Exhibit 99.1

Alcon Presents New Clinical Data and Provides Regulatory Update on PATANASE® Nasal Spray
ANAHEIM, California - November 7, 2005 – An onset of action study shows faster allergy symptom relief with Alcon’s (NYSE: ACL) olopatadine hydrochloride nasal spray 665 mcg (proposed brand name PATANASE®) when compared to mometasone furoate monohydrate nasal spray 50mcg (brand name NASONEX*) for the treatment of seasonal allergic rhinitis. The study, presented today at the annual meeting of the American College of Allergy, Asthma & Immunology (ACAAI), also demonstrated that PATANASE® nasal spray has a rapid onset of action, as early as 30 minutes.
“PATANASE® nasal spray’s fast onset of action and symptom control would be important benefits for allergy sufferers, who seek quick relief from their debilitating symptoms,” said Bob Q. Lanier, MD, medical director, Lanier Education and Research Network, and clinical professor, Division of Pediatric Allergy & Immunology, University of North Texas Health Science Center.
Study Overview and Results
The controlled allergy study included 425 patients with a history of seasonal allergic rhinitis and sensitivity to ragweed. Patients were randomized to a single intranasal dose of either PATANASE® nasal spray, NASONEX* nasal spray, or placebo spray. Patients were then exposed to 12 hours of high levels of ragweed in an environmental chamber.
The primary goal of the study was to measure total nasal symptom score (TNSS), defined as the sum of patients’ assessment of runny nose, itchy nose, stuffy nose (congestion) and sneezing, over a 12-hour period after a single dose of study medication. Secondary variables were changes from baseline in severity scores for the individual symptoms and participant assessment of global efficacy.
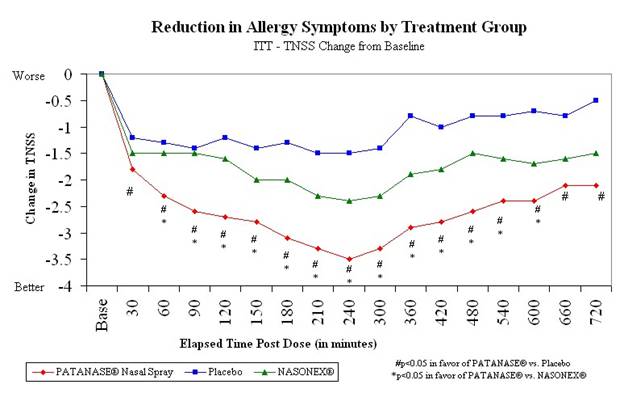
Results included:
• | PATANASE® nasal spray demonstrated an onset of action as early as 30 minutes in the treatment of seasonal allergic rhinitis. |
• | PATANASE® nasal spray demonstrated an onset of action as early as 30 minutes in the treatment of seasonal allergic rhinitis. |
• | PATANASE® nasal spray was superior to placebo spray for the overall measure of TNSS compared to baseline at all time points (30 minutes through 12 hours post dose). |
• | PATANASE® nasal spray was superior to placebo spray at all time points for stuffy nose and sneezing and 15 of 16 time points for runny nose and itchy nose. |
• | PATANASE® nasal spray was superior to NASONEX* nasal spray at 13 of 16 time points (60 minutes through 10 hours post dose) with regard to TNSS. |
• | For individual symptoms, PATANASE® nasal spray was superior to NASONEX* nasal spray at a majority of time points for stuffy nose, runny nose and itchy nose. |
• | Overall, study participants rated PATANASE® nasal spray as providing “better” symptom relief than NASONEX* nasal spray and placebo spray. |
“PATANASE® nasal spray is steroid-free and in clinical studies has demonstrated an acceptable taste profile and low sedation potential. This profile and the study’s results are encouraging as healthcare providers continue to seek future treatment options for their patients who suffer from seasonal allergic rhinitis symptoms,” said G. Michael Wall, PhD, Alcon’s senior director of otic/nasal product development.
Adverse events in this study were non-serious. These were taste perversion (2 patients) for the PATANASE® nasal spray group, headache (2 patients) for the NASONEX* group and rhinitis and increased cough (2 patients each) for the placebo group.
Regulatory Update
Alcon filed a New Drug Application (NDA) for PATANASE® nasal spray for the treatment of seasonal allergic rhinitis in December of 2004. The company said it has received correspondence from the U.S. Food and Drug Administration (FDA) requiring that the current formulation of the drug be modified to reduce or remove one of the inactive ingredients it contains before PATANASE® nasal spray can be approved. The company has developed and is testing a modified formulation, which it believes will maintain the same level of efficacy, while resolving the agency’s concerns. As previously communicated, the company anticipated it would be required to provide additional information to the FDA in order to gain approval of the drug.
“The studies we did in support of our filing showed a high level of efficacy for PATANASE® nasal spray and we look forward to meeting with the FDA in the near future to discuss items in the letter and to confirm what data and information will be required to gain approval of the new formulation. We believe the letter and our upcoming meeting will set out a pathway for approval of PATANASE® nasal spray,” said Dr. Gerald Cagle, PhD, senior vice president of research and development.
About Seasonal Allergic Rhinitis
Seasonal allergic rhinitis, also known as “hay fever”, refers to an allergic complex of symptoms caused by sensitivity to seasonal pollens. Symptoms may include congestion, sneezing, itchy nose, runny nose, watery eyes and itchy eyes.
According to ACAAI, allergies affect an estimated 40 to 50 million people in the United States. Allergies are not only bothersome, but have been linked to a variety of common and serious chronic respiratory illnesses, such as sinusitis and asthma. In addition, they may interfere with day-to-day activities or lessen the quality of life.
About Alcon
Alcon, Inc. is the world’s leading eye care company with sales exceeding $3.9 billion in 2004.
Alcon, which has been dedicated to the ophthalmic industry for over 50 years, develops, manufactures and markets pharmaceuticals, surgical equipment and devices, contact lens solutions and other vision care products that treat diseases, disorders and other conditions of the eye. For more information on Alcon, Inc., visit the company’s Web site at www.alconinc.com.
# # #
Caution Concerning Forward-Looking Statements.
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, relating principally to our ability to gain FDA approval of PATANASE® nasal spray and its expected benefits in treating seasonal allergic rhinitis. These statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performances or achievements expressed or implied by our forward-looking statements. These statements reflect the views of our management as of the date of this press release with respect to future events and are based on assumptions and subject to risks and uncertainties. Given these uncertainties, you should not place undue reliance on these forward- looking statements. Factors that might cause future results to differ include, but are not limited to, the following: we may never receive FDA approval of our NDA for PATANASE®nasal spray; approval of the NDA may take longer than we expect; treatments developed by other companies may be or may be perceived to be more effective than PATANASE®nasal spray; challenges inherent in new product marketing may adversely affect our ability to market the drug; and government regulation and legislation could have an impact on our business beyond our control. You should read this press release with the understanding that our actual future results may be materially different from what we expect. Except to the extent required under the federal securities laws and the rules and regulations promulgated by the Securities and Exchange Commission, we undertake no obligation to publicly update or revise any of these forward-looking statements, whether to reflect new information or future events or circumstances or otherwise.
For information, contact:
Alcon Laboratories Inc.
Doug MacHatton
817-551-8974
doug.machatton@alconlabs.com
Cooney/Waters Group
Jennifer Corrigan
732-382-8898
www.alconinc.com
* NASONEX is a registered trademark of Schering Corporation, Kenilworth, NJ.