
Fourth Quarter & Full Year 2019 Financial Results & Corporate Update February 25, 2020 Paratek Pharmaceuticals, Inc. (Nasdaq: PRTK): A commercial-stage biopharmaceutical company focused on the development and commercialization of novel life-saving therapies for life-threatening diseases or other public health threats for civilian, government and military use Exhibit 99.2

Q4 and Full Year 2019 Earnings Call Agenda Introduction Ben Strain, Vice President, Investor Relations & Corporate Communications Overview and Financial Highlights Evan Loh, M.D., Chief Executive Officer Fourth Quarter and Full Year 2019 Commercial Highlights Adam Woodrow, President & Chief Commercial Officer Pipeline and Future Value Drivers Randy Brenner, Chief Development & Regulatory Officer Q&A Also available for Q&A: Michael F. Bigham, Executive Chairman Sarah Higgins, Vice President of Finance, Controller and Principal Accounting Officer

Third-party industry and market information included herein has been obtained from sources believed to be reliable, but the accuracy or completeness of such information is not guaranteed by, has not been independently verified by, and should not be construed as a representation by, Paratek. The information contained in this presentation is accurate only as of the date hereof. This presentation contains forward-looking statements including statements related to our overall strategy, products, prospects, potential and expected results, including statements about the projected awareness, payor coverage, net product revenues, total revenues including assumptions related to our financial guidance, the financial impact of our BARDA contract, our anticipated cash runway, our operating expenses, our SEYSARA royalty-backed loan funded on May 1, 2019, the progression of our commercial roll out for NUZYRA, our ability to shape the future treatment paradigm for community-acquired pneumonia and serious skin infections, our plans to evaluate additional indications for NUZYRA, including NTM, and to work toward an oral-only indication in CABP, future governmental stockpiling opportunities, and our potential to further drive long-term value for all of our shareholders. All statements, other than statements of historical facts, included in this presentation are forward-looking statements, and are identified by words such as "advancing," "expect," "look forward," "anticipate," "continue," and other words and terms of similar meaning. These forward-looking statements are based upon our current expectations and involve substantial risks and uncertainties. We may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in our forward-looking statements and you should not place undue reliance on these forward-looking statements. Our actual results and the timing of events could differ materially from those included in such forward-looking statements as a result of these risks and uncertainties. These and other risk factors are discussed under "Risk Factors" and elsewhere in our Annual Report on Form 10-K for the year ended December 31, 2018 and our other filings with the Securities and Exchange Commission. We expressly disclaim any obligation or undertaking to update or revise any forward-looking statements contained herein. PARATEK® and the Hexagon Logo are registered trademarks of Paratek Pharmaceuticals, Inc. NUZYRA and its design logo are trademarks of Paratek Pharmaceuticals, Inc. All other trademarks, service marks, trade names, logos and brand names identified in this presentation are the property of their respective owners. Safe Harbor Statement

Overview & Financial Highlights Evan Loh, M.D. Chief Executive Officer Paratek Pharmaceuticals, Inc. (Nasdaq: PRTK): A commercial-stage biopharmaceutical company focused on the development and commercialization of novel life-saving therapies for life-threatening diseases or other public health threats for civilian, government and military use
Financial Highlights Full Year & Fourth Quarter 2019 Fourth Quarter 2019 Net Revenues = $9.0 million primarily driven by the launch of NUZYRA® NUZYRA net sales = $5.4 million (increase of ~74% versus 3Q 2019) Driven by increases in demand Other revenue = $3.6 million Driven by $3.0 million milestone earned from Zai Lab and royalties earned from SEYSARA Full Year 2019 Net Revenues = $16.5 million primarily driven by the launch of NUZYRA® NUZYRA net sales = $11.5 million (launched in February 2019) Other revenue = $5.0 million Driven by $3.0 million milestone earned from Zai Lab and royalties earned from SEYSARA
BARDA BioShield Contract A Unique Public-Private Partnership with Paratek
New Drug Application (NDA) Submission of Omadacycline in China Accepted by the China National Medical Products Administration (NMPA) February 2020 Zai Lab announced that NMPA has accepted its NDA for omadacycline Seeking approval for the treatment of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections Under the terms of the Zai Collaboration Agreement, Paratek is entitled to receive: A milestone payment of $6 million upon regulatory approval (anticipated in the first half of 2021) Tiered royalties at low double digit to mid-teen percentages on net revenues for sales of omadacycline in the greater China region

SEYSARA: Entered into a License Grant with Almirall for Greater China Region Greater China Region: Paratek will earn high single-digit royalties on net sales in the greater China region Almirall plans to develop sarecycline for acne in China, with a potential submission to the China National Medical Products Administration in 2023 Rest of World: Paratek and Almirall also finalized a license granting Paratek exclusive rights to develop, manufacture and commercialize saracycline outside the of the U.S. Paratek will share with Almirall any potential revenues of sarecycline outside of the U.S. and greater China region
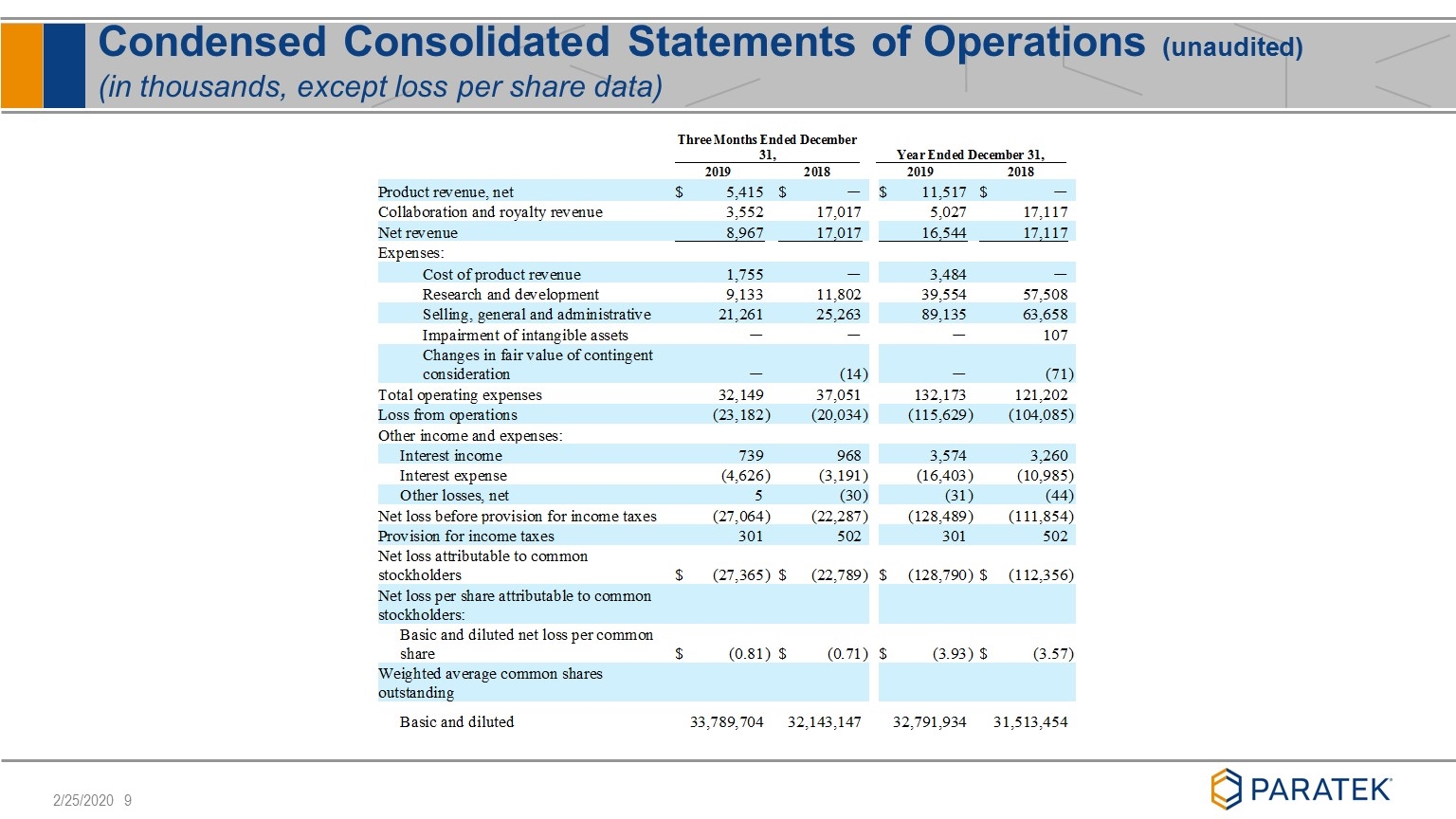
Condensed Consolidated Statements of Operations (unaudited) (in thousands, except loss per share data)

Paratek 2020 Financial Guidance Revenue Guidance Note NUZYRA US Net Product Revenue ~$66M ~$38 million of these sales coming from the initial BARDA procurement of 2,500 anthrax treatment courses The initial NUZYRA BARDA procurement is anticipated to be secured in the first half of 2020 Royalty and Collaboration Revenue and BARDA Grant Revenue $9M to $14M BARDA grant revenue consists of reimbursement associated with the post-marketing requirement clinical development activities, the anthrax development program and the onshoring of U.S. NUZYRA manufacturing 2020 Total Revenue $75M to $80M R&D and SG&A expense ~$140M Excluding the BARDA R&D and onshoring reimbursement, R&D and SG&A expense is expected to remain relatively flat when compared to 2019 R&D expense includes approximately $5 million earmarked for the start-up activities in preparation for a potential NTM registrational study
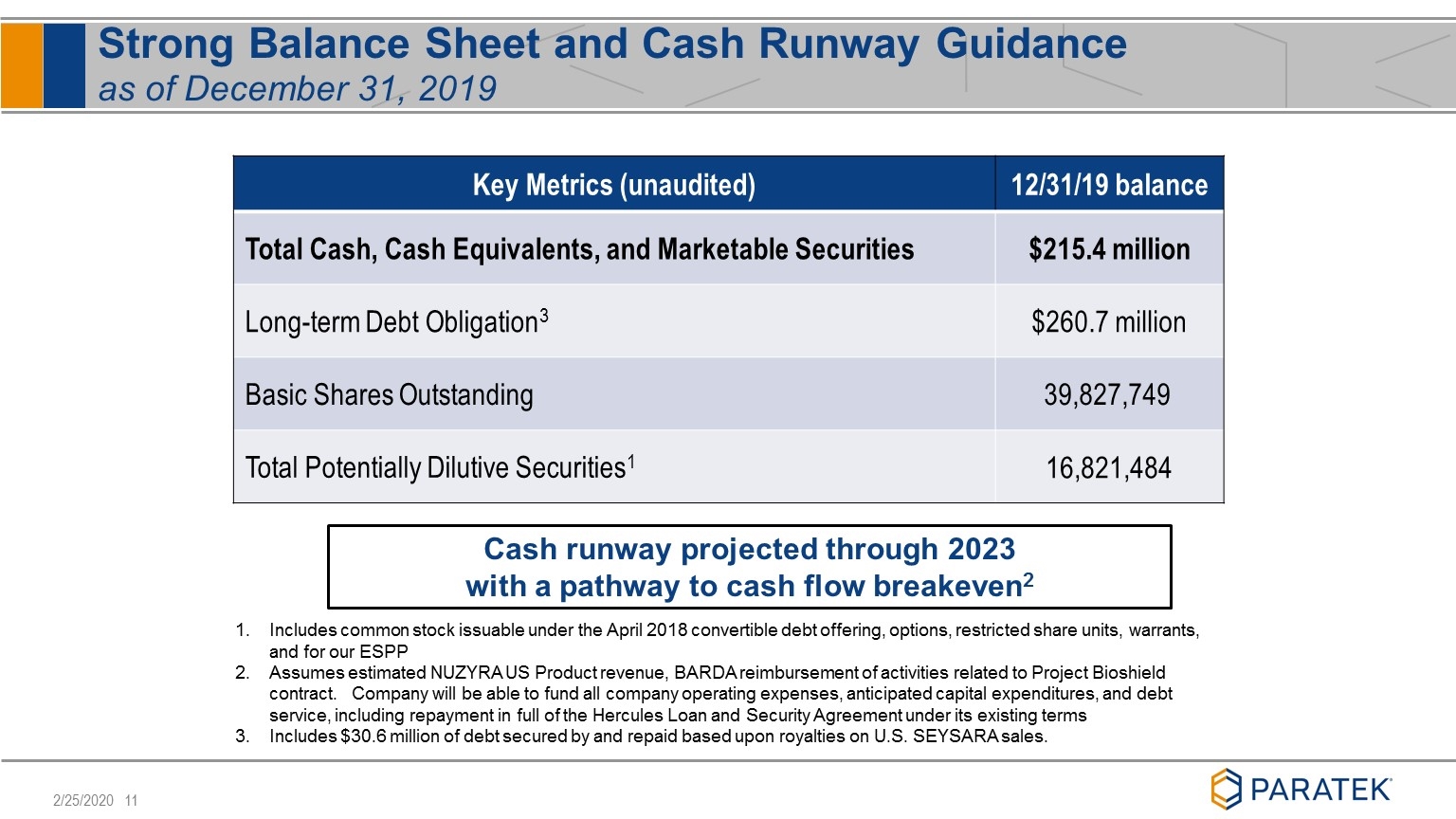
Strong Balance Sheet and Cash Runway Guidance as of December 31, 2019 Key Metrics (unaudited) 12/31/19 balance Total Cash, Cash Equivalents, and Marketable Securities $215.4 million Long-term Debt Obligation3 $260.7 million Basic Shares Outstanding 39,827,749 Total Potentially Dilutive Securities1 16,821,484 Includes common stock issuable under the April 2018 convertible debt offering, options, restricted share units, warrants, and for our ESPP Assumes estimated NUZYRA US Product revenue, BARDA reimbursement of activities related to Project Bioshield contract. Company will be able to fund all company operating expenses, anticipated capital expenditures, and debt service, including repayment in full of the Hercules Loan and Security Agreement under its existing terms Includes $30.6 million of debt secured by and repaid based upon royalties on U.S. SEYSARA sales. Cash runway projected through 2023 with a pathway to cash flow breakeven2

Fourth Quarter 2019 Commercial Highlights Adam Woodrow President & Chief Commercial Officer Paratek Pharmaceuticals, Inc. (Nasdaq: PRTK): A commercial-stage biopharmaceutical company focused on the development and commercialization of novel life-saving therapies for life-threatening diseases or other public health threats for civilian, government and military use
NUZYRA Attributes Provide A Modern-Day Solution Addressing Bacterial Resistance and the Needs of Today’s Healthcare Systems NUZYRA is a once-daily oral and IV broad spectrum antibiotic Community Acquired Bacterial Pneumonia (CABP) Acute Bacterial Skin & Skin Structure Infections (ABSSSI) High and durable clinical efficacy with favorable safety and tolerability Addresses antibiotic resistance which today is causing clinical failures with older generic antibiotics Continuity of care: Once-daily IV to oral NUZYRA has the potential to minimize hospital stay Oral only indication(s) has the potential to avoid hospitalization all together
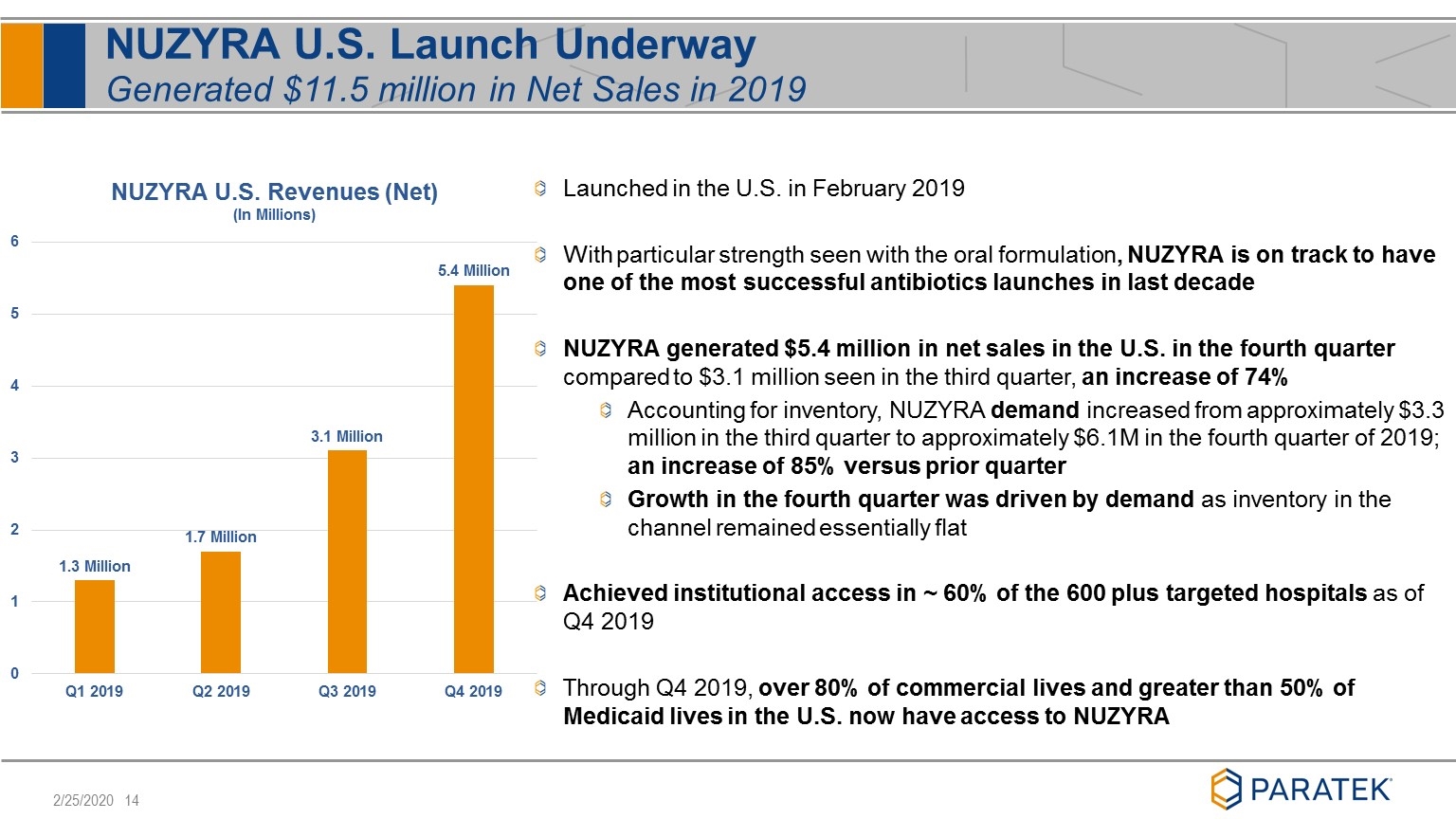
NUZYRA U.S. Launch Underway Generated $11.5 million in Net Sales in 2019 Launched in the U.S. in February 2019 With particular strength seen with the oral formulation, NUZYRA is on track to have one of the most successful antibiotics launches in last decade NUZYRA generated $5.4 million in net sales in the U.S. in the fourth quarter compared to $3.1 million seen in the third quarter, an increase of 74% Accounting for inventory, NUZYRA demand increased from approximately $3.3 million in the third quarter to approximately $6.1M in the fourth quarter of 2019; an increase of 85% versus prior quarter Growth in the fourth quarter was driven by demand as inventory in the channel remained essentially flat Achieved institutional access in ~ 60% of the 600 plus targeted hospitals as of Q4 2019 Through Q4 2019, over 80% of commercial lives and greater than 50% of Medicaid lives in the U.S. now have access to NUZYRA

Pipeline and Future Value Drivers Randy Brenner Chief Development & Regulatory Officer Paratek Pharmaceuticals, Inc. (Nasdaq: PRTK): A commercial-stage biopharmaceutical company focused on the development and commercialization of novel life-saving therapies for life-threatening diseases or other public health threats for civilian, government and military use

First-ever Bioshield award for an antibiotic for the Strategic National Stockpile Paratek was the sole recipient Valued at up to $285 million over 5 years, with potential for extension up to 10 years $77 million in reimbursement for all existing post-approval obligations $54 million for anthrax development & U.S. onshoring of manufacturing $153 million purchase of NUZYRA for the Strategic National Stockpile BARDA Project BioShield Contract Awarded To Paratek Unique & Transformative Opportunity Enabling Long-term Growth
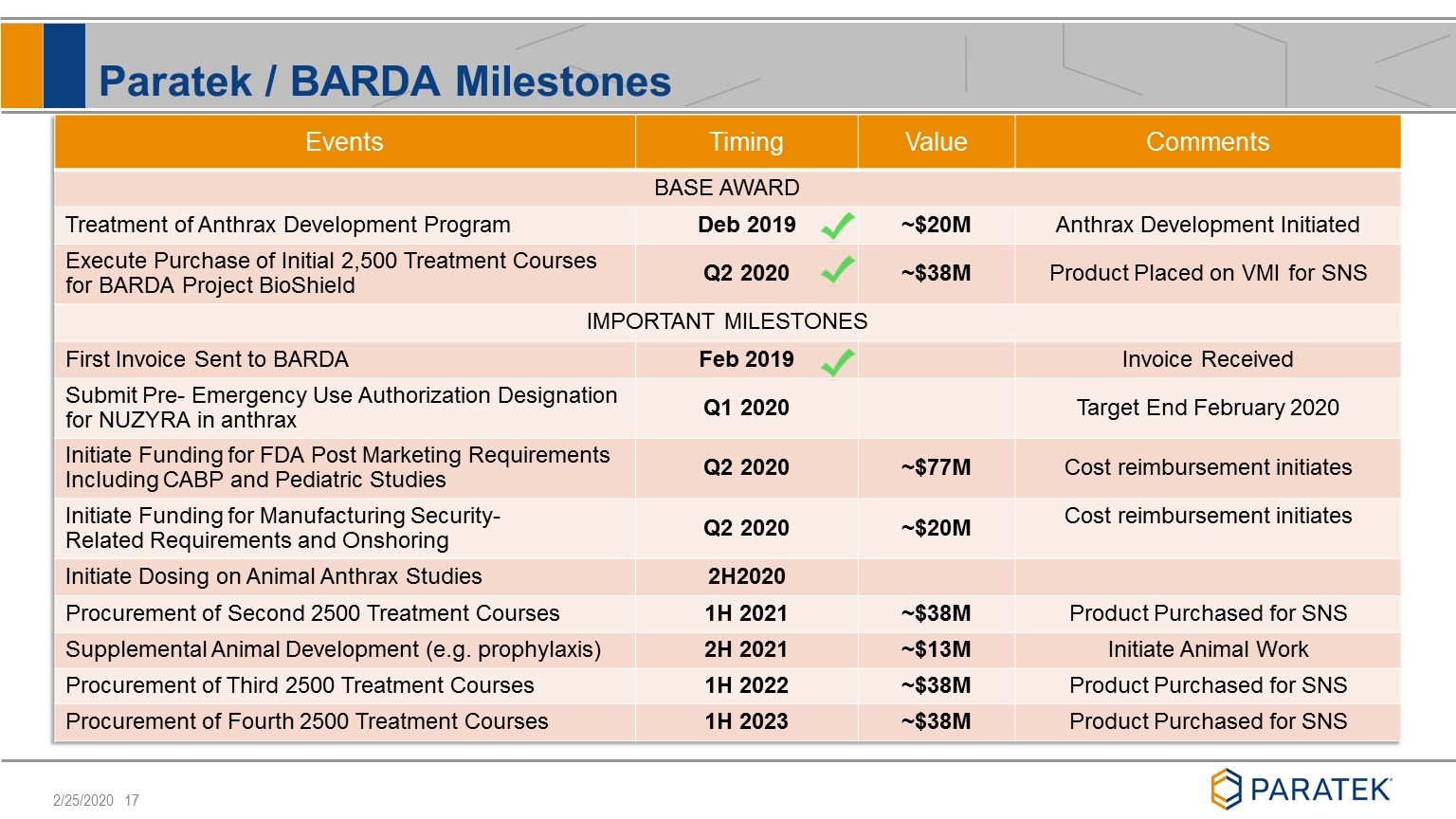
Paratek / BARDA Milestones Events Timing Value Comments BASE AWARD Treatment of Anthrax Development Program Deb 2019 ~$20M Anthrax Development Initiated Execute Purchase of Initial 2,500 Treatment Courses for BARDA Project BioShield Q2 2020 ~$38M Product Placed on VMI for SNS IMPORTANT MILESTONES First Invoice Sent to BARDA Feb 2019 Invoice Received Submit Pre- Emergency Use Authorization Designation for NUZYRA in anthrax Q1 2020 Target End February 2020 Initiate Funding for FDA Post Marketing Requirements Including CABP and Pediatric Studies Q2 2020 ~$77M Cost reimbursement initiates Initiate Funding for Manufacturing Security-Related Requirements and Onshoring Q2 2020 ~$20M Cost reimbursement initiates Initiate Dosing on Animal Anthrax Studies 2H2020 Procurement of Second 2500 Treatment Courses 1H 2021 ~$38M Product Purchased for SNS Supplemental Animal Development (e.g. prophylaxis) 2H 2021 ~$13M Initiate Animal Work Procurement of Third 2500 Treatment Courses 1H 2022 ~$38M Product Purchased for SNS Procurement of Fourth 2500 Treatment Courses 1H 2023 ~$38M Product Purchased for SNS
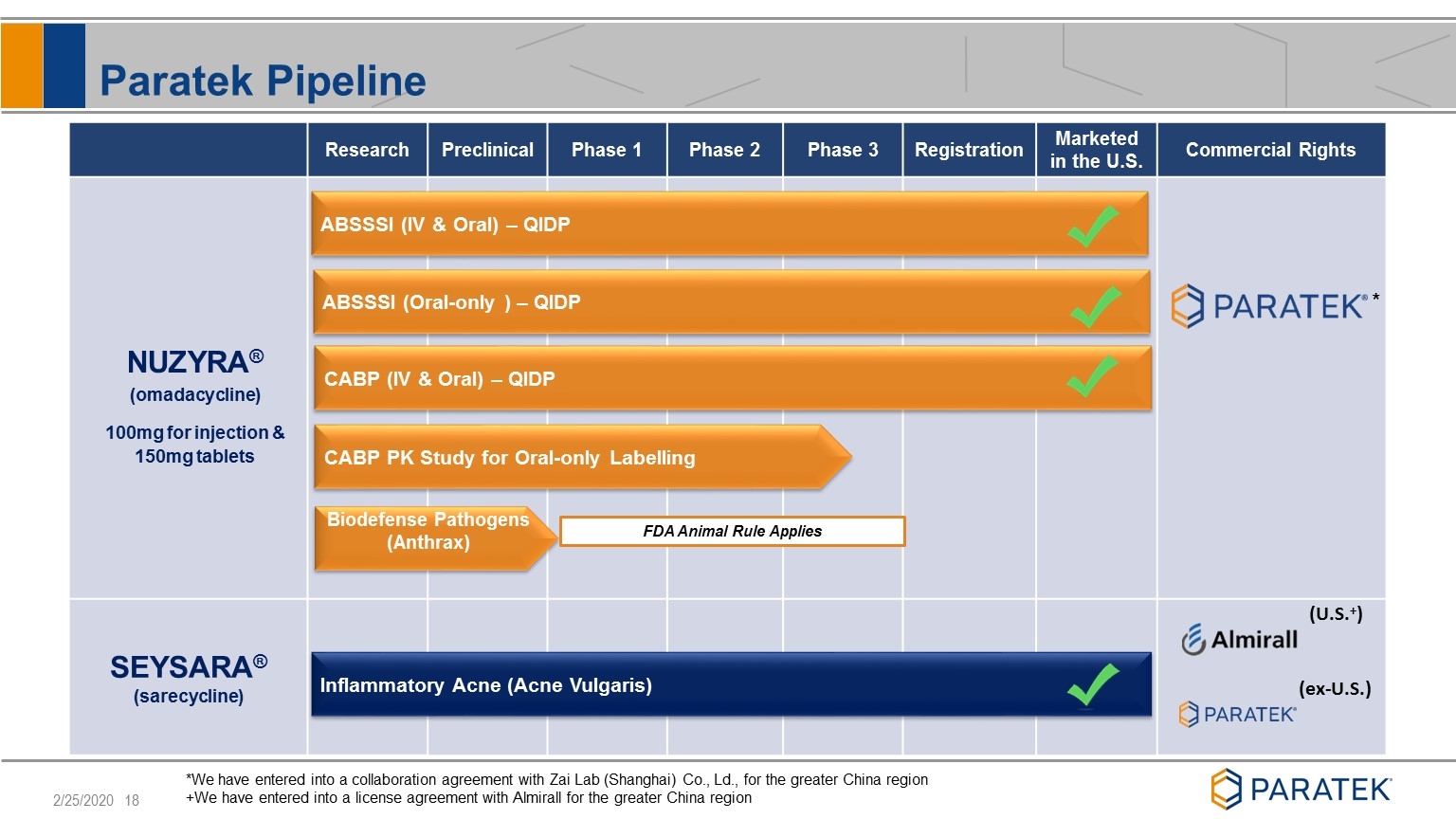
Paratek Pipeline Research Preclinical Phase 1 Phase 2 Phase 3 Registration Marketed in the U.S. Commercial Rights SEYSARA® (sarecycline) (U.S.+) (ex-U.S.) ABSSSI (IV & Oral) – QIDP CABP (IV & Oral) – QIDP Inflammatory Acne (Acne Vulgaris) *We have entered into a collaboration agreement with Zai Lab (Shanghai) Co., Ld., for the greater China region +We have entered into a license agreement with Almirall for the greater China region ABSSSI (Oral-only ) – QIDP Biodefense Pathogens (Anthrax) NUZYRA® (omadacycline) 100mg for injection & 150mg tablets FDA Animal Rule Applies CABP PK Study for Oral-only Labelling *
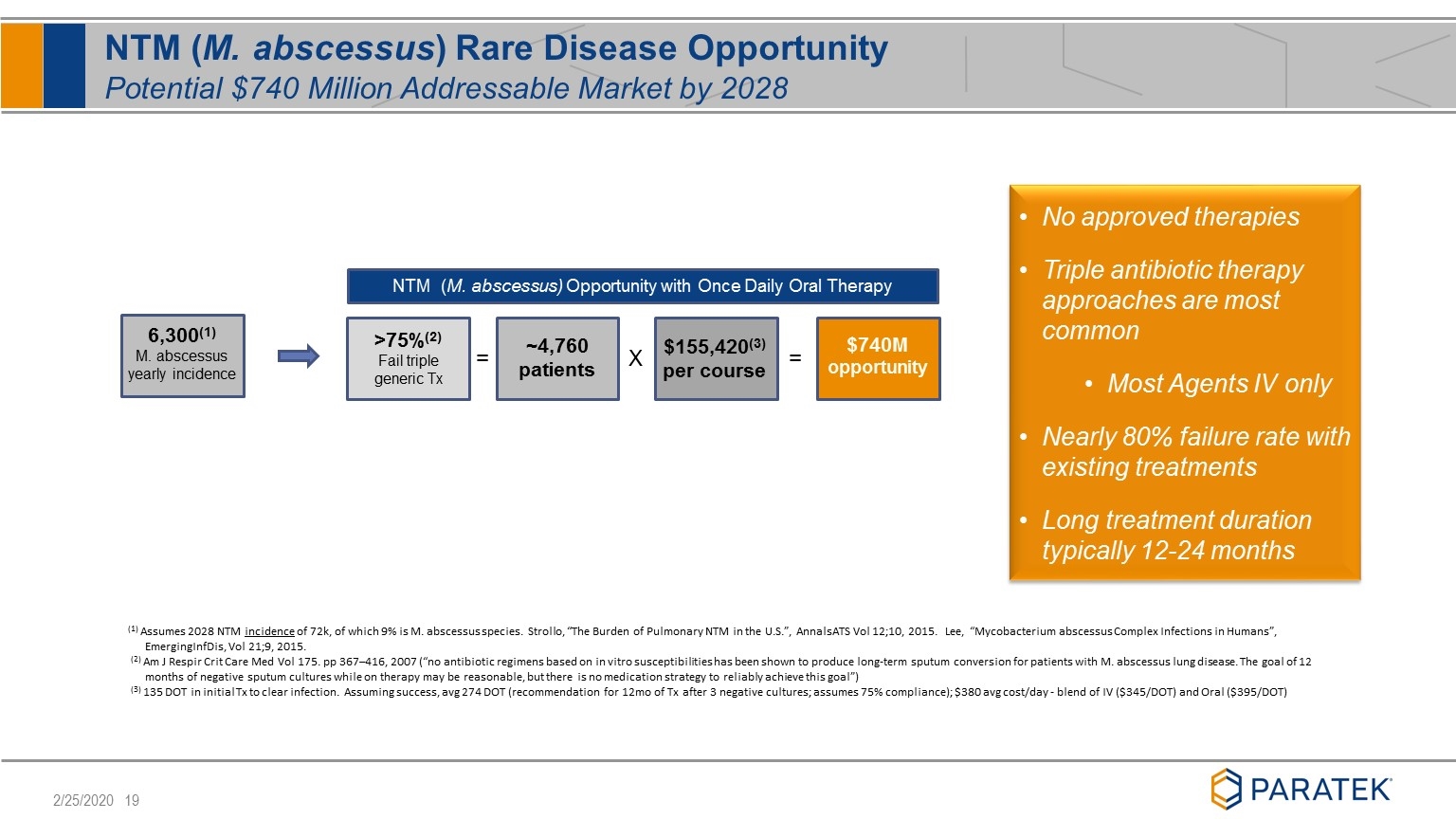
NTM (M. abscessus) Rare Disease Opportunity Potential $740 Million Addressable Market by 2028 NTM (M. abscessus) Opportunity with Once Daily Oral Therapy = X = >75%(2) Fail triple generic Tx ~4,760 patients $155,420(3) per course $740M opportunity (1) Assumes 2028 NTM incidence of 72k, of which 9% is M. abscessus species. Strollo, “The Burden of Pulmonary NTM in the U.S.”, AnnalsATS Vol 12;10, 2015. Lee, “Mycobacterium abscessus Complex Infections in Humans”, EmergingInfDis, Vol 21;9, 2015. (2) Am J Respir Crit Care Med Vol 175. pp 367–416, 2007 (“no antibiotic regimens based on in vitro susceptibilities has been shown to produce long-term sputum conversion for patients with M. abscessus lung disease. The goal of 12 months of negative sputum cultures while on therapy may be reasonable, but there is no medication strategy to reliably achieve this goal”) (3) 135 DOT in initial Tx to clear infection. Assuming success, avg 274 DOT (recommendation for 12mo of Tx after 3 negative cultures; assumes 75% compliance); $380 avg cost/day - blend of IV ($345/DOT) and Oral ($395/DOT) 6,300(1) M. abscessus yearly incidence No approved therapies Triple antibiotic therapy approaches are most common Most Agents IV only Nearly 80% failure rate with existing treatments Long treatment duration typically 12-24 months
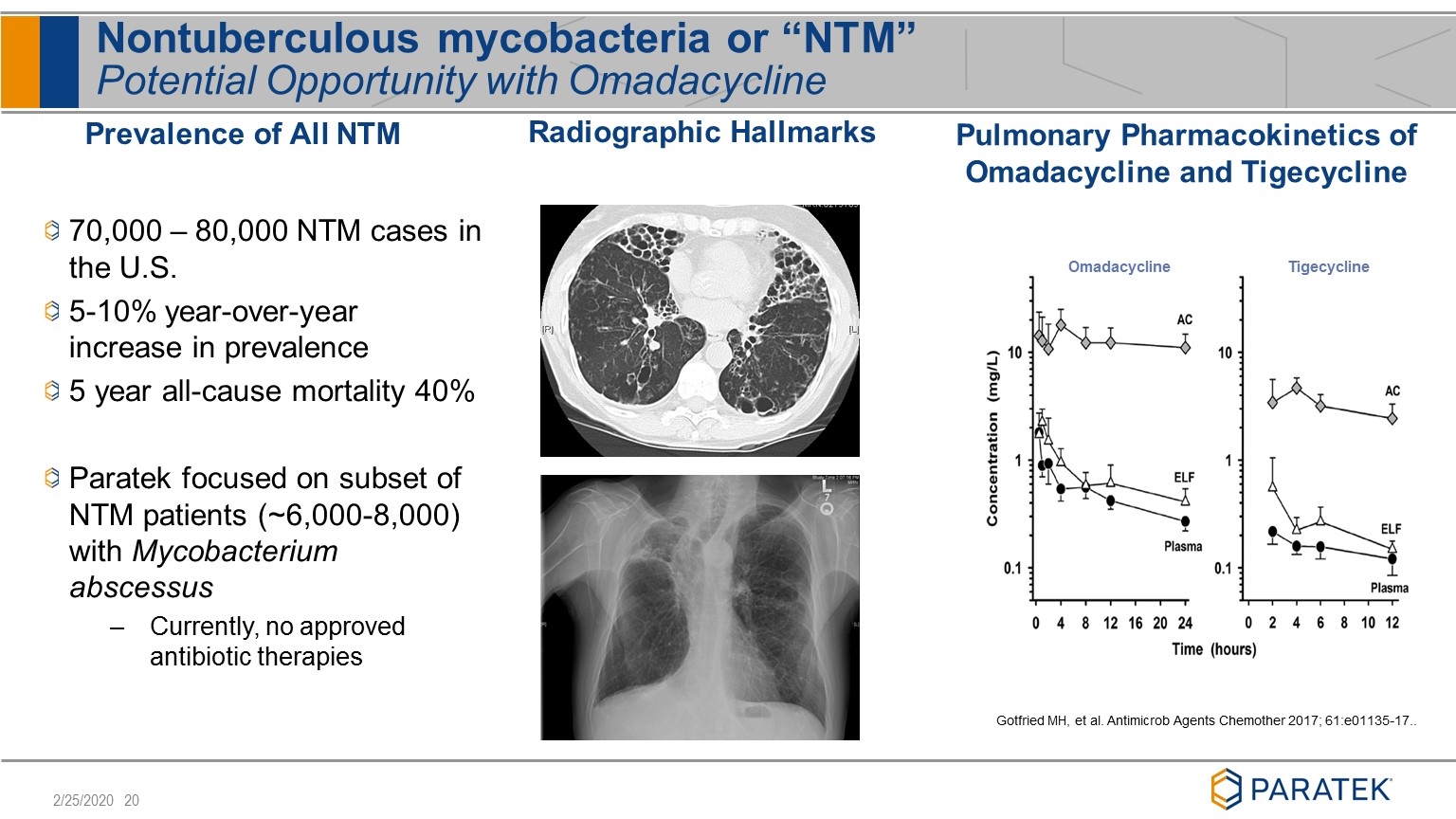
Nontuberculous mycobacteria or “NTM” Potential Opportunity with Omadacycline Prevalence of All NTM 70,000 – 80,000 NTM cases in the U.S. 5-10% year-over-year increase in prevalence 5 year all-cause mortality 40% Paratek focused on subset of NTM patients (~6,000-8,000) with Mycobacterium abscessus Currently, no approved antibiotic therapies Radiographic Hallmarks Omadacycline Tigecycline Pulmonary Pharmacokinetics of Omadacycline and Tigecycline Gotfried MH, et al. Antimicrob Agents Chemother 2017; 61:e01135-17..

Q&A Paratek Pharmaceuticals, Inc. (Nasdaq: PRTK): A commercial-stage biopharmaceutical company focused on the development and commercialization of novel life-saving therapies for life-threatening diseases or other public health threats for civilian, government and military use

Closing Remarks Evan Loh, M.D. Chief Executive Officer Paratek Pharmaceuticals, Inc. (Nasdaq: PRTK): A commercial-stage biopharmaceutical company focused on the development and commercialization of novel life-saving therapies for life-threatening diseases or other public health threats for civilian, government and military use
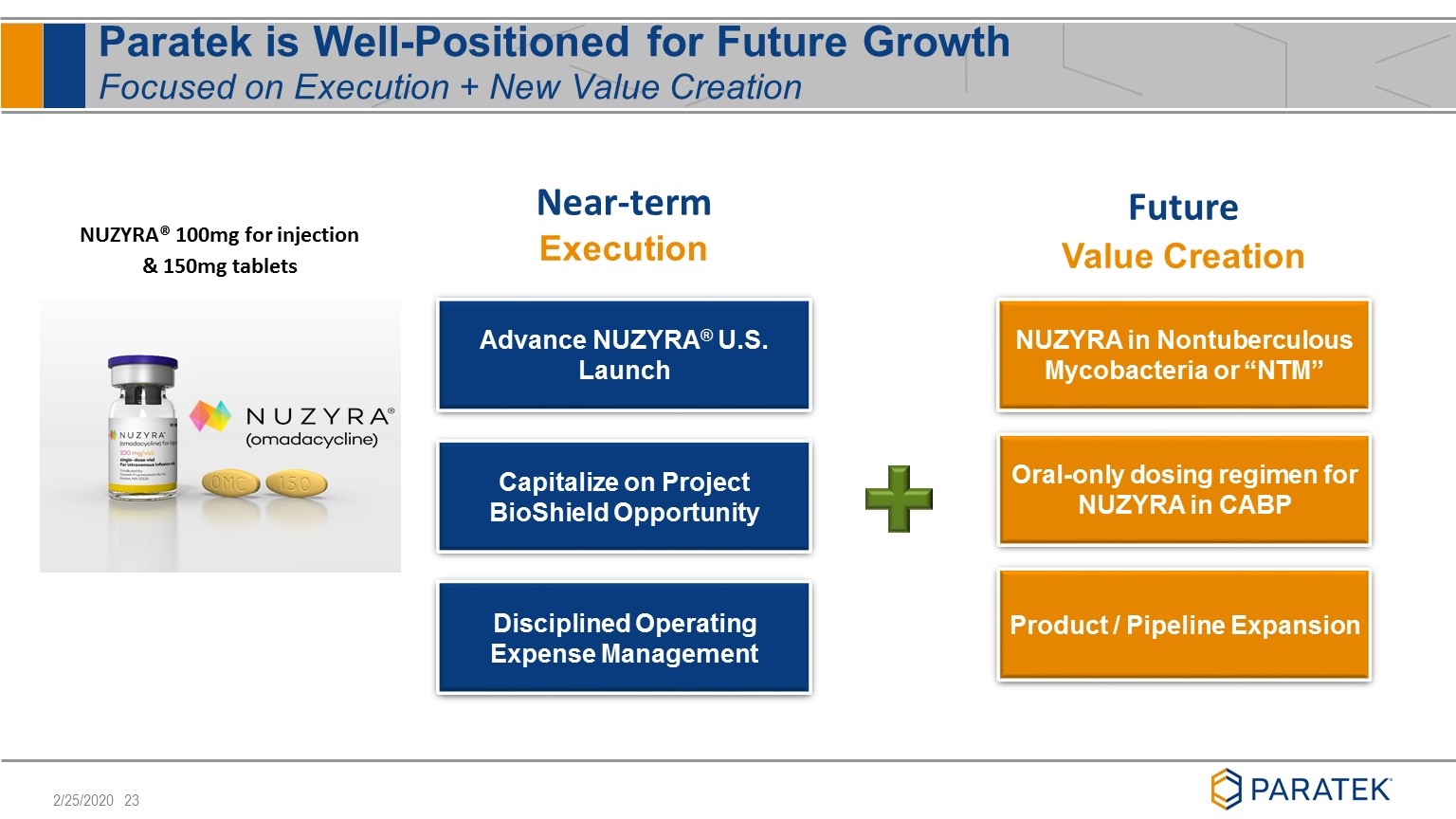
Paratek is Well-Positioned for Future Growth Focused on Execution + New Value Creation Near-term Execution Future Value Creation Advance NUZYRA® U.S. Launch Disciplined Operating Expense Management Capitalize on Project BioShield Opportunity NUZYRA in Nontuberculous Mycobacteria or “NTM” Oral-only dosing regimen for NUZYRA in CABP Product / Pipeline Expansion NUZYRA® 100mg for injection & 150mg tablets






















