Company has Already Met Significant Development Milestones with
Potential for FDA Submission by 2007
Gleevec approved
for CML in 2001
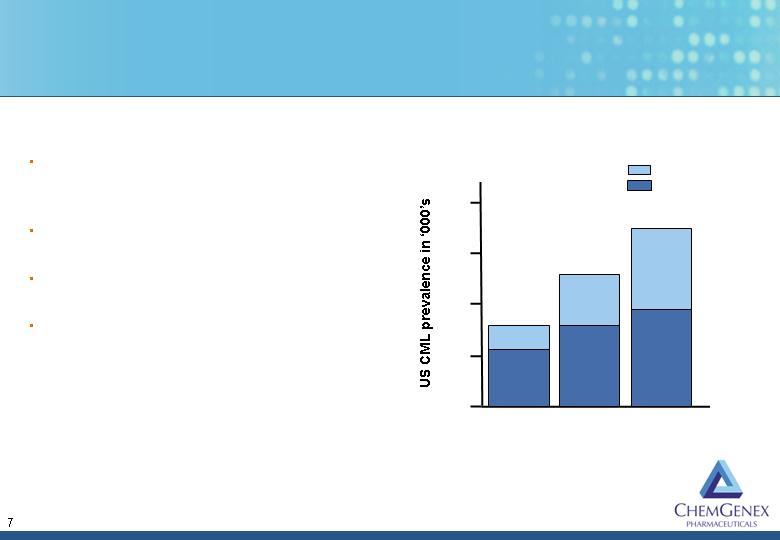
By 2004 estimates
that up to 30% of
CML patients are
resistant to
Gleevec
2004 sales of
Gleevec
US$630M for CML
US$1,400M off-
label
Research
indicates that
majority of CML
patients will
eventually develop
resistance to
Gleevec
UNMET MARKET
SAFETY &
TOXICOLOGY
INTELLECTUAL
PROPERTY
MANUFACTURING
REGULATORY
APPROVAL
OPINION
LEADERS
EFFICACY
STUDIES
Previous NCI
Phase 2 trials
have tested HHT
in ~450 patients
with manageable
safety profile
Subsequent trials
have identified no
issues
CXS has ‘use’
protection on 4
HHT patent
families from
2000-2002
Exclusive licence
of 6 HHT families
(use, purification,
analogs) from
Stragen Pharma
Freedom to
operate.
No competing IP
positions
CXS exclusively
contracted Stragen
Pharma to
manufacture
ssHHT
Stragen Pharma
can produce
ssHHT in FDA,
EMEA-approved
facilities
Ongoing dialog
with FDA on
development plans
Possible fast track
application
Orphan Drug
status granted in
Europe
Orphan Drug
status granted in
US
Ongoing CXS
clinical trials
involve world
opinion leaders
including;
Dr Kantarjian
Dr Hochhaus
Dr Goldman
Dr Cortes
Dr Guilhot
Dr Michallet
Dr Marin
Dr Marie
Dr Kantarjian is a
member of the
CXS Scientific
Advisory Board
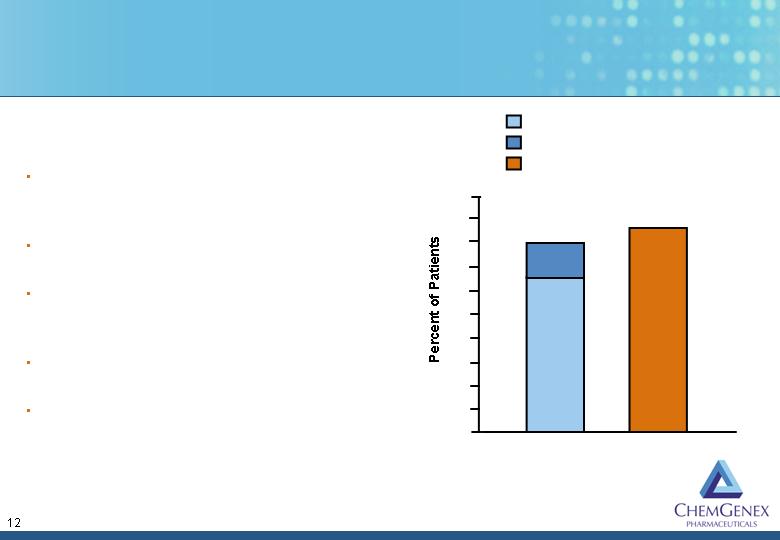
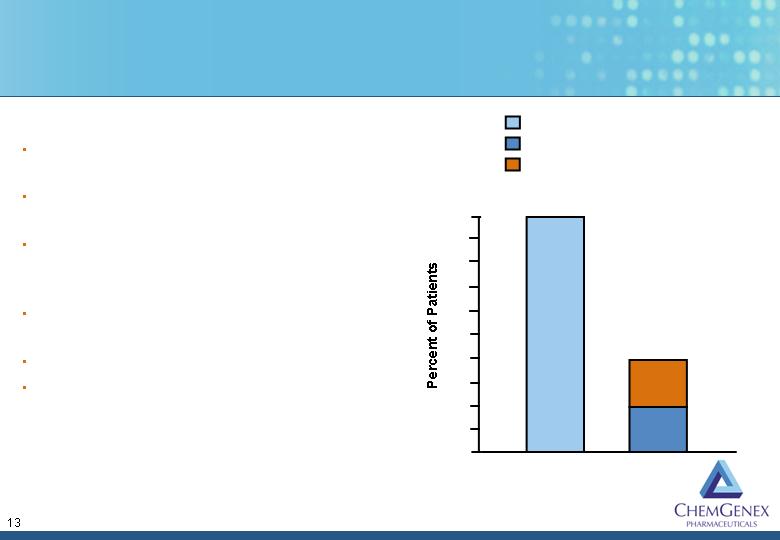
Previous CML Phase
2 studies showed:
58 patients - 88% HR
90 patients - 98% HR
CXS-Stragen CML
Phase 2 studies:
9 Gleevec-resistant
patients - 80%
reverted to chronic
stage
10 Gleevec-resistant
patients - 70%
reduction in BRC-
ABL expression
M.D. Anderson
5 Gleevec-resistant
patients – 100%
complete response
Five ongoing trials
1. Phase 2/3 in
Gleevec-resistant
CML patients
2. Phase 2/3 in CML
patients with T315I
mutation(*)
3. Phase 2 in CML
combination therapy
with Gleevec®
4. Phase 2 in AML
5. Phase 2 in MDS