
Investor Webcast: Initial Data from Phase 1a/1b Trial of Cabiralizumab/OPDIVO® and Early Efficacy Signal in Pancreatic Cancer November 8, 2017 Exhibit 99.2

This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as "may," "will," "expect," "plan," "anticipate" and similar expressions (as well as other words or expressions referencing future events or circumstances) are intended to identify forward-looking statements. These forward-looking statements reflect FivePrime's current beliefs and expectations. Each of these forward-looking statements involves risks and uncertainties. Actual results may differ from these forward-looking statements. Forward-looking statements contained in this presentation include statements about (i) the timing of initiation, progress and scope of clinical trials of cabiralizumab; and (ii) the potential use of cabiralizumab to treat patients. Many factors may cause differences between current expectations and actual results, including unexpected safety or efficacy data observed during preclinical or clinical studies, clinical site activation rates that are lower than expected, changes in expected or existing competition, failure of our collaborators to support or advance the development of cabiralizumab and unexpected litigation or other disputes. Other factors that may cause our actual results to differ from current expectations are discussed in FivePrime's filings with the U.S. Securities and Exchange Commission, including the "Risk Factors" sections contained therein. Except as required by law, we assume no obligation to update any forward-looking statements contained herein to reflect any change in expectations, even as new information becomes available. Forward-Looking Statements Disclaimer © 2017 Five Prime Therapeutics, Inc. All Rights Reserved
Durable clinical benefit in 5 of 31 patients in pancreatic cancer Including 3 independently confirmed responses in heavily pre-treated patients without microsatellite-instability (MSI) Responses associated with significant reduction in tumor marker CA19-9 Historically, pancreatic cancer without MSI does not respond to PD-1 pathway blockade BMS and FivePrime advancing cabira/OPDIVO development Enrolling 30 additional patients with pancreatic cancer in the current trial Information on BMS-sponsored ADVISE trial, which includes cabiralizumab, now available at clinicaltrials.gov (NCT03335540) Information on an additional BMS-sponsored study in pancreatic cancer will be posted on clinicaltrials.gov soon Tolerable safety profile of cabira monotherapy and cabira + Opdivo Key Takeaways Regarding Cabiralizumab/Opdivo® Combination in Pancreatic Cancer © 2017 Five Prime Therapeutics, Inc. All Rights Reserved
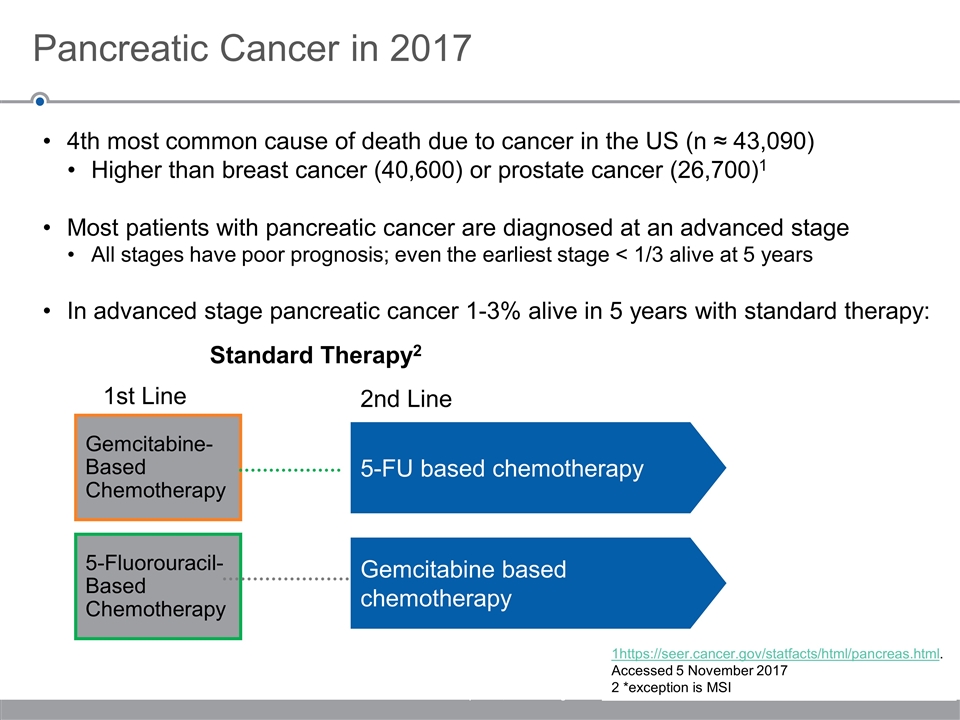
4th most common cause of death due to cancer in the US (n ≈ 43,090) Higher than breast cancer (40,600) or prostate cancer (26,700)1 Most patients with pancreatic cancer are diagnosed at an advanced stage All stages have poor prognosis; even the earliest stage < 1/3 alive at 5 years In advanced stage pancreatic cancer 1-3% alive in 5 years with standard therapy: Pancreatic Cancer in 2017 © 2017 Five Prime Therapeutics, Inc. All Rights Reserved 5-FU based chemotherapy Gemcitabine- Based Chemotherapy 5-Fluorouracil- Based Chemotherapy Gemcitabine based chemotherapy 1st Line 2nd Line Standard Therapy2 1https://seer.cancer.gov/statfacts/html/pancreas.html. Accessed 5 November 2017 2 *exception is MSI
There are no reported durable monotherapy responses in pancreatic cancer without MSI: Anti-PD-1 Anti-CTLA4 Anti-PD1 monotherapy is ineffective in pancreatic cancer except in the <1-2% of patients who have microsatellite instability-high (MSI)2 Onivyde® (liposomal irinotecan) is the most recently FDA approved agent in October 2015 2nd-line approval, in patients who have failed a gemcitabine based regimen Onivyde®/5-FU/Leucovorin1 (n=117) ORR 7.7% (USPI, 2017) PFS 3.1 months OS 6.1 months Durable responses are rare in patients with pancreatic cancer, who have a poor prognosis and few treatment options © 2017 Five Prime Therapeutics, Inc. All Rights Reserved 1 Onivyde USPI, 2017 2 Keytruda USPI, 2017
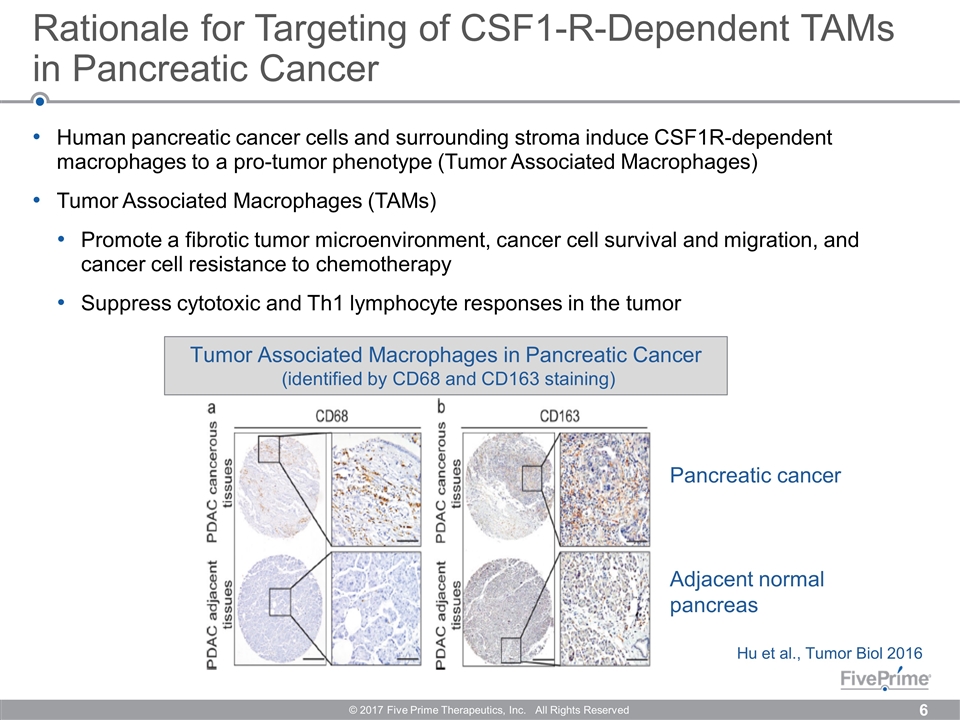
Human pancreatic cancer cells and surrounding stroma induce CSF1R-dependent macrophages to a pro-tumor phenotype (Tumor Associated Macrophages) Tumor Associated Macrophages (TAMs) Promote a fibrotic tumor microenvironment, cancer cell survival and migration, and cancer cell resistance to chemotherapy Suppress cytotoxic and Th1 lymphocyte responses in the tumor Rationale for Targeting of CSF1-R-Dependent TAMs in Pancreatic Cancer © 2017 Five Prime Therapeutics, Inc. All Rights Reserved Tumor Associated Macrophages in Pancreatic Cancer (identified by CD68 and CD163 staining) Hu et al., Tumor Biol 2016 Pancreatic cancer Adjacent normal pancreas
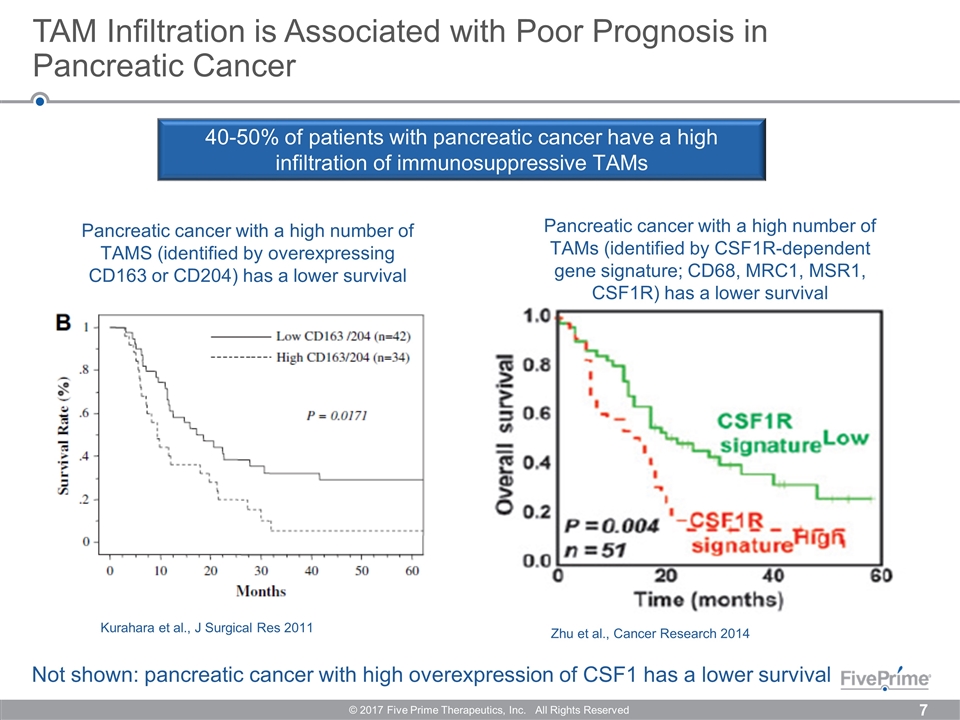
TAM Infiltration is Associated with Poor Prognosis in Pancreatic Cancer © 2017 Five Prime Therapeutics, Inc. All Rights Reserved Kurahara et al., J Surgical Res 2011 Zhu et al., Cancer Research 2014 Pancreatic cancer with a high number of TAMs (identified by CSF1R-dependent gene signature; CD68, MRC1, MSR1, CSF1R) has a lower survival Pancreatic cancer with a high number of TAMS (identified by overexpressing CD163 or CD204) has a lower survival 40-50% of patients with pancreatic cancer have a high infiltration of immunosuppressive TAMs *Ino et al., BJC 2013 Not shown: pancreatic cancer with high overexpression of CSF1 has a lower survival
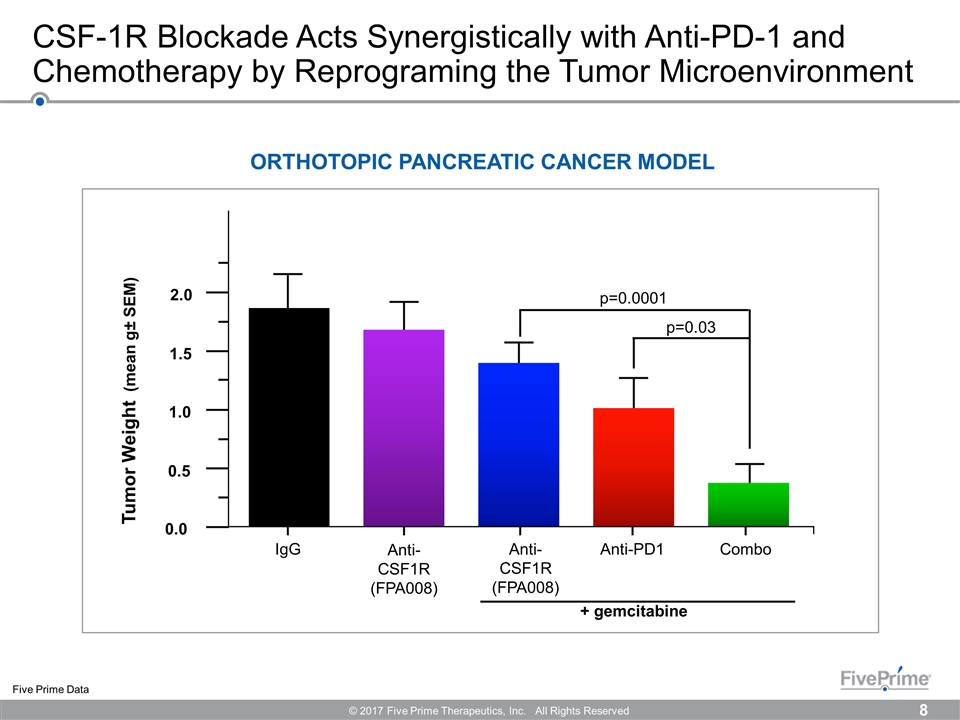
CSF-1R Blockade Acts Synergistically with Anti-PD-1 and Chemotherapy by Reprograming the Tumor Microenvironment Five Prime Data ORTHOTOPIC PANCREATIC CANCER MODEL + gemcitabine IgG Anti-PD1 Combo p=0.0001 p=0.03 0.0 0.5 1.0 1.5 2.0 Tumor Weight (mean g± SEM) Anti-CSF1R (FPA008) Anti-CSF1R (FPA008) © 2017 Five Prime Therapeutics, Inc. All Rights Reserved
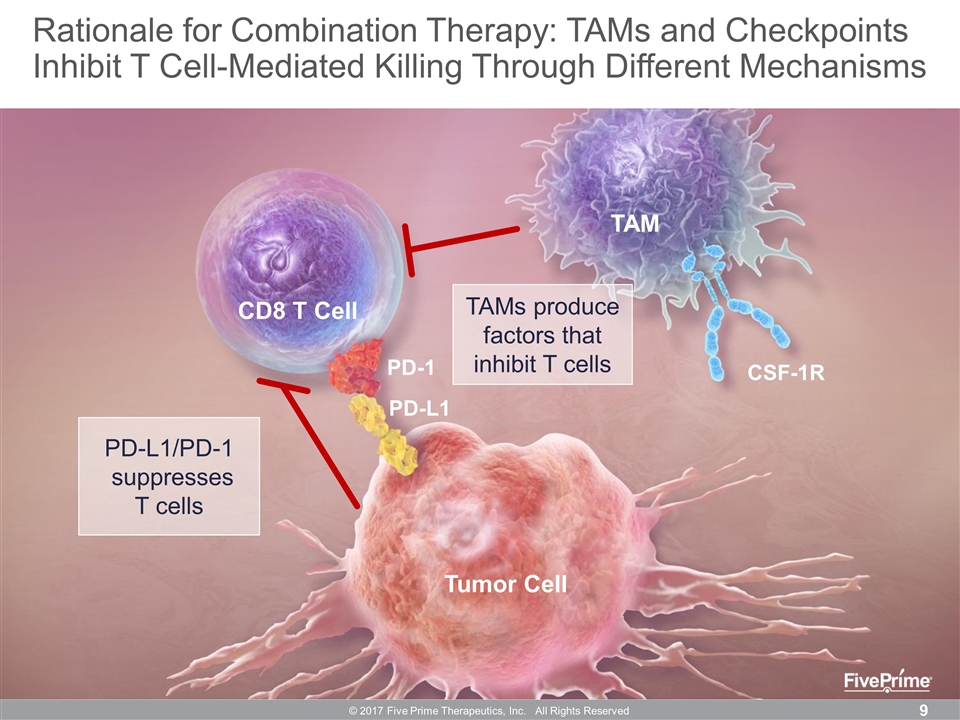
CD8 T Cell Tumor Cell PD-1 PD-L1 TAM CSF-1R PD-L1/PD-1 suppresses T cells TAMs produce factors that inhibit T cells © 2017 Five Prime Therapeutics, Inc. All Rights Reserved Rationale for Combination Therapy: TAMs and Checkpoints Inhibit T Cell-Mediated Killing Through Different Mechanisms
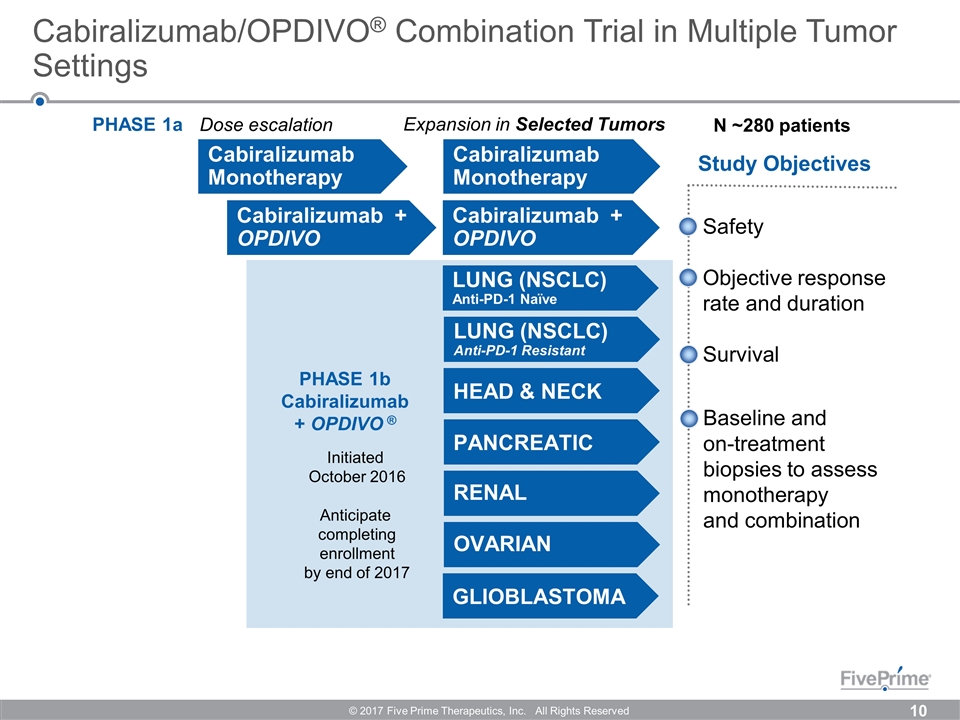
Cabiralizumab/OPDIVO® Combination Trial in Multiple Tumor Settings © 2017 Five Prime Therapeutics, Inc. All Rights Reserved PHASE 1b Cabiralizumab + OPDIVO ® Study Objectives Cabiralizumab Monotherapy N ~280 patients Safety Objective response rate and duration Survival Baseline and on-treatment biopsies to assess monotherapy and combination PANCREATIC RENAL OVARIAN GLIOBLASTOMA HEAD & NECK LUNG (NSCLC) Anti-PD-1 Naïve LUNG (NSCLC) Anti-PD-1 Resistant Expansion in Selected Tumors PHASE 1a Dose escalation Initiated October 2016 Anticipate completing enrollment by end of 2017 Cabiralizumab + OPDIVO Cabiralizumab Monotherapy Cabiralizumab + OPDIVO

Cabiralizumab +/- Nivolumab Demonstrates a Tolerable Safety Profile © 2017 Five Prime Therapeutics, Inc. All Rights Reserved *Includes pneumonitis, respiratory distress and acute respiratory distress
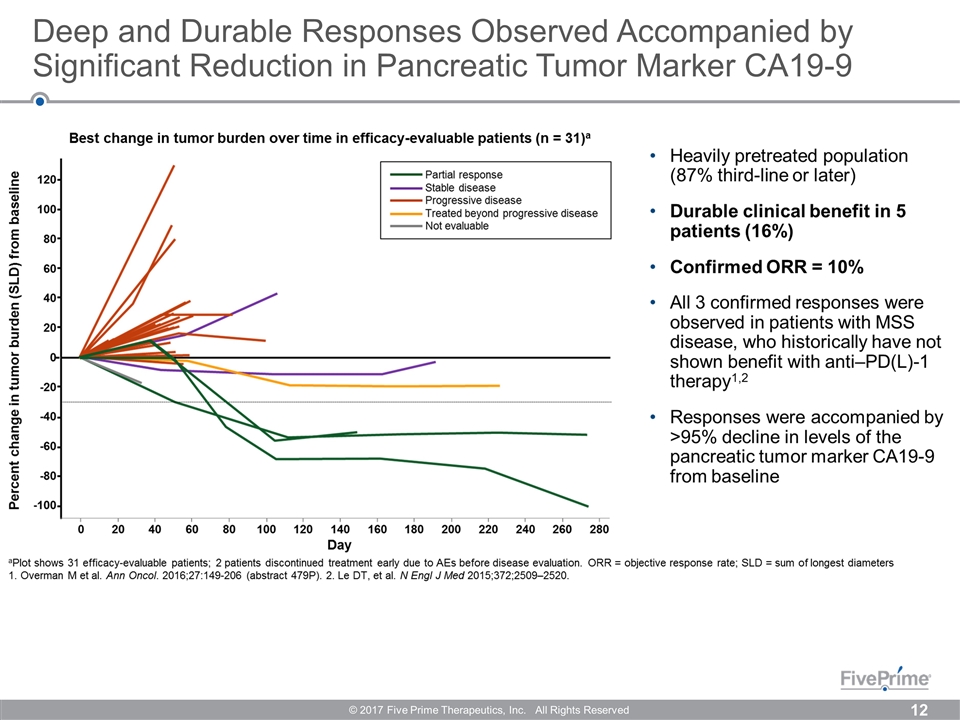
Deep and Durable Responses Observed Accompanied by Significant Reduction in Pancreatic Tumor Marker CA19-9 © 2017 Five Prime Therapeutics, Inc. All Rights Reserved
Durable clinical benefit in 5 of 31 patients in pancreatic cancer Including 3 independently confirmed responses in heavily pre-treated patients without microsatellite-instability (MSI) Responses associated with significant reduction in tumor marker CA19-9 Historically, pancreatic cancer without MSI does not respond to PD-1 pathway blockade BMS and FivePrime advancing cabira/OPDIVO development Enrolling 30 additional patients with pancreatic cancer in the current trial Information on BMS-sponsored ADVISE trial, which includes cabiralizumab, now available at clinicaltrials.gov (NCT03335540) Information on an additional BMS-sponsored study in pancreatic cancer will be posted on clinicaltrials.gov soon Tolerable safety profile of cabira monotherapy and cabira + Opdivo Conclusions © 2017 Five Prime Therapeutics, Inc. All Rights Reserved

NASDAQ:FPRX Thank You www.fiveprime.com













