
Cyto Sorbents Corporation OTCBB: CTSO An Emerging Leader in Critical Care Immunotherapy Fiscal Year 2013 Review – March 31, 2014

Safe Harbor Statement Statements in this presentation regarding CytoSorbents Corporation and its operating subsidiary CytoSorbents, Inc that are not historical facts are forward - looking statements and are subject to risks and uncertainties that could cause actual future events or results to differ materially from such statements . Any such forward - looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 . It is routine for our internal projections and expectations to change . Although these expectations may change, we are under no obligation to inform you if they do . Actual events or results may differ materially from those contained in the projections or forward - looking statements . The following factors, among others, could cause our actual results to differ materially from those described in a forward - looking statement : our history of losses ; potential fluctuations in our quarterly and annual results ; competition, inability to achieve regulatory approval for our device, technology systems beyond our control and technology - related defects that could affect the companies’ products or reputation ; risks related to adverse business conditions ; our dependence on key employees ; competition for qualified personnel ; the possible unavailability of financing as and if needed ; and risks related to protecting our intellectual property rights or potential infringement of the intellectual property rights of third parties . This list is intended to identify only certain of the principal factors that could cause actual results to differ from those discussed in the forward - looking statements . Readers are referred to a discussion of important risk factors detailed in the Company’s Form 10 - K filed with the Securities and Exchange Commission on March 31 , 2014 and other reports and documents filed from time to time by us, which are available online at www . sec . gov .

3 Conference Call Participants Dr . Phillip Chan, MD, PhD Chief Executive Officer and President Vincent Capponi , MS Chief Operating Officer Kathleen Bloch, MBA, CPA Chief Financial Officer Dr . Christian Steiner, MD Vice President of Sales and Marketing Christopher Cramer, MS, MBA Vice President of Business Development Moderator: Donna Marincas – CytoSorbents Corporation

4 Cyto Sorbents is an Emerging Leader in Critical Care Immunotherapy Leading the Prevention or Treatment of Life - Threatening Inflammation in the ICU
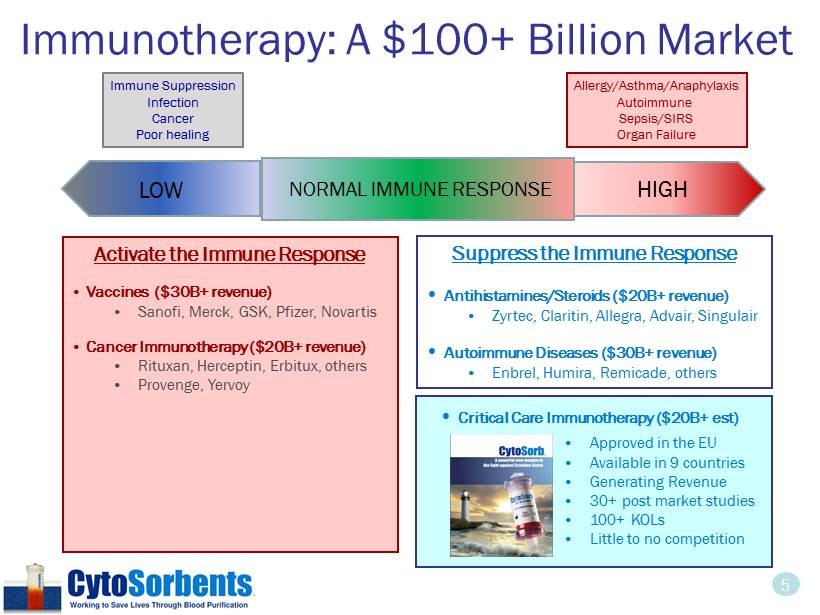
5 Immunotherapy: A $100+ Billion Market HIGH Immune Suppression Infection Cancer Poor healing Allergy/Asthma/Anaphylaxis Autoimmune Sepsis/SIRS Organ Failure Activate the Immune Response • Vaccines ($30B+ revenue) • Sanofi , Merck, GSK, Pfizer, Novartis • Cancer Immunotherapy ($20B+ revenue) • Rituxan , Herceptin, Erbitux, others • Provenge , Yervoy • Approved in the EU • Available in 9 countries • Generating Revenue • 30+ post market studies • 100+ KOLs • Little to no competition NORMAL IMMUNE RESPONSE LOW Suppress the Immune Response • Antihistamines/Steroids ($20B + revenue) • Zyrtec , Claritin, Allegra, Advair, Singulair • Autoimmune Diseases ($30B+ revenue) • Enbrel, Humira , Remicade , others • Critical Care Immunotherapy ($20B+ est)

6 What is the $20B Critical Care Opportunity? Millions of people are admitted to the intensive care unit in hospitals in the U.S. and the European Union each year with deadly inflammatory conditions • In these conditions, little exists to reduce the inflammation and actively help patients get better. Rather, patients often need to be kept alive with machines called “life support”, with the hope that their bodies heal on their own over time • Without “active” therapies, patients linger at a cost of $2,000 - 3,000 a day* with a risk of death of 1 in every 3 patients • The US alone spends nearly 1% of its GDP or ~$80 - 90 billion on critical care** * Cooper, L, et al, Crit Care Med 2004, 32(11):2247 - 2253. ** Halpern, NA, et al., Crit Care Med 2010, 38(1):65 - 71. Sepsis ARDS Burn Injury Trauma Pancreatitis Influenza Surgical

7 Cytokines Fuel the Fire of Inflammation • Cytokines are small proteins that normally help stimulate and regulate the immune system and control inflammation • Cytokines are a dual edged sword • They are required for proper immune system function • However, in mild to moderate excess, cytokines can cause or exacerbate disease (e.g. autoimmune diseases) • But cytokines in vast excess, called “cytokine storm” can lead to a massive uncontrolled systemic inflammatory response syndrome (SIRS) leading to multiple organ dysfunction syndrome (MODS) or multiple organ failure (MOF)

8 Massive Inflammation Causes Organ Failure Organ failure occurs when vital organs stop working, causing nearly half of all deaths in the ICU . Little can be done to prevent or treat it today

9 Cyto Sorb ® Removes the Fuel to the Fire • CytoSorb ® represents a powerful immunotherapy to control inflammation • Approved in the European Union as the only specifically approved cytokine filter • Clinically proven to reduce key cytokines in blood by 30 - 50% in critically ill patients • Approved for use in any situation where cytokines are elevated • Safe: More than 1,500 human treatments, no severe device related adverse events

10 The Heart of the Technology The underlying blood purification technology is based on state - of - the - art biocompatible, highly porous polymer beads that act like tiny sponges to remove harmful substances from blood • Protected by 32 issued US patents and multiple applications pending • Manufactured at our ISO 13485 certified facility in New Jersey • One of the highest grade medical sorbents on the medical market today . Each bead is about the size of a grain of salt

11 Goal: To Prevent or Treat Organ Failure Sepsis ARDS Burn Injury Trauma Pancreatitis Influenza Surgical The Potential to Revolutionize Critical Care Medicine Improve Patient Outcome and Survival Decrease Costs Of ICU and Patient Care

12 • US Dept of Health and Human Services awarded $0.5M grant (2010) for therapies that can save lives and reduce costs under the QTDP Program • NIH grant awarded $7M five year (2006 - 2010) to University of Pittsburgh and Dr. John Kellum to research CytoSorb bead for treatment of sepsis • NIH/NHLBI awarded $0.2M Phase I SBIR to advance the HemoDefend purification technology intended to improve the quality and safety of blood transfusions (2013) $15+ Million in US Government Support • DARPA awarded $3.8M five year (2012) contract as part of “Dialysis - Like Therapeutics” program to treat sepsis by removing cytokines and pathogen - derived toxins • U.S. Army awarded $1.15M SBIR contracts for trauma and burn injury research (2011 - 13) • U.S. Air Force is funding a 30 patient human pilot study in trauma valued at $3M (2013). FDA approved trial to begin this year

13 2013 Operating and Financial Highlights

14 • Huge market CytoSorb ® is sold to hospitals and critical care physicians, targeting a “need to have” $ 20 billion worldwide critical care opportunity addressing organ failure • Little to no competition • Critical care physicians understand the problem • It is a plug and play high margin disposable “razorblade” Hospital’s existing hemodialysis infrastructure is the “razor”, no new hardware • Technicians already know how to use the device • CytoSorb ® is reimbursed in Germany/Austria at > $ 500 /cartridge . Depending on the application and devices used, revenue potential per patient ~ $ 1 - 5 K • Affordable yet profitable with gross margins of 60 % +, target > 80 % • Intensive care units are highly centralized easy for a small sales force to access Cyto Sorb ® An Excellent Business Model
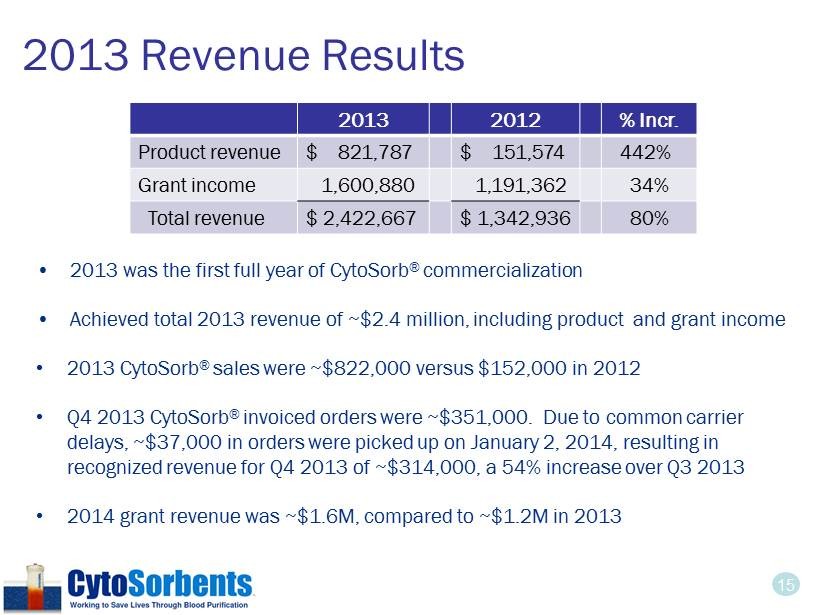
15 2013 Revenue Results • 2013 was the first full year of CytoSorb ® commercialization • Achieved total 2013 revenue of ~$2.4 million, including product and grant income • 2013 CytoSorb ® sales were ~$822,000 versus $152,000 in 2012 • Q4 2013 CytoSorb ® invoiced orders were ~$351,000. Due to common carrier delays, ~$37,000 in orders were picked up on January 2, 2014, resulting in recognized revenue for Q4 2013 of ~$314,000, a 54% increase over Q3 2013 • 2014 grant revenue was ~$1.6M, compared to ~$1.2M in 2013 2013 2012 % Incr. Product revenue $ 821,787 $ 151,574 442% Grant income 1,600,880 1,191,362 34% Total revenue $ 2,422,667 $ 1,342,936 80%

16 Continued Strong Product Growth Since Launch $13,679 $87,960 $176,098 $127,969 $203,561 $314,159 $0 $50,000 $100,000 $150,000 $200,000 $250,000 $300,000 $350,000 Q3 2012 Q4 2012 Q1 2013 Q2 2013 Q3 2013 Q4 2013 CytoSorb ® Product Sales 16

17 Record CytoSorb Sales Expected for Q1 2014 $13,679 $87,960 $176,098 $127,969 $203,561 $314,159 $530,000 $570,000 $0 $100,000 $200,000 $300,000 $400,000 $500,000 $600,000 Q3 2012 Q4 2012 Q1 2013 Q2 2013 Q3 2013 Q4 2013 Q1 2014 PROJ CytoSorb ® Product Sales 17 Maximum and Minimum Expecting Q1 2014 CytoSorb sales between $530 - $570K

18 Direct Sales: Germany, Austria, Switzerland We are currently working with 100+ KOLs in the largest and most important university and public hospitals in most major cities in Germany and many in Austria and the UK Partial List: • University of Aachen • University of Bonn • Helios Berlin Buch • Charite Mitte and Charite Virchow • Helios Erfurt • University of Goettingen • University of Greifswald • University of Hamburg - Eppendorf • University of Jena • University of Kiel • University of Leipzig • Trauma Hospital of Linz • European Medical University of Oldenburg • University of Rostock • University of Ulm • Medical University of Vienna • Vienna General Hospital (AKH) Addressable market in Germany alone: $1.0 - 1.5B

19 E.U. Approval Opens Global Distribution Signed UK, Ireland, Netherlands, Turkey, Russia and India. Expanding to other parts of Europe and other countries outside the EU that accept the CE Mark Middle East WMC Intensiv Med LLC
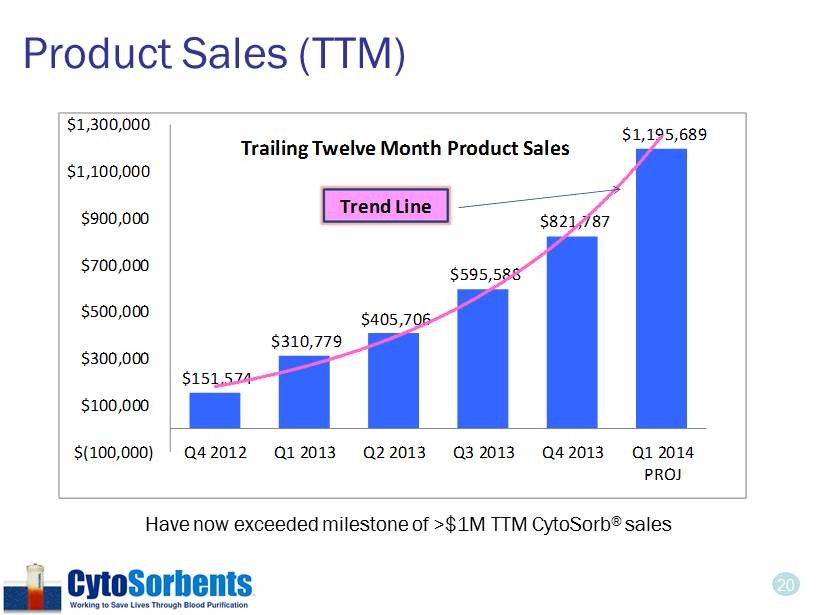
20 Product Sales (TTM) $151,574 $310,779 $405,706 $595,588 $821,787 $1,195,689 $(100,000) $100,000 $300,000 $500,000 $700,000 $900,000 $1,100,000 $1,300,000 Q4 2012 Q1 2013 Q2 2013 Q3 2013 Q4 2013 Q1 2014 PROJ Trailing Twelve Month Product Sales Trend Line Have now exceeded milestone of >$1M TTM CytoSorb ® sales

21 Strong Gross Margins • Overall 2013 product gross margins exceeded 60% • Sales prices have remained constant • Gross margins will vary based on the ratio of direct sales vs. sales to distributors, but are expected to remain very healthy • Higher scale production is expected to result in lower product costs and improve our gross margin percentage • As CytoSorb ® sales increase, gross margin on our products will help generate working capital

22 Working Capital Multiple Sources of Capital to Fund Growth • March 11, 2014 receipt of $9,451,000 from our preregistered offering has fortified our balance sheet. • Increasing Product Sales – high gross margins and increasing sales will drive us closer to cash flow breakeven • Continue to receive grant funding from DARPA, SBIR, etc. • Potential strategic partnerships $- $2,000 $4,000 $6,000 $8,000 $10,000 $12,000 Cash on Hand 3/11/2014 Financing Cash on Hand (In thousands of U.S. Dollars)

23 Investing in Growth: Catalysts for 2014

24 #1: Core Focus on Cyto Sorb ® Sales 100 + Key Opinion Leaders In Germany and Austria Driving Departmental Adoption targeting Junior and Senior Physicians Increase Sales Team + Clinical and Distributor support Reference Hospitals with Expansion to MICU, SICU, CICU, Trauma ICU 30+ Post - Market Studies Generate Clinical Data in Many Applications Orders from Existing Distributors. Further International Expansion and Reimbursement Focus

25 Sales Infrastructure Targets For 2014 Direct Sales Germany Austria Switzerland Direct Sales 5 Distributors (UK, Ireland, Netherlands, Turkey, Russia) 4 sales reps Distributor/Partner Support Rep Targets for 2014 2013 Distributors Partners Distributors Partners Biocon (India) Germany Austria Switzerland 10+ Distributors 2+ Partners 10 sales reps Clinical Support ( 1 ) Clinical Support Staff (2) Support increases in sales with increases in US Manufacturing + QA/QC Staff Reimbursement Support US Manufacturing + QA/QC Staff Reimbursement

26 “Market Pull” from Key Applications Use of CytoSorb either during or after cardiac surgery to prevent organ dysfunction and organ failure is a $500M – 1B total addressable market in the US and EU alone Cardiac Surgery Sepsis Afflicts 2.5 million people in the US and EU annually without an approved therapy, representing a $6 - 8 billion market

27 Targeting Significant Revenue Acceleration Revenue Multiplying Effect ~$ 822K 2013 Sales $M: 2014 Sales * Number of cartridges used only to graphically demonstrate the multiplier effect concept. This does not reflect revenue guidance for 2014.

28 DIVI 2013 First Ever CytoSorb Users Meeting Great energy and excitement from actual CytoSorb users in Germany and Austria • Treatment pioneers from all over Germany and Austria gathered to share their clinical experiences using CytoSorb • Because of the strong interest amongst CV surgeons, there was a dedicated track on cardiac surgery • Hospital of the University of Jena Hospital announced development of CytoSorb Registry

29 DIVI 2013 CytoSorbents Sponsored Symposium More than 120 people from Germany/Austria attended in Leipzig, Germany

30 ISICEM 2014 CytoSorbents Symposium
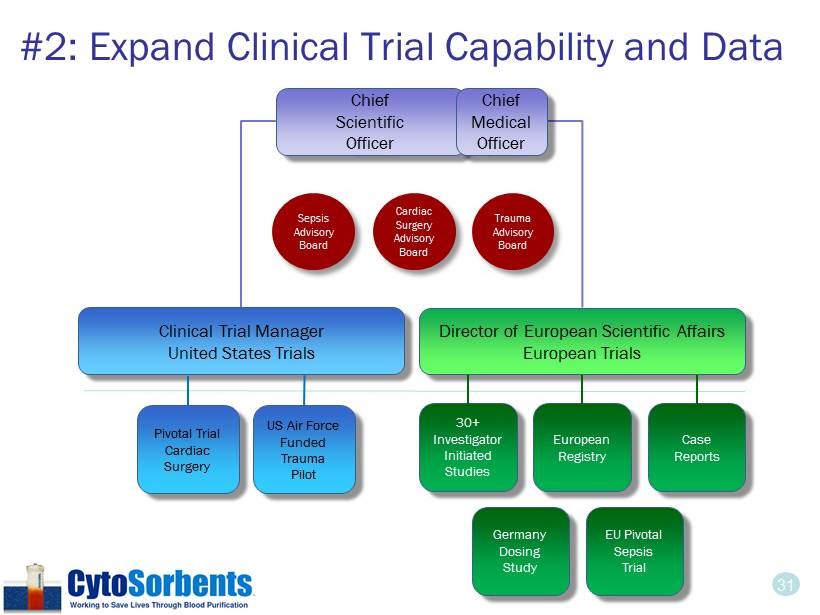
31 30+ Investigator Initiated Studies EU Pivotal Sepsis Trial Pivotal Trial Cardiac Surgery European Registry US Air Force Funded Trauma Pilot Case Reports Germany Dosing Study Clinical Trial Manager United States Trials Director of European Scientific Affairs European Trials #2: Expand Clinical Trial Capability and Data Chief Scientific Officer Trauma Advisory Board Sepsis Advisory Board Cardiac Surgery Advisory Board Chief Medical Officer
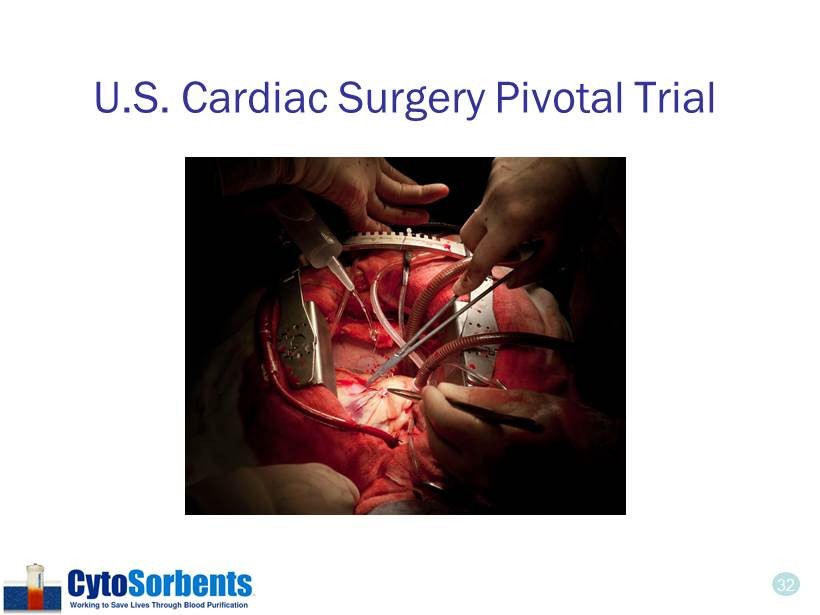
32 U.S. Cardiac Surgery Pivotal Trial

33 Rationale for U.S. Cardiac Surgery Trial The path to approval of CytoSorb® in the U.S. is potentially faster, less expensive and less risky than seeking approval for critical care applications such as sepsis • Seeing significant “market pull” for this application. 20+ heart centers in Germany and Austria. Data coming in Q2 - Q3 2014 from cardiac surgery trials • Very large markets: 500K cardiac surgeries in the U.S., 500K in the E.U., more than 1.5 million in the world each year - a potential total addressable market of $1 billion or more • CytoSorb is the only cytokine reduction technology capable of direct installment in a bypass circuit into a heart lung machine without the need for another machine • CytoSorb could completely displace leukoreduction filters used in the heart - lung machine circuit, an existing multi - million dollar market, while significantly expanding the market. • Trial is more straightforward, less expensive and less risky. Patients are much more homogenous and can be enrolled quickly, treatment and follow - up is relatively short. • Endpoint would NOT be mortality, but a clinical endpoint such as incidence of respiratory failure or acute kidney injury • Once approved in the U.S., can expand U.S. applications through a label extension strategy while driving usage in cardiac surgery outside of the U.S. where it is already approved
34 U.S. Cardiac Surgery Pivotal Trial 1) Intra - Operative Usage of CytoSorb to Prevent Post - operative C omplications • Multi - center randomized controlled trial • Enrich for cardiac surgery patients at high risk for inflammation and hemolysis and treat DURING surgery with CytoSorb in a bypass circuit • Primary endpoint: Reduction in post - operative complications (AKI vs ALI/ARDS) OR 2) Post - Operative Usage of CytoSorb to Treat Post - operative SIRS • Multi - center randomized controlled trial • CytoSorb is used AFTER surgery to treat patients who develop SIRS • Primary endpoint: Relative reduction in incidence of organ dysfunction Two potential paths to U.S. regulatory approval for CytoSorb in cardiac surgery. Expect to submit IDE during Summer 2014

35 Germany “Pivotal” Sepsis Trial
36 Treatment Alternatives Continue to Shrink Sepsis: • Early Goal Directed Therapy: Early optimization of hemodynamics and oxygenation in septic shock patients failed to demonstrate improvement in 60 - day mortality (21.0% vs 18.2% control) versus usual care in large 1,341 patient U.S. multi - center trial. This reverses the findings of a landmark 2001 study • Endotoxin removal with Toraymyxin in patients with peritonitis due to organ perforation with septic shock (ABDO - MIX Trial) showed no mortality benefit vs standard of care therapy in a 243 - patient randomized controlled multi - center trial in France (preliminary data as presented at ISICEM 2014). CytoSorb remains one of the few promising therapies in sepsis and other applications

37 Goal: Increase the Odds of Success • CytoSorb is one of the only therapies being used to treat sepsis today in Europe and elsewhere, and holds great promise in early studies. • The European Sepsis Trial and Dosing Study demonstrate that up to 24 hours of treatment is safe. In these patients with advanced sepsis and multiple organ failure, those with cytokine storm or those ≥ age 65 may have the best benefit • In actual clinical practice, there have also been many remarkable treatment successes in patients with either severe sepsis or septic shock, particularly when used early • Our goal is to ensure our pivotal sepsis study design has the best chance of success. The many sepsis studies with CytoSorb® that are being conducted, including the Dosing study, will help us get there • When ready, we plan to conduct this study in Germany, led by our major collaborators in the SepNet Trials Network

38 Critical Care, High Risk Surgery #3: Our Bead Technology Enables a Diverse and Valuable Pipeline Blood Collection & Transfusion CT Imaging, Interventional Radiology Contrast Sorb Drug Overdose, Chemo Removal Drug Sorb Beta Sorb Improving Dialysis Under Development CE Mark Approved

39 Potential for Strategic Partnerships* Cardiac Surgery Renal Dialysis Blood Transfusion Biotech and Immunotherapy Critical Care or Catheters *Companies listed here are used simply as examples of companies in these respective verticals. We make no other representations to our relationship with any of these companies .

40 Strategic Partnership: The Most Comprehensive Treatment for Sepsis Treat the Massive Inflammatory Response Treat the Primary Infection Ms. Kiran Shaw, Chairman and Managing Director of Biocon , India’s largest biotechnology company – Jan 2014 Q3 2014 earnings report: “Our recent launches of Alzumab and CytoSorb ® have done extremely well, with strong uptake by both doctors and patients.”

41 2014 and Hemo Defend ™ Two major trials are expected to be reported in 2014 that may be very important to the HemoDefend platform RECESS (Red Cell Storage Duration Study): • New Blood (≤10 days) vs Old Blood (≥21 days) in 1,696 cardiac surgery patients • Primary endpoint: Change in multiple organ dysfunction score (MODS) from pre - surgery ABLE (Age of Blood Evaluation Study): • New Blood (≤10 days) vs Standard Issue Blood (~15 - 20 days) in 2,510 critically - ill patients • Primary endpoint: 90 - day all - cause mortality • HemoDefend is designed to help keep new blood fresh and is supported by a Phase I SBIR grant from the National Heart, Lung, and Blood Institute (NHLBI) • It removes contaminants from transfused blood products that accumulate during blood storage as blood gets old that can cause transfusion reactions and adverse events such as organ failure and death. • We continue to advance development of HemoDefend towards commercialization increasing its value while de - risking the asset, with the goal of out - licensing

42 #4: Uplisting Targeted for 2H 2014 Increased institutional ownership and liquidity the goal of uplisting to NYSE or NASDAQ • Targeting major operational momentum into 2H 2014 • Revenue Growth with increase in direct and distributor sales • Fixed strategy on US Pivotal trial in cardiac surgery and/or EU sepsis trial • Data in many applications from many post - market EU studies • Potential for strategic partnerships and monetization of pipeline • New product development • Have already met with NYSE and NASDAQ • Preparing company for uplisting • Cultivating relationships with analysts and institutional investors

43 #5: CytoSorb is Being Used in a Broad Range of Applications

44 Case Report: Septic shock 57 year old male was diagnosed with gangrenous appendicitis • Deterioration of clinical course due to severe respiratory failure requiring mechanical ventilation due to suspected pneumonia and aspiration • Transferred to the ICU with septic shock requiring high doses of norepinephrine, aggressive IV hydration and antibiotics, acute respiratory distress syndrome, severe lactic acidosis, and low urine output • Began CytoSorb therapy with immediate improvement in hemodynamic stability • Weaned off of all vasopressors by Day 2 • Weaned off of mechanical ventilation by Day 5 • D id not require dialysis therapy • Transfered out of the ICU to the step down unit by Day 9.
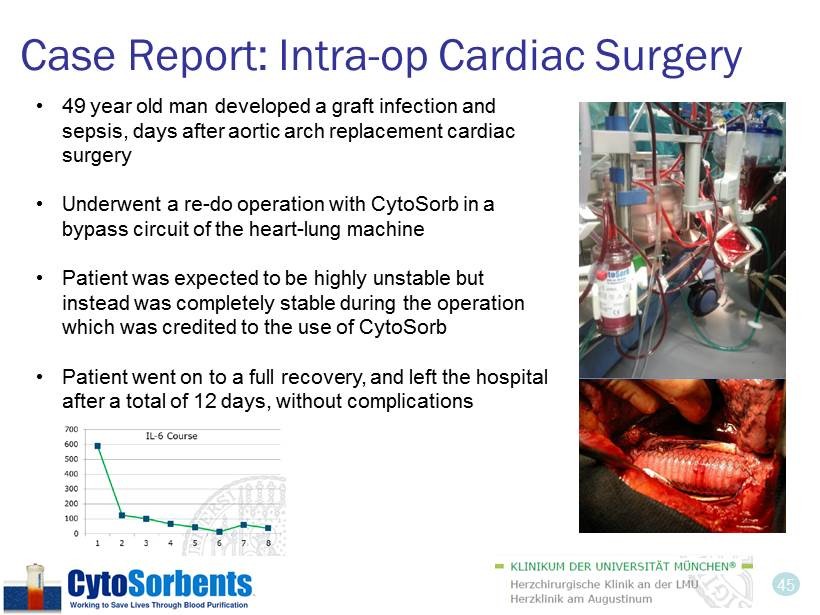
45 Case Report: Intra - op Cardiac Surgery • 49 year old man developed a graft infection and sepsis, days after aortic arch replacement cardiac surgery • Underwent a re - do operation with CytoSorb in a bypass circuit of the heart - lung machine • Patient was expected to be highly unstable but instead was completely stable during the operation which was credited to the use of CytoSorb • Patient went on to a full recovery, and left the hospital after a total of 12 days, without complications

46 Case Report: Post - op Cardiac Surgery • 83 year old man underwent quadruple bypass but on Day 8 post - op, he developed pneumonia with septic shock, respiratory and renal failure • The infection was polymicrobial with bacteremia • Treated with CytoSorb and antibiotics • Patient recovered and was discharged from the ICU by Day 7 to a respiratory weaning unit

47 Case Report: Severe Burn Injury Patient in his mid - 20‘s suffered severe burn injury with >60% total body surface area and also had severe smoke inhalation injury • Baux (Burn injury severity) Score of 103 predicted mortality of 45 - 50% • Patient was admitted to the ICU after developing shock requiring high doses of vasopressors, lung failure, and kidney injury • CytoSorb was used continuously with renal support over 8 days, changing the cartridge each day • Patient was weaned from vasopressors within 4 days • Significant improvement in kidney and lung function • Wound healing was much faster than expected • Patient currently has a good prognosis for recovery
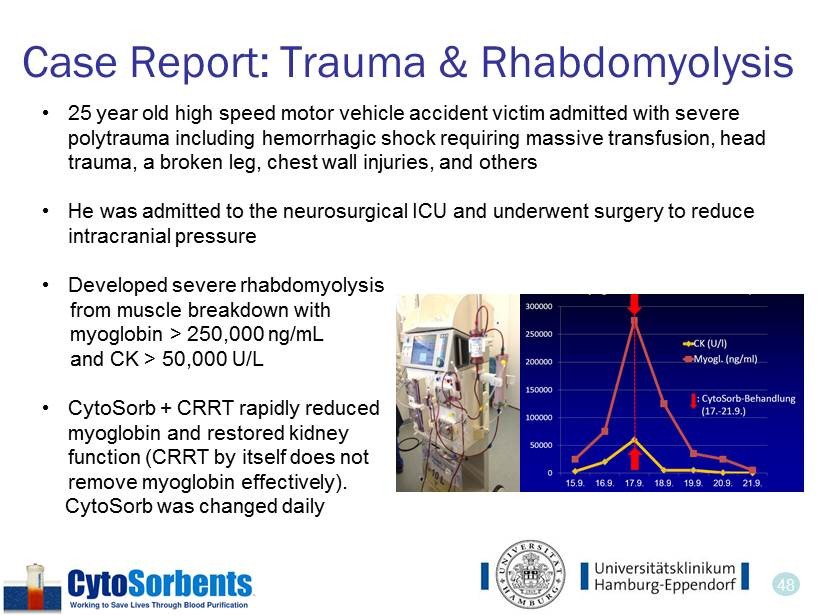
48 Case Report: Trauma & Rhabdomyolysis • 25 year old high speed motor vehicle accident victim admitted with severe polytrauma including hemorrhagic shock requiring massive transfusion, head trauma, a broken leg, chest wall injuries, and others • He was admitted to the neurosurgical ICU and underwent surgery to reduce intracranial pressure • Developed severe rhabdomyolysis from muscle breakdown with myoglobin > 250,000 ng/mL and CK > 50,000 U/L • CytoSorb + CRRT rapidly reduced myoglobin and restored kidney function (CRRT by itself does not remove myoglobin effectively). CytoSorb was changed daily

49 Case Report: Tylenol Overdose A patient overdosed on 30 grams of Tylenol (> 12 g considered to have a very high risk of death due to liver failure and other complications) • The patient subsequently developed multi - organ failure, with failure of the lungs, kidneys and liver, with collapse of the circulatory system • Patient was at high risk for bleeding events • Was stabilized within 24 hours of treatment with CytoSorb and survived

50 Case Report: Transport Stabilization CytoSorb was used with ECMO to stabilize a 58 year old man for transport from Kuwait to a Berlin ICU after a massive heart attack

51 Cyto Sorbents is Leading the Way To Prevent or Treat Life Threatening Inflammation • CytoSorb ® targets deadly inflammation in the ICU that leads to organ failure and death • It is approved in the E.U. a nd is generating international revenue • Untapped $20B market opportunity and crucial unmet medical need • Validation of the company and technology from many fronts • Unique, highly profitable product and pipeline with little to no competition • Experienced and responsible management team • Potential major catalysts in the next 6 - 9 months • Revenue Growth • Strategic Partnerships • Clinical Data • Up - listing to National Market • New Product Development • Institutional Ownership

52 Phillip P. Chan, MD, PhD - CEO 7 Deer Park Drive, Suite K Monmouth Junction, NJ 08852 pchan@cytosorbents.com Cyto Sorbents Corporation OTCBB: CTSO The Rise of An Emerging Critical Care Immunotherapy Company Q&A Session



















































