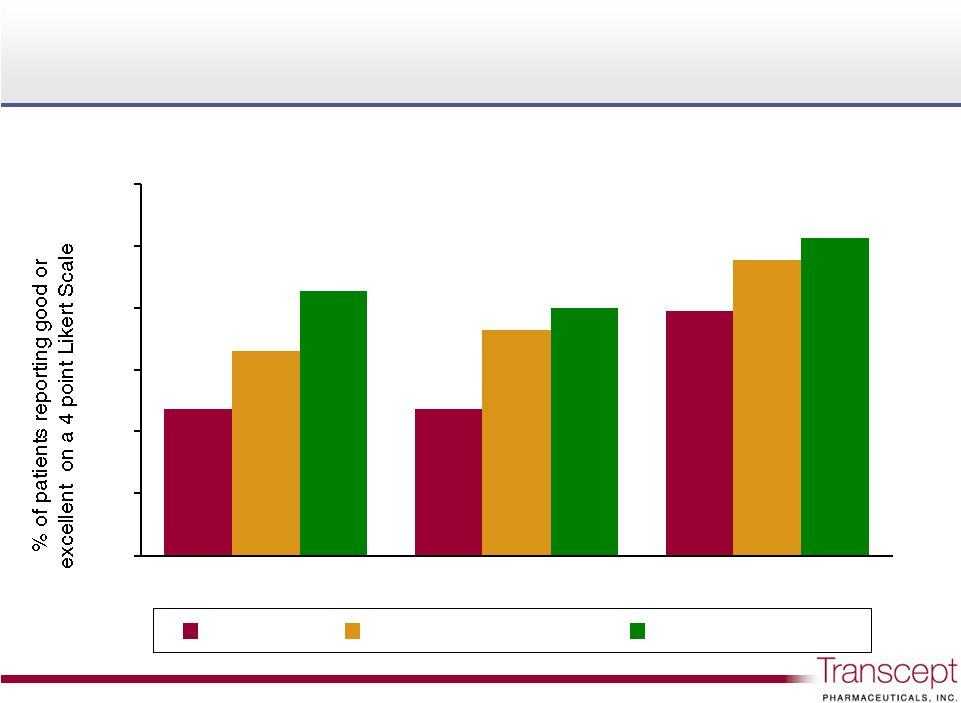
2 Safe harbor statement Safe harbor statement This slide deck contains forward-looking statements that are based upon current expectations within the meaning of the Private Securities Reform Act of 1995. We intend that such statements be protected by the safe harbor created thereby. Forward-looking statements involve risks and uncertainties and our actual results and the timing of events may differ significantly from the results discussed in the forward-looking statements. We undertake no obligation to revise or update the forward-looking statements made herein whether as a result of new information, future events or otherwise. Examples of such forward-looking statements include, but are not limited to, statements about or relating to: the submission for regulatory approval of Intermezzo with the U.S. FDA, the timing of such submission, and the scope of indications potentially covered, as well as the potential timing for marketing approval and product launch based on such submission; clinical trials with respect to our other drug candidates, including TO-2060 and TO-2061, including the anticipated dates of data availability under currently ongoing trials; the size and scope of potential markets and potential revenues for Intermezzo and our other drug candidates and expected benefits of our drug candidates. Such forward-looking statements involve risks and uncertainties, including, but not limited to, those risks and uncertainties relating to: difficulties or delays in development, obtaining regulatory approval for, and undertaking production and marketing of our drug candidates; unexpected adverse side effects or inadequate therapeutic efficacy of our drug candidates that could slow or prevent product approval; positive results in clinical trials may not be sufficient to obtain FDA approval; physician or patient reluctance to use Intermezzo, if approved; our ability to obtain additional financing if necessary; changing standards of care and the introduction of products by competitors or alternative therapies for the treatment of indications we target; the uncertainty of protection for our intellectual property, through patents, trade secrets or otherwise; and potential infringement of the intellectual property rights or trade secrets of third parties. |