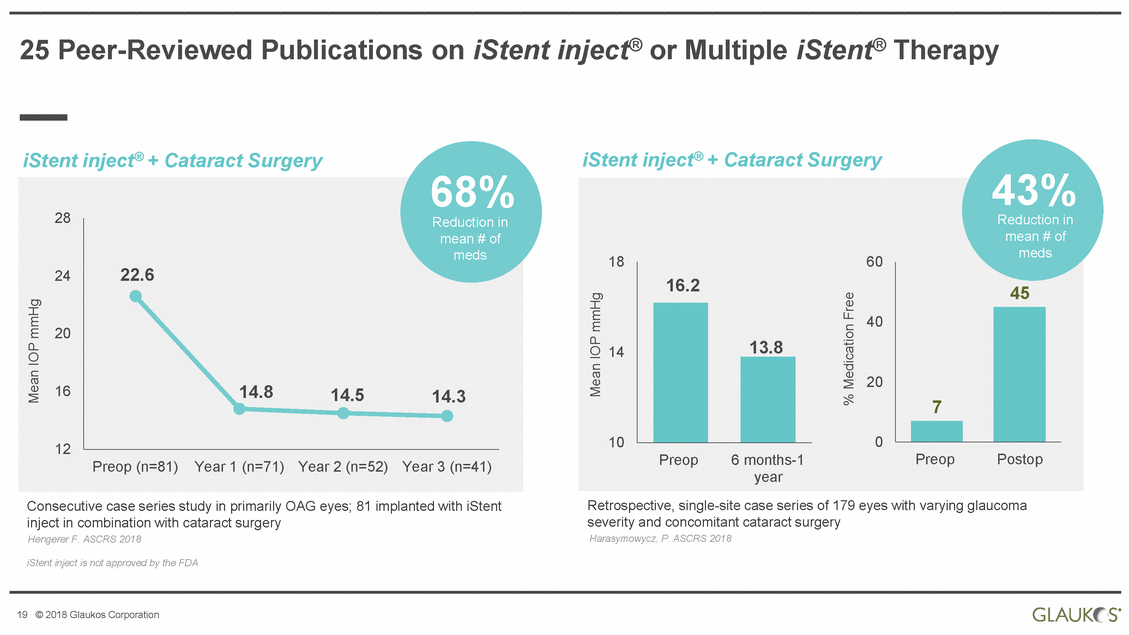
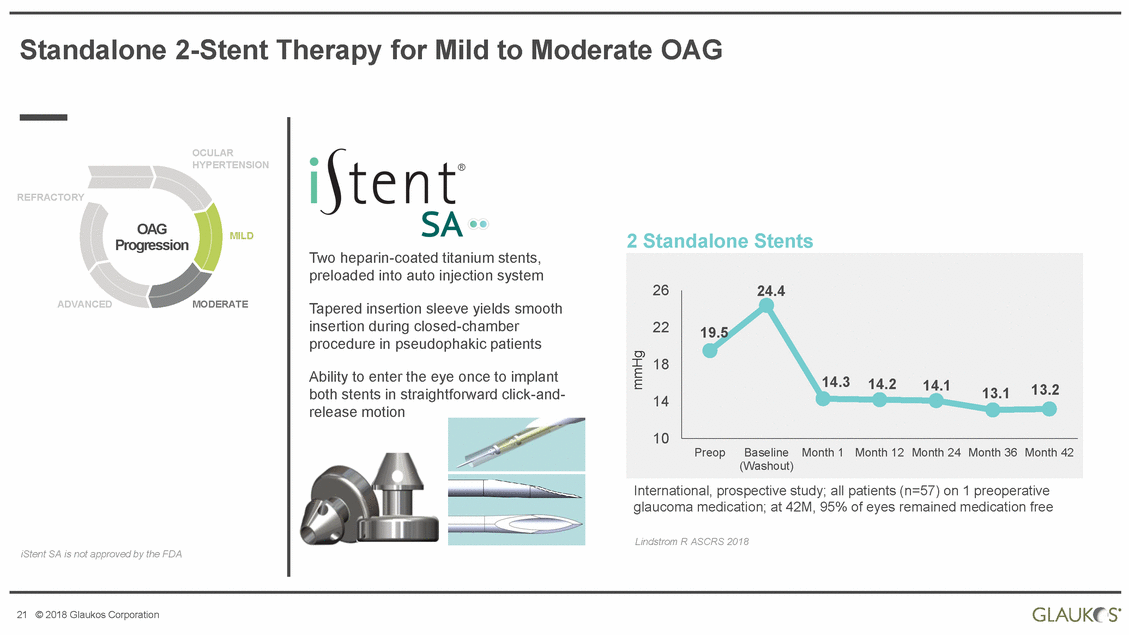
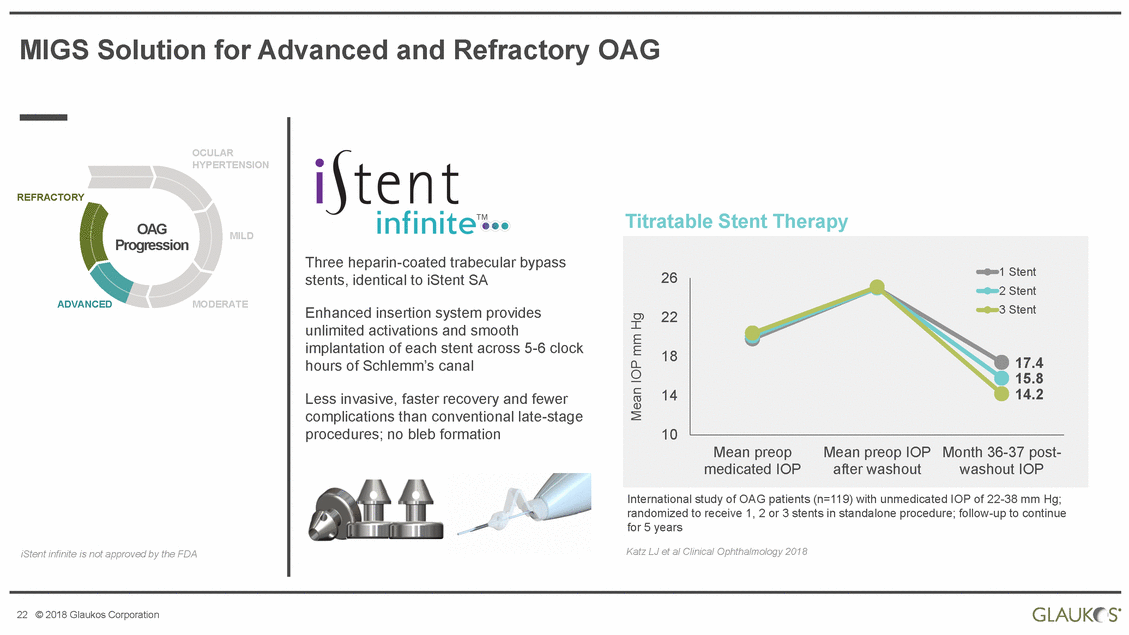
| Disclaimer All statements other than statements of historical facts included in this presentation that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements. Although we believe that we have a reasonable basis for forward-looking statements contained herein, we caution you that they are based on current expectations about future events affecting us and are subject to risks, uncertainties and factors relating to our operations and business environment, all of which are difficult to predict and many of which are beyond our control, that may cause our actual results to differ materially from those expressed or implied by forward-looking statements in this presentation. These potential risks and uncertainties include, without limitation, uncertainties about our ability to maintain profitability; our dependence on the success and market acceptance of the iStent®; our ability to leverage our sales and marketing infrastructure to increase market penetration and acceptance both in the United States and internationally of our products; our dependence on a limited number of third-party suppliers for components of our products; the occurrence of a crippling accident, natural disaster or other disruption at our primary facility, which may materially affect our manufacturing capacity and operations; maintaining adequate coverage or reimbursement by third-party payors for procedures using the iStent or other products in development; our ability to properly train, and gain acceptance and trust from, ophthalmic surgeons in the use of our products; our ability to successfully develop and commercialize additional products; our ability to compete effectively in the highly competitive and rapidly changing medical device industry and against current and future competitors (including MIGS competitors) that are large public companies or divisions of publicly traded companies that have competitive advantages; the timing, effect and expense of navigating different regulatory approval processes as we develop additional products and penetrate foreign markets; the impact of any product liability claims against us and any related litigation; the effect of the extensive and increasing federal and state regulation in the healthcare industry on us and our suppliers; the lengthy and expensive clinical trial process and the uncertainty of outcomes from any particular clinical trial; our ability to protect, and the expense and time-consuming nature of protecting, our intellectual property against third parties and competitors that could develop and commercialize similar or identical products; the impact of any claims against us of infringement or misappropriation of third party intellectual property rights and any related litigation; and the market’s perception of our limited operating history as a public company. These and other known risks, uncertainties and factors are described in detail under the caption “Risk Factors” and elsewhere in our filings with the Securities and Exchange Commission, including our Annual Report on Form 10-K for 2017 and Quarterly Report on Form 10-Q for the quarter ended March 31, 2018. Our filings with the Securities and Exchange Commission are available in the Investor Section of our website at www.glaukos.com or at www.sec.gov. In addition, information about the risks and benefits of our products is available on our website at www.glaukos.gov.All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on the forward-looking statements in this press release, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements whether as a result of new information, future events or otherwise, except as may be required under applicable securities law. 2 © 2018 Glaukos Corporation |