
September 2013 A specialty pharmaceutical company focused on the development and commercialization of proprietary products to address important therapeutic needs in the field of neuroscience NASDAQ:TSPT

Forward-looking statements This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, included in this press release regarding our strategy, future operations, future financial position, future revenues, projected expenses, prospects, plans and objectives of management are forward-looking statements. Examples of such statements include, but are not limited to, statements relating to the following: beliefs regarding our product candidate pipeline, including potential market size, risk and capital efficiency; TO-2070 offers a new and improved approach to the treatment of acute migraine; the potential for TO-2070 to be superior to existing migraine treatments, including by offering significant benefits at relatively low cost; the benefits and nature of SNBL’s nasal drug delivery system with respect to TO-2070 and as compared to existing delivery systems, including bioavailability, convenience, cost, ease of self-administration, safety and speed; the potential size and growth of the U.S. nasal drug delivery and migraine markets; migraine patient and physician behaviors; beliefs regarding the inadequacy of existing treatment options for migraine; expectations regarding the timing of FDA approval, pricing, revenue and other characteristics of potentially competing products; the occurrence and timing of our studies, receipt of data and regulatory milestones regarding TO-2070; third-party expert beliefs regarding TO-2070; and our beliefs regarding the sales of and potential market and positioning for Intermezzo®. We may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in our forward- looking statements and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions, expectations and projections disclosed in the forward-looking statements. Various important factors relating to Transcept could cause actual results or events to differ materially from the forward-looking statements that we make, including the following: achieving acceptance of Intermezzo by physicians, patients and third party payors; our dependence on our collaboration with Purdue; supplying sufficient quantities of Intermezzo from third party manufacturers and suppliers to meet anticipated market demand; the impact of competitive products and the market for Intermezzo generally; obtaining, maintaining and protecting regulatory exclusivity and intellectual property protection for Intermezzo; our ability to identify and finance additional product candidates for in-licensing or acquisition; and our ability to obtain additional funding, if needed, to support our business activities. These and other risks are described in greater detail in the "Risk Factors" section of Transcept periodic reports filed with the Securities and Exchange Commission. Forward-looking statements do not reflect the potential impact of any future in-licensing, collaborations, acquisitions, mergers, dispositions, joint ventures, or investments Transcept may enter into or make. Transcept does not assume any obligation to update any forward-looking statements, except as required by law. 2
Transcept Pharmaceuticals: Neuroscience focused specialty pharmaceutical company 3 First and only product approved for middle of the night insomnia US primary care marketing agreement: Purdue Pharma INTERMEZZO® for MOTN insomnia ~$78MM in cash as of 6/30/13; no debt Strong balance sheet Successful track record of building specialty pharma companies Successful neuroscience drug development experience through NDA approval Proven team Rapidly absorbed DHE for acute migraine Proprietary drug delivery platform Easy to use, self administered dosage form Comparatively low cost of goods expected TO-2070 for acute migraine • Large market • Low risk • Rapid and capital- efficient development
Proprietary nasal powder drug delivery technology

Nasal drug delivery Significant market opportunity U.S. nasal Rx drug delivery market estimated > $3B in 2013 Nasal cavity: potentially efficient drug delivery path – Nasal mucosa is permeable and leads to a significant vascular capillary bed – Safety advantages vs. e.g., pulmonary delivery However, previous attempts have not realized potential advantages – Liquid nasal sprays typically have short nasal residence time: • Poor bioavailability and variable absorption • Significant portion of dose is swallowed – Use of potentially irritating or toxic permeability enhancers – Stability issues – Bad taste 5

Shin Nippon Biomedical Laboratories: Optimizing nasal drug delivery Major Contract Research Organization with over 2,000 employees across the United States, Asia and Europe Established 1957 Provides comprehensive range of non-clinical and clinical development services to biopharmaceutical companies Listed on Tokyo stock exchange [Ticker: 2395.T] Leveraging expertise to diversify into other businesses Began internal development of unique, patent-protected intranasal powder drug delivery platform technology >10 years ago as part of diversification strategy 6
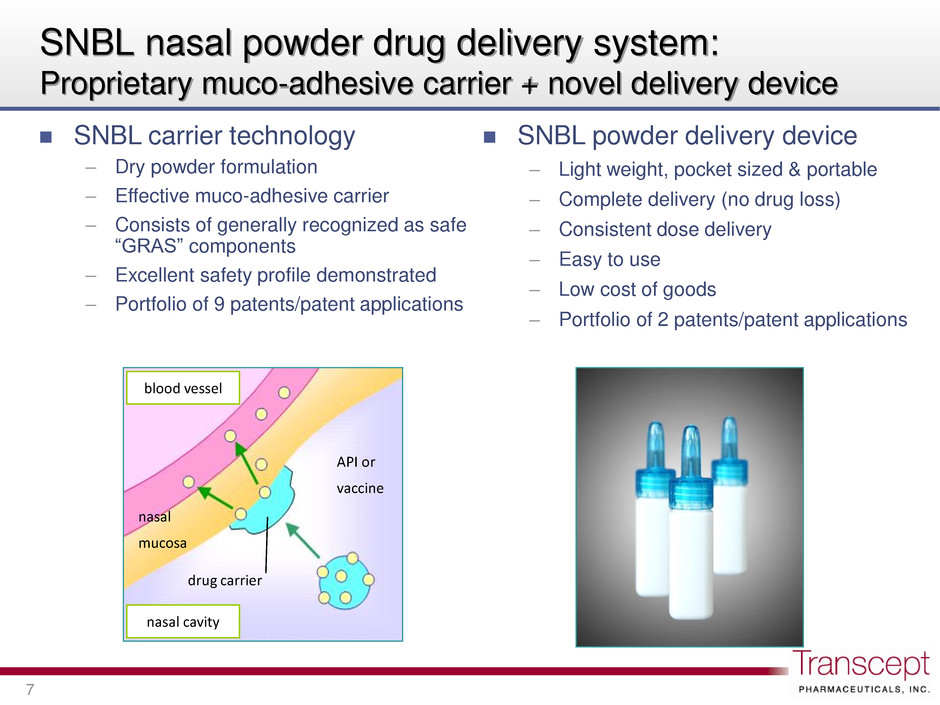
SNBL nasal powder drug delivery system: Proprietary muco-adhesive carrier + novel delivery device 7 SNBL carrier technology – Dry powder formulation – Effective muco-adhesive carrier – Consists of generally recognized as safe “GRAS” components – Excellent safety profile demonstrated – Portfolio of 9 patents/patent applications SNBL powder delivery device – Light weight, pocket sized & portable – Complete delivery (no drug loss) – Consistent dose delivery – Easy to use – Low cost of goods – Portfolio of 2 patents/patent applications blood vessel API or vaccine drug carrier nasal mucosa nasal cavity
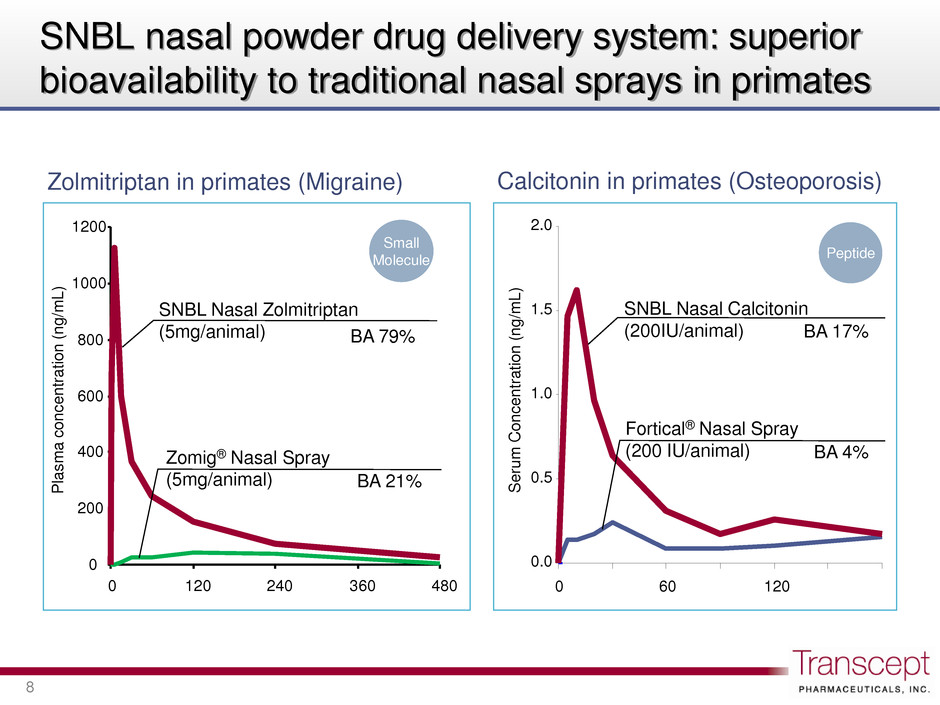
SNBL nasal powder drug delivery system: superior bioavailability to traditional nasal sprays in primates 8 Zolmitriptan in primates (Migraine) Calcitonin in primates (Osteoporosis) 0 200 400 600 800 1000 1200 0 120 240 360 480 P las m a c o n c e n tra ti o n (n g /m L ) Zomig® Nasal Spray (5mg/animal) BA 21% SNBL Nasal Zolmitriptan (5mg/animal) BA 79% Small Molecule 0.0 0.5 1.0 1.5 2.0 0 60 120 S e ru m Co n c e n tr a ti o n (n g /m L ) BA 17% SNBL Nasal Calcitonin (200IU/animal) Fortical® Nasal Spray (200 IU/animal) Peptide BA 4%

0 1 2 3 4 5 6 7 8 9 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 Zo lmi tri p ta n Co n ce n tr at io n ( n g /m L) Time (h) TRZ 0.75 mg TRZ 1.5 mg TRZ 3.0 mg Zomig NS 5 mg 9 SNBL nasal powder delivery system: Human studies confirm primate PK results SNBL zolmitriptan powder 0.75 mg SNBL nasal zolmitriptan powder vs. Zomig nasal spray (n=18) SNBL zolmitriptan powder 1.5 mg SNBL zolmitriptan powder 3.0 mg i nasal spray 5mg
TO-2070: designed for rapid and sustained relief of acute migraine
Migraine: large market with significant unmet needs 11 US and Western Europe 1-year prevalence:11% overall1 – According to a WHO survey, a day with severe migraine is as disabling as a day with quadriplegia2 In US each year: ~30MM cases; $2.6B Rx product sales3 ~80% of migraine Rx patients would be willing to try another medication4 Patients and physicians continue to look for better treatment options Patient types with unmet needs include those who: – fail to respond to triptans – suffer from severe nausea/vomiting/gastroparesis5 – present with severe allodynia (hypersensitive pain reaction) – have high-frequency migraines – are affected by menstrual migraines, and – experience significant disability. 1. Matharu, European J Neurol 2003. 2. Goadsby, NEJM 2002. 3. Decision Resources, Migraine 2013. 4. Bigal M et al. Headache 2007. 5. Krymchantowski AV et al. Cephalalgia 2006.

DHE: a trusted treatment for migraine Need for improved delivery systems 12 DHE has long been recognized as an effective treatment for migraine – Approved for use in the United States since 1946 – Frequently used by migraine specialists in triptan failures Poor oral bioavailability – Generally administered via subcutaneous (SC), intramuscular (IM) and intravenous (IV) injection Currently available liquid nasal spray – Inconvenient dosing regimen – Slow onset of relief – Inconsistent absorption – Post-nasal drip and bitter taste Pulmonary drug delivery – DHE pulmonary delivery via inhalation is currently under review by the FDA
TO-2070: a better way to deliver DHE for migraine Rapidly absorbed – Bioavailability expected to be comparable to IM and pulmonary (LevadexTM) – Superior to conventional nasal sprays Sustained efficacy – DHE is associated with lower recurrence rates versus triptans1 – Sustained high bioavailability may further support DHE’s low recurrence rate Dependable – Highly bioavailable with rapid and consistent absorption Low cost of goods 13 1. Saper, Headache 2006
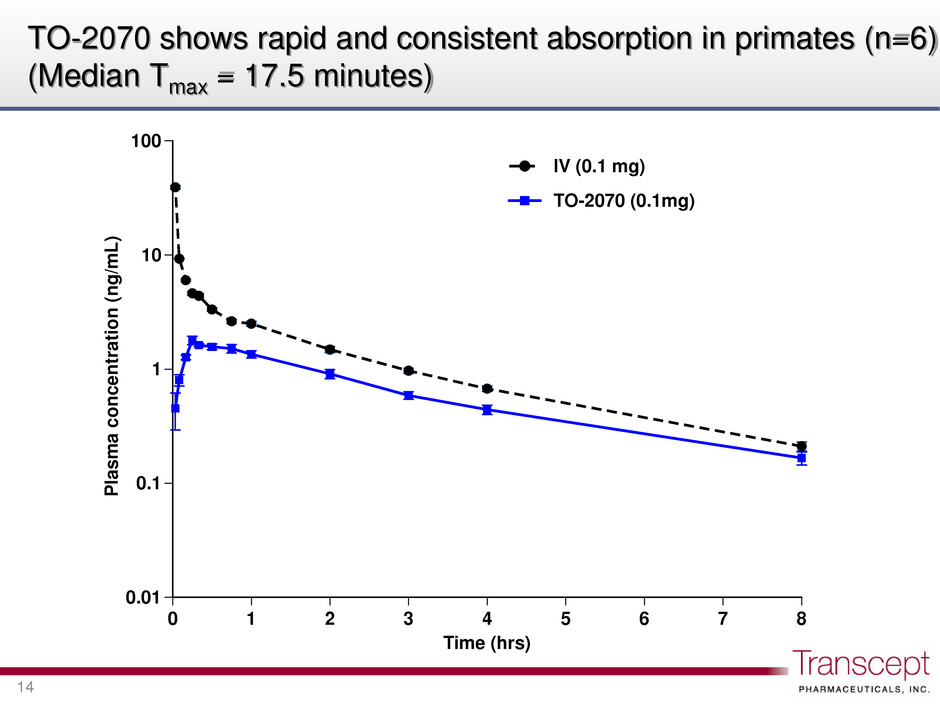
0.01 0.1 1 10 100 0 1 2 3 4 5 6 7 8 P la sm a c on ce nt ra tion (n g/ m L) Time (hrs) IV (0.1 mg) TO-2070 (0.1mg) TO-2070 shows rapid and consistent absorption in primates (n=6) (Median Tmax = 17.5 minutes) 14
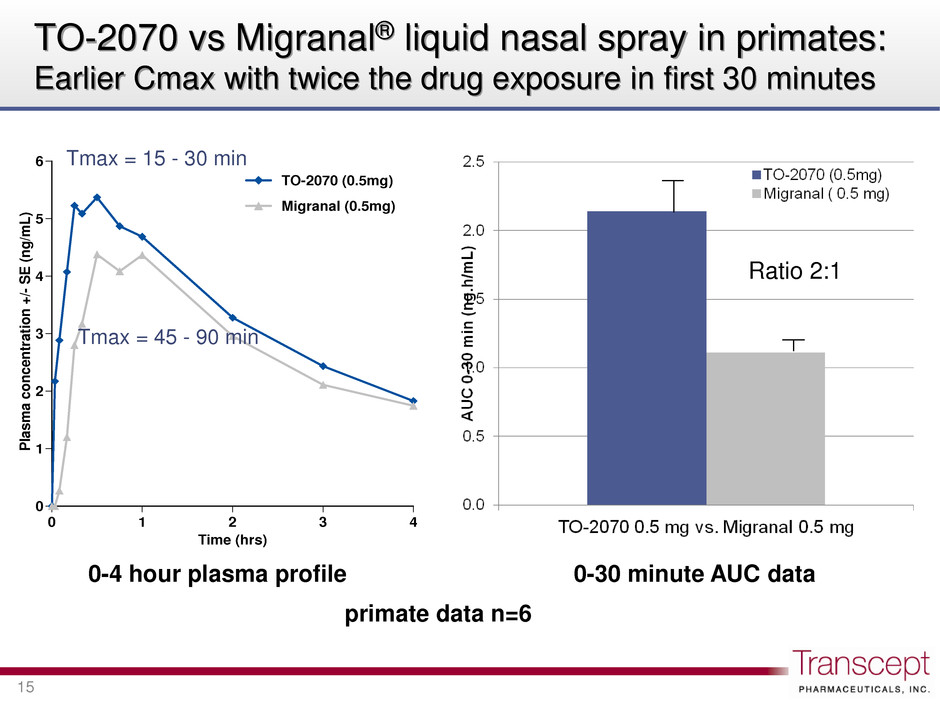
0 1 2 3 4 5 6 0 1 2 3 4 P la s m a c o n c e n tr a tion +/- S E (n g /m L ) Time (hrs) TO-2070 (0.5mg) Migranal (0.5mg) TO-2070 vs Migranal® liquid nasal spray in primates: Earlier Cmax with twice the drug exposure in first 30 minutes 15 Ratio 2:1 0-30 minute AUC data 0-4 hour plasma profile primate data n=6 Tmax = 15 - 30 min Tmax = 45 - 90 min
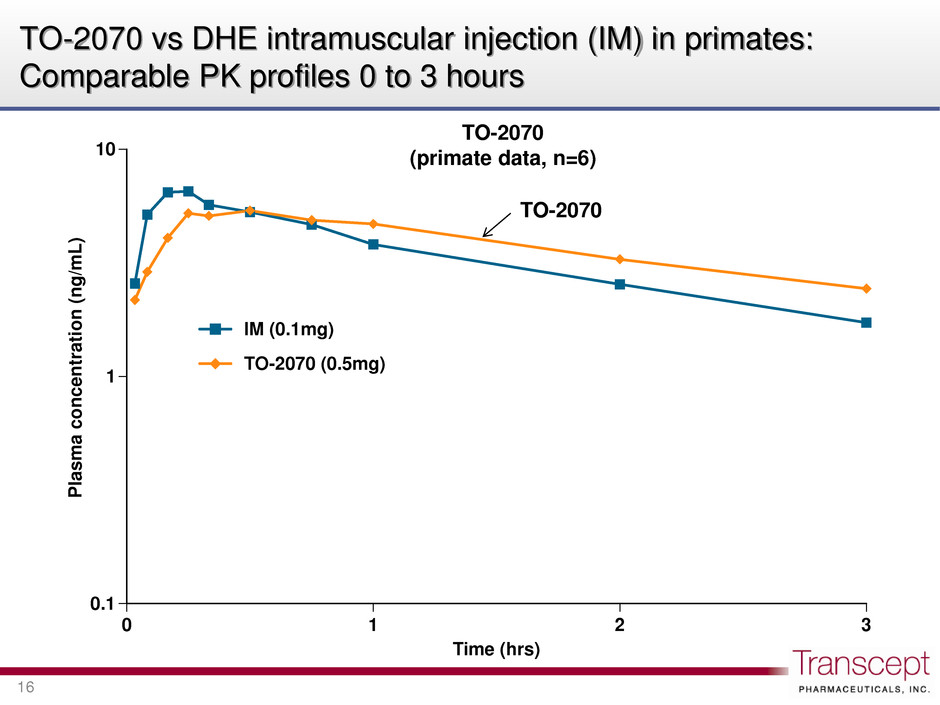
0.1 1 10 0 1 2 3 Pla sm a c on ce nt ra tion (n g/ m L) Time (hrs) IM (0.1mg) TO-2070 (0.5mg) TO-2070 vs DHE intramuscular injection (IM) in primates: Comparable PK profiles 0 to 3 hours 16 TO-2070 (primate data, n=6) TO-2070

Allergan’s Levadex (under FDA review) Developed by MAP Pharmaceuticals; acquired by Allergan for ~$1.0 B (2013) Pulmonary inhalation DHE product Approval expected mid-2014 Anticipated pricing >$75 - 100 per dose Allergan and analysts forecast $500MM in peak US sales 17 Potential drawbacks High cost of goods/price Device size Potential for pulmonary adverse effects to emerge with use in “real-world” patient populations Cumbersome and non-intuitive to use; 6 steps and manipulation of sensitive settings required for dosing
TO-2070 initial development program License agreement signed September, 2013 IND target date: 1H 2014 Human PK data expected: 2H 2014 NDA strategy to be finalized following FDA meeting Potential for 505(b)(2) regulatory pathway based on well- characterized nature of DHE 18
Initial Key Opinion Leader (KOL) feedback regarding DHE nasal powder for acute migraine

Summary of Key Opinion Leader (KOL) feedback Migraine KOLs believe that DHE is an excellent treatment for many migraine patients, but better dosage forms are needed Experts believe that the SNBL intranasal delivery technology applied to DHE offers promise for improving the management of acute migraine. This technology has the potential to offer not just another formulation, but a better one. Short time to maximum plasma concentration is a powerful message for prescribers and is generally believed to be correlated with fast onset of migraine relief. Short Tmax is not only associated with an earlier response, but a better response. 20
INTERMEZZO®

Intermezzo: the first and only prescription sleep aid approved for middle-of-the-night dosing 22

Intermezzo: the first and only sleep aid approved for middle-of-the night dosing Novel and patent-protected zolpidem formulation – Sublingual tablet – Bicarbonate-carbonate buffers Approved dose – 1.75 mg in women & patients > 65 years – 3.5 mg in men < 65 years Rapidly absorbed in both men and women Effective vs. placebo in sleep laboratory & outpatient studies Instructions to patients – Take Intermezzo “while in bed” – “When you wake up in the morning, be sure that at least 4 hours have passed since you have taken Intermezzo and you feel fully awake before driving. Do not do dangerous activities until you know how Intermezzo affects you.” 23

Intermezzo: challenges & opportunities Despite Purdue’s promotional effort and investment, INTERMEZZO has yet to achieve sales expectations / potential – For highest prescribers of hypnotics, insomnia is usually not the primary reason that patient is being seen by the physician – Low physician awareness of MOTN versus bedtime insomnia – Managed care – Targeting of optimal physician audience for direct promotion Opportunity to re-position Intermezzo as a specialty product 24
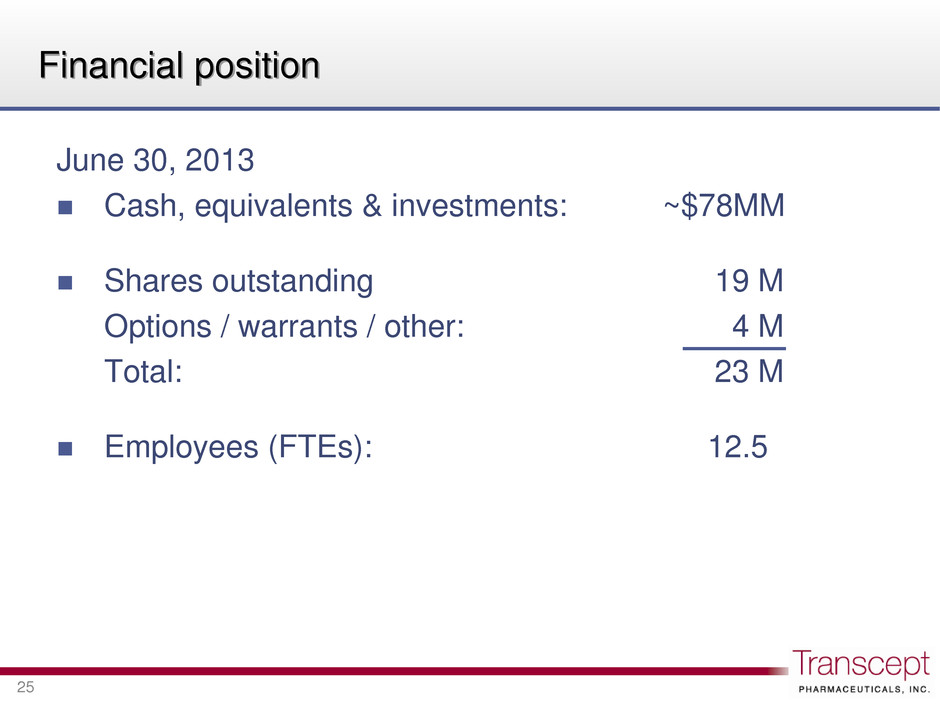
Financial position June 30, 2013 Cash, equivalents & investments: ~$78MM Shares outstanding 19 M Options / warrants / other: 4 M Total: 23 M Employees (FTEs): 12.5 25
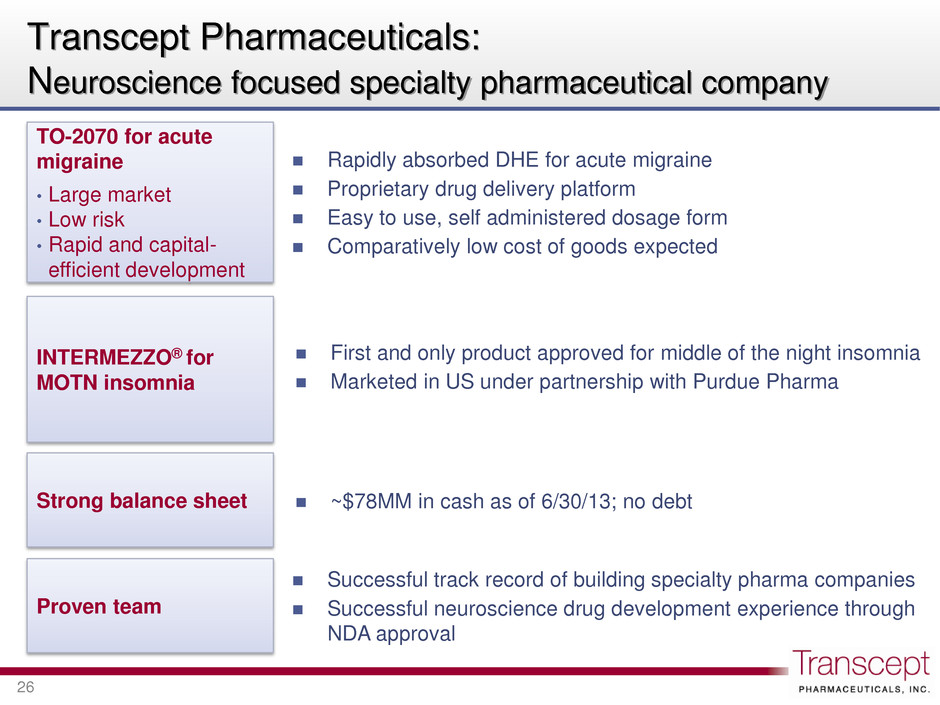
Transcept Pharmaceuticals: Neuroscience focused specialty pharmaceutical company 26 First and only product approved for middle of the night insomnia Marketed in US under partnership with Purdue Pharma INTERMEZZO® for MOTN insomnia ~$78MM in cash as of 6/30/13; no debt Strong balance sheet Successful track record of building specialty pharma companies Successful neuroscience drug development experience through NDA approval Proven team Rapidly absorbed DHE for acute migraine Proprietary drug delivery platform Easy to use, self administered dosage form Comparatively low cost of goods expected TO-2070 for acute migraine • Large market • Low risk • Rapid and capital- efficient development

A specialty pharmaceutical company focused on the development and commercialization of proprietary products to address important therapeutic needs in the field of neuroscience NASDAQ:TSPT September 2013


























