NEPHROS, INC.
The Future of Fluid Filtration
Columbia University -
Audubon Technology Center
3960 Broadway
New York, NY 10032
PH: 212.781.5113
www.nephros.com
1

NEP: Forward Looking Statements
This presentation contains certain "forward-looking statements" within the meaning of the Private Securities
Litigation Reform Act of 1995, as amended. Such statements may include statements regarding the efficacy
and intended use of Nephros' technologies, the timelines for bringing such products to market and the
availability of funding sources for continued development of such products and other statements that are not
historical facts, including statements which may be preceded by the words "intends," "may," "will," "plans,"
"expects," "anticipates," "projects," "predicts," "estimates," "aims," "believes," "hopes," "potential" or
similar words. For such statements, Nephros claims the protection of the Private Securities Litigation
Reform Act of 1995. Forward-looking statements are not guarantees of future performance, are based on
certain assumptions and are subject to various known and unknown risks and uncertainties, many of which
are beyond the control of Nephros. Actual results may differ materially from the expectations contained in
the forward-looking statements. Forward-looking statements are not guarantees of future performance, are
based on certain assumptions and are subject to various known and unknown risks and uncertainties, many
of which are beyond the control of Nephros. Actual results may differ materially from the expectations
contained in the forward-looking statements.
2

Risk Factors
Factors that may cause such differences include the risks that Nephros may not be able: (i) to
obtain funding if and when needed or on favorable terms; (ii) to continue as a going concern; (iii)
to liquidate its investments when needed to fund its operations; (iv) to maintain compliance with
the AMEX's continued listing standards; (v) to demonstrate in pre-clinical or clinical trials the
anticipated efficacy, safety or cost savings of products that appeared promising to Nephros in
research or clinical trials; (vi) to obtain appropriate or necessary governmental approvals to
achieve its business plan or effectively market its products; (vii) to have its technologies and
products accepted in current or future target markets; or (viii) to secure or enforce adequate legal
protection, including patent protection, for its products. More detailed information about Nephros
and the risk factors that may affect the realization of forward-looking statements is set forth in
Nephros' filings with the SEC, including Nephros' Annual Report on Form 10-KSB for the fiscal
year ended December 31, 2007, and Nephros' Quarterly Report on Form 10-Q for the period
ended March 31, 2008. Investors and security holders are urged to read these documents free of
charge on the SEC's website at www.sec.gov .. Nephros does not undertake to publicly update or
revise its forward-looking statements as a result of new information, future events or otherwise.
3
ESRD (Dialysis) Therapy
Water Filtration
The Nephros Package
Driving ahead with two complementary filtration
technologies, with broad market opportunities:
4
Our advanced proprietary filter
designed specifically for
advanced-therapy
hemodiafiltration (HDF)
platforms.
Unsurpassed clinical
performance in our unique
dual-stage design.
Priced comparably to current
dialyzer products; over
100,000 treatments across
Europe so far.
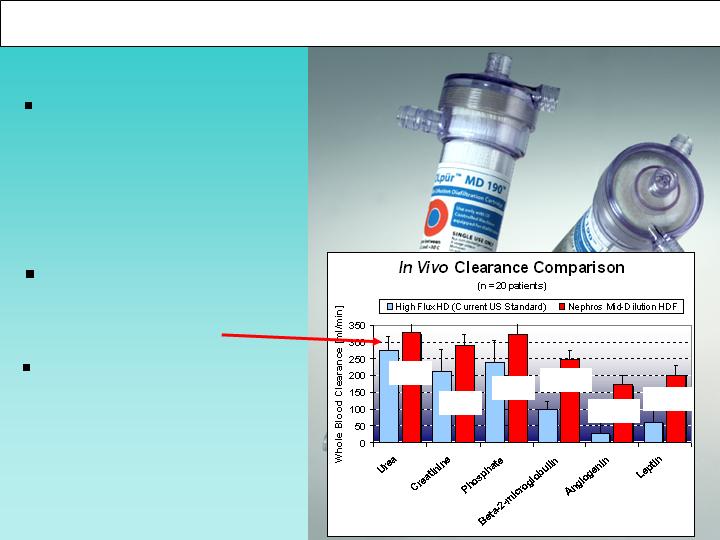
Nephros Delivers A Better Therapy: The OLpur MD Filter
18%
34%
149%
518%
221%
38%
5

Convenient add-on module
providing upgraded
hemodiafiltration capability
for standard HD machines,
with on-line real-time sterile
fluid.
Add-on
Module
Providing HDF Therapy in the US: The OLpur ™ H 2H™
Marketing “razor” for our
OLpur MD Filter
“razorblades.”
Safe, economical means to provide HDF therapy without replacing
existing dialysis machine base, a powerful economic and clinical
advantage .. US clinical trial now completed.
6

Nephros Hemodiafiltration (HDF):
Bringing a better therapy on-line…
Substantial drug
reductions
Fewer hospitalizations
Reduced infection risk
Improved patient nutrition
Improved quality of life
A 35%+
Reduction in
Mortality Risk
7

Nephros MD HDF: The Value Proposition
Appropriate for all patients
U.S. patient population includes incident (new)
patients; those with vascular access issues; pediatric
patients. Prime target markets.
Nephros is driving to be the predominant provider of
HDF therapy in the US by 2009.
ESRD patients population growth rate = 5-6% per year
HDF patients have reduced mortality risk of up to 35%
HDF patients have reduced need for certain drug
therapies; recent studies link epoetin use to cardiac
complications
MDHDF patients: a better quality of life
Baucus Medicare Improvements Act calls for bundling
of Medicare reimbursement by 2011, a key change
driving better therapy
8

Where are we today with Nephros
Hemodiafiltration?
U.S. clinical trial completed: 24 patients, 3 months using
Nephros Mid-Dilution Therapy.
Over 34,000 liters of fluid injected into patients, purified in real-
time by the Nephros H 2H module, with no adverse reactions
Now preparing FDA 510(k) submission; targeted no later than
3Q08; product launch targeted for 2Q09
New studies in Europe report that mid-dilution provides benefits
over post-dilution HDF and other therapies:
Improved middle molecule removal
Improved insulin resistance
Improved inflammatory response
Improved cardiovascular risk factors
Reduced co-morbidity risks
(for HDF generally over HD)
9

Our ESRD therapy research has
produced a cost-effective, robust
The Nephros Dual
Stage Ultrafilter (DSU)
effectively addresses a
range of biologically
contaminated water
problems…
Water Filtration Technology
10

Water Filtration Business Drivers
2 Million Hospital Acquired Infections per year in the U.S. alone, with
100,000 deaths and $30 BILLION in additional healthcare costs (over
$12,000 on average per case). Water has been identified as a primary
source of infection.
Recent study: 14 of 20 major U.S. hospitals tested positive for
legionella bacteria in the water system.
Studies indicate current approaches (heat disinfection, super-
chlorination) not 100% effective at eliminating legionella in older
hospital water systems.
States requiring hospitals to publish their infection rates.
Medicare is planning to reduce or eliminate reimbursement for certain
Hospital Acquired Infections, including legionella.
Municipal water systems continue to deteriorate; boil-water alerts on
the rise.
Disaster preparedness is high profile.
11

What does the Nephros DSU bring to the table?
Removes bacteria, fungi, viruses, parasites, and
biological toxins (including ricin, cryptosporidium,
and botulinum toxin), dramatically exceeding
purification requirements for potable water
Effective end-point filtration reduces or eliminates
the need for chlorination
Attains, and maintains, high flow rates
Provides proven durability combined with the
safety and reliability of true redundant filtration
Scalable to multiple delivery levels
12

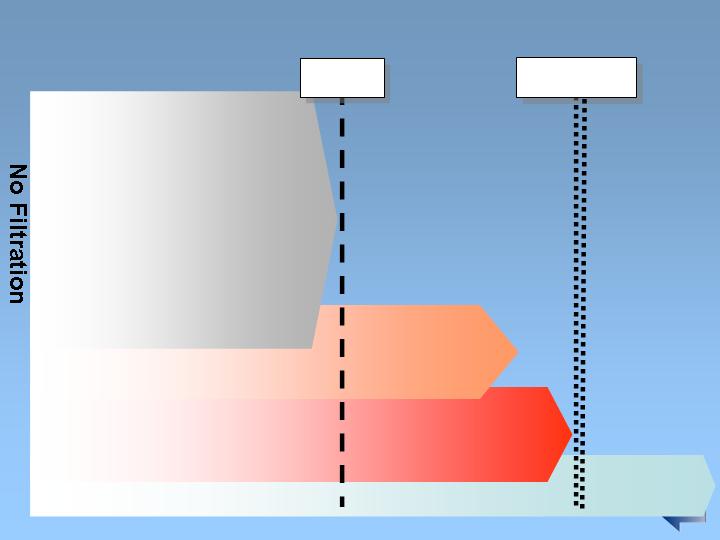
Nephros DSU substantially outperforms the
competition in endurance
Competition’s flow dropped
under the 1 gallon/minute
minimum in 30 minutes of use
Nephros DSU flow
sustained above 1.2
gallons/minute after more
than 4 weeks at 30
minutes/day
13
Water
Salts & Minerals
Clostridium Botulinum toxin
Ricin toxin
Staphylococcal enterotoxin B
Ebola virus
Smallpox; Polio
Hepatitis A, E
Giardia Lamblia
Cryptosporidium
Klebsiella pneumoniae
Bacillus anthracis
E. Coli & Salmonella
Vibrio cholerae
Legionella pneumoniae
Mycobacterium
Pseudomonas aeruginosa
BACTERIA PARASITES
Size
[um]
20
0.2
0.02
2
0.002
VIRUSES
0.2 um
Filters
Nephros
DSU
BIOLOGIC TOXINS
Nephros DSU substantially outperforms the competition in
removing bacteria, viruses and other toxins
14
The Nephros DSU:
Proven Stopping
Power
15

Nephros DSU: A New Water Filtration Standard
Reliability <<>> Purity <<>> Simplicity <<>> Adaptability
The difference between
unfiltered water and
Nephros Filtration in black
and white
16
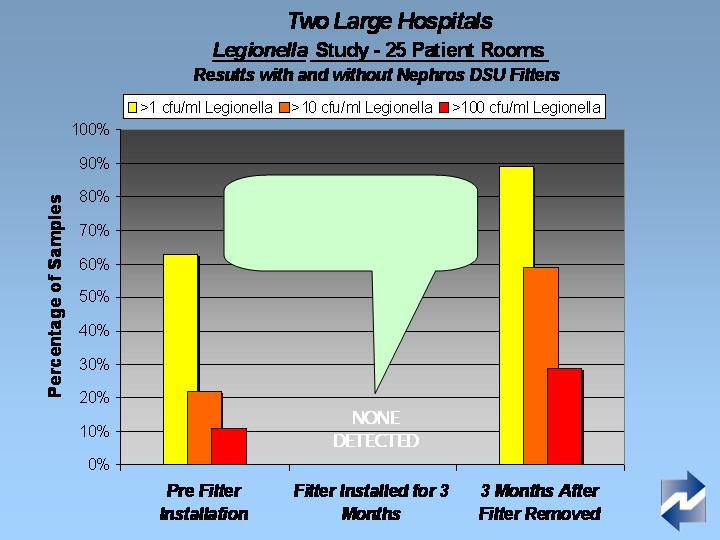
Where are we today with the Nephros DSU?
Tests at two major northeast hospitals demonstrated
product efficacy and durability
NSF testing to standard P231, microbiologically pure
water, to be completed this summer
Filter is currently in test at a major New York hospital
Currently commercializing and pursuing distribution
opportunities: hospital product launched June 15 ‘08 at
the APIC meeting, Denver
Pursuing opportunities in emergency management
$3 million military product development in progress in
conjunction with CamelBak Products and a research
university
17

The Nephros Opportunity
Complementary technologies with broad market
opportunities in ESRD therapy and water filtration
Business plan with defined goals
Management experience; a revitalized board and team
U.S. clinical trials now completed
Active distribution business with CE marked products in
Europe
Unique, advanced water filtration technology coming on-
line now
Strong and extensive patent coverage
Relative to comparables, a strong value opportunity
18

Financial Overview
19

Statement of Operations
Q1 2008
Net Revenues $387
Gross Profit $149
GP % 38.5%
Op Exp $1,925
Loss Ops ($1,776)
Other $136
Net Loss ($1,640)
Q1 2007
Net Revenue $296
Gross Profit $ 91
GP % 30.7%
Op Exp $1,609
Loss Ops ($1,518)
Other ($ 53)
Net Loss ($1,571)
20

Balance Sheet
March 31, 2008
Current Assets $3,724
L/T Assets $5,037
Current Liabs $1,532
L/T Liabs $ 0
Equity $7,229
December 31, 2007
Current Assets $9,296
L/T Assets $ 789
Current Liabs $1,329
L/T Liabs $ 0
Equity $8,756
21
NEPHROS, INC.
The Future of Fluid Filtration
Columbia University -
Audubon Technology Center
3960 Broadway
New York, NY 10032
PH: 212.781.5113
www.nephros.com
22