
Phase 2 Study of Zelicapavir in Children: RSVPEDs Topline Results December 9, 2024 Exhibit 99.2

This presentation contains forward-looking statements concerning our business, operations and financial performance and condition, as well as our plans, objectives and expectations for our research and development programs, our business and the industry in which we operate. Any statements contained herein that are not statements of historical facts may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “aim,” “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,” “due,” “estimate,” “expect,” “goal,” “intend,” “may,” “objective,” “plan,” “predict,” “potential,” “positioned,” “seek,” “should,” “target,” “will,” “would,” and other similar expressions that are predictions of or indicate future events and future trends, as well as other comparable terminology. These forward-looking statements include, but are not limited to, statements about the potential of zelicapavir, the prospects for further development and advancement of zelicapavir for the treatment of RSV, research and clinical development plans and prospects, liquidity and capital needs and other statements of expectations, beliefs, future plans and strategies, anticipated events or trends, and similar expressions. These forward-looking statements are based on our management’s current expectations, estimates, forecasts and projections about our business and the industry in which we operate and our management’s beliefs and assumptions. These forward-looking statements are not guarantees of future performance or results and involve known and unknown risks, uncertainties and other factors that are in some cases beyond our control. As a result, any or all of our forward-looking statements in this presentation may turn out to be inaccurate. Please refer to the risk factors described or referred to in “Risk Factors” in Enanta’s most recent Annual Report on Form 10-K, and other periodic reports filed with the Securities and Exchange Commission. Enanta cautions investors not to place undue reliance on the forward-looking statements contained in this presentation. These statements speak only as of the date of this presentation, and Enanta undertakes no obligation to update or revise these statements, except as may be required by law. Forward Looking Statements Disclaimer
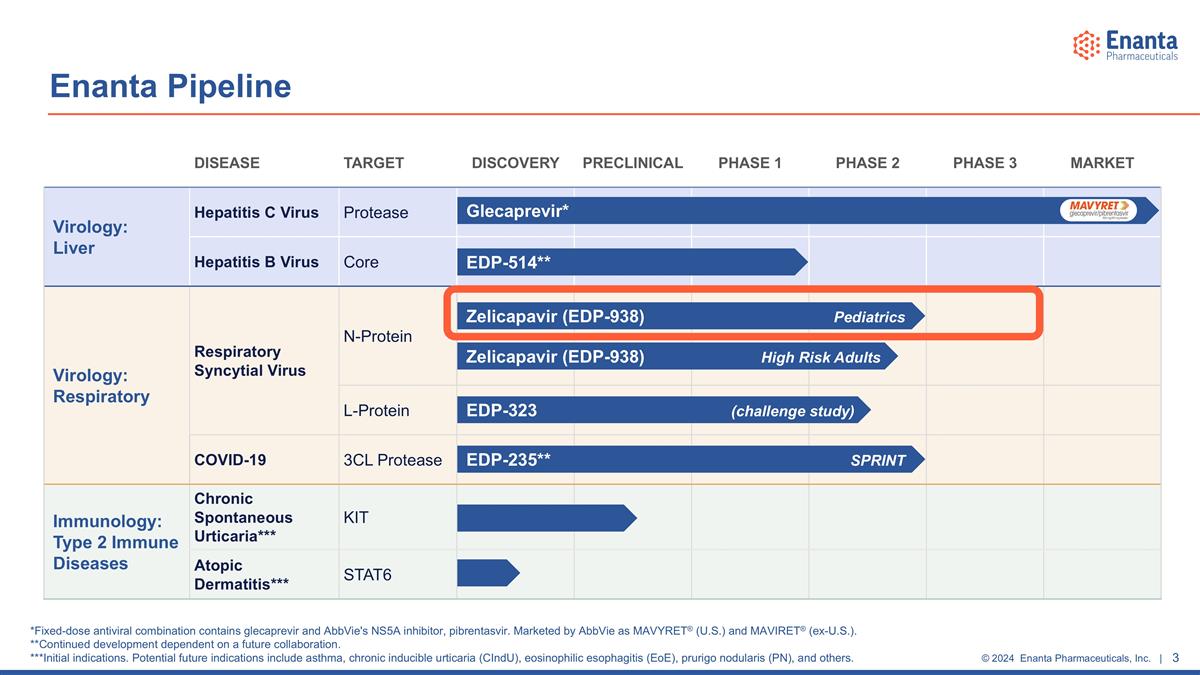
DISEASE TARGET DISCOVERY PRECLINICAL PHASE 1 PHASE 2 PHASE 3 MARKET Virology: Liver Hepatitis C Virus Protease Hepatitis B Virus Core Virology: Respiratory Respiratory Syncytial Virus N-Protein L-Protein COVID-19 3CL Protease Immunology: Type 2 Immune Diseases Chronic Spontaneous Urticaria*** KIT Atopic Dermatitis*** STAT6 Zelicapavir (EDP-938) High Risk Adults EDP-323 (challenge study) Zelicapavir (EDP-938) Pediatrics Glecaprevir* EDP-235** SPRINT Enanta Pipeline EDP-514** *Fixed-dose antiviral combination contains glecaprevir and AbbVie's NS5A inhibitor, pibrentasvir. Marketed by AbbVie as MAVYRET® (U.S.) and MAVIRET® (ex-U.S.). **Continued development dependent on a future collaboration. ***Initial indications. Potential future indications include asthma, chronic inducible urticaria (CIndU), eosinophilic esophagitis (EoE), prurigo nodularis (PN), and others.
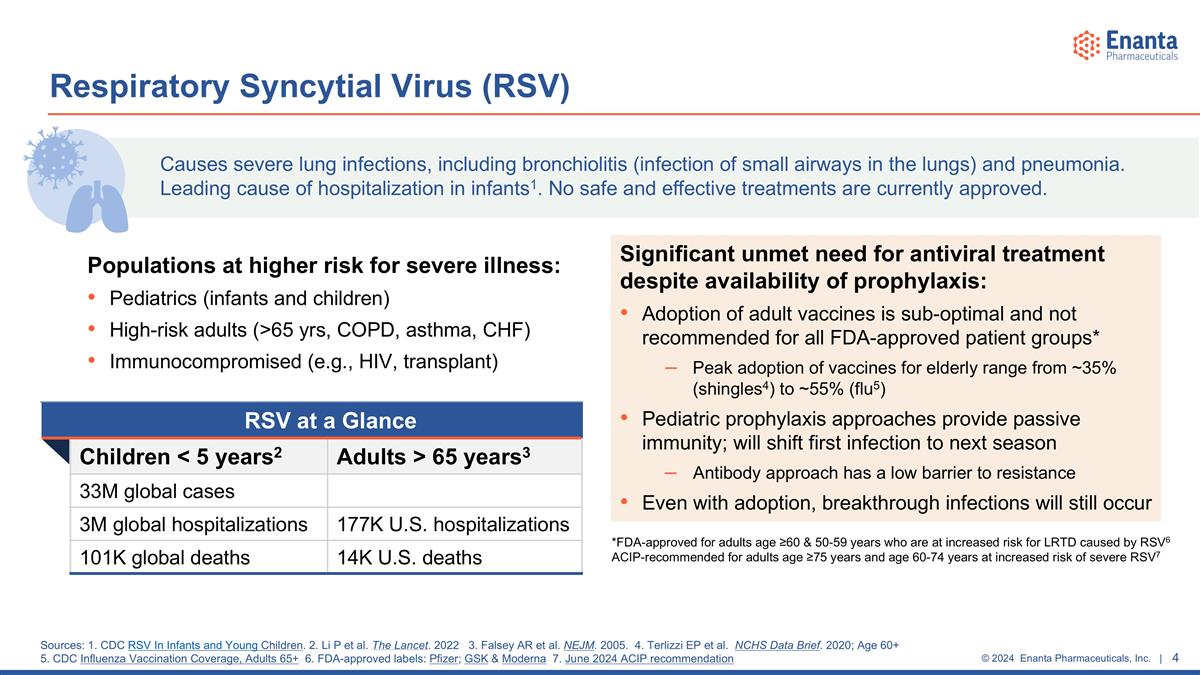
Populations at higher risk for severe illness: Pediatrics (infants and children) High-risk adults (>65 yrs, COPD, asthma, CHF) Immunocompromised (e.g., HIV, transplant) Respiratory Syncytial Virus (RSV) RSV at a Glance Children < 5 years2 Adults > 65 years3 33M global cases 3M global hospitalizations 177K U.S. hospitalizations 101K global deaths 14K U.S. deaths Causes severe lung infections, including bronchiolitis (infection of small airways in the lungs) and pneumonia. Leading cause of hospitalization in infants1. No safe and effective treatments are currently approved. Sources: 1. CDC RSV In Infants and Young Children. 2. Li P et al. The Lancet. 2022 3. Falsey AR et al. NEJM. 2005. 4. Terlizzi EP et al. NCHS Data Brief. 2020; Age 60+ 5. CDC Influenza Vaccination Coverage, Adults 65+ 6. FDA-approved labels: Pfizer; GSK & Moderna 7. June 2024 ACIP recommendation Significant unmet need for antiviral treatment despite availability of prophylaxis: Adoption of adult vaccines is sub-optimal and not recommended for all FDA-approved patient groups* Peak adoption of vaccines for elderly range from ~35% (shingles4) to ~55% (flu5) Pediatric prophylaxis approaches provide passive immunity; will shift first infection to next season Antibody approach has a low barrier to resistance Even with adoption, breakthrough infections will still occur *FDA-approved for adults age ≥60 & 50-59 years who are at increased risk for LRTD caused by RSV6 ACIP-recommended for adults age ≥75 years and age 60-74 years at increased risk of severe RSV7
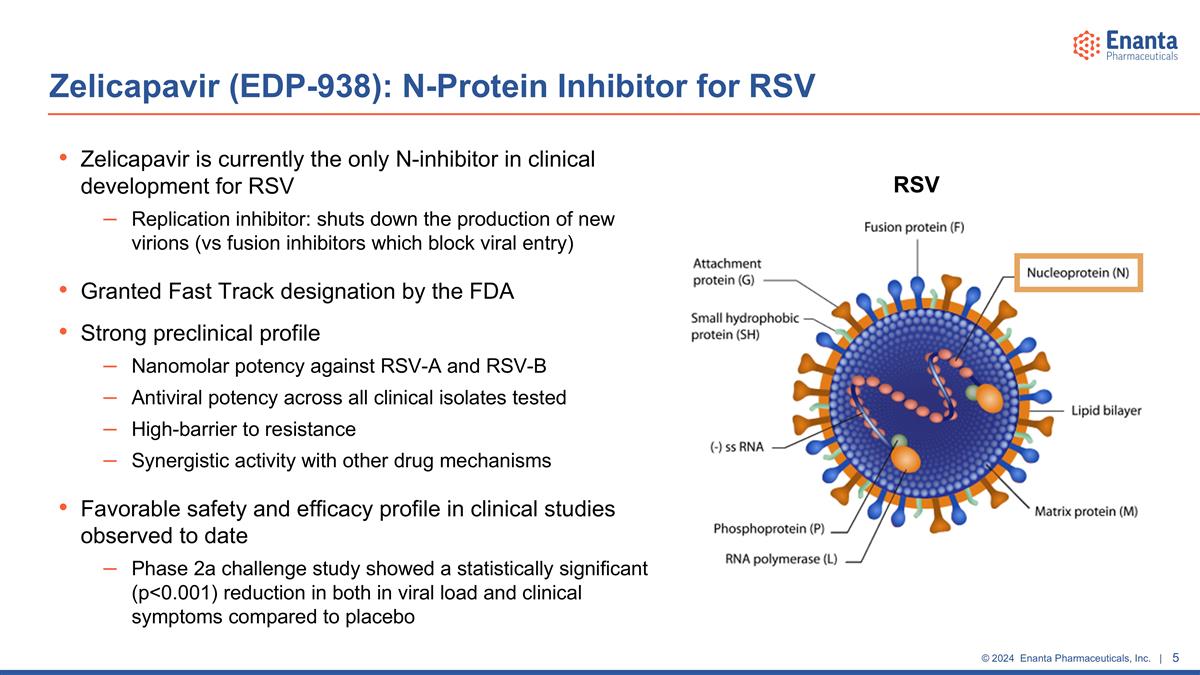
Zelicapavir is currently the only N-inhibitor in clinical development for RSV Replication inhibitor: shuts down the production of new virions (vs fusion inhibitors which block viral entry) Granted Fast Track designation by the FDA Strong preclinical profile Nanomolar potency against RSV-A and RSV-B Antiviral potency across all clinical isolates tested High-barrier to resistance Synergistic activity with other drug mechanisms Favorable safety and efficacy profile in clinical studies observed to date Phase 2a challenge study showed a statistically significant (p<0.001) reduction in both in viral load and clinical symptoms compared to placebo Zelicapavir (EDP-938): N-Protein Inhibitor for RSV RSV

Phase 2 Study of Zelicapavir in Children: RSVPEDs Topline Results A phase 2, randomized, double-blind, placebo-controlled, 2-part study to evaluate EDP‑938 regimens in Subjects aged 28 days to 36 months infected with respiratory syncytial virus
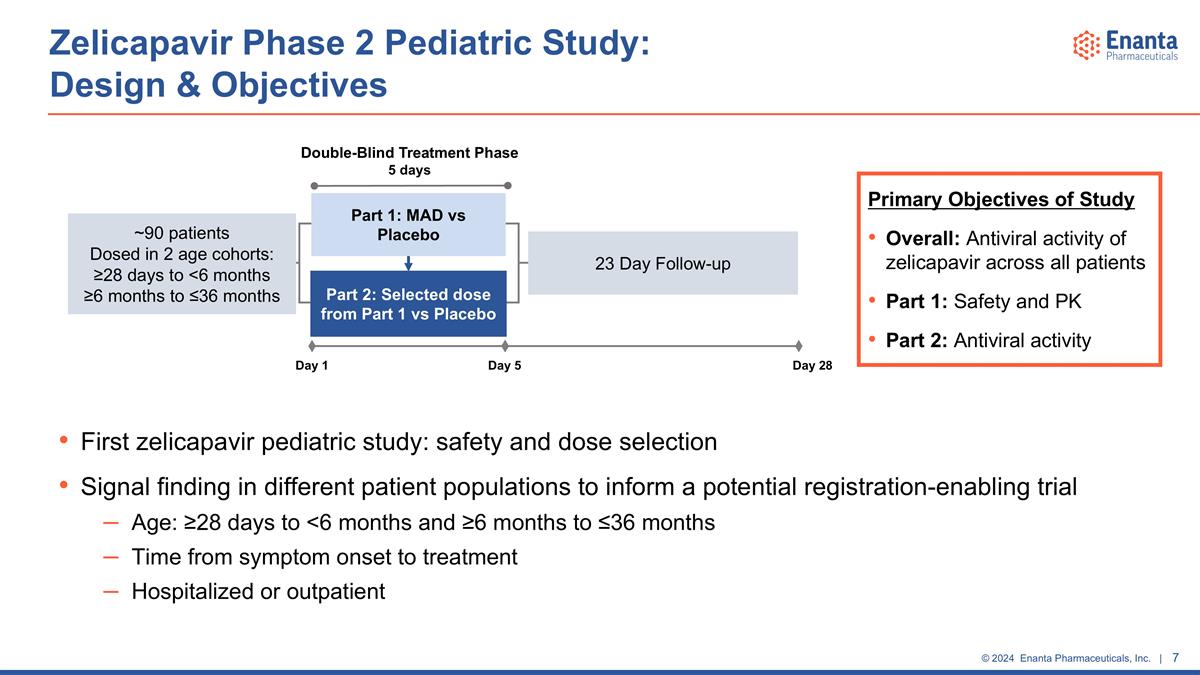
Zelicapavir Phase 2 Pediatric Study: Design & Objectives ~90 patients Dosed in 2 age cohorts: ≥28 days to <6 months ≥6 months to ≤36 months Double-Blind Treatment Phase 5 days Part 1: MAD vs Placebo Part 2: Selected dose from Part 1 vs Placebo Day 1 Day 5 Day 28 23 Day Follow-up Primary Objectives of Study Overall: Antiviral activity of zelicapavir across all patients Part 1: Safety and PK Part 2: Antiviral activity First zelicapavir pediatric study: safety and dose selection Signal finding in different patient populations to inform a potential registration-enabling trial Age: ≥28 days to <6 months and ≥6 months to ≤36 months Time from symptom onset to treatment Hospitalized or outpatient
Well tolerated, with favorable safety profile No adverse events leading to treatment discontinuation or study withdrawal Antiviral effect observed for the primary and secondary virology endpoints in overall population Viral load decline of 1.4 log at the end of treatment in Part 2 Viral load decline of 1.2 log at Day 5 observed in prespecified subset of patients randomized within 3 days of symptom onset Data support further clinical development of zelicapavir Zelicapavir Phase 2 Pediatric Study: Conclusions Primary Objectives of Study Overall: Antiviral activity of zelicapavir across all patients Part 1: Safety and PK Part 2: Antiviral activity
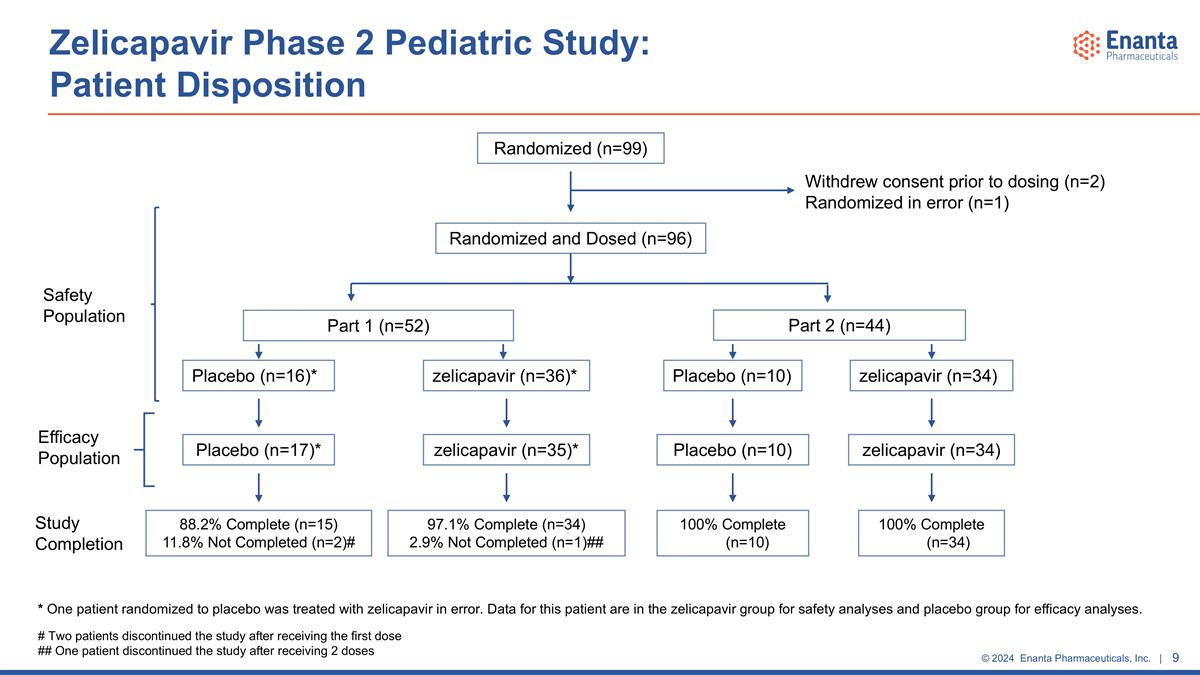
Zelicapavir Phase 2 Pediatric Study: Patient Disposition # Two patients discontinued the study after receiving the first dose ## One patient discontinued the study after receiving 2 doses * One patient randomized to placebo was treated with zelicapavir in error. Data for this patient are in the zelicapavir group for safety analyses and placebo group for efficacy analyses. Randomized (n=99) Randomized and Dosed (n=96) Withdrew consent prior to dosing (n=2) Randomized in error (n=1) Placebo (n=16)* zelicapavir (n=36)* Placebo (n=10) zelicapavir (n=34) 97.1% Complete (n=34) 2.9% Not Completed (n=1)## zelicapavir (n=35)* Placebo (n=10) zelicapavir (n=34) Part 1 (n=52) Part 2 (n=44) Placebo (n=17)* 100% Complete (n=34) 100% Complete (n=10) 88.2% Complete (n=15) 11.8% Not Completed (n=2)# Efficacy Population Safety Population Study Completion
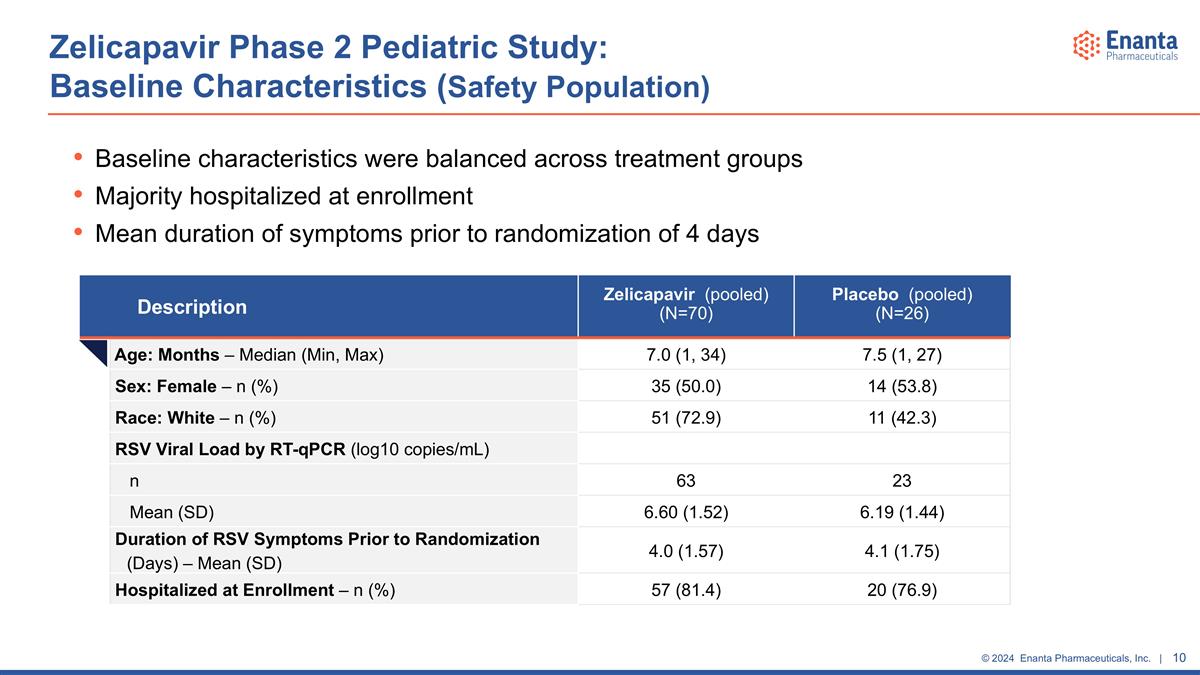
Zelicapavir Phase 2 Pediatric Study: Baseline Characteristics (Safety Population) Baseline characteristics were balanced across treatment groups Majority hospitalized at enrollment Mean duration of symptoms prior to randomization of 4 days Description Zelicapavir (pooled) (N=70) Placebo (pooled) (N=26) Age: Months – Median (Min, Max) 7.0 (1, 34) 7.5 (1, 27) Sex: Female – n (%) 35 (50.0) 14 (53.8) Race: White – n (%) 51 (72.9) 11 (42.3) RSV Viral Load by RT-qPCR (log10 copies/mL) n 63 23 Mean (SD) 6.60 (1.52) 6.19 (1.44) Duration of RSV Symptoms Prior to Randomization (Days) – Mean (SD) 4.0 (1.57) 4.1 (1.75) Hospitalized at Enrollment – n (%) 57 (81.4) 20 (76.9)
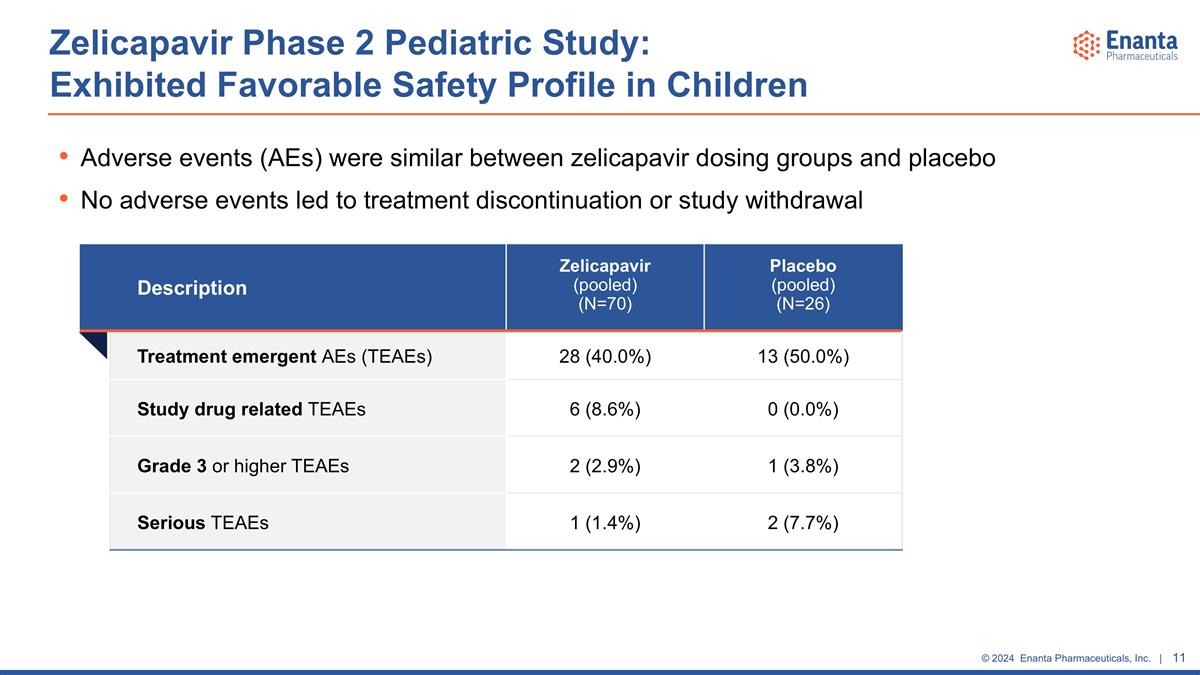
Adverse events (AEs) were similar between zelicapavir dosing groups and placebo No adverse events led to treatment discontinuation or study withdrawal Zelicapavir Phase 2 Pediatric Study: Exhibited Favorable Safety Profile in Children Description Zelicapavir (pooled) (N=70) Placebo (pooled) (N=26) Treatment emergent AEs (TEAEs) 28 (40.0%) 13 (50.0%) Study drug related TEAEs 6 (8.6%) 0 (0.0%) Grade 3 or higher TEAEs 2 (2.9%) 1 (3.8%) Serious TEAEs 1 (1.4%) 2 (7.7%)
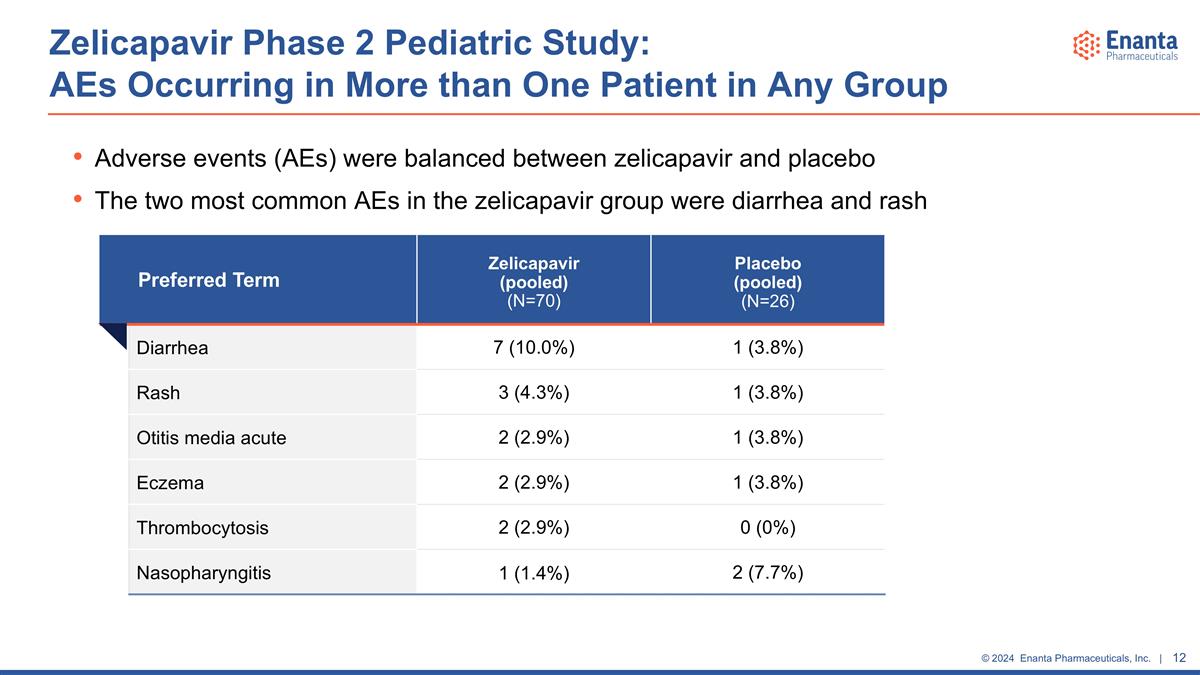
Zelicapavir Phase 2 Pediatric Study: AEs Occurring in More than One Patient in Any Group Preferred Term Zelicapavir (pooled) (N=70) Placebo (pooled) (N=26) Diarrhea 7 (10.0%) 1 (3.8%) Rash 3 (4.3%) 1 (3.8%) Otitis media acute 2 (2.9%) 1 (3.8%) Eczema 2 (2.9%) 1 (3.8%) Thrombocytosis 2 (2.9%) 0 (0%) Nasopharyngitis 1 (1.4%) 2 (7.7%) Adverse events (AEs) were balanced between zelicapavir and placebo The two most common AEs in the zelicapavir group were diarrhea and rash

Goal was to achieve similar drug exposures to the exposures proven to be efficacious in the Phase 2 adult challenge study Target drug exposures achieved across all age groups and dosing cohorts (Parts 1 and 2) A dose of 5 mg/kg was selected for age ≥ 28 days to <12 months A dose of 7.5 mg/kg was selected for age ≥12 months to ≤36 months Regardless of dose, all patients had model-predicted exposures above the efficacy threshold Exposure was similar across cohorts and all patients received a therapeutic dose Primary efficacy analyses were performed across all dosed patients from Parts 1 and 2 (n=96; efficacy population) Zelicapavir Phase 2 Pediatric Study: Achieved Target Exposure Levels in Children
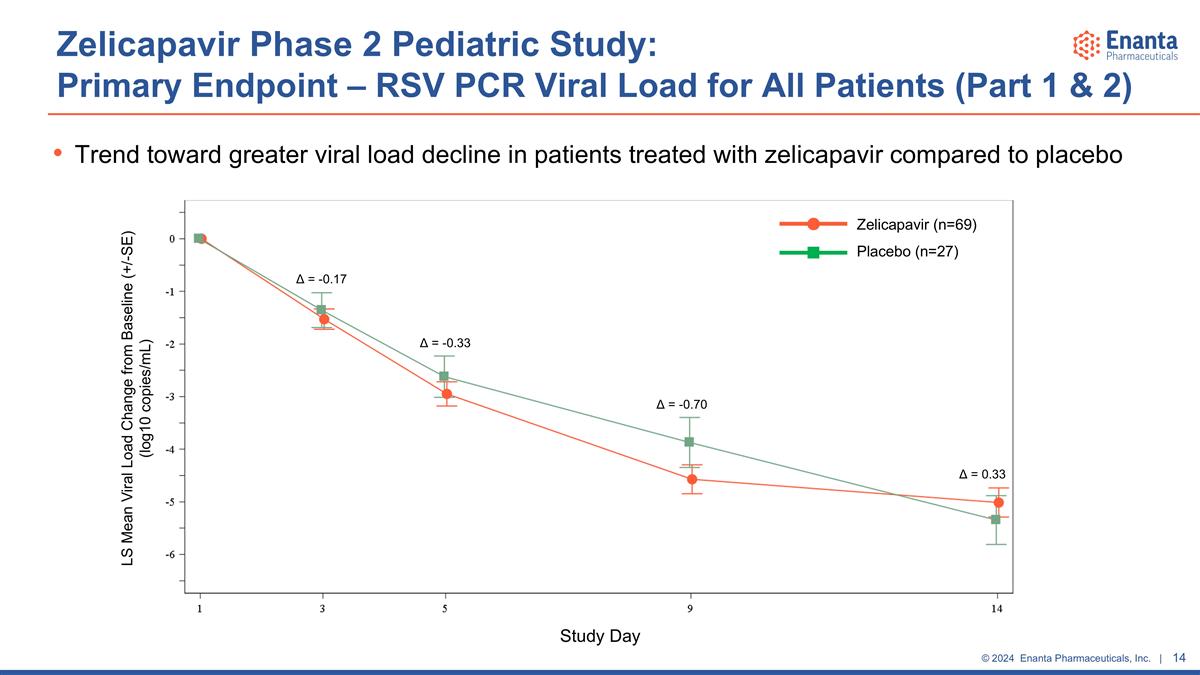
Zelicapavir Phase 2 Pediatric Study: Primary Endpoint – RSV PCR Viral Load for All Patients (Part 1 & 2) Trend toward greater viral load decline in patients treated with zelicapavir compared to placebo LS Mean Viral Load Change from Baseline (+/-SE) (log10 copies/mL) Study Day Zelicapavir (n=69) Placebo (n=27) Δ = -0.17 Δ = -0.33 Δ = -0.70 Δ = 0.33
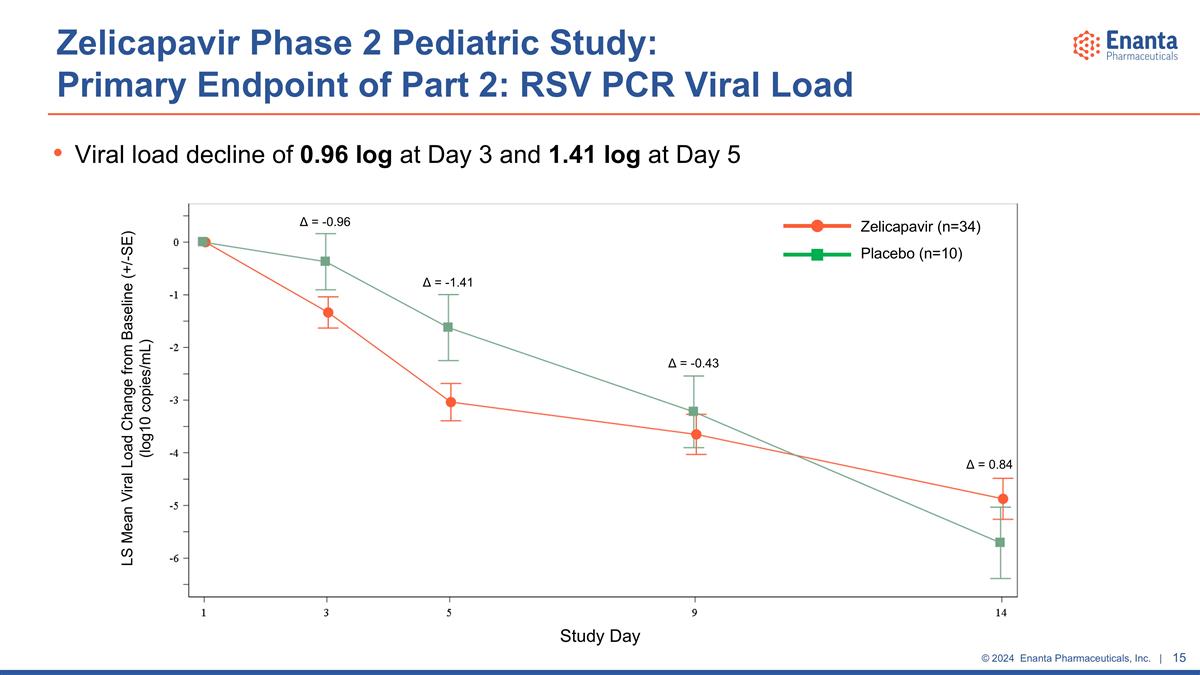
Zelicapavir Phase 2 Pediatric Study: Primary Endpoint of Part 2: RSV PCR Viral Load Viral load decline of 0.96 log at Day 3 and 1.41 log at Day 5 Zelicapavir (n=34) Placebo (n=10) LS Mean Viral Load Change from Baseline (+/-SE) (log10 copies/mL) Study Day Δ = -0.96 Δ = -1.41 Δ = -0.43 Δ = 0.84
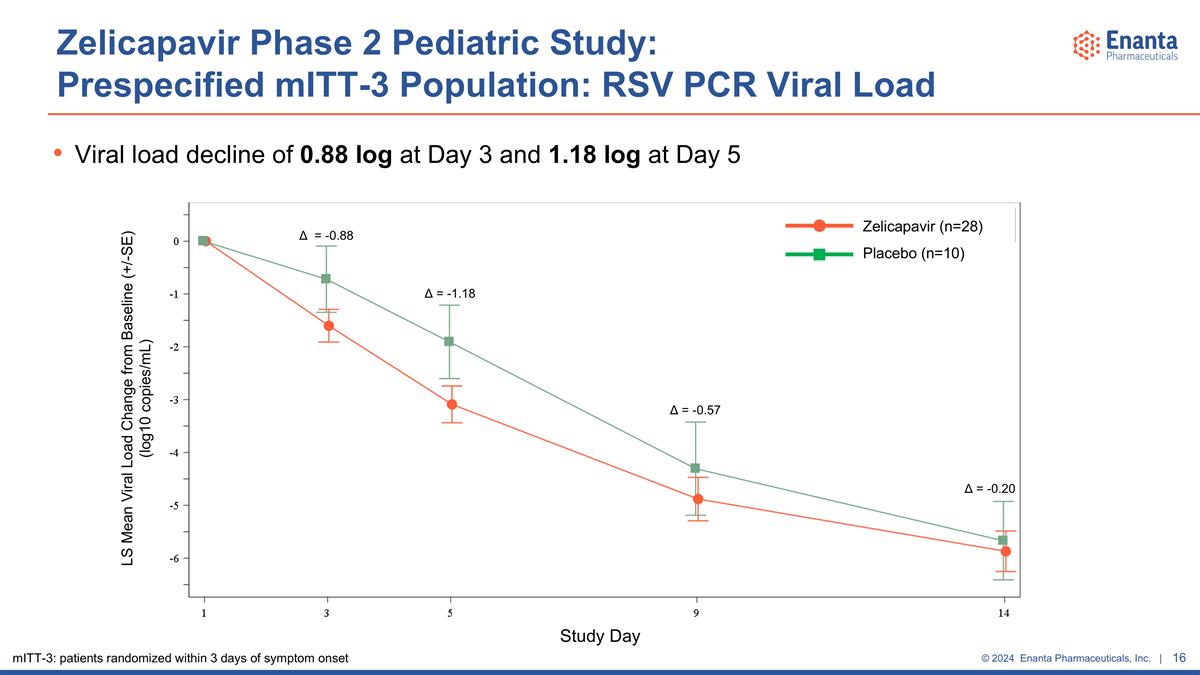
Zelicapavir Phase 2 Pediatric Study: Prespecified mITT-3 Population: RSV PCR Viral Load Viral load decline of 0.88 log at Day 3 and 1.18 log at Day 5 LS Mean Viral Load Change from Baseline (+/-SE) (log10 copies/mL) Study Day Zelicapavir (n=28) Placebo (n=10) Δ = -0.88 Δ = -1.18 Δ = -0.57 Δ = -0.20 mITT-3: patients randomized within 3 days of symptom onset
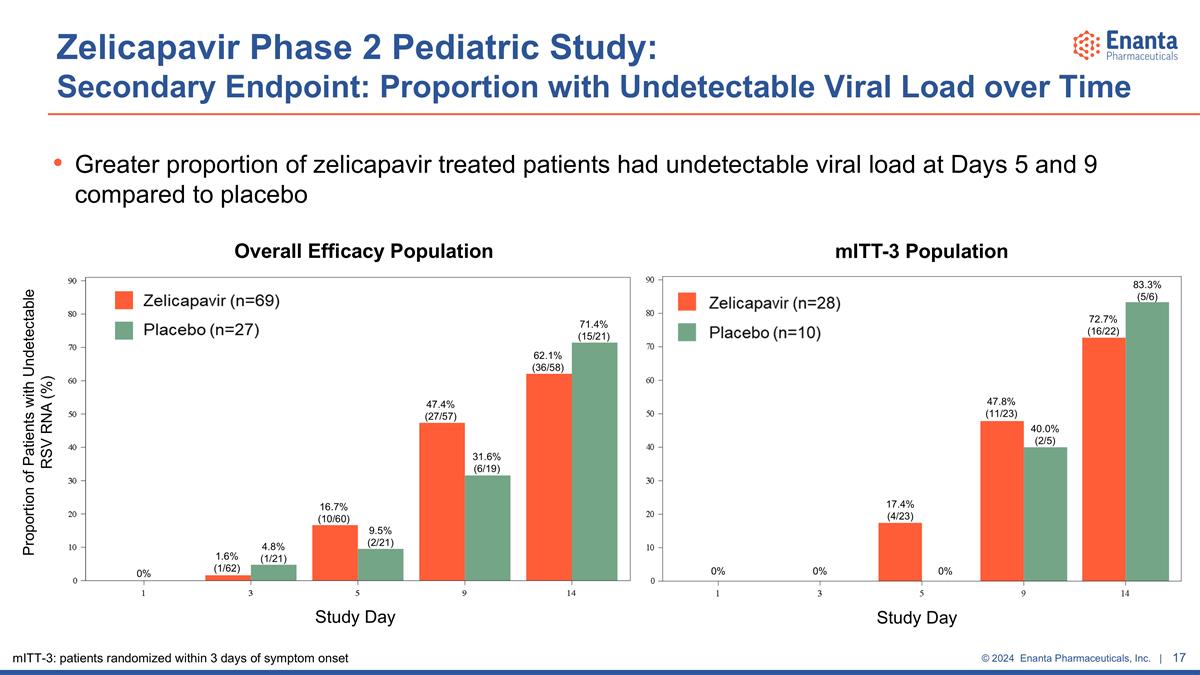
Zelicapavir Phase 2 Pediatric Study: Secondary Endpoint: Proportion with Undetectable Viral Load over Time Greater proportion of zelicapavir treated patients had undetectable viral load at Days 5 and 9 compared to placebo Proportion of Patients with Undetectable RSV RNA (%) Study Day Overall Efficacy Population mITT-3 Population Study Day 0% 1.6% (1/62) 4.8% (1/21) 16.7% (10/60) 9.5% (2/21) 47.4% (27/57) 31.6% (6/19) 62.1% (36/58) 71.4% (15/21) 17.4% (4/23) 0% 0% 47.8% (11/23) 40.0% (2/5) 72.7% (16/22) 83.3% (5/6) 0% mITT-3: patients randomized within 3 days of symptom onset
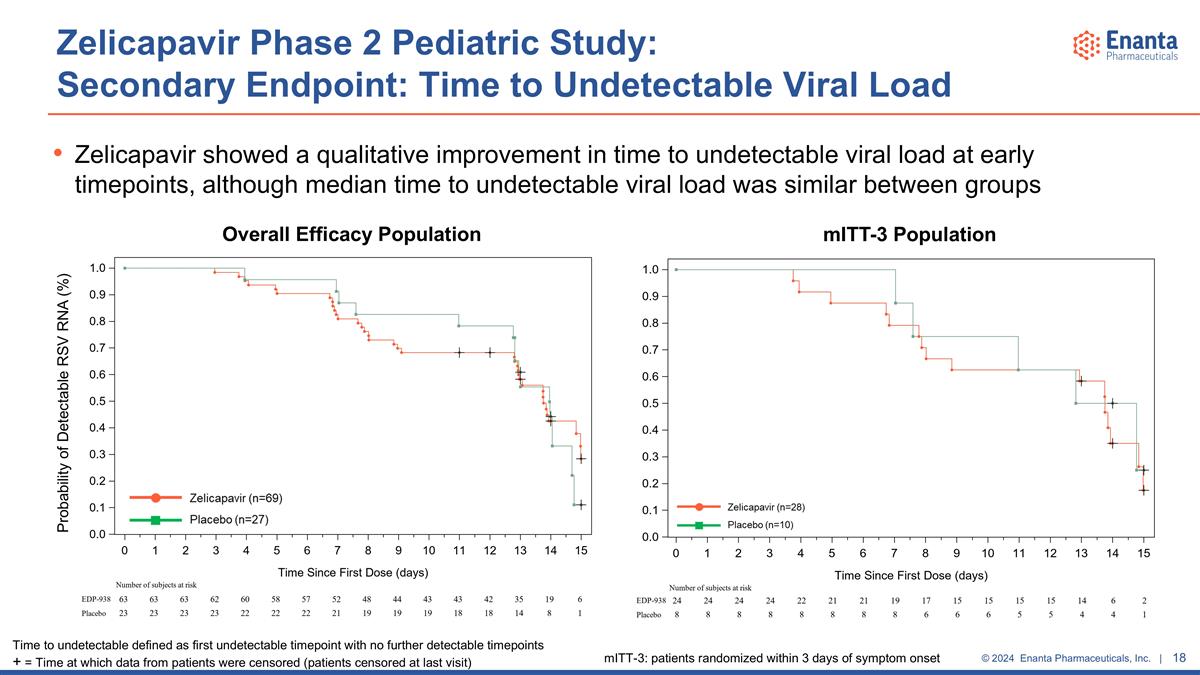
Zelicapavir Phase 2 Pediatric Study: Secondary Endpoint: Time to Undetectable Viral Load Zelicapavir showed a qualitative improvement in time to undetectable viral load at early timepoints, although median time to undetectable viral load was similar between groups Probability of Detectable RSV RNA (%) Time to undetectable defined as first undetectable timepoint with no further detectable timepoints + = Time at which data from patients were censored (patients censored at last visit) Overall Efficacy Population mITT-3 Population mITT-3: patients randomized within 3 days of symptom onset
Antiviral effect observed for the primary and secondary virology endpoints in overall population Viral load decline in Part 2 of 1.0 log at Day 3 and 1.4 log at Day 5 vs placebo Rapid and robust virology effects observed in prespecified subset of patients who were randomized within 3 days of symptom onset (mITT-3) Represents ~40% of the study population (n=38/96) Viral load decline of 0.9 log at Day 3 and 1.2 log at Day 5 vs placebo Greater proportion of patients had undetectable viral load at Days 5 & 9 vs placebo Qualitative improvement in time to undetectable viral load at early timepoints, although median time to undetectable viral load was similar between groups Improvement in AUC of change from baseline for viral load at all timepoints vs placebo Results were similar regardless of age or setting of care (outpatient & hospitalized) Zelicapavir Phase 2 Pediatric Study: Virology Summary
Zelicapavir Phase 2 Pediatric Study: Exploratory Endpoint: RSV Signs/Symptoms No validated symptom tool approved by regulatory agencies available for pediatric RSV RESOLVE-P (RESpiratory ObservabLE Reported Outcome-Pediatric) Proprietary tool in development by Enanta in alignment with regulatory agency advice Specifically designed to assess the severity of pediatric RSV infection change over time based on observations by the child’s caregiver Developed with input from caregivers, medical professionals and regulatory agencies Finalized and introduced late in the trial, so data only available from a small number of patients (n=15) ReSViNET (REspiratory Syncytial VIrus NETwork) Designed primarily for prophylaxis studies to assess disease severity at a single timepoint Publicly available pediatric tool with caregiver assessments Used as an exploratory endpoint Data available from all patients
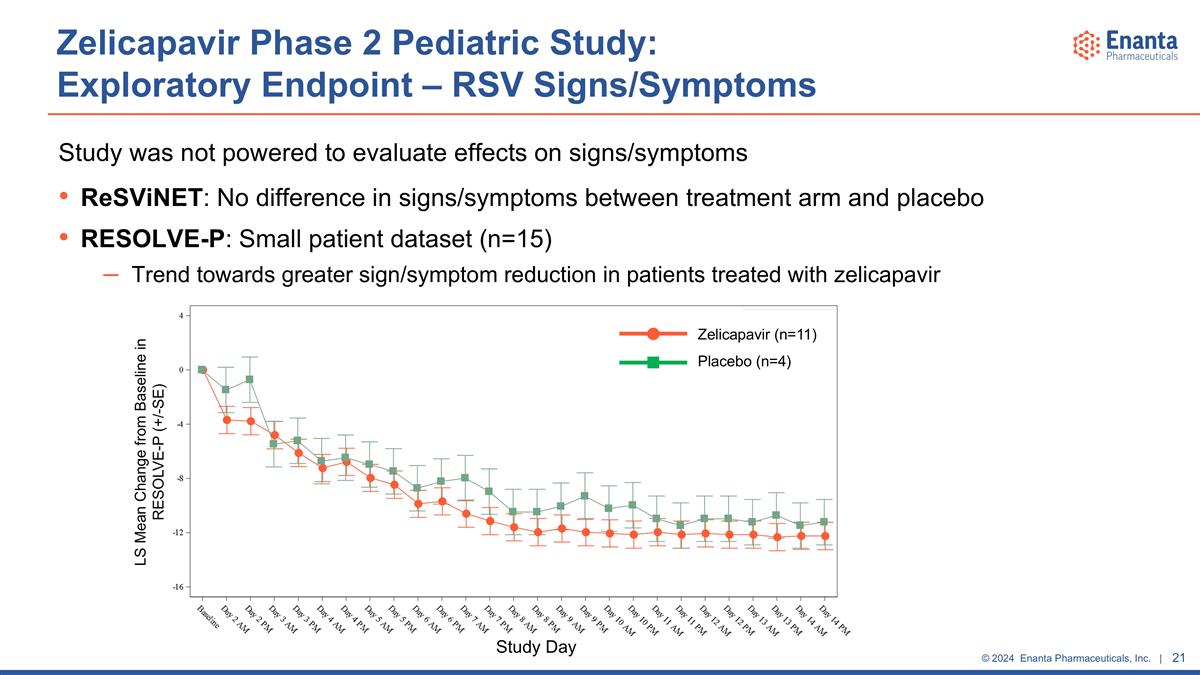
Zelicapavir Phase 2 Pediatric Study: Exploratory Endpoint – RSV Signs/Symptoms Study was not powered to evaluate effects on signs/symptoms ReSViNET: No difference in signs/symptoms between treatment arm and placebo RESOLVE-P: Small patient dataset (n=15) Trend towards greater sign/symptom reduction in patients treated with zelicapavir Study Day Zelicapavir (n=11) Placebo (n=4) LS Mean Change from Baseline in RESOLVE-P (+/-SE)
Well tolerated, with favorable safety profile No adverse events leading to treatment discontinuation or study withdrawal Antiviral effect observed for the primary and secondary virology endpoints in overall population Viral load decline of 1.4 log at the end of treatment in Part 2 Viral load decline of 1.2 log at Day 5 observed in prespecified subset of patients randomized within 3 days of symptom onset Data support further clinical development of zelicapavir Zelicapavir Phase 2 Pediatric Study: Conclusions Primary Objectives of Study Overall: Antiviral activity of zelicapavir across all patients Part 1: Safety and PK Part 2: Antiviral activity

Phase 2 Study of Zelicapavir in Children: RSVPEDs Topline Results December 9, 2024






















