©2022 Cardiovascular Systems, Inc. All Rights Reserved. Q3 FY22 Earnings Supplement May 4, 2022

Safe Harbor This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Report Act of 1995, which are provided under the protection of the safe harbor for forward-looking statements provided by that Act. For example, statements in this presentation regarding CSI’s strategy; growth; future financial measurements and investments; product development plans, milestones and introductions; geographic expansion; clinical trials and evidence; market estimates and opportunities; and developments related to the COVID-19 pandemic; are forward-looking statements. These statements involve risks and uncertainties that could cause results differ materially from those projected, including, but not limited to, those described in CSI’s filings with the Securities and Exchange Commission, including its most recent annual report on Form 10-K and subsequent quarterly and annual reports. CSI encourages you to consider all of these risks, uncertainties and other factors carefully in evaluating the forward-looking statements contained in this presentation. As a result of these matters, changes in facts, assumptions not being realized or other circumstances, CSI’s actual results may differ materially from the expected results discussed in the forward-looking statements contained in this presentation. The forward-looking statements contained in this presentation are made only as of the date of this presentation, and CSI undertakes no obligation to update them to reflect subsequent events or circumstances. 2 FORWARD LOOKING STATEMENTS FINANCIAL INFORMATION This presentation includes calculations or figures that have been prepared internally and have not been reviewed or audited by CSI’s independent registered accounting firm. Use of different methods for preparing, calculating or presenting information may lead to differences, which may be material. In addition, this presentation also includes certain non-GAAP financial measures, such as Adjusted EBITDA. Reconciliations of the non-GAAP financial measures used in this presentation to the most comparable U.S. GAAP measures for the respective periods can be found in tables in the appendix to this presentation. Non-GAAP financial measures have limitations as analytical tools and should not be considered in isolation or as a substitute for CSI's financial results prepared in accordance with GAAP.

4.9% decrease Q/Q and 11.1% decrease Y/Y Q3 FY22 Worldwide Revenues of $56.2 Million Q3 FY22 Revenue Breakdown Worldwide Peripheral $37.4 -3.9% Q/Q -11.6% Y/Y Worldwide Coronary $18.9 -6.8% Q/Q -10.1% Y/Y Worldwide Peripheral Worldwide Coronary ($ in millions, Q/Q and Y/Y represent Quarter-over-Quarter and Year-over-Year growth rates) • Omicron, staffing shortages, competition and reduction in OBL reimbursement disrupted procedure volumes • Announced several product pipeline developments, including: • FDA PMA approval of Scoreflex NC • Progress towards commercialization of IVL technology for treatment of PAD and CAD • Partnership with Innova Vascular to develop thrombectomy devices • First in-human experience with peripheral everolimus drug coated balloon • First in-human experience with Propel™ percutaneous ventricular assist device • Net loss of ($9.7M) compared to ($6.0M) in the year ago period • Cash and cash equivalents of $172.1M as of March 31, 2022 Q3 FY22 Highlights 3
Q3 Product Pipeline Milestones 4
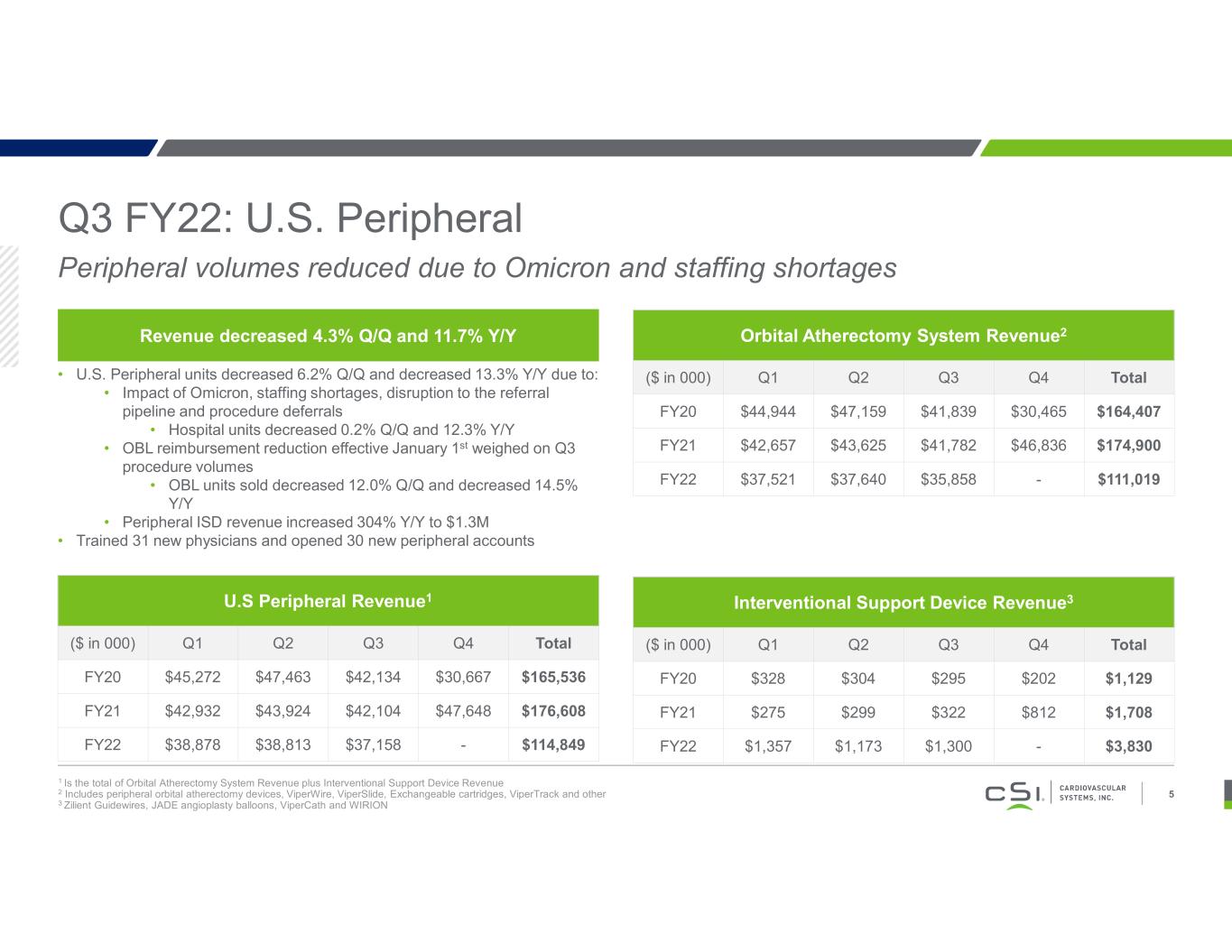
Q3 FY22: U.S. Peripheral 1 Is the total of Orbital Atherectomy System Revenue plus Interventional Support Device Revenue 2 Includes peripheral orbital atherectomy devices, ViperWire, ViperSlide, Exchangeable cartridges, ViperTrack and other 3 Zilient Guidewires, JADE angioplasty balloons, ViperCath and WIRION 5 Revenue decreased 4.3% Q/Q and 11.7% Y/Y • U.S. Peripheral units decreased 6.2% Q/Q and decreased 13.3% Y/Y due to: • Impact of Omicron, staffing shortages, disruption to the referral pipeline and procedure deferrals • Hospital units decreased 0.2% Q/Q and 12.3% Y/Y • OBL reimbursement reduction effective January 1st weighed on Q3 procedure volumes • OBL units sold decreased 12.0% Q/Q and decreased 14.5% Y/Y • Peripheral ISD revenue increased 304% Y/Y to $1.3M • Trained 31 new physicians and opened 30 new peripheral accounts U.S Peripheral Revenue1 ($ in 000) Q1 Q2 Q3 Q4 Total FY20 $45,272 $47,463 $42,134 $30,667 $165,536 FY21 $42,932 $43,924 $42,104 $47,648 $176,608 FY22 $38,878 $38,813 $37,158 - $114,849 Orbital Atherectomy System Revenue2 ($ in 000) Q1 Q2 Q3 Q4 Total FY20 $44,944 $47,159 $41,839 $30,465 $164,407 FY21 $42,657 $43,625 $41,782 $46,836 $174,900 FY22 $37,521 $37,640 $35,858 - $111,019 Interventional Support Device Revenue3 ($ in 000) Q1 Q2 Q3 Q4 Total FY20 $328 $304 $295 $202 $1,129 FY21 $275 $299 $322 $812 $1,708 FY22 $1,357 $1,173 $1,300 - $3,830 Peripheral volumes reduced due to Omicron and staffing shortages

Q3 FY22: U.S. Coronary 1 Is the total of Orbital Atherectomy System Revenue plus Interventional Support Device Revenue 2 Includes Diamondback, Coronary Guidewire, ViperSlide and other 3 Includes Sapphire angioplasty balloons, Teleport microcatheters and Scoreflex NC balloons Coronary volumes reduced due to impact of Omicron and staffing shortages 6 Revenue decreased 11.8% Q/Q and decreased 16.0% Y/Y • U.S. Coronary OAS units sold decreased 15.9% Q/Q and decreased 22.8% Y/Y as Omicron, staffing shortages and competition reduced procedure volumes • Coronary ISD revenue increased 5.2% Q/Q and 19.9% Y/Y to $2.9M and generated $939 of incremental revenue for every coronary OAS sold • Launch of Scoreflex NC Scoring PTCA Catheter in March well- received • Certified 61 new physicians and opened 8 new coronary accounts • ECLIPSE enrollment ≈1,800 as of March 31, 2022 U.S Coronary Revenue1 ($ in 000) Q1 Q2 Q3 Q4 Total FY20 $16,257 $18,497 $15,988 $9,785 $60,527 FY21 $15,899 $17,983 $17,489 $19,645 $71,016 FY22 $16,164 $16,658 $14,685 - $47,507 Orbital Atherectomy System Revenue2 ($ in 000) Q1 Q2 Q3 Q4 Total FY20 $14,669 $16,490 $14,058 $8,651 $53,868 FY21 $13,952 $15,762 $15,093 $16,781 $61,588 FY22 $13,505 $13,927 $11,813 - $39,245 Interventional Support Device Revenue3 ($ in 000) Q1 Q2 Q3 Q4 Total FY20 $1,588 $2,007 $1,930 $1,134 $6,659 FY21 $1,947 $2,221 $2,396 $2,864 $9,428 FY22 $2,659 $2,731 $2,872 - $8,262
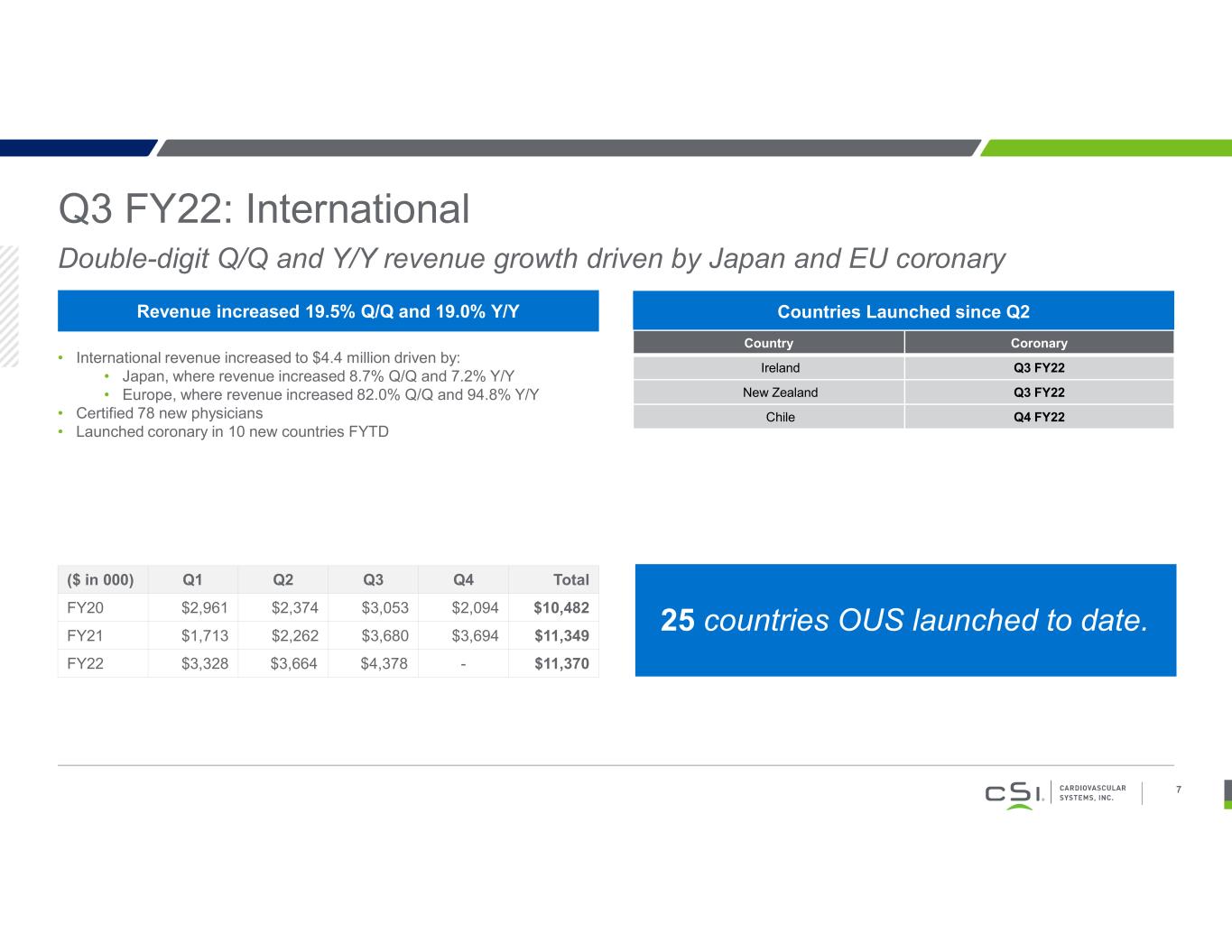
Q3 FY22: International Double-digit Q/Q and Y/Y revenue growth driven by Japan and EU coronary 7 Revenue increased 19.5% Q/Q and 19.0% Y/Y • International revenue increased to $4.4 million driven by: • Japan, where revenue increased 8.7% Q/Q and 7.2% Y/Y • Europe, where revenue increased 82.0% Q/Q and 94.8% Y/Y • Certified 78 new physicians • Launched coronary in 10 new countries FYTD ($ in 000) Q1 Q2 Q3 Q4 Total FY20 $2,961 $2,374 $3,053 $2,094 $10,482 FY21 $1,713 $2,262 $3,680 $3,694 $11,349 FY22 $3,328 $3,664 $4,378 - $11,370 Countries Launched since Q2 Country Coronary Ireland Q3 FY22 New Zealand Q3 FY22 Chile Q4 FY22 25 countries OUS launched to date.

Q3 FY22 vs. Q2 FY22 and Q3 FY21 Dollars in thousands 8 Q3 FY22 Quarter/Quarter Change Year/Year Change Worldwide Revenue $56,221 -4.9% -11.1% Worldwide Peripheral Revenue $37,370 -3.9% -11.6% Worldwide Coronary Revenue $18,851 -6.8% -10.1% U.S. Revenue $51,843 -6.5% -13.0% U.S. Peripheral Revenue $37,158 -4.3% -11.7% U.S. Coronary Revenue $14,685 -11.8% -16.0% International Revenue $4,378 19.5% 19.0% U.S. Peripheral Units - -6.2% -13.3% U.S. Coronary Units - -15.9% -22.8%

Q3 FY22: Select Financial Information Dollars in thousands, except earnings per share and shares outstanding 9 Q3 FY22 Q2 FY22 Q/Q Change Fav (Unfav) Q3 FY21 Y/Y Change Fav (Unfav) Net revenues $56,221 $59,135 ($2,914) $63,273 ($7,052) Cost of goods sold 14,790 18,073* 3,283 14,013 (777) Gross Margin 73.7% 69.4%* Increased 430 BP* 77.9% Decreased 420 BP Selling, general and administrative 41,680 40,402 (1,278) 41,442 (238) % of sales 74.1% 68.3% Increased 580 BP 65.5% Increased 860 BP Research and development 9,052 8,873 (179) 13,163 4,111 % of sales 16.1% 15.0% Increased 110 BP 20.8% Decreased 470 BP Amortization of intangible assets 346 346 - 304 (42) Loss from operations (9,647) (8,559) (1,088) (5,649) (3,998) Other (income) and expense, net (52) 345 397 292 344 Provision for income taxes 63 63 - 63 - Net loss ($9,658) ($8,967) ($691) ($6,004) ($3,654) Basic and diluted earnings per share ($0.25) ($0.23) ($0.02) ($0.15) ($0.10) Basic and diluted weighted average shares outstanding 39,287,632 39,199,593 Increased 88,039 38,911,454 Increased 376,178 *Q2 FY22 cost of goods sold includes $2.8 million one-time charge related to the recall of the WIRION embolic protection system in November 2021.

FY22: Annual Guidance 10 For the fiscal year ending June 30, 2022, CSI anticipates: Revenue of $235 million to $240 million; Gross profit as a percentage of revenues of approximately 73%; Net loss in a range of 15% to 16% of revenues; and Adjusted EBITDA loss of 4% to 5% of revenues.

Non-GAAP Financial Measures 11 CSI uses Adjusted EBITDA as a supplemental measure of performance and believes this measure facilitates operating performance comparisons from period to period and company to company by factoring out potential differences caused by depreciation and amortization expense and stock-based compensation. CSI's management uses Adjusted EBITDA to analyze the underlying trends in CSI's business, assess the performance of CSI's core operations, establish operational goals and forecasts that are used to allocate resources and evaluate CSI's performance period over period and in relation to its competitors' operating results. Additionally, CSI's management is evaluated on the basis of Adjusted EBITDA when determining achievement of their incentive compensation performance targets. Beginning with the quarter ended March 31, 2022, following correspondence from the staff of the U.S. Securities and Exchange Commission, we no longer exclude in process research and development (IPR&D) charges incurred in connection with asset acquisitions from Adjusted EBITDA or any other reported non-GAAP financial measures. For purposes of comparability, we have revised the reconciliation table above for the third quarter and full year of fiscal 2021. CSI believes that presenting Adjusted EBITDA provides investors greater transparency to the information used by CSI's management for its financial and operational decision-making and allows investors to see CSI's results "through the eyes" of management. CSI also believes that providing this information better enables CSI's investors to understand CSI's operating performance and evaluate the methodology used by CSI's management to evaluate and measure such performance. ($ in thousands) FY 2020 Q1 FY21 Q2 FY21 Q3 FY21 Q4 FY21 FY 2021 Q1 FY22 Q2 FY22 Q3 FY22 Net loss ($27,236) ($2,076) ($56) ($6,004) ($5,285) ($13,421) ($8,618) ($8,967) ($9,658) Less: Other (income) and expense, net 233 355 276 292 313 1,236 367 345 (52) Less: Provision for income taxes 231 63 63 63 63 252 136 63 63 Income (loss) from operations (26,772) (1,658) 283 (5,649) (4,909) (11,933) (8,115) (8,559) (9,647) Add: Stock-based compensation 13,612 4,907 3,877 3,704 3,742 16,230 5,672 4,240 3,892 Add: Depreciation and amortization 4,179 1,029 1,058 1,056 1,169 4,312 1,258 1,287 1,286 Adjusted EBITDA ($8,981) $4,278 $5,218 ($889) $2 $8,609 ($1,185) ($3,032) ($4,469) Use and Economic Substance of Non-GAAP Financial Measures Used by CSI and Usefulness of Such Non-GAAP Financial Measures to Investors

Investor Contact: Jack Nielsen Vice President, Investor Relations & Corporate Communications 651-202-4919 j.nielsen@csi360.com CSI®, Diamondback®, Diamondback 360®, GlideAssist®, Propel™ ViperWire®, WIRION® and ViperWire Advance® are trademarks of Cardiovascular Systems, Inc. © 2022 Cardiovascular Systems, Inc. OrbusNeich®, Teleport® and Sapphire® are trademarks of OrbusNeich Medical, Inc. Cardiovascular Systems, Inc. CSII @csi360 12












