to generate sufficient product revenue to sustain or grow our business. We may be competing with companies that currently have extensive and well-funded marketing and sales operations, particularly in the markets our biologic candidates are intended to address. Without appropriate capabilities, whether directly or through third-party collaboration partners, we may be unable to compete successfully against these more established companies.
We may attempt to form additional collaborations in the future with respect to our biologic candidates, but we may not be able to do so, which may cause us to alter our development and commercialization plans.
We may attempt to form strategic collaborations, create joint ventures or enter into licensing arrangements with third parties with respect to our programs in addition to those that we currently have that we believe will complement or augment our existing business. We may face significant competition in seeking appropriate strategic collaboration partners, and the negotiation process to secure appropriate terms is time consuming and complex. We may not be successful in our efforts to establish such a strategic collaboration for any biologic candidates and programs on terms that are acceptable, or at all. This may be because our biologic candidates and programs may be deemed to be at too early of a stage of development for collaborative effort, our research and development pipeline may be viewed as insufficient, the competitive or intellectual property landscape may be viewed as too intense or risky, and/or third parties may not view our biologic candidates and programs as having sufficient potential for commercialization, including the likelihood of an adequate safety and efficacy profile.
Any delays in identifying suitable collaboration partners and entering into agreements to develop and/or commercialize our biologic candidates could delay the development or commercialization of our biologic candidates, which may reduce their competitiveness even if they reach the market. Absent a strategic collaborator, we would need to undertake development and/or commercialization activities at our own expense. If we elect to fund and undertake development and/or commercialization activities on our own, we may need to obtain additional expertise and additional capital, which may not be available to us on acceptable terms or at all. If we are unable to do so, we may not be able to develop our biologic candidates or bring them to market and our business may be materially and adversely affected.
If the market opportunities for our biologic candidates are smaller than we believe they are, we may not meet our revenue expectations and, even if a biologic candidate receives marketing approval, our business may suffer. Because the patient populations in the market for our biologic candidates may be small, we must be able to successfully identify patients and acquire a significant market share to achieve profitability and growth.
Our estimates for the addressable patient population and our estimates for the prices we can charge for our biologic candidates may differ significantly from the actual market addressable by our biologic candidates and are based on our beliefs and estimates. These estimates have been derived from a variety of sources, including the scientific literature, patient foundations or market research, and may prove to be incorrect. Further, new studies may change the estimated incidence or prevalence of these diseases. The number of patients may turn out to be lower than expected. Additionally, the potentially addressable patient population for each of our biologic candidates may be limited or may not be amenable to treatment with our biologic candidates, and new patients may become increasingly difficult to identify or gain access to, which would adversely affect our business, financial condition, results of operations and prospects.
We face substantial competition, and our competitors may discover, develop or commercialize drugs faster or more successfully than we do.
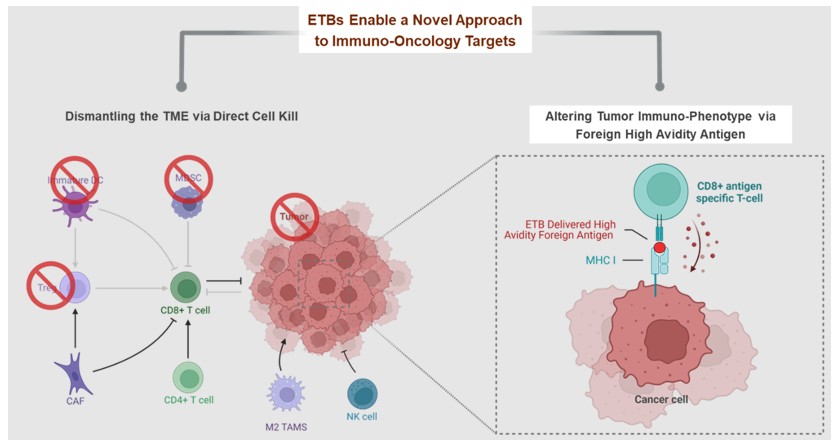
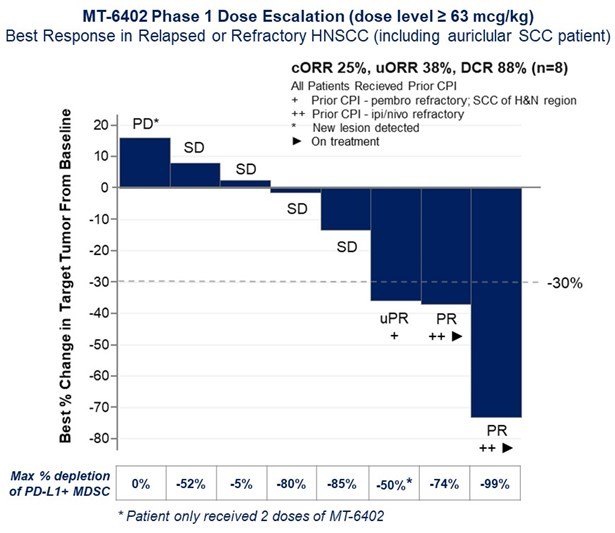
The development and commercialization of new drug products is highly competitive. We face competition from large pharmaceutical companies, specialty pharmaceutical companies, biotechnology companies, universities and other research institutions worldwide with respect to MT-6402, MT-8421, MT-0169, and the other biologic candidates that we may seek to develop or commercialize in the future. We are aware that companies including the following have products marketed or in development that could compete directly or indirectly with ETBs: Merck, Bayer, Takeda, AbbVie, Immunogen, Morphosys, Genmab, Bristol-Myers Squibb, Novartis, Regeneron, Janssen, Xencor, Amgen, AstraZeneca, Lilly, Merck KGaA, Pfizer, Sanofi, Spectrum Pharmaceuticals, Cogent Biosciences, Karyopharm, ADC Therapeutics, 2seventy bio, Gilead, GlaxoSmithKline, Incyte, TG Therapeutics, Mersana Therapeutics, Seagen, and Verastem. Our competitors may succeed in developing, acquiring or licensing technologies or biological products that are more