Table of Contents
CALCULATION OF REGISTRATION FEE
| ||||||||
Title of each class of securities to be registered(1) | Amount to be registered(1) | Proposed maximum offering price per unit | Proposed maximum aggregate offering price | Amount of registration fee(2) | ||||
Common Stock, par value $0.001 per share | 8,846,153 | $13.00 | $114,999,989 | $14,812 | ||||
| ||||||||
| ||||||||
| (1) | Includes shares of common stock that may be purchased by the underwriters pursuant to the underwriters’ option to purchase additional shares. |
| (2) | Calculated pursuant to Rule 457(r) under the Securities Act of 1933, as amended, or the Securities Act. The fee payable in connection with the offering of common stock pursuant to this prospectus supplement has been paid in accordance with Rule 456(b) under the Securities Act. |
Table of Contents
Filed Pursuant to Rule 424(b)(5)
Registration No. 333-179444
Prospectus Supplement
(To Prospectus dated February 9, 2012)
7,692,307 shares

Common stock
This is an offering of 7,692,307 shares of our common stock.
Our common stock is listed on The NASDAQ Global Select Market under the symbol “HALO.” The last reported sale price of our common stock on The NASDAQ Global Select Market on February 4, 2014 was $13.78 per share.
Per Share | Total | |||||||
Public offering price | $ | 13.00 | $ | 99,999,991.00 | ||||
Underwriting discounts and commissions | $ | 0.78 | $ | 5,999,999.46 | ||||
Proceeds to us, before expenses | $ | 12.22 | $ | 93,999,991.54 | ||||
We have granted the underwriters a 30-day option to purchase up to 1,153,846 additional shares of common stock at the public offering price less the underwriting discounts and commissions.
Investing in our common stock involves significant risks. See “Risk Factors” beginning on pageS-12.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed on the adequacy or accuracy of this prospectus supplement or the accompanying prospectus. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the shares on or about February 10, 2014.
Joint book-running managers
| J.P. Morgan | Citigroup |
Co-managers
| Piper Jaffray | BMO Capital Markets |
February 4, 2014
Table of Contents
Prospectus Supplement
| Page | ||||
| S-ii | ||||
| S-1 | ||||
| S-12 | ||||
| S-29 | ||||
| S-30 | ||||
| S-30 | ||||
| S-30 | ||||
| S-31 | ||||
Material U.S. Federal Income Tax Consequences to Non-U.S. Holders | S-32 | |||
| S-36 | ||||
| S-40 | ||||
| S-40 | ||||
| S-40 | ||||
Prospectus
| Page | ||||
| 1 | ||||
| 2 | ||||
| 6 | ||||
| 6 | ||||
| 7 | ||||
| 7 | ||||
| 8 | ||||
| 13 | ||||
| 20 | ||||
| 22 | ||||
| 23 | ||||
| 26 | ||||
| 28 | ||||
| 28 | ||||
| 28 | ||||
| 29 | ||||
No dealer, salesperson or other person is authorized to give any information or to represent anything not contained in this prospectus supplement or the accompanying prospectus. If you rely on any unauthorized information or representations, you will do so at your own risk. This prospectus supplement and the accompanying prospectus are an offer to sell only the shares offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this prospectus supplement and the accompanying prospectus is current only as of their respective dates.
S-i
Table of Contents
ABOUT THIS PROSPECTUS SUPPLEMENT
This document is in two parts. The first part is this prospectus supplement, which describes the specific terms of the offering and also adds to and updates information contained in the accompanying prospectus and the documents incorporated by reference into this prospectus supplement and the accompanying prospectus. The second part, the accompanying prospectus dated February 9, 2012, including the documents incorporated by reference, provides more general information. Generally, when we refer to this prospectus, we are referring to both parts of this document combined. To the extent there is a conflict between the information contained in this prospectus supplement, on the one hand, and the information contained in the accompanying prospectus or in any document incorporated by reference that was filed with the Securities and Exchange Commission (“SEC”) before the date of this prospectus supplement, on the other hand, you should rely on the information in this prospectus supplement. If any statement in one of these documents is inconsistent with a statement in another document having a later date—for example, a document incorporated by reference in the accompanying prospectus—the statement in the document having the later date modifies or supersedes the earlier statement. You should read this prospectus supplement and the accompanying prospectus, including the information incorporated by reference and any free writing prospectus that we have authorized for use in connection with this offering, in their entirety before making an investment decision.
Neither we nor the underwriters authorized anyone to provide you with information that is different from or in addition to the information contained or incorporated by reference in this prospectus supplement and the accompanying prospectus, along with the information contained in any free writing prospectus that we have authorized for use in connection with this offering. You should assume that the information appearing in this prospectus supplement, the accompanying prospectus, the documents incorporated by reference in this prospectus supplement and the accompanying prospectus, and any free writing prospectus that we have authorized for use in connection with this offering is accurate only as of the respective dates of those documents. Our business, financial condition, results of operations and prospects may have changed since those dates.
S-ii
Table of Contents
This summary highlights selected information appearing elsewhere or incorporated by reference in this prospectus supplement and the accompanying prospectus, and may not contain all of the information that is important to you. This prospectus supplement and the accompanying prospectus include information about the offering as well as information regarding our business. You should read this prospectus supplement and the accompanying prospectus, including the information incorporated by reference and any free writing prospectus that we have authorized for use in connection with this offering, in their entirety. If you invest in our common stock, you are assuming a high degree of risk. See “Risk Factors” beginning on page S-12.
Unless the context indicates otherwise or we expressly state to the contrary, as used in this prospectus supplement and the accompanying prospectus, the terms the “Company,” “Halozyme,” “Halozyme Therapeutics,” “we,” “us” and “our” refer to Halozyme Therapeutics, Inc., a Delaware corporation, and our operating subsidiary, Halozyme, Inc.
Overview
Halozyme is a science-driven, biopharmaceutical company committed to making molecules into medicines for patients in need. Our research focuses primarily on human enzymes that alter the extracellular matrix. The extracellular matrix is a complex matrix of proteins and carbohydrates surrounding the cell that provides structural support in tissues and orchestrates many important biological activities, including cell migration, signaling and survival. Over many years, we have developed unique technology and scientific expertise enabling us to pursue this target-rich environment for the development of therapies.
Our proprietary enzymes can be used to facilitate the delivery of injected drugs and fluids, thus enhancing the efficacy and the convenience of other drugs or can be used to alter abnormal tissue structures for clinical benefit. We have chosen to exploit our technology and expertise in a balanced way to modulate both risk and spend by: (1) developing our own proprietary products in therapeutic areas with significant unmet medical needs, such as diabetes, oncology and dermatology, and (2) licensing our technology to biopharmaceutical companies to collaboratively develop products which combine our technology with the collaborators’ proprietary compounds.
The majority of our approved product and product candidates are based on rHuPH20, a patented human recombinant hyaluronidase enzyme. rHuPH20 temporarily breaks down hyaluronic acid (“HA”)—a naturally occurring substance that is a major component of the extracellular matrix in tissues throughout the body such as skin and cartilage. We believe this temporary degradation creates an opportunistic window for the improved subcutaneous delivery of injectable biologics, such as monoclonal antibodies and other large therapeutic molecules, as well as small molecules and fluids. The HA reconstitutes its normal density within several days and, therefore, we anticipate that any effect of rHuPH20 on the architecture of the subcutaneous space is temporary. rHuPH20 can thus be applied as a drug delivery platform to increase dispersion and absorption of other injected drugs and fluids that are injected under the skin or in the muscle thereby enhancing efficacy or convenience. For example, rHuPH20 can be used to convert drugs that must be delivered intravenously into subcutaneous injections or to reduce the number of subcutaneous injections needed for effective therapy. We refer to the application of rHuPH20 to facilitate the delivery of other drugs or fluids as Enhanze™ technology. rHuPH20 is also the active ingredient in our first commercially approved product,Hylenex® recombinant (hyaluronidase human injection). Additionally, we are expanding our scientific work in the extracellular matrix by developing other enzymes and agents that target its unique aspects, giving rise to potentially new molecular entities that can be indicated in endocrinology, oncology and dermatology.
Our proprietary pipeline consists of multiple clinical stage products in diabetes, oncology and dermatology. We currently have collaborations with F. Hoffmann-La Roche, Ltd. and Hoffmann-La Roche, Inc. (“Roche”), Pfizer Inc. (“Pfizer”), Baxter Healthcare Corporation (“Baxter”), ViroPharma Incorporated (“ViroPharma”) and Intrexon Corporation (“Intrexon”), with two products approved for marketing in Europe, one product candidate
S-1
Table of Contents
which has been submitted for regulatory approval in the U.S., one product candidate which has been submitted for regulatory approval in Europe and has received a positive opinion from the EU Committee for Medicinal Products for Human Use (“CHMP”), as well as several others at various stages of development.
Our operations to date have involved: (i) building infrastructure for and staffing our operations; (ii) acquiring, developing and securing proprietary protection for our technology; (iii) developing our proprietary product pipeline; (iv) entering into and supporting our collaborations with other companies to advance licensed product candidates; and (v) selling our own approved commercial product,Hylenex recombinant. Currently, we have received only limited revenue from the sales ofHylenex recombinant, in addition to other revenues from our collaborations.
Future revenues from the sales and/or royalties of our product candidates which have not been approved or have recently been approved will depend on the ability of Halozyme and our collaborators to develop, manufacture, secure regulatory approvals for and commercialize the product candidates. We have incurred net operating losses each year since inception, with an accumulated deficit of approximately $360.1 million as of September 30, 2013.
The following are the anticipated milestones for our product development programs in 2014:
Proprietary Products
PEGPH20
| • | Complete enrollment in the on-going Phase 2 multicenter, randomized clinical trial (Study 202) evaluating PEGPH20 as a first-line therapy for patients with stage IV metastatic pancreatic cancer, in the second half of 2014. |
| • | Initiate patient enrollment in an additional solid tumor setting in the fourth quarter 2014. |
Hylenex Diabetes Program
| • | Announce top-line data from the 400 patient CONSISTENT 1 clinical trial evaluating the use ofHylenex in conjunction with rapid analog insulin in people with Type 1 diabetes using insulin pumps in the first quarter of 2014. |
| • | Submit clinical data from CONSISTENT 1 for publication at a major medical meeting in 2014. |
| • | Gain input from the U.S. Food and Drug Administration (“FDA”) on the path to secure an update to theHylenex label to include key efficacy and safety data when used as a pre-treatment in patients with diabetes using insulin pumps. |
HTI-501
| • | Announce top-line data for HTI-501 from the 36 subject Phase 2 clinical trial in cellulite in the first quarter of 2014. |
Collaboration Products
| • | Roche expects to receive approval of the marketing authorization in the EU for MabThera SC during 2014. |
| • | Baxter expects to receive a response to its amended Biologic License Application (“BLA”) for HyQvia from the FDA in mid-2014. |
S-2
Table of Contents
Product and Product Candidates
We have one marketed product and multiple product candidates targeting several indications in various stages of development. The following table summarizes our proprietary product and product candidates as well as two approved products and product candidates under development with our collaborators:
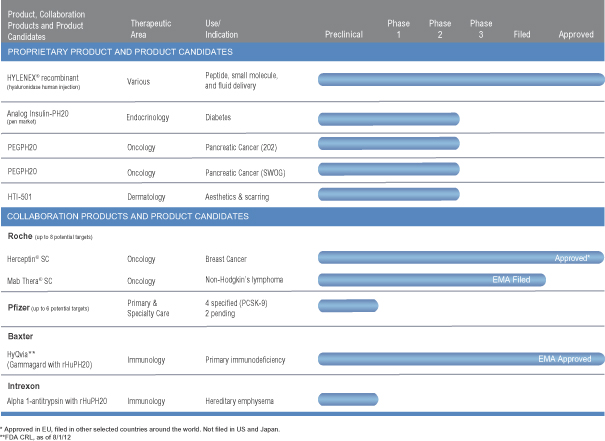
Proprietary Pipeline
Hylenex recombinant (hyaluronidase human injection)
Hylenex recombinant is a formulation of rHuPH20 that has received FDA approval to facilitate subcutaneous fluid administration for achieving hydration, to increase the dispersion and absorption of other injected drugs and, in subcutaneous urography, to improve resorption of radiopaque agents. We reintroducedHylenex recombinant to the market in December 2011 after resolution of Baxter’s voluntary recall and the return by Baxter of marketing rights to us. Upon its return to the market, our focus was to take advantage of the initial markets previously developed by Baxter. From May 2013 to September 2013,Hylenex was established as the number one prescribed HA. We are continuing to assess our commercial and strategic options for the product to address additional uses such as in connection with insulin pumps as described further below under “More Physiologic (Ultrafast) Insulin Program.”
S-3
Table of Contents
More Physiologic (Ultrafast) Insulin Program
Our most advanced proprietary program combines rHuPH20 with prandial (mealtime) insulin intended for the diabetes market. Diabetes mellitus is an increasingly prevalent, costly condition associated with substantial morbidity and mortality. Attaining and maintaining target blood sugar levels to minimize the long-term clinical risks is a key treatment goal for people living with diabetes.
The primary goal of our ultrafast insulin program is to enable a best-in-class prandial insulin product, with demonstrated clinical benefits for diabetes mellitus patients, in comparison to the current standard of care analog insulin products. Towards that goal, we pair rHuPH20 with prandial insulin to facilitate faster insulin dispersion in, and absorption from, the subcutaneous space into the vascular compartment, intended to lead to a faster insulin response and a shorter duration of action thereby potentially yielding a more physiologic insulin effect, similar to that found in non-diabetic people. A number of clinical trials investigating the various attributes of our product candidates have been completed.
We currently view two distinct opportunities to enter the prandial insulin market:
The first opportunity (what we refer to as the Continuous Subcutaneous Insulin Injection (“CSII”) market) is to pre-treat the insulin infusion site withHylenex recombinant at the time of infusion site change (once every 3 days). Pump therapy is growing in the U.S. among patients with Type 1 and Type 2 diabetes. We believe that the pre-treatment of the infusion site withHylenex recombinant could provide faster onset and shorter duration of insulin action. We currently intend to commercializeHylenex recombinant in CSII ourselves, with an initial focus on adults with Type 1 diabetes.
For the CSII market, we have published interim data from a study evaluating the use ofHylenex recombinant in analog insulin pump therapy that showed pre-administration ofHylenex recombinant provided a more physiologic profile, with what appeared to be “faster-on” and “faster-off” effects than current rapid insulin analogs. Copies of these publications can be found at http://www.halozyme.com/Technology/Journals-Abstracts-And-Posters/default.aspx. Data from the double-blind cross-over study showed that pre-treatment of the infusion site withHylenex recombinant, at the time of infusion set change, accelerated the absorption and shortened the action of mealtime insulin, provided a more consistent insulin action profile and improved post-prandial glucose control.
In preparation for commercializingHylenex recombinant in the CSII market in Type 1 diabetes for pre-administration with analog insulin, we are conducting supportive clinical studies, developing our regulatory and commercial strategy, manufacturing product and developing the administration convenience kit. In the first quarter of 2013, we initiated CONSISTENT 1, the largest of several planned studies for the CSII market. The CONSISTENT 1 study is evaluating the safety and efficacy ofHylenex recombinant in a 24 month trial conducted in over 400 Type 1 diabetic patients who were randomized 3:1 to either rapid acting analog insulin (“RAI”) delivered by CSII with Hylenex or standard CSII using RAI alone. Subjects randomized to the Hylenex group administer 150 units of Hylenex once every three days through each new infusion cannula, immediately prior to initiation of insulin delivery. The primary efficacy endpoint is comparison of change from baseline of A1C levels (A1C is a measure of average blood sugar over three months) using an industry standard non inferiority margin of 0.4%. The time point for assessment of the primary endpoint for the study was recently changed from four months to six months based on feedback we received from the FDA. Secondary endpoints for the study are hypoglycemia rates, hyperglycemia comparisons, glucose variability and safety endpoints (adverse events, local tolerability and immunogenicity). Enrollment for this trial was completed in the third quarter of 2013. We plan to communicate top line results from the CONSISTENT 1 study in the first quarter of 2014. We are currently in dialog with the FDA regarding the path for a labeling update to include key efficacy and safety data prior to initiating promotion ofHylenex recombinant for this use.
The second opportunity (what we refer to as the Multiple Daily Injection (“MDI”) market) is to combine rHuPH20 with an FDA approved RAI, e.g., insulin lispro (Humalog®) (Lispro-PH20), insulin aspart (Novolog®) (Aspart-PH20) and insulin glulisine (Apidra®) (Glulisine-PH20), (each such combination, analog-PH20), to accelerate their action. Based on the need for broad commercial reach to successfully introduce a new prandial
S-4
Table of Contents
insulin to the injection market, we believe that to maximize value, partnering with a large biotechnology or pharmaceutical company with global access to both the primary care and endocrinology markets may be required.
With regard to the MDI opportunity, we published data from two treatment studies—one in Type 1 diabetes patients and one in Type 2 diabetes patients. Copies of these publications can be found at http://www.halozyme.com/Technology/Journals-Abstracts-And-Posters/default.aspx. Both studies met their primary endpoints of A1C non-inferiority and improved post-prandial glucose control compared to patients who were treated with RAI alone. Additionally, data from the Type 1 diabetes treatment study indicated that Analog-PH20 formulations reduced hypoglycemia compared to RAI alone.
PEGPH20
We are developing PEGPH20, a new molecular entity, as a candidate for the systemic treatment of tumors that accumulate HA. PEGylation refers to the attachment of polyethylene glycol to rHuPH20, thereby creating PEGPH20. One of the novel properties of PEGPH20 is that it lasts for an extended duration in the bloodstream and, therefore, can be used to maintain therapeutic effect to treat systemic disease.
Solid malignancies often accumulate high levels of HA, including pancreatic, lung, breast, colon and prostate cancers, and therefore we believe that PEGPH20 has the potential to help patients with these types of cancer. Among solid tumors, pancreatic ductal adenocarcinoma is associated with the highest frequency of HA overexpression.
Over 100,000 patients are diagnosed with pancreatic cancer annually and are frequently not diagnosed until late stages. The pathologic accumulation of HA, along with other matrix components, creates a unique microenvironment for the growth of tumor cells compared to normal cells. We believe that depleting the HA component of the tumor architecture with PEGPH20 disrupts the tumor microenvironment, resulting in tumor growth inhibition. In addition, removal of HA rich matrix results in opening previously constricted vessels to allow anti-cancer therapies to have greater access to the tumor, which may enhance the treatment effect of complementary therapeutic modalities. Increased blood flow may also enable increased efficacy of radiotherapy treatment.
In June 2013, we presented the results from a Phase 1b clinical study of PEGPH20 in combination with gemcitabine for the treatment of patients with stage IV metastatic pancreatic cancer (Phase 1b PEGPH20 Clinical Study) at the 2013 American Society of Clinical Oncology (“ASCO”) Annual meeting. This study enrolled 28 patients with previously untreated stage IV pancreatic ductal adenocarcinoma. Patients were treated with one of three doses of PEGPH20 (1.0, 1.6 and 3.0 µg/kg twice weekly for four weeks, then weekly thereafter) in combination with gemcitabine 1000 mg/m2 administered intravenously. In this study, the overall response rate (complete response + partial response) by RECIST 1.1 criteria was 42 percent (10 of 24 patients, 95 percent CI 22 – 62 percent) for those treated at therapeutic dose levels of PEGPH20 (1.6 and 3.0 µg/kg) as assessed by an independent radiology review.
In September 2013, at the European Cancer Congress 2013, we presented exploratory post-hoc analysis of progression free survival and overall survival of a small subset of patients treated with PEGPH20 with available biopsy samples and HA scores in the Phase 1b study. Both progression free survival and overall survival were longer in patients with high levels of tumor HA compared to patients with low levels of tumor HA. The observation that patients with tumors characterized by high levels of HA may respond best to PEGPH20 has resulted in our effort to develop a companion diagnostic to enable pre-selection of these patients.
In the second quarter of 2013, we initiated a Phase 2 multicenter, randomized clinical trial evaluating PEGPH20 as a first-line therapy for patients with stage IV metastatic pancreatic cancer. Approximately 124 patients are expected to participate in the study and receive gemcitabine and nab-paclitaxel (“ABRAXANE™”)
S-5
Table of Contents
either with or without PEGPH20. The primary outcome will be to measure progression-free survival between patients administered with PEGPH20 and those who are not. We expect to complete enrollment in this study in the second half of 2014. In addition, in October 2013, SWOG, a cancer research cooperative group of more than 4,000 researchers in over 500 institutions around the world, initiated a 144 patient Phase 1b/2 randomized clinical trial of PEGPH20 in combination with modified FOLFIRINOX chemotherapy (“mFOLFIRINOX”) compared to mFOLFIRINOX treatment alone in patients with metastatic pancreatic adenocarcinoma.
HTI-501
HTI-501, an engineered drug formulation variant of cathepsin L (a lysosomal proteinase), that acts by degrading collagen, is our first conditionally-active biologic. Collagen is an abundant protein in the body, particularly in connective tissue, and is present in high amounts in the extracellular matrix in the form of collagen fibers. Collagens are a class of helical proteins that are assembled into macromolecular fibrils and fibers. The collagen fiber network provides a structural scaffolding framework in the extracellular matrix. In the skin, these collagen fibers connect the superficial epithelial tissues to the underlying connective tissues. Collagen abnormalities contribute to a number of conditions, including frozen shoulder, Dupuytren’s contracture, Peyronie’s disease and cellulite.
A conditionally active biologic is a molecule that is only active under certain physiological conditions. HTI-501 is active under mildly acidic conditions and inactive at the neutral pH normally found in the tissue. The enzyme is combined with a mildly acidic buffer and injected in its active state. The enzyme is only active locally and for a short period of time. Once the mildly acidic conditions of the HTI-501 administration have been neutralized by the body, the enzyme becomes inactive. We intend to harness this conditional activity to exert control over the duration and location of the enzyme’s therapeutic activity, potentially improving the efficacy or safety of this product candidate for both medical and aesthetic conditions.
We are exploring HTI-501 as an approach to the treatment of edematous fibrosclerotic panniculopathy, also known as cellulite. The condition affects the great majority of post-adolescent women and is prevalent in all races. We believe that the collagen fibers (“fibrous septa”) anchor the epidermis against the swelling of subcutaneous fat, which creates the dimpled appearance associated with the condition. We believe that HTI-501 deposited under the skin can release the tension in the collagenous fibrous septa and thereby smoothing the dimpled appearance of the skin. HTI-501 may also be potentially utilized as a treatment for other conditions involving collagen, such as frozen shoulder, Dupuytren’s contracture, Peyronie’s disease, keloids and hypertrophic scarring.
In September 2011, we initiated a Phase 1/2 clinical trial for HTI-501 outside the U.S. in women with moderate to severe cellulite. The Phase 1 dose-escalation portion of the trial was completed in 2012 while the ongoing Phase 2 portion of the trial is designed to assess the pharmacologic activity of HTI-501 and extend the safety assessment to multiple injections in a treatment area. In the third quarter of 2013, we completed the enrollment for the Phase 2 portion and the independent panel review of one month data.
Interim results from this trial were presented June 29, 2013 at the 9th Annual World Congress of Cosmetic Dermatology in Athens, Greece. The primary endpoint is physician assessment at Day 28, supported by secondary endpoints of subject self-evaluations and objective measurements of changes to the skin topography. The interim results from 12 of the planned 34 evaluable patients from this Phase 1/2 trial indicates pharmacologic activity at the primary 28 day observation point, with 83 percent of subjects (10 of 12) showing improvement from the pretreatment assessment, with a median improvement of 53 percent (p=.006) by the primary physician assessment. In comparison, 75 percent of subjects (9 of 12) showed improvement with a median improvement of 22 percent (p=.009) for the vehicle injection control at the same observation point. The objective measure (skin topography) for the treated area showed modest improvement in 80 percent of evaluable subjects (8 of 10) treated with HTI-501 (p=.042), but was not significantly changed for the vehicle control (p=.84) or a post-hoc evaluation of non-injected areas. To query the robustness of any study conclusions, an
S-6
Table of Contents
independent blinded panel evaluation of images will be performed on the evaluable subjects at one and six months following treatment. The HTI-501 enzyme and its formulation have been well tolerated so far in this trial at all doses and formulations tested, with no serious or severe adverse events. The most common side effects have been mild to moderate transient injection site discomfort and mild to moderate injection site bruising, resolving within about two weeks without intervention. We expect to report top line data at the three month and six month endpoints in the first quarter of 2014.
We currently do not have an investigational new drug application (“IND”) in the U.S., which would be required for us to conduct clinical trials in the U.S. for HTI-501. In order for us to file an IND, we will need to conduct significant development work including preclinical studies and manufacturing development.
Collaborations
Roche Collaboration
In December 2006, we and Roche entered into an agreement under which Roche obtained a worldwide, exclusive license to develop and commercialize product combinations of rHuPH20 with up to thirteen Roche target compounds (the “Roche Collaboration”). Roche initially had the exclusive right to apply rHuPH20 to only three pre-defined Roche biologic targets with the option to exclusively develop and commercialize rHuPH20 with ten additional targets. As of September 30, 2013, Roche has elected a total of five exclusive targets and retains the option to develop and commercialize rHuPH20 with three additional targets through the payment of annual license maintenance fees.
In September 2013, Roche launched a subcutaneous (SC) formulation of Herceptin® (trastuzumab) (Herceptin SC) in Europe for the treatment of patients with HER2-positive breast cancer. This formulation utilizes our recombinant human hyaluronidase (rHuPH20) and is administered in two to five minutes, rather than 30 to 90 minutes with the standard intravenous form. Roche received European marketing approval for Herceptin SC in August 2013. The European Commission’s approval was based on data from Roche’s Phase 3 HannaH study which showed that the subcutaneous formulation of Herceptin was associated with comparable efficacy (pathological complete response, pCR) to Herceptin administered intravenously in women with HER2-positive early breast cancer and resulted in non-inferior trastuzumab plasma levels. Overall, the safety profile in both arms of the HannaH study was consistent with that expected from standard treatment with Herceptin and chemotherapy in this setting. No new safety signals were identified. Breast cancer is the most common cancer among women worldwide. Each year, about 1.4 million new cases of breast cancer are diagnosed worldwide, and over 450,000 women will die of the disease annually. In HER2-positive breast cancer, increased quantities of the human epidermal growth factor receptor 2 (HER2) are present on the surface of the tumor cells. This is known as “HER2 positivity” and affects approximately 15% to 20% of women with breast cancer. HER2-positive cancer is a particularly aggressive form of breast cancer.
In December 2012, Roche submitted Line Extension Applications to the European Medicines Agency (EMA) for MabThera SC, Roche’s subcutaneous (SC) formulation of MabThera® (rituximab) using Halozyme’s recombinant human hyaluronidase (rHuPH20). In January 2014, the CHMP recommended that the European Commission approve MabThera SC for the treatment of patients with common forms of non-Hodgkin lymphoma (NHL). NHL is a type of cancer that affects lymphocytes (white blood cells). An estimated 66,000 new cases of NHL were diagnosed in the U.S. in 2009 with approximately 125,000 new cases reported worldwide. In December 2012, at the annual meeting of the American Society of Hematology, Roche presented positive data from the first stage of its two-stage Phase 3 clinical study investigating pharmacokinetics, efficacy and safety of MabThera SC. The primary endpoint in the first stage of the study was met, showing the MabThera SC injection resulted in non-inferior MabThera concentrations in the blood compared with IV-infused MabThera (MabThera IV).
S-7
Table of Contents
Baxter Gammagard Collaboration
GAMMAGARD LIQUID is a current Baxter product that is indicated for the treatment of primary immunodeficiency disorders associated with defects in the immune system. In September 2007, we and Baxter entered into an agreement under which Baxter obtained a worldwide, exclusive license to develop and commercialize product combinations of rHuPH20 with GAMMAGARD LIQUID (HyQvia) (the “Gammagard Collaboration”).
Baxter filed a BLA for HyQvia in the U.S. in the second quarter of 2011. On August 1, 2012, we announced that the FDA had issued a complete response letter (“CRL”) for Baxter’s HyQvia BLA. The CRL requested additional preclinical data to support the BLA. The primary issues raised in the CRL focused on non-neutralizing antibodies generated against rHuPH20 and the possible effects of these antibodies on reproduction, development and fertility. Elevated anti-rHuPH20 antibody titers were detected in the registration trial, but have not been associated with any adverse events. Pending Baxter and us providing additional preclinical data sufficient to address the regulatory questions, the FDA has requested that patients should no longer be dosed with rHuPH20 in the Baxter HyQvia program. In December 2013, we and Baxter announced that Baxter has completed submission of the amended BLA to the FDA to initiate the review process for approval of HyQvia. Baxter submitted additional preclinical data that was requested from the FDA in 2012 and expects a six-month review.
In May 2013, the European Commission granted Baxter marketing authorization in all EU Member States for the use of HyQvia (solution for subcutaneous use) as replacement therapy for adult patients with primary and secondary immunodeficiencies. This therapy offers patients the option to administer their therapy at home, in a single subcutaneous site every three to four weeks. Baxter has launched HyQvia into the first EU country in July 2013 and a number of EU countries in the second half of 2013. Baxter plans to expand the launch to other EU markets in 2014.
Pfizer Collaboration
In December 2012, we and Pfizer entered into a collaboration and license agreement, under which Pfizer has the worldwide license to develop and commercialize products combining rHuPH20 enzyme with Pfizer proprietary biologics directed to up to six targets in primary care and specialty care indications (the “Pfizer Collaboration”). Targets may be selected on an exclusive or non-exclusive basis. In September 2013, Pfizer elected the fourth therapeutic target on an exclusive basis. In December Pfizer announced that one of the targets is PCSK9
ViroPharma Collaboration
In May 2011, we and ViroPharma entered into a collaboration and license agreement under which ViroPharma obtained a worldwide exclusive license for the use of rHuPH20 enzyme in the development and commercialization of a subcutaneous injectable formulation of ViroPharma’s commercialized product, Cinryze (C1 esterase inhibitor [human]) (the “ViroPharma Collaboration”). In addition, the license provides ViroPharma with exclusivity to C1 esterase inhibitor and to hereditary angioedema, a rare, debilitating and potentially fatal genetic disease, along with three additional orphan indications.
In December 2012, ViroPharma initiated a Phase 2 double blind, multicenter, dose ranging study to evaluate the safety and efficacy of subcutaneous administration of Cinryze in combination with rHuPH20 in adolescents and adults with hereditary angioedema attacks. On August 1, 2013, ViroPharma announced that it was discontinuing the study following discussions with the Center for Biologics Evaluation and Research (“CBER”) division of the FDA as a precaution related to the emergence of an unexpected incidence and titer of non-neutralizing anti-rHuPH20 antibodies in a number of patients with the formulation being used in this study. These antibodies have not been associated with any adverse clinical effects.
S-8
Table of Contents
Intrexon Collaboration
In June 2011, we and Intrexon entered into a collaboration and license agreement under which Intrexon obtained a worldwide exclusive license for the use of rHuPH20 enzyme in the development and commercialization of a subcutaneous injectable formulation of Intrexon’s recombinant human alpha 1-antitrypsin (rHuA1AT) (the “Intrexon Collaboration”). In addition, the license provides Intrexon with exclusivity for a defined indication.
For a further discussion of the material terms of our collaboration agreements, refer to Note 4 to our consolidated financial statements in our Quarterly Report on Form 10-Q for the three and nine months ended September 30, 2013 incorporated by reference herein.
Recent Developments
Amendment to Certificate of Incorporation
In May 2013, our stockholders approved an amendment to our Certificate of Incorporation to increase our authorized number of shares of common stock from 150 million shares to 200 million shares.
Amended and Restated Loan and Security Agreement
On December 27, 2013, we entered into an Amended and Restated Loan and Security Agreement (the “Loan Agreement”) with Oxford Finance LLC, a Delaware limited liability company, and Silicon Valley Bank, a California corporation, amending and restating in its entirety the Loan and Security Agreement dated as of December 28, 2012 (the “Original Loan Agreement”). The Original Loan Agreement provided for a $30 million secured single-draw term loan facility with a maturity date of January 1, 2017. The original term loan was fully drawn at close. The Loan Agreement extends the original $30 million term loans and provides for an additional $20 million in new term loans, bringing the total term loan balance to $50 million. Upon closing of the Loan Agreement, we received approximately $19 million, net of accrued interest. The proceeds are to be used for working capital and general business requirements. The amended and restated term loan facility matures on January 1, 2018. The amended and restated term loan facility is secured by substantially all of our assets, except that the collateral does not include any equity interests in our subsidiary, Halozyme, Inc., any intellectual property (including all licensing, collaboration and similar agreements relating thereto), and certain other excluded assets. The amended and restated term loan repayment schedule provides for interest only payments for the first year, followed by consecutive equal monthly payments of principal and interest in arrears starting in February 2015 and continuing through the maturity date. The Loan Agreement provides for a 7.55% interest rate on the term loans and a final payment equal to 8.5% of the initial principal amount of the term loans, which is due when the term loans become due or upon the prepayment of the facility. Based upon preliminary estimates, as of December 31, 2013, we had approximately $71 million in cash and cash equivalents and marketable securities. This financial information is subject to completion of our year-end financial closing procedures, the preparation of our financial statements, and the completion of the audit of our financial statements as of and for the year ended December 31, 2013, and our actual results may differ from these estimates.
MabTheraSC Receives Positive CHMP Opinion
In January 2014, the CHMP recommended that the European Commission approve MabThera SC for the treatment of patients with common forms of non-Hodgkin lymphoma.
CONSISTENT 1 Clinical Trial Update
The time point for assessment of the primary endpoint for the CONSISTENT 1 clinical study evaluating the use ofHylenex recombinant in conjunction with rapid analog insulin in people with Type 1 diabetes using insulin
S-9
Table of Contents
pumps was recently changed from 4 months to 6 months basedon feedback we received from the FDA. We plan to communicate top line results from the CONSISTENT 1 study in the first quarter of 2014.
Corporate Information
We reincorporated from the State of Nevada to the State of Delaware in November 2007. Our principal offices and research facilities are located at 11388 Sorrento Valley Road, San Diego, California 92121. Our telephone number is (858) 794-8889 and our e-mail address is info@halozyme.com. Additional information about us can be found on our website at www.halozyme.com. The information on our website is not part of this prospectus supplement.
S-10
Table of Contents
Common stock offered by us in this offering | 7,692,307 shares of our common stock |
Option to purchase additional shares | We have granted the underwriters an option for a period of up to 30 days from the date of this prospectus supplement to purchase up to 1,153,846 additional shares of common stock at the public offering price less the underwriting discounts and commissions |
Common stock to be outstanding immediately after this offering | 121,679,046 shares (or 122,832,892 shares if the underwriters exercise in full their option to purchase additional shares) |
Risk factors | Investing in our common stock involves a high degree of risk. See “Risk Factors” beginning on page S-12 |
Use of proceeds | We intend to use the net proceeds from this offering to fund research and development of proprietary programs, including the potential acceleration of the PEGPH20 program, and for other general corporate purposes. See “Use of Proceeds” on page S-30 |
NASDAQ Global Select Market symbol | HALO |
The number of shares of common stock to be outstanding immediately after this offering as shown above is based on 113,986,739 shares of common stock outstanding as of September 30, 2013. This number of shares excludes, as of September 30, 2013:
| • | 7,178,512 shares of common stock issuable upon the exercise of outstanding stock options, having a weighted average exercise price of $6.92 per share; |
| • | 746,636 shares of common stock issuable upon settlement of restricted stock units; and |
| • | an aggregate of up to 7,010,395 shares of common stock reserved for future issuance under our equity incentive plans. |
Unless otherwise indicated, all information in this prospectus supplement assumes:
| • | that the underwriters do not exercise their option to purchase up to 1,153,846 additional shares of our common stock; and |
| • | no options or shares of common stock were issued after September 30, 2013, and no outstanding equity awards were exercised or vested after September 30, 2013. |
S-11
Table of Contents
An investment in our common stock involves a high degree of risk. Before deciding whether to invest in our common stock, you should consider carefully the risks described below, together with other information in this prospectus supplement, the accompanying prospectus, the information and documents incorporated by reference, and in any free writing prospectus that we have authorized for use in connection with this offering. If any of these risks actually occurs, our business, financial condition, results of operations or cash flow could be seriously harmed. This could cause the trading price of our common stock to decline, resulting in a loss of all or part of your investment. The risks and uncertainties described below are not the only ones facing us. Additional risks and uncertainties not presently known to us, or that we currently see as immaterial, may also harm our business.
Risks Related to this Offering and Our Common Stock
Our stock price is subject to significant volatility.
We participate in a highly dynamic industry which often results in significant volatility in the market price of common stock irrespective of company performance. As a result, our high and low sales prices of our common stock during the twelve months ended January 31, 2014 were $18.18 and $5.03, respectively. We expect our stock price to continue to be subject to significant volatility and, in addition to the other risks and uncertainties described elsewhere in this prospectus supplement and all other risks and uncertainties that are either not known to us at this time or which we deem to be immaterial, any of the following factors may lead to a significant drop in our stock price:
| • | the presence of competitive products to those being developed by us; |
| • | failure (actual or perceived) of our collaborators to devote attention or resources to the development or commercialization of product candidates licensed to such collaborator; |
| • | a dispute regarding our failure, or the failure of one of our third party collaborators, to comply with the terms of a collaboration agreement; |
| • | the termination, for any reason, of any of our collaboration agreements; |
| • | the sale of common stock by any significant stockholder, including, but not limited to, direct or indirect sales by members of management or our Board of Directors; |
| • | the resignation, or other departure, of members of management or our Board of Directors; |
| • | general negative conditions in the healthcare industry; |
| • | general negative conditions in the financial markets; |
| • | the failure, for any reason, to obtain regulatory approval for any of our proprietary or collaboration product candidates; |
| • | the failure, for any reason, to secure or defend our intellectual property position; |
| • | for those products that are not yet approved for commercial sale, the failure or delay of applicable regulatory bodies to approve such products; |
| • | identification of safety or patient tolerability issues; |
| • | failure of clinical trials to meet efficacy endpoints; |
| • | suspensions or delays in the conduct of clinical trials or securing of regulatory approvals; |
| • | adverse regulatory action with respect to our and our collaborators’ products and product candidates such as clinical holds, imposition of onerous requirements for approval or product recalls; |
| • | our failure, or the failure of our third party collaborators, to successfully commercialize products approved by applicable regulatory bodies such as the FDA; |
S-12
Table of Contents
| • | our failure, or the failure of our third party collaborators, to generate product revenues anticipated by investors; |
| • | problems with a bulk rHuPH20 contract manufacturer or a fill and finish manufacturer for any product or product candidate; |
| • | the sale of additional debt and/or equity securities by us; |
| • | our failure to obtain financing on acceptable terms; or |
| • | a restructuring of our operations. |
Future transactions where we raise capital may negatively affect our stock price.
We are currently a “Well-Known Seasoned Issuer” and may file automatic shelf registration statements at any time with the SEC. In addition, we currently have the ability to offer and sell additional equity, debt securities and warrants to purchase such securities, either individually or in units, under an effective automatic shelf registration statement. Sales of substantial amounts of shares of our common stock or other securities under our shelf registration statements could lower the market price of our common stock and impair our ability to raise capital through the sale of equity securities. In the future, we may issue additional options, warrants or other derivative securities convertible into our common stock.
Trading in our stock has historically been limited, so investors may not be able to sell as much stock as they want to at prevailing market prices.
Our stock has historically traded at a low daily trading volume. If low trading volume continues, it may be difficult for stockholders to sell their shares in the public market at any given time at prevailing prices.
Our rights agreement and anti-takeover provisions in our charter documents and Delaware law may make an acquisition of us more difficult.
We are party to a Rights Agreement designed to deter abusive takeover tactics and to encourage prospective acquirors to negotiate with our board of directors rather than attempt to acquire us in a manner or on terms that our board deems unacceptable, which could delay or discourage takeover attempts that stockholders may consider favorable.
In addition, anti-takeover provisions in our charter documents and Delaware law may make an acquisition of us more difficult. First, our board of directors is classified into three classes of directors. Under Delaware law, directors of a corporation with a classified board may be removed only for cause unless the corporation’s certificate of incorporation provides otherwise. Our amended and restated certificate of incorporation, as amended, does not provide otherwise. In addition, our bylaws limit who may call special meetings of stockholders, permitting only stockholders holding at least 50% of our outstanding shares to call a special meeting of stockholders. Our amended and restated certificate of incorporation, as amended, does not include a provision for cumulative voting for directors. Under cumulative voting, a minority stockholder holding a sufficient percentage of a class of shares may be able to ensure the election of one or more directors. Finally, our bylaws establish procedures, including advance notice procedures, with regard to the nomination of candidates for election as directors and stockholder proposals.
These provisions may discourage potential takeover attempts, discourage bids for our common stock at a premium over market price or adversely affect the market price of, and the voting and other rights of the holders of, our common stock. These provisions could also discourage proxy contests and make it more difficult for stockholders to elect directors other than the candidates nominated by our board of directors.
In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which may prohibit large stockholders from consummating a merger with, or acquisition of, us.
These provisions may deter an acquisition of us that might otherwise be attractive to stockholders.
S-13
Table of Contents
Management will have broad discretion as to the use of the net proceeds from this offering, and we may not use the proceeds effectively.
Our management will have broad discretion as to the application of the net proceeds and could use them for purposes other than those contemplated at the time of this offering. Our stockholders may not agree with the manner in which our management chooses to allocate and spend the net proceeds. Moreover, our management may use the net proceeds for corporate purposes that may not increase our profitability or our market value.
Investors in this offering will pay a higher price than the book value of our common stock.
You will suffer substantial dilution in the net tangible book value of the common stock you purchase in this offering because the price per share being offered hereby is substantially higher than the book value per share of our common stock. Based on the public offering price of $13.00 per share in this offering, if you purchase shares in this offering, after giving effect to the sale of 7,692,307 shares in this offering, you will suffer immediate and substantial dilution of $12.26 per share in the net tangible book value of the common stock. See “Dilution” beginning on page S-31 of this prospectus supplement for a more detailed discussion of the dilution you will incur in this offering.
Risks Related to Our Business
We have generated only minimal revenue from product sales to date; we have a history of net losses and negative cash flow, and we may never achieve or maintain profitability.
Relative to expenses incurred in our operations, we have generated only minimal revenues from product sales, licensing fees, milestone payments, bulk rHuPH20 supply payments and research reimbursements to date and we may never generate sufficient revenues from future product sales, licensing fees and milestone payments to offset expenses. Even if we ultimately do achieve significant revenues from product sales, licensing fees, research reimbursements, bulk rHuPH20 supply payments and/or milestone payments, we expect to incur significant operating losses over the next few years. We have never been profitable, and we may never become profitable. Through September 30, 2013, we have incurred aggregate net losses of approximately $360.1 million.
If our product candidates do not receive and maintain regulatory approvals, or if approvals are not obtained in a timely manner, such failure or delay would substantially impair our ability to generate revenues.
Approval from the FDA or equivalent health authorities is necessary to manufacture and market pharmaceutical products in the United States and the other countries in which we anticipate doing business have similar requirements. The process for obtaining FDA and other regulatory approvals is extensive, time-consuming, risky and costly, and there is no guarantee that the FDA or other regulatory bodies will approve any applications that may be filed with respect to any of our product candidates, or that the timing of any such approval will be appropriate for the desired product launch schedule for a product candidate. We and our collaborators attempt to provide guidance as to the timing for the filing and acceptance of such regulatory approvals, but such filings and approvals may not occur when we or our collaborators expect or at all. The FDA or other foreign regulatory agency may refuse or delay approval of our product candidates for failure to collect sufficient clinical or animal safety data and require us or our collaborators to conduct additional clinical or animal safety studies which may cause lengthy delays and increased costs to our programs. For example, we announced on August 1, 2012 that the FDA had issued a CRL for Baxter’s HyQvia BLA. The CRL requested additional preclinical data to support the BLA. The primary issues raised in the letter focused on non-neutralizing antibodies generated against rHuPH20 and the possible effects of these antibodies on reproduction, development and fertility. Elevated anti-rHuPH20 antibody titers were detected in the registration trial, but have not been associated with any adverse events. Pending Baxter and us providing additional preclinical data sufficient to address the regulatory questions, the FDA has requested that patients should no longer be dosed with rHuPH20 in the Baxter clinical studies. In view of the issues raised in the HyQvia CRL, we contacted the FDA regarding the
S-14
Table of Contents
impact on Hylenex recombinant. After reviewing the applicable data submitted by us, FDA confirmed that there was no need for actions against Hylenex recombinant or clinical programs under the Hylenex recombinant IND application(s). Subsequent to this, in August 2013, our collaborator ViroPharma announced that it was discontinuing its study of subcutaneous administration of Cinryze in combination with rHuPH20 in adolescents and adults with hereditary angioedema attacks, following discussion with FDA regarding the emergence of an unexpected incidence and titer of non-neutralizing anti-rHuPH20 antibodies in a number of patients with the formulation being used in this study. Although these antibodies have not been associated with any adverse clinical effects, we cannot assure you that they will not arise and have adverse impact on future development of rHuPH20 or future sales of Hylenex recombinant.
There can be no assurance that Baxter and we will be able to resolve the issues raised by the FDA in a timely manner which could result in a delay or failure to gain regulatory approval for the HyQvia product candidate. Furthermore, although we do not believe at this time that the issues raised by the FDA with respect to the HyQvia BLA or the ViroPharma Phase 2 study will have a significant impact on our proprietary and other collaboration product candidates, there can be no assurance that these concerns will not also be raised by the FDA or other health authorities in the future.
Only two of our collaboration product candidates has been approved for commercialization and two of our collaboration product candidates are currently in the regulatory approval process. Only one of our proprietary products has been approved for commercialization, and we have no proprietary product candidates currently in the regulatory approval process. We and our collaborators may not be successful in obtaining such approvals for any potential products in a timely manner, or at all. Refer to the risk factor titled “Our proprietary and collaboration product candidates may not receive regulatory approvals or their development may be delayed for a variety of reasons, including unsuccessful clinical trials, regulatory requirements or safety concerns” for additional information relating to the approval of product candidates.
Additionally, even with respect to products which have been approved for commercialization, in order to continue to manufacture and market pharmaceutical products, we or our collaborators must maintain our regulatory approvals. If we or any of our collaborators are unsuccessful in maintaining our regulatory approvals, our ability to generate revenues would be adversely affected.
Use of our product candidates or those of our collaborators could be associated with side effects or adverse events.
As with most pharmaceutical products, use of our product candidates or those of our collaborators could be associated with side effects or adverse events which can vary in severity (from minor reactions to death) and frequency (infrequent or prevalent). Side effects or adverse events associated with the use of our product candidates or those of our collaborators may be observed at anytime, including in clinical trials or when a product is commercialized, and any such side effects or adverse events may negatively affect our or our collaborators’ ability to obtain regulatory approval or market our product candidates. Side effects such as toxicity or other safety issues associated with the use of our product candidates or those of our collaborators could require us or our collaborators to perform additional studies or halt development or sale of these product candidates or expose us to product liability lawsuits which will harm our business. We or our collaborators may be required by regulatory agencies to conduct additional animal or human studies regarding the safety and efficacy of our pharmaceutical product candidates which we have not planned or anticipated. Furthermore, there can be no assurance that we or our collaborators will resolve any issues related to any product related adverse events to the satisfaction of the FDA or any regulatory agency in a timely manner or ever, which could harm our business, prospects and financial condition.
S-15
Table of Contents
If our contract manufacturers are unable to manufacture and supply to us bulk rHuPH20 in the quantity and quality required by us or our collaborators for use in our products and product candidates, our product development and commercialization efforts could be delayed or stopped and our collaborations could be damaged.
We have existing supply agreements with contract manufacturing organizations Avid and Cook to produce bulk rHuPH20. These manufacturers each produce bulk rHuPH20 under current cGMP for clinical uses. In addition, Avid currently produces bulk rHuPH20 forHylenex recombinant. In addition to supply obligations, Avid and Cook will also provide support for the chemistry, manufacturing and controls sections for FDA and other regulatory filings. We rely on their ability to successfully manufacture these batches according to product specifications, and Cook has relatively limited experience manufacturing bulk rHuPH20. In addition, as a result of our contractual obligations to Roche, we have been required to significantly scale up our bulk rHuPH20 production at Cook during the last three years. If Cook is unable to obtain status as an approved manufacturing facility, or if either Avid or Cook: (i) is unable to retain status as an approved manufacturing facilities; (ii) is unable to otherwise successfully scale up bulk rHuPH20 production; or (iii) fails to manufacture and supply bulk rHuPH20 in the quantity and quality required by us or our collaborators for use in our proprietary and collaboration products and product candidates for any other reason, our business will be adversely affected. In addition, a significant change in such parties’ business or financial condition could adversely affect their abilities to fulfill their contractual obligations to us. We have not established, and may not be able to establish, favorable arrangements with additional bulk rHuPH20 manufacturers and suppliers of the ingredients necessary to manufacture bulk rHuPH20 should the existing manufacturers and suppliers become unavailable or in the event that our existing manufacturers and suppliers are unable to adequately perform their responsibilities. We have attempted to mitigate the impact of supply interruption through the establishment of excess bulk rHuPH20 inventory, but there can be no assurances that this safety stock will be maintained or that it will be sufficient to address any delays, interruptions or other problems experienced by Avid and/or Cook. Any delays, interruptions or other problems regarding the ability of Avid and/or Cook to bulk rHuPH20 on a timely basis could: (i) cause the delay of clinical trials or otherwise delay or prevent the regulatory approval of proprietary or collaboration product candidates; (ii) delay or prevent the effective commercialization of proprietary or collaboration products; and/or (iii) cause us to breach contractual obligations to deliver bulk rHuPH20 to our collaborators. Such delays would likely damage our relationship with our collaborators under our key collaboration agreements, and they would have a material adverse effect on our business and financial condition.
If any party to a key collaboration agreement, including us, fails to perform material obligations under such agreement, or if a key collaboration agreement, or any other collaboration agreement, is terminated for any reason, our business could significantly suffer.
We have entered into multiple collaboration agreements under which we may receive significant future payments in the form of milestone payments, target designation fees, maintenance fees and royalties. We are dependent on our collaborators to develop and commercialize product candidates subject to our collaborations in order for us to realize any financial benefits from these collaborations. Our collaborators may not devote the attention and resources to such efforts that we would to such efforts ourselves or simultaneously develop and commercialize products in competition to those products we have licensed to them. In addition, in the event that a party fails to perform under a key collaboration agreement, or if a key collaboration agreement is terminated, the reduction in anticipated revenues could delay or suspend our product development activities for some of our product candidates, as well as our commercialization efforts for some or all of our products. Specifically, the termination of a key collaboration agreement by one of our collaborators could materially impact our ability to enter into additional collaboration agreements with new collaborators on favorable terms, if at all. In certain circumstances, the termination of a key collaboration agreement would require us to revise our corporate strategy going forward and reevaluate the applications and value of our technology.
S-16
Table of Contents
Most of our current proprietary and collaboration products and product candidates rely on the rHuPH20 enzyme, and any adverse development regarding rHuPH20 could substantially impact multiple areas of our business, including current and potential collaborations, as well as proprietary programs.
rHuPH20 is a key technological component of Enhanze technology and our most advanced proprietary and collaboration products and product candidates, including the product candidates under our Roche, Pfizer, Baxter, ViroPharma and Intrexon collaborations, our more physiologic insulin program, our PEGPH20 program and Hylenex recombinant. An adverse development for rHuPH20 (e.g., an adverse regulatory determination relating to rHuPH20, if we are unable to obtain sufficient quantities of rHuPH20, if we are unable to obtain or maintain material proprietary rights to rHuPH20 or if we discover negative characteristics of rHuPH20) would substantially impact multiple areas of our business, including current and potential collaborations, as well as proprietary programs. For example, elevated anti-rHuPH20 antibody titers have been detected in the registration trial for Baxter’s HyQvia product candidate as well as in ViroPharma’s Phase 2 clinical trial with subcutaneous Cinryze with rHuPH20, but have not been associated, in either case, with any adverse events. Baxter has submitted preclinical data to the FDA regarding the antibodies in its BLA resubmission in response to the CRL Letter received for the HyQvia BLA and is awaiting response from the FDA. ViroPharma has chosen to discontinue the Phase 2 clinical trial with subcutaneous Cinryze with rHuPH20 due to the unexpected incidence and titer of antibodies in a number of patients with the formulation being used in this study. We monitor for antibodies to rHuPH20 in our collaboration and proprietary programs, and although we do not believe at this time that the incidence of non-neutralizing anti-rHuPH20 antibodies in either the HyQvia program or the ViroPharma program will have a significant impact on our other proprietary and other collaboration product candidates, there can be no assurance that there will not be other such occurrences in our other programs or that concerns regarding these antibodies will not also be raised by the FDA or other health authorities in the future, which could result in delays or discontinuations of our development or commercialization activities or deter entry into additional collaborations with third parties.
Our proprietary and collaboration product candidates may not receive regulatory approvals or their development may be delayed for a variety of reasons, including unsuccessful clinical trials, regulatory requirements or safety concerns.
Clinical testing of pharmaceutical products is a long, expensive and uncertain process, and the failure or delay of a clinical trial can occur at any stage. Even if initial results of preclinical and nonclinical studies or clinical trial results are promising, we or our collaborators may obtain different results in subsequent trials or studies that fail to show the desired levels of safety and efficacy, or we may not, or our collaborators may not, obtain applicable regulatory approval for a variety of other reasons. Preclinical, nonclinical, and clinical trials for any of our proprietary or collaboration product candidates could be unsuccessful, which would delay or prohibit regulatory approval and commercialization of the product candidates. In the United States and other jurisdictions, regulatory approval can be delayed, limited or not granted for many reasons, including, among others:
| • | clinical results may not meet prescribed endpoints for the studies or otherwise provide sufficient data to support the efficacy of our product candidates; |
| • | clinical and nonclinical test results may reveal side effects, adverse events or unexpected safety issues associated with the use of our product candidates; |
| • | regulatory review may not find a product candidate safe or effective enough to merit either continued testing or final approval; |
| • | regulatory review may not find that the data from preclinical testing and clinical trials justifies approval; |
| • | regulatory authorities may require that we change our studies or conduct additional studies which may significantly delay or make continued pursuit of approval commercially unattractive; for example, based on FDA feedback, we recently changed the time point for assessment of the primary endpoint of non-inferiority of A1C from four months to six months in our CONSISTENT 1 trial for Hylenex recombinant for use in CSII; |
S-17
Table of Contents
| • | a regulatory agency may reject our trial data or disagree with our interpretations of either clinical trial data or applicable regulations; |
| • | the cost of clinical trials required for product approval may be greater than what we originally anticipate, and we may decide to not pursue regulatory approval for such a product; |
| • | a regulatory agency may not approve our manufacturing processes or facilities, or the processes or facilities of our collaborators, our contract manufacturers or our raw material suppliers; |
| • | a regulatory agency may identify problems or other deficiencies in our existing manufacturing processes or facilities, or the existing processes or facilities of our collaborators, our contract manufacturers or our raw material suppliers; |
| • | a regulatory agency may change its formal or informal approval requirements and policies, act contrary to previous guidance, adopt new regulations or raise new issues or concerns late in the approval process; or |
| • | a product candidate may be approved only for indications that are narrow or under conditions that place the product at a competitive disadvantage, which may limit the sales and marketing activities for such product candidate or otherwise adversely impact the commercial potential of a product. |
If a proprietary or collaboration product candidate is not approved in a timely fashion on commercially viable terms, or if development of any product candidate is terminated due to difficulties or delays encountered in the regulatory approval process, it could have a material adverse impact on our business, and we will become more dependent on the development of other proprietary or collaboration product candidates and/or our ability to successfully acquire other products and technologies. There can be no assurances that any proprietary or collaboration product candidate will receive regulatory approval in a timely manner, or at all. For example, we are currently in dialog with the FDA regarding the path for a labeling update to include key efficacy and safety data prior to initiatingHylenex recombinant for use in CSII. There can be no assurance that we will be able to gain clarity as to the FDA’s requirements or that the requirements may be satisfied by us in a commercially feasible way. If we are not successful in updating data into the Hylenex recombinant labeling, our ability to promote this use will be limited and may adversely impact our projected market for the CSII use.
We anticipate that certain proprietary and collaboration products will be marketed, and perhaps manufactured, in foreign countries. The process of obtaining regulatory approvals in foreign countries is subject to delay and failure for the reasons set forth above, as well as for reasons that vary from jurisdiction to jurisdiction. The approval process varies among countries and jurisdictions and can involve additional testing. The time required to obtain approval may differ from that required to obtain FDA approval. Foreign regulatory agencies may not provide approvals on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or jurisdictions or by the FDA.
Our third party collaborators are responsible for providing certain proprietary materials that are essential components of our collaboration product candidates, and any failure to supply these materials could delay the development and commercialization efforts for these collaboration product candidates and/or damage our collaborations.
Our development and commercialization collaborators are responsible for providing certain proprietary materials that are essential components of our collaboration product candidates. For example, Roche is responsible for producing the Herceptin and MabThera required for its subcutaneous product candidates and Baxter is responsible for producing the GAMMAGARD LIQUID for its product candidate HyQvia. If a collaborator, or any applicable third party service provider of a collaborator, encounters difficulties in the manufacture, storage, delivery, fill, finish or packaging of the collaboration product candidate or component of such product candidate, such difficulties could (i) cause the delay of clinical trials or otherwise delay or prevent the regulatory approval of collaboration product candidates; and/or (ii) delay or prevent the effective
S-18
Table of Contents
commercialization of collaboration products. Such delays could have a material adverse effect on our business and financial condition. For example, Baxter received a Warning Letter from the FDA in January 2010 regarding Baxter’s GAMMAGARD LIQUID manufacturing facility in Lessines, Belgium. The FDA indicated in March 2010 that the issues raised in the Warning Letter had been addressed by Baxter, and we do not expect these issues to impact the development of the HyQvia product candidate.
We rely on third parties to prepare, fill, finish and package our products and product candidates, and if such third parties should fail to perform, our commercialization and development efforts for our products and product candidates could be delayed or stopped.
We rely on third parties to store and ship bulk rHuPH20 on our behalf and to also prepare, fill, finish and package our products and product candidates prior to their distribution. If we are unable to locate third parties to perform these functions on terms that are acceptable to us, or if the third parties we identify fail to perform their obligations, the progress of clinical trials could be delayed or even suspended and the commercialization of approved product candidates could be delayed or prevented. For example,Hylenex recombinant was voluntarily recalled in May 2010 because a portion of theHylenex recombinant manufactured by Baxter was not in compliance with the requirements of the underlyingHylenex recombinant agreements. During the second quarter of 2011, we submitted the data that the FDA had requested to support the reintroduction ofHylenex recombinant. The FDA approved the submitted data and granted the reintroduction ofHylenex recombinant, and we reintroducedHylenex recombinant to the market in December 2011. In June 2011, we entered into a commercial manufacturing and supply agreement with Baxter, under which Baxter will fill, finish and packageHylenex recombinant product for us. Under our commercial manufacturing and supply agreement with Baxter, Baxter has agreed to fill and finishHylenex recombinant product for us for a limited period of time. The term of the commercial manufacturing and supply agreement with Baxter expires on December 31, 2015, subject to further extensions in accordance with the terms and conditions of the agreement. In June 2011, we entered into a services agreement with a third party manufacturer for the technology transfer and manufacture ofHylenex recombinant. If we are unable to receive regulatory approval for the third party manufacturer prior to the expiration of the commercial manufacturing and supply agreement with Baxter or if the new manufacturer encounters difficulties in the manufacture, fill, finish or packaging ofHylenex recombinant, our business and financial condition could be adversely effected.
If we are unable to sufficiently develop our sales, marketing and distribution capabilities or enter into successful agreements with third parties to perform these functions, we will not be able to fully commercialize our products.
We may not be successful in marketing and promoting our approved product,Hylenex recombinant or any other products we develop or acquire in the future. Our sales, marketing and distribution capabilities are very limited. In order to commercialize any products successfully, we must internally develop substantial sales, marketing and distribution capabilities or establish collaborations or other arrangements with third parties to perform these services. We do not have extensive experience in these areas, and we may not be able to establish adequate in-house sales, marketing and distribution capabilities or engage and effectively manage relationships with third parties to perform any or all of such services. To the extent that we enter into co-promotion or other licensing arrangements, our product revenues are likely to be lower than if we directly marketed and sold our products, and any revenues we receive will depend upon the efforts of third parties, whose efforts may not meet our expectations or be successful. These third parties would be largely responsible for the speed and scope of sales and marketing efforts, and may not dedicate the resources necessary to maximize product opportunities. Our ability to cause these third parties to increase the speed and scope of their efforts may also be limited. In addition, sales and marketing efforts could be negatively impacted by the delay or failure to obtain additional supportive clinical trial data for our products. In some cases, third party collaborators are responsible for conducting these additional clinical trials, and our ability to increase the efforts and resources allocated to these trials may be limited. For example, in January 2011, we and Baxter mutually agreed to terminate theHylenex Collaboration and the associated agreements.
S-19
Table of Contents
If we or our collaborators fail to comply with regulatory requirements applicable to promotion, sale and manufacturing of approved products, regulatory agencies may take action against us or them, which could significantly harm our business.
Any approved products, along with the manufacturing processes, post-approval clinical data, labeling, advertising and promotional activities for these products, are subject to continual requirements and review by the FDA, state and foreign regulatory bodies. Regulatory authorities subject a marketed product, its manufacturer and the manufacturing facilities to continual review and periodic inspections. We, our collaborators and our respective contractors, suppliers and vendors, will be subject to ongoing regulatory requirements, including complying with regulations and laws regarding advertising, promotion and sales of drug products, required submissions of safety and other post-market information and reports, registration requirements, cGMP regulations (including requirements relating to quality control and quality assurance, as well as the corresponding maintenance of records and documentation), and the requirements regarding the distribution of samples to physicians and recordkeeping requirements. Regulatory agencies may change existing requirements or adopt new requirements or policies. We, our collaborators and our respective contractors, suppliers and vendors, may be slow to adapt or may not be able to adapt to these changes or new requirements.
In particular, regulatory requirements applicable to pharmaceutical products make the substitution of suppliers and manufacturers costly and time consuming. We have minimal internal manufacturing capabilities and are, and expect to be in the future, entirely dependent on contract manufacturers and suppliers for the manufacture of our products and for their active and other ingredients. The disqualification of these manufacturers and suppliers through their failure to comply with regulatory requirements could negatively impact our business because the delays and costs in obtaining and qualifying alternate suppliers (if such alternative suppliers are available, which we cannot assure) could delay clinical trials or otherwise inhibit our ability to bring approved products to market, which would have a material adverse effect on our business and financial condition. Likewise, if we, our collaborators and our respective contractors, suppliers and vendors involved in sales and promotion of our products do not comply with applicable laws and regulations, for example off-label or false or misleading promotion, this could materially harm our business and financial condition.
Failure to comply with regulatory requirements, may result in any of the following:
| • | restrictions on our products or manufacturing processes; |
| • | warning letters; |
| • | withdrawal of the products from the market; |
| • | voluntary or mandatory recall; |
| • | fines; |
| • | suspension or withdrawal of regulatory approvals; |
| • | suspension or termination of any of our ongoing clinical trials; |
| • | refusal to permit the import or export of our products; |
| • | refusal to approve pending applications or supplements to approved applications that we submit; |
| • | product seizure; |
| • | injunctions; or |
| • | the imposition of civil or criminal penalties. |
We may wish to raise additional capital in the next twelve months and there can be no assurance that we will be able to obtain such funds.
During the next twelve months, we may wish to raise additional capital to continue the development of our product candidates or for other current corporate purposes. Our current cash reserves and expected revenues
S-20
Table of Contents
during the next few years may not be sufficient for us to continue the development of our proprietary product candidates, to fund general operations and conduct our business at the level desired. In addition, if we engage in acquisitions of companies, products or technologies in order to execute our business strategy, we may need to raise additional capital. We may raise additional capital in the future through one or more financing vehicles that may be available to us including (i) the public or private issuance of securities; (ii) new collaborative agreements; and/or (iii) expansions or revisions to existing collaborative relationships.
In view of our stage of development, business prospects, the nature of our capital structure and general market conditions, if we are required to raise additional capital in the future, the additional financing may not be available on favorable terms, or at all. If additional capital is not available on favorable terms when needed, we will be required to raise capital on adverse terms or significantly reduce operating expenses through the restructuring of our operations. If we raise additional capital, a substantial number of additional shares may be issued, and these shares will dilute the ownership interest of our current investors.
We currently have significant debt and failure by us to fulfill our obligations under the applicable loan agreements may cause the repayment obligations to accelerate.
On December 27, 2013 we entered into an Amended and Restated Loan and Security Agreement (the “Loan Agreement”) with Oxford Finance LLC, a Delaware limited liability company, and Silicon Valley Bank, a California corporation, amending and restating in its entirety the Loan and Security Agreement dated as of December 28, 2012 (the “Original Loan Agreement”). The Original Loan Agreement provided for a $30 million secured single-draw term loan facility with a maturity date of January 1, 2017. The original term loan was fully drawn at close. The Loan Agreement extends the original $30 million term loans and provides for an additional $20 million in new term loans, bringing the total term loan balance to $50 million. The amended and restated term loan facility matures on January 1, 2018. The amended and restated term loan facility is secured by substantially all of the assets of the Company and Halozyme, Inc., except that the collateral does not include any equity interests in Halozyme, Inc., any intellectual property (including all licensing, collaboration and similar agreements relating thereto), and certain other excluded assets. The Loan Agreement contains customary representations, warranties and covenants by us, as well as customary events of default and our indemnification obligations. One of the events of default is a material adverse change which is defined as a material adverse change in our business, operations or condition (financial or otherwise); a material impairment of the prospect of repayment of any portion of the loan; or a material impairment in the perfection or priority of lender’s lien in the collateral or in the value of such collateral. If we are unable to fulfill our obligations to the lenders under the applicable loan agreements, this could create a material default such that our obligation to repay the loan is accelerated which could harm our financial condition.
If proprietary or collaboration product candidates are approved for marketing but do not gain market acceptance, our business may suffer and we may not be able to fund future operations.
Assuming that our proprietary or collaboration product candidates obtain the necessary regulatory approvals for commercial sale, a number of factors may affect the market acceptance of these existing product candidates or any other products which are developed or acquired in the future, including, among others:
| • | the price of products relative to other therapies for the same or similar treatments; |
| • | the perception by patients, physicians and other members of the health care community of the effectiveness and safety of these products for their prescribed treatments relative to other therapies for the same or similar treatments; |
| • | our ability to fund our sales and marketing efforts and the ability and willingness of our collaborators to fund sales and marketing efforts; |
| • | the degree to which the use of these products is restricted by the approved product label; |
| • | the effectiveness of our sales and marketing efforts and the effectiveness of the sales and marketing efforts of our collaborators; |
S-21
Table of Contents
| • | the introduction of generic competitors; and |
| • | the extent to which reimbursement for our products and related treatments will be available from third party payors including government insurance programs (Medicare and Medicaid) and private insurers. |
If these products do not gain market acceptance, we may not be able to fund future operations, including the development or acquisition of new product candidates and/or our sales and marketing efforts for our approved products, which would cause our business to suffer.
In addition, our proprietary and collaboration product candidates will be restricted to the labels approved by FDA and applicable regulatory bodies, and these restrictions may limit the marketing and promotion of the ultimate products. If the approved labels are restrictive, the sales and marketing efforts for these products may be negatively affected.
Developing and marketing pharmaceutical products for human use involves significant product liability risks for which we currently have limited insurance coverage.
The testing, marketing and sale of pharmaceutical products involves the risk of product liability claims by consumers and other third parties. Although we maintain product liability insurance coverage, product liability claims can be high in the pharmaceutical industry, and our insurance may not sufficiently cover our actual liabilities. If product liability claims were to be made against us, it is possible that the liabilities may exceed the limits of our insurance policy, or our insurance carriers may deny, or attempt to deny, coverage in certain instances. If a lawsuit against us is successful, then the lack or insufficiency of insurance coverage could materially and adversely affect our business and financial condition. Furthermore, various distributors of pharmaceutical products require minimum product liability insurance coverage before purchase or acceptance of products for distribution. Failure to satisfy these insurance requirements could impede our ability to achieve broad distribution of our proposed products, and higher insurance requirements could impose additional costs on us. In addition, since many of our collaboration product candidates include the pharmaceutical products of a third party, we run the risk that problems with the third party pharmaceutical product will give rise to liability claims against us.
Our inability to attract, hire and retain key management and scientific personnel could negatively affect our business.
Our success depends on the performance of key management and scientific employees with relevant experience. We depend substantially on our ability to hire, train, motivate and retain high quality personnel, especially our scientists and management team. Particularly in view of the small number of employees on our staff to cover our numerous programs and key functions, if we are unable to retain existing personnel or identify or hire additional personnel, we may not be able to research, develop, commercialize or market our products and product candidates as expected or on a timely basis and we may not be able to adequately support current and future alliances with strategic collaborators.
Furthermore, if we were to lose key management personnel, we would likely lose some portion of our institutional knowledge and technical know-how, potentially causing a substantial delay in one or more of our development programs until adequate replacement personnel could be hired and trained. We currently have a severance policy applicable to all employees and a change in control policy applicable to senior executives. We have not adopted any other policies or entered into any other agreements specifically designed to motivate officers or other employees to remain with us.
We do not have key man life insurance policies on the lives of any of our employees.
Our operations might be interrupted by the occurrence of a natural disaster or other catastrophic event.
Our operations, including laboratories, offices and other research facilities, are located in three buildings in San Diego, California. We depend on our facilities and on our collaborators, contractors and vendors for the
S-22
Table of Contents
continued operation of our business. Natural disasters or other catastrophic events, interruptions in the supply of natural resources, political and governmental changes, wildfires and other fires, floods, explosions, actions of animal rights activists, earthquakes and civil unrest could disrupt our operations or those of our collaborators, contractors and vendors. Even though we believe we carry commercially reasonable business interruption and liability insurance, and our contractors may carry liability insurance that protect us in certain events, we may suffer losses as a result of business interruptions that exceed the coverage available under our and our contractors’ insurance policies or for which we or our contractors do not have coverage. Any natural disaster or catastrophic event could have a significant negative impact on our operations and financial results. Moreover, any such event could delay our research and development programs.
If we or our collaborators do not achieve projected development, clinical or regulatory goals in the timeframes we publicly announce or otherwise expect, the commercialization of our products and the development of our product candidates may be delayed and, as a result, our stock price may decline, and we may face lawsuits relating to such declines.
From time to time, we or our collaborators may publicly articulate the estimated timing for the accomplishment of certain scientific, clinical, regulatory and other product development goals. The accomplishment of any goal is typically based on numerous assumptions, and the achievement of a particular goal may be delayed for any number of reasons both within and outside of our control. If scientific, regulatory, strategic or other factors cause us to not meet a goal, regardless of whether that goal has been publicly articulated or not, our stock price may decline rapidly. For example, the announcement of the CRL received for HyQvia caused a rapid decline in our stock price. Stock price declines may also trigger direct or derivative shareholder lawsuits. As with any litigation proceeding, the eventual outcome of any legal action is difficult to predict. If any such lawsuits occur, we will incur expenses in connection with the defense of these lawsuits, and we may have to pay substantial damages or settlement costs in connection with any resolution thereof. Although we have insurance coverage against which we may claim recovery against some of these expenses and costs, the amount of coverage may not be adequate to cover the full amount or certain expenses and costs may be outside the scope of the policies we maintain. In the event of an adverse outcome or outcomes, our business could be materially harmed from depletion of cash resources, negative impact on our reputation, or restrictions or changes to our governance or other processes that may result from any final disposition of the lawsuit. Moreover, responding to and defending pending litigation significantly diverts management’s attention from our operations.
In addition, the consistent failure to meet publicly announced milestones may erode the credibility of our management team with respect to future milestone estimates.
Future acquisitions could disrupt our business and harm our financial condition.
In order to augment our product pipeline or otherwise strengthen our business, we may decide to acquire additional businesses, products and technologies. As we have limited experience in evaluating and completing acquisitions, our ability as an organization to make such acquisitions is unproven. Acquisitions could require significant capital infusions and could involve many risks, including, but not limited to, the following:
| • | we may have to issue convertible debt or equity securities to complete an acquisition, which would dilute our stockholders and could adversely affect the market price of our common stock; |
| • | an acquisition may negatively impact our results of operations because it may require us to amortize or write down amounts related to goodwill and other intangible assets, or incur or assume substantial debt or liabilities, or it may cause adverse tax consequences, substantial depreciation or deferred compensation charges; |
| • | we may encounter difficulties in assimilating and integrating the business, products, technologies, personnel or operations of companies that we acquire; |
| • | certain acquisitions may impact our relationship with existing or potential collaborators who are competitive with the acquired business, products or technologies; |
S-23
Table of Contents
| • | acquisitions may require significant capital infusions and the acquired businesses, products or technologies may not generate sufficient value to justify acquisition costs; |
| • | we may take on liabilities from the acquired company such as debt, legal liabilities or business risk which could be significant; |
| • | an acquisition may disrupt our ongoing business, divert resources, increase our expenses and distract our management; |
| • | acquisitions may involve the entry into a geographic or business market in which we have little or no prior experience; and |
| • | key personnel of an acquired company may decide not to work for us. |
If any of these risks occurred, it could adversely affect our business, financial condition and operating results. There is no assurance that we will be able to identify or consummate any future acquisitions on acceptable terms, or at all. If we do pursue any acquisitions, it is possible that we may not realize the anticipated benefits from such acquisitions or that the market will not view such acquisitions positively.
Security breaches may disrupt our operations and harm our operating results.
The wrongful use, theft, deliberate sabotage or any other type of security breach with respect to any of our information technology storage and access systems could result in disclosure or dissemination of our proprietary and confidential information that is electronically stored, including research or clinical data, resulting in a material adverse impact on our business, operating results and financial condition. Our security and data recovery measures may not be adequate to protect against computer viruses, break-ins, and similar disruptions from unauthorized tampering with our electronic storage systems. Furthermore, any physical break-in or trespass of our facilities could result in the misappropriation, theft, sabotage or any other type of security breach with respect to our proprietary and confidential information, including research or clinical data or damage to our research and development equipment and assets. Such adverse effects could be material and irrevocable to our business, operating results and financial condition.
Risks Related to Our Industry
Our products must receive regulatory approval before they can be sold, and compliance with the extensive government regulations is expensive and time consuming and may result in the delay or cancellation of product sales, introductions or modifications.
Extensive industry regulation has had, and will continue to have, a significant impact on our business. All pharmaceutical companies, including ours, are subject to extensive, complex, costly and evolving regulation by the health regulatory agencies including the FDA (and with respect to controlled drug substances, the U.S. Drug Enforcement Administration (“DEA”)) and equivalent foreign regulatory agencies and state and local/regional government agencies. The Federal Food, Drug and Cosmetic Act, the Controlled Substances Act and other domestic and foreign statutes and regulations govern or influence the testing, manufacturing, packaging, labeling, storing, recordkeeping, safety, approval, advertising, promotion, sale and distribution of our products. We are dependent on receiving FDA and other governmental approvals prior to manufacturing, marketing and shipping our products. Consequently, there is always a risk that the FDA or other applicable governmental authorities will not approve our products or may impose onerous, costly and time-consuming requirements such as additional clinical or animal testing. Regulatory authorities may require that we change our studies or conduct additional studies, which may significantly delay or make continued pursuit of approval commercially unattractive; for example, based on FDA feedback, we recently changed the time point for assessment of the primary endpoint of non-inferiority of A1C from four months to six months in our CONSISTENT 1 trial forHylenex recombinant for use in CSII. We are currently in dialog with the FDA regarding the path for a labeling update to include key efficacy and safety data prior to initiatingHylenex recombinant for use in CSII. There can be no assurance that we will be able to gain clarity as to the FDA’s requirements or that the requirements may be satisfied by us in a
S-24
Table of Contents
commercially feasible way. The FDA or other foreign regulatory agency may, at any time, halt our and our collaborators’ development and commercialization activities due to safety concerns. In addition, even if our products are approved, regulatory agencies may also take post-approval action limiting or revoking our ability to sell our products. Any of these regulatory actions may adversely affect the economic benefit we may derive from our products and therefore harm our financial condition.
Under certain of these regulations, we and our contract suppliers and manufacturers are subject to periodic inspection of our or their respective facilities, procedures and operations and/or the testing of products by the FDA, the DEA and other authorities, which conduct periodic inspections to confirm that we and our contract suppliers and manufacturers are in compliance with all applicable regulations. The FDA also conducts pre-approval and post-approval reviews and plant inspections to determine whether our systems, or our contract suppliers’ and manufacturers’ processes, are in compliance with cGMP and other FDA regulations. If we, or our contract supplier, fail these inspections, we may not be able to commercialize our product in a timely manner without incurring significant additional costs, or at all.
In addition, the FDA imposes a number of complex regulatory requirements on entities that advertise and promote pharmaceuticals including, but not limited to, standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities, and promotional activities involving the internet.
We may be subject, directly or indirectly, to various broad federal and state healthcare laws. If we are unable to comply, or have not fully complied, with such laws, we could face civil, criminal and administrative penalties, damages, monetary fines, disgorgement, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings and curtailment or restructuring of our operations, any of which could adversely affect our ability to operate.
Our business operations and activities may be directly, or indirectly, subject to various broad federal and state healthcare laws, including without limitation, anti-kickback laws, false claims laws, civil monetary penalty laws, data privacy and security laws, tracing and tracking laws, as well as transparency laws regarding payments or other items of value provided to healthcare providers. These laws may restrict or prohibit a wide range of business activities, including, but not limited to, research, manufacturing, distribution, pricing, discounting, marketing and promotion and other business arrangements. These laws may impact, among other things, our current activities with principal investigators and research subjects, as well as sales, marketing and education programs. Many states have similar healthcare fraud and abuse laws, some of which may be broader in scope and may not be limited to items or services for which payment is made by a government health care program.
Efforts to ensure that our business arrangements will comply with applicable healthcare laws may involve substantial costs. While we have adopted a healthcare corporate compliance program, it is possible that governmental and enforcement authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law interpreting applicable fraud and abuse or other healthcare laws. If our operations or activities are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to, without limitation, civil, criminal and administrative penalties, damages, monetary fines, disgorgement, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings and curtailment or restructuring of our operations, any of which could adversely affect our ability to operate.
In addition, any sales of products outside the United States will also likely subject us to foreign equivalents of the healthcare laws mentioned above, among other foreign laws.
S-25
Table of Contents
We may be required to initiate or defend against legal proceedings related to intellectual property rights, which may result in substantial expense, delay and/or cessation of the development and commercialization of our products.
We primarily rely on patents to protect our intellectual property rights. The strength of this protection, however, is uncertain. For example, it is not certain that:
| • | we will be able to obtain patent protection for our products and technologies; |
| • | the scope of any of our issued patents will be sufficient to provide commercially significant exclusivity for our products and technologies; |
| • | others will not independently develop similar or alternative technologies or duplicate our technologies and obtain patent protection before we do; and |
| • | any of our issued patents, or patent pending applications that result in issued patents, will be held valid, enforceable and infringed in the event the patents are asserted against others. |
We currently own or license several patents and also have pending patent applications applicable to rHuPH20 and other proprietary materials. There can be no assurance that our existing patents, or any patents issued to us as a result of our pending patent applications, will provide a basis for commercially viable products, will provide us with any competitive advantages, or will not face third party challenges or be the subject of further proceedings limiting their scope or enforceability. A European patent, EP1603541, claiming rHuPH20 was granted to us on November 11, 2009 with claims to the human PH20 glycoprotein, PEGylated variants, a method of producing the glycoprotein produced by recombinant methods, and pharmaceutical compositions with other agents, including antibodies, insulins, cytokines, a chemotherapeutic agent and additional therapeutic classes. A third party opposed this patent in the European Patent Office in 2010; however, the opposition has been resolved with claims maintained in amended form. Any weaknesses or limitations in our patent portfolio could have a material adverse effect on our business and financial condition. In addition, if any of our pending patent applications do not result in issued patents, or result in issued patents with narrow or limited claims, this could result in us having no or limited protection against generic or biosimilar competition against our product candidates which would have a material adverse effect on our business and financial condition.
We may become involved in interference proceedings in the U.S. Patent and Trademark Office, or other proceedings in other jurisdictions, to determine the priority, validity or enforceability of our patents. In addition, costly litigation could be necessary to protect our patent position.
We also rely on trademarks to protect the names of our products (e.g.Hylenex recombinant). We may not be able to obtain trademark protection for any proposed product names we select. In addition, product names for pharmaceutical products must be approved by health regulatory authorities such as the FDA in addition to meeting the legal standards required for trademark protection and product names we propose may not be timely approved by regulatory agencies which may delay product launch. In addition, our trademarks may be challenged by others. If we enforce our trademarks against third parties, such enforcement proceedings may be expensive.
We also rely on trade secrets, unpatented proprietary know-how and continuing technological innovation that we seek to protect with confidentiality agreements with employees, consultants and others with whom we discuss our business. Disputes may arise concerning the ownership of intellectual property or the applicability or enforceability of these agreements, and we might not be able to resolve these disputes in our favor.
In addition to protecting our own intellectual property rights, third parties may assert patent, trademark or copyright infringement or other intellectual property claims against us. If we become involved in any intellectual property litigation, we may be required to pay substantial damages, including but not limited to treble damages, attorneys’ fees and costs, for past infringement if it is ultimately determined that our products infringe a third party’s intellectual property rights. Even if infringement claims against us are without merit, defending a lawsuit takes significant time, may be expensive and may divert management’s attention from other business concerns. Further, we may be stopped from developing, manufacturing or selling our products until we obtain a license from the owner of the relevant technology or other intellectual property rights. If such a license is available at all, it may require us to pay substantial royalties or other fees.
S-26
Table of Contents
Patent protection for protein-based therapeutic products and other biotechnology inventions is subject to a great deal of uncertainty, and if patent laws or the interpretation of patent laws changes, our competitors may be able to develop and commercialize products based on our discoveries.
Patent protection for protein-based therapeutic products is highly uncertain and involves complex legal and factual questions. In recent years, there have been significant changes in patent law, including the legal standards that govern the scope of protein and biotechnology patents. Standards for patentability of full-length and partial genes, and their corresponding proteins, are changing. Recent court decisions have made it more difficult to obtain patents, by making it more difficult to satisfy the patentable subject matter requirement and the requirement of non-obviousness, have decreased the availability of injunctions against infringers, and have increased the likelihood of challenging the validity of a patent through a declaratory judgment action. Taken together, these decisions could make it more difficult and costly for us to obtain, license and enforce our patents. In addition, the Leahy-Smith America Invents Act (HR 1249) was signed into law in September 2011, which among other changes to the U.S. patent laws, changes patent priority from “first to invent” to “first to file,” implements a post-grant opposition system for patents and provides for a prior user defense to infringement. These judicial and legislative changes have introduced significant uncertainty in the patent law landscape and may potentially negatively impact our ability to procure, maintain and enforce patents to provide exclusivity for our products.
There also have been, and continue to be, policy discussions concerning the scope of patent protection awarded to biotechnology inventions. Social and political opposition to biotechnology patents may lead to narrower patent protection within the biotechnology industry. Social and political opposition to patents on genes and proteins and recent court decisions concerning patentability of isolated genes may lead to narrower patent protection, or narrower claim interpretation, for isolated genes, their corresponding proteins and inventions related to their use, formulation and manufacture. Patent protection relating to biotechnology products is also subject to a great deal of uncertainty outside the United States, and patent laws are evolving and undergoing revision in many countries. Changes in, or different interpretations of, patent laws worldwide may result in our inability to obtain or enforce patents, and may allow others to use our discoveries to develop and commercialize competitive products, which would impair our business.
If third party reimbursement and customer contracts are not available, our products may not be accepted in the market.
Our ability to earn sufficient returns on our products will depend in part on the extent to which reimbursement for our products and related treatments will be available from government health administration authorities, private health insurers, managed care organizations and other healthcare providers.
Third-party payors are increasingly attempting to limit both the coverage and the level of reimbursement of new drug products to contain costs. Consequently, significant uncertainty exists as to the reimbursement status of newly approved healthcare products. Third party payors may not establish adequate levels of reimbursement for the products that we commercialize, which could limit their market acceptance and result in a material adverse effect on our financial condition.
Customer contracts, such as with group purchasing organizations and hospital formularies, will often not offer contract or formulary status without either the lowest price or substantial proven clinical differentiation. If our products are compared to animal-derived hyaluronidases by these entities, it is possible that neither of these conditions will be met, which could limit market acceptance and result in a material adverse effect on our financial condition.
The rising cost of healthcare and related pharmaceutical product pricing has led to cost containment pressures that could cause us to sell our products at lower prices, resulting in less revenue to us.
Any of the proprietary or collaboration products that have been, or in the future are, approved by the FDA may be purchased or reimbursed by state and federal government authorities, private health insurers and other
S-27
Table of Contents
organizations, such as health maintenance organizations and managed care organizations. Such third party payors increasingly challenge pharmaceutical product pricing. The trend toward managed healthcare in the United States, the growth of such organizations, and various legislative proposals and enactments to reform healthcare and government insurance programs, including the Medicare Prescription Drug Modernization Act of 2003, could significantly influence the manner in which pharmaceutical products are prescribed and purchased, resulting in lower prices and/or a reduction in demand. Such cost containment measures and healthcare reforms could adversely affect our ability to sell our products.
In March 2010, the United States adopted the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act (the “Healthcare Reform Act”). This law substantially changes the way healthcare is financed by both governmental and private insurers, and significantly impacts the pharmaceutical industry. The Healthcare Reform Act contains a number of provisions that are expected to impact our business and operations, in some cases in ways we cannot currently predict. Changes that may affect our business include those governing enrollment in federal healthcare programs, reimbursement changes, fraud and abuse and enforcement. These changes will impact existing government healthcare programs and will result in the development of new programs, including Medicare payment for performance initiatives and improvements to the physician quality reporting system and feedback program.
Additional provisions of the Healthcare Reform Act, some of which became effective in 2011, may negatively affect our revenues in the future. For example, the Healthcare Reform Act imposes a non-deductible excise tax on pharmaceutical manufacturers or importers that sell branded prescription drugs to U.S. government programs that we believe will impact our revenues from our products. In addition, as part of the Healthcare Reform Act’s provisions closing a funding gap that currently exists in the Medicare Part D prescription drug program, we will also be required to provide a 50% discount on branded prescription drugs dispensed to beneficiaries under this prescription drug program. We expect that the Healthcare Reform Act and other healthcare reform measures that may be adopted in the future could have a material adverse effect on our industry generally and on our ability to maintain or increase our product sales or successfully commercialize our product candidates or could limit or eliminate our future spending on development projects.
Furthermore, individual states have become increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access, importation from other countries and bulk purchasing. Legally mandated price controls on payment amounts by third party payors or other restrictions could negatively and materially impact our revenues and financial condition. We anticipate that we will encounter similar regulatory and legislative issues in most other countries outside the United States.
We face intense competition and rapid technological change that could result in the development of products by others that are superior to our proprietary and collaboration products under development.
Our proprietary and collaboration products have numerous competitors in the United States and abroad including, among others, major pharmaceutical and specialized biotechnology firms, universities and other research institutions that have developed competing products. The competitors forHylenex recombinant include, but are not limited to Bausch & Lomb Inc. and Amphastar Pharmaceuticals, Inc. For our more physiologic insulin product candidates, such competitors may include Biodel Inc., Eli Lily, Sanofi Aventis, Novo Nordisk Inc. and Mannkind Corporation. These competitors may develop technologies and products that are more effective, safer, or less costly than our current or future proprietary and collaboration product candidates or that could render our technologies and product candidates obsolete or noncompetitive. Many of these competitors have substantially more resources and product development, manufacturing and marketing experience and capabilities than we do. In addition, many of our competitors have significantly greater experience than we do in undertaking preclinical testing and clinical trials of pharmaceutical product candidates and obtaining FDA and other regulatory approvals of products and therapies for use in healthcare.
S-28
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus supplement, the accompanying prospectus, the documents we have filed with the SEC that are incorporated herein by reference and any free writing prospectus that we have authorized for use in connection with this offering contain certain “forward-looking statements” within the meaning of the “safe harbor” provisions of Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and Section 27A of the Securities Act of 1933, as amended (the “Securities Act”). All statements, other than statements of historical fact, included or incorporated herein regarding our future product development and regulatory events and goals, product collaborations, our business intentions and financial estimates and results are forward-looking statements. Words such as “expect,” “anticipate,” “intend,” “plan,” “believe,” “seek,” “estimate,” “think,” “may,” “could,” “will,” “would,” “should,” “continue,” “potential,” “likely,” “opportunity” and similar expressions or variations of such words are intended to identify forward-looking statements, but are not the exclusive means of identifying forward-looking statements in this report. Additionally, statements concerning future matters such as the development or regulatory approval of new products, enhancements of existing products or technologies, timing and success of the launch of new products by us or by our collaborators, third party performance under key collaboration agreements, revenue and expense levels and other statements regarding matters that are not historical are forward-looking statements. Such statements are based on currently available operating, financial and competitive information, are not guarantees of future performance, and are subject to various risks, uncertainties and assumptions that could cause actual results to differ materially from those anticipated or implied in our forward-looking statements due to a number of factors including, but not limited to, those set forth above under the section entitled “Risk Factors” contained in this prospectus supplement.
Many of the important factors that will determine these results are beyond our ability to control or predict. You are cautioned not to put undue reliance on any forward-looking statements, which speak only as of the date such forward-looking statements are made. You should carefully read this prospectus supplement, the accompanying prospectus, and any free writing prospectus that we have authorized for use in connection with this offering, together with the information incorporated herein by reference as described under the heading “Where You Can Find Additional Information,” completely and with the understanding that our actual future results may be materially different from what we expect. Except as otherwise required by law, we do not assume any obligation to update or release any revisions to these forward-looking statements to reflect events or circumstances after the date of this prospectus supplement or to reflect the occurrence of unanticipated events.
S-29
Table of Contents
We estimate that the net proceeds to us from the sale of our common stock offered hereby will be approximately $93.7 million, after deducting underwriting discounts and commissions and estimated offering expenses payable by us (or approximately $107.8 million if the underwriters’ option to purchase additional shares is exercised in full).
We intend to use the net proceeds from this offering to fund research and development of proprietary programs, including the potential acceleration of the PEGPH20 program, and for other general corporate purposes.
Our common stock is listed on The NASDAQ Global Select Market under the symbol “HALO.” The last reported sale price for our common stock on February 4, 2014 was $13.78 per share. The table below sets forth high and low sale prices for our common stock during the periods indicated.
| 2014 | 2013 | 2012 | ||||||||||||||||||||||
| High | Low | High | Low | High | Low | |||||||||||||||||||
First Quarter (through February 4, 2014) | $ | 18.18 | $ | 13.65 | $ | 8.59 | $ | 5.14 | $ | 13.50 | $ | 9.00 | ||||||||||||
Second Quarter | — | — | $ | 8.49 | $ | 5.03 | $ | 13.05 | $ | 7.17 | ||||||||||||||
Third Quarter | — | — | $ | 12.15 | $ | 6.51 | $ | 9.92 | $ | 3.86 | ||||||||||||||
Fourth Quarter | — | — | $ | 16.36 | $ | 9.33 | $ | 7.63 | $ | 4.80 | ||||||||||||||
To date, we have paid no cash dividends to our stockholders, and we do not intend to pay cash dividends in the foreseeable future.
S-30
Table of Contents
If you purchase our common stock in this offering, your interest will be diluted to the extent of the difference between the offering price per share and the net tangible book value per share of our common stock after this offering. Our net tangible book value as of September 30, 2013 was approximately $(3.5) million, or $(0.03) per share. Net tangible book value per share is determined by dividing our total tangible assets, less total liabilities, by the number of shares of our common stock outstanding as of September 30, 2013. Dilution in net tangible book value per share represents the difference between the amount per share paid by purchasers of shares of common stock in this offering and the net tangible book value per share of our common stock immediately after this offering.
After giving effect to the sale by us of 7,692,307 shares of our common stock in this offering at the public offering price of $13.00 per share, after deducting underwriting discounts and commissions and estimated offering expenses payable by us, our as adjusted net tangible book value as of September 30, 2013 would have been approximately $90.2 million, or $0.74 per share. This would represent an immediate increase in net tangible book value of $0.77 per share to existing stockholders and an immediate dilution of $12.26 per share to investors purchasing our common stock in this offering at the public offering price. The following table illustrates this dilution on a per share basis:
Public offering price per share | $ | 13.00 | ||||||
Net tangible book value per share as of September 30, 2013 | $ | (0.03 | ) | |||||
Increase per share attributable to investors in this offering | 0.77 | |||||||
|
| |||||||
As adjusted net tangible book value per share after this offering | 0.74 | |||||||
|
| |||||||
Dilution per share to investors in this offering | $ | 12.26 | ||||||
|
|
If the underwriters exercise in full their option to purchase 1,153,846 additional shares of common stock at the public offering price of $13.00 per share, after deducting underwriting discounts and commissions and estimated offering expenses payable by us, the as adjusted net tangible book value after this offering would be $0.85 per share, representing an increase in net tangible book value of $0.88 per share to existing shareholders and immediate dilution in net tangible book value of $12.15 per share to investors participating in this offering at the public offering price.
The foregoing discussion and table are based on 113,986,739 shares of common stock outstanding as of September 30, 2013. This number of shares does not include 1,153,846 shares of common stock subject to the underwriters’ option to purchase additional shares and also excludes, as of September 30, 2013:
| • | 7,178,512 shares of common stock issuable upon the exercise of outstanding stock options, having a weighted average exercise price of $6.92 per share; |
| • | 746,636 shares of common stock issuable upon settlement of restricted stock units; and |
| • | an aggregate of up to 7,010,395 shares of common stock reserved for future issuance under our equity incentive plans. |
S-31
Table of Contents
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO NON-U.S. HOLDERS
The following summary describes the material U.S. federal income and estate tax consequences of the acquisition, ownership and disposition of our common stock acquired in this offering by a Non-U.S. Holder (as defined below). This discussion does not address all aspects of U.S. federal income and estate taxes and does not deal with foreign, state and local consequences that may be relevant to Non-U.S. Holders in light of their particular circumstances. Special rules may apply to certain Non-U.S. Holders that are subject to special treatment under the Internal Revenue Code of 1986, as amended (the “Code”), such as financial institutions, insurance companies, tax-exempt organizations, broker-dealers and traders in securities, U.S. expatriates, “controlled foreign corporations,” “passive foreign investment companies,” corporations that accumulate earnings to avoid U.S. federal income tax, persons that are subject to the Medicare Tax, persons that hold our common stock as part of a “straddle,” “hedge,” “conversion transaction,” “synthetic security” or integrated investment, partnerships and other pass-through entities, and investors in such pass-through entities. Such Non-U.S. Holders are urged to consult their own tax advisors to determine the U.S. federal, state, local and other tax consequences that may be relevant to them. Furthermore, the discussion below is based upon the provisions of the Code, and Treasury regulations, rulings and judicial decisions thereunder as of the date hereof, and such authorities may be repealed, revoked or modified, perhaps retroactively, so as to result in U.S. federal income and estate tax consequences different from those discussed below. This discussion assumes that the Non-U.S. Holder holds our common stock as a “capital asset” within the meaning of Code Section 1221.
The following discussion is for general information only and is not tax advice. Persons considering the purchase of our common stock should consult their own tax advisors concerning the U.S. federal income and estate tax consequences in light of their particular situations as well as any consequences arising under the laws of any other taxing jurisdiction, including any state, local or foreign tax consequences.
Except as otherwise described in the discussion of estate tax below, a “Non-U.S. Holder” is a beneficial holder of our common stock that is not a U.S. Holder or a partnership. A “U.S. Holder” means a beneficial holder of our common stock that is for U.S. federal income tax purposes:
| • | an individual who is a citizen or resident of the United States; |
| • | a corporation or other entity treated as a corporation for U.S. federal income tax purposes, created or organized in or under the laws of the United States or any political subdivision thereof; |
| • | an estate the income of which is subject to U.S. federal income taxation regardless of its source; or |
| • | a trust if it (i) is subject to the primary supervision of a court within the United States and one or more U.S. persons have the authority to control all substantial decisions of the trust or (ii) has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person. |
If a partnership (including any entity or arrangement treated as a partnership for U.S. federal income tax purposes) acquires our common stock, the tax treatment of a partner in the partnership will generally depend upon the status of the partner and the activities of the partnership. Persons who are partners of partnerships holding our common stock are urged to consult their tax advisors.
Distributions
Subject to the discussion below, distributions, if any, made to a Non-U.S. Holder of our common stock out of our current or accumulated earnings and profits generally will constitute dividends for U.S. tax purposes and will be subject to withholding tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. To obtain a reduced rate of withholding under a treaty, a Non-U.S. Holder generally will be required to provide us with a properly-executed IRS Form W-8BEN, or other appropriate form, certifying the Non-U.S. Holder’s entitlement to benefits under that treaty. Treasury regulations provide special rules to determine whether, for purposes of determining the applicability of a tax treaty, dividends paid to a Non-U.S. Holder that is an entity should be treated as paid to the entity or to those holding an interest in that entity. If a Non-U.S. Holder
S-32
Table of Contents
holds stock through a financial institution or other agent acting on the holder’s behalf, the holder will be required to provide appropriate documentation to such agent. The holder’s agent will then be required to provide certification to us or our paying agent, either directly or through other intermediaries.
We generally are not required to withhold tax on dividends paid to a Non-U.S. Holder that are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States if a properly-executed IRS Form W-8ECI, stating that the dividends are so connected (and are not exempt from net U.S. federal income tax under a treaty as described below), is filed with us. Effectively connected dividends will be subject to net U.S. federal income tax, generally in the same manner and at the regular rate as if the Non-U.S. Holder were a U.S. citizen or resident alien or a domestic corporation, as the case may be, unless a specific treaty exemption applies. If the Non-U.S. Holder is eligible for the benefits of a tax treaty between the United States and the holder’s country of residence, any effectively connected dividends would generally be subject to net U.S. federal income tax only if they are also attributable to a permanent establishment maintained by the holder in the United States. A corporate Non-U.S. Holder receiving effectively connected dividends may also be subject to an additional “branch profits tax”, which is imposed, under certain circumstances, at a rate of 30% (or such lower rate as may be specified by an applicable treaty) of the corporate Non-U.S. Holder’s effectively connected earnings and profits, subject to certain adjustments. If you are eligible for a reduced rate of withholding tax pursuant to a tax treaty, you may generally obtain a refund of any excess amounts currently withheld if you timely file an appropriate claim for refund with the IRS.
To the extent distributions on our common stock, if any, exceed our current and accumulated earnings and profits, they will constitute a return of capital and will first reduce your basis in our common stock, but not below zero, and then will be treated as gain from the sale of stock, subject to the tax treatment described below under the heading “Gain on Disposition of Common Stock.” Any such distribution will also be subject to the discussion below under the heading “Withholding and Information Reporting Requirements—FATCA.”
Gain on Disposition of Common Stock
A Non-U.S. Holder generally will not be subject to U.S. federal income tax with respect to gain realized on a sale or other disposition of our common stock unless (i) the gain is effectively connected with a trade or business of such holder in the United States, (ii) in the case of Non-U.S. Holders who are nonresident alien individuals, such individuals are present in the United States for 183 or more days in the taxable year of the disposition and certain other conditions are met, or (iii) we are or have been a “United States real property holding corporation” within the meaning of Code Section 897(c)(2) at any time within the shorter of the five-year period preceding such disposition or such holder’s holding period. In general, we would be a United States real property holding corporation if interests in U.S. real estate comprised at least half of our business assets. We believe that we are not, and do not anticipate becoming, a United States real property holding corporation. Even if we are treated as a United States real property holding corporation, gain realized by a Non-U.S. Holder on a disposition of our common stock will not be subject to U.S. federal income tax so long as (1) the Non-U.S. Holder owned directly, indirectly and constructively, no more than five percent of our common stock at all times within the shorter of (a) the five year period preceding the disposition or (b) the holder’s holding period and (2) our common stock is regularly traded on an established securities market. There can be no assurance that our common stock will continue to qualify as regularly traded on an established securities market.
If you are a Non-U.S. Holder described in (i) above, you will be required to pay tax on the net gain derived from the sale at generally applicable United States federal income tax rates, subject to an applicable income tax treaty providing otherwise, and corporate Non-U.S. Holders described in (i) above may be subject to the branch profits tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. If you are an individual Non-U.S. Holder described in (ii) above, you will be required to pay a flat 30% tax (or a reduced rate under an applicable income tax treaty) on the gain derived from the sale, which tax may be offset by U.S. source capital losses if you have timely filed tax returns with respect to such losses (even though you are not considered a resident of the United States).
S-33
Table of Contents
Information Reporting and Backup Withholding
Generally, we must report to the IRS the amount of dividends paid, the name and address of the recipient, and the amount, if any, of tax withheld. A similar report is sent to the holder. Pursuant to tax treaties or certain other agreements, the IRS may make its reports available to tax authorities in the recipient’s country of residence. Backup withholding will generally not apply to payments of dividends made by us or our paying agents to a Non-U.S. Holder if the holder has provided its federal taxpayer identification number, if any, or the required certification that it is not a U.S. person (which is generally provided by furnishing a properly-executed IRS Form W-8BEN), unless the payer otherwise has knowledge or reason to know that the payee is a U.S. person. The backup withholding rate is currently 28%. Backup withholding is generally not required on payments to corporations, whether domestic or foreign.
Under current U.S. federal income tax law, information reporting and backup withholding will apply to the proceeds of a disposition of our common stock effected by or through a U.S. office of a broker unless the disposing holder certifies as to its non-U.S. status or otherwise establishes an exemption. The certification procedures for claiming benefits under a tax treaty described in “—Distributions” above will satisfy the certification requirements to avoid backup withholding as well. Generally, U.S. information reporting and backup withholding will not apply to a payment of disposition proceeds where the transaction is effected outside the United States through a non-U.S. office of a non-U.S. broker. Backup withholding will apply to a payment of disposition proceeds if the broker has actual knowledge or reason to know that the holder is a U.S. person.
Backup withholding is not an additional tax. Rather, the tax liability of persons subject to backup withholding will be reduced by the amount of tax withheld. If withholding results in an overpayment of taxes, a refund may generally be obtained, provided that the required information is timely furnished to the IRS.
Withholding and Information Reporting Requirements—FATCA
Recently enacted legislation (commonly referred to as “FATCA”) will impose U.S. federal withholding tax of 30% on payments of dividends on, and gross proceeds from the sale or disposition of, our common stock if paid to a foreign entity unless (i) in the case of a foreign entity that is a “foreign financial institution” (as defined under FATCA), the foreign entity undertakes certain due diligence, reporting, withholding, and certification obligations, (ii) in the case of a foreign entity that is not a foreign financial institution, the foreign entity identifies certain of its U.S. investors, or (iii) the foreign entity is otherwise exempt under FATCA. Although this legislation is effective with respect to amounts paid after December 31, 2012, under applicable U.S. Treasury Regulations, withholding under FATCA will only apply (1) to payments of dividends on our common stock made after June 30, 2014, and (2) to payments of gross proceeds from a sale or other disposition of our common stock made after December 31, 2016. Under certain circumstances, a Non-U.S. Holder may be eligible for refunds or credits of such taxes.
Prospective investors should consult their own tax advisors regarding the possible impact of the FATCA rules on their investment in our common stock and on the entities through which they hold our common stock including, without limitation, the process and deadlines for meeting the applicable requirements to prevent the imposition of the 30% withholding tax under FATCA.
Federal Estate Tax
Common stock owned or treated as owned by an individual who is not a citizen or resident of the United States (as specifically defined for U.S. federal estate tax purposes) at the time of death is considered a U.S. situs asset includible in the individual’s gross estate for U.S. federal estate tax purposes and therefore may be subject to U.S. federal estate tax, unless an applicable estate tax treaty provides otherwise. Prospective investors are urged to consult their tax advisors regarding the U.S. federal estate tax considerations of acquiring, holding, and disposing of common stock. The test for whether an individual is a resident of the United States for federal estate tax purposes differs from the test used for U.S. federal income tax purposes. Some individuals, therefore, may be “Non-U.S. Holders” for U.S. federal income tax purposes, but not for U.S. federal estate tax purposes, and vice versa.
S-34
Table of Contents
THE PRECEDING DISCUSSION OF U.S. FEDERAL INCOME AND ESTATE TAX CONSIDERATIONS IS FOR GENERAL INFORMATION ONLY. IT IS NOT TAX ADVICE. EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS OWN TAX ADVISOR REGARDING THE TAX CONSEQUENCES OF PURCHASING, HOLDING AND DISPOSING OF OUR COMMON STOCK, INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAW.
S-35
Table of Contents
We are offering the shares of common stock described in this prospectus supplement through a number of underwriters. J.P. Morgan Securities LLC and Citigroup Global Markets Inc. are acting as jointbook-running managers of the offering and as representatives of the underwriters. We have entered into an underwriting agreement with the underwriters. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriters, and each underwriter has severally agreed to purchase, at the public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus supplement, the number of shares of common stock listed next to its name in the following table:
Name | Number of shares | |||
J.P. Morgan Securities LLC | 3,076,923 | |||
Citigroup Global Markets Inc. | 3,076,923 | |||
Piper Jaffray & Co. | 769,231 | |||
BMO Capital Markets Corp. | 769,230 | |||
|
| |||
Total | 7,692,307 | |||
|
| |||
The underwriters are committed to purchase all of the shares of common stock offered by us if they purchase any shares. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may also be increased or the offering may be terminated.
The underwriters propose to offer the shares of common stock directly to the public at the public offering price set forth on the cover page of this prospectus supplement and to certain dealers at that price less a concession not in excess of $0.468 per share. After the offering of the shares, the offering price and other selling terms may be changed by the underwriters. Sales of shares made outside of the United States may be made by affiliates of the underwriters.
The underwriters have an option to buy up to 1,153,846 additional shares of common stock from us to cover sales of shares by the underwriters which exceed the number of shares specified in the table above. The underwriters have 30 days from the date of this prospectus supplement to exercise this option. If any shares are purchased with this option, the underwriters will purchase shares in approximately the same proportion as shown in the table above. If any additional shares of common stock are purchased, the underwriters will offer the additional shares on the same terms as those on which the shares are being offered.
The underwriting fee is equal to the public offering price per share of common stock less the amount paid by the underwriters to us per share of common stock. The underwriting fee is $0.78 per share. The following table shows the per share and total underwriting discounts and commissions to be paid to the underwriters assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares.
| Without option exercise | With full option exercise | |||||||
Per Share | $ | 0.78 | $ | 0.78 | ||||
Total | $ | 5,999,999.46 | $ | 6,899,999.34 | ||||
We estimate that the total expenses of this offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding the underwriting discounts and commissions, will be approximately $315,000.
A prospectus in electronic format may be made available on the web sites maintained by one or more underwriters, or selling group members, if any, participating in the offering. The underwriters may agree to allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the representatives to the underwriters and selling group members that may make Internet distributions on the same basis as other allocations.
We have agreed that we will not (i) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise
S-36
Table of Contents
transfer or dispose of, directly or indirectly, or file with the SEC a registration statement under the Securities Act relating to, any shares of our common stock or securities convertible into or exercisable or exchangeable for any shares of our common stock, or publicly disclose the intention to make any offer, sale, pledge, disposition or filing, or (ii) enter into any swap or other arrangement that transfers, in whole or in part, any of the economic consequences of ownership of any shares of common stock or any such other securities, whether any such transaction described in clause (i) or (ii) above is to be settled by delivery of shares of common stock or such other securities, in cash or otherwise, without the prior written consent of J.P. Morgan Securities LLC and Citigroup Global Markets Inc. for a period of 90 days after the date of this prospectus supplement, other than the shares of our common stock to be sold hereunder and any shares of our common stock issued upon the exercise or vesting of awards granted under our stock-based compensation plans. Notwithstanding the foregoing, subject to certain exceptions, if (1) during the last 17 days of the 90-day restricted period, we issue an earnings release or material news or a material event relating to our company occurs; or (2) prior to the expiration of the 90-day restricted period, we announce that we will release earnings results during the 16-day period beginning on the last day of the 90-day period, the restrictions described above shall continue to apply until the expiration of the 18-day period beginning on the issuance of the earnings release or the occurrence of the material news or material event.
Our directors and executive officers have entered intolock-up agreements with the underwriters prior to the commencement of this offering pursuant to which each of these persons or entities, with limited exceptions, for a period of 90 days after the date of this prospectus supplement, may not, without the prior written consent of J.P. Morgan Securities LLC and Citigroup Global Markets Inc., (1) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any shares of our common stock or any securities convertible into or exercisable or exchangeable for our common stock (including, without limitation, common stock or such other securities which may be deemed to be beneficially owned by such directors, executive officers, and the significant stockholder in accordance with the rules and regulations of the SEC and securities which may be issued upon exercise of a stock option or warrant) or publicly disclose the intention to make any offer, sale, pledge or disposition, (2) enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of the common stock or such other securities, whether any such transaction described in clause (1) or (2) above is to be settled by delivery of common stock or such other securities, in cash or otherwise, or (3) make any demand for or exercise any right with respect to the registration of any shares of our common stock or any security convertible into or exercisable or exchangeable for our common stock.
Subject to certain conditions,the foregoing restrictions do not apply to:
| • | transfers of shares of our common stock (A) as a bona fide gift or gifts, (B) to any trust for the direct or indirect benefit of such person or the immediate family of such person or (C) by will or intestacy to legal representatives, heir or legatee of such person; |
| • | distributions of shares of common stock to members, limited partners or stockholders of such person; |
| • | transfers of shares of our common stock to such person’s affiliates or to any investment fund or other entity controlled or managed by such person; |
| • | transfers or distributions of shares of common stock acquired by such person in open market transactions after this offering; and |
| • | sales of underlying shares of our common stock to cover tax payments resulting from (i) vesting of any restricted stock awards or restricted stock units and (ii) exercise of options to purchase common stock that expire during the 90-day period referred to above. |
Our directors and executive officers may also enter into new Rule 10b5-1 trading plans as long as no trades of shares of common stock will occur under the trading plan during the restricted period and no filing or public announcement under the Exchange Act or otherwise is made in connection with entering into such a plan during the restricted period.
S-37
Table of Contents
Notwithstanding the foregoing, subject to certain exceptions, if (1) during the last 17 days of the 90-day restricted period, we issue an earnings release or material news or a material event relating to our company occurs; or (2) prior to the expiration of the 90-day restricted period, we announce that we will release earnings results during the 16-day period beginning on the last day of the 90-day period, the restrictions described above shall continue to apply until the expiration of the 18-day period beginning on the issuance of the earnings release or the occurrence of the material news or material event.
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act.
Our common stock is listed on The NASDAQ Global Select Market under the symbol “HALO.”
In connection with this offering, the underwriters may engage in stabilizing transactions, which involves making bids for, purchasing and selling shares of common stock in the open market for the purpose of preventing or retarding a decline in the market price of the common stock while this offering is in progress. These stabilizing transactions may include making short sales of the common stock, which involves the sale by the underwriters of a greater number of shares of common stock than they are required to purchase in this offering, and purchasing shares of common stock on the open market to cover positions created by short sales. Short sales may be “covered” shorts, which are short positions in an amount not greater than the underwriters’ option to purchase additional shares referred to above, or may be “naked” shorts, which are short positions in excess of that amount. The underwriters may close out any covered short position either by exercising their option to purchase additional shares, in whole or in part, or by purchasing shares in the open market. In making this determination, the underwriters will consider, among other things, the price of shares available for purchase in the open market compared to the price at which the underwriters may purchase shares through the option to purchase additional shares. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common stock in the open market that could adversely affect investors who purchase in this offering. To the extent that the underwriters create a naked short position, they will purchase shares in the open market to cover the position.
The underwriters have advised us that, pursuant to Regulation M of the Securities Act, they may also engage in other activities that stabilize, maintain or otherwise affect the price of the common stock, including the imposition of penalty bids. This means that if the representatives of the underwriters purchase common stock in the open market in stabilizing transactions or to cover short sales, the representatives can require the underwriters that sold those shares as part of this offering to repay the underwriting discount received by them.
These activities may have the effect of raising or maintaining the market price of the common stock or preventing or retarding a decline in the market price of the common stock, and, as a result, the price of the common stock may be higher than the price that otherwise might exist in the open market. If the underwriters commence these activities, they may discontinue them at any time. The underwriters may carry out these transactions on The NASDAQ Global Select Market, in theover-the-counter market or otherwise.
In addition, in connection with this offering certain of the underwriters (and selling group members) may engage in passive market making transactions in our common stock on The NASDAQ Global Select Market prior to the pricing and completion of this offering. Passive market making consists of displaying bids on The NASDAQ Global Select Market no higher than the bid prices of independent market makers and making purchases at prices no higher than these independent bids and effected in response to order flow. Net purchases by a passive market maker on each day are generally limited to a specified percentage of the passive market maker’s average daily trading volume in the common stock during a specified period and must be discontinued when such limit is reached. Passive market making may cause the price of our common stock to be higher than the price that otherwise would exist in the open market in the absence of these transactions. If passive market making is commenced, it may be discontinued at any time.
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus supplement in any jurisdiction where action for that purpose is required. The securities offered by this prospectus supplement may not be offered or sold, directly or
S-38
Table of Contents
indirectly, nor may this prospectus supplement or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus supplement comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus supplement. This prospectus supplement does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus supplement in any jurisdiction in which such an offer or a solicitation is unlawful.
This document is only being distributed to and is only directed at (i) persons who are outside the United Kingdom or (ii) to investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the “Order”) or (iii) high net worth entities, and other persons to whom it may lawfully be communicated, falling with Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”). The securities are only available to, and any invitation, offer or agreement to subscribe, purchase or otherwise acquire such securities will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of its contents.
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a Relevant Member State), from and including the date on which the European Union Prospectus Directive (the “EU Prospectus Directive”) was implemented in that Relevant Member State (the “Relevant Implementation Date”) an offer of securities described in this prospectus supplement may not be made to the public in that Relevant Member State prior to the publication of a prospectus in relation to the shares which has been approved by the competent authority in that Relevant Member State or, where appropriate, approved in another Relevant Member State and notified to the competent authority in that Relevant Member State, all in accordance with the EU Prospectus Directive, except that, with effect from and including the Relevant Implementation Date, an offer of securities described in this prospectus supplement may be made to the public in that Relevant Member State at any time:
| • | to any legal entity which is a qualified investor as defined under the EU Prospectus Directive; |
| • | to fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150 natural or legal persons (other than qualified investors as defined in the EU Prospectus Directive); or |
| • | in any other circumstances falling within Article 3(2) of the EU Prospectus Directive, provided that no such offer of securities described in this prospectus supplement shall result in a requirement for the publication by us of a prospectus pursuant to Article 3 of the EU Prospectus Directive. |
For the purposes of this provision, the expression an “offer of securities to the public” in relation to any securities in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe for the securities, as the same may be varied in that Member State by any measure implementing the EU Prospectus Directive in that Member State. The expression “EU Prospectus Directive” means Directive 2003/71/EC (and any amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State) and includes any relevant implementing measure in each Relevant Member State, and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
Certain of the underwriters and their affiliates have provided in the past to us and our affiliates and may provide from time to time in the future certain commercial banking, financial advisory, investment banking and other services for us and such affiliates in the ordinary course of their business, for which they have received and may continue to receive customary fees and commissions. In addition, from time to time, certain of the underwriters and their affiliates may effect transactions for their own account or the account of customers, and hold on behalf of themselves or their customers, long or short positions in our debt or equity securities or loans, and may do so in the future.
S-39
Table of Contents
DLA Piper LLP (US), San Diego, California will pass upon the validity of the issuance of the common stock offered by this prospectus supplement and the accompanying prospectus. The underwriters are being represented by Cooley LLP, San Diego, California.
The consolidated financial statements of Halozyme Therapeutics, Inc. appearing in Halozyme Therapeutics, Inc.’s Annual Report (Form 10-K) for the year ended December 31, 2012, and the effectiveness of Halozyme Therapeutics, Inc.’s internal control over financial reporting as of December 31, 2012 have been audited by Ernst & Young LLP, independent registered public accounting firm, as set forth in their reports thereon, included therein, and incorporated herein by reference. Such consolidated financial statements are incorporated herein by reference in reliance upon such reports given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form S-3ASR (No. 333-179444) under the Securities Act relating to the common stock offered by this prospectus supplement. This prospectus supplement is a part of that registration statement, which includes additional information not contained in this prospectus supplement.
We file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public over the Internet at the SEC’s website at http://www.sec.gov. Copies of certain information filed by us with the SEC are also available on our website at www.halozyme.com. Our website is not a part of this prospectus supplement. You may also read and copy any document we file with the SEC at its public reference room, at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1–800–SEC–0330 for further information on the operation of its Public Reference Room.
We are incorporating by reference in this prospectus supplement the documents that we file with the SEC. This means that we are disclosing important information to you by referring to these filings. The information we incorporate by reference is considered a part of this prospectus supplement, and subsequent information that we file with the SEC will automatically update and supersede this information.
Any statement contained in a document incorporated or considered to be incorporated by reference in this prospectus supplement shall be considered to be modified or superseded for purposes of this prospectus supplement to the extent that a statement contained in this prospectus supplement or in any other subsequently filed document that is considered to be incorporated by reference in this prospectus supplement modifies or supersedes such statement.
We incorporate by reference the following documents that we have filed with the SEC (other than current reports or portions thereof furnished under Item 2.02 or Item 7.01 of Form 8-K):
| • | our Quarterly Report on Form 10-Q for the quarter ended September 30, 2013, filed with the SEC on November 8, 2013; |
| • | our Quarterly Report on Form 10-Q for the quarter ended June 30, 2013, filed with the SEC on August 7, 2013; |
| • | our Quarterly Report on Form 10-Q for the quarter ended March 31, 2013, filed with the SEC on May 8, 2013; |
| • | our Annual Report on Form 10-K for the year ended December 31, 2012, filed with the SEC on March 1, 2013; |
| • | our Current Reports on Form 8-K filed with the SEC on January 4, 2013, March 28, 2013, May 17, 2013, May 24, 2013, July 3, 2013, December 18, 2013, and January 3, 2014; |
S-40
Table of Contents
| • | the portions of our definitive proxy statement on Schedule 14A, filed with the SEC on April 11, 2013, that are incorporated by reference into our Annual Report on Form 10-K for the year ended December 31, 2012; |
| • | the description of our common stock set forth in Form 8-A/A, filed with the SEC on November 20, 2007; and |
| • | the description of our stockholder rights plan set forth in Form 8-A, filed with the SEC on June 9, 2006. |
Any information in any of the foregoing documents will automatically be deemed to be modified or superseded to the extent that information in this prospectus supplement or in a later filed document that is incorporated or deemed to be incorporated herein by reference modifies or replaces such information.
We also incorporate by reference any future filings (other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that are related to such items) made with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act after the date of this prospectus supplement and prior to the termination of the offering of the common stock covered by this prospectus supplement. Information in such future filings updates and supplements the information provided in this prospectus supplement. Any statements in any such future filings will automatically be deemed to modify and supersede any information in any document we previously filed with the SEC that is incorporated or deemed to be incorporated herein by reference to the extent that statements in the later filed document modify or replace such earlier statements.
We will provide, upon written or oral request, to each person, including any beneficial owner to whom a prospectus is delivered, a copy of these filings (other than exhibits to such documents unless such exhibits are specifically incorporated by reference in any such documents) at no cost by writing to Halozyme Therapeutics, Inc., Attention: Investor Relations, 11388 Sorrento Valley Road, San Diego, CA 92121, telephone: (858) 794-8889.
S-41
Table of Contents
PROSPECTUS
HALOZYME THERAPEUTICS, INC.
Common Stock
Preferred Stock
Debt Securities
Warrants
Units
We may offer from time to time any combination of the securities described in this prospectus, either individually or in units.
This prospectus provides a general description of the securities we may offer. Each time we sell securities, we will provide specific terms of the securities offered in a supplement to this prospectus. We may also authorize one or more free writing prospectuses to be provided to you in connection with these offerings. The prospectus supplement and any related free writing prospectus may also add, update or change information contained in this prospectus. You should carefully read this prospectus, the applicable prospectus supplement and any related free writing prospectus, as well as any documents incorporated by reference before you invest in any securities. This prospectus may not be used to consummate a sale of securities unless accompanied by the applicable prospectus supplement.
Our common stock is listed on The Nasdaq Global Market under the symbol “HALO.” On February 8, 2012, the last reported sale price for our common stock was $11.28 per share. The applicable prospectus supplement will contain information, where applicable, as to any other listing on The Nasdaq Global Market or any securities market or other exchange of the securities, if any, covered by the prospectus supplement.
INVESTING IN OUR SECURITIES INVOLVES RISKS. YOU SHOULD REVIEW CAREFULLY THE RISKS AND UNCERTAINTIES DESCRIBED UNDER THE HEADING “RISK FACTORS’’ ON PAGE 6 AND CONTAINED IN THE APPLICABLE PROSPECTUS SUPPLEMENT AND ANY RELATED FREE WRITING PROSPECTUS AND UNDER SIMILAR HEADINGS IN THE OTHER DOCUMENTS THAT ARE INCORPORATED BY REFERENCE INTO THIS PROSPECTUS.
We may sell these securities directly to investors, through agents designated from time to time or to or through underwriters or dealers. For additional information on the methods of sale, you should refer to the section entitled “Plan of Distribution” in this prospectus. If any underwriters are involved in the sale of any securities with respect to which this prospectus is being delivered, the names of such underwriters and any applicable commissions or discounts will be set forth in a prospectus supplement. The price to the public of such securities and the net proceeds we expect to receive from such sale will also be set forth in a prospectus supplement.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED IF THIS PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this prospectus is February 9, 2012
Table of Contents
| Page | ||||
| 1 | ||||
| 2 | ||||
| 6 | ||||
| 6 | ||||
| 7 | ||||
| 7 | ||||
| 8 | ||||
| 13 | ||||
| 20 | ||||
| 22 | ||||
| 23 | ||||
| 26 | ||||
| 28 | ||||
| 28 | ||||
| 28 | ||||
| 29 | ||||
i
Table of Contents
This prospectus is a part of a registration statement that we filed with the Securities and Exchange Commission, or SEC, utilizing a “shelf” registration process. Under this shelf process, we may sell the securities described in this prospectus in one or more offerings. This prospectus provides you with a general description of the securities we may offer. Each time we sell securities under this shelf registration, we will provide a prospectus supplement that will contain specific information about the terms of that offering. We may also authorize one or more free writing prospectuses to be provided to you that may contain material information relating to these offerings. The prospectus supplement and any related free writing prospectus that we may authorize to be provided to you may also add, update or change information contained in this prospectus or in any documents that we have incorporated by reference into this prospectus. You should read this prospectus, any applicable prospectus supplement and any related free writing prospectus, together with the information incorporated herein by reference as described under the heading “Where You Can Find More Information.”
You should rely only on the information that we have provided or incorporated by reference in this prospectus, any applicable prospectus supplement and any related free writing prospectus that we may authorize to be provided to you. We have not authorized any dealer, salesman or other person to give any information or to make any representation other than those contained or incorporated by reference in this prospectus, any applicable prospectus supplement or any related free writing prospectus that we may authorize to be provided to you. You must not rely upon any information or representation not contained or incorporated by reference in this prospectus or the accompanying prospectus supplement. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you.
This prospectus and the accompanying supplement to this prospectus do not constitute an offer to sell or the solicitation of an offer to buy any securities other than the registered securities to which they relate, nor do this prospectus and the accompanying supplement to this prospectus constitute an offer to sell or the solicitation of an offer to buy securities in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction. You should not assume that the information contained in this prospectus, any applicable prospectus supplement or any related free writing prospectus is accurate on any date subsequent to the date set forth on the front of the document or that any information we have incorporated by reference is correct on any date subsequent to the date of the document incorporated by reference, even though this prospectus, any applicable prospectus supplement or any related free writing prospectus is delivered or securities sold on a later date.
1
Table of Contents
Prospectus Summary
This summary highlights selected information from this prospectus and does not contain all of the information that you need to consider in making your investment decision. You should carefully read the entire prospectus, including the risks of investing discussed under “Risk Factors” beginning on page 5, the information incorporated by reference, including our financial statements, and the exhibits to the registration statement of which this prospectus is a part.
Throughout this prospectus, references to “Halozyme,” the “Company,” “we,” “us,” and “our” refer to Halozyme Therapeutics, Inc. and its operating subsidiary, Halozyme, Inc.
Our Company
We are a biopharmaceutical company dedicated to developing and commercializing innovative products that advance patient care. Our research targets the extracellular matrix, an area outside the cell that provides structural support in tissues and orchestrates many important biological activities, including cell migration, signaling and survival. Over many years, we have developed unique scientific expertise that allows us to pursue this target-rich environment for the development of future therapies.
Our research focuses primarily on human enzymes that alter the extracellular matrix. Our lead enzyme, recombinant human PH20 enzyme, or rHuPH20, temporarily degrades hyaluronan, a matrix component in the skin, and facilitates the dispersion of drugs and fluids through the skin into circulation. rHuPH20 is the underlying drug delivery technology ofHylenex® recombinant (hyaluronidase human injection) for small molecules and fluids, and Enhanze™ Technology for the delivery of proprietary small and large molecules. We are also developing novel enzymes that may target other matrix structures for therapeutic benefit.
Our operations to date have involved: (i) organizing and staffing our operating subsidiary, Halozyme, Inc.; (ii) acquiring, developing and securing our technology; (iii) undertaking product development for our existing products and a limited number of product candidates; and (iv) supporting the development of partnered product candidates. We continue to increase our focus on our proprietary product pipeline and have expanded investments in our proprietary product candidates. We currently have multiple proprietary programs in various stages of research and development. In addition, we currently have collaborative partnerships with F.Hoffmann-La Roche, Ltd andHoffmann-La Roche, Inc., or Roche, Baxter Healthcare Corporation, or Baxter, ViroPharma Incorporated, or ViroPharma, and Intrexon Corporation, or Intrexon, to apply Enhanze Technology to the partners’ biological therapeutic compounds. We also had another partnership with Baxter, under which Baxter had worldwide marketing rights for our marketed product,Hylenexrecombinant (hyaluronidase human injection), or Hylenex Partnership.Hylenexrecombinant is a recombinant formulation of hyaluronidase that has received the approval from the U.S. Food and Drug Administration, or FDA, to facilitate subcutaneous fluid administration for achieving hydration; to increase the dispersion and absorption of other injected drugs; and in subcutaneous urography for improving resorption of radiopaque agents. We and Baxter mutually agreed to terminate the Hylenex Partnership in January 2011. In December 2011, we reintroducedHylenexrecombinant to the market. Our rHuPH20 technology is also being used in ICSI Cumulase®, a third party’s marketed product used forin vitro fertilization, or IVF. Currently, we have received only limited revenue from the sales ofHylenexrecombinant and active pharmaceutical ingredients, or API, to the third party that produces ICSI Cumulase, in addition to other revenues from our partnerships.
In February 2007, we and Baxter amended certain existing agreements relating toHylenexrecombinant and entered into the Hylenex Partnership for kits and formulations with rHuPH20. In October 2009, Baxter commenced the commercial launch ofHylenexrecombinant.Hylenexrecombinant was voluntarily recalled in
2
Table of Contents
May 2010, because a portion of theHylenexrecombinant manufactured by Baxter was not in compliance with the requirements of the underlyingHylenexrecombinant agreements. During the second quarter of 2011, we submitted the data that the FDA had requested to support the reintroduction ofHylenexrecombinant. The FDA has approved the submitted data and has granted the reintroduction ofHylenexrecombinant. We reintroducedHylenexrecombinant to the market in December 2011.
Effective January 7, 2011, we and Baxter mutually agreed to terminate the Hylenex Partnership and the associated agreements. In June 2011, we entered into a commercial manufacturing and supply agreement with Baxter, under which Baxter will fill and finishHylenexrecombinant for us. On July 18, 2011, we and Baxter entered into an agreement setting forth certain rights, data and assets to be transferred by Baxter to us during a transition period, or the Transition Agreement. The termination of these agreements does not affect the other relationships between the parties, including the application of our Enhanze Technology to Baxter’s GAMMAGARD LIQUID™.
We and our partners have product candidates in the research, preclinical and clinical stages, but future revenues from the sales and/or royalties of these product candidates will depend on our partners’ abilities and ours to develop, manufacture, obtain regulatory approvals for and successfully commercialize product candidates. It may be years, if ever, before we and our partners are able to obtain regulatory approvals for these product candidates. We have incurred net operating losses each year since inception, with an accumulated deficit of approximately $226.6 million as of September 30, 2011.
Sales of a substantial number of shares of our common stock pursuant to a registration statement or in connection with other transactions could lower the market price of our common stock and impair our ability to raise capital through the sale of additional equity securities. In the future, we may issue additional options, warrants or other derivative securities convertible into our common stock to fund the continued development of our product candidates, the commercialization of our products or for other general corporate purposes.
Deliatroph Pharmaceuticals, Inc., our predecessor company, was founded on February 26, 1998. In November 2007, we reincorporated from the State of Nevada to the State of Delaware. Our principal offices and research facilities are located at 11388 Sorrento Valley Road, San Diego, California 92121. Our telephone number is(858) 794-8889 and oure-mail address is info@halozyme.com. Additional information about us can be found on our website at www.halozyme.com, and in our periodic and current reports filed with the Securities and Exchange Commission (“SEC”). Copies of our current and periodic reports filed with the SEC are available at the SEC Public Reference Room at 450 Fifth Street, N.W., Washington, D.C. 20549, and online at www.sec.gov and our website at www.halozyme.com. Please note that the information on our website is not incorporated by reference in this prospectus.
The Securities We May Offer
We may offer shares of our common stock and preferred stock, various series of debt securities and warrants to purchase any of such securities, either individually or in units, from time to time under this prospectus, together with any applicable prospectus supplement and related free writing prospectus, at prices and on terms to be determined by market conditions at the time of offering. This prospectus provides you with a general description of the securities we may offer. Each time we offer a type or series of securities, we will provide a prospectus supplement that will describe the specific amounts, prices and other important terms of the securities, including, to the extent applicable:
| • | designation or classification; |
| • | aggregate principal amount or aggregate offering price; |
| • | maturity, if applicable; |
3
Table of Contents
| • | original issue discount, if any; |
| • | rates and times of payment of interest or dividends, if any; |
| • | redemption, conversion, exchange or sinking fund terms, if any; |
| • | conversion or exchange prices or rates, if any, and, if applicable, any provisions for changes to or adjustments in the conversion or exchange prices or rates and in the securities or other property receivable upon conversion or exchange; |
| • | ranking; |
| • | restrictive covenants, if any; |
| • | voting or other rights, if any; and |
| • | important United States federal income tax considerations. |
A prospectus supplement and any related free writing prospectus that we may authorize to be provided to you may also add, update or change information contained in this prospectus or in documents we have incorporated by reference. However, no prospectus supplement or free writing prospectus will offer a security that is not registered and described in this prospectus at the time of the effectiveness of the registration statement of which this prospectus is a part.
We may sell the securities directly to or through underwriters, dealers or agents. We, and our underwriters or agents, reserve the right to accept or reject all or part of any proposed purchase of securities. If we do offer securities through underwriters or agents, we will include in the applicable prospectus supplement:
| • | the names of those underwriters or agents; |
| • | applicable fees, discounts and commissions to be paid to them; |
| • | details regarding over-allotment options, if any; and |
| • | the net proceeds to us. |
Common Stock. We may offer shares of our common stock from time to time. Holders of our common stock are entitled to one vote per share on all other matters that require stockholder approval. Subject to any preferential rights of any outstanding preferred stock, holders of our common stock are entitled to dividends when and if declared by the board of directors. Our common stock is described in greater detail in this prospectus under “Description of Capital Stock — Common Stock.”
Preferred Stock. We currently have authorized 20,000,000 shares of preferred stock, $0.001 par value per share. We may offer shares of our preferred stock from time to time, in one or more series. Under our certificate of incorporation, our board of directors currently has the authority to designate up to 19,500,000 shares of preferred stock in one or more series and to fix the privileges, preferences and rights of each series of preferred stock, any or all of which may be greater than the rights of the common stock. Our board of directors has previously designated 500,000 of the 20,000,000 authorized shares of preferred stock as Series A Preferred Stock, none of which are outstanding. Our Preferred Stock is described in greater detail in this prospectus under “Description of Capital Stock — Preferred Stock.”
We will fix the rights, preferences, privileges, qualifications and restrictions of the preferred stock of each series that we sell under this prospectus and applicable prospectus supplements in the certificate of designation relating to that series. We will incorporate by reference into the registration statement of which this prospectus is a part the form of any certificate of designation that describes the terms of the series of preferred stock we are offering before the issuance of the related series of preferred stock. We urge you to read the prospectus
4
Table of Contents
supplements and any free writing prospectus that we may authorize to be provided to you related to the series of preferred stock being offered, as well as the complete certificate of designation that contains the terms of the applicable series of preferred stock.
Debt Securities. We may offer debt securities from time to time, in one or more series, as either senior or subordinated debt or as senior or subordinated convertible debt. The senior debt securities will rank equally with any other unsubordinated debt that we may have and may be secured or unsecured. The subordinated debt securities will be subordinate and junior in right of payment, to the extent and in the manner described in the instrument governing the debt, to all or some portion of our indebtedness. Any convertible debt securities that we issue will be convertible into or exchangeable for our common stock or other securities of ours. Conversion may be mandatory or at the holder’s option and would be at prescribed conversion rates.
The debt securities will be issued under one or more documents called indentures, which are contracts between us and a trustee for the holders of the debt securities. In this prospectus, we have summarized certain general features of the debt securities under “Description of Debt Securities.” We urge you, however, to read the prospectus supplements and any free writing prospectus that we may authorize to be provided to you related to the series of debt securities being offered, as well as the complete indentures that contain the terms of the debt securities. Forms of indentures have been filed as exhibits to the registration statement of which this prospectus is a part, and supplemental indentures and forms of debt securities containing the terms of debt securities being offered will be incorporated by reference into the registration statement of which this prospectus is a part from reports we file with the SEC.
Warrants. We may offer warrants for the purchase of our common stock, preferred stock and/or debt securities in one or more series, from time to time. We may issue warrants independently or together with common stock, preferred stock and/or debt securities, and the warrants may be attached to or separate from those securities.
The warrants will be evidenced by warrant certificates issued under one or more warrant agreements, which are contracts between us and an agent for the holders of the warrants. In this prospectus, we have summarized certain general features of the warrants under “Description of Warrants.” We urge you, however, to read the prospectus supplements and any free writing prospectus that we may authorize to be provided to you related to the series of warrants being offered, as well as the complete warrant agreements and warrant certificates that contain the terms of the warrants. Specific warrant agreements will contain additional important terms and provisions and will be incorporated by reference as an exhibit to the registration statement which includes this prospectus.
Units. We may offer units consisting of common stock, preferred stock, debt securities and/or warrants to purchase any of such securities in one or more series. In this prospectus, we have summarized certain general features of the units under “Description of Units.” We urge you, however, to read the prospectus supplements and any free writing prospectus that we may authorize to be provided to you related to the series of units being offered, as well as the unit agreements that contain the terms of the units. We will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference from a current report on Form8-K that we file with the SEC, the form of unit agreement and any supplemental agreements that describe the terms of the series of units we are offering before the issuance of the related series of units.
We will evidence each series of units by unit certificates that we will issue under a separate agreement. We will enter into the unit agreements with a unit agent. Each unit agent will be a bank or trust company that we select. We will indicate the name and address of the unit agent in the applicable prospectus supplement relating to a particular series of units.
THIS PROSPECTUS MAY NOT BE USED TO OFFER OR SELL ANY SECURITIES UNLESS ACCOMPANIED BY A PROSPECTUS SUPPLEMENT.
5
Table of Contents
Investing in our securities involves a high degree of risk. You should carefully consider and evaluate all of the information included and incorporated by reference or deemed to be incorporated by reference in this prospectus or the applicable prospectus supplement, including the risk factors incorporated by reference herein from our Annual Report on Form10-K for the year ended December 31, 2010 and the section labeled “Risk Factors” in Part I, Item 2, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” of our Quarterly Report on Form10-Q for the quarter ended September 30, 2011, as updated by annual, quarterly and other reports and documents we file with the SEC after the date of this prospectus and that are incorporated by reference herein or in the applicable prospectus supplement. Our business, results of operations or financial condition could be adversely affected by any of these risks or by additional risks and uncertainties not currently known to us or that we currently consider immaterial.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents incorporated by reference contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements are based on our management’s current beliefs, expectations and assumptions about future events, conditions and results and on information currently available to us. Discussions containing these forward-looking statements may be found, among other places, in the Sections entitled “Business,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” incorporated by reference from our most recent Annual Report on Form10-K and in our Quarterly Reports on Form10-Q, as well as any amendments thereto, filed with the SEC.
All statements, other than statements of historical fact, included or incorporated herein regarding our strategy, future operations, financial position, future revenues, projected costs, plans, prospectus and objectives are forward-looking statements. Words such as “expect,” “anticipate,” “intend,” “plan,” “believe,” “seek,” “estimate,” “think,” “may,” “could,” “will,” “would,” “should,” “continue,” “potential,” “likely,” “opportunity” and similar expressions or variations of such words are intended to identify forward-looking statements, but are not the exclusive means of indentifying forward-looking statements in this report. Additionally, statements concerning future matters such as the development or regulatory approval of new products, enhancements of existing products or technologies, third party performance under key collaboration agreements, revenue and expense levels and other statements regarding matters that are not historical are forward-looking statements. Such statements are based on currently available operating, financial and competitive information and are subject to various risks, uncertainties and assumptions that could cause actual results to differ materially from those anticipated or implied in our forward-looking statements due to a number of factors including, but not limited to, those set forth below under the section entitled “Risk Factors” in our most recent Annual Report on Form10-K and in our Quarterly Reports on Form10-Q, as well as any amendments thereto filed with the SEC. Given these risks, uncertainties and other factors, many of which are beyond our control, you should not place undue reliance on these forward-looking statements.
Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to revise any forward-looking statements to reflect events or developments occurring after the date of this prospectus, even if new information becomes available in the future.
6
Table of Contents
STATEMENT OF COMPUTATION OF RATIOS
The following table sets forth our ratios of earnings to fixed charges and to combined fixed charges and preferred stock dividends on a historical basis for each of the periods presented. These ratios should be read in connection with our consolidated financial statements, including the notes to those statements, incorporated by reference in this prospectus.
| Year Ended December 31, | Nine Months Ended September 30, 2011 | |||||||||||
| 2006 | 2007 | 2008 | 2009 | 2010 | ||||||||
Ratio of earnings to fixed charges(1)(2) | N/A | N/A | N/A | N/A | N/A | N/A | ||||||
Ratio of earnings to combined fixed charges and preferred stock dividends(1)(3) | N/A | N/A | N/A | N/A | N/A | N/A | ||||||
| (1) | We reported a net loss for the years ended December 31, 2006, 2007, 2008, 2009, 2010 and the nine months ended September 30, 2011 and would have needed to generate additional income of approximately $14.8 million, $23.9 million, $48.7 million, $58.4 million, $53.2 million and $1.4 million respectively, to cover our fixed charges and combined fixed charges and preferred stock dividends of approximately $45,000, $160,000, $207,000, $207,000, $232,000 and $166,000, respectively. |
| (2) | For purposes of computing the ratio of earnings to fixed charges, earnings consist of net loss plus fixed charges and fixed charges consist of interest expense and an estimate of interest within rent expense. In each of the periods presented, earnings were insufficient to cover fixed charges. |
| (3) | For purposes of computing the ratio of earnings to combined fixed charges and preferred stock dividends, earnings consist of net loss plus fixed charges. Combined fixed charges and preferred stock dividends consist of interest expense, an estimate of interest within rent expense and preferred stock dividends. For the periods presented, we had no shares of preferred stock outstanding and consequently, our ratio of earnings to combined fixed charges and preferred share dividends is the same as the ratio of earnings to fixed charges. |
Except as described in any applicable prospectus supplement and in any free writing prospectuses in connection with a specific offering, we currently intend to use the net proceeds from the sale of the securities offered hereby for operating costs, capital expenditures and for general corporate purposes, including working capital. We may also use a portion of the net proceeds to invest in or acquire businesses or technologies that we believe are complementary to our own, although we have no current plans, commitments or agreements with respect to any acquisitions as of the date of this prospectus. Pending these uses, we intend to invest the net proceeds in investment-grade, interest-bearing securities.
7
Table of Contents
As of the date of this prospectus, our certificate of incorporation authorizes us to issue 150,000,000 shares of common stock, par value $0.001 per share, and 20,000,000 shares of preferred stock, par value $0.001 per share. As of September 30, 2011, approximately 103.6 million shares of common stock were outstanding and no shares of Preferred Stock were outstanding. Our board of directors has previously designated 500,000 of the 20,000,000 authorized shares of preferred stock as Series A Preferred Stock.
The following summary describes the material terms of our capital stock. The description of our capital stock is qualified by reference to our amended and restated certificate of incorporation, as amended, our bylaws, as amended, the certificate of designation for our Series A Preferred Stock, which are incorporated by reference as exhibits into the registration statement of which this prospectus is a part.
Common Stock
The holders of common stock are entitled to one vote for each share held of record on all matters submitted to a vote of the stockholders. Subject to preferences that may be applicable to any outstanding shares of the preferred stock, the holders of common stock are entitled to receive ratably such dividends as may be declared by the board of directors out of funds legally available therefor. In the event of a liquidation, dissolution or winding up of our company, holders of the common stock are entitled to share ratably in all assets remaining after payment of liabilities and the liquidation preferences of any outstanding shares of preferred stock. Holders of common stock have no preemptive rights and no right to convert their common stock into any other securities. There are no redemption or sinking fund provisions applicable to the common stock. All outstanding shares of common stock are, and all shares of common stock to be outstanding upon the closing of this offering will be, fully paid and nonassessable.
Additional shares of authorized common stock may be issued, as authorized by our board of directors from time to time, without stockholder approval, except as may be required by applicable stock exchange requirements.
Preferred Stock
Pursuant to our Amended and Restated Certificate of Incorporation, or the Restated Certificate, our board of directors currently has the authority, without further action by the stockholders, to issue up to 19,500,000 shares of preferred stock in one or more series and to fix the designations, powers, preferences, privileges and relative participating, optional or special rights and the qualifications, limitations or restrictions thereof, including dividend rights, conversion rights, voting rights, terms of redemption and liquidation preferences, any or all of which may be greater than the rights of the common stock. The board of directors, without stockholder approval, can issue preferred stock with voting, conversion or other rights that could adversely affect the voting power and other rights of the holders of common stock. Preferred stock could thus be issued quickly with terms calculated to delay or prevent a change in control of our company or make removal of management more difficult. Additionally, the issuance of preferred stock may have the effect of decreasing the market price of the common stock and may adversely affect the voting power of holders of common stock and reduce the likelihood that common stockholders will receive dividend payments and payments upon liquidation.
Series A Preferred Stock.Holders of our Series A Preferred Stock have 1,000 votes per share of our Series A Preferred Stock and vote as a single class with the holders of our common stock on all matters submitted to a vote of stockholders. Holders of our s Series A Preferred Stock do not have cumulative voting rights. The holders of our Series A Preferred Stock have the right, subject to the rights of the holders of any shares of preferred stock to receive preferential dividends and in preference to the holders of our common stock, to receive, when and if declared by our board of directors, quarterly dividends in an amount equal to the greater of $625 or 1,000 times the dividend declared per share of the common stock. Upon our liquidation, before any payment may be made to
8
Table of Contents
holders of our common stock or shares of other preferred stock ranking junior to our Series A Preferred Stock, holders of our Series A Preferred Stock will be entitled to a preferential liquidation payment of the greater of $25,000 per share or 1,000 times the payment made per share of the common stock. Our Series A Preferred Stock is not convertible or redeemable and has no preemptive, subscription or conversion rights. Our Series A Preferred Stock was authorized for issuance in connection with the rights agreement as described below under “— Rights Agreement.” There are no shares of our Series A Preferred Stock currently outstanding.
Future Preferred Stock.Our board of directors will fix the rights, preferences, privileges, qualifications and restrictions of the preferred stock of each series that we sell under this prospectus and applicable prospectus supplements in the certificate of designation relating to that series. We will incorporate by reference into the registration statements of which this prospectus is a part the form of any certificate of designation that describes the terms of the series of preferred stock we are offering before the issuance of the related series of preferred stock. This description will include:
| • | the title and stated value; |
| • | the number of shares we are offering; |
| • | the liquidation preference per share; |
| • | the purchase price per share; |
| • | the dividend rate per share, dividend period and payment dates and method of calculation for dividends; |
| • | whether dividends will be cumulative ornon-cumulative and, if cumulative, the date from which dividends will accumulate; |
| • | our right, if any, to defer payment of dividends and the maximum length of any such deferral period; |
| • | the procedures for any auction and remarketing, if any; |
| • | the provisions for a sinking fund, if any; |
| • | the provisions for redemption or repurchase, if applicable, and any restrictions on our ability to exercise those redemption and repurchase rights; |
| • | any listing of the preferred stock on any securities exchange or market; |
| • | whether the preferred stock will be convertible into our common stock or other securities of ours, including warrants, and, if applicable, the conversion period, the conversion price, or how it will be calculated, and under what circumstances it may be adjusted; |
| • | whether the preferred stock will be exchangeable into debt securities, and, if applicable, the exchange period, the exchange price, or how it will be calculated, and under what circumstances it may be adjusted; |
| • | voting rights, if any, of the preferred stock; |
| • | preemption rights, if any; |
| • | restrictions on transfer, sale or other assignment, if any; |
| • | a discussion of any material or special United States federal income tax considerations applicable to the preferred stock; |
| • | the relative ranking and preferences of the preferred stock as to dividend rights and rights if we liquidate, dissolve or wind up our affairs; |
9
Table of Contents
| • | any limitations on issuances of any class or series of preferred stock ranking senior to or on a parity with the series of preferred stock being issued as to dividend rights and rights if we liquidate, dissolve or wind up our affairs; and |
| • | any other specific terms, rights, preferences, privileges, qualifications or restrictions of the preferred stock. |
When we issue shares of preferred stock under this prospectus, the shares will be fully paid and nonassessable and will not have, or be subject to, any preemptive or similar rights.
The General Corporation Law of the State of Delaware, the state of our incorporation, provides that the holders of preferred stock will have the right to vote separately as a class on any proposal involving fundamental changes in the rights of holders of that preferred stock. This right is in addition to any voting rights that may be provided for in the applicable certificate of designation.
Antitakeover Effects of Provisions of Charter Documents and Delaware Law
Charter Documents.Our Restated Certificate and Amended and Restated Bylaws, or Bylaws, include a number of provisions that may have the effect of deterring hostile takeovers or delaying or preventing changes in control or management of our company. First, our board of directors is classified into three classes of directors. Under Delaware law, directors of a corporation with a classified board may be removed only for cause unless the corporation’s certificate of incorporation provides otherwise. Our Restated Certificate does not provide otherwise. In addition, our Bylaws limit who may call special meetings of the stockholders, permitting only stockholders holding at least 50% of our outstanding shares to call a special meeting of stockholders. Our Restated Certificate does not include a provision for cumulative voting for directors. Under cumulative voting, a minority stockholder holding a sufficient percentage of a class of shares may be able to ensure the election of one or more directors. Finally, our Bylaws establish procedures, including advance notice procedures, with regard to the nomination of candidates for election as directors and stockholder proposals. These and other provisions of our Restated Certificate and Bylaws and Delaware law could discourage potential acquisition proposals and could delay or prevent a change in control or management of our company.
Delaware Takeover Statute.We are subject to Section 203 of the General Corporation Law of the State of Delaware, or DGCL, which regulates acquisitions of some Delaware corporations. In general, Section 203 prohibits, with some exceptions, a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years following the date of the transaction in which the person became an interested stockholder, unless:
| • | the board of directors of the corporation approved the business combination or the other transaction in which the person became an interested stockholder prior to the date of the business combination or other transaction; |
| • | upon consummation of the transaction that resulted in the person becoming an interested stockholder, the person owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding shares owned by persons who are directors and also officers of the corporation and shares issued under employee stock plans under which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| • | on or subsequent to the date the person became an interested stockholder, the board of directors of the corporation approved the business combination and the stockholders of the corporation authorized the business combination at an annual or special meeting of stockholders by the affirmative vote of at least66-2/3% of the outstanding stock of the corporation not owned by the interested stockholder. |
10
Table of Contents
Section 203 of the DGCL generally defines a “business combination” to include any of the following:
| • | any merger or consolidation involving the corporation and the interested stockholder; |
| • | any sale, transfer, pledge or other disposition of 10% or more of the corporation’s assets or outstanding stock involving the interested stockholder; |
| • | in general, any transaction that results in the issuance or transfer by the corporation of any of its stock to the interested stockholder; |
| • | any transaction involving the corporation that has the effect of increasing the proportionate share of its stock owned by the interested stockholder; or |
| • | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
In general, Section 203 defines an “interested stockholder” as any person who, together with the person’s affiliates and associates, owns, or within three years prior to the determination of interested stockholder status did own, 15% or more of a corporation’s voting stock.
Section 203 of the DGCL could depress our stock price and delay, discourage or prohibit transactions not approved in advance by our board of directors, such as takeover attempts that might otherwise involve the payment to our stockholders of a premium over the market price of our common stock.
The Rights Agreement
We are party to a Rights Agreement designed to deter abusive takeover tactics and to encourage prospective acquirors to negotiate with our board of directors rather than attempt to acquire us in a manner or on terms that our board deems unacceptable. Under the Rights Agreement, each outstanding share of common stock includes an associated preferred stock purchase right. If the rights become exercisable, each right will entitle its holder to purchase oneone-thousandth (1/1000) of a share of our Series A Preferred Stock at an exercise price of $25.00 per unit, subject to adjustment. The rights trade with all outstanding shares of the common stock. The rights will separate from the common stock and become exercisable upon the earlier of:
| • | the tenth day following a public announcement that a person or group of affiliated or associated persons has acquired or obtained the right to acquire, without approval of our board of directors, beneficial ownership of 20 percent or more of our outstanding common stock, referred to as an acquiring person; or |
| • | the tenth business day, or any later date as determined by the board of directors prior to the time that any person or group becomes an acquiring person, following the commencement of or announcement of an intention to make a tender offer or exchange offer that, if consummated, would result in the person or group becoming an acquiring person. |
Term of rights.The rights will expire on May 4, 2016, unless we extend this date or redeem or exchange the rights as described below.
Exercise after someone becomes an acquiring person.After any person or group becomes an acquiring person, each holder of a right will be entitled to receive upon exercise that number of shares of the common stock having a market value of two times the exercise price of the right. However, this right will not apply to an acquiring person, whose rights will be void.
11
Table of Contents
Upon the occurrence of certain events after someone becomes an acquiring person, each holder of a right, other than the acquiring person, will be entitled to receive, upon exercise of the right, common stock of the acquiring company having a market value equal to two times the exercise price of the right. These rights will arise only if after a person or group becomes an acquiring person:
| • | We are acquired in a merger or other business combination; or |
| • | We sell or otherwise transfer 50 percent or more of our assets or earning power. |
Adjustment.The exercise price, the number of rights outstanding and the number of preferred shares issuable upon exercise of the rights are subject to adjustment from time to time to prevent certain types of dilution.
Rights, preferences and limitations of rights.Preferred stock purchasable upon exercise of the rights will not be redeemable. Each share of preferred stock will entitle the holder to receive a preferential quarterly dividend payment of the greater of $625 or 1,000 times the dividend declared per share of the common stock. In the event of liquidation, the holders of each share of preferred stock will be entitled to a preferential liquidation payment of the greater of $25,000 per share or 1,000 times the payment made per share of the common stock. Each share of preferred stock will entitle the holder to 1,000 votes and will vote together with the common stock. Finally, in the event of any merger, consolidation or other transaction in which the common stock is exchanged, each share of the preferred stock will entitle the holder to receive 1,000 times the amount received per share of the common stock. These rights are protected by customary antidilution provisions. Because of the nature of our preferred stock’s dividend, liquidation and voting rights, the value of each oneone-thousandth interest in a share of preferred stock should approximate the value of one share of the common stock.
Exchange and redemption.After a person or group becomes an acquiring person, we may exchange the rights, in whole or in part, at an exchange ratio, subject to adjustment, of one share of common stock, or oneone-thousandth of a share of preferred stock, per right. We generally may not make an exchange after any person or group becomes the beneficial owner of 50 percent or more of the common stock.
We may redeem the rights in whole, but not in part, at a price of $0.001 per right, subject to adjustment, at any time prior to any person or group becoming an acquiring person. The redemption of the rights may be made effective at such time, on such basis and with such conditions as the board of directors in its sole discretion may establish. Once redeemed, the rights will terminate immediately, and the only right of the rights holders will be to receive the cash redemption price.
Amendments. Until the rights become nonredeemable, we may, except with respect to the redemption price of the Rights, amend the Rights Agreement in any manner. However, after the rights become nonredeemable, we may not amend the terms of the rights in any way that adversely affects the interests of the rights holders, other than the acquiring person.
Transfer Agent And Registrar
The transfer agent and registrar for our common stock is Corporate Stock Transfer Company.
Listing on The Nasdaq Global Market
Our common stock is listed on The Nasdaq Global Market under the symbol “HALO”.
12
Table of Contents
DESCRIPTION OF DEBT SECURITIES
The following description, together with the additional information we include in any applicable prospectus supplements or free writing prospectuses, summarizes the material terms and provisions of the debt securities that we may offer under this prospectus. We may issue debt securities, in one or more series, as either senior or subordinated debt or as senior or subordinated convertible debt. While the terms we have summarized below will apply generally to any future debt securities we may offer under this prospectus, we will describe the particular terms of any debt securities that we may offer in more detail in the applicable prospectus supplement or free writing prospectus. The terms of any debt securities we offer under a prospectus supplement may differ from the terms we describe below. However, no prospectus supplement shall fundamentally change the terms that are set forth in this prospectus or offer a security that is not registered and described in this prospectus at the time of its effectiveness. As of the date of this prospectus, we have no outstanding registered debt securities. Unless the context requires otherwise, whenever we refer to the “indentures,” we also are referring to any supplemental indentures that specify the terms of a particular series of debt securities.
We will issue any senior debt securities under the senior indenture that we will enter into with the trustee named in the senior indenture. We will issue any subordinated debt securities under the subordinated indenture that we will enter into with the trustee named in the subordinated indenture. We have filed forms of these documents as exhibits to the registration statement, of which this prospectus is a part, and supplemental indentures and forms of debt securities containing the terms of the debt securities being offered will be filed as exhibits to the registration statement of which this prospectus is a part or will be incorporated by reference from reports that we file with the SEC.
The indentures will be qualified under the Trust Indenture Act of 1939, as amended, or the Trust Indenture Act. We use the term “trustee” to refer to either the trustee under the senior indenture or the trustee under the subordinated indenture, as applicable.
The following summaries of material provisions of the senior debt securities, the subordinated debt securities and the indentures are subject to, and qualified in their entirety by reference to, all of the provisions of the indenture applicable to a particular series of debt securities. We urge you to read the applicable prospectus supplements and any related free writing prospectuses related to the debt securities that we may offer under this prospectus, as well as the complete indentures that contains the terms of the debt securities. Except as we may otherwise indicate, the terms of the senior indenture and the subordinated indenture are identical.
The terms of each series of debt securities will be established by or pursuant to a resolution of our board of directors and set forth or determined in the manner provided in an officers’ certificate or by a supplement indenture. Debt securities may be issued in separate series without limitation as to aggregate principal amount. We may specify a maximum aggregate principal amount for the debt securities of any series. We will describe in the applicable prospectus supplement the terms of the series of debt securities being offered, including:
| • | the title; |
| • | the principal amount being offered, and if a series, the total amount authorized and the total amount outstanding; |
| • | any limit on the amount that may be issued; |
| • | whether or not we will issue the series of debt securities in global form, and, if so, the terms and who the depositary will be; |
| • | the maturity date; |
| • | whether and under what circumstances, if any, we will pay additional amounts on any debt securities held by a person who is not a United States person for tax purposes, and whether we can redeem the debt securities if we have to pay such additional amounts; |
13
Table of Contents
| • | the annual interest rate, which may be fixed or variable, or the method for determining the rate and the date interest will begin to accrue, the dates interest will be payable and the regular record dates for interest payment dates or the method for determining such dates; |
| • | whether or not the debt securities will be secured or unsecured, and the terms of any secured debt; |
| • | the terms of the subordination of any series of subordinated debt; |
| • | the place where payments will be payable; |
| • | restrictions on transfer, sale or other assignment, if any; |
| • | our right, if any, to defer payment of interest and the maximum length of any such deferral period; |
| • | the date, if any, after which, and the price at which, we may, at our option, redeem the series of debt securities pursuant to any optional or provisional redemption provisions and the terms of those redemption provisions; |
| • | the date, if any, on which, and the price at which we are obligated, pursuant to any mandatory sinking fund or analogous fund provisions or otherwise, to redeem, or at the holder’s option, to purchase, the series of debt securities and the currency or currency unit in which the debt securities are payable; |
| • | whether the indenture will restrict our ability or the ability of our subsidiaries to: |
| • | incur additional indebtedness; |
| • | issue additional securities; |
| • | create liens; |
| • | pay dividends or make distributions in respect of our capital stock or the capital stock of our subsidiaries; |
| • | redeem capital stock; |
| • | place restrictions on our subsidiaries’ ability to pay dividends, make distributions or transfer assets; |
| • | make investments or other restricted payments; |
| • | sell or otherwise dispose of assets; |
| • | enter into sale-leaseback transactions; |
| • | engage in transactions with stockholders or affiliates; |
| • | issue or sell stock of our subsidiaries; or |
| • | effect a consolidation or merger; |
| • | whether the indenture will require us to maintain any interest coverage, fixed charge, cash flow-based, asset-based or other financial ratios; |
| • | a discussion of certain material or special United States federal income tax considerations applicable to the debt securities; |
| • | information describing any book-entry features; |
| • | provisions for a sinking fund purchase or other analogous fund, if any; |
| • | the applicability of the provisions in the indenture on discharge; |
| • | whether the debt securities are to be offered at a price such that they will be deemed to be offered at an “original issue discount” as defined in paragraph (a) of Section 1273 of the Internal Revenue Code of 1986, as amended; |
14
Table of Contents
| • | the denominations in which we will issue the series of debt securities, if other than denominations of $1,000 and any integral multiple thereof; |
| • | the currency of payment of debt securities if other than U.S. dollars and the manner of determining the equivalent amount in U.S. dollars; and |
| • | any other specific terms, preferences, rights or limitations of, or restrictions on, the debt securities, including any additional events of default or covenants provided with respect to the debt securities, and any terms that may be required by us or advisable under applicable laws or regulations. |
Conversion or Exchange Rights
We will set forth in the applicable prospectus supplement the terms on which a series of debt securities may be convertible into or exchangeable for our common stock, our preferred stock or other securities (including securities of a third-party). We will include provisions as to whether conversion or exchange is mandatory, at the option of the holder or at our option. We may include provisions pursuant to which the number of shares of our common stock, our preferred stock or other securities (including securities of a third-party) that the holders of the series of debt securities receive would be subject to adjustment.
Consolidation, Merger or Sale
Unless we provide otherwise in the prospectus supplement applicable to a particular series of debt securities, the indentures will not contain any covenant that restricts our ability to merge or consolidate, or sell, convey, transfer or otherwise dispose of all or substantially all of our assets. However, any successor to or acquirer of such assets must assume all of our obligations under the indentures or the debt securities, as appropriate. If the debt securities are convertible into or exchangeable for our other securities or securities of other entities, the person with whom we consolidate or merge or to whom we sell all of our property must make provisions for the conversion of the debt securities into securities that the holders of the debt securities would have received if they had converted the debt securities before the consolidation, merger or sale.
Events of Default under the Indenture
Unless we provide otherwise in the prospectus supplement applicable to a particular series of debt securities, the following are events of default under the indentures with respect to any series of debt securities that we may issue:
| • | if we fail to pay interest when due and payable and our failure continues for 90 days and the time for payment has not been extended; |
| • | if we fail to pay the principal, premium or sinking fund payment, if any, when due and payable at maturity, upon redemption or repurchase or otherwise, and the time for payment has not been extended; |
| • | if we fail to observe or perform any other covenant contained in the debt securities or the indentures, other than a covenant specifically relating to another series of debt securities, and our failure continues for 90 days after we receive notice from the trustee or we and the trustee receive notice from the holders of at least 25% in aggregate principal amount of the outstanding debt securities of the applicable series; and |
| • | if specified events of bankruptcy, insolvency or reorganization occur. |
We will describe in each applicable prospectus supplement any additional events of default relating to the relevant series of debt securities.
If an event of default with respect to debt securities of any series occurs and is continuing, other than an event of default specified in the last bullet point above, the trustee or the holders of at least 25% in aggregate principal amount of the outstanding debt securities of that series, by notice to us in writing, and to the trustee if
15
Table of Contents
notice is given by such holders, may declare the unpaid principal, premium, if any, and accrued interest, if any, due and payable immediately. If an event of default specified in the last bullet point above occurs with respect to us, the unpaid principal, premium, if any, and accrued interest, if any, of each issue of debt securities then outstanding shall be due and payable without any notice or other action on the part of the trustee or any holder.
The holders of a majority in principal amount of the outstanding debt securities of an affected series may waive any default or event of default with respect to the series and its consequences, except defaults or events of default regarding payment of principal, premium, if any, or interest, unless we have cured the default or event of default in accordance with the indenture. Any waiver shall cure the default or event of default.
Subject to the terms of the indentures, if an event of default under an indenture shall occur and be continuing, the trustee will be under no obligation to exercise any of its rights or powers under such indenture at the request or direction of any of the holders of the applicable series of debt securities, unless such holders have offered the trustee reasonable indemnity or security satisfactory to it against any loss, liability or expense. The holders of a majority in principal amount of the outstanding debt securities of any series will have the right to direct the time, method and place of conducting any proceeding for any remedy available to the trustee, or exercising any trust or power conferred on the trustee, with respect to the debt securities of that series, provided that:
| • | the direction so given by the holder is not in conflict with any law or the applicable indenture; and |
| • | subject to its duties under the Trust Indenture Act, the trustee need not take any action that might involve it in personal liability or might be unduly prejudicial to the holders not involved in the proceeding. |
The indentures provide that if an event of default has occurred and is continuing, the trustee will be required in the exercise of its powers to use the degree of care that a prudent person would use in the conduct of its own affairs. The trustee, however, may refuse to follow any direction that conflicts with law or the indenture, or that the trustee determines is unduly prejudicial to the rights of any other holder of the relevant series of debt securities, or that would involve the trustee in personal liability. Prior to taking any action under the indentures, the trustee will be entitled to indemnification against all costs, expenses and liabilities that would be incurred by taking or not taking such action.
A holder of the debt securities of any series will have the right to institute a proceeding under the indentures or to appoint a receiver or trustee, or to seek other remedies only if:
| • | the holder has given written notice to the trustee of a continuing event of default with respect to that series; |
| • | the holders of at least 25% in aggregate principal amount of the outstanding debt securities of that series have made written request, and such holders have offered reasonable indemnity to the trustee or security satisfactory to it against any loss, liability or expense or to be incurred in compliance with instituting the proceeding as trustee; and |
| • | the trustee does not institute the proceeding, and does not receive from the holders of a majority in aggregate principal amount of the outstanding debt securities of that series other conflicting directions within 90 days after the notice, request and offer. |
These limitations do not apply to a suit instituted by a holder of debt securities if we default in the payment of the principal, premium, if any, or interest on, the debt securities, or other defaults that may be specified in the applicable prospectus supplement.
We will periodically file statements with the trustee regarding our compliance with specified covenants in the indentures.
16
Table of Contents
The indentures provide that if a default occurs and is continuing and is actually known to a responsible officer of the trustee, the trustee must mail to each holder notice of the default within the earlier of 90 days after it occurs and 30 days after it is known by a responsible officer of the trustee or written notice of it is received by the trustee, unless such default has been cured or waived. Except in the case of a default in the payment of principal or premium of or interest on any debt security or certain other defaults specified in an indenture, the trustee shall be protected in withholding such notice if and so long as the board of directors, the executive committee or a trust committee of directors, or responsible officers of the trustee, in good faith determine that withholding notice is in the best interests of holders of the relevant series of debt securities.
Modification of Indenture; Waiver
Subject to the terms of the indenture for any series of debt securities that we may issue, we and the trustee may change an indenture without the consent of any holders with respect to the following specific matters:
| • | to fix any ambiguity, defect or inconsistency in the indenture; |
| • | to comply with the provisions described above under “Description of Debt Securities — Consolidation, Merger or Sale”; |
| • | to comply with any requirements of the SEC in connection with the qualification of any indenture under the Trust Indenture Act; |
| • | to add to, delete from or revise the conditions, limitations, and restrictions on the authorized amount, terms, or purposes of issue, authentication and delivery of debt securities, as set forth in the indenture; |
| • | to provide for the issuance of and establish the form and terms and conditions of the debt securities of any series as provided under “Description of Debt Securities — General,” to establish the form of any certifications required to be furnished pursuant to the terms of the indenture or any series of debt securities, or to add to the rights of the holders of any series of debt securities; |
| • | to evidence and provide for the acceptance of appointment hereunder by a successor trustee; |
| • | to provide for uncertificated debt securities and to make all appropriate changes for such purpose; |
| • | to add to our covenants such new covenants, restrictions, conditions or provisions for the benefit of the holders, to make the occurrence, or the occurrence and the continuance, of a default in any such additional covenants, restrictions, conditions or provisions an event of default or to surrender any right or power conferred to us in the indenture; or |
| • | to change anything that does not adversely affect the interests of any holder of debt securities of any series in any material respect. |
In addition, under the indentures, the rights of holders of a series of debt securities may be changed by us and the trustee with the written consent of the holders of at least a majority in aggregate principal amount of the outstanding debt securities of each series that is affected. However, subject to the terms of the indenture for any series of debt securities that we may issue or otherwise provided in the prospectus supplement applicable to a particular series of debt securities, we and the trustee may only make the following changes with the consent of each holder of any outstanding debt securities affected:
| • | extending the stated maturity of the series of debt securities; |
| • | reducing the principal amount, reducing the rate of or extending the time of payment of interest, or reducing any premium payable upon the redemption or repurchase of any debt securities; or |
| • | reducing the percentage of debt securities, the holders of which are required to consent to any amendment, supplement, modification or waiver. |
17
Table of Contents
Discharge
Each indenture provides that, subject to the terms of the indenture and any limitation otherwise provided in the prospectus supplement applicable to a particular series of debt securities, we can elect to be discharged from our obligations with respect to one or more series of debt securities, except for specified obligations, including obligations to:
| • | register the transfer or exchange of debt securities of the series; |
| • | replace stolen, lost or mutilated debt securities of the series; |
| • | maintain paying agencies; |
| • | hold monies for payment in trust; |
| • | recover excess money held by the trustee; |
| • | compensate and indemnify the trustee; and |
| • | appoint any successor trustee. |
In order to exercise our rights to be discharged, we must deposit with the trustee money or government obligations sufficient to pay all the principal of, any premium and interest on, the debt securities of the series on the dates payments are due.
Form, Exchange and Transfer
We will issue the debt securities of each series only in fully registered form without coupons and, unless we otherwise specify in the applicable prospectus supplement, in denominations of $1,000 and any integral multiple thereof. The indentures provide that we may issue debt securities of a series in temporary or permanent global form and as book-entry securities that will be deposited with, or on behalf of, The Depository Trust Company or another depositary named by us and identified in a prospectus supplement with respect to that series. See “Legal Ownership of Securities” below for a further description of the terms relating to any book-entry securities.
At the option of the holder, subject to the terms of the indentures and the limitations applicable to global securities described in the applicable prospectus supplement, the holder of the debt securities of any series can exchange the debt securities for other debt securities of the same series, in any authorized denomination and of like tenor and aggregate principal amount.
Subject to the terms of the indentures and the limitations applicable to global securities set forth in the applicable prospectus supplement, holders of the debt securities may present the debt securities for exchange or for registration of transfer, duly endorsed or with the form of transfer endorsed thereon duly executed if so required by us or the security registrar, at the office of the security registrar or at the office of any transfer agent designated by us for this purpose. Unless otherwise provided in the debt securities that the holder presents for transfer or exchange, we will make no service charge for any registration of transfer or exchange, but we may require payment of any taxes or other governmental charges.
We will name in the applicable prospectus supplement the security registrar, and any transfer agent in addition to the security registrar, that we initially designate for any debt securities. We may at any time designate additional transfer agents or rescind the designation of any transfer agent or approve a change in the office through which any transfer agent acts, except that we will be required to maintain a transfer agent in each place of payment for the debt securities of each series.
18
Table of Contents
If we elect to redeem the debt securities of any series, we will not be required to:
| • | issue, register the transfer of, or exchange any debt securities of that series during a period beginning at the opening of business 15 days before the day of mailing of a notice of redemption of any debt securities that may be selected for redemption and ending at the close of business on the day of the mailing; or |
| • | register the transfer of or exchange any debt securities so selected for redemption, in whole or in part, except the unredeemed portion of any debt securities we are redeeming in part. |
Information Concerning the Trustee
The trustee, other than during the occurrence and continuance of an event of default under an indenture, undertakes to perform only those duties as are specifically set forth in the applicable indenture and is under no obligation to exercise any of the powers given it by the indentures at the request of any holder of debt securities unless it is offered reasonable security and indemnity against the costs, expenses and liabilities that it might incur. However, upon an event of default under an indenture, the trustee must use the same degree of care as a prudent person would exercise or use in the conduct of his or her own affairs.
Payment and Paying Agents
Unless we otherwise indicate in the applicable prospectus supplement, we will make payment of the interest on any debt securities on any interest payment date to the person in whose name the debt securities, or one or more predecessor securities, are registered at the close of business on the regular record date for the interest.
We will pay principal of and any premium and interest on the debt securities of a particular series at the office of the paying agents designated by us, except that unless we otherwise indicate in the applicable prospectus supplement, we will make interest payments by check that we will mail to the holder or by wire transfer to certain holders. Unless we otherwise indicate in the applicable prospectus supplement, we will designate the corporate trust office of the trustee as our sole paying agent for payments with respect to debt securities of each series. We will name in the applicable prospectus supplement any other paying agents that we initially designate for the debt securities of a particular series. We will maintain a paying agent in each place of payment for the debt securities of a particular series.
All money we pay to a paying agent or the trustee for the payment of the principal of or any premium or interest on any debt securities that remains unclaimed at the end of two years after such principal, premium or interest has become due and payable will be repaid to us, and the holder of the debt security thereafter may look only to us for payment thereof.
Governing Law
The indentures and the debt securities will be governed by and construed in accordance with the laws of the State of New York, except to the extent that the Trust Indenture Act is applicable.
Ranking Debt Securities
The subordinated debt securities will be unsecured and will be subordinate and junior in priority of payment to certain other indebtedness to the extent described in a prospectus supplement. The subordinated indenture does not limit the amount of subordinated debt securities that we may issue. It also does not limit us from issuing any other secured or unsecured debt.
The senior debt securities will be unsecured and will rank equally in right of payment to all our other senior unsecured debt. The senior indenture does not limit the amount of senior debt securities that we may issue. It also does not limit us from issuing any other secured or unsecured debt.
Existing Subordinated Debt
As of December 31, 2011, the Company had no existing subordinated debt.
19
Table of Contents
The following description, together with the additional information we may include in any applicable prospectus supplements and free writing prospectuses, summarizes the material terms and provisions of the warrants that we may offer under this prospectus, which may consist of warrants to purchase common stock, preferred stock or debt securities and may be issued in one or more series. Warrants may be offered independently or together with common stock, preferred stock or debt securities offered by any prospectus supplement, and may be attached to or separate from those securities. While the terms we have summarized below will apply generally to any warrants that we may offer under this prospectus, we will describe the particular terms of any series of warrants that we may offer in more detail in the applicable prospectus supplement and any applicable free writing prospectus. The terms of any warrants offered under a prospectus supplement may differ from the terms described below. However, no prospectus supplement will fundamentally change the terms that are set forth in this prospectus or offer a security that is not registered and described in this prospectus at the time of its effectiveness.
We will issue the warrants under a warrant agreement that we will enter into with a warrant agent to be selected by us. The warrant agent will act solely as an agent of ours in connection with the warrants and will not act as an agent for the holders or beneficial owners of the warrants. We will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference from a current report on Form8-K that we file with the SEC, the form of warrant agreement, including a form of warrant certificate, that describes the terms of the particular series of warrants we are offering before the issuance of the related series of warrants. The following summaries of material provisions of the warrants and the warrant agreements are subject to, and qualified in their entirety by reference to, all the provisions of the warrant agreement and warrant certificate applicable to a particular series of warrants. We urge you to read the applicable prospectus supplement and any applicable free writing prospectus related to the particular series of warrants that we sell under this prospectus, as well as the complete warrant agreements and warrant certificates that contain the terms of the warrants.
General
We will describe in the applicable prospectus supplement the terms relating to a series of warrants, including:
| • | the offering price and aggregate number of warrants offered; |
| • | the currency for which the warrants may be purchased; |
| • | if applicable, the designation and terms of the securities with which the warrants are issued and the number of warrants issued with each such security or each principal amount of such security; |
| • | if applicable, the date on and after which the warrants and the related securities will be separately transferable; |
| • | in the case of warrants to purchase debt securities, the principal amount of debt securities purchasable upon exercise of one warrant and the price at, and currency in which, this principal amount of debt securities may be purchased upon such exercise; |
| • | in the case of warrants to purchase common stock or preferred stock, the number of shares of common stock or preferred stock, as the case may be, purchasable upon the exercise of one warrant and the price at which these shares may be purchased upon such exercise; |
| • | the effect of any merger, consolidation, sale or other disposition of our business on the warrant agreements and the warrants; |
| • | the terms of any rights to redeem or call the warrants; |
| • | any provisions for changes to or adjustments in the exercise price or number of securities issuable upon exercise of the warrants; |
20
Table of Contents
| • | the dates on which the right to exercise the warrants will commence and expire; |
| • | the manner in which the warrant agreements and warrants may be modified; |
| • | United States federal income tax consequences of holding or exercising the warrants; |
| • | the terms of the securities issuable upon exercise of the warrants; and |
| • | any other specific terms, preferences, rights or limitations of or restrictions on the warrants. |
Before exercising their warrants, holders of warrants will not have any of the rights of holders of the securities purchasable upon such exercise, including:
| • | in the case of warrants to purchase debt securities, the right to receive payments of principal of, or premium, if any, or interest on, the debt securities purchasable upon exercise or to enforce covenants in the applicable indenture; or |
| • | in the case of warrants to purchase common stock or preferred stock, the right to receive dividends, if any, or, payments upon our liquidation, dissolution or winding up or to exercise voting rights, if any. |
Exercise of Warrants
Each warrant will entitle the holder to purchase the securities that we specify in the applicable prospectus supplement at the exercise price that we describe in the applicable prospectus supplement. Unless we otherwise specify in the applicable prospectus supplement, holders of the warrants may exercise the warrants at any time up to the specified time on the expiration date that we set forth in the applicable prospectus supplement. After the close of business on the expiration date, unexercised warrants will become void.
Holders of the warrants may exercise the warrants by delivering the warrant certificate representing the warrants to be exercised together with specified information, and paying the required amount to the warrant agent in immediately available funds, as provided in the applicable prospectus supplement. We will set forth on the reverse side of the warrant certificate and in the applicable prospectus supplement the information that the holder of the warrant will be required to deliver to the warrant agent.
Upon receipt of the required payment and the warrant certificate properly completed and duly executed at the corporate trust office of the warrant agent or any other office indicated in the applicable prospectus supplement, we will issue and deliver the securities purchasable upon such exercise. If fewer than all of the warrants represented by the warrant certificate are exercised, then we will issue a new warrant certificate for the remaining amount of warrants. If we so indicate in the applicable prospectus supplement, holders of the warrants may surrender securities as all or part of the exercise price for warrants.
Enforceability of Rights by Holders of Warrants
Each warrant agent will act solely as our agent under the applicable warrant agreement and will not assume any obligation or relationship of agency or trust with any holder of any warrant. A single bank or trust company may act as warrant agent for more than one issue of warrants. A warrant agent will have no duty or responsibility in case of any default by us under the applicable warrant agreement or warrant, including any duty or responsibility to initiate any proceedings at law or otherwise, or to make any demand upon us. Any holder of a warrant may, without the consent of the related warrant agent or the holder of any other warrant, enforce by appropriate legal action its right to exercise, and receive the securities purchasable upon exercise of, its warrants.
21
Table of Contents
The following description, together with the additional information we may include in any applicable prospectus supplements, summarizes the material terms and provisions of the units that we may offer under this prospectus. While the terms we have summarized below will apply generally to any units that we may offer under this prospectus, we will describe the particular terms of any series of units in more detail in the applicable prospectus supplement. The terms of any units offered under a prospectus supplement may differ from the terms described below. However, no prospectus supplement will fundamentally change the terms that are set forth in this prospectus or offer a security that is not registered and described in this prospectus at the time of its effectiveness.
We will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference from a current report on Form8-K that we file with the SEC, the form of unit agreement that describes the terms of the series of units we are offering, and any supplemental agreements, before the issuance of the related series of units. The following summaries of material terms and provisions of the units are subject to, and qualified in their entirety by reference to, all the provisions of the unit agreement and any supplemental agreements applicable to a particular series of units. We urge you to read the applicable prospectus supplements related to the particular series of units that we sell under this prospectus, as well as the complete unit agreement and any supplemental agreements that contain the terms of the units.
General
We may issue units comprised of one or more debt securities, shares of common stock, shares of preferred stock and warrants in any combination. Each unit will be issued so that the holder of the unit is also the holder of each security included in the unit. Thus, the holder of a unit will have the rights and obligations of a holder of each included security. The unit agreement under which a unit is issued may provide that the securities included in the unit may not be held or transferred separately, at any time or at any time before a specified date.
We will describe in the applicable prospectus supplement the terms of the series of units, including:
| • | the designation and terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may be held or transferred separately; |
| • | any provisions of the governing unit agreement that differ from those described below; and |
| • | any provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units. |
The provisions described in this section, as well as those described under “Description of Capital Stock,” “Description of Debt Securities” and “Description of Warrants” will apply to each unit and to any common stock, preferred stock, debt security or warrant included in each unit, respectively.
Issuance in Series
We may issue units in such amounts and in numerous distinct series as we determine.
Enforceability of Rights by Holders of Units
Each unit agent will act solely as our agent under the applicable unit agreement and will not assume any obligation or relationship of agency or trust with any holder of any unit. A single bank or trust company may act as unit agent for more than one series of units. A unit agent will have no duty or responsibility in case of any default by us under the applicable unit agreement or unit, including any duty or responsibility to initiate any proceedings at law or otherwise, or to make any demand upon us. Any holder of a unit may, without the consent of the related unit agent or the holder of any other unit, enforce by appropriate legal action its rights as holder under any security included in the unit.
We, the unit agents and any of their agents may treat the registered holder of any unit certificate as an absolute owner of the units evidenced by that certificate for any purpose and as the person entitled to exercise the rights attaching to the units so requested, despite any notice to the contrary. See “Legal Ownership of Securities.”
22
Table of Contents
We can issue securities in registered form or in the form of one or more global securities. We describe global securities in greater detail below. We refer to those persons who have securities registered in their own names on the books that we or any applicable trustee or depositary or warrant agent maintain for this purpose as the “holders” of those securities. These persons are the legal holders of the securities. We refer to those persons who, indirectly through others, own beneficial interests in securities that are not registered in their own names, as “indirect holders” of those securities. As we discuss below, indirect holders are not legal holders, and investors in securities issued in book-entry form or in street name will be indirect holders.
Book-Entry Holders
We may issue securities in book-entry form only, as we will specify in the applicable prospectus supplement. This means securities may be represented by one or more global securities registered in the name of a financial institution that holds them as depositary on behalf of other financial institutions that participate in the depositary’s book-entry system. These participating institutions, which are referred to as participants, in turn, hold beneficial interests in the securities on behalf of themselves or their customers.
Only the person in whose name a security is registered is recognized as the holder of that security. Global securities will be registered in the name of the depositary or its participants. Consequently, for global securities, we will recognize only the depositary as the holder of the securities, and we will make all payments on the securities to the depositary. The depositary passes along the payments it receives to its participants, which in turn pass the payments along to their customers who are the beneficial owners. The depositary and its participants do so under agreements they have made with one another or with their customers; they are not obligated to do so under the terms of the securities.
As a result, investors in a global security will not own securities directly. Instead, they will own beneficial interests in a global security, through a bank, broker or other financial institution that participates in the depositary’s book-entry system or holds an interest through a participant. As long as the securities are issued in global form, investors will be indirect holders, and not legal holders, of the securities.
Street Name Holders
We may terminate a global security or issue securities that are not issued in global form. In these cases, investors may choose to hold their securities in their own names or in “street name.” Securities held by an investor in street name would be registered in the name of a bank, broker or other financial institution that the investor chooses, and the investor would hold only a beneficial interest in those securities through an account he or she maintains at that institution.
For securities held in street name, we or any applicable trustee or depositary will recognize only the intermediary banks, brokers and other financial institutions in whose names the securities are registered as the holders of those securities, and we or any such trustee or depositary will make all payments on those securities to them. These institutions pass along the payments they receive to their customers who are the beneficial owners, but only because they agree to do so in their customer agreements or because they are legally required to do so. Investors who hold securities in street name will be indirect holders, not holders, of those securities.
Legal Holders
Our obligations, as well as the obligations of any applicable trustee or third party employed by us or a trustee, run only to the legal holders of the securities. We do not have obligations to investors who hold beneficial interests in global securities, in street name or by any other indirect means. This will be the case whether an investor chooses to be an indirect holder of a security or has no choice because we are issuing the securities only in global form.
23
Table of Contents
For example, once we make a payment or give a notice to the holder, we have no further responsibility for the payment or notice even if that holder is required, under agreements with its participants or customers or by law, to pass it along to the indirect holders but does not do so. Similarly, we may want to obtain the approval of the holders to amend an indenture, to relieve us of the consequences of a default or of our obligation to comply with a particular provision of an indenture, or for other purposes. In such an event, we would seek approval only from the legal holders, and not the indirect holders, of the securities. Whether and how the holders contact the indirect holders is up to the legal holders.
Special Considerations for Indirect Holders
If you hold securities through a bank, broker or other financial institution, either in book-entry form because the securities are represented by one or more global securities or in street name, you should check with your own institution to find out:
| • | how it handles securities payments and notices; |
| • | whether it imposes fees or charges; |
| • | how it would handle a request for the holders’ consent, if ever required; |
| • | whether and how you can instruct it to send you securities registered in your own name so you can be a holder, if that is permitted in the future; |
| • | how it would exercise rights under the securities if there were a default or other event triggering the need for holders to act to protect their interests; and |
| • | if the securities are in book-entry form, how the depositary’s rules and procedures will affect these matters. |
Global Securities
A global security is a security that represents one or any other number of individual securities held by a depositary. Generally, all securities represented by the same global securities will have the same terms.
Each security issued in book-entry form will be represented by a global security that we issue to, deposit with and register in the name of a financial institution or its nominee that we select. The financial institution that we select for this purpose is called the depositary. Unless we specify otherwise in the applicable prospectus supplement, The Depository Trust Company, New York, New York, known as DTC, will be the depositary for all securities issued in book-entry form.
A global security may not be transferred to or registered in the name of anyone other than the depositary, its nominee or a successor depositary, unless special termination situations arise. We describe those situations below under “— Special Situations When A Global Security Will Be Terminated.” As a result of these arrangements, the depositary, or its nominee, will be the sole registered owner and legal holder of all securities represented by a global security, and investors will be permitted to own only beneficial interests in a global security. Beneficial interests must be held by means of an account with a broker, bank or other financial institution that in turn has an account with the depositary or with another institution that does. Thus, an investor whose security is represented by a global security will not be a legal holder of the security, but only an indirect holder of a beneficial interest in the global security.
If the prospectus supplement for a particular security indicates that the security will be issued as a global security, then the security will be represented by a global security at all times unless and until the global security is terminated. If termination occurs, we may issue the securities through another book-entry clearing system or decide that the securities may no longer be held through any book-entry clearing system.
24
Table of Contents
Special Considerations For Global Securities
As an indirect holder, an investor’s rights relating to a global security will be governed by the account rules of the investor’s financial institution and of the depositary, as well as general laws relating to securities transfers. We do not recognize an indirect holder as a holder of securities and instead deal only with the depositary that holds the global security.
If securities are issued only as global securities, an investor should be aware of the following:
| • | an investor cannot cause the securities to be registered in his or her name, and cannot obtainnon-global certificates for his or her interest in the securities, except in the special situations we describe below; |
| • | an investor will be an indirect holder and must look to his or her own bank or broker for payments on the securities and protection of his or her legal rights relating to the securities, as we describe above; |
| • | an investor may not be able to sell interests in the securities to some insurance companies and to other institutions that are required by law to own their securities innon-book-entry form; |
| • | an investor may not be able to pledge his or her interest in the global security in circumstances where certificates representing the securities must be delivered to the lender or other beneficiary of the pledge in order for the pledge to be effective; |
| • | the depositary’s policies, which may change from time to time, will govern payments, transfers, exchanges and other matters relating to an investor’s interest in the global security. We and any applicable trustee have no responsibility for any aspect of the depositary’s actions or for its records of ownership interests in the global security. We and the trustee also do not supervise the depositary in any way; |
| • | the depositary may, and we understand that DTC will, require that those who purchase and sell interests in the global security within its book-entry system use immediately available funds, and your broker or bank may require you to do so as well; and |
| • | financial institutions that participate in the depositary’s book-entry system, and through which an investor holds its interest in the global security, may also have their own policies affecting payments, notices and other matters relating to the securities. There may be more than one financial intermediary in the chain of ownership for an investor. We do not monitor and are not responsible for the actions of any of those intermediaries. |
Special Situations When A Global Security Will Be Terminated
In a few special situations described below, a global security will terminate and interests in it will be exchanged for physical certificates representing those interests. After that exchange, the choice of whether to hold securities directly or in street name will be up to the investor. Investors must consult their own banks or brokers to find out how to have their interests in securities transferred to their own names, so that they will be direct holders. We have described the rights of holders and street name investors above.
A global security will terminate when the following special situations occur:
| • | if the depositary notifies us that it is unwilling, unable or no longer qualified to continue as depositary for that global security and we do not appoint another institution to act as depositary within 90 days; |
| • | if we notify any applicable trustee that we wish to terminate that global security; or |
| • | if an event of default has occurred with regard to securities represented by that global security and has not been cured or waived. |
The applicable prospectus supplement may also list additional situations for terminating a global security that would apply only to the particular series of securities covered by the prospectus supplement. When a global security terminates, the depositary, and neither we nor any applicable trustee, is responsible for deciding the names of the institutions that will be the initial direct holders.
25
Table of Contents
We may sell the securities to or through underwriters or dealers, through agents, or directly to one or more purchasers. A prospectus supplement or supplements (and any related free writing prospectus that we may authorize to be provided to you) will describe the terms of the offering of the securities, including, to the extent applicable:
| • | the name or names of any underwriters, if any; |
| • | the purchase price of the securities and the proceeds we will receive from the sale; |
| • | any over-allotment options under which underwriters may purchase additional securities from us; |
| • | any agency fees or underwriting discounts and other items constituting agents’ or underwriters’ compensation; |
| • | any public offering price; |
| • | any discounts or concessions allowed or reallowed or paid to dealers; and |
| • | any securities exchange or market on which the securities may be listed. |
Only underwriters named in the prospectus supplement are underwriters of the securities offered by the prospectus supplement.
If underwriters are used in the sale, they will acquire the securities for their own account and may resell the securities from time to time in one or more transactions at a fixed public offering price or at varying prices determined at the time of sale. The obligations of the underwriters to purchase the securities will be subject to the conditions set forth in the applicable underwriting agreement. We may offer the securities to the public through underwriting syndicates represented by managing underwriters or by underwriters without a syndicate. Subject to certain conditions, the underwriters will be obligated to purchase all of the securities offered by the prospectus supplement. Any public offering price and any discounts or concessions allowed or reallowed or paid to dealers may change from time to time. We may use underwriters with whom we have a material relationship. We will describe in the prospectus supplement, naming the underwriter, the nature of any such relationship.
We may sell securities directly or through agents we designate from time to time. We will name any agent involved in the offering and sale of securities and we will describe any commissions we will pay the agent in the prospectus supplement. Unless the prospectus supplement states otherwise, our agent will act on a best-efforts basis for the period of its appointment.
We may authorize agents or underwriters to solicit offers by certain types of institutional investors to purchase securities from us at the public offering price set forth in the prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future. We will describe the conditions to these contracts and the commissions we must pay for solicitation of these contracts in the prospectus supplement.
We may provide agents and underwriters with indemnification against civil liabilities related to this offering, including liabilities under the Securities Act, or contribution with respect to payments that the agents or underwriters may make with respect to these liabilities. Agents and underwriters may engage in transactions with, or perform services for, us in the ordinary course of business.
All securities we offer, other than common stock, will be new issues of securities with no established trading market. Any underwriters may make a market in these securities, but will not be obligated to do so and may discontinue any market making at any time without notice. We cannot guarantee the liquidity of the trading markets for any securities.
26
Table of Contents
Any underwriter may engage in overallotment, stabilizing transactions, short covering transactions and penalty bids in accordance with Regulation M under the Exchange Act. Overallotment involves sales in excess of the offering size, which create a short position. Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. Short covering transactions involve purchases of the securities in the open market after the distribution is completed to cover short positions. Penalty bids permit the underwriters to reclaim a selling concession from a dealer when the securities originally sold by the dealer are purchased in a covering transaction to cover short positions. Those activities may cause the price of the securities to be higher than it would otherwise be. If commenced, the underwriters may discontinue any of the activities at any time.
Any underwriters who are qualified market makers on The Nasdaq Global Market may engage in passive market making transactions in the securities on The Nasdaq Global Market in accordance with Rule 103 of Regulation M, during the business day prior to the pricing of the offering, before the commencement of offers or sales of the securities. Passive market makers must comply with applicable volume and price limitations and must be identified as passive market makers. In general, a passive market maker must display its bid at a price not in excess of the highest independent bid for such security; if all independent bids are lowered below the passive market maker’s bid, however, the passive market maker’s bid must then be lowered when certain purchase limits are exceeded.
In compliance with guidelines of the Financial Industry Regulatory Authority, or FINRA, the maximum consideration or discount to be received by any FINRA member or independent broker dealer may not exceed 8% of the aggregate amount of the securities offered pursuant to this prospectus and any applicable prospectus supplement.
27
Table of Contents
DLA Piper LLP (US), San Diego, California will pass for us upon the validity of the securities being offered by this prospectus and applicable prospectus supplement, and counsel named in the applicable prospectus supplement will pass upon legal matters for any underwriters, dealers or agents.
The consolidated financial statements of Halozyme Therapeutics, Inc. appearing in Halozyme Therapeutics, Inc.’s Annual Report (Form10-K) for the year ended December 31, 2010, and the effectiveness of Halozyme Therapeutics, Inc.’s internal control over financial reporting as of December 31, 2010 have been audited by Ernst & Young LLP, independent registered public accounting firm, as set forth in their reports thereon, included therein, and incorporated herein by reference. Such consolidated financial statements are incorporated herein by reference in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We are a reporting company and file annual, quarterly and current reports, proxy statements and other information with the SEC. We have filed with the SEC a registration statement on FormS-3 under the Securities Act with respect to the securities we are offering under this prospectus. This prospectus does not contain all of the information set forth in the registration statement and the exhibits to the registration statement. For further information with respect to us and the securities we are offering under this prospectus, we refer you to the registration statement and the exhibits and schedules filed as a part of the registration statement. You may read and copy the registration statement, as well as our reports, proxy statements and other information, at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. You can request copies of these documents by writing to the SEC and paying a fee for the copying cost. Please call the SEC at1-800-SEC-0330 for more information about the operation of the Public Reference Room. The SEC maintains an internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC, where our SEC filings are also available. The address of the SEC’s web site is “http://www.sec.gov.” We maintain a website at www.halozyme.com. Information contained in or accessible through our website does not constitute a part of this prospectus.
28
Table of Contents
The SEC allows us to “incorporate by reference” information that we file with it into this prospectus, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is an important part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we filed with the SEC prior to the date of this prospectus, while information that we file later with the SEC will automatically update and supersede the information in this prospectus. We incorporate by reference into this registration statement and prospectus the documents listed below, and any future filings we will make with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of the initial registration statement but prior to effectiveness of the registration statement and after the date of this prospectus but prior to the termination of the offering of the securities covered by this prospectus (other than current reports or portions thereof furnished under Item 2.02 or Item 7.01 of Form8-K):
| • | Our Quarterly Report on Form10-Q for the quarter ended September 30, 2011; |
| • | Our Quarterly Report on Form10-Q for the quarter ended June 30, 2011; |
| • | Our Quarterly Report on Form10-Q for the quarter ended March 31, 2011; |
| • | Our Annual Report on Form10-K for the year ended December 31, 2010; |
| • | Our Current Reports on Form8-K filed on January 10, 2011, May 6, 2011, May 11, 2011, June 16, 2011, and December 12, 2011; |
| • | Our definitive proxy statement filed pursuant to Section 14 of the Exchange Act in connection with our 2011 Annual Meeting of Stockholders filed with the SEC on March 24, 2011; |
| • | The description of our common stock set forth in Form8-A/A, filed with the SEC on November 20, 2007; and |
| • | The description of our stockholder rights plan set forth in Form8-A filed with the SEC on June 9, 2006. |
We will provide to each person, including any beneficial owner, to whom a prospectus is delivered, without charge upon written or oral request, a copy of any or all of the information that has been incorporated by reference into this prospectus but not delivered with the prospectus, including exhibits that are specifically incorporated by reference into such documents. Requests should be directed to: Halozyme Therapeutics, Inc., Attention: Investor Relations, 11388 Sorrento Valley Road, San Diego, CA 92121, telephone:(858) 794-8889.
29
Table of Contents
7,692,307 Shares

Common stock
Joint book-running managers
| J.P. Morgan | Citigroup | |
Co-managers
| Piper Jaffray | BMO Capital Markets | |
February 4, 2014