Cyto Sorbents Rodman and Renshaw Presentation September 2012 OTCBB: CTSO Cyto Sorbents Corporation Working to Save Lives Through Blood Purification

Cyto Sorbents 2 Safe Harbor Statement Statements in this presentation regarding CytoSorbents Corporation and its operating subsidiary CytoSorbents, Inc that are not historical facts are forward - looking statements and are subject to risks and uncertainties that could cause actual future events or results to differ materially from such statements . Any such forward - looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 . It is routine for our internal projections and expectations to change . Although these expectations may change, we are under no obligation to inform you if they do . Actual events or results may differ materially from those contained in the projections or forward - looking statements . The following factors, among others, could cause our actual results to differ materially from those described in a forward - looking statement : our history of losses ; potential fluctuations in our quarterly and annual results ; competition, inability to achieve regulatory approval for our device, technology systems beyond our control and technology - related defects that could affect the companies’ products or reputation ; risks related to adverse business conditions ; our dependence on key employees ; competition for qualified personnel ; the possible unavailability of financing as and if needed ; and risks related to protecting our intellectual property rights or potential infringement of the intellectual property rights of third parties . This list is intended to identify only certain of the principal factors that could cause actual results to differ from those discussed in the forward - looking statements . Readers are referred to a discussion of important risk factors detailed in the Company’s Form 10 - K filed with the Securities and Exchange Commission on March 30 , 2012 and other reports and documents filed from time to time by us, which are available online at www . sec . gov .

Cyto Sorbents See Investorials Website - http://www.investorials.com/companies/ctso Investorials Video - http://www.youtube.com/watch?v= qzoH4yCW6RA Cyto Sorbents Introduction

Cyto Sorbents 4 Positive Analyst Recommendations Recent Analyst Coverage Initiation

Cyto Sorbents 5 Management Team • Phillip Chan, MD, PhD – Chief Executive Officer and President Board - certified internal medicine physician. MD/PhD from Yale School of Medicine. Internal Medicine residency at the Beth Israel Deaconess Medical Center at Harvard. Former Partner at NJTC Venture Fund heading up healthcare and life science investments for five years. Co - founder of the venture - backed medical device firm, Andrew Technologies • Robert Bartlett, MD - Chief Medical Officer World - renowned as the pioneer in extracorporeal membrane oxygenation therapy (ECMO) and the former Director of the Surgical Intensive Care Unit at University of Michigan, with extensive experience in critical care medicine including the treatment of sepsis and respiratory disease • Vincent Capponi, MS - Chief Operating Officer More than 20 years experience in the medical device, pharmaceutical and imaging fields at Upjohn, Sims Deltec and Sabratek with strengths in operations and manufacturing • Christian Steiner, MD – Vice President Sales and Marketing 12 years of experience in sales and marketing of extracorporeal therapy and critical care sales at Teraklin (MARS) and at Pulsion Medical (hemodynamic monitoring)

Cyto Sorbents 6 Medical Advisory Board John Kellum, MD Professor of Critical Care Medicine & Anesthesiology University of Pittsburgh Medical Center Chair of the Sepsis Advisory Board Emil Paganini, MD Former Section Head of Dialysis and Extracorporeal Therapy Cleveland Clinic Foundation Joseph Parrillo, MD Chief and Professor of Medicine Cooper Medical School at Rowan University Director of the Cooper Heart Institute Editor - in - Chief of the journal Critical Care Medicine Claudio Ronco, MD Director, Dialysis and Renal Transplantation Programs of St. Bartolo Hospital (Vicenza, Italy) Thomas Stewart, MD Associate Professor of Medicine and Anesthesiology Director of Critical Care Medicine Mount Sinai Hospital, University of Toronto

Cyto Sorbents 7 Cyto Sorbents Is Being Built On 3 Pillars Commercialization Research And Development Partnering

Cyto Sorbents Commercialization
Cyto Sorbents 9 Critical Care Is a Huge Market in Need • The sickest patients in the hospital are in the ICU • Unfortunately no effective treatments exist for common life - threatening illnesses such as sepsis, trauma, burn injury, lung injury and pancreatitis • Patients are kept alive with supportive care therapy but the inability to change outcome means that • More than one third of patients die • Patients linger in the ICU at a cost of ~$2,000/day • A staggering $82 billion or nearly 1% of the U.S. GDP, was spent on critical care in the US alone * Halpern, NA, et al., Crit Care Med 2010, 38(1):65 - 71. ** Cooper, L, et al, Crit Care Med 2004, 32(11):2247 - 2253.

Cyto Sorbents 10 Organ Failure is the Top Cause of Death Organ Failure occurs when vital organs stop working. It causes 50% of all deaths in the ICU Little can be done to treat or prevent it today

Cyto Sorbents 11 Potential to Revolutionize Critical Care Unlike supportive care therapies, Cyto Sorb ® targets the prevention or treatment of organ failure by reducing cytokine storm and decreasing inflammation

Cyto Sorbents Cyto Sorb ® Is An Ideal Medical Device • Safe in more than 650 human treatments • Only therapy approved in the E.U. specifically for cytokine reduction and clinically proven to reduce cytokine storm in critically ill patients • Broadly indicated for use in any clinical situation where cytokines are elevated, allowing “On - label” usage for sepsis, trauma, burns, autoimmune disease flares, lung injury, etc • Razor/razorblade business model that is “plug & play” compatible with the existing hemodialysis machine infrastructure found in most hospitals • Robust manufacturing at our ISO 13485 certified manufacturing facility with healthy gross margins > 50%, with target of > 80% with volume • One of the easiest blood purification systems to use, with little instruction needed 12

Cyto Sorbents Pieces to Commercialize Now in Place • No effective therapies for life - threatening illnesses • Aging baby boomer generation driving demand • CytoSorb is an excellent product that attacks the root cause of death • Strong reception by key opinion leaders • Few competitive alternatives • Reimbursement in Germany • Profitable Manufacturing • Many, many new uses for CytoSorb ®

Cyto Sorbents • Launched CytoSorb in June 2012 with hiring of direct sales team and establishment of CytoSorbents Europe GmbH subsidiary in Berlin • Focused on driving adoption and usage at many of the top 400 university and public hospitals in Germany, Austria and Switzerland • More than 40 key opinion leaders at different German hospitals have now agreed to evaluate Cyto Sorb with many more outside of Germany • Discussions ongoing with potential independent distributors and corporate partners to expand outside Germany 14 Formal Launch Has Begun In Germany
Cyto Sorbents Cyto Sorb Has Worldwide Market Potential 15 Middle East Europe represents a $5 - 8 billion market alone CE Mark and ISO 13485 certification will facilitate expansion outside Europe

Cyto Sorbents U.S. Is Important But Not Essential 16 CytoSorbents could be very successful with Europe alone. However, the U.S. would double the total addressable market for CytoSorb to $10 - 15 billion and we are pursuing it

Cyto Sorbents 17 Focused on Clinical Data and Research Sepsis ARDS Burns Trauma Surgery Pancreatitis Influenza Data from multiple uses will accelerate adoption and revenue

Cyto Sorbents 18 Treatment was Safe • Our European Sepsis Trial in critically ill patients demonstrated Cyto Sorb treatment was safe with no serious device related adverse events in more than 300 treatments, increasing the safe experience to more than 650 treatments overall • Treatment was well - tolerated by patients First Do No Harm Cyto Sorb
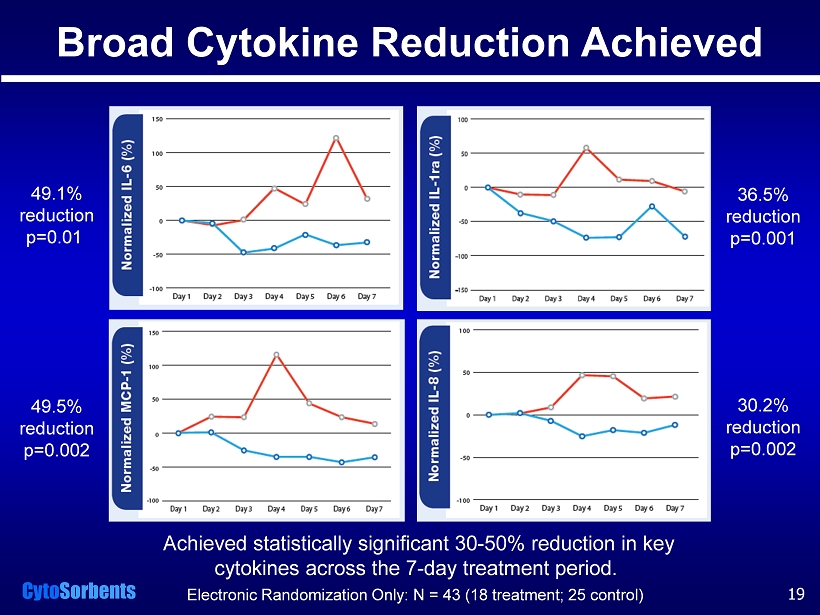
Cyto Sorbents Achieved statistically significant 30 - 50% reduction in key cytokines across the 7 - day treatment period. 49.1% reduction p=0.01 49.5% reduction p=0.002 36.5% reduction p=0.001 30.2% reduction p=0.002 Broad Cytokine Reduction Achieved Electronic Randomization Only: N = 43 (18 treatment; 25 control) 19

Cyto Sorbents Faster Recovery of Lung Function in Treated Patients with High Cytokines 20 Patients on mechanical ventilation at 28 - days (33% vs 88% control, p=0.09, N = 14; 6 treated, 8 control) (IL - 6 ≥ 1,000 pg/mL or IL - 1ra ≥ 16,000 pg/mL)

Cyto Sorbents Increased Survival in Treated Patients with High Cytokines * Statistically significant 28 - day mortality benefit (0% vs 63% control, p=0.03, n=14) but small number of patients (IL - 6 ≥ 1,000 pg/mL or IL - 1ra ≥ 16,000 pg/mL) 21 *

Cyto Sorbents Improvement in Organ Dysfunction (Age ≥ 65) 22 -30% -25% -20% -15% -10% -5% 0% % Reduction in MODS Score on Day 7 (versus Day 1) Control Treatment Cyto Sorb demonstrates a trend to benefit in a reduction of Multiple Organ Dysfunction Score (MODS) (28% reduction vs 9% reduction in control, n=21) during 7 treatment days
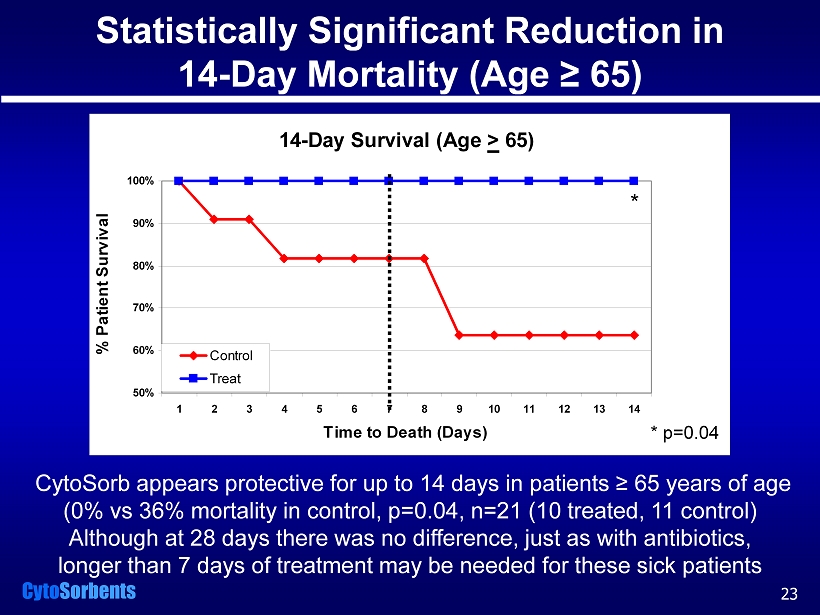
Cyto Sorbents 14-Day Survival (Age > 65) 50% 60% 70% 80% 90% 100% 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Time to Death (Days) % Patient Survival Control Treat Statistically Significant Reduction in 14 - Day Mortality (Age ≥ 65) CytoSorb appears protective for up to 14 days in patients ≥ 65 years of age (0% vs 36% mortality in control, p=0.04, n=21 (10 treated, 11 control) Although at 28 days there was no difference, just as with antibiotics, longer than 7 days of treatment may be needed for these sick patients 23 * * p=0.04
Cyto Sorbents 24 Additional Studies These intriguing early data warrant larger, prospectively designed studies • Will a “theranostics” study using high cytokine levels as an inclusion criteria yield benefit in a prospective trial? • Will more aggressive cytokine reduction in patients older than age 65 result in a 28 - day mortality benefit? • Treat for 6 hours a day for more than 7 days • Treat for more than 6 hours a day We are looking to investigate these questions in additional new studies

Cyto Sorbents 25 Cyto Sorb US Pivotal Trial Plans • In 2007, the FDA approved our IDE application to run a small sepsis trial • Our recent European Sepsis Trial suggests certain high risk groups could benefit most from CytoSorb treatment • Our current dosing study is intended to obtain more data in these high risk groups and optimize treatment parameters • With these data, we plan to meet with the FDA to modify our existing IDE application to a pivotal trial in one of these high risk groups • If the observed effect of CytoSorb continues to be robust, we hope to negotiate a much smaller, faster and less expensive trial than the 300 – 500 patient trial originally anticipated • Anticipating start date of late 2013 – early 2014

Cyto Sorbents Research and Development

Cyto Sorbents 27 Technology Overview The heart of the technology is a biocompatible, highly porous, polymer bead that can remove a wide range of toxic substances from blood and fluids based on pore capture and surface adsorption

Cyto Sorbents 28 Beads Can Be Used In Many Forms Hemoperfusion “Beads in a Bag” In - line Filter
Cyto Sorbents • CytoSorbents invests in new product development based upon its proprietary technology that is covered by 31 issued US patents and multiple applications pending • The goals are to: 29 R&D Strategy • Develop the next generation of CytoSorb polymer • Create products that address major untapped markets and monetize these through out - licensing • Fund development through both company resources and non - dilutive grant and contract funding

Cyto Sorbents rhabdomyolysis. A $1M Phase II application has been submitted and is pending • The US Dept of Health and Human Services awarded a $0.5M QTDP grant (2010) based on the potential of our technology to address a major unmet medical need and reduce healthcare costs • A $7M five year (2006 - 2010) NIH grant awarded to University of Pittsburgh to advance the use of our adsorbent bead technology for the treatment of sepsis. CytoSorbents received $0.4M 30 Leveraging Government Support • DARPA awarded a $3.8M five year (2012) contract as part of its “Dialysis - Like Therapeutics” program to treat sepsis • The US Army awarded a $100K Phase I SBIR grant (2011) for the treatment of trauma and
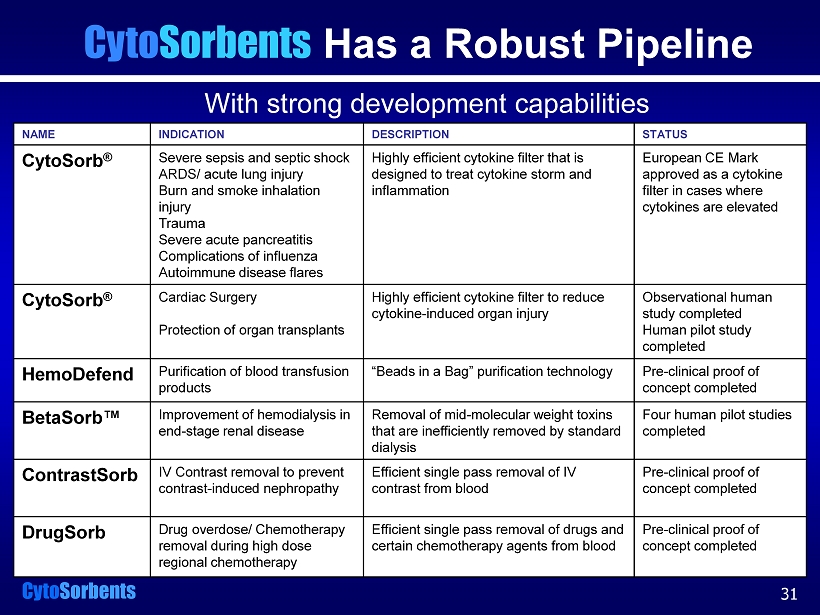
Cyto Sorbents With strong development capabilities Cyto Sorbents Has a Robust Pipeline NAME INDICATION DESCRIPTION STATUS CytoSorb ® Severe sepsis and septic shock ARDS/ acute lung injury Burn and smoke inhalation injury Trauma Severe acute pancreatitis Complications of influenza Autoimmune disease flares Highly efficient cytokine filter that is designed to treat cytokine storm and inflammation European CE Mark approved as a cytokine filter in cases where cytokines are elevated CytoSorb ® Cardiac Surgery Protection of organ transplants Highly efficient cytokine filter to reduce cytokine - induced organ injury Observational human study completed Human pilot study completed HemoDefend Purification of blood transfusion products “Beads in a Bag” purification technology Pre - clinical proof of concept completed BetaSorb™ Improvement of hemodialysis in end - stage renal disease Removal of mid - molecular weight toxins that are inefficiently removed by standard dialysis Four human pilot studies completed ContrastSorb IV Contrast removal to prevent contrast - induced nephropathy Efficient single pass removal of IV contrast from blood Pre - clinical proof of concept completed DrugSorb Drug overdose/ Chemotherapy removal during high dose regional chemotherapy Efficient single pass removal of drugs and certain chemotherapy agents from blood Pre - clinical proof of concept completed 31
Cyto Sorbents • Approximately 1 million cardiopulmonary bypass surgeries in the US and EU annually • Coronary artery bypass graft surgery • Open valve repair • Heart or lung transplantation • Cardiac defect repair • LVAD implantation • Cytokine release during the procedure is a known complication that can lead to organ dysfunction or failure after surgery • Terumo, Pall and many others already sell leukoreduction filters that try to control cytokine release by removing cells that make them • CytoSorb represents a major advance because it directly removes cytokines and has a ready ~$200 - 400M market opportunity Cyto Sorb Use During Cardiac Surgery 32

Cyto Sorbents Hemo Defend Protection for Blood Transfusions • 100M+ blood transfusions worldwide annually • Hundreds of thousands to millions of people develop transfusion reactions due to contaminants in blood products • HemoDefend beads can be placed in the blood storage bag and begin removing contaminants during storage when blood is added to the bag • Neutrally buoyant beads distribute evenly, so no mixing is needed • A macrofilter prevents bead escape with no additional equipment needed HemoDefend is a technology platform not yet approved in the US or EU 33 • Alternatively, the beads can be used as an in - line filter during transfusion • Represents a $500M+ market for packed red blood cells alone

Cyto Sorbents Contrast Sorb For Imaging & Interventional Radiology • IV contrast, used for interventional radiology (IR) and imaging procedures can cause kidney failure particularly in high risk patients (e.g diabetes, high blood pressure, others) • ~ 200M CT scans are performed worldwide each year with 70 million in the US alone, many requiring IV contrast • IR procedures such as coronary or peripheral artery stent placement, cerebral angiograms, and others require large amounts of IV contrast • 10M coronary angiograms worldwide each year • ContrastSorb achieves high single pass removal of IV contrast in vitro, targeting a $1B+ market ContrastSorb is a technology platform not yet approved in the US or EU 34

Cyto Sorbents Business Development and Partnerships

Cyto Sorbents 36 Cyto Sorbents has an active business development program Cyto Sorb ® Hemo Defend Contrast Sorb Others Potential partners include companies involved in: • Dialysis and renal therapies • Pharmaceuticals • Critical care • Advanced materials • Blood transfusion and blood purification Goal is to focus on CytoSorb while out - licensing valuable technology portfolio Unlocking Technology Value via BD

Cyto Sorbents • Cyto Sorb ® has the potential to revolutionize intensive care medicine with the goal of saving lives and reducing massive healthcare costs • Best - of - breed solution addressing a $10 - 15 billion market with little to no viable competition • Third party validation by the US Army, DARPA, Zacks, and Brean Murray • Momentum is building. Currently at an inflection point with the pieces in place to drive potential commercial success • Potential Future Catalysts • Revenue growth and increased product adoption • Greater awareness of our story and technology • Monetization of valuable technology pipeline ( Hemo Defend and others) • Non - dilutive funding from grants and other programs • Clinical data and new clinical applications • Uplisting to national stock exchange ASK YOUR DOCTOR! Cyto Sorbents Is a Major Potential Growth Story 37

Cyto Sorbents Phillip P. Chan, MD, PhD - CEO 7 Deer Park Drive, Suite K Monmouth Junction, NJ 08852 (732) 329 - 8885 ext *823 pchan@cytosorbents.com Cyto Sorbents Corporation Working to Save Lives Through Blood Purification OTCBB: CTSO Cyto Sorbents Corporation





































