UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
ANNUAL REPORT PURSUANT TO SECTIONS 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2013.
Commission file number 000-16789

ALERE INC.
(Exact Name of Registrant as Specified in Its Charter)
| | |
| Delaware | | 04-3565120 |
| (State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| |
| 51 Sawyer Road, Suite 200, Waltham, Massachusetts | | 02453 |
| (Address of principal executive offices) | | (Zip Code) |
(781) 647-3900
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Securities Exchange Act of 1934 (the “Exchange Act”):
| | |
Title of Each Class | | Name of Each Exchange on Which Registered |
| Common Stock, $0.001 per share par value | | New York Stock Exchange |
Series B Convertible Perpetual Preferred Stock, $0.001 per share par value | | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Exchange Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act of 1933. Yes þ No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ¨ No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes þ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| | | | | | |
| Large accelerated filer þ | | Accelerated filer ¨ | | Non-accelerated filer ¨ | | Smaller reporting company ¨ |
| | (Do not check if a smaller reporting company) |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No þ
The aggregate market value of the common stock held by non-affiliates of the registrant based on the closing price of the registrant’s common stock on the New York Stock Exchange on June 28, 2013 (the last business day of the registrant’s most recently completed second fiscal quarter) was $1,646,329,832.
As of February 24, 2014, the registrant had 82,505,273 shares of common stock, par value $0.001 per share, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement to be filed in connection with the registrant’s 2014 annual meeting of shareholders are incorporated by reference into Part III of this Form 10-K.
ALERE INC.
FORM 10-K
For The Fiscal Year Ended December 31, 2013
PART I
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Readers can identify these statements by forward-looking words such as “may,” “could,” “should,” “would,” “intend,” “will,” “expect,” “anticipate,” “believe,” “estimate,” “continue” or similar words. Readers should carefully review statements that contain these words because they discuss our future expectations, contain projections of our future results of operations or of our financial condition or state other “forward-looking” information. We caution investors that all such forward-looking statements involve risks and uncertainties that could cause our actual results to differ materially from any projected results or expectations that we discuss in this report. You should therefore carefully review the risk factors and uncertainties discussed in Item 1A entitled “Risk Factors,” which begins on page 18 of this report, as well as those factors identified from time to time in our periodic filings with the Securities and Exchange Commission. We undertake no obligation to update any forward-looking statements.
Unless the context requires otherwise, references in this Annual Report on Form 10-K to “we,” “us,” “our,” or our “company” refer to Alere Inc. and its subsidiaries.
GENERAL
Alere Inc. empowers individuals to take greater control of their health at home, under the supervision of their healthcare providers, by connecting innovative diagnostics in the hands of patients to their healthcare providers. A leading global provider of point-of-care diagnostics and services, we have developed a strong commercial presence in infectious disease, toxicology, cardiology and diabetes. Our products and services help healthcare practitioners make earlier, more effective treatment decisions and improve outcomes for individuals living with chronic disease. Our portfolio also includes a broad array of health information solutions that increase access to critical health data, provide clinical decision support and facilitate more comprehensive performance reporting and analysis. We believe that the integration of these solutions with our novel diagnostics and monitoring services positions us to enable customers to reduce the healthcare costs associated with managing chronic disease considerably, addressing what may be the greatest burden faced by most health systems around the world today. Our connected health approach envisions a healthcare system that unites patients with diagnostic tools at their fingertips with providers and payers in a model that provides patients with a better quality of life and improved outcomes, while simultaneously reducing healthcare costs to the system.
Our company, formerly known as Inverness Medical Innovations, Inc., was formed to acquire the women’s health and professional diagnostics businesses of its predecessor, Inverness Medical Technology, Inc., through a split-off and merger transaction, which occurred in November 2001. Since that time, we have grown our businesses through strategic acquisitions, tactical use of our intellectual property portfolio and organic growth. In July 2010, our company changed its name to Alere Inc. Our common stock is listed on the New York Stock Exchange under the symbol “ALR.”
Our principal executive offices are located at 51 Sawyer Road, Suite 200, Waltham, Massachusetts 02453 and our telephone number is (781) 647-3900. Our website is www.alere.com, and we make available through the investor center of this site, free of charge, our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports, filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, as soon as reasonably practicable after such reports are electronically filed with, or furnished to, the Securities and Exchange Commission, or the SEC. We also make our code of ethics and certain other governance documents and policies available through our website. We intend to
2
make required disclosures of amendments to our code of ethics, or waivers of a provision of our code of ethics, on the “Corporate Governance” page of our website’s investor center.
Our reportable operating segments are professional diagnostics, health information solutions and consumer diagnostics. Financial information about our reportable segments is provided in Note 17 of the Notes to Consolidated Financial Statements which are included elsewhere in this report.
Our Approach to Improving Health Outcomes
We manage the process that leads to health behavior changes and results in improved health outcomes. Our professional diagnostic devices, including point-of-care tests and connected devices, and our health information solutions, including software-based analytics, clinical decision-support tools, and accountable care and behavioral change programs, work together to allow us to improve patients’ health outcomes.
Professional Diagnostics
Our professional diagnostic solutions allow patients and their healthcare providers to work together to better manage patients’ chronic conditions over the continuum of care, from the hospital to home. Professional diagnostics are generally designed to assist medical professionals in both preventative and interventional medicine, and include testing and monitoring performed in hospitals, laboratories and doctors’ offices and, increasingly, patient self-testing, which we define as testing or monitoring performed at home under the supervision of a medical professional. Professional diagnostic products provide for qualitative or quantitative analysis of patient samples for evidence of a specific medical condition, disease state or toxicological state or to measure response to therapy. Within professional diagnostics, we focus on point-of-care, rapid diagnostic testing and the developing patient self-testing and patient self-management markets where we believe that we can directly and immediately improve patient health outcomes. We distinguish these markets from clinical diagnostic markets consisting of large, centralized laboratories offering a wide range of highly-automated laboratory services in hospital or related settings. The point-of-care market for rapid diagnostic products includes all areas where a patient is assessed or diagnosed, including hospitals, laboratories, physician offices, specialized mobile clinics, emergency rooms, rapid-response laboratories and patient health screening locations.
In the market for rapid diagnostic products, the ability to deliver faster, accurate results at competitive prices generally drives demand. While there is certainly demand for faster, more efficient automated equipment from large hospitals and major reference testing laboratories, we believe there is also growing demand by point-of-care facilities and smaller laboratories for fast, high-quality, cost-effective and potentially life-saving, self-contained diagnostic kits. As the speed and accuracy of these products improve, we believe that they will play an increasingly important role in achieving earlier diagnosis, timely intervention and therapy monitoring outside acute medical environments, especially where supplemented by the support and management services we also provide. Our current professional diagnostic products include point-of-care and laboratory tests within the following areas:
Infectious Disease. We believe that the demand for infectious disease diagnostic products is growing faster than many other segments of the immunoassay market due to the increasing incidence and awareness of certain diseases or groups of diseases, including viral hepatitis, respiratory syncytial virus (RSV), influenza, pneumonia, tuberculosis, human immunodeficiency virus (HIV) / acquired immunodeficiency syndrome (AIDS), gastrointestinal disease, vector-borne diseases such as malaria and dengue, herpes and other sexually-transmitted diseases. To meet this demand, we have continued to expand our product offerings and now offer one of the world’s largest infectious disease test menus, including tests based on leading-edge technologies that enable rapid and accurate diagnosis and monitoring of the most prevalent infectious diseases. We develop and market a wide variety of point-of-care tests for influenza A & B, RSV, strep throat, pneumonia,C. difficile, infectious
3
mononucleosis, HIV, herpes simplex virus (HSV-2), hepatitis C (HCV), hepatitis B (HBV), malaria, Lyme disease, Chlamydia,H. pylori, rubella and other infectious diseases. Our tests for infectious disease are currently sold under brand names that include Alere, Alere i, Alere Determine, Acceava, BinaxNOW, Clearview, DoubleCheckGold, Panbio, SD, TECHLAB and Alere TestPack. In January 2014, we announced the commercial availability of the Alere i Influenza A & B test in Austria, France, Spain, Switzerland, Germany, Italy and the U.K. Alere i is a rapid, instrument-based, isothermal system for the qualitative detection of infectious diseases. Our unique Alere i isothermal nucleic acid amplification technology provides molecular results in just minutes, allowing healthcare providers to make effective clinical decisions sooner than with conventional tests. Alere i remains under FDA review and is not yet available for sale in the United States.
During 2013, we also continued to expand our offerings for the diagnosis and management of HIV infection with the introduction of Alere Determine HIV-1/2 Ag/Ab Combo, the first FDA-approved rapid, point-of-care test that detects both HIV-1/2 antibodies and free HIV-1 p24 antigen. This fourth generation test has the ability to identify HIV sooner than conventional rapid tests, which rely solely on the presence of HIV-1/2 antibodies. It enables healthcare providers to diagnose HIV infection earlier, which allows individuals to seek medical care sooner. We also continued to expand the commercialization of our Alere CD4 Analyzer across Africa, Asia and Europe, as well as in South America and the Caribbean. The Alere CD4 Analyzer is one of the first point-of-care CD4 platforms that measures absolute CD4 counts. A CD4 count is a measure of the number of T-helper lymphocytes per cubic millimeter of blood, which is used to stage a patient’s HIV disease as well as monitor HIV disease progression. The Alere CD4 Analyzer provides results in 20 minutes or less, using disposable, single-use fingerstick cartridges. CD4 results delivered quickly and accurately at the point of care can improve both patient retention and access to treatment. Program data from the Alere CD4 Analyzer can be transmitted and managed using our Alere Data Point connectivity solution, which is designed to enable data transmission from analyzers in the field to a web-portal in order to assist in the management of local HIV treatment programs. The Alere CD4 Analyzer can provide an absolute CD4 count to HIV-infected patients in remote locations who may otherwise have restricted access to testing. By empowering individuals with access to this information, they can monitor their HIV drug therapy to make sure it is working, or seek medical intervention if problems arise. These are all examples of us deploying leading-edge technologies that enable rapid and accurate diagnosis and monitoring of the most prevalent infectious diseases around the world.
In addition to point-of-care products, we also offer a line of indirect fluorescent antibody, or IFA, assays for 17 viral, bacterial and autoimmune diseases, a full line of serology diagnostic products covering a broad range of disease categories and 40 enzyme-linked immunosorbent assay, or ELISA, tests for a wide variety of infectious and autoimmune diseases, as well as a full line of automated instrumentation for processing ELISA tests. We are the exclusive U.S. distributor of the AtheNA Multi-Lyte® Test System, a multiplexed, fluorescent bead-based system designed to simultaneously perform multiple assays from a single sample using just one well. It offers a simple and streamlined alternative to IFA and ELISA testing, providing improved clinical sensitivity and comparable clinical specificity in a labor-saving, automation-friendly format. Our IFA, serology and ELISA products, which generally serve the clinical diagnostics laboratory markets, are generally marketed under our Wampole brand.
Demand for certain infectious disease tests, such as influenza A & B, or flu, is significantly affected by the seasonal nature of the cold and flu season. As a result, we typically experience higher sales of our flu tests in the first and fourth quarters. Sales of our flu products also vary widely from year to year based in large part on the severity, duration and timing of the onset of the cold and flu season.
Toxicology. Drug abuse is a major global health problem, as well as a social and economic burden. In addition to being a primary cause of lost workforce productivity, family conflict and drug-related crime, abuse of illicit and prescription drugs is linked globally to the spread of HIV/AIDS, hepatitis and other blood-borne pathogens through the use of contaminated needles. This misuse of
4
drugs and drug addiction are among the costliest health problems in the United States, and increasingly abroad. As a result, employers, law enforcement officials, healthcare professionals and others expend considerable effort to ensure that their employees, patients and other constituents are free of substance abuse and misuse. This critical need creates a significant market for simple and reliable laboratory-based, point-of-care and rapid toxicology tests to detect the most commonly abused substances and an ever-evolving set of newly-formulated, synthetic toxins. Additionally, physicians and treatment centers are increasingly utilizing drug testing to identify and address signs of prescription drug misuse, whether illicit or by prescription, and more broadly, to improve outcomes in addiction medicine. Finally, both domestically and abroad, a substantial market exists for services to help employers and governments manage their workforces’ compliance with drug, alcohol and/or related fitness-for-duty health policies.
Urine and saliva-based screening and confirmation tests for drugs of abuse range from simple immunoassay tests to complex analytical procedures. The speed and sensitivity of immunoassays have made them the most widely accepted method for toxicology screening at the point of care.
We offer one of the most comprehensive lines of drugs-of-abuse tests, reagent systems and laboratory testing options available today. Our products include tests to detect alcohol, as well as various device platforms for the detection of the following illicit and prescription drugs of abuse: amphetamines/methamphetamines, cocaine, opiates, phencyclidine, tetrahydrocannabinol, acetaminophen, barbiturates, benzodiazepines, methadone, propoxyphene and tricyclic antidepressants, and a growing range of designer drugs of abuse. Our products test using urine or, for certain applications, saliva, hair or other body fluids. We believe that early detection can lead to improved health outcomes through early intervention, treatment and recovery.
Our rapid toxicology tests are sold primarily under the brands Alere Toxicology, Alere Triage, Alere iScreen and SureStep. The Alere Triage TOX Drug Screen panel sold for use with our Alere Triage MeterPro system detects the presence of many of the illicit and prescription drugs listed above at the point of care in approximately 15 minutes. It is widely used in hospital and clinical testing as a laboratory instrument to aid in the detection of drug abuse. Our Drug Detection System is an enhanced, on-site, saliva-based drug detection system utilized in roadside testing which displays results for the presence of two drugs in less than 90 seconds and six different drugs in less than five minutes. We currently sell this product only in markets outside of the United States. We believe that a significant market for this product could develop in the United States if the trend towards the decriminalization of marijuana accelerates, and if federal and state regulators develop impairment policies, as there will be an increased need for multiple forms of roadside and evidentiary tests for impaired driving.
We also offer comprehensive laboratory-based testing services throughout Europe by Alere Toxicology, and in the United States by Alere Toxicology and Redwood Toxicology Laboratory, or Redwood. Three of Alere Toxicology’s laboratories are certified to the highest standard by the U.S. Substance Abuse and Mental Health Services Administration, or SAMHSA. In addition, we are expanding our offerings in the growing market for pain management and addiction medicine services, or the monitoring and documentation of adherence to prescription drug treatment or drug abstinence plans through complex laboratory testing. Through Redwood, we offer comprehensive, low-cost laboratory testing services to multiple domestic clients, including law enforcement agencies, penal systems, insurers and employers in the United States.
We also provide automated and efficient workplace drug testing services through our eScreen business, which we acquired in 2012 and have become part of our core set of Toxicology products and solutions. The addition of the eScreen business to our portfolio of toxicology offerings helps to position us as a full-service solution provider to a broad range of domestic and foreign employers in transport, oil and gas, mining, retail and related industries that follow rigorous drug testing policies. We believe
5
that the combination of products, laboratory testing and services that we offer for drugs of abuse enhances our ability to compete in this market.
Cardiology. Cardiovascular disease encompasses a spectrum of conditions and illnesses, including high blood pressure, high cholesterol, metabolic syndrome, coronary artery disease, heart attack, heart failure and stroke. It is estimated that 83 million American adults alone have one or more types of cardiovascular disease. The worldwide cardiology point-of-care diagnostics market, including the markets for heart failure diagnostics, coronary artery disease risk assessment, coagulation testing and acute coronary syndrome, exceeds $2.0 billion. Our Alere Triage, Alere Cholestech LDX and Alere INRatio products have established us as a leader in this market. The Alere Triage System is a leading rapid diagnostic test system comprised of a portable fluorometer and various test devices that enable physicians to promote improved health outcomes through the rapid diagnosis of critical diseases and health conditions, as well as the detection of certain drugs of abuse. This aids in risk stratification of patients having critical care issues, which can reduce hospital admissions and improve clinical and economic outcomes. Alere Triage cardiovascular tests include the following:
| | • | | Alere Triage BNP Test. An immunoassay that measures B-type Natriuretic Peptide (BNP) in EDTA anticoagulated whole blood or plasma, used as an aid in the diagnosis and assessment of severity of congestive heart failure. The test is also used for risk stratification of patients with heart failure and acute coronary syndromes. We also offer a version of the Alere Triage BNP Test for use on Beckman Coulter lab analyzers. |
| | • | | Alere Triage NT-proBNP. An immunoassay for the rapid quantitative determination ofN-terminal pro-Brain Natriuretic Peptide (NT-proBNP) in EDTA anticoagulated whole blood and plasma specimens. The test is used as an aid in the diagnosis of congestive heart failure, an aid in the risk stratification of patients with acute coronary syndromes and heart failure, and an aid in the assessment of increased risk of cardiovascular events and mortality in patients at risk for heart failure who have stable coronary artery disease. Alere Triage NT-proBNP is CE marked, but not available for sale in the United States. |
| | • | | Alere Triage Cardiac Panel. An immunoassay for the quantitative determination of creatine kinase-MB (CK-MB), myoglobin and troponin I in EDTA whole blood or plasma, as an aid in determination of acute myocardial infarction, or AMI. Troponin I is highly specific for cardiac necrosis. |
| | • | | Alere Triage CardioProfilER Panel. An immunoassay panel that includes all of the markers on the Alere Triage Cardiac Panel, but adding BNP, to provide rapid, accurate results in EDTA whole blood and plasma. In addition to cardiac markers utility as an aid in the diagnosis of AMI, BNP is useful in risk stratification of patients with acute coronary syndromes and congestive heart failure. |
| | • | | Alere Triage ProfilER Shortness of Breath (S.O.B.) Panel. An immunoassay panel that includes all of the markers on the Alere Triage CardioProfiler Panel, but adding D-dimer, to provide rapid, accurate results in EDTA whole blood and plasma. In addition to cardiac markers utility as an aid in the diagnosis of AMI, d-dimer is an aid in the assessment and evaluation of patients suspected of having disseminated intravascular coagulation or thromboembolic events, including pulmonary embolism and deep vein thrombosis. |
| | • | | Alere Triage Cardio3 Panel. An immunoassay for the rapid quantitative determination ofCK-MB, troponin I and BNP in whole blood and plasma specimens. This panel is used as an aid in the diagnosis of myocardial infarction, the diagnosis and assessment of severity of congestive heart failure and the risk stratification of patients with acute coronary syndromes and heart failure. Alere Triage Cardio3 is CE marked, but not available for sale in the United States. |
| | • | | Alere Triage Cardio2 Panel. An immunoassay for the rapid quantitative determination of troponin I and BNP in whole blood and plasma specimens. This panel is used as an aid in the |
6
| | diagnosis of myocardial infarction, the diagnosis and assessment of severity of congestive heart failure and the risk stratification of patients with acute coronary syndromes and heart failure. Alere Triage Cardio2 is CE marked, but not available for sale in the United States. |
| | • | | Alere Triage Troponin I Test. An immunoassay for the quantitative determination of troponin I in whole blood and plasma specimens. The test is used as an aid in the diagnosis of myocardial infarction. Alere Triage Troponin I is CE marked, but not available for sale in the United States. |
| | • | | Alere Triage D-Dimer Test. An immunoassay for use as an aid in the assessment and evaluation of patients suspected of having disseminated intravascular coagulation or thromboembolic events, including pulmonary embolism and deep vein thrombosis. |
| | • | | Alere Triage NGAL Test. An immunoassay for use in the rapid, quantitative determination of neutrophil gelantinase-associated lipocalin (NGAL) in anticoagulated whole blood or plasma specimens. Studies have shown a link between elevated NGAL levels and the later occurrence of elevated creatinine indicative of prior acute kidney injury. Alere Triage NGAL is CE marked, but not available for sale in the United States. |
| | • | | Alere Triage CardioRenal Panel. An immunoassay panel containing NGAL and BNP. NGAL is indicated for use as an aid in the diagnosis of acute kidney injury. The BNP Test is used as an aid in the diagnosis and assessment of severity of congestive heart failure, an aid in the risk stratification of patients with congestive heart failure, and an aid in the risk stratification of patients with acute coronary syndromes. Alere Triage CardioRenal Panel is CE marked, but is not available for sale in the United States. |
Our Alere Cholestech LDX System is a small, portable point-of-care analyzer and test cassette system for testing blood glucose, cholesterol and related lipids. The Alere Cholestech LDX System makes it possible to provide a complete lipid profile with tests for total cholesterol, high-density lipoprotein cholesterol (HDL) and low-density lipoprotein cholesterol (LDL), triglycerides, and glucose. The Alere Cholestech LDX System provides results in five minutes per test cassette and is CLIA-waived, meaning the United States Food and Drug Administration, or FDA, has waived the more stringent requirements for laboratory testing applicable to moderate or high complexity laboratories based on the Alere Cholestech LDX System’s ease of use and accuracy. This waiver allows the Alere Cholestech LDX System to be marketed to physician offices and clinics, rather than hospitals or larger laboratories, and to be used in health screening by medical professionals.
Our Alere INRatio2 System is an easy-to-use, hand-held blood coagulation monitoring system for use by patients and healthcare professionals in the management of warfarin, a commonly prescribed medication used to prevent blood clots. The Alere INRatio2 System measures PT/INR, which is the patient’s blood clotting time reported pursuant to an internationally normalized ratio, to help ensure that patients at risk of blood clot formation are maintained within the therapeutic range with the proper dosage of oral anticoagulant therapy. The Alere INRatio System is 510(k) cleared by the FDA for use by healthcare professionals, as well as for patient self-testing, and is also CE marked in Europe. The system is targeted to both the professional, or point-of-care, market, as well as the patient self-testing market and utilizes small patient sample sizes.
We also offer the epoc Blood Analysis System for blood gas, electrolyte and metabolite testing. The epoc (enterprise point-of-care) platform is a point-of-care analysis system which provides wireless bedside blood gas, electrolyte and metabolite measurement testing solutions and complements our Alere Triage products in cardiology and emergency room settings. Utilizing easy to use, low-cost disposable Smart-Cards™, the epoc Blood Analysis System produces laboratory-quality results in critical and acute care settings in about 30 seconds.
7
We launched the Alere Heart Check System in Europe in 2010 and in Asia Pacific in 2011. The Alere Heart Check System provides a quantitative reading of BNP in less than 15 minutes using a fingerstick sample (12 microliters) with substantially equivalent performance to lab instruments. Initially being marketed as a point-of-care device, the Alere Heart Check System is ultimately designed for home use and is intended to enable doctors to remotely monitor BNP levels of congestive heart failure patients and adjust their therapy accordingly.
We also sell disposable, lateral flow rapid diagnostic tests for D-dimer and troponin I under our Clearview brand. These tests offer efficiency, as well as ease of use and accuracy, to clinics, hospitals and laboratories around the world.
Diabetes. We offer point-of-care diabetes products, including our Afinion Test System and our NycoCard Test System. The Afinion and NycoCard Test Systems make it possible to easily and rapidly determine the level of glycated hemoglobin, or HbA1c, in a patient’s blood at the physician’s office during the visit. HbA1c results provide information regarding the patient’s average blood sugar levels over a period of time. These systems simplify monitoring of any type of diabetes, facilitating treatment management and prevention of complications. By providing timely information regarding a patient’s blood sugar levels over time, it may also increase a patient’s motivation to comply with treatment and lifestyle changes and thereby optimize their prognosis. In June 2012, we added our CE-marked Lipid Panel, an important tool for cardiovascular disease risk assessment, to the Afinion Test System. The Afinion Test System can also measure a patient’s Albumin Creatinine Ratio, which aids in the early detection of kidney disease often present in diabetic patients. The NycoCard Test System, which is a widely distributed, low-cost product suited to countries with developing healthcare systems, includes tests for C-reactive protein, or CRP, D-Dimer and HbA1c. Physicians test for elevated levels of CRP in connection with the diagnosis, therapy and monitoring of inflammatory diseases. Information regarding the level of CRP in a patient’s bloodstream can help physicians discriminate between a serious inflammatory illness, such as pneumonia, and less severe conditions, such as acute bronchitis and other respiratory tract infections. Through our subsidiary Arriva Medical, we are a major, national mail order supplier of diabetic testing supplies, including blood glucose monitors, test strips, lancets, lancing devices, and control solutions, as well as other related medical supplies in the U.S. These products are usually covered by Medicare, Medicaid and other third-party payers.
Oncology. The Alere NMP22 BladderChek Test is the only in-office test approved by the FDA as an aid in the diagnosis of bladder cancer. The Alere NMP22 BladderChek Test is a non-invasive assay, performed on a single urine sample that detects elevated levels of NMP22 protein. The test can be performed in a physician’s office with results delivered during the patient visit, allowing a rapid, accurate and cost-effective means of aiding the detection of bladder cancer in patients at risk, when used in conjunction with standard diagnostic procedures. We also offer the Alere NMP22 Test Kit, a quantitative ELISA test designed to detect elevated levels of NMP22 protein.
Our Clearview FOB and Ultra FOB rapid tests aid in the early detection of colorectal cancer, the third most common type of cancer in men and women. Also, as a result of our November 2010 acquisition of AdnaGen, a German company specializing in the development of cancer diagnostics through the detection and analysis of circulating tumor cells, we now sell the AdnaTest ColonCancer and AdnaTest BreastCancer products, which are CE marked for the detection of circulating tumor cells.
Women’s Health. In the professional marketplace, we are a global leader in pregnancy testing. Our professional pregnancy tests are generally urine- or serum-based, CLIA-waived rapid tests in dipstick or cassette format.
Our professional women’s health products also target diseases or conditions, such as preeclampsia, rubella, pre-term labor and premature rupture of membrane, which pose unique threats to mothers and fetuses. Early detection allows for better monitoring and improved health outcomes.
8
Additionally, we offer osteoporosis therapy monitoring tests. We also market a portfolio of tests for sexually-transmitted diseases. Our women’s health products are currently sold under our Alere, Clearview and Osteomark brands.
Connected Device Technologies. We understand that fast and accurate diagnostic results alone are not likely to satisfy the future mandates of accountable care. We believe that, to be effective, diagnostic data should be actionable, comprehensive and readily accessible to patients, physicians, and payers. When this data is made available via an integrated electronic health record, or EHR, care can more easily be personalized to meet the needs of each patient, and these patients can become more effectively engaged in their own health improvement. Additionally, when an EHR is paired with robust analytical tools, healthcare providers, payers, and accountable organizations can more easily assess treatment effectiveness and quantify patient outcomes and cost savings. For these reasons, we are developing several chronic care and other health information solutions built around fast, easy, and accurate diagnostics that we expect to ultimately permit the automatic import of health data into a health information exchange, or HIE, which we believe will facilitate the sort of health interactions, analysis, and reporting that will fuel more effective delivery and quality of care.
Through Alere Informatics we offer RALS, the point-of-care industry’s leading data management solution, which is deployed in approximately 2,000 hospitals nationwide, and AegisPOC, a web-based data management solution designed for critical care settings. Our RALS systems provide bidirectional interfaces that hospitals can use to connect certain point-of-care devices measuring such physiological parameters as blood glucose, blood gases, prothrombin time and cardiac parameters to its laboratory and health information systems. These products offer fully customizable reporting and do not require device-specific data managers. AegisPOC is offered only outside the U.S.
Through Alere Connect we develop and sell remote health monitoring solutions designed to deliver streamlined, cost-effective connectivity across patient, care provider and electronic medical records. Our goal is to both improve patient outcomes and drive down healthcare costs by improving workflow efficiencies, patient compliance and care delivery. Alere Connect’s health information connectivity platform consists of cloud-based software and cellular hub hardware that can enhance care for patients in both wellness and chronic disease management programs. These solutions are FDA cleared and intended to help care management and healthcare practitioners to extend their services to a broader patient population, increasing the cost-avoidance benefits and efficiencies associated with remote health monitoring.
Our Alere Connect products include:
| | • | | HealthPAL. A small, portable cellular hub device that collects health readings from compatible medical monitors, via Bluetooth or data cable, and transmits the data to back-end clinical systems (e.g. EHR) or to a user’s personal health record. As a dedicated device for the automated transmission of health data, HealthPAL eliminates the need for a smart phone or computer to transmit and upload health data to a provider. |
| | • | | AlereMobileLink. A small, cellular hub used in chronic care management programs to allow patients to easily send collected home testing results and readings from compatible medical monitors to their healthcare providers. |
| | • | | Alere™HomeLink. A more comprehensive health information connectivity solution that links patients to their healthcare providers and EHRs. This cellular hub allows patients to easily send collected home testing results and readings from compatible medical monitors to their healthcare providers. Alere HomeLink also supports the collection of additional data from a patient through an automated question and answer format that can provide the patient’s healthcare provider with further information about the patient’s current health status. |
| | • | | AlereHealthCOM. A web-based application for healthcare professionals to remotely monitor and manage the data collected with HealthPAL, Alere MobileLink and Alere HomeLink. Alere |
9
| | HealthCOM also provides professional administration and work-flow features to assist healthcare practitioners in managing their patient populations and associated equipment inventory. |
These products enable secure data integration with a variety of online EHRs in a manner that is compliant with the requirements of the Health Insurance Portability and Accountability Act, or HIPAA. They are designed to be easily used by people of all ages and levels of technical proficiency. These products use cloud-based technology to lower implementation costs for providers, while delivering services that scale appropriately. In short, the Alere Connect platform is intended to bridge the gap between diagnostic data and devices and our health information solutions described below.
Health Information Solutions
Our health information solutions are designed to provide physicians with actionable data that allows them to make more effective decisions in real time, deliver quality care, and put the individuals they treat on a pathway to better health. Core to our strategy are our proprietary diagnostic platforms and biomarkers that provide rapid results at the point of care for the most costly chronic conditions and our health information technologies, which will ultimately enable diagnostic data to be fed directly into an information exchange that integrates patient-related data in a single EHR. We offer a variety of software-based analytics, clinical decision-support tools, and accountable care programs that enable healthcare providers to initiate earlier interventions, personalize treatment plans, lower costs by reducing hospital readmissions, and measure improvements in outcomes at both a patient and population level. With this wide range of scalable solutions, we are able to support healthcare practitioners in the transition to accountable care as well as in meeting the new pay-for-performance guidelines set by the Centers for Medicare & Medicaid Services, or CMS. Our information solutions address the data and care gaps resulting from today’s fragmented healthcare environment, but are also modular and can be easily integrated with many existing resources customers may already have in place. Our health information solutions are primarily available in the United States. We have begun offering our health information solutions in Australia, France, Germany and the United Kingdom and intend to continue the international expansion of these offerings.
Through Alere Accountable Care Solutions, or Alere ACS, formerly known as Alere Wellogic, we deliver health information solutions that help provider organizations meet the CMS “Meaningful Use” requirements and improve coordination across multiple venues of care. Alere ACS helps healthcare organizations successfully transition to and deliver value-driven care by closing gaps in today’s fragmented healthcare landscape, allowing for earlier interventions, personalized treatment, fewer hospitalizations, better coordination, and robust decision making, analysis, and reporting. Our principal offering from this group, the Alere Health Information Exchange, or Alere HIE, harmonizes multiple streams of data from disparate sources in a single patient-centered record and promotes the sharing of information among a patient’s various healthcare providers, collapsing geographic and technological barriers and enabling care providers to more effectively identify and manage high-risk patients in a broad population. Alere ACS also offers an EHR, known as Consult EHR, which gives healthcare practitioners greater visibility into a patient’s overall health status, as opposed to a more limited “snapshot”, and complies with the requirements of HIPAA. Consult EHR can also be used to manage administrative, billing and other functions more effectively and efficiently. Consult EHR is certified under the Medicare and Medicaid EHR Incentive Programs that have been authorized as part of the Health Information Technology for Economic and Clinical Health Act, or the HITECH Act, which allows clients to qualify for incentive payments by demonstrating the meaningful use of EHR technology.
Through Alere Analytics we offer a broad array of analytical and clinical decision-support tools, which are delivered on our smartPath platform. smartPath leverages our extensive library of evidence-based medical knowledge to enable real-time patient and population assessment, predictive modeling, and risk stratification. It also generates immediate recommendations for care, enabling earlier interventions and helping to reduce avoidable errors and improve overall health outcomes. The
10
smartPath platform compiles, analyzes, and enables reporting on disparate clinical data sets for hospital systems, multi-million-member insurers, government bodies, and several EHR and healthcare IT vendors. It can be easily integrated with various existing hospital and laboratory information systems. Key features of the smartPath platform include:
| | • | | combines rules and evidence-based content with actual patient data to quickly create relevant care plans; |
| | • | | uses the evidence-based knowledge and proprietary analytics to enable improved clinical decision making and efficiency; |
| | • | | applies continuously available patient- and population-level data in a manner tailored to the user; and |
| | • | | provides continuous, automated syndromic reporting that enables users to address community health issues. |
Also, our Apollo technology platform, which is the supporting infrastructure underlying many of our health improvement programs described below, integrates data from a variety of sources that include health plans, pharmacy benefit managers, point-of-care devices, and patient self-reports in a highly dynamic, interactive portal to deliver high quality patient education and engender behavior-changing communication among clinicians and patients.
All of these health information solutions, coupled with our expertise in near-patient diagnostics, enable us to offer a suite of health improvement programs, including accountable care programs, that address the three core objectives of value-driven healthcare:
| | • | | improving the patient’s care experience by raising levels of quality and satisfaction; |
| | • | | reducing the per capita costs of healthcare while maintaining a focus on the individual needs of each patient; and |
| | • | | improving the overall health of managed populations. |
Our health improvement programs are designed to address the most prevalent, costly chronic conditions, deploying real-time diagnostics data, robust analytics, and advanced decision-support capabilities to identify high-risk individuals and set personalized care plans. They are supported by trained clinicians with expertise in health coaching and who inspire individuals to overcome barriers and achieve healthy behavior change. Our data exchange solutions also help to ensure that clinicians who encounter a patient in the care pathway have real-time visibility into that patient’s health information, which helps to reduce fragmentation, create operational efficiencies, and improve care effectiveness. Our programs focused on health improvement and accountable care include:
Patient Self-testing. We offer services designed to support anticoagulation management for patients who take warfarin to control their risk for stroke and clotting disorders. Alere Home Monitoring, our patient self-testing business, assists patients in acquiring home INR monitors and with insurance coverage determinations and provides physicians with a comprehensive model that allows them to incorporate patient self-testing into their practices, and frequent self-testing may reduce the risk of bleeding and clotting. Our program has been developed to identify candidates who will benefit from self-testing protocols and who will be able to follow them successfully for a sustained period of time. The program is built around a sophisticated, web-based application that delivers patient results and other information to healthcare providers on a real-time basis, facilitating immediate therapy adjustments where appropriate and reducing the risk of serious events.
Condition and Case Management. The Alere Condition Management (Chronic Care) Program provides technology-enabled, evidence-based solutions for managing chronic and high-cost conditions, as well as improving clinical and financial outcomes. The Alere Condition Management Program
11
enables individuals with chronic conditions to better manage their health through education about their illnesses, potential complications, and the importance of therapy compliance. Our highly-trained nurses proactively contact participants to monitor their progress and compliance with the care plans set by their physicians. They also work with participants to identify potential care gaps, which occur when individuals are not treated in accordance with best practices or when they fail to follow their treatment plans. Based on an assessment of each participant’s readiness to change and the health issues to be addressed, our nurses create personalized interventions for participants that connect them with a wide range of health management solutions, devices and diagnostic tests, resulting in improved health and productivity and lower healthcare costs.
Our personalized health support model differs from more traditional models in that it applies a more disciplined approach to defining which patients could benefit from particular interactions and the best means of initiating these interactions. A second key differentiator is the use of biometric devices for participants in programs focused on higher-risk conditions. The Alere Condition Management Program currently assists individuals with the following chronic diseases or conditions: asthma, coronary artery disease, chronic obstructive pulmonary disease, diabetes, heart failure, pain, weight management and depression.
The Alere Oncology Case Management Program is the longest-running cancer management program in the United States since 1994. Our program provides services for adults diagnosed with any cancer that requires treatment beyond a single surgery, and we have developed treatment guidelines to support 42 different tumor types and more than 200 stages of cancer in a compassionate, cost-effective way.
Women’s & Children’s Health. Our Women’s and Children’s Health division delivers a wide variety of obstetrical care services that range from risk assessments focused on identifying women who may experience pregnancy complications to a neonatal program that supports early infant care management. We offer home-based obstetrical monitoring for pregnant women with medical or pregnancy-related problems that could put their health or the health of their babies at risk. We also deliver telephonic and home-based nursing services that support improved clinical outcomes. We have developed and refined these services over the years to accommodate physician care plans, with a focus on assessing patient data and providing education. Our programs empower women to make choices that promote healthy pregnancies. Our high-risk pregnancy management program revenues tend to be seasonal, decreasing with the onset of the holiday season starting on Thanksgiving. Consequently, first and fourth quarter revenues each year tend to be lower than second and third quarter revenues.
Wellness. We offer a suite of integrated health and wellness programs, tools and resources designed to empower individuals to make lasting health behavior changes and thereby reduce participant health risks and healthcare-related costs. Our health and wellness programs include screening for risk factors associated with chronic disease, particularly tobacco use, poor nutrition, physical inactivity, and chronic stress. After evaluating these risks, we deploy health coaching, administered telephonically or through web-based applications, to help individuals discover personal motivators for behavior change and setting goals based on personal desires. Our health coaches provide guidance, support and education based on individual needs. As a result, individuals become more engaged in their own healthcare, resulting in lower health risks. This approach is exemplified in our Quit For Life tobacco cessation and WeightTalk obesity programs that employ physical, psychological and behavioral strategies to empower individuals to manage their health and wellbeing.
Consumer Diagnostics
In 2007, we and affiliates of The Procter & Gamble Company, or P&G, commenced a 50/50 joint venture for the development, manufacturing, marketing and sale of existing and to-be-developed consumer diagnostic products, outside the cardiology, diabetes and oral care fields. As part of this
12
arrangement, we transferred essentially all of the assets of our consumer diagnostics business, other than our manufacturing and core intellectual property assets, to the joint venture, and P&G acquired its interest in the joint venture. Accordingly, substantially all of the consumer diagnostics business conducted by us prior to the joint venture, including all of our products targeting the worldwide over-the-counter pregnancy and fertility/ovulation test market, are now sold by the joint venture, which is an unconsolidated entity operating primarily under the name SPD Swiss Precision Diagnostics GmbH, or SPD.
As part of the SPD joint venture, we entered into a finished product purchase agreement, pursuant to which we currently manufacture and sell to SPD substantially all of the consumer diagnostic products which it sells. We also entered into certain transition and long-term services agreements with SPD, pursuant to which we provide certain operational support services to the joint venture. Our consumer diagnostics segment recognizes the revenue and costs arising from these arrangements.
Our other current consumer diagnostic products consist of our market-leading First Check brand of over-the-counter drug tests for at-home testing for up to seven illicit drugs and five prescription drugs, as well as First Check brand over-the-counter tests for cholesterol monitoring. Taking advantage of our leadership in the field of women’s health, we also sell Balance Activ Vaginal Gel directly to consumers and healthcare professionals for the effective treatment of bacterial vaginosis without antibiotics.
Methods of Distribution and Customers
We distribute our professional diagnostic products to hospitals, reference laboratories, physician offices and other point-of-care settings through an extensive worldwide distribution network. We have our own sales force in many countries, including most major markets. We also utilize third-party distributors to sell our products. Our Alere Home Monitoring business facilitates the distribution of our Alere INRatio PT/INR coagulation monitors in the United States by contacting patients who have expressed an interest or have prescriptions from their physicians and facilitating the Medicare reimbursement process for physicians and for patients monitoring at home. Our diabetes testing supplies business provides these products via mail-order to patients in the United States.
We market our health information solutions primarily to health plans (both commercial and governmental), self-insured employers and, to a lesser extent, government and governmental programs, pharmaceutical companies and physicians, through our employee sales force and channel partners.
We market and sell our First Check consumer drug testing products in the United States through retail drug stores, drug wholesalers, groceries and mass merchandisers. These products compete with other brand name drug testing products based on price, performance and brand awareness.
Manufacturing
Our primary manufacturing facilities are located in San Diego, California; Scarborough, Maine; Ottawa, Canada; Hangzhou and Shanghai, China; Jena, Germany; Matsudo, Japan; Oslo, Norway; Dundee, Scotland; and Yongin, South Korea. We also manufacture products at a number of other facilities in the United States, India, Israel, South Africa, Spain and the United Kingdom.
Our primary manufacturing facilities are ISO certified and registered with the FDA. We manufacture substantially all of our consumable diagnostic products at these facilities. We also manufacture the consumable diagnostic devices containing the diagnostic chemistry or other proprietary diagnostic technology, which are used in conjunction with our diagnostic or monitoring systems, and the digital tests and monitors that we supply to the SPD joint venture. We contract with
13
third parties to supply the electronic reader portion of these diagnostic or monitoring systems and to supply various other products that we sell, including our Alere Triage BNP Test for use on Beckman Coulter systems, a majority of our IFA tests and our TECHLAB products.
Research and Development
Our primary research and development centers are in San Diego, California; Scarborough, Maine; Jena, Germany and Cambridge and Dundee, United Kingdom. We also conduct research and development at some of our other facilities, including facilities in the United States, the United Kingdom, China, Israel, Japan and South Korea. Our research and development programs focus on the development of cardiology, infectious disease, toxicology, diabetes and metabolic syndrome products and services together with health information technologies that will facilitate connectivity and information and data management solutions. Information about research and development expenses for the last three fiscal years is provided on page F-3 of the Consolidated Financial Statements.
Global Operations
We are a global company with major manufacturing facilities in the United States, Canada, China, Germany, Japan, Norway, South Korea and the United Kingdom and significant research and development operations in the United States, Germany and the United Kingdom. Our distribution network supporting our professional diagnostics business includes offices in 32 countries.
Our professional diagnostic products are sold throughout the world. Our health information solutions are sold almost exclusively in the United States, but we now offer our health information solutions in Australia, Germany and the United Kingdom. During both 2013 and 2012, we generated approximately 61% of our net revenue from the United States, approximately 17% from Europe and approximately 22% from other locations.
For further financial information about geographic areas, see Note 17 of the “Notes to Consolidated Financial Statements” which are included elsewhere in this report.
Competition
Professional Diagnostics. Our professional diagnostics products are primarily point-of-care rapid diagnostic testing products sold within the areas of cardiology, infectious disease, toxicology, diabetes, oncology and women’s health. Competition for rapid diagnostic products is intense and is primarily based on price, quality, breadth of product line, technology and distribution capabilities. Some competitors in the market for professional rapid diagnostic products, such as BD, are large companies with substantial resources, while numerous smaller, yet aggressive companies also compete with us. We believe that no competitor, small or large, offers a portfolio of professional rapid diagnostic products as broad as ours and, as a result, our competitors differ significantly within each of our areas of focus. Automated immunoassay systems also compete with our products, depending on government regulations or when labor shortages force laboratories to automate or when the unit costs of such systems are lower and other indirect costs are not taken into account. Such systems are provided by Abbott, Siemens, Danaher, Johnson & Johnson, Roche and other large diagnostic companies.
In cardiology, the majority of diagnostic immunoassays utilized by physicians and other healthcare providers are performed by independent clinical reference laboratories and hospital-based laboratories using automated analyzers for batch testing. As a result, the primary competitors for our Alere Triage and Alere Cholestech LDX point-of-care testing systems, which consist of rapid diagnostic devices interpreted by portable electronic readers, are the large diagnostic companies identified above that produce automated immunoassay systems. We expect these large companies to continue to compete vigorously to maintain their significant market share of the cardiology testing market. Although we offer
14
our Alere Triage BNP test for use on Beckman Coulter Immunoassay Systems, our other primary cardiology products are not currently designed for automated batch testing. Our Alere Triage products, as well as our epoc Blood Analysis System, face strong competition from Abbott’s i-Stat hand-held system, and our Alere Cholestech LDX system also faces direct competition from Abaxis Medical Diagnostics, which markets its point-of-care blood laboratory systems to physician office laboratories and from Polymer Technology Systems’ CardioChek test. The primary competitor for our Alere INRatio PT/INR monitoring system is Roche who currently accounts for a majority of the domestic sales of PT/INR point-of-care and patient self-testing devices.
BD, Quidel and Meridian Bioscience are the largest competitors for our rapid diagnostic tests targeted at infectious disease and women’s health. Our HIV products, in particular, also compete with tests offered by OraSure Technologies. Newer technologies utilizing amplification techniques for analyzing molecular gene sequences, from companies such as Abbott, BD, Roche, Cepheid and Hologic, are making in-roads into the infectious disease market.
We also sell ELISA and multiplex immunoassay diagnostic testing products, as well as serology, IFA and microbiology tests, primarily targeted at infectious and autoimmune diseases. Our ELISA tests compete against large diagnostics companies similar to those named above, which manufacture automated immunoassay systems and a wide array of diagnostic products designed for processing on those systems. Other competitors, including INOVA Diagnostics, DiaSorin and Diamedix, are smaller companies that compete based on quality and service. In the United States and Canada, we focus on matching the instrumentation and product testing requirements of our customers by offering a wide selection of diagnostic products and test equipment. The markets for our serology, IFA and microbiology products are mature and competition is based primarily on price and customer service. Our main competitors in serology and microbiology testing include Remel and Biokit. Our main competitors in IFA testing are Bio-Rad Laboratories, INOVA Diagnostics and Immuno Concepts. However, products in these categories also compete to a large extent against rapid membrane and ELISA products, whose tests are often easier to perform and read and can be more precise.
Competitors for our drugs-of-abuse tests include many of the large diagnostics companies named above, which manufacture instrumented drug tests, reagents or instruments sold in a variety of formats to customers in the worldwide employment, transportation, government and clinical sectors. Additionally, in many markets in which the barriers to entry are low due to less stringent regulations, we compete with dozens of privately-held, small and emerging low-cost manufacturers of lateral flow point-of-care drug tests. Our worldwide drug testing laboratory services compete with hundreds of multi-national and regional clinical, toxicology and forensic laboratories.
In the field of diabetes, the competitors for the Afinion Test System and NycoCard Test System include Siemens Healthcare, Bio-Rad Laboratories, Roche Diagnostics, EKF and Samsung. Arriva Medical, which is our mail order diabetes testing product supply business, primarily sells products which are covered by Medicare, Medicaid and other third-party payers. Our major competitors for the sale of these products are large retail pharmacies, such as Walmart, Walgreens and CVS, independent pharmacies and a small number of mail order suppliers. Competition for reimbursed diabetes testing supplies, which represent the majority of our business, changed significantly in 2013 as a result of CMS’ decision, based on a competitive bidding process, to reimburse only 18 selected suppliers willing to accept a fixed lowered reimbursement rate. As a result of the competitive bidding process, Arriva Medical was awarded a national mail-order contract.
Generally, the competitive positions of our professional diagnostic products may be based on, among other things, being first to market with a novel product, product performance, accuracy, convenience, cost-effectiveness, the strength of our intellectual property and price, as well as on the effectiveness of our sales force and our marketing and distribution partners. Where we face competition from large diagnostic companies, these competitors have greater resources than we do. In addition, certain competitors may have more favorable competitive positions than we do, particularly in markets outside the United States.
15
We believe that our dedication to research and development and our strong intellectual property portfolio, coupled with our manufacturing capabilities, diversified product positioning, global market presence and established distribution networks, provide us with a competitive advantage in the point-of-care markets in which we compete.
Health Information Solutions. Competition in the health information space is intense due to low barriers to entry. Athenahealth, Cerner Corporation, Allscripts Healthcare Solutions, Greenway Medical Technologies, and McKesson provide health information exchange, data management and clinical support solutions that compete directly with our health information solutions. Additionally, Health Dialog, Healthways, Optum Health, Active Health and a number of smaller service providers offer products that compete with our integrated care management solutions. Our competitors and potential competitors also include health plans, self-insured employers, healthcare providers, pharmaceutical companies, pharmacy benefit management companies, case management companies and other organizations that provide services to health plans, governments, governmental programs and self-insured employers. Some of these entities, particularly health plans and self-insured employers, may be customers or potential customers and may own, acquire or establish health information service providers or capabilities for the purpose of providing health information solutions in-house. Many of these competitors are considerably larger than we are and have access to greater resources. We believe that our ability to improve clinical and financial outcomes and our technology platforms, including our Apollo system, provide us with certain competitive advantages.
Consumer Diagnostics. Our First Check tests compete against over-the-counter diagnostic tests sold primarily by Phamatech, but also by other smaller competitors. Essentially, all of our remaining consumer diagnostic product sales are to SPD, our joint venture. These products are sold by SPD in retail markets where competition is intense and based primarily on brand recognition and price. Our revenues, as well as our share of the profits from the sale of these products by SPD, are dependent upon SPD’s ability to effectively compete in these markets.
Patents and Proprietary Technology; Trademarks
We have built a strong intellectual property portfolio including patents, patent applications, copyrights, trade secrets and other intellectual property, which are intended to protect our vision of the technologies, products and services of the future. Our intellectual property portfolio includes patents and other intellectual property that we own and, in some cases, patents or other intellectual property that we license from third parties, which may be limited with respect to term and in terms of field of use or transferability and may require royalty payments. We own or license patents related to certain of our U.S. lateral flow professional and consumer diagnostics products that will expire in 2014 and 2015. Our access to these patents is not exclusive, as they have been widely licensed in various fields. We do not currently anticipate that the expiration of these patents will materially impact our business.
The medical device industry, including the diagnostic testing industry, historically has been characterized by extensive litigation regarding patents, licenses and other intellectual property rights. Litigation relating to intellectual property rights is also a risk to our health information solutions business, including our health information technologies.
We believe that our history of successfully enforcing our intellectual property rights in the United States and abroad demonstrates our resolve in enforcing our intellectual property rights, the strength of our intellectual property portfolio and the competitive advantage that we have in this area. We have incurred substantial costs, both in asserting infringement claims against others and in defending ourselves against patent infringement claims, and we expect to incur substantial litigation costs as we continue to aggressively protect our technology and defend our proprietary rights.
Finally, we believe that certain of our trademarks are valuable assets that are important to the marketing of both our products and services. We have applied for or obtained registration for many of these trademarks with the United States Patent and Trademark Office or comparable foreign agencies.
16
The medical device industry and the market for health information solutions place considerable importance on obtaining and enforcing patent, trade secret, and trademark protection for new technologies, products, services and processes. Our success therefore depends, in part, on our ability to obtain and enforce the patents and trademark registrations necessary to protect our products, to obtain and preserve our trade secrets and other confidential intellectual property and to avoid or neutralize threats to our proprietary rights from third parties. We cannot, however, guarantee our success in enforcing or maintaining our patent, trademark, trade secret and other intellectual property rights; in obtaining (including by license) future patents, trademarks, trade secrets or other intellectual property rights in a timely manner or at all; or as to the breadth or degree of protection that our patents, trade secrets, trademark registrations or other intellectual property rights might afford us. For more information regarding the risks associated with our reliance on intellectual property rights, see the discussion in Item 1A entitled “Risk Factors” on pages 18 through 38 of this report.
Government Regulation
Our businesses are subject to extensive and frequently changing federal, state, local and foreign laws and regulations. Changes in applicable laws, changes in the interpretation or application of such laws, or any failure to comply with existing or future laws, regulations or standards could have a material adverse effect on our results of operations, financial condition, business and prospects. We believe our current arrangements and practices are in material compliance with applicable laws and regulations. There can be no assurance that we are in compliance with all applicable laws and regulations or that we will be able to comply with new laws or regulations.
Our research, development and clinical programs, as well as our manufacturing and marketing operations, are subject to extensive regulation in the United States and other countries. Most notably, all of our diagnostic products, and certain of our health information technologies and solutions, sold in the United States are subject to the Federal Food, Drug and Cosmetic Act, or the FDCA, as implemented and enforced by the FDA. Our diagnostic products sold in the United States, including any imbedded or stand-alone software which has been classified by the FDA as a Class II medical device, generally require either FDA clearance to market under Section 510(k) of the FDCA, or Premarket Approval, or PMA, which may require pre-clinical and clinical trials. Certain of our health information solutions are classified by the FDA as Medical Device Data Systems, or MDDS, which are software systems distinct from Class II medical device software, that transfer, store, convert and display medical device data. MDDS do not require FDA clearance or approval to be marketed and sold, but are subject to general FDA controls, such as the Quality System Regulation. Foreign countries may require similar or more onerous approvals to manufacture or market these products. The marketing of our consumer diagnostic products is also subject to regulation by the U.S. Federal Trade Commission, or the FTC. In addition, we are required to meet regulatory requirements in countries outside the United States, which can change rapidly with relatively short notice. We must also demonstrate to the FDA that our diagnostic tests intended for home use or for use by laboratories holding a Certificate of Waiver under the Clinical Laboratory Improvement Act of 1967 and the Clinical Laboratory Amendments of 1988, or CLIA, including most physician office laboratories, are simple with a low risk of error. Foreign countries may require similar or more onerous approvals to manufacture or market our products.
CLIA extends federal oversight to many clinical laboratories, including certain of our drug testing laboratories in the United States, by requiring that they be certified to meet quality assurance, quality control and personnel standards. Laboratories also must undergo proficiency testing and are subject to inspections. Certain of our drug testing laboratories perform drug testing on employees of federal government contractors and certain other entities and are therefore regulated by SAMHSA, which has established detailed performance and quality standards that laboratories must meet to be approved to perform drug testing on employees of federal government contractors and certain other entities.
17
Certain of our clinicians, such as nurses, must comply with individual licensing requirements. We believe that all of our clinicians who are subject to licensing requirements are licensed in the jurisdiction in which they are physically present, such as the location of the call center from which they operate and, if applicable, states in which they visit or interact with patients, to the extent such licensure is required.
Under Section 6002 of the 2010 Affordable Care Act, which is commonly referred to as the Physician Payment Sunshine Act, or the Sunshine Act, we are required to collect data on and annually report to CMS certain payments or other transfers of value to physicians and teaching hospitals and annually report certain ownership and investment interests held by physicians or their immediate family members.
Many areas of our business, including but not limited to our health improvement, diabetes supply and patient self-testing services are subject to unique licensing or permit requirements by state and local health agencies. In addition, these and other areas of our business are subject to HIPAA and the HITECH Act. We are also required to obtain certification to participate in certain governmental payment programs, such as various state or federal Medicare/Medicaid programs. Some states have established Certificate of Need/Determination of Need, or CON/DON, programs regulating the expansion of healthcare operations. The failure to obtain, renew or maintain any of the required licenses, certifications or CON/DONs could adversely affect our business. We are also subject to laws regulating fraud and abuse in the healthcare industry, including anti-kickback and false claim laws.
We are also subject to a number of legal requirements relating to our international operations, including the U.S. Foreign Corrupt Practices Act and the U.K. Bribery Act, which generally prohibit engaging in certain activities to obtain or retain business or to influence a person working in an official capacity. We are also subject to the customs, export, trade sanctions and anti-boycott laws of the U.S., including those administered by the U.S. Customs and Border Protection, the Bureau of Industry and Security, the Department of Commerce and the Office of Foreign Assets Control of the Treasury Department, as well as those of other nations in which we do business. These laws may prohibit us from doing business with nationals of designated countries, including Iran, Syria and Cuba, or importing or exporting certain of our products and technologies without first obtaining a license.
For more information about the governmental regulations to which our business is subject and the risk associated with non-compliance with those regulations, see the risk factors discussed in Item 1A entitled “Risk Factors” on pages 18 through 38 of this report.
Employees
As of January 31, 2014, we had approximately 17,600 employees, including temporary and contract employees, of which approximately 8,400 employees are located in the United States. In addition, we utilize consultants specializing in areas such as research and development, risk management, regulatory compliance and marketing.
ITEM 1A. RISK FACTORS
The risks described below may materially impact your investment in our company or may in the future, and, in some cases already do, materially affect us and our business, financial condition and results of operations. You should carefully consider these factors with respect to your investment in our securities.
We face intense competition and our failure to compete effectively may negatively affect sales of our products and services.
The markets in which we operate, including the markets for medical diagnostic products and health information solutions, are rapidly evolving, and developments are expected to continue at a
18
rapid pace. Competition in these markets is intense and expected to increase as new products, services and technologies become available and new competitors enter the market. Our competitors in the United States and abroad are numerous and include, among others, diagnostic testing and medical products companies, universities and other research institutions, health information solutions providers, healthcare providers and health insurers. Many of our existing or potential competitors have substantially greater research and development capabilities, clinical, manufacturing, regulatory and marketing experience and financial and managerial resources than we do. Our sales and results of operations may be adversely affected by:
| | • | | customers’ perceptions of the comparative quality of our competitors’ products or services; |
| | • | | our ability to manufacture, in a cost-effective way, sufficient quantities of our products to meet customer demand; |
| | • | | the ability of our competitors to develop products, services and technologies that are more effective than ours or that render ours obsolete; |
| | • | | our competitors’ ability to obtain patent protection or other intellectual property rights that would prevent us from offering competing products or services; |
| | • | | the ability of our competitors to obtain regulatory approval for the commercialization of products or services more rapidly or effectively than we do; and |
| | • | | competitive pricing by our competitors, particularly in emerging markets. |
In addition, as markets for our novel products become saturated with competing products, such as for our meter-based Alere Triage BNP test, the growth rates of sales unit volume and average selling prices for those products may decline, which may adversely impact our product sales, gross margins and overall financial results. This may occur even if we are able to successfully introduce new products in these markets, and achieve market acceptance of those products, in a timely manner.
We face risks and uncertainties relating to the FDA warning letter and OIG subpoena.
On October 9, 2012, we received a warning letter from the FDA referencing inspectional observations set forth in an FDA Form 483 that we received in June 2012. The observations were the result of an inspection of our San Diego facility conducted earlier during 2012 relating to our Alere Triage products, which resulted in two recalls of certain Alere Triage products and revised release specifications for our Alere Triage meter-based products. We have submitted evidence of our completion of most of the actions we committed to in response to the FDA Form 483 and warning letter. We intend to continue to work cooperatively with the FDA in an effort to fully address each inspectional observation.
In May 2012, Alere San Diego, Inc. received a subpoena from the Office of Inspector General of the Department of Health and Human Services, or the OIG, seeking documents relating primarily to the quality control testing and performance characteristics of our Alere Triage cardiac marker devices and the Triage TOX Drug Screen manufactured at Alere San Diego. We have provided documents in response to the OIG subpoena, and the investigation is ongoing.
We cannot assure you that the government will find our efforts to resolve the FDA warning letter or the investigation initiated by the OIG subpoena to be satisfactory. We may be unable to implement corrective actions within a timeframe or in a manner satisfactory to the FDA. Failure to do so can result in enforcement proceedings by the government, which may include potential civil or criminal fines and penalties, including disgorgement of amounts earned on any legally-adulterated products; injunctive relief, which could limit, modify or constrain our ability to manufacture, market and sell our products; and exclusion from participation in government healthcare programs, such as Medicare and Medicaid. We have received inquiries from regulatory authorities outside the United States regarding the Alere Triage recalls in the United States and, in at least one case, remedial or corrective action was required. We cannot predict whether other governments’ regulatory authorities will require additional remedial or
19
corrective actions in the future. The investigation initiated by the OIG subpoena can result in civil or criminal fines or penalties, increased supervision of our business operations by the OIG, or exclusion from participation in government healthcare programs, such as Medicare and Medicaid. We are unable to predict when these matters will be resolved or what action, if any, the government will take in connection with these matters. The issues arising out of the FDA inspection and OIG subpoena may be expanded to cover other matters. We can also face product liability, third-party payer, shareholder, or other litigation. Any of these risks and uncertainties can adversely affect our revenues, results of operations, cash flows and financial condition.
Also, except for increases in manufacturing costs and decreased profitability for our Alere Triage products, we are unable to predict what impact these matters or ensuing proceedings, if any, will have on our results of operations, cash flows or financial condition. Our related efforts to improve our production and quality control processes in accordance with the revised release specifications for the Alere Triage meter-based products and to increase production to offset lower yields have increased our manufacturing costs, and our costs may increase as we implement changes to enhance our quality control processes that we or the FDA may deem necessary. Because our efforts to improve our manufacturing processes at our San Diego facility are ongoing and because we are continuing to seek to implement the remaining changes in accordance with the timelines set forth in our response to the FDA, we cannot predict the continuing impact of the final quality control release specifications on our manufacturing yields. We cannot guarantee that we will be able to manufacture all of the impacted products at cost-effective yield rates under the final release specifications, in which case, we may be required to, or we may opt to, cease production and sale of the impacted products. In any case, we expect that our ability to supply certain Alere Triage products may continue to be limited, which we expect to adversely affect revenues from sales of these products. We are unable to predict the scope or the duration of any product shortage. Our revenues and market share could continue to be adversely affected by customer decisions to switch to competing products due to product shortages or damage to our reputation resulting from these matters.
We may experience difficulties that delay or prevent our development, introduction or marketing of new or enhanced products or services.
Our success depends on our ability to effectively introduce new and competitive products and services. The development of new or enhanced products or services is a complex, costly and uncertain process and is becoming increasingly complex and uncertain in the United States. Furthermore, developing and manufacturing new products and services require us to anticipate customers’ and patients’ needs and emerging technology trends accurately. We may experience research and development, manufacturing, regulatory, marketing and other difficulties that could delay or prevent our introduction of new or enhanced products and services. The research and development process in the healthcare industry generally takes a significant amount of time from design stage to product launch. This process is conducted in various stages, and each stage presents the risk that we will not achieve our goals. We may have to abandon a product in which we have invested substantial resources. We cannot be certain that:
| | • | | any of our products or services under development will prove to be safe and effective in clinical trials; |
| | • | | we will be able to obtain, in a timely manner or at all, necessary regulatory approvals; |
| | • | | the products and services we develop can be manufactured or provided at acceptable cost and with appropriate quality; or |
| | • | | these products and services, if and when approved, can be successfully marketed. |
These factors, as well as manufacturing or distribution problems or other factors beyond our control, could delay the launch of new products or services. Any delay in the development, approval, production, marketing or distribution of a new product or service could materially and adversely affect our competitive position, our branding and our results of operations.
20
Our financial condition and results of operations may be adversely affected by international business risks.
We generate a significant percentage of our net revenue from outside the United States, and a significant number of our employees, including manufacturing, sales, support, and research and development personnel, are located outside the United States, including in Africa, Australia, Brazil, China, Germany, India, Ireland, Israel, Japan, Norway, the Philippines, South Korea, and the United Kingdom. Conducting business outside the United States subjects us to numerous risks, including:
| | • | | lost revenues as a result of macroeconomic developments, such as the current European budgetary issues, debt crisis and related European financial restructuring efforts, which may cause European governments to reduce spending and cause the value of the Euro to deteriorate, thus reducing the purchasing power of European customers; |
| | • | | decreased liquidity resulting from longer accounts receivable collection cycles typical of foreign countries; |
| | • | | lower productivity resulting from difficulties we encounter in staffing and managing sales, support, and research and development operations across many countries; |
| | • | | lost revenues or unexpected expenses resulting from difficulties associated with enforcing agreements and collecting receivables through foreign legal systems; |
| | • | | lost revenues or unexpected expenses resulting from disputes with third-party distributors of our products or from third parties claiming distribution rights to our products under foreign laws or legal systems; |
| | • | | lost revenues or unexpected expenses resulting from the imposition by foreign governments of trade barriers such as tariffs, quotas, preferential bidding, and import restrictions; |
| | • | | higher cost of sales resulting from import or export licensing requirements; |
| | • | | lost revenues or other adverse effects resulting from acts of war, terrorism, theft or other lawless conduct or otherwise resulting from economic, social or political instability in or affecting foreign countries in which we sell our products or operate; |
| | • | | lost revenues or other adverse effects resulting from international sanctions regimes; |
| | • | | adverse effects resulting from changes in foreign regulatory or other laws affecting sales of our products or our foreign operations; |
| | • | | greater tax liability resulting from international tax laws, including U.S. taxes on foreign subsidiaries; |
| | • | | increased financial accounting and reporting burdens and complexities; |
| | • | | increased costs to comply with changes in legislative or regulatory requirements; |
| | • | | lost revenues or increased expenses resulting from the failure of laws to protect our intellectual property rights; and |
| | • | | lost revenues resulting from delays in obtaining import or export licenses, transportation difficulties and delays resulting from inadequate local infrastructure. |
Our international operations subject us to varied and complex domestic, foreign and international laws and regulations. Compliance with these laws and regulations often involves significant costs or requires changes in our business practices that may reduce revenues and profitability.
We could incur additional legal compliance costs associated with our global operations and could become subject to legal penalties if we do not comply with certain regulations.
As a result of our international operations, we are subject to a number of legal requirements, including the U.S. Foreign Corrupt Practices Act, the U.K. Bribery Act and the customs, export, trade sanctions and anti-boycott laws of the U.S., including those administered by the U.S. Customs and Border Protection, the Bureau of Industry and Security, the Department of Commerce and the Office of
21
Foreign Assets Control of the Treasury Department, as well as those of other nations in which we do business. Compliance with these laws and regulations is complex and involves significant costs. In addition, our training and compliance programs and our other internal control policies may not always protect us from acts committed by our employees or agents. Any violation of these requirements by us, our employees or our agents may subject us to significant criminal and civil liability.
Because our business relies heavily on foreign operations and revenues, changes in foreign currency exchange rates and our need to convert currencies may negatively affect our financial condition and results of operations.
Our business relies heavily on our foreign operations. Eight of our ten largest manufacturing operations are located in Canada, China, Germany, Japan, Norway, South Korea and the United Kingdom, and we also have manufacturing operations in India, Israel, South Africa and Spain. We have significant research and development operations in Germany and the United Kingdom, and we conduct additional research and development activities in China, Israel, Japan and South Korea. In addition, for the year ended December 31, 2013, approximately 39% of our net revenue was derived from sales outside the United States. Because of the scope of our foreign operations and foreign sales, we face significant exposure to movements in foreign currency exchange rates. Our primary exposures are related to the operations of our European and Asia Pacific subsidiaries and our manufacturing facilities in China, Japan and South Korea. These exposures may change over time as our business practices evolve and could result in increased costs or reduced revenue and could affect our actual cash flow. Changes in the relative values of currencies occur regularly and, in some instances, may have a significant impact on our operating results. We cannot predict with any certainty changes in foreign currency exchange rates or the degree to which we can cost-effectively mitigate these risks.
Healthcare reform legislation could adversely affect our revenue and financial condition.
The Patient Protection and Affordable Care Act of 2010 (as amended by the Health Care and Education Reconciliation Act of 2010), or the ACA, makes comprehensive reforms at the federal and state level affecting the coverage and payment for healthcare services in the United States. The ACA contains many provisions designed to generate the revenues necessary to fund the coverage expansions and reduce the costs of Medicare and Medicaid. While certain provisions of the ACA took effect immediately, others have delayed effective dates. Given the scope of the changes made by the ACA and the ongoing implementation efforts, we cannot predict the impact of every aspect of the new law on our operations.
In particular, the ACA significantly alters Medicare Advantage reimbursements by setting the federal benchmark payment closer to the payments in the traditional fee-for-service Medicare program. This change could reduce our revenues from the Medicare Advantage plans for which we perform services, although the precise effect on any particular plan, much less the impact on us, is impossible to predict. Effective January 1, 2013, the ACA includes a 2.3% excise tax on the sale of certain medical devices sold outside of the retail setting. For the year ended December 31, 2013, we incurred $7.5 million in excise tax expense related to the domestic sale of our medical device products as a result of the implementation of this tax. Legislative provisions impose federal reporting requirements regarding payments or relationships between manufacturers of covered drugs, devices or biological or medical supplies, and physicians, among others.
The ACA requires that sponsors of health insurance plans maintain specified minimum medical loss ratios; these plans are customers of our health information solutions. We believe that the majority of our health information solutions would qualify as “quality improving activities”, but there have been no regulations specifically classifying our services in such a manner. If our health information solutions are not classified as “quality improving activities” under the ACA, health insurance providers will not be permitted to count expenditures on those services toward the calculation of their medical loss ratios, which may have a material adverse effect on demand for our health information solutions and the results of operations of our health information solutions business.
22
Additionally, revenues associated with our diabetes business have been impacted by the Durable Medical Equipment, Prosthetics, Orthotics and Supplies, or the DMEPOS, Competitive Bidding Program operated by the Centers for Medicare & Medicaid Services, or CMS. Under this program, Medicare no longer reimburses suppliers for certain products and services, including mail-order diabetes testing supplies, based on the Medicare fee schedule amount. Instead CMS now provides reimbursement for those products and services based on a competitive bidding process. Our Arriva business was selected through the bidding process as one of 18 recipients of a contract to have its products reimbursed by Medicare. While it limits the number of potential participants in the mail-order diabetes testing supplies market, the DMEPOS Competitive Bidding Program also requires us to sell diabetes supplies subject to Medicare reimbursement at significantly lower prices, which has had a material adverse effect on the profitability of these products.
Legislative and regulatory bodies are likely to continue to pursue healthcare reform initiatives and may continue to reduce the funding of the Medicare and Medicaid programs, including Medicare Advantage, in an effort to reduce overall healthcare spending. The ultimate impact of all of the reforms in the ACA, and its impact on us, is impossible to predict. If all of the reforms in the legislation are implemented, or if other reforms in the United States or elsewhere are adopted, those reforms may have a material adverse effect on our financial condition and results of operations.
If the results of clinical studies required to gain regulatory approval to sell our products are not available when expected, or do not demonstrate the safety and effectiveness of those products, we may be unable to sell those products.
Before we can sell certain of our products, we must conduct clinical studies intended to demonstrate that those products are safe and effective and perform as expected. The results of these clinical studies are used to obtain regulatory approval from government authorities such as the FDA. Clinical studies are experiments involving human patients having the diseases or medical conditions that the product is trying to evaluate or diagnose. Conducting clinical studies is a complex, time-consuming and expensive process. In some cases, we may spend several years completing the necessary clinical studies.
If we fail to adequately manage our clinical studies, those clinical studies and corresponding regulatory approvals may be delayed or we may fail to gain approval for our products altogether. Even if we successfully manage our clinical studies, we may not obtain favorable results and may not obtain regulatory approval. If we are unable to market and sell our new products or are unable to obtain approvals in the timeframe needed to execute our product strategies, our business and results of operations would be materially and adversely affected.
If we are unable to obtain required clearances or approvals for the commercialization of our products in the United States, we would not be able to sell those products in the United States.
Our future performance depends on, among other matters, the timely receipt of necessary regulatory approvals for new products. Regulatory approval can be a lengthy, expensive and uncertain process. In addition, regulatory processes are subject to change, and new or changed regulations can result in increased costs and unanticipated delays.
In the United States, clearance or approval to commercially distribute new medical devices is received from the FDA through clearance of a Premarket Notification 510(k), or 510(k), or through a Premarket Approval, or PMA. The FDA may deny 510(k) clearance because, among other reasons, it determines that our product is not substantially equivalent to another U.S. legally marketed device. The FDA may deny a PMA because, among other reasons, it determines that our product is not sufficiently safe or effective. As part of the clearance or approval process, if we intend to sell certain diagnostic tests for home use or for use by laboratories holding a CLIA Certificate of Waiver, including most physician office laboratories, we must generally provide data, demonstrating to the FDA’s satisfaction, that the criteria for our tests are simple with a low risk of error. Failure to obtain FDA clearance or approval would preclude commercialization in the U.S. and failure to obtain or maintain CLIA-waived
23
status for any product would preclude us from selling that product for home use or to CLIA-waived laboratories, which could materially and adversely affect our future results of operations.
Modifications or enhancements that could significantly affect safety or effectiveness, or that constitute a major change in the intended use of the device, require new 510(k) or PMA submissions. We have made modifications to some of our products since receipt of initial 510(k) clearance or PMA. With respect to several of these modifications, we filed new 510(k)s describing the modifications and received FDA 510(k) clearance. We have made other modifications to some of our products that we believe do not require the submission of new 510(k)s or PMAs. The FDA may not agree with any of our determinations not to submit a new 510(k) or PMA for any of these modifications made to our products. If the FDA requires us to submit a new 510(k) or PMA for any device modification, we may be prohibited from marketing the modified products until the new submission is cleared or approved by the FDA. As long as our San Diego facility remains subject to the FDA Warning Letter that we received in October 2012, that facility will be ineligible to receive PMA approvals. While no PMA submissions are currently pending for that facility and we do not plan any new submissions for that facility in 2014, if we are unable to resolve the Warning Letter in a timely manner, our ability to gain approval for new or enhanced products could be adversely impacted.
We are subject to regulatory approval requirements of the foreign countries in which we sell our products, and these requirements may prevent or delay us from marketing our products in those countries.
We are subject to the regulatory approval requirements for each foreign country in which we sell our products. The process for complying with these approval requirements can be lengthy and expensive. Any changes in foreign approval requirements and processes may cause us to incur additional costs or lengthen review times of our products. We may not be able to obtain foreign regulatory approvals on a timely basis, if at all, and any failure to do so may cause us to incur additional costs or prevent us from marketing our products in foreign countries, which may have a material adverse effect on our business, financial condition and results of operations. As long as our San Diego facility remains subject to the FDA warning letter that we received in October 2012, we may be unable to obtain certain export certificates from the FDA. Some foreign governments require these export certificates in order to market our products in their countries. If we are unable to obtain these certificates, we may be unable to market our products in certain foreign countries.
Our business is subject to substantial regulatory oversight and our failure to comply with applicable regulations may result in significant costs or, in certain circumstances, the suspension or withdrawal of previously obtained clearances or approvals.
Our businesses are extensively regulated by the FDA and other federal, state and foreign regulatory agencies. These regulations impact many aspects of our operations, including development, manufacturing, labeling, packaging, adverse event reporting, storage, advertising, promotion, physician interaction and record-keeping.
The FDA and foreign regulatory agencies may require post-market testing and surveillance to monitor the performance of approved products or may place conditions on any product approvals that could restrict the commercial applications of those products. The discovery of problems with a product may result in restrictions on the product, including withdrawal of the product from the market. In addition, in some cases we may sell products or provide services which are reliant on the use or commercial availability of products of third parties, including medical devices, equipment or pharmaceuticals, and regulatory restrictions placed upon any such third-party products could have a material adverse impact on the sales or commercial viability of our related products or services.
We are subject to routine inspection by the FDA and other agencies for compliance with the Quality System Regulation and Medical Device Reporting requirements in the United States and other applicable regulations worldwide. Our manufacturing facilities and those of our suppliers and distributors also are, or can be, subject to periodic regulatory inspections.
24
Under CLIA, some of our drug testing laboratories in the United States are required to be certified to meet quality assurance, quality control and personnel standards. Laboratories also must undergo proficiency testing and are subject to inspections. Our laboratories that perform drug testing on employees of federal government contractors and some other entities are regulated by the United States SAMHSA, which has established detailed performance and quality standards that laboratories must meet in order to perform this work.
Portions of our health information solutions business are subject to unique licensing or permit requirements. For example, we may be required to obtain certification to participate in governmental payment programs, such as state or federal Medicaid/Medicare programs. We may need an operating license in some states, and some states have established Certificate of Need programs regulating the expansion of healthcare operations. In addition, we believe that some of the health improvement programs offered by our health information solutions are educational in nature, do not constitute the practice of medicine or provision of healthcare and, thus, do not require that we maintain federal or state licenses to provide these services. However, it is possible that federal or state laws regarding the provision of “virtual” or telephonic medicine could be revised or interpreted to include our services, or that other laws may be enacted which require licensure or otherwise relate to our health information solutions. In that event, we may incur significant costs to comply with such laws and regulations.
We are also subject to laws relating to matters such as privacy, safe working conditions, manufacturing practices, environmental protection, fire hazard control and disposal of hazardous or potentially hazardous substances.
We may incur significant costs to comply with these laws and regulations. If we fail to comply with applicable regulatory requirements, we may be subject to fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products or injunctions against our distribution of products, termination of our service agreements by our customers, disgorgement of money, operating restrictions and criminal prosecution. Changes in applicable laws, changes in the interpretation or application of such laws, or any failure to comply with existing or future laws, regulations or standards which could have a material adverse effect on our results of operations, financial condition, business and prospects. Moreover, new laws may be enacted, or regulatory agencies may impose new or enhanced standards, that would increase our costs, as well as expose us to risks associated with non-compliance.
We are subject to healthcare fraud and abuse regulations that could result in significant liability, require us to change our business practices and restrict our operations in the future.
We are subject to laws regulating fraud and abuse in the healthcare industry, including anti-kickback and false claims laws. The Federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made under a federal healthcare program, such as Medicare or Medicaid. Many states have also adopted laws similar to the Anti-Kickback Statute. Some of these state prohibitions apply to the referral of patients for healthcare items or services reimbursed by any payer, not only the Medicare, Medicaid and Veterans Administration programs. These laws constrain the sales, marketing and other promotional activities of manufacturers of medical devices by limiting the kinds of financial arrangements, including sales programs, with hospitals, physicians, laboratories and other potential purchasers of medical devices and related services.
Other laws generally prohibit individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payers that are false or fraudulent, or are for items or services that were not provided as claimed. These laws may also be triggered by failure to return identified overpayments to a payer. Anti-kickback and false claims laws prescribe civil and/or criminal penalties for noncompliance that can be substantial including, in some
25
instances, fines, imprisonment and, within the United States, exclusion from participation in government healthcare programs. Furthermore, since we are reimbursed directly by federal healthcare programs for certain goods and services and, given that many of our customers rely on reimbursement from Medicare, Medicaid and other governmental programs to cover a substantial portion of their expenditures, our exclusion from such programs as a result of a violation of these laws could have a material adverse effect on our business, results of operations, financial condition and cash flows. The interpretation and enforcement of these laws and regulations are uncertain and subject to rapid change.
Billing and payment for healthcare services are highly regulated, and the failure to comply with applicable laws and regulations can result in civil or criminal sanctions, including exclusion from federal and state healthcare programs.
A portion of our healthcare products and services are paid for by private and governmental third-party payers, such as Medicare and Medicaid. These third-party payers typically have different and complex billing and documentation requirements that we must satisfy in order to receive payment, and they carefully audit and monitor our compliance with these requirements. We must also comply with numerous other laws applicable to billing and payment for healthcare services, including privacy laws. Failure to comply with these requirements may result in non-payment, refunds, exclusion from government healthcare programs, and civil or criminal liabilities, any of which may have a material adverse effect on our revenues and earnings. In addition, failure by third-party payers to properly process our payment claims in a timely manner could delay our receipt of payment for our products and services, which may have a material adverse effect on our cash flows.
The market for health information solutions is rapidly and continually evolving, and any such changes may impact our health information solutions business.
The market for health information solutions is rapidly and continually evolving due to factors such as changes in federal and state regulations and cost reduction pressures. We cannot predict with certainty the future growth rate or the ultimate size of the market. Our failure to manage any changes in this market may adversely affect the revenues and results of operations of our health information solutions business. The success of our health information solutions business, including our health improvement programs, depends on a number of factors. These factors include:
| | • | | our ability to differentiate our health information solutions from those of competitors; |
| | • | | the extent and timing of the acceptance of our services as a replacement for, or supplement to, traditional managed-care offerings; |
| | • | | the effectiveness of our sales and marketing efforts with customers and their participants, employees or constituents; |
| | • | | our ability to devise new and additional products and services beneficial to health plans, employers and states and their respective participants, employees or constituents; |
| | • | | our ability to obtain and retain all necessary licenses, permits and regulatory clearances and approvals related to our services and any products used as part of our services, and to deliver effective, reliable and safe services to our customers and their participants, employees or constituents; |
| | • | | our ability to achieve, measure and effectively communicate cost savings for our customers through the use of our services; and |
| | • | | our ability to obtain, retain and renew contracts with customers and potential customers with favorable pricing as competition increases and to the extent that customers attempt to provide health information solutions themselves. |
26
Increasing health insurance premiums and co-payments or high-deductible health plans may cause individuals to forgo health insurance and avoid medical attention, either of which may reduce demand for our products and services.
Health insurance premiums, co-payments and deductibles have generally increased in recent years. These increases may cause individuals to forgo health insurance, as well as medical attention. This behavior may reduce demand for our point-of-care diagnostic products and also reduce the number of lives managed by our health information solutions, including our health improvement programs.
Increased unemployment may negatively impact the collectability of uninsured accounts and patient due accounts and/or reduce total health plan populations.
Some of the contracts for our health information solutions provide reimbursement to us based on total relevant populations managed by health plans. If unemployment rates rise, our revenues under these contracts may be reduced as managed lives may decrease and may also result in a smaller percentage of our patients being covered by an employer health group while a larger percentage are covered by lower paying Medicare and Medicaid programs. One of the primary collection risks of our health information solutions business’ accounts receivable relates to uninsured patient accounts and patient accounts for which the primary insurance carrier has paid the amounts covered by the applicable insurance policy, but patient responsibility amounts (deductibles and co-payments) remain outstanding. If unemployment rates rise, these uninsured and patient due accounts could increase as a percentage of the health information solutions business’ accounts receivable. Deterioration in the collectability of these accounts could adversely affect the health information solutions business’ collection of accounts receivable, cash flows and results of operations. These financial pressures could have an adverse impact on our business.
A portion of our health information solutions fees are contingent upon performance.
Some of our existing health information solutions agreements contain savings or other guarantees, which provide that our revenues, or a portion of them, are contingent upon projected cost savings or other quality performance measures related to our health information solutions programs. There is no guarantee that we will accurately forecast cost savings and clinical outcome improvements under our health information solutions agreements or meet the performance criteria necessary to recognize potential revenues under the agreements. Additionally, untimely, incomplete or inaccurate data from our customers, or flawed analysis of such data, could have a material adverse impact on our ability to recognize revenues.
If our costs of providing health information solutions increase, we may not be able to pass these cost increases on to our customers.
Many of our health information solutions are provided pursuant to long-term contracts that we may be unable to re-negotiate. If our costs increase, we may be unable to increase our prices, which would adversely affect our overall profit margin and net income.
Demands of third-party payers, cost reduction pressures among our customers and restrictive reimbursement practices may adversely affect our revenues.
Our ability to negotiate favorable contracts with non-governmental payers, including managed-care plans, significantly affects the revenues and operating results of our health information solutions business. Our customers continue to face cost reduction pressures that may cause them to curtail their use of, or reimbursement for, health information solutions, to negotiate reduced fees or other concessions or to delay payment. Furthermore, the increasing leverage of organized buying groups among non-governmental payers may reduce market prices for our products and services, thereby
27
reducing our profitability. Reductions in price increases or the amounts received from current customers or lower pricing for our services to new customers could have a material adverse effect on the financial position, cash flows and results of operations of our health information solutions business.
In addition, the ability of our customers to obtain appropriate reimbursement for products and services from third-party payers is critical to the success of our business because it affects which products customers purchase and the prices they are willing to pay. If we develop a new product but the product is not approved for reimbursement by private and governmental third-party payers, the product may not be successful. Domestic and foreign healthcare reforms may further reduce reimbursement levels and adversely affect demand for and profitability of our products and services. These reforms, along with other cost-containment initiatives, could have a material adverse effect on our business, results of operations and financing condition.
Future reductions in state spending on preventative care programs could reduce our net revenues, net income and cash flows.
Due to the continued pressure on state budgets, many states are considering, or have enacted, cuts to existing preventative care programs. These cuts have included, or may include, elimination or reduction of coverage for some or all of our preventative care programs. As an example, 2013 funding for the Washington State Tobacco Quit Line was cut by 62%, resulting in services being dramatically reduced and supported purely by funding from the Centers for Disease Control, or CDC. We also understand that the CDC may delay its 2014 funding allocations to state agencies that provide our smoking cessation programs. During 2013, approximately 58% of the net revenue of our Alere Wellbeing business was derived from sales to state governments. Continued state budgetary pressures could lead to further reductions in funding for our services which, in turn, could have a material adverse effect on our financial position and operating results.
In addition, some states may reduce current spending on preventative care programs in order to conserve funds for use in anticipated future programs, which may or may not occur. For example, the CDC conducted successful anti-smoking campaigns in 2012 and 2013 and recently launched its 2014 campaign. We believe that, in anticipation of that ongoing campaign, many states are reducing spending on tobacco cessation programs so that they will have funds available to spend in conjunction with the CDC’s expected campaign. If the CDC cancels, delays or substantially modifies its annual campaign plans, if states do not spend the expected funds in conjunction with that campaign, or if tobacco users are reluctant to respond to the campaign, funding for our services could be negatively impacted, which could have a material adverse effect on our financial position and operating results.
We are also watching closely the impact the ACA has on state funding of our smoking cessation programs. As states perceive that more individuals are ‘insured’, and therefore would be less likely to participate in state funded smoking cessation programs, states may take the position that state funding of smoking cessation programs is no longer needed or is no longer economical.
Our data management and information technology systems are critical to maintaining and growing our business.
Our business, particularly our health information solutions business, is dependent on the effective use of information technology and, consequently, technology failure or obsolescence may negatively impact our business. In addition, data acquisition, data quality control, data privacy, data security and data analysis, which are a cornerstone of our health information solutions programs, are intense and complex processes subject to error. Untimely, incomplete or inaccurate data, flawed analysis of data or our inability to properly integrate, implement, protect and update systems could have a material adverse impact on our business and results of operations. In particular, we are relying on our integrated care management system, our health information exchange and our clinical decision-
28
support software to provide the framework and supporting infrastructure for significantly enhanced future health information solutions programs and to provide a competitive advantage. These systems and software are relatively new and may not provide these expected benefits or meet our needs or the needs of our customers or program participants.
We expect that we will need to continue to improve and further integrate our information technology systems on an ongoing basis in order to effectively run our business. If we fail to successfully manage our information technology systems, our business and operating results could be adversely affected.
Our ability to protect our information systems and electronic transmissions of sensitive data from data corruption, cyber-based attacks, security breaches or privacy violations is critical to the success of our business.
We are highly dependent on information technology networks and systems, including the Internet, to securely process, transmit and store electronic information, including personal information of our customers. Security breaches of this infrastructure, including physical or electronic break-ins, computer viruses, malware attacks by hackers and similar breaches, can cause all or portions of our websites to be unavailable, create system disruptions, shutdowns or unauthorized disclosure of confidential information. We invest in security technology to protect our data against risks of data security breaches and cyber-attacks and we have implemented solutions, processes, and procedures to help mitigate these risks, such as encryption, virus protection, security firewalls and comprehensive information security and privacy policies. However, despite our security measures, our information technology and infrastructure may be vulnerable to attacks by hackers or breached due to employee error, malfeasance or other disruptions. The age of our information technology systems, as well as the level of our protection and business continuity or disaster recovery capability, varies from site to site, and there can be no guarantee that any such plans, to the extent they are in place, will be totally effective. In addition, a security breach or privacy violation that leads to disclosure of consumer information (including personally identifiable information or protected health information) could harm our reputation, compel us to comply with disparate state breach notification laws and otherwise subject us to liability under laws that protect personal data, resulting in increased costs or loss of revenue. If we are unable to prevent further security breaches or privacy violations or implement satisfactory remedial measures, our operations could be disrupted, we may be subject to legal claims or proceedings, or we may suffer loss of reputation, financial loss and other regulatory penalties because of lost or misappropriated information, including sensitive consumer data, which could have a material adverse impact on our business, financial condition and results of operations. See Item 3 “Legal Proceedings.” While we currently expend resources to protect against cyber-attacks and security breaches, we may need to expend additional resources in the future to continue to protect against potential security breaches or to address problems caused by such attacks or any breach of our safeguards. In addition, a data security breach could distract management or other key personnel from performing their primary operational duties.
In addition, the interpretation and application of consumer and data protection laws in the United States, Europe and elsewhere are often uncertain, contradictory and in flux. It is possible that these laws may be interpreted and applied in a manner that is inconsistent with our data practices. If so, this could result in government-imposed fines or orders requiring that we change our data practices, which could have an adverse effect on our business. Complying with these various laws could cause us to incur substantial costs or require us to change our business practices in a manner adverse to our business.
Our growth is subject to global economic and political conditions, and operational disruptions at our facilities.
Our business is affected by global economic conditions and the state of the financial markets. There can be no assurance that global economic conditions and financial markets will not worsen and
29
that we will not experience any adverse effects that may be material to our consolidated cash flows, results of operations, financial positions or our ability to access capital, such as the adverse effects resulting from a prolonged shutdown in government operations in both the United States and internationally. Our business is also affected by local economic conditions, including inflation, recession, financial liquidity and currency volatility or devaluation. Political changes, some of which may be disruptive, could interfere with our supply chain, our customers and all of our activities in a particular location.
Poor economic conditions may negatively impact our toxicology business.
The high rates of unemployment that have recently affected the United States and other countries negatively impact the demand for pre-employment drug testing. Additionally, reduced government funding for drug screening programs negatively impacts the market for our toxicology tests. Finally, a portion of our domestic laboratory testing services is reimbursed by Medicare and private payers and is subject to continued downward price pressure. If any, or all, of these trends continue or accelerate, they may have a material adverse impact on the results of our toxicology business operations.
If we deliver products with defects, we may be subject to product recalls or negative publicity, our credibility may be harmed, market acceptance of our products may decrease and we may be exposed to liability.
The manufacturing and marketing of professional and consumer diagnostics involve an inherent risk of product liability claims. For example, a defect in one of our diagnostic products could lead to a false positive or false negative result, affecting the eventual diagnosis. Our product development and production are extremely complex and could expose our products to defects. Manufacturing and design defects could lead to recalls (either voluntary or required by the FDA or other government authorities) and could result in the removal of a product from the market. Defects in our products could also harm our reputation, lead to negative publicity and decrease sales of our products.
In addition, our marketing of monitoring services may cause us to be subjected to various product liability or other claims, including, among others, claims that inaccurate monitoring results lead to injury or death, or, in the case of our toxicology monitoring services, the imposition of criminal sanctions. Any product liability or other claim brought against us, regardless of merit, could be costly to defend and could result in an increase to our insurance premiums. If we are held liable for a claim, that claim could materially damage our business and financial condition.
We may experience manufacturing problems or delays due to, among other reasons, our volume and specialized processes, which could result in decreased revenue or increased costs.
The global supply of our products depends on the uninterrupted efficient operation of our manufacturing facilities. Many of our manufacturing processes are complex and involve sensitive scientific processes, including unique and often proprietary antibodies which cannot be replicated or acquired through alternative sources without undue delay or expense. Other processes present difficult technical challenges to obtain the manufacturing yields necessary to operate profitably. In addition, our manufacturing processes may require complex and specialized equipment which can be expensive to repair or replace with required lead times of up to a year.
The manufacturing of certain of our products is concentrated in one or more of our plants, with limited alternate facilities. Any event that negatively impacts our manufacturing facilities, our manufacturing systems or equipment, or our contract manufacturers or suppliers could delay or suspend shipments of products or the release of new products or could result in the delivery of inferior products. Our revenues from the affected products would decline and we could incur losses until such time as we or our contract manufacturers are able to restore our or their production processes or we are able to put in place alternative contract manufacturers or suppliers.
30
We rely on suppliers for raw materials and other products and services, and fluctuations in the availability and price of such products and services may adversely affect our business or results of operations.
We rely on numerous third parties to supply raw materials and other components for our manufacturing processes. In some cases, these raw materials and components are available only from a sole supplier. We also rely on a number of significant third-party manufacturers to produce some of our professional diagnostics products. Stringent requirements of the FDA and other regulatory authorities regarding the manufacture of our products may prevent us from quickly establishing additional or replacement sources for the raw materials, components or manufacturing services that we use or from doing so without excessive cost. As a result, a reduction or interruption in supply or an inability to secure alternative sources of raw materials, components or manufacturing services could have a material adverse effect on our business, result of operations, financial condition and cash flows.
Compliance with the SEC’s new conflict minerals rules will increase our costs and adversely affect our results of operations.
We are subject to the SEC’s new disclosure requirements for public companies that manufacture, or contract to manufacture, products for which certain minerals and their derivatives, namely tin, tantalum, tungsten and gold, known as “conflict minerals,” are necessary to the functionality or production of those products. These regulations require us to determine which of our products contain conflict minerals and, if so, to perform an extensive inquiry into our supply chain in an effort to determine whether or not such conflict minerals originate from the Democratic Republic of Congo, or DRC, or an adjoining country. We have incurred and expect to incur further additional costs to comply with these disclosure requirements, including costs related to determining the source of any of the relevant minerals used in our products. Because our supply chain is complex, the country of origin inquiry and due diligence procedures that we have implemented may not enable us to ascertain the origins of any conflict minerals that we use or determine that these minerals did not originate from the DRC or an adjoining country, which may harm our reputation. We may also face difficulties in satisfying customers who may require that our products be certified as DRC conflict-free, which could harm our relationships with these customers and lead to a loss of revenue. These new requirements could also have the effect of limiting the pool of suppliers from which we source these minerals, and we may be unable to obtain conflict-free minerals at competitive prices, which could increase our costs and adversely affect our manufacturing operations and our profitability.
We could suffer monetary damages, incur substantial costs or be prevented from using technologies important to our products as a result of pending legal proceedings.
We are involved in various legal proceedings arising out of our business. Because of the nature of our business, we may be subject at any particular time to commercial disputes, product liability claims, negligence claims or various other lawsuits arising in the ordinary course of our business, including infringement and other licensing and intellectual property claims, distributor disputes, privacy claims, employment matters or investor matters. The lawsuits we face generally seek damages, sometimes in substantial amounts, for commercial or personal injuries allegedly suffered and can include claims for punitive or other special damages. An adverse ruling or rulings in one or more such lawsuits could, individually or in the aggregate, substantially harm our sales, operations or financial performance.
31
The rights we rely upon to protect the intellectual property underlying our products may not be adequate to prevent third parties from using our technology, which would reduce a competitive advantage provided by our proprietary technology.
Our success depends in part on our ability to develop or acquire commercially valuable intellectual property rights and to enforce those rights. The degree of present and future protection for our intellectual property is uncertain and may change. The risks and uncertainties that we face with respect to our patents and other proprietary rights include the following:
| | • | | pending patent applications we have filed, or to which we have exclusive rights, may not result in issued patents or may take longer than we expect to result in issued patents; |
| | • | | patents licensed or issued to us or our customers may not provide a competitive advantage; |
| | • | | other parties may challenge patents or patent applications licensed or issued to us or our customers; |
| | • | | other companies may design around technologies we have patented, licensed or developed; and |
| | • | | all patents have a limited life, meaning at some point valuable patents will expire and we will lose the competitive advantage they provide. For example, certain patents related to our lateral flow technology expire in 2014 and 2015. |
In addition to patents, we rely on a combination of trade secrets, non-disclosure agreements and other contractual provisions and technical measures to protect our intellectual property rights. Nevertheless, these measures may not be adequate to safeguard the technology underlying our products. If these measures do not protect our rights, third parties could access our technology and our competitive advantage in the market would be reduced. In addition, employees, consultants and others who participate in the development of our products may breach their agreements with us regarding our intellectual property, and we may not have adequate remedies for the breach. We also may not be able to effectively protect our intellectual property rights in some foreign countries. For a variety of reasons, we may decide not to file for patent, copyright or trademark protection or prosecute potential infringements of our patents. Our trade secrets may also become known through other means not currently foreseen by us. Despite our efforts to protect our intellectual property, our competitors or customers may independently develop similar or alternative technologies or products that are equal or superior to our technology and products without infringing any of our intellectual property rights, or design around our proprietary technologies.
Claims by others that our products infringe their proprietary rights could adversely affect our ability to sell our products and services and could increase our costs.
Substantial litigation over intellectual property rights exists in the professional and consumer diagnostics industries and in the health information solutions marketplace. We expect that our products and services could be increasingly subject to third-party infringement claims as the number and functionality of our products grow and as we enter new and different industries and markets. Third parties may have or obtain patents which our products and services or technology may actually or allegedly infringe. Any of these third parties might assert infringement claims against us. Any litigation could result in the expenditure of significant financial resources and the diversion of management’s time and resources. In addition, litigation in which we are accused of infringement may result in negative publicity, have an impact on prospective customers, cause product delays, or require us to develop alternative technologies, make substantial payments to third parties or enter into royalty or license agreements, which may not be available on acceptable terms, or at all. If a successful claim of infringement were made against us and we could not develop non-infringing technology or license rights to the infringed or similar technology on a timely and cost-effective basis, we may be forced to stop selling current products or abandon new products under development and we could be exposed to legal actions by our customers.
32
We may need to initiate lawsuits to protect or enforce our patents and other intellectual property rights, which could be expensive and, if we lose, could cause us to lose some of our intellectual property rights, which would reduce our ability to compete.
In order to protect or enforce our patent and other intellectual property rights, we may initiate litigation or other proceedings against, or enter into negotiations or settlement discussions with, third parties. Litigation may be necessary to:
| | • | | assert claims of infringement; |
| | • | | enforce licensing terms and conditions; |
| | • | | protect our trade secrets or know-how; or |
| | • | | determine the enforceability, scope and validity of the proprietary rights of ourselves or others. |
We have initiated a number of lawsuits against competitors whom we believe to be selling products that infringe our proprietary rights. These lawsuits and any other lawsuits that we initiate in the future could be expensive, take significant time and divert management’s attention from other business concerns. Litigation can also put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing. Additionally, we may provoke third parties to assert claims against us.
Intellectual property law relating to the fields in which we operate is still evolving and, consequently, patent and other intellectual property positions in our industry are subject to change and often uncertain. We may not prevail in any of these suits or other efforts to protect our technology, and the damages or other remedies awarded, if any, may not be commercially valuable. During the course of these suits, there may be public announcements of the results of hearings, motions and other interim proceedings or developments in the litigation. If securities analysts or investors perceive any of these results to be negative, the trading prices of our securities may decline.
Our business could be materially and adversely affected as a result of the risks associated with acquisitions.
Since our inception, we acquired numerous businesses, including Axis-Shield in 2011, eScreen in 2012 and Epocal in 2013. While our business strategy no longer focuses on acquisitions, we may acquire other businesses in the future. The ultimate success of our acquisitions depends, in part, on our ability to realize the anticipated synergies, cost savings and growth opportunities from integrating acquired businesses or assets into our existing businesses. However, the acquisition and successful integration of independent businesses or assets is a complex, costly and time-consuming process, and the benefits we realize may not exceed the costs of the acquisition. The risk and difficulties associated with acquiring and integrating companies and other assets include, among others:
| | • | | the impact of the acquisition on our financial and strategic position and reputation; |
| | • | | consolidating manufacturing, research and development operations, quality systems and health information or other technology platforms, where appropriate; |
| | • | | integrating newly-acquired businesses or product lines into a uniform financial reporting system; |
| | • | | coordinating sales, distribution and marketing functions and strategies, including the integration of our current health information solutions products and services; |
| | • | | establishing or expanding manufacturing, sales, distribution and marketing functions in order to accommodate newly-acquired businesses or product lines or rationalizing these functions to take advantage of synergies; |
| | • | | preserving the important licensing, research and development, manufacturing and supply, distribution, marketing, customer and other relationships of acquired businesses; |
33
| | • | | minimizing the diversion of management’s attention from ongoing business concerns; |
| | • | | the potential loss of key employees of the acquired business; |
| | • | | coordinating geographically separate operations; and |
| | • | | regulatory and legal issues relating to the integration of legacy and newly-acquired businesses. |
These factors could have a material adverse effect on our business, results of operations or financial condition, and managing multiple acquisitions or investments at the same time could exacerbate these risks. To the extent that we issue equity securities in connection with any acquisition or investment, existing shareholders may experience dilution. Our acquisitions have often provided for future contingent payments, or earn-outs, based on the achievement of performance targets or milestones. These arrangements can impact or restrict integration of acquired businesses and can result in disputes, including litigation. Additionally, regardless of the form of consideration we pay, acquisitions and investments could negatively impact our net income and earnings per share.
If goodwill or other intangible assets that we have recorded in connection with our acquisitions of other businesses become impaired, we could have to take significant charges against earnings.
As a result of our acquisitions, we have recorded, and may continue to record, a significant amount of goodwill and other intangible assets. Under current accounting guidelines, we must assess, at least annually and potentially more frequently, whether the value of goodwill and other intangible assets has been impaired. For example, during the fourth quarters of 2011 and 2010, we determined that our goodwill related to our health information solutions business was impaired, resulting in non-cash impairment charges in the amount of approximately $383.6 million and $1.0 billion, respectively. Any further reduction or impairment of the value of goodwill or other intangible assets will result in additional charges against earnings, which could materially reduce our reported results of operations in future periods.
We do not have complete control over the operations of SPD, our 50/50 joint venture with P&G.
Because SPD is a 50/50 joint venture, we do not have complete control over its operations, including business decisions, which may impact SPD’s profitability.
Additionally, certain subsidiaries of P&G have the right, at any time upon certain material breaches by us or our subsidiaries of our obligations under the joint venture documents, to acquire all of our interest in SPD at fair market value less any applicable damages.
Our business has substantial indebtedness.
We currently have, and will likely continue to have, a substantial amount of indebtedness. Our indebtedness could, among other things, make it more difficult for us to satisfy our debt obligations, require us to use a large portion of our cash flow from operations to repay and service our debt or otherwise create liquidity problems, limit our flexibility to adjust to market conditions, place us at a competitive disadvantage and expose us to interest rate fluctuations. As of December 31, 2013, we had total debt outstanding of approximately $3.8 billion, which included approximately $2.3 billion in aggregate principal amount of indebtedness outstanding under our secured credit facility, consisting of “A” term loans (including “Delayed Draw” term loans) in the aggregate principal amount of $832.2 million, “B” term loans in the aggregate principal amount of $904.2 million, “Incremental B-1” term loans in the aggregate principal amount of $245.0 million, “Incremental B-2” term loans in the aggregate principal amount of $195.1 million and revolving credit loans in the aggregate principal amount of $170.0 million. Our secured credit facility has various final maturity dates occurring in 2016 and 2017, but if any of our 3% convertible senior subordinated notes remain outstanding on the date that is six months prior to the maturity date thereof, our secured credit facility will mature on such prior date. At
34
December 31, 2013, we also had an aggregate of approximately $1.3 billion in aggregate principal amount of indebtedness outstanding under our 7.25% senior notes and our 8.625% and 6.5% senior subordinated notes, all of which mature in 2018 or 2020, as well as $150.0 million in aggregate principal amount of indebtedness outstanding under our 3% convertible senior subordinated notes, which matures in 2016.
We expect to obtain the money to pay our expenses and pay the principal and interest on our indebtedness from cash flow from our operations and potentially from other debt or equity offerings. Accordingly, our ability to meet our obligations depends on our future performance and capital raising activities, which will be affected by financial, business, economic and other factors, many of which are beyond our control. If our cash flow and capital resources prove inadequate to allow us to pay the principal and interest on our debt and meet our other obligations, we could face substantial liquidity problems and might be required to dispose of material assets or operations, restructure or refinance our debt, which we may be unable to do on acceptable terms, and forego attractive business opportunities. In addition, the terms of our existing or future debt agreements may restrict us from pursuing any of these alternatives.
The agreements governing our indebtedness subject us to various restrictions that may limit our ability to pursue business opportunities.
The agreements governing our indebtedness subject us to various restrictions on our ability to engage in certain activities, including, among other things, our ability to:
| | • | | acquire other businesses or make investments; |
| | • | | raise additional capital; |
| | • | | incur additional debt or create liens on our assets; |
| | • | | pay dividends or make distributions or repurchase or redeem our stock or senior or subordinated debt; |
| | • | | prepay indebtedness; and |
| | • | | consolidate, merge or sell all or substantially all of our assets. |
These restrictions may limit or restrict our cash flow and our ability to pursue business opportunities or strategies that we would otherwise consider to be in our best interests.
Our secured credit facility contains certain financial and other restrictive covenants that we may not satisfy, and that, if not satisfied, could result in the acceleration of the amounts due under our secured credit facility and the limitation of our ability to borrow additional funds in the future.
The agreements governing our secured credit facility subject us to various financial and other restrictive covenants with which we must comply on an ongoing or periodic basis. These include covenants pertaining to maximum consolidated secured leverage ratios, minimum consolidated interest coverage ratios and limits on capital expenditures. If we violate any of these covenants, we may suffer a material adverse effect. Most notably, our outstanding debt under our secured credit facility could become immediately due and payable, our lenders could proceed against any collateral securing such indebtedness, and our ability to borrow additional funds in the future could be limited or terminated. Alternatively, we could be forced to refinance or renegotiate the terms and conditions of our secured credit facility, including the interest rates, financial and restrictive covenants and security requirements of the secured credit facility, on terms that may be significantly less favorable to us.
35
A default under any of the agreements governing our indebtedness could result in a default and acceleration of indebtedness under other agreements.
The agreements governing our indebtedness contain cross-default provisions whereby a default under one agreement could result in a default and acceleration of our repayment obligations under other agreements. If a cross-default were to occur, we may not be able to pay our debts or borrow sufficient funds to refinance them. Even if new financing were available, it may not be available on acceptable terms. If some or all of our indebtedness is in default for any reason, our business, financial condition and results of operations could be materially and adversely affected.
We may not be able to satisfy our debt obligations upon a change of control or fundamental change, which could limit our opportunity to enter into a change of control or fundamental change transaction.
If we undergo a change of control, as provided in our secured credit facility, our 7.25% senior notes, our 8.625% senior subordinated notes or our 6.5% senior subordinated notes, or a fundamental change or termination of trading, as provided in the 3% convertible senior subordinated notes, we may be required to repay or repurchase some or all of such indebtedness. We may not have sufficient financial resources to satisfy all of our repayment and repurchase obligations. Our failure to purchase notes as required under our 7.25% senior notes, our 8.625% senior subordinated notes, our 6.5% senior subordinated notes or our 3% convertible senior subordinated notes would constitute a default under the relevant indentures and under our secured credit facility and could have material adverse consequences for us and our stakeholders.
Our operating results may fluctuate for various reasons and, as a result, period-to-period comparisons of our results of operations will not necessarily be meaningful.
Many factors relating to our business, such as those described elsewhere in this section, make our future operating results uncertain and may cause them to fluctuate from period to period. Because our revenue and operating results are difficult to predict, we believe that period-to-period comparisons of our results of operations are not a good indicator of our future performance. If revenue declines in a quarter, our results of operations will be harmed because many of our expenses are relatively fixed. In particular, research and development, sales and marketing and general and administrative expenses are not significantly affected by variations in revenue. If our quarterly operating results fail to meet or exceed the expectations of securities analysts or investors, our stock price could drop suddenly and significantly.
Our effective tax rate may fluctuate, and we may incur obligations in tax jurisdictions in excess of amounts that have been accrued.
We are subject to income taxes in both the United States and various foreign jurisdictions, and we may take certain income tax positions on our tax returns that tax authorities may disagree with. We provide reserves for potential payments of tax to various tax authorities related to uncertain tax positions. However, the calculation of our tax liabilities involves the application of complex tax regulations to our global operations in many jurisdictions. Therefore, a dispute with a tax authority may result in a payment that is materially different from our current estimate of the tax liabilities associated with our returns.
Changes in tax laws or tax rulings could materially impact our effective tax rate. There are several proposals to reform U.S. tax rules being considered by U.S. law makers, including proposals that may reduce or eliminate the deferral of U.S. income tax on our unrepatriated earnings, potentially requiring those earnings to be taxed at the U.S. federal income tax rate, reduce or eliminate our ability to claim foreign tax credits, and eliminate various tax deductions until foreign earnings are repatriated to the U.S. Our future reported financial results may be adversely affected by tax rule changes which restrict or eliminate our ability to claim foreign tax credits or deduct expenses attributable to foreign earnings, or otherwise affect the treatment of our unrepatriated earnings.
36
We may incur losses in excess of our insurance coverage.
Our insurance coverage includes product liability, property, healthcare professional and business interruption policies. Our insurance coverage contains policy limits, specifications and exclusions. We believe that our insurance coverage is consistent with general practices within our industry. Nonetheless, we may incur losses of a type for which we are not covered by insurance or which exceed the limits of liability of our insurance policies. In that event, we could experience a significant loss which could have a material negative impact on our financial condition.
Our future success depends on our ability to recruit and retain key personnel.
Our future success depends on our continued ability to attract, hire and retain highly qualified personnel, including our executive officers and scientific, technical, sales and marketing employees, and their ability to manage growth successfully. Experienced personnel in our industry are in high demand and competition for their talents is intense. If we are unable to attract and retain key personnel, our business may be harmed. In addition, the loss of any of our key personnel, particularly key research and development personnel, could harm our business and prospects and could impede the achievement of our research and development, operation or strategic objectives.
Future sales of our common stock, including shares issuable upon conversion of our Series B Convertible Perpetual Preferred Stock, or Series B Preferred Stock, or our 3% convertible senior subordinated notes, may adversely affect the market price of our common stock.
Sales of a substantial number of shares of our common stock or other equity securities in the public market could depress the price of our common stock and impair our ability to raise capital through the sale of additional equity securities. The price of our common stock could be affected by possible sales of the substantial number of shares of our common stock potentially issuable upon conversion of our Series B Preferred Stock or our 3% convertible senior subordinated notes and by other hedging or arbitrage trading activity that may develop involving our common stock. If the conditions applicable to the conversion of our Series B Preferred Stock were satisfied, then subject to adjustment, each of the approximately 1.8 million shares of Series B Preferred Stock outstanding as of December 31, 2013 could convert into 5.7703 shares of our common stock, or approximately 10.2 million shares of our common stock. Upon certain extraordinary transactions, depending on the market price of our common stock at that time, the conversion rate could increase such that significantly more shares of common stock could be issued. Our $150.0 million in aggregate principal amount of 3% convertible senior subordinated notes is convertible into shares of our common stock at a conversion price of approximately $43.98 per share, or approximately 3.4 million shares.
The holders of our Series B Preferred Stock are entitled to receive liquidation payments in preference to the holders of our common stock.
As of December 31, 2013, the outstanding shares of our Series B Preferred Stock had an aggregate stated liquidation preference of approximately $709.8 million. Dividends accrue on the shares of Series B Preferred Stock at a rate of 3% per annum, and we have the option to pay these dividends in cash or in shares of common stock or additional shares of Series B Preferred Stock. If we pay these dividends in shares of common stock or additional shares of Series B Preferred Stock, the number of shares of common stock or Series B Preferred Stock issued will be based upon market prices at the time of such payment. Upon a liquidation of our company, the holders of shares of Series B Preferred Stock will be entitled to receive a liquidation payment prior to the payment of any amount with respect to the shares of our common stock. The amount of this preferential liquidation payment is the aggregate stated liquidation preference, plus any accrued and unpaid dividends. Because of the substantial liquidation preference to which the holders of the Series B Preferred Stock are entitled, the amount available to be distributed to the holders of our common stock upon a liquidation of our company could be substantially limited or reduced to zero.
37
The terms of the Series B Preferred Stock may limit our ability to raise additional capital through subsequent issuances of preferred stock.
For so long as any shares of Series B Preferred Stock remain outstanding, we are not permitted, without the affirmative vote or written consent of the holders of at least two-thirds of the Series B Preferred Stock then outstanding, to authorize or designate any class or series of capital stock having rights on liquidation or as to distributions (including dividends) senior to the Series B Preferred Stock. This restriction could limit our ability to plan for or react to market conditions or meet extraordinary capital needs, which could have a material adverse impact on our business.
Anti-takeover provisions in our organizational documents and Delaware law may limit the ability of our stockholders to control our policies and effect a change of control of our company and may prevent attempts by our stockholders to replace or remove our current management, which may not be in your best interests.
Provisions of our certificate of incorporation and bylaws may discourage a third party from making a proposal to acquire us, even if some of our stockholders might consider the proposal to be in their best interests, and may prevent attempts by our stockholders to replace or remove our current management. These provisions include the following:
| | • | | although we have amended our certificate of incorporation to declassify our board of directors, under Delaware law 3 continuing directors have terms ending in 2015. By preventing stockholders from voting on the election of all of our directors at our annual meetings of stockholders in 2014, the longer terms of these continuing directors may have the effect of keeping the current members of our board of directors in control for a longer period of time than stockholders may desire; and |
| | • | | subject to the rights of the holders of our Series B Preferred Stock, our certificate of incorporation authorizes our board of directors to issue shares of preferred stock without stockholder approval and to establish the preferences and rights of any preferred stock issued, which would allow the board to issue one or more classes or series of preferred stock that could discourage or delay a tender offer or change in control. |
In addition, our board of directors may in the future adopt other protective measures, such as a stockholder rights plan, which could delay, deter or prevent a change in control.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
Our corporate headquarters, together with the administrative office for our United States consumer operations, is located at 51 Sawyer Road, Suite 200, Waltham, Massachusetts. From our office in Galway, Ireland, we oversee and conduct much of our professional diagnostic products business in Europe. We also operate a shared service center in Orlando, Florida, which houses certain critical back-office and sales operations supporting our U.S. professional diagnostics operations, and a call center in Taguig City, Philippines. Our health information solutions business is headquartered in Atlanta, Georgia. These key administrative facilities are leased from third parties.
We own approximately 18.8 acres of land in San Diego, California which houses one of our eight primary manufacturing operations, as well as significant administrative and research and development operations for our professional diagnostics business. Our buildings on this property total approximately 330,000 square feet and include 167,000 square feet of manufacturing space for professional diagnostic products.
38
Our other primary manufacturing operations are in Scarborough, Maine; Hangzhou and Shanghai, China; Jena, Germany; Matsudo, Japan; Oslo, Norway; Dundee, Scotland and Yongin, South Korea. We manufacture some of our consumer and professional diagnostic products in a manufacturing facility of approximately 498,000 square feet in Hangzhou, China, which we own. The majority of our consumer diagnostic products are manufactured in a facility of approximately 133,000 square feet in Shanghai, China, which we lease. We manufacture our Alere CD4 products in a facility of approximately 159,000 square feet in Jena, Germany, which we own. We manufacture our Determine products in a leased space of approximately 32,000 square feet in Matsudo, Japan. Standard Diagnostics manufactures most of its professional diagnostic products in two facilities in Yongin, South Korea, which we own; a 51,500 square foot facility and a 37,000 square foot facility. Axis-Shield, which we acquired in late 2011, manufactures the majority of our point-of-care products for patients with diabetes in a leased space of approximately 135,000 square feet in Oslo, Norway and a leased space of approximately 54,000 square feet in Dundee, Scotland. We manufacture certain professional diagnostic products in a 64,000 square foot facility that we lease in Scarborough, Maine.
We increasingly rely on our network of toxicology laboratories to provide reliable drugs-of-abuse test results to customers. We own two SAMHSA certified laboratories in the United States, located in Gretna, Louisiana and Richmond, Virginia. We also operate toxicology laboratories in Austin, Texas; Clearwater, Florida; Santa Rosa, California; London, England and Abingdon, England, and we operate an accredited forensic laboratory in Malvern, England.
Additionally, we have facilities, which are generally leased, in various locations worldwide, including smaller manufacturing operations and laboratories, as well as research and development operations, administrative or sales offices, call centers and warehouses. We believe that adequate space for our manufacturing, testing and other operations will be available as needed.
ITEM 3. LEGAL PROCEEDINGS
Matters Relating to our San Diego Facility
On October 9, 2012, we received a warning letter from the FDA referencing inspectional observations set forth in an FDA Form 483 received in June, 2012. The observations were the result of an inspection of our San Diego facility conducted earlier during 2012 relating to our Alere Triage products, which resulted in two recalls of certain Alere Triage products and revised release specifications for our Alere Triage meter-based products. We have submitted evidence of our completion of most of the actions committed to in response to the FDA Form 483 and warning letter. We will continue to work cooperatively with the FDA in an effort to fully address each inspectional observation.
In May 2012, we also received a subpoena from the Office of Inspector General of the Department of Health and Human Services, or the OIG, seeking documents relating primarily to the quality control testing and performance characteristics of Alere Triage products. We are cooperating with the OIG and have provided documents in response to the OIG under the subpoena.
We are unable to predict when these matters will be resolved or what further action, if any, the government will take in connection with them. Except for increases in manufacturing costs and decreased profitability for our Alere Triage products, we are unable to predict what impact, if any, these matters or ensuing proceedings, if any, will have on our financial condition, results of operations or cash flows.
Matters Related to Theft of Laptop
In September 2012, a password-protected laptop containing personally identifiable information of approximately 116,000 patients was stolen from an employee of Alere Home Monitoring, or AHM. On January 24, 2013, a class action complaint was filed in the U.S. District Court for the Northern District of California against AHM, asserting claims for damages and other relief under California state law, including under California’s Confidentiality of Medical Information Act, arising out of this theft. The class
39
action has been stayed pending resolution of other class actions involving third parties based on similar circumstances. We believe that AHM has strong defenses to the claims made in the complaint, and AHM intends to defend this matter vigorously. The Office of Civil Rights of the U.S. Department of Health and Human Services, or the OCR, was notified of the inadvertent disclosure in accordance with the Breach Notification Rule under the HITECH Act, as were certain state agencies. AHM has responded to the OCR’s inquiries and continues to cooperate.
Claims in the Ordinary Course and Other Matters
Because of the nature of our business, we may be subject at any particular time to lawsuits or other claims arising in the ordinary course of our business, and we expect that this will continue to be the case in the future. Such lawsuits often seek damages, sometimes in substantial amounts. An adverse ruling in such a lawsuit could have a material adverse impact on our sales, operations or financial performance.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
40
PART II
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Unregistered Sales of Equity Securities and Use of Proceeds
On December 30, 2012, our Chief Operating Officer, Namal Nawana, was granted Restricted Stock Units, or RSUs, as follows: 5,000 RSUs to vest one (1) year after the grant date, 5,000 RSUs to vest two (2) years after the grant date, and 100,000 RSUs to vest three (3) years after the grant date. On December 30, 2013, in connection with the partial vesting of these RSUs, we issued 5,000 shares of our common stock to Mr. Nawana. We issued these shares pursuant to the exemption from registration afforded by Section 4(a)(2) of the Securities Act of 1933, as amended.
Market Information
Our common stock trades on the New York Stock Exchange (NYSE) under the symbol “ALR.” The following table sets forth the high and low sales prices of our common stock for each quarter during fiscal 2013 and 2012:
| | | | | | | | |
| | | High | | | Low | |
Fiscal 2013 | | | | | | | | |
Fourth Quarter | | $ | 36.78 | | | $ | 30.16 | |
Third Quarter | | $ | 35.38 | | | $ | 24.00 | |
Second Quarter | | $ | 29.57 | | | $ | 24.33 | |
First Quarter | | $ | 25.55 | | | $ | 18.64 | |
Fiscal 2012 | | | | | | | | |
Fourth Quarter | | $ | 22.10 | | | $ | 17.20 | |
Third Quarter | | $ | 20.54 | | | $ | 17.13 | |
Second Quarter | | $ | 26.50 | | | $ | 17.62 | |
First Quarter | | $ | 27.22 | | | $ | 21.51 | |
On February 24, 2014, there were 1,397 holders of record of our common stock.
Dividend Policy
We have never declared or paid any cash dividends on our common stock. We currently intend to retain earnings to support our growth strategy and do not anticipate paying cash dividends on our common stock in the foreseeable future. Payment of future dividends, if any, on our common stock will be at the discretion of our board of directors after taking into account various factors, including our financial condition, operating results, current and anticipated cash needs and plans for expansion. In addition, restrictive covenants under our secured credit facility and the indentures governing the terms of our senior notes and our senior subordinated notes currently prohibit or limit the payment of cash or stock dividends.
Stock Performance Graph
The following line graph compares the cumulative total stockholder return on our common stock from December 31, 2008 through December 31, 2013 with the cumulative total return of a broad equity market index and a published industry index. This graph assumes an investment of $100.00 on December 31, 2008 in our common stock, and compares its performance with the NYSE Composite Index and the Dow Jones U.S. Health Care Index, or the Current Indices. We paid no dividends on our common stock during the period covered by the graph. The Current Indices reflect a cumulative total return based upon the reinvestment of dividends of the stocks included in those indices. Measurement points are December 31, 2008 and the last trading day of each subsequent year end through December 31, 2013.
41
The comparisons shown in the graph below are based upon historical data. We caution that the stock price performance shown in the graph below is not necessarily indicative of, nor is it intended to forecast, the potential future performance of our common stock.
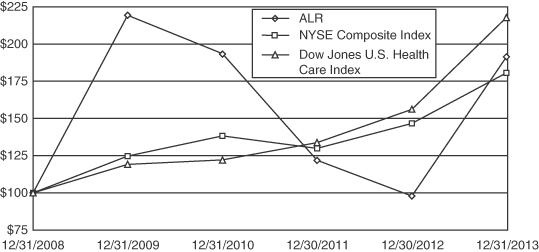
Current Indices
| | | | | | | | | | | | |
Date | | ALR | | | NYSE Composite
Index | | | Dow Jones U.S.
Healthcare Index | |
12/31/08 | | $ | 100.00 | | | $ | 100.00 | | | $ | 100.00 | |
12/31/09 | | $ | 219.51 | | | $ | 124.80 | | | $ | 119.27 | |
12/31/10 | | $ | 193.55 | | | $ | 138.34 | | | $ | 122.28 | |
12/30/11 | | $ | 122.10 | | | $ | 129.88 | | | $ | 133.82 | |
12/31/12 | | $ | 97.83 | | | $ | 146.66 | | | $ | 156.24 | |
12/31/13 | | $ | 191.43 | | | $ | 180.65 | | | $ | 217.88 | |
The performance graph above shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, or otherwise subject to the liabilities of that section. This graph will not be deemed incorporated by reference into any filing under the Securities Act, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
| ITEM 6. | SELECTED CONSOLIDATED FINANCIAL DATA |
The following tables set forth selected consolidated financial data of our company as of and for each of the years in the five-year period ended December 31, 2013 and should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our Consolidated Financial Statements and notes thereto included elsewhere in this Annual Report on Form 10-K.
On January 15, 2010, we completed the sale of our vitamins and nutritional supplements business. The sale included our entire private label and branded nutritionals businesses and represents the complete divestiture of our entire vitamins and nutritional supplements business segment. The results of the vitamins and nutritional supplements business are included in income (loss) from discontinued operations, net of tax, for all periods presented in the statement of operations data below. The assets and liabilities associated with the vitamins and nutritional supplements business have been reclassified to current classifications as assets held for sale and liabilities related to assets held for sale and, as such, have impacted working capital amounts, which are reflected in the balance sheet data section below, for all balance sheet dates presented.
42
For a discussion of certain factors that materially affect the comparability of the selected consolidated financial data or cause the data reflected herein not to be indicative of our future results of operations or financial condition, see Item 1A “Risk Factors,” Item 7 “Management’s Discussion and Analysis of Financial Condition and Results of Operation” and Notes 2(v) and 4 of our Consolidated Financial Statements included elsewhere in this report.
| | | | | | | | | | | | | | | | | | | | |
| | | For the Year Ended December 31, | |
| | | 2013 | | | 2012 | | | 2011 | | | 2010 | | | 2009 | |
| | | (in thousands, except per share data) | |
Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | |
Net product sales | | $ | 2,072,463 | | | $ | 1,913,731 | | | $ | 1,683,132 | | | $ | 1,472,403 | | | $ | 1,365,079 | |
Services revenue | | | 929,750 | | | | 876,518 | | | | 679,922 | | | | 662,185 | | | | 528,487 | |
| | | | | | | | | | | | | | | | | | | | |
Net product sales and services revenue | | | 3,002,213 | | | | 2,790,249 | | | | 2,363,054 | | | | 2,134,588 | | | | 1,893,566 | |
License and royalty revenue | | | 27,229 | | | | 28,576 | | | | 23,473 | | | | 20,759 | | | | 29,075 | |
| | | | | | | | | | | | | | | | | | | | |
Net revenue | | | 3,029,442 | | | | 2,818,825 | | | | 2,386,527 | | | | 2,155,347 | | | | 1,922,641 | |
| | | | | | | | | | | | | | | | | | | | |
Cost of net product sales | | | 1,028,520 | | | | 932,150 | | | | 795,424 | | | | 688,325 | | | | 619,503 | |
Cost of services revenue | | | 491,420 | | | | 450,999 | | | | 338,232 | | | | 325,286 | | | | 240,026 | |
| | | | | | | | | | | | | | | | | | | | |
Cost of net product sales and services revenue | | | 1,519,940 | | | | 1,383,149 | | | | 1,133,656 | | | | 1,013,611 | | | | 859,529 | |
Cost of license and royalty revenue | | | 7,763 | | | | 7,354 | | | | 7,036 | | | | 7,149 | | | | 8,890 | |
| | | | | | | | | | | | | | | | | | | | |
Cost of net revenue | | | 1,527,703 | | | | 1,390,503 | | | | 1,140,692 | | | | 1,020,760 | | | | 868,419 | |
| | | | | | | | | | | | | | | | | | | | |
Gross profit | | | 1,501,739 | | | | 1,428,322 | | | | 1,245,835 | | | | 1,134,587 | | | | 1,054,222 | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | |
Research and development | | | 160,802 | | | | 183,001 | | | | 150,165 | | | | 133,278 | | | | 112,848 | |
Sales and marketing | | | 639,834 | | | | 643,423 | | | | 565,583 | | | | 499,124 | | | | 441,646 | |
General and administrative | | | 561,227 | | | | 492,766 | | | | 399,330 | | | | 446,917 | | | | 357,033 | |
Goodwill impairment charge | | | — | | | | — | | | | 383,612 | | | | 1,006,357 | | | | — | |
Loss (gain) on dispositions, net | | | 5,124 | | | | — | | | | — | | | | — | | | | (3,355 | ) |
| | | | | | | | | | | | | | | | | | | | |
Operating income (loss) | | | 134,752 | | | | 109,132 | | | | (252,855 | ) | | | (951,089 | ) | | | 146,050 | |
Interest expense, including amortization of original issue discounts and write-off of deferred financing costs and other income (expense), net | | | (268,784 | ) | | | (230,603 | ) | | | 86,808 | | | | (116,697 | ) | | | (105,802 | ) |
| | | | | | | | | | | | | | | | | | | | |
Income (loss) from continuing operations before provision (benefit) for income taxes | | | (134,032 | ) | | | (121,471 | ) | | | (166,047 | ) | | | (1,067,786 | ) | | | 40,248 | |
Provision (benefit) for income taxes | | | (46,311 | ) | | | (30,319 | ) | | | (24,214 | ) | | | (29,931 | ) | | | 15,627 | |
| | | | | | | | | | | | | | | | | | | | |
Income (loss) from continuing operations before equity earnings of unconsolidated entities, net of tax | | | (87,721 | ) | | | (91,152 | ) | | | (141,833 | ) | | | (1,037,855 | ) | | | 24,621 | |
Equity earnings of unconsolidated entities, net of tax | | | 17,443 | | | | 13,245 | | | | 8,524 | | | | 10,566 | | | | 7,626 | |
| | | | | | | | | | | | | | | | | | | | |
Income (loss) from continuing operations | | | (70,278 | ) | | | (77,907 | ) | | | (133,309 | ) | | | (1,027,289 | ) | | | 32,247 | |
Income from discontinued operations, net of tax | | | — | | | | — | | | | — | | | | 11,397 | | | | 1,934 | |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | | (70,278 | ) | | | (77,907 | ) | | | (133,309 | ) | | | (1,015,892 | ) | | | 34,181 | |
Less: Net income attributable to non-controlling interests | | | 976 | | | | 275 | | | | 233 | | | | 1,418 | | | | 465 | |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) attributable to Alere Inc. and Subsidiaries | | | (71,254 | ) | | | (78,182 | ) | | | (133,542 | ) | | | (1,017,310 | ) | | | 33,716 | |
Preferred stock dividends | | | (21,293 | ) | | | (21,293 | ) | | | (22,049 | ) | | | (24,235 | ) | | | (22,972 | ) |
Preferred stock repurchase | | | — | | | | — | | | | 23,936 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) available to common stockholders(1) | | $ | (92,547 | ) | | $ | (99,475 | ) | | $ | (131,655 | ) | | $ | (1,041,545 | ) | | $ | 10,744 | |
| | | | | | | | | | | | | | | | | | | | |
43
| | | | | | | | | | | | | | | | | | | | |
| | | For the Year Ended December 31, | |
| | | 2013 | | | 2012 | | | 2011 | | | 2010 | | | 2009 | |
| | | (in thousands, except per share data) | |
Basic and diluted net income (loss) per common share attributable to Alere Inc. and Subsidiaries: | | | | | | | | | | | | | | | | | | | | |
Income (loss) per common share from continuing operations | | $ | (1.13 | ) | | $ | (1.23 | ) | | $ | (1.58 | ) | | $ | (12.47 | ) | | $ | 0.11 | |
Income per common share from discontinued operations | | $ | — | | | $ | — | | | $ | — | | | $ | 0.14 | | | $ | 0.02 | |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) per common share(1) | | $ | (1.13 | ) | | $ | (1.23 | ) | | $ | (1.58 | ) | | $ | (12.33 | ) | | $ | 0.13 | |
| | | | | | | | | | | | | | | | | | | | |
| |
| | | December 31, | |
| | | 2013 | | | 2012 | | | 2011 | | | 2010 | | | 2009 | |
| | | (In thousands) | |
Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 361,908 | | | $ | 328,346 | | | $ | 299,173 | | | $ | 401,306 | | | $ | 492,773 | |
Working capital | | $ | 799,228 | | | $ | 757,928 | | | $ | 669,275 | | | $ | 411,399 | | | $ | 828,944 | |
Total assets | | $ | 7,060,814 | | | $ | 7,067,928 | | | $ | 6,672,701 | | | $ | 6,330,374 | | | $ | 6,943,992 | |
Total debt | | $ | 3,843,162 | | | $ | 3,708,508 | | | $ | 3,353,495 | | | $ | 2,398,985 | | | $ | 2,149,324 | |
Other long-term obligations | | $ | 517,585 | | | $ | 594,823 | | | $ | 534,098 | | | $ | 589,822 | | | $ | 847,634 | |
Total stockholders’ equity | | $ | 2,077,966 | | | $ | 2,180,422 | | | $ | 2,229,234 | | | $ | 2,575,038 | | | $ | 3,527,555 | |
| (1) | Net income (loss) available to common stockholders and basic and diluted net income (loss) per common share are computed consistent with annual per share calculations described in Notes 2(o) and 12 of our Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K. |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Forward-Looking Statements
This Annual Report on Form 10-K, including this Item 7, contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. You can identify these statements by forward-looking words such as “may,” “could,” “should,” “would,” “intend,” “will,” “expect,” “anticipate,” “believe,” “estimate,” “continue” or similar words. You should read statements that contain these words carefully because they discuss our future expectations, contain projections of our future results of operations or of our financial condition or state other “forward-looking” information. Forward-looking statements include, without limitation, statements regarding anticipated expansion and growth in certain of our product and service offerings, the impact of our research and development activities, potential new product and technology achievements, the potential for selective divestitures of non-core assets, our ability to improve our working capital and operating margins, our ability to improve our organic revenue growth rates, the effectiveness of steps we may take to improve our operational efficiency, our ability to improve care and lower healthcare costs for both providers and patients, and our funding plans for our future working capital needs and commitments. Actual results or developments could differ materially from those projected in such statements as a result of numerous factors, including, without limitation, those risks and uncertainties set forth in Item 1A entitled “Risk Factors,” which begins on page 18 of this report, as well as those factors identified from time to time in our filings with the Securities and Exchange Commission. We do not undertake any obligation to update any forward-looking statements. This report and, in particular, the following discussion and analysis of our financial condition and results of operations, should be read in light of those risks and uncertainties and in conjunction with our accompanying consolidated financial statements and notes thereto.
44
Overview
We empower individuals to take greater control of their health at home, under the supervision of their healthcare providers, by connecting innovative diagnostics in the hands of patients to their healthcare providers. We are a leading global provider of diagnostic products that deliver rapid and accurate results at the point of care, with particular emphasis in the fields of infectious diseases, toxicology, cardiology and diabetes. Our connected device technologies and health information solutions give providers the ability to speed up and customize treatment by enabling timely access to actionable data, from both our connected devices and our personal and electronic health record solutions, as well as decision-support software and analytic tools. Through our health information solutions business, we also offer programs for chronic condition management, coagulation monitoring, smoking cessation, pregnancy management, weight loss and healthy living. Our connected health approach envisions a healthcare system that unites patients with diagnostic tools at their fingertips with providers and payers in a model that provides patients with a better quality of life and improved outcomes, while simultaneously reducing healthcare costs to the system.
Having substantially completed the foundation of our business, during 2013 we focused on re-establishing historic organic revenue growth rates in our point-of-care diagnostics business, and improving our operational efficiency with the goal of generating predictable earnings growth and sustainable, strong cash flow.
Our revenue growth has been impacted by supply challenges for certain of our Alere Triage product lines arising from an FDA inspection of our San Diego facility in 2012. During 2013, we overcame a portion of these supply challenges, and we are now consistently supplying the market with all Alere Triage products, other than our shortness-of-breath and toxicology panels. We expect to return one or both of these panels to the market during the first half of 2014. During 2013, we also continued to increase our market presence with Accountable Care Organizations with our integrated product and service offerings. Our continuing expansion in the growing markets of Asia, Latin America and Africa should also improve organic growth rates over the longer term.
In addition, we expect our new product launches in 2013, including the addition of tests for chloride and creatinine on our epoc Blood Analysis System and the Alere Determine HIV-1/2 Ag/Ab Combo test, as well as the increasingly robust contribution of previously launched products, such as our CD4 analyzer, to contribute to improved organic growth rates in the future. In January 2014, we also announced that the Alere i Influenza A & B test, the first and only commercially available molecular test to detect and differentiate influenza A and B virus in less than 15 minutes, had been launched in Austria, France, Spain, Switzerland, Germany, Italy and the U.K. The Alere i test remains under FDA review and is not yet available for sale in the United States. During 2013, we also made significant progress towards the anticipated launch of Alere Q in Africa during the first half of 2014. Alere Q enables molecular viral load testing for HIV at the point of care and applications for HCV and tuberculosis are in development for the Alere Q platform.
From late 2012 into 2013, we took important steps to build our global enterprise infrastructure, which we believe will enable us to realize more predictable earnings growth and stronger, more sustainable cash flow. During 2013, we standardized a number of our key business processes. Most notably, we began implementation of a global ERP system for many of our business units in the U.S. and Europe, which we have recently completed. We also implemented a global sourcing initiative based in the Philippines, which has enabled a more cost effective scaling of our mail order diabetes business to serve over 700,000 patients. We are already expanding this initiative to include our Alere Home Monitoring business, which we expect to result in further cost efficiencies.
We remain engaged in active and ongoing discussions with multiple parties concerning divestitures of our non-core businesses. During 2013, we completed the sale of one such non-core asset and used $27.5 million of the proceeds from the transaction to repay indebtedness. When
45
complete, we expect that these steps will not only sharpen our focus on our mission of enabling healthcare providers to improve clinical outcomes and lower costs, but will also reduce indebtedness and enhance shareholder value.
2013 Financial Highlights
| | • | | Net revenue increased by $210.6 million, or 7%, to $3.0 billion in 2013, from $2.8 billion in 2012. |
| | • | | Gross profit increased by $73.4 million, or 5%, to $1.5 billion in 2013, from $1.4 billion in 2012. |
| | • | | For the year ended December 31, 2013, we generated a net loss available to common stockholders of $92.5 million, or $1.13 per basic and diluted common share. For the year ended December 31, 2012, we generated a net loss available to common stockholders of $99.5 million, or $1.23 per basic and diluted common share. |
Results of Operations
Where discussed, results excluding the impact of foreign currency translation are calculated on the basis of local currency results, using foreign currency exchange rates applicable to the earlier comparative period. We believe presenting information using the same foreign currency exchange rates helps investors isolate the impact of changes in those rates from other trends. Our results of operations were as follows:
Year Ended December 31, 2013 Compared to Year Ended December 31, 2012
Net Product Sales and Services Revenue. Net product sales and services revenue increased by $212.0 million, or 8%, to $3.0 billion in 2013, from $2.8 billion in 2012. Net product sales and services revenue increased primarily as a result of our acquisitions which contributed an aggregate of $169.4 million of the increase. Excluding the impact of foreign currency translation, net product sales and services revenue in 2013 grew by approximately $229.4 million, or 8%, over 2012.
Net Product Sales and Services Revenue by Business Segment. Net product sales and services revenue by business segment for 2013 and 2012 is as follows (in thousands):
| | | | | | | | | | | | |
| | | 2013 | | | 2012 | | | % Increase | |
Professional diagnostics | | $ | 2,366,204 | | | $ | 2,165,216 | | | | 9 | % |
Health information solutions | | | 533,227 | | | | 535,422 | | | | — | % |
Consumer diagnostics | | | 102,782 | | | | 89,611 | | | | 15 | % |
| | | | | | | | | | | | |
Net product sales and services revenue | | $ | 3,002,213 | | | $ | 2,790,249 | | | | 8 | % |
| | | | | | | | | | | | |
Professional Diagnostics
The following table summarizes our net product sales and services revenue from our professional diagnostics business segment by groups of similar products and services for 2013 and 2012 (in thousands):
| | | | | | | | | | | | |
| | | 2013 | | | 2012 | | | % Increase
(Decrease) | |
Infectious disease | | $ | 723,213 | | | $ | 615,950 | | | | 17 | % |
Toxicology | | | 632,727 | | | | 587,261 | | | | 8 | % |
Cardiology | | | 463,281 | | | | 503,534 | | | | (8 | )% |
Diabetes | | | 225,488 | | | | 144,441 | | | | 56 | % |
Other | | | 321,495 | | | | 314,030 | | | | 2 | % |
| | | | | | | | | | | | |
Professional diagnostics net product sales and services revenue | | $ | 2,366,204 | | | $ | 2,165,216 | | | | 9 | % |
| | | | | | | | | | | | |
46
Net product sales and services revenue from our professional diagnostics business segment increased by $201.0 million, or 9%, to $2.4 billion in 2013, from $2.2 billion in 2012. Excluding the impact of foreign currency translation, net product sales and services revenue from our professional diagnostics business segment increased by $220.1 million, or 10%, comparing 2013 to 2012. Net product sales and services revenue increased primarily as a result of acquisitions, which contributed an aggregate of $167.2 million of the non-currency-adjusted increase. Also, contributing to the increase in revenue were net product sales from our North American flu-related sales which increased approximately $31.9 million, from $43.6 million in 2012 to $75.5 million 2013. Net product sales and services revenue from our professional diagnostics business segment were negatively impacted by the FDA recall matters related to our Alere Triage meter-based products. Net product sales of meter-based Triage products in the U.S. totaled $76.2 million in 2013, as compared to $150.3 million in 2012. Furthermore, net product sales and services revenue from our professional diagnostics business segment was negatively impacted by the disposition of our Spinreact operations in Spain in July 2013, for which sales totaled $15.9 million in 2013 through the date of disposition, as compared to $22.2 million in 2012, a decrease of $6.3 million. Excluding the impact of acquisitions, the increase in flu-related sales during the comparable periods, the impact of the reduction in net product sales from meter-based Triage products in the U.S. and the disposition of our Spinreact operations in Spain, the currency-adjusted organic growth for our professional diagnostics net product sales and services revenue was approximately $101.4 million, or 5%, from 2012 to 2013. This growth rate was additionally impacted by the reduction in CMS’ reimbursement rates which became effective on July 1, 2013 for our U.S. mail order diabetes business. Excluding organic revenues from our mail order diabetes business, along with all of the other impacts previously mentioned, our currency-adjusted organic growth was approximately $132.1 million, or 6.9%, from 2012 to 2013.
Within our professional diagnostics business segment, net product sales and services revenue for our cardiology business decreased by approximately $40.3 million, or 8%, to $463.3 million in 2013, from $503.5 million in 2012, primarily as a result of the impact of the FDA review of certain of our meter-based Triage products in the U.S. Net product sales and services revenue for our infectious disease business increased by approximately $107.3 million, or 17%, to $723.2 million in 2013, from $616.0 million in 2012. This change was driven principally by a growth in HIV, flu and malaria revenues during the comparable periods. Net product sales and services revenue for our toxicology business increased by approximately $45.5 million, or 8%, to $632.7 million in 2013, from $587.3 million in 2012, with our recent toxicology-related acquisitions contributing a combined net of $54.1 million of the non-currency adjusted increase. Offsetting the increase in net product sales and services revenue for our toxicology business contributed by acquisitions was a $23.6 million decrease in net product sales related to our Triage toxicology products and reductions in commercial pricing for our pain and rehab businesses implemented in the second quarter of 2012 and fourth quarter of 2013. Our diabetes net product sales and services revenue increased by approximately $81.0 million, or 56%, to $225.5 million in 2013, from $144.4 million in 2012. The increase was primarily the result of our recent acquisitions of AmMed Direct LLC, or AmMed, NationsHealth, Discount Diabetic, LLC, or Discount Diabetic, the Medicare fee-for-service assets of Liberty Medical, or the Liberty business, and Simplex Healthcare, Inc., or Simplex, which contributed a combined net $100.8 million of the non-currency adjusted increase. Included in the $225.5 million of revenue from our diabetes business for 2013 were $153.6 million of mail order diabetes sales, compared to $85.7 million for 2012.
47
Health Information Solutions
The following table summarizes our net product sales and services revenue from our health information solutions business segment by groups of similar products and services for 2013 and 2012 (in thousands):
| | | | | | | | | | | | |
| | | 2013 | | | 2012 | | | % Increase
(Decrease) | |
Condition and case management | | $ | 215,160 | | | $ | 218,378 | | | | (1 | )% |
Wellness | | | 101,642 | | | | 104,634 | | | | (3 | )% |
Women’s & children’s health | | | 113,506 | | | | 120,259 | | | | (6 | )% |
Patient self-testing services | | | 102,919 | | | | 92,151 | | | | 12 | % |
| | | | | | | | | | | | |
Health information solutions net product sales and services revenue | | $ | 533,227 | | | $ | 535,422 | | | | — | % |
| | | | | | | | | | | | |
Net product sales and services revenue from our heath information solutions business segment decreased by $2.2 million to $533.2 million in 2013, from $535.4 million in 2012. Within our health information solutions business segment, net product sales and services revenues from our condition and case management, wellness and women’s and children’s health businesses each decreased during 2013, compared to 2012, as we experienced customer terminations, lower state enrollments in wellness programs and lower revenue from homecare services in these businesses, respectively. Given the challenging contracting season, we expect weak sales in the first quarter of 2014, and then expect to resume sequential growth, adjusting for seasonality, within this segment through 2014. Our patient self-testing services net product sales and services revenue increased approximately $10.8 million, or 12%, to $102.9 million in 2013, compared to $92.2 million in 2012, principally driven by an increase in our home coagulation monitoring programs resulting from a larger patient population and a simultaneous reduction in customer attrition rates.
Consumer Diagnostics
New product sales and services revenue from our consumer diagnostics business segment increased by $13.2 million, or 15%, to $102.8 million in 2013, from $89.6 million in 2012. The increase in revenue primarily resulted from an increase in our manufacturing revenue associated with SPD, as SPD successfully launched the Clearblue Advanced Pregnancy Test with Weeks Estimator product in the U.S. during 2013. SPD sales were $178.4 million and $187.8 million during 2013 and 2012, respectively.
Net Product Sales and Services Revenue by Geographic Location. Net product sales and services revenue by geographic location for 2013 and 2012 is as follows (in thousands):
| | | | | | | | | | | | |
| | | 2013 | | | 2012 | | | % Increase | |
United States | | $ | 1,834,807 | | | $ | 1,703,929 | | | | 8 | % |
Europe | | | 506,684 | | | | 477,681 | | | | 6 | % |
Elsewhere(1) | | | 660,722 | | | | 608,639 | | | | 9 | % |
| | | | | | | | | | | | |
Net product sales and services revenue | | $ | 3,002,213 | | | $ | 2,790,249 | | | | 8 | % |
| | | | | | | | | | | | |
| (1) | Includes, among many others, the following countries: China, Japan, India, South Korea, Australia, Canada, and Brazil. |
Net product sales and services revenue of $1.8 billion and $1.7 billion generated in the United States was approximately 61% of total net product sales and services revenue for each of the years ended December 31, 2013 and 2012. The growth in net product sales and services revenue in the United States was primarily due to an increase of $71.1 million in our domestic diabetes net product sales and services revenue, resulting primarily from our recent diabetes-related acquisitions discussed above. The growth in net product sales and services revenue in all geographic regions resulted primarily from the various acquisitions and organic growth, as discussed above.
48
License and Royalty Revenue. License and royalty revenue represents license and royalty fees from intellectual property license agreements with third parties. License and royalty revenue decreased by $1.3 million, or 5%, to $27.2 million in 2013, from $28.6 million in 2012. Included in royalty revenues in 2013 was an $8.5 million one-time, up-front issuance fee associated with the license of certain of our molecular intellectual property, compared with an $11.0 million one-time, up-front issuance fee during 2012. We expect to receive ongoing royalty payments under these license agreements in future periods.
Gross Profit and Margin Percentage. Gross profit increased by $73.4 million, or 5%, to $1.5 billion in 2013, from $1.4 billion in 2012. The increase in gross profit during 2013 was largely attributed to the increase in net product sales and services revenue resulting from acquisitions. Cost of net revenue during 2013 and 2012 included amortization of $2.5 million and $4.7 million, respectively, relating to the write up of inventory to fair value in connection with certain acquisitions. Reducing gross profit for 2013 and 2012 was $7.9 million and $3.1 million, respectively, in restructuring charges.
Cost of net revenue included amortization expense of $72.2 million and $72.3 million for 2013 and 2012, respectively.
Overall gross margin percentage was 50% in 2013, compared to 51% in 2012. The decrease in gross margin principally reflects the impact of the reduction in diabetes reimbursement rates that took effect in July 2013, as well as the increased costs of manufacturing certain of our meter-based Triage products.
Gross Profit from Net Product Sales and Services Revenue, Total and by Business Segment. Gross profit from net product sales and services revenue increased by $75.2 million, or 5%, to $1.5 billion in 2013, from $1.4 billion in 2012. Gross profit from net product sales and services revenue by business segment for 2013 and 2012 is as follows (in thousands):
| | | | | | | | | | | | |
| | | 2013 | | | 2012 | | | % Increase | |
Professional diagnostics | | $ | 1,224,355 | | | $ | 1,151,325 | | | | 6 | % |
Health information solutions | | | 237,599 | | | | 236,470 | | | | — | % |
Consumer diagnostics | | | 20,319 | | | | 19,305 | | | | 5 | % |
| | | | | | | | | | | | |
Gross profit from net product sales and services revenue | | $ | 1,482,273 | | | $ | 1,407,100 | | | | 5 | % |
| | | | | | | | | | | | |
Professional Diagnostics
Gross profit from our professional diagnostics net product sales and services revenue increased by $73.0 million, or 6%, to $1.22 billion during 2013, from $1.15 billion in 2012, principally as a result of gross profit earned on revenue from acquired businesses, as discussed above. Comparing 2013 to 2012, gross profit was negatively impacted by the lower volume of our U.S. meter-based Triage product sales and a reduction in commercial pricing for our toxicology products in our pain management and addiction medicine lines of business, as discussed above. The continued impact of the FDA inspection of our San Diego facility and the related recall of certain of our meter-based Triage products also resulted in increased incremental costs during 2013, principally due to unfavorable manufacturing variances. Cost of professional diagnostics net product sales and services revenue during 2013 and 2012 included a non-cash charge of $2.5 million and $4.7 million, respectively, relating to the write-up of inventory to fair value in connection with our acquisition of Epocal, Inc., or Epocal. Reducing gross profit for 2013 and 2012 was $6.0 million and $2.3 million, respectively, in restructuring charges.
Cost of professional diagnostics net product sales and services revenue included amortization expense of $64.3 million and $64.4 million for 2013 and 2012, respectively.
49
As a percentage of our professional diagnostics net product sales and services revenue, gross profit from our professional diagnostics business was 52% in 2013, compared to 53% in 2012. The continued impact of the FDA inspection and the related product recall, discussed above, which resulted in increased incremental costs during 2013, contributed to the lower gross profit.
Health Information Solutions
Gross profit from our health information solutions net product sales and services revenue remained relatively flat at $237.6 million during 2013, as compared to $236.5 million in 2012. Reducing gross profit for 2013 and 2012 was $1.8 million and $0.8 million, respectively, in restructuring charges.
Cost of health information solutions net product sales and services revenue included amortization expense of $6.9 million and $6.7 million for 2013 and 2012, respectively.
As a percentage of our health information solutions net product sales and services revenue, gross profit from our health information solutions business was 45% in 2013, compared to 44% in 2012. The 1% increase in gross margin was primarily due to operational efficiencies realized during 2013 within the condition and case management business.
Consumer Diagnostics
Gross profit from our consumer diagnostics net product sales and services revenue increased $1.0 million, or 5%, to $20.3 million during 2013, from $19.3 million in 2012. The increase in gross profit was primarily the result of an increase in manufacturing revenue, as discussed above, and a $0.7 million charge related to our manufacturing agreement with SPD recorded during 2012.
Cost of consumer diagnostics net product sales and services revenue included amortization expense of $0.9 million and $1.2 million for 2013 and 2012, respectively.
As a percentage of our consumer diagnostics net product sales and services revenue, gross profit from our consumer diagnostics business was 20% for 2013, compared to 22% in 2012.
Research and Development Expense. Research and development expense decreased by $22.2 million, or 12%, to $160.8 million in 2013, from $183.0 million in 2012. Research and development expense during 2013 is reported net of grant funding of $6.6 million arising from the research and development funding relationship with the Bill and Melinda Gates Foundation that we entered into in February 2013. Restructuring charges associated with our various restructuring plans to integrate our newly-acquired businesses totaling $1.8 million and $1.3 million were included in research and development expense during 2013 and 2012, respectively. Amortization expense of $4.9 million and $26.9 million was included in research and development expense for 2013 and 2012, respectively. Included in the $26.9 million of amortization expense for 2012 was $19.2 million related to the write off of certain in-process research and development projects recorded in connection with the Axis-Shield acquisition during the fourth quarter of 2011 which were discontinued in 2012.
Research and development expense as a percentage of net revenue was 5% and 6% for 2013 and 2012, respectively.
Sales and Marketing Expense. Sales and marketing expense decreased by $3.6 million, or 1%, to $639.8 million in 2013, from $643.4 million in 2012. The decrease in sales and marketing expense was primarily driven by lower amortization expense during 2013, compared to 2012. Amortization expense of $228.9 million and $239.9 million was included in sales and marketing expense for 2013 and 2012, respectively. Restructuring charges associated with our various restructuring plans to integrate our newly-acquired businesses totaling $2.8 million and $2.5 million were included in sales and marketing expense during 2013 and 2012, respectively.
50
Sales and marketing expense as a percentage of net revenue was 21% and 23% for 2013 and 2012, respectively.
General and Administrative Expense. General and administrative expense increased by $68.5 million, or 14%, to $561.2 million in 2013, from $492.8 million in 2012. The increase in general and administrative expense from 2012 to 2013 was primarily attributable to a $24.6 million increase in expense related to fair value adjustments to acquisition-related contingent consideration obligations, a $4.6 million increase in amortization expense, and a $1.5 million increase in restructuring plans to integrate our newly-acquired businesses, as well as the inclusion in general and administrative expense for 2013 of $7.5 million in excise tax expense related to the domestic sale of our medical device products as a result of the 2.3% excise tax that went into effect January 1, 2013, $5.5 million of costs associated with the conduct of a contested proxy solicitation in 2013 and $6.1 million of costs associated with potential business dispositions, which were partially offset in 2013 by a $6.6 million decrease in acquisition-related costs in 2013.
General and administrative expense as a percentage of net revenue was 19% and 17% for 2013 and 2012, respectively.
Interest Expense. Interest expense includes interest charges and the amortization of deferred financing costs and original issue discounts associated with certain debt issuances. Interest expense increased by $15.1 million, or 6%, to $255.7 million in 2013, from $240.6 million in 2012. The increase is principally due to a $35.6 million loss recorded in connection with the repurchase of our 9% senior subordinated notes during 2013, which was partially offset by the effect of lower interest rates associated with our 6.5% senior subordinated notes and our 7.25% senior notes, issued in May 2013 and December 2012, respectively, compared to the higher interest rates associated with our 7.875% senior notes, which we redeemed in December 2012 and February 2013, and our 9% senior subordinated notes, which we redeemed in May and June 2013.
Interest expense in 2012 includes approximately $23.2 million of expense associated with the repurchase of substantially all of our 7.875% senior notes.
Other Income (Expense), Net. Other income (expense), net includes interest income, realized and unrealized foreign exchange losses and other income and expense. The components and the respective amounts of other income (expense), net are summarized as follows (in thousands):
| | | | | | | | | | | | |
| | | 2013 | | | 2012 | | | Increase/
(Decrease) | |
Interest income | | $ | 3,262 | | | $ | 2,028 | | | $ | 1,234 | |
Foreign exchange gain (losses), net | | | (4,016 | ) | | | (7,876 | ) | | | 3,860 | |
Other | | | (12,374 | ) | | | 15,805 | | | | (28,179 | ) |
| | | | | | | | | | | | |
Total other income (expense), net | | $ | (13,128 | ) | | $ | 9,957 | | | $ | (23,085 | ) |
| | | | | | | | | | | | |
Other expense of $12.4 million for 2013 is primarily comprised of $11.8 million of expense associated with various legal settlements, which includes a provision of $9.5 million to reflect our current estimate of the settlement and litigation costs we may incur in connection with an ongoing dispute with a customer in our U.S. toxicology business, a $5.1 million write-off of an investment and $3.3 million in losses on disposals of fixed assets, with an offsetting $8.0 million bargain purchase gain relating to our acquisition of the Liberty business.
Other income of $15.8 million for 2012 included $15.5 million of prior period royalty settlements, which included a $13.5 million final royalty termination payment received from Quidel, a net $4.2 million gain recorded on the disposal of property, plant and equipment and $1.4 million of income associated with legal settlements related to intellectual property litigation. Partially offsetting the impact of these events was the settlement of a prior year dispute with a former distributor totaling approximately $3.9 million.
51
Benefit for Income Taxes. Benefit for income taxes increased by $16.0 million to a $46.3 million benefit in 2013, from a $30.3 million benefit in 2012. The effective tax rate in 2013 was 35%, compared to 25% in 2012. The increase in the benefit for income taxes, and the corresponding effective tax rate, from 2012 to 2013 is primarily related to tax rate changes in foreign jurisdictions, U.S. research credits for 2012 and 2013, the U.S. manufacturing deduction, and the impact of the bargain purchase gain. Partially offsetting these increased benefits are contingent consideration losses not deductible for tax purposes and U.S. tax on foreign income from distributions during 2013.
The primary components of the 2013 benefit for income taxes relate to U.S. federal and state income tax benefits, including tax rate changes in foreign jurisdictions, U.S. research credits for 2012 and 2013, the U.S. manufacturing deduction, and the impact of the bargain purchase gain. These benefits are largely offset by increased provisions for changes in valuation allowances, contingent consideration losses not deductible for tax purposes, U.S. tax on foreign income from distributions during 2013, and increases in reserves for uncertain tax positions. The primary components of the 2012 benefit for income taxes relate to U.S. federal and state income tax benefits, including favorable adjustments for revaluation on contingent consideration, offset by tax provisions on foreign income, increases in certain valuation allowances, increase in reserve for uncertain tax positions, and increase for other permanent adjustments.
Equity Earnings of Unconsolidated Entities, Net of Tax. Equity earnings of unconsolidated entities are reported net of tax and include our share of earnings in entities that we account for under the equity method of accounting. Equity earnings of unconsolidated entities, net of tax, for 2013 primarily reflect the following: (i) earnings from our 50% interest in SPD in the amount of $15.0 million, (ii) earnings from our 40% interest in Vedalab S.A., or Vedalab, in the amount of $0.6 million and (iii) earnings from our 49% interest in TechLab, Inc., or TechLab, in the amount of $2.0 million. Equity earnings of unconsolidated entities, net of tax, for 2012 primarily reflect the following: (i) earnings from our 50% interest in SPD in the amount of $10.7 million, (ii) earnings from our 40% interest in Vedalab, in the amount of $0.4 million and (iii) earnings from our 49% interest in TechLab, in the amount of $2.3 million.
Net Loss Available to Common Stockholders. For 2013, we generated a net loss available to common stockholders of $92.5 million, or $1.13 per basic and diluted common share, compared a net loss available to common stockholders of $99.5 million, or $1.23 per basic and diluted common share for 2012. Net loss available to common stockholders reflects $21.3 million of preferred stock dividends paid during both 2013 and 2012. The net loss in 2013 and 2012 resulted from the various factors discussed above. See Note 12 of our Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K for the calculation of net loss per common share.
Year Ended December 31, 2012 Compared to Year Ended December 31, 2011
Net Product Sales and Services Revenue. Net product sales and services revenue increased by $427.2 million, or 18%, to $2.8 billion in 2012, from $2.4 billion in 2011. Net product sales and services revenue increased primarily as a result of our acquisitions which contributed an aggregate of $447.6 million of the increase. Excluding the impact of foreign currency translation, net product sales and services revenue in 2012 grew by approximately $460.0 million, or 19%, over 2011.
Net Product Sales and Services Revenue by Business Segment. Net product sales and services revenue by business segment for 2012 and 2011 is as follows (in thousands):
| | | | | | | | | | | | |
| | | 2012 | | | 2011 | | | % Increase
(Decrease) | |
Professional diagnostics | | $ | 2,165,216 | | | $ | 1,736,172 | | | | 25 | % |
Health information solutions | | | 535,422 | | | | 534,514 | | | | — | % |
Consumer diagnostics | | | 89,611 | | | | 92,368 | | | | (3 | )% |
| | | | | | | | | | | | |
Net product sales and services revenue | | $ | 2,790,249 | | | $ | 2,363,054 | | | | 18 | % |
| | | | | | | | | | | | |
52
Professional Diagnostics
The following table summarizes our net product sales and services revenue from our professional diagnostics business segment by groups of similar products and services for 2012 and 2011 (in thousands):
| | | | | | | | | | | | |
| | | 2012 | | | 2011 | | | % Increase
(Decrease) | |
Infectious disease | | $ | 615,950 | | | $ | 564,983 | | | | 9 | % |
Toxicology | | | 587,261 | | | | 387,209 | | | | 52 | % |
Cardiology | | | 503,534 | | | | 518,746 | | | | (3 | )% |
Diabetes | | | 144,441 | | | | 14,960 | | | | 866 | % |
Other | | | 314,030 | | | | 250,274 | | | | 25 | % |
| | | | | | | | | | | | |
Professional diagnostics net product sales and services revenue | | $ | 2,165,216 | | | $ | 1,736,172 | | | | 25 | % |
| | | | | | | | | | | | |
Net product sales and services revenue from our professional diagnostics business segment increased by $429.0 million, or 25%, to $2.2 billion in 2012, from $1.7 billion in 2011. Excluding the impact of foreign currency translation, net product sales and services revenue from our professional diagnostics business segment increased by $462.2 million, or 27%, comparing 2012 to 2011. Net product sales and services revenue increased primarily as a result of acquisitions, which contributed an aggregate of $441.4 million of the non-currency-adjusted increase. Net product sales from our North American flu-related sales decreased approximately $2.5 million, from $46.1 in 2011 to $43.6 million 2012. Net product sales and services revenue from our professional diagnostics business segment were negatively impacted by the FDA recall matters related to our Alere Triage meter-based products. Net product sales of meter-based Triage products in the U.S. totaled $150.3 million in 2012, as compared to $199.2 million in 2011. Excluding the impact of acquisitions, the decrease in flu-related sales during the comparable periods and the impact of the reduction in net product sales from meter-based Triage products in the U.S., the currency-adjusted organic growth for our professional diagnostics net product sales and services revenue was approximately $73.7 million, or 5%, from 2011 to 2012.
Within our professional diagnostics business segment, net product sales and services revenue for our cardiology business decreased by approximately $15.2 million, or 3%, to $503.5 million in 2012, from $518.7 million in 2011, primarily as a result of the FDA recall matters related to our Alere Triage meter-based products. Net product sales of meter-based Triage products in the U.S. totaled $150.3 million in 2012, as compared to $199.2 million in 2011. The decrease in sales of our meter-based Triage products was partially offset by $19.3 million in sales contributed by the acquisition of Axis-Shield. Net product sales and services revenue for our infectious disease business increased by approximately $51.0 million, or 9%, to $616.0 million in 2012, from $565.0 million in 2011, with our acquisition of Axis-Shield contributing $27.4 million of such increase. Net product sales and services revenue for our toxicology business increased by approximately $200.1 million, or 52%, to $587.3 million in 2012, from $387.2 million in 2011, with our recent acquisitions of Avee, eScreen and Amedica Biotech, Inc., or Amedica, contributing approximately $193.9 million of the increase. Our diabetes net product sales and services revenue increased by approximately $129.5 million, or 866%, to $144.4 million in 2012, from $15.0 million in 2011, with our recent acquisitions contributing nearly all of the increase.
53
Health Information Solutions
The following table summarizes our net product sales and services revenue from our health information solutions business segment by groups of similar products and services for 2012 and 2011 (in thousands):
| | | | | | | | | | | | |
| | | 2012 | | | 2011 | | | % Increase
(Decrease) | |
Condition and case management | | $ | 218,378 | | | $ | 237,938 | | | | (8 | )% |
Wellness | | | 104,634 | | | | 104,868 | | | | — | % |
Women’s & children’s health | | | 120,259 | | | | 114,287 | | | | 5 | % |
Patient self-testing services | | | 92,151 | | | | 77,421 | | | | 19 | % |
| | | | | | | | | | | | |
Health information solutions net product sales and services revenue | | $ | 535,422 | | | $ | 534,514 | | | | — | % |
| | | | | | | | | | | | |
Net product sales and services revenue from our heath information solutions business segment increased by $0.9 million to $535.4 million in 2012, from $534.5 million in 2011. Within our health information solutions business segment, our condition and case management net product sales and services revenue decreased approximately $19.6 million, or 8%, to $218.4 million in 2012, compared to $237.9 million in 2011, principally due to the increasingly competitive environment, including pricing pressures, the impact of health plans insourcing these services, and state budget pressures. Our patient self-testing services net product sales and services revenue increased approximately $14.7 million, or 19%, to $92.2 million in 2012, compared to $77.4 million in 2011, principally driven by an increase in our home coagulation monitoring programs resulting from a larger patient population and a simultaneous reduction in customer attrition rates. Higher census levels as a result of increased physician referrals led to a 5% increase in revenue from our women’s and children’s health business.
Consumer Diagnostics
Net product sales and services revenue from our consumer diagnostics business segment decreased by $2.8 million, or 3%, to $89.6 million in 2012, from $92.4 million in 2011. Net product sales by SPD were $187.8 million and $209.2 million during 2012 and 2011, respectively, with the impact of foreign currency translation accounting for substantially all of the decrease.
Net Product Sales and Services Revenue by Geographic Location. Net product sales and services revenue by geographic location for 2012 and 2011 is as follows (in thousands):
| | | | | | | | | | | | |
| | | 2012 | | | 2011 | | | % Increase | |
United States | | $ | 1,703,929 | | | $ | 1,433,372 | | �� | | 19 | % |
Europe | | | 477,681 | | | | 393,285 | | | | 21 | % |
Elsewhere(1) | | | 608,639 | | | | 536,397 | | | | 13 | % |
| | | | | | | | | | | | |
Net product sales and services revenue | | $ | 2,790,249 | | | $ | 2,363,054 | | | | 18 | % |
| | | | | | | | | | | | |
| (1) | Includes, among many others, the following countries: China, Japan, India, South Korea, Australia, Canada, and Brazil. |
Net product sales and services revenue of $1.7 billion and $1.4 billion generated in the United States was approximately 61% of total net product sales and services revenue for 2012 and 2011. The growth in net product sales and services revenue in all geographic regions resulted primarily from the various acquisitions and organic growth, as discussed above.
54
License and Royalty Revenue. License and royalty revenue represents license and royalty fees from intellectual property license agreements with third parties. License and royalty revenue increased by $5.1 million, or 22%, to $28.6 million in 2012, from $23.5 million in 2011. During 2012, we received an up-front royalty payment of $11.0 million related to a license of certain of our molecular intellectual property. We also received additional license and royalty revenue during 2012 as a result of our acquisition of Axis-Shield, which contributed approximately $3.7 million of the increase. These increases during 2012 were offset by an amendment to our license agreement with Quidel during 2011 whereby the license agreement was converted to a fully paid-up license. As a result of the amendment, we did not record royalty revenue from Quidel during 2012, as opposed to $7.5 million of royalty revenue recorded from Quidel during 2011, and do not anticipate recording royalty revenue from Quidel in the future.
Gross Profit and Margin Percentage. Gross profit increased by $182.5 million, or 15%, to $1.4 billion in 2012, from $1.2 billion in 2011. The increase in gross profit during 2012 was largely attributed to the increase in net product sales and services revenue resulting from acquisitions. Cost of net revenue during 2012 and 2011 included amortization of $4.7 million and $6.0 million, respectively, relating to the write up of inventory to fair value in connection with certain acquisitions. Reducing gross profit for 2012 and 2011 was $3.1 million and $2.9 million, respectively, in restructuring charges.
Cost of net revenue included amortization expense of $72.3 million and $63.2 million for 2012 and 2011, respectively.
Overall gross margin percentage was 51% in 2012, compared to 52% in 2011.
Gross Profit from Net Product Sales and Services Revenue, Total and by Business Segment. Gross profit from net product sales and services revenue increased by $177.7 million, or 14%, to $1.4 billion in 2012, from $1.2 billion in 2011. Gross profit from net product sales and services revenue by business segment for 2012 and 2011 is as follows (in thousands):
| | | | | | | | | | | | |
| | | 2012 | | | 2011 | | | % Increase
(Decrease) | |
Professional diagnostics | | $ | 1,151,325 | | | $ | 964,034 | | | | 19 | % |
Health information solutions | | | 236,470 | | | | 245,753 | | | | (4 | )% |
Consumer diagnostics | | | 19,305 | | | | 19,611 | | | | (2 | )% |
| | | | | | | | | | | | |
Gross profit from net product sales and services revenue | | $ | 1,407,100 | | | $ | 1,229,398 | | | | 14 | % |
| | | | | | | | | | | | |
Professional Diagnostics
Gross profit from our professional diagnostics net product sales and services revenue increased by $187.3 million, or 19%, to $1.2 billion during 2012, from $964.0 million in 2011, principally as a result of gross profit earned on revenue from acquired businesses, as discussed above. Comparing 2012 to 2011, gross profit was negatively impacted by a decrease in our meter-based Triage product sales, as discussed above. The FDA recall relating to our meter-based Triage products also resulted in incremental costs during 2012 principally due to costs of refunds made during the period, replacement products issued at no cost, unfavorable manufacturing variances and the lost margin on the reduced volume of tests sold during 2012. Cost of professional diagnostics net product sales and services revenue during 2012 and 2011 included amortization of $4.7 million and $6.0 million, respectively, relating to the write-up of inventory to fair value in connection with certain acquisitions. Reducing gross profit for both 2012 and 2011 was $2.3 million in restructuring charges.
Cost of professional diagnostics net product sales and services revenue included amortization expense of $64.4 million and $55.1 million for 2012 and 2011, respectively.
55
As a percentage of our professional diagnostics net product sales and services revenue, gross profit from our professional diagnostics business was 53% in 2012, compared to 56% in 2011. Increased revenue from our recently acquired toxicology businesses, which contribute lower-than-segment-average gross margins, and a decrease in our meter-based Triage product sales, which contribute higher-than-segment-average gross margins, contributed to the decrease in gross margin for the respective periods.
Health Information Solutions
Gross profit from our health information solutions net product sales and services revenue decreased by $9.3 million, or 4%, to $236.5 million during 2012, from $245.8 million in 2011, primarily as a result of the increasingly competitive environment, including pricing pressures, the impact of health plans insourcing less differentiated services, such as condition and case management, and state budget pressures. Reducing gross profit for 2012 and 2011 was $0.8 million and $0.7 million, respectively, in restructuring charges.
Cost of health information solutions net product sales and services revenue included amortization expense of $6.7 million for both 2012 and 2011, respectively.
As a percentage of our health information solutions net product sales and services revenue, gross profit from our health information solutions business was 44% in 2012, compared to 46% in 2011. The lower margin percentage is primarily the result of the increasingly competitive environment, including pricing pressures, the impact of health plans insourcing less differentiated services, such as condition and case management, and state budget pressures.
Consumer Diagnostics
Gross profit from our consumer diagnostics net product sales and services revenue decreased $0.3 million, or 2%, to $19.3 million during 2012, from $19.6 million in 2011.
Cost of consumer diagnostics net product sales and services revenue included amortization expense of $1.2 million and $1.4 million for 2012 and 2011, respectively.
As a percentage of our consumer diagnostics net product sales and services revenue, gross profit from our consumer diagnostics business was 22% for 2012, compared to 21% in 2011.
Research and Development Expense. Research and development expense increased by $32.8 million, or 22%, to $183.0 million in 2012, from $150.2 million in 2011. Restructuring charges associated with our various restructuring plans to integrate our newly-acquired businesses totaling $1.3 million and $0.4 million were included in research and development expense during 2012 and 2011, respectively. Amortization expense of $26.9 million and $12.6 million was included in research and development expense for 2012 and 2011, respectively. Included in the $26.9 million of amortization expense for 2012 was $19.2 million related to the write off of certain in-process research and development projects recorded in connection with the Axis-Shield acquisition during the fourth quarter of 2011. Included in the $12.6 million of amortization expense for 2011 was $7.2 million related to the write off of certain in-process research and development projects recorded in connection with the Standard Diagnostics acquisition during the first quarter of 2010.
Research and development expense as a percentage of net revenue was 6% for both 2012 and 2011.
56
Sales and Marketing Expense. Sales and marketing expense increased by $77.8 million, or 14%, to $643.4 million in 2012, from $565.6 million in 2011. The increase in sales and marketing expense primarily relates to additional spending related to newly-acquired businesses. Amortization expense of $239.9 million and $220.9 million was included in sales and marketing expense for 2012 and 2011, respectively. Restructuring charges associated with our various restructuring plans to integrate our newly-acquired businesses totaling $2.5 million and $5.0 million were included in sales and marketing expense during 2012 and 2011, respectively.
Sales and marketing expense as a percentage of net revenue was 23% and 24% for 2012 and 2011, respectively.
General and Administrative Expense. General and administrative expense increased by $93.4 million, or 23%, to $492.8 million in 2012, from $399.3 million in 2011. The increase in general and administrative expense primarily relates to additional spending related to newly-acquired businesses. Acquisition-related costs of $9.7 million and $11.5 million were included in general and administrative expense for 2012 and 2011, respectively. Included in general and administrative expense for 2012 and 2011 was $6.6 million and $14.1 million, respectively, of income recorded in connection with fair value adjustments to acquisition-related contingent consideration obligations. Amortization expense of $8.0 million and $11.2 million was included in general and administrative expense for 2012 and 2011, respectively. Restructuring charges associated with our various restructuring plans to integrate our newly-acquired businesses totaling $13.4 million and $20.0 million were included in general and administrative expense during 2012 and 2011, respectively.
General and administrative expense as a percentage of net revenue was 17% for both 2012 and 2011.
Annual Goodwill Impairment Test. We conduct our annual goodwill impairment test during the fourth quarter of each year. During the fourth quarter of 2012, Step 1 of the impairment test, as prescribed by ASC 350,Intangibles — Goodwill and Other, or ASC 350, did not indicate that the carrying value of any of the assets exceeded the fair value of the applicable reporting unit and, accordingly, we did not record any goodwill impairment charges during 2012. Step 1 of the 2011 impairment test indicated that the carrying value of the net assets of our health information solutions reporting unit exceeded the estimated fair value of the reporting unit. As a result, we were required to complete Step 2 of the impairment test, as prescribed by ASC 350, to determine the amount of the goodwill impairment charge. The Step 2 portion of the test indicated that we needed to record a goodwill impairment charge of approximately $383.6 million, which was recorded during the fourth quarter of 2011. Further details of the goodwill impairment test are disclosed in Note 2 of our Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K.
Interest Expense. Interest expense includes interest charges and the amortization of deferred financing costs and original issue discounts associated with certain debt issuances. Interest expense increased by $36.6 million, or 18%, to $240.6 million in 2012, from $204.0 million in 2011. The increase is principally due to higher interest expense recorded in connection with higher outstanding debt balances and applicable interest rates in 2012, compared to the outstanding debt balances and applicable interest rates in 2011. Interest expense in 2012 includes approximately $23.2 million of expense associated with the repurchase of substantially all of our 7.875% senior notes. Interest expense for 2011 included interest expense and amortization of fees paid for certain debt modifications totaling $32.5 million recorded in connection with the termination of our former secured credit facility and related interest rate swap agreement in 2011.
57
Other Income (Expense), Net. Other income (expense), net includes interest income, realized and unrealized foreign exchange gains and losses and other income and expense. The components and the respective amounts of other income (expense), net are summarized as follows (in thousands):
| | | | | | | | | | | | |
| | | 2012 | | | 2011 | | | Increase/
(Decrease) | |
Interest income | | $ | 2,028 | | | $ | 2,570 | | | $ | (542 | ) |
Foreign exchange gains (losses), net | | | (7,876 | ) | | | (22,870 | ) | | | 14,994 | |
Other | | | 15,805 | | | | 22,183 | | | | (6,378 | ) |
| | | | | | | | | | | | |
Other income (expense), net | | $ | 9,957 | | | $ | 1,883 | | | $ | 8,074 | |
| | | | | | | | | | | | |
The increase in foreign exchange gains (losses), net for 2012, compared to 2011, was primarily the result of a $12.7 million realized foreign currency loss associated with a cash balance established in connection with the Axis-Shield acquisition in 2011 and a $1.9 million realized foreign currency loss associated with the settlement of an acquisition-related contingent consideration obligation during 2011.
Other income of $15.8 million for 2012 included $15.5 million of prior period royalty settlements, which included a $13.5 million final royalty termination payment received from Quidel, a net $4.2 million gain recorded on the disposal of property, plant and equipment and $1.4 million of income associated with legal settlements related to intellectual property litigation. Partially offsetting the impact of these events was the settlement of a prior year dispute with a former distributor totaling approximately $3.9 million.
Other income of $22.2 million for 2011 includes $13.8 million of income associated with an amendment of our license agreement with Quidel, which also includes a settlement of prior period royalties, $5.0 million of income associated with the settlement of a dispute over certain intellectual property rights, a $4.8 million reversal of a prior period legal settlement reserve no longer deemed necessary, partially offset by approximately $1.6 million of losses recorded on disposal of fixed assets.
Gain on Sale of Joint Venture Interest. In connection with the formation of SPD in May 2007, we entered into an option agreement with P&G, pursuant to which P&G had the right, for a period of 60 days commencing on May 17, 2011, to require us to acquire all of P&G’s interest in SPD at fair market value. No gain on the proceeds that we received from P&G through the formation of SPD was recognized in our financial statements until P&G’s option to require us to purchase its interest in SPD expired. On July 16, 2011, P&G’s option to require us to acquire its interest in SPD at fair market value expired. In connection with the expiration of the option, the gain totaling approximately $288.9 million was recognized during the third quarter of 2011.
Benefit for Income Taxes. Benefit for income taxes increased by $6.1 million, to a $30.3 million benefit in 2012, from a $24.2 million benefit in 2011. The effective tax rate in 2012 was 25%, compared to 15% in 2011. The increase in the benefit for income taxes, and the corresponding effective tax rate, from 2011 to 2012, is primarily related to the rate differential on foreign earnings and the recognition of a tax benefit associated with the goodwill impairment charge recorded during 2011.
The primary components of the 2012 benefit for income taxes relate to U.S. federal and state income tax benefits, including favorable adjustments for revaluation on contingent consideration, offset by tax provisions on foreign income, increases in certain valuation allowances, increase in reserve for uncertain tax positions, and increase for other permanent adjustments. The primary components of the 2011 benefit for income taxes relate to U.S. federal and state income tax benefits and the tax benefit on foreign income, offset by losses related to the 2011 goodwill impairment that were not tax benefitted.
58
Equity Earnings in Unconsolidated Entities, Net of Tax. Equity earnings in unconsolidated entities are reported net of tax and include our share of earnings in entities that we account for under the equity method of accounting. Equity earnings in unconsolidated entities, net of tax, for 2012 primarily reflect the following: (i) earnings from our 50% interest in SPD in the amount of $10.7 million, (ii) earnings from our 40% interest in Vedalab S.A., or Vedalab, in the amount of $0.4 million and (iii) earnings from our 49% interest in TechLab, Inc., or TechLab, in the amount of $2.3 million. Equity earnings in unconsolidated entities, net of tax, for 2011 primarily reflect the following: (i) earnings from our 50% interest in SPD in the amount of $5.9 million, (ii) earnings from our 40% interest in Vedalab, in the amount of $0.7 million and (iii) earnings from our 49% interest in TechLab, in the amount of $2.0 million.
Net Loss. For 2012, we generated a net loss available to common stockholders of $99.5 million, or $1.23 per basic and diluted common share, compared a net loss available to common stockholders of $131.7 million, or $1.58 per basic and diluted common share for 2011. Net loss available to common stockholders reflects $21.3 million and $22.0 million of preferred stock dividends paid during 2012 and 2011, respectively, and $23.9 million of income associated with the repurchase of preferred stock during 2011. The net loss in 2012 and 2011 resulted from the various factors discussed above. See Note 12 of our Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K for the calculation of net loss per common share.
Liquidity and Capital Resources
Based upon our current working capital position, current operating plans and expected business conditions, we expect to fund our short- and long-term working capital needs primarily using existing cash and our operating cash flow, and we expect our working capital position to improve as we improve our future operating margins and grow our business through new product and service offerings and by continuing to leverage our strong intellectual property position. As of December 31, 2013, we had $361.9 million of cash and cash equivalents, of which $124.1 million was held by domestic subsidiaries and $237.8 million was held by foreign entities. We generally do not plan to repatriate cash held by foreign entities in the ordinary course of business due to adverse tax implications, including incremental U.S. tax liabilities and potential foreign withholding tax liabilities.
We may also utilize our secured credit facility or other new sources of financing to fund a portion of our capital needs and other commitments, including our contractual contingent consideration obligations and any future acquisitions. As of December 31, 2013, we had outstanding borrowings totaling $170.0 million under the $250.0 million revolving line of credit under our secured credit facility, leaving $80.0 million available to us for additional borrowings. Our ability to access the capital markets may be impacted by the amount of our outstanding debt and equity and the extent to which our assets are encumbered by our outstanding secured debt. The terms and conditions of our outstanding debt instruments also contain covenants which expressly restrict our ability to incur additional indebtedness and conduct other financings. As of December 31, 2013, we had $3.8 billion in outstanding indebtedness, which included $2.3 billion under our secured credit facility, including borrowings under our revolving line of credit, $450.0 million of 7.25% senior notes due 2018, $400.0 million of 8.625% senior subordinated notes due 2018, $425.0 million of 6.5% senior subordinated notes due 2020, and $150.0 million of 3% convertible senior subordinated notes due 2016. In February 2013, we redeemed the remaining $1.8 million outstanding principal amount of our 7.875% senior notes pursuant to our optional redemption right under the indenture under which the 7.875% senior notes were issued. In May 2013, we used $200.6 million of the net proceeds of our sale of our 6.5% senior subordinated notes to purchase $190.6 million outstanding principal amount of our 9% senior subordinated notes and in June 2013, we redeemed the remaining $209.4 million outstanding principal amount of our 9% senior subordinated notes. See Note 6 of our Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K for further information about our outstanding debt balances.
If the capital and credit markets experience volatility or the availability of funds is limited, we may incur increased costs associated with issuing debt instruments. In addition, it is possible that our ability
59
to access the capital and credit markets could be limited by these or other factors at a time when we would like, or need, to do so, which could have an adverse impact on our ability to refinance maturing debt and/or react to changing economic and business conditions.
Our funding plans for our working capital needs and other commitments may be adversely impacted by unexpected costs associated with integrating the operations of newly-acquired companies, executing our cost-savings strategies and prosecuting and defending our existing lawsuits and/or unforeseen lawsuits against us. We also cannot be certain that our underlying assumed levels of revenues and expenses will be realized. In addition, we intend to continue to make investments in our research and development efforts related to the substantial intellectual property portfolio we own. We may also choose to further expand our research and development efforts and may pursue the acquisition of new products and technologies through licensing arrangements, business acquisitions, or otherwise. We may also choose to make significant investment to pursue legal remedies against potential infringers of our intellectual property rights. If we decide to engage in such activities, or if our operating results fail to meet our expectations, we could be required to seek additional funding through public or private financings or other arrangements. In such event, adequate funds may not be available when needed or may be available only on terms which could have a negative impact on our business and results of operations. In addition, if we raise additional funds by issuing equity or convertible securities, dilution to then- existing stockholders may result.
Cash Flow Summary
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2013 | | | 2012 | | | 2011 | |
Net cash provided by operating activities | | $ | 244,777 | | | $ | 319,683 | | | $ | 271,253 | |
Net cash used in investing activities | | | (258,335 | ) | | | (574,191 | ) | | | (898,196 | ) |
Net cash provided by financing activities | | | 48,991 | | | | 285,745 | | | | 540,080 | |
Foreign exchange effect on cash and cash equivalents | | | (1,871 | ) | | | (2,064 | ) | | | (15,270 | ) |
| | | | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | | | 33,562 | | | | 29,173 | | | | (102,133 | ) |
Cash and cash equivalents, beginning of period | | | 328,346 | | | | 299,173 | | | | 401,306 | |
| | | | | | | | | | | | |
Cash and cash equivalents, end of period | | $ | 361,908 | | | $ | 328,346 | | | $ | 299,173 | |
| | | | | | | | | | | | |
Summary of Changes in Cash Position
Cash and cash equivalents increased $33.6 million during 2013, compared to an increase of $29.2 million during 2012. Our primary sources of cash during 2013 included $244.8 million generated by our operating activities, $460.0 million of net proceeds received in connection with long-term debt issuances, which included $425.0 million of gross proceeds received in connection with the issuance of our 6.5% senior subordinated notes, $139.0 million of net proceeds under various revolving credit facilities, which included $190.0 million borrowed, net of $42.5 million paid, against our secured credit facility revolving line-of-credit, $29.3 million of return of capital related to equity investments, $29.0 million received from the disposition of our Spinreact operations, $20.9 million of cash received from common stock issuances under employee stock option and stock purchase plans, a $10.4 million decrease related to other assets and $3.6 million in proceeds from the sale of property and equipment. Our primary uses of cash during 2013 included $471.5 million of cash payments on long-term debt, which included $400.0 million of cash payments related to the repurchase of our 9% senior subordinated notes, $176.1 million net cash paid for acquisitions, $122.2 million of capital expenditures, $42.4 million related to payments of acquisition-related contingent consideration obligations, $32.4 million related to an increase in restricted cash, $21.3 million for cash dividends paid
60
on our Series B preferred stock, $19.0 million related to tender offer consideration and call premium incurred in connection with the repurchase of our 9% senior subordinated notes, $9.8 million related to the payment of debt-related financing costs and $7.1 million for payment of capital lease obligations. Fluctuations in foreign currencies negatively impacted our cash balance by $1.9 million during 2013.
Cash and cash equivalents increased $29.2 million during 2012, compared to a decrease of $102.1 million during 2011. Our primary sources of cash during 2012 included $319.7 million generated by our operating activities, $443.2 million of net proceeds received in connection with the issuance of our 7.25% senior notes, $198.0 million of net proceeds received in connection with the “Incremental B-2” term loans under our secured credit facility, $22.4 million in proceeds from the sale of property, plant and equipment, $14.9 million from common stock issuances under employee stock option and stock purchase plans, $14.3 million of net proceeds under various revolving credit facilities, $12.7 million return of capital related to equity investments and $3.1 million from sales of marketable securities. Our primary uses of cash during 2012 included $424.6 million net cash paid for acquisitions, $311.6 million related to cash payments on long-term debt, $137.4 million of capital expenditures, $56.3 million related to an increase in other assets, $21.3 million for cash dividends paid on our Series B preferred stock, $21.0 million related to payments of acquisition-related contingent consideration obligations, $12.3 million related to a make-whole payment incurred in connection with the repurchase of our 7.875% senior notes, $7.0 million for payment of capital lease obligations and $6.2 million related to the repayment of short-term debt. Fluctuations in foreign currencies negatively impacted our cash balance by $2.1 million during 2012.
Cash and cash equivalents decreased $102.1 million during 2011, compared to a decrease of $91.5 million during 2010. Our primary sources of cash during 2011 included $271.3 million generated by our operating activities, $853.3 million of net proceeds received in connection with refinancing of our credit facilities, which included $2.1 billion of cash received in connection with entering into our new secured credit facility in June 2011, partially offset by $1.3 billion of cash payments related to the termination and repayment of our former secured credit facility and related interest rate swap agreement, $37.9 million received from common stock issuances under employee stock option and stock purchase plans, $11.5 million received from the disposition of a business and $9.2 million from sales of marketable securities. Our primary uses of cash during 2011 included $631.3 million net cash paid for acquisitions, $283.9 million related to the repurchase of our preferred and common stock, $131.6 million of capital expenditures, net of proceeds from the sale of equipment, $121.9 million net cash paid for equity method investments, which includes approximately $41.2 million paid for shares of Axis-Shield prior to our completion of the acquisition of the remaining shares outstanding, $28.3 million related to payments of acquisition-related contingent consideration obligations, $27.7 million related to an increase in other assets, which includes purchases of various licensing agreements totaling $33.0 million, $6.4 million related to an increase in restricted cash and $5.4 million for cash dividends paid on our Series B preferred stock. Fluctuations in foreign currencies negatively impacted our cash balance by $15.3 million during 2011.
Cash Flows from Operating Activities
Net cash provided by operating activities during 2013 was $244.8 million, which resulted from a net loss of $70.3 million and $99.6 million of cash used to meet net working capital requirements during the year, offset by $414.7 million of non-cash items. The $414.7 million of non-cash items included, among other items, $439.4 million related to depreciation and amortization, $35.6 million related to a loss on extinguishment of debt, $21.2 million related to non-cash stock-based compensation, $18.1 million of interest expense related to the amortization of deferred financing costs and original issue discounts, $8.6 million related to other non-cash items, $6.3 million related to the impairment of long-lived assets, $5.1 million loss from the disposition of our Spinreact operations, $3.3 million related to the impairment of intangible assets, a $3.3 million loss on the sale of fixed assets and a $2.5 million non-cash charge related to the write up of inventory to fair value in connection with the acquisition of Epocal, partially offset by a $103.6 million decrease related to changes in our deferred tax assets and
61
liabilities, which resulted in part from amortization of intangible assets, $17.4 million in equity earnings of unconsolidated entities, net of tax, and $8.0 million relating to a bargain purchase gain in connection with our acquisition of the Liberty business.
Net cash provided by operating activities during 2012 was $319.7 million, which resulted from a net loss from continuing operations of $77.9 million and $37.7 million of cash used to meet net working capital requirements during the period, offset by $435.3 million of non-cash items. The $435.3 million of non-cash items included, among other items, $456.8 million related to depreciation and amortization, a $30.6 million increase related to other non-cash items, $21.5 million of interest expense related to the amortization of deferred financing costs and original issue discounts, $15.7 million related to non-cash stock-based compensation, a $4.7 million non-cash charge related to the write-up of inventory to fair value in connection with the acquisition of Axis-Shield, $3.5 million related to the impairment of long-lived assets and $3.0 million related to the impairment of intangible assets, partially offset by a $84.6 million decrease related to changes in our deferred tax assets and liabilities, which resulted in part from amortization of intangible assets, and $13.2 million in equity earnings in unconsolidated entities.
Net cash provided by operating activities during 2011 was $271.3 million, which resulted from a net loss of $133.3 million and $74.7 million of cash used to meet net working capital requirements, offset by $479.2 million of non-cash items. The $479.2 million of non-cash items included, among other items, $391.6 million related to depreciation and amortization, a $383.6 million goodwill impairment charge related to our health management reporting unit and business segment, $37.6 million of interest expense related to the amortization of deferred financing costs and original issue discounts, $21.2 million related to non-cash stock-based compensation, a $6.0 million non-cash charge related to the write-up of inventory to fair value in connection with the acquisition of Axis-Shield and $2.9 million related to the impairment of intangible assets, partially offset by a $288.9 million gain associated with the creation of our joint venture interest in SPD, a $56.8 million decrease related to changes in our deferred tax assets and liabilities, which partially resulted from amortization of intangible assets, a $12.2 million decrease related to other non-cash items and $8.5 million in equity earnings in unconsolidated entities.
Cash Flows from Investing Activities
Our investing activities during 2013 utilized $258.3 million of cash, including $176.1 million net cash paid for acquisitions, $122.2 million of capital expenditures and an increase in our restricted cash balance of $32.4 million, which was principally driven by a $29.4 million deposit in connection with a foreign bank loan arrangement and $7.9 million of cash received from the Bill and Melinda Gates Foundation, of which $5.7 million was used to fund qualified expenditures, partially offset by a $29.3 million return of capital related to equity investments, $29.0 million in proceeds relating to the disposition of our Spinreact operations, a $10.4 million decrease in other assets and $3.6 million of proceeds from the sale of property and equipment.
Our investing activities during 2012 utilized $574.2 million of cash, including $424.6 million net cash paid for acquisitions, $137.4 million of capital expenditures and $56.3 million related to an increase in other assets, which includes a $46.0 million note receivable and purchases of various licensing agreements totaling approximately $4.3 million, partially offset by $22.4 million of proceeds from the sale of property, plant and equipment, a $12.7 million return of capital from equity investments, which included an $11.2 million return of capital from SPD, a $5.9 million decrease in our restricted cash balance and $3.1 million from sales of marketable securities.
Our investing activities during 2011 utilized $898.2 million of cash, including $631.3 million net cash paid for acquisitions, $131.6 million of capital expenditures, net of proceeds from the sale of equipment, $121.9 million net cash paid for equity method investments, which includes approximately $41.2 million paid for shares of Axis-Shield prior to our completion of the acquisition of the remaining shares outstanding, $27.7 million related to an increase in other assets, which includes purchases of
62
various licensing agreements totaling $33.0 million, an increase in our restricted cash balance of approximately $6.4 million, partially offset by $11.5 million received from the disposition of a business and $9.2 million received from sales of marketable securities.
Cash Flows from Financing Activities
Net cash provided by financing activities during 2013 was $49.0 million. Financing activities during 2013 primarily included $460.0 million of net proceeds received in connection with long-term debt issuances, which included $425.0 million of gross proceeds received in connection with the issuance of our 6.5% senior subordinated notes, $139.0 million of net proceeds under various revolving credit facilities, which included $190.0 million borrowed, net of $42.5 million paid, against our secured credit facility revolving line-of-credit, and $20.9 million of cash received from common stock issuances under employee stock option and stock purchase plans. In addition, we utilized $471.5 million of cash payments on long-term debt, which included $400.0 million of cash payments related to the repurchase of our 9% senior subordinated notes, $42.4 million for payments of acquisition-related contingent consideration obligations, $21.3 million for dividend payments related to our Series B preferred stock, $19.0 million related to tender offer consideration and call premium incurred in connection with the repurchase of our 9% senior subordinated notes, $9.8 million related to the payment of debt-related financing costs and $7.1 million for payment of capital lease obligations.
Net cash provided by financing activities during 2012 was $285.7 million. Financing activities during 2012 primarily included $648.5 million of net proceeds received in connection with long-term debt issuances, which included $443.2 million of net proceeds received in connection with the issuance of our 7.25% senior notes and $198.0 million of net proceeds received in connection with the “Incremental B-2” term loans entered under our secured credit facility, $14.9 million of cash received from common stock issuances under employee stock option and stock purchase plans and $14.3 million of net proceeds under various revolving credit facilities, which included $22.5 million borrowed against our secured credit facility revolving line-of-credit. The $443.2 million received in connection with the issuance of the 7.25% senior notes was offset by $267.4 million of cash payments related to repurchases of our 7.875% senior notes and $170.0 million used to pay down a portion of the outstanding balance under our revolving line-of-credit. In addition, we utilized $21.3 million for dividend payments related to our Series B preferred stock, $21.0 million for payments of acquisition-related contingent consideration obligations, $12.3 million related to a make-whole payment incurred in connection with the repurchase of our 7.875% senior notes, $10.1 million related to the payment of debt-related financing costs, $7.0 million for payment of capital lease obligations and $6.2 million related to the repayment of short-term debt obligations.
Net cash provided by financing activities during 2011 was $540.1 million. Financing activities during 2011 primarily included $2.1 billion of cash received in connection with entering into our new secured credit facility in June 2011 and $37.9 million of cash received from common stock issuances under employee stock option and stock purchase plans. The $2.1 billion received in connection with the secured credit facility was partially offset by $1.3 billion of cash payments related to the termination and repayment of our former secured credit facility and related interest rate swap agreement. We utilized approximately $283.9 million of cash to repurchase shares of our preferred and common stock, $28.3 million for payments of acquisition-related contingent consideration obligations, $5.4 million for dividend payments related to our Series B preferred stock and $4.2 million of principal payments on capital lease obligations.
As of December 31, 2013, we had an aggregate of $21.3 million in outstanding capital lease obligations which are payable through 2019.
63
Income Taxes
As of December 31, 2013, we had approximately $87.4 million of domestic NOL and domestic capital loss carryforwards, approximately $998.8 million of state NOL carryforwards and $254.7 million of foreign NOL and foreign capital loss carryforwards, which either expire on various dates through 2033 or can be carried forward indefinitely. As of December 31, 2013, we had approximately $70.4 million of domestic research and development, foreign tax and alternative minimum tax credits which either expire on various dates through 2033 or can be carried forward indefinitely. These loss carryforwards and tax credits may be available to reduce future federal, state and foreign taxable income, if any, and are subject to review and possible adjustment by the appropriate tax authorities. Effective January 1, 2009, we adopted a new accounting standard for business combinations. Prior to adoption of this standard, the pre-acquisition losses were applied first to reduce to zero any goodwill and other non-current intangible assets related to the acquisitions, prior to reducing our income tax expense. Upon adoption of the new accounting standard, the reduction of a valuation allowance is generally recorded to reduce our income tax expense.
Furthermore, all domestic losses and credits are subject to the Internal Revenue Service Code Sections 382 and 383 limitations, respectively, and may be limited in the event of certain cumulative changes in ownership interests of significant shareholders over a three-year period in excess of 50%. Sections 382 and 383 impose an annual limitation on the use of these losses or credits to an amount equal to the value of the company at the time of the ownership change multiplied by the long-term tax exempt rate. We have recorded a valuation allowance against a portion of the deferred tax assets related to our NOLs and credits and certain of our other deferred tax assets to reflect uncertainties that might affect the realization of such deferred tax assets, as these assets can only be realized via profitable operations.
Off-Balance Sheet Arrangements
We had no material off-balance sheet arrangements as of December 31, 2013.
Contractual Obligations
The following table summarizes our principal contractual obligations as of December 31, 2013 (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | | Payments Due by Period | |
Contractual Obligations | | Total | | | 2014 | | | 2015-2016 | | | 2017-2018 | | | Thereafter | |
Long-term debt obligations(1) | | $ | 3,823,350 | | | $ | 49,112 | | | $ | 1,186,911 | | | $ | 2,156,955 | | | $ | 430,372 | |
Capital lease obligations(2) | | | 21,262 | | | | 6,855 | | | | 10,036 | | | | 3,156 | | | | 1,215 | |
Operating lease obligations(3) | | | 231,213 | | | | 47,611 | | | | 77,160 | | | | 60,699 | | | | 45,743 | |
Pension obligations | | | 6,700 | | | | 957 | | | | 1,914 | | | | 1,914 | | | | 1,915 | |
Acquisition-related obligations(4) | | | 19,307 | | | | 5,741 | | | | 13,269 | | | | 297 | | | | — | |
Purchase obligations — capital expenditure | | | 14,798 | | | | 14,798 | | | | — | | | | — | | | | — | |
Purchase obligations — other(5) | | | 63,871 | | | | 61,652 | | | | 2,219 | | | | — | | | | — | |
Interest on debt(6) | | | 514,153 | | | | 100,635 | | | | 197,534 | | | | 174,547 | | | | 41,437 | |
Contingent consideration obligations(7) | | | 213,969 | | | | 96,747 | | | | 77,377 | | | | 34,601 | | | | 5,244 | |
| | | | | | | | | | | | | | | | | | | | |
Total | | $ | 4,908,623 | | | $ | 384,108 | | | $ | 1,566,420 | | | $ | 2,432,169 | | | $ | 525,926 | |
| | | | | | | | | | | | | | | | | | | | |
| (1) | See the description of various financing arrangements in Note 6 of our Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K. |
| (2) | See Note 8 of our Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K. |
64
| (3) | See Note 11(a) of our Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K. |
| (4) | Includes $3.8 million of deferred purchase price payments, $3.0 million of non-compete payments and $12.5 million of management incentive payments related to our acquisition of Epocal. |
| (5) | Other purchase obligations relate to inventory purchases and other operating expense commitments. |
| (6) | Includes our non-variable interest-bearing debt. See the description of various financing arrangements in Note 6 of our Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K. |
| (7) | In connection with certain of our acquisitions, additional contingent consideration may become payable to the sellers upon the satisfaction of certain performance milestones. Amounts represent the estimated fair value of these obligations. For further information pertaining to our contingent consideration arrangements see Note 11 of our Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K. |
In addition to the contractual obligations included in the table above, we recorded reserves for uncertain tax positions, including interest and penalties in the amount of $24.1 million as non-current liabilities. It is uncertain if, or when, such amounts may be settled. See disclosure regarding uncertain tax positions in Note 16 of our Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K.
Critical Accounting Policies
The Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K are prepared in accordance with accounting principles generally accepted in the United States of America, or GAAP. The accounting policies discussed below are considered by our management and our audit committee to be critical to an understanding of our financial statements because their application depends on management’s judgment, with financial reporting results relying on estimates and assumptions about the effect of matters that are inherently uncertain. Specific risks for these critical accounting policies are described in the following paragraphs. For all of these policies, management cautions that future events rarely develop exactly as forecast and the best estimates routinely require adjustment. In addition, the notes to our audited Consolidated Financial Statements for the year ended December 31, 2013, included elsewhere in this Annual Report on Form 10-K, include a comprehensive summary of the significant accounting policies and methods used in the preparation of our consolidated financial statements.
Revenue Recognition
We primarily recognize revenue when the following four basic criteria have been met: (1) persuasive evidence of an arrangement exists, (2) delivery has occurred or services rendered, (3) the fee is fixed or determinable and (4) collection is reasonably assured.
The majority of our revenue is derived from product sales. We recognize revenue upon title transfer of the products to third-party customers, less a reserve for estimated product returns and allowances. Determination of the reserve for estimated product returns and allowances is based on our management’s analyses and judgments regarding certain conditions. Should future changes in conditions prove management’s conclusions and judgments on previous analyses to be incorrect, revenue recognized for any reporting period could be adversely affected.
For products that include installation, if the installation meets the criteria to be considered a separate element, product revenue is recognized upon delivery, and installation revenue is recognized when the installation is complete. For sales that include customer-specified acceptance criteria, revenue is recognized after the acceptance criteria have been met. Certain of our products require
65
specialized installation. Revenue for these products is deferred until installation is completed. Revenue from services is deferred and recognized over the contractual period, or as services are rendered and accepted by the customer. When arrangements include multiple elements, we use objective evidence of fair value to allocate revenue to the elements, and recognize revenue when the criteria for revenue recognition have been met for each element, in accordance with authoritative guidance on multiple-element arrangements.
Additionally, we generate services revenue in connection with contracts with health plans (both commercial and governmental) and self-insured employers, whereby we provide clinical expertise through fee-based arrangements. Revenue for fee-based arrangements is recognized over the period in which the services are provided. Some contracts provide that a portion of our fees are at risk if our customers do not achieve certain financial cost savings or we do not achieve certain other clinical and operational metrics, over a period of time, typically one year. Revenue subject to refund is not recognized if (i) sufficient information is not available to calculate performance measurements or (ii) interim performance measurements indicate that we are not meeting performance targets. If either of these two conditions exists, we record the amounts as other current liabilities in the consolidated balance sheet, deferring recognition of the revenue until we establish that we are meeting the performance criteria. If we do not meet the performance targets at the end of the contractual period we are obligated under the contract to refund some or all of the at-risk fees.
We also receive license and royalty revenue from agreements with third-party licensees. Revenue from fixed-fee license and royalty agreements is recognized on a straight-line basis over the obligation period of the related license agreements. License and royalty fees that the licensees calculate based on their sales, which we have the right to audit under most of our agreements, are generally recognized upon receipt of the license or royalty payments, unless we are able to reasonably estimate the fees as they are earned. License and royalty fees that are determinable prior to the receipt thereof are recognized in the period they are earned.
Use of Estimates for Sales Returns and Other Allowances and Allowance for Doubtful Accounts
Certain sales arrangements require us to accept product returns. From time to time, we also enter into sales incentive arrangements with our retail customers, which generally reduce the sale prices of our products. As a result, we must establish allowances for potential future product returns and claims resulting from our sales incentive arrangements against product revenue recognized in any reporting period. Calculation of these allowances requires significant judgments and estimates. When evaluating the adequacy of the sales returns and other allowances, our management analyzes historical returns, current economic trends and changes in customer and consumer demand and acceptance of our products. When such analysis is not available and a right of return exists, we record revenue when the right of return is no longer applicable. Material differences in the amount and timing of our product revenue for any reporting period may result if changes in conditions arise that would require management to make different judgments or utilize different estimates.
Our total provision for sales returns and other allowances related to sales incentive arrangements amounted to $98.6 million, $67.8 million and $47.5 million, or 5%, 4% and 3%, respectively, of net product sales in 2013, 2012 and 2011, respectively, which have been recorded against product sales to derive our net product sales. Of these amounts, approximately $70.3 million, $43.7 million and $23.6 million for 2013, 2012 and 2011, respectively, represent allowances for future deductions which have been provided against our related accruals for such charges with the balance charged directly against net sales. Similarly, our management must make estimates regarding uncollectible accounts receivable balances. When evaluating the adequacy of the allowance for doubtful accounts, management analyzes specific accounts receivable balances, historical bad debts, customer concentrations, customer credit-worthiness, current economic trends and changes in our customer payment terms and patterns. Our accounts receivable balance was $548.7 million and $524.3 million, net of allowances for doubtful accounts of $76.6 million and $36.4 million, as of December 31, 2013 and 2012, respectively.
66
Inventory
We state our inventories at the lower of the actual cost to purchase or manufacture the inventory or the estimated current market value of the inventory, less cost to sell. In addition, we periodically review the inventory quantities on hand and record a provision for excess and obsolete inventory. This provision reduces the carrying value of our inventory and is calculated based primarily upon factors such as forecasts of our customers’ demands, shelf lives of our products in inventory, loss of customers and manufacturing lead times. Evaluating these factors, particularly forecasting our customers’ demands, requires management to make assumptions and estimates. Actual product and services revenue may prove our forecasts to be inaccurate, in which case we may have underestimated or overestimated the provision required for excess and obsolete inventory. If, in future periods, our inventory is determined to be overvalued, we would be required to recognize the excess value as a charge to our cost of sales at the time of such determination. Likewise, if, in future periods, our inventory is determined to be undervalued, we would have over-reported our cost of sales, or understated our earnings, at the time we recorded the excess and obsolete provision. Our inventory balance was $364.2 million and $337.1 million, net of a reserve for excess and obsolete inventory of $21.5 million and $21.8 million, as of December 31, 2013 and 2012, respectively.
Goodwill and Other Long-Lived and Intangible Assets
Our long-lived assets include property, plant and equipment, net; goodwill; other intangible assets with indefinite lives; and finite-lived intangible assets, net. As of December 31, 2013 and 2012, respectively, we had property, plant and equipment, net of $545.2 million and $534.5 million; goodwill of $3.1 billion and $3.0 billion; other intangible assets with indefinite lives of $56.7 million and $36.5 million; and finite-lived intangible assets, net of $1.7 billion and $1.8 billion.
Goodwill relates to amounts that arose in connection with our various business combinations and represents the difference between the purchase price and the fair value of the identifiable tangible and intangible net assets when accounted for using the acquisition method of accounting. Goodwill is not amortized, but is subject to periodic review for impairment.
We test goodwill and other intangible assets with indefinite lives at the reporting unit level for impairment on an annual basis and between annual tests, if events and circumstances indicate it is more likely than not that the fair value of a reporting unit is less than its carrying value. Events that would indicate impairment and trigger an interim impairment assessment include, but are not limited to, current economic and market conditions, including a decline in market capitalization, a significant adverse change in legal factors, business climate or operational performance of the business and an adverse action or assessment by a regulator.
In performing the impairment test, we utilize the two-step approach. The first step, or Step 1, requires a comparison of the carrying value of each reporting unit to its estimated fair value. To estimate the fair value of our reporting units for Step 1, we use a combination of the income approach, the market comparable approach and the market transaction approach. The income approach is based on a discounted cash flow analysis, or DCF Approach, and calculates the fair value by estimating the after-tax cash flows attributable to a reporting unit and then discounting the after-tax cash flows to a present value, using a risk-adjusted discount rate. Assumptions used in the DCF Approach require the exercise of significant judgment, including judgment about appropriate discount rates and terminal values, growth rates and the amount and timing of expected future cash flows. The forecasted cash flows are based on our most recent budget and for years beyond the budget, our estimates are based on assumed growth rates. We believe our assumptions are consistent with the plans and estimates used to manage the underlying businesses. The discount rates, which are intended to reflect the risks inherent in future cash flow projections, used in the DCF Approach are based on estimates of the weighted-average cost of capital, or WACC, of market participants relative to each respective reporting unit. The market approaches consider comparable and transactional market data based on multiples of revenue or earnings before interest, taxes, depreciation and amortization, or EBITDA, based on trading multiples of selected guideline companies and deal multiples of selected target companies.
67
If the carrying value of a reporting unit exceeds its estimated fair value, we are required to perform the second step, or Step 2, of the goodwill impairment test to measure the amount of impairment loss, if any. Step 2 of the goodwill impairment test compares the implied fair value of a reporting unit’s goodwill to its carrying value. The implied fair value of goodwill is calculated as the difference between the fair value of the reporting unit and the estimated fair value of its assets and liabilities. To the extent this amount is below the carrying value of goodwill, an impairment charge is recorded to write down the carrying value to its implied value.
Impairment charges related to goodwill have no impact on our cash balances or compliance with financial covenants under our Amended and Restated Credit Agreement.
2013 Annual Goodwill Impairment Test
We conducted our 2013 annual impairment test for our reporting units during the fourth quarter of 2013. Key assumptions (which vary by reporting unit) used in determining fair value under the DCF Approach included discount rates ranging from 11.0% to 14.0%, projected compound average revenue growth rates of 4.0% to 11.4%, and terminal value growth rates of 3.0% to 4.0%. In determining the appropriate discount rate, we considered the WACC for each reporting unit, which among other factors considers the cost of common equity capital and the marginal cost of debt of market participants. Key assumptions (which again vary by reporting unit) used in determining fair value under the market approaches were based on observed market multiples of enterprise value to revenue and EBITDA for both comparable publicly-traded companies and recent merger and acquisition transactions involving similar companies to estimate appropriate controlling basis multiples to apply to each of the reporting units. Based on the multiples implied by this market data, we selected multiples of revenue of 0.8 to 2.9 times and multiples of EBITDA of 6.4 to 10.6 times. In assessing the reasonableness of our estimated fair values of the reporting units, management compared the results of the valuation analyses against our then-current market capitalization to imply a control premium. Based on this analysis, the implied control premium was within the range of comparable industry transactions.
The Step 1 impairment test indicated the estimated fair value of the professional diagnostics, health information solutions and consumer diagnostics reporting units exceeded the carrying value of their reporting unit’s net assets as follows: by $1.6 billion, $31.5 million and $92.7 million, respectively, or 30.2%, 7.7% and 45.5%, respectively.
The estimate of fair value requires significant judgment. We based our fair value estimates on assumptions that we believe to be reasonable but that are unpredictable and inherently uncertain, including estimates of future growth rates and operating margins and assumptions about the overall economic climate and the competitive environment for our business units. There can be no assurance that our estimates and assumptions made for purposes of our goodwill and identifiable intangible asset testing as of the time of testing will prove to be accurate predictions of the future. If our assumptions regarding business plans, competitive environments or anticipated growth rates are not correct, we may be required to record goodwill and/or intangible asset impairment charges in future periods, whether in connection with our next annual impairment testing or earlier, if an indicator of an impairment is present before our next annual evaluation.
2012 Annual Goodwill Impairment Test
We conducted our 2012 annual impairment test for our reporting units during the fourth quarter of 2012. Key assumptions (which vary by reporting unit) used in determining fair value under the DCF Approach included discount rates ranging from 11.0% to 15.0%, projected compound average revenue growth rates of 3.0% to 8.1% and terminal value growth rates of 3.0% to 4.0%. The determination of the appropriate discount rate and key assumptions were the same as those in the 2013 Annual Goodwill Impairment Test above. Based on the multiples implied by this market data, we selected multiples of revenue of 0.9 to 2.4 times and multiples of EBITDA of 5.4 to 8.9 times. In assessing the
68
reasonableness of our estimated fair values of the reporting units, management compared the results of the valuation analyses against our then-current market capitalization to imply a control premium. Based on this analysis, the implied control premium was within the range of comparable industry transactions.
The Step 1 impairment test indicated the estimated fair value of the professional diagnostics, health information solutions and consumer diagnostics reporting units exceeded the carrying value of their reporting unit’s net assets as follows: by $399.9 million, $41.9 million and $53.9 million, or 7.9%, 9.4% and 27.2%, respectively.
2011 Annual Goodwill Impairment Test
We conducted our 2011 annual impairment test for our reporting units during the fourth quarter of 2011. Key assumptions (which vary by reporting unit) used in determining fair value under the DCF Approach included discount rates ranging from 11.0% to 14.5%, projected compound average revenue growth rates of 4.9% to 10.0% and terminal value growth rates of 4.0%. The determination of the appropriate discount rate and key assumptions were the same as those in the 2013 Annual Goodwill Impairment Test above Based on the multiples implied by this market data, we selected multiples of revenue of 0.8 to 2.6 times and multiples of EBITDA of 5.6 to 9.3 times. In assessing the reasonableness of our estimated fair values of the reporting units, management compared the results of the valuation analyses against our then-current market capitalization to imply a control premium. Based on this analysis, the implied control premium was within the range of comparable industry transactions.
The Step 1 impairment test indicated the estimated fair value of the professional diagnostics and consumer diagnostics reporting units exceeded the carrying value of their reporting unit’s net assets as follows: by $567.2 million and $101.9 million, or 13.5% and 53.5%, respectively.
The Step 1 impairment test indicated that the carrying value of the net assets of our health information solutions reporting unit exceeded the estimated fair value of the reporting unit. As a result, we were required to perform Step 2 of the goodwill impairment test to determine the amount, if any, of goodwill impairment charges for the health information solutions reporting unit. We completed Step 2, consistent with the procedures described above, and determined that a goodwill impairment charge in the amount of approximately $383.6 million was required. The resulting goodwill impairment charge is reflected in operating income (loss) in our accompanying Consolidated Statements of Operations.
This impairment was primarily driven by reduced future cash flow expectations from the reporting unit principally as a result of an increasingly competitive business environment for services provided by the reporting unit, including the insourcing of certain services by key customers and a reduction in spending by other customers as a result in part of the continuing difficult economic climate. Also contributing to the impairment charge was the lower valuations ascribed to similar comparable businesses in the public markets.
Valuation of Other Long-Lived Tangible and Intangible Assets
Factors we generally consider important which could trigger an impairment review on the carrying value of other long-lived tangible and intangible assets include the following: (1) significant underperformance relative to expected historical or projected future operating results, (2) significant changes in the manner of our use of acquired assets or the strategy for our overall business, (3) underutilization of our tangible assets, (4) discontinuance of product lines by ourselves or our customers, (5) significant negative industry or economic trends, (6) significant decline in our stock price for a sustained period, (7) significant decline in our market capitalization relative to net book value and (8) goodwill impairment identified during an impairment review.
69
We conduct our annual goodwill impairment test for our reporting units during the fourth quarter. The impairment test conducted during 2011 indicated there was an impairment of goodwill associated with our health information solutions reporting unit, and thus, a potential impairment of our long-lived tangible and intangible assets associated with the same reporting unit. We conducted an analysis as prescribed under ASC 360Property, Plant and Equipment,utilizing an undiscounted cash flow model. The analysis indicated there was no impairment of the long-lived tangible or intangible assets associated with our health information solutions reporting unit.
Stock-Based Compensation
Stock-based compensation expense is measured at the grant date based on the fair value of the award and is recognized as expense over the vesting period. Determining the fair value of stock-based awards at the grant date requires judgment, including estimating our stock price volatility and employee stock option exercise behaviors. If actual results differ significantly from these estimates, stock-based compensation expense and our results of operations could be materially impacted.
Our expected volatility is based upon the historical volatility of our stock. The expected term is based on the assumption that all outstanding options will be exercised at the midpoint of the vesting date and the full contractual term, including data on experience to date. As stock-based compensation expense is recognized in our consolidated statements of operations based on awards ultimately expected to vest, the amount of expense has been reduced for estimated forfeitures. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Forfeitures were estimated based on historical experience. If factors change and we employ different assumptions, the compensation expense that we record in future periods may differ significantly from what we have recorded in the current period.
Accounting for Income Taxes
As part of the process of preparing our consolidated financial statements, we are required to estimate our income taxes in each of the jurisdictions in which we operate. This process involves estimating our actual current tax exposure and assessing temporary differences resulting from differing treatment of items, such as reserves and accruals and lives assigned to long-lived and intangible assets, for tax and accounting purposes. These differences result in deferred tax assets and liabilities. We must then assess the likelihood that our deferred tax assets will be recovered through future taxable income and, to the extent we believe that recovery is not more likely than not, we must establish a valuation allowance. To the extent we establish a valuation allowance or increase this allowance in a period, we must include an expense within our tax provision.
Significant management judgment is required in determining our provision for income taxes, our deferred tax assets and liabilities and any valuation allowance recorded against our net deferred tax assets. We have recorded a valuation allowance of $83.6 million as of December 31, 2013, due to uncertainties related to the future benefits, if any, from our deferred tax assets related primarily to our foreign businesses and certain U.S. net operating losses, or NOLs, and tax credits. This is an increase of $15.0 million from the valuation allowance of $68.6 million as of December 31, 2012. The increase is primarily related to domestic state NOLs and certain foreign NOLs. The valuation allowance is based on our estimates of taxable income by jurisdiction in which we operate and the period over which our deferred tax assets will be recoverable. In the event that actual results differ from these estimates or we adjust these estimates in future periods, we may need to establish an additional valuation allowance or reduce our current valuation allowance which could materially impact our tax provision.
We established reserves for tax uncertainties that reflect the use of the comprehensive model for the recognition and measurement of uncertain tax positions. We are currently undergoing routine tax examinations by U.S. federal, various state and foreign jurisdictions. Tax authorities periodically challenge certain transactions and deductions we reported on our income tax returns. We do not expect the outcome of these examinations, either individually or in the aggregate, to have a material adverse effect on our financial position, results of operations or cash flows.
70
Loss Contingencies
In the section of this Annual Report on Form 10-K entitled “Part I, Item 3, Legal Proceedings,” we have reported on material legal proceedings, if any. Because of the nature of our business, we may be subject at any particular time to lawsuits or other claims arising in the ordinary course of our business, and we expect that this will continue to be the case in the future.
We do not accrue for potential losses on legal proceedings where our company is the defendant when we are not able to reasonably estimate our potential liability, if any, due to uncertainty as to the nature, extent and validity of the claims against us, uncertainty as to the nature and extent of the damages or other relief sought by the plaintiff and the complexity of the issues involved. Our potential liability, if any, in a particular case may become reasonably estimable and probable as the case progresses, in which case we will begin accruing for the expected loss.
Recent Accounting Pronouncements
See Note 2(v) of the Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K, regarding the impact of certain recent accounting pronouncements on our Consolidated Financial Statements.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
The following discussion about our market risk disclosures involves forward-looking statements. Actual results could differ materially from those discussed in the forward-looking statements. We are exposed to market risk related to changes in interest rates and foreign currency exchange rates. We do not use derivative financial instruments for speculative or trading purposes.
Interest Rate Risk
We are exposed to market risk from changes in interest rates primarily through our investing and financing activities. In addition, our ability to finance future acquisition transactions or fund working capital requirements may be impacted if we are not able to obtain appropriate financing at acceptable rates. To manage our interest rate exposure, our strategy is to invest in short-term, highly-liquid investments. Our investment policy also requires investment in approved instruments with an initial maximum allowable maturity of eighteen months and an average maturity of our portfolio that should not exceed six months, with at least $500,000 cash available at all times. At December 31, 2013, our short-term investments consisted of money market funds with original maturities of 90 days or less. At December 31, 2013, our short-term investments approximated market value.
At December 31, 2013, under the credit agreement for our secured credit facility we had (i) term loans in an aggregate outstanding principal amount of $2.3 billion (consisting of “A” term loans (including the “Delayed-Draw” term loans) in the aggregate principal amount of $832.2 million, “B” term loans in the aggregate principal amount of $904.2 million, “Incremental B-1” term loans in the aggregate principal amount of $245.0 million and “Incremental B-2” term loans in the aggregate principal amount of $195.1 million), (ii) $170.0 million of outstanding borrowings under our revolving line of credit and (iii) subject to our continued compliance with the credit agreement, the ability to borrow a maximum of up to an additional $80.0 million under our revolving line of credit, which includes a $50.0 million sublimit for the issuance of letters of credit. Loans can be either Base Rate Loans or Eurodollar Rate Loans at our election, and, as of December 31, 2013, interest accrues on loans and our other Obligations under the terms of the credit agreement as follows (with the terms referenced above and below in this paragraph having the meanings given to them in the credit agreement): (i) in the case of loans that are Base Rate Loans, at a rate per annum equal to the sum of the Base Rate and the Applicable Margin, each as in effect from time to time, (ii) in the case of loans that are Eurodollar Rate Loans, at a rate per annum equal to the sum of the Eurodollar Rate and the Applicable Margin, each as in effect for the applicable Interest Period, and (iii) in the case of other Obligations, at
71
a rate per annum equal to the sum of the Base Rate and the Applicable Margin for Revolving Loans that are Base Rate Loans, each as in effect from time to time. The Base Rate is a floating rate which approximates the U.S. prime rate as in effect from time to time. The Eurodollar Rate is equal to the LIBOR rate and is set for a period of one, two, three or six months at our election. Applicable Margins for our “A” term loans (including the “Delayed-Draw” term loans) and revolving line of credit loans range from (i) with respect to such loans that are Base Rate Loans, 1.75% to 2.50% and (ii) with respect to such loans that are Eurodollar Rate Loans, 2.75% to 3.50%, in each case, depending upon our consolidated secured leverage ratio (as determined under the credit agreement). Applicable Margins for our “B” term loans, “Incremental B-1” term loans and “Incremental B-2” term loans range from (i) with respect to such loans that are Base Rate Loans, 2.50% to 3.25% and (ii) with respect to such loans that are Eurodollar Rate Loans, 3.50% to 4.25%, in each case, depending upon our consolidated secured leverage ratio. Interest on “B” term loans, “Incremental B-1” term loans and “Incremental B-2” term loans based on the Eurodollar Rate is subject to a 1.00% floor with respect to the base Eurodollar Rate.
Assuming no changes in our consolidated secured leverage ratio, the effect of interest rate fluctuations on outstanding borrowings as of December 31, 2013 over the next twelve months is quantified and summarized as follows (in thousands):
| | | | |
| | | Interest Expense
Increase | |
Interest rates payable by us increase by 100 basis points | | $ | 23,464 | |
Interest rates payable by us increase by 200 basis points | | $ | 46,929 | |
Foreign Currency Risk
We face exposure to movements in foreign currency exchange rates whenever we, or any of our subsidiaries, enter into transactions with third parties that are denominated in currencies other than our, or its, functional currency. Intercompany transactions between entities that use different functional currencies also expose us to foreign currency risk. During 2013, the net impact of foreign currency changes on transactions was a loss of $4.0 million.
Gross margins of products we manufacture at our foreign plants and sell in U.S. dollars or manufacture in our U.S. plants and sell in currencies other than the U.S. dollar are also affected by foreign currency exchange rate movements. Our gross margin on total net product sales was 50.4% in 2013. If the U.S. dollar had been stronger by 1%, 5% or 10%, compared to the actual rates during 2013, our gross margin on total net product sales would have been 50.5%, 50.9% and 51.3%, respectively.
In addition, because a substantial portion of our earnings is generated by our foreign subsidiaries, whose functional currencies are other than the U.S. dollar (in which we report our consolidated financial results), our earnings could be materially impacted by movements in foreign currency exchange rates upon the translation of the earnings of such subsidiaries into the U.S. dollar.
If the U.S. dollar had been uniformly stronger by 1%, 5% or 10%, compared to the actual average exchange rates used to translate the financial results of our foreign subsidiaries, our net product sales and net income would have been impacted by approximately the following amounts (in thousands):
| | | | | | | | |
| | | Approximate
Decrease in
Net Revenue | | | Approximate
Decrease in
Net Income | |
If, during 2013, the U.S. dollar was stronger by: | | | | | | | | |
1% | | $ | (9,710 | ) | | $ | (381 | ) |
5% | | $ | (48,548 | ) | | $ | (1,903 | ) |
10% | | $ | (97,097 | ) | | $ | (3,806 | ) |
72
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements and supplementary data, except for selected quarterly financial data which are summarized below, are listed under Item 15(a) and have been filed as part of this Annual Report on Form 10-K on the pages indicated.
The following table presents selected quarterly financial data for each of the quarters in the years ended December 31, 2013 and 2012 (in thousands, except per share data):
| | | | | | | | | | | | | | | | |
| | | 2013 | |
| | | First
Quarter(2) | | | Second
Quarter(3) | | | Third
Quarter(4) | | | Fourth
Quarter(5) | |
Net revenue | | $ | 739,249 | | | $ | 763,985 | | | $ | 753,882 | | | $ | 772,326 | |
Gross profit | | $ | 364,257 | | | $ | 384,487 | | | $ | 368,646 | | | $ | 384,349 | |
Net income (loss) | | $ | 12,425 | | | $ | (60,302 | ) | | $ | (19,089 | ) | | $ | (3,312 | ) |
Net income (loss) available to common stockholders(1) | | $ | 7,200 | | | $ | (65,878 | ) | | $ | (24,815 | ) | | $ | (9,054 | ) |
Basic and diluted net income (loss) per common share attributable to Alere Inc. and Subsidiaries(1) | | $ | 0.09 | | | $ | (0.81 | ) | | $ | (0.30 | ) | | $ | (0.11 | ) |
| | | | | | | | | | | | | | | | |
| | | 2012 | |
| | | First
Quarter(6) | | | Second
Quarter(7) | | | Third
Quarter(8) | | | Fourth
Quarter(9) | |
Net revenue | | $ | 671,129 | | | $ | 700,517 | | | $ | 691,416 | | | $ | 755,763 | |
Gross profit | | $ | 353,071 | | | $ | 355,608 | | | $ | 345,775 | | | $ | 373,868 | |
Net income (loss) | | $ | 1,029 | | | $ | (12,879 | ) | | $ | (3,517 | ) | | $ | (62,540 | ) |
Net loss available to common stockholders(1) | | $ | (4,095 | ) | | $ | (18,194 | ) | | $ | (9,155 | ) | | $ | (68,031 | ) |
Basic and diluted net loss per common share attributable to Alere Inc. and Subsidiaries(1) | | $ | (0.05 | ) | | $ | (0.23 | ) | | $ | (0.11 | ) | | $ | (0.84 | ) |
| (1) | Net income (loss) available to common stockholders and basic and diluted net income (loss) per common share are computed consistent with the annual per share calculations described in Notes 2(o) and 12 of our Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K. |
| (2) | Included in net income for the first quarter of 2013 is $3.9 million related to restructuring charges associated with the decision to close various facilities, acquisition-related costs in the amount of $0.9 million recorded in accordance with ASC 805,Business Combinations, $11.0 million of expense recorded in connection with fair value adjustments to acquisition-related contingent consideration obligations in accordance with ASC 805,Business Combinations, $1.0 million of interest expense recorded in connection with fees paid for certain debt modifications and the termination of our senior secured credit facility, $4.1 million of non-cash stock-based compensation expense, $0.7 million in compensation charges associated with acquisition-related contingent consideration obligations, a $0.5 million charge associated with the write-up to fair market value of inventory acquired in connection with the acquisition of Epocal Inc. and $0.2 million of expense associated with the extinguishment of debt. |
| (3) | Included in net loss for the second quarter of 2013 is $8.1 million related to restructuring charges associated with the decision to close various facilities, acquisition-related costs in the amount of $0.4 million recorded in accordance with ASC 805,Business Combinations, $5.3 million of expense recorded in connection with fair value adjustments to acquisition-related contingent consideration obligations in accordance with ASC 805,Business Combinations, $35.6 million of loss in connection with the repurchase of our 9% senior subordinated notes, $8.1 million of bargain purchase gain associated with our acquisition of the Liberty business, $5.1 million of non-cash write-off of an investment, $0.8 million of interest expense recorded in connection with fees paid for certain debt modifications and the termination of our senior secured credit facility, $4.7 million of non-cash stock-based compensation expense, $0.5 million in compensation |
73
| | charges and $0.2 million of related interest accretion associated with acquisition-related contingent consideration obligations, and a $0.7 million charge associated with the write-up to fair market value of inventory acquired in connection with the acquisition of Epocal Inc. |
| (4) | Included in net loss for the third quarter of 2013 is $7.8 million related to restructuring charges associated with the decision to close various facilities, acquisition-related costs in the amount of $0.5 million recorded in accordance with ASC 805,Business Combinations, $2.7 million of expense recorded in connection with fair value adjustments to acquisition-related contingent consideration obligations in accordance with ASC 805,Business Combinations, $5.5 million of costs associated with the conduct of a contested proxy solicitation, $5.9 million of loss on disposition of our Spinreact operations, $0.4 million of interest expense recorded in connection with fees paid for certain debt modifications and the termination of our senior secured credit facility, $5.7 million of non-cash stock-based compensation expense, $0.8 million in compensation charges and $0.1 million of related interest accretion associated with acquisition-related contingent consideration obligations, and a $0.7 million charge associated with the write-up to fair market value of inventory acquired in connection with the acquisition of Epocal Inc. |
| (5) | Included in net loss for the fourth quarter of 2013 is $7.8 million related to restructuring charges associated with the decision to close various facilities, acquisition-related costs in the amount of $1.3 million recorded in accordance with ASC 805,Business Combinations, $1.0 million of income recorded in connection with fair value adjustments to acquisition-related contingent consideration obligations in accordance with ASC 805,Business Combinations, $6.1 million of costs associated with potential business dispositions, $0.4 million of interest expense recorded in connection with fees paid for certain debt modifications and the termination of our senior secured credit facility, $6.7 million of non-cash stock-based compensation expense, $0.8 million in compensation charges and $0.1 million of related interest accretion associated with acquisition-related contingent consideration obligations, a $0.6 million charge associated with the write-up to fair market value of inventory acquired in connection with the acquisition of Epocal Inc., and $0.1 million of costs associated with the conduct of a contested proxy solicitation, offset by an $0.8 million reduction in the loss on disposition of our Spinreact, S.A. subsidiary located in Spain. |
| (6) | Included in net loss for the first quarter of 2012 is $5.6 million related to restructuring charges associated with the decision to close various facilities, a write-off in the amount of $4.7 million relating to an inventory write-up recorded in connection with an acquisition, acquisition-related costs in the amount of $1.5 million recorded in accordance with ASC 805,Business Combinations, $5.0 million of expense recorded in connection with fair value adjustments to acquisition-related contingent consideration obligations in accordance with ASC 805,Business Combinations, $1.3 million of interest expense recorded in connection with fees paid for certain debt modifications and the termination of our senior secured credit facility and $3.9 million of non-cash stock-based compensation expense. |
| (7) | Included in net loss for the second quarter of 2012 is $1.4 million related to restructuring charges associated with the decision to close various facilities, acquisition-related costs in the amount of $3.8 million recorded in accordance with ASC 805,Business Combinations, $6.7 million of income recorded in connection with fair value adjustments to acquisition-related contingent consideration obligations in accordance with ASC 805,Business Combinations, $1.3 million of interest expense recorded in connection with fees paid for certain debt modifications and the termination of our senior secured credit facility and $4.4 million of non-cash stock-based compensation expense. |
| (8) | Included in net loss for the third quarter of 2012 is $3.3 million related to restructuring charges associated with the decision to close various facilities, acquisition-related costs in the amount of $0.8 million recorded in accordance with ASC 805,Business Combinations, $15.1 million of income recorded in connection with fair value adjustments to acquisition-related contingent consideration obligations in accordance with ASC 805,Business Combinations, $1.3 million of interest expense recorded in connection with fees paid for certain debt modifications and the termination of our senior secured credit facility and $3.6 million of non-cash stock-based compensation expense. |
74
| (9) | Included in net loss for the fourth quarter of 2012 is $10.3 million related to restructuring charges associated with the decision to close various facilities, acquisition-related costs in the amount of $3.6 million recorded in accordance with ASC 805,Business Combinations, $10.2 million of expense recorded in connection with fair value adjustments to acquisition-related contingent consideration obligations in accordance with ASC 805,Business Combinations, $23.2 million of expense associated with the repurchase of substantially all of our 7.875% senior notes, $1.0 million of interest expense recorded in connection with fees paid for certain debt modifications and the termination of our senior secured credit facility and $3.8 million of non-cash stock-based compensation expense. |
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
Not applicable.
| ITEM 9A. | CONTROLS AND PROCEDURES |
Management’s Conclusions Regarding the Effectiveness of Our Disclosure Controls and Procedures
Our management evaluated, with the participation of our Chief Executive Officer (CEO) and Chief Financial Officer (CFO), the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Securities Exchange Act of 1934, as amended) as of the end of the period covered by this Annual Report on Form 10-K. Based on this evaluation, our CEO and CFO concluded that our disclosure controls and procedures were effective to provide reasonable assurance that information required to be disclosed by the Company in reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the required time periods and that such information is accumulated and communicated to our management, including the CEO and CFO, as appropriate to allow timely decisions regarding required disclosure.
Our management understands, nonetheless, that controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and our management, necessarily, was required to apply its judgment in evaluating and implementing controls and procedures.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a-15(f) under the Securities Exchange Act of 1934, as amended. Our internal control over financial reporting is a process designed under the supervision of our CEO and CFO to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Our internal control over financial reporting includes those policies and procedures that:
| | (i) | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; |
| | (ii) | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and |
| | (iii) | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of assets that could have a material effect on our financial statements. |
75
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of our internal control over financial reporting as of December 31, 2013. In making this assessment, management used the criteria established in its 1992Internal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on this evaluation, management determined that the Company maintained effective internal control over financial reporting as of December 31, 2013.
In conducting management’s evaluation of the effectiveness of our internal control over financial reporting, management excluded 3 entities acquired in purchase business combinations during 2013 from its assessment. The acquisitions represented approximately 0.5% and 0.2% of total assets and net revenue, respectively, of the related consolidated financial statement amounts as of and for the year ended December 31, 2013. Refer to Note 4 of the accompanying consolidated financial statements for a list of the 2013 acquisitions.
The effectiveness of our internal control over financial reporting as of December 31, 2013 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which appears herein.
Changes in internal control over financial reporting
There was no change in our internal control over financial reporting that occurred during our fourth fiscal quarter of 2013 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
| ITEM 9B. | OTHER INFORMATION |
None.
76
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information regarding directors, executive officers and corporate governance included in our definitive Proxy Statement to be filed pursuant to Regulation 14A in connection with our 2014 Annual Meeting of Shareholders, or the Proxy Statement, is incorporated herein by reference.
ITEM 11. EXECUTIVE COMPENSATION
The information regarding executive compensation included in the Proxy Statement is incorporated herein by reference.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information regarding security ownership of certain beneficial owners and management and related stockholder matters included in the Proxy Statement is incorporated herein by reference.
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information regarding certain relationships and related transactions, and director independence included in the Proxy Statement is incorporated herein by reference.
| ITEM 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES |
The information regarding principal accounting fees and services included in the Proxy Statement is incorporated herein by reference.
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
1. Financial Statements.
The financial statements listed below have been filed as part of this report on the pages indicated:
2. Financial Statement Schedules.
All schedules for which provision is made in the applicable accounting regulations of the Securities and Exchange Commission have been omitted because they are inapplicable or the required information is shown in the Consolidated Financial Statements or the notes thereto included herein.
77
3. Exhibits.
Some of the agreements filed as exhibits to this Annual Report on Form 10-K contain representations and warranties that were made solely for the benefit of the parties to the agreement. These representations and warranties:
| | • | | may have been qualified by disclosures that were made to the other party or parties in connection with the negotiation of the agreements, which disclosures are not necessarily reflected in the agreements; |
| | • | | may apply standards of materiality that differ from those of investors; |
| | • | | may have constituted an allocation of risk and responsibility among the parties rather than statements of fact; and |
| | • | | were made only as of specified dates contained in the agreements and are subject to subsequent developments and changed circumstances. |
Accordingly, these representations and warranties may not describe the actual state of affairs as of the date that these representations and warranties were made or at any other time. Investors should not rely on them as statements of fact.
| | |
Exhibit No. | | Description |
| 3.1 | | Amended and Restated Certificate of Incorporation of the Company (incorporated by reference to Exhibit 3.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2012) |
| |
| 3.2 | | Amended and Restated By-laws of the Company (incorporated by reference to Exhibit 3.3 to the Company’s Current Report on Form 8-K, event date October 9, 2013, filed with the SEC on October 16, 2013) |
| |
| 4.1 | | Indenture, dated May 14, 2007, between the Company and U.S. Bank Trust National Association (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K, event date May 9, 2007, filed on May 15, 2007) |
| |
| 4.2 | | Indenture dated as of May 12, 2009 between Inverness Medical Innovations, Inc., as issuer, and U.S. Bank National Association, as trustee (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K, event date May 12, 2009, filed on May 12, 2009) |
| |
| 4.3 | | Ninth Supplemental Indenture dated September 21, 2010 to Indenture date as of May 12, 2009 among Alere Inc., as issuer, the subsidiary guarantors named therein, as guarantors, and U.S. Bank National Association, as trustee (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K, event date September 15, 2010, filed with the SEC on September 21, 2010) |
| |
| 4.4 | | Eleventh Supplemental Indenture to Indenture dated as of May 12, 2009 (relating to the Record Date Amendments and Waivers) dated as of June 16, 2011, among the Company, the subsidiary guarantors party thereto and U.S. Bank National Association, as trustee (incorporated by reference to Exhibit 4.3 to the Company’s Current Report on Form 8-K, event date June 16, 2011, filed on June 22, 2011) |
| |
| 4.5 | | Thirteenth Supplemental Indenture to Indenture dated as of May 12, 2009 (relating to the Restricted Payments Amendments and Waivers) dated as of June 16, 2011, among the Company, the subsidiary guarantors party thereto and U.S. Bank National Association, as trustee (incorporated by reference to Exhibit 4.4 to the Company’s Current Report on Form 8-K, event date June 16, 2011, filed on June 22, 2011) |
78
| | |
Exhibit No. | | Description |
| 4.6 | | Fifteenth Supplemental Indenture to Indenture dated as of May 12, 2009 (to add the guarantees of Alere Informatics, Inc., Alere Wellogic, LLC, ATS Laboratories, Inc., Avee Laboratories Inc., eScreen, Inc., Global Analytical Development LLC, Ionian Technologies Inc., Pembrooke Occupational Health, Inc., Screen Tox, Inc., and Standing Stone, Inc.) dated as of April 3, 2013 among Alere Informatics, Inc., Alere Wellogic, LLC, ATS Laboratories, Inc., Avee Laboratories Inc., eScreen, Inc., Global Analytical Development LLC, Ionian Technologies Inc., Pembrooke Occupational Health, Inc., Screen Tox, Inc., and Standing Stone, Inc., as guarantors, the Company as issuer, the other guarantor subsidiaries named therein, as guarantors, and U.S. Bank National Association, as trustee (incorporated by reference to Exhibit 4.2 to the Company’s Quarterly Report on Form 10-Q for the period ended March 31, 2013) |
| |
| 4.7 | | Sixteenth Supplemental Indenture dated as of May 24, 2013 to Indenture dated as of May 12, 2009, by and among the Company, the subsidiary guarantors named therein and U.S. Bank National Association, as trustee (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K, event date May 23, 2013, filed May 30, 2013) |
| |
| 4.8 | | Indenture dated as of August 11, 2009 between Inverness Medical Innovations, Inc., as issuer, and The Bank of New York Mellon Trust Company, N.A., as trustee (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K, event date August 11, 2009, filed on August 11, 2009) |
| |
| 4.9 | | Fifteenth Supplemental Indenture dated as of December 11, 2012 to Indenture dates as of August 11, 2009, by and among the Company, the subsidiary guarantors named therein and Bank of New York Mellon Trust Company, N.A., as trustee (incorporated by reference to Exhibit 4.2 to the Company’s Current Report on Form 8-K, event date December 11, 2012, filed on December 14, 2012) |
| |
| 4.10 | | Sixteenth Supplemental Indenture, dated April 3, 2013 (to add the guarantees of Alere Informatics, Inc., Alere Wellogic, LLC, ATS Laboratories, Inc., Avee Laboratories Inc., eScreen, Inc., Global Analytical Development LLC, Ionian Technologies Inc., Pembrooke Occupational Health, Inc., Screen Tox, Inc., and Standing Stone, Inc.) to Indenture dated as of August 11, 2009 among Alere Informatics, Inc., Alere Wellogic, LLC, ATS Laboratories, Inc., Avee Laboratories Inc., eScreen, Inc., Global Analytical Development LLC, Ionian Technologies Inc., Pembrooke Occupational Health, Inc., Screen Tox, Inc., and Standing Stone, Inc., as guarantors, the Company as issuer, the other guarantor subsidiaries named therein, as guarantors, and Bank of New York Mellon Trust Company, N.A., as trustee (incorporated by reference to Exhibit 4.6 of the Company’s Registration Statement on Form S-4 (File No. 333-187776)) |
| |
| 4.11 | | Registration Rights Agreement, dated as of December 11, 2012, by and among the Company, the guarantors named therein, and Jefferies & Company, Inc., Goldman, Sachs & Co., and Credit Suisse Securities (USA) LLC, as representatives of the Initial Purchasers (incorporated by reference to Exhibit 4.4 to the Company’s Current Report on Form 8-K, event date December 11, 2012, filed on December 14, 2012) |
| |
| 4.12 | | Registration Rights Agreement, dated as of May 24, 2013, by and among the Company, the guarantors named therein, and Goldman, Sachs & Co., Jefferies LLC and Credit Suisse Securities (USA) LLC, as representatives of the Initial Purchasers (incorporated by reference to Exhibit 4.3 to the Company’s Current Report on Form 8-K, event date May 23, 2013, filed May 30, 2013) |
| |
| +10.1 | | BNP Assay Development, Manufacture and Supply Agreement between Biosite Incorporated and Beckman Coulter, Inc. effective June 24, 2003 (incorporated by reference to Exhibit 10.22 to Annual Report of Biosite Incorporated on Form 10-K, filed on March 12, 2007) |
79
| | |
Exhibit No. | | Description |
| +10.2 | | Shareholder Agreement dated as of May 17, 2007 among Inverness Medical Switzerland GmbH, Procter & Gamble International Operations, SA and SPD Swiss Precision Diagnostics GmbH (incorporated by reference to Exhibit 10.12 to Company’s Quarterly Report on Form 10-Q for the period ended June 30, 2007) |
| |
| ‡10.3 | | Inverness Medical Innovations, Inc. 2001 Stock Option and Incentive Plan, as amended (incorporated by reference to Appendix A to the Company’s Proxy Statement filed on Schedule 14A as filed with the SEC on April 30, 2009) |
| |
| ‡10.4 | | Alere Inc. 2010 Stock Option and Incentive Plan, as amended (incorporated by reference to Appendix B to the Company’s Proxy Statement filed on Schedule 14A as filed with the SEC on June 26, 2013) |
| |
| ‡10.5 | | Rules of Alere Inc. HM Revenue and Customs Approved Share Option Plan (2007), as amended (authorized for use under the Alere Inc. 2001 Stock Option and Incentive Plan and the Alere Inc. 2010 Stock Option and Incentive Plan) (incorporated by reference to Exhibit 10.5 to the Company’s Quarterly Report on Form 10-Q for the period ended June 30, 2010) |
| |
| ‡10.6 | | Summary of Terms of Stock Option Agreements under Alere Inc. Stock Option and Incentive Plans (incorporated by reference to Exhibit 10.6 to the Company’s Annual Report on Form 10-K, for the year ended December 31, 2012) |
| |
| *‡10.7 | | Summary of Non-Employee Director Compensation |
| |
| ‡10.8 | | Alere Inc. 2001 Employee Stock Purchase Plan, as amended (incorporated by reference to Appendix C to the Company’s Proxy Statement filed on Schedule 14A as filed with the SEC on June 26, 2013) |
| |
| ‡10.9 | | Restricted Stock Unit Agreement, dated December 30, 2012, between Alere Inc. and Namal Nawana (incorporated by reference to Exhibit 10.9 to the Company’s Annual Report on Form 10-K, for the year ended December 31, 2012) |
| |
| **‡10.10 | | Consulting Agreement, dated August 30, 2009, between Inverness Medical Switzerland GmbH and Citros V.O.F. (incorporated by reference to Exhibit 10.15 to the Company’s Annual Report on Form 10-K/A, for the year ended December 31, 2011) |
| |
| **‡10.11 | | Management Consultancy Agreement, dated June 26, 2008, between Gesellschaft für Patientenhilfe DGP mbH and Leiter & Partner Unternehmensberater Partnerschaftsgesellschaft (incorporated by reference to Exhibit 10.16 to the Company’s Annual Report on Form 10-K/A, for the year ended December 31, 2011) |
| |
| **‡10.12 | | Amendment of the Contract on the Provision of Consulting, Lease and Other Services, dated April 21, 2011, between Gesellschaft für Patientenhilfe DGP mbH and Leiter & Cie. GmbH (incorporated by reference to Exhibit 10.17 to the Company’s Annual Report on Form 10-K/A, for the year ended December 31, 2011) |
| |
| 10.13 | | Purchase Agreement dated November 28, 2012 among Alere Inc., the subsidiary guarantors named therein and Jefferies & Company, Inc., Goldman, Sachs & Co. and Credit Suisse Securities (USA) LLC, as Representatives of the Initial Purchasers (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K, event date November 28, 2012, filed with the SEC on November 30, 2012) |
| |
| 10.14 | | Purchase Agreement dated May 13, 2013 among Alere Inc., the subsidiary guarantors named therein and Goldman, Sachs & Co., Jefferies LLC and Credit Suisse Securities (USA) LLC, as Representatives of the Initial Purchasers (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K, event date May 10, 2013, filed May 16, 2013) |
80
| | |
Exhibit No. | | Description |
| 10.15 | | Credit Agreement dated as of June 30, 2011 among Alere Inc., as Borrower, the Lenders and L/C Issuers party thereto, General Electric Capital Corporation, as Administrative Agent, Jefferies Finance LLC, as Syndication Agent, and Credit Suisse Securities (USA) LLC, Goldman Sachs Bank USA, DnB Nor Bank ASA and SunTrust Bank, as Co-Documentation Agents (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K, event date June 30, 2011, filed on July 7, 2011) |
| |
| 10.16 | | Guaranty and Security Agreement dated as of June 30, 2011 among Alere Inc., as Borrower, and each Grantor party thereto and General Electric Capital Corporation, as Administrative Agent (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K, event date June 30, 2011, filed on July 7, 2011) |
| |
| 10.17 | | First Amendment to Credit Agreement dated as of July 27, 2011 among Alere Inc., as Borrower, the Lenders and L/C Issuers party thereto, General Electric Capital Corporation, as Administrative Agent, Jefferies Finance LLC, as Syndication Agent, and Credit Suisse Securities (USA) LLC, Goldman Sachs Bank USA, DnB Nor Bank ASA and SunTrust Bank, as Co-Documentation Agents (incorporated by reference to Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q for the period ended June 30, 2011) |
| |
| 10.18 | | Second Amendment to Credit Agreement dated as of December 7, 2011 among Alere Inc., as Borrower, the Lenders party thereto, and General Electric Capital Corporation, as Administrative Agent (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K, event date December 7, 2011, filed on December 9, 2011) |
| |
| 10.19 | | Third Amendment to Credit Agreement dated as of March 28, 2012 among Alere Inc., as Borrower, the Lenders party thereto, and General Electric Capital Corporation, as Administrative Agent (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K, event date March 28, 2012, filed on April 2, 2012) |
| |
| 10.20 | | Fourth Amendment to Credit Agreement, dated as of March 22, 2013, among the Alere Inc., as Borrower, each of the Guarantors (as defined therein), the Lenders party thereto, and General Electric Capital Corporation, as Administrative Agent (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K, event date May 23, 2013, filed May 30, 2013) |
| |
| *21.1 | | List of Subsidiaries of the Company as of February 24, 2014 |
| |
| *23.1 | | Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm |
| |
| *31.1 | | Certification by Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act |
| |
| *31.2 | | Certification by Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act |
| |
| *32.1 | | Certification Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act |
| |
| *101 | | Interactive Data Files regarding (a) our Consolidated Statements of Operations for the Years Ended December 31, 2013, 2012 and 2011, (b) our Consolidated Statements of Comprehensive Income (Loss) for the Years Ended December 31, 2013, 2012 and 2011 (c) our Consolidated Balance Sheets as of December 31, 2013 and 2012, (d) our Consolidated Statements of Equity for the Years Ended December 31, 2013, 2012 and 2011, (e) our Consolidated Statements of Cash Flows for the Years Ended December 31, 2013, 2012 and 2011 and (f) the Notes to such Consolidated Financial Statements. |
81
| ** | The Company agrees to furnish supplementally to the Securities and Exchange Commission (“the Commission”) a copy of any omitted schedule or exhibit to this agreement upon request by the Commission. |
| + | We have omitted portions of this exhibit which have been granted confidential treatment. |
| ‡ | Management contract or compensatory plan or arrangement, or amendment thereto. |
82
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | |
| | | | ALERE INC. |
| | | |
| Date: March 3, 2014 | | | | By: | | /s/ Ron Zwanziger |
| | | | | | Ron Zwanziger |
| | | | | | Chairman, Chief Executive Officer and President |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| | | | |
Signature | | Title | | Date |
| | |
/s/ Ron Zwanziger Ron Zwanziger | | Chief Executive Officer, President and Director (Principal Executive Officer) | | March 3, 2014 |
| | |
/s/ David Teitel David Teitel | | Chief Financial Officer (Principal Financial Officer) | | March 3,��2014 |
| | |
/s/ Carla R. Flakne Carla R. Flakne | | Chief Accounting Officer (Principal Accounting Officer) | | March 3, 2014 |
| | |
/s/ Regina Benjamin Regina Benjamin | | Director | | March 3, 2014 |
| | |
/s/ Håkan Björklund Håkan Björklund | | Director | | March 3, 2014 |
| | |
/s/ Carol R. Goldberg Carol R. Goldberg | | Director | | March 3, 2014 |
| | |
/s/ John F. Levy John F. Levy | | Director | | March 3, 2014 |
| | |
/s/ Steve MacMillan Steve MacMillan | | Director | | March 3, 2014 |
| | |
/s/ Brian Markison Brian Markison | | Director | | March 3, 2014 |
| | |
/s/ Jerry McAleer Jerry McAleer | | Director | | March 3, 2014 |
| | |
/s/ Thomas McKillop Thomas McKillop | | Director | | March 3, 2014 |
83
| | | | |
Signature | | Title | | Date |
| | |
/s/ Gregg J. Powers Gregg J. Powers | | Director | | March 3, 2014 |
| | |
/s/ John A. Quelch John A. Quelch | | Director | | March 3, 2014 |
| | |
/s/ James Roosevelt, Jr. James Roosevelt, Jr. | | Director | | March 3, 2014 |
84
ALERE INC. AND SUBSIDIARIES
CONSOLIDATED FINANCIAL STATEMENTS
Table of Contents
F-1
Report of IndependentRegistered Public Accounting Firm
To the Board of Directors and Stockholders of Alere Inc.,
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of operations, of equity, of comprehensive income (loss) and of cash flows present fairly, in all material respects, the financial position of Alere Inc. and its subsidiaries at December 31, 2013 and December 31, 2012, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2013 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2013, based on criteria established in Internal Control—Integrated Framework in 1992 issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for these financial statements, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Annual Report on Internal Control over Financial Reporting. Our responsibility is to express opinions on these financial statements and on the Company’s internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
As described in Management’s Annual Report on Internal Control over Financial Reporting, management has excluded 3 entities from its assessment of internal control over financial reporting as of December 31, 2013 because they were acquired by the Company in purchase business combinations during 2013. We have also excluded these entities from our audit of internal control over financial reporting. The total assets and net revenue of these entities represented 0.5% and 0.2%, respectively, of the related consolidated financial statement amounts as of and for the year ended December 31, 2013.
/s/ PricewaterhouseCoopers LLP
Boston, Massachusetts
March 3, 2014
F-2
ALERE INC. AND SUBSIDIARIES
CONSOLIDATEDSTATEMENTS OF OPERATIONS
(in thousands, except per share amounts)
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2013 | | | 2012 | | | 2011 | |
Net product sales | | $ | 2,072,463 | | | $ | 1,913,731 | | | $ | 1,683,132 | |
Services revenue | | | 929,750 | | | | 876,518 | | | | 679,922 | |
| | | | | | | | | | | | |
Net product sales and services revenue | | | 3,002,213 | | | | 2,790,249 | | | | 2,363,054 | |
License and royalty revenue | | | 27,229 | | | | 28,576 | | | | 23,473 | |
| | | | | | | | | | | | |
Net revenue | | | 3,029,442 | | | | 2,818,825 | | | | 2,386,527 | |
| | | | | | | | | | | | |
Cost of net product sales | | | 1,028,520 | | | | 932,150 | | | | 795,424 | |
Cost of services revenue | | | 491,420 | | | | 450,999 | | | | 338,232 | |
| | | | | | | | | | | | |
Cost of net product sales and services revenue | | | 1,519,940 | | | | 1,383,149 | | | | 1,133,656 | |
Cost of license and royalty revenue | | | 7,763 | | | | 7,354 | | | | 7,036 | |
| | | | | | | | | | | | |
Cost of net revenue | | | 1,527,703 | | | | 1,390,503 | | | | 1,140,692 | |
| | | | | | | | | | | | |
Gross profit | | | 1,501,739 | | | | 1,428,322 | | | | 1,245,835 | |
Operating expenses: | | | | | | | | | | | | |
Research and development | | | 160,802 | | | | 183,001 | | | | 150,165 | |
Sales and marketing | | | 639,834 | | | | 643,423 | | | | 565,583 | |
General and administrative | | | 561,227 | | | | 492,766 | | | | 399,330 | |
Goodwill impairment charge | | | — | | | | — | | | | 383,612 | |
Loss on disposition | | | 5,124 | | | | — | | | | — | |
| | | | | | | | | | | | |
Operating income (loss) | | | 134,752 | | | | 109,132 | | | | (252,855 | ) |
Interest expense, including amortization of original issue discounts and deferred financing costs | | | (255,656 | ) | | | (240,560 | ) | | | (203,971 | ) |
Other income (expense), net | | | (13,128 | ) | | | 9,957 | | | | 1,883 | |
Gain on sale of joint venture interest | | | — | | | | — | | | | 288,896 | |
| | | | | | | | | | | | |
Loss before benefit for income taxes | | | (134,032 | ) | | | (121,471 | ) | | | (166,047 | ) |
Benefit for income taxes | | | (46,311 | ) | | | (30,319 | ) | | | (24,214 | ) |
| | | | | | | | | | | | |
Loss before equity earnings of unconsolidated entities, net of tax | | | (87,721 | ) | | | (91,152 | ) | | | (141,833 | ) |
Equity earnings of unconsolidated entities, net of tax | | | 17,443 | | | | 13,245 | | | | 8,524 | |
| | | | | | | | | | | | |
Net loss | | | (70,278 | ) | | | (77,907 | ) | | | (133,309 | ) |
Less: Net income attributable to non-controlling interests | | | 976 | | | | 275 | | | | 233 | |
| | | | | | | | | | | | |
Net loss attributable to Alere Inc. and Subsidiaries | | | (71,254 | ) | | | (78,182 | ) | | | (133,542 | ) |
Preferred stock dividends | | | (21,293 | ) | | | (21,293 | ) | | | (22,049 | ) |
Preferred stock repurchase | | | — | | | | — | | | | 23,936 | |
| | | | | | | | | | | | |
Net loss available to common stockholders | | $ | (92,547 | ) | | $ | (99,475 | ) | | $ | (131,655 | ) |
| | | | | | | | | | | | |
Basic and diluted net loss per common share attributable to Alere Inc. and Subsidiaries: | | $ | (1.13 | ) | | $ | (1.23 | ) | | $ | (1.58 | ) |
| | | | | | | | | | | | |
Weighted-average shares — basic and diluted | | | 81,542 | | | | 80,587 | | | | 83,128 | |
| | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-3
ALERE INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTSOF COMPREHENSIVE LOSS
(in thousands)
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2013 | | | 2012 | | | 2011 | |
Net loss | | $ | (70,278 | ) | | $ | (77,907 | ) | | $ | (133,309 | ) |
| | | | | | | | | | | | |
Other comprehensive income (loss), before tax: | | | | | | | | | | | | |
Changes in cumulative translation adjustment | | | (50,166 | ) | | | 54,642 | | | | (35,830 | ) |
Unrealized losses on available for sale securities | | | — | | | | (216 | ) | | | (471 | ) |
Unrealized gains on hedging instruments | | | 39 | | | | 388 | | | | 11,504 | |
Minimum pension liability adjustment | | | (415 | ) | | | (1,042 | ) | | | (3,070 | ) |
| | | | | | | | | | | | |
Other comprehensive income (loss), before tax | | | (50,542 | ) | | | 53,772 | | | | (27,867 | ) |
Income tax provision (benefit) related to items of other comprehensive income (loss) | | | (106 | ) | | | (372 | ) | | | 3,093 | |
| | | | | | | | | | | | |
Other comprehensive income (loss), net of tax | | | (50,436 | ) | | | 54,144 | | | | (30,960 | ) |
| | | | | | | | | | | | |
Comprehensive loss | | | (120,714 | ) | | | (23,763 | ) | | | (164,269 | ) |
Less: Comprehensive income attributable to non-controlling interests | | | 976 | | | | 275 | | | | 233 | |
| | | | | | | | | | | | |
Comprehensive loss attributable to Alere Inc. and Subsidiaries | | $ | (121,690 | ) | | $ | (24,038 | ) | | $ | (164,502 | ) |
| | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-4
ALERE INC. AND SUBSIDIARIES
CONSOLIDATEDBALANCE SHEETS
(in thousands, except par value amounts)
| | | | | | | | |
| | | As of December 31, | |
| | | 2013 | | | 2012 | |
| ASSETS | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 361,908 | | | $ | 328,346 | |
Restricted cash | | | 6,373 | | | | 3,076 | |
Marketable securities | | | 858 | | | | 904 | |
Accounts receivable, net of allowances of $76,643 and $36,396 at December 31, 2013 and December 31, 2012, respectively | | | 548,729 | | | | 524,332 | |
Inventories, net | | | 364,185 | | | | 337,121 | |
Deferred tax assets | | | 60,689 | | | | 67,722 | |
Prepaid expenses and other current assets | | | 129,672 | | | | 145,236 | |
| | | | | | | | |
Total current assets | | | 1,472,414 | | | | 1,406,737 | |
Property, plant and equipment, net | | | 545,164 | | | | 534,469 | |
Goodwill | | | 3,093,691 | | | | 3,048,405 | |
Other intangible assets with indefinite lives | | | 56,702 | | | | 36,451 | |
Finite-lived intangible assets, net | | | 1,684,611 | | | | 1,834,225 | |
Restricted cash | | | 29,370 | | | | — | |
Deferred financing costs, net, and other non-current assets | | | 84,073 | | | | 108,857 | |
Investments in unconsolidated entities | | | 86,830 | | | | 90,491 | |
Deferred tax assets | | | 7,959 | | | | 8,293 | |
| | | | | | | | |
Total assets | | $ | 7,060,814 | | | $ | 7,067,928 | |
| | | | | | | | |
| LIABILITIES AND EQUITY | | | | | | | | |
Current liabilities: | | | | | | | | |
Current portion of long-term debt | | $ | 49,112 | | | $ | 60,232 | |
Current portion of capital lease obligations | | | 6,855 | | | | 6,684 | |
Accounts payable | | | 187,371 | | | | 169,974 | |
Accrued expenses and other current liabilities | | | 429,848 | | | | 411,919 | |
| | | | | | | | |
Total current liabilities | | | 673,186 | | | | 648,809 | |
| | | | | | | | |
Long-term liabilities: | | | | | | | | |
Long-term debt, net of current portion | | | 3,772,788 | | | | 3,628,675 | |
Capital lease obligations, net of current portion | | | 14,407 | | | | 12,917 | |
Deferred tax liabilities | | | 329,249 | | | | 428,188 | |
Other long-term liabilities | | | 188,336 | | | | 166,635 | |
| | | | | | | | |
Total long-term liabilities | | | 4,304,780 | | | | 4,236,415 | |
| | | | | | | | |
Commitments and contingencies(Notes 8, 9 and 11) | | | | | | | | |
Stockholders’ equity: | | | | | | | | |
Series B preferred stock, $0.001 par value (liquidation preference: $709,763 at December 31, 2013 and 2012); Authorized: 2,300 shares; Issued: 2,065 shares at December 31, 2013 and 2012; Outstanding: 1,774 shares at December 31, 2013 and 2012 | | | 606,468 | | | | 606,468 | |
Common stock, $0.001 par value; Authorized: 200,000 shares; Issued: 89,666 shares at December 31, 2013 and 88,576 shares at December 31, 2012; Outstanding: 81,987 shares at December 31, 2013 and 80,897 shares at December 31, 2012 | | | 90 | | | | 89 | |
Additional paid-in capital | | | 3,319,168 | | | | 3,299,935 | |
Accumulated deficit | | | (1,636,227 | ) | | | (1,564,973 | ) |
Treasury stock, at cost, 7,679 shares at December 31, 2013 and 2012 | | | (184,971 | ) | | | (184,971 | ) |
Accumulated other comprehensive income (loss) | | | (26,562 | ) | | | 23,874 | |
| | | | | | | | |
Total stockholders’ equity | | | 2,077,966 | | | | 2,180,422 | |
Non-controlling interests | | | 4,882 | | | | 2,282 | |
| | | | | | | | |
Total equity | | | 2,082,848 | | | | 2,182,704 | |
| | | | | | | | |
Total liabilities and equity | | $ | 7,060,814 | | | $ | 7,067,928 | |
| | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-5
ALERE INC. AND SUBSIDIARIES
CONSOLIDATEDSTATEMENTS OF EQUITY
(in thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Preferred Stock | | | Common Stock | | | Additional
Paid-in
Capital | | | Accumulated
Deficit | | | Accumulated
Other
Compre-
hensive
Income
(Loss) | | | Treasury Stock,
at cost | | | Total
Stockholders’
Equity | | | Non-
controlling
Interest | | | Total
Equity | | | Redeemable
Non-
controlling
Interest | |
| | | Number of
Shares | | | $0.001
Par
Value | | | | | | | | | |
| | Number of
Shares | | | Amount | | | | | | | | Number of
Shares | | | Value | | | | | |
BALANCE, DECEMBER 31, 2010 | | | 2,091 | | | $ | 718,554 | | | | 84,928 | | | $ | 85 | | | $ | 3,232,997 | | | $ | (1,377,184 | ) | | $ | 690 | | | | 24 | | | $ | (104 | ) | | $ | 2,575,038 | | | $ | 2,688 | | | $ | 2,577,726 | | | $ | — | |
Issuance of common stock in connection with acquisitions | | | — | | | | — | | | | 832 | | | | 1 | | | | 16,183 | | | | — | | | | — | | | | — | | | | — | | | | 16,184 | | | | — | | | | 16,184 | | | | — | |
Exercise of common stock options, warrants and shares issued under employee stock purchase plan | | | — | | | | — | | | | 1,887 | | | | 2 | | | | 37,885 | | | | — | | | | — | | | | — | | | | — | | | | 37,887 | | | | — | | | | 37,887 | | | | — | |
Repurchase of common stock | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 7,655 | | | | (184,867 | ) | | | (184,867 | ) | | | — | | | | (184,867 | ) | | | — | |
Repurchase of preferred stock | | | (358 | ) | | | (123,005 | ) | | | — | | | | — | | | | — | | | | 23,935 | | | | — | | | | — | | | | — | | | | (99,070 | ) | | | — | | | | (99,070 | ) | | | — | |
Preferred stock dividends | | | 41 | | | | 10,919 | | | | — | | | | — | | | | (21,632 | ) | | | — | | | | — | | | | — | | | | — | | | | (10,713 | ) | | | — | | | | (10,713 | ) | | | — | |
Stock-based compensation expense | | | — | | | | — | | | | — | | | | — | | | | 21,215 | | | | — | | | | — | | | | — | | | | — | | | | 21,215 | | | | — | | | | 21,215 | | | | — | |
Excess tax benefits on exercised stock options | | | — | | | | — | | | | — | | | | — | | | | 3,423 | | | | — | | | | — | | | | — | | | | — | | | | 3,423 | | | | — | | | | 3,423 | | | | — | |
Minimum pension liability adjustment, net of tax | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (1,618 | ) | | | — | | | | — | | | | (1,618 | ) | | | — | | | | (1,618 | ) | | | — | |
Changes in cumulative translation adjustment, net of tax | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (35,830 | ) | | | — | | | | — | | | | (35,830 | ) | | | — | | | | (35,830 | ) | | | — | |
Unrealized gain on hedging instruments, net of tax | | | — | | | | — | | | | — | | | | — | | | | (297 | ) | | | — | | | | 6,855 | | | | — | | | | — | | | | 6,558 | | | | — | | | | 6,558 | | | | — | |
Unrealized loss on available-for-sale securities, net of tax | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (367 | ) | | | — | | | | — | | | | (367 | ) | | | — | | | | (367 | ) | | | — | |
Acquisition of non-controlling interests | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 34,936 | | | | 34,936 | | | | — | |
Purchase of subsidiary shares from non-controlling interest | | | — | | | | — | | | | — | | | | — | | | | 34,936 | | | | — | | | | — | | | | — | | | | — | | | | 34,936 | | | | (34,936 | ) | | | — | | | | 2,500 | |
Non-controlling interest dividend | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (584 | ) | | | (584 | ) | | | — | |
Redeemable non-controlling interest in subsidiaries’ income | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 3 | | | | 3 | | | | (3 | ) |
Net income (loss) | | | — | | | | — | | | | — | | | | — | | | | — | | | | (133,542 | ) | | | — | | | | — | | | | — | | | | (133,542 | ) | | | 233 | | | | (133,309 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
BALANCE, DECEMBER 31, 2011 | | | 1,774 | | | $ | 606,468 | | | | 87,647 | | | $ | 88 | | | $ | 3,324,710 | | | $ | (1,486,791 | ) | | $ | (30,270 | ) | | | 7,679 | | | $ | (184,971 | ) | | $ | 2,229,234 | | | $ | 2,340 | | | $ | 2,231,574 | | | $ | 2,497 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-6
ALERE INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF EQUITY (Continued)
(in thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Preferred Stock | | | Common Stock | | | Additional
Paid-in
Capital | | | Accumulated
Deficit | | | Accumulated
Other
Compre-
hensive
Income
(Loss) | | | Treasury Stock,
at cost | | | Total
Stockholders’
Equity | | | Non-
controlling
Interest | | | Total
Equity | | | Redeemable
Non-
controlling
Interest | |
| | | | Number of
Shares | | | $0.001
Par
Value | | | | | | | | | |
| | | Number of
Shares | | | Amount | | | | | | | | Number of
Shares | | | Value | | | | | |
BALANCE, DECEMBER 31, 2011 | | | 1,774 | | | $ | 606,468 | | | | 87,647 | | | $ | 88 | | | $ | 3,324,710 | | | $ | (1,486,791 | ) | | $ | (30,270 | ) | | | 7,679 | | | $ | (184,971 | ) | | $ | 2,229,234 | | | $ | 2,340 | | | $ | 2,231,574 | | | $ | 2,497 | |
Exercise of common stock options, warrants and shares issued under employee stock purchase plan | | | — | | | | — | | | | 862 | | | | 1 | | | | 14,923 | | | | — | | | | — | | | | — | | | | — | | | | 14,924 | | | | — | | | | 14,924 | | | | — | |
Issuance of common stock for settlement of an acquisition-related contingent consideration obligation | | | — | | | | — | | | | 67 | | | | — | | | | 1,243 | | | | — | | | | — | | | | — | | | | — | | | | 1,243 | | | | — | | | | 1,243 | | | | — | |
Preferred stock dividends | | | — | | | | — | | | | — | | | | — | | | | (21,293 | ) | | | — | | | | — | | | | — | | | | — | | | | (21,293 | ) | | | — | | | | (21,293 | ) | | | — | |
Stock-based compensation expense | | | — | | | | — | | | | — | | | | — | | | | 15,665 | | | | — | | | | — | | | | — | | | | — | | | | 15,665 | | | | — | | | | 15,665 | | | | — | |
Excess tax benefits on exercised stock options | | | — | | | | — | | | | — | | | | — | | | | (234 | ) | | | — | | | | — | | | | — | | | | — | | | | (234 | ) | | | — | | | | (234 | ) | | | — | |
Minimum pension liability adjustment, net of tax | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (756 | ) | | | — | | | | — | | | | (756 | ) | | | — | | | | (756 | ) | | | — | |
Changes in cumulative translation adjustment, net of tax | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 54,642 | | | | — | | | | — | | | | 54,642 | | | | — | | | | 54,642 | | | | — | |
Unrealized gain on hedging instruments, net of tax | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 388 | | | | — | | | | — | | | | 388 | | | | — | | | | 388 | | | | — | |
Unrealized loss on available-for-sale securities, net of tax | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (130 | ) | | | — | | | | — | | | | (130 | ) | | | — | | | | (130 | ) | | | — | |
Purchase of subsidiary shares from non-controlling interest (Note 5) | | | — | | | | — | | | | — | | | | — | | | | (35,079 | ) | | | — | | | | — | | | | — | | | | — | | | | (35,079 | ) | | | — | | | | (35,079 | ) | | | (2,433 | ) |
Non-controlling interest dividend | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (396 | ) | | | (396 | ) | | | — | |
Net income (loss) | | | — | | | | — | | | | — | | | | — | | | | — | | | | (78,182 | ) | | | — | | | | — | | | | — | | | | (78,182 | ) | | | 338 | | | | (77,844 | ) | | | (64 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
BALANCE, DECEMBER 31, 2012 | | | 1,774 | | | $ | 606,468 | | | | 88,576 | | | $ | 89 | | | $ | 3,299,935 | | | $ | (1,564,973 | ) | | $ | 23,874 | | | | 7,679 | | | $ | (184,971 | ) | | $ | 2,180,422 | | | $ | 2,282 | | | $ | 2,182,704 | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-7
ALERE INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF EQUITY (Continued)
(in thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Preferred Stock | | | Common Stock | | | Additional
Paid-in
Capital | | | Accumulated
Deficit | | | Accumulated
Other
Compre-
hensive
Income
(Loss) | | | Treasury Stock,
at cost | | | Total
Stockholders’
Equity | | | Non-
controlling
Interest | | | Total
Equity | |
| | | | Number of
Shares | �� | | $0.001
Par
Value | | | | | | | | |
| | | Number of
Shares | | | Amount | | | | | | | | Number of
Shares | | | Value | | | | |
BALANCE, DECEMBER 31, 2012 | | | 1,774 | | | $ | 606,468 | | | | 88,576 | | | $ | 89 | | | $ | 3,299,935 | | | $ | (1,564,973 | ) | | $ | 23,874 | | | | 7,679 | | | $ | (184,971 | ) | | $ | 2,180,422 | | | $ | 2,282 | | | $ | 2,182,704 | |
Issuance of common stock under employee compensation plans | | | — | | | | — | | | | 1,090 | | | | 1 | | | | 20,714 | | | | — | | | | — | | | | — | | | | — | | | | 20,715 | | | | — | | | | 20,715 | |
Preferred stock dividends | | | — | | | | — | | | | — | | | | — | | | | (21,293 | ) | | | — | | | | — | | | | — | | | | — | | | | (21,293 | ) | | | — | | | | (21,293 | ) |
Stock-based compensation expense | | | — | | | | — | | | | — | | | | — | | | | 21,210 | | | | — | | | | — | | | | — | | | | — | | | | 21,210 | | | | — | | | | 21,210 | |
Excess tax benefits on exercised stock options | | | — | | | | — | | | | — | | | | — | | | | (1,398 | ) | | | — | | | | — | | | | — | | | | — | | | | (1,398 | ) | | | — | | | | (1,398 | ) |
Minimum pension liability adjustment, net of tax | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (309 | ) | | | — | | | | — | | | | (309 | ) | | | — | | | | (309 | ) |
Changes in cumulative translation adjustment, net of tax | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (50,166 | ) | | | — | | | | — | | | | (50,166 | ) | | | — | | | | (50,166 | ) |
Unrealized gain on hedging instruments, net of tax | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 39 | | | | — | | | | — | | | | 39 | | | | — | | | | 39 | |
Non-controlling interest from acquisition | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1,788 | | | | 1,788 | |
Non-controlling interest dividend | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (164 | ) | | | (164 | ) |
Net income (loss) | | | — | | | | — | | | | — | | | | — | | | | — | | | | (71,254 | ) | | | — | | | | — | | | | — | | | | (71,254 | ) | | | 976 | | | | (70,278 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
BALANCE, DECEMBER 31, 2013 | | | 1,774 | | | $ | 606,468 | | | | 89,666 | | | $ | 90 | | | $ | 3,319,168 | | | $ | (1,636,227 | ) | | $ | (26,562 | ) | | | 7,679 | | | $ | (184,971 | ) | | $ | 2,077,966 | | | $ | 4,882 | | | $ | 2,082,848 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-8
ALERE INC. AND SUBSIDIARIES
CONSOLIDATEDSTATEMENTS OF CASH FLOWS
(in thousands)
| | | | | | | | | | | | |
| | | For The Year Ended December 31, | |
| | | 2013 | | | 2012 | | | 2011 | |
Cash Flows from Operating Activities: | | | | | | | | | | | | |
Net loss | | $ | (70,278 | ) | | $ | (77,907 | ) | | $ | (133,309 | ) |
Adjustments to reconcile net loss to net cash provided by operating activities: | | | | | | | | | | | | |
Non-cash interest expense, including amortization of original issue discounts and deferred financing costs | | | 18,091 | | | | 21,490 | | | | 37,590 | |
Depreciation and amortization | | | 439,430 | | | | 456,847 | | | | 391,576 | |
Non-cash charges for sale of inventories revalued at the date of acquisition | | | 2,504 | | | | 4,681 | | | | 6,010 | |
Non-cash stock-based compensation expense | | | 21,210 | | | | 15,665 | | | | 21,215 | |
Impairment of inventory | | | 337 | | | | 295 | | | | 445 | |
Impairment of long-lived assets | | | 6,313 | | | | 3,489 | | | | 1,549 | |
Impairment of goodwill | | | — | | | | — | | | | 383,612 | |
Impairment of intangible assets | | | 3,282 | | | | 2,988 | | | | 2,938 | |
Gain on sale of joint venture interest | | | — | | | | — | | | | (288,896 | ) |
(Gain) loss on sale of fixed assets | | | 3,320 | | | | (2,151 | ) | | | 1,577 | |
Gain on sales of marketable securities | | | — | | | | (751 | ) | | | (840 | ) |
Equity earnings of unconsolidated entities, net of tax | | | (17,443 | ) | | | (13,245 | ) | | | (8,524 | ) |
Deferred income taxes | | | (103,647 | ) | | | (84,568 | ) | | | (56,761 | ) |
Loss on extinguishment of debt | | | 35,603 | | | | 23,235 | | | | — | |
Loss on disposition | | | 5,124 | | | | — | | | | — | |
Bargain purchase gain | | | (8,023 | ) | | | — | | | | — | |
Other non-cash items | | | 8,594 | | | | 7,336 | | | | (12,247 | ) |
Changes in assets and liabilities, net of acquisitions: | | | | | | | | | | | | |
Accounts receivable, net | | | (36,449 | ) | | | (22,165 | ) | | | (39,408 | ) |
Inventories, net | | | (78,303 | ) | | | (16,791 | ) | | | (20,399 | ) |
Prepaid expenses and other current assets | | | (12,268 | ) | | | (2,526 | ) | | | (53,115 | ) |
Accounts payable | | | 13,026 | | | | (10,127 | ) | | | 6,985 | |
Accrued expenses and other current liabilities | | | 52,348 | | | | 60,416 | | | | 14,282 | |
Other non-current liabilities | | | (24,133 | ) | | | (35,543 | ) | | | 16,973 | |
Cash paid for contingent consideration | | | (13,861 | ) | | | (10,985 | ) | | | — | |
| | | | | | | | | | | | |
Net cash provided by operating activities | | | 244,777 | | | | 319,683 | | | | 271,253 | |
| | | | | | | | | | | | |
Cash Flows from Investing Activities: | | | | | | | | | | | | |
(Increase) decrease in restricted cash | | | (32,400 | ) | | | 5,911 | | | | (6,406 | ) |
Purchases of property, plant and equipment | | | (122,166 | ) | | | (137,393 | ) | | | (132,532 | ) |
Proceeds from sale of property, plant and equipment | | | 3,620 | | | | 22,390 | | | | 947 | |
Cash received from disposition | | | 29,000 | | | | — | | | | 11,491 | |
Cash paid for business acquisitions, net of cash acquired | | | (176,131 | ) | | | (424,586 | ) | | | (631,311 | ) |
Cash received from sales of marketable securities | | | 41 | | | | 3,056 | | | | 9,202 | |
Cash received from (paid for) equity method investments | | | 29,338 | | | | 12,707 | | | | (121,903 | ) |
(Increase) decrease in other assets | | | 10,363 | | | | (56,276 | ) | | | (27,684 | ) |
| | | | | | | | | | | | |
Net cash used in investing activities | | | (258,335 | ) | | | (574,191 | ) | | | (898,196 | ) |
| | | | | | | | | | | | |
Cash Flows from Financing Activities: | | | | | | | | | | | | |
Cash paid for financing costs | | | (9,845 | ) | | | (10,139 | ) | | | (74,680 | ) |
Cash paid for contingent purchase price consideration | | | (42,377 | ) | | | (20,964 | ) | | | (28,305 | ) |
Cash paid for dividends | | | (21,293 | ) | | | (21,293 | ) | | | (5,425 | ) |
Proceeds from issuance of common stock, net of issuance costs | | | 20,863 | | | | 14,924 | | | | 37,886 | |
Repurchase of preferred stock | | | — | | | | — | | | | (99,070 | ) |
Proceeds from issuance of long-term debt | | | 459,951 | | | | 648,535 | | | | 2,096,277 | |
Payments on short-term debt | | | — | | | | (6,240 | ) | | | — | |
Payments on long-term debt | | | (471,546 | ) | | | (311,612 | ) | | | (1,207,454 | ) |
Net proceeds under revolving credit facilities | | | 138,963 | | | | 14,272 | | | | 10,715 | |
Repurchase of common stock | | | — | | | | — | | | | (184,867 | ) |
Excess tax benefits on exercised stock options | | | 461 | | | | 504 | | | | 3,423 | |
Principal payments on capital lease obligations | | | (7,068 | ) | | | (7,003 | ) | | | (4,163 | ) |
Purchase of non-controlling interest | | | — | | | | (2,972 | ) | | | — | |
Other | | | (19,118 | ) | | | (12,267 | ) | | | (4,257 | ) |
| | | | | | | | | | | | |
Net cash provided by financing activities | | | 48,991 | | | | 285,745 | | | | 540,080 | |
| | | | | | | | | | | | |
Foreign exchange effect on cash and cash equivalents | | | (1,871 | ) | | | (2,064 | ) | | | (15,270 | ) |
| | | | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | | | 33,562 | | | | 29,173 | | | | (102,133 | ) |
Cash and cash equivalents, beginning of period | | | 328,346 | | | | 299,173 | | | | 401,306 | |
| | | | | | | | | | | | |
Cash and cash equivalents, end of period | | $ | 361,908 | | | $ | 328,346 | | | $ | 299,173 | |
| | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-9
ALERE INC. AND SUBSIDIARIES
NOTESTO CONSOLIDATED FINANCIAL STATEMENTS
(1) Description of Business and Basis of Presentation of Financial Information
Alere Inc. empowers individuals to take greater control of their health at home, under the supervision of their healthcare providers, by connecting innovative near-patient diagnostic tools with health information solutions. A leading global provider of point-of-care diagnostics and services, we have developed a strong commercial presence in cardiology, infectious disease, toxicology, and diabetes.
Our business is organized into three operating segments: (i) professional diagnostics, (ii) health information solutions and (iii) consumer diagnostics. The professional diagnostics segment includes an array of innovative rapid diagnostic test products and other in vitro diagnostic tests marketed to medical professionals and laboratories for detection of diseases and conditions within our areas of focus identified above. The health information solutions segment provides comprehensive, integrated programs and services focused on wellness, disease and condition management, productivity enhancement and informatics, all designed to reduce health-related costs and enhance the health and quality of life of the individuals we serve. The consumer diagnostics segment consists primarily of manufacturing operations related to our role as the exclusive manufacturer of products for SPD Swiss Precision Diagnostics, or SPD, our 50/50 joint venture with The Procter & Gamble Company, or P&G. SPD has significant operations in the worldwide over-the-counter pregnancy and fertility/ovulation test market.
Acquisitions have historically been an important part of our growth strategy. When we acquired businesses, we sought to complement existing products and services, enhance or expand our product lines and/or expand our customer base. We determined what we were willing to pay for each acquisition partially based upon our expectation that we could cost effectively integrate the products and services of the acquired companies into our existing infrastructure. In addition, we utilized existing infrastructure of the acquired companies to cost effectively introduce our products to new geographic areas. All of these factors contributed to the acquisition prices of acquired businesses that were in excess of the fair value of net assets acquired, resulting in goodwill (Note 4).
The consolidated financial statements include the accounts of Alere Inc. and its subsidiaries. Intercompany transactions and balances are eliminated and net earnings are reduced by the portion of the net earnings of subsidiaries applicable to non-controlling interests. Equity investments in which we exercise significant influence but do not control and are not the primary beneficiary are accounted for using the equity method. Investments in which we are not able to exercise significant influence over the investee and which do not have readily determinable fair values are accounted for under the cost method.
Certain amounts for prior periods have been reclassified to conform to the current period classification. These reclassifications had no effect on net income or equity.
Certain amounts presented may not recalculate directly, due to rounding.
(2) Summary of Significant Accounting Policies
(a) Use of Estimates
To prepare our financial statements in conformity with accounting principles generally accepted in the United States of America, our management must make estimates, judgments and assumptions that may affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ significantly from such estimates under different assumptions or conditions.
F-10
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(2) Summary of Significant Accounting Policies (Continued)
(b) Foreign Currencies
In general, the functional currencies of our foreign subsidiaries are the local currencies. For the purpose of consolidating the financial statements of our foreign subsidiaries, all assets and liabilities of the foreign subsidiaries are translated into U.S. dollars using the exchange rate at each balance sheet date, while the stockholders’ equity accounts are translated at historical exchange rates. Translation gains and losses that result from the conversion of the balance sheets of the foreign subsidiaries into U.S. dollars are recorded to cumulative translation adjustment, which is a component of accumulated other comprehensive income (loss) (Note 15) within stockholders’ equity. The revenue and expenses of our foreign subsidiaries are translated using the average of the rates of exchange in effect during each fiscal month.
Net realized and unrealized foreign currency exchange transaction losses of $4.0 million, $7.9 million and $22.9 million during 2013, 2012 and 2011, respectively, are included as a component of other income (expense), net in the accompanying consolidated statements of operations.
(c) Cash and Cash Equivalents
We consider all highly-liquid investments purchased with original maturities of three months or less at the date of acquisition to be cash equivalents. Cash equivalents consisted of money market funds at December 31, 2013 and 2012.
(d) Restricted Cash
We had restricted cash of $35.7 million and $3.1 million as of December 31, 2013 and 2012, respectively. Of the $35.7 million as of December 31, 2013, $29.4 million was classified as non-current on our consolidated balance sheet, as it secures a foreign bank loan arrangement that we entered into during the third quarter in 2013 and, under the terms of the loan agreement, is required to remain on deposit for two years.
(e) Marketable Securities
Securities classified as available-for-sale or trading are carried at fair value, as determined by quoted market prices at the balance sheet date. Realized gains and losses on securities are included in other income (expense), net, on a specific identification basis. Unrealized holding gains and losses (except for other than temporary impairments) on securities classified as available-for-sale, are reported in accumulated other comprehensive income, net of related tax effects. Marketable securities that are held indefinitely are classified in our accompanying consolidated balance sheets as long-term marketable securities.
(f) Inventories
Inventories are stated at the lower of cost (first-in, first-out) or market and are made up of raw material, work-in-process and finished goods. The cost elements of work-in-process and finished goods inventory consist of raw material, direct labor and manufacturing overhead. Where finished goods inventory is purchased from third-party manufacturers, the costs of finished goods inventory recorded in the financial statements represent the costs to acquire such inventory.
(g) Property, Plant and Equipment
We record property, plant and equipment at historical cost or, in the case of a business combination, at fair value on the date of the business combination. Depreciation is computed using the straight-line method based on the following estimated useful lives of the related assets: machinery,
F-11
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(2) Summary of Significant Accounting Policies (Continued)
laboratory equipment and tooling, 1-15 years; buildings, 7-61 years; leasehold improvements, lesser of the remaining term of the lease or estimated useful life of the asset; computer software and equipment, 1-10 years and furniture and fixtures, 2-16 years. Land is not depreciated. Depreciation expense related to property, plant and equipment amounted to $120.9 million, $109.7 million and $83.7 million in 2013, 2012 and 2011, respectively. Fully-depreciated property, plant and equipment that are still in use remain on the books until disposal or retirement. When property, plant and equipment are retired or disposed of, the cost and respective accumulated depreciation are removed from the books. Any gain or loss on disposal is recorded in the income statement. Expenditures for repairs and maintenance are expensed as incurred.
(h) Goodwill and Other Intangible Assets with Indefinite Lives
Goodwill relates to amounts that arose in connection with our various business combinations and represents the difference between the purchase price and the fair value of the identifiable tangible and intangible net assets when accounted for using the acquisition method of accounting. Goodwill is not amortized, but is subject to periodic review for impairment.
We test goodwill and other intangible assets with indefinite lives at the reporting unit level for impairment on an annual basis and between annual tests, if events and circumstances indicate it is more likely than not that the fair value of a reporting unit is less than its carrying value. Events that would indicate impairment and trigger an interim impairment assessment include, but are not limited to, current economic and market conditions, including a decline in market capitalization, a significant adverse change in legal factors, business climate or operational performance of the business and an adverse action or assessment by a regulator.
In performing the annual goodwill impairment test, we utilize the two-step approach. The first step, or Step 1, requires a comparison of the carrying value of each reporting unit to its estimated fair value. To estimate the fair value of our reporting units for Step 1, we use a combination of the income approach, the market comparable approach and the market transaction approach. The income approach is based on a discounted cash flow analysis, or DCF approach, and calculates the fair value by estimating the after-tax cash flows attributable to a reporting unit and then discounting the after-tax cash flows to a present value, using a risk-adjusted discount rate. Assumptions used in the DCF approach require the exercise of significant judgment, including judgment about appropriate discount rates and terminal values, growth rates and the amount and timing of expected future cash flows. The forecasted cash flows are based on our most recent budget and for years beyond the budget, our estimates are based on assumed growth rates. We believe our assumptions are consistent with the plans and estimates used to manage the underlying businesses. The discount rates, which are intended to reflect the risks inherent in future cash flow projections, used in the DCF approach are based on estimates of the weighted-average cost of capital, or WACC, of market participants relative to each respective reporting unit. The market approaches consider comparable and transactional market data based on multiples of revenue or earnings before interest, taxes, depreciation and amortization, or EBITDA based on trading multiples of selected guidelines companies and deal multiples of selected target companies.
If the carrying value of a reporting unit exceeds its estimated fair value, we are required to perform the second step, or Step 2, of the annual goodwill impairment test to measure the amount of impairment loss, if any. Step 2 of the goodwill impairment test compares the implied fair value of a reporting unit’s goodwill to its carrying value. The implied fair value of goodwill is calculated as the difference between the fair value of the reporting unit and the estimated fair value of its assets and liabilities. To the extent this amount is below the carrying value of goodwill, an impairment charge is recorded to write down the carrying value to its implied value.
F-12
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(2) Summary of Significant Accounting Policies (Continued)
Impairment charges related to goodwill have no impact on our cash balances or on compliance with financial covenants under our Amended and Restated Credit Agreement.
2013 Annual Goodwill Impairment Test
We conducted our 2013 annual goodwill impairment test for our reporting units during the fourth quarter of 2013. Key assumptions (which vary by reporting unit) used in determining fair value under the DCF approach included discount rates ranging from 11.0% to 14.0%, projected compound average revenue growth rates of 4.0% to 11.4% and terminal value growth rates of 3.0% to 4.0%. In determining the appropriate discount rate, we considered the WACC for each reporting unit, which among other factors considers the cost of common equity capital and the marginal cost of debt of market participants. Key assumptions (which again vary by reporting unit) used in determining fair value under the market approaches were based on observed market multiples of enterprise value to revenue and EBITDA for both comparable publicly-traded companies and recent merger and acquisition transactions involving similar companies to estimate appropriate controlling basis multiples to apply to each of the reporting units. Based on the multiples implied by this market data, we selected multiples of revenue of 0.8 to 2.9 times and multiples of EBITDA of 6.4 to 10.6 times. In assessing the reasonableness of our estimated fair values of the reporting units, management compared the results of the valuation analyses against our then-current market capitalization to imply a control premium. Based on this analysis, the implied control premium was within the range of comparable industry transactions.
The Step 1 impairment test indicated the estimated fair value of the professional diagnostics, health information solutions and consumer diagnostics reporting units exceeded the carrying value of their reporting unit’s net assets as follows: by $1.6 billion, $31.5 million and $92.7 million, respectively, or 30.2%, 7.7% and 45.5%, respectively.
The estimate of fair value requires significant judgment. We based our fair value estimates on assumptions that we believe to be reasonable but that are unpredictable and inherently uncertain, including estimates of future growth rates and operating margins and assumptions about the overall economic climate and the competitive environment for our business units. There can be no assurance that our estimates and assumptions made for purposes of our goodwill and identifiable intangible asset testing as of the time of testing will prove to be accurate predictions of the future. If our assumptions regarding business plans, competitive environment or anticipated growth rates are not correct, we may be required to record goodwill and/or intangible asset impairment charges in future periods, whether in connection with our next annual impairment testing or earlier, if an indicator of an impairment is present before our next annual evaluation.
2012 Annual Goodwill Impairment Test
We conducted our 2012 annual goodwill impairment test for our reporting units during the fourth quarter of 2012. Key assumptions (which vary by reporting unit) used in determining fair value under the DCF approach included discount rates ranging from 11.0% to 15.0%, projected compound average revenue growth rates of 3.0% to 8.1% and terminal value growth rates of 3.0% to 4.0%. The determination of the appropriate discount rate and key assumptions were the same as those in the 2013 Annual Goodwill Impairment Test above. Based on the multiples implied by this market data, we selected multiples of revenue of 0.9 to 2.4 times and multiples of EBITDA of 6.1 to 8.9 times. In assessing the reasonableness of our estimated fair values of the reporting units, management compared the results of the valuation analyses against our then-current market capitalization to imply a control premium. Based on this analysis, the implied control premium was within the range of comparable industry transactions.
F-13
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(2) Summary of Significant Accounting Policies (Continued)
The Step 1 impairment test indicated the estimated fair value of the professional diagnostics, health information solutions and consumer diagnostics reporting units exceeded the carrying value of their reporting unit’s net assets as follows: by $400.0 million, $41.2 million and $54.0 million, respectively, or 7.9%, 9.4% and 27.2%, respectively.
2011 Annual Goodwill Impairment Test
We conducted our 2011 annual impairment test for our reporting units during the fourth quarter of 2011. Key assumptions (which vary by reporting unit) used in determining fair value under the DCF Approach included discount rates ranging from 11.0% to 14.5%, projected compound average revenue growth rates of 4.9% to 10.0% and terminal value growth rates of 4.0%. The determination of the appropriate discount rate and key assumptions were the same as those in the 2013 Annual Goodwill Impair Test above. Based on the multiples implied by this market data, we selected multiples of revenue of 0.8 to 2.6 times and multiples of EBITDA of 5.6 to 9.3 times. In assessing the reasonableness of our estimated fair values of the reporting units, management compared the results of the valuation analyses against our then-current market capitalization to imply a control premium. Based on this analysis, the implied control premium was within the range of comparable industry transactions.
The Step 1 impairment test indicated the estimated fair value of the professional diagnostics and consumer diagnostics reporting units exceeded the carrying value of their reporting unit’s net assets as follows: by $567.2 million and $101.9 million, or 13.5% and 53.5%, respectively.
The Step 1 impairment test also indicated that the carrying value of the net assets of our health information solutions reporting unit exceeded the estimated fair value of the reporting unit. As a result, we were required to perform Step 2 of the goodwill impairment test to determine the amount, if any, of goodwill impairment charges for the health information solutions reporting unit. We completed Step 2, consistent with the procedures described above, and determined that a goodwill impairment charge in the amount of approximately $383.6 million was required. The resulting goodwill impairment charge is reflected in operating income (loss) in our accompanying consolidated statements of operations.
This impairment was primarily driven by reduced future cash flow expectations from the reporting unit principally as a result of an increasingly competitive business environment for services provided by the reporting unit, including the insourcing of certain services by key customers and a reduction in spending by other customers as a result in part of the continuing difficult economic climate. Also contributing to the impairment charge was the lower valuations ascribed to similar comparable businesses in the public markets.
(i) Impairment of Other Long-lived Tangible and Intangible Assets
Our intangible assets consist primarily of core technology, in-process research and development, patents, trademarks, trade names, customer relationships, distribution rights and non-competition agreements. The majority of our intangible assets were recorded in connection with our various business combinations. Our intangible assets are recorded at fair value at the time of their acquisition. We amortize intangible assets over their estimated useful lives.
The estimated useful lives of the individual categories of intangible assets were based on the nature of the applicable intangible asset and the expected future cash flows to be derived from the intangible asset. Amortization of intangible assets with finite lives is recognized over the shorter of the
F-14
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(2) Summary of Significant Accounting Policies (Continued)
respective lives of the agreement or the period of time the intangible assets are expected to contribute to future cash flows. We amortize our finite-lived intangible assets based on patterns on which the respective economic benefits are expected to be realized.
We evaluate long-lived tangible and intangible assets whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If indicators of impairment are present with respect to long-lived tangible and intangible assets used in operations and undiscounted future cash flows are not expected to be sufficient to recover the assets’ carrying amount, additional analysis is performed as appropriate and the carrying value of the long-lived assets is reduced to the estimated fair value, if this is lower, and an impairment loss is charged to expense in the period the impairment is identified.
We conduct our annual goodwill impairment test for our reporting units during the fourth quarter of each year. The impairment test conducted during 2011 indicated there was an impairment of goodwill associated with our health information solutions reporting unit, and thus, a potential impairment of our long-lived tangible and intangible assets associated with the same reporting unit. We conducted an analysis,utilizing an undiscounted cash flow model. The analysis indicated there was no impairment of the long-lived tangible or intangible assets associated with our health information solutions reporting unit.
(j) Acquired In-process Research and Development (IPR&D)
Acquired IPR&D represents the fair value assigned to research and development assets that we acquire as part of business combinations, and which have not been completed at the date of acquisition. The acquired IPR&D is capitalized as an intangible asset and tested for impairment at least annually until commercialization, after which time the IPR&D is amortized over its estimated useful life. We utilize a discounted probable future cash flow model on a project-by-project basis to value acquired IPR&D. Significant assumptions used in the model include the period in which material net cash inflows from significant projects are expected to commence, anticipated material changes from historical pricing, margins and expense levels and an appropriate risk adjusted discount rate applied to the project’s cash flows.
(k) Business Acquisitions
Our business acquisitions have historically been made at prices above the fair value of the assets acquired and liabilities assumed, resulting in goodwill, based on our expectations of synergies and other benefits of combining the businesses. These synergies and benefits include elimination of redundant facilities, functions and staffing; use of our existing commercial infrastructure to expand sales of the products of the acquired businesses; and use of the commercial infrastructure of the acquired businesses to expand product sales in a cost-efficient manner.
Significant judgment is required in estimating the fair value of intangible assets and in assigning their respective useful lives. The fair value estimates are based on available historical information and on future expectations and assumptions deemed reasonable by management, but are inherently uncertain.
We generally employ the income method to estimate the fair value of intangible assets, which is based on forecasts of the expected future cash flows attributable to the respective assets. Significant estimates and assumptions inherent in the valuations reflect a consideration of other marketplace participants, and include the amount and timing of future cash flows (including expected growth rates
F-15
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(2) Summary of Significant Accounting Policies (Continued)
and profitability), the underlying product life cycles, economic barriers to entry, a brand’s relative market position and the discount rate applied to the cash flows. Unanticipated market or macroeconomic events and circumstances may occur, which could affect the accuracy or validity of the estimates and assumptions.
Net assets acquired are recorded at their fair value and are subject to adjustment upon finalization of the fair value analysis. We are not aware of any information that indicates the final fair value analysis will differ materially from the preliminary estimates.
During 2013, 2012 and 2011, we expensed acquisition-related costs of $3.1 million, $9.7 million and $11.5 million, respectively, in general and administrative expense.
(l) Income Taxes
Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the year in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts that are more likely than not to be realized in the future (Note 16).
We account for uncertain tax positions using a “more-likely-than-not” threshold for recognizing and resolving uncertain tax positions. We evaluate uncertain tax positions on a quarterly basis and consider various factors, including, but not limited to, changes in tax law, the measurement of tax positions taken or expected to be taken in tax returns, the effective settlement of matters subject to audit, new audit activity and changes in facts or circumstances related to a tax position.
(m) Revenue Recognition
We primarily recognize revenue when the following four basic criteria have been met: (1) persuasive evidence of an arrangement exists, (2) delivery has occurred or services rendered, (3) the fee is fixed or determinable and (4) collection is reasonably assured.
The majority of our revenue is derived from product sales. We recognize revenue upon transfer of the title of the products to third-party customers, less a reserve for estimated product returns and allowances. Determination of the reserve for estimated product returns and allowances is based on our management’s analyses and judgments regarding certain conditions. Should future changes in conditions prove management’s conclusions and judgments on previous analyses to be incorrect, revenue recognized for any reporting period could be adversely affected.
For products that include installation, and if the installation meets the criteria to be considered a separate element, product revenue is recognized upon delivery, and installation revenue is recognized when the installation is complete. For sales that include customer-specified acceptance criteria, revenue is recognized after the acceptance criteria have been met. Certain of our products require specialized installation. Revenue for these products is deferred until installation is completed. Revenue from services is deferred and recognized over the contractual period, or as services are rendered and accepted by the customer. When arrangements include multiple elements, we use objective evidence
F-16
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(2) Summary of Significant Accounting Policies (Continued)
of fair value to allocate revenue to the elements, and recognize revenue when the criteria for revenue recognition have been met for each element, in accordance with authoritative guidance on multiple-element arrangements.
Additionally, we generate services revenue in connection with contracts with health plans (both commercial and governmental) and self-insured employers, whereby we provide clinical expertise through fee-based arrangements. Revenue for fee-based arrangements is recognized over the period in which the services are provided. Some contracts provide that a portion of our fees are at risk if our customers do not achieve certain financial cost savings or we do not achieve certain other clinical and operational metrics, over a period of time, typically one year. Revenue subject to refund is not recognized if (i) sufficient information is not available to calculate performance measurements or (ii) interim performance measurements indicate that we are not meeting performance targets. If either of these two conditions exists, we record the amounts as other current liabilities in the consolidated balance sheet, deferring recognition of the revenue until we establish that we have met the performance criteria. If we do not meet the performance targets at the end of the contractual period, we are obligated under the contract to refund some or all of the at-risk fees.
We also receive license and royalty revenue from agreements with third-party licensees. Revenue from license and royalty agreements is recognized on a straight-line basis over the obligation period of the related license agreements, or at the time when we have no further obligations. License and royalty fees that the licensees calculate based on their sales, which we have the right to audit under most of our agreements, are generally recognized upon receipt of the license or royalty payments unless we are able to reasonably estimate the fees as they are earned. License and royalty fees that are determinable prior to the receipt thereof are recognized in the period they are earned.
(n) Employee Stock-Based Compensation Arrangements
We account for share-based payments in accordance with Accounting Standards Codification, or ASC 718,Compensation — Stock Compensation. Compensation expense associated with stock options includes amortization based on the grant-date fair value estimated in accordance with the provisions of ASC 718. In addition, we record expense over the offering period in connection with shares issued under our employee stock purchase plan. Compensation expense for stock-based compensation awards includes an estimate for forfeitures and is recognized over the vesting period of the options using the straight-line method. It is our policy to recognize, through additional paid in capital, the excess or windfall tax benefits on stock option deductions, as those deductions are recognized on tax returns.
Our stock option plans provide for grants of options to employees to purchase common stock at or above the fair market value of such shares on the grant date of the award. The options generally vest over a four-year period, beginning on the date of grant, with a graded vesting schedule of 25% at the end of each of the four years. The fair value of each option grant is estimated on the date of grant primarily using a Black-Scholes option-pricing method. We use historical data to estimate the expected price volatility and the expected forfeiture rate. The contractual term of our stock option awards is ten years. The risk-free rate is based on the U.S. Treasury yield curve in effect at the time of grant with a remaining term equal to the expected term of the option. We have not made any dividend payments to common shareholders nor do we have plans to pay dividends in the foreseeable future.
F-17
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(2) Summary of Significant Accounting Policies (Continued)
(o) Net Loss per Common Share
Net loss per common share is based upon the weighted-average number of outstanding common shares and the dilutive effect of common share equivalents, such as options and warrants to purchase common stock, convertible preferred stock and convertible notes, if applicable, that are outstanding each year (Note 12).
(p) Other Operating Expenses
We expense advertising costs as incurred. In 2013, 2012 and 2011, advertising costs amounted to $11.8 million, $22.6 million and $9.9 million, respectively, and are included in sales and marketing expenses in the accompanying consolidated statements of operations.
Shipping and handling costs are included in cost of net revenue in the accompanying consolidated statements of operations. When we charge our customers for shipping and handling costs, these costs are recorded along with product revenues.
(q) Concentration of Credit Risk, Off-Balance Sheet Risks and Other Risks and Uncertainties
Financial instruments that potentially subject us to concentration of credit risk primarily consist of cash and cash equivalents and accounts receivable. We invest our excess cash primarily in high quality securities and limit the amount of our credit exposure to any one financial institution. We do not require collateral or other securities to support customer receivables; however, we perform on-going credit evaluations of our customers and maintain allowances for potential credit losses.
At December 31, 2013 and 2012, no individual customer’s accounts receivable balance was more than 10% of our aggregate accounts receivable. During 2013, 2012 and 2011, no one customer represented more than 10% of our net revenue.
We rely on a number of third parties to manufacture certain of our products. If any of our third-party manufacturers cannot, or will not, manufacture our products in the required volumes, on a cost-effective basis, in a timely manner, or at all, we will have to secure additional manufacturing capacity. Any interruption or delay in manufacturing could have a material adverse effect on our business and operating results.
(r) Financial Instruments and Fair Value of Financial Instruments
Our primary financial instruments at December 31, 2013 and 2012 consisted of cash equivalents, restricted cash, marketable securities, accounts receivable, accounts payable and debt. We apply fair value measurement accounting to value our financial assets and liabilities. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. A fair value hierarchy requires an entity to maximize the use of observable inputs, where available, and minimize the use of unobservable inputs when measuring fair value.
Described below are the three levels of inputs that may be used to measure fair value:
Level 1 Quoted prices in active markets for identical assets or liabilities.
F-18
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(2) Summary of Significant Accounting Policies (Continued)
Level 2 Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
(s) Software for Internal Use and for Resale
We may capitalize certain costs associated with the development of internal-use software, including direct materials and services. Capitalized software is amortized on a straight-line basis over its estimated useful life and is included in computer software and equipment within property, plant and equipment.
We also develop software for resale or lease to external parties and expense the costs of developing software for resale or lease incurred before establishment of technological feasibility of the underlying software. The costs incurred from establishment of technological feasibility until general release of the software are capitalized, and the capitalized software is amortized over its estimated useful life. Capitalized software for resale or lease is included in computer software and equipment within property, plant and equipment.
(t) Research and Development
Our research and development programs focus on the development of cardiology, infectious disease, toxicology, diabetes, oncology and women’s health products together with health information technologies that will facilitate connectivity and information and data management solutions. Research and development costs are expensed as incurred. Payments received from external parties to fund our research and development activities reduce the recorded research and development expenses.
(u) Leases
We lease certain facilities and equipment from external parties under operating leases. Rent expense related to operating leases is recorded in the income statement as incurred. We also lease machinery, laboratory equipment, tooling and other equipment under capital leases. In determining whether a lease is a capital or an operating lease, we estimate the expected term of the lease, which includes certain renewable options as required by lease accounting guidance. Rent deferrals, landlord incentives and rent escalations are included in calculation of minimum lease payments when performing the capital lease tests and when calculating the rent expense for operating leases.
Leased property, plant and equipment that meet the capital lease criteria are capitalized at the lower of the present value of the minimum lease payments or the fair value of the underlying asset at the inception date of the lease. Assets under capital leases are depreciated on a straight-line basis over the lease term.
Leasehold improvements are capitalized and amortized over the shorter of their estimated useful lives or the remainder of the expected term of the lease.
F-19
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(2) Summary of Significant Accounting Policies (Continued)
(v) Recent Accounting Pronouncements
Recently Issued Standards
In July 2013, the FASB issued Accounting Standards Update, or ASU No. 2013-11,Presentation of an Unrecognized Tax Benefit when a Net Operating Loss Carryforward, a Similar Tax Loss, or a Tax Credit Carryforward Exists, or ASU 2013-11. ASU 2013-11 requires an entity to present unrecognized tax benefits as a reduction to deferred tax assets when a net operating loss carryforward, similar tax loss or a tax credit carryforward exists, with limited exceptions. ASU 2013-11 is effective for fiscal years beginning on or after December 15, 2013, and for interim periods within those fiscal years. The adoption of this standard will not have a material impact on our consolidated financial statements.
Recently Adopted Standards
Effective January 1, 2013, we adopted ASU No. 2012-02,Intangibles — Goodwill and Other (Topic 350): Testing Indefinite-Lived Intangible Assets for Impairment, or ASU 2012-02. ASU 2012-02 allows an entity the option to first assess qualitative factors to determine whether the existence of events and circumstances indicates that it is more likely than not that the indefinite-lived intangible asset is impaired. If an entity concludes that it is not more likely than not that the indefinite-lived intangible asset is impaired, then the entity is not required to take further action. However, if an entity concludes otherwise, then it is required to determine the fair value of the indefinite-lived intangible asset and perform the quantitative impairment test by comparing the fair value with the carrying amount. An entity also has the option to bypass the qualitative assessment for any indefinite-lived intangible asset in any period and proceed directly to performing the quantitative impairment test. An entity will be able to resume performing the qualitative assessment in any subsequent period. The adoption of this standard is not expected to have an impact on our financial position, results of operations, comprehensive income or cash flows.
F-20
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(3) Other Balance Sheet Information
Components of selected captions in the consolidated balance sheets consist of (in thousands):
| | | | | | | | |
| | | December 31, | |
| | | 2013 | | | 2012 | |
Inventories, net: | | | | | | | | |
Raw materials | | $ | 118,571 | | | $ | 99,498 | |
Work-in-process | | | 79,559 | | | | 89,895 | |
Finished goods | | | 166,055 | | | | 147,728 | |
| | | | | | | | |
| | $ | 364,185 | | | $ | 337,121 | |
| | | | | | | | |
Property, plant and equipment, net: | | | | | | | | |
Machinery, laboratory equipment and tooling | | $ | 436,231 | | | $ | 416,311 | |
Land and buildings | | | 182,547 | | | | 176,214 | |
Leasehold improvements | | | 56,193 | | | | 45,654 | |
Computer software and equipment | | | 254,528 | | | | 217,940 | |
Furniture and fixtures | | | 35,682 | | | | 33,022 | |
| | | | | | | | |
| | | 965,181 | | | | 889,141 | |
Less: Accumulated depreciation and amortization | | | (420,017 | ) | | | (354,672 | ) |
| | | | | | | | |
| | $ | 545,164 | | | $ | 534,469 | |
| | | | | | | | |
Accrued expenses and other current liabilities: | | | | | | | | |
Compensation and compensation-related | | $ | 103,225 | | | $ | 93,467 | |
Royalty obligations | | | 25,325 | | | | 26,171 | |
Deferred revenue | | | 40,714 | | | | 38,188 | |
Income taxes payable | | | 20,690 | | | | 29,733 | |
Other taxes payable | | | 18,934 | | | | 23,038 | |
Acquisition-related obligations | | | 97,484 | | | | 79,286 | |
Other | | | 123,476 | | | | 122,036 | |
| | | | | | | | |
| | $ | 429,848 | | | $ | 411,919 | |
| | | | | | | | |
(4) Business Combinations
Acquisitions are accounted for using the acquisition method and the acquired companies’ results have been included in the accompanying consolidated financial statements from their respective dates of acquisition. During 2013, 2012 and 2011, we recorded acquisition-related costs of $3.1 million, $9.7 million and $11.5 million, respectively, in general and administrative expense.
Our business acquisitions have historically been made at prices above the fair value of the assets acquired and liabilities assumed, resulting in goodwill, based on our expectations of synergies and other benefits of combining the businesses. These synergies and benefits include elimination of redundant facilities, functions and staffing; use of our existing commercial infrastructure to expand sales of the products of the acquired businesses; and use of the commercial infrastructure of the acquired businesses to expand product sales in a cost-efficient manner.
Net assets acquired are recorded at their fair value and are subject to adjustment upon finalization of the fair value analysis. We are not aware of any information that indicates the final fair value analysis will differ materially from the preliminary estimates. The estimated useful lives of the individual categories of intangible assets were based on the nature of the applicable intangible asset and the
F-21
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(4) Business Combinations (Continued)
expected future cash flows to be derived from the intangible asset. Amortization of intangible assets with finite lives is recognized over the shorter of the respective lives of the agreement or the period of time the intangible assets are expected to contribute to future cash flows. We amortize our finite-lived intangible assets based on patterns on which the respective economic benefits are expected to be realized.
(a) Acquisitions in 2013
(i) Epocal
On February 1, 2013, we acquired Epocal, Inc., or Epocal, located in Ottawa, Canada, a provider of technologies that support blood gas and electrolyte testing at the point of care. The preliminary aggregate purchase price was approximately $248.5 million, which consisted of $151.4 million in cash, a $22.1 million settlement of a pre-existing arrangement and a contingent consideration obligation with an aggregate acquisition date fair value of $75.0 million. The operating results of Epocal are included in our professional diagnostics reporting unit and business segment. The amount allocated to goodwill from this acquisition is not deductible for tax purposes.
(ii) Other acquisitions in 2013
During the year ended December 31, 2013, we acquired the following businesses for a preliminary aggregate purchase price of $57.6 million, which included cash payments totaling $28.2 million, a $17.5 million settlement of a pre-existing arrangement, contingent consideration obligations with an aggregate acquisition date fair value of $1.3 million, deferred purchase price consideration with an acquisition date fair value of $0.8 million and an $8.0 million bargain purchase gain.
| | • | | certain assets of PT Mega Medika Mandiri, or Mega Medika, located in South Jakarta, Indonesia, a distributor of infectious disease products to the Indonesian marketplace as well as materials for vaccines to a pharmaceutical customer (Acquired January 2013) |
| | • | | Discount Diabetic, LLC, or Discount Diabetic, located in Phoenix, Arizona, a provider of blood glucose monitoring products, including diabetes testing systems and test strips and other products (Acquired April 2013) |
| | • | | the Medicare fee-for-service assets of Liberty Medical, or the Liberty business, located in Port St. Lucie, Florida, a leading mail order provider of diabetes testing supplies serving the needs of both Type 1 and Type 2 diabetic patients (Acquired April 2013) |
| | • | | 51% share in Cardio Selfcare B.V., subsequently renamed Alere Health Services B.V., or Alere Health Services, located in Ede, the Netherlands, a developer of innovative software for the healthcare industry that develops and licenses software and sells medical devices to enable patients to perform medical self-care, including thrombosis self-care (Acquired May 2013) |
| | • | | 74.9% interest in Pantech Proprietary Limited, or Pantech, located in Durban, South Africa, a supplier of rapid diagnostic test kits, including HIV, malaria, syphilis, drugs of abuse, 10 parameter urine sticks, glucometers and glucose sticks (Acquired July 2013) |
| | • | | Certain assets of Simplex Healthcare, Inc. and its subsidiaries, or Simplex, located in Tennessee, a provider of home delivery of diabetes-related medical supplies and products (Acquired November 2013) |
The operating results of Mega Medika, Discount Diabetic, the Liberty business, Alere Health Services, Pantech, and Simplex are included in our professional diagnostics reporting unit and business segment.
F-22
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(4) Business Combinations (Continued)
Our consolidated statement of operations for the year ended December 31, 2013 included revenue totaling approximately $83.0 million related to these businesses. Goodwill has been recognized in the Mega Medika, Alere Health Services, Pantech, and Simplex acquisitions and amounted to approximately $2.8 million. The goodwill related to the Mega Medika and Simplex acquisitions is deductible for tax purposes, but the goodwill related to the Pantech acquisition is not.
With respect to our acquisition of the Liberty business, the purchase price of the acquisition has been allocated to the net tangible and intangible assets acquired, with the excess of the fair value of assets acquired over the purchase price recorded as a bargain purchase gain. The $8.0 million bargain purchase gain has been recorded in other income (expense), net in our consolidated statement of operations and is not recognized for tax purposes. The bargain purchase gain resulted from our operating cost structure which we believe will allow us to operate this business more cost effectively than the sellers.
A summary of the preliminary fair values of the net assets acquired for the acquisitions consummated in 2013 is as follows (in thousands):
| | | | | | | | | | | | |
| | | Epocal | | | Other | | | Total | |
Current assets(1) | | $ | 12,089 | | | $ | 13,488 | | | $ | 25,577 | |
Property, plant and equipment | | | 1,267 | | | | 1,669 | | | | 2,936 | |
Goodwill | | | 99,836 | | | | 2,757 | | | | 102,593 | |
Intangible assets | | | 164,400 | | | | 51,180 | | | | 215,580 | |
Other non-current assets | | | 17,223 | | | | 29 | | | | 17,252 | |
| | | | | | | | | | | | |
Total assets acquired | | | 294,815 | | | | 69,123 | | | | 363,938 | |
| | | | | | | | | | | | |
Current liabilities | | | 2,627 | | | | 5,398 | | | | 8,025 | |
Non-current liabilities | | | 43,727 | | | | 6,175 | | | | 49,902 | |
| | | | | | | | | | | | |
Total liabilities assumed | | | 46,354 | | | | 11,573 | | | | 57,927 | |
| | | | | | | | | | | | |
Net assets acquired | | | 248,461 | | | | 57,550 | | | | 306,011 | |
Less: | | | | | | | | | | | | |
Contingent consideration | | | 75,000 | | | | 1,264 | | | | 76,264 | |
Settlement of pre-existing arrangements | | | 22,088 | | | | 17,500 | | | | 39,588 | |
Non-controlling interest | | | — | | | | 1,774 | | | | 1,774 | |
Bargain purchase gain | | | — | | | | 8,023 | | | | 8,023 | |
Deferred purchase price consideration | | | — | | | | 768 | | | | 768 | |
| | | | | | | | | | | | |
Cash paid | | $ | 151,373 | | | $ | 28,221 | | | $ | 179,594 | |
| | | | | | | | | | | | |
| (1) | Includes approximately $3.3 million of acquired cash. |
F-23
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(4) Business Combinations (Continued)
The following are the intangible assets acquired and their respective fair values and weighted-average useful lives (dollars in thousands):
| | | | | | | | | | | | | | | | |
| | | Epocal | | | Other | | | Total | | | Weighted-
average
Useful Life | |
Core technology and patents | | $ | 119,700 | | | $ | — | | | $ | 119,700 | | | | 20.0 years | |
Software | | | — | | | | 2,154 | | | | 2,154 | | | | 5.7 years | |
Trademarks and trade names | | | 20,500 | | | | 80 | | | | 20,580 | | | | 19.1 years | |
License agreements | | | — | | | | 620 | | | | 620 | | | | 1.5 years | |
Customer relationships | | | — | | | | 42,510 | | | | 42,510 | | | | 11.5 years | |
Other | | | — | | | | 5,816 | | | | 5,816 | | | | 3.0 years | |
In-process research and development | | | 24,200 | | | | — | | | | 24,200 | | | | N/A | |
| | | | | | | | | | | | | | | | |
Total intangible assets | | $ | 164,400 | | | $ | 51,180 | | | $ | 215,580 | | | | | |
| | | | | | | | | | | | | | | | |
(b) Acquisitions in 2012
(i) eScreen
On April 2, 2012, we acquired eScreen, Inc., or eScreen, headquartered in Overland Park, Kansas, a technology-enabled provider of employment drug screening solutions for hiring and maintaining healthier and more efficient workforces. The preliminary aggregate purchase price was approximately $295.0 million, which consisted of $271.4 million in cash and a contingent consideration obligation with an aggregate acquisition date fair value of $23.6 million. Included in our consolidated statements of operations for the year ended December 31, 2012 is revenue totaling approximately $116.7 million related to eScreen. The operating results of eScreen are included in our professional diagnostics reporting unit and business segment. The amount allocated to goodwill from this acquisition is not deductible for tax purposes.
(ii) Other acquisitions in 2012
During 2012, we acquired the following businesses for a preliminary aggregate purchase price of $199.5 million, which included cash payments totaling $147.5 million and contingent consideration obligations with aggregate acquisition date fair values of $52.0 million.
| | • | | Reatrol Comercializacao De Produtos De Saude, LDA, subsequently renamed Alere Lda, located in Vila Nova de Gaia, Portugal, a distributor of products for drugs of abuse testing (Acquired January 2012) |
| | • | | Kullgren Holding AB, or Kullgren, located in Gensta, Sweden, a company that manufactures and distributes high-quality intimacy and pharmaceutical products (Acquired February 2012) |
| | • | | Wellogic ME FZ-LLC, or Wellogic UAE, located in Dubai, United Arab Emirates, a company that provides development services to Alere Wellogic, LLC, which acquired the assets of Method Factory, Inc. (d/b/a Wellogic), or Wellogic, in December 2011 (Acquired February 2012) |
| | • | | certain assets, primarily including customer and patient lists, of AmMed Direct LLC, or AmMed, located near Nashville, Tennessee, a privately-owned mail-order provider of home-diabetes testing products and supplies (Acquired March 2012) |
F-24
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(4) Business Combinations (Continued)
| | • | | MedApps Holding Company, Inc., or MedApps, headquartered in Scottsdale, Arizona, a developer of innovative remote health monitoring solutions that deliver efficient cost-effective connectivity between patient, care provider and electronic medical records (Acquired July 2012) |
| | • | | Amedica Biotech, Inc., or Amedica, located in Hayward, California, a company focused on the development and manufacture of in vitro diagnostic tests (Acquired July 2012) |
| | • | | DiagnosisOne, Inc., or DiagnosisOne, located in Lowell, Massachusetts, a software company that provides clinical analytics technology and data-driven content to hospitals, physician groups, insurers and governments (Acquired July 2012) |
| | • | | Seelen Care Laege-og & Hospitalsartikler ApS, or Seelen, located in Holstebro, Denmark, a distributor of consumables, instruments and equipment to doctors, specialists and physiotherapists (Acquired August 2012) |
| | • | | certain assets of Diagnostik Nord, or Diagnostik, located in Schwerin, Germany, a company focused on the sale of drug screening and in vitro diagnostic medical devices and a provider of diagnostic solutions (Acquired September 2012) |
| | • | | Healthcare Connections Limited, or HCC, located in Buckinghamshire, United Kingdom, an occupational health provider specializing in employment medical programs, preventative health schemes and drug and alcohol sample collection services (Acquired November 2012) |
| | • | | the diagnostic division of Medial spol. s.r.o., subsequently renamed Alere s.r.o., located in Prague, Czech Republic, a distributor of laboratory diagnostic devices, devices operating in the point-of-care testing regime, diagnostic kits and tests for biochemistry, hematology, and microbiology (Acquired November 2012) |
| | • | | certain assets of Quantum Diagnostics, or Quantum Australia, located in Australia, an on-line medical supply company that provides a range of affordable drug and alcohol tests for personal, business and professional medical use. (Acquired November 2012) |
| | • | | certain assets of NationsHealth, Inc., or NationsHealth, headquartered in Sunrise, Florida, a privately-owned mail-order provider of diabetes home-testing products and supplies, and a share acquisition of NationsHealth’s subsidiary in the Philippines, or NationsHealth Philippines (Acquired December 2012) |
| | • | | Branan Medical Corporation, or Branan, headquartered in Irvine, California, a manufacturer of drugs of abuse testing products (Acquired December 2012) |
The operating results of Alere Lda, AmMed, MedApps, Amedica, Seelen, Diagnostik, HCC, Alere s.r.o., Quantum Australia, NationsHealth and Branan are included in our professional diagnostics reporting unit and business segment. The operating results of Wellogic UAE and DiagnosisOne are included in our health information solutions reporting unit and business segment. The operating results of Kullgren are included in our consumer diagnostics reporting unit and business segment.
Our consolidated statements of operations for the year ended December 31, 2012 included revenue totaling approximately $44.6 million related to these businesses. Goodwill has been recognized in all of these acquisitions and amounted to approximately $94.2 million. Goodwill related to the acquisitions of AmMed, Diagnostik and the US-based assets of NationsHealth, which totaled $8.8 million, is deductible for tax purposes. The goodwill related to the remaining 2012 acquisitions is not deductible for tax purposes.
F-25
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(4) Business Combinations (Continued)
A summary of the preliminary fair values of the net assets acquired for the acquisitions consummated in 2012 is as follows (in thousands):
| | | | | | | | | | | | |
| | | eScreen | | | Other | | | Total | |
Current assets(1) | | $ | 33,690 | | | $ | 13,615 | | | $ | 47,305 | |
Property, plant and equipment | | | 5,806 | | | | 3,223 | | | | 9,029 | |
Goodwill | | | 144,522 | | | | 94,219 | | | | 238,741 | |
Intangible assets | | | 204,000 | | | | 121,223 | | | | 325,223 | |
Other non-current assets | | | 9,783 | | | | 9,363 | | | | 19,146 | |
| | | | | | | | | | | | |
Total assets acquired | | | 397,801 | | | | 241,643 | | | | 639,444 | |
| | | | | | | | | | | | |
Current liabilities | | | 22,865 | | | | 5,452 | | | | 28,317 | |
Non-current liabilities | | | 79,945 | | | | 36,659 | | | | 116,604 | |
| | | | | | | | | | | | |
Total liabilities assumed | | | 102,810 | | | | 42,111 | | | | 144,921 | |
| | | | | | | | | | | | |
Net assets acquired | | | 294,991 | | | | 199,532 | | | | 494,523 | |
Less: | | | | | | | | | | | | |
Contingent consideration | | | 23,600 | | | | 52,020 | | | | 75,620 | |
| | | | | | | | | | | | |
Cash paid | | $ | 271,391 | | | $ | 147,512 | | | $ | 418,903 | |
| | | | | | | | | | | | |
| (1) | Includes approximately $3.8 million of acquired cash. |
The following are the intangible assets acquired and their respective fair values and weighted-average useful lives (dollars in thousands):
| | | | | | | | | | | | | | | | |
| | | eScreen | | | Other | | | Total | | | Weighted-
average
Useful Life | |
Core technology and patents | | $ | 93,200 | | | $ | 54,903 | | | $ | 148,103 | | | | 18.7 years | |
Trademarks and trade names | | | 17,300 | | | | 2,090 | | | | 19,390 | | | | 18.3 years | |
Customer relationships | | | 79,600 | | | | 56,885 | | | | 136,485 | | | | 18.1 years | |
Non-competition agreements | | | — | | | | 1,118 | | | | 1,118 | | | | 5.1 years | |
Other | | | 13,900 | | | | 1,327 | | | | 15,227 | | | | 9.2 years | |
In-process research and development | | | — | | | | 4,900 | | | | 4,900 | | | | N/A | |
| | | | | | | | | | | | | | | | |
Total intangible assets | | $ | 204,000 | | | $ | 121,223 | | | $ | 325,223 | | | | | |
| | | | | | | | | | | | | | | | |
(c) Acquisitions in 2011
(i) Arriva
On November 23, 2011, we acquired Arriva Medical LLC, or Arriva, located in Coral Springs, Florida, a privately-owned mail-order provider of diabetes home-testing products and supplies. The aggregate purchase price was $79.5 million, which consisted of a cash payment totaling $64.4 million and 806,452 shares of our common stock with an aggregate fair value of $15.2 million. Included in our consolidated statement of operations for the year ended December 31, 2011 is revenue totaling approximately $5.3 million related to this acquired business. The operating results of Arriva are included in our professional diagnostics reporting unit and business segment. The amount allocated to goodwill from this acquisition is deductible for tax purposes.
F-26
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(4) Business Combinations (Continued)
(ii) Axis-Shield
On November 1, 2011, we acquired Axis-Shield, located in Dundee, Scotland, a U.K. publicly traded company focused on the development and manufacture of in vitro diagnostic tests for use in clinical laboratories and at the point of care. The aggregate purchase price was $388.8 million, which consisted of cash payments totaling $279.6 million and the fair value of previously-held investment totaling $109.2 million. Included in our consolidated statement of operations for the year ended December 31, 2011 is revenue totaling approximately $36.7 million, including $1.8 million of license and royalty revenue, related to this acquired business. The operating results of Axis-Shield are included in our professional diagnostics reporting unit and business segment. We do not expect the amount allocated to goodwill to be deductible for tax purposes.
(iii) Avee
On October 3, 2011, we acquired Avee Laboratories Inc. and related companies, which we refer to collectively as Avee, located in Tampa, Florida, a privately-owned provider of drug testing services in the field of pain management. The aggregate purchase price was $120.5 million, which was paid in cash. Included in our consolidated statement of operations for the year ended December 31, 2011 is revenue totaling approximately $27.2 million related to this acquired business. The operating results of Avee are included in our professional diagnostics reporting unit and business segment. The amount allocated to goodwill from this acquisition is deductible for tax purposes.
(iv) Other acquisitions in 2011
During 2011, we acquired the following businesses for an aggregate purchase price of $198.5 million, which included cash payments totaling $139.3 million, a previously-held investment with a fair value totaling $3.9 million, 25,463 shares of our common stock with an acquisition date fair value of $1.0 million, contingent consideration obligations with an aggregate acquisition date fair value of $48.7 million, deferred purchase price consideration with an acquisition date fair value of $4.2 million and debt forgiveness with a fair value of $1.5 million.
| | • | | 90% interest in BioNote, Inc., or BioNote, headquartered in South Korea, a manufacturer of diagnostic products for the veterinary industry (Acquired January 2011). We previously owned a 10% interest in BioNote. |
| | • | | assets, including domain name, of Pregnancy.org, LLC, or Pregnancy.org, a U.S.-based company providing a website for preconception, pregnancy and newborn care content, tools and sharing (Acquired January 2011) |
| | • | | Home Telehealth Limited, subsequently renamed Alere Connected Health Limited, or Alere Connected Health, located in Cardiff, Wales, a company that focuses on delivering integrated, comprehensive services and programs to health and social care providers and insurers (Acquired February 2011) |
| | • | | Bioeasy Diagnostica Ltda., or Bioeasy, located in Belo Horizonte, Brazil, a company that markets and sells rapid diagnostic tests and systems for laboratory diagnosis, prevention and monitoring of immunological diseases and fertility (Acquired March 2011) |
| | • | | 80.92% interest in Standing Stone, Inc., or Standing Stone, located in Westport, Connecticut, a company that focuses on disease state management by enhancing the quality of care provided to patients who require long-term therapy for chronic disease management (Acquired May 2011) |
| | • | | certain assets, rights, liabilities and properties of Drug Detection Devices, Inc., or 3DL, located in Alpharetta, Georgia, a distributor that promotes, markets, distributes and sells drugs of abuse |
F-27
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(4) Business Combinations (Continued)
| | diagnostic products, including consumables, point-of-care diagnostic kits and related products and services (Acquired July 2011) |
| | • | | Colibri Medical AB, or Colibri, located in Helsingborg, Sweden, a distributor of point-of-care drugs of abuse diagnostic products primarily to the Scandinavian marketplace (Acquired July 2011) |
| | • | | Laboratory Data Systems, Inc., or LDS, located in Tampa, Florida, a provider of healthcare software products, services, consulting and solutions (Acquired August 2011) |
| | • | | certain assets, liabilities and properties of Abatek Medical LLC, or Abatek, located in Dover, New Hampshire, a distributor that promotes, markets, distributes and sells drugs of abuse diagnostic products, including consumables, point-of-care diagnostic kits and related products and services (Acquired September 2011) |
| | • | | Forensics Limited, or ROAR, located in Worcestershire, England, a company that provides forensic quality toxicology services across the United Kingdom (Acquired September 2011) |
| | • | | Mahsan Diagnostika Vertriebsgesellschaft mbH, or Mahsan, located in Reinbek, Germany, a distributor of in vitro diagnostic drugs of abuse products primarily to the German marketplace (Acquired October 2011) |
| | • | | Medical Automation Systems Inc., or MAS, located in Charlottesville, Virginia, a provider of network-based software solutions for point-of-care testing (Acquired October 2011) |
| | • | | certain assets and properties of 1 Medical Distribution, Inc., or 1 Medical, located in Worthington, Ohio, a distributor that promotes, markets, distributes and sells drugs of abuse diagnostic products, including consumables, point-of-care diagnostic kits and related products and services (Acquired November 2011) |
| | • | | Method Factory, Inc. (d/b/a Wellogic), or Wellogic, headquartered in Waltham, Massachusetts, a provider of software solutions designed to connect the healthcare community (Acquired December 2011) |
The operating results of BioNote, Bioeasy, 3DL, Colibri, LDS, Abatek, ROAR, Mahsan, MAS and 1 Medical are included in our professional diagnostics reporting unit and business segment. The operating results of Pregnancy.org, Alere Connected Health, Standing Stone and Wellogic are included in our health information solutions reporting unit and business segment.
Our consolidated statement of operations for the year ended December 31, 2011 included revenue totaling approximately $21.1 million related to these businesses. Goodwill has been recognized in all of the acquisitions, with the exception of 1 Medical, and amounted to approximately $131.3 million. Goodwill related to the acquisitions of Pregnancy.org, 3DL, Abatek, LDS and Wellogic, which totaled $32.8 million, is expected to be deductible for tax purposes. The goodwill related to the remaining 2011 acquisitions is not expected to be deductible for tax purposes.
F-28
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(4) Business Combinations (Continued)
A summary of the aggregate purchase price allocation for the acquisitions consummated in 2011 is as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | | Avee | | | Arriva | | | Axis-Shield | | | Other | | | Total | |
Current assets(1) | | $ | 10,197 | | | $ | 4,874 | | | $ | 92,666 | | | $ | 23,542 | | | $ | 131,279 | |
Property, plant and equipment | | | 5,411 | | | | 524 | | | | 50,719 | | | | 11,820 | | | | 68,474 | |
Goodwill | | | 30,409 | | | | 58,174 | | | | 136,182 | | | | 131,348 | | | | 356,113 | |
Intangible assets | | | 76,400 | | | | 27,400 | | | | 233,370 | | | | 79,454 | | | | 416,624 | |
Other non-current assets | | | — | | | | 1,196 | | | | 18,512 | | | | 13,009 | | | | 32,717 | |
| | | | | | | | | | | | | | | | | | | | |
Total assets acquired | | | 122,417 | | | | 92,168 | | | | 531,449 | | | | 259,173 | | | | 1,005,207 | |
| | | | | | | | | | | | | | | | | | | | |
Current liabilities | | | 1,927 | | | | 12,629 | | | | 44,758 | | | | 27,623 | | | | 86,937 | |
Non-current liabilities | | | — | | | | — | | | | 97,842 | | | | 30,512 | | | | 128,354 | |
| | | | | | | | | | | | | | | | | | | | |
Total liabilities assumed | | | 1,927 | | | | 12,629 | | | | 142,600 | | | | 58,135 | | | | 215,291 | |
| | | | | | | | | | | | | | | | | | | | |
Less: | | | | | | | | | | | | | | | | | | | | |
Fair value of non-controlling interest | | | — | | | | — | | | | — | | | | 2,500 | | | | 2,500 | |
| | | | | | | | | | | | | | | | | | | | |
Net assets acquired | | | 120,490 | | | | 79,539 | | | | 388,849 | | | | 198,538 | | | | 787,416 | |
Less: | | | | | | | | | | | | | | | | | | | | |
Contingent consideration | | | — | | | | — | | | | — | | | | 48,685 | | | | 48,685 | |
Fair value of previously-held equity investment | | | — | | | | — | | | | 109,231 | | | | 3,937 | | | | 113,168 | |
Fair value of common stock issued | | | — | | | | 15,183 | | | | — | | | | 1,000 | | | | 16,183 | |
Loan forgiveness | | | — | | | | — | | | | — | | | | 1,488 | | | | 1,488 | |
Deferred purchase price consideration | | | — | | | | — | | | | — | | | | 4,170 | | | | 4,170 | |
| | | | | | | | | | | | | | | | | | | | |
Cash paid | | $ | 120,490 | | | $ | 64,356 | | | $ | 279,618 | | | $ | 139,258 | | | $ | 603,722 | |
| | | | | | | | | | | | | | | | | | | | |
| (1) | Includes cash acquired of approximately $23.2 million. |
The following are the intangible assets acquired and their respective amortizable lives (dollars in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Avee | | | Arriva | | | Axis-Shield | | | Other | | | Total | | | Weighted-
average
Useful Life | |
Core technology and patents | | $ | — | | | $ | — | | | $ | 56,919 | | | $ | 19,740 | | | $ | 76,659 | | | | 10.1 years | |
Database | | | — | | | | — | | | | — | | | | 64 | | | | 64 | | | | 3.0 years | |
Trademarks and trade names | | | 1,700 | | | | 1,000 | | | | 4,145 | | | | 7,352 | | | | 14,197 | | | | 10.1 years | |
Customer relationships | | | 71,500 | | | | 23,000 | | | | 114,174 | | | | 35,051 | | | | 243,725 | | | | 12.3 years | |
Non-compete agreements | | | 3,200 | | | | 3,400 | | | | — | | | | 1,706 | | | | 8,306 | | | | 5.3 years | |
Software | | | — | | | | — | | | | — | | | | 7,400 | | | | 7,400 | | | | 10.9 years | |
Other | | | — | | | | — | | | | — | | | | 7,767 | | | | 7,767 | | | | 15.6 years | |
In-process research and development | | | — | | | | — | | | | 58,132 | | | | 374 | | | | 58,506 | | | | N/A | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total intangible assets | | $ | 76,400 | | | $ | 27,400 | | | $ | 233,370 | | | $ | 79,454 | | | $ | 416,624 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
F-29
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(5) Goodwill and Other Intangible Assets
The following is a summary of goodwill and other intangible assets as of December 31, 2013 (dollars in thousands):
| | | | | | | | | | | | | | | | |
| | | Gross
Carrying
Amount | | | Accumulated
Amortization and
Impairment Losses | | | Net
Carrying
Value | | | Weighted-
average
Useful Life | |
Amortized intangible assets: | | | | | | | | | | | | | | | | |
Core technology and patents | | $ | 1,046,970 | | | $ | 397,306 | | | $ | 649,664 | | | | 14.8 years | |
| | | | | | | | | | | | | | | | |
Other intangible assets: | | | | | | | | | | | | | | | | |
Supplier relationships | | | 18,388 | | | | 16,087 | | | | 2,301 | | | | 9.3 years | |
Trademarks and trade names | | | 266,556 | | | | 142,993 | | | | 123,563 | | | | 10.9 years | |
License agreements | | | 17,584 | | | | 13,088 | | | | 4,496 | | | | 6.9 years | |
Customer relationships | | | 1,946,481 | | | | 1,128,421 | | | | 818,060 | | | | 15.7 years | |
Manufacturing know-how | | | 17,349 | | | | 10,684 | | | | 6,665 | | | | 10.8 years | |
Other | | | 186,255 | | | | 106,393 | | | | 79,862 | | | | 7.7 years | |
| | | | | | | | | | | | | | | | |
Total other intangible assets | | | 2,452,613 | | | | 1,417,666 | | | | 1,034,947 | | | | | |
| | | | | | | | | | | | | | | | |
Total intangible assets with finite lives | | $ | 3,499,583 | | | $ | 1,814,972 | | | $ | 1,684,611 | | | | | |
| | | | | | | | | | | | | | | | |
Intangible assets with indefinite lives: | | | | | | | | | | | | | | | | |
Goodwill | | $ | 3,093,691 | | | $ | — | | | $ | 3,093,691 | | | | | |
Other intangible assets(1) | | | 56,702 | | | | — | | | | 56,702 | | | | | |
| | | | | | | | | | | | | | | | |
Total intangible assets with indefinite lives | | $ | 3,150,393 | | | $ | — | | | $ | 3,150,393 | | | | | |
| | | | | | | | | | | | | | | | |
| (1) | Primarily includes in-process research and development assets recorded in connection with certain acquisitions. |
F-30
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(5) Goodwill and Other Intangible Assets (Continued)
The following is a summary of goodwill and other intangible assets as of December 31, 2012 (dollars in thousands):
| | | | | | | | | | | | | | | | |
| | | Gross
Carrying
Amount | | | Accumulated
Amortization and
Impairment Losses | | | Net
Carrying
Value | | | Weighted-
average
Useful Life | |
Amortized intangible assets: | | | | | | | | | | | | | | | | |
Core technology and patents | | $ | 941,940 | | | $ | 326,044 | | | $ | 615,896 | | | | 14.1 years | |
| | | | | | | | | | | | | | | | |
Other intangible assets: | | | | | | | | | | | | | | | | |
Supplier relationships | | | 18,629 | | | | 15,862 | | | | 2,767 | | | | 9.3 years | |
Trademarks and trade names | | | 252,028 | | | | 116,571 | | | | 135,457 | | | | 10.3 years | |
License agreements | | | 17,507 | | | | 11,126 | | | | 6,381 | | | | 4.5 years | |
Customer relationships | | | 1,910,752 | | | | 930,542 | | | | 980,210 | | | | 15.8 years | |
Manufacturing know-how | | | 15,332 | | | | 9,607 | | | | 5,725 | | | | 12.2 years | |
Other | | | 199,880 | | | | 112,091 | | | | 87,789 | | | | 7.4 years | |
| | | | | | | | | | | | | | | | |
Total other intangible assets | | | 2,414,128 | | | | 1,195,799 | | | | 1,218,329 | | | | | |
| | | | | | | | | | | | | | | | |
Total intangible assets with finite lives | | $ | 3,356,068 | | | $ | 1,521,843 | | | $ | 1,834,225 | | | | | |
| | | | | | | | | | | | | | | | |
Intangible assets with indefinite lives: | | | | | | | | | | | | | | | | |
Goodwill | | $ | 3,048,405 | | | $ | — | | | $ | 3,048,405 | | | | | |
Other intangible assets(1) | | | 55,795 | | | | 19,344 | | | | 36,451 | | | | | |
| | | | | | | | | | | | | | | | |
Total intangible assets with indefinite lives | | $ | 3,104,200 | | | $ | 19,344 | | | $ | 3,084,856 | | | | | |
| | | | | | | | | | | | | | | | |
| (1) | Primarily includes in-process research and development assets recorded in connection with certain acquisitions. |
The estimated useful lives of the individual categories of intangible assets were based on the nature of the applicable intangible assets and the expected future cash flows to be derived from those intangible assets. Amortization of intangible assets with finite lives is recognized over the shorter of the respective lives of the underlying license agreements, if applicable, or the period of time the assets are expected to contribute to future cash flows. We amortize our finite-lived intangible assets on patterns in which the economic benefits are expected to be realized. Amortization expense of intangible assets, which in the aggregate amounted to $318.4 million, $346.9 million and $307.7 million in 2013, 2012 and 2011, respectively, is included in cost of net revenue, research and development, sales and marketing and general and administrative expenses in the accompanying consolidated statements of operations. The allocation of amortization expense to the expense categories is based on the intended usage and the expected benefits of the intangible assets in relation to the expense categories.
The following is a summary of estimated aggregate amortization expense of intangible assets for each of the five succeeding fiscal years as of December 31, 2013 (in thousands):
| | | | |
2014 | | $ | 272,516 | |
2015 | | $ | 230,224 | |
2016 | | $ | 200,310 | |
2017 | | $ | 174,540 | |
2018 | | $ | 162,468 | |
F-31
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(5) Goodwill and Other Intangible Assets (Continued)
During the fourth quarter, we perform our annual impairment tests of the carrying value of our goodwill by reporting unit. Our annual impairment review conducted during the fourth quarter of 2011 indicated that a goodwill impairment charge was required in our health information solutions business segment and reporting unit. For further discussion see Note 2(h).
Goodwill amounts for our professional diagnostics, health information solutions and consumer diagnostics reporting units are summarized as follows (in thousands):
| | | | | | | | | | | | | | | | |
| | | Professional
Diagnostics | | | Health
Information
Solutions | | | Consumer
Diagnostics | | | Total | |
Goodwill at December 31, 2011 | | $ | 2,669,601 | | | $ | 99,759 | | | $ | 51,911 | | | $ | 2,821,271 | |
Acquisitions(1) | | | 222,306 | | | | 24,604 | | | | — | | | | 246,910 | |
Fair value of non-controlling interest(2) | | | (36,040 | ) | | | — | | | | — | | | | (36,040 | ) |
Other(3) | | | 10,370 | | | | 680 | | | | 5,214 | | | | 16,264 | |
| | | | | | | | | | | | | | | | |
Goodwill at December 31, 2012 | | $ | 2,866,237 | | | $ | 125,043 | | | $ | 57,125 | | | $ | 3,048,405 | |
Acquisitions(1) | | | 87,677 | | | | (3,551 | ) | | | — | | | | 84,126 | |
Dispositions(4) | | | (14,786 | ) | | | — | | | | — | | | | (14,786 | ) |
Other(3) | | | (25,832 | ) | | | 868 | | | | 910 | | | | (24,054 | ) |
| | | | | | | | | | | | | | | | |
Goodwill at December 31, 2013 | | $ | 2,913,296 | | | $ | 122,360 | | | $ | 58,035 | | | $ | 3,093,691 | |
| | | | | | | | | | | | | | | | |
| (1) | Includes initial purchase price allocation, purchase accounting adjustments recorded to the acquired entities’ opening balance sheet and additional payments made for earn-outs and milestones achieved. |
| (2) | This is the correction of a prior period balance sheet classification error which we do not believe is material to our 2011 or 2012 annual financial statements, or any previously reported quarterly financial statements, and represents the fair value of the non-controlling interest as of December 31, 2011 related to our acquisition of Axis-Shield. |
| (3) | These amounts relate primarily to adjustments resulting from fluctuations in foreign currency exchange rates. |
| (4) | Reflects write-off related to disposition of our Spinreact operations. |
We generally expense costs incurred for the internal development of intangible assets, except for costs that are incurred to establish patents and trademarks, such as legal fees for initiating, filing and obtaining the patents and trademarks. As of December 31, 2013 and 2012, we had approximately $9.6 million and $9.7 million, respectively, of costs capitalized, net of amortization, in connection with establishing patents and trademarks which are included in other intangible assets, net, in the accompanying consolidated balance sheets. Upon the initial filing of the patents and trademarks, we commence amortization of such intangible assets over their estimated useful lives. Costs incurred to maintain the patents and trademarks are expensed as incurred.
F-32
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(6) Long-term Debt
We had the following long-term debt balances outstanding (in thousands):
| | | | | | | | |
| | | December 31,
2013 | | | December 31,
2012 | |
A term loans(1)(2) | | $ | 832,188 | | | $ | 878,438 | |
B term loans(1) | | | 904,188 | | | | 913,438 | |
Incremental B-1 term loans(1) | | | 245,000 | | | | 247,500 | |
Incremental B-2 term loans(1) | | | 195,050 | | | | 196,739 | |
Revolving line of credit(1) | | | 170,000 | | | | 22,500 | |
7.25% Senior notes | | | 450,000 | | | | 450,000 | |
7.875% Senior notes | | | — | | | | 1,809 | |
6.5% Senior subordinated notes | | | 425,000 | | | | — | |
8.625% Senior subordinated notes | | | 400,000 | | | | 400,000 | |
9% Senior subordinated notes | | | — | | | | 392,933 | |
3% Convertible senior subordinated notes | | | 150,000 | | | | 150,000 | |
Other lines of credit | | | 355 | | | | 31,957 | |
Other | | | 50,119 | | | | 3,593 | |
| | | | | | | | |
| | | 3,821,900 | | | | 3,688,907 | |
Less: Current portion | | | (49,112 | ) | | | (60,232 | ) |
| | | | | | | | |
| | $ | 3,772,788 | | | $ | 3,628,675 | |
| | | | | | | | |
| (1) | Incurred under our secured credit facility. |
| (2) | Includes “A” term loans and “Delayed Draw” term loans under our secured credit facility. |
In connection with our significant long-term debt issuances, we recorded interest expense, including amortization and write-offs of deferred financing costs and original issue discounts, in our accompanying consolidated statements of operations for the years ended December 31, 2013, 2012 and 2011, respectively, as follows (in thousands):
| | | | | | | | | | | | |
| | | 2013 | | | 2012 | | | 2011 | |
Secured credit facility(1) | | $ | 104,159 | | | $ | 104,916 | | | $ | 41,478 | |
Former secured credit facility(2) | | | — | | | | — | | | | 53,841 | (3) |
7.25% Senior notes | | | 33,906 | | | | 1,994 | | | | — | |
7.875% Senior notes | | | 137 | (4) | | | 44,994 | (5) | | | 22,291 | |
6.5% Senior subordinated notes | | | 17,384 | | | | — | | | | — | |
8.625% Senior subordinated notes | | | 37,093 | | | | 37,096 | | | | 36,437 | |
9% Senior subordinated notes | | | 54,043 | (6) | | | 41,474 | | | | 40,248 | |
3% Senior subordinated convertible notes | | | 4,984 | | | | 4,984 | | | | 4,988 | |
| | | | | | | | | | | | |
| | $ | 251,706 | | | $ | 235,458 | | | $ | 199,283 | |
| | | | | | | | | | | | |
| (1) | Includes “A” term loans, including the “Delayed-Draw” term loans; “B” term loans; “Incremental B-1” term loans; “Incremental B-2” term loans; and revolving line of credit loans. For the years ended December 31, 2013, 2012 and 2011, the amounts include $2.6 million, $5.0 million and $2.9 million, respectively, related to the amortization of fees paid for certain debt modifications. |
| (2) | Consists of loans under our former First Lien Credit Agreement and former Second Lien Credit Agreement. |
| (3) | Amount includes an approximate $29.7 million loss recorded in connection with the termination of our former secured credit facility and related interest rate swap agreement, coupled with the amortization of fees paid for certain debt modifications. |
F-33
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(6) Long-term Debt (Continued)
| (4) | Amount includes an approximate $0.2 million loss recorded in connection with the repurchase of our 7.875% senior notes. |
| (5) | Amount includes an approximate $23.2 million loss recorded in connection with the repurchase of substantially all of our 7.875% senior notes. Included in the $23.2 million is a $12.3 million make-whole payment which has been classified within cash flow from financing activities in our consolidated statement of cash flows. |
| (6) | Amount includes an approximate $35.6 million loss recorded in connection with the repurchase of our 9% senior subordinated notes. Included in the $35.6 million is $19.0 million related to tender offer consideration and call premium which has been classified within cash flow from financing activities in our consolidated statement of cash flows. |
The following describes each of the debt instruments listed above:
(a) Secured Credit Facility
On June 30, 2011, we entered into a Credit Agreement, or secured credit facility, with certain lenders, General Electric Capital Corporation as administrative agent and collateral agent, and certain other agents and arrangers, and, along with certain of our subsidiaries, a related guaranty and security agreement. On December 7, 2011, we entered into an amendment to our secured credit facility to provide an additional term loan facility for the “Incremental B-1” term loans described below (all of which we borrowed on that date). On March 28, 2012, we entered into a further amendment to our secured credit facility to provide an additional term loan facility for the “Incremental B-2” term loans described below (all of which we borrowed on that date). The secured credit facility, as amended, provides for credit facilities totaling $2.55 billion in the aggregate, consisting of term loans in the aggregate principal amount of $2.3 billion (consisting of “A” term loans (including “Delayed Draw” term loans) in the aggregate principal amount of $925.0 million, “B” term loans in the aggregate principal amount of $925.0 million, “Incremental B-1” term loans in the aggregate principal amount of $250.0 million and “Incremental B-2” term loans in the aggregate principal amount of $200.0 million), all of which we have fully drawn, and, subject to our continued compliance with the secured credit facility, a $250.0 million revolving line of credit (which includes a $50.0 million sublimit for the issuance of letters of credit).
We must repay the “A” term loans (excluding the “Delayed Draw” term loans) in eighteen consecutive quarterly installments, which began on December 31, 2011 and continue through March 31, 2016, in the amount of $7,812,500 each, and a final installment on June 30, 2016, in the amount of $484,375,000; we must repay the “Delayed-Draw” term loans included within the “A” term loans in fifteen consecutive quarterly installments, which began on September 30, 2012 and continue through March 31, 2016, in the amount of $3,750,000 each, and a final installment on June 30, 2016, in the amount of $243,750,000. We must repay the “B” term loans in twenty-two consecutive quarterly installments, which began on December 31, 2011 and continue through March 31, 2017, in the amount of $2,312,500 each, and a final installment on June 30, 2017, in the amount of $874,125,000. We must repay the “Incremental B-1” term loans in twenty-one consecutive quarterly installments, which began on March 31, 2012 and continue through March 31, 2017, in the amount of $625,000 each, and a final installment on June 30, 2017, in the amount of $236,875,000. We must repay the “Incremental B-2” term loans in twenty consecutive quarterly installments, which began on June 30, 2012 and continue through March 31, 2017, in the amount of $500,000 each, and a final installment on June 30, 2017, in the amount of $190,000,000. We have paid in full all installments of all term loans due on or before December 31, 2013. We may repay any borrowings under the revolving line of credit at any time without premium or penalty, but we must repay all such borrowings in no event later than June 30, 2016. Notwithstanding the foregoing, and subject to certain exceptions provided for in the Credit
F-34
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(6) Long-term Debt (Continued)
Agreement, in the event that any of our existing 3% convertible senior subordinated notes remain outstanding on the date that is six months prior to the maturity date thereof, then all of the term loans and revolving credit loans under the secured credit facility shall instead mature in full on such prior date.
The “A” term loans (including the “Delayed Draw” term loans) and our borrowings under the revolving credit facility bear interest at a rate per annum of, at our option, either (i) the Base Rate, as defined in the Credit Agreement, plus an applicable margin, which varies within a range from 1.75% to 2.50% depending on our consolidated secured leverage ratio, or (ii) the Eurodollar Rate, as defined in the Credit Agreement, plus an applicable margin, which varies within a range from 2.75% to 3.50% depending on our consolidated secured leverage ratio. On March 22, 2013, we entered into an amendment to the secured credit facility that provided for (among other changes) 50 basis point reductions in the interest rate margins applicable to the “B” term loans, the “Incremental B-1” term loans and the “Incremental B-2” term loans. Under the secured credit facility as so amended, the “B” term loans, the “Incremental B-1” term loans and the “Incremental B-2” term loans bear interest at a rate per annum of, at our option, either (i) the Base Rate, as defined in the Credit Agreement, plus an applicable margin, which varies within a range from 2.00% to 2.75% depending on our consolidated secured leverage ratio, or (ii) the Eurodollar Rate, as defined in the Credit Agreement, plus an applicable margin, which varies within a range from 3.00% to 3.75% depending on our consolidated secured leverage ratio. Interest on “B” term loans, “Incremental B-1” term loans and “Incremental B-2” term loans based on the Eurodollar Rate is subject to a 1.00% floor with respect to the base Eurodollar Rate. We are required to pay a fee on the unused portion of the revolving credit facility at a rate per annum of 0.50%. As of December 31, 2013, the “A” term loans (including the “Delayed-Draw” term loans), the “B” term loans, the “Incremental B-1” term loans, the “Incremental B-2” term loans and the revolving line of credit loans bore interest at the rates of 3.17%, 4.25%, 4.25%, 4.25%, and 3.17%, respectively, per annum.
We must comply with various customary financial and non-financial covenants under the Credit Agreement. The primary financial covenants consist of a maximum consolidated secured leverage ratio, a minimum consolidated interest coverage ratio and a limit on capital expenditures; we were in compliance with all such financial covenants as December 31, 2013. The primary non-financial covenants limit our ability to pay dividends or other distributions on our capital stock, to repurchase our capital stock, to conduct mergers or acquisitions, to make investments and loans, to incur future indebtedness, to place liens on assets, to prepay certain other indebtedness, to alter our capital structure and to sell assets. The covenants are subject to certain important exceptions and qualifications, which are set forth in the Credit Agreement.
The lenders under the Credit Agreement are entitled to terminated the revolving line of credit and accelerate repayment of the loans under the Credit Agreement upon the occurrence of any of various customary events of default.
As of December 31, 2013, accrued interest related to the secured credit facility amounted to $0.5 million.
(b) Former Secured Credit Facility
In connection with our entering into the secured credit facility on June 30, 2011, we repaid in full all outstanding indebtedness under and terminated our former First Lien Credit Agreement, or former
F-35
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(6) Long-term Debt (Continued)
senior secured credit facility, and our former Second Lien Credit Agreement, or former junior secured credit facility (and, collectively with the former senior secured credit facility, our former secured credit facility), each dated as of June 26, 2007, with certain lenders, General Electric Capital Corporation, as administrative agent and collateral agent, and certain other agents and arrangers, and certain related guaranty and security agreements. The aggregate outstanding principal amount of the loans repaid under our former secured credit facility in connection with the termination thereof was approximately $1.2 billion.
In August 2007, we entered into interest rate swap contracts, with an effective date of September 28, 2007, that had a total notional value of $350.0 million and an original maturity date of September 28, 2010. These interest rate swap contracts paid us variable interest at the three-month LIBOR rate, and we paid the counterparties a fixed rate of 4.85%. In March 2009, we extended our August 2007 interest rate hedge for an additional two-year period commencing in September 2010 at a one-month LIBOR rate of 2.54%. These interest rate swap contracts were entered into to convert $350.0 million of the $1.2 billion variable rate term loans under our former secured credit facility into fixed rate debt. In connection with entering into the secured credit facility on June 30, 2011, we paid $10.1 million to terminate these interest rate swap contracts which was recorded in interest expense, including amortization of original issue discounts and deferred financing costs, in our consolidated statements of operations. In January 2009, we entered into interest rate swap contracts, with an effective date of January 14, 2009, that had a total notional value of $500.0 million and a maturity date of January 5, 2011. These interest rate swap contracts paid us variable interest at the one-month LIBOR rate, and we paid the counterparties a fixed rate of 1.195%. These interest rate swap contracts were entered into to convert $500.0 million of the $1.2 billion variable rate term loan under our former secured credit facility into fixed rate debt. We did not extend the terms of these interest rate swap contracts after January 5, 2011.
(c) 7.25% Senior Notes
On December 11, 2012, we sold a total of $450.0 million aggregate principal amount of 7.25% senior notes due 2018, or the 7.25% senior notes, in a private placement to initial purchasers, who agreed to resell the notes only to qualified institutional buyers and to persons outside the United States; we sold the 7.25% senior notes at an initial offering price of 100%. Net proceeds from this offering amounted to $443.2 million, which was net of the underwriters’ commissions and offering expenses totaling $6.8 million. These notes were subsequently exchanged for new notes having substantially the same terms in an exchange offer registered under the Securities Act. We used $267.4 million of the net proceeds to purchase $248.2 million outstanding principal amount of the 7.875% senior notes as described below and $170.0 million to pay down a portion of the outstanding balance under our revolving line of credit.
The 7.25% senior notes were issued under a supplemental indenture dated as of August 11, 2009, as amended or supplemented, or the 7.25% Indenture. The 7.25% senior notes accrue interest at the rate of 7.25% per annum. Interest on the 7.25% senior notes is payable semi-annually on June 15 and December 15, beginning on June 15, 2013. The 7.25% senior notes mature on July 1, 2018, unless earlier redeemed.
We may redeem the 7.25% senior notes, in whole or part, at any time on or after December 15, 2015, by paying the principal amount of the notes being redeemed plus a declining premium, plus accrued and unpaid interest to, but excluding, the redemption date. The premium declines from
F-36
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(6) Long-term Debt (Continued)
3.625% during the twelve months on and after December 15, 2015 to 1.813% during the six months on and after December 15, 2016 to zero on and after June 15, 2017. At any time prior to December 15, 2015, we may redeem up to 35% of the aggregate principal amount of the 7.25% senior notes with money that we raise in certain equity offerings, so long as (i) we pay 107.25% of the principal amount of the notes being redeemed, plus accrued and unpaid interest to, but excluding, the redemption date; (ii) we redeem the notes within 90 days of completing such equity offering; and (iii) at least 65% of the aggregate principal amount of the 7.25% senior notes remains outstanding afterwards. In addition, at any time prior to December 15, 2015, we may redeem some or all of the 7.25% senior notes by paying the principal amount of the notes being redeemed plus a make-whole premium, plus accrued and unpaid interest to, but excluding, the redemption date.
If a change of control occurs, subject to specified conditions, we must give holders of the 7.25% senior notes an opportunity to sell their notes to us at a purchase price of 101% of the principal amount of the notes, plus accrued and unpaid interest to, but excluding, the date of the purchase.
If we, or our subsidiaries, engage in asset sales, we, or they, generally must either invest the net cash proceeds from such sales in our or their businesses within a specified period of time, prepay certain indebtedness or make an offer to purchase a principal amount of the 7.25% senior notes equal to the excess net cash proceeds, subject to certain exceptions. The purchase price of the notes would be 100% of their principal amount, plus accrued and unpaid interest.
The 7.25% Indenture provides that we and our subsidiaries must comply with various customary covenants. These covenants limit our ability, and the ability of our subsidiaries, to, among other things, incur additional debt; pay dividends on capital stock or redeem, repurchase or retire capital stock or subordinated debt; make certain investments; create liens on assets; transfer or sell assets; engage in transactions with affiliates; create restrictions on the ability of subsidiaries to pay dividends or make loans, asset transfers or other payments to us and our subsidiaries; issue capital stock of subsidiaries; engage in any business, other than our or their existing businesses and related businesses; enter into sale and leaseback transactions; incur layered indebtedness; and consolidate or merge with any person (other than certain affiliates) or transfer all or substantially all of our assets or the aggregate assets of us and our subsidiaries. These covenants are subject to certain important exceptions and qualifications, which are set forth in the 7.25% Indenture. At any time the 7.25% senior notes are rated investment grade, certain covenants will be suspended with respect to them.
The 7.25% Indenture contains customary events of default entitling the trustee or the holders of the 7.25% senior notes to declare all amounts owed pursuant to the 7.25% senior notes immediately payable if any such event of default occurs.
The 7.25% senior notes are our senior unsecured obligations and are equal in right of payment to all of our existing and future senior debt, including our borrowings under our secured credit facility. Our obligations under the 7.25% senior notes and the 7.25% Indenture are fully and unconditionally guaranteed, jointly and severally, on an unsecured senior basis by certain of our domestic subsidiaries, and the obligations of such domestic subsidiaries under their guarantees are equal in right of payment to all of their existing and future senior debt. See Note 25 for guarantor financial information.
F-37
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(6) Long-term Debt (Continued)
As of December 31, 2013, accrued interest related to the 7.25% senior notes amounted to $1.4 million.
(d) 7.875% Senior Notes
During the third quarter of 2009, we sold a total of $250.0 million aggregate principal amount of 7.875% senior notes due 2016, or the 7.875% senior notes, in two separate transactions on August 11, 2009 and September 28, 2009. Net proceeds from these offerings amounted to approximately $240.0 million, which was net of underwriters’ commissions totaling $3.7 million and original issue discount totaling $6.3 million. The 7.875% senior notes were issued under a supplemental indenture dated as of August 11, 2009, as amended or supplemented, or the 7.875% Indenture. The 7.875% senior notes accrued interest from the dates of their respective issuances at the rate of 7.875% per annum. Interest on the notes was payable semi-annually on February 1 and August 1, commencing on February 1, 2010. The terms of the notes provided that they matured on February 1, 2016, unless earlier redeemed.
In December 2012, we used $267.4 million of the net proceeds of our sale of the 7.25% senior notes to purchase $248.2 million outstanding principal amount of the 7.875% senior notes. The purchased 7.875% senior notes represented 99.3% of the total then-outstanding principal amount of the 7.875% senior notes. In February 2013, we redeemed the remaining $1.8 million outstanding principal amount of the 7.875% senior notes pursuant to our optional redemption right under the 7.875% Indenture, and we subsequently terminated the 7.875% Indenture.
(e) 6.5% Senior Subordinated Notes
In May 2013, we sold a total of $425.0 million aggregate principal amount of 6.5% senior subordinated notes due 2020, or the 6.5% senior subordinated notes, in a private placement to initial purchasers, who agreed to resell the notes only to qualified institutional buyers and to persons outside the United States. We sold the 6.5% senior subordinated notes at an initial offering price of 100%. Net proceeds from this offering amounted to $417.7 million, which were net of the underwriters’ commissions and offering expenses totaling approximately $7.3 million. These notes were subsequently exchanged for new notes having substantially the same terms in an exchange offer registered under the Securities Act. In May 2013, we used $200.6 million of the net proceeds of our sale of the 6.5% senior subordinated notes to purchase $190.6 million outstanding principal amount of our 9% senior subordinated notes pursuant to our tender offer for these notes. In June 2013, we redeemed the remaining $209.4 million outstanding principal amount of the 9% senior subordinated notes pursuant to our optional redemption right under the indenture for those notes, and we subsequently terminated that indenture.
The 6.5% senior subordinated notes were issued under a supplemental indenture dated as of May 24, 2013, or the 6.5% Indenture. The 6.5% senior subordinated notes accrue interest at the rate of 6.5% per annum. Interest on the 6.5% senior subordinated notes is payable semi-annually on June 15 and December 15, beginning on December 15, 2013. The 6.5% senior subordinated notes mature on June 15, 2020, unless earlier redeemed.
We may, at our option, redeem the 6.5% senior subordinated notes, in whole or part, at any time (which may be more than once) on or after June 15, 2016, by paying the principal amount of the notes being redeemed plus a declining premium, plus accrued and unpaid interest to (but excluding) the
F-38
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(6) Long-term Debt (Continued)
redemption date. The premium declines from 3.250% during the twelve months on and after June 15, 2016 to 1.625% during the twelve months on and after June 15, 2017 to zero on and after June 15, 2018. In addition, we may, at our option, at any time (which may be more than once) before May 24, 2015, redeem up to 10% of the aggregate principal amount of the 6.5% senior subordinated notes in each of the two twelve-month periods preceding May 24, 2015 at a redemption price of 103% of the principal amount thereof plus accrued and unpaid interest to (but excluding) the redemption date. In addition, at any time (which may be more than once) prior to June 15, 2016, we may, at our option, redeem up to 35% of the aggregate principal amount of the 6.5% senior subordinated notes with money that we raise in certain equity offerings, so long as (i) we pay 106.5% of the principal amount of the notes being redeemed, plus accrued and unpaid interest to (but excluding) the redemption date; (ii) we redeem the 6.5% senior subordinated notes within 90 days of completing such equity offering; and (iii) at least 65% of the aggregate principal amount of the 6.5% senior subordinated notes remains outstanding afterwards. In addition, at any time (which may be more than once) prior to June 15, 2016, we may, at our option, redeem some or all of the 6.5% senior subordinated notes by paying the principal amount of the 6.5% senior subordinated notes being redeemed plus a make-whole premium, plus accrued and unpaid interest to (but excluding) the redemption date.
If a change of control occurs, subject to specified conditions, we must give holders of the 6.5% senior subordinated notes an opportunity to sell their notes to us at a purchase price of 101% of the principal amount of the notes, plus accrued and unpaid interest to (but excluding) the date of the purchase.
If we or our subsidiaries engage in asset sales, we or they generally must either invest the net cash proceeds from such sales in our or their businesses within a specified period of time, repay senior indebtedness or make an offer to purchase a principal amount of the 6.5% senior subordinated notes (on a pro rata basis with respect to the 6.5% senior subordinated notes and our 8.625% senior subordinated notes) equal to the excess net cash proceeds, subject to certain exceptions. The purchase price of the 6.5% senior subordinated notes would be 100% of their principal amount, plus accrued and unpaid interest.
The 6.5% Indenture provides that we and our subsidiaries must comply with various customary covenants. These covenants limit our ability, and the ability of our subsidiaries, to, among other things, incur additional debt; pay dividends on capital stock or redeem, repurchase or retire capital stock or subordinated debt; make certain investments; create liens on assets; transfer or sell assets; engage in transactions with affiliates; create restrictions on the ability of subsidiaries to pay dividends or make loans, asset transfers or other payments to us and our subsidiaries; issue capital stock of subsidiaries; engage in any business, other than our or their existing businesses and related businesses; enter into sale and leaseback transactions; incur layered indebtedness; and consolidate or merge with any person (other than certain affiliates) or transfer all or substantially all of our assets or the aggregate assets of us and our subsidiaries. These covenants are subject to certain important exceptions and qualifications, which are set forth in the 6.5% Indenture. At any time the 6.5% senior subordinated notes are rated investment grade, certain covenants will be suspended with respect to them.
The 6.5% Indenture contains customary events of default entitling the trustee or the holders of the 6.5% senior subordinated notes to declare all amounts owed pursuant to the 6.5% senior subordinated notes immediately payable if any such event of default occurs.
The 6.5% senior subordinated notes are our senior subordinated unsecured obligations, are subordinated in right of payment to all of our existing and future senior debt, including our borrowings
F-39
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(6) Long-term Debt (Continued)
under our secured credit facility and our 7.25% senior notes, and are equal in right of payment with our 8.625% senior subordinated notes and our 3% convertible senior subordinated notes. Our obligations under the 6.5% senior subordinated notes and the 6.5% Indenture are fully and unconditionally guaranteed, jointly and severally, on a senior subordinated unsecured basis by certain of our domestic subsidiaries, and the obligations of such domestic subsidiaries under their guarantees are subordinated in right of payment to all of their existing and future senior debt. See Note 25 for guarantor financial information.
(f) 8.625% Senior Subordinated Notes
In September 2010, we sold $400.0 million aggregate principal amount of 8.625% senior subordinated notes due 2018, or the 8.625% senior subordinated notes, in a private placement to initial purchasers, who agreed to resell the notes only to qualified institutional buyers and to persons outside the United States; we sold the 8.625% subordinated notes at an initial offering price of 100%. Net proceeds from this offering amounted to $393.0 million, which was net of underwriters’ commissions totaling $7.0 million. These notes were subsequently exchanged for new notes having substantially the same terms in an exchange offer registered under the Securities Act.
The 8.625% senior subordinated notes were issued under a supplemental indenture dated as of September 21, 2010, as amended or supplemented, or the 8.625% Indenture. The 8.625% senior subordinated notes accrue interest at the rate of 8.625% per annum. Interest on the 8.625% notes is payable semi-annually on April 1 and October 1, beginning on April 1, 2011. The 8.625% subordinated notes mature on October 1, 2018, unless earlier redeemed.
We may redeem the 8.625% senior subordinated notes, in whole or part, at any time (which may be more than once) on or after October 1, 2014, by paying the principal amount of the notes being redeemed plus a declining premium, plus accrued and unpaid interest to, but excluding, the redemption date. The premium declines from 4.313% during the twelve months on and after October 1, 2014 to 2.156% during the twelve months on and after October 1, 2015 to zero on and after October 1, 2016. Prior to October 1, 2013, we may redeem, in whole or part, at any time (which may be more than once), up to 35% of the aggregate principal amount of the 8.625% senior subordinated notes with money that we raise in certain equity offerings so long as (i) we pay 108.625% of the principal amount of the notes being redeemed, plus accrued and unpaid interest to, but excluding, the redemption date; (ii) we redeem the notes within 90 days of completing such equity offering; and (iii) at least 65% of the aggregate principal amount of the 8.625% senior subordinated notes, including any 8.625% subordinated notes issued after September 21, 2010, remains outstanding afterwards. In addition, at any time prior to October 1, 2014, we may redeem some or all of the 8.625% senior subordinated notes by paying the principal amount of the notes being redeemed plus a make-whole premium, plus accrued and unpaid interest to, but excluding, the redemption date.
If a change of control occurs, subject to specified conditions, we must give holders of the 8.625% senior subordinated notes an opportunity to sell their notes to us at a purchase price of 101% of the principal amount of the notes, plus accrued and unpaid interest to, but excluding, the date of the purchase.
If we, or our subsidiaries, engage in asset sales, we, or they, generally must either invest the net cash proceeds from such sales in our or their businesses within a specified period of time, prepay senior debt or make an offer to purchase a principal amount of the 8.625% senior subordinated notes
F-40
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(6) Long-term Debt (Continued)
(on a pro rata basis with respect to the 8.625% senior subordinated notes and the 6.5% senior subordinated notes) equal to the excess net cash proceeds, subject to certain exceptions. The purchase price of the senior subordinated notes would be 100% of their principal amount, plus accrued and unpaid interest.
The 8.625% Indenture provides that we and our subsidiaries must comply with various customary covenants. These covenants limit our ability, and the ability of our subsidiaries, to, among other things, incur additional debt; pay dividends on capital stock or redeem, repurchase or retire capital stock or subordinated debt; make certain investments; create liens on assets; transfer or sell assets; engage in transactions with affiliates; create restrictions on the ability of subsidiaries to pay dividends or make loans, asset transfers or other payments to us or our subsidiaries; issue capital stock of subsidiaries; engage in any business, other than our or their existing businesses and related businesses; enter into sale and leaseback transactions; incur layered indebtedness; and consolidate or merge or transfer all or substantially all of our assets or the aggregate assets of us and our subsidiaries. These covenants are subject to certain important exceptions and qualifications, which are set forth in the 8.625% Indenture. At any time the 8.625% senior subordinated notes are rated investment grade, certain covenants will be suspended with respect to them.
The 8.625% Indenture contains customary events of default entitling the trustee or the holders of the 8.625% senior subordinated notes to declare all amounts owed pursuant the 8.625% senior subordinated notes immediately payable if any such event of default occurs.
The 8.625% senior subordinated notes are our senior subordinated unsecured obligations, are subordinated in right of payment to all of our existing and future senior debt, including our borrowings under our secured credit facility and our 7.25% senior notes, and equal in right of payment with our 6.5% senior subordinated notes and our 3% convertible senior subordinated notes. Our obligations under the 8.625% senior subordinated notes and the 8.625% Indenture are fully and unconditionally guaranteed, jointly and severally, on a senior subordinated unsecured basis by certain of our domestic subsidiaries, and the obligations of such domestic subsidiaries under their guarantees are subordinated in right of payment to all of their existing and future senior debt. See Note 25 for guarantor financial information.
As of December 31, 2013, accrued interest related to the 8.625% senior subordinated notes amounted to $8.6 million.
(g) 9% Senior Subordinated Notes
On May 12, 2009, we sold $400.0 million aggregate principal amount of 9% senior subordinated notes due 2016, or the 9% senior subordinated notes, in a public offering at an initial offering price of 96.865%. Net proceeds from this offering amounted to $379.5 million, which was net of underwriters’ commissions totaling $8.0 million and original issue discount totaling $12.5 million. The 9% senior subordinated notes were issued under a supplemental indenture dated as of May 12, 2009, as amended or supplemented, or the 9% Indenture. The 9% senior subordinated notes accrued interest from the date of their issuance at the rate of 9% per annum. Interest on the 9% senior subordinated notes was payable semi-annually on May 15 and November 15, commencing on November 15, 2009. The terms of the 9% senior subordinated notes provided that they matured on May 15, 2016, unless earlier redeemed.
F-41
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(6) Long-term Debt (Continued)
On May 24, 2013, we used $200.6 million of the net proceeds of our sale of the 6.5% senior subordinated notes to purchase $190.6 million outstanding principal amount of our 9% senior subordinated notes pursuant to our tender offer for these notes. The purchased 9% senior subordinated notes represented approximately 47.7% of the total then-outstanding principal amount of the 9% senior subordinated notes. On June 24, 2013, we redeemed the remaining $209.4 million outstanding principal amount of the 9% senior subordinated notes pursuant to our optional redemption right under the 9% Indenture, and we subsequently terminated the 9% Indenture.
(h) 3% Convertible Senior Subordinated Notes
On May 14, 2007, we sold $150.0 million principal amount of 3% convertible senior subordinated notes due 2016, or the 3% convertible senior subordinated notes, in a private placement to qualified institutional buyers, at an initial offering price of 100%. At the initial conversion price of $52.30, the 3% convertible senior subordinated notes were convertible into an aggregate of 2.9 million shares of our common stock. The conversion price was subject to adjustment one year from the date of sale. Based upon the daily volume-weighted price per share of our common stock for the thirty consecutive trading days ending May 9, 2008, the conversion price decreased from $52.30 to $43.98 in May 2008. The decrease in conversion price resulted in additional shares of our common stock becoming issuable upon conversion of the 3% convertible senior subordinated notes. The 3% convertible senior subordinated notes are now convertible into 3.4 million shares of our common stock at a conversion price of $43.98. Interest accrues at 3% per annum , compounded daily, on the outstanding principal amount of the 3% convertible senior subordinated notes and is payable in arrears on May 15th and November 15th of each year, beginning on November 15, 2007. We have paid in full all installments of interest on the 3% convertible senior subordinated notes due on or before December 31, 2013.
We may not redeem the 3% convertible senior subordinated notes prior to their stated maturity. In the event of certain fundamental changes (as defined in the indenture governing the 3% convertible senior subordinated notes) or a termination of trading of our common stock (as described in such indenture), we may be required to repurchase the 3% convertible senior subordinated notes for cash at a price equal to 100% of the unconverted principal amount thereof plus any accrued but unpaid interest. The 3% convertible senior subordinated notes are unsecured and are subordinated in right of payment to all of our existing and future senior debt, including borrowings under our secured credit facility and our senior notes, and equal in right of payment to our senior subordinated notes. The indenture governing the 3% convertible senior subordinated notes contains customary events of default entitling the trustee or the holders thereof to declare all amounts owed pursuant to the 3% convertible senior subordinated notes immediately payable if any such event of default occurs.
As of December 31, 2013, accrued interest related to the 3% convertible senior subordinated notes amounted to $0.6 million.
(i) Lines of Credit
Some of our subsidiaries maintain local lines of credit for short-term advances. Total available credit under the local lines of credit is approximately $6.1 million, of which $0.4 million was borrowed and outstanding as of December 31, 2013.
F-42
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(6) Long-term Debt (Continued)
(j) Other Debt
Included in other debt for the year ended December 31, 2013 are borrowings by certain of our subsidiaries from various financial institutions. The borrowed funds are primarily used to fund capital expenditures and working capital requirements. Interest expense on these borrowings was $2.5 million for the year ended December 31, 2013.
(k) Maturities of Long-Term Debt
The following is a summary of the maturities of long-term debt outstanding on December 31, 2013 (in thousands):
| | | | |
2014 | | $ | 49,112 | |
2015 | | | 75,211 | |
2016 | | | 1,111,700 | |
2017 | | | 1,305,717 | |
2018 | | | 851,238 | |
Thereafter | | | 430,372 | |
| | | | |
| | | 3,823,350 | |
Less: Original issue discounts | | | (1,450 | ) |
| | | | |
| | $ | 3,821,900 | |
| | | | |
(7) Fair Value Measurements
We apply fair value measurement accounting to value our financial assets and liabilities. Fair value measurement accounting provides a framework for measuring fair value under U.S. GAAP and requires expanded disclosures regarding fair value measurements. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. A fair value hierarchy requires an entity to maximize the use of observable inputs, where available, and minimize the use of unobservable inputs when measuring fair value.
Described below are the three levels of inputs that may be used to measure fair value:
Level 1 — Quoted prices in active markets for identical assets or liabilities.
Level 2 — Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
F-43
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(7) Fair Value Measurements (Continued)
The following tables present information about our assets and liabilities that are measured at fair value on a recurring basis as of December 31, 2013 and 2012, and indicates the fair value hierarchy of the valuation techniques we utilized to determine such fair value (in thousands):
| | | | | | | | | | | | | | | | |
Description | | December 31,
2013 | | | Quoted Prices in
Active Markets
(Level 1) | | | Significant Other
Observable Inputs
(Level 2) | | | Unobservable
Inputs
(Level 3) | |
Assets: | | | | | | | | | | | | | | | | |
Marketable securities | | $ | 858 | | | $ | 858 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | |
Total assets | | $ | 858 | | | $ | 858 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | |
Liabilities: | | | | | | | | | | | | | | | | |
Contingent consideration obligations(1) | | $ | 213,969 | | | $ | — | | | $ | — | | | $ | 213,969 | |
| | | | | | | | | | | | | | | | |
Total liabilities | | $ | 213,969 | | | $ | — | | | $ | — | | | $ | 213,969 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Description | | December 31,
2012 | | | Quoted Prices in
Active Markets
(Level 1) | | | Significant Other
Observable Inputs
(Level 2) | | | Unobservable
Inputs
(Level 3) | |
Assets: | | | | | | | | | | | | | | | | |
Marketable securities | | $ | 904 | | | $ | 904 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | |
Total assets | | $ | 904 | | | $ | 904 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | |
Liabilities: | | | | | | | | | | | | | | | | |
Contingent consideration obligations(1) | | $ | 176,172 | | | $ | — | | | $ | — | | | $ | 176,172 | |
| | | | | | | | | | | | | | | | |
Total liabilities | | $ | 176,172 | | | $ | — | | | $ | — | | | $ | 176,172 | |
| | | | | | | | | | | | | | | | |
| (1) | We determine the fair value of the contingent consideration obligations based on a probability-weighted approach derived from earn-out criteria estimates and a probability assessment with respect to the likelihood of achieving the various earn-out criteria. The measurement is based upon significant inputs not observable in the market. Significant increases or decreases in any of these inputs could result in a significantly higher or lower fair value measurement. Changes in the fair value of these contingent consideration obligations are recorded as income or expense within operating income in our consolidated statements of operations. See Note 11 for additional information on the valuation of our contingent consideration obligations. |
Changes in the fair value of our Level 3 contingent consideration obligations during the year ended December 31, 2013 were as follows (in thousands):
| | | | |
Fair value of contingent consideration obligations, January 1, 2013 | | $ | 176,172 | |
Acquisition date fair value of contingent consideration obligations recorded | | | 76,268 | |
Net reclassifications | | | (12 | ) |
Foreign currency adjustments | | | (51 | ) |
Payments | | | (56,432 | ) |
Present value accretion | | | 14,591 | |
Adjustments, net (income) expense | | | 3,433 | |
| | | | |
Fair value of contingent consideration obligations, December 31, 2013 | | $ | 213,969 | |
| | | | |
F-44
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(7) Fair Value Measurements (Continued)
At December 31, 2013 and 2012, the carrying amounts of cash and cash equivalents, restricted cash, receivables, accounts payable and other current liabilities approximated their estimated fair values.
The carrying amount and estimated fair value of our long-term debt were $3.8 billion and $3.9 billion, respectively, at December 31, 2013. The carrying amount and estimated fair value of our long-term debt were $3.7 billion at December 31, 2012. The estimated fair value of our long-term debt was determined using information derived from available market sources (Level 2 in the fair value hierarchy) and may not be representative of actual values that could have been or will be realized in the future.
(8) Capital Leases
The following is a schedule of the future minimum lease payments under capital leases, together with the present value of such payments as of December 31, 2013 (in thousands):
| | | | |
2014 | | $ | 6,855 | |
2015 | | | 6,551 | |
2016 | | | 3,485 | |
2017 | | | 1,883 | |
2018 | | | 1,273 | |
Thereafter | | | 1,215 | |
| | | | |
Total future minimum lease payments | | | 21,262 | |
Less: Imputed interest | | | — | |
| | | | |
Present value of future minimum lease payments | | | 21,262 | |
Less: Current portion | | | (6,855 | ) |
| | | | |
| | $ | 14,407 | |
| | | | |
At December 31, 2013, the capitalized amounts of the building, machinery and equipment and computer equipment under capital leases were as follows (in thousands):
| | | | |
Machinery, laboratory equipment and tooling | | $ | 32,256 | |
Computer equipment | | | 3,506 | |
Furniture and fixtures | | | 890 | |
Vehicles | | | 73 | |
Leasehold improvements | | | — | |
| | | | |
| | | 36,725 | |
Less: Accumulated amortization | | | (9,785 | ) |
| | | | |
| | $ | 26,940 | |
| | | | |
The amortization expense of assets recorded under capital leases is included in depreciation and amortization expense of property, plant and equipment.
F-45
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(9) Postretirement Benefit Plans
(a) Employee Savings Plans
Our company and several of our U.S.-based subsidiaries sponsor various 401(k) savings plans, to which eligible domestic employees may voluntarily contribute a portion of their income, subject to statutory limitations. In addition to the participants’ own contributions to these 401(k) savings plans, we match such contributions up to a designated level. Total matching contributions related to employee savings plans were $9.4 million, $8.8 million and $7.6 million in 2013, 2012 and 2011, respectively.
(b) U.K. Pension Plans
Our subsidiary in England, Unipath Ltd., or Unipath, has a defined benefit pension plan established for certain of its employees.
Changes in benefit obligations, plan assets, funded status and amounts recognized on the accompanying balance sheet as of and for the years ended December 31, 2013 and 2012, for our Defined Benefit Plan were as follows (in thousands):
| | | | | | | | |
| | | 2013 | | | 2012 | |
Change in projected benefit obligation | | | | | | | | |
Benefit obligation at beginning of year | | $ | 18,340 | | | $ | 14,966 | |
Interest cost | | | 754 | | | | 742 | |
Actuarial loss | | | 2,202 | | | | 2,211 | |
Benefits paid | | | (232 | ) | | | (417 | ) |
Foreign exchange impact | | | 508 | | | | 838 | |
| | | | | | | | |
Benefit obligation at end of year | | $ | 21,572 | | | $ | 18,340 | |
Change in plan assets | | | | | | | | |
Fair value of plan assets at beginning of year | | $ | 14,043 | | | $ | 11,272 | |
Actual return on plan assets | | | 2,084 | | | | 1,634 | |
Employer contribution | | | 904 | | | | 916 | |
Benefits paid | | | (232 | ) | | | (417 | ) |
Foreign exchange impact | | | 427 | | | | 638 | |
| | | | | | | | |
Fair value of plan assets at end of year | | $ | 17,226 | | | $ | 14,043 | |
| | | | | | | | |
Funded status | | $ | (4,346 | ) | | $ | (4,297 | ) |
| | | | | | | | |
Accumulated benefit obligation | | $ | 21,572 | | | $ | 18,340 | |
| | | | | | | | |
The net amounts recognized in the accompanying consolidated balance sheets are shown in current liabilities and were $4.3 million for the years ended December 31, 2013 and 2012.
The following represents the amounts recognized in other comprehensive loss for the year ended December 31, 2013:
| | | | |
Amortization of net loss | | | (199 | ) |
Amortization of prior service cost | | | (416 | ) |
New actuarial gains | | | 832 | |
Foreign exchange impact | | | 181 | |
| | | | |
Total | | $ | 398 | |
| | | | |
The amortization of prior service cost is expected to be approximately $0.4 million in 2014.
F-46
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(9) Postretirement Benefit Plans (Continued)
Amounts recognized in accumulated other comprehensive income (loss) for the years ending December 31, 2013 and 2012 are as follows:
| | | | | | | | |
| | | 2013 | | | 2012 | |
Net actuarial loss | | $ | 4,846 | | | $ | 4,098 | |
Prior service costs | | | 4,404 | | | | 4,754 | |
| | | | | | | | |
Net amount recognized | | $ | 9,250 | | | $ | 8,852 | |
| | | | | | | | |
The measurement dates used to determine plan assets and benefit obligations for the Defined Benefit Plan were December 31, 2013 and 2012.
The following table provides the weighted-average actuarial assumptions:
| | | | | | | | |
| | | 2013 | | | 2012 | |
Assumptions used to determine benefit obligations(1): | | | | | | | | |
Discount rate | | | 4.40 | % | | | 4.30 | % |
Rate of compensation increase | | | 4.15 | % | | | 3.65 | % |
Assumptions used to determine net periodic benefit cost(2): | | | | | | | | |
Discount rate | | | 4.30 | % | | | 4.90 | % |
Expected long-term return on plan assets | | | 5.15 | % | | | 5.40 | % |
Rate of compensation increase | | | 3.65 | % | | | 3.75 | % |
| (1) | The actuarial assumptions used to compute the unfunded status for the plan are based upon information available as of December 31, 2013 and 2012. |
| (2) | The actuarial assumptions used to compute the net periodic pension benefit cost are based upon the information available as of the beginning of the presented year. |
The actuarial assumptions are reviewed on an annual basis. The overall expected long-term rate of return on plan assets assumption was determined based on historical investment return rates on portfolios with a high proportion of equity securities, and was calculated using a weighted-average of the expected returns for each asset class held by the Plan. The long-term expected return on assets is net of investment expenses.
The annual net periodic benefit costs of the Defined Benefit Plan are as follows (in thousands):
| | | | | | | | | | | | |
| | | 2013 | | | 2012 | | | 2011 | |
Service cost | | $ | — | | | $ | — | | | $ | — | |
Interest cost | | | 754 | | | | 742 | | | | 773 | |
Expected return on plan assets | | | (713 | ) | | | (636 | ) | | | (731 | ) |
Amortization of net loss | | | 199 | | | | 114 | | | | 32 | |
Amortization of prior service cost | | | 416 | | | | 422 | | | | 427 | |
Curtailment loss (gain) | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
Net periodic benefit cost | | $ | 656 | | | $ | 642 | | | $ | 501 | |
| | | | | | | | | | | | |
The plan assets of the Defined Benefit Plan comprise a mix of stocks and fixed income securities and other investments. At December 31, 2013, these stocks and fixed income securities represented 74% and 26%, respectively, of the market value of the pension assets. We expect to contribute approximately £0.6 million (or $1.0 million at December 31, 2013) to the Defined Benefit Plan in 2014.
F-47
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(9) Postretirement Benefit Plans (Continued)
We expect that the benefits to be paid to plan participants will range between approximately $0.3 million and $0.4 million per year for each of the next five years and that benefits totaling $0.5 million will be paid annually for the five years thereafter.
Our overall investment strategy is to ensure the investments are spread across a range of investments varying by both investment class and geographical location which is achieved by investing largely in equity and fixed income funds. Spreading the investments in this manner reduces the risk of a decline in a particular market having a substantial impact on the whole fund. The target allocation for the plan assets is a 70% holding in equities (both in the U.K. and overseas), with the remaining assets invested in investment grade corporate bonds.
The fair values of our pension plan assets at December 31, 2013 and 2012 by asset category are presented in the following table (Level 2 in the fair value hierarchy).
| | | | | | | | |
| | | Plan Assets at
December 31, | |
Asset Category | | 2013 | | | 2012 | |
Equity securities: | | | | | | | | |
U.K. equities | | $ | 6,000 | | | $ | 4,808 | |
Overseas equities | | | 2,995 | | | | 2,532 | |
U.S. equities | | | 3,003 | | | | 2,219 | |
Debt securities — corporate bonds | | | 4,402 | | | | 4,286 | |
Other — cash | | | 826 | | | | 198 | |
| | | | | | | | |
Total plan assets | | $ | 17,226 | | | $ | 14,043 | |
| | | | | | | | |
The table above presents the fair value of our plan’s assets in accordance with the fair value hierarchy. The pension plan assets are measured using net asset value per share (or its equivalent) and are reported as a Level 2 investment above due to our ability to redeem the investment either at the balance sheet date or within limited time restrictions.
Unipath contributed $0.3 million in 2013, $0.4 million in 2012 and $0.3 million in 2011 to the Defined Contribution Plan, which was recognized as an expense in the accompanying consolidated statement of operations.
(10) Derivative Financial Instruments
We may manage our economic and transaction exposure to certain market-based risks through the use of derivative instruments. Our objective for holding derivative instruments has been to reduce volatility of net earnings and cash flows associated with changes in foreign currency exchange rates. We do not hold or issue derivative financial instruments for speculative purposes.
Foreign Currency Risk
In connection with our acquisition of Axis-Shield, we acquired a number of foreign currency forward contracts. The specific risk hedged in these contracts was the undiscounted foreign currency spot rate risk on forecasted foreign currency revenue. As of December 31, 2012, all of the acquired foreign currency forward contracts were settled. We report the effective portion of the gain or loss on a cash flow hedge as a component of other comprehensive income, and it was subsequently reclassified
F-48
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(10) Derivative Financial Instruments (Continued)
into net earnings in the period in which the hedged transaction affected net earnings or the forecasted transaction was no longer probable of occurring.
The following table summarizes the effect of the derivative instruments in our accompanying consolidated statements of operations (in thousands):
| | | | | | | | | | | | | | |
Derivative Instruments | | Location of Gain (Loss)
Recognized in Income | | Amount of Gain
(Loss) Recognized
During the Year
Ended
December 31,
2013 | | | Amount of Gain
(Loss) Recognized
During the Year
Ended
December 31,
2012 | | | Amount of Loss
Recognized
During the Year
Ended
December 31,
2011 | |
Foreign currency forward contracts | | Other comprehensive income
(loss) | | $ | — | | | $ | — | | | $ | (1,187 | ) |
| | | | | | | | | | | | | | |
Total loss | | Other comprehensive income
(loss) | | $ | — | | | $ | — | | | $ | (1,187 | ) |
| | | | | | | | | | | | | | |
(11) Commitments and Contingencies
(a) Operating Leases
We have operating lease commitments for certain of our facilities and equipment that expire on various dates through 2019. The following schedule outlines future minimum annual rental payments under these leases at December 31, 2013 (in thousands):
| | | | |
2014 | | $ | 47,611 | |
2015 | | | 40,774 | |
2016 | | | 36,386 | |
2017 | | | 32,654 | |
2018 | | | 28,045 | |
Thereafter | | | 45,743 | |
| | | | |
| | $ | 231,213 | |
| | | | |
Rent expense relating to operating leases was approximately $54.0 million, $51.2 million and $42.3 million during 2013, 2012 and 2011, respectively.
(b) Contingent Consideration Obligations
We determine the acquisition date fair value of the contingent consideration obligations based on a probability-weighted approach derived from the overall likelihood of achieving certain performance targets, including product development milestones or financial metrics. The fair value measurement is based on significant inputs not observable in the market and thus represents a Level 3 measurement, as defined in fair value measurement accounting. The resultant probability-weighted earn-out payments are discounted using a discount rate based upon the weighted-average cost of capital. At each reporting date, we revalue the contingent consideration obligations to the reporting date fair values and record increases and decreases in the fair values as income or expense in our consolidated statements of operations.
F-49
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(11) Commitments and Contingencies (Continued)
Increases or decreases in the fair values of the contingent consideration obligations may result from changes in discount periods and rates, changes in the timing and amount of earn-out criteria and changes in probability assumptions with respect to the likelihood of achieving the various earn-out criteria.
We have contractual contingent purchase price consideration obligations related to certain of our acquisitions, as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Acquisition | | Acquisition Date | | | Acquisition
Date Fair
Value | | | Maximum
Remaining
Earn-out
Potential
as of
December 31,
2013 | | | Remaining
Earn-out
Period as
of
December 31,
2013 | | | Estimated
Fair Value as
of
December 31,
2013 | | | Estimated
Fair Value as
of
December 31,
2012 | | | Payments
made
during
2013 | |
TwistDx, Inc. | | | March 11, 2010 | | | $ | 35,600 | | | $ | 124,027 | | | | 2014 – 2025 | (1) | | $ | 45,502 | | | $ | 25,100 | | | $ | 680 | |
Ionian Technologies, Inc. | | | July 12, 2010 | | | $ | 24,500 | | | | 57,500 | | | | 2014 – 2015 | | | | 29,000 | | | | 23,800 | | | | — | |
LDS | | | August 29, 2011 | | | $ | 13,000 | | | | 7,500 | | | | 2013 | | | | 7,400 | (2) | | | 6,600 | | | | — | |
ROAR | | | September 22, 2011 | | | $ | 5,463 | | | | 12,600 | | | | 2014 | | | | 2,484 | | | | 6,827 | | | | 4,606 | |
Wellogic | | | December 9, 2011 | | | $ | 18,900 | | | | 50,000 | | | | 2014 – 2019 | | | | 26,900 | | | | 23,600 | | | | — | |
eScreen | | | April 2, 2012 | | | $ | 44,500 | | | | — | | | | — | | | | — | | | | 19,300 | | | | — | |
MedApps | | | July 2, 2012 | | | $ | 13,100 | | | | 13,600 | | | | 2014 | | | | 8,200 | | | | 15,900 | | | | 7,600 | |
Amedica | | | July 3, 2012 | | | $ | 8,900 | | | | 8,100 | | �� | | 2013 | | | | 7,500 | (2) | | | 8,700 | | | | 6,945 | |
DiagnosisOne | | | July 31, 2012 | | | $ | 22,300 | | | | 33,000 | | | | 2014 – 2017 | | | | 26,600 | | | | 24,300 | | | | — | |
Epocal | | | February 1, 2013 | | | $ | 75,000 | | | | 65,500 | | | | 2014 – 2018 | | | | 47,200 | | | | — | | | | 25,000 | |
Other | | | Various | | | $ | 58,877 | | | | 23,029 | | | | 2014 – 2016 | | | | 13,183 | | | | 22,045 | | | | 11,601 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | $ | 394,856 | | | | | | | $ | 213,969 | | | $ | 176,172 | | | $ | 56,432 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (1) | The maximum earn-out period ends on the fifteenth anniversary of the acquisition date. |
| (2) | The fair value of the maximum remaining earn-out has been accrued at December 31, 2013. We expect to pay the maximum remaining earn-out during the first half of 2014. |
(c) Legal Proceedings
| | • | | Matters Relating to our San Diego Facility |
On October 9, 2012, we received a warning letter from the FDA referencing inspectional observations set forth in an FDA Form 483 received in June, 2012. The observations were the result of an inspection of our San Diego facility conducted earlier during 2012 relating to our Alere Triage products, which resulted in two recalls of certain Alere Triage products and revised release specifications for our Alere Triage meter-based products. We have submitted evidence of our completion of most of the actions we committed to in response to the FDA Form 483 and warning letter. We intend to continue to work cooperatively with the FDA in an effort to fully address each inspectional observation.
In May 2012, we also received a subpoena from the Office of Inspector General of the Department of Health and Human Services, or the OIG, seeking documents relating primarily to the quality control testing and performance characteristics of Alere Triage products. We are cooperating with the OIG and have provided documents in response to the OIG under the subpoena.
We are unable to predict when these matters will be resolved or what further action, if any, the government will take in connection with them. Except for increases in manufacturing costs and
F-50
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(11) Commitments and Contingencies (Continued)
decreased profitability for our Alere Triage products, we are unable to predict what impact, if any, these matters or ensuing proceedings, if any, will have on our financial condition, results of operations or cash flows.
| | • | | Matters Related to Theft of Laptop |
On January 24, 2013, a class action complaint was filed in the U.S. District Court for the Northern District of California against Alere Home Monitoring, or AHM, asserting claims for damages and other relief under California state law, including under California’s Confidentiality of Medical Information Act, relating to an inadvertent disclosure of personally identifiable information of approximately 116,000 patients resulting from the theft of a laptop computer from an employee of AHM. The class action has been stayed pending resolution of other class actions involving third parties based on similar circumstances. The Office of Civil Rights of the U.S. Department of Health and Human Services, or the OCR, was notified of the inadvertent disclosure in accordance with the Breach Notification Rule under the HITECH Act, as were certain state agencies. AHM has responded to the OCR’s inquiries and continues to cooperate.
| | • | | Claims in the Ordinary Course and Other Matters |
Because of the nature of our business, we may be subject at any particular time to lawsuits or other claims arising in the ordinary course of our business, and we expect that this will continue to be the case in the future. Such lawsuits often seek damages, sometimes in substantial amounts.
(12) Net Loss Per Common Share
The following tables set forth the computation of basic and diluted net loss per common share (in thousands, except per share amounts):
| | | | | | | | | | | | |
| | | 2013 | | | 2012 | | | 2011 | |
Basic and diluted net loss per common share: | | | | | | | | | | | | |
Numerator: | | | | | | | | | | | | |
Net loss | | $ | (70,278 | ) | | $ | (77,907 | ) | | $ | (133,309 | ) |
Preferred stock dividends | | | (21,293 | ) | | | (21,293 | ) | | | (22,049 | ) |
Preferred stock repurchase | | | — | | | | — | | | | 23,936 | |
Less: Net income attributable to non-controlling interest | | | 976 | | | | 275 | | | | 233 | |
| | | | | | | | | | | | |
Net loss available to common stockholders | | $ | (92,547 | ) | | $ | (99,475 | ) | | $ | (131,655 | ) |
| | | | | | | | | | | | |
Denominator: | | | | | | | | | | | | |
Weighted-average common shares outstanding — basic and diluted | | | 81,542 | | | | 80,587 | | | | 83,128 | |
| | | | | | | | | | | | |
Basic and Diluted net loss per common share attributable to Alere Inc. and Subsidiaries | | $ | (1.13 | ) | | $ | (1.23 | ) | | $ | (1.58 | ) |
| | | | | | | | | | | | |
F-51
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(12) Net Loss Per Common Share (Continued)
The following potential dilutive securities were not included in the calculation of diluted net loss per common share because the inclusion thereof would be antidilutive (in thousands):
| | | | | | | | | | | | |
| | | 2013 | | | 2012 | | | 2011 | |
Denominator: | | | | | | | | | | | | |
Options to purchase shares of common stock | | | 10,879 | | | | 10,234 | | | | 9,446 | |
Warrants | | | 4 | | | | 4 | | | | 162 | |
Conversion shares related to 3% convertible senior subordinated notes | | | 3,411 | | | | 3,411 | | | | 3,411 | |
Conversion shares related to subordinated convertible promissory notes | | | 27 | | | | 27 | | | | 27 | |
Conversion shares related to Series B convertible preferred stock | | | 10,239 | | | | 10,239 | | | | 10,239 | |
Common stock equivalents related to the settlement of deferred purchase price consideration | | | — | | | | — | | | | 142 | |
Common stock equivalents related to the settlement of a contingent consideration obligation | | | 451 | | | | — | | | | — | |
| | | | | | | | | | | | |
Total number of antidilutive potentially issuable shares of common stock excluded from diluted common shares outstanding | | | 25,011 | | | | 23,915 | | | | 23,427 | |
| | | | | | | | | | | | |
(13) Stockholders’ Equity
(a) Common Stock
As of December 31, 2013, we had 200.0 million shares of common stock, $0.001 par value, authorized, of which 7.7 million shares were held in treasury and 82.0 million shares were outstanding, 12.6 million shares were reserved for issuance upon grant and exercise of equity awards under current equity compensation plans, 1.5 million shares were reserved for issuance under our employee stock purchase plan and 4,000 shares were reserved for issuance upon exercise of outstanding warrants. We also had shares of common stock reserved for issuance upon conversion of the following securities outstanding on December 31, 2013: $150.0 million in the aggregate principal amount of 3% convertible senior subordinated notes, convertible at $43.98 per share into 3.4 million shares of our common stock; $1.7 million of subordinated convertible promissory notes, convertible at $61.49 per share into 27,647 shares of our common stock; and 1.8 million shares of our Series B convertible preferred stock, with an aggregate liquidation preference of approximately $709.8 million, convertible under certain circumstances at $69.32 per share into 10.2 million shares of our common stock.
(b) Preferred Stock
As of December 31, 2013, we had 5.0 million shares of preferred stock, $0.001 par value, authorized, of which 2.3 million shares were designated as Series B Convertible Perpetual Preferred Stock, or Series B preferred stock. In connection with our acquisition of Matria, we issued shares of the Series B preferred stock and through June 30, 2011 paid all dividends on outstanding shares of Series B preferred stock in additional shares of Series B preferred stock. Subsequent to June 30, 2011 all dividends on outstanding shares of Series B preferred stock were paid in cash. At December 31, 2013, there were 1.8 million shares of Series B preferred stock outstanding with a fair value of approximately $508.0 million.
F-52
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(13) Stockholders’ Equity (Continued)
Each share of Series B preferred stock, which has a liquidation preference of $400.00 per share, is convertible, at the option of the holder and only upon certain circumstances, into 5.7703 shares of our common stock, plus cash in lieu of fractional shares. The initial conversion price is $69.32 per share, subject to adjustment upon the occurrence of certain events, but will not be adjusted for accumulated and unpaid dividends. Upon a conversion of shares of the Series B preferred stock, we may, at our option, satisfy the entire conversion obligation in cash or through a combination of cash and common stock. Series B preferred stock outstanding at December 31, 2013 would convert into 10.2 million shares of our common stock, which are reserved. There were no conversions as of December 31, 2013.
Generally, the shares of Series B preferred stock are convertible, at the option of the holder, if during any calendar quarter beginning with the second calendar quarter after the issuance date of the Series B preferred stock, if the closing sale price of our common stock for each of 20 or more trading days within any period of 30 consecutive trading days ending on the last trading day of the immediately preceding calendar quarter exceeds 130% of the conversion price per share of common stock in effect on the last trading day of the immediately preceding calendar quarter. In addition, the shares of Series B preferred stock are convertible, at the option of the holder, in certain other circumstances, including those relating to the trading price of the Series B preferred stock and upon the occurrence of certain fundamental changes or major corporate transactions. We also have the right, under certain circumstances relating to the trading price of our common stock, to force conversion of the Series B preferred stock. Depending on the timing of any such forced conversion, we may have to make certain payments relating to foregone dividends, which payments we can make, at our option, in the form of cash, shares of our common stock, or a combination of cash and shares of our common stock.
Each share of Series B preferred stock accrues dividends at $12.00, or 3%, per annum, payable quarterly on January 15, April 15, July 15 and October 15 of each year, commencing following the first full calendar quarter after the issuance date. Dividends on the Series B preferred stock are cumulative from the date of issuance. For the years ended December 31, 2013, 2012 and 2011, Series B preferred stock dividends amounted to $21.3 million, $21.3 million and $22.0 million respectively, which reduced earnings available to common stockholders for purposes of calculating net loss per common share in each of 2013, 2012 and 2011 (Note 12). Accrued dividends are payable only if declared by our board of directors and, upon conversion by the Series B preferred stockholder, holders will not receive any cash payment representing accumulated dividends. If our board of directors declares a dividend payable, we have the right to pay the dividends in cash, shares of common stock, additional shares of Series B preferred stock or a similar convertible preferred stock or any combination thereof. The Series B preferred stock dividends for each of the years ended December 31, 2013 and 2012 were paid in cash.
The holders of Series B preferred stock have liquidation preferences over the holders of our common stock and other classes of stock, if any, outstanding at the time of liquidation. Upon liquidation, the holders of outstanding Series B preferred stock would receive an amount equal to $400.00 per share of Series B preferred stock, plus any accumulated and unpaid dividends. As of December 31, 2013, the liquidation preference of the outstanding Series B preferred stock was $709.8 million. The holders of the Series B preferred stock have no voting rights, except with respect to matters affecting the Series B preferred stock (including the creation of a senior preferred stock).
We evaluated the terms and provisions of our Series B preferred stock to determine if it qualified for derivative accounting treatment. Based on our evaluation, these securities do not qualify for derivative accounting.
F-53
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(13) Stockholders’ Equity (Continued)
(c) Share Repurchases
During the first quarter of 2011, we repurchased in the open market and privately-negotiated transactions 183,000 shares of our Series B preferred stock, which were convertible into approximately 1.1 million shares of our common stock, at a cost of approximately $49.4 million, which we paid in cash. The repurchase of the preferred stock at an average cost of $269.84 per preferred share, an amount less than the weighted-average fair value of the preferred shares at issuance, resulted in the allocation of $13.7 million of income attributable to common stockholders. Also during the first quarter of 2011, and pursuant to the same repurchase program, we repurchased 16,700 shares of our common stock at a cost of approximately $0.6 million, which we paid in cash.
During the second quarter of 2011, we repurchased in the open market and privately-negotiated transactions, 174,788 shares of our Series B preferred stock, which were convertible into approximately 1.0 million shares of our common stock, at a cost of approximately $49.7 million, which we paid in cash. The repurchase of the preferred stock at an average cost of $284.28 per preferred share, an amount less than the weighted-average fair value of the preferred shares at issuance, resulted in the allocation of $10.2 million of income attributable to common stockholders. Also during the second quarter of 2011, and pursuant to the same repurchase program, we repurchased 8,300 shares of our common stock at a cost of approximately $0.3 million, which we paid in cash.
During the third quarter of 2011, we repurchased approximately 7.6 million shares of our common stock at a cost of approximately $183.9 million, which we paid in cash.
(d) Stock Options and Awards
In 2010, we adopted the Alere Inc. 2010 Stock Option and Incentive Plan, or the 2010 Plan, which replaced our 2001 Stock Option and Incentive Plan, or the 2001 Plan. The 2010 Plan currently allows for the issuance of up to 7.2 million shares of common stock and other awards. The 2010 Plan is administered by the Compensation Committee of the Board of Directors, which selects the individuals eligible to receive awards, determines or modifies the terms and conditions of the awards granted, accelerates the vesting schedule of any award and generally administers and interprets the 2010 Plan. The 2010 Plan permits the granting of incentive and nonqualified stock options with terms of up to ten years and the granting of stock appreciation rights, restricted stock awards, unrestricted stock awards, performance share awards and dividend equivalent rights. The 2010 Plan also provides for option grants to non-employee directors and automatic vesting acceleration of all options and stock appreciation rights upon a change in control, as defined by the 2010 Plan. As of December 31, 2013 and 2012, there were 1.1 million and 1.0 million shares, respectively, available for future grant under the 2010 Plan.
The following summarizes all stock option activity during the year ended December 31, 2013 (in thousands, except exercise price):
| | | | | | | | |
| | | Options | | | Weighted-
average
Exercise Price | |
Outstanding at January 1,2013 | | | 10,650 | | | $ | 36.51 | |
Granted | | | 2,749 | | | $ | 35.64 | |
Exercised | | | (576 | ) | | $ | 22.40 | |
Canceled/expired/forfeited. | | | (1,382 | ) | | $ | 31.20 | |
| | | | | | | | |
Outstanding at December 31, 2013 | | | 11,441 | | | $ | 37.66 | |
| | | | | | | | |
Exercisable at December 31, 2013 | | | 6,799 | | | $ | 39.35 | |
| | | | | | | | |
F-54
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(13) Stockholders’ Equity (Continued)
The aggregate intrinsic value of the options outstanding at December 31, 2013 was $50.8 million. The aggregate intrinsic value of the options exercisable at December 31, 2013 was $23.6 million. The aggregate intrinsic value of stock options exercised during 2013, 2012 and 2011 was $4.5 million, $1.3 million and $17.1 million, respectively.
In addition, on December 30, 2012, we granted a restricted stock unit, or RSU, award to one of our executive employees in the amount of 110,000 units. The RSU award vests over a three-year period, however, if the executive’s employment is involuntarily terminated, without cause, within three years, the RSU award will accelerate and fully vest. The RSU award will also accelerate and fully vest if the executive terminates his employment voluntarily after one year, other than in the presence of facts or circumstances which would constitute cause for termination by us. The fair value of the RSU award is $18.37 per unit, calculated based on the closing market price of our common stock on the date of grant. The aggregate fair value of the RSU award is expensed on a straight-line basis over the recipient’s service requirement completion period of one year. All of the units underlying the RSU award have vested and 5,000 have been released as of December 31, 2013.
Based on equity awards outstanding as of December 31, 2013, there was $49.4 million of unrecognized compensation costs related to unvested share-based compensation arrangements that are expected to vest. Such costs are expected to be recognized over a weighted-average period of 1.48 years.
(e) Warrants
The following is a summary of all warrant activity during the three years ended December 31, 2013:
| | | | | | | | | | | | |
| | | Number of
Shares | | | Exercise Price | | | Weighted-
average
Exercise Price | |
| | | (in thousands) | | | | | | | |
Warrants outstanding and exercisable, December 31, 2010 | | | 243 | | | $ | 13.54 - $50.00 | | | $ | 16.00 | |
Exercised | | | (81 | ) | | $ | 13.54 - $24.00 | | | $ | 19.15 | |
| | | | | | | | | | | | |
Warrants outstanding and exercisable, December 31, 2011 | | | 162 | | | $ | 13.54 - $50.00 | | | $ | 14.44 | |
Exercised | | | (158 | ) | | $ | 13.54 | | | $ | 13.54 | |
| | | | | | | | | | | | |
Warrants outstanding and exercisable, December 31, 2012 | | | 4 | | | $ | 50.00 | | | $ | 50.00 | |
Exercised | | | — | | | | | | | | | |
| | | | | | | | | | | | |
Warrants outstanding and exercisable, December 31, 2013 | | | 4 | | | $ | 50.00 | | | $ | 50.00 | |
| | | | | | | | | | | | |
The following table presents additional information related to warrants outstanding and exercisable at December 31, 2013:
| | | | | | | | | | | | |
| | | Outstanding and Exercisable | |
Exercise Price | | Number of
Shares | | | Weighted-
average
Remaining
Contract Life | | | Weighted-
average
Exercise Price | |
| | | (in thousands) | | | | | | | |
$50.00 | | | 4 | | | | 2.50 | | | $ | 50.00 | |
F-55
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(13) Stockholders’ Equity (Continued)
The warrants included in the table above were issued in connection with the acquisition of GeneCare. There were no warrants to purchase shares of our common stock outstanding that were issued to officers and directors of our company or entities controlled by these officers and directors at December 31, 2013. All outstanding warrants have been classified in equity.
(f) Employee Stock Purchase Plan
In 2001, we adopted the 2001 Employee Stock Purchase Plan, under which eligible employees are allowed to purchase shares of our common stock at a discount through periodic payroll deductions. Purchases may occur at the end of every six-month offering period at a purchase price equal to 85% of the market value of our common stock at either the beginning or end of the offering period, whichever is lower. We may issue up to 4.0 million shares of common stock under this plan. At December 31, 2013, 2.5 million shares had been issued under this plan.
(14) Stock-based Compensation
We recorded stock-based compensation expense in our consolidated statements of operations for the years ended December 31, 2013, 2012 and 2011, respectively, as follows (in thousands):
| | | | | | | | | | | | |
| | | 2013 | | | 2012 | | | 2011 | |
Cost of net revenue | | $ | 1,126 | | | $ | 1,063 | | | $ | 1,508 | |
Research and development | | | 4,053 | | | | 3,150 | | | | 3,862 | |
Sales and marketing | | | 3,698 | | | | 3,464 | | | | 4,267 | |
General and administrative | | | 12,333 | | | | 7,988 | | | | 11,578 | |
| | | | | | | | | | | | |
| | | 21,210 | | | | 15,665 | | | | 21,215 | |
Benefit for income taxes | | | (4,190 | ) | | | (2,660 | ) | | | (4,560 | ) |
| | | | | | | | | | | | |
Stock-based compensation, net of tax | | $ | 17,020 | | | $ | 13,005 | | | $ | 16,655 | |
| | | | | | | | | | | | |
For the years ended December 31, 2013, 2012 and 2011, the presentation of our cash flows reports the excess tax benefits from the exercise of stock options as financing cash flows. For the years ended December 31, 2013, 2012 and 2011, excess tax benefits generated from option exercises amounted to $1.4 million, $0.5 million and $3.4 million, respectively.
The following assumptions were used to estimate the fair value of options granted during the years ended December 31, 2013, 2012 and 2011, using a Black-Scholes option-pricing model:
| | | | | | | | | | | | |
| | | 2013 | | | 2012 | | | 2011 | |
Risk-free interest rate | | | 1.47 | % | | | 0.83 | % | | | 1.31 | % |
Expected dividend yield | | | — | | | | — | | | | — | |
Expected life | | | 5.74 years | | | | 5.60 years | | | | 5.46 years | |
Expected volatility | | | 42 | % | | | 42 | % | | | 43 | % |
The weighted-average fair value under a Black-Scholes option pricing model of options granted to employees during 2013, 2012 and 2011 was $15.22, $7.37 and $10.95 per share, respectively. All options granted during these periods were granted at or above the fair market value on the date of grant.
F-56
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(14) Stock-based Compensation (Continued)
For the year ended December 31, 2013, we recorded compensation expense of $2.6 million related to our Employee Stock Purchase Plan. The fair value of the option component of the Employee Stock Purchase Plan shares was estimated at the date of grant using a Black-Scholes option pricing model and assumed an expected volatility of 41% and 40%, a risk-free interest rate of 0.12% and 0.09% and an expected life of 181 days and 184 days, for each of the two respective offering periods. The 2013 charge is included in the employee’s respective cost classification in the table above.
For the year ended December 31, 2012, we recorded compensation expense of $2.7 million related to our Employee Stock Purchase Plan. The fair value of the option component of the Employee Stock Purchase Plan shares was estimated at the date of grant using a Black-Scholes option pricing model and assumed an expected volatility of 44% and 47%, a risk-free interest rate of 0.06% and 0.15% and an expected life of 182 days and 184 days, for each of the two respective offering periods. In the first half of 2012 we had a second offering period to accommodate employees of a recent acquisition. The fair value of the option component of the Employee Stock Purchase Plans shares for this offering was estimated at the grant date of March 1, 2012 using a Black-Scholes option pricing model and assumed an expected volatility of 40%, a risk-free rate of 0.10%, and an expected life of 122 days. The 2012 charge is included in the employee’s respective cost classification in the table above.
For the year ended December 31, 2011, we recorded compensation expense of $2.5 million related to our Employee Stock Purchase Plan. The fair value of the option component of the Employee Stock Purchase Plan shares was estimated at the date of grant using a Black-Scholes option pricing model and assumed an expected volatility of 35% and 34%, a risk-free interest rate of 0.19% and 0.10% and an expected life of 181 days and 184 days, for each of the two respective offering periods. The 2011 charge is included in the employee’s respective cost classification in the table above.
(15) Accumulated Other Comprehensive Income (Loss)
Accumulated other comprehensive income (loss) consisted of the following (in thousands):
| | | | | | | | | | | | | | | | |
| | | Cumulative
Translation
Adjustment
(Note 2(b)) | | | Pension
Liability
Adjustment
(Note 9(b)) | | | Other(1) | | | Accumulated
Other
Comprehensive
Income (Loss) | |
Balance at December 31, 2010 | | $ | 12,705 | | | $ | (5,209 | ) | | $ | (6,806 | ) | | $ | 690 | |
Period change | | | (35,830 | ) | | | (1,618 | ) | | | 6,488 | | | | (30,960 | ) |
| | | | | | | | | | | | | | | | |
Balance at December 31, 2011 | | | (23,125 | ) | | | (6,827 | ) | | | (318 | ) | | | (30,270 | ) |
Period change | | | 54,642 | | | | (756 | ) | | | 258 | | | | 54,144 | |
| | | | | | | | | | | | | | | | |
Balance at December 31, 2012 | | | 31,517 | | | | (7,583 | ) | | | (60 | ) | | | 23,874 | |
Period change | | | (50,166 | ) | | | (309 | ) | | | 39 | | | | (50,436 | ) |
| | | | | | | | | | | | | | | | |
Balance at December 31, 2013 | | $ | (18,649 | ) | | $ | (7,892 | ) | | $ | (21 | ) | | $ | (26,562 | ) |
| | | | | | | | | | | | | | | | |
| (1) | Other represents unrealized gains (losses) on available-for-sale securities and hedging instruments. |
F-57
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(16) Income Taxes
Income (loss) before provision (benefit) for income taxes consists of the following (in thousands):
| | | | | | | | | | | | |
| | | 2013 | | | 2012 | | | 2011 | |
United States | | $ | (214,332 | ) | | $ | (158,635 | ) | | $ | (493,073 | ) |
Foreign | | | 80,300 | | | | 37,164 | | | | 327,026 | |
| | | | | | | | | | | | |
| | $ | (134,032 | ) | | $ | (121,471 | ) | | $ | (166,047 | ) |
| | | | | | | | | | | | |
Our primary temporary differences that give rise to the deferred tax asset and liability are net operating loss, or NOL, carryforwards, nondeductible reserves, and accruals and differences in basis of the tangible and intangible assets. The income tax effects of these temporary differences are as follows (in thousands):
| | | | | | | | |
| | | 2013 | | | 2012 | |
NOL and capital loss carryforwards | | $ | 137,178 | | | $ | 117,091 | |
Tax credit carryforwards | | | 70,357 | | | | 54,212 | |
Nondeductible reserves | | | 33,846 | | | | 17,036 | |
Nondeductible accruals | | | 44,023 | | | | 34,406 | |
Difference between book and tax bases of tangible assets | | | 989 | | | | 1,059 | |
Difference between book and tax bases of intangible assets | | | 25,484 | | | | 23,017 | |
Deferred revenue | | | 8,399 | | | | 5,686 | |
All other | | | 32,669 | | | | 30,071 | |
| | | | | | | | |
Gross deferred tax asset | | | 352,945 | | | | 282,578 | |
Less: Valuation allowance | | | (83,591 | ) | | | (68,555 | ) |
| | | | | | | | |
Total deferred tax assets | | | 269,354 | | | | 214,023 | |
| | | | | | | | |
Deferred tax liabilities: | | | | | | | | |
Difference between book and tax bases of tangible assets | | | 54,436 | | | | 42,835 | |
Difference between book and tax bases of intangible assets | | | 442,729 | | | | 482,955 | |
Debt | | | 18,041 | | | | 25,541 | |
Other | | | 15,365 | | | | 15,525 | |
| | | | | | | | |
Total deferred tax liability | | | 530,571 | | | | 566,856 | |
| | | | | | | | |
Net deferred tax liability | | $ | 261,217 | | | $ | 352,833 | |
| | | | | | | | |
Reported as: | | | | | | | | |
Deferred tax assets, current portion | | $ | 60,689 | | | $ | 67,722 | |
Deferred tax assets, long-term | | | 7,959 | | | | 8,293 | |
Deferred tax liabilities, current portion | | | (616 | ) | | | (660 | ) |
Deferred tax liabilities, long-term | | | (329,249 | ) | | | (428,188 | ) |
| | | | | | | | |
Net deferred tax liability | | $ | (261,217 | ) | | $ | (352,833 | ) |
| | | | | | | | |
As of December 31, 2013, we had approximately $87.4 million of domestic NOL and domestic capital loss carryforwards, approximately $998.8 million of state NOL carryforwards and $254.7 million of foreign NOL and foreign capital loss carryforwards, which either expire on various dates through 2033 or can be carried forward indefinitely. As of December 31, 2013, we had approximately $70.4 million of domestic research and development, foreign tax and alternative minimum tax credits which either expire on various dates through 2033 or can be carried forward indefinitely. These loss carryforwards and tax credits may be available to reduce future federal, state and foreign taxable
F-58
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(16) Income Taxes (Continued)
income, if any, and are subject to review and possible adjustment by the appropriate tax authorities. Section 382 imposes an annual limitation on the use of these losses to an amount equal to the value of the company at the time of the ownership change multiplied by the long-term tax exempt rate. Our domestic NOLs are subject to various Internal Revenue Service, or IRS, Code Section 382 limitations, however we forecast we will fully utilize the NOLs before their expiration. Section 383 imposes an annual limitation on the use of credits to an amount equal to the value of the company at the time of the ownership change multiplied by the long-term tax exempt rate. Our domestic credits are subject to various IRS Code Section 383 limitations. We forecast that some portion of these credits will expire before they are utilized and therefore have recorded a valuation allowance on these assets of $0.9 million. The state NOL carryforwards are subject to various statutory limitations or require certain companies to realize taxable income in order to fully utilize the NOLs.
We have recorded a valuation allowance of $83.6 million as of December 31, 2013 due to uncertainties related to the future benefits, if any, from our deferred tax assets related primarily to our foreign businesses and certain U.S. net operating losses and tax credits. This is an increase of $15.0 million from the valuation allowance of $68.6 million as of December 31, 2012. The increase is primarily related to foreign and state NOLs. The valuation allowance is based on our estimates of taxable income by the jurisdictions in which we operate and the period over which our deferred tax assets will be recoverable. In the event that actual results differ from these estimates or we adjust these estimates in future periods, we may need to establish an additional valuation allowance or reduce our current valuation allowance, which could materially impact our tax provision.
Our two China-based manufacturing subsidiaries qualify for a reduced income tax rate in 2013, 2012 and 2011. Both Chinese entities qualify as high technology status companies and the status is due to expire for 2014. It is anticipated that neither Chinese entity will qualify for high technology status in 2014 and the deferred tax rate has been increased to 25%. The prescribed statutory rate for 2013 and 2012 is 25% and the reduced rate under the high technology status is 15%. In 2010, the companies qualified under the China Tax Reform Act and the income tax rate for one of the subsidiaries was 12.5% and 11% for the other. The reduced tax rate produced a current tax expense of approximately $1.4 million in 2013. In the absence of the reduced tax rate for 2013, a tax rate of 25% would have applied and would have resulted in a current tax expense of approximately $2.4 million in 2013. The tax benefit of this rate change was approximately $1.0 million. The earnings per common share effect of the reduced tax rate is $0.01 for 2013. The reduced tax rate produced a tax expense of approximately $1.0 million in 2012. In the absence of the reduced tax rate for 2012 a tax rate of 25% would have applied and would have resulted in a tax expense of approximately $1.7 million in 2012. The earnings per common share effect of the reduced tax rate was $0.01 for 2012. The reduced tax rate produced a tax expense of approximately $0.5 million in 2011. In the absence of the reduced tax rate for 2011 a tax rate of 25% would have applied and would have resulted in a tax expense of approximately $0.8 million in 2011. The earnings per common share effect of the reduced tax rate is $0.01 for 2011.
In December 2013, our Swiss subsidiary declared a dividend to its U.S. parent against which we recorded estimated tax liabilities. The U.S. taxation of the dividend depends on the amount of available earnings and profits at the Swiss subsidiary, the calculation of which involves reviewing numerous tax computations and intercompany transactions from prior years, and estimates for certain portions of the calculation. Actual results could differ significantly from such estimates under different assumptions or conditions.
The estimated amount of undistributed earnings of our foreign subsidiaries is $244.7 million at December 31, 2013. No amount for U.S. income tax has been provided on undistributed earnings of
F-59
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(16) Income Taxes (Continued)
our foreign subsidiaries because we consider such earnings to be indefinitely reinvested. In the event of distribution of those earnings we would be subject to both U.S. income tax and foreign withholding taxes, subject to possible offset by U.S. foreign tax credits. Determination of the amount of U.S. income tax liability that would be incurred is not practicable because of the complexities associated with this hypothetical calculation.
The following table presents the components of our benefit for income taxes (in thousands):
| | | | | | | | | | | | |
| | | 2013 | | | 2012 | | | 2011 | |
Current: | | | | | | | | | | | | |
Federal | | $ | 5,523 | | | $ | 11,794 | | | $ | (1,229 | ) |
State | | | (546 | ) | | | 9,500 | | | | 4,193 | |
Foreign | | | 52,359 | | | | 32,955 | | | | 29,583 | |
| | | | | | | | | | | | |
| | | 57,336 | | | | 54,249 | | | | 32,547 | |
| | | | | | | | | | | | |
Deferred: | | | | | | | | | | | | |
Federal | | | (75,021 | ) | | | (58,292 | ) | | | (27,109 | ) |
State | | | 1,102 | | | | (15,087 | ) | | | (23,720 | ) |
Foreign | | | (29,728 | ) | | | (11,189 | ) | | | (5,932 | ) |
| | | | | | | | | | | | |
| | | (103,647 | ) | | | (84,568 | ) | | | (56,761 | ) |
| | | | | | | | | | | | |
Total benefit for income taxes | | $ | (46,311 | ) | | $ | (30,319 | ) | | $ | (24,214 | ) |
| | | | | | | | | | | | |
Benefit for income taxes in 2011 includes a benefit of $7.0 million to correct items related to periods between 2007 and 2010. We do not believe that the corrected items are material to 2011 or any previously reported quarterly or annual financial statements. As a result, we have not restated our previously issued annual or quarterly financial statements.
The following table presents a reconciliation from the U.S. statutory tax rate to our effective tax rate:
| | | | | | | | | | | | |
| | | 2013 | | | 2012 | | | 2011 | |
Statutory rate | | | 35 | % | | | 35 | % | | | 35 | % |
Effect of goodwill impairment charge | | | — | | | | — | | | | (75 | ) |
Stock-based compensation | | | (2 | ) | | | (2 | ) | | | (2 | ) |
Rate differential on foreign earnings | | | — | | | | (2 | ) | | | 42 | |
Research and development | | | 2 | | | | — | | | | 1 | |
State income taxes, net of federal benefit | | | — | | | | 3 | | | | 8 | |
Acquisition costs | | | — | | | | (2 | ) | | | (2 | ) |
Contingent consideration | | | (3 | ) | | | 6 | | | | 4 | |
Rate changes | | | 5 | | | | (2 | ) | | | 3 | |
Other permanent items | | | 4 | | | | 1 | | | | 3 | |
Section 199 deduction | | | 2 | | | | — | | | | — | |
Bargain purchase | | | 2 | | | | — | | | | — | |
Change in valuation allowance | | | (6 | ) | | | (9 | ) | | | (2 | ) |
Foreign dividends, net | | | (2 | ) | | | — | | | | — | |
Uncertain tax positions | | | (2 | ) | | | (3 | ) | | | — | |
| | | | | | | | | | | | |
Effective tax rate | | | 35 | % | | | 25 | % | | | 15 | % |
| | | | | | | | | | | | |
F-60
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(16) Income Taxes (Continued)
The 2011 goodwill impairment charge created a deferred tax impact due to the existence of goodwill deductible for tax purposes. The deferred tax is calculated based on a methodology which allocates the goodwill impairment loss proportionally to the goodwill deductible for tax purposes compared to total goodwill. The goodwill impairment allocated to goodwill deductible for tax purposes created a deferred tax asset of $35.3 million as of December 31, 2011. There has been no change to the goodwill impairment charge or the deferred tax asset in 2012 or 2013.
The rate differential on foreign earnings is 0%, (2%) and 42% for 2013, 2012 and 2011, respectively. The 2011 rate differential on foreign earnings of 42% tax benefit arose primarily because a foreign subsidiary, which is a tax resident in a low tax jurisdiction, recognized a deferred gain on the sale of a joint venture interest. The 2012 rate differential on foreign earnings of 2% tax expense resulted primarily from the tax benefit associated with lower jurisdictional tax rates offset by tax expense associated with foreign earnings taxed at the U.S. statutory rate.
State income tax benefit is 0%, 3% and 8% for 2013, 2012 and 2011, respectively. The 8% benefit in 2011, versus the 3% benefit in 2012, is primarily related to the net favorable out-of-period adjustments recorded in 2011 related to previous years. The reduction in benefit to zero in 2013 primarily relates to the reversal of deferred tax benefits in 2013 associated with state net operating loss carryforwards.
The impact on the rate in 2011 through 2013 relating to contingent consideration is due to fair value accounting used for contingent consideration not considered for tax purposes. The impact of rate changes over the same three-year period is primarily due to foreign jurisdictional rate changes as a result of newly-enacted legislation, with the 2013 impact primarily relating to legislation enacted in 2013, which reduced the U.K. tax rate. Increases in valuation allowances primarily relate to losses in foreign jurisdictions where it is not more likely than not that these losses will be utilized in future periods.
During the year ended December 31, 2013, we increased the liability for income taxes associated with uncertain tax positions by $6.9 million to a total of $25.9 million at December 31, 2013. The primary reasons for the increase are foreign tax exposures associated with certain restructurings of $2.6 million, increases for certain foreign permanent establishment exposures of $2.4 million, an increase of $0.8 million for audit exposure in certain foreign jurisdictions, an increase of $0.6 million due to reclassification of unrecognized tax benefits from other deferred tax accounts and an increase of $1.3 million for certain tax credit carry forwards. The primary decrease relates to prior periods for research expenses and credits in foreign jurisdictions of $0.9 million.
We classify $22.8 million of income tax liabilities as non-current income tax liabilities and $3.1 million as long-term deferred tax liabilities. These non-current income tax liabilities are recorded in other long-term liabilities in our consolidated balance sheet at December 31, 2013. We do not anticipate material reductions to the tax reserve over the next twelve-month period.
F-61
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(16) Income Taxes (Continued)
A reconciliation of the beginning and ending amount of liabilities for uncertain tax positions is as follows (in thousands):
| | | | |
| | | Amount | |
Balances as of January 1, 2011 | | $ | 6,160 | |
Additions for tax positions in current and prior year acquisitions | | | 189 | |
Additions for tax positions taken during current year | | | 5,814 | |
Reductions for tax positions taken during prior years | | | (26 | ) |
Reductions for tax positions in current and prior year acquisitions | | | (1,922 | ) |
Expiration of statutes of limitations or closure of tax audits | | | (684 | ) |
| | | | |
Balance as of December 31, 2011 | | | 9,531 | |
Previously recorded tax reserves not included in disclosure | | | 6,236 | |
Additions for tax positions in current and prior year acquisitions | | | 1,852 | |
Additions for tax positions taken during current year | | | 2,922 | |
Reductions for tax positions taken during prior years | | | (208 | ) |
Reductions for tax positions in current and prior year acquisitions | | | — | |
Expiration of statutes of limitations or closure of tax audits | | | (1,328 | ) |
| | | | |
Balance as of December 31, 2012 | | | 19,005 | |
Previously recorded tax reserves not included in disclosure | | | 600 | |
Additions for tax positions in current and prior year acquisitions | | | — | |
Additions for tax positions taken during current year | | | 7,211 | |
Reductions for tax positions taken during prior years | | | (900 | ) |
Reductions for tax positions in current and prior year acquisitions | | | — | |
Expiration of statutes of limitations or closure of tax audits | | | — | |
| | | | |
Balance as of December 31, 2013 | | $ | 25,916 | |
| | | | |
Interest and penalties related to income tax liabilities are included in income tax expense. The gross interest and penalties recorded in 2013 amounted to $1.3 million. The gross balance of accrued interest and penalties recorded on the consolidated balance sheet at December 31, 2013 was $1.9 million.
With limited exceptions, we are subject to U.S. federal, state and local or non-U.S. income tax audits by tax authorities for 2006 through 2012. We are currently under income tax examination by the IRS and a number of state and foreign tax authorities. We cannot currently estimate the impact of remaining audits due to the uncertainties associated with tax examinations. Management does not expect material changes in tax position as a result of these ongoing audits.
(17) Financial Information by Segment
Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision maker, or decision-making group, in deciding how to allocate resources and in assessing performance. Our chief operating decision-making group is composed of the chief executive officer and members of senior management. Our reportable operating segments are professional diagnostics, health information solutions, consumer diagnostics and corporate and other. Our operating results include license and royalty revenue which are allocated to Professional Diagnostics and Consumer Diagnostics on the basis of the original license or royalty agreement.
F-62
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(17) Financial Information by Segment (Continued)
The accounting policies of the segments are the same as those described in the summary of significant accounting policies. We evaluate performance of our operating segments based on revenue and operating income (loss). Revenues are attributed to geographic areas based on where the customer is located. Segment information for 2013, 2012 and 2011 is as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | |
2013 | | Professional
Diagnostics | | | Health
Information
Solutions | | | Consumer
Diagnostics | | | Corporate
and
Other | | | Total | |
Net revenue | | $ | 2,391,137 | | | $ | 533,227 | | | $ | 105,078 | | | $ | — | | | $ | 3,029,442 | |
Operating income (loss) | | $ | 256,087 | | | $ | (43,001 | ) | | $ | 12,122 | | | $ | (90,456 | ) | | $ | 134,752 | |
Depreciation and amortization | | $ | 348,635 | | | $ | 85,330 | | | $ | 4,330 | | | $ | 1,135 | | | $ | 439,430 | |
Restructuring charge | | $ | 12,606 | | | $ | 14,760 | | | $ | — | | | $ | — | | | $ | 27,366 | |
Stock-based compensation | | $ | — | | | $ | — | | | $ | — | | | $ | 21,210 | | | $ | 21,210 | |
Assets | | $ | 5,744,734 | | | $ | 504,645 | | | $ | 197,458 | | | $ | 613,977 | | | $ | 7,060,814 | |
Expenditures for property, plant and equipment | | $ | 86,415 | | | $ | 23,312 | | | $ | 1,645 | | | $ | 10,794 | | | $ | 122,166 | |
| | | | | | | | | | | | | | | | | | | | |
2012 | | Professional
Diagnostics | | | Health
Information
Solutions | | | Consumer
Diagnostics | | | Corporate
and
Other | | | Total | |
Net revenue | | $ | 2,189,892 | | | $ | 535,422 | | | $ | 93,511 | | | $ | — | | | $ | 2,818,825 | |
Operating income (loss) | | $ | 243,080 | | | $ | (73,432 | ) | | $ | 12,707 | | | $ | (73,223 | ) | | $ | 109,132 | |
Depreciation and amortization | | $ | 355,957 | | | $ | 96,427 | | | $ | 3,455 | | | $ | 1,008 | | | $ | 456,847 | |
Restructuring charge | | $ | 11,124 | | | $ | 9,203 | | | $ | — | | | $ | (2 | ) | | $ | 20,325 | |
Stock-based compensation | | $ | — | | | $ | — | | | $ | — | | | $ | 15,665 | | | $ | 15,665 | |
Assets | | $ | 6,214,847 | | | $ | 593,172 | | | $ | 192,748 | | | $ | 67,161 | | | $ | 7,067,928 | |
Expenditures for property, plant and equipment | | $ | 90,568 | | | $ | 41,960 | | | $ | 2,823 | | | $ | 2,042 | | | $ | 137,393 | |
| | | | | | | | | | | | | | | | | | | | |
2011 | | Professional
Diagnostics | | | Health
Information
Solutions | | | Consumer
Diagnostics | | | Corporate
and
Other | | | Total | |
Net revenue | | $ | 1,756,509 | | | $ | 534,514 | | | $ | 95,504 | | | $ | — | | | $ | 2,386,527 | |
Operating income (loss) | | $ | 248,097 | | | $ | (439,872 | ) | | $ | 10,442 | | | $ | (71,522 | ) | | $ | (252,855 | ) |
Goodwill impairment charge | | $ | — | | | $ | 383,612 | | | $ | — | | | $ | — | | | $ | 383,612 | |
Depreciation and amortization | | $ | 277,102 | | | $ | 108,383 | | | $ | 5,375 | | | $ | 716 | | | $ | 391,576 | |
Restructuring charge | | $ | 13,949 | | | $ | 13,194 | | | $ | (57 | ) | | $ | 1,196 | | | $ | 28,282 | |
Stock-based compensation | | $ | — | | | $ | — | | | $ | — | | | $ | 21,215 | | | $ | 21,215 | |
Assets | | $ | 5,826,756 | | | $ | 624,305 | | | $ | 199,422 | | | $ | 22,218 | | | $ | 6,672,701 | |
Expenditures for property, plant and equipment | | $ | 81,774 | | | $ | 46,850 | | | $ | 2,676 | | | $ | 1,232 | | | $ | 132,532 | |
F-63
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(17) Financial Information by Segment (Continued)
The following tables summarize our net revenue from the professional diagnostics and health information solutions reporting segments by groups of similar products and services for 2013, 2012 and 2011 (in thousands):
Professional Diagnostics Segment
| | | | | | | | | | | | |
| | | 2013 | | | 2012 | | | 2011 | |
Infectious disease | | $ | 723,213 | | | $ | 615,950 | | | $ | 564,983 | |
Toxicology | | | 632,727 | | | | 587,261 | | | | 387,209 | |
Cardiology | | | 463,281 | | | | 503,534 | | | | 518,746 | |
Diabetes | | | 225,488 | | | | 144,441 | | | | 14,960 | |
Other | | | 321,495 | | | | 314,030 | | | | 250,274 | |
| | | | | | | | | | | | |
Net product sales and services revenue | | | 2,366,204 | | | | 2,165,216 | | | | 1,736,172 | |
License and royalty revenue | | | 24,933 | | | | 24,676 | | | | 20,337 | |
| | | | | | | | | | | | |
Professional diagnostics net revenue | | $ | 2,391,137 | | | $ | 2,189,892 | | | $ | 1,756,509 | |
| | | | | | | | | | | | |
Health Information Solutions Segment
| | | | | | | | | | | | |
| | | 2013 | | | 2012 | | | 2011 | |
Condition and case management | | $ | 215,160 | | | $ | 218,378 | | | $ | 237,938 | |
Wellness | | | 101,642 | | | | 104,634 | | | | 104,868 | |
Women’s & children’s health | | | 113,506 | | | | 120,259 | | | | 114,287 | |
Patient self-testing services | | | 102,919 | | | | 92,151 | | | | 77,421 | |
| | | | | | | | | | | | |
Health information solutions net revenue | | $ | 533,227 | | | $ | 535,422 | | | $ | 534,514 | |
| | | | | | | | | | | | |
The following tables summarize our net revenue by geographic area for 2013, 2012 and 2011, respectively, and our long-lived tangible assets by geographic area as of December 31, 2013 and 2012, respectively (in thousands):
| | | | | | | | | | | | |
| | | 2013 | | | 2012 | | | 2011 | |
Revenue by Geographic Area: | | | | | | | | | | | | |
United States | | $ | 1,858,775 | | | $ | 1,724,704 | | | $ | 1,453,160 | |
Europe | | | 509,644 | | | | 483,857 | | | | 396,480 | |
Elsewhere(1) | | | 661,023 | | | | 610,264 | | | | 536,887 | |
| | | | | | | | | | | | |
| | $ | 3,029,442 | | | $ | 2,818,825 | | | $ | 2,386,527 | |
| | | | | | | | | | | | |
| (1) | Includes, among many others, the following countries: China, Japan, India, South Korea, Australia, Canada, and Brazil. |
| | | | | | | | |
| | | December 31, | |
| | | 2013 | | | 2012 | |
Long-lived Tangible Assets by Geographic Area: | | | | | | | | |
United States | | $ | 306,300 | | | $ | 295,786 | |
United Kingdom | | | 39,599 | | | | 37,928 | |
China | | | 30,285 | | | | 31,277 | |
Elsewhere(1) | | | 168,980 | | | | 169,478 | |
| | | | | | | | |
| | $ | 545,164 | | | $ | 534,469 | |
| | | | | | | | |
| (1) | Includes, among many others, the following countries: Germany, Norway, Sweden, South Korea, and Brazil. |
F-64
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(18) Other Arrangements
In February 2013, we entered into an agreement with the Bill and Melinda Gates Foundation, or the Gates Foundation, whereby we were awarded a grant by the Gates Foundation in the amount of $21.6 million to support the development and commercialization of a validated, low-cost, nucleic-acid assay for clinical Tuberculosis, or TB, detection and drug-resistance test cartridges and adaptation of an analyzer platform capable of operation in rudimentary laboratories in low-resource settings. In connection with this agreement, we also entered into a loan agreement with the Gates Foundation, or the Gates Loan Agreement, which provides for the making of subordinated term loans by the Gates Foundation to us from time to time, subject to the achievement of certain milestones, in an aggregate principal amount of up to $20.6 million. Funding under the Gates Loan Agreement will be used in connection with the purchase of equipment for an automated high-throughput manufacturing line and other uses as necessary for the manufacture of the TB and HIV-related products. All loans under the Gates Loan Agreement are evidenced by promissory notes that we have executed and delivered to the Gates Foundation, bear interest at the rate of 3% per annum and, except to the extent earlier repaid by us, mature and are required to be repaid in full on December 31, 2019. As of December 31, 2013, we had borrowed no amounts under the Gates Loan Agreement. As of December 31, 2013, we had received approximately $7.9 million in grant-related funding from the Gates Foundation, which was recorded as restricted cash and deferred grant funding. The deferred grant funding is classified within accrued expenses and other current liabilities on our accompanying consolidated balance sheet. As qualified expenditures are incurred under the terms of the grant, we use the deferred funding to recognize a reduction of our related qualified research and development expenditures. For the year ended December 31, 2013, we incurred approximately $6.6 million of qualified expenditures, for which we reduced our deferred grant funding balance and recorded an offset to our research and development expenses.
(19) Related Party Transactions
In November 2008, the Zwanziger Family Trust, a trust established for the benefit of the children of Ron Zwanziger, our Chairman, Chief Executive Officer and President, and the trustee of which is Mr. Zwanziger’s sister, purchased certain of our securities from third parties in market transactions. The purchase consisted of approximately $1.0 million of each of the following of our outstanding securities: our common stock, our Series B Preferred Stock and our convertible notes. To the extent we make principal and interest payments under the convertible notes in accordance with their terms, the Zwanziger Family Trust, as a holder of convertible notes, will receive its proportionate share.
In May 2007, we completed the formation of SPD, our 50/50 joint venture with P&G, for the development, manufacturing, marketing and sale of existing and to-be-developed consumer diagnostic products, outside the cardiology, diabetes and oral care fields. Upon completion of the arrangement to form the joint venture, we ceased to consolidate the operating results of our consumer diagnostic products business related to the joint venture and instead account for our 50% interest in the results of the joint venture under the equity method of accounting.
At December 31, 2013 and 2012, we had a net receivable from the joint venture of $2.1 million and $2.3 million, respectively. Included in the $2.1 million receivable balance as of December 31, 2013 is approximately $1.8 million of costs incurred in connection with our 2008 SPD-related restructuring plans. Included in the $2.3 million receivable balance as of December 31, 2012 is approximately $1.6 million of costs incurred in connection with our 2008 SPD-related restructuring plans. We have also recorded a long-term receivable totaling approximately $13.2 million and $14.6 million as of December 31, 2013 and December 31, 2012, respectively, related to the 2008 SPD-related restructuring plans. Additionally, customer receivables associated with revenue earned after the
F-65
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(19) Related Party Transactions (Continued)
formation of the joint venture was completed have been classified as other receivables within prepaid expenses and other current assets on our accompanying consolidated balance sheets in the amount of $12.4 million and $6.9 million as of December 31, 2013 and 2012, respectively. In connection with the joint venture arrangement, the joint venture bears the collection risk associated with these receivables. Sales to the joint venture under our manufacturing agreement totaled $78.0 million, $63.6 million and $71.2 million during the years ended December 31, 2013, 2012 and 2011, respectively. Additionally, services revenue generated pursuant to the long-term services agreement with the joint venture totaled $1.3 million, $1.1 million and $1.1 million during the years ended December 31, 2013, 2012 and 2011, respectively. Sales under our manufacturing agreement and long-term services agreement are included in net product sales and services revenue, respectively, in our accompanying consolidated statements of operations.
Under the terms of our product supply agreement, the joint venture purchases products from our manufacturing facilities in the U.K. and China. SPD in turn sells a portion of those tests back to us for final assembly and packaging. Once packaged, the tests are sold to P&G for distribution to third-party customers in North America. As a result of these related transactions, we have recorded $9.4 million and $7.3 million of trade receivables which are included in accounts receivable on our accompanying consolidated balance sheets as of December 31, 2013 and 2012, respectively, and $18.8 million and $21.3 million of trade accounts payable which are included in accounts payable on our accompanying consolidated balance sheets as of December 31, 2013 and 2012, respectively. During 2013 and 2012, we received $28.0 million and $11.2 million, respectively, in cash from SPD as a return of capital.
The following table summarizes our related party balances with SPD within our consolidated balance sheets (in thousands):
| | | | | | | | |
Balance Sheet Caption | | As of December 31, | |
| | 2013 | | | 2012 | |
Accounts receivable, net of allowances | | $ | 9,436 | | | $ | 7,317 | |
Prepaid expenses and other current assets | | $ | 12,417 | | | $ | 9,161 | |
Deferred financing costs, net, and other non-current assets | | $ | 13,249 | | | $ | 14,629 | |
Accounts payable | | $ | 18,811 | | | $ | 21,258 | |
(20) Valuation and Qualifying Accounts
We have established reserves against accounts receivable for doubtful accounts, product returns, discounts and other allowances. The activity in the table below includes all accounts receivable reserves. Provisions for doubtful accounts are recorded as a component of general and administrative expenses. Provisions for returns, discounts and other allowances are charged against net product sales. The following table sets forth activities in our accounts receivable reserve accounts (in thousands):
| | | | | | | | | | | | | | | | |
| | | Balance at
Beginning of
Period | | | Provision | | | Amounts
Charged
Against
Reserves | | | Balance at
End of
Period | |
Year ended December 31, 2011 | | $ | 20,381 | | | $ | 23,614 | | | $ | (19,418 | ) | | $ | 24,577 | |
Year ended December 31, 2012 | | $ | 24,577 | | | $ | 43,731 | | | $ | (31,912 | ) | | $ | 36,396 | |
Year ended December 31, 2013 | | $ | 36,396 | | | $ | 70,267 | | | $ | (30,020 | ) | | $ | 76,643 | |
F-66
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(20) Valuation and Qualifying Accounts (Continued)
We have established reserves against obsolete and slow-moving inventories. The activity in the table below includes all inventory reserves. Provisions for obsolete and slow-moving inventories are recorded as a component of cost of net product sales. The following table sets forth activities in our inventory reserve accounts (in thousands):
| | | | | | | | | | | | | | | | |
| | | Balance at
Beginning of
Period | | | Provision | | | Amounts
Charged
Against
Reserves | | | Balance at
End of
Period | |
Year ended December 31, 2011 | | $ | 12,308 | | | $ | 6,144 | | | $ | (4,843 | ) | | $ | 13,609 | |
Year ended December 31, 2012 | | $ | 13,609 | | | $ | 13,587 | | | $ | (5,374 | ) | | $ | 21,822 | |
Year ended December 31, 2013 | | $ | 21,822 | | | $ | 3,323 | | | $ | (3,671 | ) | | $ | 21,474 | |
(21) Restructuring Activities
The following table sets forth aggregate restructuring charges recorded in our consolidated statements of operations for the years ended December 31, 2013, 2012 and 2011 (in thousands):
| | | | | | | | | | | | |
Statement of Operations Caption | | 2013 | | | 2012 | | | 2011 | |
Cost of net revenue | | $ | 7,865 | | | $ | 3,113 | | | $ | 2,915 | |
Research and development | | | 1,795 | | | | 1,278 | | | | 433 | |
Sales and marketing | | | 2,771 | | | | 2,504 | | | | 4,954 | |
General and administrative | | | 14,935 | | | | 13,430 | | | | 19,980 | |
| | | | | | | | | | | | |
Total operating expenses | | | 27,366 | | | | 20,325 | | | | 28,282 | |
Interest expense, including amortization of original issue discounts and deferred financing costs | | | 311 | | | | 277 | | | | 280 | |
Equity earnings of unconsolidated entities, net of tax | | | — | | | | — | | | | 580 | |
| | | | | | | | | | | | |
Total charges | | $ | 27,677 | | | $ | 20,602 | | | $ | 29,142 | |
| | | | | | | | | | | | |
(a) 2013 Restructuring Plans
In 2013, management developed cost reduction efforts within our professional diagnostics business segment, including businesses in our United States, Europe and Asia Pacific regions. Additionally, management is continuing to take steps to improve efficiencies within our health information solutions business segment, including winding down a small portion of this business, which resulted in charges associated with the impairment of related fixed and intangible assets. The following table summarizes the restructuring activities related to our 2013 restructuring plans for the year ended December 31, 2013 (in thousands):
| | | | | | | | |
| | | Professional
Diagnostics | | | Health
Information
Solutions | |
Severance-related costs | | $ | 7,126 | | | $ | 3,267 | |
Facility and transition costs | | | 2,581 | | | | 2,955 | |
Other exit costs | | | — | | | | 17 | |
| | | | | | | | |
Cash charges | | | 9,707 | | | | 6,239 | |
Fixed asset and inventory impairments | | | 743 | | | | 1,089 | |
Intangible asset impairments | | | — | | | | 2,596 | |
Other non-cash charges | | | — | | | | (903 | ) |
| | | | | | | | |
Total charges | | $ | 10,450 | | | $ | 9,021 | |
| | | | | | | | |
F-67
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(21) Restructuring Activities (Continued)
We anticipate incurring approximately $0.4 million in additional severance and facility costs under these plans related to our health information solutions business segment through 2020, as well as $2.9 million in additional severance and facility costs under these plans related to our professional diagnostics business segment, primarily related to consolidating our toxicology businesses within the United States into one manufacturing location through 2015. As of December 31, 2013, $5.9 million in severance and exit costs under these plans remain unpaid.
(b) 2012 Restructuring Plans
In 2012, management developed cost reduction plans within our professional diagnostics business segment, including the integration of our businesses in Brazil, Europe and the United States. Additionally, management developed new plans to continue our efforts to reduce costs within our health information solutions business segment, including the termination of certain projects, which resulted in charges for the impairment of related fixed and intangible assets. The following table summarizes the restructuring activities related to our 2012 restructuring plans for the year ended December 31, 2013, 2012 and since inception (in thousands):
| | | | | | | | | | | | |
| | | Professional Diagnostics | |
| | | 2013 | | | 2012 | | | Since
Inception | |
Severance-related costs | | $ | (542 | ) | | $ | 4,732 | | | $ | 4,190 | |
Facility and transition costs | | | 82 | | | | 119 | | | | 201 | |
| | | | | | | | | | | | |
Cash charges | | | (460 | ) | | | 4,851 | | | | 4,391 | |
Fixed asset and inventory impairments | | | — | | | | 304 | | | | 304 | |
| | | | | | | | | | | | |
Total charges | | $ | (460 | ) | | $ | 5,155 | | | $ | 4,695 | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | Health Information Solutions | |
| | | 2013 | | | 2012 | | | Since
Inception | |
Severance-related costs | | $ | 2,362 | | | $ | 3,045 | | | $ | 5,407 | |
Facility and transition costs | | | 4,271 | | | | 1,234 | | | | 5,505 | |
Other exit costs | | | 179 | | | | 15 | | | | 194 | |
| | | | | | | | | | | | |
Cash charges | | | 6,812 | | | | 4,294 | | | | 11,106 | |
Fixed asset and inventory impairments | | | 75 | | | | 2,689 | | | | 2,764 | |
Intangible asset impairments | | | — | | | | 2,988 | | | | 2,988 | |
Other non-cash charges | | | (953 | ) | | | (31 | ) | | | (984 | ) |
| | | | | | | | | | | | |
Total charges | | $ | 5,934 | | | $ | 9,940 | | | $ | 15,874 | |
| | | | | | | | | | | | |
We anticipate incurring approximately $0.4 million in facility costs under these plans related primarily to our health information solutions business segment through 2020. As of December 31, 2013, $3.0 million in facility exit costs under these plans remain unpaid.
(c) Restructuring Plans Prior to 2012
In 2011, management executed a company-wide cost reduction plan which impacted our corporate and other business segment, as well as the health information solutions and professional diagnostics business segments. Management also developed plans within our professional diagnostics business segment to consolidate operating activities among certain of our U.S., European and Asia Pacific subsidiaries, including transferring the manufacturing of our Panbio products from Australia to our Standard
F-68
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(21) Restructuring Activities (Continued)
Diagnostics facility in South Korea and eliminating redundant costs among our newly-acquired Axis-Shield subsidiaries. Additionally, within our health information solutions business segment, management executed plans to further reduce costs and improve efficiencies, as well as cease operations at our GeneCare Medical Genetics Center, Inc., or GeneCare, facility in Chapel Hill, North Carolina and transfer the majority of our Quality Assured Services, Inc. operation in Orlando, Florida to our facility in Livermore, California.
In 2010, management developed several plans to reduce costs and improve efficiencies within our health information solutions and professional diagnostics business segments. Additionally in 2008, management developed and initiated plans to close our facility located in Bedford, England and transition the manufacturing operations principally to our manufacturing facilities in Shanghai and Hangzhou, China, as well as transition the business of Cholestech to our San Diego, California facility.
The following table summarizes the restructuring activities related to our 2011, 2010 and 2008 restructuring plans for the years ended December 31, 2013, 2012, 2011 and since inception (in thousands):
| | | | | | | | | | | | | | | | |
| | | Professional Diagnostics | |
| | | 2013 | | | 2012 | | | 2011 | | | Since
Inception | |
Severance-related costs | | $ | 289 | | | $ | 3,145 | | | $ | 12,047 | | | $ | 25,846 | |
Facility and transition costs | | | 1,334 | | | | 2,121 | | | | 1,310 | | | | 13,727 | |
Other exit costs | | | 58 | | | | 132 | | | | 88 | | | | 4,608 | |
| | | | | | | | | | | | | | | | |
Cash charges | | | 1,681 | | | | 5,398 | | | | 13,445 | | | | 44,181 | |
Fixed asset and inventory impairments | | | 308 | | | | 704 | | | | 534 | | | | 12,540 | |
Intangible asset impairments | | | 686 | | | | — | | | | — | | | | 686 | |
Other non-cash changes | | | — | | | | — | | | | — | | | | 64 | |
| | | | | | | | | | | | | | | | |
Total charges | | $ | 2,675 | | | $ | 6,102 | | | $ | 13,979 | | | $ | 57,471 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | Health Information Solutions | |
| | | 2013 | | | 2012 | | | 2011 | | | Since
Inception | |
Severance-related costs | | $ | — | | | $ | — | | | $ | 2,254 | | | $ | 6,901 | |
Facility and transition costs (recoveries) | | | — | | | | (807 | ) | | | 6,381 | | | | 8,010 | |
Other exit costs | | | 57 | | | | 130 | | | | 192 | | | | 569 | |
| | | | | | | | | | | | | | | | |
Cash charges (recoveries) | | | 57 | | | | (677 | ) | | | 8,827 | | | | 15,480 | |
Fixed asset and inventory impairments | | | — | | | | 85 | | | | 864 | | | | 1,114 | |
Intangible asset impairments | | | — | | | | — | | | | 2,935 | | | | 2,935 | |
Other non-cash changes | | | — | | | | — | | | | 761 | | | | 761 | |
| | | | | | | | | | | | | | | | |
Total charges (recoveries) | | $ | 57 | | | $ | (592 | ) | | $ | 13,387 | | | $ | 20,290 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | Corporate and Other | |
| | | 2013 | | | 2012 | | | 2011 | | | Since
Inception | |
Severance-related costs | | $ | — | | | $ | (3 | ) | | $ | 1,193 | | | $ | 1,190 | |
| | | | | | | | | | | | | | | | |
Cash charges (recoveries) | | | — | | | | (3 | ) | | | 1,193 | | | | 1,190 | |
Fixed asset and inventory impairments | | | — | | | | — | | | | 3 | | | | 3 | |
| | | | | | | | | | | | | | | | |
Total charges (recoveries) | | $ | — | | | $ | (3 | ) | | $ | 1,196 | | | $ | 1,193 | |
| | | | | | | | | | | | | | | | |
F-69
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(21) Restructuring Activities (Continued)
We anticipate incurring approximately $0.9 million in additional costs under these plans related to our professional diagnostics business segment, primarily related to facility exit costs. We anticipate incurring minimal additional costs under these plans related to our health information solutions business segment, primarily related to imputed interest on facility lease obligations. As of December 31, 2013, $2.2 million in cash charges remain unpaid, primarily related to facility lease obligations through 2017.
In addition to the restructuring charges discussed above, certain charges associated with the Bedford facility closure were borne by SPD, our 50/50 joint venture with P&G. Of the restructuring charges recorded by SPD, 50% has been included in equity earnings of unconsolidated entities, net of tax, in our consolidated statements of operations. The following table summarizes the 50% portion of the restructuring charges borne by SPD and included in equity earnings of unconsolidated entities, net of tax, for the years ended December 31, 2011 and since inception (in thousands):
| | | | | | | | |
| | | 2011 | | | Since
Inception | |
Severance-related costs | | $ | 30 | | | $ | 5,797 | |
Facility and transition costs | | | 479 | | | | 5,396 | |
Other exit costs | | | — | | | | 283 | |
| | | | | | | | |
Cash charges | | | 509 | | | | 11,476 | |
Fixed asset and inventory impairments (recoveries) | | | 71 | | | | 4,635 | |
| | | | | | | | |
Total charges included in equity earnings of unconsolidated entities, net of tax | | $ | 580 | | | $ | 16,111 | |
| | | | | | | | |
As of December 31, 2013, all cash charges have been paid and we do not anticipate incurring additional restructuring charges under this plan.
F-70
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(21) Restructuring Activities (Continued)
(d) Restructuring Reserves
The following table summarizes our restructuring reserves related to the plans described above, of which $7.9 million is included in accrued expenses and other current liabilities and $3.2 million is included in other long-term liabilities on our consolidated balance sheets (in thousands):
| | | | | | | | | | | | | | | | |
| | | Severance-
related
Costs | | | Facility and
Transition
Costs | | | Other Exit
Costs | | | Total | |
Balance, December 31, 2010 | | $ | 1,987 | | | $ | 4,258 | | | $ | 3,458 | | | $ | 9,703 | |
Cash charges | | | 15,494 | | | | 7,691 | | | | 280 | | | | 23,465 | |
Cash charges borne by SPD(1) | | | 60 | | | | 958 | | | | — | | | | 1,018 | |
Payments | | | (13,923 | ) | | | (7,592 | ) | | | (3,133 | ) | | | (24,648 | ) |
Currency adjustments | | | (238 | ) | | | (100 | ) | | | (12 | ) | | | (350 | ) |
| | | | | | | | | | | | | | | | |
Balance, December 31, 2011 | | | 3,380 | | | | 5,215 | | | | 593 | | | | 9,188 | |
Cash charges | | | 10,919 | | | | 2,667 | | | | 277 | | | | 13,863 | |
Payments | | | (11,118 | ) | | | (5,469 | ) | | | (248 | ) | | | (16,835 | ) |
Currency adjustments | | | (14 | ) | | | 16 | | | | — | | | | 2 | |
| | | | | | | | | | | | | | | | |
Balance, December 31, 2012 | | | 3,167 | | | | 2,429 | | | | 622 | | | | 6,218 | |
Cash charges | | | 12,502 | | | | 11,223 | | | | 311 | | | | 24,036 | |
Payments | | | (12,847 | ) | | | (5,834 | ) | | | (324 | ) | | | (19,005 | ) |
Currency adjustments | | | (114 | ) | | | 12 | | | | — | | | | (102 | ) |
| | | | | | | | | | | | | | | | |
Balance, December 31, 2013 | | $ | 2,708 | | | $ | 7,830 | | | $ | 609 | | | $ | 11,147 | |
| | | | | | | | | | | | | | | | |
| (1) | Amounts represent 100% of the charges borne by SPD. |
(22) Equity Investments
We account for the results from our equity investments under the equity method of accounting in accordance with ASC 323Investments — Equity Method and Joint Ventures,based on the percentage of our ownership interest in the business. Our equity investments primarily include the following:
(a) SPD
We have a 50/50 joint venture, called SPD, with P&G for the development, manufacturing, marketing and sale of existing and to-be-developed consumer diagnostic products, outside the cardiology, diabetes and oral care fields. We recorded earnings of $15.0 million, $10.7 million and $5.9 million for the years ended December 31, 2013, 2012 and 2011, respectively, in equity earnings of unconsolidated entities, net of tax, in our accompanying consolidated statements of operations, which represented our 50% share of SPD’s net income for the respective periods.
(b) TechLab
We own 49% of TechLab, Inc., or TechLab, a privately-held developer, manufacturer and distributor of rapid non-invasive intestinal diagnostics tests in the areas of intestinal inflammation, antibiotic-associated diarrhea and parasitology. We recorded earnings of $2.0 million, $2.3 million and $2.0 million for the years ended December 31, 2013, 2012 and 2011, respectively, in equity earnings of unconsolidated entities, net of tax, in our accompanying consolidated statements of operations, which represented our minority share of TechLab’s net income for the respective periods.
F-71
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(22) Equity Investments (Continued)
Summarized financial information for SPD and TechLab on a combined basis is as follows (in thousands):
Combined Condensed Results of Operations:
| | | | | | | | | | | | |
| | | For The Years Ended December 31, | |
| | | 2013 | | | 2012 | | | 2011 | |
Net revenue | | $ | 203,115 | | | $ | 212,955 | | | $ | 232,857 | |
| | | | | | | | | | | | |
Gross profit | | $ | 152,698 | | | $ | 139,694 | | | $ | 146,466 | |
| | | | | | | | | | | | |
Net income after taxes | | $ | 34,079 | | | $ | 26,056 | | | $ | 15,922 | |
| | | | | | | | | | | | |
Combined Condensed Balance Sheets:
| | | | | | | | |
| | | As of December 31, | |
| | | 2013 | | | 2012 | |
Current assets | | $ | 63,985 | | | $ | 79,842 | |
Non-current assets | | | 38,541 | | | | 38,991 | |
| | | | | | | | |
Total assets | | $ | 102,526 | | | $ | 118,833 | |
| | | | | | | | |
Current liabilities | | $ | 38,053 | | | $ | 45,084 | |
Non-current liabilities | | | 6,175 | | | | 6,791 | |
| | | | | | | | |
Total liabilities | | $ | 44,228 | | | $ | 51,875 | |
| | | | | | | | |
(23) Loss on Disposition
In July 2013, we sold our Spinreact operations located in Spain, which was part of our professional diagnostics reporting unit and business segment, for approximately $33.4 million in proceeds and, as a result of this transaction, we recorded a loss on disposition of $5.1 million during 2013. The financial results for our Spinreact operations are immaterial to our consolidated financial results.
(24) Supplemental Cash Flow Information
Cash Paid for Interest and Income Taxes:
During 2013, 2012 and 2011, we made cash payments for interest totaling $206.3 million, $205.0 million and $164.7 million, respectively.
During 2013, 2012 and 2011, total net cash paid for income taxes was $49.1 million, $31.9 million and $47.8 million, respectively.
F-72
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(24) Supplemental Cash Flow Information (Continued)
Non-cash Investing Activities:
During 2011, we issued shares of our common stock in connection with certain acquisitions (dollars in thousands):
| | | | | | | | | | | | |
| | | Date of Acquisition | | | Common Stock Issued | |
Company Acquired | | | Number of
Shares | | | Fair Value of
Shares | |
Arriva Medical LLC | | | November 23, 2011 | | | | 806,452 | | | $ | 15,183 | |
Pregnancy.org, LLC | | | January 28, 2011 | | | | 25,463 | | | $ | 1,000 | |
Non-cash Financing Activities:
During 2011, we recorded non-cash income (expense) to accumulated other comprehensive income (loss) of $7.3 million, representing the change in fair market value of our interest rate swap agreement.
During 2012, we issued shares of our common stock in connection with the settlement of an acquisition-related contingent consideration obligation (dollars in thousands):
| | | | | | | | |
| | | Common Stock Issued | |
Contingent Consideration Obligation | | Number of
Shares | | | Fair Value of
Shares | |
Mologic | | | 66,666 | | | $ | 1,243 | |
(25) Guarantor Financial Information
Our 7.25% senior notes due 2018, our 8.625% senior subordinated notes due 2018, and our 6.5% senior subordinated notes due 2020 are guaranteed by certain of our consolidated wholly owned subsidiaries, or the Guarantor Subsidiaries. The guarantees are full and unconditional and joint and several. The following supplemental financial information sets forth, on a consolidating basis, audited balance sheets as of December 31, 2013 and 2012, the related statements of operations, statements of comprehensive income (loss) and cash flows for each of the three years in the period ended December 31, 2013, respectively, for Alere Inc., the Guarantor Subsidiaries and our other subsidiaries, or the Non-Guarantor Subsidiaries. The supplemental financial information reflects the investments of Alere Inc. and the Guarantor Subsidiaries in the Guarantor and Non-Guarantor Subsidiaries using the equity method of accounting.
We have extensive transactions and relationships between various members of the consolidated group. These transactions and relationships include intercompany pricing agreements, intellectual property royalty agreements and general and administrative and research and development cost-sharing agreements. Because of these relationships, it is possible that the terms of these transactions are not the same as those that would result from transactions among wholly unrelated parties.
For comparative purposes, certain amounts for prior periods have been reclassified to conform to the current period classification.
F-73
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(25) Guarantor Financial Information (Continued)
CONSOLIDATING STATEMENT OF OPERATIONS
For the Year Ended December 31, 2013
(in thousands)
| | | | | | | | | | | | | | | | | | | | |
| | | Issuer | | | Guarantor
Subsidiaries | | | Non-Guarantor
Subsidiaries | | | Eliminations | | | Consolidated | |
Net product sales | | $ | — | | | $ | 894,644 | | | $ | 1,365,847 | | | $ | (188,028 | ) | | $ | 2,072,463 | |
Services revenue | | | — | | | | 852,121 | | | | 77,629 | | | | — | | | | 929,750 | |
| | | | | | | | | | | | | | | | | | | | |
Net product sales and services revenue | | | — | | | | 1,746,765 | | | | 1,443,476 | | | | (188,028 | ) | | | 3,002,213 | |
License and royalty revenue | | | — | | | | 20,611 | | | | 19,657 | | | | (13,039 | ) | | | 27,229 | |
| | | | | | | | | | | | | | | | | | | | |
Net revenue | | | — | | | | 1,767,376 | | | | 1,463,133 | | | | (201,067 | ) | | | 3,029,442 | |
| | | | | | | | | | | | | | | | | | | | |
Cost of net product sales | | | 4,459 | | | | 486,410 | | | | 703,816 | | | | (166,165 | ) | | | 1,028,520 | |
Cost of services revenue | | | 47 | | | | 475,737 | | | | 35,620 | | | | (19,984 | ) | | | 491,420 | |
| | | | | | | | | | | | | | | | | | | | |
Cost of net product sales and services revenue | | | 4,506 | | | | 962,147 | | | | 739,436 | | | | (186,149 | ) | | | 1,519,940 | |
Cost of license and royalty revenue | | | — | | | | 69 | | | | 20,732 | | | | (13,038 | ) | | | 7,763 | |
| | | | | | | | | | | | | | | | | | | | |
Cost of net revenue | | | 4,506 | | | | 962,216 | | | | 760,168 | | | | (199,187 | ) | | | 1,527,703 | |
| | | | | | | | | | | | | | | | | | | | |
Gross profit (loss) | | | (4,506 | ) | | | 805,160 | | | | 702,965 | | | | (1,880 | ) | | | 1,501,739 | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | |
Research and development | | | 21,972 | | | | 67,501 | | | | 71,329 | | | | — | | | | 160,802 | |
Sales and marketing | | | 6,340 | | | | 329,648 | | | | 303,846 | | | | — | | | | 639,834 | |
General and administrative | | | 75,185 | | | | 269,409 | | | | 216,633 | | | | — | | | | 561,227 | |
Loss on disposition | | | — | | | | — | | | | 5,124 | | | | — | | | | 5,124 | |
| | | | | | | | | | | | | | | | | | | | |
Operating income (loss) | | | (108,003 | ) | | | 138,602 | | | | 106,033 | | | | (1,880 | ) | | | 134,752 | |
Interest expense, including amortization of original issue discounts and deferred financing costs | | | (252,791 | ) | | | (25,892 | ) | | | (11,192 | ) | | | 34,219 | | | | (255,656 | ) |
Other income (expense), net | | | (10,759 | ) | | | 22,210 | | | | 9,640 | | | | (34,219 | ) | | | (13,128 | ) |
| | | | | | | | | | | | | | | | | | | | |
Income (loss) before provision (benefit) for income taxes | | | (371,553 | ) | | | 134,920 | | | | 104,481 | | | | (1,880 | ) | | | (134,032 | ) |
Provision (benefit) for income taxes | | | (154,444 | ) | | | 74,441 | | | | 34,312 | | | | (620 | ) | | | (46,311 | ) |
| | | | | | | | | | | | | | | | | | | | |
Income (loss) before equity earnings (losses) of subsidiaries and unconsolidated entities, net of tax | | | (217,109 | ) | | | 60,479 | | | | 70,169 | | | | (1,260 | ) | | | (87,721 | ) |
Equity earnings (losses) of subsidiaries, net of tax | | | 144,941 | | | | (2,948 | ) | | | — | | | | (141,993 | ) | | | — | |
Equity earnings of unconsolidated entities, net of tax | | | 1,890 | | | | — | | | | 15,470 | | | | 83 | | | | 17,443 | |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | | (70,278 | ) | | | 57,531 | | | | 85,639 | | | | (143,170 | ) | | | (70,278 | ) |
Less: Net income attributable to non-controlling interests | | | — | | | | — | | | | 976 | | | | — | | | | 976 | |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) attributable to Alere Inc. and Subsidiaries | | | (70,278 | ) | | | 57,531 | | | | 84,663 | | | | (143,170 | ) | | | (71,254 | ) |
Preferred stock dividends | | | (21,293 | ) | | | — | | | | — | | | | — | | | | (21,293 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) available to common stockholders | | $ | (91,571 | ) | | $ | 57,531 | | | $ | 84,663 | | | $ | (143,170 | ) | | $ | (92,547 | ) |
| | | | | | | | | | | | | | | | | | | | |
F-74
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(25) Guarantor Financial Information (Continued)
CONSOLIDATING STATEMENT OF OPERATIONS
For the Year Ended December 31, 2012
(in thousands)
| | | | | | | | | | | | | | | | | | | | |
| | | Issuer | | | Guarantor
Subsidiaries | | | Non-Guarantor
Subsidiaries | | | Eliminations | | | Consolidated | |
Net product sales | | $ | — | | | $ | 869,912 | | | $ | 1,187,696 | | | $ | (143,877 | ) | | $ | 1,913,731 | |
Services revenue | | | — | | | | 811,625 | | | | 64,893 | | | | — | | | | 876,518 | |
| | | | | | | | | | | | | | | | | | | | |
Net product sales and services revenue | | | — | | | | 1,681,537 | | | | 1,252,589 | | | | (143,877 | ) | | | 2,790,249 | |
License and royalty revenue | | | — | | | | 18,848 | | | | 17,958 | | | | (8,230 | ) | | | 28,576 | |
| | | | | | | | | | | | | | | | | | | | |
Net revenue | | | — | | | | 1,700,385 | | | | 1,270,547 | | | | (152,107 | ) | | | 2,818,825 | |
| | | | | | | | | | | | | | | | | | | | |
Cost of net product sales | | | 6,040 | | | | 421,403 | | | | 639,203 | | | | (134,496 | ) | | | 932,150 | |
Cost of services revenue | | | — | | | | 426,599 | | | | 30,459 | | | | (6,059 | ) | | | 450,999 | |
| | | | | | | | | | | | | | | | | | | | |
Cost of net product sales and services revenue | | | 6,040 | | | | 848,002 | | | | 669,662 | | | | (140,555 | ) | | | 1,383,149 | |
Cost of license and royalty revenue | | | — | | | | 36 | | | | 15,547 | | | | (8,229 | ) | | | 7,354 | |
| | | | | | | | | | | | | | | | | | | | |
Cost of net revenue | | | 6,040 | | | | 848,038 | | | | 685,209 | | | | (148,784 | ) | | | 1,390,503 | |
| | | | | | | | | | | | | | | | | | | | |
Gross profit (loss) | | | (6,040 | ) | | | 852,347 | | | | 585,338 | | | | (3,323 | ) | | | 1,428,322 | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | |
Research and development | | | 24,593 | | | | 71,512 | | | | 86,896 | | | | — | | | | 183,001 | |
Sales and marketing | | | 4,414 | | | | 346,620 | | | | 292,389 | | | | — | | | | 643,423 | |
General and administrative | | | 52,079 | | | | 253,985 | | | | 186,702 | | | | — | | | | 492,766 | |
| | | | | | | | | | | | | | | | | | | | |
Operating income (loss) | | | (87,126 | ) | | | 180,230 | | | | 19,351 | | | | (3,323 | ) | | | 109,132 | |
Interest expense, including amortization of original issue discounts and deferred financing costs | | | (236,320 | ) | | | (39,575 | ) | | | (13,079 | ) | | | 48,414 | | | | (240,560 | ) |
Other income (expense), net | | | (16,655 | ) | | | 40,480 | | | | 34,546 | | | | (48,414 | ) | | | 9,957 | |
| | | | | | | | | | | | | | | | | | | | |
Income (loss) before provision (benefit) for income taxes | | | (340,101 | ) | | | 181,135 | | | | 40,818 | | | | (3,323 | ) | | | (121,471 | ) |
Provision (benefit) for income taxes | | | (111,595 | ) | | | 56,188 | | | | 26,043 | | | | (955 | ) | | | (30,319 | ) |
| | | | | | | | | | | | | | | | | | | | |
Income (loss) before equity earnings (losses) of subsidiaries and unconsolidated entities, net of tax | | | (228,506 | ) | | | 124,947 | | | | 14,775 | | | | (2,368 | ) | | | (91,152 | ) |
Equity earnings (losses) of subsidiaries, net of tax | | | 148,394 | | | | (1,574 | ) | | | — | | | | (146,820 | ) | | | — | |
Equity earnings of unconsolidated entities, net of tax | | | 2,205 | | | | — | | | | 10,952 | | | | 88 | | | | 13,245 | |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | | (77,907 | ) | | | 123,373 | | | | 25,727 | | | | (149,100 | ) | | | (77,907 | ) |
Less: Net income attributable to non-controlling interests | | | — | | | | — | | | | 275 | | | | — | | | | 275 | |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) attributable to Alere Inc. and Subsidiaries | | | (77,907 | ) | | | 123,373 | | | | 25,452 | | | | (149,100 | ) | | | (78,182 | ) |
Preferred stock dividends | | | (21,293 | ) | | | — | | | | — | | | | — | | | | (21,293 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) available to common stockholders | | $ | (99,200 | ) | | $ | 123,373 | | | $ | 25,452 | | | $ | (149,100 | ) | | $ | (99,475 | ) |
| | | | | | | | | | | | | | | | | | | | |
F- 75
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(25) Guarantor Financial Information (Continued)
CONSOLIDATING STATEMENT OF OPERATIONS
For the Year Ended December 31, 2011
(in thousands)
| | | | | | | | | | | | | | | | | | | | |
| | | Issuer | | | Guarantor
Subsidiaries | | | Non-Guarantor
Subsidiaries | | | Eliminations | | | Consolidated | |
Net product sales | | $ | — | | | $ | 900,853 | | | $ | 910,100 | | | $ | (127,821 | ) | | $ | 1,683,132 | |
Services revenue | | | — | | | | 613,310 | | | | 66,612 | | | | — | | | | 679,922 | |
| | | | | | | | | | | | | | | | | | | | |
Net product sales and services revenue | | | — | | | | 1,514,163 | | | | 976,712 | | | | (127,821 | ) | | | 2,363,054 | |
License and royalty revenue | | | — | | | | 10,280 | | | | 19,152 | | | | (5,959 | ) | | | 23,473 | |
| | | | | | | | | | | | | | | | | | | | |
Net revenue | | | — | | | | 1,524,443 | | | | 995,864 | | | | (133,780 | ) | | | 2,386,527 | |
| | | | | | | | | | | | | | | | | | | | |
Cost of net product sales | | | 3,651 | | | | 410,903 | | | | 510,550 | | | | (129,680 | ) | | | 795,424 | |
Cost of services revenue | | | — | | | | 312,098 | | | | 26,134 | | | | — | | | | 338,232 | |
| | | | | | | | | | | | | | | | | | | | |
Cost of net product sales and services revenue | | | 3,651 | | | | 723,001 | | | | 536,684 | | | | (129,680 | ) | | | 1,133,656 | |
Cost of license and royalty revenue | | | — | | | | 3 | | | | 12,992 | | | | (5,959 | ) | | | 7,036 | |
| | | | | | | | | | | | | | | | | | | | |
Cost of net revenue | | | 3,651 | | | | 723,004 | | | | 549,676 | | | | (135,639 | ) | | | 1,140,692 | |
| | | | | | | | | | | | | | | | | | | | |
Gross profit (loss) | | | (3,651 | ) | | | 801,439 | | | | 446,188 | | | | 1,859 | | | | 1,245,835 | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | |
Research and development | | | 20,182 | | | | 69,292 | | | | 60,691 | | | | — | | | | 150,165 | |
Sales and marketing | | | 4,091 | | | | 336,297 | | | | 225,195 | | | | — | | | | 565,583 | |
General and administrative | | | 48,891 | | | | 228,871 | | | | 121,568 | | | | — | | | | 399,330 | |
Goodwill impairment charge | | | — | | | | 383,612 | | | | — | | | | — | | | | 383,612 | |
| | | | | | | | | | | | | | | | | | | | |
Operating income (loss) | | | (76,815 | ) | | | (216,633 | ) | | | 38,734 | | | | 1,859 | | | | (252,855 | ) |
Interest expense, including amortization of original issue discounts and deferred financing costs | | | (156,948 | ) | | | (100,636 | ) | | | (8,734 | ) | | | 62,347 | | | | (203,971 | ) |
Other income (expense), net | | | (8,813 | ) | | | 54,186 | | | | 18,857 | | | | (62,347 | ) | | | 1,883 | |
Gain on sale of joint venture interest | | | 16,309 | | | | — | | | | 272,587 | | | | — | | | | 288,896 | |
| | | | | | | | | | | | | | | | | | | | |
Income (loss) before provision (benefit) for incometaxes | | | (226,267 | ) | | | (263,083 | ) | | | 321,444 | | | | 1,859 | | | | (166,047 | ) |
Provision (benefit) for income taxes | | | (67,482 | ) | | | 20,402 | | | | 24,822 | | | | (1,956 | ) | | | (24,214 | ) |
| | | | | | | | | | | | | | | | | | | | |
Income (loss) before equity earnings of unconsolidatedentities, net of tax | | | (158,785 | ) | | | (283,485 | ) | | | 296,622 | | | | 3,815 | | | | (141,833 | ) |
Equity earnings of subsidiaries, net of tax | | | 23,524 | | | | 1,530 | | | | — | | | | (25,054 | ) | | | — | |
Equity earnings of unconsolidated entities, net of tax | | | 1,952 | | | | — | | | | 6,503 | | | | 69 | | | | 8,524 | |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | | (133,309 | ) | | | (281,955 | ) | | | 303,125 | | | | (21,170 | ) | | | (133,309 | ) |
Less: Net income attributable to non- controlling interests | | | — | | | | — | | | | 233 | | | | — | | | | 233 | |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) attributable to Alere Inc. andSubsidiaries | | | (133,309 | ) | | | (281,955 | ) | | | 302,892 | | | | (21,170 | ) | | | (133,542 | ) |
Preferred stock dividends | | | (22,049 | ) | | | — | | | | — | | | | — | | | | (22,049 | ) |
Preferred stock repurchase | | | 23,936 | | | | — | | | | — | | | | — | | | | 23,936 | |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) available to common stockholders | | $ | (131,422 | ) | | $ | (281,955 | ) | | $ | 302,892 | | | $ | (21,170 | ) | | $ | (131,655 | ) |
| | | | | | | | | | | | | | | | | | | | |
F-76
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(25) Guarantor Financial Information (Continued)
CONSOLIDATING STATEMENT OF COMPREHENSIVE INCOME (LOSS)
For the Year Ended December 31, 2013
(in thousands)
| | | | | | | | | | | | | | | | | | | | |
| | | Issuer | | | Guarantor
Subsidiaries | | | Non-Guarantor
Subsidiaries | | | Eliminations | | | Consolidated | |
Net income (loss) | | $ | (70,278 | ) | | $ | 57,531 | | | $ | 85,639 | | | $ | (143,170 | ) | | $ | (70,278 | ) |
| | | | | | | | | | | | | | | | | | | | |
Other comprehensive loss, before tax: | | | | | | | | | | | | | | | | | | | | |
Changes in cumulative translation adjustment | | | (550 | ) | | | (619 | ) | | | (48,998 | ) | | | 1 | | | | (50,166 | ) |
Unrealized gains on hedging instruments | | | — | | | | — | | | | 39 | | | | — | | | | 39 | |
Minimum pension liability adjustment | | | — | | | | — | | | | (415 | ) | | | — | | | | (415 | ) |
| | | | | | | | | | | | | | | | | | | | |
Other comprehensive loss, before tax | | | (550 | ) | | | (619 | ) | | | (49,374 | ) | | | 1 | | | | (50,542 | ) |
Income tax benefit related to items of other comprehensive loss | | | — | | | | — | | | | (106 | ) | | | — | | | | (106 | ) |
| | | | | | | | | | | | | | | | | | | | |
Other comprehensive loss, net of tax | | | (550 | ) | | | (619 | ) | | | (49,268 | ) | | | 1 | | | | (50,436 | ) |
| | | | | | | | | | | | | | | | | | | | |
Comprehensive income (loss) | | | (70,828 | ) | | | 56,912 | | | | 36,371 | | | | (143,169 | ) | | | (120,714 | ) |
Less: Comprehensive income attributable to non-controlling interests | | | — | | | | — | | | | 976 | | | | — | | | | 976 | |
| | | | | | | | | | | | | | | | | | | | |
Comprehensive income (loss) attributable to Alere Inc. and Subsidiaries | | $ | (70,828 | ) | | $ | 56,912 | | | $ | 35,395 | | | $ | (143,169 | ) | | $ | (121,690 | ) |
| | | | | | | | | | | | | | | | | | | | |
F-77
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(25) Guarantor Financial Information (Continued)
CONSOLIDATING STATEMENT OF COMPREHENSIVE INCOME (LOSS)
For the Year Ended December 31, 2012
(in thousands)
| | | | | | | | | | | | | | | | | | | | |
| | | Issuer | | | Guarantor
Subsidiaries | | | Non-
Guarantor
Subsidiaries | | | Eliminations | | | Consolidated | |
Net income (loss) | | $ | (77,907 | ) | | $ | 123,373 | | | $ | 25,727 | | | $ | (149,100 | ) | | $ | (77,907 | ) |
| | | | | | | | | | | | | | | | | | | | |
Other comprehensive income (loss), before tax: | | | | | | | | | | | | | | | | | | | | |
Changes in cumulative translation adjustment | | | (834 | ) | | | (302 | ) | | | 53,654 | | | | 2,124 | | | | 54,642 | |
Unrealized gains (losses) on available for sale securities | | | (221 | ) | | | 5 | | | | — | | | | — | | | | (216 | ) |
Unrealized gains on hedging instruments | | | 16 | | | | — | | | | 372 | | | | — | | | | 388 | |
Minimum pension liability adjustment | | | — | | | | — | | | | (1,042 | ) | | | — | | | | (1,042 | ) |
| | | | | | | | | | | | | | | | | | | | |
Other comprehensive income (loss), before tax | | | (1,039 | ) | | | (297 | ) | | | 52,984 | | | | 2,124 | | | | 53,772 | |
Income tax benefit related to items of othercomprehensive income (loss) | | | (86 | ) | | | — | | | | (286 | ) | | | — | | | | (372 | ) |
| | | | | | | | | | | | | | | | | | | | |
Other comprehensive income (loss), net of tax | | | (953 | ) | | | (297 | ) | | | 53,270 | | | | 2,124 | | | | 54,144 | |
| | | | | | | | | | | | | | | | | | | | |
Comprehensive income (loss) | | | (78,860 | ) | | | 123,076 | | | | 78,997 | | | | (146,976 | ) | | | (23,763 | ) |
Less: Comprehensive income attributable to non-controlling interests | | | — | | | | — | | | | 275 | | | | — | | | | 275 | |
| | | | | | | | | | | | | | | | | | | | |
Comprehensive income (loss) attributable to Alere Inc. and Subsidiaries | | $ | (78,860 | ) | | $ | 123,076 | | | $ | 78,722 | | | $ | (146,976 | ) | | $ | (24,038 | ) |
| | | | | | | | | | | | | | | | | | | | |
F-78
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(25) Guarantor Financial Information (Continued)
CONSOLIDATING STATEMENT OF COMPREHENSIVE INCOME (LOSS)
For the Year Ended December 31, 2011
(in thousands)
| | | | | | | | | | | | | | | | | | | | |
| | | Issuer | | | Guarantor
Subsidiaries | | | Non-Guarantor
Subsidiaries | | | Eliminations | | | Consolidated | |
Net income (loss) | | $ | (133,309 | ) | | $ | (281,955 | ) | | $ | 303,125 | | | $ | (21,170 | ) | | $ | (133,309 | ) |
| | | | | | | | | | | | | | | | | | | | |
Other comprehensive income (loss), before tax: | | | | | | | | | | | | | | | | | | | | |
Changes in cumulative translation adjustment | | | (417 | ) | | | 342 | | | | (37,825 | ) | | | 2,070 | | | | (35,830 | ) |
Unrealized gains (losses) on available for sale securities | | | (33 | ) | | | 12 | | | | (450 | ) | | | — | | | | (471 | ) |
Unrealized gains (losses) on hedging instruments | | | 11,952 | | | | — | | | | (448 | ) | | | — | | | | 11,504 | |
Minimum pension liability adjustment | | | — | | | | — | | | | (3,070 | ) | | | — | | | | (3,070 | ) |
| | | | | | | | | | | | | | | | | | | | |
Other comprehensive income (loss), before tax | | | 11,502 | | | | 354 | | | | (41,793 | ) | | | 2,070 | | | | (27,867 | ) |
Income tax provision (benefit) related to items of other comprehensive income (loss) | | | 4,650 | | | | — | | | | (1,557 | ) | | | — | | | | 3,093 | |
| | | | | | | | | | | | | | | | | | | | |
Other comprehensive income (loss), net of tax | | | 6,852 | | | | 354 | | | | (40,236 | ) | | | 2,070 | | | | (30,960 | ) |
| | | | | | | | | | | | | | | | | | | | |
Comprehensive income (loss) | | | (126,457 | ) | | | (281,601 | ) | | | 262,889 | | | | (19,100 | ) | | | (164,269 | ) |
Less: Comprehensive income attributable to non-controlling interests | | | — | | | | — | | | | 233 | | | | — | | | | 233 | |
| | | | | | | | | | | | | | | | | | | | |
Comprehensive income (loss) attributable to Alere Inc. and Subsidiaries | | $ | (126,457 | ) | | $ | (281,601 | ) | | $ | 262,656 | | | $ | (19,100 | ) | | $ | (164,502 | ) |
| | | | | | | | | | | | | | | | | | | | |
F-79
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(25) Guarantor Financial Information (Continued)
CONSOLIDATING BALANCE SHEET
December 31, 2013
(in thousands)
| | | | | | | | | | | | | | | | | | | | |
| | | Issuer | | | Guarantor
Subsidiaries | | | Non-
Guarantor
Subsidiaries | | | Eliminations | | | Consolidated | |
ASSETS | | | | | | | | | | | | | | | | | | | | |
Current assets: | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 14,801 | | | $ | 85,453 | | | $ | 261,654 | | | $ | — | | | $ | 361,908 | |
Restricted cash | | | 2,221 | | | | 2,915 | | | | 1,237 | | | | — | | | | 6,373 | |
Marketable securities | | | — | | | | 853 | | | | 5 | | | | — | | | | 858 | |
Accounts receivable, net of allowances | | | — | | | | 238,782 | | | | 309,947 | | | | — | | | | 548,729 | |
Inventories, net | | | — | | | | 168,058 | | | | 219,892 | | | | (23,765 | ) | | | 364,185 | |
Deferred tax assets | | | 5,191 | | | | 20,541 | | | | 31,451 | | | | 3,506 | | | | 60,689 | |
Prepaid expenses and other current assets | | | 512,123 | | | | (405,954 | ) | | | 23,547 | | | | (44 | ) | | | 129,672 | |
Intercompany receivables | | | 317,357 | | | | 759,497 | | | | 75,424 | | | | (1,152,278 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total current assets | | | 851,693 | | | | 870,145 | | | | 923,157 | | | | (1,172,581 | ) | | | 1,472,414 | |
Property, plant and equipment, net | | | 15,086 | | | | 288,637 | | | | 241,737 | | | | (296 | ) | | | 545,164 | |
Goodwill | | | — | | | | 1,841,377 | | | | 1,252,314 | | | | — | | | | 3,093,691 | |
Other intangible assets with indefinite lives | | | — | | | | 14,300 | | | | 42,402 | | | | — | | | | 56,702 | |
Finite-lived intangible assets, net | | | 11,006 | | | | 995,868 | | | | 677,737 | | | | — | | | | 1,684,611 | |
Restricted cash | | | — | | | | — | | | | 29,370 | | | | — | | | | 29,370 | |
Deferred financing costs, net and othernon-current assets | | | 55,207 | | | | 8,353 | | | | 20,559 | | | | (46 | ) | | | 84,073 | |
Investments in subsidiaries | | | 3,802,475 | | | | 267,824 | | | | 191,947 | | | | (4,262,246 | ) | | | — | |
Investments in unconsolidated entities | | | 29,005 | | | | — | | | | 44,637 | | | | 13,188 | | | | 86,830 | |
Deferred tax assets | | | — | | | | — | | | | 7,959 | | | | — | | | | 7,959 | |
Intercompany notes receivable | | | 1,100,746 | | | | 630,628 | | | | (741,016 | ) | | | (990,358 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total assets | | $ | 5,865,218 | | | $ | 4,917,132 | | | $ | 2,690,803 | | | $ | (6,412,339 | ) | | $ | 7,060,814 | |
| | | | | | | | | | | | | | | | | | | | |
LIABILITIES AND EQUITY | | | | | | | | | | | | | | | | | | | | |
Current liabilities: | | | | | | | | | | | | | | | | | | | | |
Current portion of long-term debt | | $ | 45,000 | | | $ | 323 | | | $ | 3,789 | | | $ | — | | | $ | 49,112 | |
Current portion of capital lease obligations | | | — | | | | 3,751 | | | | 3,104 | | | | — | | | | 6,855 | |
Accounts payable | | | 12,584 | | | | 69,076 | | | | 105,711 | | | | — | | | | 187,371 | |
Accrued expenses and other current liabilities | | | 63,990 | | | | 164,762 | | | | 201,132 | | | | (36 | ) | | | 429,848 | |
Intercompany payables | | | 728,541 | | | | 149,031 | | | | 274,707 | | | | (1,152,279 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total current liabilities | | | 850,115 | | | | 386,943 | | | | 588,443 | | | | (1,152,315 | ) | | | 673,186 | |
| | | | | | | | | | | | | | | | | | | | |
Long-term liabilities: | | | | | | | | | | | | | | | | | | | | |
Long-term debt, net of current portion | | | 3,735,137 | | | | 100 | | | | 37,551 | | | | — | | | | 3,772,788 | |
Capital lease obligations, net of current portion | | | — | | | | 5,938 | | | | 8,469 | | | | — | | | | 14,407 | |
Deferred tax liabilities | | | (43,246 | ) | | | 284,448 | | | | 88,039 | | | | 8 | | | | 329,249 | |
Other long-term liabilities | | | 19,753 | | | | 58,823 | | | | 109,806 | | | | (46 | ) | | | 188,336 | |
Intercompany notes payables | | | (774,507 | ) | | | 1,444,741 | | | | 320,125 | | | | (990,359 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total long-term liabilities | | | 2,937,137 | | | | 1,794,050 | | | | 563,990 | | | | (990,397 | ) | | | 4,304,780 | |
| | | | | | | | | | | | | | | | | | | | |
Stockholders’ equity | | | 2,077,966 | | | | 2,736,139 | | | | 1,533,488 | | | | (4,269,627 | ) | | | 2,077,966 | |
Non-controlling interests | | | — | | | | — | | | | 4,882 | | | | — | | | | 4,882 | |
| | | | | | | | | | | | | | | | | | | | |
Total equity | | | 2,077,966 | | | | 2,736,139 | | | | 1,538,370 | | | | (4,269,627 | ) | | | 2,082,848 | |
| | | | | | | | | | | | | | | | | | | | |
Total liabilities and equity | | $ | 5,865,218 | | | $ | 4,917,132 | | | $ | 2,690,803 | | | $ | (6,412,339 | ) | | $ | 7,060,814 | |
| | | | | | | | | | | | | | | | | | | | |
F-80
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(25) Guarantor Financial Information (Continued)
CONSOLIDATING BALANCE SHEET
December 31, 2012
(in thousands)
| | | | | | | | | | | | | | | | | | | | |
| | | Issuer | | | Guarantor
Subsidiaries | | | Non-
Guarantor
Subsidiaries | | | Eliminations | | | Consolidated | |
ASSETS | | | | | | | | | | | | | | | | | | | | |
Current assets: | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 3,623 | | | $ | 67,941 | | | $ | 256,782 | | | $ | — | | | $ | 328,346 | |
Restricted cash | | | — | | | | 1,680 | | | | 1,396 | | | | — | | | | 3,076 | |
Marketable securities | | | — | | | | 787 | | | | 117 | | | | — | | | | 904 | |
Accounts receivable, net of allowances | | | — | | | | 242,422 | | | | 281,910 | | | | — | | | | 524,332 | |
Inventories, net | | | — | | | | 142,465 | | | | 203,178 | | | | (8,522 | ) | | | 337,121 | |
Deferred tax assets | | | 12,193 | | | | 39,613 | | | | 13,126 | | | | 2,790 | | | | 67,722 | |
Prepaid expenses and other current assets | | | (20,636 | ) | | | 100,385 | | | | 65,520 | | | | (33 | ) | | | 145,236 | |
Intercompany receivables | | | 298,812 | | | | 1,247,637 | | | | 58,561 | | | | (1,605,010 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total current assets | | | 293,992 | | | | 1,842,930 | | | | 880,590 | | | | (1,610,775 | ) | | | 1,406,737 | |
Property, plant and equipment, net | | | 2,679 | | | | 293,635 | | | | 238,707 | | | | (552 | ) | | | 534,469 | |
Goodwill | | | — | | | | 1,859,966 | | | | 1,188,439 | | | | — | | | | 3,048,405 | |
Other intangible assets with indefinite lives | | | — | | | | 14,600 | | | | 21,851 | | | | — | | | | 36,451 | |
Finite-lived intangible assets, net | | | 24,701 | | | | 1,168,307 | | | | 641,217 | | | | — | | | | 1,834,225 | |
Deferred financing costs, net and othernon-current assets | | | 78,522 | | | | 10,451 | | | | 19,955 | | | | (71 | ) | | | 108,857 | |
Investments in subsidiaries | | | 4,114,478 | | | | 222,175 | | | | 170,393 | | | | (4,507,046 | ) | | | — | |
Investments in unconsolidated entities | | | 33,979 | | | | — | | | | 56,512 | | | | — | | | | 90,491 | |
Deferred tax assets | | | — | | | | 782 | | | | 7,511 | | | | — | | | | 8,293 | |
Intercompany notes receivable | | | 1,724,650 | | | | 722,552 | | | | 1,278 | | | | (2,448,480 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total assets | | $ | 6,273,001 | | | $ | 6,135,398 | | | $ | 3,226,453 | | | $ | (8,566,924 | ) | | $ | 7,067,928 | |
| | | | | | | | | | | | | | | | | | | | |
LIABILITIES AND EQUITY | | | | | | | | | | | | | | | | | | | | |
Current liabilities: | | | | | | | | | | | | | | | | | | | | |
Current portion of long-term debt | | $ | 45,000 | | | $ | 349 | | | $ | 14,883 | | | $ | — | | | $ | 60,232 | |
Current portion of capital lease obligations | | | — | | | | 3,217 | | | | 3,467 | | | | — | | | | 6,684 | |
Accounts payable | | | 7,993 | | | | 76,691 | | | | 85,290 | | | | — | | | | 169,974 | |
Accrued expenses and other current liabilities | | | (388,830 | ) | | | 587,119 | | | | 213,656 | | | | (26 | ) | | | 411,919 | |
Intercompany payables | | | 557,578 | | | | 803,879 | | | | 243,553 | | | | (1,605,010 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total current liabilities | | | 221,741 | | | | 1,471,255 | | | | 560,849 | | | | (1,605,036 | ) | | | 648,809 | |
| | | | | �� | | | | | | | | | | | | | | | |
Long-term liabilities: | | | | | | | | | | | | | | | | | | | | |
Long-term debt, net of current portion | | | 3,617,068 | | | | 374 | | | | 11,233 | | | | — | | | | 3,628,675 | |
Capital lease obligations, net of currentportion | | | — | | | | 5,417 | | | | 7,500 | | | | — | | | | 12,917 | |
Deferred tax liabilities | | | (5,329 | ) | | | 332,912 | | | | 100,692 | | | | (87 | ) | | | 428,188 | |
Other long-term liabilities | | | 17,678 | | | | 72,901 | | | | 76,127 | | | | (71 | ) | | | 166,635 | |
Intercompany notes payables | | | 241,421 | | | | 1,630,376 | | | | 576,684 | | | | (2,448,481 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total long-term liabilities | | | 3,870,838 | | | | 2,041,980 | | | | 772,236 | | | | (2,448,639 | ) | | | 4,236,415 | |
| | | | | | | | | | | | | | | | | | | | |
Stockholders’ equity | | | 2,180,422 | | | | 2,622,163 | | | | 1,891,086 | | | | (4,513,249 | ) | | | 2,180,422 | |
Non-controlling interests | | | — | | | | — | | | | 2,282 | | | | — | | | | 2,282 | |
| | | | | | | | | | | | | | | | | | | | |
Total equity | | | 2,180,422 | | | | 2,622,163 | | | | 1,893,368 | | | | (4,513,249 | ) | | | 2,182,704 | |
| | | | | | | | | | | | | | | | | | | | |
Total liabilities and equity | | $ | 6,273,001 | | | $ | 6,135,398 | | | $ | 3,226,453 | | | $ | (8,566,924 | ) | | $ | 7,067,928 | |
| | | | | | | | | | | | | | | | | | | | |
F-81
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(25) Guarantor Financial Information (Continued)
CONSOLIDATING STATEMENT OF CASH FLOWS
For the Year Ended December 31, 2013
(in thousands)
| | | | | | | | | | | | | | | | | | | | |
| | | Issuer | | | Guarantor
Subsidiaries | | | Non-Guarantor
Subsidiaries | | | Eliminations | | | Consolidated | |
Cash Flows from Operating Activities: | | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | $ | (70,278 | ) | | $ | 57,531 | | | $ | 85,639 | | | $ | (143,170 | ) | | $ | (70,278 | ) |
Adjustments to reconcile net income (loss) to net cash provided by operating activities: | | | | | | | | | | | | | | | | | | | | |
Equity (earnings) losses of subsidiaries, net of tax | | | (144,941 | ) | | | 2,948 | | | | — | | | | 141,993 | | | | — | |
Non-cash interest expense, including amortization of original issue discounts and deferred financing costs | | | 17,704 | | | | 311 | | | | 76 | | | | — | | | | 18,091 | |
Depreciation and amortization | | | 5,864 | | | | 254,280 | | | | 179,405 | | | | (119 | ) | | | 439,430 | |
Non-cash charges for sale of inventories revalued at the date of acquisition | | | — | | | | — | | | | 2,504 | | | | — | | | | 2,504 | |
Non-cash stock-based compensation expense | | | 8,792 | | | | 5,158 | | | | 7,260 | | | | — | | | | 21,210 | |
Impairment of inventory | | | — | | | | 26 | | | | 311 | | | | — | | | | 337 | |
Impairment of long-lived assets | | | — | | | | 5,076 | | | | 1,237 | | | | — | | | | 6,313 | |
Impairment of intangible assets | | | — | | | | 2,596 | | | | 686 | | | | — | | | | 3,282 | |
Loss on sale of fixed assets | | | — | | | | 2,175 | | | | 1,145 | | | | — | | | | 3,320 | |
Equity earnings of unconsolidated entities, net of tax | | | (1,890 | ) | | | — | | | | (15,470 | ) | | | (83 | ) | | | (17,443 | ) |
Deferred income taxes | | | (31,624 | ) | | | (11,824 | ) | | | (59,579 | ) | | | (620 | ) | | | (103,647 | ) |
Loss on extinguishment of debt | | | 35,603 | | | | — | | | | — | | | | — | | | | 35,603 | |
Loss on disposition | | | — | | | | — | | | | 5,124 | | | | — | | | | 5,124 | |
Bargain purchase gain | | | — | | | | — | | | | (8,023 | ) | | | — | | | | (8,023 | ) |
Other non-cash items | | | 5,202 | | | | (239 | ) | | | 3,631 | | | | — | | | | 8,594 | |
Changes in assets and liabilities, net of acquisitions: | | | | | | | | | | | | | | | | | | | | |
Accounts receivable, net | | | — | | | | 3,640 | | | | (40,089 | ) | | | — | | | | (36,449 | ) |
Inventories, net | | | — | | | | (51,377 | ) | | | (28,980 | ) | | | 2,054 | | | | (78,303 | ) |
Prepaid expenses and other current assets | | | (572,703 | ) | | | 508,894 | | | | 54,448 | | | | (2,907 | ) | | | (12,268 | ) |
Accounts payable | | | 4,591 | | | | (9,185 | ) | | | 17,620 | | | | — | | | | 13,026 | |
Accrued expenses and other current liabilities | | | 492,996 | | | | (423,164 | ) | | | (20,391 | ) | | | 2,907 | | | | 52,348 | |
Other non-current liabilities | | | (12,289 | ) | | | (16,715 | ) | | | 4,847 | | | | 24 | | | | (24,133 | ) |
Cash paid for contingent consideration | | | (12,437 | ) | | | — | | | | (1,424 | ) | | | — | | | | (13,861 | ) |
Intercompany payable (receivable) | | | 410,392 | | | | (251,359 | ) | | | (159,037 | ) | | | 4 | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Net cash provided by operating activities | | | 134,982 | | | | 78,772 | | | | 30,940 | | | | 83 | | | | 244,777 | |
| | | | | | | | | | | | | | | | | | | | |
Cash Flows from Investing Activities: | | | | | | | | | | | | | | | | | | | | |
Increase in restricted cash | | | (2,221 | ) | | | (1,236 | ) | | | (28,943 | ) | | | — | | | | (32,400 | ) |
Purchases of property, plant and equipment | | | (12,289 | ) | | | (54,826 | ) | | | (69,946 | ) | | | 14,895 | | | | (122,166 | ) |
Proceeds from sale of property, plant and equipment | | | — | | | | 5,186 | | | | 12,875 | | | | (14,441 | ) | | | 3,620 | |
Cash received from disposition | | | — | | | | — | | | | 29,000 | | | | — | �� | | | 29,000 | |
Cash paid for business acquisitions, net of cash acquired | | | (166,772 | ) | | | — | | | | (9,359 | ) | | | — | | | | (176,131 | ) |
Cash received (paid) from sales (purchases) of marketable securities | | | — | | | | (66 | ) | | | 107 | | | | — | | | | 41 | |
Cash received from equity method investments | | | 1,960 | | | | — | | | | 27,384 | | | | (6 | ) | | | 29,338 | |
(Increase) decrease in other assets | | | 15,269 | | | | (5,788 | ) | | | 906 | | | | (24 | ) | | | 10,363 | |
| | | | | | | | | | | | | | | | | | | | |
Net cash used in investing activities | | | (164,053 | ) | | | (56,730 | ) | | | (37,976 | ) | | | 424 | | | | (258,335 | ) |
| | | | | | | | | | | | | | | | | | | | |
Cash Flows from Financing Activities: | | | | | | | | | | | | | | | | | | | | |
Cash paid for financing costs | | | (9,845 | ) | | | — | | | | — | | | | — | | | | (9,845 | ) |
Cash paid for contingent purchase price consideration | | | (41,371 | ) | | | — | | | | (1,006 | ) | | | — | | | | (42,377 | ) |
Cash paid for dividends | | | (21,293 | ) | | | — | | | | — | | | | — | | | | (21,293 | ) |
Proceeds from issuance of common stock, net of issuance costs | | | 20,863 | | | | — | | | | — | | | | — | | | | 20,863 | |
Proceeds from issuance of long-term debt | | | 425,000 | | | | 989 | | | | 33,962 | | | | — | | | | 459,951 | |
Payments on long-term debt | | | (461,845 | ) | | | (1,288 | ) | | | (8,413 | ) | | | — | | | | (471,546 | ) |
Net proceeds (payments) under revolving credit facilities | | | 147,500 | | | | — | | | | (8,537 | ) | | | — | | | | 138,963 | |
Excess tax benefits on exercised stock options | | | 193 | | | | 200 | | | | 68 | | | | — | | | | 461 | |
Principal payments on capital lease obligations | | | — | | | | (3,813 | ) | | | (3,255 | ) | | | — | | | | (7,068 | ) |
Other | | | (18,953 | ) | | | — | | | | (165 | ) | | | — | | | | (19,118 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net cash provided by (used in) financing activities | | | 40,249 | | | | (3,912 | ) | | | 12,654 | | | | — | | | | 48,991 | |
| | | | | | | | | | | | | | | | | | | | |
Foreign exchange effect on cash and cash equivalents | | | — | | | | (618 | ) | | | (746 | ) | | | (507 | ) | | | (1,871 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | | | 11,178 | | | | 17,512 | | | | 4,872 | | | | — | | | | 33,562 | |
Cash and cash equivalents, beginning of period | | | 3,623 | | | | 67,941 | | | | 256,782 | | | | — | | | | 328,346 | |
| | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents, end of period | | $ | 14,801 | | | $ | 85,453 | | | $ | 261,654 | | | $ | — | | | $ | 361,908 | |
| | | | | | | | | | | | | | | | | | | | |
F-82
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(25) Guarantor Financial Information (Continued)
CONSOLIDATING STATEMENT OF CASH FLOWS
For the Year Ended December 31, 2012
(in thousands)
| | | | | | | | | | | | | | | | | | | | |
| | | Issuer | | | Guarantor
Subsidiaries | | | Non-Guarantor
Subsidiaries | | | Eliminations | | | Consolidated | |
Cash Flows from Operating Activities: | | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | $ | (77,907 | ) | | $ | 123,373 | | | $ | 25,727 | | | $ | (149,100 | ) | | $ | (77,907 | ) |
Adjustments to reconcile net income (loss) to net cash provided by operating activities: | | | | | | | | | | | | | | | | | | | | |
Equity (earnings) losses of subsidiaries, net of tax | | | (148,394 | ) | | | 1,574 | | | | — | | | | 146,820 | | | | — | |
Non-cash interest expense, including amortization of original issue discounts and deferred financing costs | | | 21,213 | | | | 277 | | | | — | | | | — | | | | 21,490 | |
Depreciation and amortization | | | 7,961 | | | | 266,310 | | | | 182,597 | | | | (21 | ) | | | 456,847 | |
Non-cash charges for sale of inventories revalued at the date of acquisition | | | — | | | | 1,400 | | | | 3,281 | | | | — | | | | 4,681 | |
Non-cash stock-based compensation expense | | | 4,247 | | | | 5,486 | | | | 5,932 | | | | — | | | | 15,665 | |
Impairment of inventory | | | — | | | | 5 | | | | 290 | | | | — | | | | 295 | |
Impairment of long-lived assets | | | — | | | | 2,903 | | | | 586 | | | | — | | | | 3,489 | |
Impairment of intangible assets | | | — | | | | 2,988 | | | | — | | | | — | | | | 2,988 | |
(Gain) loss on sale of fixed assets | | | 4 | | | | (2,672 | ) | | | 517 | | | | — | | | | (2,151 | ) |
Gain on sales of marketable securities | | | (751 | ) | | | — | | | | — | | | | — | | | | (751 | ) |
Equity earnings of unconsolidated entities, net of tax | | | (2,205 | ) | | | — | | | | (10,952 | ) | | | (88 | ) | | | (13,245 | ) |
Deferred income taxes | | | 20,500 | | | | (80,252 | ) | | | (23,854 | ) | | | (962 | ) | | | (84,568 | ) |
Loss on extinguishment of debt | | | 23,235 | | | | — | | | | — | | | | — | | | | 23,235 | |
Other non-cash items | | | (1,001 | ) | | | 908 | | | | 7,429 | | | | — | | | | 7,336 | |
Changes in assets and liabilities, net of acquisitions: | | | | | | | | | | | | | | | | | | | | |
Accounts receivable, net | | | — | | | | (1,641 | ) | | | (20,524 | ) | | | — | | | | (22,165 | ) |
Inventories, net | | | — | | | | (6,001 | ) | | | (13,692 | ) | | | 2,902 | | | | (16,791 | ) |
Prepaid expenses and other current assets | | | (454,780 | ) | | | 357,898 | | | | (731 | ) | | | 95,087 | | | | (2,526 | ) |
Accounts payable | | | 1,289 | | | | 3,551 | | | | (14,967 | ) | | | — | | | | (10,127 | ) |
Accrued expenses and other current liabilities | | | 346,511 | | | | (226,905 | ) | | | 35,891 | | | | (95,081 | ) | | | 60,416 | |
Other non-current liabilities | | �� | (12,373 | ) | | | (134 | ) | | | (22,966 | ) | | | (70 | ) | | | (35,543 | ) |
Cash paid for contingent consideration | | | (10,911 | ) | | | (74 | ) | | | — | | | | — | | | | (10,985 | ) |
Intercompany payable (receivable) | | | 413,479 | | | | (405,459 | ) | | | (6,858 | ) | | | (1,162 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Net cash provided by operating activities | | | 130,117 | | | | 43,535 | | | | 147,706 | | | | (1,675 | ) | | | 319,683 | |
| | | | | | | | | | | | | | | | | | | | |
Cash Flows from Investing Activities: | | | | | | | | | | | | | | | | | | | | |
Decrease in restricted cash | | | — | | | | 12 | | | | 5,899 | | | | — | | | | 5,911 | |
Purchases of property, plant and equipment | | | (2,061 | ) | | | (91,898 | ) | | | (109,396 | ) | | | 65,962 | | | | (137,393 | ) |
Proceeds from sale of property, plant and equipment | | | — | | | | 22,860 | | | | 65,747 | | | | (66,217 | ) | | | 22,390 | |
Cash paid for business acquisitions, net of cash acquired | | | (403,552 | ) | | | 1,899 | | | | (22,933 | ) | | | — | | | | (424,586 | ) |
Cash received from sales of marketable securities | | | 2,784 | | | | 269 | | | | 3 | | | | — | | | | 3,056 | |
Cash received from equity method investments | | | 1,470 | | | | — | | | | 11,237 | | | | — | | | | 12,707 | |
Increase in other assets | | | (53,189 | ) | | | (1,051 | ) | | | (2,106 | ) | | | 70 | | | | (56,276 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net cash used in investing activities | | | (454,548 | ) | | | (67,909 | ) | | | (51,549 | ) | | | (185 | ) | | | (574,191 | ) |
| | | | | | | | | | | | | | | | | | | | |
Cash Flows from Financing Activities: | | | | | | | | | | | | | | | | | | | | |
Cash paid for financing costs | | | (10,139 | ) | | | — | | | | — | | | | — | | | | (10,139 | ) |
Cash paid for contingent purchase price consideration | | | (20,116 | ) | | | (788 | ) | | | (60 | ) | | | — | | | | (20,964 | ) |
Cash paid for dividends | | | (21,293 | ) | | | — | | | | — | | | | — | | | | (21,293 | ) |
Proceeds from issuance of common stock, net of issuance costs | | | 14,924 | | | | — | | | | — | | | | — | | | | 14,924 | |
Proceeds from issuance of long-term debt | | | 648,000 | | | | — | | | | 535 | | | | — | | | | 648,535 | |
Payments on short-term debt | | | (6,240 | ) | | | — | | | | — | | | | — | | | | (6,240 | ) |
Payments on long-term debt | | | (300,155 | ) | | | (534 | ) | | | (10,923 | ) | | | — | | | | (311,612 | ) |
Net proceeds (payments) under revolving credit facilities | | | 22,500 | | | | (2 | ) | | | (8,226 | ) | | | — | | | | 14,272 | |
Excess tax benefits on exercised stock options | | | 176 | | | | 303 | | | | 25 | | | | — | | | | 504 | |
Principal payments on capital lease obligations | | | — | | | | (2,350 | ) | | | (4,653 | ) | | | — | | | | (7,003 | ) |
Purchase of non-controlling interest | | | — | | | | — | | | | (2,972 | ) | | | — | | | | (2,972 | ) |
Other | | | (12,267 | ) | | | — | | | | — | | | | — | | | | (12,267 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net cash provided by (used in) financing activities | | | 315,390 | | | | (3,371 | ) | | | (26,274 | ) | | | — | | | | 285,745 | |
| | | | | | | | | | | | | | | | | | | | |
Foreign exchange effect on cash and cash equivalents | | | 213 | | | | 474 | | | | (4,611 | ) | | | 1,860 | | | | (2,064 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | | | (8,828 | ) | | | (27,271 | ) | | | 65,272 | | | | — | | | | 29,173 | |
Cash and cash equivalents, beginning of period | | | 12,451 | | | | 95,212 | | | | 191,510 | | | | — | | | | 299,173 | |
| | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents, end of period | | $ | 3,623 | | | $ | 67,941 | | | $ | 256,782 | | | $ | — | | | $ | 328,346 | |
| | | | | | | | | | | | | | | | | | | | |
F-83
ALERE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(25) Guarantor Financial Information (Continued)
CONSOLIDATING STATEMENT OF CASH FLOWS
For the Year Ended December 31, 2011
(in thousands)
| | | | | | | | | | | | | | | | | | | | |
| | | Issuer | | | Guarantor
Subsidiaries | | | Non-Guarantor
Subsidiaries | | | Eliminations | | | Consolidated | |
Cash Flows from Operating Activities: | | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | $ | (133,309 | ) | | | (281,955 | ) | | | 303,125 | | | $ | (21,170 | ) | | $ | (133,309 | ) |
Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: | | | | | | | | | | | | | | | | | | | | |
Equity in earnings of subsidiaries, net of tax | | | (23,524 | ) | | | (1,530 | ) | | | — | | | | 25,054 | | | | — | |
Non-cash interest expense, including amortization of original issue discounts and deferred financing costs | | | 13,671 | | | | 23,473 | | | | 446 | | | | — | | | | 37,590 | |
Depreciation and amortization | | | 3,842 | | | | 260,062 | | | | 128,111 | | | | (439 | ) | | | 391,576 | |
Non-cash charges for sale of inventories revalued at the date of acquisition | | | — | | | | — | | | | 6,010 | | | | — | | | | 6,010 | |
Non-cash stock-based compensation expense | | | 5,776 | | | | 8,390 | | | | 7,049 | | | | — | | | | 21,215 | |
Impairment of inventory | | | — | | | | 172 | | | | 273 | | | | — | | | | 445 | |
Impairment of long-lived assets | | | 3 | | | | 1,331 | | | | 215 | | | | — | | | | 1,549 | |
Impairment of goodwill | | | — | | | | 383,612 | | | | — | | | | — | | | | 383,612 | |
Impairment of intangible assets | | | — | | | | 2,935 | | | | 3 | | | | — | | | | 2,938 | |
Gain on sale of joint venture interest | | | (16,309 | ) | | | — | | | | (272,587 | ) | | | — | | | | (288,896 | ) |
(Gain) loss on sale of fixed assets | | | 75 | | | | 1,655 | | | | (153 | ) | | | — | | | | 1,577 | |
Gain on sales of marketable securities | | | — | | | | — | | | | (840 | ) | | | — | | | | (840 | ) |
Equity earnings of unconsolidated entities, net of tax | | | (1,952 | ) | | | — | | | | (6,503 | ) | | | (69 | ) | | | (8,524 | ) |
Deferred income taxes | | | 35,012 | | | | (81,063 | ) | | | (8,754 | ) | | | (1,956 | ) | | | (56,761 | ) |
Other non-cash items | | | (4,286 | ) | | | 3,971 | | | | (11,932 | ) | | | — | | | | (12,247 | ) |
Changes in assets and liabilities, net of acquisitions: | | | | | | | | | | | | | | | | | | | | |
Accounts receivable, net | | | — | | | | 260 | | | | (39,668 | ) | | | — | | | | (39,408 | ) |
Inventories, net | | | — | | | | (9,458 | ) | | | (9,035 | ) | | | (1,906 | ) | | | (20,399 | ) |
Prepaid expenses and other current assets | | | 72,955 | | | | (110,333 | ) | | | (15,737 | ) | | | — | | | | (53,115 | ) |
Accounts payable | | | (233 | ) | | | (11,454 | ) | | | 18,672 | | | | — | | | | 6,985 | |
Accrued expenses and other current liabilities | | | (231,949 | ) | | | 203,333 | | | | 42,898 | | | | — | | | | 14,282 | |
Other non-current liabilities | | | 35,109 | | | | 1,636 | | | | (19,772 | ) | | | — | | | | 16,973 | |
Intercompany payable (receivable) | | | (1,512,567 | ) | | | 1,015,286 | | | | 497,281 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Net cash provided by (used in) operating activities | | | (1,757,686 | ) | | | 1,410,323 | | | | 619,102 | | | | (486 | ) | | | 271,253 | |
| | | | | | | | | | | | | | | | | | | | |
Cash Flows from Investing Activities: | | | | | | | | | | | | | | | | | | | | |
(Increase) decrease in restricted cash | | | — | | | | 48 | | | | (6,454 | ) | | | — | | | | (6,406 | ) |
Purchases of property, plant and equipment | | | 20 | | | | (67,103 | ) | | | (65,880 | ) | | | 431 | | | | (132,532 | ) |
Proceeds from sale of property, plant and equipment | | | — | | | | 292 | | | | 655 | | | | — | | | | 947 | |
Cash received from disposition | | | — | | | | — | | | | 11,491 | | | | — | | | | 11,491 | |
Cash paid for business acquisitions, net of cash acquired | | | (37,644 | ) | | | (163,379 | ) | | | (430,288 | ) | | | — | | | | (631,311 | ) |
Cash received from sales of marketable securities | | | — | | | | 145 | | | | 9,057 | | | | — | | | | 9,202 | |
Cash paid for equity method investments | | | (2,430 | ) | | | — | | | | (119,473 | ) | | | — | | | | (121,903 | ) |
(Increase) decrease in other assets | | | (24,996 | ) | | | (7,913 | ) | | | 6,379 | | | | (1,154 | ) | | | (27,684 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net cash used in investing activities | | | (65,050 | ) | | | (237,910 | ) | | | (594,513 | ) | | | (723 | ) | | | (898,196 | ) |
| | | | | | | | | | | | | | | | | | | | |
Cash Flows from Financing Activities: | | | | | | | | | | | | | | | | | | | | |
Cash paid for financing costs | | | (73,876 | ) | | | (804 | ) | | | — | | | | — | | | | (74,680 | ) |
Cash paid for contingent purchase price consideration | | | (28,305 | ) | | | — | | | | — | | | | — | | | | (28,305 | ) |
Cash paid for dividends | | | (5,425 | ) | | | — | | | | — | | | | — | | | | (5,425 | ) |
Proceeds from issuance of common stock, net of issuance costs | | | 37,886 | | | | — | | | | — | | | | — | | | | 37,886 | |
Repurchase of preferred stock | | | (99,070 | ) | | | — | | | | — | | | | — | | | | (99,070 | ) |
Proceeds from issuance of long-term debt | | | 2,100,000 | | | | 1,238 | | | | (4,961 | ) | | | — | | | | 2,096,277 | |
Payments on long-term debt | | | (10,126 | ) | | | (1,192,544 | ) | | | (4,784 | ) | | | — | | | | (1,207,454 | ) |
Net proceeds under revolving credit facilities | | | — | | | | — | | | | 10,715 | | | | — | | | | 10,715 | |
Repurchase of common stock | | | (184,867 | ) | | | — | | | | — | | | | — | | | | (184,867 | ) |
Excess tax benefits on exercised stock options | | | 1,357 | | | | 414 | | | | 1,652 | | | | — | | | | 3,423 | |
Principal payments on capital lease obligations | | | — | | | | (2,372 | ) | | | (1,791 | ) | | | — | | | | (4,163 | ) |
Other | | | (4,053 | ) | | | — | | | | (204 | ) | | | — | | | | (4,257 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net cash provided by (used in) financing activities | | | 1,733,521 | | | | (1,194,068 | ) | | | 627 | | | | — | | | | 540,080 | |
| | | | | | | | | | | | | | | | | | | | |
Foreign exchange effect on cash and cash equivalents | | | — | | | | (38 | ) | | | (16,441 | ) | | | 1,209 | | | | (15,270 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | | | (89,215 | ) | | | (21,693 | ) | | | 8,775 | | | | — | | | | (102,133 | ) |
Cash and cash equivalents, beginning of period | | | 101,666 | | | | 116,905 | | | | 182,735 | | | | — | | | | 401,306 | |
| | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents, end of period | | $ | 12,451 | | | $ | 95,212 | | | $ | 191,510 | | | $ | — | | | $ | 299,173 | |
| | | | | | | | | | | | | | | | | | | | |
F-84

