
PEGPH20: The Science & The Strategy January 7, 2015 Exhibit 99.2 1

Forward-Looking Statements All of the statements in this presentation that are not statements of historical facts constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Examples of such statements include future product development and regulatory events and goals, anticipated clinical trial results and strategies, product collaborations, our business intentions and financial estimates and results. These statements are based upon management’s current plans and expectations and are subject to a number of risks and uncertainties which could cause actual results to differ materially from such statements. A discussion of the risks and uncertainties that can affect these statements is set forth in the Company’s annual and quarterly reports filed from time to time with the Securities and Exchange Commission under the heading “Risk Factors.” The Company disclaims any intention or obligation to revise or update any forward-looking statements, whether as a result of new information, future events, or otherwise.

Halozyme: Two Potential Drivers of Future Growth and Value ENHANZE™ (rHuPH20) n CH2 CH2 OCH2 O CH3 N H O CH2 CH2 OO CH2 CH3 N H O n CH2 CH2 OO CH2 CH3 N O H n n CH2 CH2 OCH2 O CH3 N H O CH2 CH2 OO CH2 CH3 N O H n PEGylated form of rHuPH20 Investigational new drug in phase 2 development PEGPH20 3

PEGPH20 – Investigational new drug – Intended to target Hyaluronan (HA), a key component of the tumor microenvironment (TME) – Potential to increase tumor-selective access of co-administered anticancer therapy into tumors that accumulate high levels of hyaluronan (HAhigh) • Small molecule chemotherapy • Monoclonal antibody cancer therapies, including immunotherapy • Immune cells – Doubles survival in a number of animal models when combined with cancer therapy, compared to cancer therapy alone Encouraging early efficacy data in Phase 1b and 2 trials of Stage IV pancreatic ductal adenocarcinoma when co-administered with – Gemcitabine – Gemcitabine and Abraxane® PEGPH20 Goal: Improving Targeting of Co-Administered Cancer Drugs 4
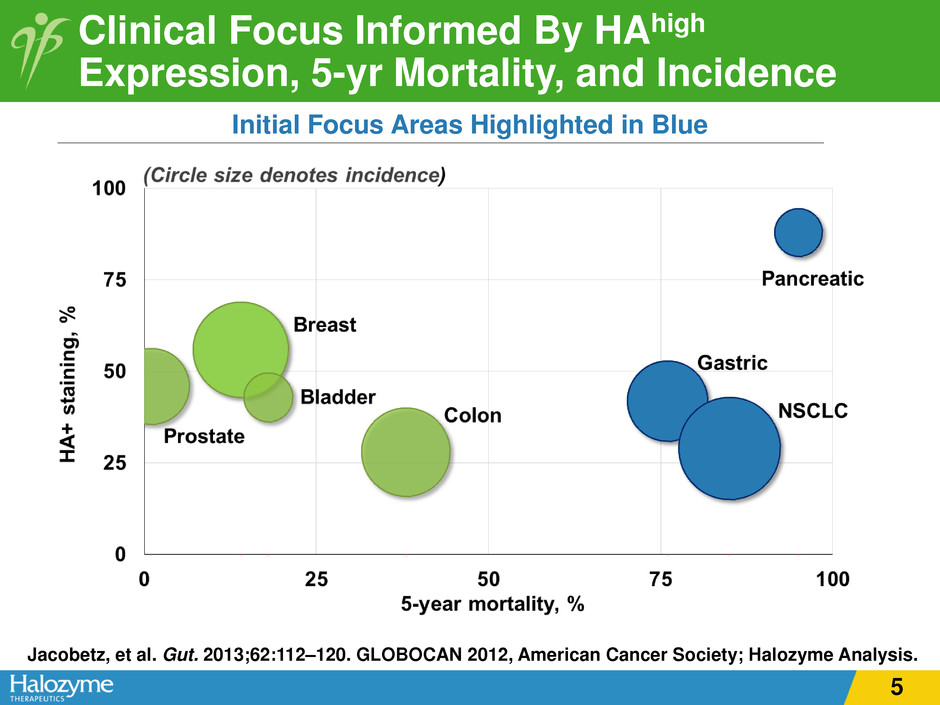
Clinical Focus Informed By HAhigh Expression, 5-yr Mortality, and Incidence Initial Focus Areas Highlighted in Blue Jacobetz, et al. Gut. 2013;62:112–120. GLOBOCAN 2012, American Cancer Society; Halozyme Analysis. 5
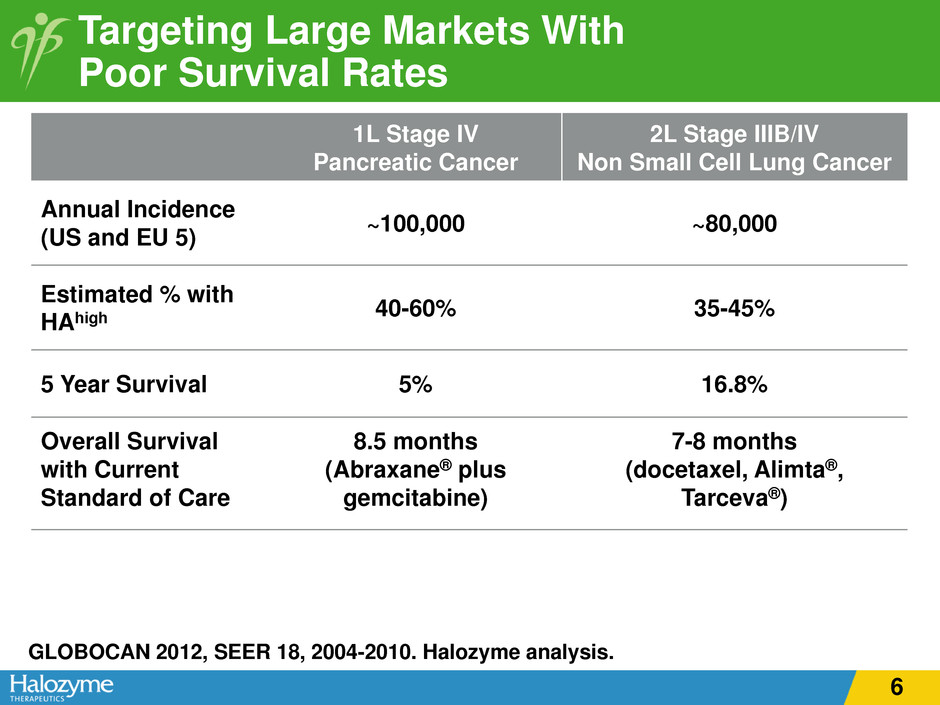
1L Stage IV Pancreatic Cancer 2L Stage IIIB/IV Non Small Cell Lung Cancer Annual Incidence (US and EU 5) ~100,000 ~80,000 Estimated % with HAhigh 40-60% 35-45% 5 Year Survival 5% 16.8% Overall Survival with Current Standard of Care 8.5 months (Abraxane® plus gemcitabine) 7-8 months (docetaxel, Alimta®, Tarceva®) Targeting Large Markets With Poor Survival Rates GLOBOCAN 2012, SEER 18, 2004-2010. Halozyme analysis. 6

2015 Financial Guidance 2015E Comments Net Revenues $85M - $95M Royalties, sales of rHuPH20 to partners, Hylenex sales and collaboration revenues Operating Expenses1 $145M - $155M R&D driven by clinical trial enrollment Cash Burn $35M - $45M Does not include new ENHANZE™ deals NOTE: 1) Includes ~$20M in non-cash expenses, stock compensation and depreciation. Cash position end 2014: approximately $135M Anticipated ENHANZE™ revenue funds operations without need for dilutive financing 7

Today’s Agenda Introduction and Objectives Helen Torley, MB, ChB, MRCP President and Chief Executive Officer Halozyme Therapeutics PEGPH20 Mechanism of Action Christopher Thanos, PhD Director, Biotherapeutics Halozyme Therapeutics Exploring Combinations of PEGPH20 With Cancer Therapies Curt Thompson, PhD Senior Director, Pharmacology Halozyme Therapeutics From Theory to Initial Clinical Experience Sunil R. Hingorani, MD, PhD Associate Member, FHCRC Director, Center for Accelerated Translation in Pancreas Cancer Clinical Development Plan Update Athena Countouriotis, MD Chief Medical Officer Halozyme Therapeutics Q&A 8

Today’s Agenda Introduction and Objectives Helen Torley, MB, ChB, MRCP President and Chief Executive Officer Halozyme Therapeutics PEGPH20 Mechanism of Action Christopher Thanos, PhD Director, Biotherapeutics Halozyme Therapeutics Exploring Combinations of PEGPH20 With Cancer Therapies Curt Thompson, PhD Senior Director, Pharmacology Halozyme Therapeutics From Theory to Initial Clinical Experience Sunil R. Hingorani, MD, PhD Associate Member, FHCRC Director, Center for Accelerated Translation in Pancreas Cancer Clinical Development Plan Update Athena Countouriotis, MD Chief Medical Officer Halozyme Therapeutics Q&A 9

PEGPH20: Mechanism of Action Christopher Thanos, PhD Director, Biotherapeutics Halozyme Therapeutics 10
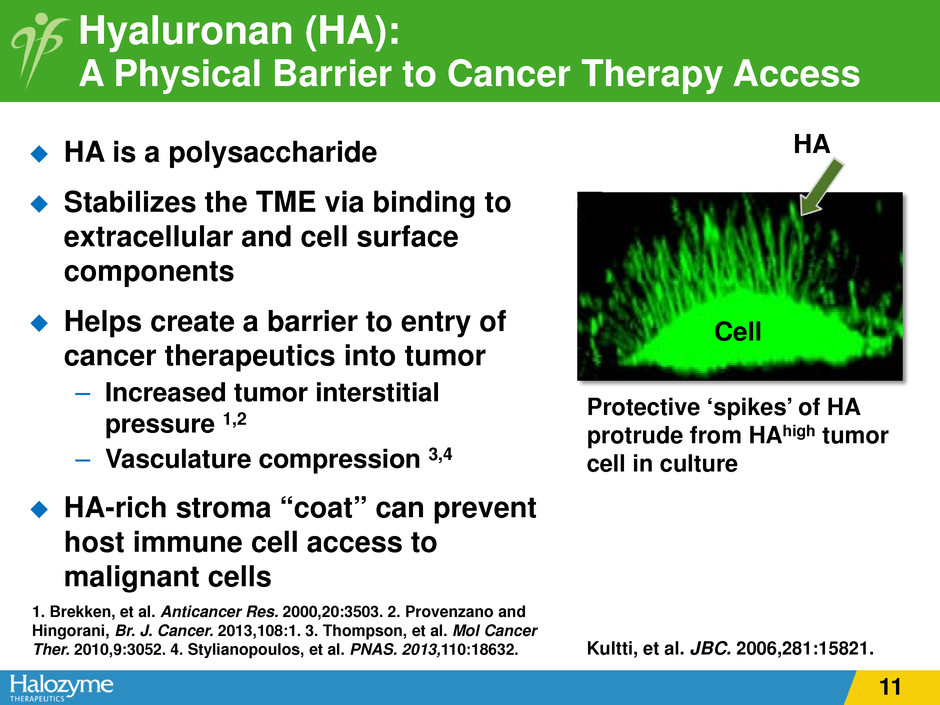
Hyaluronan (HA): A Physical Barrier to Cancer Therapy Access HA is a polysaccharide Stabilizes the TME via binding to extracellular and cell surface components Helps create a barrier to entry of cancer therapeutics into tumor – Increased tumor interstitial pressure 1,2 – Vasculature compression 3,4 HA-rich stroma “coat” can prevent host immune cell access to malignant cells Protective ‘spikes’ of HA protrude from HAhigh tumor cell in culture Cell HA 1. Brekken, et al. Anticancer Res. 2000,20:3503. 2. Provenzano and Hingorani, Br. J. Cancer. 2013,108:1. 3. Thompson, et al. Mol Cancer Ther. 2010,9:3052. 4. Stylianopoulos, et al. PNAS. 2013,110:18632. Kultti, et al. JBC. 2006,281:15821. 11
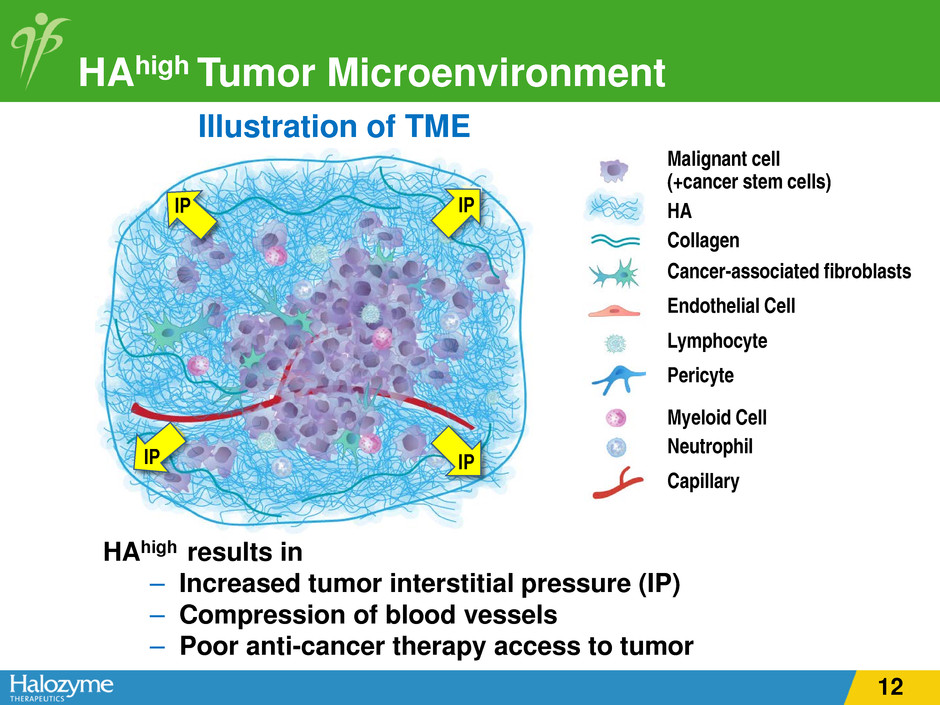
HAhigh Tumor Microenvironment Illustration of TME HAhigh results in – Increased tumor interstitial pressure (IP) – Compression of blood vessels – Poor anti-cancer therapy access to tumor Malignant cell (+cancer stem cells) HA Collagen Cancer-associated fibroblasts Endothelial Cell Lymphocyte Pericyte Myeloid Cell Neutrophil Capillary IP IP IP IP 12
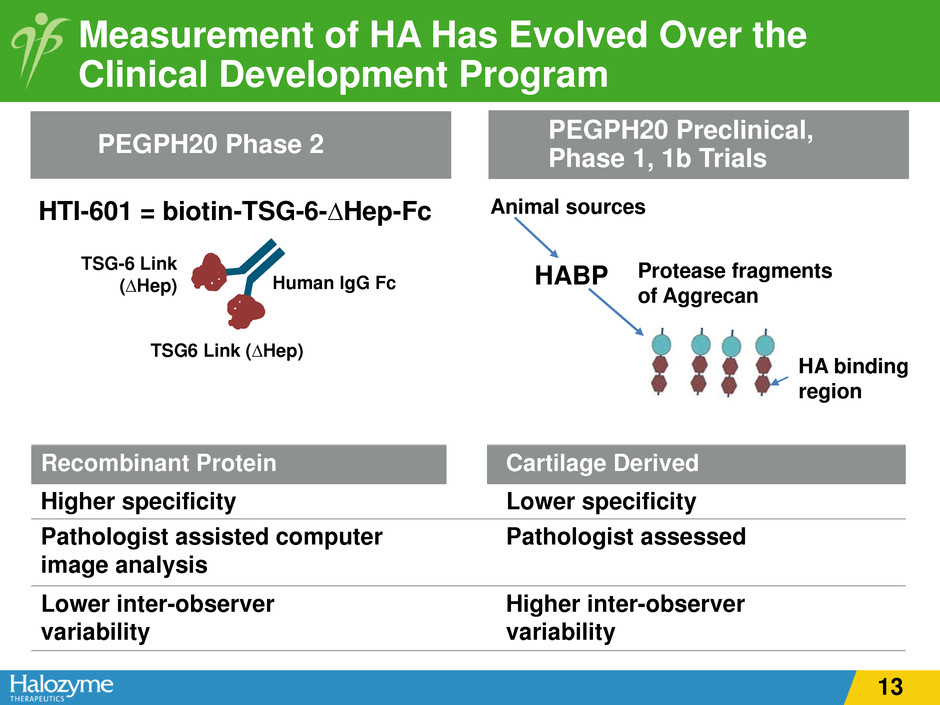
TSG6 Link (∆Hep) TSG-6 Link (∆Hep) Human IgG Fc HTI-601 = biotin-TSG-6-∆Hep-Fc Measurement of HA Has Evolved Over the Clinical Development Program Recombinant Protein Cartilage Derived Higher specificity Lower specificity Pathologist assisted computer image analysis Pathologist assessed Lower inter-observer variability Higher inter-observer variability PEGPH20 Preclinical, Phase 1, 1b TrialsPEGPH20 Phase 2 HABP Protease fragments of Aggrecan Animal sources HA binding region 13

HA Accumulation Similar In Primary Tumor and Metastasis Archived biopsies from breast adenocarcinoma samples stained with HTI 601 HAhigh Primary Breast Cancer HAhigh Paired Brain Metastasis Jadin, et al. Submitted for publication 2014. 14
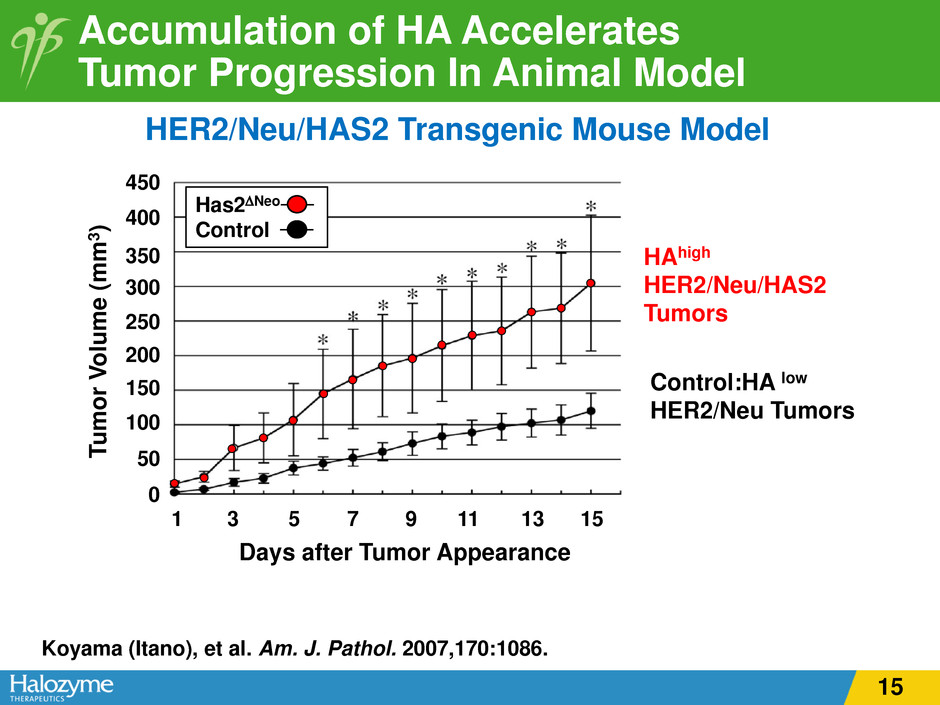
Accumulation of HA Accelerates Tumor Progression In Animal Model Koyama (Itano), et al. Am. J. Pathol. 2007,170:1086. Has2∆Neo Control 450 0 50 100 150 200 250 300 350 400 Tu m or V ol um e (m m 3 ) Days after Tumor Appearance HER2/Neu/HAS2 Transgenic Mouse Model 1 3 5 7 9 11 13 15 HAhigh HER2/Neu/HAS2 Tumors Control:HA low HER2/Neu Tumors 15
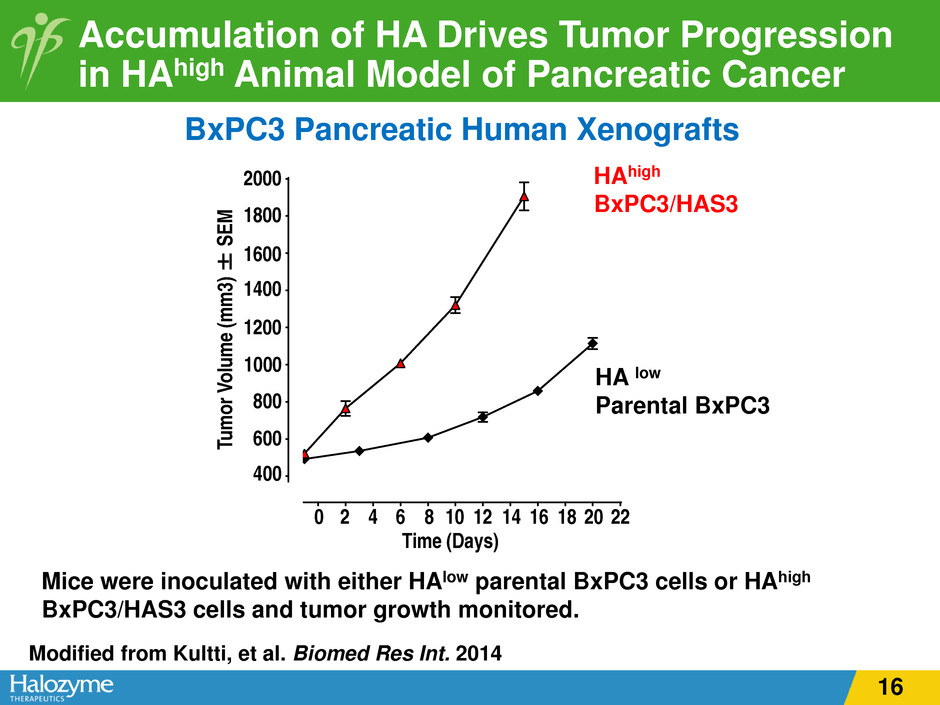
Accumulation of HA Drives Tumor Progression in HAhigh Animal Model of Pancreatic Cancer Modified from Kultti, et al. Biomed Res Int. 2014 Mice were inoculated with either HAlow parental BxPC3 cells or HAhigh BxPC3/HAS3 cells and tumor growth monitored. BxPC3 Pancreatic Human Xenografts 0 2 4 6 8 1 0 1 2 1 4 1 6 1 8 2 0 2 2 4 0 0 6 0 0 8 0 0 1 0 0 0 1 2 0 0 1 4 0 0 1 6 0 0 1 8 0 0 2 0 0 0 T i m e ( D a y s ) T u m o r V o lu m e ( m m 3 ) ± S E M H A l o w p a r e n t a l B x P C 3 H A h i g h B x P C 3 / H A S 3 Tu m or V ol um e (m m 3) ± SE M 20 180 1600 140 120 10 0 800 600 400 0 2 4 6 8 10 12 14 16 18 20 22 Time (Days) high BxPC3/HAS3 A low Parental BxPC3 16
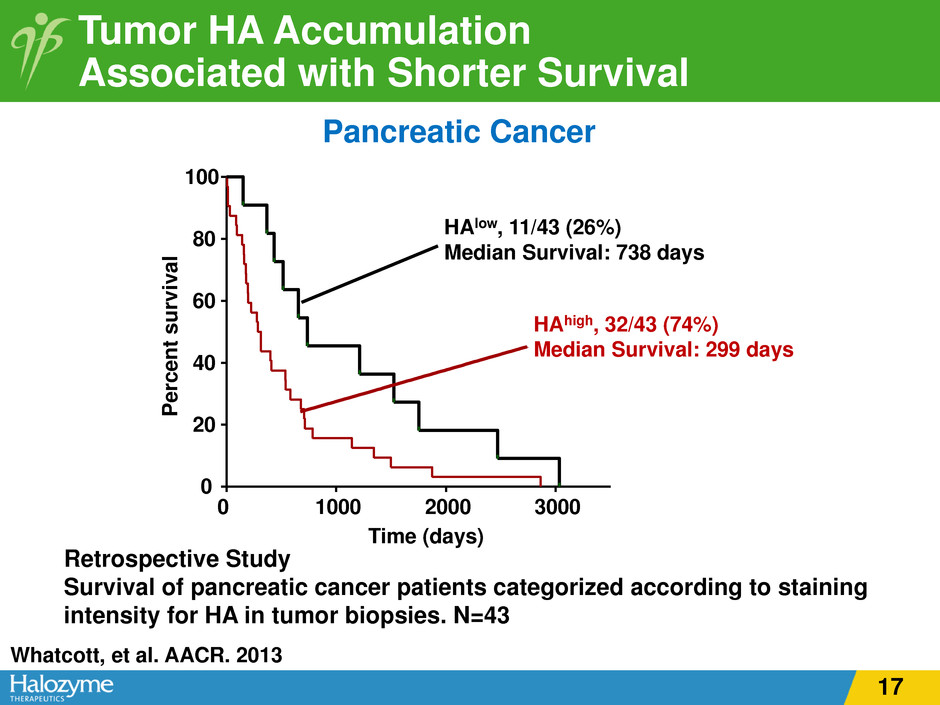
Tumor HA Accumulation Associated with Shorter Survival Pancreatic Cancer Retrospective Study Survival of pancreatic cancer patients categorized according to staining intensity for HA in tumor biopsies. N=43 HAhigh, 32/43 (74%) Median Survival: 299 days HAlow, 11/43 (26%) Median Survival: 738 days P er ce nt s ur vi va l Time (days) 0 1000 2000 3000 0 20 40 60 80 100 Whatcott, et al. AACR. 2013 17
Non-Clinical Approach: Validated Methods, Confirmation in Independent Labs Assessment of PEGPH20 (single agent and enhanced combination) antitumor activity in validated models – HAhigh human xenograft models – Patient derived (PDx) HAhigh tumor models – Genetically engineered mouse models of pancreatic ductal adenocarcinoma Independent Laboratories confirmed observations – Dr. S. Hingorani, Fred Hutchinson Cancer Center – Dr. D. Diamond, City of Hope – Dr. N. Itano, Kyoto Sangyo University – Dr. D. Tuveson, Cold Spring Harbor 18

PEGPH20-Mediated HA Depletion Selectively Impacts HAhigh Tumors In Animal Model 0 2 4 6 8 1 0 1 2 2 5 0 5 0 0 7 5 0 1 0 0 0 1 2 5 0 1 5 0 0 1 7 5 0 2 0 0 0 P E G P H 2 0 4 .5 m g /k g V e h ic le T im e (D a y s ) T u m o r V o lu m e ( m m 3 ) ± S E M NSCLC PDx Model Example 0 3 6 9 1 2 1 5 1 8 2 1 0 2 0 0 4 0 0 6 0 0 8 0 0 1 0 0 0 1 2 0 0 V e h ic le 1 0 0 % T G I P E G P H 2 0 4 .5 m g /k g T im e (D a y s ) T u m o r V o lu m e ( m m 3 ) ± S E M HAlowHAhigh Jiang, et al. Halozyme Therapeutics, Inc. unpublished data, experiments completed 2014. 19

PEGPH20-Mediated HA Removal In Animal Model Reduces Tumor Interstitial Pressure, Decompressing Vessels Normalization of Tumor IP PEGPH20-mediated Vessel Expansion -2 0 0 2 0 4 0 6 0 8 0 1 0 0 1 2 0 0 .0 0 .2 0 .4 0 .6 0 .8 1 .0 1 .2 0 .0 1 5 0 .1 5 1 .5 4 .5 1 5 C o n t r o l T im e a ft e r t r e a t m e n t (m in ) N o rm a li ze d T u m o r IF P ~40 mmHgCRPC PC3 peritibial tumors IV dose Endothelial labeling Thompson, et al. Mol. Cancer Ther. 2010,9:3052. Vehicle (24h) PEGPH20 (24h) .015 0.15 1.5 .5 15 ontrol 20

Tumor Reperfusion Post PEGPH20 Administration Results In Increased Drug Accumulation in Tumor Animal Model Osgood et al., AACR, Pancreatic Cancer Mtg., May 2014 ABRAXANE® Treated BxPC3/HAS3 Pancreatic Xenograft Tumors Mice (n≥4) received nab-paclitaxel (10 mg/kg, IV) ± PEGPH20 (1 mg/kg, IV). Mice sacrificed 1h post- nab-paclitaxel. V e h ic le P E G P H 2 0 0 1 2 3 4 5 6 7 n g /m g t is s u e , p a c li ta x e l ± S tD e v 43%↑ [paclitaxel]tum * Tumor Perfusion P E G P H 20 ( 24 h) Ve hi cl e (2 4h ) PC3 Prostate Xenograft Tumors Thompson et al. Mol Cancer Ther. 2010,9:3052. Hyperechoic microbubbles to visualize vasculature “space” or vascular area of peritibial PC3 tumors ± PEGPH20 (15 mg/kg, IV). Blue tracing is tumor area. 21

PEGPH20 Evaluated With Multiple Cancer Therapeutics Across Broad Range of Sizes In preclinical studies, PEGPH20 was shown to improve therapeutic efficacy independent of size, charge or PK of the therapeutic. 0 .1 1 1 0 1 0 0 1 0 0 0 1 0 0 0 0 1 0 0 0 0 0 S ize (n m ) monoclonal antibodies (e.g. cetuximab, trastuzumab) small molecules (e.g. gemcitabine) liposomes / nanoparticles (e.g. ABRAXANE®, DOXIL®) NK Cells (lymphocytes) shIDO-ST 22
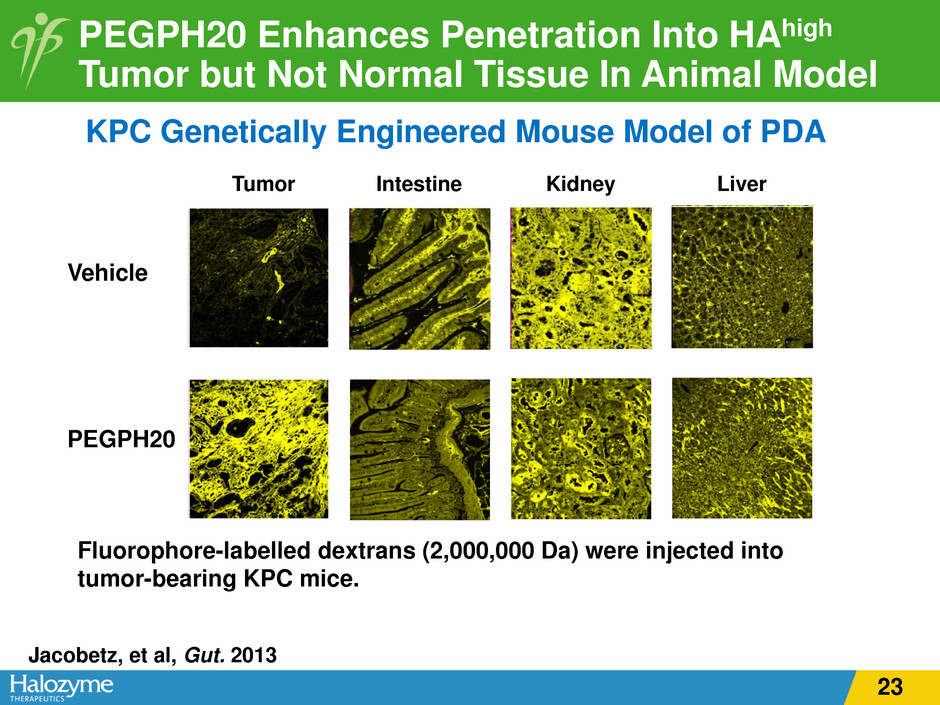
PEGPH20 Enhances Penetration Into HAhigh Tumor but Not Normal Tissue In Animal Model Fluorophore-labelled dextrans (2,000,000 Da) were injected into tumor-bearing KPC mice. Tumor Vehicle Intestine Kidney Liver PEGPH20 KPC Genetically Engineered Mouse Model of PDA Jacobetz, et al, Gut. 2013 23
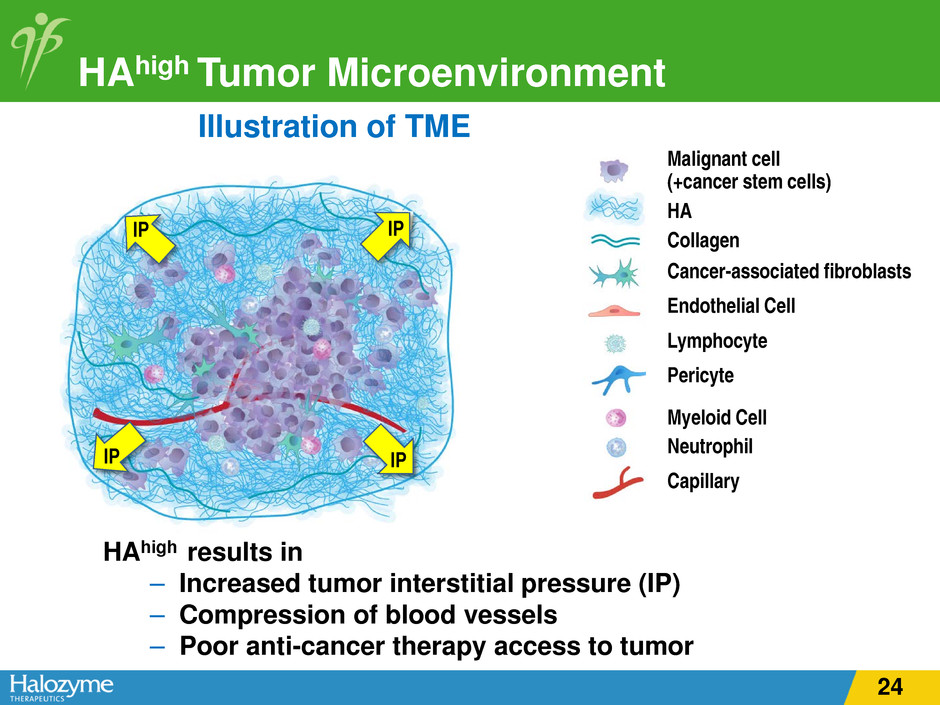
HAhigh Tumor Microenvironment Illustration of TME HAhigh results in – Increased tumor interstitial pressure (IP) – Compression of blood vessels – Poor anti-cancer therapy access to tumor Malignant cell (+cancer stem cells) HA Collagen Cancer-associated fibroblasts Endothelial Cell Lymphocyte Pericyte Myeloid Cell Neutrophil Capillary IP IP IP IP 24

PEGPH20 Has Been Shown In Animal Models to Drive Multiple Changes In the Tumor Microenvironment Illustration of TME (Post PEGPH20) Expected TME Post PEGPH20 Administration: – Reduced tumor interstitial pressure – Expansion of blood vessels Malignant cell (+cancer stem cells) HA Collagen Cancer-associated fibroblasts Endothelial Cell Lymphocyte Pericyte Myeloid Cell Neutrophil Capillary 25
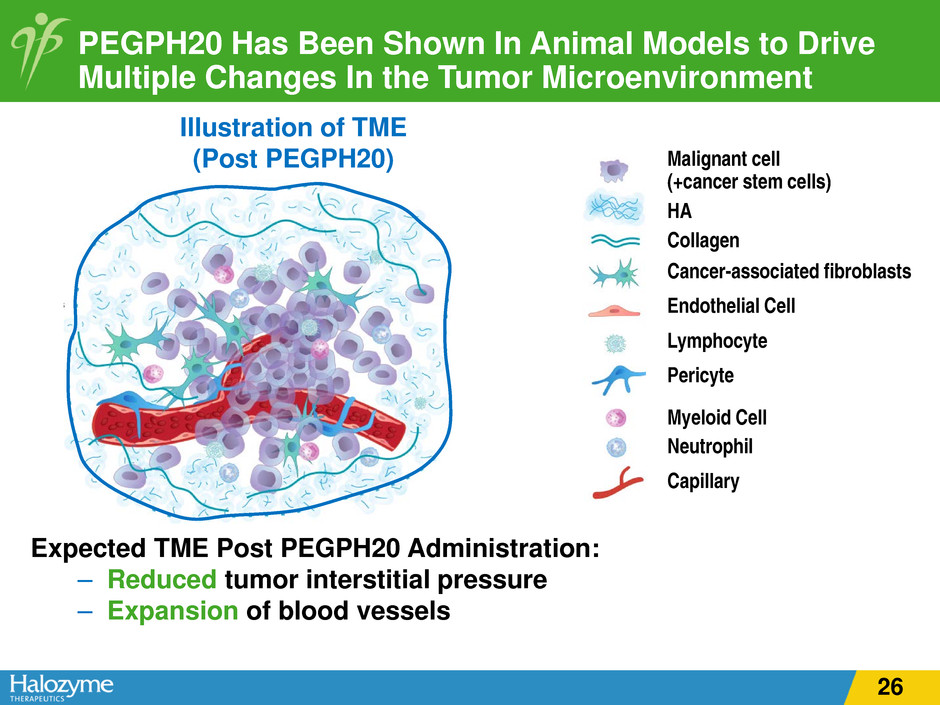
PEGPH20 Has Been Shown In Animal Models to Drive Multiple Changes In the Tumor Microenvironment Illustration of TME (Post PEGPH20) Expected TME Post PEGPH20 Administration: – Reduced tumor interstitial pressure – Expansion of blood vessels Malignant cell (+cancer stem cells) HA Collagen Cancer-associated fibroblasts Endothelial Cell Lymphocyte Pericyte Myeloid Cell Neutrophil Capillary 26

PEGPH20 Plus Chemotherapy Can Result in Increased Tumor Cell Death In Animal Models Illustration of TME (Post PEGPH20 plus Chemotherapy) Expected TME Post PEGPH20 plus Chemotherapy Administration: – Reduced tumor interstitial pressure – Expansion of blood vessels – Increased anti-cancer therapy access to tumor and increased tumor cell death Malignant cell (+cancer stem cells) HA Collagen Cancer-associated fibroblasts Endothelial Cell Lymphocyte Pericyte Myeloid Cell Neutrophil Capillary 27

PEGPH20 In Animal Models Rapidly Reverses High Tumor Pressure Allowing Increased Anti-Cancer Drug Access to Tumor Hyaluronan Degradation PEGPH20 In Animal Models Tumor Interstitial Pressure Reduced Tumor Vasculature Expansion Increased Diffusion and Delivery of Therapeutics Into Tumor/Metastases, but Not Normal Tissue 28

Today’s Agenda Introduction and Objectives Helen Torley, MB, ChB, MRCP President and Chief Executive Officer Halozyme Therapeutics PEGPH20 Mechanism of Action Christopher Thanos, PhD Director, Biotherapeutics Halozyme Therapeutics Exploring Combinations of PEGPH20 With Cancer Therapies Curt Thompson, PhD Senior Director, Pharmacology Halozyme Therapeutics From Theory to Initial Clinical Experience Sunil R. Hingorani, MD, PhD Associate Member, FHCRC Director, Center for Accelerated Translation in Pancreas Cancer Clinical Development Plan Update Athena Countouriotis, MD Chief Medical Officer Halozyme Therapeutics Q&A 29

Nonclinical Exploration of Combinations of PEGPH20 With Cancer Therapies Curt Thompson, PhD Senior Director, Pharmacology Halozyme Therapeutics 30
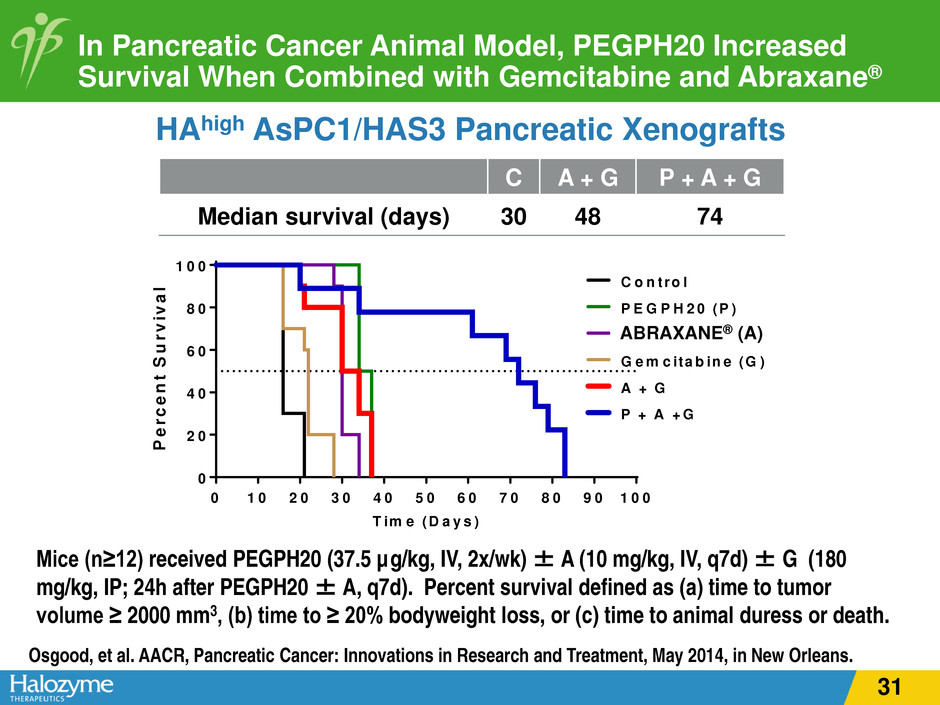
In Pancreatic Cancer Animal Model, PEGPH20 Increased Survival When Combined with Gemcitabine and Abraxane® HAhigh AsPC1/HAS3 Pancreatic Xenografts Osgood, et al. AACR, Pancreatic Cancer: Innovations in Research and Treatment, May 2014, in New Orleans. Mice (n≥12) received PEGPH20 (37.5 µg/kg, IV, 2x/wk) ± A (10 mg/kg, IV, q7d) ± G (180 mg/kg, IP; 24h after PEGPH20 ± A, q7d). Percent survival defined as (a) time to tumor volume ≥ 2000 mm3, (b) time to ≥ 20% bodyweight loss, or (c) time to animal duress or death. C A + G P + A + G Median survival (days) 30 48 74 0 1 0 2 0 3 0 4 0 5 0 6 0 7 0 8 0 9 0 1 0 0 0 2 0 4 0 6 0 8 0 1 0 0 C o n tro l P E G P H 2 0 (P ) A B A X A N E (A ) G e m c ita b in e (G ) A + G P + A + G T im e (D a y s ) P e rc e n t S u rv iv a l RAXANE® (A) 31
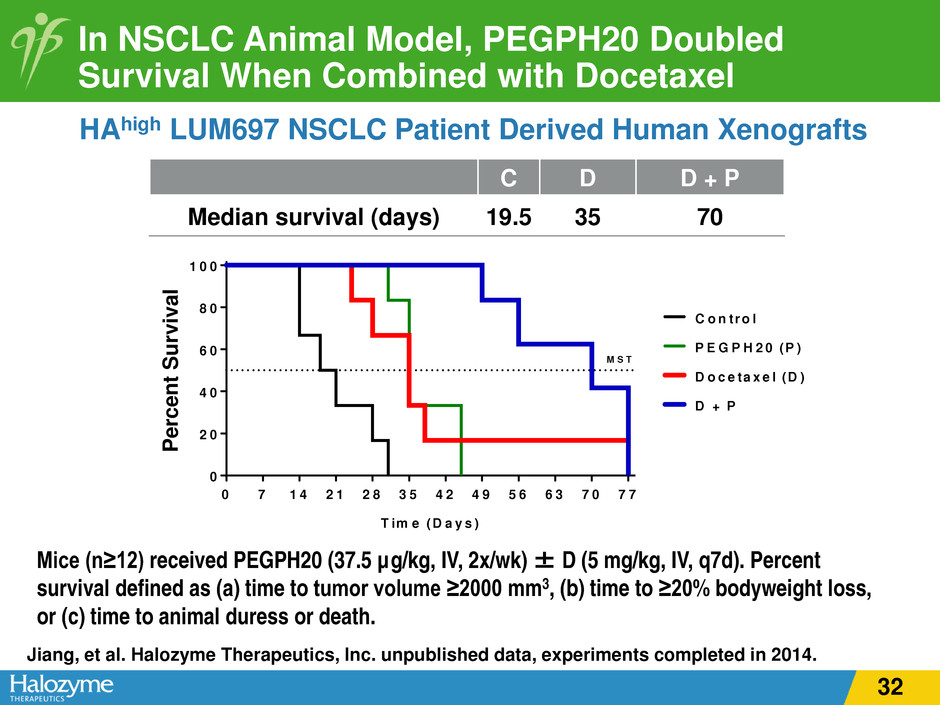
In NSCLC Animal Model, PEGPH20 Doubled Survival When Combined with Docetaxel HAhigh LUM697 NSCLC Patient Derived Human Xenografts Mice (n≥12) received PEGPH20 (37.5 µg/kg, IV, 2x/wk) ± D (5 mg/kg, IV, q7d). Percent survival defined as (a) time to tumor volume ≥2000 mm3, (b) time to ≥20% bodyweight loss, or (c) time to animal duress or death. Jiang, et al. Halozyme Therapeutics, Inc. unpublished data, experiments completed in 2014. C D D + P Median survival (days) 19.5 35 70 0 7 1 4 2 1 2 8 3 5 4 2 4 9 5 6 6 3 7 0 7 7 0 2 0 4 0 6 0 8 0 1 0 0 C o n tro l D o c e ta x e l (D ) P E G P H 2 0 (P ) D + P M S T T im e (D a y s ) P e rc e n t S u rv iv a l (% ) P er ce nt S ur vi va l 32
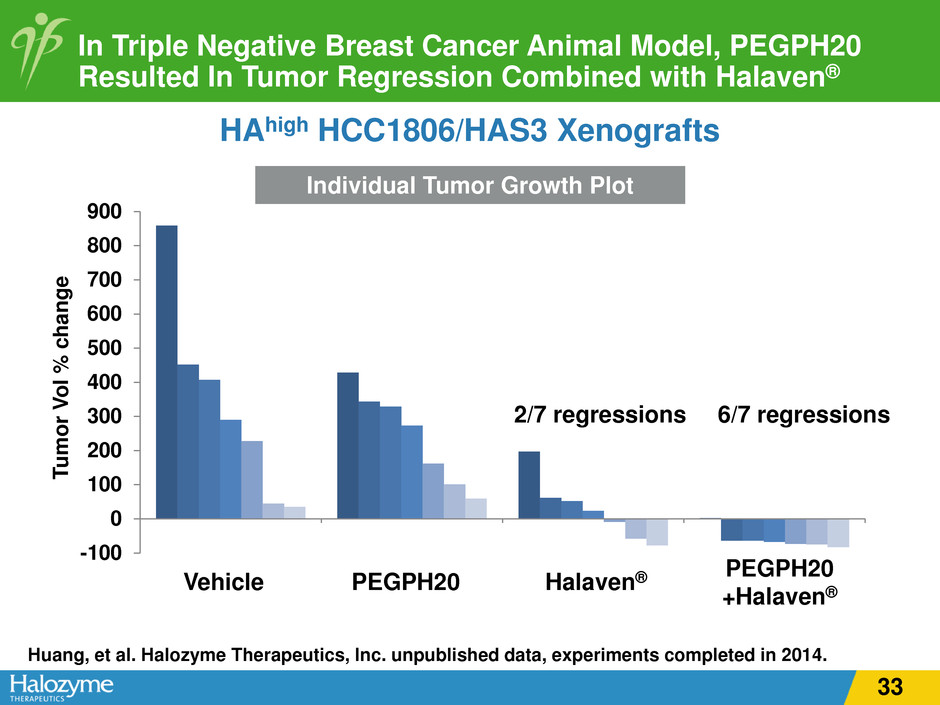
In Triple Negative Breast Cancer Animal Model, PEGPH20 Resulted In Tumor Regression Combined with Halaven® Individual Tumor Growth Plot -100 0 100 200 300 400 500 600 700 800 900 Tu m or V ol % c ha ng e PEGPH20 +Halaven® HAhigh HCC1806/HAS3 Xenografts 6/7 regressions Huang, et al. Halozyme Therapeutics, Inc. unpublished data, experiments completed in 2014. Halaven®PEGPH20Vehicle 2/7 regressions 33
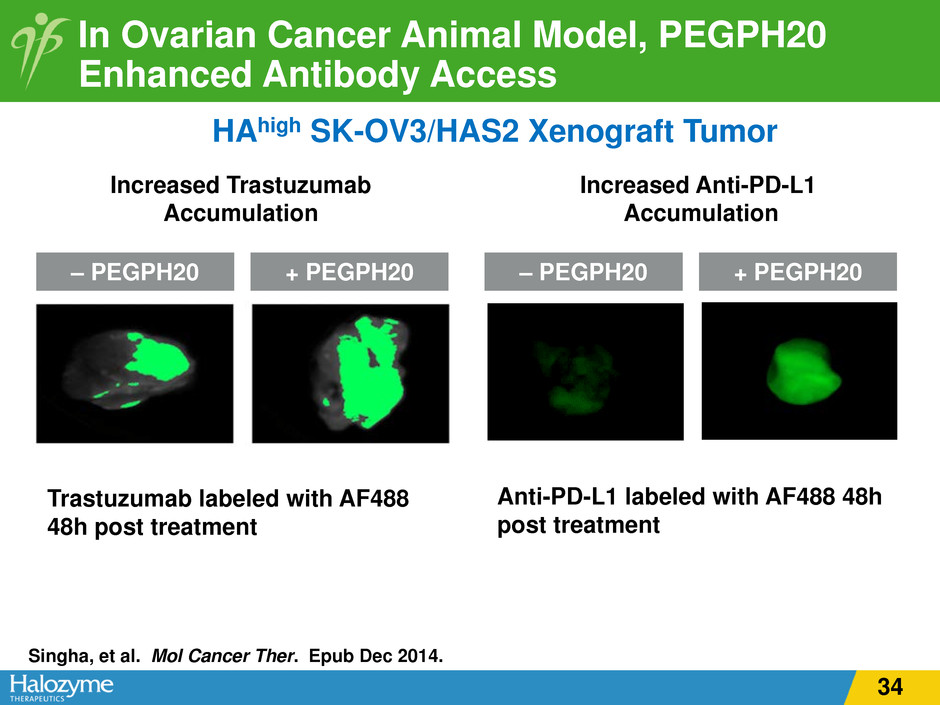
In Ovarian Cancer Animal Model, PEGPH20 Enhanced Antibody Access Trastuzumab labeled with AF488 48h post treatment Anti-PD-L1 labeled with AF488 48h post treatment Increased Trastuzumab Accumulation Singha, et al. Mol Cancer Ther. Epub Dec 2014. – PEGPH20 + PEGPH20 – PEGPH20 + PEGPH20 HAhigh SK-OV3/HAS2 Xenograft Tumor Increased Anti-PD-L1 Accumulation 34
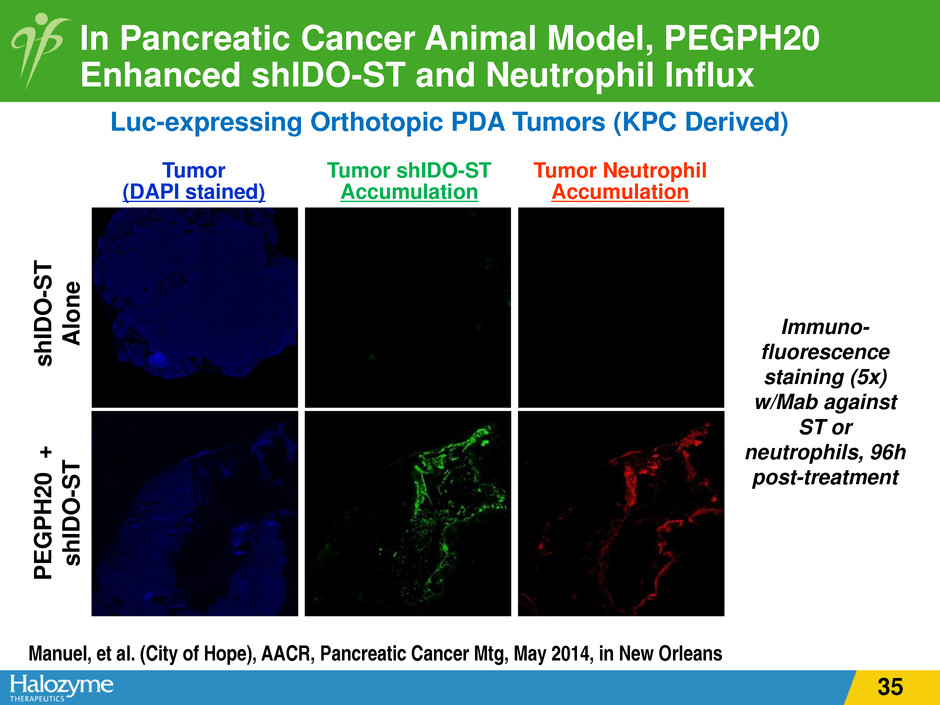
In Pancreatic Cancer Animal Model, PEGPH20 Enhanced shIDO-ST and Neutrophil Influx Luc-expressing Orthotopic PDA Tumors (KPC Derived) PE G P H 20 + sh ID O -S T sh ID O -S T A lo ne Immuno- fluorescence staining (5x) w/Mab against ST or neutrophils, 96h post-treatment Tumor (DAPI stained) Tumor Neutrophil Accumulation Tumor shIDO-ST Accumulation Manuel, et al. (City of Hope), AACR, Pancreatic Cancer Mtg, May 2014, in New Orleans 35
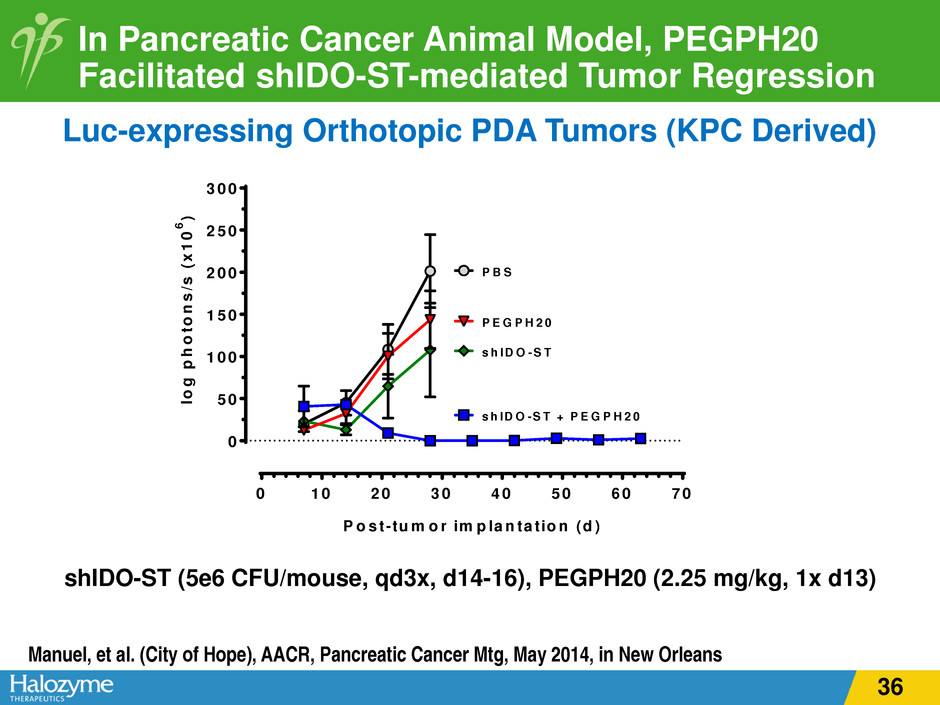
In Pancreatic Cancer Animal Model, PEGPH20 Facilitated shIDO-ST-mediated Tumor Regression shIDO-ST (5e6 CFU/mouse, qd3x, d14-16), PEGPH20 (2.25 mg/kg, 1x d13) Luc-expressing Orthotopic PDA Tumors (KPC Derived) Manuel, et al. (City of Hope), AACR, Pancreatic Cancer Mtg, May 2014, in New Orleans 0 1 0 2 0 3 0 4 0 5 0 6 0 7 0 0 5 0 1 0 0 1 5 0 2 0 0 2 5 0 3 0 0 s h ID O -S T P E G P H 2 0 s h ID O -S T + P E G P H 2 0 P B S p < 0 .0 1 , A N O V A P o s t-tu m o r im p la n ta tio n (d ) lo g p h o to n s /s ( x 1 0 6 ) 36

In Ovarian Cancer Animal Model, PEGPH20 Enhanced Immune Cell Access into HAhigh Tumor Human primary NK cells labeled with PKH26 co-injected with trastuzumab, 48h post Human primary T cells labeled with PKH26, 48h post-treatment Increased Primary T Cell Accumulation Increased Natural Killer (NK) Cell Accumulation – PEGPH20 + PEGPH20 – PEGPH20 + PEGPH20 HAhigh SK-OV3/HAS2 Xenograft tumor Singha, et al. Mol Cancer Ther. Epub Dec 2014. 37
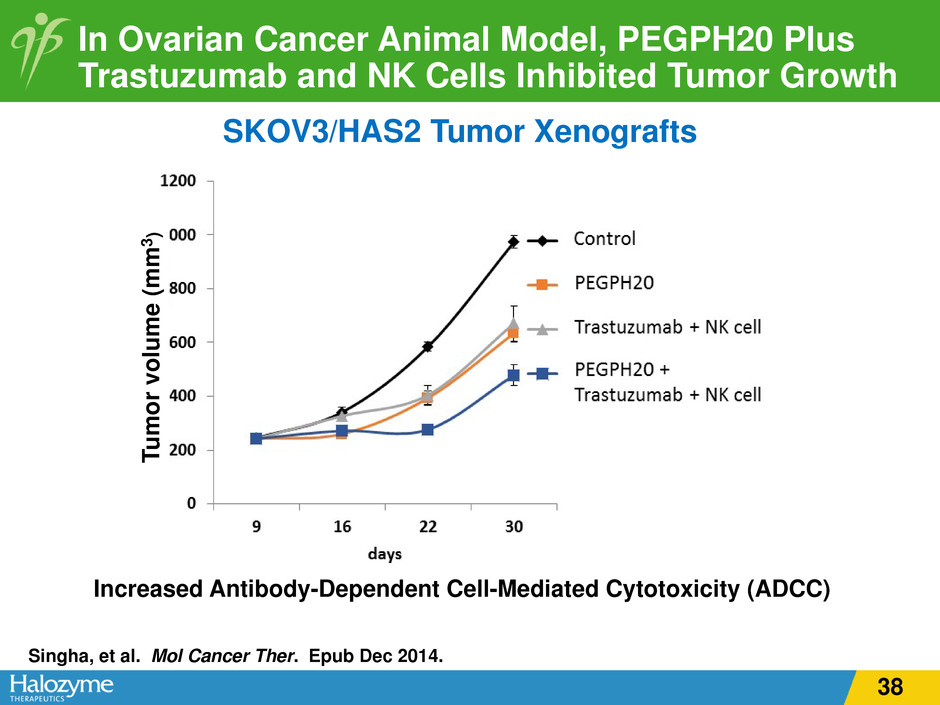
In Ovarian Cancer Animal Model, PEGPH20 Plus Trastuzumab and NK Cells Inhibited Tumor Growth Increased Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC) SKOV3/HAS2 Tumor Xenografts Tu m or v ol um e (m m 3 ) Singha, et al. Mol Cancer Ther. Epub Dec 2014. 38

PEGPH20 Nonclinical Summary PEGPH20, an investigational drug, depletes HA content of tumors in animal studies – Reduced tumor interstitial pressure resulting in increased tumor perfusion – Increased tumor access of • Small molecules • Monoclonal antibodies including immune checkpoint inhibitors • Immune cells Depletion of HA in nonclinical studies demonstrated to: – Inhibit tumor growth – Improve survival – Decrease metastasis No increase in tested cancer therapy penetration to normal tissues 39

Today’s Agenda Introduction and Objectives Helen Torley, MB, ChB, MRCP President and Chief Executive Officer Halozyme Therapeutics PEGPH20 Mechanism of Action Christopher Thanos, PhD Director, Biotherapeutics Halozyme Therapeutics Exploring Combinations of PEGPH20 With Cancer Therapies Curt Thompson, PhD Senior Director, Pharmacology Halozyme Therapeutics From Theory to Initial Clinical Experience Sunil Hingorani, MD, PhD Associate Member, FHCRC Director, Center for Accelerated Translation in Pancreas Cancer Clinical Development Plan Update Athena Countouriotis, MD Chief Medical Officer Halozyme Therapeutics Q&A 40

From Theory to Initial Clinical Experience Sunil R. Hingorani, MD, PhD Associate Member, FHCRC Director, Center for Accelerated Translation in Pancreas Cancer 41
Increasing incidence with unabated mortality Eludes detection until late stages Metastasizes early and widely during disease progression Modest survival improvement in last 3 decades Highest 1, 5, and 10 year mortalities of any cancer Sobering Realities of Pancreas Cancer 42

Pancreas Cancers are Hypoperfused: They Have a Decreased Blood Supply CT Scan MRI Scan Von Hoff, et al. Cancer Cell. 2009,16:708. 43

Pancreas Cancer as a “Solid Tumor Organ”: Multi-faceted Desmoplastic Response Cancer epithelium Stroma (everything else) Pancreas Cancer Histology 44

Changing the Game: Altering Intratumoral Physical Dynamics to Advantage in Pancreas Cancer PI > Pv and diffusion and convection limited PI < Pv and diffusion and convection favorable PI < Pv and permanent remodeling of the tumor microenvironment Provenzano, et al. Cancer Cell. 2012,21(3):418. PEGPH20Baseline Gem+PEGPH20 45

Before • Hard • Fibrotic • Hypovascular MPA#1 + GemcitabineAft r • Soft • Friable • Hypervascular Breaching the Pancreas Cancer Sanctuary: Changing Disease Biology with PEGPH20 Provenzano, et al. Cancer Cell. 2012,21(3):418. KPC Mouse Model 46
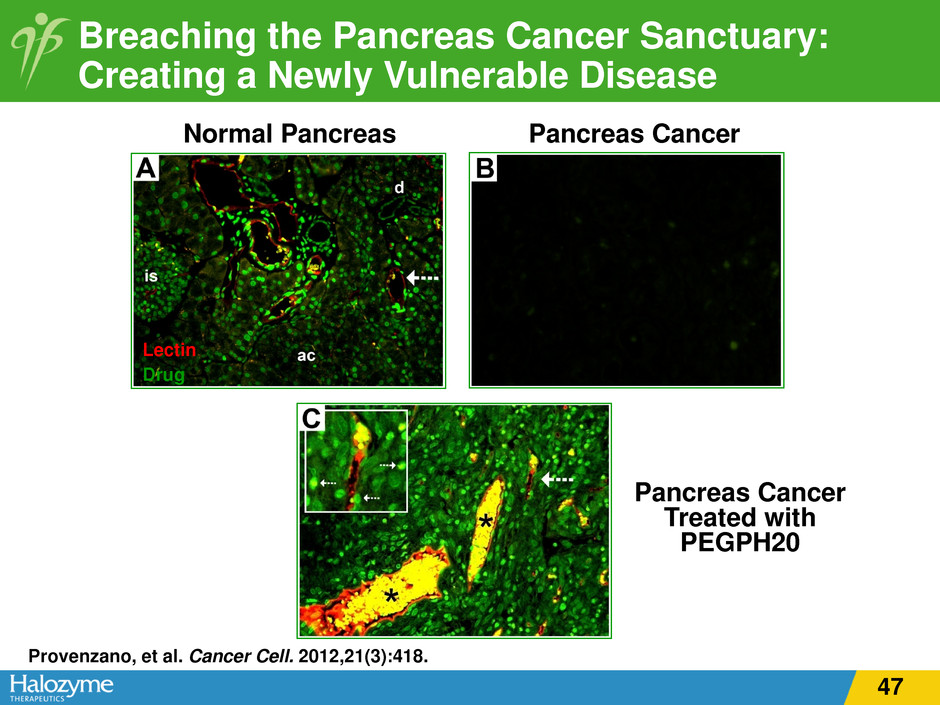
Normal Pancreas Pancreas Cancer Pancreas Cancer Treated with PEGPH20 Drug Lectin Breaching the Pancreas Cancer Sanctuary: Creating a Newly Vulnerable Disease Provenzano, et al. Cancer Cell. 2012,21(3):418. 47
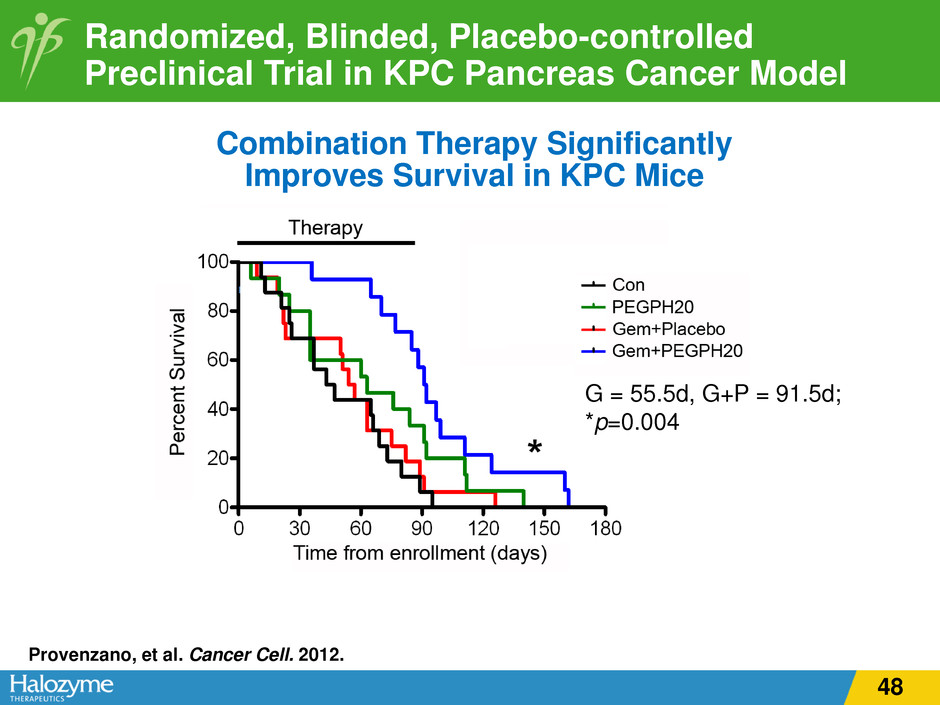
G = 55.5d, G+P = 91.5d; *p=0.004 Combination Therapy Significantly Improves Survival in KPC Mice Randomized, Blinded, Placebo-controlled Preclinical Trial in KPC Pancreas Cancer Model Provenzano, et al. Cancer Cell. 2012. 48
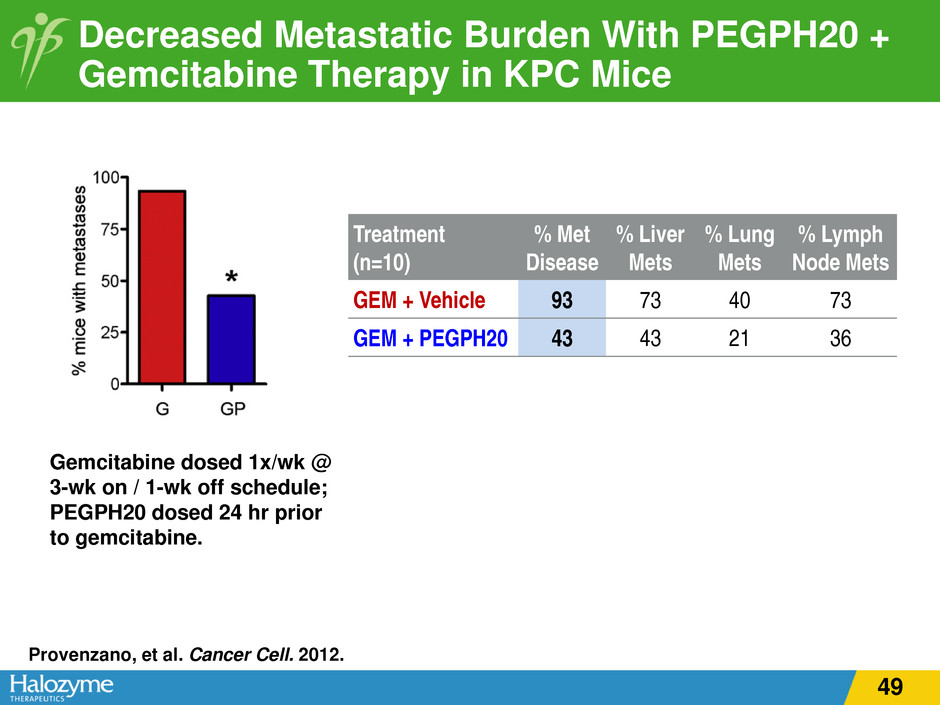
Decreased Metastatic Burden With PEGPH20 + Gemcitabine Therapy in KPC Mice Provenzano, et al. Cancer Cell. 2012. Treatment (n=10) % Met Disease % Liver Mets % Lung Mets % Lymph Node Mets GEM + Vehicle 93 73 40 73 GEM + PEGPH20 43 43 21 36 Gemcitabine dosed 1x/wk @ 3-wk on / 1-wk off schedule; PEGPH20 dosed 24 hr prior to gemcitabine. 49

Clinical Proof of Concept Demonstrated in Phase 1b Trial in Advanced Pancreatic Cancer Halozyme Study 201 Study Objectives Primary – Assess safety and tolerability of PEGPH20 in combination therapy – Establish recommended phase 2 dose (RP2D) of PEGPH20 in combination with gemcitabine in patients with Stage IV previously untreated Pancreatic Ductal Adenocarcinoma Secondary – Assess tumor response using RECIST 1.1 criteria – Assess pharmacokinetic (PK) profile and evaluate pharmacodynamic activity of PEGPH20 – Assess treatment effect based on tumor HA status 50

Study 201 PEGPH20 Treatment-Related AEs (≥10% in Total Incidence) *One Grade 5 event reported: cerebrovascular accident. Adverse Events N = 28 Grade 3/4 All Grades Incidence, % Any AE 7 (25.0) 24 (85.7) Muscle spasms 2 (7.1) 15 (53.5) Myalgia 0 11 (39.3) Arthralgia 0 8 (28.6) Edema peripheral 1 (3.6) 8 (28.6) Fatigue 1 (3.6) 7 (25.0) Pain in extremity 0 5 (17.9) Asthenia 0 3 (10.7) 51

Study 201: Example of HA Target Modulation Hingorani, et al. ASCO 2013 (abstract 4010). Post-treatment biopsy (6 wks of therapy) 0 50 100 150 200 250 300 H S co re 0 50 100 150 200 250 300 Pre-treatment biopsy 52
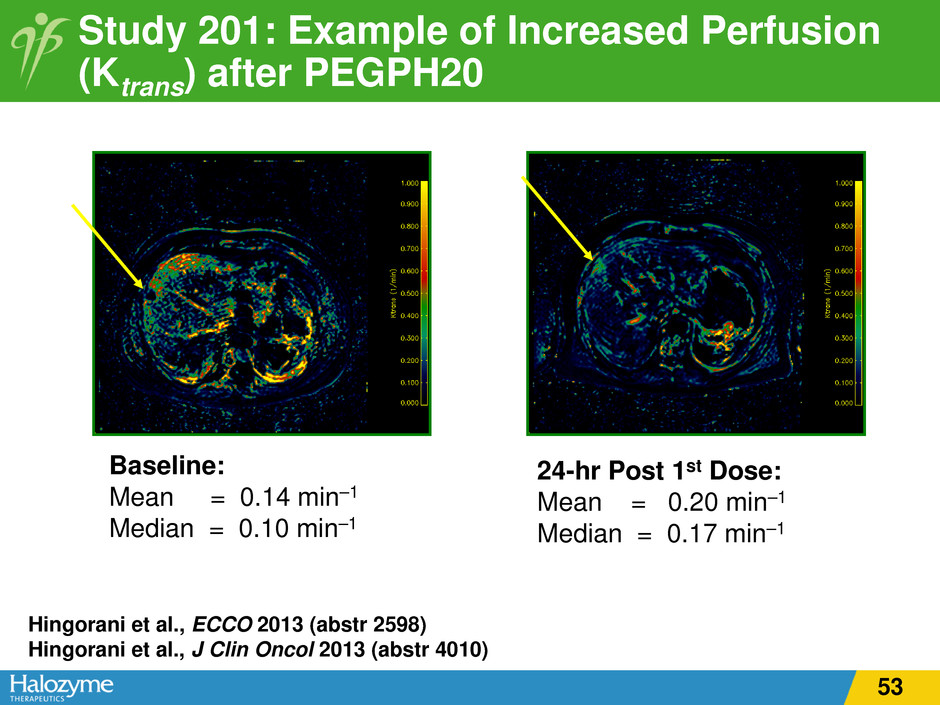
Study 201: Example of Increased Perfusion (Ktrans) after PEGPH20 Hingorani et al., ECCO 2013 (abstr 2598) Hingorani et al., J Clin Oncol 2013 (abstr 4010) Baseline: Mean = 0.14 min–1 Median = 0.10 min–1 24-hr Post 1st Dose: Mean = 0.20 min–1 Median = 0.17 min–1 53

Study 201: Example of Radiographic Response (PR) at EOC1 Baseline Sum Target L = 83.2 mm TL3 Diameter: 19.3 mm Volume: 1.5 cm3 TL3 Diameter: 10.2 mm Volume: 0.33 cm3 EOC1 Sum Target L = 56.1 mm Hingorani, et al. ASCO 2013 (abstr 4010) 54

Final Results of Study 201 Exploratory Analyses Support Potential for HAhigh as Response Predictor Single-arm Phase1b Evaluation PEGPH20+gemcitabine in Stage IV Metastatic Pancreatic Ductal Adenocarcinoma Source: Study 201 Final Report ORR% (n)* Median PFS (days) OS (days) Gem+PEGPH20 29% (7/24) 154 200 HAhigh 67% (4/6) 219 395 HAlow 27% (3/11) 108 174 0 50 100 150 200 250 300 350 0.0 0.2 0.4 0.6 0.8 1.0 219 days108 days Efficacy-evaluable=154 days HA high HA low Time (Days) P ro po rt io n of P FS 0 100 200 300 400 500 600 0.0 0.2 0.4 0.6 0.8 1.0 395 days174 days Efficacy-evaluable=200 days HA high HA low Time (Days) P ro po rt io n of s ur vi va l (*confirmed PR per RECIST1.1) Overall Survival (OS)Progression-Free Survival (PFS) 55
Pancreas cancer primary tumors and metastases effectively exclude chemotherapeutics PEGPH20 alters unfavorable hydrodynamics to promote drug penetration PEGPH20 seems well-tolerated in patients Patients with high intratumoral HA levels appear to benefit most from PEGPH20 Summary: PEGH20+Gemcitabine in Models and Patients 56

Today’s Agenda Introduction and Objectives Helen Torley, MB, ChB, MRCP President and Chief Executive Officer Halozyme Therapeutics PEGPH20 Mechanism of Action Christopher Thanos, PhD Director, Biotherapeutics Halozyme Therapeutics Exploring Combinations of PEGPH20 With Cancer Therapies Curt Thompson, PhD Senior Director, Pharmacology Halozyme Therapeutics From Theory to Initial Clinical Experience Sunil R. Hingorani, MD, PhD Associate Member, FHCRC Director, Center for Accelerated Translation in Pancreas Cancer Clinical Development Plan Update Athena Countouriotis, MD Chief Medical Officer Halozyme Therapeutics Q&A 57

Clinical Development Plan Update Athena Countouriotis, MD Chief Medical Officer Halozyme Therapeutics 58
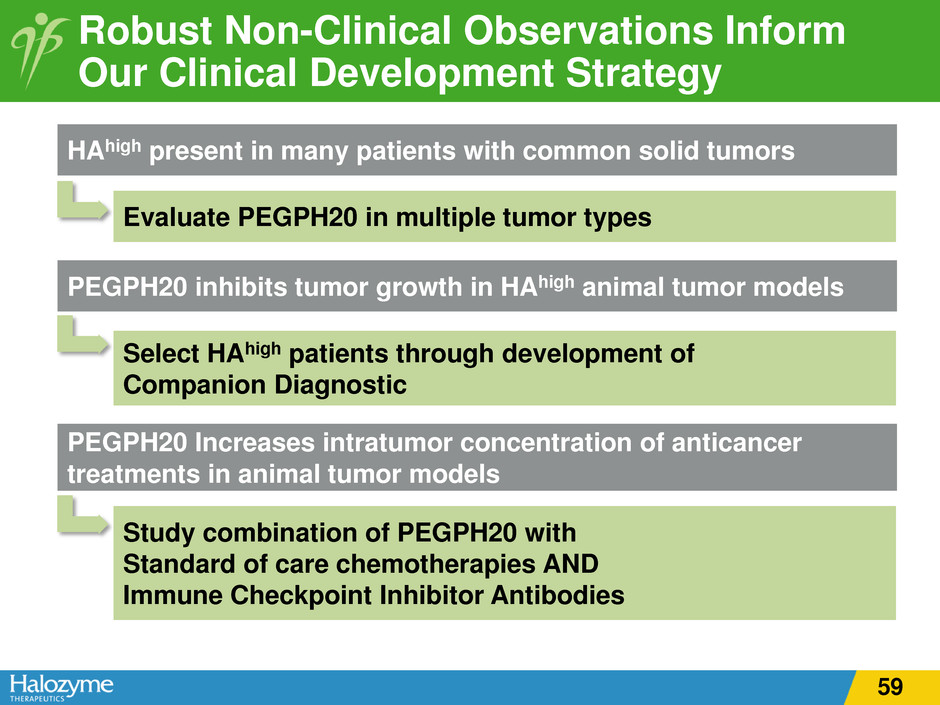
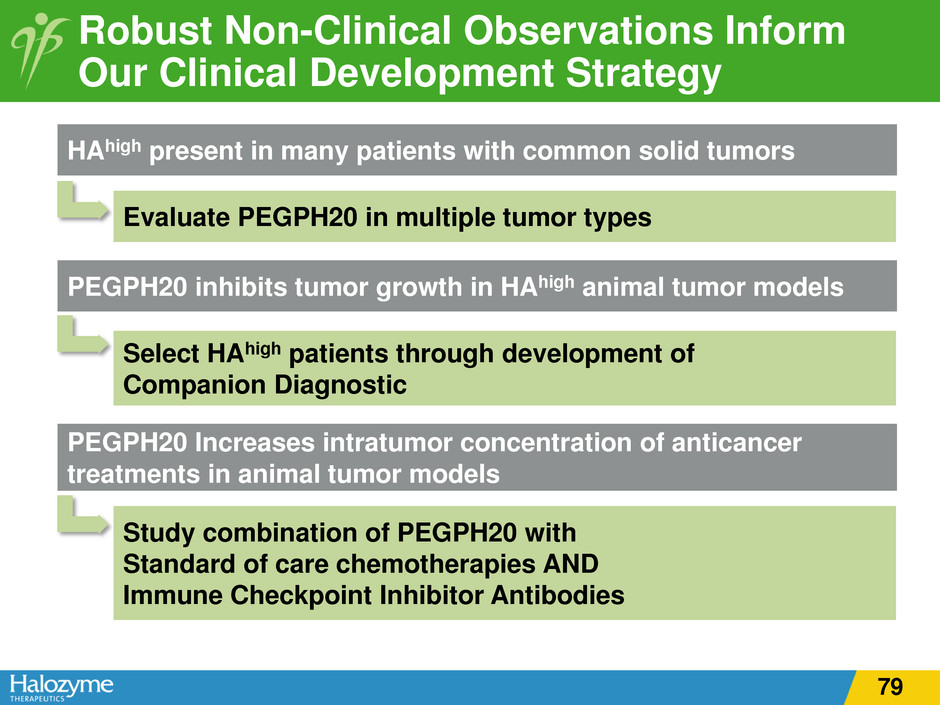
Robust Non-Clinical Observations Inform Our Clinical Development Strategy HAhigh present in many patients with common solid tumors Evaluate PEGPH20 in multiple tumor types PEGPH20 inhibits tumor growth in HAhigh animal tumor models Select HAhigh patients through development of Companion Diagnostic PEGPH20 Increases intratumor concentration of anticancer treatments in animal tumor models Study combination of PEGPH20 with Standard of care chemotherapies AND Immune Checkpoint Inhibitor Antibodies 59
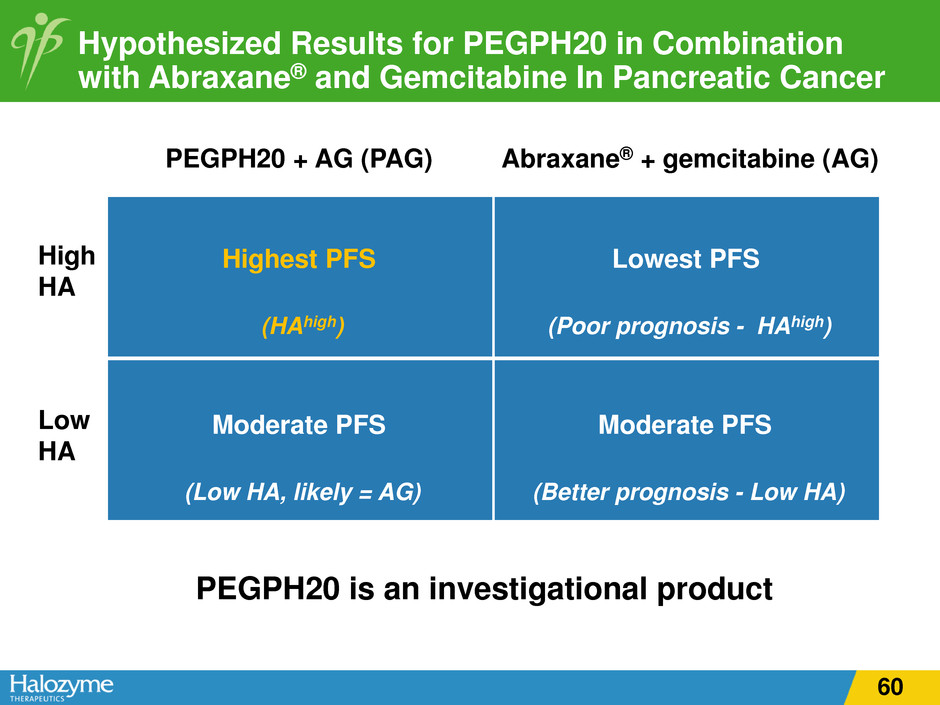
Hypothesized Results for PEGPH20 in Combination with Abraxane® and Gemcitabine In Pancreatic Cancer Abraxane® + gemcitabine (AG) PEGPH20 + AG (PAG) High HA Low HA Highest PFS (HAhigh) Lowest PFS (Poor prognosis - HAhigh) Moderate PFS (Low HA, likely = AG) Moderate PFS (Better prognosis - Low HA) PEGPH20 is an investigational product 60
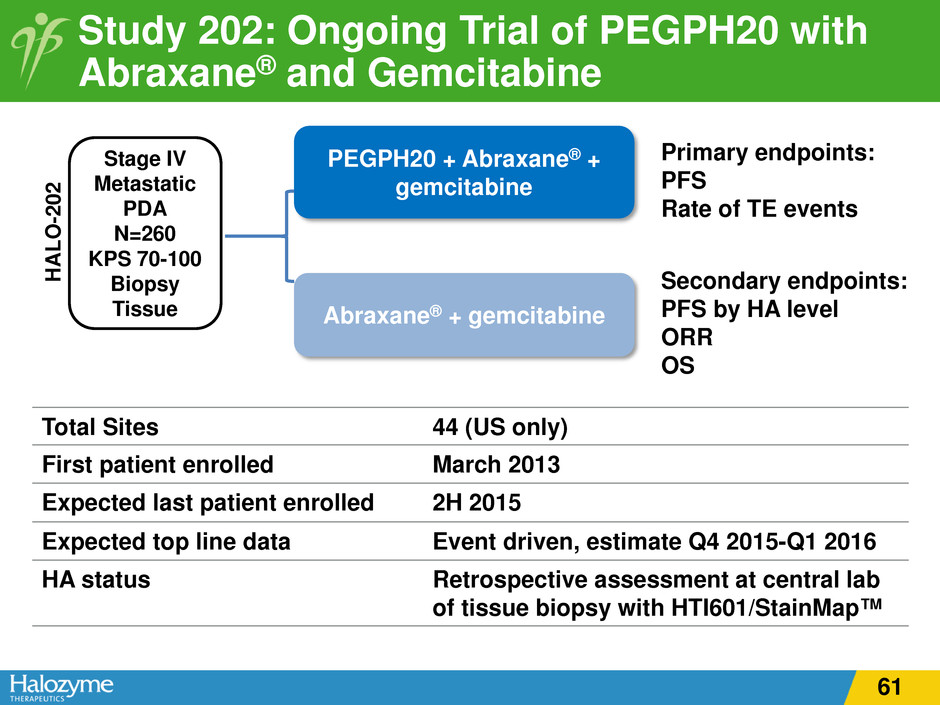
Study 202: Ongoing Trial of PEGPH20 with Abraxane® and Gemcitabine PEGPH20 + Abraxane® + gemcitabine Abraxane® + gemcitabine Stage IV Metastatic PDA N=260 KPS 70-100 Biopsy Tissue Primary endpoints: PFS Rate of TE events Secondary endpoints: PFS by HA level ORR OS H A LO -2 02 Total Sites 44 (US only) First patient enrolled March 2013 Expected last patient enrolled 2H 2015 Expected top line data Event driven, estimate Q4 2015-Q1 2016 HA status Retrospective assessment at central lab of tissue biopsy with HTI601/StainMap™ 61

Referring Institution CURRENT Central Lab FUTURE Companion Diagnostic Current Approach Designed With Goal of Meeting FDA Standards and Expectations for a Companion Diagnostic Diagnostic Biopsy Embed Process Section Stained Slide IHC-based Stain Unstained Section Digital Image Score With Partner U.S. Patent No. 8,846,034 issued for patient selection with an anti-hyaluronan agent, such as PEGPH20, diagnostic agents for detection and quantification of hyaluronan in a biological sample and combinations and kits for use in practicing the methods. Scan 62

Study 202 History and Status Clinical Hold: based on DMC finding of potential imbalance in TE events between treatment arms Protocol Amendment Add ~100 patients 2:1 PAG to AG randomization Exclude high Thromboembolic Event risk patients Add LMWH to both arms Additional primary endpoint: evaluation of TE events Stage 2 vs Stage 1 2013 2014 Mar April Aug Stage 1 Study 202: n=146 Run-in Safety/dose Enrollment Clinical Hold Protocol amendment Hold Lifted Stage 2 Study 202: n=114 Enrollment 63

Conducted to evaluate further the level of HA accumulation (ie HAhigh) to assist in the development of our companion diagnostic and our clinical development plan in pancreatic and NSCLC Important caveats – Open database, ongoing study – Data verification ongoing Interim Analysis Study 202 64
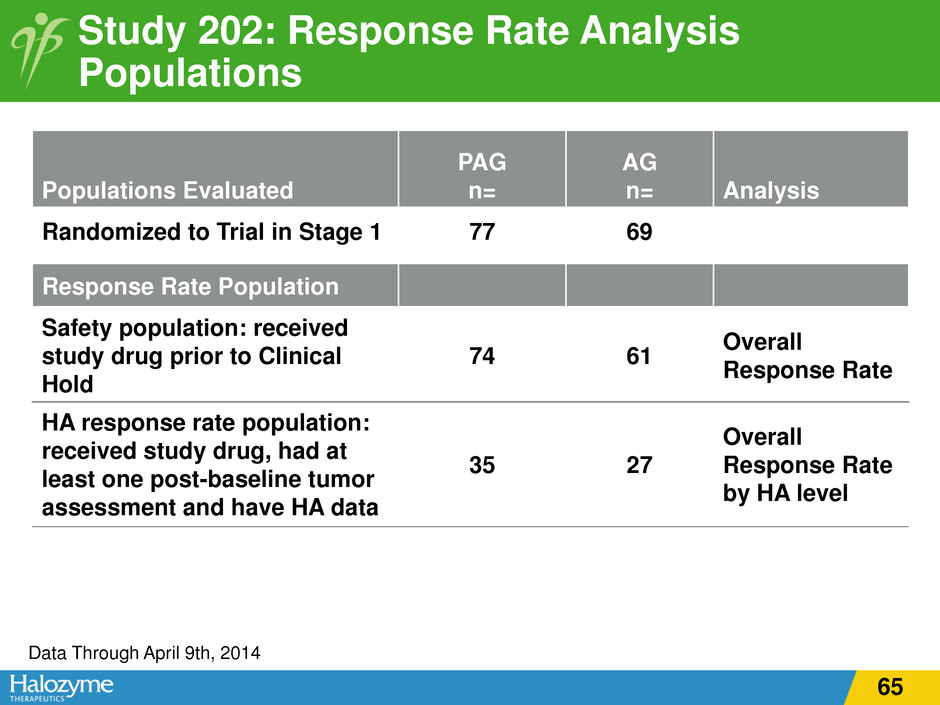
Study 202: Response Rate Analysis Populations Populations Evaluated PAG n= AG n= Analysis Randomized to Trial in Stage 1 77 69 Data Through April 9th, 2014 Response Rate Population Safety population: received study drug prior to Clinical Hold 74 61 Overall Response Rate HA response rate population: received study drug, had at least one post-baseline tumor assessment and have HA data 35 27 Overall Response Rate by HA level 65
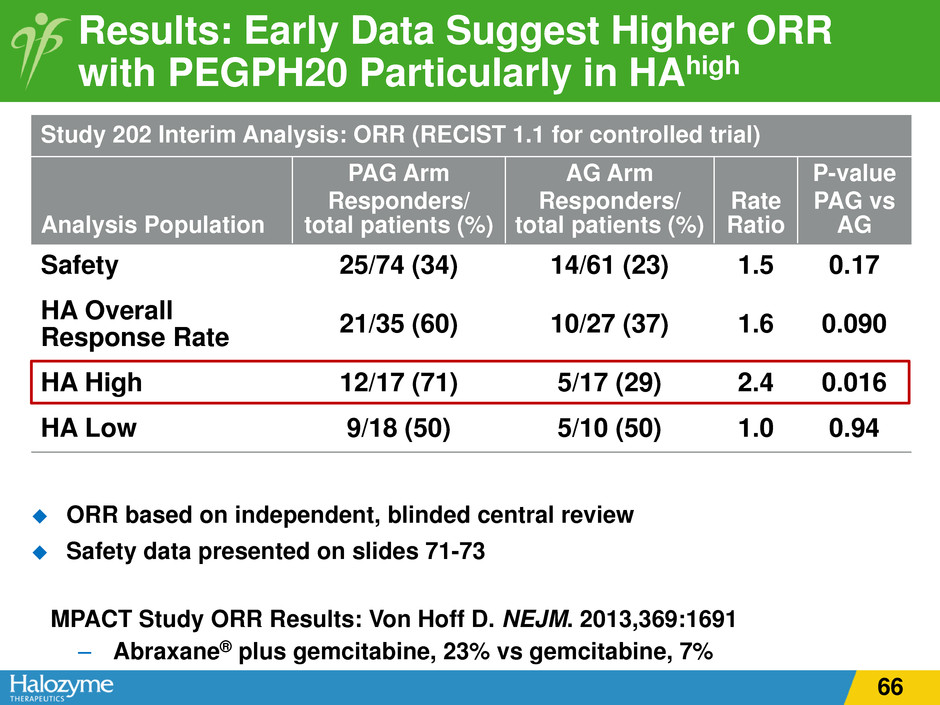
Results: Early Data Suggest Higher ORR with PEGPH20 Particularly in HAhigh ORR based on independent, blinded central review Safety data presented on slides 71-73 MPACT Study ORR Results: Von Hoff D. NEJM. 2013,369:1691 – Abraxane® plus gemcitabine, 23% vs gemcitabine, 7% Study 202 Interim Analysis: ORR (RECIST 1.1 for controlled trial) Analysis Population PAG Arm Responders/ total patients (%) AG Arm Responders/ total patients (%) Rate Ratio P-value PAG vs AG Safety 25/74 (34) 14/61 (23) 1.5 0.17 HA Overall Response Rate 21/35 (60) 10/27 (37) 1.6 0.090 HA High 12/17 (71) 5/17 (29) 2.4 0.016 HA Low 9/18 (50) 5/10 (50) 1.0 0.94 66
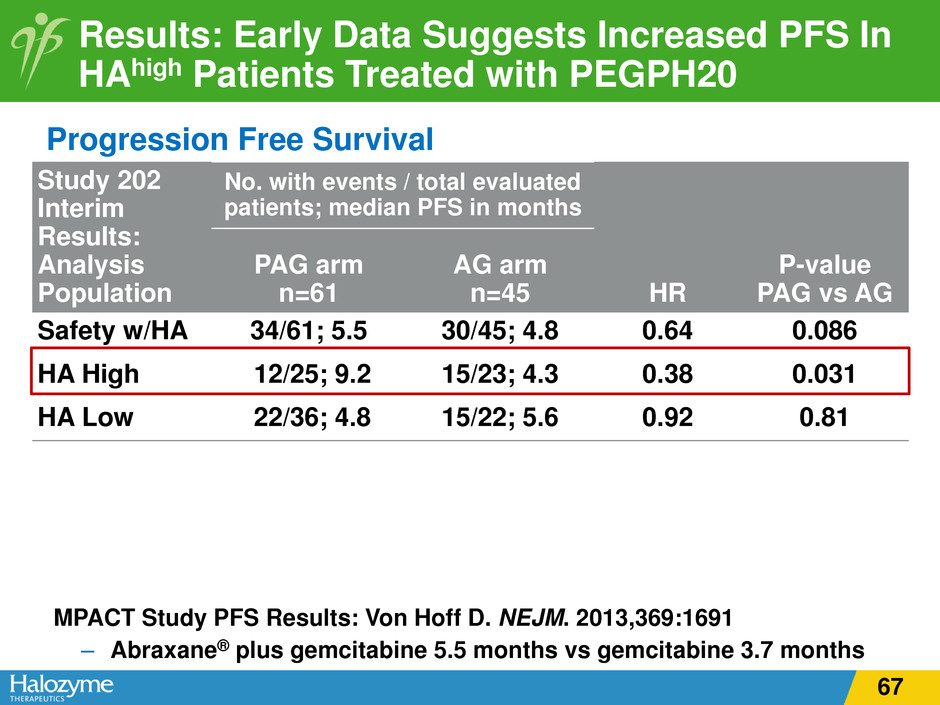
Progression Free Survival Study 202 Interim Results: Analysis Population No. with events / total evaluated patients; median PFS in months HR P-value PAG vs AG PAG arm n=61 AG arm n=45 Safety w/HA 34/61; 5.5 30/45; 4.8 0.64 0.086 HA High 12/25; 9.2 15/23; 4.3 0.38 0.031 HA Low 22/36; 4.8 15/22; 5.6 0.92 0.81 Results: Early Data Suggests Increased PFS In HAhigh Patients Treated with PEGPH20 MPACT Study PFS Results: Von Hoff D. NEJM. 2013,369:1691 – Abraxane® plus gemcitabine 5.5 months vs gemcitabine 3.7 months 67

Results: Early Data Suggests Increased PFS In HAhigh Patients Treated with PEGPH20 PFS In Patients with HAhigh K -M Est ia m te of P rogress io n Free S ur vi va l (% ) 0 10 20 30 40 50 60 70 80 90 100 Treatment Group PAG AG PAG: 9.2 MonthsAG: 4.3 Months K -M E st im at e of P ro gr es si on F re e S ur vi va l ( % ) PAG: 9.2 monthsAG: 4.3 months K- M E st im at e of Pr og re ss io n Fr ee S ur vi va l ( % ) Study Duration (months) At Risk PAG: 25 20 11 8 5 2 1 0 AG: 23 16 8 6 0 0 0 0 0 2 4 6 8 10 12 14 1 2 3 4 50 6 7 8 9 100 68

ORR: 71% PFS: 9.2 months ORR: 29% PFS: 4.3 month ORR: 50% PFS: 4.8 months ORR: 50% PFS: 5.6 months Study 202: Interim ORR and PFS Data Summary PAG AG High HA Low HA ORR data through April 9th and reflects PAG or AG treatment PFS data through December 5th and reflects PAG AG treatment in PAG arm 69
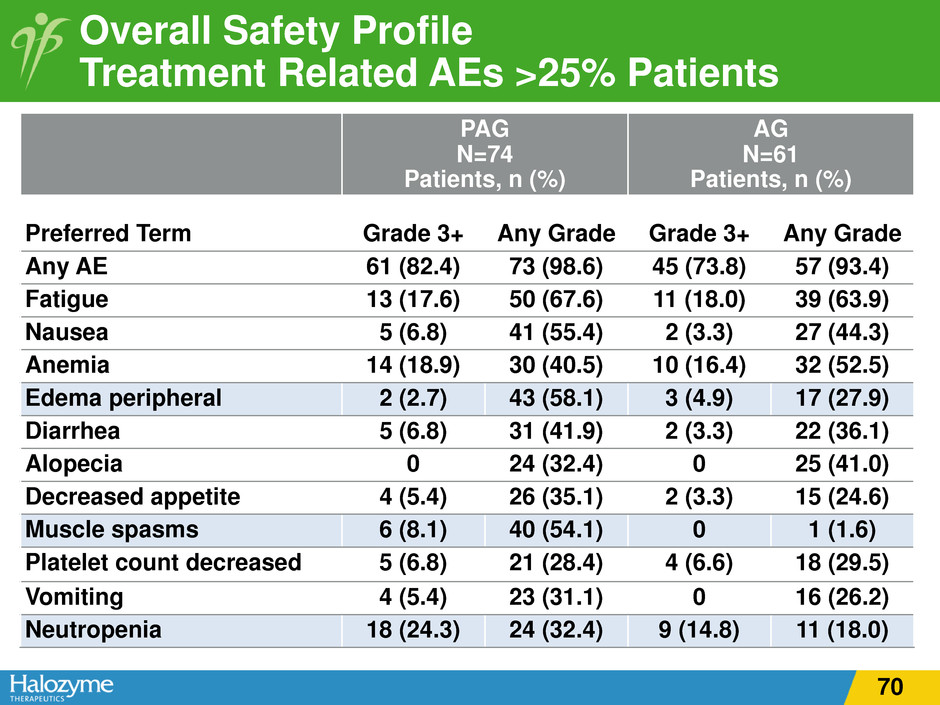
Overall Safety Profile Treatment Related AEs >25% Patients PAG N=74 Patients, n (%) AG N=61 Patients, n (%) Preferred Term Grade 3+ Any Grade Grade 3+ Any Grade Any AE 61 (82.4) 73 (98.6) 45 (73.8) 57 (93.4) Fatigue 13 (17.6) 50 (67.6) 11 (18.0) 39 (63.9) Nausea 5 (6.8) 41 (55.4) 2 (3.3) 27 (44.3) Anemia 14 (18.9) 30 (40.5) 10 (16.4) 32 (52.5) Edema peripheral 2 (2.7) 43 (58.1) 3 (4.9) 17 (27.9) Diarrhea 5 (6.8) 31 (41.9) 2 (3.3) 22 (36.1) Alopecia 0 24 (32.4) 0 25 (41.0) Decreased appetite 4 (5.4) 26 (35.1) 2 (3.3) 15 (24.6) Muscle spasms 6 (8.1) 40 (54.1) 0 1 (1.6) Platelet count decreased 5 (6.8) 21 (28.4) 4 (6.6) 18 (29.5) Vomiting 4 (5.4) 23 (31.1) 0 16 (26.2) Neutropenia 18 (24.3) 24 (32.4) 9 (14.8) 11 (18.0) 70
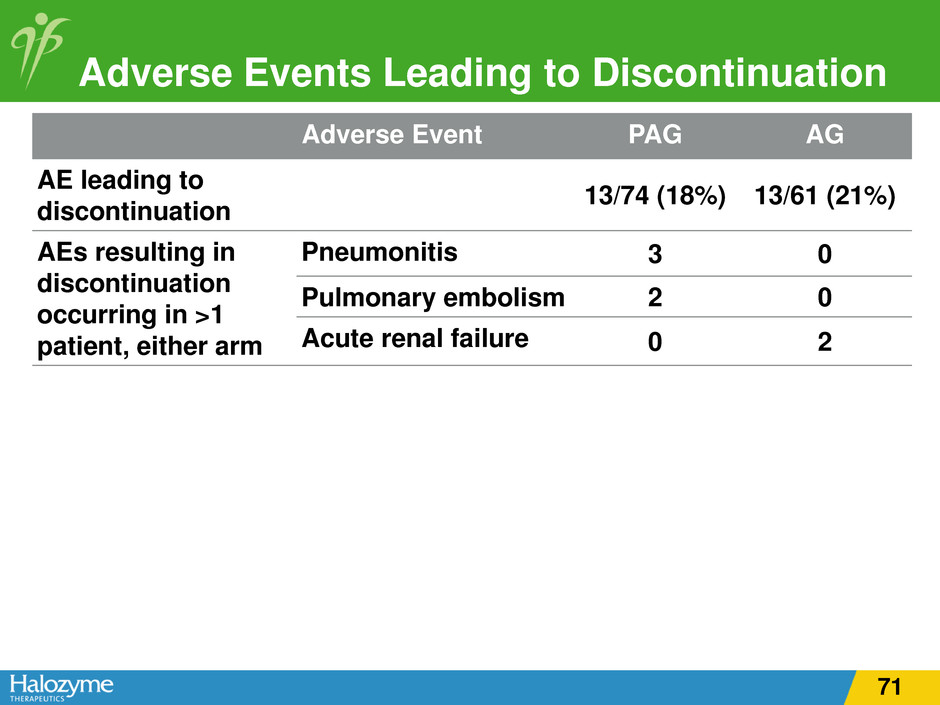
Adverse Event PAG AG AE leading to discontinuation 13/74 (18%) 13/61 (21%) AEs resulting in discontinuation occurring in >1 patient, either arm Pneumonitis 3 0 Pulmonary embolism 2 0 Acute renal failure 0 2 Adverse Events Leading to Discontinuation 71
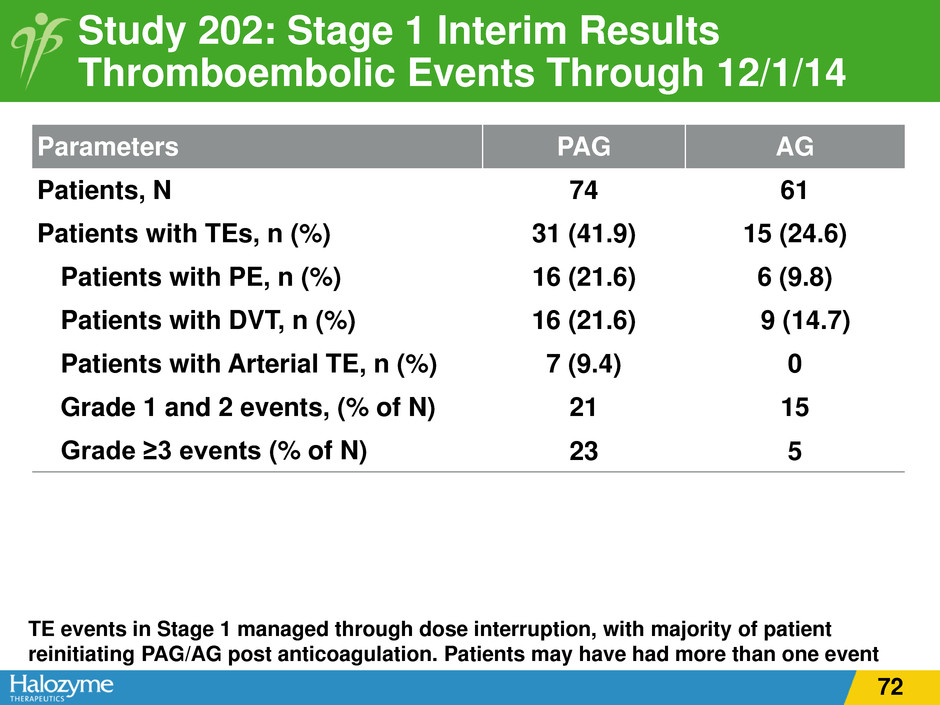
Parameters PAG AG Patients, N 74 61 Patients with TEs, n (%) 31 (41.9) 15 (24.6) Patients with PE, n (%) 16 (21.6) 6 (9.8) Patients with DVT, n (%) 16 (21.6) 9 (14.7) Patients with Arterial TE, n (%) 7 (9.4) 0 Grade 1 and 2 events, (% of N) 21 15 Grade ≥3 events (% of N) 23 5 Study 202: Stage 1 Interim Results Thromboembolic Events Through 12/1/14 TE events in Stage 1 managed through dose interruption, with majority of patient reinitiating PAG/AG post anticoagulation. Patients may have had more than one event 72

Pancreatic Cancer: Planned Next Steps Meeting with FDA (end Q1 target) – Discuss potential registration-seeking trial in HAhigh Stage 4 Pancreatic Ductal Adenocarcinoma patients Gain EMA scientific advice on proposed protocol 73

Non Small Cell Lung Cancer – Significant Unmet Medical Need Over 160K newly diagnosed 1L and 84K 2L patients annually (US & 5EU) Survival times remain low with no curative therapy – In 1st line non-biomarker population OS is 10-12 months and PFS is 4-6 months – In 2nd line OS is 7-8 months and PFS 3-4 months Immunotherapy may offer hope for some but opportunity remains to do more 2L Chemo [84,000] NSCLC [375,000] <Stage IIIB (50%) Stage IIIB/IV (50%) EGFR/ALK (20%) 1L Chemo [160,000] References: GLOBOCAN 2012 (IARC) - 3.7.2014; “Lung Cancer”, American Cancer Society, 2014. 2 Scagliotti, et al. J Clin Oncol. 2008; 26:3543-51, Halozyme analysis. PD1-Combo (PEGPH20 + PD1) PRIMAL (PEGPH20 + docetaxel) 74
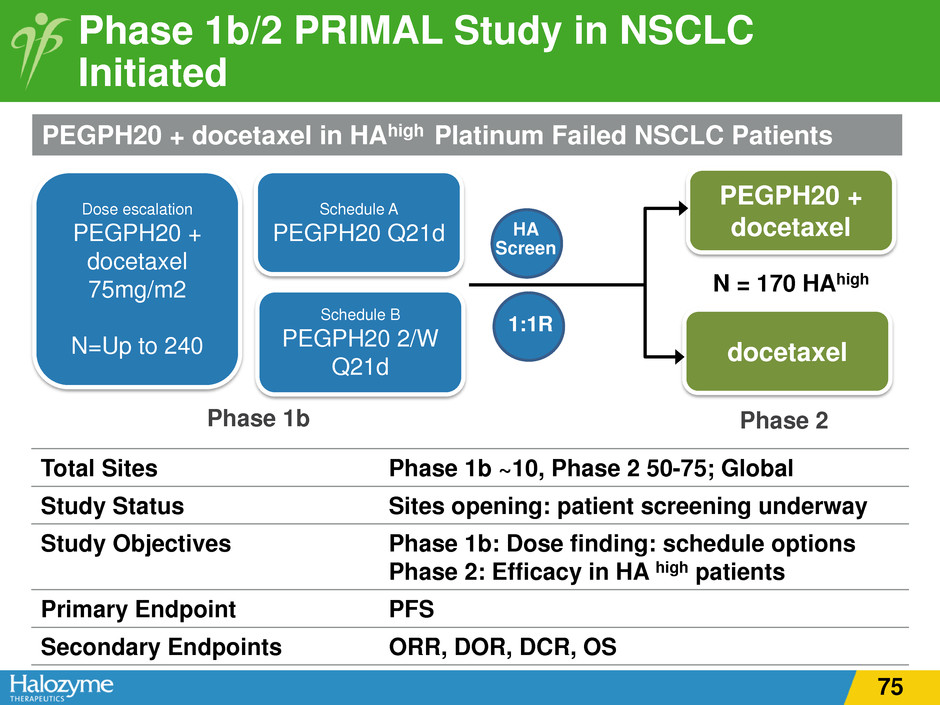
Phase 1b/2 PRIMAL Study in NSCLC Initiated Dose escalation PEGPH20 + docetaxel 75mg/m2 N=Up to 240 Schedule A PEGPH20 Q21d Schedule B PEGPH20 2/W Q21d PEGPH20 + docetaxel docetaxel N = 170 HAhigh 1:1R Phase 1b Phase 2 PEGPH20 + docetaxel in HAhigh Platinum Failed NSCLC Patients Total Sites Phase 1b ~10, Phase 2 50-75; Global Study Status Sites opening: patient screening underway Study Objectives Phase 1b: Dose finding: schedule options Phase 2: Efficacy in HA high patients Primary Endpoint PFS Secondary Endpoints ORR, DOR, DCR, OS HA Screen 75
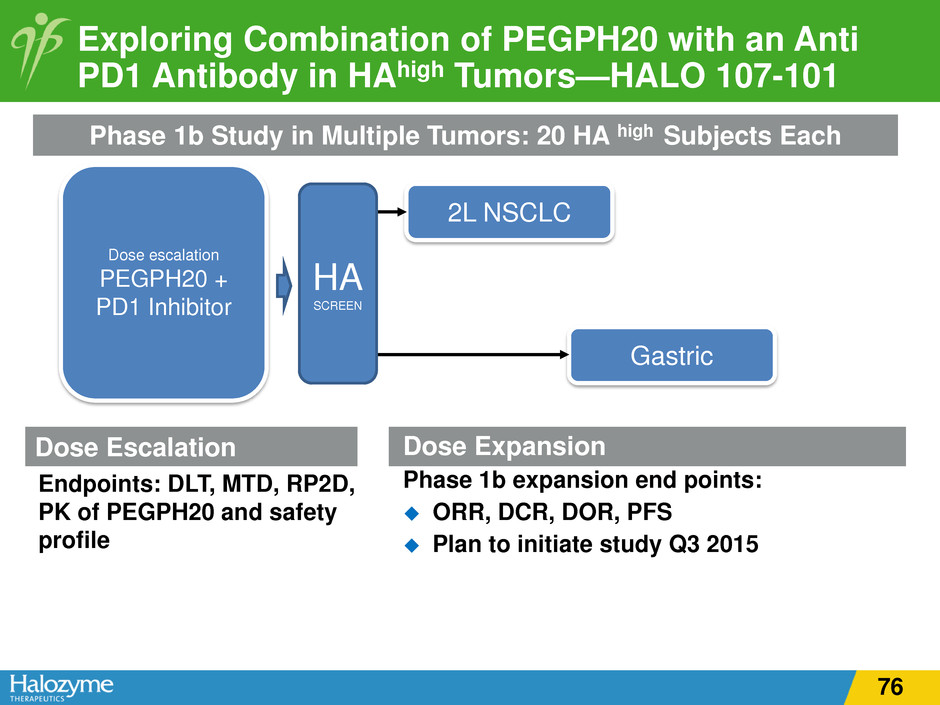
Dose Escalation Endpoints: DLT, MTD, RP2D, PK of PEGPH20 and safety profile Dose Expansion Phase 1b expansion end points: ORR, DCR, DOR, PFS Plan to initiate study Q3 2015 Exploring Combination of PEGPH20 with an Anti PD1 Antibody in HAhigh Tumors—HALO 107-101 Dose escalation PEGPH20 + PD1 Inhibitor 2L NSCLC Gastric Phase 1b Study in Multiple Tumors: 20 HA high Subjects Each HA SCREEN l ti 76

Key Projected Program and Data Milestones FDA Discussion Registration-seeking Trial Stage1 202 Efficacy and Safety Data Q1 2016 Pancreatic Cancer NSCLC Additional Cancer Q4 2015Q3 2015Q2 2015Q1 2015 Complete Enrollment 202 Stage2 Results Study 202 Target for First Patient Enrolled New Trial First Patient Enrolled PRIMAL in NSCLC PRIMAL Dose and Tolerability Readout Start Phase 2 PRIMAL Initiate Immunotherapy Trial in NSCLC 107-101 Initial Readout Immunotherapy Safety, Dose, Responses Initiate Gastric cancer arm 77

Today’s Agenda Introduction and Objectives Helen Torley, MB, ChB, MRCP President and Chief Executive Officer Halozyme Therapeutics PEGPH20 Mechanism of Action Christopher Thanos, PhD Director, Biotherapeutics Halozyme Therapeutics Exploring Combinations of PEGPH20 With Cancer Therapies Curt Thompson, PhD Senior Director, Pharmacology Halozyme Therapeutics From Theory to Initial Clinical Experience Sunil R. Hingorani, MD, PhD Associate Member, FHCRC Director, Center for Accelerated Translation in Pancreas Cancer Clinical Development Plan Update Athena Countouriotis, MD Chief Medical Officer Halozyme Therapeutics Q&A 78
Robust Non-Clinical Observations Inform Our Clinical Development Strategy HAhigh present in many patients with common solid tumors Evaluate PEGPH20 in multiple tumor types PEGPH20 inhibits tumor growth in HAhigh animal tumor models Select HAhigh patients through development of Companion Diagnostic PEGPH20 Increases intratumor concentration of anticancer treatments in animal tumor models Study combination of PEGPH20 with Standard of care chemotherapies AND Immune Checkpoint Inhibitor Antibodies 79

PEGPH20 Goal: Improving Targeting of Co-Administered Cancer Drugs Encouraging data from Phase1b and 2 pancreatic cancer trials supportive of potential for efficacy benefit in HAhigh tumors – FDA and EMA feedback sought for registration trial initiation 2015 Clinical testing underway in additional tumor – Non-small cell lung cancer • PRIMAL trial with docetaxel in 2L patients initiated • Trial with PD1 inhibitor to commence 2015 80

Question and Answer
















































































