- SVRA Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
8-K Filing
Savara (SVRA) 8-KOther Events
Filed: 3 Feb 15, 12:00am
 NYSE MKT: MSTX 2015 Canaccord Genuity Rare Disease, 2015 Canaccord Genuity Rare Disease, Biopharma One on One Day Biopharma One on One Day Brian M. Culley, CEO Brian M. Culley, CEO February 3, 2015 February 3, 2015 Exhibit 99.1 |
 NYSE MKT: MSTX 2 Forward-Looking Statements This presentation includes forward-looking statements about our business prospects, financial position, and development of MST-188 and AIR001 for therapeutic use in humans. Any statement that is not a statement of historical fact should be considered a forward-looking statement. Because forward-looking statements relate to the future, they are subject to inherent risks, uncertainties and changes in circumstances that are difficult to predict. Actual events or performance may differ materially from our expectations indicated by these forward- looking statements due to a number of factors, including, but not limited to, results of our pending and future clinical studies, the timeline for clinical and manufacturing activities and regulatory approval; our dependency on third parties to conduct our clinical studies and manufacture our clinical trial material; our ability to raise additional capital, as needed; our ability to establish and protect proprietary rights related to our product candidates; and other risks and uncertainties more fully described in our press releases and our filings with the SEC, including our annual report on Form 10-K filed with the SEC on March 26, 2014. We caution you not to place undue reliance on any of these forward-looking statements, which speak only as of the date of this presentation. We do not intend to update any forward-looking statement included in this presentation to reflect events or circumstances arising after the date of the presentation, except as may be required by law. |
 NYSE MKT: MSTX Developing vepoloxamer to improve blood flow and cell membrane integrity Sickle Cell Disease (SCD) – Phase 3 enrolling (data expected Q1’16) Acute Limb Ischemia (ALI) – Phase 2 enrolling Acute Heart Failure (ADHF) – Phase 2 initiation Q2’15 Developing AIR001 to improve cardiovascular hemodynamics and exercise tolerance Heart Failure with Preserved Ejection Fraction (HFpEF) – Phase 2a 3 Corporate Overview |
 NYSE MKT: MSTX 4 Vepoloxamer (Purified Poloxamer 188) |
 API Structure: CMC: • Large, synthesized polymer with extraction process to remove undesirable (toxic) components. • Composition of matter claims pending. Administration: • IV infusion ADME: • Rapidly and predominantly cleared by kidneys (4-8h) • Ether linkages cannot be cleaved; no drug metabolites 5 Vepoloxamer Overview HO – (CH 2 CH 2 O) 79 – (CH 2 CHO) 30 – (CH 2 CH 2 O) 79 – H CH 3 | 5 NYSE MKT: MSTX |
 NYSE MKT: MSTX No Affinity for Healthy Cell Membranes… But Adheres to Damaged Cell Membranes Core of molecule adheres to hydrophobic domains on cell surface, such as damaged membranes and adhesive proteins. 6 Vepoloxamer Mechanism of Action |
 NYSE MKT: MSTX 7 Simple biophysical mechanism improves flow and membrane integrity. Vepoloxamer Pharmacodynamics Hemorheologic Inhibits cell adhesion, reduces aggregation; improves flow. Vepoloxamer Cytoprotective Seals membranes, restores integrity (e.g. gives cells time to heal). |
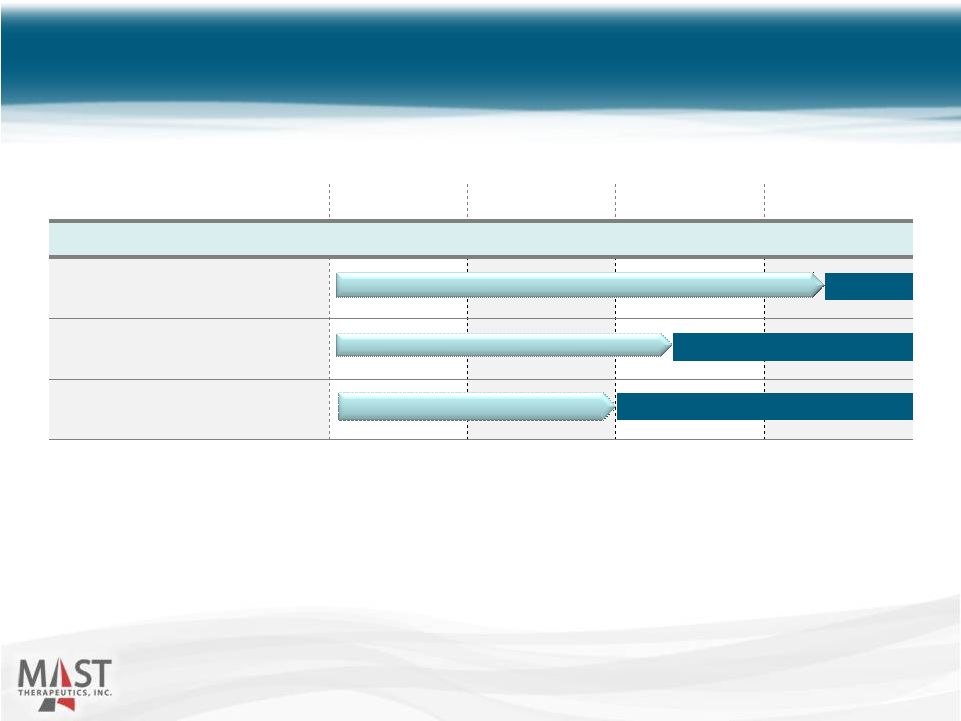 NYSE MKT: MSTX Vepoloxamer Clinical Development 8 Preclinical Phase 1 Phase 2 Phase 3 2015 Sickle Cell Disease (orphan) Acute Limb Ischemia (orphan) Acute Heart Failure Enrolling Planned initiation: 2Q 2015 Enrolling |
 NYSE MKT: MSTX 9 Sickle Cell Disease |
 NYSE MKT: MSTX 10 Overview of Sickle Cell Disease A chronic, genetic disorder and rare (orphan) disease Affects 90,000 to 100,000 people in the U.S. Characterized by severe deformation (i.e., “sickling”) of red blood cells Hallmark of disease is a “vaso-occlusive crisis” Indescribably painful condition Leading cause of hospitalization Significant unmet need No approved agents to shorten duration or severity of crisis Standard of care (hydration and analgesics) unchanged for >10 years Vaso-occlusion is associated with early death Obstructed blood flow hypoxia tissue death organ failure Average age at death; 42 years (males), 48 years (females) |
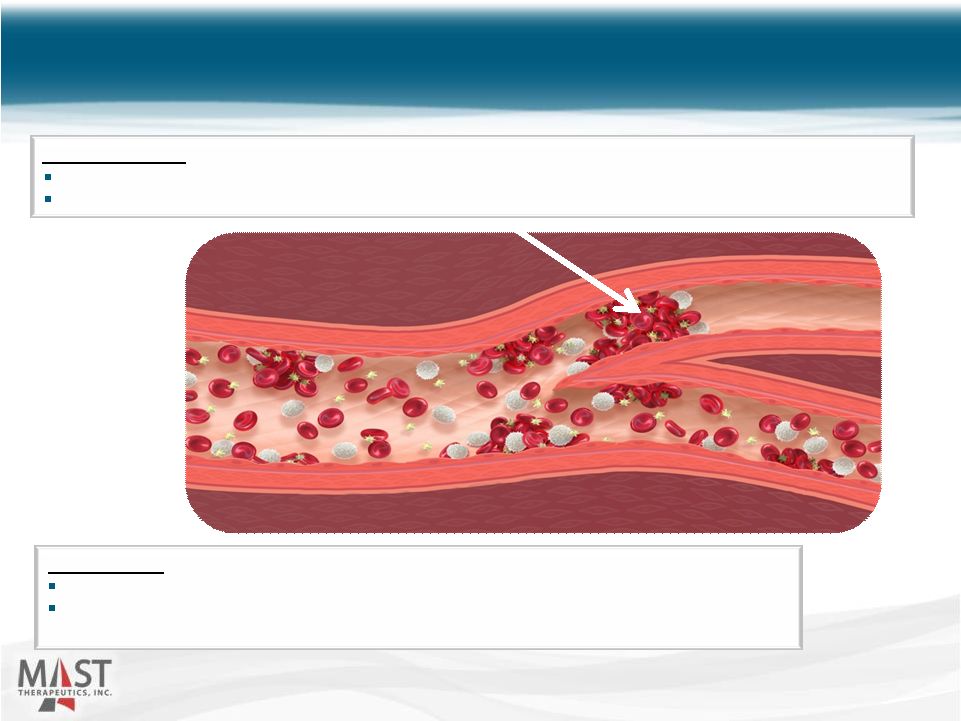 NYSE MKT: MSTX 11 Vepoloxamer: Reduces aggregation and adhesion of cells to endothelium (anti-inflammatory) Improves RBC deformability, lowers viscosity, restores flow (rheology), and reduces reperfusion injury (cytoprotection) Vaso-Occlusion: Adhesion of poorly-deformable, “sticky” cells to endothelium Entrapment of rigid, sickled cells and vessel obstruction results in ischemia and infarction Role of Vepoloxamer in Sickle Cell Disease 11 |
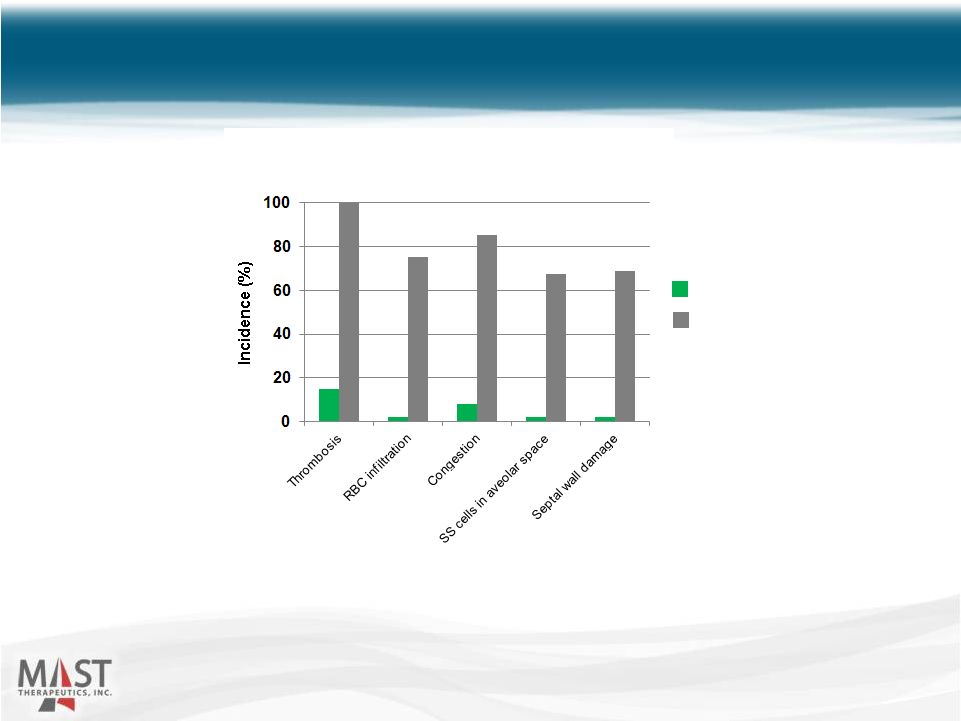 NYSE MKT: MSTX Lung pathology was compared in transgenic mice pretreated with either vepoloxamer (400 mg/kg) or saline and subject to hypoxia (5% O 2 ). (Asakura, et al.) Vepoloxamer Reduced Organ Pathology in Transgenic Sickle Mice 12 Vepoloxamer Control Lung Pathology |
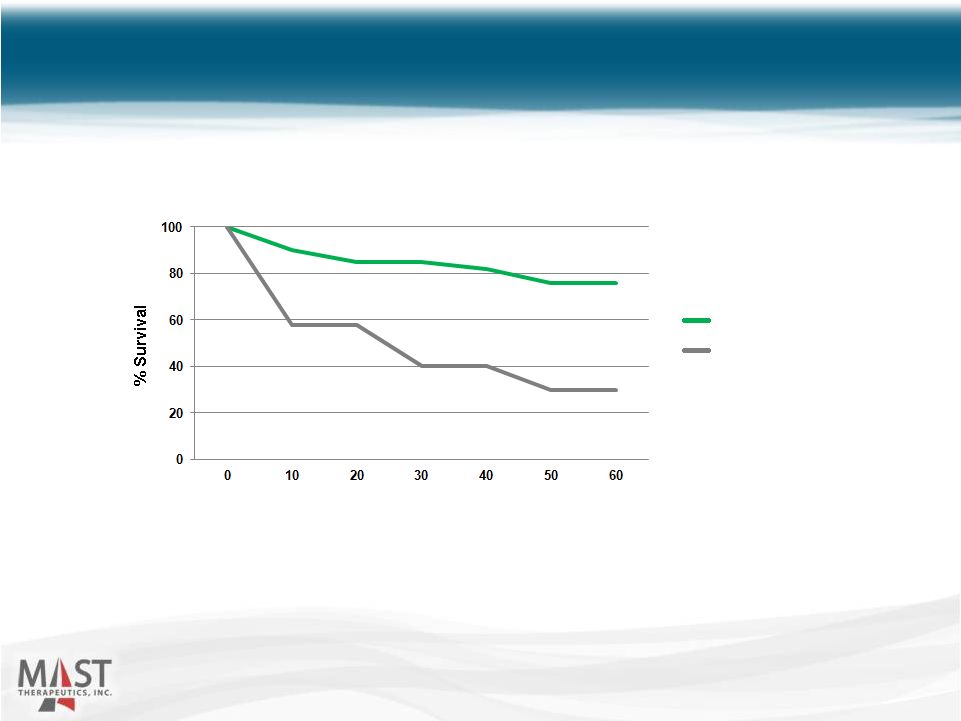 NYSE MKT: MSTX Transgenic mice pretreated with either vepoloxamer (400 mg/kg) or saline, subject to hypoxia (5% O 2 ), and monitored for survival. (Asakura, et al.) Vepoloxamer Increased Survival in Transgenic Sickle Mice 13 Vepoloxamer Control Survival of Transgenic Sickle Mice Post exposure to 5% 0 2 (60 - 100% ß S -Globin) Time (min) |
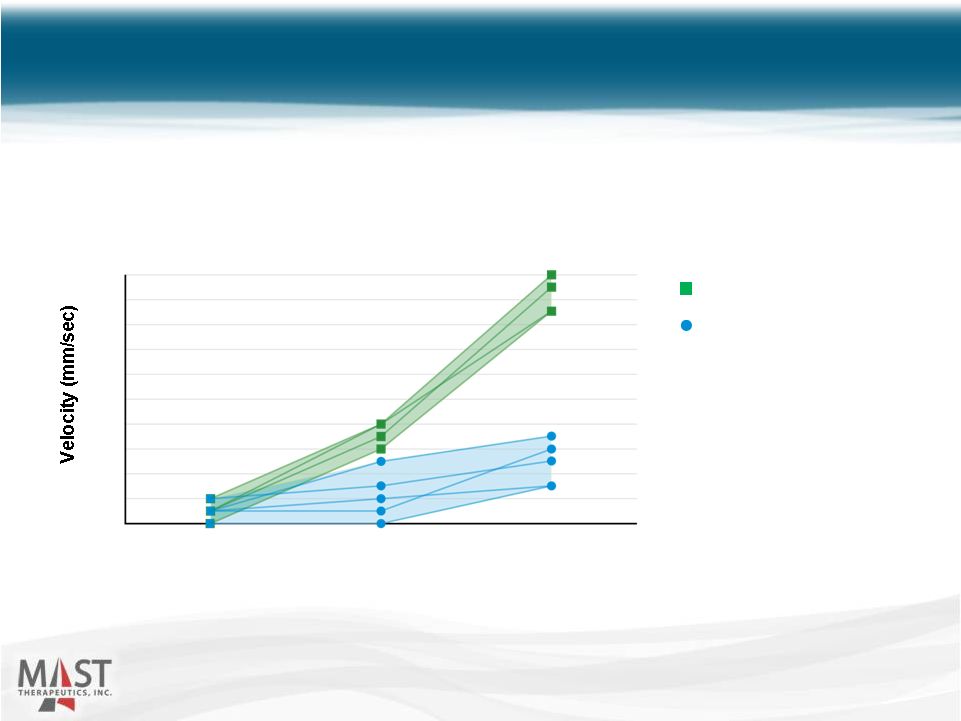 NYSE MKT: MSTX Vepoloxamer Placebo Before Infusion (Crisis Baseline) 0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 2.0 2-Hours 7-Hours After Loading Infusion After Loading Infusion Source: J. Investig. Med. 2004;52(6):402-6 (p = 0.00003) 14 Vepoloxamer improved microvascular blood flow in SCD patients in crisis 14 Vepoloxamer Improves Blood Flow Red cell velocity (mm/s) measured by video microscopy in nine sickle cell patients with vaso-occlusive crisis. |
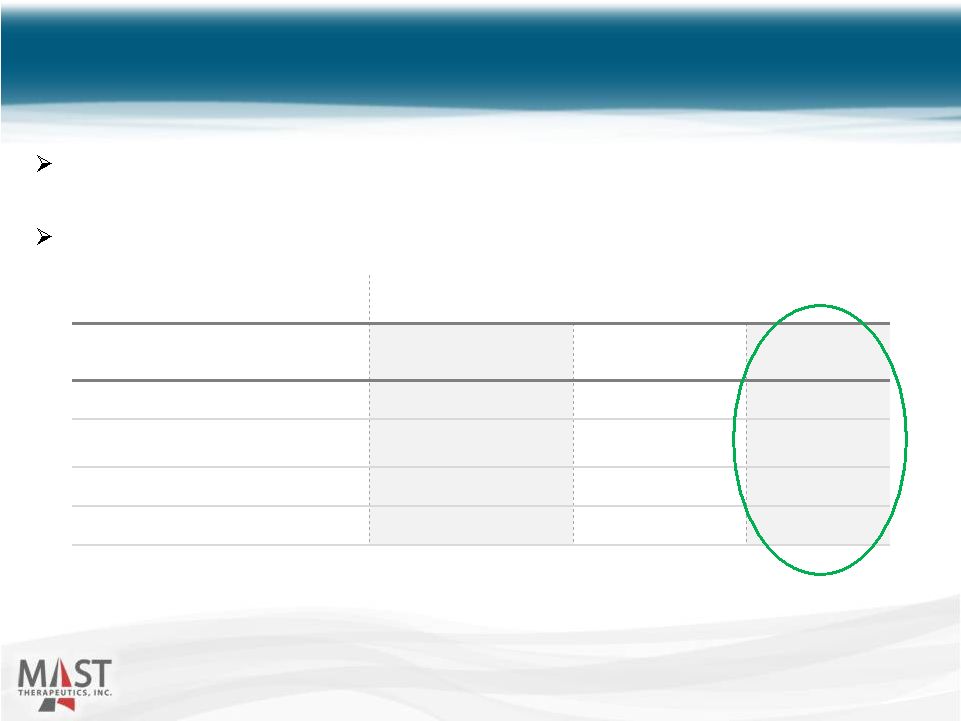 NYSE MKT: MSTX Phase 2 Study Source: Blood, September 1, 1997 – Vol 90, No. 5 * Vepoloxamer is purified poloxamer 188 15 Subjects Who Received Full Dose ± Poloxamer 188* (n=18) Placebo (n=13) p value ±± Duration of Crisis 44 hours 80 hours 0.025 Duration of Hospitalization 5 days 7 days 0.111 Total Analgesic Use 34mg 145mg 0.045 Parenteral Analgesic Use 27mg 133mg 0.022 ± Excludes patients who had drug administration errors or incomplete pain assessments (16), who withdrew consent (2) and who withdrew because of injection site pain after 15 minutes of infusion. Subjects were excluded equally (n=9) between poloxamer 188 and placebo. ±± Proportional hazards model adjusted for baseline pain. Randomized, double-blind, placebo-controlled, multi-center study in SCD patients hospitalized for crisis Significantly improved important efficacy parameters |
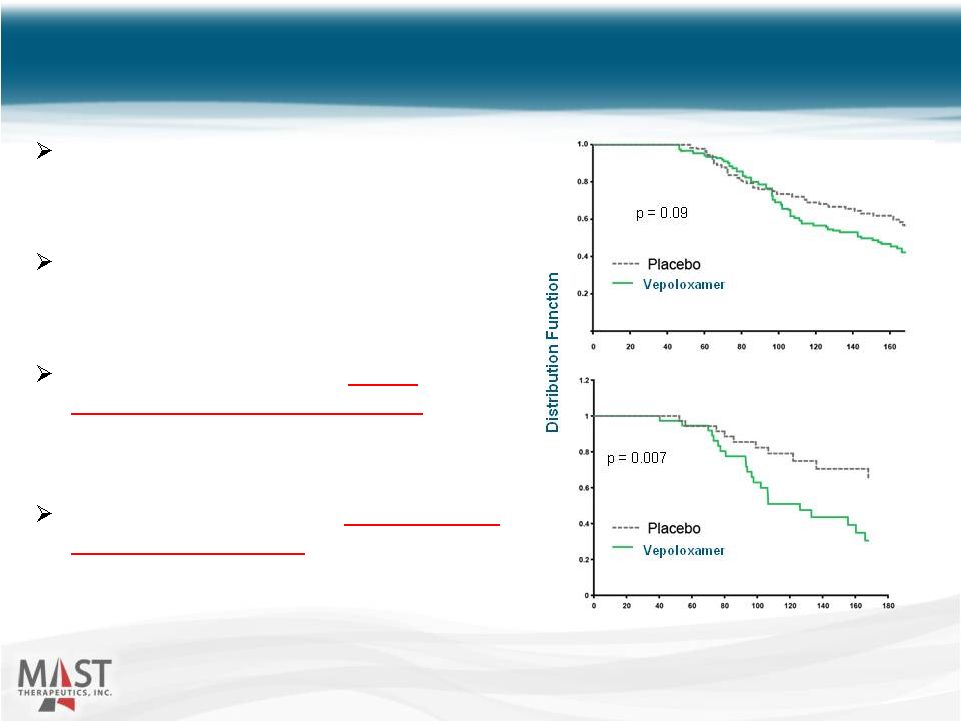 Source: JAMA, November 17, 2001 – Vol 286, No. 17 16 Randomized, double-blind, placebo- controlled, multi-center study of vepoloxamer in 350 patients with SCD. Time-to-event analysis showed consistent trend in support of earlier crisis resolution. However, prior sponsor ended enrollment at only 255 patients due to capital constraints, lowering statistical power, and, The observation period was specified to be only 168 hours, eliminating observation of any late-treatment differences (e.g. “right censoring”). Phase 3 Study 16 All Treated Patients (n=249) Hours After Randomization Children (<16 years) (n=73) NYSE MKT: MSTX |
 NYSE MKT: MSTX Randomized, Double-Blind, Placebo-Controlled, Multicenter 388 patients Standard of care +/- vepoloxamer Primary Efficacy Assessment Duration of crisis (transition off IV analgesia) No assessment of subjective pain scores Secondary Efficacy Assessments Re-hospitalization for crisis within 14 days Occurrence of acute chest syndrome Power 85% power to detect a 24-hour difference (p=0.01) 90% power to detect a 16-hour difference (p=0.05) Open-label extension Expands safety database with repeat exposures to vepoloxamer Will enroll patients who have completed treatment on EPIC 17 EPIC: Pivotal Phase 3 Study Design |
 NYSE MKT: MSTX Enrollment on-track. Top-line data expected Q1 2016. ~70 sites opened, >50 within the U.S. >33% enrolled as of Jan 6. Most Advanced New Drug in SCD Potential to be first approved drug to treat an ongoing vaso-occlusive crisis Substantial head start versus other new drugs in development for SCD Positive Factors for Regulatory Decision-Making Significant unmet need Fast Track designation Orphan Drug designation Healthcare disparity FDA declaration of SCD as an “agency priority” 18 EPIC Success Factors |
 NYSE MKT: MSTX 19 Acute Limb Ischemia (Vepoloxamer In Combination with Thrombolytics) |
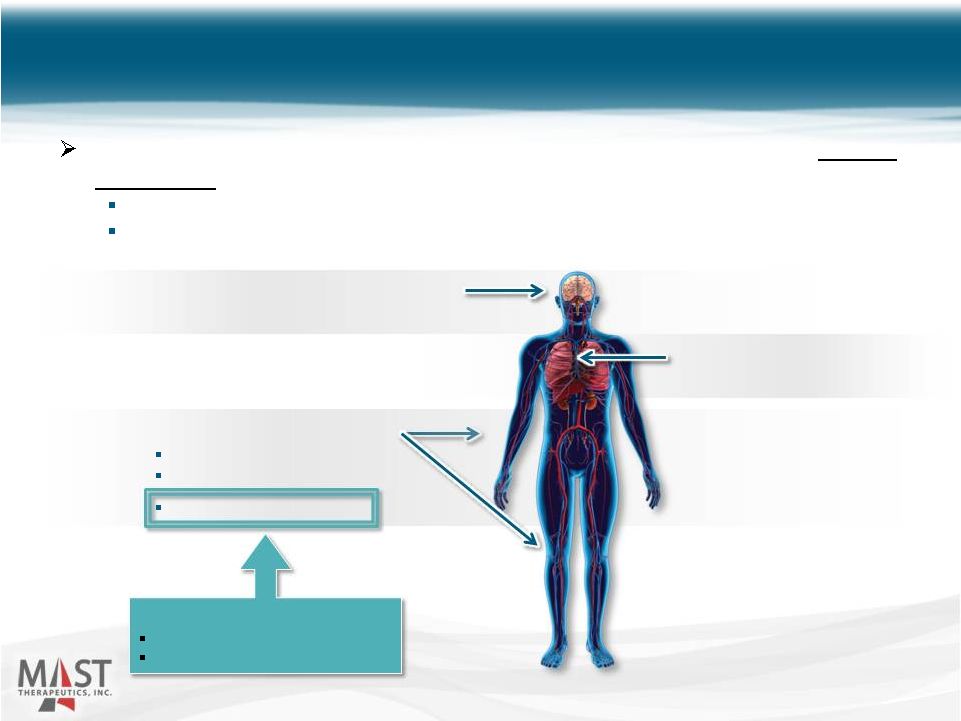 A progressive circulatory problem in which obstructed arteries reduce blood flow to tissues Thrombolytic agents (tPA) are used to treat acute complications Significant morbidity and mortality 20 Acute Ischemic Cerebrovascular Infarction (stroke) Acute Myocardial Infarction (heart attack) Peripheral Arterial Disease Intermittent Claudication Critical Limb Ischemia Acute Limb Ischemia Development Strategy: Develop initially in ALI Expand into other AD markets Overview of Occlusive Arterial Disease NYSE MKT: MSTX |
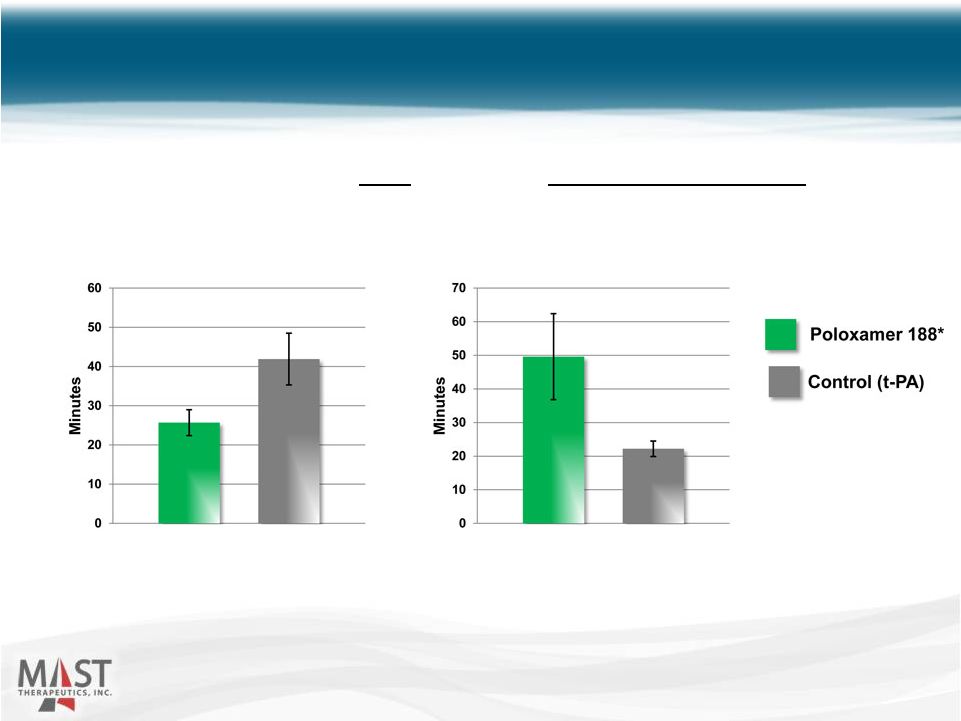 NYSE MKT: MSTX 21 Improved t-PA Effectiveness Animals randomized to t-PA (n = 10) or t-PA + poloxamer 188* (n = 10) Source: Data on file * Vepoloxamer is purified poloxamer 188 Time to Re-Occlusion Time to Reperfusion |
 NYSE MKT: MSTX 22 Parameter Poloxamer 188* Control Difference p Value N=114 Myocardial Infarct Size (median) 16% 26% 38% reduction 0.031 Myocardial Salvage (median) 13% 4% 125% increase 0.033 Ejection Fraction (median) 52% 46% 13% improvement 0.020 Incidence of Reinfarction 1% 13% 92% reduction 0.016 Synergy with Thrombolytics in Heart Attack Source: Circulation 1996; 94: 298-307 *Vepoloxamer is purified poloxamer 188 |
 NYSE MKT: MSTX 23 Clinical Proof-of-Concept Study Biomarkers Clinical outcomes Study Design Randomized, double-blind, and active-controlled (t-PA) t-PA +/- low or high dose vepoloxamer 60 subjects (20 per arm) Timing Completion of enrollment anticipated 2H 2016 ALI data can be supportive of clinical development in stroke Embolic stroke preclinical studies initiated Phase 2 Study in ALI |
 NYSE MKT: MSTX 24 Heart Failure |
 NYSE MKT: MSTX Overview of Heart Failure 25 Chronic condition characterized by decreasing heart function Heart cannot pump enough blood to meet the body’s needs Significant Unmet Medical Need Leading healthcare cost in U.S. and Europe Substantial and Growing Market Opportunity > 5 million individuals with heart failure in the U.S. Acute Decompensation Each decompensation event contributes to worsening heart failure and damage to vital organs, decreasing survival probability following the next event Vepoloxamer Membrane-sealant activity may restore weakened cardiac cell membranes, minimizing calcium overload injury Durable effect may indicate a direct improvement in cardiac function |
 NYSE MKT: MSTX In heart failure, elevated wall tension impairs lipid flow and membrane repair. This results in calcium influx and cardiac troponin leak. Vepoloxamer re-seals membranes and reduces membrane tensions, enabling lipid flow and facilitating membrane repair, thus reducing cardiac troponin and calcium overload damage. Heart Failure Development Rationale 26 |
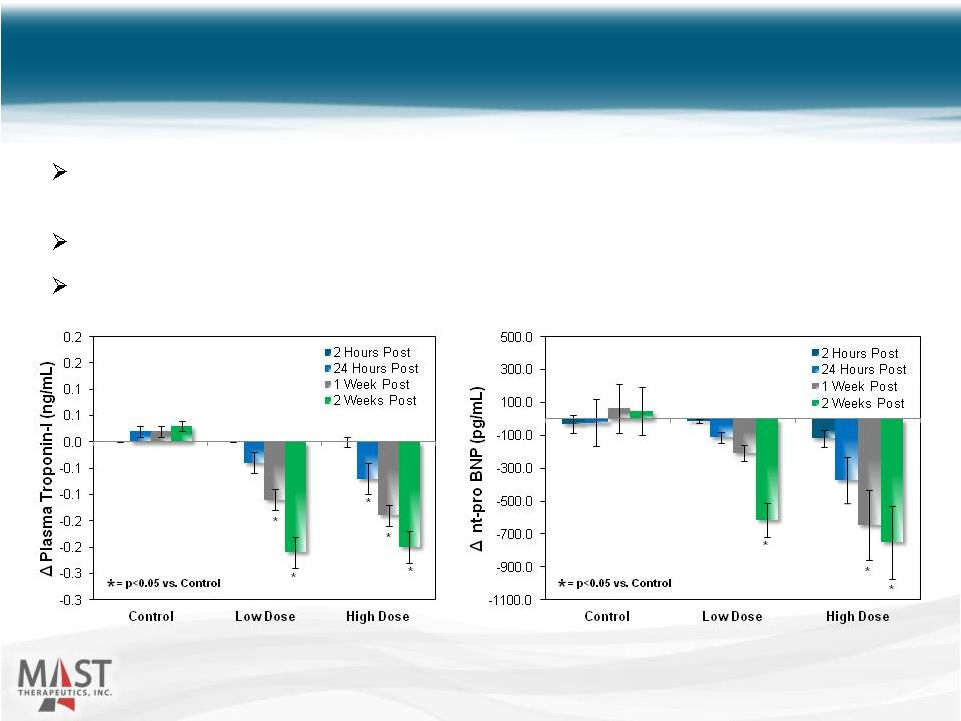 Non-clinical Model of Heart Failure 27 A single, 2h infusion improved hemodynamic parameters (LVEF, CO) and biomarkers correlated with clinical outcomes (troponin, NT-proBNP) A potentially novel mechanism, compatible with existing treatments Planning to initiate Phase 2 in acute decompensated HF in Q2 2015 Source: data on-file NYSE MKT: MSTX |
 NYSE MKT: MSTX 28 AIR001 (sodium nitrite) inhalation solution |
 NYSE MKT: MSTX AIR001 is nitrite for intermittent inhalation (via nebulizer) Beneficial effects include dilation of blood vessels and reduced inflammation Positive hemodynamic effects; reductions observed in: – pulmonary vascular resistance – pulmonary capillary wedge pressure – right atrial pressure AIR001 is being developed for Heart Failure with Preserved Ejection Fraction (HFpEF) Responsible for ~50% of heart failure hospitalizations 80% develop Pulmonary Hypertension Leads to shortness of breath, dizziness, fainting, leg swelling, etc. No approved medications 29 AIR001 |
 NYSE MKT: MSTX Three Phase 1 studies: Established MTD and safe dose level Confirmed conversion of nitrite to nitric oxide (NO) Acute improvements in hypoxia-induced pulmonary hypertension No drug-drug interaction with sildenafil One Phase 2 study: Well-tolerated, with no treatment-related serious adverse events All doses showed improvement in median pulmonary vascular resistance (PVR) & median distances obtained in the 6-minute walk test Methemoglobin levels remained normal (< 1.5%) Safety data in 124 healthy volunteers and patients with various forms of pulmonary hypertension (well-tolerated) 30 AIR001 Clinical Data |
 NYSE MKT: MSTX Supporting three institution-sponsored Phase 2a studies to: Evaluate acute hemodynamic effects of AIR001 Evaluate acute effects versus placebo on maximum oxygen consumption and exercise hemodynamics Evaluate inhaled versus intravenous administration of nitrite and safety of multiple doses of AIR001 Preliminary data anticipated 2H 2015 If positive, conduct Phase 2b proof-of-concept 31 AIR001 Clinical Development Plan |
 NYSE MKT: MSTX 32 Upcoming News & Events Initiate dosing in Phase 2a studies of AIR001 Q1 ’15 Report data from nonclinical study of vepoloxamer in embolic stroke Q1 ’15 Report data from nonclinical study of vepoloxamer in heart failure Q1 ’15 Initiate enrollment in EPIC extension study (repeat exposure) (EPIC-E) 1H ’15 Initiate enrollment in Phase 2 study of vepoloxamer in heart failure Q2 ’15 Complete enrollment in EPIC study Q4 ’15 Report data from Phase 2a study of AIR001 in HFpEF 2H ’15 Report interim safety from Phase 2 study of vepoloxamer in heart failure 2H ’15 Report EPIC study top-line data Q1 ’16 Complete enrollment in Phase 2 study of vepoloxamer in ALI 2H ’16 |
 NYSE MKT: MSTX Cash/investments at 12/31/14: $57 million Market capitalization: ~$75 million* Shares outstanding: ~159 million* Average daily volume (3 mo): ~900,000* No debt 33 * As of January 26, 2015 MSTX Financial Overview |
 NYSE MKT: MSTX A Leader in Areas of Significant Unmet Need (Vepoloxamer) Sickle Cell Disease: Most advanced new drug in development Arterial Disease: Ongoing Phase 2 in ALI with opportunity in stroke Heart Failure: Phase 2 study to begin Q2 2015 AIR001 Phase 2 program in heart failure with preserved ejection fraction Multiple phase 2a studies planned/ongoing Multiple clinical readouts anticipated within 15 months Sickle cell: Phase 3 top-line Heart failure: Phase 2 (interim safety data) HFpEF: Phase 2a studies 34 Mast Investment Summary |