- FOLD Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
8-K Filing
Amicus Therapeutics (FOLD) 8-KOther Events
Filed: 7 Sep 16, 12:00am
Exhibit 99.1
Corporate Overview September 2016

Safe Harbor This presentation contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 relating to preclinical and clinical development of our product candidates, the timing and reporting of results from preclinical studies and clinical trials, the prospects and timing of the potential regulatory approval of our product candidates, commercialization plans, financing plans, and the projected cash position for the Company. The inclusion of forward-looking statements should not be regarded as a representation by us that any of our plans will be achieved. Any or all of the forward-looking statements in this press release may turn out to be wrong and can be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties. For example, with respect to statements regarding the goals, progress, timing, and outcomes of discussions with regulatory authorities, and in particular the potential goals, progress, timing, and results of preclinical studies and clinical trials, actual results may differ materially from those set forth in this release due to the risks and uncertainties inherent in our business, including, without limitation: the potential that results of clinical or preclinical studies indicate that the product candidates are unsafe or ineffective; the potential that it may be difficult to enroll patients in our clinical trials; the potential that regulatory authorities, including the FDA, EMA, and PMDA may not grant or may delay approval for our product candidates; the potential that we may not be successful in commercializing Galafold in Europe or our other product candidates if and when approved; the potential that preclinical and clinical studies could be delayed because we identify serious side effects or other safety issues; and the potential that we will need additional funding to complete all of our studies. Further, the results of earlier preclinical studies and/or clinical trials may not be predictive of future results. With respect to statements regarding projections of the Company's cash position, actual results may differ based on market factors and the Company's ability to execute its operational and budget plans. In addition, all forward-looking statements are subject to other risks detailed in our Annual Report on Form 10-K for the year ended December 31, 2015. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, and we undertake no obligation to revise or update this news release to reflect events or circumstances after the date hereof. 2 Introduction

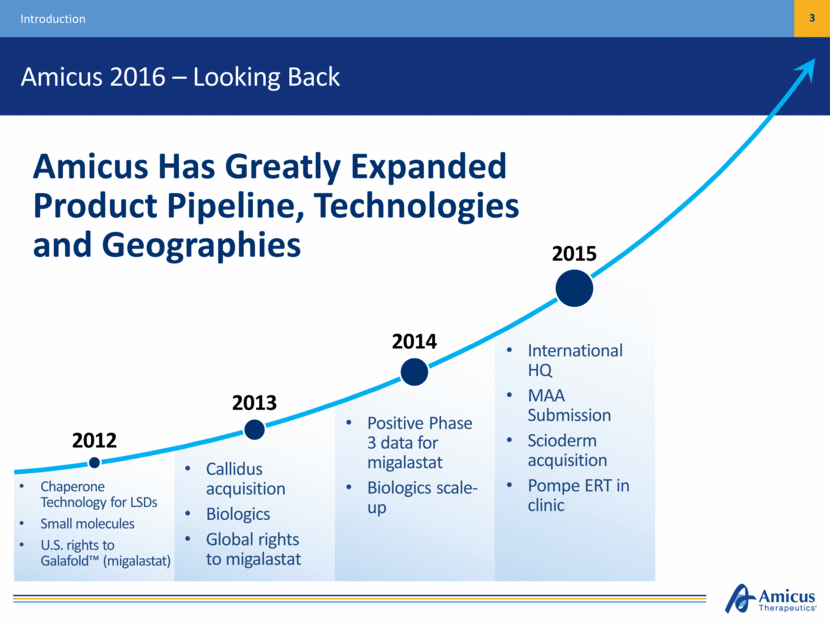
Amicus 2016 – Looking Back 3 Introduction Chaperone Technology for LSDs Small molecules U.S. rights to Galafold™ (migalastat) Callidus acquisition Biologics Global rights to migalastat Positive Phase 3 data for migalastat Biologics scale-up International HQ MAA Submission Scioderm acquisition Pompe ERT in clinic Amicus Has Greatly Expanded Product Pipeline, Technologies and Geographies 2012 2013 2014 2015
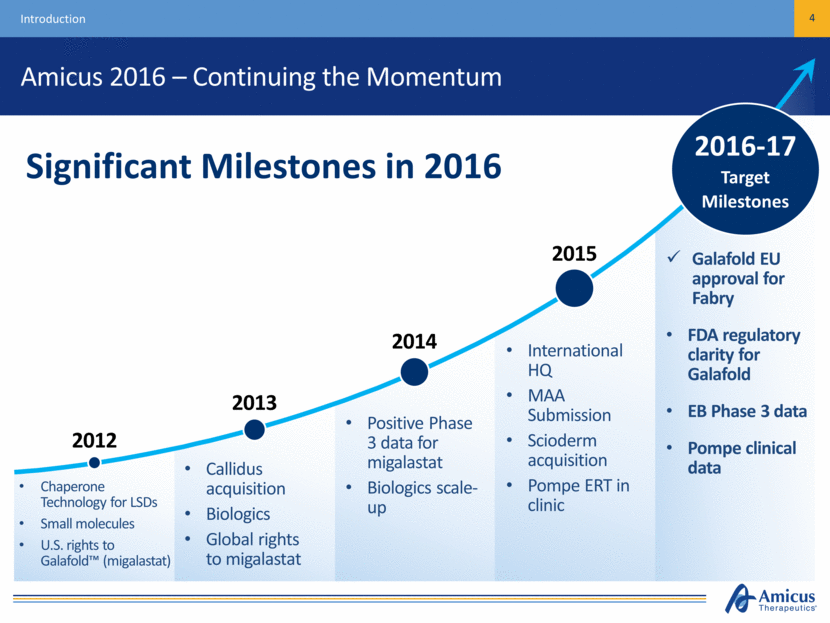
Amicus 2016 – Continuing the Momentum 4 Introduction Galafold EU approval for Fabry FDA regulatory clarity for Galafold EB Phase 3 data Pompe clinical data 2016-17 Target Milestones Significant Milestones in 2016 2015 Chaperone Technology for LSDs Small molecules U.S. rights to Galafold™ (migalastat) Callidus acquisition Biologics Global rights to migalastat Positive Phase 3 data for migalastat Biologics scale-up International HQ MAA Submission Scioderm acquisition Pompe ERT in clinic 2012 2013 2014
Amicus Vision Introduction 5 Amicus Therapeutics is a global biotechnology company at the forefront of developing advanced therapies to treat a range of devastating rare and orphan diseases

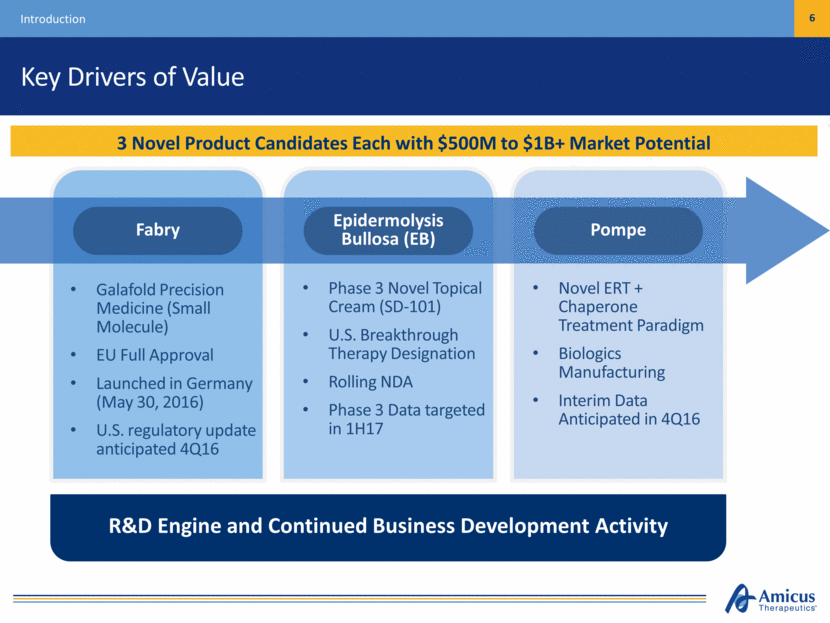
Key Drivers of Value Introduction 6 3 Novel Product Candidates Each with $500M to $1B+ Market Potential Galafold Precision Medicine (Small Molecule) EU Full Approval Launched in Germany (May 30, 2016) U.S. regulatory update anticipated 4Q16 Phase 3 Novel Topical Cream (SD-101) U.S. Breakthrough Therapy Designation Rolling NDA Phase 3 Data targeted in 1H17 Novel ERT + Chaperone Treatment Paradigm Biologics Manufacturing Interim Data Anticipated in 4Q16 R&D Engine and Continued Business Development Activity Pompe Epidermolysis Bullosa (EB) Fabry
Galafold™ (Migalastat) Precision Medicine for Fabry Disease
European Commission Granted Full Approval for Galafold Galafold: Precision Medicine for Fabry Disease 8 Galafold Indicated for Long-Term Treatment of Adults and Adolescents Aged > 16 years with a Confirmed Diagnosis of Fabry Disease and Who have an Amenable Mutation* The evaluation of EMA’s Committee for Medicinal Products for Human Use (CHMP) was based on the results of two phase III clinical trials in about 110 patients with Fabry disease who had a genetic mutation which responds to migalastat. Galafold demonstrated its efficacy compared to placebo (a dummy treatment) and to ERT in a long-term comparative study. - EMA Press Release Hard Capsules Oral Use 14 Hard Capsules *For important safety information for Galafold, including posology and method of administration, special warnings, drug interactions and adverse drug reactions, please see the European SmPC for Galafold available from the EMA website at www.ema.europa.eu The most common side effect reported in clinical trials was headache.

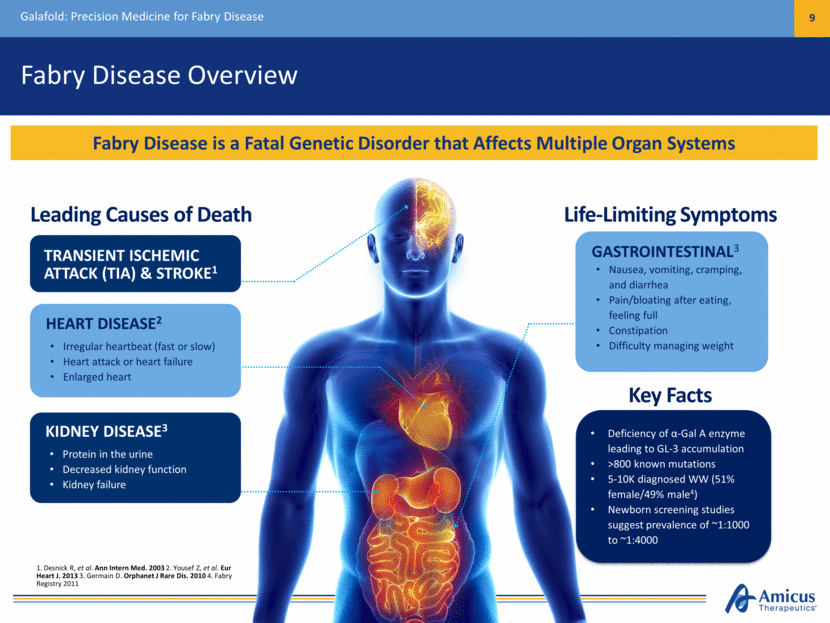
Fabry Disease Overview Galafold: Precision Medicine for Fabry Disease 9 Fabry Disease is a Fatal Genetic Disorder that Affects Multiple Organ Systems Leading Causes of Death Life-Limiting Symptoms TRANSIENT ISCHEMIC ATTACK (TIA) & STROKE1 KIDNEY DISEASE3 Protein in the urine Decreased kidney function Kidney failure HEART DISEASE2 Irregular heartbeat (fast or slow) Heart attack or heart failure Enlarged heart GASTROINTESTINAL3 Nausea, vomiting, cramping, and diarrhea Pain/bloating after eating, feeling full Constipation Difficulty managing weight Deficiency of -Gal A enzyme leading to GL-3 accumulation >800 known mutations 5-10K diagnosed WW (51% female/49% male4) Newborn screening studies suggest prevalence of ~1:1000 to ~1:4000 Key Facts 1. Desnick R, et al. Ann Intern Med. 2003 2. Yousef Z, et al. Eur Heart J. 2013 3. Germain D. Orphanet J Rare Dis. 2010 4. Fabry Registry 2011
Summary of Clinical Data Galafold: Precision Medicine for Fabry Disease 10 Favorable Efficacy and Safety Data in Two Largest Phase 3 Studies Ever Completed in Fabry Disease 1: Improvement versus placebo over 6 months in amenable patients 2: Improvement from baseline over 18+ months 3: Comparable to ERT over 18 months 4: Stabilization from baseline over 18 months with favorable comparison to natural history in literature Reduction in Cardiac Mass Left Ventricular Mass Index (LVMI) (Study 0112 and 012)* Improvement in GI Symptoms Gastrointestinal Symptoms Rating Scale (GSRS) (Study 0111)* Reduction in Disease Substrate IC GL-3 (Study 0111)* Plasma Lyso Gb-3 (Study 0112,1 and 0123)* Low Rate of Fabry-Associated Clinical Events Renal, Cardiac and Cerebro-Vascular Events (Study 0123) Stability of Kidney Function Estimated Glomerular Filtration Rate (eGFR) and Measured GFR (Study 0114 and Study 0124,3) *Analyses in this endpoint achieved statistical significance. For more complete clinical data go to amicusrx.com/posters.aspx

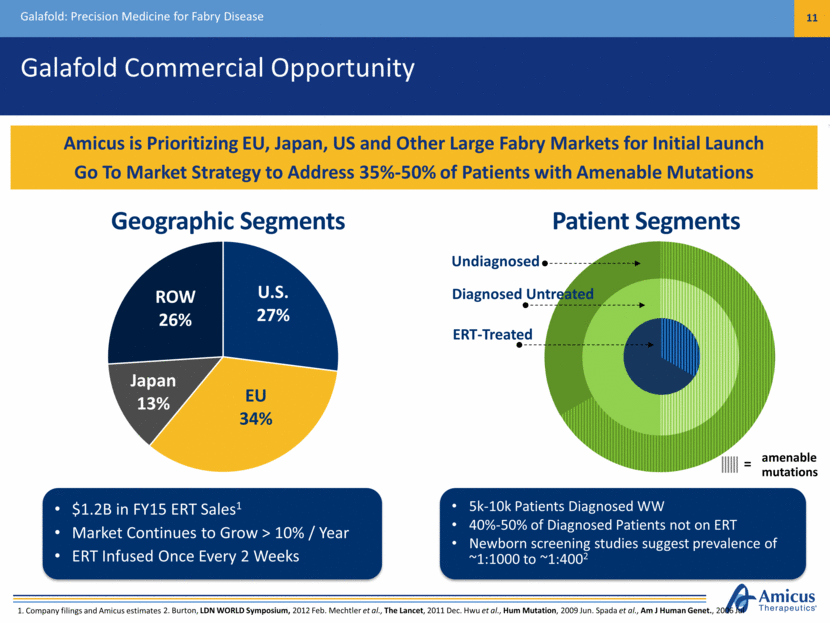
Galafold Commercial Opportunity Galafold: Precision Medicine for Fabry Disease 11 Amicus is Prioritizing EU, Japan, US and Other Large Fabry Markets for Initial Launch Go To Market Strategy to Address 35%-50% of Patients with Amenable Mutations 1. Company filings and Amicus estimates 5k-10k Diagnosed WW (40-50% of Diagnosed Patients Not on ERT Today) Market Continues to Grow > 10% / Year U.S. 27% EU 34% ROW 26% $1.2B in FY15 ERT Sales1 Market Continues to Grow > 10% / Year ERT Infused Once Every 2 Weeks Japan 13% ERT-Treated Diagnosed Untreated Undiagnosed 5k-10k Patients Diagnosed WW 40%-50% of Diagnosed Patients not on ERT Newborn screening studies suggest prevalence of ~1:1000 to ~1:4002 amenable mutations = Geographic Segments Patient Segments 2. Burton, LDN WORLD Symposium, 2012 Feb. Mechtler et al., The Lancet, 2011 Dec. Hwu et al., Hum Mutation, 2009 Jun. Spada et al., Am J Human Genet., 2006 Jul
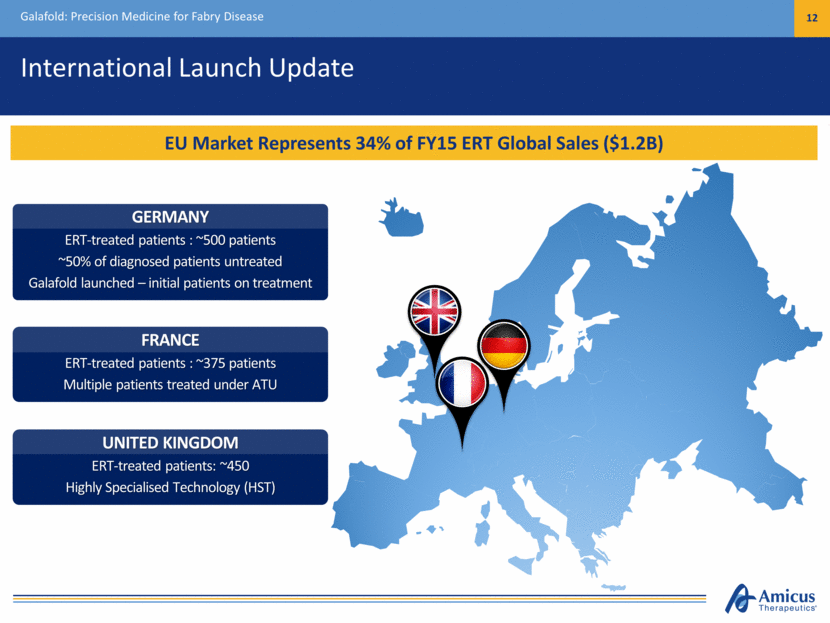
International Launch Update Galafold: Precision Medicine for Fabry Disease 12 EU Market Represents 34% of FY15 ERT Global Sales ($1.2B) UNITED KINGDOM ERT-treated patients: ~450 Highly Specialised Technology (HST) FRANCE ERT-treated patients : ~375 patients Multiple patients treated under ATU GERMANY ERT-treated patients : ~500 patients ~50% of diagnosed patients untreated Galafold launched – initial patients on treatment
EU Launch Update Galafold: Precision Medicine for Fabry Disease 13 Successful Early Days of EU Launch with Naïve and Switch Patients on Galafold – Focusing on Patient Access and Country-by-Country Reimbursement Processes LAUNCH IN GERMANY = IMPORTANT INDICATOR First Galafold Rx within 24 hours of EC approval Patient support program Experienced, high quality team Pricing dossier submitted patients (switch & naïve) on reimbursed Galafold WW (7/31/16)) 21 countries with reimbursement (commercial and EAP) 3 countries with active pricing discussions 12

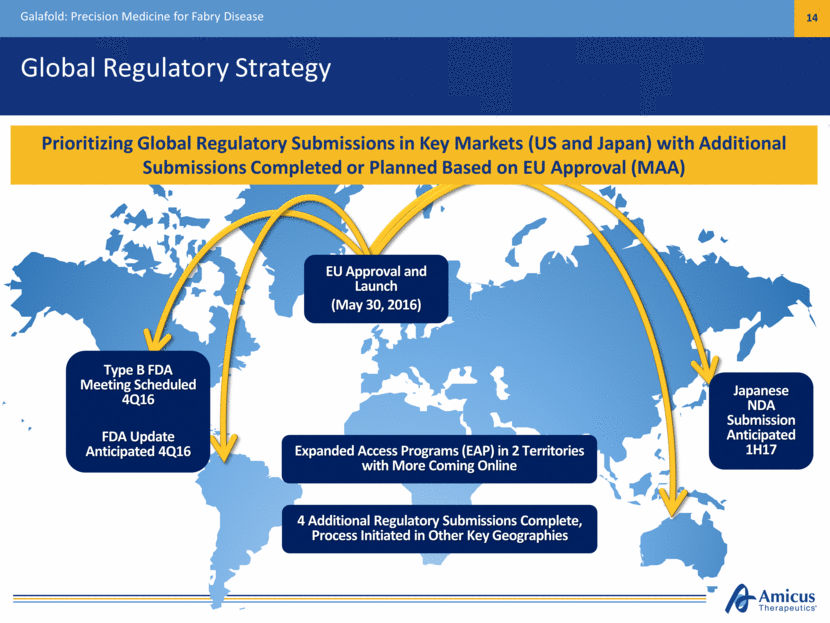
Global Regulatory Strategy Galafold: Precision Medicine for Fabry Disease 14 EU Approval and Launch (May 30, 2016) Expanded Access Programs (EAP) in 2 Territories with More Coming Online Prioritizing Global Regulatory Submissions in Key Markets (US and Japan) with Additional Submissions Completed or Planned Based on EU Approval (MAA) 4 Additional Regulatory Submissions Complete, Process Initiated in Other Key Geographies Type B FDA Meeting Scheduled 4Q16 FDA Update Anticipated 4Q16 Japanese NDA Submission Anticipated 1H17
Amicus Proprietary Fabry ERT Target Fabry ERT product profile: Improved drug targeting Co-formulation with chaperone Novel Proprietary Fabry ERT 15 Building on Biologics Capabilities and CHART Platform to Develop Differentiated Novel ERT Development status: Cell line transferred to manufacturer Preclinical data update in 2H16

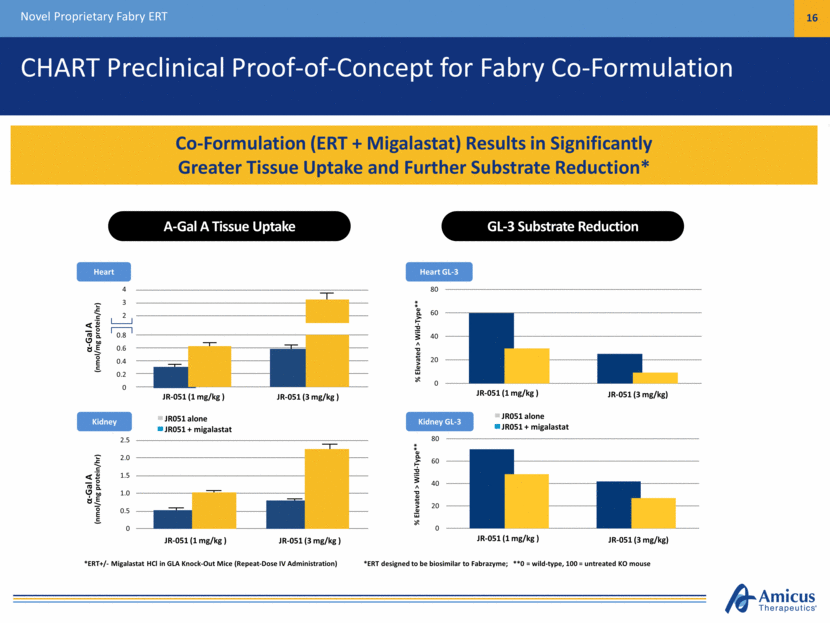
CHART Preclinical Proof-of-Concept for Fabry Co-Formulation Novel Proprietary Fabry ERT 16 Co-Formulation (ERT + Migalastat) Results in Significantly Greater Tissue Uptake and Further Substrate Reduction* Heart GL-3 *ERT designed to be biosimilar to Fabrazyme; **0 = wild-type, 100 = untreated KO mouse % Elevated > Wild-Type** % Elevated > Wild-Type** Kidney GL-3 A-Gal A Tissue Uptake GL-3 Substrate Reduction *ERT+/- Migalastat HCl in GLA Knock-Out Mice (Repeat-Dose IV Administration) JR-051 (1 mg/kg ) JR-051 (3 mg/kg) JR-051 (1 mg/kg ) JR-051 (3 mg/kg) Heart Kidney 4 3 2 0.8 0.6 0.4 0.2 0 -Gal A (nmol/mg protein/hr) JR-051 (1 mg/kg ) JR-051 (3 mg/kg ) JR051 alone JR051 + migalastat 2.5 2.0 1.5 1.0 0.5 0 -Gal A (nmol/mg protein/hr) JR-051 (1 mg/kg ) JR-051 (3 mg/kg ) JR051 alone JR051 + migalastat 0 20 40 60 80 0 20 40 60 80
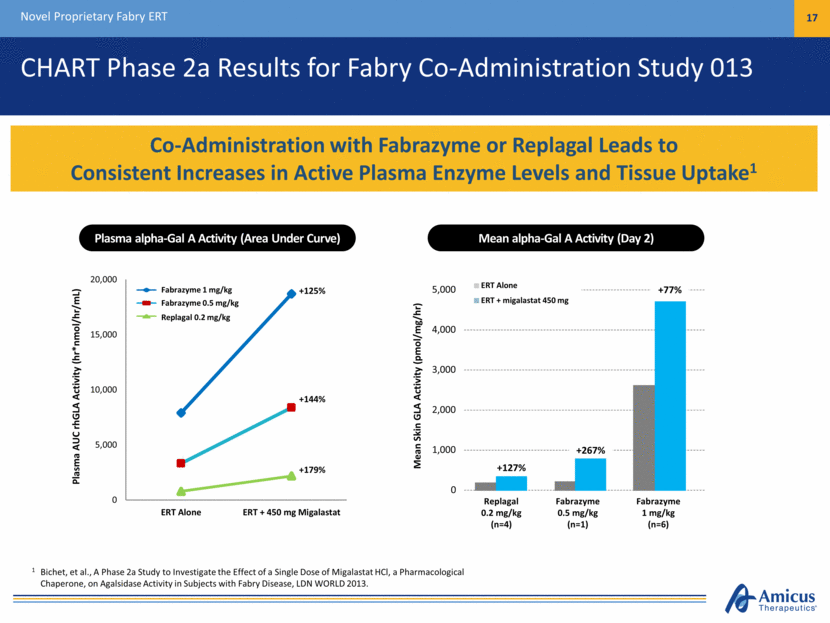
Fabrazyme 1 mg/kg Fabrazyme 0.5 mg/kg Replagal 0.2 mg/kg CHART Phase 2a Results for Fabry Co-Administration Study 013 Novel Proprietary Fabry ERT 17 Co-Administration with Fabrazyme or Replagal Leads to Consistent Increases in Active Plasma Enzyme Levels and Tissue Uptake1 1 Bichet, et al., A Phase 2a Study to Investigate the Effect of a Single Dose of Migalastat HCl, a Pharmacological Chaperone, on Agalsidase Activity in Subjects with Fabry Disease, LDN WORLD 2013. Plasma alpha-Gal A Activity (Area Under Curve) Plasma AUC rhGLA Activity (hr*nmol/hr/mL) Mean alpha-Gal A Activity (Day 2) Mean Skin GLA Activity (pmol/mg/hr) Replagal 0.2 mg/kg (n=4) Fabrazyme 0.5 mg/kg (n=1) Fabrazyme 1 mg/kg (n=6) 0 1,000 2,000 3,000 4,000 5,000 ERT Alone ERT + migalastat 450 mg +127% +267% +77% 0 5,000 10,000 15,000 20,000 ERT Alone ERT + 450 mg Migalastat +179% +144% +125%
Fabry Franchise Strategy Fabry Franchise 18 Amicus Therapeutics is Committed to Delivering the Highest Quality Therapies and Future Innovation to Find a Cure for ALL Fabry Patients ERTs ERTs Galafold Amicus ERT (ERT+Chaperone) Galafold Future Innovative Therapies Yesterday Today Tomorrow
ATB200 Novel ERT for Pompe Disease A Proprietary, Clinical-Stage Biologics Program

Pompe Disease Overview Novel ERT for Pompe Disease – ATB200 + Chaperone 20 Severe, Fatal, Genetic Disorder with Significant Unmet Medical Need 1. National Institute of Neurological Disorders and Stroke (NIH). 2. Sanofi Press Release & 10-K

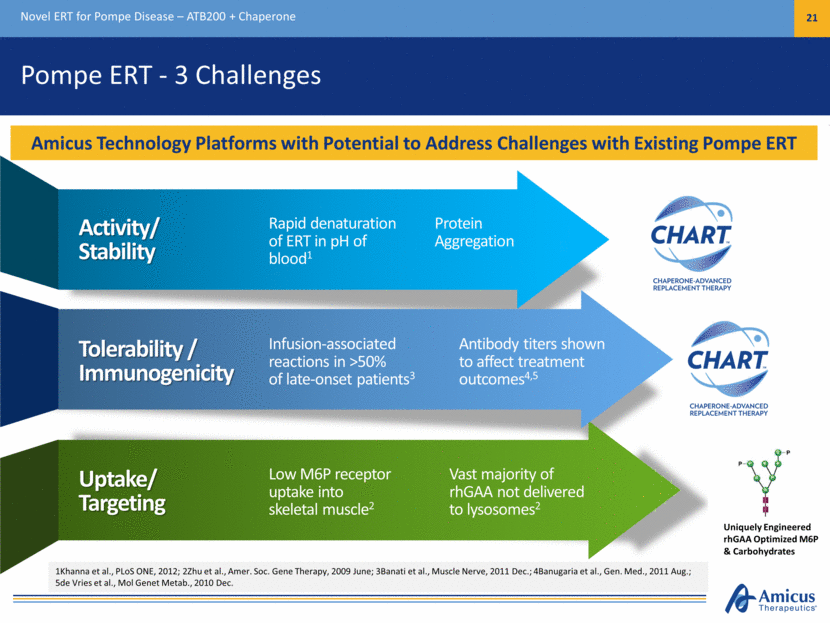
Pompe ERT - 3 Challenges Novel ERT for Pompe Disease – ATB200 + Chaperone 21 Activity/ Stability Rapid denaturation of ERT in pH of blood1 Tolerability / Immunogenicity Infusion-associated reactions in >50% of late-onset patients3 Uptake/ Targeting Low M6P receptor uptake into skeletal muscle2 Antibody titers shown to affect treatment outcomes4,5 Vast majority of rhGAA not delivered to lysosomes2 Protein Aggregation 1Khanna et al., PLoS ONE, 2012; 2Zhu et al., Amer. Soc. Gene Therapy, 2009 June; 3Banati et al., Muscle Nerve, 2011 Dec.; 4Banugaria et al., Gen. Med., 2011 Aug.; 5de Vries et al., Mol Genet Metab., 2010 Dec. Amicus Technology Platforms with Potential to Address Challenges with Existing Pompe ERT Uniquely Engineered rhGAA Optimized M6P & Carbohydrates
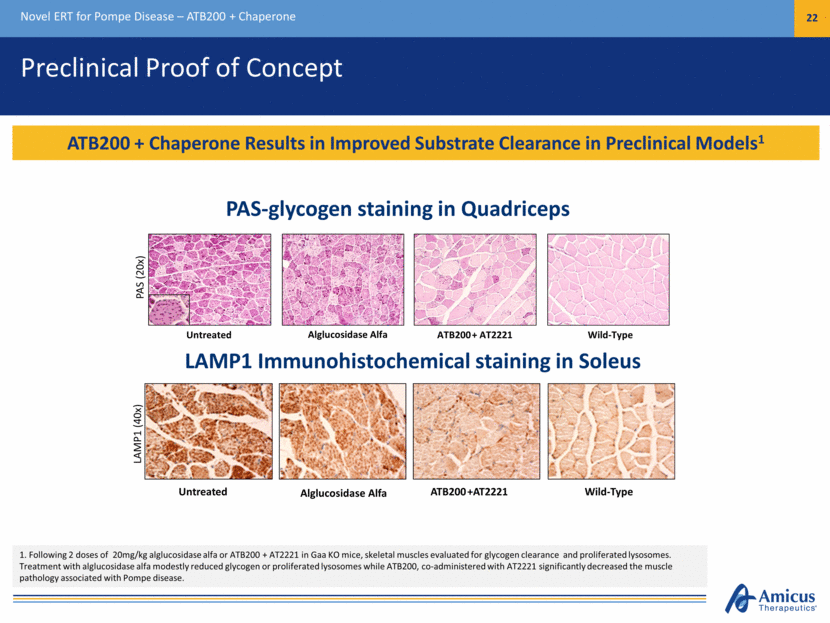
Preclinical Proof of Concept Novel ERT for Pompe Disease – ATB200 + Chaperone 22 ATB200 + Chaperone Results in Improved Substrate Clearance in Preclinical Models1 Untreated Alglucosidase Alfa ATB200 + AT2221 Wild-Type PAS (20x) Untreated Alglucosidase Alfa ATB200 +AT2221 Wild-Type LAMP1 (40x) 1. Following 2 doses of 20mg/kg alglucosidase alfa or ATB200 + AT2221 in Gaa KO mice, skeletal muscles evaluated for glycogen clearance and proliferated lysosomes. Treatment with alglucosidase alfa modestly reduced glycogen or proliferated lysosomes while ATB200, co-administered with AT2221 significantly decreased the muscle pathology associated with Pompe disease. PAS-glycogen staining in Quadriceps LAMP1 Immunohistochemical staining in Soleus
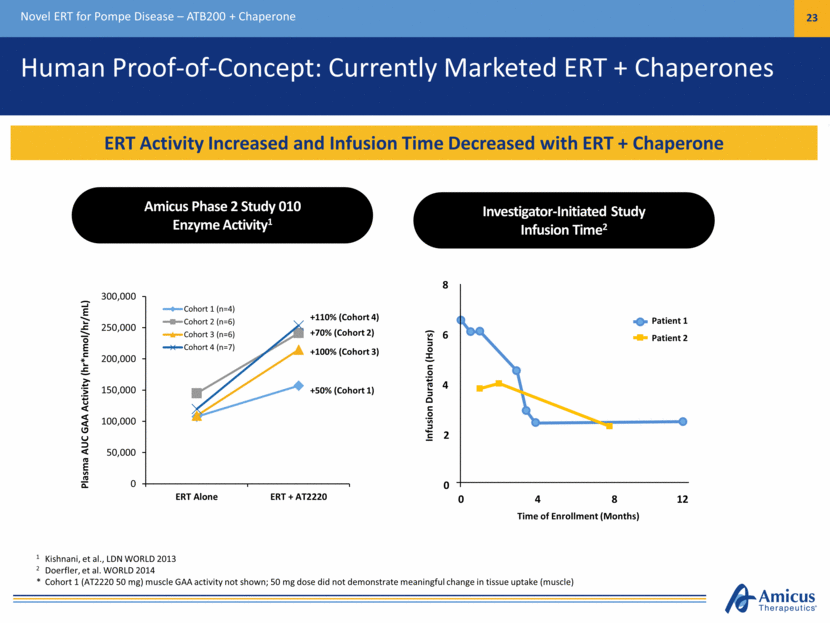
Human Proof-of-Concept: Currently Marketed ERT + Chaperones Novel ERT for Pompe Disease – ATB200 + Chaperone 23 ERT Activity Increased and Infusion Time Decreased with ERT + Chaperone 1 Kishnani, et al., LDN WORLD 2013 2 Doerfler, et al. WORLD 2014 * Cohort 1 (AT2220 50 mg) muscle GAA activity not shown; 50 mg dose did not demonstrate meaningful change in tissue uptake (muscle) Plasma AUC GAA Activity (hr*nmol/hr/mL) Time of Enrollment (Months) Infusion Duration (Hours) Patient 1 Patient 2 8 4 0 12 8 4 2 0 6 Amicus Phase 2 Study 010 Enzyme Activity1 Investigator-Initiated Study Infusion Time2 0 50,000 100,000 150,000 200,000 250,000 300,000 ERT Alone ERT + AT2220 Cohort 1 (n=4) Cohort 2 (n=6) Cohort 3 (n=6) Cohort 4 (n=7) +110% (Cohort 4) +70% (Cohort 2) +100% (Cohort 3) +50% (Cohort 1)
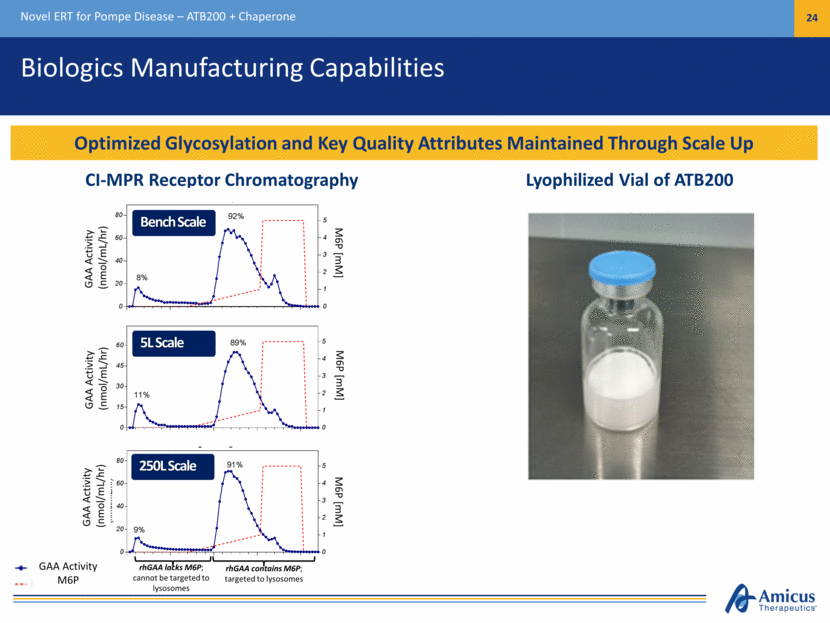
Biologics Manufacturing Capabilities Novel ERT for Pompe Disease – ATB200 + Chaperone 24 Optimized Glycosylation and Key Quality Attributes Maintained Through Scale Up rhGAA lacks M6P; cannot be targeted to lysosomes rhGAA contains M6P; targeted to lysosomes Lyophilized Vial of ATB200 GAA Activity (nmol/mL/hr) GAA Activity (nmol/mL/hr) GAA Activity (nmol/mL/hr) M6P [mM] M6P [mM] M6P [mM] CI-MPR Receptor Chromatography GAA Activity M6P Bench Scale 5L Scale 250L Scale Proof of Concept Studies 0 20 40 60 80 0 1 2 3 4 5 8% 92% GAA Activity (nmol/mL/hr) M6P [mM] 0 15 30 45 60 0 1 2 3 4 5 5L Bioreactor Run GAA Activity (nmol/mL/hr) M6P [mM] GAA Activity M6P (mM) 11% 89% 0 20 40 60 80 0 1 2 3 4 5 Engineering Run 2 GAA Activity (n mol/mL/hr) M6P [mM] 9% 91%
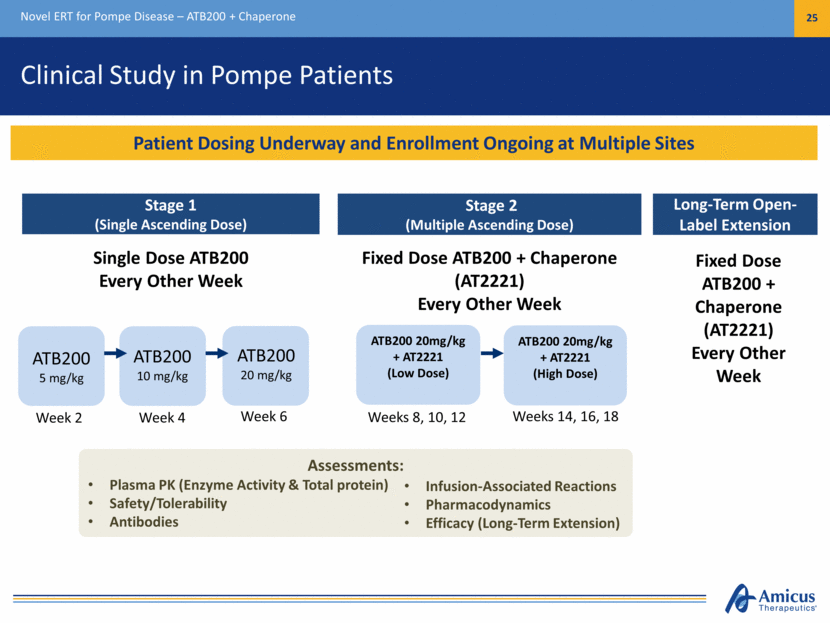
Clinical Study in Pompe Patients Novel ERT for Pompe Disease – ATB200 + Chaperone 25 Stage 2 (Multiple Ascending Dose) ATB200 20mg/kg + AT2221 (Low Dose) ATB200 20mg/kg + AT2221 (High Dose) ATB200 5 mg/kg Stage 1 (Single Ascending Dose) Single Dose ATB200 Every Other Week Fixed Dose ATB200 + Chaperone (AT2221) Every Other Week Long-Term Open-Label Extension Fixed Dose ATB200 + Chaperone (AT2221) Every Other Week Assessments: Plasma PK (Enzyme Activity & Total protein) Safety/Tolerability Antibodies Infusion-Associated Reactions Pharmacodynamics Efficacy (Long-Term Extension) ATB200 20 mg/kg ATB200 10 mg/kg Patient Dosing Underway and Enrollment Ongoing at Multiple Sites Week 2 Week 4 Week 6 Weeks 14, 16, 18 Weeks 8, 10, 12
Pompe Clinical Study ATB200-02 Data Cascade Novel ERT for Pompe Disease – ATB200 + Chaperone 26 Data in initial ambulatory ERT-switch patients (N=~4,Cohort 1) Data in ERT-naïve patients (Cohort 2) Additional extension study data (all Cohorts) A Cascade of Data Points from 4Q16 through 2017 Offer Clear Parameters to Define Success and Differentiate ATB200/AT2221 Pompe Data Cascade 4Q16 Through 2017 Additional data & initial extension data in Cohort 1 Data in non-ambulatory ERT-switch patients (Cohort 3) 18-WEEK DATA Safety / tolerability Pharmacokinetics (PK) Biomarkers Immunogenicity EXTENSION DATA Motor/pulmonary function

SD-101 for Epidermolysis Bullosa (EB) Poised to deliver pivotal data for a devastating rare disease

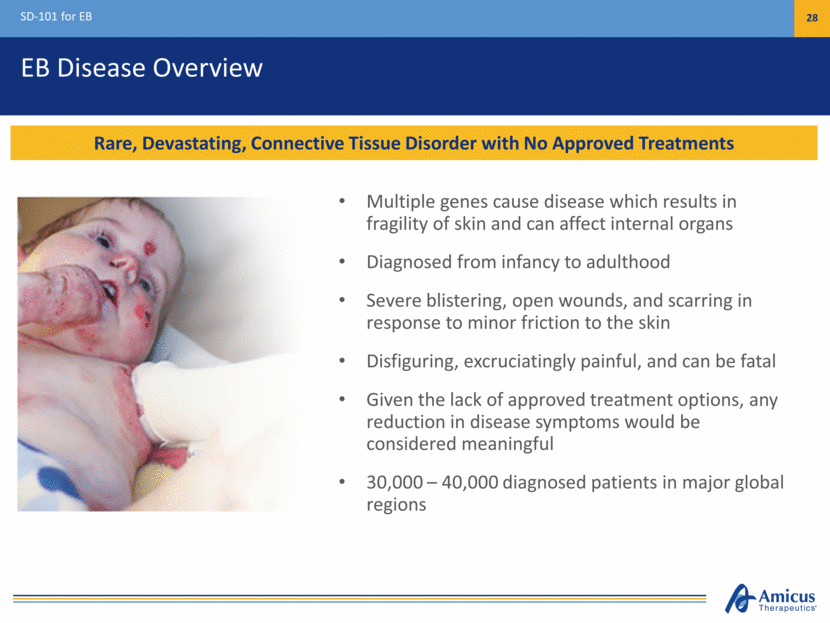
EB Disease Overview SD-101 for EB 28 Rare, Devastating, Connective Tissue Disorder with No Approved Treatments Multiple genes cause disease which results in fragility of skin and can affect internal organs Diagnosed from infancy to adulthood Severe blistering, open wounds, and scarring in response to minor friction to the skin Disfiguring, excruciatingly painful, and can be fatal Given the lack of approved treatment options, any reduction in disease symptoms would be considered meaningful 30,000 – 40,000 diagnosed patients in major global regions
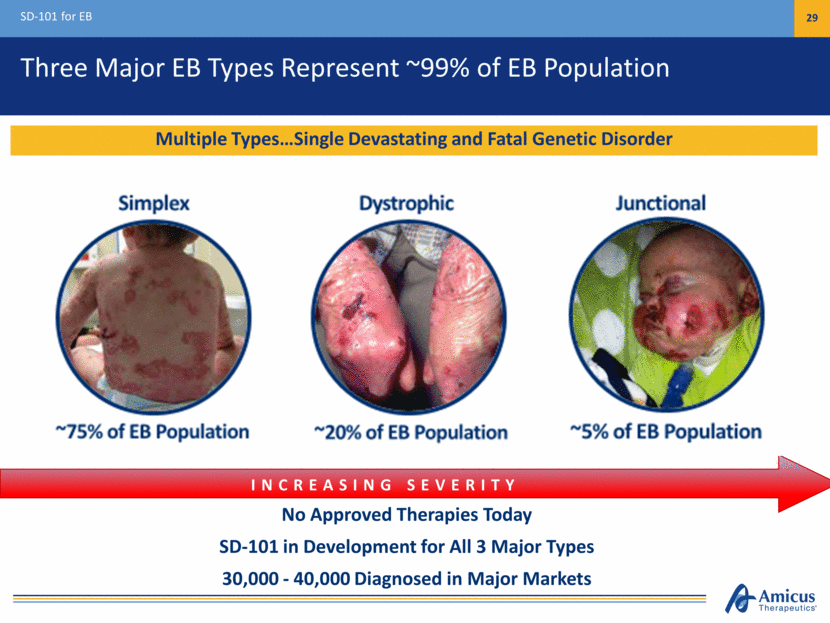
Three Major EB Types Represent ~99% of EB Population SD-101 for EB 29 No Approved Therapies Today SD-101 in Development for All 3 Major Types 30,000 - 40,000 Diagnosed in Major Markets INCREASING SEVERITY Multiple Types Single Devastating and Fatal Genetic Disorder
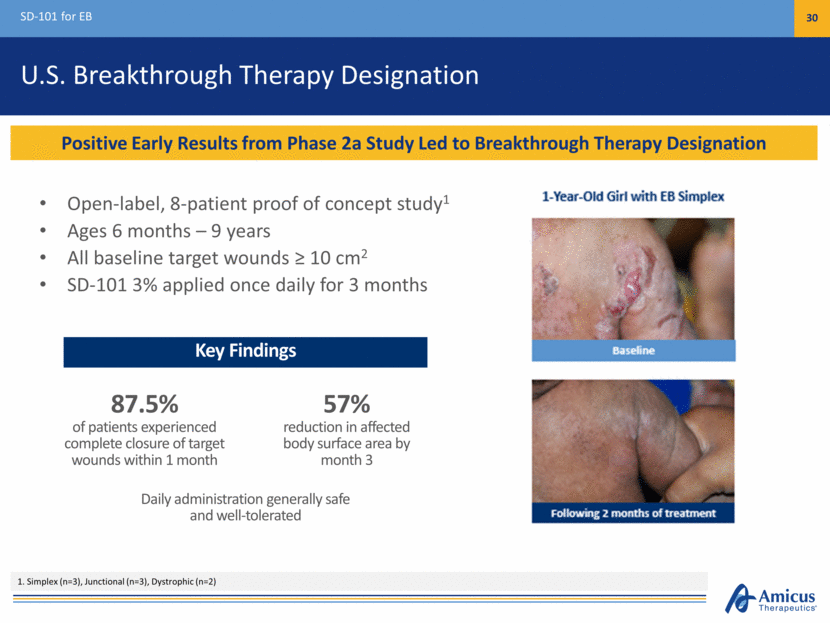
U.S. Breakthrough Therapy Designation Open-label, 8-patient proof of concept study1 Ages 6 months – 9 years All baseline target wounds > 10 cm2 SD-101 3% applied once daily for 3 months SD-101 for EB 30 Positive Early Results from Phase 2a Study Led to Breakthrough Therapy Designation 1. Simplex (n=3), Junctional (n=3), Dystrophic (n=2) Key Findings 87.5% of patients experienced complete closure of target wounds within 1 month Daily administration generally safe and well-tolerated 57% reduction in affected body surface area by month 3
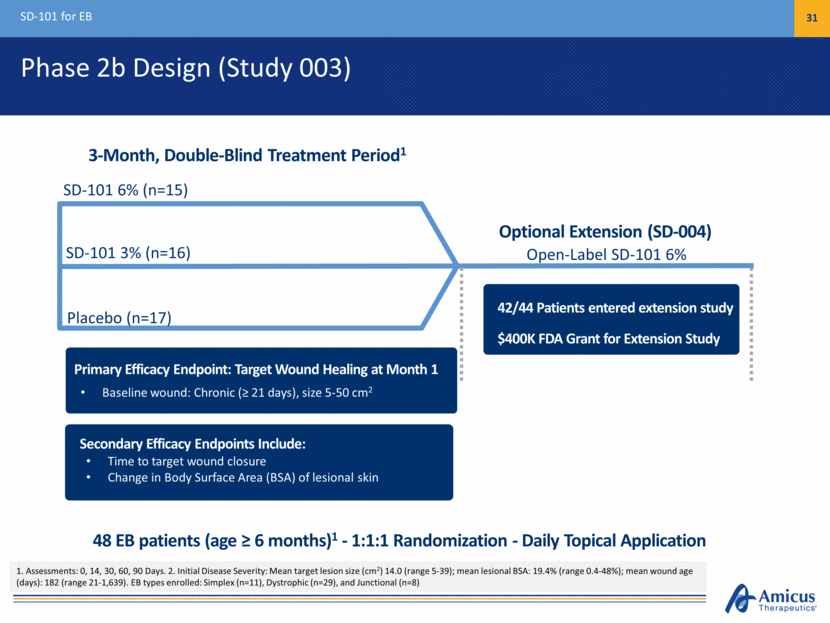
Phase 2b Design (Study 003) SD-101 for EB 31 Placebo (n=17) SD-101 6% (n=15) Open-Label SD-101 6% 3-Month, Double-Blind Treatment Period1 Primary Efficacy Endpoint: Target Wound Healing at Month 1 Baseline wound: Chronic (> 21 days), size 5-50 cm2 SD-101 3% (n=16) Secondary Efficacy Endpoints Include: Time to target wound closure Change in Body Surface Area (BSA) of lesional skin 42/44 Patients entered extension study $400K FDA Grant for Extension Study 48 EB patients (age > 6 months)1 - 1:1:1 Randomization - Daily Topical Application 1. Assessments: 0, 14, 30, 60, 90 Days. 2. Initial Disease Severity: Mean target lesion size (cm2) 14.0 (range 5-39); mean lesional BSA: 19.4% (range 0.4-48%); mean wound age (days): 182 (range 21-1,639). EB types enrolled: Simplex (n=11), Dystrophic (n=29), and Junctional (n=8) Optional Extension (SD-004)
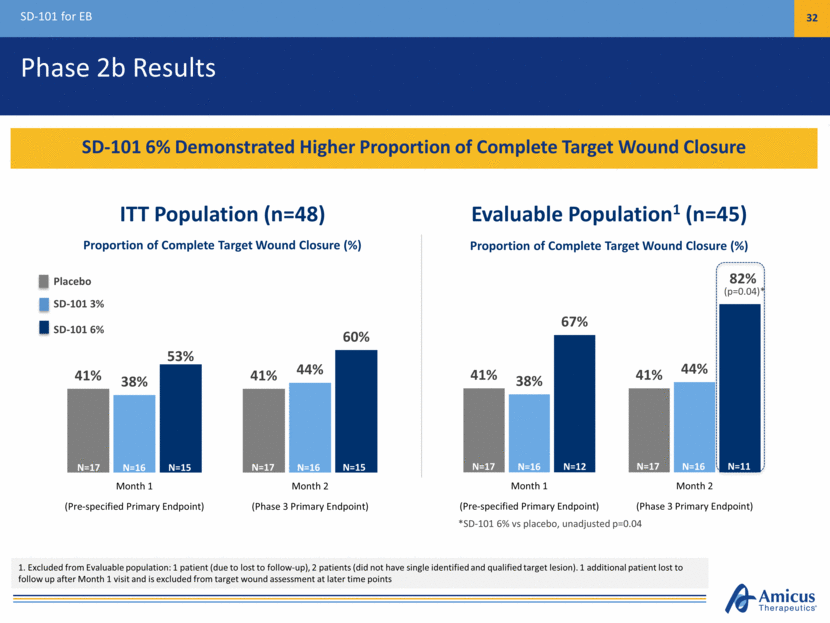
Phase 2b Results SD-101 for EB 32 SD-101 6% Demonstrated Higher Proportion of Complete Target Wound Closure *SD-101 6% vs placebo, unadjusted p=0.04 ITT Population (n=48) Evaluable Population1 (n=45) (p=0.04)* Proportion of Complete Target Wound Closure (%) Proportion of Complete Target Wound Closure (%) 1. Excluded from Evaluable population: 1 patient (due to lost to follow-up), 2 patients (did not have single identified and qualified target lesion). 1 additional patient lost to follow up after Month 1 visit and is excluded from target wound assessment at later time points N=17 N=15 N=16 N=17 N=15 N=16 N=17 N=12 N=16 N=17 N=11 N=16 Placebo SD-101 3% SD-101 6% 41% 41% 38% 44% 67% 82% Month 1 Month 2 (Pre-specified Primary Endpoint) (Phase 3 Primary Endpoint) 41% 41% 38% 44% 53% 60% Month 1 Month 2 (Pre-specified Primary Endpoint) (Phase 3 Primary Endpoint)
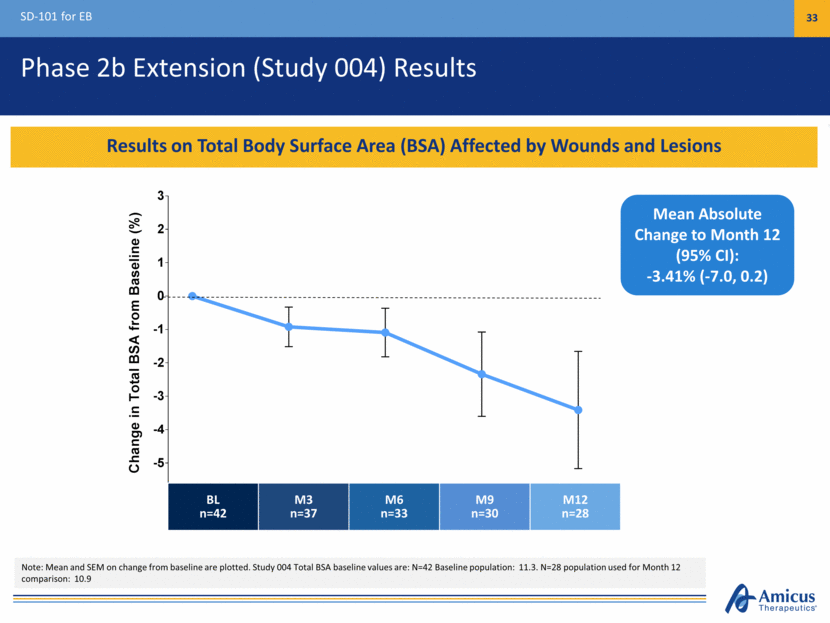
Phase 2b Extension (Study 004) Results SD-101 for EB 33 Results on Total Body Surface Area (BSA) Affected by Wounds and Lesions Mean Absolute Change to Month 12 (95% CI): -3.41% (-7.0, 0.2) BL n=42 M3 n=37 M6 n=33 M9 n=30 M12 n=28 Note: Mean and SEM on change from baseline are plotted. Study 004 Total BSA baseline values are: N=42 Baseline population: 11.3. N=28 population used for Month 12 comparison: 10.9 3 6 9 12 -5 -4 -3 -2 -1 0 1 2 3 Baseline Time, Months Change in Total BSA from Baseline ( % )
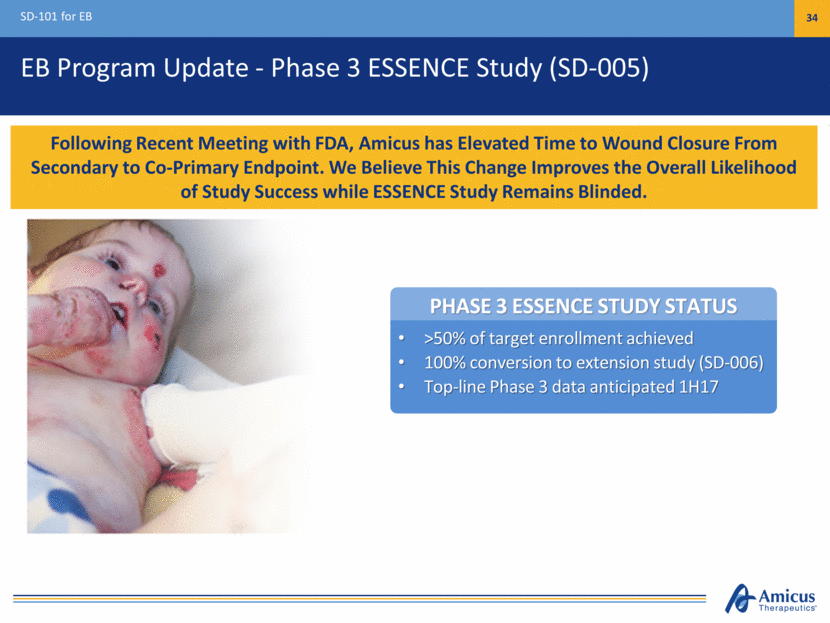
PHASE 3 ESSENCE STUDY STATUS >50% of target enrollment achieved 100% conversion to extension study (SD-006) Top-line Phase 3 data anticipated 1H17 EB Program Update - Phase 3 ESSENCE Study (SD-005) SD-101 for EB 34 Following Recent Meeting with FDA, Amicus has Elevated Time to Wound Closure From Secondary to Co-Primary Endpoint. We Believe This Change Improves the Overall Likelihood of Study Success while ESSENCE Study Remains Blinded.
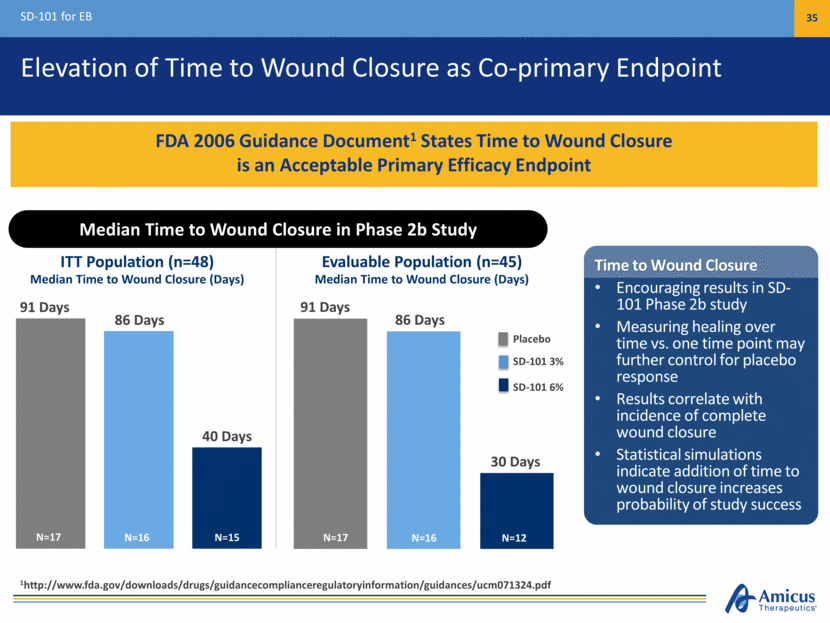
Elevation of Time to Wound Closure as Co-primary Endpoint SD-101 for EB 35 FDA 2006 Guidance Document1 States Time to Wound Closure is an Acceptable Primary Efficacy Endpoint Median Time to Wound Closure (Days) Median Time to Wound Closure (Days) 91 Days 86 Days 40 Days ITT Population (n=48) Evaluable Population (n=45) Placebo SD-101 3% SD-101 6% 91 Days 86 Days 30 Days N=17 N=15 N=16 N=17 N=12 N=16 Time to Wound Closure Encouraging results in SD-101 Phase 2b study Measuring healing over time vs. one time point may further control for placebo response Results correlate with incidence of complete wound closure Statistical simulations indicate addition of time to wound closure increases probability of study success Median Time to Wound Closure in Phase 2b Study 1http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071324.pdf
Phase 3 ESSENCE Study Design (SD-005) SD-101 for EB 36 Study Success Potentially Based on Achievement of One or Both Co-Primary Endpoints ~150 EB patients (age > 1 month) Baseline wound: Chronic (> 21 days), size >10 cm2 Placebo SD-101 6% Primary Endpoint: Target Wound Healing at Month 2 US and EU regulatory authorities agreed on primary endpoint Baseline wound: Chronic (> 21 days), size >10 cm2 Primary Endpoint: Target Wound Healing at Month 2 US and EU regulatory authorities agreed on primary endpoint Baseline wound: Chronic (> 21 days), size >10 cm2 Co-Primary Endpoints Complete closure of target wound (previously specified primary endpoint) Time to target wound closure (elevated from secondary to co-primary) Secondary Endpoints Include: Change in Body Surface Area (BSA) of lesions and blisters Patient-reported itching Patient-reported pain 3-Month, Double-Blind Treatment Period Open-Label SD-101 6% Optional Extension (SD-006) 100% Participation in Extension Study (August 1, 2016) Covariates include age of patient and size of wound at baseline Average Baseline Target Wound Size in Phase 3 Population: ~20 cm2 (August 1, 2016)
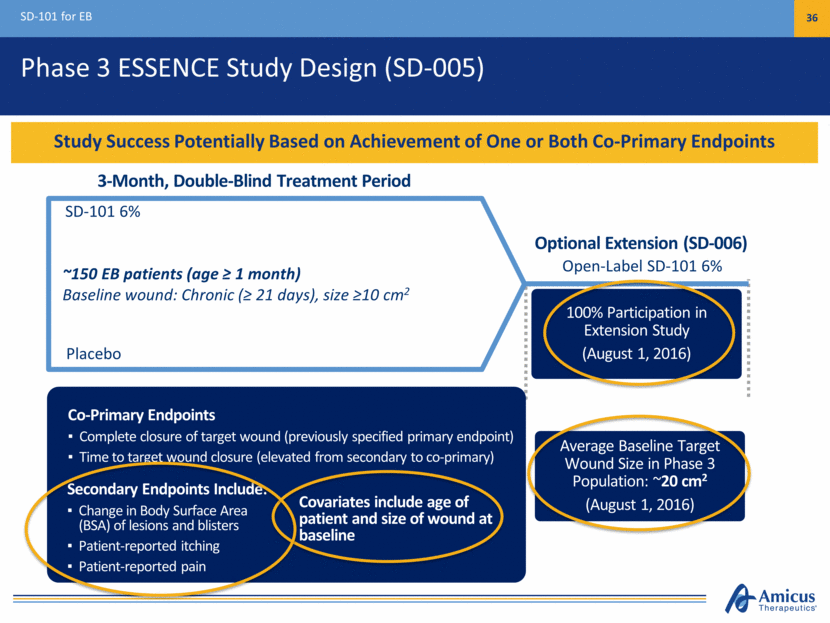
$1B+ Commercial Potential SD-101 for EB 37 KOL Feedback Supports Profound Unmet Medical Need and Broad Usage in All EB Types Diagnosed EB Patients by Geography Significant Unmet Medical Need Significant Unmet Clinical Need No approved treatments, opportunity for first-in-class Promising proof of concept in all EB types Strong Support Among Surveyed Stakeholders Physicians indicate usage in 100% patients Payers indicate support for broad reimbursement if approved Large Commercial Opportunity 30,000 – 40,000 diagnosed patients in major markets KOLs expect diagnosis rates to increase (U.S., EU3, Japan) U.S. 52% EU3 35% Japan 13%
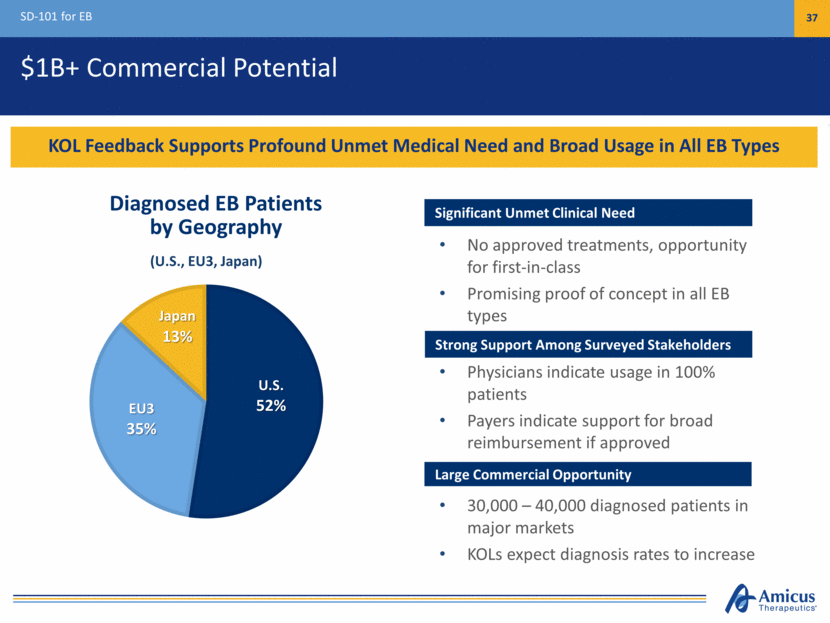
Cyclin-Dependent Kinase-Like 5 (CDKL5) Deficiency Genetic mutations in CDKL5 gene result in deficient protein essential for normal brain development Persistent, spontaneous seizures starting in infancy Severe impairment in neurological development Most affected children cannot walk, talk or care for themselves May include scoliosis, visual impairment, sensory issues, and gastrointestinal complications >1,200 documented cases worldwide1 Patient identification rising significantly CDKL5 Deficiency Program 38 Rare, Devastating, Genetic Neurological Disease with No Approved Treatments 1. LouLouFoundation.org
Strategic Fit with Amicus Vision and Biologics Pipeline CDKL5 Deficiency Program 39 New CDKL5 Program Expands Biologics Pipeline and Fits with Our Vision to Build a Leading Global Biotechnology Company Focused on Rare and Devastating Diseases CDKL5 is a rare, devastating genetic neurological disease with no approved treatment Potential first-in-class CDKL5 protein replacement therapy expands biologics pipeline Partnering with CDKL5 community to raise awareness and advance toward treatment 1 Third party market research - Michael Jasulavic, Founder of MiaMed I am confident the Company’s advancement of this program will raise CDKL5 awareness and, most importantly, increase the potential for success in developing a CDKL5 protein replacement therapy.” - Ashley R. Winslow, PhD, Director of Neurogenetics of the Orphan Disease Center at University of Pennsylvania Today there is no approved treatment for people living with CDKL5 deficiency, and the number of patients diagnosed has been increasing rapidly ” -John F. Crowley, Chairman and CEO of Amicus This CDKL5 program is an important investment in our stated strategy to expand our biologics pipeline by integrating new, innovative technologies to develop first- and best-in-class therapies for patients who are in desperate need of new treatments.”
Financial Summary Strong Balance Sheet to Invest in Rare Disease Pipeline
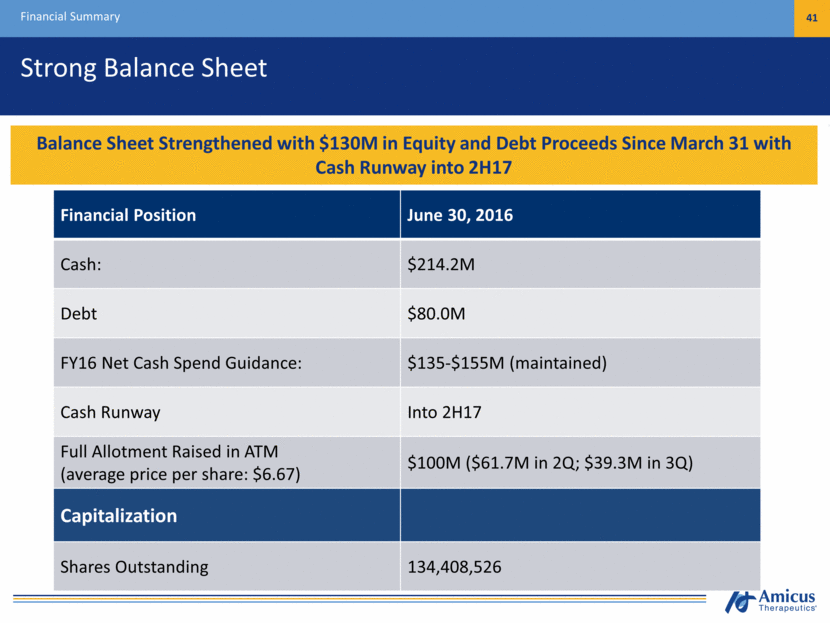
Strong Balance Sheet Financial Summary 41 Financial Position June 30, 2016 Cash: $214.2M Debt $80.0M FY16 Net Cash Spend Guidance: $135-$155M (maintained) Cash Runway Into 2H17 Full Allotment Raised in ATM (average price per share: $6.67) $100M ($61.7M in 2Q; $39.3M in 3Q) Capitalization Shares Outstanding 134,408,526 Cash Position Provides Runway Under Current Operating Plan into mid-2017 Balance Sheet Strengthened with $130M in Equity and Debt Proceeds Since March 31 with Cash Runway into 2H17
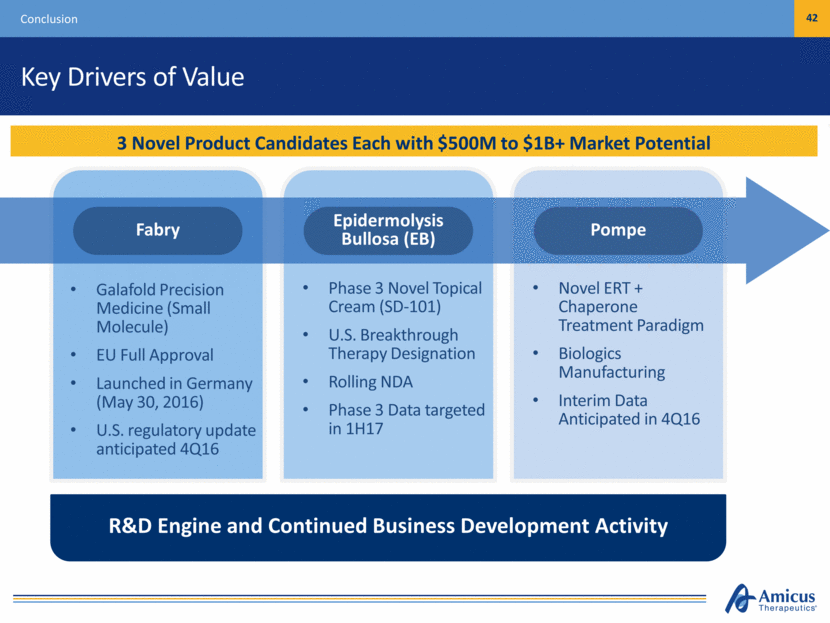
Key Drivers of Value Conclusion 42 3 Novel Product Candidates Each with $500M to $1B+ Market Potential Galafold Precision Medicine (Small Molecule) EU Full Approval Launched in Germany (May 30, 2016) U.S. regulatory update anticipated 4Q16 Phase 3 Novel Topical Cream (SD-101) U.S. Breakthrough Therapy Designation Rolling NDA Phase 3 Data targeted in 1H17 Novel ERT + Chaperone Treatment Paradigm Biologics Manufacturing Interim Data Anticipated in 4Q16 R&D Engine and Continued Business Development Activity Pompe Epidermolysis Bullosa (EB) Fabry
Thank You ©AMICUS THERAPEUTICS. CRANBURY, NJ. 2016
