
APRIL 2017 MOLECULAR TEMPLATES / THRESHOLD PHARMACEUTICALS Filed by Threshold Pharmaceuticals, Inc. Pursuant to Rule 425 Under the Securities Act of 1933 and deemed filed pursuant to Rule 14a - 12 under the Securities Exchange Act of 1934 Subject Company: Threshold Pharmaceuticals, Inc. Commission File Number: 001 - 32979

Forward - Looking Statements 2 Except for statements of historical fact, the statements in this presentation are forward - looking statements, including statemen ts regarding the proposed merger with Threshold Pharmaceuticals; the future development of Molecular Templates proprietary Engineered Toxin Body (ETB) tec hnology; the future development of Threshold’s evofosfamide ; our expected cash levels and financing plans, including with respect to the Longitude equity commitment, which is subject to future conditions; and the anticipated timing thereof. These statements constitute "forward - looking statements" w ithin the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act and are usually identified by the use of words such as "anticipates," "believes," "estimates," "expects," "intends," "may," "plans," "projects," "seeks," "should," "will," and variations of such words or sim ila r expressions. These forward - looking statements reflect our current views about our plans, intentions, expectations, strategies and prospects, which are b ase d on the information currently available to us and on assumptions we have made. Although we believe that our plans, intentions, expectations, strategies and pr ospects as reflected in or suggested by those forward - looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved. Furthermore, actual results may differ materially from those described in the forward - looking statements a nd will be affected by a variety of risks and factors that are beyond our control. These statements involve risks and uncertainties that can cause ac tua l results to differ materially from those in such forward - looking statements. Important factors that may cause actual results to differ materially from the re sults discussed in the forward - looking statements include risks and uncertainties, including the (1) inability to complete the proposed merger; (2) liquidit y a nd trading market for shares prior to and following the consummation of the proposed merger and proposed financing; (3) costs and potential litigation ass oci ated with the proposed merger; (4) a failure to satisfy the conditions to the closing of the proposed investment by Longitude Capital; (5) the failu re by Molecular or Threshold to secure and maintain relationships with collaborators; (6) risks relating to clinical trials and other uncertainties of produc t c andidate development; (7) risks relating to the commercialization, if any, of Molecular’s or Threshold’s proposed product candidates (such as marketing, regulatory, product liability, supply, competition, and other risks); (8) dependence on the efforts of third parties; and (9) dependence on intellectual property. Fur ther information regarding these and other risks is included under the heading "Risk Factors" in Threshold's Annual Report on Form 10 - K, which has been filed wit h the Securities and Exchange Commission on March 27, 2017 and is available from the SEC's website (www.sec.gov) and in other filings that Thresho ld will make with the SEC in connection with the proposed transactions, including the registration statement and the proxy statement/prospectus describ ed above under "Important Information and Where to Find It." Existing and prospective investors are cautioned not to place undue reliance on these forw ard - looking statements, which speak only as of the date hereof. The statements made in this press release speak only as of the date stated herein, and subs equ ent events and developments may cause our expectations and beliefs to change. Unless otherwise required by applicable securities laws, we do no t intend, nor do we undertake any obligation, to update or revise any forward - looking statements contained in this news release to reflect subsequen t information, events, results or circumstances or otherwise.

Important Information for Investors and Stockholders 3 Important Information for Investors and Stockholders This communication may be deemed to be solicitation material in respect of the proposed transaction between Threshold Pharmac eut icals, Inc. (Threshold) and Molecular Templates, Inc. (Molecular Templates) and Molecular Templates stockholders. In connection with the proposed transaction betwe en Threshold and Molecular Templates and its stockholders, Threshold will file with the Securities and Exchange Commission (SEC) a registration statement containing a pr oxy statement of Threshold that will also constitute a prospectus of Threshold. Threshold will mail the proxy statement/prospectus to Threshold stockholders, and the s ecu rities may not be sold or exchanged until the registration statement becomes effective. THRESHOLD URGES INVESTORS AND STOCKHOLDERS TO READ THE PROXY STATEMENT/PROSPECTUS REGARDING THE PROPOSED TRANSACTION WHEN IT BECOMES AVAILABLE, AS WELL AS OTHER DOCUMENTS FILED OR THAT WILL BE FILED WITH THE SEC, BECAUSE THEY CONTAIN OR WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION. This communication is not a substitute for the registration statement, definitive proxy statement/prospectus or any other documents that Threshold may file with the SE C or send to Threshold stockholders in connection with the proposed transaction. Before making any voting decision, investors and security holders are urged to read th e registration statement, proxy statement/prospectus and all other relevant documents filed or that will be filed with the SEC in connection with the propose d t ransaction as they become available because they will contain important information about the proposed transaction and related matters. You may obtain free copies of the proxy statement/prospectus and all other documents filed or that will be filed with the SEC re garding the proposed transaction at the website maintained by the SEC www.sec.gov. Once they are filed, copies of the registration statement and proxy statement/prospec tus will be available free of charge on Threshold's website at www.thresholdpharm.com or by contacting Threshold's Investor Relations at 510.703.9491 or by mail at Inve stor Relations, Threshold Pharmaceuticals Inc., 170 Harbor Way, Suite 300, South San Francisco, California 94080. Participants in Solicitation Threshold, Molecular Templates and their respective directors and executive officers may be deemed to be participants in the sol icitation of proxies from the holders of Threshold common stock in connection with the proposed transaction. Information about Threshold's directors and executive off ice rs is set forth in Threshold's definitive proxy statement for its 2016 annual meeting, which was filed with the SEC on April 29, 2016. Other information regarding the int erests of such individuals, as well as information regarding Molecular Templates' directors and executive officers and other persons who may be deemed participants in the proposed transaction, will be set forth in the proxy statement/prospectus, which will be included in Threshold's registration statement when it is filed with the SEC . Y ou may obtain free copies of these documents as described in the preceding paragraph. Non - Solicitation This communication shall not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an of fer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualifica tio n under the securities laws of any such jurisdiction. No public offer of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securi tie s Act of 1933, as amended.

Threshold and Molecular Templates : Advantages of New Company Development of Molecular Templates proprietary Engineered Toxin Body (ETB) technology o Lead compound MT - 3724 initiating phase II program in NHL in 2017 o Development of multiple pipeline compounds and large pharma collaborations expected in 2017 Combined company to continue development of evofosfamide o Evofosfamide is safe with monotherapy activity across multiple indications o Phase I study planned at MD Anderson in combination with Ipilumumab New company to have robust cash balance o >$60M in cash at closing of transaction (3Q17) • Additional $11M in non - dilutive CPRIT financing available for CD38 program • Planned $40M financing coincident with closing of transaction • Sufficient to fund company to 3Q19 through multiple milestones 4

Evofosfamide Combination development with checkpoint inhibitor Ipilimumab

Evofosfamide : continued development in new company Evofosfamide did not meet primary endpoint in two phase III trials (MAESTRO and TH - CR - 406/ SARCO21) o The MAESTRO study in pancreatic adenocarcinoma did not demonstrate a statistically significant improvement in overall survival (OS) for evofosfamide+gemcitabine compared to gemcitabine alone (p=0.0589) o Meaningful improvement seen in subgroup of 116 patients from Japan with risk of death reduced by 48% in evofosfamide arm • Discussions with the Japanese regulatory authorities underway regarding potential registration pathways for evofosfamide for treatment of pancreatic cancer Translational data at MD Anderson suggest evofosfamide may improve efficacy of Ipilumumab o Evofosfamide to be tested in combination with ipilumumab at MD Anderson o Four indications planned for phase I study • Metastatic or locally advanced prostate cancer • Metastatic pancreatic cancer • Melanoma • HPV negative squamous cell carcinoma of the head and neck 6

The ETB scaffold Proprietary platform with novel mechanism leveraging powerful biology

Engineered Toxin Bodies (ETBs) are comprised of an antibody fragment binding domain genetically fused to a proprietarily engineered Shiga - Like Toxin A - Subunit (SLT - A) Molecular Templates I Engineered Toxin Body (ETB) platform Platform Capabilities Forced Receptor Internalization (1 st generation ETB) • Ability to self - internalize into cells even against non - or poorly internalizing targets; ability to decouple internalization • Enzymatic inactivation of protein synthesis that is not reliant on tumor microenvironment De - Immunized and Optimized Toxin Scaffold (2 nd generation ETB) • Selective De - Immunization; B - cell and CD4 epitopes removed; CD8 epitopes preserved to allow for tumor cross priming • Enhanced efficacy; cell kill potency increased by 10 - 100x over 1 st gen Next - Gen Immuno - Oncology (3 rd generation ETB) • Ability to self - navigate to cytosol; delivery of foreign class 1 antigens for processing / presentation by target cells • Potential for synergy with checkpoint inhibitors 1 st generation ETB (MT - 3724) starts potential pivotal study in 1Q18 • Clear monotherapy activity in heavily pre - treated DLBCL patients • Excellent safety and tolerability 2 nd generation ETB programs enter clinic in 2018 • Hematologic malignancy – CD38 • Solid tumors – PD - L1, Her2 Additional 2 nd generation ETB programs under development • CD45, CD79b, BCMA, CD123, B7 - H3, CD70, LMP - 1 3rd generation ETB program enters clinic in 2018 • Adds proprietary immuno - oncology dimension to ETB platform Multiple Clinical Programs 8

Shiga - Like Toxin A (SLT - A) has well defined biological properties Unique SLT - A Biological Properties o Can force receptor internalization o Self - routes to the cytosol o Irreversibly and enzymatically inactivates ribosomes activity to destroy target cells • Novel intracellular mechanism of cell - kill aimed at major tumor requirement (protein synthesis) • Exceptionally potent SLT produced by certain E. coli strains o SLT is 99% identical to Shiga toxin SLT comprised of A and B subunits B - subunit binds CD77 receptor o CD77 is a glycosphingolipid that does not normally internalize A - subunit (SLT - A) internalizes, self - routes to cytosol, and inactivates ribosome Shiga - Like Toxin (SLT) 9 A B
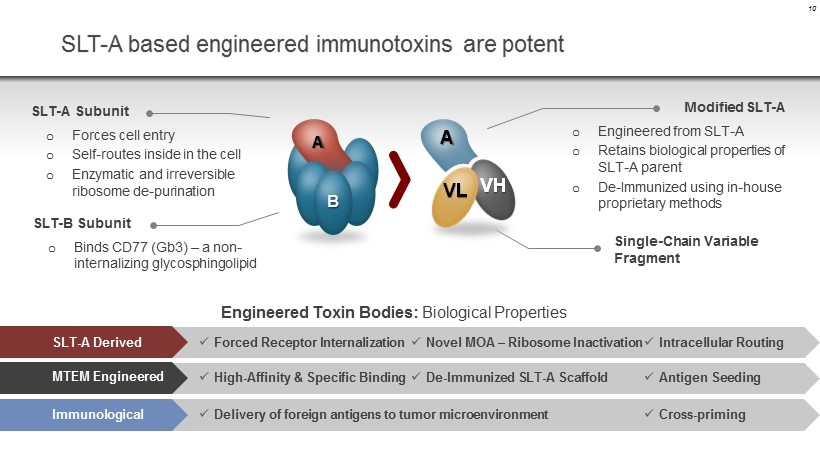
SLT - A based engineered immunotoxins are potent SLT - A Subunit o Forces cell entry o Self - routes inside in the cell o Enzymatic and irreversible ribosome de - purination SLT - B Subunit o Binds CD77 (Gb3) – a non - internalizing glycosphingolipid Modified SLT - A o Engineered from SLT - A o Retains biological properties of SLT - A parent o De - Immunized using in - house proprietary methods Engineered Toxin Bodies: Biological Properties A VL VH HIGH POTENCY A VL VH REDUCED IMMUNOGENICITY ANTIGEN SEEDING A VL VH Single - Chain Variable Fragment SLT - A Derived x Forced Receptor Internalization x Novel MOA – Ribosome Inactivation x Intracellular Routing MTEM Engineered x High - Affinity & Specific Binding x De - Immunized SLT - A Scaffold x Antigen Seeding Immunological x Delivery of foreign antigens to tumor microenvironment x Cross - priming 10 A B
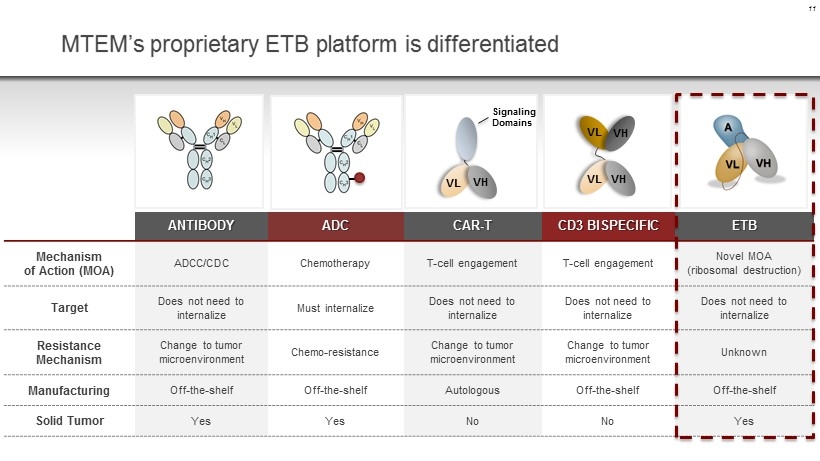
MTEM’s proprietary ETB platform is differentiated ANTIBODY ADC CAR - T CD3 BISPECIFIC ETB Mechanism of Action (MOA) ADCC/CDC Chemotherapy T - cell engagement T - cell engagement Novel MOA (ribosomal destruction) Target Does not n eed to internalize Must internalize Does not need to internalize Does not need to internalize Does not need to internalize Resistance Mechanism Change to tumor microenvironment Chemo - resistance Change to tumor microenvironment Change to tumor microenvironment Unknown Manufacturing Off - the - shelf Off - the - shelf Autologous Off - the - shelf Off - the - shelf Solid Tumor Yes Yes No No Yes 11 VL VH VL VH VL VH Signaling Domains

MT - 3724 1 st generation scaffold

MT - 3724, a 1 st generation ETB, is a novel approach to target CD20 Rituximab o Binds CD20 (does not internalize) o Destroys tumor cells by ADCC/CDC Bexxar & Zevalin o Bind CD20 (do not internalize) o Destroys tumor cells by localized radiation MT - 3724 o 1 st generation ETB o Binds CD20; forces internalization into cell o Cell - kill by enzymatic ribosomal destruction 13 • ~20,000 patients/yr with NHL die in US each year • These patients fail current CD20 therapies • However, >90% of patients retain CD20 expression after failure • CD20 does not internalize so ADCs not feasible

MT - 3724 has potent in vitro and in vivo activity in pre - clinical models In vitro activity against CD20+ cells Cell Line CD20 CD 50 ( nM ) Raji + 0.36 Daudi + 1.49 Jeno - 1 + 1.53 ST - 486 + 1.70 NHL Rituxan Fail A + 0.1 NHL Rituxan Fail B + 5.0 Jurkat Neg. 1412 BC - 1 Neg. 2051 Untreated Mice MT - 3724 (2 mg/kg) 1 cycle of treatment (2 wks of M - W - F treatment) ; Orthotopic Xenograft of Raji (CD20+) Lymphoma Cells, Tumor Imaging of Untreated Mice 10 Days Post Tumor Inoculation (Molecular Imaging) Disseminated NHL Model • Potent and specific against CD20+ cell lines • Efficacious against cells from patients who failed Rituxan • All untreated mice dead by day 16 • No treated mice dead by day 16

Overview of MT - 3724 phase I trial in NHL patients Baseline Characteristics 15 Monotherapy 3+3 Dose - Escalation Study in NHL o Failed chemo and CD20 antibody therapy, all subtypes o 1st cycle: 2 weeks on (M - W - F), 2 weeks off of dosing o Subsequent cycles are 2 weeks on (M - W - F), 1 week off o MTD is assessed from cycle 1 o Efficacy scans are conducted after cycle 2 and 4 o Patients could originally only receive up to 5 cycles of drug: new amendment to allow continued dosing o Conducted at 3 sites (MSK, MDACC, UNC) Characteristics All Doses (N = 21) Median Age, years (range) 67 (34 - 78) Prior Therapies, median (range) 4 (1 - 11) < 4, n (%) 5 (24%) ≥ 4, n (%) 13 (76%) NHL Subtype, n (%) DLBCL 11 (52%) FL 3 (38%) MCL 2 (10%)
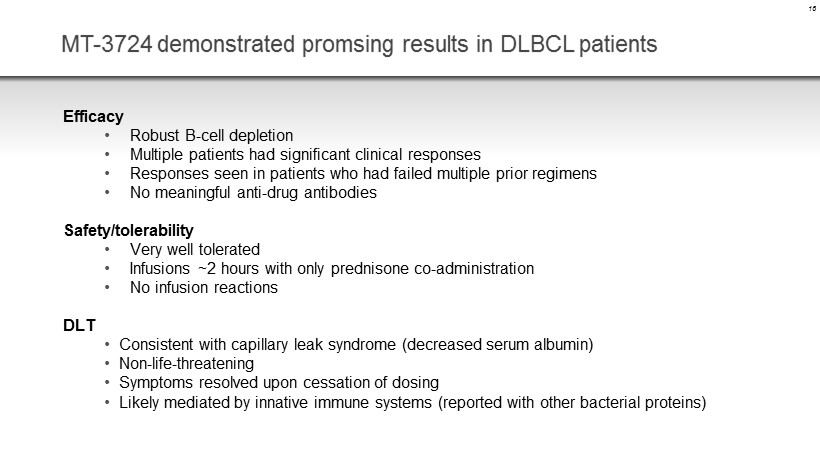
MT - 3724 demonstrated promsing results in DLBCL patients Efficacy • Robust B - cell depletion • Multiple patients had significant clinical responses • Responses seen in patients who had failed multiple prior regimens • No meaningful anti - drug antibodies Safety/tolerability • Very well tolerated • Infusions ~2 hours with only prednisone co - administration • No infusion reactions DLT • Consistent with capillary leak syndrome (decreased serum albumin) • Non - life - threatening • Symptoms resolved upon cessation of dosing • Likely mediated by innative immune systems (reported with other bacterial proteins) 16
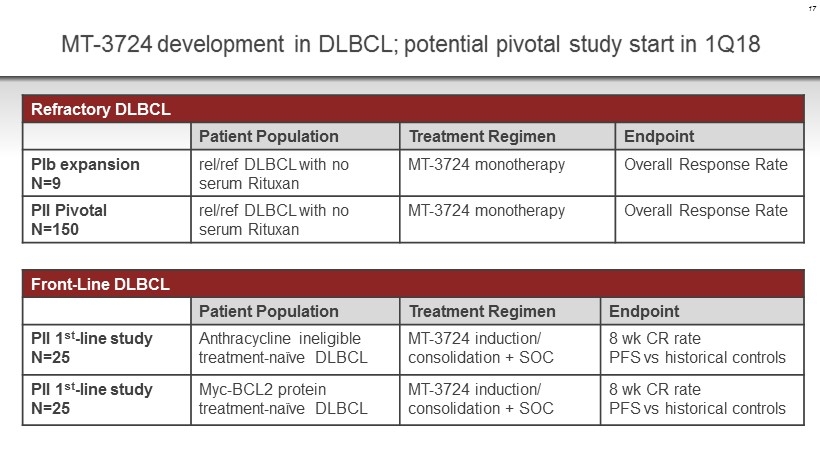
MT - 3724 development in DLBCL; potential pivotal study start in 1Q18 17 Refractory DLBCL Patient Population Treatment Regimen Endpoint PIb expansion N=9 rel /ref DLBCL with no serum Rituxan MT - 3724 monotherapy Overall Response Rate PII Pivotal N=150 rel /ref DLBCL with no serum Rituxan MT - 3724 monotherapy Overall Response Rate Front - Line DLBCL Patient Population Treatment Regimen Endpoint PII 1 st - line study N=25 Anthracycline ineligible treatment - naïve DLBCL MT - 3724 induction/ consolidation + SOC 8 wk CR rate PFS vs historical controls PII 1 st - line study N=25 Myc - BCL2 protein treatment - naïve DLBCL MT - 3724 induction/ consolidation + SOC 8 wk CR rate PFS vs historical controls
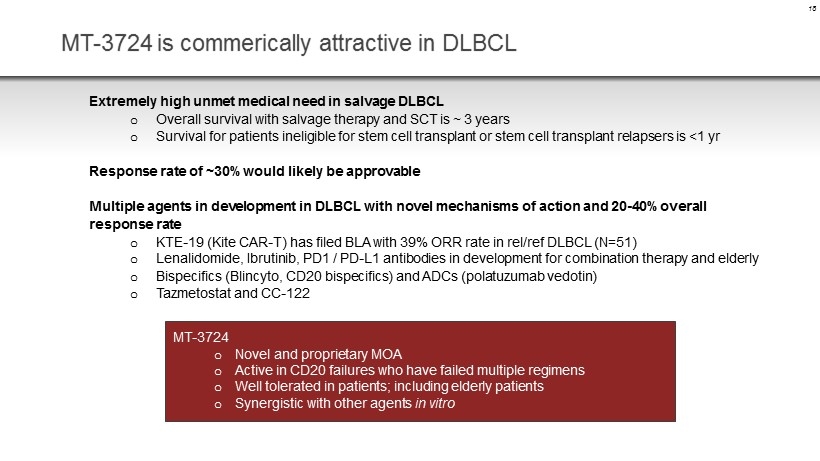
MT - 3724 is commerically attractive in DLBCL Extremely high unmet medical need in salvage DLBCL o Overall survival with salvage therapy and SCT is ~ 3 years o Survival for patients ineligible for stem cell transplant or stem cell transplant relapsers is <1 yr Response rate of ~30% would likely be approvable Multiple agents in development in DLBCL with novel mechanisms of action and 20 - 40% overall response rate o KTE - 19 (Kite CAR - T) has filed BLA with 39% ORR rate in rel /ref DLBCL (N=51) o Lenalidomide, Ibrutinib, PD1 / PD - L1 antibodies in development for combination therapy and elderly o Bispecifics ( Blincyto , CD20 bispecifics ) and ADCs ( polatuzumab vedotin ) o Tazmetostat and CC - 122 18 MT - 3724 o Novel and proprietary MOA o Active in CD20 failures who have failed multiple regimens o Well tolerated in patients; including elderly patients o Synergistic with other agents in vitro

2 nd gen. ETB scaffold De - immunized and higher potency

2 nd generation ETB scaffold overview 1 st generation • High Potency A VL VH HIGH POTENCY A VL VH REDUCED IMMUNOGENICITY ANTIGEN SEEDING A VL VH • Improved engineering of molecule (fit between SLTA and scFv) • Engineered scaffold for reduced innate (safety) and adaptive (potency) immunity Higher Potency and De - Immunized 20 2 nd generation • Higher Potency • De - immunized

MT - 4019: 2 nd generation ETB targeting CD38 entering clinic in 2018 21 MT - 4019 CD38 is an ectoenzyme central to disease o Daratumumab has unprecedented single agent activity Daratumumab failures retain CD38 expression o Patients who progress on Daratumumab therapy show increase in CD55/CD59 levels o CD55 and CD59 are complement inhibiting proteins CD38 is poorly - internalizing o ADCs are not a viable strategy to target CD38 MT - 4019 overview o 2 nd generation ETB o Picomolar activity against CD38+ cells o Dramatically reduced ADA and innate response in murine and NHP model o Activity in low CD38, high CD55/CD59 - expressing ( Darzalex - resistant ) cells o Synergistic activity with IMiDs and proteasome inhibitors

MT - 4019 has picomolar activity and reduced ADA response 22 Cell Line Type CD38 Expression (MFI) CD55/CD59 Expression CD 50 H929 Multiple myeloma 55,000 >50,000 16pM Daudi B - lymphoblast 166,000 <20,000 58pM ST486 B - lymphoblast 129,000 <20,000 41pM MOLP - 8 Multiple myeloma 146,000 >50,000 228pM BC3 B - lymphocyte 79,000 <20,000 180pM IM - 9 Multiple myeloma - ND >>100nM HDLM - 2 B - lymphoblast - ND >>100nM L1236 B - lymphoblast - ND >>100nM • MT - 4019 has potent activity in high CD55/CD59 expression cell lines which are Darzalex resistant • MT - 4019 has activity in cells with low CD38 levels • MT - 4019 has same antigen binding domain as MT - 4000 but uses de - immunized scaffold • MT - 4019 generates much lower immune response in murine models
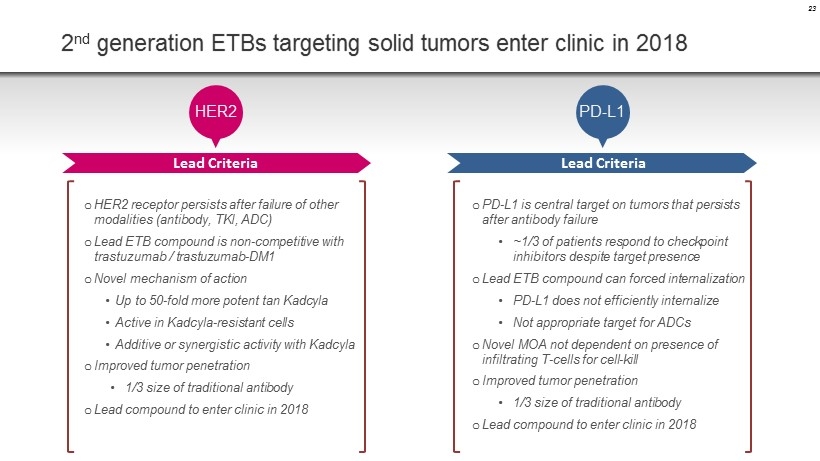
2 nd generation ETBs targeting solid tumors enter clinic in 2018 23 HER2 o HER2 receptor persists after failure of other modalities ( antibody , TKI, ADC) o Lead ETB compound is non - competitive with trastuzumab / trastuzumab - DM1 o Novel mechanism of action • Up to 50 - fold more potent tan Kadcyla • Active in Kadcyla - resistant cells • Additive or synergistic activity with Kadcyla o Improved tumor penetration • 1/3 size of traditional antibody o Lead compound to enter clinic in 2018 Lead Criteria PD - L1 Lead Criteria o PD - L1 is central target on tumors that persists after antibody failure • ~1/3 of patients respond to checkpoint inhibitors despite target presence o Lead ETB compound can forced internalization • PD - L1 does not efficiently internalize • Not appropriate target for ADCs o Novel MOA not dependent on presence of infiltrating T - cells for cell - kill o Improved tumor penetration • 1/3 size of traditional antibody o Lead compound to enter clinic in 2018

3 rd gen. ETB scaffold Antigen Seeding

3 rd generation ETB scaffold adds immuno - oncology activity to platform A VL VH HIGH POTENCY A VL VH REDUCED IMMUNOGENICITY ANTIGEN SEEDING A VL VH “Antigen Seeding” capability represents the next generation immuno - oncology approach Higher Potency and De - Immunized Next - Gen Immune Oncology 25 • Improved engineering of molecule (fit between SLTA and scFv) • Engineered scaffold for reduced innate (safety) and adaptive (potency) immunity 1 st generation • High Potency 2 nd generation • Higher Potency • De - immunized 3 rd generation • Higher Potency • De - immunized • Antigen seeding

SLT - A scaffold can deliver payload of MHC - I antigens for cell surface presentation 3 rd generation ETB scaffold incorporates immuno - oncology activity Nucleus Golgi Endoplasmic Reticulum Endosome Cytosol Cleaved Antigen ETB with Class I Viral Antigen Target Cytotoxic T - Cells TCR MHC - I Antigen/MHC - I Complex o Fusion of Class I viral dominant antigen to Engineered Toxin Body o Create high frequency & high avidity effector T - cell response to tumor o Dual mechanism of cell - kill x Potential to recruit high - avidity T - cell response to tumor without sacrificing SLTA - mediated cell - kill Antigen Seeding Technology (AST) 26

CMV - specific CD8+ T - cells undergo unique progressive expansion (memory inflation) and accumulate in periphery o Up to 20% of T - cells in elderly hosts are specific to pp65 CMV - specific CD8+ T - cells may be resistant to exhaustion (decreased PD - 1, CD160, CD244 expression) o CMV - specific CD8+ T - cells in CLL patients are not exhausted whereas non - CMV specific T - cells are exhausted Lead 3 rd gen. ETB adds antigen seeding to PD - L1 targeting construct Detection of CMV MHC Class I Complex on Intoxicated Cells L1236 (PD - L1+) cell count CMV - pp65 TCR multimer - PE - PBS treated cells - PD - L1 ETB no antigen - PD - L1 ETB/ CMV antigen TCR Specific Detection of Surface MHC I/ CMV antigen complex 27 o Soluble TCR to pp65 pMHC Detects Expression on Intoxicated PD - L1+ Cells o TCR - Specific Reporter Cell Line Detects Expression on Intoxicated PD - L1+ Cells o Antigen Seeding Complements Protein Inhibition Mechanism of Action - Antigen seeding occurs before cell death - Antigen seeding could be combined with checkpoint inhibitors

CMC Off - the - shelf, scalable ETB manufacturing

ETB GMP manufacturing is efficient and scalable GMP Expression System & Format – Engineered BL21 derived E. Coli – Optimized for soluble expression – 24 hour fermentation process – In - house PD to scale from 5L to 300L reactor – 7x GMP campaigns at 300L scale with MT - 3724 – Two - step affinity purification with MT - 3724 – One - step purification for 2 nd - and 3 rd - gen ETBS – 24 months of stability (on - going) at - 20C with MT - 3724 – Expected COGs of <10%

Milestones Next 18 months
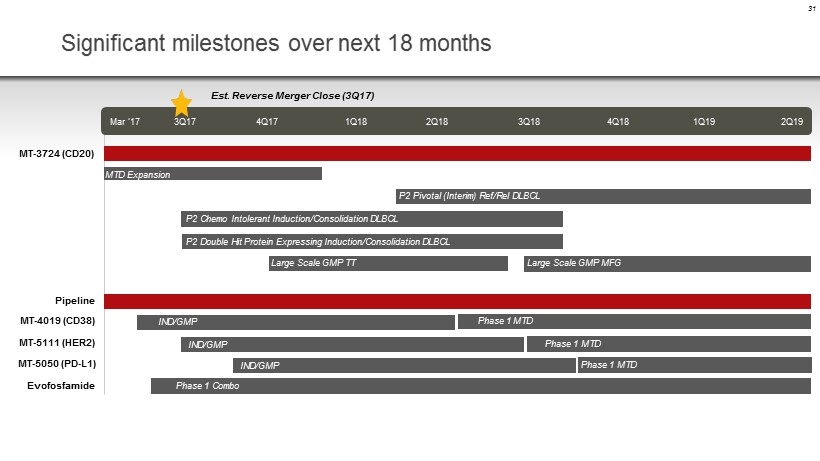
31 Significant milestones over next 18 months Mar ‘17 3Q17 4Q17 1Q18 2Q18 3Q18 4Q18 1Q19 2Q19 MT - 3724 (CD20) P2 Chemo Intolerant Induction/Consolidation DLBCL P2 Pivotal (Interim) Ref/ Rel DLBCL Stop Gap P2 GMP MFG Pipeline IND/GMP Phase 1 MTD IND/GMP P2 Double Hit Protein Expressing Induction/Consolidation DLBCL Large Scale GMP TT MTD Expansion Large Scale GMP MFG Large Scale GMP MFG IND/GMP Phase 1 MTD Phase 1 MTD Phase 1 Combo Est. Reverse Merger Close (3Q17) MT - 4019 (CD38) MT - 5111 (HER2) Evofosfamide MT - 5050 (PD - L1)

Summary Publicly traded oncology company

MTEM is a clinical stage oncology company with a distinctive platform Lead compound (MT - 3724) has novel mechanism of action, good safety, and monotherapy activity o Proof - of - concept for internalization of non - internalizing receptors (CD20) o Novel MOA is active in patients refractory to chemotherapy o Safety profile is differentiated and should be combinable with chemotherapy o No signs of neutralizing antibodies in DLBCL patients o Establishes scalable GMP process for all ETBs 2 nd generation ETBs are more potent with reduced immunogenicity o MT - 4019 targets CD38, a validated target, with extremely high potency and enters the clinic in 2018 o Constructs targeting solid tumors (Her2 and PD - 1) will also enter the clinic in 2018 3 rd generation ETBs add novel immuno - oncology dimension to 2 nd generation constructs o Antigen seeding allows for recruitment of high avidity CMV - specific T - cells to the tumor o Antigen seeding can be added to any ETB Lead discovery underway against broad universe of non - internalizing receptors 33

Evofosfamide : continued development in new company Evofosfamide did not meet primary endpoint in two phase III trials (MAESTRO and TH - CR - 406/ SARCO21) o The MAESTRO study in pancreatic adenocarcinoma did not demonstrate a statistically significant improvement in overall survival (OS) for evofosfamide+gemcitabine compared to gemcitabine alone (p=0.0589) o Meaningful improvement seen in subgroup of 116 patients from Japan with risk of death reduced by 48% in evofosfamide arm • Discussions with the Japanese regulatory authorities underway regarding potential registration pathways for evofosfamide for treatment of pancreatic cancer Translational data at MD Anderson suggest evofosfamide may improve efficacy of Ipilumumab o Evofosfamide to be tested in combination with ipilumumab at MD Anderson o Four indications planned for phase I study • Metastatic or locally advanced prostate cancer • Metastatic pancreatic cancer • Melanoma • HPV negative squamous cell carcinoma of the head and neck 34

































