Exhibit 99.1
Exhibit 99.1
Transcept PHARMACEUTICALS, INC.
A specialty pharmaceutical company focused on the development and commercialization of proprietary products to address important therapeutic needs in the field of neuroscience
August 2010
Forward looking statements
This slide deck contains forward-looking statements based upon current expectations within the meaning of the Private Securities Reform Act of 1995. Such statements involve risks and uncertainties. Actual results may differ significantly from that discussed in the forward-looking statements.
Examples of such statements include, but are not limited to: plans to resubmit the Intermezzo® NDA; Intermezzo® being the first commercially available sleep aid in the United States in its target indication; plans for a highway driving study of Intermezzo® and studies of TO-2061; and the ability of Transcept to resubmit the Intermezzo® NDA with content to warrant FDA approval; the cost of planned studies and the sufficiency of cash resources to address the FDA Complete Response Letter issues and fund currently planned operations and studies; the ability of Transcept to obtain and maintain patent protection, regulatory exclusivity and payor reimbursement for its product candidates; the satisfaction of conditions under our collaboration agreement with Purdue to obtain payments thereunder; plans of Transcept to exercise the option to co-promote Intermezzo® and to develop a specialty sales force; plans of Purdue to prioritize Intermezzo® commercialization; plans to out-license commercialization of Intermezzo® outside the U.S.; reimbursement potential for TO-2061; and the ability of Transcept to identify potential products to complement any co-promotion of Intermezzo®.
Various important factors could cause actual results or events to differ materially from the forward-looking statements that Transcept makes, including risks related to the following: our ability to carry out planned studies successfully and in a timely manner; new information arising out of clinical trial results; the FDA’s view of the sufficiency of the planned resubmission to support review and approval of the Intermezzo® NDA; unforeseen expenses related to FDA approval and the business of Transcept generally; a decision by Purdue to terminate the Collaboration Agreement, before or even if the Intermezzo® NDA is approved; obtaining, maintaining and protecting the intellectual property rights and payor reimbursement for our product candidates; dependence on third parties to manufacture product candidates for our planned studies and carry out clinical trials; and the ability of Transcept to obtain additional funding, if needed, to support its business activities.
These and other risks are described in greater detail in the “Risk Factors” section of Transcept periodic reports filed with the SEC. Forward-looking statements do not reflect the potential impact of any future in-licensing, collaborations, acquisitions, mergers, dispositions, joint ventures, or investments Transcept may enter into or make. We undertake no obligation to revise or update the forward-looking statements made herein whether as a result of new information, future events or otherwise.
2
Transcept Pharmaceuticals: company profile
Public listing through reverse merger in Jan 2009 – Nasdaq:TSPT
Intermezzo® (zolpidem tartrate sublingual tablet): lead product candidate
U.S. primary care partnership with Purdue Pharma, psychiatry co-promote option
Strong balance sheet: $83.1M of cash, cash equivalents and marketable securities on Mar 31, 2010
Management team with consistent history of pharmaceutical product development and commercial success
3
Transcept Pharmaceuticals: key corporate events
Intermezzo®: Preclinical program and two Phase 3 studies completed: 2004-2008
Intermezzo® NDA submitted: September 2008
U.S. commercial partnership: Purdue, Intermezzo® primary care marketing and sales: July 2009
Intermezzo® Complete Response Letter received from FDA: October 2009
Transcept plans to resubmit the Intermezzo® NDA in Q1 2011 for six-month FDA Class 2 review
4
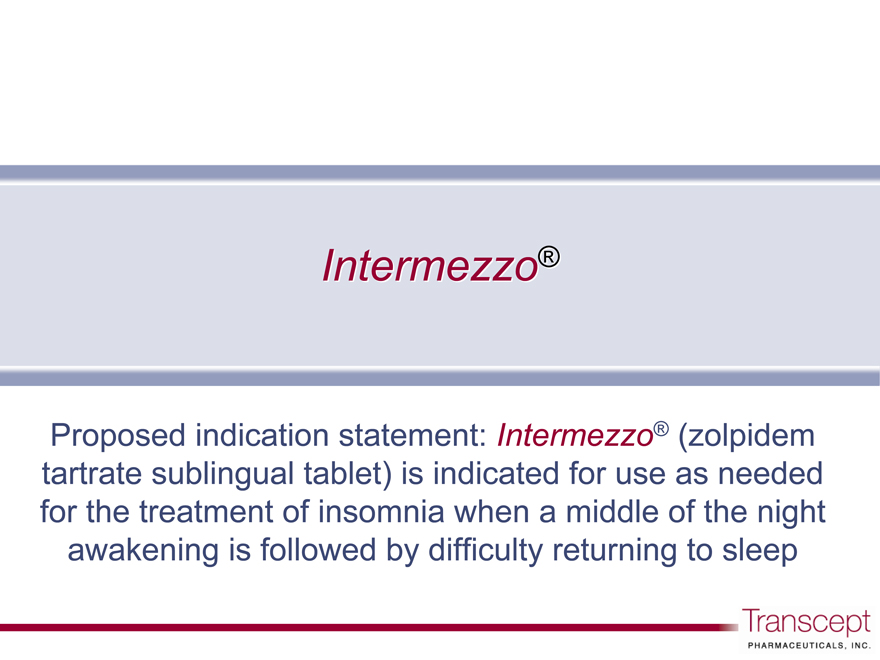
Intermezzo®
Proposed indication statement: Intermezzo® (zolpidem tartrate sublingual tablet) is indicated for use as needed for the treatment of insomnia when a middle of the night awakening is followed by difficulty returning to sleep
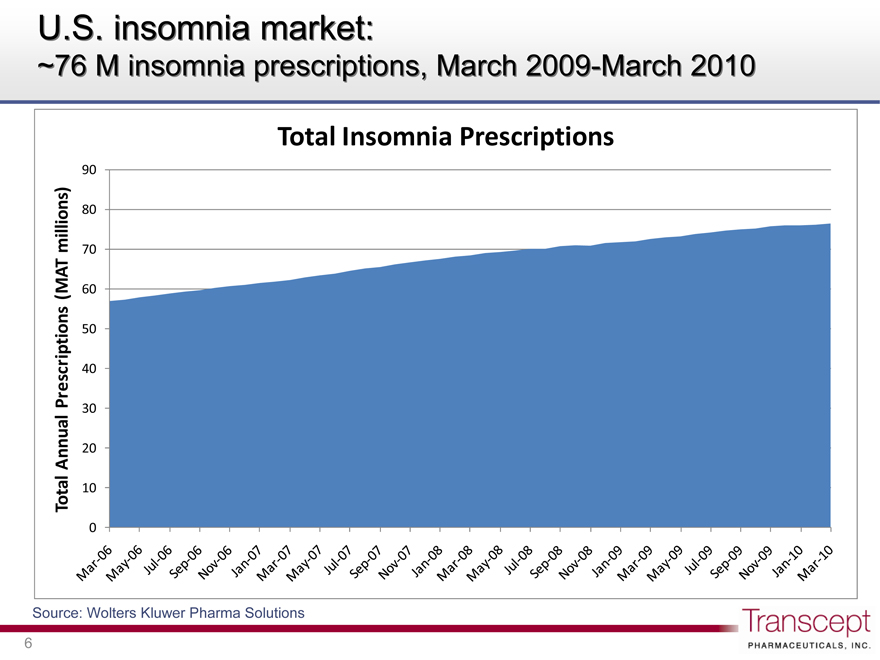
U.S. insomnia market:
~76 M insomnia prescriptions, March 2009-March 2010
Total Insomnia Prescriptions
Total Annual Prescriptions (MAT millions)
0,10,20,30,40,50,60,70,80,90
Mar-06 May-06 Jul-06 Sep-06 Nov-06 Jan-07 Mar-07 May-07 Jul-07 Sep-07 Nov-07 Jan-08 Mar-08 May-08 Jul-08 Sep-08 Nov-08 Jan-09 Mar-09 May-09 Jul-09 Sep-09 Nov-09 Jan-10 Mar-10
Source: Wolters Kluwer Pharma Solutions
6
Stanford Sleep Epidemiology Research Center:
middle of the night awakening: #1 insomnia symptom
Large scale population based sleep epidemiology study*, ~9,000 subjects over 4 years
35% report awakenings at least 3x per week
>90% report awakenings persist more than six months
Fewer than 25% of the middle of the night awakening group reported difficulty going to sleep at bedtime
10.9% of study subjects who experienced middle of the night awakenings consulted a physician
*Maurice M. Ohayon, MD, DSc, PhD: Nocturnal Awakenings and comorbid disorders in the American general population, J of Psych Research (2008)
7
Intermezzo®: no product currently indicated for prn treatment of middle of the night awakening
Middle of the night awakenings typically did not occur every night, even in a more severely affected patient group*
Commonly prescribed 7 to 8 hour sleep aids require bedtime prophylactic dosing before an awakening occurs and are not designed for use in the middle of the night
An ideal therapeutic would:
Be used at the time patients need help returning to sleep, not every night in advance of a problem that may not occur
Return patients to sleep rapidly
Use a low enough dose to avoid next day residual effects
* Transcept Pharmaceuticals Phase 3 outpatient study, n = 294
8
|
d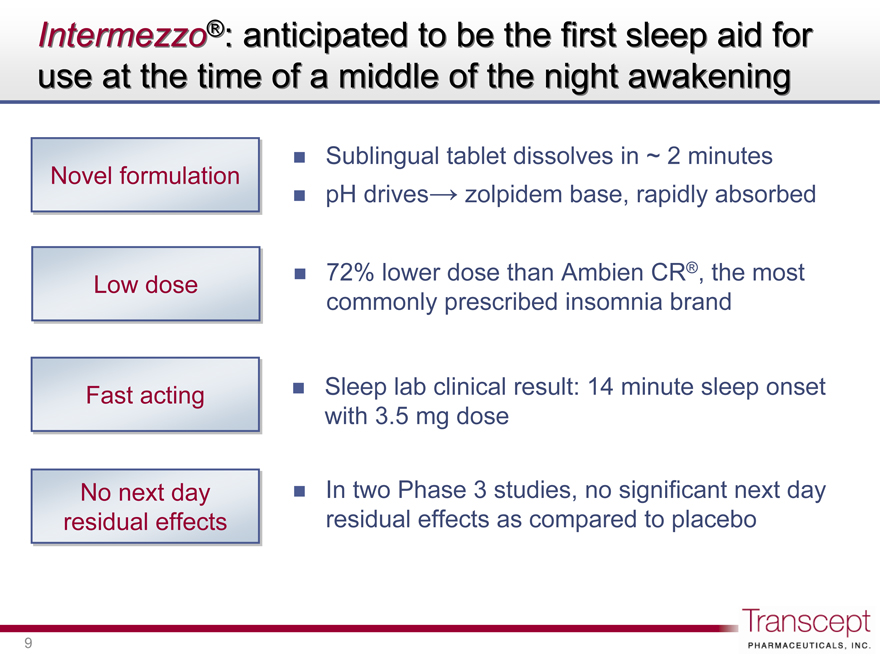 |
Intermezzo®: anticipated to be the first sleep aid for use at the time of a middle of the night awakening
Novel formulation
Sublingual tablet dissolves in ~ 2 minutes
pH drives zolpidem base, rapidly absorbed
Low dose
72% lower dose than Ambien CR®, the most commonly prescribed insomnia brand
Fast acting
Sleep lab clinical result: 14 minute sleep onset with 3.5 mg dose
No next day residual effects
In two Phase 3 studies, no significant next day residual effects as compared to placebo
9
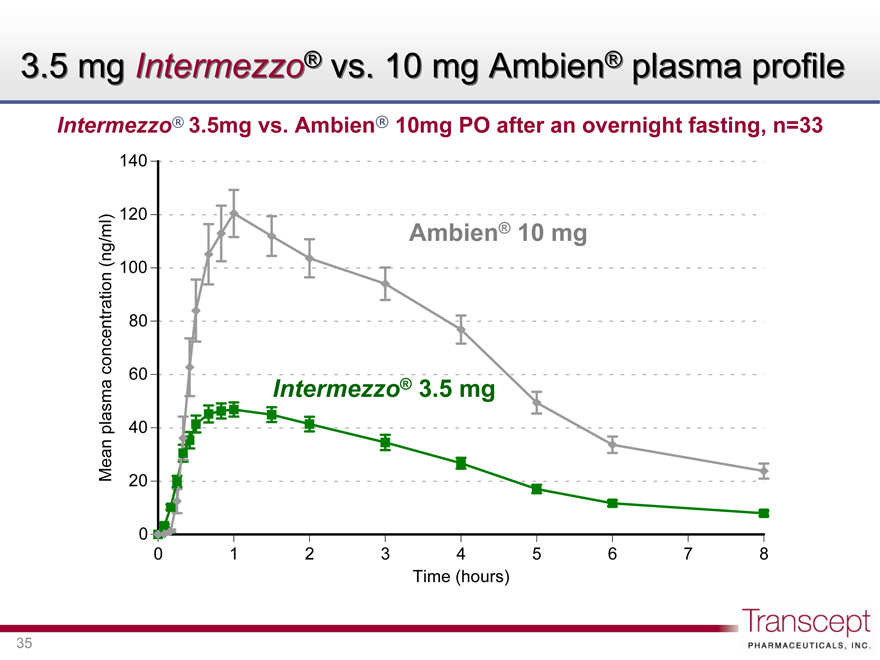
Intermezzo® 3.5 mg delivered greater early zolpidem bioavailability than a ~3x higher Ambien® dose
PK comparison study: Intermezzo® 3.5mg vs. Ambien 10mg PO n=33
Area under the curve (AUC) (ng.hr/mL)
Intermezzo® 3.5 mg
Ambien® 10 mg
Time (min)
0 1 2 3 4
0 5 10 15 20 25
9.3x 2.9x 1.4x
10
Regulatory Review
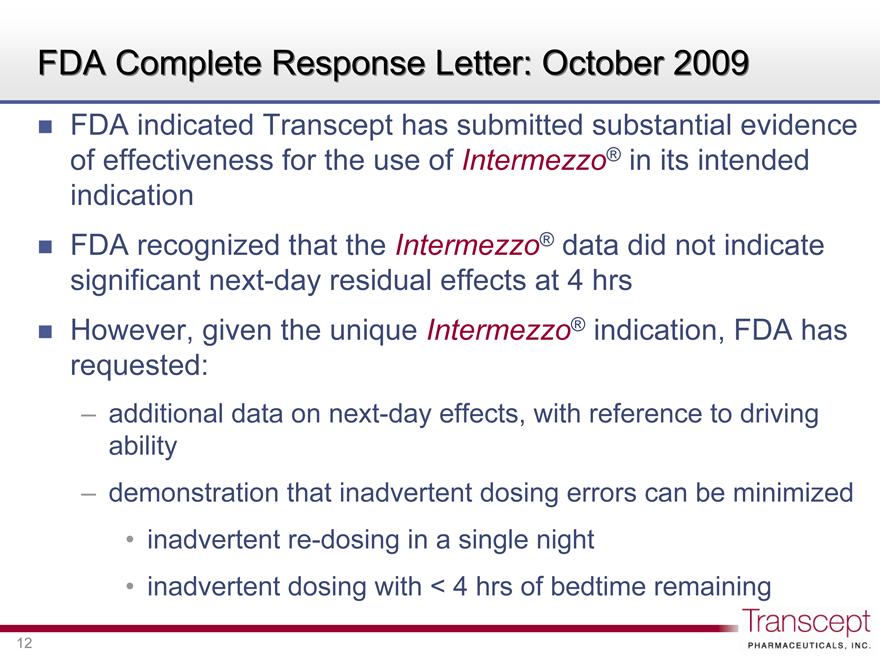
FDA Complete Response Letter: October 2009
FDA indicated Transcept has submitted substantial evidence of effectiveness for the use of Intermezzo® in its intended indication
FDA recognized that the Intermezzo® data did not indicate significant next-day residual effects at 4 hrs
However, given the unique Intermezzo® indication, FDA has requested:
additional data on next-day effects, with reference to driving ability
demonstration that inadvertent dosing errors can be minimized
inadvertent re-dosing in a single night
inadvertent dosing with < 4 hrs of bedtime remaining
12
Actions to address FDA Complete Response Letter
Inadvertent re-dosing in a single night
FDA has indicated that redesigned unit dose packaging appears to reduce the potential for inadvertent re-dosing in a single night.
Inadvertent dosing with < 4 hours bedtime remaining
FDA has agreed to a highway driving study to assess the effect of Intermezzo® on next day driving ability beginning at three and four hours after MOTN dosing. Study has begun.
FDA has agreed to consider as part of the overall Intermezzo® NDA the Transcept contention that a pre-approval in-use study could not accurately measure patient ability to avoid inadvertent dosing errors.
13
Redesigned unit dose packaging for NDA resubmission
Prototype package: product is not approved by FDA
Prototype package: product is not approved by FDA
Prototype package only: Intermezzo® is not approved by the U.S. Food and Drug Administration.
14
Highway driving study overview
Approximately 40 healthy adult volunteers
Highway driving over a one-hour period
Single-center (Maastricht), double-blind, randomized, placebo-controlled crossover design
Key comparisons:
Intermezzo® 3.5mg vs. placebo, 4 hours post-dose
Intermezzo® 3.5mg vs. placebo, 3 hours post-dose
Zopiclone 7.5mg vs. placebo (positive control)
Key measure of driving impairment: standard deviation of lateral position (a measurement of ‘weaving’) as compared to placebo
15
U.S. Commercial Partnership: Purdue Pharma
Purdue Pharma:
Intermezzo® U.S. primary care marketing partner
> $2.5 billion 2009 revenue, leading pain therapeutic franchise, significant U.S. primary care marketing and sales presence
Intermezzo®: an important new product opportunity for Purdue
Purdue team actively engaged with Transcept to address issues raised in FDA Complete Response Letter
17
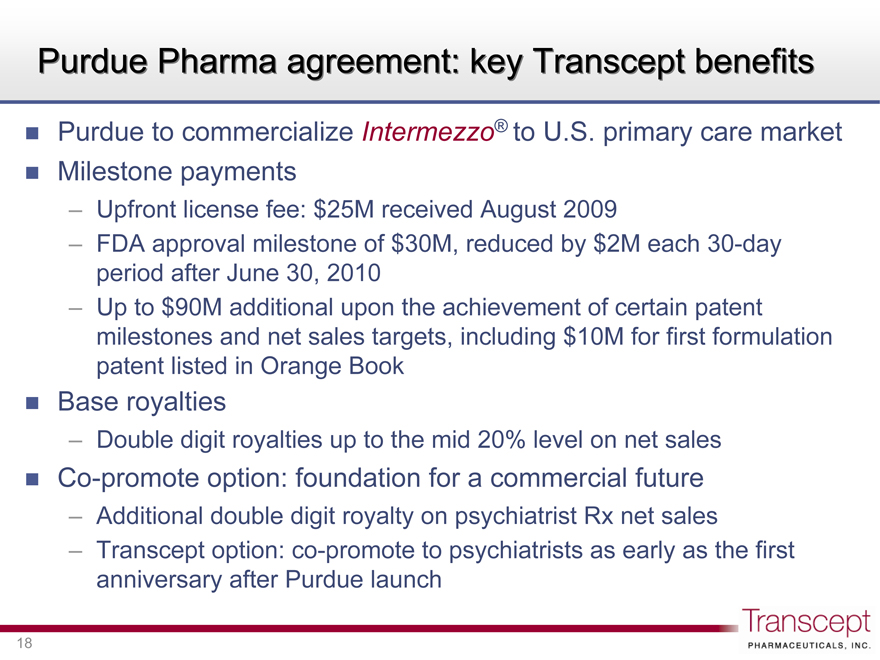
Purdue Pharma agreement: key Transcept benefits
Purdue to commercialize Intermezzo® to U.S. primary care market
Milestone payments
Upfront license fee: $25M received August 2009
FDA approval milestone of $30M, reduced by $2M each 30-day period after June 30, 2010
Up to $90M additional upon the achievement of certain patent milestones and net sales targets, including $10M for first formulation patent listed in Orange Book
Base royalties
Double digit royalties up to the mid 20% level on net sales
Co-promote option: foundation for a commercial future
Additional double digit royalty on psychiatrist Rx net sales
Transcept option: co-promote to psychiatrists as early as the first anniversary after Purdue launch
18
Intermezzo®: Intellectual property
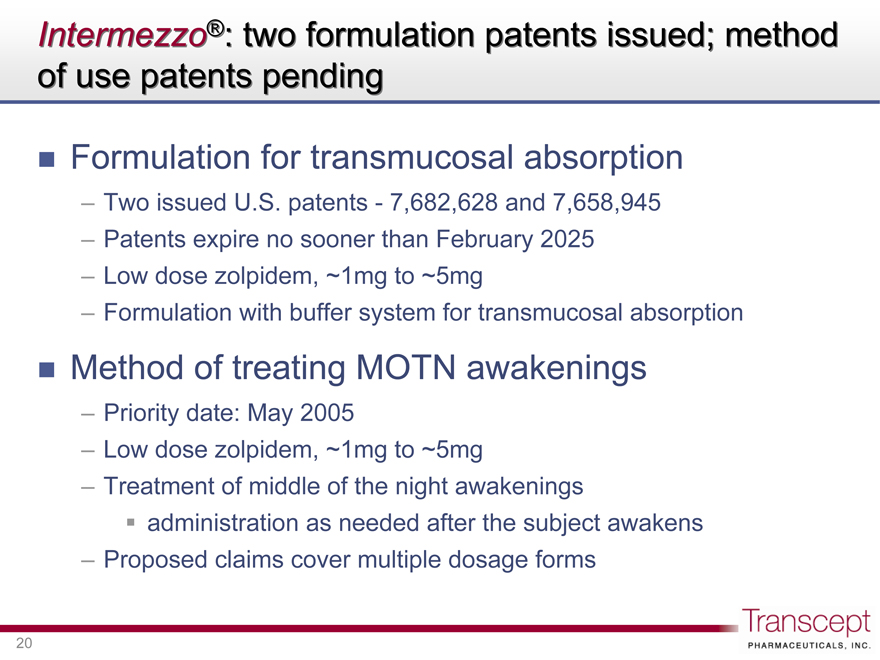
Intermezzo®: two formulation patents issued; method of use patents pending
Formulation for transmucosal absorption
Two issued U.S. patents - 7,682,628 and 7,658,945
Patents expire no sooner than February 2025
Low dose zolpidem, ~1mg to ~5mg
Formulation with buffer system for transmucosal absorption
Method of treating MOTN awakenings
Priority date: May 2005
Low dose zolpidem, ~1mg to ~5mg
Treatment of middle of the night awakenings
administration as needed after the subject awakens
Proposed claims cover multiple dosage forms
20
Pipeline development
TO-2061: ultra low dose ondansetron as adjunctive therapy in patients with obsessive compulsive disorder who have not adequately responded to SSRI treatment
22
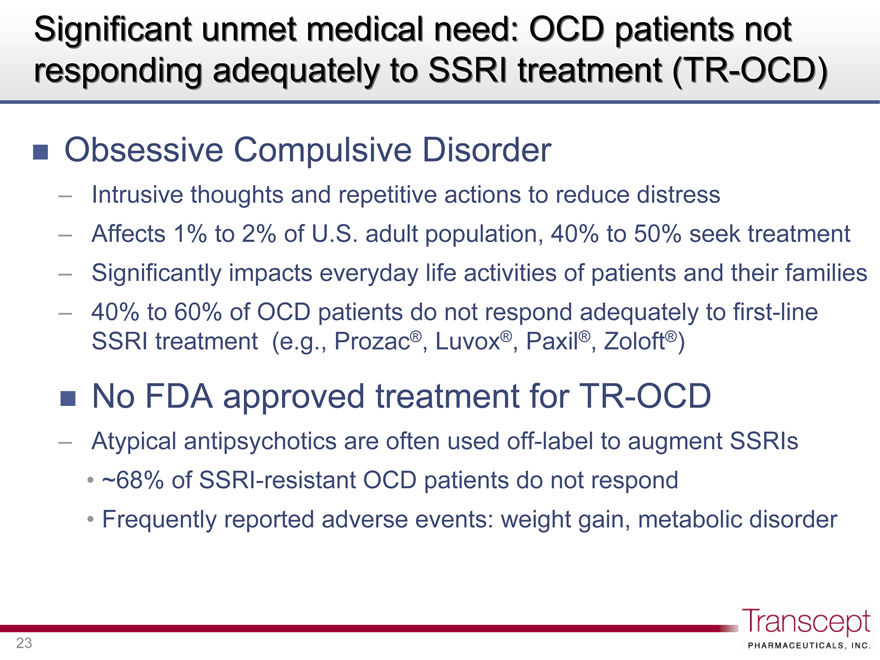
Significant unmet medical need: OCD patients not responding adequately to SSRI treatment (TR-OCD)
Obsessive Compulsive Disorder
Intrusive thoughts and repetitive actions to reduce distress
Affects 1% to 2% of U.S. adult population, 40% to 50% seek treatment
Significantly impacts everyday life activities of patients and their families
40% to 60% of OCD patients do not respond adequately to first-line SSRI treatment (e.g., Prozac®, Luvox®, Paxil®, Zoloft®)
No FDA approved treatment for TR-OCD
Atypical antipsychotics are often used off-label to augment SSRIs
~68% of SSRI-resistant OCD patients do not respond
Frequently reported adverse events: weight gain, metabolic disorder
23
Pilot studies: ultra low dose adjunctive ondansetron therapy in TR-OCD
Ondansetron (5-HT3 antagonist approved as Zofran®)
Affects serotonin and dopamine pathways
Typical daily Zofran® doses of 16 mg to 24 mg during chemotherapy
Two 12 week open-label adjunctive therapy studies with ondansetron titrated to 0.5 mg BID dose
Pilot Study A*: Adjunctive ondansetron therapy in patients who responded poorly to at least 12 weeks of SSRIs combined with an atypical antipsychotic, n=14
Pilot Study B: Adjunctive ondansetron therapy in patients who responded poorly to at least 12 weeks of SSRI treatment, n=21
* Pallanti et al.: Ondansetron Augmentation in Treatment-Resistant Obsessive-Compulsive Disorder, CNS Drugs (2009)
24
Pilot studies: ultra low dose adjunctive ondansetron therapy in TR-OCD
Results
OCD symptoms improved over baseline in patients who had responded poorly to SSRI monotherapy and SSRIs in combination with atypical antipsychotics
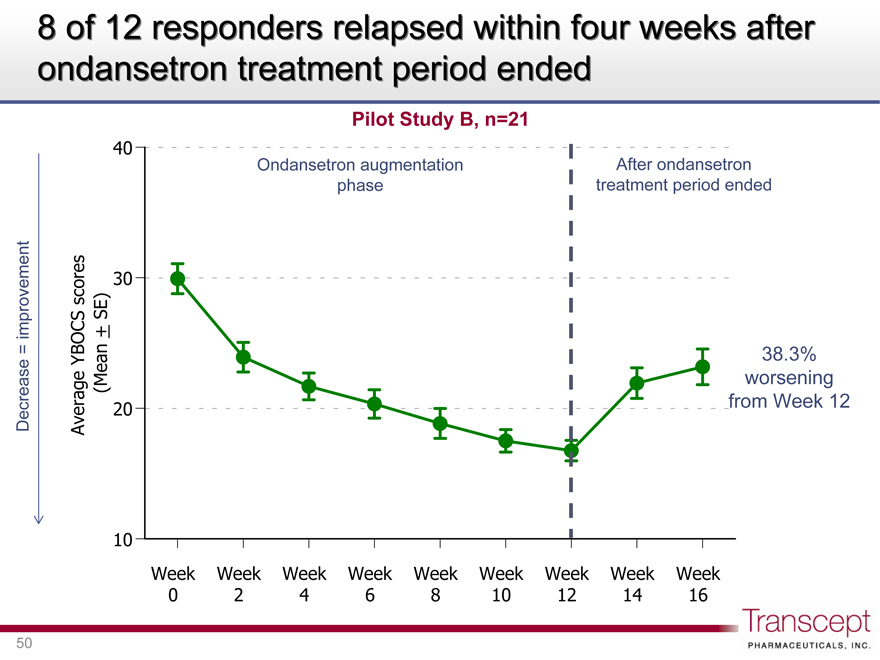
In Pilot Study B, where relapse data were collected, most patients who responded to adjunctive ondansetron therapy relapsed after ondansetron treatment period ended
Low-dose ondansetron was well tolerated (no dropouts due to AE’s)
25
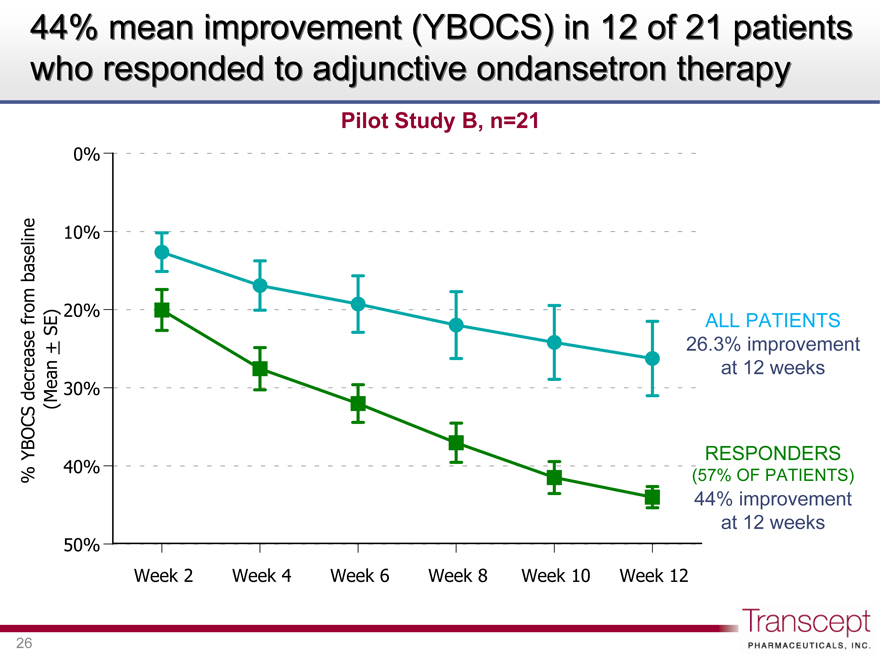
44% mean improvement (YBOCS) in 12 of 21 patients who responded to adjunctive ondansetron therapy
Pilot Study B, n=21
YBOCS% decrease from baseline (Mean ± SE)
ALL PATIENTS 26.3% improvement at 12 weeks
RESPONDERS
(57% OF PATIENTS)
44% improvement at 12 weeks
0% 10% 20% 30% 40% 50%
Week 2 Week 4 Week 6 Week 8 Week 10 Week 12
26
TO-2061: Development overview
Regulatory highlights
IND accepted Q2 2010: opening PK study may proceed
505b2 NDA pathway
PK studies: Planned for completion ~H1 2011
Double-blind, placebo-controlled study of SSRI adjunctive therapy with ultra low dose ondansetron in TR-OCD patients, n~150
If successful, this study could potentially be submitted as one of two pivotal Phase 3 studies
Top-line results: preliminary estimation H2 2012
Modest investment
<$10M incremental expense estimated through Phase 2 results
27
Commercial strategy
Intellectual property
Method of use patent application filed, priority date May 19, 2009
Ondansetron, up to ~1.5 mg/day
Pending claims for treating TR-OCD with ondansetron augmentation of SSRIs
Managed markets: positive initial reception
Managed care survey: 7 payer organizations with ~108M total patient lives
Positive initial response to product concept
Payers acknowledged the unmet medical need
All payers expect Tier 3 formulary coverage
Tablet splitting unlikely given the very low proposed dose of 0.5 mg BID and the 8x-16x higher doses of available ondansetron formulations
Strategic fit: psychiatry
~87% of patient visits for OCD were to psychiatrists in 2009*
Complementary to existing Intermezzo® psychiatry co-promote option with Purdue
* SDI Physician Drug and Diagnosis Audit
28
Financial Overview
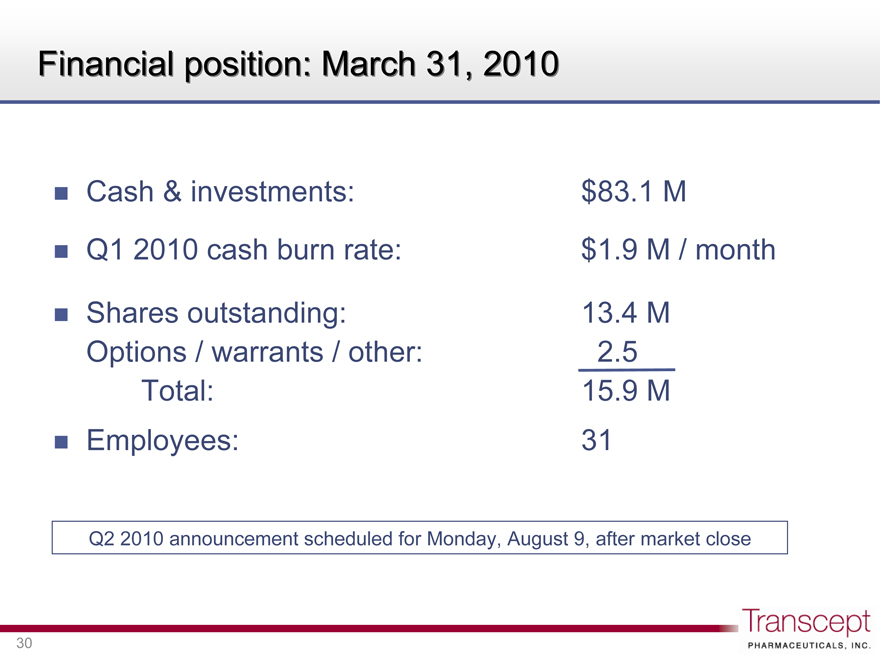
Financial position: March 31, 2010
Cash & investments: $83.1 M
Q1 2010 cash burn rate: $1.9 M / month
Shares outstanding: 13.4 M
Options / warrants / other: 2.5
Total: 15.9 M
Employees: 31
Q2 2010 announcement scheduled for Monday, August 9, after market close
30
Key Transcept goals and activities
Completed Intermezzo® US marketing partnership, July 09
Patent coverage: two Intermezzo® formulation patents issued
Proposal to FDA on Intermezzo® NDA resubmission, Feb 10
FDA agreement on highway driving study design, March 10
Begin highway driving study, June 2010
Resubmit Intermezzo® NDA first quarter 2011, Class 2 review
Begin ultra low-dose ondansetron development program
Develop ex-U.S. Intermezzo® regulatory strategy
31
Transcept Pharmaceuticals, Inc.
Intermezzo® is a registered trademark of Transcept Pharmaceuticals, Inc.
Ambien® is a registered trademark of sanofi-aventis
Zofran® and Paxil® are registered trademarks of The GlaxoSmithKline Group of Companies
Prozac® is a registered trademark of Eli Lilly & Co.
Luvox® is a registered trademark of Solvay Pharmaceuticals, Inc.
Zoloft® is a registered trademark of Pfizer Inc.
Intermezzo® Phase 1 Studies
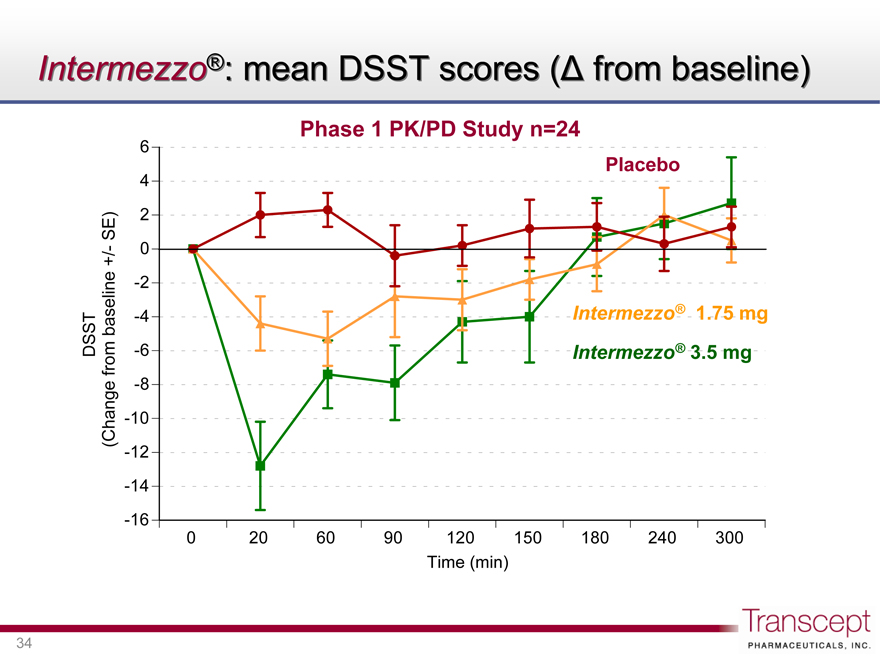
Intermezzo®: mean DSST scores (D from baseline)
Phase 1 PK/PD Study n=24
DSST (Change from baseline +/- SE)
Placebo
Intermezzo® 1.75 mg
Intermezzo® 3.5 mg
Time (min)
6 4 2 0 -2 -4 -6 -8 -10 -12 -14 -16
0 20 60 90 120 150 180 240 300
34
3.5 mg Intermezzo® vs. 10 mg Ambien® plasma profile
Intermezzo® 3.5mg vs. Ambien® 10mg PO after an overnight fasting, n=33
Mean plasma concentration (ng/ml)
140 120 100 80 60 40 20 0
0 1 2 3 4 5 6 7 8
Ambien® 10 mg
Intermezzo 3.5 mg
Time (hours)
35
Intermezzo® Sleep Lab Study
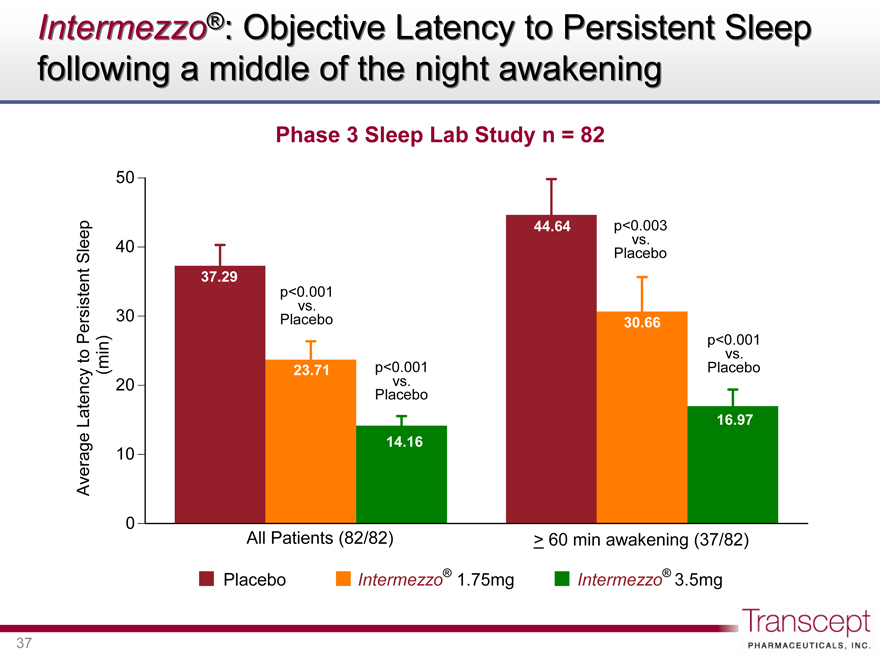
Intermezzo®: Objective Latency to Persistent Sleep following a middle of the night awakening
Phase 3 Sleep Lab Study n = 82
Average Latency to Persistent Sleep (min)
50 40 30 20 10 0
p<0.001 vs. Placebo
p<0.001 vs. Placebo
37.29
23.71
14.16
p<0.003 vs. Placebo
p<0.001 vs. Placebo
44.64
30.66
16.97
All Patients (82/82)
> 60 min awakening (37/82)
Placebo Intermezzo® 1.75mg Intermezzo® 3.5mg
37
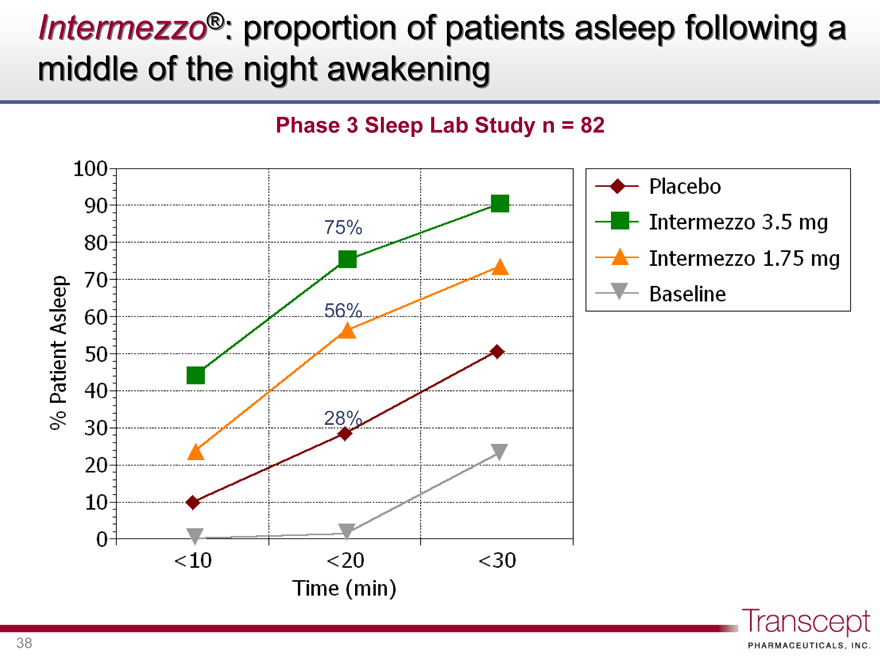
Intermezzo®: proportion of patients asleep following a middle of the night awakening
Phase 3 Sleep Lab Study n = 82
75% 56% 28%
% Patient Asleep
0 10 20 30 40 50 60 70 80 90 100
<10 <20 <30
Time (min)
Placebo
Intermezzo 3.5 mg
Intermezzo 1.75 mg
Baseline
38
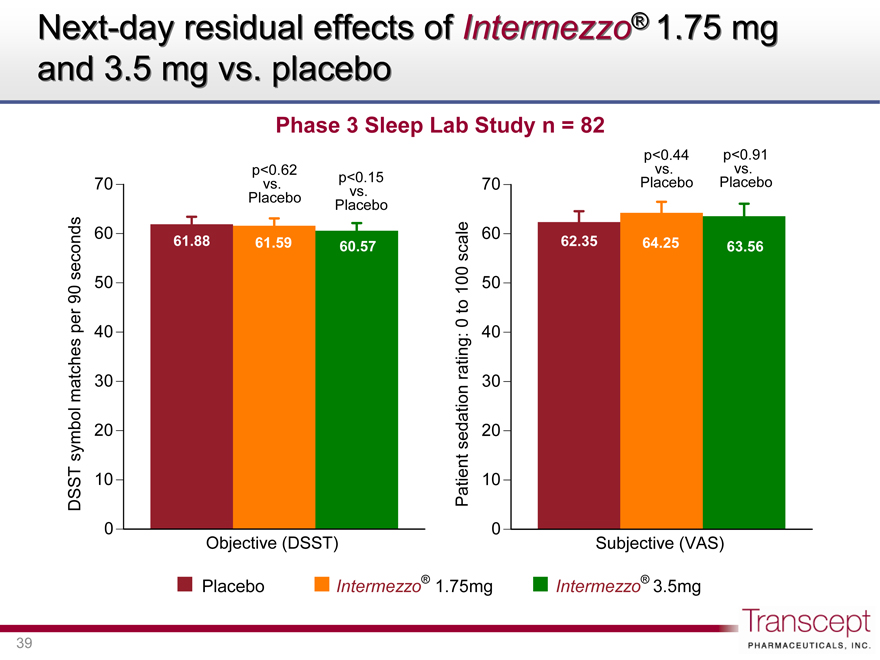
Next-day residual effects of Intermezzo® 1.75 mg and 3.5 mg vs. placebo
Phase 3 Sleep Lab Study n = 82
p<0.62 vs. Placebo
p<0.15 vs. Placebo
DSST symbol matches per 90 seconds
70 60 50 40 30 20 10 0
61.88 61.59 60.57
Objective (DSST)
p<0.44 vs. Placebo
p<0.91 vs. Placebo
Patient sedation rating: 0 to 100 scale
70 60 50 40 30 20 10 0
62.35 64.25 63.56
Subjective (VAS)
Placebo Intermezzo® 1.75mg Intermezzo® 3.5mg
39
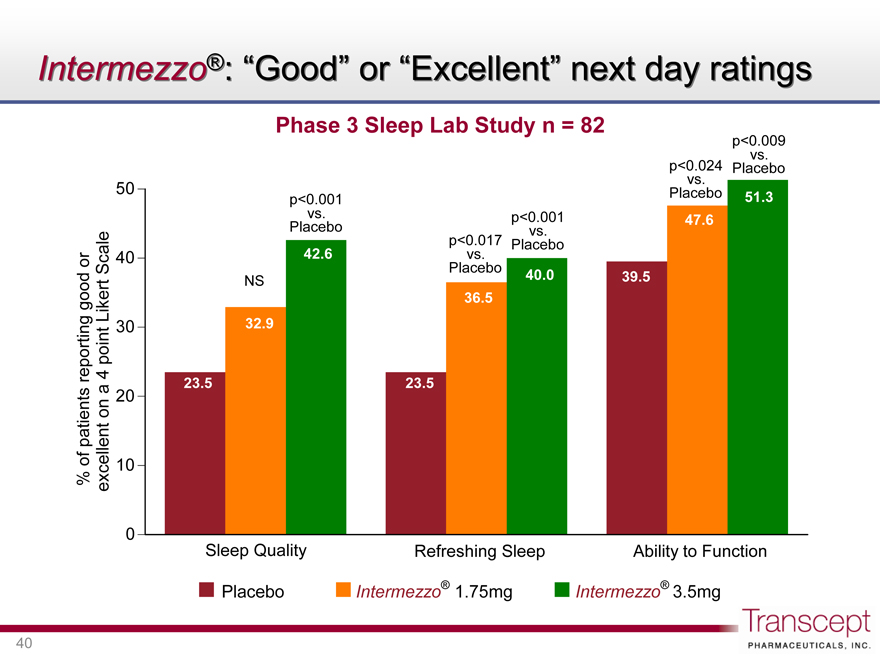
Intermezzo®: “Good” or “Excellent” next day ratings
Phase 3 Sleep Lab Study n = 82
% of patients reporting good or excellent on a 4 point Likert Scale
50 40 30 20 10 0
NS
p<0.001 vs. Placebo
42.6
32.9
23.5
p<0.017 vs. Placebo
p<0.001 vs. Placebo
40.0
36.5
23.5
p<0.024 vs. Placebo
p<0.009 vs. Placebo
51.3
47.6
39.5
Sleep Quality Refreshing Sleep Ability to Function
Placebo Intermezzo® 1.75mg Intermezzo® 3.5mg
40
Intermezzo® Outpatient Study
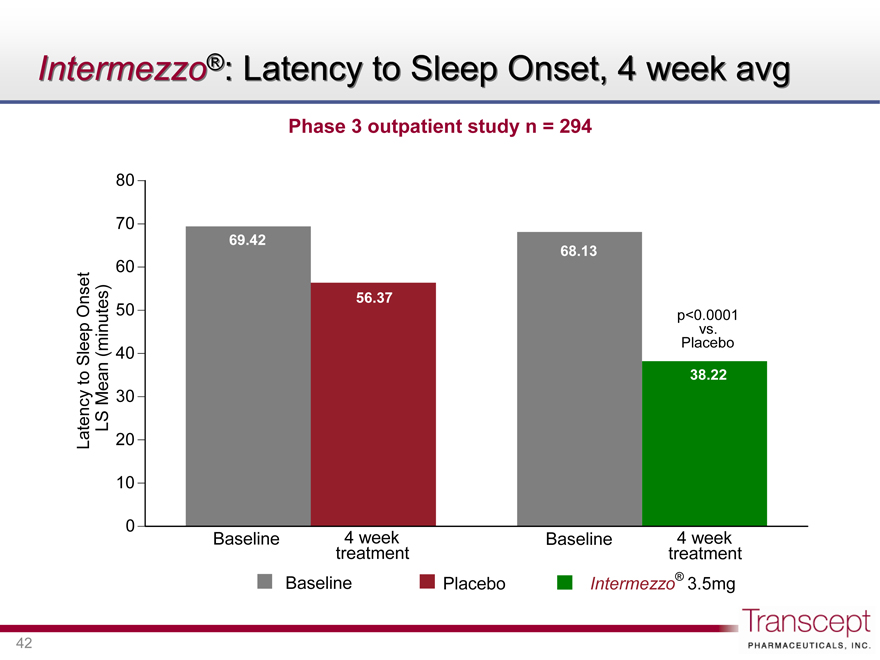
Intermezzo®: Latency to Sleep Onset, 4 week avg
Phase 3 outpatient study n = 294
Latency to Sleep Onset LS Mean (minutes)
80 70 60 50 40 30 20 10 0
69.42
56.37
Baseline
4 week treatment
p<0.0001 vs. Placebo
68.13
38.22
Baseline
4 week treatment
Baseline Placebo Intermezzo® 3.5mg
42
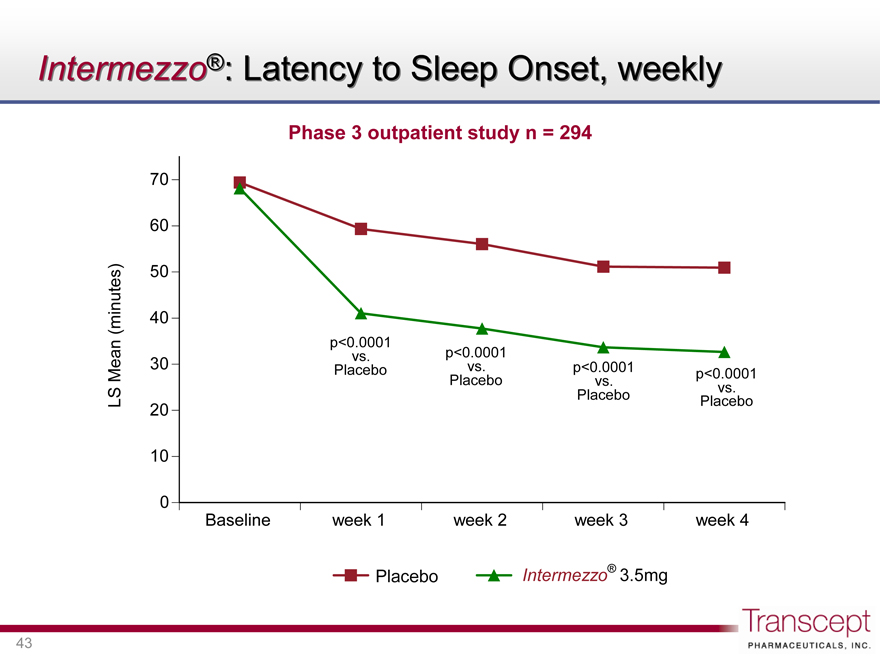
Intermezzo®: Latency to Sleep Onset, weekly
Phase 3 outpatient study n = 294
LS Mean (minutes)
70 60 50 40 30 20 10 0
p<0.0001 vs. Placebo
p<0.0001 vs. Placebo
p<0.0001 vs. Placebo
p<0.0001 vs. Placebo
Baseline week 1 week 2 week 3 week 4
Placebo Intermezzo® 3.5mg
43
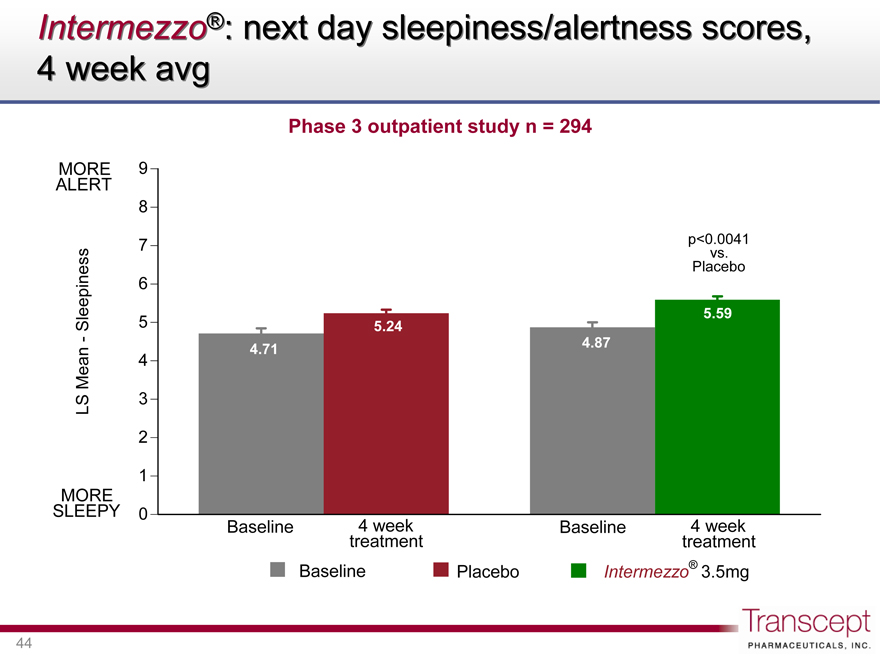
Intermezzo®: next day sleepiness/alertness scores, 4 week avg
Phase 3 outpatient study n = 294
ALERT MORE
LS Mean—Sleepiness
MORE SLEEPY
9 8 7 6 5 4 3 2 1 0
5.24
4.71
Baseline
4 week treatment
p<0.0041 vs. Placebo
5.59
4.87
Baseline
4 week treatment
Baseline Placebo Intermezzo® 3.5mg
44
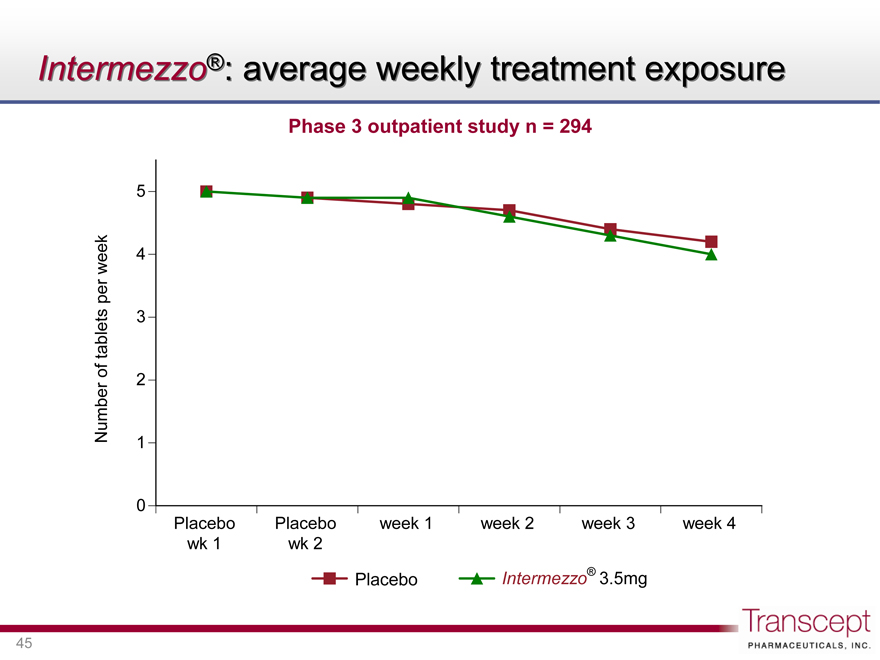
Intermezzo®: average weekly treatment exposure
Phase 3 outpatient study n = 294
Number of tablets per week
5 4 3 2 1 0
Placebo wk 1 Placebo wk 2 week 1 week 2 week 3 week 4
Placebo Intermezzo® 3.5mg
45
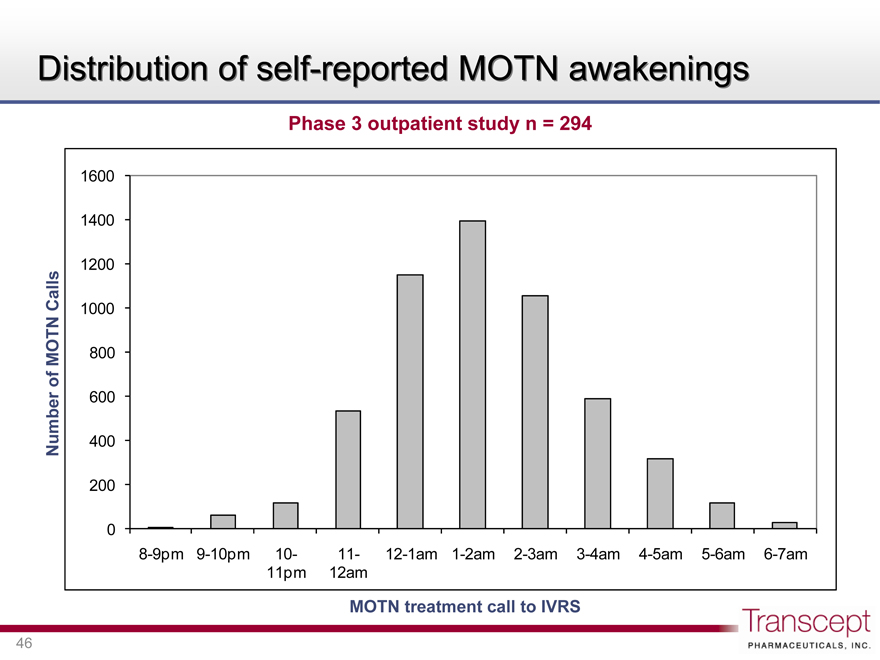
Distribution of self-reported MOTN awakenings
Phase 3 outpatient study n = 294
Number of MOTN Calls
1600 1400 1200 1000 800 600 400 200 0
8-9pm 9-10pm 10-11-pm 11-12am 12-1am 1-2am 2-3am 3-4am 4-5am 5-6am 6-7am MOTN treatment call to IVRS
46
Intermezzo® highway driving study
Study schematic
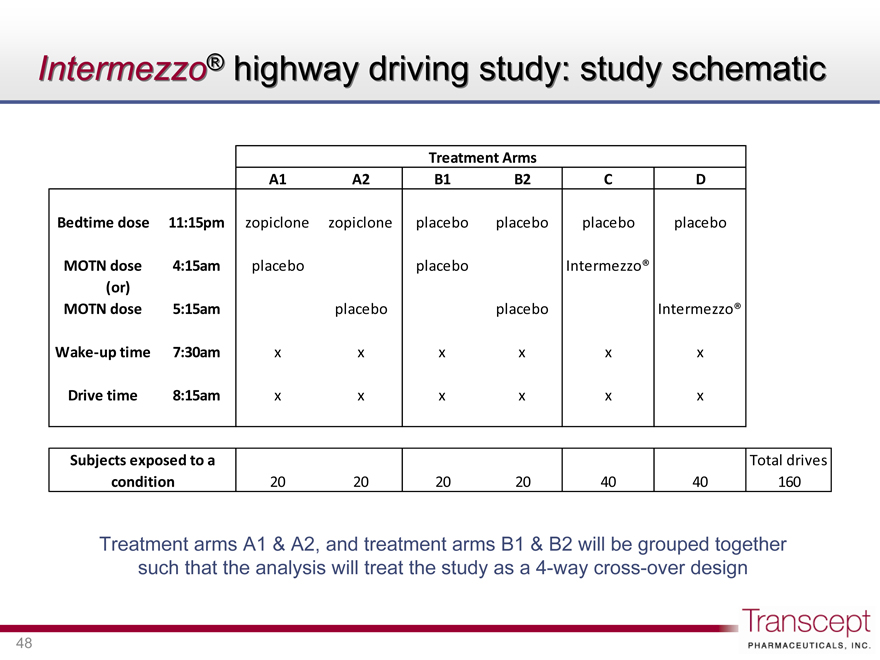
Intermezzo® highway driving study: study schematic
Treatment Arms
Bedtime dose
MOTN dose (or) MOTN dose
Wake-up time
Drive time
11:15pm 4:15am 5:15am 7:30am 8:15am
A1 zopiclone placebo x x
A2 zopiclone placebo x x
B1 placebo placebo x x
B2 placebo placebo x x
C placebo Intermezzo® x x
D placebo Intermezzo® x x
Subjects exposed to a condition
Total drives
20 20 20 20 40 40 160
Treatment arms A1 & A2, and treatment arms B1 & B2 will be grouped together such that the analysis will treat the study as a 4-way cross-over design
48
TO-2061 Pilot Study B
8 of 12 responders relapsed within four weeks after ondansetron treatment period ended
Pilot Study B, n=21
Ondansetron augmentation phase
After ondansetron treatment period ended
38.3% worsening from Week 12
Decrease = improvement
Average YBOCS scores (Mean ± SE)
40 30 20 10
Week 0 Week 2 Week 4 Week 6 Week 8 Week 10 Week 12 Week 14 Week 16
50