QuickLinks -- Click here to rapidly navigate through this documentAs filed with the Securities and Exchange Commission on November 16, 2001
Registration No. 333-
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
NeoGenesis Pharmaceuticals, Inc.
(Exact name of Registrant as specified in its charter)
| Delaware | | 8731 | | 04-3357990 |
| (State or other jurisdiction of incorporation or organization) | | (Primary Standard Industrial Classification Code Number) | | (I.R.S. Employer Identification No.) |
840 Memorial Drive
Cambridge, MA 02139
(617) 868-1500
(Address, including zip code, and telephone number, including
area code, of Registrant's principal executive offices)
Satish Jindal, Ph.D.
Chief Executive Officer
NeoGenesis Pharmaceuticals, Inc.
840 Memorial Drive
Cambridge, MA 02139
(617) 868-1500
(Name, address, including zip code, and telephone number,
including area code, of agent for service)
copies to:
John J. Concannon III, Esq.
Bingham Dana LLP
150 Federal Street
Boston, Massachusetts 02110
Telephone (617) 951-8000
Telecopy (617) 951-8736 | | Gerald S. Tanenbaum, Esq.
Cahill Gordon & Reindel
80 Pine Street
New York, New York 10005-1702
Telephone (212) 701-3000
Telecopy (212) 269-5420 |
Approximate date of commencement of proposed sale to public:As soon as practicable after the effective date hereof.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act, check the following box. / /
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. / /
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. / /
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. / /
If delivery of the prospectus is expected to be made pursuant to Rule 434, please check the following box. / /
CALCULATION OF REGISTRATION FEE
|
Title of Each Class of Securities to be Registered
| | Proposed Maximum Aggregate
Offering Price (1)
| | Amount of
Registration Fee (2)
|
|---|
|
| Common Stock, $.001 par value per share | | $115,000,000 | | $28,750 |
|
- (1)
- Estimated solely for the purpose of calculating the amount of the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended.
- (2)
- Calculated pursuant to Rule 457(a) based on an estimate of the proposed maximum aggregate offering price.
THE REGISTRANT HEREBY AMENDS THIS REGISTRATION STATEMENT ON SUCH DATE OR DATES AS MAY BE NECESSARY TO DELAY ITS EFFECTIVE DATE UNTIL THE REGISTRANT SHALL FILE A FURTHER AMENDMENT WHICH SPECIFICALLY STATES THAT THIS REGISTRATION STATEMENT SHALL THEREAFTER BECOME EFFECTIVE IN ACCORDANCE WITH SECTION 8(A) OF THE SECURITIES ACT OF 1933 OR UNTIL THE REGISTRATION STATEMENT SHALL BECOME EFFECTIVE ON SUCH DATE AS THE COMMISSION, ACTING PURSUANT TO SAID SECTION 8(A), MAY DETERMINE.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and we are not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
Subject to completion, dated November , 2001
Prospectus
shares
Common stock
This is an initial public offering of common stock by NeoGenesis Pharmaceuticals, Inc. We are selling shares of common stock. The estimated initial public offering price is between $ and $ per share.
We intend to apply for listing of our common stock on The Nasdaq National Market under the symbol NGPI.
|
| | Per share
| | Total
|
|---|
|
| Initial public offering price | | $ | | | $ | |
Underwriting discounts |
|
$ |
|
|
$ |
|
Proceeds to NeoGenesis, before expenses |
|
$ |
|
|
$ |
|
|
We have granted the underwriters an option for a period of 30 days to purchase up to additional shares of our common stock.
Investing in our common stock involves a high degree of risk. See "Risk factors" beginning on page 6.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
JPMorgan
Bear, Stearns & Co. Inc.
SG Cowen
, 2001
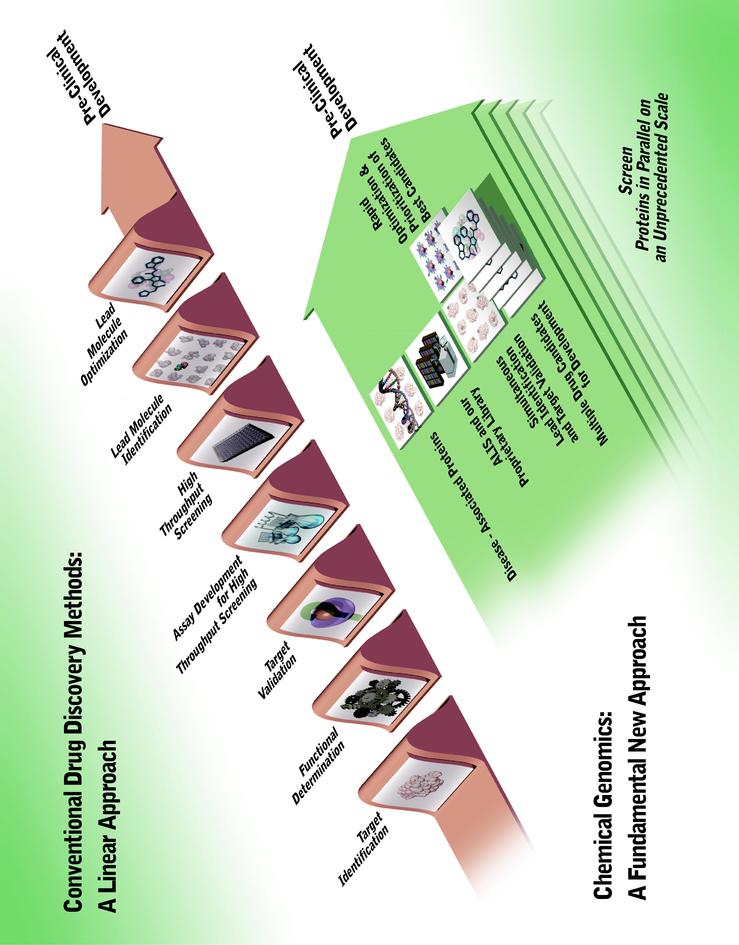
This diagram compares conventional drug discovery methods to our chemical genomics approach for the discovery of small molecule drugs.
© NeoGenesis Pharmaceuticals, Inc. 2001
Table of contents
| | Page
|
|---|
| Prospectus summary | | 1 |
| Risk factors | | 6 |
| Forward-looking statements | | 20 |
| Use of proceeds | | 21 |
| Dividend policy | | 21 |
| Capitalization | | 22 |
| Dilution | | 23 |
| Selected financial data | | 25 |
| Management's discussion and analysis of financial condition and results of operations | | 26 |
| Business | | 32 |
| Management | | 47 |
| Principal stockholders | | 55 |
| Certain transactions | | 57 |
| Description of capital stock | | 61 |
| Shares eligible for future sale | | 65 |
| U.S. Federal tax considerations for non-U.S. holders | | 67 |
| Underwriting | | 70 |
| Legal matters | | 73 |
| Experts | | 73 |
| Where you can find more information | | 74 |
| Index to financial statements | | F-1 |
"ALIS", "Nano-ALIS", "NeoGenesis", "NeoMorph" and "QSC" are our trademarks. All other trademarks or tradenames referred to in this prospectus are the property of their respective owners.
Prospectus summary
This summary highlights selected information contained elsewhere in this prospectus. This summary does not contain all the information you should consider before investing in shares of our common stock. You should read this entire prospectus carefully, including "Risk factors" beginning on page 6 and our financial statements and the notes to those financial statements beginning on page F-1, before making an investment decision.
NeoGenesis Pharmaceuticals, Inc.
We are focused on the discovery of small molecule drugs on a proteome and genome-wide scale. We believe that we are the only company currently capable of delivering small molecule drug candidates for virtually every medically important protein encoded by the human genome. We believe the rate at which we are forming discovery alliances validates the vast opportunity presented by the convergence of our technologies with genomics and proteomics. By using our chemical genomics technologies, we believe that, over time, we will derive economic benefit from hundreds of drug candidates at levels of participation ranging from single digit royalties to full ownership.
Our success has motivated leading biotechnology and pharmaceutical companies to enter into collaborations with us. Since May 2001, we have entered into seven new collaborations, including the expansion of our existing relationships with Biogen, Inc. and Schering-Plough Ltd. Our expanded relationships have further validated our technologies and increased the overall value of our portfolio of collaborations. Current collaborators include Biogen, Celltech R&D Limited, Immusol Incorporated, Merck & Co., Inc., Mitsubishi Pharma Corporation, Oxford GlycoSciences (UK) Limited, Schering-Plough and Tularik Inc.
The human genome was sequenced to gain insight into human health and to better understand the relationship between genes and diseases, referred to as genomics, and between proteins and diseases, referred to as proteomics. Proteomic and genomic efforts are expected to identify approximately 20,000 to 30,000 proteins associated with disease, of which in excess of 5,000 are expected to be potential drug targets that the industry can exploit for small molecule drug development, which is the process of developing an orally administered drug.
Conventional drug discovery technologies are limited in their ability to validate and prioritize disease-associated proteins as small molecule drug targets. In addition, conventional methods cannot increase proportionally, or scale, to efficiently exploit the wealth of information being generated from genomics and proteomics for the discovery and development of small molecule drug candidates. Moreover, given the current research and development budgets of pharmaceutical and biotechnology companies, we believe that commercial exploitation of thousands of disease-associated proteins using conventional technologies would be economically prohibitive.
Our solution
We believe that we provide a comprehensive solution for the discovery of small molecule drugs by exploiting virtually all disease-associated proteins in the human genome. Our chemical genomics technologies represent a fundamental new approach that overcomes the scaling and
1
associated limitations inherent in conventional drug discovery methods. Our industrialized chemical genomics platform is designed to scale to the genome. We believe it will:
- •
- screen disease-associated proteins in parallel on an unprecedented scale and in an unbiased fashion;
- •
- prioritize disease-associated proteins amenable to drug discovery and confirm their roles as validated drug targets for the treatment of disease;
- •
- significantly increase the number of novel small molecules directed to each disease-associated protein;
- •
- deliver drug candidates for newly validated drug targets identified from the expanding inventory of disease-associated proteins;
- •
- deliver drug candidates for known drug targets for which conventional drug discovery methods have failed;
- •
- accelerate the process of drug discovery and development; and
- •
- increase the likelihood that drug candidates will be developed successfully.
We have developed an integrated, proprietary suite of drug discovery technologies that form our scalable chemical genomics platform. At the heart of our solution is the seamless integration of our Automated Ligand Identification System, or ALIS, a powerful, scalable and broadly-applicable screening system, with our large, proprietary library of diverse, medicinally relevant small molecules. Our solution permits the discovery and development of drug candidates by identifying specific small molecules that bind to proteins. By evaluating the small molecules that bind to disease-associated proteins in relevant biological tests, or assays, our chemical genomics technologies simultaneously validate a disease-associated protein as a useful drug target and deliver small molecule drug candidates for pre-clinical and clinical development.
We believe that our solution will yield a larger inventory of small molecule drug candidates for clinical trials with a higher rate of success than achieved by conventional methods and will establish the standard for small molecule drug discovery and development.
Our strategy
Our goal is to derive economic benefit from virtually every disease-associated protein in the human genome. We plan to use our chemical genomics technologies to exploit these proteins, which include members of all medically important protein families.
Key elements of our strategy include:
- •
- broad collaborations with pharmaceutical and biotechnology companies that provide research and development funding, milestone payments and royalties;
- •
- strategic collaborations with pharmaceutical and biotechnology companies in which we retain substantial commercial rights; and
- •
- internal drug discovery, development and commercialization.
2
To accomplish our strategy we will continue to:
- •
- increase our annual screening capacity from 72 proteins to approximately 500 proteins by the end of 2003;
- •
- enhance ALIS technology;
- •
- expand our screening library;
- •
- advance our medicinal chemistry capabilities;
- •
- develop drug candidates with a greater likelihood of success; and
- •
- develop a robust intellectual property estate.
We were formed on March 13, 1997, as a Delaware corporation. Our principal executive offices are located at 840 Memorial Drive, Cambridge, Massachusetts 02139, and our telephone number is (617) 868-1500. Our web site address iswww.neogenesis.com. Information contained in our web site is not a part of this prospectus.
3
The offering
Common stock offered by NeoGenesis: shares
Common stock to be outstanding after this offering: shares
Proposed Nasdaq National Market symbol: NGPI
Use of proceeds
For research and development activities relating to our collaborations and internal drug development programs, the scaling and further development of our drug discovery technologies and other general corporate purposes. See "Use of proceeds" on page 21 for more information on our use of the net proceeds from this offering.
The number of shares of our common stock that will be outstanding after this offering is based on the number of shares outstanding as of October 31, 2001 and excludes:
- •
- 2,646,875 shares of our common stock issuable upon the exercise of stock options outstanding as of October 31, 2001 with a weighted average exercise price of $0.49 per share, of which options to purchase 720,500 shares of our common stock were then exercisable, and
- •
- 1,074,000 shares of our common stock reserved for future grant under our 1997 stock option plan, as amended, as of October 31, 2001.
Unless specifically stated otherwise, the information in this prospectus:
- •
- assumes no exercise of the underwriters' over-allotment option;
- •
- assumes an initial public offering price of $ per share, the midpoint of the initial public offering price range indicated on the cover of this prospectus;
- •
- reflects the automatic conversion of all shares of our preferred stock outstanding as of October 31, 2001 into 14,359,145 shares of our common stock upon the completion of this offering;
- •
- reflects the automatic conversion of a $2.0 million convertible note payable into shares of our common stock upon the completion of this offering;
- •
- reflects a five-for-one split of our common and preferred stock outstanding as of October 5, 2000; and
- •
- reflects the filing, prior to the completion of this offering, of our Fourth Amended and Restated Certificate of Incorporation, referred to in this prospectus as our certificate of incorporation, and the adoption of our Amended and Restated By-Laws, referred to in this prospectus as our by-laws, implementing the provisions described under "Description of capital stock."
4
Summary financial data
The summary financial data set forth below should be read in conjunction with our financial statements and the related notes to our financial statements and "Management's discussion and analysis of financial condition and results of operations" contained elsewhere in this prospectus. The unaudited pro forma basic and diluted net loss per share data give effect to the conversion of our convertible preferred stock from the date of original issuance. The unaudited pro forma balance sheet data reflect 5,846,625 shares of our common stock outstanding at September 30, 2001, the automatic conversion of all outstanding shares of our preferred stock into 14,341,075 shares of our common stock upon the completion of this offering and the automatic conversion of a $2.0 million convertible note payable into shares of our common stock upon the completion of this offering. The pro forma as adjusted balance sheet data reflect the pro forma adjustments and the sale of the shares of our common stock offered by this prospectus at an assumed initial public offering price of $ per share after deducting the underwriting discount and estimated offering expenses payable by us.
| |
|---|
| | Period from
inception
(March 13,
1997)
through
December 31,
1997
| |
| |
| |
| |
| |
| |
|---|
| | Year ended December 31,
| | Nine months ended
September 30,
| |
|---|
(in thousands, except share and per share data)
| |
|---|
| | 1998
| | 1999
| | 2000
| | 2000
| | 2001
| |
|---|
| |
|---|
| Statements of operations data: | | | | | | | | | | | | | |
| Total revenue | | $ — | | $ 568 | | $ 1,867 | | $ 1,594 | | $ 759 | | $ 2,647 | |
| Total operating expenses | | 1,169 | | 3,817 | | 4,848 | | 6,899 | | 4,740 | | 11,043 | |
| | |
| |
| Loss from operations | | (1,169 | ) | (3,249 | ) | (2,981 | ) | (5,305 | ) | (3,981 | ) | (8,396 | ) |
| | |
| |
| Net loss | | $(1,169 | ) | $(3,276 | ) | $(3,139 | ) | $(5,390 | ) | $(4,046 | ) | $(7,661 | ) |
| | |
| |
| |
| |
| |
| |
| |
| | |
| |
| Net loss per common share: | | | | | | | | | | | | | |
| | Basic and diluted | | $ (0.59 | ) | $ (1.13 | ) | $ (0.86 | ) | $ (1.14 | ) | $ (0.91 | ) | $ (1.40 | ) |
| | |
| |
| |
| |
| |
| |
| |
| | Unaudited pro forma basic and diluted | | | | | | | | $ (0.55 | ) | | | $ (0.45 | ) |
| | | | | | | | |
| | | |
| |
| | |
| |
Shares used in computing basic and diluted net loss per common share |
|
1,983,811 |
|
2,905,651 |
|
3,665,247 |
|
4,722,259 |
|
4,424,509 |
|
5,466,846 |
|
| | |
| |
| |
| |
| |
| |
| |
| Shares used in computing unaudited pro forma basic and diluted net loss per common share | | | | | | | | 9,844,493 | | | | 17,103,824 | |
| | | | | | | | |
| | | |
| |
| |
|
September 30, 2001
(in thousands)
| | Actual
| | Pro forma
| | Pro forma
as adjusted
|
|---|
|
| Balance sheet data: | | | | | | | |
| Cash and cash equivalents | | $ | 37,896 | | $37,896 | | $ |
| Working capital | | | 33,423 | | 33,423 | | |
| Total assets | | | 44,083 | | 44,083 | | |
| Long-term liabilities, less current maturities | | | 2,547 | | 528 | | |
| Convertible preferred stock | | | 54,250 | | — | | — |
| Total stockholders' equity (deficit) | | $ | (19,033 | ) | $37,236 | | $ |
|
5
Risk factors
You should carefully consider the risks described below and all other information contained in this prospectus before making an investment decision. If any of the following risks, as well as other risks and uncertainties that we have not yet identified or that we currently think are immaterial, actually occur, our business, financial condition and results of operations could be materially and adversely affected. In that event, the trading price of shares of our common stock could decline and you may lose part or all of your investment.
Risks related to our business
Our success depends on our ability to apply our chemical genomics technologies on an industrial scale.
Our success as a company depends on our ability to rapidly screen a large number of proteins to identify potential small molecule drug candidates. In order to accomplish this successfully, we will be required to implement our chemical genomics technologies on a larger scale, which will include building and implementing additional ALIS workstations and hiring additional qualified personnel.
Our technologies may fail to scale to the level necessary to successfully pursue our business strategy and sustain our growth. In addition, our technologies will have to scale to meet the anticipated demand of our collaboration agreements in the screening of proteins supplied to us by our collaborators. We do not have experience in scaling our technologies to meet these expected demands. If we are unable to effectively scale our technologies, we may be in breach of our existing collaboration agreements and may not be able to enter into new collaboration agreements in the future. In addition, we may not be able to pursue our internal drug discovery efforts.
Our chemical genomics technologies are unproven and may not be commercially successful.
The use of chemical genomics for drug discovery is unproven. Our current focus is to develop our chemical genomics technologies to probe the human genome in an effort to identify small molecule compounds that might be useful as drugs. Our chemical genomics technologies may not be successful because of, among other reasons, the size and complexity of the undertaking, the uncertainty that our library contains relevant and novel small molecule compounds, the possibility that using small molecule compounds to identify drugs against all disease-associated proteins is not a useful way to study biological processes such as disease or the possibility that our collaborators may adopt alternative technologies of our competitors.
We may not identify small molecule drug candidates that we or our collaborators may develop into commercial drugs.
We may not identify small molecule drug candidates with the most commercial potential. If we or our collaborators fail to allocate resources towards those drug candidates with the most commercial potential, if any, our business will be materially affected. Further, our collaborators may elect not to develop products arising out of our collaborative arrangements or to devote sufficient resources to the development, manufacturing, marketing or sale of these products, if any. If any of these events occur, we may not achieve our business objectives.
6
Our success will depend on our ability to grow and to manage our growth.
We are an early stage company. We have experienced and expect to continue to experience growth in the number of our employees and the scope of our operating and financial systems. We expect our growth will continue to place a significant strain on our operational, managerial, human and financial resources. Our ability to compete effectively will depend, in large part, on our ability to hire, train and retain additional management and professional, scientific and technical personnel and our ability to expand, improve and effectively use our operating, management, business development and financial systems to accommodate our expanded operations. The physical expansion of our operations may lead to significant costs and may divert our management and business development resources. If we fail to effectively anticipate, implement or manage the changes required to sustain our growth, we may not be able to compete successfully.
We have a history of substantial net losses and cannot predict when we will become profitable, if at all.
We were incorporated in March 1997 and have a limited operating history upon which an investor may evaluate our operations and future prospects. We have incurred net losses since our inception, including net losses of $5.4 million for the year ended December 31, 2000 and $7.7 million for the nine months ended September 30, 2001. As of September 30, 2001, we had an accumulated deficit of $20.6 million. We expect to continue to incur significant operating losses in future periods and cannot predict when we will become profitable, if at all. We expect to make substantial expenditures to further develop our drug discovery programs and technologies. We expect that our rate of spending will accelerate as a result of the increased costs and expenses associated with our collaborations and with our internal drug discovery programs and technologies. In particular, under some of our recent collaboration agreements, we are required to bear up to 50% of the costs associated with the development of small molecule drug candidates identified in the collaboration and we will be required to bear 100% of the costs associated with the development of small molecule drug candidates that we choose to pursue independently. We expect to enter into similar collaboration agreements in the future. While we expect our revenues to be derived from milestone payments, royalties and other fees under our existing and possible future collaborative arrangements, our revenue and profit potential is unproven and our limited operating history makes our future operating results difficult to predict. Additionally, our quarterly revenues and operating results are difficult to predict and may fluctuate significantly from quarter to quarter for numerous reasons, including the timing of the receipt of milestone payments and royalties, both of which are not within our control. Consequently, an investor in our common stock may be unable to evaluate our future operations or drug products, if any.
We will require funds in addition to the proceeds of this offering that we may not be able to raise.
We will require substantial funds to pursue our business strategy. Additional financing may not be available when we need it or, if available, it may not be on terms that are favorable to us. If we are unable to obtain adequate funding on a timely basis, we may be required to cease one or more of our drug discovery programs. We could be required to seek funds through arrangements with collaborators or others that may require us to relinquish certain rights to our technologies or drug candidates, which we would otherwise pursue on our own.
7
Our collaboration agreements are of limited scope and duration and if we are unable to expand the scope of our existing collaborations or enter into new ones, our revenues will be negatively impacted and our drug discovery programs may be slowed or halted.
Our collaboration agreements are of limited scope and duration and may be terminated by our collaborators on little or no notice. We may not develop long-term relationships with our collaborators that could lead to expanded or additional collaborations. If we are unable to maintain or expand our existing collaborations or enter into new ones, we may have reduced revenues and be forced to slow or halt our drug discovery programs. In addition, each collaboration will involve the negotiation of terms that may be unique to each collaborator. These business development efforts may take an extended period of time and require significant attention of our management and may not result in collaborations.
Our existing collaboration agreements are, and we would expect any future collaboration agreements to be, based on our success in using our chemical genomics technologies. Under our collaboration agreements, we expect to receive revenue for the research and development of products based on achievement of specific milestones and the development and commercialization of successful products. Achieving these milestones will depend, in part, on the efforts of our collaborators as well as our own efforts. If we or any of our collaborators fail to meet specific milestones, then the collaboration can be terminated, which could result in a decrease in our future revenues.
Conflicts with our collaborators and strategic partners could harm our business.
Disagreements with our existing or future collaborators or strategic partners, such as clinical development partners, manufacturing partners or sales and marketing partners, could develop over rights to our intellectual property with respect to our drug discovery programs. Any conflict with our collaborators or strategic partners could reduce our ability to enter into future collaboration or strategic partnering arrangements and negatively impact our relationship with existing collaborators and strategic partners, which could reduce our revenues and delay or terminate our drug discovery programs.
If we are unable to obtain patent protection for our discoveries, most significantly the small molecule drug candidates that we identify and our chemical genomics technologies, our business will be adversely affected.
Our success depends on our ability to obtain patent protection for the small molecule drug candidates that we may develop and for our chemical genomics technologies. Our patent positions and those of other pharmaceutical and biotechnology companies are generally uncertain and involve complex legal, scientific and factual questions. There is significant uncertainty both in the United States and abroad regarding the patentability of drug discovery processes and the scope of patent protection available for our drug discovery processes. There is a substantial backlog of biotechnology patent applications at the U.S. Patent and Trademark Office and no clear policy has emerged regarding the breadth of claims covered in biotechnology patents. The biotechnology patent situation outside the United States is even more uncertain and is currently undergoing review and revision in many countries. These proposals and other changes in patent laws in the United States and other countries may result in changes in, or interpretations of, the patent laws that will adversely affect our patent
8
position. If we are unable to obtain patent protection for our discoveries, most significantly the small molecule compounds that we identify and our chemical genomics technologies, our business will be adversely affected.
It is difficult and costly to protect our intellectual property rights and we cannot ensure their protection.
The protection of our intellectual property, including obtaining patents for the small molecule compounds that we may develop and our chemical genomics technologies, is a costly and time-consuming process. We may fail to secure patent protection relating to any of our existing or future drug candidates, discoveries or technologies despite the expenditure of considerable resources. Our success depends in part on the establishment of patent protection for small molecule compounds, methods of treatment, proteins and related technologies we discover or invent. Consequently, we intend to continue our efforts in applying for patent protection and prosecuting pending and future patent applications. Historically, such efforts have required the expenditure of considerable time and money, and we expect that we will be required to expend significant amounts to pursue our intellectual property strategy. If future changes in U.S. or foreign patent laws complicate or hinder our efforts to obtain patent protection, the costs associated with patent prosecution may increase significantly.
We rely on trade secret protection to protect our confidential and proprietary information. However, trade secrets are generally difficult to protect. While we have entered into confidentiality agreements with our employees and our collaborators who are provided data or materials, we may not be able to prevent their disclosure of these data or materials. Others may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets or disclose our technologies. We may not be able to protect our trade secrets.
With respect to our software and the specific content of our library of small molecule compounds, we rely primarily on a combination of copyright, trademark and trade secret laws, confidentiality procedures and contractual provisions to protect our proprietary rights. There can be no assurance that our trademarks or copyrights will offer any protection or that they will not be challenged, invalidated or circumvented. Furthermore, there can be no assurance that others will not develop technologies that are similar or superior to our existing technologies or the content of our library. Unauthorized parties may attempt to copy aspects of our software, our technologies or our library or to obtain and use information that we regard as proprietary. Policing unauthorized use of our materials or information may be difficult. In addition, the laws of some foreign countries do not protect proprietary rights as fully as do U.S. laws. There can be no assurance that our means of protecting our proprietary rights in the U.S. or abroad will be adequate.
To the extent we are unable to protect our intellectual property, our investment in our chemical genomics technologies and compositions of small molecule compounds may not yield the benefits we expect. The degree of patent protection afforded to pharmaceutical inventions is uncertain and any patents that may be issued concerning our potential products and services will be subject to this uncertainty. There can be no assurance that other parties will not develop competitive products and services outside the protection that may be afforded by the claims of our patents.
9
We may be unable to develop any of the small molecule compounds in our library that we discover using our drug discovery technologies because we may be unable to obtain certain rights from third parties at a reasonable price.
Our strategy depends upon identifying small molecule compounds that are suitable as drugs for virtually all disease-associated proteins in the human genome. We cannot assure you that a disease-associated protein with which any specific small molecule drug candidate interacts or the use of that small molecule compound as a therapy for disease is not or will not be subject to the intellectual property rights of third parties. Furthermore, we cannot assure you that any specific small molecule drug candidate's role in disease or the tests used to determine whether the small molecule compound is useful as a medical treatment is not or will not be subject to the intellectual property rights of third parties. There are many issued patents and published patent applications that disclose proteins, methods of treating diseases associated with such proteins, tests for measuring the utility of small molecule compounds and individual therapeutic approaches against diseases associated with such proteins. If any of our activities with respect to a specific small molecule compound are subject to the intellectual property rights of third parties, we will not be able to commercially develop that small molecule compound without obtaining a license or challenging the applicable patent or other intellectual property right. We cannot assure you that a license will be available on commercially reasonable terms, if at all, or that we could successfully challenge any applicable intellectual property right.
We may be unable to commercialize any particular small molecule compound in our library or any potential small molecule drug candidate identified from using our library.
Our success relies, in part, on the novelty of the small molecule compounds in our library or small molecule compounds that we develop based upon our library. We have not undertaken a comprehensive review of all ten million small molecules of our current library to determine whether each small molecule is either novel or patentable. We cannot assure you that any specific small molecule compound in our library or any small molecule drug candidate we may later select for further analysis is not subject to a patent held by another party or even that we would have exclusive rights to either the small molecule compound itself or its use as a drug. Chemists often claim small molecule compounds by reference to large classes of small molecule compounds identified by chemical formula or characteristics. In addition, many patents claim the process by which intellectual property rights to small molecule compounds are made, or may claim small molecule compounds by describing the process by which they are made. It is probable that some subsets of the small molecule compounds in our library are or will be covered by patents owned by third parties. Furthermore, the best drug candidates selected from our library or even the best small molecule compound to treat any specific condition resulting from or relating to a disease-associated protein may be subject to patents owned by third parties. We may be unable to commercialize a portion of our library or specific drug candidates we discover without a license, which we cannot assure that we will be able to obtain on commercially acceptable terms, if at all. If we do not have or cannot obtain exclusivity with respect to any small molecule compound we select from our library or any specific drug candidate, the likelihood of it being developed as a drug decreases dramatically.
10
The availability of novel genomic and proteomic data may decrease in the future and may adversely affect our ability to discover novel small molecule compounds.
We rely on the continuing availability of existing genomic and proteomic data and the continuous generation of new genomic and proteomic data for the discovery of new disease- associated proteins. Our strategy relies on the assumption that thousands of new disease-associated proteins are yet to be discovered. Since many companies and government or public agencies are analyzing the genomic and proteomic data that is currently publicly available and are generating additional publicly available information, it has become increasingly difficult for other entities to be the first to discover novel proteins through the analysis of this data. Companies and government or public agencies have already mapped and made available significant portions of the genome, and the flow of novel genomic and proteomic data will likely decrease significantly in the future and may impact our ability or the ability of our collaborators to establish a proprietary patent position.
We may incur substantial costs or lose important rights as a result of litigation or other proceedings relating to patent and other intellectual property rights.
The defense and prosecution of intellectual property rights, U.S. Patent and Trademark Office interference proceedings and related legal and administrative proceedings in the United States and elsewhere involve complex legal and factual questions. As a result, these proceedings are costly and time-consuming and their outcome is uncertain. Litigation may be necessary to:
- •
- assert claims of infringement;
- •
- enforce our issued and licensed patent and our pending patent applications;
- •
- protect trade secrets or know-how; or
- •
- determine the enforceability, scope and validity of the proprietary rights of others.
If we become involved in any litigation, interference or other administrative proceedings, we will incur substantial expense and it will divert the efforts of our technical and management personnel. An adverse determination may subject us to significant liabilities or require us to seek licenses that may not be available from third parties on commercially reasonable terms, if at all. We or our collaborators may be restricted or prevented from developing and commercializing our drug product candidates, if any, in the event of an adverse determination in a judicial or administrative proceeding, or if we fail to obtain necessary licenses. Any of these outcomes could harm our business.
We are aware that, in several countries, another entity has filed patent applications relating to a technique for creating a mass-encoded combinatorial library. We believe that the method described in those applications is different from the method that we employ, and at this time, no patents on this method have been issued to this other entity. If valid patents were to be issued to this other entity in countries that are important to our business strategy and if it were deemed that we infringed on those patents, we would be required to alter our method or obtain a license in order to expand and use our mass-encoded library. If we were unable to obtain a license on commercially acceptable terms, our ability to conduct our business would be significantly impaired.
In addition, some of our competitors have, or are affiliated with companies having, substantially greater resources than we and those competitors may be able to sustain the costs
11
of complex patent litigation to a greater degree and for longer periods of time than we. Uncertainties resulting from the initiation and continuation of any patent or related litigation could have a material adverse effect on our ability to compete in the marketplace pending resolution of the disputed matters.
As is commonplace in the biotechnology and pharmaceutical industry, we employ individuals who were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. To the extent our employees are involved in research areas which are similar to those areas in which they were involved at their former employers, we may be subject to claims that such employees and/or we have inadvertently or otherwise used or disclosed the alleged trade secrets or other proprietary information of the former employers. Litigation may be necessary to defend against such claims, which could result in substantial costs and be a distraction to management and which may have a material adverse effect on us, even if we are successful in defending such claims.
Our patent applications may not result in issued patents that are enforceable.
We have two issued U.S. patents and ten U.S. patent applications pending. In addition, we have five patent applications pending elsewhere. There can be no assurance that additional patents will be issued to us or our licensors as a result of their pending applications or that, if issued, such patents will be sufficiently broad to afford protection against competitors with similar technologies. There can be no assurance that any patents issued to us or our collaborative partners, or for which we have license rights, will not be challenged, invalidated or circumvented, or that the rights granted thereunder will provide competitive advantages to us.
To date, we have not developed any drug products and may not be able to successfully develop any drug products.
A part of our business strategy is to develop and commercialize small molecule drug candidates discovered through our collaborations and that we discover independently. Our ability to develop and commercialize drug products in the future will depend on our:
- •
- successfully completing pre-clinical and clinical testing;
- •
- obtaining necessary regulatory approvals;
- •
- effectively deploying sales and marketing resources;
- •
- producing and manufacturing drug products;
- •
- successfully obtaining intellectual property protection for our drug products; and
- •
- entering into arrangements with third parties to provide any of these functions.
Each of these will require significant expenditures of our resources, including financial and managerial resources, and may not result in a successful drug product. Our inability to develop effectively any drug product will have a negative impact on our business.
12
Pre-clinical development and clinical trials for our potential small molecule drug candidates, if any, are expensive and time consuming and their outcome is uncertain.
Before we can obtain regulatory approval for the commercial sale of any drug candidate that we wish to develop, we will be required to complete pre-clinical development and extensive clinical trials in humans to demonstrate its safety and efficacy. Each of these clinical trials requires the investment of substantial expense and time. In addition, under some of our collaboration agreements, we will be required to share in the expense incurred by our collaborators in obtaining regulatory approval for the potential drug products developed.
Success in pre-clinical development and early clinical trials does not ensure that large-scale trials will be successful nor does it predict final results. Acceptable results in early trials may not be repeated in later trials. A number of companies in the pharmaceutical and biotechnology industry have suffered significant setbacks in advanced clinical trials, even after promising results in earlier clinical trials. Negative or inconclusive results or adverse medical events during a clinical trial could cause that trial to be redone or terminated. In addition, failure to construct appropriate clinical trial protocols could result in the test or control group experiencing a disproportionate number of adverse events and could cause a clinical trial to be redone or terminated.
We currently have no experience in conducting pre-clinical and clinical development activities.
We currently have no experience in conducting pre-clinical and clinical development activities. In order to successfully develop and commercialize our drug products, if any, we will be required to develop the internal capability to conduct pre-clinical and clinical development activities. In the event we are unable to do so, we may rely in large part on our collaborators and third-party clinical research organizations to design and conduct most of these activities. Our inability to contract for any necessary clinical activities on acceptable terms would impair or delay our ability to complete our drug development programs, which could adversely affect our business. If we rely on collaborators and third parties for pre-clinical and clinical development activities, it may reduce our control over these activities and may make us dependent upon these parties. We may choose to, or may be required to, suspend, repeat or terminate our clinical trials if they are not conducted in accordance with regulatory requirements, the results are negative or inconclusive or the clinical trials are not well designed.
Since we may be subject to extensive and uncertain government regulatory requirements, we may be unable to obtain government approval of any drugs in a timely manner.
Any drugs that we may develop will be subject to an extensive regulatory approval process by the U.S. Food and Drug Administration, or FDA, and comparable agencies in other countries. The regulation of new drugs is extensive and the required pre-clinical and clinical testing is lengthy and expensive. We may not obtain FDA approvals in a timely manner, or at all. We or our collaborators may encounter significant delays or excessive costs in our efforts to secure necessary approvals or licenses. Even if FDA regulatory approvals are obtained, the FDA extensively regulates manufacturing, labeling, distributing, marketing, promotion and advertising after drug approval. Regulatory requirements ultimately imposed on these and other areas could adversely affect our ability to clinically test, manufacture and market drugs that we develop, if any.
13
We will rely on third-party manufacturers to produce our drugs, if any.
We do not currently have the ability to manufacture drugs and, initially, would likely rely on contract manufacturers to produce our drug candidates, if any, for use in our clinical trials. If our contract manufacturers fail to deliver the required quantities of our drug candidates for clinical use on a timely basis, with sufficient quality and at commercially reasonable prices, and we fail to find replacement manufacturers or to develop our own manufacturing capabilities, we may be unable to continue development and production of our drug candidates, if any.
In addition, contract manufacturers have a limited number of facilities in which our drug product candidates can be produced. Contract manufacturers may not perform or may discontinue their business for the time required by us to successfully produce and market our drug product candidates, if any.
We currently have no marketing capability.
We have no internal capability to market drug products, if any, that we develop. In order to successfully commercialize our drugs, if any, we may be required to develop an internal sales force. In the event we are unable to do so, we will rely on our collaborators or contract with other third parties to market any drugs that we may develop internally. Our collaborators or other third parties may not be successful in marketing our drug products, if any, and we may not be able to establish a successful marketing force.
Catastrophic damage to our facility could impair our business.
While some of our electronic data is stored off-site, all of our equipment and operations are located in our headquarters in Cambridge, Massachusetts. If this facility were to be damaged or destroyed, we would be unable to conduct our business for an indefinite period of time. Further, our library of small molecule compounds against which we screen potential drug targets is stored in this location and no back-up library exists. If these small molecule compounds or the equipment in which they are stored were compromised, it could take years to generate a replacement library, which would substantially impair our business.
We may seek to expand our business through possible acquisitions and may have difficulty in identifying targets and integrating those targets with our business and operations.
In the future, we may from time to time seek to expand our business through acquisitions. Our acquisition of companies and businesses and our expansion of operations would involve risks such as the following:
- •
- the potential inability to identify target companies best suited to our business strategy;
- •
- the potential inability to successfully integrate acquired operations and businesses and to realize anticipated synergies, economies of scale or other expected values;
- •
- unanticipated costs associated with the acquisition;
- •
- adverse effects on existing business relationships, particularly our collaborators;
- •
- potential loss of the acquired organization's or our own key employees;
14
- •
- the incurrence of expenses related to transactions that may or may not be consummated; and
- •
- the inability to effectively manage and coordinate operations at multiple venues, which, among other things, could divert our management's attention from other important business matters.
In addition, any acquisition of companies and businesses or expansion of operations could result in dilutive issuances of equity securities, the incurrence of additional debt, large one-time write-offs and the creation of goodwill or other intangible assets that could negatively affect our financial condition and the reported results of our operations. Furthermore, we cannot guarantee that the terms of any acquisition will be favorable to us.
Risks related to our industry
The pharmaceutical and biotechnology industry is highly concentrated and any further consolidation could reduce the number of our potential collaborators and may affect our ability to increase our revenue and achieve our business strategies.
We believe there are a limited number of large pharmaceutical and biotechnology companies and these companies represent the market for our drug discovery programs. The number of our potential collaborators could decline even further through consolidation among or growth of these companies. If the number of our potential collaborators declines, their relative negotiating power with us may increase. If we are unable to enter additional collaborations on commercially acceptable terms, we may not be able to increase our revenue or achieve our business strategies.
Failure to attract, retain and motivate skilled personnel may have an adverse effect on our business strategies.
Our success depends on our continued ability to attract, retain and motivate highly qualified management and scientific personnel and our ability to develop and maintain important relationships with leading academic and other research institutions and scientists. Competition for personnel and relationships in our industry is intense. If we lose the services of our management or scientific personnel or our relationships with academic and other research institutions and scientists are compromised, it could significantly delay our drug discovery programs and impede the achievement of our research and development objectives.
At present, the scope of our needs is largely limited to the expertise of personnel who are able to work with our chemical genomics technologies. In the future, we will need to hire additional personnel as we continue to expand our internal drug discovery programs since these activities will require additional expertise in disciplines applicable to the products we hope to develop and commercialize. For example, we may need to hire qualified sales and marketing personnel to commercialize any drug products we develop. Qualified personnel will also be required to conduct pre-clinical and clinical trials. Further, our business strategy will require that our management team expand to incorporate additional expertise. We do not know if we will be able to attract, retain or motivate the required personnel to achieve our business strategies.
15
We face substantial competition with respect to discovering, developing and commercializing drug products.
The biotechnology and pharmaceutical industry is highly competitive. Many of our competitors are substantially larger than we are and have substantially greater capital resources, research and development staffs and facilities than we have. Furthermore, many of our competitors are more experienced than we are in drug discovery, development and commercialization. In addition, our competitors may discover, develop and commercialize drug products that render non-competitive or obsolete our chemical genomics technologies or the drug targets, if any, that we or our collaborators seek to develop and commercialize. We also may experience competition from companies that have acquired or may acquire technologies from universities or other research institutions.
Healthcare reform and restrictions on reimbursements may limit our financial returns.
Our ability or the ability of our collaborators to commercialize drug products, if any, may depend in part on the extent to which government health administration authorities, private health insurers and other organizations will reimburse consumers for the cost of these products. These third parties are increasingly challenging both the need for and the price of new drug products. Significant uncertainty exists as to the reimbursement status of newly approved therapeutics. Adequate third party reimbursement may not be available for our own or our collaborator's drug products to enable us or them to maintain price levels sufficient to realize an appropriate return on their and our investments in research and product development. We cannot predict the effect that changes in the healthcare system may have on our business and these changes adversely effect our business.
Risks related to this offering
Our stock price will likely be volatile and your investment could decline in value.
The trading price of our common stock is likely to be volatile and subject to wide price fluctuations in response to various factors, including:
- •
- actual or anticipated variations in our quarterly operating results;
- •
- introductions or announcements of technological innovations or new products by us, our collaborators or our competitors;
- •
- disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to patent our technologies;
- •
- changes in our financial estimates by securities analysts;
- •
- conditions or trends in the pharmaceutical and biotechnology industries;
- •
- additions or departures of key personnel;
- •
- the loss of a significant customer or collaborator;
- •
- announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments;
- •
- regulatory developments in the United States and abroad;
- •
- public concern or opinion as to new drug discovery techniques; and
- •
- economic and political factors.
16
In addition, the stock market in general, and the market for biotechnology and pharmaceutical companies in particular, have experienced significant price and volume fluctuations that have often been unrelated or disproportionate to operating performance. These broad market and industry factors may seriously harm the market price of our common stock, regardless of our operating performance. In the past, following periods of volatility in the market price of a company's securities, securities class action litigation has often been instituted. A securities class action suit against us could result in potential liabilities, substantial costs and the diversion of our management's attention and resources, regardless of whether we win or lose.
There may not be an active, liquid trading market for our common stock.
Prior to this offering, there has been no public market for our common stock. An active, liquid trading market for our common stock may not develop or be maintained following this offering. As a result, you may not be able to sell your shares of our common stock quickly or at the market price. The initial public offering price of our common stock was determined by negotiation between us and representatives of the underwriters based upon a number of factors and may not be indicative of prices that will prevail following the completion of this offering. The market price of our common stock may decline below the initial public offering price and you may not be able to resell your shares of our common stock at or above the initial offering price.
Future sales of our common stock may depress our stock price.
The market price of our common stock could decline as a result of sales of substantial amounts of our common stock in the public market after the closing of this offering, or the perception that these sales could occur. In addition, these factors could make it more difficult for us to raise funds through future offerings of common stock. There will be shares of our common stock outstanding immediately after this offering, based on the number of shares of our common stock outstanding as of October 31, 2001. All of the shares of our common stock sold in this offering will be freely transferable without restriction or further registration under the Securities Act of 1933, except for any shares of our common stock purchased by our executive officers, directors, principal stockholders and some related parties.
The remaining shares of our common stock will be eligible for sale in the public market as follows:
- •
- shares of our common stock after 180 days from the date of this prospectus, and
- •
- shares of our common stock at various times after 180 days from the date of this prospectus.
All of our officers, directors and stockholders have agreed with our underwriters to be bound by a 180-day lock-up agreement that prohibits these holders from selling or transferring their stock except in limited circumstances. The lock-up agreements signed by our stockholders are only contractual agreements and J.P. Morgan Securities Inc., on behalf of the underwriters, at their discretion, can waive the restrictions of the lock-up agreement at an earlier time without prior notice or announcement and allow stockholders to sell their shares of our common stock. If the restrictions of the lock-up agreement are waived, shares of our common stock will be available for sale into the market, subject only to applicable securities rules and regulations, which would likely reduce the market price for shares of our common stock.
17
After this offering, we intend to register 7,125,000 shares of our common stock that are reserved for issuance upon the exercise of options granted or reserved for grant under our stock option plan and our employee stock purchase plan. Once we register these shares of our common stock, stockholders can sell them in the public market upon issuance, subject to restrictions under the securities laws. In addition, some of our existing stockholders will be entitled to register their shares of our common stock after this offering.
You will experience immediate and substantial dilution as a result of this offering and may experience additional dilution in the future.
You will pay a price per share that substantially exceeds the per share value of our tangible assets after subtracting our total liabilities. As a result, if we were to distribute our net tangible assets to our stockholders immediately following this offering, you would receive less than the amount you paid for your shares of our common stock. In addition, purchasers of shares of our common stock in this offering will have contributed approximately % of the aggregate price paid by all purchasers of our stock, but will own only approximately % of the shares of our common stock outstanding after the offering based on the number of shares of our common stock outstanding as of October 31, 2001. If the holders of outstanding options exercise those options at prices below the initial public offering price, you will incur further dilution. We may also acquire other companies or technologies or finance strategic alliances by issuing equity, which may result in additional dilution to our stockholders.
Our executive officers, directors and principal stockholders own a large percentage of our voting common stock and could limit new stockholders from influencing corporate decisions.
Immediately after this offering, our executive officers, directors, current principal stockholders and their respective affiliates will beneficially own, in the aggregate, approximately % of our outstanding common stock. These stockholders, as a group, would be able to control substantially all matters requiring approval by our stockholders, including mergers, sales of assets, the election of all directors and approval of other significant corporate transactions in a manner with which you may not agree or that may not be in the best interest of other stockholders.
Our management will have broad discretion in the use of net proceeds from this offering and may not use them effectively.
As of the date of this prospectus, we cannot specify with certainty the amounts we will spend on particular uses from the net proceeds we will receive from this offering. Our management will have broad discretion in the application of the net proceeds but currently intends to use the net proceeds as described in "Use of proceeds." The failure by our management to apply these funds effectively could affect our ability to continue to develop our business.
18
Delaware law and our charter documents contain provisions that could discourage or prevent a potential takeover, even if the transaction would benefit our stockholders and could depress our stock price.
We are incorporated in Delaware. Certain provisions of our certificate of incorporation and bylaws and the provisions of Delaware General Corporation Law could have the effect of delaying, deferring or preventing an acquisition of us, even if an acquisition would be beneficial to our stockholders. These provisions include:
- •
- authorizing the board of directors to issue additional preferred stock;
- •
- prohibiting cumulative voting in the election of directors;
- •
- limiting the persons who may call special meetings of stockholders;
- •
- maintaining a classified board of directors;
- •
- prohibiting stockholder action by written consent;
- •
- establishing advance notice requirements for nominations for election to the board of directors and for proposing matters that can be acted on by stockholders at stockholder meetings; and
- •
- prohibiting a combination with any holder of 15% or more of our capital stock until the holder has held the stock for three years unless, among other things, the board of directors approves the transaction.
19
Forward-looking statements
This prospectus includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934. We have based these forward-looking statements on our current expectations and projections about future events. These forward-looking statements include statements about the following:
- •
- our chemical genomics technologies and the implications of their preliminary results against groups of proteins;
- •
- our ability to rapidly find a small molecule candidate for virtually every disease-associated protein in the human genome on an industrial scale;
- •
- the commercialization of small molecule drug candidates identified with our collaborators;
- •
- our expectations regarding the expansion of existing collaborations and development of new collaborations;
- •
- the protection of intellectual property rights in our drug discovery and development efforts;
- •
- anticipated operating losses, future revenues, capital expenditures and the need for additional financing;
- •
- the success of our internal drug discovery programs and technologies; and
- •
- the revenue to be derived from our collaborative efforts.
The forward-looking statements are principally contained in the sections entitled "Prospectus summary," "Risk factors," "Business" and "Management's discussion and analysis of financial condition and results of operations." These statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. We discuss many of these risks in greater detail under the heading "Risk factors." In some cases, you can identify forward-looking statements by terminology such as "may," "will," "should," "could," "would," "expect," "plan," "anticipate," "believe," "estimate," "project," "predict," "intend," "potential" or the negative of such terms or other similar expressions. These statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties. Given these uncertainties, you should not place undue reliance on these forward-looking statements.
You should read this prospectus and the documents that we reference in this prospectus and have filed as exhibits to the registration statement, of which this prospectus is part, completely and with the understanding that our actual future results may be materially different from what we expect. We may not update these forward-looking statements, even though our situation may change in the future, unless we have obligations under the Federal securities laws to update and disclose material developments related to previously disclosed information. We qualify all of our forward-looking statements by these cautionary statements.
20
Use of proceeds
Our net proceeds from this offering are estimated to be $ million, or $ million if the underwriters' over-allotment option is exercised in full, based on an assumed initial public offering price of $ per share and after deducting the underwriting discount and estimated offering expenses payable by us.
We intend to use the net proceeds of this offering primarily for research and development activities relating to our collaborations and internal drug development programs, the scaling and further development of our drug discovery technologies and other general corporate and working capital purposes, which may include investing in complementary products, licensing additional technologies or making potential acquisitions. We have no current agreements or understandings relating to potential acquisitions and are not currently engaged in any negotiations with respect to future transactions.
The foregoing use of the net proceeds from this offering represents our current intentions based upon our present plans and business condition. We retain broad discretion in the allocation and use of the net proceeds of this offering and a change in our plans or business condition could result in the application of the net proceeds from this offering in a manner other than as described in this prospectus. Pending the uses described above, we intend to invest the net proceeds from this offering in short-term, investment grade, interest-bearing securities.
Dividend policy
We have never declared or paid cash dividends on our capital stock. We currently intend to retain our future earnings, if any, to support the growth and development of our business and we do not anticipate paying any cash dividends in the foreseeable future. Any future determination to pay dividends will be at the discretion of our board of directors and will depend on our financial condition, results of operations, capital requirements and other factors that our board of directors may deem relevant.
21
Capitalization
The following table shows our capitalization as of September 30, 2001:
- •
- on an actual basis;
- •
- on a pro forma basis to reflect the automatic conversion of all outstanding shares of our preferred stock into 14,341,075 shares of our common stock upon the completion of this offering and the automatic conversion of a $2.0 million convertible note payable into shares of our common stock upon the completion of this offering; and
- •
- on a pro forma as adjusted basis to reflect the pro forma adjustments and the sale of the shares of our common stock offered by this prospectus at an assumed initial public offering price of $ per share after deducting the underwriting discount and estimated offering expenses payable by us.
This table does not include:
- •
- 2,741,875 shares of our common stock issuable upon the exercise of stock options outstanding as of September 30, 2001 with a weighted average exercise price of $0.47 per share, of which options to purchase 769,500 shares of our common stock were then exercisable, and
- •
- 1,074,000 shares of our common stock reserved for future grant under our 1997 Stock Option Plan as of September 30, 2001.
You should read this table in conjunction with "Management's discussion and analysis of financial condition and results of operations" and our financial statements and related notes appearing elsewhere in this prospectus.
|
September 30, 2001
(in thousands, except share data)
| | Actual
| | Pro forma
| | Pro forma
as adjusted
|
|---|
|
| Cash and cash equivalents | | $ | 37,896 | | $37,896 | | $ |
| | |
|
| Long-term debt and capital lease obligations, less current maturities | | | 528 | | 528 | | 528 |
| Convertible note payable | | | 2,019 | | — | | — |
| Convertible preferred stock, $.001 par value per share — 14,359,145 shares authorized, 14,341,075 shares issued and outstanding, actual, and no shares authorized, issued and outstanding pro forma and pro forma as adjusted | | | 54,250 | | — | | — |
| Stockholders' equity (deficit): | | | | | | | |
| | Preferred stock, $.001 par value per share — no shares authorized, issued and outstanding, actual, 5,000,000 shares authorized and no shares issued and outstanding, pro forma and pro forma as adjusted | | | — | | — | | — |
| | Common stock, $.001 par value per share — 24,021,645 shares authorized, 5,846,625 shares issued and outstanding, actual, 95,000,000 shares authorized, issued and outstanding pro forma and 95,000,000 shares authorized, shares issued and outstanding pro forma as adjusted | | | 6 | | 20 | | |
| | Deferred compensation | | | (1,568 | ) | (1,568 | ) | (1,568) |
| | Additional paid-in capital | | | 3,164 | | 59,419 | | |
| | Accumulated deficit | | | (20,635 | ) | (20,635 | ) | (20,635) |
| | |
|
| | | | Total stockholders' equity (deficit) | | | (19,033 | ) | 37,236 | | |
| | |
|
| | | | | Total capitalization | | $ | 37,764 | | $37,764 | | $ |
| | |
| |
| |
|
|
22
Dilution
The pro forma net tangible book value of our common stock as of September 30, 2001 was $ million, or $ per share. The pro forma net tangible book value per share of our common stock is the difference between our tangible assets and our liabilities, divided by the number of shares of our common stock outstanding as of September 30, 2001, after giving effect to the automatic conversion of all outstanding shares of our preferred stock into 14,341,075 shares of our common stock upon the completion of this offering and the automatic conversion of a $2.0 million convertible note payable into shares of our common stock upon the completion of this offering. For new investors in our common stock, dilution is the per share difference between the initial public offering price of our common stock and the pro forma net tangible book value of our common stock immediately after completing this offering. Dilution in this case results from the fact that the per share offering price of our common stock is substantially in excess of the per share price paid by our current stockholders.
As of September 30, 2001, after giving effect to the sale of the shares of our common stock offered by the prospectus at an assumed initial public offering price of $ per share and after deducting the underwriting discount and estimated offering expenses payable by us, the pro forma net tangible book value per share of our common stock would have been $ per share. Therefore, new investors in our common stock would have paid $ for a share of common stock having a pro forma net tangible book value of approximately $ per share after this offering. That is, their investment would have been diluted by approximately $ per share. At the same time, our current stockholders would have realized an increase in pro forma net tangible book value of $ per share after this offering without further cost or risk to themselves. The following table illustrates this per share dilution:
|
| Assumed initial public offering price per share | | | | | $ | |
| | Pro forma net tangible book value per share as of September 30, 2001 | | $ | | | | |
| | Increase in pro forma net tangible book value per share attributable to investors in this offering | | | | | | |
| | |
| | | |
| Pro forma net tangible book value per share after this offering | | | | | $ | |
| | | | | |
|
| Dilution per share to new investors | | | | | $ | |
| | | | | |
|
|
The following table sets forth, on a pro forma as adjusted basis as of September 30, 2001, the number of shares of our common stock purchased, the total consideration paid and the average price per share paid by existing and new stockholders, including $2.0 million relating to the convertible note payable, before deducting the underwriting discount and our offering expenses payable by us:
| | | |
| | Shares purchased
| | Total consideration
| |
| |
|
|---|
| | Average price
per share
|
|---|
| | Number
| | Percent
| | Amount
| | Percent
|
|---|
|
| Existing stockholders | | | | % | | $ | 56,837,656 | | % | | | | $ | |
| New investors | | | | | | | | | | | | | | |
| | |
|
| | Total | | | | 100% | | $ | | | 100% | | | | $ | |
| | |
| |
| |
| |
| | | |
|
|
23
The foregoing discussion and tables assume no exercise of any outstanding stock options or warrants. As of September 30, 2001, there were options outstanding to purchase a total of 2,741,875 shares of our common stock with a weighted average exercise price of $0.47 per share and a warrant outstanding to purchase a total of 18,070 shares of our series C preferred stock with an exercise price of $2.2126 per share. To the extent that any of these options or warrants are exercised, your investment will be further diluted. In addition, on November 9, 2001, we granted options to purchase a total of 665,500 shares of our common stock with an exercise price of $5.00 per share under our 1997 Stock Option Plan, as amended.
24
Selected financial data
The following selected financial data should be read in conjunction with "Management's discussion and analysis of financial condition and results of operations" and our financial statements and the related notes to those statements included elsewhere in this prospectus. The statements of operations data for the years ended December 31, 1998, 1999 and 2000 and the balance sheet data as of December 31, 1999 and 2000 are derived from our audited financial statements included elsewhere in this prospectus. The statement of operations data for the period from inception (March 13, 1997) through December 31, 1997 and the balance sheet data as of December 31, 1997 and 1998 are derived from our audited financial statements not included in this prospectus. The statements of operations data for the nine months ended September 30, 2000 and 2001 and the balance sheet data as of September 30, 2001 have been derived from our unaudited financial statements, which are included elsewhere in this prospectus. In our opinion, these unaudited interim financial statements include all adjustments, consisting of only normal recurring adjustments that are necessary for a fair presentation of our financial position and results of operations for those periods. Operating results for the nine month period ended September 30, 2001 are not necessarily indicative of the results to be expected for the fiscal year ending December 31, 2001.
| |
|---|
| | Period from
inception
(March 13,
1997)
through
December 31,
1997
| |
| |
| |
| |
| |
| |
|---|
| | Year ended December 31,
| | Nine months ended September 30,
| |
|---|
(in thousands, except share
and per share data)
| |
|---|
| | 1998
| | 1999
| | 2000
| | 2000
| | 2001
| |
|---|
| |
|---|
| Statements of operations data: | | | | | | | | | | | | | | |
| Total revenue | | $ | — | | $ 568 | | $ 1,867 | | $ 1,594 | | $ 759 | | $ 2,647 | |
| | |
| |
| Operating expenses: | | | | | | | | | | | | | | |
| | Research and development | | | 485 | | 2,427 | | 3,632 | | 5,093 | | 3,566 | | 7,817 | |
| | Selling, general, and administrative | | | 684 | | 1,390 | | 1,169 | | 1,644 | | 1,167 | | 2,068 | |
| | Stock-based compensation | | | — | | — | | 47 | | 162 | | 7 | | 1,158 | |
| | |
| |
| | | | Total operating expenses | | | 1,169 | | 3,817 | | 4,848 | | 6,899 | | 4,740 | | 11,043 | |
| | |
| |
| Interest income (expense), net | | | — | | (27 | ) | (158 | ) | (85 | ) | (65 | ) | 735 | |
| | |
| |
| Net loss | | $ | (1,169 | ) | $(3,276 | ) | $(3,139 | ) | $(5,390 | ) | $(4,046 | ) | $(7,661 | ) |
| | |
| |
| |
| |
| |
| |
| |
| | |
| |
| Net loss per common share: | | | | | | | | | | | | | | |
| | Basic and diluted | | $ | (0.59 | ) | $ (1.13 | ) | $ (0.86 | ) | $ (1.14 | ) | $ (0.91 | ) | $ (1.40 | ) |
| | |
| |
| |
| |
| |
| |
| |
| | Unaudited pro forma basic and diluted | | | | | | | | | $ (0.55 | ) | | | $ (0.45 | ) |
| | |
| |
| | Shares used in computing basic and diluted net loss per common share | | | 1,983,811 | | 2,905,651 | | 3,665,247 | | 4,722,259 | | 4,424,509 | | 5,466,846 | |
| | |
| |
| |
| |
| |
| |
| |
| | Shares used in computing unaudited pro forma basic and diluted net loss per common share | | | | | | | | | 9,844,493 | | | | 17,103,824 | |
| | | | | | | | | |
| | | |
| |
| |
| |
|---|
| | December 31,
| | September 30,
| |
|---|
(in thousands)
| | 1997
| | 1998
| | 1999
| | 2000
| | 2001
| |
|---|
| |
|---|
| Balance sheet data: | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 454 | | $ | 54 | | $ | 85 | | $ | 18,579 | | $ | 37,896 | |
| Working capital (deficit) | | | 88 | | | (1,032 | ) | | (1,694 | ) | | 16,104 | | | 33,423 | |
| Total assets | | | 864 | | | 1,567 | | | 1,474 | | | 20,173 | | | 44,083 | |
| Long-term liabilities, less current maturities | | | 1,417 | | | 1,226 | | | 1,102 | | | 125 | | | 2,547 | |
| Convertible preferred stock | | | 250 | | | 3,500 | | | 5,867 | | | 29,855 | | | 54,250 | |
| Total stockholders' equity (deficit) | | $ | (1,168 | ) | $ | (4,444 | ) | $ | (7,466 | ) | $ | (12,656 | ) | $ | (19,033 | ) |
| |
25
Management's discussion and analysis of financial condition and results of operations
You should read the following discussion and analysis in conjunction with "Selected financial data" and our financial statements and notes included elsewhere in this prospectus.
Overview
We are focused on the discovery of small molecule drugs on a proteome and genome-wide scale. We believe that we provide a comprehensive solution for the discovery of small molecule drugs by exploiting virtually all disease-associated proteins in the human genome. We have developed an integrated, proprietary suite of drug discovery technologies that form our scalable chemical genomics platform. At the heart of our solution is the seamless integration of our Automated Ligand Identification System, or ALIS, a powerful, scalable and broadly- applicable screening system, with our large, proprietary library of diverse, medicinally relevant, small molecules. We believe that our solution will yield a larger inventory of small molecule drug candidates for clinical trials with a higher rate of success than achieved by conventional methods and will establish the standard for small molecule drug discovery and development.
Since May 2001, we have entered into seven new collaborations, including the expansion of our existing relationships with Biogen, Inc. and Schering-Plough Ltd. Current collaborators include Biogen, Celltech R&D Limited, Immusol Incorporated, Merck & Co., Inc., Mitsubishi Pharma Corporation, Oxford GlycoSciences (UK) Limited, Schering-Plough and Tularik Inc.
We were incorporated in 1997 in Delaware and are headquartered in Cambridge, Massachusetts. Since inception, we have devoted substantially all of our efforts to the development of our chemical genomics technologies and to the discovery of small molecule drug candidates for our collaborators and for internal drug discovery programs. We have incurred net losses since our inception, including net losses of $5.4 million for the year ended December 31, 2000 and $7.7 million for the nine months ended September 30, 2001. We have an accumulated deficit as of September 30, 2001 of $20.6 million. We have not achieved profitability on a quarterly or annual basis and we expect to incur significant additional losses over the next several years. We expect cumulative losses to increase primarily due to research and development activities relating to our collaborations and internal drug development programs, the scaling and further development of our drug discovery technologies and other general corporate purposes. We anticipate that our quarterly results will fluctuate for the foreseeable future. Therefore, period-to-period comparisons should not be relied upon as predictive of the results in future periods. Our sources of working capital have been equity financings, collaboration revenue, equipment lease financings and interest earned on investments.
Our collaboration revenue is primarily derived from long-term contracts with biotechnology and pharmaceutical companies. These contracts may include payments for screening and related services, technology access and license fees, research and development milestones and royalties. We follow the provisions of the Securities and Exchange Commission's Staff Accounting Bulletin No. 101 (SAB 101),Revenue Recognition. In accordance with SAB 101, we recognize revenue related to screening and related services as they are performed, so long as there is persuasive evidence of an arrangement, the fee is fixed or determinable and collection
26
of the related receivable is probable. Amounts received for technology access or license fees are deferred and recognized as services are performed over the performance period of the contract. Amounts received for milestones will be recognized when they are earned as long as the milestones are deemed to be substantive. Royalty revenue will be recognized when earned. We have not recognized any milestone or royalty revenue to date. Amounts received prior to satisfying our revenue recognition criteria are recorded as deferred revenue in the accompanying balance sheets.
Our grant revenue has been derived from a Small Business Innovation Research grant. Under the terms of our SBIR grant award, we expect to receive $950,000 in reimbursement for certain costs and fixed asset purchases. Amounts received for reimbursement of incurred costs are recognized as revenue upon cash receipt. Amounts received for reimbursement of fixed asset purchases have been netted against the cost of the asset.
Research and development expenses primarily consist of salaries and related expenses for personnel and consulting services. Other research and development expenses include fees paid to consultants and outside service providers, and the costs of materials used in screening, research and development, information technology and facility costs. We charge all research and development expenses to operations as incurred.
Selling, general and administrative expenses primarily consist of salaries and related costs for personnel in business development, administration, finance, accounting and human resources. Other costs include facility costs and professional fees for legal and accounting services.
We have granted stock options to employees and non-employees at prices deemed below fair market value on the dates of grant. As a result, we have recorded deferred compensation expense which represents, in the case of employees, the difference between the option exercise price and the deemed fair value of our common stock. In the case of non-employees, deferred compensation represents the fair market value of the options granted computed using the Black-Scholes option pricing model.
As of September 30, 2001, the deferred compensation balance was $1.3 million and $0.3 million for employees and non-employees, respectively. Deferred compensation for employees will be recognized over the remaining vesting period of the related option. Deferred compensation related to non-employees will be recognized over the vesting period of the related option, however, the amount recognized is subject to change based on changes in the fair value of our common stock.
For purposes of our statements of operations, we have allocated stock-based compensation expense to the appropriate expense category based on the nature of the service provided to us by each individual. We have continued to grant stock options after September 30, 2001 and we may record additional deferred compensation in the period from October 1, 2001 through the closing of this offering.
Results of operations
Nine months ended September 30, 2001 and September 30, 2000
Total revenue: Total revenue increased $1.8 million to $2.6 million for the nine months ended September 30, 2001 from $0.8 million for the nine months ended September 30, 2000.
27
Collaboration revenue increased $1.9 million to $2.5 million for the nine months ended September 30, 2001 from $0.6 million for the nine months ended September 30, 2000. The increase in collaboration revenue was primarily due to an increase in the number of our new collaboration agreements and related services. As a result of collaborations entered into during the nine months ended September 30, 2001, our deferred revenue increased to $5.0 million from $1.2 million as of December 31, 2000.
Research and development: Research and development expenses increased $4.2 million to $7.8 million for the nine months ended September 30, 2001 from $3.6 million for the nine months ended September 30, 2000. The increase was primarily due to additional expenditures for salary and related costs of $2.2 million, increased facility costs of $0.5 million and increased supply costs for compound manufacturing of $0.5 million for the nine months ended September 30, 2001.
Selling, general and administrative: Selling, general and administrative expenses increased $0.9 million to $2.1 million for the nine months ended September 30, 2001 from $1.2 million for the nine months ended September 30, 2000. The increase was primarily attributable to increased legal costs of $0.3 million related to business development activities and patent costs in 2001 and $0.2 million related to increased salary and related costs for the nine months ended September 30, 2001.
Stock-based compensation: Stock-based compensation increased to $1.2 million for the nine months ended September 30, 2001 from $6,700 for the nine months ended September 30, 2000. The increase was the result of stock-based compensation expense of $0.5 million related to option grants to employees with an exercise price below the deemed fair value of our common stock as determined for financial reporting purposes and $0.5 million related to option grants to non-employees for the nine months ended September 30, 2001. In addition, we recorded $0.1 million of stock-based compensation expense related to the acceleration of vesting of certain options related to a terminated employee for the nine months ended September 30, 2001.
Interest income (expense), net: Interest income (expense), net increased $0.8 million to $0.7 million for the nine months ended September 30, 2001 from $(0.1) million for the nine months ended September 30, 2000. The increase was primarily due to an increase in interest income resulting from a higher average cash balance during the nine months ended September 30, 2001.
Years ended December 31, 2000 and 1999
Total revenue: Total revenue decreased by $0.3 million to $1.6 million for the year ended December 31, 2000 from $1.9 million for the year ended December 31, 1999. This decrease was due to a decrease in collaboration revenue partially offset by an increase in grant revenue. Collaboration revenue decreased $0.4 million to $1.4 million for the year ended December 31, 2000 from $1.8 million for the year ended December 31, 1999, primarily due to the completion of one of our collaborations that resulted in recognized revenue of $1.0 million in 1999. As a result of collaborations entered into during the year ended December 31, 2000, deferred revenue increased to $1.2 million as of December 31, 2000 from $0.1 million as of December 31, 1999.
28
Research and development: Research and development expenses increased $1.5 million to $5.1 million for the year ended December 31, 2000 from $3.6 million for the year ended December 31, 1999. The increase was primarily attributable to increases in salary and related costs of $1.1 million and increased supply costs of $0.2 million for compound manufacturing during the year ended December 31, 2000.
Selling, general and administrative: Selling, general and administrative expenses increased $0.4 million to $1.6 million for the year ended December 31, 2000 from $1.2 million for the year ended December 31, 1999. The increase was primarily attributable to increased legal costs of $0.2 million related to business development activities and patent costs and increased salary and related costs of $0.1 million during the year ended December 31, 2000.
Stock-based compensation: Stock-based compensation increased to $0.2 million for the year ended December 31, 2000 from $47,000 for the year ended December 31, 1999. The increase was the result of stock-based compensation expense of $0.1 million related to option grants to employees with an exercise price below the deemed fair value of our common stock as determined for financial reporting purposes and $28,000 related to option grants to non-employees during the year ended December 31, 2000.
Interest income (expense), net: Interest income (expense), net decreased to $(0.1) million for the year ended December 31, 2000 from $(0.2) million for the year ended December 31, 1999. The decrease was primarily due to the repayment of notes payable to stockholders during 2000, which resulted in lower interest expense for the year ended December 31, 2000, partially offset by an increase in interest income due to a higher average cash balance during 2000.
Years ended December 31, 1999 and 1998
Total revenue: Total revenue increased $1.3 million to $1.9 million for the year ended December 31, 1999 from $0.6 million for the year ended December 31, 1998. Collaboration revenue increased $1.2 million to $1.8 million for the year ended December 31, 1999 from $0.6 million for the year ended December 31, 1998. This increase was due to the completion of one of our collaborations that resulted in recognized revenue of $1.0 million for the year ended December 31, 1999.
Research and development: Research and development expense increased $1.2 million to $3.6 million for the year ended December 31, 1999 from $2.4 million for the year ended December 31, 1998. The increase was primarily attributable to increased salary and related costs of $0.8 million and increased facilities costs of $0.4 million for the year ended December 31, 1999.
Selling, general and administrative: Selling, general and administrative expenses decreased $0.2 million to $1.2 million for the year ended December 31, 1999 from $1.4 million for the year ended December 31, 1998. The decrease was primarily attributable to a decrease in business development expenses of $0.2 million for the year ended December 31, 1999.
Stock-based compensation: Stock-based compensation increased to $47,000 for the year ended December 31, 1999 from $0 for the year ended December 31, 1998. Stock-based compensation expense for the year ended December 31, 1999 was primarily related to option grants to employees with an exercise price below the deemed fair value of our common stock as determined for financial reporting purposes.
29
Interest income (expense), net: Interest income (expense), net increased $0.1 million to $(0.2) million for the year ended December 31, 1999 from $(27,000) for the year ended December 31, 1998. The increase was primarily due to interest expense associated with notes payable to stockholders during the year ended December 31, 1999.
Liquidity and capital resources
Since inception, we have financed our operations primarily through the private placement of preferred stock, collaboration agreements, loans with our directors and stockholders and a convertible note payable with a strategic partner. We have received $56.4 million in aggregate net proceeds from sales of our preferred stock, issuances of our common stock through exercise of options and issuance of a convertible note payable through September 30, 2001. As of September 30, 2001, we had cash and cash equivalents of approximately $37.9 million. Our funds are currently invested in money market funds and institutional bonds.
During the nine months ended September 30, 2001, operations used cash of $3.1 million. Our operating activities used cash of $3.6 million for the year ended December 31, 2000, $3.3 million for the year ended December 31, 1999, and $2.5 million for the year ended December 31, 1998. The use of cash in all periods primarily resulted from our losses from operations and changes in our working capital accounts.
Our investing activities used cash of $3.0 million for the nine months ended September 30, 2001. Our investment activities during 2001 consisted of purchases of property and equipment and the issuance of a $0.5 million letter of credit to our landlord in connection with an amendment to our facility lease. Our investing activities used cash to purchase property and equipment of $0.3 million for the year ended December 31, 2000, $0.1 million for the year ended December 31, 1999 and $0.7 million for the year ended December 31, 1998.
In the nine months ended September 30, 2001, our financing activities provided cash of $25.4 million, which consisted of sales of preferred stock to private investors and strategic partners and the issuance of a $2.0 million convertible note payable to a strategic partner. Our financing activities consisted primarily of the sales of preferred stock to private investors and provided us with cash of $22.4 million for the year ended December 31, 2000, $3.5 million for the year ended December 31, 1999, and $2.8 million for the year ended December 31, 1998.
In August 2001, we entered into a $3.5 million equipment facility lease with an outside third party for the funding of capital equipment. We had $3.5 million available under this agreement as of September 30, 2001. As of October 31, 2001, we have drawn approximately $0.8 million to fund capital purchases.
Based on our current plans, we believe that our cash and cash equivalents, together with the estimated net proceeds from this offering, will be sufficient to meet our capital requirements for at least the next 24 months. Our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement that involves risks and uncertainties, and actual results could vary materially. The factors described above will impact our future capital requirements and the adequacy of our available funds. We may be required to raise additional funds through public or private financings, or other arrangements. We cannot be certain that such additional funding, if necessary, will be available on terms attractive to us, or at all. Furthermore, any additional equity financing may be
30
dilutive to existing stockholders and debt financing, if available, may involve restrictive covenants. Our failure to raise capital when needed could have a material adverse effect on our business.
Tax loss carryforwards
As of December 31, 2000, we had Federal net operating loss carryforwards of $11.0 million and research and development credit carryforwards of $0.5 million. The net operating loss and tax credit carryforwards will expire at various dates, beginning in 2012, if not used. Under the provisions of the Internal Revenue Code, substantial changes in our ownership may limit the amount of net operating loss carryforwards that could be utilized annually in the future to offset taxable income. A full valuation allowance has been established in our financial statements to reflect the uncertainty of our ability to use available tax loss carryforwards and other deferred tax assets.
Quantitative and qualitative disclosure about market risk
As of September 30, 2001, we had cash and cash equivalents of $37.9 million consisting of cash and highly liquid investments. Decreases in interest rates over time will reduce our interest income from short-term investments. Our outstanding capital lease obligations are all at fixed interest rates and therefore, have minimal exposure to changes in interest rates.
Recent accounting pronouncements
In July 2001, the Financial Accounting Standards Board (FASB) issued SFAS No. 141,Business Combinations, and SFAS No. 142,Goodwill and Other Intangible Assets. Statement No. 141 requires that all business combinations initiated after June 30, 2001 be accounted for using the purchase method of accounting. Statement No. 142 discusses how intangible assets that are acquired should be accounted for in financial statements upon their acquisition and also how goodwill and other intangible assets should be accounted for after they have been initially recognized in the financial statements. Beginning on January 1, 2002, with the adoption of Statement No. 142, goodwill and certain purchased intangibles existing on June 30, 2001, will no longer be subject to amortization over their estimated useful life. Rather, the goodwill and certain purchased intangibles will be subject to an annual assessment for impairment based on fair value. The provisions of Statement No. 142 are required to be applied starting with fiscal years beginning after December 15, 2001. We do not expect the adoption of these statements to have a material impact on our financial position or results of operations.
In August 2001, the FASB issued SFAS No. 144,Accounting for the Impairment or Disposal of Long-Lived Assets, which supersedes SFAS No. 121,Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets to Be Disposed Of. SFAS No. 144 further refines the requirements of SFAS No. 121 that companies (1) recognize an impairment loss only if the carrying amount of a long-lived asset is not recoverable based on its undiscounted future cash flows and (2) measure an impairment loss as the difference between the carrying amount and fair value of the asset. In addition, SFAS No. 144 provides guidance on accounting and disclosure issues surrounding long-lived assets to be disposed of by sale. We do not expect the adoption of this statement to have a material impact on our financial position or results of operations.
31
Business
Overview
We are focused on the discovery of small molecule drugs on a proteome and genome-wide scale. We believe that we are the only company currently capable of delivering small molecule drug candidates for virtually every medically important protein encoded by the human genome. We believe the rate at which we are forming discovery alliances validates the vast opportunity presented by the convergence of our technologies with genomics and proteomics. By using our chemical genomics technologies, we believe that, over time, we will derive economic benefit from hundreds of drug candidates at levels of participation ranging from single digit royalties to full ownership.
Our success has motivated leading biotechnology and pharmaceutical companies to enter into collaborations with us. Since May 2001, we have entered into seven new collaborations, including the expansion of our existing relationships with Biogen and Schering-Plough. Our expanded relationships have further validated our technologies and increased the overall value of our portfolio of collaborations. Current collaborators include Biogen, Celltech, Immusol, Merck, Mitsubishi Pharma, Oxford Glycosciences, Schering-Plough and Tularik.
Chemical genomics
Chemical genomics is a methodology for deriving small molecule drug candidates from the wealth of information produced by the sequencing of the human genome. Chemical genomics uses small molecules to probe, modulate, validate, and prioritize disease-associated proteins while simultaneously assessing the suitability of these small molecules as drug candidates. Representing a fundamental shift from conventional drug discovery methods, we believe chemical genomics will become the standard for future small molecule drug discovery. A successful chemical genomics drug discovery and development platform will:
- •
- screen disease-associated proteins in parallel on an unprecedented scale and in an unbiased fashion;
- •
- prioritize disease-associated proteins amenable to drug discovery and confirm their roles as validated drug targets for the treatment of disease;
- •
- significantly increase the number of novel small molecules directed to each disease-associated protein;
- •
- deliver drug candidates for newly validated drug targets identified from the expanding inventory of disease-associated proteins;
- •
- deliver drug candidates for known drug targets for which conventional drug discovery methods have failed;
- •
- accelerate the process of drug discovery and development; and
- •
- increase the likelihood that drug candidates will be developed successfully.
32
Industry background and opportunity
The human genome was sequenced to gain insight into human health and to better understand the relationship among genes, proteins and disease. While genomic and proteomic efforts are yielding many disease-associated proteins as potential drug targets, the wealth of information being generated is on a scale that surpasses the ability of conventional drug discovery technologies to effectively translate this information to small molecule drugs. Small molecule drugs offer many advantages, including oral administration, lower cost of production and improved patient compliance.
To date, small molecule drugs developed by the pharmaceutical industry are directed towards fewer than 500 drug targets. Industry sources estimate that the size of the global pharmaceutical market in 2000 was approximately $354 billion, a majority of which is small molecule drugs.
Many pharmaceutical and biotechnology companies are using genomics and proteomics in an attempt to enhance their product pipelines. These efforts are expected to identify approximately 20,000 to 30,000 disease-associated proteins of which in excess of 5,000 are expected to be potential drug targets that the industry can exploit for small molecule drug development. It is expected that the discovery of these new disease-associated proteins will enable the creation of entirely new classes of drugs. However, conventional drug discovery methods are inadequate to determine the function of these disease-associated proteins and validate them as drug targets. Moreover, conventional methods lack the scalability necessary to discover small molecule candidates for the many validated drug targets expected to emerge.
To fully exploit this rapidly expanding inventory of disease-associated proteins, we believe an integrated drug discovery platform that can be applied at a scale matching the number of disease-associated proteins identified by proteomics and genomics is required. The company that develops and implements such a platform should have at its disposal a large inventory of drug candidates that can be commercialized at its choice through collaborations or internal development. We believe that chemical genomics is currently the only drug discovery strategy that can identify drug candidates on a proteome and genome-wide scale.
Limitations of conventional drug discovery methods
We believe conventional drug discovery methods will not scale adequately to exploit the opportunities provided by the genomics and proteomics revolution. It is estimated that, on average, validating a drug target, discovering and optimizing a drug candidate and progressing the candidate up to clinical trials costs in excess of $250 million and requires approximately six to seven years to complete. Industry sources state that a significant portion of this time and cost arises from inadequacies in the methods used to identify and prioritize both disease-associated proteins and drug candidates for clinical development. We estimate that developing drug candidates for just 10% of newly identified drug targets using conventional drug discovery and development methods would exceed the combined capacity and research and development budgets of the ten largest pharmaceutical companies for 2000. As a result, the commercial exploitation of thousands of disease-associated proteins using these conventional drug discovery methods would be economically prohibitive.
33
The conventional small molecule drug discovery process up to clinical trials consists of the following linear steps:
- •
- target identification;
- •
- function determination;
- •
- target validation;
- •
- assay development for high throughput screening;
- •
- high throughput screening;
- •
- lead molecule identification;
- •
- lead molecule optimization; and
- •
- pre-clinical development of a drug candidate.
Conventional methods begin with target identification, which is the comprehensive biological assessment of a protein's potential association with disease after it has been implicated by genomics and proteomics. Conventional small molecule drug discovery generally requires up-front knowledge of a protein's function. However, recent efforts have determined function for only a small fraction of disease-associated proteins. Following the determination of protein function, biological and genetic methods are used to validate a disease-associated protein as a drug target. However, conventional methods for target validation do not directly determine if a drug target is amenable to small molecule drug discovery. Limitations in determining protein function and target validation by conventional methods lead to the frequent delay and failure of small molecule drug discovery and development, resulting in the unnecessary waste of time and cost.
Once protein function is determined, conventional methods rely on the large-scale screening of potential drug compounds in biological assays. Before this process, known as high throughput screening, or HTS, can commence, a specific assay for each disease-associated protein must be developed. Failure to successfully develop an appropriate HTS assay means that many disease-associated proteins will not be exploited. Since HTS assays are biased to known protein functions, they can fail to identify novel drug candidates unrelated to the known function. In addition, HTS assays do not have sufficient throughput to ensure that a small molecule drug candidate can be identified for each disease-associated protein. Limitations of conventional HTS methods severely constrain the number and diversity of drug candidates that can be identified.
The conventional drug discovery process is further limited by shortcomings in chemical libraries used for HTS. Often, these libraries lack the diversity and size to identify drug candidates for every disease-associated protein, and many library compounds have poor pharmaceutical properties. In addition, we believe conventional libraries are comprised of many compounds that are present in the public domain and cannot be patented. Frequently, the compounds identified from these libraries require the efforts of many chemists over several years to optimize drug properties to create a compound that is suitable for pre-clinical development, and a large number of small molecule drug candidates still fail due to problems with selectivity, pharmacokinetics, bioavailability and toxicity.
34
We believe that conventional drug discovery methods are inadequate to exploit fully the opportunities provided by genomics and proteomics. A fundamental new approach to drug discovery is required to capitalize on this opportunity.
NeoGenesis solution
We believe that we provide a comprehensive solution for the discovery of small molecule drugs by exploiting virtually all disease-associated proteins encoded by the human genome. Our chemical genomics strategy represents a fundamental new approach that overcomes the scaling and associated limitations inherent in conventional drug discovery methods. Our strategy simultaneously validates disease-associated proteins as drug targets and delivers small molecule drug candidates for pre-clinical and clinical development.
The diagram below compares conventional drug discovery methods to our chemical genomics approach for the discovery of small molecule drugs. We believe that our solution will yield a larger inventory of small molecule drug candidates for clinical trials with a greater likelihood of success than achieved by conventional drug discovery processes.
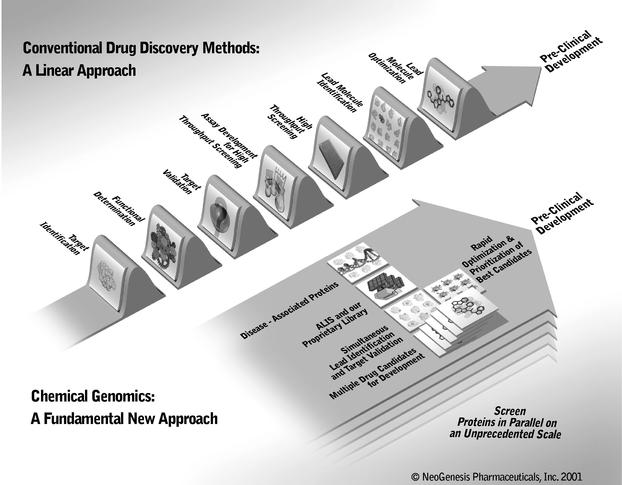
Our chemical genomics platform is based upon an integrated, proprietary suite of drug discovery technologies. We believe our platform is uniquely scalable and will be capable of screening thousands of disease-associated proteins against many millions of drug-like small molecules. At the heart of our solution is the seamless integration of our Automated Ligand
35
Identification System, or ALIS, a powerful, scalable and broadly-applicable screening system, with our large library of diverse, medicinally relevant, mass-encoded small molecules. We believe that an essential benefit of ALIS is that it is unbiased and does not require functional information or development of a function-specific assay in order to screen a disease-associated protein. Our solution also incorporates a rapid medicinal chemistry optimization capability used to advance drug candidates to the clinic.
We believe our technologies have been validated by the results we have obtained for our collaborators. Since October 2000, we have entered into collaborative relationships with eight leading pharmaceutical and biotechnology companies. We have discovered approximately three to five diverse small molecules for each of 24 of the 25 disease-associated proteins that we have screened using our technologies, including candidates for drug targets where conventional methods have failed. Two of our collaborators selected small molecules we identified for lead optimization, the next step in their drug development process. In addition, two of our initial collaborators have entered into new agreements that expand the scope and nature of our relationship, including the utilization of our rapid medicinal chemistry optimization capability.
Corporate strategy
Our goal is to derive economic benefit from virtually every disease-associated protein in the human genome. We plan to use our chemical genomics technologies to exploit these proteins, which include members of all medically important protein families.
Key elements of our strategy include:
Broad collaborations with pharmaceutical and biotechnology companies that provide research and development funding, milestone payments and royalties. Based on the strength of our technologies and our proven success, we have established a number of revenue generating collaborations with leading pharmaceutical and biotechnology companies, including Biogen, Celltech, Merck, Mitsubishi Pharma, Oxford GlycoSciences, Schering-Plough and Tularik. We intend to pursue collaborations with many additional companies. We expect to leverage our success in generating small molecule drug candidates by broadening our collaborative relationships across research and development departments within a single company and by expanding the scope of our existing collaborations. We have already been successful in expanding our relationships with Biogen and Schering-Plough.
Strategic collaborations with pharmaceutical and biotechnology companies in which we retain substantial commercial rights. The success of our chemical genomics platform is affording us the opportunity to enter into strategic collaborations in which we retain a substantial portion of the economic benefit from commercial development of drugs that we discover. For example, we are to share equally the costs and any profits resulting from the development of any drugs from our collaboration with Immusol and some drugs from our collaboration with Tularik.
Internal drug development and commercialization. We are exploiting our chemical genomics technologies to generate a pipeline of drug candidates that we intend to develop as commercial products either internally or with a strategic partner. As a part of this effort, we are currently screening a number of drug targets and have already identified small molecules that bind to several of these targets. We are also in negotiations with respected academic
36
institutions to gain access to proteins in medically important protein families. In the future, we expect to utilize up to one quarter of our screening capacity and associated drug discovery and development resources to support our internal efforts.
To accomplish our strategy we will continue to:
Increase our screening capacity. Industry sources have indicated that genomic and proteomic efforts are expected to identify in excess of 5,000 potential drug targets. We currently have an annual capacity to screen 72 proteins, which we believe is equal to or greater than the capacity of any individual biotechnology or pharmaceutical company. We intend to increase our annual screening capacity to approximately 500 proteins by the end of 2003.
Enhance ALIS technology. We are committed to continually enhancing our chemical genomics platform by incorporating the latest technological advances in order to maintain and increase our competitive superiority over conventional drug discovery technologies. For example, we recently developed our proprietary Nano-ALIS technology to reduce protein consumption during our screening process by a factor of ten. We expect to incorporate Nano-ALIS technology into our platform in the second half of 2002. In addition to our own internal efforts, we continue to identify and evaluate technologies that we may license or acquire from third parties.
Expand our screening library. We intend to expand our current screening library by preparing additional diverse, medicinally relevant compounds. We will accomplish this by building upon previous experience and continually targeting existing and newly discovered, medically important protein families. Over the last six months, we have added approximately 1.2 million compounds to our library, targeting one important protein family.
Advance our medicinal chemistry capabilities. We have used our technologies to perform rapid optimization of compounds using mixtures, accelerating the drug optimization process beyond the capabilities of conventional medicinal chemistry methods that prepare compounds one at a time. In two of our collaborations, we demonstrated the ability to increase binding affinity for a compound greater than ten-fold during which time conventional methods failed entirely. We intend to scale our proprietary rapid optimization process to match the throughput of ALIS by the end of 2002.
Develop drug candidates with a greater likelihood of clinical success. We are applying ALIS to identify and eliminate compounds from our library that interact with proteins known to be responsible for metabolism, clearance, toxicity and other factors that contribute to failure of drug candidates in pre-clinical and clinical development. We believe that this will result in delivery of higher quality drug candidates with a greater likelihood of clinical success and will improve the quality of our library.
Develop a robust intellectual property estate. As we continue to scale our operations and discover novel drug candidates, we intend to apply for broad composition-of-matter patents on these drug candidates. In addition, we intend to file patent applications that claim new methods for treating diseases involving the proteins validated by our chemical genomics technologies. We have also applied for, and intend to continue to apply for, broad patents for our technologies, such as those embodied by ALIS.
37
Collaborations
We have entered into collaborations with leading pharmaceutical and biotechnology companies to discover and develop small molecule drug candidates. We expect these collaborations to fund a portion of our internal drug discovery and development efforts. Since May 2001, we have entered into seven new collaborations, including the expansion of our existing relationships with Biogen and Schering-Plough. In addition, our two most recent collaborations provide for the equal sharing of the costs of product development and any profits resulting from the development of any drugs from our collaboration with Immusol and some drugs from our collaboration with Tularik.
Under our current collaborations, we expect to screen approximately 240 different proteins. The table below summarizes our current collaborations:
|
Collaborator
| | Effective date
of agreement
| | Initial term
|
|---|
|
| Immusol | | October 2001 | | 3 years |
| Tularik | | August 2001 | | less than 1 year |
| Schering-Plough | | August 2001 | | 2 years |
| Celltech | | July 2001 | | 4.5 years |
| Mitsubishi Pharma | | July 2001 | | 1 year |
| Biogen | | June 2001 | | 39 months |
| Oxford GlycoSciences | | June 2001 | | 3 years |
| Merck | | November 2000 | | 1 year |
|
Immusol Incorporated
In October 2001, we entered into a drug discovery collaboration agreement with Immusol Incorporated under which we agreed to screen a number of proteins supplied to us by Immusol. Immusol and NeoGenesis are to share equally in both the costs of product development and the profits and losses from any product sales if drugs result from the collaboration. The collaboration has an initial term of three years.
Tularik Inc.
In August 2001, we entered into a drug discovery collaboration agreement with Tularik Inc. under which we agreed to screen a number of proteins supplied to us by Tularik. In the initial phase of this collaboration, Tularik will evaluate the small molecule compounds that we identify for a limited number of their proprietary proteins. Thereafter, Tularik can expand the collaboration to include a larger number of proteins that we will screen on their behalf. Tularik maintains the option to develop some or all of the small molecule compounds we identify. If Tularik chooses to develop any of these small molecule compounds, we will assign our rights to those compounds to Tularik and it is obligated to use commercially reasonable efforts to develop and commercialize products. Subject to some restrictions, we retain the rights to use small molecule compounds that we identify for Tularik for purposes unrelated to the collaboration. Tularik pays us fees to screen some of these proteins and has agreed to pay us an additional fee if we conduct optimization on their behalf. In addition, Tularik has also agreed to pay us additional amounts per screened protein if it initiates an internal medicinal chemistry program for resultant small molecule compounds. Tularik is required to make
38
milestone payments to us based upon various pre-clinical and clinical development activities and to pay us royalties on applicable product sales, if any.
Some of the proteins that are evaluated in both the initial phase and the expanded phase will be designated as "shared" prior to their screening. The screening and optimization fees and milestone payments do not apply to shared proteins. Tularik and NeoGenesis will share equally in both the costs of product development and any profits and losses from any product sales if drugs result from the collaboration. The initial phase of the collaboration has a term of less than one year and the expanded phase, if any, will have a term of three years.
Schering-Plough Ltd.
In August 2001, we expanded our existing relationship with Schering-Plough through drug discovery agreements under which we agreed to screen a number of proteins supplied to us by Schering-Plough. Schering-Plough maintains the option to develop a specific number of the small molecule compounds we identify. If Schering-Plough chooses to develop any of these small molecule compounds, we will assign the rights to those compounds to Schering-Plough and it is obligated to use commercially reasonable efforts to develop and commercialize products. Schering-Plough pays us fees to screen these proteins and has agreed to pay us an additional fee if we conduct optimization on their behalf. Schering-Plough is required to make milestone payments to us based upon various pre-clinical and clinical development activities and to pay us royalties on applicable product sales, if any. As part of the collaboration, Schering-Plough made an investment in our series E preferred stock. The collaboration has an initial term of two years and Schering-Plough may extend the collaboration for three additional one-year periods. Schering-Plough may terminate the collaboration upon 180 days notice at any time following August 2, 2002.
Celltech R&D Limited
In July 2001, we entered into a chemical genomics collaboration with Celltech R&D Limited under which we agreed to screen a number of proteins supplied to us by Celltech. Celltech maintains the option to develop a specific number of the small molecule compounds we identify. If Celltech chooses to develop any of these small molecule compounds, we will assign the rights to those compounds to Celltech and it is obligated to use commercially reasonable efforts to develop and commercialize products. Celltech pays us fees to screen these proteins and has agreed to pay us an additional fee if we conduct optimization on their behalf. Celltech is required to make milestone payments to us based upon various clinical development activities and is required to pay us royalties on applicable product sales, if any. As part of the collaboration, Celltech made an investment in our series E preferred stock. The collaboration has an initial term that expires December 31, 2005. Celltech may terminate the agreement upon 90 days notice any time following January 11, 2003.
Mitsubishi Pharma Corporation
In July 2001, we entered into a drug discovery collaboration agreement with Mitsubishi Pharma Corporation, or MPC, under which we have agreed to identify small molecule compounds for a protein supplied to us by MPC. MPC maintains the option to develop a specific number of the small molecule compounds we identify. If MPC chooses to develop any of these small molecule compounds, we will assign the rights to those compounds to MPC and it is obligated to use
39
commercially reasonable efforts to develop and commercialize products. MPC paid us a fee to screen these proteins and has agreed to pay us an additional fee if we conduct optimization on their behalf. MPC is required to make milestone payments to us based upon various pre-clinical and clinical development activities and to pay us royalties on applicable product sales, if any. The collaboration has an initial term of one year and MPC may extend the program for an additional year, subject to mutually acceptable terms.
Biogen, Inc.
In June 2001, we expanded our existing relationship with Biogen, Inc. Under this drug discovery collaboration, we agreed to screen a number of proteins supplied to us by Biogen. Biogen maintains the option to develop some or all of the small molecule compounds we identify. If Biogen chooses to develop any of these small molecule compounds, we will assign the rights to those compounds to Biogen and it is obligated to use commercially reasonable efforts to develop and commercialize products. Subject to some restrictions, we retain the rights to use small molecule compounds that we identify for Biogen for purposes unrelated to the collaboration. Biogen will pay us an additional fee if we conduct optimization on their behalf. Biogen is required to make milestone payments to us based upon various pre-clinical and clinical development activities and has agreed to pay us royalties on applicable product sales, if any. Biogen also made us an interest-bearing loan that will convert into our common stock upon the closing of this offering. The collaboration has an initial term of 39 months and is subject to extension by mutual agreement.
Oxford GlycoSciences (UK) Limited
In June 2001, we entered into a chemical genomics collaboration agreement with Oxford GlycoSciences (UK) Limited, or OGS, under which we agreed to screen a number of proteins supplied to us by OGS. OGS maintains the option to develop a specific number of the small molecule compounds we identify. If OGS chooses to develop any of these small molecule compounds, we will assign the rights to those compounds to OGS and it is obligated to use commercially reasonable efforts to develop and commercialize products. OGS pays us fees to screen these proteins and has agreed to pay us an additional fee if we conduct optimization on its behalf. OGS is required to make milestone payments to us based upon various pre-clinical and clinical development activities and to pay us royalties on applicable product sales, if any. In addition, OGS paid us an up-front technology access fee and made an investment in our series E preferred stock. The collaboration has an initial term of three years and OGS may extend the collaboration for an additional year.
Merck & Co., Inc.
In November 2000, we entered into a drug discovery collaboration agreement with Merck & Co., Inc. under which we agreed to screen a number of proteins supplied to us by Merck. Merck, at its sole discretion, may elect to develop some or all of the small molecule compounds we identify. If Merck chooses to develop any of these small molecule compounds, we will assign the rights to those compounds to Merck and it is obligated to use commercially reasonable efforts, consistent with its usual practices, to develop and commercialize products. Merck paid us a fee to screen these proteins and has agreed to pay us an additional fee if we conduct optimization on their behalf. Merck is required to make milestone payments to us
40
based upon various pre-clinical and clinical development activities and to pay us royalties on applicable product sales, if any. The collaboration has an initial term of one year.
Our technologies
We have developed an integrated, proprietary suite of drug discovery technologies that form our scalable chemical genomics platform. We believe that our industrialized platform is capable of identifying small molecule drug candidates that interact, or bind, tightly to virtually every disease-associated protein. By evaluating these small molecules in disease models, our chemical genomics technologies simultaneously validate disease-associated proteins while discovering small molecule drug candidates. Chemical genomics represents a fundamental shift from conventional drug discovery methods and we believe it will establish the standard for future drug discovery.
Key elements of our integrated technologies include:
- •
- Library design: proprietary computational methods to design a small molecule library capable of binding to virtually every disease-associated protein;
- •
- Library production: chemical techniques to synthesize a library comprised of over 10 million small molecules, stored as approximately 5,000 mixtures, each containing approximately 2,000 mass encoded compounds;
- •
- Protein screening: a screening engine that can be scaled to eventually screen virtually all disease-associated proteins against our library using proprietary analytical hardware and software systems to identify small molecules which bind tightly and specifically to a disease-associated protein;
- •
- Drug candidate optimization: a method to rapidly optimize small molecules as drug development candidates; and
- •
- Chemical genomics information management: comprehensive information management tools to utilize the wealth of information about small molecule drug candidates and their interaction with disease-associated proteins.
Library design
Our primary tool for chemical library design is called Quantized Surface Complementarity, or QSC. QSC is a computational model capable of operating on thousands of proteins and is unconstrained by existing library design methods. QSC identifies surface domains, more commonly called binding pockets, and a large number of chemical templates that match those binding pockets. Mixtures of small molecule compounds, based upon these chemical templates, are then selected for synthesis after we assess their medicinal relevance, synthetic tractability and uniqueness through a combination of software and medicinal chemistry guidelines.
Library production
We synthesize small molecule compounds as mixtures in a format that enables ALIS to efficiently screen proteins. Our proprietary NeoMorph library, consisting of over 10 million diverse small molecules, is stored as approximately 5,000 mixtures, each containing approximately 2,000 mass-encoded compounds. We use proprietary software to ensure that
41
each small molecule compound within a mixture can be uniquely identified by its mass. This obviates the need for artificial tagging methods, which adds preparation time and cost, and may interfere with the screening process.
Protein screening
Our Automated Ligand Identification System, or ALIS, uses our proprietary mass-encoded library to perform ultra-high capacity, micro-volume, affinity-based screening that we believe can be scaled to the genome. Each of our fully automated ALIS workstations can screen a total of 12 proteins per year against our entire library of small molecules at a rate of approximately 300,000 compounds per day. ALIS permits the identification of drug candidates without any previous knowledge of protein function and can identify small molecules that bind to both soluble and membrane proteins. ALIS is also being applied to identify and eliminate compounds or library members that interact with proteins known to be responsible for metabolism, clearance, toxicity and other factors that contribute to drug development failure.
The following diagram illustrates the five steps of the ALIS process:
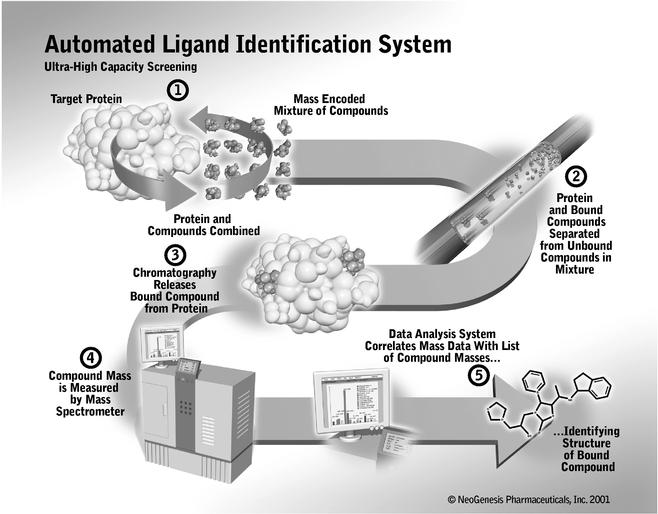
Step 1. A protein is combined with a mass-encoded mixture of small molecule compounds from our library. These compounds interact with the protein and some of these compounds may bind.
42
Step 2. The protein and any small molecule compounds that are bound to it, or protein-compound complexes, are separated from unbound small molecule compounds by micro-volume size exclusion chromatography.
Step 3. Protein-compound complexes are subjected to high-resolution reverse-phase liquid chromatography under high temperature. These conditions unfold the protein and release the small molecule compounds.
Step 4. The mass of each released small molecule compound is measured by an electrospray mass spectrometer.
Step 5. A proprietary data analysis system identifies the chemical structure of each of the released compounds by correlating the mass spectral data with the list of masses of compounds from the mixture.
This process may be repeated for a particular protein for every mixture in the library.
Drug candidate optimization
The chemical synthesis methods we use to prepare the small molecule compounds in our screening library can also be utilized to prepare variants, or analogs, of the compounds identified by ALIS. These well-developed methods enable the rapid synthesis of thousands of analogs of every compound existing in the screening library, meaning that billions of potential drug candidates can be easily accessed for drug optimization efforts. When a candidate has been identified by ALIS, we rapidly prepare large numbers of compounds in mixtures for further evaluation by ALIS. As a result, we believe we are able to identify compounds that bind to the protein with improved affinity more rapidly than conventional medicinal chemistry methods where compounds are synthesized one at a time. After improved compound analogs have been identified, we conduct additional evaluations to assess their effectiveness as drug candidates. Further optimization may be undertaken using our rapid procedures in combination with conventional medicinal chemistry methods until a compound qualifies as a drug development candidate.
Chemical genomics information management
We have designed and built a proprietary information management system to store and query information that is produced by our chemical genomics platform. This system captures the unique information related to the interaction of our small molecule compounds with proteins that we screen. Our chemical genomics information management system relies on sophisticated data analysis algorithms, database systems and user interface tools to develop information that will be critical for continual library design optimization, improved screening efficiency and rapid compound optimization. We believe that this information will assist in the intelligent development of a broad range of small molecule drugs.
Intellectual property
We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary rights are covered by valid and enforceable patents or trademarks, or are effectively maintained as trade secrets. Accordingly, patents, trademarks and other proprietary rights are an essential element of our business strategy. To date we have
43
been issued two U.S. patents that claim important aspects of our proprietary ALIS and NeoMorph technologies. In addition, we have pending ten U.S. patent applications covering various aspects of our technologies, including combinatorial chemistry methods, QSC computational chemistry methods and screening methods. International applications relating to these technologies also have been or are expected to be filed.
As the scaling of our operations yields novel drug candidates, we intend to apply for broad composition-of-matter patents on these drug candidates. In addition, we intend to file patent applications claiming new methods for treating diseases involving the proteins validated by our chemical genomics technologies. We have also applied for, and intend to continue to apply for, patents relating to our technologies, such as those embodied by ALIS.
We are party to various agreements that give us rights to use technologies developed in our collaborative research and development programs. These collaborations are intended to lead to the identification of small molecule drug candidates and the validation of drugs targets, with respect to which our collaborators, or we, either individually or jointly in accordance with the terms of our agreement, may seek patent protection.
As a general matter, obtaining patents in the biotechnology and pharmaceutical field is highly uncertain and involves complex legal, scientific and factual matters. Obtaining a patent in the United States in the biotechnology and pharmaceutical field can be expensive and can, and often does, require several years to complete. Failure to receive patents under the applications that we have filed and may file from time to time could be harmful to us. Our patent filings in the United States may be subject to interference or re-examination proceedings. The defense and prosecution of interference and re-examination proceedings and related legal and administrative proceedings in the United States involve complex legal and factual questions.
We also file patent applications outside of the United States. The laws of some foreign countries may not protect our proprietary rights to the same extent as do the laws of the United States. Third parties may attempt to oppose the issuance of our patents in foreign countries by way of opposition proceedings. Additionally, if an opposition proceeding is initiated against any of our patent filings in a foreign country, that proceeding could have an adverse effect on the corresponding patents that are issued or pending in the United States. If we become involved in any interference, re-examination, opposition or litigation proceedings in the United States or foreign countries regarding patent or other proprietary rights, those proceedings may result in substantial cost to us, regardless of the outcome. In addition, these proceedings may have a material adverse affect on our ability to develop, manufacture, market or license our technologies or products, or to maintain or form strategic alliances.
Although we plan to aggressively prosecute our patent applications and defend our patents against third-party infringement, we cannot assure you that any of our patent applications will result in the issuance of patents or that, if issued, such patents will not be challenged, invalidated or circumvented. Moreover, we cannot assure you that our patents, to the extent they are or will be issued, will provide us protection against competitors with other technologies. Our technologies and potential products may conflict with patents that have been or may be granted to competitors, universities or others. In particular, patent applications or patents for innovative and broadly applicable technologies, such as our ALIS system, are sometimes challenged by third parties as obvious, or as obvious extensions of technologies previously developed by those third parties.
44
As the biotechnology industry expands and more patents are issued, the risk increases that our technologies and potential products may give rise to claims that they infringe the patents of others. Third parties claiming infringement of their proprietary rights could bring legal actions against us claiming damages and seeking to enjoin our use or commercialization of a product or our use of a technology. We cannot assure you that such claims will not be brought against us in the future. If any actions based on these claims are successful, in addition to any potential liability for damages, we could be required to obtain a license in order to continue to use a technology or to manufacture or market a product, or could be required to cease using those products or technologies. Any claim, with or without merit, could result in costly litigation and divert the efforts and attention of our scientific and management personnel. We cannot assure you that we would prevail in any action or that any license required under any patent would be made available or would be made available on acceptable terms.
We are aware that another entity has filed patent applications in several countries, relating to a technique for creating a mass-encoded combinatorial library. We believe that the method described in those applications is different from the method that we employ, and at this time, no patents on this method have been issued to this other entity. If valid patents were to issue to this other entity in countries that are important to our business strategy and if it were deemed that we infringe on those patents, we would be required to alter our method or obtain a license in order to expand and use our mass-encoded library. If we were unable to alter our method or to obtain a license on commercially acceptable terms, if at all, our ability to conduct our business would be significantly impaired.
In addition to patent protection, we rely upon trade secrets, proprietary know-how and continuing technological advances to develop and maintain our competitive position. To maintain the confidentiality of our trade secrets and proprietary information, all of our employees are required to enter into and adhere to an invention and non-disclosure agreement and non-competition and non-solicitation agreement, as a condition of employment. Additionally, our employment-confidentiality and invention-assignment agreement requires that our employees do not bring to NeoGenesis, or use without proper authorization, any third-party proprietary technology. We also require all of our consultants and collaborators that have access to proprietary property to execute confidentiality and invention rights agreements in our favor before beginning their relationship with us. While such arrangements are intended to enable us to better control the use and disclosure of our proprietary property and provide for our ownership of proprietary technology developed on our behalf, they may not provide us with meaningful protection for such property and technology in the event of unauthorized use or disclosure.
Competition
The development and commercialization of new drugs is competitive and we will face competition from pharmaceutical and biotechnology companies, academic and scientific institutions, governmental agencies and public and private research organizations. Our competitors may develop products or other technologies that are more effective, safer or less costly than any that we might develop, or may obtain FDA approval for their products more rapidly than we may obtain approval for ours. In addition, we may face competition based on other factors, including size, diversity and ease-of-use of compound libraries, speed and cost of
45
identifying and optimizing potential small molecule compounds and patent positions from companies offering one or more technology components of the discovery process.
Most pharmaceutical and biotechnology companies have internal research and development departments and could provide significant competition should any of the products we develop compete with their existing or future drugs. Many of our competitors have greater financial, operational, sales and marketing resources than us. In addition, we compete with these entities, as well as academic and research institutions, contract research companies and other firms, to hire qualified scientists and other personnel.
Facilities
We lease and occupy 27,992 square feet of laboratory and office space at a single location on two floors in Cambridge, Massachusetts. Our lease expires in April 2007 and includes an option for us to extend for an additional period of five years. The base rent for the extension period, if exercised, would be set at fair market rent.
We believe that our existing facilities are adequate for our current needs and that we will be able to obtain additional space, as needed, on commercially reasonable terms.
Legal proceedings
We are not a party to any legal proceedings.
Employees
As of September 30, 2001, we had 80 full-time employees, including 38 Ph.D.s. Of our full time employees, 64 are in research and development, seven are in engineering and informatics, six are in corporate administration and three are in business development. None of our employees is represented by a labor union, and we have never experienced a work stoppage, slowdown or strike. Our management considers its relationship with our employees to be excellent and employee turnover to be low.
46
Management
Executive officers, key employees and directors
Our executive officers, key employees and directors and their respective ages and positions are as follows:
|
Name
| | Age
| | Position
|
|---|
|
| Satish Jindal, Ph.D. | | 46 | | Chief Executive Officer, Chief Scientific Officer and Director |
Allen H. Michels (1) |
|
61 |
|
Chairman of the Board, President and Chief Operating Officer |
David M. Hunter |
|
57 |
|
Vice President, Finance, and Chief Financial Officer |
Steven P. Adams, Ph.D. |
|
48 |
|
Vice President, Research and Development |
Seth N. Birnbaum |
|
28 |
|
Vice President, Systems Engineering |
Daniel L. Flynn, Ph.D. |
|
47 |
|
Vice President, Chemistry |
Peter T. Lomedico, Ph.D. |
|
52 |
|
Vice President, Strategic Alliances |
Christopher J. Ainley |
|
43 |
|
Director |
Francis J. Bullock, Ph.D. (2) |
|
64 |
|
Director |
Joseph M. Davie, M.D., Ph.D. (1) |
|
62 |
|
Director |
Thomas B. Hallowell (2) |
|
53 |
|
Director |
Theodore G. Johnson (1) |
|
69 |
|
Director |
Michael B. Sheffery, Ph.D. (1)(2) |
|
51 |
|
Director |
|
- (1)
- Member of compensation committee
- (2)
- Member of audit committee
Executive officers
Satish Jindal, Ph.D., is one of our founders and is our Chief Executive Officer, Chief Scientific Officer and a director. Dr. Jindal has served as our Chief Executive Officer since November 2001 and previously held the position of President from our inception in March 1997 to November 2001. Dr. Jindal has been our Chief Scientific Officer since March 1997. He previously held the position of senior director of drug discovery at Applied BioSystems, a subsidiary of Applera Corporation (formerly known as PerSeptive Biosystems) from March 1994 to September 1996. Dr. Jindal was a postdoctoral fellow at the Whitehead Institute for Biomedical Research at the Massachusetts Institute of Technology. Dr. Jindal received a Ph.D. from Punjab University in India.
Allen H. Michels is one of our founders and is our Chairman of the Board, President and Chief Operating Officer. Mr. Michels had served as Chairman of our board of directors since our inception in March of 1997 and as our President and Chief Operating Officer since November 2001. He previously held the position of Chief Executive Officer from our inception in March
47
1997 to November 2001. Mr. Michels, who has considerable experience in high growth companies, has recently, as planned, exchanged roles with Satish Jindal, Ph.D., another founder, and will now dedicate his energies to rapid scaling-up of our drug discovery infrastructure. Prior to founding NeoGenesis, Mr. Michels was the founder, chief executive officer, director and consultant of Virtual Machine Works from 1994 to 1996. He was also founder, chief executive officer and director for Ardent Computer from 1985 to 1990, for Chronologic Simulation from 1992 to 1994 and for Convergent Technologies from 1979 to 1985. From 1967 to 1979 he was a general manager of Intel Corporation, Digital Equipment Corporation and North American Phillips. Mr. Michels received a B.S. from the Illinois Institute of Technology.
David M. Hunter is our Vice President, Finance and Chief Financial Officer. Mr. Hunter has served as our Vice President, Finance, and Chief Financial Officer since April 2000. He was previously the vice president, finance and chief financial officer of Micrion Corporation from April 1984 to January 2000 and has held senior level financial positions at several companies in a variety of industries, including software and manufacturing. Mr. Hunter is a certified public accountant and a member of the AICPA and the Financial Executives Institute. Mr. Hunter received a M.B.A. from Columbia University.
Steven P. Adams, Ph.D., is our Vice President, Research and Development. Dr. Adams has served as our Vice President, Research and Development since July 2001. He served as our Vice President, Drug Discovery and Development from March 2001 to July 2001. He previously held the positions of senior director of medicinal chemistry and senior director of drug discovery and evaluation at Biogen, Inc. from April 1992 to March 2001. Dr. Adams also served as a consultant for Geminx Biotechnologies Inc. from November 2000 to February 2001 and for Activx Biosciences from October 2000 to January 2001. Dr. Adams was Head of Biological Chemistry and a Science Fellow, Corporate Research Division at Monsanto Company, G.D. Searle Unit, from 1980 to 1992. Dr. Adams received a Ph.D. in Organic Chemistry from the University of Wisconsin.
Seth N. Birnbaum is one of our founders and is our Vice President, Systems Engineering. He has served as our Vice President, Systems Engineering since March 2001. From our inception in March 1997 to February 2001, Mr. Birnbaum held the positions of Director of Engineering and ALIS Operations, Director of Engineering and ALIS Project and Laboratory Engineer. Mr. Birnbaum previously held the position of Lead Project Engineer, Diversified Technologies and served as an automation consultant to the Scripps Institute from August 1996 to May 1997. He was previously the founder, vice president, engineering and member of the board of Trakus, Inc. from 1996 to 1997. Mr. Birnbaum received a B.S. in Bio-mechanical Engineering at Massachusetts Institute of Technology.
Key employees
Daniel L. Flynn, Ph.D., is our Vice President, Chemistry. Dr. Flynn has been our Vice President, Chemistry since October 1, 2001. He was previously the senior director of chemistry at Millennium Pharmaceuticals, Inc. from December 1999 to September 2001 and director, medicinal chemistry at Amgen, Inc. from October 1998 to November 1999. He also held the positions of director of medicinal chemistry, combinatorial chemistry and research fellow at Monsanto Company, G.D. Searle Unit, from 1988 to 1998. He was the founder and president of
48
The Medicinal & Bioorganic Chemistry Foundation. Dr. Flynn was a postdoctoral fellow at Indiana University and received a Ph.D. in Medicinal Chemistry from the University of Kansas.
Peter T. Lomedico, Ph.D., is our Vice President, Strategic Alliances. Dr. Lomedico has been our Vice President, Strategic Alliances since November 2001. He previously worked at CuraGen Corporation as vice president, discovery research from September 1999 to October 2001 and Genome Therapeutics Corporation as vice president, human genetics from January 1996 to August 1999. From January 1994 to December 1995, Dr. Lomedico served as research director and co-founder of Morphogenesis, Inc. From September 1980 to December 1993, Dr. Lomedico conducted genomics and drug discovery research in various positions at Hoffmann-LaRoche, Inc. Dr. Lomedico received his B.S. from Villanova University, his Ph.D. from the University of Texas at Houston and was a postdoctoral research fellow under Professor W. Gilbert at Harvard University.
Directors
Christopher J. Ainley has served as a director of NeoGenesis since September 1997. Mr. Ainley has been managing director and portfolio manager of the Mid-Cap Equity Fund and the Small-Cap Growth Fund of Trust Company of the West since October 1994. Mr. Ainley had previously managed investment funds at Putnam Investments from August 1987 to March 1992 and Morgan Guaranty Bank from April 1992 to September 1994. He received a M.B.A. from Harvard Business School. Mr. Ainley is the brother-in-law of Mr. Michels.
Francis J. Bullock, Ph.D., has served as a director of NeoGenesis since June 1999. Mr. Bullock has been senior consultant at Arthur D. Little, Inc. since September 1993. He was previously the senior vice president of research operations at the Schering-Plough Research Institute. He also held the position of head of the metabolic disease and CNS research at Abbott Laboratories. He is a member of board of directors of Genzyme Transgenics Corporation and Array Biopharma Inc. Dr. Bullock received a Ph.D. from Harvard University.
Joseph M. Davie, M.D., Ph.D., has served as a director of NeoGenesis since February 2001. Dr. Davie was a vice president and senior vice president, research at Biogen, Inc. from April 1993 to July 2000. From July 1987 to January 1993, Dr. Davie held several positions at G.D. Searle & Co., including president, research and development and senior vice president, science and technology. Previously, Dr. Davie served as professor and head of the Department of Microbiology and Immunology at Washington University School of Medicine. He is a member of the board of directors of Targeted Genetics Corporation. Dr. Davie received an A.B., M.A. and Ph.D. in Bacteriology from Indiana University and a M.D. from Washington University School of Medicine.
Thomas B. Hallowell has served as a director of NeoGenesis since October 1998. Mr. Hallowell has been investment counselor at Essex Street Associates, a private investment company since January 1990. He received his B.A. degree from Harvard College. Mr. Hallowell is a co-founder of the Stoneridge Children's Montessori School, Beverly, Massachusetts.
Theodore G. Johnson has served as a director of NeoGenesis since October 1998. Mr. Johnson served as executive vice president of Digital Equipment Corporation from 1980 to 1982. He retired from Digital in December 1982. He was previously the executive vice president of Sales and Service of Digital Equipment Corporation from 1965 to 1980. He is a member of the board
49
of directors at Gensym Corporation. Mr. Johnson received a M.B.A. from Harvard Business School.
Michael B. Sheffery, Ph.D., has served as a director of NeoGenesis since December 2000. Dr. Sheffery has been the managing general partner of OrbiMed Advisors, LLC since January 1998. He previously held the position of senior biotechnology industry analyst at Mehta & Isaly from April 1996 to December 1997. Prior to that, Dr. Sheffery was Head of the Laboratory of Gene Structure and Expression at Memorial Sloan-Kettering Cancer Center. Dr. Sheffery received a Ph.D. in Molecular Biology from Princeton University.
Election of directors
Following this offering, our board of directors will be divided into three classes, with members of each class serving for a staggered three-year term. Messrs. Hallowell and Johnson and Dr. Bullock will serve in the class whose term expires in 2004; Messrs. Michels and Ainley and Dr. Sheffery will serve in the class whose term expires in 2005; and Drs. Jindal and Davie will serve in the class whose term expires in 2006. Upon the expiration of the term of a class of directors, directors in that class will be elected for three-year terms at the annual meeting of stockholders in the year in which their term expires.
Compensation of directors
We reimburse our directors for reasonable out-of-pocket expenses incurred in attending meetings of our board of directors. Our board members do not receive compensation for their services as members of our board. On February 1, 1999, we granted Dr. Bullock an option to purchase 150,000 shares of our common stock at an exercise price of $0.08 per share. On February 9, 2001, we granted Dr. Davie an option to purchase 30,000 shares of our common stock at an exercise price of $1.49 per share.
Board committees
Our board of directors has established a compensation committee and an audit committee. The compensation committee, which consists of Drs. Davie (Chairman) and Sheffery and Messrs. Michels and Johnson, reviews executive salaries, administers bonuses, incentive compensation and stock plans, and approves the salaries and other benefits of our officers. In addition, the compensation committee consults with our management regarding our benefit plans and compensation policies and practices.
The audit committee, which consists of Mr. Hallowell (Chairman) and Drs. Sheffery and Bullock, reviews the professional services provided by our independent accountants, the independence of our accountants from our management, our annual financial statements and our system of internal accounting controls. The audit committee also reviews other matters with respect to our accounting, auditing and financial reporting practices and procedures as it may find appropriate or may be brought to its attention.
Compensation committee interlocks and insider participation
Prior to the appointment of the compensation committee, our full board of directors was responsible for the functions of a compensation committee. No interlocking relationship exists between any member of our board of directors or our compensation committee and any
50
member of our board of directors or compensation committee of any other company, and none of these interlocking relationships have existed in the past.
Executive compensation
The following table sets forth information, for the fiscal year ended December 31, 2000, with respect to the compensation paid and shares of our common stock underlying options granted to our:
- •
- Chief Executive Officer, and
- •
- our four most highly compensated executive officers who received annual compensation in excess of $100,000, who, together with our Chief Executive Officer, we refer to in this prospectus as the Named Executive Officers.
Summary compensation table
|
| | Annual compensation (1)
| | Long-term
compensation
awards
|
|---|
Name and principal position
| | Salary
| | Bonus
| | Restricted
stock award(s)
| | Securities
underlying
options(#)
|
|---|
|
Allen H. Michels,
Chief Executive Officer (2) | | $240,000 | | — | | — | | — |
Satish Jindal, Ph.D.,
President, Chief Scientific Officer (3) | | 200,833 | | — | | — | | — |
George R. Lenz, Ph.D.,
Vice President, Research and Development (4) | | 161,200 | | — | | — | | 137,500 |
David M. Hunter,
Vice President, Finance, and Chief Financial Officer | | 109,819 | | — | | — | | 500,000 |
Seth N. Birnbaum,
Vice President, Systems Engineering | | 105,400 | | — | | — | | — |
|
- (1)
- In accordance with the rules of the Securities and Exchange Commission, the compensation does not include medical, group life or other benefits which are available to our salaried employees, and perquisites and other benefits, securities or property which do not exceed the lesser of $50,000 or 10% of the person's salary and bonus shown on the table.
- (2)
- Mr. Michels became our President and Chief Operating Officer in November 2001.
- (3)
- Dr. Jindal became our Chief Executive Officer in November 2001.
- (4)
- Our current Vice President, Research and Development, Dr. Adams, joined NeoGenesis in March 2001.
51
Stock options
The following table contains information concerning the grant of options to purchase shares of our common stock to each of the Named Executive Officers during the fiscal year ended December 31, 2000.
Option grants in 2000
|
| | Individual grants
| |
| |
|
|---|
| | Potential realizable
value at assumed annual rates of
for stock appreciation
option term (2)
|
|---|
| |
| | Percent of
total
options
granted to
employees
in 2000
| |
| |
|
|---|
| | Number of
securities
underlying
options
granted(1)
| |
| |
|
|---|
| | Exercise
price per
share
| |
|
|---|
| | Expiration
date
|
|---|
Name
| | 5%
| | 10%
|
|---|
|
| Allen H. Michels | | — | | — | | — | | — | | — | | — |
| Satish Jindal, Ph.D. | | — | | — | | — | | — | | — | | — |
| George R. Lenz, Ph.D. | | — | | — | | — | | — | | — | | — |
| David M. Hunter | | 500,000 | | 29.34% | | $0.306 | | 5/11/10 | | $96,221 | | $243,843 |
| Seth N. Birnbaum | | — | | — | | — | | — | | — | | — |
|
- (1)
- Shares of our common stock underlying options generally vest over a four-year period, with 25% of the shares vesting on the anniversary of the grant date, and the remainder of the shares vesting in equal annual installments over the following three years. The shares of our common stock underlying the option granted to Mr. Hunter will also vest immediately upon a merger, consolidation or other acquisition event resulting in a change in control of NeoGenesis.
- (2)
- The 5% and 10% assumed annual rates of compounded stock price appreciation are mandated by the rules of the Securities and Exchange Commission and do not represent an estimate or projection of our future stock prices. The actual value realized may be greater or less than the potential realizable values set forth in the table.
Option values at December 31, 2000
The following table contains information concerning option holdings for the year ended December 31, 2000 with respect to each of the Named Executive Officers.
|
| | Number of shares
underlying unexercised options at fiscal
year end (1)
| | Value of unexercised
in-the-money options at
fiscal year end (2)
|
|---|
Name
| | Exercisable
| | Unexercisable
| | Exercisable
| | Unexercisable
|
|---|
|
| Allen H. Michels | | — | | — | | — | | — |
| Satish Jindal, Ph.D. | | — | | — | | — | | — |
| George R. Lenz, Ph.D. | | 50,000 | | 87,500 | | $ | | $ |
| David M. Hunter | | — | | 500,000 | | $ | | $ |
| Seth N. Birnbaum | | — | | — | | — | | — |
|
- (1)
- "Exercisable" refers to those options which were both exercisable and vested, while "Unexercisable" refers to those options which were unvested.
- (2)
- Value is determined by subtracting the exercise price per share from the initial public offering price of $ per share and multiplying the result by the number of shares of our common stock underlying the options.
52
Employment agreements
We have not entered into employment agreements with any of our employees. Since May 1997 our policy is to extend written offers of employment to all of our employees. As a condition to employment, each new employee is required to sign our standard offer letter and Invention and Non-Disclosure Agreement and Non-Competition and Non-Solicitation Agreement.
In March 2000, we offered employment to David M. Hunter, our Chief Financial Officer. Under the terms of our offer letter to him, we offered Mr. Hunter the position of Vice President of Finance and Chief Financial Officer and we agreed to pay Mr. Hunter $145,000 per year. The board granted Mr. Hunter stock options to purchase 500,000 shares of our common stock. As a condition to Mr. Hunter's employment, he was required to sign our standard Invention and Non-Disclosure Agreement and Non-Competition and Non-Solicitation Agreement. In July 2000, Mr. Hunter's salary was increased to $155,150 per year.
In January 2001, we offered employment to Steven P. Adams, Ph.D., our Vice President, Research and Development. Under the terms of our offer letter to him, we agreed to pay Dr. Adams $175,000 per year. We agreed to grant Dr. Adams stock options to purchase 200,000 shares of our common stock. As a condition to Dr. Adams' employment, he was required to sign our standard Invention and Non-Disclosure Agreement and Non-Competition and Non-Solicitation Agreement.
Benefit plans
1997 Stock Option Plan
Our 1997 Stock Option Plan, as amended, provides for the grant of incentive stock options and nonqualified stock options to our employees, directors and consultants.
In November 2001, our board of directors approved an amendment to our 1997 Stock Option Plan. The maximum number of shares of our common stock that may be subject to incentive stock options granted under the 1997 Stock Option Plan, as amended, is 6,625,000 shares. As of November 9, 2001, there were 1,908,500 shares of our common stock available for future grant under the 1997 Stock Option Plan, as amended, and there were options to purchase 3,312,375 shares of our common stock outstanding, of which 720,500 were vested.
Upon the completion of this offering, options to purchase 408,750 shares of our common stock will become fully exercisable as a result of shares of our common stock being listed on The Nasdaq National Market under the terms of some of the stock options agreements that we entered into with our employees under our 1997 Stock Option Plan, as amended.
Our board of directors has authorized our compensation committee to administer our 1997 Stock Option Plan, as amended, including the granting of options to our executive officers. Subject to any applicable limitations contained in our 1997 Stock Option Plan, as amended, our board of directors, our compensation committee or executive officers to whom our board of directors delegates authority, as the case may be, selects the recipients of awards and determines:
- •
- the number of shares of our common stock covered by options and the dates upon which any option grants vest and become exercisable;
53
- •
- the exercise price of options; and
- •
- the duration of options.
Generally, options under our 1997 Stock Option Plan, as amended vest over a four year period from the date of grant. In the event of a merger, consolidation or other acquisition event resulting in a change in control of NeoGenesis, each outstanding option granted to specified executives shall become fully exercisable. Our compensation committee may in its discretion accelerate the vesting of any options at any time. In the event of a consolidation, merger, or sale or liquidation of all or substantially all of NeoGenesis' assets or upon the compensation committee's vote, the board of directors or the compensation committee, may in its discretion, provide that the outstanding options under the 1997 Stock Option Plan, as amended:
- •
- remain outstanding and upon the exercise of the option, the holder of the option will receive shares of our common stock, other securities or property that the holder of the option would have received had he or she exercised prior to the change in control;
- •
- be exercised in full prior to the change in control; or
- •
- be cancelled upon a thirty day notice to the holder of the option.
Our board of directors may amend, modify, suspend or terminate our 1997 Stock Option Plan, as amended at any time, subject to applicable law and the rights of holders of outstanding options. Our 1997 Stock Option Plan, as amended will terminate in April 1, 2007, unless our board of directors terminates it prior to that time.
401(k) plan
During 1999, we adopted an employee savings and retirement plan, qualified under Section 401(k) of the Internal Revenue Code, covering all of our employees. Pursuant to the 401(k) plan, employees may elect to reduce their current compensation by up to the statutorily prescribed annual limit and have the amount of the reduction contributed to the 401(k) plan.
2002 Employee Stock Purchase Plan
We adopted our 2002 Employee Stock Purchase Plan prior to this offering, which shall become effective as of July 1, 2002. Our 2002 Employee Stock Purchase Plan allows our employees to purchase shares of our common stock through payroll deductions. Our 2002 Employee Stock Purchase Plan is intended to qualify as an employee stock purchase plan under Section 423 of the Internal Revenue Code. The initial offering period will start on July 1, 2002 and will end on December 31, 2002, unless otherwise determined by our board of directors. Subsequent offerings under our 2002 Employee Stock Purchase Plan are planned to start on January 1 and July 1 of each year and end on June 30 and December 31 of each year. During each offering period, an eligible employee may select a rate of payroll deduction from 1% to 20% of compensation, up to an aggregate of $25,000 in any calendar year. The purchase price for shares of our common stock purchased under our 2002 Employee Stock Purchase Plan is 85% of the lesser of the fair market value of the shares of our common stock on the first day or the last day of the offering period. An aggregate of 500,000 shares of our common stock have initially been reserved for issuance under our 2002 Employee Stock Purchase Plan.
54
Principal stockholders
The following table provides information regarding the beneficial ownership of our common stock as of October 31, 2001, and as adjusted to reflect the completion of the sale of our common stock offered in this prospectus by:
- •
- each person who beneficially owns more than 5% of the outstanding shares of our common stock;
- •
- each of the Named Executive Officers;
- •
- each of our directors; and
- •
- all of our executive officers and directors as a group.
The amounts and percentages of common stock beneficially owned are reported on the basis of regulations of the Securities and Exchange Commission governing the determination of beneficial ownership of securities. Under the rules of the Commission, a person is deemed to be a "beneficial owner" of a security if that person has or shares "voting power," which includes the power to vote or to direct the voting of such security, or "investment power," which includes the power to dispose of or to direct the disposition of such security. A person is also deemed to be a beneficial owner of any securities of which that person has a right to acquire beneficial ownership within 60 days. Under these rules, more than one person may be deemed a beneficial owner of the same securities and a person may be deemed to be the beneficial owner of securities as to which such person has no economic interest.
The number of shares of our common stock that will be outstanding before this offering reflects 20,300,770 shares of our common stock outstanding as of October 31, 2001, the automatic conversion of all outstanding shares of our preferred stock into 14,359,145 shares of our common stock upon the completion of this offering and the automatic conversion of a $2.0 million convertible note payable into shares of our common stock upon the completion of this offering. The number of shares of our common stock that will be outstanding after this offering reflects each of the foregoing and the sale of the shares of our common stock offered by this prospectus.
|
|---|
| |
| | Percentage of shares of our
common stock
|
|---|
| | Number of shares of
our common stock
beneficially owned
|
|---|
Name of beneficial owner
| | Before offering
| | After offering
|
|---|
|
|---|
| Thomas B. Hallowell (1) | | 3,462,660 | | % | | % |
| Freya Fanning & Co. (2) | | 3,319,790 | | % | | % |
| Michael B. Sheffery, Ph.D. (3) | | 2,554,979 | | % | | % |
| Caduceus Private Investments, LP (4) | | 1,965,616 | | % | | % |
| Celltech R&D Limited (5) | | 1,675,042 | | % | | % |
| Allen H. Michels | | 1,620,000 | | % | | % |
| Satish Jindal, Ph.D. | | 1,262,000 | | % | | % |
| Peter B. Loring (6) | | 1,221,155 | | % | | % |
| Theodore G. Johnson (7) | | 691,560 | | % | | % |
| Seth N. Birnbaum | | 487,500 | | % | | % |
| Christopher J. Ainley | | 308,855 | | % | | % |
| George R. Lenz, Ph.D. (8) | | 250,000 | | % | | % |
| Francis J. Bullock, Ph.D. (9) | | 150,000 | | * | | * |
| David M. Hunter | | 125,000 | | * | | * |
| Joseph M. Davie, M.D., Ph.D. | | — | | — | | — |
| All executive officers and directors as a group (12 persons) (10) | | 11,774,435 | | % | | % |
|
- *
- Less than 1% of the outstanding shares of our common stock.
55
- (1)
- Includes 3,319,790 shares of our common stock owned by Freya Fanning & Co, a trust of which Mr. Hallowell is the trustee and 60,000 shares of our common stock held by the Key Trust of which Mr. Hallowell is a trustee. Mr. Hallowell disclaims beneficial ownership of the shares of our common stock held by Freya Fanning & Co. and the Key Trust, except to the extent of his pecuniary interest therein. Mr. Hallowell's address is c/o Essex Street Associates, 400 Essex Street, P.O. Box 5600, Beverly Farms, Massachusetts 01915.
- (2)
- Freya Fanning & Co.'s address is c/o Essex Street Associates, 400 Essex Street, P.O. Box 5600, Beverly Farms, Massachusetts 01915.
- (3)
- Consists entirely of 1,965,616 shares of our common stock held by Caduceus Private Investments, L.P. of which OrbiMed Capital LLC is the sole general partner, 46,606 shares of our common stock held by OrbiMed Associates LLC of which OrbiMed Advisors LLC is the managing member and 542,757 shares of our common stock held by Finsbury Worldwide Pharmaceutical Trust PLC for which OrbiMed Advisors LLC acts as investment advisor. Dr. Sheffery is a General Partner of OrbiMed Advisors LLC. Dr. Sheffery disclaims beneficial ownership of these shares of our common stock except to the extent of his pecuniary interest therein. Dr. Sheffery's address is c/o OrbiMed Advisors LLC, 767 Third Avenue, 30th Floor, New York, New York 10017-2023.
- (4)
- Caduceus Private Investments, LP's address is c/o OrbiMed Advisors LLC, 767 Third Avenue, 6th Floor, New York, New York 10017-2023.
- (5)
- Celltech R&D Limited's address is 208 Bath Road, Slough, Berkshire, SL1 3WE, England.
- (6)
- Consists of 81,570 shares of our common stock held by Mr. Loring, 1,049,005 shares of our common stock held by the NeoGenesis Drug Discovery Inc. Series D Preferred Investment Trust, or NDD Trust, of which Peter Loring is a trustee and 90,580 shares held by William B. Perkins and Peter B. Loring, as Trustees for Constantin von Wentzel Trust, or CVW Trust. Mr. Loring disclaims beneficial ownership of the shares of our common stock held by the NDD Trust and the CVW Trust, except to the extent of his pecuniary interest therein. Mr. Loring's address is c/o Loring, Wolcott & Coolidge Office, 230 Congress Street, Boston, Massachusetts 02110-2437.
- (7)
- Includes 300,000 shares of our common stock held by TGJ Trust of which Mr. Johnson is the trustee and includes 50,000 shares of our common stock held by the Wolverine Limited Partnership of which Mr. Johnson is a limited partner.
- (8)
- Includes 50,000 shares of our common stock issuable upon the exercise of options exercisable within 60 days of October 31, 2001.
- (9)
- Consist entirely of shares of our common stock issuable upon the exercise of options exercisable within 60 days of October 31, 2001.
- (10)
- Includes an aggregate of 200,000 shares of our common stock issuable upon the exercise of options within 60 days of October 31, 2001.
56
Certain transactions
Preferred stock issuances
Series A financing
In June 1997, we issued an aggregate of 1,250,000 shares of our series A convertible preferred stock to several individual investors and several of our existing stockholders, including 500,000 shares of our series A convertible preferred stock to Allen H. Michels, our President, Chief Operating Officer and Chairman of our board of directors, 250,000 shares of our series A convertible preferred stock to Christopher J. Ainley, a member of our board, and 250,000 shares of our series A convertible preferred stock to Theodore G. Johnson, a member of our board, at a purchase price of $0.20 per share for a total cash consideration to us of $250,000.
Series B financing
In March 1998, we issued an aggregate of 1,182,765 shares of our series B convertible preferred stock to several individual investors and several of our existing stockholders, including 153,925 shares of our series B convertible preferred stock to Theodore G. Johnson, 40,785 shares of our series B convertible preferred stock to Christopher J. Ainley, 81,570 shares of our series B convertible preferred stock to Freya Fanning & Co. and 81,570 shares of our series B convertible preferred stock to Peter B. Loring, at a purchase price of $1.2259 per share, for a total cash consideration to us of $1.5 million.
Series C financing
In April 1998, we issued an aggregate of 903,920 shares of our series C convertible preferred stock to one institutional investor, Freya Fanning & Co., at a purchase price of $2.2126 per share for a total cash consideration to us of $2.0 million. In January and May 1999 and July 2000, we issued an aggregate of 3,337,135 shares of our series C convertible preferred stock to several of our existing stockholders at a purchase price of $2.2126 per share for a total cash consideration to us of $7.4 million. In these two years, we sold 1,863,795 shares of our series C convertible preferred stock to Freya Fanning & Co., 45,195 shares of our series C convertible preferred stock to Theodore G. Johnson, 82,870 shares of our series C convertible preferred stock to Thomas B. Hallowell, 1,061,355 shares of our series C convertible preferred stock to Peter B. Loring, trustee of the NeoGenesis Drug Discovery, Inc. Series D Preferred Investment Trust and 90,580 shares of our series C convertible preferred stock to William B. Perkins and Peter B. Loring, as Trustees for the Constantin von Wentzel Trust.
Warrants to purchase shares of series C convertible preferred stock
In October 1998, we issued warrants to purchase an aggregate of 196,810 shares of our series C convertible preferred stock to several of our existing stockholders at an exercise price of $2.2126. All of the warrants have been exercised for a total cash consideration to us of approximately $435,460. In this transaction, we issued a warrant to purchase 18,070 shares of our series C convertible preferred stock to Christopher J. Ainley, a warrant to purchase 136,810 shares of our series C convertible preferred stock to Freya Fanning & Co. and a warrant to purchase 41,930 shares of our series C convertible preferred stock to Theodore G. Johnson.
57
Series D financing
In December 2000 and January 2001, we issued an aggregate of 4,623,487 shares of our series D convertible preferred stock to several institutional and individual investors and several existing stockholders at a purchase price of $4.9746 per share, for a total cash consideration to us of $23.0 million. In the December 2000 transaction, we sold 333,695 shares of our series D convertible preferred stock to Freya Fanning & Co., 100,510 shares of our series D convertible preferred stock to Theodore G. Johnson and 1,965,616 shares of our series D convertible preferred stock to Caduceus Private Investments, LP.
Series E financing
In July 2001 and August 2001, we issued an aggregate of 3,517,588 shares of our series E convertible preferred stock to three of our collaborators, including Celltech R&D Limited, at a purchase price of $5.97 per share, for a total cash consideration to us of $21.0 million.
Loans from investors and members of the board of directors
In April 1998, we borrowed an amount equal to $756,757 from Freya Fanning & Co. The principal amount, along with interest accruing at a rate of 8.0% per year, was due on December 31, 2000. In April 1998, we borrowed an amount equal to $100,000 from Christopher J. Ainley. The principal amount, along with interest accruing at a rate of 8.0% per year, was due on December 31, 2000. We also borrowed an amount equal to $232,000 from Theodore G. Johnson in April 1998. The principal amount, along with interest accruing at a rate of 8.0% per year, was due on December 31, 2000.
In addition, we also borrowed $400,000 from Freya Fanning & Co. in August 2000. The principal amount, along with interest accruing at a rate of 7.0% per year, was due on August 31, 2001. We borrowed $400,000 from Peter B. Loring in August 2000. The principal amount, along with interest accruing at a rate of 7.0% per year, was due on August 31, 2001. We have paid all amounts that we have borrowed from these persons on or prior to each of the due dates.
Rights and restrictions of preferred stock
Upon the completion of this offering, each share of our preferred stock will be converted into one share of our common stock. When outstanding shares of our preferred stock convert into common stock upon the completion of this offering, all rights and preferences of the previously outstanding preferred stock, including any dividend rights, liquidation preferences and special voting rights, will terminate and be of no further force and effect. Notwithstanding the conversion, the holders of the preferred stock, except for the holders of the series E convertible preferred stock, will be entitled to "piggyback" and demand registration rights with respect to the shares of our common stock into which the shares of our preferred stock convert. See "Description of capital stock."
Common stock, options and warrant issuances
From our inception in March 1997 through October 31, 2001, we have issued an aggregate 5,941,625 shares of our common stock in exchange for cash or services. The following table
58
presents information regarding our issuances of common stock to our executive officers and directors.
|
|---|
Name
| | Shares of our common stock
|
|---|
|
|
|---|
| Satish Jindal, Ph.D. | | 1,270,000 |
| Allen H. Michels | | 1,120,000 |
| Seth N. Birnbaum | | 512,500 |
| George R. Lenz, Ph.D. | | 200,000 |
| David M. Hunter | | 125,000 |
| Thomas B. Hallowell, as trustee of Key Trust | | 60,000 |
| Theodore G. Johnson | | 50,000 |
|
From our inception in March 1997 through November 9, 2001, we have granted options to purchase shares of our common stock to our executive officers and directors under our 1997 Stock Option Plan, as amended, as listed below.
|
|---|
Name
| | Options to purchase shares
of our common stock
| | Average exercise price
|
|---|
|
|---|
| David M. Hunter | | 500,000 | | $ 0.31 |
| George R. Lenz, Ph.D. | | 250,000 | | 0.05 |
| Steven P. Adams, Ph.D. | | 200,000 | | 1.49 |
| Francis J. Bullock, Ph.D. | | 125,000 | | 0.08 |
| Satish Jindal, Ph.D. | | 100,000 | | 5.00 |
| Joseph M. Davie, M.D., Ph.D. | | 30,000 | | 1.49 |
|
Agreements with directors and executive officers
In August 2001, we loaned $250,000 to Satish Jindal, Ph.D., our Chief Executive Officer. This loan is forgivable over a five year period.
In addition, we have extended written offers of employment to Mr. Hunter and Dr. Adams. For a more detailed description of these offers, see "Management—Employment agreements."
Change in control and indemnification
Our certificate of incorporation limits the liability of our directors for monetary damages arising from a breach of their fiduciary duties as directors, except to the extent otherwise required by Delaware law. Such limitation of liability does not affect the availability of equitable remedies such as injunctive relief or rescission.
Our by-laws provide that we may indemnify our directors and officers to the fullest extent permitted by Delaware law.
We have not entered into any indemnification agreements with our directors and officers and our directors and officers have the right to be indemnified to the extent permitted by Delaware law and our by-laws, as amended.
59
Future transactions
We believe that all of the transactions that we consummated with parties that may be deemed to be affiliated with us, as set forth above, were made on terms no less favorable to us than could have been obtained from unaffiliated third parties. We will require that all future transactions with parties affiliated with us, including loans between us and our officers, directors, principal stockholders and their affiliates, be approved by a majority of our board of directors, including a majority of independent and disinterested directors, and that such transactions be on terms no less favorable to us than could be obtained from unaffiliated third parties.
60
Description of capital stock
General
We are authorized to issue 95,000,000 shares of our common stock, $.001 par value per share, and 5,000,000 shares of our preferred stock, $.001 par value per share. The following description of our capital stock is subject to and qualified in its entirety by our certificate of incorporation and by-laws, each as amended and restated, and by the provisions of applicable Delaware law.
Common stock
As of October 31, 2001, there were shares of our common stock outstanding, assuming the automatic conversion of all outstanding shares of our preferred stock into 14,359,145 shares of our common stock upon the completion of this offering and the automatic conversion of a $2.0 million convertible note payable into shares of our common stock upon the completion of this offering. These shares of our common stock were held of record by approximately 79 stockholders.
Holders of our common stock are entitled to one vote per share for each share held of record on all matters to be voted upon by the stockholders. Accordingly, holders of a majority of the shares of our common stock entitled to vote in any election of directors may elect all of the directors standing for election. Subject to preferences that may be applicable to any outstanding preferred stock, holders of our common stock are entitled to receive ratably such dividends as may be declared from time to time by our board of directors out of funds legally available for that purpose. In the event of a liquidation, dissolution or winding up of NeoGenesis, the holders of our common stock are entitled to share ratably in all assets remaining after payment of liabilities, subject to prior distribution rights of preferred stock, if any, then outstanding. The holders of our common stock have no cumulative voting rights, preemptive or conversion rights or other subscription rights. There are no redemption or sinking fund provisions applicable to our common stock. The rights, preferences and privileges of holders of our common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of our preferred stock which we may designate and issue in the future. We have received full payment for all outstanding shares of our common stock and cannot require our shareholders to make further payments on the stock.
Preferred stock
Our board of directors has the authority, without action by the stockholders, to designate and issue preferred stock in one or more series and to designate the rights, preferences and privileges of each series, which may be greater than the rights of our common stock. It is not possible to state the actual effect of the issuance of any shares of our preferred stock upon the rights of holders of shares of our common stock until our board of directors determines the specific rights of the holders of such shares of preferred stock. The effects, however, might include, among other things:
- •
- restriction of dividends on our common stock;
- •
- dilution of the voting power of our common stock;
61
- •
- impairment of the liquidation rights of our common stock; or
- •
- delay and prevention of a change in control of NeoGenesis without further action by the stockholders.
Registration rights
As of October 31, 2001, the holders of approximately 10,841,557 shares of our common stock had demand registration rights with respect to those shares under the Securities Act. We granted these rights under the terms of an investor rights agreement, dated as of December 27, 2000, to investors that participated in some of our preferred stock financings, some of whom are affiliates or directors of NeoGenesis. If requested to do so by holders of over 50% of the total number of shares of our common stock issued upon the conversion of our series A preferred stock, series B preferred stock, series C preferred stock and series D preferred stock, we will be required, subject to limitations relating to the timing of the request, to file a registration statement under the Securities Act covering all registrable shares having a market value of at least $5.0 million that are requested to be registered. We are required to effect up to two of these demand registrations. Similarly, if requested to do so by holders of over 50% of the total number of shares of our common stock issued upon the conversion of our series D preferred stock, we will be required, subject to limitations relating to the timing of the request, to file a registration statement under the Securities Act covering all registrable shares having a market value of at least $5.0 million that are requested to be registered. We are required to effect up to two of these demand registrations. We will bear all fees, costs and expenses of any of these demand registrations other than underwriting discounts and commissions.
Once we are eligible to register shares using a short-form registration statement, we will be required, if requested to do so by any holder or holders of shares of our common stock issued upon the conversion of our preferred stock, to register shares having a market value of at least $1.0 million. In any six-month period, we are not required to effect more than one short-form registration requested by holders of shares of our common stock issued upon the conversion of our series A preferred stock, series B preferred stock and series C preferred stock and we are not required to effect more than one short-form registration requested by holders of shares of our common stock issued upon the conversion of our series D preferred stock. We will bear all fees, costs and expenses of such registrations other than underwriting discounts and commissions.
In addition, holders of our common stock issued upon the conversion of our series A preferred stock, series B preferred stock, series C preferred stock and series D preferred stock have piggyback registration rights. If we propose to register any of our securities under the Securities Act other than in connection with our employee benefit plans or a corporate reorganization, then, subject to limitations based on the number of shares to be registered and the terms on which they are to be sold, the holders of registrable shares may require us to include all or a portion of their shares in such registration, although the managing underwriter of any such offering has the right to limit the number of shares in such registration. We will bear all fees, costs and expenses of such registrations other than underwriting discounts and commissions.
62
Delaware anti-takeover law and certain charter and by-law provisions
We are subject to Section 203 of the Delaware General Corporation Law, an anti-takeover law. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in any "business combination" with an "interested stockholder" for a period of three years following the date the person became an interested stockholder, unless the "business combination" or the transaction in which the person became an interested stockholder is approved in a prescribed manner. Generally, a "business combination" includes a merger, asset or stock sale or other transaction resulting in a financial benefit to the interested stockholder. Generally, an "interested stockholder" is a person who, together with affiliates and associates, owns or within three years prior to the determination of interested stockholder status, did own, 15% or more of a corporation's voting stock. The existence of this provision may have an anti-takeover effect with respect to transactions not approved in advance by our board of directors, including discouraging attempts that might result in a premium over the market price for the shares of our common stock held by stockholders.
Our amended and restated certificate of incorporation and by-laws provide for the division of our board of directors into three classes, as nearly equal in size as possible, with staggered three-year terms. Our by-laws also provide that vacancies on our board of directors may only be filled by a vote of a majority of the board of directors then in office. Furthermore, any director elected by the stockholders, or by the board of directors to fill a vacancy, may be removed only for cause by a vote of a majority of the voting power of the shares of our common stock entitled to vote for the election of directors. These provisions of our amended and restated certificate of incorporation and by-laws could make it more difficult for a third party to acquire, or discourage a third party from attempting to acquire, control of NeoGenesis and therefore may limit the price that certain investors might be willing to pay in the future for shares of our common stock.
Our amended and restated certificate of incorporation and by-laws further provide that any action required or permitted to be taken by our stockholders may be taken only at a duly called annual or special meeting of the stockholders and not by unanimous written consent of stockholders in lieu of a meeting. In addition, a special meeting of our stockholders can only be called by our Chairman of the board of directors, our President or a majority of our board of directors. These provisions could have the effect of delaying stockholder actions that are favored by the holders of a majority of our outstanding voting securities until the next annual stockholders' meeting, particularly because special meetings of the stockholders may only be called by our board of directors or our chief executive officer. These provisions may also discourage another person or entity from making a tender offer for our stock, because such person or entity, even if it acquired a majority of our outstanding voting securities, would be able to take action as a stockholder (such as electing new directors or approving a merger) only at a duly called stockholders meeting.
Our amended and restated certificate of incorporation provides that the affirmative vote of 67.0% of our outstanding voting stock is required to amend or repeal some provisions in the certificate of incorporation relating to preferred stock, election and authority of directors, indemnification of officers and directors, evaluation of tender offer, merger and other acquisition proposals by our board of directors, limitations on stockholder meetings and this
63
super majority provision. These provisions could have the effect of deterring another person or entity from seeking to acquire NeoGenesis.
Limitation of liability and indemnification
Our amended and restated certificate of incorporation incorporates provisions permitted under the Delaware General Corporation Law relating to the liability of directors. The provisions eliminate a director's liability for monetary damages for a breach of fiduciary duty, including gross negligence, except in circumstances involving certain wrongful acts, such as breach of director's duty of loyalty or acts or omissions that involve intentional misconduct or a knowing violation of law. These provisions do not eliminate a director's duty of care nor do they prevent recourse against directors through equitable remedies such as injunctive relief. Moreover, the provisions do not apply to claims against a director for violations of certain laws, including federal securities laws.
Our amended and restated certificate of incorporation also contains provisions to indemnify our directors, officers, employees or other agents to the fullest extent permitted by the Delaware General Corporation Law. These provisions may have the practical effect in certain cases of eliminating the ability of our stockholders to collect monetary damages from directors.
Transfer agent and registrar
The transfer agent and registrar for our common stock will be American Stock Transfer & Trust Company.
Listing
We intend to apply for an application for our common stock to be quoted on The Nasdaq National Market under the symbol NGPI.
64
Shares eligible for future sale
Sales of restricted shares
Upon the completion of this offering, based upon the number of shares of our common stock outstanding as of October 31, 2001, and assuming the automatic conversion of all outstanding shares of our preferred stock into 14,359,145 shares of our common stock upon the completion of this offering and the automatic conversion of a $2.0 million convertible note payable into shares of our common stock upon the completion of this offering, we will have shares of our common stock outstanding. Of these shares, shares of our common stock to be sold in this offering will be freely tradable without restriction or further registration under the Securities Act of 1933, as amended, except that any shares of our common stock purchased by our affiliates, as that term is defined in Rule 144 under the Securities Act, may generally only be sold in compliance with the limitations of Rule 144 described below.
The remaining shares of our common stock outstanding upon completion of this offering are deemed "restricted shares" under Rule 144 or Rule 701 under the Securities Act. None of these restricted shares of our common stock will be eligible for sale in the public market on the date of this prospectus. Upon expiration of the lock-up agreements described below, 180 days after the date of this prospectus, an additional shares of our common stock will be eligible for sale in the public market pursuant to Rule 144.
In general, under Rule 144, a stockholder who has beneficially owned his or her restricted shares of our common stock for at least one year is entitled to sell, within any three-month period, a number of shares of our common stock that does not exceed the greater of:
- •
- one percent of the then outstanding shares of our common stock (approximately shares of our common stock immediately after the completion of this offering); or
- •
- the average weekly trading volume in our common stock on The Nasdaq National Market during the four calendar weeks preceding the date on which notice of such sale is filed, provided certain requirements concerning availability of public information, manner of sale and notice of sale are satisfied.
In addition, our affiliates must comply with the restrictions and requirements of Rule 144, other than the one-year holding period requirement, in order to publicly sell shares of our common stock which are not restricted securities. A stockholder who is not one of our affiliates and has not been our affiliate for at least three months prior to the sale and who has beneficially owned restricted shares of our common stock for at least two years may resell the shares without limitation. In meeting the one- and two-year holding periods described above, a holder of restricted shares of our common stock can include the holding periods of a prior owner who was not our affiliate. The one- and two-year holding periods described above do not begin to run until the full purchase price or other consideration is paid by the person acquiring the restricted shares of our common stock from the issuer or one of our affiliates.
65
Rule 701 provides that currently outstanding shares of our common stock acquired under our employee compensation plans may be resold beginning 90 days after the date of this prospectus by:
- •
- persons, other than our affiliates, subject only to the manner of sale provisions of Rule 144; and
- •
- our affiliates under Rule 144, without compliance with its one-year minimum holding period, subject to certain limitations.
Options
Rule 701 also provides that the shares of our common stock acquired upon the exercise of currently outstanding options or pursuant to other rights granted under our 1997 Stock Option Plan, as amended, may be resold beginning 90 days after the date of this prospectus by:
- •
- persons, other than our affiliates, subject only to the manner of sale provisions of Rule 144; and
- •
- our affiliates under Rule 144, without compliance with its one-year minimum holding period, subject to certain limitations.
At October 31, 2001 approximately 2,124,625 shares of our common stock were issued or issuable pursuant to vested options under our 1997 stock option plan, as amended, of which all vested options are subject to lock-up agreements with the underwriters. These shares will become eligible for sale in the public market in accordance with Rule 701 under the Securities Act beginning 180 days after the date of this prospectus.
Following the date of this prospectus, we intend to file one or more registration statements on Form S-8 under the Securities Act to register up to 7,125,000 shares of our common stock issuable under our 1997 Stock Option Plan, as amended and our 2002 Employee Stock Purchase Plan. These registration statements will become effective upon filing.
Lock-up agreements
We and our executive officers, directors and stockholders have agreed that, during the period beginning from the date of this prospectus and continuing to and including the date 180 days after the date of this prospectus, none of us will, directly or indirectly:
- •
- offer, sell, offer to sell, contract to sell or otherwise dispose of any shares of our common stock or any of our securities which are substantially similar to the common stock, including but not limited to any securities that are convertible into or exchangeable for, or that represent the right to receive, common stock or any such substantially similar securities; or
- •
- enter into any swap, option, future, forward or other agreement that transfers, in whole or in part, the economic consequence of ownership of common stock or any securities substantially similar to the common stock,
other than pursuant to employee stock option plans existing on the date of this prospectus, without the prior written consent of J.P. Morgan Securities Inc. In addition, our executive officers, directors and stockholders have agreed that, without the prior written consent of J.P. Morgan Securities Inc. on behalf of the underwriters, they will not, from the date of this prospectus and through the period ending 180 days after the date of this prospectus, make any demand for, or exercise any right with respect to, the registration of any shares of our common stock or any of our securities which are substantially similar to the common stock.
66
U.S. Federal tax considerations for non-U.S. holders
The following is a general discussion of certain material U.S. federal income and estate tax consequences of the ownership and disposition of our common stock by a beneficial owner thereof that is a "Non-U.S. Holder." A "Non-U.S. Holder" is a person or entity that, for U.S. federal income tax purposes, is a non-resident alien individual, a foreign corporation or a foreign estate or trust. The test for whether an individual is a resident of the U.S. for federal estate tax purposes differs from the test used for federal income tax purposes. Some individuals, therefore, may be "Non-U.S. Holders" for purposes of the federal income tax discussion below, but not for purposes of the federal estate tax discussion, andvice versa.
This discussion is based on the U.S. Internal Revenue Code of 1986, as amended, judicial decisions and administrative regulations and interpretations in effect as of the date of this prospectus, all of which are subject to change, including changes with retroactive effect. This discussion does not address all aspects of U.S. federal income and estate taxation that may be relevant to Non-U.S. Holders in light of their particular circumstances (including, without limitation, Non-U.S. Holders who are pass-through entities or who hold their common stock through pass-through entities) and does not address any tax consequences arising under the laws of any state, local or non-U.S. jurisdiction. Prospective holders should consult their tax advisors with respect to the federal income and estate tax consequences of holding and disposing of our common stock in light of their particular situations and any consequences to them arising under the laws of any state, local or non-U.S. jurisdiction.
Dividends
Subject to the discussion below, dividends, if any, paid to a Non-U.S. Holder of our common stock out of our current or accumulated earnings and profits generally will be subject to withholding tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. To obtain a reduced rate of withholding under a treaty, a Non-U. S. Holder generally will be required to provide us with a properly-executed IRS Form W-8BEN certifying the Non-U.S. Holder's entitlement to benefits under that treaty. Treasury Regulations provide special rules to determine whether, for purposes of determining the applicability of a tax treaty, dividends paid to a Non-U.S. Holder that is an entity should be treated as paid to the entity or to those holding an interest in that entity.
There will be no withholding tax on dividends paid to a Non-U.S. Holder that are effectively connected with the Non-U.S. Holder's conduct of a trade or business within the United States if a properly-executed IRS Form W-8ECI, stating that the dividends are so connected, is filed with us. Instead, the effectively connected dividends will be subject to regular U.S. income tax, generally in the same manner as if the Non-U.S. Holder were a U.S. citizen or resident alien or a domestic corporation, as the case may be, unless a specific treaty exemption applies. A corporate Non-U.S. Holder receiving effectively connected dividends may also be subject to an additional "branch profits tax", which is imposed, under certain circumstances, at a rate of 30% (or such lower rate as may be specified by an applicable treaty) of the corporate Non-U.S. Holder's effectively connected earnings and profits, subject to certain adjustments.
67
Gain on disposition of common stock
A Non-U.S. Holder generally will not be subject to U.S. federal income tax with respect to gain realized on a sale or other disposition of our common stock unless (i) the gain is effectively connected with a trade or business of such holder in the United States and a specific treaty exemption does not apply to eliminate the tax, (ii) if a tax treaty would otherwise apply to eliminate the tax, the gain is attributable to a permanent establishment of the Non-U.S. Holder in the U.S., (iii) in the case of Non-U.S. Holders who are nonresident alien individuals and hold our common stock as a capital asset, such individuals are present in the United States for 183 or more days in the taxable year of the disposition and certain other conditions are met, (iv) the Non-U.S. Holder is subject to tax pursuant to the provisions of the Code regarding the taxation of U.S. expatriates, or (v) we are or have been a "United States real property holding corporation" within the meaning of Code Section 897(c)(2) at any time within the shorter of the five-year period preceding such disposition or such holder's holding period. We believe that we are not, and do not anticipate becoming, a United States real property holding corporation. Even if we are treated as a United States real property holding corporation, gain realized by a Non-U.S. Holder on a disposition of our common stock will not be subject to U.S. federal income tax so long as (i) the Non-U.S. Holder is considered to have beneficially owned no more than five percent of our common stock at all times within the shorter of (a) the five year period preceding the disposition or (b) the holder's holding period and (ii) our common stock is regularly traded on an established securities market. There can be no assurance that our common stock will continue to qualify as regularly traded on an established securities market.
Information reporting requirements and backup withholding
Generally, we must report to the U.S. Internal Revenue Service the amount of dividends paid, the name and address of the recipient, and the amount, if any, of tax withheld. A similar report is sent to the holder. Pursuant to tax treaties or certain other agreements, the U.S. Internal Revenue Service may make its reports available to tax authorities in the recipient's country of residence.
Backup withholding will generally not apply to payments of dividends made by us or our paying agents to a Non-U.S. Holder if the holder has provided its federal taxpayer identification number, if any, or the required certification that it is not a U.S. person (which is generally provided by furnishing a properly-executed IRS Form W-8BEN), unless the payer otherwise has knowledge that the payee is a U.S. person.
Under current U.S. federal income tax law, information reporting and backup withholding imposed at a rate of 30.5% (30% after December 31, 2001 and subject to periodic reductions through 2006) will apply to the proceeds of a disposition of our common stock effected by or through a U.S. office of a broker unless the disposing holder certifies as to its non-U.S. status or otherwise establishes an exemption. Generally, U.S. information reporting and backup withholding will not apply to a payment of disposition proceeds where the transaction is effected outside the United States through a non-U.S. office of a non-U.S. broker. However, U.S. information reporting requirements (but not backup withholding) will apply to a payment of disposition proceeds where the transaction is effected outside the United States by or through an office outside the United States of a broker that fails to maintain documentary evidence that the holder is a Non-U.S. Holder and that certain conditions are met, or that the
68
holder otherwise is entitled to an exemption, and the broker is (i) a U.S. person, (ii) a foreign person which derived 50% or more of its gross income for certain periods from the conduct of a trade or business in the United States, (iii) a "controlled foreign corporation" for U.S. federal income tax purposes, or (iv) a foreign partnership (a) at least 50% of the capital or profits interest in which is owned by U.S. persons, or (b) that is engaged in a U.S. trade or business. Backup withholding will apply to a payment of disposition proceeds if the broker has actual knowledge that the holder is a U.S. person.
Backup withholding is not an additional tax. Rather, the tax liability of persons subject to backup withholding will be reduced by the amount of tax withheld. If withholding results in an overpayment of taxes, a refund may be obtained, provided that the required information is furnished to the U.S. Internal Revenue Service.
Federal estate tax
An individual Non-U.S. Holder who is treated as the owner of, or has made certain lifetime transfers of, an interest in our common stock will be required to include the value thereof in his gross estate for U.S. federal estate tax purposes, and may be subject to U.S. federal estate tax unless an applicable estate tax treaty provides otherwise.
69
Underwriting
J.P. Morgan Securities Inc. is acting as the sole book running manager for this offering.
We and the underwriters named below have entered into an underwriting agreement covering the common stock to be offered in this offering. J.P. Morgan Securities Inc., Bear, Stearns & Co. Inc. and SG Cowen Securities Corporation, are acting as representatives of the underwriters. Each underwriter has agreed to purchase the number of shares of common stock set forth opposite its name in the following table.
|
|---|
Name
| | Number of shares
|
|---|
|
|---|
| J.P. Morgan Securities Inc. | | |
| Bear, Stearns & Co. Inc. | | |
| SG Cowen Securities Corporation | | |
| | | |
| | | |
| | | |
| | | |
| | |
|
| Total | | |
|
The underwriting agreement provides that if the underwriters take any of the shares presented in the table above, then they must take all of these shares. No underwriter is obligated to take any shares allocated to a defaulting underwriter except under limited circumstances.
The underwriters are offering the shares of common stock, subject to the prior sale of shares, and when, as and if such shares are delivered to and accepted by them. The underwriters will initially offer to sell shares to the public at the initial public offering price shown on the cover page of this prospectus. The underwriters may sell shares to securities dealers at a discount of up to $ per share from the initial public offering price. Any such securities dealers may resell shares to certain other brokers or dealers at a discount of up to $ per share from the initial public offering price. After the initial public offering, the underwriters may vary the public offering price and other selling terms.
If the underwriters sell more shares than the total number shown in the table above, the underwriters have the option to buy up to an additional shares of common stock from us to cover such sales. They may exercise this option during the 30-day period from the date of this prospectus. If any shares are purchased with this option, the underwriters will purchase shares in approximately the same proportion as shown in the table above.
The following table shows the per share and total underwriting discounts that we will pay to the underwriters. These amounts are shown assuming both no exercise and full exercise of the underwriters' option to purchase additional shares.
|
|---|
| | Without overallotment exercise
| | With overallotment exercise
|
|---|
|
|---|
| Per share | | $ | | | $ | |
| Total | | $ | | | $ | |
|
The representatives have advised us that, on behalf of the underwriters, they may make short sales of our common stock in connection with this offering, resulting in the sale by the
70
underwriters of a greater number of shares than they are required to purchase pursuant to the underwriting agreement. The short position resulting from those short sales will be deemed a "covered" short position to the extent that it does not exceed the shares subject to the underwriters' over-allotment option and will be deemed a "naked" short position to the extent that it exceeds that number. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the trading price of the common stock in the open market that could adversely affect investors who purchase shares in this offering. The underwriters may reduce or close out their covered short position either by exercising the over-allotment option or by purchasing shares in the open market. In determining which of these alternatives to pursue, the underwriters will consider the price at which shares are available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. Any "naked" short position will be closed out by purchasing shares in the open market. Similar to the other stabilizing transactions described below, open market purchases made by the underwriters to cover all or a portion of their short position may have the effect of preventing or retarding a decline in the market price of our common stock following this offering. As a result, our common stock may trade at a price that is higher than the price that otherwise might prevail in the open market.
The representatives have advised us that, pursuant to Regulation M under the Securities Act of 1933, they may engage in transactions, including stabilizing bids or the imposition of penalty bids, that may have the effect of stabilizing or maintaining the market price of the shares of common stock at a level above that which might otherwise prevail in the open market. A "stabilizing bid" is a bid for or the purchase of shares of common stock on behalf of the underwriters for the purpose of fixing or maintaining the price of the common stock. A "penalty bid" is an arrangement permitting the representatives to claim the selling concession otherwise accruing to an underwriter or syndicate member in connection with the offering if the common stock originally sold by that underwriter or syndicate member is purchased by the representatives in the open market pursuant to a stabilizing bid or to cover all or part of a syndicate short position. The representatives have advised us that stabilizing bids and open market purchases may be effected on The Nasdaq National Market, in the over-the-counter market or otherwise and, if commenced, may be discontinued at any time.
One or more of the underwriters may facilitate the marketing of this offering online directly or through one of its affiliates. In those cases, prospective investors may view offering terms and a prospectus online and, depending upon the particular underwriter, place orders online or through their financial advisor.
We estimate that the total expenses of this offering, excluding underwriting discounts, will be approximately $ .
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act of 1933.
We and our executive officers, directors and certain stockholders have agreed that, during the period beginning from the date of this prospectus and continuing to and including the date 180 days after the date of this prospectus, none of us will, directly or indirectly, offer, sell, offer to sell, contract to sell or otherwise dispose of any shares of common stock or any of our securities which are substantially similar to the common stock, including but not limited to any
71
securities that are convertible into or exchangeable for, or that represent the right to receive, common stock or any such substantially similar securities or enter into any swap, option, future, forward or other agreement that transfers, in whole or in part, the economic consequence of ownership of common stock or any securities substantially similar to the common stock, other than pursuant to employee stock option plans existing on the date of this prospectus, without the prior written consent of J.P. Morgan Securities Inc.
At our request, the underwriters have reserved shares of common stock for sale to our directors, officers and employees who have expressed an interest in participating in this offering. We expect these persons to purchase no more than 10% of the common stock offered in this offering. The number of shares available for sale to the general public will be reduced to the extent such persons purchase such reserved shares.
We intend to apply to have the common stock listed on The Nasdaq National Market under the symbol NGPI.
It is expected that delivery of the shares will be made to investors on or about , 2002.
There has been no public market for our common stock prior to this offering. We and the underwriters will negotiate the initial offering price. In determining the price, we and the underwriters expect to consider a number of factors in addition to prevailing market conditions, including:
• the history of and prospects for our industry and for biotechnology companies generally;
• an assessment of our management;
• our present operations;
• our historical results of operations;
• the trend of our revenues and earnings; and
• our earnings prospects.
We and the underwriters will consider these and other relevant factors in relation to the price of similar securities of generally comparable companies. Neither we nor the underwriters can assure investors that an active trading market will develop for our common stock, or that our common stock will trade in the public market at or above the initial offering price.
72
Legal matters
The validity of the common stock offered hereby will be passed upon for NeoGenesis by Bingham Dana LLP, Boston, Massachusetts. John J. Concannon III, Esq., a partner at Bingham Dana LLP, is the Secretary of NeoGenesis. As of the date of this prospectus, individual attorneys at Bingham Dana beneficially own a total of 129,102 shares of our common stock. Legal matters in connection with this offering will be passed upon for the underwriters by Cahill Gordon & Reindel, New York, New York.
Experts
The financial statements as of December 31, 1999 and 2000 and for each of the three years in the period ended December 31, 2000, included in this prospectus and elsewhere in the registration statement, have been audited by Arthur Andersen LLP, independent public accountants, as indicated in their report with respect thereto, and are included herein in reliance upon the authority of said firm as experts in giving said report.
73
Where you can find more information
We have filed with the Securities and Exchange Commission a registration statement on Form S-1, including exhibits and schedules, under the Securities Act with respect to the shares of our common stock to be sold in the offering. This prospectus does not contain all of the information set forth in the registration statement. For further information with respect to us and the shares to be sold in the offering, reference is made to the registration statement and the exhibits and schedules attached to the registration statement. Statements contained in this prospectus as to the contents of any contract, agreement or other document referred to are not necessarily complete. As a result of this offering, we will become subject to the information and reporting requirements of the Securities Exchange Act of 1934, as amended, and will file annual, quarterly and current reports, proxy statements and other information with the Securities and Exchange Commission.
You may read and copy all or any portion of the registration statement or any reports, statements or other information that we file at the Securities and Exchange Commission's Public Reference Room at 450 Fifth Street, N.W., Washington, D.C. 20549. You can request copies of these documents, upon payment of a duplicating fee, by writing to the Securities and Exchange Commission. Please call the Securities and Exchange Commission at 1-800-SEC-0330 for further information on the operation of the Public Reference Room. Our Securities and Exchange Commission filings, including the registration statement, are also available to you on the Securities and Exchange Commission's web site http://www.sec.gov.
74
NeoGenesis Pharmaceuticals, Inc.
Index to financial statements
| | Page
|
|---|
| Report of Independent Public Accountants | | F-2 |
Balance sheets as of December 31, 1999 and 2000, September 30, 2001 (unaudited) and pro forma as of September 30, 2001 (unaudited) |
|
F-3 |
Statements of operations for the years ended December 31, 1998, 1999 and 2000, and for the nine months ended September 30, 2000 and 2001 (unaudited) |
|
F-4 |
Statements of convertible preferred stock and stockholders' equity (deficit) for the years ended December 31, 1998, 1999 and 2000, and for the nine months ended September 30, 2001 and pro forma September 30, 2001 (unaudited) |
|
F-5 |
Statements of cash flows for the years ended December 31, 1998, 1999 and 2000, and for the nine months ended September 30, 2000 and 2001 (unaudited) |
|
F-8 |
Notes to financial statements |
|
F-9 |
F–1
Report of Independent Public Accountants
To the Board of Directors of
NeoGenesis Pharmaceuticals, Inc.:
We have audited the accompanying balance sheets of NeoGenesis Pharmaceuticals, Inc. (the Company) as of December 31, 1999 and 2000, and the related statements of operations, stockholders' equity (deficit) and cash flows for each of the three years in the period ended December 31, 2000. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with auditing standards generally accepted in the United States. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 1999 and 2000, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2000 in conformity with accounting principles generally accepted in the United States.
Boston, Massachusetts
October 30, 2001
F–2
| |
|---|
| | December 31,
| | September 30,
| | Pro forma
September 30,
| |
|---|
| | 1999
| | 2000
| | 2001
| | 2001
| |
|---|
| |
| |
| | (unaudited)
| | (unaudited)
| |
|---|
| |
|---|
| Assets | | | | | | | | | | | |
| Current assets: | | | | | | | | | | | |
| | Cash and cash equivalents | | $ | 85,165 | | $ | 18,579,014 | | $37,895,726 | | $37,895,726 | |
| | Accounts receivable | | | 50,000 | | | 300,000 | | 1,694,283 | | 1,694,283 | |
| | Prepaid expenses and other current assets | | | 141,529 | | | 74,186 | | 152,523 | | 152,523 | |
| | |
| |
| | | | Total current assets | | | 276,694 | | | 18,953,200 | | 39,742,532 | | 39,742,532 | |
| | |
| |
| Property and equipment, net (Note 3) | | | 1,073,069 | | | 1,095,846 | | 3,655,348 | | 3,655,348 | |
| | |
| |
| Restricted cash | | | — | | | — | | 485,000 | | 485,000 | |
| Note receivable from officer (Note 4) | | | — | | | — | | 200,000 | | 200,000 | |
| Other assets | | | 123,842 | | | 123,842 | | — | | — | |
| | |
| |
| | | | Total other assets | | | 123,842 | | | 123,842 | | 685,000 | | 685,000 | |
| | |
| |
| | | | Total assets | | $ | 1,473,605 | | $ | 20,172,888 | | $44,082,880 | | $44,082,880 | |
| | |
| |
| |
| |
| |
| | |
| |
| Liabilities, convertible preferred stock and stockholders' equity (deficit) | | | | | | | | | | | |
| Current liabilities: | | | | | | | | | | | |
| | Current maturities of capital lease obligations | | $ | 79,944 | | $ | 149,785 | | $ 281,419 | | $ 281,419 | |
| | Current maturities of long-term debt | | | 66,359 | | | 45,356 | | 127,561 | | 127,561 | |
| | Accounts payable | | | 116,160 | | | 300,905 | | 431,564 | | 431,564 | |
| | Accrued expenses | | | 238,486 | | | 293,135 | | 290,611 | | 290,611 | |
| | Notes payable — stockholders (Note 7) | | | 1,338,757 | | | 800,000 | | — | | — | |
| | Deferred rent | | | 60,017 | | | 62,271 | | 169,165 | | 169,165 | |
| | Deferred revenue | | | 71,250 | | | 1,197,500 | | 5,019,476 | | 5,019,476 | |
| | |
| |
| | | | Total current liabilities | | | 1,970,973 | | | 2,848,952 | | 6,319,796 | | 6,319,796 | |
| | |
| |
| Advances pending conversion to series C preferred stock | | | 1,000,000 | | | — | | — | | — | |
| | |
| |
| Capital lease obligations, net of current maturities | | | 97,603 | | | 124,947 | | 422,571 | | 422,571 | |
| | |
| |
| Convertible note payable | | | — | | | — | | 2,018,545 | | — | |
| | |
| |
| Long-term debt, net of current maturities | | | 4,197 | | | — | | 105,266 | | 105,266 | |
| | |
| |
| Commitments and contingencies (Note 10) | | | | | | | | | | | |
| Convertible preferred stock, $.001 par value — | | | | | | | | | | | |
| | 14,359,145 shares authorized; no shares authorized pro forma | | | | | | | | | | | |
| | Issued and outstanding — 4,175,875 shares, 10,041,683 shares and 14,341,075 shares at December 31, 1999 and 2000, and September 30, 2001, respectively, no shares pro forma (liquidation value of $54,679,237 at September 30, 2001) | | | 5,866,775 | | | 29,854,507 | | 54,249,992 | | — | |
| Stockholders' equity (deficit): | | | | | | | | | | | |
| | Preferred stock, $.001 par value — | | | | | | | | | | | |
| | | no shares authorized issued and outstanding, actual, 5,000,000 shares authorized and no shares issued and outstanding pro forma | | | — | | | — | | — | | — | |
| | Common stock, $.001 par value — | | | | | | | | | | | |
| | | 24,021,645 shares authorized — 95,000,000 shares authorized pro forma | | | | | | | | | | | |
| | | Issued and outstanding — 4,580,000 shares, 5,242,625 shares, 5,846,625 shares, and shares at December 31, 1999 and 2000, September 30, 2001 and September 30, 2001 pro forma, respectively | | | 4,580 | | | 5,243 | | 5,847 | | 20,188 | |
| | Deferred compensation | | | (1,926 | ) | | (425,032 | ) | (1,568,575 | ) | (1,568,575 | ) |
| | Additional paid-in capital | | | 115,085 | | | 738,262 | | 3,164,040 | | 59,418,236 | |
| | Accumulated deficit | | | (7,583,682 | ) | | (12,973,991 | ) | (20,634,602 | ) | (20,634,602 | ) |
| | |
| |
| | | | Total stockholders' equity (deficit) | | | (7,465,943 | ) | | (12,655,518 | ) | (19,033,290 | ) | 37,235,247 | |
| | |
| |
| | | | Total liabilities, convertible preferred stock and stockholders' equity (deficit) | | $ | 1,473,605 | | $ | 20,172,888 | | $44,082,880 | | $44,082,880 | |
| | |
| |
| |
| |
| |
| |
The accompanying notes are an integral part of these financial statements.
F–3
| |
|---|
| | Year ended December 31,
| | Nine months ended September 30,
| |
|---|
| | 1998
| | 1999
| | 2000
| | 2000
| | 2001
| |
|---|
| |
| |
| |
| | (unaudited)
| |
|---|
| |
|---|
| Revenue: | | | | | | | | | | | | | | | | |
| | Collaboration | | $ | 567,772 | | $ | 1,803,478 | | $ | 1,423,750 | | $ | 646,250 | | $ | 2,520,524 | |
| | Grant | | | — | | | 63,221 | | | 170,088 | | | 112,745 | | | 126,776 | |
| | |
| |
| | | | Total revenue | | | 567,772 | | | 1,866,699 | | | 1,593,838 | | | 758,995 | | | 2,647,300 | |
| | |
| |
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Research and development(1) | | | 2,427,388 | | | 3,631,675 | | | 5,092,598 | | | 3,566,406 | | | 7,816,983 | |
| | Selling, general and administrative(1) | | | 1,389,877 | | | 1,169,113 | | | 1,644,088 | | | 1,167,038 | | | 2,068,569 | |
| | Stock-based compensation | | | — | | | 46,834 | | | 162,362 | | | 6,646 | | | 1,157,743 | |
| | |
| |
| | | | Total operating expenses | | | 3,817,265 | | | 4,847,622 | | | 6,899,048 | | | 4,740,090 | | | 11,043,295 | |
| | |
| |
| | | | Loss from operations | | | (3,249,493 | ) | | (2,980,923 | ) | | (5,305,210 | ) | | (3,981,095 | ) | | (8,395,995 | ) |
| | |
| |
Interest income |
|
|
16,000 |
|
|
13,381 |
|
|
50,441 |
|
|
27,604 |
|
|
826,858 |
|
| Interest expense | | | (42,232 | ) | | (171,413 | ) | | (135,540 | ) | | (92,493 | ) | | (91,474 | ) |
| | |
| |
| | | | Net loss | | $ | (3,275,725 | ) | $ | (3,138,955 | ) | $ | (5,390,309 | ) | $ | (4,045,984 | ) | $ | (7,660,611 | ) |
| | |
| |
| |
| |
| |
| |
| | |
| |
Net loss per common share (Note 2(m)): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Basic and diluted | | $ | (1.13 | ) | $ | (0.86 | ) | $ | (1.14 | ) | $ | (0.91 | ) | $ | (1.40 | ) |
| | |
| |
| |
| |
| |
| |
| | Pro forma basic and diluted | | | | | | | | $ | (0.55 | ) | | | | $ | (0.45 | ) |
| | | | | | | | |
| | | | |
| |
| | |
| |
| Shares used in computing net loss per common share: | | | | | | | | | | | | | | | | |
| | Basic and diluted | | | 2,905,651 | | | 3,665,247 | | | 4,722,259 | | | 4,424,509 | | | 5,466,846 | |
| | |
| |
| |
| |
| |
| |
| | Pro forma basic and diluted | | | | | | | | | 9,844,493 | | | | | | 17,103,824 | |
| | | | | | | | |
| | | | |
| |
| | |
| |
(1) Excludes non-cash stock-based compensation, as follows (Note 11(g)): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Research and development | | $ | — | | $ | 46,834 | | $ | 143,555 | | $ | 6,646 | | $ | 1,012,411 | |
| | Selling, general and administrative | | | — | | | — | | | 18,807 | | | — | | | 145,332 | |
| | |
| |
| | | | Total stock-based compensation | | $ | — | | $ | 46,834 | | $ | 162,362 | | $ | 6,646 | | $ | 1,157,743 | |
| | |
| |
| |
| |
| |
| |
| |
The accompanying notes are an integral part of these financial statements.
F–4
NeoGenesis Pharmaceuticals, Inc.
Statements of convertible preferred stock and stockholders' equity (deficit)
| |
|---|
| | Convertible preferred stock
| | Common stock
| |
| |
| |
| |
| |
|---|
| |
| | Additional
paid-in
capital
| |
| | Total stockholders'
(deficit)
equity
| |
|---|
| | Number of
shares
| | Carrying
value
| | Number of
shares
| | $.001 Par
value
| | Deferred
compensation
| | Accumulated
deficit
| |
|---|
| |
|---|
| Balance, December 31, 1997 | | 1,250,000 | | | $ 250,000 | | 5,000,000 | | | $5,000 | | $ | — | | $ | (4,000 | ) | $ | (1,169,002 | ) | $ | (1,168,002 | ) |
| | Sale of series B convertible preferred stock | | 40,784 | | | 50,000 | | — | | | — | | | — | | | — | | | — | | | — | |
| | Issuance of series B preferred stock upon the conversion of an advance from stockholder | | 1,141,981 | | | 1,400,000 | | — | | | — | | | — | | | — | | | — | | | — | |
| | Sale of series C convertible preferred stock, net of issuance costs of $200,010 | | 903,920 | | | 1,800,003 | | — | | | — | | | — | | | — | | | — | | | — | |
| | Net loss | | — | | | — | | — | | | — | | | — | | | — | | | (3,275,725 | ) | | (3,275,725 | ) |
| | |
| |
| Balance, December 31, 1998 | | 3,336,685 | | | 3,500,003 | | 5,000,000 | | | 5,000 | | | — | | | (4,000 | ) | | (4,444,727 | ) | | (4,443,727 | ) |
| | Sale of series C convertible preferred stock, net of issuance costs of $146,470 | | 839,190 | | | 2,366,772 | | — | | | — | | | — | | | — | | | — | | | — | |
| | Repurchase and retirement of restricted common stock | | — | | | — | | (462,500 | ) | | (463 | ) | | — | | | — | | | — | | | (463 | ) |
| | Issuance of warrants in conjunction with debt financing | | — | | | — | | — | | | — | | | — | | | 53,365 | | | — | | | 53,365 | |
| | Exercise of common stock options | | — | | | — | | 42,500 | | | 43 | | | — | | | 16,960 | | | — | | | 17,003 | |
| | Compensation expense related to modification of stock options | | — | | | — | | — | | | — | | | — | | | 38,974 | | | — | | | 38,974 | |
| | Deferred compensation related to issuance of stock options granted to non-employees | | — | | | — | | — | | | — | | | (9,786 | ) | | 9,786 | | | — | | | — | |
| | Amortization of deferred compensation | | — | | | — | | — | | | — | | | 7,860 | | | — | | | — | | | 7,860 | |
| | Net loss | | — | | | — | | — | | | — | | | — | | | — | | | (3,138,955 | ) | | (3,138,955 | ) |
| | |
| |
| Balance, December 31, 1999 | | 4,175,875 | | $ | 5,866,775 | | 4,580,000 | | $ | 4,580 | | $ | (1,926 | ) | $ | 115,085 | | $ | (7,583,682 | ) | $ | (7,465,943 | ) |
F–5
| |
Balance, December 31, 1999 |
|
4,175,875 |
|
$ |
5,866,775 |
|
4,580,000 |
|
$ |
4,580 |
|
$ |
(1,926 |
) |
$ |
115,085 |
|
$ |
(7,583,682 |
) |
$ |
(7,465,943 |
) |
| | Sale of series C convertible preferred stock | | 2,041,548 | | | 3,847,914 | | — | | | — | | | — | | | — | | | — | | | — | |
| | Issuance of series C convertible preferred stock upon conversion of advances from stockholders | | 451,957 | | | 1,000,000 | | — | | | — | | | — | | | — | | | — | | | — | |
| | Issuance of series C convertible preferred stock in exchange for services | | 4,440 | | | 9,824 | | — | | | — | | | — | | | — | | | — | | | — | |
| | Repurchase of series B convertible preferred stock | | (652,560 | ) | | (800,000 | ) | — | | | — | | | — | | | — | | | — | | | — | |
| | Sale of series D convertible preferred stock, net of issuance costs of $70,000 | | 4,020,423 | | | 19,929,994 | | — | | | — | | | — | | | — | | | — | | | — | |
| | Exercise of common stock options | | — | | | — | | 662,625 | | | 663 | | | — | | | 37,709 | | | — | | | 38,372 | |
| | Deferred compensation related to issuance of stock options to employees and non-employees | | — | | | — | | — | | | — | | | (585,468 | ) | | 585,468 | | | — | | | — | |
| | Amortization of deferred compensation | | — | | | — | | — | | | — | | | 162,362 | | | — | | | — | | | 162,362 | |
| | Net loss | | — | | | — | | — | | | — | | | — | | | — | | | (5,390,309 | ) | | (5,390,309 | ) |
| | |
| |
| Balance, December 31, 2000 | | 10,041,683 | | $ | 29,854,507 | | 5,242,625 | | $ | 5,243 | | $ | (425,032 | ) | $ | 738,262 | | $ | (12,973,991 | ) | $ | (12,655,518 | ) |
F–6
Balance, December 31, 2000 |
|
10,041,683 |
|
$ |
29,854,507 |
|
5,242,625 |
|
$ |
5,243 |
|
$ |
(425,032 |
) |
$ |
738,262 |
|
$ |
(12,973,991 |
) |
$ |
(12,655,518 |
) |
| | Sale of series C convertible preferred stock upon exercise of warrants | | 178,740 | | | 395,481 | | — | | | — | | | — | | | — | | | — | | | — | |
| | Sale of series D convertible preferred stock | | 603,064 | | | 3,000,002 | | — | | | — | | | — | | | — | | | — | | | — | |
| | Sale of series E convertible preferred stock | | 3,517,588 | | | 21,000,002 | | — | | | — | | | — | | | — | | | — | | | — | |
| | Exercise of common stock options | | — | | | — | | 604,000 | | | 604 | | | — | | | 124,492 | | | — | | | 125,096 | |
| | Compensation expense related to modification of stock option | | — | | | — | | — | | | — | | | — | | | 146,883 | | | — | | | 146,883 | |
| | Deferred compensation related to stock options granted to employees and non-employees | | — | | | — | | — | | | — | | | (2,154,403 | ) | | 2,154,403 | | | — | | | — | |
| | Amortization of deferred compensation | | — | | | — | | — | | | — | | | 1,010,860 | | | — | | | — | | | 1,010,860 | |
| | Net loss | | — | | | — | | — | | | — | | | — | | | — | | | (7,660,611 | ) | | (7,660,611 | ) |
| | |
| |
| Balance, September 30, 2001 (unaudited) | | 14,341,075 | | | 54,249,992 | | 5,846,625 | | | 5,847 | | | (1,568,575 | ) | | 3,164,040 | | | (20,634,602 | ) | | (19,033,290 | ) |
| | Conversion of convertible note payable into common stock (unaudited) | | — | | | — | | — | | | — | | | — | | | 2,018,545 | | | — | | | 2,018,545 | |
| | Conversion of convertible preferred stock into common stock (unaudited) | | (14,341,075 | ) | | (54,249,992 | ) | 14,341,075 | | | 14,341 | | | — | | | 54,235,651 | | | — | | | 54,249,992 | |
| | |
| |
| Pro forma balance, September 30, 2001 (unaudited) | | — | | $ | — | | — | | $ | 20,188 | | $ | (1,568,575 | ) | $ | 59,418,236 | | $ | (20,634,602 | ) | $ | 37,235,247 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
The accompanying notes are an integral part of these financial statements.
F–7
| |
|---|
| | Year ended December 31,
| | Nine months ended September 30,
| |
|---|
| | 1998
| | 1999
| | 2000
| | 2000
| | 2001
| |
|---|
| |
| |
| |
| | (unaudited)
| |
|---|
| |
|---|
| Cash flows from operating activities: | | | | | | | | | | | | | | | | |
| | Net loss | | $ | (3,275,725 | ) | $ | (3,138,955 | ) | $ | (5,390,309 | ) | $ | (4,045,984 | ) | $ | (7,660,611 | ) |
| | Adjustments to reconcile net loss to net cash used in operating activities — | | | | | | | | | | | | | | | | |
| | | Depreciation and amortization | | | 168,308 | | | 374,236 | | | 416,364 | | | 291,534 | | | 646,886 | |
| | | Stock-based compensation | | | — | | | 46,834 | | | 162,362 | | | 6,646 | | | 1,157,743 | |
| | | Issuance of series C preferred stock warrants | | | — | | | 53,365 | | | — | | | — | | | — | |
| | | Issuance of series C preferred stock for services | | | — | | | — | | | 9,824 | | | 9,824 | | | — | |
| | | Gain on disposal of fixed assets | | | — | | | (14,644 | ) | | — | | | — | | | — | |
| | | Deferred rent | | | 44,238 | | | 15,779 | | | 2,254 | | | — | | | 106,894 | |
| | | Changes in operating assets and liabilities — | | | | | | | | | | | | | | | | |
| | | | Accounts receivable | | | — | | | (22,500 | ) | | (250,000 | ) | | 50,000 | | | (1,394,283 | ) |
| | | | Prepaid expenses and other current assets | | | (199,557 | ) | | 30,528 | | | 67,343 | | | 30,409 | | | (28,337 | ) |
| | | | Other assets | | | — | | | (569 | ) | | — | | | — | | | 123,842 | |
| | | | Accounts payable | | | 452,284 | | | (485,771 | ) | | 184,745 | | | 140,985 | | | 130,659 | |
| | | | Accrued expenses | | | (66,859 | ) | | 98,342 | | | 54,649 | | | 140,158 | | | 16,021 | |
| | | | Deferred revenue | | | 374,728 | | | (303,478 | ) | | 1,126,250 | | | 878,750 | | | 3,821,976 | |
| | |
| |
| | | | | Net cash used in operating activities | | | (2,502,583 | ) | | (3,346,833 | ) | | (3,616,518 | ) | | (2,497,678 | ) | | (3,079,210 | ) |
| | |
| |
| Cash flows from investing activities: | | | | | | | | | | | | | | | | |
| | Increase in restricted cash | | | — | | | — | | | — | | | — | | | (485,000 | ) |
| | Issuance of note receivable to officer | | | — | | | — | | | — | | | — | | | (250,000 | ) |
| | Purchase of property and equipment | | | (739,038 | ) | | (117,450 | ) | | (257,090 | ) | | (232,428 | ) | | (2,236,917 | ) |
| | |
| |
| | | | | Net cash used in investing activities | | | (739,038 | ) | | (117,450 | ) | | (257,090 | ) | | (232,428 | ) | | (2,971,917 | ) |
| | |
| |
| Cash flows from financing activities: | | | | | | | | | | | | | | | | |
| | Proceeds from sale of convertible preferred stock | | | 1,850,003 | | | 2,366,772 | | | 23,777,908 | | | 4,847,914 | | | 24,395,485 | |
| | Proceeds from advances from stockholders | | | — | | | 1,000,000 | | | — | | | — | | | — | |
| | Proceeds from (repayment of) note payable — stockholders | | | 1,088,757 | | | 250,000 | | | (538,757 | ) | | (450,000 | ) | | (800,000 | ) |
| | Repayment of long-term debt | | | (75,439 | ) | | (73,031 | ) | | (25,200 | ) | | (23,137 | ) | | (77,530 | ) |
| | Issuance of convertible note payable | | | — | | | — | | | — | | | — | | | 2,000,000 | |
| | Payments under capital lease obligations | | | (21,724 | ) | | (64,389 | ) | | (84,866 | ) | | (61,327 | ) | | (275,212 | ) |
| | Proceeds from exercise of stock options and warrants | | | — | | | 17,003 | | | 38,372 | | | 27,618 | | | 125,096 | |
| | Repurchase of preferred stock/common stock | | | — | | | (463 | ) | | (800,000 | ) | | (800,000 | ) | | — | |
| | |
| |
| | | | | Net cash provided by financing activities | | | 2,841,597 | | | 3,495,892 | | | 22,367,457 | | | 3,541,068 | | | 25,367,839 | |
| | |
| |
| Change in cash and cash equivalents | | $ | (400,024 | ) | $ | 31,609 | | $ | 18,493,849 | | $ | 810,962 | | $ | 19,316,712 | |
| Cash and cash equivalents, beginning of period | | | 453,580 | | | 53,556 | | | 85,165 | | | 85,165 | | | 18,579,014 | |
| | |
| |
| Cash and cash equivalents, end of period | | $ | 53,556 | | $ | 85,165 | | $ | 18,579,014 | | $ | 896,127 | | $ | 37,895,726 | |
| | |
| |
| |
| |
| |
| |
| | |
| |
| Supplemental disclosure of cash flow information: | | | | | | | | | | | | | | | | |
| | Cash paid for interest | | $ | 18,460 | | $ | 30,645 | | $ | 226,387 | | $ | 22,045 | | $ | 92,874 | |
| | |
| |
| |
| |
| |
| |
| Supplemental disclosure of non-cash investing and financing activities: | | | | | | | | | | | | | | | | |
| | Property and equipment purchased under capital lease obligations or financed from notes payable | | $ | 334,676 | | $ | 144,256 | | $ | 182,050 | | $ | — | | $ | 969,471 | |
| | |
| |
| |
| |
| |
| |
| | Property and equipment purchased under SBIR grant award | | $ | — | | $ | — | | $ | 280,757 | | $ | 280,757 | | $ | 92,901 | |
| | |
| |
| |
| |
| |
| |
| | Conversion of advances from stockholder into 1,141,981 shares of series B convertible preferred stock | | $ | 1,400,000 | | $ | — | | $ | — | | $ | — | | $ | — | |
| | |
| |
| |
| |
| |
| |
| | Conversion of advances from stockholder into 451,957 shares of series C convertible preferred stock | | $ | — | | $ | — | | $ | 1,000,000 | | $ | — | | $ | — | |
| | |
| |
| |
| |
| |
| |
| |
The accompanying notes are an integral part of these financial statements.
F–8
1. Organization and nature of business
NeoGenesis Pharmaceuticals, Inc. (NeoGenesis or the Company) was incorporated on March 13, 1997, as a Delaware corporation under the name NeoGenesis Drug Discovery, Inc. The Company changed its name to NeoGenesis Pharmaceuticals, Inc. on November 14, 2001. The Company is currently focused on the discovery of novel small molecule drugs on a proteome and genome-wide scale.
The Company is subject to a number of risks associated with companies in the biotechnology industry. Principal among these are the risks associated with the Company's dependence on collaborative arrangements, development by the Company or its competitors of new technological innovations, dependence on key personnel, protection of proprietary technology, compliance with the U.S. Food and Drug Administration and other governmental regulations and approval requirements, as well as the ability to grow the Company's business and obtaining adequate financing to fund this growth.
2. Summary of significant accounting policies
The accompanying financial statements reflect the application of certain accounting policies as described in this note and elsewhere in the accompanying financial statements and notes.
- (a)
- Use of estimates in the preparation of financial statements
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates.
- (b)
- Unaudited interim financial information
The accompanying balance sheet as of September 30, 2001, and the statements of operations and cash flows for the nine months ended September 30, 2000 and 2001, and the statement of convertible preferred stock and stockholders' equity (deficit) for the nine months ended September 30, 2001 are unaudited, but in the opinion of management, include all adjustments, consisting only of normal recurring adjustments, necessary for a fair presentation of results for these interim periods. Certain information and footnote disclosures normally included in financial statements prepared in accordance with accounting principles generally accepted in the United States have been omitted, although the Company believes that the disclosures included are adequate to make the information presented not misleading. The results of operations for the nine months ended September 30, 2001, are not necessarily indicative of the results expected for the entire fiscal year. The data disclosed in the notes to these financial statements for these periods is unaudited.
F–9
- (c)
- Unaudited pro forma presentation
The unaudited pro forma balance sheet and the unaudited pro forma statement of convertible preferred stock and stockholders' equity (deficit) as of September 30, 2001, reflect the automatic conversion of all outstanding shares of series A, series B, series C, series D and series E convertible preferred stock into 14,341,075 shares of common stock upon the closing of the Company's proposed initial public offering (IPO). Additionally, all principal and interest outstanding under the Company's convertible note payable, $2,018,545 at September 30, 2001, will automatically convert into shares of the Company's common stock based on the price per share of the IPO.
- (d)
- Cash and cash equivalents
The Company considers all highly liquid instruments with original maturities of 90 days or less at the time of purchase to be cash equivalents. Cash equivalents are carried at cost which approximate their fair market value. As of December 31, 1999 and 2000, cash equivalents principally consisted of money market funds. As of September 30, 2001, cash equivalents principally consisted of money market funds and institutional bonds.
- (e)
- Depreciation and amortization
The Company provides for depreciation by charging to operations on a straight-line basis amounts estimated to allocate the cost of assets over the estimated useful lives of the related assets, as follows:
|
|---|
Asset classification
| | Estimated useful life
|
|---|
|
|---|
| Leasehold improvements | | Life of lease |
| Laboratory and office equipment | | 3-5 years |
| Equipment purchased under capital lease obligations | | Life of lease |
| Software | | 3 years |
| Furniture and fixtures | | 5 years |
|
- (f)
- Long-lived assets
In accordance with Statement of Financial Accounting Standards (SFAS) No. 121,Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets to Be Disposed Of, the Company continually evaluates whether events or circumstances have occurred that indicate that the carrying value of these assets may be impaired. The Company believes there has been no significant impairment of its long-lived assets as of each of the balance sheet dates presented.
- (g)
- Revenue recognition
The Company's collaboration revenue is primarily derived from long-term contracts with biotechnology and pharmaceutical companies. These contracts may include payments for screening and related services, technology access and license fees, research and
F–10
development milestones and royalties. The Company follows the provisions of the Securities and Exchange Commission's Staff Accounting Bulletin No. 101 (SAB 101),Revenue Recognition. In accordance with SAB 101, the Company recognizes revenue related to screening and related services as they are performed, so long as there is persuasive evidence of an arrangement, the fee is fixed or determinable and collection of the related receivable is probable.
Amounts received for technology access or license fees are deferred and recognized as services are performed over the performance period of the contract. Amounts received for milestones will be recognized when they are earned as long as the milestone is deemed to be substantive. Royalty revenue will be recognized when earned. The Company has not recognized any milestone or royalty revenue to date.
Amounts received prior to satisfying the above revenue recognition criteria are recorded as deferred revenue in the accompanying balance sheets. The Company expects that a majority of its deferred revenue balance as of September 30, 2001 will be recognized during the year ended December 31, 2002.
Grant revenues are derived from a Small Business Innovation Research (SBIR) grant. Under the terms of the Company's SBIR grant award, the Company will receive $950,000 in reimbursement for certain costs and fixed asset purchases. Amounts received for reimbursement of incurred costs are recognized as revenue upon cash receipt. Amounts received for reimbursement of fixed asset purchases have been netted against the cost of the asset. Fixed asset reimbursements amounted to $280,757 during the year ended December 31, 2000, and $92,901 during the nine months ended September 30, 2001. No such purchases were made in 1999.
- (h)
- Research and development
Research and development expenses primarily consist of salaries and related expenses for personnel and consulting services. Other research and development expenses include fees paid to consultants and outside service providers, the costs of materials used in screening and research and development, information technology and facilities costs. The Company charges all research and development expenses to operations as incurred.
- (i)
- Stock-based compensation
The Company's employee stock option plans are accounted for using the intrinsic value-based method of Accounting Principles Board (APB) Opinion No. 25,Accounting for Stock Issued to Employees, and related interpretations. The Company uses the fair value method of SFAS No. 123,Accounting for Stock-Based Compensation (SFAS No. 123), and Emerging Issues Task Force No. 96-18,Accounting for Stock Appreciation Rights and Other Variable Stock Option or Award Plans (EITF 96-18), to account for non-employee stock-based compensation.
F–11
- (j)
- Concentration of credit risk and significant customers
SFAS No. 105,Disclosure of Information about Financial Instruments with Off-Balance-Sheet Risk and Financial Instruments with Concentrations of Credit Risk, requires disclosure of any significant off-balance-sheet risks and credit risk concentrations. The Company has no significant off-balance-sheet risk. Financial instruments, which subject the Company to credit risk, principally consist of cash, cash equivalents, accounts receivable and a note receivable from an officer. The Company maintains the majority of its cash balances with high quality financial institutions. One customer represented 100% of total accounts receivable at December 31, 1999, while a different customer represented 100% of total accounts receivable at December 31, 2000. As of September 30, 2001, two customers represented 100% of total accounts receivable. During the year ended December 31, 1999, three customers, each of which individually represented greater than 10% of total revenue, represented approximately 80% of total revenue. During the year ended December 31, 2000, four customers, each of which individually represented greater than 10% of total revenue, represented 88% of total revenue. During the nine months ended September 30, 2000, five customers, each of which individually represented greater than 10% of total revenue, represented approximately 87% of total revenue. During the nine months ended September 30, 2001, three customers, each of which individually represented greater than 10% of total revenue, represented approximately 82% of total revenue.
- (k)
- Fair value of financial instruments
SFAS No. 107,Disclosures about Fair Value of Financial Instruments, requires disclosure of the fair value of financial instruments. The Company has estimated the fair value of financial instruments using available market information and appropriate valuation methodologies. The carrying value of cash, cash equivalents, accounts receivable, a note receivable from an officer and accounts payable approximate fair market value due to the short-term nature of these financial instruments. The carrying value of the Company's capital lease obligations, long-term debt and a convertible note payable also approximates fair value based on comparable market terms and conditions.
- (l)
- Comprehensive income (loss)
Comprehensive income (loss) is defined as the change in net assets of the Company during a period from transactions generated from non-owner sources. It includes all changes in equity during a period except those resulting from investments by owners and distributions to owners. The Company had no other components of comprehensive loss other than its net loss for all periods presented.
- (m)
- Net loss per common share and pro forma net loss per common share
Basic and diluted net loss per common share are presented in conformity with SFAS No. 128,Earnings per Share, for all periods presented. In accordance with the Securities and Exchange Commission's Staff Accounting Bulletin No. 98, the Company has determined
F–12
there were no nominal issuances of the Company's stock prior to the Company's IPO. In accordance with SFAS No. 128, basic and diluted net loss per common share has been computed by dividing the weighted average number of shares of common stock outstanding during the period, less restricted common shares subject to repurchase.
Restricted common stock, options and warrants to purchase 2,729,310, 3,264,310, 3,571,685, 3,552,460 and 2,759,945 shares of common stock for the years ended December 31, 1998, 1999 and 2000, and for the nine months ended September 30, 2000 and 2001, respectively, have not been included in the computation of diluted net loss per common share as their effects would have been anti-dilutive.
The Company's historical capital structure is not indicative of its capital structure after the proposed IPO due to the anticipated automatic conversion of all shares of convertible preferred stock into shares of common stock concurrent with the closing of the Company's proposed IPO. Accordingly, pro forma net loss per common share is presented for the year ended December 31, 2000 and the nine-month period ended September 30, 2001.
Pro forma basic and diluted net loss per common share is computed by dividing the net loss for the period by the pro forma weighted average number of unrestricted common shares outstanding, assuming the conversion of the Company's series A, B, C, D and E convertible preferred stock into shares of the Company's common stock which will occur upon the closing of the Company's proposed IPO, as if such conversion occurred at the date of the original issuance of the shares of convertible preferred stock. Pro forma diluted net loss per common share excludes options to purchase 3,571,685 and 2,759,945 shares of common stock for the year ended December 31, 2000 and the nine months ended September 30, 2001, respectively, and a warrant to purchase 18,070 shares of series C convertible preferred stock as their effect would be anti-dilutive. Pro forma basic and diluted net loss per common share does not include the common shares issuable upon the automatic conversion of the convertible note payable in connection with the Company's proposed IPO. These common shares have been excluded as the note holder does not have any right to convert the note prior to the IPO or the maturity date (Note 9).
F–13
The following table sets forth the shares used in computing basic and diluted net loss per common share and shares used in computing pro forma basic and diluted net loss per common share:
|
|---|
| | Year ended December 31,
| | Nine months ended September 30,
|
|---|
| | 1998
| | 1999
| | 2000
| | 2000
| | 2001
|
|---|
|
|---|
| Historical: | | | | | | | | | | |
| | Weighted average common shares outstanding | | 5,000,000 | | 4,591,309 | | 4,763,939 | | 4,680,896 | | 5,466,846 |
| | Less—Weighted average restricted common shares outstanding | | (2,094,349 | ) | (926,062 | ) | (41,680 | ) | (256,387 | ) | — |
| | |
|
| | Shares used in computing basic and diluted net loss per common share | | 2,905,651 | | 3,665,247 | | 4,722,259 | | 4,424,509 | | 5,466,846 |
| | |
| |
| |
| |
| |
|
| Pro Forma: | | | | | | | | | | |
| | Shares used in computing historical basic and diluted net loss per common share | | | | | | 4,722,259 | | | | 5,466,846 |
| | Weighted average number of shares assumed upon the conversion of convertible preferred stock | | | | | | 5,122,234 | | | | 11,636,978 |
| | |
|
| | Shares used in computing pro forma basic and diluted net loss per common share | | | | | | 9,844,493 | | | | 17,103,824 |
| | | | | | |
| | | |
|
|
- (n)
- Derivative instruments and hedging activities
SFAS No. 133,Accounting for Derivative Instruments and Hedging Activities, establishes accounting and reporting standards for derivative instruments, including certain derivative instruments embedded in other contracts, and for hedging activities. SFAS No. 133, as amended by SFAS No. 137 and SFAS No. 138, is effective for all fiscal quarters of fiscal years beginning after June 15, 2000. The Company's adoption of SFAS No. 133 during fiscal 2000 did not have an impact on its financial position or results of operations.
- (o)
- Segment information
SFAS No. 131,Disclosures About Segments of an Enterprise and Related Information, establishes standards for reporting information regarding operating segments and for related disclosures about products and services and geographical areas.
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision maker, or decision making group, in making decisions regarding resource allocation and assessing performance. To date, the Company has viewed its operations and manages its
F–14
business as principally one operating segment, which is drug discovery and development. As of September 30, 2001, all of the Company's assets are located in the United States.
For the years ended December 31, 1998, 1999, 2000, and the nine months ended September 30, 2000, all revenue recognized was derived from customers within the United States. For the nine months ended September 30, 2001, customers in the United States, Switzerland, United Kingdom and Japan accounted for 79%, 15%, 5% and 1% of the Company's revenue recognized, respectively.
- (p)
- Recent accounting pronouncements
In July 2001, the Financial Accounting Standards Board (FASB) issued SFAS No. 141,Business Combinations, and SFAS No. 142,Goodwill and Other Intangible Assets. Statement No. 141 requires that all business combinations initiated after June 30, 2001 be accounted for using the purchase method of accounting. Statement No. 142 discusses how intangible assets that are acquired should be accounted for in financial statements upon their acquisition and also how goodwill and other intangible assets should be accounted for after they have been initially recognized in the financial statements. Beginning on January 1, 2002, with the adoption of Statement No. 142, goodwill and certain purchased intangibles existing on June 30, 2001, will no longer be subject to amortization over their estimated useful life. Rather the goodwill and certain purchased intangibles will be subject to an annual assessment for impairment based on fair value. The provisions of Statement No. 142 are required to be applied starting with fiscal years beginning after December 15, 2001. The Company does not expect adoption of these statements to have a material impact on its financial position or results of operations.
In August 2001, the FASB issued SFAS No. 144, Accounting for the Impairment or Disposal of Long-Lived Assets, which supercedes SFAS No. 121,Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets to Be Disposed Of. SFAS No. 144 further refines the requirements of SFAS No. 121 that companies (1) recognize an impairment loss only if the carrying amount of a long-lived asset is not recoverable based on its undiscounted future cash flows and (2) measure an impairment loss as the difference between the carrying amount and fair value of the asset. In addition, SFAS No. 144 provides guidance on accounting and disclosure issues surrounding long-lived assets to be disposed of by sale. The Company does not expect adoption of this statement to have a material impact on its financial position or results of operations.
F–15
3. Property and equipment
Property and equipment consist of the following at:
|
|---|
| | December 31,
| | September 30,
|
|---|
| | 1999
| | 2000
| | 2001
|
|---|
|
|---|
| Leasehold improvements | | $ | 613,724 | | $ | 613,724 | | $ 828,210 |
| Laboratory and office equipment | | | 455,857 | | | 619,616 | | 2,511,095 |
| Equipment under capital lease obligations | | | 252,487 | | | 403,793 | | 1,108,263 |
| Software | | | 221,704 | | | 225,149 | | 390,566 |
| Construction-in-progress | | | — | | | 118,182 | | 254,700 |
| Furniture and fixtures | | | 51,337 | | | 53,786 | | 147,804 |
| | |
|
| | | | 1,595,109 | | | 2,034,250 | | 5,240,638 |
| Less — accumulated depreciation and amortization | | | (522,040 | ) | | (938,404 | ) | (1,585,290) |
| | |
|
| | | $ | 1,073,069 | | $ | 1,095,846 | | $3,655,348 |
| | |
| |
| |
|
|
4. Note receivable from officer
In August 2001, the Company loaned to an officer $250,000, which will be forgiven over a five-year period commencing in August 2002, contingent on the officer's continued employment with the Company. The outstanding balance as of September 30, 2001 is $250,000. The note is unsecured and bears interest at 5.75% per annum.
5. Income taxes
The Company provides for federal and state income taxes in accordance with SFAS No. 109,Accounting for Income Taxes. Under SFAS No. 109, deferred tax assets and liabilities are recognized based on temporary differences between the financial statement and tax bases of assets and liabilities. Deferred income taxes are based on enacted rates and laws applicable to the periods in which differences are expected to reverse. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized.
No provision for federal or state income taxes has been recorded, as the Company has incurred a net loss for all periods presented.
At December 31, 2000, the Company had federal net operating loss carryforwards of approximately $11,045,000, which will begin to expire in 2012. The Company also had tax credit carryforwards of approximately $496,000, which will begin to expire in 2012 unless previously utilized. The United States Tax Reform Act of 1986 contains provisions that may limit the Company's net operating loss carryforwards available to be used in any given year in the event of significant changes in the ownership interests of significant stockholders, as defined.
F–16
Significant components of the Company's net deferred tax asset are as follows:
| |
|---|
December 31,
| | 1999
| | 2000
| |
|---|
| |
|---|
| Deferred tax assets: | | | | | | | |
| | Net operating loss carryforwards | | $ | 2,829,740 | | $ | 4,417,976 | |
| | Research credit carryforward | | | 260,047 | | | 495,751 | |
| | Temporary differences | | | 70,587 | | | 137,318 | |
| | |
| |
| | | | Total deferred tax assets | | | 3,160,374 | | | 5,051,045 | |
| | | | Valuation allowance | | | (3,160,374 | ) | | (5,051,045 | ) |
| | |
| |
| Net deferred tax asset | | $ | — | | $ | — | |
| | |
| |
| |
| |
SFAS No. 109 requires that a valuation allowance be recorded when it is more likely than not that some portion or all of the deferred tax assets will not be realized. Accordingly, since the Company cannot be assured of realizing the deferred tax asset, a full valuation allowance has been provided.
6. Accrued expenses
Accrued expenses consist of the following at:
|
|---|
| | December 31,
| | September 30,
|
|---|
| | 1999
| | 2000
| | 2001
|
|---|
| |
| |
| | (unaudited)
|
|---|
|
|---|
| Professional services | | $ | 37,929 | | $ | 189,163 | | $200,515 |
| Accrued interest | | | 110,793 | | | 19,945 | | — |
| Other | | | 89,764 | | | 84,027 | | 90,096 |
| | |
|
| | | $
| 238,486
| | $
| 293,135
| | $290,611
|
|
7. Notes payable—stockholders
As of December 31, 1999, the Company had short-term notes payable to three preferred stockholders totaling $1,338,757. All notes bore interest at 8.0% per annum and were repaid in full during 2000. In connection with these notes, the Company issued warrants to purchase 196,810 shares of series C convertible preferred stock (see Note 11(e)).
As of December 31, 2000, the Company had short-term notes payable to two preferred stockholders totaling $800,000. These loans were issued in connection with the Company's repurchase of its series B convertible preferred stock from another preferred stockholder. Both notes bore interest at 7.0% per annum and were repaid in August 2001.
F–17
8. Long-term debt
In June and July 1998, the Company issued two notes payable to two vendors for the purchase of certain scientific software licenses. The notes bore interest at 4.6% and 8.0% per annum, respectively, and were repaid in full during 2001.
In July 2001, the Company issued a note payable of $265,000 for the purchase of certain equipment. The note is payable in 24 equal monthly installments beginning in July 2001 and bears interest at 11.0% per year.
Long-term debt consists of the following at:
|
|---|
| | December 31,
| | September 30,
|
|---|
| | 1999
| | 2000
| | 2001
|
|---|
|
|---|
| Notes payable for software licenses | | $ | 70,556 | | $ | 45,356 | | $ — |
| Note payable for equipment | | | — | | | — | | 232,827 |
| | |
|
| | | | 70,556 | | | 45,356 | | 232,827 |
| Less — current maturities | | | (66,359 | ) | | (45,356 | ) | (127,561) |
| | |
|
| | | $
| 4,197
| | $
| —
| | $ 105,266
|
|
9. Convertible note payable
On June 29, 2001, the Company received $2,000,000 from a customer under a convertible note payable in connection with a collaboration agreement. The convertible note payable bears interest at 3.75% per year and is repayable on its maturity date of June 29, 2005, at the option of the Company, in either cash or the Company's common stock. Upon successful completion of an IPO, the outstanding principal and interest under this note payable will automatically convert into shares of common stock at a conversion price equal to the price per share at which the common stock is sold in the IPO.
10. Commitments and contingencies
- (a)
- Capital and operating leases
The Company leases office and laboratory space under operating lease agreements that expire at various dates through April 2007. During 1999, the Company sublet a portion of its leased premises to an unrelated entity, as a tenant at will. The sublet agreement ceased at the end of 2000. The Company has also entered into capital lease agreements for equipment purchases with obligations through July 2004 at interest rates ranging from 8.13% to 24.03% per year. Rent expense included in the accompanying statements of operations was approximately $469,000, $503,000, and $338,000 for the years ended December 31, 1998, 1999, and 2000, respectively, net of sublease income of approximately $65,000 and $32,000 for the years ended December 31, 1999 and 2000, respectively. There
F–18
was no sublease income for the year ended December 31, 1998. Rent expense for the nine months ended September 30, 2000 and 2001, was approximately $238,000 and $751,000, respectively, net of sublease income of approximately $31,000 for the nine months ended September 30, 2000. There was no sublease income for the nine months ended September 30, 2001.
Future minimum capital and operating lease commitments as of December 31, 2000 are as follows:
|
|---|
| | Capital leases
| | Operating leases
|
|---|
|
|---|
| Year ended December 31, | | | | | | |
| | 2001 | | $ | 168,635 | | $ | 509,824 |
| | 2002 | | | 89,850 | | | 429,419 |
| | 2003 | | | 54,350 | | | 329,672 |
| | |
|
| | Total future minimum lease payments | | | 312,835 | | $ | 1,268,915 |
| | | | | |
|
| Less — amounts representing interest | | | 38,103 | | | |
| | |
| | | |
| | Capital lease obligation at December 31, 2000 | | | 274,732 | | | |
| Less — current maturities | | | 149,785 | | | |
| | |
| | | |
| | Capital lease obligation, net of current maturities | | $ | 124,947 | | | |
| | |
| | | |
|
In April 2001, the Company entered into an amendment to its facilities lease to occupy additional space in its current location. The term of both the current lease and the amended lease has been extended until April 30, 2007. The amended total future lease commitments for the quarter ended December 31, 2001, years ended December 31, 2002, 2003, 2004, 2005, 2006, and thereafter are approximately $439,000, $1,428,000, $1,561,000, $2,081,000, $2,155,000, $2,155,000, and $718,000, respectively.
F–19
In connection with the facilities lease amendment entered into in April 2001, the Company was required to issue a letter of credit in the amount of $485,000. The required amount of the letter of credit is collateralized by a certificate of deposit that is held by the bank issuing the letter of credit. This amount is included as "Restricted cash" in long-term assets in the accompanying balance sheet as the deposit will not be refunded until April 2007.
- (b)
- SBIR grants
Costs under U.S. government contracts are subject to audit by the appropriate U.S. government agency. Management believes that cost disallowances, if any, arising from audits of costs charged to government contracts through September 30, 2001, would not have a material effect on its financial position or results of operations.
11. Convertible preferred stock and stockholders' equity (deficit)
- (a)
- Board of directors' and stockholders' actions
The Company's board of directors and stockholders approved the following on November 9, 2001:
• Amendment to the certificate of incorporation increasing the number of authorized shares of common stock, $.001 par value per share, from 24,021,645 shares to 85,640,855 shares.
• Also pursuant to the amendment to the certificate of incorporation and subject to the closing of the Company's IPO, the number of authorized shares of common stock, $.001 par value per share, will be increased to 95,000,000 and the number of authorized shares of preferred stock, $.001 par value per share, will be decreased to 5,000,000. The Board of Directors will have the authority to issue such shares of preferred stock in one or more series and fix the relative rights and preferences without vote or action by the stockholders.
• An increase in the number of shares issuable under the NeoGenesis Pharmaceuticals, Inc. 1997 Stock Option Plan (the 1997 Plan) from 5,125,000 shares to 6,625,000 shares.
• Upon the closing of the Company's IPO, the Company will adopt the NeoGenesis Pharmaceuticals, Inc. 2002 Employee Stock Purchase Plan (the ESPP Plan) under which an aggregate of 500,000 shares of common stock, subject to automatic increase as described in the ESPP Plan, may be issued.
- (b)
- Common stock
The Company has authorized 24,021,645 shares of common stock, $.001 par value of which 5,846,625 shares are issued and outstanding, as of September 30, 2001. As previously discussed, the Company's board of directors has approved an increase in the number of authorized shares of common stock from 24,021,645 shares to 85,640,855 shares and has reserved a total of 6,625,000 shares of common stock for issuance under the 1997 Stock
F–20
Plan, of which 1,309,125 shares have been issued and are outstanding as of September 30, 2001. In addition, the Company has reserved a total of 14,359,145 shares for the conversion of 14,341,075 shares of series A, series B, series C, series D and series E convertible preferred stock and warrants to purchase 18,070 shares of series C convertible preferred stock.
- (c)
- Stock split
In October 2000, the Company effected a five-for-one stock split of its common and preferred stock. All common and preferred share and per share amounts in the accompanying financial statements have been retroactively adjusted to reflect these stock splits.
- (d)
- Preferred stock
The Company has authorized 14,359,145 shares of convertible preferred stock, $.001 par value, and designated five series of convertible preferred stock as of September 30, 2001: 1,250,000 shares of series A preferred stock, 530,205 shares of series B convertible preferred stock, 4,437,865 shares of series C convertible preferred stock, 4,623,487 shares of series D convertible preferred stock and 3,517,588 shares of series E convertible preferred stock.
On December 27, 2000, the Company authorized the issuance and sale of up to 4,623,487 shares of its series D preferred stock. The Company designated and sold a total of 4,020,423 shares of series D preferred stock for gross proceeds of approximately $20,000,000 in December 2000. On January 2, 2001, the Company sold an additional 603,064 shares of series D preferred stock for gross proceeds of approximately $3,000,000.
In June 2001, the Company authorized the issuance and sale of up to 3,517,588 shares of its series E preferred stock. The Company designated and sold a total of 3,517,588 shares of series E preferred stock to three of its collaborators for gross proceeds of approximately $21,000,000, during the months of July and August 2001.
F–21
|
|---|
| | December 31,
| | September 30,
| | Pro forma
September 30,
|
|---|
| | 1999
| | 2000
| | 2001
| | 2001
|
|---|
| |
| |
| | (unaudited)
| | (unaudited)
|
|---|
|
|---|
| Series A preferred stock, $.001 par value | | | | | |
| | Authorized, issued and outstanding — 1,250,000 shares at December 31, 1999 and 2000 and September 30, 2001, and no shares authorized, issued or outstanding pro forma (Liquidation value of $250,000 at September 30, 2001) | | $ | 250,000 | | $ | 250,000 | | $ 250,000 | | $ | — |
| Series B preferred stock, $.001 par value | | | | | |
| | Authorized — 1,182,765 shares at December 31, 1999, 503,205 shares authorized at December 31, 2000 and September 30, 2001 | | | | | | | | | | | |
| | Issued and outstanding — 1,182,765, 530,205, and 530,205 shares at December 31, 1999 and 2000 and September 30, 2001 and no shares authorized, issued or outstanding pro forma (Liquidation value of $650,000 at September 30, 2001) | | | 1,450,000 | | | 650,000 | | 650,000 | | | — |
| Series C preferred stock, $.001 par value | | | | | |
| | Authorized — 4,437,865 shares at December 31, 1999 and 2000 and September 30, 2001 | | | | | | | | | | | |
| | Issued and outstanding — 1,743,110, 4,241,055, and 4,419,795 shares at December 31, 1999 and 2000 and September 30, 2001 and no shares authorized, issued or outstanding pro forma (Liquidation value of $9,779,238 at September 30, 2001) | | | 4,166,775 | | | 9,024,513 | | 9,419,994 | | | — |
| Series D preferred stock, $.001 par value | | | | | |
| | Authorized — 4,623,487 at December 31, 2000 and September 30, 2001 | | | | | | | | | | | |
| | Issued and outstanding — 4,020,423 and 4,623,487 shares at December 31, 2000 and September 30, 2001 and no shares authorized, issued or outstanding pro forma (Liquidation value of $22,999,999 at September 30, 2001) | | | — | | | 19,929,994 | | 22,929,996 | | | — |
| Series E preferred stock, $.001 par value | | | | | |
| | Authorized — 3,517,588 shares at September 30, 2001 | | | | | | | | | | | |
| | Issued and outstanding — 3,517,588 at September 30, 2001 and no shares authorized, issued or outstanding pro forma (Liquidation value of $21,000,000 at September 30, 2001) | | | — | | | — | | 21,000,002 | | | — |
| | |
|
| | | $
| 5,866,775
| | $
| 29,854,507
| | $54,249,992
| | $
| —
|
|
F–22
The rights, preferences, and privileges of the series A, series B, series C, series D, and series E preferred stock are listed below.
- (i)
- Dividends
The Company shall not declare or pay any dividends on shares of common stock unless the holders of the preferred stock then outstanding receive an amount equal to the dividends declared or paid on common stock.
- (ii)
- Conversion
Each share of series A, series B, series C, series D and series E preferred stock is convertible at the option of the holder, at any time and from time to time into one share of common stock adjusted for certain dilutive events, as defined. In addition, all shares of series A, series B, series C and series D preferred stock shall be automatically converted into shares of common stock immediately prior to the consummation of an IPO resulting in gross proceeds to the Company of at least $10,000,000 or $15,000,000, in the case of the series D preferred stock, and at a price per share at least twice the conversion price per share. The conversion price for the series A, series B, series C and series D preferred stock is equal to the purchase price or $0.20, $1.2259, $2.2126 and $4.9746, respectively. Each share of series E preferred stock shall automatically convert into shares of common stock immediately prior to the consummation of an initial public offering.
- (iii)
- Voting rights
The series A, series B, series C and series D preferred stockholders are entitled to vote on all matters with the common stockholders as if they were one class of stock. The series A, series B, series C and series D preferred stockholders are entitled to the number of votes equal to the number of shares of common stock into which each share of those series of preferred stock are then convertible. The series E preferred stockholders generally have no voting rights until June 30, 2004.
- (iv)
- Liquidation
In the event of any voluntary or involuntary liquidation, dissolution or winding-up, including the sale of all or substantially all of the assets, of the Company, as defined, the holders of the series A, series B, series C and series D preferred stock then outstanding will be entitled to be paid an amount equal to $0.20, $1.2259, $2.2126, and $4.9746 per share, respectively, plus any dividends declared but unpaid on such shares prior to any payment to the series E preferred stockholders and common stockholders. Amounts remaining after payment to the series A, series B, series C and series D preferred stockholders, if any, will be distributed to the series E preferred stockholders in an amount equal to $5.97 per share plus any dividends declared but unpaid on such shares prior to any payment to the common stockholders. After this
F–23
payment to the series E preferred stockholders, the series E preferred stockholders are not entitled to any further distribution of assets. Amounts remaining after these payments, if any, will be shared among the series D preferred stockholders and common stockholders on a proportional basis as if the series D preferred stock had been converted to common stock until the series D preferred stockholders have received total distributions equal to 2.5 times their liquidation preference amounts.
- (e)
- Warrants
During 1998, the Company issued warrants to purchase 196,810 shares of series C preferred stock at $2.2126 per share to certain stockholders in connection with short-term loans made by these shareholders to the Company. The value of these warrants, $53,365, was calculated using the Black-Scholes option pricing model and has been recorded as a component of interest expense for the year ended December 31, 1999. These warrants were fully exercisable upon grant. In September 2001, certain stockholders exercised warrants to purchase 178,740 shares of series C preferred stock for total proceeds of approximately $395,000 to the Company. The remaining warrants to purchase 18,070 shares of series C preferred stock were exercised on October 11, 2001.
- (f)
- Stock option plan
In 1997, the Company adopted the 1997 Stock Option Plan, under which, as amended, 5,125,000 shares of the Company's common stock were reserved for issuance. The 1997 Stock Option Plan provides for the grant of incentive stock options, non-qualified stock options and common stock purchases. All options granted under the 1997 Stock Option Plan expire not later than 10 years from the date of grant. Options generally vest over four years, with one-fourth of the shares vesting after one year and the remainder vesting annually over the next three years. The exercise price of incentive stock options must be equal to at least the fair market value of the Company's common stock on the date of grant.
F–24
|
|---|
| | Number
of shares
| | Range of
exercise
prices
| | Weighted
average
exercise price
|
|---|
|
|---|
| Outstanding, December 31, 1997 | | 765,000 | | $ | 0.01 | | $0.01 |
| | Canceled | | (82,500 | ) | | 0.01 | | 0.01 |
| | |
|
| Outstanding, December 31, 1998 | | 682,500 | | $ | 0.01 | | $0.01 |
| | Granted | | 1,767,500 | | | 0.01-0.31 | | 0.13 |
| | Exercised | | (42,500 | ) | | 0.08 | | 0.08 |
| | Canceled | | (33,750 | ) | | 0.08 | | 0.08 |
| | |
|
| Outstanding, December 31, 1999 | | 2,373,750 | | $ | 0.01-0.31 | | $0.10 |
| | Granted | | 1,704,000 | | | 0.31 | | 0.31 |
| | Exercised | | (662,625 | ) | | 0.01-0.31 | | 0.06 |
| | Canceled | | (40,250 | ) | | 0.08-0.31 | | 0.19 |
| | |
|
| Outstanding, December 31, 2000 | | 3,374,875 | | $ | 0.01-0.31 | | $0.21 |
| | Granted | | 557,500 | | | 1.49-1.97 | | 1.57 |
| | Exercised | | (604,000 | ) | | 0.08-0.31 | | 0.21 |
| | Canceled | | (586,500 | ) | | 0.01-1.49 | | 0.26 |
| | |
|
| Outstanding, September 30, 2001 (unaudited) | | 2,741,875
| | | $0.01-$1.97
| | $0.47
|
| | |
|
| Exercisable, September 30, 2001 (unaudited) | | 769,500
| | | $0.01-$0.31
| | $0.08
|
| | |
|
| Exercisable, December 31, 2000 | | 925,000
| | | $0.01-$0.31
| | $0.08
|
| | |
|
| Exercisable, December 31, 1999 | | 1,021,875
| | | $0.01-$0.31
| | $0.02
|
| | |
|
| Exercisable, December 31, 1998 | | 183,125
| | | $0.01
| | $0.01
|
| | |
|
| Options available for grant, September 30, 2001 (unaudited) | | 1,074,000
| | | | | |
|
F–25
The following table summarizes information about options outstanding at September 30, 2001:
|
|---|
| | Outstanding
| | Exercisable
|
|---|
Range of
exercise
prices
| | Number of
shares
| | Weighted
average
remaining
contractual
life
(in years)
| | Weighted
average
exercise
price
| | Number of
shares
| | Weighted
average
exercise
price
|
|---|
|
|---|
| $0.01 — $0.08 | | 868,750 | | 7.13 | | $0.03 | | 640,000 | | $0.03 |
| 0.22 — 0.31 | | 1,317,125 | | 8.50 | | 0.31 | | 129,500 | | 0.31 |
| 1.49 — 1.97 | | 556,000 | | 9.42 | | 1.57 | | — | | — |
| | |
|
| | | 2,741,875 | | 8.25 | | $0.47 | | 769,500 | | $0.08 |
| | |
| |
| |
| |
| |
|
|
The Company has elected to account for its stock-based compensation in accordance with the provisions of APB Opinion No. 25, but will disclose the pro forma effects on net income (loss) as if the fair value of the options had been expensed.
The Company has computed the pro forma disclosure for its stock compensation plan for employees during the years ended December 31, 1999 and 2000 using the Black-Scholes option pricing model under the fair value method prescribed by SFAS No. 123. The assumptions and certain weighted average information for years ended December 31, 1999 and 2000 were as follows:
|
|---|
December 31,
| | 1999
| | 2000
|
|---|
|
|---|
| Risk-free interest rate | | 4.72—5.94% | | 5.61—6.51% |
| Expected dividend yield | | — | | — |
| Expected lives | | 7 years | | 7 years |
| Expected volatility | | 80% | | 80% |
| Weighted average grant date fair value of options granted below fair market value | | $0.08 | | $2.33 |
| Weighted average grant date fair value of options granted equal to fair market value | | $0.13 | | $0.24 |
|
For purposes of pro forma disclosures, the estimated fair value of the options to employees is amortized over the vesting period, which is generally four years. Had compensation costs of options to employees been determined based on the fair value at the grant dates as
F–26
prescribed by SFAS No. 123, the effect for the years ended December 31, 1998, 1999 and 2000 would have been as follows:
| |
|---|
Year ended December 31,
| | 1998
| | 1999
| | 2000
| |
|---|
| |
|---|
| Net loss: | | | | | | | | | | |
| | As reported | | $ | (3,275,725 | ) | $ | (3,138,955 | ) | $ | (5,390,309 | ) |
| | Pro forma | | | (3,276,786 | ) | | (3,167,033 | ) | | (5,465,122 | ) |
Basic and diluted net loss per common share: |
|
|
|
|
|
|
|
|
|
|
| | As reported | | | $(1.13 | ) | | $(0.86 | ) | | $(1.14 | ) |
| | Pro forma | | | (1.13 | ) | | (0.87 | ) | | (1.16 | ) |
| |
- (g)
- Stock-based compensation
In connection with stock options granted to employees during the year ended December 31, 2000 and the nine months ended September 30, 2001, the Company recorded aggregate deferred compensation of approximately $1,819,000, which represents the difference between the option exercise price and the deemed fair value of the common stock as determined for financial reporting purposes. The deferred compensation related to employees will be recorded as an expense over the vesting period of the underlying stock options using the method prescribed by FASB Interpretation No. 28 (FIN 28),Accounting for Stock Appreciation Rights and Other Variable Stock Option or Award Plans. The Company has recorded stock-based
compensation expense related to the amortization of deferred compensation of approximately $28,000 and $497,000 during the year ended December 31, 2000 and for the nine months ended September 30, 2001, respectively, for these employee grants. Based on the grant of stock options through September 30, 2001, the Company expects to recognize additional stock-based compensation of approximately $223,000, $588,000, $312,000, $145,000, and $26,000 during the three months ended December 31, 2001 and the years ended December 31, 2002, 2003, 2004, and 2005, respectively.
In connection with stock options granted to non-employees through September 30, 2001, the Company has recorded aggregate deferred compensation of approximately $921,000, which represents the fair value of non-employee grants. In accordance with EITF 96-18, at the end of each financial reporting period prior to vesting, the value of these options (as calculated using the Black-Scholes option pricing model) will be remeasured using the then current fair value of the Company's common stock. At that point, deferred compensation and the non-cash compensation recognized during that period will be adjusted accordingly. Stock-based compensation expense related to these non-employee options for the years ended December 31, 1999 and 2000 and the nine months ended September 30, 2001 was $7,900, $135,000 and $513,000, respectively.
In May 2001, the Company approved the acceleration of vesting of certain options related to a terminated employee. Vesting for approximately 31,250 options resulted in a non-cash
F–27
stock-based compensation charge of approximately $147,000 during the nine months ended September 30, 2001.
12. Employee benefit plan
During 1999, the Company adopted the NeoGenesis Pharmaceuticals, Inc. 401(k) Plan and Trust (401(k) Plan). The 401(k) Plan covers all eligible employees. Employees must complete three months of service and be 21 years of age or older as of the plan's entry dates. Participants may contribute up to 20% of their compensation, not to exceed the maximum allowable by Internal Revenue Service regulations. The 401(k) Plan currently does not provide for employer matching contributions, and as such, the Company did not contribute for any of the periods presented.
F–28
shares
Common stock
Prospectus
JPMorgan
Bear, Stearns & Co. Inc.
SG Cowen
, 2001
You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with information different from that contained in this prospectus. We are offering to sell, and seeking offers to buy, shares of our common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of our common stock.
No action is being taken in any jurisdiction outside the United States to permit a public offering of the common stock or possession or distribution of this prospectus in that jurisdiction. Persons who come into possession of this prospectus in jurisdictions outside the United States are required to inform themselves about and to observe any restrictions as to this offering and the distribution of this prospectus applicable to that jurisdiction.
Until , 2002, all dealers that buy, sell or trade in our common stock, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers' obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the fees and expenses in connection with the issuance and distribution of the securities being registered (excluding the underwriting discount). Except for the Securities and Exchange Commission registration fee and the National Association of Securities Dealers, Inc. filing fee, all amounts are estimates.
| Securities and Exchange Commission Registration Fee | | $ | 28,750 |
| National Association of Securities Dealers, Inc. Filing Fee | | | 12,000 |
| Nasdaq National Market Fees | | | * |
| Fees of Registrar and Transfer Agent | | | * |
| Legal Fees and Expenses | | | * |
| Accounting Fees and Expenses | | | * |
| Printing Expenses | | | * |
| Blue Sky Qualification Fees and Expenses | | | * |
| Transfer and Registrar Fee | | | * |
| Miscellaneous | | | * |
| | |
|
| Total | | $ | * |
| | |
|
- *
- To be provided by amendment
Item 14. Indemnification of directors and officers
Section 145 of the Delaware General Corporation law empowers a Delaware corporation to indemnify its officers and directors and certain other persons to the extent and under the circumstances set forth therein.
The form of amended and restated certificate of incorporation of the Registrant and the amended and restated by-laws of the Registrant, copies of the forms of which will be filed as Exhibits 3.1 and 3.2, respectively, provide for indemnification of officers and directors of the Registrant and certain other persons against liabilities and expenses incurred by any of them in certain stated proceedings and under certain stated conditions.
The above discussion of the Registrant's fourth amended and restated certificate of incorporation, amended and restated by-laws and Section 145 of the Delaware General Corporation Law is not intended to be exhaustive and is qualified in its entirety by the forms of such fourth amended and restated certificate of incorporation and amended and restated by-laws and the Delaware statute.
The Registrant will agree to indemnify the underwriters and their controlling persons, and the underwriters will agree to indemnify the Registrant and its controlling persons, including directors and executive officers of the Registrant, against certain liabilities, including liabilities under the Securities Act. Reference is made to the form of the Underwriting Agreement that will be filed as part of the Exhibits hereto.
For information regarding the Registrant's undertaking to submit to adjudication the issue of indemnification for violation of the securities laws, see Item 17 hereof.
II–1
Item 15. Recent Sales of Unregistered Securities
Set forth in chronological order is information regarding shares of common stock and preferred stock, warrants to acquire preferred stock and indebtedness issued and options granted by the Registrant since March 13, 1997, the date of the Registrant's inception. Further included is the consideration, if any, received by the Registrant for such shares, warrants and indebtedness and information relating to the section of the Securities Act of 1933, as amended (the "Securities Act"), or rule of the Securities and Exchange Commission under which exemption from registration was claimed.
From its inception in March 1997 to November 9, 2001, the Registrant has entered into stock option agreements with certain employees, officers and consultants to the Registrant pursuant to the Registrant's 1997 Stock Option Plan, as amended, covering approximately 4,151,000 shares of its common stock, of which 1,404,125 shares of common stock have been issued by the Registrant upon exercise of such options. The purchase price under the options is $.01 to $5.00 based on the fair market value of the common stock on the date of grant. The grants and sales were made in reliance upon Rule 701 promulgated under the Securities Act and are deemed to be exempt transactions as sales of an issuer's securities pursuant to a written plan or contract relating to the compensation of such individuals and upon Section 4(2) of the Securities Act as transactions not involving any public offering.
From the Registrant's inception in March 1997 through October 31, 2001, 4,537,500 shares of Common Stock have been issued in exchange for services and cash. These sales were made in reliance upon Section 4(2) of the Securities Act, as transactions by an issuer not involving a public offering.
In June 1997, the Registrant was initially financed through the sale of an aggregate of 1,250,000 shares of Series A Convertible Preferred Stock at $0.20 per share for a total sale price of $250,000. These sales were made in reliance upon Section 4(2) of the Securities Act, as transactions by an issuer not involving a public offering.
In March 1998, the Registrant sold an aggregate of 1,182,765 shares of Series B Convertible Preferred Stock at $1.2259 per share for a total sale price of approximately $1.5 million. In August 2000, the Company repurchased 652,560 shares of Series B Convertible Preferred Stock from one stockholder for a repurchase price of $1.2259 per share. These sales were made in reliance upon Section 4(2) of the Securities Act, as transactions by an issuer not involving a public offering.
For the period from April 1998 through July 2000, the Registrant sold an aggregate of 4,241,055 shares of Series C Convertible Preferred Stock at $2.2126 per share for a total sale price of approximately $9.4 million. These sales were made in reliance upon Section 4(2) of the Securities Act, as transactions by an issuer not involving a public offering.
In October 1998, the Registrant issued warrants to purchase an aggregate of 196,810 shares of Series C Convertible Preferred Stock to its current stockholders at an exercise price of $2.2126. All of the warrants to purchase shares of Series C Convertible Preferred Stock have been exercised and the Company has received $435,460 from the exercise. These sales were made in reliance upon Section 4(2) of the Securities Act, as transactions by an issuer not involving a public offering.
II–2
For the period from December 2000 through January 2001, the Registrant sold an aggregate of 4,623,487 shares of Series D Convertible Preferred Stock at $4.9746 per share for a total sale price of approximately $23.0 million. These sales were made in reliance upon Section 4(2) of the Securities Act, as transactions by an issuer not involving a public offering.
In June 2001, the Registrant issued a convertible promissory note in the amount of $2.0 million to Biogen, Inc., which is automatically convertible into shares of its common stock upon the closing of this offering at a price equal to the initial public offering price. This sale was made in reliance upon Section 4(2) of the Securities Act, as a transaction by an issuer not involving a public offering.
For the period from July through August 2001, the Registrant sold an aggregate of 3,517,588 shares of Series E Convertible Preferred Stock at $5.97 per share for a total sale price of $21.0 million. These sales were made in reliance upon Rule 506 of Regulation D, promulgated under the Securities Act, and Section 4(2) of the Securities Act, as transactions by an issuer not involving a public offering.
The recipients of securities in each of the above transactions represented their intentions to acquire the securities for investment only and not with a view to or for sale in connection with any distributions thereof and appropriate legends were affixed to the instruments representing such securities issued in such transactions.
II–3
Item 16. Exhibits and Financial Statement Schedules
(a) Exhibits
EXHIBIT INDEX
Exhibit Number
| | Description
|
|---|
| 1* | | Form of Underwriting Agreement |
| 3.1 | | Fourth Amended and Restated Certificate of Incorporation of the Registrant, to be effective upon the closing of this offering |
| 3.2 | | Form of Amended and Restated By-Laws of the Registrant, to be effective upon the closing of this offering |
| 3.3 | | Form of Fifth Amended and Restated Certificate of the Registrant, to be filed promptly following the closing of this offering |
| 4.1* | | Specimen certificate for shares of the Registrant's Common Stock |
| 5* | | Opinion of Bingham Dana LLP, counsel to the Registrant |
| 10.1* | | 1997 Stock Option Plan, as amended |
| 10.2 | | 2002 Employee Stock Purchase Plan, to be effective upon the closing of this offering |
| 10.3 | | Preferred Stock Purchase Agreement, dated December 27, 2000, by and among the registrant and the other parties thereto |
| 10.4 | | Investor Rights Agreement, dated December 27, 2000, by and among the registrant and the other parties thereto, as amended. |
| 10.5 | | Right of First Refusal, Co-Sale and Waiver Agreement, dated December 27, 2000, by and among the registrant and the other parties thereto |
| 10.6 | | Indenture of Lease by and between RiverTech Associates LLC and the registrant, dated as of September 26, 1997 and the First Lease Amendment dated as of April 1, 2001 |
| 10.7† | | Agreement by and between the registrant and Merck & Co., Inc., a New Jersey corporation, dated as of November 15, 2000 |
| 10.8† | | Agreement by and between the registrant and Oxford GlycoScienses (UK) Limited, as company incorporated under the laws of England and Wales, dated as of June 18, 2001 |
| 10.9† | | Agreement by and between the registrant and Biogen, Inc., a Massachusetts corporation, dated as of June 29, 2001 |
| 10.10† | | Agreement by and between the registrant and Mitsubishi Pharma Corporation (formerly known as Mitsubishi-Tokyo Pharmaceuticals, Inc.), a corporation organized under the laws of Japan, dated as of July 4, 2001 |
| 10.11† | | Agreement by and between the registrant and Celltech R&D Limited, dated as of July 11, 2001 |
| 10.12† | | Agreement by and between the registrant and Schering-Plough Ltd., a Swiss corporation, dated as of August 2, 2001 |
| 10.13† | | Agreement by and between the registrant and Schering Corporation, a New Jersey corporation, dated as of August 2, 2001 |
| 10.14† | | Agreement by and between the registrant and Tularik Inc., a Delaware corporation, dated as of August 30, 2001 |
| 10.15† | | Agreement by and between the registrant and Immusol Incorporated, a California corporation, dated as of October 18, 2001 |
| 23.1* | | Consent of Bingham Dana LLP (included in Exhibit 5) |
| 23.2 | | Consent of independent auditors, Arthur Andersen LLP |
| 24.1 | | Power of Attorney (included on signature page) |
- *
- To be filed by amendment to this registration statement.
- †
- Confidential treatment requested as to certain portions, which portions are omitted and filed separately with the Securities and Exchange Commission.
II–4
(b) Financial Statement Schedules
Schedule II—Valuation and Qualifying Accounts is included in this Registration Statement. All other schedules have been omitted because they are not required or because the required information is given in the financial statements or notes to those statements.
Item 17. Undertakings
The undersigned Registrant hereby undertakes to provide to the underwriters at the closing specified in the Underwriting Agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
The undersigned Registrant hereby undertakes that:
- (1)
- For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
- (2)
- For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initialbona fide offering thereof.
II–5
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the Registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of Cambridge, Commonwealth of Massachusetts, on this 16th day of November, 2001.
| | | NEOGENESIS PHARMACEUTICALS, INC. |
|
�� |
By: |
/s/ SATISH JINDAL, PH.D.
Satish Jindal, Ph.D.
Chief Executive Officer |
POWER OF ATTORNEY
Each person whose signature appears below hereby appoints each of Satish Jindal, Ph.D., Allen H. Michels, David M. Hunter, and John J. Concannon III severally, acting alone and without the other, as his true and lawful attorney-in-fact with the authority and full power of substitution and resubstitution, for such person and in such person's name, place and stead, in any and all capacities, to execute in the name of each such person, any and all amendments (including without limitation, post-effective amendments) to this Registration Statement on Form S-1, to sign any and all additional registration statements relating to the same offering of securities as this Registration Statement that are filed pursuant to Rule 462(b) of the Securities Act, and to file such registration statements with the Securities and Exchange Commission, together with any exhibits thereto and other documents therewith, necessary or advisable to enable the Registrant to comply with the Securities Act, and any rules, regulations and requirements of the Securities and Exchange Commission in respect thereof, which amendments may make such other changes in the Registration Statement as the aforesaid attorney-in-fact executing the same deems appropriate.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed below by the following persons in the capacities and on the dates indicated:
Signature
| | Title
| | Date
|
|---|
/s/ SATISH JINDAL, PH.D.
Satish Jindal, Ph.D. |
|
Chief Executive Officer, Chief Scientific Officer and Director (Principal Executive Officer) |
|
November 16, 2001 |
/s/ ALLEN H. MICHELS
Allen H. Michels |
|
President, Chief Operating Officer and Chairman of the Board |
|
November 16, 2001 |
/s/ DAVID M. HUNTER
David M. Hunter |
|
Chief Financial Officer (Principal Accounting Officer and Principal Financial Officer), Treasurer and Vice President—Finance |
|
November 16, 2001 |
II–6
/s/ CHRISTOPHER J. AINLEY
Christopher J. Ainley |
|
Director |
|
November 16, 2001 |
/s/ FRANCIS J. BULLOCK, PH.D.
Francis J. Bullock, Ph.D. |
|
Director |
|
November 16, 2001 |
/s/ JOSEPH M. DAVIE, M.D., PH.D.
Joseph M. Davie, M.D., Ph.D. |
|
Director |
|
November 16, 2001 |
/s/ THOMAS B. HALLOWELL
Thomas B. Hallowell |
|
Director |
|
November 16, 2001 |
/s/ THEODORE G. JOHNSON
Theodore G. Johnson |
|
Director |
|
November 16, 2001 |
/s/ MICHAEL B. SHEFFERY, PH.D.
Michael B. Sheffery, Ph.D. |
|
Director |
|
November 16, 2001 |
II–7
EXHIBIT INDEX
Exhibit Number
| | Description
|
|---|
| 1* | | Form of Underwriting Agreement |
| 3.1 | | Fourth Amended and Restated Certificate of Incorporation of the Registrant, to be effective upon the closing of this offering |
| 3.2 | | Form of Amended and Restated By-Laws of the Registrant, to be effective upon the closing of this offering |
| 3.3 | | Form of Fifth Amended and Restated Certificate of the Registrant, to be filed promptly following the closing of this offering |
| 4.1* | | Specimen certificate for shares of the Registrant's Common Stock |
| 5* | | Opinion of Bingham Dana LLP, counsel to the Registrant |
| 10.1* | | 1997 Stock Option Plan, as amended |
| 10.2 | | 2002 Employee Stock Purchase Plan, to be effective upon the closing of this offering |
| 10.3 | | Preferred Stock Purchase Agreement, dated December 27, 2000, by and among the registrant and the other parties thereto |
| 10.4 | | Investor Rights Agreement, dated December 27, 2000, by and among the registrant and the other parties thereto |
| 10.5 | | Right of First Refusal, Co-Sale and Waiver Agreement, dated December 27, 2000, by and among the registrant and the other parties thereto |
| 10.6 | | Indenture of Lease by and between RiverTech Associates LLC and the registrant, dated as of September 26, 1997 and the First Lease Amendment dated as of April 1, 2001 |
| 10.7† | | Agreement by and between the registrant and Merck & Co., Inc., a New Jersey corporation, dated as of November 15, 2000 |
| 10.8† | | Agreement by and between the registrant and Oxford GlycoScienses (UK) Limited, as company incorporated under the laws of England and Wales, dated as of June 18, 2001 |
| 10.9† | | Agreement by and between the registrant and Biogen, Inc., a Massachusetts corporation, dated as of June 29, 2001 |
| 10.10† | | Agreement by and between the registrant and Mitsubishi Pharma Corporation (formerly known as Mitsubishi-Tokyo Pharmaceuticals, Inc.), a corporation organized under the laws of Japan, dated as of July 4, 2001 |
| 10.11† | | Agreement by and between the registrant and Celltech R&D Limited, dated as of July 11, 2001 |
| 10.12† | | Agreement by and between the registrant and Schering-Plough Ltd., a Swiss corporation, dated as of August 2, 2001 |
| 10.13† | | Agreement by and between the registrant and Schering Corporation, a New Jersey corporation, dated as of August 2, 2001 |
| 10.14† | | Agreement by and between the registrant and Tularik Inc., a Delaware corporation, dated as of August 30, 2001 |
| 10.15† | | Agreement by and between the registrant and Immusol Incorporated, a California corporation, dated as of October 18, 2001 |
| 23.1* | | Consent of Bingham Dana LLP (included in Exhibit 5) |
| 23.2 | | Consent of independent auditors, Arthur Andersen LLP |
| 24.1 | | Power of Attorney (included on signature page) |
- *
- To be filed by amendment to this registration statement.
- †
- Confidential treatment requested as to certain portions, which portions are omitted and filed separately with the Securities and Exchange Commission.
QuickLinks
CALCULATION OF REGISTRATION FEETable of contentsProspectus summaryThe offeringSummary financial dataRisk factorsForward-looking statementsUse of proceedsDividend policyCapitalizationDilutionSelected financial dataManagement's discussion and analysis of financial condition and results of operationsBusinessManagementSummary compensation tableOption grants in 2000Principal stockholdersCertain transactionsDescription of capital stockShares eligible for future saleU.S. Federal tax considerations for non-U.S. holdersUnderwritingLegal mattersExpertsWhere you can find more informationReport of Independent Public AccountantsNeoGenesis Pharmaceuticals, Inc. Statements of convertible preferred stock and stockholders' equity (deficit)PART II INFORMATION NOT REQUIRED IN PROSPECTUSEXHIBIT INDEXSIGNATURESPOWER OF ATTORNEYEXHIBIT INDEX