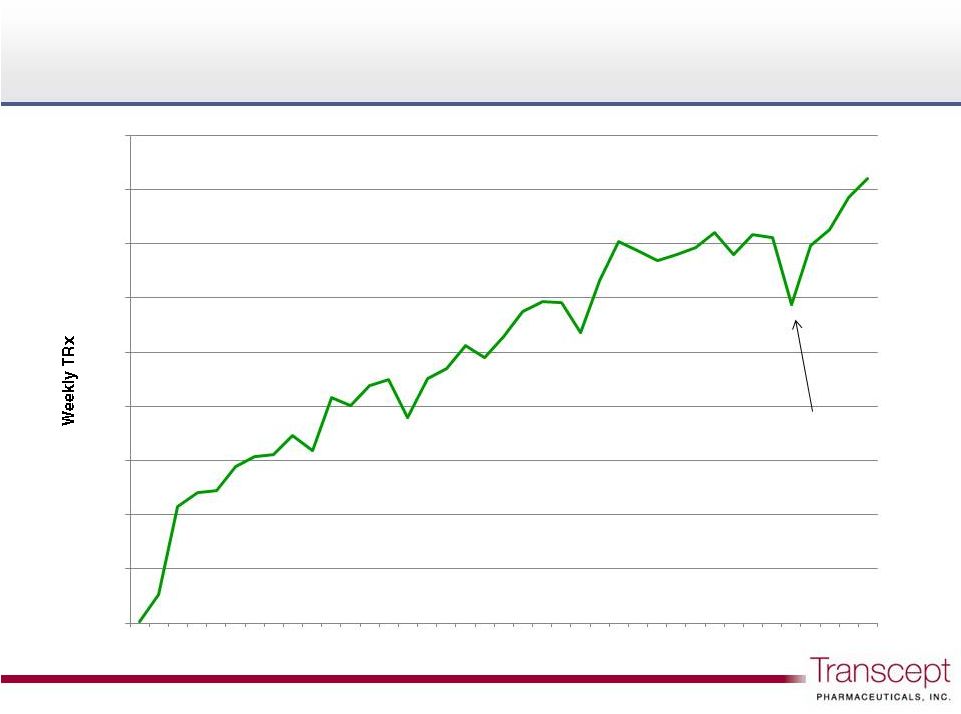
This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, included in this presentation are forward-looking statements. Examples of such statements include our expectations regarding the potential market for Intermezzo® and the potential market size for a middle of the night sleep aid; our expectations regarding an ideal therapeutic; Purdue plans to invest approximately $100 million to support sales and marketing over the first 12 months of commercialization of Intermezzo in the U.S., including the size, timing and nature of such sales and marketing plans; the success of the commercial launch of Intermezzo by Purdue in the U.S., including continued growth in the number of Intermezzo prescriptions; our eligibility for and the receipt and size of royalty payments from Purdue pursuant to our Collaboration Agreement, and our expectations regarding future co-promote and royalty opportunities; intellectual property protection for Intermezzo being obtained and maintained; plans for the Phase 2 study of TO-2061, including the expected timing of receipt of final clinical trial results; and our strategy to build a specialty sales and marketing organization, in-license or acquire additional product candidates, and determine the future development path for TO-2061. All of these forward-looking statements are based on estimates and assumptions by our management that, although we believe to be reasonable, are inherently uncertain. Forward-looking statements involve risks and uncertainties, including, but not limited to, achieving acceptance of Intermezzo by physicians, patients and third-party payors; the impact of competitive products and the market for Intermezzo generally; our dependence on Purdue’s commercialization efforts, including our reliance on Purdue to set the future pricing of Intermezzo and on our Collaboration Agreement with Purdue; obtaining, maintaining and protecting marketing and other regulatory exclusivity and intellectual property protection for Intermezzo and TO-2061, as well as protection from generic versions of our products; competitive product commercialization; manufacturing and supply risks for Intermezzo; adverse results from our clinical trial of TO-2061; adverse patent decisions at the USPTO or in court; our intent and ability to carry out plans to promote Intermezzo to psychiatrists in the United States through our co-promotion option; our intent and ability to successfully in-license or acquire additional product candidates; and variability in the business of Transcept generally. These and other risks are described in greater detail in the "Risk Factors" section of Transcept periodic reports filed with the Securities and Exchange Commission. Forward-looking statements do not reflect the potential impact of any future in-licensing, collaborations, acquisitions, mergers, dispositions, joint ventures, or investments Transcept may enter into or make. Transcept does not assume any obligation to update any forward-looking statements, except as may be required by law. Forward looking statements Forward looking statements 2 |