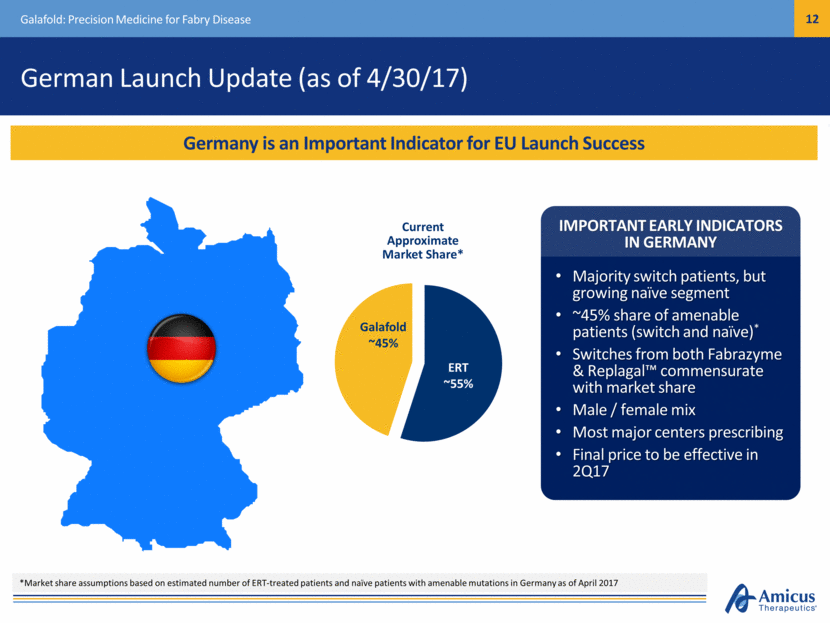
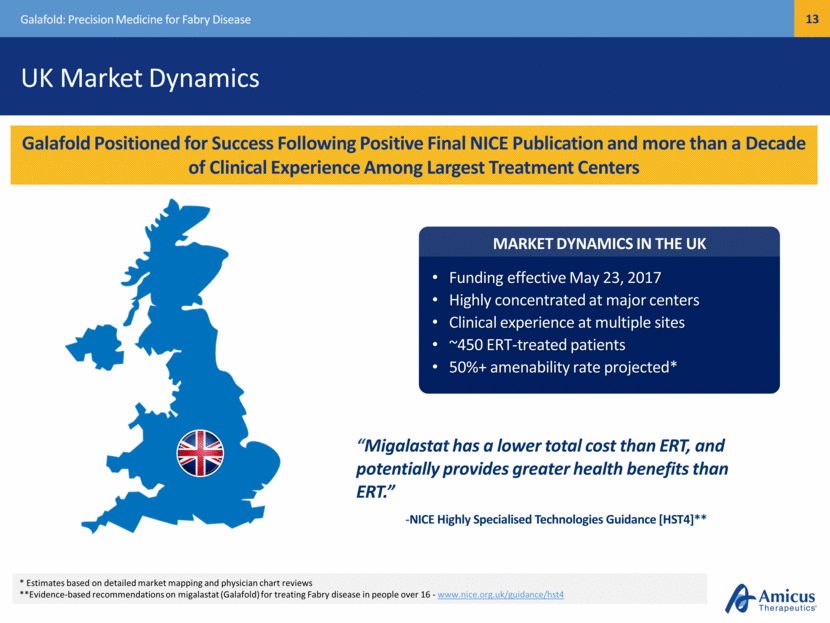
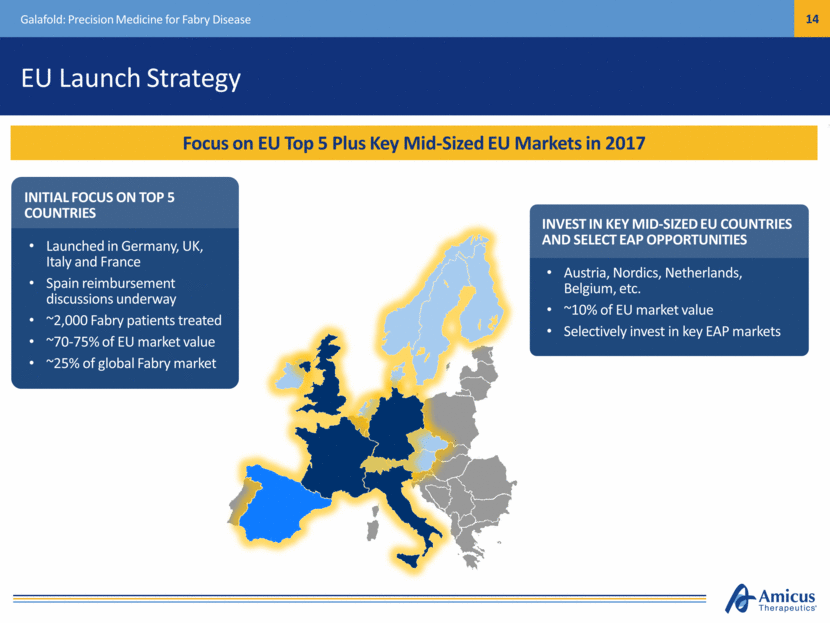
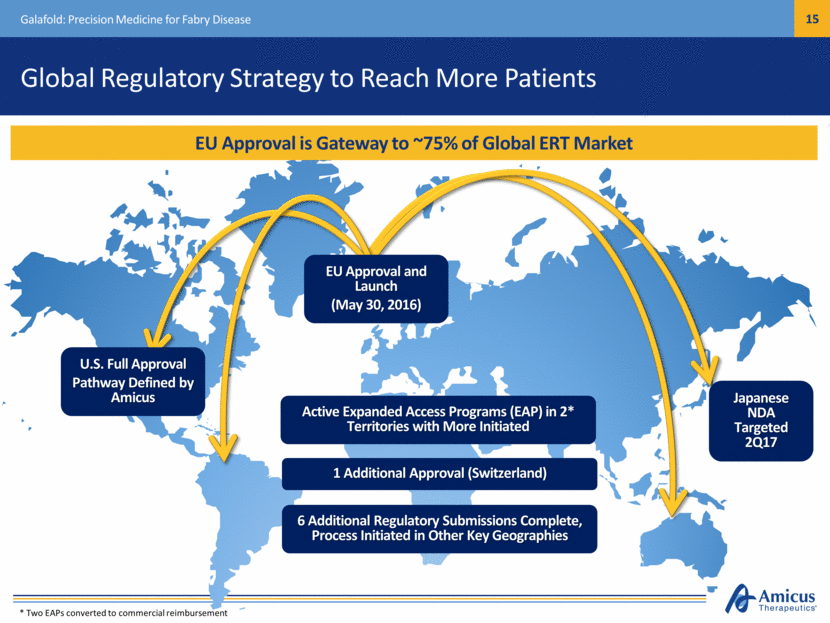
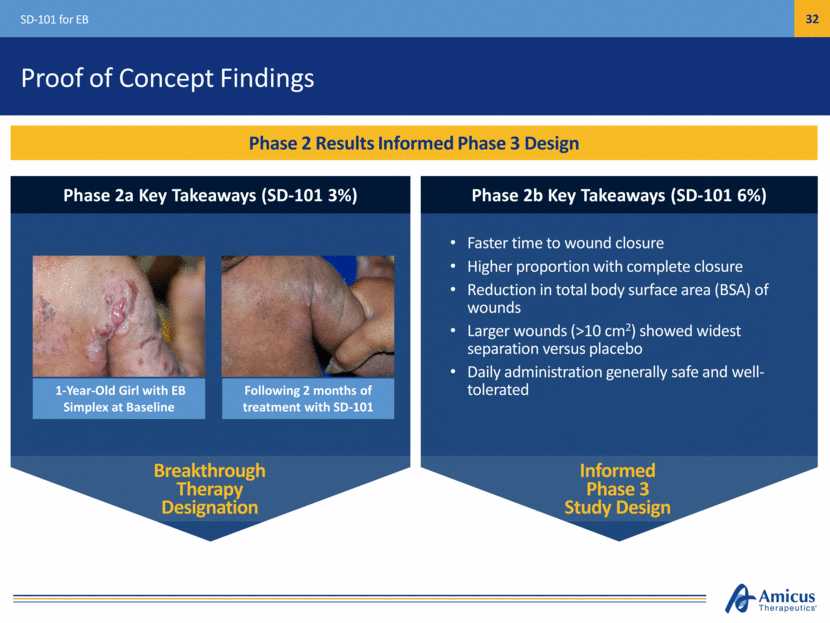
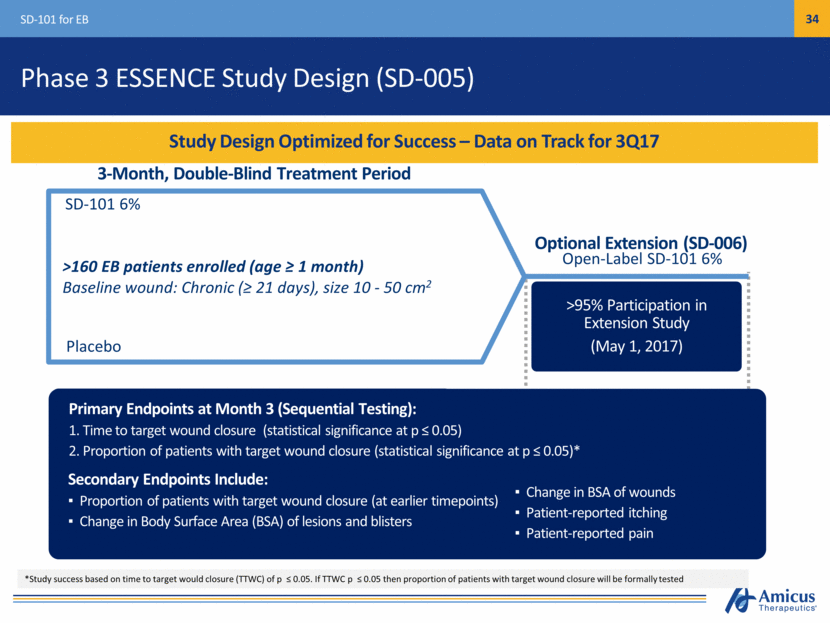
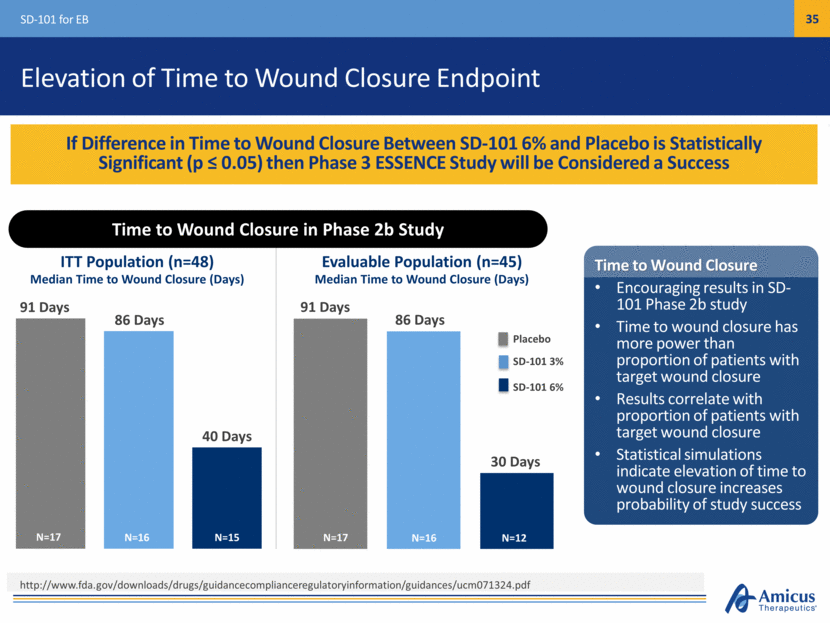
Safe Harbor This presentation contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 relating to preclinical and clinical development of our product candidates, the timing and reporting of results from preclinical studies and clinical trials, the prospects and timing of the potential regulatory approval of our product candidates, commercialization plans, financing plans, and the projected cash position for the Company. In particular, this presentation relates to the preclinical and preliminary clinical data from a global Phase 1/2 study (ATB200-02) to investigate ATB200/AT2221. The inclusion of forward-looking statements arising from this preliminary data and study should not be regarded as a representation by us that any of our plans will be achieved. Any or all of the forward-looking statements in this presentation may turn out to be wrong and can be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties. For example, with respect to statements regarding the goals, progress, timing, and outcomes of discussions with regulatory authorities, and in particular the potential goals, progress, timing, and results of preclinical studies and clinical trials, actual results may differ materially from those set forth in this release due to the risks and uncertainties inherent in our business, including, without limitation: the potential that results of clinical or preclinical studies indicate that the product candidates are unsafe or ineffective; the potential that it may be difficult to enroll patients in our clinical trials; the potential that regulatory authorities, including the FDA, EMA, and PMDA, may not grant or may delay approval for our product candidates; the potential that we may not be successful in commercializing Galafold in Europe or our other product candidates if and when approved; the potential that preclinical and clinical studies could be delayed because we identify serious side effects or other safety issues; and the potential that we will need additional funding to complete all of our studies. Further, the results of earlier preclinical studies and/ or clinical trials may not be predictive of future results for any of our product candidates, including ATB200/AT2221 and SD-101. The preliminary data and Phase 1/2 study investigating ATB200/AT2221 discussed herein is inherently preliminary and early in the study, derived from a limited patient set, and later trial results with this patient set or others may not be consistent with these preliminary results. With respect to statements regarding projections of our cash position, actual results may differ based on market factors and our ability to execute operational and budget plans. In addition, all forward-looking statements are subject to other risks detailed in our previous filings with the SEC and in our Annual Report on Form 10-K for the year ended December 31, 2016. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, and we undertake no obligation to revise or update this presentation to reflect events or circumstances after the date hereof. Introduction