YM BIOSCIENCES INC.
AMENDED ANNUAL INFORMATION FORM
YEAR ENDED JUNE 30, 2005
September 8, 2005
EXPLANATORY NOTE: This Annual Information Form has been amended to incorporate by reference the previously filed business acquisition report dated June 28, 2005 in respect of the acquisition of DELEX Therapeutics Inc. on May 2, 2005.
YM BIOSCIENCES INC.
ANNUAL INFORMATION FORM
TABLE OF CONTENTS
| DOCUMENTS INCORPORATED BY REFERENCE | 2 | |||
| GLOSSARY OF TERMS AND PROPER NAMES | 3 | |||
| FORWARD LOOKING STATEMENTS | 7 | |||
| CORPORATE STRUCTURE | 7 | |||
| GENERAL DEVELOPMENT OF THE BUSINESS | 8 | |||
| NARRATIVE DESCRIPTION OF THE BUSINESS (the “Business”) | 10 | |||
| DIVIDENDS | 47 | |||
| CAPITAL STRUCTURE | 47 | |||
| COMMON SHARES | 47 | |||
| MARKET FOR SECURITIES | 48 | |||
| DIRECTORS AND OFFICERS | 48 | |||
| AUDIT FEES | 54 | |||
| LEGAL PROCEEDINGS | 54 | |||
| TRANSFER AGENT AND REGISTRAR | 54 | |||
| MATERIAL CONTRACTS | 54 | |||
| ADDITIONAL INFORMATION | 55 |
DOCUMENTS INCORPORATED BY REFERENCE
YM BioSciences’ “Management’s Discussion and Analysis of Financial Condition and Results of Operations” (the “MD&A”) and the audited consolidated balance sheets as at June 30, 2005 and June 30, 2004, and the audited consolidated statements of earnings and retained earnings and changes in financial position for each of the years in the three year period ended June 30, 2005 (the “Financial Statements”) previously filed.
The MD&A and the Financial Statements, in their entirety, are incorporated by reference in, and form part of, this Annual Information Form. The previously filed business acquisition report dated June 28, 2005 in respect of the acquisition of Delex Therapeutics Inc. on May 2, 2005 is also incorporated by reference in, and forms part of, this Annual Information Form.
All of the documents referred to above have been filed via SEDAR (System for Electronic Document Analysis and Retrieval) and are available to the public at SEDAR’s website, www.sedar.com. Further information may also be found on the Corporation’s website www.ymbiosciences.com.
-2-
GLOSSARY OF TERMS AND PROPER NAMES
This glossary contains general terms used in the discussion of the biopharmaceutical industry, as well as specific technical terms used in the descriptions of our technology and business.
Abgenix - Abegnix Incorporated
Active Immunotherapy - Deliberate stimulation of the patient's own immune response through administration of antigens with or without immunological adjuvants. Therapeutic cancer vaccines are considered Active Specific Immunotherapy agents because the body is stimulated to make its own antibodies specific for the tumor cells
Adjuvant - Substance added to a vaccine to enhance its immunogenicity (i.e. its ability to stimulate an immune response)
Affinity - Binding strength of an antibody to a target
Amgen - Amgen Incorporated
Antisense Drug - Short spans of nucleic acid (DNA or RNA) used to disrupt the expression of disease related genetic code
Aphton - Aphton Corporation
ASCO - The American Society of Clinical Oncology
AZ/AstraZeneca - AstraZeneca PLC
Autocrine - Used herein to describe a hormonal pathway characterized by the production of a biologically active substance by a cell; the substance then binds to receptors on that same cell to initiate a cellular response
Autocrine loop - A self-sustaining process built on a self-feeding positive feedback cycle. Refers to the ability of a substance to act on the same cell that produced it
BMS - Bristol Myers Squibb Company
Cancer Vaccine - Vaccines or candidate vaccines designed to treat cancer, using pure or extracted tumor-specific antigens or using the patient's own whole tumor cells as the source of antigens - see “Active Immunotherapy”
CBQ - Centro de Bioactivos Quimicos (Center for Bioactive Chemicals), Santa Clara, Cuba
cDNA - Cloned copies of mRNA - the essential messenger element of the genes in the DNA that help in the coding of proteins
cGMP - current good manufacturing practices, as mandated from time to time by Health Canada and the FDA
CTA - Clinical Trial Application - an application made for Health Canada, the Canadian health regulatory authority for clinical trial
Chemopotentiator - A substance that enhances the activity of a chemotherapy agent
Chimeric - A chimeric antibody consists mainly of human protein, but the portion of the antibody that binds to the target is still mouse protein
CIM - Centro de Inmunologia Molecular (Center for Molecular Immunology), Havana, Cuba
-3-
CIMAB - a Cuban company responsible for commercializing products developed at CIM
Cisplatin - Approved chemotherapeutic agent
c-myc - Cellular gene involved in proliferation, commonly deregulated in cancer
CTA - Clinical Trial Application - previously known as an Investigational New Drug application which must be filed and accepted by the regulatory agency of Health Canada before each phase of human clinical trials may begin
Cyclophosphamide - Approved chemotherapeutic agent
Cytoprotective - Having the capacity to protect cells
Cytostatic - Having capacity to arrest the growth of cells
Cytotoxic - Having capacity to kill cells
Cytotoxic T cell response - Killing the tumor cell by activated tumor-specific T cells
DELEX - DELEX Therapeutics Inc.
Doxorubicin - Approved chemotherapeutic agent
E. coli - A common bacterial strain often used as a host for recombinant protein production
Eli Lilly - Eli Lilly and Company
EMEA - The Europe Agency for the Evaluation of Medicinal Products - the European health regulatory authority
Epidermal Growth Factor - A growth factor known to be involved in regulation of epithelial cell growth
Epithelial - Derived from epithelium which is the layer of cells forming the epidermis of the skin and the surface layer of the serous and mucous membranes
Estramustine - An approved chemotherapeutic agent
Extracellular domain (ECD) - The portion of a cell surface protein located outside the cell
5-FU - See Fluorouracil
Fluorouracil (5-Fluorouracil, 5-FU) - Approved chemotherapeutic agent
Fusion protein - Two or more proteins genetically engineered to be produced as a single protein
Genentech - Genentech Incorporated
Genmab - Genmab A/S
Genta - Genta Incorporated
Glioma - A form of brain cancer involving the malignant transformation of a glial cell
GMP - good manufacturing practices, i.e. guidelines established by the governments of various countries, including Canada and the United States, to be used as a standard in accordance with the World Health Organization's Certification Scheme on the quality of pharmaceutical products
-4-
GnRH - Gonadotrophin Releasing Hormone; controlling the circulating levels of the sex hormones
HER-1 positive tumors - Tumors expressing/producing the EGF receptor
Hormone-refractory - Term used to indicate that a tumor is no longer responsive to hormone therapy
Humanized - The process whereby an antibody derived from murine cells is altered to resemble a human antibody. Humanized antibodies are less likely to cause allergic reactions when given to humans but retain the biological activity of the original murine form
ImClone - ImClone Systems Incorporated
IND - Investigational New Drug application which must be filed and accepted by the FDA before each phase of human clinical trials may begin
Irinotecan - An approved chemotherapeutic agent
In vivo - In the living body or organism. A test performed on a living organism
ISIS - ISIS Pharmaceuticals
KFDA - Korea Food and Drug Administration - the Korean health regulatory authority
Ligand - Used herein to describe a protein or peptide that binds to a particular receptor
Lorus - Lorus Therapeutics Inc.
Merck - Merck KGaA
Metastatic - A term used to describe a cancer where tumor cells have migrated from the primary tumor to a secondary site (e.g. from prostate to bone)
Mitoxantrone - An approved chemotherapy agent
Monoclonal antibody (“MAb”) - Antibodies of exceptional purity and specificity derived from hybridoma cells (cells which are fused cells, generally MAb produced in mice, that secrete MAbs)
Murine - Derived from mouse cells
NCE - A new chemical entity
NCIC - The National Cancer Institute of Canada
Neoplastic - New and abnormal growth of tissue (neoplasm), which may be benign or cancerous
NSCLC - Non Small Cell Lung Cancer
OFAC - US Department of the Treasury Office of Foreign Asset & Control
Oncogene - A gene that induces or promotes uncontrolled cell growth
Oncoscience - Oncoscience AG
Orange Book - A reference to the Hatch/Waxman Act
-5-
Orphan Drug - A drug aimed at treating a condition with an incidence of less than 200,000 per year in the United States (often given a seven year market exclusivity by the FDA
OSI - OSI Pharmaceuticals, Inc.
Overall Survival - For patients who have died, overall survival was calculated in months from the day of randomization to date of death. Otherwise, survival was censored at the last day the patient is known alive
P64k - Outer membrane protein of N. meningitides
Passive Immunotherapy - Immunologically active material transferred into the patient as a passive recipient. Monoclonal antibodies are considered Passive Immunotherapy since antibodies are generated outside the body and given to the patient
pGp - P-Glycoprotein. A pumping mechanism that removes noxious substances from the cell
pGp inhibitor - Inhibitor of the activity of P-Glycoprotein
P. haemolytica - A bacterium causing respiratory disease in cattle and sheep
Phosphorylation - Addition/donation of a phosphate group to a particular amino acid which can lead to tumor growth
Prednisone - An approved standard anti-inflammatory
Resection - The process of tumor removal
Roche - F.Hoffmann-LaRoche Ltd.
TAP - TAP Pharmaceuticals
Taxol - An approved chemotherapeutic agent
Taxotere - An approved chemotherapeutic agent
TGFα - Transforming growth factor alpha
Th 1 - T helper cell type 1 (generally involved in stimulating a cell-mediated immune response)
Therapeutic vaccine - An approach to the treatment of cancer utilizing “active immunotherapy”
Titers - Term used to express levels of circulating antibodies
Tyrosine kinase - An enzyme that catalyzes the phosphorylation of tyrosine residues in proteins with nucleotides as phosphate donors
Upregulation - Increased production of an RNA transcript or a protein by a cell
Vinyl furan - A chemical polymer
Yttrium 90 - A radioisotope used in the treatment of disease
-6-
FORWARD LOOKING STATEMENTS
Statements contained herein that are not based on historical fact, including without limitation statements containing the words "believes," "may," “likely,” "plans," "will," "estimate," "continue," "anticipates," "intends," "expects" and similar expressions, constitute "forward-looking statements" within the meaning of the US Private Securities Litigation Reform Act of 1995. Such forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause the actual results, events or developments to be materially different from any future results, events or developments expressed or implied by such forward-looking statements. Such factors include, without limitation, changing market conditions, our ability to obtain patent protection and protect our intellectual property rights, commercialization limitations imposed by intellectual property rights owned or controlled by third parties, intellectual property liability rights and liability claims asserted against us, the successful and timely completion of clinical studies, the impact of competitive products and pricing, new product development, uncertainties related to the regulatory approval process, product development delays, our ability to attract and retain business partners and key personnel, future levels of government funding, our ability to obtain the capital required for research, operations and marketing and other risks detailed from time-to-time in the Corporation’s ongoing quarterly filings, annual information forms and annual reports. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. In light of these risks, uncertainties and assumptions, the forward-looking events discussed in this annual information form might not occur.
Unless otherwise indicated, or the context requires otherwise, the information appearing in this annual information form is stated as at June 30, 2005 and references in this annual information form to “$” or “dollars” are to Canadian dollars. Information contained on our website is not part of this annual information form.
CORPORATE STRUCTURE
YM BioSciences Inc. (“YM BioSciences” or “YM” or the “Corporation”) was incorporated under the laws of the Province of Ontario on August 17, 1994. On February 7, 2001 the Corporation changed its name to YM BioSciences Inc. and on December 11, 2001 was continued into the Province of Nova Scotia under the Nova Scotia Companies Act.
The head office and principal place of business of the Corporation is 5045 Orbitor Drive, Building 11, Suite 400, Mississauga, Ontario, L4W 4Y4. The registered head office of YM BioSciences is 1959 Upper Water Street, Suite 800, Halifax, Nova Scotia, B3J 2X2
ORGANIZATIONAL STRUCTURE
The Corporation currently has three material subsidiaries, shown in the following diagram:
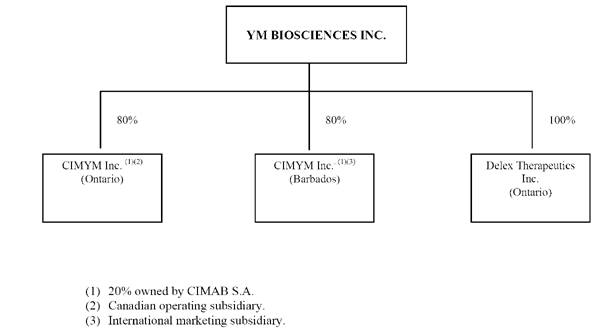
-7-
CIMYM is 80% owned by the Corporation and 20% owned by CIMAB. It is a Canadian operating subsidiary incorporated under the laws of Ontario.
CIMYM (Barbados) 80% owned by the Corporation and 20% owned by CIMAB. It is an international marketing subsidiary incorporated under the laws of Barbados.
DELEX is 100% owned by the Corporation. On May 2, 2005, the Corporation acquired all of the issued and outstanding shares of capital stock of DELEX. YM issued to the DELEX shareholders common shares of YM in consideration for their DELEX shares and additional shares of YM in consideration for working capital in DELEX. See “General Development of the Business” for more details regarding the acquisition. DELEX is now a Canadian operating subsidiary of YM incorporated under the laws of Ontario.
Unless otherwise noted, “YM BioSciences”, “YM”, and the “Corporation” includes YM BioSciences Inc. and its subsidiaries.
GENERAL DEVELOPMENT OF THE BUSINESS
The Corporation was founded in 1994 to acquire rights to develop drug products. The Corporation is principally focused on products for the treatment of patients with cancer.
In 1995, the Corporation secured our first drug licenses and our initial financing. The Corporation initially licensed a range of drug products at various stages of assessment and development, including certain of the Corporation's current anti-cancer products. In 1998, the Corporation decided to concentrate on anti-cancer products. The Corporation has used funds raised in our initial financing and subsequent financings in 1997, 1999, 2000, 2002, 2003 and 2004 to advance certain of our licensed drug products through clinical trials in Canada, the United States and Europe, and to expand our portfolio initially of anti-cancer products by licensing additional drug and cancer-related products in later stages of development. In addition, the Corporation has licensed certain drug products that were in pre-clinical. See “Narrative Description of the Business - Products in Clinical Development” and “- Products in Pre-Clinical Development”.
The Corporation has four product candidates currently in the clinical stage of development:
o TESMILIFENE is a small molecule chemopotentiator that has been clinically demonstrated to augment the anti-tumor activity of the cytotoxic drug families of anthracyclines and taxanes and preclinically of other families of chemotherapy. In a Phase III trial the drug was used in combination with doxorubicin and demonstrated a greater than 50% increase in survival in women with metastatic and recurrent breast cancer compared with the patients treated with doxorubicin alone.
o NIMOTUZUMAB (previously known as TheraCIM hR3), a humanized monoclonal antibody, targeting the protein known as Epidermal Growth Factor Receptor ("EGFr"), is designed to treat epithelial cancers and to be administered prior to, simultaneously with, or subsequent to, chemotherapy and radiotherapy. In various Phase II trials, the drug has doubled the reported complete response rate to radiation in head-and-neck tumors and demonstrated clinical benefit in adult and pediatric glioma. The drug has been approved for sale in the People’s Republic of China, Argentina and Columbia for nasopharangeal cancer in the first case and for head and neck cancer in the latter two.
-8-
o AeroLEF™, a proprietary formulation of both free and liposome-encapsulated fentanyl administered by pulmonary inhalation, is being developed for the treatment of severe and moderate acute pain and cancer pain. AeroLEF™ has been developed to provide both rapid onset and extended relief while recognizing the need for interpersonal variability as well as inter-episode variability in the amount of drug needed. The drug has completed a Phase IIa trial and cleared for initiatiation of a randomized Phase IIb trial.
o NORELINTM is a therapeutic vaccine designed to stimulate the production of antibodies against GnRH in patients, resulting in reduced production of hormones that may cause or contribute to the growth of certain sex-hormone dependent cancers. It has completed a Phase I/II safety and immunogenicity trial in hormone-sensitive prostate cancer suggesting that the approach may be better tolerated than currently available therapies.
o RADIOTHERACIM is a radiolabelled humanized MAb, targeting the EGFr, that completed a pilot trial for the treatment of brain cancers. In published results from that clinical study in patients with glioma the product appeared to increase survival compared with historical results with standard radiation therapy. No additional clinical trials are planned at this time.
The Corporation also has interests in therapeutic cancer vaccines in the pre-clinical stage of development, namely TGFα Cancer Vaccine and HER-1 Cancer Vaccine. The Corporation's license for the TGFα Vaccine and the HER-1 Vaccine is suspended under the terms of the out-licensing agreement in July 2004 between the Corporation, our subsidiary CIMYM, Inc., a Barbados corporation ("CIMYM (Barbados)"), CIMAB and Tarcanta Inc. and Tarcanta, Ltd. (collectively, "Tarcanta"), two wholly-owned subsidiaries of California-based CancerVax Corporation ("CancerVax") relating to Tarcanta licensing TGFα and HER-1 from CIMAB. In connection with the out-licensing agreement, CancerVax has announced that it has received a license from the Office of Foreign Asset Control of the United States Department of Treasury ("OFAC" or "Treasury") authorizing Tarcanta to enter into the transactions with CIMAB and the Corporation. See "Business - Licensing Arrangements - Out-Licensing".
On May 2, 2005, the Corporation acquired DELEX Therapeutics Inc. (“DELEX”), a private clinical stage company developing inhalation-delivered products to treat severe or moderate pain including cancer pain. DELEX’s lead product is AeroLEFTM, a proprietary formulation of opioids for the treatment of acute and breakthrough pain that has completed preliminary efficacy trials and is positioned to undergo further Phase II efficacy trials in 2005.
Through this acquisition, DELEX became a wholly-owned subsidiary of the Corporation. Prior to the acquisition, DELEX’s principal shareholders, in addition to its founders and management, were New Generation Biotech (Equity) Fund, a fund managed by Genesys Capital Partners, BDC Venture Capital, a corporation owned by the Business Development Bank of Canada, and the Eastern Technology Seed Investment Fund Limited Partnership. In consideration for their shares and the accompanying working capital in DELEX, YM BioSciences issued to the DELEX shareholders 1,587,302 common shares upon closing of the transaction. In addition, the Corporation issued 4,603,174 to be held in escrow for the benefit of DELEX shareholders. Of the escrowed common shares an aggregate of 1,825,396 will be released in four tranches of approximately 456,349 common shares each of two in November 2005, May 2006, and November 2006, and May 2007 following closing and up to 2,777,779 common shares in escrow will be released only if specific milestones are achieved, including the receipt of an IND for the planned Phase IIb trial, the conclusion of a strategic partnership for further development and commercialization of the product, and upon the initiation of Phase III trials. On receipt of US regulatory approval, if any, for AeroLEFTM or any product using DELEX’s technology, the Corporation will make an additional payment to the DELEX shareholders of $4.75 million in cash and/or common shares at the Corporation’s option.
In addition to the above consideration, DELEX employees were granted an aggregate of 437,000 stock options under the Corporation’s stock option plan.
There is no indication of any public takeover offers by third parties in respect of the YM's shares or by YM in respect of other companies' shares which have occurred during the last and current financial year.
-9-
NARRATIVE DESCRIPTION OF THE BUSINESS (the “Business”)
The Corporation is a biopharmaceutical company engaged in the development of products primarily for the treatment of patients with cancer. YM generally in-licenses substances designed for anti-cancer use in order to advance them along the regulatory and clinical pathways toward commercial approval. The Corporation's licenses generally cover the major market countries of the developed world (including Canada, the United States, Japan and Europe) or are world-wide. The Corporation uses our expertise to manage and perform what we believe are the most critical aspects of the drug development process which include the design and conduct of clinical trials, the development and execution of strategies for the protection and maintenance of intellectual property rights and the interaction with drug regulatory authorities internationally. YM concentrates on drug development and does not engage in drug discovery, avoiding the significant investment of time and capital that is generally required before a compound is identified as appropriate forclinical trials. YM has in-licensed certain preclinical products which have been related to its clinical programs. YM both conducts and out-sources clinical trials and out-sources the manufacture of clinical materials to third parties.
The Corporation's current portfolio of products in clinical development includes three anti-cancer agents (a small molecule, a vaccine and a monoclonal antibody) in a number of formulations currently targeting nine different tumors and/or stages of cancer as well as a proprietary inhalation delivery system for fentanyl to treat acute pain including cancer pain. The Corporation also has a financial interest in two additional anti-cancer immunotherapies in pre-clinical development. The Corporation intends to license the rights to manufacture and market our drug products to other pharmaceutical companies in exchange for license fees and royalty payments and to continue to seek other in-licensing opportunities in pursuing our business strategy. The Corporation does not currently intend to manufacture or market products although we may, if the opportunity is available on terms that are considered attractive, participate in ownership of manufacturing facilities or retain marketing or co-development rights to specific products.
RISK FACTORS
Risks Related To Our Business
WE ARE IN THE EARLY STAGES OF DEVELOPMENT AND, AS A RESULT, ARE UNABLE TO PREDICT WHETHER WE WILL BE ABLE TO PROFITABLY COMMERCIALIZE OUR PRODUCTS.
The Corporation was founded in 1994 and none of the products, licensed or owned, have received regulatory approval for sale in any of the Corporation’s licensed territories. Accordingly, the Corporation has not generated any revenues from the commercialization of our products. A significant commitment of resources to conduct clinical trials and additional development will be required to commercialize most of the products. There can be no assurance that the products will meet applicable regulatory standards, be capable of being produced in commercial quantities at reasonable cost or be successfully marketed, or that the investment made by the Corporation in the commercialization of the products will be recovered through sales, license fees or related royalties.
WE HAVE A LACK OF REVENUES AND A HISTORY OF LOSSES AND, THEREFORE, ARE UNABLE TO PREDICT THE EXTENT OF ANY FUTURE LOSSES OR WHEN WE WILL BECOME PROFITABLE.
Up to June 30, 2005, the Corporation recognized approximately $748,000 from the out-licensing of our licensed products TGFα and HER-1 from Tarcanta and tesmilifene from Shin Poong. Since incorporation and up to June 30, 2005, YM has an accumulated deficit of $60.8 million. The Corporation expects expenditures and the accumulated deficit to increase as we proceed with our commercialization programs until such time as any sales, license fees and royalty payments generate sufficient revenues to fund our continuing operations.
WE ARE DEPENDENT ON OTHERS FOR THE MANUFACTURE, DEVELOPMENT AND SALE OF OUR PRODUCTS. IF WE ARE UNABLE TO ESTABLISH OR MANAGE COLLABORATIONS IN THE FUTURE, THERE COULD BE A DELAY IN THE MANUFACTURE, DEVELOPMENT AND SALE OF OUR PRODUCTS.
-10-
We do not conduct any of our own basic research. Basic research on a particular product licensed to us or acquired by us is conducted by other biopharmaceutical companies, scientific and academic institutions and hospitals, or scientists affiliated with those institutions. Once the basic research is complete, the Corporation generally enters into license agreements to in-license the right to develop and market the products. The Corporation has negotiated certain in-licensing agreements with: the University of Manitoba, CancerCare Manitoba, Vincent Research and Consulting, CIMAB, Biostar Inc., the Veterinary Infectious Disease Organization ("VIDO") (a division of the University of Saskatchewan), the University of Saskatchewan, and DELEX technology was developed at Dalhousie University. See "Business - Licensing Arrangements - In-Licensing".
The Corporation enters into arrangements with, and is dependent on others with, respect to the manufacture, development and sale of our in-licensed products. Product development includes, but is not limited to, pre-clinical testing, clinical testing, regulatory approvals and the development of additional regulatory and marketing information. The Corporation's ability to successfully develop and commercialize our in-licensed or acquired products is dependent on our ability to make arrangements with others on commercially acceptable terms. The product development process may be delayed or terminated if the Corporation cannot secure or maintain such arrangements. The Corporation does not have long-term, material, third-party manufacture, formulation or supply agreements, except with respect to two of the Corporation's licensed products (TheraCIM and RadioTheraCIM) which are expected to be manufactured by CIMAB or a related party, subject to certain terms and conditions of the licensing agreements between the Corporation and CIMAB. The Corporation has entered into an agreement with Pharm-Olam International, Ltd. ("POI") in connection with clinical testing of tesmilifene.
The Corporation expects to enter into out-licensing agreements with others with respect to manufacturing and marketing our products. The Corporation may retain co-development and marketing rights if management deems it appropriate to do so. At this time, the Corporation has entered into four out-licensing agreements.
The Corporation entered into the first out-licensing agreement through our subsidiary, CIMYM Inc., an Ontario corporation ("CIMYM"). On November 12, 2003, CIMYM out-licensed the rights for TheraCIM in most of Europe to Oncoscience AG (“Oncoscience”) of Germany. Under the terms of the agreement, CIMYM is entitled to receive up to US$30 million as a share of any amounts received by Oncoscience in relation to the development or sublicensing of the product and as a royalty on initial net sales. After CIMYM has received US$30 million, CIMYM continues to receive royalties on net sales but at a lesser percentage.
The Corporation and CIMYM (Barbados) entered into the second out-licensing agreement with Tarcanta and CIMAB relating to Tarcanta licensing TGFα and HER-1 from CIMAB. CancerVax has announced that it has received a license from Treasury authorizing Tarcanta to enter into the transactions with CIMAB and the Corporation. On July 13, 2004, the Corporation, CIMYM (Barbados), CIMAB and Tarcanta entered into a License, Development, Manufacturing and Supply Agreement. By the terms of this agreement with Tarcanta, the 2001 CIMYM License has been suspended until such time, if at all, there is a default under the agreement with Tarcanta. Under the terms of the new agreement and in consideration for the suspension of the 2001 CIMYM License, the Corporation is entitled to receive an aggregate payment of $1,000,000 which is payable in four equal installments, the final payment due December 31, 2005. In addition, under the new agreement the Corporation may receive 35% of an aggregate of $16,350,000 in milestone payments to be paid by Tarcanta upon the successful completion of certain research and development activities. The Corporation has no continuing involvement in these research and development activities and has no future obligations under the development plan established by the out-licensing arrangement between CIMAB and Tarcanta. Finally, the Corporation retains an interest in the revenues from the manufacture and marketing of the drugs or from their sub-licensing. See "Business - Licensing Arrangements - Out-Licensing" and see "Business - Licensing Arrangements - In-Licensing - Licenses for TheraCIM, RadioTheraCIM, TGFα and HER-1".
The Corporation entered into an out-licensing agreement with Kuhnil Pharmaceutical Company (“Kuhnil”) of Seoul, Korea to expand the development program for nimotuzumab (TheraCIM-hR3), its monoclonal antibody against the EGF receptor, for specific population of patients with non-small cell lung cancer (NSCLC). Kuhnil will join the Joint Development Team, already comprised of YM, Oncoscience and CIMAB management, to oversee the development of nimotuzumab. Kuhnil will fund Korean development costs and provide an undisclosed amount of up-front, milestone and royalty payments. In addition, Kuhnil will launch bridging studies in the local population as required by the Korean health regulatory authority, KFDA, in order to support all indications currently under study by CIMYM and Oncoscience to permit them to be launched in Korea and other Asian countries. In Europe, Oncoscience is currently awaiting advice from EMEA for two pivotal trials with nimotuzumab in glioma where the drug is currently undergoing a phase II monotherapy trial in pancreatic cancer.
-11-
The Corporation entered into an agreement with Shin Poong Pharmaceutical Company (“Shin Poong”) of Seoul, Korea to expand the development program for its lead drug, tesmilifene, into gastric cancer. The two companies will form a Joint Development Team to oversee the development of tesmilifene in gastric cancer. Shin Poong will fully fund development costs and provide an undisclosed amount of up-front, milestone and royalty payments. In addition, Shin Poong will launch a bridging study in the local population in calendar 2005 in order to allow the breast cancer indication currently under study by YM to be launched in Korea and other Asian countries. Shin Poong recently completed recruitment for a gastric cancer study with its proprietary taxane drug, Padexol®.
There can be no assurance that the Corporation will be successful in maintaining our relationships with research institutions or others or in negotiating additional in-licensing or out-licensing agreements on terms acceptable to the Corporation or that any such arrangements will be successful. In addition, there can be no assurance that the arrangements between the Corporation and others will prevent other parties from entering into arrangements with such entities for the development or commercialization of similar products or that the parties with whom the Corporation has such arrangements will not be pursuing alternative technologies or developing products either on their own or in collaboration with others, including the Corporation's competitors. If the Corporation does not establish sufficient in-licensing and out-licensing arrangements, we could encounter delays in product introductions or could find that the development, manufacture or sale of our licensed products could be materially adversely affected.
WE HAVE NO EXPERIENCE IN COMMERCIAL MANUFACTURING OF OUR PRODUCTS AND MAY ENCOUNTER PROBLEMS OR DELAYS IN MAKING ARRANGEMENTS FOR PRODUCTS TO BE COMMERCIALLY MANUFACTURED, WHICH COULD RESULT IN DELAYED DEVELOPMENT, REGULATORY APPROVAL AND MARKETING.
The Corporation has not commercially launched any of its licensed or owned products and has no commercial manufacturing experience. To be successful, the products must be manufactured in commercial quantities in compliance with regulatory requirements and at acceptable costs. The Corporation does not have and does not intend to acquire facilities for the production of the products although we may invest in the ownership of production facilities if appropriate opportunities are available.
Two of the Corporation's licensed products (namely, TheraCIM and RadioTheraCIM) are currently manufactured at commercial scale, but in small quantities for testing, by the relevant licensor (namely, CIMAB) or a related party, subject to certain terms and conditions of the licensing agreements between the Corporation and CIMAB. Currently these expectations are being met. There can be no assurance, however, that such entities will be able to develop adequate manufacturing capabilities for additional commercial scale quantities in a commercially reasonable manner.
Three other products of the Corporation which are currently manufactured, and finished and filled, in small quantities for testing, by third parties are tesmilifene and NorelinTM which are licensed and AeroLEFTM which is owned by the Corporation. The manufacturing process for these products is such that the Corporation expects that commercial quantities can be manufactured. If current suppliers cannot manufacture commercial quantities or the Corporation otherwise experiences a problem with current suppliers, it will be necessary for the Corporation to obtain these products from new suppliers. The Corporation does not have supply agreements with the third party suppliers of tesmilifene, NorelinTM or AeroLEFTM, but such suppliers have produced quantities for the Corporation, or in the case of AeroLEFTM for DELEX, to specification on purchase order. The Corporation expects that we could find new suppliers, if necessary. There can be no assurance, however, that the Corporation or our licensors will be able to reach satisfactory arrangements with our current suppliers or, if necessary, new suppliers or that such arrangements will be successful.
All manufacturing facilities must comply with applicable regulations in their jurisdiction or where products are to be sold. In addition, production of the licensed and owned products may require raw materials for which the sources and amount of supply are limited. An inability to obtain adequate supplies of such raw materials could significantly delay the development, regulatory approval and marketing of the Corporation's licensed and owned products.
-12-
WE ARE DEPENDENT ON DEVICES FROM THIRD PARTIES IN ORDER TO SUCCESSFULLY COMMERCIALIZE AEROLEFTM
Device use will be an important element for successful commercialization of AeroLEFTM in both the inpatient and outpatient settings.
We have selected the AeroEclipse(R) inhalation device for our Phase II clinical studies for the inpatient indications for AeroLEFTM and anticipate using the AeroEclipse(R) for our planned Phase III clinical studies for the inpatient market opportunity. Material changes to the AeroEclipse(R) device by the manufacturer, Trudell Medical, or a decision to change to an alternative inhalation device would likely delay the initiation of Phase III clinical trials. A change of devices after the initiation of Phase III studies, however, would require additional clinical trials bridging studies and could significantly delay the commercialization of AeroLEFTM. Currently DELEX purchases the AeroEclipse(R) and it does not have a defined supply agreement.
While inpatient use of AeroLEFTM, in the hospital or hospice setting, may be achieved with existing equipment such as the AeroEclipse(R), outpatient use will require a portable nebulization device. Several devices currently exist and are available for use with approved respiratory agents (bronchodilators, antibiotics, steroids). The Company has an active development program to evaluate and identify the best devices for use with AeroLEFTM and other pipeline products. Although the Company would prefer to access these devices on an arms-length basis from the manufacturer, it will explore the benefits of a strategic partnership that could provide for custom adaptations to optimize product delivery, lower prices or other benefits.
THE DRUG ENFORCEMENT ADMINISTRATION (“DEA”) LIMITS THE AVAILABILITY OF THE ACTIVE INGREDIENTS IN CERTAIN OF OUR CURRENT DRUG CANDIDATES AND, AS A RESULT, OUR QUOTA MAY NOT BE SUFFICIENT TO COMPLETE CLINICAL TRIALS, OR TO MEET COMMERCIAL DEMAND OR MAY RESULT IN CLINICAL DELAYS.
The DEA regulates chemical compounds as Schedule I, II, III, IV and V substances, with Schedule I substances considered to present the highest risk of substance abuse and Schedule V substances the lowest risk. Certain active ingredients in AeroLEFTM, such as fentanyl, are listed by the DEA as Schedule II under the Controlled Substances Act of 1970. Consequently, their manufacture, research, shipment, storage, sale and use are subject to a high degree of oversight and regulation. For example, all Schedule II drug prescriptions must be signed by a physician, physically presented to a pharmacist and may not be refilled without a new prescription. Further, the amount of Schedule II substances we can obtain for clinical trials and commercial distribution is limited by the DEA and our quota may not be sufficient to complete clinical trials or meet commercial demand. There is a risk that DEA regulations may interfere with the supply of the drugs used in our clinical trials, and, in the future, our ability to produce and distribute our products in the volume needed to meet commercial demand.
WE ARE DEPENDENT ON LICENSES FROM THIRD PARTIES AND THE MAINTENANCE OF LICENSES IS NECESSARY FOR OUR SUCCESS.
We have obtained our rights to the licensed products under license agreements from various third party licensors as follows:
| (a) | License Agreement between CIMYM Inc. and CIMAB SA, January 24, 2001 with respect to TGFα and HER-1, which agreement has been suspended in accordance with the terms of the Tarcanta out-licensing agreement (See "Business - Licensing Agreements - Out-Licensing"); |
| (b) | License Agreement between YM BioSciences Inc. (formerly known as York Medical Inc.), University of Manitoba and The Manitoba Cancer Treatment and Research Foundation, carrying on its undertaking as Cancercare Manitoba, dated November 2, 2000 with respect to tesmilifene; |
| (c) | License Agreement between YM BioSciences Inc. (formerly known as York Medical Inc.) and Biostar Inc. dated October 11, 2000 with respect to NorelinTM; and |
| (d) | License Agreement, as amended between YM BioSciences Inc. (formerly known as Yorkton Medical Inc.) and CIMAB SA, dated May 3, 1995 with respect to TheraCIM and RadioTheraCIM. |
-13-
The above listed license agreements are more fully described under the heading "Business Overview - Licensing Arrangements - In-Licensing". The Corporation owns AeroLEFTM and therefore is not subject to a license in respect of it.
The Corporation is dependent upon the licenses for our rights to the licensed products and commercialization of the licensed products. While the Corporation believes we are in compliance with our obligations under the licenses, certain licenses may be terminated or converted to non-exclusive licenses by the licensors if there is a breach of the terms of the licenses. There can be no assurance that the licenses are enforceable or will not be terminated or converted. The termination or conversion of the licenses or the inability of the Corporation to enforce our rights under the licenses would have a material adverse effect on the Corporation as the Corporation would not have products to develop. To the extent that management considers a particular license to be material to the undertaking of the Corporation, the Corporation has entered into a signed license agreement for that license. Terms of certain remaining licenses are to be determined at a later date. The in-license agreements to which the Corporation is currently a party require the Corporation to maintain and defend the patent rights that we in-license against third parties.
Although the Corporation's current in-licenses are governed by the laws of Saskatchewan, Manitoba, Ontario and Canada, and Barbados, the enforcement of certain of them may necessitate pursuing legal proceedings and obtaining orders in other jurisdictions, including the United States and the Republic of Cuba. There can be no assurance that a court judgment or order obtained in one jurisdiction will be enforceable in another. In international venture undertakings it is standard practice to attorn to a neutral jurisdiction to seek remedy for unresolved commercial disputes. These arrangements are usually negotiated as part of the original business agreement. In the case of the out-license agreements by the Corporation, the parties have agreed that the proper laws of the contracts are variously, Ontario or Canada and England.
One of our products is licensed from a developing state. As is the case in many developing states, the commercial legal environment in Cuba is in a formative stage and may be subject to greater political risk. It is possible that the Corporation may not be able to enforce our legal rights in Cuba or against Cuban entities to the same extent as it would in a country with a more developed commercial and legal system. Termination of the Corporation's license arrangements or difficulties in enforcement of such arrangements could have a material adverse effect on the Corporation's ability to continue development of our licensed products.
As described under "Business of the Corporation - Licensing Arrangements", the Corporation has a number of license agreements with CIMAB. CIMAB is an institution of the Government of Cuba that purportedly operates at arms-length from the state bureaucracy with regard to its business, scientific and administrative decision-making. It is akin to a "crown corporation" in Canada. CIMAB's management is purportedly both autonomous and responsible for the success of their business decisions. Despite the fact that CIMAB's management is purportedly both autonomous and responsible for business decisions and that the license agreements with the Corporation declare Ontario as proper law, because of the fact that CIMAB is a state-owned entity, the Corporation will not be able to force CIMAB to comply with any judgment if CIMAB or the Government of Cuba refuses to comply.
WE HAVE ADVANCED FUNDS TO OUR JOINT VENTURE SUBSIDIARIES WHICH WE ARE ONLY ENTITLED TO RECOVER WHEN THE JOINT VENTURE'S NET INCOME EXCEEDS THE AMOUNT OF CUMULATIVE ADVANCES.
YM and CIMAB entered into a funding agreement with CIMYM in November 1995 (the "Funding Agreement") in connection with the 1995 CIMYM License. The Funding Agreement provides that YM will arrange for the appropriate studies and clinical trials for the licensed products held by CIMYM and will fund the cost of such studies and trials provided that doing so would not be commercially or scientifically unreasonable. Accordingly, YM makes the final determination as to whether or not a clinical trial expense is justified with respect to any given product.
-14-
CIMYM (Barbados) was incorporated in Barbados in May 1996 to market the licensed products under the 1995 CIMYM License outside of Canada. YM provides funding to CIMYM (Barbados) under similar terms and conditions as funding to CIMYM, except that while CIMYM has a payable outstanding for the amounts advanced by YM to CIMYM, CIMYM (Barbados) has issued redeemable preferred shares to YM for the amounts advanced to CIMYM (Barbados).
YM is entitled to be reimbursed for all funds we provide pursuant to the Funding Agreement out of revenue generated from the exploitation of the CIMYM License, subject to the successful development of the licensed products and adequate generation of revenue. There can be no assurance, however, that the Corporation will be able to recover the advances, as the Corporation is not entitled to recover such advances unless and until the joint venture's net income exceeds the amount of the cumulative advances.
As at June 30, 2005, YM has advanced $26.7 million to CIMYM and CIMYM (Barbados), collectively. Accordingly, the Corporation has set up a reserve in full against the other joint venture partners share of the advances. All advances have been expensed and, therefore, any reimbursement of such advances would be considered to be income by the Corporation.
WE ARE RELIANT ON LICENSORS FOR RESEARCH ON NEW PRODUCTS.
The Corporation does not conduct our own basic research with respect to the identification of new products. Instead, the Corporation relies upon research and development work conducted by others as a primary source for new products. While the Corporation expects that we will be able to continue to identify licensable products or research suitable for licensing and commercialization by the Corporation, there can be no assurance that this will occur.
WE CONDUCT OUR BUSINESS INTERNATIONALLY AND ARE SUBJECT TO LAWS AND REGULATIONS OF SEVERAL COUNTRIES WHICH MAY AFFECT OUR ABILITY TO ACCESS REGULATORY AGENCIES AND MAY AFFECT THE ENFORCEABILITY AND VALUE OF OUR LICENSES.
The Corporation has conducted clinical trials in more than 20 countries including Canada, the United Kingdom, India, Russia and the United States and intends to, and may, conduct future clinical trials in these and other jurisdictions. There can be no assurance that any sovereign government, including Canada's, will not establish laws or regulations that will be deleterious to the interests of the Corporation. There is no assurance that the Corporation, as a foreign corporation, will continue to have access to the regulatory agencies in any jurisdiction where we might want to conduct clinical trials or obtain final regulatory approval, and there can be no assurance that the Corporation will be able to enforce our licenses in foreign jurisdictions. Governments have, from time to time, established foreign exchange controls which could have a material adverse effect on the Corporation and our financial condition, since such controls may limit the Corporation's ability to flow funds into a country to meet our obligations under in-licensing agreements and to flow funds out of a country which the Corporation may be entitled to, in the form of royalty and milestone payments, under out-licensing agreements. In addition, the value of the Corporation's licenses will depend upon no punitive or prohibitive legislation in respect of biological materials.
WE ALSO CONDUCT OUR BUSINESS INTERNATIONALLY IN THAT WE CURRENTLY LICENSE PRODUCTS AND TECHNOLOGIES FROM SOURCES IN CANADA AND CUBA. WE HAVE PREVIOUSLY, AND INTEND TO, AND MAY, LICENSE PRODUCTS FROM SOURCES IN OTHER JURISDICTIONS.
The Corporation has licensed products, namely TheraCIM and RadioTheraCIM, from an academic institute in Cuba, namely CIM. The United States has maintained an embargo against Cuba, administered by Treasury. The laws and regulations establishing the embargo have been amended from time to time, most recently by the passage of the Cuban Liberty and Democratic Solidarity Act (the "Helms-Burton Bill"). The embargo applies to almost all transactions involving Cuba or Cuban enterprises, and it bars from such transactions any US persons unless such persons obtain specific licenses from Treasury authorizing their participation in the transactions. There is Canadian legislation (the Foreign Extraterritorial Measures Act) which provides generally that judgments against Canadian companies under the Helms-Burton Bill will not be enforced in Canada. The US embargo could have the effect of limiting the Corporation's access to US capital, US finance, US customers and US suppliers. In particular, the Corporation's products licensed from Cuban sources, noted above, are likely to be prohibited from sale in the United States unless Treasury issues a license or the embargo is lifted.
-15-
The Corporation's licensed rights to the TGFα Vaccine and the HER-1 Vaccine are suspended under the terms of the out-licensing agreement between the Corporation, CIMYM (Barbados), CIMAB and Tarcanta relating to Tarcanta licensing TGFα and HER-1 from CIMAB. In connection with the out-licensing agreement, CancerVax has announced that it has received a license from Treasury authorizing Tarcanta to enter into the transactions with CIMAB and the Corporation. See "Business - Licensing Arrangements - Out-Licensing" and see "Business - Licensing Arrangements - In-Licensing - Licenses for TheraCIM, RadioTheraCIM, TGFα and HER-1".
The Helms-Burton Bill authorizes private lawsuits for damages against anyone who "traffics" in property confiscated, without compensation, by the Government of Cuba from persons who at the time were, or have since become, nationals of the United States. The Corporation does not own any real property in Cuba and, to the best of the Corporation's knowledge, and based upon the advice of the Cuban government, none of the properties of the scientific centers of the licensors from where the licensed products were developed and are or may be manufactured was confiscated by the Government of Cuba from persons who at the time were, or have since become, nationals of the United States. However, there can be no assurance that the Corporation's understanding in this regard is correct. The Corporation does not intend to traffic in confiscated property.
Risks Related To Our Financial Results And Need For Financing
WE MAY BE A "PASSIVE FOREIGN INVESTMENT COMPANY" WHICH COULD RESULT IN ADVERSE US TAX CONSEQUENCES FOR US INVESTORS.
The Corporation may be deemed to be a "Passive Foreign Investment Company" ("PFIC"). A PFIC is a non-US corporation that meets an income test and/or an asset test. The income test is met if 75% or more of a corporation's gross income is "passive income" (generally, dividends, interest, rents, royalties, and gains from the disposition of assets producing passive income) in any taxable year. The asset test is met if at least 50% of the average value of a corporation's assets produce, or are held for the production of, passive income. Based on our current income, assets and activities, the Corporation may be a PFIC. See "United States Federal Income Tax Considerations - US Holders". As a result, a US Holder of the Corporation's common shares could be subject to increased tax liability, possibly including an interest charge, upon the sale or other disposition of the US Holder's common shares or upon the receipt of "excess distributions".
WE MAY NOT BE ABLE TO OBTAIN NECESSARY FUNDING FROM SALES OR LICENSE FEES OR ROYALTIES AND, AS A RESULT, MAY NEED TO TRY TO OBTAIN FUTURE CAPITAL THROUGH THE PUBLIC MARKET OR PRIVATE FINANCING WHICH MAY NOT BE AVAILABLE ON ACCEPTABLE TERMS.
The Corporation may require additional funding for the commercialization of our products, licensed and owned, and we will require additional funds if new products are licensed or acquired and put into development. The amount of additional funding required depends on the status of each project or new opportunity at any given time. The Corporation's business strategy is to in-license rights to promising drug products, further develop those products by progressing the products toward regulatory approval by conducting and managing clinical trials, and finally to out-license rights to manufacture and/or market resulting drug products to other pharmaceutical firms in exchange for royalties and license fees. Due to the in- and out-licensing arrangements and the Corporation's dependence on others for the manufacture, development and sale of our in-licensed products, the Corporation does not have consistent monthly or quarterly expenditures and cannot determine the amount and timing of required additional funding with any certainty. As at June 30, 2005 the Corporation had cash and short-term deposits totaling $30,568,845 and payables of $3,825,615. Management expects that the current cash reserves will be sufficient to fund the Corporation's development program beyond the fiscal year ending June 30, 2006.
-16-
The Corporation assesses our additional funding needs on a project-by-project basis from time-to-time. To the extent that the Corporation is unable to fund our expenditures from sales, license fees and royalties, it may be necessary to reconsider the continuation of existing projects or entering into new projects, or require the Corporation to access either the public markets or private financings whenever conditions permit. In addition, the Corporation has no established bank financing arrangements and there can be no assurance that the Corporation will be able to establish such arrangements on satisfactory terms. Such financing, if required and completed, may have a dilutive effect on the holders of our common shares. There is no assurance that such financing will be available if required, or that it will be available on favorable terms.
OUR OPERATING RESULTS AND STOCK PRICE MAY FLUCTUATE SIGNIFICANTLY.
The trading price of the Corporation's common shares, as with many emerging biopharmaceutical companies, is likely to be highly volatile. Factors such as the efficacy of the Corporation's products or the products of the Corporation's competitors, announcements of technological innovations by the Corporation or our competitors, governmental regulations, developments in patents or other proprietary rights of the Corporation, our licensors or our competitors, litigation, fluctuations in the Corporation's operating results, the Corporation being thinly capitalized, market conditions for biopharmaceutical stocks and general market and economic conditions could have a significant impact on the future trading price of the common shares. In addition, the Corporation's common shares are highly volatile since it may take years before any of our licensed products will receive final regulatory approval to be marketed in Canada, the United States or other territories.
Since September 30, 2004, our common shares have been listed for trading on the American Stock Exchange. However, there can be no assurance that an active trading market in our shares in the US will be sustained. Similarly, there can be no assurance that an active trading market in our shares on the TSX or AIM will be sustained.
Risks Related To Our Industry
IF OUR PRE-CLINICAL AND CLINICAL TESTING OF DRUG PRODUCTS DO NOT PRODUCE SUCCESSFUL RESULTS, WE WILL NOT BE ABLE TO COMMERCIALIZE OUR PRODUCTS.
Each of the Corporation's products, licensed or owned, must be subjected to additional pre-clinical and/or clinical testing in order to demonstrate the safety and efficacy of the Corporation's products in humans. The Corporation's ability to commercialize our products will depend on the success of currently ongoing pre-clinical and clinical trials and subsequent pre-clinical and clinical trials that have not yet begun.
The Corporation is not able to predict the results of pre-clinical and clinical testing of drug products, including the Corporation's products. It is not possible to predict, based on studies or testing in laboratory conditions or in animals, whether a drug product will prove to be safe or effective in humans.
In addition, success in one stage of testing is not necessarily an indication that the particular drug product will succeed in later stages of testing and development. There can be no assurance that the pre-clinical or clinical testing of the Corporation's products will yield satisfactory results that will enable the Corporation to progress toward commercialization of such products. Unsatisfactory results may cause material adverse affects on the Corporation's business, financial condition or results of operations as it could result in the Corporation having to reduce or abandon future testing or commercialization of particular drug products.
-17-
IF OUR COMPETITORS DEVELOP AND MAINTAIN THEIR TECHNOLOGICAL CAPABILITIES BETTER THAN THE CORPORATION, WE MAY NOT BE ABLE TO REMAIN COMPETITIVE IF DEFICIENCIES IN OUR TECHNOLOGICAL CAPABILITIES DELAY PRE-CLINICAL AND CLINICAL TRIALS OF OUR PRODUCTS.
The Corporation's success depends in part on developing and maintaining a competitive position in the development and commercialization of our products, licensed or owned, and technological capabilities in our areas of expertise. The biotechnology and pharmaceutical industries are subject to rapid and substantial technological change. While the Corporation will seek to expand our technological capabilities in order to remain competitive, there can be no assurance that developments by others will not render the Corporation's products non-competitive or that the Corporation or our licensors will be able to keep pace with technological developments. Competitors have developed technologies that could be the basis for competitive products. Some of those products may have an entirely different approach or means of accomplishing the desired therapeutic effect than the Corporation's products and may be more effective or less costly than the Corporation's products. In addition, other forms of medical treatment may offer competition to the products. The Corporation's technological capabilities and competitiveness and the success of the Corporation's competitors and their products and technologies, could have a material adverse impact on the future pre-clinical and clinical trials of the Corporation's products, including the Corporation's ability to obtain the necessary regulatory approvals for the conduct of such clinical trials.
IF OUR COMPETITORS DEVELOP AND MARKET PRODUCTS THAT ARE MORE EFFECTIVE THAN OUR EXISTING PRODUCT CANDIDATES OR ANY PRODUCTS THAT WE MAY DEVELOP, OR OBTAIN MARKETING APPROVAL BEFORE WE DO, OUR PRODUCTS MAY BE RENDERED OBSOLETE OR UNCOMPETITIVE.
Technological competition from pharmaceutical companies, biotechnology companies and universities is intense and is expected to increase. Many competitors and potential competitors of the Corporation have substantially greater product development capabilities and financial, scientific, marketing and human resources than the Corporation. The Corporation's future success depends in part on our ability to maintain a competitive position, including our ability to further progress our products, licensed or owned, through the necessary pre-clinical and clinical trials towards regulatory approval for sale and commercialization. Other companies may succeed in commercializing products earlier than the Corporation is able to commercialize our products or in developing products that are more effective than our products. The Corporation considers its main competitors to be: Genentech, Inc. ("Genentech"), Lorus Therapeutics Inc., ISIS Pharmaceuticals and Eli Lilly and Company with respect to tesmilifene; Aphton Corporation ("Aphton"), TAP Pharmaceuticals and AstraZeneca PLC ("AstraZeneca") with respect to NorelinTM; and Abgenix Inc. ("Abgenix"), Amgen Inc. ("Amgen"), Genmab A/S ("Genmab"), ImClone Systems Inc. ("ImClone"), Bristol-Myers Squibb Company ("BMS"), Merck KGaA ("Merck"), OSI Pharmaceuticals, Inc. ("OSI"), F.Hoffmann-LaRoche Ltd. ("Roche"), Genentech and AstraZeneca with respect to TheraCIM and RadioTheraCIM. The main competitors for the AeroLEFTM product are Cephalon, Inc. (“Cephalon”), Endo Pharmaceuticals Holdings Inc. (“Endo”), LAB International Inc. (“LAB”), Alexza Molecular Delivery Corporation (“Alexza”), Aradigm Corporation (“Aradigm”), Barr Pharmaceuticals, Inc. (“Barr”), CeNeS Pharmaceuticals plc (“CeNeS”) and Alza Corporation (“Alza”).
While the Corporation will seek to expand our technological capabilities in order to remain competitive, there can be no assurance that research and development by others will not render our products obsolete or uncompetitive or result in treatments or cures superior to our products, or that our products will be preferred to any existing or newly developed technologies.
WE ARE SUBJECT TO EXTENSIVE GOVERNMENT REGULATION THAT INCREASES THE COST AND UNCERTAINTY ASSOCIATED WITH GAINING FINAL REGULATORY APPROVAL OF OUR PRODUCT CANDIDATES.
Securing final regulatory approval for the manufacture and sale of human therapeutic products in Canada and the Corporation's other territories, including the United States, is a long and costly process that is controlled by that particular territory's national regulatory agency. The national regulatory agency in Canada is Health Canada ("Health Canada"), and in the United States it is the United States Health and Human Services Food and Drug Administration ("FDA"). See "Regulatory Approvals" for a description of approval processes in Canada and the United Sates. Other national regulatory agencies have similar regulatory approval processes, but each national regulatory agency has its own approval processes. Approval in either Canada or the United States does not assure approval by other national regulatory agencies, although often test results from one country may be used in applications for regulatory approval in another country.
-18-
Prior to obtaining final regulatory approval to market a drug product, every national regulatory agency has a variety of statutes and regulations which govern the principal development activities. These laws require controlled research and testing of products, government review and approval of a submission containing pre-clinical and clinical data establishing the safety and efficacy of the product for each use sought, approval of manufacturing facilities including adherence to GMP during production and storage, and control of marketing activities, including advertising and labeling.
None of the Corporation's products have been completely developed or tested and, therefore, we are not yet in a position to seek final regulatory approval to market any of our products, licensed or owned. To date we have obtained various regulatory approvals to develop and test our products. Currently the Corporation is conducting an international Phase III trial of tesmilifene in metastatic and recurrent breast cancer in 700 patients. The Corporation has received regulatory approvals for the tesmilifene study in several countries, including Canada and the United States, and approval is pending in a few other countries. In addition, TheraCIM has been approved for testing in Canada and Europe and has been designated an orphan drug in Europe and by the FDA in the United States. Finally, DELEX has conducted Phase I and II trials in Canada of AeroLEFTM. See "Products in Clinical Development".
Two of the Corporation's products, namely TheraCIM and RadioTheraCIM which are being developed in Canada and Europe are also being separately developed or tested in Cuba. Cuba is among several nations which have been identified by the US Department of State as being a state sponsoring terrorism and as such the US Government has put in place certain anti-terrorism controls against Cuba. Although as of the date of this filing such anti-terrorism controls have not had any adverse affect on the operations of the Corporation, because of the anti-terrorism controls and the Helms-Burton Bill there is no assurance that the Corporation will be able to successfully complete clinical testing in the United States and obtain final regulatory approval or a license from OFAC in order to successfully commercialize our Cuban sourced products in that jurisdiction. There can be no assurance that the licensed products will be successfully commercialized. The process of completing clinical testing and obtaining final regulatory and other US government approvals to market the licensed products is likely to take a number of years for most of the licensed products and require the expenditure of substantial resources. Any failure to obtain, or a delay in obtaining, such approvals could adversely affect the Corporation's ability to develop the product and delay commercialization of the product. Further, there can be no assurance that the Corporation's licensed products will prove to be safe and effective in clinical trials under the standards of the regulations in the Corporation's territories or receive applicable regulatory approvals from applicable regulatory bodies.
CHANGES IN GOVERNMENT REGULATIONS ALTHOUGH BEYOND OUR CONTROL COULD HAVE AN ADVERSE EFFECT ON OUR BUSINESS.
The Corporation has, or has had, licenses with, or clinical trials at, various academic organizations, hospitals and companies in Canada, Cuba, Italy, the United States and the United Kingdom and numerous other countries and depends upon the validity of our licenses and access to the data for the timely completion of clinical research in those jurisdictions. Any changes in the drug development regulatory environment or shifts in political attitudes of a government are beyond the control of the Corporation and may adversely affect our business.
Two of the Corporation's products, namely TheraCIM and RadioTheraCIM which are being developed in Canada and Europe are also being separately developed or tested in Cuba. Cuba is among several nations which have been identified by the US Department of State as being a state sponsoring terrorism and as such the US Government has put in place certain anti-terrorism controls against Cuba. Although as of the date of this filing such anti-terrorism controls have not had any adverse affect on the operations of the Corporation, because of the anti-terrorism controls and the Helms-Burton Bill there is no assurance that the Corporation will be able to successfully complete our clinical testing and obtain final regulatory approval in order to successfully commercialize our Cuban sourced products.
The business of the Corporation may also be affected in varying degrees by such factors as government regulations with respect to intellectual property, regulation or export controls. Such changes remain beyond the control of the Corporation and the effect of any such changes cannot be predicted.
These factors could have a material adverse effect on the Corporation's ability to further develop our licensed products.
-19-
Risks Related To Intellectual Property And Litigation
OUR SUCCESS DEPENDS UPON OUR ABILITY TO PROTECT OUR INTELLECTUAL PROPERTY AND OUR PROPRIETARY TECHNOLOGY.
The Corporation's success will depend, in part, on the ability of the Corporation and our licensors to obtain patents, maintain trade secrets protection, and operate without infringing on the proprietary rights of third parties or having third parties circumvent the Corporation's rights. Certain licensors and the institutions that they represent, and in certain cases, the Corporation on behalf of the licensors and the institutions that they represent, have filed and are actively pursuing certain applications for Canadian and foreign patents. The patent position of pharmaceutical and biotechnology firms is uncertain and involves complex legal and financial questions for which, in some cases, important legal principles are largely unresolved. There can be no assurance that the patent applications made in respect of the licensed products will result in the issuance of patents, that the term of a patent will be extendable after it expires in due course, that the licensors or the institutions that they represent will develop additional proprietary products that are patentable, that any patent issued to the licensors or the Corporation will provide the Corporation with any competitive advantages, that the patents of others will not impede the ability of the Corporation to do business or that third parties will not be able to circumvent the patents obtained in respect of the licensed products. The cost to the Corporation of obtaining and maintaining patents is high. Furthermore, there can be no assurance that others will not independently develop similar products which duplicate any of the licensed products, or, if patents are issued, design around the patent for the product. There can be no assurance that processes or products of the Corporation's licensors or the Corporation do not or will not infringe upon the patents of third parties, or that the scope of patents issued to the Corporation's licensors or the Corporation will successfully prevent third parties from developing similar and competitive products.
Much of the Corporation's know-how and technology may not be patentable, though they may constitute trade secrets. There can be no assurance, however, that the Corporation will be able to meaningfully protect our trade secrets. To help protect our rights, the Corporation requires employees, consultants, advisors and collaborators to enter into confidentiality agreements. There can be no assurance that these agreements will provide meaningful protection for the Corporation's trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure.
Further, the Corporation's business may be materially adversely affected by competitors who independently develop competing technologies, especially if no patents or only narrow patents are obtained by the Corporation in respect of our licensed products.
The Corporation maintains patents in connection with tesmilifene, NorelinTM, TheraCIM and AeroLEFTM. The following is a description of the Corporation's key current and pending patents in connection with these drug products.
TESMILIFENE
YM is the exclusive licensee to patents and patent applications from the University of Manitoba for tesmilifene. Patents that claim the use of tesmilifene in combination with chemotherapeutic agents have issued in the United States, Europe, Japan, Canada and Australia. US patent 5,859,065 broadly claims the use of tesmilifene and structurally related analogs in combination with any chemotherapeutic for the treatment of any cancer. Although the twenty-year term of this patent expires in December 2010, YM plans to take full advantage of patent terms extensions of up to five additional years granted under the Patent Term Restoration Act in the United States. Other issued patents US 6,284,799 and US 5,747,543 expire in 2014 and 2015 respectively.
In addition to these granted patents, YM is also exclusively licensed to patent applications relevant to the current clinical development program. Patent applications, based upon WO 03/039526 and WO 03/037318, have been nationalized not only in the United States, Western Europe, and Japan but also in emerging markets, including China, India, Asia, and Eastern Europe. These international patent applications claim the use of tesmilifene in patient subpopulations that benefit from the chemopotentiating and cytoprotective properties of the drug. Patents resulting from these patent filings will expire in November 2022.
-20-
In addition to patent protection, YM intends to rely upon the available term of data exclusivity in the US and other countries for an NCE. Furthermore, full advantage will be taken of the Orange Book provisions in the United States and equivalent provision in Canada and other countries, as a means for delaying generic competition.
NORELINTM
YM has a license to human therapeutic applications of this GnRH vaccine based on a leukotoxin-derived but non-leukotoxic carrier protein, to which multimeric units of GnRH are coupled at each flank. By eliciting an antibody response to GnRH, NorelinTM is designed to block GnRH from reaching its receptors in the pituitary gland.
The NorelinTM patent estate is extensive, and includes four key US patents covering various aspects of NorelinTM as a composition of matter, the carrier component of the NorelinTM vaccine, as well as production of NorelinTM as a recombinant product. A key US patent is US 5,837,268, which covers the particular NorelinTM sequence, its formulation as a vaccine, and its end-use, and subject to any term restoration, will expire in 2012. Other key US patents are US 5,422,110; US 5,708,155; and US 5,837,268. All of the key patents are owned by the University of Saskatchewan and licensed to YM, through Biostar (See "Licensing Arrangements").
In addition, YM has more recently applied for our own patents covering the NorelinTM formulation and dosing regimen that was the subject of our clinical trials. Patents resulting from these applications will not expire until 2024.
The Corporation is aware of US patent #6,303,123 owned by Aphton relating to the use of GnRH immunogenic conjugates to treat GnRH-dependent diseases, including prostatic hypertrophy, and is developing a strategy for addressing this patent should it prove relevant to the Corporation's commercial activities with NorelinTM.
There can be no assurance that litigation or other proceedings will not be commenced seeking to challenge patent protection or patent applications of the Corporation's licensors, or that any such challenges will not be successful. The cost of litigation to uphold the validity and prevent infringement of patents related to the Corporation's licensed drug products may be significant. In addition, it is possible that others may claim rights in YM's licensed drug products, patents or patent applications. These other persons could bring legal actions against the Corporation, our licensors or our customers or licensees claiming damages and seeking to enjoin them from using, manufacturing and marketing the affected products or processes. If any such action were successful, in addition to any potential liability for damages, the Corporation could be required to obtain a license in order to continue to develop, use, license or market the affected product or process. There can be no assurance that the Corporation would prevail in any such action or that any required license would be made available or, if available, would be available on acceptable terms.
THERACIM
CIMYM is the exclusive licensee for particular territories including the United States under a patent estate that includes composition of matter coverage for Nimotuzumab, and further includes coverage for TheraCIM-based formulations and end-uses in the treatment of EGFR-dependent cancers. The composition of matter patents are granted in the United States, in Europe, are allowable in Japan, and are pending in Canada.
CIMYM's key US patent, US 5,891,996 expires in November 2015, and term extensions of up to five years may be available under the Patent Term Restoration Act. The same term and extension apply also to the key European patent, EP 712863.
The Corporation is aware of US patent #5,770,195 granted to Genentech, Inc. ("Genentech"), for the anti-cancer use of EGFr MAbs in combination with a cytotoxic agent. The Corporation is also aware of US patents granted to others in this field. In April 2001 Rorer International (Overseas) ("Rorer") was issued the US patent #6,217,866 which includes claims to any antibody targeting the EGFr administered with any anti-neoplastic agent. A counterpart patent has been granted in Europe. The Corporation has filed an opposition to the grant of the European patent. The Corporation believes that the Rorer patents are licensed to ImClone. Management is aware that inventorship of the Rorer patent is currently being challenged. In the event that the challenge is successful , management believes we will be able to obtain licenses under such patents on commercially reasonable terms, though there can be no assurance thereof. In addition, the Corporation is aware of a separate series of national patent applications filed by ImClone, and represented by EP1080113, claiming the anti-cancer use of radiation in combination with any inhibitor of any receptor tyrosine kinase that is involved in the genesis of tumors. ImClone is also reported to have filed a PCT application covering the use of EGFr MAbs to treat patients having tumors that do not respond to treatment with conventional therapies. The Corporation is also challenging ImClone's claims in respect of the radiation-related patent applications by having filed additional prior art at the relevant national patent offices. The outcome of these challenges cannot be predicted, and there can be no assurance that the Corporation will succeed in challenging the validity or scope of patent claims by ImClone or any other patent applicant.
-21-
The manufacturing of TheraCIM may fall within the scope of process patents owned by Protein Design Labs Inc., Genentech, and the Medical Research Council of the United Kingdom. Management is aware that some of these process patents are currently being challenged by companies other than YM. In the event any of the applicable process patents are upheld, management believes we will be able to obtain licenses under such patents on commercially reasonable terms, though there can be no assurance thereof.
There may also be risks related to TheraCIM and the Corporation's other licenses for drug products originating from Cuba, namely RadioTheraCIM, TGFα Vaccine and HER-1 Cancer Vaccine. Cuba is a socialist country and, under the current patent law, ownership of the inventions of the Cuban inventors for which patent applications have been filed rests with the State. The material license agreements for the Corporation's Cuban sourced products are as follows:
| (a) | License Agreement between CIMYM Inc. and CIMAB SA, January 24, 2001 with respect to TGFα and HER-1, which agreement has been suspended in accordance with the terms of the Tarcanta out-licensing agreement (See "Business - Licensing Agreements - Out-Licensing"); and |
| (b) | License Agreement between YM BioSciences Inc. (formerly known as Yorkton Medical Inc.) and CIMAB SA, dated May 3, 1995, as amended, with respect to TheraCIM and RadioTheraCIM. |
The above listed license agreements are more fully described under the heading "Business Overview - Licensing Arrangements - In-Licensing".
AEROLEFTM
The AeroLEFTM product is described in four patent families. DELEX owns key patents, expiring in 2014, claiming a method of administering systemic analgesia by inhaling free and liposome-encapsulated opiod analgesic. North American coverage includes a reissued US patent and a pending application in Canada. DELEX owns two US applications with counterpart PCT applications, expiring in 2024, claiming the formulation for use in a method comprised of continuously inhaling the formulation to deposit at least one rapid-onset opiod and one sustained-effect opiod in the lungs. A pending PCT application entitled “Stable Compositions” claims the liposomal composition and other physical characteristics.
The Corporation is aware of US patents owned by Phares Pharmaceutical Research NV related to liposome compositions. These patents expire in 2008 and are not expected to hamper the Corporation’s commercial activities.
THE CORPORATION'S POTENTIAL INVOLVEMENT IN INTELLECTUAL PROPERTY LITIGATION COULD NEGATIVELY AFFECT THE CORPORATION'S BUSINESS.
The Corporation's future success and competitive position depend in part upon the Corporation's ability to maintain our intellectual property portfolio. There can be no assurance that any patents will be issued on any existing or future patent applications. Even if such patents are issued, there can be no assurance that any patents issued to or licensed to the Corporation will not be challenged. The Corporation's ability to establish and maintain a competitive position may be achieved in part by prosecuting claims against others who the Corporation believes are infringing the Corporation's rights and by defending claims brought by others who believe that we are infringing their rights. In addition, enforcement of the Corporation's patents in foreign jurisdictions will depend on the legal procedures in those jurisdictions. Even if such claims are found to be invalid, the Corporation's involvement in intellectual property litigation could have a material adverse effect on our ability to out-license any products that are the subject of such litigation. In addition, the Corporation's involvement in intellectual property litigation could result in significant expense to it, which could materially adversely affect the use or licensing of related intellectual property and divert the efforts of the Corporation's valuable technical and management personnel from their principal responsibilities, whether or not such litigation is resolved in the Corporation's favor.
-22-
PRODUCT LIABILITY CLAIMS ARE AN INHERENT RISK OF OUR BUSINESS, AND IF OUR CLINICAL TRIAL AND PRODUCT LIABILITY INSURANCE PROVE INADEQUATE, PRODUCT LIABILITY CLAIMS MAY HARM OUR BUSINESS.
Human therapeutic products involve an inherent risk of product liability claims and associated adverse publicity. The Corporation currently maintains clinical trial liability insurance with an ultimate net loss value of up to $5 million per claim and a policy aggregate of $10 million. The Corporation currently has no other product liability insurance and there can be no assurance that we will be able to obtain or maintain product liability insurance on acceptable terms or with adequate coverage against potential liabilities. Such insurance is expensive, difficult to obtain and may not be available in the future on acceptable terms, or at all. An inability to obtain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims could have a material adverse effect on the Corporation by preventing or inhibiting the commercialization of our products, licensed and owned, if a product is withdrawn or a product liability claim is brought against the Corporation.
Risks Related to Being A Canadian Entity For Foreign Investors
AS WE ARE A CANADIAN COMPANY, THERE MAY BE LIMITATIONS ON THE ENFORCEMENT OF CERTAIN CIVIL LIABILITIES AND JUDGMENTS OBTAINED IN THE UNITED STATES AGAINST US.
The Corporation is incorporated under the laws of the province of Nova Scotia, Canada, and our assets are located outside of the United States. Many of the Corporation's directors and officers and certain of the experts named elsewhere in this annual information form are residents of Canada. All or a substantial portion of our assets and the assets of these persons are located outside of the United States. As a result, it may be difficult for shareholders to enforce a US judgment in Canada or other non-US jurisdictions or to succeed in a lawsuit in a non-US jurisdiction based only on violations of US securities laws.
THE CORPORATION IS GOVERNED BY THE CORPORATE LAWS IN NOVA SCOTIA, CANADA, WHICH IN SOME CASES HAVE A DIFFERENT EFFECT ON SHAREHOLDERS THAN THE CORPORATE LAWS IN DELAWARE, UNITED STATES.
The material differences between the Nova Scotia Companies Act (“NSCA”) as compared to the Delaware General Corporation Law (“DGCL”) which may be of most interest to shareholders include the following: (i) for material corporate transactions (such as amalgamations, other extraordinary corporate transactions, amendments to the memorandum of association and amendments to the articles of association) the NSCA generally requires three-quarter majority vote by shareholders which in most instances requires a confirmatory resolution by a majority of the shareholders (and, in addition, especially where the holders a class of shares is being affected differently from others, approval will be required by holders of two-thirds of the shares of such class voting in a meeting called for the purpose), whereas DGCL generally only requires a majority vote of shareholders for similar material corporate transactions; (ii) quorum for shareholders meetings is not prescribed under the NSCA and is only 5% under the Corporation's articles of association, whereas under DGCL quorum is when the holders of a majority of the shares entitled to vote are present; and (iii) the Corporation's articles of association require a special resolution and the Corporations Miscellaneous Provisions Act (Nova Scotia) requires three-quarters of all shareholders entitled to vote to pass a resolution for one or more directors to be removed, whereas DGCL only requires the affirmative vote of a majority of the shareholders.
-23-
BUSINESS OVERVIEW
OVERVIEW
The Corporation is a biopharmaceutical company engaged in the development of products primarily for the treatment of patients with cancer. YM generally in-licenses substances designed for anti-cancer use in order to advance them along the regulatory and clinical pathways toward commercial approval. The Corporation's licenses generally cover the major market countries of the developed world (including Canada, the United States, Japan and Europe) or are world-wide. The Corporation uses our expertise to manage and perform what we believe are the most critical aspects of the drug development process which include the design and conduct of clinical trials, the development and execution of strategies for the protection and maintenance of intellectual property rights and the interaction with drug regulatory authorities internationally. YM concentrates on drug development and does not engage in drug discovery, avoiding the significant investment of time and capital that is generally required before a compound is identified clinical trials. YM has in-licensed certain preclinical products which have been related to its clinical programs. YM both conducts and out-sources clinical trials and out-sources the manufacture of clinical materials to third parties.
The Corporation's current portfolio of products in clinical development includes three anti-cancer agents (a small molecule, a vaccine and a monoclonal antibody) in a number of formulations currently targeting nine different tumors and/or stages of cancer as well as a proprietary inhalation delivery system for fentanyl to treat acute pain incluing cancer pain. The Corporation also has a financial interest in two additional anti-cancer immunotherapies in pre-clinical development. The Corporation intends to license the rights to manufacture and market our drug products to other pharmaceutical companies in exchange for license fees and royalty payments and to continue to seek other in-licensing opportunities in pursuing our business strategy. The Corporation does not currently intend to manufacture or market products although we may, if the opportunity is available on terms that are considered attractive, participate in ownership of manufacturing facilities or retain marketing or co-development rights to specific products.
BUSINESS STRATEGY
The Corporation is principally focused on development of products for the treatment of cancer or cancer-related conditions. YM's strategy is to license rights to promising products, further develop those products by conducting and managing clinical research and trials and progressing the products toward regulatory approval, and sub-license or out-license rights to manufacture and/or market resulting drug products to other pharmaceutical firms in exchange for royalties and license fees. The Corporation seeks to use our product development capabilities to bridge discoveries and research from scientific/academic institutions or other biopharmaceutical companies, on the one hand, with commercial manufacturing and marketing of biopharmaceutical products, on the other hand.
The main elements of the Corporation's business strategy are described below:
Identification of Product Candidates:. The Corporation directly performs scientific evaluation and market assessment of biopharmaceutical products and research developed by scientific/academic institutions and other biopharmaceutical companies. As part of this process, the Corporation evaluates the related scientific research and pre-clinical and clinical research, if any, and the intellectual property rights in such products and research, with a view to determining the therapeutic and commercial potential of the applicable product candidates.
In-Licensing: Upon identifying a promising biopharmaceutical product, the Corporation seeks to negotiate a license to the rights for the product from the holder of those rights, the developer or researcher. The terms of such licenses vary, but generally the Corporation's goal is to secure licenses that permit us to engage in further development, clinical trials, intellectual property protection (on behalf of the licensor or otherwise) and further licensing of manufacturing and marketing rights to any resulting products. This process of securing license rights to products is commonly known as "in-licensing".
-24-
Further Development: Upon in-licensing a cancer-related product, the Corporation's strategy is to apply our skills and expertise to progress the products toward regulatory approval and commercial production and sale in major markets. These activities include implementing intellectual property protection and registration strategies, performing or having performed for it, pre-clinical research and testing, the formulating or reformulating of drug products, making regulatory submissions, performing or managing clinical trials in target jurisdictions, and undertaking or managing the collection, collation and interpretation of clinical and field data and the submission of such data to the relevant regulatory authorities in compliance with applicable protocols and standards.
Out-Licensing: The Corporation generally plans to further license manufacturing and marketing rights to our licensed products to other pharmaceutical firms. This is commonly known as "out-licensing". Under the Corporation's business model, licensees would be expected, to the extent necessary, to participate in the remaining clinical development required to obtain final regulatory approval for the product. The Corporation expects that out-licensing would result in a pharmaceutical company or other licensee marketing or manufacturing the product in return for licensing fees in addition to royalties on any sales of the product. Management believes this model is consistent with current biotechnology and pharmaceutical industry licensing practices. In addition, although out-licensing is a primary strategy of the Corporation, the Corporation may retain co-development or marketing rights to particular products or territories. To date, the Corporation has out-licensed one of our products in certain European countries, our two anti-cancer pre-clinical products to two wholly-owned subsidiaries of a United States corporation and one of our products in South Korea. See "Business - Licensing Arrangements - Out-Licensing".
The Corporation actively searches for new product opportunities using the relationships of our management and advisory team and continuous monitoring of the academic and biotechnology environment in cancer treatment developments. The Corporation's staff analyses and evaluates opportunities and continuously reviews them. In addition, the Corporation has existing rights of first refusal in certain of our existing license agreements for certain additional products and extensions to existing products. The Corporation intends to seek other in-licensing opportunities in pursuing our business strategy.
CANCER AND CANCER THERAPEUTIC MARKET
According to Millennium Research Group, Inc. (June 2001) it was estimated that worldwide 10.1 million people are diagnosed with some type of cancer annually and in North America there were approximately 1.1 million new cases in 2000. The Millennium Research Group, Inc. projects that the incidence of new cases is likely to increase by 0.27% per year between 2000 and 2005. According to the American Cancer Society, in the United States cancer is the second leading cause of disease-related death, behind cardiovascular disease which it is predicted to surpass in the next few years. The principal reasons for this projection appear to be the aging population in more developed countries, environmental issues related to industrial development, and improvements in the treatment of cardiovascular disease. North America, Europe and Japan are the principal markets for cancer therapies because of the established healthcare and payor systems.
The principal types of cancer in the United States, accounting for approximately 55% of the incidence of all cancers, based on management's analysis, are prostate (17%), breast (16%), lung (13%) and colorectal (8%). These four types of cancer are also responsible for the highest combined mortality, accounting for approximately 50% of all cancer deaths in the United States. Bladder, ovarian, brain and oral cancer, as well as lymphoma, leukemia and melanoma account for the majority of the balance of cancer deaths. The incidence of a particular cancer varies greatly between continents, principally because of diet and habit.
Surgery, radiation and chemotherapy remain the principal effective treatments for cancer. Although there is an ongoing debate about the value of chemotherapeutics with regards to prolongation of life, their palliative value has resulted in significant improvements in quality of life for cancer sufferers. In addition, although the reason is not clearly understood, current cancer drugs are effective in only a subpopulation of individuals with the same disease. Notwithstanding this, revenues in the global oncology market were reported to be approximately US$20 billion in 2003 (see http://www.bioscorpio.com/dmhc1926.htm), and are expected to increase to over US$45 billion by 2011. The use of cancer therapies is forecast to increase as diagnostic methods improve (as already demonstrated in prostate cancer) and, particularly, as more effective treatments are developed.
-25-
Numerous new approaches to cancer are currently in clinical trials. As targets become validated and technologies improve, research is beginning to yield therapeutic approaches that appear to be more effective than existing ones. Monoclonal antibodies were first described in 1978, and are now beginning to yield commercially viable therapeutic products, such as Rituxan(R), the first monoclonal treatment for cancer, approved by the FDA in 1998. The Corporation is aware of only five naked monoclonal antibodies approved in the United States for the treatment of cancer, Rituxan(R), Campath(R), Herceptin(R), Avastin(R) and Erbitux(R) although many more are in development. A second approach to cancer treatment, therapeutic cancer vaccines, has been under development for many years, and the first such vaccine, Melacine(R), was approved in 1999 in Canada.
The Corporation is also developing a novel formulation of the opiod, fentanyl, for the treatment of severe and moderate pain, including cancer pain. In the United States cancer pain is suffered by more than 1.5 million patients. We are aware of numerous other companies that are pursuing approaches to the delivery of fentanyl including Cephalon, Inc. (“Cephalon”), Endo Pharmaceuticals Holdings Inc. (“Endo”), LAB International Inc. (“LAB”), Alexza Molecular Delivery Corporation (“Alexza”), Aradigm Corporation (“Aradigm”), Barr Pharmaceuticals, Inc. (“Barr”), CeNeS Pharmaceuticals plc (“CeNeS”) and Alza Corporation (“Alza”).
PRODUCTS IN CLINICAL DEVELOPMENT
The Corporation’s current portfolio of products in clinical development includes three anti-cancer agents (a small molecule, a vaccine and a monoclonal antibody) in a number of formulations targeting eight different tumors and/or stages of cancer as well as product for the treatment of pain, including cancer pain (AeroLEFTM). The Corporation has also out-licensed two additional anti-cancer immunotherapies in pre-clinical development. A number of the Coporation’s products involve newer approaches to the treatment of cancer and include two formulations of a monoclonal antibody, TheraCIM and RadioTheraCIM, and an anti-cancer therapeutic vaccine, NorelinTM, currently in clinical development as well as two anti-cancer therapeutic vaccines, HER-1 and TGFα, in preclinical development. The Corporation's lead product, tesmilifene, is a chemical that has been clinically reported to enhance the activity of certain known chemotherapeutics. The Corporation's drug products target some of the most common cancer indications, including breast, prostate (early-stage as well as metastatic disease), and non-small cell lung cancer. YM is also pursuing several smaller cancer indications including head-and-neck cancer, brain cancers and certain indications with orphan drug designations. The Corporation expects, based on clinical trials done to date, to develop all of our clinical stage candidates beyond their respective initial indications.
TESMILIFENE
BACKGROUND:
Tesmilifene is a small molecule anti-cancer drug with multiple modes of action that appears to enhance the activity of traditional chemotherapy agents. Its chemical designation is N,N-diethyl-2-[4-(phenylmethyl)phenoxy]ethanamine hydrochloride. It has demonstrated synergistic effects with anthracyclines in late-stage clinical trials and with taxanes, 5-FU vinca alkaloids and platins in earlier-stage clinical and pre-clinical studies.
CLINICAL EXPERIENCE AND DEVELOPMENT PLANS:
Tesmilifene has been administered to more than 650 cancer patients and demonstrated to be well tolerated. The product has been approved by either or both of the FDA and Health Canada for use in numerous clinical trials including:
| (a) | Phase I/II study of tesmilifene alone and in combination with doxorubicin in patients with metastatic and recurrent breast cancer; |
| (b) | Phase I/II study of tesmilifene in combination with various anti-neoplastic agents; |
| (c) | Phase I/ II study of tesmilifene in combination with cyclophosphamide in patients with hormone-refractory prostate cancer; |
| (d) | Phase II trial of tesmilifene plus doxorubicin in patients with metastatic and recurrent breast cancer; |
| (e) | Phase II pilot study of mitoxantrone/prednisone plus tesmilifene in patients with symptomatic hormone-refractory metastatic prostate cancer; |
-26-
| (f) | Phase II combination study of tesmilifene with doxorubicin and taxol in advanced breast cancer; |
| (g) | Randomized Phase III trial of tesmilifene plus doxorubicin in patients with metastatic and recurrent breast cancer; |
| (h) | Phase II combination study of tesmilifene with various taxanes in first-line metastatic and recurrent breast cancer; and |
| (i) | Randomized Phase III trial of tesmilifene plus epirubicin and cyclophosphamide in patients with metastatic and recurrent breast cancer. |
In October, 2003 the FDA provided clearance to the Corporation to initiate a Phase III trial, patient events design and endpoints of which were subject to a positive review by the FDA in March, 2003 under a process known as Special Protocol Assessment ("SPA"). An SPA is intended to provide official evaluation of, and agreement with, a protocol and endpoints to form the basis of a new drug application. In November, 2003 the Corporation received approval, from the FDA to apply an "adaptive design" to the pivotal trial for which the SPA had approved the protocol. This adaptive design, which in the case of YM's pivotal trial provides for "sequential analysis", permits the independent Data Safety Monitoring Board ("DSMB") to review the status of the patients in the trial and to conclude, at any point during the trial, whether the trial should be stopped because of sufficient evidence of the effect of tesmilifene; continued for the purpose of increasing the numbers of the patients in the trial; or stopped because of the absence of any effect (futility) of the drug in patients with metastatic and recurrent breast cancer. This sequential analysis can be applied at any point during the trial. The FDA has advised the Corporation that the first interim analysis of the data generated under this process that it is prepared to review to satisfy its requirements for approval may take place only after 192 patient events (deaths) have occurred in the patient population of the trial. Sequential analysis differs significantly from the classical trial design which requires enrollment of the full number of patients contemplated in the original protocol prior to which no review of the patients may take place except with a considerable statistical penalty being paid by the sponsor for the trial results. Under a sequential analysis a positive outcome would permit shortened time to approval, and thus to market.
The Corporation has initiated the above-mentioned international Phase III trial of tesmilifene in metastatic and recurrent breast cancer in 700 patients. The FDA has agreed that the trial may be stopped after 192 events have been recorded provided that certain results have been achieved, as determined by the DSMB.
In March 2004, the Corporation entered into a Clinical Research Services Agreement with Pharm-Olam International (“POI”), a clinical research organization ("CRO"), to conduct this Phase III trial internationally. POI in turn is contracting with others to perform services and to recruit and treat patients. The contract with POI is payable over the next few years and payments due are dependent on the number of patients recruited, number of countries trials are conducted in, the length of time over which particular clinical trials are to be conducted and the time for completion of all Phase III clinical trials. The Corporation is liable for certain payment of clinical services costs, data management costs and pass through costs. The agreement will terminate after POI has completed all services thereunder, if the parties mutually consent, or may be terminated by either party in the event of certain defaults by the other party. In the event the Corporation terminates the agreement without cause and prior to the study under the agreement being completed, then the Corporation must pay POI a termination fee of 10% of the remaining compensation, if any is still owed for clinical services costs and data management costs under the agreement at the time of the termination.
In January 2005, the Corporation and Shin Poong Pharmaceutical Company of Seoul, Korea (“Shin Poong”) formed a joint development team to oversee the expansion of the development program for tesmilifene into gastric cancer. Shin Poong will fully fund development costs and will provide up-front, milestone and royalty payments. In addition, Shin Poong expects to launch a bridging study in the local population in order to allow the breast cancer indication currently under study by the Corporation to be launched in Korea and other Asian countries.
-27-
In May 2005, the Corporation, through a subsidiary, partnered with Kuhnil to expand the development program for TheraCIM-hR3, its monoclonal antibody against the EGF receptor, for a specific population of patients with non-small cell lung cancer.
The SouthWest Oncology Group, a US National Cancer Institute-supported cancer clinical trials cooperative group, advised the Corporation that it proposes to undertake a randomized Phase III trial in which tesmilifene is expected to be combined with mitoxantrone (Novantrone(R))/prednisone and compared to results in patients to be treated with taxotere/estramustine/calcitriol for advanced, metastatic, hormone-refractory prostate cancer.
The Corporation completed a US/Canadian Phase II trial of tesmilifene in 29 patients in combination with mitoxantrone (Novantrone(R))/prednisone for the treatment of metastatic, hormone-refractory prostate cancer. Preliminary results from this trial were presented at the annual meeting of the American Society of Clinical Oncology ("ASCO") in May 2002. Those data demonstrated an objective reduction in pain in 75% of patients receiving tesmilifene/mitoxantrone/prednisone compared with 29% in previous studies who received mitoxantrone/prednisone alone and a decrease in PSA in 59% of patients compared with 33% in previous studies. Objective pain reduction is measured using a specific pain-related questionnaire and by discontinuance or reduction of treatment with analgesics.
The National Cancer Institute of Canada ("NCIC") and BMS, the then-licensee of tesmilifene, designed and conducted a global, open-label, randomized Phase III study of tesmilifene/doxorubicin versus doxorubicin alone in metastatic and recurrent breast cancer with tumor response and progression-free-survival as primary endpoints and overall survival as a secondary endpoint. A planned interim analysis failed to demonstrate improvement in tumor response and progression-free-survival and BMS terminated all clinical development. However, the 305 patients then enrolled in the study were followed by NCIC for analysis of the secondary endpoint, overall survival.
At the 2001 ASCO meeting, approximately two years after the decision by BMS to terminate development, the NCIC reported that an increase in overall survival of greater than 50% was seen in those patients who had received the tesmilifene/doxorubicin combination compared with patients receiving doxorubicin alone (23.6 months vs. 15.6 months; p<0.03, as adjusted). Results of the trial have been published in a major oncology journal.
MANUFACTURING:
Tesmilifene is a small molecule that is inexpensive and simple to manufacture through a two-step chemical process. The tesmilifene active drug substance is currently manufactured at Fabbrica Italiana Sintetici in Italy and is formulated into final drug product by Chesapeake Biological Laboratories Inc. in the United States. The Corporation understands that both of these suppliers operate facilities meeting GMP standards. The Corporation does not have supply agreements with these suppliers, but both have produced quantities for the Corporation to specification on a purchase order basis. The Corporation has not at this time engaged in detailed discussions regarding commercial scale manufacturing of tesmilifene, however, the Corporation believes that numerous pharmaceutical chemical manufacturers worldwide would be able to manufacture this compound at commercial scale.
INTELLECTUAL PROPERTY:
Aspects of tesmilifene, including its anti-cancer uses, are the subject of patents that have issued in the United States, Europe, Japan, Canada and Australia. Tesmilifene's cytoprotective end-use is the subject of other patents granted or pending in major markets. In addition, the drug's use in combination with anti-cancer agents to enhance the survival of cancer patients is the subject of patent filings in the US and many other major and minor markets. The Corporation has obtained our rights to such patents principally under a license agreement with University of Manitoba and CancerCare Manitoba and also by assignment from our consultant, Vincent Research & Consulting Inc. See "Business - Licensing Arrangements".
-28-
The scope of patent coverage and the patent term differ by country. In the United States, the Corporation relies on three layers of basic patent protection. A key patent among these is US 5,859,065, having claims relating to the use of tesmilifene and certain structurally related analogs in combination with any chemotherapeutic for the treatment of any cancer. The twenty year term of this patent expires in December 2010, but extensions of up to five additional years may be available under the Patent Term Restoration Act in the United States. We intend to take full advantage of the available term extension. In addition, in the United States, the Corporation is licensed under two granted patents with related coverage, namely, US 6,284,799 expiring February 2014 and US 5,747,543 expiring May 2015.
In addition to being licensed under these granted patents, YM is also licensed under numerous pending patents relevant to our clinical development program. These include national filings based on WO03/039526 and WO03/037318. This series of patent applications is focused on the survival advantage demonstrated following analysis of the phase III tesmilifene trial, and relates to a selection of patient subpopulations that will most benefit from the chemopotentiating and cytoprotective properties of tesmilifene. Patents resulting from these patent filings will expire in November 2022 in the United States and most other major markets.
The tesmilifene patent estate licensed to YM includes still other patents pending for particular chemotherapeutic/tesmilifene combination therapies adapted for improved efficacy in the treatment of breast and prostate cancers, among others.
In addition to patents, YM intends to rely on the available term of data exclusivity in the US and other countries, given that we believe tesmilifene qualifies as an NCE. Furthermore, full advantage will be taken of the Orange Book provisions in the United States and equivalent provisions in Canada and other countries, as a means for delaying generic competition.
COMPETITIVE POSITION:
The primary competition for tesmilifene is other enhancers of standard chemotherapies and possibly the market reduction for those chemotherapies from the introduction of new drugs for tesmilifene's target conditions. Competition appears to be principally from antisense drugs and pGp inhibitors.
Avastin from Genentech is being developed as an inhibitor of vascular endothelial growth factor (VEGF) and its activity, while a different approach, could be competitive with tesmilifene.
Antisense drugs (including Genasense from Genta Incorporated, GTI 2501 from Lorus Therapeutics Inc. and ISIS 2503 from ISIS Pharmaceuticals) have the potential to become competitive for tesmilifene as chemopotentiators.
To the knowledge of the Corporation only one pGp inhibitor, Zosuquidar-LY335979 from Eli Lilly and Company, continues in clinical development.
The development of new drugs for metastatic and recurrent breast cancer could reduce the size of the market for currently used chemotherapeutics which tesmilifene is demonstrated to enhance. To the knowledge of the Corporation there are more than 300 studies in breast cancer currently underway.
THERACIM AND RADIOTHERACIM
BACKGROUND:
TheraCIM, targeting the EGFr, is a humanized MAb. The EGFr is present at high concentrations on the surface of many cancer cells and it is postulated that the binding of ligands to this receptor is important in the continuing growth of cancer cells. TheraCIM appears to block this binding resulting in the potential for inhibition of cell growth or, possibly, cell destruction by the immune system. Improved tumor responses have been reported when EGFr targeting MAbs are combined with other anti-cancer treatments.
-29-
The Corporation's EGFr MAb is being developed in the following formulations:
| TheraCIM | Naked Antibody | Administered in combination with conventional radiation therapy. |
| RadioTheraCIM | Radiolabelled Antibody | For local injection directly into tumor resection cavities, such as in the treatment of brain cancers. |
The discussion below on Manufacturing and Competitive Position refers to TheraCIM.
CLINICAL EXPERIENCE - THERACIM:
In October 2000, the Corporation initiated a Phase II clinical trial of TheraCIM in patients with locally advanced head-and-neck cancer completed in the first quarter of 2003. Twenty-four fully evaluable patients were recruited in five sites across Canada. The side effects seen to date in this study appear to be less severe than those previously noted with the current standard-of-care, chemoradiation, with no apparent loss of effectiveness of the combination therapy. TheraCIM appears to sensitize tumors to the degree that patients receiving the antibody appear to have in excess of twice the response rate to radiation than that reported for patients receiving radiation alone.
A previous Phase I/II trial for which recruitment was completed in the third quarter of calendar 2002 and conducted by the Corporation's licensor, CIMAB, resulted in 24 fully evaluable patients receiving TheraCIM with radiation. This trial demonstrated a significant benefit compared to radiation alone (greater than 60% complete response compared to approximately 30% complete response expected from radiation alone). Results of the trial have been published in a major oncology journal.
In July 2001, YM received approval from Health Canada, to initiate a Phase I/II study of Nimotuzumab in conjunction with radiotherapy in patients with brain cancer resulting from metastases from non-small-cell lung cancer. YM has postponed implementing this trial while we evaluate the results of trials of competitive approaches to treatment of this condition.
In June 2004, the Corporation was advised that Oncosicence, the Corporation’s licensee, has enrolled the first patient in a 47-patient rolling Phase I/II trial in pediatric glioma, a form of brain cancer. In November 2004, the Corporation was advised that Oncoscience initiated thePhase I/II trial in Europe in patients with metastatic pancreatic cancer.
The Corporation has also been advised that recruitment for a randomized Phase III, 84 patient study in head-and-neck cancer with TherCIM together with radiation being conducted by the Corporation’s licensor. A CIMAB-sponsored single-arm Phase II glioma study of the drug with radiation is also ongoing.
In August 2004, TheraCIM was designated an orphan drug by the European Medicines Agency for the Evaluation of Medical Products (EMEA) in Europe and in November 2004 by the FDA in the United States.
In December 2004, preliminary results were released from a randomized Phase II pivotal trial assessing the efficacy and safety of TheraCIM h-R3 (nimotuzumab), combined with radiation compared to radiation alone in locally advanced Stage 3-4, nasopharyngeal carcinoma, a subject of head-and-neck cancer. Of the 130 patients in the intent-to-treat analysis, those in the combination arm were reported to have a 90.6% Complete Response compared to 51.5% in the radiation-alone group. “Complete Response” is defined as the elimination of tumour at the primary site, locoregional lymph nodes and distant metastases. The study was conducted by the Corporation’s licensor, CIMAB and Biotech Pharmaceuticals Limited, CIMAB’s joint-venture partner in China. We are advised that the product was approved for sale in China in April 2005.
-30-
In January 2005, Oncoscience announced that the Phase II trial in children with brain cancer (glioma) utilizing the EGF receptor monoclonal antibody (h-R3) as a monotherapy achieved its clinical endpoint and that six of 17 evaluable patients (35.3%) enjoyed clinical benefit. Data from that trial were presented at the European High-Grade Glioma Meeting in February 2005. Oncoscience is proposing to conduct a new study which will be a randomized Phase III trial comparing radiation to radiation plus h-R3 as a first-line therapy following surgery in children with pontine glioma. Oncoscience advised that the trial is expected to enroll approximately 50 patients which it plans to initiate following regulatory approval. Oncoscience anticipates regulatory approval for pivotal trial in glioma in adults which is expected to enroll between 110 and 150 patients and be initiated in the fourth quarter of 2005 if regulatory approval is received.
In March 2005, the FDA approved the use of TheraCIM h-R3 as a monotherapy in the treatment of a child with advanced glioma under an IND. The application to the FDA which resulted in the issuance of the IND to the investigator was prompted by the release of data from the Phase II monotherapy trial sponsored by Oncoscience in pediatric glioma presented in Europe in February 2005.
In May 2005, data were presented at the 2005 Annual Meeting of the American Society of Clinical Oncology (ASCO) on 24 evaluable adult patients treated with antibody in conjunction with radiation for high-grade malignant glioma. Complete response was achieved by 16.7%, partial response by 20.8%, 66.8% achieved disease stabilization for a total 87.5% objective response rate.
CLINICAL EXPERIENCE - RADIOTHERACIM:
In May 2003, data were presented at ASCO, the American Society of Clinical Oncology's annual meeting, of an investigator-led trial utilizing an Yttrium-90-labeled version of TheraCIM in 45 patients, post-excision, with glioma. It was concluded that this method is feasible and warrants further study. Since the trial is physician-sponsored, neither the Corporation nor CIMAB, the supplier of the RadioTheraCIM, control the conduct of the trial. There are no additional trials in planning for this approval.
MANUFACTURING:
Currently, CIMAB, supplies TheraCIM and RadioTheraCIM in quantities sufficient to facilitate the clinical development of these products. It is expected that CIMAB will manufacture and supply, or will contract for the manufacture and supply of commercial quantities of TheraCIM and RadioTheraCIM in accordance with the current licensing agreements at such time and stage of product development as commercial quantities of these products are required. There is a risk that CIMAB may experience difficulties obtaining or producing commercially viable quantities of these products. Product from CIMAB's manufacturing plant has been approved for use in a clinical trial in Canada and Europe. The plant operates according to GMP principles and its cGMP compliance status has been reviewed on behalf of the Corporation by industry experts. However, the facility has not been validated by a non-Cuban regulatory agency and the Corporation recognizes that the manufacturing facility has to continue to meet GMP standards in order to supply product for commercial use. Consequently, in 1999, the Corporation entered into a collaboration with the Biotechnology Research Institute ("BRI") of the National Research Council of Canada in order to fund the development of a manufacturing process to produce clinical grade material on a commercial scale. This collaboration yielded promising results, and CIMAB’s scale-up process was accepted by Health Canada for commercial scale manufacturing process which is now required to provide data required to satisfy applicable regulatory requirements.
The Corporation's license agreement for TheraCIM contemplates manufacturing of the product by CIMAB or a supplier contracted by CIMAB. Should CIMAB agree to alternative manufacturing arrangements, such as a sub-licensee of CIMYM manufacturing the product, the loss of manufacturing benefits to CIMAB may be reflected in a lower license fee and royalty payable to CIMYM than if manufacturing remains with CIMAB. See "Business - Licensing Arrangements".
-31-
MARKETING:
TheraCIM and RadioTheraCIM are licensed by the Corporation from a Cuban source, CIMAB, and as such are likely to be prohibited from sale in the United States unless OFAC issues a license or the US embargo against Cuba is lifted.
INTELLECTUAL PROPERTY:
Aspects of TheraCIM, including claims to the antibody and its formulation, are the subject of patents that have issued in the United States and Canada and a patent application that has been granted in Europe. In addition, the combination of any EGF-based passive immunization (such as TheraCIM) together with any EGF-based active immunization is the subject of Patent Cooperation Treaty ("PCT") and United States patent applications. CIMYM is the exclusive licensee under a patent estate that includes composition of matter coverage for Nimotuzumab, and further includes coverage for TheraCIM-based formulations and end-uses in the treatment of EGFr-dependent cancers. The composition of matter patents are granted in the United States, in Europe, are allowable in Japan, and are pending in Canada. The Corporation has obtained our rights to such patents under a license agreement with CIMAB, the company responsible for the commercialization of products developed at CIM and CBQ. See "Business - Licensing Arrangements".
CIMYM's key US patent, US 5,891,996 expires in November 2015, and term extensions of up to five years may be available under the Patent Term Restoration Act. The same term and extension apply also to the key European patent, EP 712863.
The Corporation is aware of US patent #5,770,195 granted to Genentech, for the anti-cancer use of EGFr MAbs in combination with a cytotoxic agent. The Corporation is also aware of US patents granted to others in this field. In April 2001, Rorer International (Overseas) ("Rorer") was issued the US patent #6,217,866 which includes claims to any antibody targeting the EGFr administered with any anti-neoplastic agent. A counterpart patent has been granted in Europe. The Corporation has filed an opposition to the grant of the European patent. The Corporation believes that the Rorer patents are licensed to ImClone. In addition, the Corporation is aware of a separate series of national patent applications filed by ImClone, and represented by EP1080113, claiming the anti-cancer use of radiation in combination with any inhibitor of any receptor tyrosine kinase that is involved in the genesis of tumors. ImClone is also reported to have filed a PCT application covering the use of EGFr MAbs to treat patients having tumors that do not respond to treatment with conventional therapies. The Corporation is also challenging ImClone's claims in respect of the radiation-related patent applications by filing additional prior art at the relevant national patent offices. The outcome of these challenges cannot be predicted, and there can be no assurance that the Corporation will succeed in challenging the validity or scope of patent claims by ImClone or any other patent applicant. The Corporation has not incurred material expenses in connection with the challenges to ImClone's radiation-related patent application. See "Risk Factors - Patents and Proprietary Rights".
The manufacturing of TheraCIM may fall within the scope of process patents owned by Protein Design Labs Inc., Genentech, and the Medical Research Council of the United Kingdom. Management is aware that some of these process patents are currently being challenged by companies other than YM. In the event any of the applicable process patents are upheld, management believes we will be able to obtain licenses under such patents on commercially reasonable terms, though there can be no assurance thereof.
COMPETITIVE POSITION:
To the knowledge of the Corporation, other companies that are involved in the development of monoclonal antibody cancer therapeutics directly related to the Corporation's efforts include Abgenix/Amgen, Genmab, ImClone/BMS, and Merck.
The Corporation understands that OSI, in concert with Genentech and Roche, and AstraZeneca, have small molecules designed to target the tyrosine kinase domains of EGF receptors. The Corporation understands that Iressa(R), from AstraZeneca, has been approved in thirty-five countries, including Japan and the United States for third line monotherapy of NSCLC. OSI reported that it has positive survival data in a Phase III monotherapy study in treatment refractory NSCLC.
-32-
OSI's product, TarcevaTM, is reported to be in co-development with Roche and Genentech and is reported to be in numerous trials in various indications including Phase III registration studies. TarcevaTM has been approved in the United States for NSCLC. See "- Competition".
Erbitux(R), developed by ImClone/BMS and Merck, is approved in the United States, Canada, Germany, Austria and Switzerland for metastatic colorectal cancer in combination with irinotecan in irinotecan-refractory patients. Management understands that Erbitux(R) is under review by other regulatory agencies including EMEA, the European regulatory agency.
NORELIN TM
BACKGROUND:
Originally developed by Biostar Inc. ("Biostar"), NorelinTM is an active specific immunotherapy agent that harnesses the immune system to block the activity of the master hormone GnRH, which controls the production of both male and female sex hormones. These hormones bind to receptors in malignant cancer cells and promote the growth and spread of cancer. By eliciting an antibody response to GnRH, NorelinTM is designed to block GnRH from reaching its receptors in the pituitary gland, intending to reduce the amount of sex hormones in circulation and is expected to reduce their effect on tumor growth. NorelinTM consists of an adjuvant combined with the immunogen, the drug substance IPS-21, a proprietary carrier protein that is a non-toxic fragment of P. haemolytica, flanked by eight copies of GnRH on both ends. Extensive testing by Biostar of IPS-21 and product formulations was carried out in numerous domestic and laboratory species, using a range of adjuvants and doses. In pre-clinical testing, NorelinTM has been effective in inducing an antibody response to GnRH, which in turn reduced sex hormones to sterilization levels in the pre-clinical animal models assessed. In addition, a significant anti-tumor effect has been demonstrated in several animal models.
CLINICAL EXPERIENCE:
In 2002, YM obtained a ”No Objection” for a CTA for NorelinTM and a safety and immunogenicity study in patients with hormone-sensitive prostate cancer was initiated in the third quarter of calendar 2002. Hormone-dependent prostate cancer is characterized by elevated levels of testosterone, which fuels tumor growth, and elevated levels of PSA. The disease is currently managed by lowering the testosterone and PSA with pharmaceuticals or surgery. The trial enrolled patients in two stages. In the first stage (results reported in June of 2003), 12 patients were enrolled and treated with the Corporation proprietary formulation of NorelinTM and were followed for 90 days. In the second stage, four patients were enrolled starting in mid-2004 and followed for 180 days before determining whether or not they responded. YM originally planned to enroll a total of 12 patients in the second stage, but voluntarily terminated enrollment of the trial when delays in trial implementation resulted in clinical supplies of the drug exceeding stability time-limits. The drug substance demonstrated excellent stability for four years, more than sufficient to consider it commercializable.
Patients from the first stage whose testosterone dropped significantly at 90 days were advanced into a long-term booster program. Seven of the 12 enrolled patients developed anti-GnRH antibodies and two went on to achieve castrate levels of testosterone by day 120. Those two patients received booster doses for approximately two years and are in long-term follow-up. They remain at castration levels with normalized PSA and have not demonstrated any sign of disease progression at >2.5 years as at June 30, 2005.
Three patients from the first stage and three patients from the second stage received at least five doses of the vaccine and these patients were followed for 120 days. All responded to the vaccine, developing antibodies and achieving testosterone suppression. Three of these patients achieved complete reduction of testosterone to castration levels.
-33-
Adverse events reported included injection site pain in 8/16 (50%) patients, hot flashes 3/16 (19%), nausea 2/16 (13%), decreased libido 1/16 (6%) and headache 1/16 (6%). There was no testosterone flare and no bone pain or worsening of symptoms reported.
The data are sufficiently compelling to warrant further clinical investigation which will require the manufacture of additional drug product. The evaluation of the costs of manufacturing are currently underway.
MANUFACTURING:
Unlike MAbs, NorelinTM can be produced in a bacterial host such as E. coli. Numerous production facilities are available in North America and elsewhere. The Corporation does not have a supply agreement with any particular supplier, but this drug has been produced in suitable quantities for the Corporation to specification on a purchase order basis. The drug substance was manufactured by Diosynth Inc., located in North Carolina, US, and the current drug product has been manufactured under cGMP conditions by the University of Iowa's Pharmaceutical Services Division, located in Iowa, US The Corporation is aware of US patent #6,303,123 owned by Aphton relating to the use of GnRH immunogenic conjugates to treat GnRH-dependent diseases, including prostatic hypertrophy, and is developing a strategy for addressing this patent should it prove relevant to the Corporation's commercial activities with NorelinTM.
INTELLECTUAL PROPERTY:
Aspects of NorelinTM, including claims to the fusion protein, its synthesis and its formulation, are the subject of patents that have issued in the United States, and patent applications are pending in a number of other major markets. The NorelinTM patent estate is extensive, and includes four key US patents covering various aspects of NorelinTM as a composition of matter, the carrier component of the NorelinTM vaccine, as well as production of NorelinTM as a recombinant product. A key US patent is US 5,837,268, which covers the particular NorelinTM sequence, its formulation as a vaccine, and its end-use, and subject to any term restoration, will expire in 2012.
The Corporation has obtained our rights to such patents under a license agreement with Biostar. See "Business - Licensing Arrangements".
COMPETITIVE POSITION:
Although the Corporation is aware of numerous products in development for prostate cancer, the Corporation is aware of only three competing products in the GnRH vaccine field. Of the four products in development (including the Corporation's product), to the knowledge of the Corporation, a product by Aphton appears to be the most advanced, having reportedly completed Phase I testing and having reportedly commenced Phase II testing. The Corporation believes that the competitive vaccines are based on chemical synthesis and/or classical conjugation techniques, unlike NorelinTM which is produced in a bacterial host. As a result, the Corporation believes those competitive vaccines are complex mixtures of proteins that would be expected to be more difficult and expensive to produce than NorelinTM.
These vaccine products will seek to compete with existing treatments. Two existing products designed to induce chemical castration in the treatment of prostate cancer have been approved for marketing and have been in use for a number of years. These products, Lupron by TAP Pharmaceuticals and Zoladex by AstraZeneca, have a strong market presence.
-34-
AEROLEFTM
BACKGROUND:
AeroLEFTM is a proprietary formulation of fentanyl, an opioid analgesic, that is administered by inhalation and permits self-titration by patients. The development of AeroLEFTM as a combination of pulmonarily-delivered free and liposomal dosage form takes advantage of (1) the lung’s large absorptive surface and thin barrier to absorption to permit rapid transport of the free fentanyl fraction (loading dose) into the systemic circulation and (2) the capacity of liposomes to function as reservoirs for the regulated release over time of the encapsulated fentanyl. AeroLEFTM is being developed to provide both rapid and extended opiod analgesic levels for patients with severe and moderate acute pain and cancer pain.
CLINICAL EXPERIENCE:
The clinical experience with AeroLEFTM includes two Phase I studies with healthy volunteers and one Phase II acute pain study in post-surgical patients carried out in Canada under IND submissions approved by Health Canada.
In the first Phase I study, both single and multi-dose regimens of AeroLEFTM dosing with nebulization resulted in therapeutic plasma concentrations detected during the dosing session and peak plasma fentanyl concentrations recorded at approximately the end of the 10-15 minute dosing session. Similar mean peak plasma fentanyl concentrations of 2.53 and 2.23 ng/ml were observed in the single dose and multi-dose arms, respectively. The mean plasma concentration of fentanyl was maintained within the target therapeutic range (0.5-2.0 ng/ml) for just over four hours in the AeroLEFTM treatments groups. By comparison, in the intravenous fentanyl arm of the study, the mean peak plasma fentanyl concentration was 2.80 ng/ml at about 5 minutes but the duration above 0.5 ng/ml was only until one hour post-dosing.
In a second Phase I study involving healthy volunteer subjects, multiple doses (up to 4 doses) of AeroLEFTM were administered with various commercially available nebulizer devices. The most favourable pharmacokinetic-pharmacodynamic profile was achieved following delivery of AeroLEFTM with the breath-actuated AeroEclipse® nebulizer from Trudell Medical. Based on these results, the AeroEclipse® device was selected for delivery of AeroLEFTM in the Phase II study.
The preliminary results of a Phase II acute pain study using AeroLEFTM as the primary postoperative analgesic were reported at the annual meeting of the American Society of Anesthesiologists in October 2004. The study involved a unique “dose-to-effective-analgesia” following patient-controlled administration of AeroLEFTM as the primary analgesic treatment in adult patients experiencing severe and moderate pain following elective knee surgery. This Phase II study demonstrated that 95% of patients successfully achieved analgesia via self-titration with AeroLEFTM as the primary medication. Eighteen (18) subjects rapidly achieved perceptible analgesia soon after commencing nebulization (median 2.7 min) and continued self-titration to a median time to effective/adequate analgesia of 17 minutes.
MANUFACTURING:
The AeroLEFTM manufacturing process involves a two-step, controlled process that produces liposomal formulations with the target particle size and the target ratio of free and encapsulated fentanyl. AeroLEFTM clinical product supply for the two Phase I studies and the Phase II study was contracted to OctoPlus b.v., based in the Netherlands. During 2004, the manufacturing process was transferred to Dalton Pharma Services of Toronto, Ontario for production of AeroLEFTM to support future clinical trials and development activities. As AeroLEFTM advances to Phase III trials, the Company plans to engage a second contract manufacturer in the United States to provide pivotal trial materials and commercial supply. Fentanyl, the active pharmaceutical ingredient in the AeroLEFTM formulation, is commercially available from various vendors licensed to synthesize and market the controlled substance.
-35-
INTELLECTUAL PROPERTY:
The AeroLEFTM product is described in four patent families. DELEX owns key patents, expiring in 2014, claiming a method of administering systemic analgesia by inhaling free and liposome-encapsulated opiod analgesic. North American coverage includes a reissued US patent and a pending application in Canada. DELEX owns two US applications with counterpart PCT applications, expiring in 2024, claiming the formulation for use in a method comprised of continuously inhaling the formulation to deposit at least one rapid-onset opiod and one sustained-effect opiod in the lungs. A pending PCT application entitled “Stable Compositions” claims the liposomal composition and other physical characteristics.
The corporation is aware of US patents owned by Phares Pharmaceutical Research NV related to liposome compositions. These patents expire in 2008 and are not expected to hamper the Corporation’s commercial activities.
COMPETITIVE POSITION:
The opioid analgesic market which includes products based on morphine, fentanyl, oxycodone, or hydromorphone is currently dominated by several pharmaceuticals companies such as Johnson & Johnson Inc., Abbott Laboratories, Baxter International Inc., AstraZeneca PLC, Purdue Pharma L.P., Cephalon, Inc., Endo Pharmaceutical Holdings Inc., and Organon International. The fentanyl segment of the opioid analgesic market includes three approved routes of administration:
| (a) | intravenous administration of fentanyl citrate available as generic products from various suppliers |
| (b) | transdermal administration via the Duragesic® patch |
| (c) | transmucosal administration via the Actiq® lollipop |
Several competitors are developing non-invasive alternatives for enhanced delivery of fentanyl, including Alza Corporation (IONSYS for transdermal delivery), Cephalon (Oravescent Fentanyl for transmucosal delivery), Endo (Rapinyl for sublingual delivery), Aradigm (pulmonary delivery), Alexza (pulmonary delivery) and LAB (pulmonary delivery). All the competitors, to our knowledge, will deliver fixed dosage forms of the drug.
PRODUCTS IN PRE-CLINICAL DEVELOPMENT
TGFα CANCER VACCINE
BACKGROUND:
Human epidermal growth factors and their receptors are known to play an important role in both normal cell proliferation and in neoplastic growth. The EGFr is overexpressed in many human epithelial malignancies, including breast, bladder, ovarian, colon, lung, brain and esophageal cancer. In some tumors, EGFr overexpression is an indicator of a poor clinical prognosis. There are a number of ligands (proteins or peptides produced in the human body) that can bind to the EGFr and are postulated to promote cancer growth. These include EGF, TGFα, amphiregulin, heparin-binding EGF-like growth factor and betacellulin.
RATIONALE:
The most common ligand for the EGFr in human tissues is TGFα, which is often overexpressed in human epithelial malignancies. With this ligand, the EGFr forms a well-defined autocrine loop and there are numerous reports demonstrating the influence of the TGFα/EGFr system in human tumors. Increased production in the body of either EGFr or TGFα have been identified as early events in the progression of head-and-neck cancers. The autocrine loop EGFr/TGFα has also been found to be important for the growth of human renal carcinoma cells. Furthermore, studies with both brain tumor cell lines and primary tumor tissues suggest that the TGFα and the EGFr function as an important autocrine loop in supporting proliferation of brain tumors. The relationship between TGFα and oncogenes is also established. One example is the relationship between TGFα and the oncogene c-myc. Overexpression of these genes is demonstrated in human cancers, suggesting that their interaction may be a critical step in malignant growth. Taken as a whole, these studies suggest that overexpression of EGFr and its ligand TGFα is frequent in human tumors.
-36-
INTELLECTUAL PROPERTY:
Aspects of the TGFα Vaccine, including claims for vaccines containing TGFα, are the subject of patent applications that have been filed in all major markets including the United States. The TGFα Vaccine would also fall under the scope of the passive/active immunization claims described in connection with TheraCIM, above. The Corporation has obtained our rights to such patent applications under a license agreement with CIMAB. See "Business - Licensing Arrangements".
CURRENT STATUS:
A TGFα/P64k fusion protein has been produced in E. coli and semi-purified. Immunized mice have mounted an anti-TGFα antibody response. A murine tumor model depending on TGFα expression for in vivo growth is currently under development.
HER-1 BASED CANCER VACCINE
BACKGROUND:
As described above, the EGF/EGFr system is an attractive target for cancer therapy. The EGFr is overexpressed in many malignant tumors of epithelial origin, which include breast, bladder, ovarian, colon, lung, brain and esophageal cancer. EGFr expression in human breast tumors has been correlated with a poor prognosis. Furthermore it has been demonstrated that expression of EGFr in breast tumor metastases is frequently elevated compared to the primary tumor, which suggests the involvement of EGFr in the metastatic process, though there can be no assurance thereof.
Although several MAbs against the EGFr, both naked and those associated with drugs, toxins or radioisotopes, are being evaluated for cancer immunotherapy, active specific immunotherapy with the EGFr itself has not, to the knowledge of the Corporation, been tested.
RATIONALE:
The HER-1 Vaccine project is aimed at developing a cancer vaccine composed of the extracellular domain of the human EGFr, presented in a Th1-pattern-inducing vehicle. An antibody response would block the interaction between EGFr and its ligands, provoking a cytostatic effect, but tumor shrinkage could also be induced by a cytotoxic T cell ("CTL") response.
INTELLECTUAL PROPERTY:
Aspects of the HER-1 Vaccine, including claims for vaccines containing HER-1, are the subject of patent applications that have been filed in the major markets including the United States. The HER-1 Vaccine would also fall under the scope of the passive/active immunization claims described in connection with TheraCIM, above. The Corporation has obtained our rights to such patent applications under a license agreement with CIMAB. See "Business - Licensing Arrangements".
The Corporation's license for the HER-1 Based Cancer Vaccine is suspended under the terms of the out-licensing agreement between the Corporation, CIMYM (Barbados), CIMAB and Tarcanta relating to Tarcanta licensing TGFα and HER-1 from CIMAB. In connection with the out-licensing agreement, CancerVax has announced that it has received a license from Treasury authorizing Tarcanta to enter into the transactions with CIMAB and the Corporation. See "Business - Licensing Arrangements - Out-Licensing" and see "Business - Licensing Arrangements - In-Licensing - Licenses for TheraCIM, RadioTheraCIM, TGFα and HER-1".
-37-
CURRENT STATUS:
In the product, the cDNAs encoding the extra-cellular domain ("ECD") of the human and murine EGFr have been cloned into expression vectors, and stable cell lines secreting these ECDs appear to have been established. Mice were immunized with the ECD of the murine EGFr in different immunogenic preparations. Specific T cell proliferation and antibody titers above 1/1000 were obtained without severe toxicity. Pre-clinical tumor challenge experiments are ongoing.
The Corporation's license for the TGFα Cancer Vaccine and the HER-1 Based Cancer Vaccine is suspended under the terms of the out-licensing agreement between the Corporation, CIMYM (Barbados), CIMAB and Tarcanta relating to Tarcanta licensing TGFα and HER-1 from CIMAB. Under the terms of the new agreement and in consideration for the suspension of the 2001 CIMYM License, the Corporation is entitled to receive an aggregate payment of $1,000,000 which is payable in four equal installments, the final payment due December 31, 2005. In addition, under the new agreement the Corporation may receive 35% of an aggregate of $16,350,000 in milestone payments. Finally, the Corporation retains an interest in the revenues from the manufacture and marketing of the drugs or from their sub-licensing. In connection with the out-licensing agreement, CancerVax has announced that it has received a license from Treasury authorizing Tarcanta to enter into the transactions with CIMAB and the Corporation. See "Business - Licensing Arrangements - Out-Licensing" and see "Business - Licensing Arrangements - In-Licensing - Licenses for TheraCIM, RadioTheraCIM, TGFα and HER-1".
The biopharmaceutical industry is intensely competitive. Many companies, including other biopharmaceutical companies and biotechnology companies, are actively engaged in activities similar to those of the Corporation, including research and development of drugs for the treatment of cancer. More specifically, competitors for the development of new therapeutic products to treat cancer also focus on MAb-based cancer therapeutics, cancer vaccines and other approaches that are based on both active and passive immunotherapies and small molecule discovery and development. A 2001 survey by the Pharmaceutical Research and Manufacturers of America ("PhRMA") listed 399 new treatments for cancer that are currently being tested by researchers.
To the knowledge of the Corporation, other companies that are involved in the development of monoclonal antibody cancer therapeutics directly related to the Corporation's efforts include Abgenix/Amgen, Genmab, ImClone/BMS, and Merck. The Corporation understands that OSI in concert with Genentech and Roche and AstraZeneca has small molecules designed to target the tyrosine kinase domains of EGF receptors. Iressa(R) has been approved in twenty countries, including Japan and the United States for third line monotherapy of NSCLC. OSI reported that is has positive survival data in a phase III monotherapy study in treatment refractory NSCLC. TarcevaTM has been approved in the United States for NSCLC. Erbitux(R) is approved in United States, Austria, Canada, Germany and Switzerland for metastatic colorectal in combination with irinotecan in irinotecan-refractory patients. Erbitux(R) is under review by other regulatory agencies including EMEA the European regulatory agency.
Several competitors are developing non-invasive alternatives for enhanced delivery of fentanyl, including Alza Corporation (IONSYS for transdermal delivery), Cephalon (Oravescent Fentanyl for transmucosal delivery), Endo (Rapinyl for sublingual delivery), Aradigm (pulmonary delivery), Alexza (pulmonary delivery) and LAB (pulmonary delivery).
The Corporation expects to encounter significant competition for the pharmaceutical products we are developing and plan to develop in future. Many of the Corporation's competitors have substantially greater financial and other resources, larger research and development capabilities and more extensive marketing and manufacturing organizations than the Corporation. In addition, some such companies have considerable experience in pre-clinical testing, clinical trials and other regulatory approval procedures. There are also academic institutions, governmental agencies and other research organizations which are conducting research in areas in which the Corporation is working and they may also market commercial products, either on their own or through collaborative efforts. If any of these competitors were to complete clinical trials, obtain required regulatory approvals and commence commercial sales of their products before the Corporation they may achieve a significant competitive advantage.
-38-
CLINICAL, PRE-CLINICAL AND BASIC RESEARCH
The Corporation, designs, funds and manages clinical and some pre-clinical research, and may support, but does not conduct, basic research. The Corporation manages the development of products that we in-license through our own team of clinical, regulatory, licensing and business development executives and through a number of research and medical collaborations. The Corporation is responsible for filing applications with the relevant authorities for regulatory approval for clinical trials and conducts, or has conducted on our behalf, clinical trials to progress products in development toward regulatory approval and possible out-licensing for commercial sale. The Corporation's current licenses generally provide that the Corporation will conduct, or cause to be conducted, the tests and clinical studies necessary to progress products in development toward regulatory approval with a view to obtaining the approval for sale of the licensed drug from appropriate regulatory authorities. The Corporation has received regulatory approvals for clinical trials in a number of countries, including in Canada, the United States, the United Kingdom, Europe and South Africa from Phase I through Phase III. Some basic research is conducted at the facilities of the Corporation's licensors, and the Corporation pays for certain amounts of this research.
LICENSING ARRANGEMENTS
IN-LICENSING
LICENSE FOR TESMILIFENE
In November 2000, YM was granted an exclusive worldwide license by the University of Manitoba and The Manitoba Cancer Treatment and Research Foundation (now CancerCare Manitoba) (the "Original Licensor") for all products and formulations of tesmilifene pursuant to which the Corporation undertook the responsibility for the clinical development of the product and its commercialization.
The Corporation must pay to the Original Licensor a specified minority percentage of revenues received from sub-licensing the product, after our recovery of certain specified development and attributed overhead costs. If the Corporation manufactures and sells tesmilifene itself rather than through sub-licensing, the Corporation must pay a specified lesser minority percentage of net sales, after our recovery of certain specified development and attributed overhead costs, to the Original Licensor. Management believes these royalties are consistent with general industry practice for similar arrangements. No royalties have been paid to date, and future royalties cannot be quantified because they are dependent on net sales, net royalties and net revenues which have not yet materialized. There can be no assurance as to if or when the Corporation may sell the licensed product nor enter into sub-licensing arrangements for the product. Under the terms of this license agreement, the Corporation has paid US$300,000 over the years 2000, 2001 and 2002 for sponsored research. The Corporation must make reasonable efforts to ensure that the licensed product is efficiently marketed and distributed by November 2005. The Corporation may sub-license the product. This license agreement shall be in force as long as any patents thereunder are valid, or until such time as the license agreement is terminated by either party because of a default by the other party, by either party if the other party enters into liquidation or reorganization proceedings or receivership or bankruptcy, or by YM on 90 days written notice if there are no sub-licensees. In 2003, the Corporation acquired certain additional patent rights for the use of tesmilifene from Vincent Research and Consulting in exchange for a small share of YM's future royalty revenues. Management of YM does not consider the agreement with Vincent Research and Consulting to be material to YM as of the date hereof.
LICENSE FOR NORELINTM
In October 2000, YM secured the exclusive, sub-licensable, worldwide license to the human therapeutic rights to NorelinTM from Biostar. The license is non-exclusive with respect to diagnostic applications for P. haemolytica antibodies and excludes applications related to infectious diseases. Pursuant to the license, the Corporation paid 75,000 of our common shares and 37,500 warrants were granted to purchase our common shares. The warrants granted to Biostar were granted at a price of US$9.00 per common share and expired on October 11, 2004. Finally, pursuant to the license, the Corporation is required to pay Biostar an amount equal to the lesser of (a) either two or four percent (depending on the nature of the product) of net sales, and (b) 10 percent of any sub-licensing revenue received by the Corporation. No such royalty payments have been paid to date, and future royalties cannot be quantified because they are dependent on net sales and sub-licensing arrangements which have not yet materialized. There can be no assurance as to if or when the Corporation may have net sales or enter into sub-licensing arrangements for the licensed products. This license agreement shall be in force as long as any patents thereunder are valid, or until such time as the license agreement is terminated by either party because of a default by the other party, by either party if the other party enters into liquidation or reorganization proceedings or receivership or bankruptcy, or by YM on 90 days written notice if there are no sub-licensees. Notwithstanding the foregoing, any sub-license will be terminated upon the termination of the underlying license between Biostar and the University of Saskatchewan. YM has been advised that certain rights to technology under the license depend on patents and patent applications, the prosecution and maintenance of which are funded by third parties pursuant to agreements with the Veterinary Infectious Disease Organization ("VIDO"), a division of the University of Saskatchewan. If such parties purport to abandon any such applications or patents, VIDO has the obligation to provide Biostar with the opportunity to fund the prosecution and maintenance of such applications and patents, if VIDO chooses not to do so itself. Similarly, Biostar has the obligation to provide YM with the opportunity to fund the prosecution and maintenance of such applications and patents, if Biostar chooses not to do so itself.
-39-
LICENSES FOR THERACIM, RADIOTHERACIM, TGFΑ AND HER-1.
(i) The 1995 CIMYM License
In May 1995, YM acquired an exclusive, sub-licensable license (as amended, the "1995 CIMYM License") from CIMAB, acting on behalf of CIM, to products for passive immunotherapy of cancer directed toward EGF and EGFr as targets, including hR3, a humanized MAb targeting the EGFr. CIMAB is the company responsible for the commercialization of products developed at CIM. The 1995 CIMYM License is in respect of Europe, Canada, the United States, Japan, Australia, Taiwan, Singapore, Thailand, Hong Kong, South Korea, Malaysia, Indonesia and the Philippines. As a term of the 1995 CIMYM License, YM has a right of first refusal with respect to licensing any other products derived from the EGF and EGFr programs of CIMAB except its anti-EGFr monoclonal antibody for psoriasis in Europe.
Pursuant to the 1995 CIMYM License, in 1995 the Corporation incorporated CIMYM and assigned the 1995 CIMYM License to CIMYM. Pursuant to the terms of the 1995 CIMYM License, CIMAB acquired a 20% interest in CIMYM as partial consideration for the 1995 CIMYM License. In addition to that 20% equity interest in CIMYM, CIMAB is entitled to receive 10% of net revenues received by CIMYM. In addition, YM and CIMYM, pursuant to the terms of the 1995 CIMYM License, paid US$2,750,000 for certain product development costs for TheraCIM and US$330,000 for certain product development costs for RadioTheraCIM.
The terms of the 1995 CIMYM License provide for CIMYM to conduct or cause to be conducted pre-clinical and clinical trials to evaluate the licensed products and to work with CIMAB to select sites, develop protocols and instruct investigators for pre-clinical and clinical trials. CIMYM is to decide after the end of each stage of trials whether to proceed with further development or to terminate the 1995 CIMYM License with respect to that product. In addition, the 1995 CIMYM License provides that, where commercially reasonable, CIMYM shall file applications for regulatory approval to market the licensed products in the applicable territory. Pursuant to the 1995 CIMYM License, CIMAB has the right, subject to certain terms and conditions, to supply the related drug substances (i.e., TheraCIM and RadioTheraCIM) for commercial sale. CIMAB shall sell the product manufactured by it in Cuba to CIMYM at 85% of the sales price that CIMYM sets for the sale of the product to sub-licensees, thereby entitling CIMYM to the 15% difference. CIMYM shall use its best efforts to obtain a sub-license agreement in which CIMAB retains the right to manufacture the product. YM will be responsible for any failure of CIMYM to fulfill its obligations under the 1995 CIMYM License. This license agreement shall be in force as long as any patents thereunder are valid, or until such time as the license agreement is terminated by either party because of a default by the other party, or by CIMYM with written notice within 90 days after the end of a stage of pre-clinical trials or after each stage of clinical trials.
In connection with the 1995 CIMYM License, CIMYM entered into an international sales, marketing, manufacturing and administrative agreement with CIMYM (Barbados) pursuant to which CIMYM (Barbados) acquired the rights to market TheraCIM outside Canada (see "Arrangements with Subsidiaries"). CIMAB owns a corresponding 20% interest in CIMYM (Barbados).
-40-
(ii) The 2001 CIMYM License
In 2001, CIMYM (Barbados) acquired an exclusive, sub-licensable license (the "2001 CIMYM License") from CIMAB to two active immunotherapy products described as HER-1 Vaccine and TGFα Vaccine. CIMAB has the right to consent to any sub-licensee, such consent not to be unreasonably withheld. The 2001 CIMYM License is in respect of Europe, Canada, the United States, Japan, Australia, New Zealand, Taiwan, Singapore, Thailand, Hong Kong, South Korea, Malaysia, Indonesia and the Philippines. Under the 2001 CIMYM License, CIMYM (Barbados) has a right of first refusal with respect to licensing all other products derived from the EGF active immunotherapy program of CIM.
The terms of the 2001 CIMYM License provide for CIMYM (Barbados) to conduct or cause to be conducted pre-clinical and clinical trials to evaluate the licensed products, and to work with CIMAB to select sites, develop protocols and instruct investigators for pre-clinical and clinical trials. CIMYM (Barbados) is to decide after the end of each stage of trials whether to proceed with further development or to terminate the 2001 CIMYM License with respect to that product. In addition, the 2001 CIMYM License provides that, where commercially reasonable, CIMYM (Barbados) shall file applications for regulatory approval to market the licensed products in the applicable territory. Pursuant to the 2001 CIMYM License, subject to certain terms and conditions, CIMAB has the right to supply the related drug substance (i.e., TGFα and HER-1) for commercial sale, unless sub-licensees require manufacturing rights. CIMAB shall sell the product manufactured by it in Cuba to CIMYM (Barbados) at either 90%, if there are no positive net revenues, or 100% of the best available price for the manufacture, supply and delivery of such product in Havana, Cuba.
If the Corporation elects to proceed with the license, the Corporation would be expected to make milestone and research and development payments, the amount of such payments to be negotiated between the Corporation and CIMAB. On signing of the 2001 CIMYM License, CIMYM (Barbados) paid CIMAB US$125,000 for product develop costs.
The terms of the 2001 CIMYM License have been suspended pursuant to a License, Development, Manufacturing and Supply agreement entered into by the Corporation and its subsidiary, CIMYM (Barbados), Tarcanta and CIMAB on July 13, 2004. By the terms of this agreement with Tarcanta, the 2001 CIMYM License has been suspended until such time, if at all, there is a default under the agreement with Tarcanta. See "Business - Licensing Arrangements - Out-Licensing".
As at September 30, 2004 YM has advanced US$24 million to CIMYM and CIMYM (Barbados), collectively, for the licensing and development of the products licensed by CIMYM. Under the terms of the 1995 and 2001 CIMYM Licenses, YM was given the right to recover all funds advanced to CIMYM and CIMYM (Barbados), collectively, from either CIMYM and CIMYM (Barbados). To the extent that the net revenues of CIMYM are less than or equal to the advanced amounts, YM is only permitted to recover such advances from 30% of the net revenues. At this time none of the advances have been repaid. There have been no revenues to date.
In connection with each of the 1995 CIMYM License and the 2001 CIMYM License, it is expected that, notwithstanding that CIMAB owns 20% of the voting securities of each of CIMYM and CIMYM (Barbados), it could, based on the terms of the relevant license, receive approximately 40% of the overall economic return from commercialization of the related drug products. This could occur, for example, if CIMAB manufactures the licensed product.
AEROLEFTM
The DELEX technology that constitutes the platform known as Rose-DS, or where AeroLEFTM is based, is owned not licensed.
OUT-LICENSING
The Corporation generally plans to out-license our licensed drugs to pharmaceutical companies for manufacturing and marketing under license, although we may retain co-development or marketing rights if management considers it appropriate to do so. Under the Corporation's business model, licensees would be expected, to the extent necessary, to participate in the remaining clinical development required to obtain final regulatory approval for the product. The Corporation expects that out-licensing would result in a pharmaceutical company or other licensee marketing or manufacturing the product in return for licensing fees and royalties on the sale of the product. Management believes this model is consistent with current biotechnology and pharmaceutical industry licensing practices.
-41-
The Corporation's objectives in seeking to out-license products include:
o obtaining long term revenue streams from royalty payments on the sale of the products;
o providing access to the resources and experience of large pharmaceutical companies;
o obtaining up-front payments for product sub-licensing rights; and
o minimizing development expenditures through cost sharing programmes (especially late-stage clinical trials and regulatory approval applications).
The Corporation believes that out-licensing arrangements could be attractive to pharmaceutical corporations because they would provide the prospective partner with access to new products without the initial research risk or earlier clinical development costs. Since partners are expected to be sought only at the later stages of a product's development, the Corporation anticipates that prospective licensees would view the Corporation's products as having a reduced risk of failure to achieve regulatory approval.
YM does not intend to develop our own manufacturing, marketing or distribution programmes although we may wish to participate in ownership of manufacturing facilities if appropriate opportunities become available. The Corporation intends to remain principally focused on the identification, further development and commercialization of in-licensed products.
THERACIM:
On November 12, 2003, the Corporation's subsidiary, CIMYM, licensed the rights for TheraCIM (known as “Theraloc” in Europe) in most of Europe to Oncoscience of Germany. Under the terms of the agreement, CIMYM is entitled to receive up to US$30 million as a share of any amounts received by Oncoscience in relation to development or sublicensing of the product and as a royalty on initial net sales. After CIMYM has received US$30 million, CIMYM continues to receives royalties on net sales but at a lesser percentage. Oncoscience has agreed to use diligent and reasonable efforts to develop and commercially exploit TheraCIM in the licensed territory. No amounts or royalties have been received as of the date hereof by CIMYM from Oncoscience, since no sublicensing fees or net sales amounts have been received by Oncoscience. This license agreement may be terminated by either party in the event of specified breaches and insolvency events, if a Phase II trial of TheraCIM has not commenced in Europe within two years of licensing, or if certain regulatory approvals for marketing TheraCIM in Europe have not been obtained within five years. In addition, Oncoscience may terminate the agreement at any time on 90 days notice.
TGFΑ CANCER VACCINE AND HER-1 BASED CANCER VACCINE:
On July 13, 2004, the Corporation and its subsidiary, CIMYM (Barbados), have entered into a License, Development, Manufacturing and Supply Agreement with Tarcanta, two wholly-owned subsidiaries of CancerVax, and CIMAB relating to Tarcanta licensing TGFα and HER-1 from CIMAB. CancerVax has announced that it has received a license from OFAC authorizing Tarcanta to enter into the transactions with CIMAB and the Corporation. By the terms of this agreement with Tarcanta, the 2001 CIMYM License has been suspended until such time, if at all, there is a default under the agreement with Tarcanta. Under the terms of the new agreement and in consideration for the suspension of the 2001 CIMYM License, the Corporation is entitled to receive an aggregate payment of $1,000,000 which is payable in four equal installments, the final payment due December 31, 2005. In addition, under the new agreement the Corporation may receive 35% of an aggregate of $16,350,000 in milestone payments to be paid by Tarcanta upon the successful completion of certain research and development activities. The Corporation has no continuing involvement in these research and development activities and has no future obligations under the development plan established by the out-licensing arrangement between CIMAB and Tarcanta. Finally, the Corporation retains an interest in the revenues from the manufacture and marketing of the drugs or from their sub-licensing. Tarcanta has agreed to undertake further clinical development of the licensed drugs, and acquired an exclusive license to market and sell the products in North America, Europe and certain other jurisdictions. This agreement is terminable by a party in certain circumstances including in the event of specified breaches and insolvency events. CIMAB has the right to terminate if reasonable efforts are not made to file submissions for clinical trials for the licensed products, or if the first regulatory approval for marketing the licensed products is not received within 12 years. In addition, Tarcanta may terminate the agreement at any time on 180 days notice. In the event of early termination, the 2001 CIMYM License would be reinstated.
-42-
TESMILIFENE
In January 2005, the Corporation licensed the rights to tesmilifene to Shin Poong Pharmaceutical Company of Seoul, Korea (“Shin Poong”). They formed a joint development team to oversee the expansion of the development program for tesmilifene into gastric cancer. Shin Poong will fully fund development costs and will provide up-front, milestone and royalty payments. In addition, Shin Poong expects to launch a bridging study in the local population in order to allow the breast cancer indication currently under study by the Corporation to be launched in South Korea.
THERACIM-HR3
In May 2005, the Corporation licensed the rights to TheraCIM-hR3 to Kuhnil. They formed a joint development team to oversee the expansion of the development program for TheraCIM-hR3 (nimotuzumab), its monoclonal antibody against the EGF receptor, for a specific population of patients with non-small cell lung cancer. Kuhnil will fully fund development costs and will provide up-front, milestone and royalty payments.
REGULATORY APPROVAL
Securing final regulatory approval for the manufacture and sale of human therapeutic products in Canada and the Corporation's other territories, including the United States, is a long and costly process that is controlled by that particular territory's national regulatory agency. The national regulatory agency in Canada is Health Canada, and in the United States it is the FDA. Other national regulatory agencies have similar regulatory approval processes, but each national regulatory agency has its own approval processes. Approval in either Canada or the United States does not assure approval by other national regulatory agencies, although often test results from one country may be used in applications for regulatory approval in another country.
Prior to obtaining final regulatory approval to market a drug product, every national regulatory agency has a variety of statutes and regulations which govern the principal development activities. These laws require controlled research and testing of products, government review and approval of a submission containing pre-clinical and clinical data establishing the safety and efficacy of the product for each use sought, approval of manufacturing facilities including adherence to GMP during production and storage, and control of marketing activities, including advertising and labeling.
None of the Corporation's products has been completely developed or tested and, therefore, we are not yet in a position to seek final regulatory approval to market any of our in-licensed products. To date we have obtained various regulatory approvals to develop and test our in-licensed products. Currently the Corporation is conducting an international Phase III trial of tesmilifene in metastatic and recurrent breast cancer in 700 patients. The Corporation has received regulatory approvals for the tesmilifene study in several countries, including Canada and the United States, and approval is pending in a few other countries. In addition, TheraCIM has been designated an orphan drug in Europe and by the FDA in the United States. See "Products in Clinical Development".
-43-
CANADIAN APPROVAL PROCESS
The manufacture, distribution and consumption of medical products, drugs and equipment is regulated by a variety of industry-specific statutes and regulations in Canada and the countries to which YM has rights for the licensed products. Drugs sold in Canada are regulated by the Food and Drugs Act (Canada) and the regulations made under that Act.
Even though a drug, medical product or device may be approved for use in another jurisdiction, it may not be sold in Canada until approved by Health Canada. Outside Canada, the regulatory approval process for the manufacture and sale of pharmaceuticals varies from country to country and the time required may be longer or shorter than that required by Health Canada.
The Food and Drug Regulations require licensing of manufacturing facilities, carefully controlled research and testing of products, governmental review and approval of test results prior to marketing of therapeutic products, and adherence to GMP, as defined by each licensing jurisdiction, during production.
The principal activities which must be completed prior to obtaining approval for marketing of a therapeutic drug product are essentially the same in Canada as in most major markets of the world and are as follows:
o Pre-clinical Animal Studies. Pre-clinical studies are conducted in animals to test pharmacology and toxicology and to do formulation work based on in vivo results.
o Phase I Clinical Trials. Phase I clinical trials consist of testing a product in a small number of humans for its safety (toxicity), dose tolerance and pharmacokinetic properties.
o Phase II Clinical Trials. Phase II clinical trials usually involve a larger patient population than is required for Phase I trials and are conducted to evaluate the efficacy of a product in patients having the disease or medical condition for which the product is indicated. These trials also serve to further identify side effects and risks in a larger group of patients.
o Phase III Clinical Trials. Phase III clinical trials involve conducting tests in an expanded patient population at geographically dispersed test sites (multi-center trials) in a controlled and/or uncontrolled environment to gather information about clinical safety and efficacy. These trials also generate information from which the overall benefit-risk relationship of the drug can be determined and provide a basis for drug labeling.
Two key factors influencing the progression of clinical trials are the rate at which patients can be recruited into clinical trials and whether effective treatments are currently available for the disease the drug is intended to treat. Patient recruitment is largely dependent upon the incidence and severity of the disease and the alternative treatments available, as well as alternate research studies.
A Clinical Trial Application must be filed and accepted by either the Therapeutic Products Directorate ("TPD") or the Biologics and Genetic Therapies Directorate ("BGTD") of Health Canada before each phase of human clinical trials may begin. The CTA application must contain specified information including the results of the pre-clinical or clinical tests completed at the time of the CTA application. In addition, since the method of manufacture may affect the efficacy and safety of a drug, information on chemistry and manufacturing methods must be presented. Health Canada conducts inspections to determine compliance with GMP. Good manufacturing practices and quality control procedures must be in place.
Upon completion of all clinical studies, the results are submitted to the TPD or BGTD as part of a New Drug Submission ("NDS"). A notice of compliance ("NOC") which permits marketing of the product typically takes between 12 and 24 months from the date a NDS is submitted.
Even after marketing approval has been obtained, further studies may be required to provide additional data on safety and efficacy in order to gain approval for the use of a drug as a treatment for clinical indications other than those for which the product was initially tested. Also, Health Canada conducts post-market surveillance programmes to monitor a product's side effects. Results of post-marketing programmes may limit or expand the further marketing of products. A serious safety or efficacy problem involving an approved drug or medical device may result in Health Canada action requiring withdrawal of the product from the market.
-44-
UNITED STATES APPROVAL PROCESS
In the United States, the FDA, a federal government agency, is responsible for the drug approval process. The FDA's mission is to ensure that all medications on the market are safe and are effective. The FDA's approval process examines potential drugs; only those that meet strict requirements are approved.
The drug approval process begins with the discovery of a potential drug. Pharmaceutical companies then test the drug extensively. A description of the different stages in the drug approval process in the US follows.
Stage 1: Preclinical Research
After an experimental drug is discovered, research is conducted to help determine its potential for treating or curing an illness. This is called preclinical research. Animal studies are conducted to determine if there are any harmful effects of the drug and to help understand how the drug works. Information from these experiments is submitted to the FDA in an Investigational New Drug Application. The FDA reviews information in an IND Application and decides if the drug is safe to study in humans.
Stage 2: Clinical Research
In Stage 2, the experimental drug is studied in humans. The studies are known as clinical trials. Clinical trials are carefully designed and controlled experiments in which the experimental drug is administered to patients to test its safety and to determine the effectiveness of an experimental drug. The four general phases of clinical research are described below.
Phase I clinical studies are generally conducted with healthy volunteers who are not taking other medicines; patients with the illness that the drug will treat are not tested at this stage. Ultimately, Phase I studies demonstrate how an experimental drug affects the body of a healthy individual. Phase I consists of a series of small studies consisting of "tens" of volunteers. Tests are done on each volunteer throughout the study to see how the person's body processes, responds to, and is affected by the drug. Low doses and high doses of the drug are usually studied, resulting in the determination of the safe dosage range in volunteers by the end of Phase I. This information will determine whether the drug proceeds to Phase II.
Phase II clinical studies are conducted in order to determine how an experimental drug affects people who have the disease to be treated. Phase II usually consists of a limited number of studies that help determine the drug's short-term safety, side effects, and general effectiveness. The studies in Phase II are often controlled investigations, involving comparison between the experimental drug and a placebo, or between the experimental drug and an existing drug. Information gathered in Phase II studies will determine whether the drug proceeds to Phase III.
Phase III studies consists of numerous clinical trials that are used to more fully investigate the nature of the drug. These trials differ from Phase II trials because a larger number of patients are studied (sometimes in the thousands) and because the studies are usually of longer duration. As well, Phase III studies can include patients who have more than one illness and are taking medications in addition to the experimental drug used in the study. Therefore, the patients in Phase III studies more closely reflect the general population. The information from Phase III forms the basis for most of the drug's initial labeling, which will guide physicians on how to use the drug.
Phase IV studies are conducted after a drug is approved. Companies often conduct Phase IV studies to more fully understand how their drug compares to other drugs. Also, the FDA may require additional studies after the drug is approved. FDA-required Phase IV studies often investigate the drug in specific types of patients that may not have been included in the Phase III studies. FDA-required Phase IV studies can also involve very large numbers of patients to further assess the drug's safety.
-45-
Stage 3: FDA Review and Approval
Following Phase III, the pharmaceutical company prepares reports of all studies conducted on the drug and submits the reports to the FDA in a New Drug Application ("NDA"). The FDA reviews the information in the NDA to determine if the drug is safe and effective for its intended use. Occasionally, the FDA will ask experts for their opinion of the drug; this occurs at advisory committee meetings. If the FDA determines that the drug is safe and effective, the drug will be approved.
Stage 4: Marketing
After the FDA has approved the experimental drug, the pharmaceutical company can make it available to physicians and their patients. A company may also continue to conduct research to discover new uses for the drug. Each time a new use for a drug is discovered, the drug is once again subject to the entire FDA approval process before it an be marketed for that purpose.
ARRANGEMENTS WITH SUBSIDIARIES
YM and CIMAB entered into that certain Funding Agreement with CIMYM in November 1995 in connection with the 1995 CIMYM License. The Funding Agreement provides that YM will arrange for the appropriate studies and clinical trials for the licensed products held by CIMYM and will fund the cost of such studies and trials provided that doing so would not be commercially or scientifically unreasonable. Accordingly, YM makes the final determination as to whether or not a clinical trial expense is justified with respect to any given product. YM is entitled to be reimbursed for all funds we provide pursuant to the Funding Agreement out of revenue generated from the exploitation of the relevant license, subject to the successful development of the licensed products and adequate generation of revenue.
YM and CIMAB, contemporaneously with the assignment of the 1995 CIMYM License, entered into a joint-venture shareholders agreement (the "Shareholders Agreement") with CIMYM relating to its operation. Pursuant to the Shareholders Agreement, CIMYM is required to include nominees of CIMAB both as board members and as members of operating management. The Shareholder Agreement provides that: (i) issued and outstanding shares of CIMYM may not be sold or transferred without the consent of both YM and CIMAB; (ii) the issue of additional shares of CIMYM shall first be offered to each of YM and CIMAB in proportion to their holdings, and thereafter, with the consent of both YM and CIMAB, to any other person; and (iii) the boards of directors of CIMYM will consist of five directors, three of whom are nominees of YM and two of whom are nominees of CIMAB. All material and out-of-the-ordinary-course-of-business contracts of CIMYM, including those relating to the borrowing of money, issuing guarantees, entering into non arm's-length agreements, paying dividends and pledging of property, must be approved by four-fifths of the Board of directors.
CIMYM (Barbados) was incorporated in Barbados in May 1996 to market the licensed products under the 1995 CIMYM License outside of Canada. YM and CIMAB have entered into a joint-venture shareholder agreement (the "Barbados Shareholders Agreement") with CIMYM (Barbados) relating to its operation. The terms of the Barbados Shareholders Agreement are consistent with the Shareholders Agreements, except that the boards of directors of CIMYM (Barbados) consists of a majority of directors nominated by YM. Material and out-of-the-ordinary-course-of-business contracts and approval for the strategic marketing plan and annual budget must be approved by a vote of the majority of directors, including the affirmative vote of at least one nominee of YM and one nominee of CIMAB. YM provides funding to CIMYM (Barbados) under similar terms and conditions as funding to CIMYM. All earnings of CIMYM (Barbados) are to be paid annually to the shareholders as dividends unless a change in such policy is approved by a majority of the directors, including one nominee of each of YM and CIMAB.
Pursuant to international sales, marketing, manufacturing and administrative agreements dated as of July 4, 1996, CIMYM sub-licensed certain of its respective rights to the licensed product under the 1995 CIMYM License to CIMYM (Barbados) in exchange for certain royalty payments.
Under the current arrangements, CIMYM (Barbados) will arrange for the out-licensing of the licensed products in all relevant territories except Canada. CIMYM remains responsible for all elements of commercializing the licensed products within Canada, and for the cost of commercializing the licensed products outside of Canada up to the point of out-licensing.
-46-
PROPERTY, PLANTS AND EQUIPMENT
FACILITIES
The Corporation currently occupies 5,800 square feet of space in Mississauga, Ontario pursuant to a sub-lease agreement dated July 31, 1997 (the "Sub-Lease") and a lease amending and extension agreement dated February 1, 2003 (the "Lease Amending Agreement"), such Lease Amending Agreement extended the initial terms of the Sub-Lease for a term of five years commencing on February 1, 2003 and expiring on January 31, 2008. The average annual costs, including operating expenses, are approximately $120,000.
DELEX occupies 7,200 square feet of space at 6535 Millcreek Drive in Mississauga Ontario pursuant to a lease amending and extension agreement dated March 1, 2005 and expiring on the last day of February 2006. Annual rent is $45,000 under this lease.
There are no environmental issues associated with the facilities and the Corporation currently has no plans to construct, expand or improve the facilities.
EQUIPMENT AND OTHER PROPERTY
As at June 30, 2005, the Corporation owned tangible fixed assets with a book value of approximately $227,000, consisting primarily of office and lab equipment.
EMPLOYEES
As of June 30, 2005, the Corporation employed 14 permanent employees. Each of the employees are located at the Corporation's head office. Other than administrative staff, the employees conduct the Corporation's licensing and product development activities. As a result of the acquisition of DELEX, there are 13 additional employees.
DIVIDENDS
The Corporation has not paid any dividends since its incorporation. The Corporation will consider paying dividends in future as its operational circumstances may permit having regard to, among other things, the Corporation’s earnings, cash flow and financial requirements. It is the current policy of the board of directors to retain all earnings to finance the Corporation’s business plan.
CAPITAL STRUCTURE
The authorized share capital of the Corporation consists of 500,000,000 common shares without nominal or par value, 500,000,000 Class A non-voting common shares without nominal or par value, 500,000,000 Class A preferred shares without nominal or par value and 500,000,000 Class B preferred shares, issuable in series, without nominal or par value. As at June 30, 2005, there were 41,362,066 common shares, no Class A non-voting common shares and no preferred shares outstanding.
COMMON SHARES
All of the common shares rank equally as to voting rights, participation in a distribution of the assets of the Corporation on a liquidation, dissolution or winding-up of the Corporation and the entitlement to dividends. The holders of the common shares are entitled to receive notice of all meetings of shareholders and to attend and vote the common shares at the meetings. Each common share carries with it the right to one vote.
In the event of the liquidation, dissolution or winding-up of the Corporation, the holders of the common shares will be entitled, subject to the rights, privileges, restrictions and conditions attaching to any other class of shares of the Corporation, to receive, on a pro rata basis, share for share, with the Class A non-voting common shares, all of the remaining property of the Corporation. There are no pre-emptive or conversion rights and no provisions for redemption, retraction, purchase for cancellation or surrender or sinking or purchase funds.
-47-
MARKET FOR SECURITIES
The Corporation has been listed on the Toronto Stock Exchange (“TSX”) and the Alternative Investment Market (“AIM”) operated by the London Stock Exchange since June 11, 2002. Initially, the Corporation listed our Class B Preferred Shares, Series 1. On June 12, 2003, the Class B Preferred Shares, Series 1, were automatically converted on a one-for-one basis into the common shares, which became listed on the TSX and the AIM on that date.
The Corporation's common shares have traded on the TSX since June 12, 2003 under the symbol “YM”, were admitted to trading on the AIM on June 12, 2003 under the symbol “YMBA” and have been traded on the American Stock and Options Exchange (“AMEX”) since October 1, 2004 under the symbol “YMI”.
DIRECTORS AND OFFICERS
| Name | Position | Period Served |
David G.P. Allan Toronto, Canada | Chairman, Chief Executive Officer and Director | Since 1994 |
Thomas I.A. Allen (1)(2)(3) Toronto, Canada | Director | Since 1996 |
Mark Entwistle (3) Ottawa, Canada | Director | Since 1997 |
John Friedman(3) New York, USA | Director | Since 2004 |
Henry Friesen (1) Winnipeg, Manitoba | Director | Since 2001 |
Paul M. Keane Mississauga, Ontario | Officer | Since 1996 |
Vincent Salvatori Victoria, British Columbia | Officer | Since 2002 |
Len Vernon Nobleton, Ontario | Officer | Since 1997 |
Julius Vida (2) Greenwich, USA | Director | Since 2001 |
Gilbert Wenzel Zurich, Switzerland | Director | Since 2001 |
Tryon M. Williams (1)(2) London, England | Director | Since 1995 |
| (1) | Member of Audit Committee. |
| (2) | Member of Corporate Governance and Nominating Committee. |
| (3) | Member of Compensation Committee. |
-48-
SHARE OWNERSHIP OF DIRECTORS AND EXECUTIVE OFFICERS
The following table sets out details of our shares and options that are directly or indirectly held by directors and executive officers as at June 30, 2005, based on 41,362,066 common shares issued and outstanding on such date.
| Name | Number of Common Shares | Percentage of Common Shares Outstanding | Common Shares Held Under Option | Exercise Price | Expiration Date |
| David G.P. Allan | 669,659 | 1.6% | 703,250 | $1.75 - $4.50 | 2007 - 2015 |
| Thomas I.A. Allen | — | — | 113,160 | $1.75 - $4.50 | 2007 - 2015 |
| Mark Entwistle | — | — | 88,160 | $1.75 - $4.50 | 2007 - 2015 |
| John Friedman | — | — | 57,500 | $2.10 - $3.15 | 2014 - 2015 |
| Henry Friesen | — | — | 88,160 | $1.75 - $4.50 | 2011 - 2015 |
| Paul M. Keane | — | — | 224,000 | $1.75 - $4.50 | 2007 - 2015 |
| Diana Pliura | — | — | 265,000 | $3.15 | 2015 |
| Vincent Salvatori | — | — | 85,000 | $1.75 - $3.15 | 2008 - 2015 |
| Len Vernon | — | — | 190,000 | $1.75 - $4.50 | 2008 - 2015 |
| Julius Vida | — | — | 83,160 | $1.75 - $4.50 | 2011 - 2015 |
| Gilbert Wenzel | — | — | 83,160 | $1.75 - $4.50 | 2011 - 2015 |
| Tryon M. Williams | 20,100 | * | 128,098 | $1.75 - $4.50 | 2007 - 2015 |
| * | Less than one percent |
As of the date of hereof, the directors and senior officers of YM BioSciences as a group beneficially owned or controlled, directly or indirectly, 689,759 common shares of the Corporation, representing approximately 1.7% of the issued and outstanding voting shares of the Corporation.
DAVID G.P. ALLAN - CHAIRMAN, CHIEF EXECUTIVE OFFICER AND DIRECTOR
Mr. Allan has been Chief Executive Officer of the Corporation since April 1998 and Chairman of the Board of directors of the Corporation since August 1994. In 1992 Mr. Allan created the Knowledge-Based Industries Group for a Canadian investment bank where he was Executive Director until 1998. Mr. Allan is a former governor of The Toronto Stock Exchange, a former member of the Canadian Healthcare Licensing Association and of the Awards Selection Committee for the Networks of Centres of Excellence in Canada.
THOMAS I.A. ALLEN, Q.C., F.C.I.ARB - DIRECTOR
Mr. Allen was called to the Bar in Ontario and began practicing law in 1965 concentrating on securities law and corporate law. He was a member of senior management of Gordon Capital Corporation, and its merchant bank, Gordon Investment Corporation, from mid 1989 until early 1994. Mr. Allen was counsel to Davies, Ward & Beck from 1994 until 1996 when he joined the Toronto office of the law firm Ogilvy Renault. Mr. Allen is Chairman of the Accounting Standards Oversight Council of Canada, a member of the Advisory Board of the Office of the Superintendent of Financial Institutions of Canada and a director of Bema Gold Corporation, Mundoro Mining Inc., GEAC Computer Corporation, Unisphere Waste Conversion Inc., and Middlefield Bancorp Limited. Mr Allen has been a director of the Corporation since December 1996.
-49-
MARK ENTWISTLE, M.A. - DIRECTOR
Prior to founding his own consulting practice in 1997 in international trade, political business intelligence and strategic communications, Mr. Entwistle was an Ambassador for Canada in the Caribbean from 1993 to 1997. Mr. Entwistle was previously a career diplomat with the Canadian Department of Foreign Affairs and International Trade in a variety of embassy positions from 1982 to 1997, and served as Press Secretary and Director of Communications to the Prime Minister of Canada from 1991-1993. He is a Fellow of the Canadian Defence and Foreign Affairs Institute. Mr. Entwistle has been a director of the Corporation since October 1997.
JOHN FRIEDMAN - DIRECTOR
Mr. Friedman launched the Easton Capital Group (“Easton”) in 1993, with Easton Capital Corporation. In 1999, Easton Hunt Capital Partners was added to the Group. Prior to Easton, Mr. Friedman was a founder of Atrium Capital Corporation, which he helped manage from 1991-1993, and also the founder and Managing General Partner of Security Pacific Capital Investors from 1989 through 1991. Security Pacific Capital Investors was a $200-million private equity fund geared towards expansion financings and recapitalizations. Prior to joining Security Pacific, Mr. Friedman was a Managing Director and Partner at E.M. Warburg, Pincus & Co., Inc., where he spent eight and a half years from 1981-1989. Prior thereto, he worked at Shearson Loeb Rhoades and was an attorney with Sullivan and Cromwell from 1978 through 1980. He holds a JD degree from Yale Law School and a BA degree from Yale College. Mr. Friedman currently serves on the Boards of Conor Medsystems, Renovis, Acorda Therapeutics, Comverse Technology, Trellis Bioscience, YM BioSciences Inc., Assistive Technology, and ModelWire, Inc., and is on the President's Council at the Cold Spring Harbor Laboratory. Mr. Friedman has been a director of the Corporation since April 2004.
HENRY FRIESEN, O.C., M.D., F.R.S.C. - DIRECTOR
Dr. Friesen was most recently Chair, Genome Canada from 2000 until July 2005, a $600 million budget non-profit organization that supports national genomics to benefit Canadian science and industry. From 1991 to 2000 Dr. Friesen was President of the Medical Research Council of Canada and was instrumental in transforming it into the Canadian Institute of Health Research, an organization with an annual budget of over $650 million per year dedicated to supporting Canadian researchers as well as industry participants. Dr. Friesen is noted for his discoveries about the human hormone prolactin and as Head of the Department of Physiology and Professor of Medicine at the University of Manitoba. Dr. Friesen is a Fellow of the Royal Society of Canada, a Companion of the Order of Canada and also sits on the Board of directors of Sanofi Pasteur Canada and Spectral Diagnostics Inc. Dr. Friesen has been a director of the Corporation since November 2001.
PAUL M. KEANE, M.D., F.R.C.P.C., F.A.C.P., F.R.C. PATH - DIRECTOR, MEDICAL AFFAIRS
Dr. Keane has been an officer of the Corporation since January 1996. Dr. Keane was Director of Clinical Research at Miles Canada Inc. (now Bayer Canada) from 1989 to 1995, prior to which he was Professor of Medicine at University of Calgary and Professor of Pathology at McMaster University. Dr. Keane has authored numerous scientific publications in peer review journals, has acted as a reviewer of research proposals for the Medical Research Council of Canada and has acted in an editorial capacity for a number of scientific journals.
VINCENT SALVATORI, PH.D. - EXECUTIVE VICE PRESIDENT
Dr. Salvatori has been an officer of the Corporation since December 2002. Dr. Salvatori is an experienced drug development executive with an accomplished background in the pharmaceutical and biotechnology industry. He has more than 20 years of experience in all aspects of drug development, corporate operations and external collaborations. Dr. Salvatori most recently held the position of Senior Vice President of Clinical Operations for Bioniche Life Sciences Inc. from May 1998 to July 2002. He was previously at StressGen Biotechnologies Corporation from January 1995 to April 1998 where he held the positions of Chief Operating Officer and Vice President of Research and Development, subsequently appointed to Senior Vice President. In this capacity, Dr. Salvatori was responsible for corporate operations, strategic management and clinical/regulatory development. Prior to joining StressGen, Dr. Salvatori was the Senior Director of Program Management at QLT PhotoTherapeutics Inc. from June 1990 to December 1994 and held various positions at Boehringer Ingelheim (Canada) Ltd. from April 1982 to June 1990.
-50-
LEN VERNON, B.SC., C.A. - DIRECTOR, FINANCE AND ADMINISTRATION
Mr. Vernon earned a B.Sc. in 1968 and was awarded his C.A. in 1972 with Clarkson Gordon & Co. (now Ernst & Young llp). He has held senior financial positions with a number of organizations both public and private. Prior to joining YM as an officer in July 1997, Mr. Vernon was an independent consultant working with senior management in a variety of industries. Prior to 1992 he was Vice-president, Finance and Administration of Unitel Inc. (now Allstream Inc.) a major Canadian telecommunications company.
JULIUS VIDA, PH.D., M.B.A. - DIRECTOR
Dr. Vida has been the President of Vida International Pharmaceutical Consultants, a consulting firm advising pharmaceutical and biotechnology companies, since 1993. Previously Dr. Vida was Director of Licensing and subsequently Vice President, Business Development, Licensing and Strategic Planning at Bristol-Myers Squibb, from 1975 to 1993. Dr. Vida is a director of a number of biotechnology firms including Medarex, Inc., Orphan Medical, Inc., ALS, Inc., FibroGen, Inc., OsteoScreen, Inc., Spectrum Pharmaceuticals Inc., Albachem, LTD. (UK) and SWITCH Biotech AG, Inc. Dr. Vida has been a director of the Corporation since September 2001.
GILBERT WENZEL, PH.D. - DIRECTOR
Dr. Wenzel is currently President and Chief Executive Officer of Quisisana AG, a business development firm focused on pharmaceuticals. Prior to founding Quisisana in January 2003, Dr. Wenzel joined Novartis Group, a global pharmaceutical manufacturer, in November 2000 where he served as Head of Strategic Planning and a Member of its Executive Committee until January 2003. Prior to joining Novartis in November 2000, Dr. Wenzel spent 15 years with McKinsey & Co., an international management consulting firm, and was a member of the European Leadership Group of its Pharma/Healthcare Sector and of the European New Venture Initiative. From 1981 to 1985, Dr. Wenzel was at Hoechst AG in Germany and developed global strategies for generics and over-the-counter medicines. Dr. Wenzel has been a director of the Corporation since March 2001.
TRYON M. WILLIAMS, B.SC. (MATH) - DIRECTOR
Mr. Williams is the Chairman, CEO and director of CellStop Systems, Inc., an automobile electronics manufacturer, and Chief Executive Officer and director of Bingo.com, Inc., an internet technology company. Since 1993, Mr. Williams has been Adjunct Professor, Sauder School of Business, The University of British Columbia. Mr. Williams is also a director of Infowave Software, Inc. and several other private corporations. Mr. Williams has been a director of the Corporation since November 1995.
CLINICAL AND SCIENTIFIC ADVISORY BOARD
The Corporation maintains a Clinical and Scientific Advisory Board (“CSAB”) composed of internationally recognized clinicians and scientists. Management meets with members of the CSAB periodically to review operational aspects of the Corporation's clinical and scientific programme and make recommendations with regard to the perceived trends and direction of medical and biopharmaceutical technologies and the industry generally. Each member of the CSAB has signed a confidentiality agreement with the Corporation. CSAB members receive honoraria paid by the Corporation of varying amounts per year. The current composition of the CSAB is as follows:
-51-
LORNE J. BRANDES, B.SC., M.D., C.R.C.P.C.
Professor, Departments of Medicine and Pharmacology/Therapeutics, University of Manitoba, Winnipeg, Manitoba, Canada; Section of Hematology/Oncology, CancerCare Manitoba, Winnipeg, Manitoba, Canada. Dr. Brandes has been an advisor since November 2000.
ROBERT S. KERBEL, PH.D.
Professor of Medical Biophysics, University of Toronto, Toronto, Ontario, Canada; Canada Research Chair in Molecular Medicine; Director, Molecular and Cell Biology Research, Sunnybrook and Women's College Health Science Centre, Toronto, Ontario, Canada. Dr. Kerbel has been an advisor since April 1999.
AGUSTIN LAGE DAVILA M.D. PH.D.
Director, Centro de Inmunologia Molecular, Havana, Cuba; Professor of Medicine, University of Havana. Dr. Davila was a director of the Corporation until his resignation on May 28, 2002 at which time he became an advisor.
RAYMOND M. REILLY, PH.D.
Associate Professor, Departments of Medical Imaging and Pharmaceutical Sciences, University of Toronto, Toronto, Ontario, Canada; Associate Scientist, Department of Medical Imaging, University Health Network, Toronto, Ontario, Canada. Dr. Reilly was an advisor since December 1998.
NICLAS STIERNHOLM, PH.D.
Chief Executive Officer, Trillium Therapeutics Inc., Toronto, Ontario, Canada. Dr. Stiernholm was an executive vice-president of the Corporation until he resigned in December 2002 at which time he became an advisor.
MARK VINCENT, M.D., M.R.C.P., F.R.C.P.C. - CHAIR
Associate Professor, Department of Oncology, University of Western Ontario, London, Ontario, Canada; Staff Medical Oncologist, London Regional Cancer Centre, London, Ontario, Canada. Dr. Vincent has been an advisor since October 1998.
DANIEL D. VON HOFF, M.D., F.A.C.P.
Professor of Medicine, University of Arizona and Executive Vice President, Translational Genomics Research Institute and Director, Translational Drug Development Program, Tucson, Arizona, United States. Dr. Von Hoff has been an advisor since July 2001.
BOARD PRACTICES
The directors of the Corporation are elected and retired in rotation. The Board of Directors is divided into three equal classes (Class I, Class II and Class III) with the term of office of the directors of one class expiring each year. Each director holds office for a term ending on the date of the third annual general meeting of our shareholders following the annual general meeting of shareholders at which he was elected or until he resigns or is removed from office in accordance with the Corporation's memorandum of association and articles of association. For the purposes of implementing this structure, the current Class I directors will hold office until the 2005 annual general meeting of our shareholders, the current Class II directors will hold office until the 2006 annual general shareholders meeting and the current Class III directors will hold office until the 2007 annual general shareholders meeting. Messrs. Friedman and Vida are Class I directors; Messrs. Entwhile, Friesen and Wenzel are Class II directors; and Messrs. Allen, Allan and Williams are Class III directors.
-52-
No director has a service contract with us. Each director has formally consented to serve as a director and signed a confidentially agreement with us.
From time to time the Board appoints, and empowers, committees to carry out specific functions on behalf of the Board. The following describes the current committees of the Board and their members:
AUDIT COMMITTEE
The members of the Corporation's Audit Committee are Thomas I.A. Allen, Henry Friesen, and Tryon M. Williams.
The principal functions of the Audit Committee are to appoint, compensate and oversee the external auditors; to review and approve annual and quarterly financial statements and all legally required continuous and public disclosure documents containing financial information about the Corporation before they are submitted to the Board of Directors for approval; to review and approve the adequacy of internal accounting controls and the quality of financial reporting procedures and systems; to examine the presentation and impact of key financial and other significant risks that may be material to the Corporation's financial reporting; and to review and approve the nature and scope of the annual audit and review the results of the external auditor's examination. The Audit Committee reports its findings with respect to such matters to the Board of Directors.
CORPORATE GOVERNANCE AND NOMINATING COMMITTEE
The members of the Corporation's Corporate Governance and Nominating Committee are Thomas I.A. Allen, Julius Vida and Tryon M. Williams.
The mandate of the Corporation's Corporate Governance and Nominating Committee is to develop and monitor the Corporation's system of corporate governance in the context of the Toronto Stock Exchange Report on Corporate Governance, and the rules and regulations promulgated by the Ontario Securities Commission and the Securities and Exchange Commission, including reviewing the mandate of the Board of Directors and its committees; periodically reviewing and evaluating the performance of all directors, committees and the Board as a whole; selecting new candidates for Board memberships, making recommendations to the Board and ensuring that appropriate orientation and education programmes are available for new Board members; establishing procedures to ensure that the Board may meet independent of Management and reviewing annually the membership and chairs of all committees. All members of this Committee are unrelated and non-executive directors of the Corporation.
COMPENSATION COMMITTEE
The members of the Corporation's Compensation Committee are Thomas I.A. Allen, John Friedman and Mark Entwistle.
The mandate of the Compensation Committee is to establish and monitor the Corporation's policies for attracting, retaining, developing and motivating senior employees. The compensation policies are designed to support the Corporation's strategic objectives, ensure that incentive programmes are designed to motivate senior managers to achieve or exceed corporate objectives and to enhance shareholder value and to ensure that there is reasonable consistency in the application of the compensation policies. The Corporation's responsibilities include reviewing annually the performance of the Chief Executive Officer (or more frequently if deemed necessary by the Compensation Committee), setting the Chief Executive Officer's compensation and, in consultation with the Chief Executive Officer, establishing his personal objectives, reviewing the performance and approving the compensation of executive officers of the Corporation on the recommendation of the Chief Executive Officer, establishing incentive compensation programmes and monitoring their effectiveness and developing and documenting the compensation policy and philosophy of the Corporation for approval by the Board of Directors. All members of this Committee are unrelated and non-executive directors of the Corporation.
-53-
AUDIT FEES
During the years ended June 30, 2004 and 2005, the Corporation paid the following fees to its external auditors:
Service | Fees Incurred | |
2005 | 2004 | |
| Audit- Fees | $385,000 | $193,000 |
| Audit-Related Fees | Nil | Nil |
| Tax Fees | $12,000 | $35,100 |
| All Other Fees | Nil | Nil |
Total Fees Paid | $397,000 | $228,100 |
Tax fees consist of fees for tax compliance services and tax advisory services.
The Audit Committee follows policy and procedure guidelines prescribed from time to time by the US Securities Exchange Commission in connection with the pre-approval of non-audit services.
LEGAL PROCEEDINGS
The Corporation is not a party to any material pending legal or arbitration proceedings and is not aware of any material contemplated legal proceedings to which we may be a party except for a claim by a minor shareholder of DELEX Therapeutics Inc..
TRANSFER AGENT AND REGISTRAR
The registrar and transfer agent for the Corporation's common shares in Canada is CIBC Mellon Trust Company at its principal offices in Toronto, Canada and in the United States is Mellon Investor Services LLC at its principal offices in Ridgefield Park, New Jersey.
MATERIAL CONTRACTS
Except for contracts entered into in the ordinary course of business, the only material contracts which the Corporation entered into prior to the date hereof as follows:
| (a) | 2005 Amended and Restated Stock Option Plan. See “Share Ownership of Directors and Executive Officers - Stock Option Plan”. |
| (b) | Merger Agreement dated as of April 12, 2005 among YM, 2069044 Ontario Limited (“2069044”), being a wholly-owned subsidiary of YM, DELEX, the Business Development Bank of Canada, New Generation Biotech (Equity) Fund Inc., and Eastern Technology Seed Investment Fund Limited Partnership, pursuant to which YM acquired DELEX. See “General Development of the Business”. |
| (c) | Debt Assignment Agreements each dated as of May 2, 2005 between YM and each of the Business Development Bank of Canada, New Generation Biotech (Equity) Fund Inc., and Eastern Technology Seed Investment Fund Limited Partnership, pursuant to which YM acquired the outstanding debt of DELEX in connection with purchase of DELEX. See “General Development of the Business”. |
| (d) | Escrow Agreement dated as of May 2, 2005 among YM, the Business Development Bank of Canada, New Generation Biotech (Equity) Fund Inc., Eastern Technology Seed Investment Fund Limited Partnership and Equity Transfer Services Inc., pursuant to which certain common shares of YM are held in escrow for the benefit of the former DELEX shareholders to be released in tranches over time and upon completion of certain milestones. See “General Development of the Business”. |
-54-
| (e) | Clinical Research Services Agreement between YM and Pharm-Olam International, Ltd. (“POI”), dated March 10, 2004. The Corporation has contracted with POI to do a Phase III clinical trial with tesmilifene in metastatic and recurrent breast cancer. POI in turn is contracting with others to perform services and to recruit and treat patients. The contract with POI is payable over the next few years depending on the recruitment of patients. |
| (f) | Lease Amending and Extension Agreement between 1411029 Ontario Limited and YM BioSciences Inc. dated January 15, 2003. See “Property, Plants and Equipment - Facilities”. |
| (g) | Development and License Agreement between CIMYM Inc., CIMAB SA and Oncoscience AG, dated November 5, 2003. See “Business - Licensing Arrangements - Out-Licensing - TheraCIM”. |
| (h) | Licensing Bonus Pool Plan dated March 31, 2004. See “Compensation - Licensing Bonus Pool”. |
| (i) | License, Development, Manufacturing and Supply Agreement between YM BioSciences Inc., CIMYM, Inc., Tarcanta, Inc., Tarcanta, Ltd. and CIMAB dated July 13, 2004. “Business - Licensing Arrangements - Out-Licensing”. |
| (j) | Clinical Research Services Agreement between YM and POI dated December 2004 relating to a pharmacokinetics clinical trial of tesmilifene with an anthracycline, involving 30 patients at two sites, at an expected cost of £194,527 ($448,000). Either party may cancel the contract with 30 days' notice; in which case, the Corporation would pay for the cost to date plus a penalty equal to 10% of the remainder of the contract price. |
| (k) | Clinical Research Services Agreement between YM and POI dated June 2005 relating to a pharmacokinetics clinical trial of tesmilifene with a taxane, involving 30 patients at two sites, at an expected cost of £344,000 ($756,000). Either party may cancel the contract with 30 days' notice; in which case, the Corporation would pay for the cost to date plus a penalty equal to 10% of the remainder of the contract price. |
In the ordinary course of our business, the Corporation enters into licenses for products which we develop, however, because of the immateriality of such licenses to the Corporation, they are not referenced here. The licenses for these products are more fully described in this annual information form under the heading “Business Overview - Licensing Arrangements”.
ADDITIONAL INFORMATION
Additional information, including directors’ remuneration and indebtedness, principal holders of the Corporation’s securities, options to purchase securities and interests of insiders in material transactions, if any, is contained in the Corporation’s information circular for its most recent annual meeting of shareholders that involved the election of directors and that additional financial information is provided in the Corporation’s comparative financial statements for its most recently completed year.
When securities of the Corporation are in the course of distribution pursuant to a short form prospectus, or when a preliminary short form prospectus has been filed in respect of the Corporation’s securities, the Corporation will provide the following documents to any person or company upon request to the Corporate Secretary of the Corporation:
| 1. | a copy of this Annual Information Form, together with a copy of any document or the pertinent pages of any document incorporated by reference in this Annual Information Form; |
| 2. | a copy of the Financial Statements of the Corporation, together with the accompanying auditors’ report as well as copies of any subsequent interim financial statements that the Corporation has filed; |
| 3. | a copy of the Corporation’s information circular in respect of its most recent annual meeting of shareholders that involved the election of directors or one copy of annual filing prepared in stead of that information circular, as appropriate; and |
| 4. | a copy of any other document that is incorporated by reference into the preliminary short form prospectus or the short form prospectus. |
-55-
At any other time, a copy of the documents referred to in subsections 1, 2, 3 and 4 above may be obtained from the Corporate Secretary of the Corporation, however, a reasonable fee may be charged if the request is made by a person or company who is not an a shareholder of the Corporation.
All requests for the above-mentioned documents must be addressed to:
YM BioSciences Inc.
5045 Orbitor Drive
Building 11, Suite 400
Mississauga, Ontario
L4W 4Y4
Attention: Secretary
Telephone: (905) 629-9761
Fax: (905) 629-4959
e-mail: ir@ymbiosciences.com
Web Page: www. ymbiosciences.com
-56-
Schedule “A”
YM BIOSCIENCES INC.
AUDIT COMMITTEE MANDATE
1. General
The board of directors (the “Board”) of YM BioSciences Inc. (the “Corporation”) has delegated the responsibilities, authorities and duties described below to the audit committee (the “Audit Committee”). For the purpose of these terms of reference, the term “Corporation” shall include the Corporation and its subsidiaries.
The Audit Committee shall be directly responsible for overseeing the accounting and financial reporting processes of the Corporation and audits of the financial statements of the Corporation, and the Audit Committee shall be directly responsible for the appointment, compensation, and oversight of the work of any registered external auditor employed by the Corporation (including resolution of disagreements between management of the Corporation and the external auditor regarding financial reporting) for the purpose of preparing or issuing an audit report or related work. In so doing, the Audit Committee will comply with all applicable Canadian and United States securities laws, rules and guidelines, any applicable stock exchange requirements or guidelines and any other applicable regulatory rules.
2. Members
The Audit Committee shall be composed of a minimum of three members. Members of the Audit Committee shall be appointed by the Board. Each member shall serve until such member’s successor is appointed, unless that member resigns or is removed by the Board or otherwise ceases to be a director of the Corporation. The Board shall fill any vacancy if the membership of the Committee is less than three directors. The Chair of the Committee may be designated by the Board or, if it does not do so, the members of the Committee may elect a Chair by vote of a majority of the full Committee membership.
All members of the Audit Committee must satisfy the independence, financial literacy and experience requirements of applicable Canadian and United States securities laws, rules and guidelines, any applicable stock exchange requirements or guidelines and any other applicable regulatory rules. In particular:
| (a) | each member shall be “independent” and “financially literate” within the meaning of Multilateral Instrument 52-110 “Audit Committees”; |
| (b) | at least one member must be “financially sophisticated” under the rules of the American Stock Exchange; and |
| (c) | at least one member must be an “audit committee financial expert” within the meaning of that term under the United States Securities Exchange Act of 1934, as amended, and the rules adopted by the United States Securities and Exchange Commission thereunder. |
3. Meetings
The Audit Committee shall meet at least quarterly at such times and at such locations as the Chair of the Audit Committee shall determine, provided that meetings shall be scheduled so as to permit the timely review of the Corporation’s quarterly and annual financial statements and related management discussion and analysis. The external auditor or any two members of the Audit Committee may also request a meeting of the Audit Committee. The Chair of the Audit Committee shall hold in camera sessions of the Audit Committee, without management present, at every meeting.
-57-
The Audit Committee shall submit the minutes of all meetings to the Board, and when requested to, shall discuss the matters discussed at each Audit Committee meeting with the Board. NTD: Confirm how much procedural detail to include.
4. Committee Charter
The Audit Committee shall have a written charter that sets out its mandate and responsibilities and the Audit Committee shall review and reassess the adequacy of such charter at least annually or otherwise, as it deems appropriate, and propose recommended changes to the Board.
5. Duties of the Audit Committee:
The Audit Committee shall have the following duties:
Financial Information and Reporting
| 1. | The Audit Committee shall review with management and the external auditor, and recommend to the Board for approval, the annual and interim financial statements of the Corporation and related financial reporting, including management’s discussion and analysis and earnings press releases. |
| 2. | The Audit Committee shall review with management and the external auditor, and recommend to the Board for approval, any financial statements of the Corporation which have not previously been approved by the Board and which are to be included in a prospectus or other public disclosure document of the Corporation. |
| 3. | The Audit Committee shall consider and be satisfied that adequate policies and procedures are in place for the review of the Corporation’s disclosure of financial information extracted or derived from the Corporation’s financial statements (other than disclosure referred to in clause (a)(i) above), and periodically assess the adequacy of such procedures. |
Internal Controls
| 4. | The Audit Committee shall review, as appropriate, the Corporation’s internal system of audit controls and the results of internal audits. |
| 5. | The Audit Committee shall establish procedures for the receipt, retention and treatment of any complaint regarding accounting, internal accounting controls or auditing matters; and the confidential, anonymous submissions by employees of concerns regarding questionable accounting or auditing matters. |
External Auditors
| 6. | The Audit Committee shall be directly responsible for overseeing the work of the external auditor engaged for the purpose of preparing or issuing an auditor’s report or performing other audit, review or attest services for the Corporation, including the resolution of disagreements between management and the external auditor regarding financial reporting. |
| 7. | The external auditor shall report directly to the Audit Committee and the Audit Committee should have a clear understanding with the external auditor that such external auditor must maintain an open and transparent relationship with the Audit Committee, and that the ultimate accountability of the external auditor is to the shareholders of the Corporation. |
| 8. | The Audit Committee shall recommend to the Board the external auditor to be nominated for the purpose of preparing or issuing an auditor’s report or performing other audit, review or attest services for the Corporation; and the compensation of the external auditor. |
-58-
| 9. | The Audit Committee will ensure the rotation of partners on the audit engagement team of the external auditor in accordance with applicable law. |
| 10. | The Audit Committee shall meet with the external auditor, as the Audit Committee may deem appropriate, to consider any matter which the Audit Committee or external auditor believes should be brought to the attention of the Board or the shareholders of the Corporation. |
| 11. | The Audit Committee shall meet with the external auditor, as the Audit Committee may deem appropriate, to review and discuss a report from the external auditor at least quarterly regarding: |
| (a) | all critical accounting policies and practices to be used |
| (b) | all alternative treatments within generally accepted accounting principles for policies and practices related to material items that have been discussed with management, including the ramifications of the use of such alternative disclosures and treatments, and the treatment preferred by the external auditor, and |
| (c) | other material written communications between the external auditor and management, such as any management letter or schedule of unadjusted differences. |
Pre Approval of Non-Audit Services
| 12. | The Audit Committee shall pre-approve all non-audit services to be provided to the Corporation or its subsidiary entities by the Corporation’s external auditor. |
Complaints procedure
| 13. | The Audit Committee shall establish procedures for the receipt, retention and treatment of complaints received by the Corporation regarding accounting, internal accounting controls, or auditing matters; and the confidential, anonymous submission by employees of the Corporation of concerns regarding questionable accounting or auditing matters. |
| 14. | The Audit Committee shall review and approve the Corporation’s hiring policies regarding partners, employees and former partners and employees of the present and former external auditor of the Corporation. |
Reporting
| 15. | The Audit Committee shall report regularly to the Board about any issues that arise with respect to the quality or integrity of the Corporation’s financial statements, the Corporation’s compliance with legal or regulatory requirements, the performance and independence of the external auditor, or the internal audit function. |
6. Authority to engage independent counsel and advisors
The Audit Committee has the authority to engage independent counsel and other advisors as it determines necessary to carry out its duties, to set and pay the compensation for any advisors employed by the audit committee, and to communicate directly with the internal and external auditors.
The Corporation shall provide appropriate funding, as determined by the Audit Committee, in its capacity as a committee of the board of directors, for payment of compensation (a) to the external auditors employed by the issuer for the purpose of rendering or issuing an audit report, and (b) to any advisers employed by the Audit Committee.
-59-