
BURCON NUTRASCIENCE CORPORATION
1946 West Broadway
Vancouver, B.C.
V6J 1Z2
Telephone: (604) 733-0896
Facsimile: (604) 733-8821
ANNUAL INFORMATION FORM
FOR THE YEAR ENDED MARCH 31, 2018
June 18, 2018
TABLE OF CONTENTS
(i)
(ii)
(iii)
PRELIMINARY NOTES
Effective Date of Information
All information in this Annual Information Form ("AIF") is as of March 31, 2018 unless otherwise indicated.
Forward Looking Statements
This AIF contains certain "forward-looking statements" and "forward-looking information" as defined under applicable Canadian and US securities laws (collectively, "forward-looking statements") which may include, but are not limited to, statements with respect to possible events, conditions, acquisitions, or results of operations that are based on assumptions about future conditions and courses of action and include future oriented financial information with respect to prospective results of operations, financial position or cash flows that is presented either as a forecast or a projection, and also include, but are not limited to, statements with respect to the future financial and operating performance of the Company. All statements, other than statements of historical fact, are forward-looking statements. When used in this AIF the words "estimate", "budget", "project", "believe", "anticipate", "intend", "expect", "plan", "projects", "predict", "may", "should", "will", or the negatives of these words or other variations thereof and comparable terminology or statements that certain actions, events or results "may", "could", "would", "might" or "will" be taken, occur or be achieved are intended to identify forward-looking statements. The forward-looking statements pertain to, among other things:
- continued development of the Company's products and business;
- the Company's growth strategy;
- production costs and pricing of CLARISOY® soy protein, Peazazz® pea protein, Puratein®, Supertein® and Nutratein® canola protein isolates;
- marketing strategies for the Company's soy, pea and canola proteins;
- development of commercial applications for soy, pea and canola protein proteins;
- ability to produce proteins and protein isolates in commercial quantities with sufficient grade and quality at cost-effective prices;
- construction , commissioning and operation of production facilities;
- future protection of intellectual property and improvements to existing processes and products;
- input and other costs; and
- liquidity and working capital.
The forward-looking statements are based on a number of key expectations and assumptions made by management of the Company, including, but not limited to:
- the Company's ability to obtain required regulatory approvals;
- the Company's or its licensing partner's ability to generate new sales;
- the Company's or its licensing partner's ability to produce, deliver and sell the expected product volumes at the expected prices;
- the Company's ability to control costs;
- the Company's ability to obtain and maintain intellectual property rights and trade secret protection;
- market acceptance and demand for the Company's products;
- the successful execution of the Company's business plan;
- achievement of current timetables for product development programs and sales;
- the availability and cost of labour and supplies;
- the availability of additional capital; and
- general economic and financial market conditions.
Although the Company believes that the factors and assumptions used to develop the forward-looking statements are reasonable, undue reliance should not be placed on such forward-looking statements. The forward-looking statements reflect the Company's current views with respect to future events based on currently available information and are inherently subject to risks and uncertainties. Many factors, both known and unknown, could cause actual results, performance or achievements to be materially different from the results, performance or achievements that are or may be expressed or implied by such forward-looking statements contained in this AIF, including, but not limited to:
- the condition of the global economy;
- market acceptance of the Company's products;
- changes in product pricing;
- changes in the Company's customers' requirements, the competitive environment and related market conditions;
- delays in the construction, commissioning and operation of production facilities;
- product development delays;
- changes in the availability or price of labour and supplies;
- the Company's ability to attract and retain business partners, suppliers, employees and customers;
- changing food or feed ingredient industry regulations;
- the Company's access to funding and its ability to provide the capital required for product development, operations and marketing efforts, and working capital requirements; and
- the Company's ability to protect its intellectual property.
Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those anticipated, believed, estimated or expected. The Company cautions readers not to place undue reliance on any such forward-looking statements, which speak only as of the date made. Although the Company has attempted to identify important factors that could cause actual results to differ materially from forward-looking statements, there may be other factors that cause results not to be as anticipated, estimated, described or intended. The Company disclaims any obligation subsequently to revise any forward-looking statements to reflect events or circumstances after the date of such statements or to reflect changes in assumptions or the occurrence of anticipated or unanticipated events, except as required by law.
The Company qualifies all the forward-looking statements contained in this AIF by the foregoing cautionary statements.
Material risk factors that could cause actual results to differ materially from the forward-looking information are contained under the heading "Risk Factors" beginning on page 52.
Currency
All dollar amounts in this AIF are expressed in Canadian dollars, unless otherwise indicated.
Glossary
Certain terms used herein are defined in the attached Glossary.
CORPORATE STRUCTURE
Burcon NutraScience Corporation ("Burcon" or the "Company") was incorporated under the Business Corporations Act (Yukon) on November 3, 1998 under the name "Burcon Capital Corp." and extra-provincially registered in British Columbia on February 5, 1999. Burcon changed its name to "Burcon NutraScience Corporation" on October 18, 1999. The head office of Burcon is located at 1946 West Broadway, Vancouver, B.C., V6J 1Z2. The registered office of Burcon is located at Suite 200, Financial Plaza, 204 Lambert Street, Whitehorse, Yukon, Y1A 3T2.
INTERCORPORATE RELATIONSHIPS
Burcon owns 100% of the issued and outstanding shares of its subsidiary, Burcon NutraScience (MB) Corp. ("Burcon-MB") which was incorporated under the Corporations Act (Manitoba) on February 28, 1992 under the name B.M.W. Canola Inc. Its name was changed to Burcon NutraScience (MB) Corp. on May 30, 2000.
GENERAL DEVELOPMENT OF THE BUSINESS
Burcon was formed in November 1998 as a venture capital pool corporation whose principal business was to identify and evaluate assets, properties or businesses for acquisition. On October 8, 1999 Burcon acquired Burcon-MB.
Since October 1999, Burcon has raised gross proceeds of approximately $74.8 million through the sale of equity securities on both a public and private basis, the exercise of stock options and share purchase warrants, through rights offerings to existing shareholders and issuance of convertible securities. The proceeds have been used, and will continue to be used, to fund research, development, regulatory recognition and commercialization of Burcon's patented and patent-pending protein extraction and purification technologies. Burcon's technologies not only enable the production of plant proteins and protein isolates but also relate to applications of the proteins produced therefrom into products, including food and beverages.
Burcon's common shares were listed on the Toronto Stock Exchange (the "TSX") in June 2009. Prior thereto, Burcon's common shares were listed on the TSX Venture Exchange (the"TSXV"). The Company's common shares are also listed on the Frankfurt Stock Exchange under the symbol "BNE".
On October 27, 2011, Burcon's common shares commenced trading on The NASDAQ Global Market ("NASDAQ Global Market") under the symbol "BUR". On June 8, ,2017, the Company received a letter from the Listings Qualifications Department of the Nasdaq Stock Market LLC ("NASDAQ") notifying the Company that it was not in compliance with Listing Rule 5450(b)(2), which requires the listed securities of the Company to maintain a minimum market value of US$50 million. The Company had not met the requirement for a period of 30 consecutive business days prior to receipt of the NASDAQ letter. On August 21, 2017, the Company received a second letter from NASDAQ notifying the Company that it was not in compliance with Listing Rule 5450(a)(1), which requires the listed securities of the Company to maintain a minimum bid price of US$1 per share. The Company had not met the requirement for a period of 30 consecutive business days prior to receipt of the second NASDAQ letter. The receipt of the two NASDAQ letters did not result in the immediate delisting of the Company's common shares from the NASDAQ Global Market. The Company had a compliance period of 180 calendar days or until December 5, 2017 and February 19, 2018, to regain compliance with NASDAQ's minimum market value of listed securities requirement and minimum bid price requirement, respectively. On December 6, 2017, the Company received notification from NASDAQ stating the Company did not meet the December 5, 2017 deadline to regain compliance with NASDAQ's minimum market value of listed securities requirement. NASDAQ stated that the Company's common shares would be delisted from the NASDAQ Global Market at the opening of business on December 15, 2017 unless the Company submitted a request to appeal the determination to the NASDAQ hearing Panel (the "Panel") by December 13, 2017. On the same day, the Company received a further letter from NASDAQ notifying the Company that it was not in compliance with Listing Rule 5450(b)(2)(C), which requires the listed securities of the Company to maintain a minimum market value of publicly held shares of US$15 million. The Company had not met the requirement for a period of 30 consecutive business days prior to receipt of the second NASDAQ letter on December 6, 2017. The Company submitted an appeal to the Panel together with a plan for regaining compliance with the various continued listing requirements.
On February 5, 2018 the Company received notification from the Panel granting the Panel's approval for the Company to transfer its listing for its common shares from the NASDAQ Global Market to The NASDAQ Capital Market ("NASDAQ Capital Market"). Trading on the Company's common shares on the NASDAQ Capital Market became effective on February 7, 2018. The Panel subjected the continued listing of the Company's shares on the NASDAQ Capital Market to certain conditions, including closing its 2018 Rights Offering (defined below) and having shareholders' equity of over US$2.5 million on or before February 16, 2018. On February 14, 2018, Burcon announced that the 2018 Rights Offering had closed. However, because the 2018 Rights Offering was not fully subscribed, the Company was required to provide additional submissions to the Panel in support of its compliance plan. On April 24, 2018, the Company withdrew its appeal of the delisting. The board of directors of Burcon determined that it was in the overall best interest of the Company to withdraw the appeal of the delisting. The decision was based on several factors, including the board's assessment of the probability of the Company regaining compliance with the continued listing requirements, an analysis of the benefits of continued listing weighed against the onerous regulatory burden and significant costs associated with maintaining the continued listing. On April 27, 2018, the Company's common shares were suspended from trading on the NASDAQ Capital Market. The Company filed a Form 25 (Notification of Removal from Listing and/or Registration under Section 12(b) of the Securities Exchange Act of 1934) with the United States Securities and Exchange Commission (the "SEC") on June 4, 2018 to delist the Company's common shares from the NASDAQ Capital Market and to deregister its common shares under Section 12(b) of the Securities Exchange Act of 1934, as amended (the "Exchange Act"). The delisting became effective on June 14, 2018 and the deregistration will become effective ninety days from June 4, 2018. On June 15, 2018, the Company filed a Form 15 with the SEC to suspend its reporting obligations under Section 15(d) of the Exchange Act. The Company's reporting obligations with the SEC were suspended upon the filing of the Form 15 and shall remain suspended for as long as the Company continues to meet the criteria for such suspension on the first day of any subsequent fiscal year. The common shares of Burcon are quoted for trading in the United States on the OTC Pink Open Market operated by OTC Markets Group, under the ticker "BUROF".
On March 23, 2015, Burcon announced that it would be offering rights (the "2015 Rights Offering") to holders of its common shares of record at the close of business on April 2, 2015 (the "2015 Record Date"). Pursuant to the 2015 Rights Offering, each holder of common shares on the 2015 Record Date received one transferable right for each common share held. Every 22 rights entitled a holder to purchase one common share at a price of $2.26 per share. Burcon announced on May 1, 2015 that it had completed the Rights Offering. The 2015 Rights Offering was fully subscribed and Burcon issued 1,552,044 common shares at a price of $2.26 per common share for aggregate gross proceeds to Burcon of $3,507,619.
Each of PT International Development Corporation Limited ("PT International"), E-Concept Ltd. ("E-Concept") and I-Global Ltd. ("I-Global") acted as guarantors of the 2015 Rights Offering, having agreed to purchase from Burcon such number of common shares available to be purchased, but not otherwise subscribed for, that would result in a minimum of 1,552,044 common shares being issued under the 2015 Rights Offering (the "2015 Standby Commitment"). As the 2015 Rights Offering was over-subscribed, PT International, E-Concept and I-Global were not required to fulfill their respective obligations under the 2015 Standby Commitment. However, to Burcon's knowledge, each of PT International, E-Concept and I-Global did exercise its basic subscription privilege under the 2015 Rights Offering in order to maintain its respective proportionate ownership interest in Burcon.
As compensation for providing the 2015 Standby Commitment, each of PT International, E-Concept and I-Global received non-transferrable common share purchase warrants (the "2015 Standby Warrants") entitling PT International to acquire up to 198,429 common shares, E-Concept to acquire up to 104,220 common shares and I-Global to acquire up to 85,362 common shares. The exercise price under the 2015 Standby Warrants was $2.26 per common share. The 2015 Standby Warrants had a term expiring two years after the closing of the 2015 Rights Offering, being April 30, 2017. In accordance with the policies of the TSX, the issuance of the 2015 Standby Warrants to each of PT International, E-Concept and I-Global was subject to shareholder approval. Shareholder approval was received at Burcon's annual and special meeting held on September 3, 2015. Upon completion of the 2016 Rights Offering (as defined below), the exercise price of the 2015 Standby Warrants was adjusted effective immediately after the 2016 Record Date (as defined below). After the adjustment, the exercise price of the 2015 Standby Warrants was reduced to $2.25 per common share. PT International, E-Concept and I-Global did not exercise their 2015 Standby Warrants prior to the expiry and such warrants lapsed on April 30, 2017.
On April 7, 2016, Burcon announced that it had entered into a convertible note purchase agreement pursuant to which it would issue a convertible note (the "Note") to Large Scale Investments Limited (the "Lender"), a wholly-owned subsidiary of PT International, for the principal amount of $2,000,000 (the "Principal Amount").
Funding by the Lender and the issuance of the Note occurred on May 12, 2016. The Note bears interest at a rate of 8% per annum, calculated daily, compounded monthly. Interest will accrue on the Principal Amount and will be payable on the earlier of three years from the issue of the Note, the occurrence of an event of default as set out in the Note, or voluntary prepayment by Burcon (the "Maturity Date").
The Lender may convert the Principal Amount in whole or in part into common shares in the capital of Burcon at any time commencing on or after July 1, 2016 and up to and including the Maturity Date. When issued, the conversion price of the Note was $4.01 per common share, which represented a premium of approximately 24% over the volume weighted average trading price of the common shares on the TSX for the 5 trading days immediately before April 7, 2016 (the "Conversion Price"). Burcon also has the right, before the Maturity Date, upon written notice to the Lender of not less than thirty (30) days, to prepay in cash all or any portion of the Principal Amount by paying to the Lender an amount equal to the Principal Amount to be prepaid multiplied by 110%. At any time on or after July 1, 2016 and up to the end of such 30-day notice period, the Lender will have the right to convert the Principal Amount in full or in part, into common shares at the Conversion Price. The Note was and any common shares issued upon the conversion of the Note will be subject to a four month hold period under applicable Canadian securities laws. Upon completion of the 2018 Rights Offering (as defined below), the Conversion Price of the Note was adjusted effective immediately after the 2018 Record Date (as defined below). After the adjustment, the Conversion Price was reduced to $3.94 per common share.
The payment of the Principal Amount and all accrued and unpaid interest thereon will be subordinated in right of payment to any amount owing in respect of secured indebtedness of Burcon. Subject to prior TSX approval and the consent of the Lender, Burcon may pay any interest that is due and payable under the Note through the issuance of common shares at a conversion price equal to the volume weighted average trading price of the common shares on the TSX for the 5 trading days immediately prior to the date such interest is due and payable.
On October 24, 2016, Burcon announced that it would be offering rights (the "2016 Rights Offering") to holders of its common shares of record at the close of business on November 3, 2016 (the "2016 Record Date"). Pursuant to the 2016 Rights Offering, each holder of common shares on the 2016 Record Date received one transferable right for each common share held. Every 18 rights entitled a holder to purchase one common share at a price of $2.58 per share. Burcon announced on December 1, 2016 that it had completed the Rights Offering. The 2016 Rights Offering was fully subscribed and Burcon issued 1,990,708 common shares at a price of $2.58 per common share for aggregate gross proceeds to Burcon of $5,136,027.
PT International and Allan Yap, Burcon's Chairman and Chief Executive Officer, acted as guarantors of the 2016 Rights Offering, having agreed to purchase from Burcon such number of common shares available to be purchased, but not otherwise subscribed for, that would result in 1,990,708 of common shares being issued under the 2016 Rights Offering (the "2016 Standby Commitment"). As the 2016 Rights Offering was over-subscribed, PT International and Allan Yap were not required to fulfill their respective obligations under the 2016 Standby Commitment. However, to Burcon's knowledge, each of PT International and Allan Yap did exercise their basic subscription privilege under the 2016 Rights Offering in order to maintain their respective proportionate ownership interest in Burcon.
As compensation for providing the 2016 Standby Commitment, each of PT International and Allan Yap received non-transferrable common share purchase warrants (the "2016 Standby Warrants") entitling PT International to acquire up to 253,815 common shares and Allan Yap to acquire up to 243,862 common shares. The exercise price under the 2016 Standby Warrants is $2.58 per common share. The 2016 Standby Warrants will expire two years after the closing of the 2016 Rights Offering, being November 30, 2018. In accordance with the policies of the TSX, the issuance of the 2016 Standby Warrants to each of PT International and Allan Yap was subject to shareholder approval. Shareholder approval was received at Burcon's annual and special meeting held on September 7, 2017. Upon completion of the 2018 Rights Offering (as defined below), the exercise price of the 2016 Standby Warrants was adjusted effective immediately after the 2018 Record Date (as defined below). After the adjustment, the exercise price of the 2016 Standby Warrants was reduced to $2.54 per common share.
On January 5, 2018, Burcon announced that it would be offering rights (the "2018 Rights Offering") to holders of its common shares of record at the close of business on January 16, 2018 (the "2018 Record Date"). Pursuant to the 2018 Rights Offering, each holder of common shares on the 2018 Record Date received one transferable right for each common share held. Every 4 rights entitled a holder to purchase one common share at a price of $0.57 per share. Burcon announced on February 14, 2018 that it had completed the Rights Offering. The 2018 Rights Offering was not fully subscribed and Burcon issued 6,114,361 common shares at a price of $0.57 per common share for aggregate gross proceeds to Burcon of $3,485,186.
Allan Yap, Burcon's Chairman and Chief Executive Officer, acted as guarantor of the 2018 Rights Offering, having agreed to purchase from Burcon such number of common shares available to be purchased, but not otherwise subscribed for, that would result in 4,728,397 of common shares being issued under the 2018 Rights Offering (the "2018 Standby Commitment"). As the total number of common shares subscribed for under the 2018 Rights Offering exceeded the number of common shares guaranteed by Allan Yap, Mr. Yap was not required to fulfill his obligations under the 2018 Standby Commitment.
As compensation for providing the 2018 Standby Commitment, Allan Yap is entitled to receive non-transferrable common share purchase warrants (the "2018 Standby Warrants") entitling him to acquire up to 1,182,099 common shares. The exercise price under the 2018 Standby Warrants is $0.69 per common share. The 2018 Standby Warrants will expire two years after the closing of the 2018 Rights Offering, being February 13, 2020. In accordance with the policies of the TSX, the issuance of the 2018 Standby Warrants to Allan Yap is subject to shareholder approval. Shareholder approval will be sought at Burcon's annual and special meeting, which is expected to be held in September, 2018.
The Company's fiscal year end is March 31. During fiscal years 2016 to 2018, Burcon raised a total of approximately $12.5 million in capital as follows:
- In April 2015, Burcon completed the 2015 Rights Offering and raised gross proceeds of approximately $3.5 million as described above.
- In March 2016, PT International exercised the 2014 Standby Warrants for gross proceeds of approximately $335,000.
- In November 2016, Burcon completed the 2016 Rights Offering and raised gross proceeds of approximately $5.1 million as described above.
- In February, 2018, Burcon completed the 2018 Rights Offering and raised gross proceeds of approximately $3.5 million as described above.
In addition to the capital raised, Burcon issued the Note for $2.0 million in May 2016 as described above.
The proceeds raised from the transactions described above have been used and will continue to be used to:
- further develop Burcon's protein extraction and purification technologies and pursue new related products;
- pursue and develop new applications from the functional attributes of Burcon's proteins;
- fund Burcon's patent activities;
- fund the activities associated with Burcon's obligations under the License and Production Agreement with ADM, for the commercialization of Burcon's CLARISOY® soy protein;
- supporting ADM in connection with its commercialization of CLARISOY® soy protein;
- fund the activities associated with identifying, negotiating terms and securing a strategic alliance for the commercialization of Burcon's Peazazz® pea protein;
- fund the activities associated with efforts relating to identifying a strategic partner for the commercialization of Burcon's canola protein isolates and other proteins;
- fund the design, engineering and construction of an initial semi-works facility for the commercial production of Peazazz® pea protein; and
- provide general working capital.
Soy
Soy protein isolate is used as a functional ingredient or fortifier in a wide variety of food products including protein shakes, power bars, soups and sauces, meats and meat analogs, and breads and baked goods. In addition to enhancing the protein content of foods, soy protein isolates are used by food manufacturers for their functional applications. These applications include the ability to emulsify, whip, bind and add viscosity to foods. See "Description of the Business".
Burcon has developed technologies to extract and purify soy protein from a variety of soy materials. These technologies encompass various processes to produce a soy protein which Burcon has branded as "CLARISOY®". One process for producing CLARISOY® soy protein results in a unique soy protein which is 100% soluble and transparent in applications with a pH of 4.0 and below. ADM has branded this soy protein as CLARISOY® 100. CLARISOY® 100 is specifically designed to enable beverage manufacturers to meet the demand for great-tasting, nutritionally enhanced beverages targeted to the ever-growing number of health and wellness minded consumers. Unique to any other proteins on the market, CLARISOY® 100 is the only vegetable-based protein that offers clarity and complete protein nutrition for low pH beverage systems. Potential beverage applications for CLARISOY® 100 include: sports nutrition beverages, citrus-based drinks, fruit-flavoured beverages, lemonades, powdered beverage mixes, fruit juice blends and fortified waters.
Another process for producing CLARISOY® soy protein allows for the production of a soy protein specifically processed for use in beverage systems with a pH of less than 4.0 with cloud systems or beverages neutralized to a pH of 7.0 or higher. ADM has branded this soy protein CLARISOY® 150. Due to its clean flavour and high solubility in higher pH ranges, CLARISOY® 150 allows for greater use of soy protein in mildly flavoured neutral beverages such as meal replacement, weight management products and in numerous non-beverage applications such as foods and nutritional products.
On March 4, 2011, Burcon, Burcon-MB and ADM entered into the License and Production Agreement for the worldwide, exclusive production, marketing and sale by ADM of soy protein products using Burcon's CLARISOY® soy protein technology. See "Material Contracts".
On June 18, 2012, Burcon announced that ADM had constructed and was operating a commercial-scale production plant in Decatur, Illinois to produce CLARISOY® 100, the first product to launch in ADM's line of CLARISOY® soy proteins.
On June 26, 2012, Burcon announced that ADM would launch CLARISOY® 150, the first extension of the CLARISOY® product line at the opening of the 2012 Institute of Food Technologists Annual Meeting and Food Expo ("IFT Expo") in Las Vegas on June 26, 2012.
On October 22, 2012, Burcon announced that ADM earned the Best Beverage Ingredient Concept prize for CLARISOY® soy protein at the 2012 InterBev Awards ceremony in Las Vegas, Nevada.
On December 19, 2012, Burcon announced that it had been notified by ADM of the first commercial sale of CLARISOY® soy protein produced by ADM.
On August 13, 2013, Burcon announced that the Canadian Institute of Food Science and Technology recognized the development and introduction of CLARISOY® as a "significant innovation" with its 2013 Food Innovation Award, and highlighted CLARISOY® as the world's first vegetable-based protein that offers clarity and high quality protein nutrition in low pH food systems. Burcon also announced that ADM launched CLARISOY® 120 in a powdered mix prototype called "Pineapple Shakeup" at the IFT Expo in July 2013. CLARISOY® 120 is designed for powdered drinks and drink mixes allowing for easy and rapid dispersibility when added to a liquid.
On March 6, 2014, Burcon announced that it received written notice from ADM that ADM intends to expand to full-scale commercial production of CLARISOY® soy protein pursuant to the License and Production Agreement. ADM's intention to expand commercial production of CLARISOY® ensures that its production capacity meets the required obligations under the License and Production Agreement to retain its exclusive license for CLARISOY®.
On June 23, 2014, Burcon announced that ADM would be demonstrating CLARISOY® 170, the newest product in the CLARISOY® portfolio, at the 2014 IFT Expo in New Orleans. CLARISOY® 170 is formulated to be ideal for dairy protein replacement, which could include neutral beverage applications with pH of 7.0 or higher.
At the 2015 Institute of Food Technologists Annual Meeting & Food Expo in Chicago, ADM sampled a high protein smoothie product which included 17 grams of protein per 8 ounce serving, where the primary protein source was CLARISOY®. At the same show, ADM demonstrated a 100% dairy-free vegan rich vanilla soft serve where CLARISOY® was the sole protein source. For customers seeking a convenient, delicious and nutritious beverage, ADM showcased a dairy-free cold brew coffee that featured CLARISOY® containing, non-dairy creamer, adding 5 grams of soy protein to the beverage.
These applications demonstrate how CLARISOY® is well-suited for adding protein, nutrition and functionality to everyday products, and how it excels particularly in beverage applications due to its clean favor and smooth mouthfeel. These applications can help meet consumer demand for good-tasting, convenient products featuring plant-based protein and also provide solutions for customers looking to replace dairy protein for products that appeal to consumers who choose a vegan or other healthy lifestyles. ADM's focus on dairy replacement using CLARISOY® not only provides a price-stable and sustainable ingredient for food and beverage manufacturers, but also addresses the large consumer base that is lactose intolerant or sensitive to dairy products.
At the 2016 Institute of Food Technologists Annual Meeting & Food Expo in Chicago, ADM showcased three unique products that emphasize the current trends of convenient wellness, plant-based protein ingredients and free-from. ADM sampled a high protein vegan smoothie which contained 17 grams of protein per 8 ounce serving, where the primary protein source was CLARISOY™. ADM also demonstrated a non-dairy Greek yogurt that contained 12 grams of protein per 150 gram serving, in which only CLARISOY® was used as the sole protein source. Targeting the convenient ready-to-drink beverage market, ADM showcased a drinkable yogurt beverage that contained 4 grams of CLARISOY® soy protein per serving.
At the 2017 Institute of Food Technologists Annual Meeting & Food Expo in Las Vegas, ADM featured two products containing CLARISOY® soy protein and other ADM ingredients: Plant Power Frappe - a cold-brewed coffee containing 10 grams of soy protein where the primary protein source was CLARISOY®; and Bourbon Barrel Coffee Creamer - a low calorie, non-dairy creamer where CLARISOY® was gain the primary protein source.
ADM has also launched an energy drink suitable for vegans made with CLARISOY®. This product is available in the United States and European markets.
ADM continues to market CLARISOY® as a versatile soy protein ingredient well-suited to boosting the nutritional and functional profile of many different products. CLARISOY®, having clean flavor and high solubility, is exceptional in beverage products including ready-to-drink beverages. Customers that are looking for plant-based protein ingredients to formulate vegan or non-dairy products will find CLARISOY® to be an attractive protein source. Not only is CLARISOY® suitable for high protein fortification products such as meal replacements and sport nutrition smoothies but is also suitable for casual wellness products that require a bit of protein boost.
On December 17, 2015, Burcon announced that it expected ADM's first full-scale commercial CLARISOY® production facility to be operational by mid-2016. On November 8, 2016, Burcon announced that ADM had successfully commissioned the first full-scale CLARISOY® production facility.
Since March 2013 to the date of this AIF, Burcon has been granted fourteen U.S. patents covering its soy protein composition and extraction and purification processes.
Pea
Pea protein is increasing in popularity as a plant-based protein ingredient which can be used in a wide variety of food products. One of the reasons is that pea protein is able to deliver functionality and protein nutrition to products without the issues of allergenicity and genetic modification that may be present with other proteins. Pea proteins currently available in the market are sold for use in a variety of food products including: snacks and cereals; diet products (high protein foods); gluten-free and vegetarian and vegan foods as well as in nutritional supplements such as meal replacement shakes.
In November 2011, Burcon announced that it had developed a novel pea protein which it has branded as "Peazazz®". Peazazz® is 100% soluble and transparent in low pH solutions with clean flavour characteristics. It is heat stable permitting hot fill applications. See "Description of the Business".
On January 29, 2013, Burcon announced that it had commenced building a Peazazz® semi-works production facility to produce Peazazz® pea protein at small commercial scale in Winnipeg, Manitoba to provide market development quantities to customers for product and market development activities.
On June 25, 2013, Burcon announced that it had completed, on schedule, the construction of its new semi-works plant in Winnipeg for Peazazz®. The Peazazz® semi-works plant utilizes commercial-scale equipment capable of producing the tonnage amounts required by food and beverage makers looking to conduct full-scale, real world market evaluations of Peazazz® in their consumer products.
In July 2013, Burcon officially launched Peazazz® pea protein at the 2013 IFT Expo. Burcon demonstrated two products: Peach Mango Rhythm, made from 30% real juice, with all natural flavors and containing five grams of Peazazz® pea protein per 250ml serving and Vanilla Jazz, a neutral pH milk-style beverage with a faint hint of vanilla flavoring, fortified with vitamins and minerals and five grams of Peazazz® pea protein per 250ml serving.
On August 23, 2013, Burcon announced that the startup and commissioning of the Peazazz® pea protein semi-works plant had been completed, allowing it to begin producing sample quantities for shipment to interested parties who had signed material transfer agreements ("MTAs") with Burcon. While the semi-works plant utilizes commercial-scale equipment, it is only used to produce the amounts of Peazazz® needed for targeted market development activities with certain prospective customers. By functioning as a model for potential manufacturing partners to emulate, the plant can also ultimately shorten their time-to-market. Burcon has entered into a number of MTAs with parties interested in Peazazz®. Such parties include major food and beverage makers, suppliers and potential industry production and sales partners. Since August 2013, Burcon has shipped samples of Peazazz® pea protein to various key potential multi-national production and/or distribution partners and undertook applications work in response to requests from certain potential commercialization partners. Burcon continued discussions with certain multi-national food ingredient providers about a royalty or a joint operations agreement for Peazazz® during fiscal 2018. As of the date of this AIF, discussions are still on-going with these parties.
Canola
Burcon's technologies allow it to extract and purify three types of canola protein isolates from canola meal, a co-product (together with canola oil) of the canola seed crushing industry. Burcon has branded these protein isolates under the trade names "Puratein®", "Supertein®" and "Nutratein®".
The goal of Burcon's research is to develop its patented and patent-pending processes to utilize inexpensive oilseed meals, such as canola meal, for the production of purified plant proteins that exhibit nutritional, functional or nutraceutical profiles. Burcon expects that Puratein® canola protein isolate and Supertein® canola protein isolate will participate and compete with soy, dairy, and egg proteins in the expanding global protein ingredient market, with potential uses in prepared foods, nutritional supplements and personal care products. Nutratein®, having an excellent amino acid profile, is expected to be suitable in nutritional supplements, meal replacement products, high-protein foods and beverages and high-value animal feed.
During fiscal 2018, Burcon's key focus was on its pea protein commercialization efforts. However, Burcon continued to work on securing interest from potential partners to establish the commercial value of all three of its canola proteins. Burcon's goal is to work with food and beverage manufacturers to establish the value of Burcon's proteins in their food products, as well as to work with upstream canola processors to demonstrate how Burcon's technologies can add value to their canola meal products.
Specialty Proteins and Phytochemical Extractions
Burcon's extraction and purification technologies can also be used to produce specialty proteins such as flax and hemp proteins. Burcon's core extraction and purification technology is versatile and can be adapted to process a range of oilseed and non-oilseed meals to produce high-value protein products for use in the food and beverage industries.
The demand for plant proteins in the protein market continues to grow and as such, Burcon believes that there may be niche market opportunities for its specialty protein ingredients. Burcon plans to explore these opportunities in the near future.
In February 2018, Burcon applied for accreditation from Health Canada's Office of Controlled Substances to conduct research for the future commercial production of purified cannabinoid extracts. Burcon submitted an application for a Controlled Drugs and Substances Dealers License to Health Canada and intends to also pursue partnering opportunities with growers and suppliers of hemp and cannabis input materials. The Office of Controlled Substances commits to a service delivery standard of 180 business days for the issuance of a decision on an application for a new dealer's licence for controlled substances, from the receipt of a complete application. If Burcon receives the license, it expects to apply its extensive experience in phytochemical extraction technologies to develop technologies for the production of highly-purified cannabis-derived compounds.
DESCRIPTION OF THE BUSINESS
The protein ingredient industry continues to experience rapid growth, with plant proteins in particular experiencing high demand. This increase in demand for plant proteins is fuelled in part by scientific advances, changing demographics as well as by the public's changing perception of the safety of animal-based products. External issues such as melamine tampering/contamination, mad cow disease, E. coli, swine flu, avian flu and the growing use of antibiotics in animal production, as well as demographic trends are all combining to produce significant demand for plant proteins.
Two major attributes are relevant to the commercial value of protein as an ingredient: functional value and nutritional value.
Functional Value
Proteins possess a wide range of attributes essential to the structure and textural integrity of food products. These relevant properties include: solubility, viscosity, water-binding, gelation, cohesion, adhesion, elasticity, emulsification, foaming, whipping, fat-binding, film forming and flavour-enhancing qualities.
In weighing the commercial potential of any protein ingredient, functional utility is at least as important as nutritional value. For example, although the nutritional value of wheat protein is comparatively low, (the Protein Digestibility Corrected Amino Acid Score ("PDCAAS") of whole wheat is 0.40, only wheat protein-called gluten-will make a traditional loaf of bread. Thus, the functionality of wheat protein makes it a staple in the North American diet. At the
top end of the functional scale, egg white protein will whip, coagulate, and form films. Such functional versatility makes egg white one of the most valuable food proteins. Certain of Burcon's proteins can be made to mimic many of egg's functions, and in certain instances can outperform egg.
Nutritional Value
Proteins are organic compounds made up of carbon, hydrogen, oxygen and nitrogen. It is the presence of the nitrogen that sets proteins apart from other nutrients. Nitrogen is essential to human life, but since we have no other source of nitrogen-unlike plants, we are unable to absorb it as a nutrient from the ground-one of the most important roles of dietary protein is to bring nitrogen into the body.
Proteins are made up of sub-units called amino acids. There are twenty dietary amino acids, typically subdivided into two categories: non-essential amino acids, which can be made within the body, and essential amino acids which must come from diet.
Amino acids supplied from dietary protein are needed for synthesis of body proteins in muscle, organs, bone and skin, and for synthesis of enzymes, certain hormones, antibodies and a host of bodily processes.
The essential amino acids are lysine, methionine + cysteine, threonine, tryptophan, leucine, isoleucine, valine, phenylalanine, arginine and histidine (adults do not require a dietary supply of arginine).
A diet deficient in one or more of the essential amino acids impairs growth in children, causes adults to lose muscle mass, and lowers the body's resistance to a variety of diseases. Extreme protein deficiency can be a cause of death. An adequate daily supply of high-quality protein is essential to optimal growth and health.
The nutritional supplements industry has seen rapid growth in the use of protein ingredients over the past ten years. Protein bars, once consumed only by endurance athletes, are now widely available and protein-rich meal-replacement products and dietary supplements have become supermarket staples and are sold in large quantities through all the major multi-level marketing companies. Protein supplements are also increasingly and successfully being promoted to the expanding market of geriatric consumers. Potential nutritional applications for protein isolates include power shakes, protein bars, protein powders and any other concentrated protein supplement.
Soy
The soybean had its beginnings in China. Chinese historical documents suggest that soybeans have been a diet staple in Asia since the 11th Century BC.
According to the Soyfoods Association of North America, the soybean was introduced to North America around the 1760s. Today, soybeans are grown in many parts of the world with the United States being the largest producer of this crop, followed by Brazil, Argentina and China. The United States Department of Agriculture ("USDA") notes that the forecasted value of U.S. soybean production in 2017 was approximately US$41‡ billion, similar to the previous year, which was up 17% from the year before, which represented the second-highest value among U.S. produced crops, behind corn. Production of soybeans has remained stable in the United States. U.S. soybean farmers planted approximately 90.1 million acres‡§ of soy in 2017, which represented approximately an 8% increase from 2016. The USDA Foreign Agricultural Service estimated that approximately 336.7‡ million metric tons of soybeans were produced during 2017/2018, with U.S. production accounting for approximately 119‡ million metric tons or 35%.
Soybeans are similar in size and colour to peas and are primarily cultivated for their oil and protein. Soybeans are the largest single source of edible oil and accounted for approximately 59%§ of the world's total oilseed production in 2017. In addition to being a source of oil and protein, soybean meal is used in animal feed for the production of meat and eggs. Soy flour is used in the commercial baking industry while soy hulls are processed to make breads, cereal and snacks.
Each soybean is comprised of approximately 40% protein, 35% carbohydrate (including fiber), 20% oil, and 5% ash.
Soy Protein
Commercially sold soy protein is available in predominantly three forms: soy flour, soy concentrates and soy protein isolates. After cracking and dehulling the soybean, soy processors roll them into flakes. Oil from the soybean flakes is removed and then the flakes are dried. The defatted flakes are then further processed into soy protein.
Soy protein isolate is the purest of the three forms of soy protein and contains over 90% protein, on a moisture free basis. Soy protein isolates are relatively neutral in flavour and odour and are used primarily by the food industry. Today, soy protein isolate is used in a variety of food applications, including as a protein replacement for dairy proteins in food or in products such as protein shakes, power bars, soups and sauces, meat analogs, breads and baked goods. Soy protein isolates are desired by food manufacturers for their functional applications. These applications include the ability to emulsify, whip, bind and add viscosity to foods.
____________________
‡ Source: United States Department of Agriculture website.
§ Source: www.soystats.com
In addition to its functional attributes, soy protein isolate provides nutritional enhancement to foods. Soy protein contains all the essential amino acids required for human nutrition.
Numerous studies have been conducted on the health benefits of soy protein. In October 1999, the FDA approved a health claim for soy protein and its role in reducing the risk of coronary heart disease. Food manufacturers may label foods containing soy protein by stating that "Diets low in saturated fat and cholesterol that include 25 grams of soy protein daily may reduce the risk of heart disease. One serving of (name of food) provides __ grams of soy protein." To qualify for the claim, the food must contain per serving:
- 6.25 grams of soy protein;
- low fat (less than 3 grams);
- low in saturated fat (less than 1 gram);
- low in cholesterol (less than 20 milligrams); and
- sodium value of less than 480 milligrams for individual foods, less than 720 milligrams if considered a main dish, and less than 960 milligrams if considered a meal.
In March 2015, after a meta-analysis of scientific studies, Health Canada's Food Directorate concluded that scientific evidence exists to support a health claim about soy protein and blood cholesterol lowering. The evidence supports a direction of effect towards a reduction in total and LDL cholesterol levels when soy protein is consumed. Foods containing soy protein may state on its label, "[Serving size] of (brand name) [name of food] supplies/provides X% of the daily amount of soy protein shown to help reduce/lower cholesterol." The daily amount referred to is 25 grams of soy protein. For example, "150g of tofu supplies 70% of the daily amount of soy protein shown to help lower cholesterol".
The quest for healthier lifestyles has led consumers to search for healthier alternatives to animal protein. The FDA's and Health Canada's approval of a health claim for soy protein has fuelled soy protein's increasing popularity and general acceptance among consumers. These factors, along with the desire by consumers for food producers to find eco-friendly ways to produce food for humans, are expected to sustain market demand for soy protein isolates. Burcon intends to participate in this growing market through its CLARISOY® soy protein.
CLARISOY®
In November 2008, Burcon announced that it had developed a soy protein which it branded as CLARISOY®. Burcon has developed technologies to extract and purify soy protein from a variety of soy materials. These technologies encompass various processes to produce CLARISOY® soy protein. One process for producing CLARISOY® soy protein results in a soy protein that is 100% soluble, transparent and heat stable in applications with a pH of 4.0 and below. ADM has branded this soy protein as CLARISOY™ 100. CLARISOY® soy protein is specifically designed to enable beverage manufacturers to meet the demand for great-tasting, nutritionally enhanced beverages targeted to the ever-growing number of health and wellness minded consumers. ADM is currently marketing CLARISOY® as an economical, high-quality plant-based dairy alternative that provides greater cost stability and comparable nutrition. Food and beverage manufacturers who are looking to manage cost and product margins should be able to rely on CLARISOY® soy protein as a reliable source of plant-based protein with uncompromising taste, nutrition and performance. CLARISOY® soy protein is expected to be an ideal protein ingredient to replace or partially replace dairy protein in food, drinks and snacks without affecting taste.
Another process for producing CLARISOY® soy protein allows for the production of a soy protein specifically processed for use in beverage systems with a pH of less than 4.0 with cloud systems or beverages neutralized to a pH of 7.0 or higher. ADM has branded this soy protein CLARISOY® 150. Due to its clean flavour and high solubility in higher pH ranges, CLARISOY® 150 allows for greater use of soy protein in mildly flavoured neutral beverages such as meal replacement, weight management products and in numerous non-beverage applications such as foods and nutritional products.
ADM launched CLARISOY™ 120 in a powdered mix prototype called "Pineapple Shakeup" at the IFT Expo in July 2013. CLARISOY™ 120 is designed for powdered drinks and drink mixes allowing for easy and rapid dispersibility when added to a liquid.
ADM launched CLARISOY® 170, at the 2014 IFT Expo in New Orleans. CLARISOY® 170 is formulated to be ideal for dairy protein replacement, which could include neutral beverage applications with pH of 7.0 or higher.
ADM also launched CLARISOY® 180 as part of its CLARISOY® line of soy protein specifically designed for neutral applications and dairy replacements. CLARISOY® 180 is formulated for high protein replacement beverages at neutral pH.
The following is a table showing the CLARISOY® product lines marketed by ADM as of the date of this AIF:
Product Line | Product Characteristics | Transparent | Applications |
CLARISOY®100 | High viscosity | Yes | Low pH beverage systems |
CLARISOY®110 | Low viscosity | Yes | Low pH shots High protein meal replacement beverages Collagen replacement |
CLARISOY®120 | Agglomerated High viscosity | Yes | Powdered Low Protein Low pH beverages |
CLARISOY®150 | High viscosity | No | Low pH beverage systems Coffee creamers |
CLARISOY®170 | High viscosity | No | Neutral pH for dairy protein replacement |
CLARISOY®180 | Low viscosity | No | Neutral pH for high protein replacement beverages |
Based on the recommendations of the Joint Expert Consultation of the Food and Agricultural Organization ("FAO") and World Health Organization ("WHO") in 1989, the FDA and the FAO/WHO adopted in 1993 the PDCAAS as the preferred method for measuring the quality of a protein based on the amino acid requirements of humans. The PDCAAS method for evaluating protein quality is based on the needs of humans. The quality of a protein is based on the amino acid requirements of a 2 to 5 year old child, which is considered to be the most nutritionally demanding age group, other than infants. After adjusting for digestibility, the protein quality rankings of a specific protein evaluated under the PDCAAS method are compared to a standard amino acid profile with the highest possible score being a 1.0. A PDCAAS score of 1.0 means that, after digestion of the protein, it provides 100% or more of all the essential amino acids required. Proteins with a PDCAAS of 1.0 include egg and cow's milk.
The PDCAAS scoring system has since been updated by the FAO/WHO/United Nations University ("UNU") in 2002, altering the reference amount of specific amino acids and also dividing the requirement by age groups of children 1-2 years and 3-10 years. In the Report of a Joint FAO/WHO expert consultation on protein and amino acid requirements in human nutrition, the FAO/WHO/UNU came to the conclusion that previous reports considerably overestimated the protein requirements. Despite the foregoing, the FDA has neither formally adopted the updated levels recommended in the 2002 report nor advised food companies to use these updated levels when calculating PDCAAS values.
Based on the PDCAAS method, Burcon's CLARISOY® soy protein has a score of 0.98 and 1.00 under the 1989 FAO/WHO pattern and the 2002 FAO/WHO/UNU pattern, respectively, suggesting that Burcon's CLARISOY® soy protein is a good quality protein source.
Soy Protein Production
Processes used in the production of common and traditional soy protein isolates involve the use of harsh caustics and acids to separate the soy proteins from the other constituents of defatted soy. This harsh chemical treatment causes some denaturation of the soy proteins resulting in a lack of solubility and stability in aqueous products and, notably, a lack of solubility in acid beverages (juices, sport energy drinks, fortified waters, flavoured waters etc.) and produces soy proteins with substantial flavour compounds imparting beany or earthy flavours. Burcon has developed and filed applications to obtain patent protection for novel processes allowing for the production of uniquely soluble soy proteins with clean flavour characteristics.
Pursuant to the terms of the License and Production Agreement (see "Material Contracts"), Burcon has licensed its CLARISOY® soy protein technology to ADM, on an exclusive basis, to use, market and sell the products (the "Soy Products") that use the CLARISOY® technology. Under the License and Production Agreement, ADM is the primary party responsible for the commercialization efforts for CLARISOY®, including:
- developing the necessary process engineering for scaling up the production of the Soy Products;
- developing applications for the Soy Products; and
- designing, building and commissioning an initial production facility to manufacture the Soy Products.
During the term of the license under the License and Production Agreement, Burcon will continue to refine its protein extraction and purification technology for soy protein in its Winnipeg Technical Centre.
On March 6, 2014, Burcon announced that it received written notice from ADM that ADM intends to expand to full-commercial scale production of CLARISOY® soy protein pursuant to the License and Production Agreement ("Full Commercial Production"). On November 8, 2016, Burcon announced that ADM had successfully commissioned the first full-scale CLARISOY® production facility.
Pea
Field pea, or Pisum sativum in Latin, is part of the legume family and was one of the earliest cultivated food crops. A pea is most commonly the green or yellow small spherical seed inside a peapod that contains multiple peas. The pea plant is grown in cool-weather conditions in many parts of the world, including Canada, Europe and temperate regions of Asia.
Peas are consumed as a vegetable worldwide for their high nutritional value and health benefits. Not only are peas high in protein, fibre, starch, vitamins and minerals, but they are also non-allergenic and environmentally sustainable. As part of the legume family, pea plants have the ability to lock in nitrogen from the atmosphere and store it in their root nodules. This nitrogen-fixation ability allows producers to use less fertilizer and replenish the soil with nitrogen, making peas a much desired sustainable crop.
In November 2011, Burcon announced that it had developed a novel pea protein which it has branded as "Peazazz®", its first technology platform to extract added-value proteins from a non-oilseed source. Burcon's technology extracts and purifies a novel pea protein from field peas. Both the nutritional and functional characteristics of pea protein allow for a host of great tasting food and beverage product applications as well as for use in nutritional supplements.
Pea protein is increasing in popularity as a plant-based protein ingredient which can be used in a wide variety of food products. One of the reasons is that pea protein is able to deliver functionality and protein nutrition to products without the issues of allergenicity and genetic modification that may be present with other proteins. Pea proteins currently available in the market are sold for use in a variety of food products including: snacks and cereals; diet products (high protein foods); gluten-free and vegetarian and vegan foods as well as in nutritional supplements such as meal replacement shakes.
Peazazz®
Peazazz® pea protein is uniquely soluble and clean-tasting pea protein that is suitable for dairy alternative food and beverages. Peazazz® has clean flavour characteristics and is well suited for use in low and neutral pH beverages as well as a variety of other healthy and great tasting food and beverage product applications. Its valuable nutritional and functional characteristics make Peazazz® an attractive product to companies looking for an alternative plant protein ingredient.
Pea is a widely accepted and consumed vegetable, recognized for its nutritional value and health benefits. Burcon has successfully extracted added-value proteins that contain essential amino acids into a white powder that can easily be incorporated into a variety of foods and beverages including dairy alternative products, dry-blended beverages, ready-to-drink beverages, protein bars and crisps, weight management and meal replacement products, and vegetarian and vegan foods.
Burcon's Peazazz® pea protein is non-allergenic and can be produced from a non-GMO source. Consumers are increasingly looking for clean-label and "free-from" products. Burcon's Peazazz® pea protein is dairy-free, soy-free, gluten-free, allergen and GMO-free.
Pea proteins currently available on the market are sold for use in a variety of food products including: snacks and cereals; high-protein weight management products; gluten-free and vegetarian and vegan foods. Burcon is not aware of any pea protein isolate in the market that is clean-tasting with superior solubility like Peazazz®. Burcon expects the introduction of Peazazz® pea protein to be able to gain a share of the pea protein market, as well as expand the pea protein market to include (what it previously could not) a broader range of product applications such as low pH beverages.
On January 29, 2013, Burcon announced that it had commenced building a Peazazz® semi-works production facility to produce Peazazz® pea protein at small commercial scale in Winnipeg, Manitoba to provide market development quantities to customers for product and market development activities.
On June 25, 2013, Burcon announced that it had completed, on schedule, the construction of its new semi-works plant in Winnipeg for Peazazz®. The Peazazz® semi-works plant utilizes commercial-scale equipment capable of producing the tonnage amounts required by food and beverage makers looking to conduct full-scale, real world market evaluations of Peazazz® in their consumer products. The plant also supports Burcon's ongoing discussions with companies who are potential partners with Burcon for the production and marketing of Peazazz®.
In July 2013, Burcon officially launched Peazazz® pea protein at the 2013 IFT Expo. Burcon demonstrated two products: Peach Mango Rhythm, made from 30% real juice, with all natural flavors and containing five grams of Peazazz® pea protein per 250ml serving and Vanilla Jazz, a neutral pH milk-style beverage with a faint hint of vanilla flavoring, fortified with vitamins and minerals and five grams of Peazazz® pea protein per 250ml serving.
On August 23, 2013, Burcon announced that the start-up and commissioning of the Peazazz® pea protein semi-works plant had been completed, allowing it to begin producing sample quantities for shipment to interested parties who had signed MTAs with Burcon. While the semi-works plant utilizes commercial-scale equipment, it will only be used to produce the amounts of Peazazz® needed for targeted market development activities with certain prospective customers. By functioning as a model for potential manufacturing partners to emulate, the plant can also ultimately shorten their time-to-market.
Burcon has entered into a number of MTAs with parties interested in Peazazz®. Such parties include major food and beverage makers, suppliers and potential industry production and sales partners. Since August 2013, Burcon has shipped samples of Peazazz® pea protein to various key potential multi-national production and/or distribution partners and undertook applications work in response to requests from certain potential commercialization partners. During fiscal 2018, Burcon continued discussions with certain multi-national food ingredient providers about a royalty or a joint operations agreement for Peazazz®. As of the date of this AIF, discussions are still on-going with these parties.
Canola
Canola is the North American name for the enhanced variation of rapeseed first developed and introduced in 1974 when a Canadian researcher bred a "double low" variety of rapeseed with reduced levels of the two negative elements naturally occurring in rapeseed: erucic acid and glucosinolates. This type of rapeseed is known in Europe and parts of Asia as rapeseed or oilseed rape and has become the world's second largest oilseed crop. The growth of rapeseed as an international crop can be attributed to three factors: the ability to grow rapeseed in temperate climates; favourable production costs; and a beneficial fatty acid profile for the oil, which is high in monounsaturates.
Each canola plant produces yellow flowers which produce pods that are similar in shape to pea pods and about 1/5th the size. Within the pods are tiny round seeds that are crushed to obtain canola oil. After the oil is removed through processing at a canola crushing plant, the remainder of the seed (approximately 60% by weight) is canola meal. Canola meal is the raw material from which Burcon intends to extract protein commercially to produce Puratein® canola protein isolate, Supertein® canola protein isolate and Nutratein® canola protein isolate. Canola meal is comprised of approximately 35% protein. Canola meal is in abundant and relatively inexpensive supply and is sold almost exclusively as an animal feed ingredient; however, its protein value, even in feed applications, is limited by the presence of a large amount of fiber and other anti-nutritional factors naturally present in canola seed. Burcon's extraction process separates the protein from the fiber and from most of the naturally occurring anti-nutritional factors.
In the past, numerous attempts have been made at finding an economically viable method to extract canola protein from canola meal. There is a significant amount of scientific publications describing various methods to do so, most of which publications also underscore numerous reasons for the scientific interest in obtaining canola protein isolate, including, amongst others: a unique amino acid profile, rich in sulfur containing amino acids; an abundant source of protein; and two distinct protein fractions. However, none of the existing technologies described in the scientific literature is commercially applied at present. Major drawbacks of the existing technologies, which often use alkaline extraction followed by isoelectric precipitation, include the insufficient purity of the canola protein isolate, unacceptable colour and taste of the canola protein products as well as the resulting protein's limitations regarding functionality. Phenolics that are naturally present in canola oxidize readily in alkaline conditions causing dark coloration of the final protein product.
Burcon's canola protein extraction process does not use harsh chemicals but rather is based primarily on making use of physical separation and purification techniques. At the core of Burcon's canola protein isolate production process is a micelle formation step, which separates the two naturally occurring proteins in canola: napin and cruciferin. Processing of these two fractions results in the cruciferin-rich canola protein isolate Puratein® canola protein isolate and the napin-rich canola protein isolate Supertein® canola protein isolate. Burcon has also developed Nutratein® canola protein isolate, which consists of a blend of the two fractions.
Canola Protein
Potential nutritional applications for canola protein isolates include power shakes, protein bars, protein powders and any other concentrated protein supplement.
Based on the PDCAAS method, the PDCAAS scores for Burcon's canola proteins are as follows:
Canola Protein Isolate | FAO/WHO 1989 mg/g protein (2-5 years old) | FAO/WHO/UNU 2002 mg/g protein (3-10 years old) |
Puratein® | 0.60 | 0.72 |
Supertein® | 0.71 | 0.91 |
Nutratein® | 0.90 | 1.00 |
Burcon's canola protein has a score in the range of 0.60 to 0.90 under the 1989 FAO/WHO pattern and a score in the range of 0.72 - 1.00 under the 2002 FAO/WHO/UNU pattern, suggesting that Burcon's canola protein is a good quality protein source.
Puratein®
Puratein® canola protein isolate's key functionalities are emulsifying, gelling and binding. Puratein® canola protein isolate has potential in a wide variety of food types.
Puratein® canola protein isolate is a canola protein isolate comprised mainly of globulin proteins. The functional properties of Puratein® canola protein isolate include emulsification, gel formation, thickening, formation of heat-stable foams, and water- and ingredient-binding. Applications for Puratein® canola protein isolate include dressings and sauces, meat substitutes, baked goods and protein bars. Puratein® canola protein isolate has good taste characteristics with no off-flavours.
Supertein®
Supertein® canola protein isolate is a highly soluble canola protein isolate comprised principally of albumin proteins. The functional properties of Supertein® canola protein isolate include solubility, the ability to form transparent solutions, foaming and nutrition. Applications for Supertein® canola protein isolate include beverages, confectionery, aerated desserts, and protein bars, among many others. Supertein® canola protein isolate has a slightly sweet taste with no off-flavours.
The exceptional cysteine content of canola protein (rapeseed protein) has long been of interest to nutritional scientists. A potential link between canola protein's high cysteine content and disease prevention has been reported in a study in the British Journal of Nutrition entitled "Rapeseed protein inhibits the initiation of insulin resistance by a high-saturated fat, high-sucrose diet in rats" by Mariotti F., Hermier D., Sarrat C., Magné J., Fénart E., Evrard J., et al 2008 Nov; 100(5):984-91. The study's aim was to determine whether rapeseed protein, described by the study's authors as "an emergent cysteine-rich protein" could inhibit the onset of the metabolic syndrome. The main finding of the study "is that rapeseed protein substituted for milk protein inhibited the onset of insulin resistance in rats fed the high-saturated fat, high-sucrose diet". The authors further noted that rapeseed protein mitigated certain factors associated with metabolic syndrome: "The study's result highlights the importance of the type of protein as a major component of diet quality, in terms of cardiovascular and diabetic risks."Supertein® canola protein isolate is rich in sulfur-containing amino acids and particularly rich in cysteine. The typical cysteine content of Burcon's Supertein® canola protein isolate is nearly double that of whey protein, which is recognized for its high cysteine content. The findings in the study reported in the British Journal of Nutrition suggest that Supertein® may have potential applications in the prevention of metabolic syndrome.
During fiscal 2018, Burcon's key focus was on its pea protein commercialization efforts. However, Burcon continued to work on securing interest from potential partners to establish the commercial value of its canola protein isolates. Burcon's goal is to continue to work with food and beverage manufacturers to establish the value of Burcon's Puratein® and Supertein® canola proteins in their food products.
Nutratein®
Nutratein® is a canola protein isolate comprised of a mixture of globulin and albumin proteins. Nutratein® is a fine powder that has good solubility across a broad pH range. Nutratein® has an excellent amino acid profile and its PDCAAS score makes it a suitable in nutritional supplements, meal replacement products, high-protein foods and beverages and initially participate as a protein ingredient in high-value animal feed. Burcon continues to work on pursuing an animal nutrition application with companies in the animal feed industry with the intention of using Nutratein® canola protein isolate to replace or partially replace dairy protein in certain high-value animal feed applications. Applications in human nutrition include nutritional supplements, meal replacement products, protein bars, meat applications, baked goods or nutritional beverages. Nutratein® canola protein isolate is bland in taste with no off-flavours.
Research and Development
Burcon has designed and built a large-scale pilot production facility, complete with an analytical laboratory, for the development and small-scale production of proteins from various plant sources. On January 29, 2013, Burcon announced that it had commenced building a Peazazz® semi-works production facility to produce Peazazz® pea protein at commercial scale in Winnipeg, Manitoba. On June 25, 2013, Burcon announced that it had completed, on schedule, the construction of its new semi-works plant in Winnipeg for Peazazz®. The Peazazz® semi-works plant utilizes commercial-scale equipment and will be capable of producing the tonnage amounts required by food and beverage makers looking to conduct full-scale market evaluations of Peazazz® in their consumer products. Start-up and commissioning of the plant was completed in August 2013. During fiscal 2018, the semi-works plant continued to produce samples to provide to potential strategic partners for evaluation as well as conduct work required to support Burcon's intellectual property portfolio.
Burcon has over 18 years of experience in developing high-quality vegetable protein ingredients and has successfully developed CLARISOY® soy protein and is developing Peazazz® pea protein, three unique canola protein isolates, Supertein®, Puratein® and Nutratein® canola protein isolates and other specialty proteins such as flax and hemp. After years of research on developing these products and the associated extraction technologies and in running large-scale pilot operations to a standard that meets Burcon's expectations of commercial quality, Burcon has amassed significant experience and know-how.
Objectives
For fiscal 2019, Burcon's main objective will continue to be to further the development and commercialization of its products, with its primary focus on commercializing its Peazazz® pea protein technology.
Burcon's pea protein product development and commercialization objectives include:
- Identifying additional multi-national food ingredient providers to secure a royalty or a joint operations agreement for Peazazz®;
- Continuing to operate the Peazazz® semi-works facility to produce sample products to
- supply the potential strategic alliance partners with sufficient product development quantities of Peazazz® samples so that such potential partners can conduct full-scale, real-world testing;
- demonstrate to such major protein ingredient participants and food and beverage makers that Burcon's Peazazz® technology is scalable and produces a consistent quality product;
- Conducting further research to develop additional applications for Peazazz® pea protein into food products; and
- Continuing to file patent applications to protect the Peazazz® pea protein extraction process as well as the composition of Peazazz® pea protein and applications for Peazazz® pea protein into food products.
In addition, Burcon will also:
- continue to refine its protein extraction and purification technologies, develop new technologies and related products;
- conduct research to develop extraction and purification technologies for the production of highly-purified cannabis-derived products, if the respective license is granted by Health Canada;
- further strengthen and expand its core intellectual property portfolio;
- support ADM in connection with its commercialization of CLARISOY® soy protein products;
- explore opportunities for acquiring or licensing into Burcon, novel technologies that will complement or enhance Burcon's intellectual property portfolio and business initiatives;
- pursue product development agreements with major food, beverage and nutritional product companies to develop improved or novel applications for Supertein®, Puratein® and Nutratein® canola protein isolates as well as Burcon's other specialty proteins into their products;
- pursue a strategic alliance with a potential partner in connection with the development of a commercial facility for the production, marketing and sale of Burcon's canola protein isolates and Burcon's other specialty proteins; and
- pursue activities to support the expansion of Burcon's investor base by raising awareness about Burcon through various media channels, analyst coverage and investor relations.
Marketing Strategies
Burcon's CLARISOY® soy protein, Peazazz® pea protein, Puratein®, Supertein® and Nutratein® canola protein isolates are expected to compete with soy, whey, milk and egg proteins in the expanding global multi-billion dollar protein ingredient market. Burcon's proteins are expected to specifically compete with whey protein isolates, casein/caseinates and dried egg-white as well as with existing traditional soy and pea proteins.
Food Business News reported the following average prices for the major protein ingredients that Burcon expects its products to compete with:
Whey protein isolate US $5.10 /pound1 CDN $15.41 /kg. 2
Casein/caseinates US $2.76 /pound3 CDN $7.92 /kg. 2
Dried Egg-white US $5.10 /pound3 CDN $14.64 /kg. 2
Soy and pea protein prices are not readily available to the public. However, through discussions with industry participants and market data research, Burcon management estimates that soy protein isolate and pea protein isolate prices are approximately as follows:
Soy protein isolate US $3.50 /pound CDN $10.04 /kg.2
Pea protein isolate US $3.00 /pound CDN $8.61 /kg. 2
Notes:
1. Price based on last available listing in Food Business News (November 22, 2011 edition). There is no current available price listing for whey protein isolate (90% edible).
2. Conversion into Canadian dollars made by Burcon, based on Bank of Canada exchange rate of C$1.00 = US$0.7680 on May 29, 2018.
3. Prices based on the May 1, 2018 edition of Food Business News for the week ending April 27, 2018.
Burcon expects the average selling price of Peazazz® pea protein to achieve an average price level that would equate to a premium over current pea pricing and at a discount to some animal-based proteins. Burcon expects the cost to produce Peazazz® pea protein to be materially consistent with the cost to produce traditional pea protein isolates.
Pursuant to the License and Production Agreement, ADM has the sole discretion in developing the sales and marketing strategies for CLARISOY® soy protein. Given ADM's extensive experience in the sale and marketing of soy protein products, Burcon expects that CLARISOY® will be strategically priced amongst ADM's current product portfolio and other competing protein products in the global market.
Plant Protein
According to Frost & Sullivan, the global protein-ingredient market is expected to grow at a compounded annual growth rate (CAGR) of 7.7% to reach $31.5 billion by 2018. The global protein ingredient market is currently dominated by soy, whey and casein. This combined market segment alone is projected to grow at 12% CAGR to reach $21.3 billion by 2018. Soy-derived proteins are the largest segment in the plant protein market, accounting for 53.4 percent volume share. Wheat and pea proteins are second and third, respectively. Demand outweighs supply in the plant protein market and volume is expected to grow steadily at 5.3% CAGR.
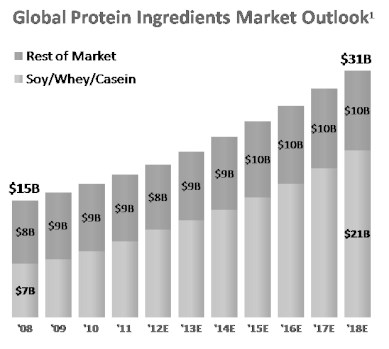
1) Based on current market prices and using volume projections from Frost & Sullivan Report, "Strategic Insight into the Global Plant Protein Ingredients Market," May 2012. Volume outlook based on 2011 base year.
Plant proteins continue to experience significant sales growth in North America and Western Europe for a variety of reasons. Changing demographics is one reason for this trend as nutrition-conscious baby boomers become aware of the health benefits of protein in general and, more specifically, of the benefit of plant proteins over certain animal proteins. Food manufacturers in turn are motivated by simple economics to prefer inexpensive plant proteins over their more costly animal counterparts.
Near-vegetarians, a growing group of consumers who choose meatless meals regularly but not exclusively, are also contributing to the trend favoring plant proteins. Responding to this new demographic, food manufacturers are taking advantage of the functional merits of plant proteins to create meat-free, high-protein foods.
While the demand for plant proteins is being driven by the health and wellness trend in the developed world, a possibly even larger force shaping the global protein ingredient industry is the growth of the middle class consumer in the developing world. During the past number of years, there has been a marked increase in demand for, and the price of, all protein ingredients. The growth of the middle class in the BRIC countries (Brazil, Russia, India and China) has been noted by many observers as being the key factor in that demand increase. As consumers evolve from a subsistence living to earning incomes where - in their respective countries - they can be classified as middle class, they invariably spend a large portion of their new income on food.
Casein and whey, the two major dairy protein ingredients, have experienced a decline in price in recent years due to surplus in the global supply. This, in turn, has pressured major soy protein producers, led by Solae LLC, now DuPont, to lower the price of their soy proteins in order to compete with the dairy protein ingredient suppliers. Dairy prices have since stabilized and the market is beginning to see an uptrend in prices as stronger global demand meets the supply surplus.
Wellness consumers are not only looking for products using plant-based ingredients but are also becoming more aware of the "what's in" and "what's not" trends in their food and beverage products. The "free-from" trend is an extension of the health and wellness trend, where consumers are seeking out products which use ingredients that are, for example, free from major allergens, artificial flavours, genetically modified organisms (GMO) or pesticides and herbicides. There are an increasing number of product launches with claims such as "dairy-free", "soy-free" and "gluten-free". More often than not, consumers are now checking the nutrition label and ingredients list on a product before making a decision to buy it.
In addition to the health and wellness trend in the developed world and the expanding middle class in the developing world, plant proteins are gaining popularity from the recognition that they offer a more environmentally friendly alternative to animal derived proteins. Consumers are considering the environmental footprint a product makes when making their purchase decisions. Production of animal proteins is viewed as less "environmentally economic" when compared to the production of plant proteins. Producers must feed plant protein to animals in order to produce animal proteins and animals are not efficient converters, pound for pound, of the proteins they consume. There is also a growing awareness of the large amount of greenhouse gases generated globally through livestock production. As a result of these factors, consumers are looking to food manufacturers to find more eco-friendly ways to produce food for humans.
Lastly, health concerns caused by melamine tampering/contamination, swine flu, E. coli, Asian bird flu and mad cow disease have provoked consumer concern that animal-based protein products may be unsafe.
Soy Protein
Markets & Markets estimated that in 2015, the soy protein market generated revenues of approximately US$7.11 billion and projects revenues to reach about US$10.12 billion by 2020, growing at a CAGR of 7.3%. Only 2% of soymeal is further processed into soy protein and products for use in human food applications. The other 98% of soymeal is used as animal feed. Soy proteins used in meat alternative and dairy replacement segments are in demand and growing at a high CAGR of 7.6% and 7.8%, respectively.
As discussed above, ADM has the sole discretion in developing the sales and marketing strategies for CLARISOY® soy protein pursuant to the License and Production Agreement. Given ADM's extensive experience in the sale and marketing of soy protein products, Burcon expects that CLARISOY® will be strategically priced amongst ADM's current product portfolio and other competing protein products in the global market.
Pea Protein
The following chart illustrates the annual global production of dried peas (in tonnes) from 2010 to 2016 as estimated by the FAO:

All figures in tonnes
Global production of dried peas in 2016 was estimated at 14.3 million tonnes, up 19% from 2015 with Canada being the largest producer in the world at 4.6 million tonnes. A portion of peas are further processed into different components: proteins, fibres and starch. The separation of pea components can be based on a dry-milling or an aqueous separation process and the use of organic solvents is not required.
There is currently no publicly available data on the size of the global pea protein market. Burcon management believes that a small fraction of the global production of dried peas is further processed into pea protein concentrates and pea protein isolates. Major producers of pea protein have expanded their production capacity to meet the growing demand. Frost & Sullivan estimates that the growth of the pea protein market would outpace other plant protein markets at a high CAGR of 5.7% due to pea protein's universal applicability in all food and beverage products. The unique functionality of Burcon's Peazazz® pea protein and its clean flavor profile could be major factors in expanding and growing the vegetable protein market.
Canola Protein
The initial market for Nutratein® canola protein isolate is anticipated to be in the high-value animal feed sector. Nutratein® canola protein isolate is expected to be a nutritional and functional protein ingredient that offers a compelling alternative source of protein for animal and fish feed manufacturers. Nutratein® has high nutritional and functional attributes that make it a suitable replacement for the costly whey protein concentrates in animal feed. The initial market for Puratein® canola protein isolate and Supertein® canola protein isolate is anticipated to be food and nutrition processors that target: the baking industry; meat analogue manufacturers (e.g. veggie-burgers); beverage processors (non-dairy fortified drinks); sport nutrition manufacturers; and prepared foods manufacturers incorporating whole egg, dried egg white or certain dairy proteins.
The overall strategic intent is to build Nutratein®, Puratein® and Supertein® canola protein isolates as global products through an alliance with a development partner. Burcon continues to work with food and beverage manufacturers and animal feed companies to establish the value of all three of Burcon's canola proteins in potential market applications.
Intellectual Property
Patents
In October 1999, Burcon acquired the shares of Burcon-MB. At the time of the acquisition, Burcon-MB held patents and applications covering the protein micellar mass process for extracting and producing a canola protein isolate. Since the acquisition, Burcon has focused on developing its protein extraction and purification processes and seeking patent protection for its developments. Through Burcon-MB, Burcon has filed patent applications in various countries over its inventions. Burcon's patent applications can be grouped into three categories:
- applications to protect additional novel protein extraction and purification technologies;
- applications to protect the uses of Puratein®, Supertein® and Nutratein® canola protein isolates, CLARISOY® soy protein, Peazazz® pea protein, and other plant proteins, for example, as functional food and beverage ingredients; and
- applications to protect the "signature characteristics" of Puratein®, Supertein® and Nutratein® canola protein isolates, CLARISOY® soy protein, Peazazz® pea protein and other plant proteins.
As of the date of this AIF, Burcon's patents and patent applications cover over 50 distinct inventions. Burcon has filed applications for most of its inventions internationally under the Patent Cooperation Treaty of the World Intellectual Property Organization. As at the date of this AIF, Burcon holds 247 issued patents in various countries, including patents covering composition of matter and a number of key processes and uses of Burcon's products as functional food and beverage ingredients, 65 of which have been issued in the U.S. Burcon holds patents or has filed patent applications in: Australia, Brazil, Canada, China, Hong Kong, India, Japan, the European Union, Mexico, New Zealand, Russia, South Africa, South Korea and the United States. Burcon currently has over 270 patent applications that are being reviewed by the patent offices in various countries, 41 of which are U.S. patent applications.
Granted U.S. Patents
Burcon holds 65 issued patents in the United States relating to soy protein, canola protein, flax protein and pea protein. Although the initial protein micellar mass canola protein isolate patents acquired from Burcon-MB expired in 2016 and 2017, Burcon holds patents covering improvements made by Burcon to the protein extraction and purification technologies. These new inventions include:
Soy
- processes to extract and purify soy protein from various soy material to produce soy protein products that are soluble in acidic medium and produces heat stable solutions suitable for protein fortification of sport drinks and other beverages;
- protection covering the composition and signature characteristics of Burcon's CLARISOY® soy protein;
- alternative processes for producing soy protein products;
Canola
- improving the quality of the input meal prior to the purification and extraction process, which results in better protein quality and higher protein yield. The residual oil from canola meal is traditionally recovered using a solvent called hexane. After the residual oil has been extracted, the hexane is recovered by heating the canola meal to a high temperature of about 110°C to 140°C. Burcon has been granted 2 U.S. patents covering a low-temperature process of recovering the hexane at temperatures below 100°C and below 50°C, respectively, to provide a desolventized meal that allows for improved protein recovery. Burcon also holds a U.S. patent to protect novel processing conditions in oil seed meal preparation and the production of a tailored meal in place of conventional canola meal. Through tailoring the input meal, including the low-temperature process, Burcon is able to produce canola protein isolates with superior organoleptic properties (improved colour, taste, aroma and mouth feel);
- process improvements to produce canola protein isolate efficiently and to obtain higher yields of canola protein isolate;
- processes for reducing phytic acid in the production of protein isolates from oilseed meals. Phytic acid is a naturally occurring anti-nutritional component found in oilseed meals such as canola meal and soybean meal;
- protection covering important processing conditions for producing Supertein® canola protein isolate as well as for the preparation of a highly refined Supertein® canola protein isolate;
- protection covering the composition of the dominant species of protein in Burcon's Puratein® canola protein isolate. Puratein® is a cruciferin-rich canola protein isolate comprised principally of globulin proteins, allowing it to have unique functional qualities;
- protection covering the process for producing Nutratein® canola protein isolate. Nutratein® is rich in both of the two major storage proteins found in canola: napin and cruciferin, making it a suitable ingredient for animal feed and aquaculture applications;
- processes to improve the final colour profile of Puratein® canola protein isolate and Supertein® canola protein isolate;
- applications for the uses of canola protein isolate as a functional food and beverage ingredient;
- the use of canola protein isolate as a flavour enhancer in a food product;
- alternative processes for producing canola protein isolates;
- protection covering the process for producing protein isolates using mustard seed as a starting material;
Pea
- protection covering the process for producing pea protein isolates having reduced astringency in low pH solutions; and
Flax
- processes for the production of flax protein isolates with unique protein profiles.
Patent Strategy
Burcon believes that it has developed a dynamic patent portfolio by seeking protection for new technologies as well as further protecting current technologies. In addition, Burcon has filed patent applications to cover alternative extraction technologies, which, in Burcon's opinion, would not be commercially viable. Such filings have been made as part of Burcon's defensive strategy to gain as much protection in the protein extraction and purification space as possible. Burcon will continue its research and development to further refine its processes and make new discoveries. The Company will continue to file additional patent applications to protect these discoveries.
In an effort to conserve cash resources, Burcon made the decision to abandon certain non-core canola patents and canola patent applications during fiscal year 2014 which it deemed to be non-essential or redundant for the purposes of achieving its strategic objectives by not paying annuities or maintenance payments when due.
Trade-marks
Burcon has obtained trade-mark registrations for "Nutratein", "Supertein" and "Peazac" in Canada, as well as "Puratein", "Peazazz", and the slogan "A New World in Protein" in Canada and the United States. The Company's application to registered "Peazac" in the United States has recently been allowed. Burcon had previously obtained trademark registrations for "CLARISOY" in the United States and Canada. In June 2011, Burcon and ADM entered into a trademark assignment and license agreement pursuant to which Burcon assigned its ownership of the CLARISOY® trademark to ADM. In return, Burcon has obtained a worldwide, non-exclusive, royalty-free license to use the CLARISOY® trademark for corporate marketing and promotion of Burcon, conducting research or development of CLARISOY® soy protein products and compliance with the requirements of applicable law. Burcon also has the option to re-acquire the CLARISOY® trademark if the license under the License and Production Agreement is terminated.
Facilities
Burcon's head office is located at 1946 West Broadway, Vancouver, British Columbia, Canada in leased office space. Through Burcon-MB, Burcon leases the premises where the Winnipeg Technical Centre is located at market rental rates. These premises are located at 1388 Waller Avenue, Winnipeg, Manitoba, Canada. The lease will expire on August 31, 2021. The premises include a 10,333 square foot facility in a light industrial park. Burcon owns the equipment in this facility which includes tanks of up to 20,000 litre capacity, membrane systems, centrifuges, filter presses, various dryers and laboratory analytical equipment. Burcon operates exclusively and independently within these facilities under the immediate direction of its management. Certain services such as laboratory testing and analysis which cannot be conducted internally are contracted out as necessary.
Personnel
As of March 31, 2018, Burcon-MB had 13 employees and/or contractors with varying degrees of technical expertise who perform the duties relating to the operation of the research laboratory and pilot plant in Winnipeg. Additionally, as of March 31, 2018, Burcon had 7 employees and/or contractors responsible for accounting, administration, corporate development, investor and public relations, legal and research and development activities who were predominantly located at Burcon's head office in Vancouver.
Competitive Conditions
ADM has licensed Burcon's soy protein technology and has commissioned its first full-scale CLARISOY® commercial production facility. Burcon is now working on securing one or more strategic partners to commercialize its Peazazz® pea protein, Puratein® canola protein isolate, Supertein® canola protein isolate and Nutratein® canola protein isolate.
The protein ingredient market is a global industry dominated by a few relatively large participants. Burcon recognizes that the selective use of alliances and partnerships can lower certain risks and can be the fastest and most profitable approach to maximizing revenues and cash flow. With this understanding, Burcon entered into the License and Production Agreement with ADM to commercialize Burcon's soy protein ingredients. See "Material Contracts".
ADM is a public company with annual revenues of approximately US$60.8 billion (fiscal 2017). It is a multinational company that produces among other things, ethanol, high fructose corn syrup, soy flour, soy protein concentrate, soy protein isolate and other specialty ingredients. It is currently one of the world's largest processor of oilseed crops.
Burcon recognizes that, in addition to ADM, there are other large industry participants with significant resources that dominate the plant protein ingredient industry. Two of the other major industry participants that sell plant protein ingredients to the food and beverage industries include Cargill Inc. ("Cargill") and DuPont.
Cargill is the U.S.'s largest private company with annual revenues of US$107.2 billion (2016). Cargill is an international producer, marketer, processor and distributor of agricultural, food, financial and industrial products and is one of the world's largest canola crushers.
DuPont, through its wholly-owned subsidiary DuPont Nutrition and Health, is a world leader in soy-based proteins and specialty ingredients for the food, beverage, meat and nutritional products industries. E.I. du Pont de Nemours and Company ("DuPont")(NYSE: DD) began in 1802 as a chemical company. In August 2017, Dow Chemical and DuPont successfully completed a merger to create the world's largest chemical company. DowDupont is a multinational company specializing in chemicals, energy, agriculture and specialty food ingredients with annual revenues at approximately US$62.5 billion (fiscal 2017). Through the previous acquisitions of Danisco and DuPont Protein Technologies, DowDuPont is now one of the largest participants in protein ingredients, specialty ingredients, food and beverage ingredients, enzymes and bio-based solutions.
The pea protein industry outside of China is dominated by three major participants: Roquette Freres, Cosucra Groupe Warcoing and Nutri-Pea Limited. Based in France, Roquette Freres is a private company which produces more than 700 by-products from the starch extracted from corn, wheat, potatoes and peas. It has grown to become the second largest producer of starch in Europe and fifth largest producer in the world. Roquette Freres is currently the largest participant in the pea protein industry.
Cosucra Groupe Warcoing is a Belgian group of independent companies dedicated to the development, production and promotion of natural ingredients from chicory and yellow pea. Cosucra's line of products includes pea protein isolate, pea fibre, pea hull fibre and pea native starch.
Puris Foods, formerly World Food Processing, is a new entrant to the pea protein industry. Puris has been processing pulse crops in Iowa, US, since 1985 and has recently expanded capacity to include downstream production of pea protein products. In January 2018, Cargill entered into a joint venture agreement with Puris to invest and expand Puris' pea protein production to include a second commercial facility.
Based in Manitoba, Canada, Nutri-Pea Limited is a privately owned company specializing in the manufacture of food ingredients derived from Canadian yellow field peas. Nutri-Pea extracts fibre, starch and protein products from yellow field peas.
Rising commodity prices have had a noticeable impact on the global aquaculture and livestock farming sectors in recent years, as through their direct impact on feed costs as well as on energy costs. These rising input prices have in turn been one of the factors that has increased the cost to produce animal proteins (egg protein products as well as the dairy proteins, casein and whey). Burcon anticipates that under commercial production levels, it will be able to produce its plant protein isolates at a cost level which will make them significantly competitive with animal proteins.
Burcon offers a value proposition for both the multibillion-dollar oilseed crushing industry which produces enormous volumes of canola meal and soybean meal that currently sell as relatively low-margin animal feed. Burcon has the technology and know-how to add value to these oilseed meals by extracting unique and potentially valuable food proteins. The unique nutritional and functional characteristics of Puratein® canola protein isolate, Supertein® canola protein isolate, Nutratein® canola protein isolate, CLARISOY® soy protein and Peazazz® pea protein differentiate them from the existing plant-based proteins (predominantly soy) as well as from the existing animal based proteins (casein, whey and egg). Burcon has also developed specialty proteins, including flax and hemp. Burcon anticipates that all these unique attributes will make the proteins valuable to the food and beverage industry.
For the branded consumer product companies and food ingredient companies, the value proposition comes from both the novel properties of Burcon's proteins as well as the inherent first-mover advantage. Exclusivity through patent protection and the first-mover advantage could add significant value to Burcon's opportunity in an industry where first-movers dominate, and market share changes slowly. For example, the iconic sport beverage brand Gatorade owned 83% of the sport beverage market in the United States in 2007, down only 10 percentage points from its 93% share which it enjoyed at its peak two decades earlier (source: Advertising Age magazine April 21, 2008). Gatorade still held a large share of the market in 2013 (69.5%) while its next closest competitor, Powerade, owned 28.8% of the market. Although the sport beverage industry has expanded to include a growing number of other competitors, Gatorade's early market share advantage shows that the exclusivity and first-mover advantage could create an opportunity for food and beverage processors to brand or co-brand multiple new products fortified with Burcon's proteins.
See also "Risk Factors".
Environmental Matters
Burcon's extraction processes use no harsh chemicals and emit no noxious odours or significant waste products. Biodegradable, natural and/or recyclable input materials, end-products and by-products are used and, therefore, are expected to present no significant environmental risk. As such, Burcon does not foresee any financial and operational effects of environmental protection or requirements on the capital expenditures, earnings and the competitive position of Burcon in the current financial year or in the foreseeable future.
Burcon's processes, as demonstrated to date, are expected to work equally well on input materials from both genetically modified ("GM") and non-GM sources.
Regulatory Approval For Marketing CLARISOY®
Food-grade soy protein isolate first became available on October 2, 1959 with the dedication of Central Soya's edible soy isolate, Promine D, production facility in Chicago. An edible soy isolate and edible spun soy fiber has also been available since 1960 from the Ralston Purina Company in St. Louis, where they had originally developed the technology. In 1987, Protein Technologies, Inc. ("PTI") became the world's leading maker of isolated soy protein and was subsequently acquired by DuPont.
Soy protein isolate is a highly refined or purified form of soy protein with a minimum protein content of 90% on a moisture-free basis. It is made from defatted soy flour which has had most of the non-protein components, fats and carbohydrates removed. Because of this, it has a neutral flavour and will cause less gas due to bacterial flatulence.
Soy protein isolates are mainly used to improve the texture of meat products, but are also used to increase protein content, enhance flavour, and as an emulsifier.
Pure soy protein isolate is used mainly by the food industry. It is sometimes available in health stores or in the pharmacy section of the supermarket. It is usually found combined with other food ingredients.
In 1995, the New England Journal of Medicine (Vol. 333, No. 5) published a report from the University of Kentucky entitled, "Meta-Analysis of the Effects of Soy Protein Intake on Serum Lipids." It was financed by the PTI division of DuPont, The Solae Company, St. Louis. This meta-analysis concluded that soy protein is correlated with significant decreases in serum cholesterol, Low Density Lipoprotein LDL (bad) cholesterol and triglyceride concentrations. However, High Density Lipoprotein HDL (good) cholesterol did not increase. Soy phytoestrogens (isoflavones: genistein and daidzein) adsorbed onto the soy protein were suggested as the agent reducing serum cholesterol levels. On the basis of this research, PTI, in 1998, filed a petition with FDA for a health claim that soy protein may reduce cholesterol and the risk of heart disease.
In October 1999, the FDA approved a health claim for soy protein and its role in reducing the risk of coronary heart disease. Food manufacturers may label foods containing soy protein by stating that "Diets low in saturated fat and cholesterol that include 25 grams of soy protein daily may reduce the risk of heart disease. One serving of (name of food) provides __ grams of soy protein." One serving (1 cup or 240 mL) of soy milk, for instance, contains 6 or 7 grams of soy protein.
In March 2015, after a meta-analysis of scientific studies, Health Canada's Food Directorate concluded that scientific evidence exists to support a health claim about soy protein and blood cholesterol lowering. The evidence supports a direction of effect towards a reduction in total and LDL cholesterol levels when soy protein is consumed. Foods containing soy protein may state on its label, "[Serving size] of (brand name) [name of food] supplies/provides X% of the daily amount of soy protein shown to help reduce/lower cholesterol." The daily amount referred to is 25 grams of soy protein. For example, "150g of tofu supplies 70% of the daily amount of soy protein shown to help lower cholesterol".
While soy protein and soy protein isolates themselves have not been granted GRAS status by the FDA, they are widely used in food and nutritional applications including infant formula. As a result, Burcon does not anticipate any regulatory process for its CLARISOY® soy protein. However, there can be no assurance that the FDA will not require companies producing and selling soy protein isolates to meet additional regulatory requirements in the future.
Regulatory Approval For Marketing Peazazz®
Peas were one of the earliest cultivated food crops and have a long history of safe consumption in human foods. They are widely accepted and consumed as a vegetable in our daily diets. Although pea protein is a relatively new vegetable-based protein ingredient, it is commercially available and used by the food industry.
Despite peas and pea protein being widely accepted and consumed, Burcon has, in the process of discussions with potential strategic partners, been informed by certain major food and beverage manufacturers that they require all of its procured ingredients to be GRAS approved to ensure consistent quality safety in their end products. Burcon has successfully obtained self-affirmed GRAS status for its Peazazz® pea protein products and has made its submission to the FDA for GRAS notification on June 15, 2018. Similar to Burcon's GRAS notification for its canola proteins, the FDA will review Burcon's submission and either respond with further challenges to Burcon's safety claims or respond with a no objection letter. See "Obtaining Regulatory Approval for Marketing Puratein®, Supertein® and Nutratein®". Peas have had a substantial history of consumption for food use by a significant number of consumers. Therefore, Burcon does not expect the regulatory process for Peazazz® to be as extensive as the one undertaken for Burcon's canola proteins.
Obtaining Regulatory Approval For Marketing Puratein®, Supertein® and Nutratein®
Canola meal is currently used as a protein ingredient in dairy, beef, swine and poultry rations and is recognized for its consistent quality and value. Canola meal's nutritional value, even in animal feed applications, is limited by the presence of a large amount of fiber and other anti-nutritional factors such as glucosinolates. Glucosinolates are the sulphur compounds that give mustard its sharp taste. Analysis of Puratein® canola protein isolate and Supertein® canola protein isolate conducted by independent testing laboratories has indicated very low levels of glucosinolates. Puratein® and Supertein® canola protein isolates have numerous potential applications, primarily as a food ingredient and as a personal care product ingredient. Burcon is initially pursuing animal and feed applications for Nutratein® canola protein isolate. Moreover, Nutratein® may have potential applications as a food ingredient also. See "Description of the Business". In order to market Puratein® canola protein isolate and Supertein® canola protein isolate as a food ingredient, approvals must be obtained from the applicable regulatory authorities in the countries currently targeted for commercialization. Initially, Burcon believes these countries are the United States, the countries comprising the European Union and Canada. In order to market Nutratein® canola protein isolate as a potential human food and/or animal feed ingredient, approvals must be obtained from the applicable regulatory authorities in the countries currently targeted for commercialization. Initially, Burcon expects the target markets are the United States and Canada.
United States
Puratein® and Supertein®
During fiscal 2008, Burcon, in conjunction with ADM, pursued regulatory recognition for Puratein® canola protein isolate and Supertein® canola protein isolate.
A substance may be "generally recognized as safe" or "GRAS" as a food ingredient based on two principles: it is a prior sanctioned substance, meaning that it has been used in food before 1958; or it is determined to be GRAS by scientific experts based on scientific procedures. Accordingly, because canola meal (and canola protein contained therein) does not have a history of safe use in human foods, the determination that Puratein® canola protein isolate and Supertein® canola protein isolate are GRAS must be based on scientific procedures.
Scientific studies were conducted during fiscal 2008 and based on those studies, Burcon and ADM prepared a dossier of data that included scientific information about canola, how canola is grown, handled and processed, Burcon's protein extraction process and finally, the intended uses of the proteins in foods and beverages. A panel of qualified experts in the fields of food safety, toxicology, nutritional sciences, food allergies and pediatric nutrition reviewed the dossier to which it also had input and affirmed unanimously that the proteins are safe for their intended uses. In October 2008, Burcon's Puratein® canola protein isolate and Supertein® canola protein isolate achieved self-affirmed GRAS status.
Substances that are GRAS under conditions of their intended use are exempted from the usual Federal Food, Drug, and Cosmetic Act ("FFDCA") food additive tolerance requirements.
For a substance to be GRAS, the scientific data and information about the use of the substance must be widely known and there is a consensus among qualified experts that the data and information establish that the substance is safe under the conditions of its intended use.
When a use of a substance does not qualify for the GRAS exemption, then the substance is considered to be a food additive under the FFDCA. Use of the substance is subject to the premarket approval mandated by the FFDCA. For a food additive, privately held data and information about the use of a substance are sent by the proponent to the FDA, which evaluates the data and information to determine whether the data and information establishes that the substance is safe under the conditions of its intended use. If found unsafe, the FDA may take enforcement action to stop distribution of the food substance and foods containing it on the grounds that such foods are or contain an unlawful food additive.
A GRAS designation typically exists in one of three forms:
1. Self-affirmed. The manufacturer of the substance has performed all necessary research, including the formation of an expert panel to review safety concerns, and is prepared to use these findings to defend its product's GRAS status.
2. FDA-pending. The manufacturer has performed all the aforementioned due diligence, and submitted to the FDA for GRAS approval.
3. No comment. The FDA has reviewed a product's GRAS notification claim and responded with "no comment"; i.e., no further challenges on the product's GRAS status.
To enhance consumer acceptance of Puratein® canola protein isolate and Supertein® canola protein isolate, Burcon and ADM chose to pursue GRAS notification for Puratein® canola protein isolate and Supertein® canola protein isolate. GRAS notification is a voluntary procedure whereby a company informs the FDA of its determination that the use of a substance is GRAS.
During fiscal 2010, Burcon's scientists collaborated with ADM in the preparation and review of the manuscripts for the publication of the toxicology studies conducted in fiscal 2008 as part of the GRAS self-affirmation process. On August 20, 2009 and November 3, 2009, Burcon announced the publication of the Puratein® and Supertein® toxicology studies, respectively, in peer-reviewed journals. The publication of these scientific studies forms a significant part of the GRAS notification process.
On January 19, 2010, Burcon announced that it had filed a formal notification in accordance with the FDA proposed regulation 62FR 18938, having determined, based on a review of the data referenced in the notification, that Burcon's Puratein® canola protein isolate and Supertein® canola protein isolate are GRAS for their intended use as an ingredient in a variety of food and beverage applications and in addition, that both substances are exempt from premarket approval requirements of the Food, Drug and Cosmetic Act (the "GRAS Notification"). In response to comments from the FDA, Burcon modified and resubmitted the GRAS Notification in February 2010. In a letter dated April 1, 2010, the FDA formally acknowledged receipt of the GRAS Notification.
After the FDA acknowledges receipt of the GRAS notice, it then evaluates whether the submitted notice provides a sufficient basis for the GRAS determination and whether information in the notice or otherwise available to the FDA, raises issues that lead the FDA to question whether use of the substance is GRAS. Following the evaluation and within 180 days, the FDA responds in one of 3 ways:
1. the FDA does not question the basis for the notifier's GRAS determination;
2. the FDA concludes that the notice does not provide a sufficient basis for a GRAS determination (for example, the notice does not include appropriate data and information, or because the available data and information raise questions about safety of the notified substance); or
3. the FDA has, at the notifier's request, ceased to evaluate the GRAS notice.
A substance is GRAS notified when, after reviewing the GRAS notification, the FDA responds with a no-objection letter if it is satisfied with the submission.
On August 30, 2010, Burcon announced that the FDA issued a no objection letter with respect to Puratein® and Supertein® canola protein isolates. This response indicates that the FDA has no objection to the conclusion that Puratein® and Supertein® are Generally Recognized as Safe (GRAS) among qualified experts for use alone or together as an ingredient in dairy products, grain products, fruit and vegetable juices and beverages, salad dressings, meal replacements and nutritional bars.
Nutratein®
Human Food
Burcon's Nutratein® canola protein extraction process allows for the production of a canola protein that is rich in both of the two major storage proteins found in canola: napin and cruciferin. Nutratein® is a blended canola protein isolate that consists of the napin-rich protein fraction (Supertein®) and the cruciferin-rich protein fraction (Puratein®) of canola.
The FDA has issued a no objection letter with respect to Puratein® and Supertein® canola protein isolates. This response indicates that the FDA has no objection to the conclusion that Puratein® and Supertein® are GRAS for their intended uses in human food.
Burcon's management believes that a substantial equivalence claim, based on the similarities to Puratein® and Supertein® canola protein isolates, can be made in regard to Nutratein® canola protein isolate being Generally Recognized as Safe (GRAS) for its intended uses in human food applications. In order to obtain a substantial equivalence claim, Burcon is required to submit to the FDA a summary of its scientific and regulatory assessment of the food in the form of a New Protein Consultation (NPC) document. The summary will include information regarding Nutratein® such as protein characterization, compositional analysis, protein uses and levels of intake and the applicant's conclusion on the safety and nutritional assessment of the protein.
Following the submission, the FDA responds to the applicant by letter indicating one of the following:
1. the FDA does not question the basis for the applicant's determination that the product is not materially different in any respect relevant to food safety from other canola protein isolate varieties;
2. the FDA requires additional information on this protein product in order to determine a safety conclusion; or
3. the FDA has, at the applicant's request, ceased to evaluate the NPC.
Animal Feed
Nutratein® canola protein isolate is a nutritional and functional protein ingredient that offers a compelling alternative source of protein for animal feed manufacturers. Animal feed, and in particular, high-valued feed replacers are currently dominated by the use of high-cost whey protein concentrates ("WPC"). Burcon's Nutratein® canola protein isolate has the nutritional value that could compete with WPC.
Although Nutratein® canola protein isolate is extracted from canola meal which is currently being used as a protein ingredient in livestock feed, regulatory approvals for Nutratein® as a novel feed ingredient will still be required.
The regulatory approval process in the United States for Nutratein® canola protein isolate as an animal feed ingredient requires a submission of a GRAS Notification to the FDA's Center for Veterinary Medicine ("CVM"), Division of Animal Feeds, indicating scientific evidence of Nutratein®'s safe and intended uses in animal feed. The regulatory process is similar to the GRAS Notification process for Burcon's Puratein® and Supertein® canola protein isolates. A substance is GRAS notified when, after reviewing the GRAS notification, the FDA responds with a no-objection letter if it is satisfied with the submission.
The CVM regulates the manufacture and distribution of food additives and drugs that will be given to animals. These include animals from which human foods are derived, as well as food additives and drugs for pet (or companion) animals.
Europe
Puratein® and Supertein®
Where a new ingredient has not been used to a significant degree in human food in the EU market prior to May 1997, the ingredient is regarded as a novel food ingredient and would be regulated under the 1997 Regulation (EC) No 258/97 concerning Novel Foods and Novel Food Ingredients.
Food supplement products are marketed in Europe as foodstuffs or medicines depending on the indication of the product. A "foodstuff" is defined as any substance or product, whether processed, partially processed or unprocessed, intended to be ingested by humans. The use of Puratein® canola protein isolate and Supertein® canola protein isolate in pill or powder form for use in supplement type products (e.g. energy bars and drinks) would categorize the ingredients as a foodstuff in Europe, provided that no health related claims were declared.
According to the EU novel food regulations, a novel food application must contain a general description of the novel food, including technical data and categorization. The application should outline the rationale justifying that Puratein® canola protein isolate and Supertein® canola protein isolate is in fact "novel" as prescribed under the regulations. The EU has formulated a Scientific Committee for Food which evaluates various classes and subclasses of novel foods. Puratein® canola protein isolate and Supertein® canola protein isolate fall under two subclasses depending on whether it was obtained from genetically or non-genetically modified canola meal.
The European Commission has revised its novel food approval process, where approval in one member state is valid in all member states unless an objection is raised. Previously, approval from all member states was required that resulted in a processing time of more than three years. Following a no-objection response from the member states, the novel food ingredient can be marketed in all EU countries. Burcon will therefore be required to meet all requirements prescribed under the EU's novel foods regulation for marketing Puratein® canola protein isolate and Supertein® canola protein isolate.
Canada
Puratein®, Supertein® & Nutratein®
The manufacture and sale of Puratein®, Supertein® and Nutratein® canola protein isolates is subject to compliance with regulatory regimes in Canada that require a manufacturer to demonstrate a product's safety as a food.
These activities are governed by the federal Food and Drugs Act and Regulations, which are administered by the Food Directorate, Health Products and Food Branch of Health Canada, a Canadian government agency. The Food and Drug Regulations require a manufacturer to notify the Food Directorate in writing of its intention to sell or advertise for sale of a novel food.
In Canada, each of Puratein®, Supertein® and Nutratein® canola protein isolate is considered a "novel food", meaning a food that does not have a history of safe use in humans or that has been manufactured by a process that has not been previously applied to it and which causes it to undergo a major change.
The regulatory pathway to product acceptance requires a submission of a Safety Assessment Data Package to Health Canada that includes details such as novel extraction process, nutritional, toxicology and allergenicity considerations. The package must also include specific information on the novel food's intended use and directions for its preparation, as well as the text of all labels to be used in connection with the novel food.
The steps toward obtaining approval as a novel food include the following:
- preliminary consultation and meetings with Health Canada officials to review the characteristics and make-up of the product and obtain guidance on the Safety Assessment Data Package;
- toxicology tests conducted by recognized research and testing facilities to provide evidence of the safety of the product; and
- submission to Health Canada Food Directorate of a Novel Food Notification that includes a description of the product, its development and intended uses; details of the procedures for manufacturing; packaging and storing the product; information regarded expected levels of consumption; and results of the toxicology tests;
- safety assessment of the novel food by the relevant bureau of the Food Directorate and presented to the Food Rulings Committee for consideration; and
- Health Canada issues a no objection letter to the sale of novel food product as human food in Canada as specified in the notification.
Review of the Novel Food Notification by Health Canada will determine whether the toxicology test information is sufficient to establish safety of the product or whether any additional testing is required. Although Burcon has investigated the process for approval of Puratein® canola protein isolate and Supertein® canola protein isolate as novel foods in Canada, the Company does not intend to pursue regulatory approval as a food ingredient for these canola proteins in Canada until Burcon has secured a strategic partner for Puratein®, Supertein® and Nutratein®. However, there is no assurance that Health Canada will approve the product or will not require further testing.
Nutratein®
Animal Feed
In accordance with the federal Feeds Act and Regulations, an application for a novel feed ingredient such as Nutratein® canola protein isolate is submitted to the Canadian Food Inspection Agency ("CFIA"), Animal Feed Division which approves feed ingredients and registers feed products in order to verify that feeds meet:
• standards for safety and usefulness, and
• regulatory requirements for labelling, prior to importation, manufacture or being offered for sale to livestock producers and other customers.
The safety and efficacy evaluations are conducted by staff of the Animal Feed Division. The review of a submission to register a novel feed or ingredient involves a complete assessment of product safety (to livestock, workers, food and the environment), a review of data supporting the intended purpose of the product, an assessment of the product's compliance with regulatory standards and a review of the label for compliance with the Feeds Regulations.
Estimated Timelines
Obtaining "novel food" status in Europe and Canada for Puratein® and Supertein® could take between 1 1/2 and 2 1/2 years. The entire process for obtaining a GRAS substantially equivalent status for Nutratein® canola protein isolate in the United States - from submission to receiving a no objection letter - could take between one to two years. These estimates are based on the assumption that there are no major issues raised by the applicable regulatory authorities. See "Risk Factors". Burcon's Supertein® and Puratein® canola protein isolates have now been GRAS-notified in the United States. Burcon has determined that it will postpone the process for pursuing regulatory approval of Puratein® and Supertein® in Canada and Europe and Nutratein® in the United States and Canada until Burcon has secured a strategic partner for these products and the terms of a strategic alliance have been determined with a strategic partner.
Risk Factors
Patents and Proprietary Rights
Burcon's success will depend, in part, on its ability to obtain patents, maintain trade secret protection and operate without infringing on the proprietary rights of others or having others infringe on its rights. Burcon has filed applications for most of its inventions internationally under the Patent Cooperation Treaty of the World Intellectual Property Organization. As at the date of this AIF, Burcon has been granted a total of 247 patents in various countries including patents covering composition of matter and a number of key processes for producing and using Burcon's soy, pea, canola and flax protein products as functional food and beverage ingredients. Of those patents, 65 have been granted in the United States. Countries in which Burcon holds issued patents or has filed patent applications are: Australia, Brazil, Canada, China, Hong Kong, India, Japan, the European Union, Mexico, New Zealand, Russia, South Africa, South Korea and the United States. Currently, Burcon has over 270 patent applications that are being reviewed by the patent offices in those countries.
The patent positions of food processing and manufacturing businesses, including Burcon's, are uncertain and involve complex legal and factual questions for which important legal issues are largely unresolved. For example, the coverage claimed in a patent application can be significantly reduced before a patent is issued. There can be no assurance that Burcon's pending patent applications will result in the issuance of patents, that Burcon will develop additional proprietary products that are patentable, that any patents issued to Burcon will provide it with adequate protection or any competitive advantages, that such patents will not be successfully challenged by any third parties or that the patents of others will not impede Burcon's ability to commercialize its technology. Furthermore, there can be no assurance that others will not independently develop products or technologies similar to Burcon's or, if patents are issued to Burcon, design around any patented products developed by Burcon.
Publication of discoveries in the scientific or patent literature often lag behind actual discoveries. As a consequence, Burcon cannot be certain that it was the first creator of inventions covered by issued patents or pending patent applications or that it was the first to file patent applications for such inventions. Moreover, Burcon might have to participate in interference proceedings declared by the United States Patent and Trademark Office or other proceedings outside the United States, including oppositions, to determine priority of invention or patentability. An unfavourable outcome in an interference or opposition proceeding could preclude Burcon from selling products using the technology or require Burcon to obtain license rights from prevailing third parties. There is no guarantee that any prevailing party would offer Burcon a license or that Burcon could acquire any license made available to it on commercially acceptable terms. There can be no assurance that the patents that Burcon has received or may be able to obtain in the future would be held valid or enforceable by a court or that a competitor's technology or product would be found to infringe such patents.
Part of Burcon's intellectual property is in the form of trade secrets and know-how and may not be protected by patents. There can be no assurance that Burcon will be able to protect its trade secrets. To help protect Burcon's rights, Burcon requires its employees, consultants, advisors and collaborators to enter into confidentiality agreements. There can be no assurance that these agreements will provide meaningful protection for Burcon's trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure.
Protection of Intellectual Property is Expensive
Burcon's future success and competitive position depends in part on its ability to obtain and maintain certain proprietary intellectual property rights used in its principal product candidates. Any such success may be achieved in part by prosecuting claims against others who it believes are infringing its rights and by defending claims of intellectual property infringement brought by its competitors and others. Burcon's involvement in any such intellectual property litigation could result in significant expense incurred by Burcon, adversely affecting the development of product candidates or sales of such challenged product or intellectual property and diversion of efforts of Burcon's technical and management personnel, whether or not such litigation is resolved in Burcon's favour. Some of Burcon's competitors may be able to sustain the costs of complex patent litigation more effectively than Burcon because they have substantially greater resources.
Uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on Burcon's ability to continue its operations. In the event of an adverse outcome as a defendant in any such litigation, Burcon may, among other things, be required to:
- cease the development, manufacture, use or sale of product candidates or products that infringe upon the intellectual property of others;
- expend significant resources to design around a patent or to develop or acquire non-infringing intellectual property;
- discontinue processes incorporating infringing technology; or
- obtain licenses to the infringing intellectual property.
No assurance can be provided that Burcon would be successful in such development or in the acquisition of non-infringing technology or that such licenses for such infringing technology would be available upon reasonable terms, if at all. Any such development, acquisition or license could require the expenditure of substantial time and other resources and could have a material adverse effect on Burcon's business and financial results. If Burcon does not obtain such licenses, it could encounter delays in the introduction of products or could find that the development, manufacture or sale of products requiring such licenses could be prohibited.
Should third parties file patent applications, or be issued patents claiming technology also claimed by Burcon in pending applications, Burcon may be required to participate in interference proceedings with the United States Patent and Trademark Office, or other proceedings outside the United States, including oppositions, to determine priority of invention or patentability, which could result in substantial cost to Burcon even if the eventual outcome were favourable to Burcon.
In an effort to conserve cash resources, Burcon decided to abandon certain non-core canola patents and canola patent applications during fiscal year 2014 which it deemed to be non-essential or redundant for the purposes of achieving its strategic objectives. In addition, Burcon continues to review its portfolio of patent applications on an on-going basis to ensure that it is meeting its objectives.
The Timeline for Development and Commercialization of New Food Products Can Be Long
Burcon acquired the initial canola protein extraction technology from Burcon-MB in October 1999. Since then, it has conducted research and development on a number of plant proteins. On June 18, 2012, Burcon announced that ADM has begun commercial production of CLARISOY® soy protein. On December 17, 2015, Burcon announced that it expects ADM's first full-scale commercial CLARISOY® production facility to be operational by mid-2016. On November 8, 2016, Burcon announced that ADM had successfully commissioned the first full-scale CLARISOY® production facility. However, there can be no assurance that ADM will be able to sell a sufficient amount of CLARISOY® to provide meaningful royalties to Burcon. In addition, Burcon has not commercialized any of its other products, and accordingly, has not begun to market or generate significant revenues from the commercialization of these products. There can be no assurance that any of its products will: meet applicable food regulatory standards; obtain required regulatory approvals in countries where such approvals have yet to be sought; be capable of being produced in commercial quantities at reasonable costs; or be successfully marketed; or that the investment made in such potential products will be recouped through sales or related royalties. With the exception of CLARISOY® soy protein, none of Burcon's potential products are commercially available as a food ingredient for human consumption. Although Burcon is in discussions with certain potential partners for Peazazz® pea protein, no strategic alliance has yet been formed. The rising popularity of pea proteins has resulted in more companies entering the market to produce pea proteins that could compete with Burcon's Peazazz® pea proteins. Burcon must secure a strategic partner for its canola protein isolates. If Burcon is unable to secure an alternative strategic partner for its canola protein isolates then the commercialization of its products may be delayed or unsuccessful. Even if Burcon commercializes a product or products, its business strategy may not be successful.
Burcon Has a History of Net Losses and Negative Operating Cash Flow and May Never Achieve Profitability
Burcon has accumulated net losses of approximately $89 million from its date of incorporation through March 31, 2018. On December 19, 2012, Burcon announced that it had been notified by ADM of the first commercial sale of CLARISOY® soy protein produced by ADM. However, Burcon has reported minimal royalty revenue during fiscal 2013 to 2018. Although Burcon expects to receive royalty payments from ADM pursuant to the License and Production Agreement, the magnitude of future royalty payments cannot be ascertained at this time. In the absence of a definitive time for when sales of products will be significant, Burcon expects its accumulated net losses will increase as it continues to commercialize its products, its research and development and its product application trials. Burcon expects to continue to incur substantial losses for the foreseeable future. Burcon cannot predict if it will ever achieve profitability and, if it does, it may not be able to sustain or increase its profitability.
Burcon's ability to achieve and maintain profitability will depend on, among other things, the market's acceptance of any of its products that receive regulatory approval. The commercial success of any of Burcon's products will depend on whether:
- they receive public and industry acceptance as a food ingredient and dietary supplement; and
- they may be sold at competitive prices or are able to obtain sufficient royalty revenue from licensing which adequately exceeds Burcon's production (or business) costs.
Market Conditions
During fiscal year 2018, Burcon completed a rights offering of its common shares in February 2018. Although Burcon has sufficient funds to operate until October 2018, it may need to raise capital in the near future in order to meet its business objectives. However, the inherent risk in investing in companies such as Burcon may make it difficult for the Company to obtain capital and financing for its operations. There can be no assurance that additional financing will be available on acceptable terms, if at all.
Financing Requirements
Since acquiring Burcon-MB on October 8, 1999, Burcon has raised gross proceeds of approximately $74.8 million from the sale or issuance of equity securities. Developing Burcon's products and conducting product application trials is capital intensive. As at the March 31, 2018 balance sheet date, Burcon had $3.4 million in cash and cash equivalents. Including the net proceeds of $3.4 million from the rights offering in February 2018, management estimates that these cash resources are sufficient to continue the current level of operations until October 2018. If Burcon does not receive sufficient royalties from ADM under the License and Production Agreement, Burcon may need to raise additional capital to fund its objectives and operations beyond this date. The Note is expected to mature on May 12, 2019 upon which the interest accrued and the Principal Amount will be payable unless PT International has converted the Principal Amount into common shares of the Company. There can be no assurance that PT International will convert the Principal Amount of the Note into common shares. Additional financing may not be available on acceptable terms, if at all. If Burcon raises funds by issuing more equity securities, holders of common shares will experience dilution. If Burcon is unable to raise additional funds when it needs them, it may be required to delay, reduce or eliminate some or all of its development programs and some or all of its product application trials. Burcon may also be forced to license technologies to others that it would prefer to develop internally.
Product and Market Related Risks
The long-term success of Puratein®, Supertein® and Nutratein® canola protein isolates, CLARISOY® soy protein and Peazazz® pea protein hinges upon market acceptance by food and feed ingredient manufacturers and suppliers in numerous product applications. Burcon has not yet secured a strategic partner for its technology for producing Puratein®, Supertein® and Nutratein® canola protein isolates or Peazazz® pea protein. If Burcon is unable to secure a strategic partner to assist in establishing credibility, market acceptance of and the commercialization of its products may be delayed or unsuccessful. The commercial products manufactured using Burcon's protein and extraction technologies must exhibit certain functional and nutritional characteristics to garner any market share in the industries that are targeted. There can be no assurance that Burcon's products will meet industry standards. Even though Puratein® , Supertein® and Nutratein® canola protein isolates, CLARISOY® soy protein and Peazazz® pea protein may be found to be functionally acceptable in product applications, there is no assurance that they will obtain market acceptance and within a reasonable time frame. Burcon's products have only been produced in small scale batches, and the majority of food or feed ingredient manufacturers will require a substantial testing phase and demonstration of consistent delivery and production capabilities for commercialization. Without this capability, market acceptance of Puratein®, Supertein®, and Nutratein® canola protein isolates, and Peazazz® pea protein may be delayed.
There are many large companies in the marketplace that manufacture and produce mature and well-known protein ingredients that have been used for many years. These companies also possess far greater financial, marketing and human resources than Burcon. Products such as dried egg white and soy protein isolate have been used in the food processing industry for years with successful results. These protein ingredients are proven to be functional, technologically sound, readily available and reliable. Burcon recognizes that it must devote resources and energy over a long period of time to develop these markets as they tend to be quite conservative. Food companies rely on taste, appearance and health appeal to sell their products and they are unlikely to accept even a lower priced product without comparable or superior functionality. Major companies in the food processing industry have invested hundreds of millions of dollars in brand and product development and will avoid ingredients or processes that may be of questionable or unproven benefit.
Consumer Acceptance
There is a continuing public issue regarding food products derived from genetically modified organisms ("GMOs"). Genetic modification, where a plant's genetic makeup is altered by insertion, deletion or reversal of genes, often from an entirely different organism, should not be confused with traditional plant breeding techniques which have been used for generations to selectively breed plants with desirable traits. In fact, canola is a variation of rapeseed developed by Canadian plant breeders using traditional techniques.
The GMO debate centres on the issue of whether food products derived from GMOs pose potential health risks to consumers and/or the environment. Burcon's processes for extracting a protein isolate from canola meal and soy are equally effective with starting materials from either GM or non-GM sources and can also utilize oilseed meals other than canola or soy. Therefore, if Burcon chooses to use starting materials from a GMO source, the resultant protein isolate may be less acceptable to some consumers.
Government Regulatory Approval
The approval, manufacture and sale of food ingredients in Canada, the United States and Europe, such as Burcon's products, are governed by regulatory regimes in those countries which require a manufacturer to be able to demonstrate a product's safety. In order to obtain approval to market a product, a manufacturer may be required to undertake controlled research and testing, which will be subject to government review and approval. There is a risk that government approval may not be received in a timely fashion or at all. See "Obtaining Regulatory Approval for Marketing Puratein®, Supertein® and Nutratein®".
Rapid Technological Change
The food processing industry is subject to rapid and substantial technological change. There can be no assurance that developments by others will not render Burcon's products or technology non-competitive or that Burcon will be able to keep pace with technological developments.
Significant Competition
Technological competition among food industry participants is intense and is expected to increase. Many competitors and potential competitors of Burcon have substantially greater product development capabilities and financial, scientific, marketing, and human resources than Burcon. Other companies may succeed in developing products earlier than Burcon, obtaining regulatory approvals for such products more rapidly than Burcon or in developing products that are more effective than those proposed to be developed by Burcon. While Burcon will seek to expand its technological capabilities in order to remain competitive, there can be no assurance that research and development by others will not render Burcon's technology or products obsolete or non-competitive.
Lack of Commercial Manufacturing Experience
Burcon has not yet manufactured any products in substantial quantity. To be successful, Burcon's products must be manufactured in commercial quantities in compliance with regulatory requirements and at acceptable cost. In order to manufacture its products in commercial quantities, if it elects to do so, Burcon will need to develop its own manufacturing facilities or contract with third parties to manufacture its products. Developing its own commercial scale manufacturing facilities will require Burcon to raise substantial funds and to retain additional management and technical personnel who have manufacturing experience. No assurance can be given that Burcon will be able to make the transition to commercial production.
Ability to Hire and Retain Key Personnel
Burcon is highly dependent on its senior management and scientific and technical personnel. The competition for qualified personnel in the food industry is intense, and Burcon relies heavily on its ability to attract and retain qualified managerial, scientific and technical personnel. In addition, Burcon's ability to manage growth effectively will require it to continue to implement and improve its management systems and to recruit and train new employees. There can be no assurance that Burcon will be able to attract and retain skilled and experienced personnel.
Reliance on Key Personnel
Burcon is dependent on certain members of its management and the loss of the services of one or more of these individuals could adversely affect the Company. Neither Burcon nor Burcon-MB has purchased key man insurance on behalf of any member of Burcon's and/or Burcon-MB's senior management.
Product Liability
Food products involve an inherent risk of product liability claims and associated adverse publicity. There can be no assurance that Burcon will be able to obtain or maintain product liability insurance on acceptable terms or with adequate coverage against potential liabilities. Such insurance is expensive and may not be available in the future on acceptable terms, or at all. The inability to obtain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims could prevent or inhibit the commercialization of Burcon's potential products.
Nasdaq Listing
On June 8, 2017, the Company received a letter from the Listings Qualifications Department of the Nasdaq Stock Market LLC ("NASDAQ") notifying the Company that it was not in compliance with Listing Rule 5450(b)(2), which requires the listed securities of the Company to maintain a minimum market value of US$50 million. The Company had not met the requirement for a period of 30 consecutive business days prior to receipt of the NASDAQ letter. On August 21, 2017, the Company received a second letter from NASDAQ notifying the Company that it was not in compliance with Listing Rule 5450(a)(1), which requires the listed securities of the Company to maintain a minimum bid price of US$1 per share. The Company had not met the requirement for a period of 30 consecutive business days prior to receipt of the second NASDAQ letter. The receipt of the two NASDAQ letters did not result in the immediate delisting of the Company's common shares from the NASDAQ Global Market. The Company had a compliance period of 180 calendar days or until December 5, 2017 and February 19, 2018, to regain compliance with NASDAQ's minimum market value of listed securities requirement and minimum bid price requirement, respectively. On December 6, 2017, the Company received notification from NASDAQ stating the Company did not meet the December 5, 2017 deadline to regain compliance with NASDAQ's minimum market value of listed securities requirement. NASDAQ stated that the Company's common shares would be delisted from the NASDAQ Global Market at the opening of business on December 15, 2017 unless the Company submitted a request to appeal the determination to the NASDAQ hearing Panel (the "Panel") by December 13, 2017. On the same day, the Company received a further letter from NASDAQ notifying the Company that it was not in compliance with Listing Rule 5450(b)(2)(C), which requires the listed securities of the Company to maintain a minimum market value of publicly held shares of US$15 million. The Company had not met the requirement for a period of 30 consecutive business days prior to receipt of the second NASDAQ letter dated December 6, 2017. The Company submitted an appeal to the Panel together with a plan for regaining compliance with the various continued listing requirements.
On February 5, 2018 the Company received notification from the Panel granting the Panel's approval for the Company to transfer its listing for its common shares from the NASDAQ Global Market to The NASDAQ Capital Market ("NASDAQ Capital Market"). Trading on the Company's common shares on the NASDAQ Capital Market became effective on February 7, 2018. The Panel subjected the continued listing of the Company's shares on the NASDAQ Capital Market to certain conditions, including closing its 2018 Rights Offering (defined below) and having shareholders' equity of over US$2.5 million on or before February 16, 2018. Because the 2018 Rights Offering was not fully subscribed, the Company was required to provide additional submissions in support of its compliance plan. On April 24, 2018, the Company withdrew its appeal of the delisting. The board of directors of Burcon determined that it was in the overall best interest of the Company to withdraw the appeal of the delisting. The decision was based on several factors, including the board's assessment of the probability of the Company regaining compliance with the continued listing requirements, an analysis of the benefits of continued listing weighed against the onerous regulatory burden and significant costs associated with maintaining the continued listing. On April 27, 2018, the Company's common shares were suspended from trading on the NASDAQ Capital Market. The Company filed a Form 25 (Notification of Removal from Listing and/or Registration under Section 12(b) of the Securities Exchange Act of 1934) with the United States Securities and Exchange Commission (the "SEC") on June 4, 2018 to delist the Company's common shares from the NASDAQ Capital Market and to deregister its common shares under Section 12(b) of the Securities Exchange Act of 1934, as amended (the "Exchange Act"). The delisting became effective on June 14, 2018 and the deregistration will become effective ninety days from June 4, 2018. On June 15, 2018, the Company filed a Form 15 with the SEC to suspend its reporting obligations under Section 15(d) of the Exchange Act. The Company's reporting obligations with the SEC were suspended upon the filing of the Form 15 and shall remain suspended for as long as the Company continues to meet the criteria for such suspension on the first day of any subsequent fiscal year. The common shares of Burcon are quoted for trading in the United States on the OTC Pink Open Market operated by OTC Markets Group, under the ticker "BUROF".
The delisting of Burcon's common shares from the Nasdaq Capital Market could negatively impact Burcon because it: (i) could reduce the liquidity, and possibly the market price, of our common shares; (ii) could reduce the number of US investors willing to hold or acquire our common shares, which could negatively impact Burcon's ability to raise equity financing; and (iii) would limit Burcon's ability to use certain types of a registration statements in the United States to offer and sell freely tradable securities, thereby preventing the Company from accessing the US public capital markets.
DIVIDEND RECORD AND POLICY
There are no restrictions that could prevent Burcon from paying dividends provided that Burcon has retained earnings from which such dividends can be paid. Burcon has not declared any dividends on its Common Shares. The Company's directors have determined that dividends will not be paid until a number of years after it receives revenues from the commercial production of its products, and will only be paid if the directors believe that to do so would be in the best interests of the Company and its shareholders.
DESCRIPTION OF CAPITAL STRUCTURE
The authorized share capital of Burcon consists of an unlimited number of Common Shares without par value. Each holder of Common Shares is entitled to one vote in respect of each Common Share held by such holder at meetings of shareholders. In the event of the liquidation, dissolution or winding-up of the Company, whether voluntary or involuntary or any other distribution of its assets among its shareholders, the holders of Common shares will be entitled to receive the remaining property or assets of the Company available for distribution pro rata, in proportion to the number of Common Shares held. As at June 18, 2018, 43,941,536 Common Shares were issued and outstanding. In addition, the Company has 3,595,549 outstanding incentive options to purchase Common Shares. As part of the 2018 Rights Offering, a guarantor received warrants to purchase up to 1,182,099 Common Shares. The issuance of these warrants is subject to shareholder approval, which will be sought at the Company's annual meeting expected to be held in September 2018. The Company also has outstanding warrants to purchase up to 497,677 Common Shares issued to certain guarantors pursuant to the 2016 Rights Offering. See "General Development of the Business".
MARKET FOR SECURITIES
The Common Shares have been listed and trade on the TSX under the symbol "BU" since June 18, 2009 and NASDAQ since October 27, 2011. As disclosed above, the Company's Common Shares were delisted from the NASDAQ Capital Market effective June 14, 2018. See "General Development of the Business". Prior to their listing on the TSX, the Common Shares were listed and traded on the TSXV under the symbol "BU". The following table sets forth, for the periods indicated, the reported high and low closing prices and total volume of trading of the Common Shares on the TSX (Canadian dollars):
| TORONTO STOCK EXCHANGE** |
Calendar Period | High (C$) | Low (C$) | Total Volume |
April 2017 | 2.08 | 1.89 | 386,389 |
May 2017 | 1.99 | 1.30 | 969,657 |
June 2017 | 1.60 | 1.30 | 422,401 |
____________________
** Source: Stockwatch.com
July 2017 | 1.38 | 1.00 | 502,121 |
August 2017 | 1.02 | 0.66 | 632,332 |
September 2017 | 0.89 | 0.61 | 1,608,276 |
October 2017 | 0.79 | 0.63 | 369,593 |
November 2017 | 0.69 | 0.50 | 959,892 |
December 2017 | 0.92 | 0.54 | 2,628,723 |
January 2018 | 0.74 | 0.56 | 1,150,551 |
February 2018 | 0.66 | 0.56 | 1,664,153 |
March 2018 | 0.70 | 0.52 | 575,644 |
PRIOR SALES
During the fiscal year ended March 31, 2018, the Company issued the following common shares:
Number of common shares | Issue Price per common share | Date of Issue | Description of Issuance |
6,114,361 | $0.57 | February 13, 2018 | Common shares issued pursuant to a rights offering circular dated January 5, 2018. |
The following securities convertible into common shares were issued during the fiscal year ended March 31, 2018:
Options
Number of Options | Exercise Price | Date of Issue | Description of Issuance |
452,000 | $0.69 | January 3, 2018 | Options granted pursuant to the Company's Amended and Restated 2001 Share Option Plan. |
Warrants
Number of Warrants | Exercise Price | Date of Issue | Description of Issuance |
1,182,099 | $0.69 | February 13, 2018 | Compensation warrants issued to a guarantor for the standby commitment provided pursuant to the rights offering circular dated January 5, 2018. |
DIRECTORS AND OFFICERS
Directors and Officers
The following chart sets out the name, province or state and country of residence of each director and officer of the Company, each such person's principal occupation during the past five years, the period of time each has served as a director or officer of the Company and the Common Shares beneficially owned or controlled by each of them as at the date of this AIF. A biography of each director and officer, which includes a five year history of employment, follows under "Biographies of Directors and Officers". The term of office of each director will expire at the conclusion of the Company's next annual meeting.
Name, Position and Municipality of Residence | Principal Occupation During the Previous Five Years | Period as a Director of the Company | Common Shares Held | Options Held |
Allan Yap, Chairman of the Board, Chief Executive Officer and Director, Hong Kong, China | Chairman and Chief Executive Officer of Burcon | Since November 3, 1998 | 1,566,719 | 285,844 |
Johann F. Tergesen, President, Chief Operating Officer British Columbia, Canada | President and Chief Operating Officer of Burcon | From November 3, 1998 to September 12, 2012 | 705,913 | 407,659 |
Name, Position and Municipality of Residence | Principal Occupation During the Previous Five Years | Period as a Director of the Company | Common Shares Held | Options Held |
Rosanna Chau, Director,
Hong Kong, China | Director of certain subsidiaries of PT International Development Corporation Limited ("PT International") (formerly known as ITC Corporation Limited) (investment holding); Deputy Chairman and Executive Director of PT International until December 28, 2017 | Since November 3, 1998 | 560,532 | 165,844 |
David Lorne John Tyrrell, Director, Alberta, Canada | Director, Li Ka Shing Institute of Virology & Distinguished University Professor, University of Alberta since April 2010; Glaxo SmithKline Chair in Virology, Department of Medical Microbiology and Immunology, University of Alberta since 2004; Chief Scientific Officer of KMT (biotechnology company in Edmonton) from 2004 to 2017; Professor of Medical Microbiology & Immunology, University of Alberta since 1982 | Since December 1, 2009 | 78,531†† | 185,844 |
____________
†† 1,918 of these Shares are held by Kathleen Tyrrell (daughter) and 3,193 of these Shares are held by spouse, Lee Ann Tyrrell.
Alan Chan, Director, Hong Kong, China | Executive Director of ITC Properties Group Ltd. ("ITC Properties") (property development and investment) since March 2010; Executive Director of PT International (investing holding) from March 2009 to March 2017; Alternate (non-executive) director to Dr. Chan Kwok Keung, Charles of PYI Corporation Limited ("PYI") from July 2010 to September 2014; Executive director of PYI from November 2011 to July 2016; Non-executive director of PYI from July 2016 to April 2017 | Since April 20, 2010 | NIL | 210,844 |
J. Douglas Gilpin, Director, Alberta, Canada | Consultant, providing corporate governance, corporate director and business advisory services | Since September 1, 2011 | NIL | 165,844 |
Peter H. Kappel, Director, British Columbia, Canada | Corporate Director | Since January 28, 2016 | 166,591‡‡ | 82,502 |
____________________
‡‡ 10,292 of these Common Shares are held by Philip Kappel (son) and 31,246 of these Common Shares are held by Stefanie Kappel (spouse).
David Ju, Director, British Columbia, Canada | Real Estate Investment and Development | Since December 21, 2017 | NIL | 30,000 |
Jade Cheng,
Chief Financial Officer and Treasurer,
British Columbia, Canada | Chief Financial Officer and Treasurer of Burcon; Controller of Burcon Group Limited (investment company); Director and President of Burcon Group Limited since July 2007 | n/a | 215,681 | 335,727 |
Randy Willardsen, Senior Vice-President,
Process, California, United States of America | Senior Vice-President, Process of Burcon; President, Willardsen Consulting & Engineering, Inc. (agriculture and biotech industries consulting services) | n/a | 382,008 | 386,659 |
Dorothy Law, Senior Vice-President, Legal, Corporate Secretary,
British Columbia, Canada | Senior Vice-President, Legal of Burcon since September 2009; Corporate Secretary of Burcon since September 2000; Director, Secretary and Corporate Counsel of Burcon Group Limited (investment company) | From December 1998 to April 2010 | 293,502 | 335,727 |
Martin Schweizer, Vice-President, Technical Development, Manitoba, Canada | Vice President, Technical Development of Burcon since September 2009 | n/a | 84,747 | 275,727 |
TOTAL SECURITIES | | | 4,054,224 | 2,868,221 |
Committees
Burcon does not have an executive committee of its directors. Burcon has an audit committee, a corporate governance committee and a nominating and compensation committee. The members of the audit committee consist of J. Douglas Gilpin, David Ju and Peter H. Kappel. The members of the corporate governance committee consist of Lorne Tyrrell, J. Douglas Gilpin and Peter H. Kappel. The members of the nominating and compensation committee consist of Peter H. Kappel, David Ju and Lorne Tyrrell.
Aggregate Ownership of Securities
As at the date of this AIF, directors and officers of Burcon as a group, beneficially owned, directly or indirectly, 4,054,224 or 9.23% of the issued and outstanding Common Shares of the Company. Directors and officers of Burcon and its subsidiaries held options to acquire an additional 2,868,221 Common Shares.
Biographies of Directors and Officers
Allan Yap - Director, Chairman and Chief Executive Officer
Dr. Yap has over 30 years of experience in finance, investment and banking and holds an Honorary degree of Doctor of Laws. Dr. Yap is an executive director and the chairman of Master Glory Group Ltd. and Rosedale Hotel Holdings Limited, both of which are companies whose shares are listed on the main board of The Stock Exchange of Hong Kong Limited. He is the chairman and chief executive officer of China Enterprises Limited, a company whose shares are traded on the OTC Market in the United States of America. He is also the executive chairman of Hanwell Holdings Limited and Tat Seng Packaging Group Ltd., both of which are companies whose shares are listed on the Singapore Exchange Limited.
Johann F. Tergesen - President and Chief Operating Officer
Mr. Tergesen was one of the founding shareholders of Burcon Capital Corp., which acquired B.M.W. Canola Inc. in October of 1999 and subsequently changed its name to Burcon NutraScience Corporation. Mr. Tergesen was a director of Burcon from November 3, 1998 to September 12, 2012. Prior to his role as president and C.O.O. of Burcon, Mr. Tergesen was vice president and treasurer of BurCon Properties Limited, a real estate development and ownership company with assets in excess of $3 billion. Mr. Tergesen has been with the Burcon group of companies since December of 1995. Mr. Tergesen holds a B.A. in economics from the University of Winnipeg, an M.B.A. from McGill University, and is a member of the Chartered Professional Accountants of British Columbia.
Rosanna Chau - Director
Ms. Chau has over 37 years of experience in international corporate management, strategic investments and finance. Throughout her career, she has served on the board of directors of twelve publicly-listed companies spanning multiple industries, including property, hotel, bio-tech, construction and building materials, pharmaceuticals, entertainment, and consumer electronic products, and geographical locations, including Hong Kong, Mainland China, Macau, the United States, Canada, Singapore, Australia and Europe. Ms. Chau holds a Bachelor's Degree in Commerce from the University of Alberta and a Master's Degree in Commerce from the University of New South Wales and has been awarded the Certificate in Traditional Chinese Medicine: A Way to Health at the Chinese University of Hong Kong. She has professional accounting qualifications and experience in different jurisdictions and is a fellow member of the Hong Kong Institute of Certified Public Accountants and the CPA Australia and a member of the Chartered Professional Accountants of British Columbia.
David Lorne John Tyrrell - Director
D. Lorne Tyrrell is the Glaxo SmithKline Chair in Virology in the Department of Medical Microbiology and Immunology at the University of Alberta. Since 1986, he has focused his research on viral hepatitis. Supported by Canadian Institute of Health Research and Glaxo Canada, Dr. Tyrrell's work on the development of antiviral therapy resulted in the licensing of the first oral antiviral agent to treat chronic hepatitis B infection - lamivudine - in 1998. Dr. Tyrrell holds more than 50 international patents for his studies on viral hepatitis. Dr. Tyrrell was Dean of the Faculty of Medicine and Dentistry from 1994 - 2004 at the University of Alberta and is currently the Chair of the Board of Directors of the Gairdner Foundation. The Canada Gairdner International Awards recognizes excellence in medical science research globally. Dr. Tyrrell has received numerous prestigious awards including the Gold Medal of the Canadian Liver Foundation (2000), the FNG Starr Award of the Canadian Medical Association (2004), the Principal Award of the Manning Awards Foundation (2005) and the Queen Elizabeth II Diamond Jubilee Medal (2012). Dr. Tyrrell was appointed Officer of the Order of Canada in 2002. In April 2010, Dr. Tyrrell was appointed as the inaugural director of the Li Ka Shing Institute of Virology at the University of Alberta. On April 28, 2011, Dr. Tyrrell was inducted to the Canadian Medical Hall of Fame. In 2015, he was awarded the Canada Council for the Arts Killam Prize in Health Sciences.
Alan Chan - Director
Mr. Chan is an executive director of ITC Properties Group Limited ("ITC Properties"). At ITC Properties, Mr. Chan is involved with the investment and development of commercial, hospitality and residential projects. In addition, he is the lead in developing new policies for green and sustainable practices throughout the group. Mr. Chan is also currently an advisor to BEE Inc. (Bisagni Environmental Enterprise). Prior to joining ITC Properties, Mr. Chan worked in the Investment Banking Division of Goldman Sachs Group with a focus on capital raising, mergers & acquisitions and strategic advisory for financial institutions in Greater China and Southeast Asia. Mr. Chan is a graduate of Duke University majoring in Political Science - International Relations and minoring in Philosophy and Economics.
J. Douglas Gilpin - Director
Douglas Gilpin, FCPA, FCA, ICD.D., retired from the partnership of KPMG LLP in 1999. In 2008, Mr. Gilpin received a Life Service Award from The Institute of Chartered Accountants of Alberta in recognition of 40 years experience in delivering professional services to business and the community. During his 18 years tenure as a partner in Advisory Services at KPMG he served as an audit engagement partner. He was a member of the KPMG's National Quality Assurance, the National Financial Institutions and Insurance Groups and he was the Quality Assurance Partner for the Edmonton office for 10 years. Mr. Gilpin has consulted on corporate governance, including compliance with the Sarbanes Oxley Act 404 and National Instrument 52-109 reporting for issuers listed on the TSX. Mr. Gilpin has served as a director of Canada Health Infoway ("CHI"), Afexa Life Sciences Inc. ("Afexa"), Alberta Innovates (formerly Alberta Innovates Technology Futures) ("AITF") and on the Board of the Health Quality Council of Alberta ("HQCA"). He was the chair of the governance committee and a member of the audit committee of CHI, and was the Chair of the audit committee for each of AITF and HQCA. He currently is a director and chair of the audit committee of The Institute of Health Economics. Mr. Gilpin is executive chair of The Inspections Group Inc., a privately owned company that performs safety code inspection services, building, plumbing and gas and electrical inspections in compliance with the Safe Codes Act of Alberta. Mr. Gilpin is a member of the Institute of Corporate Directors and received his ICD.D. designation from the Institute in 2011. Mr. Gilpin was elected as Fellow of the Institute of Chartered Accountants of Alberta in 2012.
Peter H. Kappel - Director
Mr. Kappel is a former investment banker who now manages a private investment portfolio. A former chartered accountant with KPMG in Vancouver and Frankfurt, he made the transition to investment banking with JP Morgan (New York/Frankfurt) after business school. He also served in senior roles at Nomura, Dresdner Kleinwort Wasserstein, Calyon and DVB Bank in London. In the latter three, he was the Managing Director in charge of their respective European Securitisation businesses. He was responsible for many ground breaking transactions, a regular speaker at ABS conferences and a founding and former executive committee member of the European Securitisation Forum of the Bond Market Association. He holds an MBA from the Institut Européen d'Administration des Affaires ("INSEAD"), a Bachelor of Arts (Honours) degree in Economics from the University of Victoria and received his Chartered Accountant designation through the Institute of Chartered Accountants of British Columbia. Mr. Kappel is on the Board of Partnerships British Columbia, where he serves as Audit Committee Chair.
David Ju - Director
Mr. Ju is a Vice President of Concord Pacific Group, a Canadian multi-industry investment group with a diverse portfolio of businesses in Canada, the United Kingdom and Hong Kong. Mr. Ju has extensive experience in financial services and large-scale real estate development projects in both the United States and Canada. A Chartered Accountant by training, Mr. Ju was a former member of PricewaterhouseCoopers in Vancouver and New York, where he consulted on transactions, and advised on corporate governance and assurance matters, including compliance with the Sarbanes Oxley Act 404 for clients listed on both the Toronto Stock Exchange as well as the New York Stock Exchange. Mr. Ju holds a Bachelor's Degree in Business Administration from Simon Fraser University and is a member of the Chartered Professional Accountants of British Columbia.
Jade Cheng - Chief Financial Officer and Treasurer
Ms. Cheng has been with the Burcon group of companies since July 1995, holding various senior financial positions, including Vice-President, Group Audit and Corporate Secretary of BurCon Properties Limited until its merger with its subsidiary, Oxford Properties Group Inc., in May 1998. Prior thereto, Ms. Cheng was a Manager with the General Practice group of the Vancouver office of Coopers & Lybrand (now PricewaterhouseCoopers LLP), Chartered Accountants. Ms. Cheng holds a Bachelor's Degree in Economics and a Master's Degree in Business Administration from the University of British Columbia and is a member of the Chartered Professional Accountants of British Columbia.
Randy Willardsen - Senior Vice-President, Process
Mr. Willardsen has over 31 years of experience in the fields of membrane filtration and food, dairy and biotechnology processes. Mr. Willardsen was the founder of Separation Technology, Inc., a leading supplier of membrane-based purification equipment and related services to the food industry with particular emphasis on dairy and beverage applications. Mr. Willardsen was also co-founder of both Inprotech Corporation, a supplier of high quality whey proteins to the U.S. market, and BioPlex Nutrition, a nutritional supplement company focused on formulated protein supplements. With BioPlex, he served as technical director, and oversaw manufacturing of all products until the company was sold in 1999. Most recently, Mr. Willardsen founded Gallo Protein, a partnership with Joseph Gallo Farms to produce highly purified whey protein isolates.
Mr. Willardsen has worked with Burcon since April of 2001, and holds a Masters degree in Food Science and Nutrition from the University of Minnesota.
Dorothy Law - Senior Vice-President, Legal and Corporate Secretary
Ms. Law was a director of Burcon from December 1998 to April 2010. Ms. Law joined the Burcon group of companies in August 1997 and acted as corporate counsel. Prior thereto, she was an associate at the law firm of Lang Michener LLP (now McMillan LLP), practising primarily in the areas of securities, corporate and commercial law. Ms. Law was called to the British Columbia Bar and admitted as a member of the Law Society of British Columbia in August 1996. Ms. Law holds a Bachelor of Laws degree and a Bachelor of Commerce degree from the University of British Columbia. Ms. Law was also admitted as a solicitor of the High Court of Hong Kong in May 1999. She is a non-practising member of the Law Society of Hong Kong.
Martin Schweizer - Vice President, Technical Development
Dr. Schweizer joined Burcon in May 2002 as a process-engineering specialist. He relocated from Nancy, France, where he earned his doctorate at the Institut National Polytechnique de Lorraine, with an emphasis on the enzymatic hydrolysis of rapeseed proteins. Prior to his Ph.D. work, Dr. Schweizer completed a chemical engineering degree (Dipl.-Ing) at the University of Karlsruhe, Germany, where he specialized in food process engineering and water technology. He has over 20 years of experience in research and development and his main expertise lies in the fractionation and purification of biochemical compounds using current state of the art technology such as membrane filtration, liquid chromatography and various extraction technologies, both aqueous and solvent based. Since January 2003, Dr. Schweizer has overseen Burcon's research and development efforts at its Winnipeg Technical Centre.
Cease Trade Orders, Bankruptcies, Penalties or Sanctions
Other than as set out below, none of the directors or executive officers:
a) is, as at the date of the AIF, or was within 10 years before the date of the AIF, a director or chief executive officer or chief financial officer of any company (including Burcon) that:
i) was the subject of an order (as defined in National Instrument 51-102F2) that was issued while the director or executive officer was acting in the capacity as director, chief executive officer or chief financial officer; or
ii) was subject to an order that was issued after the director or executive officer ceased to be a director, chief executive officer, or chief financial officer, and which resulted from an event that occurred while that person was acting in the capacity as a director, chief executive officer, or chief financial officer.
Except as set out below, none of the directors, executive officers or a shareholder holding a sufficient number of securities of the Company to affect materially the control of the Company,
a) is at the date hereof, or has been within 10 years before the date of this AIF, a director or executive officer of any company (including Burcon) that while that person was acting in that capacity, or within a year of that person ceasing to act in that capacity, became bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency or was subject to or instituted any proceedings, arrangement or compromise with creditors or had a receiver, receiver manager or trustee appointed to hold its assets;
b) has, within the 10 years before this AIF, become bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency, or become subject to or instituted any proceedings, arrangement or compromise with creditors, or had a receiver, receiver manager or trustee appointed to hold the assets of the director, executive officer or shareholder;
c) has been subject to any penalties or sanctions imposed by a court relating to securities legislation or by a securities regulatory authority or has entered into a settlement agreement with a securities regulatory authority; or
d) has been subject to any other penalties or sanctions imposed by a court or regulatory body that would likely be considered important to a reasonable investor in making an investment decision.
Ms. Rosanna Chau was the Deputy Chairman and Executive Director of PT International (formerly ITC Corporation Limited ("ITC")), a company whose shares are listed on The Stock Exchange of Hong Kong Limited, up to December 28, 2017. On November 15, 2005 the Securities and Futures Commission (the "SFC") of Hong Kong criticized the board of directors of ITC for breaching Rule 21.3 of the Code on Takeovers and Mergers (the "Takeovers Code") in respect of the dealing in the securities of Hanny Holdings Limited ("Hanny", now known as Master Glory Group Limited) by ITC during an offer period without the consent of the Executive Director of the Corporate Finance Division of the SFC. Rule 21.3 of the Takeovers Code restricts share dealings and transactions by an offeror and parties acting in concert with it during securities exchange offers. Hanny was involved in a securities exchange offer announced in April 2005. Since ITC held over 20% of the shares of Hanny, it was presumed to be acting in concert with Hanny under the Takeovers Code. Ms. Rosanna Chau was a director of ITC at that time.
Mr. J. Douglas Gilpin was a director of ViRexx Medical Corp. ("ViRexx") until his resignation from the board of directors of ViRexx on September 14, 2008. On November 18, 2008, trading in ViRexx shares was suspended by the Alberta Securities Commission for failure to file interim unaudited financial statements, interim management discussion and analysis, and certification of interim filings for the interim period ended September 30, 2008. Similar cease trade orders were issued by the securities regulatory authorities of British Columbia, Ontario and Quebec. On November 21, 2008, ViRexx announced that on October 16, 2008, ViRexx filed a Notice of Intention to make a proposal to its creditors pursuant to the Bankruptcy and Insolvency Act (Canada). On December 11, 2008, ViRexx announced the granting of a court order to reorganize ViRexx and to approve the proposal in the proposal proceedings under the Bankruptcy and Insolvency Act (Canada) and the Business Corporations Act (Alberta). On December 23, 2008, ViRexx announced that in accordance with the order for reorganization in such proposal proceedings, Paladin Labs Inc. had become the sole shareholder of ViRexx and that ViRexx would be taking steps necessary to cease being a reporting issuer in Canada and the United States.
Conflicts of Interest
Ms. Rosanna Chau was the Deputy Chairman and Executive Director of PT International up to December 28, 2017 and Mr. Alan Chan was an executive director of PT International until March 2017. Mr. Chan is the son of Dr. Chan Kwok Keung, Charles, who held approximately 67.96% of the issued share capital of PT International until January 24, 2017. PT International indirectly owns, as at the date of this AIF, approximately 22.45% of the issued and outstanding shares of Burcon.
In connection with the Note as described in "General Development of the Business", the Lender is a wholly-owned subsidiary of PT International who in turn is an insider and related party of Burcon. The issuance of the Note to the Lender (the "Transaction") is considered a "related party transaction" pursuant to Multilateral Instrument 61-101 - Protection of Minority Security Holders in Special Transactions ("MI 61-101") and the following disclosure is provided in accordance with s. 5.2 thereof.
The material terms of the Transaction are summarized in the section "General Development of the Business". The purpose and business reason for the Transaction was to raise funds so that Burcon could continue to meet its working capital requirements and use the funds for the purposes described above. PT International currently holds 9,866,568 Common Shares, representing approximately 22.45% of the Common Shares outstanding as of June 18, 2018. Assuming full conversion of the Principal Amount at the Conversion Price, PT International would acquire, indirectly through the Lender, an additional 507,614 Common Shares or 1.15% of the Common Shares outstanding as of June 18, 2018. The Purchase Agreement and the issuance of the Note was unanimously approved by the directors of Burcon present at a board meeting held on March 30, 2016, with Mr. Alan Chan and Ms. Rosanna Chau, who were also directors of PT International at the time, abstaining from participating in the vote. Other than the Purchase Agreement and the Note to be issued thereunder described above, Burcon has not entered into any agreement with an interested party or a joint actor with an interested party (as such terms are defined in MI 61-101) in connection with the Transaction.
Burcon relied on the exemptions available under s. 5.5(a) and s. 5.7(a) of MI 61-101 from the formal valuation and minority shareholder approval requirements, respectively, on the basis that at the time the Transaction was agreed to (that is, April 7, 2016), the fair market value of the Note (being $2,000,000) did not exceed 25% of Burcon's market capitalization (being $115,739,782.50 based on 35,832,750 Common Shares outstanding as of April 7, 2016 and the five day VWAP of the Common Shares on the TSX immediately prior to April 7, 2016 of $3.23).
The Company rents its head office premises from and shares certain office equipment with Burcon Group Limited ("Burcon Group"), an indirect wholly-owned subsidiary of PT International. Jade Cheng and Dorothy Law, officers of the Company, are the directors of Burcon Group. During fiscal 2018, Burcon paid $76,299 (2017 - $80,241) to Burcon Group for the rental charges. In addition, administrative services are contracted through a management agreement with Burcon Group. For the year ended March 31, 2018, Burcon was charged $520 (2017 - $1,488) by Burcon Group for these services. From April 1, 2017 to March 31, 2018, professional services provided by Burcon to Burcon Group provided income to Burcon of $13,188 (2017 - $14,446). These transactions, occurring in the normal course of operations, are measured at the exchange amount, which is the amount of consideration established and agreed to by the related parties.
TRANSFER AGENTS AND REGISTRARS
The Company's co-transfer agents and co-registrars for its Common Shares are Computershare Investors Services Inc. at its principal transfer offices in Vancouver, British Columbia and Toronto, Ontario and Computershare Trust Company N.A. at its principal transfer office in Denver, Colorado.
MATERIAL CONTRACTS
Burcon is a party to the following material contracts, copies of which are available on SEDAR at www.sedar.com:
Standby Commitment Agreement with Allan Yap
On January 5, 2018, Burcon entered into a standby commitment agreement with Allan Yap in connection with the 2018 Standby Commitment. See "General Development of the Business".
Standby Commitment Agreement with PT International and Allan Yap
On October 24, 2016, Burcon entered into a standby commitment agreement with PT International and Allan Yap in connection with the 2016 Standby Commitment. See "General Development of the Business".
Convertible Note Purchase Agreement with Large Scale Investments Limited
On April 7, 2016, Burcon announced that it had entered into the Purchase Agreement with Large Scale Investments Limited (the "Lender"), a wholly-owned subsidiary of PT International, pursuant to which it will issue the Note for the Principal Amount.
Funding by the Lender and the issuance of the Note occurred on May 12, 2016. The Note bears interest at a rate of 8% per annum, calculated daily, compounded monthly. Interest will accrue on the Principal Amount and will be payable on the earlier of three years from the date of issuance of the Note, the occurrence of an event of default as set out in the Note or voluntary prepayment by Burcon (the "Maturity Date").
The Lender may convert the Principal Amount in whole or in part into common shares in the capital of Burcon at any time commencing on or after July 1, 2016 and up to and including the Maturity Date at a conversion price of $4.01 per common share (the "Conversion Price"), subject to adjustment in certain circumstances. The Conversion Price represents a premium of approximately 24% over the volume weighted average trading price ("VWAP") of the common shares on the Toronto Stock Exchange (the "TSX") for the 5 trading days immediately before April 7, 2016. Burcon also has the right, before the Maturity Date, upon written notice to the Lender of not less than 30 days, to prepay in cash all or any portion of the Principal Amount by paying to the Lender an amount equal to the Principal Amount to be prepaid multiplied by 110%. At any time on or after July 1, 2016 and up to the end of such 30-day notice period, the Lender will have the right to convert the Principal Amount in full or in part, into common shares at the Conversion Price. The Note and any common shares issued upon the conversion of the Note will be subject to a four month hold period under applicable Canadian securities laws. Upon completion of the 2018 Rights Offering, the Conversion Price of the Note was adjusted effective immediately after the 2018 Record Date. After the adjustment, the Conversion Price was reduced to $3.94 per common share.
The payment of the Principal Amount and all accrued and unpaid interest thereon will be subordinated in right of payment to any amount owing in respect of secured indebtedness of Burcon. Subject to prior TSX approval and the consent of the Lender, Burcon may pay any interest that is due and payable under the Note through the issuance of common shares at a conversion price equal to the VWAP of the common shares on the TSX for the 5 trading days immediately prior to the date such interest is due and payable.
Standby Commitment Agreement with PT International, E-Concept and I-Global
On March 23, 2015, Burcon entered into a standby commitment agreement with PT International, E-Concept and I-Global in connection with the 2015 Standby Commitment. See "General Development of the Business".
License and Production Agreement with ADM
On March 4, 2011, Burcon and Burcon's wholly-owned subsidiary, Burcon NutraScience (MB) Corp. (together referred to for this section only, as "Burcon") entered into a license and production agreement (the "License and Production Agreement") with ADM. Pursuant to the License and Production Agreement, Burcon has granted to ADM an exclusive, royalty-bearing, worldwide license (the "License") to use and exploit Burcon's soy protein technology solely to make, have made, use, market and sell soy protein products (the "Soy Products") that use, incorporate or are derived from any Burcon technology. ADM has agreed to use reasonable commercial efforts to design, build and commission an initial production facility (the "Semi-works Production Facility") within a specified amount of time after it receives permit approval from the US Environmental Protection Agency ("EPA Approval Date") to manufacture the Soy Products. ADM will also, within a time specified under the License and Production Agreement, provide written notice to Burcon to advise whether it will or will not increase its annual production capacity of the Products beyond the capacity of the Semi-works Production Facility ("Full Commercial Production"). The License and Production Agreement provides each party the right to convert the exclusive license to a non-exclusive license under certain conditions.
In consideration of the License, ADM will pay to Burcon running royalties based on the net revenue (as defined in the License and Production Agreement) in relation to the sale of the Soy Products which fall within the scope of the Burcon Technology. ADM will pay quarterly royalties (the "Pre-production Royalty") to Burcon from the EPA Approval Date until the first bona fide arms' length sale of Products manufactured in the Semi-works Production Facility. Once such sale in the Semi-works Production Facility occurs, ADM will pay to Burcon royalties based on a percentage of net revenue from the sale of Products (the "Semi-works Production Royalty"). If ADM expands production to Full Commercial Production the royalty rate will be reduced to a lower percentage rate (the "Full Commercial Production Royalty"). The Full Commercial Production Royalty rate will be further reduced if ADM expands production and sale into certain geographic regions or if ADM achieves a pre-defined further expanded level of production capacity. The Semi-works Production Royalty and the Full Commercial Production Royalty rates may also be reduced if the exclusive license is converted to a non-exclusive license or if certain Burcon patents do not grant within a specified time.
If the exclusive license is converted to a non-exclusive license, Burcon will be entitled to make, have made, use, market and sell the Soy Products on a non-exclusive basis and to grant any such rights to any other person. ADM will grant to Burcon an irrevocable, non-exclusive, royalty bearing license, with a right to sublicense, to use ADM Improvements (as defined in the License and Production Agreement) to make, have made, use, market or sell the Soy Products worldwide. If the license is converted to a non-exclusive license and Burcon chooses to use ADM improvements, the aggregate royalties payable by Burcon to ADM in any year will not exceed the aggregate royalties payable by ADM to Burcon in the same year.
Under the License and Production Agreement, Burcon will be responsible for filing, prosecution and maintenance of Burcon patent rights in certain countries. Burcon will also be responsible for defending any action in which the validity of any Burcon patent right is raised in any jurisdiction.
Royalties payable under the License will terminate on the later of the date of expiry of the last to expire of the Burcon Patent Rights (as defined in the License and Production Agreement) and twenty years from March 4, 2011. Since March 4, 2011, Burcon has filed additional patent applications to seek important commercial protection for the production and use of CLARISOY®. ADM has elected to include these applications to the License and, if granted could lengthen the royalty term under the License and Production Agreement to at least the year 2035.
INTERESTS OF EXPERTS
The Company's auditors are PricewaterhouseCoopers LLP, Chartered Professional Accountants, who have prepared an independent auditor's report dated June 18, 2018 in respect of the Company's consolidated financial statements as at March 31, 2018 and March 31, 2017 and for the years then ended. PricewaterhouseCoopers LLP has advised that they are independent with respect to the Company within the meaning of the Chartered Professional Accountants of British Columbia code of Professional Conduct.
AUDIT COMMITTEE AND DISCLOSURE UNDER NATIONAL INSTRUMENT 52-110
Under National Instrument 52-110 ("NI 52-110"), Burcon is required to disclose in its AIF certain information concerning the composition of its audit committee and its auditor. The audit committee carries out the various responsibilities set forth in its charter, a copy of which is attached to this AIF as Schedule "A".
Composition of the Audit Committee
The audit committee of Burcon is comprised of J. Douglas Gilpin, David Ju and Peter H. Kappel. Mr. Gilpin is the chair of the audit committee. All members of the audit committee are financially literate. Under NI 52-110, an individual is "financially literate" if he has the ability to read and understand a set of financial statements that present a breadth and level of complexity of accounting issues that are generally comparable to the breadth and complexity of the issues that can reasonably be expected to be raised by Burcon's financial statements. The Board has determined that J. Douglas Gilpin, a member of the audit committee, qualifies as an audit committee financial expert as that term is defined by the NASDAQ listing standards applicable to the Company during fiscal 2018. Mr. Gilpin was an audit engagement partner in Advisory Services at KPMG LLP (chartered accountants) for 18 years until 1999. Mr. Kappel holds a Bachelor of Arts (Honours) degree in Economics from the University of Victoria and received his Chartered Accountant designation through the Institute of Chartered Accountants of British Columbia. Mr. Kappel is on the Board of Partnerships British Columbia, where he serves as Audit Committee Chair. Mr. Ju became a member of the audit committee on January 3, 2018. Mr. Ju is a Chartered Accountant and is a member of the Chartered Professional accountants of British Columbia. A member of the audit committee is "independent" if the member has no direct or indirect material relationship with Burcon, which could, in the view of Burcon's board of directors, reasonably interfere with the exercise of a member's independent judgement. All the members of the audit committee are independent. The Board has determined that each of the audit committee members is independent, as that term is defined by the NASDAQ listing standards applicable to the Company during fiscal 2018.
Audit Committee Oversight
During the most recently completed financial year, all recommendations of the audit committee with respect to financial reporting and to nomination or compensation of Burcon's external auditor were adopted by the board of directors.
Pre-Approval Policies and Procedures
The charter of the audit committee requires pre-approval of non-audit services provided by the external auditor of Burcon. The auditor was engaged to provide certain tax return review services during the years ended March 31, 2018 and 2017. These services were pre-approved by the audit committee.
External Auditor Service Fees
Fees billed by PricewaterhouseCoopers LLP ("PwC") to Burcon for professional services relating to the last two fiscal years are outlined in the following table.
Nature of Services | Fees billed by auditor for the fiscal year ended March 31, 2018 | Fees billed by auditor for the fiscal year ended March 31, 2017 |
Audit Fees1 | $66,000 | $66,000 |
Audit-Related Fees2 | $59,500 | $47,000 |
Tax Fees3 | $7,398 | $5,000 |
All Other Fees4 | Nil | Nil |
Total | $132,898 | $118,000 |
Notes:
(1) "Audit Fees" include the aggregate fees billed by PwC relating to the respective fiscal year. Included in Fiscal 2018's and Fiscal 2017's Audit Fees are additional fees billed for services rendered in connection with the new IFRS Standards and the Note, respectively.
(2) "Audit-Related Fees" include the aggregate fees billed for the respective fiscal year for assurance and related services by PwC that are not reported under "Audit Fees". Of the $59,500 incurred in Fiscal 2018, $47,000 related to quarterly reviews of each of the Company's interim financial statements and $12,500 related to services rendered in connection with the 2018 Rights Offering. Fiscal 2017's fees related to quarterly reviews of the Company's interim financial statements.
(3) "Tax Fees" include the aggregate fees billed for the respective fiscal year for professional services rendered by PwC for tax compliance and tax advice.
(4) "All Other Fees" include the aggregate fees billed for the respective fiscal year for products and services provided by PwC, other than the services reported under "Audit Fees", "Audit-Related Fees" and "Tax Fees".
ADDITIONAL INFORMATION
Additional information relating to Burcon can be found on the SEDAR website at www.sedar.com.
Additional information, including directors' and officers' remuneration and indebtedness, principal holders of Burcon's securities and securities authorized for issuance under equity compensation plans, is contained in Burcon's Management Proxy Circular dated July 21, 2017 for its most recent annual meeting of shareholders that involved the election of directors.
Additional financial information is provided in Burcon's financial statements and MD&A for its most recently completed financial year ended March 31, 2018.
GLOSSARY
In this AIF, the following terms have the meanings set forth herein:
ADM | Archer-Daniels-Midland Company |
| | |
Affiliate | has the meaning set out in the Canada Business Corporations Act |
| | |
albumin | refers generally to any protein with water solubility, which is moderately soluble in concentrated salt solutions, and experiences heat coagulation (protein denaturation). Substances containing albumin, such as egg white, are called albuminoids |
| | |
BMW | B.M.W. Canola Inc. |
| | |
Board | the Board of Directors of the Company |
| | |
Burcon or Company | Burcon NutraScience Corporation |
| | |
Burcon-MB | Burcon Nutrascience (MB) Corp. (formerly B.M.W. Canola Inc.) |
| | |
CLARISOY® | the trade-marked brand name for Burcon's soy protein. CLARISOY® is a trademark of ADM and is licensed to Burcon |
Common Share or Shares | a common share in the capital of the Company |
| | |
E-Concept | E-Concept Limited |
| | |
erucic acid | fatty acids contained in canola, but not considered essential for human growth |
| | |
FAO | the Food and Agricultural Organization |
| | |
FDA | the United States Food and Drug Administration |
| | |
globulin | this generic term encompasses a heterogeneous series of families of proteins, with larger molecules and less soluble in pure water than albumin. Globular proteins, or spheroproteins are one of the two main protein classes, comprising "globe"-like proteins that are more or less soluble in aqueous solutions (where they form colloidal solutions) |
| | |
glucosinolates | the sulphur compounds that give mustards their sharp taste |
| | |
GM | genetically modified |
| | |
GMO | genetically modified organism |
| | |
GRAS | generally recognized as safe |
| | |
I-Global | I-Global Ltd. |
| | |
ITC | ITC Corporation Limited (now PT International Development Corporation Limited) |
| | |
License and Production Agreement | The License and Production Agreement dated March 4, 2011 made among Burcon, Burcon-MB and ADM |
| | |
NASDAQ | Nasdaq Stock Market LLC |
| | |
NASDAQ Global Market | The NASDAQ Global Market |
| | |
NASDAQ Capital Market | The NASDAQ Capital Market |
| | |
Nutratein® | the trade-marked brand name for Burcon's blended cruciferin-rich and napin-rich canola protein isolates |
PDCAAS | protein digestibility corrected amino acid score |
| | |
Peazac® | the trade-marked brand name for one of Burcon's pea protein |
| | |
Peazazz® | the trade-marked brand name for Burcon's pea protein isolate |
| | |
PT International | PT International Development Corporation Limited (formerly ITC Corporation Limited) |
| | |
Puratein® | the trade-marked brand name for Burcon's cruciferin-rich canola protein isolate |
| | |
Supertein® | the trade-marked brand name for Burcon's napin-rich canola protein isolate |
| | |
TSX | Toronto Stock Exchange |
| | |
TSXV | TSX Venture Exchange |
| | |
US or United States | the United States of America |
| | |
Winnipeg Technical Centre | the premises where Burcon's research laboratory and pilot plant operations are located |
SCHEDULE "A"
BURCON NUTRASCIENCE CORPORATION ("BURCON")
AUDIT COMMITTEE CHARTER
General Functions, Authority and Role
The purpose of the audit committee is to oversee the accounting and financial reporting process of Burcon and the audits of its financial statements, and thereby assist the Board of Directors of Burcon in monitoring (1) the integrity of the financial statements of Burcon, (2) compliance by Burcon with legal and regulatory requirements related to financial reporting, (3) the performance of Burcon's external auditors, and (4) the performance of Burcon's internal controls and financial reporting process.
The audit committee has the power to conduct or authorize investigations into any matters within its scope of responsibilities, with full access to its auditors and its legal advisors and to all books, records, facilities and personnel of Burcon. In connection with such investigations or otherwise in the course of fulfilling its responsibilities under this charter, the audit committee has the authority to independently retain special legal, accounting, or other consultants to advise it, and may request any officer or employee of Burcon, its independent legal counsel or independent auditor to attend a meeting of the audit committee or to meet with any members of, or consultants to, the audit committee. Burcon shall provide appropriate funding, as determined by the audit committee, for payment of (i) compensation to the external auditor to prepare and issue an audit report or perform other audit, review or attest services for Burcon, (ii) compensation to any outside advisors employed by the audit committee, and (iii) ordinary administrative expenses of the audit committee that are necessary or appropriate in carrying out the audit committee's duties.
Burcon's independent auditor is ultimately accountable to the audit committee, who, in its capacity as a committee of Burcon's board of directors, is directly responsible for appointing, retaining and determining the appropriate compensation of the external auditor. The audit committee must also oversee the work of the external auditor engaged for the purpose of preparing or issuing an audit report or performing other audit, review or attest services for Burcon, including resolution of disagreements between management and the external auditor regarding financial reporting. The external auditor must report directly to the audit committee. In the course of fulfilling its specific responsibilities hereunder, the audit committee must maintain free and open communication between Burcon's external auditors, Board of Directors and Burcon management. The responsibilities of a member of the audit committee are in addition to such member's duties as a member of the Board of Directors.
Membership
The audit committee of the board of directors of Burcon shall consist of a minimum of three directors. Members of the audit committee shall be directors appointed by the board of directors and may be removed by the board of directors at its discretion.
All members of the audit committee shall satisfy the independence and audit committee composition requirements of all applicable corporate and securities laws and stock exchange listing standards; provided, however, that one or more members may be non-independent within the meaning of all applicable regulations.
All members of the audit committee must be financially literate, i.e. have the ability to read and understand a set of financial statements that present a breadth and level of complexity of accounting issues that are generally comparable to the breadth and complexity of the issues that can reasonably be expected to be raised by Burcon's financial statements. At least one member shall be a financial expert.
The members of the audit committee shall elect, amongst themselves, one member to act as chairperson on an annual basis.
Responsibilities
The audit committee is responsible for:
- reviewing Burcon's interim and annual financial statements and management's discussion and analysis related thereto, and all annual and interim earnings press releases before they are publicly disclosed;
- ensuring that adequate procedures are in place for the review of Burcon's public disclosure of financial information extracted or derived from Burcon's financial statements, other than management's discussion and analysis and annual and interim earnings press releases, and periodically reviewing and updating such procedures;
- establishing procedures for the receipt, retention and treatment of complaints received by Burcon regarding accounting, internal accounting controls, or auditing matters;
- establishing procedures for the confidential, anonymous submission by employees of Burcon of concerns regarding questionable accounting or auditing matters;
- reviewing and approving Burcon's hiring policies regarding partners, employees and former partners and employees of the present and former auditor of Burcon;
- reviewing with management, Burcon's major financial risk exposures and the steps management has taken to monitor and control such exposures;
- assessing risk areas and policies to manage risk;
- overseeing the work of Burcon's external auditors engaged for the purpose of preparing or issuing an audit report or related work;
- ensuring Burcon's external auditors report directly to the audit committee throughout the term of their appointment;
- ensuring Burcon's external auditors provide a formal written statement delineating all relationships between the external auditor and Burcon, actively engaging in a dialogue with the external auditor with respect to any disclosed relationships or services that may impact the objectivity and independence of the external auditor and for taking, or recommending that Burcon's full board of directors take, appropriate action to oversee the independence of the external auditor;
- pre-approving all non-audit services to be provided to Burcon or Burcon's subsidiaries by Burcon's external auditor;
- recommending to Burcon's board of directors the external auditor to be nominated for the purpose of preparing or issuing an auditor's report (or any related work), as well as the compensation to be paid to the external auditor;
- annually reviewing and reassessing the adequacy of this charter and recommend any proposed changes to the Board of Directors for approval;
- reporting committee actions to the Board of Directors with such recommendations as the committee may deem appropriate; and
- providing copies of meeting of the audit committee to the Board of Directors.
The audit committee does the following main things to discharge these responsibilities:
- meeting with management and the external auditors at least two times per year;
- meeting separately with each of management and the external auditors several times per year as required;
- reviewing and approving the annual audit scope and the annual audit plan proposed by the auditors;
- reviewing carefully and acting on all internal control points raised by the auditors in correspondence with management; and
- discussing Burcon's compliance with tax and financial reporting rules as issues arise.


