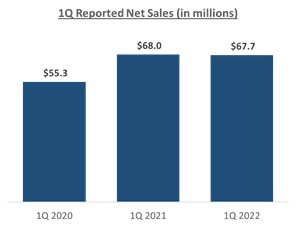
effective on January 1, 2022. Despite an impact to customer ordering patterns and competitive trialing activities in the first quarter, as expected, we’ve been very pleased with the execution of our commercial strategies and the resiliency of our combo-cataract iStent® franchise in the face of the reimbursement headwinds thus far in 2022.
Our commercial team continues to successfully train and educate our current and prospective surgeon customers on the favorable long-term risk-benefit profile of our iStent family of technologies and advance Micro-Invasive Glaucoma Surgery (MIGS) towards the standard of care for glaucoma.
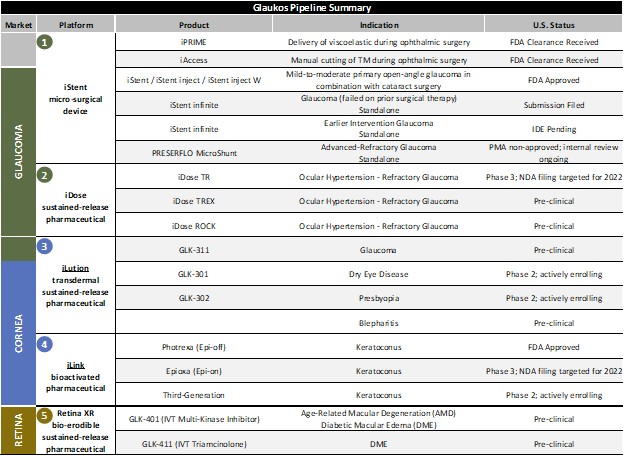
We remain focused on maximizing the access to our sight-saving technologies and overall care for glaucoma patients domestically. One such new technology is iAccess™, our new trabecular trephine device, for which we commenced full U.S. commercial launch activities in the latter part of the first quarter. Initial surgeon feedback on precision goniotomy with iAccess has been positive with surgeons most commonly citing the technology’s differentiated tissue-sparing, minimally-invasive approach to creating an extensive opening to Schlemm’s canal via numerous ectomies across multiple clock hours.
While we acknowledge that we may continue to face near-term headwinds in combination-cataract glaucoma domestically based on the cuts in professional reimbursement for trabecular stents that remain substantially below more invasive alternatives, we continue to feel confident in our ability to execute our strategy as we continue to bring complementary technologies to market in the quarters ahead. At the same time, we will remain prudent as it relates to forward guidance as we navigate the year ahead.
International Glaucoma
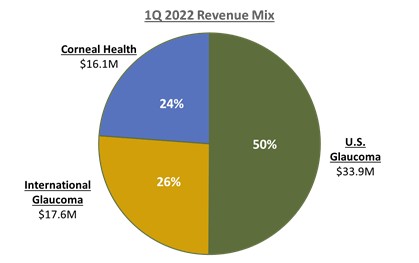
Our first quarter International Glaucoma net revenues were approximately $17.6 million, representing year-over-year growth of 28%, or 35% on a constant currency basis, versus 1Q 2021, which included a one-time unfavorable catch-up provision associated with government rebates in France.
Growth internationally during the first quarter was broad-based, but similar to the U.S., we did experience intermittent COVID disruptions during January and early February in particular across several key European and Asia-Pacific markets. Early launch activities of the PRESERFLO® MicroShunt® in Australia and Canada continue to go well and our overall performance in key markets such as Japan and France highlighted the strong quarter. We remain early in our penetration of the international opportunity and continue to make significant investments in our commercial sales and market access efforts in existing markets globally while selectively pursuing geographic expansion opportunistically.
Corneal Health
Our first quarter Corneal Health net revenues were approximately $16.1 million, representing year-over-year growth of 13% versus 1Q 2021.
The first quarter performance was driven by U.S. Photrexa® sales of $13.1 million along with a continued trend of healthy new U.S. Photrexa account starts, partially offset again by COVID-related headwinds and sporadic reimbursement volatility. Moving forward, our focus remains on executing our commercial and market development strategies and building upon the strong momentum we are experiencing within this emerging growth franchise for Glaukos.