
Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above
Link to searchable text of slide shown above

Link to searchable text of slide shown above
Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above
Link to searchable text of slide shown above
Link to searchable text of slide shown above

Link to searchable text of slide shown above
Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above
Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above
Link to searchable text of slide shown above

Link to searchable text of slide shown above
Link to searchable text of slide shown above
Link to searchable text of slide shown above
Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above
Searchable text section of graphics shown above
[GRAPHIC]
[LOGO]
CEO’s Report
November 16, 2005
A Year of Outstanding Success – All Milestones Met
Initiate Phase 2b study on Quinamed in prostate | | Initiated November 2004 |
and ovarian cancers | | Expanded September 2005 |
| | |
Initiate Phase 2 study with Ceflatonin + Gleevec treating CML | | Initiated April 2005 |
patients in accelerated phase | | |
| | |
Initiate Phase 2b study with Ceflatonin treating CML patients | | Initiated October 2005 |
who have developed resistance to Gleevec | | |
| | |
Build pipeline with in-license or product acquisition | | Licensed CXS299 February 2005 |
| | |
Strengthen Board of Directors and Scientific Advisory Board | | Mr Burns and Mr Bradfield appointed July 2005 |
| | |
Complete ADR listing on the NASDAQ in the US | | Listed on NASDAQ June 2005 |
[LOGO]
Success Has Been Recognised Internationally by Frost & Sullivan
2005
[LOGO]
| Growth Strategy Leadership Award |
| Australia & New Zealand Biotech Industry |
“This award is presented in recognition of the company’s overall growth in terms of financial position, technology innovation and formation of strategic alliances and partnerships.”
[LOGO]
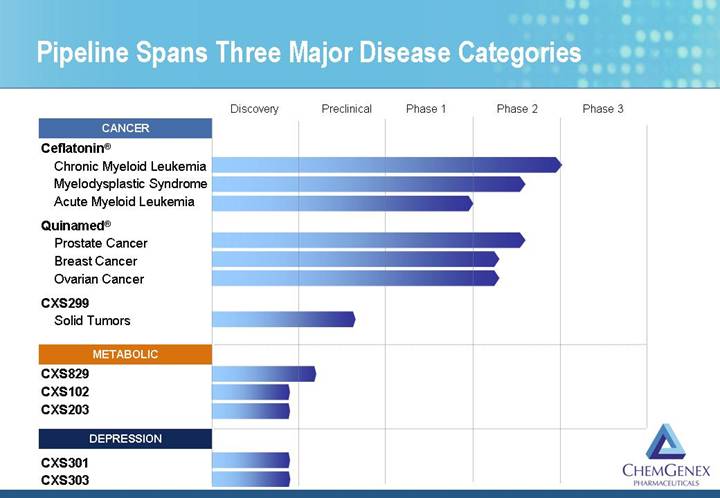
Pipeline Spans Three Major Disease Categories
[CHART]
ChemGenex’s Technology Base and Corporate Platform Identifies New Therapeutic Targets and Supports Clinical Programs
| Disease-
specific animal
models | |
Human DNA
collections | | Genomics-
dedicated
supercomputer |
| Personalized
Medicine | |
Biomarker
Discovery | | Experienced
clinical
development
team |
| Novel targets
for complex
diseases | |
A Platform to Develop, Partner and Commercialize
[GRAPHIC]
Corporate Leadership Provides Broad Base of Expertise
• | CEO and Managing Director: | | Greg Collier |
| | | |
• | President: | | Dennis Brown |
| | | |
• | VP Business Development: | | Harry Pedersen |
| | | |
• | VP Operations: | | James Campbell |
| | | |
• | Finance & Administration: | | Rick Merrigan |
| | | Tina Herbert |
| | | |
• | Senior Scientific Directors: | | Paul Zimmet |
| | John Blangero |
| | Ken Walder |
| | Shawnya Michaels |
Two Cancer Compounds in Clinical Trials
• Ceflatonin® (HHT)
• Chronic myeloid leukemia (CML)
• Myelodysplastic syndrome (MDS)
• Acute myeloid leukemia (AML)
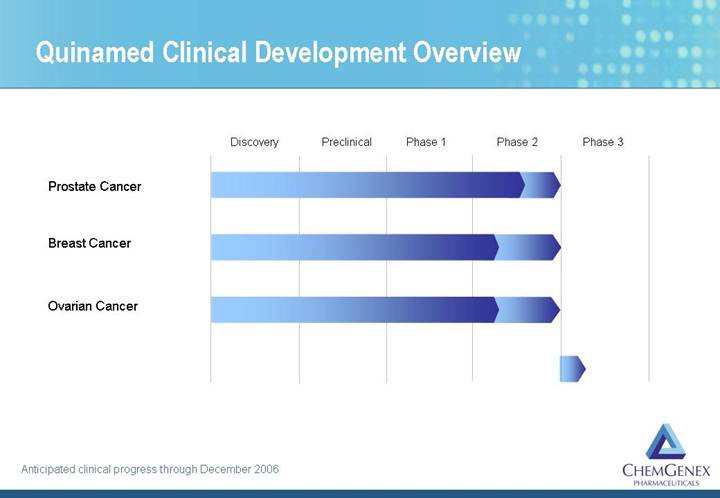
• Quinamed® (amonafide dihydrochloride)
• Prostate cancer
• Breast cancer
• Ovarian cancer
Gleevec® Dominates the Chronic Myeloid Leukemia Market
• BCR-ABL kinase inhibitor
• CML sales ~US$630 million in 2004
• Off label sales of ~US$1,300 million in 2004
• Gleevec® fails to eliminate residual disease
• Residual cells are part of the leukemic stem cell compartment
• A substantial fraction of patients develop acquired resistance to Gleevec®
Dynamics of chronic myeloid leukaemia. Michor, F et al., Nature 435, 1267-1270, June 2005
“The idea of magic bullets is great, but in practice it’s probably not going to be the right approach for complex diseases”
“Forget drugs carefully designed to hit one particular molecule – a better way of treating complex diseases such as cancer may be to aim for several targets at once”
Playing Dirty. Franz, S., Nature 437, 942-943, October 13 2005
UNMET MARKET | | SAFETY & | | INTELLECTUAL | | MANUFACTURING | | EFFICACY | | OPINION | | REGULATORY |
| | TOXICOLOGY | | PROPERTY | | | | STUDIES | | LEADERS | | APPROVAL |
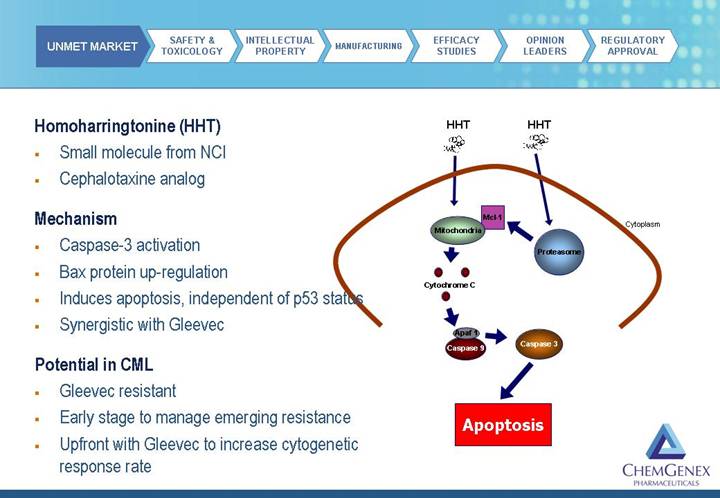
Homoharringtonine (HHT)
• Small molecule from NCI
• Cephalotaxine analog
Mechanism
• Caspase-3 activation
• Bax protein up-regulation
• Induces apoptosis, independent of p53 status
• Synergistic with Gleevec
Potential in CML
• Gleevec resistant
• Early stage to manage emerging resistance
• Upfront with Gleevec to increase cytogenetic response rate
[GRAPHIC]
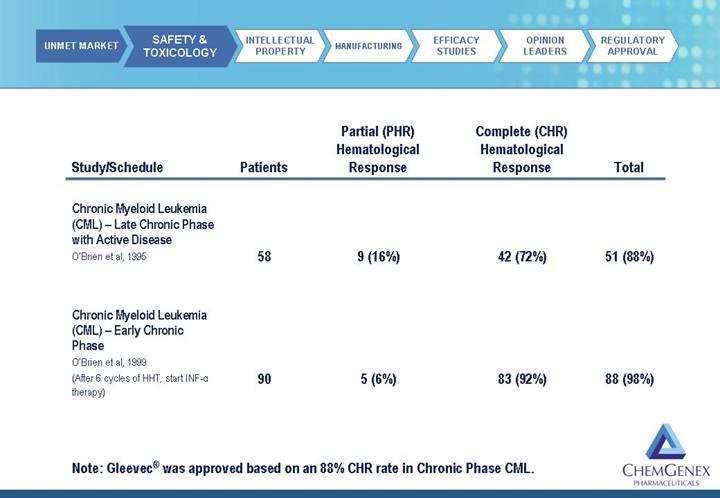
Study/Schedule | | Patients | | Partial (PHR)
Hematological
Response | | Complete (CHR)
Hematological
Response | | Total | |
| | | | | | | | | |
Chronic Myeloid Leukemia
(CML) – Late Chronic Phase
with Active Disease | | | | | | | | | |
O’Brien et al, 1995 | | 58 | | 9 (16%) | | 42 (72%) | | 51 (88%) | |
| | | | | | | | | |
Chronic Myeloid Leukemia
(CML) – Early Chronic
Phase | | | | | | | | | |
O’Brien et al, 1999
(After 6 cycles of HHT, start INF- therapy) therapy) | | 90 | | 5 (6%) | | 83 (92%) | | 88 (98%) | |
Note: Gleevec® was approved based on an 88% CHR rate in Chronic Phase CML.
• Stragen Pharma Partnership Will Accelerate Clinical and Commercial Development
• Stragen: based in Geneva, Switzerland
• provides production and global supply of Ceflatonin®
• Manages regulatory approvals within Europe
• ChemGenex is responsible for global clinical development
• Registration and marketing in North America and Asia-Pacific
• Combined assets produce broad patent portfolio and longevity
• JV to market ChemGenex-branded Ceflatonin® in Europe
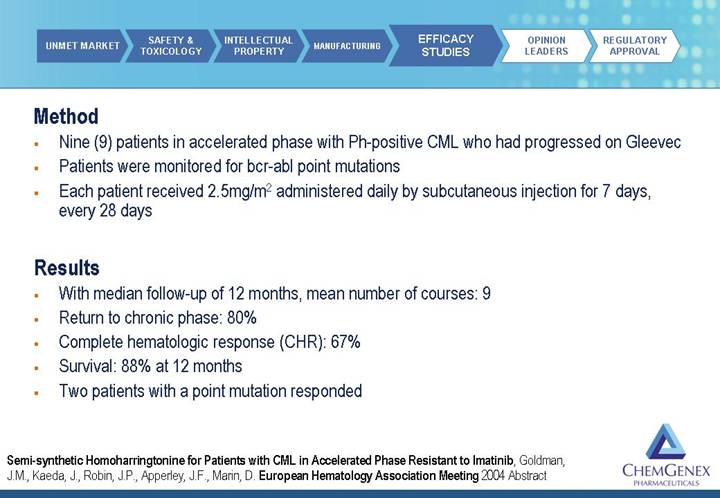
Method
• Nine (9) patients in accelerated phase with Ph-positive CML who had progressed on Gleevec
• Patients were monitored for bcr-abl point mutations
• Each patient received 2.5mg/m2 administered daily by subcutaneous injection for 7 days, every 28 days
Results
• With median follow-up of 12 months, mean number of courses: 9
• Return to chronic phase: 80%
• Complete hematologic response (CHR): 67%
• Survival: 88% at 12 months
• Two patients with a point mutation responded
Semi-synthetic Homoharringtonine for Patients with CML in Accelerated Phase Resistant to Imatinib, Goldman, J.M., Kaeda, J., Robin, J.P., Apperley, J.F., Marin, D. European Hematology Association Meeting 2004 Abstract
Method
• Ten (10) patients with CML who had achieved >35% Ph-negativity on imatinib were included
• All patients had been treated at > 400mg/day at least 2 years and had reached a BCR-ABL transcript plateau
• Patients were monitored for bcr-abl point mutations
• Initial dosing was 2.5 mg/m2 administered daily by subcutaneous injection for 1 day, every 28 days
• Dose was escalated by adding 1 day of treatment every 2 days, upto 7 days every 28 days
Results
• Decline in BCR-ABL transcript levels: 70%
• Decline in BCR-ABL transcripts of greater than 1 log: 50%
• Two (2) patients with p-loop mutations responded
Phase 1/2 Trial of Adding Semi-synthetic Homoharringtonine in CML Patients Who Have Achieved Partial or Complete Cytogenetic Response on Imatinib. Marin, D., Kaeda, J.S., Andreasson, C., Saunders, S.M., Bua, M., Olavarria, E., Goldman, J.M., Apperley, J.F., Cancer . 2005;103-9;1850-1855.
• Positive phase 2 data as a single agent in CML
• 88% response rate - late chronic phase
• 98% response rate - early chronic phase
• Positive phase 2 data as single agent in accelerated phase CML resistant to Gleevec®
• 80% return to chronic from accelerated phase
• 67% complete hematologic response rate – accelerated phase
• Positive phase 2 data in-combination with Gleevec®
• 70% decline in BCR-ABL transcripts
• 50% at least a 1 log decrease in BCR-ABL transcripts
• Patients with p-loop mutations responded
Clinical Advisor | | Affiliation |
| | |
Hagop Kantarjian, M.D. | | M.D. Anderson Cancer Center/Houston |
Chairman, Leukemia Department | | |
| | |
Prof. Andreas Hochhaus, M.D. | | Heidelberg University |
Professor of Internal Medicine | | |
| | |
John Goldman, M.D. | | Imperial College London/Hammersmith Hospital |
Professor of Hematology | | |
| | |
David Marin, M.D. | | Imperial College London/Hammersmith Hospital |
Professor of Hematology | | |
| | |
Jean-Pierre Marie, M.D. | | University of Paris |
Professor of Hematology/Oncology | | |
• CML Resistant to High-Dose Gleevec®
• Patients resistant to abl-kinase inhibitor therapy
• Chronic, accelerated and blast phases
• Simon two-stage design
• 40-85 patients
• CML Patients with the T315I bcr-abl point mutations
• Chronic, accelerated and blast phases
• Simon two-stage design
• Up to an additional 86 patients in second phase
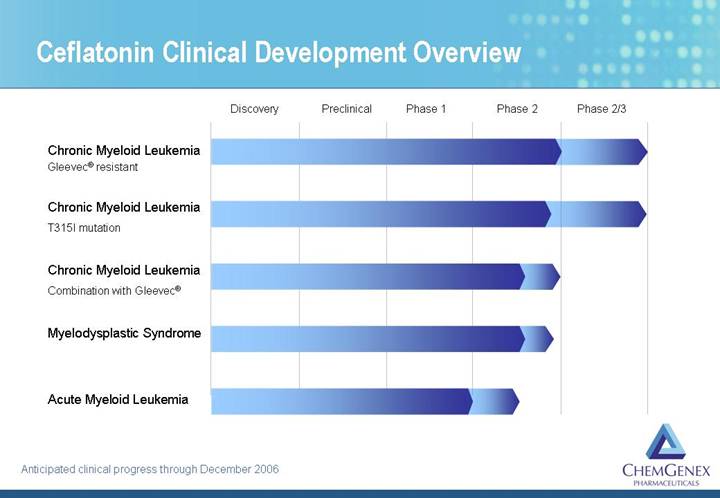
Ceflatonin Clinical Development Overview
[CHART]
Anticipated clinical progress through December 2006
Two Cancer Compounds in Clinical Trials
• Ceflatonin® (HHT)
• Chronic myeloid leukemia (CML)
• Myelodysplastic syndrome (MDS)
• Acute myeloid leukemia (AML)
• Quinamed® (amonafide dihydrochloride)
• Prostate cancer
• Breast cancer
• Ovarian cancer
Quinamed® is Company’s Second Clinical Stage Program
Amonafide dihydrochloride
• Small molecule
• Substituted isoquinoline
Mechanism of Action
• Topoisomerase II inhibitor
• Affects ADP-ribosylation and EGFR pathway
Phase 2 Active
• 25% response rate in breast cancer
• 12% response rate in prostate cancer
Clinical Strategy to Bring Quinamed® to Market
• Development of amonafide salts
• Improved infusion regimes (weekly versus daily)
• Dosage determined with understanding of patient’s NAT2 genotype
• Metabolite limits clearance of amonafide, increases side effects
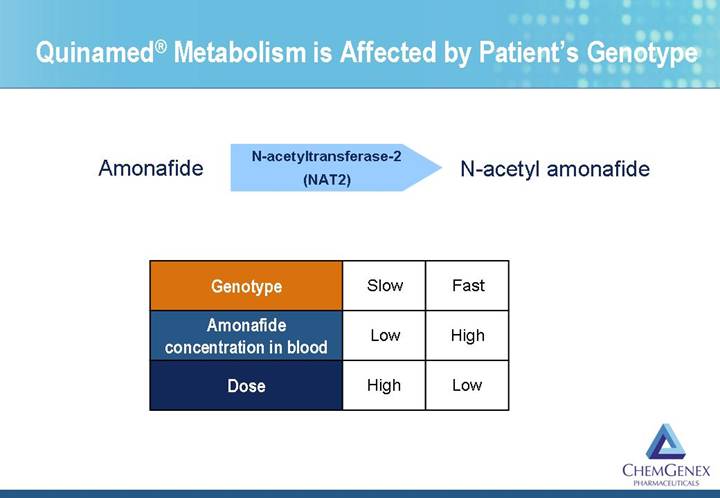
Quinamed® Metabolism is Affected by Patient’s Genotype
Amonafide | N-acetyltransferase-2
(NAT2) | N-acetyl amonafide |
Genotype | | Slow | | Fast | |
| | | | | |
Amonafide
concentration in blood | | Low | | High | |
| | | | | |
Dose | | High | | Low | |
Quinamed® Phase 1 Trial Synopsis - Completed in 2004
• Established maximum tolerated dose (MTD) by patient genotype
• 32 patients enrolled
• Anti-cancer activity observed
• Manageable side effects
• Data presented at ASCO in June 2004
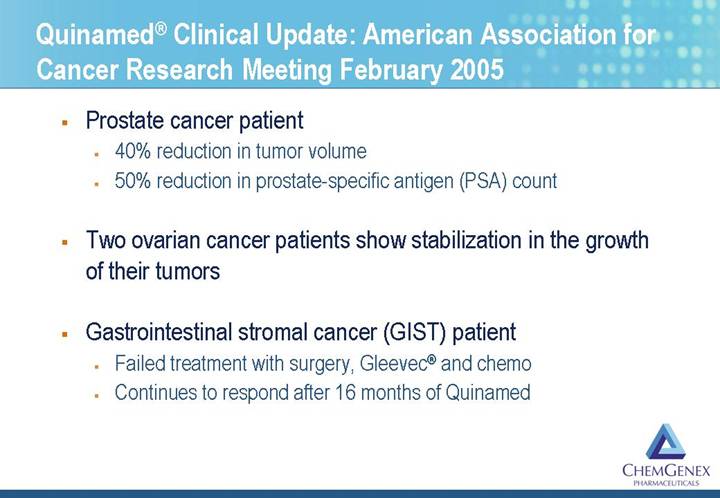
Quinamed® Clinical Update: American Association for Cancer Research Meeting February 2005
• Prostate cancer patient
• 40% reduction in tumor volume
• 50% reduction in prostate-specific antigen (PSA) count
• Two ovarian cancer patients show stabilization in the growth of their tumors
• Gastrointestinal stromal cancer (GIST) patient
• Failed treatment with surgery, Gleevec® and chemo
• Continues to respond after 16 months of Quinamed
Quinamed Clinical Development Overview
[CHART]
Anticipated clinical progress through December 2006
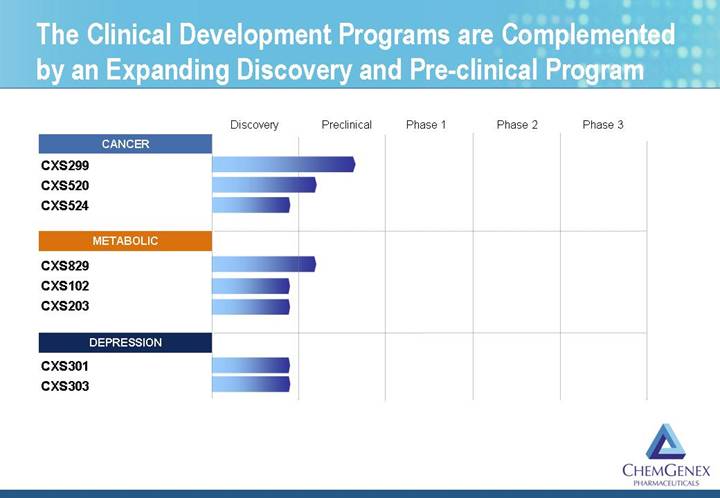
The Clinical Development Programs are Complemented by an Expanding Discovery and Pre-clinical Program
[CHART]
CXS299 is a Novel Targeted Therapy for Resistant Solid Tumors
• There are several approved platinum II agents at market
• Cisplatin (BMS)
• Carboplatin (BMS)
• Oxaliplatin (Sanofi-Aventis)
• CXS299 is a novel platinum (IV) anti-cancer compound licensed from the M. D. Anderson Cancer Center
• In vivo data indicate that CXS299 overcomes resistance to existing platinum (II) therapeutics
• If verified in the clinic, the improved activity of CXS299 will offer new hope to cancer patients who are refractory to current therapies
SEPS1 is a Significant New Discovery in Inflammation and Chronic Complex Diseases
• A ChemGenex-discovered protein that is a critical regulator of inflammation
• Published in Nature Genetics, October 9, 2005
• Inflammation underpins many major complex diseases including cancer, cardiovascular disease, diabetes, obesity and Alzheimer’s disease
• Gene variation shows strongest reported association with inflammation markers
• Significant opportunities for diagnostic and therapeutic development
CXS829 is a New Obesity Therapeutic Candidate
• A newly-discovered ion channel involved in the transmission of satiation from the stomach to the brain
• Located on chromosome 17 in a region linked with obesity
• Gene variation shows strong evidence of association with obesity
• Non-specific inhibitor causes significant reduction in food intake
Proprietary Targets Have Potential Application Across a Range of Diseases
• CXS203
• Novel diabetes target
• Mitochondrial protein involved in structural integrity
• Human studies show gene variant strongly associated with predisposition to diabetes
ChemGenex is a Balanced Investment Opportunity With Significant Clinical Progress Expected In 2006
[CHART]
Upcoming Milestones
Clinical
• Clinical data presentation at ASH
• Initiate Phase 2/3 study in CML Patients with the T315I bcr-abl point mutation
• Guidance from FDA SPA
• Expansion of Phase 2/3 study in CML Patients CML Resistant to High-Dose Gleevec
• Phase 2 data for Ceflatonin in MDS and AML
• Phase 2 data for Quinamed in prostate, ovarian and breast cancers
Corporate
• Evaluation of potential in-licensing opportunities
• New partnership opportunities
Contacts
USA
3475 Edison Way, Suite M
Menlo Park, CA 94025
Tel: +1 650 474 9800
Fax: +1 650 474 9808
Australia
1 Pigdons Road
Geelong, VIC 3217
Tel: +61 3 5227 2752
Fax: +61 3 5227 1322
chemgenex@chemgenex.com
www.chemgenex.com
Safe Harbor Statement
Certain statements made herein that use the words “estimate,” ‘project,” “intend,” “expect,” “believe,” and similar expressions are intended to identify forward-looking statements within the meaning of the US Private Securities Litigation Reform Act of 1995. These forward-looking statements involve known and unknown risks and uncertainties which could cause the actual results, performance or achievements of the company to be materially different from those which may be expressed or implied by such statements, including, among others, risks or uncertainties associated with the development of the company’s technology, the ability to successfully market products in the clinical pipeline, the ability to advance promising therapeutics through clinical trials, the ability to establish our fully integrated technologies, the ability to enter into additional collaborations and strategic alliances and expand current collaborations and obtain milestone payments, the suitability of internally discovered genes for drug development, the ability of the company to meet its financial requirements, the ability of the company to protect its proprietary technology, potential limitations on the company’s technology, the market for the company’s products, government regulation in Australia and the United States, changes in tax and other laws, changes in competition and the loss of key personnel. These statements are based on our management’s current expectations and are subject to a number of uncertainties that could change the results described in the forward looking statements. Investors should be aware that there are no assurances that results will not differ from those projected.



































