grant to CPRIT a nonexclusive, irrevocable, royalty-free, perpetual, worldwide license, solely for academic, research and other non-commercial purposes, under the Project Results and to exploit any necessary additional intellectual property rights, subject to certain exclusions.
Molecular will pay to CPRIT, during the term of the CPRIT Agreement, certain payments equal to a percentage of revenue ranging from the low-to mid-single digits. These payments will continue up to and until CPRIT receives an aggregate amount of 400% of the sum of all monies paid to Molecular by CPRIT under the CPRIT Agreement. If Molecular is required to obtain a license from a third party to sell any such product, the revenue sharing percentages may be reduced. In addition, once Molecular pays CPRIT 400% of the monies it has received under the CPRIT Agreement, it will continue to pay CPRIT a revenue sharing percentage of 0.5%.
The CPRIT Agreement will terminate, with certain obligations extending beyond termination, on the earlier of (a) May 31, 2019 or (b) the occurrence of any of the following events: (i) by mutual written consent of the parties, (ii) by CPRIT for an Event of Default (as defined in the CPRIT Agreement) by Molecular, (iii) by CPRIT if allocated funds should become legally unavailable during the term of the CPRIT Agreement and CPRIT is unable to obtain additional funds or (iv) by Molecular for convenience. CPRIT may approve a no cost extension for the CPRIT Agreement for a period not to exceed six months after the termination date if additional time is required to ensure adequate completion of the approved project, subject to the terms and conditions of the CPRIT Agreement.
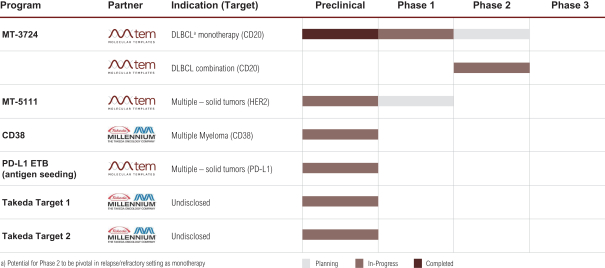
MT-3724 Phase II Combination Study
In the third quarter of 2018, Molecular initiated a Phase II combination study of its lead candidate, MT-3724, with Gemcitabine and Oxaliplatin (GEMOX) in patients with advanced DLBCL. The study is a multi-center, open-label two part study that will evaluate the safety and tolerability of MT-3724 in combination with GEMOX in relapsed or refractory CD20 positive Non-Hodgkin B-cell Lymphoma patients. Molecular intends to enroll up to 64 subjects for four 28-day treatment cycles. Molecular expects to initiate a second combination trial, MT-3724 with Revlimid.
Corporate Information and History
We were originally incorporated in the State of Delaware in October 2001 under the name “Threshold Pharmaceuticals, Inc.” On August 1, 2017, Molecular Templates, Inc., formerly known as Threshold Pharmaceuticals, Inc. (NASDAQ: THLD) (“Threshold”), completed its business combination with then-private Molecular Templates, Inc. (“Private Molecular” formerly D5 Pharma Inc.) in accordance with the terms of the Agreement and Plan of Merger and Reorganization, dated as of March 16, 2017, by and among Threshold, Trojan Merger Sub, Inc. (“Merger Sub”), and Private Molecular (the “Merger Agreement”), pursuant to which Merger Sub merged with and into Private Molecular, with Private Molecular surviving as a wholly owned subsidiary of Threshold (the “Merger”). On August 1, 2017, in connection with, and prior to the completion of, the Merger, Threshold effected a 11:1 reverse stock split of its common stock and on August 1, 2017, immediately after completion of the Merger, Threshold changed its name to “Molecular Templates, Inc.” (NASDAQ: MTEM).
Our corporate headquarters are located at 9301 Amberglen Boulevard, Suite 100, Austin, Texas 78729 and telephone number is (512) 869-1555. We maintain a website at www.mtem.com, to which we regularly post copies of our press releases as well as additional information about us. The information contained on, or that can be accessed through, our website is not a part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
All brand names or trademarks appearing in this prospectus are the property of their respective holders. Use or display by us of other parties’ trademarks, trade dress, or products in this prospectus is not intended to, and does not, imply a relationship with, or endorsements or sponsorship of, us by the trademark or trade dress owners. As used herein, the words “Molecular Templates,” “Molecular,” “MTEM,” the “Company,” “we,” “us,” and “our” refer to Molecular Templates, Inc. and our subsidiaries. Our subsidiary Molecular Templates OpCo, Inc. was incorporated in Delaware in February 2009.