Exhibit 99.1

GYNOID LIPODYSTROPHY (CELLULITE): A PHASE 1/ 2, VEHICLE-CONTROLLED STUDY TO EVALUATE SAFETY, TOLERABILITY AND PHARMACOLOGIC EFFECT OF SUBCUTANEOUSLY INJECTED RHUCAT-L IN HEALTHY FEMALE SUBJECTS (PRELIMINARY INTERIM RESULTS) DR. FRANCISCO PEREZ ATAMOROS DERMATOLOGIST amdac@iwm.com.mx

Public Disclosure Francisco Perez-Atamoros, M.D. is the independent owner of Centro Dermatologico Tennyson where the study is being conducted. Dr Perez-Atamoros is the Principal Investigator for the study. Materials and monetary support for the study are provided by Halozyme Therapeutics, Inc. THIS IS NOT A COMMERCIAL PRODUCT.
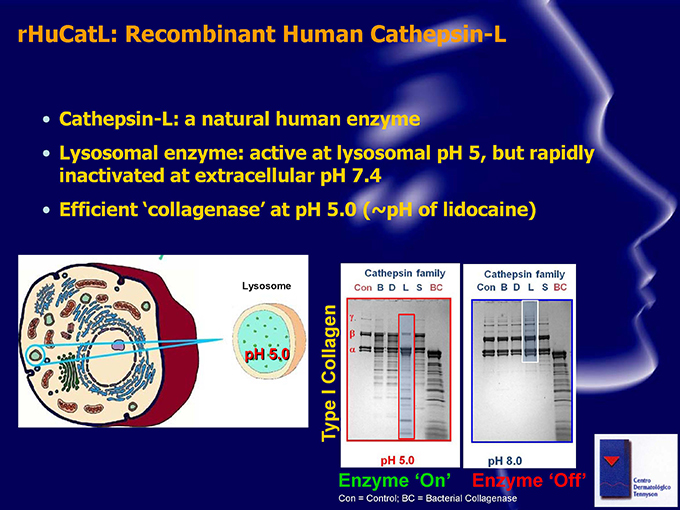 rHuCatL: Recombinant Human Cathepsin-L Cathepsin-L: a natural human enzyme Lysosomal enzyme: active at lysosomal pH 5, but rapidly inactivated at extracellular pH 7.4 Efficient collagenase’ at pH 5.0 (~pH of lidocaine) Lysosome pH 5.0 Collagen I Type Enzyme ‘On’ Enzyme Off
rHuCatL: Recombinant Human Cathepsin-L Cathepsin-L: a natural human enzyme Lysosomal enzyme: active at lysosomal pH 5, but rapidly inactivated at extracellular pH 7.4 Efficient collagenase’ at pH 5.0 (~pH of lidocaine) Lysosome pH 5.0 Collagen I Type Enzyme ‘On’ Enzyme Off
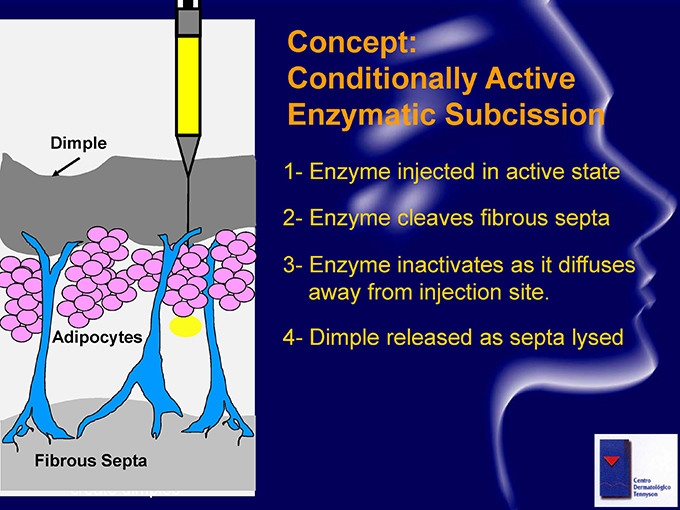
Concept: Conditionally Active Enzymatic Subcission 1- Enzyme injected in active state 2- Enzyme cleaves fibrous septa 3- Enzyme inactivates as it diffuses away from injection site. 4- Dimple released as septa lysed

Phase 2, Protocol Healthy female subjects aged 18-45 with moderate to severe cellulite Double-blind randomization (right/left) for active and vehicle injection; 1cc injections into 5 dimples within 10x10 cm2 areas on each side Primary efficacy assessment is at Day 28, with additional assessments before and at Days 2, 7, 14, and at 3 and 6 months following treatment Primary endpoint is physician assessment, with secondary endpoints of subject self-evaluation and objective measures of skin topography

Safety Observations Safety profile to date: No serious or severe Adverse Events No dose-limiting toxicities Common mild to moderate transient pain at injection sites (generally bilateral) Common mild to moderate injection site bruising (typically one side) , which resolves within approximately 7-14 days Other treatment-related AEs included related mild injection site reactions (such as erythema, pruritus) Study drug and vehicle have been well tolerated
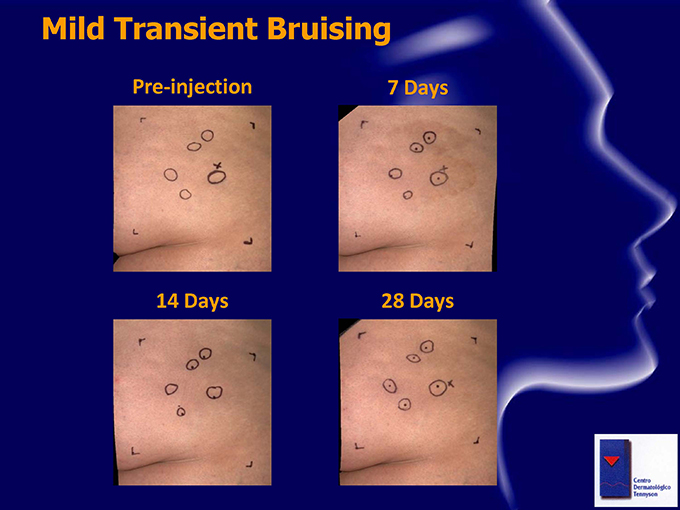
Mild Transient Bruising Pre-injection 7 Days 14 Days 28 Days
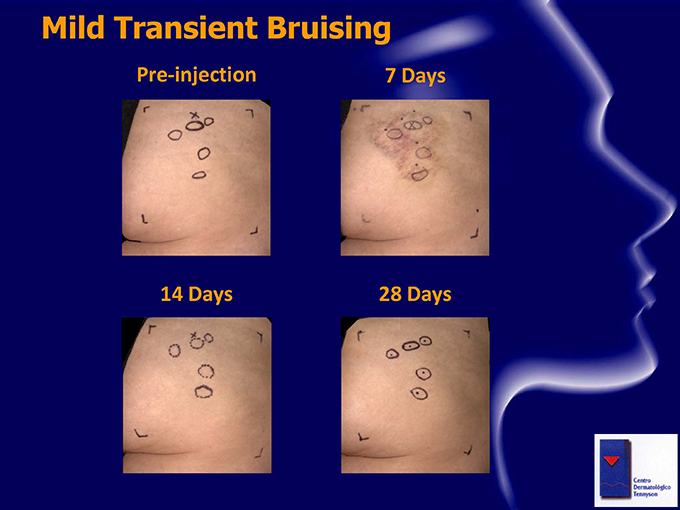
Mild Transient Bruising Pre-injection 7 Days 14 Days 28 Days
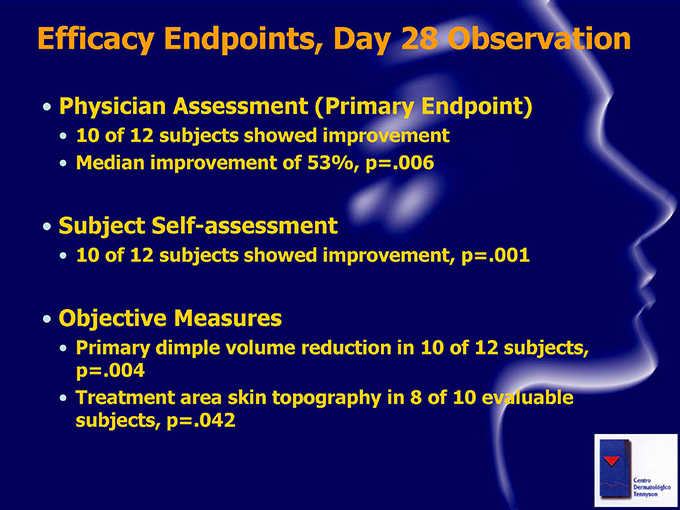
Efficacy Endpoints, Day 28 Observation Physician Assessment (Primary Endpoint) 10 of 12 subjects showed improvement Median improvement of 53%, p=.006 Subject Self-assessment 10 of 12 subjects showed improvement, p=.001 Objective Measures Primary dimple volume reduction in 10 of 12 subjects, p=.004 Treatment area skin topography in 8 of 10 evaluable subjects, p=.042
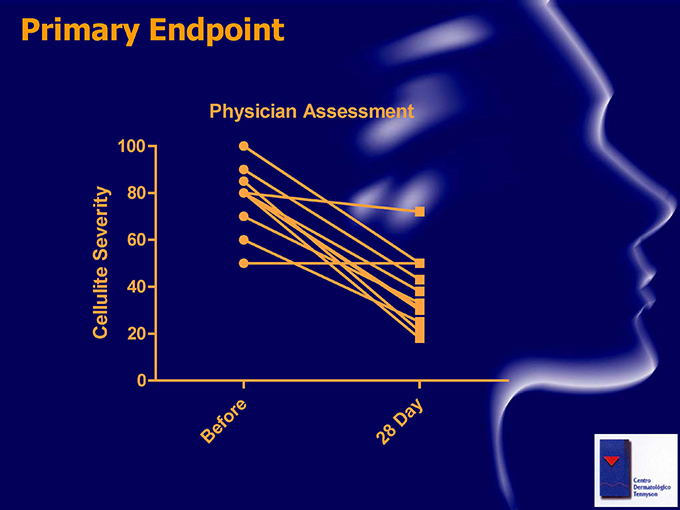
Primary Endpoint Physician Assessment 100 80 Severity 60 Cellulite 40 20 0 or e ay D ef B 28
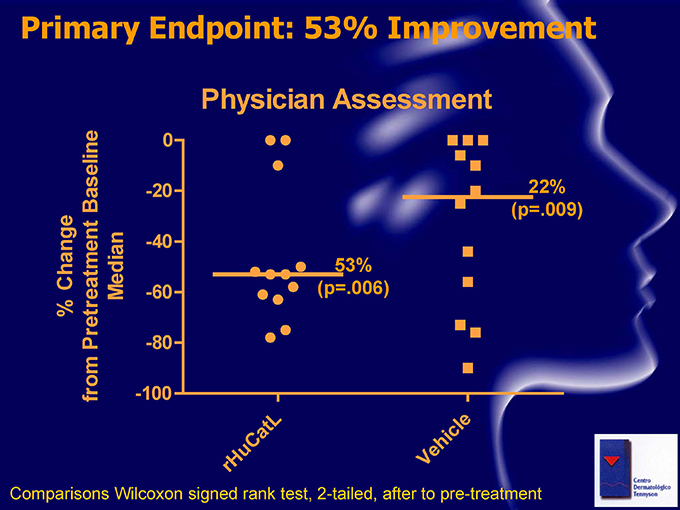
Primary Endpoint: 53% Improvement Physician Assessment 0 Baseline -20 22% (p.009) -40 Change 53% Median -60 (p.006) % Pretreatment -80 from -100 t L ic le Ca h u e r H V Comparisons Wilcoxon signed rank test, 2-tailed, after to pre-treatment
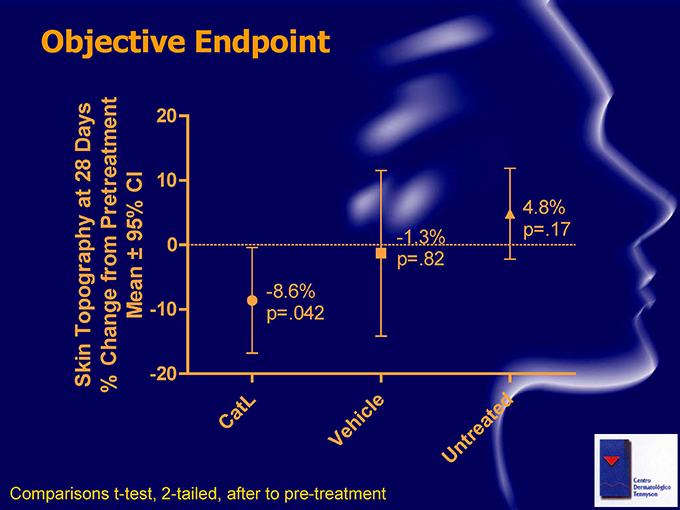
Objective Endpoint Days 20 28 CI 10 at 4.8% Pretreatment 95% -1.3% p=.17 0 from p=.82 -8.6% Topography Mean -10 p=.042 Change Skin -20 % L le ed at ic C h at e r e V nt U Comparisons t-test, 2-tailed, after to pre-treatment
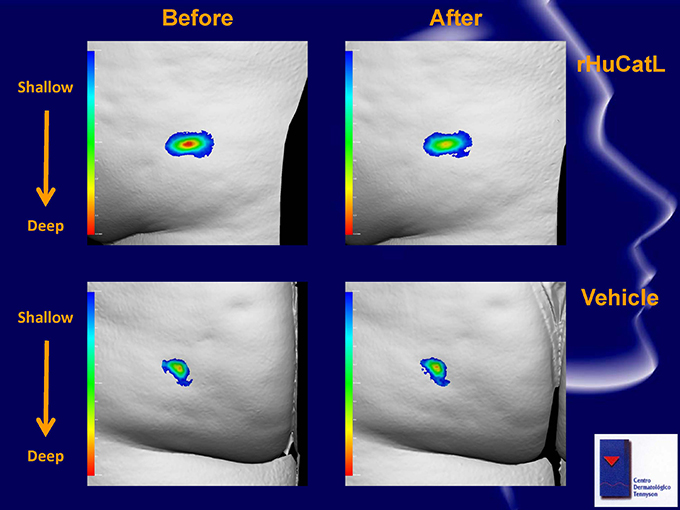
Before After rHuCatL Shallow Deep Vehicle Shallow Deep
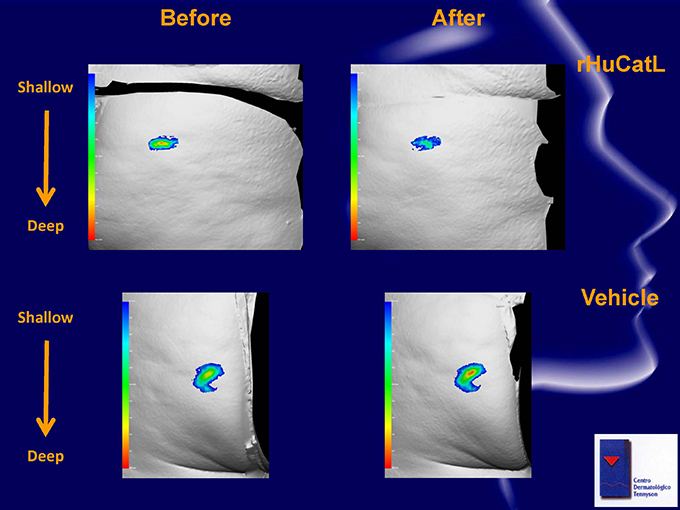
Before After rHuCatL Shallow Deep Vehicle Shallow Deep

Positive Effect Can Be Seen In 1st Week Pre-injection 7 Days 14 Days 28 Days
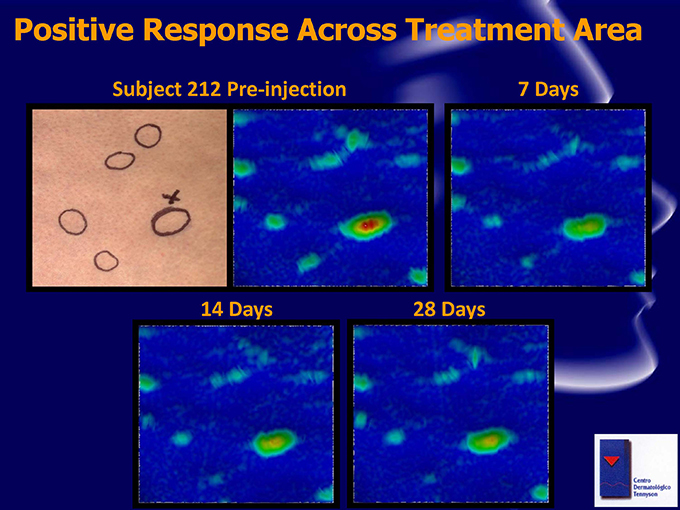
Positive Response Across Treatment Area Subject 212 Pre-injection 7 Days 14 Days 28 Days
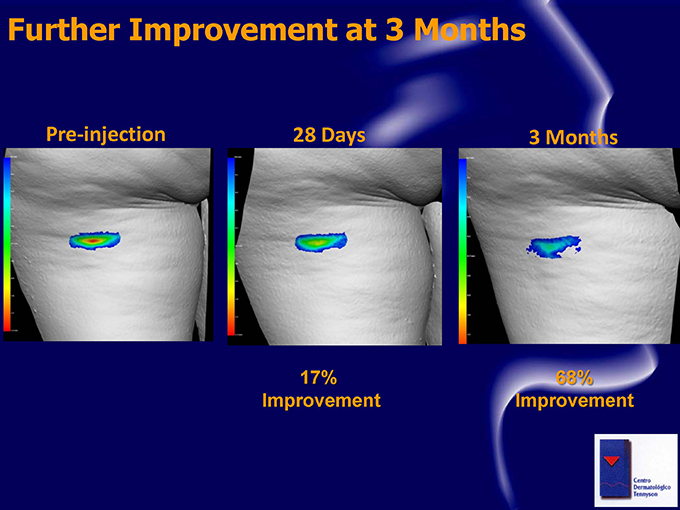
Further Improvement at 3 Months Pre-injection 28 Days 3 Months 17% 68% Improvement Improvement
Conclusions rHuCatL is being developed as a convenient, controlled enzymatic treatment for cellulite Interim data (N12) show a favorable response in 80% of subjects, median improvement is 53% 28 days after treatment Some subjects show further improvement 3 and 6 months after treatment Study drug has been well tolerated. The most common side effects were mild to moderate transient injection site pain and bruising