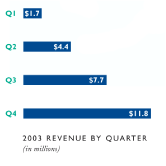| | | | | |
| | | OMB APPROVAL |
| | |
|
| | | OMB Number: | | 3235-0059 |
| | | Expires: | | February 28, 2006 |
| | | Estimated average burden
hours per response | 12.75 |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of the Securities
Exchange Act of 1934 (Amendment No. )
| |
| | Filed by the Registrant x |
| | Filed by a Party other than the Registrant o |
| |
| | Check the appropriate box: |
| |
| | o Preliminary Proxy Statement |
| | o Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
| | o Definitive Proxy Statement |
| | x Definitive Additional Materials |
| | o Soliciting Material Pursuant to §240.14a-12 |
Pharmion Corporation
(Name of Registrant as Specified In Its Charter)
(Name of Person(s) Filing Proxy Statement, if other than the Registrant) Payment of Filing Fee (Check the appropriate box):
| |
| | x No fee required. |
| | o Fee computed on table below per Exchange Act Rules 14a-6(i)(4) and 0-11. |
| |
| | 1) Title of each class of securities to which transaction applies: |
| |
| | 2) Aggregate number of securities to which transaction applies: |
| |
| | 3) Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): |
| |
| | 4) Proposed maximum aggregate value of transaction: |
| |
| | o Fee paid previously with preliminary materials. |
| |
| | o Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| |
| | 1) Amount Previously Paid: |
| |
| | 2) Form, Schedule or Registration Statement No.: |
| |
| SEC 1913 (02-02) | Persons who are to respond to the collection of information contained in this form are not required to respond unless the form displays a currently valid OMB control number. |
This filing includes supplemental material that was included when we mailed our definitive proxy materials. This information does not impact our definitive proxy materials and is provided only for supplemental purposes.

2003 was a pivotal year for Pharmion.
TO OUR SHAREHOLDERS:
2003 was a pivotal year for Pharmion. In addition to the achievement of major milestones for our two lead development product candidates, which included our submission to the U.S. Food and Drug Administration of a new drug application for Vidaza™, the initiation of named patient and compassionate use sales in Europe for Thalidomide Pharmion 50mg™ and the approval of thalidomide in Australia and New Zealand, we completed our initial public offering (IPO) in November. We want to thank our investors for giving us the opportunity to prove ourselves — and also to let you know that this is a responsibility that we take very seriously.
Our focus is on delivering innovative products for clinicians and the patients they treat — and we believe that if we do this well, we, and our investors, will see tangible results. In this letter, I will briefly describe our strategies, goals and the milestones investors can look for to determine how successful we are in achieving our objectives. Our enclosed Form 10-K can provide you with greater detail.
STRONG FINANCIAL PROGRESS IN 2003
Net sales for 2003 totaled $25.5 million, including thalidomide sales of $15.6 million, which represented a fourfold increase over total net sales of $4.7 million for 2002. Sales growth was driven by the Company’s initiation of named patient and compassionate use sales of thalidomide during the second quarter of 2003.
Pharmion reported a 2003 net loss of $(50.1) million compared to a net loss of $(34.7) million in 2002. On a GAAP basis, Pharmion reported a net loss per share attributable to common stockholders of $(14.70) for 2003 compared to $(57.58) for 2002. The pro forma net loss per share attributable to common stockholders would have been ($2.66) for the full year of 2003 compared to $(2.47) for 2002, had the conversion of Pharmion’s outstanding shares of redeemable convertible preferred stock taken place on January 1, 2002. The actual conversion took place in connection with Pharmion’s initial public offering on November 12, 2003.
We ended 2003 with $88.5 million in cash and cash equivalents, which included $76.2 million in net proceeds from our IPO. Earlier in 2003, we raised $14 million in convertible debt, which was converted into shares of common stock in March 2004, adding 1.3 million shares, or approximately five percent, to our total shares outstanding.
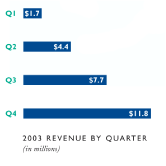
| | | | | | | | | | | | | | | |
| Pharmion Corporation | | • 2525 28th Street | | • Boulder, CO 80301 | | • U S A | | / | | Tel: 720.564.9100 | | Fax: 720.564.9191 | | www.pharmion.com |

We achieved a number of significant regulatory and commercial milestones during the year, including the following:
| • | | Commenced named patient and compassionate use sales of thalidomide in Europe and other international markets; |
| |
| • | | Established the Pharmion Risk Management Program (PRMP™), now offered in more than 20 languages, modeled after Celgene Corporation’s U.S. program (S.T.E.P.S.™) which has been used successfully for more than five years to provide for the safe distribution and appropriate use of thalidomide; |
| |
| • | | Launched Thalidomide Pharmion 50mg in Australia and New Zealand following marketing approval in those countries for the treatment of relapsed/refractory multiple myeloma, which represent the first approvals worldwide for thalidomide in that indication; |
| |
| • | | Acquired Laphal Développement, which significantly expanded the scope of Pharmion’s efforts to obtain marketing approval and commercialize thalidomide in Europe and other international markets; |
| |
| • | | Submitted our New Drug Application (NDA) for Vidaza, which was accepted for filing with Priority Review status by the U.S. Food and Drug Administration in February 2004; and |
| |
| • | | Initiated a confirmatory clinical trial comparing the effect of Vidaza to conventional care options on survival in patients with Myelodysplastic Syndromes (MDS), which we believe represents the largest study to date for this disease. |
These accomplishments reinforce our corporate strategy, which I’ll describe for those of you less familiar with us. Pharmion is a pharmaceutical company focused on acquiring, developing and commercializing innovative products for the treatment of hematology and oncology patients in the U.S., Europe and other international markets. We believe there are significant opportunities within this market, and that we are in a unique position to take advantage of these opportunities based on the depth of our global regulatory expertise and commercial capabilities. Our strategy has four key components, including:
| 1. | | Focusing on the hematology and oncology markets, where there is significant unmet medical need, the potential for expedited regulatory reviews and a targeted physician population that can be addressed with a relatively small sales force. |
| |
| 2. | | Seeking to acquire not only commercial products, but development candidates that help us build a strong pipeline; to date, we have licensed four products. |
| |
| 3. | | Utilizing our significant global regulatory and development expertise to bring products to market in the U.S., Europe and many additional international markets. |
| |
| 4. | | Leveraging our U.S. and international commercial organization, which includes a direct sales force in the U.S., Europe and Australia, and distributor partners in 20 additional countries, to realize the full potential of our products. |
While this represents a long-term strategy that we believe is just beginning to bear fruit, we have identified a number of goals for 2004 that serve as milestones for our success. These 2004 goals include the following:
| • | | The FDA approval and launch of Vidaza in the U.S., which, if approved, would be the first drug available in the U.S. for the treatment of MDS; |
| |
| • | | The continued growth of named patient and compassionate use sales of thalidomide in Europe and other international markets, with an ongoing commitment to the safe distribution and use of the drug; |
| |
| • | | Commencement of the regulatory approval process in Europe for Vidaza, and the initiation of named patient and compassionate use sales in Europe to address the unmet medical need for patients with MDS; and |
| |
| • | | The acceleration of our search for new products and product candidates that will expand our pipeline. |
In summary, we made substantial progress in 2003, and we look forward to building on that progress in 2004. We could not have accomplished all that we have without the support of our employees worldwide, as well as our shareholders, our board members, and the clinicians and patients who use our products. We have built a tremendous team at Pharmion and I am honored by their commitment and energized by the work we have before us.
Best regards,
Patrick J. Mahaffy
May 5, 2004
| | | |
| 2000 | | Pharmion incorporated in Delaware |
| | | |
| 2001 | | Licensed azacitidine (now Vidaza) from Pharmacia
Licensed thalidomide from Celgene |
| | | |
| 2002 | | Licensed Refludan® from Schering AG
Licensed Innohep® from LEO Pharma
Relaunched Innohep in the U.S. |
| | | |
| 2003 | | Commenced sales of thalidomide in EU for compassionate use
Thalidomide Pharmion 50mg approved in Australia and NZ
Raised $76.2M in IPO
Submitted NDA for Vidaza |
Pharmion is a pharmaceutical company focused on acquiring, developing and and commercializing innovative products or the treatment of hematology and oncology patients in the U.S., Europe and other international markets.
SHAREHOLDER INFORMATION
| | | | | |
| | Pharmion Corporate
| | |
Board of Directors
| | Headquarters
| | Shareholder Inquiries
|
| | | | |
PATRICK J. MAHAFFY
President and Chief Executive Officer
Pharmion Corporation
JUDITH A. HEMBERGER, PH.D.
Executive Vice President
and Chief Operating Officer
Pharmion Corporation
JAMES BLAIR, PH.D.
General Partner
Domain Associates, L.L.C.
CAM L. GARNER
Former Chairman and Chief Executive Officer
Dura Pharmaceuticals, Inc.
DR. THORLEF SPICKSCHEN
Former Chairman and Chief Executive Officer
BASF Pharma/Knoll AG
BRIAN G. ATWOOD
Co-Founder
Versant Ventures
M. JAMES BARRETT, PH.D.
General Partner
New Enterprise Associates
JAY MOORIN
Co-Founder and Partner
ProQuest
| | 2525 28th Street
Boulder, Colorado 80301
(720) 564-9100 (phone)
(720) 564-9191 (fax)
www.pharmion.com
Annual Meeting
The Pharmion annual meeting of shareholders will be held on Wednesday, June 2, 2004 at 8:30 a.m. at the Hotel Boulderado, 2115 13th Street, Boulder, Colorado 80302.
Transfer Agent and Registrar
American Stock Transfer
& Trust Company
59 Maiden Lane
New York, New York 10038
Independent Auditors
Ernst & Young LLP
Denver, Colorado
Investor Relations
Breanna Burkart
or Anna Sussman
720.564.9150
ir@pharmion.com
| | Inquiries related to stock transfers or lost certificates should be directed to American Stock Transfer & Trust Company,
(800) 937-5449 or
(212) 936-5100.
Forward-Looking Statements
This letter, particularly the discussion of our outlook, contains statements about expected future events that are forward-looking and subject to risks and uncertainties. Factors that could cause actual results to differ and vary materially from expectations can be found in Pharmion’s with the SEC. Forward-looking statements speak only as of the date on which they are made, and Pharmion undertakes no obligation to update publicly or revise any forward-looking statement, whether as a result of new information, future developments or otherwise. |
Executive Officers | | | | |
| | | | | |
PATRICK J. MAHAFFY | | | | |
| President and Chief Executive Officer | | | | |
| | | | | |
JUDITH A. HEMBERGER, PH.D.
Executive Vice President
and Chief Operating Officer | | | | |
| | | | | |
ERLE T. MAST | | | | |
| Chief Financial Officer | | | | |
| | | | | |
GILLIAN C. IVERS-READ | | | | |
| Vice President, Clinical Development | | | | |
| and Regulatory Affairs | | | | |
| | | | | |
MICHAEL COSGRAVE | | | | |
| Vice President, | | | | |
| International Commercial Operations | | | | |