QuickLinks -- Click here to rapidly navigate through this document
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| (Mark One) | |
ý |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2007 |
OR |
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to |
Commission file number: 001-33277
SYNTA PHARMACEUTICALS CORP.
(Exact name of registrant as specified in its charter)
Delaware
(State or other jurisdiction of
incorporation or organization) | | 04-3508648
(I.R.S. Employer
Identification No.) |
45 Hartwell Avenue
Lexington, Massachusetts
(Address of principal executive offices) |
|
02421
(Zip Code) |
Registrant's telephone number, including area code(781) 274-8200
Securities registered pursuant to Section 12(b) of the Exchange Act:
Title of each class
| | Name of each exchange on which registered
|
|---|
| Common Stock, $0.0001 Par Value Per Share | | The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Exchange Act:
None.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer o | | Accelerated filer o | | Non-accelerated filer ý
(Do not check if a smaller reporting company) | | Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No ý
The aggregate market value of the registrant's common stock held by non-affiliates of the registrant (without admitting that any person whose shares are not included in such calculation is an affiliate), computed by reference to the price at which the common stock was last sold on June 29, 2007, the last business day of the registrant's most recently completed second fiscal quarter, was $145,161,182.
As of March 14, 2008 the registrant had 33,873,538 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
The following documents (or parts thereof) are incorporated by reference into the following parts of this Annual Report on Form 10-K: Certain information required in Part III of this Annual Report on Form 10-K is incorporated from the registrant's Proxy Statement for the 2008 Annual Meeting of Stockholders to be held on June 11, 2008.
PART I
Item 1. BUSINESS
Overview
We are a biopharmaceutical company focused on discovering, developing, and commercializing small molecule drugs to extend and enhance the lives of patients with severe medical conditions, including cancer and chronic inflammatory diseases. We have a unique chemical compound library, an integrated discovery engine, and a diverse pipeline of clinical- and preclinical-stage drug candidates with distinct mechanisms of action and novel chemical structures. We have three drug candidates in clinical trials, one drug candidate in preclinical studies, and one program undergoing lead optimization. We discovered and developed each of our drug candidates internally using our compound library and discovery capabilities. At present, other than our lead drug candidate, elesclomol, we retain all rights to each of our drug candidates and programs, across all geographic markets and therapeutic indications. We have entered into a partnership with GlaxoSmithKline, or GSK, for the joint development and commercialization of elesclomol.
Our Lead Drug Candidate, Elesclomol (formerly, STA-4783)
Our most advanced clinical-stage drug candidate, elesclomol, is a novel, injectable, small molecule compound that triggers apoptosis, or programmed cell death, in cancer cells, which we believe has potential for the treatment of a broad range of cancer types.
In September 2006, we announced positive results for elesclomol in combination with paclitaxel, a leading chemotherapeutic agent, in a double-blind, randomized, controlled, multicenter Phase 2b clinical trial in patients with stage IV metastatic melanoma. We believe that this is the first blinded clinical trial of a drug candidate for the treatment of metastatic melanoma in 30 years to meet its primary endpoint with statistical significance. In November 2006, we received Fast Track designation from the U.S. Food and Drug Administration, or FDA, for the development of elesclomol for the treatment of metastatic melanoma. In December 2007, we received orphan drug designation for elesclomol in this indication in the United States from the FDA. Orphan drug status is designed to encourage biotechnology and pharmaceutical companies to develop drugs for rare diseases affecting fewer than 200,000 people in the United States. Assuming that elesclomol is approved by the FDA, we will be entitled to seven years of market exclusivity for elesclomol for the treatment of patients with metastatic melanoma.
Based on the results of our Phase 2b trial, we initiated a global, pivotal Phase 3 clinical trial of elesclomol in metastatic melanoma, called the SYMMETRY trial, in the third quarter of 2007. The SYMMETRY trial is being conducted under the terms of a Special Protocol Assessment, or SPA, agreed to by the FDA. The SPA process provides for a written agreement between a clinical trial sponsor and the FDA that the proposed design and planned analyses of the clinical trial is sufficient to support regulatory approval of a drug candidate, unless public health concerns unrecognized at the time of the protocol assessment become evident. The SYMMETRY trial is enrolling patients with stage IV metastatic melanoma who have not received prior chemotherapy but who may have already been treated with non-chemotherapeutic agents, such as biologics. Approximately 630 patients will be enrolled in the blinded, randomized, controlled study, which generally mirrors the design of our Phase 2b trial and will be conducted at approximately 150 centers worldwide.
As with our prior Phase 2b trial, patients enrolled in the SYMMETRY trial will be randomized to receive either elesclomol plus paclitaxel or paclitaxel alone. The dosage of each agent, the dosing schedule, and the primary endpoint—progression free survival, or PFS—are the same as in our prior Phase 2b trial. The SYMMETRY trial increases the total number of patients enrolled from the prior Phase 2b trial and includes central review of radiology scans, stratification to ensure balance between
1
treatment and control arms, and a no-crossover design for facilitating the assessment of overall survival, or OS.
Based on our current enrollment projections and event rate targets, we expect to complete enrollment and initiate the primary endpoint analysis of the SYMMETRY trial by the end of 2008. Assuming that the results of the PFS analysis are positive, we plan to submit a new drug application, or NDA, to the FDA in the first half of 2009. If actual enrollment or event rates differ from our current projections, our target dates for completing the PFS analysis and submitting the NDA will likely change.
In October 2007, we entered into a collaborative development, commercialization and license agreement with GSK for elesclomol (hereinafter referred to as the GSK Agreement), under which we are eligible to receive up to $1.01 billion in milestones and other payments, as well as share 40-50% of the profits and losses from sales in the United States and receive double-digit tiered royalties from sales outside of the United States. Under the terms of the GSK Agreement, the companies will jointly develop and commercialize elesclomol in the United States, and GSK will have exclusive responsibility for the development and commercialization of elesclomol outside the United States. Pursuant to the agreement, we received a non-refundable upfront cash payment of $80 million in November 2007. We are also eligible to receive potential pre-commercial milestone payments from GSK of up to $585 million, which include both payments for operational progress, such as trial initiation and enrollment, and payments for positive clinical and regulatory outcomes, such as regulatory approval. In addition, we are eligible to receive up to $300 million in potential commercial milestone payments from GSK based on achieving certain net sales thresholds.
�� Our Phase 2b clinical trial of elesclomol enrolled a total of 81 metastatic melanoma patients at 21 centers in the United States. This clinical trial was conducted in a double-blind, randomized, controlled fashion and compared the effects of elesclomol in combination with paclitaxel, a widely used chemotherapy, versus paclitaxel alone. The primary endpoint for assessing efficacy was PFS. PFS is calculated for each patient by measuring the time from the patient's assignment to a treatment group in the trial until a PFS event, which is the earlier of tumor progression or death. In published guidelines and actions related to clinical trials conducted by other companies, the FDA has previously indicated PFS is an acceptable endpoint for registration in metastatic melanoma and other cancer types.
In our Phase 2b trial, elesclomol plus paclitaxel demonstrated a statistically significant improvement in PFS compared to treatment with paclitaxel alone. In the intent-to-treat analysis, which includes all 81 patients, median PFS increased from 1.8 months for patients treated with paclitaxel alone to 3.7 months for patients treated with elesclomol plus paclitaxel. The percentage of patients who survived and were free of tumor progression at six months more than doubled from 15% for patients treated with paclitaxel alone to 35% for patients treated with elesclomol plus paclitaxel. The statistical significance of the improvement in PFS is described by aP-value, which measures the probability that the difference is due to chance alone. AP-value of less than 0.05 is considered statistically significant and unlikely due to chance. TheP-value in this analysis was 0.035.
In the per-protocol analysis of the trial results, which includes the 77 patients who could be evaluated for efficacy as specified in the trial protocol, median PFS increased from 1.8 months for patients treated with paclitaxel alone to 4.4 months for patients treated with elesclomol plus paclitaxel. The percentage of patients who survived and were free of tumor progression at six months more than doubled from 15% for patients treated with paclitaxel alone to 37% for patients treated with elesclomol plus paclitaxel. TheP-value in this analysis was 0.017.
A recently published meta-analysis by Korn et al. of 42 clinical trials incorporating 2,100 patients with stage IV metastatic melanoma showed a median PFS of 1.7 months and a six month PFS rate of
2
14.5%. Results for patients in the control arm of our Phase 2b trial—a median PFS of 1.8 months and a six month PFS rate of 15%—are consistent with the Korn et al. data and other historical data.
In addition to a statistically significant result for the primary endpoint, PFS, we observed a positive trend for the secondary endpoint, tumor response rate, which measures the percentage of patients who have experienced a substantial decrease in tumor size as defined by the industry standard Response Evaluation Criteria in Solid Tumors, or RECIST, criteria. Patients who received elesclomol plus paclitaxel showed a 15% tumor response rate, versus a 4% tumor response rate for patients who received paclitaxel alone. While the positive trend in the secondary endpoint for this trial was encouraging, it did not reach statistical significance. Our Phase 2b trial did not include a sufficient number of patients to detect this level of difference with statistical significance. In contrast, our Phase 3 trial will enroll a sufficient number of patients to detect this level of difference with statistical significance.
Our Phase 2b trial also included a planned analysis of OS, measuring the time from each patient's random treatment assignment until death from any cause. However, at the time that we performed this analysis, most patients were still alive and as a consequence, the results we obtained were not meaningful. After concluding the planned study, we filed a protocol amendment permitting collection of further OS data. We analyzed these data after those patients not known to have died had been followed for more than two years. The results of this further analysis demonstrated a median OS of 11.9 months for patients randomly assigned to elesclomol plus paclitaxel versus 7.8 months for patients randomly assigned to paclitaxel alone. As with the increased tumor response rate, the improvement in median OS was encouraging, but did not achieve statistical significance. Our Phase 2b trial did not have sufficient numbers of patients to detect this level of difference with statistical significance. In contrast, our Phase 3 trial has been designed to have sufficient statistical power to detect a difference from nine months to 12 months in OS.
In addition to an encouraging OS difference between the two arms of our Phase 2b trial, we believe that the 11.9 month median OS result and the one year OS rate of 49% in the patients who received elesclomol plus paclitaxel compare favorably with survival data from melanoma trials reported by others. As described in a 2006 paper by Tarhini and Agarwala, prior clinical trials in a similar patient population have shown median OS of six to nine months, and no current therapy has shown an OS benefit. The Korn et al. publication reported a median OS of 6.2 months and a one year survival rate of 25.5%.
Our Phase 2b trial included both first-line patients, those that had not received prior chemotherapy, and second-line patients, those that had received one prior chemotherapy regimen. We explored the effect on PFS in those two subgroups. While we saw a PFS benefit from treatment with elesclomol in both groups of patients, the benefit was greater in the first-line group. The second-line patient population (N=49) experienced an improvement in median PFS from 1.8 months to 2.6 months; the first-line patient population (N=32) experienced an improvement in median PFS from 1.8 months to 7.1 months. While these subset analyses are based on a smaller number of patients than the overall trial, the pronounced benefit in the first-line population did achieve statistical significance, with aP-value of 0.02. Together with our medical advisors, we decided to conduct the Phase 3 trial in the first-line patient population, which we believe is the most likely to show the greatest benefit from treatment with elesclomol.
We submitted the initial investigational new drug application, or IND, for elesclomol in September 2002. Including the patients treated in the Phase 2b metastatic melanoma clinical trial, we have treated a total of approximately 300 patients at over 50 medical centers in the United States and Canada with elesclomol. Elesclomol has been well tolerated, with toxicities of the elesclomol plus paclitaxel combination generally similar to those of paclitaxel alone, and the incidences of individual severe adverse events generally less than 10%.
3
Our Other Drug Candidates and Research Programs
Elesclomol was discovered and developed internally by our scientists, using our chemical compound library and our biology, chemistry, and pharmaceutical development capabilities. In addition to elesclomol, we have discovered and developed three other drug candidates currently in clinical or preclinical development, each of which has a distinct chemical structure, mechanism of action, and market opportunity. We also have one program in the lead optimization stage of discovery and other programs in earlier stages of discovery.
STA-9090. STA-9090 is a novel, injectable, small molecule drug candidate we are developing for the treatment of cancer. STA-9090 inhibits heat shock protein 90, or Hsp90, a chaperone protein that regulates the activity of numerous signaling proteins that trigger uncontrolled proliferation in cancer cells, in particular kinase proteins. Examples of kinase proteins include c-Kit, Bcr-Abl, Her2, EGFR, and others that are the targets of approved direct kinase inhibitors such as Gleevec, Herceptin, Tarceva, and Erbitux. We believe that inhibiting kinases indirectly, by disrupting the chaperone activities of Hsp90, provides two advantages: first, a means to simultaneously attack multiple cancer-promoting kinases; and, second, an ability to kill tumor cells with mutated kinases that have lost responsiveness to a direct kinase inhibitor. We have shown in preclinical experiments that STA-9090 is significantly more potent against certain types of cancer cells than Gleevec, as well as the two Hsp90 inhibitors furthest along in clinical development, 17-AAG and 17-DMAG. STA-9090 is further differentiated from these Hsp90 inhibitors because it is a novel chemical structure that is not a derivative or analog of the natural product geldanamycin. We believe that this creates a distinct activity profile for STA-9090 and is a competitive advantage. We are currently conducting two Phase 1 studies to identify the maximum tolerated dose of STA-9090 based on once- and twice-a-week intravenous dosing schedules, respectively. In addition to an evaluation of safety and tolerability, patients in these studies will be assessed for biological activity based on biomarker responses and clinical response rates based on the RECIST criteria. We intend to initiate a third STA-9090 Phase 1 trial in hematologic cancers in the second half of 2008.
STA-9584. STA-9584 is a novel, injectable, small molecule compound that disrupts the blood vessels that supply tumors with oxygen and essential nutrients. In preclinical experiments, STA-9584 has shown strong anti-tumor activity in a broad range of cancer models, including prostate, lung, breast, melanoma, and lymphoma. In preclinical testing, STA-9584 has been shown to act against established tumor vessels, a mechanism that is differentiated from the mechanism of anti-angiogenesis inhibitors such as Avastin, which prevents the formation of new tumor vessels. This program is currently in preclinical development.
Apilimod (STA-5326). Apilimod is a novel, orally administered, small molecule drug candidate we are developing for the treatment of autoimmune and other chronic inflammatory diseases. Apilimod inhibits the production of the cytokines interleukin-12, or IL-12, and interleukin-23, or IL-23, and thereby down-regulates the inflammation pathways that underlie certain autoimmune and inflammatory diseases. We submitted the initial IND for apilimod in March 2003. We are currently conducting a Phase 2a clinical trial of apilimod in patients with rheumatoid arthritis, or RA, and sponsoring a Phase 2a clinical trial in patients with gastrointestinal manifestations of common variable immunodeficiency, or CVID. Both the RA and CVID Phase 2a studies completed initial enrollment. Based on the data we have reviewed to date from the CVID trial and a strategic review of the apilimod program, we have decided to complete the ongoing CVID trial, but not to further pursue this indication for apilimod. The preliminary results of the first 22 patients in the RA trial showed encouraging biomarker and clinical signals suggesting activity of apilimod in this indication. We have
4
elected to enroll an additional cohort in the RA Phase 2a trial to explore a higher dose of apilimod. We expect to complete enrollment of this higher dose cohort in the second half of 2008.
CRAC ion channel inhibitor. We have developed novel, small molecule inhibitors of calcium release activated calcium, or CRAC, ion channels expressed on immune cells. The CRAC ion channel is the primary route for calcium entry into T cells and other immune cells, regulating multiple immune cell processes important for initiating and maintaining an inflammatory immune response. We have demonstrated in preclinical experiments that our CRAC ion channel inhibitors selectively inhibit the production of critical pro-inflammatory cytokines, such as interleukin-2, or IL-2, and TNFa by immune cells, and that these compounds are effective in multiple animal models of immune diseases, including models of arthritis. This program is in the lead optimization stage of discovery.
Our Drug Candidate Pipeline
The following table summarizes our most advanced drug candidates currently in clinical or preclinical development:
| | Product Candidate
| | Disease
| | Stage
| | Status
| | Worldwide
Commercial Rights
|
|---|
| Oncology | | Elesclomol (formerly STA-4783)
Oxidative stress inducer | | Metastatic melanoma | | Phase 2b | | Completed—met primary endpoint | | Synta and GSK share U.S. commercial rights
GSK has exclusive rights outside U.S. |
|
|
|
|
|
|
Phase 3 |
|
Expect to submit NDA 1H 2009 |
|
|
|
|
|
|
Additional cancers |
|
Phase 2 |
|
Expect to initiate in 2008 |
|
|
|
|
STA-9090
Hsp90 inhibitor |
|
Cancer |
|
Phase 1 |
|
Two Phase 1 trials ongoing |
|
Synta |
|
|
STA-9584
Vascular disrupting agent |
|
Cancer |
|
Preclinical development |
|
Ongoing |
|
Synta |
Inflammatory Diseases |
|
Apilimod (STA-5326) Oral IL-12/23 inhibitor |
|
Rheumatoid arthritis |
|
Phase 2a |
|
Expect to complete enrollment in 2H 2008 |
|
Synta |
|
|
Oral CRAC ion channel inhibitor |
|
Autoimmune diseases, transplant |
|
Lead optimization |
|
Ongoing |
|
Synta |
In the above table, lead optimization indicates that compounds have shown activity, selectivity, and efficacy inin vivo models, as well as an acceptable preliminary safety profile. These compounds are being optimized for potency, drug-like properties, and safety before entering into preclinical development. Preclinical development activities include manufacturing, formulation, and full toxicology studies in preparation for a Phase 1 clinical trial. Phase 1 indicates initial clinical safety testing and pharmacological profiling in healthy volunteers, with the exception that Phase 1 clinical trials in oncology are typically performed in patients with cancer. Phase 2 involves efficacy testing and continued safety testing in patients with a specific disease, and may include separate Phase 2a and Phase 2b clinical trials. Phase 2a clinical trials typically test the drug candidate in a small number of patients and are designed to provide early information on drug safety and efficacy. Phase 2b clinical trials typically involve larger numbers of patients and comparison with placebo, standard treatments, or other active comparators. Phase 3 indicates a confirmatory study of efficacy and safety in a larger patient population, and typically involves comparison with placebo, standard treatments, or other active comparators.
5
Oncology Programs
We have two clinical-stage programs and one preclinical-stage program in oncology:
- •
- Elesclomol. Our most advanced clinical-stage drug candidate, elesclomol, has achieved positive results in a double-blind, randomized, controlled, multicenter Phase 2b clinical trial in patients with stage IV metastatic melanoma. We are conducting the SYMMETRY trial, a global, pivotal Phase 3 clinical trial in metastatic melanoma, under the terms of an SPA agreement with the FDA. We have entered into a partnership with GSK to jointly develop and commercialize elesclomol.
- •
- STA-9090. STA-9090, our novel, small molecule Hsp90 inhibitor, is in two Phase 1 clinical trials.
- •
- STA-9584. STA-9584, our novel small molecule compound that disrupts the blood vessels that supply tumors with oxygen and essential nutrients, is in preclinical development.
Oncology Background
Cancers are diseases characterized by abnormal and uncontrolled cell growth and division, typically leading to tumor formation. As a tumor grows, it can directly disrupt organ function at its site of origin. In addition, cancer cells can also spread to other organs, such as the brain, bones and liver, by a process called metastasis. The growth of metastatic tumors at these new sites can disrupt the function of these other organs. There are many kinds of cancer, but all are characterized by uncontrolled growth of abnormal cells.
The World Health Organization estimates that more than 11 million people are diagnosed with cancer every year worldwide, and seven million people die from the disease annually. The American Cancer Society estimates that approximately 1.4 million people in the United States will be diagnosed with cancer in 2008, and approximately 566,000 people will die from the disease.
Anti-cancer agents are the second largest therapeutic class of pharmaceuticals in the world, with global sales of $34.6 billion in 2006.
Melanoma
Melanoma is the deadliest type of skin cancer and is the sixth most commonly diagnosed cancer in the United States. The National Cancer Institute has estimated that the prevalence of melanoma in the United States, or the number of patients alive who have been diagnosed with the disease, currently is more than 660,000. The American Cancer Society estimates that in 2008 the incidence, or number of newly diagnosed cases, of melanoma in the United States will be approximately 62,500, with 8,400 deaths from the disease. According to a December 2006 Datamonitor report, the incidence of melanoma has doubled every decade for the past 40 years, faster than any other cancer type, and is currently the fifth and sixth leading cause of global cancer mortality within males and females, respectively.
Melanoma is classified into four stages, which are based on well-defined criteria, including characteristics of the primary tumors, involvement of the regional lymph nodes, and the extent and location of metastases. When melanoma is discovered and treated in the early stages, where the cancer is confined to a local area, patients have a relatively high rate of survival. For example, stage I patients have a five-year survival rate of between 90 and 95%. Once melanoma has advanced to stage III, where the cancer has spread to the regional lymph nodes, or stage IV, where the cancer has spread to distant organs, the prognosis for patients is much worse, with five-year survival rates less than 20%. We are unaware of any reliable industry survey data specifically for the prevalence of metastatic melanoma in the United States or worldwide. Commonly used estimates assume that 5-10% of all patients diagnosed
6
have metastatic disease, which estimates the prevalence of metastatic melanoma at approximately 30,000 to 60,000 patients in the United States.
For early stage melanoma, surgical removal of the primary melanoma lesion is the standard of care. Surgical removal may also be performed to remove distant skin metastases, lymph nodes or other organs to which the cancer has spread. Sometimes interferon alpha-2b is administered to patients as an adjuvant to surgery to reduce the rate of disease relapse. This is the only drug approved by the FDA for use in such a role.
For metastatic melanoma, treatment options are limited. Single-agent chemotherapy has typically shown PFS of less than two months. Randomized trials comparing combination chemotherapy against single agent chemotherapy have shown significant toxicity with no significant improvement in survival. Dacarbazine, also known as DTIC, has been one of the most studied drugs in this setting, either alone or in combination, and is the only FDA-approved chemotherapy for the treatment of metastatic melanoma. However, when DTIC is used as a single agent, it has been shown to have limited clinical benefits. Various other single-agent chemotherapies such as temozolomide, fotemustine and oblimersen have been tested against or in combination with DTIC. Response rates from controlled studies have typically been between 6% to 25% with median time to progression/ PFS of 1.8 to 2.4 months. Immunotherapy with IL-2 has been approved by the FDA based on longer duration responses than typically observed with chemotherapy, but these responses occur only in a small subset of patients, and treatment with IL-2 is accompanied by severe toxicities. No agents other than DTIC or IL-2 have been approved by the FDA for the treatment of metastatic melanoma. Therefore, we believe there is an urgent need in metastatic melanoma for additional therapies demonstrating meaningful clinical benefit, favorable safety profiles, and broad patient applicability.
Taxanes
The class of drugs known as taxanes is the market-leading class of chemotherapeutic drugs, with over $2 billion in worldwide sales in 2005. Approved taxanes include Taxol, a formulation of paclitaxel first approved in 1992 and marketed by Bristol-Myers Squibb, which achieved peak sales of approximately $1.6 billion in 2000 before patent expiry; Taxotere (docetaxel), which is marketed by Sanofi-Aventis and had global sales of approximately 1.5 billion euros in 2006; Abraxane, a paclitaxel protein conjugate marketed by Abraxis Pharmaceutical Partners; and several generic versions of paclitaxel. Taxanes have shown efficacy across a wide range of cancer types and have been approved by the FDA for the treatment of prostate, ovarian, breast, and non-small cell lung cancers, as well as Kaposi's sarcoma. Additionally, we believe taxanes are prescribed off-label for other cancer types, including metastatic melanoma, head and neck, uterine, stomach, esophageal, and bladder. In metastatic melanoma, the response rate of single agent paclitaxel has been reported as less than 20%. A study published in 2002 inCancer Investigation showed that combining DTIC and paclitaxel for the treatment of metastatic melanoma was not superior to using either agent alone. Other anti-cancer agents that are sometimes added to taxanes in an attempt to improve efficacy include Paraplatin, a formulation of carboplatin marketed by Bristol-Myers Squibb. While in some cases the addition may increase treatment efficacy, carboplatin has been shown to add substantial toxicity. As a result, we believe there is a significant opportunity for agents that can enhance the anti-tumor effects of taxanes without adding undesirable side effects.
7
Our Lead Clinical Development Program—Elesclomol
Elesclomol is a novel, small molecule drug candidate that induces programmed cell death in a wide variety of cancer cell typesin vitro, and has demonstrated anti-cancer activity in a broad range of preclinical cancer models. We believe that the anti-cancer activity of elesclomol is due to its ability to directly increase oxidative stress, as measured by the level of reactive oxygen species, or ROS, inside cancer cells. Because cancer cells have an elevated level of oxidative stress relative to non-cancer cells, we believe that the increase in ROS induced by elesclomol causes cancer cells to exceed a breaking point that triggers tumor cell death, while causing minimal damage to normal cells. In our preclinical models, we have observed anti-cancer activity of elesclomol both as a single agent and in combination with widely used anti-cancer therapies, such as paclitaxel, docetaxel, gemcitabine, and rituximab.
We have completed six clinical trials with elesclomol in cancer patients, in which a total of approximately 300 patients have been treated at over 50 medical centers in the United States and Canada. Based on the positive results observed in our Phase 2b clinical trial in metastatic melanoma, we initiated a global, pivotal Phase 3 clinical trial in metastatic melanoma in the third quarter of 2007, the SYMMETRY trial. The protocol is being conducted under an SPA agreed to by the FDA. The SPA process may result in a written agreement between a clinical trial sponsor and the FDA that the design and planned analyses of the clinical trial will support regulatory approval, unless public health concerns unrecognized at the time of the protocol assessment become evident. However, the approval decision may be made on the basis of a number of factors, including the degree of clinical benefit, and the FDA is not obligated to approve elesclomol as a result of the SPA, even if the clinical outcome is positive.
Elesclomol has also received Fast Track designation from the FDA for the treatment of metastatic melanoma. The FDA grants Fast Track designation for drug candidates intended to treat serious or life threatening conditions and that demonstrate the potential to address unmet medical needs. Fast Track designation can facilitate the development of a drug candidate and expedite its review by allowing for more frequent and timely meetings with the FDA and submission of an NDA on a rolling basis. However, Fast Track designation does not alter the standards for approval of a drug candidate, including the need for clinical trials that demonstrate safety and efficacy, nor does it mean that the FDA will expedite approval of a drug candidate. In addition, Fast Track designation does not increase the likelihood of approval of a drug candidate.
In December 2007, we also received orphan drug designation for elesclomol for metastatic melanoma in the United States from the FDA. Orphan drug status is designed to encourage biotechnology and pharmaceutical companies to develop drugs for rare diseases affecting fewer than 200,000 people in the United States. Assuming that elesclomol is approved by the FDA, we will be entitled to seven years of market exclusivity for elesclomol for the treatment of patients with metastatic melanoma. In October 2007, we entered into a partnership with GSK for the joint development and commercialization of elesclomol.
Our Phase 2b Clinical Trial in Metastatic Melanoma
Our Phase 2b clinical trial enrolled a total of 81 metastatic melanoma patients at 21 centers in the United States. This clinical trial was conducted in a double-blind, randomized, controlled fashion and compared the effects of elesclomol in combination with paclitaxel, the most widely used taxane, versus paclitaxel alone. The primary endpoint for assessing efficacy was PFS. PFS is considered an acceptable endpoint for registration in metastatic melanoma and other cancer types, as supported by the current FDA draft guidance set forth inClinical Trial Endpoints for the Approval of Cancer Drugs and Biologics issued in April 2005, and by the EMEA guidance set forth in the draft of Appendix 1Methodological Considerations for Using Progression-Free Survival (PFS) as Primary Endpoint in Confirmatory Trials for
8
Registration issued in July 2006 to theGuideline on the Evaluation of Anti-cancer Medicinal Products in Man, which became effective in June 2006.
In September 2006, we presented the results from our Phase 2b clinical trial at the joint meeting of Perspectives in Melanoma X and the Third International Melanoma Research Congress, held in The Netherlands. Patients who received elesclomol plus paclitaxel showed a statistically significant improvement in PFS compared to those who received paclitaxel alone. Consistent with safety data for elesclomol gathered from other clinical trials, elesclomol was well tolerated in this clinical trial, with toxicities of the elesclomol plus paclitaxel combination generally similar to those of paclitaxel alone.
The primary objective of our Phase 2b clinical trial was to assess the efficacy in stage IV metastatic melanoma patients of once-weekly treatment of elesclomol plus paclitaxel versus paclitaxel alone, based on the endpoint of PFS. Secondary endpoints were objective response rate, duration of tumor responses, and studies of adverse events and laboratory abnormalities. Once-weekly treatments of elesclomol (213 mg/m2) plus paclitaxel (80 mg/m2) or paclitaxel alone (80 mg/m2) were delivered for three weeks, followed by one week with no treatment. Investigators were permitted to repeat these four-week cycles until disease progression. Tumor assessments were performed at baseline and every other cycle thereafter.
Disease progression and tumor response were defined based on industry standard RECIST criteria, which are the unified response assessment criteria agreed to by the World Health Organization, United States National Cancer Institute, and European Organisation for Research and Treatment of Cancer. RECIST defines disease progression and tumor response based on an assessment of target and non-target lesions. A 20% or greater increase in the sum of the greatest diameters in target lesions, or unequivocal progression in non-target lesions, or the appearance of a new lesion is defined as disease progression. A reduction in the sum of the diameters of at least 30% as compared to baseline is defined as a partial response, or PR. A complete disappearance of target and non-target lesions (and the normalization of any tumor markers) constitutes a complete response, or CR. Both PRs and CRs must be confirmed by repeat assessments at least four weeks after the PR or CR was first documented. A response assessment of stable disease indicates that a CR, a PR or disease progression has not occured at that timepoint. Non-progression refers to an assessment of CR, PR, or stable disease. Objective response rate is typically defined as the sum of PR and CR assessments.
In this clinical trial, we enrolled patients who had received up to one prior chemotherapy treatment. An unlimited number of prior immunotherapy treatments were also allowed, provided that a period of four weeks subsequent to the last treatment elapsed prior to trial entry. Patients with Eastern Cooperative Oncology Group, or ECOG, performance status greater than 2 were excluded, as were patients with any brain metastases. The ECOG performance status is a standard patient assessment tool used in determining the care of cancer patients. Patients with an ECOG score of 3 or 4 are significantly disabled by their disease and are often excluded from clinical trials.
Two-thirds of patients were assigned to treatment with elesclomol plus paclitaxel, with the remaining one-third of patients assigned to treatment with paclitaxel alone. We chose this 2:1 weighting ratio to contribute more productively to the safety database for elesclomol than an even randomization, while still allowing for a statistical comparison of treatment effects. Patients who progressed on paclitaxel alone were given the option to crossover to elesclomol plus paclitaxel and were then treated until further progression.
The intent-to-treat analysis, which includes all 81 randomly assigned patients, showed that patients assigned to elesclomol plus paclitaxel experienced a statistically significant increase in PFS, with a
9
P-value of 0.035. The median PFS in this analysis increased from 1.8 months for patients assigned to paclitaxel alone to 3.7 months for patients assigned to elesclomol plus paclitaxel. The percentage of patients who survived and were free of tumor progression at six months more than doubled from 15% for patients assigned to paclitaxel alone to 35% for patients assigned to elesclomol plus paclitaxel. The hazard ratio for PFS in this analysis was 0.58, indicating that patients assigned to elesclomol plus paclitaxel had a 42% reduction in the risk of disease progression or death relative to patients assigned to paclitaxel alone.
The objective response rate, counting complete and partial responses, was 15.1% for patients assigned to elesclomol plus paclitaxel versus 3.6% for patients assigned to paclitaxel alone (P-value=0.153). This result showed an encouraging trend but did not reach statistical significance. We were able to obtain complete progression data on only three of the nine patients that were responders in the trial, and as a result had insufficient data to perform an analysis on duration of response.
The table below summarizes the median PFS, the PFS at six months, the hazard ratio, and the objective response rates for the intent-to-treat population.
| |
| | Elesclomol + Paclitaxel
N=53
| | Paclitaxel alone
N=28
| | P-value(1)
| | Hazard ratio(2)
|
|---|
| Intent-to-treat | | PFS: | | | | | | 0.035 | | 0.583 |
| | analysis (N=81) | | • Median (months) | | 3.68 | | 1.84 | | | | |
| | | • At 6 months (% of patients) | | 35 | % | 15 | % | | | |
| | | Objective response rate(3) | | 15.1 | % | 3.6 | % | 0.153 | | |
- (1)
- P-value measures the probability that the difference is due to chance alone. AP-value of less than 0.05 is considered statistically significant and unlikely to be due to chance alone.
- (2)
- Hazard ratio is an estimate of comparative risk between the two treatment groups. A hazard ratio of 1 can be interpreted as no decrease in risk, while a hazard ratio of 0.58 can be thought of as a 42% reduction in risk of occurrence for the event as compared to the control group.
- (3)
- Objective response rate is defined as the sum of complete and partial tumor response rates, as assessed by RECIST.
The figure below shows the Kaplan-Meier plots of PFS in this clinical trial for the intent-to-treat population.

10
In the per-protocol analysis of the trial results, which includes the 77 patients who could be evaluated for efficacy as specified in the trial protocol, median PFS increased from 1.8 months for patients treated with paclitaxel alone to 4.4 months for patients treated with elesclomol plus paclitaxel. The percentage of patients who survived and were free of tumor progression at six months more than doubled from 15% for patients treated with paclitaxel alone to 37% for patients treated with elesclomol plus paclitaxel. TheP-value in this analysis was 0.017.
This Phase 2b trial also included a planned OS analysis, measuring the time from each patient's random treatment assignment until death from any cause. However, at the time that we performed this analysis, most patients were still alive and as a consequence, the results we obtained were not meaningful. After concluding the planned study, we filed a protocol amendment permitting collection of further OS data. We analyzed these data after those patients not known to have died had been followed for more than two years. The results of this further analysis demonstrated a median OS of 11.9 months for patients randomly assigned to elesclomol plus paclitaxel versus 7.8 months for patients randomly assigned to paclitaxel alone. As with the increased tumor response rate, the improvement in OS was encouraging, but did not achieve statistical significance. Our Phase 2b trial did not have sufficient numbers of patients to detect this level of difference with statistical significance.
As is common in Phase 2 trials focused on PFS, our Phase 2b trial used a crossover design, in which patients who were initially randomized to the paclitaxel control arm were eligible to crossover and receive elesclomol plus paclitaxel after their disease had progressed. As a result, the paclitaxel control arm of our study included both patients who eventually received elesclomol and patients who never received elesclomol. The crossover design makes it more difficult to compare in this trial OS in patients who received elesclomol with OS in patients who never received elesclomol. Therefore, we believe it is also helpful to consider survival times in studies reported in the medical literature. The 11.9 month median OS result and the one year OS rate of 49% in the patients who received elesclomol plus paclitaxel compare favorably with these historical data. As described in a 2006 paper by Tarhini and Agarwala, prior clinical trials in a similar patient population have shown median OS of six to nine months, and no current therapy has shown an OS benefit. The Korn et al. publication reported a median OS of 6.2 months and a one year survival rate of 25.5%. Our SYMMETRY Phase 3 trial does not employ a crossover design in order to provide a clear comparison of OS between the paclitaxel alone control arm and the elesclomol plus paclitaxel treatment arm.
Elesclomol was well tolerated in this clinical trial. As shown in the table below, the incidence of any specific high severity adverse event, as reported by investigators, was less than 10%. We believe this compares favorably with treatments for metastatic melanoma such as the CVD regimen (cisplatin, vinblastine, and DTIC) or the Dartmouth regimen (DTIC, cisplatin, carmustine, and tamoxifen) that have reported substantially greater incidences of high severity adverse events. The incidence of such events that occurred in 2% or more of the patients treated with elesclomol plus paclitaxel was as follows:
Grade 3 or Higher Adverse Events(1)(2)
| | Elesclomol + Paclitaxel (N=52)
| | Paclitaxel (N=28)
| |
|---|
| Neutropenia(3) | | 4(7.7 | %) | 0(0 | %) |
| Back pain | | 2(3.8 | %) | 2(7.1 | %) |
| Fatigue | | 2(3.8 | %) | 2(7.1 | %) |
| Neuropathy(4) | | 2(3.8 | %) | 1(3.6 | %) |
- (1)
- As specified in the clinical trial protocol, the patient population for evaluating safety includes only those patients who received at least one treatment with elesclomol plus paclitaxel or paclitaxel alone. This represents 80 of the total 81 patients enrolled in the trial.
11
- (2)
- Grade refers to the National Cancer Institute's Common Terminology Criteria, or CTC, for adverse events. The CTC are commonly used in cancer clinical trials and are based on a 5-point severity scale with the following classifications: mild=1, moderate=2, severe=3, life-threatening=4, and fatal=5.
- (3)
- Neutropenia is an abnormal decrease in a type of white blood cells.
- (4)
- Neuropathy is abnormal or diminished nerve sensation.
The adverse events seen across all severity grades in this clinical trial were typical of those expected from paclitaxel alone. The most common adverse events seen in the elesclomol plus paclitaxel group included fatigue, alopecia, constipation, nausea, hypoaesthesia, arthralgia, insomnia, diarrhea, and anemia.
Our Phase 2b trial included both patients who had received no prior chemotheraphy and patients who had received one prior regimen of chemotherapy, in order to help assess which group might benefit the most and help us design future clinical trials. In the analysis of these groups, we used the same definition for what type of treatment constitutes prior chemotherapy as is now being used in our Phase 3 trial. This definition was agreed to with the FDA in our SPA process. Although the prior chemotherapy subset analysis was performed post hoc and relies upon a relatively small number of patients, and must therefore be interpreted cautiously, we saw an especially pronounced benefit from treatment with elesclomol in the group that had not received any prior chemotherapy, also called the first-line or chemotherapy-naïve group. The median PFS more than tripled for first-line patients randomly assigned to elesclomol plus paclitaxel (N=24; 7.1 months) versus first-line patients randomly assigned to paclitaxel alone (N=8; 1.8 months). The hazard ratio describing the difference in PFS between the two groups was 0.315, denoting a 68.5% reduction in the risk of disease progression or death for first-line patients randomly assigned to elesclomol plus paclitaxel relative to first-line patients randomly assigned to paclitaxel alone. This difference had aP-value of .019. Based on the encouraging results for the first-line patient group, we have designed the SYMMETRY Phase 3 trial to enroll only first-line, chemotherapy-naïve metastatic melanoma patients.
The results are illustrated in the table below.
Prior chemotherapy treatment
| |
| | Elesclomol + Paclitaxel
| | Paclitaxel alone
|
|---|
None
(N=32) | | Median PFS | | 7.1 months | | 1.8 months |
| | | Median OS | | 15.9 months | | 10.0 months |
| | | Objective response rate | | 21%(5/24) | | 0%(0/8) |
One
(N=49) |
|
Median PFS |
|
2.8 months |
|
1.8 months |
| | | Median OS | | 9.0 months | | 7.8 months |
| | | Objective response rate | | 10%(3/29) | | 5%(1/20) |
We also observed that the results for treatment with paclitaxel alone in patients who have received no prior chemotherapy are comparable to results previously reported for patients treated with DTIC alone who had received no prior chemotherapy. For example, in a 771-patient, randomized clinical trial comparing treatment with DTIC versus DTIC plus oblimersen in patients with no prior chemotherapy, which was published in theJournal of Clinical Oncology in October 2006, the median PFS in patients who were treated with DTIC alone was 1.6 months.
12
Results From the Lead-in, Phase 2a Stage of the Trial
This clinical trial employed a two-stage, lead-in design, with an open-label, single-arm Phase 2a stage prior to the commencement of the blinded, randomized, controlled Phase 2b stage. The objective of the Phase 2a stage was to evaluate the safety of elesclomol plus paclitaxel, determine the recommended dose level, and to assess whether it demonstrated sufficient activity to warrant further study. A total of 31 patients were enrolled in this stage, of which 28 were treated at what was determined to be the elesclomol recommended dose level (213 mg/m2). Of these 28 patients, four achieved an objective response as assessed by RECIST, and an additional 11 achieved stable disease, for a total non-progression rate of 15 out of 28 (54%). This met the pre-specified efficacy criteria, supporting the decision to proceed with enrolling the 81 additional patients for the Phase 2b stage of the trial. The addition of elesclomol to paclitaxel was well tolerated on the weekly schedule. Median PFS was 5.2 months and median OS was 13.4 months in the 28 patients that received the 213 mg/m2 dose level.
Our Phase 3 SYMMETRY Trial
Based on the results of our Phase 2b trial, in the third quarter of 2007, we initiated a global, pivotal Phase 3 clinical trial of elesclomol in first-line, stage IV melanoma patients called the SYMMETRY trial. The SYMMETRY trial is being conducted under the terms of an SPA agreement with the FDA. The SPA process may result in a written agreement between a clinical trial sponsor and the FDA that the design and planned analyses of the clinical trial is sufficient to support regulatory approval. The agreement is binding on the FDA unless public health concerns that were not recognized at the time of the protocol assessment become evident. However, the FDA is not obligated to approve elesclomol as a result of the SPA, even if the clinical outcome is positive. The SYMMETRY trial is enrolling patients with stage IV metastatic melanoma who have not received prior chemotherapy but who may have already been treated with non-chemotherapeutic agents such as biologics. Approximately 630 patients will be enrolled in the blinded, randomized, controlled study, which will be conducted at approximately 150 centers worldwide. Patients will be randomized (1:1) to elesclomol (213 mg/m2) plus paclitaxel (80 mg/m2) or paclitaxel alone (80 mg/m2) and will receive three weekly treatments and one week without treatment per each four week cycle. If tolerated, treatment will continue until disease progression. Patients will be stratified according to lactate dehydrogenase, or LDH, levels (elevated or normal), M-grade status (Mla/b or Mlc), prior treatment history (zero or one prior regimen with biologics or other non-chemotherapies), and reason for discontinuation of prior treatment (disease progression or other). LDH is an enzyme that is normally present throughout the body, but blood levels of LDH become elevated when tissue damage occurs. Elevated LDH levels in melanoma patients are associated with a poorer disease prognosis and a decreased survival rate compared to normal LDH levels. Similarly, M-grade status is a measure of spread of disease and is considered to be a prognostic factor for OS in melanoma. By stratifying patients for these prognostic factors in addition to prior treatment history and reason for discontinuation, the Phase 3 trial design seeks to evenly balance patients with similar disease status across the treatment and control arms of the trial. Responses will be assessed using industry standard RECIST criteria at baseline and at a minimum every other cycle, with radiology scans being assessed by independent, blinded reviewers at a central site.
The control arm treatment, the combination arm treatment, the doses, the schedule, and the primary endpoint—PFS—are the same as in the Phase 2b trial. This trial increases the total number of patients enrolled from the prior trial and includes central review of radiology scans, stratification to ensure balance between treatment arms, and a no-crossover design for facilitating the assessment of OS. In addition, the SYMMETRY Phase 3 clinical trial is only enrolling patients who have not received prior chemotherapy, while the prior Phase 2b trial enrolled both chemotherapy-naïve patients as well as patients who received one prior treatment with chemotherapy.
13
There are two planned analyses for PFS, which is the primary endpoint of the SYMMETRY Phase 3 trial:
- •
- An interim analysis to assess safety and non-futility will be conducted by an independent Data Monitoring Committee.
- •
- The final analysis for PFS will be conducted after two criteria have been satisfied: a prespecified minimum number of PFS events, approximately 160 events, has occurred and full trial enrollment has been completed. At the time of the final analysis for PFS, a first interim analysis will also be performed for OS, a secondary endpoint.
Following the PFS analyses, two additional analyses for OS are planned in the SYMMETRY trial: a second interim analysis and a final OS analysis.
The SYMMETRY trial has been designed with at least 90% power to detect a statistically significant improvement in PFS, as well as 80% power to detect a difference in OS. Projections and powering assumptions are based on detecting an improvement of three to five months in PFS (hazard ratio 0.60), and nine to 12 months in OS (hazard ratio 0.75), respectively. These limits correspond to a minimum of approximately 160 PFS events and 390 OS events. Secondary endpoints in addition to OS include response rate, clinical benefit rate (defined as complete response, partial response, or stable disease at 24 weeks), and duration of response.
Additional Clinical Trial Results
We completed a Phase 1 clinical trial of elesclomol in combination with paclitaxel in October 2004. This clinical trial, which enrolled 35 patients, was designed to assess the safety, pharmacokinetics, and efficacy of elesclomol with paclitaxel in a broad cancer patient population. The combination of elesclomol plus paclitaxel was well tolerated, with minimal toxicity attributed to elesclomol at all doses tested. Partial response or stable disease was observed in several cancer types, including melanoma, ovarian, Kaposi's sarcoma, angiosarcoma, parotid gland adenocarcinoma, colorectal, pancreatic and paraganglioma. In some patients, these cancers had previously progressed to more advanced stages during treatment with paclitaxel alone.
Based on the promising signs of activity and safety results we observed in our Phase 1 clinical trial, we initiated Phase 2 clinical trials in malignant melanoma, soft tissue sarcoma, and non-small cell lung cancer. Together these trials have enrolled approximately 300 patients at over 50 medical centers throughout the United States and Canada. These trials were designed to assess response rates, non-progression rates, and PFS, and to further expand the safety database for elesclomol.
We completed a Phase 2 clinical trial of elesclomol in 84 patients with soft tissue sarcoma in 2005, the results of which were inconclusive. We designed this two-stage Phase 2 clinical trial to assess activity based on response rate and non-progression rate, or NPR. This clinical trial utilized a single-arm design. All patients received weekly treatments of the combination of paclitaxel (80 mg/m2) and elesclomol (213 mg/m2) for three weeks, followed by one week off-treatment. These four-week cycles were repeated until the earlier of disease progression, or a minimum of four months. We enrolled patients with soft tissue sarcoma who had failed at least one prior chemotherapy treatment. In the first stage, 30 eligible patients were evaluated for objective response or disease stabilization after three months and met the predefined criteria for expansion of enrollment. Upon completion of the trial, the Kaplan-Meier estimate of NPR at three months was 35%, with a 95% confidence interval of between 24.3% and 45.8%. A recent publication by Van Glabbeke et al. proposed a criterion of NPR at three months >=40% to suggest drug activity in this indication. Given that the observed confidence interval includes 40%, this result did not definitively establish evidence of clinical activity or lack thereof. The observed safety profile of elesclomol plus paclitaxel was acceptable. Pending the results of our SYMMETRY Phase 3 trial of elesclomol in malignant melanoma and further investigation of different
14
drug combinations, we may consider future development of elesclomol in sarcoma, based on a different elesclomol dose, dosing schedule or drug combination regimen.
We completed a Phase 2 clinical trial of elesclomol in 103 patients with non-small cell lung cancer in 2005. We designed this two-stage trial to compare the effect of a standard first-line lung cancer combination therapy, paclitaxel and carboplatin, with the effect of this same combination therapy plus elesclomol. Patients included in this study were diagnosed with either stage IIIb or stage IV non-small cell lung cancer and had not received prior chemotherapy. The objective of the first stage, open-label portion was to determine the recommended dose for the second stage. In the second stage, patients were randomly assigned either to receive elesclomol plus paclitaxel and carboplatin, or to receive paclitaxel and carboplatin alone. Patients received one treatment of paclitaxel and carboplatin, with or without elesclomol, every three weeks. These three-week cycles were repeated until the earlier of disease progression or completion of six cycles. Efficacy was assessed using RECIST, and the primary endpoint in this clinical trial was time-to-progression. No improvement was observed in time-to-progression between elesclomol plus paclitaxel plus carboplatin, compared to paclitaxel plus carboplatin. In comparison to patients in our Phase 2b metastatic melanoma trial, patients in this clinical trial received both a less frequent dose of elesclomol (once every three weeks compared to once a week for three weeks), and a lower total dose of elesclomol during each monthly cycle (266 mg/m2 compared to 639 mg/m2). Pending the results of our SYMMETRY Phase 3 trial of elesclomol in malignant melanoma and further investigation of different drug combinations, we may consider future development of elesclomol in non-small cell lung cancer, based on a different elesclomol dose, dosing schedule or combination regimen.
Safety Results from all Clinical Trials to Date with Elesclomol
In order to assess the safety profile of elesclomol based on all of the clinical trials completed to date, we collected and integrated the adverse event data for all 352 subjects who participated in the six clinical trials conducted with elesclomol, including the Phase 2b melanoma trial.
Of the 352 subjects in these trials, 298 received the elesclomol plus paclitaxel combination. Of these 298 subjects, 239 received elesclomol in combination with paclitaxel, and 59 received elesclomol in combination with paclitaxel and carboplatin. All participating subjects suffered from solid tumor cancers.
The following table presents the most recent findings of grade 3 or higher adverse events across all clinical trials that were reported in³3% of subjects in the elesclomol plus paclitaxel treatment group.
Grade 3 or Higher Adverse Events
| | Elesclomol plus Paclitaxel
(N = 239)(1)
| | Paclitaxel Alone (N = 30)(2)
|
|---|
| Neutropenia | | 15 (6%) | | 0 |
| Anemia | | 8 (3%) | | 1 (3%) |
| DVT | | 8 (3%) | | 1 (3%) |
| Fatigue | | 8 (3%) | | 2 (7%) |
| Hyperglycemia | | 8 (3%) | | 1 (3%) |
| Dyspnea | | 7 (3%) | | 1 (3%) |
| Hypophosphatemia | | 7 (3%) | | 1 (3%) |
| Leukopenia | | 6 (3%) | | 0 |
| Extremity Pain | | 6 (3%) | | 0 |
- (1)
- Of the 239 patients, 224 received the same or higher dose of elesclomol plus paclitaxel as we used in the Phase 2b melanoma trial. Of these 224 patients, 201 patients were on the same once per
15
week schedule as in the Phase 2b melanoma trial and 23 patients were on a once every three week schedule.
- (2)
- Includes the 28 patients in the control arm of the Phase 2b melanoma trial.
Consistent with the results observed in our melanoma Phase 2b trial, there was a small increase in observations of neutropenia: 6% of elesclomol plus paclitaxel subjects versus 0% of the paclitaxel alone subjects. Frequencies of other grade 3 or higher adverse events were similar for the two treatment groups, and in some cases, occurred at slightly lower frequencies in the elesclomol plus paclitaxel group. In addition, we did not observe any clinically relevant trends in any of the other hematology, serum chemistry, or urinalysis testing on these patients.
Frequencies of adverse events of all grades of severity were comparable between the two groups. Types of adverse events that were reported as occurring in at least 20% of subjects who received elesclomol plus paclitaxel were as follows, for the combination and for paclitaxel alone, respectively: asthenic conditions (54% versus 53%), nausea and vomiting symptoms (44% versus 53%), alopecias (44% versus 53%), musculoskeletal and connective tissue signs and symptoms (36% versus 43%), edema (27% versus 20%), gastrointestinal atonic and hypomotility disorders (24% versus 30%), non-infective diarrhea (23% versus 17%), peripheral neuropathies (23% versus 23%), anemias (21% versus 20%), appetite disorders (21% versus 20%), joint related signs and symptoms (21% versus 10%), and coughing and associated symptoms (21% versus 27%). Asthenic conditions generally refers to lack of strength or weakness throughout or in a particular area of the body. Edema is swelling caused by fluid accumulation in bodily tissues. Gastrointestinal atonic and hypomotility disorders generally refer to muscle weakness and decreased movement, respectively, in the gastrointestinal tract. Anemia is the abnormal reduction in red blood cells.
We believe the integrated analysis of adverse event data from all 239 subjects who received the elesclomol plus paclitaxel combination shows that elesclomol plus paclitaxel was well tolerated and that the adverse events and laboratory results were similar to those expected for paclitaxel alone.
Elesclomol Mechanism of Action
Elesclomol is a novel, injectable small molecule that we believe rapidly and potently induces the generation of ROS in cancer cells, increasing the level of oxidative stress in cancer cell and ultimately leading to cancer cell death by apoptosis (programmed cell death).
ROS is a collective term used to describe chemical species that are produced as byproducts of normal oxygen metabolism and include superoxide, hydrogen peroxide, and the hydroxyl radical. In normal cells, ROS are produced at low levels and are effectively neutralized by the cells' antioxidant system. In contrast, cancer cells produce elevated levels of ROS due to their increased metabolic activity, resulting in oxidative stress. Sustained levels of ROS that exceed the cells' antioxidant capacity can readily induce cell death by apoptosis. We believe that oxidative stress is one of the most fundamental differences between cancer cells and normal cells, and that this difference causes cancer cells to be particularly vulnerable to agents that can selectively elevate ROS.
We believe the evidence that the primary mechanism of action of elesclomol is through induction of ROS is strong. This evidence includes:
- •
- Gene transcript profiles of cancer cells before versus after application of elesclomol show the characteristic signatures of an immediate, potent oxidative stress response. This response includes the rapid induction of heat shock protein genes such as heat shock protein 70, or Hsp70, metallothioneins, antioxidants, and other stress response genes.
- •
- Direct cellular measurements of specific ROS agents, such as hydrogen peroxide, show strong time-dependent and dose-dependent induction by elesclomol.
- •
- The effects of elesclomol are eliminated by applying antioxidants known to reduce ROS, or inhibitors that block the generation of ROS.
16
Once ROS levels in cancer cells exceed the breaking point, cell death occurs through apoptosis from the intrinsic mitochondrial pathway. Apoptotic cell death through the mitochondrial pathway involves the oxidation of cardiolipin, release of cytochrome c from the mitochondria, and activation of the caspase cascade. By increasing ROS and activating the intrinsic mitochondrial apoptosis pathway, we believe that in addition to inducing apoptosis as a single agent, elesclomol can enhance the anti-cancer activity of other chemotherapeutic agents that act through the same pathway. We have shown in preclinicalin vivo models that elesclomol significantly enhanced the anti-tumor activity of paclitaxel, rituximab, and gemcitabine, while adding minimal additional toxicity. These results have been demonstrated in a variety of animal models of cancer, including breast, lung, lymphoma, colorectal, cervical carcinoma and melanoma.
Our preclinical safety studies showed that the addition of elesclomol added little or no toxicity to that seen with paclitaxel alone, and that elesclomol has a relatively high therapeutic index, or margin between effective dose and toxic dose. We believe that the favorable safety profile that has been observed preclinically and clinically with elesclomol is due to the pronounced difference between cancer cells and normal cells in their respective ability to recover from such an increase in oxidative stress.
Elevated oxidative stress is one of the most fundamental features that differentiates cancer cells from normal cells. By taking advantage of this fundamental difference, we believe elesclomol offers the potential for a novel anti-cancer approach that is broadly effective across cancer types in conjunction with ROS-sensitive chemotherapeutics such as paclitaxel, while maintaining an attractive safety profile.
Additional Cancer Types for Future Clinical Development
Based on the activity seen in a broad range of tumor models in preclinical experiments, and our understanding of the mechanism of action, which is not specific to melanoma, we believe that elesclomol has the potential to treat many forms of cancer. We prioritize our clinical development plans based on a number of criteria, including scientific rationale and degree of unmet medical need. Based on these criteria, we believe there are several attractive opportunities for the further clinical development of elesclomol, including:
- •
- Cancers having elevated levels of ROS. We believe that cancer types having elevated levels of oxidative stress may be particularly susceptible to the increase in ROS caused by treatment with elesclomol. In addition to melanoma, other solid tumor cancer types known to have high levels of oxidative stress include breast, prostate, ovarian, and pancreatic. Hematologic cancers, such as leukemias, are also known to have elevated levels of ROS, and may represent attractive potential development opportunities.
- •
- Cancers in which we have observed signs of activity of elesclomol in our Phase 1 clinical trial. In our Phase 1 clinical trial, partial response or disease stabilization was observed in several cancer types, including melanoma, ovarian, Kaposi's sarcoma, angiosarcoma, parotid gland adenocarcinoma, colorectal, pancreatic and paraganglioma. In particular, one patient with a history of recurrent ovarian cancer had a documented partial response to treatment with elesclomol plus paclitaxel after having failed multiple prior chemotherapeutic regimens. This patient received a special protocol exception from the FDA in order to continue on elesclomol plus paclitaxel beyond the end of the clinical trial and received a total of eight cycles of treatment. We believe that these cancer types may warrant further exploration.
- •
- Adjuvant treatment of earlier-stage melanoma. Adjuvant therapy with interferon alfa-2b, an immunotherapy marketed as Intron A by Schering-Plough, is FDA-approved for use following surgical removal of melanoma to reduce the likelihood of disease recurrence. We believe the safety profile, the results from our Phase 2b trial in malignant melanoma, and the mechanism of action of elesclomol suggest exploring usage of elesclomol in earlier-stage melanoma patients.
17
We are evaluating these opportunities with our partner, GSK, and expect to announce plans to initiate Phase 2 clinical trials in one or more of these indications in 2008.
New Formulations
To date, except for a human bridging study utilizing the salt form of elesclomol, all of our clinical trials have been conducted using the first formulation of elesclomol that we developed, a free acid form. We intend to continue to use this formulation in our SYMMETRY Phase 3 clinical trial of elesclomol for metastatic melanoma, as well as for our initial commercial product if elesclomol is approved. The free acid form of elesclomol is a powder that is dissolved in the paclitaxel-Cremophor solution, diluted in a saline infusion bag and co-administered via the same infusion line. In order to use the free acid form of elesclomol with other oncology products, including taxanes other than paclitaxel, it must be dissolved in an organic solvent, such as Cremophor, that may cause additional toxicities due to the presence of the organic solvent.
We have developed a second, water-soluble form of elesclomol, a sodium salt formulation, that does not require dissolving with an organic solvent such as Cremophor. This sodium salt formulation may be more easily used with other taxanes and other oncology products that are formulated differently than paclitaxel, or potentially used as a single agent without need for an organic solvent. In 2005, we conducted a human bridging study using this salt form and observed pharmacokinetic equivalence between the salt and free acid forms of elesclomol. We intend to explore the use of this new salt form of elesclomol in future clinical trials both as a single agent, and in combination with other anti-cancer agents. We expect to begin clinical trials with this new salt form in the second half of 2008.
Other Oncology Programs
STA-9090 and Our Hsp90 Inhibitor Program
We are using our internal chemistry and drug optimization expertise in the area of heat shock proteins to develop novel synthetic small molecule inhibitors of Hsp90 for the treatment of cancer. STA-9090 is a novel chemical entity that selectively inhibits the activity of Hsp90. This program is currently in Phase 1 clinical development, with two Phase 1 trials ongoing to explore once- and twice-a-week dosing regimens, respectively. We intend to initiate a third STA-9090 Phase 1 trial in hematologic cancers in the second half of 2008.
Hsp90 is a chaperone protein that regulates the folding, stability, and function of numerous signaling proteins that trigger uncontrolled proliferation in cancer cells. Many of the proteins that require Hsp90 for their folding and activity are kinases that regulate tumor survival, proliferation, and angiogenesis. These include well-recognized cancer targets such as Bcr-Abl, Her2, EGFR, c-Kit, c-Met, Flt3, and BRAF, which are the targets of approved anti-cancer drugs such as Gleevec, Herceptin, Tarceva, and Erbitux, all of which are direct inhibitors of these kinase proteins. We believe that inhibiting kinases indirectly, by disrupting the chaperone activities of Hsp90, provides two advantages: first, a means to simultaneously attack multiple cancer-promoting kinases; and, second, an ability to kill tumor cells with mutated kinases that have lost responsiveness to direct kinase inhibitors. Furthermore, because cancer cells have far greater levels of active Hsp90 than normal cells, we believe that inhibitors of Hsp90 may selectively halt proliferation of tumor cells and thereby cause cancer cell death.
A number of companies have programs targeting inhibition of Hsp90 for the treatment of various forms of cancer. Based on results from experiments we conducted in both cell models and preclinical animal models, we believe that our lead compound, STA-9090, displays substantially higher potency than competing Hsp90 inhibitors in development. In addition to the higher potency of STA-9090 in certain cancer types, these experiments also demonstrated that STA-9090 may be active against cancer cell types for which other Hsp90 inhibitors have not shown activity. We believe these findings suggest a potential competitive advantage for STA-9090 in treating those cancers.
18
To our knowledge, the Hsp90 inhibitors that are furthest along in clinical development are 17-AAG, or tanespimycin, and 17-DMAG, or alvespimycin. These compounds are being developed by Kosan Biosciences for several cancer types including multiple myeloma, breast cancer, and melanoma. Recently, Kosan announced that it plans to discontinue development of alvespimycin in favor of tanespimycin. Both of these compounds are derivatives of the natural product, geldanamycin, and have been observed to have certain serious side effects, including liver toxicities. In contrast, STA-9090 is a novel small molecule compound that is not a geldanamycin derivative or analog. In addition, while 17-AAG and 17-DMAG have complex routes of synthesis, STA-9090 has a relatively simple route of synthesis.
In the figures below we illustrate what we believe are the two key potential advantages of our Hsp90 inhibitor, STA-9090: improved potency and the activity against cancers that have developed resistance to kinase inhibitors.
Improved potency. One of the several kinases that we have observed in preclinical testing to be more sensitive to STA-9090 than to other Hsp90 inhibitors is c-Kit. c-Kit plays a critical role in several cancer types including gastrointestinal stromal tumors, or GIST, acute myelogenous leukemia, or AML, and mastocytomas. The c-Kit gene is often mutated in cancers and can drive uncontrolled cancer cell proliferation. Inhibition of Hsp90 leads to the degradation and loss of c-Kit. In preclinical testing we have found that STA-9090 is more effective in causing the loss of c-Kit relative to other Hsp90 inhibitors such as 17-AAG and 17-DMAG. This loss of c-Kit leads to the death of those cancer types that depend upon c-Kit for their growth and survival. The figure below shows the result of anin vitro experiment we conducted comparing the activity of STA-9090 against human AML tumor cells with the two leading Hsp90 inhibitors, 17-AAG and 17-DMAG, and with the Bcr-Abl and c-Kit kinase inhibitor Gleevec. This figure shows that STA-9090 was 25-fold to 170-fold more effective in tumor cell killing than these other agents in this experiment, as measured by the IC50 (the dose that killed 50% of tumor cells).

Activity against cancers that develop resistance to kinase inhibitors. In patients who are treated for cancers with kinase inhibitors such as Gleevec, an initial period of responding to treatment can be followed by a relapse, in which the disease rapidly worsens and no longer responds to further treatment with that kinase inhibitor. This relapse is believed to be due to the appearance of new mutations in the target kinase. In contrast to direct kinase inhibitors, STA-9090 is an indirect kinase inhibitor that acts
19
by inhibiting Hsp90 rather than the kinases themselves. STA-9090 therefore has the potential to be effective in inhibiting both the original and the mutant kinases. The figure below illustrates this point. In anin vitro experiment, a tumor cell line with a Gleevec-resistant mutation in c-Kit is no longer killed by Gleevec. In contrast, STA-9090 demonstrates potent killing of these cells. This figure also shows that STA-9090 is substantially more potent than the competing Hsp90 inhibitors, 17-AAG or 17-DMAG, in this model, as with the previous model.

In addition to the activity shown in cancer cells in the figures above, we have shown that STA-9090 is more potent than 17-AAG in a range of additional cancer cell models as well as in multiple preclinical animal models of human cancer types including lung, prostate carcinoma, breast, gastric, melanoma, lymphoma, multiple myeloma, acute myelogenous leukemia, and chronic myeloid leukemia.
We believe that our preclinical data suggest the potential for using STA-9090 to treat patients whose cancers have relapsed following treatment with small molecule kinase inhibitors such as Gleevec, Sutent, or Tarceva. In addition, we believe that knowledge of which cancer-causing proteins are most susceptible to treatment with STA-9090 will help us to focus our clinical development on cancer types most likely to respond to treatment with our drug candidate.
STA-9584—Our Vascular Disrupting Agent
STA-9584 is a novel anti-cancer agent with a dual mechanism of action: STA-9584 disrupts the vessels feeding tumors, which can choke off the supply of oxygen and nutrients, and, in addition, STA-9584 directly causes tumor cell death by inhibiting microtubules, which are cellular structures that play an important role in cell division and proliferation. STA-9584 has demonstrated strong activity in a range of animal models of human tumors, including prostate, lung, breast, melanoma, and lymphoma. This program is in preclinical development.
Because rapidly growing cancer cells have a high demand for oxygen and nutrients, tumors cause new blood vessels to grow in order to supply those needs. Those new vessels differ from normal blood vessels in that they are fragile and weak, forming disorganized and tortuous networks. We believe that drugs that disrupt tumor vessels, or tumor vasculature, could therefore starve tumor cells of oxygen and nutrients, leading to the rapid death of these cells, including tumor cells resistant to other therapies. Vascular disruption contrasts with anti-angiogenic approaches, such as the proposed mechanism of action of approved cancer drugs such as Avastin, which inhibit the growth of new tumor blood vessels but are not believed to affect established tumor vasculature.
20
To our knowledge, of the drug candidates in the category of vascular disrupting agents, combretastatin is one of the most advanced in development. We believe the dual mechanism of action of STA-9584 represents an important difference from combretastatin, in that STA-9584 both disrupts tumor vasculature and directly kills tumor cells through inhibiting microtubules. Consistent with this dual mechanism, we have observed in our preclinical models that STA-9584 causes tumor cell death throughout the tumor, both at the tumor core and rim, whereas vascular disrupting agents such as combretastatin cause tumor cell death primarily at the core of tumors, where the demand for oxygen and nutrients is most pronounced.
We believe the high potency of STA-9584 and acceptable therapeutic index in our preclinical models make this compound a promising candidate for treatment of a wide range of solid-tumor cancers. An example of the potency of STA-9584 is shown in the figure below, in which STA-9584 leads to complete tumor elimination in a preclinical model of prostate cancer. In this preclinical study, PC-3 human prostate cancer cells were implanted subcutaneously into nude mice. Once tumors reached over 100 mm3 in size, mice were treated with a placebo control or STA-9584 by intravenous injection once per week. Three doses of STA-9584 caused the regression of tumors.

Inflammatory Disease Programs
We have the following two inflammatory disease programs in development:
- •
- Apilimod (STA-5326). Apilimod is our novel, orally administered, small molecule drug candidate that inhibits the production of the cytokines IL-12 and IL-23, which are believed to be important regulators of the biological processes underlying certain autoimmune and inflammatory diseases. We are currently conducting a Phase 2a clinical trial in patients with RA and sponsoring a Phase 2a clinical trial in patients with CVID. Both the RA and CVID Phase 2a studies completed initial enrollment. Based on the data we have reviewed to date from the CVID trial and a strategic review of the apilimod program, we have decided to complete the ongoing CVID trial, but not to further pursue this indication for apilimod. The preliminary results of the first 22 patients in the RA trial showed encouraging biomarker and clinical signals suggesting possible activity of apilimod in this indication. We have elected to enroll an additional cohort in the RA Phase 2a trial to explore a higher dose of apilimod. We expect to complete enrollment of this higher dose cohort in the second half of 2008.
- •
- CRAC ion channel inhibitors. We are developing inhibitors of CRAC, ion channels expressed on immune cells, for the treatment of autoimmune diseases, transplant rejection, asthma, and
21
Inflammatory Disease Background
Inflammatory diseases are typically caused by aberrant activity of the immune system. The immune system normally protects the body from injury and infection, but in autoimmune diseases it attacks and damages the body's own tissues. Major autoimmune diseases include rheumatoid arthritis, psoriasis, Crohn's disease, and multiple sclerosis. Together, these diseases afflict over seven million people in the United States and over 21 million people worldwide.
Despite the availability of numerous therapeutic options for these diseases, inflammatory diseases remain major causes of impairment of daily activities, reduced quality of life, significant disability, and sometimes death. Current therapeutic treatments for chronic inflammatory diseases have the potential to cause musculoskeletal, endocrinologic, neurologic, and metabolic side effects, which can limit their long-term use. The limitations of conventional treatments, together with a growing understanding of the pathogenesis of inflammatory diseases, have stimulated significant interest in the development of targeted immune modulators for the management of chronic inflammatory diseases.
Apilimod (STA-5326) and Our Oral IL-12/23 Inhibitor Program
We believe we have discovered the first oral, small molecule, selective inhibitors of the cytokines IL-12 and IL-23. The IL-12 cytokine is an important "master switch" that triggers the immune response of the T cell known as T helper type 1, or Th1. T cells play a critical role in the coordination of the body's immune response, and while Th1 cells are normally involved in the body's defense against intracellular attack by bacteria and other micro-organisms, an overactive Th1 response can lead to various autoimmune or inflammatory diseases including Crohn's disease, psoriasis, RA, multiple sclerosis, and CVID. The IL-23 cytokine is critical to the generation of the T cells which produce other pro-inflammatory proteins believed to be important to maintaining the immune response. We believe that the Phase 2 clinical trial results observed with anti-IL-12/23 antibody therapies validate the inhibition of IL-12/23 activity as a promising approach for the treatment of inflammatory and autoimmune diseases.
We have conducted or sponsored 11 Phase 1 and Phase 2 clinical trials with our lead compound, apilimod, also designated STA-5326, or its salt form, apilimod mesylate, also designated STA-5326m. Our blinded, randomized clinical trials for apiliomd in Crohn's disease and psoriasis did not achieve their primary endpoints, and the preliminary data we have seen from the open label Phase 2a CVID trial do not demonstrate a high degree of clinical benefit. Following a strategic review of this program, we decided not to pursue further development of apilimod in these indications at this time. Our biomarker study in RA showed promising signs of activity, and we have elected to enroll an additional cohort to explore a higher dose of apilimod.
We believe that the collective evidence from our trials and from trials with other agents that target IL-12 and IL-23 show that this mechanism represents a promising therapeutic approach. Based on our data, we believe that the pharmaceutical properties of our first-generation compound may not be optimal for treating these indications. Pending the results from our RA study, we may elect to pursue such indications in the future with other compounds that offer improved pharmaceutical properties.
RA is a chronic autoimmune disease that is primarily characterized by joint synovial inflammation that can lead to long-term joint damage, chronic pain, loss of function and disability. Over two million people suffer from the disease in the United States. We are currently conducting a randomized, placebo-controlled Phase 2a clinical trial of apilimod in RA patients with moderate to severe disease.
22
All patients in this clinical trial are to be treated with methotrexate, a commonly used drug to treat RA, in addition to receiving either apilimod or placebo. The primary endpoint of this trial is based on an assessment of markers of inflammation in joint tissue after four to eight weeks of treatment. We believe that tissue assessments will provide an objective measure that will allow conclusions regarding potential efficacy to be based on a smaller number of patients. The preliminary results of the first 22 patients in this trial showed encouraging biomarker and clinical signals suggesting activity of apilimod in this indication. We have elected to enroll an additional cohort in the RA Phase 2a trial to explore a higher dose of apilimod. We expect to complete enrollment of this additional cohort in the second half of 2008.
Psoriasis is a chronic, inflammatory skin disorder that is characterized by thickened, red areas of skin that are covered with scales. The area of skin affected can range from discrete, localized patches, to extensive areas of the body. The joints, nails, and mucous membranes may also be affected by the disease. Chronic plaque psoriasis is the most common form of psoriasis. This disease involves the formation of plaques, which are circular-to-oval, elevated, and often scaly skin lesions that contain swollen blood vessels and infiltrating immune cells. Affected areas are characterized by itching, swelling, and pain, all of which can impair daily activities and sleep.
We conducted two complementary Phase 2 clinical trials of apilimod for the treatment of moderate to severe chronic plaque psoriasis. In each of these trials patients were treated for 12 consecutive weeks. One psoriasis trial was an open-label Phase 2a clinical trial designed to assess the biological response to apilimod through histological studies of skin biopsies. While the data showed signs of activity, as assessed both histologically and clinically, strong clinical benefit was not demonstrated. Another psoriasis trial was a double-blind, randomized, placebo-controlled, multicenter Phase 2b clinical trial of 212 patients. Despite observing a difference between apilimod and placebo, the primary endpoint of the trial was not achieved, and the magnitude of clinical benefit did not warrant advancement into Phase 3 clinical trials at the doses and with the formulation tested.
Crohn's disease is a chronic inflammatory bowel disease characterized by inflammation at points throughout the length of the gastrointestinal, or digestive, tract. Symptoms can be severe and include abdominal pain, frequent diarrhea and intestinal bleeding. In addition, patients with Crohn's disease may experience malnutrition and an increased risk of colorectal cancer.
We initiated three Phase 2 clinical trials in moderate-to-severe Crohn's disease: a 73-patient Phase 2a clinical trial, a planned 282-patient Phase 2b clinical trial and a planned 12-patient biomarker trial. The Phase 2a clinical trial was an open-label, dose-escalating study to assess the safety, pharmacokinetics, and efficacy of apilimod. In this trial, a capsule formulation containing the free base form of apilimod was studied. Promising signs of activity were observed. In the Phase 2b study, we switched formulation to a tablet containing the mesylate form of apilimod. This Phase 2b study was a double-blind, randomized, placebo-controlled, multicenter clinical trial with two treatment arms and one placebo arm. As specified in the protocol, an interim analysis was performed after half the patients expected to be enrolled in the trial had completed treatment. This analysis indicated a low likelihood of achieving the primary endpoint in the trial, and thus, the Phase 2b and biomarker trials were terminated at that point.
CRAC Ion Channel Inhibitors
Ion channels have proven to be very attractive targets for small molecule drug development. Examples of successful ion channel modulating drugs include Norvasc, which is marketed by Pfizer for
23
the treatment of hypertension, and Ambien, which is marketed by Sanofi-Aventis for the treatment of insomnia. Ion channel modulators developed to date target channels on excitable cells, which are cells that transmit electrical signals, such as muscle cells and nerve cells, and have been primarily developed for treating cardiac or central nervous system conditions. While ion channels in excitable cells are involved in the electrical signaling of those cells, ion channels are also known to play an important role in the signaling pathways and function of certain non-excitable cell types, such as immune cells.
We are developing small molecule inhibitors of CRAC, ion channels expressed on immune cells. The CRAC ion channel is the primary route for calcium entry into T cells and mast cells. Calcium entry regulates multiple immune cell processes, including T cell proliferation and cytokine secretion, which are important for initiating and sustaining an inflammatory immune response. The relevance of inhibiting this biological pathway has been validated by the clinical and market success of the calcineurin inhibitors, cyclosporin and tacrolimus, in treating autoimmune diseases and transplant rejection. The calcineurin inhibitors, however, act on both immune and non-immune cell types and have substantial toxicities. By more selectively inhibiting the same biological pathway, therapies that inhibit CRAC ion channels offer the potential of modulating the immune system with fewer toxicities. Such therapies may hold promise for treating immune disorders such as RA, psoriasis, multiple sclerosis, transplant rejection, allergy, or asthma.
We have discovered a family of novel, small molecule, orally administered CRAC ion channel inhibitors that are both selective and highly potent. We have demonstrated in preclinical experiments that these compounds inhibit the production by immune cells of multiple critical pro-inflammatory cytokines, such as IL-1, IL-2, IL-6, and TNFa, which are critical to immune disorders such as RA and transplant rejection. We have also demonstrated that some of these compounds inhibit mast cell degranulation and the release of histamines, which is believed to be important for the treatment of allergy and asthma. We have shown that our compounds are effective in multiple animal models of immune diseases, including models of arthritis. This program is in the lead optimization stage.
Our Drug Discovery Capabilities
Our drug discovery approach is based on the close integration and rapid cycle times among our chemistry, biology, and pharmaceutical development groups. Drug candidates are typically identified using novel chemical structures from our chemical compound library in cell-based assays that are designed to preserve the complexity of biological signaling. Earlyin vivo testing and a rapid optimization process allow us to generate a high number of promising leads from our screening hits, improve the profiles of our compounds, and, in some cases, discover novel pathways or mechanisms of action with the potential to define entirely new categories of treatment.
Our approach integrates the following capabilities and resources:
- •
- Unique chemical compound library. Our chemical library contains over 100,000 small molecules and numerous plant extracts collected from universities, non-profit institutions, other organizations, and commercial sources. Many of our compounds are proprietary and not available from commercial sources. This library represents a diverse and distinct set of chemical structures that was not generated using combinatorial chemistry and continues to be a valuable source of lead compounds for drug discovery. We are continuing our compound collection efforts. In addition, for each of our discovery programs we build focused libraries dedicated to particular drug targets. We have modeled the three-dimensional structure of most of our compounds, allowing us to use computer-based, orin silico, screening to identify new drug candidates.
- •
- Broad set of screening assays. We have high throughput screening capabilities linked to our chemical library that facilitate the rapid identification of new drug candidates. We have developed a wide variety of biochemical and cell-basedin vitro assays designed to identify
24
promising compounds for treating cancer, immune disorders and other diseases, which form the basis of our initial screening efforts. In addition to assays for identifying new compounds, we have also developed assays we use for early optimization of safety and pharmacokinetic properties.
- •
- Robust in vivo testing capabilities. We have substantialin vivo testing facilities that we use for evaluating the safety, efficacy, and pharmaceutical properties of our compounds, including absorption, distribution, metabolism, elimination, and toxicology properties. These facilities are equipped for detailed experimental measurements and surgical tasks, such as the rodent microsurgery we use for sophisticated toxicology assessments. We have experience with a wide range of animal models of disease, including multiple models in cancer, inflammatory diseases and metabolic diseases. We believe the ability to complete early testing of compoundsin vivo, internally and without dependencies on third parties, is a valuable advantage in our ability to rapidly optimize the pharmaceutical properties of our most promising compounds.
- •
- Multi-functional chemistry capabilities. We possess a full range of chemistry capabilities, including medicinal chemistry, analytical chemistry, physical chemistry, process development and computational chemistry. Our approach to medicinal chemistry applies the rigorous exploration of permutations of biologically active molecular components to optimize lead compounds. Our in-house process development capability of characterizing and specifying manufacturing processes for our compounds allows us to reduce dependencies on third parties and is an important advantage in our ability to successfully commercialize our drug candidates.
- •
- Methods for novel target elucidation and validation. Our scientists use expression profiling, RNA interference, affinity purification, proteomics, electrophysiology, and other methods to identify the therapeutic intervention points of novel, promising compounds.
Manufacturing
Our drug candidates and preclinical compounds are small molecules that can be readily synthesized by processes that we have developed. Utilizing our medicinal chemistry and process development capabilities, we have developed manufacturing processes to produce the active pharmaceutical ingredient, or API, for our drug candidates. We also have the internal capability to synthesize small molecule compounds in quantities of up to several hundred grams for use in our preclinical studies, including proof-of-concept studies in animal models, early pharmacokinetic assays, initial toxicology studies, and formulation development. We currently contract with third parties for the synthesis of all materials used in our clinical trials and rely on third party manufacturers for the supply of our drug candidates in bulk quantities and for the production of suitable dosage forms.
The starting materials and reagents required for synthesizing our drug candidates and preclinical compounds are commercially available from multiple sources. We have established a quality control and quality assurance program, including a set of standard operating procedures, analytical methods, and specifications, designed to ensure that our drug candidates are manufactured in accordance with the FDA's current Good Manufacturing Practices, or cGMP, and other applicable domestic and foreign regulations. We have selected manufacturers that we believe comply with cGMP and other applicable regulatory standards. We do not currently expect to manufacture cGMP material internally for our clinical trials nor undertake the commercial scale manufacture of our drug candidates after approval. We are discussing with our current suppliers and other third party manufacturers the long-term supply and manufacture of these and other drug candidates we may develop.
We are currently working with two contract manufacturers to produce elesclomol in its free acid form, which is the API that is being used in the SYMMETRY Phase 3 clinical trial of elesclomol for
25
metastatic melanoma. We intend to use one of these manufacturers as the primary supplier of elesclomol API and the other as a backup API manufacturer for the SYMMETRY trial and other clinical trials of elesclomol that we may initiate. We have contracts with each of these manufacturers to produce elesclomol API in quantities we believe will be sufficient for our current clinical trial needs, and we believe that they have already successfully produced elesclomol API in the quantities and to the specifications needed for the SYMMETRY Phase 3 trial. If additional API is required and the primary manufacturer we choose to provide elesclomol API should become unavailable to us for any reason, we believe the backup manufacturer will be able to provide us with sufficient elesclomol API with little or no delays. If both of these manufacturers should become unavailable, we believe that there are a number of potential replacements, as our processes are not technically complex nor manufacturer-specific. However, we may incur some added cost and delay in identifying or qualifying such replacements, including delays associated with transferring the process to the new manufacturer and conducting API manufacturing runs.
We are using several different manufacturers for various process steps in the preparation of elesclomol drug product. Although we believe that most of these steps are routine and can be accomplished by other possible manufacturers, the powder filling step involves highly specialized processing, including the automated filling of vials with elesclomol API in a sterile environment. We believe that our selected manufacturer for this step may be one of a limited number of third party contract manufacturers currently capable of conducting this process on our behalf. We have entered into an agreement with this third-party manufacturer for the SYMMETRY Phase 3 clinical trial of elesclomol for metastatic melanoma and other manufacturing runs required for NDA submission to the FDA.
Under the terms of our agreement with GSK, GSK is responsible for commercial manufacturing of elesclomol API and drug product.
Sales and Marketing
We currently have limited marketing, sales or distribution capabilities. In order to commercialize any of our drug candidates, we must develop these capabilities internally or through collaboration with third parties. In selected therapeutic areas where we feel that any approved products can be commercialized by a specialty sales force that calls on a limited and focused group of physicians, we currently plan to participate in the commercialization of these drug candidates. In therapeutic areas that require a large sales force selling to a large and diverse prescribing population, we currently plan to partner our drug candidates for commercialization.
In our partnership with GSK, we have retained rights to co-commercialize and co-promote our lead oncology drug candidate, elesclomol, in the United States. While the primary diagnosing physicians for melanoma are dermatologists and primary care physicians, care of patients with metastatic melanoma is referred to oncologists, surgical oncologists and dermatological oncologists. In the United States, oncology is a highly concentrated specialty, with approximately 650 community cancer programs and oncology private practices and approximately 9,000 oncologists in private practice. We believe this concentration of target physicians can be effectively addressed by a relatively small specialty sales force.
We have begun to build the commercial infrastructure necessary to bring elesclomol to market in collaboration with our partner, GSK. In addition to a specialty sales force, sales management, internal sales support, and an internal marketing group, we will need to establish capabilities to manage key accounts, such as managed care organizations, group purchasing organizations, specialty pharmacies, and government accounts including Veterans Affairs and the Department of Defense. Outside the United States, GSK has exclusive rights to commercialize elesclomol.
26
Competition
The development and commercialization of new drugs is highly competitive. We will face competition with respect to all drug candidates we may develop or commercialize in the future from pharmaceutical and biotechnology companies worldwide. The key competitive factors affecting the success of any approved product will be its efficacy, safety profile, price, method of administration and level of promotional activity. The efficacy and safety profile of our drug candidates relative to competitors will depend upon the results of our clinical trials and experience with the approved product in the commercial marketplace.
Elesclomol. If approved for the treatment of metastatic melanoma, elesclomol may compete with:
- •
- Drugs that are approved by the FDA for the treatment of metastatic melanoma. Currently, in the United States, there are only two drugs approved for the treatment of metastatic melanoma: dacarbazine/DTIC and the injectable protein IL-2. In addition, interferon alfa-2b, also an injectable protein, is the only drug approved for use as an adjuvant to surgery to prevent relapse of melanoma.
- •
- Drugs that are not approved for the treatment of metastatic melanoma, but are used off-label either alone or in combination to treat the disease, including taxanes, temozolomide, vincristine, carmustine, melphalan, and platinum-chemotherapeutics, such as cisplatin and carboplatin.
- •
- Compounds in development for metastatic melanoma. Compounds in clinical development may be grouped into six categories: (1) the kinase inhibitors such as Nexavar, being developed by Bayer and Onyx; Sutent, being developed by Pfizer; and ispinesib, being developed by Cytokinetics and GSK; (2) the anti-CTLA-4 monoclonal antibodies, ipilimumab and tremelumimab; (3) the anti-integrin volociximab; (4) injectable angiogenesis inhibitors, such as Avastin; (5) cancer vaccines such as M-Vax and MDX-1379; and (6) derivatives, analogs, or reformulations of known chemotherapies, such as Abraxane, or other chemotherapies.
Apilimod. If approved, apilimod is expected to compete against the currently approved therapies for the treatment of chronic inflammatory diseases, including:
- •
- large-molecule, injectable TNF-antagonists, including: Remicade, marketed by Johnson & Johnson; Enbrel, marketed by Amgen and Wyeth Pharmaceuticals; and Humira, marketed by Abbott Laboratories; and
- •
- broadly immunosuppressive small molecule agents including corticosteroids and azathioprine.
Apilimod may also compete with CNTO-1275 currently in clinical trials and ABT-874 currently awaiting approval, two injectable antibody-based clinical candidates targeting IL-12 that are being developed by Johnson & Johnson and Abbott Laboratories, respectively. We expect that as an oral, small molecule drug, apilimod may prove competitive relative to current and future biologic therapies in manufacturing costs and convenience of administration. We are not aware of any orally administered, selective inhibitors of IL-12 production in clinical trials. Other novel, oral agents in development for inflammatory diseases represent potential competition to apilimod. These include chemokine inhibitors, oral fumarates, and calcineurin inhibitors.
STA-9090. If approved, STA-9090 may compete against the currently approved therapies for the treatment of cancers and other cancer treatments currently under development. In particular, STA-9090 may compete with 17-AAG, being developed by Kosan, and other agents that inhibit Hsp90, including Hsp90 inhibitors from Medimmune/Infinity, BiogenIdec, Novartis/Vernalis, Pfizer/Serenex, and Astex.
STA-9584. If approved, STA-9584 may compete with the currently approved therapies for the treatment of cancers, and other cancer treatments currently under development, including other vascular disrupting agents, such as ABT-751, being developed by Abbott Laboratories; AS1404, being
27
developed by Novartis/Antisoma; CA4P, being developed by Oxigene; EXEL-0999, being developed by Exelixis; and ZD6126, being developed by Angiogene.
Many of our potential competitors have substantially greater financial, technical, and personnel resources than us. In addition, many of these competitors have significantly greater commercial infrastructures. Our ability to compete successfully will depend largely on our ability to leverage our experience in drug discovery, development and commercialization to:
- •
- discover and develop medicines that are superior to other products in the market;
- •
- attract high-quality scientific, product development, and commercial personnel;
- •
- obtain patent and/or proprietary protection for our medicines and technologies;
- •
- obtain required regulatory approvals;
- •
- selectively commercialize certain drug candidates in indications treated by specialist physicians; and
- •
- selectively partner with pharmaceutical companies in the development and commercialization of certain drug candidates.
Patents and Proprietary Rights
Our success depends in part on our ability to obtain and maintain proprietary protection for our drug candidates, technology, and know-how, to operate without infringing on the proprietary rights of others, and to prevent others from infringing our proprietary rights. Our policy is to seek to protect our proprietary position by, among other methods, filing U.S. and foreign patent applications related to our proprietary technology, inventions, and improvements that are important to the development of our business. We also rely on trade secrets, know-how, continuing technological innovation, and in-licensing opportunities to develop and maintain our proprietary position.
As of March 14, 2008, our patent portfolio had a total of 602 patents and patent applications worldwide, including specific patent filings with claims to the composition-of-matter and methods of use of elesclomol and apilimod. We own or have exclusively licensed a total of 23 issued U.S. patents and 94 U.S. patent applications, as well as 485 foreign counterparts to these patents and patent applications. With respect to elesclomol, we have two issued U.S. patents that claim the chemical structure of elesclomol that expire no earlier than 2022. Both of these issued U.S. patents also claim related chemical structures, pharmaceutical compositions, and methods for treating a subject with cancer. In addition, we have filed several U.S. patent applications that have the potential to extend the patent life of elesclomol, including U.S. patent applications claiming aspects of the treatment regimen for metastatic melanoma which, if issued, would expire no earlier than 2026. We have also filed a U.S. patent application claiming the salt form of elesclomol which, if issued, would expire no earlier than 2025.
With respect to apilimod, we have two issued U.S. patents that claim the chemical structure of apilimod and methods for treating specific disorders using apilimod, respectively. These patents expire no earlier than 2021.
We have pending U.S. patent applications covering compositions-of-matter, methods of treatment and other aspects of our STA-9090, STA-9584 and our CRAC ion channel program. The patent term of our U.S. patents may potentially be extended under applicable law or regulations, such as the Patent Term Restoration Act. Counterpart filings to these patents and patent applications have been made in a number of other jurisdictions, including Europe and Japan.
We have also in-licensed various technologies to complement our ongoing clinical and research programs. These licenses generally extend for the term of the related patent and contain customary
28
royalty, termination, and other provisions. We have license agreements with Beth Israel Deaconess Medical Center and The Queen's Medical Center, Inc. that provide us with the exclusive commercial right to certain patent filings made by Beth Israel and Queen's Medical in the field of ion channels. We do not believe that these license agreements are currently material to our business. We have exclusive license rights to a patent filing made by Dana-Farber Cancer Institute covering combinations of ingredients that could potentially relate to our elesclomol/taxane combination therapy, should such patent claims issue. We would owe nominal royalty payments to Dana-Farber if any of the claims which ultimately issue under a patent or that are pending in an application from this patent filing cover a commercial product. We also have a non-exclusive license to a U.S. patent assigned to Columbia University that could potentially cover a possible aspect of the elesclomol mechanism. This license is not royalty bearing unless we include specific mechanism language on the label of any approved product, in which case a nominal royalty would be owed.
Government Regulation and Product Approval
Government authorities in the United States, at the federal, state and local level, and other countries extensively regulate, among other things, the research, development, testing, manufacture, labeling, packaging, promotion, storage, advertising, distribution, marketing and export and import of products such as those we are developing. Our drugs must be approved by the FDA through the NDA process before they may be legally marketed in the United States.
United States Government Regulation
In the United States, the FDA regulates drugs under the Federal Food, Drug and Cosmetic Act, or the FDCA, and implementing regulations. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to administrative or judicial sanctions. These sanctions could include:
- •
- the FDA's refusal to approve pending applications;
- •
- license suspension or revocation;
- •
- withdrawal of an approval;
- •
- a clinical hold;
- •
- warning letters;
- •
- product recalls;
- •
- product seizures;
- •
- total or partial suspension of production or distribution; or
- •
- injunctions, fines, civil penalties or criminal prosecution.
Any agency or judicial enforcement action could have a material adverse effect on us. The process of obtaining regulatory approvals and the subsequent substantial compliance with appropriate federal, state, local, and foreign statutes and regulations require the expenditure of substantial time and financial resources.
The process required by the FDA before a drug may be marketed in the United States generally involves the following:
- •
- completion of preclinical laboratory tests according to Good Laboratory Practices;
- •
- submission of an IND, which must become effective before human clinical trials may begin;
29
- •
- performance of adequate and well-controlled human clinical trials according to Good Clinical Practices to establish the safety and efficacy of the proposed drug for its intended use;
- •
- submission to the FDA of an NDA;
- •
- satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with current cGMP, to assure that the facilities, methods and controls are adequate to preserve the drug's identity, strength, quality and purity; and
- •
- FDA review and approval of the NDA.
Once a pharmaceutical candidate is identified for development, it enters the preclinical testing stage. Preclinical tests include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies. An IND sponsor must submit the results of the preclinical tests, together with manufacturing information and analytical data, to the FDA as part of the IND. Some preclinical or nonclinical testing may continue even after the IND is submitted. In addition to including the results of the preclinical studies, the IND will also include a protocol detailing, among other things, the objectives of the clinical trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated if the first phase lends itself to an efficacy determination. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, specifically places the sponsor on clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before clinical trials can begin.
All clinical trials must be conducted under the supervision of one or more qualified investigators in accordance with good clinical practice regulations. These regulations include the requirement that all research subjects provide informed consent. Further, an institutional review board, or IRB, at each institution participating in the clinical trial must review and approve the plan for any clinical trial before it commences at that institution. Each new clinical protocol must be submitted to the FDA as part of the IND. Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and more frequently if serious adverse events occur.
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
- •
- Phase 1. The drug is initially introduced into healthy human subjects or patients with the disease and tested for safety, dosage tolerance, pharmacokinetics, pharmacodynamics, absorption, metabolism, distribution and elimination. In the case of some products for severe or life-threatening diseases, especially when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients.
- •
- Phase 2. Involves studies in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage.
- •
- Phase 3. Clinical trials are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical study sites. These studies are intended to establish the overall risk-benefit ratio of the product and provide, if appropriate, an adequate basis for product labeling.
30
Phase 1, Phase 2, and Phase 3 testing may not be completed successfully within any specified period, if at all. The FDA or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. In addition, an IRB can suspend or terminate approval of a clinical trial at its institutions for several reasons, including if the clinical trial is not being conducted in accordance with the IRB's requirements or if the drug has been associated with unexpected serious harm to patients.
During the development of a new drug, sponsors are given an opportunity to meet with the FDA at certain points. These points are prior to submission of an IND, at the end of Phase 2, and before an NDA is submitted. These meetings can provide an opportunity for the sponsor to share information about the data gathered to date, for the FDA to provide advice, and for the sponsor and FDA to reach agreement on the next phase of development. Sponsors typically use the end of Phase 2 meeting to discuss their Phase 2 clinical results and present their plans for the pivotal Phase 3 clinical trial that they believe will support approval of the new drug. If a Phase 2 clinical trial is the subject of discussion at an end of Phase 2 meeting with the FDA, a sponsor may be able to request a SPA, the purpose of which is to reach agreement with the FDA on the design of the Phase 3 clinical trial protocol design and analysis that will form the primary basis of an efficacy claim. If such an agreement is reached, it will be documented and made part of the administrative record, and it will be binding on the FDA unless public health concerns unrecognized at the time of protocol assessment are evident, and may not be changed except under a few specific circumstances.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the drug and finalize a process for manufacturing the product in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the drug candidate and the manufacturer must develop methods for testing the quality, purity and potency of the final drugs. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the drug candidate does not undergo unacceptable deterioration over its shelf-life.
The results of product development, preclinical studies and clinical studies, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the drug, results of chemical studies and other relevant information are submitted to the FDA as part of an NDA requesting approval to market the product. The submission of an NDA is subject to the payment of user fees, but a waiver of such fees may be obtained under specified circumstances. The FDA reviews all NDAs submitted before it accepts them for filing. It may request additional information rather than accept a NDA for filing. In this event, the NDA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing.
Once the submission is accepted for filing, the FDA begins an in-depth review. The FDA may refuse to approve an NDA if the applicable regulatory criteria are not satisfied or may require additional clinical or other data. Even if such data are submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. The FDA reviews an NDA to determine, among other things, whether a product is safe and effective for its intended use and whether its manufacturing is cGMP-compliant to assure and preserve the product's identity, strength, quality and purity. Before approving an NDA, the FDA will inspect the facility or facilities where the product is manufactured and tested.
Satisfaction of FDA requirements or similar requirements of state, local and foreign regulatory authorities typically takes at least several years and the actual time required may vary substantially, based upon, among other things, the indication and the type, complexity and novelty of the product. Government regulation may delay or prevent marketing of potential products for a considerable period of time and impose costly procedures upon our activities. Success in early stage clinical trials does not assure success in later stage clinical trials. Data obtained from clinical activities are not always
31
conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all. Even if a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial application of the product. Further, even after regulatory approval is obtained, later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. Delays in obtaining, or failures to obtain, regulatory approvals for any drug candidate could substantially harm our business and cause our stock price to drop significantly. In addition, we cannot predict what adverse governmental regulations may arise from future U.S. or foreign governmental action.
The FDA has various programs, including Fast Track, priority review, and accelerated approval, that are intended to expedite or simplify the process for reviewing drugs, and/or provide for approval on the basis of surrogate endpoints. Even if a drug qualifies for one or more of these programs, we cannot be sure that the FDA will not later decide that the drug no longer meets the conditions for qualification or that the time period for FDA review or approval will be shortened. Generally, drugs that may be eligible for these programs are those for serious or life-threatening conditions, those with the potential to address unmet medical needs, and those that offer meaningful benefits over existing treatments. Fast Track designation applies to the combination of the product and the specific indication for which it is being studied. Although Fast Track and priority review do not affect the standards for approval, the FDA will attempt to facilitate early and frequent meetings with a sponsor of a Fast Track designated drug and expedite review of the application for a drug designated for priority review. Drugs that receive an accelerated approval may be approved on the basis of adequate and well-controlled clinical trials establishing that the drug product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit or on the basis of an effect on a clinical endpoint other than survival or irreversible morbidity. As a condition of approval, the FDA may require that a sponsor of a drug receiving accelerated approval perform post-marketing clinical trials. We have applied for and received Fast Track designation from the FDA for elesclomol for the treatment of metastatic melanoma. However, there can be no assurance that elesclomol will be reviewed or approved more expeditiously than would otherwise have been the case.
Depending upon the timing, duration and specifics of FDA approval of the use of our drugs, some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product's approval date. The patent term restoration period is generally one-half the time between the effective date of an IND, and the submission date of an NDA, plus the time between the submission date of an NDA and the approval of that application. Only one patent applicable to an approved drug is eligible for the extension and the extension must be applied for prior to expiration of the patent. The United States Patent and Trademark Office, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, we intend to apply for restorations of patent term for some of our currently owned or licensed patents to add patent life beyond their current expiration date, depending on the expected length of clinical trials and other factors involved in the submission of the relevant NDA.
32
Market exclusivity provisions under the FDCA also can delay the submission or the approval of certain applications. The FDCA provides a five-year period of non-patent marketing exclusivity within the United States to the first applicant to gain approval of an NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an abbreviated new drug application, or ANDA, or a 505(b)(2) NDA submitted by another company for another version of such drug where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement. The FDCA also provides three years of marketing exclusivity for an NDA, 505(b)(2) NDA or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example, for new indications, dosages, or strengths of an existing drug. This three-year exclusivity covers only the conditions associated with the new clinical investigations and does not prohibit the FDA from approving ANDAs for drugs containing the original active agent. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA; however, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the preclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
Under the Orphan Drug Act, the FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making available in the United States a drug for this type of disease or condition will be recovered from sales in the United States for that drug. Orphan drug designation must be requested before submitting an NDA. After the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the same drug for the same indication, except in very limited circumstances, for seven years. Orphan drug exclusivity, however, also could block the approval of one of our products for seven years if a competitor obtains approval of the same drug as defined by the FDA or if our drug candidate is determined to be contained within the competitor's product for the same indication or disease.
We have been granted orphan drug designation from the FDA for elesclomol for the treatment of metastatic melanoma and plan to apply for orphan drug designation for other elesclomol indications and for other drug candidates that meet the criteria for orphan designation. We may not be awarded orphan drug status for elesclomol in indications other than melanoma or for any of our other drug candidates or indications. In addition, obtaining FDA approval to market a product with orphan drug exclusivity may not provide us with a material commercial advantage.
Section 505A of the FDCA, as amended by the FDA Amendments Act of 2007, permits certain drugs to obtain an additional six months of exclusivity, if the sponsor submits information requested in writing by the FDA, or a Written Request, relating to the use of the drug in children. The FDA may not issue a Written Request for studies on unapproved or approved indications or where it determines
33
that information relating to the use of a drug in a pediatric population, or part of the pediatric population, may not produce health benefits in that population.
We have not requested or received a Written Request for such pediatric studies, although we may ask the FDA to issue a Written Request for such studies in the future. To receive the six-month pediatric market exclusivity, we would have to receive a Written Request from the FDA, conduct the requested studies in accordance with a written agreement with the FDA or, if there is no written agreement, in accordance with commonly accepted scientific principles, and submit reports of the studies. The FDA will accept the reports upon its determination that the studies were conducted in accordance with and are responsive to the original Written Request or commonly accepted scientific principles, as appropriate, and that the reports comply with the FDA's filing requirements. The FDA may not issue a Written Request for such studies or accept the reports of the studies.
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory standards is not maintained or if problems occur after the product reaches the market. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further FDA review and approval. In addition, the FDA may require testing and surveillance programs to monitor the effect of approved products that have been commercialized, and the FDA has the power to prevent or limit further marketing of a product based on the results of these post-marketing programs.
Any drug products manufactured or distributed by us pursuant to FDA approvals are subject to continuing regulation by the FDA, including, among other things:
- •
- record-keeping requirements;
- •
- reporting of adverse experiences with the drug;
- •
- providing the FDA with updated safety and efficacy information;
- •
- drug sampling and distribution requirements;
- •
- notifying the FDA and gaining its approval of specified manufacturing or labeling changes;
- •
- complying with certain electronic records and signature requirements; and
- •
- complying with FDA promotion and advertising requirements.
Drug manufacturers and their subcontractors are required to register their establishments with the FDA and some state agencies, and are subject to periodic unannounced inspections by the FDA and some state agencies for compliance with cGMP and other laws.
We rely, and expect to continue to rely, on third parties for the production of clinical and commercial quantities of our products. Future FDA and state inspections may identify compliance issues at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to correct.
From time to time, legislation is drafted, introduced and passed in Congress that could significantly change the statutory provisions governing the approval, manufacturing and marketing of products regulated by the FDA. In addition, FDA regulations and guidance are often revised or reinterpreted by the agency in ways that may significantly affect our business and our products. It is impossible to predict whether legislative changes will be enacted, or FDA regulations, guidance or interpretations changed or what the impact of such changes, if any, may be.
34
Foreign Regulation
In addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we must obtain approval by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country.
Under European Union regulatory systems, we may submit marketing authorization applications either under a centralized or decentralized procedure. The centralized procedure, which is compulsory for medicines produced by biotechnology or those medicines intended to treat AIDS, cancer, neurodegenerative disorders, or diabetes and optional for those medicines which are highly innovative, provides for the grant of a single marketing authorization that is valid for all European Union member states. The decentralized procedure provides for mutual recognition of national approval decisions. Under this procedure, the holder of a national marketing authorization may submit an application to the remaining member states. Within 90 days of receiving the applications and assessments report each member state must decide whether to recognize approval. If a member state does not recognize the marketing authorization, the disputed points are eventually referred to the European Commission, whose decision is binding on all member states.
As in the United States, we may apply for designation of a product as an orphan drug for the treatment of a specific indication in the European Union before the application for marketing authorization is made. Orphan drugs in Europe enjoy economic and marketing benefits, including up to 10 years of market exclusivity for the approved indication unless another applicant can show that its product is safer, more effective or otherwise clinically superior to the orphan-designated product.
Reimbursement
Sales of pharmaceutical products depend in significant part on the availability of third-party reimbursement. Third-party payors include government health administrative authorities, managed care providers, private health insurers and other organizations. We anticipate third-party payors will provide reimbursement for our products. However, these third-party payors are increasingly challenging the price and examining the cost-effectiveness of medical products and services. In addition, significant uncertainty exists as to the reimbursement status of newly approved healthcare products. We may need to conduct expensive pharmacoeconomic studies in order to demonstrate the cost-effectiveness of our products. Our product candidates may not be considered cost-effective. It is time consuming and expensive for us to seek reimbursement from third-party payors. Reimbursement may not be available or sufficient to allow us to sell our products on a competitive and profitable basis.
The passage of the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or the MMA, imposes new requirements for the distribution and pricing of prescription drugs for Medicare beneficiaries, and includes a major expansion of the prescription drug benefit under a new Medicare Part D. Medicare Part D went into effect on January 1, 2006. Under Part D, Medicare beneficiaries may enroll in prescription drug plans offered by private entities which will provide coverage of outpatient prescription drugs. Part D plans include both stand-alone prescription drug benefit plans and prescription drug coverage as a supplement to Medicare Advantage plans. Unlike Medicare Part A and B, Part D coverage is not standardized. Part D prescription drug plan sponsors are not required to pay for all covered Part D drugs, and each drug plan can develop its own drug formulary that identifies which drugs it will cover and at what tier or level. However, Part D prescription drug formularies must include drugs within each therapeutic category and class of covered Part D drugs, though not necessarily all the drugs in each category or class. Any formulary used by a
35
Part D prescription drug plan must be developed and reviewed by a pharmacy and therapeutic committee.
It is not clear what effect the MMA will have on the prices paid for currently approved drugs and the pricing options for new drugs approved after January 1, 2006. Government payment for some of the costs of prescription drugs may increase demand for products for which we receive marketing approval. However, any negotiated prices for our products covered by a Part D prescription drug plan will likely be lower than the prices we might otherwise obtain. Moreover, while the MMA applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own payment rates. Any reduction in payment that results from the MMA may result in a similar reduction in payments from non-governmental payors.
We expect that there will continue to be a number of federal and state proposals to implement governmental pricing controls and limit the growth of healthcare costs, including the cost of prescription drugs. At the present time, Medicare is prohibited from negotiating directly with pharmaceutical companies for drugs. However, Congress is currently considering passing legislation that would lift the ban on federal negotiations. While we cannot predict whether such legislative or regulatory proposals will be adopted, the adoption of such proposals could have a material adverse effect on our business, financial condition and profitability.
In addition, in some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, the European Union provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products.
Employees
We believe that our success will depend greatly on our ability to identify, attract, and retain capable employees. As of March 14, 2008, we had 175 full time employees, including a total of 70 employees who hold M.D. or Ph.D. degrees. 135 of our employees are primarily engaged in research and development activities, and 40 are primarily engaged in general and administrative activities. Our employees are not represented by any collective bargaining unit, and we believe our relations with our employees are good.
Company History and Available Information
We commenced operations in July 2001. In September 2002, we acquired Principia Associates, Inc., which had previously acquired Shionogi BioResearch Corp., a U.S.-based drug discovery subsidiary of the Japanese pharmaceutical company, Shionogi & Co., Ltd. In this acquisition, we acquired a unique chemical compound library, an integrated set of drug discovery capabilities, and a pipeline of preclinical and research programs. Since 2002, we have been advancing these programs into later stages of development; discovering and developing additional drug candidates; and expanding our management and scientific teams and capabilities to support more advanced stages of drug development and commercialization.
Our principal executive offices are located at 45 Hartwell Avenue, Lexington, Massachusetts 02421, and our telephone number is (781) 274-8200. Our website address is www.syntapharma.com. The information contained on our website is not incorporated by reference into, and does not form any part of, this Annual Report on Form 10-K. We have included our website address as a factual reference and
36
do not intend it to be an active link to our website. Our trademarks include Synta Pharmaceuticals, our corporate logo, SYMMETRY and the SYMMETRY logo. Other service marks, trademarks and trade names appearing in this Annual Report on Form 10-K are the property of their respective owners. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and all amendments to those reports, are available free of charge through the Investors section of our website as soon as reasonably practicable after such materials have been electronically filed with, or furnished to, the Securities and Exchange Commission.
Item 1A. RISK FACTORS
If any of the following risks occurs, our business, business prospects, financial condition, results of operations, or cash flows could be materially harmed.
Risks Related to Our Financial Position and Need for Additional Capital
We have incurred significant losses since our inception, and we expect to incur losses for the foreseeable future and may never reach profitability.
Since inception we have incurred significant operating losses and, as of December 31, 2007, we had an accumulated deficit of $300.1 million. We expect to continue to incur significant operating expenses and capital expenditures and anticipate that our expenses and losses will increase substantially in the foreseeable future as we:
- •
- complete the SYMMETRY trial, our pivotal Phase 3 clinical trial of elesclomol for the treatment of metastatic melanoma that was initiated in the third quarter of 2007 and potentially initiate Phase 2 clinical trials of elesclomol in additional cancer types;
- •
- begin to perform and fund pre-commercialization activities, and establish sales and marketing functions and commercial manufacturing arrangements for elesclomol, consistent with our obligations under our collaborative development, commercialization and license agreement, or the GSK Agreement, with GlaxoSmithKline, or GSK;
- •
- complete the current Phase 2a clinical trial of apilimod for the treatment of rheumatoid arthritis, or RA and possibly initiate Phase 2 clinical trials of apilimod in additional inflammatory disease indications;
- •
- initiate additional Phase 3 clinical trials of elesclomol and one or more Phase 3 clinical trials of apilimod, if supported by Phase 2 results;
- •
- complete the Phase 1 clinical trials of STA-9090 that were initiated in the fourth quarter of 2007, initiate additional Phase 1 trials and initiate any later-stage clinical trials, if supported by Phase 1 results;
- •
- complete preclinical development of STA-9584 and initiate clinical trials, if supported by positive preclinical data;
- •
- advance our CRAC ion channel inhibitor program into clinical trials, if supported by positive preclinical data;
- •
- discover, develop, and seek regulatory approval for backups of our current drug candidates and other new drug candidates;
- •
- identify additional compounds or drug candidates and acquire rights from third parties to those compounds or drug candidates through licenses, acquisitions or other means;
- •
- commercialize any approved drug candidates;
- •
- hire additional clinical, scientific, and management personnel; and
37
- •
- add operational, financial, and management information systems and personnel.
We must generate significant revenue to achieve and maintain profitability. Even if we succeed in developing and commercializing one or more of our drug candidates, we may not be able to generate sufficient revenue and we may never be able to achieve or maintain profitability.
Our operating history may make it difficult to evaluate the success of our business to date and to assess our future viability.
We commenced operations in July 2001 and are a development-stage company. Our operations to date have been limited to organizing and staffing our company, acquiring, developing, and securing our technology, and undertaking preclinical studies and clinical trials of our drug candidates. We have not yet demonstrated an ability to obtain regulatory approval, formulate and manufacture a commercial-scale product, or conduct sales and marketing activities necessary for successful product commercialization. Consequently, any predictions about our future success or viability may not be as accurate as they could be if we had a longer operating history or had previously discovered, developed, and/or commercialized an approved product.
If we fail to obtain the capital necessary to fund our operations, we will be unable to successfully develop and commercialize our lead drug candidates.
Although we have raised substantial capital to date, we may require additional capital in order to complete clinical development and commercialize our drug candidates, elesclomol, apilimod, STA-9090, and STA-9584, and to conduct the research and development and clinical and regulatory activities necessary to bring other drug candidates to market. We initiated the SYMMETRY trial, our global, pivotal Phase 3 clinical trial of elesclomol for the treatment of metastatic melanoma, in the third quarter of 2007, and we expect the remaining costs necessary for the new drug application, or NDA, submission, including the cost of the clinical trial, clinical drug supplies, registration manufacturing and regulatory activities necessary to compile the NDA submission, together with the costs of related nonclinical toxicology and other testing to support the trial, will be in the range of $60 million to $70 million. We may not have sufficient capital, however, to fully fund certain other activities, including activities related to the continued clinical development of our other lead drug candidates and advancement of our other programs. Our future capital requirements will depend on many factors that are currently unknown to us, including:
- •
- our ability to fulfill our obligations and otherwise maintain our agreement with GSK;
- •
- the progress and results of the SYMMETRY trial, our global, pivotal Phase 3 clinical trial of elesclomol for the treatment of metastatic melanoma that was initiated in the third quarter of 2007;
- •
- the progress and results of any additional Phase 2 clinical trials of elesclomol in other cancer types we may initiate;
- •
- the costs of performing and funding pre-commercialization activities, and establishing sales and marketing functions and commercial manufacturing arrangements for elesclomol, consistent with our obligations under our agreement with GSK;
- •
- the progress and results of the current Phase 2a clinical trial of apilimod for the treatment of RA and any future Phase 2 clinical trials we may initiate for other inflammatory disease indications;
- •
- the progress and results of any additional Phase 3 clinical trials of elesclomol in other cancer types and any Phase 3 clinical trials of apilimod we may initiate in the future based on the results of Phase 2 clinical trials;
38
- •
- the progress and results of our Phase 1 clinical trials of STA-9090 initiated in the fourth quarter of 2007, any additional Phase 1 clinical trials of STA-9090 and any later-stage clinical trials we may initiate in the future based on the results of the Phase 1 clinical trials;
- •
- the results of our preclinical studies and testing of STA-9584 and our CRAC ion channel inhibitor program, and our decision to initiate clinical trials, if supported by the preclinical results;
- •
- the costs, timing, and outcome of regulatory review of elesclomol, apilimod, STA-9090 and our preclinical drug candidates;
- •
- the scope, progress, results, and cost of preclinical development, clinical trials, and regulatory review of any new drug candidates we may discover or acquire;
- •
- the costs of preparing, filing, and prosecuting patent applications and maintaining, enforcing, and defending intellectual property-related claims;
- •
- our ability to establish additional strategic collaborations and licensing or other arrangements on terms favorable to us;
- •
- the costs to satisfy our obligations under potential future collaborations; and
- •
- the timing, receipt, and amount of sales or royalties, if any, from elesclomol, apilimod, STA-9090, STA-9584, and our other potential products.
Our funding requirements will depend on a number of factors, including:
- •
- the progress of our research and development programs, including the completion of preclinical studies and clinical trials for our current drug candidates and the results from these studies and trials;
- •
- the number of drug candidates we advance into later-stage clinical trials and the scope of our research and development programs;
- •
- our ability to discover additional drug candidates using our drug discovery technology and advance them into clinical development;
- •
- the costs involved in preparing, filing, prosecuting, maintaining and enforcing patent claims for our drug discovery technology and drug candidates and avoiding infringing the intellectual property of others;
- •
- the time and costs involved in obtaining regulatory approvals for our drug candidates;
- •
- our ability to establish and maintain collaborative arrangements, including our agreement with GSK;
- •
- the potential in-licensing of other products or technologies or the acquisition of complementary businesses;
- •
- the cost of manufacturing, marketing and sales activities, if any; and
- •
- the timing, receipt and amount of revenue, if any, from our drug candidates.
There can be no assurance that additional funds will be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available on a timely basis, we may be required to:
- •
- terminate, significantly modify or delay our research and development programs;
- •
- reduce our planned commercialization efforts; or
39
- •
- obtain funds through collaborators that may require us to relinquish rights to our technologies or drug candidates that we might otherwise seek to develop or commercialize independently.
Based on our current operating plans, we expect our existing funds to be sufficient to fund operations through at least 2008. Payment to us by GSK of milestones for our operational progress and achievement of certain success criteria leading to the approval by the FDA of elesclomol for the treatment of metastatic melanoma could extend our cash availability, as could payments of milestones in connection with the development of elesclomol in other cancer indications and achievement of certain net sales thresholds. Based on our current operating plans, we expect to receive between $40 million and $50 million in operational progress milestone payments, under our agreement with GSK, in 2008. However, our operating plans may change as a result of many factors currently unknown to us, and we may need additional funds sooner than planned. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans.
Raising additional capital may cause dilution to existing stockholders, restrict our operations or require us to relinquish rights.
We may seek the additional capital necessary to fund our operations through public or private equity offerings, debt financings, and collaborative and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, existing stockholders' ownership interests will be diluted and the terms may include liquidation or other preferences that adversely affect their rights as a stockholder. Pursuant to the terms of our collaboration with GSK, GSK may, subject to our agreement, purchase up to $45 million of our common stock in two separate tranches upon the future achievement of specified development and regulatory milestones. In the first tranche, GSK would be obligated, at our sole discretion, to purchase $25 million of our common stock. In the second tranche, which is subject to agreement by both GSK and us, GSK would purchase $20 million of our common stock. The per share purchase price under each tranche is at a specified premium. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring additional debt, making capital expenditures, or declaring dividends. If we raise additional funds through collaboration and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or drug candidates, or grant licenses on terms that are not favorable to us.
Risks Related to the Development and Regulatory Approval of Our Drug Candidates
Our success is largely dependent on the success of our lead drug candidate, elesclomol, as well as our other drug candidates, and we cannot be certain that we will be able to obtain regulatory approval for or successfully commercialize any of these drug candidates.
We have invested a significant portion of our time and financial resources in the development of our lead drug candidate, elesclomol, for the treatment of cancer. We have also invested a significant amount of time and financial resources in the development of our other drug candidates, apilimod, STA-9090 and STA-9584. We anticipate that our success will depend largely on the receipt of regulatory approval and successful commercialization of these drug candidates. The future success of these drug candidates will depend on several factors, including the following:
- •
- our ability to provide acceptable evidence of their safety and efficacy;
- •
- receipt of marketing approval from the Food and Drug Administration, or FDA, and any similar foreign regulatory authorities;
- •
- successful formulation of an efficacious and commercially viable form of apilimod;
40
- •
- obtaining and maintaining commercial manufacturing arrangements with third-party manufacturers or establishing commercial-scale manufacturing capabilities;
- •
- establishing an internal sales force or collaborating with pharmaceutical companies or contract sales organizations to market and sell any approved drug; and
- •
- acceptance of any approved drug in the medical community and by patients and third-party payors.
Many of these factors are beyond our control. Accordingly, there can be no assurance that we will ever be able to generate revenues through the sale of an approved product.
If we do not obtain the required regulatory approval, we will be unable to market and sell our drug candidates.
Elesclomol, apilimod, STA-9090, STA-9584, and any other drug candidates we may discover or acquire and seek to commercialize are subject to extensive governmental regulations relating to development, clinical trials, manufacturing, and commercialization. Rigorous preclinical testing and clinical trials and an extensive regulatory approval process are required to be successfully completed in the United States and in many foreign jurisdictions before a new drug can be sold. Satisfaction of these and other regulatory requirements is costly, time consuming, uncertain, and subject to unanticipated delays. The time required to obtain approval by the FDA is unpredictable but typically exceeds five years following the commencement of clinical trials, depending upon the complexity of the drug candidate. We initiated clinical development of elesclomol, apilimod and STA-9090 in 2002, 2003 and 2007, respectively, and thus far, these drug candidates have been studied in only a relatively small number of patients. We initiated the SYMMETRY trial, our global, pivotal Phase 3 clinical trial of elesclomol for the treatment of metastatic melanoma, in the third quarter of 2007. Apilimod is currently in Phase 2a clinical trials for the treatment of RA. We initiated two Phase 1 clinical trials of STA-9090 in the fourth quarter of 2007. STA-9584 is in preclinical development.
We have limited experience in conducting and managing the clinical trials necessary to obtain regulatory approvals, including approval by the FDA. In connection with the clinical trials of elesclomol, apilimod, STA-9090 and STA-9584 and any other drug candidate we may seek to develop in the future, we face risks that:
- •
- the drug candidate may not prove to be efficacious;
- •
- the dosing of the drug candidate in a particular clinical trial may not be at an optimal level (for example, we are uncertain whether the Phase 2 clinical trial results for elesclomol in sarcoma and non-small cell lung cancer and Phase 2 clinical trial results for apilimod in psoriasis and Crohn's disease were the result of suboptimal dosing amounts and/or dosing schedules);
- •
- patients may die or suffer other adverse effects for reasons that may or may not be related to the drug candidate being tested;
- •
- the results may not confirm the positive results of earlier clinical trials; and
- •
- the results may not meet the level of statistical significance required by the FDA or other regulatory agencies for marketing approval.
Of the large number of drugs in development, only a small percentage result in the submission of an NDA to the FDA and even fewer are approved for commercialization. Furthermore, even if we do receive regulatory approval to market a commercial product, any such approval may be subject to limitations on the indicated uses for which we may market the product.
41
We will need to demonstrate the safety and efficacy of elesclomol in one or more Phase 3 clinical trials in order to obtain FDA approval for use in the treatment of metastatic melanoma, and there can be no assurance that elesclomol will achieve positive results in further clinical testing.
Positive results in early clinical trials of a drug candidate may not be replicated in later clinical trials. A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in late-stage clinical trials even after achieving promising results in earlier-stage development. Although our Phase 2b clinical trial of elesclomol for the treatment of metastatic melanoma achieved the primary endpoint of increasing progression-free survival, or PFS, there can be no assurance that the SYMMETRY trial, our global, pivotal Phase 3 trial for the treatment of metastatic melanoma, will achieve positive results. A number of factors could contribute to a lack of positive results in the SYMMETRY trial. For example, in our Phase 2b clinical trial, the majority of patients had been treated with prior chemotherapy, whereas our SYMMETRY trial will enroll only patients who have received no prior treatment with chemotherapy. In addition, the clinical investigators involved in the Phase 2b clinical trial used their judgment to determine when a patient's melanoma had progressed, using the criteria defined in the trial protocol and, among other factors, either CT or magnetic resonance imaging scans of a patient's tumors. In some past clinical trials by other companies involving similar subjective judgments, it has been reported that the variation among clinical trial sites in determining progression contributed to positive results. In the SYMMETRY trial, we will use a single centralized radiological reading center to review all patient scans, which could cause the results of our SYMMETRY trial to differ from those observed in our Phase 2b clinical trial.
In the SYMMETRY trial, we will seek to stratify, or evenly allocate to each trial arm, patients having certain strong prognostic factors, such as elevated lactate dehydrogenase, or LDH, levels. However, we may not be able to effectively stratify all such prognostic factors evenly. Although we found that patients with elevated LDH were evenly distributed between the elesclomol plus paclitaxel arm and the paclitaxel control arm in our Phase 2b clinical trial, we noted that the M-grade distribution of patients was uneven. M-grade is a measure of the degree of metastasis, or spread of the disease. In our Phase 2b clinical trial, 53% of the patients in the elesclomol plus paclitaxel group were classified by the clinical investigator as M1c, the most advanced stage of metastatic melanoma, compared to 75% in the paclitaxel alone group. However, we believe that M-grade distribution between the treatment and control arms did not impact the positive results of that trial. The median PFS for M1c patients who received elesclomol plus paclitaxel was 3.7 months versus 1.8 months for M1c patients who received paclitaxel alone. This result suggests a PFS benefit regardless of M-grade status of the patient. Further, a statistical analysis that we conducted evaluating the impact of multiple variables, including LDH levels, liver metastases and M-grade classification, showed that, firstly, investigator-reported M-grade was not a prognostic factor in this study, and secondly, that the M-grade distribution between the two arms did not contribute to the positive outcome of this clinical trial. Furthermore, published results from historical trials show that the degree of metastasis may not be prognostic for PFS, although it appears to be prognostic for OS, as described, for example, in the recent Korn et al. paper. Despite these analyses, however, we cannot provide complete assurance that the M-grade distribution did not have an impact on the Phase 2b trial results or that if evenly distributed in a future trial, that the clinical trial results would not be altered. We also recently analyzed the Phase 2b data in a post hoc fashion by each patient's prior chemotherapy status. The median PFS for chemotherapy-naïve patients who received elesclomol plus paclitaxel (N=24) was 7.1 months versus 1.8 months for chemotherapy-naïve patients assigned to the paclitaxel control arm (N=8). We noted that patients who had at least one prior chemotherapy had a lesser PFS benefit: elesclomol plus paclitaxel (N=29) versus paclitaxel alone (N=20) of 2.8 months versus 1.8 months median PFS, respectively. We have selected chemotherapy-naïve patients only as the population for our Phase 3 clinical trial and therefore, do not expect these differences to negatively impact the likelihood of success of our Phase 3 trial. However, we can give no assurances that the Phase 2b trial results were not influenced by these differences.
42
If we do not receive positive results in our SYMMETRY trial, we may not be able to obtain regulatory approval or commercialize elesclomol for this indication and our development of elesclomol for other indications may be delayed or cancelled.
Even if our SYMMETRY trial of elesclomol for the treatment of metastatic melanoma achieves the primary endpoint of increasing PFS, the FDA may not find the increase to be clinically meaningful or the FDA might still require us to establish an overall survival benefit prior to registration.
The primary endpoint of our recently-completed Phase 2b clinical trial of elesclomol for treating metastatic melanoma was PFS, and PFS is also the primary endpoint of our global, pivotal Phase 3 SYMMETRY trial of elesclomol for the treatment of metastatic melanoma. PFS, which measures for each patient the time from assignment to a treatment group until the earlier of tumor progression or death, is an endpoint that the FDA and/or its Oncologic Drug Advisory Committee, or ODAC, have previously indicated may be acceptable for registration in melanoma and other cancer types in clinical trials by other companies. However, no therapy for the treatment of melanoma has been approved to date based on a PFS endpoint. In our initial meeting and later discussions with the FDA on the design of our SYMMETRY trial for elesclomol, the FDA accepted our use of PFS as the primary endpoint in this trial and overall survival, or OS, as the secondary endpoint, although the FDA noted that the magnitude of an increase in PFS would need to be clinically meaningful in order to support approval of elesclomol based on the PFS endpoint. We can give no assurances, however, that the FDA or any other regulatory body will not require a different primary endpoint, such as OS, or additional efficacy endpoints for registration. If the FDA requires a different or any additional efficacy endpoints, we may be required to conduct larger or longer Phase 3 clinical trials than currently planned to achieve a statistically significant result to enable approval of elesclomol for the treatment of metastatic melanoma.
Further, we applied to the FDA for Special Protocol Assessment, or SPA, of the SYMMETRY trial of elesclomol for the treatment of metastatic melanoma. The SPA process may result in a written agreement between a clinical trial sponsor and the FDA that the design and planned analyses of the clinical trial will support regulatory approval, unless public health concerns unrecognized at the time of the protocol assessment become evident. Following discussions with the FDA, we received a response letter stating that the FDA has completed its review of our SPA application and has determined that the design and planned analyses of our study adequately address the objectives necessary to support a regulatory submission. However, the approval decision may be made based on a number of factors, including the degree of clinical benefit, and the FDA is not obligated to approve elesclomol as a result of the SPA, even if the clinical outcome is positive. Therefore, we cannot provide assurance that positive results in the SYMMETRY trial will be sufficient for FDA approval of elesclomol.
In addition, in order to detect a statistically significant result in our SYMMETRY trial for the primary endpoint of PFS, we believe that we will need to enroll and evaluate between 250 and 300 patients. However, based on our communications with the FDA and our medical advisors, we intend to use OS as a secondary endpoint, and estimate that we will need to enroll approximately 630 patients to detect a statistically significant benefit in this endpoint. We plan to conduct the final analysis for the PFS primary endpoint after two criteria have been satisfied: a prespecified minimum number of PFS events have occurred and full enrollment has been completed. Although we do not currently expect any delay in the availability of the PFS results beyond that point, there can be no assurance that future discussions with the FDA will not result in further delay of the analysis or in the release of this data. In addition, even if the SYMMETRY trial shows statistically and clinically meaningful benefits in the PFS primary endpoint, the FDA may decide to wait to review data relative to the OS secondary endpoint before considering elesclomol for approval. In our Phase 2b trial of elesclomol for metastatic melanoma, during a post-hoc analysis of patients as originally randomized, we noted an improvement in median OS for patients randomized to the elesclomol plus paclitaxel arm (median OS = 11.9 months)
43
as compared to those patients randomized to the paclitaxel alone arm (median OS = 7.8 months), but the difference did not achieve statistical significance. Although we are encouraged by the improvement in OS we observed in our Phase 2b clinical trial of elesclomol for metastatic melanoma, we note that OS was not a pre-defined endpoint of that trial, the analysis we performed was not prospectively defined and the results might have been influenced by a number of confounding factors, including the cross-over design of the trial, prior treatments and further treatments received following treatment on our trial. We can give no assurance that we will obtain positive OS data in the SYMMETRY trial that are sufficient to achieve the secondary endpoint of the trial, or establish an OS benefit trend at all. If the FDA were to approve elesclomol based on the data from the PFS endpoint and the results of the OS secondary endpoint are not positive, the FDA may limit the use of elesclomol or even withdraw it from the market.
If the FDA requires additional clinical data prior to registration, we may need to conduct more, larger or longer Phase 3 clinical trials than currently planned.
Prior to approving a new drug, the FDA typically requires that the efficacy of the drug be demonstrated in two double-blind, controlled studies. In light of the unmet medical need in metastatic melanoma and the results of our Phase 2b clinical trial, we believe that we will be required to conduct only a single Phase 3 clinical trial of elesclomol. However, the FDA has indicated that the trial must provide compelling evidence of clinically meaningful benefit in order to warrant consideration for marketing approval, and the FDA has noted that a trial that is merely statistically positive may not provide sufficient evidence to support an NDA filing or approval of a drug candidate. If the FDA determines that the results of our SYMMETRY trial do not have a clinically meaningful benefit, or if the FDA requires us to conduct additional Phase 3 clinical trials of elesclomol prior to seeking approval, we will incur significant additional development costs and commercialization of elesclomol may be prevented or delayed.
If the current formulation and method of administering elesclomol is not commercially feasible, we may not be able to commercialize elesclomol without reformulation and conducting additional clinical trials.
To date, other than a human bridging study of a salt form of elesclomol, all of our clinical trials have been and are being conducted using the free acid form of elesclomol, which we intend to continue to use in our clinical trials planned for 2008, as well as in our initial commercial product. Because this free acid form of elesclomol is not water soluble, prior to administration, it must be dissolved in an organic solvent. In the completed Phase 2b clinical trial in metastatic melanoma, this was achieved by combining the elesclomol with a volume of organic solvent included in the paclitaxel solution and agitating the resulting mixture with a sonication machine for up to 45 minutes. Once the elesclomol was fully dissolved, the resulting solution was added to the remaining paclitaxel solution, and the combined elesclomol/paclitaxel solution was administered to the patient. We have improved the process for preparing the active pharmaceutical ingredient, or API, and drug product of elesclomol, such that elesclomol can now be dissolved in the paclitaxel solution without sonication. We believe these improved procedures replicate the results of the prior methods and are suitable for preparing drug product for clinical trials and commercialization. These improved procedures will be used in our SYMMETRY trial and any Phase 2 clinical trials that we may initiate in additional cancer indications using the free acid form of elesclomol. We have taken steps to ensure that the medical personnel responsible for formulating elesclomol are properly trained to carry out the new dissolution process. Although we believe that the changes in the procedures for preparing and dissolving elesclomol prior to administration will not affect the efficacy or pharmaceutical properties of the treatment, there can be no assurance that the results of future trials will not be affected by these changes. In addition, in order to use the free acid form of elesclomol with other oncology products, including taxanes other than paclitaxel, it must be dissolved in an organic solvent, which may cause additional toxicity due to the presence of the organic solvent.
44
We have developed a water-soluble salt form of elesclomol that does not need to be dissolved in an organic solvent and therefore may be used more easily with other oncology products or potentially, as a stand alone agent without need for an organic solvent. We intend to explore the use of this new salt form of elesclomol in future clinical trials. However, it is also our intention to use the free acid form of elesclomol in our initial commercial product. If the free acid form does not prove to be commercially feasible and we are required to commercialize the salt form of elesclomol, it will require additional formulation development efforts and clinical studies which would delay the commercialization of this drug candidate.
While we believe elesclomol may have applicability to a broad range of solid tumor cancers, including tumor types other than melanoma, our clinical trials of elesclomol in non-small cell lung cancer and soft tissue sarcoma have shown negative or inconclusive results.
Based on our understanding of the mechanism of action and the preclinical activity we have seen with elesclomol, which included showing activity in a broad range of cancer types, we intend to conduct clinical trials of elesclomol in a number of other cancer indications in addition to melanoma. In addition to our Phase 2b clinical trial in metastatic melanoma, we have also conducted Phase 2 clinical trials of elesclomol in sarcoma and non-small cell lung cancer. The results of the soft tissue sarcoma clinical trial did not definitively establish evidence of clinical activity. In the non-small cell lung cancer clinical trial, no improvement was observed in time-to-progression between combination treatment with elesclomol and a standard first-line combination therapy. Although we are currently analyzing these data further to assess future development of elesclomol in sarcoma and non-small cell lung cancer, including assessing the possibility for a potential future clinical trial in non-small cell lung cancer at a more frequent dosing schedule and higher dose than previously tested, there can be no assurances that we will continue the development of elesclomol in these indications or that elesclomol will prove effective in and be approved for treating these or other forms of cancer.
Because our drug candidates are in an early stage of development, there is a high risk of failure, and we may never succeed in developing marketable products or generating product revenue.
We have no drug candidates that have received regulatory approval for commercial sale. We do not expect to have any commercial products on the market until at least 2009, if at all. We are exploring human diseases at the cellular level and attempting to develop drug candidates that intervene with cellular processes. Drug development is an uncertain process that involves trial and error, and we may fail at numerous stages along the way. Success in preclinical studies of a drug candidate may not be predictive of similar results in humans during clinical trials, and successful results from early or small clinical trials of a drug candidate may not be replicated in later and larger clinical trials. For example, although preclinical data and Phase 2a clinical trial results suggested that apilimod had activity in psoriasis and Crohn's disease, our Phase 2b clinical trials of apilimod in those indications did not demonstrate clinical benefit. Accordingly, the results from preclinical studies and the completed and ongoing clinical trials for our drug candidates may not be predictive of the results we may obtain in later stage clinical trials.
If clinical trials for our drug candidates, including elesclomol and apilimod, are prolonged or delayed, we may be unable to commercialize our drug candidates on a timely basis, which would require us to incur additional costs and delay our receipt of any revenue from potential product sales.
We cannot predict whether we will encounter problems with any of our completed, ongoing or planned clinical trials that will cause us or any regulatory authority to delay or suspend those clinical trials or delay the analysis of data derived from them. A number of events, including any of the following, could delay the completion of our ongoing and planned clinical trials and negatively impact
45
our ability to obtain regulatory approval for, and to market and sell, a particular drug candidate, including our clinical drug candidates elesclomol and apilimod:
- •
- conditions imposed on us by the FDA or any foreign regulatory authority regarding the scope or design of our clinical trials;
- •
- delays in obtaining, or our inability to obtain, required approvals from institutional review boards or other reviewing entities at clinical sites selected for participation in our clinical trials;
- •
- insufficient supply or deficient quality of our drug candidates or other materials necessary to conduct our clinical trials;
- •
- delays in obtaining regulatory agency agreement for the conduct of our clinical trials;
- •
- lower than anticipated enrollment and retention rate of subjects in clinical trials;
- •
- negative or inconclusive results from clinical trials, or results that are inconsistent with earlier results, that necessitate additional clinical studies (for example, due to patient-to-patient pharmacokinetic variability);
- •
- serious and unexpected drug-related side effects experienced by patients in clinical trials; or
- •
- failure of our third-party contractors to comply with regulatory requirements or otherwise meet their contractual obligations to us in a timely manner.
Commercialization of our drug candidates may be delayed by the imposition of additional conditions on our clinical trials by the FDA or the requirement of additional supportive studies by the FDA. In addition, clinical trials require sufficient patient enrollment, which is a function of many factors, including the size of the patient population, the nature of the trial protocol, the proximity of patients to clinical sites, the availability of effective treatments for the relevant disease, the conduct of other clinical trials that compete for the same patients as our clinical trials, and the eligibility criteria for our clinical trials. For example, competing trials for melanoma treatments or the emergence of new approved therapies may make it more difficult to enroll patients in our SYMMETRY trial on the schedule currently planned. We are aware of other ongoing clinical trials of drug candidates for the treatment of metastatic melanoma, including Nexavar, Sutent, Avasba, Avastin, ipilimumab, and tremelumimab. Enrollment efforts and future results with respect to these trials could also adversely impact patient enrollment in our SYMMETRY trial. We have had satisfactory patient enrollment in our completed clinical trials. However, in our SYMMETRY trial, we expect to enroll approximately 630 patients with stage IV metastatic melanoma, which is significantly more patients than we enrolled in our completed Phase 2b clinical trial for elesclomol. Initiation of the SYMMETRY trial in certain geographical regions has been slower than we expected, which increases the risk that full trial enrollment may be delayed beyond our initial goal. To increase the likelihood of our meeting the overall enrollment timeline for this trial, we are taking several specific actions, including potentially increasing the target number of clinical sites. Despite these efforts, if patient enrollment remains below our initial projections, the completion of this trial will be delayed. Delays in patient enrollment can result in increased costs and longer development times. Our failure to enroll patients in our clinical trials could delay the completion of the clinical trial beyond our current expectations. In addition, the FDA could require us to conduct clinical trials with a larger number of subjects than we have projected for any of our drug candidates. We may not be able to enroll a sufficient number of patients in a timely or cost-effective manner. Furthermore, enrolled patients may drop out of our clinical trials, which could impair the validity or statistical significance of the clinical trials.
We do not know whether our clinical trials will begin as planned, will need to be restructured, or will be completed on schedule, if at all. Delays in our clinical trials will result in increased development costs for our drug candidates. In addition, if our clinical trials are delayed, our competitors may be able
46
to bring products to market before we do and the commercial viability of our drug candidates, including our drug candidates elesclomol and apilimod, could be limited.
Failure to comply with foreign regulatory requirements governing human clinical trials and marketing approval for drugs could prevent us from selling our drug candidates in foreign markets, which may adversely affect our operating results and financial condition.
The requirements governing the conduct of clinical trials, product licensing, pricing, and reimbursement for marketing our drug candidates outside the United States vary greatly from country to country and may require additional testing. While GSK has exclusive responsibility to develop elesclomol outside the United States, we also expect that our future clinical development of apilimod, STA-9090 and other drug candidates will involve a number of clinical trials in foreign jurisdictions, particularly in Europe. We have no experience in obtaining foreign regulatory approvals. The time required to obtain approvals outside the United States may differ from that required to obtain FDA approval. We or GSK may not obtain foreign regulatory approvals on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other countries or by the FDA. Failure to comply with these regulatory requirements or obtain required approvals could impair our and GSK's ability to develop foreign markets for our drug candidates and may have a material adverse effect on our results of operations and financial condition.
Our drug candidates will remain subject to ongoing regulatory review even if they receive marketing approval, and if we fail to comply with continuing regulations, we could lose these approvals and the sale of any approved commercial products could be suspended.
Even if we receive regulatory approval to market a particular drug candidate, the manufacturing, labeling, packaging, adverse event reporting, storage, advertising, promotion, and record keeping related to the product will remain subject to extensive regulatory requirements. If we fail to comply with the regulatory requirements of the FDA and other applicable domestic and foreign regulatory authorities or previously unknown problems with any approved commercial products, manufacturers, or manufacturing processes are discovered, we could be subject to administrative or judicially imposed sanctions, including:
- •
- restrictions on the products, manufacturers, or manufacturing processes;
- •
- untitled or warning letters;
- •
- civil or criminal penalties;
- •
- fines;
- •
- injunctions;
- •
- product seizures or detentions;
- •
- import bans;
- •
- voluntary or mandatory product recalls and related publicity requirements;
- •
- suspension or withdrawal of regulatory approvals;
- •
- total or partial suspension of production; and
- •
- refusal to approve pending applications for marketing approval of new products or supplements to approved applications.
47
If side effects increase or are identified during the time our drug candidates are in development or after they are approved and on the market, we may be required to perform lengthy additional clinical trials, change the labeling of any such products, or withdraw any such products from the market, any of which would hinder or preclude our ability to generate revenues.
In our completed Phase 2b clinical trial of elesclomol for metastatic melanoma, there were four patients with possible or probable drug-related serious adverse events related to treatment with elesclomol. The first event involved a patient who developed lichenoid dermatitis, a severe rash-like condition, which was considered by the investigator to be possibly related to treatment. The second event involved a patient who experienced atrial fibrillation with rapid ventricular response. This event was also considered by the investigator to be possibly related to treatment. The third event involved an infection which, despite a normal absolute neutrophil count was considered by the investigator to be possibly related to treatment. The fourth event involved severe dehydration that was considered by the investigator to be probably related to treatment. If the incidence of these events increases or if other effects are identified after any of our drug candidates are approved and on the market:
- •
- regulatory authorities may withdraw their approvals;
- •
- we may be required to reformulate any such products, conduct additional clinical trials, make changes in labeling of any such products, or implement changes to or obtain new approvals of our or our contractors' manufacturing facilities;
- •
- we may experience a significant drop in the sales of the affected products;
- •
- our reputation in the marketplace may suffer; and
- •
- we may become the target of lawsuits, including class action suits.
Any of these events could harm or prevent sales of the affected products or could substantially increase the costs and expenses of commercializing and marketing any such products.
We have also observed significant toxicities in preclinical animal studies of our clinical drug candidate, STA-9090. If significant toxicities occur at a clinical dose of STA-9090 which is not sufficiently efficacious, we may not be able to demonstrate an adequate therapeutic index to obtain regulatory approval for STA-9090.
While we choose to test our drug candidates in specific clinical indications based in part on our understanding of their mechanisms of action, our understanding may be incorrect or incomplete and, therefore, our drugs may not be effective against the diseases tested in our clinical trials.
Our rationale for selecting the particular therapeutic indications for each of our drug candidates is based in part on our understanding of the mechanism of action of these drug candidates. However, our understanding of the drug candidate's mechanism of action may be incomplete or incorrect, or the mechanism may not be clinically relevant to the diseases treated. In such cases, our drug candidates may prove to be ineffective in the clinical trials for treating those diseases.
We deal with hazardous materials and must comply with environmental laws and regulations, which can be expensive and restrict how we do business.
Our activities involve the controlled storage, use, and disposal of hazardous materials, including cytotoxic agents, genotoxic agents, infectious agents, corrosive, explosive and flammable chemicals, and various radioactive compounds. We are subject to federal, state, and local laws and regulations governing the use, manufacture, storage, handling, and disposal of these hazardous materials. Although we believe that our safety procedures for the handling and disposing of these materials comply with the standards prescribed by these laws and regulations, we cannot eliminate the risk of accidental contamination or injury from these materials.
48
In the event of an accident, state or federal authorities may curtail our use of these materials, and we could be liable for any civil damages that result, which may exceed our financial resources and may seriously harm our business. We currently maintain insurance covering hazardous waste clean up costs in an amount of up to $250,000 per site. Because we believe that our laboratory and materials handling policies and practices sufficiently mitigate the likelihood of materials liability or third-party claims, we currently carry no insurance covering such claims. While we believe that the amount of insurance we carry is sufficient for typical risks regarding our handling of these materials, it may not be sufficient to cover pollution conditions or other extraordinary or unanticipated events. Additionally, an accident could damage, or force us to shut down, our operations. In addition, if we develop a manufacturing capacity, we may incur substantial costs to comply with environmental regulations and would be subject to the risk of accidental contamination or injury from the use of hazardous materials in our manufacturing process.
Risks Related to Our Dependence on Third Parties
We have recently entered into an agreement with GSK relating to the development and commercialization of elesclomol. If this agreement is unsuccessful or terminated by GSK for any reason, our ability to commercialize elesclomol on a timely basis, or at all, could be affected and our business could be materially harmed.
On October 8, 2007, we entered into a Collaborative Development, Commercialization and License Agreement with GSK for the joint development and commercialization of elesclomol. We do not have a history of working together with GSK and cannot predict the success of this collaboration. The agreement involves a complex allocation of responsibilities, costs and benefits and provides for milestone payments to us upon the achievement of specified operational progress, positive clinical and regulatory outcomes and sales milestones.
With respect to responsibilities and control over decisions, we and GSK have established a series of joint committees which will be responsible for the development and commercialization of elesclomol. We have the right, but not the obligation to participate in these various joint governance committees. Under the committee structure, if the committees are unable to reach a decision, the matter is referred to senior executives of each of the parties. Each party has ultimate decision making authority with respect to a specified set of issues. For certain other specified issues, the matter must be resolved by consensus of the parties, and for all other issues, the matter must be resolved through arbitration. Accordingly, GSK's failure to devote sufficient resources to the development and commercialization of elesclomol or the failure of the parties to reach consensus on the conduct of development or commercialization activities with respect to elesclomol may delay its clinical development, which could lead to the delay in payment of clinical and regulatory milestones under the collaboration agreement and may delay commercialization of elesclomol.
In addition, the agreement provides that GSK may terminate the agreement upon not less than three months' written notice at any time prior to the date of the first commercial sale of an elesclomol product and not less than six months' written notice at any time on and after such date, in which case GSK may be obligated in certain circumstances to make additional payments to us.
Loss of GSK as a collaborator in the development or commercialization of elesclomol, any dispute over the terms of, or decisions regarding, the agreement, or any other adverse developments in our relationship with GSK could result in our inability to fully develop and/or commercialize elesclomol, or at all, and could materially harm our business and could accelerate our need for additional capital.
49
We rely on third parties to conduct our clinical trials, and those third parties may not perform satisfactorily, including failing to meet established deadlines for the completion of such clinical trials.
We do not have the ability to independently conduct clinical trials for our drug candidates, and we rely on third parties such as contract research organizations, medical institutions, and clinical investigators to perform this function. Our reliance on these third parties for clinical development activities reduces our control over these activities. Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. To date, our contract research organizations and other similar entities with which we are working have performed well; however, if these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may be delayed in obtaining regulatory approvals for our drug candidates and may be delayed in our efforts to successfully commercialize our drug candidates for targeted diseases.
We have no manufacturing capacity and depend on third-party manufacturers to produce our clinical trial drug supplies.
We do not currently operate manufacturing facilities for clinical or commercial production of elesclomol, apilimod or STA-9090, or any of our preclinical drug candidates. We have limited experience in drug manufacturing, and we lack the resources and the capabilities to manufacture any of our drug candidates on a clinical or commercial scale. As a result, we currently rely on third-party manufacturers to supply, store, and distribute drug supplies for our clinical trials and anticipate future reliance on a limited number of third-party manufacturers until we increase the number of manufacturers with whom we contract. Any performance failure on the part of our existing or future manufacturers could delay clinical development or regulatory approval of our drug candidates or commercialization of any approved products, producing additional losses and depriving us of potential product revenue.
Our drug candidates require precise, high quality manufacturing. Failure by our contract manufacturers to achieve and maintain high manufacturing standards could result in patient injury or death, product recalls or withdrawals, delays or failures in testing or delivery, cost overruns, or other problems that could seriously hurt our business. Contract manufacturers may encounter difficulties involving production yields, quality control, and quality assurance. These manufacturers are subject to ongoing periodic unannounced inspection by the FDA and corresponding state and foreign agencies to ensure strict compliance with current Good Manufacturing Practice, or cGMP, and other applicable government regulations and corresponding foreign standards; however, we do not have control over third-party manufacturers' compliance with these regulations and standards.
If for some reason our contract manufacturers cannot perform as agreed, we may be unable to replace such third-party manufacturers in a timely manner and the production of our drug candidates would be interrupted, resulting in delays in clinical trials and additional costs. Switching manufacturers may be difficult because the number of potential manufacturers is limited and the FDA must approve any replacement manufacturer prior to manufacturing our drug candidates. Such approval would require new testing and compliance inspections. In addition, a new manufacturer would have to be educated in, or develop substantially equivalent processes for, production of our drug candidates after receipt of FDA approval. It may be difficult or impossible for us to find a replacement manufacturer on acceptable terms quickly, or at all.
50
We are using a single manufacturer for the supply of elesclomol powder-filled vials for the SYMMETRY trial, our global, pivotal Phase 3 clinical trial for the treatment of metastatic melanoma and potentially, for commercial supply, and the failure of this manufacturer to supply sufficient quantities of elesclomol powder-filled vials could have a material adverse effect on our business.
We are using a single manufacturer for the supply of elesclomol powder-filled vials for the SYMMETRY trial, our global, pivotal Phase 3 clinical trial for the treatment of metastatic melanoma and potentially, for commercial supply, if approved. This process involves highly specialized processing, including the automated filling of vials with elesclomol under sterile conditions. We believe that this manufacturer may be one of a limited number of third-party contract manufacturers currently capable of conducting this process on our behalf. We have entered into a clinical supply agreement and a quality agreement with this manufacturer for the production of elesclomol drug product, which we believe will satisfy our manufacturing requirements for the SYMMETRY trial and additional Phase 2 clinical trials of elesclomol for other cancer indications. Although the clinical supply agreement notes that the parties have a mutual desire to enter into good faith negotiations for commercial supply services, if circumstances allow, there are no terms in this contract relating to commercial supply of elesclomol, and we cannot assure that we will be able to enter into a commercial supply agreement with this manufacturer on commercially reasonable terms, or at all. Any performance failure on the part of this manufacturer or the failure to enter an appropriate commercial supply agreement on reasonable terms in the future, assuming GSK decides to contract with this manufacturer or other circumstances so require, could delay clinical development, regulatory approval or commercialization of elesclomol, which could have a material adverse effect on our business. Moreover, although we believe we have identified a suitable backup manufacturer for elesclomol powder-filled vials, neither GSK nor we have an agreement with this manufacturer for producing this product and there can be no assurance that we will be able to enter into such an agreement on favorable terms, if at all.
We anticipate continued reliance on third-party manufacturers if we are successful in obtaining marketing approval from the FDA and other regulatory agencies for any of our drug candidates.
To date, our drug candidates have been manufactured in relatively small quantities for preclinical testing and clinical trials by third-party manufacturers. If the FDA or other regulatory agencies approve any of our drug candidates for commercial sale, we expect that we would continue to rely, at least initially, on third-party manufacturers to produce commercial quantities of our approved drug candidates. These manufacturers may not be able to successfully increase the manufacturing capacity for any of our approved drug candidates in a timely or economic manner, or at all. Significant scale-up of manufacturing may require additional validation studies, which the FDA must review and approve. If they are unable to successfully increase the manufacturing capacity for a drug candidate, particularly elesclomol, or we are unable to establish our own manufacturing capabilities, the commercial launch of any approved products may be delayed or there may be a shortage in supply.
If we do not establish additional collaborations, we may have to alter our development plans.
Our drug development programs and potential commercialization of our drug candidates will require substantial additional cash to fund expenses. Although we have established a collaboration with GSK relating to the joint development and commercialization of elesclomol, our strategy also includes potentially selectively collaborating with leading pharmaceutical and biotechnology companies to assist us in furthering development and potential commercialization of some of our other drug candidates. We may enter into one or more of such collaborations in the future, especially for target indications in which the potential collaborator has particular therapeutic expertise or that involve a large, primary care market that must be served by large sales and marketing organizations or for markets outside of North America. We face significant competition in seeking appropriate collaborators and these collaborations are complex and time-consuming to negotiate and document. We may not be able to
51
negotiate collaborations on acceptable terms, or at all. If that were to occur, we may have to curtail the development of a particular drug candidate, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of our sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on acceptable terms, or at all. If we do not have sufficient funds, we will not be able to bring our drug candidates to market and generate product revenue.
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell our drug candidates, we may be unable to generate product revenue or co-commercialize elesclomol under our arrangement with GSK.
Although we have entered into a collaborative development, commercialization and license agreement with GSK for elesclomol, we do not currently have an organization for the sales, marketing, and distribution of pharmaceutical products. In order to co-commercialize elesclomol in the United States under our arrangement with GSK or market any other products that may be approved by the FDA, we must build our sales, marketing, managerial, and other non-technical capabilities or make arrangements with third parties to perform these services. If we are unable to establish adequate sales, marketing, and distribution capabilities, whether independently or with third parties, our share in elesclomol profits with GSK may be diminished or we may not be able to generate product revenue and we may not become profitable.
Risks Related to Our Intellectual Property
If our patent position does not adequately protect our drug candidates or any future products, others could compete against us more directly, which would harm our business.
As of March 14, 2008, our patent portfolio consisted of a total of 602 patents and patent applications worldwide. We own or license a total of 23 issued U.S. patents and 94 U.S. patent applications, as well as 485 foreign patents and patent applications. We have issued U.S. composition-of-matter patents claiming the chemical structures of elesclomol and apilimod.
Our commercial success will depend in part on our ability to obtain additional patents and protect our existing patent position as well as our ability to maintain adequate protection of other intellectual property for our technologies, drug candidates, and any future products in the United States and other countries. If we do not adequately protect our intellectual property, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our business and ability to achieve profitability. The laws of some foreign countries do not protect our proprietary rights to the same extent as the laws of the United States, and we may encounter significant problems in protecting our proprietary rights in these countries.
The patent positions of biotechnology and pharmaceutical companies, including our patent position, involve complex legal and factual questions, and, therefore, validity and enforceability cannot be predicted with certainty. Patents may be challenged, deemed unenforceable, invalidated, or circumvented. We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary technologies, drug candidates, and any future products are covered by valid and enforceable patents or are effectively maintained as trade secrets.
In addition, although we do not believe that any of the patents or patent applications that we currently license are material to our business, we may in the future license intellectual property that is material to us. In such cases, we may be dependent upon the licensors to obtain, maintain and enforce patent protection for the licensed intellectual property. These licensors may not successfully prosecute patent applications or may fail to maintain issued patents. The licensors may also determine not to
52
pursue litigation against other companies that infringe the patents, or may pursue such litigation less aggressively than we would. If any of the foregoing occurs, and the terms of any such future license do not allow us to assume control of patent prosecution, maintenance and enforcement, any competitive advantage we may have due to the license may be diminished or eliminated.
The degree of future protection for our proprietary rights is uncertain, and we cannot ensure that:
- •
- we or our licensors were the first to make the inventions covered by each of our pending patent applications;
- •
- we or our licensors were the first to file patent applications for these inventions;
- •
- others will not independently develop similar or alternative technologies or duplicate any of our technologies;
- •
- any of our or our licensors' pending patent applications will result in issued patents;
- •
- any of our or our licensors' patents will be valid or enforceable;
- •
- any patents issued to us or our licensors and collaborators will provide a basis for commercially viable products, will provide us with any competitive advantages or will not be challenged by third parties;
- •
- we will develop additional proprietary technologies or drug candidates that are patentable; or
- •
- the patents of others will not have an adverse effect on our business.
Although third parties may challenge our rights to, or the scope or validity of our patents, to date we have not received any communications from third parties challenging our patents or patent applications covering our drug candidates.
We typically file for patent protection first on the composition-of-matter of our drug candidates and also claim their activities and methods for their production and use to the extent known at that time. As we learn more about the mechanisms of action and new methods of manufacture and use of these drug candidates, we generally file additional patent applications for these new inventions. Although our patents may prevent others from making, using, or selling similar products, they do not ensure that we will not infringe the patent rights of third parties. For example, because we sometimes identify the mechanism of action or molecular target of a given drug candidate after identifying its composition-of-matter and therapeutic use, we may not be aware until the mechanism or target is further elucidated that a third party has an issued or pending patent claiming biological activities or targets that may cover our drug candidate. If such a patent exists or is granted in the future, we cannot provide assurances that a license will be available on commercially reasonable terms, or at all.
We may be unable to adequately prevent disclosure of trade secrets and other proprietary information.
We rely on trade secrets to protect our proprietary technologies, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. We rely in part on confidentiality agreements with our employees, consultants, outside scientific collaborators, sponsored researchers, and other advisors to protect our trade secrets and other proprietary information. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover our trade secrets and proprietary information. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
53
Litigation or other proceedings or third-party claims of intellectual property infringement would require us to spend time and money and could prevent us from developing or commercializing our drug candidates.
Our commercial success will depend in part on not infringing upon the patents and proprietary rights of other parties and enforcing our own patents and proprietary rights against others. Certain of our research and development programs are in highly competitive fields in which numerous third parties have issued patents and patent applications with claims closely related to the subject matter of our programs. We are not currently aware of any litigation or other proceedings or claims by third parties that our drug candidates, technologies or methods infringe their intellectual property.
However, while it is our practice to conduct freedom to operate searches and analyses, we cannot guarantee that we have identified every patent or patent application that may be relevant to the research, development or commercialization of our drug candidates. In the case of patent applications, we assess the likelihood of claims in pending, third party patent applications being allowed which may interfere with our freedom to operate relative to our drug candidates. We cannot provide assurances that our assessments in this regard will be correct and that patent claims covering our drug candidates that were assessed a low likelihood of issuance by us will not issue to a third party in the future. Moreover, there can be no assurance that third parties will not assert against us patents that we believe are not infringed by us or are invalid. For example, we are aware of a U.S. patent and a related European patent that claim generic chemical structures, pharmaceutical formulations and methods of treatment relating to compounds similar to STA-9090 and a U.S. patent that claims methods of treating certain cancers using heat shock protein 90, or Hsp90, inhibitors. The claims of these patents may be relevant to the commercialization of our drug candidate, STA-9090. However, based on our analysis of these patents, we do not believe that the manufacture, use, importation or sale of STA-9090 would infringe any valid claim of these patents. However, we cannot guarantee that these patents would not be asserted against us and, if asserted, that a court would find these patents to be invalid or not infringed.
In the event of a successful infringement action against us with respect to any third party patent rights, we may be required to:
- •
- pay substantial damages;
- •
- stop developing, commercializing, and selling the infringing drug candidates or approved products;
- •
- stop utilizing the infringing technologies and methods in our drug candidates or approved products;
- •
- develop non-infringing products, technologies, and methods; and
- •
- obtain one or more licenses from other parties, which could result in our paying substantial royalties or our granting of cross licenses to our technologies.
We may not be able to obtain licenses from other parties at a reasonable cost, or at all. If we are not able to obtain necessary licenses at a reasonable cost, or at all, we could encounter substantial delays in product introductions while we attempt to develop alternative technologies, methods, and products, which we may not be able to accomplish.
We may be subject to claims that we have wrongfully hired an employee from a competitor or that we or our employees have wrongfully used or disclosed alleged confidential information or trade secrets of their former employers.
As is commonplace in our industry, we employ individuals who were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although no claims against us are currently pending, we have previously been subject to a claim by an
54
alleged competitor that a prospective employee we sought to hire was bound by an ongoing non-competition obligation which prevented us from hiring this employee. We may be subject in the future to claims that our employees or prospective employees are subject to a continuing obligation to their former employers (such as non-competition or non-solicitation obligations) or claims that our employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Risks Related to the Commercialization of Our Drug Candidates
If physicians and patients do not accept our future products or if the markets for indications for which any drug candidate is approved is smaller than expected, we may be unable to generate significant revenue, if any.
Even if elesclomol, apilimod, STA-9090, STA-9584 or any other drug candidates we may develop or acquire in the future obtain regulatory approval, they may not gain market acceptance among physicians, healthcare payors, patients, and the medical community. Physicians may elect not to recommend these drugs for a variety of reasons including:
- •
- timing of market introduction of competitive products, including other melanoma treatments currently in development (such as Nexavar, Sutent, ispinesib, ipilimumab, tremelumimab, volociximab, M-Vax and MDX-1379, as well as forms of chemotherapy);
- •
- demonstration of clinical safety and efficacy compared to other products;
- •
- cost-effectiveness;
- •
- availability of reimbursement from managed care plans and other third-party payors;
- •
- convenience and ease of administration;
- •
- prevalence and severity of adverse side effects;
- •
- other potential advantages of alternative treatment methods; and
- •
- ineffective marketing and distribution support of our products.
If our approved drugs fail to achieve market acceptance, we may not be able to generate significant revenue and our business would suffer.
In addition, we have initiated a Phase 3 clinical trial for our most advanced clinical-stage candidate, elesclomol, in patients with stage IV metastatic melanoma. We currently estimate that there are relatively few people with metastatic melanoma in the United States. Accordingly, even if we are successful in obtaining regulatory approval to market elesclomol for this indication, the market for this indication may not be sufficient to generate significant revenue and our business would suffer.
If the government and third-party payors fail to provide adequate coverage and reimbursement rates for our future products, if any, our revenue and prospects for profitability will be harmed.
In both domestic and foreign markets, our sales of any future products will depend in part upon the availability of reimbursement from third-party payors. Such third-party payors include government health programs such as Medicare, managed care providers, private health insurers, and other organizations. These third-party payors are increasingly attempting to contain healthcare costs by demanding price discounts or rebates and limiting both coverage and the amounts that they will pay for new drugs, and, as a result, they may not cover or provide adequate payment for our drugs. We might need to conduct post-marketing studies in order to demonstrate the cost-effectiveness of any future products to such payors' satisfaction. Such studies might require us to commit a significant amount of
55
management time and financial and other resources. Our future products might not ultimately be considered cost-effective. Adequate third-party reimbursement might not be available to enable us to maintain price levels sufficient to realize an appropriate return on investment in product development.
U.S. and foreign governments continue to propose and pass legislation designed to reduce the cost of healthcare. For example, in some foreign markets, the government controls the pricing and profitability of prescription pharmaceuticals. In the United States, we expect that there will continue to be federal and state proposals to implement similar governmental controls. In addition, recent changes in the Medicare program and increasing emphasis on managed care in the United States will continue to put pressure on pharmaceutical product pricing. Cost control initiatives could decrease the price that we would receive for any products in the future, which would limit our revenue and profitability. Accordingly, legislation and regulations affecting the pricing of pharmaceuticals might change before our drug candidates are approved for marketing. Adoption of such legislation could further limit reimbursement for pharmaceuticals.
For example, the Medicare Prescription Drug Improvement and Modernization Act of 2003, or MMA, changes the way Medicare will cover and pay for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly and disabled and introduced new reimbursement methodologies, based on average sales prices for drugs that are administered in an in-patient setting or by physicians, such as elesclomol, if approved. In addition, this legislation provides authority for limiting the number of drugs that will be covered in any therapeutic class. Although we do not know what the full impact of the new reimbursement methodologies will have on the prices of new drugs, we expect that there will be added pressure to contain and reduce costs. These cost reduction initiatives and other provisions of this legislation could decrease the coverage and price that we receive for any approved products and could seriously harm our business. While the MMA applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates, and any reduction in reimbursement that results from the MMA may result in a similar reduction in payments from private payors.
If a successful product liability claim or series of claims is brought against us for uninsured liabilities or in excess of insured liabilities, we could be forced to pay substantial damage awards.
The use of any of our drug candidates in clinical trials, and the sale of any approved products, might expose us to product liability claims. We currently maintain product liability insurance coverage in an amount of up to $10.0 million, which we believe is adequate for our clinical trials currently in progress. We monitor the amount of coverage we maintain as the size and design of our clinical trials evolve and intend to adjust the amount of coverage we maintain accordingly. However, there can be no assurance that such insurance coverage will fully protect us against some or all of the claims to which we might become subject. We might not be able to maintain adequate insurance coverage at a reasonable cost or in sufficient amounts or scope to protect us against potential losses. In the event a claim is brought against us, we might be required to pay legal and other expenses to defend the claim, as well as uncovered damages awards resulting from a claim brought successfully against us. Furthermore, whether or not we are ultimately successful in defending any such claims, we might be required to direct financial and managerial resources to such defense and adverse publicity could result, all of which could harm our business.
If we inadvertently violate the guidelines pertaining to promotion and advertising of our clinical candidates or approved products, we may be subject to disciplinary action by the FDA's Division of Drug Marketing, Advertising, and Communications or other regulatory bodies.
The FDA's Division of Drug Marketing, Advertising, and Communications, or DDMAC, is responsible for reviewing prescription drug advertising and promotional labeling to ensure that the information contained in these materials is not false or misleading. There are specific disclosure
56
requirements and the applicable regulations mandate that advertisements cannot be false or misleading or omit material facts about the product. Prescription drug promotional materials must present a fair balance between the drug's effectiveness and the risks associated with its use. Most warning letters from DDMAC cite inadequate disclosure of risk information.
DDMAC prioritizes its actions based on the degree of risk to the public health, and often focuses on newly introduced drugs and those associated with significant health risks. There are two types of letters that DDMAC typically sends to companies which violate its drug advertising and promotional guidelines: notice of violation letters, or untitled letters, and warning letters. In the case of an untitled letter, DDMAC typically alerts the drug company of the violation and issues a directive to refrain from future violations, but does not typically demand other corrective action. A warning letter is typically issued in cases that are more serious or where the company is a repeat offender. Although we have not received any such letters from DDMAC, we may inadvertently violate DDMAC's guidelines in the future and be subject to a DDMAC untitled letter or warning letter, which may have a negative impact on our business.
Risks Related to Our Industry
We may not be able to keep up with the rapid technological change in the biotechnology and pharmaceutical industries, which could make any future approved products obsolete and reduce our revenue.
Biotechnology and related pharmaceutical technologies have undergone and continue to be subject to rapid and significant change. Our future will depend in large part on our ability to maintain a competitive position with respect to these technologies. Our competitors may render our technologies obsolete by advances in existing technological approaches or the development of new or different approaches, potentially eliminating the advantages in our drug discovery process that we believe we derive from our research approach and proprietary technologies. In addition, any future products that we develop, including our clinical drug candidates, elesclomol, apilimod and STA-9090, and our preclinical drug candidate, STA-9584, may become obsolete before we recover expenses incurred in developing those products, which may require that we raise additional funds to continue our operations.
Our market is subject to intense competition. If we are unable to compete effectively, our drug candidates may be rendered noncompetitive or obsolete.
We are engaged in segments of the pharmaceutical industry that are highly competitive and rapidly changing. Many large pharmaceutical and biotechnology companies, academic institutions, governmental agencies, and other public and private research organizations are pursuing the development of novel drugs that target cancer and chronic inflammatory diseases. We face, and expect to continue to face, intense and increasing competition as new products enter the market and advanced technologies become available. In addition to currently approved drugs, there are a significant number of drugs that are currently under development and may become available in the future for the treatment of cancer and chronic inflammatory diseases. We would expect our drug candidates to compete with marketed drugs and drug candidates currently under development, including the following:
- •
- Elesclomol. If approved, we would expect elesclomol to compete with currently approved drugs for the treatment of metastatic melanoma, including dacarbazine/DTIC marketed by Bayer, and generic versions thereof, the injectable protein interleukin 2, or IL-2, marketed by Chiron, and the injectable protein interferon alfa-2b, marketed by Schering-Plough. Elesclomol may also compete with drug candidates currently in clinical development by other companies, including: (1) kinase inhibitors such as Nexavar, being developed by Bayer and Onyx, Sutent, being developed by Pfizer, and ispinesib, being developed by Cytokinetics and GSK; (2) the
57
anti-CTLA-4 monoclonal antibodies, ipilimumab and tremelumimab; (3) the anti-integrin volociximab; (4) injectable angiogenesis inhibitors, such as Avastin; (5) cancer vaccines such as M-Vax and MDX-1379; and (6) derivatives, analogs, or reformulations of known chemotherapies, such as Abraxane, or other cytotoxic chemotherapies. In addition, elesclomol may compete against drugs not currently approved for the treatment of metastatic melanoma, but which are commonly used off-label to treat this disease, such as taxanes, temozolomide, vincristine, carmustine, melphalan, and platinum-chemotherapeutics, such as cisplatin and carboplatin.
- •
- Apilimod. If approved, we would expect apilimod to compete with other treatments of chronic inflammatory diseases, including (1) large-molecule, injectable TNFa antagonists, such as Remicade, marketed by Johnson & Johnson, Enbrel, marketed by Amgen and Wyeth Pharmaceuticals, and Humira, marketed by Abbott Laboratories, (2) broadly immunosuppressive small molecule agents, including corticosteroids, methotrexate, and azathioprine, and (3) injectible antibodies targeting IL-12, including CNTO-1275 currently in clinical trials and ABT-874 currently awaiting approval, being developed by Johnson & Johnson and Abbott Laboratories, respectively.
- •
- STA-9090. If approved, we would expect STA-9090 to compete with the currently approved therapies for the treatment of cancers, and other cancer treatments currently under development, including 17-AAG, being developed by Kosan, and other agents that inhibit Hsp90, including Hsp90 inhibitors being developed by AstraZenica/Medimmune/Infinity, BiogenIdec, Novartis/Vernalis, and Astex.
- •
- STA-9584. If approved, we would expect STA-9584 to compete with the currently approved therapies for the treatment of cancers, and other cancer treatments currently under development, including other vascular disrupting agents, such as ABT-751, being developed by Abbott Laboratories; AS1404, being developed by Novartis/Antisoma, CA4P, being developed by Oxigene, EXEL-0999, being developed by Exelixis, and ZD6126, being developed by Angiogene.
Many of our competitors have:
- •
- significantly greater financial, technical and human resources than we have and may be better equipped to discover, develop, manufacture and commercialize drug candidates;
- •
- more extensive experience in preclinical testing and clinical trials, obtaining regulatory approvals and manufacturing and marketing pharmaceutical products;
- •
- drug candidates that have been approved or are in late-stage clinical development; and/or
- •
- collaborative arrangements in our target markets with leading companies and research institutions.
Competitive products may render our products obsolete or noncompetitive before we can recover the expenses of developing and commercializing our drug candidates. Furthermore, the development of new treatment methods and/or the widespread adoption or increased utilization of any vaccine for the diseases we are targeting could render our drug candidates noncompetitive, obsolete or uneconomical. If we successfully develop and obtain approval for our drug candidates, we will face competition based on the safety and effectiveness of our drug candidates, the timing of their entry into the market in relation to competitive products in development, the availability and cost of supply, marketing and sales capabilities, reimbursement coverage, price, patent position and other factors. If we successfully develop drug candidates but those drug candidates do not achieve and maintain market acceptance, our business will not be successful.
58
Risks Related to Employee Matters and Managing Growth
Our future success depends on our ability to retain our chief executive officer and other key executives and to attract, retain, and motivate qualified personnel.
We are highly dependent on Safi R. Bahcall, Ph.D., our President and Chief Executive Officer, and the other principal members of our executive and scientific teams. All of the agreements with these principal members of our executive and scientific teams provide that employment is at-will and may be terminated by the employee at any time and without notice. Although we do not have any reason to believe that we may lose the services of any of these persons in the foreseeable future, the loss of the services of any of these persons might impede the achievement of our research, development, and commercialization objectives. Recruiting and retaining qualified scientific personnel and possibly sales and marketing personnel will also be critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific personnel from universities and research institutions. We do not maintain "key person" insurance on any of our employees. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us.
We expect to expand our development, clinical research, and marketing capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We expect to experience significant growth in the number of our employees and the scope of our operations. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational, and financial systems, expand our facilities, and continue to recruit and train additional qualified personnel. Due to our limited resources, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The physical expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.
If we make strategic acquisitions, we will incur a variety of costs and might never realize the anticipated benefits.
All of our acquisitions to date have been of related parties. Accordingly, we have very limited experience in independently identifying acquisition candidates and integrating the operations of truly independent acquisition candidates with our company. Currently we are not a party to any acquisition agreements, nor do we have any understanding or commitment with respect to any such acquisition. If appropriate opportunities become available, however, we might attempt to acquire approved products, additional drug candidates, or businesses that we believe are a strategic fit with our business. If we pursue any transaction of that sort, the process of negotiating the acquisition and integrating an acquired product, drug candidate, or business might result in operating difficulties and expenditures and might require significant management attention that would otherwise be available for ongoing development of our business, whether or not any such transaction is ever consummated. Moreover, we might never realize the anticipated benefits of any acquisition. Future acquisitions could result in potentially dilutive issuances of equity securities, the incurrence of debt, contingent liabilities, or impairment expenses related to goodwill, and impairment or amortization expenses related to other intangible assets, which could harm our financial condition.
59
Risks Related to Our Common Stock
Our stock price has been and is likely to continue to be volatile and the market price of our common stock may drop.
Prior to our February 2007 initial public offering, there was not a public market for our common stock. There is a limited history on which to gauge the volatility of our stock price; however, since our common stock began trading on The NASDAQ Global Market on February 6, 2007 through December 31, 2007, our stock price has fluctuated from a low of $4.93 to a high of $11.25. In addition, the stock market has recently experienced significant volatility, particularly with respect to pharmaceutical, biotechnology, and other life sciences company stocks. The volatility of pharmaceutical, biotechnology, and other life sciences company stocks often does not relate to the operating performance of the companies represented by the stock. Some of the factors that may cause the market price of our common stock to fluctuate include:
- •
- progress in and results from the SYMMETRY trial, our global, pivotal Phase 3 clinical trial of elesclomol for the treatment of metastatic melanoma;
- •
- plans for, progress in, and results from any other future clinical trials of elesclomol;
- •
- results of our current Phase 2a or any future clinical trials of apilimod we may initiate;
- •
- results of our current Phase 1 clinical trials of STA-9090, and results from any other future clinical trials of STA-9090;
- •
- results of clinical trials conducted by others on drugs that would compete with our drug candidates;
- •
- failure or delays in advancing STA-9584 or our CRAC ion channel inhibitor program, or other drug candidates we may discover or acquire in the future, into clinical trials;
- •
- failure or discontinuation of any of our research programs;
- •
- developments relating to our agreement with GSK or any future collaborations we may enter into;
- •
- issues in manufacturing our drug candidates or approved products;
- •
- regulatory developments or enforcement in the United States and foreign countries;
- •
- developments or disputes concerning patents or other proprietary rights;
- •
- introduction of technological innovations or new commercial products by us or our competitors;
- •
- changes in estimates or recommendations by securities analysts, if any cover our common stock;
- •
- public concern over our drug candidates or any approved products;
- •
- litigation;
- •
- future sales of our common stock;
- •
- general market conditions;
- •
- changes in the structure of healthcare payment systems;
- •
- failure of any of our drug candidates, if approved, to achieve commercial success;
- •
- economic and other external factors or other disasters or crises;
- •
- period-to-period fluctuations in our financial results; and
- •
- overall fluctuations in U.S. equity markets.
60
These and other external factors may cause the market price and demand for our common stock to fluctuate substantially, which may limit or prevent investors from readily selling their shares of common stock and may otherwise negatively affect the liquidity of our common stock. In addition, in the past, when the market price of a stock has been volatile, holders of that stock have instituted securities class action litigation against the company that issued the stock. If any of our stockholders brought a lawsuit against us, we could incur substantial costs defending the lawsuit. Such a lawsuit could also divert the time and attention of our management.
Insiders have substantial control over us which could delay or prevent a change in corporate control or result in the entrenchment of management and/or the board of directors.
Our directors, executive officers and principal stockholders, together with their affiliates and related persons, beneficially own, in the aggregate, approximately 48% of our outstanding common stock. These stockholders, if acting together, may have the ability to determine the outcome of matters submitted to our stockholders for approval, including the election and removal of directors and any merger, consolidation, or sale of all or substantially all of our assets. In addition, these persons, acting together, may have the ability to control the management and affairs of our company. Accordingly, this concentration of ownership may harm the market price of our common stock by:
- •
- delaying, deferring, or preventing a change in control;
- •
- entrenching our management and/or the board of directors;
- •
- impeding a merger, consolidation, takeover, or other business combination involving us; or
- •
- discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us.
Provisions of our charter, bylaws, and Delaware law may make an acquisition of us or a change in our management more difficult.
Certain provisions of our restated certificate of incorporation and restated bylaws could discourage, delay, or prevent a merger, acquisition, or other change in control that stockholders may consider favorable, including transactions in which stockholders might otherwise receive a premium for their shares. These provisions also could limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock. Stockholders who wish to participate in these transactions may not have the opportunity to do so. Furthermore, these provisions could prevent or frustrate attempts by our stockholders to replace or remove our management. These provisions:
- •
- allow the authorized number of directors to be changed only by resolution of our board of directors;
- •
- establish a classified board of directors, providing that not all members of the board of directors be elected at one time;
- •
- authorize our board of directors to issue without stockholder approval blank check preferred stock that, if issued, could operate as a "poison pill" to dilute the stock ownership of a potential hostile acquirer to prevent an acquisition that is not approved by our board of directors;
- •
- require that stockholder actions must be effected at a duly called stockholder meeting and prohibit stockholder action by written consent;
- •
- establish advance notice requirements for stockholder nominations to our board of directors or for stockholder proposals that can be acted on at stockholder meetings;
- •
- limit who may call stockholder meetings; and
61
- •
- require the approval of the holders of 80% of the outstanding shares of our capital stock entitled to vote in order to amend certain provisions of our restated certificate of incorporation and restated bylaws.
In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which may, unless certain criteria are met, prohibit large stockholders, in particular those owning 15% or more of our outstanding voting stock, from merging or combining with us for a prescribed period of time.
We do not anticipate paying cash dividends, and accordingly, our stockholders must rely on stock appreciation for any return on their investment.
We currently intend to retain our future earnings, if any, to fund the development and growth of our business. As a result, capital appreciation, if any, of our common stock will be the sole source of gain on an investment in our common stock for the foreseeable future.
Item 1B. UNRESOLVED STAFF COMMENTS
Not applicable.
Item 2. PROPERTIES
Our operations are based primarily in Lexington, Massachusetts, which is located approximately 10 miles west of Boston, Massachusetts. We currently lease a total of 68,730 square feet of office and laboratory space in Lexington and 15,000 square feet of office and laboratory space in the neighboring town of Bedford, Massachusetts. We lease the following properties:
Location
| | Approximate
Square Feet
| | Use
| | Lease
Expiration Date
|
|---|
45 Hartwell Avenue
Lexington, Massachusetts |
|
24,420 |
|
Office and Laboratory |
|
Nov. 2011 |
91 Hartwell Avenue
Lexington, Massachusetts |
|
21,830 |
|
Office |
|
August 2009 |
125 Hartwell Avenue
Lexington, Massachusetts |
|
22,480 |
|
Office and Laboratory |
|
Nov. 2011 |
45-47 Wiggins Avenue
Bedford, Massachusetts |
|
15,000 |
|
Office and Laboratory |
|
Oct. 2011 |
We believe our facilities are adequate for our current needs.
Item 3. LEGAL PROCEEDINGS
We are currently not a party to any material legal proceedings.
Item 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
No matters were submitted to a vote of security holders during the fourth quarter of the year ended December 31, 2007.
62
PART II
Item 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock began trading on The NASDAQ Global Market on February 6, 2007 under the symbol "SNTA." Prior to that time, there was no established public trading market for our common stock. The following table sets forth the high and low sales prices of our common stock as quoted on The NASDAQ Global Market for the periods indicated.
2007:
| | High
| | Low
|
|---|
| First Quarter (from February 6, 2007) | | $ | 10.10 | | $ | 8.07 |
| Second Quarter | | | 10.27 | | | 7.92 |
| Third Quarter | | | 9.86 | | | 4.93 |
| Fourth Quarter | | | 11.25 | | | 6.31 |
Stockholders
As of March 14, 2008, there were approximately 149 stockholders of record of the 33,873,538 outstanding shares of our common stock.
Dividends
We have never paid or declared any cash dividends on our common stock. We currently intend to retain all available funds and any future earnings to fund the development and expansion of our business, and we do not anticipate paying any cash dividends in the foreseeable future. Any future determination to pay dividends will be at the discretion of our board of directors and will depend on our financial condition, results of operations, capital requirements, and other factors that our board of directors deems relevant. In addition, the terms of any future debt or credit facility may preclude us from paying dividends.
Unregistered Sales of Securities
During the year ended December 31, 2007, we sold 2,750 shares of common stock to employees or former employees through the exercise of options that were not registered under the Securities Act. These shares were issued pursuant to written compensatory plans or arrangements with our employees, directors and consultants, in reliance on the exemption from registration provided by Rule 701 under the Securities Act.
Issuer Purchases of Equity Securities
None.
Use of Proceeds from Registered Securities
The Registration Statement on Form S-1 (Reg. No. 333-138894) in connection with our initial public offering was declared effective by the Securities and Exchange Commission on February 6, 2007. In our initial public offering, we sold 5,000,000 shares of our common stock at an initial public offering price per share of $10.00. As of December 31, 2007, all of the net proceeds of the offering had been used to fund operations. There had been no material change in the planned use of proceeds from our initial public offering as described in our final prospectus dated February 6, 2007 filed with the SEC pursuant to Rule 424(b)(4).
63
Stock Performance Graph
The following graph compares the cumulative total stockholder return on our common stock from February 6, 2007 (the first trading date following our initial public offering) to December 31, 2007 with the cumulative total return of (i) the NASDAQ Market Index and (ii) the NASDAQ Biotechnology Index. This graph assumes the investment of $100.00 on February 6, 2007 in our common stock, the NASDAQ Market Index and the NASDAQ Biotechnology Index, and assumes any dividends are reinvested. We have not paid any dividends on our common stock, and we do not include dividends in the representation of our performance. The stock price performance on the graph below does not necessarily indicate future price performance.
COMPARISON OF CUMULATIVE TOTAL RETURN
AMONG SYNTA PHARMACEUTICALS CORP.,
NASDAQ BIOTECH AND NASDAQ MARKET INDEX
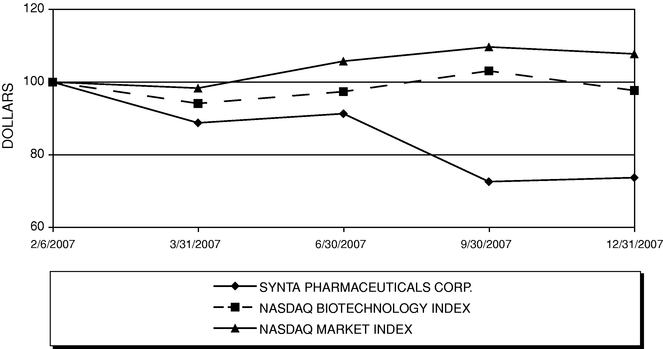
| | 2/06/2007
| | 3/31/2007
| | 6/30/2007
| | 9/30/2007
| | 12/31/2007
|
|---|
| Synta Pharmaceuticals Corp. | | $ | 100.00 | | $ | 88.78 | | $ | 91.31 | | $ | 72.61 | | $ | 73.71 |
| NASDAQ Biotech | | $ | 100.00 | | $ | 94.11 | | $ | 97.37 | | $ | 103.07 | | $ | 97.68 |
| NASDAQ Market Index | | $ | 100.00 | | $ | 98.33 | | $ | 105.74 | | $ | 109.66 | | $ | 107.75 |
The information in this section shall not be deemed "soliciting material" or to be "filed" with the Securities and Exchange Commission, and is not to be incorporated by reference in any filing of Synta Pharmaceuticals Corp. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Annual Report on Form 10-K and irrespective of any general incorporation language in those filings.
64
Item 6. SELECTED FINANCIAL DATA
The following table sets forth our selected consolidated financial data and has been derived from our audited consolidated financial statements. Consolidated balance sheets as December 31, 2007 and 2006, as well as consolidated statements of operations for the years ended December 31, 2007, 2006, and 2005, and the report thereon are included elsewhere in this Annual Report on Form 10-K. The information below should be read in conjunction with our audited consolidated financial statements (and notes thereon) and "Management's Discussion and Analysis of Financial Condition and Results of Operations," included below in Item 7.
| | Years ended December 31,
| |
|---|
| | 2007
| | 2006
| | 2005
| | 2004
| | 2003
| |
|---|
| Consolidated Statement of Operations Data: | | | | | | | | | | | | | | | | |
| Collaboration revenue | | $ | 743 | | $ | — | | $ | — | | $ | — | | $ | — | |
| Grant revenue | | | — | | | — | | | — | | | 173 | | | 1,304 | |
| | |
| |
| |
| |
| |
| |
| Total revenues | | | 743 | | | — | | | — | | | 173 | | | 1,304 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating expenses | | | | | | | | | | | | | | | | |
| | Research and development | | | 52,025 | | | 50,503 | | | 59,901 | | | 38,136 | | | 24,337 | |
| | In-process research and development | | | | | | — | | | — | | | 1,583 | | | — | |
| | General and administrative | | | 14,934 | | | 8,648 | | | 11,279 | | | 7,383 | | | 5,261 | |
| | |
| |
| |
| |
| |
| |
| | Total operating expenses | | | 66,959 | | | 59,151 | | | 71,180 | | | 47,102 | | | 29,598 | |
| | |
| |
| |
| |
| |
| |
| Loss from operations | | | (66,216 | ) | | (59,151 | ) | | (71,180 | ) | | (46,929 | ) | | (28,294 | ) |
| Investment income, net | | | 2,721 | | | 1,881 | | | 2,317 | | | 995 | | | 416 | |
| | |
| |
| |
| |
| |
| |
| Net loss | | | (63,495 | ) | | (57,270 | ) | | (68,863 | ) | | (45,934 | ) | | (27,878 | ) |
| Convertible preferred stock dividends | | | — | | | 1,859 | | | — | | | — | | | — | |
| Convertible preferred stock beneficial conversion charge | | | 58,585 | | | — | | | — | | | — | | | — | |
| | |
| |
| |
| |
| |
| |
| Net loss attributable to common stockholders | | $ | (122,080 | ) | $ | (59,129 | ) | $ | (68,863 | ) | $ | (45,934 | ) | $ | (27,878 | ) |
| | |
| |
| |
| |
| |
| |
| Basic and diluted net loss attributable to common stockholders per share | | $ | (3.76 | ) | $ | (2.66 | ) | $ | (3.09 | ) | $ | (2.46 | ) | $ | (1.86 | ) |
| Weighted average shares used in computing basic and diluted net loss per common share | | | 32,466 | | | 22,265 | | | 22,253 | | | 18,704 | | | 15,024 | |
| | As of December 31,
| |
|---|
| | 2007
| | 2006
| | 2005
| | 2004
| | 2003
| |
|---|
| Consolidated Balance Sheet Data: | | | | | | | | | | | | | | | | |
| Cash, cash equivalents and marketable securities | | $ | 115,577 | | $ | 46,824 | | $ | 62,057 | | $ | 124,968 | | $ | 76,226 | |
| Working capital | | | 96,225 | | | 36,081 | | | 48,476 | | | 113,147 | | | 73,564 | |
| Total assets | | | 122,649 | | | 54,789 | | | 71,210 | | | 132,019 | | | 80,387 | |
| Capital lease obligations, net of current portion | | | 2,815 | | | 3,170 | | | 4,259 | | | 1,188 | | | — | |
| Deferred collaboration revenue, net of current portion(1) | | | 74,166 | | | — | | | — | | | — | | | — | |
| Convertible preferred stock | | | — | | | 41,820 | | | — | | | — | | | — | |
| Common stock | | | 3 | | | 2 | | | 2 | | | 2 | | | 2 | |
| Additional paid-in capital | | | 324,946 | | | 234,807 | | | 239,029 | | | 238,930 | | | 144,154 | |
| Accumulated deficit | | | (300,053 | ) | | (236,558 | ) | | (179,288 | ) | | (110,425 | ) | | (64,491 | ) |
| Total stockholders' equity (deficit) | | | 24,896 | | | (1,747 | ) | | 52,477 | | | 117,956 | | | 76,891 | |
- (1)
- In October 2007, we entered into the GSK Agreement with GSK for elesclomol. See Notes 2 and 8 in the accompanying consolidated financial statements.
65
Item 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
This Management's Discussion and Analysis of Financial Condition and Results of Operations should be read together with the consolidated financial statements, related notes and other financial information included elsewhere in this Annual Report on Form 10-K.
Overview
We are a biopharmaceutical company focused on discovering, developing, and commercializing small molecule drugs to extend and enhance the lives of patients with severe medical conditions, including cancer and chronic inflammatory diseases. We have a unique chemical compound library, an integrated discovery engine, and a diverse pipeline of clinical- and preclinical-stage drug candidates with distinct mechanisms of action and novel chemical structures. We have three drug candidates in clinical trials, one drug candidate in preclinical studies, and one program in the lead optimization stage of discovery, as well as other programs in earlier stages of discovery. We discovered and developed each of our drug candidates internally using our compound library and discovery capabilities. At present, other than our lead drug candidate, elesclomol, we retain all rights to each of our drug candidates and programs, across all geographic markets and therapeutic indications. We have entered into a partnership with GlaxoSmithKline, or GSK, for the joint development and commercialization of elesclomol.
Our Lead Drug Candidate, Elesclomol (formerly, STA-4783)
Our most advanced clinical-stage drug candidate, elesclomol, is a novel, injectable, small molecule compound that triggers apoptosis, or programmed cell death, in cancer cells, which we believe has potential for the treatment of a broad range of cancer types.
In September 2006, we announced positive results for elesclomol in combination with paclitaxel, a leading chemotherapeutic agent, in a double-blind, randomized, controlled, multicenter Phase 2b clinical trial in patients with stage IV metastatic melanoma. We believe that this is the first blinded clinical trial of a drug candidate for the treatment of metastatic melanoma in 30 years to meet its primary endpoint with statistical significance. In November 2006, we received Fast Track designation from the U.S. Food and Drug Administration, or FDA, for the development of elesclomol for the treatment of metastatic melanoma. In December 2007, we received orphan drug designation for elesclomol in this indication in the United States from the FDA. Orphan drug status is designed to encourage biotechnology and pharmaceutical companies to develop drugs for rare diseases affecting fewer than 200,000 people in the United States. Assuming that elesclomol is approved by the FDA, we will be entitled to seven years of market exclusivity for elesclomol for the treatment of patients with metastatic melanoma.
Based on the results of our Phase 2b trial, we initiated a global, pivotal Phase 3 clinical trial of elesclomol in metastatic melanoma, called the SYMMETRY trial, in the third quarter of 2007. The SYMMETRY trial is being conducted under the terms of a Special Protocol Assessment, or SPA, agreed to by the FDA. The SPA process provides for a written agreement between a clinical trial sponsor and the FDA that the proposed design and planned analyses of the clinical trial is sufficient to support regulatory approval of a drug candidate, unless public health concerns unrecognized at the time of the protocol assessment become evident. The SYMMETRY trial is enrolling patients with stage IV metastatic melanoma who have not received prior chemotherapy but who may have already been treated with non-chemotherapeutic agents, such as biologics. Approximately 630 patients will be enrolled in the blinded, randomized, controlled study, which generally mirrors the design of our Phase 2b trial and will be conducted at approximately 150 centers worldwide.
66
As with our prior Phase 2b trial, patients enrolled in the SYMMETRY trial will be randomized to receive either elesclomol plus paclitaxel or paclitaxel alone. The dosage of each agent, the dosing schedule, and the primary endpoint—progression free survival, or PFS—are the same as in our prior Phase 2b trial. The SYMMETRY trial increases the total number of patients enrolled from the prior Phase 2b trial and includes central review of radiology scans, stratification to ensure balance between treatment and control arms, and a no-crossover design for facilitating the assessment of overall survival, or OS.
Based on our current enrollment projections and event rate targets, we expect to complete enrollment and initiate the primary endpoint analysis of the SYMMETRY trial by the end of 2008. Assuming that the results of the PFS analysis are positive, we plan to submit a new drug application, or NDA, to the FDA in the first half of 2009. If actual enrollment or event rates differ from our current projections, our target dates for completing the PFS analysis and submitting the NDA will likely change.
In October 2007, we entered into a collaborative development, commercialization and license Agreement with GSK for elesclomol, under which we are eligible to receive up to $1.01 billion in milestones and other payments, as well as share 40-50% of the profits and losses from sales in the United States and receive double-digit tiered royalties from sales outside of the United States. Under the terms of the GSK Agreement, the companies will jointly develop and commercialize elesclomol in the United States, and GSK will have exclusive responsibility for the development and commercialization of elesclomol outside the United States. Pursuant to the agreement, we received a non-refundable upfront cash payment of $80 million in November 2007. We are also eligible to receive potential pre-commercial milestone payments from GSK of up to $585 million, which include both payments for operational progress, such as trial initiation and enrollment, and payments for positive clinical and regulatory outcomes, such as regulatory approval. Of the $585 million in potential payments, $135 million are related to the development in metastatic melanoma and up to $450 million are related to the development of elesclomol in other cancer indications. In addition, we are eligible to receive up to $300 million in potential commercial milestone payments from GSK based on achieving certain net sales thresholds. We will take the lead role and fund, up to a specified amount, all activities related to seeking FDA approval of elesclomol for the treatment of metastatic melanoma. We will also fund early clinical development of elesclomol in two other cancer indications. All other worldwide development costs will be shared, with us responsible for a modest proportion of those costs. In the United States, our share of the operating profits and losses from the commercialization and sales of elesclomol will be 40-50%, with the percentage increasing as the level of annual sales increases. We may elect not to participate in co-commercialization, in which case we would earn royalties in lieu of profit-sharing. Outside of the United States, we will receive double-digit tiered royalties. Under the GSK Agreement, GSK may, subject to our agreement, purchase up to $45 million of our common stock in two separate tranches upon the achievement of specified development and regulatory milestones. In the first tranche, GSK would be obligated to buy $25 million of our common stock at our sole discretion. We attributed $260,000 of value to this option to require GSK to purchase our common stock. The second tranche of $20 million of common stock would be subject to the agreement of both us and GSK. The per share purchase price under each tranche would be at a specified premium. GSK may terminate the agreement upon not less than three months' written notice at any time prior to the date of the first commercial sale of an elesclomol product and upon not less than six months' written notice at any time on and after such date, in which case GSK may be obligated in certain circumstances to make additional payments to us. Under the GSK Agreement, we have the right, but not the obligation to participate in various joint governance committees. The agreement was subject to the Hart-Scott-Rodino Act and has received clearance by the U.S. government.
67
Our Other Oncology Drug Candidates and Research Programs
STA-9090. STA-9090 is a novel, injectable, small molecule drug candidate we are developing for the treatment of cancer. STA-9090 inhibits heat shock protein 90, or Hsp90, a chaperone protein that regulates the activity of numerous signaling proteins that trigger uncontrolled proliferation in cancer cells, in particular kinase proteins. Examples of kinase proteins include c-Kit, Bcr-Abl, Her2, EGFR, and others that are the targets of approved direct kinase inhibitors such as Gleevec, Herceptin, Tarceva, and Erbitux. We believe that inhibiting kinases indirectly, by disrupting the chaperone activities of Hsp90, provides two advantages: first, a means to simultaneously attack multiple cancer-promoting kinases; and, second, an ability to kill tumor cells with mutated kinases that have lost responsiveness to a direct kinase inhibitor. We have shown in preclinical experiments that STA-9090 is significantly more potent against certain types of cancer cells than Gleevec, as well as the two Hsp90 inhibitors furthest along in clinical development, 17-AAG and 17-DMAG. STA-9090 is further differentiated from these Hsp90 inhibitors because it is a novel chemical structure that is not a derivative or analog of the natural product geldanamycin. We believe that this creates a distinct activity profile for STA-9090 and is a competitive advantage. We are currently conducting two Phase 1 studies to identify the maximum tolerated dose of STA-9090 based on once- and twice-a-week intravenous dosing schedules, respectively. In addition to an evaluation of safety and tolerability, patients in these studies will be assessed for biological activity based on biomarker responses and clinical response rates based on the RECIST criteria.
STA-9584. STA-9584 is a novel, injectable, small molecule compound that disrupts the blood vessels that supply tumors with oxygen and essential nutrients. In preclinical experiments, STA-9584 has shown strong anti-tumor activity in a broad range of cancer models, including prostate, lung, breast, melanoma, and lymphoma. In preclinical testing, STA-9584 has been shown to act against established tumor vessels, a mechanism that is differentiated from the mechanism of anti-angiogenesis inhibitors such as Avastin, which prevents the formation of new tumor vessels. This program is currently in preclinical development.
Autoimmune and Inflammatory Diseases
Apilimod (STA-5326). Apilimod is a novel, orally administered, small molecule drug candidate we are developing for the treatment of autoimmune and other chronic inflammatory diseases. Apilimod inhibits the production of the cytokines interleukin-12, or IL-12, and interleukin-23, or IL-23, and thereby down-regulates the inflammation pathways that underlie certain autoimmune and inflammatory diseases. We are currently conducting a Phase 2a clinical trial of apilimod in patients with rheumatoid arthritis, or RA. The preliminary results of the first 22 patients in the RA trial showed encouraging biomarker and clinical signals suggesting activity of apilimod in this indication. We have elected to enroll an additional cohort in the RA Phase 2a trial to explore a higher dose of apilimod. We expect to complete enrollment of this higher dose cohort in the second half of 2008.
CRAC ion channel inhibitor. We have developed novel, small molecule inhibitors of calcium release activated calcium, or CRAC, ion channels expressed on immune cells. The CRAC ion channel is the primary route for calcium entry into T cells and other immune cells, regulating multiple immune cell processes important for initiating and maintaining an inflammatory immune response. We have demonstrated in preclinical experiments that our CRAC ion channel inhibitors selectively inhibit the production of critical pro-inflammatory cytokines, such as interleukin-2, or IL-2, and TNFa by immune cells, and that these compounds are effective in multiple animal models of immune diseases, including models of arthritis. This program is in the lead optimization stage of discovery.
68
Initial Public Offering
In February 2007, we raised $50.0 million in gross proceeds from the sale of 5,000,000 shares of our common stock in our initial public offering, or the IPO, at $10.00 per share. The net offering proceeds to us after deducting approximately $5.3 million in expenses for underwriters' discounts, fees and commissions, legal, accounting, printing, listing and filing fees, and miscellaneous expenses were approximately $44.7 million. All outstanding shares of our Series A convertible preferred stock and $1.9 million in accumulated dividends on the Series A convertible preferred stock were converted into 6,278,765 shares of common stock upon the completion of the IPO. In accordance with Emerging Issues Task Force, or EITF, No. 98-5,Accounting for Convertible Securities with Beneficial Conversion Features or Contingently Adjustable Conversion Ratios, and EITF No. 00-27,Application of Issue No. 98-5 to Certain Convertible Instruments, we recorded a non-cash beneficial conversion charge of approximately $58.6 million in February 2007 in connection with the contingent adjustable conversion feature of the Series A convertible preferred stock.
We were incorporated in March 2000 and commenced operations in July 2001. Since that time, we have been principally engaged in raising capital and in the discovery and development of novel drug candidates.
Since our inception, we have had no revenues from product sales. We have funded our operations principally with $195.4 million in net proceeds from private placements of our common stock, $40.0 million in net proceeds from a private placement of our Series A convertible preferred stock, $44.7 million in net proceeds from our initial public offering, and an $80 million non-refundable upfront payment under the GSK Agreement, which, together with the exercise of common stock warrants and options, provided aggregate net cash proceeds of approximately $361.4 million through December 31, 2007.
We have devoted substantially all of our capital resources to the research and development of our drug candidates. We have never been profitable and, as of December 31, 2007, we had an accumulated deficit of $300.1 million. We expect to incur significant and increasing operating losses for the foreseeable future as we advance our drug candidates from discovery through preclinical development and clinical trials and seek regulatory approval and eventual commercialization. In addition to these increasing research and development expenses, we expect general and administrative costs to increase in connection with additional headcount, public-company requirements and compliance, commercial development and medical community relations, as we, together with GSK, prepare for the potential launch of elesclomol. We will need to generate significant revenues to achieve profitability and may never do so.
Financial Operations Overview
We have not yet generated any product revenue and do not expect to generate any product revenue for the foreseeable future. We will seek to generate revenue from product sales and from future collaborative or strategic relationships, which could include research and development, milestone payments, profit sharing and royalties. In October 2007, we entered into the GSK Agreement with GSK for our lead drug candidate, elesclomol. The $80 million non-refundable upfront payment we received from GSK in November 2007, together with the $260,000 estimated value of an option to require GSK to purchase $25 million of our common stock, is being recognized as collaboration revenue using the time-based model over the estimated performance period, the 15-year period through the earliest expiration date of the related patents, which we estimate to be the effective life of the GSK Agreement (see Revenue Recognition in the Critical Accounting Policies and Estimates section). In 2007, we recognized $743,000 of collaboration revenue under the GSK Agreement. In the future, we expect any revenue we generate will fluctuate from quarter-to-quarter as a result of the timing and amount of
69
payments received under the GSK Agreement and from future collaborations or strategic relationships, and the amount and timing of payments we receive upon the sale of our drug candidates, to the extent any are successfully commercialized.
Research and development expense consists of costs incurred in connection with developing and advancing our drug discovery technology and identifying and developing our drug candidates. We charge all research and development expenses to operations as incurred.
Our research and development expense consists of:
- •
- internal costs associated with research, preclinical and clinical activities;
- •
- payments to third party contract research organizations, investigative sites and consultants in connection with our preclinical and clinical development programs;
- •
- costs associated with drug formulation and supply of drugs for clinical trials;
- •
- personnel related expenses, including salaries, stock-based compensation, benefits and travel; and
- •
- overhead expenses, including rent and maintenance of our facilities, and laboratory and other supplies.
We do not know if we will be successful in developing our drug candidates. While expenses associated with the completion of our current clinical programs are expected to be substantial and increase, we believe that accurately projecting total program-specific expenses through commercialization is not possible at this time. The timing and amount of these expenses will depend upon the costs associated with potential future clinical trials of our drug candidates, and the related expansion of our research and development organization, regulatory requirements, advancement of our preclinical programs and product manufacturing costs, many of which cannot be determined with accuracy at this time based on our stage of development. This is due to the numerous risks and uncertainties associated with the duration and cost of clinical trials, which vary significantly over the life of a project as a result of unanticipated events arising during clinical development, including with respect to:
- •
- the number of clinical sites included in the trial;
- •
- the length of time required to enroll suitable subjects;
- •
- the number of subjects that ultimately participate in the trials; and
- •
- the efficacy and safety results of our clinical trials and the number of additional required clinical trials.
Our expenditures are subject to additional uncertainties, including the terms and timing of regulatory approvals and the expense of filing, prosecuting, defending or enforcing any patent claims or other intellectual property rights. In addition, we may obtain unexpected or unfavorable results from our clinical trials. We may elect to discontinue, delay or modify clinical trials of some drug candidates or focus on others. A change in the outcome of any of the foregoing variables in the development of a drug candidate could mean a significant change in the costs and timing associated with the development of that drug candidate. For example, if the FDA or other regulatory authority were to require us to conduct clinical trials beyond those that we currently anticipate, or if we experience significant delays in any of our clinical trials, we would be required to expend significant additional financial resources and time on the completion of clinical development. Additionally, future commercial and regulatory factors beyond our control will evolve and therefore impact our clinical development programs and plans over time.
70
Despite this uncertainty, however, our development strategy for our lead clinical-stage drug candidate, elesclomol, is currently based on a number of assumptions that allow us to make broad estimates of certain clinical trial expenses. We initiated the SYMMETRY trial, our global, pivotal Phase 3 clinical trial of elesclomol for the treatment of metastatic melanoma, in the third quarter of 2007, and we expect the remaining costs necessary for the NDA submission, including the cost of the clinical trial, clinical drug supplies, registration manufacturing and regulatory activities necessary to compile the NDA submission, together with the costs of related nonclinical toxicology and other testing to support the trial, will be in the range of $60 million to $70 million. We do not expect to receive regulatory approval of any of our drug candidates until 2009 at the earliest, if at all.
Beyond our three lead drug candidates, we anticipate that we will select drug candidates and research projects for further development on an ongoing basis in response to their preclinical and clinical success, as well as commercial potential.
General and administrative expense consists primarily of salaries and related expenses for personnel in executive, finance, business and commercial development, investor and medical community relations, human resources and administrative functions. Other costs include stock-based compensation costs, directors' and officers' liability insurance premiums, legal costs of pursuing patent protection of our intellectual property, fees for general legal, accounting, public-company requirements and compliance, and other professional services, as well as overhead-related costs not otherwise included in research and development. We anticipate increases in costs of commercial development and medical community relations, as we, together with GSK, prepare for the potential launch of elesclomol.
Convertible preferred stock dividends consisted of cumulative but undeclared dividends that were payable on our Series A convertible preferred stock. The Series A convertible preferred stock accrued dividends at 8% per year. All outstanding shares of our Series A convertible preferred stock and the $1.9 million in accumulated dividends were converted into 6,278,765 shares of our common stock upon completion of the IPO in February 2007.
Critical Accounting Policies and Estimates
Our management's discussion and analysis of our financial condition and results of operations are based on our financial statements which have been prepared in accordance with U.S. generally accepted accounting principles, or GAAP. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reported periods. We are required to make estimates and judgments with respect to accrued expenses, acquisitions and stock-based compensation. We base our estimates on historical experience, known trends and events, and various other factors that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources and the reported amounts of revenues and expenses. Actual results may differ from these estimates under different assumptions or conditions.
We believe the following accounting policies and estimates are most critical to aid you in understanding and evaluating our reported financial results.
71
Our principal sources of revenue may include upfront payments, development milestone payments, reimbursements of development costs, profit sharing payments, sales milestones and royalties from our collaborations. We recognize revenue from these sources in accordance with Staff Accounting Bulletin 104, "Revenue Recognition", or SAB 104, Emerging Issues Task Force, or EITF, No. 99-19, "Reporting Revenue Gross as a Principal Versus Net as an Agent", or EITF No. 99-19, and EITF No. 00-21, "Revenue Arrangements with Multiple Deliverables", or EITF No. 00-21. The application of EITF No. 00-21 requires subjective analysis and requires management to make estimates and assumptions about whether deliverables within multiple- element arrangements are separable from the other aspects of the contractual arrangement into separate units of accounting and to determine the fair value to be allocated to each unit of accounting.
We entered into the GSK Agreement with GSK in October 2007. We evaluated the multiple deliverables within the GSK Agreement in accordance with the provisions of EITF No. 00-21 to determine whether the delivered elements that are our obligation have value to GSK on a stand-alone basis and whether objective reliable evidence of fair value of the undelivered items exists. Deliverables that meet these criteria are considered a separate unit of accounting. Deliverables that do not meet these criteria are combined and accounted for as a single unit of accounting. The appropriate recognition of revenue is then applied to each separate unit of accounting.
Our deliverables under the GSK Agreement, including the related rights and obligations, contractual cash flows and performance periods, are more fully described in Note 8 in the accompanying consolidated financial statements, and are considered a single unit of accounting.
The GSK Agreement consists of the following key funding streams: a non-refundable upfront payment, product development milestone payments, reimbursements of certain development costs, sales milestone payments, profit sharing payments and product royalty payments. The cash flows associated with the single unit of accounting from the development portion of the GSK Agreement are recognized as revenue using a time-based model. Under this model, cash flow streams are recognized as revenue over the estimated performance period. Upon receipt of cash payments, revenue is recognized to the extent the accumulated service time, if any, has occurred. The remainder is deferred and recognized as revenue ratably over the remaining estimated performance period. A change in the period of time expected to complete the deliverable is accounted for as a change in estimate on a prospective basis. Revenue is limited to amounts that are nonrefundable and that GSK is contractually obligated to pay us.
The $80 million non-refundable upfront payment we received from GSK in November 2007, together with the $260,000 estimated value of an option to require GSK to purchase $25 million of our common stock, is being recognized as collaboration revenue using the time-based model over the estimated performance period, the 15-year period through the earliest expiration date of the related patents, which we estimate to be the effective life of the GSK Agreement. We are also recognizing product development milestone payments and reimbursements of development costs as collaboration revenue using the time-based model over the same performance period through November 2022. Based on the guidance of EITF No. 99-19, we have determined that we are acting as a principal under the GSK Agreement and, as such, have recorded these amounts as collaboration revenue. In 2007, we recognized $743,000 of collaboration revenue under the GSK Agreement.
Profit sharing payments are based upon a formula that provides for a range of 40-50% of net profits earned on U.S. sales of products included in the GSK Agreement. Royalty revenues are based upon a percentage of sales in non-U.S. territories. Profit sharing payments and royalties from the sales of products included in the GSK Agreement will be recorded on the accrual basis when results are reliably measurable, collectability is reasonably assured and all other revenue recognition criteria are
72
met. Sales milestones, which are based upon the achievement of certain agreed-upon sales thresholds, will be recorded when the respective sales threshold is achieved and collectability is reasonably assured.
Consistent with our policy on revenue recognition, deferred collaboration revenue represents cash received in advance for licensing fees, option fees, consulting, research and development contracts and related cost sharing and supply agreements. Such payments are reflected as deferred collaboration revenue until revenue can be recognized under our revenue recognition policy. Deferred collaboration revenue is classified as current if management believes we will complete the earnings process and be able to recognize the deferred amount as revenue within 12 months of the balance sheet date. At December 31, 2007, total deferred collaboration revenue was approximately $79.5 million, of which $5.4 million was current and will be recognized as revenue during 2008.
As part of the process of preparing financial statements, we are required to estimate accrued expenses. This process involves identifying services which have been performed on our behalf, and estimating the level of service performed and the associated cost incurred for such service as of each balance sheet date in our financial statements. Given our current business, the primary area of uncertainty concerning accruals which could have a material effect on our business is with respect to service fees paid to contract manufacturers in conjunction with the production of clinical drug supplies and to contract research organizations in connection with our preclinical studies and clinical trials. In connection with all of the foregoing service fees, our estimates are most affected by our understanding of the status and timing of services provided. The majority of our service providers, including contract research organizations, invoice us in arrears for services performed. In the event that we do not identify some costs which have begun to be incurred, or we under or over estimate the level of services performed or the costs of such services in a given period, our reported expenses for such period would be too low or too high. We currently reflect the over or under accrual of expenses directly in our operations in the period the amount was determined.
Our arrangements with contract research organizations in connection with clinical trials often provide for payment prior to commencing the project or based upon predetermined milestones throughout the period during which services are expected to be performed. We recognize expense relating to these arrangements based on the various services provided over the estimated time to completion. The date on which services commence, the level of services performed on or before a given date, and the cost of such services are often determined based on subjective judgments. We make these judgments based upon the facts and circumstances known to us based on the terms of the contract or our ongoing monitoring of service performance. In the years ended December 31, 2007, 2006 and 2005, respectively, we had arrangements with multiple contract research organizations whereby these organizations commit to performing services for us over multiple reporting periods. We currently recognize and plan to continue to recognize the expenses associated with these arrangements based on our expectation of the timing of the performance of components under these arrangements by these organizations. Generally, these components consist of the costs of setting up the trial, monitoring the trial, closing the trial and preparing the resulting data.
With respect to financial reporting periods presented in this Annual Report on Form 10-K, and based on our receipt of invoices from our third party providers, the timing of our actual costs incurred have not differed materially from our estimated timing of such costs. In light of the foregoing, we do not believe our estimates of future expenses and our practice of making judgments concerning the accrual of expenses are reasonably likely to change in the future. There were no changes in our estimates and accruals for contract service fees that had a material effect on our net losses in the years ended December 31, 2007, 2006 and 2005, respectively.
73
Effective January 1, 2006, we adopted Statement of Financial Accounting Standards, or SFAS, No. 123R,Share-Based Payment, or SFAS No. 123R, for stock-based awards to employees, using the modified prospective method of transition for awards granted after January 17, 2005 (valued using the fair value method), and using the prospective method for awards granted prior to January 17, 2005 (valued using the minimum value method). Therefore, compensation cost recognized in the years ended December 31, 2007 and 2006 includes: (1) compensation costs related to the vesting of stock options granted after January 17, 2005 but prior to January 1, 2006, based on the grant date fair value method estimated in accordance with the provisions of SFAS No. 123,Accounting for Stock-Based Compensation, or SFAS No. 123, adjusted for estimated forfeitures, (2) compensation costs related to the continued vesting of nonvested restricted stock awards granted prior to January 1, 2006, and (3) compensation costs for all share-based payments granted or modified subsequent to January 1, 2006, based on the provisions of SFAS No. 123R.
We continue to use the Black-Scholes option pricing model as the most appropriate valuation method for our option grants. The Black-Scholes model requires inputs for risk-free interest rate, dividend yield, volatility and expected lives of the options. Since we do not have a significant history of stock trading activity, expected volatility is based on historical data from several public companies similar in size and value to us. We will continue to use a weighted average approach using historical volatility and other similar public entity volatility information until historical volatility of our common stock is relevant to measure expected volatility for future option grants. We estimate the forfeiture rate based on historical data. Our options generally vest 25% after one year of service and quarterly over three years thereafter. Based on an analysis of historical forfeitures, we applied a forfeiture rate of 10% to all options that vest upon completion of the first year of service following the date of grant. The analysis will be re-evaluated at least annually and the forfeiture rate will be adjusted as necessary. The risk-free interest rate for periods within the contractual life of the option is based on the U.S. Treasury yield curve in effect at the time of the grant. The expected lives for options granted represents the period of time that options granted are expected to be outstanding. Since January 1, 2006, we have used the simplified method for determining the expected lives of options.
For awards with graded vesting, we allocate compensation costs under SFAS No. 123R on a straight-line basis over the requisite service period. Accordingly, we amortized the fair value of each option over each option's service period, which is generally the vesting period.
We account for stock options issued to non-employees in accordance with the provisions of SFAS No. 123 and EITF No. 96-18,Accounting for Equity Instruments that are Issued to Other than Employees, or in Conjunction with Selling Goods or Services, which requires valuing and remeasuring such stock options to the current fair value until the performance date has been reached.
Certain of our options granted to non-employees that are fully vested and no longer subject to a performance requirement are subject to EITF Issue No. 00-19,Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company's Own Stock, which requires the stock options held by certain non-employee consultants to be accounted for as liability awards. The fair value of these vested and unexercised awards was recognized as liability awards starting in April 2007 following the registration of stock options under Form S-8, using the Black-Scholes model. As of December 31, 2007, a liability of $1,343,000 was reflected in the balance sheet as other current liabilities. The fair value of the award is re-measured at each financial statement reporting date until the options are exercised or expire. When and if non-employee consultants exercise their options or the options expire, the corresponding liability will be reclassified to equity. As of December 31, 2007, vested stock options to acquire 312,911 shares of common stock held by non-employee consultants remained unexercised.
74
Our net loss for the years ended December 31, 2007 and 2006 includes $5.4 million and $4.8 million, respectively, of compensation costs and no income tax benefit related to our stock-based compensation arrangements for employee and non-employee awards. As of December 31, 2007, the total amount of unrecognized stock-based compensation expense is $12.6 million, which will be recognized over a weighted average period of 4.0 years.
Consolidated Results of Operations
Year Ended December 31, 2007 Compared with Year Ended December 31, 2006
Revenue
| | Year Ended December 31,
| | 2007 to 2006
Change
|
|---|
| | 2007
| | 2006
| | $
| | %
|
|---|
| | (dollars in millions)
| |
|
|---|
| Revenues | | $ | 0.7 | | $ | — | | $ | 0.7 | | — |
In October 2007, we entered into a collaborative development, commercialization and license agreement with GSK for elesclomol. Under the terms of the GSK Agreement, the companies will jointly develop and commercialize elesclomol in the United States, and GSK will have exclusive responsibility for the development and commercialization of elesclomol outside the United States. The $80 million non-refundable upfront payment we received from GSK in November 2007, together with the $260,000 estimated value of an option to require GSK to purchase $25 million of our common stock, is being recognized as collaboration revenue using the time-based model over the estimated performance period, the 15-year period through the earliest expiration date of the related patents, which we estimate to be the effective life of this agreement (see Notes 2 and 8 in the accompanying consolidated financial statements).
| | Year Ended December 31,
| | 2007 to 2006 Change
| |
|---|
| | 2007
| | 2006
| | $
| | %
| |
|---|
| | (dollars in millions)
| |
| |
|---|
| Clinical-stage drug candidates | | | | | | | | | | | | |
| | Elesclomol | | $ | 32.0 | | $ | 9.6 | | $ | 22.4 | | 233 | % |
| | Apilimod | | | 1.3 | | | 16.8 | | | (15.5 | ) | (92 | )% |
| | STA-9090 | | | 7.0 | | | 12.3 | | | (5.3 | ) | (43 | )% |
| | |
| |
| |
| |
| |
| Total clinical-stage drug candidates | | | 40.3 | | | 38.7 | | | 1.6 | | 4 | % |
| Early stage and discontinued programs | | | 11.7 | | | 11.8 | | | (0.1 | ) | (1 | )% |
| | |
| |
| |
| |
| |
| Total research and development | | $ | 52.0 | | $ | 50.5 | | $ | 1.5 | | 3 | % |
| | |
| |
| | | | | | |
In the year ended December 31, 2007, costs incurred under our elesclomol program increased by $22.4 million over the year ended December 31, 2006, including a $13.7 million increase for personnel costs, related research supplies, operational overhead and stock compensation, and an $8.7 million increase for external costs. These increases were principally due to start-up expenses incurred in connection with the SYMMETRY trial, our global, pivotal Phase 3 clinical trial of elesclomol for the treatment of metastatic melanoma, which was initiated in the third quarter of 2007, offset by non-recurring external costs incurred in 2006 in connection with the completion of the Phase 2b clinical trial for metastatic melanoma.
In the year ended December 31, 2007, costs incurred in connection with apilimod for the treatment of Crohn's disease decreased by $15.5 million over the year ended December 31, 2006,
75
including a $6.8 million decrease for personnel costs, related research supplies, operational overhead and stock compensation, and an $8.7 million decrease for non-recurring external costs. These decreases were principally due to the completion of the Phase 2b clinical trial in June 2006.
In the year ended December 31, 2007, costs incurred under our STA-9090 program decreased by $5.3 million over the year ended December 31, 2006, including a $2.9 million decrease for personnel costs, related research supplies, operational overhead and stock compensation, and a $2.4 million decrease for external costs. These decreases were principally due to the advancement of the program from preclinical development into clinical development upon the filing of an investigational new drug application in the third quarter of 2007 and the initiation of two Phase 1 clinical trials in the fourth quarter of 2007.
In addition, in the year ended December 31, 2007, costs incurred under our early-stage and discontinued programs decreased by $0.1 million over the year ended December 31, 2006, including a $0.6 million increase for personnel costs, related research supplies, operational overhead and stock compensation, offset by a $0.7 million decrease for external costs.
| | Year Ended December 31,
| | 2007 to 2006 Change
| |
|---|
| | 2007
| | 2006
| | $
| | %
| |
|---|
| | (dollars in millions)
| |
| |
|---|
| General and administrative | | $ | 14.9 | | $ | 8.6 | | $ | 6.3 | | 73 | % |
The increase in general and administrative expense principally resulted from increases of $1.8 million for personnel costs and related overhead in connection with increased headcount, and $4.4 million in external professional fees, including investor and medical community relations, public-company reporting and compliance requirements and increased director and officer insurance premiums following completion of our IPO in February 2007, and intellectual property and general legal fees, as well as a $0.1 million increase in stock-based compensation.
| | Year Ended December 31,
| | 2007 to 2006 Change
| |
|---|
| | 2007
| | 2006
| | $
| | %
| |
|---|
| | (dollars in millions)
| |
| |
|---|
| Investment income, net | | $ | 2.7 | | $ | 1.9 | | $ | 0.8 | | 42 | % |
The increase in net investment income was principally due to the higher average cash balances resulting from the net cash proceeds of $44.7 million raised from the sale of our common stock in the IPO in February 2007 and the $80 million non-refundable upfront payment received from GSK in November 2007.
| | Year Ended December 31,
| | 2007 to 2006 Change
| |
|---|
| | 2007
| | 2006
| | $
| | %
| |
|---|
| | (dollars in millions except for net loss per share)
| |
|---|
| Net loss | | $ | (63.5 | ) | $ | (57.3 | ) | $ | (6.2 | ) | (11 | )% |
Basic and diluted net loss per share attributable to common stockholders |
|
$ |
(3.76 |
) |
$ |
(2.66 |
) |
|
|
|
|
|
76
The increase in the basic and diluted net loss per share attributable to common stockholders was principally due to the non-cash beneficial conversion charge of approximately $58.6 million that was recognized in February 2007 in connection with the contingent adjustable conversion feature of the Series A convertible preferred stock that converted into common stock upon the completion of the IPO in February 2007, offset in part by an increase in the number of weighted average common shares outstanding resulting from the sale of 5,000,000 shares of common stock and the conversion of the Series A preferred stock and accumulated dividends into 6,278,765 shares of common stock in connection with the IPO.
Year Ended December 31, 2006 Compared with Year Ended December 31, 2005
Revenue
There were no revenues in the years ended December 31, 2006 and 2005.
| | Year Ended December 31,
| | 2006 to 2005 Change
| |
|---|
| | 2006
| | 2005
| | $
| | %
| |
|---|
| | (dollars in millions)
| |
| |
|---|
| Clinical-stage drug candidates | | | | | | | | | | | | |
| | Elesclomol | | $ | 9.6 | | $ | 14.0 | | $ | (4.4 | ) | (31 | )% |
| | Apilimod | | | 16.8 | | | 27.5 | | | (10.7 | ) | (39 | )% |
| | STA-9090 | | | 12.3 | | | 4.6 | | | 7.7 | | 167 | % |
| | |
| |
| |
| |
| |
| Total clinical-stage drug candidates | | | 38.7 | | | 46.1 | | | (7.4 | ) | (16 | )% |
| Early stage and discontinued programs | | | 11.8 | | | 13.8 | | | (2.0 | ) | (14 | )% |
| | |
| |
| |
| |
| |
| Total research and development | | $ | 50.5 | | $ | 59.9 | | $ | (9.4 | ) | (16 | )% |
| | |
| |
| | | | | | |
In the year ended December 31, 2006, costs incurred under our elesclomol program decreased by $4.4 million over the year ended December 31, 2005, including a $5.2 million decrease for non-recurring external costs incurred in 2005 and in the first half of 2006 in connection with the completion of certain clinical trials, offset by a $0.8 million increase for personnel costs, related research supplies, operational overhead and stock compensation.
In the year ended December 31, 2006, costs incurred in connection with apilimod decreased by $10.7 million over the year ended December 31, 2005, including a $4.0 million decrease for personnel costs, related research supplies, operational overhead and stock compensation, and a $6.7 million decrease for non-recurring external costs incurred in 2005 in connection with the completion of early-stage clinical trials.
In the year ended December 31, 2006, costs incurred under our STA-9090 program increased by $7.7 million over the year ended December 31, 2005, including a $4.8 million increase for personnel costs, related research supplies, operational overhead and stock compensation, and a $2.9 million increase for external costs. These increases were principally due to the advancement of the program from the discovery phase into preclinical development.
In addition, in the year ended December 31, 2006, costs incurred under our early-stage and discontinued programs decreased by $2.0 million over the year ended December 31, 2005, including a $0.2 million increase for personnel costs, related research supplies, operational overhead and stock compensation, offset by a $2.2 million decrease for external costs.
77
| | Year Ended December 31,
| | 2006 to 2005 Change
| |
|---|
| | 2006
| | 2005
| | $
| | %
| |
|---|
| | (dollars in millions)
| |
| |
|---|
| General and administrative | | $ | 8.6 | | $ | 11.3 | | $ | (2.7 | ) | (24 | )% |
The decrease in general and administrative expense was principally due to $2.4 million incurred in connection with the filing of a Registration Statement on Form S-1 with the Securities and Exchange Commission in 2005 relating to an initial public offering of our common stock. We determined that we would not complete the planned offering and withdrew the filing in June 2005. The related costs were expensed in the year ended December 31, 2005 as we did not reactivate and complete the offering within 90 days of the withdrawal of the filing. This decrease was also due to decreases of $0.6 million for personnel costs and related overhead due principally to decreased headcount and $0.3 million in external professional fees, principally for general legal and other consulting services, offset by an increase in stock-based compensation of $0.6 million principally related to the net effect of the increased expense in connection with implementation of SFAS No. 123R less the impact of the conclusion of vesting of certain non-employee options in 2005.
| | Year Ended December 31,
| | 2006 to 2005 Change
| |
|---|
| | 2006
| | 2005
| | $
| | %
| |
|---|
| | (dollars in millions)
| |
| |
|---|
| Investment income, net | | $ | 1.9 | | $ | 2.3 | | $ | (0.4 | ) | (17 | )% |
The decrease in investment income was principally due to a decrease in average cash balances as a result of the use of existing cash resources during 2005 and 2006, prior to the net cash proceeds of $40.0 million raised from the sale of our Series A convertible preferred stock in June 2006.
Series A convertible preferred stock dividends were $1.9 million for the year ended December 31, 2006 due to the issuance of the Series A convertible preferred stock in June 2006. The Series A convertible preferred stock dividends accrued at the rate of 8% per year.
| | Year Ended December 31,
| | 2006 to 2005 Change
| |
|---|
| | 2006
| | 2005
| | $
| | %
| |
|---|
| | (dollars in millions except for net loss per share)
| |
|---|
| Net loss | | $ | (57.3 | ) | $ | (68.9 | ) | $ | 11.6 | | 17 | % |
Basic and diluted net loss per share attributable to common stockholders |
|
$ |
(2.66 |
) |
$ |
(3.09 |
) |
|
|
|
|
|
The decreases in net loss and basic and diluted net loss per share attributable to common stockholders were principally due to the completion of several clinical trials in 2005 and in the first half of 2006.
78
Liquidity and Capital Resources
We have incurred significant operating losses since our inception. We have funded our operations principally with $195.4 million in net proceeds from private placements of our common stock, $40.0 million in net proceeds from a private placement of our Series A convertible preferred stock, $44.7 million in net proceeds from the IPO, and the $80 million non-refundable upfront payment under the GSK Agreement, which, together with the exercise of common stock warrants and options, provided aggregate net cash proceeds of approximately $361.4 million through December 31, 2007. We have also generated funds from government grant revenues, equipment lease financings and investment income.
As of December 31, 2007, we had cash and cash equivalents of $115.6 million, an increase of $68.8 million from $46.8 million as of December 31, 2006. This increase principally reflects $44.7 million of net proceeds from our IPO, an $80 million non-refundable upfront payment under the GSK Agreement and our net loss of $63.5 million during the year ended December 31, 2007, as adjusted for non-cash charges for depreciation and stock-based compensation, and changes in working capital.
In October 2007, we entered into the GSK Agreement with GSK and received a non-refundable upfront cash payment of $80 million in November 2007. We are also eligible to receive potential pre-commercial milestone payments from GSK of up to $585 million, which include both payments for operational progress, such as trial initiation and enrollment, and payments for positive clinical and regulatory outcomes, such as regulatory approval. Of the $585 million in potential payments, $135 million are related to the development in metastatic melanoma and up to $450 million are related to the development of elesclomol in other cancer indications. In addition, we are eligible to receive up to $300 million in potential commercial milestone payments from GSK based on achieving certain net sales thresholds. Based on our current operating plans, we expect to receive between $40 million and $50 million in operational progress milestone payments in 2008.
Under our equipment lease agreement, we may periodically directly lease, or sell and lease back up to a maximum outstanding balance of $6.0 million of equipment and leasehold improvements. In June 2007, this agreement was extended through June 2008. As of December 31, 2007, approximately $1.4 million was available under this revolving lease line for future property and equipment expenditures.
The following table provides information regarding our cash position, cash flows and capital expenditures for the years ended December 31, 2007, 2006 and 2005 (in millions).
| | Year Ended December 31,
| |
|---|
| | 2007
| | 2006
| | 2005
| |
|---|
| | (dollars in millions)
| |
|---|
| Cash, cash equivalents and marketable securities | | $ | 115.6 | | $ | 46.8 | | $ | 62.1 | |
| Working capital | | | 96.2 | | | 36.1 | | | 48.5 | |
Cash flows provided by (used in): |
|
|
|
|
|
|
|
|
|
|
| | Operating activities | | | 27.2 | | | (53.0 | ) | | (61.9 | ) |
| | Investing activities | | | 10.8 | | | 23.6 | | | 39.2 | |
| | Financing activities | | | 43.9 | | | 39.3 | | | 3.8 | |
| Capital expenditures (included in investing activities) | | | (2.4 | ) | | (1.6 | ) | | (4.9 | ) |
79
Our operating activities provided cash of $27.2 million in the year ended December 31, 2007, including the $80 million non-refundable upfront payment received under the GSK Agreement in November 2007, offset by $52.8 million in the use of cash in operating activities. Our operating activities used cash of $53.0 million and $61.9 million in the years ended December 31, 2006 and 2005, respectively. The use of cash in all of these periods principally resulted from our losses from operations, as adjusted for non-cash charges for depreciation and stock-based compensation, and changes in our working capital accounts.
Our investing activities provided cash of $10.8 million, $23.6 million and $39.2 million in the years ended December 31, 2007, 2006 and 2005, respectively. Our investing activities in 2007 included sales and maturities of marketable securities in our investment portfolio in the amount of $28.1 million, offset by the purchases of marketable securities in the amount of $15.0 million and purchases of property and equipment in the amount of $2.4 million. Our investing activities in 2006 included sales and maturities of marketable securities in our investment portfolio in the amount of $143.4 million, offset by the purchases of marketable securities in the amount of $118.2 million and purchases of property and equipment in the amount of $1.6 million. Our investing activities in 2005 included sales and maturities of marketable securities in our investment portfolio in the amount of $228.4 million, offset by the purchases of marketable securities in the amount of $184.4 million and purchases of property and equipment in the amount of $4.9 million, including a research and development expansion of one of our facilities.
Our financing activities provided $43.9 million, $39.3 million and $3.8 million in the years ended December 31, 2007, 2006 and 2005, respectively. In February 2007, we raised net cash proceeds of $44.7 million from the sale of 5,000,000 shares of common stock in the IPO. In June 2006, we raised gross proceeds of $40.0 million from the sale of 8,000,000 shares of our Series A convertible preferred stock. We raised $2.0 million, $1.4 million and $4.7 million in proceeds from the sale and lease-back of property and equipment in the years ended December 31, 2007, 2006 and 2005, respectively. We repaid $2.6 million, $2.1 million and $1.1 million in capital equipment leases in the years ended December 31, 2007, 2006 and 2005, respectively. In January 2007, we repurchased 29,046 shares of our previously restricted common stock in the amount of $0.3 million from certain officers and non-officer employees in order to fund the minimum statutory tax withholding requirements related to the vesting of 80,000 shares of restricted common stock.
The following tables summarize our contractual obligations at December 31, 2007 and the effects such obligations are expected to have on our liquidity and cash flows in future periods (in millions).
Contractual Obligations (as of December 31, 2007)
| | Total
| | 2008
| | 2009 through 2010
| | 2011 through 2012
| | More than 5 years
|
|---|
| Capital lease obligations(1) | | $ | 5.9 | | $ | 2.8 | | $ | 2.8 | | $ | 0.3 | | $ | — |
| Operating lease obligations | | | 6.7 | | | 2.0 | | | 3.3 | | | 1.4 | | | — |
| Research and development contracts | | | 36.1 | | | 26.4 | | | 9.7 | | | — | | | — |
| Consulting | | | 0.2 | | | 0.1 | | | 0.1 | | | — | | | — |
| Purchase obligations | | | 0.2 | | | 0.1 | | | 0.1 | | | — | | | — |
| | |
| |
| |
| |
| |
|
| Total | | $ | 49.1 | | $ | 31.4 | | $ | 16.0 | | $ | 1.7 | | | — |
| | |
| |
| |
| |
| |
|
- (1)
- Including scheduled interest payments.
Research and development contracts principally include contracts for human clinical studies, animal studies and clinical manufacturing. The future research and development contract obligations in the table of Contractual Obligations above assume that each of the studies and related manufacturing
80
contracts is completed as planned. In the event a study or manufacturing contract is terminated prior to planned completion by mutual agreement between the contractor and us, the amount paid under such contracts may be less than the amounts presented.
Under various license agreements, substantially all of which are related to our early-stage discovery programs, we may be obligated to pay up to an aggregate of $3.9 million if specified development and commercialization milestones are met, as follows (in thousands). These amounts are not included in the table of Contractual Obligations above.
Milestone
| | Amount
|
|---|
| Phase 1 clinical trials | | $ | 150 |
| Phase 2 clinical trials | | | 250 |
| Phase 3 clinical trials | | | 350 |
| Completion of Phase 3 clinical trials | | | 75 |
| FDA new drug approval | | | 1,875 |
| European market approval | | | 500 |
| Other | | | 650 |
| | |
|
| Total | | $ | 3,850 |
| | |
|
We expect to incur substantial expenses and generate significant operating losses as we continue to advance our drug candidates into preclinical studies and clinical trials and as we:
- •
- complete the SYMMETRY trial, our global, pivotal Phase 3 clinical trial of elesclomol for the treatment of metastatic melanoma, that was initiated in the third quarter of 2007, and initiate Phase 2 clinical trials of elesclomol in other cancer types;
- •
- begin to perform and fund pre-commercialization activities, and establish sales and marketing functions and commercial manufacturing arrangements for elesclomol, consistent with our obligations under our agreement with GSK;
- •
- complete the current Phase 2a clinical trial of apilimod for the treatment of RA, and possibly initiate Phase 2 clinical trials of apilimod in other inflammatory disease indications;
- •
- initiate additional Phase 3 clinical trials of elesclomol in other cancer types and one or more Phase 3 clinical trials of apilimod, if supported by Phase 2 results;
- •
- complete two Phase 1 clinical trials of STA-9090 that were initiated in the fourth quarter of 2007, initiate additional Phase 1 trials and initiate any later-stage additional clinical trials, if supported by Phase 1 results;
- •
- complete preclinical development of STA-9584 and initiate clinical trials, if supported by positive preclinical data;
- •
- advance our CRAC ion channel inhibitor program into clinical trials, if supported by positive preclinical data;
- •
- discover, develop, and seek regulatory approval for backups of our current drug candidates and other new drug candidates;
- •
- identify additional compounds or drug candidates and acquire rights from third parties to those compounds or drug candidates through licenses, acquisition or other means;
- •
- commercialize any approved drug candidates;
81
- •
- hire additional clinical, scientific, and management personnel; and
- •
- add operational, financial, and management information systems and personnel.
Our funding requirements will depend on a number of factors, including:
- •
- the progress of our research and development programs, including the completion of preclinical studies and clinical trials for our current drug candidates and the results from these studies and trials;
- •
- the number of drug candidates we advance into later-stage clinical trials and the scope of our research and development programs;
- •
- our ability to discover additional drug candidates using our drug discovery technology and advance them into clinical development;
- •
- the costs involved in preparing, filing, prosecuting, maintaining and enforcing patent claims for our drug discovery technology and drug candidates and avoiding infringing the intellectual property of others;
- •
- the time and costs involved in obtaining regulatory approvals for our drug candidates;
- •
- our ability to establish and maintain collaborative arrangements, including our agreement with GSK;
- •
- the potential in-licensing of other products or technologies or the acquisition of complementary businesses;
- •
- the cost of manufacturing, marketing and sales activities, if any; and
- •
- the timing, receipt and amount of revenue, if any, from our drug candidates.
We do not anticipate that we will generate product revenue for the next several years. We expect our continuing operating losses to result in increases in cash used in operations over the next several years. Based on our current operating plans, we expect our existing funds will be sufficient to fund operations through at least 2008. Payment to us by GSK of milestones for our operational progress and achievement of certain success criteria leading to the approval by the FDA of elesclomol for the treatment of metastatic melanoma could extend our cash availability, as could payments of milestones in connection with the development of elesclomol in other cancer indications and achievement of certain net sales thresholds. Based on our current operating plans, we expect to receive between $40 million and $50 million in operational progress milestone payments, under our agreement with GSK, in 2008. However, we may require significant additional funds earlier than we currently expect in order to conduct additional clinical trials and seek regulatory approval of our drug candidates. Because of the numerous risks and uncertainties associated with the development and commercialization of our drug candidates, we are unable to estimate the amounts of increased capital outlays and operating expenditures associated with our current and anticipated clinical trials.
Additional funding may not be available to us on acceptable terms or at all. In addition, the terms of any financing may adversely affect the holdings or the rights of our stockholders. For example, if we raise additional funds by issuing equity securities or by selling debt securities, if convertible, further dilution to our existing stockholders may result. To the extent our capital resources are insufficient to meet our future capital requirements, we will need to finance our future cash needs through public or private equity offerings, collaboration agreements, debt financings or licensing arrangements.
If adequate funds are not available, we may be required to terminate, significantly modify or delay our research and development programs, reduce our planned commercialization efforts, or obtain funds through collaborators that may require us to relinquish rights to our technologies or drug candidates
82
that we might otherwise seek to develop or commercialize independently. We may elect to raise additional funds even before we need them if the conditions for raising capital are favorable.
Cash, Cash Equivalents and Marketable Securities
As of December 31, 2007, we had cash and cash equivalents of $115.6 million consisting of cash deposited in a highly rated financial institution in the United States and in short-term money market funds. Subsequent to year-end we transferred our invested funds to a short-term U.S. Treasury money market fund. The primary objective of our investment activities is to preserve our capital for the purpose of funding operations and we do not enter into investments for trading or speculative purposes. We believe that we did not have material exposure to high-risk investments, such as mortgage-backed securities, auction rate securities or other special investment vehicles, or SIV's, within our money-market fund investments. We also believe that we do not have any material exposure to changes in fair value as a result of changes in interest rates. Declines in interest rates, however, would reduce future investment income.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements or relationships with unconsolidated entities of financial partnerships, such as entities often referred to as structured finance or special purpose entities.
Tax Loss Carryforwards
In 2005 and in 2007, we performed analyses to determine if there were changes in ownership, as defined by Section 382 of the Internal Revenue Code, that would limit our ability to utilize certain net operating loss and tax credit carryforwards. We determined that we experienced a change in ownership, as defined by Section 382, in connection with the acquisition of Principia Associates, Inc. on September 20, 2002, but did not experience a change in ownership upon the effectiveness of our IPO. As a result, the utilization of our federal tax net operating loss carryforwards generated prior to the ownership change is limited. As of December 31, 2007 we have net operating loss carryforwards for U.S. federal tax purposes of approximately $259.1 million, after taking into consideration net operating losses expected to expire unused as a result of this limitation, and the remainder will expire in varying amounts through 2027 unless utilized. In addition, as of December 31, 2007, we have state net operating loss carryforwards of approximately $243.6 million, which will expire through 2011 unless utilized. The utilization of these net operating loss carryforwards may be further limited as we experience future ownership changes as defined in Section 382 of the Internal Revenue Code.
Recently Issued Accounting Pronouncements
In December 2007, the Financial Accounting Standards Board or the FASB, issued SFAS No. 141R,Business Combinations, or SFAS No. 141R. The pronouncement establishes principles and requirements for how an acquirer recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed, any noncontrolling interest in the acquiree and the goodwill acquired. The pronouncement also establishes disclosure requirements to enable the evaluation of the nature and financial effects of the business combination. SFAS No. 141R is effective for fiscal years beginning after December 15, 2008. We are currently evaluating SFAS No. 141R and the impact it may have on our results of operations or financial position.
In December 2007, the FASB issued SFAS No. 160,Noncontrolling Interests in Consolidated Financial Statements—an Amendment of ARB No. 51,or SFAS No. 160. The pronouncement establishes accounting and reporting standards pertaining to ownership interests in subsidiaries held by parties other than the parent, the amount of net income attributable to the parent and to the noncontrolling
83
interest, changes in a parent's ownership interest, and the valuation of any retained noncontrolling equity investment when a subsidiary is deconsolidated. The pronouncement also establishes disclosure requirements that identify and distinguish between the interests of the parent and the interests of the noncontrolling owners. SFAS No. 160 is effective for fiscal years beginning on or after December 15, 2008. We are currently evaluating SFAS No. 160 and the impact it may have on our results of operations or financial position.
In June 2007, the EITF issued EITF No. 07-03,Accounting for Nonrefundable Advance Payments for Goods or Services to Be Used in Future Research and Development Activities, or EITF No. 07-03, which provides guidance for upfront payments related to goods and services of research and development activities. EITF No. 07-03 is effective for fiscal years beginning after December 15, 2007. We do not believe the adoption of EITF No. 07-03 will have a material impact on our overall financial position or results of operations.
In February 2007, the FASB issued SFAS No. 159,The Fair Value Option for Financial Assets and Financial Liabilities, or SFAS No. 159, including an amendment of SFAS No. 115, which permits entities to choose to measure many financial instruments and certain other items at fair value that are not currently required to be measured at fair value. SFAS No. 159 is effective for us beginning in 2008. We do not believe the adoption of SFAS No. 159 will have a material impact on our overall financial position or results of operations.
In September 2006, the FASB issued SFAS No. 157,Fair Value Measurements, or SFAS No. 157, which provides guidance for using fair value to measure assets and liabilities. The pronouncement clarifies (1) the extent to which companies measure assets and liabilities at fair value; (2) the information used to measure fair value; and (3) the effect that fair value measurements have on earnings. SFAS No. 157 will apply whenever another standard requires (or permits) assets or liabilities to be measured at fair value. SFAS No. 157 will be applicable to us for fiscal years beginning after November 15, 2007. In February 2008, the FASB issued SFAS No. 157-1 and No. 157-2 which delay the effective date of SFAS No. 157 for one year for certain non-financial assets and liabilities and removes certain leasing transactions from its scope. We do not believe the adoption of SFAS No. 157 will have a material impact on our overall financial position or results of operations.
In July 2006, the FASB issued FASB Interpretation No. 48,Accounting for Uncertainty in Income Taxes—an interpretation of FAS 109, or Interpretation No. 48. This interpretation clarifies the accounting for uncertainty in income taxes recognized in a company's financial statements in accordance with FASB Statement No. 109,Accounting for Income Taxes. This interpretation prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken in a tax return. It also provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure and transition. Interpretation No. 48 is effective for fiscal years beginning after December 15, 2006. We adopted Interpretation No. 48 effective January 1, 2007 and its adoption had no impact on our consolidated results of operations and financial position.
Certain Factors That May Affect Future Results of Operations
The Securities and Exchange Commission encourages companies to disclose forward-looking information so that investors can better understand a company's future prospects and make informed investment decisions. This Annual Report on Form 10-K contains such "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995.
Words such as "may," "anticipate," "estimate," "expects," "projects," "intends," "plans," "believes" and words and terms of similar substance used in connection with any discussion of future operating or financial performance, identify forward-looking statements. All forward-looking statements are management's present expectations of future events and are subject to a number of risks and
84
uncertainties that could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to those set forth under the heading "Risk Factors" contained in Item 1A of this Annual Report on Form 10-K.
In light of these assumptions, risks and uncertainties, the results and events discussed in the forward-looking statements contained in this Annual Report on Form 10-K or in any document incorporated by reference might not occur. Stockholders are cautioned not to place undue reliance on the forward-looking statements, which speak only as of the date of this Annual Report on Form 10-K. We are not under any obligation, and we expressly disclaim any obligation, to update or alter any forward-looking statements, whether as a result of new information, future events or otherwise. All subsequent forward-looking statements attributable to Synta or to any person acting on its behalf are expressly qualified in their entirety by the cautionary statements contained or referred to in this section.
Item 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Interest Rate Sensitivity. As of December 31, 2007, we had cash and cash equivalents of $115.6 million consisting of cash deposited in a highly rated financial institution in the United States and in short-term money market funds. Subsequent to year-end we transferred our invested funds to a short-term U.S. Treasury money market fund. The primary objective of our investment activities is to preserve our capital for the purpose of funding operations and we do not enter into investments for trading or speculative purposes. We believe that we did not have material exposure to high-risk investments such as mortgage-backed securities, auction rate securities or other special investment vehicles, or SIV's, within our money-market fund investments. We believe that we do not have any material exposure to changes in fair value as a result of changes in interest rates. Declines in interest rates, however, would reduce future investment income. During the year ended December 31, 2007, we had investment income of $3.2 million. If overall interest rates fell by 10% during the year ended December 31, 2007, our interest income would have decreased by less than $0.3 million, assuming consistent investment levels.
Capital Market Risk. We currently have no product revenues and depend on funds raised through other sources. One possible source of funding is through further equity offerings. Our ability to raise funds in this manner depends upon capital market forces affecting our stock price.
Item 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The information required by this Item 8 is included at the end of this Annual Report on Form 10-K beginning on page F-1.
Item 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
Item 9A(T). CONTROLS AND PROCEDURES
(a) Evaluation of Disclosure Controls and Procedures. Our principal executive officer and principal financial officer, after evaluating the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended) as of the end of the period covered by this Annual Report on Form 10-K, have concluded that, based on such evaluation, our disclosure controls and procedures were adequate and effective. In designing and evaluating our disclosure controls and procedures, our management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and our management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
85
(b) Changes in Internal Controls. There were no changes in our internal control over financial reporting, identified in connection with the evaluation of such internal control that occurred during the fourth quarter of our last fiscal year, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management's Report On Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended. Our internal control system was designed to provide reasonable assurance to our management and board of directors regarding the preparation and fair presentation of published financial statements. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2007. In making this assessment, it used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework. Based on our assessment we believe that, as of December 31, 2007, our internal control over financial reporting is effective based on those criteria.
This Annual Report on Form 10-K does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management's report was not subject to attestation by our registered public accounting firm pursuant to temporary rules of the Securities and Exchange Commission that permit us to provide only management's report in this Annual Report.
Item 9B. OTHER INFORMATION
Not applicable.
86
PART III
Item 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The response to this item is incorporated by reference from the discussion responsive thereto under the captions "Management," "Section 16(a) Beneficial Ownership Reporting Compliance" and "Code of Conduct and Ethics" in our Proxy Statement for the 2008 Annual Meeting of Stockholders to be held on June 11, 2008.
We have adopted a code of conduct and ethics that applies to all of our directors, officers and employees. This code is publicly available on our website atwww.syntapharma.com. Amendments to the code of conduct and ethics or any grant of a waiver from a provision of the code requiring disclosure under applicable Securities and Exchange Commission and The NASDAQ Stock Market rules will be disclosed in a Current Report on Form 8-K.
Item 11. EXECUTIVE COMPENSATION
The response to this item is incorporated by reference from the discussion responsive thereto under the captions "Compensation Discussion and Analysis," "Executive Compensation," "Management—Committees of the Board of Directors and Meetings," "Management—Compensation Committee Interlocks and Insider Participation" and "Compensation Committee Report" in our Proxy Statement for the 2008 Annual Meeting of Stockholders to be held on June 11, 2008.
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The response to this item is incorporated by reference from the discussion responsive thereto under the captions "Security Ownership of Certain Beneficial Owners and Management" and "Executive Compensation—Equity Compensation Plan Information" in our Proxy Statement for the 2008 Annual Meeting of Stockholders to be held on June 11, 2008.
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The response to this item is incorporated by reference from the discussion responsive thereto under the caption "Certain Relationships and Related Person Transactions," "Management—The Board of Directors" and "Management—Director Independence" in our Proxy Statement for the 2008 Annual Meeting of Stockholders to be held on June 11, 2008.
Item 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The response to this item is incorporated by reference from the discussion responsive thereto under the caption "Independent Public Accountants" in our Proxy Statement for the 2008 Annual Meeting of Stockholders to be held on June 11, 2008.
87
PART IV
| Item 15. | | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
Item 15(a) |
|
The following documents are filed as part of this Annual Report on Form 10-K: |
Item 15(a)(1) and (2) |
|
The Consolidated Financial Statements beginning on page F-1 are filed as part of this Annual Report on Form 10-K. Other financial statement schedules have not been included because they are not applicable or the information is included in the financial statements or notes thereto. |
Item 15(a)(3) |
|
Exhibits |
The following is a list of exhibits filed as part of this Annual Report on Form 10-K.
Exhibit Number
| | Description of Exhibit
|
|---|
| 3.1(1) | | Restated Certificate of Incorporation of the Registrant. (3.2) |
3.2(1) |
|
Restated Bylaws of the Registrant. (3.4) |
4.1(1) |
|
Form of Common Stock Certificate. (4.1) |
4.2.1(1) |
|
Amended and Restated Investor Rights Agreement, dated December 13, 2002, by and among the Registrant and certain stockholders of the Registrant. (4.2.1) |
4.2.2(1) |
|
First Amendment, dated January 11, 2005, to the Amended and Restated Investor Rights Agreement, dated December 13, 2002, by and among the Registrant and certain stockholders of the Registrant. (4.2.2) |
4.2.3(1) |
|
Second Amendment, dated January 31, 2007, to the Amended and Restated Investor Rights Agreement, dated December 13, 2002, by and among the Registrant and certain stockholders of the Registrant. (4.2.3) |
10.1(1)* |
|
2001 Stock Plan. (10.1) |
10.2(1)* |
|
2006 Stock Plan. (10.2) |
10.2(a)(1)* |
|
Form of incentive stock option agreement under 2006 Stock Plan. (10.2(a)) |
10.2(b)(1)* |
|
Form of nonqualified stock option agreement under 2006 Stock Plan. (10.2(b)) |
10.2(c)(1)* |
|
Form of restricted stock agreement under 2006 Stock Plan. (10.2(c)) |
10.2(d)(1)* |
|
Form of nonqualified stock option agreement for directors under 2006 Stock Plan. (10.2(d)) |
10.2(e)(1)* |
|
Form of restricted stock agreement for directors under 2006 Stock Plan. (10.2(e)) |
10.3(1)* |
|
Director Compensation Policy. (10.3) |
10.4* |
|
Non-Qualified Stock Option Agreement, dated February 27, 2008, by and between the Registrant and Keith R. Gollust. |
10.5(1) |
|
Duffy Hartwell Limited Partnership Commercial Lease, dated November 4, 1996, by and between Duffy Hartwell Limited Partnership and Shionogi BioResearch Corp., as amended by First Amendment to Commercial Lease, dated August 30, 2006. (10.5) |
88
10.6(1) |
|
Lease of 125 Hartwell Avenue, Lexington, MA, dated October 26, 1992, by and between Fuji ImmunoPharmaceuticals Corp. and 125 Hartwell Trust, as amended by First Amendment dated January 31, 1993, Second Amendment dated October 1, 1997, Third Amendment dated November 1, 2002, Assignment and Assumption of Lease and Consent of Release by Landlord and Fourth Amendment of Lease, dated July 9, 2004, Fifth Amendment, dated October 22, 2004 and Sixth Amendment, dated August 1, 2005. (10.6) |
10.6.1 |
|
Seventh Amendment, dated November 26, 2007, to Lease of 125 Hartwell Avenue, Lexington, MA, dated October 26, 1992, by and between the Registrant, as successor-by-assignment, and 125 Hartwell Trust. |
10.7(1) |
|
Lease, dated January 13, 2005, by and between the Registrant and Mortimer B. Zuckerman and Edward H. Linde, Trustees of 91 Hartwell Avenue Trust, as extended on August 14, 2006. (10.7) |
10.7.1 |
|
First Amendment to Lease, dated as of September 7, 2007, to Lease, dated January 13, 2005, by and between the Registrant and Mortimer B. Zuckerman and Edward H. Linde, Trustees of 91 Hartwell Avenue Trust. |
10.8(1) |
|
Pinnacle Properties Management, Inc. Standard Form Commercial Lease, dated May 31, 1999, by and between 6-8 Preston Court, L.L.C. and Asiana Pharmaceuticals Corporation, as amended by Amendment to Lease #1, dated July 31, 2000, Amendment to Lease #2, dated November 26, 2001, and Amendment to Lease #3, dated December 2003, and as assigned to the Registrant by Assignment and Assumption of Lease and Landlord's Consent, dated May 25, 2005, and Subordination, Non-Disturbance and Attornment Agreement, dated May 25, 2005. (10.8) |
10.9(1) |
|
Master Lease Agreement, dated November 10, 2004, by and between the Registrant and General Electric Capital Corporation, as amended by Letter Agreement, dated June 24, 2005, and as extended by Letter Agreement, dated November 29, 2006. (10.9) |
10.9.1 |
|
Extension, dated as of June 29, 2007, of Master Lease Agreement, dated November 10, 2004, by and between the Registrant and General Electric Capital Corporation, as amended. |
10.10(1)* |
|
Letter Agreement, dated April 18, 2005, by and between the Registrant and Safi R. Bahcall, Ph.D. (10.13) |
10.11(1)* |
|
Letter Agreement, dated October 12, 2002, by and between the Registrant and Dr. Keizo Koya. (10.14) |
10.12(1)* |
|
Letter Agreement, dated January 22, 2003, by and between the Registrant and Dr. James Barsoum. (10.15) |
10.13(1)* |
|
Letter Agreement, dated April 15, 2004, by and between the Registrant and Dr. Jeremy Chadwick. (10.16) |
10.14(1)* |
|
Letter Agreement, dated February 19, 2004, by and between the Registrant and Keith Ehrlich. (10.17) |
10.15(1)* |
|
Letter Agreement, dated January 14, 2003, by and between the Registrant and Wendy E. Rieder. (10.18) |
10.16(1)* |
|
Letter Agreement, dated March 24, 2005, by and between the Registrant and Eric W. Jacobson. (10.19) |
89
10.17(1)* |
|
Letter Agreement, dated February 27, 2006, by and between the Registrant and Martin D. Williams. (10.20) |
10.18(1)* |
|
Agreement and Release, dated January 14, 2005, by and between the Registrant and Lan Bo Chen, Ph.D. (10.22) |
10.19(1)* |
|
Consulting Agreement, dated April 18, 2005, by and between the Registrant and Lan Bo Chen, Ph.D. (10.23) |
10.19.1* |
|
Amendment to Consulting Agreement, dated March 23, 2007, by and between the Registrant and Lan Bo Chen, Ph.D. |
10.20(1)* |
|
Form of Indemnification Agreement between the Registrant and its directors and executive officers. (10.26) |
10.21(1) |
|
Lease Agreement, dated December 14, 2006, by and between ARE-MA Region No. 24, LLC and the Registrant. (10.27) |
10.22(2)* |
|
Summary of compensation arrangements applicable to the Registrant's Named Executive Officers (2006 bonus and 2007 salary increases). (10.27) |
10.23* |
|
Summary of bonus arrangements applicable to the Registrant's Named Executive Officers. |
10.24** |
|
Collaborative Development, Commercialization and License Agreement, dated October 8, 2007 by and between the Registrant and GlaxoSmithKline. |
21.1(2) |
|
List of Subsidiaries. (21.1) |
23.1 |
|
Consent of KPMG LLP, Independent Registered Public Accounting Firm. |
31.1 |
|
Certification of Principal Executive Officer under Section 302 of the Sarbanes-Oxley Act of 2002. |
31.2 |
|
Certification of Principal Accounting and Financial Officer under Section 302 of the Sarbanes-Oxley Act of 2002. |
32.1 |
|
Certification of the Principal Executive Officer and the Principal Accounting and Financial Officer under Section 906 of the Sarbanes-Oxley Act of 2002. |
- *
- Management contract, compensatory plan or arrangement.
- **
- Confidential portions of these documents have been filed separately with the Securities and Exchange Commission pursuant to a request for confidential treatment.
- (1)
- Incorporated by reference from the Registrant's Registration Statement on Form S-1, as amended (Registration No. 333-138894), initially filed with the Securities and Exchange Commission on November 22, 2006.
- (2)
- Incorporated by reference from the Registrant's Annual Report on Form 10-K for the year ended December 31, 2006 (File No. 001-33277).
90
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | SYNTA PHARMACEUTICALS CORP. |
Date: March 20, 2008 |
|
By: |
/s/ SAFI R. BAHCALL, PH.D.
Safi R. Bahcall, Ph.D.
President and Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities indicated below and on the dates indicated.
Signatures
| | Title
| | Date
|
|---|
|
|
|
|
|
/s/ SAFI R. BAHCALL, PH.D.
Safi R. Bahcall, Ph.D. | | President, Chief Executive Officer and Director (principal executive officer) | | March 20, 2008 |
/s/ KEITH S. EHRLICH, C.P.A.
Keith S. Ehrlich, C.P.A. |
|
Vice President, Finance and Administration, Chief Financial Officer (principal accounting and financial officer) |
|
March 20, 2008 |
/s/ KEITH R. GOLLUST
Keith R. Gollust |
|
Chairman of the Board |
|
March 20, 2008 |
/s/ LAN BO CHEN, PH.D.
Lan Bo Chen, Ph.D. |
|
Director |
|
March 20, 2008 |
/s/ BRUCE KOVNER
Bruce Kovner |
|
Director |
|
March 20, 2008 |
/s/ WILLIAM REARDON, C.P.A.
William Reardon, C.P.A. |
|
Director |
|
March 20, 2008 |
/s/ ROBERT N. WILSON
Robert N. Wilson |
|
Director |
|
March 20, 2008 |
91
INDEX TO FINANCIAL STATEMENTS
SYNTA PHARMACEUTICALS CORP.
Years ended December 31, 2007, 2006, and 2005
| | Page
|
|---|
Report of Independent Registered Public Accounting Firm |
|
F-2 |
| Consolidated Financial Statements: | | |
| | Balance Sheets | | F-3 |
| | Statements of Operations | | F-4 |
| | Statements of Stockholders' Equity (Deficit) and Comprehensive Loss | | F-5 |
| | Statements of Cash Flows | | F-6 |
| | Notes to Financial Statements | | F-7 |
F-1
Report of Independent Registered Public Accounting Firm
The Board of Directors
Synta Pharmaceuticals Corp.:
We have audited the accompanying consolidated balance sheets of Synta Pharmaceuticals Corp. (the Company) as of December 31, 2007 and 2006, and the related consolidated statements of operations, stockholders' equity (deficit) and comprehensive loss, and cash flows for each of the years in the three-year period ended December 31, 2007. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Synta Pharmaceuticals Corp. as of December 31, 2007 and 2006, and the results of their operations and their cash flows for each of the years in the three-year period ended December 31, 2007 in conformity with U.S. generally accepted accounting principles.
As discussed in Note 2 to the consolidated financial statements, the Company adopted Statement of Financial Accounting Standard (SFAS) No. 123R,Share-Based Payment, effective January 1, 2006.
/s/ KPMG LLP
Boston, Massachusetts
March 19, 2008
F-2
SYNTA PHARMACEUTICALS CORP.
Consolidated Balance Sheets
(in thousands, except share and per share amounts)
| | December 31, 2007
| | December 31, 2006
| |
|---|
| Assets | | | | | | | |
| Current assets: | | | | | | | |
| | Cash and cash equivalents | | $ | 115,577 | | $ | 33,687 | |
| | Restricted cash | | | 83 | | | 540 | |
| | Marketable securities available-for-sale | | | — | | | 13,137 | |
| | Prepaid expenses and other current assets | | | 1,337 | | | 263 | |
| | |
| |
| |
| | | | Total current assets | | | 116,997 | | | 47,627 | |
| | Property and equipment, net | | | 5,576 | | | 6,067 | |
| | Deferred offering costs | | | — | | | 963 | |
| | Other assets | | | 76 | | | 132 | |
| | |
| |
| |
| | | | Total assets | | $ | 122,649 | | $ | 54,789 | |
| | |
| |
| |
| Liabilities and Stockholders' Equity (Deficit) | | | | | | | |
| Current liabilities: | | | | | | | |
| | Accounts payable | | $ | 2,488 | | $ | 2,632 | |
| | Accrued expenses | | | 9,184 | | | 6,127 | |
| | Capital lease obligations—current | | | 2,406 | | | 2,330 | |
| | Deferred collaboration revenue—current | | | 5,351 | | | — | |
| | Other current liabilities | | | 1,343 | | | — | |
| | Deferred grant revenue | | | — | | | 457 | |
| | |
| |
| |
| | | | Total current liabilities | | | 20,772 | | | 11,546 | |
| | |
| |
| |
| | Deferred collaboration revenue—long-term | | | 74,166 | | | — | |
| | Capital lease obligations—long-term | | | 2,815 | | | 3,170 | |
| | |
| |
| |
| | | | Total long-term liabilities | | | 76,981 | | | 3,170 | |
| | |
| |
| |
| | | | Total liabilities | | | 97,753 | | | 14,716 | |
| | |
| |
| |
| Convertible preferred stock, at redemption value: | | | | | | | |
| | Series A convertible preferred stock, $0.0001 par value per share. Authorized: no shares at December 31, 2007 and 8,000,000 shares at December 31, 2006; no shares issued and outstanding at December 31, 2007 and 8,000,000 shares issued and outstanding at December 31, 2006 | | | — | | | 41,820 | |
| | |
| |
| |
Stockholders equity (deficit): |
|
|
|
|
|
|
|
| | Preferred stock, par value $0.0001 per share. | | | | | | | |
| | | Authorized: 5,000,000 shares at December 31, 2007 and no shares at December 31, 2006; no shares issued and outstanding at December 31, 2007 and at December 31, 2006 | | | — | | | — | |
| | Common stock, par value $0.0001 per share. | | | | | | | |
| | | Authorized: 100,000,000 shares at December 31, 2007 and 158,000,000 shares at December 31, 2006; 33,875,948 shares issued and outstanding at December 31, 2007 and 22,564,068 shares issued and outstanding at December 31, 2006 | | | 3 | | | 2 | |
| | Additional paid-in-capital | | | 324,946 | | | 234,807 | |
| | Accumulated other comprehensive income | | | — | | | 2 | |
| | Accumulated deficit | | | (300,053 | ) | | (236,558 | ) |
| | |
| |
| |
| | | | Total stockholders' equity (deficit) | | | 24,896 | | | (1,747 | ) |
| | |
| |
| |
| | | | Total liabilities and stockholders' equity (deficit) | | $ | 122,649 | | $ | 54,789 | |
| | |
| |
| |
See accompanying notes to consolidated financial statements.
F-3
SYNTA PHARMACEUTICALS CORP.
Consolidated Statements of Operations
(in thousands, except share and per share amounts)
| | Years ended December 31
| |
|---|
| | 2007
| | 2006
| | 2005
| |
|---|
| Collaboration revenue | | $ | 743 | | $ | — | | | — | |
| | |
| |
| |
| |
| Operating expenses: | | | | | | | | | | |
| | Research and development | | | 52,025 | | | 50,503 | | | 59,901 | |
| | General and administrative | | | 14,934 | | | 8,648 | | | 11,279 | |
| | |
| |
| |
| |
| | | | Total operating expenses | | | 66,959 | | | 59,151 | | | 71,180 | |
| | |
| |
| |
| |
| | | | Loss from operations | | | (66,216 | ) | | (59,151 | ) | | (71,180 | ) |
| Other income: | | | | | | | | | | |
| | Investment income, net | | | 2,721 | | | 1,881 | | | 2,317 | |
| | |
| |
| |
| |
| | | | Net loss | | | (63,495 | ) | | (57,270 | ) | | (68,863 | ) |
| Convertible preferred stock dividends | | | — | | | 1,859 | | | — | |
| Convertible preferred stock beneficial conversion charge | | | 58,585 | | | — | | | — | |
| | |
| |
| |
| |
| | | | Net loss attributable to common stockholders | | $ | (122,080 | ) | $ | (59,129 | ) | $ | (68,863 | ) |
| | |
| |
| |
| |
Basic and diluted weighted average common shares outstanding |
|
|
32,466,006 |
|
|
22,265,242 |
|
|
22,253,423 |
|
| Basic and diluted net loss attributable to common stockholders per share | | $ | (3.76 | ) | $ | (2.66 | ) | $ | (3.09 | ) |
See accompanying notes to consolidated financial statements.
F-4
SYNTA PHARMACEUTICALS CORP.
Consolidated Statements of Stockholders' Equity (Deficit) and Comprehensive Loss
(in thousands, except share amounts)
| | Common stock
| |
| |
| | Accumulated
other
comprehensive
income (loss)
| |
| |
| |
| |
|---|
| | Additional
paid-in
capital
| | Deferred
compensation
| |
| | Total
stockholders'
equity (deficit)
| | Comprehensive
loss
| |
|---|
| | Shares
| | Amount
| | Accumulated deficit
| |
|---|
| Balance at December 31, 2004 | | 22,550,699 | | $ | 2 | | $ | 238,930 | | $ | (10,435 | ) | $ | (116 | ) | $ | (110,425 | ) | $ | 117,956 | | $ | (46,083 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| |
Issuance of restricted common shares |
|
96,589 |
|
|
— |
|
|
1,425 |
|
|
(1,425 |
) |
|
— |
|
|
— |
|
|
— |
|
|
|
|
| | Forfeitures of restricted common shares | | (40,000 | ) | | — | | | (881 | ) | | 743 | | | — | | | — | | | (138 | ) | | | |
| | Exercise of stock warrants | | 67,138 | | | — | | | 134 | | | — | | | — | | | — | | | 134 | | | | |
| | Issuance of stock options for services | | — | | | — | | | 201 | | | (201 | ) | | — | | | — | | | — | | | | |
| | Forfeitures of stock options for services | | — | | | — | | | (329 | ) | | 329 | | | — | | | — | | | — | | | | |
| | Remeasurement of stock options for services | | — | | | — | | | (451 | ) | | 451 | | | — | | | — | | | — | | | | |
| | Compensation expense related to stock options for services | | — | | | — | | | — | | | 1,142 | | | — | | | — | | | 1,142 | | | | |
| | Compensation expense related to issuance of stock options and restricted stock below fair value | | — | | | — | | | — | | | 2,171 | | | — | | | — | | | 2,171 | | | | |
| | Unrealized gains on marketable securities | | — | | | — | | | — | | | — | | | 75 | | | — | | | 75 | | | 75 | |
| | Net loss | | — | | | — | | | — | | | — | | | — | | | (68,863 | ) | | (68,863 | ) | | (68,863 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance at December 31, 2005 | | 22,674,426 | | | 2 | | | 239,029 | | | (7,225 | ) | | (41 | ) | | (179,288 | ) | | 52,477 | | | (68,788 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| |
Eliminate deferred stock compensation |
|
— |
|
|
— |
|
|
(7,225 |
) |
|
7,225 |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
| | Convertible preferred stock dividends | | — | | | — | | | (1,859 | ) | | — | | | — | | | — | | | (1,859 | ) | | | |
| | Forfeitures of restricted common shares | | (127,500 | ) | | — | | | — | | | — | | | — | | | — | | | — | | | | |
| | Issuance of common shares for services | | 4,875 | | | — | | | 69 | | | — | | | — | | | — | | | 69 | | | | |
| | Issuance of restricted common shares | | 12,142 | | | — | | | — | | | — | | | — | | | — | | | — | | | | |
| | Exercise of stock options | | 125 | | | — | | | 2 | | | — | | | — | | | — | | | 2 | | | | |
| | Compensation expense related to stock options for services | | — | | | — | | | 4,791 | | | — | | | — | | | — | | | 4,791 | | | | |
| | Unrealized gains on marketable securities | | — | | | — | | | — | | | — | | | 43 | | | — | | | 43 | | | 43 | |
| | Net loss | | — | | | — | | | — | | | — | | | — | | | (57,270 | ) | | (57,270 | ) | | (57,270 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance at December 31, 2006 | | 22,564,068 | | | 2 | | | 234,807 | | | — | | | 2 | | | (236,558 | ) | | (1,747 | ) | | (57,227 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| | Issuance of common shares in IPO, net | | 5,000,000 | | | — | | | 44,660 | | | — | | | — | | | — | | | 44,660 | | | | |
| | Conversion of convertible preferred stock | | 6,278,765 | | | 1 | | | 41,819 | | | — | | | — | | | — | | | 41,820 | | | | |
| | Issuance of restricted common shares | | 15,661 | | | — | | | — | | | — | | | — | | | — | | | — | | | | |
| | Repurchase of previously restricted common shares | | (29,046 | ) | | — | | | (290 | ) | | — | | | — | | | — | | | (290 | ) | | | |
| | Exercise stock options | | 51,500 | | | — | | | 136 | | | — | | | — | | | — | | | 136 | | | | |
| | Forfeitures of restricted common shares | | (5,000 | ) | | — | | | — | | | — | | | — | | | — | | | — | | | | |
| | Issuance of common stock purchase obligation | | — | | | — | | | (260 | ) | | — | | | — | | | — | | | (260 | ) | | | |
| | Compensation expense related to stock options for services | | — | | | — | | | 5,924 | | | — | | | — | | | — | | | 5,924 | | | | |
| | Reclassification of vested stock options granted to non-employee consultants to liabilities | | — | | | — | | | (1,850 | ) | | — | | | — | | | — | | | (1,850 | ) | | | |
| | Unrealized losses on marketable securities | | — | | | — | | | — | | | — | | | (2 | ) | | — | | | (2 | ) | | (2 | ) |
| | Net loss | | — | | | — | | | — | | | — | | | — | | | (63,495 | ) | | (63,495 | ) | | (63,495 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance at December 31, 2007 | | 33,875,948 | | $ | 3 | | $ | 324,946 | | | — | | $ | — | | $ | (300,053 | ) | $ | 24,896 | | $ | (63,497 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
See accompanying notes to consolidated financial statements.
F-5
SYNTA PHARMACEUTICALS CORP.
Consolidated Statements of Cash Flows
(in thousands)
| | Years ended December 31
| |
|---|
| | 2007
| | 2006
| | 2005
| |
|---|
| Cash flows from operating activities: | | | | | | | | | | |
| | Net loss | | $ | (63,495 | ) | $ | (57,270 | ) | $ | (68,863 | ) |
| | Adjustments to reconcile net loss to net cash provided by (used in) operating activities: | | | | | | | | | | |
| | | Expense deferred offering costs | | | — | | | — | | | 1,085 | |
| | | Other stock-related compensation expense | | | 5,417 | | | 4,791 | | | 3,175 | |
| | | Depreciation and amortization | | | 3,351 | | | 3,655 | | | 2,455 | |
| | | Changes in operating assets and liabilities: | | | | | | | | | | |
| | | | Restricted cash | | | 457 | | | (83 | ) | | — | |
| | | | Prepaid expenses and other current assets | | | (1,074 | ) | | 173 | | | 161 | |
| | | | Other assets | | | 56 | | | 1 | | | (17 | ) |
| | | | Accounts payable | | | (144 | ) | | (729 | ) | | 476 | |
| | | | Accrued expenses | | | 3,854 | | | (3,523 | ) | | (354 | ) |
| | | | Deferred revenue | | | 78,800 | | | — | | | — | |
| | |
| |
| |
| |
| | | | | Net cash provided by (used in) operating activities | | | 27,222 | | | (52,985 | ) | | (61,882 | ) |
| | |
| |
| |
| |
| Cash flows from investing activities: | | | | | | | | | | |
| | Purchases of marketable securities | | | (15,014 | ) | | (118,204 | ) | | (184,365 | ) |
| | Sales and maturities of marketable securities | | | 28,149 | | | 143,358 | | | 228,424 | |
| | Purchases of property and equipment | | | (2,350 | ) | | (1,580 | ) | | (4,883 | ) |
| | |
| |
| |
| |
| | | | | Net cash provided by investing activities | | | 10,785 | | | 23,574 | | | 39,176 | |
| | |
| |
| |
| |
| Cash flows from financing activities: | | | | | | | | | | |
| | Proceeds from issuances of common stock and exercise of common stock warrants, net | | | 44,660 | | | — | | | 134 | |
| | Proceeds from issuance of convertible preferred stock, net | | | — | | | 39,961 | | | — | |
| | Proceeds from exercise of stock options | | | 136 | | | 2 | | | — | |
| | Repurchase of restricted common stock | | | (290 | ) | | — | | | — | |
| | Proceeds from sale—leaseback of property and equipment | | | 1,994 | | | 1,412 | | | 4,745 | |
| | Payment of capital lease obligations | | | (2,617 | ) | | (2,086 | ) | | (1,100 | ) |
| | |
| |
| |
| |
| | | | | Net cash provided by financing activities | | | 43,883 | | | 39,289 | | | 3,779 | |
| | |
| |
| |
| |
| | | | | Net increase (decrease) in cash and cash equivalents | | | 81,890 | | | 9,878 | | | (18,927 | ) |
| Cash and cash equivalents at beginning of period | | | 33,687 | | | 23,809 | | | 42,736 | |
| | |
| |
| |
| |
| Cash and cash equivalents at end of period | | $ | 115,577 | | $ | 33,687 | | $ | 23,809 | |
| | |
| |
| |
| |
| Supplemental disclosure of noncash investing and financing activities: | | | | | | | | | | |
| | Acquisition of equipment under capital leases | | $ | 2,338 | | $ | 1,412 | | $ | 5,549 | |
| | Convertible preferred stock beneficial conversion charge | | $ | 58,585 | | | — | | | — | |
| | Convertible preferred stock dividends | | | — | | $ | 1,859 | | | — | |
| | Conversion of preferred stock | | $ | 41,820 | | | — | | | — | |
| | Issuance of common stock purchase obligation | | $ | 260 | | | — | | | — | |
Supplemental disclosure of cash flow information: |
|
|
|
|
|
|
|
|
|
|
| | Cash paid for interest | | $ | 536 | | $ | 574 | | $ | 274 | |
See accompanying notes to consolidated financial statements.
F-6
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements
(1) Nature of Business
The Company was incorporated in March 2000 and commenced operations in July 2001. The Company is a biopharmaceutical company focusing on discovering, developing and commercializing small molecule drugs that address severe medical conditions, including cancer and chronic inflammatory diseases.
The Company is subject to risks common to emerging companies in the drug development and pharmaceutical industry including, but not limited to, uncertainty of product development and commercialization, lack of marketing and sales history, dependence on key personnel, uncertainty of market acceptance of products, product liability, uncertain protection of proprietary technology, potential inability to raise additional financing and compliance with the Food and Drug Administration (FDA) and other government regulations.
In February 2007, the Company sold 5,000,000 shares of its common stock at $10.00 per share in an initial public offering (IPO), resulting in net proceeds of approximately $44.7 million (see Note 5).
In October 2007, the Company and GlaxoSmithKline (GSK) entered into a collaborative development, commercialization and license agreement for elesclomol. Under the terms of the agreement (the GSK Agreement), the Company received a non-refundable upfront cash payment of $80 million in November 2007 (see Note 8).
The Company has incurred significant operating losses since its inception and, as a result, at December 31, 2007 had an accumulated deficit of $300.1 million. Operations have been funded principally through the sale of common stock and convertible preferred stock, the upfront payment from GSK, and capital leases. At December 31, 2007, the Company had approximately $115.6 million in cash and cash equivalents.
Based on the Company's current operating plans, it expects its existing funds will be sufficient to fund operations through at least 2008. Payment to the Company by GSK of milestones for operational progress and achievement of certain success criteria leading to the approval by the FDA of elesclomol for the treatment of metastatic melanoma could extend the Company's cash availability, as could payments of milestones in connection with the development of elesclomol in other cancer indications and achievement of certain net sales thresholds. However, the Company may require significant additional funds earlier than it currently expects to conduct additional clinical trials and seek regulatory approval of its drug candidates. No assurances can be made that future capital will be available on terms acceptable to the Company to support its long-term liquidity needs.
Beginning in the fourth quarter of 2007, the Company was no longer a development-stage enterprise when it began recognizing revenue under the GSK Agreement.
(2) Summary of Significant Accounting Policies
The consolidated financial statements include the financial statements of the Company and its wholly owned subsidiaries. All significant intercompany balances and transactions have been eliminated in consolidation.
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect
F-7
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(2) Summary of Significant Accounting Policies (Continued)
certain reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting periods. Significant items subject to such estimates and assumptions include long-term contract accruals, recoverability of long-lived and deferred tax assets, valuation of acquired in-process research and development, measurement of stock-based compensation, and the fair value of the Company's common stock. The Company bases its estimates on historical experience and various other assumptions that management believes to be reasonable under the circumstances. Changes in estimates are recorded in the period in which they become known. Actual results could differ from those estimates.
Cash equivalents include money market funds and marketable securities. The Company considers all highly liquid investments with original maturities of three months or less at the date of purchase to be cash equivalents. Changes in cash and cash equivalents may be affected by shifts in investment portfolio maturities, as well as actual cash disbursements to fund operations.
The Company considers its marketable securities available-for-sale in accordance with SFAS No. 115,Accounting for Certain Investments in Debt and Equity Securities. Marketable securities consist of investments in high-grade corporate, government and government agency obligations that are classified as available-for-sale. Since these securities are available to fund current operations they are classified as current assets on the consolidated balance sheets. Marketable securities are stated at fair value, including accrued interest, with their unrealized gains and losses included as a component of accumulated other comprehensive income (loss), which is a separate component of stockholders' equity, until such gains and losses are realized. The fair value of these securities is based on quoted market prices. If a decline in value is considered other-than-temporary, based on available evidence, the unrealized loss is transferred from accumulated other comprehensive income (loss) to the consolidated statement of operations. Realized gains and losses are determined on the specific identification method.
During the years ended December 31, 2007, 2006 and 2005, the Company recorded no realized gains and losses on marketable securities, and there were no unrealized gains and losses as of December 31, 2007. There were no charges to write down marketable securities in 2007 and 2006.
Financial instruments that potentially subject the Company to a concentration of credit risk consist of money market funds and marketable securities. Deposits with banks may exceed the amount of insurance provided on such deposits. Generally, these deposits may be redeemed upon demand and, therefore, bear minimal risk. Marketable securities consist of investments in high-grade corporate, government and government agency obligations. The Company's policy for investments in marketable securities, approved by the board of directors, establishes guidelines relating to diversification and maturities that allows the Company to manage risk.
As of December 31, 2007, the Company had cash and cash equivalents of $115.6 million consisting of cash deposited in a highly rated financial institution in the United States and in short-term money market funds. Subsequent to year-end, the Company transferred its invested funds to a short-term U.S.
F-8
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(2) Summary of Significant Accounting Policies (Continued)
Treasury money market fund. The primary objective of the Company's investment activities is to preserve its capital for the purpose of funding operations and the Company does not enter into investments for trading or speculative purposes. The Company believes that it did not have material exposure to high-risk investments, such as mortgage-backed securities, auction rate securities or other special investment vehicles, or SIV's, within its money-market fund investments. The Company also believes that it does not have any material exposure to changes in fair value as a result of changes in interest rates. Declines in interest rates, however, would reduce future investment income.
The carrying amounts of the Company's financial instruments, which include cash equivalents, marketable securities, and capital lease obligations, approximate their fair values.
Property equipment and software is carried at cost and depreciated using the straight-line method over the estimated useful lives of the related assets, which range from three to five years. Leasehold improvements are amortized over the lesser of the lease term or estimated useful life.
Research and development costs are expensed as incurred in accordance with SFAS No. 2,Accounting for Research and Development Costs. Research and development costs are comprised of costs incurred in performing research and development activities, including salaries, benefits, facilities, research-related overhead, clinical trial costs, contracted services, technology acquisition license fees, and other external costs.
Costs to secure and defend patents are expensed as incurred and are classified as general and administrative expense in the Company's consolidated statements of operations. Patent expenses were approximately $2,515,000, $1,561,000 and $1,598,000 for the years ended December 31, 2007, 2006 and 2005, respectively.
The Company accounts for income taxes in accordance with SFAS No. 109,Accounting for Income Taxes. Deferred tax assets and liabilities are determined based on differences between financial reporting and income tax basis of assets and liabilities, as well as net operating loss carryforwards, and are measured using the enacted tax rates and laws that are expected to be in effect when the differences reverse. Deferred tax assets may be reduced by a valuation allowance to reflect the uncertainty associated with their ultimate realization.
The Company accounts for the impairment and disposition of long-lived assets in accordance with SFAS No. 144,Accounting for the Impairment or Disposal of Long-Lived Assets (SFAS No. 144). In accordance with SFAS No. 144, management assesses the potential impairments of its long-lived assets whenever events or changes in circumstances indicate that an asset's carrying value may not be
F-9
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(2) Summary of Significant Accounting Policies (Continued)
recoverable. If the carrying value exceeds the undiscounted future cash flows estimated to result from the use and eventual disposition of the asset, the Company writes down the asset to its estimated fair value. Management believes that no long-lived assets were impaired as of December 31, 2007 and 2006.
The Company's principal sources of revenue may include up front payments, development milestone payments, reimbursements of development costs, profit sharing payments, sales milestones and royalties from its collaborations. The Company recognizes revenue from these sources in accordance with Staff Accounting Bulletin (SAB) 104, "Revenue Recognition", or SAB 104, Emerging Issues Task Force (EITF) No. 99-19, "Reporting Revenue Gross as a Principal Versus Net as an Agent", or EITF No. 99-19, and EITF No. 00-21, "Revenue Arrangements with Multiple Deliverables", or EITF No. 00-21. The application of EITF No. 00-21 requires subjective analysis and requires management to make estimates and assumptions about whether deliverables within multiple-element arrangements are separable from the other aspects of the contractual arrangement into separate units of accounting and to determine the fair value to be allocated to each unit of accounting.
The Company entered into the GSK Agreement with GSK in October 2007. The Company evaluated the multiple deliverables within the GSK Agreement in accordance with the provisions of EITF No. 00-21 to determine whether the delivered elements that are the obligation of the Company have value to GSK on a stand-alone basis and whether objective reliable evidence of fair value of the undelivered items exists. Deliverables that meet these criteria are considered a separate unit of accounting. Deliverables that do not meet these criteria are combined and accounted for as a single unit of accounting. The appropriate recognition of revenue is then applied to each separate unit of accounting.
The Company's deliverables under the GSK Agreement, including the related rights and obligations, contractual cash flows and performance periods, are more fully described in Note 8 and are considered a single unit of accounting.
The GSK Agreement consists of the following key funding streams: an upfront payment, product development milestone payments, reimbursements of certain development costs, sales milestone payments, profit sharing payments and product royalty payments. The cash flows associated with the single unit of accounting from the development portion of the GSK Agreement are recognized as revenue using a time-based model. Under this model, cash flow streams are recognized as revenue over the estimated performance period. Upon receipt of cash payments, revenue is recognized to the extent the accumulated service time, if any, has occurred. The remainder is deferred and recognized as revenue ratably over the remaining estimated performance period. A change in the period of time expected to complete the deliverable is accounted for as a change in estimate on a prospective basis. Revenue is limited to amounts that are nonrefundable and that GSK is contractually obligated to pay to the Company.
The $80 million non-refundable upfront payment the Company received from GSK in November 2007, together with the $260,000 fair value of an option to require GSK to purchase $25 million of the Company's common stock, is being recognized as collaboration revenue using the time-based model over the estimated performance period, the 15-year period through the earliest expiration date of the related patents, which the Company estimates to be the effective life of the GSK Agreement. The
F-10
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(2) Summary of Significant Accounting Policies (Continued)
Company is also recognizing product development milestone payments and reimbursements of development costs as collaboration revenue using the time-based model over the same performance period through November 2022. Based on the guidance of EITF No. 99-19, the Company has determined that it is acting as a principal under the GSK Agreement and, as such, records these amounts as collaboration revenue. In 2007, the Company recognized $743,000 of collaboration revenue under the GSK Agreement.
Profit sharing payments are based upon a formula that provides for a range of 40-50% of net profits earned on U.S. sales of products included in the GSK Agreement. Royalty revenues are based upon a percentage of sales in non-U.S. territories. Profit sharing payments and royalties from the sales of products included in the GSK Agreement will be recorded on the accrual basis when results are reliably measurable, collectibility is reasonably assured and all other revenue recognition criteria are met. Sales milestones, which are based upon the achievement of certain agreed-upon sales thresholds, will be recorded when the respective sales threshold is achieved and collectibility is reasonably assured.
Consistent with the Company's policy on revenue recognition, deferred collaboration revenue represents cash received in advance for licensing fees, option fees, consulting, research and development contracts and related cost sharing and supply agreements. Such payments are reflected as deferred collaboration revenue until revenue can be recognized under the Company's revenue recognition policy. Deferred collaboration revenue is classified as current if management believes the Company will complete the earnings process and be able to recognize the deferred amount as revenue within 12 months of the balance sheet date. At December 31, 2007, total deferred collaboration revenue was approximately $79.5 million, of which $5.4 million was current and will be recognized as revenue during 2008.
Prior to January 1, 2006, the Company applied the intrinsic-value-based method of accounting prescribed by Accounting Principles Board (APB) Opinion No. 25,Accounting for Stock Issued to Employees,and related interpretations including Financial Accounting Standards Board (FASB) Interpretation No. 44,Accounting for Certain Transactions involving Stock Compensation, an Interpretation of APB Opinion No. 25, in accounting for its employee stock options. Under this method, compensation expense is generally recorded on the date of grant only if the estimated fair value of the underlying stock exceeds the exercise price. Given the absence of an active market for the Company's common stock prior to the IPO, the board of directors historically has determined the estimated fair value of common stock on the dates of grant based on several factors, including progress against regulatory, clinical and product development milestones, sales of common stock to outside investors and the likelihood of achieving a liquidity event such as an initial public offering or sale of the Company. As a result, the Company recorded deferred compensation charges for the difference between the estimated fair value of the common stock and the exercise price of options granted at the date of grant. Compensation expense is recognized over the vesting period on a straight-line basis.
SFAS No. 123,Accounting for Stock-Based Compensation (SFAS No. 123) and SFAS No. 148,Accounting for Stock-Based Compensation—Transition and Disclosure, an amendment of FASB Statement
F-11
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(2) Summary of Significant Accounting Policies (Continued)
No. 123, established accounting and disclosure requirements using a fair-value-based method of accounting for stock-based employee compensation plans. As permitted by existing accounting standards, the Company elected to continue to apply the intrinsic-value-based method of accounting described above, for options granted through December 31, 2005. The following table illustrates the effect on net loss attributable to common stockholders as if the fair-value-based method had been applied to all outstanding and unvested awards for the year ended December 31, 2005, prior to the adoption of SFAS No. 123(R),Share-Based Payment on January 1, 2006 (in thousands, except per share amounts).
| | Year ended
December 31, 2005
| |
|---|
| Net loss attributable to common stockholders, as reported | | $ | (68,863 | ) |
| Add: stock-based employee compensation expense determined under the fair value method | | | (4,172 | ) |
| Deduct: stock-based employee compensation expense included in reported net loss | | | 2,034 | |
| | |
| |
| Pro forma net loss attributable to common stockholders | | $ | (71,001 | ) |
| | |
| |
| Basic and diluted net loss attributable to common stockholders per common share, as reported | | $ | (3.09 | ) |
| Basic and diluted net loss attributable to common stockholders per common share, pro forma | | $ | (3.19 | ) |
For the years ended December 31, 2007, 2006 and 2005, the fair value of each employee stock option award was estimated on the date of grant based on the fair value method using the Black-Scholes option pricing valuation model with the following weighted average assumptions:
| | Years ended December 31,
| |
|---|
| | 2007
| | 2006
| | 2005
| |
|---|
| Risk-free interest rate | | 4.6 | % | 4.63 | % | 3.91 | % |
| Expected life in years | | 6.25 years | | 6.25 years | | 5 years | |
| Volatility | | 75 | % | 75 | % | 70 | % |
| Expected dividend yield | | — | | — | | — | |
| Weighted average grant-date fair value | | $6.11 | | $9.80 | | $13.40 | |
Effective January 1, 2006, the Company adopted SFAS No. 123(R) using the modified prospective method of transition for employee stock option awards granted after January 17, 2005 (valued using the fair value method), and using the prospective method for awards granted prior to January 17, 2005 (valued using the minimum value method). Therefore, compensation cost recognized in the years ended December 31, 2007 and 2006 includes: (a) compensation costs related to the vesting of employee stock options granted after January 17, 2005 but prior to January 1, 2006, based on the grant date fair value method estimated in accordance with the provisions of SFAS No. 123 adjusted for estimated forfeitures (b) compensation costs related to the continued vesting of nonvested restricted stock awards granted prior to January 1, 2006, and (c) compensation costs for all share-based payments granted or modified subsequent to January 1, 2006, based on the provisions of SFAS No. 123(R).
F-12
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(2) Summary of Significant Accounting Policies (Continued)
Prior to the adoption of SFAS No. 123(R), the Company presented its unamortized portion of deferred compensation cost for nonvested stock options in the consolidated statement of stockholders' equity (deficit) and comprehensive loss with a corresponding credit to additional paid-in capital. Upon the adoption of SFAS No. 123(R), these amounts were offset against each other. Under SFAS No. 123(R), an equity instrument is not considered to be issued until the instrument vests. As a result, compensation costs are recognized over the requisite service period with an offsetting credit to additional paid-in capital, and the deferred compensation balance of $7,225,000 at January 1, 2006 was netted against additional paid-in capital during the first quarter of 2006.
The Company uses the Black-Scholes option pricing model as the most appropriate valuation method for its option grants. The Black-Scholes model requires inputs for risk-free interest rate, dividend yield, volatility and expected lives of the options. Since the Company has a limited history of stock activity, expected volatility is based on historical data from several public companies similar in size and value to the Company. The Company will continue to use a weighted average approach using historical volatility and other similar public entity volatility information until historical volatility of the Company is relevant to measure expected volatility for future option grants. The Company estimates the forfeiture rate based on historical data. Based on an analysis of historical forfeitures, the Company has applied a forfeiture rate of 10% to all options that vest upon completion of the first year of service following the date of grant. The analysis will be re-evaluated at least annually and the forfeiture rate will be adjusted as necessary. The risk-free rate for periods within the contractual life of the option is based on the U.S. Treasury yield curve in effect at the time of the grant. The expected lives for options granted represents the period of time that options granted are expected to be outstanding. Since January 1, 2006 the Company has used the simplified method for determining the expected lives of options.
For awards with graded vesting, the Company allocates compensation costs under SFAS No. 123(R) on a straight-line basis over the requisite service period. The Company amortized the fair value of each option over each option's service period, which is generally the vesting period.
The Company's net loss for the years ended December 31, 2007 and 2006 includes $5,417,000 and $4,791,000, respectively, of compensation costs and no income tax benefit related to the Company's stock-based compensation arrangements for employee and nonemployee awards. As of December 31, 2007, the total amount of unrecognized stock-based compensation expense is $12,649,000 and will be recognized over a weighted average period of 4.0 years.
The Company accounts for stock options issued to non-employees in accordance with the provisions of SFAS No. 123 and EITF No. 96-18,Accounting for Equity Instruments that are Issued to Other than Employees, or in Conjunction with Selling Goods or Services, which requires valuing and remeasuring such stock options to the current fair value until the performance date has been reached.
Certain of the Company's options granted to non-employees that are fully vested and no longer subject to a performance requirement are subject to EITF Issue No. 00-19,Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company's Own Stock, which requires the stock options held by certain non-employee consultants to be accounted for as liability awards. The fair value of these vested and unexercised awards was recognized as liability awards starting in April 2007 following the registration of stock options under Form S-8, using the Black-Scholes model. As of December 31, 2007, a liability of $1,343,000 was reflected in the consolidated balance sheet as other current liabilities. The fair value of the award is re-measured at each financial statement reporting date until the options are exercised or expire. When and if non-employee consultants exercise their Company options or the Company options expire, the corresponding liability will be reclassified to equity. As of December 31, 2007, vested stock options to acquire 312,911 shares of common stock held by non-employee consultants remained unexercised.
F-13
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(2) Summary of Significant Accounting Policies (Continued)
The following table outlines the details of recognized and unrecognized expense for these stock-based compensation arrangements (in thousands):
| | Stock compensation
expense for
the years ended
| |
|
|---|
| | Unrecognized stock compensation expense as of
December 31, 2007
|
|---|
| | 2007
| | 2006
|
|---|
| Employee stock options | | $ | 4,045 | | $ | 2,752 | | $ | 9,394 |
| Repriced employee stock options | | | 139 | | | 407 | | | 147 |
| Employee options issued below fair value | | | 10 | | | 60 | | | 17 |
| Non-employee stock options | | | (444 | ) | | 272 | | | 75 |
| Restricted stock | | | 1,667 | | | 1,300 | | | 3,016 |
| | |
| |
| |
|
| | | $ | 5,417 | | $ | 4,791 | | $ | 12,649 |
| | |
| |
| |
|
Stock-based compensation expense is allocated as follows (in thousands):
| | Years ended December 31,
|
|---|
| | 2007
| | 2006
| | 2005
|
|---|
| Research and development | | $ | 3,902 | | $ | 3,372 | | $ | 2,397 |
| General and administrative | | | 1,515 | | | 1,419 | | | 778 |
| | |
| |
| |
|
| | Total | | $ | 5,417 | | $ | 4,791 | | $ | 3,175 |
| | |
| |
| |
|
Certain of the employee stock options granted by the Company are structured to qualify as incentive stock options (ISOs). Under current tax regulations, the Company does not receive a tax deduction for the issuance, exercise or disposition of ISOs if the employee meets certain holding requirements. If the employee does not meet the holding requirements, a disqualifying disposition occurs, at which time the Company will receive a tax deduction. The Company does not record tax benefits related to ISOs unless and until a qualifying disposition occurs. In the event of a disqualifying disposition, the entire tax benefit is recorded as a reduction of income tax expense. The Company has not recognized any income tax benefit for the share-based compensation arrangement due to the fact that the Company does not believe it is more likely than not it will recognize any deferred tax assets from such compensation cost recognized in the current period.
SFAS No. 130,Reporting Comprehensive Income, requires that all components of comprehensive income (loss) be disclosed in the consolidated financial statements. Comprehensive income (loss) is defined as the change in equity of a business enterprise during a period from transactions, and other events and circumstances from non-owner sources. Changes in unrealized gains and losses on marketable securities represents the only difference between the Company's net loss and comprehensive loss.
The Company has adopted SFAS No. 131,Disclosure About Segments of an Enterprise and Related Information, which requires companies to report selected information about operating segments, as well
F-14
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(2) Summary of Significant Accounting Policies (Continued)
as enterprise-wide disclosures about products, services, geographical area, and major customers. Operating segments are determined based on the way management organizes its business for making operating decisions and assessing performance. The Company has only one operating segment, the discovery, development and commercialization of drug products.
Net loss per share is computed based on the guidance of SFAS No. 128,Earnings Per Share, requiring companies to report both basic net loss per common share, which is computed using the weighted average number of common shares outstanding during the period, and diluted net loss per common share, which is computed using the weighted average number of common shares outstanding and the weighted average dilutive potential common shares outstanding using the treasury stock method. However, for all periods presented, diluted net loss per share is the same as basic net loss per share as the inclusion of weighted average shares of unvested restricted common stock and common stock issuable upon the exercise of stock options and warrants and conversion of convertible preferred stock would be anti-dilutive.
The following table summarizes securities outstanding as of each year-end which were not included in the calculation of diluted net loss per share as their inclusion would be anti-dilutive.
| | December 31
|
|---|
| | 2007
| | 2006
| | 2005
|
|---|
| Common stock options | | 3,880,277 | | 3,044,343 | | 2,948,927 |
| Nonvested restricted common stock | | 157,832 | | 291,073 | | 415,454 |
| Convertible preferred stock | | — | | 2,092,931 | | — |
The convertible preferred stock and accrued dividends had been reflected as being converted into common stock using a $20.00 per share conversion factor. In February 2007, in connection with the IPO, all outstanding shares of the convertible preferred stock and accrued dividends were converted into common stock upon the completion of the IPO (see Note 5).
In December 2007, the Financial Accounting Standards Board or FSAB, issued SFAS No. 141R,Business Combinations, or SFAS No. 141R. The pronouncement establishes principles and requirements for how an acquirer recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed, any noncontrolling interest in the acquiree and the goodwill acquired. The pronouncement also establishes disclosure requirements to enable the evaluation of the nature and financial effects of the business combination. SFAS No. 141R is effective for fiscal years beginning after December 15, 2008. The Company is currently evaluating SFAS No. 141R and the impact it may have on its results of operations or financial position.
In December 2007, the FASB issued SFAS No. 160,Noncontrolling Interests in Consolidated Financial Statements—an Amendment of ARB No. 51, or SFAS No. 160. The pronouncement establishes accounting and reporting standards pertaining to ownership interests in subsidiaries held by parties other than the parent, the amount of net income attributable to the parent and to the noncontrolling interest, changes in a parent's ownership interest, and the valuation of any retained noncontrolling
F-15
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(2) Summary of Significant Accounting Policies (Continued)
equity investment when a subsidiary is deconsolidated. The pronouncement also establishes disclosure requirements that identify and distinguish between the interests of the parent and the interests of the noncontrolling owners. SFAS No. 160 is effective for fiscal years beginning on or after December 15, 2008. The Company is currently evaluating SFAS No. 160 and the impact it may have on its results of operations or financial position.
In June 2007, the EITF issued EITF No. 07-03,Accounting for Nonrefundable Advance Payments for Goods or Services to Be Used in Future Research and Development Activities, or EITF No. 07-03, which provides guidance for upfront payments related to goods and services of research and development activities. EITF No. 07-03 is effective for fiscal years beginning after December 15, 2007. The Company does not believe the adoption of EITF No. 07-03 will have a material impact on its overall financial position or results of operations.
In February 2007, the FASB issued SFAS No. 159,The Fair Value Option for Financial Assets and Financial Liabilities, or SFAS No. 159, including an amendment of SFAS No. 115, which permits entities to choose to measure many financial instruments and certain other items at fair value that are not currently required to be measured at fair value. SFAS No. 159 is effective for the Company beginning in 2008. The Company does not believe the adoption of SFAS No. 159 will have a material impact on its overall financial position or results of operations.
In September 2006, the FASB issued SFAS No. 157,Fair Value Measurements, or SFAS No. 157, which provides guidance for using fair value to measure assets and liabilities. The pronouncement clarifies (1) the extent to which companies measure assets and liabilities at fair value; (2) the information used to measure fair value; and (3) the effect that fair value measurements have on earnings. SFAS No. 157 will apply whenever another standard requires (or permits) assets or liabilities to be measured at fair value. SFAS No. 157 will be applicable to us for fiscal years beginning after November 15, 2007. In February 2008, the FASB issued SFAS No. 157-1 and No. 157-2 which delay the effective date of SFAS No. 157 for one year for certain non-financial assets and liabilities and removes certain leasing transactions from its scope. The Company does not believe the adoption of SFAS No. 157 will have a material impact on its overall financial position or results of operations.
In July 2006, the FASB issued FASB Interpretation No. 48,Accounting for Uncertainty in Income Taxes—an interpretation of FAS 109, or Interpretation No. 48. This interpretation clarifies the accounting for uncertainty in income taxes recognized in a company's financial statements in accordance with FASB Statement No. 109,Accounting for Income Taxes. This interpretation prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken in a tax return. It also provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure and transition. Interpretation No. 48 is effective for fiscal years beginning after December 15, 2006. The Company adopted this Interpretation No. 48 effective January 1, 2007 and its adoption had no impact on its consolidated results of operations and financial position.
F-16
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(3) Cash, Cash Equivalents and Marketable Securities
A summary of cash and cash equivalents and available-for-sale marketable securities held by the Company as of December 31, 2007 and 2006 is as follows:
| | December 31, 2007
|
|---|
| | Cost
| | Unrealized
gains
| | Unrealized
losses
| | Fair
value
|
|---|
| | (in thousands)
|
|---|
| Cash and cash equivalents: | | | | | | | | | | | | |
| | Cash and money market funds | | $ | 115,577 | | | — | | | — | | $ | 115,577 |
| Marketable securities: | | | | | | | | | | | | |
| | Corporate bonds: | | | | | | | | | | | | |
| | | Due within 1 year | | | — | | | — | | | — | | | — |
| | |
| |
| |
| |
|
| | | | Total cash, cash equivalents and marketable securities | | $ | 115,577 | | $ | — | | $ | — | | $ | 115,577 |
| | |
| |
| |
| |
|
| | December 31, 2006
|
|---|
| | Cost
| | Unrealized
gains
| | Unrealized
losses
| | Fair
value
|
|---|
| | (in thousands)
|
|---|
| Cash and cash equivalents: | | | | | | | | | | | | |
| | Cash and money market funds | | $ | 33,687 | | $ | — | | $ | — | | $ | 33,687 |
| Marketable securities: | | | | | | | | | | | | |
| | Corporate bonds: | | | | | | | | | | | | |
| | | Due within 1 year | | | 13,135 | | | 2 | | | — | | | 13,137 |
| | |
| |
| |
| |
|
| | | | Total cash, cash equivalents and marketable securities | | $ | 46,822 | | $ | 2 | | $ | — | | $ | 46,824 |
| | |
| |
| |
| |
|
(4) Property and Equipment
Property and equipment consist of the following at December 31:
| | 2007
| | 2006
| |
|---|
| | (in thousands)
| |
|---|
| Laboratory equipment | | $ | 10,110 | | $ | 8,352 | |
| Leasehold improvements | | | 4,238 | | | 3,854 | |
| Computers and software | | | 1,961 | | | 1,414 | |
| Furniture and fixtures | | | 791 | | | 677 | |
| | |
| |
| |
| | | | 17,100 | | | 14,297 | |
| Less accumulated depreciation and amortization | | | (11,524 | ) | | (8,230 | ) |
| | |
| |
| |
| | | $ | 5,576 | | $ | 6,067 | |
| | |
| |
| |
Depreciation and amortization expenses of property and equipment were approximately $3,351,000, $3,655,000 and $2,455,000 for the years ended December 31, 2007, 2006 and 2005, respectively. The net book value and accumulated depreciation of equipment under capital lease was $4,155,000 and $5,254,000 and $4,050,000 and $3,020,000, at December 31, 2007, and 2006, respectively.
F-17
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(5) Stockholders' Equity
In June 2006, the Company's stockholders approved an increase in the number of authorized shares of common stock from 150,000,000 shares to 158,000,000 shares and 8,000,000 shares of preferred stock all of which were designated as shares of Series A Convertible Preferred Stock, each share having a $0.0001 par value.
Each common stockholder is entitled to one vote for each share of stock held. The common stock will vote together with all other classes and series of stock of the Company as a single class on all actions to be taken by the Company's stockholders. Each share of common stock is entitled to receive dividends, as and when declared by the Company's board of directors.
The Company has never declared cash dividends on its common stock and does not expect to do so in the foreseeable future.
In January 2007, the Board of Directors and the stockholders of the Company approved (i) a 1-for-4 reverse stock split, which was effected on February 2, 2007, subject to a reduction for fractional shares that were paid for in cash, (ii) an adjustment of the authorized common shares to 100,000,000 and the authorized preferred shares to 5,000,000, which became effective upon the completion of the IPO, and (iii) an adjustment in the number of common shares reserved under the 2006 Stock Plan to 2,500,000. All share data shown in the accompanying consolidated financial statements has been retroactively restated to reflect the reverse split. The reverse stock split did not alter the par value of the common stock and the preferred stock, which is $0.0001 per share, or modify any voting rights or other terms of the common stock.
In February 2007, the Company raised $50.0 million in gross proceeds from the sale of 5,000,000 shares of its common stock in the IPO at $10.00 per share. The net offering proceeds after deducting approximately $5.3 million in expenses for underwriters' discounts, fees and commissions, legal, accounting, printing, listing and filing fees, and miscellaneous expenses were approximately $44.7 million. As of December 31, 2006, the Company had incurred approximately $1.0 million in deferred IPO costs related to this offering, which were paid in 2007.
In June 2006, the Company sold 8,000,000 shares of its Series A Convertible Preferred Stock (the Preferred Stock) at a price of $5.00 per share resulting in gross proceeds of $40 million. The Preferred Stock accrued a cumulative annual dividend of 8% of its purchase price, and was automatically convertible into shares of the Company's common stock upon completion of an IPO. The number of shares of common stock into which each share of Preferred Stock was convertible was determined by dividing the Preferred Stock purchase price plus all accrued dividends by the lesser of $20.00 or 66.6667% of the offering price to the public of the IPO.
In February 2007, all outstanding shares of the Preferred Stock and $1.9 million in accumulated dividends on the Preferred Stock were converted into 6,278,765 shares of common stock upon the completion of the IPO.
F-18
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(5) Stockholders' Equity (Continued)
In accordance with EITF No. 98-5,Accounting for Convertible Securities with Beneficial Conversion Features or Contingently Adjustable Conversion Ratios, and EITF No. 00-27,Application of Issue No. 98-5 to Certain Convertible Instruments, the Company recorded a non-cash beneficial conversion charge of approximately $58.6 million in February 2007 in connection with the contingent adjustable conversion feature of the Preferred Stock.
During 2005 and 2004, the Company sold and issued 87,500 and 365,000 restricted shares of common stock, respectively, to its officers and certain employees at par value, of which 5,000, 127,500 and 40,000 of these restricted shares were forfeited in 2007, 2006 and 2005, respectively. Holders of 260,000 of the restricted shares employed by the Company in January 2007 became vested in 50% of the restricted stock. The remaining 50% vests upon the earlier of January 2009 or the approval of the Company's first new drug application (NDA) by the FDA. Holders of 25,000 shares of the restricted shares employed by the Company in January 2008 became vested in 50% of the restricted stock. The remaining 50% vests upon the earlier of January 2010 or the approval of the Company's first NDA by the FDA. During 2007, 2006 and 2005, the Company sold and issued 15,661, 12,142 and 9,089 shares of restricted stock, respectively, at par value to certain members of its Board of Directors in connection with their annual director fees. These restricted shares vest over the service periods. Compensation expense recognized for restricted shares was approximately $1,667,000, $1,300,000 and $1,916,000 in the years ended December 31, 2007, 2006 and 2005, respectively. The remaining unrecognized compensation expense on restricted stock at December 31, 2007 was $3,016,000. The weighted average period over which the balance is expected to be recognized is 1.1 years.
(6) Stock Option Plans
In March 2006, the Company terminated the 2001 Stock Plan and adopted the Synta Pharmaceuticals Corp. 2006 Stock Plan (the 2006 Stock Plan). The 2006 Stock Plan provides for the grant of incentive stock options, nonstatutory stock options and nonvested stock to employees, officers, directors and consultants to the Company. As of December 31, 2007, a total of 2,500,000 shares of common stock had been reserved for issuance under the 2006 Stock Plan. In February 2008, the board of directors increased the number of shares of common stock reserved for issuance to 3,800,000 under an "evergreen" provision, which provides for an annual increase based on the lesser of 1,300,000 shares, 5% of the Company's then outstanding shares of common stock, or such other amount as the board of directors may determine. The administration of the 2006 Stock Plan is under the general supervision of the board of directors. The exercise price of the stock options is determined by the board of directors, provided that incentive stock options are granted at not less than fair market value of the common stock on the date of grant and expire no later than ten years from the date the option is granted. Options generally vest over four years.
As of December 31, 2007, the Company had options outstanding to purchase 2,742,576 shares of its common stock, had outstanding 150,000 restricted shares of common stock and had no shares available for future issuance under the 2001 Stock Plan.
As of December 31, 2007, the Company had options outstanding to purchase 1,062,701 shares of its common stock, had outstanding 7,832 restricted shares of common stock and had available 1,409,496 shares available for future issuance under the 2006 Stock Plan.
F-19
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(6) Stock Option Plans (Continued)
As of December 31, 2007, the Company had options outstanding to purchase 75,000 shares of its common stock that were granted outside of the 2001 Stock Plan and 2006 Stock Plan.
In February 2006, the Company's board of directors authorized the amendment of 933,075 stock options outstanding as of March 1, 2006 for active employees, board of directors and consultants under the 2001 Stock Option Plan having an exercise price of $16.00 and above to provide for such options to have an amended exercise price equal to the then fair value of $14.00 per share. The amendment affected 159 option holders, of which 150 were employees. The amendment was accounted for in the same manner as the cancellation of existing options and the grant of new options. The Company recognized compensation expense, in the amount of approximately $269,000, to reflect the incremental compensation for vested options in connection with the re-pricing and, $139,000 and $138,000, respectively, of additional compensation in the years ended December 31, 2007 and 2006, respectively, to reflect the amortization of the incremental compensation for the unvested options. As of December 31, 2007, the total amount of unrecognized additional stock-based compensation expense in connection with the amended shares is $147,000 and will be recognized over a weighted average period of 2.1 years.
The Company's share-based compensation plan provides for awards of restricted shares of common stock to officers, other employees and non-employee directors. Restricted stock awards are subject to forfeiture if employment terminates during the prescribed retention period (see Note 5).
The following table summarizes stock option activity during the years ended December 31, 2007, 2006 and 2005:
| | 2007
| | 2006
| | 2005
|
|---|
| | Options
available
for grant
| | Shares
| | Weighted
average
exercise
price of
shares
under
plan
| | Options
available
for grant
| | Shares
| | Weighted
average
exercise
price of
shares
under
plan
| | Options
available
for grant
| | Shares
| | Weighted
average
exercise
price of
shares
under
plan
|
|---|
| Outstanding at January 1 | | 2,326,358 | | 3,044,343 | | $ | 11.89 | | 382,992 | | 2,948,927 | | $ | 13.92 | | 876,402 | | 2,512,106 | | $ | 11.80 |
| Granted | | (1,098,259 | ) | 1,082,598 | | | 8.82 | | (763,126 | ) | 750,984 | | | 14.00 | | (801,160 | ) | 704,571 | | | 22.00 |
| Exercised | | — | | (51,500 | ) | | 2.64 | | — | | (125 | ) | | 16.00 | | — | | — | | | — |
| Cancelled(1) | | 87,647 | | (195,164 | ) | | 11.57 | | 300,242 | | (655,443 | ) | | 15.84 | | 307,750 | | (267,750 | ) | | 15.32 |
| Additional shares reserved(2) | | 93,750 | | — | | | — | | 2,406,250 | | — | | | — | | — | | — | | | — |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
|
| Outstanding at December 31 | | 1,409,496 | | 3,880,277 | | $ | 11.21 | | 2,326,358 | | 3,044,343 | | $ | 11.88 | | 382,992 | | 2,948,927 | | $ | 13.92 |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
|
| Exercisable at December 31 | | | | 2,467,882 | | $ | 11.66 | | | | 2,011,393 | | $ | 10.88 | | | | 1,747,635 | | $ | 10.80 |
- (1)
- In March 2006, the Company terminated the 2001 Stock Plan and cancelled the then 93,472 shares reserved for future issuance.
Options cancelled subsequent to the March 2006 termination of the 2001 Stock Plan do not return to the pool of options available for future issuance.
Includes the effect of stock option cancellations for the period prior to termination of the 2001 Stock Plan of 277,593 shares.
Includes the effect of non-vested restricted stock cancellations for the period prior to termination of the 2001 Stock Plan of 112,500 shares.
F-20
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(6) Stock Option Plans (Continued)
Includes the effect of stock option cancellations under the 2006 Stock Plan of 2,375 shares.
- (2)
- In March 2006, the Company adopted the 2006 Stock Plan and authorized 2,406,250 shares for future issuance. In January 2007, the Company authorized the increase in shares reserved for future issuance from 2,406,250 to 2,500,000.
Included in the Company's stock options outstanding at December 31, 2007 are 332,180 options issued to non-employee consultants with a weighted average exercise price of $9.16 of which 312,911 are vested. The compensation expense is recorded over the respective vesting periods and is subject to variable accounting treatment prior to vesting, whereby the Company remeasures the fair value of the options at the end of each reporting period. Changes in the fair value may result in an expense or a credit in each reporting period. Compensation expense related to these options was approximately $(444,000), $272,000 and $1,142,000 for the years ended December 31, 2007, 2006 and 2005, respectively.
The following table summarizes information about outstanding and exercisable stock options at December 31, 2007:
| | Options Outstanding
| | Options Exercisable
|
|---|
Exercise price
| | Number
outstanding
| | Weighted
average
remaining
contractual
life (years)
| | Weighted
average
exercise
price
per share
| | Aggregate
intrinsic
value
| | Number
exercisable
| | Weighted
average
remaining
contractual
life
| | Weighted
average
exercise
price
per share
| | Aggregate
intrinsic
value
|
|---|
| $ 2.00 | | 116,012 | | 3.89 | | $ | 2.00 | | $ | 545,256 | | 116,012 | | 3.89 | | $ | 2.00 | | $ | 545,256 |
| 6.07-8.88 | | 796,902 | | 9.26 | | | 8.54 | | | — | | — | | — | | | — | | | — |
| 10.00-10.84 | | 1,598,593 | | 5.56 | | | 10.83 | | | — | | 1,425,493 | | 5.13 | | | 10.93 | | | — |
| 14.00 | | 1,368,770 | | 7.34 | | | 14.00 | | | — | | 926,377 | | 7.07 | | | 14.00 | | | — |
| | |
| |
| |
| |
| |
| |
| |
| |
|
| | | 3,880,277 | | 6.90 | | $ | 11.21 | | $ | 545,256 | | 2,467,882 | | 5.80 | | $ | 11.66 | | $ | 545,256 |
| | |
| |
| |
| |
| |
| |
| |
| |
|
In April 2006, stock options to purchase 125 shares of the Company's common stock were exercised, resulting in proceeds of $2,000.
Between January 2007 through October 2007, stock options to purchase 51,500 shares of the Company's common stock were exercised, resulting in proceeds of $136,000, and having an intrinsic value of approximately $366,000 based on the closing price of the Company's common stock on the dates of these stock option exercises.
F-21
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(6) Stock Option Plans (Continued)
The following table summarizes restricted stock activity during the years ended December 31, 2007, 2006 and 2005:
| | 2007
| | 2006
| | 2005
|
|---|
| | Shares
| | Weighted
average
grant date
fair value
| | Shares
| | Weighted
average
grant date
fair value
| | Shares
| | Weighted
average
grant date
fair value
|
|---|
| Outstanding at January 1 | | 291,073 | | $ | 21.15 | | 415,454 | | $ | 20.31 | | 365,000 | | $ | 22.00 |
| Granted | | 15,661 | | | 8.30 | | 12,142 | | | 14.00 | | 96,589 | | | 14.76 |
| Vested | | (143,902 | ) | | 20.92 | | (9,023 | ) | | 14.00 | | (6,135 | ) | | 22.00 |
| Cancelled | | (5,000 | ) | | 22.00 | | (127,500 | ) | | 18.08 | | (40,000 | ) | | 22.00 |
| | |
| |
| |
| |
| |
| |
|
| Outstanding at December 31 | | 157,832 | | $ | 20.05 | | 291,073 | | $ | 21.15 | | 415,454 | | $ | 20.31 |
| | |
| |
| |
| |
| |
| |
|
In January 2007, the Company repurchased 29,046 shares of its previously restricted common stock from certain officers and non-officer employees in order to fund the minimum statutory tax withholding requirements related to the vesting of 80,000 shares of restricted common stock. In June 2007, these treasury shares were retired.
(7) Accrued Expenses
Accrued expenses consist of the following at December 31:
| | 2007
| | 2006
|
|---|
| | (in thousands)
|
|---|
| Contracted research costs | | $ | 3,517 | | $ | 3,052 |
| Compensation and benefits | | | 3,165 | | | 1,196 |
| Professional fees | | | 1,721 | | | 1,451 |
| Other | | | 781 | | | 428 |
| | |
| |
|
| | | $ | 9,184 | | $ | 6,127 |
| | |
| |
|
(8) Collaborative Development, Commercialization and License Agreement
In October 2007, the Company and GSK entered into the GSK Agreement for elesclomol. Under the terms of the agreement, the companies will jointly develop and commercialize elesclomol in the United States, and GSK will have exclusive responsibility for the development and commercialization of elesclomol outside the United States. Pursuant to the agreement, the Company received a non-refundable upfront cash payment of $80 million in November 2007. The Company is also eligible to receive potential pre-commercial milestone payments from GSK of up to $585 million, which include both payments for operational progress, such as trial initiation and enrollment, and payments for positive clinical and regulatory outcomes, such as regulatory approval. Of the $585 million in potential payments, $135 million are related to the development in metastatic melanoma and $450 million are related to the development of elesclomol in other cancer indications. In addition, the Company is eligible to receive up to $300 million in potential commercial milestone payments from GSK based on
F-22
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(8) Collaborative Development, Commercialization and License Agreement (Continued)
achieving certain net sales thresholds. The Company will take the lead role and fund, up to a specified amount, all activities related to seeking FDA approval of elesclomol for the treatment of metastatic melanoma. The Company will also fund early clinical development of elesclomol in two other cancer indications. All other worldwide development costs will be shared, with the Company responsible for a modest proportion of those costs. In the United States, the Company's share of the operating profits and losses from the commercialization and sales of elesclomol will be 40-50%, with the percentage increasing as the level of annual sales increases. The Company may elect not to participate in co-commercialization, in which case the Company would earn royalties in lieu of profit sharing. Outside of the United States, the Company will receive double-digit tiered royalties. Under the GSK Agreement, GSK may, subject to the agreement of the Company, purchase up to $45 million of the Company's common stock in two separate tranches upon the achievement of specified development and regulatory milestones. In the first tranche, GSK would be obligated to buy $25 million of the Company's common stock at the sole discretion of the Company. The Company attributed $260,000 of value to this option to require GSK to purchase our common stock. The second tranche of $20 million of common stock would be subject to the agreement of both the Company and GSK. The per share purchase price under each tranche would be at a specified premium. GSK may terminate the agreement upon not less than three months' written notice at any time prior to the date of the first commercial sale of an elesclomol product and upon not less than six months' written notice at any time on and after such date, in which case GSK may be obligated in certain circumstances to make additional payments to the Company. Under the GSK Agreement, the Company has the right, but not the obligation to participate in various joint governance committees. The agreement was subject to the Hart-Scott-Rodino Act and has received clearance by the U.S. government (see Note 2).
(9) Income Taxes
Differences between the actual tax benefit and tax benefit computed using the United States federal income tax rate is as follows:
| | Years ended December 31
| |
|---|
| | 2007
| | 2006
| | 2005
| |
|---|
| | (in thousands)
| |
|---|
| Income tax benefit at statutory rate | | $ | (21,588 | ) | $ | (19,472 | ) | $ | (23,414 | ) |
| Stock-based compensation | | | 716 | | | 579 | | | — | |
| Tax credits | | | (1,647 | ) | | (1,743 | ) | | (2,232 | ) |
| Other | | | 42 | | | 40 | | | 33 | |
| Change in valuation allowance | | | 22,477 | | | 20,596 | | | 25,613 | |
| | |
| |
| |
| |
| | Income tax benefit | | $ | — | | $ | — | | $ | — | |
| | |
| |
| |
| |
F-23
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(9) Income Taxes (Continued)
The effects of temporary differences that give rise to significant portions of deferred tax assets and deferred tax liabilities at December 31, are presented below:
| | 2007
| | 2006
| |
|---|
| | (in thousands)
| |
|---|
| Deferred tax assets: | | | | | | | |
| | Federal and state net operating loss carryforwards | | $ | 103,359 | | $ | 80,157 | |
| | Federal and state research and experimentation credits | | | 9,886 | | | 8,310 | |
| | Licenses | | | 601 | | | 663 | |
| | Depreciation and amortization | | | 1,851 | | | 1,867 | |
| | Deferred compensation | | | 4,943 | | | 3,609 | |
| | Other | | | 936 | | | 743 | |
| | |
| |
| |
| | | Deferred tax assets | | | 121,576 | | | 95,349 | |
| Less valuation allowance | | | (121,576 | ) | | (95,349 | ) |
| | |
| |
| |
| | | Net deferred tax assets | | $ | — | | $ | — | |
| | |
| |
| |
The valuation allowance for deferred tax assets was approximately $121,576,000 and $95,349,000 as of December 31, 2007 and 2006, respectively. The increase in the total valuation allowance for the years ended December 31, 2007 and 2006 was approximately $26,227,000 and $24,345,000, respectively. The Company has established valuation allowances against its deferred tax assets because management believes that, after considering all of the available objective evidence, both historical and perspective, the realization of the deferred tax assets does not meet the "more likely than not" criteria under SFAS No. 109.
In 2005 and February 2007, the Company performed analyses to determine if there were changes in ownership, as defined by Section 382 of the Internal Revenue Code, that would limit its ability to utilize certain net operating loss and tax credit carryforwards. The Company determined that it experienced an ownership change, as defined by Section 382, in connection with its acquisition of Principia Associates, Inc. on September 20, 2002, but did not experience a change in ownership upon the effectiveness of the Company's IPO. As a result, the utilization of the Company's federal tax net operating loss carryforwards generated prior to the ownership change is limited. As of December 31, 2007, the Company has net operating loss carryforwards for U.S. federal tax purposes of approximately $259,076,000, after taking into consideration net operating losses expected to expire unused as a result of Section 382 limitations, and the remainder will expire in varying amounts through 2027 unless utilized. At December 31, 2007, the Company has state net operating loss carryforwards of approximately $243,596,000, which will expire through 2011 unless utilized. The utilization of these net operating loss carryforwards may be further limited if the Company experiences future ownership changes as defined in Section 382 of the Internal Revenue Code. At December 31, 2007, the Company had approximately $8,117,000 and $2,681,000, respectively, in federal and state research and development credits which expire through 2027 and 2022, respectively.
The Company is currently open to examination under the statute of limitations by the Internal Revenue Service and state jurisdictions for the tax years ended 2000 through 2006. Carryforward tax attributes generated in years past may still be adjusted upon future examination if they have or will be used in a future period. The Company is currently not under examination by the Internal Revenue Service or any other jurisdictions for any tax years. The Company adopted the provisions of FIN 48 in
F-24
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(9) Income Taxes (Continued)
the first quarter of 2007. The Company has not recorded any interest and penalties on any unrecognized tax benefits since its inception.
(10) Commitments and Contingencies
The Company leases its research and office facilities under non-cancelable operating leases with terms expiring through 2011. Each of these leases contains renewal options ranging from one to five years.
In September 2007, the Company renewed a lease for one of its research and office facilities for an eighteen-month term with a fourteen-month renewal option.
In November 2007, the Company renewed a lease for one of its research and office facilities for a four-year term and two three-year renewal options.
The Company subleased laboratory and office space from its scientific founder, who is a major shareholder of the Company, under a tenant-at-will arrangement. This lease was assumed by the Company in May 2005. In January 2007, the Company entered into an early termination agreement for this research and office facility under which the Company was obligated to pay the landlord $68,000 for termination fees and expenses.
In November 2004, the Company entered into an agreement for a revolving property and equipment lease line of credit which was amended in 2005. Under the amended agreement, the Company may periodically directly lease, or sell and lease-back, up to $6.0 million of property and equipment, with payment periods of 36 or 48 months and a $1.00 purchase option at the end of each lease period. The lease rates are based upon a fixed base interest rate plus the respective prevailing 36- or 48-month U.S. Treasury Bill interest rates at the time of each funding. The leases are accounted for as capital leases. In June 2007, the agreement was extended through June 2008. As of December 31, 2007, the Company sold and leased back under this agreement an aggregate of approximately $9.5 million of its previously purchased property and equipment, of which approximately $3.1 million and $6.4 million were capitalized and are being paid over 36 and 48 months, respectively. As a result, the Company recorded net deferred gains of approximately $316,000, which is being amortized over the applicable lease periods, and as of December 31, 2007 approximately $65,000 in net deferred gains was unamortized. As of December 31, 2007, approximately $1.4 million was available under this lease line for future property and equipment expenditures. The Company also leases certain vehicles and equipment under various other non-cancellable capital and operating leases.
F-25
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(10) Commitments and Contingencies (Continued)
Future minimum payments, excluding operating costs and taxes, under the Company's capital and non-cancellable operating leases, are approximately as follows (in thousands):
| | Capital leases
| | Operating leases
|
|---|
| Years ended December 31, | | | | | | |
| | 2008 | | $ | 2,828 | | $ | 1,987 |
| | 2009 | | | 1,939 | | | 1,836 |
| | 2010 | | | 853 | | | 1,480 |
| | 2011 | | | 311 | | | 1,351 |
| | 2012 | | | — | | | — |
| | |
| |
|
| Total minimum lease payments | | | 5,931 | | $ | 6,654 |
| | | | | |
|
| Less: amount representing interest | | | (710 | ) | | |
| | |
| | | |
| | Present value of minimum capital lease payments | | | 5,221 | | | |
| | Less current portions of capital lease obligations | | | (2,406 | ) | | |
| | |
| | | |
| Capital lease obligations—long term | | $ | 2,815 | | | |
| | |
| | | |
Rent expense was approximately $2,307,000, $1,914,000 and $2,217,000, for the years ended December 31, 2007, 2006 and 2005, respectively, including rent paid for the lease from its scientific founder in the amount of approximately $96,000 in 2005.
License Agreements
Queen's Medical Center
In March 2003, the Company entered into an exclusive, royalty-bearing license agreement with Queen's Medical Center (QMC) for certain technology related to ion channel technologies. Under the terms of the agreement, if certain milestones are met, the Company is obligated to make cash payments of up to an aggregate of $1.0 million. If commercialization is achieved, the Company will be required to pay royalties to QMC on the net sales of any product using the licensed technologies. In the event the Company grants a sublicense of the licensed technology, the Company is obligated to compensate QMC a percentage of all fees received from the sublicense.
Through December 31, 2007, no milestone, royalty, or sublicense payments had been earned by or paid to QMC.
The Company acquired two exclusive licenses from Beth Israel Deaconess Medical Center (Beth Israel) relating primarily to monoclonal antibodies and ion channel technologies. Under the terms of the licenses, if certain milestones are met, the Company is required to make cash payments up to an aggregate of $2.0 million. If commercialization is achieved, the Company will be required to pay royalties on the net sales of any product using the licensed technologies. In the event the Company grants a sublicense of the licensed technologies, the Company is obligated to compensate Beth Israel a percentage of all fees received from the sublicense.
The Company also assumed an exclusive license with Beth Israel to specific know-how relating to certain calcium channels. Under the terms of the agreement, if certain milestones are met, the
F-26
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(10) Commitments and Contingencies (Continued)
Company is required to make cash payments up to an aggregate of $800,000. If commercialization is achieved, the Company will be required to pay royalties on the net sales of any product using the licensed know-how.
Through December 31, 2007, no milestone, royalty or sublicense payments had been earned by or paid to Beth Israel.
In July 2002, the Company entered into an exclusive license agreement with Dana-Farber Cancer Institute (DFCI) for certain patent rights relating to the use of immune system modulators with other agents for use against cancer. Under the terms of the agreement, if certain milestones are met, the Company is required to make cash payments up to an aggregate of $600,000. If commercialization is achieved, the Company will be required to pay nominal royalties on the net sales of any product using the licensed technologies.
Through December 31, 2007, no milestone, royalty or sublicense payments had been earned by or paid to DFCI.
In October 2002, the Company entered into a consulting agreement with an SAB member for scientific advisory services which was amended in October 2003. Under the amended consulting agreement, the term was four years from the effective date of the amendment, and in exchange for a one-time payment of $400,000, the parties agreed to eliminate a one-time bonus payment to the SAB member based on the achievement of a certain performance milestone that was included in the original agreement. In addition to an annual consulting fee, the consultant was entitled to a bonus payment of a portion of any up-front or milestone payments received by the Company related to certain calcium channel technology during the four-year term of the amended agreement. In April 2007, the Company further amended this consulting agreement for a two-year term from the effective date of the amendment. In addition to the annual consulting fee, the consultant is entitled to potential bonus payments upon the Company entering into a partnership for certain calcium channel technology and upon the filing of an investigational new drug application (IND) with the FDA for a drug candidate developed under such a partnership.
As permitted under Delaware law, the Company's Certificate of Incorporation and Bylaws provide that the Company will indemnify certain of its officers and directors for certain claims asserted against them in connection with their service as an officer or director. The maximum potential amount of future payments that the Company could be required to make under these indemnification provisions is unlimited. However, the Company has purchased a directors' and officers' liability insurance policy that reduces its monetary exposure and enables it to recover a portion of any future amounts paid. The Company believes the estimated fair value of these indemnification arrangements is minimal.
The Company customarily agrees in the ordinary course of its business to indemnification provisions in agreements with clinical trials investigators in its drug development programs, in sponsored research agreements with academic and not-for-profit institutions, in various comparable
F-27
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(10) Commitments and Contingencies (Continued)
agreements involving parties performing services for the Company in the ordinary course of business, and in its real estate leases. The Company has agreed to indemnify GSK and its affiliates under the GSK Agreement against losses incurred or imposed as a direct result of claims arising out of the manufacture, use or sale by the Company of any product, except with respect to claims or losses that result from a breach of the GSK Agreement by, or the gross negligence or willful misconduct of, GSK. The Company also expects to agree to certain indemnification provisions in any future drug discovery and development collaboration agreements. With respect to the Company's clinical trials and sponsored research agreements, these indemnification provisions typically apply to any claim asserted against the investigator or the investigator's institution relating to personal injury or property damage, violations of law or certain breaches of the Company's contractual obligations arising out of the research or clinical testing of the Company's compounds or drug candidates. With respect to lease agreements, the indemnification provisions typically apply to claims asserted against the landlord relating to personal injury or property damage caused by the Company, to violations of law by the Company or to certain breaches of the Company's contractual obligations. The indemnification provisions appearing in collaboration agreements are similar, but in addition provide some limited indemnification for its collaborator in the event of third-party claims alleging infringement of intellectual property rights. In each of the cases above, the term of these indemnification provisions generally survives the termination of the agreement, although the provision has the most relevance during the contract term and for a short period of time thereafter. The maximum potential amount of future payments that the Company could be required to make under these provisions is generally unlimited. The Company purchased insurance policies covering personal injury, property damage and general liability that reduce its exposure for indemnification and would enable it in many cases to recover a portion of any future amounts paid. The Company has never paid any material amounts to defend lawsuits or settle claims related to these indemnification provisions. Accordingly, the Company believes the estimated fair value of these indemnification arrangements is minimal.
(11) Related Party Transactions
In January 2005, the Company entered into an Agreement and Release with its scientific founder, who is a board member, whereby all outstanding matters regarding various oral understandings and arrangements between the scientific founder and the Company were resolved, including arrangements relating to (1) the assignment by the scientific founder of the benefit of his interests, if any, resulting from the Company's acquisition of the net assets of Cancer Genomics, Inc., Kava Pharmaceuticals, Inc. and SinglePixel Biomedical, Inc. (collectively, CKS), (2) the scientific founder's assignment of inventions, non-competition, non-solicitation and confidentiality agreements with the Company, and (3) a release by the scientific founder of any and all claims that the scientific founder may have had against the Company. Pursuant to this agreement, the Company is paying the scientific founder $500,000, payable in $25,000 installments quarterly for five years. The full amount of the obligation was charged to research and development expense in 2005.
The Company paid its scientific founder and a member of the board consulting fees of approximately $25,000 per month in January and February 2007 pursuant to a consulting agreement dated April 18, 2005. In March 2007, the Company amended the consulting agreement to reduce the fee from $25,000 to $10,000 per month. Total consulting fees paid in 2007, 2006 and 2005 were approximately $150,000, $300,000 and $300,000, respectively.
F-28
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(12) Retirement Plan
In 2003, the Company implemented a 401(k) retirement plan (the Synta 401(k) Plan) in which substantially all of its permanent employees are eligible to participate. Participants may contribute a percentage of their annual compensation to the plan, subject to statutory limitations. The Company may declare discretionary matching contributions to the Synta 401(k) Plan.
In April 2006, the Company began matching participants' contributions up to 50% of the first 6% of the employee's salary. The match is subject to a three-year equally graded vesting schedule and any forfeitures will be applied to reduce the Company's contributions. Company contributions for the years ended December 31, 2007 and 2006 were approximately $411,000 and $236,000, respectively, subject to forfeitures.
(13) Research Grant Contracts
In 2003, the Company was awarded a $500,000 government contract with DARPA to perform research services associated with performance enhancement. Through December 31, 2006, the Company had recognized approximately $43,000 of research grant revenue for services performed under the terms of the contract, which expired in September 2004, and had recorded deferred revenue of approximately $457,000, which represented advance payments received under this contract. The advance payments were deposited in a separate non-interest-bearing account and were recorded as restricted cash as of December 31, 2006. In 2007, the Company returned the unused funds to DARPA.
(14) Initial Public Offering Costs
During 2005 and 2004, the Company incurred $2,389,000 of costs in connection with its planned initial public offering of common stock, of which $1,084,000 was deferred at December 31, 2004. Following the Company's filing of its Registration Statement on Form S-1 with the Securities and Exchange Commission in 2005, the Company determined that it would not complete the planned offering and withdrew its filing. The Company did not reactivate and complete its offering within 90 days of the withdrawal of the filing and, accordingly, these costs were expensed in 2005.
F-29
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(15) Quarterly Financial Data (unaudited)
The following tables present a summary of quarterly results of operations for 2007 and 2006:
| | Three Months Ended
| |
|---|
| | March 31,
2007
| | June 30,
2007
| | September 30,
2007
| | December 31,
2007
| |
|---|
| | (in thousands, except per share data)
| |
|---|
| Net loss attributable to common stockholders | | $ | (74,940 | ) | $ | (16,741 | ) | $ | (14,875 | ) | $ | (15,524 | ) |
| | |
| |
| |
| |
| |
| Basic and diluted net loss attributable to common stockholders per share | | $ | (2.61 | ) | $ | (0.50 | ) | $ | (0.44 | ) | $ | (0.46 | ) |
| Basic and diluted weighted average number of common shares outstanding | | | 28,767,605 | | | 33,658,536 | | | 33,661,613 | | | 33,708,862 | |
| | Three Months Ended
| |
|---|
| | March 31,
2006
| | June 30,
2006
| | September 30,
2006
| | December 31,
2006
| |
|---|
| | (in thousands, except per share data)
| |
|---|
| Net loss attributable to common stockholders | | $ | (16,211 | ) | $ | (14,891 | ) | $ | (14,733 | ) | $ | (13,294 | ) |
| | |
| |
| |
| |
| |
| Basic and diluted net loss attributable to common stockholders per share | | $ | (0.73 | ) | $ | (0.67 | ) | $ | (0.66 | ) | $ | (0.60 | ) |
| Basic and diluted weighted average number of common shares outstanding | | | 22,219,025 | | | 22,224,772 | | | 22,226,964 | | | 22,230,033 | |
F-30
QuickLinks
PART IPART IIPART IIIPART IVItem 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULESSIGNATURESINDEX TO FINANCIAL STATEMENTSReport of Independent Registered Public Accounting FirmSYNTA PHARMACEUTICALS CORP. Consoldated Balance SheetsSYNTA PHARMACEUTICALS CORP. Consoldated Statements of OperationsConsolidated Statements of Stockholders' Equity (Deficit) and Comprehensive LossSYNTA PHARMACEUTICALS CORP. Consoldated Statements of Cash FlowsSYNTA PHARMACEUTICALS CORP. Notes to Consolidated Financial Statements




