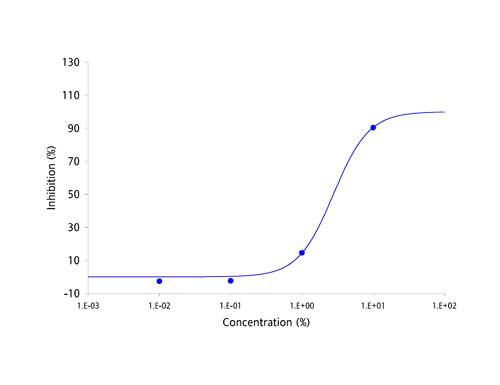
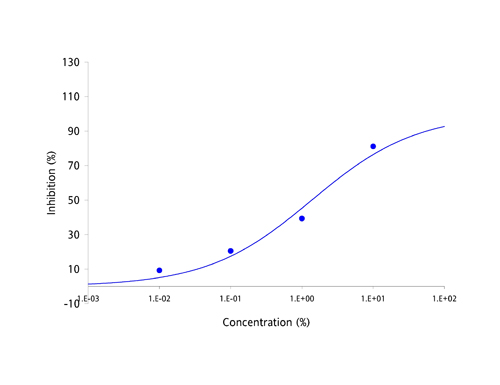
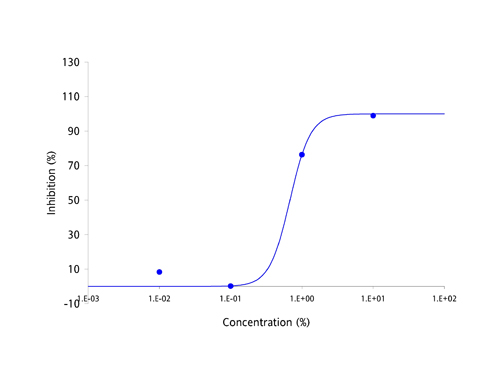
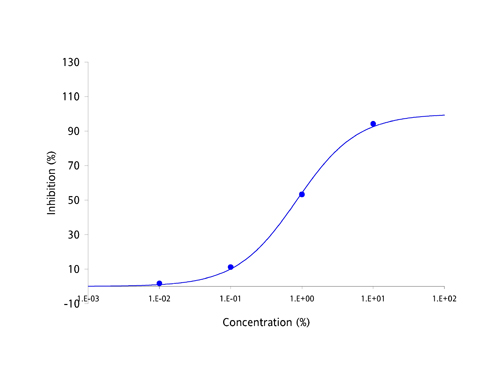
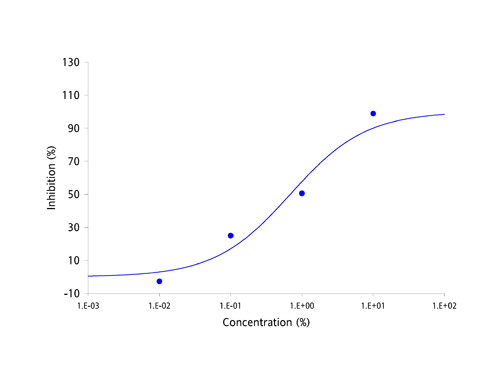
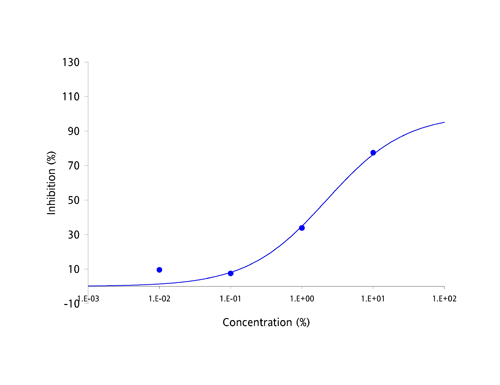
| Antibodies have been the focus of medical and pharmaceutical research for several decades because they are part of the body's natural defense mechanisms and are highly specific in their actions. However, manufacturing antibodies for drug use is a complex and costly process, which has resulted in the current round of antibody based drugs being amongst the most expensive medicines known to date. Apart from cost, the other great challenge facing developers of antibody-based drugs has been deliverability. Antibodies are large and complex proteins, which can only be administered by slow infusion directly into the bloodstream. This dramatically increases both cost and risk of administration and can result in large amounts of regular unproductive time. The protein-protein interaction between antibodies and their targets are not restricted to just diseases. Molecules which bind to multiple targets are in constant use to shuttle molecules around the body via the bloodstream. These binding proteins have the potential to be harnessed in exactly the same way as antibodies, as a treatment for a broad range of diseases and disease-causing molecules. The MPL Group has focused on antibody-like protein fragments for some time and has developed a novel drug development platform and pipeline based on these proteins. Adaptation of antibody-like protein fragments into drug formulations has enormous clinical and commercial potential. In order to overcome the delivery problems usually associated with large and complex antibodies, the scientific team focused on a range of topically deliverable protein fragments with specific biological activities. The Group creates its antibody-like fragments by specifically cleaving well established food-chain plasma proteins using a proprietary and patented process that provides both high yields and low production costs. The antibody-like fragments have now been studied for their specific biological activities and have yielded an impressive range of cytokine-specific Intellectual Property (IP). Some 40 different receptors and enzymes mediation properties have been identified to date, including, but not limited to: - TNF-α antagonism
- Inhibition of TACE
- Caspase inhibition
- Cox-1 and Cox-2 inhibition
- Bradykinin antagonism
- Cannabinoid receptors
- Chemokine CCR receptors
| | | These same biological mechanisms are associated with a number of commercially important sectors in which the topical delivery route of active ingredients derived from the platform provide a sustainable competitive advantage.- Arthritis and Joint Degeneration
- Inflammatory
- Skin Disease
- Cosmetic and Aesthetic Surgery
- Musculo-skeletal Pain and Inflammation
- Photo-damage and Photoaging
- Peripheral Neuropathies
DELIVERABILITY While antibodies usually face challenges of high manufacturing cost and patient unfriendly delivery routes due to their complexity and molecular size, the antibody-like fragments of the MPL Group are relatively small and stable molecules that can be manufactured using standard pharmaceutical processing techniques. Our small antibody-like fragments are applicable to a wider than usual application base that includes through-the-skin delivery, local targeting, non-systemic applications as well as potential applications in oral and subcutaneous administration. The potential of transdermally delivered antibody-like protein fragments creates a broad range of therapeutic and commercial possibilities in the over-the counter treatments across the cosmetic as well as therapeutic markets. SAFETY A number of years ago, the Group made the decision to utilize plasma proteins that were an established part of the normal human food chain. This was a critical decision, as it implies a high level of intrinsic compatibility and safety in humans. However, the most compelling evidence of long term safety and efficacy in humans came from the very first product which have now been successfully marketed as a pharmaceutical product in Australia for over 18 years. This topically applied OTC product is approved for the temporary relief of arthritic and muscular pain and inflammation and has an unblemished safety and efficacy record. As the antibody-like fragments are extracted and refined from the same protein source, the Group has good reason to have a high level of confidence that clinical safety and efficacy will emerge as one of the primary benefits of all products and processes of the MPL Group. THE FUTURE Globally, billions of dollars have been expended in the research of antibodies and means of production and delivery. A number of highly successful drugs have emerged over recent years which has made antibody-type drug discovery an attractive space for product research and development. The MPL Group is determined to lead the development of high volume, medium value drug products targeting global and expanding unmet medical needs with a family of antibody- like fragment therapeutics. The MPL Group has the technology, the intellectual property, the scientific lead and the ambition to make this a reality. |