
Halozyme Therapeutics, Inc. Thinking Outside the Cell™ October 2013 Gregory Frost Chief Executive Officer Exhibit 99.1

2 <#> Safe Harbor All of the statements in this presentation that are not statements of historical facts constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements and all references to financial estimates are based upon management’s current plans and expectations and are subject to a number of risks and uncertainties which could cause actual results to differ materially from such statements. While the Company believes that the assumptions concerning future events are reasonable, it cautions that there are inherent difficulties in anticipating or predicting certain important factors. A discussion of these factors, including risks and uncertainties, is set forth in the Company’s annual and quarterly reports filed from time to time with the Securities and Exchange Commission. The Company disclaims any intention or obligation to revise or update any forward-looking statements, whether as a result of new information, future events, or otherwise.
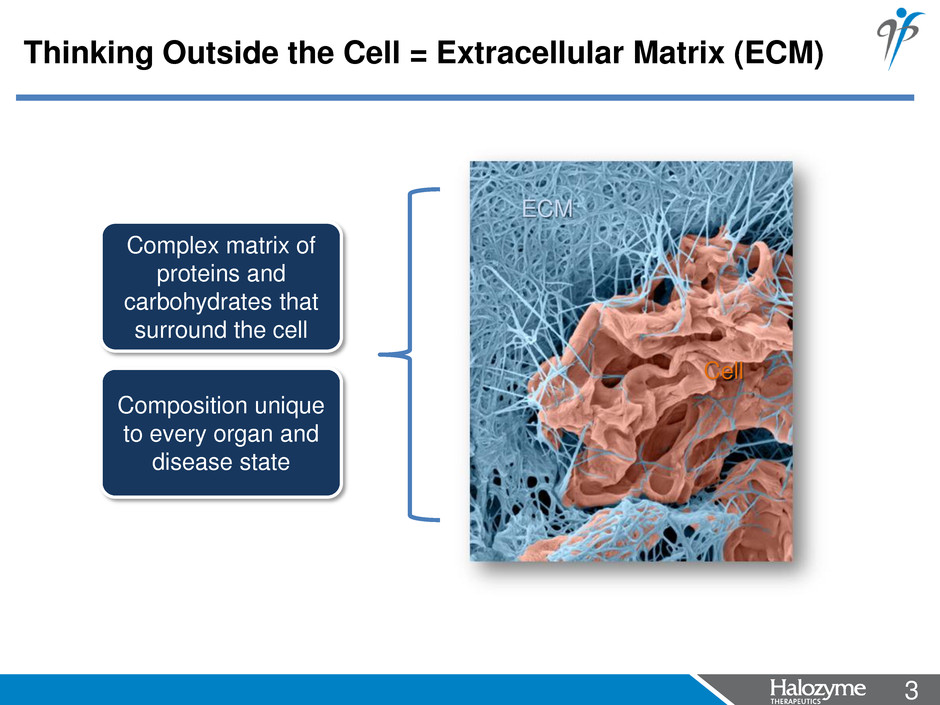
3 <#> Thinking Outside the Cell = Extracellular Matrix (ECM) Complex matrix of proteins and carbohydrates that surround the cell Composition unique to every organ and disease state Cell ECM
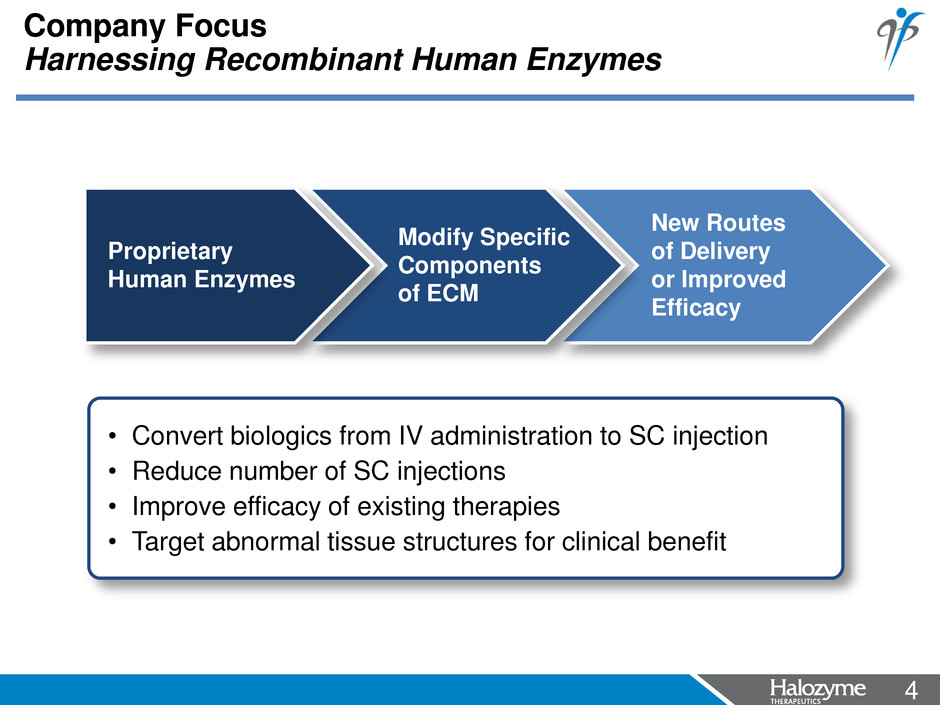
4 <#> New Routes of Delivery or Improved Efficacy Company Focus Harnessing Recombinant Human Enzymes Modify Specific Components of ECM Proprietary Human Enzymes • Convert biologics from IV administration to SC injection • Reduce number of SC injections • Improve efficacy of existing therapies • Target abnormal tissue structures for clinical benefit
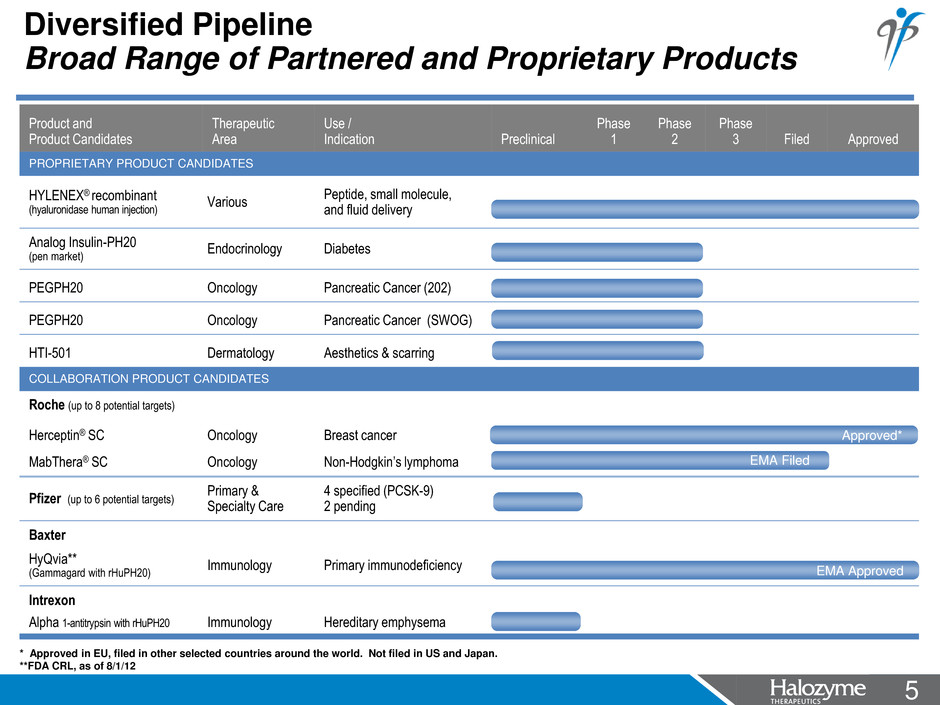
5 <#> Product and Product Candidates Therapeutic Area Use / Indication Preclinical Phase 1 Phase 2 Phase 3 Filed Approved PROPRIETARY PRODUCT CANDIDATES HYLENEX® recombinant (hyaluronidase human injection) Various Peptide, small molecule, and fluid delivery Analog Insulin-PH20 (pen market) Endocrinology Diabetes PEGPH20 Oncology Pancreatic Cancer (202) PEGPH20 Oncology Pancreatic Cancer (SWOG) HTI-501 Dermatology Aesthetics & scarring COLLABORATION PRODUCT CANDIDATES Roche (up to 8 potential targets) Herceptin® SC Oncology Breast cancer MabThera® SC Oncology Non-Hodgkin’s lymphoma Pfizer (up to 6 potential targets) Primary & Specialty Care 4 specified (PCSK-9) 2 pending Baxter HyQvia** (Gammagard with rHuPH20) Immunology Primary immunodeficiency Intrexon Alpha 1-antitrypsin with rHuPH20 Immunology Hereditary emphysema Diversified Pipeline Broad Range of Partnered and Proprietary Products * Approved in EU, filed in other selected countries around the world. Not filed in US and Japan. **FDA CRL, as of 8/1/12 EMA Approved Approved* EMA Filed

6 <#> Partnered Programs Near-Term Growth Engine • Alpha 1 antitrypsin (pre-clinical) • IV to SC • First 2 exclusive targets for Primary & Specialty Care (PCSK-9) • Pfizer elected 3rd and 4th exclusive targets since deal signing • IV to SC or improved dosing frequency UP TO 2 ADDITIONAL TARGETS Royalties from top-line sales plus milestones drive profitability • IV to SC • European Launch 3Q2013 • IV to SC • EMA filing 4Q 2012 UP TO 6 ADDITIONAL TARGETS • IV to SC dosing • European Launch 3Q2013 • Program delayed with CRL in US, potential to resubmit by EOY
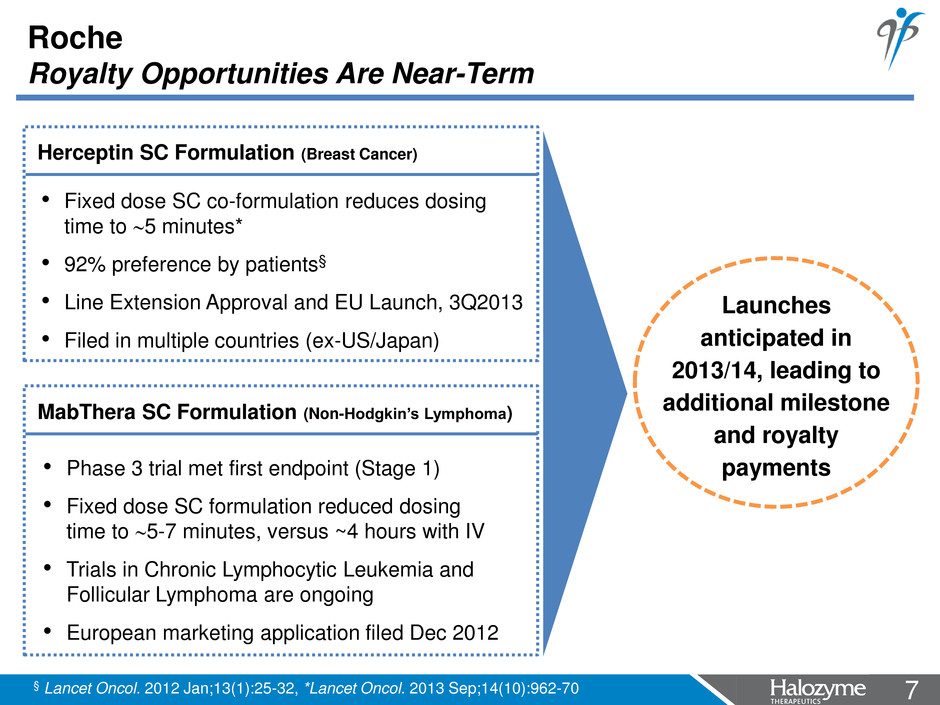
7 <#> Roche Royalty Opportunities Are Near-Term • Fixed dose SC co-formulation reduces dosing time to 5 minutes* • 92% preference by patients§ • Line Extension Approval and EU Launch, 3Q2013 • Filed in multiple countries (ex-US/Japan) Herceptin SC Formulation (Breast Cancer) MabThera SC Formulation (Non-Hodgkin’s Lymphoma) • Phase 3 trial met first endpoint (Stage 1) • Fixed dose SC formulation reduced dosing time to 5-7 minutes, versus ~4 hours with IV • Trials in Chronic Lymphocytic Leukemia and Follicular Lymphoma are ongoing • European marketing application filed Dec 2012 Launches anticipated in 2013/14, leading to additional milestone and royalty payments § Lancet Oncol. 2012 Jan;13(1):25-32, *Lancet Oncol. 2013 Sep;14(10):962-70
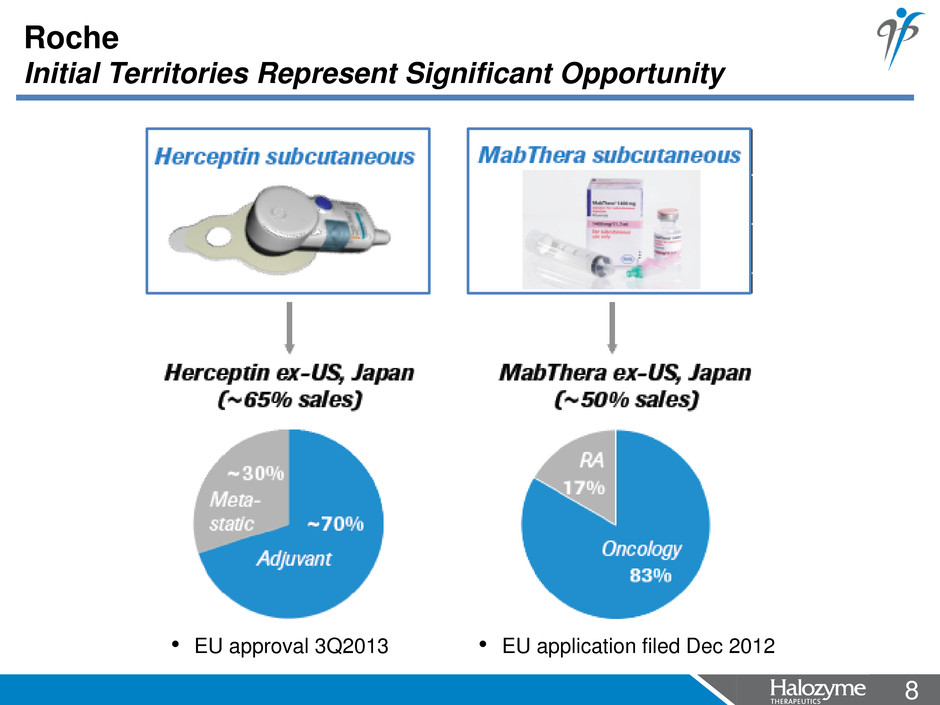
8 <#> Roche Initial Territories Represent Significant Opportunity • EU approval 3Q2013 • EU application filed Dec 2012
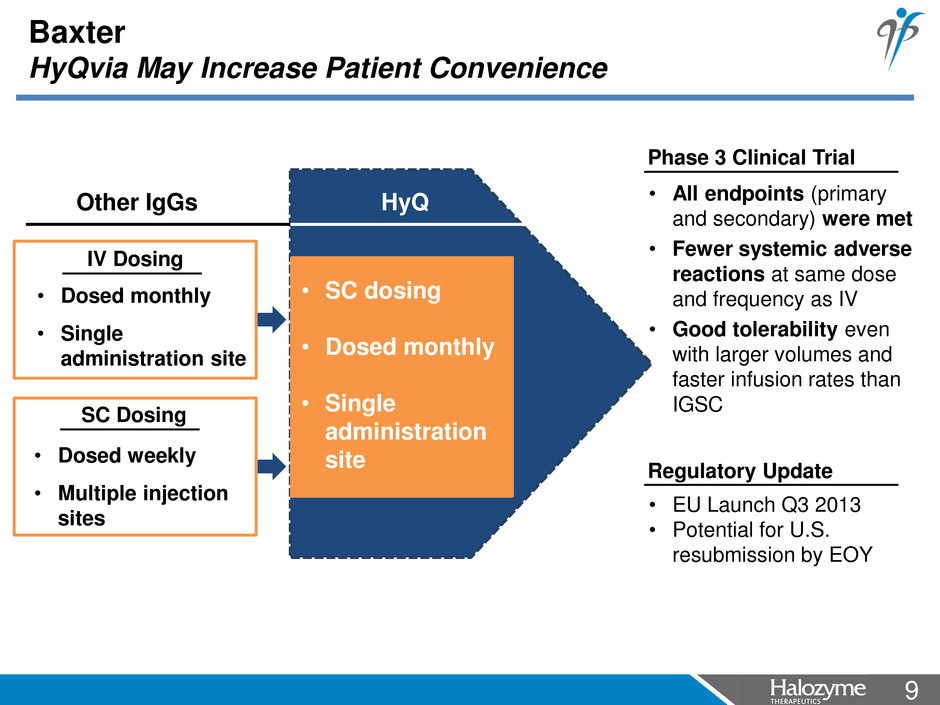
9 <#> Baxter HyQvia May Increase Patient Convenience • SC dosing • Dosed monthly • Single administration site Other IgGs HyQ • Dosed monthly • Single administration site IV Dosing • Dosed weekly • Multiple injection sites SC Dosing • All endpoints (primary and secondary) were met • Fewer systemic adverse reactions at same dose and frequency as IV • Good tolerability even with larger volumes and faster infusion rates than IGSC • EU Launch Q3 2013 • Potential for U.S. resubmission by EOY Phase 3 Clinical Trial Regulatory Update
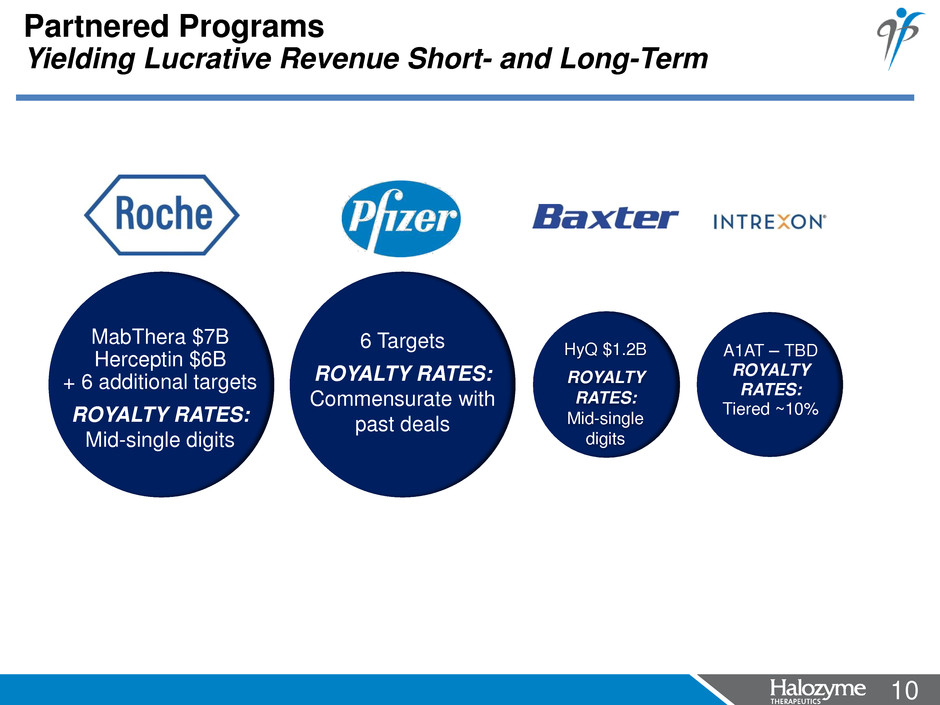
10 <#> Partnered Programs Yielding Lucrative Revenue Short- and Long-Term 6 Targets ROYALTY RATES: Commensurate with past deals MabThera $7B Herceptin $6B + 6 additional targets ROYALTY RATES: Mid-single digits HyQ $1.2B ROYALTY RATES: Mid-single digits A1AT – TBD ROYALTY RATES: Tiered ~10%
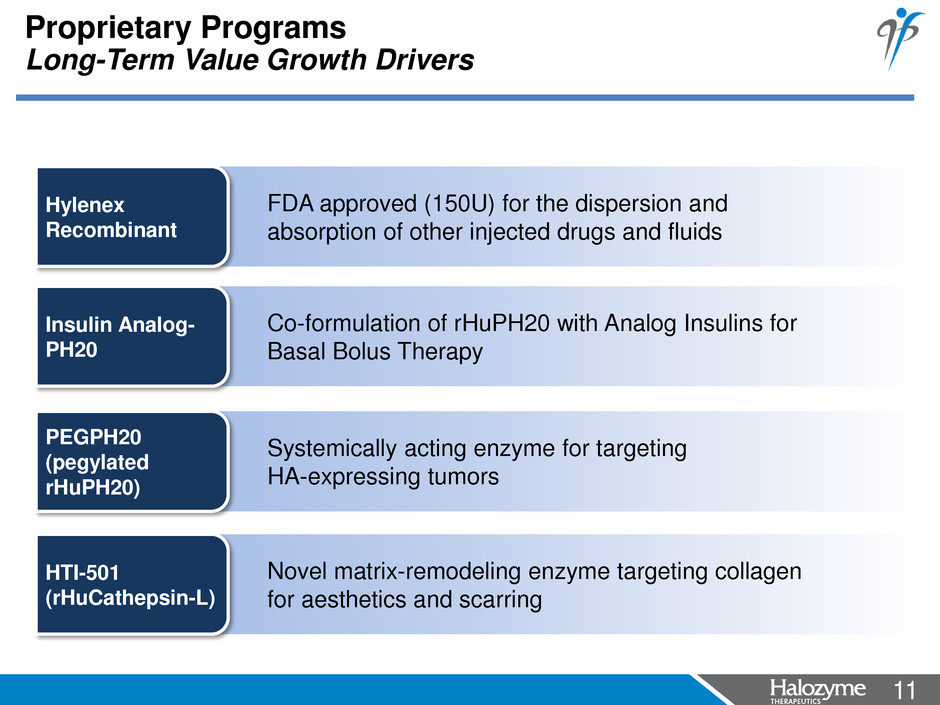
11 <#> Novel matrix-remodeling enzyme targeting collagen for aesthetics and scarring HTI-501 (rHuCathepsin-L) Systemically acting enzyme for targeting HA-expressing tumors PEGPH20 (pegylated rHuPH20) FDA approved (150U) for the dispersion and absorption of other injected drugs and fluids Hylenex Recombinant Proprietary Programs Long-Term Value Growth Drivers Co-formulation of rHuPH20 with Analog Insulins for Basal Bolus Therapy Insulin Analog- PH20
12 <#> First Proprietary Product: HYLENEX recombinant (hyaluronidase human injection) Indicated to increase dispersion and absorption of other injected drugs and fluids • Establishing base with hospital and surgery center markets – Regional anesthesia – Hydration and extravasation – Captured >50% market share within first 16 months of launch • Building opportunity in insulin pumps – Facilitate SC absorption of insulin for patients with diabetes on insulin pump therapy – Completed enrollment of Phase 4 trial in Q3 2013
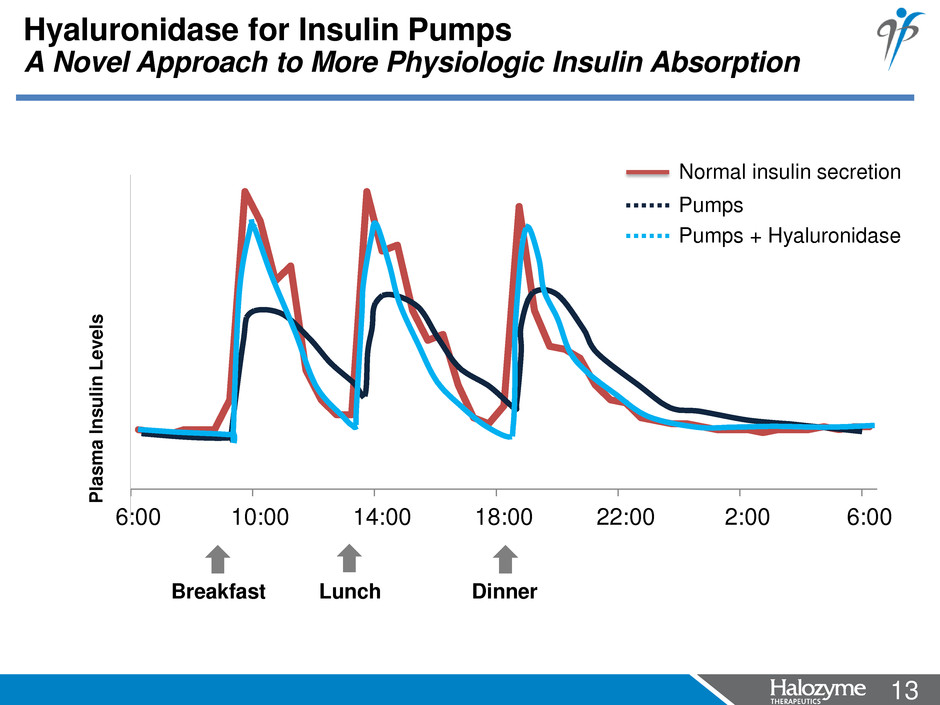
13 <#> 6:00 10:00 14:00 18:00 22:00 2:00 6:00 Hyaluronidase for Insulin Pumps A Novel Approach to More Physiologic Insulin Absorption P lasma I n s u li n L e v e ls Pumps Pumps + Hyaluronidase Normal insulin secretion Breakfast Lunch Dinner
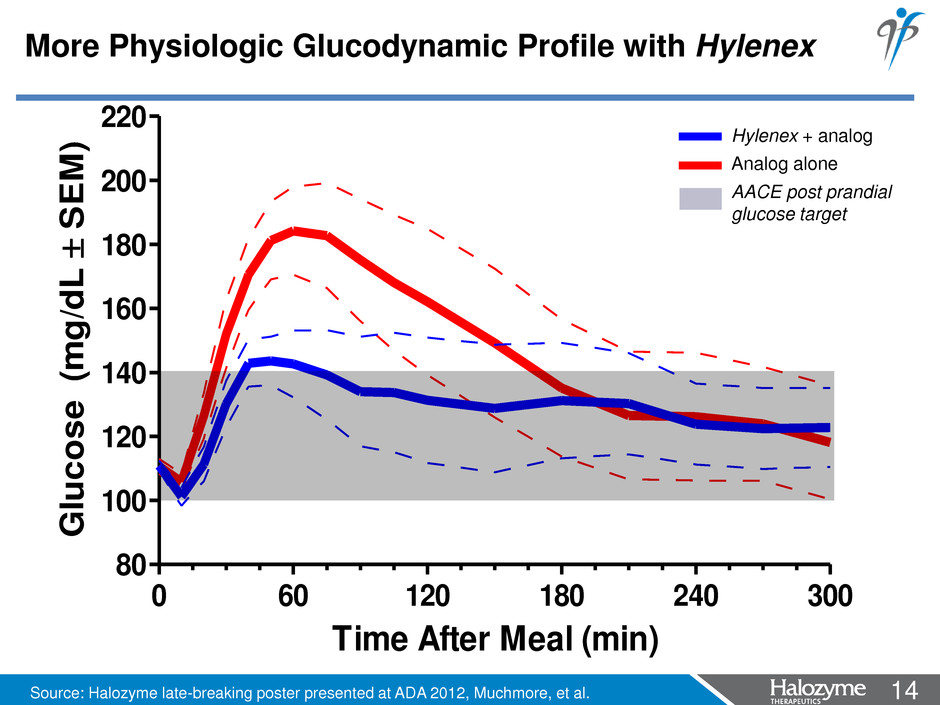
14 <#> 0 60 120 180 240 300 80 100 120 140 160 180 200 220 Time After Meal (min) G lu co se ( m g/ dL SE M ) Hylenex + analog Analog alone AACE post prandial glucose target More Physiologic Glucodynamic Profile with Hylenex Source: Halozyme late-breaking poster presented at ADA 2012, Muchmore, et al.
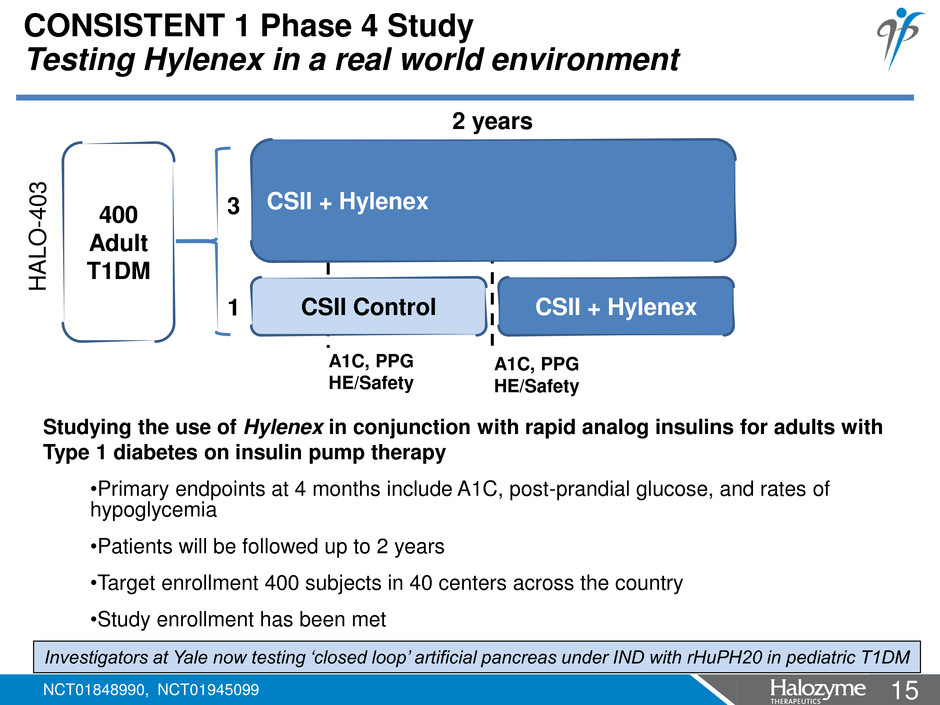
15 <#> CONSISTENT 1 Phase 4 Study Testing Hylenex in a real world environment CSII + Hylenex CSII Control CSII + Hylenex 2 years A1C, PPG HE/Safety A1C, PPG HE/Safety 400 Adult T1DM 3 1 Studying the use of Hylenex in conjunction with rapid analog insulins for adults with Type 1 diabetes on insulin pump therapy •Primary endpoints at 4 months include A1C, post-prandial glucose, and rates of hypoglycemia •Patients will be followed up to 2 years •Target enrollment 400 subjects in 40 centers across the country •Study enrollment has been met Investigators at Yale now testing ‘closed loop’ artificial pancreas under IND with rHuPH20 in pediatric T1DM NCT01848990, NCT01945099 HA L O -4 0 3
16 <#> Additional studies in relevant patient populations Phase 4 Clinical Trials Multiple device options Device Approvals New fill and finish lines Manufacturing Scale Up Hylenex in Insulin Pumps Three Steps to Commercial Readiness
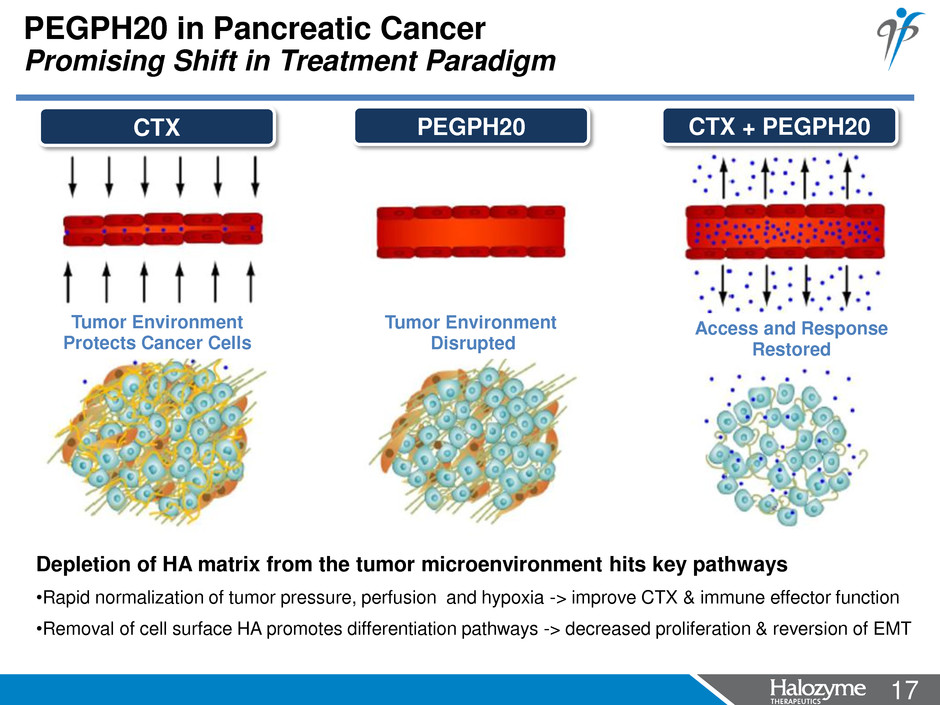
17 <#> PEGPH20 in Pancreatic Cancer Promising Shift in Treatment Paradigm Access and Response Restored CTX + PEGPH20 Tumor Environment Disrupted PEGPH20 Tumor Environment Protects Cancer Cells CTX Depletion of HA matrix from the tumor microenvironment hits key pathways •Rapid normalization of tumor pressure, perfusion and hypoxia -> improve CTX & immune effector function •Removal of cell surface HA promotes differentiation pathways -> decreased proliferation & reversion of EMT
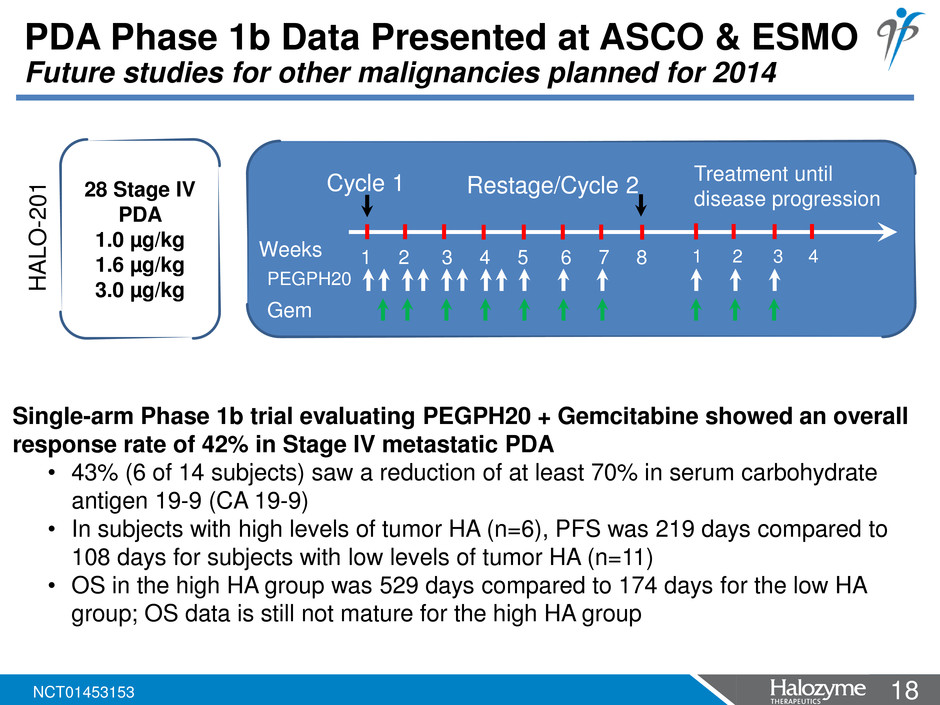
18 <#> PDA Phase 1b Data Presented at ASCO & ESMO Future studies for other malignancies planned for 2014 1 2 3 4 5 6 7 8 Weeks Restage/Cycle 2 Cycle 1 Treatment until disease progression PEGPH20 Gem 1 2 3 4 28 Stage IV PDA 1.0 µg/kg 1.6 µg/kg 3.0 µg/kg Single-arm Phase 1b trial evaluating PEGPH20 + Gemcitabine showed an overall response rate of 42% in Stage IV metastatic PDA • 43% (6 of 14 subjects) saw a reduction of at least 70% in serum carbohydrate antigen 19-9 (CA 19-9) • In subjects with high levels of tumor HA (n=6), PFS was 219 days compared to 108 days for subjects with low levels of tumor HA (n=11) • OS in the high HA group was 529 days compared to 174 days for the low HA group; OS data is still not mature for the high HA group HA L O -2 0 1 NCT01453153
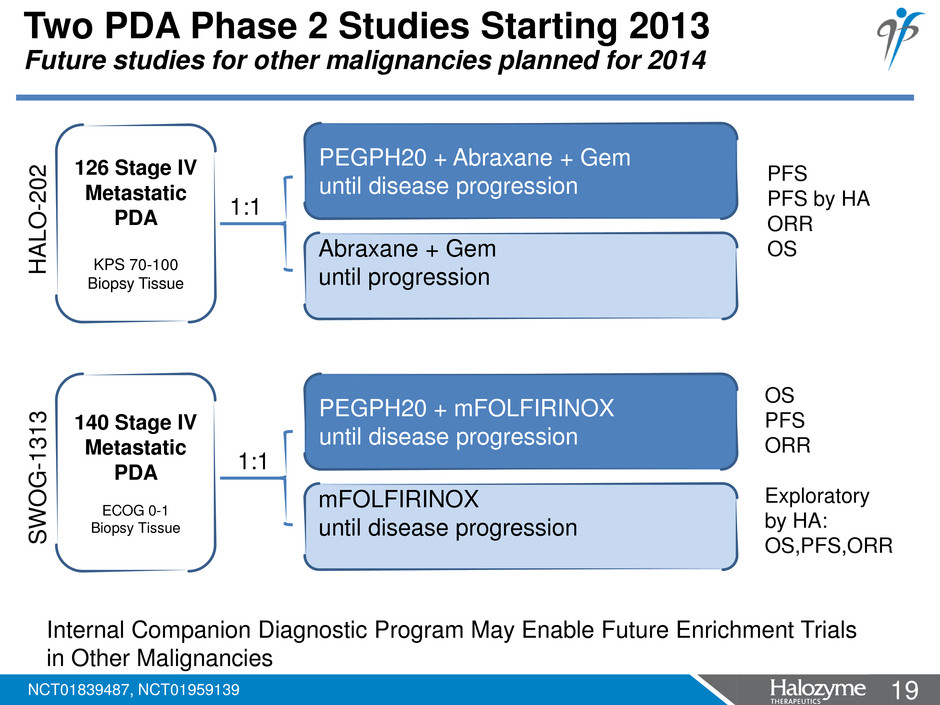
19 <#> Two PDA Phase 2 Studies Starting 2013 Future studies for other malignancies planned for 2014 PEGPH20 + Abraxane + Gem until disease progression Abraxane + Gem until progression 126 Stage IV Metastatic PDA KPS 70-100 Biopsy Tissue 1:1 PEGPH20 + mFOLFIRINOX until disease progression mFOLFIRINOX until disease progression 140 Stage IV Metastatic PDA ECOG 0-1 Biopsy Tissue 1:1 PFS PFS by HA ORR OS OS PFS ORR Exploratory by HA: OS,PFS,ORR HA L O -2 0 2 SW O G -1 3 1 3 Internal Companion Diagnostic Program May Enable Future Enrichment Trials in Other Malignancies NCT01839487, NCT01959139
20 <#> HTI-501 (rHuCAT-L) A Novel Conditionally Active Biologic Multiple conditions involve collagen pathologies • Cellulite, keloid scars, Dupuytren’s contracture HTI-501 (recombinant human cathepsin-L) • Targeted digestion of collagen through unique mechanism • Extracellular pH provides focal control of collagen degradation Clinical program • Cellulite first target • Phase 1 complete – well tolerated at all doses • 36 patient RP2 enrollment completed July 2013 • Independent panel review of 1 month data complete • 3 and 6 month response 1Q2014
21 <#> Financials Well Positioned for 2013 and Beyond 2012 2013 2014+ $76M in cash June 30, 2013 $45-50M 2013 estimated net cash burn Partner Royalties start 2013 Cash flow positive expected as early as end of 2014 Well-financed to support proprietary product launches and advance pipeline
22 <#> Advance proprietary programs • Advance clinical trials for PEGPH20 • Complete HTI-501 Phase 2 clinical trial Secure revenue streams from existing channels • Support EMA filings and launch of Herceptin SC and MabThera SC • Advance insulin pump commercialization Strategies to Ensure Success Poised for a Pivotal Year in 2013 Grow partnerships • Advance SC programs with Pfizer • Pursue new partnerships • $45-50M expected cash burn 2013 • Driving toward positive cash flow by end of 2014 • Maintaining fiscal discipline Manage business toward positive cash flow

A Phase 1b Multicenter International Clinical Trial of Gemcitabine Combined with PEGPH20 (PEGylated Recombinant Human Hyaluronidase) in Patients with Stage IV Previously Untreated Pancreatic Cancer
25 <#> Introduction to Hyaluronan (HA) • A glycosaminoglycan (GAG) polymer of 2,000-25,000 repeating disaccharide units • HA cross-links tumor extracellular matrix via binding to other proteoglycans • Within a tumor microenvironment (TME) several cell types can generate HA, including stromal and malignant cells • Each disaccharide unit of HA can coordinate up to 15 H2O molecules resulting in a 1,000-fold expanded hydrocolloidal gel and altering the TME: • Increased interstitial pressure • Compression of vasculature • A hypoxic, treatment-resistant tumor phenotype
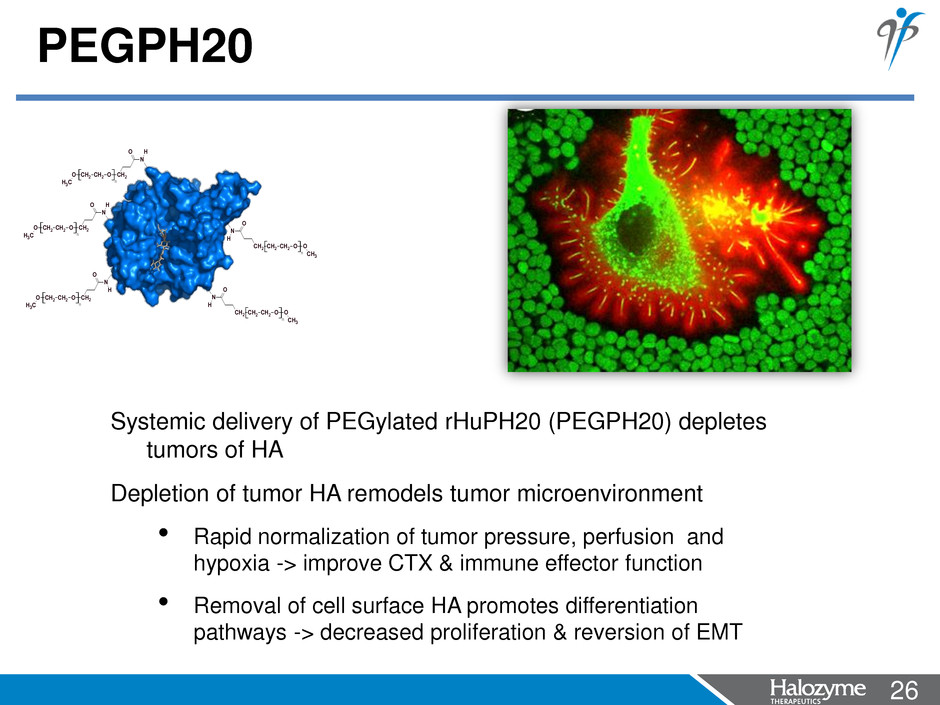
26 <#> PEGPH20 n CH2 CH2 OCH2 O CH3 N H O CH2 CH2 OO CH2 CH3 N H O n CH2 CH2 OO CH2 CH3 N O H n n CH2 CH2 OCH2 O CH3 N H O CH2 CH2 OO CH2 CH3 N O H n Systemic delivery of PEGylated rHuPH20 (PEGPH20) depletes tumors of HA Depletion of tumor HA remodels tumor microenvironment • Rapid normalization of tumor pressure, perfusion and hypoxia -> improve CTX & immune effector function • Removal of cell surface HA promotes differentiation pathways -> decreased proliferation & reversion of EMT
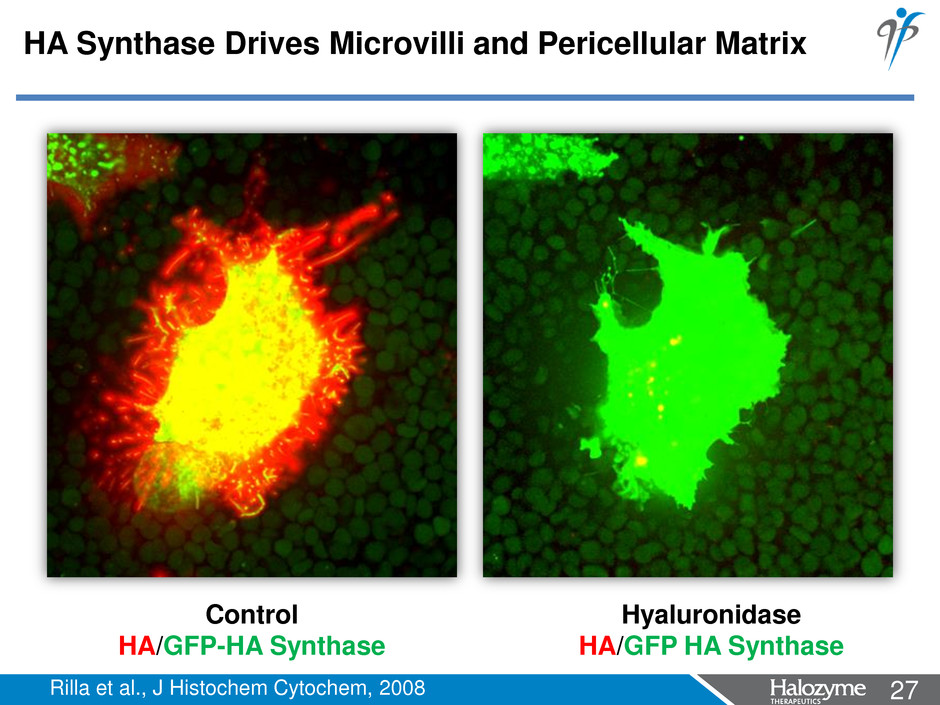
27 <#> Hyaluronidase HA/GFP HA Synthase Control HA/GFP-HA Synthase Rilla et al., J Histochem Cytochem, 2008 HA Synthase Drives Microvilli and Pericellular Matrix

28 <#> Clinical Trial Objectives Primary • Establish the recommended phase 2 dose (RP2D) of PEGPH20 in combination with gemcitabine in patients with Stage IV previously untreated PDAC Secondary • Assess the safety and tolerability of PEGPH20 in combination therapy • Assess tumor response using RECIST 1.1 criteria • Assess the pharmacokinetic (PK) profile and evaluate the pharmacodynamic activity of PEGPH20 • Assess the treatment effect based on tumor HA status

29 <#> Clinical Trial Design • Phase 1b, open-label, multicenter (US and Russia), dose-escalation clinical trial of PEGPH20 in combination with gemcitabine in treatment naïve stage IV pancreatic patients • 3-6 patients were to be enrolled in the dose escalation cohort and 14 patients were to be enrolled in the expansion cohort when RP2D is identified • Patients were treated at one of 3 dose levels (1.0, 1.6 and 3.0 µg/kg twice weekly for 4 weeks, then weekly thereafter) in combination with gemcitabine 1000 mg/m2 IV • Response assessments were performed every 8 weeks • Identification of the recommended phase 2 dose (RP2D) based on safety data from evaluable subjects in Cycle 1 • Dexamethasone (8 mg) was administered orally 1 hour pre and 8 - 12 hours post-PEGPH20
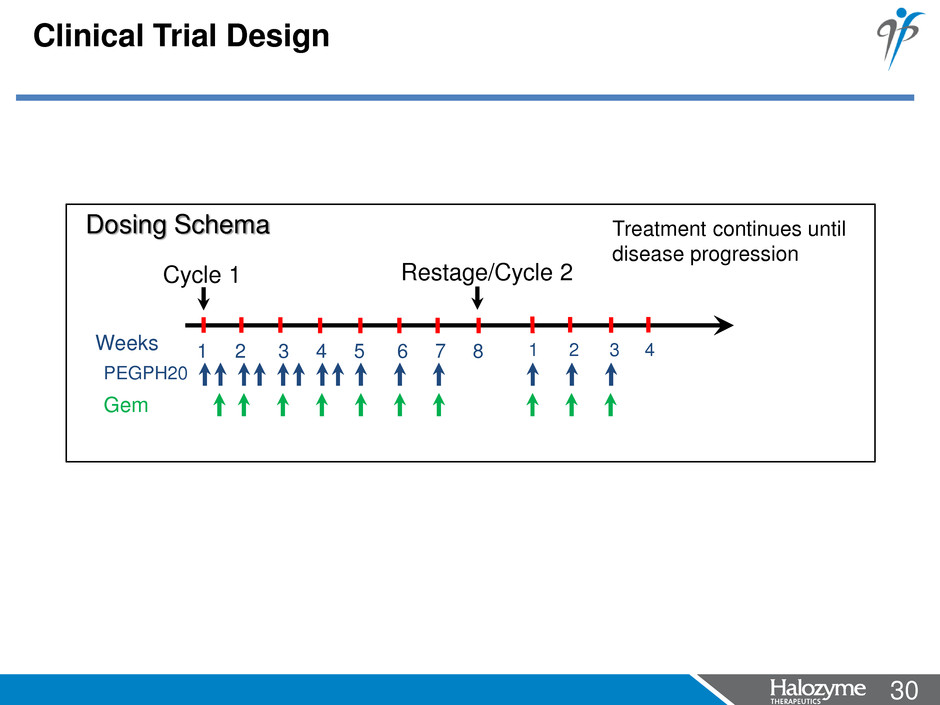
30 <#> Clinical Trial Design 1 2 3 4 5 6 7 8 Weeks Dosing Schema Restage/Cycle 2 Cycle 1 Treatment continues until disease progression PEGPH20 Gem 1 2 3 4

31 <#> Patient Selection Key eligibility criteria: • Newly diagnosed, previously untreated, histologically-confirmed Stage IV PDA with documented disseminated neoplasm to the liver and/or the lung. • One or more metastatic tumors measurable on CT scan per RECIST 1.1 criteria • Acceptable liver and renal functions and hematologic parameters • Karnofsky Performance Status ≥ 70%
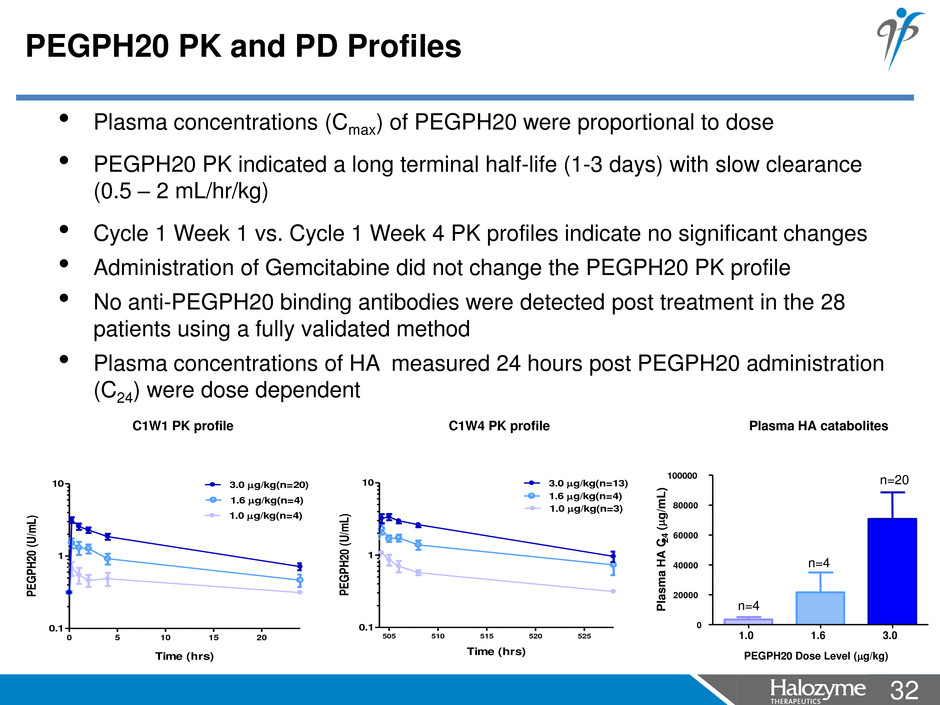
32 <#> PEGPH20 PK and PD Profiles • Plasma concentrations (Cmax) of PEGPH20 were proportional to dose • PEGPH20 PK indicated a long terminal half-life (1-3 days) with slow clearance (0.5 – 2 mL/hr/kg) • Cycle 1 Week 1 vs. Cycle 1 Week 4 PK profiles indicate no significant changes • Administration of Gemcitabine did not change the PEGPH20 PK profile • No anti-PEGPH20 binding antibodies were detected post treatment in the 28 patients using a fully validated method • Plasma concentrations of HA measured 24 hours post PEGPH20 administration (C24) were dose dependent C1W1 PK profile C1W4 PK profile Plasma HA catabolites 0 5 10 15 20 0.1 1 10 1.6 g/kg(n=4) 3.0 g/kg(n=20) 1.0 g/kg(n=4) Time (hrs) PE G PH 20 (U /m L) 505 510 515 520 525 0.1 1 10 1.6 g/kg(n=4) 3.0 g/kg(n=13) 1.0 g/kg(n=3) Time (hrs) PE G PH 20 (U /m L) 1.0 1.6 3.0 0 20000 40000 60000 80000 100000 n=4 n=20 n=4 PEGPH20 Dose Level (g/kg) Pl a s m a H A C 2 4 ( g /m L )
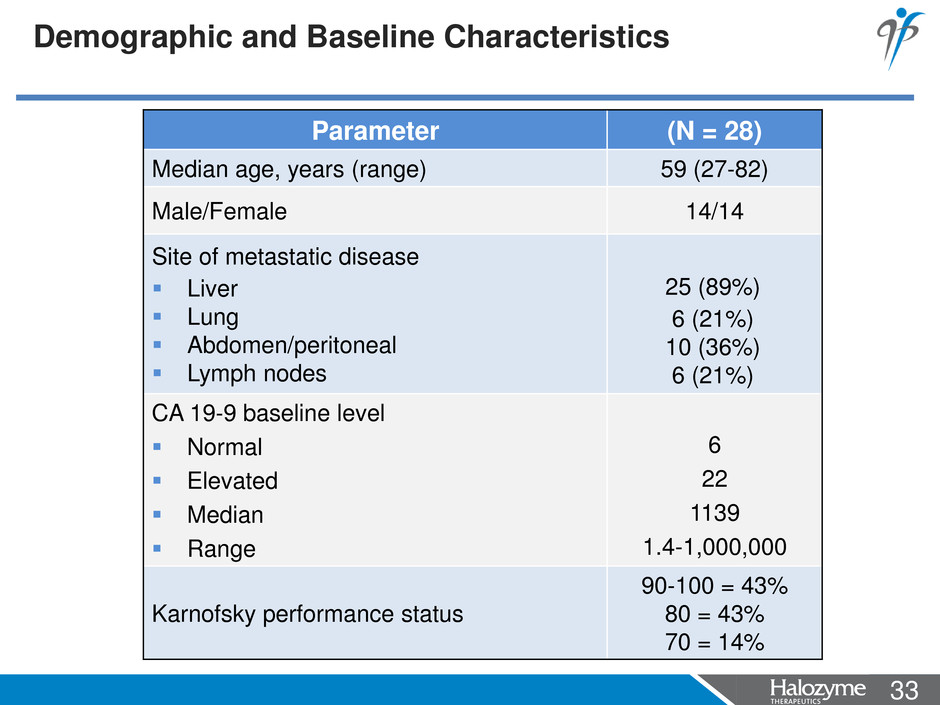
33 <#> Demographic and Baseline Characteristics Parameter (N = 28) Median age, years (range) 59 (27-82) Male/Female 14/14 Site of metastatic disease Liver Lung Abdomen/peritoneal Lymph nodes 25 (89%) 6 (21%) 10 (36%) 6 (21%) CA 19-9 baseline level Normal Elevated Median Range 6 22 1139 1.4-1,000,000 Karnofsky performance status 90-100 = 43% 80 = 43% 70 = 14%
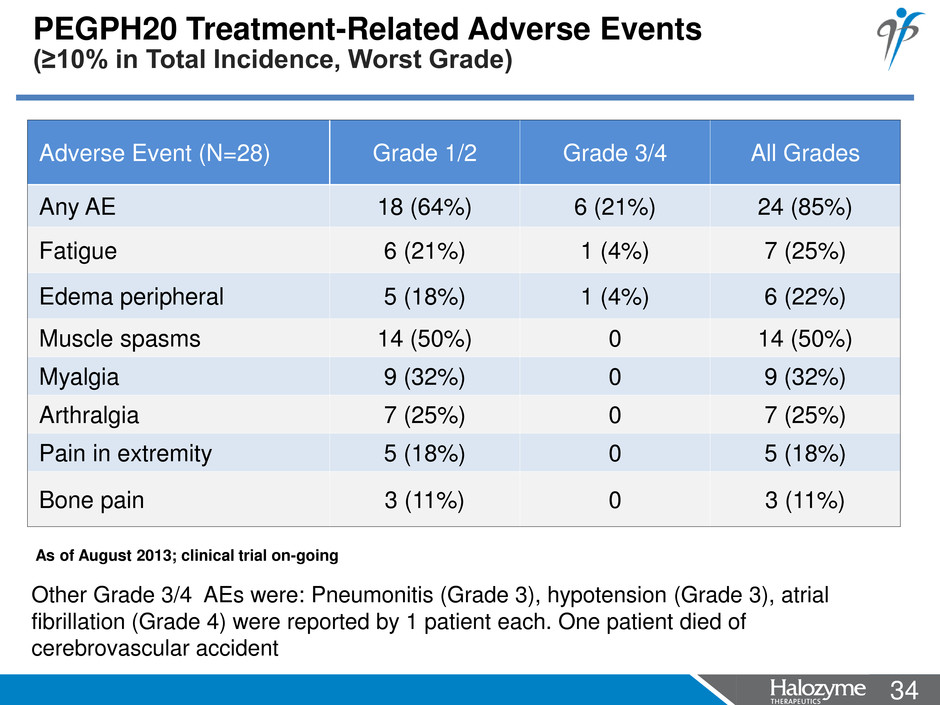
34 <#> PEGPH20 Treatment-Related Adverse Events (≥10% in Total Incidence, Worst Grade) Adverse Event (N=28) Grade 1/2 Grade 3/4 All Grades Any AE 18 (64%) 6 (21%) 24 (85%) Fatigue 6 (21%) 1 (4%) 7 (25%) Edema peripheral 5 (18%) 1 (4%) 6 (22%) Muscle spasms 14 (50%) 0 14 (50%) Myalgia 9 (32%) 0 9 (32%) Arthralgia 7 (25%) 0 7 (25%) Pain in extremity 5 (18%) 0 5 (18%) Bone pain 3 (11%) 0 3 (11%) Other Grade 3/4 AEs were: Pneumonitis (Grade 3), hypotension (Grade 3), atrial fibrillation (Grade 4) were reported by 1 patient each. One patient died of cerebrovascular accident As of August 2013; clinical trial on-going
35 <#> Efficacy Assessment • Response was assessed by independent central radiology using RECIST 1.1 criteria • Baseline core biopsy was optional in the dose escalation phase and required in the expansion cohort • Exploratory analyses were conducted to assess the combination treatment effect based on tumor biopsy HA presentation
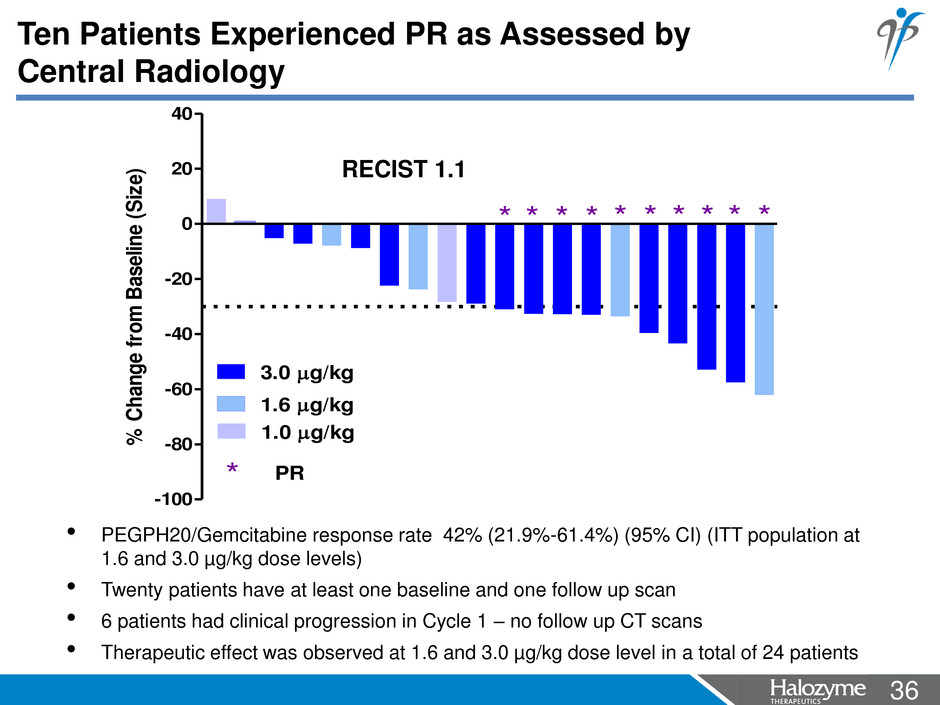
36 <#> Ten Patients Experienced PR as Assessed by Central Radiology • PEGPH20/Gemcitabine response rate 42% (21.9%-61.4%) (95% CI) (ITT population at 1.6 and 3.0 µg/kg dose levels) • Twenty patients have at least one baseline and one follow up scan • 6 patients had clinical progression in Cycle 1 – no follow up CT scans • Therapeutic effect was observed at 1.6 and 3.0 µg/kg dose level in a total of 24 patients -100 -80 -60 -40 -20 0 20 40 * ** ** ** * ** * PR 1.0 g/kg 3.0 g/kg 1.6 g/kg % C ha ng e fro m B as eli ne (S ize ) RECIST 1.1
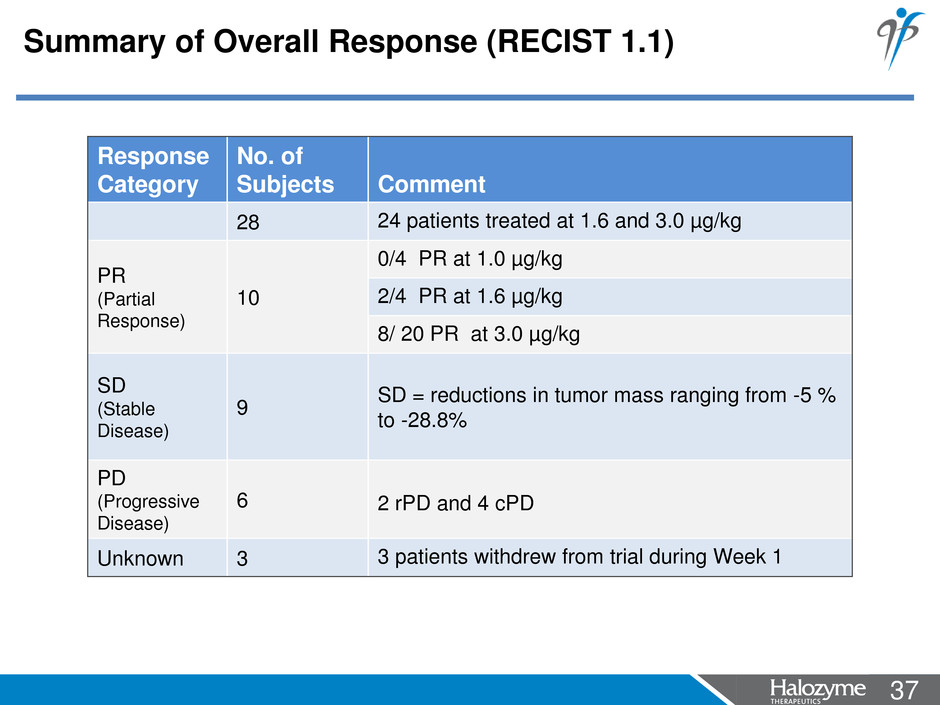
37 <#> Response Category No. of Subjects Comment 28 24 patients treated at 1.6 and 3.0 µg/kg PR (Partial Response) 10 0/4 PR at 1.0 µg/kg 2/4 PR at 1.6 µg/kg 8/ 20 PR at 3.0 µg/kg SD (Stable Disease) 9 SD = reductions in tumor mass ranging from -5 % to -28.8% PD (Progressive Disease) 6 2 rPD and 4 cPD Unknown 3 3 patients withdrew from trial during Week 1 Summary of Overall Response (RECIST 1.1)

38 <#> PEGPH20 + Gemcitabine Treatment Reduced Serum CA19-9 -100 -80 -60 -40 -20 0 20 40 60 80 100 120 140 160 180 200 ** * * * * 3.0 g/kg 1.0 g/kg 1.6 g/kg * PR * Ch an ge fr om B as eli ne ( % ) • Elevated CA19-9 is defined as >37 U/mL • 12 of 14 patients experienced CA19-9 response • 6/14 (43%) patients achieved ≥ 70% reduction CA19-9 Baseline Normal (N) 6 Elevated (N) 22 Median (U/mL) 1139 Range (U/mL) 1.4-1,000,000
39 <#> HA Assessment in Tumor Biopsy • Baseline core biopsy was optional in the dose escalation phase and required in the expansion cohort • The method of HA scoring was prospectively defined for tumor-cell associated and stromal associated tissue • Staining was semi-quantified by an independent board certified pathologist using H-score method of the central pathology lab • Exploratory analysis were conducted to assess the combination treatment effect based on HA presentation in tumor biopsy
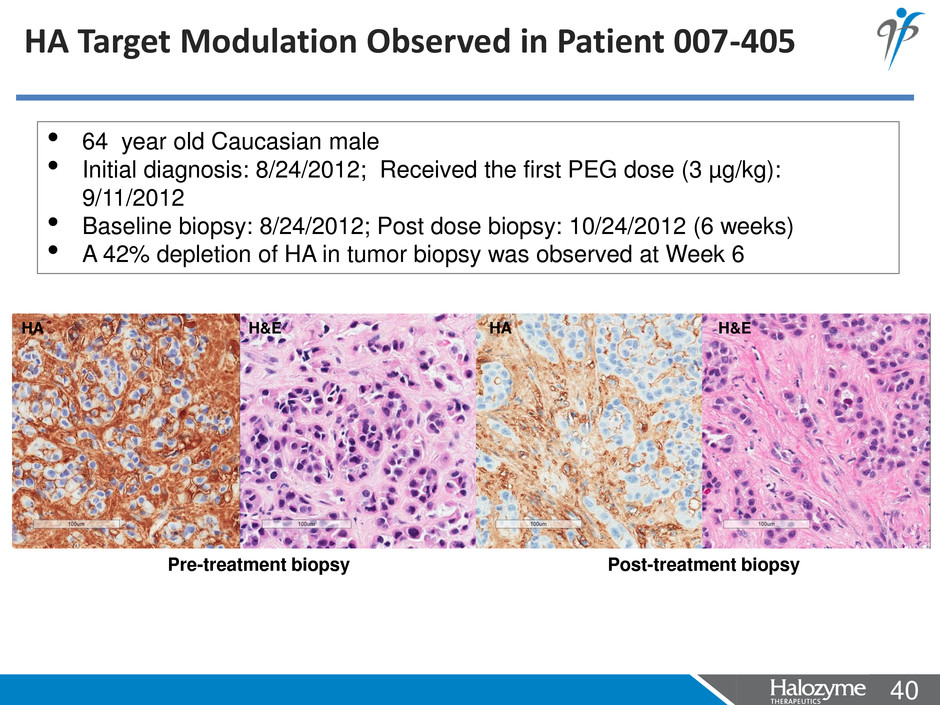
40 <#> HA Target Modulation Observed in Patient 007-405 • 64 year old Caucasian male • Initial diagnosis: 8/24/2012; Received the first PEG dose (3 µg/kg): 9/11/2012 • Baseline biopsy: 8/24/2012; Post dose biopsy: 10/24/2012 (6 weeks) • A 42% depletion of HA in tumor biopsy was observed at Week 6 HA H&E Pre-treatment biopsy Post-treatment biopsy HA H&E
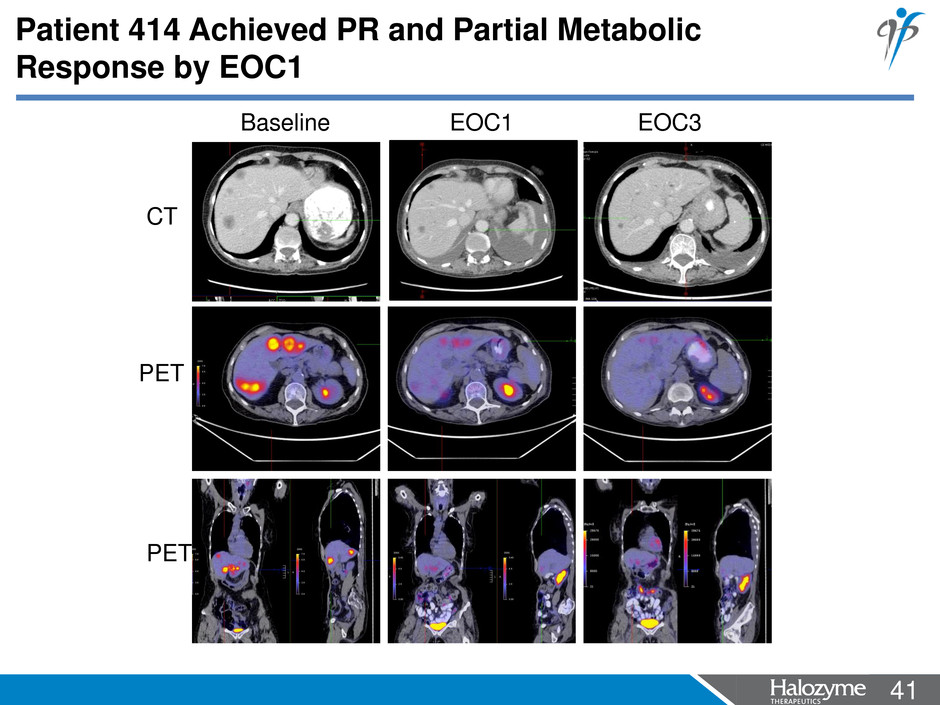
41 <#> PET CT Baseline EOC1 EOC3 PET Patient 414 Achieved PR and Partial Metabolic Response by EOC1
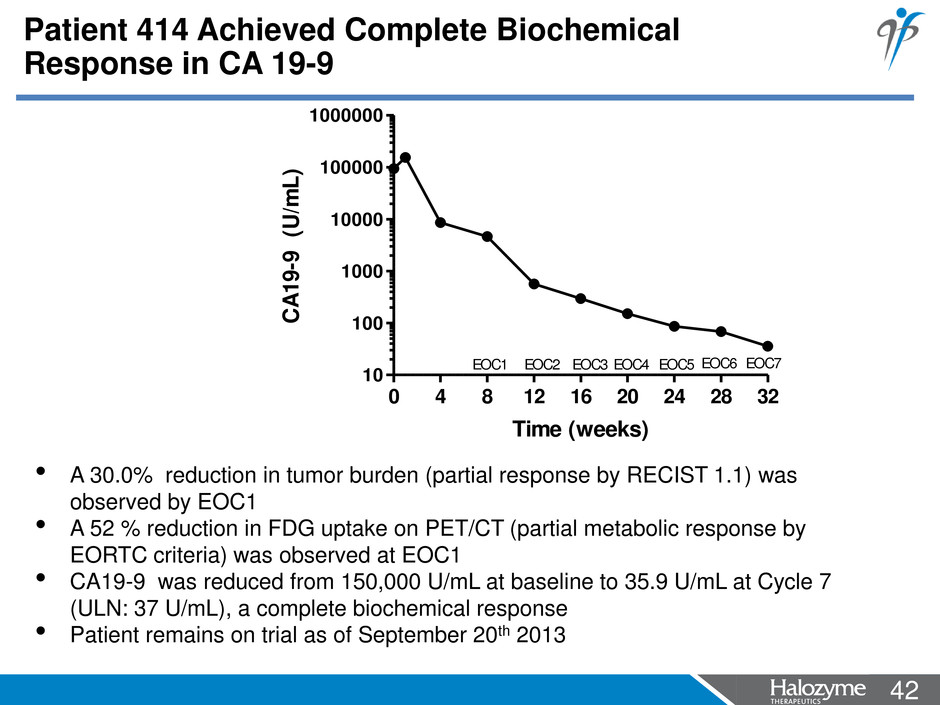
42 <#> • A 30.0% reduction in tumor burden (partial response by RECIST 1.1) was observed by EOC1 • A 52 % reduction in FDG uptake on PET/CT (partial metabolic response by EORTC criteria) was observed at EOC1 • CA19-9 was reduced from 150,000 U/mL at baseline to 35.9 U/mL at Cycle 7 (ULN: 37 U/mL), a complete biochemical response • Patient remains on trial as of September 20th 2013 0 4 8 12 16 20 24 28 32 10 100 1000 10000 100000 1000000 EOC1 EOC2 EOC3 EOC4 EOC5 EOC7EOC6 Time (weeks) CA 19 -9 ( U/ m L) Patient 414 Achieved Complete Biochemical Response in CA 19-9
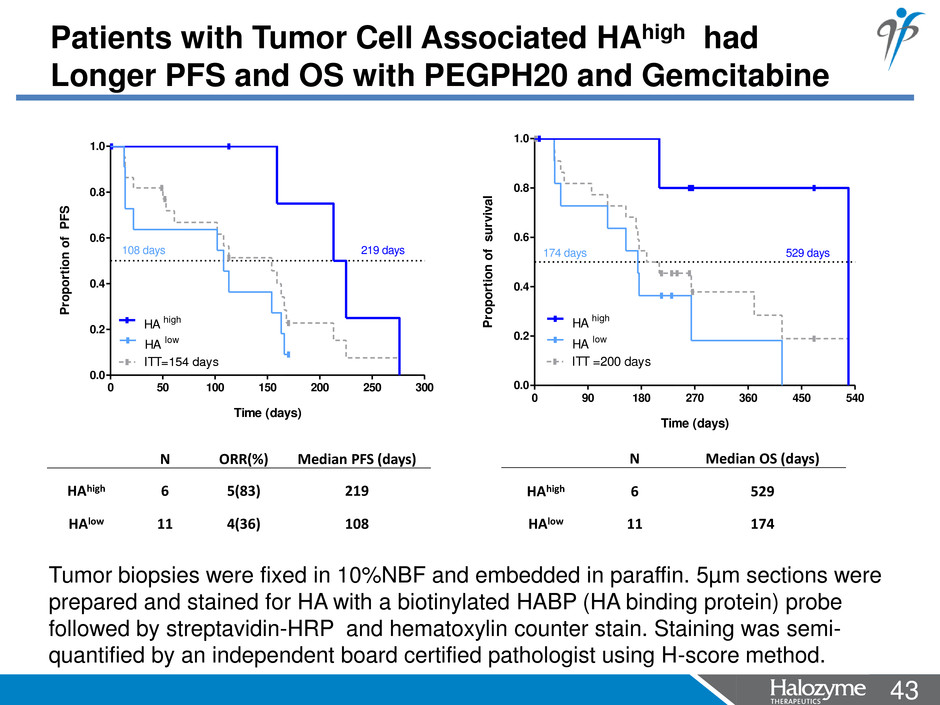
43 <#> Tumor biopsies were fixed in 10%NBF and embedded in paraffin. 5µm sections were prepared and stained for HA with a biotinylated HABP (HA binding protein) probe followed by streptavidin-HRP and hematoxylin counter stain. Staining was semi- quantified by an independent board certified pathologist using H-score method. N ORR(%) Median PFS (days) HAhigh 6 5(83) 219 HAlow 11 4(36) 108 N Median OS (days) HAhigh 6 529 HAlow 11 174 0 50 100 150 200 250 300 0.0 0.2 0.4 0.6 0.8 1.0 ITT=154 days HA high HA low 219 days108 days Time (days) Pr op or tio n of P FS 0 90 180 270 360 450 540 0.0 0.2 0.4 0.6 0.8 1.0 ITT =200 days HA high HA low 529 days174 days Time (days) P ro po rt io n of s ur vi va l Patients with Tumor Cell Associated HAhigh had Longer PFS and OS with PEGPH20 and Gemcitabine
44 <#> • 28 patients were enrolled and received at least one dose of study drug. 24 patients were treated at either 1.6 or 3.0 µg/kg; RP2D is 3.0 µg/kg • MSEs (muscle spasm, myalgia and arthralgia) were the most common treatment-related adverse events and restricted to grade 1 and 2; chronic therapy (up to 9 Cycles, ~ 10 months) was well tolerated • PK analysis demonstrated sustained exposure of PEGPH20 with slow plasma clearance (0.5 – 2.0 mL/hr/kg) and long terminal half-life (2-3 days) • No anti-PEGPH20 antibodies were detected post treatment at any time post treatment (up to 10 months) • Response rate at 1.6 and 3.0 µg/kg was 42% (21.9%-61.4%, 95% CI) • CA19-9 reduction of ≥70% was achieved in 6/14 (43%) patients with available CA19-9 levels pre and post treatment • PFS and OS were longer in patients with high tumor-cell associated HA compared to those with low tumor-cell associated HA • Tumor biopsy HA levels may prove predictive for treatment effect Summary
45 <#> • PEGPH20 in combination with gemcitabine is well tolerated in stage IV PDA patients • Confirmation of combination treatment effect (ORR, PFS and OS) based on HA levels will be further evaluated in the on-going randomized phase 2 studies where biopsy is a requirement for patient enrollment • The prognostic significance of tumor-cell associated HA may be of clinical utility if confirmed in the on-going randomized phase 2 studies • Preliminary efficacy data is encouraging. Further combination therapies with PEGPH20 in PDA and other HA positive tumor indications are warranted • A randomized phase 2 clinical trial of PEGPH20 in combination with nab- paclitaxel and gemcitabine for the treatment of stage IV PDA patients is currently enrolling in the US (clinicaltrial.gov identifier:NCT01839487). • A randomized phase 2 clinical trial of PEGPH20 in combination with mFolfirinox in stage IV PDA patients with good performance status was recently activated (clinicaltrial.gov identifier:NCT01959139) Conclusion

46 <#> Patients and their supporting families Sunil R Hingorani1MD, PhD, William P Harris2MD, Joseph Thaddeus Beck3MD, Boris A Berdov4MD, Stephanie A Wagner5MD, Eduard M Pshevlotsky6MD, Sergei A Tjulandin7MD, Oleg A Gladkov8MD, Randall F Holcombe9MD, Lisa Schechet10, Ping Jiang 10MD, Joy Zhu10MD, PhD, Craig E Devoe11MD. Institutions: 1Fred Hutchinson Cancer Research Center, 2Seattle Cancer Care Alliance, 3Highlands Oncology Group, 4Medical Radiological Research Center, 5Indiana University Melvin and Bren Simon Cancer Center, 6Omsk Regional Budget Medical Institution: Clinical Oncological Center, 7Russian Oncology Research Center n.a.N.N. Blokhin, 8Chelyabinsk Regional Clinical Oncology Center, 9Mount Sinai School of Medicine, 10Halozyme Therapeutics, 11Monter Cancer Center Acknowledgement













































