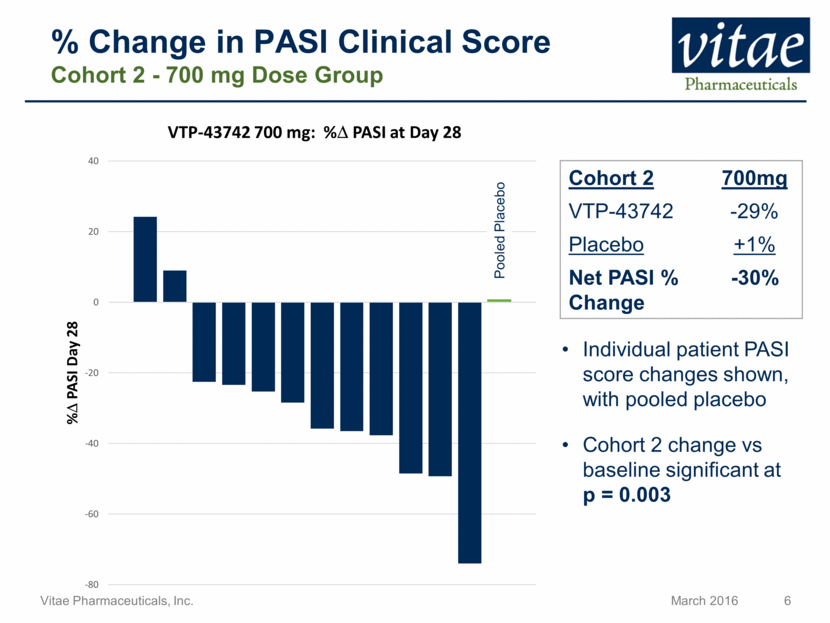
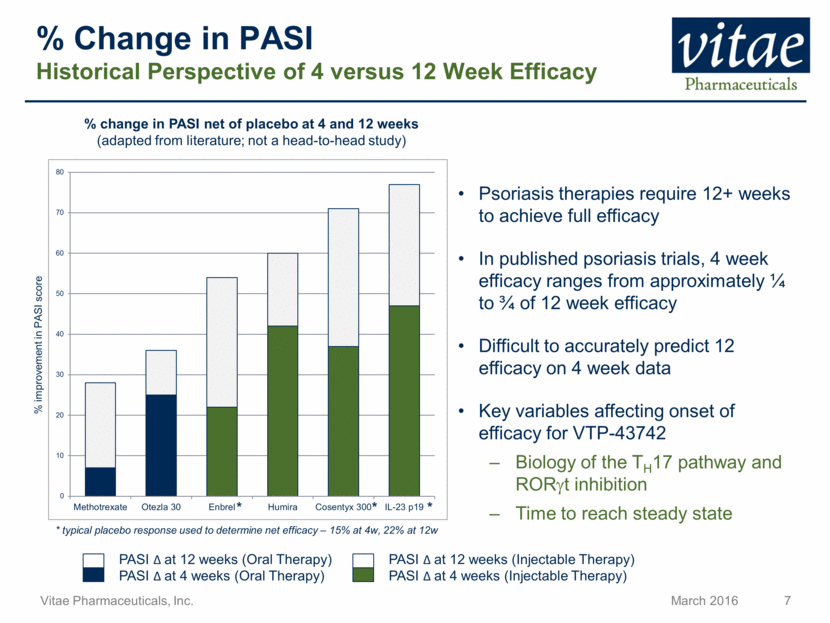
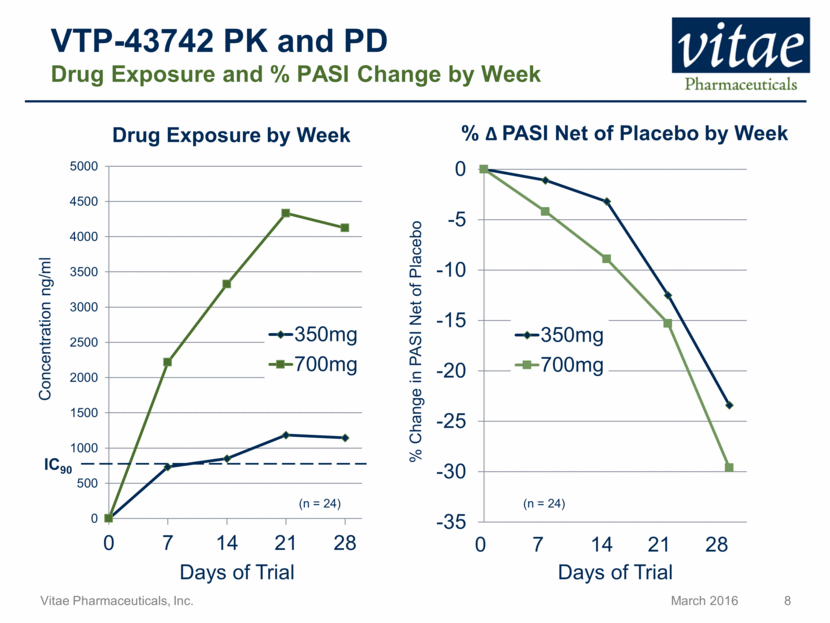
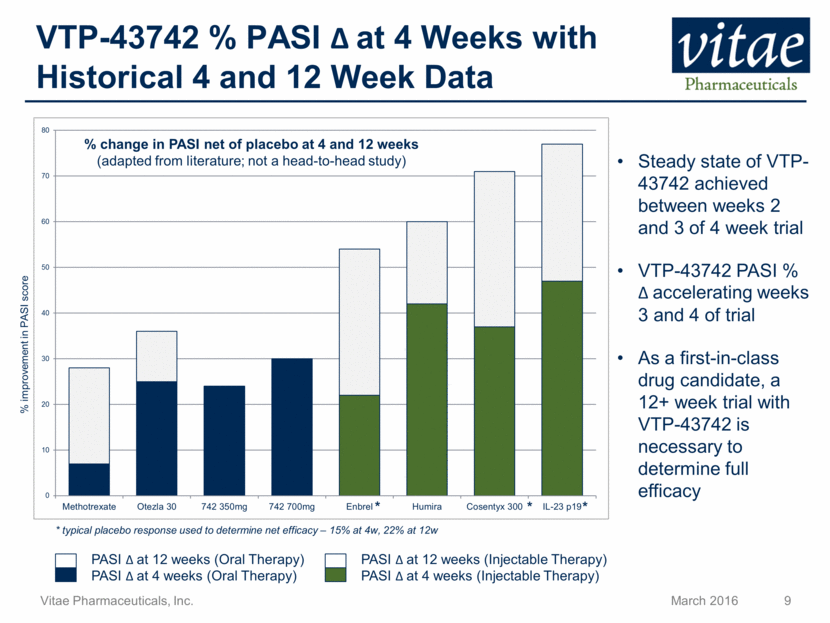
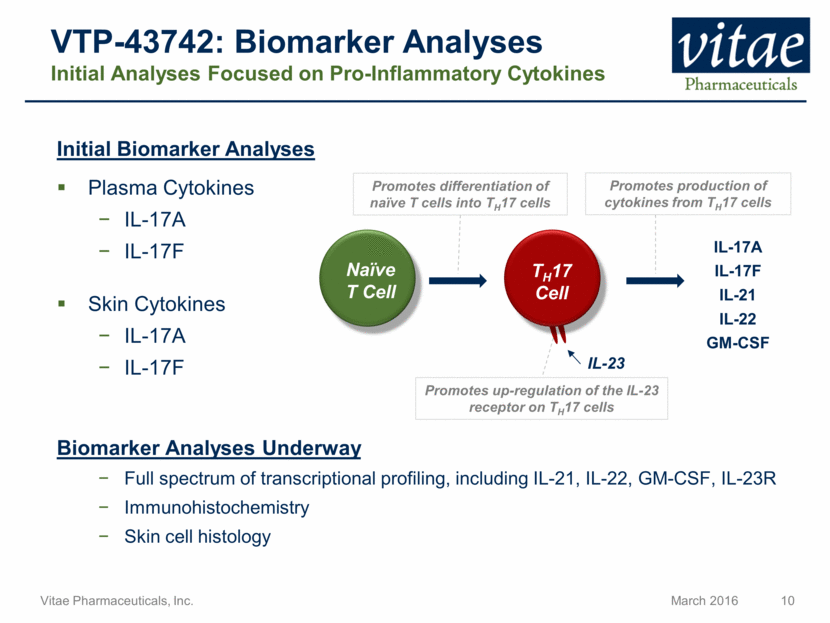
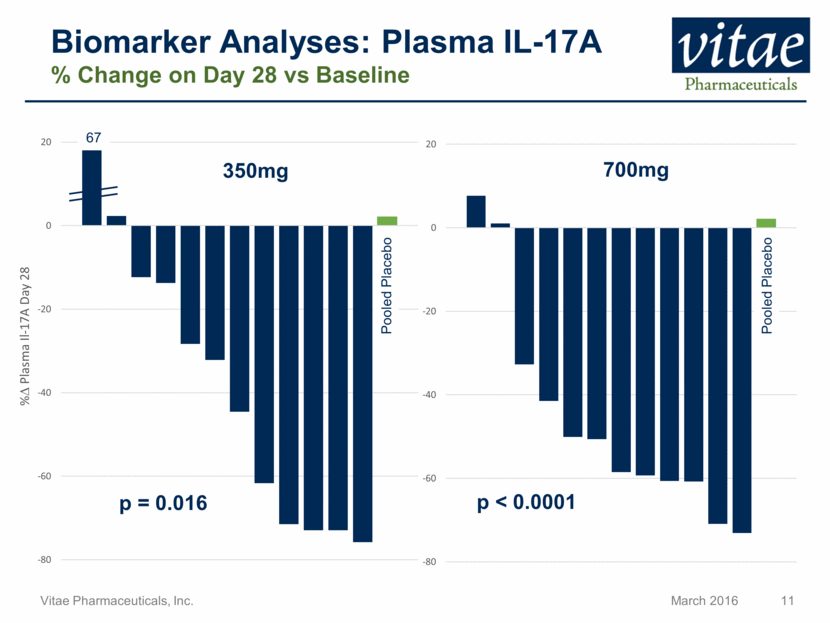
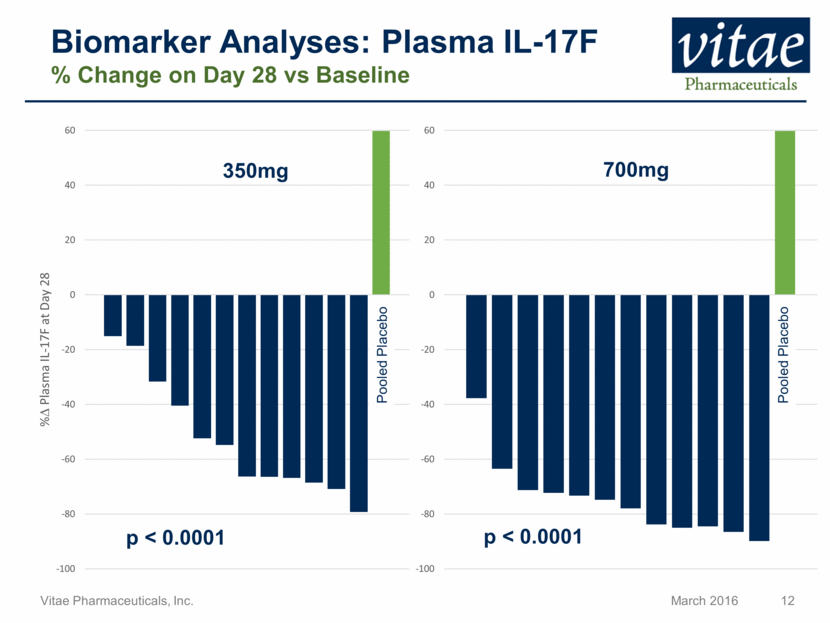
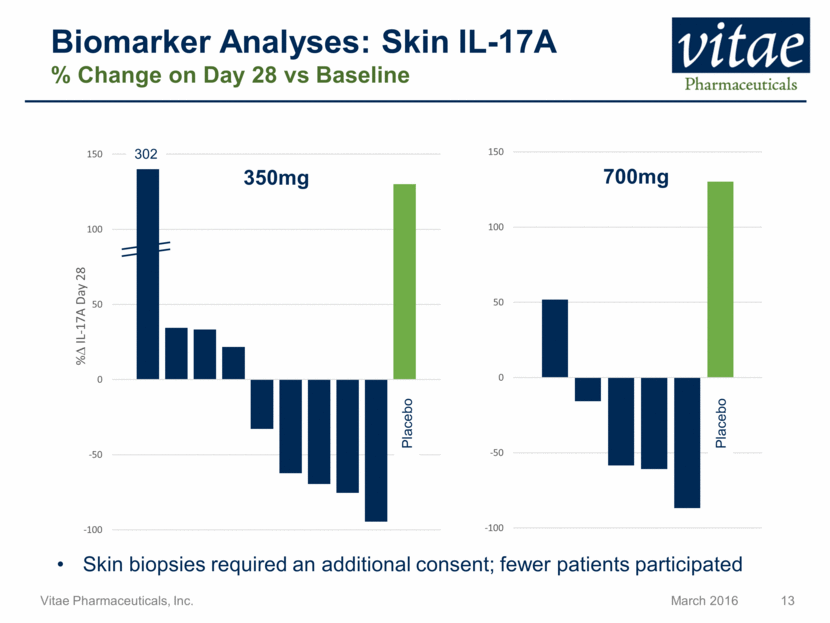
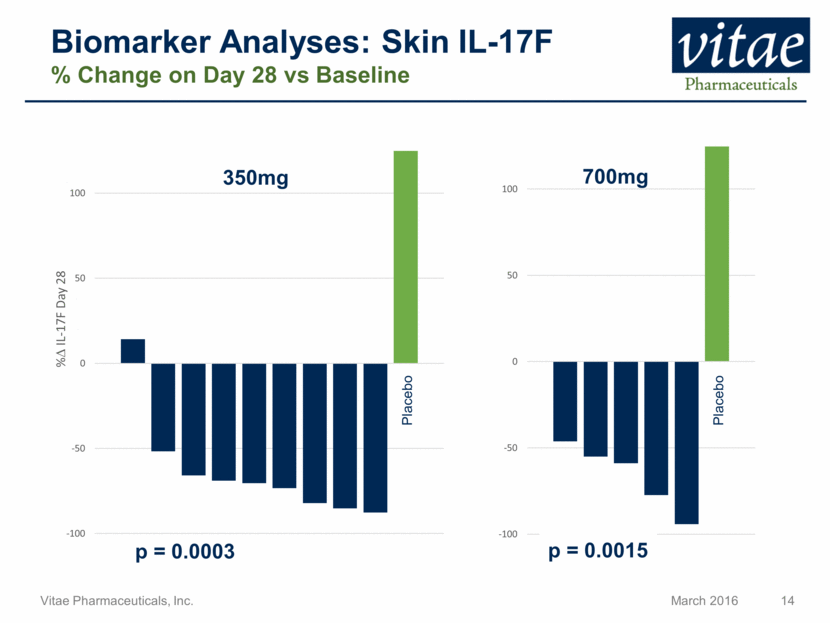
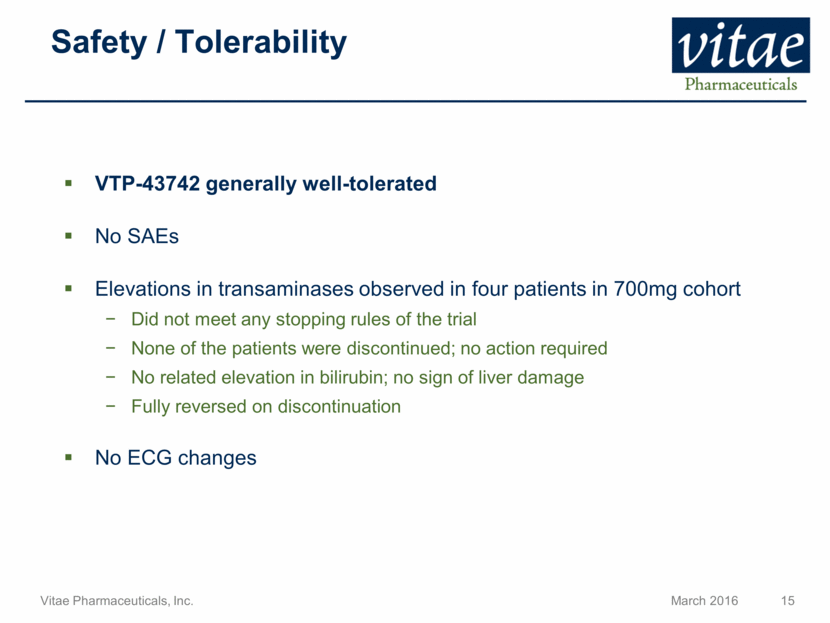
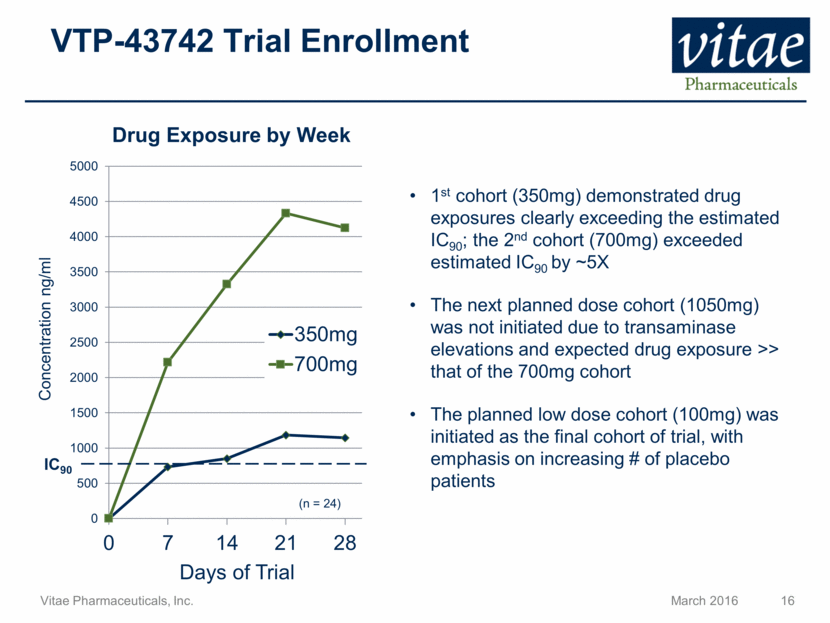
Disclaimer Regarding Forward Looking Statements This presentation includes statements that are, or may be deemed, “forward-looking statements”, within the meaning of Section 27A of the Securities Act of 1933, as amended. All statements, other than statements of historical facts, included in this presentation regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans and objectives of management are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “target,” “design,” “estimate,” “predict,” “opportunity,” “proposition,” “strategy,” “potential,” “plan” or the negative of these terms and similar expressions intended to identify forward-looking statements. These statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties, including the clinical plans for VTP-43742, the potential 12 week efficacy for VTP-43742 and the Company’s projected cash runway. Actual results may be materially different. Certain information in this presentation is from previously published data and literature from prior psoriasis trials of other compounds. Extrapolation of VTP-43742 4 week data to 12 week data is not indicative of any actual clinical results or future trial outcomes. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Forward-looking statements include, but are not limited to, statements about: the timing and success of preclinical studies and clinical trials; the ability to obtain and maintain regulatory approval of our product candidates; the scope, progress, expansion and costs of developing and commercializing our product candidates; our expectations regarding our expenses and revenue; the sufficiency of our cash resources and needs for additional financing; our ability to adequately manufacture our product candidates and the raw materials utilized therein; our ability to obtain and maintain intellectual property protection for our product candidates; our expectations regarding competition; the size and growth of the potential markets for our product candidates and the ability to serve those markets; the rate and degree of market acceptance of any of our product candidates; our anticipated growth strategies; the anticipated trends and challenges in our business and the market in which we operate; our ability to establish and maintain development partnerships; our ability to attract or retain key personnel; our expectations regarding federal, state and foreign regulatory requirements; regulatory developments in the United States and foreign countries and other factors that are described in the "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" sections of our Annual Report on Form 10-K for the year ended December 31, 2015 which has been filed with the Securities and Exchange Commission (SEC) and is available on the SEC's website at www.sec.gov. All written and verbal forward-looking statements attributable to Vitae or any person acting on its behalf are expressly qualified in their entirety by the cautionary statements contained or referred to herein. Vitae cautions investors not to rely too heavily on the forward-looking statements Vitae makes or that are made on its behalf. The information in this presentation is provided only as of the date of this presentation, and Vitae undertakes no obligation, and specifically declines any obligation, to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise.