
“Genetic Technologies for Targeted Therapeutics”
CEO’s Presentation
Dr Greg Collier
November 29 2006
CXSP
NASDAQ
LISTED
CXS
ASX
LISTED
>

Report Card for 2006
Dr Ian Nisbet appointed as Vice President of Oncology
November, 2006
Fast Track status granted by FDA for Ceflatonin® in CML
patients with the T315I mutation
November, 2006
Progressed diabetes discovery program to compound
screening
June, 2006
Launched Phase 2/3 study of Ceflatonin® in CML patients
with T315I mutation
June, 2006
Completed A$15M financing
May, 2006
Ceflatonin® granted USA Orphan Drug status for treatment
of CML
March, 2006
Expanded phase 2 study of Quinamed® to Prostate, Breast
and Ovarian Cancers
September, 2005
>

Strong Leadership Team
Ken Walder PhD,
Shawnya Michaels BS,
Jeremy Jowett DPhil,
John Blangero, PhD
Senior Directors of Research
Regulatory Affairs Consultant
Luana Staiger, BS
European Medical Director
Annie-Claude Benichou, MD
Consultant Medical Director
Richard S. Schwartz, MD
Senior Director of Finance
Tina Herbert, MBA
Vice President of Oncology
Ian Nisbet, PhD
Chief Financial Officer and Company Secretary
Rick Merrigan, MBA
Vice President of Operations
James Campbell, PhD, MBA
President and Executive Director
Dennis Brown, PhD
CEO and Managing Director
Greg Collier PhD
>

World Opinion Leaders Directing Clinical Development
Versailles University Hospital
Professor of Hematology
Philippe Rousselot, MD
Lille University Hospital
Professor of Hematology
Thierry Facon, MD
Our Lady of Mercy, the Bronx
Director, Cancer Center
Peter Wiernik, MD
Imperial College London
Professor of Hematology
David Marin, MD
Hospital Edouard Herriot, Lyon
Professor of Hematology
Mauricette Michallet, MD
University of Poitiers
Professor of Hematology
François Guilhot, MD
M.D. Anderson/Houston
Chief, Chronic Leukemia Dept
Jorge Cortes, MD
Heidelberg University
Professor of Internal Medicine
Prof. Andreas Hochhaus, MD
M.D. Anderson/Houston
Chairman, Leukemia Dept
Hagop Kantarjian, MD
>
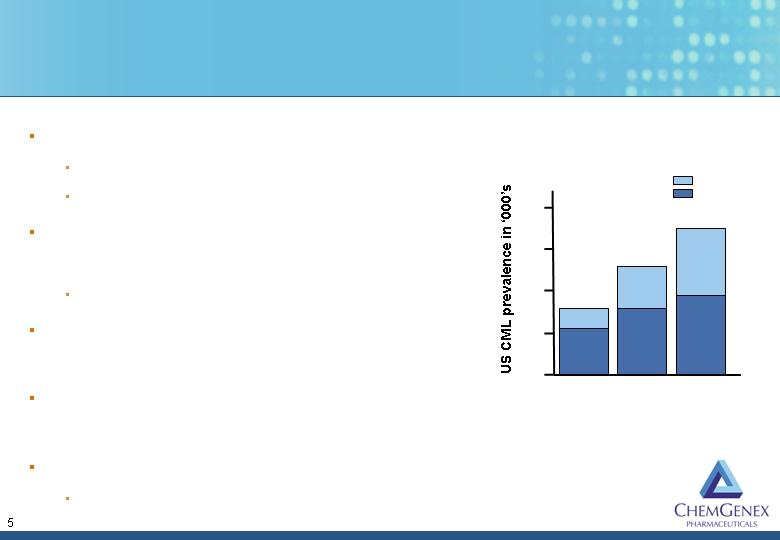
Tyrosine Kinase Inhibitors Have Revolutionized CML Treatment
But Resistance is Increasing
Gleevec approved in 2001
US$2.2 billion in 2005
50-90% of patients still Ph+ while on Gleevec
Abl-kinase point mutations associated with
Gleevec resistance arise in patients over time
Lead to 70-80% of treatment failures
Sprycel approved in 2006 for Gleevec-
resistant patients
Neither Gleevec or Sprycel are effective in
CML patients with the T315I mutation
T315I mutation is a growing and unmet clinical problem
Known TKIs create selective pressure for mutation
2003
2005
2007
20
30
60
Baseline
TKI effect
Approximately 4,600 new
cases per year in the US
40
50
>
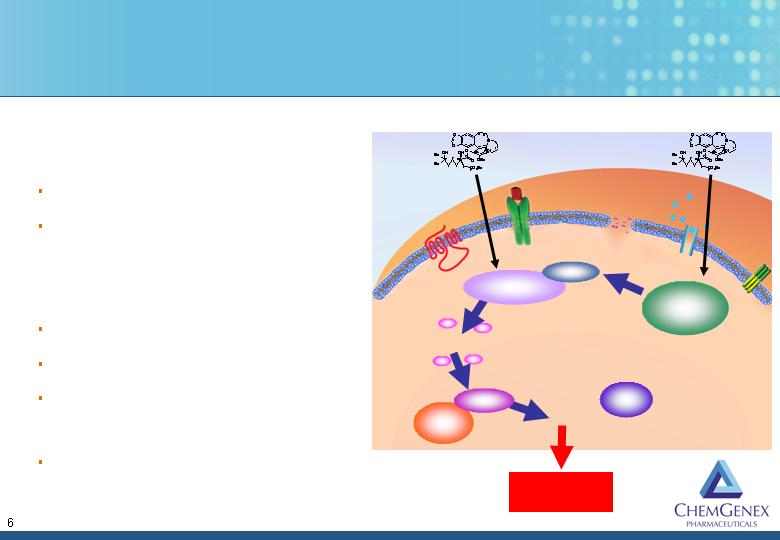
Ceflatonin Induces Apoptosis in Myeloid Cells
Homoharringtonine (HHT)
Small molecule from NCI
Cephalotaxine analog
Mechanism
Caspase-3 activation
Bax protein up-regulation
Induces apoptosis, independent of
p53 status
Synergistic with tyrosine kinase
inhibitors (TKI’s)
HHT
HHT
Apoptosis
Proteosome
Caspase 3
Caspase 9
Cytochrome C
Mitochondria
Mcl-1
Apaf 1
>

Ceflatonin Has Demonstrated Clinical Efficacy
Active as a single-agent in CML
88% response rate - late chronic phase
98% response rate - early chronic phase
Active as a single-agent in both chronic and accelerated-phase CML resistant to Gleevec®
80% return to chronic from accel. Phase
67-100% complete hematologic response rates
40% complete cytogenetic response rate
Eliminated p-loop mutations in two patients
88% survival at 12 months
Active in-combination with Gleevec® to increase cytogenetic response
70% decline in BCR-ABL transcripts
50% at least a 1 log decrease in BCR-ABL transcripts
Two patients with partial response, achieved a complete cytogenetic response
Patients with p-loop mutations responded
Manageable safety and toxicology profile (>450 patients)
>

Clinical Trial Protocol for CML Patients with the T315I
Mutation - Chronic, Accelerated and Blast Phases
Patients confirmed as being T315I positive
Initiation phase
Patients receive 2.5mg/m2 administered daily by subcutaneous
injection for 14 d, every 28 d
Up to 6 cycles of induction therapy
Clinical end-points
Complete hematologic response (3 months for Chronic and
Accelerated phase patients, 2 months for Blast phase patients)
Complete cytogenetic response
Ongoing molecular mutation testing
Maintenance phase
2.5 mg/m2 administered daily by subcutaneous injection for 7 days
every 28 days
>

Ceflatonin Clinical Trial Progress
Predicted completion of enrolment
H2, 2007
Several additional sites proposed
H1, 2007
5 additional centres; Boston, Buffalo,
Heidelberg, Hamburg, London
December, 2006
7 active centres; Houston, Bronx, Los
Angeles, Poiters, Lyon, Lille, Versailles
November, 2006
First patient enrolled
September, 2006
Study initiated
June, 2006
>
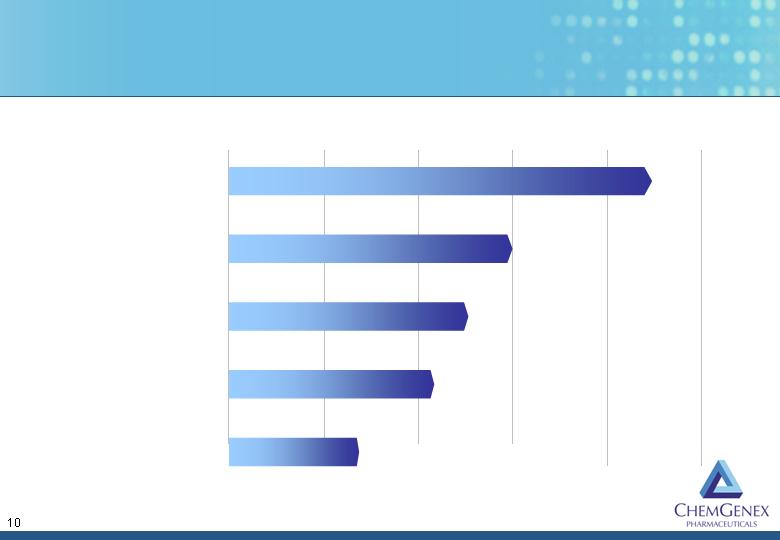
Ceflatonin Leads the Competition in T315I+ CML
Discovery Preclinical Phase 1 Phase 2 Registration
Directed
ChemGenex – Ceflatonin
Merck/Vertex – VX-680
Astex Therapeutics – AT9283
Exelixis – XL228
Novartis/SGX
>
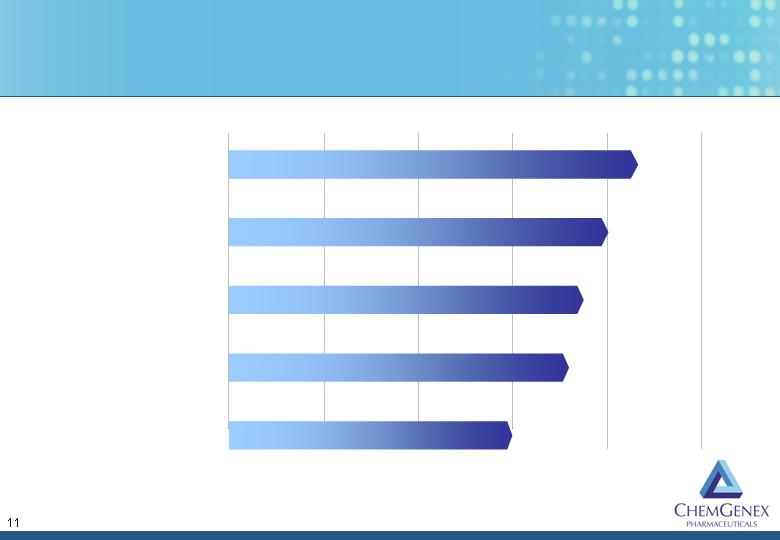
Ceflatonin Has Potential Applications Beyond T315I+ CML
Discovery Preclinical Phase 1 Phase 2 Registration
Directed
Chronic Myeloid Leukemia
T315I mutation
Chronic Myeloid Leukemia
TKI resistant
Chronic Myeloid Leukemia
HHT + TKI for minimal residual
disease
Myelodysplastic Syndrome
Acute Myeloid Leukemia
>

Quinamed is Company’s Second Clinical Stage Program
Amonafide dihydrochloride
Small molecule
Substituted isoquinoline
Mechanism of Action
Topoisomerase II inhibitor
Affects ADP-ribosylation and EGFR pathway
Established anti-tumor activity
Robust phase II data shows efficacy in major markets, Prostate,
Breast& Ovarian Cancer
Single agent or combination therapy
>

Development Strategy to Bring Quinamed to Market
Development of amonafide salts
Improved infusion regimes (weekly versus daily)
Dosage determined by patient’s NAT2 genotype
Genotype
Amonafide
concentration in blood
Dose
Slow
Low
High
Fast
High
Low
Amonafide
N-acetyltransferase-2
(NAT2)
N-acetyl amonafide
>

Quinamed Clinical Trial Progress
Phase 1 trial completed in 2004
MTD determined
Manageable side effects
Efficacy in prostate, ovarian and breast cancer
Phase 2a trial to be completed in 2006
50 patients treated
Results expected in Q1 2007
>
A Robust Pipeline Spanning Significant Chronic Diseases
Discovery Preclinical Phase 1 Phase 2 Phase 3
Ceflatonin®
Chronic Myeloid Leukemia
Myelodysplastic Syndrome
Acute Myeloid Leukemia
Quinamed®
Prostate Cancer
Breast Cancer
Ovarian Cancer
CXS299
Solid Tumors
CXS1192
CXS1821
CXS1662
CXS1473
CANCER
METABOLIC
>

Report Card for 2006
Dr Ian Nisbet appointed as Vice President of Oncology
November, 2006
Fast Track status granted by FDA for Ceflatonin® in CML
patients with the T315I mutation
November, 2006
Progressed diabetes discovery program to compound
screening
June, 2006
Launched Phase 2/3 study of Ceflatonin® in CML patients
with T315I mutation
June, 2006
Completed A$15M financing
May, 2006
Ceflatonin® granted USA Orphan Drug status for treatment
of CML
March, 2006
Expanded phase 2 study of Quinamed® to Prostate, Breast
and Ovarian Cancers
September, 2005
>

Upcoming Milestones and Investment Highlights
Scheduled completion of enrollment for
Ceflatonin T315I+ study
H2, 2007
Initiation of phase 2b trial for Quinamed
H2, 2007
Preliminary data from Ceflatonin T315I+ study
H1, 2007
Pre-clinical opportunities for partnering in
diabetes and metabolic diseases
H1, 2007
Phase 2a data for Quinamed expected
Q1, 2007
Preliminary data from ongoing studies at ASH
December, 2006
>

Safe Harbor Statement
Certain statements made herein that use the words “estimate,” ‘project,” “intend,” “expect,” “believe,” and similar
expressions are intended to identify forward-looking statements within the meaning of the US Private Securities
Litigation Reform Act of 1995. These forward-looking statements involve known and unknown risks and
uncertainties which could cause the actual results, performance or achievements of the company to be materially
different from those which may be expressed or implied by such statements, including, among others, risks or
uncertainties associated with the development of the company’s technology, the ability to successfully market
products in the clinical pipeline, the ability to advance promising therapeutics through clinical trials, the ability to
establish our fully integrated technologies, the ability to enter into additional collaborations and strategic alliances
and expand current collaborations and obtain milestone payments, the suitability of internally discovered genes for
drug development , the ability of the company to meet its financial requirements, the ability of the company to
protect its proprietary technology, potential limitations on the company’s technology, the market for the company’s
products, government regulation in Australia and the United States, changes in tax and other laws, changes in
competition and the loss of key personnel. These statements are based on our management’s current
expectations and are subject to a number of uncertainties that could change the results described in the forward
looking statements. Investors should be aware that there are no assurances that results will not differ from th
ose projected.

















