Table of Contents
SEC File No. 333-155787
The information in this preliminary prospectus supplement is not complete and may be changed. This preliminary prospectus supplement and the accompanying prospectus are not an offer to sell these securities, and we are not soliciting an offer to buy these securities, in any jurisdiction where the offer or sale is not permitted.
Preliminary Prospectus Supplement dated June 22, 2009
(to Prospectus dated November 28, 2008)

| PER SHARE | TOTAL | |||||||
| Public offering price | $ | $ | ||||||
| Underwriting discounts and commissions | $ | $ | ||||||
| Proceeds to Halozyme (before expenses) | $ | $ | ||||||
| iii | ||||
| iii | ||||
| S-1 | ||||
| S-7 | ||||
| S-19 | ||||
| S-20 | ||||
| S-21 | ||||
| S-22 | ||||
| S-23 | ||||
| S-27 | ||||
| S-30 | ||||
| S-32 | ||||
| S-32 | ||||
| S-32 | ||||
| S-33 | ||||
Prospectus | ||||
| 1 | ||||
| 1 | ||||
| 5 | ||||
| 19 | ||||
| 20 | ||||
| 20 | ||||
| 20 | ||||
| 25 | ||||
| 34 | ||||
| 36 | ||||
| 38 | ||||
| 42 | ||||
| 43 | ||||
| 44 | ||||
| 44 | ||||
| 44 | ||||
| 45 | ||||
ii
Table of Contents
iii
Table of Contents
S-1
Table of Contents
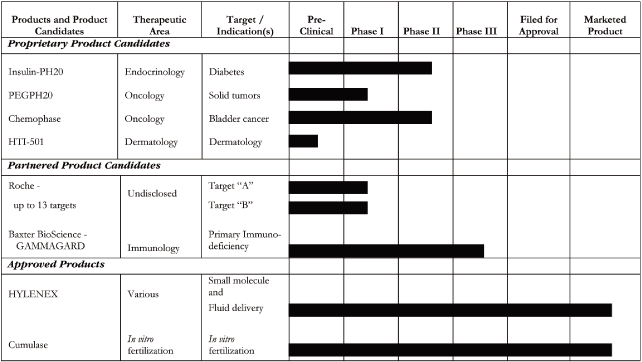
S-2
Table of Contents
S-3
Table of Contents
S-4
Table of Contents
S-5
Table of Contents
Common stock offered by us | 5,200,000 shares | |
Common stock outstanding after the offering | 88,416,967 shares | |
NASDAQ Global Market symbol | “HALO” |
S-6
Table of Contents
S-7
Table of Contents
S-8
Table of Contents
S-9
Table of Contents
| • | FDA review may not find a product candidate safe or effective enough to merit either continued testing or final approval; | ||
| • | FDA review may not find that the data from preclinical testing and clinical trials justifies approval, or they may require additional studies that would make it commercially unattractive to continue pursuit of approval; | ||
| • | the FDA may reject our trial data or disagree with our interpretations of either clinical trial data or applicable regulations; | ||
| • | the cost of a clinical trial may be greater than what we originally anticipate, and we may decide to not pursue FDA approval for such a trial; | ||
| • | the FDA may not approve our manufacturing processes or facilities, or the processes or facilities of our contract manufacturers or raw material suppliers; | ||
| • | the FDA may change its formal or informal approval requirements and policies, act contrary to previous guidance, or adopt new regulations; or | ||
| • | the FDA may approve a product candidate for indications that are narrow or under conditions that place the product at a competitive disadvantage, which may limit our sales and marketing activities or otherwise adversely impact the commercial potential of a product. |
S-10
Table of Contents
| • | restrictions on our products or manufacturing processes; | ||
| • | warning letters; | ||
| • | withdrawal of the products from the market; | ||
| • | voluntary or mandatory recall; | ||
| • | fines; | ||
| • | suspension or withdrawal of regulatory approvals; | ||
| • | suspension or termination of any of our ongoing clinical trials; | ||
| • | refusal to permit the import or export of our products; | ||
| • | refusal to approve pending applications or supplements to approved applications that we submit; | ||
| • | product seizure; or | ||
| • | injunctions or the imposition of civil or criminal penalties. |
S-11
Table of Contents
| • | we may have to issue convertible debt or equity securities to complete an acquisition, which would dilute our stockholders and could adversely affect the market price of our common stock; | ||
| • | an acquisition may negatively impact our results of operations because it may require us to amortize or write down amounts related to goodwill and other intangible assets, or incur or assume substantial debt or liabilities, or it may cause adverse tax consequences, substantial depreciation or deferred compensation charges; | ||
| • | we may encounter difficulties in assimilating and integrating the business, products, technologies, personnel or operations of companies that we acquire; | ||
| • | certain acquisitions may impact our relationship with existing or potential partners who are competitive with the acquired business, products or technologies; | ||
| • | acquisitions may require significant capital infusions and the acquired businesses, products or technologies may not generate sufficient value to justify acquisition costs; | ||
| • | an acquisition may disrupt our ongoing business, divert resources, increase our expenses and distract our management; | ||
| • | acquisitions may involve the entry into a geographic or business market in which we have little or no prior experience; and | ||
| • | key personnel of an acquired company may decide not to work for us. |
| • | the price of products relative to other therapies for the same or similar treatments; | ||
| • | the perception by patients, physicians and other members of the health care community of the effectiveness and safety of these products for their prescribed treatments; | ||
| • | our ability to fund our sales and marketing efforts and the ability and willingness of our partners to fund sales and marketing efforts; | ||
| • | the degree to which the use of these products is restricted by the product label approved by the FDA; |
S-12
Table of Contents
| • | the effectiveness of our sales and marketing efforts and the effectiveness of the sales and marketing efforts of our partners; and | ||
| • | the introduction of generic competitors. |
S-13
Table of Contents
S-14
Table of Contents
| • | our patents and pending patent applications cover products and/or technology that we invented first; | ||
| • | we were the first to file patent applications for these inventions; | ||
| • | others will not independently develop similar or alternative technologies or duplicate our technologies; | ||
| • | any of our pending patent applications will result in issued patents; and | ||
| • | any of our issued patents, or patent pending applications that result in issued patents, will be held valid and infringed in the event the patents are asserted against others. |
S-15
Table of Contents
S-16
Table of Contents
| • | our failure, or the failure of one of our third party partners, to comply with the terms of our collaboration agreements; | ||
| • | the termination, for any reason, of any of our collaboration agreements; | ||
| • | the sale of common stock by any significant stockholder, including, but not limited to, direct or indirect sales by members of management or our Board of Directors; |
S-17
Table of Contents
| • | general negative conditions in the healthcare industry; | ||
| • | general negative conditions in the financial markets; | ||
| • | the failure, for any reason, to obtain FDA approval for any of our proprietary or partnered product candidates; | ||
| • | the failure, for any reason, to secure or defend our intellectual property position; | ||
| • | for those products that are approved by the FDA, the failure of the FDA to approve such products in a timely manner consistent with the FDA’s historical approval process; | ||
| • | the suspension of any clinical trial due to safety or patient tolerability issues; | ||
| • | the suspension of any clinical trial due to market and/or competitive conditions; | ||
| • | our failure, or the failure of our third party partners, to successfully commercialize products approved by the FDA; | ||
| • | our failure, or the failure of our third party partners, to generate product revenues anticipated by investors; | ||
| • | problems with an API contract manufacturer or a fill and finish manufacturer for any product or product candidate; | ||
| • | the sale of additional debt and/or equity securities by us; and | ||
| • | the departure of key personnel. |
S-18
Table of Contents
S-19
Table of Contents
| • | on an actual basis; and | ||
| • | on a pro forma as adjusted basis to reflect this offering. |
| March 31, 2009 | ||||||||
| (unaudited) | ||||||||
| (dollars in thousands) | ||||||||
| Actual | As Adjusted | |||||||
| Stockholders’ equity: | ||||||||
| Preferred stock, par value $0.001 per share; 20,000,000 shares authorized; no shares issued and outstanding | $ | — | $ | — | ||||
| Common stock, par value $0.001 per share; 150,000,000 shares authorized; 82,946,637 shares issued and outstanding | 83 | 88 | ||||||
| Additional paid-in capital | 132,277 | 169,612 | ||||||
| Accumulated deficit | (128,436 | ) | (128,436 | ) | ||||
| Total stockholders’ equity | $ | 3,924 | 41,264 | |||||
| • | 7,870,737 shares of common stock issuable upon exercise of options outstanding as of March 31, 2009, at a weighted average exercise price of $3.35 per share; and | ||
| • | 1,927,715 shares of common stock issuable upon the exercise of warrants outstanding as of March 31, 2009, at a weighted average exercise price of $2.25 per share. |
S-20
Table of Contents
| Assumed public offering price per share | $ | 7.52 | ||||||
| Net tangible book value per share as of March 31, 2009 | $ | 0.05 | ||||||
| Increase in net tangible book value per share attributable to new investors | $ | 0.42 | ||||||
| As adjusted net tangible book value per share after this offering | $ | 0.47 | ||||||
| Dilution per share to new investors | $ | 7.05 | ||||||
| • | 7,870,737 shares of common stock issuable upon exercise of options outstanding as of March 31, 2009, at a weighted average exercise price of $3.35 per share; and | ||
| • | 1,927,715 shares of common stock issuable upon the exercise of warrants outstanding as of March 31, 2009, at an exercise price of $2.25 per share. |
S-21
Table of Contents
| High | Low | |||||||
Fiscal Year 2009 | ||||||||
| Second quarter (through June 19, 2009) | $ | 7.89 | $ | 5.13 | ||||
| First quarter | $ | 6.29 | $ | 4.10 | ||||
Fiscal Year 2008 | ||||||||
| Fourth quarter | $ | 7.29 | $ | 2.60 | ||||
| Third quarter | $ | 8.26 | $ | 5.35 | ||||
| Second quarter | $ | 6.62 | $ | 4.75 | ||||
| First quarter | $ | 7.25 | $ | 4.19 | ||||
Fiscal Year 2007 | ||||||||
| Fourth quarter | $ | 9.46 | $ | 6.00 | ||||
| Third quarter | $ | 10.50 | $ | 7.49 | ||||
| Second quarter | $ | 11.00 | $ | 8.00 | ||||
| First quarter | $ | 9.70 | $ | 6.75 | ||||
S-22
Table of Contents
Applicable to Non-U.S. Holders
| • | does not purport to be a complete analysis of all of the potential tax considerations that may be applicable to an investor as a result of the investor’s particular tax situation; | ||
| • | is based on the Internal Revenue Code of 1986, as amended (the “Code”), the existing applicable United States federal income tax regulations promulgated or proposed under the Code, which we refer to as the “Treasury Regulations,” judicial authority and currently effective published rulings and administrative pronouncements, each as of the date hereof and each of which are subject to change at any time, possibly with retroactive effect, and differing interpretations; | ||
| • | is applicable only to beneficial owners of common stock who hold their common stock as a “capital asset,” within the meaning of section 1221 of the Code; | ||
| • | does not address all aspects of United States federal income taxation that may be relevant to holders in light of their particular circumstances or who are subject to special treatment under United States federal income tax laws, including but not limited to: |
| o | certain former citizens and long-term residents of the United States; | ||
| o | “controlled foreign corporations” and “passive foreign investment companies”; | ||
| o | partnerships, other pass-through entities and investors in these entities; and | ||
| o | investors that expect to receive dividends or realize gain in connection with the investors’ conduct of a United States trade or business, permanent establishment or fixed base. |
| • | does not discuss any possible applicability of any United States state or local taxes, non-United States taxes or any United States federal tax other than the income tax, including, but not limited to, the federal gift tax and estate tax. |
S-23
Table of Contents
| • | an individual citizen or resident of the United States; | ||
| • | a corporation, or other entity treated as an association taxable as a corporation, that is organized in or under the laws of the United States, any state thereof or the District of Columbia; | ||
| • | an estate the income of which is subject to United States federal income taxation regardless of its source; or | ||
| • | a trust if it (1) is subject to the primary supervision of a court within the United States and one or more United States persons have the authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable the Treasury Regulations to be treated as a United States person. |
S-24
Table of Contents
| • | the gain is effectively connected with a trade or business of the non-U.S. holder in the United States (and, if required by an applicable income tax treaty, is attributable to a U.S. permanent establishment of the non-U.S. holder or, in the case of an individual, a fixed base); | ||
| • | the non-U.S. holder is an individual who is present in the United States for 183 days or more in the taxable year of that disposition, and certain other conditions are met; or | ||
| • | we are or have been a “United States real property holding corporation” for United States federal income tax purposes at any time during the shorter of the five-year period preceding such disposition and the non-U.S. holder’s holding period in the common stock. |
S-25
Table of Contents
S-26
Table of Contents
| Name | Number of Shares | |
| Jefferies & Company, Inc. | 5,200,000 |
| Per Share | Total | |||||||
| Public offering price | $ | $ | ||||||
| Underwriting discounts and commissions paid by us | $ | $ | ||||||
| Proceeds to us, before expenses | $ | $ | ||||||
S-27
Table of Contents
| • | offer, pledge, announce the intention to sell, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any common stock or any securities convertible into or exercisable or exchangeable for common stock (including without limitation, common stock which may be deemed to be beneficially owned by the undersigned in accordance with the rules and regulations of the Securities and Exchange Commission and securities which may be issued upon exercise of a stock option or warrant), or | ||
| • | enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of the common stock, whether any such transaction described herein or above is to be settled by delivery of common stock or such other securities, in cash or otherwise; or | ||
| • | make any demand for or exercise any right with respect to, the registration of any common stock or any security convertible into or exercisable or exchangeable for common stock. |
S-28
Table of Contents
S-29
Table of Contents
S-30
Table of Contents
S-31
Table of Contents
S-32
Table of Contents
| • | Our Annual Report on Form 10-K for the fiscal year ended December 31, 2008, filed on March 13, 2009; | ||
| • | Our Quarterly Report on Form 10-Q for the quarter ended March 31, 2009, filed on May 8, 2009. | ||
| • | Our Current Reports on Form 8-K, filed on January 9, 2009, January 26, 2009, February 9, 2009 and April 15, 2009; | ||
| • | Our definitive proxy statement filed pursuant to Section 14 of the Exchange Act in connection with our 2009 Annual Meeting of Stockholders filed on April 2, 2009; | ||
| • | The description of our common stock set forth in Form 8-A/A filed on November 20, 2007. |
S-33
Table of Contents
Debt Securities,
Warrants and Units
Prospectus | ||||
| 1 | ||||
| 1 | ||||
| 5 | ||||
| 19 | ||||
| 20 | ||||
| 20 | ||||
| 20 | ||||
| 25 | ||||
| 34 | ||||
| 36 | ||||
| 38 | ||||
| 42 | ||||
| 43 | ||||
| 44 | ||||
| 44 | ||||
| 44 | ||||
| 45 |
Table of Contents
1
Table of Contents
2
Table of Contents
| • | designation or classification; | ||
| • | aggregate principal amount or aggregate offering price; | ||
| • | maturity, if applicable; | ||
| • | original issue discount, if any; | ||
| • | rates and times of payment of interest or dividends, if any; | ||
| • | redemption, conversion, exchange or sinking fund terms, if any; | ||
| • | conversion or exchange prices or rates, if any, and, if applicable, any provisions for changes to or adjustments in the conversion or exchange prices or rates and in the securities or other property receivable upon conversion or exchange; | ||
| • | ranking; | ||
| • | restrictive covenants, if any; | ||
| • | voting or other rights, if any; and | ||
| • | important United States federal income tax considerations. |
| • | the names of those underwriters or agents; |
3
Table of Contents
| • | applicable fees, discounts and commissions to be paid to them; | ||
| • | details regarding over-allotment options, if any; and | ||
| • | the net proceeds to us. |
4
Table of Contents
5
Table of Contents
6
Table of Contents
7
Table of Contents
8
Table of Contents
| • | FDA officials may not find a product candidate safe or effective enough to merit either continued testing or final approval; | ||
| • | FDA officials may not find that the data from pre-clinical testing and clinical trials justifies approval, or they may require additional studies that would make it commercially unattractive to continue pursuit of approval; | ||
| • | FDA may reject our trial data or disagree with our interpretations of either clinical trial data or applicable regulations; | ||
| • | the cost of a clinical trial may be greater than what we originally anticipate, and we may decide to not pursue FDA approval for such a trial; | ||
| • | the FDA may not approve our manufacturing processes or facilities, or the processes or facilities of our contract manufacturers or raw material suppliers; | ||
| • | the FDA may change its formal or informal approval requirements and policies, act contrary to previous guidance, or adopt new regulations; or | ||
| • | the FDA may approve a product candidate for indications that are narrow or under conditions that place the product at a competitive disadvantage, which may limit our sales and marketing activities or otherwise adversely impact the commercial potential of a product. | ||
| • | If the FDA does not approve our product candidates in a timely fashion on commercially viable terms, or if we terminate development of any of our product candidates due to difficulties or delays encountered in the regulatory approval process, it will have a material adverse impact on our business and we will be dependent on the development of our other product candidates and/or our ability to successfully acquire other products and technologies. We may not receive regulatory approval of our Chemophase product candidate or any other product candidates, in a timely manner, or at all. |
9
Table of Contents
| • | restrictions on our products or manufacturing processes; | ||
| • | warning letters; | ||
| • | withdrawal of the products from the market; | ||
| • | voluntary or mandatory recall; | ||
| • | fines; | ||
| • | suspension or withdrawal of regulatory approvals; | ||
| • | suspension or termination of any of our ongoing clinical trials; | ||
| • | refusal to permit the import or export of our products; | ||
| • | refusal to approve pending applications or supplements to approved applications that we submit; | ||
| • | product seizure; or |
10
Table of Contents
| • | injunctions or the imposition of civil or criminal penalties. |
| • | the price of our products relative to other therapies for the same or similar treatments; | ||
| • | the perception by patients, physicians and other members of the health care community of the effectiveness and safety of our products for their prescribed treatments; | ||
| • | our ability to fund our sales and marketing efforts; | ||
| • | the degree to which the use of our products is restricted by the product label approved by the FDA; | ||
| • | the effectiveness of our sales and marketing efforts; and | ||
| • | the introduction of generic competitors. |
11
Table of Contents
12
Table of Contents
| • | our failure, or the failure of one of our third party partners, to comply with the terms of our collaboration agreements; | ||
| • | the termination, for any reason, of any of our collaboration agreements; | ||
| • | the sale of common stock by any significant stockholder, including, but not limited to, direct or indirect sales by members of management or our Board of Directors; |
13
Table of Contents
| • | general negative conditions in the healthcare industry; | ||
| • | general negative conditions in the financial markets; | ||
| • | the failure, for any reason, to obtain FDA approval for any of our product candidates; | ||
| • | the failure, for any reason, to secure or defend our intellectual property position; | ||
| • | for those products that are approved by the FDA, the failure of the FDA to approve such products in a timely manner consistent with the FDA’s historical approval process; | ||
| • | the suspension of any clinical trial due to safety or patient tolerability issues; | ||
| • | the suspension of any clinical trial due to market and/or competitive conditions; | ||
| • | our failure, or the failure of our third party partners, to successfully commercialize products approved by the FDA; | ||
| • | our failure, or the failure of our third party partners, to generate product revenues anticipated by investors; | ||
| • | problems with an API contract manufacturer or a fill and finish manufacturer for any product or product candidate; | ||
| • | the sale of additional debt and/or equity securities by us; and | ||
| • | the departure of key personnel. |
14
Table of Contents
15
Table of Contents
| • | our patents and pending patent applications cover products and/or technology that we invented first; | ||
| • | we were the first to file patent applications for these inventions; | ||
| • | others will not independently develop similar or alternative technologies or duplicate our technologies; | ||
| • | any of our pending patent applications will result in issued patents; and | ||
| • | any of our issued patents, or patent pending applications that result in issued patents, will be held valid and infringed in the event the patents are asserted against others. |
16
Table of Contents
| • | we may have to issue convertible debt or equity securities to complete an acquisition, which would dilute our stockholders and could adversely affect the market price of our common stock; | ||
| • | an acquisition may negatively impact our results of operations because it may require us to incur large one-time charges to earnings, amortize or write down amounts related to goodwill and other intangible assets, or incur or assume substantial debt or liabilities, or it may cause adverse tax consequences, substantial depreciation or deferred compensation charges; | ||
| • | we may encounter difficulties in assimilating and integrating the business, products, technologies, personnel or operations of companies that we acquire; | ||
| • | certain acquisitions may disrupt our relationship with existing customers who are competitive with the acquired business, products or technologies; | ||
| • | acquisitions may require significant capital infusions and the acquired businesses, products or technologies may not generate sufficient revenue to offset acquisition costs; | ||
| • | an acquisition may disrupt our ongoing business, divert resources, increase our expenses and distract our management; | ||
| • | acquisitions may involve the entry into a geographic or business market in which we have little or no prior experience; and | ||
| • | key personnel of an acquired company may decide not to work for us. |
17
Table of Contents
18
Table of Contents
19
Table of Contents
| Nine Months | ||||||||||||||||||||||||
| Ended | ||||||||||||||||||||||||
| Year Ended December 31, | September 30, | |||||||||||||||||||||||
| 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | |||||||||||||||||||
| Ratio of earnings to fixed charges | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||
| Ratio of earnings to combined fixed charges and preferred stock dividends | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||||||
| Deficiency of earnings available to cover fixed charges | $ | 2.1 | $ | 9.1 | $ | 13.3 | $ | 14.8 | $ | 23.9 | $ | 31.8 | ||||||||||||
| Deficiency of earnings available to cover combined fixed charges and preferred stock dividends | $ | 2.1 | $ | 9.1 | $ | 13.3 | $ | 14.8 | $ | 23.9 | $ | 31.8 | ||||||||||||
20
Table of Contents
| • | the title and stated value; |
21
Table of Contents
| • | the number of shares we are offering; | ||
| • | the liquidation preference per share; | ||
| • | the purchase price per share; | ||
| • | the dividend rate per share, dividend period and payment dates and method of calculation for dividends; | ||
| • | whether dividends will be cumulative or non-cumulative and, if cumulative, the date from which dividends will accumulate; | ||
| • | our right, if any, to defer payment of dividends and the maximum length of any such deferral period; | ||
| • | the procedures for any auction and remarketing, if any; | ||
| • | the provisions for a sinking fund, if any; | ||
| • | the provisions for redemption or repurchase, if applicable, and any restrictions on our ability to exercise those redemption and repurchase rights; | ||
| • | any listing of the preferred stock on any securities exchange or market; | ||
| • | whether the preferred stock will be convertible into our common stock or other securities of ours, including warrants, and, if applicable, the conversion period, the conversion price, or how it will be calculated, and under what circumstances it may be adjusted; | ||
| • | whether the preferred stock will be exchangeable into debt securities, and, if applicable, the exchange period, the exchange price, or how it will be calculated, and under what circumstances it may be adjusted; | ||
| • | voting rights, if any, of the preferred stock; | ||
| • | preemption rights, if any; | ||
| • | restrictions on transfer, sale or other assignment, if any; | ||
| • | a discussion of any material or special United States federal income tax considerations applicable to the preferred stock; | ||
| • | the relative ranking and preferences of the preferred stock as to dividend rights and rights if we liquidate, dissolve or wind up our affairs; |
22
Table of Contents
| • | any limitations on issuances of any class or series of preferred stock ranking senior to or on a parity with the series of preferred stock being issued as to dividend rights and rights if we liquidate, dissolve or wind up our affairs; and | ||
| • | any other specific terms, rights, preferences, privileges, qualifications or restrictions of the preferred stock. |
| • | the board of directors of the corporation approved the business combination or the other transaction in which the person became an interested stockholder prior to the date of the business combination or other transaction; | ||
| • | upon consummation of the transaction that resulted in the person becoming an interested stockholder, the person owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding shares owned by persons who are directors and also officers of the corporation and shares issued under employee stock plans under which employee participants do not have |
23
Table of Contents
| the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or | |||
| • | on or subsequent to the date the person became an interested stockholder, the board of directors of the corporation approved the business combination and the stockholders of the corporation authorized the business combination at an annual or special meeting of stockholders by the affirmative vote of at least 66-2/3% of the outstanding stock of the corporation not owned by the interested stockholder. |
| • | any merger or consolidation involving the corporation and the interested stockholder; | ||
| • | any sale, transfer, pledge or other disposition of 10% or more of the corporation’s assets or outstanding stock involving the interested stockholder; | ||
| • | in general, any transaction that results in the issuance or transfer by the corporation of any of its stock to the interested stockholder; | ||
| • | any transaction involving the corporation that has the effect of increasing the proportionate share of its stock owned by the interested stockholder; or | ||
| • | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
24
Table of Contents
| • | the title; |
25
Table of Contents
| • | the principal amount being offered, and if a series, the total amount authorized and the total amount outstanding; | |
| • | any limit on the amount that may be issued; | |
| • | whether or not we will issue the series of debt securities in global form, and, if so, the terms and who the depositary will be; | |
| • | the maturity date; | |
| • | whether and under what circumstances, if any, we will pay additional amounts on any debt securities held by a person who is not a United States person for tax purposes, and whether we can redeem the debt securities if we have to pay such additional amounts; | |
| • | the annual interest rate, which may be fixed or variable, or the method for determining the rate and the date interest will begin to accrue, the dates interest will be payable and the regular record dates for interest payment dates or the method for determining such dates; | |
| • | whether or not the debt securities will be secured or unsecured, and the terms of any secured debt; | |
| • | the terms of the subordination of any series of subordinated debt; | |
| • | the place where payments will be payable; | |
| • | restrictions on transfer, sale or other assignment, if any; | |
| • | our right, if any, to defer payment of interest and the maximum length of any such deferral period; | |
| • | the date, if any, after which, and the price at which, we may, at our option, redeem the series of debt securities pursuant to any optional or provisional redemption provisions and the terms of those redemption provisions; | |
| • | the date, if any, on which, and the price at which we are obligated, pursuant to any mandatory sinking fund or analogous fund provisions or otherwise, to redeem, or at the holder’s option, to purchase, the series of debt securities and the currency or currency unit in which the debt securities are payable; | |
| • | whether the indenture will restrict our ability or the ability of our subsidiaries to: |
| • | incur additional indebtedness; | ||
| • | issue additional securities; |
26
Table of Contents
| • | create liens; | ||
| • | pay dividends or make distributions in respect of our capital stock or the capital stock of our subsidiaries; | ||
| • | redeem capital stock; | ||
| • | place restrictions on our subsidiaries’ ability to pay dividends, make distributions or transfer assets; | ||
| • | make investments or other restricted payments; | ||
| • | sell or otherwise dispose of assets; | ||
| • | enter into sale-leaseback transactions; | ||
| • | engage in transactions with stockholders or affiliates; | ||
| • | issue or sell stock of our subsidiaries; or | ||
| • | effect a consolidation or merger; |
| • | whether the indenture will require us to maintain any interest coverage, fixed charge, cash flow-based, asset-based or other financial ratios; | |
| • | a discussion of certain material or special United States federal income tax considerations applicable to the debt securities; | |
| • | information describing any book-entry features; | |
| • | provisions for a sinking fund purchase or other analogous fund, if any; | |
| • | the applicability of the provisions in the indenture on discharge; | |
| • | whether the debt securities are to be offered at a price such that they will be deemed to be offered at an “original issue discount” as defined in paragraph (a) of Section 1273 of the Internal Revenue Code of 1986, as amended; | |
| • | the denominations in which we will issue the series of debt securities, if other than denominations of $1,000 and any integral multiple thereof; | |
| • | the currency of payment of debt securities if other than U.S. dollars and the manner of determining the equivalent amount in U.S. dollars; and | |
| • | any other specific terms, preferences, rights or limitations of, or restrictions on, the debt securities, including any additional events of default or covenants provided with |
27
Table of Contents
| respect to the debt securities, and any terms that may be required by us or advisable under applicable laws or regulations. |
| • | if we fail to pay interest when due and payable and our failure continues for 90 days and the time for payment has not been extended; | |
| • | if we fail to pay the principal, premium or sinking fund payment, if any, when due and payable at maturity, upon redemption or repurchase or otherwise, and the time for payment has not been extended; | |
| • | if we fail to observe or perform any other covenant contained in the debt securities or the indentures, other than a covenant specifically relating to another series of debt securities, and our failure continues for 90 days after we receive notice from the trustee or we and the trustee receive notice from the holders of at least 25% in aggregate principal amount of the outstanding debt securities of the applicable series; and | |
| • | if specified events of bankruptcy, insolvency or reorganization occur. |
28
Table of Contents
| • | the direction so given by the holder is not in conflict with any law or the applicable indenture; and | |
| • | subject to its duties under the Trust Indenture Act, the trustee need not take any action that might involve it in personal liability or might be unduly prejudicial to the holders not involved in the proceeding. |
| • | the holder has given written notice to the trustee of a continuing event of default with respect to that series; |
29
Table of Contents
| • | the holders of at least 25% in aggregate principal amount of the outstanding debt securities of that series have made written request, and such holders have offered reasonable indemnity to the trustee or security satisfactory to it against any loss, liability or expense or to be incurred in compliance with instituting the proceeding as trustee; and | |
| • | the trustee does not institute the proceeding, and does not receive from the holders of a majority in aggregate principal amount of the outstanding debt securities of that series other conflicting directions within 90 days after the notice, request and offer. |
| • | to fix any ambiguity, defect or inconsistency in the indenture; | |
| • | to comply with the provisions described above under “Description of Debt Securities — Consolidation, Merger or Sale”; | |
| • | to comply with any requirements of the SEC in connection with the qualification of any indenture under the Trust Indenture Act; | |
| • | to add to, delete from or revise the conditions, limitations, and restrictions on the authorized amount, terms, or purposes of issue, authentication and delivery of debt securities, as set forth in the indenture; | |
| • | to provide for the issuance of and establish the form and terms and conditions of the debt securities of any series as provided under “Description of Debt Securities — General,” to establish the form of any certifications required to be furnished |
30
Table of Contents
| pursuant to the terms of the indenture or any series of debt securities, or to add to the rights of the holders of any series of debt securities; | ||
| • | to evidence and provide for the acceptance of appointment hereunder by a successor trustee; | |
| • | to provide for uncertificated debt securities and to make all appropriate changes for such purpose; | |
| • | to add to our covenants such new covenants, restrictions, conditions or provisions for the benefit of the holders, to make the occurrence, or the occurrence and the continuance, of a default in any such additional covenants, restrictions, conditions or provisions an event of default or to surrender any right or power conferred to us in the indenture; or | |
| • | to change anything that does not adversely affect the interests of any holder of debt securities of any series in any material respect. |
| • | extending the stated maturity of the series of debt securities; | |
| • | reducing the principal amount, reducing the rate of or extending the time of payment of interest, or reducing any premium payable upon the redemption or repurchase of any debt securities; or | |
| • | reducing the percentage of debt securities, the holders of which are required to consent to any amendment, supplement, modification or waiver. |
| • | register the transfer or exchange of debt securities of the series; | |
| • | replace stolen, lost or mutilated debt securities of the series; | |
| • | maintain paying agencies; |
31
Table of Contents
| • | hold monies for payment in trust; | |
| • | recover excess money held by the trustee; | |
| • | compensate and indemnify the trustee; and | |
| • | appoint any successor trustee. |
| • | issue, register the transfer of, or exchange any debt securities of that series during a period beginning at the opening of business 15 days before the day of mailing of a notice of redemption of any debt securities that may be selected for redemption and ending at the close of business on the day of the mailing; or |
32
Table of Contents
| • | register the transfer of or exchange any debt securities so selected for redemption, in whole or in part, except the unredeemed portion of any debt securities we are redeeming in part. |
33
Table of Contents
| • | the offering price and aggregate number of warrants offered; | ||
| • | the currency for which the warrants may be purchased; |
34
Table of Contents
| • | if applicable, the designation and terms of the securities with which the warrants are issued and the number of warrants issued with each such security or each principal amount of such security; | ||
| • | applicable, the date on and after which the warrants and the related securities will be separately transferable; | ||
| • | in the case of warrants to purchase debt securities, the principal amount of debt securities purchasable upon exercise of one warrant and the price at, and currency in which, this principal amount of debt securities may be purchased upon such exercise; | ||
| • | in the case of warrants to purchase common stock or preferred stock, the number of shares of common stock or preferred stock, as the case may be, purchasable upon the exercise of one warrant and the price at which these shares may be purchased upon such exercise; | ||
| • | the effect of any merger, consolidation, sale or other disposition of our business on the warrant agreements and the warrants; | ||
| • | the terms of any rights to redeem or call the warrants; | ||
| • | any provisions for changes to or adjustments in the exercise price or number of securities issuable upon exercise of the warrants; | ||
| • | the dates on which the right to exercise the warrants will commence and expire; | ||
| • | the manner in which the warrant agreements and warrants may be modified; | ||
| • | United States federal income tax consequences of holding or exercising the warrants; | ||
| • | the terms of the securities issuable upon exercise of the warrants; and | ||
| • | any other specific terms, preferences, rights or limitations of or restrictions on the warrants. |
| • | in the case of warrants to purchase debt securities, the right to receive payments of principal of, or premium, if any, or interest on, the debt securities purchasable upon exercise or to enforce covenants in the applicable indenture; or | ||
| • | in the case of warrants to purchase common stock or preferred stock, the right to receive dividends, if any, or, payments upon our liquidation, dissolution or winding up or to exercise voting rights, if any. |
35
Table of Contents
36
Table of Contents
| • | the designation and terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may be held or transferred separately; | ||
| • | any provisions of the governing unit agreement that differ from those described below; and | ||
| • | any provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units. |
37
Table of Contents
38
Table of Contents
| • | how it handles securities payments and notices; | ||
| • | whether it imposes fees or charges; | ||
| • | how it would handle a request for the holders’ consent, if ever required; | ||
| • | whether and how you can instruct it to send you securities registered in your own name so you can be a holder, if that is permitted in the future; | ||
| • | how it would exercise rights under the securities if there were a default or other event triggering the need for holders to act to protect their interests; and |
39
Table of Contents
| • | if the securities are in book-entry form, how the depositary’s rules and procedures will affect these matters. |
| • | an investor cannot cause the securities to be registered in his or her name, and cannot obtain non-global certificates for his or her interest in the securities, except in the special situations we describe below; | ||
| • | an investor will be an indirect holder and must look to his or her own bank or broker for payments on the securities and protection of his or her legal rights relating to the securities, as we describe above; |
40
Table of Contents
| • | an investor may not be able to sell interests in the securities to some insurance companies and to other institutions that are required by law to own their securities in non-book-entry form; | ||
| • | an investor may not be able to pledge his or her interest in the global security in circumstances where certificates representing the securities must be delivered to the lender or other beneficiary of the pledge in order for the pledge to be effective; | ||
| • | the depositary’s policies, which may change from time to time, will govern payments, transfers, exchanges and other matters relating to an investor’s interest in the global security. We and any applicable trustee have no responsibility for any aspect of the depositary’s actions or for its records of ownership interests in the global security. We and the trustee also do not supervise the depositary in any way; | ||
| • | the depositary may, and we understand that DTC will, require that those who purchase and sell interests in the global security within its book-entry system use immediately available funds, and your broker or bank may require you to do so as well; and | ||
| • | financial institutions that participate in the depositary’s book-entry system, and through which an investor holds its interest in the global security, may also have their own policies affecting payments, notices and other matters relating to the securities. There may be more than one financial intermediary in the chain of ownership for an investor. We do not monitor and are not responsible for the actions of any of those intermediaries. |
| • | if the depositary notifies us that it is unwilling, unable or no longer qualified to continue as depositary for that global security and we do not appoint another institution to act as depositary within 90 days; | ||
| • | if we notify any applicable trustee that we wish to terminate that global security; or | ||
| • | if an event of default has occurred with regard to securities represented by that global security and has not been cured or waived. |
41
Table of Contents
| • | the name or names of any underwriters, if any; | ||
| • | the purchase price of the securities and the proceeds we will receive from the sale; | ||
| • | any over-allotment options under which underwriters may purchase additional securities from us; | ||
| • | any agency fees or underwriting discounts and other items constituting agents’ or underwriters’ compensation; | ||
| • | any public offering price; | ||
| • | any discounts or concessions allowed or reallowed or paid to dealers; and | ||
| • | any securities exchange or market on which the securities may be listed. |
42
Table of Contents
43
Table of Contents
44
Table of Contents
| • | Our Quarterly Report on Form 10-Q for the quarter ended September 30, 2008; | ||
| • | Our Quarterly Report on Form 10-Q for the quarter ended June 30, 2008; | ||
| • | Our Quarterly Report on Form 10-Q for the quarter ended March 31, 2008; | ||
| • | Our Annual Report on Form 10-K for the year ended December 31, 2007; | ||
| • | Our Current Reports on Form 8-K filed on February 8, 2008; March 19, 2008; April 21, 2008; August 21, 2008; and November 7, 2008; | ||
| • | Our definitive proxy statement filed pursuant to Section 14 of the Exchange Act in connection with our 2008 Annual Meeting of Stockholders filed with the SEC on April 3, 2008; and | ||
| • | The description of our common stock set forth in Form 8-A/A, filed with the SEC on November 20, 2007. |
SECURITIES ACT LIABILITY
45
Table of Contents
