
Cyto Sorbents Corporation OTCBB: CTSO An Emerging Leader in Critical Care Immunotherapy Biotech Showcase Presentation January 15, 2014

Safe Harbor Statement Statements in this presentation regarding CytoSorbents Corporation and its operating subsidiary CytoSorbents, Inc that are not historical facts are forward - looking statements and are subject to risks and uncertainties that could cause actual future events or results to differ materially from such statements . Any such forward - looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 . It is routine for our internal projections and expectations to change . Although these expectations may change, we are under no obligation to inform you if they do . Actual events or results may differ materially from those contained in the projections or forward - looking statements . The following factors, among others, could cause our actual results to differ materially from those described in a forward - looking statement : our history of losses ; potential fluctuations in our quarterly and annual results ; competition, inability to achieve regulatory approval for our device, technology systems beyond our control and technology - related defects that could affect the companies’ products or reputation ; risks related to adverse business conditions ; our dependence on key employees ; competition for qualified personnel ; the possible unavailability of financing as and if needed ; and risks related to protecting our intellectual property rights or potential infringement of the intellectual property rights of third parties . This list is intended to identify only certain of the principal factors that could cause actual results to differ from those discussed in the forward - looking statements . Readers are referred to a discussion of important risk factors detailed in the Company’s Form 10 - K filed with the Securities and Exchange Commission on April 3 , 2013 and other reports and documents filed from time to time by us, which are available online at www . sec . gov .

3 Cyto Sorbents Overview CytoSorbents is a publicly - traded critical care immunotherapy company specializing in the removal of toxic substances from blood
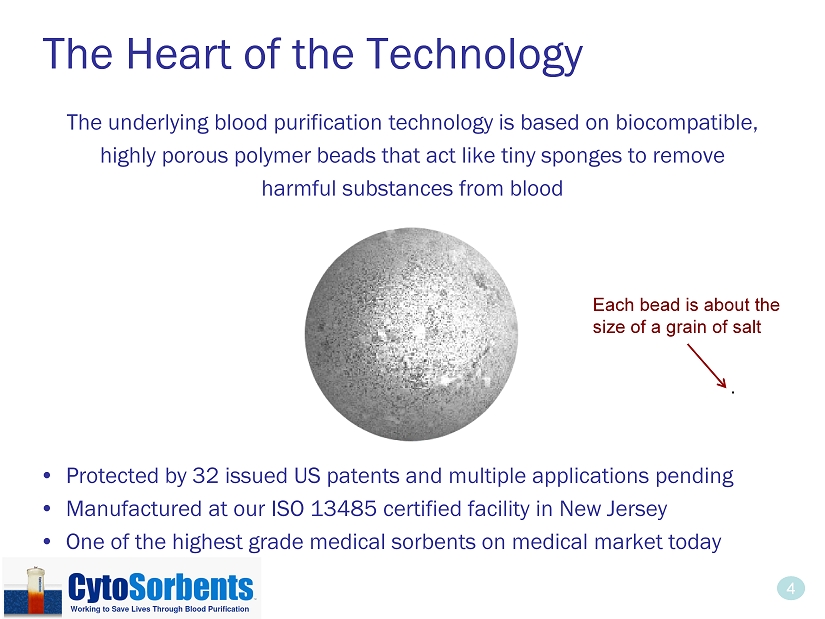
4 The Heart of the Technology The underlying blood purification technology is based on biocompatible, highly porous polymer beads that act like tiny sponges to remove harmful substances from blood • Protected by 32 issued US patents and multiple applications pending • Manufactured at our ISO 13485 certified facility in New Jersey • One of the highest grade medical sorbents on medical market today . Each bead is about the size of a grain of salt

5 Critical Care, High Risk Surgery Our Bead Technology Enables a Versatile Product Portfolio Blood Collection & Transfusion CT Imaging, Interventional Radiology Contrast Sorb Drug Overdose, Chemo Removal Drug Sorb Beta Sorb Improving Dialysis Under Development CE Mark Approved

6 Management Team Phillip Chan, MD, PhD – Chief Executive Officer and President Board - certified Internist . MD/PhD from Yale School of Medicine . Internal Medicine residency at the Beth Israel Deaconess Medical Center at Harvard . Former Partner at NJTC Venture Fund leading life science investments for 5 years . Co - founder of Andrew Technologies, commercializing its HydraSolve™ lipoplasty device in the U . S . Robert Bartlett, MD - Chief Medical Officer World - renowned as the pioneer in extracorporeal membrane oxygenation therapy (ECMO) – used worldwide in ICUs in refractory lung failure, and former Director of the Surgical Intensive Care Unit at University of Michigan, with extensive experience in critical care medicine including the treatment of sepsis and respiratory disease Vincent Capponi, MS - Chief Operating Officer 20 + years experience in the medical device, pharmaceutical and imaging fields . Led regulatory approval for the heparin flush syringe, used worldwide in hospitals, and managed manufacturing of > 1 million units/week Kathleen Bloch, MBA, CPA – Chief Financial Officer 20 + years as CFO of private and public companies . Former Laureate Biopharma CFO, a contract biopharmaceutical manufacturer Christian Steiner, MD – Vice President of Sales and Marketing 13 + years experience in sales and marketing of extracorporeal therapy and critical care sales at Teraklin for MARS, the first liver failure dialysis technology, and at Pulsion Medical (hemodynamic monitoring) Christopher Cramer, MS, MBA – Vice President of Business Development 15 + years experience in business development and commercial experience . Former Senior Director of New Venture Development at Johnson & Johnson, and previously at PwC Consulting

7 Leading Advisors in Critical Care John Kellum, MD University of Pittsburgh Mitchell Cohen, MD University of San Francisco (UCSF) Raul Coimbra, MD, PhD University of San Diego (UCSD) Ronald Maier, MD University of Washington, Seattle Ernest Moore, MD University of Colorado Emil Paganini, MD Cleveland Clinic Foundation Joseph Parrillo, MD Hackensack Heart and Vascular Hospital Claudio Ronco, MD St. Bartolo Hospital, Vincenza, Italy

8 • US Dept of Health and Human Services awarded $0.5M grant (2010) for therapies that can save lives and reduce costs under the QTDP Program • NIH grant awarded $7M five year (2006 - 2010) to University of Pittsburgh and Dr. John Kellum to research CytoSorb bead for treatment of sepsis • NIH/NHLBI awarded $0.2M Phase I SBIR to advance the HemoDefend purification technology intended to improve the quality and safety of blood transfusions (2013) $15+ Million in US Government Support • DARPA awarded $3.8M five year (2012) contract as part of “Dialysis - Like Therapeutics” program to treat sepsis by removing cytokines and pathogen - derived toxins • U.S. Army awarded $1.15M SBIR contracts for trauma and burn injury research (2011 - 13) • U.S. Air Force is funding a 30 patient human pilot study in trauma valued at $3M (2013). FDA approved trial to begin this year

9 The Critical Care Opportunity

10 Cyto Sorbents is working to save lives by targeting uncontrolled inflammation through blood purification

11 Inflammation Plays a Major Role in Nearly Every Known Disease • Life threatening conditions like sepsis & trauma • Autoimmune diseases like rheumatoid arthritis, inflammatory bowel, psoriasis, and lupus • Heart disease, peripheral artery disease • Cancer, cancer cachexia, graft vs host disease • Neurodegenerative diseases such as Alzheimer’s, multiple sclerosis (MS), Parkinson’s • Many, many others others Uncontrolled inflammation wreaks havoc on the body and can be deadly

12 Cytokines Fuel the Fire of Inflammation • Cytokines are small proteins that normally help stimulate and regulate the immune system and control inflammation • Cytokines are a dual edged sword • They are required for proper immune system function • However, in mild to moderate excess, cytokines can cause or exacerbate disease (e.g. autoimmune diseases) • Anti - cytokine approach validated by $25 billion in worldwide sales of specific anti - cytokine therapies such as Enbrel (Amgen), Remicade (J&J) and Humira (AbbVie) • But cytokines in vast excess, often called “cytokine storm” can lead to a massive uncontrolled systemic inflammatory response syndrome (SIRS) leading to multiple organ dysfunction syndrome (MODS) or multiple organ failure (MOF)
13 Massive Inflammation Leads to Organ Failure Organ failure occurs when vital organs stop working, causing nearly half of all deaths in the ICU , but little can be done to treat or prevent it today

14 Cyto Sorb ® Removes the Fuel to the Fire • CytoSorb ® is the only specifically approved extracorporeal cytokine filter in the E.U. • Clinically proven to reduce key cytokines in blood by 30 - 50% in critically ill patients • Approved for use in any situation where cytokines are elevated • Safe: More than 1,300 human treatments with no serious device related adverse events

15 How Is It Used? Place a temporary dialysis catheter in a major vein Connect the device to a standard dialysis machines found in hospitals worldwide Pump blood out of the body and through the cartridge The polymer beads directly contact blood and remove unwanted or toxic substances “Purified” blood is pumped back into the patient Can treat 20 - 30 total blood volumes per 6 hr treatment Each treatment uses a new cartridge Plug and Play Compatible with a Hospital’s Existing Infrastructure
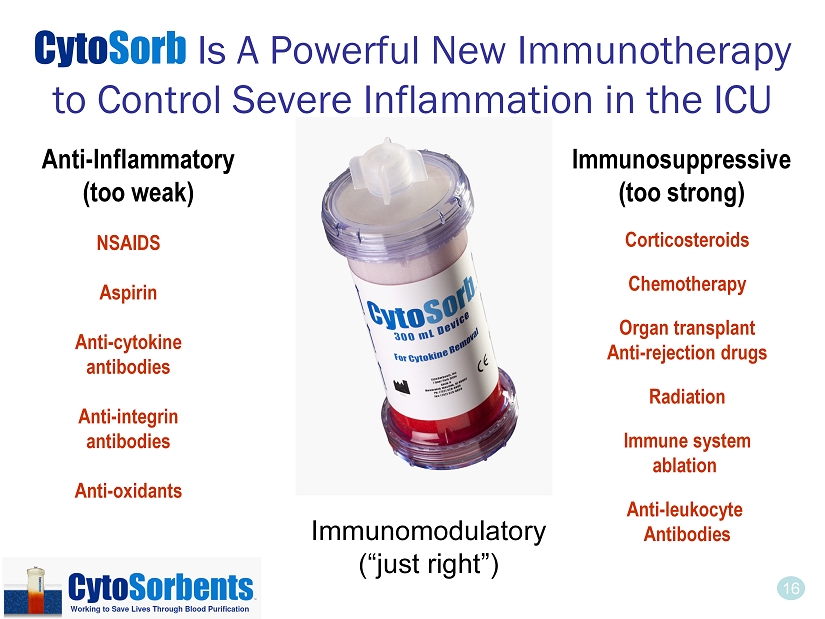
16 Cyto Sorb Is A Powerful New Immunotherapy to Control Severe Inflammation in the ICU NSAIDS Aspirin Anti - cytokine antibodies Anti - integrin antibodies Anti - oxidants Anti - Inflammatory (too weak) Immunosuppressive (too strong) Immunomodulatory (“just right”) Corticosteroids Chemotherapy Organ transplant Anti - rejection drugs Radiation Immune system ablation Anti - leukocyte Antibodies

17 The Goal: To Prevent or Treat Organ Failure Sepsis ARDS Burn Injury Trauma Pancreatitis Influenza Surgical Critical Care: $10 - 15B Opportunity Improve Patient Outcome and Survival Decrease Costs Of ICU and Patient Care
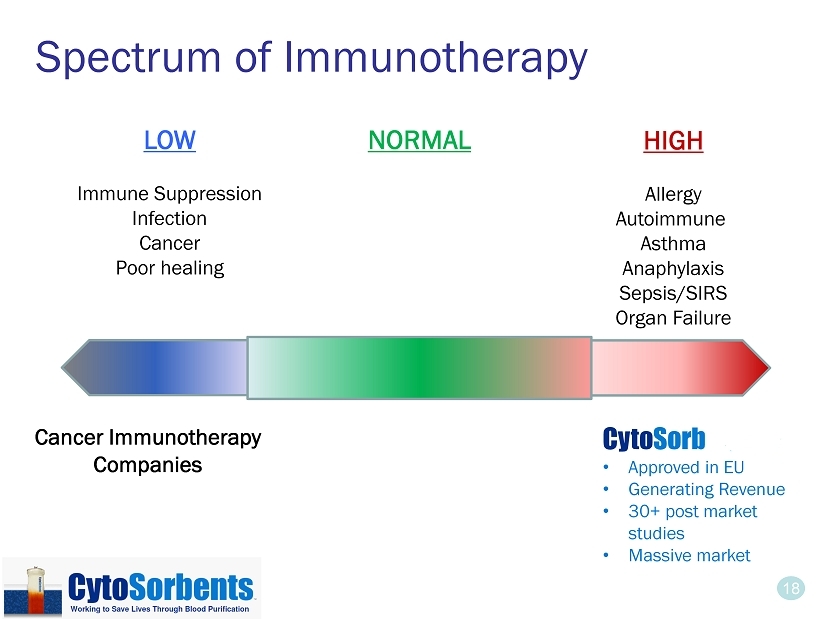
18 Spectrum of Immunotherapy LOW Immune Suppression Infection Cancer Poor healing HIGH Allergy Autoimmune Asthma Anaphylaxis Sepsis/SIRS Organ Failure Cancer Immunotherapy Companies Cyto Sorb - $65M • Approved in EU • Generating Revenue • 30+ post market studies • Massive market NORMAL

19 Competition 19 No Approved Therapies in the EU or US to Prevent or Treat Organ Failure Sepsis ARDS and ALI Burn injury Trauma Severe Acute Pancreatitis Cardiopulmonary bypass Complications of surgery Organ transplant Many others Organ failure remains one of the biggest unmet needs in all of modern medicine

20 Blood Purification for Sepsis: Competition 20 Criteria Cyto Sorb Septex Ultraflux CPFA PMMA AN69 ST Toraymyxin CytoPherx Manufacturer Cyto Sorbents Gambro Fresenius Bellco Toray Gambro Toray CytoPherx Used for sepsis treatment in EU? √ √ √ √ √ √ √ î Specific approval as a cytokine filter? √ î î î î î î î Broad cytokine removal proven in a randomized RCT? √ √ î î * î î î î RCT data showing improvement in mortality in sepsis? √ î î î * î î √ î Platform Independent? √ î î î √ √ √ î Easy setup - single circuit? √ î î î î î √ î Expansive binding capacity? √ î î √ î î √ î CYTOKINE REMOVAL ENDOTOXIN ACTIVATED CELLS * Pending published data from COMPACT trial

21 Operating and Financial Highlights

22 Huge market . CytoSorb ® is sold to hospitals and critical care physicians, targeting a “need to have” $ 10 - 15 billion critical care opportunity addressing organ failure Little competition . CytoSorb is the only specifically approved cytokine filter Critical care physicians understand the problem . Very little education is needed It is a plug and play high margin disposable “razorblade” with the hospital’s existing hemodialysis “razor” infrastructure . No new hardware is needed Technicians already know how to use the device . Standard hemoperfusion CytoSorb ® is reimbursed in Germany/Austria at > $ 500 /cartridge or > $ 3 - 5 , 000 /patient Very profitable with gross margins of 60 % + . With scale, can drive GMs > 80 % • Gamma sterilized . Shelf life : 3 years at room temperature Intensive care units are highly centralized – easy for a small sales force to access An Excellent Business Model

23 Direct Sales: Germany, Austria, Switzerland We are currently working with KOLs in the largest and most important university and public hospitals in most major cities in Germany and many in Austria and the UK Partial List: • University of Aachen • University of Bonn • Helios Berlin Buch • Charite Mitte and Charite Virchow • Helios Erfurt • University of Goettingen • University of Greifswald • University of Hamburg - Eppendorf • University of Jena • University of Kiel • University of Leipzig • Trauma Hospital of Linz • European Medical University of Oldenburg • University of Rostock • University of Ulm • Medical University of Vienna • Vienna General Hospital (AKH)
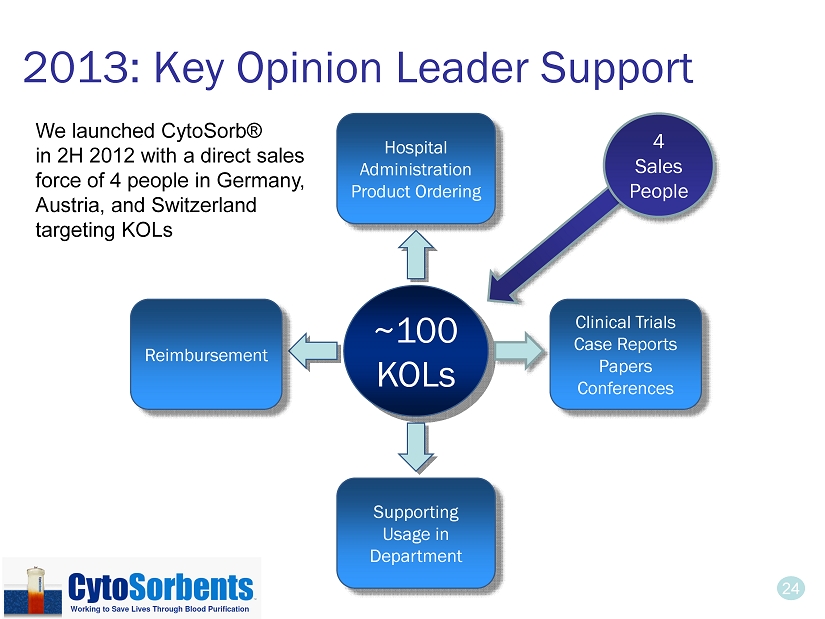
24 2013: Key Opinion Leader Support ~100 KOLs Hospital Administration Product Ordering Clinical Trials Case Reports Papers Conferences Supporting Usage in Department Reimbursement 4 Sales People We launched CytoSorb® in 2H 2012 with a direct sales force of 4 people in Germany, Austria, and Switzerland targeting KOLs

25 2014: Drive Departmental Usage Junior and Senior Physicians Nursing and Technical Staff We are starting this phase now Key Opinion Leader
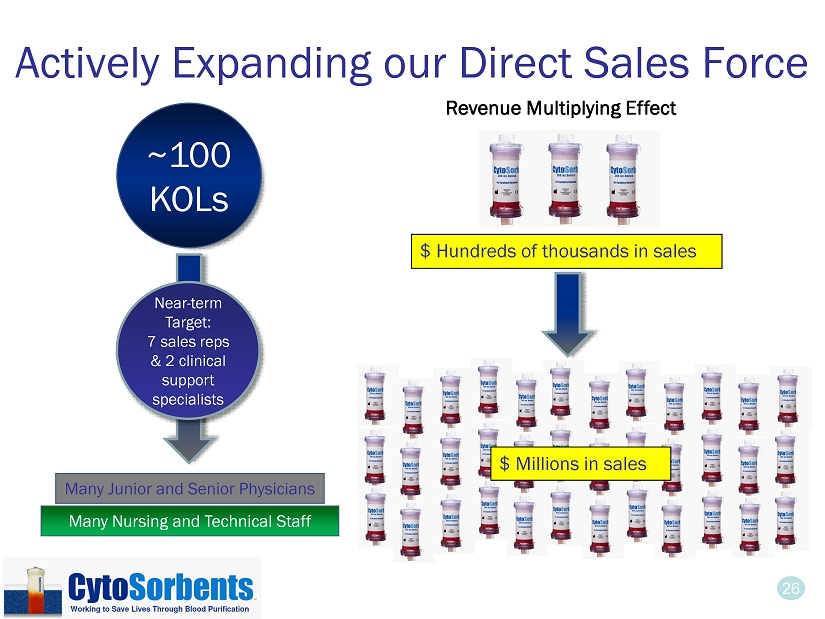
26 Actively Expanding our Direct Sales Force Many Junior and Senior Physicians Many Nursing and Technical Staff ~100 KOLs Near - term Target: 7 sales reps & 2 clinical support specialists $ Millions in sales $ Hundreds of thousands in sales Revenue Multiplying Effect
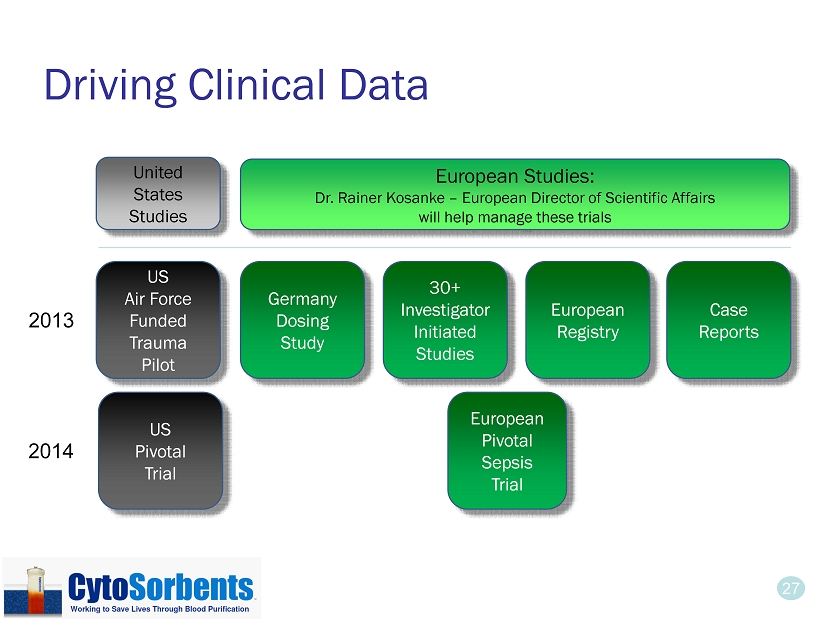
27 Driving Clinical Data 27 30+ Investigator Initiated Studies European Pivotal Sepsis Trial US Pivotal Trial European Registry US Air Force Funded Trauma Pilot Case Reports Germany Dosing Study 2013 2014 United States Studies European Studies: Dr. Rainer Kosanke – European Director of Scientific Affairs will help manage these trials

28 Sepsis ARDS Burns Trauma Surgery Pancreatitis Influenza Life Support Kidney & Liver Failure 30+ Investigator Initiated Studies • Initial data from several expected in 1H 2014
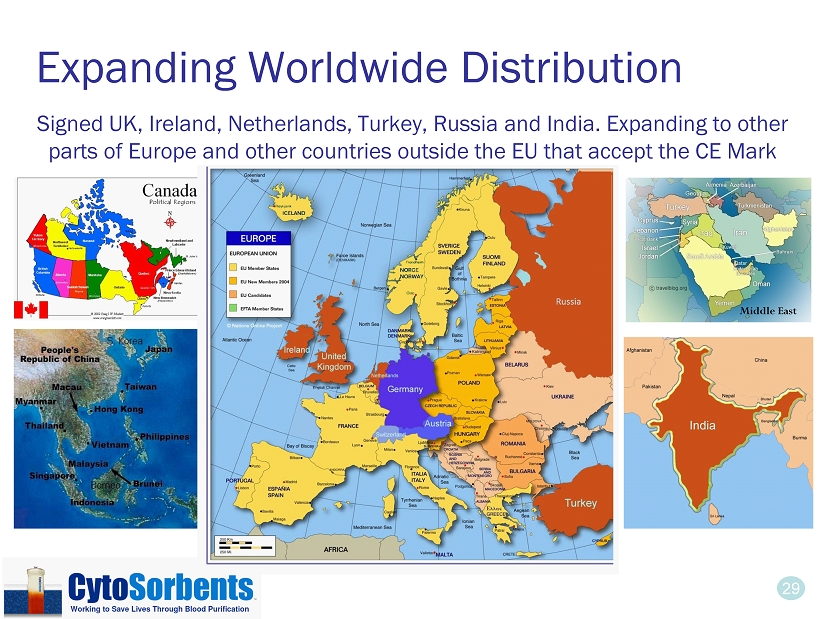
29 Expanding Worldwide Distribution Signed UK, Ireland, Netherlands, Turkey, Russia and India. Expanding to other parts of Europe and other countries outside the EU that accept the CE Mark Middle East

30 Strategic Partnership with Biocon The Most Comprehensive Treatment for Sepsis Treat the Massive Inflammatory Response Treat the Primary Infection Focus is initially on exclusive distribution in India and emerging markets

31 2013 Financials • First full year of CytoSorb ® commercialization • Expect to report ~$2.4 million in 2013 revenue • Total CytoSorb® sales for 2013 are expected to be greater than $840,000 • Q4 2013 product sales are projected to be more than $330,000, an increase of more than 60% over the previous quarter, and 275% increase from Q4 2012 • 2014 revenue from grants is projected to be approximately $1.6M, compared to approximately $1.2M in 2013

32 Strong Product Growth Since Introduction $13,679 $87,960 $176,098 $127,969 $203,561 $330,000 $0 $50,000 $100,000 $150,000 $200,000 $250,000 $300,000 $350,000 $400,000 Q3 2012 Q4 2012 Q1 2013 Q2 2013 Q3 2013 Q4 2013 PROJ CytoSorb ® Product Sales $360,000 High and low estimates 32
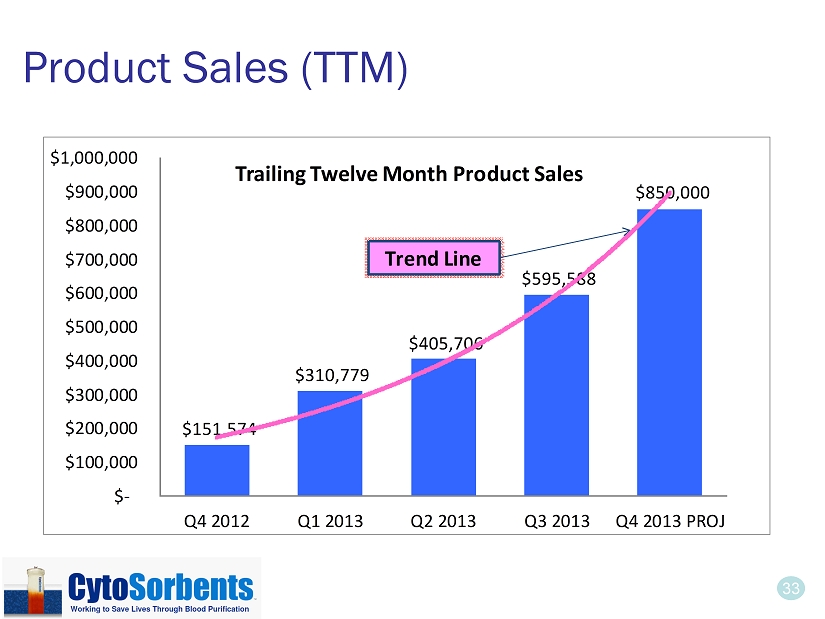
33 Product Sales (TTM) $151,574 $310,779 $405,706 $595,588 $850,000 $- $100,000 $200,000 $300,000 $400,000 $500,000 $600,000 $700,000 $800,000 $900,000 $1,000,000 Q4 2012 Q1 2013 Q2 2013 Q3 2013 Q4 2013 PROJ Trailing Twelve Month Product Sales Trend Line

34 Strong Gross Margins • For the three months ended December 31, 2013 product gross margins are expected to exceed 60% • Sales prices have remained constant through Q4 2013 • Gross margins will vary based on the ratio of direct sales vs. sales to distributors, but are expected to remain very healthy

35 Major Catalyst Is Expected To Be Sales Product revenues have been growing rapidly. Based on the foundation we laid in 2013, we believe we now have the formula in place for growth. • Strong key opinion leader support • Positive clinical experiences • Increasing orders and reorders • A growing sales and clinical support team to more efficiently capture sales • Clear evidence that our marketing efforts with junior and senior physicians in critical care departments are bearing fruit • New clinical data from a multitude of clinical studies that may drive usage • New markets like cardiac surgery where we are seeing a high level of interest • Existing distributors coming on - line, with new distribution being added • Reimbursement in key markets • Strong interest from potential strategic partners

36 Cyto Sorbents Poised for Growth Cyto Sorb ® is a unique immunotherapy product that may revolutionize critical care medicine, save lives, and reduce costs Blockbuster potential: Massive market, major unmet need, could save lives, reduce costs Approved and generating revenue Validation of our company and technology from many fronts: x Biocon, DARPA, US Army, US Air Force, NIH/NHLBI, scientific advisors, analysts Unique, highly profitable product and pipeline with little to no competition Led by an experienced and responsible management team Undiscovered at a fully - diluted market cap of ~$65M Potential major catalysts in the next 6 - 9 months including revenue growth, partnerships, clinical data, up - listing, institutional ownership, and more
37 Phillip P. Chan, MD, PhD - CEO 7 Deer Park Drive, Suite K Monmouth Junction, NJ 08852 pchan@cytosorbents.com Working to Save Lives Through Blood Purification OTCBB: CTSO




































