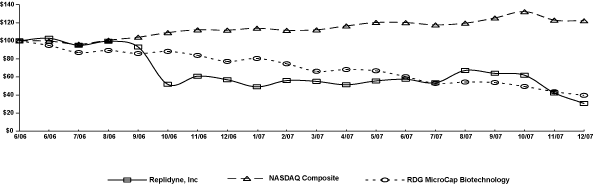
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
| | | |
| þ | | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | | For the fiscal year ended December 31, 2007 |
or |
o | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number:000-52082
REPLIDYNE, INC.
(Exact name of registrant as specified in its charter)
| | | |
| Delaware | | 84-1568247 |
| (State of Incorporation) | | (I.R.S. Employer I.D. No.) |
| | | |
1450 Infinite Drive
Louisville, Colorado
(Address of Principal Executive Offices) | | 80027
(Zip Code) |
303-996-5500
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| |
| Common Stock, One-tenth of One Cent ($0.001) Par Value Per Share | NASDAQ Global Market |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes o No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 ofRegulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of thisForm 10-K or any amendment to thisForm 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” inRule 12b-2 of the Exchange Act. (Check one):
| | | | | | | |
Large accelerated filero | | Accelerated filerþ | | Non-accelerated filer o
(Do not check if a smaller reporting company) | | Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined inRule 12b-2 of the Exchange Act). Yes o No þ
As of February 14, 2008, there were 27,059,110 shares of the registrant’s common stock outstanding and the approximate aggregate market value of such shares held by non-affiliates of the registrant (based upon the closing sale price of such shares on the NASDAQ Global Market on June 29, 2007 of $5.80 per share) was $95 million. Shares of the registrant’s common stock held by each current executive officer and director and by each stockholder who is known by the registrant to own 10% or more of the outstanding common stock have been excluded from this computation in that such persons may be deemed to be affiliates of the registrant. Share ownership information of certain persons known by the registrant to own greater than 10% of the outstanding common stock for purposes of the preceding calculation is based solely on information on Schedule 13D or Schedule 13G filed with the Commission and is as of December 31, 2007. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
DOCUMENTS INCORPORATED BY REFERENCE:
Portions of the Registrant’s Definitive Proxy Statement to be filed with the Commission pursuant to Regulation 14A in connection with the 2008 Annual Meeting are incorporated herein by reference into Part III of this report, which such proxy statement will be filed with the Commission within 120 days after registrant’s fiscal year ended December 31, 2007. Other references incorporated are listed in the exhibit list in Part IV of this report.
PART I
ITEM 1. BUSINESS
Special Note Regarding Forward Looking Statements
This report contains plans, intentions, objectives, estimates and expectations that constitute forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, which are subject to the “safe harbor” created by those sections. Forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “could,” “would,” “expect,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “predicts,” “potential” and similar expressions intended to identify forward-looking statements. Examples of these statements include, but are not limited to, statements regarding the following: the timing and implications of obtaining regulatory approval of any of our product candidates; the progress of our research programs, including clinical testing; our ability to identify new product candidates; the potential of any product candidates to lead to the development of commercial products; our anticipated timing for initiation or completion of our clinical trials for any of our product candidates and expectations regarding future results of such trials; other statements regarding our future product development activities and plans to develop or acquire and commercialize product candidates, regulatory strategies and clinical strategies, including our intent to develop or seek regulatory approval for our product candidates in specific indications; our future expenditures for research and development and the conduct of clinical trials; the ability of our third-party manufacturing parties to support our requirements for drug supply; the extent to which our intellectual property rights may protect our technology and product candidates; the size and growth of the potential markets for our product candidates and our plans to develop our sales and marketing capabilities to serve those markets; the rate and degree of market acceptance of any future products; the success of competing drugs that are or become available; our plans and ability to enter into collaboration arrangements; any statements regarding our future financial performance, results of operations or sufficiency of capital resources to fund our operating requirements; and any other statements that are other than statements of historical fact. Our actual results could differ materially from those discussed in these forward-looking statements due to a number of factors, including the success and timing of preclinical studies and clinical trials; our ability to obtain a new partner for faropenem medoxomil on acceptable terms; our ability to obtain and maintain regulatory approval of product candidates and the labeling under any approval that may be obtained; plans to develop and commercialize product candidates; the loss of key scientific or management personnel; the size and growth of the potential markets for our product candidates and our ability to serve those markets; regulatory developments in the U.S. and foreign countries; the rate and degree of market acceptance of any future products; the accuracy of our estimates regarding expenses, future revenues and capital requirements; our ability to obtain and maintain intellectual property protection for our product candidates; the successful development of our sales and marketing capabilities; the success of competing drugs that are or become available; and the performance of third party manufacturers. These and additional risks and uncertainties are described more fully by us in Part I, Item 1A and Part II, Item 7 of this report and in our other filings with the Commission. You should not place undue reliance on these forward-looking statements, which apply only as of the date of this report. You should read this report completely and with the understanding that our actual future results may be materially different from what we expect. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future.
Overview
We are a biopharmaceutical company focused on discovering, developing, in-licensing and commercializing innovative anti-infective products. Our most advanced product candidate, faropenem medoxomil, is a novel oral, community antibiotic for which we are currently seeking a development and commercialization partner. Our second product candidate, REP3123, is a new, narrow spectrum antibacterial agent for the treatment of
2
Clostridium difficile, orC. difficile, bacteria andC. difficile-associated disease, an increasing health care concern among elderly and hospitalized patients. We are also pursuing the development of other novel compounds that inhibit bacterial DNA replication, which we believe represents a potentially promising drug target in antibiotic development.
In December 2005, we submitted a new drug application, or NDA, for faropenem medoxomil based on 11 Phase III studies for the following adult indications: acute bacterial sinusitis; community-acquired pneumonia; acute exacerbation of chronic bronchitis; and uncomplicated skin and skin structure infections. In October 2006, the FDA issued a non-approvable letter with respect to our NDA citing the need for further clinical studies for all indications, including studies using a superiority design for acute bacterial sinusitis and acute exacerbation of chronic bronchitis, more extensive microbiologic confirmation and consideration of alternate dosing regimens. A superiority design trial requires demonstrating that a product candidate is superior to placebo. Historically, all of our trials were conducted using a non-inferiority design, which required these trials to demonstrate that a product candidate is not significantly less effective than an approved treatment. On January 22, 2008, we received a Warning Letter from the FDA related to our NDA filed in December 2005 for faropenem medoxomil citing certain conditions found by the FDA during their review of our role as the applicant of the NDA. Specifically, the Warning Letter noted that certain raw data, descriptions and analysis supporting clinical trials included in the NDA were not available for the FDA’s review and had not been obtained or reviewed by us prior to submission of the NDA. We intend to respond to the Warning Letter within the time limits required by the FDA.
The focus of our activities following receipt of the non-approvable letter from the FDA has been to clarify the approval process for faropenem medoxomil in the treatment of community respiratory tract infections. We do not expect to pursue the indication for uncomplicated skin and skin structure infections unless we enter into a collaboration with a partner that wishes to do so. Based on the FDA’s recommendations in the non-approvable letter, as well as our ongoing discussions with the FDA, we understand that at least two approved clinical studies using faropenem medoxomil for the treatment of community-acquired pneumonia will be required for approval in this indication. If we or a future partner seek approval for faropenem medoxomil to treat acute bacterial sinusitis and acute exacerbation of chronic bronchitis in addition to community-acquired pneumonia, the faropenem medoxomil adult program may be anchored on at least two clinical trials for the treatment of community-acquired pneumonia with single clinical trials using a superiority clinical trial design in acute bacterial sinusitis and acute exacerbation of chronic bronchitis. We have completed a special protocol assessment, or SPA, for the design of a Phase III clinical trial of faropenem medoxomil compared to placebo for the treatment of acute bacterial sinusitis. We plan to continue our ongoing Phase III placebo-controlled clinical trial for treatment of acute exacerbation of chronic bronchitis with faropenem medoxomil which is intended to meet the FDA’s requirements. Until we have secured a partner for the faropenem medoxomil program, which cannot be assured, we plan to limit our faropenem medoxomil clinical activities to the ongoing Phase III placebo-controlled clinical trial for the treatment of acute exacerbation of chronic bronchitis. If we are delayed in securing or are unable to secure a partner for the faropenem medoxomil program, we may elect to discontinue our development activities on this program, including to discontinue the Phase III placebo-controlled clinical trial for the treatment of acute exacerbation of chronic bronchitis. We have licensed all rights to faropenem medoxomil from Asubio Pharma Co., Ltd., or Asubio Pharma, in the U.S. and Canada. In addition, we have the sole negotiation right to license such rights for the rest of the world, except Japan.
We are also developing REP3123, our investigational narrow spectrum antibacterial agent, to treatC. difficile,bacteria andC. difficile-associated disease.C. difficileis a Gram-positive bacterium that causes diarrhea and other intestinal conditions, such as colitis, and is a major cause of morbidity among the elderly and hospitalized patients. People generally contractC. difficile-associated disease through the ingestion ofC. difficile spores after coming into contact with a contaminated item or surface. These spores then germinate, grow and multiply in the digestive tract. Inin vitro preclinical studies, REP3123 displayed an ability to inhibit growth of theC. difficilebacterium and prevent the bacterium from forming the spores that allow it to be spread from person to person, but without inhibiting other key organisms that are essential for normal intestinal functioning. Also in preclinical studies, REP3123 exhibited signs it may be able to stop the
3
production of destructive intestinal toxins caused byC. difficilebacteria. These results suggest that REP3123 has the potential to reduceC. difficile-associated disease outbreak and relapse rates through reducing the presence ofC. difficilespores and reduce the severity of, or possibly even prevent,C. difficile-associated disease through inhibiting the growth of or stopping production of toxins caused byC. difficilebacteria. We retain worldwide rights to REP3123.
We have also developed assays that identify compounds that inhibit bacterial DNA replication. The compounds may be useful to treat bacterial infections. We believe that bacterial DNA replication is an attractive target system for new antibacterial drugs because it is an essential cellular process and stalled DNA replication can trigger cell death. Our assays allow for efficient screening of large libraries of small molecules and are designed to mimic the bacterial DNA replication systems of numerous bacteria, with the goal of identifying novel inhibitors of bacterial DNA replication. We have identified compounds that are able to inhibit bacterial DNA replication in these assays. We believe that the novel mechanism of action of our technology may reduce the risk that bacteria will develop resistance to drugs based on this technology. We are currently optimizing the initial inhibitors identified in the assays.
Strategy
Our goal is to discover, develop, in-license and commercialize novel anti-infective compounds that address unmet medical needs resulting from growing resistance to existing drug products. Key elements of our strategy are:
| | |
| | • | Maximize the commercial potential for faropenem medoxomil by securing a development and commercialization partner. If approved, we believe that faropenem medoxomil may become a leading community antibiotic and a preferred branded oral beta-lactam in adult and pediatric markets due to its safety profile and spectrum of activity. We are seeking a partner for the development and commercialization of faropenem medoxomil in the territories available to us under our license agreement. We plan to limit our faropenem medoxomil clinical trial activities to the ongoing Phase III placebo-controlled clinical trial for treatment of acute exacerbation of chronic bronchitis until we have secured a partner for the faropenem medoxomil program, which cannot be assured. |
| |
| | • | Advance development of our novel anti-infective products. We intend to advance our pipeline of novel anti-infective product candidates by continuing to pursue discovery research programs. We have an active program to develop a treatment forC. difficilebacterial infections andC. difficile-associated disease,which are major causes of morbidity among elderly and hospitalized patients and are diseases for which existing therapies have significant limitations. We also plan to use our DNA replication inhibition expertise to develop anti-infective products with novel mechanisms of action. |
| |
| | • | Accelerate growth through acquiring or in-licensing additional products or product candidates that augment our research and development pipeline or through pursuing strategic alternatives. We maintain a strong business development capability that will continue to pursue product candidates that augment our research and development pipeline. In executing these initiatives we expect to consider strategic alternatives that could involve a merger or the acquisition of some or all of our assets and reduce our current focus on the development of anti-infective product candidates. |
We continuously reassess all of our research and development efforts, including those for the anti-infective product candidates described above. At any time, we may expand, delay, terminate or dispose of all or any portion of our research and development programs or we may develop or acquire rights to new product candidates.
Antibiotic Market Background and Opportunity
Bacterial infections occur when bacteria that naturally exist in the body or that are inhaled, ingested or otherwise acquired are not controlled by the immune system. The antibiotics used to treat these infections are classified as either broad spectrum or narrow spectrum. Broad spectrum antibiotics are typically oral antibiotics used to treat community-acquired infections, whereas narrow spectrum antibiotics are typically intravenous
4
antibiotics used to treat specific bacteria in the hospital setting, with the exception of penicillin. According to IMS Health, the annual worldwide market for antibiotics was $28.0 billion in 2006, which includes U.S. sales of $7.7 billion for oral antibiotics. The U.S. oral antibiotics market in 2006 was comprised of $6.2 billion for the adult market and $1.5 billion for the pediatric market. IMS Health estimates that in 2006, beta-lactams had a 49% market share of the adult oral antibiotic market representing over 90 million prescriptions and a 75% market share of the pediatric oral antibiotic market representing over 40 million prescriptions. We believe that faropenem medoxomil’s safety profile and activity against many common bacterial infections suggest its potential to become a leading branded oral beta-lactam antibiotic.
We believe the two primary factors that drive a physician’s choice to prescribe an oral antibiotic to treat community-acquired infections are the drug’s effectiveness against a particular type of bacterial infection and the drug’s safety profile. We believe that an antibiotic with good efficacy and an excellent safety profile may be used in preference to a more powerful antibiotic that has the risk of serious side effects, especially in non life-threatening infections. As a patient’s condition becomes more serious, the physician may be more willing to expose that patient to a potentially increased risk of side effects and safety issues to obtain the benefit of a drug that may be more potent against the bacteria that caused the infection.
Oral antibiotics are classified as either first- or second-line therapies for each disease state by key opinion leader physicians who write the antibiotic treatment guidelines, such as those published by the Sinus and Allergy Health Partnership and American Academy of Pediatrics. First-line therapy includes both branded and generic antibiotics and constitutes a larger market than second-line therapy which currently is comprised primarily of branded products.
According to IMS Health, over 90% of all bacterial infections that occurred in 2006 were classified as upper respiratory tract infections, lower respiratory tract infections and uncomplicated skin and skin structure infections. There are three primary classes of oral antibiotics that are prescribed to treat respiratory tract and skin infections; the beta-lactam class, the macrolide/ketolide class, and the quinolone class. Each class has a distinctive chemical structure that is shared by the various antibiotics included in that class.
Beta-lactam antibiotics have been the most widely prescribed antibiotics for more than 50 years. This class of antibiotics is well known for favorable efficacy, safety and tolerability. Since the introduction of penicillin in 1942, only two other sub-classes of beta-lactams have been introduced: cephalosporins (1974) and carbapenems (1985). Carbapenems are only available in intravenous form for use in the hospital setting. Therefore, if approved, the introduction of the penem sub-class will represent the first oral community beta-lactam sub-class introduction in more than 30 years.
The penem sub-class of beta-lactam antibiotics has structural features that resemble a fusion of the penicillin and cephalosporin core structures. An advantage of penems is their ability to resist degradation by commonly encountered beta-lactamase enzymes. Bacteria commonly become resistant to beta-lactam antibiotics by producing beta-lactamase enzymes that inactivate the antibiotic. Beta-lactamase enzymes are known to destroy some of the penicillin and cephalosporin antibiotics, which can result in resistance to those sub-classes of beta-lactam antibiotics.
Beta-lactam antibiotics are effective against a range of common bacterial infections and do not exhibit many of the safety issues common with the macrolide/ketolide and quinolone classes. The beta-lactam class is recommended as first-line therapy for treating acute bacterial sinusitis and uncomplicated skin and skin structure infections in adults. According to the Infectious Disease Society of America, macrolides are a preferred treatment for acute exacerbation of chronic bronchitis while quinolones are a preferred treatment for community-acquired pneumonia.
5
The following table shows the prescriptions and percentage use of each class of oral antibiotics in 2006 for common adult indications:
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | Adult
| | | Drug Class Share of Indication | |
| | | | | Oral Market
| | | Beta-
| | | Macrolides/
| | | | | | Other
| |
Bacterial Infection Type | | Indication | | Prescriptions | | | Lactams | | | Ketolides | | | Quinolones | | | Antibiotic | |
| | | | | (in millions) | | | | | | | | | | | | | |
| |
| Upper Respiratory Tract Infections | | Acute Bacterial Sinusitis | | | 34.2 | | | | 48 | % | | | 32 | % | | | 16 | % | | | 4 | % |
| | | Acute Bacterial Otitis Media | | | 8.2 | | | | 72 | % | | | 18 | % | | | 6 | % | | | 4 | % |
| | | Tonsillitis/Pharyngitis | | | 17.8 | | | | 66 | % | | | 28 | % | | | 3 | % | | | 3 | % |
| Lower Respiratory Tract Infections | | Acute Exacerbation of Chronic | | | | | | | | | | | | | | | | | | | | |
| | | Bronchitis | | | 29.3 | | | | 19 | % | | | 51 | % | | | 21 | % | | | 9 | % |
| | | Community-Acquired Pneumonia | | | 6.5 | | | | 12 | % | | | 31 | % | | | 55 | % | | | 2 | % |
| Skin Infections | | Uncomplicated Skin & Skin Structure Infections | | | 31.8 | | | | 64 | % | | | 7 | % | | | 13 | % | | | 16 | % |
| Total | | | | | | | | | 41 | % | | | 26 | % | | | 22 | % | | | 11 | % |
Source: IMS Health
The safety profile of the beta-lactam class has been particularly important in the pediatric market. The beta-lactam class is recommended by the American Academy of Pediatrics as first-line therapy for acute bacterial otitis media, tonsillitis/pharyngitis and acute bacterial sinusitis in pediatric patients. Ketolides and quinolones are currently not approved for pediatric indications. The following table shows the prescriptions and percentage use of each class of oral antibiotics in 2006 for common pediatric indications:
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | Pediatric
| | | Drug Class Share of Indication | |
| | | | | Oral Market
| | | Beta-
| | | | | | | | | Other
| |
Bacterial Infection Type | | Indication | | Prescriptions | | | Lactams | | | Macrolides | | | Quinolones | | | Antibiotic | |
| | | | | (in millions) | |
| |
| Upper Respiratory Tract Infections | | Acute Bacterial Sinusitis | | | 4.4 | | | | 85 | % | | | 14 | % | | | 1 | % | | | 1 | % |
| | | Acute Bacterial Otitis Media | | | 20.8 | | | | 89 | % | | | 9 | % | | | 0 | % | | | 2 | % |
| | | Tonsillitis/Pharyngitis | | | 7.7 | | | | 90 | % | | | 9 | % | | | 0 | % | | | 1 | % |
| Lower Respiratory Tract Infections | | Acute Exacerbation of Chronic | | | | | | | | | | | | | | | | | | | | |
| | | Bronchitis | | | 3.1 | | | | 43 | % | | | 54 | % | | | 0 | % | | | 3 | % |
| | | Community-Acquired Pneumonia | | | 1.5 | | | | 55 | % | | | 44 | % | | | 1 | % | | | 0 | % |
| Skin Infections | | Uncomplicated Skin & Skin | | | | | | | | | | | | | | | | | | | | |
| | | Structure Infections | | | 2.9 | | | | 81 | % | | | 8 | % | | | 1 | % | | | 10 | % |
| Total | | | | | | | | | 82 | % | | | 14 | % | | | 1 | % | | | 3 | % |
Source: IMS Health
We believe that in addition to efficacy and safety, prescribing decisions in the pediatric market are also significantly affected by the tolerability and taste of the antibiotic. Because the efficacy of many antibiotics depends on the patient taking the full course of therapy at the prescribed times, a patient’s discontinuation of therapy or refusal to take the drug due to tolerability issues can result in prolongation of the infection and possibly serious complications.
We believe that three key factors are creating significant opportunities for new branded antibiotics that are more effective, better tolerated and safer than existing therapies:
| | |
| | • | Emergence of drug-resistant bacteria. Over the past several decades, many of the most prevalent bacteria that cause adult and pediatric respiratory and skin infections have developed resistance to currently marketed antibiotics. If bacteria are resistant, the infection can become difficult or impossible to treat and may lead to serious complications, including death. The two most prevalent bacteria in respiratory infections includeStreptococcus pneumoniae, orS. pneumoniaeandHaemophilus influenzae, |
6
| | |
| | | orH. influenzae. According to the 2006 PROTEKT U.S. surveillance study, designed to track antibiotic resistance, more than 29% of the Streptococcus species are resistant to at least one of the drugs most commonly used to treat these infections. Further, following the introduction in February 2000 of the heptavalent pneumococcal pediatric vaccine Prevnar®, the emergence of non-vaccine serotypes has been observed. In October 2007, theJournal of the American Medical Association focused attention onS. pneumoniae serotype 19A, a serotype not included in the heptavalent vaccine, and the limited treatment options available to pediatric patients due to this serotype’s resistance to many antibiotics commonly used in children. The rate ofH. influenzaeresistance to at least one of the drugs most commonly used to treat infections caused by these bacteria has reached 30%, as reported in the 2005Journal of Clinical Infectious Disease. The U.S. Centers for Disease Control has stated that antibiotic resistance is now among that organization’s top concerns. |
| | |
| | • | Tolerability. Many current oral antibiotics have been associated with tolerability issues that cause patients extreme discomfort and poor compliance that can lead to product failures. The most widely reported adverse event among leading oral antibiotics is diarrhea. The prescribing label for two of the leading oral beta-lactam antibiotics for use in adults, Augmentin® and Omnicef®, lists diarrhea incidence levels of approximately 15%. |
| |
| | • | Safety. Many of the common oral antibiotics in the quinolone and macrolide/ketolide classes are burdened with safety issues such as hepatotoxicity (drug related liver damage), heart rhythm abnormalities, photosensitivity (increased sensitivity to sunlight), hypoglycemia (low blood sugar), hyperglycemia (high blood sugar) or rash. Macrolide and ketolide antibiotics are also associated with clinically meaningful drug/drug interactions with frequently prescribed drugs such as cholesterol lowering agents. To date, four of the nine quinolone antibiotics that have been marketed have been withdrawn from the market due to safety concerns. Additionally, in February 2007 the labeled indication for Ketek®, a ketolide, was amended to remove approval for the treatment of acute bacterial sinusitis and acute exacerbations of chronic bronchitis after the FDA determined that drug’s safety profile, specifically related to hepatoxicity, no longer justified approval for these indications. |
7
Our Product Candidates and Development Programs
Our current product candidate and development program portfolio consists of the following:
| | | | | |
Product Candidate | | Target Indications | | Development Status |
| |
Faropenem Medoxomil | | | | |
| 600 mg dose | | Acute exacerbation of chronic bronchitis | | Phase III clinical trial ongoing |
| | | Acute bacterial sinusitis | | Special protocol assessment for design of placebo-controlled Phase III clinical trial completed in October 2007 |
| | | Community-acquired Pneumonia | | Discussion on design of Phase III clinical trials ongoing with the FDA |
|
Faropenem Medoxomil | | | | |
| Oral liquid formulation | | Acute bacterial otitis media (pediatric) | | Phase II clinical trial completed |
|
REP3123 | | C. difficilebacteria andC. difficile -associated disease | | Preclinical studies completed. IND targeted for the second half of 2008 |
|
REP8839 | | Impetigo
Skin and wound infections | | Phase I studies completed — development program on hold |
|
DNA Replication Inhibition | | Novel mechanism of action antibiotic targeting Gram-positive bacteria maintaining oral bioavailability and bactericidal activity | | Discovery |
Faropenem Medoxomil Program
Faropenem medoxomil is a member of the penem class of beta-lactam antibiotics. If approved by the FDA, it would be the first oral penem available outside of Japan. We believe that with its broad spectrum of activity, potency and safety and tolerability profile, faropenem medoxomil would be appropriate for use as a first-line therapy for the treatment of community-acquired respiratory tract and skin infections in adult primary care and pediatric settings. The following characteristics differentiate faropenem medoxomil from existing beta-lactam antibiotics:
| | |
| | • | First oral penem available in the U.S. If approved by the FDA, faropenem medoxomil would represent the first new sub-class of beta-lactams (penems) to be introduced in oral form for community use in more than 30 years. Over the years many bacteria have developed resistance to older beta-lactam antibiotics. Penems intrinsically are able to resist degradation by beta-lactamase enzymes. Because faropenem medoxomil is a first product in a new class of antibiotics, its introduction should not be burdened with resistance issues at the levels associated with other existing antibiotics. |
| |
| | • | Potency profile. In vitrostudies have indicated that faropenem medoxomil is four times more active than Augmentin® (amoxicillin/clavulanate) againstS. pneumoniae,including those strains that have evolved resistance to penicillin or amoxicillin. Faropenem medoxomil is also generally twice as active as Augmentin® againstH. influenzae,including those strains that have evolved resistance to other beta-lactam antibiotics.In vitro potency does not always correlate to clinical efficacy. |
8
| | |
| | • | Safety profile. Due to its safety profile, we believe that faropenem medoxomil would be appropriate as a first-line treatment for common respiratory and skin infections in the primary care setting. We believe that faropenem medoxomil would allow physicians to reserve quinolones for second-line therapy, reducing quinolone resistance and improving the risk-to-benefit ratio for individual patients. Unlike carbapenems, faropenem medoxomil has a low potential for neurotoxicity. In Phase III clinical testing, faropenem medoxomil has not exhibited the potentially serious safety issues that affect the macrolide/ketolide and quinolone classes of antibiotics. |
| |
| | • | Tolerability profile. In the Phase II and Phase III clinical studies referenced in our December 2005 NDA that were completed at the 300 mg, twice per day dose, the overall incidence of diarrhea was less than 5% in over 5,000 patients treated with faropenem medoxomil. This rate of incidence compares favorably with the incidence of diarrhea reported with other commonly used beta-lactam antibiotics. We anticipate using the 600 mg, twice per day dose in future clinical trials in adult settings. |
Faropenem medoxomil is a prodrug form of the parent compound faropenem and was initially discovered by Suntory Limited, now known as Asubio Pharma. Faropenem medoxomil is metabolized by the body to release faropenem sodium, a drug that has been approved and sold in Japan by Asubio Pharma since 1997. Since then, it is estimated that more than 69 million prescriptions have been written. Prodrugs are designed to improve the amount of drug reaching the bloodstream in which the prodrug molecule is separated by the body’s natural metabolic enzymes into its active component and an inactive component. In clinical pharmacology studies, approximately 72% to 84% of an orally administered dose of faropenem medoxomil was absorbed into the bloodstream and then rapidly converted to the active parent compound faropenem, resulting in three to four times greater bioavailability compared to faropenem sodium.
Preclinical Data. In preclinical studies, faropenem medoxomil has exhibited broad spectrum activity that includes bacteria commonly associated with respiratory infections (S. pneumoniae, H. influenzaeandMoraxella catarrhalis, orM. catarrhalis) and uncomplicated skin structure and skin infections (methicillin-susceptibleS. aureusandStreptococcus pyogenes, orS. pyogenes). The following table shows the antibacterial activities of faropenem medoxomil and other antibiotics against these common respiratory and skin bacterial pathogens inin vitrostudies. The MIC(90) value shown is the minimum inhibitory concentration of drug required to inhibit growth of 90% of the bacterial isolates within a given population. The lower the MIC(90) value for a given drug the more potent it is against the population of bacteria.
| | | | | | | | | | | | | | | | | | | | | |
| | | MIC(90) (mg/mL) | |
| | | Faropenem | | | Augmentin | | | Omnicef | | | Zithromax | | | Levaquin | |
| |
Respiratory Pathogens | | | | | | | | | | | | | | | | | | | | |
S. pneumoniae | | | | | | | | | | | | | | | | | | | | |
| Penicillin-susceptible | | | 0.008 | | | | 0.03 | | | | 0.12 | | | | 0.25 | | | | 1 | |
| Penicillin-intermediate | | | 0.25 | | | | 1 | | | | 4 | | | | m512 | | | | 1 | |
| Penicillin-resistant | | | 1 | | | | 4 | | | | >4 | | | | m512 | | | | 1 | |
H. influenzae | | | | | | | | | | | | | | | | | | | | |
b-Lactamase-positive | | | 0.5 | | | | 2 | | | | 0.5 | | | | 4 | | | | 0.015 | |
b-Lactamase-negative | | | 1 | | | | 1 | | | | 1 | | | | 4 | | | | 0.015 | |
M. catarrhalis | | | | | | | | | | | | | | | | | | | | |
b-Lactamase-positive | | | 0.5 | | | | 0.5 | | | | 0.25 | | | | 0.06 | | | | 0.06 | |
b-Lactamase-negative | | | 0.12 | | | | 0.03 | | | | 0.12 | | | | 0.06 | | | | 0.06 | |
| | | | | | | | | | | | | | | | | | | | | |
Skin Pathogens | | | | | | | | | | | | | | | | | | | | |
S. aureus | | | | | | | | | | | | | | | | | | | | |
| Methicillin-susceptible | | | 0.12 | | | | 1 | | | | 0.5 | | | | >64 | | | | 0.25 | |
| Methicillin-resistant | | | >32 | | | | >32 | | | | — | | | | >64 | | | | — | |
S. pyogenes | | | 0.03 | | | | 0.015 | | | | 0.03 | | | | 0.25 | | | | 1 | |
9
Faropenem Medoxomil for the Adult Market. We submitted an NDA for faropenem medoxomil to the FDA in December 2005 seeking approval for four indications: acute bacterial sinusitis, community-acquired pneumonia, acute exacerbation of chronic bronchitis and uncomplicated skin and skin structure infections. In October 2006, the FDA issued a non-approvable letter for all indications included in the NDA. In the non-approvable letter, the FDA recommended further clinical studies for all indications, including studies using a superiority design for the indications of acute bacterial sinusitis and acute exacerbation of chronic bronchitis, more extensive microbiologic confirmation and consideration of alternate dosing regimens. The FDA did not raise any safety concerns or chemistry, manufacturing or controls issues related to the product. However, in January 2008 we received a Warning Letter from the FDA pursuant to completion of the FDA’s review of clinical trials performed in connection with the NDA for faropenem medoxomil filed in December 2005. The Warning Letter noted that we failed to make available certain underlying data and analyses from clinical trials performed by Bayer Corporation, as the previous licensee of faropenem medoxomil, and incorporated by us into the NDA for FDA review and failed to adequately verify and ensure the integrity of clinical data or information included in the NDA relevant to the evaluation of faropenem medoxomil safety and effectiveness derived from certain clinical sites. We intend to respond to the Warning Letter within the time limits required by the FDA. We are currently conducting a Phase III placebo-controlled clinical trial for treatment of acute exacerbation of chronic bronchitis with faropenem medoxomil.
Clinical Overview. Regulatory requirements for the approval of new drugs can change over time. Historically, the FDA and foreign regulatory authorities have not required clinical trials using a superiority design, including placebo-controlled clinical trials, for the approval of antibiotics but instead have relied on non-inferiority studies. In a non-inferiority study, a drug candidate is compared with an approved antibiotic treatment and it must be shown that the product candidate is not significantly less effective than the approved treatment. All efficacy studies upon which our NDA was based were designed as non-inferiority studies. In September 2005, the FDA informed us that it would likely require clinical trials using a superiority design such as a placebo-controlled trial prior to approving faropenem medoxomil for acute exacerbation of chronic bronchitis. Nevertheless, the FDA agreed to review our application for this indication and accepted the NDA for filing. In completing their review of the NDA, the FDA established the requirement for superiority studies for approval for this indication as well as for acute bacterial sinusitis. In October 2007, we completed a special protocol assessment with the FDA for the design of a placebo-controlled clinical trial for the treatment of acute bacterial sinusitis with faropenem medoxomil. Later in October 2007, the FDA issued a Draft Guidance for Industry titled “Acute Bacterial Sinusitis: Developing Drugs for Treatment”, which guidance generally encompassed the protocols described in our special protocol assessment for this indication.
The clinical trials that supported our NDA submitted in December 2005 were conducted by Bayer as a previous licensee of faropenem medoxomil. The primary study objective in most of these studies was to demonstrate that faropenem medoxomil was non-inferior to a control antibiotic treatment approved for use in the U.S. Faropenem medoxomil was shown to be non-inferior in eight of nine randomized controlled studies and similar results were demonstrated in two uncontrolled studies. The definition of statistical non-inferiority was met if there was less than 5% probability (a 95% confidence interval) that faropenem medoxomil was 10% worse than the standard treatment. The choice of a 10% delta conformed to standards for establishing non-inferiority of antimicrobial agents that had previously been approved. Efficacy evaluation, including clinical and microbiologic responses, was determined by physician assessment and bacterial cultures. The clinical outcome analysis was first conducted for subjects who met all the protocol defined criteria or rules (the “clinically evaluable population”) and subsequently on all treated subjects (the “intent-to-treat population”).
The Phase III clinical trials included in our December 2005 NDA were all conducted using a 300 mg, twice per day, dose. In January 2006, we initiated a placebo-controlled Phase III clinical trial for the acute exacerbation of chronic bronchitis indication using the 600 mg, twice per day, dose. We had previously evaluated the potential for adverse events with the 600 mg, twice per day, dose in a Phase I study and a Phase II study conducted in 2005. In the Phase I study, the 600 mg, twice per day, dose was directly compared to a 300 mg, twice per day, dose, both administered for seven days. In the Phase II study, a 600 mg, twice per day dose was compared to a 300 mg, twice per day, dose seven day treatment course in patients with acute
10
bacterial sinusitis. In both trials, the adverse events were similar in both type and frequency. Based on the results of these two studies, together with prior Phase I studies that included increased doses of faropenem medoxomil higher than 600 mg, we believe that the incidence and severity of adverse events are unlikely to be substantially higher with the 600 mg, twice per day, dose than previously observed with the 300 mg, twice per day, dose.
Clinical Studies for Acute Bacterial Sinusitis. The efficacy of faropenem medoxomil in subjects with acute bacterial sinusitis was evaluated in three Phase III studies at a 300 mg, twice per day dose. In two comparative studies, whereseven-day andten-day courses of faropenem medoxomil were compared to cefuroxime axetil, the primary endpoints were met and statistical non-inferiority was demonstrated. The third study was an open-label (no comparative control treatment) trial in which all subjects received faropenem medoxomil after undergoing a needle aspiration of the sinus cavity in order to obtain a direct sinus specimen to culture for bacterial pathogens. The clinical and microbiologic outcomes were consistent with the comparative studies.
Clinical Studies for Community-Acquired Pneumonia. The efficacy of faropenem medoxomil in subjects with community-acquired pneumonia was evaluated in four Phase III studies at a 300 mg, twice per day, dose. In three comparative studies, the primary endpoints were met and non-inferiority was demonstrated for10-day therapy with faropenem medoxomil compared to10-day therapy with amoxicillin/clavulanate,14-day therapy with cefpodoxime and10-day therapy with amoxicillin. The fourth study was an open-label trial in which bacterial samples were collected for culture. The clinical and microbiologic outcomes were consistent with the comparative studies at a dose of 300 mg taken two times per day. In the clinical trials included in our December 2005 NDA, evaluable microbiologic specimens were obtained approximately 9% to 21% of the time. Based on the non-approvable letter we received to our NDA in October 2006, in future clinical trials a higher rate of microbiologic specimens for microbiologic confirmation of both bacteriologic disease and bacteriologic clearance will be required.
Clinical Studies for Acute Exacerbation of Chronic Bronchitis. The efficacy of faropenem medoxomil in acute exacerbation of chronic bronchitis was evaluated in two comparative, non-inferiority Phase III studies. The primary endpoints were met in both studies and statistical non-inferiority was demonstrated forfive-day faropenem medoxomil compared tofive-day azithromycin andseven-day clarithromycin, both macrolide antibiotics. In January 2006, we initiated a placebo-controlled Phase III clinical trial in this indication using the 600 mg, twice per day, dose. As of March 5, 2008, we had enrolled 372 patients of a target enrollment of approximately 610 patients in this clinical trial.
Clinical Studies for Uncomplicated Skin and Skin Structure Infections. The efficacy of faropenem medoxomil in subjects with uncomplicated skin and skin structure infections was evaluated in two Phase III studies. The results of one study met the protocol-specified criterion for non-inferiority of faropenem medoxomil to amoxicillin/clavulanate. A second study did not demonstrate non-inferiority of faropenem medoxomil to cephalexin. When we pooled the data from the two studies, the eradication rates for the key pathogens in this indication,S. aureusandS. pyogenes,were high (greater than 90%) and were similar for faropenem medoxomil and the comparators. The focus of our current faropenem medoxomil activities is to clarify the approval process for faropenem medoxomil in the treatment of community respiratory tract infections. We do not intend to pursue development of faropenem medoxomil for uncomplicated skin and skin structure infections unless we enter into a collaboration with a partner who wishes to do so.
Other Studies. Three Phase III studies for other indications were also initiated, two in tonsillitis/pharyngitis and one in uncomplicated urinary tract infections.
The efficacy offive-day treatment with faropenem medoxomil in subjects with tonsillitis/pharyngitis was evaluated in one Phase III study. The comparator was a10-day treatment with penicillin VK. Another study was discontinued shortly after enrollment began. In the completed study, afive-day treatment with faropenem medoxomil did not demonstrate non-inferiority relative to the comparator. The bacteriological cure rate was 87% in the faropenem medoxomil treated patients and 94% in the penicillin VK patients. We believe that this difference may be related to the shorter course of therapy in the faropenem medoxomil arm. Multiple published reports suggest that shorter course therapy with penicillin is associated with lower bacteriological
11
cure rates in this indication. We currently do not intend to conduct additional studies in adults for this indication.
The efficacy offive-day treatment with faropenem medoxomil in subjects with uncomplicated urinary tract infections was studied in one Phase III study. The comparator wasfive-day treatment with trimethoprim-sulfamethoxazole. In this study,five-day treatment with faropenem medoxomil did not demonstrate non-inferiority relative to the comparator. The clinical cure rate was 86% in the faropenem medoxomil treated patients and 96% in the trimethoprim-sulfamethoxazole patients. We believe that this difference may be related at least in part to factors specific to the kidneys. There is an enzyme in the kidneys known to degrade carbapenem antibiotics as well as faropenem, resulting in decreased drug concentrations in the region of the infection. We do not consider this indication to be an important commercial opportunity for a beta-lactam antibiotic such as faropenem medoxomil. We currently do not intend to conduct additional studies in this indication.
Safety and Tolerability Data. We believe that faropenem medoxomil has a favorable safety and tolerability profile. The pharmacokinetics of faropenem medoxomil following oral administration were evaluated in 27 Phase I studies, three Phase II studies and one Phase III study. Faropenem medoxomil was well absorbed, rapidly converted to faropenem and reached maximum plasma concentrations approximately one hour after administration. Single doses of faropenem medoxomil up to 3,000 mg and multiple doses up to 3,750 mg per day were administered without notable safety issues.
We evaluated faropenem medoxomil in a Phase I study to determine whether there was any potential of faropenem medoxomil to prolong QT interval, a measure of electrocardiac function, which has been problematic for the quinolone and macrolide classes of antibiotics. This “Thorough QT” study, required for all new drug applications, demonstrated that faropenem medoxomil does not cause any electrocardiographic abnormalities, including QT interval prolongation.
In Phase III clinical testing, faropenem medoxomil exhibited the activity and safety profile typical of beta-lactam antibiotics with improved tolerability. The Phase III studies have accrued a safety database comprising 3,461 patients in respiratory tract infection indications and 4,863 patients in all Phase III studies. Faropenem medoxomil has been administered to over 5,000 people including all Phase I, Phase II and Phase III studies. The most common adverse events involved the gastrointestinal tract, including diarrhea, nausea or abdominal pain, or the central nervous system, including headaches and dizziness.
We believe that the safety profile of faropenem medoxomil is similar to that of penicillins and cephalosporins. Unlike some carbapenems, faropenem medoxomil showed no proconvulsant effects in animal models. There was only one incident of convulsion in the faropenem medoxomil clinical studies (a rate of 0.02%), which the treating physician did not attribute to faropenem medoxomil. In comparison with amoxicillin/clavulanate, faropenem medoxomil produced lower rates of adverse events, including gastrointestinal events and liver enzyme abnormalities. Unlike macrolides/ketolides and quinolones, faropenem medoxomil was not associated with hepatotoxicity, heart rhythm abnormalities, photosensitivity, hypoglycemia or hyperglycemia.
In the Phase II and Phase III clinical studies referenced in our December 2005 NDA, the overall incidence of diarrhea was less than 5% in over 5,000 patients treated with faropenem medoxomil. We believe the safety and tolerability profile of faropenem medoxomil make it a promising agent to be used as a first-line antibiotic in the community setting.
Ongoing Clinical Development. We have engaged in discussions with the FDA to determine the clinical trial designs and regulatory requirements that will be required for faropenem medoxomil to be approved in the U.S. for treatment of community respiratory tract infections. Based on the FDA’s recommendations in the non-approvable letter, as well as our discussions with the FDA, we understand that at least two approved clinical studies using faropenem medoxomil for the treatment of community-acquired pneumonia will be required for approval in this indication. If approval is sought for faropenem medoxomil to treat acute bacterial sinusitis and acute exacerbation of chronic bronchitis in addition to community-acquired pneumonia, the faropenem medoxomil adult program may be anchored on at least two clinical trials for the treatment of community-
12
acquired pneumonia with single clinical trials using a superiority clinical trial design in acute bacterial sinusitis and acute exacerbation of chronic bronchitis. We have completed an SPA for the design of a placebo-controlled Phase III clinical trial for the treatment of acute bacterial sinusitis with faropenem medoxomil. We plan to continue our ongoing Phase III placebo-controlled clinical trial for treatment of acute exacerbation of chronic bronchitis with faropenem medoxomil, which is intended to meet the FDA’s requirements. We plan to limit our faropenem medoxomil clinical trial activities to the ongoing Phase III placebo-controlled clinical trial for the treatment of exacerbation of chronic bronchitis until we have secured a partner for the faropenem medoxomil program. If we are delayed in securing or are unable to secure a partner for the faropenem medoxomil program, we may elect to discontinue our development activities on this program, including to discontinue the Phase III placebo-controlled clinical trial for the treatment of acute exacerbation of chronic bronchitis. We further understand that clinical trials for community respiratory indications will include a requirement for minimum levels of microbiologic confirmation of physician assessed clinical outcomes. Future clinical trials of faropenem medoxomil in adult settings are expected to be conducted using the 600 mg, twice per day, dose of faropenem medoxomil. Clinical trials at the 600 mg, twice per day dose will need to accumulate a safety database of clinical trial participants using faropenem medoxomil of approximately 1,500 patients.
Placebo-controlled Acute Exacerbation of Chronic Bronchitis Study. We have an ongoing Phase III trial in acute exacerbation of chronic bronchitis. The clinical trial is a placebo-controlled clinical trial of approximately 610 patients with a primary end point to demonstrate the efficacy, as assessed by the treating physician, of treatment with faropenem medoxomil over placebo. The initial comparators we selected were placebo and Ketek (telithromycin). On December 26, 2006, we announced that we had stopped enrollment in this trial to exclude the Ketek comparator arm. Ketek had initially been included in the study to generate secondary data points to a product we had projected as a competitor product to faropenem medoxomil. We based our decision to exclude Ketek on the findings of a joint Advisory Meeting of the FDA’s Anti-Infective Drug and Drug Safety and Risk Management committees held on December 14 and 15, 2006 that recommended to the FDA that the risks of using Ketek outweigh the benefits of using the drug for treatment of acute exacerbation of chronic bronchitis. This recommendation was adopted by the FDA on February 12, 2007. Following required communication with investigational review boards overseeing the clinical trial sites, we re-initiated this trial without the Ketek comparator arm. Through March 5, 2008 we had enrolled 372 patients into this study and anticipate completing enrollment in the second half of 2008.
In this study, we are using a 600 mg, twice per day dose. Study subjects are taking two 300 mg tablets at each dose. We anticipate using a single 600 mg tablet in commercial settings, which will require that we demonstrate bioequivalence of the two dosage forms. We have developed a single 600 mg prototype tablet. The duration of therapy is five days. We believe that this higher dose may offer the potential for greater efficacy than the lower dose.
We have corresponded with the FDA regarding our ongoing development work in treating acute exacerbations of chronic bronchitis. Based on this correspondence, we believe that the results of this single study may support filing for approval to treat this indication as a component of a clinical trials package to treat community respiratory tract infections that includes two clinical trials using faropenem medoxomil for the treatment of community-acquired pneumonia. Because the FDA has not issued formal guidance regarding the design or conduct of placebo-controlled studies for this indication, there can be no assurance that the FDA will accept such a filing or grant approval even if the results obtained from our study meet the primary endpoint(s) defined in our protocol.
Faropenem Medoxomil for the Pediatric Market
We are developing a faropenem medoxomil oral liquid formulation for pediatric use. Faropenem medoxomil has performed wellin vitroagainst many common pediatric pathogens. We believe that the well-known safety of beta-lactam antibiotics and the tolerability profile of faropenem medoxomil demonstrated in extensive clinical testing in adults make faropenem medoxomil a promising candidate for the pediatric market.
13
Formulation Development. For pediatric indications, it is important that faropenem medoxomil be available as an oral liquid formulation. For example, the majority of patients being treated for acute bacterial otitis media are less than three years old and require an oral liquid formulation. Any oral liquid formulation should have both a competitive taste profile and the requisite stability. Like many other medications, the active ingredient in faropenem medoxomil is bitter. However, we have developed a prototype oral liquid formulation that we believe has a competitive taste profile for use in future Phase III clinical trials.
Phase II Acute Bacterial Otitis Media Clinical Trial Completed. We have completed a Phase II clinical trial for treatment of acute bacterial otitis media with an oral liquid formulation of faropenem medoxomil. The study, which was completed at sites in Israel and Costa Rica, was not conducted under our U.S. Investigational New Drug Application, or IND, for faropenem medoxomil. The Phase II clinical trial studied over 300 pediatric patients at four different doses, administered twice daily, and was designed to determine the dosage for use in Phase III clinical trials. The clinical trial used a double tap design in which middle ear fluid was obtained both prior to and during treatment through tympanocentesis, then submitted for culture, which provided microbiologic confirmation of the effectiveness of faropenem medoxomil in eradicating bacteria from middle ear fluid. The study met its primary endpoint of generating sufficient data to permit dose selection for future Phase III clinical trials in acute bacterial otitis media in that there was a demonstrated dose response in bacteriological eradication of pathogens from the middle ear. All doses examined were well tolerated and there was no clear dose effect to tolerability. We believe that these study results will provide the information to permit dose selection in future Phase III clinical trials.
Regulatory Guidance for the Design of Future Clinical Studies for Treatment of Acute Bacterial Otitis Media. Our plans to initiate additional clinical trials using faropenem medoxomil for pediatric indications are dependent on our securing a partner for the faropenem medoxomil program. After both the assessment of Phase II clinical trial results and consultation with the FDA, we believe we are in a position to design Phase III clinical trials using an improved oral liquid formulation to support an NDA for acute bacterial otitis media in children. In January 2008, the FDA issued a Draft Guidance for Industry titled “Acute Bacterial Otitis Media: Developing Drugs for Treatment”. The Draft Guidance for Industry outlines the FDA’s recommendation that only superiority clinical trials are recommended for acute bacterial otitis media clinical studies. Superiority clinical trials may include double blinded, placebo-controlled studies with a background of optimized antimicrobial therapy, delayed versus immediate therapy, dose response using higher to lower doses and superiority of the study drug to another drug. Additionally, the Draft Guidance asserts the need for microbiologic confirmation of bacterial infections through tympanocentesis in at least one superiority clinical trial. The details of any study design will be determined through an interactive process with the FDA.
Methionyl tRNA Synthetase Inhibitor Program
Our methionyl tRNA synthetase inhibitor program includes REP3123, our investigational narrow spectrum antibacterial agent to treatC. difficile bacteria andC. difficile-associated disease, and REP8839, our topical antibiotic agent to treat skin and wound infections.
We acquired the worldwide rights to the methionyl tRNA synthetase inhibitor program from GlaxoSmithKline, or GSK, in June 2003 in exchange for 4,000,000 shares of our Series B convertible preferred stock at a deemed fair value of $1.25 per share and a final milestone payment of $1.5 million in June 2006. As part of this asset purchase, we acquired certain patents and patent applications and other program intellectual property, supporting material and related license rights. We retain the worldwide rights to this program and have no royalty or other ongoing financial obligations to GSK.
REP3123 and REP8839 are inhibitors of methionyl tRNA synthetase, an enzyme that plays an essential role in protein synthesis. Inhibition of methionyl tRNA synthetase results in reduced protein synthesis and attenuation of bacterial growth. REP3123 and REP8839 are members of a novel group of structurally-related molecules that selectively inhibit the activity of methionyl tRNA synthetase. Methionyl tRNA synthetase is a specific aminoacyl tRNA synthetase responsible for the attachment of the amino acid methionine to its cognate tRNA. Aminocyl tRNA synthetases are enzymes that play an essential role in protein biosyntheses by attaching
14
amino acids to specific carrier molecules, called tRNAs, that then carry the amino acid to the ribosome and donate it to the growing polypeptide chain.
Clostridium difficile Program. C. difficileis a spore-forming Gram-positive bacterium present in the intestinal tract. Toxin-producing strains ofC. difficilecan result inC. difficile-associated disease, or CDAD, that can manifest itself in a wide spectrum of clinical conditions, ranging from mild diarrhea to colitis (severe inflammation of the colon), where serious complications can occur. Oral vancomycin is the only antibiotic that is currently approved by the FDA for the treatment ofC. difficile-associated disease. Metronidazole is also used extensively in clinical practice following reports of its efficacy inC. difficile-associated disease. However, recent studies have noted relatively high and growing incidence of treatment failure and relapse following treatment using each of vancomycin and metronidazole therapy. Furthermore, widespread vancomycin use raises resistance concerns. As a result, there are limited clinical options for the treatment ofC. difficile-associated disease. In addition, there are few new drugs in development to treat this condition and a need exists for the development of new agents to address this emerging problem.
Patients taking antibiotics are at risk of developingC. difficile-associated disease. Antibiotics alter the normal flora in the intestinal tract, causing it to be more susceptible to overgrowth ofC. difficile. Another risk factor forC. difficile-associated disease is prolonged hospitalization. The spores ofC. difficilecan be extremely difficult to eradicate in the hospital setting and recentlyC. difficile infection has been associated with health care worker transmissions to patients not receiving antibiotics. People generally contractC. difficile-associated disease through the ingestion ofC. difficilespores after coming into contact with a contaminated item or surface. These spores then germinate, grow and multiply in the digestive tract. This factor is believed to contribute to the relapse rate of those treated forC. difficile-associated disease with current therapies of approximately15-20 percent.
As a result of these characteristics, in recent yearsC. difficile-associated disease has emerged as a significant health concern among elderly and hospitalized patients. In addition, the incidence and severity ofC. difficile-associated disease is increasing worldwide along with the emergence of epidemic strains ofC. difficilewith increased virulence. In U.S. hospitals, IMS Health estimate that there are more than 250,000 cases ofC. difficile-associated disease per year, prolonging hospital stays and associated health care costs. The health care costs for treatment of patients withC. difficile-associated disease have been estimated to exceed $1.1 billion annually.
We are developing REP3123, our investigational narrow spectrum antibacterial agent, to treatC. difficilebacteria andC. difficile-associated disease. Inin vitropreclinical studies, REP3123 has displayed an ability to inhibit growth of theC. difficilebacterium and prevent the bacterium from forming the spores that allow it to be spread from person to person, but without inhibiting other key organisms that are essential for normal intestinal functioning. Also, in preclinical studies, REP3123 exhibited signs it may be able to stop the production of destructive intestinal toxins caused byC. difficilebacteria. These results suggest that REP3123 has the potential to reduceC. difficile-associated disease outbreak and relapse rates through reducing the presence ofC. difficilespores and reduce the severity of, or possibly even prevent,C. difficile-associated disease through inhibiting the growth of or stopping production of toxins caused byC. difficilebacteria. We anticipate filing an IND with respect to a candidate from our REP3123 program before the end of 2008.
REP8839 Program. During 2007 we were developing REP8839 as a topical agent for the treatment of skin and wound infections, including MRSA infections. Our initial target indication was the treatment of impetigo, one of the most common skin infections among children. In December 2007 we announced that we had suspended further development work on REP8839 due to the incremental investment that would be required to optimize the formulation of REP8839 and the size of the initial target market being assessed. Preclinical studies have indicated that REP8839 exhibits potent activity against major skin pathogens such asS. aureusandS. pyogenes,including strains ofS. aureusthat are resistant to methicillin, vancomycin, linezolid or mupirocin.
15
DNA Replication Inhibitors Program.
DNA replication is one of the essential steps in bacterial growth. To reproduce and perpetuate an infection, bacteria must first replicate their DNA. Two copies must be made so that one can be passed to each daughter cell. DNA replication is a highly coordinated process. Inhibition of any step from the assembly of protein complexes to the dissociation of the replication machinery, offers the potential of interrupting bacterial growth and providing the basis for a new class of antibacterial drugs.
Despite the complexity of the replication system, we have developed robust high-throughput screening strategies through which we were able to identify compounds that interfere with the replication process.
We are advancing a lead series of novel DNA replication inhibitors identified from our proprietary compound collection. These inhibitors use a novel mechanism of action to block an essential step in the DNA replication process.
Based on current preclinical data, our lead series of compounds exhibits a novel mechanism of action that may block DNA replication and exhibits oral bioavailability and bactericidal activity against all major classes of antibiotic-resistant Gram-positive bacteria, including clinically-relevant resistant phenotypes such as methicillin-resistantS. aureus(MRSA), vancomycin-resistant enterococci (VRE) and penicillin-resistantS. pneumoniae(PRSP). Based on the preclinical results to date, we anticipate identifying an IND candidate from within our lead series before the end of 2008.
Research and Development Programs
Research and development expenditures made to advance our product candidates and other research efforts during the last three fiscal years were as follows (in thousands):
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2007 | | | 2006 | | | 2005 | |
| |
| Faropenem medoxomil | | $ | 29,231 | | | $ | 23,266 | | | $ | 24,744 | |
| REP8839 | | | 4,550 | | | | 8,363 | | | | 3,589 | |
| Other research and development | | | 9,532 | | | | 6,666 | | | | 847 | |
| | | | | | | | | | | | | |
| | | $ | 43,313 | | | $ | 38,295 | | | $ | 29,180 | |
| | | | | | | | | | | | | |
Our Former Collaboration with Forest Laboratories
In February 2006, we entered into a collaboration and commercialization agreement with Forest Laboratories to be our exclusive partner for the development and marketing of faropenem medoxomil in the U.S. On May 7, 2007, our collaboration and commercialization agreement with Forest Laboratories was terminated. This termination followed the issuance in October 2006 of a non-approvable letter by the FDA for our faropenem medoxomil NDA that was submitted to the FDA in December 2005. As a result of the termination, we reacquired all rights to faropenem medoxomil previously granted to Forest Laboratories. Under the agreement, we received $60 million in upfront and milestone payments and throughout the term of the agreement, we generated approximately $14.6 million of contract revenue for funded activities related to the development of faropenem medoxomil. There were no penalty fees incurred by either us or Forest Laboratories in connection with the termination of the agreement and no amounts previously received by us under the agreement are refundable.
Sales and Marketing
As a community antibiotic, faropenem medoxomil would be primarily marketed in the U.S. to primary care practitioners, which for adults, include family practice, general practice and internal medicine physicians, physician assistants and nurse practitioners and for children, include pediatricians and primary care practitioners. We do not anticipate building sales capabilities to serve the primary care or pediatric markets within the U.S. or outside the U.S. and will seek a partner with respect to these sales activities.
16
Our License Agreement with Asubio Pharma
We entered into a license agreement with Daiichi Suntory Pharma (now Daiichi Asubio Pharma Co., Ltd.) that was effective in March 2004. Under this agreement, we have an exclusive license to, with the right to sublicense, Asubio Pharma’s patent rights and know-how to develop and commercialize all forms of faropenem medoxomil for adult and pediatric use in the U.S. and Canada. The license includes rights to all clinical and other data related to faropenem medoxomil generated by Asubio Pharma and prior licensees, other than rights to manufacture faropenem.
We also have a sole negotiation right to develop and commercialize faropenem medoxomil in the rest of the world, excluding Japan, until two years following the commercial introduction of faropenem medoxomil in the U.S. or Canada. Our license does not include the rights to other forms of faropenem, such as faropenem sodium, but Asubio Pharma has agreed not to license or market any other form of faropenem for use in the U.S. or Canada.
In consideration for our licenses, we paid Asubio Pharma an initial license fee of $3.8 million comprised of $0.6 million paid in 2003 and $3.2 million paid in 2004. In December 2005, we submitted our first NDA for adult use of faropenem medoxomil and, at that time, we recorded research and development expense in the amount of $2.1 million for the first milestone due to Asubio Pharma under this agreement. In February 2006, in conjunction with our entering into the license agreement with Forest Laboratories, this milestone payment was increased to $3.2 million. The increased milestone amount of $1.1 million was accounted for as research and development expense in 2006 when the modified terms of the license were finalized. Under the modified license agreement we are further obligated to make future payments of (i) up to ¥375 million (approximately $3.3 million as of December 31, 2007) upon initial FDA approval, (ii) ¥500 million (approximately $4.5 million as of December 31, 2007) upon a product launch and (iii) up to ¥750 million (approximately $6.7 million as of December 31, 2007) in subsequent milestone payments for faropenem medoxomil. If we terminate our license agreement with Asubio Pharma, or if there is an intolerable delay in the commercial launch of faropenem medoxomil, as defined, we will be obligated to pay a termination fee of up to ¥375 million (approximately $3.3 million as of December 31, 2007). Additionally, we are responsible for royalty payments to Asubio Pharma based upon net sales of faropenem medoxomil.
Our license agreement with Asubio Pharma extends until the last relevant patent expires or 12 years after the first commercial sale of faropenem medoxomil in the territory, whichever is later. Each party has the right to terminate the agreement in the event of the bankruptcy or dissolution of the other party or a material breach of the agreement. We may also terminate the license agreement upon six months written notice in the event we conclude that development of faropenem medoxomil, preparation or submission of applications or registrations with respect thereto are to be canceled due to issues of safety or efficacy or it becomes no longer commercially reasonable to commercialize the product.
In periods after we or our licensee have marketed faropenem medoxomil in the U.S. for at least twelve months, if we substantially fail to meet our goals under our sales and marketing plan over a period of two years, then we must make certain payments to Asubio Pharma or Asubio Pharma may convert our license to a non-exclusive license, in which case we would be required to grant Asubio Pharma a license to use the information and know-how we have developed under this agreement.
Under certain circumstances, we may be required to make certain payments to Asubio Pharma upon termination of the agreement.
Manufacturing
We obtain the drug substance, or active pharmaceutical ingredient, faropenem medoxomil, from Nippon Soda Company Ltd., or Nippon Soda. As a penem antibiotic, faropenem medoxomil requires dedicated manufacturing facilities for the manufacture of drug substance and drug product. For many years, beta-lactams have been produced separately in segregated facilities due to concerns about allergic reactions to these types of antibiotics. During development, faropenem medoxomil was manufactured by Nippon Soda in a segregated
17
building at its Takaoka facility in Japan and Bayer manufactured the faropenem medoxomil tablet internally for its clinical studies.
In anticipation of commercial production, Nippon Soda expanded and equipped a new facility located in Nihongi, Japan. The Nihongi facility is presently being used for the manufacture of faropenem sodium for the Japanese market. Faropenem medoxomil is produced from faropenem sodium by converting it into an ester prodrug form. We have a requirements contract for the supply of faropenem medoxomil at the Nihongi facility. Nippon Soda is obliged to supply all of our requirements of faropenem medoxomil and we are obligated to purchase all faropenem medoxomil requirements from Nippon Soda. We have the right to transfer manufacturing to a third party, with Nippon Soda’s cooperation, if Nippon Soda cannot assure supply and in certain other circumstances. In the case of such a transfer, Nippon Soda will be required to grant us the necessary licenses, including the right to sublicense, under its intellectual property to manufacture faropenem medoxomil. Nippon Soda has patent protection for certain aspects of the manufacturing process through 2014. After a commercial launch of faropenem medoxomil, the parties have agreed to certain minimum purchase requirements and pricing. In accordance with our supply agreement with Asubio Pharma and Nippon Soda, as a result of the non-approvable letter received from the FDA in October 2006 and subsequent activities related to the development of faropenem medoxomil, we recorded delay compensation fees of $0.9 million in the year ended December 31, 2007 and delay compensation fees of $0.9 million and an initial order cancellation fee of $0.6 million in the year ended December 31, 2006. These amounts were recorded as research and development expense. If commercial launch of faropenem medoxomil is further delayed, we may incur additional delay compensation fees of up to ¥105 million ($0.9 million as of December 31, 2007) for 2008 and up to ¥280 million annually ($2.5 million as of December 31, 2007) for all periods following January 1, 2009. If we terminate this agreement, abandon the development or commercialization of faropenem medoxomil or are unable to notify Nippon Soda of the faropenem medoxomil launch go date, as defined, by July 1, 2009, we will be obligated to pay Nippon Soda prorated delay compensation fees through the effective date of termination and reimburse Nippon Soda for up to ¥65 million ($0.6 million as of December 31, 2007) in engineering costs. The term of this agreement is for the life of the Asubio Pharma patents on faropenem medoxomil or 12 years after commercial launch, whichever is longer. We believe that the capacity of this plant is sufficient to provide commercial quantities of faropenem medoxomil for the next several years.
In 2005, we and MEDA Manufacturing GmbH (formerly Tropon GmbH), or MEDA, entered into a supply agreement for production of finished 300 mg adult tablets of faropenem medoxomil, which was amended as to certain terms in 2006. Beginning in 2006, we became obligated to make annual minimum purchases of 300 mg adult tablets from MEDA of €2.3 million (approximately $3.4 million at December 31, 2007). If in any year we did not satisfy our minimum purchase commitments, we were required to pay MEDA the shortfall amount. Fifty percent (50%) of the shortfall amount, if applicable, may have been credited against future drug product purchases. We were required to buy all of our requirements for 300 mg adult oral faropenem medoxomil tablets from MEDA until cumulative purchases exceed €22 million (approximately $32.4 million at December 31, 2007). This agreement was amended in March 2006 such that our obligations with respect to all purchase commitments and facility decontamination costs were suspended and deemed satisfied by Forest Laboratories pursuant to an agreement between MEDA and Forest Laboratories. Under our agreement with Forest Laboratories, we remained liable for any shortfall amount in 2006 that may not have been credited against future drug product purchases. In 2006, we incurred $1.5 million relating to our portion of the 2006 shortfall in minimum purchases under these agreements. The amount was accounted for as research and development expense in 2006. In May 2007, concurrent with Forest Laboratories termination of its supply agreements with MEDA, the previously suspended provisions in our agreements with MEDA were no longer suspended and our obligations with respect to purchase commitments and facility decontamination costs were no longer waived. In April 2007, we provided notice to MEDA of our termination of the supply agreement in accordance with the termination provisions of the agreement as future clinical development of faropenem medoxomil adult tablets would use 600 mg dosing. As a result of this notice occurring before the termination date of our collaboration agreement with Forest Laboratories, and as Forest Laboratories, under the terms of the collaboration agreement, was responsible for supply chain management of faropenem medoxomil, including obligations under the MEDA agreement, through May 7, 2007 (the term of the collaboration agreement), we have not accrued for any minimum purchase or termination fees under this
18
agreement. MEDA has indicated that it disputes our right to terminate the agreement on the basis indicated in our notice of termination. We believe that we had the right to terminate the agreement. However, if it is determined that we have obligations to MEDA beyond May 7, 2007 under the agreement, then additional costs may be incurred which may include additional amounts for minimum future drug purchases that were not made and a termination fee for decontamination of MEDA’s facility of up to €1.7 million ($2.5 million as of December 31, 2007).
We have built a small scale drug product manufacturing facility at our Louisville, Colorado site. The facility is used for the manufacture of development batches (oral tablets and liquid suspensions) and for the manufacture of clinical supplies. The facility is dedicated exclusively for faropenem medoxomil manufacturing and will not be used for other product classes.
We currently have a small internal manufacturing group. For our discovery programs, we generally conduct research and development scale manufacturing in-house or use contract manufacturers. We use contract manufacturers for scale up of preclinical and clinical quantities of product. We anticipate using contract manufacturers for commercial scale quantities of product when this is commercially feasible.
Government Regulation and Product Approval
Regulation by governmental authorities in the U.S. and other countries is a significant factor in the development, manufacture and marketing of pharmaceuticals and antibiotics. All of our products will require regulatory approval by governmental agencies prior to commercialization. In particular, pharmaceutical drugs are subject to rigorous preclinical testing and clinical trials and other pre-marketing approval requirements by the FDA and regulatory authorities in other countries. In the U.S., various federal, and, in some cases, state statutes and regulations, also govern or impact the manufacturing, safety, labeling, storage, record-keeping and marketing of pharmaceutical products. The lengthy process of seeking required approvals and the continuing need for compliance with applicable statutes and regulations require the expenditure of substantial resources. Regulatory approval, if and when obtained for any of our product candidates, may be limited in scope, which may significantly limit the indicated uses for which our product candidates may be marketed. Further, approved drugs and manufacturers are subject to ongoing review and discovery of previously unknown problems that may result in restrictions on their manufacture, sale or use or in their withdrawal from the market.
Before testing any compounds with potential therapeutic value in human subjects in the U.S., we must satisfy stringent government requirements for preclinical studies. Preclinical testing includes bothin vitroandin vivolaboratory evaluation and characterization of the safety and efficacy of a drug and its formulation. Preclinical testing results obtained from studies in several animal species, as well as data fromin vitrostudies, are submitted to the FDA as part of an IND and are reviewed by the FDA prior to the commencement of human clinical trials. These preclinical data must provide an adequate basis for evaluating both the safety and the scientific rationale for the initial trials in human volunteers.
In order to test a new drug in humans in the U.S., an IND must be filed with the FDA. The IND will become effective automatically 30 days after receipt by the FDA, unless the FDA raises concern or questions about the conduct of the trials as outlined in the IND prior to that time. In this case, the IND sponsor and the FDA must resolve any outstanding concerns before clinical trials can proceed.
Upon request, the FDA will evaluate an SPA submitted by a sponsor company. An SPA evaluation must be specifically requested by a sponsor and be submitted for each specific protocol individually. The SPA submission should include the protocol detail, enough information for the FDA to assess the role of the protocol within the overall clinical development plan, supporting documentation, questions to the FDA from the sponsor and the specific regulatory action anticipated through the conduct of the study such as approval of an indication or a labeling claim. If the SPA is accepted for review, the FDA anticipates responding to the assessment within 45 days. However, if an FDA question or response requires the SPA to be revised, it is considered to be re-submitted thereby re-initiating the 45 day review period. FDA guidance documents suggest a 90 day total review period due to the anticipated need for revisions. If a clinical trial has commenced prior to an SPA being approved by the FDA, it will not qualify for SPA review.
19
Clinical trials are typically conducted in three sequential phases, Phases I, II and III, with Phase IV trials potentially conducted after initial marketing approval. These phases may be compressed, may overlap or may be omitted in some circumstances.
| | |
| | • | Phase I. After an IND becomes effective, Phase I human clinical trials may begin. These trials evaluate a drug’s safety profile and the range of safe dosages that can be administered to healthy volunteersand/or patients, including the maximum tolerated dose that can be given to a trial subject with the target disease or condition. Phase I trials also determine how a drug is absorbed, distributed, metabolized and excreted by the body and the duration of its action. |
| |
| | • | Phase II. Phase II clinical trials are typically designed to evaluate the potential effectiveness of the drug in patients and to further ascertain the safety of the drug at the dosage given in a larger patient population. |
| |
| | • | Phase III. In Phase III clinical trials, the drug is usually tested in one or more controlled, randomized trials comparing the investigational new drug to an approved form of therapy or placebo in an expanded and well defined patient population and at multiple clinical sites. The goal of these trials is to obtain definitive statistical evidence of safety and effectiveness of the investigational new drug regimen as compared to a placebo or an approved standard therapy in defined patient populations with a given disease and stage of illness. |
| |
| | • | Phase IV. Phase IV clinical trials are studies required of or agreed to by a sponsor that are conducted after the FDA has approved a product for marketing. These studies are used to gain additional experience from the treatment of patients in the intended therapeutic indication and to document a clinical benefit in the case of drugs approved under accelerated approval regulations. If the FDA approves a product while a company has ongoing clinical trials that were not necessary for approval, a company may be able to use the data from these clinical trials to meet all or part of any Phase IV clinical trial requirement. These clinical trials are often referred to as Phase III/IV post approval clinical trials. Failure to promptly conduct Phase IV clinical trials could result in withdrawal of approval for products approved under accelerated approval regulations. |
After completion of Phase I, II and III clinical trials, if there is substantial evidence that the drug is safe and effective, an NDA is prepared and submitted for the FDA to review. The NDA must contain all of the essential information on the drug gathered to that date, including data from preclinical and clinical trials, and the content and format of an NDA must conform to all FDA regulations and guidelines. Accordingly, the preparation and submission of an NDA is a significant undertaking for a company.
The FDA reviews all submitted NDAs before it accepts them for filing and may request additional information from the sponsor rather than accepting an NDA for filing. In this case, the NDA must be re-submitted with the additional information and, again, is subject to review before filing. Once the submission is accepted for filing, the FDA begins an in-depth review of the NDA. Most NDAs are reviewed by the FDA within 10 months of submission. The review process is often significantly extended by the FDA through requests for additional information and clarification. The FDA may refer the application to an appropriate advisory committee, typically a panel of clinicians, for review, evaluation and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendation but typically considers it strongly. If the FDA evaluations of both the NDA and the manufacturing facilities are favorable, the FDA may issue either an approval letter or an approvable letter, the latter of which usually contains a number of conditions that must be satisfied in order to secure final approval. If the FDA’s evaluation of the NDA submission or manufacturing facility is not favorable, the FDA may refuse to approve the NDA or issue a non-approvable letter.
Any products we manufacture or distribute under FDA approvals are subject to pervasive and continued regulation by the FDA, including record-keeping requirements and reporting of adverse experiences. Drug manufacturers and their subcontractors are required to register with the FDA and, where appropriate, state agencies, and are subject to periodic unannounced inspections by the FDA and state agencies for compliance
20
with cGMP regulations which impose procedural and documentation requirements upon us and any third party manufacturers we utilize.
The FDA closely regulates the marketing and promotion of drugs. A company can make only those claims relating to safety and efficacy that are approved by the FDA. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties. Physicians may prescribe legally available drugs for uses that are not described in the product’s labeling and that differ from those tested by us and approved by the FDA. Such off-label uses are common across medical specialties. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, restrict manufacturer’s communications on the subject of off-label use.
The FDA’s policies may change and additional government regulations may be enacted that could prevent or delay regulatory approval of our product candidates or approval of new indications after the initial approval of our existing products. We cannot predict the likelihood, nature or extent of adverse governmental regulations that might arise from future legislative or administrative action, either in the U.S. or abroad.
We will also be subject to a wide variety of foreign regulations governing the development, manufacture and marketing of our products. Whether or not FDA approval has been obtained, approval of a product by the comparable regulatory authorities of foreign countries must still be obtained prior to manufacturing or marketing the product in those countries. The approval process varies from country to country and the time needed to secure approval may be longer or shorter than that required for FDA approval. We cannot assure you that clinical trials conducted in one country will be accepted by other countries or that approval in one country will result in approval in any other country.
The Drug Price Competition and Patent Term Restoration Act of 1984, also known as the Hatch-Waxman Act, provides five years of “new chemical entity,” or NCE, marketing exclusivity, to the first applicant who obtains approval of an NDA for a product that does not contain an active ingredient found in any other FDA approved product. If the FDA approves our NDA for faropenem, we will likely be entitled to five years of NCE exclusivity for faropenem. This exclusivity period would not prevent the submission by a generic competitor of an abbreviated new drug application, or by a branded competitor of a new drug application under Section 505(b)(2) of the Federal Food, Drug and Cosmetic Act, for a compound that contains faropenem medoxomil as the active ingredient as early as four years following the FDA’s approval of our NDA for faropenem medoxomil. Such a competitor would likely be required to conduct clinical trials to bring a faropenem medoxomil product, other than faropenem medoxomil, to market in the U.S., though the competitor may be able to rely in part on the FDA’s prior findings of safety and efficacy of faropenem. Similarly, data exclusivity in Europe provides a period of up to 10 years from the date a product is granted marketing approval, during which the regulatory authorities are not permitted to cross-refer to the data submitted by the original applicant for approval when reviewing an application from a generic manufacturer of the same approved product. Data exclusivity does not prevent a generic manufacturer from filing for regulatory approval of the same or similar drug, even in the same indication for which that drug was previously approved in Europe, based upon data generated independently by that manufacturer.
Intellectual Property
The proprietary nature of, and protection for, our product candidates, processes and know-how are important to our business. We seek patent protection in the U.S. and internationally for our product candidates and other technology. Our policy is to patent or in-license the technology, inventions and improvements that we consider important to the development of our business. In addition, we use license agreements to selectively convey to others rights to our own intellectual property. We also rely on trade secrets, know-how and continuing innovation to develop and maintain our competitive position. We cannot be sure that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications filed by us in the future, nor can we be sure that any of our existing patents or any patents granted to us in the future will be commercially useful in protecting our technology.
21
We have licensed two U.S. patents from Asubio Pharma covering the faropenem medoxomil composition of matter and a process for making faropenem medoxomil. Both of these patents expire on November 3, 2015. The Canadian equivalent of these patents expires in August 2011. The U.S. and Canadian patents are licensed to us and we have the sole negotiation right to license such rights in the rest of the world, excluding Japan. We believe that patent term extension under Hatch-Waxman Act should be available to extend our patent exclusivity for faropenem medoxomil to at least 2020 in the U.S. We plan to pursue development of alternative formulations of faropenem medoxomil, such as a pediatric formulation. We have not controlled and do not control the prosecution of the patents licensed from Asubio Pharma. We cannot be certain that such prosecution efforts have been or will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents.
Asubio Pharma also owns patents related to faropenem sodium composition of matter that expire in 2008 in the U.S. and have expired in the rest of the world. We do not have a license to the faropenem sodium patents but our agreement with Asubio Pharma specifies that it will not license any form of faropenem for use in the U.S. or Canada.
We acquired worldwide rights to the methionyl tRNA synthetase inhibitor program from GSK in June 2003. Our agreement with GSK included the assignment of patents and patent applications to us relating to small molecule methionyl tRNA synthetase inhibitors and the targets initially used to identify the inhibitors. We have filed additional patent applications directed to small molecule methionyl tRNA synthetase, uses, production methods and the like. We have two issued U.S. patents that cover REP8839 and additional patent applications directed to REP8839 and combinations of REP8839 and mupirocin. As of December 31, 2006, we have 13 issued U.S. patents, 13 pending U.S. patent applications, 1 issued foreign patent and 30 pending foreign patent applications related to the methionyl tRNA synthetase programs including the REP8839 program. These patents expire from 2017 to 2025.
We have filed 4 pending U.S. patent applications, two provisional patent applications, and four pending foreign patent applications directed to composition of matter and methods of use related to our REP3123 program that expire in 2027.
We have begun to file patent applications directed to compounds that inhibit DNA replication that have been identified through our in-house screening efforts. We also own a portfolio of patents related to the DNA replication targets and drug screening methods to identify inhibitors of DNA replication. As of December 31, 2007, we have 1 issued U.S. patent, 8 pending U.S. patent applications, 3 issued foreign patents and 16 pending foreign patent applications related to our bacterial DNA replication program. These patents expire from 2021 to 2027.
Competition
The oral anti-infective marketplace has traditionally been one of the most competitive within the pharmaceutical industry due to the large number of products competing for market share and significant levels of commercial resources being utilized to promote brands. In addition, our ability to compete may be affected because in some cases insurers and other third-parties may seek to encourage the use of generic products. This may have the effect of making branded products less attractive, from a cost perspective, to buyers. Among the products with which we will directly compete, we expect to differentiate on the basis of greater potency, improved resistance profile, enhanced safety and tolerability. Although we expect to face competition in the future, we do not expect the level of competition from branded products to be as intense as it has been in prior years due to the recent and ongoing exclusivity expiration of many major brands. Furthermore, we believe the pipeline of new oral antibiotics to treat community-acquired respiratory tract infections in development is weak, with a limited number of products currently in Phase III development. Several pharmaceutical and biotechnology companies are actively engaged in research and development related to new generations of antibiotics. We cannot predict the basis upon which we will compete with new products marketed by others. Many of our competitors have substantially greater financial, operation, sales and marketing and research and development resources than we have.
22
Employees
As of December 31, 2007, we had 53 full time employees, 23 of whom hold Ph.D., M.D. or Pharm.D. degrees. Of our total employees, 28 were engaged in discovery research, 9 in clinical and regulatory affairs, 5 in commercial and corporate development and 11 in support administration, including finance, information systems, facilities and human resources. We consider our relationship with our employees to be good.
Corporate Information
We were incorporated under the laws of the state of Delaware on December 6, 2000. Our principal executive offices are located at 1450 Infinite Drive, Louisville, Colorado 80027, and our telephone number is(303) 996-5500. Our web site address ishttp://www.replidyne.com. The information contained in, or that can be accessed through, our website is not part of this report and should not be considered part of this report.
Web Availability
We make available free of charge through our web site,www.replidyne.com, our annual report onForm 10-K and other reports required under the Securities Exchange Act of 1934, as amended, as soon as reasonably practicable after such reports are filed with, or furnished to, the Securities and Exchange Commission (the “SEC”). These documents are also available through the SEC’s website atwww.sec.gov. Certain of our corporate governance policies, including the charters for the Board of Directors’ audit, compensation and nominating and corporate governance committees and our code of ethics, corporate governance guidelines and whistleblower policy can be found at our website. We will provide to any person without charge, upon request, a copy of any of the foregoing materials. Any such request must be made in writing to Replidyne, Inc., 1450 Infinite Drive, Louisville, CO 80027, Attn: Investor Relations.
You should carefully consider the risks described below, which we believe are the material risks of our business. Our business could be harmed by any of these risks. The trading price of our common stock could decline due to any of these risks, and you may lose all or part of your investment. In assessing these risks, you should also refer to the other information contained in our SEC filings, including our financial statements and related notes. Additional risks and uncertainties not presently known to us or that we currently deem immaterial also may impair our business operations. We are relying upon the safe harbor for all forward-looking statements in this annual report, and any such statements made by or on behalf of the Company are qualified by reference to the following cautionary statements, as well as to those set forth elsewhere in this Report.
Risks Related to our Business
We have received both a non-approvable letter and a Warning Letter from the FDA for our NDA filed in December 2005 for faropenem medoxomil, our most advanced product candidate, and we are currently evaluating our development program for faropenem medoxomil and do not currently know if faropenem medoxomil will ever receive regulatory approval, which is necessary before it can be commercialized.
If we do not receive regulatory approval for faropenem medoxomil and we are not able to commercialize faropenem medoxomil, we will not generate revenue for several years, if at all, and we may never generate sufficient revenue to achieve and sustain profitability. We need approval from the FDA prior to marketing our product candidates in the U.S. In December 2005, we submitted our first NDA to the FDA for use of faropenem medoxomil in four adult clinical indications. In October 2006, the FDA issued a non-approvable letter for all four indications in our NDA and recommended further clinical studies and microbiologic evaluation for all indications. We are in the planning stages with respect to our faropenem medoxomil clinical trials program and have only a single ongoing clinical trial, which clinical trial is studying the use of faropenem medoxomil for treatment of acute exacerbation of chronic bronchitis. Further clinical development of faropenem medoxomil for any indications will require us to complete additional and more extensive clinical trials, which will be costly and time consuming. Such further development will require that we address the
23
items identified in the Warning Letter described below to the FDA’s satisfaction, which results have not been reviewed to date. The amount and timing of the increased costs related to our clinical trials is difficult to predict due to the uncertainty inherent in the timing of clinical trial initiations, the rate of patient enrollment and the novel design of future trials. However, we expect that at least two to three years will be required to complete additional clinical trials. If we continue our clinical development program for faropenem medoxomil, we may not obtain necessary approvals from the FDA even if our trials demonstrate the effectiveness of faropenem medoxomil for any indication. The data we collect from any additional clinical trials with larger patient populations may not demonstrate sufficient safety and efficacy to support regulatory approval of faropenem medoxomil, in which case we would experience potentially significant delays in, or be required to abandon, development of that product candidate. If we continue our clinical development program for faropenem medoxomil, we will have fewer resources to devote to the research and development of other potential product candidates and development stage programs. If we decide to terminate any further development of faropenem medoxomil, we will be dependent upon the success of the other product candidates in our pipeline or other compounds we may in-license and the size of the potential markets for such other product candidates may not be as significant as the potential markets for faropenem medoxomil. All of our other existing product candidates and development stage programs are in Phase I clinical development or preclinical development.
On January 22, 2008, we received a Warning Letter from the Division of Scientific Investigation of the FDA, or DSI, informing us of objectionable conditions found during its investigation of our role as applicant for our NDA for faropenem medoxomil. The FDA’s observations were based on its establishment inspection reports following on site inspections in conjunction with the FDA’s review of our NDA. Specifically the DSI cited that we failed to make available the underlying raw data from the investigation for the FDA’s audit and failed to provide the FDA adequate descriptions and analyses of any other data or information relevant to the evaluation of the safety and effectiveness of faropenem medoxomil obtained or otherwise received by us from any source derived from clinical investigations. The clinical trials that supported our NDA were conducted by Bayer as a previous licensee of faropenem medoxomil. If we are unable to sufficiently establish that the clinical trials used in our NDA were conducted in accordance with FDA regulations, we may be subject to enforcement action by the FDA or required to complete additional trials before we are able to obtain FDA approval for faropenem medoxomil, which would be costly and time consuming and could potentially result in the termination of further development of faropenem medoxomil and cause us not to be able to enter our other product candidates into clinical trials.
Even if we obtain FDA approval for faropenem medoxomil, it may not cover all of the clinical indications for which we seek approval. Also, an approval might contain significant limitations with respect to conditions of use in the form of narrow indications, incomplete activity against key bacterial pathogens, warnings, precautions or contra-indications. We cannot predict if or when we might again seek regulatory review of faropenem medoxomil for any indication or of any of our other product candidates.
The FDA has substantial discretion in the approval process and may either refuse to accept an application for substantive review or may conclude after review of our data that our application is insufficient to allow approval of a product candidate. If the FDA does not accept or approve our application, it may require that we conduct additional clinical, preclinical or manufacturing validation studies and submit that data before it will reconsider our application. Depending on the extent of these or any other studies, approval of any application that we submit may be delayed by several years, or may require us to expend more resources than we have available. It is also possible that additional studies, if performed and completed, may not be considered sufficient by the FDA to approve our application for any particular indication for which we are seeking approval. In addition, the FDA has and is likely to continue to seek the advice of experts on specific topics by convening advisory committees from time to time. In April 2008, the FDA is scheduled to convene an Anti-Infective Drug’s Advisory Committee to discuss the issues relating to the identification of an appropriate noninferiority margin for an active controlled clinical trials for the treatment of community-acquired pneumonia. If the Advisory Committee were to recommend a noninferiority margin within the noninferiority margins contemplated in our clinical trials planning for this indication, or the adoption of superiority studies in the indication, we would have to assess the cost required to complete these expanded or novel clinical trials. As
24
our clinical trials program for community respiratory tract infections may be anchored in community-acquired pneumonia, this recommendation, if adopted by the FDA, would significantly increase the costs of developing faropenem medoxomil for the treatment of this indication. If any of these outcomes occur, we may be forced to abandon our application for approval, which might cause us to cease development of faropenem medoxomil.
Faropenem medoxomil has been in-licensed from another pharmaceutical company, Asubio Pharma Co., Ltd., or Asubio Pharma. A previous licensee, Bayer AG, or Bayer, completed extensive preclinical studies and Phase II and Phase III clinical trials for a particular dosage of faropenem medoxomil. We may rely on some of the data from these preclinical studies and clinical trials in a future application or submission to the FDA for approval to market faropenem medoxomil. We may seek to rely on some of the data from these preclinical studies and clinical trials in a future application or submission to the FDA for approval to market faropenem medoxomil. If we are unable to address the items identified in the Warning Letter received from the FDA or there are any problems with these previous preclinical studies or clinical trials, including problems with the design or statistical analysis of such pre-clinical studies or clinical trials, this could cause our application for regulatory approval to be delayed or rejected, in which case we might need to conduct additional trials.
Because of the termination of our collaboration with Forest Laboratories to develop and commercialize faropenem medoxomil, we are seeking a new partner. If we do not obtain a new partner on acceptable terms, we likely will not be able to develop and commercialize faropenem medoxomil for adult or pediatric indications or generate any future revenue from faropenem medoxomil.
On May 7, 2007, Forest Laboratories exercised their right to terminate our development and commercialization agreement, under which Forest Laboratories had been granted an exclusive sublicense for the development and sale of faropenem medoxomil for all indications in the U.S. and a right of first refusal to extend the territory to include Canada. As a result of the termination we have reacquired all rights to faropenem medoxomil previously granted to Forest Laboratories.
We are currently seeking another partner or partners to assist us in the development and commercialization of faropenem medoxomil. We face competition in our search for partners with whom we may collaborate. Further, faropenem medoxomil has previously been licensed to other licensees who have opted not to develop and commercialize the product. As a result, we may not be successful in finding another partner on acceptable terms, or at all, and any failure to obtain a new partner on acceptable terms may adversely affect faropenem medoxomil development, commercialization and potential future sales. Identifying a new partner and entering into a collaboration agreement with it could cause delays in obtaining regulatory approvals and commercializing faropenem medoxomil, which would negatively impact our business. If we are delayed or do not identify a new partner for the development and commercialization of faropenem medoxomil, we will not commence further clinical trials of it beyond the ongoing clinical trial for the treatment of acute exacerbation of chronic bronchitis and may choose to halt our ongoing clinical trial for the exacerbation of chronic bronchitis. If it is determined that we have ceased development or commercialization of faropenem medoxomil or are unable to notify Nippon Soda of the faropenem medoxomil launch go date, as defined, by July 1, 2009 in accordance with the definitions contained in our license and supply agreements with Asubio Pharma and Nippon Soda Company Ltd., or Nippon Soda, we will incur a license termination fee of ¥375 million (approximately $3.3 million as of December 31, 2007), prorated delay compensation fees to Nippon Soda through the effective date of termination and reimburse Nippon Soda for up to ¥65 million ($0.6 million as of December 31, 2007) in engineering costs.
If we fail to enter into new strategic collaborations, we may have to reduce or delay our rate of product development and commercialization and/or increase our expenditures.
Our business model is based in part upon entering into strategic collaborations for discoveryand/or development of some of our product candidates. Our strategy to develop and commercialize our products includes entering into various relationships with pharmaceutical or biotechnology companies to advance our programs. We may not be able to negotiate any of our collaborations on acceptable terms. If we are not able to establish collaborative arrangements, we may have to reduce or delay further development of some of our
25
programsand/or increase our expenditures and undertake the development activities at our own expense. If we are not able to establish and maintain strategic collaborations on acceptable terms:
| | |
| | • | the development of our current or future product candidates may be reduced in scope, terminated or delayed which would require us to further reduce the number of our employees; |
| |
| | • | our cash expenditures related to development of our current or future product candidates would increase significantly; |
| |
| | • | we may be required to hire additional employees or otherwise develop expertise, such as sales and marketing expertise, for which we have not budgeted; |
| |
| | • | we will bear all of the risk related to the development of each of our current and future product candidates; and |
| |
| | • | we may be unable to meet demand for any future products that we may develop. |
In this event, we would likely be required to limit the size or scope of one or more of our programs.
Securing a strategic partner to develop and commercialize our product candidates may require us to relinquish valuable rights and will render us dependent on the efforts of any future partners, over which we would have limited control, and if our collaborations are unsuccessful, our potential to develop and commercialize product candidates and to generate future revenue from our product candidates would be significantly reduced.
In order to secure a strategic partner to develop and commercialize our product candidates, we may be required to relinquish valuable rights to our potential products or proprietary technologies. If we are able to identify and reach agreement with collaborators for our product candidates, those relationships will be subject to a number of risks, including:
| | |
| | • | collaborators may not pursue further development and commercialization of compounds resulting from collaborations or may elect not to renew research and development programs; |
| |
| | • | collaborators may delay clinical trials, under fund a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials, or require the development of a new formulation of a product candidate for clinical testing; |
| |
| | • | a collaborator with marketing and distribution rights to one or more of our product candidates may not commit sufficient resources to the marketing and distribution of any future products, limiting our potential revenues from the commercialization of these products; |
| |
| | • | disputes may arise delaying or terminating the research, development or commercialization of our product candidates, or result in significant litigation or arbitration; |
| |
| | • | strategic partners could develop drugs which compete with our future products, if any; |
| |
| | • | strategic partners could turn their focus away from anti-infective products and community respiratory tract infection indications; |
| |
| | • | strategic partners could fail to effectively manage manufacturing relationships with suppliers; |
| |
| | • | contracts with strategic partners may not provide significant protection or may be difficult to enforce if a strategic partner fails to perform; and |
| |
| | • | if an arrangement with a strategic partner expires or is terminated, we may not be able to replace it or the terms on which we replace it may be unacceptable. |
If as a result of our financial condition or other factors we enter into a strategic collaboration while a drug candidate program is in early preclinical development, we may not generate as much near or long-term revenue from such program as we could have generated if we had the resources to further independently develop such program. In addition, if we raise additional funds through licensing arrangements, it may be
26
necessary to relinquish potentially valuable rights to our potential products or proprietary technologies, or grant licenses on terms that are not favorable to us.
The type of trials that the FDA is recommending for faropenem medoxomil will be novel in design without formally approved guidance and may require alternative dosing regimens.
In the non-approval letter we received in October 2006, the FDA indicated that it recommends conducting additional large-scale clinical trials at alternate doses for all indications covered by our NDA, including superiority designed studies, which will be costly, difficult and time consuming to conduct. All efficacy studies upon which our NDA was based were designed as non-inferiority studies. In addition, dosages of 300 mg, twice per day, used in these studies were determined by the prior licensee of faropenem medoxomil, Bayer. Historically, the FDA and foreign regulatory authorities have not required superiority studies, such as placebo-controlled clinical trials, for approval of antibiotics but instead have relied on non-inferiority studies. In a non-inferiority study, a drug candidate is compared with an approved antibiotic and it must be shown that the drug product candidate is not less effective than the approved treatment within a defined non-inferiority margin. In a superiority study, a drug candidate is compared either with an approved antibiotic treatment or placebo and it must be shown that the drug candidate is more effective than the approved treatment or placebo, as the case may be. Although the FDA has indicated that superiority designed trials will be required for some indications and has issued Draft Guidances to Industry regarding acute bacterial sinusitis and acute bacterial otitis media, there is no formally approved guidance on the design of these studies.
Conducting placebo-controlled trials for antibiotics is expected to be time consuming and expensive and can be difficult to complete. Institutional review boards may not grant approval for placebo-controlled trials because of ethical concerns about denying some participating patients access to any antibiotic therapy during the course of the trial. It may be difficult to enroll patients in placebo-controlled trials even if institutional review board approval is obtained because certain patients would receive no or delayed therapy during the course of the trial. Although we are currently conducting a placebo-controlled trial for acute exacerbation of chronic bronchitis, we have not completed any placebo-controlled trials for faropenem medoxomil for any indications. We may not be able to show a statistically significant advantage over placebo or another control treatment in any trials that we are able to complete. These factors could delay for several years or ultimately prevent commercialization of faropenem medoxomil for any indications for which the FDA requires superiority designed trials. Demonstration of superiority of a drug candidate over an approved antibiotic is likely to be difficult and require a large number of patients because clinical success rates for most approved antibiotics that would serve as appropriate comparisons are high, typically 70% to 90%.
If we choose, after discussion with the FDA, to pursue additional clinical trials in an effort to gain approval from the FDA for faropenem medoxomil, then our ongoing development programs for faropenem medoxomil will be lengthy and expensive. The amount of time and cost associated with these trials are difficult to predict due to the uncertainty inherent in the timing of clinical trial initiations, the rate of patient enrollment and details of future trial designs. In addition, the guidance we receive from the FDA in future meetings with them will influence the number, size and duration of planned and unplanned trials. Even if clinical trials show our product candidates to be safe and effective in treating their target conditions, we do not expect to be able to record commercial sales of any of our product candidates until at least 2011. Even if we conduct these trials in accordance with FDA recommendations and achieve protocol defined end points, faropenem medoxomil may not be approved.
Further delays in clinical testing or approval could result in increased costs to us and delay our ability to generate revenue.
We may experience delays in clinical testing of our product candidates. We currently plan to limit our faropenem medoxomil clinical trial activities to completion of the ongoing Phase III placebo-controlled clinical trial for treatment of acute exacerbation of chronic bronchitis while we seek a partner for the faropenem medoxomil program. Even in this trial, we temporarily stopped enrollment to exclude Ketek®. We had included Ketek as a comparator in the clinical trial to generate secondary data points versus a product projected to be a competitor product to faropenem medoxomil. We based our decision to exclude Ketek on the
27
findings of a joint Advisory Meeting of the FDA’s Anti-Infective Drug and Drug Safety and Risk Management committees held on December 14 and 15, 2006 that recommended to the FDA that the risks of using Ketek outweigh the benefits of using the drug for treatment of acute exacerbation of chronic bronchitis. This recommendation was adopted by the FDA on February 12, 2007. Following required communication with investigational review boards overseeing the clinical trial sites, we re-initiated this trial without the Ketek comparator arm in February 2007. We do not know whether potential future clinical trials will begin on time, will need to be redesigned or will be completed on schedule, if at all. Clinical trials can be delayed for a variety of reasons, including delays in obtaining regulatory approval to commence a trial, in reaching agreement on acceptable clinical trial terms with prospective sites, in obtaining institutional review board or ministry of health approval at each site or country in which we seek to conduct clinical trials, in recruiting patients to participate in a trial, or in obtaining sufficient supplies of clinical trial materials. Many factors affect patient enrollment, including the size and nature of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the trial, the design of the clinical trial, competing clinical trials, clinicians’ and patients’ perceptions as to the potential advantages of the drug being studied in relation to other available therapies, including any new drugs that may be approved for the indications we are investigating, and whether the clinical trial design involves comparison to placebo. Our antibiotics treat bacterial infections which tend to be seasonal in nature. As a result, during certain times of the year, it is difficult to find patients to enroll in our trials. Prescribing physicians would also face ethical issues associated with enrolling patients in clinical trials of our product candidates over existing antibiotics that have established safety and efficacy profiles or in placebo-controlled trials. These ethical issues may be even more pronounced in conducting clinical trials of antibiotics in children. Any delays in completing our clinical trials will increase our costs, slow down our product development and approval process and delay our ability to generate revenue or seek approval of faropenem medoxomil.
The success of our strategy to identify a new partner for the faropenem medoxomil program will depend in part on our ability to obtain FDA regulatory clarity for the process of developing an oral liquid formulation of faropenem medoxomil for pediatric use.
The development of faropenem medoxomil for pediatric use is an important component of the faropenem medoxomil program. We have developed a prototype oral liquid formulation, completed a Phase II clinical trial in acute bacterial otitis media (middle ear infection) and are considering the design of future studies in acute bacterial otitis media and tonsillitis/pharyngitis. Our ability to identify a new partner for this product candidate for pediatric use is subject to various risks, including the following:
| | |
| | • | It is unusual for the FDA to approve a drug for pediatric use that has not been approved for adult use. As a result, in the event that we abandon further development of faropenem medoxomil for adult use, it may be difficult to obtain FDA approval for a pediatric indication. |
| |
| | • | In January 2008, the FDA issued a Draft Guidance for Industry titled “Acute Bacterial Sinusitis: Developing Drugs for Treatment” that described clinical trial design for treatment of acute bacterial otitis media in pediatric patients. By the terms of the FDA Authorization Act of 2007, the FDA is required to issue final approval guidelines for developers of antibiotics for this indication within twelve months of its enactment, or approximately September 2008. Delays in understanding the pediatric clinical trials program required for the approval of faropenem medoxomil for treating pediatric patients could result in our inability to identify a partner for this program and could delay initiation of pivotal clinical trials, its potential commercial launch and our ability to generate future revenue. |
| |
| | • | In January 2008, we received a Warning Letter from the FDA in connection with the NDA we filed for faropenem medoxomil in December 2005. If we are unable to address the conditions identified in the Warning Letter, we may not be able to use clinical data contained in the NDA to support future clinical trials of faropenem medoxomil in pediatric patients which would result in significant delays in the faropenem medoxomil pediatric development program. |
| |
| | • | Preclinical testing and clinical trials are protracted, expensive and uncertain processes. It may take us and any future collaboration partner several years to complete the testing and trials, and failure can |
28
| | |
| | | occur at any stage of the process. Success in preclinical testing and early clinical trials does not ensure that later clinical trials will be successful. These risks are potentially more pronounced in clinical tests involving children. |
| | |
| | • | We have completed only one Phase II clinical trial in children with acute bacterial otitis media to date and this clinical trial was not completed under the U.S. IND for faropenem medoxomil. A clinical trial conducted by Bayer for tonsillitis/pharyngitis in adults and adolescents did not meet its primary endpoint. |
| |
| | • | Any NDA or other marketing authorization applications that we may file might be denied by the FDA and analogous foreign regulators. |
| |
| | • | Any regulatory approval we ultimately obtain may be limited or subject to post-approval commitments that render the product not commercially viable. |
| |
| | • | This product candidate, even if found to be safe and effective, might be difficult to develop into a commercially viable drug or to manufacture on a large scale. It may also prove to be economically unfeasible to market commercially. |
| |
| | • | Competitors may develop and market superior drugs or be more effective in marketing equivalent drugs. |
| |
| | • | Even if this product candidate is successfully developed and effectively marketed, the size of the market may be smaller than expected or may decrease over time, such that our sales revenue is less than initially contemplated. |
Any failure to obtain regulatory approval of faropenem medoxomil for pediatric use would have a material and adverse impact on our ability to successfully partner the faropenem medoxomil program and would significantly reduce the revenues that we might generate from faropenem medoxomil.
All of the Phase III clinical trials of faropenem medoxomil included in our NDA submitted in December 2005 were conducted using a 300 mg, twice per day, dose. We expect that future clinical trials will be conducted at the 600 mg, twice per day, dose. If the incidence of adverse events from use of faropenem medoxomil at the 600 mg, twice per day, dose is significantly higher than that observed in completed clinical studies at the 300 mg, twice per day, dose we may not be able to generate future revenue from faropenem medoxomil.
The Phase III clinical trials included in our December 2005 NDA were all conducted using a 300 mg, twice per day, dose. The dose was selected by the previous licensee of faropenem medoxomil. We expect that future clinical trials will be conducted at the alternate 600 mg, twice per day, dose. In January 2006, we initiated a Phase III clinical trial for the acute exacerbation of chronic bronchitis indication using the higher dose. We have previously evaluated the potential for adverse events with the 600 mg, twice per day, dose in a Phase I study and a Phase II study conducted in 2005. In the Phase I study, the 600 mg, twice per day, dose was directly compared to a 300 mg, twice per day, dose, both administered for seven days. In the Phase II study, a 600 mg, twice per day, dose for five day treatment course was compared to a 300 mg, twice per day, dose seven day treatment courses in patients with acute bacterial sinusitis. In both trials, the adverse events were similar in both type and frequency. If there is an increased level of adverse events observed for faropenem medoxomil 600 mg, twice per day as compared to 300 mg, twice per day, it will likely reduce future potential product revenue from faropenem medoxomil.
We have limited experience in acquiring or in-licensing product candidates, and integrating third parties’ products, businesses and technologies into our business infrastructure. If we determine that future acquisition or in-licensing opportunities are desirable and do not successfully execute on and integrate such targets, we may incur costs and disruptions to our business and we may be unable to grow our business.
A key element of our strategy is to acquire or in-license product candidates and integrate third party products, businesses and technologies into our business infrastructure. These efforts include potential licensing
29
and acquisition transactions. To date, we have in-licensed rights to each of our product candidates. In addition to our internal drug development efforts, we may seek to expand our product pipeline and technologies by acquiring or in-licensing products, businesses or technologies that we believe are a strategic fit with our business and complement our existing product candidates, research programs and technologies.
If we decide not to pursue the development of faropenem medoxomil for any or all indications, then we may devote substantial additional time and energy to the pursuit of strategic opportunities, including potential licensing and acquisition transactions. These transactions may include new anti-infective products or product candidates as well as products or product candidates outside of the anti-infective area. The success of this strategy depends upon our ability to identify, select and acquire the right pharmaceutical product candidates and products on terms that are acceptable to us. Proposing, negotiating and implementing an economically viable product acquisition or license is a lengthy and complex process. Other companies, including those with substantially greater financial, marketing and sales resources, may compete with us for the acquisition or license of product candidates and approved products. We may not be able to acquire or license the rights to additional product candidates and approved products on terms that we find acceptable, or at all.
Any product candidate we license or acquire may require additional development efforts prior to commercial sale, including extensive clinical testing and approval by the FDA and applicable foreign regulatory authorities. All product candidates are prone to the risks of failure inherent in pharmaceutical product development, including the possibility that the product candidate will not be shown to be sufficiently safe and effective for approval by regulatory authorities. In addition, we cannot ensure that any such products that are approved will be manufactured or produced economically, successfully commercialized or widely accepted in the marketplace.
In addition, future acquisitions may entail numerous operational and financial risks including:
| | |
| | • | exposure to unknown liabilities; |
| |
| | • | disruption of our business and diversion of our management’s time and attention to the development of acquired products or technologies; |
| |
| | • | incurrence of substantial debt or dilutive issuances of securities to pay for acquisitions; |
| |
| | • | higher than expected acquisition and integration costs; |
| |
| | • | difficulties in and costs of combining the operations and personnel of any acquired businesses with our operations and personnel; |
| |
| | • | impairment of relationships with key suppliers or customers of any acquired businesses due to changes in management and ownership; and |
| |
| | • | inability to retain key employees of any acquired businesses. |
Finally, we may devote resources to potential acquisitions or in-licensing opportunities that are never completed or fail to realize the anticipated benefits of such efforts.
Our drug discovery approach and technologies and our product candidates other than faropenem medoxomil are unproven and in very early stages of development, which may not allow us to establish or maintain a clinical development pipeline or successful collaborations, and may never result in the discovery or development of commercially viable products.
Because we do not currently know when or if we will continue clinical development of faropenem medoxomil for certain adult indications or any other indications, we are more dependent on the potential success of our internal discovery research programs and product candidates other than faropenem medoxomil. Development of REP8839, one of our product candidates that has completed its Phase I clinical trials, was suspended by us due to the incremental investment that would be required to optimize the formulation of REP8839 and the niche initial target market being addressed by the product. As a significant part of our growth strategy, we intend to develop and commercialize additional products and product candidates through our discovery research program. A significant portion of the research that we are conducting involves new and
30
unproven technologies, and may not result in the discovery or development of commercially viable products. Research programs to identify new disease targets and product candidates require substantial technical, financial and human resources whether or not we ultimately identify any candidates. Our research programs may initially show promise in identifying potential product candidates, yet fail to yield product candidates for clinical development. The process of successfully discovering product candidates is expensive, time-consuming and unpredictable, and the historical rate of failure for drug candidates is extremely high. Data from our current research programs may not support the clinical development of our lead compounds or other compounds from these programs, and we may not identify any compounds suitable for recommendation for clinical development. Moreover, any compounds we recommend for clinical development may not be effective or safe for their designated use, which would prevent their advancement into clinical trials and impede our ability to maintain or expand our clinical development pipeline. If we are unable to identify new product candidates or advance our lead compounds into clinical development, we may not be able to establish or maintain a clinical development pipeline or generate product revenue. Our ability to identify new compounds and advance them into clinical development also depends upon our ability to fund our research and development operations, and we cannot be certain that additional funding will be available on acceptable terms, or at all. If we continue our clinical development program for faropenem medoxomil for certain adult indications or any other indications we will have fewer resources to devote to the further research and development of other product candidates, such as REP3123, or potential product candidates identified through our discovery research program. There is no guarantee that we will be able to successfully advance any product candidates identified through our discovery research program into clinical trials or successfully develop any product candidate we advance into clinical trials for commercial sale. In addition, the size of the potential markets for such other product candidates may not be as attractive as the potential markets for faropenem medoxomil. If we are unable to develop suitable potential product candidates through internal research programs or are not able to advance the development of our early stage product candidates such as REP3123, our business will suffer and we may be unable to grow our business.
We are at an early stage of development as a company, with no current sources of revenue, and we may never generate future revenue or become profitable.
We are a biopharmaceutical company that emerged from the development stage in February 2006 and have a limited operating history. Currently, we have no products approved for commercial sale and, to date, we have not generated any revenue from product sales. Our ability to generate revenue depends heavily on:
| | |
| | • | our ability to obtain a new collaboration partner for faropenem medoxomil on acceptable terms; |
| |
| | • | obtaining U.S. and foreign regulatory approvals for our most advanced product candidate, faropenem medoxomil; |
| |
| | • | successfully developing or obtaining a collaboration partner for our anti-bacterial agent addressingC. difficilebacteria andC. difficile-associated disease, REP3123, or our inhibition of DNA replication program; and |
| |
| | • | successfully commercializing any product candidates for which we receive FDA approval. |
Our existing product candidates and development programs will require extensive additional clinical evaluation, regulatory approval, significant marketing efforts and substantial investment before they can provide us with any revenue. If we do not receive regulatory approval for and successfully commercialize faropenem medoxomil, we will be unable to generate any royalty revenue from product sales for many years, if at all. If we are unable to generate revenue, we will not become profitable, and we may be unable to continue our operations.
We have incurred significant operating losses since inception and anticipate that we will incur continued losses for the foreseeable future.
We have experienced significant operating losses since our inception in December 2000. At December 31, 2007, we had an accumulated deficit of approximately $109.3 million. We have generated no revenue from
31
product sales to date. We have funded our operations to date principally from the sale of our securities and payments by Forest Laboratories under our former collaboration agreement. As a result of the October 2006 FDA non-approval letter for our December 2005 NDA for faropenem medoxomil and the termination of our Forest Laboratories collaboration agreement in May 2007, our prospects for near term future revenues are substantially uncertain. We expect to continue to incur substantial additional operating losses for the next several years as we pursue our clinical trials and research and development efforts. Because of the numerous risks and uncertainties associated with developing and commercializing antibiotics, we are unable to predict the extent of any future losses. We may never have any significant future revenue or become profitable on a sustainable basis.
If we fail to obtain additional financing, we may be unable to complete the development and commercialization of faropenem medoxomil and other product candidates, or continue our research and development programs.
Our operations have consumed substantial amounts of cash since inception. We currently expect to spend substantial amounts to:
| | |
| | • | continue the clinical development of faropenem medoxomil while we seek a partner for this program; |
| |
| | • | continue our research and development programs; |
| |
| | • | license or acquire additional product candidates; and |
| |
| | • | launch and commercialize any product candidates for which we receive regulatory approval, including building our own sales force to address certain markets. |
We do not expect that our current capital resources will be sufficient to fund the complete development of our faropenem medoxomil product candidate and any product candidates generated from our discovery research program. To date, our sources of cash have been limited primarily to the proceeds from the sale of our securities and payments by Forest Laboratories under our former collaboration agreement. As a result of the termination of our Forest Laboratories collaboration agreement on May 7, 2007, our prospects for near term future revenues are substantially uncertain. We are currently seeking a new collaboration partner for faropenem medoxomil and using our cash and cash equivalents, short-term investments and interest earned on these balances toward the funding necessary to support our planned activities. If we cannot find a new partner on acceptable terms or if the funds provided from existing resources are insufficient to satisfy our future capital needs, or if we develop, in-license or acquire additional products or product candidates or pursue additional applications for our product candidates, we may seek to sell additional equity or debt securities. We cannot be certain that additional funding will be available on acceptable terms, or at all. To the extent that we raise additional funds by issuing equity securities, our stockholders may experience significant dilution. Any debt financing, if available, may involve restrictive covenants, such as limitations on our ability to incur additional indebtedness, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we are unable to raise additional capital when required or on acceptable terms, we may have to significantly delay, scale back or discontinue the developmentand/or commercialization of one or more of our product candidates. We also may be required to:
| | |
| | • | seek collaborators for our product candidates at an earlier stage than otherwise would be desirable and on terms that are less favorable than might otherwise be available; and |
| |
| | • | relinquish or license on unfavorable terms our rights to technologies or product candidates that we otherwise would seek to develop or commercialize ourselves. |
32
We have limited manufacturing capabilities and will depend on third parties to manufacture faropenem medoxomil and future products. If we cannot develop adequate manufacturing internally or identify suitable third party manufacturers, or these manufacturers fail to meet our requirements and strict regulatory standards, we may be unable to develop or commercialize our products.
We do not have the capability to manufacture commercial quantities of faropenem medoxomil drug substance. If we decide to pursue additional large scale clinical trials for faropenem medoxomil or if our other product candidates advance into full scale clinical trials, we may not have the capability to manufacture quantities of faropenem medoxomil or such other product candidates for our clinical trials. We originally engaged Nippon Soda and MEDA as our sole suppliers of faropenem medoxomil drug substance and faropenem medoxomil tablets, respectively. Pursuant to the terms of our former collaboration agreement with Forest Laboratories, Forest Laboratories had agreed to assume responsibility for supply chain management for faropenem medoxomil and entered into a direct relationship with both Nippon Soda and MEDA as its sole supplier of faropenem medoxomil drug substance. However, following termination of our agreement with Forest Laboratories, the Nippon Soda and MEDA obligations reverted directly to us. Further, in connection with our determination that future clinical development of faropenem medoxomil would be completed using the 600 mg tablet as compared to the 300 mg tablet used in the clinical trials included in the December 2005 NDA for which the FDA issued a non-approval letter in April 2007, we notified MEDA that we would terminate the agreement to manufacture 300 mg tablets. We are contractually bound to purchase all of our requirements for bulk drug substance from Nippon Soda and expect Nippon Soda will be the sole supplier of faropenem medoxomil drug substance for the foreseeable future. Nippon Soda may terminate this supply agreement for a number of reasons, such as:
| | |
| | • | an uncured material breach of the supply agreement by us; |
| |
| | • | our liquidation or insolvency; |
| |
| | • | in some circumstances, following a change of control; or |
| |
| | • | our failure to notify Nippon Soda of a launch go date, as defined, for faropenem medoxomil in the U.S. and Canada by July 1, 2009. |
Nippon Soda will be subject to ongoing periodic unannounced inspections by the FDA and corresponding state agencies for compliance with good manufacturing practices regulations, or cGMPs, and similar foreign standards. We do not have control over compliance by Nippon Soda with these regulations and standards.
Nippon Soda has only a single facility located in Nihongi, Japan that can readily manufacture commercial quantities of faropenem medoxomil. If that facility were to be damaged or destroyed, we would have no readily available source of supply. Nippon Soda has not yet manufactured faropenem medoxomil at commercial scale on a consistent basis, nor has Nippon Soda completed the manufacturing process validations that are part of the regulatory requirements prior to obtaining marketing approval for faropenem medoxomil. We may not be able to identify a suitable third party manufacturer to manufacture 600 mg faropenem medoxomil tablets or, if we do identify a manufacturer for 600 mg faropenem medoxomil tablets, we may not be able to obtain acceptable terms.
Reliance on a third party manufacturer entails risk, to which we would not be subject if we manufactured products ourselves, including:
| | |
| | • | reliance on the third party for regulatory compliance and quality assurance; |
| |
| | • | delays or failure to manufacture sufficient quantities needed for clinical trials in accordance with our specifications or to deliver such quantities on the dates we require; |
| |
| | • | the possible breach of the manufacturing agreement by the third party because of factors beyond our control; and |
| |
| | • | the possibility of termination or non-renewal of the agreement by the third party because of our breach of the manufacturing agreement or based on its own business priorities, and the non-approvable letter |
33
| | |
| | | we recently received from the FDA for our NDA for faropenem medoxomil may adversely influence the business priorities of our current suppliers. |
Any of these factors could cause delay or suspension of clinical trials, regulatory submissions, required approvals or commercialization of faropenem medoxomil and our other product candidates under development or cause us to incur higher costs and could prevent us from commercializing our product candidates successfully. If we obtain regulatory approval for faropenem medoxomil and our contract manufacturers fail to deliver the required commercial quantities of bulk drug substance or finished product on a timely basis and at commercially reasonable prices and we are unable to find one or more replacement manufacturers capable of production at a substantially equivalent cost, in substantially equivalent volumes and quality, and on a timely basis, we would likely be unable to meet demand for faropenem medoxomil and we would lose potential revenue. It may take several years to establish an alternative source of supply for faropenem medoxomil and to have any such new source approved by the FDA, especially because faropenem medoxomil requires dedicated manufacturing facilities.
If the FDA does not approve Nippon Soda’s facility, we may be unable to develop or commercialize faropenem medoxomil.
We rely on Nippon Soda to manufacture faropenem medoxomil drug substance and currently have no plans to develop our own manufacturing facility. The facilities used by our contract manufacturer to manufacture our product candidates must be approved by the FDA. Nippon Soda’s facility has undergone its initial inspection by the FDA as part of the faropenem medoxomil NDA review. Although no FDA Form 483 observations were noted by the FDA site inspector, if Nippon Soda cannot successfully manufacture material that conforms to our specifications and strict regulatory requirements, Nippon Soda will not be able to maintain FDA approval for its manufacturing facility. If the FDA does not maintain approval of this facility for the manufacture of faropenem medoxomil, we may need to find alternative manufacturing facilities, which would result in significant delay of up to several years in obtaining approval for and manufacturing faropenem medoxomil. In addition, our contract manufacturer will be subject to ongoing periodic unannounced inspections by the FDA and corresponding state and foreign agencies for compliance with cGMPs and similar regulatory requirements. These regulations cover all aspects of the manufacturing, testing, quality control and record-keeping relating to our product candidates. We do not have control over Nippon Soda’s compliance with these regulations and standards. Failure by Nippon Soda to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, failure to grant approval to market our product candidates, delays, suspension or withdrawals of approvals, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect our business. In addition, we have no control over Nippon Soda’s ability to maintain adequate quality control, quality assurance and qualified personnel. Failure by our contract manufacturer to comply with or maintain any of these standards could adversely affect our ability to develop, obtain regulatory approval for or market our product candidates.
Any of our product candidates that are in clinical trials or that we advance into clinical trials are subject to extensive regulation, which can be costly and time consuming, cause unanticipated delays, or prevent the receipt of the required approvals to commercialize our product candidates.
The clinical development, manufacturing, labeling, storage, record-keeping, advertising, promotion, export, marketing and distribution of any of our product candidates currently in clinical trials or that we advance into clinical trials are subject to extensive regulation by the FDA in the U.S. and by comparable governmental authorities in foreign markets. Currently, we are developing faropenem medoxomil for adult and pediatric use and we have completed preclinical testing of REP3123. In the U.S. and in many foreign jurisdictions, rigorous preclinical testing and clinical trials and an extensive regulatory review process must be successfully completed before a new drug can be sold. Satisfaction of these and other regulatory requirements is costly, time consuming, uncertain and subject to unanticipated delays. Clinical testing is expensive, can take many years to complete and its outcome is uncertain. Failure can occur at any time during the clinical trial process. The results of preclinical studies and early clinical trials of our product candidates may not be predictive of the results of later-stage clinical trials. Product candidates in later stages of clinical trials may
34
fail to show the desired safety and efficacy traits despite having progressed through initial clinical testing. The time required to obtain approval by the FDA is unpredictable but typically takes many years following the commencement of clinical trials, depending upon numerous factors. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change. We have not obtained regulatory approval for any product candidate.
Our product candidates may fail to receive regulatory approval for many reasons, including the following:
| | |
| | • | we may be unable to demonstrate to the satisfaction of the FDA or comparable foreign regulatory authorities that a product candidate is safe and effective for a particular indication; |
| |
| | • | the results of clinical trials may not meet the level of statistical significance required by the FDA or other regulatory authorities for approval; |
| |
| | • | the FDA or other regulatory authorities may disagree with the design of our clinical trials; |
| |
| | • | we may be unable to demonstrate that a product candidate’s benefits outweigh its risks; |
| |
| | • | we may be unable to demonstrate that the product candidate presents an advantage over existing therapies, or over placebo in any indications for which the FDA requires a placebo-controlled trial; |
| |
| | • | the FDA or comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials; |
| |
| | • | the data collected from clinical trials of our product candidates may not be sufficient to support the submission of a new drug application or to obtain regulatory approval in the U.S. or elsewhere; |
| |
| | • | the FDA or comparable foreign regulatory authorities may fail to approve the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; |
| |
| | • | we may not be able to satisfactorily address the objectionable conditions identified in the Warning Letter we received from the FDA in January 2008; and |
| |
| | • | the approval policies or regulations of the FDA or comparable foreign regulatory authorities may change. |
The FDA or comparable foreign regulatory authorities might decide that our data is insufficient for approval and require additional clinical trials or other studies. Furthermore, even if we do receive regulatory approval to market a commercial product, any such approval may be subject to limitations on the indicated uses for which we may market the product. It is possible that none of our existing product candidates or any product candidates we may seek to develop in the future will ever obtain the appropriate regulatory approvals necessary for us or our collaborators to begin selling them.
Also, recent events have raised questions about the safety of marketed drugs and may result in increased cautiousness by the FDA in reviewing new drugs based on safety, efficacy or other regulatory considerations and may result in significant delays in obtaining regulatory approvals and more stringent product labeling requirements. Further, the FDA has been granted new authority to require additional clinical trials of license holders of pharmaceutical products, including post approval clinical trials, and modify previously approved product labels under the FDA Amendments Act of 2007 that was enacted September 2007. Any delay in obtaining, or inability to obtain, applicable regulatory approvals would prevent us from commercializing our product candidates.
If we fail to attract and keep senior management and key scientific personnel, we may be unable to successfully develop our product candidates, conduct our clinical trials and commercialize our product candidates.
Our success depends in part on our continued ability to attract, retain and motivate highly qualified management, clinical and scientific personnel and on our ability to develop and maintain important relationships with leading academic institutions, clinicians and scientists. We are highly dependent upon our senior management and scientific staff, particularly Kenneth Collins, our President and Chief Executive Officer,
35
Roger Echols, M.D., our Chief Medical Officer, Pete Letendre, PharmD., our Chief Commercial Officer Nebojsa Janjic, Ph.D., our Chief Scientific Officer, Mark Smith, our Chief Financial Officer, and Donald Morrissey, our Senior Vice President of Corporate Development. The loss of services of any of Mr. Collins, Dr. Echols, Dr. Letendre, Dr. Janjic, Mr. Smith or Mr. Morrissey could delay or prevent the successful completion of our strategy or development of our product candidates. In addition, we only recently formed our clinical and regulatory group, the services of which we highly depend upon to conduct our clinical programs and obtain regulatory approvals.
Competition for qualified personnel in the biotechnology and pharmaceutical fields is intense. We will need to hire additional personnel as we expand our clinical development and commercial activities. In addition, we may be required to grant significant amounts of share-based compensation to certain individuals to attract them, which could increase the related non-cash compensation expense. We may not be able to attract and retain qualified personnel on acceptable terms. We do not carry “key person” insurance covering any members of our senior management. Each of our officers and key employees may terminate his or her employment at any time without notice and without cause or good reason.
We currently have no sales organization. If we are unable to establish a direct sales force in the U.S. to promote our product candidates, the commercial opportunity for our product candidates may be diminished.
We currently have no sales organization. If our most advanced product candidate, faropenem medoxomil, is approved by the FDA, we will require a partner to market the product. If we elect to rely on third parties to sell our product candidates in the U.S., we may receive less revenue than if we sold our product candidates directly. In addition, we may have little or no control over the sales efforts of those third parties. In the event we are unable to develop our own sales force or collaborate with a third party to sell our product candidates, we may not be able to commercialize our product candidates which would negatively impact our ability to generate revenue.
The commercial success of our product candidates will depend upon attaining significant market acceptance of these products among physicians, patients, health care payors and the medical community.
None of our product candidates has been commercialized for any indication. Even if approved for sale by the appropriate regulatory authorities, physicians may not prescribe our product candidates, in which case we would not generate revenue or become profitable. Market acceptance of our most advanced product candidate, faropenem medoxomil, and any future product candidates by physicians, health care payors and patients will depend on a number of factors, including:
| | |
| | • | the clinical indications for which the product candidate is approved; |
| |
| | • | acceptance by physicians and patients of each product candidate as a safe and effective treatment; |
| |
| | • | perceived advantages over alternative treatments; |
| |
| | • | the cost of treatment in relation to alternative treatments, including numerous generic antibiotics; |
| |
| | • | the extent to which the product candidate is approved for inclusion on formularies of hospitals and managed care organizations; |
| |
| | • | the extent to which bacteria develop resistance to the product candidate, thereby limiting its efficacy in treating or managing infections; |
| |
| | • | whether the product candidate is designated under physician treatment guidelines as a first-line therapy or as a second- or third-line therapy for particular infections; |
| |
| | • | the availability of adequate reimbursement by third parties; |
| |
| | • | relative convenience and ease of administration; and |
| |
| | • | prevalence and severity of side effects. |
36
Even if faropenem medoxomil ultimately obtains regulatory approval, many of the above factors may be adversely impacted by the historical difficulty of obtaining any such approval and may create a negative perception among physicians and health care payors of the advantages or efficacy of faropenem medoxomil.
If lawsuits or arbitration proceedings arising as a result of termination of collaboration or other commercial contracts are successfully brought against us, we may incur substantial liabilities and may be unable to commercialize our product candidates.
Between February 6, 2007 and May 7, 2007, we operated under the termination provisions of our collaboration agreement with Forest Laboratories. On April 27, 2007, under the termination provisions of our agreement with Forest Laboratories, we terminated our agreement with MEDA for the manufacture of 300 mg tablets of faropenem medoxomil. MEDA has indicated to us that it disputes our right to terminate the agreement on the basis indicated in our notice of termination. We believe we have acted in accordance with the terms of these and other commercial agreements. However, if it is determined that we have obligations to MEDA beyond May 7, 2007 under the agreement, then we may incur additional costs. Consistent with our position that we had the right to terminate this agreement and that Forest Laboratories is responsible for all supply chain obligations through May 7, 2007, we have not accrued for any minimum purchases after that date or termination fees, including potential plantde-contamination expenses, under this agreement.
The interpretation of the terms of our collaboration and commercial agreements may be the subject of disagreement between us and our collaborators and other commercial partners that could result in lawsuitsand/or arbitration proceedings. If former partners or other parties to our commercial contracts are successful in lawsuits or arbitration proceedings, we may incur judgments against us that could have a material impact on our financial position and limit our ability to complete development of and launch commercially our product candidates.
If our product candidates are unable to compete effectively with generic and branded antibiotics, our commercial opportunity will be reduced or eliminated.
If approved, our most advanced product candidate, faropenem medoxomil, will compete against both generic and branded community antibiotic therapies. The market for such products is very competitive and includes generic products, such as amoxicillin/clavulanate and cefdinir, and established branded products, such as Zithromax®, Ketek® and Levaquin®, which are marketed by major pharmaceutical companies, all of which have significantly greater financial resources and expertise in research and development, preclinical testing, conducting clinical trials, obtaining regulatory approvals, manufacturing and marketing approved products than we do. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large, established companies.
Over the next several years, our future products, if any, will face more competition in the form of generic versions of branded products competition that will lose their patent exclusivity. Many of the currently branded antibiotics will be sold as generics before we expect to be able to commercially launch faropenem medoxomil. Generic antibiotic therapies typically are sold at lower prices than branded antibiotics and are preferred by managed care providers of health services. As a result, managed care may place different constraints on formulary status and reimbursement at the time we expect to be able to commercially launch faropenem medoxomil. If we are unable to demonstrate to physicians that, based on experience, clinical data, side-effect profiles and other factors, our products are preferable to these generic antibiotic therapies, we may have limited revenue potential due to formulary status. Our commercial opportunity will also be reduced or eliminated if our competitors develop and commercialize generic or branded antibiotics that are safer, more effective, have fewer side effects or are less expensive than our product candidates.
Asubio Pharma owns a portfolio of patents related to faropenem compounds, including the faropenem parent compound, medoxomil and other faropenem prodrugs. We have licensed from Asubio Pharma the patents to faropenem medoxomil and other faropenem prodrugs. These patents may not prevent competitors from developing other faropenem drugs that are not covered by the Asubio Pharma patents. Beginning in 2008, when the Asubio Pharma patents related to the faropenem parent compound expire, competitors may
37
submit NDAs seeking approval of antibiotics containing the faropenem parent compound as the active ingredient. These applications would have to contain full reports of safety and efficacy data conducted by or for the applicants and could not in any way rely upon the safety and efficacy data utilized in the approval of faropenem medoxomil. In addition, as early as four years after the approval of a faropenem medoxomil NDA, if any, competitors could also file NDA’s seeking approval of faropenem drugs that would likely require the applicant to conduct clinical trials in order to bring the product to market in the U.S., though the FDA may allow the applicant to rely in part on the FDA’s prior findings of safety and efficacy of faropenem medoxomil.
If product liability lawsuits are successfully brought against us or any future collaboration partners, we may incur substantial liabilities and may be required to limit commercialization of our product candidates.
We face an inherent risk of product liability lawsuits related to the testing of our product candidates, and will face an even greater risk if product candidates are introduced commercially. An individual may bring a liability claim against us if one of our product candidates causes, or merely appears to have caused, an injury. We have agreed to indemnify Nippon Soda from product liability claims under our commercial arrangement. If we cannot successfully defend ourselves against the product liability claim, we may incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| | |
| | • | decreased demand for our product candidates; |
| |
| | • | injury to our reputation; |
| |
| | • | withdrawal of clinical trial participants; |
| |
| | • | significant litigation costs; |
| |
| | • | substantial monetary awards to or costly settlement with patients; |
| |
| | • | product recalls; |
| |
| | • | loss of revenue; and |
| |
| | • | the inability to commercialize our product candidates. |
We are highly dependent upon consumer perceptions of us, the faropenem medoxomil brand and the safety and quality of our products. We could be adversely affected if we or the faropenem medoxomil brand is subject to negative publicity. We could also be adversely affected if any of our products or any similar products distributed by other companies prove to be, or are asserted to be, harmful to consumers. Also, because of our dependence upon consumer perceptions, any adverse publicity associated with illness or other adverse effects resulting from consumers’ use or misuse of our products or any similar products distributed by other companies could have a material adverse impact on our results of operations.
We have global clinical trial liability insurance that covers our clinical trials up to a $10.0 million annual aggregate limit. Our current or future insurance coverage may prove insufficient to cover any liability claims brought against us. We intend to expand our insurance coverage to include the sale of commercial products if marketing approval is obtained for our product candidates. In addition, because of the increasing costs of insurance coverage, we may not be able to maintain insurance coverage at a reasonable cost or obtain insurance coverage that will be adequate to satisfy any liability that may arise.
We may be required to suspend or discontinue clinical trials due to side effects or other safety risks that could preclude approval of our product candidates.
Our clinical trials may be suspended at any time for a number of reasons. We may voluntarily suspend or terminate our clinical trials if at any time we believe that they present an unacceptable risk to participants. In addition, regulatory agencies may order the temporary or permanent discontinuation of our clinical trials at any time if they believe that the clinical trials are not being conducted in accordance with applicable regulatory requirements or that they present an unacceptable safety risk to participants.
38
Many antibiotics can produce significant side effects. Side effects associated with many current antibiotics include kidney and liver toxicities, heart rhythm abnormalities, photosensitivity, rash, and excessive flushing of the skin and central nervous system toxicities, such as seizures. In clinical trials, side effects of faropenem medoxomil have included gastrointestinal disorders (such as diarrhea, nausea and vomiting), nervous system disorders (such as dizziness and headaches), as well as infections and infestations (such as pneumonia and vaginal mycosis). Later clinical trials in a larger patient population could reveal other side effects. These or other side effects could interrupt, delay or halt clinical trials of our product candidates and could result in the FDA or other regulatory authorities stopping further development of or denying approval of our product candidates for any or all targeted indications. Even if we believe our product candidates are safe, our data is subject to review by the FDA, which may disagree with our conclusions. Moreover, we could be subject to significant liability if any volunteer or patient suffers, or appears to suffer, adverse health effects as a result of participating in our clinical trials.
We rely on third parties to conduct our clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval for or commercialize our product candidates.
We have agreements with third-party contract research organizations to provide monitors for and to manage data for our on-going clinical programs. We and our contract research organizations are required to comply with current Good Clinical Practices, or GCPs, regulations and guidelines enforced by the FDA for all of our products in clinical development. The FDA enforces GCPs through periodic inspections of trial sponsors, principal investigators and trial sites. If we or our contract research organizations fail to comply with applicable GCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA may require us to perform additional clinical trials before approving our marketing applications. We cannot ensure that, upon inspection, the FDA will determine that any of our clinical trials comply with GCPs. In addition, our clinical trials must be conducted with product produced under cGMP regulations, and will require a large number of test subjects. Our failure to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process.
Our contract research organizations have the right to terminate their agreements with us in the event of an uncured material breach. In addition, some of our contract research organizations have an ability to terminate their respective agreements with us if it can be reasonably demonstrated that the safety of the subjects participating in our clinical trials warrants such termination, if we make a general assignment for the benefit of our creditors, or if we are liquidated. If any of our relationships with these third-party contract research organizations terminate, we may not be able to enter into arrangements with alternative contract research organizations. If contract research organizations do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols, regulatory requirements, or for other reasons, our clinical trials may be extended, delayed or terminated, and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. As a result, our financial results and the commercial prospects for our product candidates would be harmed, our costs could increase and our ability to generate revenue could be delayed.
Our ability to pursue the development and commercialization of our product candidates depends upon the continuation of our licenses from third parties.
Our license agreement with Asubio Pharma provides us with an exclusive license to develop and sell any products with the compound faropenem medoxomil as an active ingredient for any indication in the U.S. and Canada. Either we or Asubio Pharma may terminate the license agreement immediately upon the bankruptcy or dissolution of the other party or upon a breach of any material provision of the agreement if the breach is not cured within 60 days following written notice. We are currently in discussions with Asubio Pharma regarding the future development plans for faropenem medoxomil. If there is any dispute between us and Asubio Pharma regarding our rights or obligations under the license agreement, including diligence obligations, the achievement of milestones or interpretation of other material provisions, we risk litigation and our
39
business may be adversely affected. If our license agreement with Asubio Pharma were terminated, we would lose our rights to develop and commercialize faropenem medoxomil.
If we fail to gain and maintain approval for our product candidates in international markets, our market opportunities will be limited.
Sales of our product candidates outside of the U.S. will be subject to foreign regulatory requirements governing clinical trials and marketing approval. Even if the FDA grants marketing approval for a product candidate, comparable regulatory authorities of foreign countries must also approve the manufacturing or marketing of the product candidate in those countries. Approval in the U.S., or in any other jurisdiction, does not ensure approval in other jurisdictions. Obtaining foreign approvals could result in significant delays, difficulties and costs for us and require additional trials and additional expenses. Regulatory requirements can vary widely from country to country and could delay the introduction of our products in those countries. Clinical trials conducted in one country may not be accepted by other countries and regulatory approval in one country does not mean that regulatory approval will be obtained in any other country. None of our product candidates is approved for sale in international markets and we do not have experience in obtaining regulatory approval in international markets. If we fail to comply with these regulatory requirements or to obtain and maintain required approvals, our target market will be reduced and our ability to generate revenue will be diminished.
We may not be able to enter into acceptable agreements to market and commercialize our product candidates in international markets.
If appropriate regulatory approvals are obtained, we intend to commercialize our product candidates in international markets through collaboration arrangements with third parties. If we decide to sell our product candidates in international markets, we may not be able to enter into any arrangements on favorable terms or at all. In addition, these arrangements could result in lower levels of income to us than if we marketed our product candidates entirely on our own. If we are unable to enter into a marketing arrangement for our product candidates in international markets, we may not be able to develop an effective international sales force to successfully commercialize those products in international markets. If we fail to enter into marketing arrangements for our products and are unable to develop an effective international sales force, our ability to generate revenue would be limited.
Even if we receive regulatory approval for our product candidates, we will be subject to ongoing significant regulatory obligations and oversight.
If we receive regulatory approval to sell our product candidates, the FDA and foreign regulatory authorities may impose significant restrictions on the indicated uses or marketing of such products, or impose ongoing requirements for post-approval studies. Following any regulatory approval of our product candidates, we will be subject to continuing regulatory obligations, such as safety reporting requirements, and additional post-marketing obligations, including regulatory oversight of the promotion and marketing of our products. If we become aware of previously unknown problems with any of our product candidates here or overseas or at our contract manufacturers’ facilities, a regulatory agency may impose restrictions on our products, our contract manufacturers or on us, including requiring us to reformulate our products, conduct additional clinical trials, make changes in the labeling of our products, implement changes to, or obtain re-approvals of, our contract manufacturers’ facilities, or withdraw the product from the market. In addition, we may experience a significant drop in the sales of the affected products, our reputation in the marketplace may suffer and we may become the target of lawsuits, including class action suits. Moreover, if we fail to comply with applicable regulatory requirements, we may be subject to fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution. Any of these events could harm or prevent sales of the affected products or could substantially increase the costs and expenses of commercializing and marketing these products.
40
Our corporate compliance program cannot guarantee that we are in compliance with all potentially applicable regulations.
The development, manufacturing, pricing, marketing, sales, and reimbursement of our product candidates, together with our general operations, are subject to extensive regulation by federal, state and other authorities within the U.S. and numerous entities outside of the U.S. If we fail to comply with any of these regulations, we could be subject to a range of regulatory actions, including suspension or termination of clinical trials, the failure to approve a product candidate, restrictions on our product candidates or manufacturing processes, withdrawal of products from the market, significant fines, or other sanctions or litigation, and exclusion of our products from the Medicare/Medicaid payment system. As a publicly traded company we are subject to significant regulations, including the Sarbanes-Oxley Act of 2002, some of which have only recently been adopted, and all of which are subject to change. While we have developed and instituted a corporate compliance program based on what we believe are the current best practices and continue to update the program in response to newly implemented or changing regulatory requirements, we cannot ensure that we are or will be in compliance with all potentially applicable regulations. For example, we cannot assure that in the future our management will not find a material weakness in connection with its annual review of our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act. We also cannot ensure that we could correct any such weakness to allow our management to assess the effectiveness of our internal control over financial reporting as of the end of our fiscal year in time to enable our independent registered public accounting firm to attest that such assessment will have been fairly stated in our annual reports filed with the Securities and Exchange Commission or attest that we have maintained effective internal control over financial reporting as of the end of our fiscal year. If we fail to comply with the Sarbanes-Oxley Act or any other regulations we could be subject to a range of consequences, including restrictions on our ability to sell equity or otherwise raise capital funds, significant fines, enforcement or other civil or criminal actions by the Securities and Exchange Commission or delisting by the NASDAQ Global Market or other sanctions or litigation. In addition, if we disclose any material weakness in our internal control over financial reporting or other consequence of failing to comply with applicable regulations, this may cause our stock price to decline.
Reimbursement may not be available for our product candidates, which could diminish our sales or affect our ability to sell any future products profitably.
Market acceptance and sales of our product candidates will depend on reimbursement policies and may be affected by future health care reform measures. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which drugs they will pay for and establish reimbursement levels. We cannot be sure that reimbursement will be available for any of our product candidates. Also, we cannot be sure that reimbursement amounts will not reduce the demand for, or the price of, our products. We have not commenced efforts to have our product candidates reimbursed by government or third-party payors. If reimbursement is not available or is available only to limited levels, we may not be able to commercialize our products.
In both the U.S. and certain foreign jurisdictions, there have been a number of legislative and regulatory changes to the health care system that could impact our ability to sell our products profitably. In particular, the Medicare Modernization Act of 2003 added an outpatient prescription drug benefit to Medicare, which became effective on January 1, 2006. Drug benefits under this provision are administered through private plans that negotiate price concessions from pharmaceutical manufacturers. We cannot be certain that faropenem medoxomil will successfully be placed on the list of drugs covered by particular health plans or plan formularies, nor can we predict the negotiated price for faropenem medoxomil, which will be determined by market factors. With respect to Medicaid, the Deficit Reduction Act of 2005 made several changes to the way pharmacies are reimbursed under Medicaid, most of which went into effect on January 1, 2007. These changes could lead to reduced drug prices. Many states have also created preferred drug lists and include drugs on those lists only when the manufacturers agree to pay a supplemental rebate. If faropenem medoxomil or our other product candidates are not included on these preferred drug lists, physicians may not be inclined to prescribe them to their Medicaid patients.
41
As a result of legislative proposals and the trend towards managed health care in the U.S., third-party payors are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement of new drugs. They may also refuse to provide any coverage of uses of approved products for medical indications other than those for which the FDA has granted market approvals. As a result, significant uncertainty exists as to whether and how much third-party payors will reimburse patients for their use of newly-approved drugs, which in turn will put pressure on the pricing of drugs. The availability of numerous generic antibiotics at lower prices than branded antibiotics, such as faropenem medoxomil, if it were approved for commercial introduction, can also be expected to substantially reduce the likelihood of reimbursement for faropenem medoxomil. We expect to experience pricing pressures in connection with the sale of our products due to the trend toward managed health care, the increasing influence of health maintenance organizations and additional legislative proposals.
We may need to modify the size of our organization, and we may experience difficulties in managing either growth or restructuring.
We are a small company with 53 full time employees as of December 31, 2007. As our development and commercialization plans and strategies develop, we may need to either reduce or expand the size of our employee base for managerial, operational, sales, financial and other reasons. In December 2007, we undertook an organizational restructuring that reduced the number of employees in the clinical, commercial, administrative and research functions by 27 employees. Future growth would impose significant added responsibilities on members of management, including the need to identify, recruit, maintain and integrate additional employees. Future restructuring activities may involve significant changes to our drug development and growth strategies, our commercialization plans and other operational matters, including a significant reduction in our employee base. Any future restructuring activity could result in disruption to our business, adversely affecting the morale of our employees and making it more difficult to retain qualified personnel. Also, our management may have to divert a disproportionate amount of its attention away from our day-to-day activities and devote a substantial amount of time to managing either growth or restructuring activities. Our future financial performance and our ability to commercialize our product candidates and to compete effectively will depend, in part, on our ability to effectively manage any future growth or restructuring, as the case may be. To that end, we must be able to:
| | |
| | • | manage our development efforts effectively; |
| |
| | • | manage our clinical trials effectively; |
| |
| | • | integrate additional management, administrative, manufacturing and sales and marketing personnel, or reorganize these personnel; |
| |
| | • | maintain sufficient administrative, accounting and management information systems and controls; and |
| |
| | • | hire and train additional or replacement qualified personnel. |
We may not be able to accomplish these tasks, and our failure to accomplish any of them could harm our financial results.
Risks Related to our Intellectual Property
It is difficult and costly to protect our proprietary rights, and we may not be able to ensure their protection.
Our commercial success will depend in part on obtaining and maintaining patent protection and trade secret protection of our product candidates, and the methods used to manufacture them, as well as successfully defending these patents against third-party challenges. Our ability to protect our product candidates from unauthorized making, using, selling, offering to sell or importation by third-parties is dependent upon the extent to which we have rights under valid and enforceable patents or trade secrets that cover these activities.
As of December 31, 2007, we have exclusively licensed from Asubio Pharma two issued U.S. patents; one issued foreign patent and one pending U.S. patent application covering faropenem medoxomil, a pro-drug of faropenem. The two issued U.S. patents covering faropenem medoxomil also cover other potential prodrugs
42
of faropenem but do not cover all potential faropenem-based antibiotic compounds. We do not and have not had any control over the filing or prosecution of these patents or patent applications. We cannot be certain that such prosecution efforts have been or will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents. In addition, our enforcement of these faropenem medoxomil patents or defense of any claims asserting the invalidity of these patents would be subject to the cooperation of Asubio Pharma. Although Asubio Pharma has agreed to cooperate with us in such efforts, if requested, we cannot be assured that Asubio Pharma would devote sufficient efforts to cooperate with us in these circumstances.
The patent positions of pharmaceutical and biotechnology companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in biotechnology patents has emerged to date in the U.S. The biotechnology patent situation outside the U.S. is even more uncertain. Changes in either the patent laws or in interpretations of patent laws in the U.S. and other countries may diminish the value of our intellectual property. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in our licensed patents, our patents or in third-party patents.
Asubio Pharma owns a portfolio of patents related to faropenem compounds, including the faropenem parent compound, faropenem medoxomil and other faropenem prodrugs. We have licensed from Asubio Pharma the patents to faropenem medoxomil and other faropenem prodrugs. These patents may not prevent competitors from developing other faropenem drugs that are not covered by the Asubio Pharma patents. Beginning in 2008, when the Asubio Pharma patents expire, competitors may submit NDAs seeking approval of antibiotics containing the faropenem parent compound as the active ingredient. These applications would have to contain full reports of safety and efficacy data conducted by or for the applicants and could not in any way rely upon the safety and efficacy data utilized in the approval of faropenem medoxomil. In addition, as early as four years after the approval of a faropenem medoxomil NDA, if any, generic and branded competitors could also file NDAs seeking approval of faropenem drugs that would likely require the applicant to conduct clinical trials in order to bring the product to market in the U.S., though the FDA may allow the applicant to rely in part on the FDA’s prior findings of safety and efficacy of faropenem medoxomil. To the extent that any competitor relies on any of the findings of safety or efficacy with respect to faropenem medoxomil, the competitor will have to certify that its compound either does not infringe our patents or that our patents are invalid.
The degree of future protection for our proprietary rights is uncertain because legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep our competitive advantage. For example:
| | |
| | • | others may be able to make compounds that are similar to our product candidates but that are not covered by the claims of our licensed patents, or for which we are not licensed under our license agreements; |
| |
| | • | we or our licensors might not have been the first to make the inventions covered by our pending patent application or the pending patent applications and issued patents of our licensors; |
| |
| | • | we or our licensors might not have been the first to file patent applications for these inventions; |
| |
| | • | others may independently develop similar or alternative technologies or duplicate any of our technologies; |
| |
| | • | it is possible that our pending patent applications will not result in issued patents; |
| |
| | • | our issued patents and the issued patents of our licensors may not provide us with any competitive advantages, or may be held invalid or unenforceable as a result of legal challenges by third-parties; |
| |
| | • | we may not develop additional proprietary technologies that are patentable; or |
| |
| | • | the patents of others may have an adverse effect on our business. |
43
We also may rely on trade secrets to protect our technology, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. Although we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors, outside scientific collaborators and other advisors may unintentionally or willfully disclose our information to competitors. Enforcing a claim that a third-party illegally obtained and is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the U.S. are sometimes less willing to protect trade secrets. Moreover, our competitors may independently develop equivalent knowledge, methods and know-how.
We may incur substantial costs as a result of litigation or other proceedings relating to patent and other intellectual property rights and we may be unable to protect our rights to, or use, our technology.
If we choose to go to court to stop someone else from using the inventions claimed in our patents or our licensed patents, that individual or company has the right to ask the court to rule that these patents are invalidand/or should not be enforced against that third-party. These lawsuits are expensive and would consume time and other resources even if we were successful in stopping the infringement of these patents. In addition, there is a risk that the court will decide that these patents are not valid and that we do not have the right to stop the other party from using the inventions. There is also the risk that, even if the validity of these patents is upheld, the court will refuse to stop the other party on the ground that such other party’s activities do not infringe our rights to these patents.
Furthermore, a third-party may claim that we or our manufacturing or commercialization partners are using inventions covered by the third-party’s patent rights and may go to court to stop us from engaging in our normal operations and activities, including making or selling our product candidates. These lawsuits are costly and could affect our results of operations and divert the attention of managerial and technical personnel. There is a risk that a court would decide that we or our commercialization partners are infringing the third-party’s patents and would order us or our partners to stop the activities covered by the patents. In addition, there is a risk that a court will order us or our partners to pay the other party damages for having violated the other party’s patents. We have indemnified our commercial partners against patent infringement claims. The biotechnology industry has produced a proliferation of patents, and it is not always clear to industry participants, including us, which patents cover various types of products or methods of use. The coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform. If we are sued for patent infringement, we would need to demonstrate that our products or methods of use either do not infringe the patent claims of the relevant patentand/or that the patent claims are invalid, and we may not be able to do this. Proving invalidity, in particular, is difficult since it requires a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents.
Because some patent applications in the U.S. may be maintained in secrecy until the patents are issued, because patent applications in the U.S. and many foreign jurisdictions are typically not published until eighteen months after filing, and because publications in the scientific literature often lag behind actual discoveries, we cannot be certain that others have not filed patent applications for technology covered by our licensors’ issued patents or our pending applications or our licensors’ pending applications, or that we or our licensors were the first to invent the technology. Our competitors may have filed, and may in the future file, patent applications covering technology similar to ours. Any such patent application may have priority over our or our licensors’ patent applications and could further require us to obtain rights to issued patents covering such technologies. If another party has filed a U.S. patent application on inventions similar to ours, we may have to participate in an interference proceeding declared by the U.S. Patent and Trademark Office to determine priority of invention in the U.S. The costs of these proceedings could be substantial, and it is possible that such efforts would be unsuccessful, resulting in a loss of our U.S. patent position with respect to such inventions.
Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to raise the funds necessary to continue our operations.
44
Risks Related to Ownership of our Common Stock
The market price of our common stock is highly volatile.
Prior to June 28, 2006, there was no public market for our common stock. We cannot assure you that an active trading market for our common stock will exist at any time. You may not be able to sell your shares quickly or at the market price if trading in our common stock is not active. The trading price of our common stock has been highly volatile and could be subject to wide fluctuations in price in response to various factors, many of which are beyond our control, including:
| | |
| | • | announcement of FDA approval or non-approval of our product candidates, or specific label indications for their use, or delays in the FDA review process; |
| |
| | • | actions taken by regulatory agencies with respect to our product candidates, clinical trials, manufacturing process or sales and marketing activities; |
| |
| | • | termination of significant agreements; |
| |
| | • | changes in laws or regulations applicable to our products, including but not limited to, clinical trial requirements for approvals; |
| |
| | • | the success of our development efforts and clinical trials; |
| |
| | • | the success of our efforts to acquire or in-license additional products or product candidates; |
| |
| | • | developments concerning our collaborations, including but not limited to, those with our sources of manufacturing supply and our commercialization partners; |
| |
| | • | actual or anticipated variations in our quarterly operating results; |
| |
| | • | announcements of technological innovations by us, our collaborators or our competitors; |
| |
| | • | new products or services introduced or announced by us or our commercialization partners, or our competitors and the timing of these introductions or announcements; |
| |
| | • | actual or anticipated changes in earnings estimates or recommendations by securities analysts; |
| |
| | • | conditions or trends in the biotechnology and biopharmaceutical industries; |
| |
| | • | announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments; |
| |
| | • | general economic and market conditions and other factors that may be unrelated to our operating performance or the operating performance of our competitors; |
| |
| | • | changes in the market valuations of similar companies; |
| |
| | • | sales of common stock or other securities by us or our stockholders in the future; |
| |
| | • | additions or departures of key scientific or management personnel; |
| |
| | • | developments relating to proprietary rights held by us or our competitors; |
| |
| | • | disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; |
| |
| | • | trading volume of our common stock; and |
| |
| | • | sales of our common stock by us or our stockholders. |
In addition, the stock market in general and the market for biotechnology and biopharmaceutical companies in particular have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of those companies. These broad market and industry factors may seriously harm the market price of our common stock, regardless of our operating performance. In the past, following periods of volatility in the market, securitiesclass-action litigation has often been instituted
45
against companies. Such litigation, if instituted against us, could result in substantial costs and diversion of management’s attention and resources, which could materially adversely affect our business and financial condition.
We are at risk of securities class action litigation or may become subject to stockholder activism efforts that each could cause material disruption to our business.
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biotechnology and biopharmaceutical companies have experienced significant stock price volatility in recent years. Further, certain influential institutional investors and hedge funds have taken steps to involve themselves in the governance and strategic direction of certain companies that were perceived to be operating sub-optimally due to governance or strategic related disagreements with such stockholders. Our stock price decreased significantly following our announcement that the FDA had issued a non-approvable letter for our most advanced product candidate, faropenem medoxomil. If we face such litigation or stockholder activism efforts due to this development or any future development affecting us, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
Our principal stockholders and management own a significant percentage of our stock and are able to exercise significant influence over matters subject to stockholder approval.
Our executive officers, directors and principal stockholders, together with their respective affiliates, currently own a significant percentage of our voting stock, including shares subject to outstanding options and warrants, and we expect this group will continue to hold a significant percentage of our outstanding voting stock. Accordingly, these stockholders will likely be able to have a significant impact on the composition of our board of directors and continue to have significant influence over our operations. This concentration of ownership could have the effect of delaying or preventing a change in our control or otherwise discouraging a potential acquirer from attempting to obtain control of us, which in turn could have a material and adverse effect on the market value of our common stock.
We incur significant costs as a result of operating as a public company, and our management is required to devote substantial time to compliance initiatives.
As a public company, we incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, the Sarbanes-Oxley Act, as well as rules subsequently implemented by the Securities and Exchange Commission and the NASDAQ Global Market, have imposed various requirements on public companies, including requiring establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. Our management and other personnel devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations have increased our legal and financial compliance costs and made some activities more time-consuming and costly. For example, these rules and regulations have made it more difficult and more expensive for us to obtain director and officer liability insurance coverage.
The Sarbanes-Oxley Act requires, among other things, that we maintain effective internal controls for financial reporting and disclosure controls and procedures. In particular, we must perform system and process evaluation and testing of our internal controls over financial reporting to allow management and our independent registered public accounting firm to report on the effectiveness of our internal controls over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act. Our testing, or the subsequent testing by our independent registered public accounting firm, may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses. Our compliance with Section 404 may require that we incur substantial accounting expense and expend significant management efforts. We currently do not have an internal audit group, and have had to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge. Moreover, if we are not able to comply with the requirements of Section 404 in a timely manner, or if we or our independent registered public accounting firm identifies deficiencies in our internal controls over financial reporting that are deemed to be
46
material weaknesses, the market price of our stock could decline and we could be subject to sanctions or investigations by NASDAQ, the SEC or other regulatory authorities, which would require additional financial and management resources.
Substantial sales of our common stock in the public market could cause our stock price to fall.
Sales of a substantial number of shares of our common stock in the public market or the perception that these sales might occur, could depress the market price of our common stock and could impair our ability to raise capital through the sale of additional equity securities. We are unable to predict the effect that sales may have on the prevailing market price of our common stock.
Certain holders of shares of our common stock and warrants to purchase shares of our common stock are entitled to rights with respect to the registration of their shares under the Securities Act. Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares purchased by affiliates. Any sales of securities by these stockholders could have a material adverse effect on the trading price of our common stock.
Future sales and issuances of our common stock or rights to purchase common stock, including pursuant to our equity incentive plans, could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
We expect that significant additional capital will be required in the future to continue our planned operations. To the extent we raise additional capital by issuing equity securities; our stockholders may experience substantial dilution. We may sell common stock in one or more transactions at prices and in a manner we determine from time to time. If we sell common stock in more than one transaction, stockholders who purchase stock may be materially diluted by subsequent sales. Such sales may also result in material dilution to our existing stockholders, and new investors could gain rights superior to existing stockholders. Pursuant to our 2006 Equity Incentive Plan, our management is authorized to grant stock options to our employees, directors and consultants, and our employees are eligible to participate in our 2006 Employee Stock Purchase Plan. The number of shares available for future grant under our 2006 Equity Incentive Plan can, subject to approval of our board of directors, increase each April 1 by the lesser of five percent of the number of total outstanding shares of our common stock on December 31 of the preceding year or 1,325,448 shares, subject to the ability of our board of directors to reduce such increase. Additionally, the number of shares reserved for issuance under our 2006 Employee Stock Purchase Plan can, subject to approval of our board of directors, increase each April 1 by the lesser of one percent of the number of total outstanding shares of our common stock on December 31 of the prior year or 101,957 shares, subject to the ability of our board of directors to reduce such increase. In addition, we also have warrants outstanding to purchase shares of our common stock. Our stockholders will incur dilution upon exercise of any outstanding stock options or warrants.
All of the shares of common stock sold in our initial public offering are freely tradable without restrictions or further registration under the Securities Act of 1933, as amended, except for any shares purchased by our affiliates as defined in Rule 144 under the Securities Act. Rule 144 defines an affiliate as a person who directly, or indirectly through one or more intermediaries, controls, or is controlled by, or is under common control with, us and would include persons such as our directors and executive officers.
Our ability to utilize our net operating loss carryforwards and certain other tax attributes may be limited.
Under Section 382 of the Internal Revenue Code, if a corporation undergoes an “ownership change” (generally defined as a greater than 50% change (by value) in its equity ownership over a three-year period), the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes to offset its post-change income may be limited. We believe that, based on an analysis of historical equity transactions under the provisions of Section 382, ownership changes have occurred at two points since our inception. These ownership changes will limit the annual utilization of our net operating losses in future periods. We do not believe, however, that these ownership changes will result in the loss of any of our net
47
operating loss carryforwards existing on the date of each of the ownership changes. We may also experience ownership changes in the future as a result of subsequent shifts in our stock ownership, and such changes may result in the loss of net operating loss carryforwards on such ownership change date.
Some provisions of our charter documents and Delaware law may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our stockholders.
Provisions in our certificate of incorporation and bylaws, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us, even if doing so would benefit our stockholders. These provisions include:
| | |
| | • | authorizing the issuance of “blank check” preferred stock, the terms of which may be established and shares of which may be issued without stockholder approval; |
| |
| | • | limiting the removal of directors by the stockholders; |
| |
| | • | prohibiting stockholder action by written consent, thereby requiring all stockholder actions to be taken at a meeting of our stockholders; |
| |
| | • | eliminating the ability of stockholders to call a special meeting of stockholders; and |
| |
| | • | establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon at stockholder meetings. |
In addition, we are subject to Section 203 of the Delaware General Corporation Law, which generally prohibits a Delaware corporation from engaging in any of a broad range of business combinations with an interested stockholder for a period of three years following the date on which the stockholder became an interested stockholder, unless such transactions are approved by our board of directors. This provision could have the effect of delaying or preventing a change of control, whether or not it is desired by or beneficial to our stockholders.
| |
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
Not applicable.
Our facilities currently consist of approximately 52,000 square feet of laboratory and office facilities located at our headquarters in Louisville, Colorado, which is leased until September 2011, and approximately 8,000 square feet of office facilities for our clinical and regulatory group in Milford, Connecticut, which is leased until May 2010.
We believe that these facilities are adequate to meet our current needs. We believe that if additional space beyond the space currently under lease is needed in the future, such space will be available on commercially reasonable terms as needed.
| |
| ITEM 3. | LEGAL PROCEEDINGS |
We are not currently subject to any material pending legal proceedings.
| |
| ITEM 4. | SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS |
Not applicable.
48
Executive Officers of the Registrant
Our executive officers are as follows:
| | | | | | | |
Name | | Age | | Position |
| |
| Kenneth J. Collins | | | 61 | | | President, Chief Executive Officer and Director |
| Roger M. Echols, M.D. | | | 59 | | | Chief Medical Officer |
| Nebojsa Janjic, Ph.D. | | | 47 | | | Chief Scientific Officer and Secretary |
| Peter W. Letendre, Pharm.D. | | | 50 | | | Chief Commercial Officer |
| Donald J. Morrissey, Jr. | | | 42 | | | Senior Vice President, Corporate Development |
| Mark L. Smith | | | 46 | | | Chief Financial Officer and Treasurer |
Kenneth J. Collinshas served as our President, Chief Executive Officer and a member of the board of directors since January 2002. From 1997 to 2001, Mr. Collins served as President of Pegasus Technology Ventures, a firm that advised and raised seed capital for early stage life sciences companies. From 1995 to 1996, Mr. Collins served as Chief Financial Officer and a member of the board of directors of Quark, Inc., a developer of desktop publishing software. Mr. Collins served as an Executive Vice President from 1992 to 1994 and Chief Financial Officer from 1983 to 1994 of Synergen, Inc., a biotechnology company. Mr. Collins holds a B.S. from the University of Notre Dame and an M.B.A. from the Harvard Business School.
Roger M. Echols, M.D. has served as our Chief Medical Officer since January 2005. From 1997 to 2004, Dr. Echols served as Vice President of Infectious Disease Clinical Research and Development at Bristol Myers Squibb. He served as Medical Director at Immunex Corporation from 1996 to 1997 and as Medical Director at Bayer Corporation from 1989 to 1996. Prior to joining the pharmaceutical industry, Dr. Echols was Head of the Division of Infectious Diseases at Albany Medical College and an attending physician at Albany Medical Center. Dr. Echols holds a B.A. from Yale University and an M.D. from Tufts University School of Medicine and trained in internal medicine and infectious diseases at the University of New Mexico.
Nebojsa Janjic, Ph.D. has served as our Secretary since December 2000 and as our Chief Scientific Officer since June 2005. Dr. Janjic joined us at inception and served as our Senior Vice President and Vice President, Research and Development until June 2005. From 1992 to 1999, Dr. Janjic held various positions at NeXstar Pharmaceuticals, Inc., a biotechnology company, most recently serving as Senior Director, Drug Discovery. Dr. Janjic holds B.S. and Ph.D. degrees from the University of Washington and completed postdoctoral training at the Scripps Research Institute.
Peter W. Letendre, Pharm.D. has served as our Chief Commercial Officer since March 2005. From October 2002 until February 2005, Dr. Letendre held various positions at Abbott Laboratories, most recently as Vice President and General Manager of the anti-infective division from October 2002 until July 2004. From August 1990 to September 2002, Dr. Letendre held a number of marketing positions with SmithKline Beecham and GlaxoSmithKline Pharmaceuticals, including Marketing Director for the diabetes and metabolism division from 2000 to 2002. From 1988 to 1990, Dr. Letendre served as the Associate Dean of Clinical Practice at Southeastern University of the Health Sciences. Dr. Letendre holds B.S. and Doctor of Pharmacy degrees from the Massachusetts College of Pharmacy and Allied Health Sciences.
Donald J. Morrissey, Jr. has served as our Senior Vice President, Corporate Development since March 2006 and, prior to that, as Vice President, Corporate Development and General Counsel since 2002. From 1997 to 2002, Mr. Morrissey held various positions with Caliper Technologies, most recently as Vice President, Legal Affairs and Business Development from September 2001 to November 2002. From 1992 to 1997, Mr. Morrissey was a business attorney with Cooley Godward LLP. Mr. Morrissey holds a B.A. from the University of Colorado and a J.D. from the University of Southern California Law School.
Mark L. Smithhas served as our Chief Financial Officer and Treasurer since March 2006. From August 1999 to March 2006, Mr. Smith held financial executive capacities at Nabi Biopharmaceuticals, including serving as Senior Vice President, Finance, Chief Financial Officer and Chief Accounting Officer from 2001 to March 2006. From 1998 to 1999, Mr. Smith served as Vice President of Finance and Administration and Chief Financial Officer of Neuromedical Systems, Inc. From 1996 to 1998, Mr. Smith served in various financial
49
executive capacities at Genzyme Corporation. From 1991 to 1996, Mr. Smith held various positions at Genetrix, Inc., most recently as its Chief Financial Officer. Before joining Genetrix, Inc., Mr. Smith practiced with the accounting firm of PricewaterhouseCoopers LLP in both the U.S. and Australia. Mr. Smith holds a B.A. in Accounting from the Canberra College of Advanced Education in Australia.
PART II
| |
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Price Range of Common Stock and Dividend Policy
The following table sets forth the high and low sales prices for our common stock (based uponintra-day trading) as reported by the NASDAQ Global Market:
| | | | | | | | | |
| | | Common Stock | |
| | | High | | | Low | |
| |
Fiscal Year Ended December 31, 2007 | | | | | | | | |
| First quarter | | $ | 6.28 | | | $ | 4.28 | |
| Second quarter | | | 6.07 | | | | 5.10 | |
| Third quarter | | | 7.50 | | | | 5.23 | |
| Fourth quarter | | | 6.66 | | | | 3.05 | |
Fiscal Year Ended December 31, 2006 | | | | | | | | |
| Second quarter | | $ | 10.25 | | | $ | 9.66 | |
| Third quarter | | | 10.86 | | | | 8.40 | |
| Fourth quarter | | | 10.74 | | | | 4.80 | |
The number of record holders of our common stock on February 14, 2008 was approximately 92. No cash dividends have been previously paid on our common stock and none are anticipated in 2008.
Comparative Stock Performance Graph
The comparative stock performance graph below compares the cumulative total stockholder return (assuming reinvestment of dividends, if any) from investing $100 on June 28, 2006, the date on which our common stock was first publicly traded, in each of (i) our common stock, (ii) the NASDAQ Composite Index and (iii) the RDG MicroCap Biotechnology Index; except that, in the case of the NASDAQ Composite Index and the RDG MicroCap Biotechnology Index, the stock performance graph below reflects an investment date of May 31, 2006.
50
COMPARISON OF 18 MONTH CUMULATIVE TOTAL RETURN*
Among Replidyne, Inc, The NASDAQ Composite Index
And The RDG MicroCap Biotechnology Index
| |
| * | $100 invested on 6/28/06 in stock or 5/31/06 in index-including reinvestment of dividends. Fiscal year ending December 31. |
Recent Sales of Unregistered Securities
None
Issuer Purchases of Equity Securities.
| | | | | | | | | | | | | | | | | |
| | | | | | | | | Maximum Number (or
|
| | | | | | | Total Number of
| | Approximate Dollar
|
| | | | | | | Shares Purchased as
| | Value) of Shares
|
| | | | | | | Part of Publicly
| | That may Yet be
|
| | | Total Number of
| | Average Price
| | Announced Plans or
| | Purchased Under the
|
Period | | Shares Purchased | | Paid per Share | | Programs | | Plans or Programs |
| |
| 10/31/07 | | | 5,778 | (1) | | $ | 0.61 | | | | None | | | | Not Applicable | |
| 12/19/07 | | | 237 | (2) | | $ | 5.48 | | | | None | | | | Not Applicable | |
| | |
| (1) | | Repurchase of unvested restricted stock from an employee at cost. |
| |
| (2) | | Shares acquired in payment of tax liabilities pursuant to the partial vesting of a restricted stock award issued to an Employee under our 2006 Equity Incentive Plan. The tax liabilities were paid in 2007 and 2008. |
The information included under the heading “Comparative Stock Performance Graph” in this Item 5 of our annual report onForm 10-K shall not be deemed to be “soliciting material” or subject to Regulation 14A or 14C, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the Exchange Act), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act.
51
| |
| ITEM 6. | SELECTED FINANCIAL DATA |
The following selected financial data should be read together with our financial statements and accompanying notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” appearing elsewhere in this Annual Report. The selected financial data in this section is not intended to replace our financial statements and the accompanying notes. Historical results are not necessarily indicative of operating results to be expected in the future. All amounts in the following table are expressed in thousands, except for per share data.
The selected financial data presented below for each year in the five years ended December 31, 2007, are derived from our financial statements, which have been audited by KPMG LLP, independent registered public accounting firm, and are qualified by reference to such Financial Statements and Notes thereto. The statements of operations data for the years ended December 31, 2007, 2006 and 2005 and the balance sheet data as of December 31, 2007 and 2006 are derived from our audited financial statements appearing elsewhere in this Annual Report onForm 10-K. The statements of operations data for the years ended December 31, 2004 and 2003 and the balance sheet data as of December 31, 2005, 2004 and 2003 are derived from our audited financial statements not included in this Annual Report.
| | | | | | | | | | | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2007 | | | 2006 | | | 2005 | | | 2004 | | | 2003 | |
| | | (in thousands, except per share amounts) | |
| |
Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | |
| Revenue | | $ | 58,571 | | | $ | 15,988 | | | $ | 441 | | | $ | 834 | | | $ | 726 | |
| Costs and expenses: | | | | | | | | | | | | | | | | | | | | |
| Research and development | | | 43,313 | | | | 38,295 | | | | 29,180 | | | | 16,282 | | | | 12,331 | |
| Sales, general and administrative | | | 13,020 | | | | 12,187 | | | | 5,329 | | | | 2,994 | | | | 2,155 | |
| | | | | | | | | | | | | | | | | | | | | |
| Total costs and expenses | | | 56,333 | | | | 50,482 | | | | 34,509 | | | | 19,276 | | | | 14,486 | |
| | | | | | | | | | | | | | | | | | | | | |
| Income (loss) from operations | | | 2,238 | | | | (34,494 | ) | | | (34,068 | ) | | | (18,442 | ) | | | (13,760 | ) |
| Other income (expense), net | | | 5,454 | | | | 5,245 | | | | 399 | | | | (797 | ) | | | (190 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Net income (loss) | | | 7,692 | | | | (29,249 | ) | | | (33,669 | ) | | | (19,239 | ) | | | (13,950 | ) |
| Preferred stock dividends and accretion | | | — | | | | (5,391 | ) | | | (7,191 | ) | | | (3,560 | ) | | | (1,294 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Net income (loss) attributable to common stockholders | | $ | 7,692 | | | $ | (34,640 | ) | | $ | (40,860 | ) | | $ | (22,799 | ) | | $ | (15,244 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Basic net income (loss) attributable to common stockholders per share(1): | | $ | 0.29 | | | $ | (2.49 | ) | | $ | (39.20 | ) | | $ | (30.55 | ) | | $ | (20.82 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Diluted net income (loss) attributable to common stockholders per share(1): | | $ | 0.28 | | | $ | (2.49 | ) | | $ | (39.20 | ) | | $ | (30.55 | ) | | $ | (20.82 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | As of December 31, | |
| | | 2007 | | | 2006 | | | 2005 | | | 2004 | | | 2003 | |
| | | (in thousands) | |
| |
Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
| Cash, cash equivalents and short-term investments | | $ | 90,266 | | | $ | 125,567 | | | $ | 59,420 | | | $ | 27,018 | | | $ | 692 | |
| Working capital | | | 80,440 | | | | 68,147 | | | | 50,755 | | | | 24,409 | | | | (1,657 | ) |
| Total assets | | | 94,690 | | | | 135,561 | | | | 63,579 | | | | 30,067 | | | | 4,169 | |
| Long-term debt, net of current portion and discount | | | — | | | | — | | | | — | | | | 84 | | | | 1,208 | |
| Accumulated deficit | | | (109,288 | ) | | | (116,980 | ) | | | (83,107 | ) | | | (42,235 | ) | | | (20,105 | ) |
| Preferred stock | | | — | | | | — | | | | 136,815 | | | | 69,447 | | | | 20,058 | |
| Total stockholders’ equity (deficit) | | | 82,404 | | | | 71,372 | | | | (82,632 | ) | | | (42,202 | ) | | | (20,115 | ) |
52
| | |
| (1) | | Please see Note 2 to our financial statements for an explanation of the method used to calculate the net loss attributable to common stockholders per share and the number of shares used in the computation of the per share amounts. |
| |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
You should read the following discussion and analysis together with our financial statements and the notes to those statements included elsewhere in this report. This discussion contains forward-looking statements that involve risks and uncertainties. As a result of many factors, such as those set forth under Part I, Item 1A and elsewhere in this report, our actual results may differ materially from those anticipated in these forward-looking statements. See “Special Note Regarding Forward-Looking Statements” under Part I, Item 1.
Overview
We are a biopharmaceutical company focused on discovering, developing, in-licensing and commercializing innovative anti-infective products. Our most advanced product candidate, faropenem medoxomil, is a novel oral, community antibiotic for which we are currently seeking a development and commercialization partner. Our second product candidate, REP3123, is a new narrow spectrum antibacterial agent for the treatment ofClostridium difficile, orC. difficile, bacteria andC. difficile-associated disease, an increasing health care concern among elderly and hospitalized patients. We are also pursuing the development of other novel compounds that inhibit bacterial DNA replication, which we believe represents a potentially promising drug target in antibiotic development.
In December 2005, we submitted a new drug application, or NDA, for faropenem medoxomil based on 11 Phase III studies for the following adult indications: acute bacterial sinusitis; community-acquired pneumonia; acute exacerbation of chronic bronchitis; and uncomplicated skin and skin structure infections. In October 2006, the FDA issued a non-approvable letter with respect to our NDA citing the need for further clinical studies for all indications, including studies using a superiority design for acute bacterial sinusitis and acute exacerbation of chronic bronchitis, more extensive microbiologic confirmation and consideration of alternate dosing regimens. A superiority design trial requires demonstrating that a product candidate is superior to placebo. Historically, all of our trials were conducted using a non-inferiority design, which required these trials to demonstrate that a product candidate is not significantly less effective than an approved treatment. On January 22, 2008, we received a Warning Letter from the FDA related to our NDA filed in December 2005 for faropenem medoxomil citing certain conditions found by the FDA during their review of our role as the applicant of the NDA. Specifically, the Warning Letter noted that certain raw data, descriptions and analysis supporting clinical trials included in the NDA were not available for the FDA’s review and had not been obtained or reviewed by us prior to submission of the NDA. We intend to respond to the Warning Letter within the time limits required by the FDA.
The focus of our activities following receipt of the non-approvable letter from the FDA has been to clarify the approval process for faropenem medoxomil in the treatment of community respiratory tract infections. We do not expect to pursue the indication for uncomplicated skin and skin structure infections unless we enter into a collaboration with a partner that wishes to do so. Based on the FDA’s recommendations in the non-approvable letter, as well as our ongoing discussions with the FDA, we understand that at least two approved clinical studies using faropenem medoxomil for the treatment of community-acquired pneumonia will be required for approval in this indication. If we or a future partner seek approval for faropenem medoxomil to treat acute bacterial sinusitis and acute exacerbation of chronic bronchitis in addition to community-acquired pneumonia, the faropenem medoxomil adult program may be anchored on at least two clinical trials for the treatment of community-acquired pneumonia with single clinical trials using a superiority clinical trial design in acute bacterial sinusitis and acute exacerbation of chronic bronchitis. We have completed a special protocol assessment, or SPA, for the design of a Phase III clinical trial of faropenem medoxomil compared to placebo for the treatment of acute bacterial sinusitis. We plan to continue our ongoing Phase III placebo-controlled clinical trial for treatment of acute exacerbation of chronic bronchitis with
53
faropenem medoxomil which is intended to meet the FDA’s requirements. Until we have secured a partner for the faropenem medoxomil program, which cannot be assured, we plan to limit our faropenem medoxomil clinical activities to the ongoing Phase III placebo-controlled clinical trial for the treatment of acute exacerbation of chronic bronchitis. If we are delayed in securing or are unable to secure a partner for the faropenem medoxomil program, we may elect to discontinue our development activities on this program, including to discontinue the Phase III placebo-controlled clinical trial for the treatment of acute exacerbation of chronic bronchitis. We have licensed all rights to faropenem medoxomil from Asubio Pharma Co., Ltd., or Asubio Pharma, in the U.S. and Canada. In addition, we have the sole negotiation right to license such rights for the rest of the world, except Japan.
We are also developing REP3123, our investigational narrow spectrum antibacterial agent to treatC. difficilebacteria andC. difficile -associated disease.C. difficileis a Gram-positive bacterium that causes diarrhea and other intestinal conditions, such as colitis, and is a major cause of morbidity among the elderly and hospitalized patients. People generally contractC. difficile-associated disease through the ingestion ofC. difficile spores after coming into contact with a contaminated item or surface. These spores then germinate, grow and multiply in the digestive tract. Inin vitro preclinical studies, REP3123 displayed an ability to inhibit growth of theC. difficilebacterium and prevent the bacterium from forming the spores that allow it to be spread from person to person, but without inhibiting other key organisms that are essential for normal intestinal functioning. Also in preclinical studies, REP3123 exhibited signs it may be able to stop the production of destructive intestinal toxins caused byC. difficilebacteria. These results suggest that REP3123 has the potential to reduceC. difficile-associated disease outbreak and relapse rates through reducing the presence ofC. difficilespores and reduce the severity of, or possibly even prevent,C. difficile-associated disease through inhibiting the growth of or stopping production of toxins caused byC. difficilebacteria. We retain worldwide rights to REP3123.
We have also developed assays that identify compounds that inhibit bacterial DNA replication. The compounds may be useful to treat bacterial infections. We believe that bacterial DNA replication is an attractive target system for new antibacterial drugs because it is an essential cellular process and stalled DNA replication can trigger cell death. Our assays allow for efficient screening of large libraries of small molecules and are designed to mimic the bacterial DNA replication systems of numerous bacteria, with the goal of identifying novel inhibitors of bacterial DNA replication. We have identified compounds that are able to inhibit bacterial DNA replication in these assays. We believe that the novel mechanism of action of our technology may reduce the risk that bacteria will develop resistance to drugs based on this technology. We are currently optimizing the initial inhibitors identified in the assays.
We had also been developing REP8839, a topical antibiotic that had exhibited activity in preclinical studies againstS. aureus, including methicillin resistantS. aureusor MRSA, and mupirocin resistant strains ofS. aureus.As a result of prioritizing our preclinical programs, in December 2007, we suspended the development of REP8839 due to the incremental investment required to optimize the formulation and the niche market opportunity for its initial target indication of treatment of impetigo.
We have incurred significant operating losses since our inception on December 6, 2000, and, as of December 31, 2007, we had an accumulated deficit of $109 million. We have generated no revenue from product sales to date. We have funded our operations to date principally from the sale of our securities and payments received from Forest Laboratories under our former collaboration and commercialization agreement. Although we reported net income for the year ended December 31, 2007 as a result of the termination of our agreement with Forest Laboratories, as discussed below, we expect to incur substantial operating losses for the next several years as we pursue our clinical trials and research and development efforts.
Former Collaboration with Forest Laboratories
In February 2006, we entered into a collaboration and commercialization agreement with Forest Laboratories to be our exclusive partner for the development and marketing of faropenem medoxomil in the U.S. On May 7, 2007, Forest Laboratories exercised its right to terminate this agreement. The termination followed issuance in October 2006 of a non-approvable letter by the FDA for our NDA for faropenem
54
medoxomil. As a result, we reacquired all rights to faropenem medoxomil previously granted to Forest Laboratories. There were no penalty fees incurred by either us or Forest Laboratories in connection with the termination of the agreement and no amounts previously received by us under the agreement are refundable. We received $60 million in upfront and milestone payments and approximately $14.6 million of contract revenue for funded activities related to the development of faropenem medoxomil from Forest Laboratories during the period of our collaboration.
In accordance with our revenue recognition policy for upfront and milestone payments received under collaboration and commercialization agreements, we had recognized revenue in prior periods for the payments received from Forest Laboratories on a straight-line basis over a period of approximately 15 years, which was the estimated period of benefit. These upfront and milestone payments received are non-refundable. As no further obligations existed beyond the termination date of May 7, 2007, we recognized the remaining unamortized deferred upfront and milestone fees of approximately $55 million as revenue on that date. We also received reimbursements from Forest Laboratories for research and development and sales and marketing activities during 2007. These amounts have been recorded as revenue. This treatment reflected our role as principal in these transactions whereby we were responsible for selecting vendors, performing significant duties and bearing credit risk.
Comparison of Years Ended December 31, 2007 and 2006
Revenue. We recognized $58.6 million in revenue during 2007 compared to $16.0 million in 2006. The increase was due to the recognition of previously deferred revenue as a result of the termination of our collaboration and commercialization agreement with Forest Laboratories in 2007. Revenue recognized during 2007 included $56.2 million of license revenue, representing the unamortized portion of $60 million in upfront and milestone payments we received under our collaboration agreement with Forest Laboratories, as compared to $3.8 million of license revenue recognized in 2006. Revenue recognized during 2007 also included $2.4 million of contract revenue for funded activity under our former collaboration and commercialization agreement with Forest Laboratories, as compared to $12.2 million of contract revenue recognized in 2006. Due to the termination of our collaboration and commercialization agreement with Forest Laboratories, our prospects for other near term future revenues are substantially uncertain. Our ability to generate future revenue depends heavily on our ability to obtain a new collaboration partner for faropenem medoxomil on acceptable terms, which cannot be assured.
Research and Development Expense. Research and development expenses were $43.3 million for 2007 as compared to $38.3 million in 2006. Research and development expenditures made to advance our product candidates and other research efforts during 2007 and 2006 were as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| | | Year Ended
| | | | |
| | | December 31, | | | Change | |
| | | 2007 | | | 2006 | | | $ | | | % | |
| |
| Faropenem medoxomil | | $ | 29,231 | | | $ | 23,266 | | | $ | 5,965 | | | | 26 | % |
| REP8839 | | | 4,550 | | | | 8,363 | | | | (3,813 | ) | | | (46 | )% |
| Other research and development | | | 9,532 | | | | 6,666 | | | | 2,866 | | | | 43 | % |
| | | | | | | | | | | | | | | | | |
| | | $ | 43,313 | | | $ | 38,295 | | | $ | 5,018 | | | | 13 | % |
| | | | | | | | | | | | | | | | | |
Costs to support our faropenem medoxomil program were $6.0 million higher in 2007 as compared to 2006. The increase primarily reflects expenditures related to increased external clinical trial activity and clinical trial preparations with a clinical research organization of $10.4 million. This increase was partially offset by a $1.4 million decrease in preclinical research and outside services, a $1.2 million decrease in contingent supply agreement fees and a $1.1 million decrease in program acquisition fees. Research and development activities in 2007 were focused on the ongoing Phase III clinical trial for the treatment of acute exacerbation of chronic bronchitis as well as planning activities in preparation for potential future Phase III clinical trials for the treatment of acute bacterial sinusitis and community-acquired pneumonia. Research and development activities in 2006 were focused on the Phase III placebo-controlled acute exacerbation of chronic
55
bronchitis clinical trial as well as the Phase II clinical trial in pediatric patients with acute bacterial otitis media which results were reported in the first quarter of 2007.
In 2007, costs to support our REP8839 program decreased by $3.8 million as compared to 2006 primarily reflecting decreased clinical and preclinical development costs of $2.0 million. This program was suspended in December 2007 due to the incremental investment required to optimize the formulation compared to the niche market opportunity represented by the product candidate’s initial target indication of impetigo. Additionally, in 2006 we incurred $1.5 million under our June 2003 purchase agreement with GlaxoSmithKline PLC, or GSK to complete the purchase of the inhibition of tRNA synthetase technology underlying REP8839 and REP3123.
In 2007, other research and development costs increased by $2.9 million as compared to 2006. Costs of internal research and development personnel and related costs increased by $2.1 million as we increased the activity levels of our research and development personnel in support of ourC. difficileprogram, or REP3123, and DNA replication inhibition program. Other costs in support of these programs included external preclinical research, consulting and other services that increased by $0.4 million in 2007 compared to 2006.
Clinical development timelines, likelihood of success and associated costs are uncertain and therefore vary widely. Although we focused primarily on faropenem medoxomil for the treatment of community-acquired respiratory tract infections in 2006 and 2007, we anticipate that we will make determinations as to which research and development projects to pursue and how much funding to direct toward each project on an on-going basis in response to the guidance we receive through meetings with the FDA regarding each intended indication for faropenem medoxomil and the scientific and clinical success of each of our product candidates and research and development programs.
Due to the risks inherent in the clinical trial process, development completion dates and costs will vary significantly for each product candidate and are difficult to estimate. The lengthy regulatory approval process for our current and potential product candidates requires substantial additional resources. Any failure by us to obtain, or any delay in obtaining, regulatory approvals for our product candidates could cause the costs of our research and development to increase and have a material adverse effect on our results of operations. We cannot be certain when any cash flows from our current product candidates will commence.
Selling, General and Administrative Expenses. Selling, general and administrative expenses were $13.0 million for 2007, compared to $12.2 million for 2006. In 2007, we incurred incremental personnel costs of $0.9 million associated with personnel hired during 2006 to support our commercial, finance and administrative activities, compensation costs of $0.5 million related to our organizational restructuring announced in December 2007 and increased costs associated with the adoption of SFAS 123(R),Share-Based Paymentof $0.7 million. We also incurred increased legal, accounting and insurance fees resulting from our first full year of compliance with Section 404 of the Sarbanes-Oxley Act. These increases were partially offset by reductions in market research costs of $1.4 million primarily related to the faropenem medoxomil program. In 2008, we expect that selling, general and administrative expenses will be lower than 2007 levels.
Investment Income, net. Investment income was $5.5 million for 2007, compared to $6.0 million for 2006. The decrease was primarily due to lower overall cash available for investing in 2007. In 2006, we received cash of $60 million under our former collaboration and commercialization agreement with Forest Laboratories and $44.5 million in net proceeds from our initial public offering.
Interest Expense. In 2006 we incurred interest expense of $14 thousand. The equipment loan and security agreement was paid in full in 2006.
Other Expense, net. Other expense was $0.1 million for 2007, compared to $0.7 million in 2006. The decrease was primarily due to $0.4 million lower foreign currency losses associated with our foreign currency denominated payables and $0.1 million in losses to adjust derivatives in 2006 to market value.
Comparison of Years Ended December 31, 2006 and 2005
Revenue. Revenue was $16 million for the year ended December 31, 2006, as compared to $0.4 million for the year ended December 31, 2005. The increase was due to revenue generated from our collaboration and
56
commercialization agreement with Forest Laboratories which began in 2006. Revenue recognized during 2006 includes $3.8 million of license revenue, representing a portion of the upfront and milestone payments totaling $60 million, which was being recognized in our financial statements as of December 31, 2006 as revenue over the estimated period of performance of approximately 14 years, and $12.2 million of contract revenue for funded activity under our collaboration and commercialization agreement with Forest Laboratories. Revenue recognized in 2005 consists solely of license revenue generated from a research and development project that was completed in 2005.
Research and Development Expense. Research and development expenses were $38.3 million for the year ended December 31, 2006 compared to $29.2 million for the year ended December 31, 2005. Research and development expenditures made to advance our product candidates and other research efforts during 2006 and 2005 were as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| | | Year Ended
| | | | |
| | | December 31, | | | Change | |
| | | 2006 | | | 2005 | | | $ | | | % | |
| |
| Faropenem medoxomil | | $ | 23,266 | | | $ | 24,744 | | | $ | (1,478 | ) | | | (6 | )% |
| REP8839 | | | 8,363 | | | | 3,589 | | | | 4,774 | | | | 133 | % |
| Other research and development | | | 6,666 | | | | 847 | | | | 5,819 | | | | 687 | % |
| | | | | | | | | | | | | | | | | |
| | | $ | 38,295 | | | $ | 29,180 | | | $ | 9,115 | | | | 31 | % |
| | | | | | | | | | | | | | | | | |
Costs incurred for the development of faropenem medoxomil were lower in 2006 compared to 2005 primarily reflecting decreased external clinical trial activity of $2.5 million, a $1.6 million decrease in costs of our internal research and development personnel and related costs and a $1 million decrease in expense incurred under our license agreement with Asubio Pharma. These decreases were partially offset by $2.9 million of supply agreement contingencies that were recognized on October 20, 2006 when the FDA issued a non-approvable letter for the NDA we filed for faropenem medoxomil. During 2006, we continued to support our ongoing placebo controlled Phase III trial among patients with acute exacerbation of chronic bronchitis and our Phase II dose ranging clinical trial among pediatric patients with acute bacterial otitis media. During 2005, in addition to the thorough QT study completed for faropenem medoxomil in connection with our NDA submission we incurred significant external clinical research organization expenses supporting preparation of the NDA for faropenem medoxomil that was filed with the FDA in December 2005.
In 2006, costs to support our REP8839 program increased by $4.8 million compared to 2005 following initiation of our Phase I clinical trials program for this compound in July 2006, which resulted in increased external clinical trial costs of $1.9 million and internal personnel costs of $0.7 million. In 2006 we also incurred $1.5 million under our June 2003 purchase agreement with GlaxoSmithKline PLC, or GSK, due upon filing of our IND related to REP8839 with the FDA that was accounted for as research and development expense. We have no further financial obligations due to GSK under this agreement.
In 2006, other research and development costs increased by $5.8 million compared to 2005. Costs of internal research and development personnel and related costs increased by $2.2 million as we increased our research and development personnel in support of our expanded development activities specifically related to ourC. difficileprogram and DNA replication inhibition program. Other costs in support of these activities included external preclinical research, consulting, services and chemicals, compounds and laboratory costs that increased by $2 million.
Selling, General and Administrative Expenses. Selling, general and administrative expenses were $12.2 million for the year ended December 31, 2006, as compared to $5.3 million for the year ended December 31, 2005. The increase was primarily due to increased personnel and related costs of $4.3 million which resulted from additional staff required to support our commercial organization and administrative and finance personnel, costs of recruiting and relocating personnel, costs associated with the initial adoption of SFAS 123(R),Share-Based Payment, of $0.8 million, as well as $0.8 million in additional legal, accounting, insurance and other professional costs related to compliance obligations associated with being a public
57
company. Market research expenses also increased by $1 million, principally related to market research associated with faropenem medoxomil and REP8839.
Investment Income, net. Investment income was $6 million for the year ended December 31, 2006, as compared to $0.7 million for the year ended December 31, 2005. The increase was primarily due to higher overall cash available for investing following receipt of $60 million under our collaboration and commercialization agreement with Forest Laboratories in the first quarter of 2006 and $44.5 million in net proceeds from our initial public offering completed in the third quarter of 2006.
Interest Expense. Interest expense was $14 thousand for the year ended December 31, 2006, as compared to $0.1 million for the year ended December 31, 2005. The decrease was due to payment in full of our equipment loan and security agreement during the first quarter of 2006.
Other Expense, net. Other expense was $0.7 million for the year ended December 31, 2006, as compared to $0.2 million for the year ended December 31, 2005. The increase was primarily due to the recognition of approximately $0.4 million in foreign currency losses associated with our foreign currency denominated payables.
Liquidity and Capital Resources
As of December 31, 2007, we had $90.3 million in cash, cash equivalents and short-term investments. While we reported net income for the year ended December 31, 2007, we have accumulated significant operating losses since our inception in 2000 and as of December 31, 2007 we had an accumulated deficit of $109.3 million. We have funded our operations to date principally from private placements of equity securities and convertible notes totaling $121.5 million, receipt of payments from Forest Laboratories under our former collaboration and commercialization agreement totaling $74.6 million and net proceeds received from our initial public offering of $44.5 million.
As described above, our collaboration and commercialization agreement with Forest Laboratories was terminated on May 7, 2007, and as a result, our prospects for other near term future revenues are uncertain. Our ability to generate future revenue depends heavily on our ability to obtain a new collaboration partner for faropenem medoxomil on acceptable terms.
In October 2006, the FDA issued a non-approvable letter for our NDA for faropenem medoxomil that had been filed in December 2005. According to the non-approvable letter, the FDA recommends further clinical studies for all four indications that were the subject of the NDA including studies using superiority design for the indications of acute bacterial sinusitis and acute exacerbation of chronic bronchitis, additional microbiologic testing and consideration of alternate dosing regimens. We are discussing clinical plans with the FDA including the number of clinical trials needed for each indication, and currently expect that at least two to three years will be required for completion of the clinical studies. We do not intend to initiate additional clinical trials using faropenem medoxomil beyond the ongoing placebo-controlled Phase III clinical trial for acute exacerbation of chronic bronchitis until we have secured a partner for this program, which cannot be assured. We continue to evaluate the impact this FDA action will have on our liquidity and capital resources including costs of additional clinical trials and delays in product launch.
In 2004, we entered into a license agreement with Asubio Pharma to develop and commercialize faropenem medoxomil in the U.S. and Canada and we have the sole negotiation right to license such rights for the rest of the world except Japan which was modified in December 2005. Under the modified license agreement we are further obligated to future payments of up to ¥375 million (approximately $3.3 million as of December 31, 2007) upon filing of a new NDA at a higher dose of faropenem medoxomil than was studied in the prior NDA and up to ¥1,250 million (approximately $11.1 million as of December 31, 2007) in subsequent regulatory and commercial milestone payments for faropenem medoxomil. If we terminate our license agreement with Asubio Pharma, or if there is an intolerable delay in the commercial launch of faropenem medoxomil, as defined, we will be obligated to pay a termination fee of up to ¥375 million (approximately $3.3 million as of December 31, 2007). Additionally, we are responsible for royalty payments to Asubio Pharma based upon net sales of faropenem medoxomil. The license term extends to the later of: (i) the
58
expiration of the last to expire of the licensed patents owned or controlled by Asubio Pharma or (ii) 12 years after the first commercial launch of faropenem medoxomil. We have recorded payments made to date as research and development expense, as faropenem medoxomil has not been approved by the FDA.
Under a supply agreement entered into in December 2004 between Asubio Pharma, Nippon Soda and us, we are obligated to purchase, and Nippon Soda is obligated to supply, all our commercial requirements of the active pharmaceutical ingredient in faropenem medoxomil. During the three years following placement of an initial purchase order by us, which has not occurred, with Nippon Soda, we are obligated to make certain annual minimum purchase commitments to be determined initially by us and Nippon Soda at the time of a commercial launch. Since full commercial launch of faropenem medoxomil has been delayed, we are currently obligated to pay Nippon Soda escalating annual delay compensation fees of up to ¥280 million (approximately $2.5 million as of December 31, 2007) per year, which commenced on July 1, 2007. As a result of the non-approvable letter we received from the FDA in October 2006 and subsequent activities related to the development of faropenem medoxomil, we recorded delay compensation fees of $0.9 million in the year ended December 31, 2007 and delay compensation fees of $0.9 million and an initial order cancellation fee of $0.6 million in the year ended December 31, 2006. These amounts were recorded as research and development expense. If commercial launch of faropenem medoxomil is further delayed or if we are unable to obtain a collaboration partner for faropenem medoxomil under our current expected timeframe, we may incur additional delay compensation fees of up to ¥105 million ($0.9 million as of December 31, 2007) for 2008 and up to ¥280 million annually ($2.5 million as of December 31, 2007) for all periods following January 1, 2009. If we terminate this agreement, abandon the development or commercialization of faropenem medoxomil or are unable to notify Nippon Soda of the faropenem medoxomil launch go date, as defined, by July 1, 2009, we will be obligated to pay Nippon Soda prorated delay compensation fees through the effective date of termination and reimburse Nippon Soda for up to ¥65 million ($0.6 million as of December 31, 2007) in engineering costs. We continue to evaluate amounts which may become payable to Asubio Pharma and Nippon Soda under the terms of the agreement, and adjust our accrual accordingly.
In April 2005, we entered into a supply agreement for production of 300 mg adult tablets of faropenem medoxomil with MEDA, which was amended in March 2006. Beginning in 2006, we became obligated to make annual minimum purchases of MEDA’s product of €2.3 million (approximately $3.4 million as of December 31, 2007). If in any year we did not satisfy this minimum purchase commitment, we were required to pay MEDA the shortfall amount. Fifty percent (50%) of the shortfall amount, if applicable, would have been credited against future drug product purchases. We were required to buy all of our requirements for adult oral faropenem medoxomil tablets from MEDA until cumulative purchases exceeded €22 million (approximately $32.4 million at December 31, 2007). Upon termination of the agreement, under certain circumstances, we would have been obligated to pay up to €1.7 million (approximately $2.5 million as of December 31, 2007) in facility decontamination costs incurred by MEDA. In March 2006 when the agreement was amended, our obligations with respect to all purchase commitments and facility decontamination costs were suspended and deemed satisfied by Forest Laboratories pursuant to an agreement between MEDA and Forest Laboratories. Under our agreement with Forest Laboratories, we remained responsible for any shortfall amount in 2006 that may not be credited against future drug product purchases. In May 2007, following termination of our collaboration agreement with Forest Laboratories and the termination by Forest Laboratories of its supply agreement with MEDA, all previously suspended provisions in our direct agreement with MEDA were no longer suspended. In April 2007, we provided MEDA notice of termination of the supply agreement in accordance with the terms of the agreement. We believe that supply chain obligations, including fees that may arise from this agreement with MEDA, incurred through May 7, 2007 are the responsibility of Forest Laboratories under the commercialization and collaboration agreement. MEDA has indicated to us that it disputes our right to terminate the agreement on the basis indicated in our notice of termination. We believe that we had the right to terminate the agreement. However, if it is determined that we have obligations to MEDA beyond May 7, 2007 under the agreement, we may incur additional minimum purchase commitmentsand/or decontamination costs. We incurred expenses of $0.8 million and $1.5 million under this agreement in 2007 and 2006, respectively.
59
In May 2007, we entered into an arrangement with an investment bank to assist us in identifying a licensing partner for our faropenem medoxomil program and to provide other investment banking services. Under the terms of the agreement, we may incur transaction fees of up to $6 million based on the value of a license or strategic transaction as defined.
We have entered into employment agreements with our chief executive officer and other named executive officers that provide for base salary, eligibility for bonuses and other generally available benefits. The employment agreements provide that we may terminate the named executive officer employment at any time with or without cause. If a named executive officer is terminated by us without cause or such officer resigns for good reason, then the named executive officer is entitled to receive a severance package consisting of salary continuation for a period of twelve months from the date of termination among other benefits. If such termination occurs one month before or thirteen months following a change of control, then the executive is entitled to salary continuation for a period of twelve months (or eighteen months with respect to Mr. Collins and Dr. Janjic) from the date of termination and acceleration of vesting of all of the executive’s outstanding unvested options to purchase our common stock among other benefits. In addition, during 2007 we established a severance benefit plan that defines termination benefits for all eligible employees, as defined, not under an employment contract, if the employee is terminated without cause. Under this plan, employees whose employment is terminated without cause are provided a severance benefit of between nine and eighteen weeks pay, based on grade level, plus an additional two weeks pay for each year of service.
We have not yet commercialized our product candidates or generated any revenue from product sales. We anticipate that we will continue to incur substantial net losses in the next several years as we develop our products, conduct and complete clinical trials, pursue additional product candidates, expand our clinical development team and corporate infrastructure and prepare for the potential commercial launch of our product candidates including faropenem medoxomil. We do not anticipate generating any product related revenue until we obtain FDA approval for faropenem medoxomil and we or a future partner launches the product, which may not occur.
The pace and outcome of our clinical development programs and the progress of our discovery research program are difficult to predict. These projects may require several years and substantial expenditures to complete and may ultimately be unsuccessful. If we enter into additional third party collaborations or acquire new product candidates, the timing and amounts of any related licensing cash flows or expenses are likely to be highly variable. As a result, we anticipate that our quarterly results will fluctuate for the foreseeable future. In view of this variability and of our limited operating history, we believe that period-to-period comparisons of our operating results are not meaningful and you should not rely on them as indicative of our future performance.
Based on the current status of our product development and commercialization plans, we believe that our current cash, cash equivalents, short-term investments and interest earned on these balances will be sufficient to satisfy our anticipated cash needs for working capital and capital expenditures through at least the next 12 months. This forecast of the period in which our financial resources will be adequate to support operations is a forward-looking statement and involves risks, uncertainties and assumptions. Our actual results and the timing of selected events may differ materially from those anticipated as a result of many factors, including but not limited to those discussed under “Risk Factors” in Part I, Item 1A of this annual report.
Our future capital uses and requirements depend on a number of factors, including but not limited to the following:
| | |
| | • | the rate of progress and cost of our preclinical studies, clinical trials and other research and development activities; |
| |
| | • | our ability to obtain a new partner for development and commercialization of faropenem medoxomil on acceptable terms; |
| |
| | • | the scope and number of clinical development and research programs we pursue; |
| |
| | • | the costs, timing and outcomes of regulatory approvals; |
| |
| | • | the costs of establishing or contracting for marketing and sales capabilities, including the establishment of our own sales force; |
60
| | |
| | • | the extent to which we acquire or in-license new products, technologies or businesses; |
| |
| | • | the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; and |
| |
| | • | the terms and timing of any additional collaborative, strategic partnership or licensing agreements that we may establish. |
If our available cash, cash equivalents, short-term investments and interest earned on these balances are insufficient to satisfy our liquidity requirements, or if we develop additional products or pursue additional applications for our products or conduct additional clinical trials beyond those currently contemplated, we may seek to sell additional equity or debt securities or acquire a credit facility. The sale of additional equity may result in additional dilution to our stockholders. If we raise additional funds through the issuance of debt securities, those securities could have rights senior to those of our common stock and could contain covenants that would restrict our operations. We may require additional capital beyond our currently forecasted amounts. Any such required additional capital may not be available on reasonable terms, if at all. If we are unable to obtain additional financing, we may be required to modify our planned research, development and commercialization strategy, which could adversely affect our business.
Our future contractual obligations, including financing costs, at December 31, 2007, include the following (in thousands):
| | | | | | | | | | | | | | | | | | | | | |
| | | Payments Due by Period | |
| | | | | | Less Than
| | | 1-3
| | | 3-5
| | | Over
| |
| | | Total | | | 1 Year | | | Years | | | Years | | | 5 Years | |
| |
| Operating lease obligations(1) | | $ | 2,759 | | | $ | 737 | | | $ | 1,508 | | | $ | 514 | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | |
| MEDA Purchase Commitments(2) | | $ | 770 | | | $ | 770 | | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | |
| Nippon Soda Delay Compensation(3) | | $ | 7,795 | | | $ | 935 | | | $ | 6,860 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | |
| | |
| (1) | | Operating lease obligations represent future minimum rental commitments for non-cancelable operating leases for our office and laboratory facilities in Colorado and Connecticut. |
| |
| (2) | | Purchase obligations represent annual minimum purchase requirements of adult tablets of faropenem medoxomil with MEDA under our April 2005 supply agreement, through the termination of this agreement on May 11, 2007. This amount was paid in the first quarter of 2008. |
| |
| (3) | | Delay compensation assumes, for this purpose only, that a full commercial launch of an approved faropenem medoxomil drug does not occur for three years and the agreement is not terminated. |
The table above reflects only payment obligations that are fixed and determinable, based on certain of the assumptions described in the footnotes to the table. The table above does not include information with respect to the following contractual obligations because the amounts of the obligations are not currently determinable:
| | |
| | • | contractual obligations for clinical trials; |
| |
| | • | royalty obligations, which would be payable based on any future sales of faropenem medoxomil; |
| |
| | • | amounts due to Asubio Pharma under our license agreement, which amounts are uncertain as to timing and dependent on the achievement of milestones or termination of the agreement; and |
| |
| | • | contingent amounts that may become due under supply agreements, including minimum purchase commitments not yet established, the extent of delay compensation amounts determined based on the timing of a commercial launch and fees that may become due in termination. |
We enter into agreements with clinical research organizations and other vendors related to our clinical trials. Certain payments are made based upon the number of patients enrolled. For the years ended December 31, 2007 and 2006, we incurred external costs of approximately $20.4 million and $11.4 million, respectively, associated with conducting our clinical trials. At this time, due to the variability associated with
61
these agreements, we are unable to estimate the future patient enrollment costs we will incur and therefore have excluded these costs from the table above.
Under our license agreement with Asubio Pharma, we are obligated to future payments of (i) up to ¥375 million (approximately $3.3 million as of December 31, 2007) upon filing of an NDA at a higher dose and up to ¥1,250 million (approximately $11.1 million as of December 31, 2007) in subsequent regulatory and commercial milestone payments for faropenem medoxomil. Additionally, we are responsible for royalty payments to Daiichi Asubio based upon net sales of faropenem medoxomil.
Under our supply agreement with Nippon Soda, we are obligated to purchase, and Nippon Soda is obligated to supply, all our commercial requirements of the faropenem medoxomil active pharmaceutical ingredient. During the three years following placement of an initial purchase order by us with Nippon Soda, which has not occurred, we are obligated to make certain annual minimum purchase commitments to be determined initially by us and Nippon Soda at the time of a commercial launch. Since full commercial launch of an approved faropenem drug has been delayed, we are currently obligated for certain annual delay compensation to Nippon Soda up to ¥280 million (approximately $2.5 million as of December 31, 2007). If we terminate this agreement, abandon the development or commercialization of faropenem medoxomil or are unable to notify Nippon Soda of the faropenem medoxomil launch go date, as defined, by July 1, 2009, we will be obligated to pay Nippon Soda prorated delay compensation fees through the effective date of termination and reimburse Nippon Soda for up to ¥65 million ($0.6 million as of December 31, 2007) in engineering costs.
Critical Accounting Policies and Estimates
This discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the U.S. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, contingent assets and liabilities, revenues, expenses and related disclosures. Actual results may differ from these estimates. Our significant accounting policies are described in Note 2 of “Notes to Financial Statements” included elsewhere in this annual report. We believe the following accounting policies affect our more significant judgments and estimates used in the preparation of our financial statements.
Revenue Recognition. We have generated revenue through research, license, collaboration and commercialization agreements. These arrangements can contain multiple elements, including non-refundable upfront fees, payments for reimbursement of research and commercialization costs, non-refundable payments associated with achieving specific milestones, and royalties based on specified percentages of net product sales.
In determining when to recognize revenue related to upfront and milestone payments under these arrangements we apply the revenue recognition criteria as outlined in the Emerging Issues Task Force (EITF) IssueNo. 00-21,Revenue Arrangements with Multiple Deliverables(EITF 00-21). In applying these criteria, we consider a variety of factors to determine the appropriate method of revenue recognition, including whether the elements of the arrangement are separable, whether payments received are subject to refund or forfeiture, whether there are determinable fair values and whether there is a unique earnings process associated with each element of an arrangement.
When a payment is specifically tied to a separate earnings process and the amount to be received is fixed and determinable, revenue is recognized when the performance obligation associated with the payment is completed. Performance obligations typically consist of significant and substantive milestones. Revenues from milestone payments may be considered separable from funding for research, development or commercial activities because of the uncertainty surrounding the achievement of the milestones. Accordingly, these payments could be recognized as revenue when the performance milestone is achieved as described inEITF 00-21. In circumstances where we cannot identify a separate earnings process related to an upfront or milestone payment, we record deferred revenue and recognize revenue ratably over the period of expected benefit, which is generally the unexpired contract term.
Revenues derived from reimbursement of expenses for research, development and commercial activities under our collaboration and commercialization agreements are recorded in compliance with EITF Issue
62
No. 99-19,Reporting Revenue Gross as Principal Versus Net as an Agent(EITF 99-19). In accordance with the criteria established byEITF 99-19, in transactions where we act as principal, with discretion to choose suppliers, bear credit risk and perform a substantive part of the services, revenue is recorded at the gross amount of the reimbursement. Costs associated with these reimbursements are reflected as a component of operating expenses in our statements of operations.
Under our former agreement with Forest Laboratories entered into in February 2006, we recorded the initial $50 million upfront payment received in February 2006 as deferred revenue and were recognizing this amount into revenue ratably over the expected term of the agreement. In addition, we received a development milestone payment of $10 million in March 2006. Due to this milestone being achieved within one month of entering into the collaboration and commercialization agreement with Forest Laboratories, we could not identify a separate earnings process related to this milestone payment and were recognizing revenue related to this payment over the expected term of the agreement. In February 2007, we and Forest Laboratories announced that our agreement would terminate, and as a result, we reacquired all U.S. adult and pediatric rights previously granted to Forest Laboratories. As no further obligations exist beyond May 7, 2007, the effective date of the termination, we recognized the remaining unamortized deferred revenue balance as revenue in the second quarter of 2007.
We have also received amounts from Forest Laboratories as reimbursement for certain research and development. We believe that, as it relates to these activities, we act as the principal, performing a substantive part of the services directly, having the discretion to choose our suppliers and bearing all credit risk associated with the performance of these activities. We therefore have recorded these amounts as revenue in accordance with our revenue recognition policy. See Note 2 to our financial statements for more information about our revenue recognition policies.
Clinical Trial and Other Accrued Expenses. As part of the process of preparing our financial statements, we are required to estimate accrued expenses. This process involves identifying services that third parties have performed on our behalf and estimating the level of service performed and the associated cost incurred on these services as of each balance sheet date in our financial statements. We are party to agreements which include provisions that require payments to the counterparty under certain circumstances. We develop estimates of liabilities using our judgement based upon the facts and circumstances known and accounts for these estimates in accordance with accounting principles involving accrued expenses generally accepted in the U.S. In regards to our clinical trials, we record expenses based on estimates of the services received and efforts expended pursuant to contracts with clinical research organizations (CROs) and other third party vendors associated with our clinical trials. We contract with third parties to perform a range of clinical trial activities in the ongoing development of our product candidates. The terms of these agreements vary and may result in uneven payments. Payments under these contracts depend on factors such as the achievement of certain defined milestones, the successful enrollment of patients and other events. The objective of our clinical trial accrual policy is to match the recording of expenses in our financial statements of the actual services received and efforts expended. In doing so, we rely on information from CROs and our clinical operations group regarding the status of our clinical trials to calculate our accrual for clinical expenses at the end of each reporting period. Our estimates and assumptions could differ significantly from the amounts that we actually may incur.
Share-Based Compensation. Effective January 1, 2006, we adopted Statement of Financial Accounting Standards No. 123(R),Share-Based Payment(SFAS 123(R)), which requires compensation costs related to share-based transactions, including employee stock options, to be recognized in the financial statements based on fair value. SFAS 123(R) revises SFAS 123, as amended,Accounting for Stock-Based Compensation, and supersedes Accounting Principles Board Opinion No. 25,Accounting for Stock Issued to Employees. We adopted SFAS 123(R) using the prospective method. Under this method, compensation cost is recognized for all share-based awards granted or modified on or after January 1, 2006.
We selected the Black-Scholes option pricing model as the most appropriate valuation method for option grants with serviceand/or performance conditions. The Black-Scholes model requires inputs for risk-free interest rate, dividend yield, volatility and expected lives of the options. Since the Company has a limited history of stock activity, expected volatility is based on historical data from several public companies similar in size and value to
63
us. We will continue to use a weighted average approach using historical volatility and other similar public entity volatility information until our historical volatility is relevant to measure expected volatility for future option grants. We estimate the forfeiture rate based on historical data. Based on an analysis of historical forfeitures, we applied an annual forfeiture rate of 4.48% during 2007. The forfeiture rate is re-evaluated on a quarterly basis. The risk-free rate for periods within the contractual life of the option is based on the U.S. Treasury yield curve in effect at the time of the grant. The expected lives for options granted represents the period of time that options granted are expected to be outstanding and is derived from historical exercise behavior.
During 2007, we estimated the fair value of option grants as of the date of grant using the Black-Scholes option pricing model with the following weighted-average assumptions. Expected volatility was estimated to be 75%. The weighted average risk free interest rate was 4.46% and the dividend yield was 0.00%. The weighted average expected lives for each individual vesting tranche under the graded vesting attribution method discussed below was estimated to be 3.05 years.
We had a choice of two attribution methods for allocating compensation costs under SFAS No. 123(R): the “straight-line” method, which allocates expense on a straight-line basis over the requisite service period of the last separately vesting portion of an award, or the “graded vesting attribution method”, which allocates expense on a straight-line basis over the requisite service period for each separately vesting portion of the award as if the award was, in substance, multiple awards. We chose the graded vesting attribution method and accordingly, amortize the fair value of each option over each option’s vesting period (requisite service period).
Deferred Tax Asset Valuation Allowance. In establishing a valuation allowance on our deferred tax assets we are required to make significant estimates and judgments about our future operating results. Our ability to realize deferred tax assets depends on our future taxable income as well as limitations on utilization primarily of net operating losses and tax credits. We are required to reduce our deferred tax assets by a valuation allowance if it is more likely than not that some portion or all of our deferred tax asset will not be realized. Although we reported net income for the year ended December 31, 2007 as a result of the termination of our agreement with Forest Laboratories, we expect to incur substantial operating losses for the next several years as we pursue our clinical trials and research and development efforts. Accordingly, we have recorded a full valuation allowance on our net deferred tax assets since inception due to uncertainties related to our ability to realize deferred tax assets in the foreseeable future. See Note 11 to our financial statements.
Recent Accounting Pronouncements
In September 2006, the Financial Accounting Standards Board (FASB) issued Statement of Financial Accounting Standard (SFAS) No. 157,Fair Value Measurements(SFAS 157). SFAS 157 defines fair value, establishes a framework for measuring fair value in applying generally accepted accounting principles, and expands disclosures about fair value measurements. SFAS 157 applies whenever an entity is measuring fair value under other accounting pronouncements that require or permit fair value measurement. SFAS 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007; however, the FASB provided a one year deferral for implementation of the standard for non-financial assets and liabilities. We do not expect that the adoption of SFAS 157 will have a material impact on our financial statements.
In June 2007, the FASB ratified EITF IssueNo. 07-03,Accounting for Advance Payments for Goods or Services to Be Used in Future Research and Development Activities(EITF 07-03). The scope ofEITF 07-03 is limited to nonrefundable advance payments for goods and services to be used or rendered in future research and development activities pursuant to an executory contractual arrangement. This issue provides that nonrefundable advance payments for goods or services that will be used or rendered for future research and development activities should be deferred and capitalized. Such amounts should be recognized as an expense as the related goods are delivered or the related services are performed. We will be required to adoptEITF 07-03 for new contracts entered into in 2008. We do not expect that the adoption ofEITF 07-03 will have a material impact on our financial statements.
In December 2007, the FASB ratified EITF IssueNo. 07-01, Accounting for Collaborative Arrangements(EITF 07-01).EITF 07-01 defines collaborative arrangements and establishes reporting requirements for transactions between participants in a collaborative arrangement and between participants in the arrangement
64
and third parties.EITF 07-01 also establishes the appropriate income statement presentation and classification for joint operating activities and payments between participants, as well as disclosures related to these arrangements.EITF 07-01 is effective for fiscal years beginning after December 15, 2008. We do not expect that the adoption ofEITF 07-01 will have an impact on our financial statements.
| |
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Our exposure to market risk is primarily limited to our cash, cash equivalents, and short-term investments. We have attempted to minimize risk by investing in quality financial instruments, primarily money market funds, federal agency notes, commercial paper, bank and corporate debt securities and U.S. treasury notes, with no security having an effective duration in excess of two years. The primary objective of our investment activities is to preserve our capital for the purpose of funding operations while at the same time maximizing the income we receive from our investments without significantly increasing risk. To achieve these objectives, our investment policy allows us to maintain a portfolio of cash equivalents and short-term investments in a variety of marketable securities, including U.S. government, money market funds and under certain circumstances, derivative financial instruments. Our cash and cash equivalents as of December 31, 2007 included a liquid money market account. The securities in our investment portfolio are classified as available-for-sale or held-to-maturity and are, due to their short-term nature, subject to minimal interest rate risk.
65
| |
| ITEM 8. | FINANCIAL STATEMENTS |
Replidyne, Inc.
Index to Financial Statements
| | | | | |
| | | Page(s) |
| |
| | | 67 | |
| | | 69 | |
| | | 70 | |
| | | 71 | |
| | | 73 | |
| | | 74 | |
66
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders
Replidyne, Inc.:
We have audited the accompanying balance sheets of Replidyne, Inc. as of December 31, 2007 and 2006, and the related statements of operations, stockholders’ equity (deficit), preferred stock and comprehensive income (loss), and cash flows for each of the years in the three-year period ended December 31, 2007. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Replidyne, Inc. as of December 31, 2007 and 2006, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2007, in conformity with U.S. generally accepted accounting principles.
As discussed in note 2 to the accompanying financial statements, the Company adopted Statement of Financial Accounting Standards No. 123(R),Share-Based Payment, effective January 1, 2006.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Replidyne, Inc.’s internal control over financial reporting as of December 31, 2007, based on criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated March 13, 2008 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
KPMG LLP
Boulder, Colorado
March 13, 2008
67
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Replidyne, Inc.:
We have audited Replidyne, Inc.’s internal control over financial reporting as of December 31, 2007, based on criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Replidyne, Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting (Item 9A). Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Replidyne, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2007, based on criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the balance sheets of Replidyne, Inc. as of December 31, 2007 and 2006, and the related statements of operations, stockholders’ equity (deficit), preferred stock and comprehensive income (loss), and cash flows for each of the years in the three-year period ended December 31, 2007, and our report dated March 13, 2008 expressed an unqualified opinion on those financial statements.
KPMG LLP
Boulder, Colorado
March 13, 2008
68
REPLIDYNE, INC.
BALANCE SHEETS
(in thousands, except par value)
| | | | | | | | | |
| | | December 31, | |
| | | 2007 | | | 2006 | |
| |
ASSETS |
| Current assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 43,969 | | | $ | 24,091 | |
| Short-term investments | | | 46,297 | | | | 101,476 | |
| Receivable from Forest Laboratories | | | — | | | | 4,634 | |
| Prepaid expenses and other current assets | | | 2,429 | | | | 2,079 | |
| | | | | | | | | |
| Total current assets | | | 92,695 | | | | 132,280 | |
| Property and equipment, net | | | 1,905 | | | | 3,170 | |
| Other assets | | | 90 | | | | 111 | |
| | | | | | | | | |
| Total assets | | $ | 94,690 | | | $ | 135,561 | |
| | | | | | | | | |
| |
LIABILITIES AND STOCKHOLDERS’ EQUITY |
| Current liabilities: | | | | | | | | |
| Accounts payable and accrued expenses | | $ | 12,255 | | | $ | 7,957 | |
| Deferred revenue | | | — | | | | 56,176 | |
| | | | | | | | | |
| Total current liabilities | | | 12,255 | | | | 64,133 | |
| Other long-term liabilities | | | 31 | | | | 56 | |
| | | | | | | | | |
| Total liabilities | | | 12,286 | | | | 64,189 | |
| | | | | | | | | |
| Commitments and contingencies | | | | | | | | |
| Stockholders’ equity: | | | | | | | | |
| Common stock, $0.001 par value. Authorized 100,000 shares; issued 27,085 and 27,010 shares; outstanding 27,077 and 26,979 shares at December 31, 2007 and 2006, respectively | | | 27 | | | | 27 | |
| Treasury stock, $0.001 par value; 8 and 31 shares at December 31, 2007 and 2006, respectively, at cost | | | (1 | ) | | | (2 | ) |
| Additional paid-in capital | | | 191,570 | | | | 188,334 | |
| Accumulated other comprehensive income (loss) | | | 96 | | | | (7 | ) |
| Accumulated deficit | | | (109,288 | ) | | | (116,980 | ) |
| | | | | | | | | |
| Total stockholders’ equity | | | 82,404 | | | | 71,372 | |
| | | | | | | | | |
| Total liabilities and stockholders’ equity | | $ | 94,690 | | | $ | 135,561 | |
| | | | | | | | | |
See notes to financial statements.
69
REPLIDYNE, INC.
STATEMENTS OF OPERATIONS
(in thousands, except per share amounts)
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2007 | | | 2006 | | | 2005 | |
| |
| Revenue | | $ | 58,571 | | | $ | 15,988 | | | $ | 441 | |
| | | | | | | | | | | | | |
| Costs and expenses: | | | | | | | | | | | | |
| Research and development | | | 43,313 | | | | 38,295 | | | | 29,180 | |
| Sales, general and administrative | | | 13,020 | | | | 12,187 | | | | 5,329 | |
| | | | | | | | | | | | | |
| Total costs and expenses | | | 56,333 | | | | 50,482 | | | | 34,509 | |
| | | | | | | | | | | | | |
| Income (loss) from operations | | | 2,238 | | | | (34,494 | ) | | | (34,068 | ) |
| Investment income, net | | | 5,535 | | | | 5,953 | | | | 722 | |
| Interest expense | | | — | | | | (14 | ) | | | (100 | ) |
| Other expense, net | | | (81 | ) | | | (694 | ) | | | (223 | ) |
| | | | | | | | | | | | | |
| Net income (loss) | | | 7,692 | | | | (29,249 | ) | | | (33,669 | ) |
| Preferred stock dividends and accretion | | | — | | | | (5,391 | ) | | | (7,191 | ) |
| | | | | | | | | | | | | |
| Net income (loss) attributable to common stockholders | | $ | 7,692 | | | $ | (34,640 | ) | | $ | (40,860 | ) |
| | | | | | | | | | | | | |
| Net income (loss) attributable to common stockholders per share — basic | | $ | 0.29 | | | $ | (2.49 | ) | | $ | (39.20 | ) |
| | | | | | | | | | | | | |
| Net income (loss) attributable to common stockholders per share — diluted | | $ | 0.28 | | | $ | (2.49 | ) | | $ | (39.20 | ) |
| | | | | | | | | | | | | |
| Weighted average common shares outstanding — basic | | | 26,730 | | | | 13,908 | | | | 1,042 | |
| | | | | | | | | | | | | |
| Weighted average common shares outstanding — diluted | | | 27,666 | | | | 13,908 | | | | 1,042 | |
| | | | | | | | | | | | | |
See notes to financial statements.
70
REPLIDYNE, INC.
COMPREHENSIVE INCOME (LOSS)
(in thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Preferred Stock | | | Stockholders’ Equity (Deficit) | |
| | | Series A
| | | | | | | | | Series C
| | | Series D
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Redeemable
| | | Series B
| | | Redeemable
| | | Redeemable
| | | | | | | | | | | | | | | | | | | | | Accumulated
| | | | | | Total
| |
| | | Convertible
| | | Convertible
| | | Convertible
| | | Convertible
| | | | | | | | | | | | | | | Additional
| | | Deferred
| | | Other
| | | | | | Stockholders’
| |
| | | Preferred Stock | | | Preferred Stock | | | Preferred Stock | | | Preferred Stock | | | Common Stock | | | Treasury Stock | | | Paid-In
| | | Stock-Based
| | | Comprehensive
| | | Accumulated
| | | Equity
| |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Compensation | | | Income (Loss) | | | Deficit | | | (Deficit) | |
| |
| Balances, January 1, 2005 | | | 13,000 | | | $ | 15,886 | | | | 4,000 | | | $ | 5,630 | | | | 36,800 | | | $ | 47,931 | | | | — | | | $ | — | | | | 790 | | | $ | 1 | | | | (31 | ) | | $ | (2 | ) | | $ | — | | | $ | (7 | ) | | $ | 41 | | | $ | (42,235 | ) | | $ | (42,202 | ) |
| Issuance of common stock upon exercise of stock options | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1,108 | | | | 1 | | | | — | | | | — | | | | 290 | | | | — | | | | — | | | | — | | | | 291 | |
| Issuance of Series D redeemable, convertible preferred stock in August 2005 for cash, net of issuance costs of $2,323 | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 34,722 | | | | 60,177 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Stock-based compensation related to stock option grants to an employee | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 54 | | | | — | | | | — | | | | — | | | | 54 | |
| Stock-based compensation related to stock option grants to non-employees | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1 | | | | — | | | | — | | | | — | | | | 1 | |
| Reclassification of warrants on redeemable preferred stock | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (345 | ) | | | — | | | | — | | | | (12 | ) | | | (357 | ) |
| Amortization of deferred stock-based compensation | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 3 | | | | — | | | | — | | | | 3 | |
| Accretion of offering costs on redeemable preferred stock | | | — | | | | 14 | | | | — | | | | — | | | | — | | | | 24 | | | | — | | | | 169 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (207 | ) | | | (207 | ) |
| Non-cash dividends on preferred stock | | | — | | | | 1,040 | | | | — | | | | 400 | | | | — | | | | 3,680 | | | | — | | | | 1,864 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (6,984 | ) | | | (6,984 | ) |
| Realized gain on available-for-sale equity securities | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (41 | ) | | | — | | | | (41 | ) |
| Unrealized gain on available-for-sale equity securities | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 479 | | | | — | | | | 479 | |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (33,669 | ) | | | (33,669 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (33,231 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balances, December 31, 2005 | | | 13,000 | | | | 16,940 | | | | 4,000 | | | | 6,030 | | | | 36,800 | | | | 51,635 | | | | 34,722 | | | | 62,210 | | | | 1,898 | | | | 2 | | | | (31 | ) | | | (2 | ) | | | — | | | | (4 | ) | | | 479 | | | | (83,107 | ) | | | (82,632 | ) |
| Issuance of Series C preferred stock upon exercise of stock purchase warrants | | | — | | | | — | | | | — | | | | — | | | | 80 | | | | 100 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 183 | | | | — | | | | — | | | | — | | | | 183 | |
| Issuance of common stock upon exercise of stock options | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 214 | | | | — | | | | — | | | | — | | | | 176 | | | | — | | | | — | | | | — | | | | 176 | |
| Reclassification of warrants on redeemable preferred stock | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 446 | | | | — | | | | — | | | | — | | | | 446 | |
| Issuance of common stock under employee stock purchase plan | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 43 | | | | — | | | | — | | | | — | | | | 221 | | | | — | | | | — | | | | — | | | | 221 | |
| Issuance of common stock in initial public offering, net of offering costs | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 5,006 | | | | 5 | | | | — | | | | — | | | | 44,534 | | | | — | | | | — | | | | — | | | | 44,539 | |
| Changes in restricted stock, net | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 79 | | | | — | | | | — | | | | — | | | | 79 | |
| Reclassification of deferred stock based compensation | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (4 | ) | | | 4 | | | | — | | | | — | | | | — | |
| Stock-based compensation expense related to options granted | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1,180 | | | | — | | | | — | | | | — | | | | 1,180 | |
| Accretion of offering costs on redeemable preferred stock | | | — | | | | 5 | | | | — | | | | — | | | | — | | | | 9 | | | | — | | | | 231 | | | | — | | | | — | | | | — | | | | — | | | | (246 | ) | | | — | | | | — | | | | — | | | | (246 | ) |
| Non-cash dividends on preferred stock | | | — | | | | 528 | | | | — | | | | 203 | | | | — | | | | 1,873 | | | | — | | | | 2,541 | | | | — | | | | — | | | | — | | | | — | | | | (522 | ) | | | — | | | | — | | | | (4,624 | ) | | | (5,146 | ) |
| Conversion of preferred stock into common stock upon initial public offering | | | (13,000 | ) | | | (12,930 | ) | | | (4,000 | ) | | | (5,000 | ) | | | (36,880 | ) | | | (45,980 | ) | | | (34,722 | ) | | | (60,578 | ) | | | 18,067 | | | | 18 | | | | — | | | | — | | | | 124,470 | | | | — | | | | — | | | | — | | | | 124,488 | |
| Settlement of accrued dividends on preferred stock with common stock upon initial public offering | | | — | | | | (4,543 | ) | | | — | | | | (1,233 | ) | | | — | | | | (7,637 | ) | | | — | | | | (4,404 | ) | | | 1,782 | | | | 2 | | | | — | | | | — | | | | 17,817 | | | | — | | | | — | | | | — | | | | 17,819 | |
| Reversal of unrealized gain on available-for-sale equity securities, net | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (486 | ) | | | — | | | | (486 | ) |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (29,249 | ) | | | (29,249 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (29,735 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balances, December 31, 2006 | | | — | | | $ | — | | | | — | | | $ | — | | | | — | | | $ | — | | | | — | | | $ | — | | | | 27,010 | | | $ | 27 | | | | (31 | ) | | $ | (2 | ) | | $ | 188,334 | | | $ | — | | | $ | (7 | ) | | $ | (116,980 | ) | | $ | 71,372 | |
(continued)
See notes to financial statements.
71
REPLIDYNE, INC.
STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT), PREFERRED STOCK, AND
COMPREHENSIVE INCOME (LOSS) — (continued)
(in thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Preferred Stock | | | Stockholders’ Equity (Deficit) | |
| | | Series A
| | | | | | | | | Series C
| | | Series D
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Redeemable
| | | Series B
| | | Redeemable
| | | Redeemable
| | | | | | | | | | | | | | | | | | | | | Accumulated
| | | | | | Total
| |
| | | Convertible
| | | Convertible
| | | Convertible
| | | Convertible
| | | | | | | | | | | | | | | Additional
| | | Deferred
| | | Other
| | | | | | Stockholders’
| |
| | | Preferred Stock | | | Preferred Stock | | | Preferred Stock | | | Preferred Stock | | | Common Stock | | | Treasury Stock | | | Paid-In
| | | Stock-Based
| | | Comprehensive
| | | Accumulated
| | | Equity
| |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Compensation | | | Income (Loss) | | | Deficit | | | (Deficit) | |
| |
| Balances, December 31, 2006 | | | — | | | $ | — | | | | — | | | $ | — | | | | — | | | $ | — | | | | — | | | $ | — | | | | 27,010 | | | $ | 27 | | | | (31 | ) | | $ | (2 | ) | | $ | 188,334 | | | $ | — | | | $ | (7 | ) | | $ | (116,980 | ) | | $ | 71,372 | |
| Issuance of common stock upon exercise of stock options | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 52 | | | | — | | | | — | | | | — | | | | 64 | | | | — | | | | — | | | | — | | | | 64 | |
| Issuance of common stock under employee stock purchase plan | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 68 | | | | — | | | | — | | | | — | | | | 282 | | | | — | | | | — | | | | — | | | | 282 | |
| Release of restrictions on restricted stock | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 115 | | | | — | | | | — | | | | — | | | | 115 | |
| Vested shares of restricted stock returned to the company for taxes | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (2 | ) | | | (8 | ) | | | — | | | | — | | | | — | | | | — | | | | (8 | ) |
| Return of unvested restricted stock by employee upon termination | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (33 | ) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Stock-based compensation expense related to options granted | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 2,784 | | | | — | | | | — | | | | — | | | | 2,784 | |
| Issuance of restricted stock to an employee | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 13 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Unrealized gain on available-for-sale equity securities, net | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 103 | | | | — | | | | 103 | |
| Retirement of treasury stock | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (58 | ) | | | — | | | | 58 | | | | 9 | | | | (9 | ) | | | — | | | | — | | | | — | | | | — | |
| Net income | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 7,692 | | | | 7,692 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 7,795 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balances, December 31, 2007 | | | — | | | $ | — | | | | — | | | $ | — | | | | — | | | $ | — | | | | — | | | $ | — | | | | 27,085 | | | $ | 27 | | | | (8 | ) | | $ | (1 | ) | | $ | 191,570 | | | $ | — | | | $ | 96 | | | $ | (109,288 | ) | | $ | 82,404 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
See notes to financial statements.
72
REPLIDYNE, INC.
STATEMENTS OF CASH FLOWS
(in thousands)
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2007 | | | 2006 | | | 2005 | |
| |
| Cash flows from operating activities: | | | | | | | | | | | | |
| Net income (loss) | | $ | 7,692 | | | $ | (29,249 | ) | | $ | (33,669 | ) |
| Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: | | | | | | | | | | | | |
| Depreciation | | | 1,474 | | | | 1,418 | | | | 1,258 | |
| Stock-based compensation | | | 2,784 | | | | 1,180 | | | | 58 | |
| Amortization of debt discount and issuance costs | | | — | | | | 9 | | | | 35 | |
| Amortization of discounts and premiums on short-term investments | | | 779 | | | | (744 | ) | | | (469 | ) |
| Other | | | 15 | | | | 105 | | | | 28 | |
| Changes in operating assets and liabilities: | | | | | | | | | | | | |
| Receivable from Forest Laboratories | | | 4,634 | | | | (4,634 | ) | | | — | |
| Prepaid expenses and other current assets | | | (349 | ) | | | (1,695 | ) | | | (182 | ) |
| Other assets | | | 21 | | | | 150 | | | | (288 | ) |
| Accounts payable and accrued expenses | | | 4,435 | | | | (518 | ) | | | 6,996 | |
| Deferred revenue | | | (56,175 | ) | | | 56,175 | | | | (307 | ) |
| Other long-term liabilities | | | (25 | ) | | | (25 | ) | | | 81 | |
| | | | | | | | | | | | | |
| Net cash provided by (used in) operating activities | | | (34,715 | ) | | | 22,172 | | | | (26,459 | ) |
| | | | | | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | | | | | |
| Purchases of short-term investments classified as available-for-sale | | | (26,803 | ) | | | (169,827 | ) | | | (157,281 | ) |
| Purchases of short-term investments classified as held-to-maturity | | | (74,870 | ) | | | (60,854 | ) | | | — | |
| Maturities of short-term investments classified as available-for-sale | | | 59,489 | | | | 147,504 | | | | 125,500 | |
| Maturities of short-term investments classified as held-to-maturity | | | 96,686 | | | | 36,916 | | | | — | |
| Proceeds from sale of property and equipment | | | 7 | | | | 45 | | | | 1 | |
| Acquisitions of property and equipment | | | (232 | ) | | | (1,214 | ) | | | (1,570 | ) |
| | | | | | | | | | | | | |
| Net cash provided by (used in) investing activities | | | 54,277 | | | | (47,430 | ) | | | (33,350 | ) |
| | | | | | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | | | | | |
| Principal payments on debt | | | — | | | | (169 | ) | | | (1,173 | ) |
| Proceeds from issuance of common stock from the exercise of stock options and under the employee stock purchase plan | | | 346 | | | | 397 | | | | 291 | |
| Proceeds from repayment of principal on notes receivable from officers | | | — | | | | 356 | | | | — | |
| Proceeds from exercise of preferred stock warrants | | | — | | | | 100 | | | | — | |
| Proceeds from sale of common stock from initial public offering, net of underwriters discount and offering costs | | | — | | | | 44,539 | | | | — | |
| Bank overdraft | | | — | | | | (227 | ) | | | 227 | |
| Purchase of unvested restricted stock from employees upon termination | | | (30 | ) | | | — | | | | — | |
| Proceeds from sale of Series D redeemable convertible preferred stock, net | | | — | | | | — | | | | 60,177 | |
| | | | | | | | | | | | | |
| Net cash provided by financing activities | | | 316 | | | | 44,996 | | | | 59,522 | |
| | | | | | | | | | | | | |
| Net increase (decrease) in cash and cash equivalents | | | 19,878 | | | | 19,738 | | | | (287 | ) |
| Cash and cash equivalents: | | | | | | | | | | | | |
| Beginning of year | | | 24,091 | | | | 4,353 | | | | 4,640 | |
| | | | | | | | | | | | | |
| End of year | | $ | 43,969 | | | $ | 24,091 | | | $ | 4,353 | |
| | | | | | | | | | | | | |
| Supplemental cash flow information: | | | | | | | | | | | | |
| Cash paid for interest | | $ | — | | | $ | 15 | | | $ | 75 | |
| | | | | | | | | | | | | |
| Notes receivable issued to officers for the exercise of stock options | | $ | — | | | $ | — | | | $ | 356 | |
| | | | | | | | | | | | | |
| Reclassification of warrants from accrued liabilities to equity | | $ | — | | | $ | 629 | | | $ | — | |
| | | | | | | | | | | | | |
See notes to financial statements.
73
REPLIDYNE, INC.
NOTES TO FINANCIAL STATEMENTS
| |
| (1) | Business and Organization |
Replidyne, Inc. (Replidyne or the Company) is a biopharmaceutical company focused on discovering, developing, in-licensing and commercializing anti-infective products. The Company’s most advanced product candidate, faropenem medoxomil, is a novel oral community antibiotic for which the Company submitted a New Drug Application (NDA) with the U.S. Food and Drug Administration (FDA) in December 2005 for treatment of acute bacterial sinusitis, community-acquired pneumonia, acute exacerbation of chronic bronchitis, and uncomplicated skin and skin structure infections in adults. In October 2006, the FDA issued a non-approvable letter for the NDA. According to the non-approvable letter, the FDA recommends further clinical studies for all indications included in the NDA, additional microbiologic confirmation and consideration of alternate dosing of faropenem medoxomil.
The Company’s research and development product pipeline also includes REP3123, an investigational narrow-spectrum antibacterial agent for the treatment ofClostridium difficile(C. difficile) bacteria andC. difficile-associated disease (CDAD), and its bacterial DNA replication inhibitor technology. Additionally, the Company had also been developing REP8839, a topical antibiotic for the treatment of skin and wound infections, including methicillin-resistantStaphylococcus aureus(MRSA) infections. As a result of prioritizing its preclinical programs in December 2007, the Company suspended the development of REP8839 due to the incremental investment required to optimize the formulation and the niche market opportunity for its initial indication of treating impetigo.
In February 2006, the Company entered into a collaboration and commercialization agreement with Forest Laboratories Holding Limited (Forest Laboratories) for the commercialization, development and distribution of faropenem medoxomil in the U.S. Under this agreement, in 2006 the Company received nonrefundable upfront and milestone payments of $60 million and during the term of the agreement received $14.6 million of contract revenue from funded activities related to the development of faropenem medoxomil. On May 7, 2007, the collaboration and commercialization agreement with Forest Laboratories terminated. As a result, the Company reacquired all rights to faropenem medoxomil previously granted to Forest Laboratories and recognized as revenue in 2007 all remaining unamortized deferred revenue under this agreement totaling $55 million.
| |
| (2) | Summary of Significant Accounting Policies |
Accounting Estimates in the Preparation of Financial Statements. The preparation of financial statements in conformity with accounting principles generally accepted in the U.S. requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from these estimates.
Cash and Cash Equivalents. The Company considers all highly liquid investments purchased with maturities of 90 days or less when acquired to be cash equivalents. Cash equivalents are carried at amortized cost, which approximates fair value.
Short-Term Investments. Short-term investments are investments purchased with maturities of longer than 90 days held at a financial institution. At December 31, 2007, contractual original maturities of the Company’s short-term investments were less than two years for investments classified as available-for-sale and less than one year for investments classified as held-to-maturity. At December 31, 2007, the current weighted average days to maturity was approximately thirteen months for investments classified as available-for-sale and approximately two months for investments classified as held-to-maturity.
Management determines the classification of securities at purchase based on its intent. In accordance with SFAS No. 115,Accounting for Certain Investments in Debt and Equity Securities, the Company classifies its securities as held-to-maturity or available-for-sale. Held-to-maturity securities are those which the Company
74
REPLIDYNE, INC.
NOTES TO FINANCIAL STATEMENTS — (continued)
has the positive intent and ability to hold to maturity and are reported at amortized cost. Available-for-sale securities are those the Company may decide to sell if needed for liquidity, asset/liability management, or other reasons.
Available-for-sale securities are recorded at estimated fair value. The estimated fair value amounts are determined by the Company using available market information. Unrealized holding gains and losses on available-for-sale securities are excluded from earnings and are reported as a separate component of other comprehensive income or loss until realized. Cost is adjusted for amortization of premiums and accretion of discounts from the date of purchase to maturity. Such amortization is included in investment income and other. Realized gains and losses and declines in value judged to be other than temporary on available-for-sale securities are also included in investment income and other. The cost of securities sold is based on the specific-identification method. A decline in the market value of any available-for-sale security below cost that is deemed to be other than temporary results in a reduction in carrying amount to fair value. The impairment is charged to earnings and a new cost basis for the security is established. To determine whether an impairment is other than temporary, the Company considers whether it has the ability and intent to hold the investment until a market price recovery and considers whether evidence indicating the cost of the investment is recoverable outweighs evidence to the contrary. Evidence considered in this assessment includes the reasons for the impairment, the severity and duration of the impairment, changes in value subsequent to period end, and forecasted performance of the investee. No impairments were recorded as a result of this analysis during 2007, 2006 or 2005. The Company’s investments were classified as follows at December 31, 2007 and 2006 (in thousands):
| | | | | | | | | |
| | | December 31, | |
| | | 2007 | | | 2006 | |
| |
| Available-for-sale securities — recorded at fair value | | $ | 16,213 | | | $ | 49,525 | |
| Held-to-maturity securities — recorded at amortized cost | | | 30,084 | | | | 51,951 | |
| | | | | | | | | |
| Total short-term investments | | $ | 46,297 | | | $ | 101,476 | |
| | | | | | | | | |
The following is a summary of the types of short-term investments classified as available-for-sale securities (in thousands):
| | | | | | | | | | | | | | | | | |
| | | December 31,
| | | December 31,
| |
| | | 2007 | | | 2006 | |
| | | Amortized
| | | Estimated
| | | Amortized
| | | Estimated
| |
| | | Cost | | | Fair Value | | | Cost | | | Fair Value | |
| |
| U.S. government agencies | | $ | 3,998 | | | $ | 4,005 | | | $ | 40,599 | | | $ | 40,601 | |
| U.S. bank and corporate notes | | | 12,119 | | | | 12,208 | | | | 8,933 | | | | 8,924 | |
| | | | | | | | | | | | | | | | | |
| | | $ | 16,117 | | | $ | 16,213 | | | $ | 49,532 | | | $ | 49,525 | |
| | | | | | | | | | | | | | | | | |
Unrealized holding gains and losses on available-for-sale securities as of December 31, 2007 were $0.1 million and $7 thousand, respectively. Unrealized holding gains and losses on available-for-sale securities as of December 31, 2006 were $5 thousand and $12 thousand, respectively. Net unrealized holding gains or losses are recorded in accumulated other comprehensive income or loss.
75
REPLIDYNE, INC.
NOTES TO FINANCIAL STATEMENTS — (continued)
The following is a summary of short-term investments classified as held-to-maturity securities (in thousands):
| | | | | | | | | | | | | | | | | |
| | | December 31,
| | | December 31,
| |
| | | 2007 | | | 2006 | |
| | | Amortized
| | | Estimated
| | | Amortized
| | | Estimated
| |
| | | Cost | | | Fair Value | | | Cost | | | Fair Value | |
| |
| U.S. bank and corporate notes | | $ | 30,084 | | | $ | 30,091 | | | $ | 42,962 | | | $ | 42,951 | |
| U.S. government agencies | | | — | | | | — | | | | 8,989 | | | | 8,985 | |
| | | | | | | | | | | | | | | | | |
| | | $ | 30,084 | | | $ | 30,091 | | | $ | 51,951 | | | $ | 51,936 | |
| | | | | | | | | | | | | | | | | |
Unrealized holding gains and losses on held-to-maturity investments as of December 31, 2007 were $10 thousand and $3 thousand, respectively. Unrealized holding gains and losses on held-to-maturity investments as of December 31, 2006 were $3 thousand and $18 thousand, respectively.
Concentrations of Credit Risk. Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash, cash equivalents and short-term investments. The Company has established guidelines to limit its exposure to credit risk by placing investments with high credit quality financial institutions, diversifying its investment portfolio, and making investments with maturities that maintain safety and liquidity.
Property and Equipment. Property and equipment are recorded at cost, less accumulated depreciation and amortization. Depreciation is computed using the straight-line method over the estimated useful lives of the assets, generally three to seven years. Leasehold improvements are amortized over the shorter of the life of the lease or the estimated useful life of the assets. Repairs and maintenance costs are expensed as incurred.
Long-Lived Assets and Impairments.The Company periodically evaluates the recoverability of its long-lived assets in accordance with SFAS No. 144,Accounting for the Impairment or Disposal of Long-Lived Assets, and, if appropriate, reduces the carrying value whenever events or changes in business conditions indicate the carrying amount of the assets may not be fully recoverable. SFAS No. 144 requires recognition of impairment of long-lived assets in the event the net book value of such assets exceeds the fair value less costs to sell such assets. The Company has not yet generated positive cash flows from operations on a sustained basis, and such cash flows may not materialize for a significant period in the future, if ever. Additionally, the Company may make changes to its business plan that will result in changes to the expected cash flows from long-lived assets. As a result, it is reasonably possible that future evaluations of long-lived assets may result in impairment.
Accrued Expenses. As part of the process of preparing its financial statements, the Company is required to estimate accrued expenses. This process involves identifying services that third parties have performed on the Company’s behalf and estimating the level of service performed and the associated cost incurred on these services as of each balance sheet date in the Company’s financial statements. Examples of estimated accrued expenses include contract service fees, such as amounts due to clinical research organizations, professional service fees, such as attorneys and independent accountants, and investigators in conjunction with preclinical and clinical trials, and fees payable to contract manufacturers in connection with the production of materials related to product candidates. Estimates are most affected by the Company’s understanding of the status and timing of services provided relative to the actual level of services incurred by the service providers. The date on which certain services commence, the level of services performed on or before a given date, and the cost of services is often subject to judgment. Additionally, the Company is a party to agreements which include provisions that require payments to the counterparty under certain circumstances. The Company develops estimates of liabilities using its judgment based upon the facts and circumstances known and accounts for these estimates in accordance with accounting principles involving accrued expenses generally accepted in the U.S.
76
REPLIDYNE, INC.
NOTES TO FINANCIAL STATEMENTS — (continued)
Segments. The Company operates in one segment. Management uses one measure of profitability and does not segment its business for internal reporting purposes.
Share-Based Compensation. Effective January 1, 2006, the Company adopted SFAS No. 123(R),Share-Based Payment, using the prospective method of transition. Under that transition method, compensation cost recognized after adoption includes: (a) compensation costs for all share-based payments granted prior to January 1, 2006, based on the intrinsic value method prescribed by Accounting Principles Board (APB) Opinion No. 25,Accounting for Stock Issued to Employees,and (b) compensation cost for all share-based payments granted or modified subsequent to January 1, 2006, based on the grant date fair value estimated in accordance with the provisions of SFAS No. 123(R).
The Company selected the Black-Scholes option pricing model as the most appropriate valuation method for option grants with serviceand/or performance conditions. The Black-Scholes model requires inputs for risk-free interest rate, dividend yield, volatility and expected lives of the options. Since the Company has a limited history of stock activity, expected volatility is based on historical data from several public companies similar in size and nature of operations to the Company. The Company will continue to use historical volatility and other similar public entity volatility information until its historical volatility is relevant to measure expected volatility for future option grants. The Company estimates the forfeiture rate based on historical data. Based on an analysis of historical forfeitures, the Company applied an annual forfeiture rate of 4.48% during 2007 and applied an annual forfeiture rate of 6.97% during 2006. The forfeiture rate is re-evaluated on a quarterly basis. The risk-free rate for periods within the contractual life of the option is based on the U.S. Treasury yield curve in effect at the time of the grant for a period commensurate with the expected term of the grant. The expected term (without regard to forfeitures) for options granted represents the period of time that options granted are expected to be outstanding and is derived from the contractual terms of the options granted and historical option exercise behaviors.
For options granted during 2007, the Company estimated the fair value of option grants as of the date of grant using the Black-Scholes option pricing model with the following weighted average assumptions. Expected volatility was estimated to be 75%. The weighted average risk-free interest rate was 4.46%, and the dividend yield was 0.00%. The weighted average expected lives for each individual vesting tranche under the graded vesting attribution method discussed below was estimated to be 3.05 years.
For certain options granted during 2006, the Company estimated the fair value of option grants as of the date of grant using the Black-Scholes option pricing model with the following weighted average assumptions. Expected volatility was estimated to be 75%. The weighted average risk-free interest rate was 4.58%, and the dividend yield was 0.00%. The weighted average expected lives for each individual vesting tranche under the graded vesting attribution method discussed below was estimated to be 2.18 years.
During 2006, the Company also issued options which vest over the earlier to be achieved service or market condition. In determining the estimated fair value of these option awards on the date of grant, the Company elected to use a binomial lattice option pricing model together with Monte Carlo simulation techniques using the following weighted average assumptions during 2006: risk-free interest rate of 5.08%, expected dividend yield of 0%, expected volatility of 75%, forfeiture rate of 6.97%, suboptimal exercise factor of 2, and post-vesting exit rate of 6.97%. An expected life of 7.01 years was derived from the model.
The lattice model requires inputs for risk-free interest rate, dividend yield, volatility, contract term, average vesting period, post-vest exit rate and suboptimal exercise factor. Both the fair value and expected life are outputs from the model. The risk-free interest rate was determined based on the yield available on U.S. Treasury Securities over the life of the option. The dividend yield and volatility factor was determined in the same manner as described above for the Black-Scholes model. The lattice model assumes that employees’ exercise behavior is a function of the option’s remaining vested life and the extent to which the option is in-the-money. The lattice model estimates the probability of exercise as a function of the suboptimal exercise
77
REPLIDYNE, INC.
NOTES TO FINANCIAL STATEMENTS — (continued)
factor and the post-vesting exit rate. The suboptimal exercise factor and post-vesting exit rate were based on actual historical exercise behavior.
The Company had a choice of two attribution methods for allocating compensation costs under SFAS No. 123(R): the “straight-line” method, which allocates expense on a straight-line basis over the requisite service period of the last separately vesting portion of an award, or the “graded vesting attribution method”, which allocates expense on a straight-line basis over the requisite service period for each separately vesting portion of the award as if the award was, in substance, multiple awards. The Company chose the graded vesting attribution method and accordingly, amortizes the fair value of each option over each option’s vesting period (requisite service period).
Employee stock options granted by the Company are generally structured to qualify as “incentive stock options” (ISOs). Under current tax regulations, the Company does not receive a tax deduction for the issuance, exercise or disposition of ISOs if the employee meets certain holding requirements. If the employee does not meet the holding requirements, a disqualifying disposition occurs, at which time the Company will receive a tax deduction. The Company does not record tax benefits related to ISOs unless and until a disqualifying disposition occurs. In the event of a disqualifying disposition, the entire tax benefit is recorded as a reduction of income tax expense. The Company has not recognized any income tax benefit or related tax asset for share-based compensation arrangements as the Company does not believe, based on its history of operating losses, that it is more likely than not it will realize any future tax benefit from such compensation cost recognized since inception of the Company.
Under SFAS 123(R), the estimated fair value of share-based compensation, including stock options granted under the Company’s Equity Incentive Plan and discounted purchases of common stock by employees under the Employee Stock Purchase Plan, is recognized as compensation expense. The estimated fair value of stock options is expensed over the requisite service period as discussed above. Compensation expense under the Company’s Employee Stock Purchase Plan is calculated based on participant elected contributions and estimated fair values of the common stock and the purchase discount at the date of the offering. See Note 10 for further information on share-based compensation under these plans. Share-based compensation included in the Company’s statement of operations was as follows (in thousands):
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2007 | | | 2006 | | | 2005 | |
| |
| Research and development | | $ | 1,234 | | | $ | 385 | | | $ | 58 | |
| Sales, general and administrative | | | 1,550 | | | | 795 | | | | — | |
| | | | | | | | | | | | | |
| | | $ | 2,784 | | | $ | 1,180 | | | $ | 58 | |
| | | | | | | | | | | | | |
SFAS No. 123(R) was applied only to awards granted or modified after the required effective date of January 1, 2006. Awards granted prior to the Company’s implementation of SFAS No. 123(R) are accounted for under the recognition and measurement provisions of APB Opinion No. 25 and related interpretations.
Stock-Based Compensation under APB No. 25. Prior to January 1, 2006, the Company applied the intrinsic-value-based method of accounting prescribed by Accounting Principles Board (APB) Opinion No. 25,Accounting for Stock Issued to Employees, and related interpretations, including Financial Accounting Standards Board (FASB) Interpretation No. 44,Accounting for Certain Transactions involving Stock Compensation, an interpretation of APB Opinion No. 25, in accounting for its employee stock options. Under this method, compensation expense is generally recorded on the date of grant only if the estimated fair value of the underlying stock exceeds the exercise price. Given the absence of an active market for the Company’s common stock prior to its initial public offering, the board of directors historically determined the estimated fair value of common stock on the dates of grant based on several factors, including progress against regulatory, clinical and product development milestones; sales of redeemable convertible preferred stock and
78
REPLIDYNE, INC.
NOTES TO FINANCIAL STATEMENTS — (continued)
the related liquidation preference associated with such preferred stock; progress toward establishing a collaborative development and commercialization partnership for faropenem medoxomil; changes in valuation of comparable publicly-traded companies; overall equity market conditions; and the likelihood of achieving a liquidity event such as an initial public offering or sale of the Company. The Company also considered the guidance set forth in the American Institute of Certified Public Accountants Practice Guide,Valuation of Privately Held-Company Equity Securities Issued As Compensation.In addition, the Company obtained independent valuations of its common stock at September, November and December 2005. These independent valuations supported the fair value of the Company’s common stock established by the board of directors in 2005. Based on these factors, during 2005 the Company valued its common stock and set exercises prices for common stock options at each date of grant within the range of $0.61 to $1.32 per share.
SFAS No. 123,Accounting for Stock-Based Compensation, and SFAS No. 148,Accounting for Stock-Based Compensation — Transition and Disclosure, an amendment of FASB Statement No. 123, established accounting and disclosure requirements using a fair-value-based method of accounting for stock-based employee compensation plans. As permitted by existing accounting standards, the Company elected to continue to apply the intrinsic-value-based method of accounting described above, for options granted through December 31, 2005. The following table illustrates the effect on net loss as if the fair-value-based method had been applied to all outstanding and unvested awards in the year ended December 31, 2005, prior to the adoption of SFAS 123(R), on January 1, 2006 (in thousands, except per share data):
| | | | | |
| Net loss attributable to common stockholders, as reported | | $ | (40,860 | ) |
| Add: stock-based employee compensation expense included in reported net loss attributable to common stockholders | | | 57 | |
| Deduct: total stock-based employee compensation expense determined under fair value based method for all awards | | | (98 | ) |
| | | | | |
| Pro forma net loss attributable to common stockholders | | $ | (40,901 | ) |
| | | | | |
| Net loss attributable to common stockholders per share — basic and diluted, as reported | | $ | (39.20 | ) |
| | | | | |
| Pro forma net loss attributable to common stockholders per share — basic and diluted | | $ | (39.24 | ) |
| | | | | |
Prior to January 1, 2006, the fair value of each employee stock option award was estimated on the date of grant based on the minimum value method using the Black-Scholes option pricing valuation model. For options granted during 2005, the Company used the following weighted average assumptions: weighted average risk-free interest rate of 4.19%; dividend yield of 0.00%; expected life of 5 years and volatility, under the minimum value method, of .0001%.
Clinical Trial Expenses. The Company records clinical trial expenses based on estimates of the services received and efforts expended pursuant to contracts with clinical research organizations (CROs) and other third party vendors associated with its clinical trials. The Company contracts with third parties to perform a range of clinical trial activities in the ongoing development of its product candidates. The terms of these agreements vary and may result in uneven payments. Payments under these contracts depend on factors such as the achievement of certain defined milestones, the successful enrollment of patients and other events. The objective of the Company’s clinical trial accrual policy is to match the recording of expenses in its financial statements to the actual services received and efforts expended. In doing so, the Company relies on information from CROs and its clinical operations group regarding the status of its clinical trials to calculate the accrual for clinical expenses at the end of each reporting period.
Net Income (Loss) Per Share. Net income (loss) per share is computed using the weighted average number of shares of common stock outstanding and is presented for basic and diluted net income (loss) per share. Basic net income (loss) per share is computed by dividing net income (loss) attributable to common
79
REPLIDYNE, INC.
NOTES TO FINANCIAL STATEMENTS — (continued)
stockholders by the weighted average number of common shares outstanding during the period, excluding common stock subject to vesting provisions. Diluted net income (loss) per share is computed by dividing net income (loss) attributable to common stockholders by the weighted average number of common shares outstanding during the period increased to include, if dilutive, the number of additional common shares that would have been outstanding if the potential common shares had been issued or restrictions lifted on restricted stock. The dilutive effect of common stock equivalents such as outstanding stock options, warrants and restricted stock is reflected in diluted net income (loss) per share by application of the treasury stock method.
The following table sets forth the computation of basic and diluted net income (loss) per share (amounts in thousands, except per share amounts):
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2007 | | | 2006 | | | 2005 | |
| |
| Numerator: | | | | | | | | | | | | |
| Net income (loss) | | $ | 7,692 | | | $ | (29,249 | ) | | $ | (33,669 | ) |
| Preferred stock dividends and accretion | | | — | | | | (5,391 | ) | | | (7,191 | ) |
| | | | | | | | | | | | | |
| | | $ | 7,692 | | | $ | (34,640 | ) | | $ | (40,860 | ) |
| | | | | | | | | | | | | |
| Denominator: | | | | | | | | | | | | |
| Weighted-average shares outstanding, excluding unvested restricted stock | | | 26,730 | | | | 13,908 | | | | 1,042 | |
| Effect of dilutive securities | | | 936 | | | | — | | | | — | |
| | | | | | | | | | | | | |
| Denominator for diluted earnings per share | | | 27,666 | | | | 13,908 | | | | 1,042 | |
| | | | | | | | | | | | | |
| Basic income (loss) earnings per share | | $ | 0.29 | | | $ | (2.49 | ) | | $ | (39.20 | ) |
| | | | | | | | | | | | | |
| Diluted income (loss) earnings per share | | $ | 0.28 | | | $ | (2.49 | ) | | $ | (39.20 | ) |
| | | | | | | | | | | | | |
Potentially dilutive securities representing approximately 1.5 million, 2.5 million and 19.4 million shares of common stock for the years ended December 31, 2007, 2006 and 2005, respectively, were excluded from the computation of diluted earnings per share for these periods because their effect would have been antidilutive. Potentially dilutive securities include stock options, warrants, shares to be purchased under the employee stock purchase plan, restricted stock and shares which would be issued under convertible preferred stock.
Fair Value of Financial Instruments. The carrying amounts of financial instruments, including cash and cash equivalents, receivables from Forest Laboratories, and accounts payable approximate fair value due to their short-term maturities.
Revenue Recognition. The Company’s commercial collaboration agreements can contain multiple elements, including nonrefundable upfront fees, payments for reimbursement of research costs, payments for ongoing research, payments associated with achieving specific milestones and royalties based on specified percentages of net product sales, if any. The Company applies the revenue recognition criteria outlined in Emerging Issues Task Force (EITF) IssueNo. 00-21,Revenue Arrangements with Multiple Deliverables(EITF 00-21), in accounting for upfront and milestone payments under the agreement. In applying the revenue recognition criteria withinEITF 00-21, the Company considers a variety of factors in determining the appropriate method of revenue recognition under these arrangements, such as whether the elements are separable, whether there are determinable fair values and whether there is a unique earnings process associated with each element of a contract.
Where the Company does not believe that an upfront fee or milestone payment is specifically tied to a separate earnings process, revenues are recognized ratably over the estimated term of the agreement. When
80
REPLIDYNE, INC.
NOTES TO FINANCIAL STATEMENTS — (continued)
the Company’s obligations under such arrangements are completed, any remaining deferred revenue is recognized.
Payments received by the Company for the reimbursement of expenses for research, development and commercial activities under commercial collaboration and commercialization agreements are recorded in accordance with EITF Issue99-19,Reporting Revenue Gross as Principal Versus Net as an Agent(EITF 99-19). PerEITF 99-19, in transactions where the Company acts as principal, with discretion to choose suppliers, bears credit risk and performs a substantive part of the services, revenue is recorded at the gross amount of the reimbursement. Costs associated with these reimbursements are reflected as a component of operating expenses in the Company’s statements of operations.
Research and Development. Research and development costs are expensed as incurred. These costs consist primarily of salaries and benefits, licenses to technology, supplies and contract services relating to the development of new products and technologies, allocated overhead, clinical trial and related clinical manufacturing costs, and other external costs.
The Company is currently producing clinical and commercial grade product in its facilities and through third parties. Prior to filing for regulatory approval of its products for commercial sale, and such approval being assessed as probable, these costs are expensed as incurred to research and development.
Comprehensive Income (Loss). The Company applies the provisions of SFAS No. 130,Reporting Comprehensive Income, which establishes standards for reporting comprehensive income or loss and its components in financial statements. The Company’s comprehensive income (loss) is comprised of its net income or loss and unrealized gains and losses on securities available-for-sale. For the year ended December 31, 2007 comprehensive income was $7.8 million and for the years ended December 31, 2006 and 2005, the Company reported comprehensive losses of $29.7 million and $33.2 million, respectively.
Income Taxes. The Company accounts for income taxes pursuant to SFAS No. 109,Accounting for Income Taxes, which requires the use of the asset and liability method of accounting for deferred income taxes. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. A valuation allowance is recorded to the extent it is more likely than not that a deferred tax asset will not be realized. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in operations in the period that includes the enactment date.
Based on an analysis of historical equity transactions under the provisions of Section 382 of the Internal Revenue Code, the Company believes that ownership changes have occurred at two points since its inception. These ownership changes limit the annual utilization of the Company’s net operating losses in future periods. The Company does not believe that these ownership changes will result in the loss of any of its net operating loss carryforwards existing on the date of each ownership change. The Company’s only significant deferred tax assets are its net operating loss carryforwards. The Company has provided a valuation allowance for its entire net deferred tax asset since its inception as, due to uncertainty as to future utilization of its net operating loss carryforwards, due primarily to its history of operating losses, the Company has concluded that it is more likely than not that its deferred tax asset will not be realized.
FASB Interpretation No. 48 (FIN 48),Accounting for Uncertainty in Income Taxes — An Interpretation of FASB Statement No. 109,defines a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. FIN 48 also provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure and transition. At the adoption date of January 1, 2007, the Company had no unrecognized tax benefits which would affect its effective tax rate if recognized. At December 31, 2007, the Company has no
81
REPLIDYNE, INC.
NOTES TO FINANCIAL STATEMENTS — (continued)
unrecognized tax benefits. The Company classifies interest and penalties arising from the underpayment of income taxes in the statements of operations as general and administrative expenses. As of December 31, 2007, the Company has no accrued interest or penalties related to uncertain tax positions. The tax years 2003 to 2006 federal returns remain open to examination, and the tax years 2002 to 2006 remain open to examination by other taxing jurisdictions to which we are subject.
Recent Accounting Pronouncements. In September 2006, the FASB issued Statement of Financial Accounting Standard (SFAS) No. 157,Fair Value Measurements(SFAS 157). SFAS 157 defines fair value, establishes a framework for measuring fair value in applying generally accepted accounting principles, and expands disclosures about fair value measurements. SFAS 157 applies whenever an entity is measuring fair value under other accounting pronouncements that require or permit fair value measurement. SFAS 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007, but the FASB provided a one year deferral for implementation of the standard for non-financial assets and liabilities. The Company does not expect that the adoption of SFAS 157 will have a material impact on its financial statements.
In June 2007, the FASB ratified EITF IssueNo. 07-03,Accounting for Advance Payments for Goods or Services to Be Used in Future Research and Development Activities(EITF 07-03). The scope ofEITF 07-03 is limited to nonrefundable advance payments for goods and services to be used or rendered in future research and development activities pursuant to an executory contractual arrangement. This issue provides that nonrefundable advance payments for goods or services that will be used or rendered for future research and development activities should be deferred and capitalized. Such amounts should be recognized as an expense as the related goods are delivered or the related services are performed. The Company will be required to adoptEITF 07-03 for new contracts entered into in 2008. The Company does not expect that the adoption ofEITF 07-03 will have a material impact on its financial statements.
In December 2007, the FASB ratified EITF IssueNo. 07-01, Accounting for Collaborative Arrangements(EITF 07-01).EITF 07-01 defines collaborative arrangements and establishes reporting requirements for transactions between participants in a collaborative arrangement and between participants in the arrangement and third parties.EITF 07-01 also establishes the appropriate income statement presentation and classification for joint operating activities and payments between participants, as well as disclosures related to these arrangements.EITF 07-01 is effective for fiscal years beginning after December 15, 2008. The Company does not expect that the adoption ofEITF 07-01 will have a material impact on its financial statements.
| |
| (3) | Property and Equipment |
Property and equipment at December 31, 2007 and 2006 consist of the following (in thousands):
| | | | | | | | | |
| | | December 31, | |
| | | 2007 | | | 2006 | |
| |
| Equipment | | $ | 5,011 | | | $ | 4,760 | |
| Furniture and fixtures | | | 700 | | | | 820 | |
| Leasehold improvements | | | 2,220 | | | | 2,195 | |
| | | | | | | | | |
| | | | 7,931 | | | | 7,775 | |
| Less accumulated depreciation and amortization | | | (6,026 | ) | | | (4,605 | ) |
| | | | | | | | | |
| Property and equipment, net | | $ | 1,905 | | | $ | 3,170 | |
| | | | | | | | | |
For the years ended December 31, 2007, 2006 and 2005 depreciation and amortization expense was $1.5 million, $1.4 million and $1.3 million, respectively.
82
REPLIDYNE, INC.
NOTES TO FINANCIAL STATEMENTS — (continued)
| |
| (4) | Agreement with Forest Laboratories Holdings Limited |
In February 2006, the Company entered into a collaboration and commercialization agreement with Forest Laboratories for the commercialization, development and distribution of faropenem medoxomil in the U.S. In October 2006, the Company received a non-approvable letter from the FDA for the NDA it submitted for faropenem medoxomil in December 2005. According to the non-approvable letter, the FDA recommended further clinical studies for all indications included in the NDA, additional microbiologic confirmation and consideration of alternate dosing of faropenem medoxomil. In May 2007, the collaboration and commercialization agreement with Forest Laboratories was terminated. In accordance with the terms of the agreement, following the termination, all of Forest Laboratories’ rights and licenses with respect to faropenem medoxomil have ceased.
The Company received $60 million in upfront and milestone payments from Forest Laboratories in 2006, which the Company was recognizing into revenue through 2020, the then estimated term of the agreement. Effective May 7, 2007, the termination date of the agreement with Forest Laboratories, the Company recognized all remaining deferred revenue related to the upfront and milestone payments of approximately $55 million.
| |
| (5) | Accounts Payable and Accrued Expenses |
Accounts payable and accrued expenses at December 31, 2007 and 2006 consist of the following (in thousands):
| | | | | | | | | |
| | | December 31, | |
| | | 2007 | | | 2006 | |
| |
| Accounts payable — trade | | $ | 4,553 | | | $ | 3,223 | |
| Accrued employee compensation | | | 2,692 | | | | 1,313 | |
| Accrued clinical trial costs | | | 1,227 | | | | 894 | |
| Accrued contingent supply agreement fees | | | 2,641 | | | | 882 | |
| Other accrued expenses | | | 1,142 | | | | 1,645 | |
| | | | | | | | | |
| | | $ | 12,255 | | | $ | 7,957 | |
| | | | | | | | | |
| |
| (6) | Commitments and Contingencies |
Operating Leases. The Company has entered into a74-month sub-lease agreement for its Colorado corporate office and laboratory facility and a60-month lease agreement for its Connecticut office facility. These lease agreements include rent concessions and escalating rent payments throughout the term of the lease. The rent expense related to these leases is recorded monthly on a straight-line basis in accordance with U.S. generally accepted accounting principles. Additionally, the Company received leasehold incentives which have been recorded as a deferred credit and are being amortized monthly on a straight-line basis to rent expense over the term of the lease.
83
REPLIDYNE, INC.
NOTES TO FINANCIAL STATEMENTS — (continued)
At December 31, 2007, future minimum lease payments under the Company’s noncancelable operating leases are as follows (in thousands):
| | | | | |
| For the Year Ending December 31, | | | | |
| 2008 | | $ | 737 | |
| 2009 | | | 779 | |
| 2010 | | | 729 | |
| 2011 | | | 514 | |
| | | | | |
| Total future minimum lease payments | | $ | 2,759 | |
| | | | | |
During the years ended December 31, 2007, 2006 and 2005 the Company recognized $0.7 million, $0.6 million and $0.6 million in rent expense, respectively.
Indemnifications. The Company has agreements whereby it indemnifies directors and officers for certain events or occurrences while the director or officer is, or was, serving in such capacity at the Company’s request. The maximum potential amount of future payments the Company could be required to make under these indemnification agreements is unlimited.
Employment Agreements. The Company has entered into employment agreements with its chief executive officer and other named executive officers that provide for base salary, eligibility for bonuses and other generally available benefits. The employment agreements provide that the Company may terminate the named executive officer employment at any time with or without cause. If a named executive officer is terminated by the Company without cause or such officer resigns for good reason, then the named executive officer is entitled to receive a severance package consisting of salary continuation for a period of twelve months from the date of termination among other benefits. If such termination occurs one month before or thirteen months following a change of control, then the executive is entitled to salary continuation for a period of twelve months (or eighteen months with respect to Mr. Collins and Dr. Janjic) from the date of termination and acceleration of vesting of all of the executive’s outstanding unvested options to purchase the Company’s common stock among other benefits. In addition, during 2007 the Company established a severance benefit plan that defines termination benefits for all eligible employees, as defined, not under an employment contract, if the employee is terminated without cause. Under this plan, employees whose employment is terminated without cause are provided a severance benefit of between nine and eighteen weeks pay, based on grade level, plus an additional two weeks pay for each year of service.
Asubio Pharma License Agreement. In 2004, the Company entered into a license agreement with Asubio Pharma Co., Ltd., or Asubio Pharma to develop and commercialize faropenem medoxomil in the U.S. and Canada for adult and pediatric use, which was amended as to certain terms in 2006. The Company has an exclusive option to license rights to faropenem medoxomil for the rest of the world excluding Japan. The Company bears the cost of and manages development, regulatory approvals and commercialization efforts. Asubio Pharma is entitled to upfront fees, milestone payments and royalties.
In consideration for the license, in 2003 and 2004 the Company paid Asubio Pharma an initial license fee of ¥400 million ($3.8 million). In December 2005, the Company submitted its first NDA for adult use of faropenem medoxomil and, at that time, recorded an accrual in the amount of ¥250 million ($2.1 million) for the first milestone due to Asubio Pharma under this agreement. This amount was expensed to research and development in 2005 and paid in 2006. In February 2006, this milestone payment was increased to ¥375 million (approximately $3.2 million). The increased milestone amount of ¥125 million ($1.1 million) was accounted for as research and development expense in the quarter ended March 31, 2006 when the modified terms of the license were finalized. Under the modified license agreement the Company is further obligated to make future payments of up to ¥375 million (approximately $3.3 million at December 31, 2007) upon filing of an NDA at a higher dose and up to ¥1,250 million (approximately $11.1 million at
84
REPLIDYNE, INC.
NOTES TO FINANCIAL STATEMENTS — (continued)
December 31, 2007) in subsequent regulatory and commercial milestone payments for faropenem medoxomil. If it is determined that the Company has ceased development or commercialization of faropenem medoxomil as defined, or the Company terminates its license agreement with Asubio Pharma, it will be obligated to pay a termination fee of up to ¥375 million (approximately $3.3 million as of December 31, 2007). Additionally, the Company is responsible for royalty payments to Asubio Pharma based upon net sales of faropenem medoxomil. The license term extends to the later of: (i) the expiration of the last to expire of the licensed patents owned or controlled by Asubio Pharma or (ii) 12 years after the first commercial launch of faropenem medoxomil. The Company has recorded payments made to date as research and development expense, as faropenem medoxomil has not been approved by the FDA.
Asubio Pharma and Nippon Soda Supply Agreement. Under a supply agreement entered into in December 2004 between Asubio Pharma, Nippon Soda Company Ltd., or Nippon Soda, and the Company, the Company is obligated to purchase, and Nippon Soda is obligated to supply, all of the Company’s commercial requirements of the active pharmaceutical ingredient in faropenem medoxomil for the U.S. and Canadian markets. During the three years following placement of an initial purchase order by the Company, which has not occurred, with Nippon Soda, the Company becomes obligated to make certain annual minimum purchases of drug substance to be determined initially by the Company and Nippon Soda at the time of a commercial launch. Since full commercial launch of faropenem medoxomil has been delayed, the Company is currently obligated to pay Nippon Soda escalating annual delay compensation fees of up to ¥280 million (approximately $2.5 million as of December 31, 2007) per year, which commenced on July 1, 2007. As a result of the non-approvable letter the Company received from the FDA in October 2006 and subsequent activities related to the development of faropenem medoxomil, the Company recorded delay compensation fees of $0.9 million in the year ended December 31, 2007 and delay compensation fees of $0.9 million and an initial order cancellation fee of $0.6 million in the year ended December 31, 2006. These amounts were recorded as research and development expense. If commercial launch of faropenem medoxomil is further delayed or if the Company is unable to obtain a collaboration partner for faropenem medoxomil under its current expected timeframe, the Company may incur additional delay compensation fees of up to ¥105 million ($0.9 million as of December 31, 2007) for 2008 and up to ¥280 million annually ($2.5 million as of December 31, 2007) for all periods following January 1, 2009. If the Company terminates this agreement, abandons the development or commercialization of faropenem medoxomil or is unable to notify Nippon Soda of the faropenem medoxomil launch go date, as defined, by July 1, 2009, the Company will be obligated to pay Nippon Soda prorated delay compensation fees through the effective date of termination and reimburse Nippon Soda for up to ¥65 million ($0.6 million as of December 31, 2007) in engineering costs. As of December 31, 2007, the Company has accrued $1.9 million in delay compensation under this agreement, $0.9 million of which is based upon the Company’s expectations as to the timing of activities related to the faropenem medoxomil program. The Company continues to evaluate amounts which may become payable to Asubio Pharma and Nippon Soda under the terms of the agreement, and adjusts its accrual accordingly.
MEDA Supply Agreement. In 2005, the Company and MEDA Manufacturing GmbH (formerly Tropon GmbH), or MEDA, entered into a supply agreement for production of 300 mg adult tablets of faropenem medoxomil, which was amended as to certain terms in 2006. Beginning in 2006, the Company became obligated to make annual minimum purchases of 300 mg adult tablets from MEDA of €2.3 million (approximately $3.4 million at December 31, 2007). If in any year the Company did not satisfy its minimum purchase commitments, the Company was required to pay MEDA the shortfall amount. Fifty percent (50%) of the shortfall amount, if applicable, may be credited against future drug product purchases. The Company was required to buy all of its requirements for 300 mg adult oral faropenem medoxomil tablets from MEDA until cumulative purchases exceed €22 million (approximately $32.4 million at December 31, 2007). The agreement provided that, upon termination, up to €1.7 million (approximately $2.5 million at December 31, 2007) would be payable for decontamination fees.
85
REPLIDYNE, INC.
NOTES TO FINANCIAL STATEMENTS — (continued)
This agreement was amended in March 2006 such that the Company’s obligations with respect to all purchase commitments and facility decontamination costs were suspended and deemed satisfied by Forest Laboratories pursuant to an agreement between MEDA and Forest Laboratories. Under its agreement with Forest Laboratories, the Company remained liable for any shortfall amount in 2006 that may not have been credited against future drug product purchases. In 2006, the Company incurred $1.5 million relating to its portion of the 2006 shortfall in minimum purchases under these agreements. The amount was accounted for as research and development expense in 2006. In May 2007, concurrent with Forest Laboratories’ termination of its supply agreements with MEDA, the previously suspended provisions in the Company’s agreements with MEDA were no longer suspended, and the Company’s obligations with respect to purchase commitments and facility decontamination costs were no longer waived. In April 2007, the Company provided notice to MEDA of its termination of the supply agreement in accordance with the termination provisions of the agreement as future clinical development of faropenem medoxomil adult tablets would use 600 mg dosing. As this notice occurred before the termination date of the Company’s collaboration agreement with Forest Laboratories, the Company believes that Forest Laboratories, under the terms of the collaboration agreement, was responsible for supply chain obligations related to faropenem medoxomil, including minimum purchase commitments and decontamination obligations under the MEDA agreement, through May 7, 2007 (the term of the collaboration agreement). At December 31, 2007, the Company accrued for minimum purchase fees and interest through date of termination of its agreement with MEDA. MEDA has indicated that it disputes the Company’s right to terminate the agreement on the basis indicated in its notice of termination. The Company believes that it terminated the agreement in accordance with its terms. If it is determined that the Company has obligations to MEDA beyond May 7, 2007 under the agreement, then additional costs may be incurred which may include additional amounts for minimum future drug purchases that were not made and for decontamination of MEDA’s facility.
Other. The Company entered into an arrangement with an investment bank to assist the Company in identifying a licensing partner for its faropenem medoxomil program and to provide other investment banking services. Under the terms of the agreement, the Company may incur transaction fees of up to $6 million based on the value of a license or strategic transaction as defined.
During the fourth quarter of 2007, the Company announced plans to restructure its operations to align critical resources with strategic priorities. As a result, the Company reduced its headcount, primarily in the administrative, clinical, commercial and regulatory functions. The aggregate charge to the Company’s net earnings to restructure its operations was $1.4 million. The restructuring costs related primarily to employee severance and benefits which are expected to be paid in 2008. All expenses are recorded as operating expenses in the Company’s statement of operations for the year ended December 31, 2007.
| |
| (8) | Employee Benefit Plans |
The Company has a 401(k) plan and matches an amount equal to 50 percent of the employee’s current contributions, limited to $2 thousand per participant annually. The Company commenced its matching contribution program in 2006 and contributed $0.1 million during each of the years ended December 31, 2007 and 2006.
The Company’s Certificate of Incorporation, as amended and restated on July 3, 2006, authorizes the Company to issue 105,000,000 shares of $0.001 par value stock which is comprised of 100,000,000 shares of common stock and 5,000,000 shares of preferred stock. Each share of common stock is entitled to one vote on each matter properly submitted to the stockholders of the Company for their vote. The holders of common
86
REPLIDYNE, INC.
NOTES TO FINANCIAL STATEMENTS — (continued)
stock are entitled to receive dividends when and as declared or paid by the board of directors, subject to prior rights of the Preferred Stockholders, if any.
Common Stock Warrants. In connection with the issuance of debt and convertible notes in 2002 and 2003, the Company issued warrants to certain lenders and investors to purchase shares of the Company’s then outstanding redeemable convertible preferred stock. The warrants were initially recorded as liabilities at their fair value. In July 2006, upon completion of the Company’s initial public offering, all outstanding preferred stock warrants were automatically converted into common stock warrants and reclassified to equity at the then current fair value. As of December 31, 2007 and 2006, warrants for the purchase of 53,012 shares of common stock were outstanding and exercisable with exercise prices ranging from $4.90 to $6.13 per share.
| |
| (10) | Share-Based Compensation |
Stock Option Plan. The Company’s Equity Incentive Plan, as amended (the Option Plan), provides for issuances of up to 7,946,405 shares of common stock for stock option grants. Options granted under the Option Plan may be either incentive or nonqualified stock options. Incentive stock options may only be granted to Company employees, including its officers. Nonqualified stock options may be granted to Company employees, which include its officers, directors, and consultants to the Company. Generally, options granted under the Option Plan expire ten years from the date of grant and vest over four years: 25% on the first anniversary from the grant date and ratably in equal monthly installments over the remaining 36 months. This plan is considered a compensatory plan and subject to the provisions of SFAS No. 123(R).
Stock options outstanding at December 31, 2007, changes during the year then ended and options exercisable at December 31, 2007 are presented below (share amounts in thousands):
| | | | | | | | | | | | | | | | | |
| | | | | | | | | Weighted
| | | | |
| | | | | | Weighted
| | | Average
| | | | |
| | | Number
| | | Average
| | | Remaining
| | | Aggregate
| |
| | | of
| | | Exercise
| | | Contractual
| | | Intrinsic
| |
| | | Shares | | | Price | | | Term (Years) | | | Value | |
| | | | | | | | | | | | (in millions) | |
| |
| Options outstanding at January 1, 2007 | | | 2,068 | | | $ | 4.10 | | | | | | | | | |
| Granted | | | 1,150 | | | | 5.36 | | | | | | | | | |
| Exercised | | | (52 | ) | | | 1.23 | | | | | | | | | |
| Forfeited | | | (286 | ) | | | 6.46 | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Options outstanding at December 31, 2007 | | | 2,880 | | | | 4.42 | | | | 8.37 | | | $ | (3.8 | ) |
| | | | | | | | | | | | | | | | | |
| Options exercisable at December 31, 2007 | | | 823 | | | $ | 3.56 | | | | 7.75 | | | $ | (0.4 | ) |
| | | | | | | | | | | | | | | | | |
87
REPLIDYNE, INC.
NOTES TO FINANCIAL STATEMENTS — (continued)
Additional information regarding outstanding common stock options as of December 31, 2007 is presented below (in thousands, except for exercise price and weighted average data):
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | Stock Options
| | | | | | | |
| | | | | | Outstanding | | | | | | | |
| | | | | | Weighted
| | | | | | | | | | |
| | | | | | Average
| | | | | | Stock Options
| |
| | | | | | Remaining
| | | | | | Exercisable | |
| | | Number of
| | | Contractual
| | | Exercise
| | | Number of
| | | Exercise
| |
| Exercise Price | | Shares | | | Life (Years) | | | Price | | | Shares | | | Price | |
| |
| $ 0.49 | | | 21 | | | | 5.03 | | | $ | 0.49 | | | | 21 | | | $ | 0.49 | |
| 0.61 | | | 413 | | | | 7.08 | | | | 0.61 | | | | 270 | | | | 0.61 | |
| 1.32 | | | 37 | | | | 7.77 | | | | 1.32 | | | | 23 | | | | 1.32 | |
| 3.19 | | | 861 | | | | 8.05 | | | | 3.19 | | | | 273 | | | | 3.19 | |
| 5.20 | | | 172 | | | | 8.19 | | | | 5.20 | | | | 80 | | | | 5.20 | |
| 5.35 | | | 907 | | | | 9.18 | | | | 5.35 | | | | — | | | | — | |
| 5.40 | | | 10 | | | | 9.58 | | | | 5.40 | | | | — | | | | — | |
| 5.46 | | | 57 | | | | 9.36 | | | | 5.46 | | | | — | | | | — | |
| 5.54 | | | 27 | | | | 9.36 | | | | 5.54 | | | | — | | | | — | |
| 6.11 | | | 5 | | | | 9.79 | | | | 6.11 | | | | — | | | | — | |
| 6.18 | | | 32 | | | | 8.96 | | | | 6.18 | | | | 8 | | | | 6.18 | |
| 8.97 | | | 141 | | | | 8.37 | | | | 8.97 | | | | 64 | | | | 8.97 | |
| 9.00 | | | 20 | | | | 8.76 | | | | 9.00 | | | | 8 | | | | 9.00 | |
| 9.38 | | | 16 | | | | 8.78 | | | | 9.38 | | | | 5 | | | | 9.38 | |
| 9.51 | | | 10 | | | | 8.79 | | | | 9.51 | | | | 4 | | | | 9.51 | |
| 9.64 | | | 50 | | | | 8.79 | | | | 9.64 | | | | 14 | | | | 9.64 | |
| 9.82 | | | 1 | | | | 8.69 | | | | 9.82 | | | | 1 | | | | 9.82 | |
| 10.00 | | | 93 | | | | 8.51 | | | | 10.00 | | | | 45 | | | | 10.00 | |
| 10.03 | | | 7 | | | | 8.62 | | | | 10.03 | | | | 7 | | | | 10.03 | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | 2,880 | | | | | | | $ | 4.42 | | | | 823 | | | $ | 3.56 | |
| | | | | | | | | | | | | | | | | | | | | |
The weighted average grant date fair value of options granted during the years ended December 31, 2007, 2006 and 2005 was $2.75, $2.52 and $0.15 per share, respectively. The total intrinsic value of options exercised during 2007, 2006 and 2005 was $0.2 million, $0.5 million, and $0.2 million, respectively.
Restricted Shares of Common Stock. Historically, the Company had granted options for shares of common stock that were eligible to be exercised prior to vesting, provided that the shares issued upon such exercise are subject to restrictions which will be released consistent with the original option vesting period. In the event of termination of the service of an employee, the Company may repurchase all unvested shares from the optionee at the original issue price. Options granted under the Option Plan expire no later than 10 years from the date of grant.
A summary of the changes in these restricted shares of common stock during 2007 is presented below (in thousands):
| | | | | |
| Restricted, non-vested shares outstanding at December 31, 2006 | | | 400 | |
| Shares vested upon release of restrictions | | | (151 | ) |
| Restricted stock repurchased upon termination | | | (26 | ) |
| | | | | |
| Restricted, non-vested shares outstanding at December 31, 2007 | | | 223 | |
| | | | | |
88
REPLIDYNE, INC.
NOTES TO FINANCIAL STATEMENTS — (continued)
As of December 31, 2007, restrictions on approximately 145,000 of these shares will be released at an accelerated rate if an NDA for faropenem medoxomil is approved by the FDA.
Stock Based Compensation — Stock Options. During the years ended December 31, 2007, 2006 and 2005, the Company recognized $2.6 million, $1.1 million and $0.1 million of stock based compensation for employee awards, respectively. As of December 31, 2007, the Company had $2.6 million of total unrecognized compensation costs (net of expected forfeitures) from options granted under the Option Plan to be recognized over a weighted average remaining period of approximately 1.77 years.
Employee Stock Purchase Plan. The Company has reserved 305,872 shares of common stock for issuance under its Employee Stock Purchase Plan (the Purchase Plan). The Purchase Plan allows eligible employees to purchase common stock of the Company at the lesser of 85% of its market value on the offering date or the purchase date as established by the Board of Directors. Employee purchases are funded through after-tax payroll deductions, which participants can elect from one percent to twenty percent of compensation, subject to the federal limit. The Purchase Plan is considered a compensatory plan and subject to the provisions of SFAS No. 123(R). To date, 111,679 shares have been issued pursuant to the Purchase Plan. During the years ended December 31, 2007 and 2006, the Company recognized $0.2 million and $39 thousand in share-based compensation expense under SFAS No. 123(R) related to the Purchase Plan, respectively.
SFAS No. 109 requires that a valuation allowance be provided if it is more likely than not that some portion or all of the Company’s deferred tax assets will not be realized. The Company’s ability to realize the benefit of its deferred tax assets will depend on the generation of future taxable income through profitable operations. Due to the uncertainty of future profitable operations, the Company has recorded a full valuation allowance against its net deferred tax assets.
The Company has had no provision for income taxes since inception due to its net operating losses.
The income tax effects of temporary differences that give rise to significant portions of the Company’s net deferred tax assets are as follows (in thousands):
| | | | | | | | | |
| | | 2007 | | | 2006 | |
| |
| Deferred tax assets: | | | | | | | | |
| Net operating loss carryforwards | | $ | 32,632 | | | $ | 36,702 | |
| Research and experimentation credits | | | 4,540 | | | | 2,383 | |
| Depreciation and amortization | | | 681 | | | | 455 | |
| Accrued expenses and other | | | 739 | | | | 505 | |
| | | | | | | | | |
| Total deferred tax assets | | | 38,592 | | | | 40,045 | |
| Valuation allowance | | | (38,592 | ) | | | (40,045 | ) |
| | | | | | | | | |
| Net deferred tax assets | | $ | — | | | $ | — | |
| | | | | | | | | |
89
REPLIDYNE, INC.
NOTES TO FINANCIAL STATEMENTS — (continued)
The benefit for income taxes differs from the amount computed by applying the United States of America federal income tax rate of 35% to the loss before income taxes as follows (in thousands):
| | | | | | | | | | | | | |
| | | December 31, | |
| | | 2007 | | | 2006 | | | 2005 | |
| |
| U.S. federal income tax benefit at statutory rates | | $ | 2,692 | | | $ | (10,237 | ) | | $ | (11,784 | ) |
| State income tax benefit, net of federal impact | | | 250 | | | | (951 | ) | | | (1,094 | ) |
| Non-deductable expenses | | | 588 | | | | 235 | | | | 39 | |
| Research and experimentation credits | | | (2,157 | ) | | | (940 | ) | | | (905 | ) |
| Other items | | | 81 | | | | 69 | | | | 12 | |
| Change in valuation allowance | | | (1,454 | ) | | | 11,824 | | | | 13,732 | |
| | | | | | | | | | | | | |
| | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | |
At December 31, 2007, the Company had approximately $85 million of net operating loss carryforwards and approximately $4.5 million of research and experimentation credits which may be used to offset future taxable income. The carryforwards will expire in 2020 through 2027. The Internal Revenue Code places certain limitations on the annual amount of net operating loss carryforwards that can be utilized if certain changes in the Company’s ownership occur. The Company believes, based on an analysis of historical equity transactions under the provisions of Section 382, that ownership changes have in fact occurred at two points since its inception. These ownership changes will limit the annual utilization of the Company’s net operating losses in future periods. The Company does not believe, however, that these ownership changes will result in the loss of any of its net operating loss carryforwards existing on the date of the ownership changes.
| |
| (12) | Selected Quarterly Financial Data (unaudited) |
The following is a summary of the quarterly results of operations for the years ended December 31, 2007 and 2006 (unaudited, in thousands, except for income (loss) per share data):
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | Net Income
| | | Basic Net Income
| | | Diluted Net Income
| | | | |
| | | | | | | | | (Loss)
| | | (Loss) Attributable
| | | (Loss) Attributable
| | | | |
| | | | | | | | | Attributable
| | | to Common
| | | to Common
| | | | |
| | | | | | Net Income
| | | to Common
| | | Stockholders
| | | Stockholders
| | | | |
| | | Revenue | | | (Loss) | | | Stockholders | | | per Share | | | per Share | | | | |
| |
| Year ended December 31, 2007: | | | | | | | | | | | | | | | | | | | | | | | | |
| First quarter | | $ | 2,925 | | | $ | (8,552 | ) | | $ | (8,552 | ) | | $ | (0.32 | ) | | $ | (0.32 | ) | | | | |
| Second quarter | | | 55,646 | | | | 45,490 | | | | 45,490 | | | | 1.71 | | | | 1.65 | | | | | |
| Third quarter | | | — | | | | (12,303 | ) | | | (12,303 | ) | | | (0.46 | ) | | | (0.46 | ) | | | | |
| Fourth quarter | | | — | | | | (16,943 | ) | | | (16,943 | ) | | | (0.63 | ) | | | (0.63 | ) | | | | |
| Year ended December 31, 2006: | | | | | | | | | | | | | | | | | | | | | | | | |
| First quarter | | $ | 2,877 | | | $ | (7,702 | ) | | $ | (10,355 | ) | | $ | (7.21 | ) | | $ | (7.21 | ) | | | | |
| Second quarter | | | 4,045 | | | | (6,208 | ) | | | (8,862 | ) | | | (5.79 | ) | | | (5.79 | ) | | | | |
| Third quarter | | | 3,679 | | | | (5,722 | ) | | | (5,806 | ) | | | (0.23 | ) | | | (0.23 | ) | | | | |
| Fourth quarter | | | 5,387 | | | | (9,617 | ) | | | (9,617 | ) | | | (0.36 | ) | | | (0.36 | ) | | | | |
On January 22, 2008, the Company received a Warning Letter from the FDA. The Warning Letter was issued pursuant to the completion of the FDA’s review of clinical trials performed in connection with the December 2005 NDA filed by the Company in support of faropenem medoxomil 300 mg tablets twice per day dose, in respect of which the FDA issued a non-approvable letter in October 2006. The Company intends to respond to the Warning Letter within the time limits required by the FDA
90
| |
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| |
| ITEM 9A. | CONTROLS AND PROCEDURES |
Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of our disclosure controls and procedures, as such term is defined underRule 13a-15(e) promulgated under the Securities Exchange Act of 1934, as amended (the Exchange Act). Based on this evaluation, our principal executive officer and our principal financial officer concluded that our disclosure controls and procedures were effective as of the end of the period covered by this annual report.
Management Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Our internal control over financial reporting was designed to provide reasonable assurance to our management and board of directors regarding the preparation and fair presentation of published financial statements. Internal control over financial reporting is promulgated under the Exchange Act as a process designed by, or under the supervision of, our principal executive and principal financial officers and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
| | |
| | • | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect our transactions and dispositions of our assets; |
| |
| | • | Provide reasonable assurance that our transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and |
| |
| | • | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition or disposition of our assets that could have a material effect on the financial statements. |
Internal control over financial reporting, no matter how well designed, has inherent limitations and may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with policies or procedures may deteriorate. Therefore, even effective internal control over financial reporting can only provide reasonable assurance with respect to financial statement preparation and presentation.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2007. In making this assessment, it used the criteria based on the framework set forth by the Committee of Sponsoring Organizations of the Treadway Commission in “Internal Control — Integrated Framework”. Based on our assessment we believe that, as of December 31, 2007, our internal control over financial reporting is effective based on those criteria.
The effectiveness of our internal control over financial reporting as of December 31, 2007 has been audited by KPMG LLP, an independent registered public accounting firm, as stated in their report which is included herein.
91
No Changes in Internal Control over Financial Reporting
There were no changes in our internal controls over financial reporting during the quarter ended December 31, 2007 that have materially affected, or are reasonably likely to materially affect, our internal controls over financial reporting.
| |
| ITEM 9B. | OTHER INFORMATION |
None.
PART III
| |
| ITEM 10. | DIRECTORS AND EXECUTIVE OFFICERS OF THE REGISTRANT |
We have adopted a Code of Business Conduct and Ethics (the “Code”) that applies to all of our employees (including executive officers) and directors. The Code is available on our website at www.replidyne.com under the heading “Investor Information”. We intend to satisfy the disclosure requirement regarding any waiver of a provision of the Code applicable to any executive officer or director, by posting such information on such website. We shall provide to any person without charge, upon request, a copy of the Code. Any such request must be made in writing to Replidyne, Inc.,c/o Investor Relations, 1450 Infinite Drive, Louisville, CO 80027.
Except for information relating to executive officers under the heading “Executive Officers of the Registrant,” which can be found in Part I following Item 4, all additional information required by this item will be contained in our definitive Proxy Statement (“Proxy Statement”) for our 2008 Annual Meeting of Stockholders to be held on May 8, 2008 under the headings “Election of Directors” and “Section 16(a) Beneficial Ownership Reporting Compliance” and is incorporated herein by reference.
| |
| ITEM 11. | EXECUTIVE COMPENSATION |
The information required by this item will be contained in the Proxy Statement under the headings “Executive Compensation” and “Information Regarding the Board of Directors and Corporate Governance” and is incorporated herein by reference.
| |
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by this item will be contained in the Proxy Statement under the headings “Securities Authorized for Issuance under Equity Compensation Plans” and “Security Ownership of Certain Beneficial Owners and Management” and is incorporated herein by reference.
| |
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS |
The information required by this item will be contained in the Proxy Statement under the heading “Transactions with Related Persons” and “Information Regarding the Board of Directors and Corporate Governance” and is incorporated herein by reference.
| |
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The information required by this item will be contained in the Proxy Statement under the heading “Ratification of Selection of Independent Auditors” and is incorporated herein by reference.
92
PART IV
| |
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
a. Financial Statements. The following financial statements are submitted as part of this report:
| | |
| | | Reports of Independent Registered Public Accounting Firm,
Balance Sheets at December 31, 2007 and 2006,
Statements of Operations for 2007, 2006 and 2005,
Statements of Stockholders’ Equity (Deficit), Preferred Stock and Comprehensive Income (Loss)
for 2007, 2006 and 2005,
Statements of Cash Flows for 2007, 2006 and 2005,
Notes to Financial Statements. |
b. Financial Statement Schedules
No financial statement schedules are included because they are not required or the information is included in the financial statements or notes thereto.
c. Exhibits
| | | | | | | | | |
Exhibit
| | | | |
Number | | Note | | Description of Document |
| |
| | 3 | .1 | | | (1) | | | Restated Certificate of Incorporation. |
| | 3 | .2 | | | (1) | | | Amended and Restated Bylaws. |
| | 4 | .1 | | | (1) | | | Reference is made to exhibits 3.1 and 3.2. |
| | 4 | .2 | | | (1) | | | Specimen Common Stock Certificate. |
| | 4 | .3 | | | (1) | | | Form of Warrant to purchase shares of Series A Convertible Preferred Stock (together with schedule prepared in accordance with Instruction 2 to Item 601 ofRegulation S-K). |
| | 4 | .4 | | | (1) | | | Form of Warrant to purchase shares of Series C Preferred Stock (together with schedule prepared in accordance with Instruction 2 to Item 601 ofRegulation S-K). |
| | 4 | .5 | | | (1) | | | Fourth Amended and Restated Stockholders’ Agreement, dated August 17, 2005, between the Registrant and certain of its stockholders, as amended March 7, 2006. |
| | 10 | .1+ | | | (1) | | | Form of Indemnification Agreement for Directors. |
| | 10 | .2+ | | | (1) | | | Form of Indemnification Agreement for Executive Officers. |
| | 10 | .3+ | | | (1) | | | 2006 Equity Incentive Plan. |
| | 10 | .4+ | | | (1) | | | Form of Option Grant Notice and Form of Option Agreement under 2006 Equity Incentive Plan. |
| | 10 | .5+ | | | (1) | | | 2006 Employee Stock Purchase Plan. |
| | 10 | .6+ | | | (1) | | | Form of Offering Document under 2006 Employee Stock Purchase Plan. |
| | 10 | .7+ | | | (1) | | | Employment Agreement, dated April 3, 2006, between the Registrant and Kenneth J. Collins. |
| | 10 | .7.1+ | | | (3) | | | Amendment, dated June 15, 2007, to Employment Agreement, dated April 3, 2006, between the Registrant and Kenneth J. Collins. |
| | 10 | .8+ | | | (1) | | | Employment Agreement, dated April 3, 2006, between the Registrant and Nebojsa Janjic, Ph.D. |
| | 10 | .8.1+ | | | (3) | | | Amendment, dated June 15, 2007, to Employment Agreement, dated April 3, 2006, between the Registrant and Nebojsa Janjic, Ph.D. |
| | 10 | .9+ | | | (1) | | | Employment Agreement, dated April 3, 2006, between the Registrant and Peter Letendre, Pharm.D. |
| | 10 | .9.1+ | | | (3) | | | Amendment, dated June 15, 2007, to Employment Agreement, dated April 3, 2006, between the Registrant and Peter Letendre, Pharm.D. |
| | 10 | .10+ | | | (1) | | | Employment Agreement, dated April 3, 2006, between the Registrant and Roger M. Echols, M.D. |
93
| | | | | | | | | |
Exhibit
| | | | |
Number | | Note | | Description of Document |
| |
| | 10 | .10.1+ | | | (3) | | | Amendment, dated June 15, 2007, to Employment Agreement, dated April 3, 2006, between the Registrant and Roger M. Echols, M.D. |
| | 10 | .11+ | | | (1) | | | Employment Agreement, dated April 3, 2006, between the Registrant and Mark Smith. |
| | 10 | .11.1+ | | | (3) | | | Amendment, dated June 15, 2007, to Employment Agreement, dated April 3, 2006, between the Registrant and Mark Smith. |
| | 10 | .12+ | | | (1) | | | Employment Agreement, dated April 3, 2006, between the Registrant and Donald Morrissey. |
| | 10 | .13+ | | | (1) | | | Summary of Director Compensation Program. |
| | 10 | .14* | | | (1) | | | License Agreement, dated March 15, 2004, between the Registrant and Daiichi Suntory Pharma Co., Ltd. |
| | 10 | .14.1* | | | (1) | | | Amendment, dated April 5, 2005, to License Agreement, dated March 15, 2004, between the Registrant and Daiichi Suntory Pharma Co., Ltd. |
| | 10 | .14.2* | | | (1) | | | Second Amendment, dated February 10, 2006, to License Agreement, dated March 15, 2004, between the Registrant and Daiichi Suntory Pharma Co., Ltd. |
| | 10 | .15* | | | (1) | | | Supply Agreement, dated December 20, 2004, among the Registrant, Daiichi Suntory Pharma Co., Ltd. and Nippon Soda Co., Ltd. |
| | 10 | .16 | | | (1) | | | Lease Agreement, dated March 22, 2005, by and between the Registrant and Crown Milford LLC. |
| | 10 | .17 | | | (1) | | | Lease Agreement, dated October 25, 2005, by and between the Registrant and Triumph 1450 LLC. |
| | 10 | .18* | | | (1) | | | Collaboration and Commercialization Agreement, dated February 10, 2006, between the Registrant and Forest Laboratories Holdings Limited. |
| | 10 | .19+ | | | (2) | | | Replidyne Inc. Variable Incentive Bonus Plan for Calendar Year 2007. |
| | 23 | .1 | | | | | | Consent of KPMG LLP. |
| | 24 | .1 | | | | | | Power of Attorney (included on signature page hereto). |
| | 31 | .1 | | | | | | Certification of principal executive officer required byRule 13a-14(a). |
| | 31 | .2 | | | | | | Certification of principal financial officer required byRule 13a-14(a). |
| | 32 | .1 | | | | | | Section 1350 Certification. |
| | |
| + | | Indicates management contract or compensatory plan. |
| |
| * | | Confidential treatment has been granted with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission. |
| |
| (1) | | Incorporated by reference to the same numbered exhibit filed with our Registration Statement onForm S-1(File No. 333-133021), as amended, declared effective June 29, 2006. |
| |
| (2) | | Incorporated by reference to our Current Report onForm 8-K filed on March 9, 2007. |
| |
| (3) | | Incorporated by reference to our Current Report onForm 8-K filed on June 19, 2007. |
94
SIGNATURES
Pursuant to the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
REPLIDYNE, INC.
Kenneth Collins
President and Chief Executive Officer
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons in the capacities indicated and on the dates indicated.
| | | | | | | |
Signature | | Title | | Date |
| |
/s/ Kenneth J. Collins
Kenneth J. Collins | | President, Chief Executive Officer and Member of the Board of Directors (Principal Executive Officer) | | March 14, 2008 |
| | | | | |
/s/ Mark L. Smith
Mark L. Smith | | Chief Financial Officer, Treasurer, (Principal Financial and Accounting Officer) | | March 14, 2008 |
| | | | | | | |
| | | | | | | |
| | | | | |
/s/ Kirk K. Calhoun
Kirk K. Calhoun | | Member of the Board of Directors | | March 14, 2008 |
| | | | | |
/s/ Edward Brown
Edward Brown | | Member of the Board of Directors | | March 14, 2008 |
| | | | | |
/s/ Geoffrey Duyk
Geoffrey Duyk, MD, Ph.D. | | Member of the Board of Directors | | March 14, 2008 |
| | | | | |
/s/ Christopher D. Earl
Christopher D. Earl, Ph.D. | | Member of the Board of Directors | | March 14, 2008 |
| | | | | |
/s/ Augustine Lawlor
Augustine Lawlor | | Member of the Board of Directors | | March 14, 2008 |
| | | | | |
/s/ Daniel J. Mitchell
Daniel J. Mitchell | | Member of the Board of Directors | | March 14, 2008 |
95
| | | | | | | | | |
Exhibit
| | | | |
Number | | Note | | Description of Document |
| |
| | 3 | .1 | | | (1) | | | Restated Certificate of Incorporation. |
| | 3 | .2 | | | (1) | | | Amended and Restated Bylaws. |
| | 4 | .1 | | | (1) | | | Reference is made to exhibits 3.1 and 3.2. |
| | 4 | .2 | | | (1) | | | Specimen Common Stock Certificate. |
| | 4 | .3 | | | (1) | | | Form of Warrant to purchase shares of Series A Convertible Preferred Stock (together with schedule prepared in accordance with Instruction 2 to Item 601 ofRegulation S-K). |
| | 4 | .4 | | | (1) | | | Form of Warrant to purchase shares of Series C Preferred Stock (together with schedule prepared in accordance with Instruction 2 to Item 601 ofRegulation S-K). |
| | 4 | .5 | | | (1) | | | Fourth Amended and Restated Stockholders’ Agreement, dated August 17, 2005, between the Registrant and certain of its stockholders, as amended March 7, 2006. |
| | 10 | .1+ | | | (1) | | | Form of Indemnification Agreement for Directors. |
| | 10 | .2+ | | | (1) | | | Form of Indemnification Agreement for Executive Officers. |
| | 10 | .3+ | | | (1) | | | 2006 Equity Incentive Plan. |
| | 10 | .4+ | | | (1) | | | Form of Option Grant Notice and Form of Option Agreement under 2006 Equity Incentive Plan. |
| | 10 | .5+ | | | (1) | | | 2006 Employee Stock Purchase Plan. |
| | 10 | .6+ | | | (1) | | | Form of Offering Document under 2006 Employee Stock Purchase Plan. |
| | 10 | .7+ | | | (1) | | | Employment Agreement, dated April 3, 2006, between the Registrant and Kenneth J. Collins. |
| | 10 | .7.1+ | | | (3) | | | Amendment, dated June 15, 2007, to Employment Agreement, dated April 3, 2006, between the Registrant and Kenneth J. Collins. |
| | 10 | .8+ | | | (1) | | | Employment Agreement, dated April 3, 2006, between the Registrant and Nebojsa Janjic, Ph.D. |
| | 10 | .8.1+ | | | (3) | | | Amendment, dated June 15, 2007, to Employment Agreement, dated April 3, 2006, between the Registrant and Nebojsa Janjic, Ph.D. |
| | 10 | .9+ | | | (1) | | | Employment Agreement, dated April 3, 2006, between the Registrant and Peter Letendre, Pharm.D. |
| | 10 | .9.1+ | | | (3) | | | Amendment, dated June 15, 2007, to Employment Agreement, dated April 3, 2006, between the Registrant and Peter Letendre, Pharm.D. |
| | 10 | .10+ | | | (1) | | | Employment Agreement, dated April 3, 2006, between the Registrant and Roger M. Echols, M.D. |
| | 10 | .10.1+ | | | (3) | | | Amendment, dated June 15, 2007, to Employment Agreement, dated April 3, 2006, between the Registrant and Roger M. Echols, M.D. |
| | 10 | .11+ | | | (1) | | | Employment Agreement, dated April 3, 2006, between the Registrant and Mark Smith. |
| | 10 | .11.1+ | | | (3) | | | Amendment, dated June 15, 2007, to Employment Agreement, dated April 3, 2006, between the Registrant and Mark Smith. |
| | 10 | .12+ | | | (1) | | | Employment Agreement, dated April 3, 2006, between the Registrant and Donald Morrissey. |
| | 10 | .13+ | | | (1) | | | Summary of Director Compensation Program. |
| | 10 | .14* | | | (1) | | | License Agreement, dated March 15, 2004, between the Registrant and Daiichi Suntory Pharma Co., Ltd. |
| | 10 | .14.1* | | | (1) | | | Amendment, dated April 5, 2005, to License Agreement, dated March 15, 2004, between the Registrant and Daiichi Suntory Pharma Co., Ltd. |
| | 10 | .14.2* | | | (1) | | | Second Amendment, dated February 10, 2006, to License Agreement, dated March 15, 2004, between the Registrant and Daiichi Suntory Pharma Co., Ltd. |
| | 10 | .15* | | | (1) | | | Supply Agreement, dated December 20, 2004, among the Registrant, Daiichi Suntory Pharma Co., Ltd. and Nippon Soda Co., Ltd. |
| | 10 | .16 | | | (1) | | | Lease Agreement, dated March 22, 2005, by and between the Registrant and Crown Milford LLC. |
| | 10 | .17 | | | (1) | | | Lease Agreement, dated October 25, 2005, by and between the Registrant and Triumph 1450 LLC. |
| | | | | | | | | |
Exhibit
| | | | |
Number | | Note | | Description of Document |
| |
| | 10 | .18* | | | (1) | | | Collaboration and Commercialization Agreement, dated February 10, 2006, between the Registrant and Forest Laboratories Holdings Limited. |
| | 10 | .19+ | | | (2) | | | Replidyne Inc. Variable Incentive Bonus Plan for Calendar Year 2007. |
| | 23 | .1 | | | | | | Consent of KPMG LLP. |
| | 24 | .1 | | | | | | Power of Attorney (included on signature page hereto). |
| | 31 | .1 | | | | | | Certification of principal executive officer required byRule 13a-14(a). |
| | 31 | .2 | | | | | | Certification of principal financial officer required byRule 13a-14(a). |
| | 32 | .1 | | | | | | Section 1350 Certification. |
| | |
| + | | Indicates management contract or compensatory plan. |
| |
| * | | Confidential treatment has been granted with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission. |
| |
| (1) | | Incorporated by reference to the same numbered exhibit filed with our Registration Statement onForm S-1(File No. 333-133021), as amended, declared effective June 29, 2006. |
| |
| (2) | | Incorporated by reference to our Current Report onForm 8-K filed on March 9, 2007. |
| |
| (3) | | Incorporated by reference to our Current Report onForm 8-K filed on June 19, 2007. |