Safety and Tolerability in the ITT Population
The number of treatment emergent adverse events (TEAEs), including serious adverse events, occurred with similar frequency and severity in the active arms compared to placebo. The number of subjects discontinuing due to TEAEs was as follows: continuous dosing arm; n=2, intermittent dosing arm; n=1, placebo arm; n=1.
The Company plans to meet with the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) in the coming months to discuss these data and the path forward.
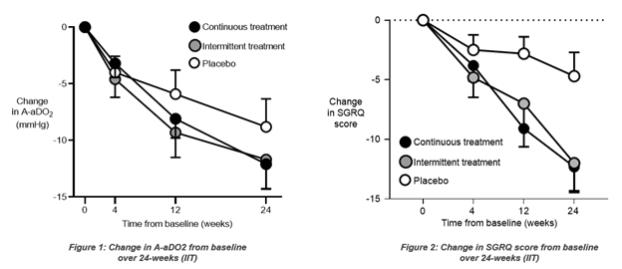
“Disappointingly, with the placebo effect stronger than anticipated, the study did not meet its primary endpoint,” said Rob Neville, Chief Executive Officer, Savara. “However, we remain encouraged about the results of IMPALA, most notably the significant improvement in SGRQ, the consistency of trends and improvements seen across the endpoints and the favorable safety profile. We are preparing to meet with the FDA and EMA to discuss the results from this study and to determine our options to seek approval based on the current data, and potentially conduct an additional study incorporating the learnings from IMPALA. It is with much gratitude that we acknowledge the patients participating in the study. It is on their behalf that we will continue to pursue our goal of bringing this important therapy to market.”
The Company intends to submit the full data set from IMPALA to a peer-reviewed scientific journal or to an upcoming medical meeting for consideration.
Conference Call and Webcast
The Company will host a conference call today at 5:30 p.m. Eastern Time (ET) / 2:30 p.m. Pacific Time (PT) to discuss these results. Shareholders and other interested parties may access the conference call by dialing (855)239-3120 from the U.S., (855)669-9657 from Canada, and (412)542-4127 from elsewhere outside the U.S. and request the “Savara Inc.” call. A live webcast of the conference call will be available online in the Investors section of Savara’s website at https://www.savarapharma.com/investors/events-presentations/.
Approximately one hour after the call, a replay of the webcast will be available on Savara’s website for 30 days, and a telephone replay will be available through June 19, 2019 by dialing (877)344-7529 from the U.S., (855)669-9658 from Canada and (412)317-0088 from elsewhere outside the U.S. and entering the replay access code 10132445.
About the IMPALA Phase 3 Clinical Study
The IMPALA study is a randomized, double-blind, placebo-controlled study designed to compare the efficacy and safety of Molgradex with placebo in patients with aPAP. The study is being conducted in 18 countries worldwide. Patients were randomized to receive treatment for 24 weeks in one of three arms: 1) Molgradex 300 µg administered once daily continuously over 24 weeks, 2) Molgradex 300 µg, and matching placebo, administered once daily in7-day intermittent cycles of each, or 3) inhaled placebo administered once daily continuously over 24 weeks. At the end of the24-week double-blind period, all treatment arms roll into a24-week open-labelfollow-on period and receive Molgradex 300 ug administered daily in7-day intermittent cycles. The primary endpoint in the study is the absolute change from baseline in