the cost of health care, CMS projects that Medicare program funding will grow to $1.1 trillion by 2022.
Initially, Medicare was offered only on a fee-for-service basis. Under the Medicare fee-for-service payment system, an individual can choose any licensed physician enrolled in Medicare and use the services of any hospital, health care provider or facility certified by Medicare. CMS reimburses providers, based on a fee schedule, if Medicare covers the service and CMS considers it “medically necessary.”
Fee-for-service Medicare is paid according to a physician fee schedule, or PFS, set each year by CMS in accordance with formulas mandated by Congress. CMS is required to limit the growth in spending under the PFS by a predetermined sustained growth rate, or SGR. If implemented as mandated, the SGR would result in significant payment reductions under the PFS. For 2013 it would be approximately 27%. Every year since 2003 Congress has delayed application of the SGR but we cannot predict whether they will continue to do so. There is pressure for Congress to implement a permanent solution to the SGR reductions. We cannot predict whether the SGR will be repealed or if another formula would be substituted and what form that might take. Repeal of the SGR could be offset by further reductions in Medicare payments.
Medicare Advantage.Medicare Advantage is a Medicare health plan program developed and administered by the CMS as an alternative to the original fee-for-service Medicare program. Under the Medicare Advantage program, Medicare beneficiaries may choose to receive benefits under a managed care health plan that provides benefits at least comparable to those offered under the original Medicare fee-for-service payment system in exchange for which the health plan receives a monthly per patient premium payment from CMS. The Medicare Advantage monthly premium varies based on the county in which the member resides, and is adjusted to reflect the demographics and estimated risk profile of the members that enroll. Once a person is authorized by CMS to participate in Medicare Advantage, health plans compete for enrollment based on benefit design differences such as co-payments or deductibles, availability of preventive care, attractiveness of and access to a network of hospitals, physicians and ancillary providers and premium contribution or, most often in Medicare Advantage plans, the absence of any monthly premium. As described above under “Industry”, in certain parts of the country, many health plans that provide Medicare Advantage benefits subcontract with integrated medical systems such as HCP to transfer the responsibility for managing patient care.
One of CMS’s primary directives in establishing the Medicare Advantage program was to make it more attractive for health plans to enroll members with higher intensity illnesses. To accomplish this and in addition to base rate increases, CMS adopted a risk adjustment payment system for Medicare health plans in which the
S-176
Table of Contents
participating health plans’ premiums are adjusted based on the actual illness burden of the members that enroll. The model bases a portion of the total CMS reimbursement payments on various clinical and demographic factors, including hospital inpatient diagnoses, additional diagnosis data from ambulatory treatment settings, hospital outpatient department and physician visits, gender, age and Medicaid eligibility. CMS requires that all managed care companies capture, collect and submit the necessary diagnosis code information to CMS twice a year for reconciliation with CMS’s internal database. Medical providers, such as HCP, provide this diagnosis code information to health plan customers for submission to CMS. Under this system, the risk-adjusted portion of the total CMS payment to the Medicare Advantage plans will equal the local rate set forth in the traditional demographic rate book, adjusted to reflect the plan members’ gender, age and morbidity. See “—Government Regulations” below.
Most Medicare beneficiaries have the option to enroll in private health insurance plans that contract with Medicare under the Medicare Advantage program. The share of Medicare beneficiaries in such plans has risen rapidly in recent years; it reached approximately 25% in 2011 from approximately 13% in 2004. Plan costs for the standard benefit package can be significantly lower or higher than the corresponding cost for beneficiaries in the traditional Medicare fee-for-service payment program, but prior to the Health Reform Acts, private plans were generally paid a higher average amount, and they used the additional payments to reduce enrollee cost-sharing requirements, provide extra benefits, and/or reduce Medicare premiums. These enhancements were valuable to enrollees but also resulted in higher Medicare costs overall and higher premiums for all Medicare Part B beneficiaries and not just those enrolled in Medicare Advantage plans. The Health Reform Acts require that future payments to plans be based on “benchmarks” in a range of 95% to 115% of local fee-for-service Medicare costs, with bonus amounts payable to plans meeting high quality-of-care standards. In addition, beginning in 2014, health plans offering Medicare Advantage will be required to spend at least 85% of their premium dollars on medical care, the so-called medical loss ratio, or MLR. Since HCP is not a health plan it is not subject to the 85% MLR floor. However, payments health plans make to HCP will apply in full towards the health plans’ 85% MLR requirement. If HCP’s administrative costs combined with a plan’s other administrative costs aggregate to more than 15% of the total premium dollars the plan receives, the plan will either be required to reduce its administrative costs or increase the amount expended for MLR.
Medicaid.Medicaid is a federal entitlement program administered by the states that provides health care and long-term care services and support to low-income Americans. Medicaid is funded jointly by the states and the federal government. The federal government guarantees matching funds to states for qualifying Medicaid expenditures based on each state’s federal medical assistance percentage, which is calculated annually and varies inversely with average personal income in the state. Subject to federal rules, each state establishes its own eligibility standards, benefit packages, payment rates and program administration within broad federal statutory and regulatory guidelines. Every state Medicaid program must balance a number of potentially competing demands, including the need for quality care, adequate provider access, and cost-effectiveness. In an effort to improve quality and provide more uniform and cost-effective care, many states have implemented Medicaid managed care programs to improve access to coordinated health care services, including preventative care, and to control health care costs. Under Medicaid managed care programs, a health plan receives capitation payments from the state. The health plan, in turn, arranges for the provision of health care services by contracting with a network of medical providers, such as HCP. HCP has capitation contracts to manage approximately 25,000 Medicaid managed care members in its southern California market.
Commercial Payors
According to the Robert Wood Johnson Foundation, in 2009, approximately 61% of non-elderly U.S. citizens received their health care benefits through their employer, which contracted with health plans to administer these health care benefits. Patients enrolled in health plans offered through an employment setting are generally referred to as commercial members. Nationally, commercial health plan enrollment was approximately 162 million as of 2010. Under the Health Reform Acts, beginning in 2014, many uninsured individuals and many individuals who receive their health insurance benefits through small employers may purchase their health care
S-177
Table of Contents
benefits through insurance exchanges in which health plans compete directly for individual or small group members enrollment. HCP derives a significant amount of its revenues from commercial payors; however, these payors represent a disproportionately small share of HCP’s operating profits.
Whether in the Medicare Advantage, commercial or Medicaid market, managed care health plans seek to provide a coordinated and efficient approach to managing the health care needs of their enrolled populations. By negotiating with providers, such as pharmacies, hospitals and physicians, and indirectly trying to influence physicians’ behavior through various incentive and penalty schemes, managed care companies attempt to enhance their profitability by limiting their medical costs. These health plans have shown success in mitigating certain components of medical cost, but we believe they are limited by their indirect relationship with physicians, who in the aggregate direct most of their patients’ health care costs. We believe that physician-led and professionally-managed integrated medical systems such as HCP’s have a greater opportunity to influence cost and improve quality due to the close coordination of care at the most effective point of contact with the patient—the primary care physician.
Capitation
There are a number of different models under which an integrated medical system receives payment for managing and providing health care services to its members.
Capitation Structure. Under traditional fee-for-service reimbursement, physicians are paid a specified fee for services they provide during a patient visit. Under this structure, physician compensation is solely related to the volume of patient visits and procedures performed, thus offering limited financial incentive to focus on cost containment and preventative care.
Under capitation, payors pay a fixed amount per enrolled member, thereby subcontracting a significant portion of the responsibility for managing patient care to physicians. Global capitation represents a prospective budget from which the provider system then manages care-related expenses including payments to affiliated providers outside the group, such as hospitals and specialists. Compared to traditional fee-for-service models, We believe that capitation arrangements better align provider incentives with both quality and efficiency of care for a population of patients. We believe that this approach improves the quality of the experience for patients and the potential profitability for efficient care providers.
Since premiums paid represent a significant amount per person, the revenue and, when costs are effectively managed, profit opportunity available to an integrated medical system under a capitated arrangement can be significant. This is particularly the case for patients with multiple diseases and senior members. We believe that the advantages, savings and efficiencies made possible by the capitated model are most pronounced when the care demands of the population are the most severe and require the most coordination, such as for the senior population or patients with chronic, complex and follow-on diseases. While organized coordination of care is central to the capitated model, it is also well suited to the implementation of preventative care and disease management over the long-term since physicians have a financial incentive to improve the overall health of their population.
The inherent risk in assumption of global care risk can lead to losses if a number of individual patients’ medical costs exceed the expected amount. This risk is especially significant to individual practitioners or smaller physician groups who lack the scale required to spread the risk over a broad population. HCP has the scale, comprehensive medical delivery resources, significant infrastructure to support practicing physicians, and demonstrated care management know-how to spread the risk of losses over a large patient population.
Global Model.In Florida and Nevada, HCP contracts directly with health plans under global capitation arrangements that include hospital services because state law permits HCP to assume financial responsibility for both professional and institutional services. Because of the capitation to HCP, and HCP’s assumption of nearly the entire professional and institutional risk, HCP’s health plan customers function primarily to support it in processing claims and undertaking marketing and sales efforts to enroll members.
S-178
Table of Contents
Risk-Share Model. In California, HCP utilizes a capitation model in several different forms. While there are variations specific to each arrangement, HCP generally contracts with health plans to receive a PMPM fee for professional (physician) services and assumes the financial responsibility for professional services only. In some cases, the health plans separately enter into capitation contracts with third parties (typically hospitals) who receive directly a portion of the PMPM fee and assume contractual financial responsibility for institutional (hospital) services. In other cases, the health plan does not pay any portion of the PMPM fee to the hospital, but rather simply administers claims for hospital expenses itself. In both cases, HCP is responsible for managing the care dollars associated with both the professional and institutional services provided for the PMPM fee, but in the case of institutional services and as a result of its managed care-related administrative services agreements with hospitals, HCP recognizes the surplus of institutional revenues less institutional expense as HCP revenues.
HCP’s Competitive Strengths
We believe that HCP distinguishes itself through its ability to demonstrably improve medical outcomes and patient satisfaction while effectively managing costs. HCP achieves this result through the following key strengths:
| • | Clinically based utilization management models.HCP’s clinical leadership and affiliated group and network physicians devote significant efforts to ensuring that HCP’s members receive the most appropriate care in the most appropriate setting. HCP believes this results in significant differences compared to a typical unmanaged patient population. For example, during fiscal 2010, HCP’s inpatient acute bed days in California were 864 days per 1,000 members for its Medicare Advantage members, as compared to an average of 1,706 days per 1,000 patients for Medicare’s fee-for-service program during the same period. Similarly, HCP’s 30 day “all cause” hospital re-admission rate in California during fiscal 2010 was 14%, which HCP believes was lower than the Medicare fee-for-service benchmark. HCP has achieved similarly favorable outcomes in Nevada and Florida when compared to benchmarks. |
| • | Service commitment. HCP is committed to maximizing its patients’ satisfaction levels with HCP and their physicians. HCP regularly conducts comprehensive satisfaction surveys of its members and actively monitors survey results at the individual physician level. In its most recent survey conducted during the second quarter of 2012, 91.6% of patients surveyed gave their HCP physician top satisfaction scores. We believe that HCP’s high rates of patient satisfaction lead to greater member retention. Because of the number of HCP commercial health plan customers, if an employer changes health plans, members can often move to another plan and still retain their participation with HCP. HCP believes the longevity of the patient-physician relationship provides it with additional leverage with the health plans and helps to ensure the stability of the relationship between the health plans and HCP. |
| • | Long standing relationships with health plans. We believe that HCP’s scale, combined with its strong reputation and high quality patient care, makes it an attractive partner for health plans compared to smaller provider groups that may have a higher risk of default and may not have the same resources to devote to integrated care techniques. We believe that HCP is a leader in managing global capitation arrangements by assuming both professional (physician) and institutional (hospital) risk and has the critical mass necessary to diversify these risks across a large membership base. HCP’s scale and resources enable it to invest in continuous innovation to improve the clinical outcomes of its members. We believe that health plans in the regions in which it operates appreciate HCP’s ability to manage global risk because these arrangements eliminate the volatility of medical costs, the largest cost component for health plans. HCP, or its predecessor companies, have longstanding relationships with its health plan customers, with these relationships having an average tenure of approximately 20 years. For example, HCP has had a relationship spanning approximately 28 years with UnitedHealthcare, one of HCP’s largest customers. HCP also provides care to a significant portion of Humana’s Medicare Advantage membership in the central Florida region. HCP is not aware of any health plan customer that has not renewed its contract with HCP. |
S-179
Table of Contents
| • | Proprietary database of long-tenured patient data. HCP has nearly three decades of experience in managing complex disease cases for its population of patients. As a result, HCP has developed a rich dataset of patient care experiences and outcomes which permits HCP to proactively monitor and intervene in improving the care of its members. HCP uses this proprietary database to: |
| • | identify patients with high-cost or high-utilization disease categories; |
| • | provide direct feedback to their physicians and other care-givers with point of care reminders and other notifications of patient’s needs; |
| • | reduce variation in practice patterns, provide immediate feedback to physicians and improve the overall quality of care; |
| • | benchmark HCP’s performance across its organization and against published metrics to establish a best practices approach to health care; and |
| • | accurately model historical utilization and cost patterns and, from that, seek to project future patterns, allowing HCP to better assess risk and negotiate health plan contracts. |
| • | Experienced management team.HCP’s senior management team possesses substantial experience within the healthcare industry, with average experience of nearly 35 years. The management team has overseen significant growth in its business and demonstrated the ability to produce strong financial performance. HCP’s senior management team is expected to continue with HCP after the Merger. |
| • | Strong financial performance.Consistent revenue and EBITDA growth over the prior 14 years, coupled with negative working capital and low maintenance capital expenditures over this period of less than one percent of revenue, have enabled HCP to achieve attractive historical cash flows. In the twelve months ended June 30, 2012, HCP generated cash flows from operating activities of $512 million. HCP’s ability to generate strong and consistent cash flow from operations has enabled it to invest in its operations and pursue attractive growth opportunities. |
| • | Scalable and portable business model. We believe that HCP’s strong clinical outcomes, reputation with health plans and health care providers and its ability to successfully manage complex regulatory, reimbursement, clinical and operating environments associated with practicing medicine are key reasons that medical groups and IPAs are interested in joining HCP’s network. HCP has the capacity to extend its network and systems to encompass additional medical groups and IPAs with only limited incremental capital expenditures. |
HCP’s Strategy
HCP intends to continue to increase its membership, and generate incremental revenue and earnings opportunities in existing and new markets. HCP expects to accomplish this through pursuing the following activities:
Continue to Provide High Quality Care to Patients While Minimizing Costs.HCP intends to continue to improve quality care and strong medical outcomes for its patients while managing health care costs and minimizing the level of unnecessary care by investing in the following programs and initiatives:
| • | Integrated care teams. HCP has re-engineered the patient care process to enhance the patient care experience through the use of integrated care teams. These include care teams of physicians, nurses and medical assistants who have direct contact with and deep personal knowledge of a panel of assigned patients. Patients have direct phone and/or email access to these teams for appointments and information flow. Teams are supported by a multi-disciplined support center, 24 hours a day, seven days per week, that handles customer service issues, claims and benefit questions as well as medical questions and the triaging of medical conditions to the appropriate resource after office hours. |
S-180
Table of Contents
| • | Disease management programs.HCP proactively manages its patients with specific disease conditions, including chronic obstructive pulmonary disease, chronic kidney disease, ESRD and diabetes, among others, through a combination of direct clinical intervention and treatment, and patient education. These programs are designed to reduce the escalation of the severity of the medical conditions, thereby reducing hospital admissions and medical claims costs, as well as improving the overall quality of life for patients with these conditions. |
| • | Hospitalists.HCP utilizes hospitalists in all of its markets to more efficiently use HCP’s primary care physicians and to provide more individualized and focused attention for hospitalized patients. These specifically trained physicians monitor and manage on a 24 hours a day, seven days per week basis all aspects of care during a patient’s hospital stay, in many cases on-site at the hospital. We believe this results in more efficient, and generally shorter hospital stays, as well as reduced levels of readmissions. |
| • | Comprehensive care centers.HCP offers comprehensive care centers that are typically located within existing medical clinics and practice locations. These comprehensive care centers provide customized interventions for high-risk patients with multiple chronic diseases. These comprehensive services are designed to prevent these chronic disease conditions from becoming more severe. |
| • | Home care program.The most ill, highest risk patient population typically accounts for a disproportionate level of hospitalizations and emergency room visits. HCP’s home care program brings personalized care to its most frail and ill patients in their home. This program is designed to reduce inpatient acute admissions and emergency room visits for the patients under HCP’s care. |
| • | Same or next day access.Most physicians who depend on fee-for-service reimbursement have fully booked schedules so that when a patient calls with symptoms that are troublesome, but not life-threatening, the patient may be told to go to the emergency room, an extremely high cost and inefficient setting for delivery of care. To mitigate this problem, HCP keeps open a significant block of its physicians’ schedules for same or next day access. This allows patients with non life-threatening problems to be seen in a physician’s office on the same or next day after they call. We believe this program not only improves the quality of care, but also enhances patient satisfaction and retention. |
| • | Urgent care centers.HCP owns and operates freestanding urgent care centers to provide access for patients who require immediate care. These centers create a more appropriate clinical alternative to emergency room visits, which are typically expensive and may lead to unnecessary inpatient admissions. |
Organically Grow by Adding Physicians, Physician Groups and IPAs in Existing and Adjacent Markets. Consistent with HCP’s historical growth model, HCP plans to continue to organically grow its network in and adjacent to its existing markets by adding physicians, physician groups and IPAs, particularly those with strong senior enrollment and an acceptance of integrated care management and evidence-based medicine techniques. We believe that HCP’s strong relationships with many leading health plans, extensive provider networks, and reputation for providing quality care, make it an attractive partner for a wide range of physician groups and IPAs. We believe that there are many of these physician groups and IPAs in its existing and adjacent markets that have experience in managed care. As such, HCP believes that the growth opportunity from organically adding physician groups and IPAs is significant in its primary and adjacent markets.
Opportunistically Expand into New Markets. HCP intends to continue to expand its business model into new markets in a disciplined and opportunistic manner. HCP has acquired or has become affiliated with a number of medical groups, IPAs and physician practices in the past and is currently reviewing a number of acquisitions and affiliation candidates of various sizes both within and outside its existing geographic markets. If a significant portion of the opportunities currently being reviewed were consummated, HCP could be required to raise up to $1 billion in additional financing.
S-181
Table of Contents
Pursue New Product Offerings. HCP also intends to pursue new product offerings. In HCP’s existing markets, HCP intends to contract with health plans that undertake to manage the care of members who are dually eligible for both Medicare and Medicaid benefits, and who are currently receiving care through a traditional fee-for-service model. Health plans receive a higher premium from CMS for dual-eligible patients under a Medicare Advantage program, as these patients typically have higher medical costs. For example, these patients experience 80% higher medical costs than the average Medicare patient and have a 47% higher rate of diabetes; over half of these patients are under treatment for five or more chronic conditions. As a result of CMS authorized demonstration projects, several states are exploring enrolling these dual-eligible patients in managed care plans, and California announced an intention to launch a demonstration project in 2013. Given the high level of chronic disease states among this population and the higher associated costs, HCP believes there is a sizeable new revenue opportunity to apply its integrated care management model to serving dual-eligible patients. In addition, HCP has been selected by CMS Innovation Center to be among the 32 “Pioneer Accountable Care Organizations,” in each of HCP’s three markets. HCP is the only such Pioneer ACO in more than one state. Pioneer ACOs contract with CMS on a direct basis, not through health plans, to manage the care of Medicare fee-for-service patients attributable to these organizations. The Pioneer ACO program presents an opportunity for HCP to bring the benefit of its integrated care programs to a fee-for-service patient population. Because Medicare fee-for-service is not part of HCP’s health plan customers business, this new product offering will not compete with HCP’s customers.
Provider Network
HCP provides complete medical care through a network of participating physicians and other health care professionals. Through its group model, HCP employs, directly and through its affiliated physician groups, approximately 374 affiliated group full-time primary care physicians who practice in clinics that are operated by HCP. Through its IPA model, HCP contracts with approximately 1,454 additional network primary care physicians who provide care for HCP’s members in an independent office setting. These physicians are complemented by a network of several thousand specialists and ancillary providers and 139 network hospitals that provide specialty or institutional care to the patients of HCP’s affiliated physicians, physician groups and IPAs.
In order to comply with local regulations prohibiting the corporate practice of medicine, many of HCP’s group physicians are employed by affiliated medical groups with which HCP has entered into long-term management agreements, while, in other regions, the physicians are employed directly by HCP. The largest of these HCP managed medical groups is HCPAMG, which employs, directly or indirectly, 590 full-time primary care physicians, specialists and hospitalists. See “—Government Regulations—Corporate Practice of Medicine and Fee Splitting” below.
HCP does not own hospitals, although hospitals are an essential part of its provider network. In most cases, however, HCP contracts or otherwise aligns with hospitals to manage the utilization, readmission and cost of hospital services.
Most HCP patients receive specialty care through HCP’s network based on referrals made by their primary care physician. These specialists may be reimbursed based on capitation, case rates or on a discounted fee-for-service rate.
A typical fee-for-service primary care physician might treat approximately 30 patients per day. In contrast, HCP group physicians typically see 18 to 20 patients per day, which we believe is a more appropriate benchmark to ensure there is sufficient time to understand all of the patients’ clinical needs. HCP care teams, including nurses, engage in outreach to patients in order help monitor the fragile and high risk patients, and help improve adherence to physicians’ care plans. During these visits, HCP’s physicians, nurses and educators use the time to educate patients and manage their health care needs. The goal of this preventative care delivery model is to keep patients healthy. Education improves self-management and compliance which allows the patient to recognize
S-182
Table of Contents
early signs of their disease and seek appropriate care. We believe this translates into earlier intervention, which in turn leads to fewer emergency room visits, fewer hospital admissions and fewer hospital bed days (the most expensive location for health care).
This clinical model seeks to provide early diagnosis of disease or deterioration in a chronic and complex condition and provide preventive care to maintain optimal health and avert unnecessary hospitalization. Clinic-based case managers and hospitalists coordinate with the primary care physicians to ensure that patients are receiving proper care whether they are in the clinic, in the hospital or are not regularly accessing health care. Physicians and case managers encourage patients to regularly visit the clinics in order to enhance their day-to-day health and diagnose any illness or deterioration in condition as early as possible.
Information Technology and Clinical Data Management
HCP’s information technology system, including HCP’s electronic health record and data warehouse, is designed to support the HCP delivery model with data-driven opportunities to improve the quality and cost effectiveness of the care received by its members. Using informatics technology, HCP has created disease registries that track large numbers of patients with defined medical conditions. HCP applies the data from these registries to manage the care for patients with similar medical conditions which we believe leads to a better medical outcome. We believe its approach to using this data is effective because the information is communicated by the patient’s physician rather than the health plan or disease management companies.
HCP employs a wide variety of other information applications in order to service IPA and network providers using web connectivity. The “HCP Connect!” on-line portal provides web-based eligibility, referrals, electronic claims submission and explanation of benefits, and other communication vehicles for individual physician offices. The success of this suite of applications has enhanced HCP’s ability to manage its IPA networks, and has resulted in significant back-office efficiencies for HCP and its affiliated physician groups. HCP has further expanded its ability to share key utilization and clinical data with its internal and contracted physicians and specialists through the Physician Information Portal and the Clinical Viewer. Through these secure web portals, a physician is able to obtain web-based, point of care information regarding a patient, including diagnosis history, provide quality indicators, historical risk-adjustment coding information, pharmacy medication history, and other key information. In addition to its web-portals geared towards physicians, HCP has recently introduced a patient on-line portal to enable HCP’s patients to securely view their own clinical information, schedule physician appointments and interact electronically with their physicians. HCP believes these tools help to lead to high quality clinical outcomes, create internal efficiencies, and enhance the satisfaction of its affiliated physicians and patients.
In addition, HCP uses its data to carefully track high utilizing patients through an automated data warehouse and mining system. HCP filters the data warehouse to identify and reach out to patients with high-utilization patterns who are inefficiently using resources such as visiting an emergency room when either a same-day appointment or urgent care center would be more appropriate and satisfactory for the member. High utilizing patients are identified and tracked as part of HCP’s electronic health record by their physician and HCP’s care management staff. Specific care plans are attached to each of these patients and tracked carefully for full compliance. The objective is to proactively manage their care at times when these patients are either not compliant with the care plan or when changing circumstances require care managers to develop new and more suitable care plans. By using these resources, HCP has achieved improvements in quality of care, satisfaction and cost.
S-183
Table of Contents
Government Regulations
In addition to the laws and regulations to which DaVita is subject, HCP’s internal operations and contractual relationships with healthcare providers such as hospitals, other healthcare facilities, and healthcare professionals are subject to extensive and increasing regulation by numerous federal, state, and local government entities. These laws and regulations often are interpreted broadly and enforced aggressively by multiple government agencies, including the Office of Inspector General, or OIG, the U.S. Department of Justice, and various state authorities. Many of these laws and regulations are the same as those that impact DaVita. For example:
| • | HCP’s financial relationships with healthcare providers including physicians and hospitals could subject HCP to sanctions and penalties under the federal anti-kickback statute; |
| • | The referral of Medicare patients by HCP-affiliated physicians for the provision of designated health services, or DHS, may subject the parties to sanctions and penalties under the federal Stark Law; |
| • | HCP’s financial relationships and those of its affiliated physicians may subject the parties to penalties and sanction under state fraud and abuse law; |
| • | HCP’s submission of claims to governmental payors such as the Medicare and Medicaid programs and submission of data to Medicare Advantage plans for services provided by its affiliated physicians and clinical personnel may subject HCP to sanctions and penalties under the federal False Claims Act, or FCA; and |
| • | HCP’s handling of electronic protected health information may subject HCP to sanctions and penalties under HIPAA, and state medical privacy laws which often include penalties and restrictions that are more severe than those which arise under HIPAA. |
A finding that claims for services were not covered or not payable, or the imposition of sanctions associated with a violation of any of these healthcare laws and regulations, could result in criminal or civil penalties and exclusion from participation in Medicare, Medicaid and other federal and state healthcare programs and could have a material adverse effect on HCP’s business, financial condition and results of operations. HCP cannot guarantee that its arrangements or business practices will not be subject to government scrutiny or be found to violate certain healthcare laws. Government audits, investigations and prosecutions, even if HCP is ultimately found to be without fault, can be costly and disruptive to HCP’s business. Moreover, changes in healthcare legislation or government regulation may restrict HCP’s existing operations, limit the expansion of HCP’s business or impose additional compliance requirements and costs, any of which could have a material adverse effect on HCP’s business, financial condition and results of operations.
The following includes brief descriptions of some, but not all, of the laws and regulations that, in addition to those described in relation to DaVita, affect HCP’s business.
Licensing, Certification, Accreditation and Related Laws and Guidelines.
HCP clinical personnel are subject to numerous federal, state and local licensing laws and regulations, relating to, among other things, professional credentialing and professional ethics. Since HCP clinical personnel perform services in medical office settings, hospitals and other types of healthcare facilities, HCP may indirectly be subject to laws applicable to those entities as well as ethical guidelines and operating standards of professional trade associations and private accreditation commissions, such as the American Medical Association and the Joint Commission. There are penalties for non-compliance with these laws and standards, including loss of professional license, civil or criminal fines and penalties, loss of hospital admitting privileges, federal health care program disenrollment, loss of billing privileges, and exclusion from participation in various governmental and other third-party healthcare programs.
S-184
Table of Contents
Professional Licensing Requirements.
HCP’s clinical personnel including physicians must satisfy and maintain their professional licensing in the states where they practice medicine. Activities that qualify as professional misconduct under state law may subject them to sanctions, including the loss of their licenses and could, possibly, subject HCP to sanctions as well. Some state boards of medicine impose reciprocal discipline, that is, if a physician is disciplined for having committed professional misconduct in one state where he or she is licensed, another state where he or she is also licensed may impose the same discipline even though the conduct occurred in another state. Therefore, if an HCP-affiliated physician is licensed in multiple states, sanctions or loss of licensure in one state may result in sanction or the loss of licensure in another state. Professional licensing sanctions may also result in exclusion from participation in governmental healthcare programs, such as Medicare and Medicaid, as well as other third-party programs.
Corporate Practice of Medicine and Fee Splitting.
Two states in which HCP operates, California and Nevada, which represented 76% of HCP’s revenues for 2011, have laws that prohibit business entities, such as HCP and its subsidiaries, from practicing medicine, employing physicians to practice medicine, exercising control over medical decisions by physicians, known collectively as the corporate practice of medicine. These states also prohibit entities from engaging in certain arrangements, such as fee-splitting, with physicians. In some states these prohibitions are expressly stated in a statute or regulation, while in other states the prohibition is a matter of judicial or regulatory interpretation. In California, a violation of the corporate practice of medicine prohibition constitutes the unlawful practice of medicine, which is a public offense punishable by fines and other criminal penalties. In addition, any physician who participates in a scheme that violates California’s corporate practice of medicine prohibition may be punished for aiding and abetting a lay entity in the unlawful practice of medicine. In Nevada, violation of the corporate practice of medicine rules by a lay entity also constitutes the unlawful practice of medicine. This violation is a felony punishable by fines and other criminal penalties. Physicians in Nevada can similarly be punished for aiding and abetting in the unlicensed practice of medicine.
In California and Nevada, where the corporate practice of medicine is prohibited, HCP operates by maintaining long-term management contracts with multiple affiliated professional organizations that, in turn, employ or contract with physicians to provide those professional medical services required by the enrollees of the payors with which the professional organizations contract. Under these management arrangements, HCP performs only non-medical administrative services, does not represent that it offers medical services, and does not exercise influence or control over the practice of medicine by the physicians or the affiliated physician groups with which it contracts. For example, in California, HCP has a full-service management contract with HCPAMG. HCPAMG is owned by California-licensed physicians and professional medical corporations and contracts with physicians to provide professional medical services. In Nevada, HCP’s Nevada subsidiaries have similar management arrangements with Nevada professional corporations that employ and contract with physicians to provide professional medical services.
Some of the relevant laws, regulations, and agency interpretations in California and Nevada have been subject to limited judicial and regulatory interpretation. Moreover, state laws are subject to change. Regulatory authorities and other parties, including HCP’s affiliated physicians, may assert that, despite the management contracts under which HCP operates, HCP is engaged in the prohibited corporate practice of medicine or that HCP’s arrangements constitute unlawful fee-splitting. If this were to occur, HCP could be subject to civil or criminal penalties, HCP’s contracts could be found legally invalid and unenforceable (in whole or in part), or HCP could be required to restructure its contractual arrangements.
S-185
Table of Contents
If HCP were required to restructure its management arrangements in California or Nevada due to determination that a corporate practice of medicine violation existed, such a restructuring might include revisions of the management services agreements, which might include a modification of the management fee, and/or establishing an alternative structure, such as obtaining a California Knox-Keene license (as described below) or its Nevada equivalent which would permit HCP to contract with a physician network without violating the corporate practice of medicine prohibition.
The Knox-Keene Act.
The California Department of Managed Health Care, or DMHC, licenses and regulates health care service plans, or HCSPs, such as health plans pursuant to the Knox-Keene Act. In addition to administering the Knox-Keene Act’s various patient’s rights protections for HCSP-enrolled individuals, the DMHC is responsible for ensuring the financial sustainability over time of HCSPs and other regulated entities. As such, the DMHC is charged with continually monitoring the financial health of regulated entities. The DMHC’s Division of Financial Oversight conducts examinations of the fiscal and administrative affairs of licensed HCSPs to protect consumers and providers from potential insolvencies. Financial examination reviews include examinations of cash flow, premium receivables, intercompany transactions and medical liabilities. The examination also ensures that there is adequate tangible net equity, or TNE, as determined according to calculations included in the Knox-Keene Act . The TNE regulations for organizations holding a Knox-Keene license vary depending on circumstances, but generally require any licensee to have on hand in cash or cash equivalents 130% of the greater of (1) One Million Dollars ($1,000,000); (2) the sum of 2% of the first One Million Five Hundred Thousand Dollars ($1,500,000) of annualized premium revenues plus 1% of annualized premium revenues in excess of One Million Five Hundred Thousand Dollars ($1,500,000); or (3) the sum of 8% of the first One Million Five Hundred Thousand Dollars ($1,500,000) of annualized health care expenditures (except those paid on a capitated basis or managed hospital payment basis); plus 4% of the annualized health care expenditures, except those paid on a capitated basis or managed hospital payment basis, which are in excess of One Million Five Hundred Thousand Dollars ($1,500,000); plus 4% of annualized hospital expenditures paid on a managed hospital payment basis.
The DMHC interprets the Knox-Keene Act to apply to both HCSPs and downstream contracting entities, including provider groups, that enter into “global risk contracts” with licensed HCSPs. A “global risk contract” is a health care services contract in which a downstream contracting entity agrees to provide both professional (e.g., medical group) services and institutional (e.g., hospital) services subject to an at-risk or capitated reimbursement methodology. According to DMHC, entities that accept global risk must obtain a limited Knox-Keene license.
Under a limited Knox-Keene license, entities may enter into global risk contracts with other licensed HCSPs. Holders of limited licenses must comply with the same financial requirements as HCSPs with full licenses, including demonstrating specific levels of TNE, but are granted waivers from meeting marketing and other terms of full Knox-Keene licensure. The consequences of operating without a license include civil penalties, criminal penalties and the issuance of cease and desist orders.
HCP does not hold a limited Knox-Keene license. Instead of operating under such a license which would allow HCP to directly enter risk contracts with HCSPs for the provision of both professional and institutional services, HCP utilizes arrangements with hospital and its affiliated physician organizations. If (i) DMHC were to determine that HCP has been inappropriately taking global risk for institutional and professional services as a result of its various hospital and physician arrangements without having a limited Knox-Keene license or (ii) the California Board of Medicine were to conclude that the current HCP physician arrangements present a violation of the corporate practice of medicine, HCP may be required to obtain a limited Knox-Keene license to resolve such violations and HCP could be subject to civil and criminal penalties. Alternatively, HCP might voluntarily elect to obtain a limited Knox-Keene license for various reasons including to permit it to contract directly with HCSPs, to simplify its current contractual and financial structure and to facilitate expansion into new markets. If HCP were to obtain a limited Knox-Keene license, one of the primary impacts would be the TNE requirements described above.
S-186
Table of Contents
Although obtaining such a limited Knox-Keene license would ameliorate risks under the Knox-Keene Act and California’s corporate practice of medicine prohibition, there are disadvantages associated with obtaining such a license. These disadvantages include: (1) regulatory oversight of operations, (2) the need to seek approval for all material business changes, (3) significant requirements to maintain certain TNE levels, and (4) other operating limitations imposed by the Knox-Keene Act and its regulations.
Competition
HCP operates in a highly competitive environment and competes with medical groups and individual physicians in its markets. HCP competes with other primary care groups or physicians contracted with the health plans for membership. The health plans contract with care providers on the basis of costs, reputation, scope, efficiency and stability. The individual members select a primary care physician at the time of membership with the health plan. Location, name recognition, quality indicators and other factors go into that decision. For example, HCP competes with both Permanente Medical Group, which is the exclusive provider for Kaiser, and Heritage Provider Network in California; however, HCP’s principal competitors for members and health plan contracts vary by market.
Customers
While HCP promotes its physicians, programs and services, HCP does not “sell” to patients. Representatives from health plans with whom HCP contracts, and field marketing personnel, make the “sales” of enrolling patients. HCP contributes to the success of these sales efforts through relevant and differentiated messaging, materials and events that highlight the value of HCP’s accomplishments, services and effective care delivery. In this way, HCP’s marketing department and the health plans and field marketers are each responsible for their core competencies.
S-187
Table of Contents
Name | Position | |
Kent J. Thiry | Chairman of the Board and Chief Executive Officer | |
Javier J. Rodriguez | President | |
James K. Hilger | Interim Chief Financial Officer and Chief Accounting Officer | |
Dennis L. Kogod | Chief Operating Officer | |
Allen R. Nissenson, M.D. FACP | Chief Medical Officer | |
Laura A. Mildenberger | Chief People Officer | |
David T. Shapiro | Chief Compliance Officer | |
Kim M. Rivera | Chief Legal Officer and Corporate Secretary | |
LeAnne M. Zumwalt | Group Vice President |
Kent J. Thiry, age 56, became our chairman of the Board of Directors and chief executive officer in October 1999. From June 1997 until he joined us, Mr. Thiry was chairman of the board and chief executive officer of Vivra Holdings, Inc., which was formed to operate the non-dialysis business of Vivra Incorporated, or Vivra, after Gambro AB acquired the dialysis services business of Vivra in June 1997. From September 1992 to June 1997, Mr. Thiry was the president and chief executive officer of Vivra, a provider of renal dialysis and other healthcare services. From April 1992 to August 1992, Mr. Thiry was president and co-chief executive officer of Vivra, and from September 1991 to March 1992, he was president and chief operating officer of Vivra. From 1983 to 1991, Mr. Thiry was associated with Bain & Company, first as a consultant, and then as vice president. Mr. Thiry previously served on the board of Varian Medical Systems, Inc. from August 2005 to February 2009 and served as the non-executive chairman of Oxford Health Plans, Inc. until it was sold to UnitedHealth Group in July 2004. As a member of management, Mr. Thiry provides significant industry-specific experience and unique expertise regarding the company’s business and operations as well as executive leadership and management experience.
Javier J. Rodriguez, age 42, became our president in February 2012. Mr. Rodriguez served as our senior vice president from April 2006 to February 2012. From December 2003 to April 2006, Mr. Rodriguez served as our vice president value management, operations. From August 1999 to December 2003, Mr. Rodriguez served as our vice president of payor contracting. From July 1998 to August 1999, Mr. Rodriguez served as divisional financial analyst. Prior to joining DaVita, Mr. Rodriguez worked for Baxter Healthcare Corporation in Finance from 1995 to 1996. He also served as Director of Operations for CBS Marketing Inc. in Mexico City.
James K. Hilger, age 50, became our interim chief financial officer in April 2012 in connection with Mr. Borgen’s resignation from the company and continues to serve as our chief accounting officer, a position he has held since April 2010. Prior to April 2010, Mr. Hilger served as our vice president and controller since May 2006, after having served as our vice president, finance beginning in September 2005. Mr. Hilger was our acting chief financial officer from November 2007 through February 2008. From September 2003 until joining us, Mr. Hilger served as vice president, finance and administration and chief financial officer of Pyramid Breweries, a brewer of specialty beverages. From December 1998 to July 2003, Mr. Hilger served in positions as chief executive officer and chief financial officer of WorldCatch, Inc., a seafood industry company. From 1987 until joining WorldCatch, Inc., Mr. Hilger held a variety of senior financial positions in the food industry. Mr. Hilger began his career in public accounting with Ernst & Whinney.
Dennis L. Kogod, age 53, became our chief operating officer in January 2009 and prior to that, he served as our president-west beginning in October 2005. From January 2004 until joining us, Mr. Kogod served as president and chief operating officer-west of Gambro Healthcare, Inc., which we acquired in October 2005. From July 2000 to January 2004, Mr. Kogod served as president, west division of Gambro Healthcare, Inc. From June 1999 to July 2000, Mr. Kogod was president of Teleflex Medical Group, a medical original equipment manufacturer of medical delivery systems. From January 1996 to June 1999, Mr. Kogod was corporate vice
S-188
Table of Contents
president of Teleflex Surgical Group, a surgical device and service organization. Mr. Kogod served on the board of directors of Arbios Systems, Inc., a medical device and cell-based therapy company.
Allen R. Nissenson, MD, FACP, age 65, became our chief medical officer in August 2008. He is an emeritus professor of medicine at the David Geffen School of Medicine at UCLA, where he served as director of the dialysis program from 1977 to 2008 and associate dean from 2005 to 2008. Dr. Nissenson was the president of the Southern California End-Stage Renal Disease Network from 2005 to 2007. Dr. Nissenson was the president of the National Anemia Action Council from 2001 to 2007. Dr. Nissenson was the president of the Renal Physicians Association from 1999 to 2001.
Laura A. Mildenberger, age 54, became our chief people officer in July 2008, having joined us in October 2001 as vice president of operations. Prior to joining us, Ms. Mildenberger served as vice president of operations for the western U.S. for Matrix Rehabilitation, a physical therapy outpatient company, from March 2000 to October 2001. From 1993 to 2000, Ms. Mildenberger served as a general manager for NovaCare Outpatient Rehabilitation, a provider of physical and occupation therapy services. From 1988 to 1993, Ms. Mildenberger was the executive vice president/principal of Worker Rehabilitation Services, a multi-site physical rehabilitation company. Ms. Mildenberger began her career as an occupational therapist at the Mayo Clinic.
David T. Shapiro, age 42, became our chief compliance officer in October 2008 having joined us in March 2008 as the deputy chief compliance officer. Additionally, from October of 2008 until July 2011, Mr. Shapiro served as senior vice president. Prior to joining us, Mr. Shapiro was counsel at the Pepper Hamilton law firm from March 2007 through February 2008, during which time he represented health care clients in government investigations and compliance issues. From October 2003 through March 2007, Mr. Shapiro served as a trial attorney with the Civil Frauds Section of the United States Department of Justice. From June 1999 through October 2003, Mr. Shapiro was an attorney with the law firm Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C. in Washington, DC.
Kim M. Rivera, age 43, became our chief legal officer and secretary in July 2011, and prior to that served as our vice president, general counsel and corporate secretary since January 2010. From February 2006 to November 2009, Ms. Rivera served as vice president and associate general counsel of The Clorox Company, a consumer products company. From August 2004 to February 2006, Ms. Rivera served as vice president law and chief litigation counsel to Rockwell Automation, Inc., a provider of industrial automation control and information solutions. From November 1999 to August 2004, she served as general counsel to Rockwell’s Automation Control and Information Group. Prior to joining Rockwell, Ms. Rivera was an attorney at the law firm of Jones Day.
LeAnne M. Zumwalt, age 53, became our group vice president in July 2011. From January 2000 to July 2011, Ms. Zumwalt served as vice president and currently oversees our public policy and government relations and our purchasing functions. Ms. Zumwalt has served in various capacities with us and served as our vice president investor relations from January 2000 through October 2009. From 1997 to 1999, Ms. Zumwalt served as chief financial officer of Vivra Specialty Partners, a privately held health care service and technology firm. From 1991 to 1997, Ms. Zumwalt held various executive positions at Vivra Incorporated, a publicly held provider of dialysis services. Prior to joining Vivra Incorporated, Ms. Zumwalt was a senior manager at Ernst & Young, LLP. Ms. Zumwalt serves on the board of directors of The Advisory Board Company.
S-189
Table of Contents
Name | Position | |
Dr. Robert Margolis | Chairman and Chief Executive Officer | |
Matthew Mazdyasni | Executive Vice President and Chief Financial and Administrative Officer | |
Dr. William Chin | Executive Medical Director | |
Zan Calhoun | Chief Operating Officer |
Dr. Margolis, age 66, has been the Chairman and Chief Executive Officer of HCP since 1982 and the managing partner of HCP Medical Group since he founded its predecessor entity in 1975. Upon the closing of the Merger, Dr. Margolis will be appointed to the Company’s board and will serve as the Co-Chairman of the Company’s Board of Directors. Dr. Margolis serves on the boards of directors of the Martin Luther King Hospital, the National Committee for Quality Assurance, the California Association of Physician Groups, and the California Hospital Medical Center, Los Angeles. Dr. Margolis also serves as a member of the Executive Management Advisory Board at UCLA’s School of Public Health, a member of HealthCare Policy Advisory Council for Harvard Medical School, and a member of the advisory board of the USC Schaeffer Center for Health Policy and Economics. Dr. Margolis previously served as the chairman of the boards of directors of the American Medical Group Association, the National Committee for Quality Assurance, and the Unified Medical Group Association. Dr. Margolis has a national reputation in the managed care industry with over 40 years of industry experience. He works extensively on issues of quality improvement, pay for performance and access to care issues.
Mr. Mazdyasni, age 55, joined HCP in 1982 and currently serves as its Executive Vice President and Chief Financial and Administrative Officer. Mr. Mazdyasni serves on the board of directors of the California Association of Physician Groups and California Medical Group Insurance Company, Risk Retention Group. Mr. Mazdyasni is also a member of Advisory Council of University of Southern California Sol Price School of Public Policy and the California Associate of Physician Group Public Policy Committee.
Dr. Chin, age 71, has been the Executive Medical Director of HCP since 1992 and is one of its founding partners. Prior to founding HCP, Dr. Chin was President of Huntington Medical Group from 1980 to 1992.
Mr. Calhoun, age 65, joined HCP in 2004 and currently serves as its Chief Operating Officer. Prior to joining HCP, Mr. Calhoun was the Chief Executive Officer of OAO Healthcare Solutions, a healthcare software company in southern California, from 2003 to 2004.
S-190
Table of Contents
DESCRIPTION OF OTHER INDEBTEDNESS
Senior Secured Credit Facility
We have an outstanding $3,000,000,000 Senior Secured Credit Agreement, or Credit Agreement. As of June 30, 2012, the Credit Agreement consists of a five year $350,000,000 revolving line of credit, a five year $925,000,000 Term Loan A, approximately $198,000,000Term Loan A-2 and a six year $1,723,750,000 Term Loan B. We also have the right to request an increase to the borrowing capacity to a total aggregate principal amount of not more than $4,000,000 subject to bank participation. The revolving line of credit and the Term Loan A bears interest at LIBOR plus an interest rate margin of 2.50%. The interest rate margin is subject to adjustment depending upon DaVita’s leverage ratio and can range from 2.25% to 2.75%. The Term Loan A requires annual principal payments of $50,000,000 in 2012, $100,000,000 in 2013, and $150,000,000 in 2014, with the balance of $650,000,000 due in 2015. The Term Loan A-2 requires annual principal payments of $2,000,000 with a balance of $191,500,000 due in 2016, and bears interest at LIBOR (floor of 1.00%) plus an interest rate margin of 3.50% subject to a rating based step-down to 3.25%. The Term Loan B bears interest at LIBOR (floor of 1.50%) plus 3.00% subject to a ratings based step-down to 2.75%. The Term Loan B requires annual principal payments of $17,500,000 in each year from 2011 through 2015 with the balance of $1,662,500,000 due in 2016. The borrowings under the Credit Agreement are guaranteed by substantially all of our direct and indirect wholly-owned domestic subsidiaries and are secured by substantially all of DaVita’s and our guarantors’ assets. The Credit Agreement contains customary affirmative and negative covenants such as various restrictions on investments, acquisitions, the payment of dividends, redemptions and acquisitions of capital stock, capital expenditures and other indebtedness, as well as limitations on the amount of tangible net assets in non-guarantor subsidiaries. However, many of these restrictions will not apply as long as the our leverage ratio is below 3.50:1.00. In addition, the Credit Agreement requires compliance with financial covenants including an interest coverage ratio and a leverage ratio that determines the interest rate margins as described above.
2018 and 2020 Notes
We have $775,000,000 aggregate principal amount of 6 3/8% senior notes due 2018 and $775,000,000 aggregate principal amount of 6 5/8% senior notes due 2020 (collectively the 2018 and 2020 Notes). The 2018 and 2020 Notes pay interest on May 1 and November 1 of each year. The 2018 and 2020 Notes are unsecured senior obligations and rank equally to other unsecured senior indebtedness. The 2018 and 2020 Notes are guaranteed by substantially all of our direct and indirect wholly owned domestic subsidiaries. We may redeem some or all of the 6 5/8% senior notes at any time on or after November 1, 2013 at certain redemption prices and may redeem some or all of the 6 5/8% senior notes at any time on or after November 1, 2014 at certain redemption prices.
Amendment of Senior Secured Credit Facilities
We launched a syndication process pursuant to which we are seeking to enter into amended senior secured credit facilities, which we are planning to enter into prior to the closing of the Merger. We anticipate that the amended senior secured credit facilities will provide for an aggregate additional borrowing capacity of $3,000 million (with a right by the Company to request an increase in the borrowing capacity in an amount not to exceed the greater of (a) $1,000 million and (b) an additional amount so long as our senior secured leverage ratio does not exceed 3.50:1.00, subject to lender participation), comprised of a new five year Term Loan A-3 facility in an aggregate principal amount of $1,350 million, and a new seven year Term Loan B-2 facility in an aggregate principal amount of $1,650 million. We expect that our amended senior secured credit facilities will be guaranteed by a substantial portion of our direct and indirect wholly-owned domestic subsidiaries and will be secured by substantially all of our and our subsidiary guarantors’ assets. In particular, these facilities will be secured by first priority pledges of 100% of the equity interests owned by us and our subsidiary guarantors in domestic subsidiaries and up to 65% of the equity interests of our direct wholly-owned foreign subsidiaries.
S-191
Table of Contents
We expect that our amended senior secured credit facilities will contain customary affirmative and negative covenants such as various restrictions on investments, acquisitions, the payment of dividends, redemptions and acquisitions of capital stock, capital expenditures and other indebtedness, as well as limitations on the amount of tangible net assets in non-guarantor subsidiaries. In addition, we expect that the amended senior secured credit facilities will require compliance on a quarterly basis with certain financial covenants.
S-192
Table of Contents
We will issue $1,000 million aggregate principal amount of % senior notes due 2022, or Notes, under an Indenture dated as of the Issue Date, or Indenture, among us, as issuer, the Subsidiary Guarantors party thereto and The Bank of New York Mellon Trust Company, N.A. as trustee, or Trustee. The terms of the Notes include those expressly set forth in the Indenture and those made part of the Indenture by reference to the Trust Indenture Act of 1939, as amended. The Indenture does not limit the aggregate principal amount of Notes that may be issued thereunder, although the aggregate principal amount of Notes to be issued in this offering will be limited to $1,000 million. We may issue an unlimited principal amount of additional Notes under the Indenture having substantially identical terms and conditions as the Notes issued on the Issue Date, or Additional Notes. We will only be permitted to issue Additional Notes if at the time of issuance we are in compliance with the covenants contained in the Indenture. Any Additional Notes will be part of the same series as the Notes that we are currently offering and will vote on all matters with the registered holders, or Holders, of the other Notes.
This “Description of Notes” is intended to be a useful overview of some of the provisions of the Notes and the Indenture. Since this description is only a summary, you should refer to the Indenture and the Escrow Agreement for a complete description of the obligations of the Company and the Subsidiary Guarantors and your rights.
You will find the definitions of capitalized terms used in this description under the heading “Certain definitions.” For purposes of this description, unless otherwise expressly stated or the context otherwise requires, references to the “Company,” “DaVita,” “we,” “our,” and “us” and similar references refer only to DaVita Inc. and not to its subsidiaries; references to a “Holder” refer to a registered holder of any Notes.
General
Maturity and interest. The Notes will mature on , 2022; interest on the Notes will be payable semi-annually in arrears and will:
| • | accrue at the rate of % per annum, |
| • | accrue from the most recent interest payment date or, if no interest has been paid on the Notes, from the Issue Date, |
| • | be payable in cash semi-annually in arrears on and (commencing on , 2013 with respect to the Notes issued in this offering) to the Holders of record on the and , respectively, immediately preceding such interest payment dates, and |
| • | be computed on the basis of a 360-day year comprised of twelve 30-day months. |
Ranking. The Notes will be our unsecured senior obligations. The Notes:
| • | will rank equally in right of payment with all of our other existing and future unsecured indebtedness that is not, by its terms, expressly subordinated in right of payment to the Notes (including our outstanding 6 3/8% Senior Notes due 2018 and 6 5/8% Senior Notes due 2020), |
| • | will be senior in right of payment to all of our existing, if any, and future unsecured indebtedness that is, by its terms, expressly subordinated in right of payment to the Notes, |
| • | will be effectively subordinated to all of our existing and future secured indebtedness to the extent of the value of the assets securing such indebtedness, including indebtedness under our Senior Credit Agreement, |
| • | will be effectively subordinated to all debt and other liabilities, including trade payables, of our Subsidiaries, and |
S-193
Table of Contents
| • | will not be guaranteed or otherwise supported, directly or indirectly, by the assets profits or cash flow of certain affiliated physician groups that are consolidated with HCP for financial reporting purposes, as affiliated physician groups would not be Subsidiaries of the Company. |
Similarly, a Subsidiary Guarantor’s Note Guarantee of the Notes will be the unsecured and unsubordinated obligation of such Subsidiary Guarantor and:
| • | will rank equally in right of payment with all existing and future unsecured indebtedness of such Subsidiary Guarantor that is not, by its terms, expressly subordinated in right of payment to such Note Guarantee (including, if applicable, its guarantee of our 6 3/8% Senior Notes due 2018 and 6 5/8% Senior Notes due 2020), |
| • | will be senior in right of payment to all existing and future unsecured indebtedness of such Subsidiary Guarantor that is, by its terms, expressly subordinated in right of payment to such Note Guarantee, and |
| • | will be effectively subordinated to all of the existing, if any, and future secured indebtedness of such Subsidiary Guarantor (including its guarantee of our obligations under our Senior Credit Agreement) to the extent of the value of the assets securing such indebtedness. |
As of June 30, 2012, after giving pro forma effect to the Financings and the Merger as if they had occurred on that date, the Company and the Subsidiary Guarantors would have had total secured debt of approximately $5,650 million and approximately $284 million of additional secured debt available to be borrowed under our amended senior secured credit facilities (after giving effect to outstanding letters of credit of approximately $66 million), and the Notes and the Note Guarantees would have been structurally subordinated to $510 million of liabilities, including $64 million of indebtedness and the rest being primarily trade payables, of the Subsidiaries that are not Subsidiary Guarantors.
HCP provides services to certain affiliated physician groups that are not owned by HCP, will not constitute “Subsidiaries” (as defined in the Indenture) and will not guarantee the Notes, even though the accounts of these groups are consolidated with the financial statements of HCP and would be consolidated with the financial statements of the Company following the Merger. Pursuant to management agreements between HCP and these affiliated Physician Groups, a substantial portion of the aggregate net revenues of these groups is payable to Subsidiaries of HCP and will be payable to entities that will be Subsidiary Guarantors as compensation for management and administrative services under management services agreements. See “HCP Business—Government Regulations—Corporate Practice of Medicine and Fee Splitting.” As of June 30, 2012, after giving pro forma effect to the Financing and the Merger as if they had occurred on that date, our consolidated balance sheet would have included third party liabilities of these Physician Groups, in the amount of approximately $305 million and assets of these Physician Groups in the amount of approximately $510 million after elimination of intercompany receivables (or approximately 3% of our consolidated total assets at that date). The pro forma consolidated net operating revenues and Adjusted EBITDA of DaVita for the twelve months ended June 30, 2012, giving effect to the Financing and the Merger as if they had occurred on July 1, 2011, would have been $9,929 million and $2,167 million, respectively. The pro forma consolidated net operating revenues and Adjusted EBITDA of the Company, excluding Physician Groups and the Company’s existing Subsidiaries that are not Guarantors, for the twelve months ended June 30, 2012, giving effect to the Financing and the Merger as if they had occurred on July 1, 2011, would have been $6,623 million and $1,744 million, respectively. Substantially all of the difference between pro forma consolidated Adjusted EBITDA of $2,167 million and the pro forma consolidated Adjusted EBITDA excluding HCP’s Physician Groups and DaVita’s existing Subsidiaries that are not Subsidiary Guarantors of $1,744 million for the twelve months ended June 30, 2012 is attributable to the exclusion of the existing DaVita Subsidiaries that are not Subsidiary Guarantors. The consolidated net operating revenues and Adjusted EBITDA of HCP for the twelve months ended June 30, 2012 were $2,564 million and $561 million, respectively. Excluding HCP’s Physician Groups, but inclusive of the management fees earned by HCP from the Physician Groups of $725 million, the net operating revenue and Adjusted EBITDA of HCP for the twelve months ended June 30, 2012 would have been $1,731 million and $557 million, respectively. Excluding the management fees earned by HCP from the Physician Groups, HCP net operating revenue for the twelve months ended June 30, 2012 would have been $1,006 million.
S-194
Table of Contents
Payments on the Notes; paying agent and registrar. We will pay principal of, premium, if any, and interest on the Notes at the office or agency designated by the Company in the Borough of Manhattan, The City of New York, except that we may, at our option, pay interest on the Notes by check mailed to Holders of the Notes at their registered address as they appear in the registrar’s books. We will designate the corporate trust office of the Trustee in New York, New York to act as our initial paying agent and registrar for the Notes. We may, however, change the paying agent or registrar or designate the Company or any of its Restricted Subsidiaries to act as paying agent or registrar for the Notes without prior notice to the Holders of Notes.
We will pay principal of, premium, if any, and interest on, Notes in global form, or Global Notes, registered in the name of or held by The Depository Trust Company, or DTC, or its nominee in immediately available funds to DTC or its nominee, as the case may be, as the registered Holder of such Global Notes.
Transfer and exchange. A Holder may transfer or exchange Notes in accordance with the Indenture. The registrar and the Trustee may require a Holder, among other things, to furnish appropriate endorsements and transfer documents. No service charge will be imposed by the Company, the Trustee or the registrar for any registration of transfer or exchange of Notes, but the Company may require a Holder to pay a sum sufficient to cover any transfer tax or other governmental taxes and fees required by law or permitted by the Indenture. The Company is not required to transfer or exchange any Note selected for redemption or tendered for repurchase and not withdrawn pursuant to a Change of Control Offer or Asset Disposition Offer. Also, the Company is not required to transfer or exchange any Note during a period beginning at the opening of business 15 days before the mailing of notice of redemption of Notes and ending at the close of business on the day of such mailing or register the transfer or exchange of any Note selected for redemption in whole or in part except the unredeemed portion of any Note redeemed in part.
The registered Holder of a Note will be treated as the owner of it for all purposes. Only registered Holders will have rights under the Indenture.
Defaulted interest. The Company will pay interest (including post-petition interest in any proceeding under any bankruptcy law) on overdue principal and, to the extent such payments are lawful, interest on overdue installments of interest, without regard to any applicable grace periods, at the rate of 2.0% per annum in excess of the interest rate applicable to the Notes.
Form of Notes; book-entry notes. The Notes will be issued in fully registered form, in minimum denominations of $2,000 in principal amount and multiples of $1,000 in principal amount in excess thereof and will be issued in the form of one or more Global Notes in book-entry form registered in the name of a depository, or Depository, or its nominee, which will be considered the registered Holder of those Global Notes for all purposes under the Indenture. The initial Depository will be DTC. Investors will not have the right to exchange beneficial interests in Global Notes for Notes in physical form. Notes in physical form will be issued in exchange for beneficial interests in the Global Notes only in the limited circumstances described elsewhere in this prospectus supplement under “Book-entry, Delivery and Form.”
Escrow of proceeds; release conditions
Unless the Merger shall have been consummated simultaneously with the consummation of the offering of the Notes contemplated hereby, the Escrow Proceeds will be placed by the Company in escrow until the earliest of (i) the date on which the Company delivers to the Escrow Agent the Officers’ Certificate referred to in the second succeeding paragraph, (ii) the Escrow End Date and (iii) the date on which the Company delivers notice to the Escrow Agent to the effect set forth in clause (ii) under “Special mandatory redemption”. The terms of the escrow will be set forth in the Escrow Agreement, pursuant to which the Company will deposit with the Escrow Agent on the Issue Date the net proceeds (after deducting the underwriting discount) from the offering of the Notes, together with additional cash and Eligible Escrow Investments, in an amount sufficient (as reasonably determined by the Company taking into account investment income therefrom and proceeds thereof) to redeem the Notes forcash at a
S-195
Table of Contents
redemption price equal to the aggregate issue price of the Notes sold on the Issue Date, together with accrued and unpaid interest on such Notes from the Issue Date or the most recent date to which interest has been paid or duly provided for on the Notes, as the case may be, up to but not including the Business Day after theEscrow End Date (collectively, referred to as Escrow Proceeds). The Company will grant the Trustee, for the benefit of the noteholders, subject to any lien of the Escrow Agent, a first priority security interest in the escrow account and all deposits and investment property therein to secure the payment of Special Mandatory Redemption;provided,however, that such lien and security interest shall automatically be released and terminate at such time as the Escrow Proceeds are released from the escrow. The Escrow Agent will invest the Escrow Proceeds in such Eligible Escrow Investments as the Company may from time to time direct in writing.
The Notes will be secured only by a pledge of the Escrow Proceeds and only for so long as the Escrow Proceeds remain in escrow.
The Company will only be entitled to direct the Escrow Agent to release Escrow Proceeds (in which case the Escrow Proceeds will be paid to or as directed by the Company) upon delivery to the Escrow Agent, on or prior to the Escrow End Date, of an Officers’ Certificate certifying that the following conditions have been or, substantially concurrently with the release of the Escrow Proceeds, will be satisfied (the date of delivery of such certificate to the Escrow Agent is hereinafter called the Release Date:
(1) (A) all conditions precedent to the consummation of the Merger will have been satisfied or waived in accordance with the terms of the Merger Agreement (other than the payment of Closing Merger Consideration (as defined in the Merger Agreement) and other than those conditions that by their terms are to be satisfied substantially concurrently with the consummation of Merger) and (B) the Merger will be consummated on substantially the terms described in this prospectus supplement substantially concurrently with the release of funds on deposit with the Escrow Agent; and
(2) no Default or Event of Default shall have occurred and be continuing under the Indenture;
(3) all conditions precedent to the execution and delivery of the amendment to the Senior Credit Agreement (other than the release of the Escrowed Proceeds) have been satisfied or waived and prior to or substantially concurrently with the release of the funds from the Escrow Account, the amendment to the Senior Credit Agreement will be effective and the term loans to be drawn in connection with the Merger will be able to be drawn upon by the Company on the Release Date; and
(4) Health Care Partners Holdings LLC and its subsidiaries that are required to guarantee the Senior Credit Agreement shall have, by supplemental indenture or joinder, as applicable, effective upon the Escrow Release Date, become, or substantially concurrently with the Release shall become, parties to the Indenture and other transaction documents as Subsidiary Guarantors.
Special mandatory redemption
If (i) the Escrow Agent has not received the Officers’ Certificate described above under “Escrow of proceeds; release conditions” on or prior to the Escrow End Date, or (ii) the Company notifies the Escrow Agent in writing that the Company will not pursue the consummation of the Merger, then the Escrow Agent shall, without the requirement of notice to or action by the Company, the Trustee or any other Person, release the Escrow Proceeds (including investment earnings thereon and proceeds thereof) to the Trustee and the Trustee shall apply (or cause a paying agent to apply) such proceeds to redeem the Notes on the Business Day following the date of the release of the Escrow Proceeds to the Trustee, or Special Mandatory Redemption Date, at a redemption price, or Special Mandatory Redemption Price, equal to 100% of the issue price of the Notes, plus accrued and unpaid interest from the Issue Date or the most recent date to which interest has been paid or duly provided for on the Notes, as the case may be, to, but excluding, the Special Mandatory Redemption Date. On the Special Mandatory Redemption Date, the Trustee will pay to the Company any Escrow Proceeds in excess of the amount necessary to effect the Special Mandatory Redemption.
Optional redemption
Except as described above under “Special mandatory redemption” and below, the Notes are not redeemable at our option until , 2017. On and after , 2017, we may at our option redeem the Notes, in whole or
S-196
Table of Contents
from time to time in part, upon not less than 30 or more than 60 days’ notice, at the following redemption prices (expressed as a percentage of principal amount) plus accrued and unpaid interest, if any, on the Notes to be redeemed to the applicable redemption date, if redeemed during the twelve-month period beginning on of the years indicated below:
Year | Percentage | |||
2017 | % | |||
2018 | % | |||
2019 | % | |||
2020 and thereafter | 100.000 | % | ||
Prior to , 2015, we may, at our option, on any one or more occasions, upon not less than 30 or more than 60 days’ notice, redeem up to 35% of the original aggregate principal amount of Notes (including the original aggregate principal amount of any Additional Notes) issued under the Indenture with the Net Cash Proceeds of one or more Equity Offerings at a redemption price (expressed as a percentage of the principal amount thereof) of %plusaccrued and unpaid interest, if any, to the redemption date;providedthat
(1) at least 65% of the original aggregate principal amount of the Notes (including the original aggregate principal amount of any Additional Notes) issued under the Indenture remains outstanding after each such redemption; and
(2) the redemption date occurs within 90 days after the closing of such Equity Offering (for purposes of clarity, in the event that there are two or more closings for any Equity Offering, then each such closing shall be deemed a separate “closing” for purposes of the foregoing provisions of this clause (2) with respect to the securities issued at such closing).
In addition, the Notes may be redeemed, in whole or from time to time in part, at any time prior to , 2017, at our option, upon not less than 30 nor more than 60 days’ notice, at a redemption price equal to 100% of the principal amount of the Notes redeemed plus the Applicable Premium on those Notes as of, and accrued and unpaid interest, if any, on those Notes to, the applicable redemption date.
Selection and notice
If the optional redemption date for any Notes is on or after an interest payment record date and on or before the related interest payment date, the accrued and unpaid interest, if any, will be paid to the Person in whose name such Note is registered at the close of business on such record date, and no additional interest will be payable to Holders whose Notes are subject to redemption by the Company on such redemption date.
In the case of any partial redemption of the Notes, selection of those Notes for redemption will be made by the Trustee in accordance with the procedures of the Depository in compliance with the requirements of the principal national securities exchange, if any, on which the Notes are listed or, if the Notes are not listed, then on a pro rata basis, by lot or by such other method as the Trustee in its sole discretion will deem to be fair and appropriate and in accordance with the procedures of the Depository;provided that Notes may be redeemed in part only in integral multiples of $1,000 and the remaining principal amount of any Note must not be less than $2,000. So long as the notes are represented by a Global Security registered in the name of DTC, neither the Trustee or any of its agents shall have any responsibility for any actions taken or not taken by the Depositary. If any Note is to be redeemed in part only, the notice of redemption relating to such Note will state the portion of the principal amount thereof to be redeemed. A new Note in principal amount equal to the unredeemed portion thereof will be issued in the name of the Holder thereof upon cancellation of the original Note.
Except under the circumstances set forth above under the heading “Special mandatory redemption”, the Company is not required to make mandatory redemption payments or sinking fund payments with respect to the Notes.
S-197
Table of Contents
Note Guarantees
The Company’s obligations under the Notes and the Indenture will be jointly and severally guaranteed by each Restricted Subsidiary that Guarantees any Indebtedness (including Bank Indebtedness under the Senior Credit Agreement) of the Company or any other Restricted Subsidiary (other than a Guarantee by a Foreign Subsidiary of Indebtedness of another Foreign Subsidiary or a Guarantee by a Receivable Subsidiary) and each other Restricted Subsidiary that the Company may, at its option, otherwise cause to become a Subsidiary Guarantor of the Notes pursuant to the terms of the Indenture.
Not all of our Subsidiaries will be required to guarantee the Notes. Unrestricted Subsidiaries, Foreign Subsidiaries, our Receivables Subsidiaries and Restricted Subsidiaries that do not Guarantee Indebtedness of us or any Restricted Subsidiary will not be required to be Subsidiary Guarantors. In the event of a bankruptcy, liquidation or reorganization of any of these non-guarantor Subsidiaries, these non-guarantor Subsidiaries will generally be required to pay the holders of their debts and their trade creditors before they will be able to distribute any of their assets to us.
As of June 30, 2012, after giving pro forma effect to the Financings and the Merger as if they had occurred on that date, the Company and the Subsidiary Guarantors would have had total secured debt of approximately $5,650 million and approximately $284 million of additional secured debt available to be borrowed under our amended senior secured credit facilities (after giving effect to outstanding letters of credit of approximately $66 million), and the Notes and the Note Guarantees would have been structurally subordinated to $510 million of liabilities, including $64 million of indebtedness and the rest being primarily trade payables, of the Subsidiaries that are not Subsidiary Guarantors.
HCP provides services to certain Physician Groups that are not owned by HCP, will not constitute Subsidiaries and will not guarantee the notes, even though the accounts of these groups are consolidated with the financial statements of HCP and would be consolidated with the financial statements of the Company following the Merger. Pursuant to management agreements between HCP and these Physician Groups, a substantial portion of the aggregate net revenues of these groups is payable to Subsidiaries of HCP and will be payable to entities that will be Subsidiary Guarantors as compensation for management and administrative services under Management Services Agreements. See “HCP’s Business—Government Regulations—Corporate Practice of Medicine and Fee Splitting.” As of June 30, 2012, after giving pro forma effect to the Financing and the Merger as if they had occurred on that date, our consolidated balance sheet would have included third party liabilities of these Physician Groups, in the amount of approximately $305 million and assets of these Physician Groups in the amount of approximately $510 million after elimination of intercompany receivables (or approximately 3% of our consolidated total assets at that date). The pro forma consolidated net operating revenues and Adjusted EBITDA of DaVita for the twelve months ended June 30, 2012, giving effect to the Financing and the Merger as if they had occurred on July 1, 2011, would have been $9,929 million and $2,167 million, respectively. The pro forma consolidated net operating revenues and Adjusted EBITDA of the Company, excluding Physician Groups and the Company’s existing Subsidiaries that are not Guarantors, for the twelve months ended June 30, 2012, giving effect to the Financing and the Merger as if they had occurred on July 1, 2011, would have been $6,623 million and $1,744 million, respectively. Substantially all of the difference between pro forma consolidated Adjusted EBITDA of $2,167 million and the pro forma consolidated Adjusted EBITDA excluding HCP’s Physician Groups and DaVita’s existing Subsidiaries that are not Subsidiary Guarantors of $1,744 million for the twelve months ended June 30, 2012 is attributable to the exclusion of the existing DaVita Subsidiaries that are not Subsidiary Guarantors. The consolidated net operating revenues and Adjusted EBITDA of HCP for the twelve months ended June 30, 2012 were $2,564 million and $561 million, respectively. Excluding HCP’s Physician Groups, but inclusive of the management fees earned by HCP from the Physician Groups of $725 million, the net operating revenue and Adjusted EBITDA of HCP for the twelve months ended June 30, 2012 would have been $1,731 million and $557 million, respectively. Excluding the management fees earned by HCP from the Physician Groups, HCP net operating revenue for the twelve months ended June 30, 2012 would have been $1,006 million.
S-198
Table of Contents
The Indenture will permit the Incurrence of certain additional Indebtedness by our Subsidiaries that are not Subsidiary Guarantors.
The Indenture will provide that under certain circumstances, the Company will be permitted to designate any of its Subsidiaries as “Unrestricted Subsidiaries” under the Indenture. The effect of designating a Subsidiary as an “Unrestricted Subsidiary” will be that:
| • | an Unrestricted Subsidiary will not be subject to many of the restrictive covenants in the Indenture, |
| • | a Subsidiary that is a Subsidiary Guarantor of the Notes issued under the Indenture and that is designated as an Unrestricted Subsidiary will be released from its Note Guarantee and its obligations under the Indenture, and |
| • | the assets, income, cash flow and other financial results of an Unrestricted Subsidiary will not be consolidated with those of the Company for purposes of calculating compliance with the restrictive covenants contained in the Indenture. |
The obligations of each Subsidiary Guarantor under its Note Guarantee and the Indenture will be limited to the maximum amount as will, after giving effect to all other contingent and fixed liabilities of such Subsidiary Guarantor (including, without limitation, any Guarantees under the Senior Credit Agreement and its Guarantees of the Existing Notes) that are relevant under federal or state bankruptcy, fraudulent conveyance, fraudulent transfer or similar laws, and after giving effect to any collections from, rights to receive contribution from, or payments made by or on behalf of, any other Subsidiary Guarantor in respect of the obligations of such Subsidiary Guarantor under its Note Guarantee and the Indenture, result in the obligations of such Subsidiary Guarantor under its Note Guarantee and the Indenture not constituting a fraudulent conveyance or fraudulent transfer under such laws.
A Subsidiary Guarantor shall be released from its obligations under its Note Guarantee and its obligations under the Indenture:
(1) in the event of a sale or other disposition of all or substantially all of the assets of such Subsidiary Guarantor, by way of merger, consolidation or otherwise, or a sale or other disposition of all of the equity interests of such Subsidiary Guarantor then held by the Company and the Restricted Subsidiaries,
(2) if such Subsidiary Guarantor is designated as an Unrestricted Subsidiary under the Indenture or otherwise ceases to be a Restricted Subsidiary under the Indenture, in each case in accordance with the provisions of the Indenture, upon effectiveness of such designation or when it first ceases to be a Restricted Subsidiary under the Indenture, respectively, or
(3) if such Subsidiary Guarantor no longer Guarantees any other Indebtedness of the Company or any Restricted Subsidiary of the Company (except for Guarantees of other Indebtedness of the Company or any Restricted Subsidiary of the Company that are released contemporaneously with the release of such Subsidiary Guarantor’s Note Guarantee);provided that a Subsidiary Guarantor shall not be permitted to be released from its Note Guarantee if it is an obligor with respect to Indebtedness that would not, under the “Limitation on indebtedness” covenant, be permitted to be Incurred by a Restricted Subsidiary that is not a Subsidiary Guarantor.
Change of control
If a Change of Control occurs, unless the Company has exercised its right to redeem all of the Notes issued as described under “Optional redemption,” or “Special mandatory redemption” each Holder of Notes will have the right to require the Company to repurchase all or any part (in integral multiples of $1,000,provided that the remaining principal amount of any Note repurchased in part must not be less than $2,000) of such Holder’s Notes at a purchase price in cash equal to 101% of the principal amount of the Notes plus accrued and unpaid interest, if any, to the Change of Control Payment Date.
S-199
Table of Contents
Within 30 days following any Change of Control, unless the Company has exercised its right to redeem all of the then outstanding Notes as described under “Optional redemption,” the Company will mail a notice, or Change of Control Offer, to each Holder of Notes, with a copy to the Trustee, stating:
(1) that a Change of Control has occurred and that such Holder has the right to require the Company to purchase such Holder’s Notes at a purchase price in cash equal to 101% of the principal amount of such Notesplusaccrued and unpaid interest, if any, to the Change of Control Payment Date referred to below, or Change of Control Payment;
(2) the repurchase date (which shall be no earlier than 30 days nor later than 60 days after the date such notice is mailed), or Change of Control Payment Date; and
(3) the procedures determined by the Company, consistent with the Indenture, that a Holder must follow in order to have its Notes repurchased.
On the Change of Control Payment Date, the Company will, to the extent lawful:
(1) accept for payment all Notes or portions of Notes (in integral multiples of $1,000,provided that the remaining principal amounts of any Note repurchased in part must not be less than $2,000) properly tendered and not withdrawn pursuant to the Change of Control Offer;
(2) deposit with the paying agent an amount equal to the Change of Control Payment in respect of all Notes or portions of Notes so tendered; and
(3) deliver or cause to be delivered to the Trustee the Notes so accepted together with an Officers’ Certificate stating the aggregate principal amount of Notes or portions of Notes being purchased by the Company.
The paying agent will promptly mail to each Holder of Notes so tendered the Change of Control Payment for such Notes, and the Trustee will promptly authenticate and mail (or cause to be transferred by book entry) to each Holder a new Note equal in principal amount to any unpurchased portion of the Notes surrendered, if any;providedthat each such new Note will be in a minimum principal amount of $2,000 or an integral multiple of $1,000 in excess thereof.
If the Change of Control Payment Date is on or after an interest payment record date and on or before the related interest payment date, the accrued and unpaid interest, if any, will be paid to the Persons in whose names the Notes are registered at the close of business on such record date, and no additional interest will be payable to Holders who tender pursuant to the Change of Control Offer.
The Change of Control provisions described above will be applicable whether or not any other provisions of the Indenture are applicable. Except as described above with respect to a Change of Control, the Indenture does not contain provisions that permit the Holders to require that the Company repurchase or redeem the Notes issued under the Indenture in the event of a takeover, recapitalization or similar transaction.
The Company will not be required to make a Change of Control Offer upon a Change of Control if a third party makes the Change of Control Offer in the manner, at the times and otherwise in compliance with the requirements set forth in the Indenture applicable to a Change of Control Offer made by the Company and purchases all Notes that are validly tendered and not withdrawn under such Change of Control Offer.
The Company will comply, to the extent applicable, with the requirements of Section 14(e) of the Exchange Act and any other securities laws or regulations in connection with the repurchase of Notes pursuant to this covenant. To the extent that the provisions of any securities laws or regulations conflict with provisions of the Indenture, the Company will comply with the applicable securities laws and regulations and will not be deemed to have breached its obligations described in the Indenture by virtue of the conflict.
If a Change of Control Offer is made, there can be no assurance that we will have available funds sufficient to pay for all or any of the Notes that might be delivered by Holders seeking to accept the Change of Control
S-200
Table of Contents
Offer. In addition, certain agreements governing our existing and future Indebtedness (including our Senior Credit Agreement) restrict or may restrict our ability to purchase Notes upon a Change of Control and also provide or may provide that some change of control events with respect to us constitute a default under those agreements. In the event a Change of Control occurs at a time when we are prohibited from purchasing Notes pursuant to such agreements, we could seek the consent of the holders of such Indebtedness to the purchase of Notes or could attempt to refinance the borrowings that contain the prohibition. If we do not obtain a consent or repay the borrowings, we will remain prohibited from purchasing Notes. In the case, our failure to purchase tendered Notes would constitute an Event of Default under the Indenture which may, in turn, constitute a default under our other debt agreements.
The amount required to be escrowed by the Company as described above under “Escrow of proceeds; release conditions” is less than the amount required to pay for all of the Notes that might be delivered by Holders seeking to accept the Change of Control Offer.
The Change of Control provisions described above may deter certain mergers, tender offers and other takeover attempts involving the Company by increasing the capital required to effectuate such transactions. The definition of “Change of Control” includes a disposition of all or substantially all of the property and assets of the Company and its Restricted Subsidiaries taken as a whole to any Person. Although there is a limited body of case law interpreting the phrase “substantially all,” there is no precise established definition of the phrase under applicable law. Accordingly, in certain circumstances there may be uncertainty as to whether a particular transaction would involve a disposition of “substantially all” of the assets of the Company and its Restricted Subsidiaries. As a result, it may be unclear as to whether a Change of Control has occurred and whether a Holder of Notes may require the Company to make an offer to repurchase the Notes as described above.
Certain covenants
Limitation on indebtedness
The Indenture will provide that the Company will not, and will not permit any of its Restricted Subsidiaries to, Incur any Indebtedness (including Acquired Indebtedness);provided,however, that the Company and any Restricted Subsidiary may Incur Indebtedness (including Acquired Indebtedness) if, after giving effect thereto, or Coverage Ratio Exception:
(1) the Consolidated Coverage Ratio for the Company and its Restricted Subsidiaries is at least 2.00 to 1.00; and
(2) no Default or Event of Default will have occurred or be continuing or would occur as a consequence of Incurring the Indebtedness or transactions relating to such Incurrence.
The Indenture will provide that the first paragraph of this covenant will not prohibit the Incurrence of the following Indebtedness, or Permitted Indebtedness:
(1) Indebtedness of the Company or any Restricted Subsidiary Incurred pursuant to the Senior Credit Facilities (with letters of credit being deemed to have a principal amount equal to the maximum potential liability thereunder to the Company and its Restricted Subsidiaries) or a Qualified Receivables Transaction in an aggregate principal amount Incurred pursuant to this clause (1) at any time outstanding not to exceed $4,000.0 million, less the aggregate principal amount of all principal repayments with the proceeds from Asset Dispositions utilized in accordance with clause (3)(a)(i) of the first paragraph under the “Limitation on sales of assets and subsidiary stock” covenant that permanently reduce the commitments thereunder;
(2) Guarantees by the Company or any Subsidiary Guarantor of Indebtedness Incurred in accordance with the provisions of the Indenture or existing on the Issue Date, or Guarantees by a Foreign Subsidiary of Indebtedness of a Foreign Subsidiary incurred in accordance with the provisions of the Indenture;providedthat in the event such Indebtedness that is being Guaranteed by the Company or a Subsidiary Guarantor is a
S-201
Table of Contents
Subordinated Obligation relative to the Notes or a Guarantor Subordinated Obligation relative to the Note Guarantees of the Notes, then the related Guarantee shall be subordinated in right of payment to the Notes or the Note Guarantees thereof, as the case may be;
(3) Indebtedness of the Company owing to and held by any Restricted Subsidiary or Indebtedness of a Restricted Subsidiary owing to and held by the Company or any other Restricted Subsidiary;provided,however,
(i) any subsequent issuance or transfer of Capital Stock or any other event which results in any such Indebtedness being beneficially held by a Person other than the Company or a Restricted Subsidiary of the Company; and
(ii) any sale or other transfer of any such Indebtedness to a Person other than the Company or a Restricted Subsidiary of the Company;
shall be deemed, in each case, to constitute an Incurrence of such Indebtedness by the Company or such Subsidiary, as the case may be, not permitted by this clause (3);
(4) Indebtedness represented by (a) the Notes issued on the Issue Date and the Note Guarantees thereof, and (b) any Indebtedness (other than the Indebtedness described in clauses (1), (2), (3), (5), (7), (8), (9), (10) and (17)) outstanding on the Issue Date;
(5) Indebtedness under Hedging Obligations that are Incurred in the ordinary course of business (and not for speculative purposes) (1) for the purpose of fixing or hedging interest rate risk with respect to any Indebtedness Incurred without violation of the Indenture,provided that the notional principal amount of such Hedging Obligations at the time Incurred does not exceed the principal amount of the Indebtedness to which such Hedging Obligations relate; or (2) for the purpose of fixing or hedging currency exchange rate risk,provided that the underlying Currency Agreements with respect to such Hedging Obligations do not increase the Indebtedness of the Company and its Restricted Subsidiaries outstanding other than as a result of fluctuations in foreign currency exchange rates or by reason of fees, indemnities and compensation payable thereunder;
(6) the Incurrence by the Company or any of its Restricted Subsidiaries of Indebtedness with respect to property or other assets other than Capital Stock or other Investments, in each case to the extent Incurred for the purpose of financing or refinancing all or any part of the purchase price or cost of acquisition, construction or improvements of property or other assets used or useful in the business of the Company or any of its Restricted Subsidiaries, in an aggregate principal amount not to exceed at any time outstanding the greater of (a) $250.0 million and (b) 7.5% of Total Tangible Assets at that time;
(7) Indebtedness Incurred in respect of workers’ compensation claims, self-retention or self-insurance obligations, unemployment insurance, performance, release, appeal, surety and similar bonds and related reimbursement obligations and completion guarantees or similar instruments provided or Incurred by the Company or a Restricted Subsidiary in the ordinary course of business and obligations in connection with participation in government reimbursement or other programs or other similar requirements (in each case, other than for an obligation for money borrowed);
(8) Indebtedness arising from agreements of the Company or a Restricted Subsidiary providing for indemnification, contribution, earnout, adjustment of purchase price or similar obligations, in each case, Incurred or assumed in connection with the acquisition or disposition of any business, assets or Capital Stock of a Restricted Subsidiary;providedthat any amount of such obligations included on the face of the balance sheet of the Company or any Restricted Subsidiary shall not be permitted under this clause (8);
(9) Indebtedness arising from the honoring by a bank or other financial institution of a check, draft or similar instrument drawn against insufficient funds in the ordinary course of business,provided,however, that such Indebtedness is extinguished within five Business Days of Incurrence;
(10) shares of Preferred Stock of a Restricted Subsidiary issued to the Company or another Restricted Subsidiary;providedthat any subsequent transfer of any Capital Stock or any other event which results in
S-202
Table of Contents
any such Restricted Subsidiary ceasing to be a Restricted Subsidiary or any other subsequent transfer of any such shares of Preferred Stock (except to the Company or another Restricted Subsidiary) shall be deemed, in each case, to be an issuance of Preferred Stock not permitted by this clause (10);
(11) Indebtedness of the Company or a Restricted Subsidiary to the extent the net proceeds thereof are promptly deposited to effect defeasance or covenant defeasance of the Notes as described below under “Defeasance” or to effect discharge of the Indenture as described below under “Satisfaction and discharge,” so long as the other conditions thereunder have been satisfied in full;
(12) Refinancing Indebtedness with respect to Indebtedness Incurred pursuant to the Coverage Ratio Exception or pursuant to this clause (12) or Incurred or referred to in clause (4) above;
(13) Guarantees given by the Company or any Restricted Subsidiary in respect of Indebtedness of any Special Purpose Licensed Entity which obligations, when aggregated with the aggregate amount of all then outstanding Investments made under clause (12) of the definition of “Permitted Investment,” do not exceed $150.0 million at any time outstanding;
(14) (a) Indebtedness, including Acquired Indebtedness, of the Company or any Subsidiary Guarantor incurred in connection with, or in anticipation or contemplation of, an acquisition or merger by the Company or such Subsidiary Guarantor of property used or useful in a Permitted Business (whether through the direct purchase of assets or the purchase of Capital Stock of, or merger or consolidation with, any Person owning such assets);provided that the Consolidated Coverage Ratio for the Company and its Restricted Subsidiaries determined on apro forma basis for the Incurrence of such Indebtedness (and the application of the proceeds therefrom), either (A) would have been at least 2.00 to 1 or (B) would have been greater than such Consolidated Coverage Ratio immediately prior to such acquisition; and
(b) Acquired Indebtedness Incurred by the debtor thereof prior to the time that the debtor thereunder was acquired (whether by merger, consolidation, acquisition of Capital Stock or otherwise) by the Company or any of its Restricted Subsidiaries, or prior to the time that the related asset or property was acquired by the Company or any of its Restricted Subsidiaries, and was not Incurred in connection with, or in anticipation or contemplation of, such acquisition, and Refinancing Indebtedness thereof, in an aggregate amount not to exceed $200.0 million at any time outstanding;
(15) Indebtedness Incurred in connection with any Sale/Leaseback Transaction;providedthat the aggregate outstanding amount of all such Indebtedness under this clause (15) does not exceed $50.0 million at any time outstanding;
(16) Indebtedness of Restricted Subsidiaries that are not Subsidiary Guarantors in an aggregate amount not to exceed $250.0 million at any time outstanding;
(17) Indebtedness under the Existing Notes outstanding on the Issue Date and any Guarantees of the Existing Notes;
(18) Indebtedness arising under any cash pooling arrangement to the extent that the net value thereof (determined by subtracting the borrowings and other withdrawals therefrom from the amount of cash deposited therein) is positive (for purposes of clarity, it is understood and agreed that, if the net value of any cash pooling arrangement is negative, then any Indebtedness attributable to such negative balance shall not be permitted under this clause (18) but may be Incurred under the Coverage Ratio Exception or any other clause of “Permitted Indebtedness” to the extent permitted thereby); and
(19) in addition to the items referred to in clauses (1) through (18) above, Indebtedness of the Company and its Restricted Subsidiaries in an aggregate outstanding principal amount which, when taken together with the principal amount of all other Indebtedness Incurred pursuant to this clause (19) and then outstanding (including any renewals, extensions, substitutions, refinancings or replacements of such Indebtedness), will not exceed $350.0 million at any time outstanding.
S-203
Table of Contents
For purposes of determining compliance with, and the outstanding principal amount of any particular Indebtedness Incurred pursuant to and in compliance with, this covenant:
(1) subject to clause (2) below, in the event that Indebtedness meets the criteria of more than one of the types of Indebtedness described in the first and second paragraphs of this covenant, the Company, in its sole discretion, will be permitted to classify such item of Indebtedness on the date of Incurrence and may later reclassify all or a portion of such item of Indebtedness in any manner that complies with this covenant, and only be required to include the amount and type of such Indebtedness in one of such paragraphs (or, in the case of the second paragraph of this covenant, one of the clauses in such second paragraph);
(2) all Indebtedness Incurred or outstanding under the Senior Credit Facilities on October 20, 2010 shall be deemed Incurred under the Senior Credit Facilities on October 20, 2010 under clause (1) of Permitted Indebtedness and not the Coverage Ratio Exception or any of the other clauses under “Permitted Indebtedness”;
(3) Guarantees of, or obligations in respect of letters of credit relating to, Indebtedness which is otherwise included in the determination of a particular amount of Indebtedness shall not be included as long as Incurred by a Person that could have Incurred such Indebtedness;
(4) if obligations in respect of letters of credit are Incurred pursuant to the Senior Credit Facilities and are being treated as Incurred pursuant to the first or second paragraph above and the letters of credit relate to other Indebtedness, then such other Indebtedness shall not be included;
(5) the principal amount of any Disqualified Stock of the Company or a Restricted Subsidiary, or Preferred Stock of a Restricted Subsidiary that is not a Subsidiary Guarantor, will be equal to the greater of its voluntary or involuntary liquidation preference and its maximum fixed repurchase price (not including, in either case, any redemption or repurchase premium);
(6) Indebtedness permitted by this covenant need not be permitted solely by reference to one provision permitting such Indebtedness but may be permitted in part by one such provision and in part by one or more other provisions of this covenant permitting such Indebtedness;
(7) the amount of Indebtedness issued at a price that is less than the principal amount thereof will be equal to the amount of the liability in respect thereof determined in accordance with GAAP; and
(8) the principal amount of any Indebtedness outstanding in connection with a Qualified Receivables Transaction is the Receivables Transaction Amount relating to such Qualified Receivables Transaction (which amount shall not include dispositions of self-pay receivables in the ordinary course of business, which the Company or any of its Restricted Subsidiaries believes in good faith cannot be paid in full).
Accrual of interest, accrual of dividends, the accretion of accreted value, the payment of interest in the form of additional Indebtedness and the payment of dividends in the form of additional shares of Preferred Stock or Disqualified Stock will not be deemed to be an Incurrence of Indebtedness for purposes of this covenant. The amount of any Indebtedness outstanding as of any date shall be (i) the accreted value thereof in the case of any Indebtedness issued with original issue discount and (ii) the principal amount or, in the case of Preferred Stock or Disqualified Stock, the greater of the voluntary or involuntary liquidation preference and the maximum fixed repurchase price thereof, together with any interest thereon that is more than 30 days past due, in the case of any other Indebtedness.
In addition, the Company will not permit any of its Unrestricted Subsidiaries to Incur any Indebtedness or issue any shares of Disqualified Stock (other than Non-Recourse Debt and other than Indebtedness of a Receivables Subsidiary in respect of a Qualified Receivables Transaction). If on any date an Unrestricted Subsidiary becomes a Restricted Subsidiary, any Indebtedness of such Subsidiary outstanding at such time shall be deemed to be Incurred by a Restricted Subsidiary as of such date (and, if such Indebtedness is not permitted to be Incurred as of such date under this “Limitation on indebtedness” covenant, the Company shall be in default of this covenant).
S-204
Table of Contents
For purposes of determining compliance with any U.S. dollar-denominated restriction on the Incurrence of Indebtedness, the U.S. dollar-equivalent principal amount of Indebtedness denominated in a foreign currency shall be calculated based on the relevant currency exchange rate in effect on the date such Indebtedness was Incurred, or, in the case of revolving credit Indebtedness, the date such Indebtedness was first committed;provided that if such Indebtedness is Incurred to refinance other Indebtedness denominated in a foreign currency, and such refinancing would cause the applicable U.S. dollar-dominated restriction to be exceeded if calculated at the relevant currency exchange rate in effect on the date of such refinancing, such U.S. dollar-dominated restriction shall be deemed not to have been exceeded so long as the principal amount of such refinancing Indebtedness does not exceed the principal amount of such Indebtedness being refinanced. Notwithstanding any other provision of this covenant, the maximum amount of Indebtedness that the Company and its Restricted Subsidiaries may Incur pursuant to this covenant shall not be deemed to be exceeded solely as a result of fluctuations in the exchange rate of currencies. The principal amount of any Indebtedness Incurred to refinance other Indebtedness, if Incurred in a different currency from the Indebtedness being refinanced, shall be calculated based on the currency exchange rate applicable to the currencies in which such refinanced Indebtedness and refinancing Indebtedness are denominated that is in effect on the date of such refinancing. In the event that any other provision (including, without limitation, any other covenant or any defined term) of the Indenture requires the calculation of the principal amount of any Indebtedness, such calculation shall, unless otherwise expressly stated or the context otherwise requires, be made in a manner consistent with the third paragraph, the fourth paragraph and this sixth paragraph of this “Limitation on indebtedness” covenant, mutatis mutandis.
Limitation on layering
The Indenture will provide that the Company will not, and will not permit any Subsidiary Guarantor to, directly or indirectly, Incur any Indebtedness that is or purports to be by its terms (or by the terms of any agreement governing such Indebtedness) subordinated to any other Indebtedness of the Company or of such Subsidiary Guarantor, as the case may be, unless such Indebtedness is also by its terms (or by the terms of any agreement governing such Indebtedness) made expressly subordinate to the Notes or the Note Guarantee of such Subsidiary Guarantor with respect to such Notes, to the same extent and in the same manner as such Indebtedness is subordinated to such other Indebtedness of the Company or such Subsidiary Guarantor, as the case may be.
For purposes of the foregoing, no Indebtedness will be deemed to be subordinated or junior in right of payment to any other Indebtedness of the Company or any Subsidiary Guarantor solely by virtue of being unsecured or secured by a junior priority Lien or by virtue of the fact that the holders of such Indebtedness have entered into intercreditor agreements or similar arrangements giving one or more of such holders priority over the other holders in the collateral held by them.
Limitation on restricted payments
The Indenture will provide that the Company will not, and will not permit any of its Restricted Subsidiaries, directly or indirectly, to:
(1) declare or pay any dividend or make any distribution (whether made in cash, securities or other property) on or in respect of its Capital Stock (including any payment in connection with any merger or consolidation involving the Company or any of its Restricted Subsidiaries) except:
(a) dividends or distributions payable in Capital Stock of the Company (other than Capital Stock that is Disqualified Stock) or in options, warrants or other rights to purchase such Capital Stock of the Company; and
(b) dividends or distributions payable to the Company or a Restricted Subsidiary (and if such Restricted Subsidiary is not a Wholly-Owned Restricted Subsidiary, to its other holders of common Capital Stock on apro rata basis);
(2) purchase, redeem, retire or otherwise acquire for value any Capital Stock of the Company or any direct or indirect parent of the Company held by Persons other than the Company or a Restricted Subsidiary
S-205
Table of Contents
(other than in exchange for Capital Stock of the Company (other than Capital Stock that is Disqualified Stock) or in exchange for options, warrants, or other such rights to purchase such Capital Stock of the Company));
(3) purchase, repurchase, redeem, defease or otherwise acquire or retire for value, prior to scheduled maturity, scheduled repayment or scheduled sinking fund payment, any Subordinated Obligations or Guarantor Subordinated Obligations (other than (x) such Subordinated Obligations or Guarantor Subordinated Obligations purchased in anticipation of satisfying a sinking fund obligation, principal installment or final maturity, in each case due within one year of the date of purchase, repurchase, redemption, defeasance or other acquisition or retirement and (y) such Subordinated Obligations or Guarantor Subordinated Obligations held by the Company or any Restricted Subsidiary); or
(4) make any Restricted Investment in any Person
(any such dividend, distribution, purchase, redemption, repurchase, defeasance, other acquisition, retirement or Restricted Investment referred to in clauses (1) through (4) shall be referred to herein as a “Restricted Payment”), if at the time the Company or such Restricted Subsidiary makes such Restricted Payment:
(a) a Default or Event of Default shall have occurred and be continuing (or would result therefrom); or
(b) the Company is not able to Incur an additional $1.00 of Indebtedness pursuant to the Coverage Ratio Exception after giving effect, on apro formabasis, to such Restricted Payment; or
(c) the aggregate amount of such Restricted Payment and all other Restricted Payments declared or made subsequent to October 20, 2010 (excluding Restricted Payments permitted by clauses (2)(ii), (3), (4), (8), (9), (10) and (11) below) would exceed the sum, or Restricted Payments Basket, of (without duplication):
(i) 50% of Consolidated Net Income for the period (treated as one accounting period) from October 1, 2010 to the end of the most recent fiscal quarter ending prior to the date of such Restricted Payment for which financial statements are in existence (or, in case such Consolidated Net Income is a deficit,minus100% of such deficit);plus
(ii) 100% of the aggregate Net Cash Proceeds and the Fair Market Value of Qualified Proceeds received by the Company from the issue or sale of its Capital Stock (other than Capital Stock that is Disqualified Stock) or other capital contributions to the common equity of the Company subsequent to October 20, 2010 (other than (x) Net Cash Proceeds received from an issuance or sale of such Capital Stock to a Subsidiary of the Company or an employee stock ownership plan or similar trust to the extent such sale to an employee stock ownership plan or similar trust is financed by loans from or Guaranteed by the Company or any Restricted Subsidiary unless such loans have been repaid with cash on or prior to the date of determination and (y) Net Cash Proceeds received by the Company from the issue or sale of its Capital Stock to the extent used to redeem Notes in compliance with the provisions of the second paragraph of “Optional redemption”);plus
(iii) the amount by which Indebtedness of the Company or its Restricted Subsidiaries is reduced on the Company’s balance sheet upon the conversion or exchange (other than by a Subsidiary of the Company) subsequent to October 20, 2010 of any Indebtedness of the Company or its Restricted Subsidiaries issued after October 20, 2010 convertible or exchangeable for Capital Stock (other than Disqualified Stock) of the Company (less the amount of any cash, or the Fair Market Value of any other property, distributed by the Company or its Restricted Subsidiaries upon such conversion or exchange);plus
(iv) the amount equal to the net reduction in Restricted Investments made by the Company or any of its Restricted Subsidiaries in any Person resulting from:
(A) repurchases or redemptions of such Restricted Investments by such Person, proceeds realized upon the sale of such Restricted Investment to an unaffiliated purchaser, repayments of
S-206
Table of Contents
loans or advances or other transfers of assets (including by way of dividend or distribution) by such Person to the Company or any Restricted Subsidiary (other than expressly for reimbursement of tax payments) not to exceed the aggregate amount of all such Restricted Investments made since October 20, 2010; or
(B) the redesignation of Unrestricted Subsidiaries as Restricted Subsidiaries (valued in each case as provided in the definition of “Investment”) not to exceed, in the case of any Unrestricted Subsidiary, the amount of Investments previously made since the October 20, 2010 by the Company or any Restricted Subsidiary in such Unrestricted Subsidiary,
which amount in each case under this clause (iv) was included in the calculation of the amount of Restricted Payments;provided,however, that no amount will be included under clause (iv)(A) of this paragraph to the extent it is already included in Consolidated Net Income.
The Indenture will provide that the provisions of the preceding paragraph will not prohibit:
(1) the payment of any dividend within 60 days after the date of declaration of such dividend if the dividend would have been permitted on the date of declaration;
(2) if no Default or Event of Default under the Indenture shall have occurred and be continuing, the acquisition, retirement, defeasance or purchase of any shares of Capital Stock of the Company either (i) solely in exchange for shares of Capital Stock of the Company (other than Disqualified Stock) or (ii) through the application of the net proceeds of a substantially concurrent sale for cash (other than to a Subsidiary of the Company) of shares of Capital Stock of the Company (other than Capital Stock that is Disqualified Stock) (providedthat the amount of net proceeds so applied shall not be applied toward the Restricted Payments Basket);
(3) if no Default or Event of Default under the Indenture shall have occurred and be continuing, the acquisition, making of any principal payment, redemption, defeasance or other retirement of any Subordinated Obligations either (i) solely in exchange for shares of Capital Stock of the Company (other than Capital Stock that is Disqualified Stock), (ii) through the application of net proceeds of a substantially concurrent sale for cash (other than to a Subsidiary of the Company) of (a) shares of Capital Stock of the Company (other than Capital Stock that is Disqualified Stock) (providedthat the amount of net proceeds so applied shall not be applied toward the Restricted Payments Basket), (b) Refinancing Indebtedness permitted to be Incurred pursuant to the “Limitation on indebtedness” covenant or (c) “Refinancing Indebtedness” as defined in, and that was permitted to be incurred and, prior to the date of the Indenture, was incurred pursuant to Section 4.10, of the indentures pursuant to which the Existing Notes were issued, (iii) upon a Change of Control or in connection with an Asset Disposition to the extent required by the agreement governing such Subordinated Obligations but only if the Company shall have complied with the “Change of control” covenant or the “Limitations on sale of assets and subsidiary stock” covenant, as applicable, and, if applicable, purchased all Notes validly tendered pursuant to the relevant offer prior to acquiring, paying, redeeming, defeasing or otherwise retiring such Subordinated Obligations or (iv) to the extent such Subordinated Obligations constitute Acquired Indebtedness not Incurred in connection with or in anticipation or contemplation of the underlying acquisition or merger or the applicable Person becoming a Restricted Subsidiary;
(4) so long as no Default or Event of Default shall have occurred and be continuing, repurchases by the Company of Common Stock of the Company from officers, directors and employees of the Company or any of its Subsidiaries or their authorized representatives upon the death, disability or termination of employment of such employees or termination of their seat on the board of the Company, in an aggregate amount not to exceed $15.0 million in any calendar year;
(5) repurchases of Capital Stock deemed to occur upon exercise of stock options, warrants or other convertible or exercisable securities if such Capital Stock represents a portion of the exercise or conversion price thereof;
S-207
Table of Contents
(6) so long as no Default or Event of Default shall have occurred and be continuing, payments to holders of the Company’s Capital Stock in lieu of issuance of fractional shares of its Capital Stock or to dissenting shareholders if required by applicable law;
(7) the distribution of Capital Stock of an Unrestricted Subsidiary of the Company to holders of Capital Stock of the Company;
(8) so long as no Default or Event of Default shall have occurred and shall be continuing, purchases or other acquisitions or retirements by the Company or any of its Restricted Subsidiaries of the Company’s Common Stock for aggregate consideration not to exceed $1,200.0 million;
(9) so long as no Default or Event of Default shall have occurred and be continuing, the making of any Restricted Payments if, at the time of the making of such payments, and after giving pro forma effect thereto (including, without limitation, the Incurrence of any Indebtedness to finance such payment), the Consolidated Total Leverage Ratio would not exceed 3.50 to 1.00;
(10) the purchase, redemption, defeasance or other retirement of the Existing Notes on or after October 20, 2010 to the extent such purchase, redemption, defeasance or other retirement would constitute a Restricted Payment; and
(11) additional Restricted Payments not to exceed $500.0 million in the aggregate since October 20, 2010.
The amount of all Restricted Payments (other than cash) shall be the Fair Market Value on the date of such Restricted Payment of the asset(s) or securities proposed to be paid, transferred or issued by the Company or such Restricted Subsidiary, as the case may be, pursuant to such Restricted Payment. If the Company or a Restricted Subsidiary makes a Restricted Payment which, at the time of the making of such Restricted Payment, would in the good faith determination of the Company be permitted under the provisions of the Indenture, such Restricted Payment shall be deemed to have been made in compliance with the Indenture notwithstanding any subsequent adjustments or restatements made in good faith to the Company’s financial statements.
As of the Issue Date, the Company would have been able to make up to approximately $154 million of Restricted Payments under the provisions of the Indenture described in clause (c) of the first paragraph above.
Limitation on liens
The Indenture will provide that the Company will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly, create, incur or suffer to exist any Lien (other than Permitted Liens) of any nature whatsoever against any assets or property of the Company or any Restricted Subsidiary (including Capital Stock of Restricted Subsidiaries), whether owned on the Issue Date or acquired after that date, which Lien secures Indebtedness or trade payables, unless contemporaneously therewith:
(1) in the case of any Lien securing an obligation that ranks pari passu with the Notes or a Note Guarantee thereof, effective provision is made to secure such Notes or such Note Guarantee, as the case may be, at least equally and ratably with or prior to such obligation with a Lien on the same collateral; and
(2) in the case of any Lien securing an obligation that is subordinated in right of payment to the Notes or a Note Guarantee, effective provision is made to secure the Notes or Note Guarantee, as the case may be, with a Lien on the same collateral that is prior to the Lien securing such subordinated obligation;
in each case, for so long as such obligation is secured by such Lien.
S-208
Table of Contents
Limitation on restrictions on distributions from restricted subsidiaries
The Indenture will provide that the Company will not, and will not permit any Restricted Subsidiary to, create or otherwise cause or permit to exist or become effective any consensual encumbrance or consensual restriction on the ability of any Restricted Subsidiary to:
(1) pay dividends or make any other distributions on its Capital Stock or pay any Indebtedness or other obligations owed to the Company or any Restricted Subsidiary (it being understood that the priority of any Preferred Stock in receiving dividends or liquidating distributions prior to dividends or liquidating distributions being paid on Common Stock shall not be deemed a restriction on the ability to make distributions on Capital Stock);
(2) make any loans or advances to the Company or any Restricted Subsidiary (it being understood that the subordination of loans or advances made to the Company or any Restricted Subsidiary to other Indebtedness Incurred by the Company or any Restricted Subsidiary shall not be deemed a restriction on the ability to make loans or advances); or
(3) transfer any of its property or assets to the Company or any Restricted Subsidiary (it being understood that such transfers shall not include any type of transfer described in clause (1) or (2) above).
The Indenture will provide that the preceding provisions will not prohibit:
(i) any encumbrance or restriction pursuant to an agreement in effect at or entered into on the Issue Date, including, without limitation, the Indenture, the Notes issued thereunder and the Guarantees thereof, the Existing Notes and the Guarantees thereof and the related indentures and the Senior Credit Facilities, in each case, in effect on such date;
(ii) any encumbrance or restriction with respect to a Restricted Subsidiary pursuant to an agreement relating to any Capital Stock or Indebtedness Incurred by a Restricted Subsidiary on or before the date on which such Restricted Subsidiary was acquired (whether by merger, consolidation, acquisition of Capital Stock or otherwise) by the Company or a Restricted Subsidiary (other than Capital Stock or Indebtedness that was Incurred as consideration in, or to provide all or any portion of the funds utilized to consummate, the transaction or series of related transactions pursuant to which such Restricted Subsidiary became a Restricted Subsidiary was acquired by the Company or in contemplation of the transaction) and outstanding on such date;providedthat any such encumbrance or restriction shall not extend to any assets or property of the Company or any other Restricted Subsidiary other than the assets and property so acquired and property acquired by such Restricted Subsidiary after its date of acquisition;
(iii) any encumbrance or restriction with respect to a Restricted Subsidiary pursuant to an agreement effecting an amendment, restatement, modification, renewal, increase, refunding, replacement or refinancing of an agreement referred to in clause (i) or (ii) of this paragraph or this clause (iii);provided,however, that the encumbrances and restrictions with respect to such Restricted Subsidiary contained in any such agreement, amendment, restatement, modification, renewal, increase, refunding, replacement or refinancing are not, in the good faith judgment of the Company’s Board of Directors, materially less favorable, taken as a whole, to the Holders of the Notes; than the encumbrances and restrictions contained in such agreements referred to in clause (i) or (ii) of this paragraph on the Issue Date or the date such Restricted Subsidiary became a Restricted Subsidiary or was acquired (whether by merger, consolidation, acquisition of Capital Stock or otherwise) by the Company or a Restricted Subsidiary, whichever is applicable;
(iv) (a) purchase money obligations for property acquired in the ordinary course of business, (b) Capitalized Lease Obligations permitted under the Indenture, (c) industrial revenue bonds or (d) operating leases, in each case, that impose encumbrances or restrictions of the nature described in clause (3) of the first paragraph of this covenant on the property so acquired;
(v) any restriction with respect to a Restricted Subsidiary (or any of its property or assets) imposed pursuant to an agreement entered into for the direct or indirect sale or disposition (whether by sale, merger,
S-209
Table of Contents
consolidation, acquisition of Capital Stock or otherwise) of the Capital Stock or assets of such Restricted Subsidiary (or the property or assets that are subject to such restriction) pending the closing of such sale or disposition;
(vi) customary non-assignment provisions in leases and other agreements entered into by the Company or any Restricted Subsidiary in the ordinary course of business;
(vii) encumbrances or restrictions arising or existing by reason of applicable law or any applicable rule, regulation or order;
(viii) customary encumbrances or restrictions existing under or by reason of provisions in joint venture, partnership (limited or general), limited liability company or similar agreements required in connection with the entering into of such transaction;
(ix) customary restrictions imposed on the transfer, licensing, sub-licensing and assignment of intellectual property and of intellectual property licenses;
(x) restrictions relating to any Lien permitted under the Indenture imposed by the holder of such Lien;
(xi) any other Indebtedness or contractual requirements Incurred with respect to a Qualified Receivables Transaction relating exclusively to the assets that are the subject of the Qualified Receivables Transaction;
(xii) in the case of Restricted Subsidiaries that are not Subsidiary Guarantors, restrictions imposed under instruments governing Indebtedness Incurred pursuant to the definition of “Permitted Indebtedness”;
(xiii) in the case of any Restricted Subsidiary that is not a Subsidiary Guarantor, restrictions under the constitutive documents governing such Subsidiary: (A) with respect to Subsidiaries, existing on the Issue Date; and (B) with respect to Subsidiaries created or acquired after the Issue Date: (1) prohibiting such Subsidiary from Guaranteeing Indebtedness of the Company or another Subsidiary; (2) on dividend payments and other distributions solely to permitpro ratadividends and other distributions in respect of any Capital Stock of such Subsidiary; (3) limiting transactions with the Company or another Subsidiary to those with terms that are fair and reasonable to such Subsidiary and no less favorable to such Subsidiary than could have been obtained in an arm’s-length transaction with an unrelated third party; and (4) limiting such Subsidiary’s ability to transfer assets or Incur Indebtedness without the consent of the holders of the Capital Stock of such Subsidiary; and
(xiv) any encumbrances or restrictions imposed by any amendments, restatements, modifications, renewals, increases, restrictions, encumbrances, refundings, replacements or refinancings of the contracts, instruments or obligations referred to in clauses (i) through (xiii) above;provided that such amendments, restatements, modifications, renewals, increases, restrictions, encumbrances, refundings, replacements or refinancings are, in the good faith judgment of the Company’s Board of Directors, no more materially restrictive with respect to such encumbrances and restrictions than those prior to such amendment or refinancing.
Limitation on sales of assets and subsidiary stock
The Indenture will provide that the Company will not, and will not permit any of its Restricted Subsidiaries to, make any Asset Disposition unless:
(1) the Company or such Restricted Subsidiary, as the case may be, receives consideration (both cash and non-cash) equal to not less than the Fair Market Value (such Fair Market Value to be determined on the date of contractually agreeing to such Asset Disposition) of the shares and assets subject to such Asset Disposition;
(2) at least 75% of the consideration from such Asset Disposition received by the Company or such Restricted Subsidiary, as the case may be, is in the form of cash or Cash Equivalents or Replacement Assets. For purposes of this clause (2), each of the following shall be deemed to be cash:
(a) any liabilities (as shown on the face of the Company’s or such Restricted Subsidiary’s then most recent balance sheet), of the Company or any Restricted Subsidiary (other than contingent
S-210
Table of Contents
liabilities and Subordinated Obligations) that are assumed by the transferee of any such assets pursuant to a customary novation agreement that releases the Company or such Restricted Subsidiary from further liability; and
(b) any securities, notes or other obligations received by the Company or any such Restricted Subsidiary from such transferee that are converted by the Company or such Restricted Subsidiary into cash (to the extent of the cash received in that conversion) within 180 days of the closing of such Asset Disposition; and
(c) any Designated Noncash Consideration received by the Company or any Restricted Subsidiary in such Asset Disposition having an aggregate Fair Market Value (as determined in good faith by the Board of Directors of the Company), taken together with all other Designated Noncash Consideration received pursuant to this clause (c) that is at that time outstanding, not to exceed $100.0 million (with the Fair Market Value of each item of Designated Noncash Consideration being measured at the time received without giving effect to subsequent changes in value); and
(3) an amount equal to 100% of the Net Available Cash from such Asset Disposition:
(a)first, is applied by the Company or such Restricted Subsidiary, as the case may be,
(i) to the extent the Company or any Restricted Subsidiary, as the case may be, elects (or is required by the terms of any Bank Indebtedness) to prepay, repay or purchase such Bank Indebtedness of the Company or of a Restricted Subsidiary within 365 days from the date of such Asset Disposition (such period referred to as the Application Period), unless to the extent such Net Available Cash is otherwise used in accordance with clause (ii);provided,however, that, in connection with any prepayment, repayment or purchase of any such Indebtedness pursuant to this clause (a), the Company or such Restricted Subsidiary will retire such Indebtedness and will cause the related commitment (if any) to be permanently reduced in an amount equal to the principal amount so prepaid, repaid or purchased, or
(ii) to the extent the Company or any Restricted Subsidiary, as the case may be, elects, to invest in Replacement Assets within the applicable Application Period; and
(b)second, to the extent of the balance of the Net Available Cash after application in accordance with (a) above (such balance referred to as the Excess Proceeds), is applied by the Company or such Restricted Subsidiary, as the case may be, toward an offer to purchase Notes issued under the Indenture as set forth in the next succeeding paragraph;
provided,however, that pending the final application of any such Net Available Cash in accordance with clause (a) or clause (b) above, the Company and its Restricted Subsidiaries may temporarily reduce Indebtedness or otherwise invest such Net Available Cash in any manner not prohibited by the Indenture.
The Indenture provides that on the 366th day after an Asset Disposition (or such earlier date, if any, as the Board of Directors of the Company or such Restricted Subsidiary determines that the Net Available Cash will not be applied in accordance with clause (3)(a) of the first paragraph of this covenant), if the aggregate amount of Excess Proceeds exceeds $50.0 million, the Company will be required to make an offer, or Asset Disposition Offer, to all Holders of Notes issued under the Indenture and, to the extent required by the terms of other Senior Indebtedness, to all holders of other Senior Indebtedness outstanding with similar provisions requiring the Company to make an offer to purchase such Senior Indebtedness with the proceeds from any Asset Disposition, or Pari Passu Notes, to purchase the maximum principal amount of such Notes and any such Pari Passu Notes to which the Asset Disposition Offer applies that may be purchased out of the Excess Proceeds, at an offer price in cash in an amount equal to 100% of the principal amount of such Notes and Pari Passu Notes plus accrued and unpaid interest to the date of purchase, in accordance with the procedures set forth in the Indenture or the agreements governing the Pari Passu Notes, as applicable, in integral multiples of $1,000 in principal amount (provided that the unpurchased portion of any Note shall not be less than $2,000 in principal amount) or, in the case of Pari Passu Notes, in such other integral multiples as maybe specified in the agreements governing the
S-211
Table of Contents
Pari Passu Notes. To the extent that the aggregate amount of such Notes and Pari Passu Notes so validly tendered and not properly withdrawn pursuant to an Asset Disposition Offer is less than the Excess Proceeds, the Company may use any remaining Excess Proceeds for general corporate purposes, subject to other covenants contained in the Indenture. If the aggregate principal amount of such Notes and Pari Passu Notes validly tendered and not properly withdrawn pursuant to an Asset Disposition Offer exceeds the amount of Excess Proceeds, the Trustee in accordance with the applicable procedures of the Depository shall select such Notes and the holders, trustees or similar representatives, as the case may be, of Pari Passu Notes shall select the Pari Passu Notes to be purchased on a pro rata basis on the basis of the aggregate principal amount of such tendered Notes and Pari Passu Notes. Upon completion of such Asset Disposition Offer, the amount of Excess Proceeds shall be reset at zero.
Each Asset Disposition Offer will remain open for a period of 20 Business Days following its commencement, except to the extent that a longer period is required by applicable law, or Asset Disposition Offer Period. No later than five Business Days after the termination of the Asset Disposition Offer Period, or Asset Disposition Purchase Date, the Company will purchase the principal amount of Notes and Pari Passu Notes required to be purchased pursuant to this covenant, or Asset Disposition Offer Amount, or, if less than the Asset Disposition Offer Amount has been so validly tendered, all such Notes and Pari Passu Notes validly tendered in response to the Asset Disposition Offer.
If the Asset Disposition Purchase Date is on or after an interest record date and on or before the related interest payment date, any accrued and unpaid interest will be paid to the Person in whose name a Note is registered at the close of business on such record date, and no additional interest will be payable to Holders who tender Notes pursuant to the Asset Disposition Offer.
On or before the Asset Disposition Purchase Date, the Company will, to the extent lawful, accept for payment, on apro ratabasis to the extent necessary, the Asset Disposition Offer Amount of Notes and Pari Passu Notes or portions of such Notes and Pari Passu Notes so validly tendered and not properly withdrawn pursuant to the Asset Disposition Offer, or if less than the Asset Disposition Offer Amount has been validly tendered and not properly withdrawn, all such Notes and Pari Passu Notes so validly tendered and not properly withdrawn, in integral multiples of $1,000 in principal amount (provided that the unpurchased portion of any Note shall not be less than $2,000 in principal amount) or, in the case of Pari Passu Notes, in such other integral multiples as may be specified in the agreements governing such Pari Passu Notes. The Company will deliver to the Trustee an Officers’ Certificate stating that such Notes or portions thereof were accepted for payment by the Company in accordance with the terms of this covenant and, in addition, the Company will deliver all certificates and notes required, if any, by the agreements governing the Pari Passu Notes. The Company or the paying agent, as the case may be, will promptly (but in any case not later than five Business Days after termination of the Asset Disposition Offer Period) mail or deliver to each tendering Holder of Notes or holder or lender of Pari Passu Notes, as the case may be, an amount equal to the purchase price of the Notes or Pari Passu Notes so validly tendered and not properly withdrawn by such holder or lender, as the case may be, and accepted by the Company for purchase, and the Company will promptly issue a new Note, and the Trustee, upon delivery of an Officers’ Certificate from the Company, will authenticate and mail or deliver such new Note to such Holder, in a principal amount equal to any unpurchased portion of the Note surrendered;providedthat each such new Note will be in a principal amount of $2,000 or an integral multiple of $1,000 in excess thereof. In addition, the Company will take any and all other actions required by the agreements governing the Pari Passu Notes. Any Note not so accepted will be promptly mailed or delivered by the Company to the Holder thereof. The Company will publicly announce the results of the Asset Disposition Offer on or promptly following the Asset Disposition Purchase Date.
In the event of the transfer of substantially all (but not all) of the property and assets of the Company and its Restricted Subsidiaries as an entirety to a Person in a transaction permitted under the “Merger and consolidation” covenant, which transaction does not constitute a Change of Control, the successor company shall be deemed to have sold the properties and assets of the Company and its Restricted Subsidiaries not so transferred for purposes of this covenant, and shall comply with the provisions of this covenant with respect to such deemed sale as if it were
S-212
Table of Contents
an Asset Disposition. In addition, the Fair Market Value of such properties and assets of the Company or its Restricted Subsidiaries deemed to be sold shall be deemed to be Net Available Cash for purposes of this covenant.
The Company will comply, to the extent applicable, with the requirements of Rule 14(e) of the Exchange Act and any other securities laws or regulations in connection with the repurchase of Notes pursuant to the Indenture. To the extent that the provisions of any securities laws or regulations conflict with provisions of this covenant, the Company will comply with the applicable securities laws and regulations and will not be deemed to have breached its obligations under the Indenture by virtue of any conflict.
If the Company is required to make an Asset Disposition Offer, such offer may be subject to restrictions arising out of the terms of its existing and future Indebtedness or other limitations comparable to the restrictions and limitations that may apply to offers to purchase the Notes following a Change of Control. See “Change of control” and “Risk Factors—Risks Relating to Investment in the Notes.” Accordingly, there can be no assurance that the Company will be able to make an Asset Sale Offer or to pay for any Notes tendered for purchase pursuant to such offer.
Limitation on affiliate transactions
The Indenture will provide that the Company will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly, enter into or conduct any transaction (including the purchase, sale, lease or exchange of any property or the rendering of any service) with any Affiliate of the Company, or Affiliate Transaction, involving aggregate consideration in excess of $10.0 million unless:
(1) the terms of such Affiliate Transaction are no less favorable, taken as a whole, to the Company or such Restricted Subsidiary, as the case may be, than those that could be obtained in a comparable transaction at the time of such transaction in arm’s-length dealings with a Person who is not such an Affiliate; and
(2) in the event such Affiliate Transaction involves an aggregate consideration in excess of $35.0 million, the terms of such transaction have been approved by a majority of the members of the Board of Directors of the Company and by a majority of the members of such Board of Directors having no personal stake in such transaction, if any (and such majority or majorities, as the case may be, determines that such Affiliate Transaction satisfies the criteria in clause (1) above).
The preceding paragraph will not apply to:
(1) any Restricted Payment permitted to be made pursuant to the “Limitation on restricted payments” covenant or any Investment described in the definition of “Permitted Investments”;
(2) any issuance of securities, or other payments, awards or grants in cash, securities or otherwise pursuant to, or the funding of, employment agreements and other compensation arrangements, options to purchase Capital Stock of the Company, stock purchase, ownership or option plans, long-term incentive plans, stock appreciation rights plans, participation plans or similar employee or director benefits plans provided on behalf of directors, officers, consultants and employees of the Company and its Subsidiaries, in each case, as approved by the Board of Directors of the Company;
(3) loans or advances to employees, consultants, officers or directors in the ordinary course of business of the Company or any of its Restricted Subsidiaries (including for travel, entertainment, moving or relocation) or Guarantees in respect thereof or otherwise made on their behalf (including payment on any such Guarantees) made in compliance with applicable law but in any event not to exceed $15.0 million in the aggregate outstanding (without giving effect to the forgiveness of any such loan) at any one time with respect to all loans or advances made since the Issue Date;
(4) any transaction between the Company and a Restricted Subsidiary or between Restricted Subsidiaries, and Guarantees issued by the Company or a Restricted Subsidiary for the benefit of the
S-213
Table of Contents
Company, a Restricted Subsidiary and/or a Special Purpose Licensed Entity, as the case may be, in accordance with the “Limitation on indebtedness” covenant and the “Limitation on liens” covenant;
(5) the payment of reasonable and customary fees to directors, and indemnity provided on behalf of, directors, officers, employees or consultants, of the Company or any of its Subsidiaries;
(6) the performance of obligations of the Company or any of its Restricted Subsidiaries under the terms of any agreement to which the Company or any of its Restricted Subsidiaries is a party as of or on the Issue Date, as these agreements may be amended, modified, supplemented, extended or renewed from time to time;provided,however, that any future amendment, modification, supplement, extension or renewal entered into after the Issue Date will be permitted to the extent that its terms are not more materially disadvantageous, taken as a whole, to the Holders of the Notes than the terms of the agreements in effect on the Issue Date;
(7) transactions entered into by the Company or any of its Restricted Subsidiaries in the ordinary course of business (including, without limitation, management contracts and payments pursuant to management contracts) with any Person (including, without limitation, any joint venture, limited or general partnership, limited liability company or similar business entity) that owns or has any rights to use property or assets used or useful in a Permitted Business;
(8) sales of Receivables, or participations therein, in connection with any Qualified Receivables Transaction; and
(9) transactions relating to any cash pooling arrangement.
Conduct of business
The Indenture will provide that the Company will not, and will not permit any Restricted Subsidiary to, engage in any other business that is not a Permitted Business.
SEC reports
The Indenture will provide that, notwithstanding that the Company may not be subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act, to the extent permitted by the Exchange Act, the Company will file or furnish with the SEC, and make available to the Trustee and the registered Holders of the Notes issued under the Indenture, the annual reports and the information, documents and other reports (or copies of such portions of any of the foregoing as the SEC may by rules and regulations prescribe) that are specified in Sections 13 and 15(d) of the Exchange Act within the time periods specified therein or in the relevant forms. In the event that the Company is not permitted to file such reports, documents and information with the SEC pursuant to the Exchange Act, the Company will nevertheless make available such Exchange Act information to the Trustee and the Holders of the Notes issued under the Indenture as if the Company were subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act within the time periods specified therein.
If the Company has designated any of its Subsidiaries as Unrestricted Subsidiaries, or if the consolidated financial statements include the accounts of the Physician Groups, then the quarterly and annual financial information required by the preceding paragraph shall include a reasonably detailed presentation, (a) in the footnotes to the financial statements and (b) in Management’s Discussion and Analysis of Results of Operations and Financial Condition, of the financial condition and results of operations of the Company and its Restricted Subsidiaries. The quarterly and annual financial information required by the preceding paragraph above shall reflect the adjustments necessary to eliminate the accounts of any Unrestricted Subsidiaries and any Physician Groups and Subsidiaries thereof (which may be in footnote form only) from such consolidated financial statements.
For purposes of this covenant, the Company and the Subsidiary Guarantors will be deemed to have furnished the reports to the Trustee and the Holders of Notes as required by this covenant if they have filed or
S-214
Table of Contents
furnished such reports with the SEC via the EDGAR (or successor or similar) filing system and such reports are publicly available.
Merger and consolidation
The Indenture will provide that the Company will not consolidate with or merge with or into, or convey, transfer or lease all or substantially all its assets to, any Person,unless:
(1) the resulting, surviving or transferee Person, or Successor Company, will be a corporation organized and existing under the laws of the United States of America, any State of the United States or the District of Columbia and the Successor Company (if not the Company) will expressly assume, by supplemental indenture, executed and delivered to the Trustee, in form reasonably satisfactory to such Trustee, all the obligations of the Company under the Notes issued under the Indenture;
(2) immediately after giving effect to such transaction (and treating any Indebtedness that becomes an obligation of the Successor Company or any Subsidiary of the Successor Company as a result of such transaction as having been Incurred by the Successor Company or such Subsidiary at the time of such transaction), no Default or Event of Default shall have occurred and be continuing;
(3) immediately after giving effect to such transaction and any related financing, the Successor Company would be able to Incur at least an additional $1.00 of Indebtedness pursuant to the Coverage Ratio Exception;
(4) each Subsidiary Guarantor (unless it is the other party to the transactions above, in which case clause (1) shall apply or unless the Company is the Successor Company) shall have by supplemental indenture confirmed that its Note Guarantee under the Indenture shall apply to such Successor Company’s obligations in respect of the Indenture and the Notes issued under the Indenture; and
(5) the Company shall have delivered to the Trustee an Officers’ Certificate and an Opinion of Counsel, each stating that such consolidation, merger or transfer and such supplemental indentures (if any) comply with the Indenture.
For purposes of this covenant, the sale, lease, conveyance, assignment, transfer, or other disposition of all or substantially all of the properties and assets of one or more Subsidiaries of the Company, which properties and assets, if held by the Company instead of such Subsidiaries, would constitute all or substantially all of the properties and assets of the Company on a consolidated basis, shall be deemed to be the transfer of all or substantially all of the properties and assets of the Company.
Notwithstanding the foregoing, the sale, conveyance, assignment, transfer or other disposition of assets of any Subsidiary in connection with a Qualified Receivables Transaction that complies with the other provisions of the Indenture shall not constitute the sale, conveyance, assignment, transfer or other disposition of all or substantially all the assets of the Company or such Subsidiary for purposes of this “Merger and consolidation” covenant.
The predecessor Company will be released from its obligations under the Indenture and the Successor Company will succeed to, and be substituted for, and may exercise every right and power of, the Company under the Indenture, but, in the case of a lease of all or substantially all its assets, the predecessor Company will not be released from the obligation to pay the principal of and interest on the Notes.
Although there is a limited body of case law interpreting the phrase “substantially all,” there is no precise established definition of the phrase under applicable law. Accordingly, in certain circumstances there may be a degree of uncertainty as to whether a particular transaction would involve “all or substantially all” of the property or assets of a Person. As a result, it may be unclear as to whether or not the “Merger and consolidation” covenant applies to a particular sale or other transfer of properties.
Notwithstanding the preceding clause (3), (v) the Company may effect a reorganization described in the proviso to the definition of “Change of Control,” (w) any Restricted Subsidiary may consolidate with, merge
S-215
Table of Contents
with or into or transfer all or part of its properties and assets to the Company or a Subsidiary Guarantor, (x) any Restricted Subsidiary that is not a Subsidiary Guarantor may consolidate with, merge with or into or transfer all or part of its properties and assets to a Restricted Subsidiary that is not a Subsidiary Guarantor, (y) the Company may merge with or into an Affiliate incorporated solely for the purpose of reincorporating the Company in another jurisdiction and (z) the Company may consolidate with, merge with or into or transfer all or part of its properties and assets to a Subsidiary Guarantor.
In addition, the Indenture will provide that the Company will not permit any Subsidiary Guarantor to consolidate with or merge with or into any Person (other than another Subsidiary Guarantor or the Company) and will not permit the conveyance, transfer or lease of substantially all of the assets of any Subsidiary Guarantor to any Person (other than another Subsidiary Guarantor or the Company)unless:
(1) (a) the resulting, surviving or transferee Person will be a corporation, partnership, trust or limited liability company organized and existing under the laws of the United States of America, any State of the United States or the District of Columbia and such Person (if not such Subsidiary Guarantor) will expressly assume, by supplemental indenture, executed and delivered to the Trustee, all the obligations of such Subsidiary Guarantor under its Note Guarantee; (b) immediately after giving effect to such transaction (and treating any Indebtedness that becomes an obligation of the resulting, surviving or transferee Person or any Restricted Subsidiary as a result of such transaction as having been Incurred by such Person or such Restricted Subsidiary at the time of such transaction), no Default of Event of Default shall have occurred and be continuing; and (c) the Company will have delivered to the Trustee an Officers’ Certificate and an Opinion of Counsel, each stating that such consolidation, merger or transfer and such supplemental indentures comply with the Indenture; or
(2) the transaction is made in compliance with the “Limitation on sales of assets and subsidiary stock” covenant.
Notwithstanding the foregoing, the Merger and the related transactions shall be permitted under the Indenture.
Future subsidiary guarantors
The Indenture will provide that the Company will not permit any Restricted Subsidiary to Guarantee the payment of any Indebtedness of the Company or any Indebtedness of any other Restricted Subsidiary (other than a Guarantee by a Foreign Subsidiary of Indebtedness of a Foreign Subsidiary or a Guarantee by a Receivables Subsidiary), unless such Restricted Subsidiary simultaneously executes and delivers a supplemental indenture pursuant to which such Restricted Subsidiary will unconditionally Guarantee, on a joint and several basis, the full and prompt payment of the principal of, premium, if any, and interest on the Notes issued under the Indenture and all other obligations under the Indenture on a senior basis;providedthat, if such Indebtedness is by its express terms subordinated in right of payment to the Notes or a Note Guarantee, any Guarantee of such Restricted Subsidiary with respect to such Indebtedness shall be subordinated in right of payment to such Restricted Subsidiary’s Note Guarantee substantially to the same extent as such Indebtedness is subordinated to the Notes or such Note Guarantee, as the case may be.
The obligations of a Subsidiary Guarantor under its Note Guarantee will be limited as necessary to prevent its Note Guarantee from constituting a fraudulent conveyance or fraudulent transfer under applicable law. See “—Note Guarantees” above.
The Indenture will provide that any Subsidiary Guarantor shall be released from its obligations under its Note Guarantee of the Notes and its obligations under the Indenture upon certain circumstances. For additional information, see “—Note Guarantees” above and “—Defeasance” and “—Satisfaction and discharge” below.
Covenant termination
If on any date following the Release Date (i) the Notes have Investment Grade Ratings from both of the Rating Agencies, (ii) no Default or Event of Default under the Indenture has occurred and is continuing and
S-216
Table of Contents
(iii) the Company has delivered an officers’ certificate to the Trustee certifying that the conditions set forth in clauses (i) and (ii) above are satisfied (the occurrence of the events described in the foregoing clauses (i), (ii) and (iii) being collectively referred to as a Covenant Termination Event, the Company and its Restricted Subsidiaries will no longer be subject to, and will be permanently released from their obligations under, the following provisions of the Indenture:
| • | “—Limitation on indebtedness”; |
| • | “—Limitation on restricted payments”; |
| • | “—Limitation on restrictions on distributions from restricted subsidiaries”; |
| • | “—Limitation on sales of assets and subsidiary stock”; |
| • | “—Limitation on affiliate transactions”; |
| • | “—Conduct of business”; and |
| • | clause (3) of the first paragraph of “—Merger and consolidation,” |
and no failure by the Company or any Subsidiary to comply with any of the foregoing provisions shall constitute a Default or Event of Default under the Indenture.
There can be no assurance that the Notes will ever achieve or maintain Investment Grade Ratings.
Events of default
Under the Indenture, each of the following is an Event of Default under the Indenture:
(1) default in any payment of interest on any Note issued and outstanding under the Indenture when due, continued for 30 days;
(2) default in the payment of principal of or premium, if any, on any Note issued and outstanding under the Indenture when due at its Stated Maturity, upon optional redemption, upon required repurchase, upon declaration or otherwise;
(3) failure by the Company or any Subsidiary Guarantor to comply with its obligations under the “Merger and consolidation” covenant;
(4) failure by the Company to comply for 30 days after written notice with any of its obligations under the covenant described under “Change of control” above or under the covenants described under “Certain covenants” above (in each case, other than a failure to purchase Notes issued and outstanding under the Indenture, which will constitute an Event of Default under clause (2) above, and other than a failure to comply with the “Merger and consolidation” covenant, which is covered by clause (3));
(5) failure by the Company to comply for 60 days after written notice with its other agreements contained in the Indenture;
(6) default under any mortgage, indenture or instrument under which there may be issued or by which there may be secured or evidenced any Indebtedness for money borrowed by the Company or any of its Restricted Subsidiaries (or the payment of which is Guaranteed by the Company or any of its Restricted Subsidiaries), other than Indebtedness owed to the Company or a Restricted Subsidiary, whether such Indebtedness or Guarantee exists on, or is created after, the Issue Date, which default:
(a) is caused by a failure to pay principal at final maturity of such Indebtedness prior to the expiration of the grace period provided in such Indebtedness, or payment default; or
(b) results in the acceleration of such Indebtedness prior to its final maturity, or cross-acceleration provision;
and, in each case, the principal amount of any such Indebtedness, together with the principal amount of any other such Indebtedness under which there has been a payment default or the maturity of which has been so accelerated, aggregates $75.0 million or more;
S-217
Table of Contents
(7) certain events of bankruptcy, insolvency or reorganization of the Company or a Significant Subsidiary, or bankruptcy provisions;
(8) failure by the Company or any Significant Subsidiary to pay the uninsured portion of final judgments aggregating in excess of $75.0 million, which judgments are not paid, discharged or stayed for a period of 60 days, or judgment default provision; or
(9) any Note Guarantee of a Subsidiary Guarantor under the Indenture that is a Significant Subsidiary ceases to be in full force and effect (except as contemplated by the terms of the Indenture) or is declared null and void in a judicial proceeding or any Subsidiary Guarantor under the Indenture that is a Significant Subsidiary or group of Subsidiary Guarantors under the Indenture that taken together would constitute a Significant Subsidiary denies or disaffirms its or their, as the case may be, obligations under the Indenture or its Note Guarantee or their Note Guarantees, as the case may be.
However, a default under clauses (4) and (5) of this paragraph will not constitute an Event of Default until the Trustee or the Holders of 25% in principal amount of the Notes outstanding under the Indenture notify the Company of the default and the Company does not cure such default within the time specified in clauses (4) and (5) of this paragraph after receipt of such notice.
If an Event of Default (other than an Event of Default with respect to the Company of the type described in clause (7) above) occurs and is continuing, the Trustee by notice to the Company, or the Holders of at least 25% in principal amount of Notes outstanding under the Indenture by notice to the Company and the Trustee, may, and the Trustee at the request of such Holders shall, declare the principal of, premium, if any, and accrued and unpaid interest, if any, on all the Notes to be due and payable. Upon such a declaration, such principal, premium and accrued and unpaid interest will be due and payable immediately. In the event of a declaration of acceleration of the Notes because an Event of Default described in clause (6) under “Events of default” has occurred and is continuing, the declaration of acceleration of the Notes shall be automatically annulled if the default or payment default triggering such Event of Default pursuant to clause (6) shall be remedied or cured by the Company or a Restricted Subsidiary or waived by the holders of the relevant Indebtedness within 60 days after the declaration of acceleration with respect thereto and if (1) the annulment of the acceleration of the Notes would not conflict with any judgment or decree of a court of competent jurisdiction and (2) all existing Events of Default, except nonpayment of principal, premium or interest on the Notes that became due solely because of the acceleration of the Notes, have been cured or waived. If an Event of Default with respect to the Company of the type described in clause (7) above occurs and is continuing, the principal of, premium, if any, and accrued and unpaid interest on all the Notes will become and be immediately due and payable without any declaration or other act on the part of the Trustee or any Holders. The Holders of a majority in principal amount of the outstanding Notes under the Indenture may waive all past defaults (except with respect to nonpayment of principal, premium or interest) and rescind any such acceleration with respect to the Notes and its consequences if (1) rescission would not conflict with any judgment or decree of a court of competent jurisdiction and (2) all existing Events of Default under the Indenture, other than the nonpayment of the principal of, premium, if any, and interest on the Notes that have become due solely by such declaration of acceleration, have been cured or waived.
Subject to the provisions of the Indenture relating to the duties of the Trustee thereunder, if an Event of Default occurs and is continuing under the Indenture, the Trustee will be under no obligation to exercise any of the rights or powers under the Indenture at the request, order or direction of any of the Holders unless such Holders have offered to such Trustee reasonable indemnity or security against any loss, liability or expense. Except to enforce the right to receive payment of principal, premium, if any, or interest when due, no Holder may pursue any remedy with respect to the Indenture or any Notes issued thereunder unless:
(1) such Holder has previously given the Trustee notice that an Event of Default under the Indenture is continuing;
(2) Holders of at least 25% in principal amount of Notes outstanding under the Indenture have requested the Trustee to pursue the remedy;
S-218
Table of Contents
(3) such Holders have offered the Trustee security or indemnity reasonably satisfactory to the Trustee against any loss, liability or expense;
(4) the Trustee has not complied with such request within 60 days after the receipt of the request and the offer of security or indemnity; and
(5) the Holders of a majority in principal amount of Notes outstanding under the Indenture have not given the Trustee a direction that, in the opinion of the Trustee, is inconsistent with such request within such 60-day period.
Subject to certain restrictions, the Holders of a majority in principal amount of Notes outstanding under the Indenture are given the right to direct the time, method and place of conducting any proceeding for any remedy available to the Trustee or of exercising any trust or power conferred on the Trustee. The Indenture provides that in the event an Event of Default under the Indenture has occurred and is continuing, the Trustee will be required in the exercise of its powers to use the degree of care that a prudent person would use in the conduct of its own affairs. The Trustee, however, may refuse to follow any direction that conflicts with law or the Indenture or that the Trustee determines is unduly prejudicial to the rights of any other Holder of Notes issued under the Indenture or that would involve the Trustee in personal liability. Prior to taking any action under the Indenture, the Trustee will be entitled to indemnification satisfactory to it in its sole discretion against all losses and expenses caused by taking or not taking such action.
The Indenture provides that if a Default under the Indenture occurs and is continuing and the Trustee receives actual notice of such Default to the Trustee, the Trustee must mail to each Holder of Notes issued under the Indenture notice of the Default within 90 days after it occurs. Except in the case of a Default in the payment of principal of, premium, if any, or interest on any Notes, the Trustee may withhold notice if and so long as the board of directors or a committee of trust officers of the Trustee in good faith determines that withholding notice is in the interests of such Holders. In addition, the Company is required to deliver to the Trustee, within 120 days after the end of each fiscal year, a certificate indicating whether the signers thereof know of any Default under the Indenture that occurred during the previous year. The Company also is required to deliver to the Trustee, within 30 days after the occurrence thereof, written notice of any events which would constitute certain Defaults under the Indenture, their status and what action the Company is taking or proposing to take in respect thereof.
Amendments and waivers
Subject to certain exceptions, the Indenture and the Notes may be amended or supplemented with the consent of the Holders of a majority in principal amount of Notes outstanding under the Indenture (including without limitation, consents obtained in connection with a purchase of, or tender offer or exchange offer for, such Notes) and, subject to certain exceptions, any past default or compliance with any provisions may be waived with the consent of the Holders of a majority in principal amount of outstanding Notes (including, without limitation, consents obtained in connection with a purchase of, or tender offer or exchange offer for, such Notes). However, the Indenture shall provide that, without the consent of each Holder of an outstanding Note affected, no amendment, supplement or waiver may, among other things:
(1) reduce the amount of Notes whose Holders must consent to an amendment;
(2) reduce the stated rate of or extend the stated time for payment of interest on any Note;
(3) reduce the principal of or extend the Stated Maturity of any Note;
(4) reduce the premium payable upon the redemption of any Note or change the time at which any Note may be redeemed as described above under “Optional redemption,” whether through an amendment or waiver of provisions in the covenants, definitions or otherwise;
(5) make any Note payable in money other than that stated in such Note;
(6) impair the right of any Holder of any Note to receive payment of principal, premium, if any, and interest on such Holder’s Notes on or after the due dates therefor or to institute suit for the enforcement of any payment on or with respect to such Holder’s Notes;
S-219
Table of Contents
(7) make any change in the amendment provisions of the Indenture which require each Holder’s consent or in the waiver provisions;
(8) make any change to the ranking of the Notes or the Note Guarantees that adversely affects the rights of any Holder of the Notes; or
(9) release any Subsidiary Guarantor from any of its obligations under its Note Guarantee, except as permitted by the Indenture.
Notwithstanding the foregoing, without the consent of any Holder, the Company, the Subsidiary Guarantors and the Trustee may amend the Indenture and the Notes to:
(1) cure any ambiguity, omission, defect or inconsistency;
(2) provide for the assumption by a successor corporation of the obligations of the Company under the Indenture or the assumption by a corporation, partnership, trust or limited liability company of the obligations of a Subsidiary Guarantor under the Indenture;
(3) provide for uncertificated Notes in addition to or in place of certificated Notes (providedthat the uncertificated Notes are issued in registered form for purposes of Section 163(f) of the Code;
(4) add Subsidiary Guarantors (or other guarantors) or Note Guarantees (or other Guarantees) with respect to the Notes, or release a Subsidiary Guarantor (or any other such guarantor) or any Note Guarantee (or other Guarantee) in accordance with the applicable provisions of the Indenture;
(5) secure the Notes or the Note Guarantees (or any other Guarantee) thereof;
(6) add to the covenants of the Company for the benefit of the Holders of such Notes or surrender any right or power conferred upon the Company;
(7) make any change that does not materially adversely affect the rights of any Holder of such Notes;
(8) comply with any requirement of the SEC in order to effect or maintain the qualification of the Indenture under the Trust Indenture Act;
(9) release a Subsidiary Guarantor from its obligations under its Note Guarantee (or release any other guarantor from its obligations under its Guarantee) or the Indenture in accordance with the applicable provisions of the Indenture;
(10) provide for the appointment of a successor trustee;provided that such successor trustee is otherwise qualified and eligible to act as such under the terms of the Indenture; or
(11) conform any provision of the Indenture to this “Description of Notes.”
The consent of the Holders is not necessary under the Indenture to approve the particular form of any proposed amendment or supplement. It is sufficient if such consent approves the substance of the proposed amendment or supplement. A consent to any amendment, supplement or waiver under the Indenture by any Holder of Notes given in connection with a tender of such Holder’s Notes will not be rendered invalid by such tender. After an amendment under the Indenture becomes effective, the Company is required to mail to the Holders of Notes issued thereunder a notice briefly describing such amendment. However, the failure to give such notice to all such Holders, or any defect in the notice will not impair or affect the validity of the amendment or supplement.
Defeasance
The Company at any time may terminate all obligations under the Indenture and the Notes issued thereunder, or legal defeasance, except for certain obligations, including those respecting the defeasance trust and obligations to register the transfer or exchange of the Notes, to replace mutilated, destroyed, lost or stolen Notes
S-220
Table of Contents
and to maintain a registrar and paying agent in respect of the Notes. If the Company exercises its legal defeasance option, any Note Guarantees and all obligations of the Subsidiary Guarantors under the Indenture will terminate.
The Company at any time may terminate its obligations under the Indenture described under “Change of control” and clause (3) of the “Merger and consolidation” covenant and under all of the other covenants described under “Certain covenants,” or covenant defeasance, and no failure by the Company or any Subsidiary to comply with any of the foregoing provisions shall constitute a Default or Event of Default under the Indenture.
The Company may exercise its legal defeasance option notwithstanding its prior exercise of its covenant defeasance option. If the Company exercises its legal defeasance option, payment of the Notes may not be accelerated because of an Event of Default with respect to the Notes. If the Company exercises its covenant defeasance option, payment of the Notes may not be accelerated because of an Event of Default specified in clause (4), (5), (6), (7) (with respect only to Significant Subsidiaries), (8) or (9) under “Events of default” above or because of the failure of the Company to comply with clause (3) of the “Merger and consolidation” covenant above or because of any failure to purchase Notes as described under “Change of control” or “Limitation on sales of assets and subsidiary stock.”
In order to exercise either defeasance option under an Indenture, the Company must irrevocably deposit in trust, or defeasance trust, with the Trustee money or U.S. Government Obligations for the payment of principal, premium, if any, and interest on the Notes outstanding under the Indenture to redemption or maturity, as the case may be, and must comply with certain other conditions, including delivery to the Trustee of an Opinion of Counsel (subject to customary exceptions and exclusions) reasonably acceptable to the Trustee to the effect that Holders of such Notes will not recognize income, gain or loss for Federal income tax purposes as a result of such deposit and defeasance and will be subject to Federal income tax on the same amounts and in the same manner and at the same times as would have been the case if such deposit and defeasance had not occurred. In the case of legal defeasance only, such Opinion of Counsel must be based on a ruling of the Internal Revenue Service or change subsequent to the date of the Indenture in applicable Federal income tax law.
Satisfaction and discharge
The Indenture will be discharged and will cease to be of further effect (except as to rights of registration of transfer or exchange of Notes issued thereunder and certain other provisions) as to all outstanding Notes issued thereunder when either
(1) all the Notes that have been authenticated and delivered (except lost, stolen or destroyed Notes which have been replaced or paid and Notes for whose payment money has been deposited in trust or segregated and held in trust by the Company and thereafter repaid to the Company or discharged from this trust) have been delivered to the Trustee for cancellation, or
(2) (a) all the Notes not delivered to the Trustee for cancellation otherwise (i) have become due and payable, (ii) will become due and payable, or may be called for redemption, within one year or (iii) have been called for redemption pursuant to the provisions described under “Optional redemption,” and, in any case, the Company has irrevocably deposited or caused to be deposited with the Trustee as trust funds, in trust solely for the benefit of the Holders of such Notes, U.S. legal tender, U.S. Government Obligations or a combination thereof, in such amounts as will be sufficient (without consideration of any reinvestment of interest) to pay and discharge the entire Indebtedness (including all principal and accrued interest) on such Notes not theretofore delivered to the Trustee for cancellation,
(b) the Company has paid all sums payable by it under the Indenture, and
(c) the Company has delivered irrevocable instructions to the Trustee to apply the deposited money toward the payment of such Notes at maturity or on the date of redemption, as the case may be.
In addition, the Company must deliver an Officers’ Certificate and an Opinion of Counsel stating that all conditions precedent to satisfaction and discharge have been complied with. If the Indenture is discharged as aforesaid, all Note Guarantees and all obligations of the Subsidiary Guarantors under the Indenture will terminate.
S-221
Table of Contents
No personal liability of directors, officers, employees and stockholders
No director, officer, employee, incorporator, stockholder, partner or member of, or owner of an equity interest in, of the Company or any Subsidiary Guarantor, as such, shall have any liability for any obligations of the Company under any Notes, the Indenture, any Note Guarantee or for any claim based on, in respect of, or by reason of, such obligations or their creation. Each Holder by accepting a Note waives and releases all such liability. The waiver and release are part of the consideration for issuance of the Notes.
Concerning the trustee
The Bank of New York Mellon Trust Company, N.A. is the Trustee under the Indenture and has been appointed by the Company as registrar and paying agent with regard to the Notes.
Governing law
The Indenture provides that it and the Notes and Note Guarantees will be governed by, and construed in accordance with, the laws of the State of New York.
Certain definitions
“2010 Transactions” means the issuance of the Existing Notes on the October 20, 2010, the entering into of the Senior Credit Agreement on October 20, 2010, the tender offer for and/or repurchase, redemption or other retirement of the Company’s outstanding 6 5/8% Senior Notes due 2013 and the Company’s outstanding 7 1/4% Senior Subordinated Notes due 2015 and the application of the proceeds from the issuance of the Existing Notes and borrowings under the Senior Credit Agreement in connection with a tender offer for and/or repurchase, redemption or other retirement of the Company’s outstanding 6 5/8% Senior Notes due 2013 and the Company’s outstanding 7 1/4% Senior Subordinated Notes due 2015 and otherwise as set forth under “Use of Proceeds” in the prospectus supplement dated October 5, 2010 relating to the original issuance of the Existing Notes on October 20, 2010.
“Acquired Indebtedness” means Indebtedness (i) of a Person or any of its Subsidiaries existing at the time such Person becomes a Restricted Subsidiary or (ii) assumed in connection with the acquisition of assets from such Person, in each case whether or not Incurred by such Person in connection with, or in anticipation or contemplation of, such Person becoming a Restricted Subsidiary or such acquisition. Acquired Indebtedness shall be deemed to have been Incurred, with respect to clause (i) of the preceding sentence, on the date such Person becomes a Restricted Subsidiary and, with respect to clause (ii) of the preceding sentence, on the date of consummation of such acquisition of assets.
“Additional Notes” means Notes issued after the Issue Date in accordance with the Indenture.
“Affiliate” of any specified Person means any other Person, directly or indirectly, controlling or controlled by or under direct or indirect common control with such specified Person. For the purposes of this definition, “control” when used with respect to any Person means the power to direct the management and policies of such Person, directly or indirectly, whether through the ownership of voting securities, by contract or otherwise; and the terms “controlling” and “controlled” have meanings correlative to the foregoing.
“Applicable Premium” means, with respect to any Note to be redeemed on any redemption date, the greater of:
(1) 1.0% of the then outstanding principal amount of such Note; and
(2) the excess, if any, of:
(a) the present value at such redemption date of (i) the redemption price of such Note at , 2017 (such redemption price being set forth in the table appearing in the first paragraph under the
S-222
Table of Contents
caption “—Optional Redemption”)plus (ii) all required interest payments due on such Note through , 2017 (excluding accrued but unpaid interest to such redemption date), computed using a discount rate equal to the Treasury Rate as of such redemption date plus 50 basis points; over
(b) the then outstanding principal amount of such Note.
“Asset Disposition” means any direct or indirect sale, lease (other than an operating lease entered into in the ordinary course of business), transfer, issuance or other disposition (other than a license or sub-license entered into in the ordinary course of business), or a series of related sales, leases, transfers, issuances or dispositions that are part of a common plan, of shares of Capital Stock of a Subsidiary (other than directors’ qualifying shares), property or other assets (each referred to for the purposes of this definition as a, or disposition, by the Company or any of its Restricted Subsidiaries, including any disposition by means of a merger, consolidation or similar transaction.
Notwithstanding the preceding, the following items shall not be deemed to be Asset Dispositions:
(1) a sale, lease, transfer, issuance or other disposition (including, without limitation, by merger, consolidation or sale or other transfer of Capital Stock) by a Restricted Subsidiary to the Company or by the Company or a Restricted Subsidiary to a Restricted Subsidiary;
(2) the sale or other disposition of cash and cash equivalents in the ordinary course of business;
(3) a disposition of inventory in the ordinary course of business;
(4) a disposition of obsolete or worn out equipment or equipment that is disposed of in each case in the ordinary course of business;
(5) transactions permitted under the “Merger and consolidation” covenant;
(6) an issuance of Capital Stock by a Restricted Subsidiary to the Company or to a Restricted Subsidiary;
(7) for purposes of the “Limitation on sales of assets and subsidiary stock” covenant only, the making of a Permitted Investment (other than a Permitted Investment to the extent such transaction results in the receipt of cash or Cash Equivalents by the Company or its Restricted Subsidiaries) or a Restricted Payment made in accordance with the “Limitation on restricted payments” covenant;
(8) any sale, lease, transfer or other disposition (including, without limitation, by merger, consolidation or sale or other transfer of Capital Stock) of assets (including without limitation the Capital Stock of Subsidiaries) with an aggregate Fair Market Value of less than $50.0 million per transaction or series of related transactions;
(9) the creation of any Permitted Lien and dispositions in connection with Permitted Liens;
(10) dispositions of receivables in connection with the compromise, settlement or collection thereof in the ordinary course of business or in bankruptcy or similar proceedings;
(11) the issuance by a Restricted Subsidiary of Preferred Stock that is permitted by the “Limitation on indebtedness” covenant;
(12) any sale, transfer, issuance or other disposition or distribution of Capital Stock in, or Indebtedness or other securities of, an Unrestricted Subsidiary;
(13) the licensing or sublicensing of intellectual property or other general intangibles and licenses, leases or subleases of other property to the extent not materially interfering with the business of the Company and its Restricted Subsidiaries taken as a whole;
(14) sales or other dispositions of assets or property pursuant to Sale/Leaseback Transactions entered into in compliance with the “Limitation on indebtedness” covenant;
(15) sales or other dispositions of Receivables and related assets or an interest therein of the type specified in the definition of “Qualified Receivables Transaction” in a Qualified Receivables Transaction; and
S-223
Table of Contents
(16) the disposition of all or substantially all of the assets of the Company in a manner permitted pursuant to the “Merger and consolidation” covenant or any disposition that constitutes a Change of Control pursuant to the Indenture.
“Attributable Indebtedness” in respect of a Sale/Leaseback Transaction means, as at the time of determination, the present value (discounted at the interest rate assumed in making calculations in accordance with Accounting Standards Codification Topic 840 “Leases”) of the total obligations of the lessee for rental payments during the remaining term of the lease included in such Sale/Leaseback Transaction (including any period for which such lease has been extended).
“Average Life” means, as of the date of determination, with respect to any Indebtedness or Preferred Stock, the quotient obtained by dividing (1) the sum of the products of the numbers of years from the date of determination to the dates of each successive scheduled principal payment of such Indebtedness or redemption or similar payment with respect to such Preferred Stock multiplied by the amount of such payment by (2) the sum of all such payments.
“Bank Indebtedness” means any and all amounts, whether outstanding on the Issue Date or Incurred after the Issue Date, in respect of the Senior Credit Facilities and any related notes, collateral documents, letters of credit and Guarantees and any Interest Rate Agreement entered into in connection with the Senior Credit Facilities, including principal, premium, if any, interest (including interest accruing on or after the filing of any petition in bankruptcy or for reorganization of the Company at the rate specified therein whether or not a claim for post-filing interest is allowed in such proceedings), fees, charges, expenses, reimbursement obligations, Guarantees and all other amounts payable thereunder or in respect thereof.
“Board of Directors” means, as to any Person, the board of directors or similar body of such Person or any duly authorized committee thereof. For purposes of clarity, it is understood and agreed that references to a majority or other percentage or portion of the Board of Directors of any Person means a majority or such other percentage or portion of the board of directors or similar body of such Person or of any duly authorized committee thereof.
“Business Day” means each day that is not a Saturday, Sunday or other day on which banking institutions in New York, New York are authorized or required by law to close.
“Capital Stock” of any Person means any and all shares, interests, rights to purchase, warrants, options, participation or other equivalents of or interests in (however designated) equity of such Person, including any Preferred Stock and limited liability or partnership interests (whether general or limited), but excluding any debt securities convertible into such equity.
“Capitalized Lease Obligations” means an obligation that is required to be classified and accounted for as a capitalized lease for financial reporting purposes in accordance with GAAP, and the amount of Indebtedness represented by such obligation will be the capitalized amount of such obligation at the time any determination thereof is to be made as determined in accordance with GAAP, and the Stated Maturity thereof will be the date of the last payment of rent or any other amount due under such lease prior to the first date such lease may be terminated without penalty.
“Cash Equivalents” means:
(1) securities with maturities of one year or less from the date of acquisition, issued, fully guaranteed or insured by the United States of America or any agency or instrumentality thereof;
(2) securities with maturities of one year or less from the date of acquisition issued, fully guaranteed or insured by any State of the United States of America or any political subdivision thereof rated at least AA-
S-224
Table of Contents
by S&P or Aa3 by Moody’s, or carrying an equivalent rating by a nationally recognized rating agency if both of the two named rating agencies cease publishing ratings of investments;
(3) certificates of deposit, time deposits, overnight bank deposits, demand deposits or other deposits, bankers’ acceptances and repurchase agreements issued by or in a Qualified Issuer having maturities of 270 days or less from the date of acquisition;
(4) commercial paper of an issuer rated at least A-2 by S&P or P-2 by Moody’s, or carrying an equivalent rating by a nationally recognized rating agency if both of the two named rating agencies cease publishing ratings of investments, and having maturities of 270 days or less from the date of acquisition;
(5) money market accounts or funds, a substantial portion of the assets of which constitute Cash Equivalents described in clauses (1) through (4) above, with, issued by or managed by Qualified Issuers;
(6) money market accounts or funds, a substantial portion of the assets of which constitute Cash Equivalents described in clauses (1) through (4) above, which money market accounts or funds have net assets of not less than $500.0 million and have the highest rating available of either S&P or Moody’s, or carrying an equivalent rating by a nationally recognized rating agency if both of the two named rating agencies cease publishing ratings of investments;
(7) money market accounts or funds rated at least AA by S&P and at least Aa by Moody’s;
(8) auction rate securities rated not less than AAA by S&P and not less than Aaa by Moody’s;
(9) securities with maturities of one year or less from the date of acquisition issued by, and any certificates of deposit, time deposits, overnight bank deposits, demand deposits, or other accounts issued by or with, a bank or other financial institution to the extent insured by the Federal Deposit Insurance Corporation or any similar or successor entity; and
(10) in the case of Foreign Subsidiaries of the Company, substantially similar instruments to those set forth in clauses (1) through (9) above.
“Change of Control” means:
(1) any “person” or “group” of related persons (as such terms are used in Sections 13(d) and 14(d) of the Exchange Act) is or becomes the beneficial owner (as defined in Rules 13d-3 and 13d-5 under the Exchange Act), except that such person or group shall be deemed to have “beneficial ownership” of all shares that any such person or group has the right to acquire, whether such right is exercisable immediately or only after the passage of time, directly or indirectly, of more than 35% of the total voting power of the Voting Stock of the Company (or its successor by merger, consolidation or purchase of all or substantially all of its assets) (for the purposes of this clause, such person or group shall be deemed to beneficially own any Voting Stock of the Company held by a parent entity, if such person or group “beneficially owns” (as defined above), directly or indirectly, more than 35% of the voting power of the Voting Stock of such parent entity); or
(2) the first day on which a majority of the members of the Board of Directors of the Company are not Continuing Directors; or
(3) the sale, lease, transfer, conveyance or other disposition (other than by way of merger or consolidation), in one or a series of related transactions, of all or substantially all of the assets of the Company and its Restricted Subsidiaries taken as a whole to any “person” (as such term is used in Sections 13(d) and 14(d) of the Exchange Act), excluding any such transaction that complies with the “Merger and consolidation” covenant; or
(4) the adoption by the stockholders of the Company of a plan or proposal for the liquidation or dissolution of the Company;
provided that notwithstanding the foregoing, the occurrence of a reorganization that results in all the Capital Stock of the Company being held by a Parent Entity shall not result in a Change of Control provided that the shareholders of the Parent Entity immediately after such reorganization are substantially the same as the shareholders of the Company (with substantially equivalent ownership percentages) immediately preceding such reorganization.
S-225
Table of Contents
“Code” means the Internal Revenue Code of 1986, as amended.
“Common Stock” means with respect to any Person, any and all shares, interests or other participations in, and other equivalents (however designated and whether voting or nonvoting) of such Person’s common stock whether or not outstanding on the Issue Date, and includes, without limitation, all series and classes of such common stock.
“Consolidated Coverage Ratio” means as of any date of determination, with respect to any Person, the ratio of (x) the aggregate amount of Consolidated EBITDA of such Person for the period of the most recent four consecutive fiscal quarters ending prior to the date of such determination for which financial statements are in existence to (y) Consolidated Fixed Charges for such four fiscal quarters;provided,however, that:
(1) if the Company or any Restricted Subsidiary:
(a) has Incurred any Indebtedness since the beginning of such period that remains outstanding on such date of determination or if the transaction giving rise to the need to calculate the Consolidated Coverage Ratio is an Incurrence of Indebtedness, Consolidated EBITDA and Consolidated Fixed Charges for such period will be calculated after giving effect on apro formabasis to such Indebtedness as if such Indebtedness had been Incurred on the first day of such period (except that in making such computation, the amount of Indebtedness under any revolving credit facility drawn for working capital purposes in the ordinary course of business outstanding on the date of such calculation will be deemed to be (i) the average daily balance of such Indebtedness during such four fiscal quarters or such shorter period for which such facility was outstanding or (ii) if such facility was created after the end of such four fiscal quarters, the average daily balance of such Indebtedness during the period from the date of creation of such facility to the date of such calculation) and the discharge of any other Indebtedness repaid, repurchased, defeased or otherwise discharged with the proceeds of such new Indebtedness as if such discharge had occurred on the first day of such period; or
(b) has repaid, repurchased, defeased or otherwise discharged any Indebtedness since the beginning of the period that is no longer outstanding on such date of determination or if the transaction giving rise to the need to calculate the Consolidated Coverage Ratio involves a discharge of Indebtedness (in each case other than Indebtedness Incurred under any revolving credit facility unless such Indebtedness has been permanently repaid and the related commitment terminated), Consolidated EBITDA and Consolidated Fixed Charges for such period will be calculated after giving effect on apro formabasis to such discharge of such Indebtedness, including with the proceeds of such new Indebtedness, as if such discharge had occurred on the first day of such period;
(2) if since the beginning of such period the Company or any Restricted Subsidiary will have made any asset sale or other disposition or if the transaction giving rise to the need to calculate the Consolidated Coverage Ratio is such an asset sale or other disposition:
(a) the Consolidated EBITDA for such period will be reduced by an amount equal to the Consolidated EBITDA (if positive) directly attributable to the assets which are the subject of such asset sale or other disposition for such period or increased by an amount equal to the Consolidated EBITDA (if negative) directly attributable thereto for such period; and
(b) Consolidated Fixed Charges for such period will be reduced by an amount equal to the Consolidated Fixed Charges directly attributable to any Indebtedness of the Company or any Restricted Subsidiary repaid, repurchased, defeased or otherwise discharged with respect to the Company and its continuing Restricted Subsidiaries in connection with such asset sale or other disposition for such period (or, if the Capital Stock of any Restricted Subsidiary is sold, the Consolidated Fixed Charges for such period directly attributable to the Indebtedness of such Restricted Subsidiary to the extent the Company and its continuing Restricted Subsidiaries are no longer liable for such Indebtedness after such sale);
(3) if since the beginning of such period the Company or any Restricted Subsidiary (by merger, consolidation, acquisition of Capital Stock or otherwise) will have made an Investment in any Restricted
S-226
Table of Contents
Subsidiary (or any Person which becomes a Restricted Subsidiary or is merged or consolidated with or into the Company) or an acquisition of assets, including any acquisition of assets occurring in connection with a transaction causing a calculation to be made hereunder, which constitutes all or substantially all of a company, division, operating unit, segment, business, group of related assets or line of business, Consolidated EBITDA and Consolidated Fixed Charges for such period will be calculated after givingproformaeffect thereto (including the Incurrence of any Indebtedness) as if such Investment or acquisition occurred on the first day of such period; and
(4) if since the beginning of such period any Person (that subsequently became a Restricted Subsidiary or was merged with or into the Company or any Restricted Subsidiary since the beginning of such period) will have Incurred any Indebtedness or discharged any Indebtedness or made any asset sale or other disposition or any Investment or acquisition of assets that would have required an adjustment pursuant to clause (2) or (3) above if made by the Company or a Restricted Subsidiary during such period, Consolidated EBITDA and Consolidated Fixed Charges for such period will be calculated after givingpro formaeffect thereto as if such transaction occurred on the first day of such period.
For purposes of this definition, wheneverpro formaeffect is to be given to any calculation under this definition, thepro formacalculations (includingpro formaexpense and cost reductions calculated on a basis consistent with Regulation S-X under the Securities Act) will be determined in good faith by a responsible financial or accounting officer of the Company;provided that suchpro formacalculations may include operating expense reductions for such period resulting from the transaction which is being givenpro formaeffect that have been realized or for which the steps necessary for realization have been taken or are reasonably expected to be taken within one year following any such transaction (which operating expense reductions are reasonably expected to be sustainable). If any Indebtedness bears a floating rate of interest and is being givenpro formaeffect, the interest expense on such Indebtedness will be calculated as if the rate in effect on the date of determination had been the applicable rate for the entire period (taking into account any Interest Rate Agreement applicable to such Indebtedness if such Interest Rate Agreement has a remaining term in excess of 12 months). If any Indebtedness that is being givenpro formaeffect bears an interest rate at the option of the Company, the interest rate shall be calculated by applying such optional rate chosen by the Company.
“Consolidated Debt Expense” means, for any period, without duplication, the total debt expense of the Company and its consolidated Restricted Subsidiaries, computed on a consolidated basis, whether paid or accrued, and included in debt expense as set forth on the statement of operations of the Company,plus, to the extent not included in such debt expense and without duplication:
(1) interest expense attributable to Capitalized Lease Obligations and the interest portion of rent expense associated with Attributable Indebtedness in respect of the relevant lease giving rise thereto, determined as if such lease were a capitalized lease in accordance with GAAP and the interest component of any deferred payment obligations;
(2) amortization of debt discount and debt issuance cost (provided that any amortization of bond premium will be credited to reduce Consolidated Debt Expense unless, pursuant to GAAP, such amortization of bond premium has otherwise reduced Consolidated Debt Expense);
(3) non-cash interest expense;
(4) commissions, discounts and other fees and charges owed with respect to letters of credit and bankers’ acceptance financing;
(5) interest expense on Indebtedness of another Person that is Guaranteed by such Person or one of its Restricted Subsidiaries or secured by a Lien on assets of such Person or one of its Restricted Subsidiaries;
(6) cash costs associated with Hedging Obligations (including amortization of fees but excluding mark-to-market charges or adjustments);provided,however, that if Hedging Obligations result in net
S-227
Table of Contents
benefits rather than costs, such benefits shall be credited to reduce Consolidated Debt Expense unless, pursuant to GAAP, such net benefits are otherwise reflected in Consolidated Net Income;
(7) the consolidated interest expense of such Person and its Restricted Subsidiaries that was capitalized during such period; and
(8) the cash contributions to any employee stock ownership plan or similar trust or stock option plan to the extent such contributions are used by such plan or trust to pay interest or fees to any Person (other than the Company and its Restricted Subsidiaries) in connection with Indebtedness incurred by such plan or trust.
For the purpose of calculating the Consolidated Coverage Ratio in connection with the Incurrence of any Indebtedness described in the final paragraph of the definition of “Indebtedness,” the calculation of Consolidated Debt Expense shall include all interest expense (including any amounts described in clauses (1) through (8) above) relating to any Indebtedness of the Company or any Restricted Subsidiary described in the final paragraph of the definition of “Indebtedness.”
For purposes of the foregoing, total debt expense will be determined (i) after giving effect to any net payments made or received by the Company and its Subsidiaries with respect to Interest Rate Agreements, (ii) exclusive of amounts classified as other comprehensive income in the balance sheet of the Company and (iii) exclusive of the write-off of deferred financing costs. Notwithstanding anything to the contrary contained herein, commissions, discounts, yield and other fees and charges Incurred in connection with any transaction pursuant to which the Company or its Restricted Subsidiaries may sell, convey or otherwise transfer or grant a security interest in any accounts receivable or related assets shall be included in Consolidated Debt Expense.
“Consolidated EBITDA” for any period means, without duplication, the Consolidated Net Income for such period,plusthe following, to the extent deducted or taken into account in calculating such Consolidated Net Income:
(1) Consolidated Fixed Charges;
(2) Consolidated Income Taxes;
(3) consolidated expenses for valuation adjustments or impairment charges;
(4) consolidated depreciation or amortization expense;
(5) expenses and charges relating to non-controlling interests and equity income in consolidated Subsidiaries; and
(6) other non-cash charges reducing Consolidated Net Income (excluding any such non-cash charge to the extent it represents an accrual of or reserve for cash charges in any future period or amortization of a prepaid cash expense that was paid in a prior period not included in the calculation).
Notwithstanding the preceding sentence, clauses (2) through (6) relating to amounts of a Restricted Subsidiary of a Person will be added to Consolidated Net Income to compute Consolidated EBITDA of such Person only to the extent (and in the same proportion) that the net income (loss) of such Restricted Subsidiary was included in calculating the Consolidated Net Income of such Person and, to the extent the amounts set forth in clauses (2) through (6) are in excess of those necessary to offset a net loss of such Restricted Subsidiary or if such Restricted Subsidiary has net income for such period included in Consolidated Net Income, only if a corresponding amount would be permitted at the date of determination to be dividended to the Company by such Restricted Subsidiary without prior approval (that has not been obtained), pursuant to the terms of its charter and all agreements, instruments, judgments, decrees, orders, statutes, rules and governmental regulations applicable to that Restricted Subsidiary or its stockholders.
“Consolidated Fixed Charges” means, on a consolidated basis and without duplication,
(1) Consolidated Debt Expense,plus
S-228
Table of Contents
(2) the product of (a) all dividends paid or payable, in cash, Cash Equivalents or indebtedness, or accrued during such period on any series of Disqualified Stock of the Company or on Preferred Stock of its Restricted Subsidiaries payable to a party other than the Company or a Restricted Subsidiary, times (b) a fraction, the numerator of which is one and the denominator of which is one minus the then current combined federal, state, provincial and local statutory tax rate of such Person, expressed as a decimal, in each case, on a consolidated basis and in accordance with GAAP.
“Consolidated Income Taxes” means, with respect to the Company and its consolidated Restricted Subsidiaries for any period, on a consolidated basis and without duplication, taxes imposed upon the Company or other payments required to be made by the Company by any governmental authority which taxes or other payments are calculated by reference to the income or profits of the Company or the Company and its consolidated Restricted Subsidiaries (to the extent such income or profits were included in computing Consolidated Net Income for such period), other than income taxes attributable to extraordinary, unusual or nonrecurring gains or losses or taxes attributable to sales or dispositions outside the ordinary course of business.
“Consolidated Net Income” means, for any period, the net income (loss) of the Company and its consolidated Restricted Subsidiaries determined on a consolidated basis in accordance with GAAP;provided,however, that there will not be included in such Consolidated Net Income:
(1) any net income (loss) of any Person if such Person is not a Restricted Subsidiary, except that:
(a) subject to the limitations contained in clauses (3) through (9) below, the Company’s equity in the net income of any such Person for such period will be included in such Consolidated Net Income up to the aggregate amount of cash actually distributed by such Person during such period to the Company or a Restricted Subsidiary as a dividend or other distribution (subject, in the case of a dividend or other distribution to a Restricted Subsidiary, to the limitations contained in clause (2) below); and
(b) the Company’s equity in a net loss of any such Person for such period will be included in determining such Consolidated Net Income;
(2) any net income (but not loss) of any Restricted Subsidiary if such Subsidiary is subject to restrictions, directly or indirectly, by operation of the terms of its charter, any contract or agreement, operation of law or otherwise, on the payment of dividends or the making of distributions by such Restricted Subsidiary, directly or indirectly, to the Company, except that:
(a) subject to the limitations contained in clauses (3) through (9) below, the Company’s equity in the net income of any such Restricted Subsidiary for such period will be included in such Consolidated Net Income up to the aggregate amount of cash that could have been distributed by such Restricted Subsidiary (excluding the effect of restrictions relating to the Senior Credit Facilities permitted pursuant to clauses (i) and (iii) of the second paragraph of the “Limitation on restrictions on distributions from restricted subsidiaries” covenant) during such period to the Company or another Restricted Subsidiary as a dividend (subject, in the case of a dividend to another Restricted Subsidiary, to the limitation contained in this clause); and
(b) for the avoidance of doubt, the Company’s equity in a net loss of any such Restricted Subsidiary for such period will be included in determining such Consolidated Net Income;
(3) any gain (loss) realized upon the sale or other disposition of any property, plant or equipment of the Company or its consolidated Restricted Subsidiaries (including pursuant to any Sale/Leaseback Transaction) which is not sold or otherwise disposed of in the ordinary course of business and any gain (loss) realized upon the sale or other disposition of any Capital Stock of any Person;
(4) any gain or loss arising from the early extinguishment of any Indebtedness in connection with the 2010 Transactions, including the amortization or write-off of debt issuance costs or debt discount in connection with the 2010 Transactions;
(5) any non-cash compensation charges arising from the grant of, issuance, vesting or repricing of stock, stock options or other equity-based awards or any amendment, modification, substitution or change of any such stock, stock options or other equity-based awards;
S-229
Table of Contents
(6) the cumulative effect of a change in accounting principles;
(7) any fees, expenses or charges related to the 2010 Transactions;
(8) any extraordinary or nonrecurring gain (or extraordinary or nonrecurring loss), together with any related provision for taxes on any such extraordinary or nonrecurring gain (or the tax effect of any such extraordinary or nonrecurring loss), realized by the Company or any Restricted Subsidiary during such period; and
(9) gains and losses due solely to fluctuations in currency values.
For purposes of this definition of “Consolidated Net Income,” “nonrecurring” means any gain or loss as of any date that is not reasonably likely to recur within the two years following such date;providedthat if there was a gain or loss similar to such gain or loss within the two years preceding such date, such gain or loss shall not be deemed nonrecurring.
“Consolidated Total Leverage Ratio” means, as of any date of determination, with respect to the Company and its consolidated Restricted Subsidiaries, the ratio of (x) the aggregate amount of all Indebtedness of the Company and its consolidated Restricted Subsidiaries, or Consolidated Total Indebtedness, as of the last day of the period of the most recent four consecutive fiscal quarters ending prior to the date of determination for which financial statements are in existence to (y) the aggregate amount of Consolidated EBITDA of the Company and its consolidated Restricted Subsidiaries for such period, all calculated on consolidated basis in accordance with GAAP. For purposes of calculating the Consolidated Total Leverage Ratio, Consolidated EBITDA shall, if necessary, be calculated on a pro forma basis in a manner consistent with the proviso to the first sentence of the definition of “Consolidated Coverage Ratio”; and Consolidated Total Indebtedness shall, if necessary, be calculated on a pro forma basis as follows:
if the Company or any Restricted Subsidiary:
(a) has Incurred any Indebtedness since the last day of the applicable four quarter period that remains outstanding on the applicable date of determination or if the transaction giving rise to the need to calculate the Consolidated Total Leverage Ratio includes the Incurrence of Indebtedness, Consolidated Total Indebtedness will be calculated after giving effect on a pro forma basis to such Indebtedness as if such Indebtedness had been Incurred on the last day of such period and the discharge of any other Indebtedness repaid, repurchased, defeased or otherwise discharged with the proceeds of such new Indebtedness as if such discharge had occurred on the last day of such period; or
(b) has repaid, repurchased, defeased or otherwise discharged any Indebtedness since the last day of such period that is no longer outstanding on such date of determination or if the transaction giving rise to the need to calculate the Consolidated Total Leverage Ratio includes a discharge of Indebtedness, Consolidated Total Indebtedness will be calculated after giving effect on a pro forma basis to such discharge of such Indebtedness, including with the proceeds of such new Indebtedness, as if such discharge had occurred on the last day of such period.
All such pro forma calculations shall be made in a manner consistent with the second paragraph of the definition of “Consolidated Coverage Ratio,” but without giving effect to the proviso to the first sentence of such second paragraph. In addition, the calculation of the Consolidated Total Leverage Ratio shall be made in a manner consistent with the third paragraph, the fourth paragraph and the sixth paragraph under the “Limitation on indebtedness” covenant above, mutatis mutandis.
“Continuing Directors” means, as of any date of determination, any member of the board of directors of the Company who: (1) was a member of such board of directors on the Issue Date; or (2) was nominated for election or elected to such board of directors with the approval of a majority of the Continuing Directors who were members of such board at the time of such nomination or election.
“Coverage Ratio Exception” has the meaning ascribed to such term in the first paragraph of the “Limitation on indebtedness” covenant.
S-230
Table of Contents
“Currency Agreement” means in respect of a Person any foreign exchange contract, currency swap agreement, currency futures contract, currency option contract or other similar currency agreement or arrangements as to which such Person is a party or a beneficiary.
“Default” means any event which is, or after notice or passage of time or both would be, an Event of Default under the Indenture.
“Designated Noncash Consideration” means the Fair Market Value (as determined in good faith by the Board of Directors) of noncash consideration received by the Company or any Restricted Subsidiary in connection with an Asset Disposition that is designated as Designated Noncash Consideration pursuant to an Officers’ Certificate setting forth the basis of such valuation, less the amount of cash or Cash Equivalents received in connection with a subsequent sale or other transfer of such Designated Noncash Consideration.
“Disqualified Stock” means with respect to any Person, any Capital Stock of such Person which by its terms (or by the terms of any security into which it is convertible or for which it is exchangeable) or upon the happening of any event:
(1) matures or is mandatorily redeemable pursuant to a sinking fund obligation or otherwise;
(2) is convertible or exchangeable for Indebtedness or Disqualified Stock (excluding Capital Stock which is convertible or exchangeable solely at the option of the Company or a Restricted Subsidiary); or
(3) is redeemable at the option of the holder of the Capital Stock in whole or in part,
in each case on or prior to the date that is 91 days after the earlier of the date (a) of the Stated Maturity of the Notes or (b) on which there are no Notes outstanding;providedthat only the portion of Capital Stock which so matures or is mandatorily redeemable, is so convertible or exchangeable or is so redeemable at the option of the holder thereof prior to such date will be deemed to be Disqualified Stock;provided,further, that any Capital Stock that would constitute Disqualified Stock solely because the holders thereof have the right to require the Company to repurchase such Capital Stock upon the occurrence of a change of control or asset sale (each defined in a substantially identical manner to the corresponding definitions in the Indenture) shall not constitute Disqualified Stock if the terms of such Capital Stock (and all such securities into which it is convertible or for which it is exchangeable) provide that the Company may not repurchase or redeem any such Capital Stock (and all such securities into which it is convertible or for which it is exchangeable) pursuant to such provision prior to compliance by the Company with the provisions of the Indenture described under the caption “Change of control” or the “Limitation on sales of assets and subsidiary stock” covenant, as the case may be, and such repurchase or redemption complies with the “Limitation on restricted payments” covenant.
“Eligible Escrow Investments” means (1) U.S. Government Obligations maturing no later than the Business Day preceding Escrow End Date and (2) securities representing an interest or interests in money market funds registered under the Investment Company Act of 1940 whose shares are registered under the Securities Act as investing exclusively in direct obligations of the United States.
“Equity Offering” means an offering for cash (generating gross proceeds of not less than $100.0 million) by the Company (to the extent such offering is not on behalf of selling stockholders) of its Common Stock, or options, warrants or rights with respect to its Common Stock, other than public offerings with respect to the Company’s Common Stock, or options, warrants or rights, registered on Form S-4 or S-8 or any successors thereto.
“Escrow Agent” means The Bank of New York Mellon Trust Company, N.A., as escrow agent under the Escrow Agreement or any successor escrow agent as set forth in the Escrow Agreement.
“Escrow Agreement” means the Escrow Agreement to be dated as of the Issue Date, among the Company, the Trustee and the Escrow Agent, as amended, supplemented, modified, extended, renewed, restated or replaced in whole or in part from time to time.
S-231
Table of Contents
“Escrow End Date” means November 30, 2012;providedthat the Issuer may elect to extend the Escrow End Date for an additional 30 days on no more than 3 occasions so long as (i) two Business days prior to the scheduled Escrow End Date it provides written notice to the Escrow Agent and the Trustee and has issued a press release stating that it has extended the Escrow End Date, (ii) the Company has deposited cash or Eligible Escrow Investments into escrow with the Escrow Agent, to be held pursuant to the terms of the Escrow Agreement, in an amount sufficient to fund the redemption price due on the latest permitted date for the revised Special Mandatory Redemption in respect of all outstanding Notes and has certified that such amount will be satisfactory for such purpose and (iii) the Termination Date (as defined in the Merger Agreement) has been extended to match the extended Escrow End Date.
“Exchange Act” means the United States Securities Exchange Act of 1934, as amended, and the rules and regulations of the SEC promulgated thereunder.
“Existing Notes” means the Company’s outstanding 6 3/8% Senior Notes due 2018 and the Company’s outstanding 6 5/8% Senior Notes due 2020.
“Fair Market Value” means, with respect to any asset, the price (after taking into account any liabilities relating to such asset) that would be negotiated in an arm’s-length transaction for cash between a willing seller and a willing and able buyer, neither of which is under any compulsion to complete the transaction. Fair Market Value (other than of any asset with a public trading market) (x) of $75.0 million or less shall be determined by Senior Management or the Board of Directors of the Company, in each case, acting reasonably and in good faith and (y) in excess of $75.0 million shall be determined by the Board of Directors of the Company acting reasonably and in good faith and shall be evidenced by a board resolution delivered to the Trustee.
“Foreign Subsidiary” means any Restricted Subsidiary that is not organized under the laws of the United States of America or any state thereof or the District of Columbia and any Subsidiary of such Restricted Subsidiary.
“GAAP” means generally accepted accounting principles in the United States of America as in effect on October 20, 2010, including those set forth in the opinions and pronouncements of the Accounting Principles Board of the American Institute of Certified Public Accountants and statements and pronouncements of the Financial Accounting Standards Board or in such other statements by such other entity as approved by a significant segment of the accounting profession. All ratios and computations based on GAAP contained in the Indenture will be computed in conformity with GAAP.
“Guarantee” means any obligation, contingent or otherwise, of any Person directly or indirectly guaranteeing any Indebtedness of any other Person and any obligation, direct or indirect, contingent or otherwise, of such Person:
(1) to purchase or pay (or advance or supply funds for the purchase or payment of) such Indebtedness of such other Person (whether arising by virtue of partnership arrangements, or by agreement to keep-well, to purchase assets, goods, securities or services, to take-or-pay, or to maintain financial statement conditions or otherwise); or
(2) entered into for purposes of assuring in any other manner the obligee of such Indebtedness of the payment thereof or to protect such obligee against loss in respect thereof (in whole or in part);
provided,however, that the term “Guarantee” will not include endorsements for collection or deposit in the ordinary course of business or undertakings customary in a Qualified Receivables Transaction. The term “Guarantee” used as a verb has a corresponding meaning.
“Guarantor Subordinated Obligation” means, with respect to a Subsidiary Guarantor, any Indebtedness of such Subsidiary Guarantor which is expressly subordinate in right of payment to the obligations of such Subsidiary Guarantor under its Note Guarantee with respect to the Notes pursuant to a written agreement.
S-232
Table of Contents
“Hedging Obligations” of any Person means the obligations of such Person pursuant to any Interest Rate Agreement or Currency Agreement.
“Incur” means issue, create, assume, Guarantee, incur or otherwise become liable for;provided,however, that any Indebtedness or Capital Stock of a Person existing at the time such Person becomes a Restricted Subsidiary (whether by merger, consolidation, acquisition or otherwise) will be deemed to be Incurred by such Restricted Subsidiary at the time it becomes a Restricted Subsidiary;providedthat solely for purposes of determining compliance with the “Limitation on indebtedness” covenant (i) amortization of debt discount or the accretion of principal with respect to a non-interest bearing or other discount security and (ii) unrealized losses or charges in respect of Hedging Obligations (including those resulting from the application of Accounting Standards Codification Topic “Derivation and Hedging”), in each case will be deemed not to be an Incurrence of Indebtedness; and the terms “Incurred” and “Incurrence” have meanings correlative to the foregoing.
“Indebtedness” means, with respect to any Person on any date of determination (without duplication):
(1) all obligations in respect of indebtedness of such Person for borrowed money;
(2) all obligations of such Person evidenced by bonds, debentures, notes or other similar instruments;
(3) all obligations of such Person in respect of letters of credit, bankers’ acceptances or other similar instruments (including reimbursement obligations with respect thereto except to the extent such reimbursement obligation relates to a trade payable and such obligation is satisfied within 30 days of Incurrence);
(4) all obligations of such Person to pay the deferred and unpaid purchase price of property or services (except trade payables and other accrued liabilities arising in the ordinary course of business in connection with obtaining goods, materials or services);
(5) Capitalized Lease Obligations and all Attributable Indebtedness of such Person;
(6) with respect to any Subsidiary that is not a Subsidiary Guarantor, any Preferred Stock (but excluding, in each case, any accrued dividends);
(7) all Indebtedness of other Persons secured by a Lien on any asset of such Person, whether or not such Indebtedness is assumed by such Person;provided,however, that the amount of such Indebtedness will be the lesser of (a) the Fair Market Value of such asset at such date of determination and (b) the amount of such Indebtedness of such other Persons;
(8) all Indebtedness of other Persons to the extent Guaranteed by such Person (for purposes of clarity, it is understood and agreed that, if a person Guarantees only a portion of the Indebtedness of another Person, then only the portion of such Indebtedness so guaranteed shall be deemed Indebtedness of the Person Guaranteeing such Indebtedness);
(9) all obligations of such Person under Currency Agreements and Interest Rate Agreements (the amount of any such obligations to be equal at any time to the termination value of such agreement or arrangement giving rise to such obligation that would be payable by such Person at such time);
(10) all net obligations of such Person under conditional sale or other title retention agreements relating to assets purchased by such Person;
(11) all outstanding Disqualified Stock issued by such Person with the amount of Indebtedness represented by such Disqualified Stock being equal to the greater of its voluntary or involuntary liquidation preference and its maximum fixed repurchase price (not including, in either case, any redemption or repurchase premium); and
(12) to the extent not otherwise included in this definition, the Receivables Transaction Amount outstanding relating to a Qualified Receivables Transaction entered into by such Person.
For purposes hereof, the “maximum fixed repurchase price” of any Disqualified Stock which does not have a fixed repurchase price shall be calculated in accordance with the terms of such Disqualified Stock as if such
S-233
Table of Contents
Disqualified Stock were purchased on any date on which Indebtedness shall be required to be determined pursuant to the Indenture, and if such price is based upon, or measured by, Fair Market Value, the Fair Market Value of such Disqualified Stock.
The amount of Indebtedness of any Person at any date will be the outstanding balance at such date of all unconditional obligations as described above and the maximum liability, upon the occurrence of the contingency giving rise to the obligation, of any contingent obligations at such date.
In addition, “Indebtedness” of any Person shall include Indebtedness described above in this definition that would not appear as a liability on the balance sheet of such Person if:
(1) such Indebtedness is the obligation of a partnership or joint venture that is not a Restricted Subsidiary, or a Joint Venture;
(2) such Person or a Restricted Subsidiary of such Person is a general partner of the Joint Venture, or a General Partner; and
(3) there is recourse, by contract or operation of law, with respect to the payment of such Indebtedness to property or assets of such Person or a Restricted Subsidiary of such Person;
and then such Indebtedness shall be included in an amount not to exceed:
(a) the lesser of (i) the net assets of the General Partner and (ii) the amount of such obligations to the extent that there is recourse, by contract or operation of law, to the property or assets of such Person or a Restricted Subsidiary of such Person; or
(b) if less than the amount determined pursuant to clause (a) immediately above, the actual amount of such Indebtedness that is recourse to such Person or a Restricted Subsidiary of such Person, if the Indebtedness is evidenced by a writing and is for a determinable amount.
“Interest Rate Agreement” means with respect to any Person any interest rate protection agreement, interest rate future agreement, interest rate option agreement, interest rate swap agreement, interest rate cap agreement, interest rate collar agreement, interest rate hedge agreement or other similar agreement or arrangement as to which such Person is party or a beneficiary.
“Investment” means, with respect to any Person, all investments by such Person in other Persons (including Affiliates) in the form of any direct or indirect advance, loan (other than advances or extensions of credit to customers or trade receivables in the ordinary course of business) or other extensions of credit (including by way of Guarantee or similar arrangement, but excluding any debt or extension of credit represented by a bank deposit other than a time deposit) or capital contribution to (by means of any transfer of cash or other property to others or any payment for property or services for the account or use of others), or any purchase or acquisition of Capital Stock, Indebtedness or other similar instruments issued by, such Person and all other items that are or would be classified as investments on a balance sheet prepared in accordance with GAAP;providedthat none of the following will be deemed to be an Investment:
(1) endorsements of negotiable instruments and documents in the ordinary course of business; and
(2) an acquisition of assets, Capital Stock or other securities by the Company or a Subsidiary for consideration to the extent such consideration consists of Common Stock of the Company.
For purposes of the “Limitation on restricted payments” covenant,
(1) “Investment” will include the portion (proportionate to the Company’s equity interest in a Restricted Subsidiary to be designated as an Unrestricted Subsidiary) of the Fair Market Value of the net assets of such Restricted Subsidiary at the time that such Restricted Subsidiary is designated an Unrestricted Subsidiary;provided,however, that upon a redesignation of such Subsidiary as a Restricted Subsidiary, the
S-234
Table of Contents
Company will be deemed to continue to have a permanent “Investment” in an Unrestricted Subsidiary in an amount (if positive) equal to (a) the Company’s “Investment” in such Subsidiary at the time of such redesignation less (b) the portion (proportionate to the Company’s equity interest in such Subsidiary) of the Fair Market Value of the net assets of such Subsidiary at the time that such Subsidiary is so re-designated a Restricted Subsidiary;
(2) any property transferred to or from an Unrestricted Subsidiary will be valued at its Fair Market Value at the time of such transfer; and
(3) if the Company or any Restricted Subsidiary sells or otherwise disposes of any Capital Stock of any Restricted Subsidiary such that, after giving effect to any such sale or disposition, such entity is no longer a Subsidiary of the Company, the Company shall be deemed to have made an Investment on the date of any such sale or distribution equal to the Fair Market Value of the Capital Stock of that entity not sold or disposed of.
“Investment Grade Rating” means a rating equal to or higher than Baa3 (or the equivalent) by Moody’s and BBB- (or the equivalent) by S&P.
“Issue Date” means the date on which any Notes are first issued under the Indenture.
“Lien” means any mortgage, pledge, security interest, encumbrance, lien or charge of any kind (including any conditional sale or other title retention agreement or lease in the nature thereof).
“Management Services Agreement” means each of those certain agreements by and between the Company or one of its Subsidiaries (or any Physician Group with an existing agreement with the Company or one of its Subsidiaries for the provision of management services) and any Physician Group pursuant to which the Company or one of its Subsidiaries (or Physician Group) provides management services to such Physician Group; and, directly or indirectly, receives a management or similar fee for such services.
“Merger” means the Merger of Seismic Acquisition LLC with and into Healthcare Partners Holdings, LLC as contemplated by the Merger Agreement and all other transactions contemplated thereby.
“Merger Agreement” means Agreement and Plan of Merger, by and among DaVita Inc., Seismic Acquisition LLC and the other parties signatory thereto dated as of May 20, 2012 and, unless otherwise expressly stated or the context otherwise requires, as the same may be amended, supplemented or modified from time to time.
“Moody’s” means Moody’s Investors Service, Inc. or any successor to the rating agency business thereof.
“Net Available Cash” from an Asset Disposition means cash payments received (including any cash payments received by way of deferred payment of principal pursuant to a note or installment receivable or otherwise and net proceeds from the sale or other disposition of any securities received as consideration, but only as and when received, but excluding any other consideration received in the form of assumption by the acquiring Person of Indebtedness or other obligations relating to the properties or assets that are the subject of such Asset Disposition or received in any other non-cash form) therefrom, in each case net of:
(1) all legal, accounting, investment banking, title and recording tax expenses, commissions and other fees and expenses incurred, and all Federal, state, provincial, foreign and local taxes required to be paid or accrued as a liability under GAAP (after taking into account any available tax credits or deductions and any tax sharing agreements), in connection with or as a consequence of such Asset Disposition;
(2) all payments made on any Indebtedness which is secured by any assets subject to such Asset Disposition, in accordance with the terms of any Lien upon such assets, or which must by its terms, or in order to obtain a necessary consent to such Asset Disposition, or by applicable law be repaid out of the proceeds from such Asset Disposition;
(3) all payments made to discharge any severance liabilities arising in connection with such Asset Disposition;
S-235
Table of Contents
(4) all distributions and other payments required to be made to holders of non-controlling interests in Subsidiaries or in joint ventures, limited or general partnerships, limited liability companies or similar business entities or other Persons as a result of such Asset Disposition; and
(5) the deduction of appropriate amounts to be provided by the seller as a reserve, in accordance with GAAP, against any liabilities associated with the assets disposed of in such Asset Disposition and retained by the Company or any Restricted Subsidiary after such Asset Disposition.
“Net Cash Proceeds,” with respect to any issuance or sale of Capital Stock, means the cash proceeds of such issuance or sale net of attorneys’ fees, accountants’ fees, underwriters’ or placement agents’ fees, listing fees, discounts or commissions and brokerage, consultant and other fees and charges actually incurred in connection with such issuance or sale and net of taxes paid or payable as a result of such issuance or sale (after taking into account any available tax credit or deductions and any tax sharing arrangements).
“Nominee Agreement” means, with respect to any Physician Group, any agreement granting the Company or one of its Subsidiaries direct or indirect rights with respect to transfers of equity interests in such Physician Group.
“Non-Recourse Debt” means Indebtedness of a Person:
(1) as to which neither the Company nor any Restricted Subsidiary (a) provides any Guarantee or credit support of any kind (including any undertaking, Guarantee, indemnity, agreement or instrument that would constitute Indebtedness other than any undertakings, indemnities, agreements or instruments which are excluded from the definition of “Guarantee”) or (b) is directly or indirectly liable (as a guarantor or otherwise); and
(2) as to which the lenders have been notified in writing that they will not have any recourse to the stock or assets of the Company or any of its Restricted Subsidiaries (other than the Capital Stock of or other ownership interests in any Unrestricted Subsidiaries).
“Note Guarantee” means, individually, any Guarantee of payment of the Notes by a Subsidiary Guarantor pursuant to the terms of the Indenture and any supplemental indentures thereto, and, collectively, all such Guarantees. Each such Note Guarantee will be in the form prescribed by the Indenture.
“Officer” means the Chairman of the Board, the Chief Executive Officer, the President, the Chief Financial Officer, any Vice President, the Treasurer or the Secretary of the Company. Officer of any Subsidiary Guarantor has a correlative meaning.
“Officers’ Certificate” means a certificate signed by two Officers or by an Officer and either an Assistant Treasurer or an Assistant Secretary of the Company.
“Opinion of Counsel” means a written opinion reasonably acceptable to the Trustee from legal counsel. The counsel may be an employee of or counsel to the Company.
“Parent Entity” means, for purposes of the proviso to the definition of “Change of Control,” a newly created entity having, at the time of consummation of a reorganization transaction permitted by such proviso, no assets with a Fair Market Value in excess of $1.0 million (other than Capital Stock of the Company and its Subsidiaries) and no liabilities with a Fair Market Value in excess of $1.0 million, in each case that would be reflected on an unconsolidated balance sheet of such entity at such time.
“Permitted Business” means the businesses engaged in by the Company and its Subsidiaries on October 20, 2010 as described in the prospectus supplement dated October 5, 2010 relating to the original issuance of the Existing Notes, the related prospectus dated September 30, 2010 and the documents incorporated and deemed to
S-236
Table of Contents
be incorporated by reference in such prospectus supplement or prospectus, and businesses of the types that are reasonably related thereto or that are reasonable extensions thereof and, without limitation to the foregoing, any and all healthcare services businesses and any businesses reasonably related thereto or that are reasonable extensions thereof. For purposes of clarity, it is understood and agreed that a business engaged in by a Person other than the Company and its Subsidiaries is a “Permitted Business” so long as it is the type of business described in the preceding sentence.
“Permitted Indebtedness” has the meaning ascribed to such term in the second paragraph of the “Limitation on indebtedness” covenant.
“Permitted Investment” means an Investment by the Company or any Restricted Subsidiary in:
(1)(a) the Company or a Restricted Subsidiary or a Person which will, upon the making of such Investment, become a Restricted Subsidiary, and (b) any Investment deemed to be made upon the designation of an Unrestricted Subsidiary as a Restricted Subsidiary;
(2) another Person if as a result of such Investment such other Person is merged or consolidated with or into, or transfers or conveys all or substantially all its assets to, the Company or a Restricted Subsidiary;
(3) cash and Cash Equivalents;
(4) payroll, travel, moving, entertainment and similar advances to cover matters that are expected at the time of such advances ultimately to be treated as expenses for accounting purposes and that are made in the ordinary course of business;
(5) Guarantees issued in accordance with the “Limitation on indebtedness” covenant;
(6) Capital Stock, obligations or securities received in settlement of debts created in the ordinary course of business and owing to the Company or any Restricted Subsidiary or in satisfaction of judgments or pursuant to any plan of reorganization or similar arrangement upon the bankruptcy or insolvency of a debtor;
(7) Investments made as a result of the receipt of non-cash consideration from an Asset Disposition that was made pursuant to and in compliance with the “Limitation on sales of assets and subsidiary stock” covenant or a Sale/Leaseback Transaction;
(8)(a) Investments in existence on the Issue Date and any extension, modification or renewal of any such investments existing on the Issue Date, but only to the extent not involving additional advances, contributions or other Investments (of cash or otherwise) or other increases thereof or Guarantees (other than as a result of the accrual or accretion of interest or original issue discount or the issuance by such investee of pay-in-kind securities, in each case, pursuant to the terms of such Investment as in effect on the Issue Date), (b) solely for purposes of the definition of “Restricted Investments,” Investments in existence on October 20, 2010 and any extension, modification or renewal of any such Investments existing on October 20, 2010, but only to the extent not involving additional advances, contributions or other Investments (of cash or otherwise) or other increases thereof or Guarantees (other than as a result of the accrual or accretion of interest or original issue discount or the issuance by such investee of pay-in-kind securities, in each case pursuant to the terms of such Investment as in effect on October 20, 2010) and (c) Investments made pursuant to the Merger Agreement and any related agreement;
(9) Currency Agreements, Interest Rate Agreements and related Hedging Obligations, which transactions or obligations are both Incurred in compliance with the “Limitation on indebtedness” covenant and of the type described in clause (5) of the definition of “Permitted Indebtedness”;
(10) Investments by the Company or any of its Restricted Subsidiaries, together with all other Investments pursuant to this clause (10), in an aggregate amount at the time of such Investment not to exceed $100.0 million outstanding at any one time (with the Fair Market Value of such Investment being measured at the time made and without giving effect to subsequent changes in value);
(11) any Investment received in exchange for the Capital Stock of an Unrestricted Subsidiary and Investments owned by an Unrestricted Subsidiary upon its redesignation as a Restricted Subsidiary;
S-237
Table of Contents
(12) Investments of the Company or any Restricted Subsidiary in any Special Purpose Licensed Entity which, when aggregated with the aggregate amount of all obligations Guaranteed pursuant to clause (13) of the definition of “Permitted Indebtedness,” shall not exceed $150.0 million at any time outstanding;
(13) Investments by the Company or a Restricted Subsidiary in connection with a Qualified Receivables Transaction;
(14) Investments in any Person to the extent such Investments consist of prepaid expenses, negotiable instruments held for collection, and lease, workers’ compensation, performance and similar deposits made in the ordinary course of business by the Company or any Restricted Subsidiary;
(15) any Investment by the Company or a Restricted Subsidiary in a Permitted Business having an aggregate Fair Market Value, taken together with all other Investments made pursuant to this clause (15) that are at that time outstanding, not to exceed $250.0 million;
(16) any Eligible Escrow Investments made as contemplated by the provisions described under “Escrow of proceeds; release conditions”; and
(17) Investments pursuant to any Permitted Physician Group Loan.
“Permitted Liens” means, with respect to any Person:
(1) Liens securing Indebtedness under one or more Senior Credit Facilities or other Indebtedness Incurred in accordance with the Limitation on indebtedness covenant in an aggregate principal amount outstanding that does not exceed the greater of (A) the aggregate principal amount of Indebtedness permitted to be outstanding under clause (1) of the definition of “Permitted Indebtedness” and (B) the maximum principal amount such that the Secured Indebtedness Leverage Ratio would not exceed 3.5 to 1.0, in each case calculated on a pro forma basis at the time any Indebtedness secured by a Lien pursuant to this clause (1) is Incurred and after giving effect to the Incurrence of such Indebtedness and the application of the proceeds therefrom;
(2) pledges or deposits by such Person under workmen’s compensation laws, unemployment insurance laws, social security laws or similar legislation or regulations or deposits in connection with bids, tenders, contracts (other than for the payment of Indebtedness) or leases to which such Person is a party, or deposits to secure public or statutory obligations of such Person or deposits of cash or United States government bonds to secure surety or appeal bonds to which such Person is a party, or deposits as security for contested taxes or import or customs duties or for the payment of rent, or deposits or other security securing liabilities to insurance carriers under insurance or self-insurance arrangements in each case Incurred in the ordinary course of business;
(3) Liens imposed by law, including carriers’, warehousemen’s, materialmen’s, repairmen’s and mechanics’ Liens, in each case for sums not more than 60 days past due or being contested in good faith by appropriate proceedings if a reserve or other appropriate provisions, if any, as shall be required by GAAP shall have been made in respect thereof;
(4) Liens for taxes, assessments or other governmental charges not yet subject to penalties for non-payment or which are being contested in good faith by appropriate proceedings provided appropriate provisions, if any, required pursuant to GAAP have been made in respect thereof;
(5) Liens in favor of issuers of surety, indemnity, bid, warranty, release, appeal or performance bonds or letters of credit or bankers’ acceptances issued, and completion guarantees provided for, pursuant to the request of and for the account of such Person in the ordinary course of its business;provided,however, that such letters of credit do not constitute an obligation for money borrowed;
(6) encumbrances, ground leases, easements or reservations of, or rights of others for, licenses, rights of way, sewers, electric lines, telegraph and telephone lines and other similar purposes, or zoning, building codes or other restrictions (including, without limitation, minor defects or irregularities in title and similar
S-238
Table of Contents
encumbrances) as to the use of real properties or Liens incidental to the conduct of the business of such Person or to the ownership of its properties which do not in the aggregate materially adversely affect the value of said properties or materially impair their use in the operation of the business of such Person;
(7) Liens securing Hedging Obligations so long as the related Indebtedness is, and is permitted to be under the Indenture, secured by a Lien on the same property securing such Hedging Obligation;
(8) leases, licenses, subleases and sublicenses of assets (including, without limitation, real property and intellectual property rights) which do not materially interfere with the ordinary conduct of the business of the Company or any of its Restricted Subsidiaries;
(9) judgment Liens not giving rise to an Event of Default so long as such Lien is adequately bonded or appropriate reserves have been established as required by GAAP, if any;
(10) Liens for the purpose of securing the payment of all or a part of the purchase price of, or Capitalized Lease Obligations, purchase money obligations or other payments Incurred to finance the acquisition, improvement or construction of, assets or property acquired or constructed in the ordinary course of business;providedthat:
(a) the aggregate principal amount of Indebtedness secured by such Liens is otherwise permitted to be Incurred under the Indenture and does not exceed the cost of the assets or property so acquired or constructed; and
(b) such Liens are created within 180 days after the completion of the construction or acquisition of such assets or property and do not encumber any other assets or property of the Company or any Restricted Subsidiary other than such assets or property and assets affixed or appurtenant thereto or proceeds thereof;
(11) banker’s Liens, rights of set-off or similar rights and remedies as to deposit accounts or other funds maintained with a depositary institution;providedthat:
(a) such deposit account is not a dedicated cash collateral account and is not subject to restrictions against access by the Company in excess of those set forth by regulations promulgated by the Federal Reserve Board; and
(b) such deposit account is not intended by the Company or any Restricted Subsidiary to provide collateral to the depository institution;
(12) Liens arising from Uniform Commercial Code financing statement filings regarding operating leases entered into by the Company and its Restricted Subsidiaries in the ordinary course of business;
(13)(a) Liens existing on the Issue Date and (b) Liens existing pursuant to the Escrow Agreement;
(14) Liens on property or assets (including improvements, accessions and proceeds in respect thereof) or shares of Capital Stock of a Person at the time such Person becomes a Restricted Subsidiary;provided,however, that such Liens are not created, Incurred or assumed in connection with, or in contemplation of, such other Person becoming a Restricted Subsidiary;provided further,however, that any such Lien may not extend to any other property owned by the Company or any Restricted Subsidiary;
(15) Liens on property or assets (including improvements, accessions and proceeds in respect thereof) at the time the Company or a Restricted Subsidiary acquired such property or assets, including any acquisition by means of a merger or consolidation with or into the Company or any Restricted Subsidiary;provided,however, that such Liens are not created, Incurred or assumed in connection with, or in contemplation of, such acquisition;provided further,however, that such Liens may not extend to any other property owned by the Company or any Restricted Subsidiary;
(16) Liens securing Indebtedness or other obligations of a Restricted Subsidiary owing to the Company or another Restricted Subsidiary;
(17) Liens securing the Notes and any Guarantees thereof;
S-239
Table of Contents
(18) Liens securing Refinancing Indebtedness Incurred to refinance, refund, replace, amend, extend or modify Indebtedness that was previously so secured not in violation of the Indenture;providedthat any such Lien is limited to all or part of the same property or assets (plusimprovements, accessions, proceeds or dividends or distributions in respect thereof) that secured (or, under the written arrangements under which the original Lien arose, could secure) the Indebtedness being refinanced or is in respect of property that is the security for a Permitted Lien hereunder;
(19) any interest or title of a lessor under any Capitalized Lease Obligation or operating lease;
(20) Liens in favor of the Company or a Restricted Subsidiary;
(21) Liens under industrial revenue, municipal or similar bonds;
(22) Liens in connection with dispositions of self-pay receivables in the ordinary course of business, which the Company or any of its Restricted Subsidiaries believe in good faith cannot be paid in full;
(23) Liens securing Indebtedness Incurred pursuant to clause (16) of the definition of “Permitted Indebtedness”;provided,however, that such Liens do not extend to the assets or property of the Company or any Subsidiary Guarantor;
(24) Liens on assets that are the subject of a Qualified Receivables Transaction;
(25) customary non-assignment provisions in leases and other agreements entered into by the Company or any Restricted Subsidiary in the ordinary course of business;
(26) (x) Liens securing Indebtedness Incurred pursuant to Sale/Leaseback Transactions entered into in compliance with clause (15) of the definition of “Permitted Indebtedness,” but only to the extent that such Liens attach to the assets or property being financed pursuant to such Sale/Leaseback Transactions and do not encumber any other assets or property of the Company or its Restricted Subsidiaries, and (y) Liens securing Indebtedness Incurred in connection with any Sale/Leaseback Transaction entered into in respect of the Company’s headquarters facility (including, without limitation, land, building, improvements and related assets) in Denver, Colorado in an aggregate principal amount not to exceed $125.0 million at any time outstanding (it being understood that, to the extent that Indebtedness of the type described in this clause (y) exceeds $125.0 million, then the amount in excess of $125.0 million may be secured by other Permitted Liens or otherwise in a manner that complies with the “Limitation on liens” covenant);
(27) Liens and setoff rights securing obligations in respect of, or arising in connection with, cash pooling arrangements so long as any Indebtedness under any such cash pooling arrangement complies with the “Limitation on indebtedness” covenant; and
(28) in addition to the items referred to in clauses (1) through (27) above, Liens securing Indebtedness of the Company and its Restricted Subsidiaries in an aggregate principal amount which, when taken together with the aggregate principal amount of all other Indebtedness of the Company and its Restricted Subsidiaries secured by Liens Incurred pursuant to this clause (28) and then outstanding, will not exceed $150.0 million.
“Permitted Physician Group Loans” means loans or advances to any Physician Group which funds may be used contemporaneously to finance the acquisition of the equity interests or assets of one or more additional Physician Groups and any Subsidiaries thereof (excluding Subsidiaries organized or acquired in contemplation of such transaction);provided that (1) immediately before and immediately after giving pro forma effect to any such loan or advance, no Default shall have occurred and be continuing, (2) any additional Physician Group acquired as an entity pursuant to the foregoing shall enter into a Management Services Agreement with the Company or any of its Subsidiaries (or a Physician Group with a Management Services Agreement with the Company or any of its Subsidiaries) and Nominee Agreements and (3) any acquisition of an additional Physician Group shall be consummated in compliance with all applicable laws in all material respects.
“Person” means any individual, corporation, partnership, joint venture, association, joint-stock company, trust, unincorporated organization, limited liability company, government or any agency or political subdivision thereof or any other entity.
S-240
Table of Contents
“Physician Groups” means HealthCare Partners Affiliates Medical Group, Seismic Medical Group, HealthCare Partners Medical Group (Bacchus), Ltd., JSA Professional Association, Healthcare Partners Medical Group, Inc., Physician Associates of the Greater San Gabriel Valley, a Medical Group Inc., Northridge Medical Group, Inc., Talbert Medical Group, Inc. and any other professional corporation, limited liability company, partnership or other entity that, directly or indirectly, provides or arranges medical services and (i) provides or arranges such services in a state that only permits the equity interests of such entity to be held by one or more licensed physicians or licensed professionals or professional entities and (ii) has entered into a Management Services Agreement and Nominee Agreements.
“Preferred Stock” as applied to the Capital Stock of any corporation, means Capital Stock of any class or classes (however designated) which is preferred as to the payment of dividends, or as to the distribution of assets upon any voluntary or involuntary liquidation or dissolution of such corporation, over shares of Capital Stock of any other class of such corporation.
“Qualified Issuer” means any commercial bank that has a combined capital and surplus in excess of $500.0 million.
“Qualified Proceeds” means assets that are used or useful in, or Capital Stock of any Person engaged in, a Permitted Business.
“Qualified Receivables Transaction” means any sale, factoring or securitization transaction involving Receivables that may be entered into by the Company or any of its Restricted Subsidiaries pursuant to which the Company or any of its Restricted Subsidiaries may sell, convey or otherwise transfer, or may grant a security interest in, any Receivables (whether existing on the Issue Date or arising thereafter) of the Company or any of its Restricted Subsidiaries, and any assets related thereto including, without limitation, all collateral securing such Receivables, all bank accounts specifically designated for the collection of such Receivables, all contracts and all guarantees or other obligations in respect of such Receivables, the proceeds of such Receivables and other assets which are customarily transferred, or in respect of which security interests are customarily granted, in connection with sales, factoring or securitizations involving Receivables.
“Rating Agencies” means Moody’s and S&P.
“Receivable” means a right to receive payment arising from a sale or lease of goods or the performance of services by a Person pursuant to an arrangement with another Person pursuant to which such other Person is obligated to pay for goods or services under terms that permit the purchase of such goods and services on credit and all proceeds thereof and rights (contractual or otherwise) and collateral related thereto and shall include, in any event, any items of property that would be classified as an account receivable of the Company or any of its Subsidiaries or an “account,” “chattel paper,” “payment intangible” or “instrument” under the Uniform Commercial Code as in effect in the State of New York and any “supporting obligations” or “proceeds” as so defined of any such items.
“Receivables Subsidiary” means any Subsidiary formed for the purpose of facilitating or entering into one or more Qualified Receivables Transactions, and that engages only in activities reasonably related or incidental thereto.
“Receivables Transaction Amount” means (a) in the case of any Receivables securitization (but excluding any sale or factoring of Receivables), the amount of obligations outstanding under the legal documents entered into as part of such Receivables securitization on any date of determination that would be characterized as principal if such Receivables securitization were structured as a secured lending transaction rather than as a purchase and (b) in the case of any sale or factoring of Receivables, the cash purchase price paid by the buyer in connection with its purchase of Receivables (including any bills of exchange) less the amount of collections received in respect of such Receivables and paid to such buyer, excluding any amounts applied to purchase fees
S-241
Table of Contents
or discount or in the nature of interest, in each case as determined in good faith and in a consistent and commercially reasonable manner by the Company.
“refinance” means to refinance, repay, prepay, replace, exchange, renew, extend or refund; “refinanced” and “refinances” shall have correlative meanings.
“Refinancing Indebtedness” means Indebtedness that is Incurred to refinance (including pursuant to any defeasance or discharge mechanism) any Indebtedness existing on the Issue Date or Incurred in compliance with the Indenture, including Indebtedness that refinances Refinancing Indebtedness;provided,however, that:
(1) (a) if the Stated Maturity of the Indebtedness being refinanced, or Refinanced Indebtedness, is earlier than the Stated Maturity of the Notes, the Refinancing Indebtedness has a Stated Maturity no earlier than the Stated Maturity of the Refinanced Indebtedness or (b) if the Stated Maturity of the Refinanced Indebtedness is later than the Stated Maturity of the Notes, the Refinancing Indebtedness has a Stated Maturity at least 91 days later than the Stated Maturity of the Notes;
(2) the Refinancing Indebtedness has an Average Life at the time such Refinancing Indebtedness is Incurred that is equal to or greater than the Average Life of the Refinanced Indebtedness;
(3) such Refinancing Indebtedness is Incurred in an aggregate principal amount (or if issued with original issue discount, an aggregate issue price) that is equal to or less than the sum of the aggregate principal amount (or if issued with original issue discount, the aggregate accreted value) then outstanding of the Refinanced Indebtedness (plus, without duplication, any additional Indebtedness Incurred to pay interest or dividends owed thereon, any reasonable premium (or premium required to be paid pursuant to the instruments governing such Refinancing Indebtedness) paid to the holders of the Refinanced Indebtedness and reasonable fees and expenses Incurred in connection therewith);
(4) if the Refinanced Indebtedness is subordinated in right of payment to the Notes or the Note Guarantees, such Refinancing Indebtedness is subordinated in right of payment to the Notes or the Note Guarantees thereof, as the case may be, on terms at least as favorable to the Holders of the Notes as those contained in the documentation governing the Refinanced Indebtedness;
(5) the obligor of Refinancing Indebtedness is the same Person as the obligor of the Refinanced Indebtedness; and
(6) the proceeds of the Refinancing Indebtedness shall be used substantially concurrently with the Incurrence thereof to redeem or refinance (including pursuant to any defeasance or discharge mechanism) the Refinanced Indebtedness, unless the Refinanced Indebtedness is not then due and is not redeemable or prepayable at the option of the obligor thereof or is redeemable or prepayable only with notice or lapse of time, in which case such proceeds shall be held in a segregated account until the Refinanced Indebtedness becomes due or redeemable or prepayable or such notice or time period lapses and then shall be used to refinance the Refinanced Indebtedness;providedthat in any event the Refinanced Indebtedness shall be redeemed or refinanced within one year of the Incurrence of the Refinancing Indebtedness.
“Replacement Assets” means:
(1) other properties or assets to replace the properties or assets that were the subject of the Asset Disposition;
(2) properties and assets that are or will be used or useful in businesses of the Company or its Restricted Subsidiaries or a Permitted Business; or
(3) any Permitted Business or Capital Stock of a Person operating in a Permitted Business to the extent not otherwise prohibited by the Indenture.
“Restricted Investment” means any Investment other than a Permitted Investment.
“Restricted Payment” has the meaning ascribed to such term in the first paragraph of the “Limitation on restricted payments” covenant.
S-242
Table of Contents
“Restricted Payments Basket” has the meaning ascribed to such term in the first paragraph of the “Limitation on restricted payments” covenant.
“Restricted Subsidiary” means any Subsidiary of the Company other than an Unrestricted Subsidiary.
“S&P” means Standard & Poor’s Ratings Services or any successor to the rating agency business thereof.
“Sale/Leaseback Transaction” means an arrangement relating to property owned on the date of the Indenture or thereafter acquired whereby the Company or a Restricted Subsidiary transfers such property to a Person and the Company or a Restricted Subsidiary leases it from such Person.
“SEC” means the United States Securities and Exchange Commission.
“Secured Indebtedness” means any Indebtedness of the Company or any of its Restricted Subsidiaries for borrowed money that is secured by a Lien on any property of the Company or any of its Restricted Subsidiaries and which Lien arises under any instrument or agreement to which the Company or any of its Restricted Subsidiaries is a party or by which any of them is bound.
“Secured Indebtedness Leverage Ratio” means, as of any date of determination, with respect to the Company and its consolidated Restricted Subsidiaries, the ratio of (x) the aggregate amount of all Secured Indebtedness of the Company and its consolidated Restricted Subsidiaries (“Consolidated Total Secured Indebtedness”) as of the last day of the period of the most recent four consecutive fiscal quarters ending prior to the date of determination for which financial statements are in existence to (y) the aggregate amount of Consolidated EBITDA of the Company and its consolidated Restricted Subsidiaries for such period, all calculated on consolidated basis in accordance with GAAP. For purposes of calculating the Secured Indebtedness Leverage Ratio, Consolidated EBITDA shall, if necessary, be calculated on a pro forma basis in a manner consistent with the proviso to the first sentence of the definition of “Consolidated Coverage Ratio”; and Consolidated Total Secured Indebtedness shall, if necessary, be calculated on a pro forma basis as follows:
if the Company or any Restricted Subsidiary:
(a) has Incurred any Indebtedness since the last day of the applicable four quarter period that remains outstanding on the applicable date of determination or if the transaction giving rise to the need to calculate the Secured Indebtedness Leverage Ratio includes the Incurrence of Indebtedness, Consolidated Total Secured Indebtedness will be calculated after giving effect on a pro forma basis to such Indebtedness as if such Indebtedness had been Incurred on the last day of such period and the discharge of any other Indebtedness repaid, repurchased, defeased or otherwise discharged with the proceeds of such new Indebtedness as if such discharge had occurred on the last day of such period; or
(b) has repaid, repurchased, defeased or otherwise discharged any Indebtedness since the last of such period that is no longer outstanding on such date of determination or if the transaction giving rise to the need to calculate the Secured Indebtedness Leverage Ratio includes a discharge of Indebtedness, Consolidated Total Secured Indebtedness will be calculated after giving effect on a pro forma basis to such discharge of such Indebtedness, including with the proceeds of such new Indebtedness, as if such discharge had occurred on the last day of such period.
All such pro forma calculations shall be made in a manner consistent with the second paragraph of the definition of “Consolidated Coverage Ratio.” In addition, the calculation of the Secured Indebtedness Leverage Ratio shall be made in a manner consistent with the third paragraph, the fourth paragraph and the sixth paragraph under the “Limitation on indebtedness” covenant above, mutatis mutandis.
“Securities Act” means the United States Securities Act of 1933, as amended, and the rules and regulations of the SEC promulgated thereunder.
“Senior Credit Agreement” means the Credit Agreement, dated as of October 20, 2010, as supplemented by that certain Increase Joinder Agreement dated as of August 20, 2011, and as further amended prior to or
S-243
Table of Contents
substantially concurrently with the consummation of the Merger, among the Company, the guarantors party thereto, the several banks and other financial institutions or entities from time to time lenders thereunder and JPMorgan Chase Bank, N.A., as administrative agent and collateral agent, including any related letters of credit, Guarantees, collateral documents, instruments and agreements executed in connection therewith, and in each case as the same may be amended, restated, modified, renewed, refunded, replaced or refinanced in whole or in part from time to time (including increasing the amount loaned thereunder, extending the maturity of any Indebtedness thereunder or contemplated thereby or deleting, adding or substituting one or more parties thereto or with different parties (whether or not such added, substituted or different parties are banks or other institutional lenders)).
“Senior Credit Facilities” means, with respect to the Company or any Restricted Subsidiary, one or more debt or credit facilities (including the Senior Credit Agreement), commercial paper facilities, indentures or other financing arrangements providing for revolving credit loans, term loans, letters of credit or other Indebtedness, including any notes, mortgages, guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements or refundings thereof and any debt or credit facilities, commercial paper facilities, indentures or other financing arrangements that replace, refund or refinance any part of any such debt or credit facilities, commercial paper facilities, indentures, other financing arrangements, loans, letters of credit or other Indebtedness, whether by or with the same or any other agents, lenders or group of lenders, investors or other providers of financing. For the avoidance of doubt, (x) Indebtedness Incurred under the Senior Credit Facilities after October 10, 2010 and the Existing Notes issued on October 20, 2010 (and in each case any Guarantees thereof and related Hedging Obligations) shall not be deemed to be Indebtedness under the Senior Credit Facilities Incurred or outstanding on the Release Date and (y) Indebtedness in respect of debt securities and Qualified Receivables Transactions and other Indebtedness, and Guarantees thereof and Hedging Obligations relating thereto, may, at the Company’s option, be Incurred after October 20, 2010 under the Coverage Ratio Exception or any clause of the definition of “Permitted Indebtedness” (so long as the Indebtedness in respect of such debt securities or Qualified Receivables Transactions or other Indebtedness, as the case may be, is permitted to be Incurred thereunder) and, the Company may, at its option, classify and reclassify Indebtedness in respect of any such debt securities and Qualified Receivables Transactions and other Indebtedness and Guarantees and Hedging Obligations in respect thereof, in whole or in part, as being or not being Senior Credit Facilities at the date of Incurrence and from time to time thereafter.
“Senior Indebtedness” means whether outstanding on the Issue Date or thereafter issued, created, Incurred or assumed, all amounts payable by the Company under or in respect of Indebtedness of the Company, including premiums and accrued and unpaid interest (including interest accruing on or after the filing of any petition in bankruptcy or for reorganization relating to the Company at the rate specified in the documentation with respect thereto whether or not a claim for post-filing interest is allowed in such proceeding) and fees relating thereto;provided,however, that Senior Indebtedness will not include:
(1) any Indebtedness Incurred in violation of the Indenture;
(2) any obligation of the Company to any Subsidiary;
(3) any liability for Federal, state, foreign, local or other taxes owed or owing by the Company;
(4) any accounts payable or other liability to trade creditors arising in the ordinary course of business (including Guarantees thereof or instruments evidencing such liabilities);
(5) any Indebtedness, Guarantee or obligation of the Company that is expressly subordinate or junior in right of payment to any other Indebtedness, Guarantee or obligation of the Company, including, without limitation, any Subordinated Obligations; or
(6) any Capital Stock.
“Senior Management” means the Chairman of the Board (if an officer), President, Chief Executive Officer, Chief Operating Officer or Chief Financial Officer of the Company.
S-244
Table of Contents
“Significant Subsidiary” means any Restricted Subsidiary that would be a “Significant Subsidiary” of the Company within the meaning of Rule 1-02 under Regulation S-X promulgated by the SEC.
“Special Purpose Licensed Entity” means any Person in a business related to any business of the Company and the Restricted Subsidiaries that (i) the Company and its Restricted Subsidiaries are prohibited from engaging in directly under applicable law, including provisions of state law (a) prohibiting the ownership of healthcare facilities by public companies, (b) prohibiting the corporate practice of medicine or (c) otherwise restricting the ability of the Company or one of its Restricted Subsidiaries to acquire directly a required license to operate a healthcare facility, and (ii) has entered into a transaction or series of transactions with the Company or any of its Restricted Subsidiaries under which:
(x) the Company or any of its Restricted Subsidiaries provides management, administrative or consulting services to the Special Purpose Licensed Entity;
(y) the owners of the Special Purpose Licensed Entity are prohibited from transferring any of their interests in the Special Purpose Licensed Entity without the consent of the Company or one of its Restricted Subsidiaries; and
(z) the Company or one of its Subsidiaries has the right to require the owners of the Special Purpose Licensed Entity to transfer all of their interests in the Special Purpose Licensed Entity to a Person designated by the Company or one of its Restricted Subsidiaries.
“Stated Maturity” means, with respect to any security or Indebtedness, the date specified in such security or the instrument or agreement pursuant to which such Indebtedness was incurred, as the case may be, as the fixed date on which the payment of principal of such security or Indebtedness is due and payable, including pursuant to any mandatory redemption provision, but shall not include any contingent obligations to repay, redeem or repurchase any such principal prior to the date originally scheduled for the payment thereof.
“Subordinated Obligation” means any Indebtedness of the Company which is subordinate or junior in right of payment to the Notes pursuant to a written agreement.
“Subsidiary” of any Person means (a) any corporation, association or other business entity (other than a partnership, joint venture, limited liability company or similar entity) of which more than 50% of the total ordinary voting power of shares of Capital Stock entitled (without regard to the occurrence of any contingency) to vote in the election of directors, managers or trustees thereof (or persons performing similar functions) or (b) any partnership, joint venture, limited liability company or similar business entity of which more than 50% of the capital accounts, distribution rights, total equity and voting interests or general or limited partnership interests, as applicable, is, in the case of clauses (a) and (b), at the time owned or controlled, directly or indirectly, by (1) such Person, (2) such Person and one or more Subsidiaries of such Person or (3) one or more Subsidiaries of such Person. Unless otherwise specified herein, each reference to a Subsidiary will refer to a Subsidiary of the Company.
“Subsidiary Guarantor” means each Subsidiary of the Company in existence on the Issue Date that provides a Note Guarantee of the Notes on the Issue Date and any other Restricted Subsidiary that provides a Note Guarantee of the Notes in accordance with the Indenture;providedthat upon the release or discharge of such Person from its Note Guarantee in accordance with the Indenture, such Person ceases to be a Subsidiary Guarantor.
“Total Tangible Assets” means as of any date, the total amount of tangible assets of the Company and the Restricted Subsidiaries on a consolidated basis at the end of the fiscal quarter immediately preceding such date.
“Treasury Rate” means, as of the applicable redemption date for any Notes, the yield to maturity as of such redemption date of United States Treasury securities with a constant maturity (as compiled and published in the
S-245
Table of Contents
most recent Federal Reserve Statistical Release H.15(519) that has become publicly available at least two Business Days prior to such redemption date (or, if such Statistical Release is no longer published, any publicly available source of similar market data)) most nearly equal to the period from such redemption date to , 2017;provided,however, that if the period from such redemption date to , 2017 is less than one year, the weekly average yield on actively traded United States Treasury securities adjusted to a constant maturity of one year will be used.
“Unrestricted Subsidiary” means:
(1) any Subsidiary of the Company that at the time of determination shall be designated an Unrestricted Subsidiary by the Board of Directors of the Company in the manner provided below; and
(2) any Subsidiary of an Unrestricted Subsidiary.
The Board of Directors of the Company may designate any Subsidiary of the Company (including any newly acquired or newly formed Subsidiary or a Person becoming a Subsidiary through merger or consolidation or Investment therein) to be an Unrestricted Subsidiary only if:
(x) (1) such Subsidiary or any of its Subsidiaries does not own any Capital Stock or Indebtedness of or have any Investment in, or own or hold any Lien on any property of, any other Subsidiary of the Company which is not a Subsidiary of the Subsidiary to be so designated or otherwise an Unrestricted Subsidiary;
(2) all the Indebtedness of such Subsidiary and its Subsidiaries shall, at the date of designation, and will at all times thereafter, consist of Non-Recourse Debt;
(3) such designation and the Investment of the Company in such Subsidiary complies with the “Limitation on restricted payments” covenant;
(4) such Subsidiary, either alone or in the aggregate with all other Unrestricted Subsidiaries, does not operate, directly or indirectly, all or substantially all of the business of the Company and its Subsidiaries;
(5) such Subsidiary is a Person with respect to which neither the Company nor any of its Restricted Subsidiaries has any direct or indirect obligation:
(a) to subscribe for additional Capital Stock of such Person; or
(b) to maintain or preserve such Person’s financial condition or to cause such Person to achieve any specified levels of operating results; and
(6) on the date such Subsidiary is designated an Unrestricted Subsidiary, such Subsidiary is not a party to any agreement, contract, arrangement or understanding with the Company or any Restricted Subsidiary with terms, taken as a whole, substantially less favorable to the Company than those that might have been obtained from Persons who are not Affiliates of the Company; or
(y) with respect to any Receivables Subsidiary, such Subsidiary is designated as an Unrestricted Subsidiary in accordance with the following paragraph.
Any such designation by the Board of Directors of the Company shall be evidenced to the Trustee by filing with the Trustee a resolution of the Board of Directors of the Company giving effect to such designation and an Officers’ Certificate certifying that such designation complies with the foregoing conditions. If, at any time, any Unrestricted Subsidiary (other than a Receivables Subsidiary) would fail to meet the foregoing requirements as an Unrestricted Subsidiary, it shall thereafter cease to be an Unrestricted Subsidiary for purposes of the Indenture and any Indebtedness of such Subsidiary shall be deemed to be Incurred as of such date.
The Board of Directors of the Company may designate any Unrestricted Subsidiary to be a Restricted Subsidiary;providedthat immediately after giving effect to such designation, no Default or Event of Default shall have occurred and be continuing or would occur as a consequence thereof and the Company could Incur at
S-246
Table of Contents
least $1.00 of additional Indebtedness under the Coverage Ratio Exception on apro formabasis taking into account such designation.
“U.S. Government Obligations” means securities that are (a) direct obligations of the United States of America for the timely payment of which its full faith and credit is pledged or (b) obligations of a Person controlled or supervised by and acting as an agency or instrumentality of the United States of America the timely payment of which is unconditionally guaranteed as a full faith and credit obligation of the United States of America, which in either case, are not callable or redeemable at the option of the issuer thereof, and shall also include a depositary receipt issued by a bank (as defined in Section 3(a)(2) of the Securities Act), as custodian with respect to any such U.S. Government Obligations or a specific payment of principal of or interest on any such U.S. Government Obligations held by such custodian for the account of the holder of such depositary receipt;providedthat (except as required by law) such custodian is not authorized to make any deduction from the amount payable to the holder of such depositary receipt from any amount received by the custodian in respect of the U.S. Government Obligations or the specific payment of principal of or interest on the U.S. Government Obligations evidenced by such depositary receipt.
“Voting Stock” of a corporation means all classes of Capital Stock of such corporation then outstanding and normally entitled to vote in the election of directors.
“Wholly-Owned Restricted Subsidiary” means a Restricted Subsidiary, all of the Capital Stock of which (other than directors’ qualifying shares) is owned by the Company or another Wholly-Owned Restricted Subsidiary.
S-247
Table of Contents
The notes initially will be represented by one or more permanent global certificates in definitive, fully registered form, which we refer to as the Global Notes. The Global Notes will be deposited upon issuance with The Depository Trust Company, New York, New York, or DTC, as depository, or Depository, and registered in the name of a nominee of DTC.
The Global Notes
DTC has advised us that pursuant to procedures established by it (i) upon the issuance of the Global Notes, DTC or its custodian will credit, on its internal system, the principal amount at maturity of the individual beneficial interests represented by such Global Notes to the respective accounts of persons who have accounts with DTC and (ii) ownership of beneficial interests in the Global Notes will be shown on, and the transfer of such ownership will be effected only through, records maintained by DTC or its nominee (with respect to interests of participants) and the records of participants (with respect to interests of persons other than participants). Ownership of beneficial interests in the Global Notes will be limited to persons who have accounts with DTC (“participants”) or persons who hold interests through participants. Investors may hold their interests in the Global Notes directly through DTC if they are participants in such system, or indirectly through organizations that are participants in such system.
So long as DTC, or its nominee, is the registered owner or holder of the Global Notes, DTC or such nominee, as the case may be, will be considered the sole owner or holder of the notes represented by such Global Notes for all purposes under the indenture governing the notes. No beneficial owner of an interest in the Global Notes will be able to transfer that interest except in accordance with DTC’s procedures, in addition to those provided for under the indenture with respect to the notes.
Payments of the principal of, premium, if any, and interest on, the Global Notes will be made to DTC or its nominee, as the case may be, as the registered owner of the Global Notes. None of the Company, any guarantor, the trustee or any paying agent under the indenture governing the notes will have any responsibility or liability for any aspect of the records relating to or payments made on account of beneficial ownership interests in the Global Notes or for maintaining, supervising or reviewing any records relating to such beneficial ownership interests.
DTC has advised us that its present practice is, upon receipt of any payment of principal, premium, if any, and interest on the Global Notes, to credit immediately participants’ accounts with payments in amounts proportionate to their respective beneficial interests in the principal amount of the Global Notes as shown on the records of DTC. Payments by participants to owners of beneficial interests in the Global Notes held through such participants will be governed by standing instructions and customary practice, as is now the case with securities held for the accounts of customers registered in the names of nominees for such customers. Such payments will be the responsibility of such participants.
Transfers between participants in DTC will be effected in the ordinary way through DTC’s same-day funds system in accordance with DTC rules and will be settled in same-day funds. Holders will not be entitled to receive notes in physical form except under the limited circumstances set forth below.
DTC has advised us that it will take any action permitted to be taken by a holder of notes, including the presentation of notes for exchange as described below, only at the direction of one or more participants to whose account interests in the Global Notes are credited and only in respect of such portion of the aggregate principal amount of notes as to which such participant or participants has or have given such direction.
DTC has advised us as follows: DTC is a limited purpose trust company organized under the laws of the State of New York, a member of the Federal Reserve System, a “clearing corporation” within the meaning of the
S-248
Table of Contents
Uniform Commercial Code and a “clearing agency” registered pursuant to the provisions of Section 17A of the Securities Exchange Act of 1934, as amended. DTC was created to hold securities for its participants and facilitate the clearance and settlement of securities transactions between participants through electronic book-entry changes in accounts of its participants, thereby eliminating the need for physical movement of certificates. Participants include securities brokers and dealers, banks, trust companies and clearing corporations and certain other organizations. Indirect access to the DTC system is available to others such as banks, brokers, dealers and trust companies that clear through or maintain a custodial relationship with a participant, either directly or indirectly, or indirect participants.
Although DTC has agreed to the foregoing procedures in order to facilitate transfers of interests in the Global Notes among participants of DTC, it is under no obligation to perform such procedures, and such procedures may be discontinued at any time. Neither we nor the trustee will have any responsibility for the performance by DTC or its participants or indirect participants of their respective obligations under the rules and procedures governing their operations.
Clearstream. Clearstream is incorporated under the laws of Luxembourg as a professional depositary. Clearstream holds securities for its participating organizations, or Clearstream Participants, and facilitates the clearance and settlement of securities transactions between Clearstream Participants through electronic book-entry changes in accounts of Clearstream Participants, thereby eliminating the need for physical movement of certificates. Clearstream interfaces with domestic markets in several countries. As a professional depositary, Clearstream is subject to regulation by the Luxembourg Monetary Institute. Clearstream Participants are financial institutions around the world, including securities brokers and dealers, banks, trust companies, clearing corporations and certain other organizations, and may include the underwriters. Indirect access to Clearstream is also available to others, such as banks, brokers, dealers and trust companies that clear through or maintain a custodial relationship with a Clearstream Participant either directly or indirectly.
Distributions with respect to notes held beneficially through Clearstream will be credited to cash accounts of Clearstream Participants in accordance with its rules and procedures to the extent received by DTC for Clearstream.
Euroclear. Euroclear was created to hold securities for participants of Euroclear, or Euroclear Participants, and to clear and settle transactions between Euroclear Participants through simultaneous electronic book-entry delivery against payment, thereby eliminating the need for physical movement of certificates and any risk from lack of simultaneous transfers of securities and cash. Euroclear is operated by Euroclear Bank S.A./N.V., or Euroclear Operator, under contract with Euroclear Clearance Systems S.C., a Belgian cooperative corporation, or Cooperative. All operations are conducted by the Euroclear Operator, and all Euroclear securities clearance accounts and Euroclear cash accounts are accounts with the Euroclear Operator, not the Cooperative. The Cooperative establishes policy for Euroclear on behalf of Euroclear Participants. Euroclear Participants include banks (including central banks), securities brokers and dealers and other professional financial intermediaries and may include the underwriters. Indirect access to Euroclear is also available to other firms that clear through or maintain a custodial relationship with a Euroclear Participant, either directly or indirectly.
The Euroclear Operator is regulated and examined by the Belgian Banking Commission.
Links have been established among DTC, Clearstream and Euroclear to facilitate the initial issuance of the notes sold outside of the U.S. and cross-market transfers of the notes associated with secondary market trading.
Although DTC, Clearstream and Euroclear have agreed to the procedures provided below in order to facilitate transfers, they are under no obligation to perform these procedures, and these procedures may be modified or discontinued at any time.
S-249
Table of Contents
Clearstream and Euroclear will record the ownership interests of their participants in much the same way as DTC, and DTC will record the total ownership of each of the U.S. agents of Clearstream and Euroclear, as participants in DTC. When notes are to be transferred from the account of a DTC participant to the account of a Clearstream participant or a Euroclear participant, the purchaser must send instructions to Clearstream or Euroclear through a participant at least one day prior to settlement. Clearstream or Euroclear, as the case may be, will instruct its U.S. agent to receive notes against payment. After settlement, Clearstream or Euroclear will credit its participant’s account. Credit for the notes will appear on the next day (European time).
Because settlement is taking place during New York business hours, DTC participants will be able to employ their usual procedures for sending notes to the relevant U.S. agent acting for the benefit of Clearstream or Euroclear participants. The sale proceeds will be available to the DTC seller on the settlement date. As a result, to the DTC participant, a cross-market transaction will settle no differently than a trade between two DTC participants.
When a Clearstream or Euroclear participant wishes to transfer notes to a DTC participant, the seller will be required to send instructions to Clearstream or Euroclear through a Clearstream or Euroclear participant at least one business day prior to settlement. In these cases, Clearstream or Euroclear will instruct its U.S. agent to transfer these notes against payment for them. The payment will then be reflected in the account of the Clearstream or Euroclear participant the following day, with the proceeds back valued to the value date, which would be the preceding day, when settlement occurs in New York, if settlement is not completed on the intended value date, that is, the trade fails, proceeds credited to the Clearstream or Euroclear participant’s account will instead be valued as of the actual settlement date.
You should be aware that you will only be able to make and receive deliveries, payments and other communications involving the notes through Clearstream and Euroclear on the days when those clearing systems are open for business. Those systems may not be open for business on days when banks, brokers and other institutions are open for business in the U.S. In addition, because of time zone differences there may be problems with completing transactions involving Clearstream and Euroclear on the same business day as in the U.S.
Notes in physical form will be issued in exchange for beneficial interests in the Global Notes only if:
| • | the Depository notifies us that it is unwilling or unable to continue as Depository for the Global Notes and a successor Depository is not appointed by us within 90 days of such notice, or |
| • | an Event of Default (as defined) with respect to the notes has occurred and is continuing and the note registrar has received a written request from the Depository to issue notes in physical form. |
S-250
Table of Contents
U.S. FEDERAL INCOME TAX CONSIDERATIONS
The following summary of the U.S. federal income tax consequences of the purchase, ownership and disposition of the notes is based upon the Internal Revenue Code of 1986, as amended, or Code, existing and proposed Treasury regulations promulgated thereunder, or Treasury Regulations, and administrative and judicial interpretations thereof, all as currently in effect, all of which are subject to change (including changes in effective dates and retroactive changes) or possible differing interpretations. This summary deals only with notes held as capital assets and does not purport to deal with persons in special tax situations, such as financial institutions, insurance companies, real estate investment trusts, regulated investment companies, dealers in securities or currencies, partnerships or other pass-through entities, former citizens or residents of the U.S., tax exempt organizations, holders that are subject to the alternative minimum tax provisions of the Code, persons holding notes as a hedge against currency risks or part of a “straddle,” “hedge,” “conversion,” or other integrated transaction for tax purposes, or U.S. Holders (as defined below) whose functional currency is not the U.S. dollar. It also does not deal with persons other than original purchasers of the notes (except where otherwise specifically noted). This discussion does not address the effect of any other U.S. federal taxes (such as gift and estate taxes), the tax considerations arising under the laws of any foreign, state or local jurisdiction, or any reporting requirements of or other tax consequences under the Treasury Regulations relating to certain tax shelter transactions.
Persons considering the purchase of the notes should consult their own tax advisors concerning the application of U.S. federal income tax laws to their particular situations as well as any consequences of the purchase, ownership and disposition of the notes arising under the laws of any other taxing jurisdiction or tax treaty.
As used herein, the term “U.S. Holder” means a beneficial owner of a note that is for U.S. federal income tax purposes (i) a citizen or resident of the U.S., (ii) a corporation (including an entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the U.S., any state thereof or the District of Columbia, (iii) an estate, the income of which is subject to U.S. federal income taxation regardless of its source, or (iv) a trust if a court within the U.S. is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have the authority to control all substantial decisions of the trust. Notwithstanding the preceding sentence, to the extent provided in U.S. Treasury Regulations, certain trusts which were in existence on August 20, 1996 and were treated as U.S. persons under the Code and applicable Treasury Regulations thereunder prior to such date that elect to continue to be so treated also shall be considered U.S. Holders. As used herein, the term “non-U.S. Holder” means a beneficial owner of a note that is not a U.S. Holder or a partnership. If a partnership (including for this purpose any entity treated as a partnership for U.S. federal income tax purposes) is the beneficial owner of any note, the treatment of a partner in that partnership will generally depend upon the status of such partner and the activities of such partnership. Prospective purchasers that are partnerships or that hold the notes through a partnership or similar pass-through entity should consult their tax advisors regarding the U.S. federal income tax consequences of the purchase, ownership, or disposition of the notes.
Treatment of the notes as indebtedness
We intend to take the position that, under current law and interpretations thereof, the notes will be classified for U.S. federal income tax purposes as indebtedness. Pursuant to the terms of the notes, we will agree and each holder will agree (by its acceptance of a beneficial ownership interest in a note) to treat the notes as indebtedness for all U.S. federal income tax purposes. No assurance can be given, however, that the Internal Revenue Service, or IRS, will not challenge such position or, if challenged, that such a challenge will not be successful. If the IRS were to assert successfully that the notes should be treated as equity for U.S. federal income tax purposes, the tax treatment of the notes would differ from that described below. The remainder of this discussion assumes that the notes will be classified as indebtedness for U.S. federal income tax purposes.
S-251
Table of Contents
U.S. Holders
Payments of interest. Payments of interest on a note generally will be taxable to a U.S. Holder as ordinary interest income at the time such payments are accrued or are received, in accordance with the U.S. Holder’s regular method of tax accounting.
We will be obligated to pay additional interest on the notes under certain circumstances described in “Description of Notes—General—Defaulted interest.” Under the Treasury Regulations, the possibility of additional payments on a debt instrument may be disregarded for purposes of determining the amount of interest or original issue discount income to be recognized by the holder in respect of the debt instrument (or the timing of such recognition) if the likelihood of the payments, as of the date the debt instrument is issued, is remote or the amount of potential payments is incidental. We believe that the likelihood of any such payments of additional interest is remote. Therefore, although the matter is not free from doubt, any additional interest should be taxable as ordinary income at the time it is accrued or received, in accordance with the U.S. Holder’s regular method of tax accounting. Our determination is binding on a holder unless such holder discloses its contrary position in the manner required by applicable Treasury Regulations. Our determination is not, however, binding on the IRS and it is possible that the IRS might take a different position from that described above, in which case the timing and amount of income inclusion may be different from that described above. U.S. Holders should consult their tax advisors about the treatment of the possibility of payments of additional interest.
Disposition of a note. Upon the sale, exchange, redemption, retirement or other taxable disposition of a note, a U.S. Holder generally will recognize taxable gain or loss equal to the difference between the amount realized on the sale, exchange, redemption, retirement or other taxable disposition of a note (other than amounts representing accrued and unpaid interest, which will be taxable as such) and such U.S. Holder’s adjusted tax basis in the note. A U.S. Holder’s adjusted tax basis in a note generally will equal such U.S. Holder’s initial investment in the note. Such gain or loss generally will be capital gain or loss and will be long-term capital gain or loss if the note had been held at the time of disposition for more than one year. Long-term capital gains of certain taxpayers will be taxed at preferential rates. The deductibility of capital losses is subject to limitations.
Medicare tax.For taxable years beginning after December 31, 2012, a 3.8% tax will be imposed on the net investment income (which includes interest and gains from a disposition of a note) of certain individuals, trusts and estates. Prospective investors in the notes should consult their tax advisors regarding the possible applicability of this tax to an investment in the notes.
Non-U.S. Holders
Payments of interest.A non-U.S. Holder that is an individual or corporation (or an entity treated as a corporation for federal income tax purposes) holding the notes on its own behalf will not be subject to U.S. federal income taxes on payments of interest on a note, unless such non-U.S. Holder is (i) a direct or indirect 10% or greater shareholder of DaVita, (ii) a controlled foreign corporation related to DaVita, or (iii) a bank receiving interest described in section 881(c)(3)(A) of the Code. To qualify for the exemption from taxation, the Withholding Agent, as defined below, must have received a statement from the individual or corporation that:
| • | is signed under penalties of perjury by the beneficial owner of the note, |
| • | certifies that such owner is not a U.S. Holder, and |
| • | provides the beneficial owner’s name and address. |
A “Withholding Agent” is the last U.S. payor (or a non-U.S. payor that is a qualified intermediary, U.S. branch of a foreign person, or withholding foreign partnership) in the chain of payment prior to payment to a non-U.S. Holder (which itself is not a Withholding Agent). Generally, this statement is made on an IRS Form W-8BEN, or W-8BEN, which is effective for the remainder of the year of signature plus three full calendar years unless a change in circumstances makes any information on the form incorrect. Notwithstanding the
S-252
Table of Contents
preceding sentence, a W-8BEN with a U.S. taxpayer identification number will remain effective until a change in circumstances makes any information on the form incorrect, provided that the Withholding Agent reports at least annually to the beneficial owner on IRS Form 1042-S. The beneficial owner must inform the Withholding Agent within 30 days of such change and furnish a new W-8BEN. A noteholder that is not an individual or corporation (or an entity treated as a corporation for U.S. federal income tax purposes) holding the notes on its own behalf may have substantially increased reporting requirements. In particular, in the case of notes held by a foreign partnership (or foreign trust), the partners (or beneficiaries) rather than the partnership (or trust) will be required to provide the certification discussed above, and the partnership (or trust) will be required to provide certain additional information.
A non-U.S. Holder whose income with respect to its investment in a note is effectively connected with the conduct of a U.S. trade or business would generally be taxed as if the non-U.S. Holder was a U.S. person provided the non-U.S. Holder provides to the Withholding Agent an IRS Form W-8ECI. In addition, if the non-U.S. Holder is a corporation, any effectively connected income will generally be subject to a “branch profits tax” at a rate of 30% (or a reduced rate under an applicable treaty).
Certain securities clearing organizations, and other entities who are not beneficial owners, may be able to provide a signed statement to the Withholding Agent. However, in such case, the signed statement may require a copy of the beneficial owner’s W-8BEN (or the substitute form).
Disposition of a note.Generally, a non-U.S. Holder will not be subject to U.S. federal income taxes on any amount that constitutes gain upon the sale, exchange, redemption, retirement or other taxable disposition of a note, unless (i) such non-U.S. Holder is an individual who is present in the U.S. for 183 days or more in the taxable year of the disposition and such gain is derived from sources within the U.S. and certain other conditions are met or (ii) such gain is effectively connected with the conduct of a U.S. trade or business of the non-U.S. Holder (and, if a treaty applies, such gain is attributable to a permanent establishment or fixed base maintained by such holder in the U.S.). In addition, if the non-U.S. Holder is a corporation, any such effectively connected gain will generally be subject to a “branch profits tax” at a rate of 30% (or a reduced rate under an applicable treaty).
Alternative characterization of the notes. As discussed above, we intend to take the position that the notes will be classified for U.S. federal income tax purposes as indebtedness. If, however, the IRS were to assert successfully that the notes should be treated as equity for U.S. federal income tax purposes, this alternative characterization may result in material adverse tax consequences to non-U.S. Holders. In particular, interest paid on the notes and distributions with respect to the notes would be treated as distributions with respect to our stock. Consequently, non-U.S. Holders would be subject to withholding at a rate of 30% (or such lower rate as may be specified in an applicable tax treaty) to the extent distributions are characterized as dividends from sources within the U.S. for U.S. federal income tax purposes.
Information reporting and backup withholding
Backup withholding of U.S. federal income tax may apply to payments made in respect of the notes to registered owners that are not “exempt recipients” and that fail to provide certain identifying information (such as the registered owner’s taxpayer identification number) in the required manner. Generally, individuals are not exempt recipients, whereas corporations and certain other entities generally are exempt recipients. Payments made in respect of the notes to a U.S. Holder must be reported to the IRS, unless the U.S. Holder is an exempt recipient or establishes an exemption.
Generally, compliance with the identification procedures described above under “—Non-U.S. Holders—Payments of interest” would establish an exemption from backup withholding for those non-U.S. Holders that are not exempt recipients. However, we may be required to report annually to the IRS and to the non-U.S. Holder the amount of, and the tax withheld with respect to, any interest paid to the non-U.S. Holder, regardless of whether
S-253
Table of Contents
any tax was actually withheld, and proceeds from sales or other dispositions of the notes. Copies of these information returns may also be made available to the tax authorities of the country in which you reside under the provisions of a specific treaty or agreement.
Upon the sale or other disposition of a note to (or through) a broker, the broker must report the sale and withhold on the entire purchase price, unless either (i) the broker determines that the seller is a corporation or other exempt recipient or (ii) the seller certifies that such seller is a non-U.S. Holder (and certain other conditions are met). Certification of the registered owner’s non-U.S. status would generally be made on a W-8BEN under penalties of perjury, although in certain cases it may be possible to submit other documentary evidence.
Any amounts withheld under the backup withholding rules from a payment to a beneficial owner would be allowed as a refund or a credit against such beneficial owner’s U.S. federal income tax provided the required information is furnished to the IRS.
Foreign account tax compliance
On March 18, 2010, the Hiring Incentives to Restore Employment Act, or HIRE Act, was signed into law. Under certain circumstances, the HIRE Act will impose a withholding tax of 30% on payments of U.S. source income on, and the gross proceeds from a disposition of, the notes made to certain foreign entities unless various information reporting requirements are satisfied. These rules generally would apply to payments made after December 31, 2012. However, under the HIRE Act, the withholding and reporting requirements generally will not apply to payments made on, or gross proceeds from a disposition of, debt instruments outstanding as of March 18, 2012, or Grandfather Date.
Despite the December 31, 2012 date set forth in the HIRE Act, the IRS has issued preliminary guidance indicating that the withholding tax on U.S. source income will not be imposed with respect to payments made prior to January 1, 2014 and that the withholding tax on gross proceeds from a disposition of debt instruments will not be imposed with respect to payments made prior to January 1, 2015. In addition, the IRS has released proposed regulations that would extend the Grandfather Date to January 1, 2013. These proposed regulations would be effective once finalized. Prospective investors should consult their tax advisors regarding the HIRE Act.
S-254
Table of Contents
Subject to the terms and conditions in the underwriting agreement among us, the guarantors and the underwriters, we have agreed to sell to each underwriter, and each underwriter severally and not jointly has agreed to purchase from us, the principal amount of notes set forth opposite that underwriter’s name:
Underwriters | Principal amount of notes | |||||
J.P. Morgan Securities LLC | $ | |||||
Barclays Capital Inc. | ||||||
Merrill Lynch, Pierce, Fenner & Smith Incorporated | ||||||
Credit Suisse Securities (USA) LLC | ||||||
Goldman, Sachs & Co. | ||||||
Morgan Stanley & Co. LLC | ||||||
SunTrust Robinson Humphrey, Inc. | ||||||
Wells Fargo Securities, LLC | ||||||
Credit Agricole Securities (USA) Inc. | ||||||
Mitsubishi UFJ Securities (USA), Inc. | ||||||
Scotia Capital (USA) Inc. | ||||||
SMBC Nikko Capital Markets Limited | ||||||
| $ | 1,000,000,000 | |||||
The obligations of the underwriters under the underwriting agreement, including their agreement to purchase notes from us, are several and not joint. The underwriting agreement provides that the underwriters have agreed to purchase all of the notes if any of them are purchased. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
The underwriters initially propose to offer the notes to the public at the public offering price that appears on the cover page of this prospectus. The underwriters may offer the notes to selected dealers at the public offering price minus a concession of up to % of the principal amount. In addition, the underwriters may allow, and those selected dealers may reallow, a concession of up to % of the principal amount to certain other dealers. After the initial offering, the underwriters may change the public offering price and any other selling terms. The underwriters may offer and sell notes through certain of their affiliates.
The following table shows the underwriting discounts and commissions to be paid by us to the underwriters in connection with this offering (expressed as a percentage of the principal amount of the notes).
| Paid by us | ||||
Per note | % | |||
In the underwriting agreement, we have agreed that:
| • | We will not, for a period of 30 days after the date of this prospectus, without first obtaining the prior written consent of J.P. Morgan Securities LLC, directly or indirectly, sell, offer, contract or grant any option to sell, pledge or transfer or otherwise dispose of, or announce the offering of, any debt securities that are substantially similar to the notes or securities exchangeable for or convertible into debt securities that are substantially similar to the notes, except for the notes sold to the underwriters pursuant to the underwriting agreement. |
| • | We will indemnify the several underwriters against certain liabilities, including liabilities under the Securities Act, or contribute to payments that the underwriters may be required to make in respect of those liabilities. |
S-255
Table of Contents
The notes are a new issue of securities and there is currently no established trading market for the notes. We do not intend to apply for the notes to be listed on any securities exchange or to arrange for the notes to be quoted on any quotation system. The underwriters have advised us that they intend to make a market in the notes, but they are not obligated to do so and they may discontinue any market making at any time in their sole discretion. Accordingly, we cannot assure you that a liquid trading market will develop for the notes, that you will be able to sell your notes at a particular time or that the prices that you receive when you sell will be favorable.
The underwriters also may impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because such underwriter or its affiliates have repurchased notes sold by or for the account of such underwriter in stabilizing or short covering transactions.
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a Relevant Member State), each underwriter has represented and agreed that with effect from and including the date on which the Prospectus Directive is implemented in that Relevant Member State, or Relevant Implementation Date, it has not made and will not make an offer of our securities which are the subject of this prospectus to the public in that Relevant Member State other than:
| • | to any legal entity which is a qualified investor as defined in the Prospectus Directive; |
| • | to fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150, natural or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject to obtaining the prior con-sent of J.P. Morgan Securities LLC for any such offer; or |
| • | in any other circumstances falling within Article 3(2) of the Prospectus Directive, |
provided that no such offer of notes shall require us or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Directive.
For the purposes of this provision, the expression “an offer of securities to the public” in relation to any notes in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe for the securities, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State, the expression “Prospectus Directive” means Directive 2003/71/EC (and amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State), and includes any relevant implementing measure in the Relevant Member State and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
We have not authorized and do not authorize the making of any offer of notes through any financial intermediary on our behalf, other than offers made by the underwriters with a view to the final placement of the notes as contemplated in this prospectus. Accordingly, no purchaser of the notes, other than the underwriters, is authorized to make any further offer of the notes on behalf of us or the underwriters.
Each underwriter has agreed that:
(a) it has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in investment activity (within the meaning of Section 21 of the FSMA) received by it in connection with the issue or sale of the notes in circumstances in which Section 21(1) of the FSMA does not apply to us or the guarantors; and
(b) it has complied and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the notes in, from or otherwise involving the United Kingdom.
The notes may not be offered or sold by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), or (ii) to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571,
S-256
Table of Contents
Laws of Hong Kong) and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a “prospectus” within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), and no advertisement, invitation or document relating to the notes may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to notes which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder.
This prospectus supplement does not constitute an issue prospectus pursuant to Article 652a or Article 1156 of the Swiss Code of Obligations and the notes will not be listed on the SIX Swiss Exchange. Therefore, this prospectus supplement may not comply with the disclosure standards of the listing rules (including any additional listing rules or prospectus schemes) of the SIX Swiss Exchange. Accordingly, the notes may not be offered to the public in or from Switzerland, but only to a selected and limited circle of investors who do not subscribe to the notes with a view to distribution. Any such investors will be individually approached by the underwriters from time to time.
This prospectus supplement relates to an Exempt Offer in accordance with the Offered Securities Rules of the Dubai Financial Services Authority, or DFSA. This prospectus supplement is intended for distribution only to persons of a type specified in the Offered Securities Rules of the DFSA. It must not be delivered to, or relied on by, any other person. The DFSA has no responsibility for reviewing or verifying any documents in connection with Exempt Offers. The DFSA has not approved this prospectus supplement nor taken steps to verify the information set forth herein and has no responsibility for the prospectus supplement. The notes to which this prospectus supplement relates may be illiquid and/or subject to restrictions on their resale. Prospective purchasers of the notes offered should conduct their own due diligence on the notes. If you do not understand the contents of this prospectus supplement you should consult an authorized financial advisor.
The securities have not been and will not be registered under the Financial Instruments and Exchange Law of Japan (the Financial Instruments and Exchange Law) and each underwriter has agreed that it will not offer or sell any securities, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan (which term as used herein means any person resident in Japan, including any corporation or other entity organized under the laws of Japan), or to others for re-offering or resale, directly or indirectly, in Japan or to a resident of Japan, except pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the Financial Instruments and Exchange Law and any other applicable laws, regulations and ministerial guidelines of Japan.
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the notes may not be circulated or distributed, nor may the notes be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore, or SFA, (ii) to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the notes are subscribed or purchased under Section 275 by a relevant person which is: (a) a corporation (which is not an accredited investor) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or (b) a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary is an accredited investor, shares, debentures and units of shares and debentures of that corporation or the beneficiaries’ rights and interest in that trust shall not be transferable for 6 months after that corporation or that trust has acquired the notes under Section 275 except: (1) to an institutional investor under Section 274 of the
S-257
Table of Contents
SFA or to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA; (2) where no consideration is given for the transfer; or (3) by operation of law.
We expect that delivery of the notes will be made against payment therefor on or about , 2012 which will be the tenth business day following the pricing of the notes (such settlement being herein referred to as “T+ ”). Under Rule 15c6-1 under the Exchange Act, trades in the secondary market generally are required to settle in three business days, unless the parties to any such trade expressly agree otherwise. Accordingly, you should be aware that the trading of the notes on the date of pricing or the next succeeding business days may be affected by the T+ settlement.
In connection with the offering of the notes, the underwriters may engage in overallotment, stabilizing transactions and syndicate covering transactions. Overallotment involves sales in excess of the offering size, which creates a short position for the underwriters. Stabilizing transactions involve bids to purchase the notes in the open market for the purpose of pegging, fixing or maintaining the price of the notes. Syndicate covering transactions involve purchases of the notes in the open market after the distribution has been completed in order to cover short positions. Stabilizing transactions and syndicate covering transactions may cause the price of the notes to be higher than it would otherwise be in the absence of those transactions. If the underwriters engage in stabilizing or syndicate covering transactions, they may discontinue them at any time.
The underwriters and their respective affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. Certain of the underwriters and their respective affiliates have provided, and may in the future provide, a variety of these services to the issuer and to persons and entities with relationships with the issuer, for which they received or will receive customary fees and expenses.
If any of the underwriters or their respective affiliates have a lending relationship with us, certain of those underwriters or their respective affiliates routinely hedge, and certain other of those underwriters or their respective affiliates are likely to hedge, their credit exposure to us consistent with their customary risk management policies. Typically, these underwriters and their affiliates would hedge such exposure by entering into transactions which consist of either the purchase of credit default swaps or the creation of short positions in our securities, including potentially the notes offered hereby. Any such credit default swaps or short positions could adversely affect future trading prices of the notes offered hereby.
In the ordinary course of their various business activities, the underwriters and their respective affiliates, officers, directors and employees may purchase, sell or hold a broad array of investments and actively trade securities, derivatives, loans, commodities, currencies, credit default swaps and other financial instruments for their own account and for the accounts of their customers, and such investment and trading activities may involve or relate to assets, securities and/or instruments of the issuer (directly, as collateral securing other obligations or otherwise) and/or persons and entities with relationships with the issuer. The underwriters and their respective affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express independent research views in respect of such assets, securities or instruments and may at any time hold, or recommend to clients that they should acquire, long and/or short positions in such assets, securities and instruments.
Additionally, certain of the underwriters and their affiliates are lenders and agents under our senior secured credit facilities and are acting as arrangers for the amendment to our senior secured credit facilities. In addition, affiliates of certain of the underwriters may be lenders with respect to HCP’s existing debt and may receive a portion of the proceeds of the additional borrowings under our amended senior secured credit facilities in connection with the repayment of HCP’s existing debt upon consummation of the Merger. Certain affiliates of the underwriters have in the past and may continue to act in the future as agents and or lenders under our
S-258
Table of Contents
amended senior secured credit facilities. JPMorgan Chase Bank, N.A, an affiliate of J.P. Morgan Securities LLC, is administrative agent under our senior secured credit facilities. J.P. Morgan Securities LLC is acting as financial advisor to DaVita in connection with its acquisition of HCP. Some of the underwriters and their affiliates have engaged in, and may in the future engage in, investment banking and other commercial dealings in the ordinary course of business with us or our affiliates. They have received, or may in the future receive, customary fees and commissions for these transactions.
SMBC Nikko Capital Markets Limited is not a U.S. registered broker-dealer and, therefore, intends to participate in the offering outside of the United States and, to the extent that the offering is within the United States, as facilitated by an affiliated U.S. registered broker-dealer, SMBC Nikko Securities America, Inc., or SMBC Nikko-SI, as permitted under applicable law. To that end, SMBC Nikko Capital Markets Limited and SMBC Nikko-SI have entered into an agreement pursuant to which SMBC Nikko-SI provides certain advisory and/or other services with respect to this offering. In return for the provision of such services by SMBC Nikko-SI, SMBC Nikko Capital Markets Limited will pay to SMBC Nikko-SI a mutually agreed-fee.
S-259
Table of Contents
The following is a summary of certain considerations associated with the purchase of the Notes by employee benefit plans that are subject to Title I of the Employee Retirement Income Security Act of 1974, as amended , or ERISA, plans, individual retirement accounts and other arrangements that are subject to Section 4975 of the Internal Revenue Code of 1986, as amended, or Code, or provisions under any federal, state, local, non-U.S. or other laws or regulations that are similar to such provisions of ERISA or the Code (collectively, hereinafter referred to as the Similar Laws, and entities whose underlying assets are considered to include “plan assets,” pursuant to 29 C.F.R. Section 2510.3-101, as modified by Section 3(42) of ERISA, of such plans, accounts and arrangements, each a Plan.
General Fiduciary Matters
ERISA and the Code impose certain duties on persons who are fiduciaries of a Plan subject to Title I of ERISA or Section 4975 of the Code, or an ERISA Plan, and prohibit certain transactions involving the assets of an ERISA Plan and its fiduciaries or other interested parties. Under ERISA and the Code, any person who exercises any discretionary authority or control over the administration of such an ERISA Plan or the management or disposition of the assets of such an ERISA Plan, or who renders investment advice for a fee or other compensation to such an ERISA Plan, is generally considered to be a fiduciary of the ERISA Plan.
In considering an investment in the Notes of a portion of the assets of any Plan, a Plan fiduciary should determine whether the investment is in accordance with the documents and instruments governing the Plan and the applicable provisions of ERISA, the Code or any Similar Laws relating to a fiduciary’s duties to the Plan including, without limitation, the prudence, diversification, delegation of control and prohibited transaction provisions of ERISA, the Code and any other applicable Similar Laws.
Prohibited Transaction Issues
Section 406 of ERISA and Section 4975 of the Code prohibit ERISA Plans from engaging in specified transactions involving plan assets with persons or entities who are “parties in interest,” within the meaning of ERISA, or “disqualified persons,” within the meaning of Section 4975 of the Code, unless an exemption is available. A party in interest or disqualified person who engages in a non-exempt prohibited transaction may be subject to excise taxes and other penalties and liabilities under ERISA and the Code. In addition, the fiduciary of the ERISA Plan that engages in such a non-exempt prohibited transaction may be subject to penalties and liabilities under ERISA and the Code. The acquisition and/or holding of Notes by an ERISA Plan with respect to which the issuer or the underwriters or their affiliates may be considered a party in interest or a disqualified person may constitute or result in a direct or indirect prohibited transaction under Section 406 of ERISA and/or Section 4975 of the Code, unless the investment is acquired and is held in accordance with an applicable statutory, class or individual prohibited transaction exemption. In this regard, the U.S. Department of Labor has issued prohibited transaction class exemptions, or PTCEs, that may apply to the acquisition and holding of the Notes. These class exemptions include, without limitation, PTCE 84-14 respecting transactions determined by independent qualified professional asset managers, PTCE 90-1 respecting insurance company pooled separate accounts, PTCE 91-38 respecting bank collective investment funds, PTCE 95-60 respecting life insurance company general accounts and PTCE 96-23 respecting transactions determined by in-house asset managers, although there can be no assurance that all of the conditions of any such exemptions will be satisfied. In addition to the foregoing, the Pension Protection Act of 2006 provides a statutory exemption (Section 408(b)(17) of ERISA and Section 4975(d)(20) of the Code) for transactions between an ERISA Plan and a person that is a party in interest and/or a disqualified person (other than a fiduciary or an affiliate that, directly or indirectly, has or exercises discretionary authority or control or renders investment advice with respect to the assets involved in the transaction) solely by reason of providing services to the Plan or by relationship to a service provider, provided that the ERISA Plan fiduciary has made a determination that there is adequate consideration for the transaction.
S-260
Table of Contents
Because of the foregoing, the Notes should not be purchased or held by any person investing “plan assets” of any Plan, unless such purchase will not constitute a non-exempt prohibited transaction under ERISA and the Code or similar violation of any applicable Similar Laws.
Representation
Accordingly, by acceptance of a Note, each purchaser and subsequent transferee of a Note will be deemed to have represented and warranted that either (i) no portion of the assets used by such purchaser or transferee to acquire and hold the Notes constitutes assets of any Plan or (ii) the purchase and holding of the Notes by such purchaser or transferee will not constitute a non-exempt prohibited transaction under Section 406 of ERISA or Section 4975 of the Code or similar violation under any applicable Similar Laws.
The foregoing discussion is general in nature and is not intended to be all-inclusive. Due to the complexity of these rules and the penalties that may be imposed upon persons involved in non-exempt prohibited transactions, it is particularly important that fiduciaries or other persons considering purchasing the Notes (and holding the Notes) on behalf of, or with the assets of, any Plan, consult with their counsel regarding the potential applicability of ERISA, Section 4975 of the Code and any Similar Laws to such investment and whether an exemption would be applicable to the purchase and holding of the Notes.
Certain legal matters in connection with the notes offered hereby will be passed upon for us by Kim M. Rivera, the Company’s Chief Legal Officer and Corporate Secretary, and Sidley Austin LLP, San Francisco, California, special counsel to the Company. Cahill Gordon & ReindelLLP, New York, New York will act as counsel for the underwriters, and certain regulatory matters will be passed upon for the underwriters by Winston & Strawn LLP, Washington, D.C. Ms. Rivera participates in various employee benefit plans offered by DaVita and as of June 30, 2012 held 5,000 restricted stock units (none of which were vested) and 112,500 stock-settled stock appreciation rights (22,500 of which were vested).
The consolidated financial statements and financial statement schedule of DaVita Inc. as of December 31, 2011 and 2010, and for each of the years in the three-year period ended December 31, 2011, and management’s assessment of the effectiveness of internal control over financial reporting as of December 31, 2011 have been incorporated by reference herein and in the registration statement in reliance upon the reports of KPMG LLP, independent registered public accounting firm incorporated by reference herein, and upon the authority of said firm as experts in accounting and auditing. The audit report covering the consolidated financial statements refers to the Company’s adoption of accounting standards updates 2011-07Health Care Entities—Presentation and Disclosure of Patient Service Revenue, Provision for Bad Debts, and the Allowance for Doubtful Accounts and 2011-05 as amended by 2011-12Comprehensive Income—Presentation of Comprehensive Income, which were applied retrospectively for all periods presented effective January 1, 2012.
The consolidated financial statements of HealthCare Partners Holdings, LLC and Affiliates at December 31, 2011 and 2010, and for each of the three years in the period ended December 31, 2011, appearing in this Prospectus Supplement and Registration Statement of DaVita Inc. have been audited by Ernst & Young LLP, independent auditor, as set forth in their report thereon appearing elsewhere herein, are included in reliance upon such reports given on the authority of such firm as experts in accounting and auditing.
The SEC allows us to “incorporate by reference” information into this prospectus supplement and the accompanying prospectus. This means that we can disclose important information to you by referring you to another document that DaVita has filed separately with the SEC that contains that information. The information
S-261
Table of Contents
incorporated by reference is considered to be part of this prospectus supplement and the accompanying prospectus. Information that DaVita files with the SEC after the date of this prospectus supplement and that is incorporated by reference in this prospectus supplement will automatically modify and supersede the information included or incorporated by reference in this prospectus supplement and the accompanying prospectus to the extent that the subsequently filed information modifies or supersedes the existing information. We incorporate by reference (other than any portions of any such documents that are not deemed “filed” under the Securities Exchange Act of 1934, as amended, or the Exchange Act, in accordance with the Exchange Act and applicable SEC rules):
| • | Annual Report on Form 10-K for the year ended December 31, 2011; |
| • | Quarterly Reports on Form 10-Q for the quarters ended March 31, 2012 and June 30, 2012; and |
| • | Current Reports on Form 8-K filed with the SEC on February 16, 2012, April 16, 2012, May 21, 2012 (solely with respect to Items 1.01 and 9.01), June 14, 2012, July 3, 2012, July 6, 2012, July 9, 2012, August 10, 2012 and August 13, 2012; and |
| • | any future filings we make with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act until we sell all of the securities offered by this prospectus supplement. |
We will provide free of charge a copy of any or all of the information that has been incorporated by reference in this prospectus if you write to or call us at the following:
DaVita Inc.
1551 Wewatta Street
Denver, Colorado 80202
Telephone: (888) 484-7505
Attention: Investor Relations
WHERE YOU CAN FIND MORE INFORMATION
DaVita Inc. is subject to the informational reporting requirements of the Exchange Act and, in accordance with these requirements, it files annual, quarterly and current reports, proxy statements and other information with the SEC. Such reports, proxy statements and other information may be inspected and copied at the SEC’s Public Reference Room, 100 F Street, N.E., Washington, D.C. 20549. Information on the operation of the Public Reference Room may be obtained by calling 1-800-SEC-0330. DaVita Inc.’s SEC filings are available to the public at the SEC’s website at http://www.sec.gov. You may also inspect information that we file with The New York Stock Exchange at the offices of the New York Stock Exchange at 20 Broad Street, New York, New York 10005.
The address of our internet site ishttp://www.davita.com. We make available free of charge on or through our internet site DaVita Inc.’s annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, proxy statements and amendments to those reports and proxy statements filed or furnished pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file this material with, or furnish it to, the SEC. Any internet addresses provided in this prospectus are for informational purposes only and are not intended to be hyperlinks. In addition, the information on our internet site is not a part of, and is not incorporated or deemed to be incorporated by reference in, this prospectus or any accompanying prospectus supplement or other offering materials. Accordingly, no information in our or any of these other internet addresses is included herein or incorporated or deemed to be incorporated by reference herein.
S-262
Table of Contents
INDEX TO FINANCIAL STATEMENTS OF
HEALTHCARE PARTNERS HOLDINGS, LLC AND AFFILIATES
| Page | ||||
Audited Consolidated Financial Statements | ||||
| F-2 | ||||
Consolidated Balance Sheets as of December 31, 2011 and 2010 | F-3 | |||
Consolidated Statements of Income for the Years Ended December 31, 2011, 2010 and 2009 | F-4 | |||
Consolidated Statements of Members’ Equity for the Years Ended December 31, 2011, 2010 and 2009 | F-5 | |||
Consolidated Statements of Cash Flows for the Years Ended December 31, 2011, 2010 and 2009 | F-6 | |||
| F-7 | ||||
Unaudited Consolidated Financial Statements | ||||
Consolidated Balance Sheets as of June 30, 2012 and December 31, 2011 | F-30 | |||
Consolidated Statements of Income for the Three and Six Months Ended June 30, 2012 and 2011 | F-31 | |||
| F-32 | ||||
Consolidated Statements of Cash Flows for the Six Months ended June 30, 2012 and 2011 | F-33 | |||
| F-34 | ||||
F-1
Table of Contents
Report of Independent Auditors
The Members
HealthCare Partners Holdings, LLC and Affiliates
We have audited the accompanying consolidated balance sheets of HealthCare Partners Holdings, LLC and Affiliates (the Company) as of December 31, 2011 and 2010, and the related consolidated statements of income, members’ equity, and cash flows for the years ended December 31, 2011, 2010 and 2009. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with auditing standards generally accepted in the United States. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of HealthCare Partners Holdings, LLC and Affiliates at December 31, 2011 and 2010, and the consolidated results of their operations and their cash flows for the years ended December 31, 2011, 2010 and 2009, in conformity with U.S. generally accepted accounting principles.
/s/ Ernst & Young LLP
Los Angeles, California
June 29, 2012
F-2
Table of Contents
HEALTHCARE PARTNERS HOLDINGS, LLC AND AFFILIATES
(In Thousands)
| December 31 | ||||||||
| 2011 | 2010 | |||||||
Assets | ||||||||
Current assets: | ||||||||
Cash and cash equivalents | $ | 394,521 | $ | 361,099 | ||||
Investments | 175,098 | 179,333 | ||||||
Accounts receivable—patients, net | 22,297 | 17,280 | ||||||
Accounts receivable—health plans | 99,570 | 79,896 | ||||||
Funds on deposit with third party | 63,638 | 66,688 | ||||||
Prepaid expenses and other current assets | 30,949 | 43,135 | ||||||
Current deferred tax assets, net | 6,709 | 9,322 | ||||||
|
|
|
| |||||
Total current assets | 792,782 | 756,753 | ||||||
Property and equipment, net | 75,848 | 66,893 | ||||||
Notes receivable from related parties, less current portion | 7,812 | 8,345 | ||||||
Goodwill | 278,565 | 270,143 | ||||||
Intangible assets, net | 157,389 | 142,493 | ||||||
Other assets | 53,525 | 41,829 | ||||||
|
|
|
| |||||
Total assets | $ | 1,365,921 | $ | 1,286,456 | ||||
|
|
|
| |||||
Liabilities and members’ equity | ||||||||
Current liabilities: | ||||||||
Medical claims and related payables | $ | 94,406 | $ | 78,927 | ||||
Other medical payables | 136,703 | 116,743 | ||||||
Accounts payable—health plans | 21,048 | 21,087 | ||||||
Accounts payable and accrued expenses | 206,934 | 177,084 | ||||||
Current maturities of long-term debt and capital leases | 29,575 | 2,835 | ||||||
|
|
|
| |||||
Total current liabilities | 488,666 | 396,676 | ||||||
Long-term debt and capital leases, less current maturities | 526,776 | 214,918 | ||||||
Noncurrent deferred tax liability, net | 64,613 | 36,677 | ||||||
Other liabilities | 97,798 | 72,169 | ||||||
|
|
|
| |||||
Total liabilities | 1,177,853 | 720,440 | ||||||
Members’ equity: | ||||||||
Preferred equity | — | 85,149 | ||||||
Common equity | 188,068 | 480,867 | ||||||
|
|
|
| |||||
| 188,068 | 566,016 | |||||||
|
|
|
| |||||
Total liabilities and members’ equity | $ | 1,365,921 | $ | 1,286,456 | ||||
|
|
|
| |||||
See accompanying notes.
F-3
Table of Contents
HEALTHCARE PARTNERS HOLDINGS, LLC AND AFFILIATES
Consolidated Statements of Income
(In Thousands)
| Year Ended December 31 | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
Operating revenues: | ||||||||||||
Medical revenues | $ | 2,375,119 | $ | 2,048,566 | $ | 1,730,698 | ||||||
Other operating revenues | 46,747 | 39,899 | 46,095 | |||||||||
|
|
|
|
|
| |||||||
Total operating revenues | 2,421,866 | 2,088,465 | 1,776,793 | |||||||||
Operating expenses: | ||||||||||||
Medical expenses | 1,165,757 | 1,034,139 | 930,157 | |||||||||
Hospital expenses | 247,636 | 222,352 | 211,527 | |||||||||
Clinic support and other operating costs | 307,544 | 262,563 | 225,516 | |||||||||
General and administrative expenses | 206,928 | 178,043 | 136,291 | |||||||||
Depreciation and amortization | 30,636 | 28,615 | 26,036 | |||||||||
|
|
|
|
|
| |||||||
Total expenses | 1,958,501 | 1,725,712 | 1,529,527 | |||||||||
Equity in earnings of unconsolidated joint ventures | 24,607 | 15,100 | 11,549 | |||||||||
|
|
|
|
|
| |||||||
Operating income | 487,972 | 377,853 | 258,815 | |||||||||
Other income (expense): | ||||||||||||
Interest income | 6,376 | 5,888 | 5,568 | |||||||||
Interest expense | (15,614 | ) | (5,421 | ) | (5,632 | ) | ||||||
Gain on sale of fixed assets | 3 | 80 | — | |||||||||
Gain on sale of investments, net | 1,317 | 41 | 1,794 | |||||||||
|
|
|
|
|
| |||||||
Total other income (expense), net | (7,918 | ) | 588 | 1,730 | ||||||||
|
|
|
|
|
| |||||||
Income before income taxes | 480,054 | 378,441 | 260,545 | |||||||||
Provision for income taxes | 71,465 | 48,564 | 40,258 | |||||||||
|
|
|
|
|
| |||||||
Net income | $ | 408,589 | $ | 329,877 | $ | 220,287 | ||||||
|
|
|
|
|
| |||||||
See accompanying notes.
F-4
Table of Contents
HEALTHCARE PARTNERS HOLDINGS, LLC AND AFFILIATES
Consolidated Statements of Members’ Equity
(In Thousands)
| Preferred Equity | Common Equity | Total Equity | ||||||||||
Balance at January 1, 2009 | $ | 81,757 | $ | 143,224 | $ | 224,981 | ||||||
Net income | 5,250 | 215,037 | 220,287 | |||||||||
Distributions to members | (3,669 | ) | (108,189 | ) | (111,858 | ) | ||||||
Share-based compensation | — | 6,619 | 6,619 | |||||||||
|
|
|
|
|
| |||||||
Balance at December 31, 2009 | 83,338 | 256,691 | 340,029 | |||||||||
Net income | 5,250 | 324,627 | 329,877 | |||||||||
Other comprehensive loss | — | (122 | ) | (122 | ) | |||||||
Distributions to members | (3,439 | ) | (107,703 | ) | (111,142 | ) | ||||||
Share-based compensation | — | 7,374 | 7,374 | |||||||||
|
|
|
|
|
| |||||||
Balance at December 31, 2010 | 85,149 | 480,867 | 566,016 | |||||||||
Net income | 71 | 408,518 | 408,589 | |||||||||
Other comprehensive income | — | 368 | 368 | |||||||||
Distributions to members | — | (211,002 | ) | (211,002 | ) | |||||||
Share-based compensation | — | 7,527 | 7,527 | |||||||||
Repurchase of Class A Units | (75,220 | ) | (464,780 | ) | (540,000 | ) | ||||||
Tax liability assumed to repurchase Class A Units | — | (37,019 | ) | (37,019 | ) | |||||||
Issuance of Class B Units | (10,000 | ) | 10,000 | — | ||||||||
Repurchase of vested options | — | (6,411 | ) | (6,411 | ) | |||||||
|
|
|
|
|
| |||||||
Balance at December 31, 2011 | $ | — | $ | 188,068 | $ | 188,068 | ||||||
|
|
|
|
|
| |||||||
See accompanying notes.
F-5
Table of Contents
HEALTHCARE PARTNERS HOLDINGS, LLC AND AFFILIATES
Consolidated Statements of Cash Flows
(In Thousands)
| Year Ended December 31 | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
Operating activities | ||||||||||||
Net income | $ | 408,589 | $ | 329,877 | $ | 220,287 | ||||||
Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||||||
Depreciation and amortization | 30,636 | 28,615 | 26,036 | |||||||||
Amortization of loan fees | 3,144 | 678 | 721 | |||||||||
Gain on sale of investments | (1,317 | ) | (41 | ) | (1,794 | ) | ||||||
Share-based compensation | 7,527 | 7,374 | 6,619 | |||||||||
Deferred taxes | (6,471 | ) | (3,493 | ) | (937 | ) | ||||||
Changes in operating assets and liabilities: | ||||||||||||
Accounts receivable—patients, net | (5,017 | ) | (456 | ) | (3,567 | ) | ||||||
Prepaid expenses and other current assets | 14,275 | (35,654 | ) | (2,787 | ) | |||||||
Other assets | (13,749 | ) | (10,801 | ) | (1,697 | ) | ||||||
Accounts payable, accrued compensation, and other liabilities | 55,994 | 36,895 | 19,154 | |||||||||
Medical claims and capitation payable | 35,439 | 46,408 | 17,846 | |||||||||
Accounts payable—health plans | (19,714 | ) | (56,346 | ) | 6,007 | |||||||
|
|
|
|
|
| |||||||
Net cash provided by operating activities | 509,336 | 343,056 | 285,888 | |||||||||
Investing activities | ||||||||||||
Net purchases of equipment, furniture and fixtures | (23,182 | ) | (21,424 | ) | (11,843 | ) | ||||||
Acquisition of medical practices, net of cash acquired | (39,819 | ) | (30,667 | ) | (18,805 | ) | ||||||
Purchases of marketable securities | (103,048 | ) | (285,680 | ) | — | |||||||
Sales of marketable securities | 107,688 | 109,993 | 3,119 | |||||||||
Proceeds from notes receivable from related parties | 1,872 | 2,129 | 246 | |||||||||
|
|
|
|
|
| |||||||
Net cash used in investing activities | (56,489 | ) | (225,649 | ) | (27,283 | ) | ||||||
Financing activities | ||||||||||||
Payments on long term debt and short term borrowings | (247,012 | ) | (2,780 | ) | (3,230 | ) | ||||||
Proceeds from debt issuance | 585,000 | — | — | |||||||||
Distributions to members | (211,002 | ) | (111,142 | ) | (111,858 | ) | ||||||
Repurchase of vested options | (6,411 | ) | — | — | ||||||||
Repurchase of Class A Units | (540,000 | ) | — | — | ||||||||
Other | — | — | 130 | |||||||||
|
|
|
|
|
| |||||||
Net cash used in financing activities | (419,425 | ) | (113,922 | ) | (114,958 | ) | ||||||
|
|
|
|
|
| |||||||
Net increase in cash and cash equivalents | 33,422 | 3,485 | 143,647 | |||||||||
Cash and cash equivalents—beginning of year | 361,099 | 357,614 | 213,967 | |||||||||
|
|
|
|
|
| |||||||
Cash and cash equivalents—end of year | $ | 394,521 | $ | 361,099 | $ | 357,614 | ||||||
|
|
|
|
|
| |||||||
Income taxes paid | $ | 56,705 | $ | 53,200 | $ | 43,102 | ||||||
|
|
|
|
|
| |||||||
Interest paid | $ | 12,316 | $ | 4,580 | $ | 4,912 | ||||||
|
|
|
|
|
| |||||||
Noncash activities: | ||||||||||||
Contribution of assets to Magan joint venture | $ | — | $ | 1,030 | $ | 9,126 | ||||||
Contingent consideration | $ | — | $ | 20,400 | $ | 2,559 | ||||||
Redemption of Class A Units | $ | 10,000 | $ | — | $ | — | ||||||
Tax liability assumed to repurchase Class A Units | $ | 37,019 | $ | — | $ | — | ||||||
See accompanying notes.
F-6
Table of Contents
HEALTHCARE PARTNERS HOLDINGS, LLC AND AFFILIATES
Notes to Consolidated Financial Statements
December 31, 2011
1. Organization and Business
HealthCare Partners Holdings, LLC (HCPH) is a California limited liability company that was established on February 23, 2005 in connection with a reorganization of its subsidiaries. HCPH, together with its affiliated physician groups and subsidiaries, is a patient and physician-focused, integrated health care delivery and management company, providing coordinated outcomes – based medical care in a cost-effective manner. Through capitation contracts with some of the nation’s leading health plans, HCPH has approximately 667,000 patients enrolled with health maintenance organizations (HMOs) under its care in southern California, central and south Florida and Las Vegas, Nevada. HealthCare Partners, LLC, a California limited liability company and a wholly-owned subsidiary of HCPH (HCP LLC), provides various non-medical management and administrative services, facilities, and equipment to affiliated physician-related organizations, including medical groups, independent practice associations (IPAs), and other similar organizations under long-term management services agreements. HCP LLC’s largest management service agreement is with one of HCPH’s affiliated physician groups, HealthCare Partners Affiliates Medical Group (HCPAMG). See Note 2 for additional information.
HCPAMG was formed in 1994 and is organized as a California general partnership with 30 general partners. The majority of the partners of HCPAMG are also members of HCPH. HCPAMG and its affiliates provide managed health care and related services through regional delivery systems and a joint venture (see Note 6) to approximately 586,000 enrollees in southern California under contracts with various HMOs and to privately insured individuals. Pursuant to the terms of the management services agreement between HCP LLC and HCPAMG, HCP LLC earns a management fee from HCPAMG equal to a percentage of HCPAMG’s adjusted gross revenue.
JSA Holdings, Inc., a Delaware corporation and a wholly-owned subsidiary of HCP LLC (JSAH), is a holding company that, through various subsidiaries, operates a management services organization with an integrated medical group and IPA providing physician practice management and administrative services; managed health care; retail pharmacies; and other related health care services to approximately 81,000 HMO enrollees in Florida and Nevada.
Certain entities in the consolidated group that are organized as corporations file separate corporate tax returns. A provision for income taxes is included in the consolidated financial statements for those entities (see Note 12). No provision for income taxes has been made in the consolidated financial statements for the limited liability companies or partnerships as taxes on the profits and losses for those entities are the responsibility of the individual partners and limited liability company members.
2. Summary of Significant Accounting Policies
Principles of Consolidation
Accounting Standards Codification (ASC) Section 810-10-15-14 stipulates that, generally, any entity which by design, any of the following conditions exist a) insufficient equity to finance its activities without additional subordinated financial support or b) equity holders that, as a group, lack the characteristics which evidence a controlling financial interest, is considered a Variable Interest Entity (VIE). Entities, in which a majority voting interest is owned or where HCP LLC determines that it is the primary beneficiary of a VIE through a qualitative analysis that identifies which variable interest holder has the controlling financial interest in the VIE, are included in these consolidated financial statements. The variable interest holder who has both (1) the power to direct the activities of the VIE that most significantly impact the VIE’s economic performance and (2) the obligation to absorb losses of or the right to receive benefits from the VIE which could potentially be significant
F-7
Table of Contents
to the VIE, has the controlling financial interest and is the primary beneficiary. In performing the analysis, management considered all relevant facts and circumstances, including: the design and activities of the VIE, the terms of the contracts the VIE has entered into, the nature of the VIE’s variable interests issued and how they were negotiated with or marketed to potential investors, and which parties participated significantly in the design or redesign of the entity.
The financial statements of HCPAMG are consolidated with HCP LLC. HCP LLC determined that HCPAMG qualifies as a variable interest entity and HCP LLC has a variable interest in HCPAMG through its management services agreement. HCP LLC engages, on an exclusive authority basis, to provide all non-medical management and administrative services to HCPAMG. The management services agreement commenced in February 2005 and continues for 20 years. Pursuant to the management services agreement, HCPAMG is solely responsible for all aspects of the practice of medicine and provision of patient care. HCPAMG provides professional medical services to the HCP LLC – managed medical facilities and IPAs that are located in California under a management services agreement, and employs physicians or contracts with various other independent physicians and physician groups to provide the professional medical services in California. HCP LLC obtains professional medical services from HCPAMG in California, rather than provide such services directly or through subsidiaries, in order to comply with California’s prohibition against the corporate practice of medicine. HCP LLC provides non-medical, technical and administrative services to HCPAMG for which it receives a management fee, per the management services agreement. Through the management services agreement, HCP LLC has exclusive authority over all non-medical decision making related to the ongoing business operations of HCPAMG.
Pursuant to the management services agreement with HCPAMG, HCP LLC determines the annual budget of HCPAMG and makes all physician employment decisions. HCPAMG has de minimis equity and working capital as, through the management services agreement, all of HCPAMG’s cash flows are transferred to HCP LLC. As such, HCP LLC has determined that HCPAMG is a variable interest entity, and that HCP LLC is the primary beneficiary, and consequently, HCP LLC has consolidated the revenue and expenses of HCPAMG. HCPAMG recognized $1.4 billion of revenue and expenses for the year ended December 31, 2011, $1.1 billion of revenue and expenses for the year ended December 31, 2010, and $0.9 billion of revenue and expenses for the year ended December 31, 2009. The cash flows of HCPAMG are included in the accompanying consolidated statements of cash flows.
The creditors of HCPAMG do not have recourse to HCP LLC general credit and there are no other arrangements that could expose HCP LLC to losses. However, HCPAMG is managed to recognize no net income or net loss and, therefore, HCP LLC may be required to provide financial support to cover any operating expenses in excess of operating revenues. HCPAMG and its wholly owned subsidiaries total assets and liabilities as of December 31, 2011 are $198.8 million and $183.1 million, respectively. HCPAMG and its wholly owned subsidiaries total assets and liabilities as of December 31, 2010 are $190.7 million and $178.6 million, respectively.
HCP LLC, through its wholly-owned subsidiary, HealthCare Partners Nevada, LLC, a Nevada limited liability company (HCPNV), also consolidates the financial statements of Amir Bacchus, MD Fremont Medical Center, Ltd (Bacchus-Fremont), a professional medical corporation providing medical services through facilities in Las Vegas, Nevada. Bacchus-Fremont has entered into a 20-year management services agreement with HCPNV, and a stock restriction agreement, under which the sole shareholder of Bacchus-Fremont is restricted from transferring or selling the shares other than to a designee of HCPNV. Upon certain events set forth in such management services agreement, the shares held by the sole shareholder will be automatically transferred to a designee of HCPNV for a nominal amount. HCP LLC has determined that Bacchus-Fremont qualifies as a variable interest entity and that it has a variable interest in Bacchus-Fremont through the management services agreement.
The consolidated financial statements include the accounts and operating results of HCPH, HCP Blocker Corporation (see Note 10), HCP LLC and its wholly-owned subsidiaries, HCPAMG and its wholly-owned subsidiaries, Bacchus-Fremont, and HCPMG, Inc., a California professional corporation (HCPMGI) a payroll/staff leasing company which provides clinical and administrative staff services to HCP LLC and HCPAMG. The wholly-owned subsidiaries of HCP LLC include JSAH, HCPNV, DNH Medical Management, Inc. (d/b/a/ the
F-8
Table of Contents
Camden Group), a California professional corporation, Northridge Medical Services Group, Inc., a California corporation (NMSG), Talbert Health Services, Inc., a California professional corporation, HealthCare Partners ASC-L.B., LLC, a California limited liability company (HCP ASC-L.B., and previously known as HealthCare Partners Medical Plan, LLC), and HealthCare Partners South Florida. a Florida limited liability company (HCP So FL). The wholly owned subsidiaries of HCPAMG include Talbert Medical Group, Inc. and subsidiaries (TMG), Northridge Medical Group, Inc. (NMG) and Physician Associates of the Greater San Gabriel Valley, Inc. (PA), all of which are controlled by HCP LLC via Nominee Agreements with HCP LLC and HCPAMG, which, among other things, provides HCP LLC the ability to control the sole director of each of the subsidiaries, provides for limitations on the ability of HCPAMG to sell or transfer the stock without concurrence of HCP LLC and provides that any dividends issued by each subsidiary shall be payable to HCP LLC. The operating results of acquisitions are included from the date of acquisition. The entities are collectively referred to as “the Company.” All significant intercompany accounts and transactions have been eliminated in consolidation. As discussed in Note 6, HCPAMG also has a 50% interest in Magan Medical Group (Magan), a joint venture with Magan Medical Clinic, Inc., and HCP LLC has a 67% interest in California Medical Group Insurance Company Risk Retention Group, an Arizona corporation (CMGI). These investments are accounted for using the equity method.
Use of Estimates
The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements. Estimates also affect the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates.
The most significant areas requiring the use of estimates include settlements under risk-sharing programs, medical receivables, determination of allowances for uncollectible accounts and retroactive premium adjustments, estimates of medical claims and related payables, valuation of goodwill, valuation of long-lived and intangible assets, estimates of self-insured claims reserves, and assessment of uncertain income tax positions.
Reclassifications
Certain prior year information has been reclassified to conform to the current year presentation. Changes occurred in accounts receivable-health plans and accounts payable-health plans in order to distinguish accounts payable from accounts receivable.
Medical Revenues and Cost Recognition
Professional Capitation Revenue
The Company’s medical group affiliates are licensed to contract with HMOs to provide physician services in California and to provide both hospital and physician services under global risk capitation contracts in Florida and Nevada. Medical revenues consist primarily of fees for medical services provided by the medical group entities under capitated contracts with various HMOs or under fee-for-service type arrangements with privately insured individuals, and revenues under risk-sharing programs. Capitation revenue under HMO contracts is prepaid monthly based on the number of enrollees electing physicians affiliated with one of the medical group entities as their health care provider, regardless of the level of actual medical services utilized. Capitation revenue is reported as revenue in the month in which enrollees are entitled to receive health care. A portion of the capitation revenue pertaining to Medicare enrollees is subject to possible retroactive premium risk adjustments based on their individual acuity. Due to lack of sufficient data to project the amount of such retroactive adjustments, the Company records any corresponding retroactive revenues in the year of receipt. During 2011, 2010, and 2009, the Company recorded approximately $14.3 million, $19.5 million and $15.5 million, respectively, of additional revenue related to prior year premium risk adjustments. Fee-for-service revenues (including patient co-pays) are recorded when the services are provided.
F-9
Table of Contents
Hospital Risk Share Revenue
Depending on the state regulation regarding global risk capitation, revenues may be received by HCPH or an independent hospital with which HCPH contracts. In the Florida and Nevada service markets, the global capitation revenue is recorded by HCPH with the corresponding cost of medical care reported by HCPH as hospital expenses. In California, the independent hospitals receive the global capitation revenues. The revenues are used to pay medical claims for the related enrollees. HCPH is entitled to any residual amounts and bears the risk of any deficits. In all cases, an estimate is made for the cost of medical services that have been rendered and where no medical claim has been received (IBNR).Under risk-sharing programs, the medical groups share in the risk for hospitalization services and earn additional incentive revenues or incur penalties based on the utilization of hospital services. Estimated shared-risk receivables from the HMOs are recorded based upon hospital utilization and associated costs incurred by assigned HMO enrollees, including an estimate of IBNR compared to budgeted funding. Differences between actual contract settlements and estimated receivables are recorded in the year of final settlement. During 2011, 2010, and 2009, the Company recorded favorable changes in estimates related to its prior year shared risk settlements in the amount of $37.5 million, $31.6 million and $20.0 million, respectively, as a result of lower than expected claim costs. The medical groups also receive other incentive payments from health plans based on specified performance and quality criteria. These amounts are accrued when earned and the amounts can be reasonably estimated, and are included in medical revenues. The Company also earns revenues from retail pharmacies, management fees, and health care consulting services. These amounts are included in other operating revenue when services are provided.
Medical Costs
The medical groups are responsible for the medical services the affiliated physicians and contracted hospitals provide to assigned HMO enrollees. The Company provides medical services to health plan enrollees through a network of contracted providers under sub-capitation and fee-for-service arrangements, company-operated clinics and staff physicians. Medical costs for professional and institutional services rendered by employed and contracted providers are recorded as medical expenses and hospital expenses, respectively, in the consolidated statements of income. Costs for operating medical clinics, including the salaries of non-medical personnel and support costs, are recorded in clinic support and other operating costs.
An estimate of amounts due to contracted physicians, hospitals, and other professional providers is included in medical claims and related payables in the accompanying consolidated balance sheets. Medical claims payable include claims reported as of the balance sheet date and estimates of IBNR. Such estimates are developed using actuarial methods and are based on many variables, including the utilization of health care services, historical payment patterns, cost trends, product mix, seasonality, changes in membership, and other factors. The estimation methods and the resulting reserves are continually reviewed and updated. Many of the medical contracts are complex in nature and may be subject to differing interpretations regarding amounts due for the provision of various services. Such differing interpretations may not come to light until a substantial period of time has passed following the contract implementation. Any adjustments to reserves are reflected in current operations. The Company recorded favorable changes in estimates to prior year medical claims and related payables balances in 2011, 2010, and 2009 totaling $5.3 million, $5.1 million, and $4.2 million, respectively.
Laws and regulations governing the Medicare and Medicaid programs are complex and subject to interpretation. The Company believes that they are in compliance with all applicable laws and regulations and are not aware of any pending or threatened investigations involving allegations of potential wrongdoing. While no such regulatory inquiries have been made, compliance with such laws and regulations can be subject to future government review and interpretation, as well as significant regulatory action, including fines, penalties, and exclusion from the Medicare and Medicaid programs.
F-10
Table of Contents
Fee for Service Revenue
The Company provides medical services to patients on a fee for service basis. Revenues are recorded at the time of service along with an estimate for contractually required discounts (contractual allowances). These estimates are based on historical collection trends for categories of payors. Estimates are made monthly for uncollectible accounts based on the payor mix of open accounts receivable and the length of time the account has been outstanding. At December 31, 2011, the Company had total outstanding patient accounts receivable of $47.6 million, with contractual allowances of $21.1 million and reserves for uncollectible accounts of $4.2 million. At December 31, 2010, the Company had total outstanding patient accounts receivable of $41.4 million, with contractual allowances of $23.6 million and reserves for uncollectible accounts of $0.5 million.
Share-Based Compensation
HCPH issues warrants and options to purchase common member units to the Company’s employees as part of the compensation program. The Company accounts for share-based compensation under ASC 718, Stock Compensation, which requires the measurement and recognition of compensation expense for all share-based payment awards made to employees and non-employee directors based on their estimated fair values on the date of grant. The warrants vest based on performance objectives and the related expense is recorded over the service period when achievement is probable. The options vest based on continuous service, and the related expense is recorded ratably over the service period (which is generally the vesting period), from the date of grant.
Medical Malpractice Liability Insurance
The Company maintains medical malpractice insurance through various independent and related-party insurance companies. The Company purchased its primary medical malpractice coverage from CMGI, in which the Company holds a 67% equity interest with other medical providers (see Note 6). Insurance coverage is on a claims-made basis with individual claim deductibles ranging from $0 to $500,000 and a maximum insurance limit per claim of $1.0 million. The annual aggregate limits are $3.0 million per insured individual and per medical group.
In August 2010, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) 2010-24,Healthcare Entities(Topic 954):Presentation of Insurance Claims and Recoveries, which modifies the presentation of insurance claims and related insurance recoveries. The update clarifies that health care entities should no longer net insurance recoveries against related claim liability; the claim liability should be determined without consideration of insurance recoveries. The Company adopted ASU 2010-24 on January 1, 2011 and did not elect to retrospectively apply the new presentation to its 2010 consolidated financial statements. The adoption of this ASU resulted in the establishment of an insurance recoverable, primarily recorded in other noncurrent assets of $4.2 million and an increase in other liabilities of $4.2 million as of December 31, 2011.
The Company estimates its accrual for medical malpractice claims, including IBNR, based upon each medical group’s separate claims experience using a 4% discount rate. The medical malpractice accrual, gross of anticipated insurance recoveries of $4.2 million, is $16.1 million as of December 31, 2011. The medical malpractice accrual, net of anticipated insurance recoveries, is $11.4 million as of December 31, 2010. The medical malpractice accrual is included in other noncurrent liabilities, except for the current portion, which is included in accounts payable and accrued expenses.
Workers’ Compensation Insurance
The Company maintains various workers’ compensation policies with deductibles ranging from $0 to $500,000 per claim and annual aggregate coverage for cumulative claims up to $4.5 million. Accruals for uninsured
F-11
Table of Contents
claims, deductibles, and IBNR totaled $6.8 million and $6.5 million at December 31, 2011 and 2010, respectively, and are estimated based upon the Company’s claims experience using a 4% discount rate. These estimates are included in other noncurrent liabilities except for the current portion, which is included in accounts payable and accrued expenses.
Cash Equivalents
Cash equivalents consist of money market mutual funds and certificates of deposit with remaining maturities, on the acquisition date, of three months or less.
Funds on Deposit with a Third Party
The Company has established a risk sharing arrangement with a local hospital, wherein the Company shares in any surplus or deficit. One of the terms of this agreement is the establishment of a segregated investment fund to ensure adequate cash to pay IBNR. The Company and hospital monitor the reserve balance to maintain the adequacy of funds on deposit. The Company has recorded $63.6 million and $66.7 million as of December 31, 2011 and 2010, respectively, in funds on deposit with a third party. See Note 5 (“Fair Value Measurements”).
Investments
The Company has determined that all investments held are available for sale. Accordingly, such investments are carried at fair value with unrealized gains and losses excluded from earnings and reported as a separate component of equity in other comprehensive income. The Company also holds auction rate securities, which are recorded as other noncurrent assets at an estimated fair value of $3.0 million at December 31, 2011 and 2010. In addition, at December 31, 2010, the Company held auction rate securities of $3.7 million, included as a current asset in investments due to the anticipated call of the security in 2011. Due to the lack of an active market, the estimated fair value of these auction rate securities was based on independent appraisals and discounted cash flow valuation models, which took into consideration the collateral underlying these securities, credit risks related to the securities and the issuers, interest rate spreads and illiquidity factors.
Investment income consists of interest, which is recognized on an accrual basis. Interest income on mortgage-backed and asset-backed securities is determined using the effective yield method based on estimated prepayments.
Gains and losses with respect to dispositions of investments are based on the specific-identification method.
Goodwill and Intangible Assets
Goodwill is not amortized, but is subject to an impairment test annually or more frequently if certain indicators of impairment are present. As of December 31, 2011, the Company early adopted ASU 2011-08,Testing Goodwill for Impairment. As such, the Company assessed qualitative factors to determine whether it is more likely than not that the fair value of the reporting units is less than the carrying value. As a result of this assessment, it was determined that performing the two-step impairment test was not necessary. The Company’s analysis indicated that goodwill had not been impaired as of December 31, 2011 and 2010.
Identifiable intangible assets with definite useful lives are amortized over periods between 2 and 26 years (see Note 8). Intangible assets are measured for impairment when events or changes in business conditions suggest that the carrying value of an asset may not be recovered. No intangible assets were deemed to be impaired at December 31, 2011 and 2010.
Property and Equipment
Building, equipment, furniture, and leasehold improvements are stated at cost less accumulated depreciation. Expenditures for maintenance and repairs are charged against operations as incurred. Leasehold improvements
F-12
Table of Contents
are amortized over the shorter of their useful lives or the term of the associated leases. Amortization of assets recorded under capital leases is included in depreciation and amortization expense and is computed using the straight-line method over the useful lives or lease terms, if shorter. Depreciation of equipment and furniture is computed using the straight-line method over the useful lives of the assets which range from five to seven years. Buildings are depreciated based on lives ranging from 16 to 25 years.
Fair Values of Financial Instruments
Financial instruments consist mainly of cash and cash equivalents, investments, auction rate securities, accounts and notes receivable, medical claims and related payables, accounts payable and accrued expenses and long-term debt. The fair values of cash and cash equivalents, accounts and notes receivable, medical claims and related payables, and accounts payable and accrued expenses approximate their carrying amounts due to their short-term nature. The estimated fair values for available for sale securities, excluding auction rate securities, generally represent quoted market prices for securities traded in the public marketplace or estimated values for securities not traded in the public marketplace. See Note 5 for further information. The estimated fair values for auction rate securities generally represent valuation models utilizing unobservable inputs, such as estimated repurchase scenarios. Management believes that the term loan’s carrying value approximates fair value given it was borrowed during the current year. Self-insured liabilities are recorded at the estimated present value of claims obligations using appropriate discount rates.
Concentrations of Credit Risk
Financial instruments which potentially subject the Company to concentrations of credit risk consist principally of cash and cash equivalents, investments, medical-related and other receivables, and notes receivable from related parties.
Investments are managed under documented investment guidelines established by the Board of Directors which limit the amounts that can be invested in any one issuer or industry. At December 31, 2011 and 2010, the Company invested $340.9 million and $324.4 million, respectively, in a portfolio of highly liquid money market securities comprised of high grade tax exempt municipal bonds, and approximately $3.0 million and $6.7 million, respectively, in auction rates securities. The money market fund shares trade at a net asset value (NAV) of $1 per share, which represents the price at which investors buy (bid price) and sell (redemption price) fund shares from and to the fund companies. The Company monitors the value of the funds periodically for potential indicators of impairment.
The NAV is computed using the closing market prices of the portfolio’s securities. Although the funds seek to preserve the value of the investment at $1 per share, it is possible to lose principal if the underlying securities suffer losses. The money market funds can be withdrawn at any time without restriction. The auction rate securities are uninsured and are held in two portfolios backed by life insurance policies and high grade short-term investments. These debt securities are serviced by cash flows from the underlying instruments. The Company also has cash in financial institutions which are insured by the Federal Deposit Insurance Corporation (FDIC) at up to $250,000 for each qualifying account. At various times throughout the year the Company has cash in financial institutions which exceed the FDIC insurance limit. Management reviews the financial condition of these financial institutions on a periodic basis and does not believe this concentration of cash results in a high level of risk for the Company. The Company’s credit risk with respect to medical-related and other receivables is limited, as a majority of the receivables are due from large HMOs or insurance companies. During 2011 and 2010, the Company received 70.0% of its medical revenues from three health plans and in 2009, 72.6% of its medical revenues were received from three health plans. Management does not believe that there are any significant credit risks associated with these organizations. The notes receivable from related parties are fully secured by real property of adequate value.
F-13
Table of Contents
Advertising Costs
The Company expenses advertising and promotional costs as incurred as clinic support and other operating costs or as general and administrative expenses. In 2011, 2010, and 2009, the Company incurred advertising and promotional expenses totaling $4.1 million, $2.0 million and $1.6 million, respectively.
Adoption of New Accounting Pronouncements
In September 2011, the FASB issued ASU Number 2011-08,Intangibles—Goodwill and Other, which modifies the guidance related to testing goodwill for impairment. This guidance provides entities with an option to assess qualitative factors to determine whether it is more-likely-than-not that the fair value of the reporting unit is less than its carrying amount. If, after the assessment, an entity determines that it is not more-likely-than-not that the fair value is less than its carrying amount, then performing the two-step goodwill impairment test is not necessary. If an entity concludes otherwise, then the entity is required to perform the first step of the two-step impairment by calculating the fair value of the reporting unit. An entity has the option to bypass the qualitative assessment for any reporting unit in any period and proceed directly to performing the first step of the two-step goodwill impairment test. This guidance is effective for interim and annual goodwill impairment tests beginning after December 15, 2011, and early adoption is permitted. The Company early adopted ASU 2011-08 as of December 31, 2011. The adoption of this accounting standards update did not have a material impact on the Company’s financial position, results of operations, or cash flows.
New Accounting Pronouncements Not Yet Adopted
In May 2011, the FASB issued ASU Number 2011-04,Fair Value Measurement, which modifies the fair value measurement and disclosure guidance. This guidance results in new disclosures primarily related to Level 3 measurements, including quantitative disclosure about unobservable inputs and assumptions, a description of the valuation processes, and a narrative description of the sensitivity of the fair value to changes in unobservable inputs. This guidance is effective for annual periods beginning after December 15, 2011, and early adoption is not permitted. The adoption of this accounting standards update will not have a material impact on the Company’s financial position, results of operations, or cash flows.
In July 2011, the FASB issued an accounting standards update modifying the presentation and disclosure of patient service revenue, provision for bad debts, and the allowance for doubtful accounts. The guidance changes the presentation on the statement of operations by requiring the reclassification of the provision for bad debts associated with patient service revenues from an operating expense to a deduction from patient service revenue (net of contractual allowances and discounts). Additionally, the amendment requires disclosures regarding the entity’s policy for recognizing patient service revenue and assessing bad debts. Qualitative and quantitative information about changes in the allowance for doubtful accounts is required.
This guidance is effective for interim and annual periods beginning after December 15, 2012, and should be applied retrospectively to all prior periods presented. The adoption of this accounting standards update will not have a material impact on the statement of financial position, income statement, or cash flows.
In June 2011, the FASB issued ASU Number 2011-05,Presentation of Comprehensive Income, which changed the disclosure requirements for the presentation of other comprehensive income (OCI) in the financial statements, including eliminating the option to present OCI in the statement of stockholders’ equity. OCI and its components will be required to be presented for both interim and annual periods either in a single financial statement, the statement of comprehensive income, or in two separate but consecutive financial statements consisting of a statement of income followed by a separate statement presenting OCI. This standard is required to be applied retrospectively beginning January 1, 2012, except for certain provisions for which adoption was delayed.
F-14
Table of Contents
3. Acquisitions
Outcome Based Delivery Systems, LLC
On October 1, 2011, JSAH, HCP So FL, HCPNV, and Bacchus-Fremont acquired the assets of Outcome Based Delivery Systems, LLC (OBDS). OBDS was a physician group practice and management services organization serving a fee-for-service patient population and approximately 4,600 senior HMO enrollees in Florida and Nevada. The acquisition included contracts with employed physicians, health maintenance organizations, and affiliated physicians, as well as non-compete agreements with former owners. The total acquisition consideration amounted to $23.0 million, plus adjustments for certain prepaid items and physician tail insurance coverage obligations totaling $0.4 million. There was no contingent consideration as part of the acquisition. $2.3 million of the acquisition consideration was deposited into an escrow account with an escrow agent to secure certain obligations of the seller for a one year period.
Fair value as of the acquisition date of the non-compete agreements, customer relationships, provider networks, and trade names was determined by our management. Management considers multiple factors including an analysis performed by third-party valuation specialists. The amortization period for the customer relationships, trade names, provider networks and non-compete agreements are 10, 15, 5 and 5 years, respectively.
Specialty Medical Centers
On March 1, 2011, HCPNV acquired the assets of Specialty Medical Centers for $8.7 million. Located in Pahrump, Nevada, Specialty Medical Centers provides primary care and medical diagnostic services to patients through seven clinics in the southern Nevada area. The Company recorded goodwill, customer relationships and provider network of $3.7 million, $3.4 million and $1.3 million, respectively related to this acquisition. The amortization period for the customer relationships and provider networks are 10 and 5 years, respectively.
Talbert Medical Group
On May 1, 2010, HCPAMG and HCP LLC acquired all outstanding shares of Talbert Medical Group, Inc. (TMG) and their subsidiary entities, Talbert Surgical Associates, LLC, Mosaic Management Services, Inc., Talbert Health Plan Services, Inc. and Talbert Health Plan, Inc. for $28.8 million cash consideration. These companies provide managed health care services to approximately 78,500 managed care enrollees in Southern California. The selling shareholders are also eligible to receive up to an additional $28.0 million over a two year period, contingent upon attainment of certain performance criteria. The Company has estimated a fair value of $19.9 million for the contingent consideration at the acquisition date and has included this amount as purchase consideration. The Company recorded the current portion of this amount in accounts payable and accrued expenses and the noncurrent portion in other liabilities at December 31, 2010. In 2011, the Company paid contingent consideration totaling $8.1 million, reduced the outstanding contingent consideration to $10.4 million and reduced operating expenses by $1.4 million. As a stock purchase, the goodwill and a significant portion of the intangible assets acquired are not deductible for income tax purposes. Future tax liabilities related to the fair value of the identifiable intangible assets in excess of the tax deductible amounts have been recorded as deferred tax liabilities on the acquisition date (see Note 12). The acquisition agreement provides for a final working capital settlement, which is not expected to result in significant adjustments to the purchase consideration.
Other Acquisitions
The Company also made acquisitions of various physician practices for total consideration of $7.8 million for 2011. Consideration for 2011 acquisitions was allocated primarily to customer relationships and goodwill.
F-15
Table of Contents
A summary of the acquisitions is as follows (dollars in thousands):
| 2011 | ||||
Acquisition consideration: | ||||
Cash consideration, net of cash acquired | $ | 39,819 | ||
Note payable | 150 | |||
Contingent consideration | — | |||
|
| |||
Aggregate purchase consideration | 39,969 | |||
Allocation of purchase price: | ||||
Tangible assets (excluding cash), net of liabilities assumed | 932 | |||
Amortizable intangibles: | ||||
Trade names | 678 | |||
Provider network | 2,327 | |||
Non-compete agreements | 9,604 | |||
Customer relationships | 13,565 | |||
|
| |||
Total identifiable assets | 27,106 | |||
Net deferred tax liabilities on book-tax basis difference in assets acquired | — | |||
Goodwill | 12,863 | |||
|
| |||
Total acquired assets | $ | 39,969 | ||
|
| |||
4. Investments
The following tables summarize the Company’s investments as of the dates indicated (dollars in thousands):
| December 31, 2011 | ||||||||||||||||
| Cost or Amortized Cost | Gross Unrealized | Estimated Fair Value | ||||||||||||||
| Gains | Losses | |||||||||||||||
Municipal bonds | $ | 117,149 | $ | 430 | $ | (39 | ) | $ | 117,540 | |||||||
Corporate bonds | 36,625 | 108 | (286 | ) | 36,447 | |||||||||||
Asset and mortgage backed bonds | 10,177 | 13 | (7 | ) | 10,183 | |||||||||||
U.S. Treasury bonds | 8,086 | 5 | — | 8,091 | ||||||||||||
Government related bonds | 2,201 | 19 | — | 2,220 | ||||||||||||
Agency bonds | 617 | — | — | 617 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total investments | $ | 174,855 | $ | 575 | $ | (332 | ) | $ | 175,098 | |||||||
|
|
|
|
|
|
|
| |||||||||
| December 31, 2010 | ||||||||||||||||
| Cost or Amortized Cost | Gross Unrealized | Estimated Fair Value | ||||||||||||||
| Gains | Losses | |||||||||||||||
Municipal bonds | $ | 127,871 | $ | 48 | $ | (218 | ) | $ | 127,701 | |||||||
Corporate bonds | 29,474 | 89 | (48 | ) | 29,515 | |||||||||||
Asset and mortgage backed bonds | 11,147 | 3 | (22 | ) | 11,128 | |||||||||||
U.S. Treasury bonds | 251 | 1 | — | 252 | ||||||||||||
Government related bonds | 6,091 | 28 | (18 | ) | 6,101 | |||||||||||
Agency bonds | 898 | 12 | — | 910 | ||||||||||||
Auction rate security | 3,726 | — | — | 3,726 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total investments | $ | 179,458 | $ | 181 | $ | (306 | ) | $ | 179,333 | |||||||
|
|
|
|
|
|
|
| |||||||||
F-16
Table of Contents
Expected maturities differ from contractual maturities because borrowers may have the right to call or prepay obligations with or without call or prepayment penalties. The contractual maturities of investments as of December 31, 2011 are summarized below (dollars in thousands):
| Cost or Amortized Cost | Estimated Fair Value | |||||||
Due in one year or less | $ | 49,823 | $ | 49,904 | ||||
Due one year through five years | 98,273 | 98,428 | ||||||
Due after five years through ten years | 6,276 | 6,276 | ||||||
Due after ten years | 10,306 | 10,307 | ||||||
Asset and mortgaged backed bonds | 10,177 | 10,183 | ||||||
|
|
|
| |||||
| $ | 174,855 | $ | 175,098 | |||||
|
|
|
| |||||
Gain on sale of investments, net, is primarily comprised of realized gains and minimal realized losses for the years ended December 31, 2011, 2010 and 2009.
At each reporting date, the Company performs an evaluation of impaired investments to determine if the unrealized losses are other-than-temporary. The Company determines whether it intends to sell, or if it is more-likely-than-not that it will be required to sell, impaired securities. For all impaired debt securities for which there is no intent or expected requirement to sell, the evaluation considers whether it is likely the amortized cost value will be recovered. The Company did not identify any other-than-temporary impairment in investments for the years ended December 31, 2011 and December 31, 2010.
The following table shows the Company’s gross unrealized losses and estimated fair value, aggregated by investment category and length of time that individual securities have been in a continuous unrealized loss position (dollars in thousands):
| December 31, 2011 | ||||||||||||||||||||||||
| Less than 12 Months | 12 Months or More | Total | ||||||||||||||||||||||
| Estimated Fair | Unrealized | Estimated Fair | Unrealized | Estimated Fair | Unrealized | |||||||||||||||||||
| Value | Losses | Value | Losses | Value | Losses | |||||||||||||||||||
Municipal bonds | $ | 9,748 | $ | (39 | ) | $ | 410 | $ | — | $ | 10,158 | $ | (39 | ) | ||||||||||
Corporate bonds | 11,359 | (251 | ) | 3,078 | (35 | ) | 14,437 | (286 | ) | |||||||||||||||
Asset and mortgage backed bonds | 3,667 | (4 | ) | 1,023 | (3 | ) | 4,690 | (7 | ) | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
| $ | 24,774 | $ | (294 | ) | $ | 4,511 | $ | (38 | ) | $ | 29,285 | $ | (332 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
| December 31, 2010 | ||||||||||||||||||||||||
| Less than 12 Months | 12 Months or More | Total | ||||||||||||||||||||||
| Estimated Fair | Unrealized | Estimated Fair | Unrealized | Estimated Fair | Unrealized | |||||||||||||||||||
| Value | Losses | Value | Losses | Value | Losses | |||||||||||||||||||
Municipal bonds | $ | 61,259 | $ | (218 | ) | $ | — | $ | — | $ | 61,259 | $ | (218 | ) | ||||||||||
Corporate bonds | 12,623 | (48 | ) | — | — | 12,623 | (48 | ) | ||||||||||||||||
Asset and mortgage backed bonds | 5,695 | (22 | ) | — | — | 5,695 | (22 | ) | ||||||||||||||||
Government related bonds | 3,335 | (18 | ) | — | — | 3,335 | (18 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
| $ | 82,912 | $ | (306 | ) | $ | — | $ | — | $ | 82,912 | $ | (306 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
The Company held no investments at December 31, 2009.
F-17
Table of Contents
5. Fair Value Measurements
A three-level valuation hierarchy is used to classify inputs into the measurement of assets and liabilities at fair value. The following is a brief description of those three levels:
| • | Level 1: Observable inputs such as quoted prices (unadjusted) in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities. |
| • | Level 2: Inputs other than quoted prices are observable for the asset or liability, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active. |
| • | Level 3: Unobservable inputs that are used when little or no market data is available and reflect the reporting entity’s own assumptions. These include pricing models, discounted cash flow methodologies and similar techniques that use significant unobservable inputs. |
Assets and liabilities measured at fair value are based on one or more of three valuation techniques noted in the tables below:
| (a) | Market approach. Prices and other relevant information generated by market transactions involving identical or comparable assets or liabilities. |
| (b) | Cost approach. Amount that would be required to replace the service capacity of an asset (replacement cost). |
| (c) | Income approach. Techniques to convert future amounts to a single present amount based on market expectations (including present value techniques, option-pricing, and excess earnings models). |
The Company held the following financial assets and liabilities measured at estimated fair value on a recurring basis (dollars in thousands)
| Estimated Fair Value Measurements at Reporting Date Using | Valuation Technique (a,b,c) | |||||||||||||||||||
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||||||
December 31, 2011 | ||||||||||||||||||||
Assets: | ||||||||||||||||||||
Investments: | ||||||||||||||||||||
Municipal bonds | $ | 117,540 | $ | — | $ | 117,540 | $ | — | a | |||||||||||
Corporate bonds | 36,447 | — | 36,447 | — | a | |||||||||||||||
Asset and mortgage-backed bonds | 10,183 | — | 10,183 | — | a | |||||||||||||||
U.S. Treasury bonds | 8,091 | — | 8,091 | — | a | |||||||||||||||
Government related bonds | 2,220 | — | 2,220 | — | a | |||||||||||||||
Agency bonds | 617 | — | 617 | a | ||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
| 175,098 | — | 175,098 | — | |||||||||||||||||
Funds on deposit with third party | 63,638 | — | 63,638 | — | a | |||||||||||||||
Other investments, auction rate securities | 2,950 | — | — | 2,950 | c | |||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Total assets measured at fair value | $ | 241,686 | $ | — | $ | 238,736 | $ | 2,950 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Liabilities: | ||||||||||||||||||||
Contingent consideration | $ | 10,408 | $ | — | $ | — | $ | 10,408 | c | |||||||||||
|
|
|
|
|
|
|
| |||||||||||||
F-18
Table of Contents
| Estimated Fair Value Measurements at Reporting Date Using | Valuation Technique (a,b,c) | |||||||||||||||||||
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||||||
December 31, 2010 | ||||||||||||||||||||
Assets: | ||||||||||||||||||||
Investments: | ||||||||||||||||||||
Municipal bonds | $ | 127,701 | $ | — | $ | 127,701 | $ | — | a | |||||||||||
Corporate bonds | 29,515 | — | 29,515 | — | a | |||||||||||||||
Asset and mortgage-backed bonds | 11,128 | — | 11,128 | — | a | |||||||||||||||
U.S. Treasury bonds | 252 | — | 252 | — | a | |||||||||||||||
Government related bonds | 6,101 | — | 6,101 | — | a | |||||||||||||||
Agency bonds | 910 | — | 910 | a | ||||||||||||||||
Auction rate securities | 3,726 | — | — | 3,726 | c | |||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
| 179,333 | — | 175,607 | 3,726 | |||||||||||||||||
Funds on deposit with third party | 66,688 | — | 66,688 | — | a | |||||||||||||||
Other investments, auction rate securities | 2,950 | — | — | 2,950 | c | |||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Total assets measured at fair value | $ | 248,971 | $ | — | $ | 242,295 | $ | 6,676 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Liabilities: | ||||||||||||||||||||
Contingent consideration | $ | 20,533 | $ | — | $ | — | $ | 20,533 | c | |||||||||||
|
|
|
|
|
|
|
| |||||||||||||
6. Magan Joint Venture and Other Investment
The Company has a 50% interest in a joint venture, Magan, and a 67% interest in CMGI, which are accounted for using the equity method. The carrying value of Magan was $3.9 million and $7.8 million at December 31, 2011 and 2010, respectively, and is included in other assets. HCP LLC provides management services to Magan and earned management fees of approximately $10.0 million, $9.3 million and $7.0 million in 2011, 2010 and 2009, respectively, which are included in other operating revenue. HCPAMG’s allocation of earnings from Magan was $24.6 million, $15.1 million and $11.5 million in 2011, 2010 and 2009, respectively, and is recorded as equity earnings of unconsolidated joint ventures. The joint venture engages in practice of medicine that constitutes a component of the Company’s principal revenue-generating activities; therefore, earnings from the joint venture are included in operating income.
As of December 31, 2010, the Company had contributed a total of $10.3 million of goodwill and intangible assets to Magan. The goodwill and intangibles represent approximately 25,000 managed care enrollees whose primary care physician practice is in a defined geography. In exchange for the joint venture partner’s 50% interest in the contributed assets, the Company received a note in the amount of $4.6 million, bearing interest at prime plus 1%. The outstanding balance of the note receivable was $0 and $3.4 million at December 31, 2011 and 2010, respectively.
HCP LLC holds a 67% ownership interest in CMGI, which was initially formed with three other medical groups from the southern California area. Two of the four members of the board of directors of CMGI are executives of HCP LLC. CMGI, domiciled in Arizona, allocates profits and losses based on the ownership interests of its investor groups. The Company accounts for this investment using the equity method as discussed in Note 2. The carrying value of CMGI is included in other assets. CMGI currently provides professional medical malpractice coverage to the Company. Premiums paid to CMGI for 2011 and 2010 coverage totaled $0.6 million and $1.2 million, respectively, net of refunds received of $1.4 million and $1.5 million in 2011 and 2010, respectively.
F-19
Table of Contents
Summarized financial information for the Company’s joint ventures as of and for the year ended December 31 included (dollars in thousands):
| 2011 | 2010 | |||||||
Assets | $ | 25,912 | $ | 28,747 | ||||
Liabilities | 13,827 | 17,435 | ||||||
Net income | 49,557 | 32,566 | ||||||
7. Property and Equipment
Building, equipment, furniture and improvements at December 31 consist of the following (dollars in thousands):
| 2011 | 2010 | |||||||
Land | $ | 10,684 | $ | 10,684 | ||||
Buildings | 10,271 | 10,271 | ||||||
Equipment | 96,813 | 88,129 | ||||||
Furniture and fixtures | 16,006 | 14,791 | ||||||
Leasehold improvements | 47,674 | 40,567 | ||||||
Building and equipment under capital leases | 1,114 | 710 | ||||||
Construction in process | 6,514 | 3,001 | ||||||
|
|
|
| |||||
| 189,076 | 168,153 | |||||||
Less accumulated depreciation | (113,228 | ) | (101,260 | ) | ||||
|
|
|
| |||||
| $ | 75,848 | $ | 66,893 | |||||
|
|
|
| |||||
Depreciation expense was $15.0 million, $13.5 million and $13.1 million in 2011, 2010, and 2009, respectively.
8. Goodwill and Intangible Assets
Goodwill
The following is a summary of changes in the Company’s recorded goodwill during 2010 and 2011 (dollars in thousands):
Balance at January 1, 2010 | $ | 227,078 | ||
Sale of PA assets to Magan Medical Clinic | (515 | ) | ||
Contribution of PA assets to Magan Medical Group | (515 | ) | ||
Acquisition of TMG | 35,960 | |||
Acquisition of Rainbow Medical Centers | 2,936 | |||
Acquisition of Advanced Medical Centers | 5,194 | |||
Other | 5 | |||
|
| |||
Balance at December 31, 2010 | 270,143 | |||
Acquisition of Specialty Medical Centers | 3,668 | |||
Acquisition of OBDS | 7,448 | |||
Acquisition of other medical partners | 1,747 | |||
Other | (4,441 | ) | ||
|
| |||
Balance at December 31, 2011 | $ | 278,565 | ||
|
|
F-20
Table of Contents
Intangible Assets
Identifiable intangible assets at December 31, 2011, consist of (dollars in thousands):
| Estimated Useful Life | Balance | Accumulated Amortization | Net Book Value | |||||||||||||
Customer relationships | 10—26 years | $ | 162,380 | $ | (36,398 | ) | $ | 125,982 | ||||||||
Non-compete agreements | 2—5 years | 25,670 | (15,764 | ) | 9,906 | |||||||||||
Trade names | 15 years | 25,562 | (7,441 | ) | 18,121 | |||||||||||
Software system | 5 years | 1,267 | (1,267 | ) | — | |||||||||||
Provider network | 5 years | 4,975 | (1,595 | ) | 3,380 | |||||||||||
|
|
|
|
|
| |||||||||||
Total identifiable intangible assets, net | $ | 219,854 | $ | (62,465 | ) | $ | 157,389 | |||||||||
|
|
|
|
|
| |||||||||||
Identifiable intangible assets at December 31, 2010, consist of (dollars in thousands):
| Estimated Useful Life | Balance | Accumulated Amortization | Net Book Value | |||||||||||
Customer relationships | 10—26 years | $ | 144,659 | $ | (25,198 | ) | $ | 119,461 | ||||||
Non-compete agreements | 2—5 years | 16,036 | (13,090 | ) | 2,946 | |||||||||
Trade names | 15 years | 24,298 | (5,213 | ) | 19,085 | |||||||||
Software system | 5 years | 1,267 | (1,056 | ) | 211 | |||||||||
Provider network | 5 years | 1,575 | (785 | ) | 790 | |||||||||
|
|
|
|
|
| |||||||||
Total identifiable intangible assets, net | $ | 187,835 | $ | (45,342 | ) | $ | 142,493 | |||||||
|
|
|
|
|
| |||||||||
Amortization expense was $15.6 million, $15.1 million and $13.0 million in 2011, 2010, and 2009, respectively. Amortization of intangible assets over the next five years will be approximately (dollars in thousands):
2012 | $ | 16,407 | ||
2013 | 16,114 | |||
2014 | 15,944 | |||
2015 | 15,615 | |||
2016 | 14,758 | |||
Thereafter | 78,551 | |||
|
| |||
| $ | 157,389 | |||
|
|
9. Debt and Capitalized Leases
Long-Term Debt and Capital Leases
On January 6, 2011, the Company paid its existing credit facility in full and entered into a new credit facility which includes a term loan in the amount of $585.0 million and a revolving line of credit in the amount of $15.0 million. The loan is collateralized by all of the properties of HCP LLC, HCP LLC’s equity interest in its subsidiaries, and HCPH’s equity interest in HCP LLC. Principal payments on the term loan are due quarterly until maturity on January 6, 2016. In addition, there are mandatory prepayments based on certain formulas of excess cash flow as defined in the agreement. The term loan bears monthly interest, based on the election of HCP LLC, at either the “Eurodollar Rate” (defined as the British Bankers Association LIBOR rate) plus a margin ranging from 1.50% to 2.50%, or the “Base Rate” (defined as the higher of the Federal Funds Rate plus 0.5% or the bank’s prime rate) plus a margin ranging from 0.50% to 1.50%. Interest is paid monthly.
The revolving line of credit matures on January 6, 2016, and bears interest at the Eurodollar Rate or the Base Rate plus an applicable margin as defined. There were no amounts outstanding on the revolving line of credit as
F-21
Table of Contents
of December 31, 2011 and 2010. However, the Company has several standby letters of credit in a collective amount of $5.8 million, primarily to secure medical service obligations and workers’ compensation claim liabilities. There were no amounts outstanding under these letters of credit at December 31, 2011 and 2010.
Each credit facility contains customary covenants, including restrictive financial covenants. As of December 31, 2011 and 2010, HCP LLC was in compliance with all covenants under the prevailing credit facility. Management believes, given that the debt was issued in 2011 and there has been a lack of other changes, that the carrying value approximates fair value.
Approximately $6.1 million of costs incurred in the debt financing was capitalized and is being amortized to interest expense ratably over the life of the loan. Debt issuance costs of $4.9 million (net of accumulated amortization of $1.2 million) and $1.9 million (net of accumulated amortization of $2.8 million) are included in other assets as of December 31, 2011 and 2010, respectively. The unamortized balance related to the prior credit facility was expensed in 2011 upon discharge of the term loan.
Long-term debt and capital lease obligations at December 31 consist of the following (dollars in thousands):
| 2011 | 2010 | |||||||
Bank term loan payable at Eurodollar Rate plus margin factor (2.05% at December 31, 2011 and 2.00% at December 31, 2010) | $ | 555,750 | $ | 217,070 | ||||
Capitalized lease obligation | 481 | 470 | ||||||
Other long-term debt | 120 | 213 | ||||||
|
|
|
| |||||
| 556,351 | 217,753 | |||||||
Less current maturities | (29,575 | ) | (2,835 | ) | ||||
|
|
|
| |||||
| $ | 526,776 | $ | 214,918 | |||||
|
|
|
| |||||
At December 31, 2011, maturities of debt and capital lease obligations are as follows (dollars in thousands):
2012 | $ | 29,575 | ||
2013 | 29,412 | |||
2014 | 43,880 | |||
2015 | 43,875 | |||
2016 | 409,500 | |||
Thereafter | 109 | |||
|
| |||
| $ | 556,351 | |||
|
|
The scheduled maturities exclude mandatory prepayments based on excess cash flows.
10. Capital Structure
At December 31, 2011 and 2010, the Company has authorized 1,000 and 24,088,677 Class A Preferred Units (Class A Units), respectively, and 116,084,729 and 141,119,856 Class B Common Units (Class B Units), respectively. At December 31, 2011, the Company had outstanding 1,000 Class A Units held by HCP Blocker Corporation, a wholly-owned subsidiary of HCPH, and 100,131,969 Class B units. At December 31, 2010, 24,088,677 Class A units and 99,695,419 Class B units were outstanding.
Net income of HCPH is allocated such that the shareholders of Class A Units are allocated the Class A Yield equal to 3% of the liquidation preference amount, totaling $119.5 million for the period from January 6 through December 31, 2011, and the Class A Yield equal to 7% of the liquidation preference amount, totaling $75 million
F-22
Table of Contents
for the year ended December 31, 2010, and the period from January 1 through January 5, 2011. Remaining net income or loss is allocated to holders of Class B units based on their pro rata ownership interest of total outstanding Class B units.
Paid-in capital from the Class A owners and any undistributed net income allocations to Class A Units are recorded as preferred equity. Paid-in capital from the Class B owners and any undistributed net income or loss is recorded as common equity.
Redeemable Preferred Units
On March 2, 2005, Summit Partners, LP and Affiliates (Summit) acquired 24,088,677 Class A Units, representing 19.6% of total outstanding units, in exchange for cash consideration of $75.0 million. On January 6, 2011, HCPH entered into an agreement with Summit (Redemption Agreement) to repurchase from Summit all of its outstanding Class A Units (Class A Unit Redemption) for total consideration of $587.0 million. Total consideration consists of $540.0 million in cash, 436,550 Class B Units valued at $10.0 million, and an assumed tax liability of $37.0 million. The Class A Unit Redemption consisted of repurchasing 18,855,176 Class A Units from various Summit partnerships and acquiring all of the outstanding shares of HCP Blocker Corporation (Blocker Corp.), a C-corporation which held the remaining 5,233,501 HCPH Class A Units. As a part of the Redemption Agreement, an investment fund affiliated with Summit Partners indemnified HCPH for unpaid tax liabilities, if any, of Blocker Corp. existing as of January 6, 2011. Pursuant to the Amended and Restated Operating Agreement (Operating Agreement) executed on January 6, 2011, HCPH revised the rights and privileges of Class A Units. Class A units receive an annual net income allocation equal to 3% of the $119.5 million liquidation preference (Class A Yield) of which approximately 40% is distributable in cash. Undistributed Class A earnings are payable upon a redemption event as described below.
Upon occurrence of any of the following events, the Class A owners have the option to require HCPH to repurchase the Class A units upon: (1) a change in control resulting when the executive members no longer own 50% of the HCPH units, (2) sale of all or substantially all of HCPH’s assets and (3) dissolution or liquidation of HCPH. At any time on or after the day that is after April 1, 2022, but prior to the occurrence of the Sale of HCPH or Initial Public Offering, the Class A owners may request that the Class A Units be redeemed. The repurchase price of the Class A Units is the liquidation preference of $119.5 million plus any undistributed Class A Yield. An amendment of the Operating Agreement that affects the rights of the Class A Units must be approved by the holder(s) of such Units.
Prior to the Class A Unit Redemption, Class A Units received an annual net income allocation equal to 7% of the $75.0 million preferred investment (Class A Yield), of which approximately 70% was distributable in cash. Undistributed Class A earnings are included in the Preferred Capital account balance and are payable upon a redemption event as described below. The Class A units may be converted to Class B units at the owner’s option, based on the then prevailing conversion price.
Options
As of December 31, 2011 and 2010, 5,409,500 and 6,395,000 Class B options were outstanding under the employee equity incentive plan, of which 3,610,275 and 3,505,400 options were vested and exercisable, respectively (see Note 11). The weighted-average remaining contractual life on the outstanding options was 7.3 years.
Warrants
In connection with the JSAH acquisition, HCPH issued immediately exercisable warrants, with an exercise price of $7.28 per unit, to certain selling shareholders for the purchase of 418,000 Class B units. These units were valued at $0.6 million based on the Black-Scholes option pricing model and were included in the purchase consideration. At December 31, 2010, 1,383,000 warrants were vested. These warrants expired on June 30, 2011;
F-23
Table of Contents
however, the two holders of the warrants, who are executives in the Company’s Nevada unit, had taken legal action to nullify the stated warrant expiration date, which the Company contested. At December 31, 2011, no warrants were outstanding. In May 2012, the Company resolved this dispute (see Note 19).
11. Share-Based Compensation
Common Membership Unit Options
In 2008, HCPH adopted the Amended and Restated 2008 Membership Interest Option and Purchase Plan (the Plan), which provides for the award of options to purchase common membership units in HCPH to the Company’s officers, clinician, and non-clinician employees. The Plan provides for the issuance of up to 15,952,760 membership units in HCPH. There are no equity awards with market conditions.
During the year ended December 31, 2008, the first year that options were granted, 5,648,500 options were granted at an exercise price of $12.94, which vest over five years. In 2010, 829,000 options were granted at an exercise price of $12.02 with a five year vesting period; and in 2011, 272,500 options were granted at an exercise price of $18.40 with a five year vesting period. At December 31, 2011 and 2010, 3,610,275 and 3,505,400 options had vested and were exercisable, respectively. In 2011, 1,160,000 vested options were repurchased from option holders based on a market value of $18.40 per option. No options have been exercised. The weighted-average remaining contractual life on the outstanding options was 7.3 years. For the years ended December 31, 2011, 2010 and 2009, the Company recorded $7.5 million, $7.4 million, and $6.6 million respectively, of share-based compensation expense related to the Plan, which is reported primarily in medical expenses and in general and administrative expenses in the consolidated statements of income. As of December 31, 2011, there was $11.0 million of unrecognized compensation cost related to the unvested common membership unit options, which will be recognized over a period of four years.
The fair value of the common membership unit options is affected by assumptions regarding a number of complex and subjective variables, including the fair value of HCPH common member units, expected price volatility, forfeiture rate, risk-free interest rate, expected dividends and the projected exercise and post-vesting employment termination behavior of employees. The Black-Scholes valuation model is used to estimate the fair value of common membership unit options at the grant date.
In determining the fair value of an HCPH member unit, the Board of Directors considered numerous factors, including recent cash sales of HCPH common units to third-party investors, new business and economic developments affecting the Company, and independent appraisals using discounted cash flow analyses and revenue and earnings multiples for comparable publicly traded companies. The risk-free interest rate is based on the implied yield on U.S. Treasury zero-coupon issues for the expected option term. The expected volatility is based on the average historical volatility of common stock of publicly traded health care organizations with a business model, size, market capitalization, financial leverage and life cycle similar to that of the Company. The expected term represents the period of time in which the options granted are expected to be outstanding. Under the “short-cut” or simplified method, the expected term is estimated as the midpoint between the vesting date and the end of the contractual term.
F-24
Table of Contents
Assumptions used to estimate the value of common membership unit options granted are as follows:
| 2011 | 2010 | |||
Risk-free interest rate | 2.59% | 3.01% | ||
Expected volatility | 41.0% | 44.0% | ||
Expected forfeiture rate | 10% | 10% | ||
Expected option life (in years) | 6.4 years | 6.4 years | ||
Expected dividend yield | 0% | 0% | ||
Grant date weighted-average fair value | $8.21 | $5.75 |
No common membership unit options were granted in 2009.
The dividend yield was estimated at 0%, as HCPH has not paid and does not anticipate paying cash distributions in the foreseeable future related to common membership units that are outside of normal earnings distributions.
12. Income Taxes
The components of the provision for income taxes are as follows (dollars in thousands):
| Year Ended December 31 | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
Current: | ||||||||||||
Federal | $ | 68,842 | $ | 47,630 | $ | 37,445 | ||||||
State | 9,094 | 4,427 | 3,750 | |||||||||
|
|
|
|
|
| |||||||
| 77,936 | 52,057 | 41,195 | ||||||||||
Deferred: | ||||||||||||
Federal | (4,951 | ) | (3,166 | ) | (1,784 | ) | ||||||
State | (1,520 | ) | (327 | ) | 847 | |||||||
|
|
|
|
|
| |||||||
| (6,471 | ) | (3,493 | ) | (937 | ) | |||||||
|
|
|
|
|
| |||||||
Total income tax expense | $ | 71,465 | $ | 48,564 | $ | 40,258 | ||||||
|
|
|
|
|
| |||||||
The Camden Group, HCPMGI, PA, NMG, TMG, Bacchus-Fremont and JSAH (collectively, the taxable entities) are taxed as C-corporations under the Internal Revenue Code and applicable state laws, and each files separate stand-alone corporate income tax returns.
HCPH, HCP LLC and HCPAMG are classified as partnerships for tax purposes, and as such they are not taxpaying entities. Rather, the owners of these entities pay taxes on their allocable share of net income from the entities.
The reported income tax provision differs from the amounts that would have resulted had the reported income before income taxes been taxed at the U.S. federal statutory rate. The principal reasons for the differences between the amounts provided and those that would have resulted from the application of the U.S. federal statutory tax rate are income included in pre-tax income from non-taxpaying entities (e.g., HCPH, HCP LLC and HCPAMG), nondeductible items, and state taxes.
F-25
Table of Contents
The tax effects of temporary differences that give rise to significant portions of the federal and state deferred tax assets and liabilities at December 31 for the taxable entities are comprised of the following (dollars in thousands):
| 2011 | 2010 | |||||||
Current deferred tax assets (liabilities): | ||||||||
State taxes | $ | (216 | ) | $ | (8 | ) | ||
Accrued professional liability | 439 | 558 | ||||||
Accrued expenses | 4,275 | 5,394 | ||||||
Deferred revenue | 130 | 83 | ||||||
Allowance for doubtful accounts | 634 | 475 | ||||||
Net operating loss carryforward | 1,447 | 2,820 | ||||||
|
|
|
| |||||
Total current deferred tax assets, net | 6,709 | 9,322 | ||||||
|
|
|
| |||||
Noncurrent deferred tax assets (liabilities): | ||||||||
Net operating loss carryforward | 675 | 397 | ||||||
State taxes | (592 | ) | — | |||||
Accrued expenses | 3,572 | 1,623 | ||||||
Accrued professional liability | — | 958 | ||||||
Allowance for doubtful accounts | 12 | 13 | ||||||
Deferred revenue | — | 81 | ||||||
Share-based payments | 425 | 1,516 | ||||||
Transaction costs | 218 | 218 | ||||||
Depreciation and amortization | (1,795 | ) | (1,776 | ) | ||||
Intangible assets | (30,047 | ) | (39,645 | ) | ||||
Tax liability assumed to repurchase Class A units | (37,019 | ) | — | |||||
Valuation allowance | (62 | ) | (62 | ) | ||||
|
|
|
| |||||
Total noncurrent deferred tax liability, net | (64,613 | ) | (36,677 | ) | ||||
|
|
|
| |||||
Net deferred tax liability | $ | (57,904 | ) | $ | (27,355 | ) | ||
|
|
|
| |||||
The taxable entities account for income taxes using the liability method, which is an asset and liability approach that requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in financial statements or tax returns. Under this method, deferred income tax balances reflect the effects of temporary differences between their book and tax bases, as well as from net operating loss carryforwards, and are stated at enacted tax rates expected to be in effect when taxes are actually paid or recovered. Deferred income tax assets represent amounts available to reduce income taxes payable on taxable income in future years. The Company evaluates the recoverability of these future tax deductions by assessing the adequacy of future expected taxable income from all sources, including reversal of taxable temporary differences, forecasted operating earnings and available tax planning strategies. To the extent the Company does not consider it more-likely-than-not that a deferred tax asset will be recovered, a valuation allowance is established. There is $0 and $0.1 million valuation allowance for deferred tax assets as of December 31, 2011 and 2010, respectively.
As of December 31, 2011, TMG, NMG, PA and Bacchus-Fremont, collectively, have federal and state net operating loss carryforwards of approximately $4.9 million and $5.9 million, respectively. Any unused operating loss carryforwards will begin to expire in 2030.
As of December 31, 2011 and 2010, the Company has unrecognized tax benefits of approximately $35.0 million and $18.6 million, respectively, for which the deductibility is uncertain. These amounts include interest and penalties of $7.8 million and $4.7 million at December 31, 2011 and 2010, respectively. The Company recognizes interest and penalties accrued related to unrecognized tax benefits as a component of the provision for income taxes in the consolidated statements of income.
F-26
Table of Contents
The open tax years that may be audited by the federal and/or state tax authorities include 2007–2011. There are no unresolved federal or state tax examinations.
13. Leases
The Company leases equipment and certain office space under various noncancelable operating leases with unrelated parties. Certain leases contain renewal options for various terms, and certain lease payments may increase for annual cost-of-living adjustments. Certain clinic and office space is leased on a long-term operating basis from various partnerships, which are owned by certain partners or shareholders of HCPH and senior management of the Company. Four of these leases provide for annual cost-of-living adjustments.
Future minimum lease payments under non-cancelable operating leases are as follows (dollars in thousands):
| Unrelated Parties | Related Parties | Total | ||||||||||
2012 | $ | 32,196 | $ | 5,443 | $ | 37,639 | ||||||
2013 | 31,014 | 5,559 | 36,573 | |||||||||
2014 | 29,587 | 4,428 | 34,015 | |||||||||
2015 | 27,120 | 2,719 | 29,839 | |||||||||
2016 | 21,511 | 2,719 | 24,230 | |||||||||
Thereafter | 28,654 | 4,909 | 33,563 | |||||||||
|
|
|
|
|
| |||||||
| $ | 170,082 | $ | 25,777 | $ | 195,859 | |||||||
|
|
|
|
|
| |||||||
Rental expense under leases with unrelated and related parties amounted to $32.7 million and $5.3 million, respectively, in 2011, $30.0 million and $5.4 million, respectively, in 2010, and $23.1 million and $5.0 million in 2009, and are included in clinic support and general and administrative expenses on the consolidated statements of income.
14. Retirement Plans
The Company sponsors various 401(k) retirement plans covering substantially all of its employees. Eligible employees may contribute a portion of their compensation per year subject to defined maximums. The plans provide for multiple employer specific matching contribution schedules ranging from 0% to 6% of employee salary contributions. The Company may, at their discretion, make a contribution to the plans which are not a matching contribution. Total expenses related to employer contributions to the plans totaled approximately $2.8 million in 2011, $1.6 million in 2010 and $1.9 million in 2009.
The Company sponsors a non-qualified deferred compensation program (the Program) for certain clinicians and executive employees. Under the Program, employee-designated deferrals of salary are withheld by the Company. An amount equal to the withholding is invested at the direction of the employee in a portfolio of phantom investments selected from the investments available under the Program, which are tracked by an administrator. With a portion of the withholding, the Company purchases life insurance policies on each of the participating clinicians and executives with the Company named as beneficiary of the policies. The Program permits the Company to make specified contributions to senior partners and discretionary contributions to other participants.
Deferred compensation liabilities, including gains and losses on phantom investments, amounted to $34.1 million and $26.5 million at December 31, 2011 and 2010, respectively, and are classified in other noncurrent liabilities. The cash surrender value of the life insurance policies, which amounted to $34.1 million and $26.4 million at December 31, 2011 and 2010, respectively, is recorded in other noncurrent assets. The Company recorded $5.0 million, $3.9 million and $3.8 million for the years ended December 31, 2011, 2010 and 2009, respectively, for a company sponsored contribution to the deferred compensation on behalf of senior partners and other plan participants.
F-27
Table of Contents
Effective January 1, 2006, the Company introduced an additional retirement plan, the Cash Balance Retirement Plan (Cash Balance Plan). The Cash Balance Plan is a contributory defined benefit pension plan covering clinicians and qualifying non-clinician employees of HCP LLC and HCPAMG. During 2008, the Company elected to terminate the Cash Balance Plan. As required by the Cash Balance Plan, the balances for all participants became fully vested and employee participation was frozen upon the decision to terminate the Plan. The Company received approval from the Internal Revenue Service to terminate the Cash Balance Plan and distributed all account balances in 2011.
The funding status of the Cash Balance Plan at December 31 is as follows:
| 2011 | 2010 | |||||||
| (In Thousands) | ||||||||
Projected benefit obligation | $ | — | $ | (13,060 | ) | |||
Fair value of plan assets | — | 12,486 | ||||||
|
|
|
| |||||
Funded status of the plan | $ | — | $ | (574 | ) | |||
|
|
|
| |||||
At December 31, 2010, the accumulated benefit obligation equaled the projected benefit obligation. As the Cash Balance Plan had been terminated, the Company’s future costs were limited to the extent that guaranteed investment earnings on undistributed account balances exceeded investment earnings on plan assets. The underfunded balance at December 31, 2010 was included in accounts payable and accrued expenses, as the amount was funded in the following year. Total net periodic pension cost was $0.9 million, $0.6 million and $0.3 million in 2011, 2010, and 2009, respectively. The Cash Balance Plan made distributions totaling $13.4 million, $0.5 million and $0.3 million in 2011, 2010 and 2009, respectively.
15. Accounts Payable and Accrued Expenses
Accounts payable and accrued liabilities at December 31 consist of the following (dollars in thousands):
| 2011 | 2010 | |||||||
Trade accounts payable | $ | 66,982 | $ | 56,618 | ||||
Accrued compensation | 93,141 | 69,493 | ||||||
Accrued contracted physician bonuses | 16,098 | 11,645 | ||||||
Amounts due Magan Medical Group | 2,629 | 12,093 | ||||||
Practice acquisition liabilities | 8,822 | 11,690 | ||||||
Other | 19,262 | 15,545 | ||||||
|
|
|
| |||||
Total | $ | 206,934 | $ | 177,084 | ||||
|
|
|
| |||||
16. Other Liabilities
Other liabilities at December 31 consist of the following (dollars in thousands):
| 2011 | 2010 | |||||||
Deferred compensation liabilities | $ | 39,032 | $ | 30,895 | ||||
Reserve for uncertain tax positions | 34,972 | 18,639 | ||||||
Reserve for medical malpractice claims | 12,417 | 8,344 | ||||||
Other | 11,377 | 14,291 | ||||||
|
|
|
| |||||
Total | $ | 97,798 | $ | 72,169 | ||||
|
|
|
| |||||
F-28
Table of Contents
17. Commitments and Contingencies
HCPH and its affiliates operate in a regulated and litigious industry. As a result, various lawsuits, claims and legal and regulatory proceedings have been instituted or asserted against HCPH related to patient treatment. Additionally, HCPH and its affiliates are subject to claims and suits arising in the ordinary course of business, including claims for personal injuries, unpaid wages, or wrongful termination. In certain of these actions the claimants may seek punitive damages against HCPH and its affiliates which may not be covered by insurance. It is management’s opinion that the ultimate resolution of these pending claims and legal proceedings will not have a material adverse effect on HCPH’s results of operations or financial position. See also Note 10.
18. Note Receivable from Related Parties
HCP LLC holds a note receivable from California Primary Physicians Property Group (CPPPG), a California general partnership and a related party. The promissory note bears interest at 8.2% per annum and has fixed monthly principal and interest payments of $0.1 million. The note, which matures on January 1, 2013, is collateralized by certain real property owned by CPPPG for which fair value is estimated to be in excess of the outstanding amount of $8.2 million at December 31, 2011, and $8.5 million at December 31, 2010.
19. Subsequent Events
Based on management’s review of subsequent events through June 29, 2012, the date the financial statements were available for issuance, the following significant events occurred: HCP LLC paid cash distributions to its members of $206.3 million subsequent to December 31, 2011, including $30.2 million related to 2011 earnings.
On May 20, 2012, HCPH entered into a definitive merger agreement (Merger) to be acquired by DaVita, Inc. (DaVita). HCPH’s members must still vote to approve the Merger. Should the Merger be approved the Company will survive the Merger as a wholly owned subsidiary of DaVita. The Merger is expected to consummate in the fourth quarter of 2012. However, HCPH cannot predict the actual timing of the completion of the Merger.
On May 20, 2012, the Company and two executives of the Company’s Nevada unit reached agreement whereby their legal action against the Company, as discussed in Note 10, is to be dismissed in exchange for $10.0 million to be paid upon consummation of the Merger, and $20.0 million to be paid over the next eight years contingent upon attainment of certain performance milestones.
F-29
Table of Contents
HealthCare Partners Holdings, LLC and Affiliates
(In Thousands)
| June 30, 2012 | December 31, 2011 | |||||||
| (Unaudited) | ||||||||
Assets | ||||||||
Current assets: | ||||||||
Cash and cash equivalents | $ | 355,145 | $ | 394,521 | ||||
Investments | 180,261 | 175,098 | ||||||
Accounts receivable – patients, net | 31,996 | 22,297 | ||||||
Accounts receivable – health plans | 139,752 | 99,570 | ||||||
Funds on deposit with third party | 68,715 | 63,638 | ||||||
Prepaid expenses and other current assets | 37,337 | 30,949 | ||||||
Current deferred tax assets, net | 7,062 | 6,709 | ||||||
|
|
|
| |||||
Total current assets | 820,268 | 792,782 | ||||||
Property and equipment, net | 78,801 | 75,848 | ||||||
Notes receivable from related parties, less current portion | 7,791 | 7,812 | ||||||
Goodwill | 287,704 | 278,565 | ||||||
Intangible assets, net | 157,835 | 157,389 | ||||||
Other assets | 62,083 | 53,525 | ||||||
|
|
|
| |||||
Total assets | $ | 1,414,482 | $ | 1,365,921 | ||||
|
|
|
| |||||
Liabilities and members’ equity | ||||||||
Current liabilities: | ||||||||
Medical claims and related payables | $ | 95,315 | $ | 94,406 | ||||
Other medical payables | 132,257 | 136,703 | ||||||
Accounts payable – health plans | 25,712 | 21,048 | ||||||
Accounts payable and accrued expenses | 196,652 | 206,934 | ||||||
Current maturities of long-term debt and capital leases | 29,537 | 29,575 | ||||||
|
|
|
| |||||
Total current liabilities | 479,473 | 488,666 | ||||||
Long-term debt and capital leases, less current maturities | 512,029 | 526,776 | ||||||
Noncurrent deferred tax liability, net | 68,246 | 64,613 | ||||||
Other liabilities | 106,940 | 97,798 | ||||||
|
|
|
| |||||
Total liabilities | 1,166,688 | 1,177,853 | ||||||
Members’ equity: | ||||||||
Common equity | 247,794 | 188,068 | ||||||
|
|
|
| |||||
Total liabilities and members’ equity | $ | 1,414,482 | $ | 1,365,921 | ||||
|
|
|
| |||||
See accompanying notes.
F-30
Table of Contents
HealthCare Partners Holdings, LLC and Affiliates
Consolidated Statements of Income (Unaudited)
(In Thousands)
| Three Months Ended June 30 | Six Months Ended June 30 | |||||||||||||||
| 2012 | 2011 | 2012 | 2011 | |||||||||||||
| (Unaudited) | ||||||||||||||||
Operating revenues: | ||||||||||||||||
Medical revenues | $ | 642,302 | $ | 582,990 | $ | 1,295,137 | $ | 1,158,610 | ||||||||
Other operating revenue | 14,365 | 11,225 | 28,120 | 21,948 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total operating revenues | 656,667 | 594,215 | 1,323,257 | 1,180,558 | ||||||||||||
Operating expenses: | ||||||||||||||||
Medical expenses | 312,677 | 285,242 | 619,928 | 569,652 | ||||||||||||
Hospital expenses | 77,148 | 61,351 | 155,446 | 121,652 | ||||||||||||
Clinic support and other operating costs | 83,454 | 76,077 | 165,175 | 147,707 | ||||||||||||
General and administrative expenses | 55,079 | 49,882 | 109,806 | 100,982 | ||||||||||||
Depreciation and amortization | 8,500 | 7,738 | 15,938 | 15,885 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total expenses | 536,858 | 480,290 | 1,066,293 | 955,878 | ||||||||||||
Equity in earnings of unconsolidated joint ventures | 6,534 | 3,755 | 11,453 | 8,746 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Operating income | 126,343 | 117,680 | 268,417 | 233,426 | ||||||||||||
Other income (expense): | ||||||||||||||||
Interest income | 1,818 | 2,271 | 3,472 | 3,367 | ||||||||||||
Interest expense | (3,145 | ) | (3,600 | ) | (6,311 | ) | (9,036 | ) | ||||||||
Loss on sale of fixed assets | — | (24 | ) | (29 | ) | (25 | ) | |||||||||
Gain (loss) on sale of investments | 11 | (3 | ) | 46 | 1,281 | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Total other income, net | (1,316 | ) | (1,356 | ) | (2,822 | ) | (4,413 | ) | ||||||||
|
|
|
|
|
|
|
| |||||||||
Income before income taxes | 125,027 | 116,324 | 265,595 | 229,013 | ||||||||||||
Provision for income taxes | 13,401 | 11,462 | 32,930 | 36,907 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Net income | $ | 111,626 | $ | 104,862 | $ | 232,665 | $ | 192,106 | ||||||||
|
|
|
|
|
|
|
| |||||||||
See accompanying notes.
F-31
Table of Contents
HealthCare Partners Holdings, LLC and Affiliates
Consolidated Statements of Comprehensive Income (Unaudited)
(In Thousands)
| Three Months Ended June 30 | Six Months Ended June 30 | |||||||||||||||
| 2012 | 2011 | 2012 | 2011 | |||||||||||||
| (Unaudited) | ||||||||||||||||
Net income | $ | 111,626 | $ | 104,862 | $ | 232,665 | $ | 192,106 | ||||||||
Other comprehensive income: | ||||||||||||||||
Unrealized gain on investments | (49 | ) | 533 | 295 | 678 | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Other comprehensive income | (49 | ) | 533 | 295 | 678 | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Comprehensive income | $ | 111,577 | $ | 105,395 | $ | 232,960 | $ | 192,784 | ||||||||
|
|
|
|
|
|
|
| |||||||||
See accompanying notes.
F-32
Table of Contents
HealthCare Partners Holdings, LLC and Affiliates
Consolidated Statements of Cash Flows (Unaudited)
(In Thousands)
Six Months Ended June 30 | ||||||||
| 2012 | 2011 | |||||||
| (Unaudited) | ||||||||
Operating activities | ||||||||
Net income | $ | 232,665 | $ | 192,106 | ||||
Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||
Depreciation and amortization | 15,938 | 15,885 | ||||||
Amortization of loan fees | 612 | 612 | ||||||
Gain on sale of investments | (46 | ) | (1,281 | ) | ||||
Share-based compensation | 3,702 | 3,696 | ||||||
Deferred taxes | 3,280 | (8,483 | ) | |||||
Changes in operating assets and liabilities: | ||||||||
Accounts receivable – patients, net | (9,699 | ) | (2,972 | ) | ||||
Prepaid expenses and other current assets | (11,450 | ) | (482 | ) | ||||
Other assets | (9,120 | ) | (8,182 | ) | ||||
Accounts payable, accrued compensation, and other liabilities | (2,773 | ) | 1,835 | |||||
Medical claims and capitation payable | (3,536 | ) | 35,453 | |||||
Accounts receivable/payable – health plans | (35,517 | ) | (47,528 | ) | ||||
|
|
|
| |||||
Net cash provided by operating activities | 184,056 | 180,659 | ||||||
Investing activities | ||||||||
Net purchases of equipment, furniture, and fixtures | (10,400 | ) | (11,158 | ) | ||||
Acquisition of medical practices, net of cash acquired | (16,512 | ) | (9,088 | ) | ||||
Purchases of marketable securities | (55,739 | ) | (50,309 | ) | ||||
Sales of marketable securities | 50,916 | 55,018 | ||||||
Proceeds from notes receivable from related parties | 192 | 206 | ||||||
Other | (170 | ) | — | |||||
|
|
|
| |||||
Net cash used in investing activities | (31,713 | ) | (15,331 | ) | ||||
Financing activities | ||||||||
Payments on long-term debt and short-term borrowings | (14,785 | ) | (232,011 | ) | ||||
Proceeds from debt issuance | — | 585,000 | ||||||
Distributions to members | (176,934 | ) | (150,398 | ) | ||||
Repurchase of Class A Units | — | (540,000 | ) | |||||
Repurchase of vested options | — | (6,411 | ) | |||||
|
|
|
| |||||
Net cash used in financing activities | (191,719 | ) | (343,820 | ) | ||||
|
|
|
| |||||
Net decrease in cash and cash equivalents | (39,376 | ) | (178,492 | ) | ||||
Cash and cash equivalents – beginning of period | 394,521 | 361,099 | ||||||
|
|
|
| |||||
Cash and cash equivalents – end of period | $ | 355,145 | $ | 182,607 | ||||
|
|
|
| |||||
Noncash activities: | ||||||||
Redemption of Class A Units with Class B Units | $ | — | $ | 10,000 | ||||
Tax liability assumed to repurchase Class A Units | $ | — | $ | 37,019 | ||||
See accompanying notes.
F-33
Table of Contents
HealthCare Partners Holdings, LLC and Affiliates
Notes to Consolidated Financial Statements (Unaudited)
June 30, 2012
1. Organization and Business
HealthCare Partners Holdings, LLC (HCPH or the Company) is a California limited liability company that was established on February 23, 2005, in connection with a reorganization of its subsidiaries. HCPH, together with its affiliated physician groups and subsidiaries, is a patient and physician-focused integrated health care delivery and management company providing coordinated, outcomes-based medical care in a cost-effective manner. Through capitation contracts with some of the nation’s leading health plans, HCPH has approximately 669,400 patients enrolled with health maintenance organizations (HMOs) under its care in southern California (including Magan, see Note 2), central and south Florida, and southern Nevada. HealthCare Partners, LLC, a California limited liability company and a wholly owned subsidiary of HCPH (HCP LLC), provides various nonmedical management and administrative services, facilities, and equipment to affiliated physician-related organizations, including medical groups, independent practice associations (lPAs), and other similar organizations under long-term management services agreements. HCP LLC’s largest management service agreement is with one of HCPH’s affiliated physician groups, HealthCare Partners Affiliates Medical Group (HCPAMG). See Note 2 for additional information.
HCPAMG was formed in 1994 and is organized as a California general partnership with 30 general partners. The majority of the partners of HCPAMG are also members of HCPH. HCPAMG and its affiliates provide managed health care and related services through regional delivery systems and a joint venture (see Note 6) to approximately 580,800 enrollees in Southern California under contracts with various HMOs and to privately insured individuals. HCP LLC earns a management fee from HCPAMG equal to a percentage of HCPAMG’s adjusted gross revenue, as defined by the management services agreement.
JSA Holdings, Inc., a Delaware corporation and a wholly owned subsidiary of HCP LLC (JSAH), is a holding company that, through various subsidiaries, operates a management services organization with an integrated medical group and IPA providing physician practice management and administrative services, managed health care, retail pharmacies, and other related health care services to approximately 88,600 HMO enrollees in Florida and Nevada.
On May��20, 2012, HCPH entered into a definitive merger agreement (Merger) to be acquired by DaVita, Inc. (DaVita). The Company’s members must still vote to approve the Merger. Should the Merger be approved, the Company will become a wholly owned subsidiary of DaVita. The Merger is expected to consummate in the fourth quarter of 2012. However, the Company cannot predict the actual timing of the completion of the Merger.
2. Summary of Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the accounts and operating results of HCPH, HCP Blocker Corporation (HCP Blocker) (see Note 10), HCP LLC and its wholly-owned subsidiaries (including JSAH), HCPAMG and its wholly-owned subsidiaries, HealthCare Partners Medical Group (Bacchus), Ltd. (HCPMG (Bacchus)), and HCPMG, Inc. (HCPMGI) a payroll / staff leasing company which provides clinical and administrative staff services to HCP LLC and HCPAMG. The entities are collectively referred to as “the Company.” In the opinion of management, all adjustments considered necessary for a fair presentation of the results as of the dates and for the interim periods presented have been included; such adjustments consist of normal recurring adjustments. All significant intercompany accounts and transactions have been eliminated in consolidation. The consolidated results of operations for the current interim period are not necessarily indicative of the results for the entire year ending December 31, 2012. Financial information related to subsidiaries acquired
F-34
Table of Contents
HealthCare Partners Holdings, LLC and Affiliates
Notes to Consolidated Financial Statements (Unaudited) (continued)
2. Summary of Significant Accounting Policies (continued)
during any year is included only for the periods subsequent to their acquisition. As discussed in Note 6, HCPAMG also has a 50% interest in Magan Medical Group (Magan), a joint venture with Magan Medical Clinic, Inc., and HCP LLC has a 67% interest in California Medical Group Insurance Company Risk Retention Group, an Arizona corporation (CMGI). These investments are accounted for using the equity method.
The financial statements of HCPAMG are consolidated with HCP LLC, as HCP LLC has determined that HCPAMG qualifies as a variable interest entity in accordance with Accounting Standards Codification (ASC) Section 810-10-15-14 through its long-term, exclusive management services agreement. Pursuant to the agreement, HCPAMG is solely responsible for all aspects of the practice of medicine and provision of patient care. HCP LLC provides non-medical, technical, and administrative services to HCPAMG, for which it receives a management fee per the management services agreement. Through the management services agreement, HCP LLC has exclusive authority over all nonmedical decision making related to the ongoing business operations of HCPAMG. HCPAMG has deminimis equity and working capital as, through the management services agreement, all of HCPAMG’s cash flows are transferred to HCP LLC. As such, HCP LLC has determined that HCPAMG is a variable interest entity and that HCP LLC is the primary beneficiary. Consequently, HCP LLC has consolidated the revenue and expenses of HCPAMG. HCPAMG recognized $747.0 million of revenue and expenses for the six months ended June 30, 2012, and $681.7 million of revenue and expenses for the six months ended June 30, 2011.
The creditors of HCPAMG do not have recourse to HCP LLC general credit and there are no other arrangements that could expose HCP LLC to losses. However, HCPAMG is managed to recognize no net income or net loss and, therefore, HCP LLC may be required to provide financial support to cover any operating expenses in excess of operating revenues. As of June 30, 2012, HCPAMG and its wholly owned subsidiaries had total assets of $205.0 million and total liabilities of $189.8 million.
HCP LLC, through its wholly-owned subsidiary, HealthCare Partners Nevada, LLC, a Nevada limited liability company (HCPNV), also consolidates the financial statements of HCPMG (Bacchus), a professional medical corporation providing medical services through facilities in southern Nevada. HCPMG (Bacchus) has entered into a 20-year management services agreement with HCPNV and a stock restriction agreement, under which the sole shareholder of HCPMG (Bacchus) is restricted from transferring or selling the shares other than to a designee of HCP LLC. HCP LLC has determined that HCPMG (Bacchus) qualifies as a variable interest entity and that it has a variable interest in HCPMG (Bacchus) through the management services agreement.
The unaudited consolidated interim financial statements have been prepared under the assumption that users of the interim consolidated financial statements have either read or have access to the consolidated financial statements for the fiscal year ended December 31, 2011. Accordingly, certain disclosures that would substantially duplicate the disclosures contained in the December 31, 2011 consolidated financial statements have been omitted. These unaudited consolidated interim financial statements should be read in conjunction with the December 31, 2011 consolidated financial statements.
Use of Estimates
The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements. Estimates also affect the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates.
F-35
Table of Contents
HealthCare Partners Holdings, LLC and Affiliates
Notes to Consolidated Financial Statements (Unaudited) (continued)
2. Summary of Significant Accounting Policies (continued)
The most significant areas requiring the use of estimates include settlements under risk-sharing programs, medical receivables, determination of allowances for uncollectible accounts and retroactive premium adjustments, estimates of medical claims and related payables, estimates of self-insured claims reserves, and assessment of uncertain income tax positions.
Medical Revenues and Cost Recognition
Professional Capitation Revenue
The Company’s medical group affiliates are licensed to contract with HMOs to provide physician services in California and to provide both hospital and physician services under global risk capitation contracts in Florida and Nevada. Medical revenues consist primarily of fees for medical services provided by the medical group entities under capitated contracts with various HMOs or under fee-for-service type arrangements with privately insured individuals, and revenues under risk-sharing programs. Capitation revenue under HMO contracts is prepaid monthly based on the number of enrollees electing physicians affiliated with one of the medical group entities as their health care provider, regardless of the level of actual medical services utilized. Capitation revenue is reported as revenue in the month in which enrollees are entitled to receive health care. A portion of the capitation revenue pertaining to Medicare enrollees is subject to possible retroactive premium risk adjustments based on their individual acuity. Due to lack of sufficient data to project the amount of such retroactive adjustments, the Company records any corresponding retroactive revenues in the year of receipt.
Hospital Risk Share Revenue
Depending on the state regulation regarding global risk capitation, revenues may be received by HCPH or an independent hospital with which the Company contracts. In the Florida and Nevada service markets, the global capitation revenue is recorded by the Company, with the corresponding cost of institutional care reported by the Company as hospital expenses. In California, the independent hospitals receive the global capitation revenues. The revenues are used to pay medical claims for the related enrollees. The Company is entitled to any residual amounts and bears the risk of any deficits. In all cases, an estimate is made for the cost of medical services that have been rendered and where no medical claim has been received (IBNR).
Under risk-sharing programs, the medical groups share in the risk for hospitalization services and earn additional incentive revenues or incur penalties based on the utilization of hospital services. Estimated shared-risk receivables from the HMOs are recorded based upon hospital utilization and associated costs incurred by assigned HMO enrollees, including an estimate of costs related to IBNR, compared to funding. Differences between actual contract settlements and estimated receivables are recorded in the year of final settlement. The Company recorded favorable changes in estimates related to its prior year shared risk settlements totaling $7.2 million and $3.7 million in the quarters ended June 30, 2012 and 2011, respectively, and $4.0 million and $3.0 million in the quarters ended March 31, 2012 and 2011, respectively, as a result of lower than expected claims costs. The medical groups also receive other incentive payments from health plans based on specified performance and quality criteria. These amounts are accrued when earned and the amounts can be reasonably estimated, and are included in medical revenues. The Company also earns revenues from retail pharmacies, management fees, and health care consulting services. These amounts are included in other operating revenue when services are provided.
Fee-for-Service Revenue
The Company provides medical services to patients on a fee-for-service basis. Revenues are recorded at the time of service along with an estimate for contractually required discounts (contractual allowances). These estimates
F-36
Table of Contents
HealthCare Partners Holdings, LLC and Affiliates
Notes to Consolidated Financial Statements (Unaudited) (continued)
2. Summary of Significant Accounting Policies (continued)
are based on historical collection trends for categories of payors. Estimates are made monthly for uncollectible accounts based on the payor mix of open accounts receivable and the length of time the account has been outstanding. At June 30, 2012, the Company had total outstanding patient accounts receivable of $53.4 million, with contractual allowances of $17.9 million and reserves for uncollectible accounts of $3.5 million. At December 31, 2011, the Company had total outstanding patient accounts receivable of $47.6 million, with contractual allowances of $21.1 million and reserves for uncollectible accounts of $4.2 million.
Medical Costs
The medical groups are responsible for the medical services the affiliated physicians and contracted hospitals provide to assigned HMO enrollees. The Company provides medical services to health plan enrollees through a network of contracted providers under sub-capitation and fee-for-service arrangements, Company-operated clinics, and staff physicians. Medical costs for professional services rendered by employed and contracted providers are recorded as medical expenses. Medical costs for institutional services covered by our Florida and Nevada global capitation contracts are recorded as hospital expenses. Costs for operating medical clinics, including the salaries of nonmedical personnel and support costs, are recorded in clinic support and other operating costs.
An estimate of amounts due to contracted physicians, hospitals, and other professional providers is included in medical claims and related payables in the accompanying consolidated balance sheets. Medical claims payable include claims reported as of the balance sheet date and estimates of IBNR. Such estimates are developed using actuarial methods and are based on many variables, including the utilization of health care services, historical payment patterns, cost trends, product mix, seasonality, changes in membership, and other factors. The estimation methods and the resulting reserves are continually reviewed and updated. Many of the medical contracts are complex in nature and may be subject to differing interpretations regarding amounts due for the provision of various services. Such differing interpretations may not come to light until a substantial period of time has passed following the contract implementation. Any adjustments to reserves are reflected in current operations. The Company recorded favorable changes in estimates to prior year medical claims and related payables balances totaling $0 and $1.3 million in the quarters ended June 30, 2012 and 2011, respectively, and $5.0 million and $1.3 million in the quarters ended March 31, 2012 and 2011, respectively.
Laws and regulations governing the Medicare and Medicaid programs are complex and subject to interpretation. The Company believes that it is in compliance with all applicable laws and regulations and is not aware of any pending or threatened investigations involving allegations of potential wrongdoing. While no such regulatory inquiries have been made, compliance with such laws and regulations can be subject to future government review and interpretation, as well as significant regulatory action, including fines, penalties, and exclusion from the Medicare and Medicaid programs.
Cash Equivalents
Cash equivalents consist of money market mutual funds and certificates of deposit with remaining maturities, on the acquisition date, of three months or less.
Funds on Deposit With a Third Party
The Company has established a risk-sharing arrangement with a local hospital wherein the Company shares in any surplus or deficit. One of the terms of this agreement is the establishment of a segregated investment fund to
F-37
Table of Contents
HealthCare Partners Holdings, LLC and Affiliates
Notes to Consolidated Financial Statements (Unaudited) (continued)
2. Summary of Significant Accounting Policies (continued)
ensure adequate cash to pay medical claims. The Company and hospital monitor the reserve balance to maintain the adequacy of funds on deposit.
Investments
The Company has determined that all investments held are available-for-sale. Accordingly, such investments are carried at fair value, with unrealized gains and losses excluded from earnings and reported as a separate component of equity in other comprehensive income. The Company also holds auction rate securities, which are recorded as other noncurrent assets at an estimated fair value of $3.0 million at June 30, 2012, and December 31, 2011. Due to the lack of an active market, the estimated fair value of these auction rate securities was based on independent appraisals and discounted cash flow valuation models, which took into consideration the collateral underlying these securities, credit risks related to the securities and the issuers, interest rate spreads, and illiquidity factors.
Investment income consists of interest, which is recognized on an accrual basis. Interest income on mortgage-backed and asset-backed securities is determined using the effective-yield method based on estimated prepayments. Gains and losses with respect to dispositions of investments are based on the specific-identification method.
Goodwill
Goodwill is not amortized, but is subject to an impairment test annually or more frequently if certain indicators of impairment are present. As of January 1, 2011, the Company early-adopted Accounting Standards Update (ASU) 2011-08,Testing Goodwill for Impairment. As such, the Company assessed qualitative factors to determine whether it is more likely than not that the fair value of the reporting units is less than the carrying value. As a result of this assessment, it was determined that performing the two-step impairment test was not necessary.
Fair Values of Financial Instruments
Financial instruments consist mainly of cash and cash equivalents, investments, auction rate securities, accounts and notes receivable, medical claims and related payables, accounts payable and accrued expenses, and long-term debt. The fair values of cash and cash equivalents, accounts and notes receivable, medical claims and related payables, and accounts payable and accrued expenses approximate their carrying amounts due to their short-term nature. The estimated fair values for investments, excluding auction rate securities, generally represent quoted market prices for securities traded in the public marketplace or estimated values for securities not traded in the public marketplace. See Note 5 for further information. The estimated fair values for auction rate securities generally represent valuation models utilizing unobservable inputs, such as estimated repurchase scenarios. Self-insured liabilities are recorded at the estimated present value of claims obligations using appropriate discount rates.
Recent Accounting Pronouncements
In May 2011, the Financial Accounting Standards Board (FASB) issued ASU Number 2011-04,Fair Value Measurement, which modifies the fair value measurement and disclosure guidance. This guidance results in new disclosures primarily related to Level 3 measurements, including quantitative disclosure about unobservable inputs and assumptions, a description of the valuation processes, and a narrative description of the sensitivity of the fair value to changes in unobservable inputs. This guidance is effective for reporting periods (including
F-38
Table of Contents
HealthCare Partners Holdings, LLC and Affiliates
Notes to Consolidated Financial Statements (Unaudited) (continued)
2. Summary of Significant Accounting Policies (continued)
interim periods) beginning after December 15, 2011, and the Company adopted this guidance effective March 31, 2012. The adoption of this ASU did not have a material impact on the Company’s financial position, results of operations, or cash flows, but changed certain disclosures.
In June 2011, the FASB issued ASU Number 2011-05,Presentation of Comprehensive Income, which changed the disclosure requirements for the presentation of other comprehensive income (OCI) in the financial statements, including eliminating the option to present OCI in the statement of stockholders’ equity. OCI and its components will be required to be presented for both interim and annual periods either in a single financial statement, the statement of comprehensive income, or in two separate but consecutive financial statements consisting of a statement of income followed by a separate statement presenting OCI. The Company adopted this ASU effective March 31, 2012.
In July 2011, the FASB issued ASU Number 2011-07,Presentation and Disclosure of Patient Services Revenue, Provision for Bad Debts, and the Allowance for Doubtful Accounts for Health Care Entities, which modifies the presentation and disclosure of patient service revenue, provision for bad debts, and the allowance for doubtful accounts. The guidance changes the presentation on the statement of operations by requiring the reclassification of the provision for bad debts associated with patient service revenues from an operating expense to a deduction from net patient service revenue. Additionally, the amendment requires disclosures regarding the Company’s policy for recognizing patient service revenue and assessing bad debts. Qualitative and quantitative information about changes in the allowance for doubtful accounts is required. This guidance is effective for annual periods beginning after December 15, 2012, and interim and annual periods thereafter, and should be applied retrospectively to all prior periods presented. The adoption of this accounting standards update will not have a material impact on the statements of financial position, income, or cash flows.
3. Acquisitions
On March 30, 2012, HCPNV and HCPMG (Bacchus) entered into an agreement to purchase the assets of Cardiovascular Consultants of Nevada for $15.0 million cash consideration. The acquisition included contracts with employed physicians, clinic assets at six southern Nevada locations, and noncompete agreements with the former owners. This amount includes a $5.0 million deposit into an escrow account with an escrow agent to secure employment obligations of the seller for 2012.
The preliminary estimated fair value of assets and liabilities acquired is as follows (in thousands):
| Six Months Ended June 30, 2012 | ||||
Tangible assets | $ | 575 | ||
Intangible assets | 7,500 | |||
Goodwill | 8,585 | |||
Liabilities assumed | (1,660 | ) | ||
|
| |||
| $ | 15,000 | |||
|
| |||
Purchase accounting will be finalized upon completion of an independent evaluation.
4. Investments
The Company has determined that all investments held are available-for-sale securities. Accordingly, such investments are carried at fair value with unrealized gains and losses excluded from earnings and reported as a
F-39
Table of Contents
HealthCare Partners Holdings, LLC and Affiliates
Notes to Consolidated Financial Statements (Unaudited) (continued)
4. Investments (continued)
separate component of equity in other comprehensive income. The following tables summarize the Company’s investments as of the dates indicated (dollars in thousands):
| June 30, 2012 | ||||||||||||||||
| Cost or Amortized Cost | Gross Unrealized | Estimated Fair Value | ||||||||||||||
| Gains | Losses | |||||||||||||||
Municipal bonds | $ | 117,417 | $ | 407 | $ | (23 | ) | $ | 117,801 | |||||||
Corporate bonds | 36,937 | 162 | (58 | ) | 37,041 | |||||||||||
Asset and mortgage-backed bonds | 17,291 | 31 | (9 | ) | 17,313 | |||||||||||
U.S. Treasury bonds | 6,415 | — | — | 6,415 | ||||||||||||
Government-related bonds | 1,668 | 24 | (1 | ) | 1,691 | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Total investments | $ | 179,728 | $ | 624 | $ | (91 | ) | $ | 180,261 | |||||||
|
|
|
|
|
|
|
| |||||||||
| December 31, 2011 | ||||||||||||||||
| Cost or Amortized Cost | Gross Unrealized | Estimated Fair Value | ||||||||||||||
| Gains | Losses | |||||||||||||||
Municipal bonds | $ | 117,149 | $ | 430 | $ | (39 | ) | $ | 117,540 | |||||||
Corporate bonds | 36,625 | 108 | (286 | ) | 36,447 | |||||||||||
Asset and mortgage-backed bonds | 10,177 | 13 | (7 | ) | 10,183 | |||||||||||
U.S. Treasury bonds | 8,086 | 5 | — | 8,091 | ||||||||||||
Government-related bonds | 2,201 | 19 | — | 2,220 | ||||||||||||
Agency bonds | 617 | — | — | 617 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total investments | $ | 174,855 | $ | 575 | $ | (332 | ) | $ | 175,098 | |||||||
|
|
|
|
|
|
|
| |||||||||
The contractual maturities of investments as of June 30, 2012, are summarized below (dollars in thousands):
| Cost or Amortized Cost | Estimated Fair Value | |||||||
Due in one year or less | $ | 54,397 | $ | 54,505 | ||||
Due one year through five years | 96,673 | 97,076 | ||||||
Due after five years through ten years | 3,876 | 3,876 | ||||||
Due after ten years | 7,491 | 7,491 | ||||||
Asset and mortgaged-backed bonds | 17,291 | 17,313 | ||||||
|
|
|
| |||||
| $ | 179,728 | $ | 180,261 | |||||
|
|
|
| |||||
Expected maturities differ from contractual maturities because borrowers may have the right to call or prepay obligations with or without call or prepayment penalties.
F-40
Table of Contents
HealthCare Partners Holdings, LLC and Affiliates
Notes to Consolidated Financial Statements (Unaudited) (continued)
4. Investments (continued)
Realized gains and losses based on specific identification method for the six months ended June 30, 2012 and 2011 (in thousands):
| Six Months Ended June 30 | ||||||||
| 2012 | 2011 | |||||||
Realized gains | $ | 53 | $ | 1,294 | ||||
Realized losses | (7 | ) | (13 | ) | ||||
|
|
|
| |||||
Net realized gains | $ | 46 | $ | 1,281 | ||||
|
|
|
| |||||
At each reporting date, the Company performs an evaluation of impaired investments to determine if the unrealized losses are other than temporary. The Company determines whether it intends to sell, or if it is more likely than not that it will be required to sell, impaired securities. For all impaired debt securities for which there is no intent or expected requirement to sell, the evaluation considers whether it is likely the amortized cost value will be recovered. The Company did not identify any other-than-temporary impairment in investments for the six months ended June 30, 2012 and 2011.
The following table shows the Company’s gross unrealized losses and estimated fair value, aggregated by investment category and length of time that individual securities have been in a continuous unrealized loss position (dollars in thousands):
| June 30, 2012 | ||||||||||||||||||||||||
| Less than 12 Months | 12 Months or More | Total | ||||||||||||||||||||||
| Estimated Fair | Unrealized | Estimated Fair | Unrealized | Estimated Fair | Unrealized | |||||||||||||||||||
| Value | Losses | Value | Losses | Value | Losses | |||||||||||||||||||
Municipal bonds | $ | 7,076 | $ | (14 | ) | $ | 5,191 | $ | (9 | ) | $ | 12,267 | $ | (23 | ) | |||||||||
Corporate bonds | 16,948 | (27 | ) | 15,536 | (31 | ) | 32,484 | (58 | ) | |||||||||||||||
Asset and mortgage-backed bonds | 2,291 | (9 | ) | — | — | 2,291 | (9 | ) | ||||||||||||||||
U.S. Treasury bonds | 1,300 | (1 | ) | — | — | 1,300 | (1 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
| $ | 27,615 | $ | (51 | ) | $ | 20,727 | $ | (40 | ) | $ | 48,342 | $ | (91 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
| December 31, 2011 | ||||||||||||||||||||||||
| Less than 12 Months | 12 Months or More | Total | ||||||||||||||||||||||
| Estimated Fair | Unrealized | Estimated Fair | Unrealized | Estimated Fair | Unrealized | |||||||||||||||||||
| Value | Losses | Value | Losses | Value | Losses | |||||||||||||||||||
Municipal bonds | $ | 9,748 | $ | (39 | ) | $ | 410 | $ | — | $ | 10,158 | $ | (39 | ) | ||||||||||
Corporate bonds | 11,359 | (251 | ) | 3,078 | (35 | ) | 14,437 | (286 | ) | |||||||||||||||
Asset and mortgage-backed bonds | 3,667 | (4 | ) | 1,023 | (3 | ) | 4,690 | (7 | ) | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
| $ | 24,774 | $ | (294 | ) | $ | 4,511 | $ | (38 | ) | $ | 29,285 | $ | (332 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
F-41
Table of Contents
HealthCare Partners Holdings, LLC and Affiliates
Notes to Consolidated Financial Statements (Unaudited) (continued)
5. Fair Value Measurements
A three-level valuation hierarchy is used to classify inputs into the measurement of assets and liabilities at fair value. The following is a brief description of those three levels:
| • | Level 1: Observable inputs such as quoted prices (unadjusted) in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities. |
| • | Level 2: Inputs other than quoted prices that are observable for the asset or liability, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active. |
| • | Level 3: Unobservable inputs that are used when little or no market data is available and reflect the reporting entity’s own assumptions. These include pricing models, discounted cash flow methodologies, and similar techniques that use significant unobservable inputs. |
Assets and liabilities measured at fair value are based on one or more of three valuation techniques noted in the tables below:
| (a) | Market approach. Prices and other relevant information generated by market transactions involving identical or comparable assets or liabilities. |
| (b) | Cost approach. Amount that would be required to replace the service capacity of an asset (replacement cost). |
| (c) | Income approach. Techniques to convert future amounts to a single present amount based on market expectations (including present value techniques, option-pricing, and excess earnings models). |
The Company held the following financial assets and liabilities measured at estimated fair value on a recurring basis (dollars in thousands):
| Estimated Fair Value Measurements at Reporting Date Using | Valuation Technique (a,b,c) | |||||||||||||||||
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||||
June 30, 2012 | ||||||||||||||||||
Assets: | ||||||||||||||||||
Investments: | ||||||||||||||||||
Municipal bonds | $ | 117,801 | $ | — | $ | 117,801 | $ | — | a | |||||||||
Corporate bonds | 37,041 | — | 37,041 | — | a | |||||||||||||
Asset and mortgage-backed bonds | 17,313 | — | 17,313 | — | a | |||||||||||||
U.S. Treasury bonds | 6,415 | — | 6,415 | — | a | |||||||||||||
Government-related bonds | 1,691 | — | 1,691 | — | a | |||||||||||||
|
|
|
|
|
|
|
| |||||||||||
| 180,261 | — | 180,261 | — | |||||||||||||||
Funds on deposit with third party | 68,715 | — | 68,715 | — | a | |||||||||||||
Other assets, auction rate securities | 2,950 | — | — | 2,950 | c | |||||||||||||
|
|
|
|
|
|
|
| |||||||||||
Total assets measured at fair value | $ | 251,926 | $ | — | $ | 248,976 | $ | 2,950 | ||||||||||
|
|
|
|
|
|
|
| |||||||||||
Liabilities: | ||||||||||||||||||
Contingent consideration | $ | 10,408 | $ | — | $ | — | $ | 10,408 | c | |||||||||
|
|
|
|
|
|
|
| |||||||||||
F-42
Table of Contents
HealthCare Partners Holdings, LLC and Affiliates
Notes to Consolidated Financial Statements (Unaudited) (continued)
5. Fair Value Measurements (continued)
| Estimated Fair Value Measurements at Reporting Date Using | Valuation Technique (a,b,c) | |||||||||||||||||
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||||
December 31, 2011 | ||||||||||||||||||
Assets: | ||||||||||||||||||
Investments: | ||||||||||||||||||
Municipal bonds | $ | 117,540 | $ | — | $ | 117,540 | $ | — | a | |||||||||
Corporate bonds | 36,447 | — | 36,447 | — | a | |||||||||||||
Asset and mortgage-backed bonds | 10,183 | — | 10,183 | — | a | |||||||||||||
U.S. Treasury bonds | 8,091 | — | 8,091 | — | a | |||||||||||||
Government-related bonds | 2,220 | — | 2,220 | — | a | |||||||||||||
Agency bonds | 617 | — | 617 | — | a | |||||||||||||
|
|
|
|
|
|
|
| |||||||||||
| 175,098 | — | 175,098 | — | |||||||||||||||
Funds on deposit with third party | 63,638 | — | 63,638 | — | a | |||||||||||||
Other assets, auction rate securities | 2,950 | — | — | 2,950 | c | |||||||||||||
|
|
|
|
|
|
|
| |||||||||||
Total assets measured at fair value | $ | 241,686 | $ | — | $ | 238,736 | $ | 2,950 | ||||||||||
|
|
|
|
|
|
|
| |||||||||||
Liabilities: | ||||||||||||||||||
Contingent consideration | $ | 10,408 | $ | — | $ | — | $ | 10,408 | c | |||||||||
|
|
|
|
|
|
|
| |||||||||||
The auction rate securities classified as Level 3 investments represent securities in less liquid markets and require significant management assumptions when determining fair value. Due to the lack of an active market with active fair value indicators, the Company utilizes multiple valuation scenarios based on discounted cash flow models, which include probabilities for principal repayment, dividend rates, and estimates of occurrence, in conjunction with a review of the financial strength of the security issuer.
The following table presents quantitative information about the inputs and valuation methodologies the Company uses for material fair value measurements of auction-rate securities classified in Level 3 of the fair value hierarchy as of June 30, 2012 (dollars in thousands):
| Fair Value at | Valuation | Unobservable | Range (Weighted-Average) | |||||||||||||||||
| June 30, 2012 | Technique | Input | Minimum | Maximum | Weighted | |||||||||||||||
Auction-rate securities | $ | 2,950 | Discounted cash flow | Probability of principal return | — | % | 94 | % | 65 | % | ||||||||||
The estimated fair value of the liability for contingent consideration of $10.4 million as of June 30, 2012, and December 31, 2011, represents a remaining milestone payment for an acquisition completed in 2010. The fair value of the liability was estimated based on Management’s probability assessments of projected performance of the acquired entity, by comparing the certain base year quality and financial performance measures to the target (low-case) and maximum (high-case) results using the income approach to determine the estimated fair value of the contingent earn-out consideration. Due to the short-term nature of the scheduled payment, no discounting was applied.
F-43
Table of Contents
HealthCare Partners Holdings, LLC and Affiliates
Notes to Consolidated Financial Statements (Unaudited) (continued)
5. Fair Value Measurements (continued)
The following table presents quantitative information about the inputs and valuation methodology the Company uses for material fair value measurements of contingent consideration classified in Level 3 of the fair value hierarchy as of June 30, 2012 (dollars in thousands):
| Fair Value at June 30, 2012 | Valuation | Unobservable Input | Weighted- Average | |||||||||
Contingent consideration | $ | 10,408 | Income approach | Performance earnout – probability of low case | 97 | % | ||||||
Performance earnout – probability of high case | 87 | % | ||||||||||
There were no changes to the fair value of assets measured on a recurring basis using significant unobservable inputs (Level 3) in the six months ended June 30, 2012.
6. Equity Method Investments
The Company has a 50% interest in a joint venture, Magan, and a 67% interest in CMGI, which are accounted for using the equity method. The carrying value of Magan was $4.4 million and $3.9 million at June 30, 2012 and December 31, 2011, respectively, and is included in other assets. HCP LLC provides management services to Magan and earned management fees of approximately $5.1 million and $5.0 million in the six months ended June 30, 2012 and 2011, respectively, which are included in other operating revenue. The Company’s equity earnings from Magan were $11.5 million and $8.7 million in the six months ended June 30, 2012 and 2011, respectively, and are recorded as equity earnings. The joint venture engages in the practice of medicine, which constitutes a component of the Company’s principal revenue-generating activities; therefore, earnings from the joint venture are included in operating income.
HCP LLC holds a 67% ownership interest in CMGI, which was initially formed with three other medical groups from the Southern California area. Two of the four members of the Board of directors of CMGI are executives of HCP LLC. CMGI, domiciled in Arizona, allocates profits and losses based on the ownership interests of its investor groups. The Company accounts for this investment using the equity method. The carrying value of CMGI is $2.0 million at June 30, 2012 and December 31, 2011, and is included in other assets. The Company recorded no equity earnings in the six months ended June 30, 2012 and 2011. CMGI currently provides professional medical malpractice coverage to the Company.
Summarized financial information for the Company’s joint ventures as of and for the six months ended June 30 included (dollars in thousands):
| June 30, 2012 | December 31, 2011 | |||||||
Assets | $ | 27,465 | $ | 25,912 | ||||
Liabilities | 14,805 | 13,827 | ||||||
| Six Months Ended June 30 | ||||||||
| 2012 | 2011 | |||||||
Net income | $ | 23,479 | $ | 17,858 | ||||
F-44
Table of Contents
HealthCare Partners Holdings, LLC and Affiliates
Notes to Consolidated Financial Statements (Unaudited) (continued)
7. Debt and Capitalized Leases
Long-Term Debt and Capital Leases
On January 6, 2011, the Company paid its existing credit facility in full and entered into a new credit facility that includes a term loan in the amount of $585.0 million and a revolving line of credit in the amount of $15.0 million. The loan is collateralized by all of the properties of HCP LLC, HCP LLC’s equity interest in its subsidiaries, and HCPH’s equity interest in HCP LLC. Principal payments on the term loan are due quarterly until maturity on January 6, 2016. In addition, there are mandatory prepayments based on certain formulas of excess cash flow as defined in the agreement. No mandatory prepayments were required as of June 30, 2012. The term loan bears monthly interest, based on the election of HCP LLC, at either the “Eurodollar Rate” (defined as the British Bankers Association LIBOR rate) plus a margin ranging from 1.50% to 2.50%, or the “Base Rate” (defined as the higher of the Federal Funds Rate plus 0.5% or the bank’s prime rate) plus a margin ranging from 0.50% to 1.50%. Interest is paid monthly.
The revolving line of credit matures on January 6, 2016, and bears interest at the Eurodollar Rate or the Base Rate plus an applicable margin as defined. There were no amounts outstanding on the revolving line of credit as of June 30, 2012, and December 31, 2011. However, the Company has several standby letters of credit totaling $17.0 million, primarily to secure performance obligations of the Company’s Medicare Accountable Care Organizations with the Center for Medicare and Medicaid Services and workers’ compensation claim liabilities.
Each credit facility contains customary covenants, including restrictive financial covenants. As of June 30, 2012, HCP LLC was in compliance with all covenants.
Approximately $6.1 million of costs incurred in the debt financing was capitalized and is being amortized to interest expense ratably over the life of the loan. The net capitalized debt issuance costs of $4.3 million (net of accumulated amortization of $1.8 million) and $4.9 million (net of accumulated amortization of $1.2 million) are included in other assets as of June 30, 2012, and December 31, 2011, respectively. The unamortized balance related to the prior credit facility was expensed during the six months ended June 30, 2011, upon discharge of the term loan.
Long-term debt and capital lease obligations at June 30, 2012 and 2011, consist of the following (dollars in thousands):
| June 30, 2012 | December 31, 2011 | |||||||
Bank term loan payable at Eurodollar Rate plus margin factor (1.99% at June 30, 2012, and 2.05% at December 31, 2011) | $ | 541,125 | $ | 555,750 | ||||
Capitalized lease obligation | 332 | 481 | ||||||
Other long-term debt | 109 | 120 | ||||||
|
|
|
| |||||
| 541,566 | 556,351 | |||||||
Less current maturities | (29,537 | ) | (29,575 | ) | ||||
|
|
|
| |||||
| $ | 512,029 | $ | 526,776 | |||||
|
|
|
| |||||
F-45
Table of Contents
HealthCare Partners Holdings, LLC and Affiliates
Notes to Consolidated Financial Statements (Unaudited) (continued)
8. Accounts Payable and Accrued Expenses
Accounts payable and accrued liabilities at June 30, 2012, and December 31, 2011, consist of the following (dollars in thousands):
| June 30, 2012 | December 31, 2011 | |||||||
Trade accounts payable | $ | 65,524 | $ | 66,982 | ||||
Accrued compensation | 79,742 | 93,141 | ||||||
Accrued contracted physician bonuses | 19,024 | 16,098 | ||||||
Amounts due Magan Medical Group | 4,715 | 2,629 | ||||||
Practice acquisition liabilities | 10,408 | 10,408 | ||||||
Other | 17,239 | 17,676 | ||||||
|
|
|
| |||||
Total | $ | 196,652 | $ | 206,934 | ||||
|
|
|
| |||||
9. Other Liabilities
Other liabilities at June 30, 2012, and December 31, 2011, consist of the following (dollars in thousands):
| June 30, 2012 | December 31, 2011 | |||||||
Deferred compensation liabilities | $ | 43,504 | $ | 39,032 | ||||
Reserve for uncertain tax positions | 38,313 | 34,972 | ||||||
Reserve for medical malpractice claims | 12,353 | 12,417 | ||||||
Other | 12,770 | 11,377 | ||||||
|
|
|
| |||||
Total | $ | 106,940 | $ | 97,798 | ||||
|
|
|
| |||||
10. Capital Structure
At June 30, 2012, and December 31, 2011, the Company authorized 1,000 and 24,088,677 Class A Preferred Units (Class A Units) and 116,084,729 Class B Common Units (Class B Units), respectively. At June 30, 2012, and December 31, 2011, the Company had outstanding 1,000 Class A Units held by HCP Blocker, a wholly owned subsidiary of HCPH, which are eliminated in consolidation, and 100,131,969 Class B Units.
On March 2, 2005, Summit Partners, LP and Affiliates (Summit) acquired 24,088,677 Class A Units, representing 19.6% of total outstanding units, in exchange for cash consideration of $75.0 million. On January 6, 2011, HCPH entered into an agreement with Summit (Redemption Agreement) to repurchase from Summit all of its outstanding Class A Units (Class A Unit Redemption) for total consideration of $587.0 million. Total consideration consisted of $540.0 million in cash, 436,550 Class B Units valued at $10.0 million, and an assumed tax liability of $37.0 million. The Class A Unit Redemption consisted of repurchasing 18,855,176 Class A Units from various Summit partnerships and acquiring all of the outstanding shares of HCP Blocker, a C-corporation, which held the remaining 5,233,501 Class A Units.
As a part of the Redemption Agreement, an investment fund affiliated with Summit indemnified HCPH for unpaid tax liabilities, if any, of HCP Blocker existing as of January 6, 2011.
F-46
Table of Contents
HealthCare Partners Holdings, LLC and Affiliates
Notes to Consolidated Financial Statements (Unaudited) (continued)
10. Capital Structure (continued)
Pursuant to the Amended and Restated Operating Agreement (Operating Agreement) executed on January 6, 2011, HCPH revised the rights and privileges of Class A Units held by HCP Blocker. Class A Units receive an annual net income allocation equal to 3% of the $119.5 million liquidation preference (Class A Yield), of which approximately 40% is distributable in cash. The liquidation preference, including the undistributed Class A Yield, was $124.8 million and $121.2 million at June 30, 2012 and 2011, respectively. Remaining net income or loss is allocated to the holders of Class B Units based on their pro rata ownership interest in the total outstanding Class B Units.
Warrants
In connection with the JSAH acquisition, HCPH issued immediately exercisable warrants, with an exercise price of $7.28 per unit, to certain selling shareholders for the purchase of 418,000 Class B Units. These units were valued at $0.6 million based on the Black-Scholes option-pricing model and were included in the purchase consideration. These warrants expired on June 30, 2011; however, the two holders of the warrants, who are executives in the Company’s Nevada unit, had taken legal action to nullify the stated warrant expiration date. In May 2012, the Company and two executives of the Company’s Nevada unit reached an agreement whereby their legal action against the Company is to be dismissed in exchange for $10 million to be paid upon consummation of the merger with DaVita, Inc., and $20 million to be paid over the next eight years contingent upon attainment of certain performance milestones. At June 30, 2012, no warrants were outstanding.
11. Income Taxes
HCPMGI, HCPMG (Bacchus), HCP Blocker, JSAH, and certain HCPAMG wholly-owned subsidiaries (collectively, the taxable entities) are taxed as C-corporations under the Internal Revenue Code and applicable state laws, and each files separate stand-alone corporate income tax returns.
HCPH, HCP LLC, HCPNV, and HCPAMG are classified as partnerships for tax purposes, and as such they are not taxpaying entities. Rather, the owners of these entities pay taxes on their allocable share of net income from the entities.
Income tax expense for interim periods is measured using an estimated tax rate for the annual period. The reported income tax provision differs from the amounts that would have resulted had the reported income before income taxes been taxed at the U.S. federal statutory rate. The principal reasons for the differences between the amounts provided and those that would have resulted from the application of the U.S. federal statutory tax rate are income included in pre-tax income from nontaxpaying entities (e.g., HCPNV, HCP LLC, and HCPAMG), nondeductible items, and state taxes.
As of June 30, 2012, and December 31, 2011, the Company has unrecognized tax benefits of approximately $38.3 million and $35.0 million, respectively, for which the deductibility is uncertain. These amounts include interest and penalties of $8.5 million and $7.8 million at June 30, 2012, and December 31, 2011, respectively. The Company recognizes interest and penalties accrued related to unrecognized tax benefits as a component of the provision for income taxes in the consolidated statements of income.
12. Commitments and Contingencies
HCPH and its affiliates operate in a regulated and litigious industry. As a result, various lawsuits, claims, and legal and regulatory proceedings have been instituted or asserted against HCPH related to patient treatment.
F-47
Table of Contents
HealthCare Partners Holdings, LLC and Affiliates
Notes to Consolidated Financial Statements (Unaudited) (continued)
12. Commitments and Contingencies (continued)
Additionally, HCPH and its affiliates are subject to claims and suits arising in the ordinary course of business, including claims for personal injuries, unpaid wages, or wrongful termination. In certain of these actions, the claimants may seek punitive damages against HCPH and its affiliates that may not be covered by insurance. It is management’s opinion that the ultimate resolution of these pending claims and legal proceedings will not have a material adverse effect on HCPH’s results of operations or financial position.
13. Subsequent Events
Based on management’s review of subsequent events through August 13, 2012, the date the financial statements were available for issuance, the following significant events occurred:
On July 24, 2012, HCP LLC entered into a definitive agreement to acquire 100% of the ownership interests of a medical group organization with operations in an area of the western United States in which the Company does not currently maintain a presence, for cash consideration of $73.1 million and earn-out consideration of up to $70.0 million contingent upon attainment of certain performance based criteria. The transaction, which is subject to shareholder and regulatory approval, is anticipated to close on or about September 1, 2012.
F-48
Table of Contents
PROSPECTUS

Debt Securities
and Guarantees
We may offer and sell from time to time, any of the securities listed above, in one or more series.
This prospectus contains a general description of the securities we may offer for sale. The specific terms of these securities will be contained in one or more supplements to this prospectus. The prospectus supplements may add, update or change information contained in this prospectus. You should read this prospectus and any prospectus supplement, as well as the documents incorporated and deemed to be incorporated by reference in this prospectus, carefully before you invest.
Investing in our securities involves risks. See “Risk Factors” on page 5 of this prospectus.
This prospectus may not be used to offer to sell any securities unless accompanied by a prospectus supplement.
We may sell any securities on a continuous or delayed basis directly to investors or through underwriters, dealers or agents designated from time to time. For additional information on the methods of sale, you should refer to the section entitled “Plan of Distribution” in this prospectus. If any underwriters, dealers or agents are involved in the sale of any securities, the applicable prospectus supplement will set forth the names of such underwriters, dealers or agents and any applicable commissions or discounts. The price to the public of such securities and the net proceeds we expect to receive from such sale will also be set forth in the applicable prospectus supplement.
Neither the Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is August 13, 2012.
Table of Contents
Prospectus
| Page | ||||
| 1 | ||||
| 2 | ||||
| 3 | ||||
| 5 | ||||
| 5 | ||||
| 5 | ||||
| 6 | ||||
| 6 | ||||
| 6 | ||||
| 6 | ||||
| 7 | ||||
| 7 | ||||
We have not authorized anyone to provide any information other than that contained or incorporated by reference in this prospectus and the applicable prospectus supplement or in any related free writing prospectus we authorize that supplements this prospectus. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. You should not assume that the information in this prospectus or any applicable prospectus supplement is accurate as of any date other than the date on the cover of the applicable document. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy by anyone in any jurisdiction in which such offer or solicitation is not authorized, or in which the person is not qualified to do so or to any person to whom it is unlawful to make such offer or solicitation.
Table of Contents
This prospectus is part of an automatic shelf registration statement that we filed with the Securities and Exchange Commission, or the SEC, as a “well-known seasoned issuer” as defined in Rule 405 under the Securities Act of 1933, as amended. Under the automatic shelf registration process, we may, at any time and from time to time, in one or more offerings, sell debt securities, including any related guarantees, under this prospectus. This prospectus provides you with a general description of the securities we may offer. Each time we offer debt securities, we will provide a prospectus supplement that will contain specific information about the terms of those securities and the offering. The prospectus supplement may also add, update or change the information in this prospectus. Please carefully read this prospectus and the applicable prospectus supplement, together with the documents incorporated and deemed to be incorporated by reference in this prospectus and the additional information described below under the heading “Where You Can Find More Information.”
As allowed by SEC rules, this prospectus does not contain all the information you can find in the registration statement or the exhibits to the registration statement. For further information, we refer you to the registration statement, including its exhibits and schedules. Statements contained in this prospectus about the provisions or contents of any contract, agreement or any other document referred to are not necessarily complete. For each of these contracts, agreements or documents filed as an exhibit to the registration statement, we refer you to the actual exhibit for a more complete description of the matters involved.
As used in this prospectus, unless stated otherwise or the context requires otherwise, “DaVita,” “the Company,” “we,” “us” and “our” refer to DaVita Inc. and its subsidiaries.
1
Table of Contents
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public from the SEC’s website athttp://www.sec.gov. You may also read and copy any document we file with the SEC at the SEC’s Public Reference Room located at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the Public Reference Room. We also file certain reports and other information with the New York Stock Exchange, or the NYSE, on which our common stock is traded. Copies of such material can be inspected at the offices of the New York Stock Exchange, 20 Broad Street, New York, New York 10005. Information about us, including our SEC filings, is also available through our website athttp://www.davita.com. However, information on our website is not a part of this prospectus or any accompanying prospectus supplement.
The SEC allows us to “incorporate by reference” in this prospectus information that we file with the SEC, which means that we are disclosing important business and financial information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus, and information that we file later with the SEC will automatically update and supersede information contained in documents filed earlier with the SEC or contained in this prospectus. This prospectus incorporates by reference the documents filed by us listed below and any future filings we make with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, prior to the termination of the offering under this prospectus; provided, however, that we are not incorporating, in each case, any documents or information deemed to have been furnished and not filed in accordance with SEC rules:
| • | Annual Report on Form 10-K for the year ended December 31, 2011; |
| • | Quarterly Reports on Form 10-Q for the quarters ended March 31, 2012 and June 30, 2012; and |
| • | Current Reports on Form 8-K filed with the SEC on February 16, 2012, April 16, 2012, May 21, 2012 (solely with respect to Items 1.01 and 9.01), June 14, 2012, July 3, 2012, July 6, 2012, July 9, 2012 and August 10, 2012. |
We will provide free of charge a copy of any or all of the information that has been incorporated by reference in this prospectus if you write to or call us at the following:
DaVita Inc.
1551 Wewatta Street
Denver, Colorado 80202
Telephone: (888) 484-7505
Attention: Investor Relations
2
Table of Contents
This prospectus and the documents deemed to be incorporated by reference in this prospectus contains or may contain statements that are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended and Section 21E of the Securities Exchange Act of 1934, as amended. We intend these forward-looking statements to be covered by the safe harbor provisions for such statements contained in these documents. All statements that do not concern historical facts are forward-looking statements and include, among other things, statements about our expectations, beliefs, intentions and/or strategies for the future. These forward-looking statements include statements regarding our future operations, financial condition and prospects, expectations for treatment growth rates, revenue per treatment, expense growth, levels of the provision for uncollectible accounts receivable, operating income, cash flow, operating cash flow, estimated tax rates, capital expenditures, the development of new centers and center acquisitions, government and commercial payment rates, revenue estimating risk and the impact of our related level of indebtedness on our financial performance, including earnings per share and the anticipated timing of the closing of the HCP transaction. These statements can sometimes be identified by the use of forward looking words such as “may,” “believe,” “will,” “should,” “could,” “would,” “expect,” “project,” “estimate,” “anticipate,” “plan,” “continue,” “seek,” “forecast,” or “intend” or other similar words or expressions of the negative thereof.
These statements involve substantial known and unknown risks and uncertainties that could cause our actual results to differ materially from those described in the forward-looking statements, including, but not limited to:
| • | risks resulting from uncertainties associated with governmental regulations, |
| • | general economic and other market conditions, |
| • | competition, |
| • | accounting estimates, the variability of our cash flows, |
| • | the concentration of profits generated from commercial payor plans, |
| • | continued downward pressure on average realized payment rates from commercial payors, which may result in the loss of revenue or patients, |
| • | a reduction in the number of patients under higher-paying commercial plans, |
| • | a reduction in government payment rates under the Medicare ESRD program or other government-based programs, the impact of health care reform legislation that was enacted in the United States in March 2010, |
| • | changes in pharmaceutical or anemia management practice patterns, payment policies or pharmaceutical pricing, |
| • | our ability to maintain contracts with physician medical directors, |
| • | legal compliance risks, including our continued compliance with complex government regulations, |
| • | current or potential investigations by various governmental entities and related government or private-party proceedings, the impact of our agreement in principal to settle all allegations relating to claims arising out of the previously disclosed litigation filed in 2002 in the U.S. District Court in the Eastern District of Texas to resolve the federal program claims regarding EPO relating to historical EPO practices dating back to 1997, |
| • | continued increased competition from large and medium-sized dialysis providers that compete directly with us, the emergence of new models of care introduced by the government or private sector, such as accountable care organizations, independent practice association and integrated delivery systems, and changing affiliation models for physicians, such as employment by hospitals, that may erode our patient base and reimbursement rates, and |
3
Table of Contents
| • | our ability to complete any acquisitions, mergers or dispositions that we might be considering or announce, including the HCP transaction, or integrate and successfully operate any business we may acquire, expansion of our operations and services to markets outside the United States, or to businesses outside of dialysis. |
The forward-looking statements included or incorporated by reference in this prospectus are only made as of the date of this prospectus or the respective document incorporated by reference herein, as applicable. Except as required by law, we undertake no obligation to update or revise these statements, whether as a result of changes in underlying factors, new information, future events or otherwise. See “Where You Can Find More Information.”
4
Table of Contents
We are a leading provider of kidney dialysis services primarily in the United States for patients suffering from chronic kidney failure, also known as end stage renal disease, or ESRD. ESRD is the stage of advanced kidney impairment that requires continued dialysis treatments or a kidney transplant to sustain life. Dialysis is the removal of toxins, fluids and salts from the blood of ESRD patients by artificial means. As of June 30, 2012, we provided dialysis and administrative services through a network of 1,884 outpatient dialysis centers located in the United States throughout 43 states and the District of Columbia, serving a total of approximately 149,000 patients. Our centers offer outpatient hemodialysis treatments and other ESRD-related services such as the administration of physician-prescribed pharmaceuticals, including erythropoietin, or EPO, vitamin D analogs and iron supplements. We also provide services for home dialysis patients, vascular access, disease management services and laboratory services related to ESRD. As of June 30, 2012, we also provided acute inpatient dialysis services in approximately 960 hospitals and related laboratory services throughout the United States.
In addition, as of June 30, 2012, we provided dialysis and administrative services to a total of 19 outpatient dialysis centers located in four countries outside of the United States. Our international dialysis operations are currently in a start-up phase in which we primarily commenced operations during the fourth quarter of 2011.
Our principal executive offices are located at 1551 Wewatta Street, Denver, CO 80202, and our telephone number is (303) 405-2100.
An investment in our debt securities involves significant risks. Before purchasing any securities, you should carefully consider and evaluate all of the information included and incorporated by reference in this prospectus or the applicable prospectus supplement, including the risk factors incorporated by reference herein from our Quarterly Report on Form 10-Q for the quarter ended June 30, 2012, as updated by subsequent annual, quarterly and other reports and documents we file with the SEC that are incorporated by reference herein or in the applicable prospectus supplement. Our business, financial condition, results of operations or liquidity could be adversely affected by any of these risks.
The risks and uncertainties we describe are not the only ones we face. Additional risks and uncertainties not known to us or that we deem immaterial may also impair our business or operations. Any adverse effect on our business, financial condition, results of operations or liquidity could result in a decline in the value of the debt securities and the loss of all or part of your investment.
Unless otherwise specified in a prospectus supplement accompanying this prospectus, the net proceeds from the sale of the securities to which this prospectus relates will be used for general corporate purposes. General corporate purposes may include repayment of debt, acquisitions, additions to working capital, capital expenditures and investments in our subsidiaries. Net proceeds may be temporarily invested prior to use.
5
Table of Contents
RATIO OF EARNINGS TO FIXED CHARGES
Our ratios of earnings to fixed charges for each of the periods indicated are set forth below. The information set forth below should be read together with the financial statements and the accompanying notes incorporated by reference into this prospectus. See “Where You Can Find More Information.”
| Six Months Ended June 30, 2012 | Year Ended December 31, | |||||||||||||||||||||||
| 2011 | 2010 | 2009 | 2008 | 2007 | ||||||||||||||||||||
Ratio of earnings to fixed charges (1) | 3.20 | 3.31 | 3.44 | 3.58 | 3.01 | 2.92 | ||||||||||||||||||
| (1) | The ratio of earnings to fixed charges is computed by dividing earnings by fixed charges. Earnings for this purpose is defined as pretax income from continuing operations adjusted by adding back fixed charges expensed during the period less pre-tax net income attributable to noncontrolling interests. Fixed charges include debt expense (interest expense and the amortization of deferred financing costs), the estimated interest component of rent expense on operating leases, and capitalized interest. |
DESCRIPTION OF DEBT SECURITIES
The debt securities will be our direct unsecured general obligations and will be senior debt securities, which will rank equally with any of our other unsecured senior debt. The debt securities will be issued under one or more separate indentures between us and a designated trustee. We will include in a prospectus supplement the specific terms of each series of senior debt securities being offered. In addition, the material terms of any indenture, which will govern the rights of the holders of our senior debt securities will be set forth in the applicable prospectus supplement.
One or more registrants may issue from time to time guarantees for the benefit of holders of our debt securities. Any such guarantees will, unless otherwise specified in the applicable prospectus supplement, include the following terms and conditions, plus any additional terms specified in the accompanying prospectus supplement.
A guarantee will provide that the guarantor unconditionally guarantees the due and punctual payment of the principal, interest (if any), premium (if any) and all other amounts due under the applicable debt securities when the same shall become due and payable, whether at maturity, pursuant to mandatory or optional repayments, by acceleration or otherwise, in each case after any applicable grace periods or notice requirements, according to the terms of the applicable underlying securities. Any guarantee shall be unconditional irrespective of the validity or enforceability of the applicable underlying security, any change or amendment thereto or any other circumstances that may otherwise constitute a legal or equitable discharge or defense of a guarantor. However, the guarantors will not waive presentment or demand of payment or notice with respect to the applicable underlying security unless otherwise provided in the accompanying prospectus supplement.
We may sell the securities covered by this prospectus in any of the following ways:
| • | directly to one or more purchasers; |
| • | through underwriters, dealers or agents; or |
| • | through a combination of any of these methods of sale. |
We will identify the specific plan of distribution, including any direct purchasers or any underwriters, dealers or agents and their compensation in a prospectus supplement.
6
Table of Contents
Unless otherwise specified in the prospectus supplement accompanying this prospectus, Kim M. Rivera, the Company’s Chief Legal Officer and Corporate Secretary, and Sidley Austin LLP, San Francisco, California, special counsel to the Company, will pass upon certain legal matters for us with respect to the securities. Ms. Rivera participates in various employee benefit plans offered by DaVita and as of June 30, 2012, held 5,000 restricted stock units (none of which were vested) and 112,500 stock-settled stock appreciation rights (22,500 of which were vested).
The consolidated financial statements and financial statement schedule of DaVita Inc. as of December 31, 2011 and 2010, and for each of the years in the three-year period ended December 31, 2011, and management’s assessment of the effectiveness of internal control over financial reporting as of December 31, 2011 have been incorporated by reference herein and in the registration statement in reliance upon the reports of KPMG LLP, independent registered public accounting firm incorporated by reference herein, and upon the authority of said firm as experts in accounting and auditing. The audit report covering the consolidated financial statements refers to the Company’s adoption of accounting standards updates 2011-07Health Care Entities – Presentation and Disclosure of Patient Service Revenue, Provision for Bad Debts, and the Allowance for Doubtful Accounts and 2011-05 as amended by 2011-12Comprehensive Income – Presentation of Comprehensive Income, which were applied retrospectively for all periods presented effective January 1, 2012.
7
Table of Contents
