
Fourth Quarter and Full-Year 2018 Financial Results and Corporate Update February 27, 2019 Recognizing the serious threat of bacterial infections, Paratek is dedicated to providing solutions that enable positive outcomes and lead to better patient stories. Exhibit 99.2

Q4 & FY 2018 Earnings Call Agenda Introduction Ben Strain, Executive Director, Investor Relations & Corporate Communications Fourth Quarter Overview and Business Update Michael F. Bigham, Chief Executive Officer and Chairman of the Board Commercial Update Adam Woodrow, Chief Commercial Officer Financial Update Douglas W. Pagán, Chief Financial Officer Closing Remarks Michael F. Bigham, Chief Executive Officer and Chairman of the Board Also Available for Q&A: Evan Loh, M.D., President, COO & CMO

Third-party industry and market information included herein has been obtained from sources believed to be reliable, but the accuracy or completeness of such information is not guaranteed by, has not been independently verified by, and should not be construed as a representation by, Paratek. The information contained in this presentation is accurate only as of the date hereof. “Paratek” and the Paratek logo are trademarks and service marks of Paratek. All other trademarks, service marks, trade names, logos and brand names identified in this presentation are the property of their respective owners. This presentation contains forward-looking statements including statements related to our overall strategy, products, prospects, potential and expected results, including statements about the projected net product revenues of NUZYRA™, our anticipated cash runway, our expected borrowings under our royalty-backed loan agreement with Healthcare Royalty Partners III, L.P., the progression of our commercial roll out for NUZYRA™, the prospects of our U.S. launch of NUZYRA™, our ability to shape the future treatment paradigm for serious community-acquired pneumonia and skin infections, our plans to evaluate additional indications for NUZYRA™, including UTI, and to work toward an oral-only indication in CABP, our potential to further drive long-term[, transformative] value for all of our shareholders, our expectations regarding the duration of our patent protection exclusivity for NUZYRA™ and our plans to obtain regulatory approval of omadacycline in the European Union . All statements, other than statements of historical facts, included in this press release are forward-looking statements, and are identified by words such as "advancing," "expect," "look forward," "anticipate," "continue," and other words and terms of similar meaning. These forward-looking statements are based upon our current expectations and involve substantial risks and uncertainties. We may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in our forward-looking statements and you should not place undue reliance on these forward-looking statements. Our actual results and the timing of events could differ materially from those included in such forward-looking statements as a result of these risks and uncertainties. These and other risk factors are discussed under "Risk Factors" and elsewhere in our Annual Report on Form 10-K for the year ended December 31, 2017, our Form 10-Q filed for the quarter ended September 30, 2018 and our other filings with the Securities and Exchange Commission. We expressly disclaim any obligation or undertaking to update or revise any forward-looking statements contained herein. PARATEK® and the Hexagon Logo are registered trademarks of Paratek Pharmaceuticals, Inc. NUZYRA and its design logo are trademarks of Paratek Pharmaceuticals, Inc. Safe Harbor Statement

Fourth Quarter & FY 2018 Overview & Business Update Michael F. Bigham, Chief Executive Officer & Chairman of the Board Recognizing the serious threat of bacterial infections, Paratek is dedicated to providing solutions that enable positive outcomes and lead to better patient stories.
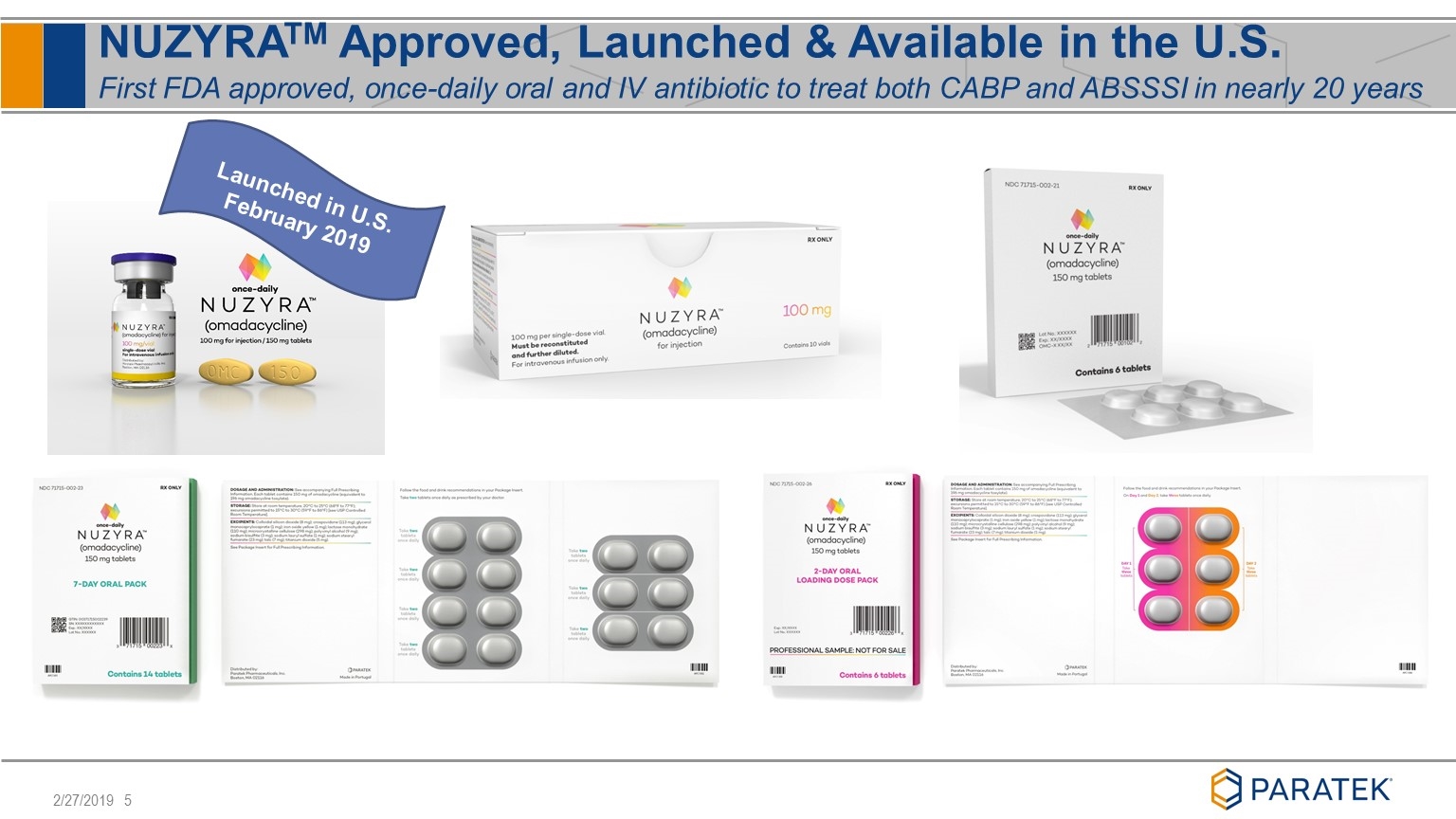
NUZYRATM Approved, Launched & Available in the U.S. First FDA approved, once-daily oral and IV antibiotic to treat both CABP and ABSSSI in nearly 20 years Launched in U.S. February 2019

The New England Journal of Medicine Published results from the OPTIC and OASIS-1 Phase 3 clinical trials of NUZYRA Publication affirmation of the potential positive clinical impact NUZYRA can play in supporting the battle against the growing health challenge of antibiotic resistance Once-daily oral and IV NUZYRA safe and effective in adults with pneumonia and skin infections, demonstrating clinical activity against relevant pneumonia- and skin-associated drug resistant bacteria Highlights The New England Journal of Medicine Published February 7, 2019 N Engl J Med 2019; 380:517-527; DOI: 10.1056/NEJMoa1800201 (Link to Article) N Engl J Med 2019; 380:528-538; DOI: 10.1056/NEJMoa1800170 (Link to Article)
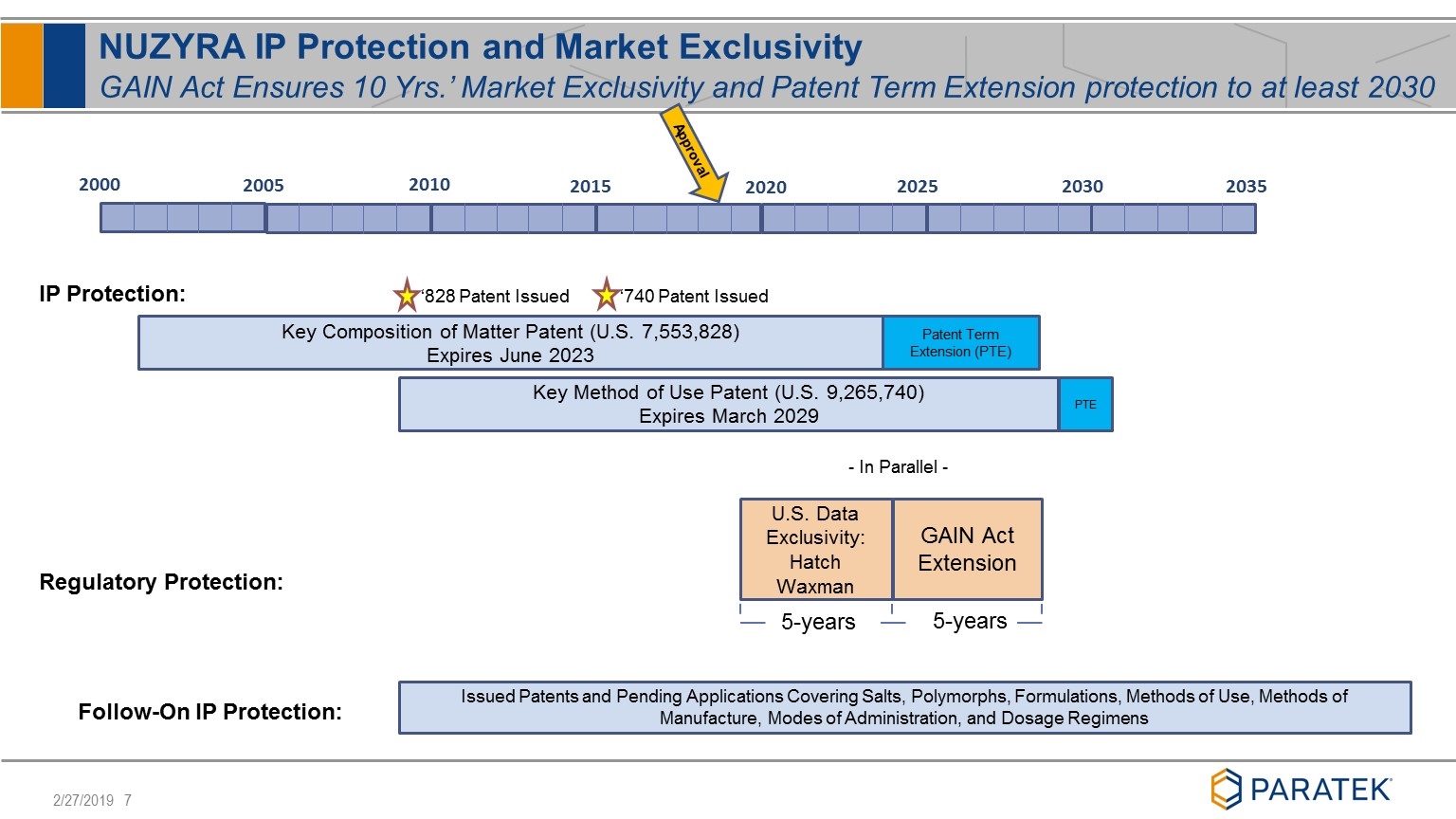
NUZYRA IP Protection and Market Exclusivity GAIN Act Ensures 10 Yrs.’ Market Exclusivity and Patent Term Extension protection to at least 2030 Patent Term Extension (PTE) Key Composition of Matter Patent (U.S. 7,553,828) Expires June 2023 2000 2005 2010 2015 2020 2025 2030 ‘828 Patent Issued Approval GAIN Act Extension - In Parallel - U.S. Data Exclusivity: Hatch Waxman 5-years 5-years IP Protection: Regulatory Protection: Issued Patents and Pending Applications Covering Salts, Polymorphs, Formulations, Methods of Use, Methods of Manufacture, Modes of Administration, and Dosage Regimens Follow-On IP Protection: 2035 PTE Key Method of Use Patent (U.S. 9,265,740) Expires March 2029 ‘740 Patent Issued
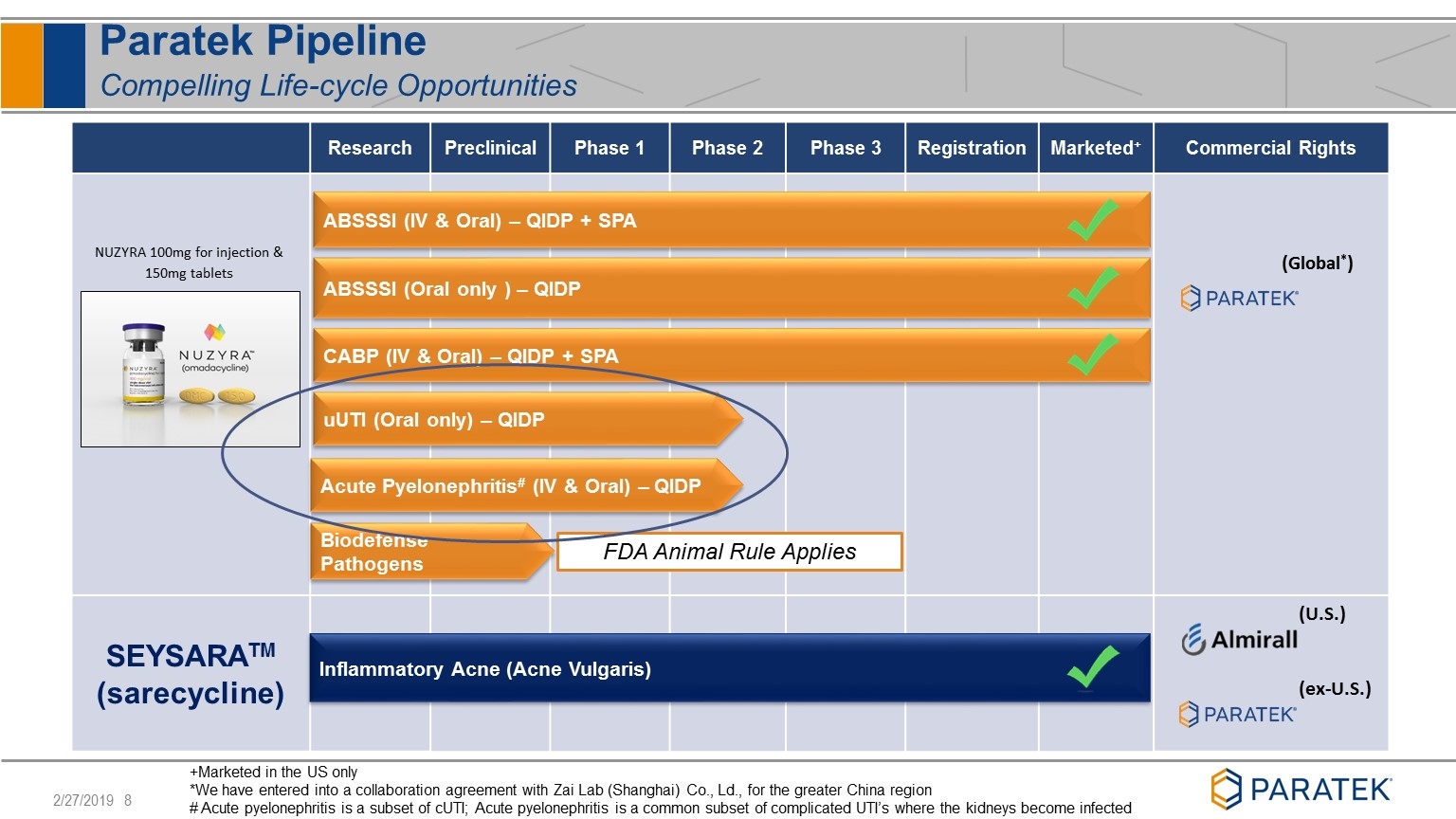
Paratek Pipeline Compelling Life-cycle Opportunities Research Preclinical Phase 1 Phase 2 Phase 3 Registration Marketed+ Commercial Rights SEYSARATM (sarecycline) (U.S.) (ex-U.S.) (Global*) uUTI (Oral only) – QIDP ABSSSI (IV & Oral) – QIDP + SPA CABP (IV & Oral) – QIDP + SPA Inflammatory Acne (Acne Vulgaris) +Marketed in the US only *We have entered into a collaboration agreement with Zai Lab (Shanghai) Co., Ld., for the greater China region # Acute pyelonephritis is a subset of cUTI; Acute pyelonephritis is a common subset of complicated UTI’s where the kidneys become infected ABSSSI (Oral only ) – QIDP Biodefense Pathogens NUZYRA 100mg for injection & 150mg tablets FDA Animal Rule Applies Acute Pyelonephritis# (IV & Oral) – QIDP
Strengthening the Balance Sheet Summary of Deal terms: Non-recourse, SEYSARA royalty-backed loan with HealthCare Royalty Partners Principal amount of $32.5 million, to be funded May 1, 2019 Interest rate 12% per annum paid quarterly Royalty payments in excess of accrued interest on the loan will be used to repay principal until the balance is fully repaid Once the loan has been paid back in full, the value will revert back to the benefit of Paratek
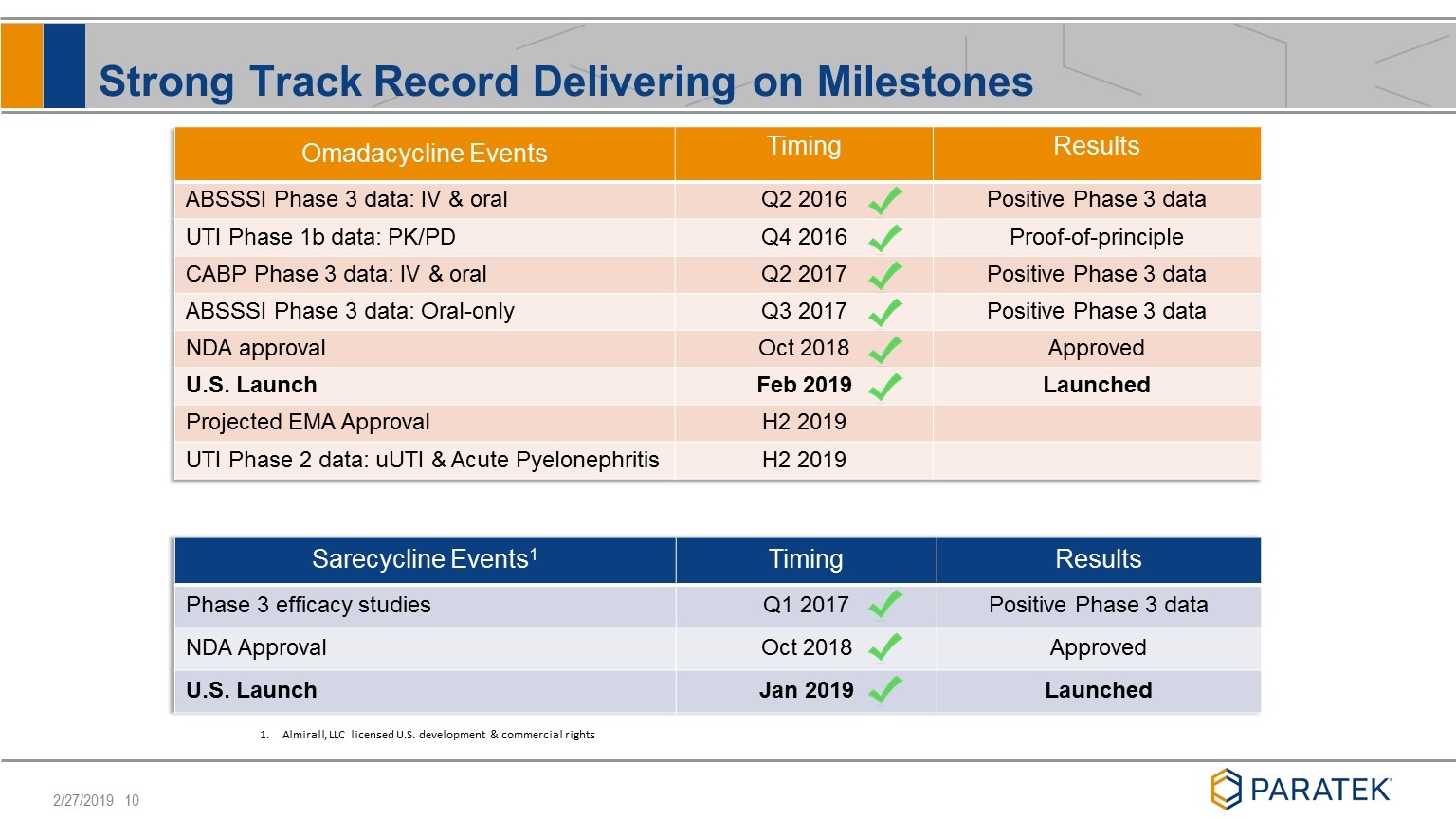
Strong Track Record Delivering on Milestones Omadacycline Events Timing Results ABSSSI Phase 3 data: IV & oral Q2 2016 Positive Phase 3 data UTI Phase 1b data: PK/PD Q4 2016 Proof-of-principle CABP Phase 3 data: IV & oral Q2 2017 Positive Phase 3 data ABSSSI Phase 3 data: Oral-only Q3 2017 Positive Phase 3 data NDA approval Oct 2018 Approved U.S. Launch Feb 2019 Launched Projected EMA Approval H2 2019 UTI Phase 2 data: uUTI & Acute Pyelonephritis H2 2019 Sarecycline Events1 Timing Results Phase 3 efficacy studies Q1 2017 Positive Phase 3 data NDA Approval Oct 2018 Approved U.S. Launch Jan 2019 Launched Almirall, LLC licensed U.S. development & commercial rights

Commercial Update Adam Woodrow, Chief Commercial Officer Recognizing the serious threat of bacterial infections, Paratek is dedicated to providing solutions that enable positive outcomes and lead to better patient stories.
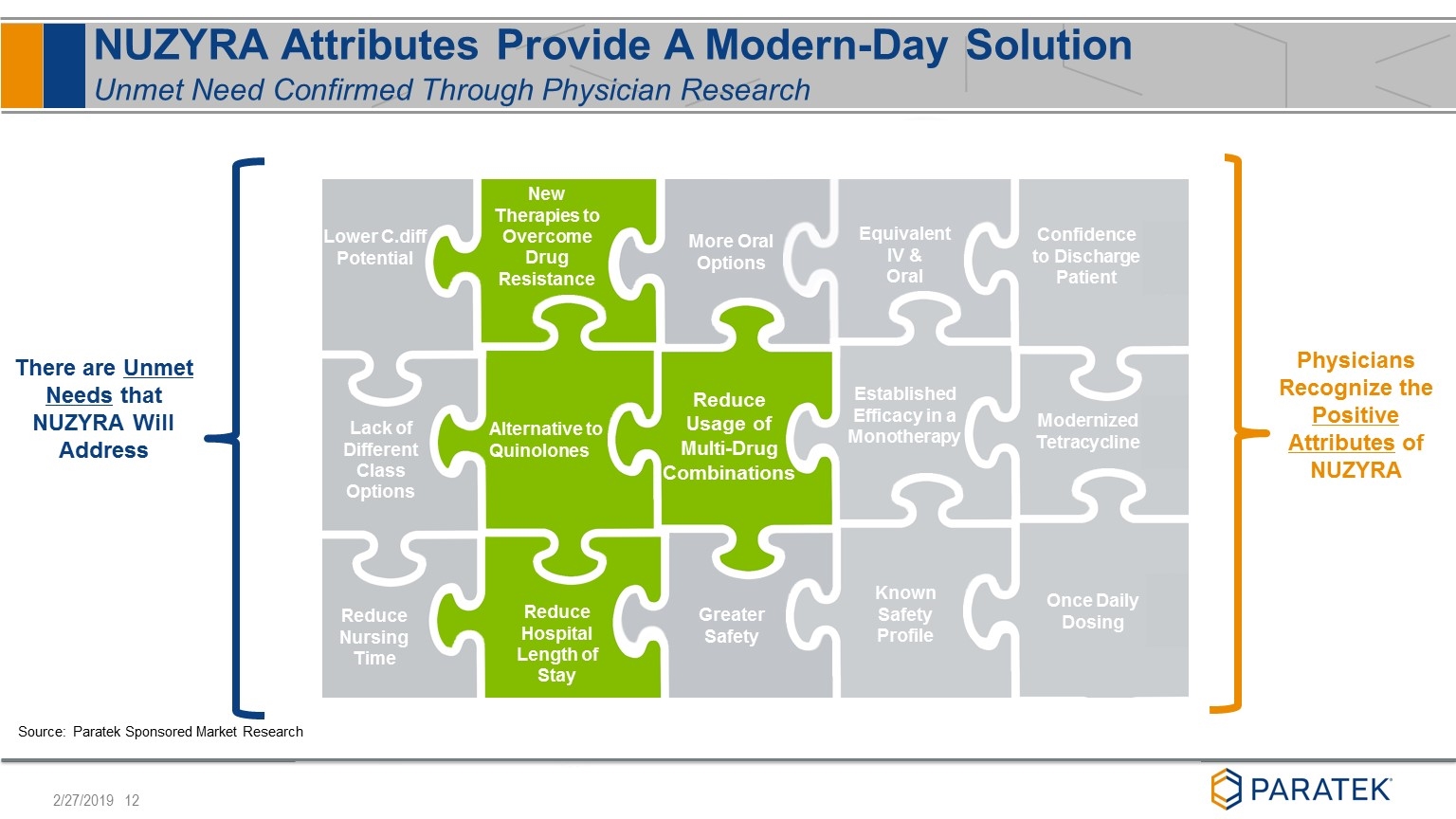
More Oral Options New Therapies to Overcome Drug Resistance Greater Safety Alternative to Quinolones There are Unmet Needs that NUZYRA Will Address Reduce Hospital Length of Stay Reduce Nursing Time Lack of Different Class Options Reduce Usage of Multi-Drug Combinations NUZYRA Attributes Provide A Modern-Day Solution Unmet Need Confirmed Through Physician Research Known Safety Profile Established Efficacy in a Monotherapy Equivalent IV & Oral Physicians Recognize the Positive Attributes of NUZYRA Modernized Tetracycline Confidence to Discharge Patient Once Daily Dosing Reduce Nursing Time Lower C.diff Potential Source: Paratek Sponsored Market Research Reduce Usage of Multi-Drug Combinations
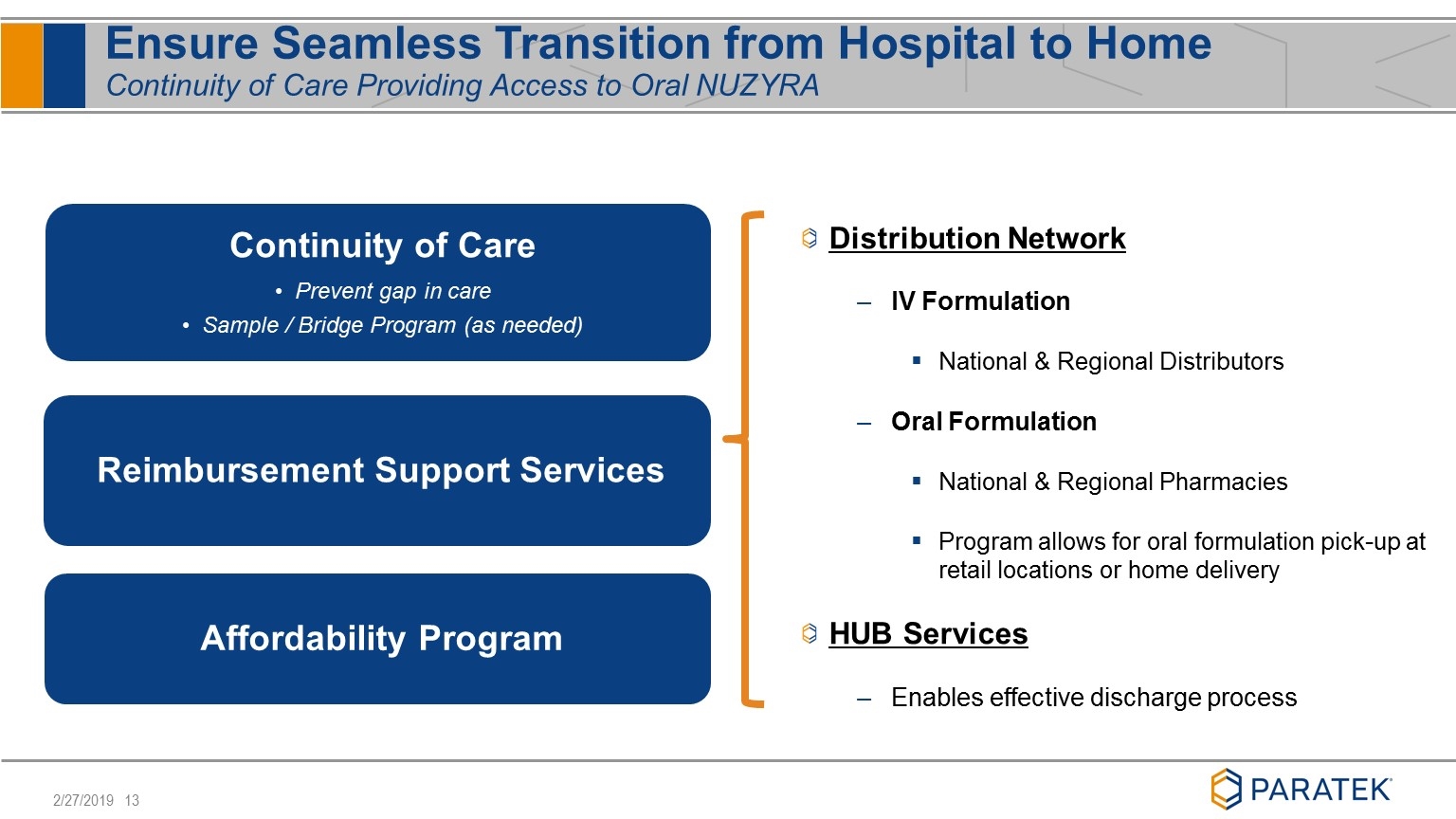
Ensure Seamless Transition from Hospital to Home Continuity of Care Providing Access to Oral NUZYRA Continuity of Care Prevent gap in care Sample / Bridge Program (as needed) Reimbursement Support Services Affordability Program 3 Distribution Network IV Formulation National & Regional Distributors Oral Formulation National & Regional Pharmacies Program allows for oral formulation pick-up at retail locations or home delivery HUB Services Enables effective discharge process
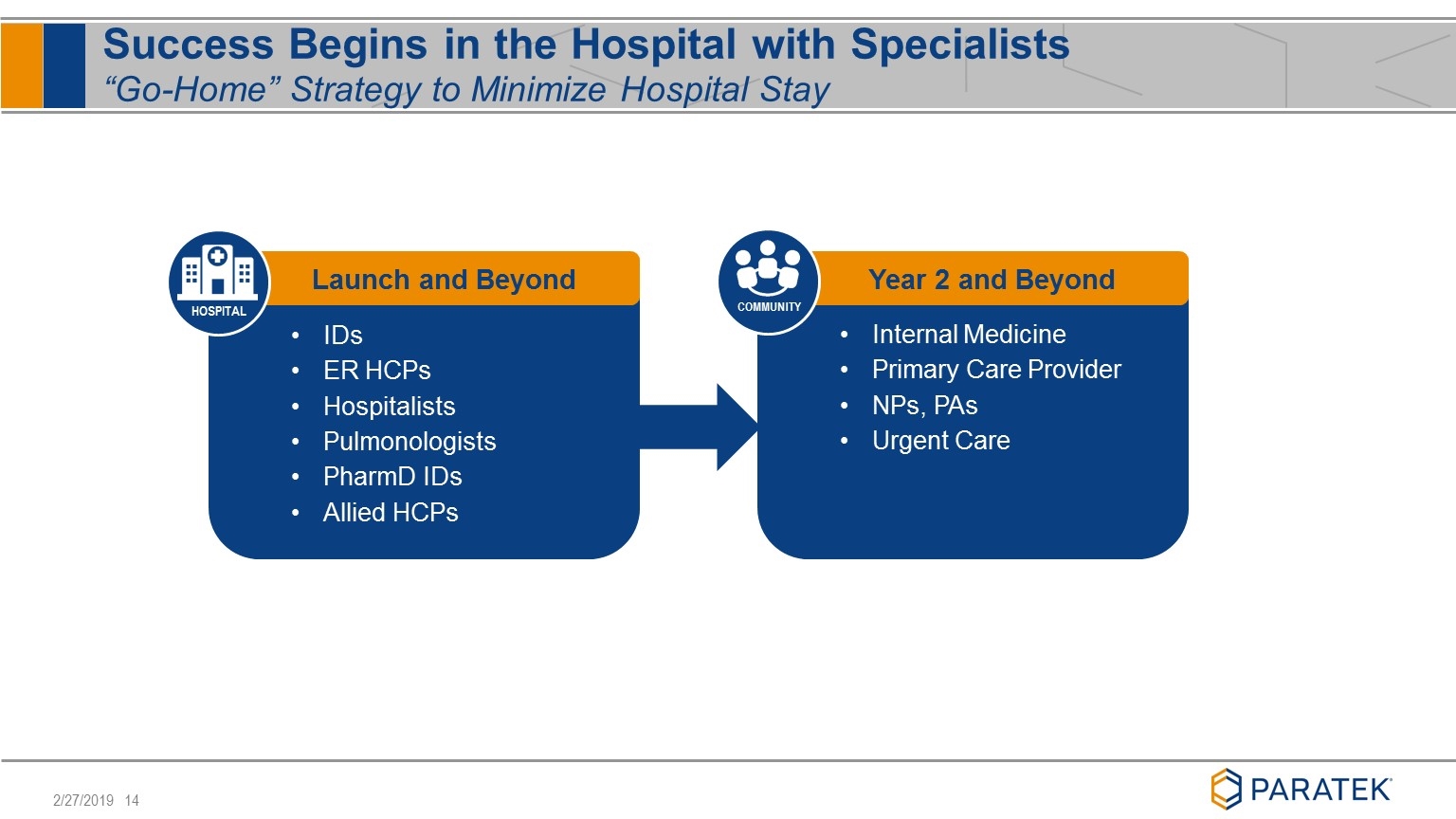
Success Begins in the Hospital with Specialists “Go-Home” Strategy to Minimize Hospital Stay IDs ER HCPs Hospitalists Pulmonologists PharmD IDs Allied HCPs Launch and Beyond HOSPITAL Internal Medicine Primary Care Provider NPs, PAs Urgent Care Year 2 and Beyond COMMUNITY
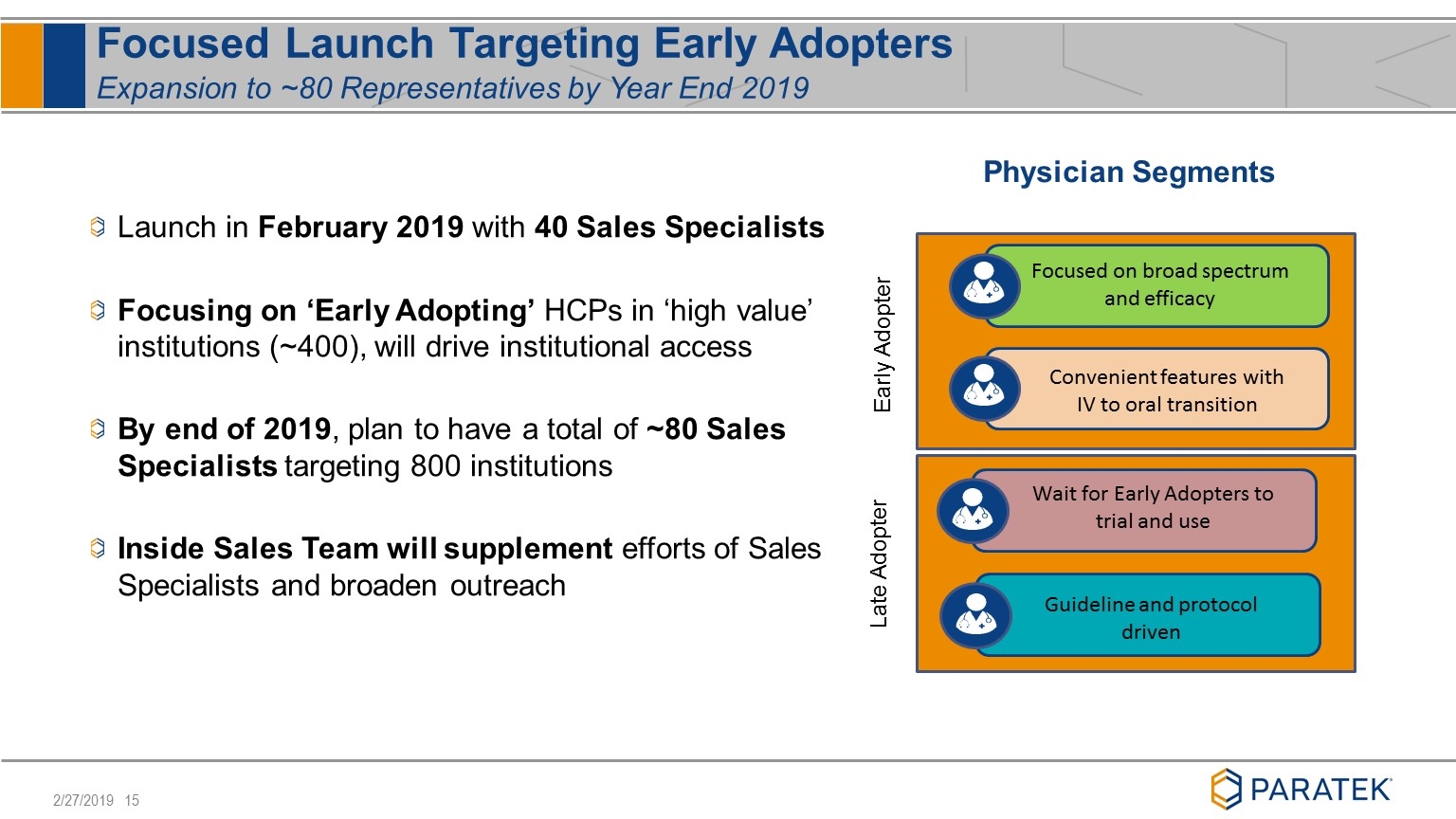
Focused Launch Targeting Early Adopters Expansion to ~80 Representatives by Year End 2019 Physician Segments Guideline and protocol driven Wait for Early Adopters to trial and use Convenient features with IV to oral transition Focused on broad spectrum and efficacy Launch in February 2019 with 40 Sales Specialists Focusing on ‘Early Adopting’ HCPs in ‘high value’ institutions (~400), will drive institutional access By end of 2019, plan to have a total of ~80 Sales Specialists targeting 800 institutions Inside Sales Team will supplement efforts of Sales Specialists and broaden outreach Early Adopter Late Adopter

Additional Tools to Support NUZYRA Adoption

Influencers: IDs PharmD IDs Pharmacy Directors Microbiologists = Field Force Has Two Simultaneous Objectives Institutional Access + Demand Generation Adoption Formulary/Protocols Specialty Access & Buying + Institutional Access Prescribers: IDs ER Hospitalists Pulmonologists Trial & Usage Demand Generation

Early Indicators to Track Performance 1 Covered Lives Institutional Access 3 months Post-Launch 33% of covered lives under contract 12 months Post-Launch 66% of covered lives under contract 12 months Post-Launch 70% of 800 targeted institutions

Financial Update Douglas W. Pagán, Chief Financial Officer Recognizing the serious threat of bacterial infections, Paratek is dedicated to providing solutions that enable positive outcomes and lead to better patient stories.
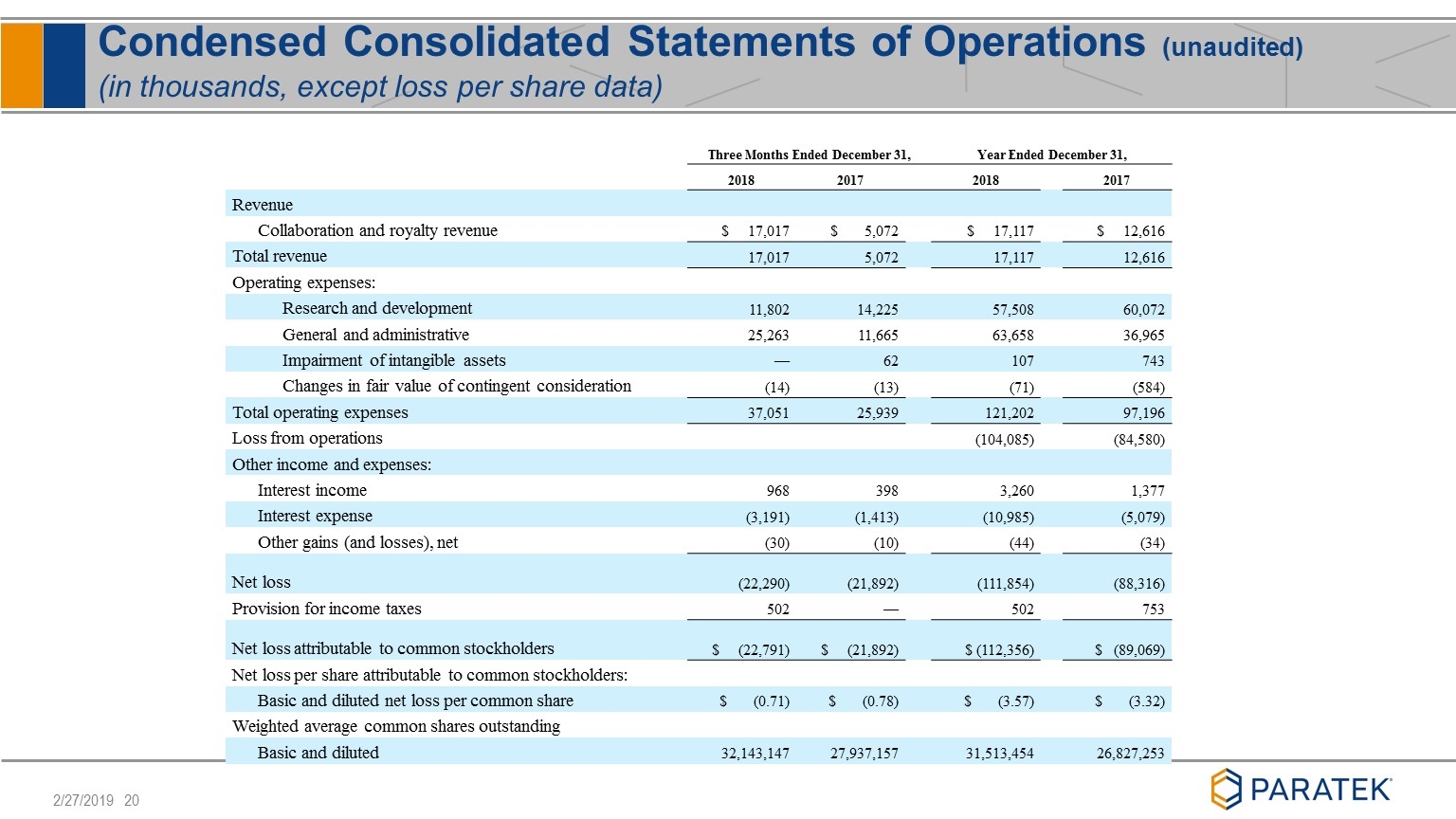
Condensed Consolidated Statements of Operations (unaudited) (in thousands, except loss per share data) Three Months Ended December 31, Year Ended December 31, 2018 2017 2018 2017 Revenue Collaboration and royalty revenue $ 17,017 $ 5,072 $ 17,117 $ 12,616 Total revenue 17,017 5,072 17,117 12,616 Operating expenses: Research and development 11,802 14,225 57,508 60,072 General and administrative 25,263 11,665 63,658 36,965 Impairment of intangible assets — 62 107 743 Changes in fair value of contingent consideration (14) (13) (71) (584) Total operating expenses 37,051 25,939 121,202 97,196 Loss from operations (104,085) (84,580) Other income and expenses: Interest income 968 398 3,260 1,377 Interest expense (3,191) (1,413) (10,985) (5,079) Other gains (and losses), net (30) (10) (44) (34) Net loss (22,290) (21,892) (111,854) (88,316) Provision for income taxes 502 — 502 753 Net loss attributable to common stockholders $ (22,791) $ (21,892) $ (112,356) $ (89,069) Net loss per share attributable to common stockholders: Basic and diluted net loss per common share $ (0.71) $ (0.78) $ (3.57) $ (3.32) Weighted average common shares outstanding Basic and diluted 32,143,147 27,937,157 31,513,454 26,827,253
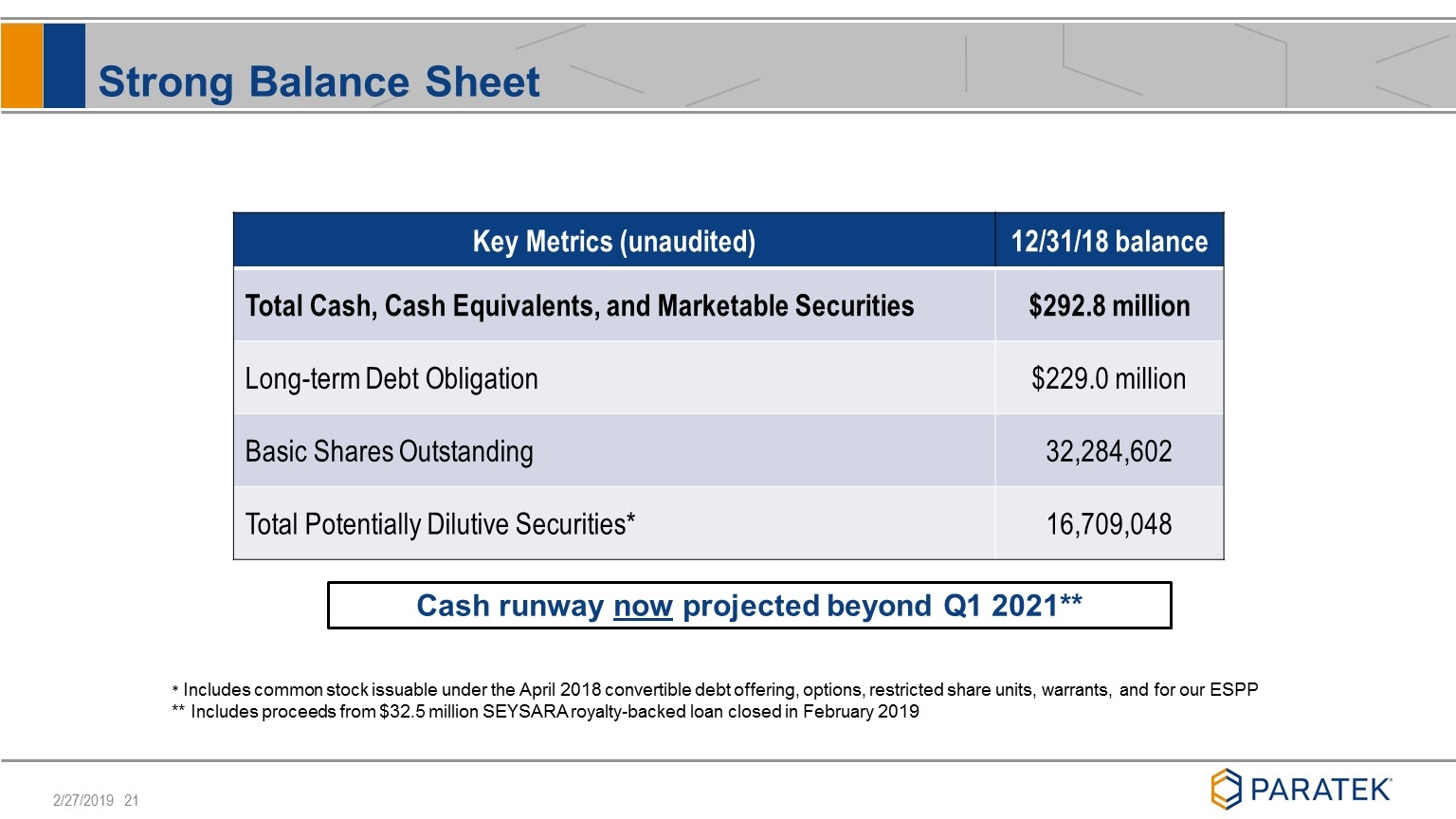
Strong Balance Sheet Key Metrics (unaudited) 12/31/18 balance Total Cash, Cash Equivalents, and Marketable Securities $292.8 million Long-term Debt Obligation $229.0 million Basic Shares Outstanding 32,284,602 Total Potentially Dilutive Securities* 16,709,048 * Includes common stock issuable under the April 2018 convertible debt offering, options, restricted share units, warrants, and for our ESPP ** Includes proceeds from $32.5 million SEYSARA royalty-backed loan closed in February 2019 Cash runway now projected beyond Q1 2021**
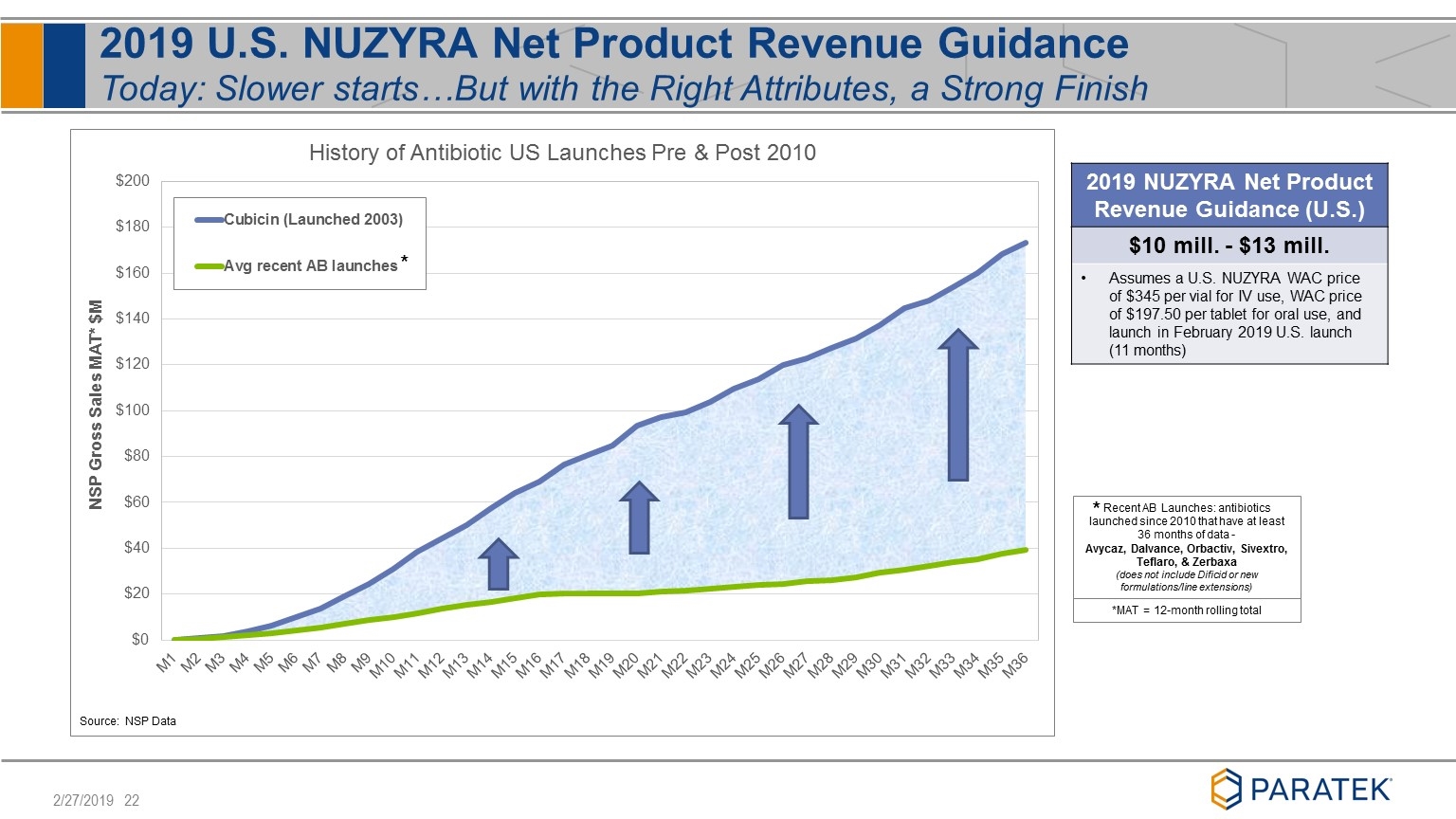
2019 U.S. NUZYRA Net Product Revenue Guidance Today: Slower starts…But with the Right Attributes, a Strong Finish Recent AB Launches: antibiotics launched since 2010 that have at least 36 months of data - Avycaz, Dalvance, Orbactiv, Sivextro, Teflaro, & Zerbaxa (does not include Dificid or new formulations/line extensions) *MAT = 12-month rolling total 2019 NUZYRA Net Product Revenue Guidance (U.S.) $10 mill. - $13 mill. Assumes a U.S. NUZYRA WAC price of $345 per vial for IV use, WAC price of $197.50 per tablet for oral use, and launch in February 2019 U.S. launch (11 months) * *

Closing Remarks Michael F. Bigham, Chief Executive Officer & Chairman of the Board Recognizing the serious threat of bacterial infections, Paratek is dedicated to providing solutions that enable positive outcomes and lead to better patient stories.
Well-Positioned for Future Growth Focused on Execution + New Value Creation Near-term Focus New Value Creation Launch of NUZYRA Prudent Operating Expense Management Non-Dilutive Sources of Capital Life-cycle Opportunities for NUZYRA Bio-Defense Product / Pipeline Expansion























